Significance
In addition to mediating protein traffic from the endoplasmic reticulum to the Golgi apparatus, coat protein complex II (COPII) vesicles also act constructively as a membrane source for autophagosome biogenesis during autophagy to maintain cellular homeostasis in eukaryotes. Nonetheless, the participation and underlying mechanism of the COPII machinery in the autophagic pathway and autophagosome biogenesis remain elusive in plants. Here, we identified and characterized a distinct population of COPII vesicles consisting of a unique AtSar1d-AtRabD2a nexus, which is required for autophagosome biogenesis through mechanistic connection with autophagy-related (ATG) machinery in Arabidopsis. Our results broadened the understandings of the mechanism underlying COPII function in eukaryotic autophagy and manifested the functional diversity of specific COPII paralogs in plant autophagy and autophagosome biogenesis.
Keywords: autophagy, coat protein complex II, Sar1, Rab GTPase, plant stress
Abstract
In eukaryotes, secretory proteins traffic from the endoplasmic reticulum (ER) to the Golgi apparatus via coat protein complex II (COPII) vesicles. Intriguingly, during nutrient starvation, the COPII machinery acts constructively as a membrane source for autophagosomes during autophagy to maintain cellular homeostasis by recycling intermediate metabolites. In higher plants, essential roles of autophagy have been implicated in plant development and stress responses. Nonetheless, the membrane sources of autophagosomes, especially the participation of the COPII machinery in the autophagic pathway and autophagosome biogenesis, remains elusive in plants. Here, we provided evidence in support of a novel role of a specific Sar1 homolog AtSar1d in plant autophagy in concert with a unique Rab1/Ypt1 homolog AtRabD2a. First, proteomic analysis of the plant ATG (autophagy-related gene) interactome uncovered the mechanistic connections between ATG machinery and specific COPII components including AtSar1d and Sec23s, while a dominant negative mutant of AtSar1d exhibited distinct inhibition on YFP-ATG8 vacuolar degradation upon autophagic induction. Second, a transfer DNA insertion mutant of AtSar1d displayed starvation-related phenotypes. Third, AtSar1d regulated autophagosome progression through specific recognition of ATG8e by a noncanonical motif. Fourth, we demonstrated that a plant-unique Rab1/Ypt1 homolog AtRabD2a coordinates with AtSar1d to function as the molecular switch in mediating the COPII functions in the autophagy pathway. AtRabD2a appears to be essential for bridging the specific AtSar1d-positive COPII vesicles to the autophagy initiation complex and therefore contributes to autophagosome formation in plants. Taken together, we identified a plant-specific nexus of AtSar1d-AtRabD2a in regulating autophagosome biogenesis.
Autophagy is a conserved catabolic process characterized by the de novo generation of a double-membrane structure called an autophagosome with a fundamental function in the bulk turnover of cytoplasmic components, including proteins, RNAs, and organelles. Genetic studies in yeast have elucidated the molecular machinery of autophagy, whereby 42 autophagy-related (ATG) genes have been identified (1–3). These ATG genes are highly conserved among eukaryotes but often have multiple isoforms in other higher organisms, in particular in sessile plants. Albeit increasing understanding on the molecular function of Atg proteins in acting hierarchically on the phagophore assembly site (PAS) to produce autophagosomes, the origin of the autophagosomal membrane remains unclear in higher eukaryotes. Furthermore, the dedication of other membranes and machineries in the autophagy pathway remains under investigation.
Plant autophagy is known to play important roles in the sessile lifestyle of plants, participating in seed germination, seedling establishment, plant development, hormone responses, lipid metabolism, and reproductive development (4). Plant autophagy research is advancing with findings not only on the counterparts of the yeast/mammalian Atg proteins but also dealing with some plant-unique factors functioning in different steps of autophagosome biogenesis, thereby uncovering novel mechanisms that might or might not be conserved in nonplant species (5). More interestingly, higher plants possess multiple protein isoforms of ATG machinery, whose functional heterogeneity in the autophagy pathway has only recently been unveiled (6).
The coat protein complex II (COPII) machinery consists of five cytosolic components: the small GTPase Sar1, the inner coat protein dimer Sec23-Sec24, and the outer coat proteins Sec13-Sec31. These proteins are essential for COPII-coated vesicle formation, which buds from specialized regions of the ER, namely ER exit sites (ERESs) (7). Under nutrient-rich conditions, COPII vesicles mediate anterograde ER to Golgi transport. However, increasing evidence from yeast and mammals suggests that the COPII machinery or even COPII vesicles themselves may contribute to autophagosome formation when cells are starved for nutrients (8–16). Gene duplication events have occurred substantially in sessile plants during evolution, and the importance of distinct paralogs in environmental stress adaptation during plant development has been implied (17). Arabidopsis encodes multiple COPII paralogs in its genome, including five Sar1s, seven Sec23s, three Sec24s, two Sec13s, and two Sec31s (17). Increasing numbers of studies have pinpointed the functional diversity and importance of distinct COPII paralogs in ER protein export (18–23). Nonetheless, the mechanism by which COPII vesicles are redirected to the autophagy pathway upon nutrient starvation, and their roles in autophagosome biogenesis, remains unclear. Furthermore, the participation of specific COPII paralogs in autophagy regulation remains unknown in plants.
Here, we report on a role of a specific Sar1 homolog, AtSar1d, that modulates plant autophagosome biogenesis in concert with AtRabD2a. Large-scale proteomic analysis of the ATG interactome has revealed possible mechanistic connections between the ATG machinery and specific COPII components in plants. Cellular and biochemical analyses have shown that the dominant negative (DN) mutant of AtSar1d (AtSar1dDN) specifically perturbs YFP-ATG8 vacuolar degradation upon autophagic induction. Consistently, a transfer DNA (T-DNA) insertion mutant of AtSar1d exhibited starvation-related phenotypes. Notably, AtSar1d regulates autophagosome progression through specific recognition of ATG8e by a previously uncharacterized noncanonical motif. We further identify a plant-unique Rab1/Ypt1 homolog AtRabD2a that colocalizes with AtSar1d and ATG8 upon starvation by transient expression in Arabidopsis protoplasts. A DN mutant of AtRabD2a (AtRabD2aNI) perturbs autophagy flux, while AtRabD2a is indispensable for bridging the AtSar1d-positive COPII vesicles with the ATG1 complex, thus contributing to autophagosome biogenesis in plants. Our study therefore unequivocally demonstrates that the plant-specific COPII machinery regulates autophagosome biogenesis and sheds light on the evolutionary importance of gene duplication events in the plant autophagy pathway.
Results
The ATG Interactome Reveals a Functional Role of the COPII Vesiculating Machinery in Plant Autophagy.
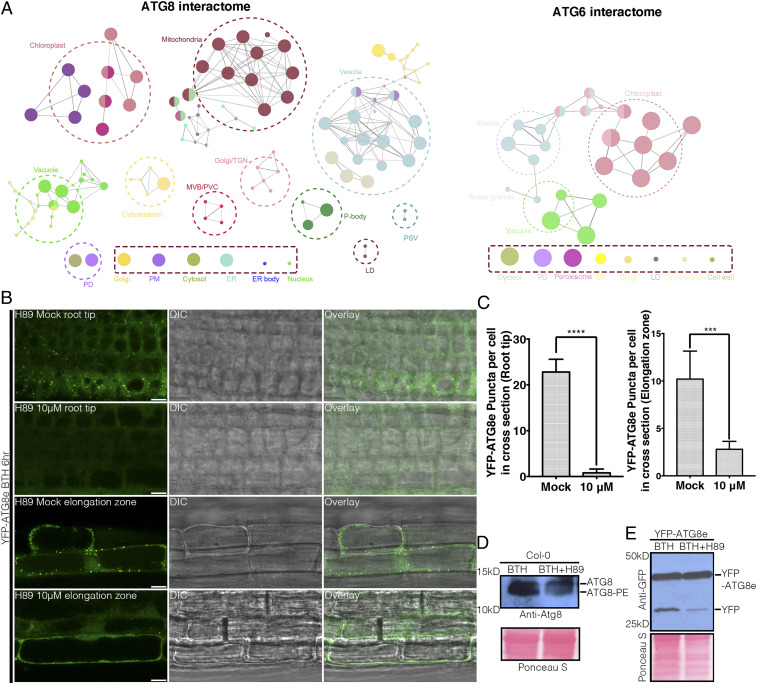
In yeast, a forward proteomic approach has demonstrated that the autophagic machinery and ERES components share various functionally related protein interactors (8). However, the roles of the early secretory pathway, especially ERES and the COPII machinery, in autophagosome formation remain unknown in higher plants. To address this, we performed green fluorescent protein (GFP)-trap–based pull-down assays and large-scale proteomic analyses using Arabidopsis transgenic plants expressing YFP-ATG6 and YFP-ATG8 upon nutrient deprivation. A total of 157 interactors of ATG6 and 1,527 interactors of ATG8 have been identified, while 127 proteins mutually interacted with both ATG6 and ATG8 (SI Appendix, Fig. S1A). Gene ontology enrichments and ClueGO network analysis showed that a variety of vesicle-related machinery components are potential ATG interactors (Fig. 1A). Intriguingly, multiple specific COPII and COPI paralogs were identified in the ATG6 and ATG8 interactome (SI Appendix, Fig. S1B). To verify the reliability of these pull-down and proteomic assays, we performed transient expression assays in Arabidopsis protoplasts. Indeed, the potential ATG6 interactor AtSec23f and its close homolog AtSec23b showed a colocalization with ATG6 (SI Appendix, Fig. S2A). Furthermore, yeast two-hybrid (Y2H) and coimmunoprecipitation (Co-IP) assays validated the interaction between ATG6 and AtSec23b or AtSec23f, respectively (SI Appendix, Fig. S2 B and C). To demonstrate the involvement of the COPII machinery in plant autophagosome biogenesis, we applied the serine/threonine kinase inhibitor H89 to abolish the membrane association of Sar1, thereby inhibiting COPII polymerization by interfering with the recruitment of the inner COPII coat protein Sec23/24. Low concentrations of H89, which exhibited little effect on ER protein export, strongly inhibited autophagosome formation upon autophagy induction by benzothiadiazole (BTH) treatment (Fig. 1 B and C and SI Appendix, Fig. S3), an analog of salicylic acid which has been shown to trigger autophagy (24). ATG8 lipidation and YFP-ATG8e vacuolar turnover were also inhibited significantly upon a low-dose application of H89 followed by BTH treatment (Fig. 1 D and E). Similarly, H89 inhibited autophagosome biogenesis upon nutrient deprivation as demonstrated by confocal analysis, ATG8 lipidation, and a YFP-ATG8e turnover assay (SI Appendix, Fig. S4). Taken together, this proves a molecular link between the COPII machinery and autophagy in plants.
Fig. 1.
The early secretory pathway regulates plant autophagy (A) Transgenic Arabidopsis seedlings that were 5 d old expressing ATG6-YFP and YFP-ATG8e were subjected to GFP-trap following by liquid chromatography–tandem mass spectrometry analysis to identify potential interactors. Gene ontology enrichments and the ClueGO network were used for an ATG6 and ATG8e interactor analysis based on cellular components. PD, plasmodesmata; PM, plasma membrane; TGN, trans-Golgi network; ER, endoplasmic reticulum; LD, lipid droplet; PSV, protein storage vacuole; MVB/PVC, multivesicular body/prevacuolar compartment. (B) Transgenic Arabidopsis seedlings that were 5 d old expressing YFP-ATG8e were subjected to BTH with or without 10 μM H89 (a serine/threonine kinase inhibitor that abolishes membrane recruitment of Sar1 GTPase) before confocal imaging. (Scale bars, 10 μm.) (C) Quantification of autophagosome numbers in B with at least three replicates. (D) Wild-type 5 d old Arabidopsis seedlings were treated with or without 10 μM H89 and BTH before protein extraction to detect the ATG8 lipidation using Atg8 antibodies. (E) Transgenic Arabidopsis seedlings that were 5 d old expressing YFP-ATG8e were treated with or without 10 μM H89 and BTH followed by protein extraction and immunoblot detection of ATG8 vacuole turnover using GFP antibodies. BTH, benzothiadiazole. H89, N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulphonamide.
Functional Heterogeneity of Distinct Sar1 Paralogs in the Plant Autophagy Pathway.
Arabidopsis encodes multicopies of COPII paralogs in its genome while increasing evidences have suggested their importance in plant development and stress responses (17). However, functional roles of specific COPII components in regulating autophagy pathway and autophagosome formation remain unknown in plants. Since our proteomic analysis identified several specific COPII paralogs in the ATGs interactome, we therefore asked which of the specific Sar1 paralogs exhibited a distinct function in regulating autophagy. Using transient expression assays, we showed that AtSar1d and AtSec23f were substantially colocalized with the bona fide autophagy marker ATG8e upon long-term culture for over 24 h (SI Appendix, Fig. S5). To elucidate the possible function of AtSar1d in autophagy, we generated double transgenic Arabidopsis plants coexpressing YFP-ATG8e/AtSar1d-mRFP or mCherry-ATG8e/AtSar1c-GFP. Consistent with the transient expression data, AtSar1d substantially colocalized with ATG8e upon autophagy induction as compared with AtSar1c in planta (Fig. 2 A and C). Further, we generated transgenic plants coexpressing mCherry-ATG8e and the DN mutant of AtSar1d or AtSar1c driven by the dexamethasone (DEX) inducible promoter. Strikingly, AtSar1dDN showed strong inhibition on autophagic flux upon DEX induction with BTH and ConcA treatment (Fig. 2 B and D). In sharp contrast, transgenic plants expressing AtSar1cDN merely perturbed the vacuolar delivery of mCherry-ATG8e upon DEX induction in the presence of BTH and ConcA (Fig. 2 B and D). Similarly, AtSar1dDN exhibited strong inhibition on vacuolar delivery of mCherry-ATG8e upon nitrogen starvation (SI Appendix, Fig. S6). Consistent with the confocal analysis, immunoblot confirmed that AtSar1dDN, but not AtSar1cDN, perturbed mCherry-ATG8e vacuolar turnover in the transgenic plants (Fig. 2 E and F). Transmission electron microscopy (TEM) analysis further confirmed the defects in autophagosome biogenesis in AtSar1dDN transgenic plants upon BTH and ConcA treatment, while AtSar1cDN merely perturbed the autophagosome formation in plants (SI Appendix, Fig. S7). Likewise, AtSar1a, which has been shown to play a unique function in ER protein export upon stress, exhibited little inhibition on autophagosome formation and vacuolar degradation of autophagic flux upon BTH treatment (SI Appendix, Fig. S8). Albeit a transient expression assay using leaf protoplasts isolated from AtSar1dDN mutants showed that AtSar1dDN could also partially inhibit ER export of vacuolar cargo Aleurain-mRFP upon long-time DEX induction under normal growth condition, AtSar1dDN strongly perturbed autophagosome formation upon autophagic induction, while the ER export remained normal (SI Appendix, Fig. S9). Taken together, AtSar1d specifically regulates autophagosome biogenesis in Arabidopsis.
Fig. 2.
AtSar1d regulates autophagosome biogenesis in Arabidopsis (A) Transgenic Arabidopsis seedlings coexpressing AtSar1d-mRFP/YFP-ATG8e or AtSar1c-GFP/mCherry-ATG8e were subjected to mock or autophagy inductions before confocal laser scanning microscopy observation. (Scale bars, 10 μm.) (B) Transgenic Arabidopsis seedlings coexpressing the DN mutants of AtSar1d (AtSar1dDN) or AtSar1c (AtSar1cDN) under the control of the DEX-inducible promoter and mCherry-ATG8e were subjected to autophagy inductions by BTH and ConcA treatments followed by confocal imaging. (Scale bars, 10 μm.) (C) Quantification of colocalization ratio in A. (D) Quantification of vacuolar turnover of ATG8e in B. (E) Transgenic Arabidopsis seedlings that were 5 d old coexpressing Dex::AtSar1cDN-GFP or Dex::AtSar1dDN-GFP with mCherry-ATG8e were treated with BTH (+B) or DEX and BTH (+D+B) followed by protein extraction and subsequent immunoblot analysis using red fluorescent protein (RFP)/GFP antibodies for ATG8 vacuolar turnover analysis. The bands of the mCherry core represented the vacuolar turnover of ATG8, as full-length mCherry-ATG8 would be delivered to vacuole and degraded into mCherry. (F) Quantification of ratio of mCherry-ATG8e in E was done by calculating the full-length mCherry-ATG8e over the total amount of mCherry and mCherry-ATG8e. The intensity of bands was quantified using ImageJ. ConcA, Concanamycin A.
AtSar1d Is Indispensable for Nutrient Starvation in Plants.
Plant autophagy is essential for cells to survive in nutrient-limited conditions, whereas perturbation of the ATG core machinery gives rise to a defective phenotype during plant development (25). To further confirm the bona fide function of AtSar1d in the plant autophagy pathway, an AtSar1d T-DNA insertion mutant sar1d-1 was obtained and sequenced by next-generation sequencing (SI Appendix, Fig. S10 A and B). Under long-day conditions, the sar1d-1 mutant had an indistinguishable phenotype to Col-0 and atg7-1 (SI Appendix, Fig. S10C). Nonetheless, compared to Col-0, the sar1d-1 mutant plants exhibited a defective phenotype upon nitrogen starvation, which is similar to atg7-1 mutants (Fig. 3A). In contrast, overexpression of AtSar1d-GFP can complement the defects in sar1d-1 mutants upon starvation (Fig. 3A). Chlorophyll content measurements further confirmed these observations (Fig. 3B). Our results showed that the AtSar1d mutant exhibited an autophagy-related phenotype, and AtSar1d may specifically participate in the autophagy pathway.
Fig. 3.
Perturbation of AtSar1d exhibits nutrient starvation-related phenotypes (A) Phenotypic analysis of Col-0, atg7-1, and sar1d-1 mutants and the AtSar1d-GFP/sar1d-1 complement line upon nitrogen starvation. (B) Quantification of chlorophyll contents in A with at least three replicate experiments.
AtSar1d Regulates Autophagosome Progression by Recognition of the ATG Machinery.
To further elucidate the underlying mechanism of AtSar1d in plant autophagosome biogenesis, we floral dipped YFP-ATG8e into Col-0 and sar1d-1 mutant plants, respectively. Under normal conditions, YFP-ATG8e mainly displayed a cytosolic pattern in both Col-0 and sar1d-1 mutant plants (Fig. 4 A and B). Upon BTH treatment, in YFP-ATG8e plants in the Col-0 background, substantial numbers of positive punctae were formed, while less punctate structures emerged in sar1d-1 mutants (Fig. 4 A and B). Notably, the number of mature ring-like autophagosomes decreased significantly in sar1d-1 mutants upon autophagic induction compared to the Col-0 plants (Fig. 4 A and C). Consistently, less ATG8e-positive punctae and mature autophagosomes formed in sar1d-1 mutant plants upon nitrogen starvation (SI Appendix, Fig. S11). Indeed, TEM analysis showed that more autophagosomes of smaller size were found, while the autophagic flux into the vacuole was decreased in sar1d-1 mutants upon BTH treatment (Fig. 4 D–F). Three-dimensional (3D)-TEM analysis further confirmed these observations (Fig. 4G and Movie S1). In yeast, phosphorylation of sec24 reprograms COPII vesicles for the autophagy pathway, while the sec24 mutant does not perturb the ATG protein assembly at the PAS (11). We therefore investigated whether the sar1d-1 mutant would perturb the ATG assembly and phagophore membranes expanding in Arabidopsis. A transient expression assay using leaf protoplasts isolated from the sar1d-1 mutant showed that the ATG1 complex component ATG13a as well as the transmembrane protein ATG9 remained sufficiently colocalized with ATG8e, suggesting that the mutation of AtSar1d did not perturb the ATG assembly (SI Appendix, Fig. S12). A recent study using a COPII vesicle-labeling system in yeast suggested COPII vesicles as a membrane source for autophagosomes (14). Notably, transient expression analysis using Arabidopsis protoplasts showed that AtSar1d associated with the ER network and colocalized with the bona fide ER exit site marker AtSec24a (SI Appendix, Fig. S13A) (19). Upon prolonged culture, AtSar1d-eYFP showed substantial colocalization with mCherry-ATG8e, while few colocalizations of AtSar1d-eYFP with mCherry-ATG8e were observed in cells overexpressing the Sar1 guanine nucleotide exchange factor Sec12, whose overexpression could inhibit COPII vesicles budding (SI Appendix, Fig. S13 B and C). Live-cell imaging further revealed that AtSar1d-eYFP puncta transported from ER to ATG8e positive structures (SI Appendix, Fig. S13D and Movie S2). Our results therefore demonstrated that AtSar1d-positive COPII vesicles may act as a membrane source and are crucial for autophagosome progression.
Fig. 4.
Autophagosome progression is perturbed with accumulation of smaller size autophagosomes (APs) in the sar1d-1 mutant (A) Arabidopsis seedlings expressing YFP-ATG8e under Col-0 or the sar1d-1 mutant background were subjected to mock or autophagy induction by BTH before confocal laser scanning microscopy observation. (Scale bars, 10 μm.) (B) Quantification of APs in A with at least three replicates. (C) Quantification of mature APs in A with at least three replicates. (D) TEM thin section analysis of high-pressure frozen 5 d old Col-0 or sar1d-1 mutant Arabidopsis root tips upon BTH and ConcA treatment. (Scale bars, 500 nm.) Arrowheads indicated examples of autophagic bodies in vacuole. AP, Autophagosome; V, Vacuole. (E) Quantification of autophagic bodies in vacuole in D with at least three replicates. (F) Quantification of autophagosome sizes in D with at least three replicates. (G) 3D electron tomography analysis of autophagosomal structures in the sar1d-1 mutant upon BTH treatment. (Scale bars, 500 nm.)
In mammals, a cross-talk between the autophagic PI3K and the COPII machinery generates ER–Golgi intermediate compartment–derived COPII vesicles, which are active as a membrane source for LC3 lipidation and are essential for autophagosome biogenesis (10, 16). Interestingly, a recent study revealed that the plant Sar1 paralog may contain a noncanonical motif to interact with ATG8 (26). Indeed, our proteomic analysis demonstrated that AtSar1d is a potential candidate of ATG8 interactors, and this was further confirmed by Co-IP analysis under normal and autophagy-induced conditions (Fig. 5A and SI Appendix, Fig. S14). To elaborate the mechanistic connection between AtSar1d and ATG8, we performed homology modeling between AtSar1d and ATG8e. Interestingly, a flexible region, which is variable among all Arabidopsis Sar1 paralogs in the C terminus, is in close proximity to the potential interaction surface with ATG8e (Fig. 5 B and C and SI Appendix, Fig. S15). Consistent with the Co-IP results, AtSar1d but not AtSar1c can be recruited onto the ER by CNX-mCherry-ATG8e (Fig. 5D), suggesting that AtSar1d can interact with ATG8e. Further mutagenesis analysis demonstrated that in the swopping of the variable region in AtSar1d to AtSar1c, AtSar1d(D-C) failed to result in recruitment onto the ER (Fig. 5D). These results are consistent with the structural prediction because glycine does not contain a large side chain, whereas an amino acid with a large side chain may hinder the interaction with ATG8e. Co-IP assays and fluorescence resonance energy transfer (FRET) analysis further demonstrated that the variable region is essential for the interaction with ATG8 (Fig. 5 E and F and SI Appendix, Fig. S14C). Taken together, we have unveiled a region on AtSar1d for ATG8 interaction, which is essential for a specific COPII paralog to manifest autophagosome biogenesis.
Fig. 5.
AtSar1d interacts specifically with ATG8 (A) Coimmunoprecipitation analysis of YFP (negative control) and various AtSar1 paralogs (AtSar1a, AtSar1c, AtSar1b, and AtSar1d) with 3HA-ATG8e using Arabidopsis protoplasts. (B) Sequence alignment of Arabidopsis, human, rodent, worm, and yeast Sar1 homologs. The red triangle indicates the predicted amino acid essential for ATG8e interaction. (C) Structure modeling indicates that the variant region in AtSar1d face to the interaction interface with ATG8, and the individual amino acids within the variant regions were labeled. (D) Recruitment assay using CNX-mCherry-ATG8e with AtSar1c, AtSar1d, or their swopping mutant. (Scale bars, 10 μm) (E) Coimmunoprecipitation analysis of AtSar1d or the swopping mutant with ATG8e. (F) FRET analysis of AtSar1c, AtSar1d, or their swopping mutant with ATG8e in Arabidopsis protoplasts.
A Plant-Unique Complex Redirects the Specific COPII Population to Phagophore and Regulates Autophagosome Biogenesis.
Although increasing numbers of studies have shed light on the role of the COPII machinery in autophagosome biogenesis in eukaryotes, less is known about how COPII vesicles can be redistributed to the PAS for autophagosome initiation or progression upon stress. Rab GTPases and the SNARE complex are essential for vesicle docking and fusion with target membranes (27). In yeast and mammals, Ypt1 and Rab1 have been demonstrated to function in COPII-mediated membrane traffic between the ER and Golgi. Interestingly, recent studies revealed an alternative function for Ypt1 and Rab1 in the autophagy pathway through interacting with ATG1 (28). However, Rab1-related proteins in Arabidopsis are divided into two putative subclasses by sequence comparison, Rab-D1 and Rab-D2, which are conserved in monocots and dicots (29) (SI Appendix, Fig. S16A). The existence of Rab GTPases and their underlying mechanisms in mediating COPII vesicle fusion with the PAS remain a missing puzzle in plants. Using a transient expression system, we screened for potential Rab GTPases that may function as a link between COPII and PAS. Surprisingly, among the close homologs of plant Rab1 proteins, only AtRabD2a exhibited a substantial colocalization with both ATG8e and AtSar1d after long-term incubation in protoplasts (SI Appendix, Fig. S16 B–D). In contrast, AtRabD2b and AtRabD2c showed little colocalization with ATG8e (SI Appendix, Fig. S16 E and F).
In mammals, Rab1 can interact with the COPII inner coat protein Sec23 and guide the COPII vesicles to the Golgi apparatus. Similarly, AtRabD2a colocalized with the Sec23 homologs AtSec23b and AtSec23c in plant cells (SI Appendix, Fig. S17A), whereas Co-IP and FRET analysis demonstrated an interaction between AtRabD2a and AtSec23b or AtSec23c (SI Appendix, Fig. S17 B and C). We further generated transgenic AtRabD2a plants driven by the DEX-inducible promoter. Upon BTH and Conc A treatment, the AtRabD2a-positive punctae are delivered to the vacuole, which is similar to other autophagy-related proteins (SI Appendix, Fig. S18). To confirm the essential role of AtRabD2a in regulating autophagy, we generated transgenic plants coexpressing an inactive guanosine diphosphate-bound form of AtRabD2a (AtRabD2aNI) controlled by the DEX-inducible promoter and mCherry-ATG8e. Notably, autophagosome formation as well as autophagic flux to the vacuole are strongly inhibited in AtRabD2aNI mutant plants upon BTH or BTH and ConcA treatments (Fig. 6 A and C). Similarly, AtRabD2aNI exhibited strong inhibition on vacuolar delivery of mCherry-ATG8e upon nitrogen starvation (SI Appendix, Fig. S19). Consistently, TEM analysis showed a decrease in the number of autophagic bodies in the vacuole in AtRabD2aNI mutant plants under BTH and ConcA conditions (Fig. 6 B and D). Consistent with a previous study (29), a transient expression assay using AtRabD2aNI leaf protoplasts showed that AtRabD2aNI could also inhibit ER export of Aleurain-mRFP upon long-time DEX induction under normal growth condition, while the AtRabD2aNI specifically inhibited autophagosome biogenesis upon autophagic induction, whereas the ER export remained normal (SI Appendix, Fig. S20).
Fig. 6.
AtRabD2a functions as a molecular switch and specifically regulates autophagosome biogenesis (A) Expression of the DN form of AtRabD2a (AtRabD2aNI) perturbed the autophagic flux of ATG8e into the vacuole upon autophagic induction by BTH treatments. (Scale bars, 10 μm.) (B) TEM analysis of the autophagic flux in the AtRabD2aNI mutant upon autophagic induction by BTH and ConcA treatments. (Scale bars, 1 μm.) (C) Quantification of the number of autophagic bodies in A. (D) Quantification of the number of autophagic bodies in B.
In yeast, Ypt1 can interact with ATG1 to mediate autophagosome initiation (30). We therefore hypothesized that AtRabD2a may interact with ATG1 to dock AtSar1d-positive COPII vesicles to the phagophore for autophagosome progression. Indeed, AtRabD2a exhibited colocalization with ATG1a and ATG13a in Arabidopsis protoplasts (Fig. 7A). Co-IP analysis and FRET assays further demonstrated the bona fide interaction between AtRabD2a and ATG1a or ATG13a (Fig. 7 B and C). On the contrary, AtRabD2b and AtRabD2c showed little interaction and colocalization with ATG1a and ATG13a (SI Appendix, Fig. S21). Our results therefore indicated that AtRabD2a can interact specifically with the ATG1 complex component ATG1a and ATG13a, which is previously uncharacterized in plants (Fig. 7). To conclude, we identified a specific Rab GTPase AtRabD2a, which can coordinate with AtSar1d to form a plant-specific nexus to modulate autophagosome biogenesis via interaction with the ATG1 complex.
Fig. 7.
AtRabD2a specifically interacts with ATG1 complex in planta. (A) Coexpression of AtRabD2a with ATG1a or ATG13a, respectively, in Arabidopsis protoplasts. (Scale bars, 10 μm.) (B) Coimmunoprecipitation between AtRabD2a and ATG1a or ATG13a. (C) FRET analysis of the interaction between AtRabD2a and ATG1a or ATG13a.
Discussion
Autophagy plays essential roles in multiple developmental processes in higher eukaryotes. Knowledge about the molecular mechanisms underlying autophagosome biogenesis, the membrane origins, and the specific roles of other membranes in autophagosome formation are gradually accumulating. Although essential for anterograde vesicular traffic between the ER and the Golgi, an unexpected role for the COPII machinery in regulating autophagosome biogenesis has been discovered in yeast and animals (8–16). Unlike animals, sessile plants are tightly regulated by phytohormones to adapt to changing environmental conditions during development. The importance of autophagy has been recognized in seed germination, seedling establishment, plant growth, stress responses, metabolism, and reproductive development (4). Nonetheless, the origin of PAS and the membrane source of plant autophagy remain largely elusive. Furthermore, recent studies have revealed that higher plants possess some unique components in modulating autophagosome formation, thus broadening our knowledge on the basis of autophagy pathway in higher eukaryotes (5).
In this study, we unveiled a plant-specific AtSar1d-AtRabD2a nexus in modulating autophagosome biogenesis upon stress. We have identified multiple COPII paralogs as the interactors of ATG6 and ATG8 under stress conditions via large-scale proteomic analyses (Fig. 1A and SI Appendix, Fig. S1). In agreement with yeast and animals, our results prove the involvement of COPII machinery in the autophagy pathway. Surprisingly, a specific COPII paralog AtSar1d identified from proteomic analysis exhibits a distinct function in autophagosome formation (Fig. 2 and SI Appendix, Figs. S6–S9), suggesting that higher plants may utilize unique strategies in regulating autophagosome biogenesis, probably through the operation of plant-specific paralogs. Consistently, T-DNA insertion mutants of AtSar1d displayed a defective phenotype upon starvation (Fig. 3). Unexpectedly, cellular analysis revealed that a smaller size of autophagosomes were formed in the AtSar1d mutant (Fig. 4 and SI Appendix, Fig. S11), while the ATG assembly on phagophore was not interrupted (SI Appendix, Fig. S12), indicating that AtSar1d-positive COPII vesicles contribute to autophagosome progression (SI Appendix, Fig. S13). In yeast and mammals, the specificity of the COPII paralog Lst1/SEC24C function in ER-phagy has been demonstrated upon ER stress (31). However, exactly how Lst1/SEC24C regulates ER-phagy formation remains unknown. Intriguingly, a recent study indicated that Sar1 may bind to ATG8 independent of an LC3-interacting region (LIR) docking site in plants (26). Nonetheless, plant Sar1 paralogs do not contain a canonical LIR motif. Our results showed that AtSar1 paralogs can bind to ATG8, while AtSar1d exhibited a strong affinity to ATG8 under normal or autophagy-induced conditions (Fig. 5A and SI Appendix, Fig. S14). Surprisingly, AtSar1d contains a variable region, which is indispensable for ATG8 interaction in vivo (Fig. 5 B–F and SI Appendix, Fig. S15). Therefore, our study has not only unveiled the mechanism underlying specific COPII paralog function in modulating autophagosome biogenesis but has also identified a previously uncharacterized motif for ATG8 interaction, albeit with its underlying mechanism remaining to be investigated in future study.
Rab GTPases and SNARE complexes are essential for vesicle docking and fusion with target membranes (27). Recent studies have revealed that several Rab GTPases function at different steps during autophagosome formation via interacting with ATG proteins (2). Nonetheless, the role of Rab GTPase in plant autophagosome biogenesis remains a puzzle. Using AtSar1d and ATG8e as bait, we screened out AtRabD2a coordinating with AtSar1d to redirect specific COPII populations to the phagophore for autophagosome formation (SI Appendix, Fig. S16). AtRabD2a can interact with COPII machinery and ATG1 complexes specifically (Fig. 7 and SI Appendix, Fig. S17), serving as a link between COPII and autophagy. Consistently, AtRabD2a mutants perturbed autophagosome formation upon stress (Fig. 6 and SI Appendix, Figs. S19 and S20). We therefore first identified a Rab GTPase function in plant autophagy and revealed a specific AtSar1d-AtRabD2a nexus function in regulating autophagosome biogenesis. Albeit increasing studies proved the bona fide role of COPII in autophagy pathway, the components involved in this process remained largely under investigation. Up to now, only a few specific COPII components or related proteins have been characterized to function in the autophagy pathway (13, 31). Our large-scale proteomic analysis has identified several other COPII components besides AtSar1d, such as Sec24-like CEF and AtSec23f. Indeed, we have shown that AtSec23f could interact and colocalize with ATG6 (SI Appendix, Fig. S2). It will be of great interest to identify additional components such as SNARE proteins in this specific type of COPII vesicles in future studies to further understand the roles of the COPII machinery in regulating the autophagy pathway.
During evolution, gene duplication events occurred substantially in monocot and dicot plants, with their functional redundancy or diversity remaining largely unclear. The COPII vesiculating machinery likewise contains multiple copies in the plant genome, which has been postulated to function in response to hormones or environmental stresses (17). Our study has revealed that higher plants use a unique strategy in regulating autophagy via manipulating specific protein paralogs, which contain sequence specificity. This research has also shed light on the evolutionary importance of gene duplication events in fundamental processes in sessile organisms.
Materials and Methods
Additional materials and methods including plasmid construction, plant materials, plant growth, chemical or stress treatments, antibodies, Arabidopsis protoplasts transient expression, the Arabidopsis leaf protoplast transient expression assay, confocal microscopy and acceptor photobleaching FRET analysis, TEM analysis, 3D tomography analysis, protein extraction and Western blot, RT-PCR, immunoprecipitation-mass spectrometry, yeast two-hybrid assay, immunoprecipitation, structure modeling, gene ontology enrichment and network analysis, and quantification analysis are described in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31670179 and 91854201), the Research Grants Council of Hong Kong (AoE/M-05/12, CUHK14130716, 14102417, 14100818, 14101219, 14104716, 14177217, C4012-16E, C4041-18E, C4033-19E, C4002-17G, C4002-20W, and RIF R4005-18), and The Chinese University of Hong Kong Research Committee to L.J. and K.-B.W.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021293118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Nakatogawa H., Suzuki K., Kamada Y., Ohsumi Y., Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N., Komatsu M., Autophagy: Renovation of cells and tissues. Cell 147, 728–741 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N., A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 20, 521–527 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Michaeli S., Galili G., Genschik P., Fernie A. R., Avin-Wittenberg T., Autophagy in plants–What’s new on the menu? Trends Plant Sci. 21, 134–144 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y., Li B., Lin Y., Jiang L., The interplay between endomembranes and autophagy in plants. Curr. Opin. Plant Biol. 52, 14–22 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zess E. K., et al., N-terminal β-strand underpins biochemical specialization of an ATG8 isoform. PLoS Biol. 17, e3000373 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanetti G., Pahuja K. B., Studer S., Shim S., Schekman R., COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 14, 20–28 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Graef M., Friedman J. R., Graham C., Babu M., Nunnari J., ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell 24, 2918–2931 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan D., et al., The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc. Natl. Acad. Sci. U.S.A. 110, 19432–19437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge L., Zhang M., Schekman R., Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. eLife 3, e04135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis S., et al., Sec24 phosphorylation regulates autophagosome abundance during nutrient deprivation. eLife 5, e21167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge L., et al., Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep. 18, 1586–1603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong Y. T., et al., The ULK1-FBXW5-SEC23B nexus controls autophagy. eLife 7, e42253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shima T., Kirisako H., Nakatogawa H., COPII vesicles contribute to autophagosomal membranes. J. Cell Biol. 218, 1503–1510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara N., et al., Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol. Biol. Cell 12, 3690–3702 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge L., Melville D., Zhang M., Schekman R., The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. eLife 2, e00947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung K. P., Zeng Y., Jiang L., COPII paralogs in plants: Functional redundancy or diversity? Trends Plant Sci. 21, 758–769 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y., et al., Unique COPII component AtSar1a/AtSec23a pair is required for the distinct function of protein ER export in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 112, 14360–14365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faso C., et al., A missense mutation in the Arabidopsis COPII coat protein Sec24A induces the formation of clusters of the endoplasmic reticulum and Golgi apparatus. Plant Cell 21, 3655–3671 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conger R., et al., Evidence for the involvement of the Arabidopsis SEC24A in male transmission. J. Exp. Bot. 62, 4917–4926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi J., et al., MAIGO5 functions in protein export from Golgi-associated endoplasmic reticulum exit sites in Arabidopsis. Plant Cell 25, 4658–4675 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu X., Chatty P. R., Roeder A. H., Endomembrane trafficking protein SEC24A regulates cell size patterning in Arabidopsis. Plant Physiol. 166, 1877–1890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka Y., Nishimura K., Kawamukai M., Oshima A., Nakagawa T., Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis. Planta 238, 561–575 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Zhuang X., et al., ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 114, E426–E435 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Signorelli S., Masclaux-Daubresse C., Moriyasu Y., Van den Ende W., Bassham D. C., Editorial: Sugars and autophagy in plants. Front. Plant Sci. 10, 1190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall R. S., Hua Z., Mali S., McLoughlin F., Vierstra R. D., ATG8-Binding UIM proteins define a new class of autophagy adaptors and receptors. Cell 177, 766–781.e24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahn R., Lang T., Südhof T. C., Membrane fusion. Cell 112, 519–533 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Hurley J. H., Young L. N., Mechanisms of autophagy initiation. Annu. Rev. Biochem. 86, 225–244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinheiro H., et al., Genetic evidence that the higher plant Rab-D1 and Rab-D2 GTPases exhibit distinct but overlapping interactions in the early secretory pathway. J. Cell Sci. 122, 3749–3758 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., et al., Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc. Natl. Acad. Sci. U.S.A. 110, 9800–9805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui Y., et al., A COPII subunit acts with an autophagy receptor to target endoplasmic reticulum for degradation. Science 365, 53–60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.