Abstract
Background
Household transmission is responsible for the subsequent outbreak of community-acquired COVID-19. The aim of this study was to elucidate the household transmission mode and to further estimate effective and basic reproductive number with and without non-pharmaceutical interventions (NPIs).
Methods
A total of 26 households with 39 family clusters between January, 2020 and February, 2021 in Taiwan were enrolled for analysis. The Becker's chain binomial model was used to analyze the probabilities of being infected and escaping from SARS-COV-2 before and after January 1st, 2021, which were further converted to estimating basic reproductive numbers in the absence of NPIs. The likelihood of leading to the subsequent community-acquired outbreak given NPIs was further assessed.
Results
The secondary attack rate was 46.2%. Given the saturated Greenwood model selected as the best fitted model, the probability of being infected and escaping from COVID-19 within household was estimated as 44.4% (95% CI: 5.0%–53.7%) and 55.7% (95% CI: 46.3%–65.0%), respectively. In the second period of early 2021, the infected probability was increased to 58.3% (95% CI: 12.7%–90.0%) and the escape probability was lowered to 41.7% (95% CI: 0.0%–86.9%). The corresponding basic reproductive numbers (R0) increased from 4.29 in the first period to 6.73 in the second period without NPIs. However, none of subsequent community-acquired outbreak was noted in Taiwan given very effective NPIs in both periods.
Conclusion
The proposed method and results are useful for designing household-specific containment measures and NPIs to stamp out a large-scale community-acquired outbreak as demonstrated in Taiwan.
Keywords: Chain binomial model, COVID-19, Household transmission
Introduction
A novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection emerged in Wuhan, Hubei Province, China.1 , 2 It spreads without delay globally through international human mobility from January 2020. The disease caused by SARS-CoV-2 virus was later named as coronavirus disease 2019 (COVID-19). The 2020 outbreak of this novel virus was not only a large threat to global health but also provides an example of how we are able to provide guidance for infection control measures given different transmission modes.
The majority of the COVID-19 cases are originated from cluster infection in a closed setting such as household. In Taiwan, the first household transmission cluster was noted in Changhua, which was caused by an imported COVID-19 case who transmitted the disease to her husband as a first domestic infected case.3 Many other clustered infection episodes due to household exposure have been reported.2 , 4 Assessing the majority of transmissibility of SARS-CoV-2 through household with human-to-human close contact provides an opportunity to understand the force of transmission given the virulence of SARS-COV-2 and host response to infection.
Chan and colleagues first reported SARS-CoV-2 person-to-person transmission within household in China.5 However, the secondary attack rates of COVID-19 within households are highly variable and ranged from 4% to 55% have been reported in a meta-analysis of household transmission.6 A large variation on the secondary attack rate is two-fold. The first can be attributed to the methods on the identification of COVID-19 cases. The use of symptom-based screening method for the identification of COVID-19 cases in household clusters can result in an underestimated secondary attack rate due to the neglect of asymptomatic cases or presymptomatic cases. The second reason is due to the lapse of generations of household transmission using the theoretical household transmission model like the Becker model applied to household infection of influenza.7 , 8 There are limited studies addressing the detailed mechanisms of COVID-19 spreading in household such as the Greenwood model9 and the Reed-Frost model,10 representing the two extremes of pathogen transmission in household by saturated mode and close-contact mode, respectively. Elucidating the predominate mode on the transmission of COVID-19 and identifying the protective and risk factors in household by using these two extremes may not only prevent the household transmission but also forestall a large-scale community-acquired outbreak. By comparing the basic reproductive numbers in the clustered outbreaks of households and that in community, the effectiveness on the containment measures of NPIs can be assessed.
In this study, we thus first aimed to elucidate the household transmission by the Greenwood model and the Reed-Frost model. The roles of individual characteristics and viral variant on household transmission were further assessed on basis of the established transmission model. On the basis of the force of COVID-19 transmission in household, we further assessed the effectiveness of containment measures of non-pharmaceutical interventions (NPIs) in Taiwan by comparing the effective and basic reproductive number stemming from these empirical results.
Materials and Methods
Data source
In Taiwan, the first case of COVID-19 causing subsequent household transmission was diagnosed on January 25th, 2020 and the first family cluster case was reported on January 28th, 2020. From January 28th, 2020 to February 28th, 2021, a total of 39 events of family clusters occurred involved with 26 households were reported in Taiwan. The policy of quarantine and isolation followed by the laboratory confirmation of COVID-19 cases and the detailed reports on the temporal sequences of these household clustered cases provides a good chance for the elucidation of transmission mode within household.
Data for the following analysis were derived from the reports of Taiwan Central Epidemic Command Center (CECC) and Taiwan Centers for Disease Control. For minimizing the spread of COVID-19, the guidance for the surveillance of infected case and its potential contacts was provided by CECC. After surveillance, the close contacts were advised to undergo SARS-CoV-2 testing for symptomatic contacts. The contact information on exposure history and close contact identification for family members were collected.
The eligible family contacts were identified by history of contact from SARS-COV-2 confirmed cases. All index patients and infected contacts in household were confirmed by SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR). Following the identification of index case, a 14-day quarantine at home for the family contacts was mandatory and monitored at daily basis by local health care centers. If the quarantined family contacts who had symptoms during the quarantined period, they were referred to undergo RT-PCR testing. Uninfected household contacts were those who had no symptoms or had symptoms but with at least twice tested negative.
A total of 26 households with 39 family clusters between January 28, 2020 and February 28, 2021were enrolled in the current analysis. The family cluster infection is illustrated in Fig. 1 . Case A, a business man, was a frequent traveler between Mainland China and Taiwan. He was infected and regraded as an index case and infected his parents (Case B and Case C) by close contact (Fig. 1 (a) and (d)). He was living together with his wife (Case D) and daughter (Case E) but both of them were escaped from infection (Fig. 1 (b) and (d)). The infected Case B was living with another son's family. He became another index case. Two of other four family members were infected by Case B (Fig. 1 (c) and 1(d)). The available information on age, gender, and household size for family index cases and close contact family members were collected.
Figure 1.
An Illustration for the family clusters.
Statistical analysis
The Becker's chain binomial model7 incorporating two underlying assumptions on the modes of SARS-CoV-2 spread was used to elucidate the spread of household. The spread of SARS-CoV-2 within a household would assume to follow binomial distribution with infected probability p and escape probability q at epidemic chains. The infected probability for a susceptible subject exposed to different numbers of the infective is assume to be p, and the escape probability is q followed the concept proposed by Greenwood.9
The details of chain binominal model are elaborated as follows. Let there be St (number of the susceptible) of generation t exposed to the infective It (number of the infective) of generation t, the probability of the infective x (x = 0,1,2, …) of generation t+1 (It+1) is estimated as follows:
| (1) |
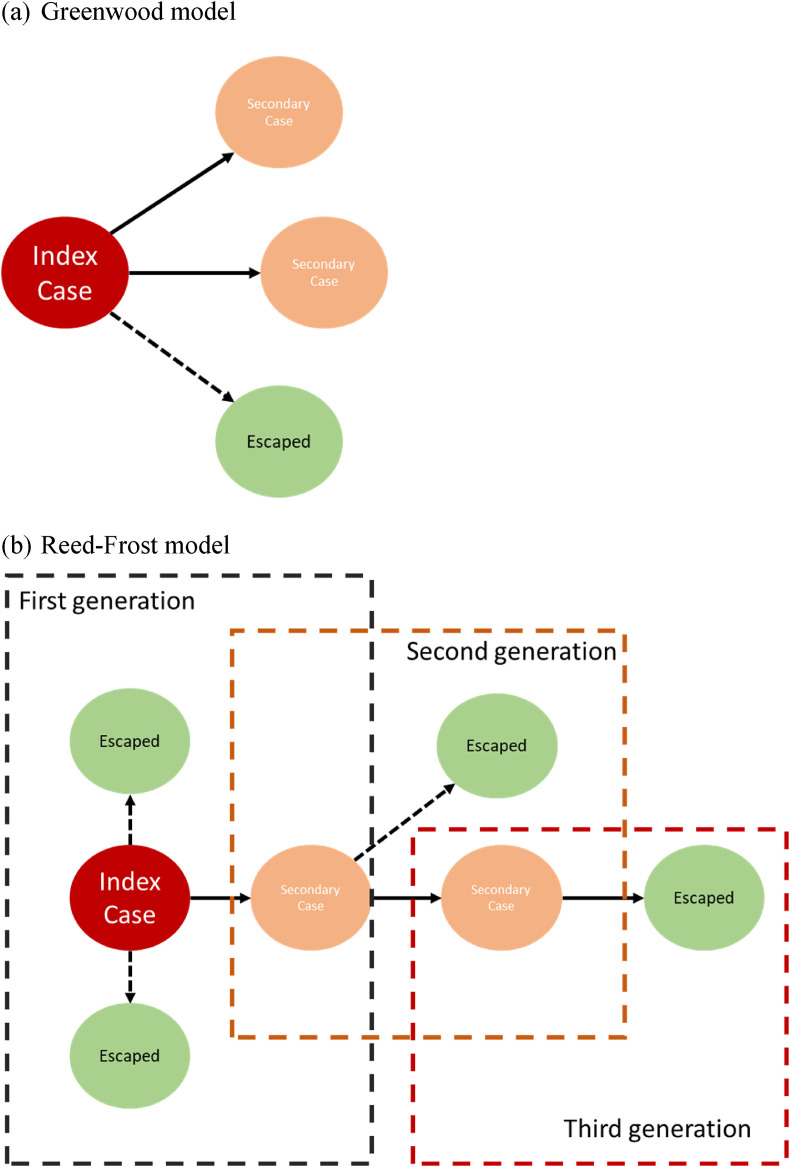
where qi denotes the probability that a given susceptible escapes infection when exposed to the infective i of a special generation. p i is equal to 1 –q i. There are two simplified models. One is the Reed-Frost model (q i = ) and another is the Greenwood model (q i = q). The Greenwood model is suitable for the infection under the environment saturated with infectious agent. The Reed-Frost model is suitable for the infection via close person-to-person contact.
Fig. 2 shows the transmission of SARS-CoV-2 by using the Greenwood model (Fig. 2 (a)) and the Reed-Frost model (Fig. 2(b)) in a household with size four and one index case. For the Greenwood model (Fig. 2 (a)), the probability of escape from is the same for all of the household members as long as there is a least one infectives. Furthermore, due to such a transmission mechanism, the clustered cases would be expected. Regarding the Reed-Frost transmission model (Fig. 2(b)), the transmission of pathogen can occur only after effective contact between susceptible household members and the infectious one. In this scenario, single case rather than clustered cases would be expected. Note that in both transmission models illustrated in Fig. 2, the number of secondary cases are 2. However, the secondary attack rate of 67% (2/3) can only be applied to the Greenwood model in Fig. 2(a). For the Reed-Frost model, the secondary attack rate should be taken into account the history of three generations for the transmission, which gives the figure of 40% (2/6). Based on the empirical data on household transmission of SARS-CoV-2, we applied the Greenwood model and the Reed-Frost model detailed above to estimate the escape probability with the relative merit of two built models assessed by the Akaike information criterion (AIC).
Figure 2.
The spread of SARS-CoV-2 within the household for Greenwood and Reed-Frost model.
By applying an exponential distribution, the estimated results on the escape probability can be transformed into daily transmission rate given the infectious period of 7 days. As the new viral variants was spreading in the end of 2020 and some family clusters came from a remarkable nosocomial infection starting in January, 2021, the contact rate per day, daily transmission rate, and infectious period were used to estimate the basic reproductive number (R0) in two study periods, before 2021 and after 2021. The formula is written as follows.
| R0= (contact rate per day) × daily transmission probability × infective period | (2) |
Here, contact rate 7.74 per day was abstracted from Hsu et al.8 The duration of infectiveness was assumed as 7 days. On the basis of the estimated results of the basic reproductive number (R0) stemming from the force of COVID-19 spreading in household, we further assess the effectiveness of NPIs by comparing the effective reproductive number in community with the estimated results.
A logistic regression model was used to assess the effect of age, gender, and household size on infected probability for SARS-CoV-2. All statistical analysis was performed with SAS 9.4 software.
Results
From 21 January, 2020 to 28 February, 2021, 26 households from these 18 index cases of SARS-COV-2 in Taiwan were enrolled in this study. Table 1 shows the 39 family cluster events (the last column of Table 1, Frequency of clusters) occurred in 26 household reported in Taiwan during study period. The mode of transmission including the number index case and secondary case and the total number of COVID-19 cases for each type of family cluster events were also listed in Table 1. The household size distribution, ranged from 2 to 11, with mean household sized of 3.72 (Std:1.73), and median of 4.0 (IQR = 3). Secondary transmission of SARS-CoV-2 developed in 47 of 145 household contacts. The overall household secondary attack rate was 46.2%. The household secondary attack rate in the period after 2021 was higher (11/20, 55.0%) than the period before 2021 (36/82, 43.9%).
Table 1.
Distribution of family clustered events of SARS-CoV-2 infection in Taiwan.
| Size of Family cluster | Total reported cases | Transmission mode |
Frequency of clusters | |
|---|---|---|---|---|
| Index case | Secondary case | |||
| Before 2021 | ||||
| 2 | 2 | 1 | 1 | 11 |
| 3 | 1 | 1 | 0 | 1 |
| 3 | 2 | 1 | 1 | 4 |
| 3 | 3 | 1 | 2 | 3 |
| 4 | 1 | 1 | 0 | 1 |
| 4 | 2 | 1 | 1 | 5 |
| 4 | 3 | 1 | 2 | 1 |
| 4 | 4 | 4 | 0 | 1 |
| 5 | 2 | 1 | 1 | 5 |
| 6 | 3 | 1 | 2 | 1 |
| 11 | 3 | 2 | 1 | 1 |
| After 2021 | ||||
| 4 | 1 | 1 | 0 | 1 |
| 5 | 3 | 1 | 2 | 3 |
| 6 | 6 | 1 | 5 | 1 |
Household transmission of COVID-19
We applied Becker's chain binomial model, and estimated the infected and escaped probabilities. Table 2 shows the estimated results based on the Greenwood model and the Reed-Frost model. With the saturated Greenwood model, the infected probability was estimated as 44.4% (95% CI: 5.0%–53.7%) for the susceptible who had contacted with index case within a household. The corresponding escape probability was estimated as 55.7% (95% CI: 46.3%–65.0%). With Reed-Frost method, the infected probability estimated as 40.0% (95% CI: 31.4%–48.6%). The estimated escape probability is 60.0% (95% CI: 51.4%–68.6%). The AIC was smaller using the Greenwood method (102.1) than the Reed-Frost method (130.9). The Greenwood method was further applied to calculating the infection probabilities of different time periods. The estimated household infected probability was higher in period of transmission after 2021 (58.3%, 95% CI: 13.0%–90.0%) than before 2021 (42.7%, 95% CI: 32.8%–52.6%) (Table 3 ).
Table 2.
Estimated results on household infected probability and escape probability by two methods.
| Transmission model | Estimate | 95% C.I. | AIC |
|---|---|---|---|
| Greenwood model | |||
| Infected probability | 0.444 | (0.350,0.537) | 102.1 |
| Escape probability | 0.557 | (0.463,0.650) | |
| Reed-Frost model | |||
| Infected probability | 0.400 | (0.314,0.486) | 130.9 |
| Escape probability | 0.600 | (0.514,0.686) | |
Table 3.
Estimated results on household infected probability and escape probability by two pandemic periods.
| Transmission model | Before 2021 |
After 2021 |
||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Infected probability | 0.427 | (0.328, 0.526) | 0.583 | (0.127, 0.900) |
| Escape probability | 0.573 | (0.474, 0.672) | 0.417 | (0.100, 0.822) |
Basic reproductive number without NPIs
Using the Greenwood method, the escape probability was 57.3% and 41.7% before and after 2021. The transmission probability per contact by the escape probability were 0.079% and 0.125% in the two periods, before and after 2021. The basic reproductive number was estimated as 4.29 in the period before 2021. After 2021, the estimated basic reproductive number was 6.73.
Effective reproductive number given NPIs in Taiwan
An illustration of the potential community-acquired infection by household cluster was presented as follows. Owing to this household cluster with 11 family members in Changhua, Taiwan, the Changhua government initiated the contact tracing to identify 665 contacts of these family members within 36 h. On the basis of this contact tracing, the contact rate was estimated as 22 per day. These majority of contacts were from clinics, restaurants, workplaces, and other social contacts. Among 665 contacts, four contacts were identified as confirmed COVID-19 cases. The transmission probability was thus estimated as 0.006 (4/665). The reproductive number was thus estimated as 0.92 (contact rate [22 per day] × transmission probability [0.6%] × infective period [7 days]).
Table 4 shows the impact of risk factors of household contacts associated with SARS-CoV-2 infection. Male and older family members are more likely to be infected but the results were not statistically significant. The inverse association between household size and infection was not statistically significant after controlling age and gender (adjusted odds ratio: 0.98, 95% CI: 0.71–1.36).
Table 4.
Characteristics of household contacts in multivariable logistic analysis.
| Characteristics | Adjusted Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| ≥50 years old vs. <50 years old | 1.04 | (0.27–3.96) | 0.9550 |
| Male vs. Female | 1.33 | (0.37–4.84) | 0.6636 |
| Household size | 0.98 | (0.71–1.36) | 0.9028 |
Discussion
We report the SARS-CoV-2 transmission data of 26 households with infected and uninfected members in Taiwan. The secondary attack rate was 46.2% among household contacts. The 55.0% of household secondary attack rate in the period after 2021 was higher than 43.9% in the period before 2021. The household-member secondary attack rate in our study was higher than those from China (16.3%),4 USA (28%),11 and report from meta-analysis (16.6%).12 These household secondary attack rates by COVID-19 were also generally higher than 10.2% of household secondary attack rate by SARS in 2003.13 It does exist the considerable heterogeneity among these investigations of COVID-19 household infection. The multiple reasons including the definition of the close contacts within households (close relatives or living in same household), age distribution and the relationships between contacts, and testing protocols (symptom-based approach or comprehensive testing for all contacts) might result in the variation of estimated household transmission rates. The rapid contact tracing with testing for all possible contacts in family could be the major reason for higher household secondary attack rate in Taiwan.
However, these non-model empirically-based estimates are not the actual household transmission rate. We therefore estimated the escape probability of contracting SARS-CoV-2 with a series of chain binomial models when the susceptible subject is in contact with a family member who has already been infected and diagnosed with COVID-19. As the pathogen transmitted in household, two extremes in the spread of disease, namely saturated infection (Greenwood transmission model) and close contact transmission (Reed-Frost transmission model) may occur. While the former is mainly due to aerosol transmission such as common cold, the close contact mode of the latter is like HIV and sex-transmitted diseases, the transmission of most of pathogens can lie between these two extremes.14 The adequate model here is the Greenwood method in our household transmission analysis has also been confirmed by using a formal statistical comparison. There are several practical implications for these results on the mechanism of COVID-19 spreading. First, the saturated transmission model of COVID-19 in household (Greenwood model) explained the high attack rate of revealed in the early clustered outbreak, not only in household but also in confined environment such as cruise ship and mass gathering events. This finding underscores the necessity in the containment measures such as face mask for preventing disease transmission through this mode to spread. Second, although the estimated results on the escaped probabilities were close between the two modes (Greenwood model, 55.7%; Reed-Frost model, 60.0%), the biological mechanism of disease spreading inherited in these two modes implied different scales of outbreak. For an infectious disease with the transmission model of saturated infection like COVID-19, large scale outbreaks are expected and a strict implementation of containment measures and NPIs is required for preventing and mitigating these events.
The basic reproductive number could be further estimated as 4.29 and 6.73 for the period before 2021 and after 2021, respectively. The basic reproductive number larger than one indicates the spread of COVID-19 with the occurrence of outbreak. These findings suggest that without NPIs, household settings can remarkably increase the likelihood of SARS-CoV-2 transmission. Although household transmission can be a source of community-acquired outbreak, this high likelihood of SARS-CoV-2 transmission in the family clusters in Taiwan did not result in any further outbreak in community. The basic reproductive number was estimated as 0.92 in community. The reproductive number less than one indicates that the likelihood of community-acquired outbreak was very low because of the implementation of successful containment measures and NPIs in Taiwan.
Very few studies estimated the household transmission of COVID-19 by the chain binomial approach. Hsu et al. applied the chain binomial approach to analyze household data on influenza epidemics in Taiwan. The estimated escape probability and basic reproductive numbers within households were approximately 8.3% and 2.56, respectively,8 which are lower than current report for SARS-CoV-2. Although both COVID-19 and influenza viruses are respiratory diseases and transmitted by contact and droplets, the attack rate of COVID-19 could be attributed to higher basic reproductive rates and progression rate to severe syndrome, longer incubation period and shorter serial interval. The 5.7 of high basic reproductive number was reported in cruise ship with close-setting environment.15 These evidences show that SARS-CoV-2 could spread rapidly in confined environment with close contacts.
After 2021, the major household transmission came from an index case who acquired his COVID-19 by nosocomial infection.16 The index case is an imported COVID-19 patient from USA, who arrived at Taiwan on December 27, 2020. The patient was symptomatic on December 29, 2020 and was transferred to a hospital in northern Taiwan for isolation. The patient was later proved as a COVID-19 with the B.1.1.7 variant by using standard RT-PCR test and genomic sequencing test in the hospital. The first case of the nosocomial infection was a doctor caring for the index case, who was symptomatic on January 8, 2021. Following the transmission of SARS-CoV-2 from patient to health care worker, a series of cases was evolved through the either the symptom-based surveillance or the contact tracing process. Up to February 28, 2021, a total of 10 cases infected by health care worker from household transmission were identified.
Notably, a 1.44-fold (0.583/0.427) higher infection probability in later pandemic period (after 2021) than early pandemic period (before 2021) was found in this study. The basic reproductive number was also higher in the later period. As we know, more frequent infected cases with viral variants have been detected after year of 2021. Davies et al. reported that new SARS-CoV-2 mutations resulting 56% more cases arising than previously observed viral variants combined.17 The higher transmissibility in 2021 could be explained by new SARS-CoV-2 variant of concern (VOC).
In the secondary transmission group in family cluster, the risk of household transmission was higher for male and older family member but was not statistically significant. The trends are consistent with other household transmission study.18
Regarding on the high transmission rate in households, we suggested that family members at risk of infection by COVID-19 such as health care workers or travelers who were exposed to high risk areas should wear facial mask combined with personal hygiene to reduce the risk of transmission. The regular disinfection of the bathroom and toilet is also important. These NPIs are unable to be lifted, as the effects of new vaccines on reducing the transmission on SARS-CoV-2, particularly viral variants have not been clear for the time being. Given several restricted containments and mitigation plans, there is no community-acquired outbreak during COVID-19 pandemic but also no more family cluster infection was observed after March, 2021 in Taiwan.
There are several limitations in the current study. The information on personal protection behaviors such as mask wearing, hand washing and social distancing and relationship to index cases are not collected. We are not able to examine whether household transmission differed by personal protection behaviors and relationships. The comorbidities of family members may affect the transmission of COVID-19 in household, which are also difficult to be corrected.
In conclusion, the higher household transmission of SARS-CoV-2 for the period of early 2021 with the emergence of viral variants was observed. The basic reproductive number in household in the absence of NPIs was more than 3. Although the household transmission is responsible for the subsequent outbreak of community-acquired COVID-19 outbreak, the effective reproductive number less than 1 in community demonstrated that the low likelihood of the subsequent community-acquired outbreak given the effectiveness of NPIs.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by Ministry of Science and Technology, Taiwan (MOST 108-2118-M-038 -002 -MY3; MOST 109-2327-B-002-009).
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;26;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y.C., Liao C.H., Chang C.F., Chou C.C., Lin Y.R. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N Engl J Med. 2020 Mar 12;382(11):1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W., Zhang B., Lu J., Liu S., Chang Z., Peng C. Characteristics of household transmission of COVID-19. Clin Infect Dis. 2020 Nov 5;71(8):1943–1946. doi: 10.1093/cid/ciaa450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. (Lon- don, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh W.C., Naing L., Chaw L., Rosledzana M.A., Alikhan M.F., Jamaludin S.A. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PloS One. 2020 Oct 8;15(10) doi: 10.1371/journal.pone.0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker N.G. Chapman and Hall; London: 1989. Analysis of infectious disease data. [Google Scholar]
- 8.Hsu C.Y., Yen A.M., Chen L.S., Chen H.H. Surveillance of influenza from household to community in Taiwan. Trans R Soc Trop Med Hyg. 2014 Apr;108(4):213–220. doi: 10.1093/trstmh/tru023. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood M. On the statistical measure of infectious disease. J Hyg. 1931;31:336–351. doi: 10.1017/s002217240001086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost W.H. Some conceptions of epidemics in general. Am J Epidemiol. 1976;103:141–151. doi: 10.1093/oxfordjournals.aje.a112212. [DOI] [PubMed] [Google Scholar]
- 11.Lewis N.M., Chu V.T., Ye D., Conners E.E., Gharpure R., Laws R.L. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis. 2020 Aug 16:ciaa1166. [Google Scholar]
- 12.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020 Dec 1;3(12) doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson-Clark S.D., Deeks S.L., Gournis E., Hay K., Bondy S., Kennedy E. Household transmission of SARS, 2003. CMAJ (Can Med Assoc J) 2006;175(10):1219–1223. doi: 10.1503/cmaj.050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas J.C., Weber D.J. Oxford University Press; 2001. Epidemiologic methods for the study of infectious diseases. [Google Scholar]
- 15.Lai C.C., Hsu C.Y., Jen H.H., Yen A.M., Chan C.C., Chen H.H. The bayesian susceptible-exposed-infected-recovered model for the outbreak of COVID-19 on the diamond princess cruise ship. Stoch Environ Res Risk Assess. 2021 Jan 26:1–15. doi: 10.1007/s00477-020-01968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taiwan CDC. https://covid19.mohw.gov.tw/en/cp-5142-59029-206.html, available access on 21 April, 2021.
- 17.Davies N.G., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J., Pearson C.A.B. medRxiv; 2020. Estimated transmissibility and severity of novel SARS-CoV-2 variant of concern 202012/01 in England. [Google Scholar]
- 18.Wang Y., Tian H., Zhang L., Zhang M., Guo D., Wu W. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5(5) doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]