Abstract
Background
Depression and anxiety are the most frequent indication for which antidepressants are prescribed. Long‐term antidepressant use is driving much of the internationally observed rise in antidepressant consumption. Surveys of antidepressant users suggest that 30% to 50% of long‐term antidepressant prescriptions had no evidence‐based indication. Unnecessary use of antidepressants puts people at risk of adverse events. However, high‐certainty evidence is lacking regarding the effectiveness and safety of approaches to discontinuing long‐term antidepressants.
Objectives
To assess the effectiveness and safety of approaches for discontinuation versus continuation of long‐term antidepressant use for depressive and anxiety disorders in adults.
Search methods
We searched all databases for randomised controlled trials (RCTs) until January 2020.
Selection criteria
We included RCTs comparing approaches to discontinuation with continuation of antidepressants (or usual care) for people with depression or anxiety who are prescribed antidepressants for at least six months. Interventions included discontinuation alone (abrupt or taper), discontinuation with psychological therapy support, and discontinuation with minimal intervention. Primary outcomes were successful discontinuation rate,relapse (as defined by authors of the original study), withdrawal symptoms, and adverse events. Secondary outcomes were depressive symptoms, anxiety symptoms, quality of life, social and occupational functioning, and severity of illness.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane.
Main results
We included 33 studies involving 4995 participants. Nearly all studies were conducted in a specialist mental healthcare service and included participants with recurrent depression (i.e. two or more episodes of depression prior to discontinuation). All included trials were at high risk of bias. The main limitation of the review is bias due to confounding withdrawal symptoms with symptoms of relapse of depression. Withdrawal symptoms (such as low mood, dizziness) may have an effect on almost every outcome including adverse events, quality of life, social functioning, and severity of illness.
Abrupt discontinuation
Thirteen studies reported abrupt discontinuation of antidepressant.
Very low‐certainty evidence suggests that abrupt discontinuation without psychological support may increase risk of relapse (hazard ratio (HR) 2.09, 95% confidence interval (CI) 1.59 to 2.74; 1373 participants, 10 studies) and there is insufficient evidence of its effect on adverse events (odds ratio (OR) 1.11, 95% CI 0.62 to 1.99; 1012 participants, 7 studies; I² = 37%) compared to continuation of antidepressants, without specific assessment of withdrawal symptoms. Evidence about the effects of abrupt discontinuation on withdrawal symptoms (1 study) is very uncertain.
None of these studies included successful discontinuation rate as a primary endpoint.
Discontinuation by "taper"
Eighteen studies examined discontinuation by "tapering" (one week or longer). Most tapering regimens lasted four weeks or less.
Very low‐certainty evidence suggests that "tapered" discontinuation may lead to higher risk of relapse (HR 2.97, 95% CI 2.24 to 3.93; 1546 participants, 13 studies) with no or little difference in adverse events (OR 1.06, 95% CI 0.82 to 1.38; 1479 participants, 7 studies; I² = 0%) compared to continuation of antidepressants, without specific assessment of withdrawal symptoms. Evidence about the effects of discontinuation on withdrawal symptoms (1 study) is very uncertain.
Discontinuation with psychological support
Four studies reported discontinuation with psychological support. Very low‐certainty evidence suggests that initiation of preventive cognitive therapy (PCT), or MBCT, combined with "tapering" may result in successful discontinuation rates of 40% to 75% in the discontinuation group (690 participants, 3 studies). Data from control groups in these studies were requested but are not yet available.
Low‐certainty evidence suggests that discontinuation combined with psychological intervention may result in no or little effect on relapse (HR 0.89, 95% CI 0.66 to 1.19; 690 participants, 3 studies) compared to continuation of antidepressants. Withdrawal symptoms were not measured. Pooling data on adverse events was not possible due to insufficient information (3 studies).
Discontinuation with minimal intervention
Low‐certainty evidence from one study suggests that a letter to the general practitioner (GP) to review antidepressant treatment may result in no or little effect on successful discontinuation rate compared to usual care (6% versus 8%; 146 participants, 1 study) or on relapse (relapse rate 26% vs 13%; 146 participants, 1 study). No data on withdrawal symptoms nor adverse events were provided.
None of the studies used low‐intensity psychological interventions such as online support or a changed pharmaceutical formulation that allows tapering with low doses over several months. Insufficient data were available for the majority of people taking antidepressants in the community (i.e. those with only one or no prior episode of depression), for people aged 65 years and older, and for people taking antidepressants for anxiety.
Authors' conclusions
Currently, relatively few studies have focused on approaches to discontinuation of long‐term antidepressants. We cannot make any firm conclusions about effects and safety of the approaches studied to date. The true effect and safety are likely to be substantially different from the data presented due to assessment of relapse of depression that is confounded by withdrawal symptoms. All other outcomes are confounded with withdrawal symptoms. Most tapering regimens were limited to four weeks or less. In the studies with rapid tapering schemes the risk of withdrawal symptoms may be similar to studies using abrupt discontinuation which may influence the effectiveness of the interventions. Nearly all data come from people with recurrent depression.
There is an urgent need for trials that adequately address withdrawal confounding bias, and carefully distinguish relapse from withdrawal symptoms. Future studies should report key outcomes such as successful discontinuation rate and should include populations with one or no prior depression episodes in primary care, older people, and people taking antidepressants for anxiety and use tapering schemes longer than 4 weeks.
Plain language summary
Stopping long‐term antidepressants in people with depression or anxiety
Review question
We aimed to find out if it is effective and safe to stop antidepressants for people with depression or anxiety who have been taking them for six months or longer.
We compared different approaches for stopping long‐term antidepressants versus continuation. We looked at benefits (e.g. successful discontinuation rate) and harms, such as return of the depressive or anxiety episode (relapse), side effects, and withdrawal symptoms (i.e. symptoms people experience when stopping an antidepressant).
Background
Antidepressants are widely used for depression and anxiety. Guidelines recommend that an antidepressant should be continued for at least six months after people start to feel better, and for at least two years if they have had two or more periods of depression. Many people take antidepressants for much longer, and as they can cause unpleasant side effects, long‐term use puts people at risk of harm that may outweigh the benefits.
Study characteristics
Our search up until January 2020 found 33 studies, which included 4995 adult participants. Most people in these studies had recurrent depression (two or more episodes of depression before stopping antidepressants), and most were recruited from specialist mental healthcare services. In 13 studies, the antidepressant was stopped abruptly; in 18 studies, the antidepressant was stopped gradually over several weeks ("tapering"); in four studies, psychological therapy support was also offered; and in one study, stopping was prompted by a letter to the GP with guidance on tapering. Most tapering schemes lasted four weeks or less.
Key results
We found very low‐certainty evidence suggesting that abrupt stopping may lead to higher risk of relapse and there was insufficient evidence of its effect on occurrence of side effects compared to continuation of the antidepressant.
We found very low‐certainty evidence suggesting that "tapering" over a few weeks may lead to higher risk of a return and again may have little or no effect on side effects compared to continuation.
We found evidence of very low to low certainty to suggest that stopping the antidepressant in combination with providing preventive cognitive therapy (PCT), or MBCT, was possible for 40% to 75% of participants in the group tapering the antidepressant and may show no difference in effects on relapse.
We found low‐certainty evidence suggesting that a prompt letter and guidance on tapering sent to the GP may have no effect on the number of people who stop their antidepressant.
We were unable to draw conclusions about withdrawal symptoms after abrupt or gradual stopping of an antidepressant, as this generally was not assessed.
None of the studies used very slow tapering schemes beyond a few weeks, tapered liquid forms of antidepressants, or used tapering strips (to allow tapering with very low doses).
None of the identified studies investigated stopping combined with providing supportive therapy such as online support or self‐help therapy.
Certainty of evidence
Overall, the certainty of evidence was low to very low. This means we have limited or little confidence in the results, and new research is likely to change our conclusions. The main reasons for this assessment of evidence certainty were that trials did not distinguish between symptoms of relapse of depression and symptoms of withdrawal. Also, most studies used no tapering or very "rapid" tapering schedules (four weeks or less), and nearly all studies included people with recurrent depression (more than two episodes).
Conclusions
We found few studies that examined stopping long‐term antidepressants. We are uncertain if the approaches for stopping long‐term antidepressants studied to date are effective and safe in people with recurrent depression. People should discuss with their doctor when they want to stop their antidepressant.
Future studies should include people in primary care with only one or no earlier episodes of depression, older people, and people taking antidepressants for anxiety. Studies should taper antidepressants slowly while taking care to distinguish withdrawal symptoms from relapse.
Summary of findings
Summary of findings 1. Abrupt discontinuation compared to continuation of long‐term antidepressants for depressive and anxiety disorders in adults.
| Abrupt discontinuation compared to continuation of long‐term antidepressants for depressive and anxiety disorders in adults | ||||||
| Patient or population: participants with depressive or anxiety disorders Setting: primary care and specialist mental healthcare services Intervention: abrupt discontinuation Comparison: continuation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with continuation of long‐term antidepressants | Risk difference with abrupt discontinuation | |||||
| Successful discontinuation rate | None of the studies reported successful discontinuation rate | |||||
| Relapse (as defined by study authors) (HR) Follow‐up from 24 weeks to 80 weeks Studies without psychological support |
Low riska (3 studies) | HR 2.09 (1.59 to 2.74) | 1373 (10 RCTs) | ⊕⊝⊝⊝ VERY LOWb | Studies did not distinguish relapse from symptoms of withdrawal | |
| 100 per 1000 | 98 more per 1000 (from 54 more to 151 more) | |||||
| Moderate risk (4 studies) | ||||||
| 285 per 1000 | 219 more per 1000 (from 128 more to 316 more) | |||||
| High risk (3 studies) | ||||||
| 564 per 1000 | 260 more per 1000 (from 169 more to 333 more) | |||||
| Withdrawal symptoms Assessed with: proportion of participants with a withdrawal syndrome based on DESS Scale Follow‐up 2 weeks |
In one study, there was no evidence of an effect on the incidence of withdrawal symptoms between abrupt discontinuation and continuation | 182 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc | Withdrawal syndrome was defined as increase of 4 or more on the DESS Scale (regardless of severity) during the first 2 weeks after discontinuation There were no differences in the proportions of participants with withdrawal syndrome based on DESS scores between the abrupt discontinuation group (31/146; 21.2%) and the continuation group (4/36; 11.1%) (P ≥ 0.06). |
||
| Adverse events Follow‐up: from 4 to 100 weeks |
216 per 1000 | 18 more per 1000 (from 70 fewer to 138 more) | OR 1.11 (0.62 to 1.99) | 1012 (7 RCTs) |
VERY LOWd | Pooling possible for 7 of 10 studies that measured adverse events. Studies did not distinguish between adverse events and withdrawal symptoms |
| Depressive symptoms Assessed with: HAM‐D scale Follow‐up: range 40 to 80 weeks |
Mean HAM‐D total score at endpoint: 9.9 (1 study) | MD 0.44 higher (1.12 lower to 2 higher) | ‐ | 330 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWe | Pooling possible for 3 of 7 studies that measured depressive symptoms Higher score indicates more severe depressive symptoms |
| Anxiety symptoms Assessed with 4 different scales Follow‐up: range 28 to 80 weeks |
In 2 studies (n = 235), there were no differences in anxiety symptoms between abrupt discontinuation and continuation of antidepressants A third study (n = 204) showed that antidepressant continuation improved anxiety symptoms |
‐ | 439 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWf | Data could not be pooled due to variability of outcome measures One study used our prioritised outcome HAM‐D Scale |
|
| Quality of life | None of the studies reported quality of life | |||||
| Social and occupational functioning | None of the studies reported social and occupational functioning | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DESS: Discontinuation‐Emergent Signs and Symptoms; HAM‐D: Hamilton Rating Scale for Depression; HR: hazard ratio; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAssumed risk calculated as the proportion of participants on antidepressants with the outcome (relapse) in the included studies and divided into low (cut‐off < 20%), moderate (cut‐off < 40%), and high risk (≥ 40%), multiplied by 1000.
bDowngraded by two levels for risk of bias (poor description of randomisation and blinding and withdrawal confounding bias) and by one level for indirectness (majority of participants with recurrent depression; one study included panic disorder).
cDowgraded by one level due to imprecision (single study with small number of participants), by one level for risk of bias (poor description of randomisation and blinding, severity of withdrawal symptoms not scored in the outcome; and relatively short period for observing withdrawal symptoms), and by one level for indirectness (study included participants with single or recurrent depressive disorder but did not report numbers of participants with single or recurrent disorder or previous number of episodes)
dDowngraded by one level for risk of bias (poor description of randomisation process and blinding, attrition bias and withdrawal confounding bias), by one level for imprecision (wide 95% confidence interval, which includes the null effect of no difference), and by one level for indirectness (studies included participants with recurrent depressive disorder).
eDowngraded by one level for risk of bias (poor description of randomisation and blinding, attrition bias, and withdrawal confounding bias), by one level for imprecision (wide 95% confidence interval, which includes the null effect of no difference between treatments), and by one level for indirectness (participants with recurrent disorder).
fDowngraded by one level for imprecision (no pooling and small number of participants), by one level for risk of bias (confounding withdrawal bias, poor description of randomisation and blinding), and by one level for indirectness (only one of the three studies included participants with anxiety disorder (panic disorder)).
Summary of findings 2. Discontinuation by "tapering" compared to continuation (or usual care) for depressive and anxiety disorders in adults.
| Discontinuation by "tapering" compared to continuation (or usual care) for depressive and anxiety disorders in adults | ||||||
| Patient or population: participants with depressive or anxiety disorders Setting: primary care and specialist mental healthcare services Intervention: discontinuation by "tapering" over 1 week or longer Comparison: continuation of antidepressant use | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with continuation (or usual care) | Risk with discontinuation by "tapering" | |||||
| Successful discontinuation rate Studies without co‐intervention |
None of the studies reported successful discontinuation rate | |||||
| Relapse (as defined by study authors) (HR) Follow‐up: range 24 to 156 weeks Studies without co‐intervention |
Low riska (8 studies) | HR 2.97 (2.24 to 3.93) | 1546 (13 RCTs) | ⊕⊝⊝⊝ VERY LOWb | Studies did not distinguish relapse from symptoms of withdrawal | |
| 59 per 1000 | 107 more per 1000 (from 69 more to 154 more) | |||||
| Moderate risk (3 studies) | ||||||
| 256 per 1000 | 308 more per 1000 (from 211 more to 409 more) | |||||
| High risk (2 studies) | ||||||
| 448 per 1000 | 381 more per 1000 (from 288 more to 455 more) | |||||
| Withdrawal symptoms Assessed with proportion of participants with a withdrawal syndrome based on DESS Scale Follow‐up: 2 weeks |
In one study, there was no evidence of an effect on the incidence of withdrawal symptoms between "tapering" discontinuation and continuation | 176 (1 RCTs) | ⊕⊝⊝⊝ VERY LOWc | Withdrawal syndrome was defined as increase ≥ 4 on the DESS Scale (regardless of severity) during the first 2 weeks after discontinuation There were no differences in the proportions of participants with withdrawal syndrome based on DESS scores between the discontinuation group "tapered" over one week (30/139; 21.6%) and the continuation group (4/36; 11.1%) (P ≥ 0.06) |
||
| Adverse events Follow‐up: 4 to 156 weeks |
418 per 1000 | 14 more per 1000 (from 47 fewer to 80 more) | OR 1.06 (0.82 to 1.38) | 1479 (7 RCTs) |
⊕⊝⊝ VERY LOWd |
Pooling possible for 7 of 10 studies that measured adverse events. Studies did not distinguish between adverse events and withdrawal symptoms |
| Depressive symptoms Assessed with HAM‐D Scale Follow‐up: range 28 to 76 weeks |
Mean HAM‐D total endpoint score was 5.8 to 9.9 (5 studies) | MD 3.50 higher (2.31 higher to 4.86 higher) | ‐ | 1017 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWe | Higher score indicates more severe depressive symptoms Pooling possible for 6 of 12 studies that measured depressive symptoms |
| Anxiety symptoms Assessed with HAM‐A Scale Follow‐up: range 24 to 52 weeks |
Mean HAM‐A total endpoint score was 4.5 to 6.5 (3 studies) | MD 3.53 higher (1.92 higher to 5.14 higher) | ‐ | 526 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWf | Pooling possible for 3 of 4 studies that measured anxiety symptoms Higher score indicates more and more severe anxiety symptoms |
| Quality of life Assessed with SF‐36 subscales Follow‐up: range 34 to 76 weeks |
Mean QoL physical health score ranged from 74.3 to 86.2 (3 studies) | MD 2.08 lower (5.66 lower to 1.49 higher) | ‐ | 502 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWg | Pooling possible for 3 of 7 studies that measured quality of life Lower score indicates greater impairment of functioning |
| Mean QoL social functioning score ranged from 70.6 to 80.6 (3 studies) | MD 6.44 lower (12.10 lower to 0.77 lower) | ‐ | ||||
| Mean QoL emotional functioning score ranged from 65.7 to 73.2 (3 studies) | MD 18.81 lower (26.66 lower to 10.97 lower) | ‐ | ||||
| Social and occupational functioning Assessed with SAS‐SR Scale Follow‐up: range 34 to 76 weeks |
Mean social and occupational functioning total endpoint score ranged from 1.79 to 1.87 (3 studies) | MD 0.19 higher (0.11 higher to 0.28 higher) | ‐ | 502 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWh | Pooling possible for 3 of 7 studies that measured social and occupational functioning Higher score indicates greater impairment of functioning |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBT: cognitive‐behavioural therapy; CI: confidence interval; DESS: Discontinuation‐Emergent Signs and Symptoms; GAD: General Anxiety Disorder Scale; HAM‐A: Hamilton Anxiety Scale; HAM‐D: Hamilton Rating Scale for Depression; HR: hazard ratio; MBCT: mindfulness‐based cognitive therapy; MD: mean difference; IPT: interpersonal therapy; OR: odds ratio; QoL: quality of life; RCT: randomised controlled trial; PCT: preventive cognitive therapy; SAS‐SR: Social Adjustment Scale Self‐Report; SF‐36: Short Form 36‐Item Health Survey. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAssumed risk calculated as proportion of participants on placebo with outcome (relapse) in included studies divided into low (cut‐off < 20%), moderate (cut‐off < 40%), and high risk (≥ 40%), multiplied by 1000.
bDowngraded by two levels for risk of bias (poor description of randomisation and blinding, withdrawal confounding bias) and by one level for indirectness (three studies included participants with panic disorder; the other studies included recurrent depression or chronic depression).
cDowngraded by one level due to imprecision (single study with small number of participants), by one level for risk of bias (poor description of randomisation and blinding, severity of withdrawal symptoms not scored in the outcome, and relatively short period for monitoring of withdrawal symptoms), and by one level for indirectness (study included participants with single or recurrent depressive disorder but did not report numbers of participants with single or recurrent disorder)
dDowngraded by two levels for risk of bias (poor description of randomisation and blinding, high risk of attrition bias, confounding withdrawal bias (withdrawal symptoms may be misdiagnosed as adverse events), and by one level for indirectness (three studies reported only serious adverse events).
eDowngraded by two levels for risk of bias (poor description of randomisation and blinding, attrition bias, and confounding withdrawal bias (low mood as withdrawal symptom may be misdiagnosed as depressive symptoms by use of HAM‐D Scale or other clinical scales) and by one level for indirectness (five of six studies included recurrent or chronic depression).
fDowngraded by two levels for risk of bias (poor description of randomisation and binding, attrition bias, and confounding withdrawal bias) and by one level for indirectness (two studies included participants with generalised anxiety disorder; one study included recurrent depression).
gDowngraded by one level for risk of bias (poor description of blinding outcome assessors, attrition bias, and withdrawal confounding bias (withdrawal symptoms may impair the quality of life measures)) and by one level for indirectness (studies included participants with recurrent depression or chronic/double depression) and imprecision (wide 95% CI).
hDowngraded by two levels for risk of bias (poor description of blinding of outcome assessors, attrition bias, and withdrawal confounding bias) and by one level for indirectness (studies included participants with recurrent or chronic/double depression).
Summary of findings 3. Discontinuation with high‐intensity psychological interventions compared to continuation for depressive disorders in adults.
| Discontinuation with high‐intensity psychological interventions compared to continuation for depressive disorders in adults | ||||||
| Patient or population: participants with long‐term antidepressants for depressive disorders Setting: primary care or specialist mental healthcare services Intervention: discontinuation by tapering with high‐intensity psychological support Comparison: continuation of antidepressant use | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with continuation (usual care) | Risk with discontinuation with high‐intensity psychological interventions | |||||
| Successful discontinuation rate: proportion (%) of participants who successfully stopped use of antidepressants at the end of the trial Follow‐up: 64 to 104 weeks |
‐ | 690 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa |
Data could not be pooled from 3 studies due to insufficient data reported Intervention included PCT and MBCT. Discontinuation of antidepressants with support from psychotherapy in the discontinuation group varied from 40% after 6 months in Bockting 2018, to over 59% of participants after 24 months in Kuyken 2015, to 75% after 6 months in Kuyken 2008. |
||
| Risk of relapse (as defined by study authors) (HR) Follow‐up: 64 to 104 weeks |
556 per 1000b | 41 fewer per 1000 (from 113 to 38 more) | HR 0.89 (0.66 to 1.19) | 690 (3 RCTs) | ⊕⊕⊝⊝ LOWc | Studies did not distinguish relapse from symptoms of withdrawal |
| Withdrawal symptoms | None of the studies reported withdrawal | |||||
| Adverse events | 3 studies reported no differences in adverse events | 690 (3 RCTs) | ⊕⊝⊝ ⊝ VERY LOWd |
Data could not be pooled due to insufficient data reported Kuyken 2015 (n = 424) reported 3 non‐fatal and 2 fatal serious adverse events (deaths) in each treatment group, and these were considered probably not related to the intervention or the trial. Kuyken 2008 (n = 123) reported that no serious adverse events were recorded through the oversight of the Trial Steering Committee. Bockting 2018 (n= 185; split 143) reported only suicide data. There were 2 suicide attempts (1 in the PCT with tapering group and 1 in the continuation group) |
||
| Depressive symptoms Assessed with HAM‐D Scale Follow‐up: 64 to 104 weeks |
Mean HAM‐D total endpoint score ranged from 4.7 to 8.69 | MD 0.42 lower (1.82 lower to 0.89 higher) | ‐ | 484 (2 RCTs) | ⊕⊕⊝⊝ LOWe | |
| Anxiety symptoms | None of the studies reported anxiety symptoms | |||||
| Quality of life Assessed with WHO QoL‐BREF subscales Follow‐up: range 64 to 104 weeks |
Mean QoL physical health total endpoint score ranged from 14.9 to 22.93 (2 studies) |
MD 0.22 lower (2.16 lower to 1.73 higher) | ‐ | 455 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWf | Intervention included MBCT. |
| Mean QoL psychological health total endpoint score ranged from 13.1 to 17.36 (2 studies) |
MD 0.37 higher (0.75 lower to 1.49 higher) | |||||
| Mean QoL social relationships total endpoint score ranged from 9.66 to 13.9 (2 studies) | MD 0.05 higher (0.56 lower to 0.66 higher) | |||||
| Social and occupational functioning | None of the studies reported social and occupational functioning | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAM‐D: Hamilton Rating Scale for Depression; HR: hazard ratio; MBCT: mindfulness‐based cognitive therapy; MD: mean difference; PCT: preventive cognitive therapy; RCT: randomised controlled trial; WHO QoL‐BREF: World Health Organization Cross‐Cultural Comparisons of Quality of Life. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for bias (withdrawal confounding bias) and by one level for imprecision (no pooling due to insufficient information) and indirectness (studies included participants with recurrent depression in remission).
bAssumed risk calculated as the proportion of participants on antidepressant with the outcome relapse/recurrence in the three included studies and divided into low, moderate, and high risk, multiplied by 1000.
cDowngraded by one level for bias (risk of withdrawal confounding bias; studies did not use very slow tapering regimens or low doses) and by one level for indirectness (studies included participants with recurrent depression in remission).
dDowngraded by one level for risk of bias (withdrawal confounding bias; adverse events may include withdrawal symptoms), by one level for imprecision (no meta‐analysis possible; small number of events and insufficient data supported authors' conclusions) and by one level for indirectness (studies measured only serious adverse events).
eDowngraded by one level due to risk of bias (withdrawal confounding bias) and by one level due to indirectness (studies included recurrent depressive disorder).
fDowngraded by one level due to risk of bias (withdrawal confounding bias, attrition bias, performance bias; outcome assessed with self‐report questionnaire and likely to be influenced by lack of blinding of participants), by one level due to imprecision (wide 95% confidence interval, which includes the null effect), and by one level due to indirectness (studies included participants with recurrent depressive disorder).
Summary of findings 4. Discontinuation of long‐term antidepressant with minimal intervention compared to usual care for depressive and anxiety disorders in adults.
| Discontinuation of long‐term antidepressant with minimal intervention compared to usual care for depressive and anxiety disorders in adults | ||||||
| Patient or population: participants with long‐term antidepressants for depressive or anxiety disorders Setting: primary care (GP practices) Intervention: discontinuation with minimal intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with discontinuation of long‐term antidepressant with minimal intervention | |||||
| Successful discontinuation rate: proportion (%) of participants who successfully stopped use of antidepressants at the end of the trial Defined as no antidepressant use during the preceding 6 months and absence of a depressive or anxiety disorder Follow‐up: 52 weeks |
In one study, there was no evidence of an effect on the antidepressant discontinuation rate | ‐ | 146 (1 RCT) | ⊕⊕⊝⊝ LOWa |
Intervention included a patient‐specific letter to the GP with a recommendation to discontinue the antidepressant and tapering advice Successful discontinuation was defined by study authors as no antidepressant use during the preceding 6 months and absence of a depressive or anxiety disorder In the intervention group, 6% (95% CI 2 to 14) successfully stopped their antidepressant compared to 8% (95% CI 4 to 16) who spontaneously stopped in the usual care group after 1 year (P = 0.6) |
|
| Relapse rate Follow‐up: 52 weeks |
In one study, there was no evidence of an effect on relapse rate | ‐ | 146 (1 RCT) | ⊕⊕⊝⊝ LOWb | In the tapering advice group, 26% participants relapsed compared to 13% in the usual care group (P = 0.05) . Study did not distinguish relapse from symptoms of withdrawal |
|
| Withdrawal symptoms | One study measured withdrawal symptoms but did not analyse or report data | |||||
| Adverse events | Not measured | |||||
| Depressive symptoms Assessed with CESD scale Follow‐up: 52 weeks |
In one study, there was no effect on depressive symptoms | 106 (1 RCT) | ⊕⊝⊝⊝ LOWc | Study authors reported a higher mean CESD score in the tapering group (n = 51) (mean CESD total endpoint score 13.7 (SD 8.9)) compared to the usual care group (n = 55) (mean CESD total endpoint score 12.6 (SD 7.9)) at the end of the trial but no difference between groups (P = 0.51) (higher CES‐D score means increased intense symptom severity) | ||
| Anxiety symptoms Assessed with PAS scale Follow‐up: 52 weeks |
In one study, there was no effect on anxiety symptoms | 104 (1 RCT) | ⊕⊝⊝⊝ LOWd | Study authors measured the severity of illness in patients with panic disorder by using the Panic and Agoraphobic Scale (PAS). Study authors reported a higher mean PAS score in the tapering group (n = 50) (mean PAS 4.1 (SD 7.2)) compared to the usual care group (n = 51) (mean PAS 3.6 (SD 7.1)) at the end of the study but no differences between groups (P = 0.71) (higher PAS scores indicating greater severity) | ||
| Quality of life Assessed with QALY by using EQ‐5D Follow‐up: 52 weeks |
In one study, there was no evidence of an impact on the quality of life | ‐ | 146 (1 RCT) | ⊕⊝⊝⊝ VERY LOWe | Participants in the tapering advice (n = 70) group had a mean of 0.70 QALYs (SD 0.25) and those in the usual care group (n = 76) had a mean of 0.72 QALYs (SD 0.26). There was no difference between discontinuation with tailored recommendation and usual care in quality of life (mean difference (with multiple imputation for missing values) ‐0.02, 95% CI ‐0.05 to 0.10; higher scores indicate better quality of life)). Study authors reported 0.07 as the minimally important difference for the EQ‐5D | |
| Social and occupational functioning | Study did not report social and occupational functioning | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CESD: Center for Epidemiological Studies Depression Scale; CI: confidence interval; DESS: Discontinuation‐Emergent Signs and Symptoms; EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions; GP: general practitioner; PAS: Panic and Agoraphobic Scale; QALY: quality‐adjusted life‐year; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for imprecision (single study with a small number of participants) and by one level for risk of bias (withdrawal confounding bias; withdrawal symptoms may be misclassified as relapse and may lead to restart of the antidepressant).
bDowngraded by one level for withdrawal confounding bias (study measured DESS symptoms but did not analyse data) and by one level for imprecision (single study with a small number of participants).
cDowngraded by one level due to risk of bias (withdrawal confounding bias, attrition bias) and by one level due to imprecision (a single study).
dDowngraded by one level due to risk of bias (withdrawal confounding bias, attrition bias) and by one level due to imprecision (a single study).
eDowngraded by one level for imprecision (a single study with a small number of participants) and by two levels for risk of bias (withdrawal confounding bias, high number of missing values, risk of performance bias).
Background
Description of the condition
Antidepressant use
Antidepressants can be divided into various classes based on their slightly different mechanisms of action (see Table 5). They act, with a few exceptions, by enhancing transmission of the chemical messengers dopamine, noradrenaline (norepinephrine), adrenaline (epinephrine), and serotonin, which are thought to be involved in mood regulation in the brain. However, the exact mechanism of action of antidepressants is still not known (Harmer 2017).
1. Different classes of antidepressants.
| Classes of antidepressants | Examples |
| A. Major classes of antidepressants | |
| Selective serotonin reuptake inhibitors (SSRIs) | citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline |
| Serotonin‐noradrenaline reuptake inhibitors (SNRIs) | duloxetine, venlafaxine, desvenlafaxine, milnacipran, levomilnacipran |
| Noradrenaline reuptake inhibitors (NARIs) | reboxetine |
| Tricyclic antidepressants (TCAs) and related | amitriptyline, clomipramine, dosulepin, doxepin, imipramine, nortriptyline, maprotiline |
| Noradrenaline‐dopamine reuptake inhibitors (NDRIs) | bupropion |
| Monoamine oxidase inhibitors (MAOIs) | phenelzine, moclobemide, tranylcypromine |
| B. Other drugs used to treat depression | |
| Melatonergic antidepressants | agomelatine |
| Noradrenergic and specific serotonergic antidepressant (NaSSA) and related drugs | mirtazapine, mianserin |
| Serotonin antagonist and reuptake inhibitors (SARIs) | trazodone |
| Multi‐modal serotonin reuptake inhibitor and receptor blocker | vortioxetine, vilazodone |
| Hypericum perforatum (St John's Wort) | |
The consumption of antidepressants has increased significantly in most countries since 2000 and continues to rise (OECD 2017). The highest use per population in the Convention on the Organisation for Economic Co‐operation and Development (OECD) Health Report is seen in Iceland, Australia, Portugal, UK, Sweden, Canada, and Belgium (OECD 2017). About 12.7% of Belgian adults take antidepressants daily (RIZIV 2014), with similar rates in the UK (16%; DHSC 2018), as well as in many other European countries (Gusmão 2013). In the USA, the rate is about 12.7% (Pratt 2018), and in Australia, about 16.8% of adults take antidepressants daily (Brett 2017).
Similar to most prescribed medicines, antidepressants can have side effects and adverse effects. Very common (more than 10%) adverse events ('harms') for all antidepressants include sleep disturbance, sexual dysfunction, and gastrointestinal problems. More serious rare adverse effects (0.01% to 0.1%) are higher risk of agitation and suicide at the beginning of treatment, or when dosage is increased, and gastrointestinal bleeding, and in older people, low sodium in the blood with risk of agitation and confusion and increased risk of fracture (BCFI/CBIP 2018). All antidepressants are also associated with side effects related to their mechanism of action. For example, very common side effects (> 10%) of tricyclic agents (TCAs) are blurred vision, constipation, and dry mouth due to their anticholinergic properties. Other common problematic anticholinergic effects (1% to 10%) include increased intraocular pressure, urinary retention, postural hypotension, dizziness, and negative impact on cognition in older people. However, these rates have been derived from short‐term regulatory studies; independent studies have found much higher rates of adverse events with long‐term use (Bet 2013). Patients also commonly report adverse effects on mood and emotion, such as feeling emotionally numb and what they described as feeling “addicted” (Cartwright 2016; Davies 2019). Long‐term antidepressant use may impair patients’ autonomy and may increase their dependence on medical help and medication (Kendrick 2015). Typical antidepressant withdrawal symptoms or discontinuation symptoms, such as flu‐like symptoms, insomnia, nausea, imbalance or vertigo, sensory disturbances, hyperarousal (anxiety and agitation), and suicidality (Valuck 2009), can occur when doses of any antidepressant are stopped, missed, or reduced.
A previous Cochrane Review on antidepressant treatment for depression in primary care suggested that for one person to experience side effects that led that person to stop taking the antidepressant drug (i.e. number needed to treat for an additional harmful outcome (NNTH)), between 4 and 30 people had to be treated with a TCA, and between 20 and 90 people with a selective serotonin reuptake inhibitor (SSRI) (Arroll 2009). In older people, antidepressants tend to pose greater risk for adverse events because of coexisting disease and drug‐drug interactions related to other medications taken regularly (Kok 2017).
Indications for antidepressants
Depressive disorders
The most prevalent condition that warrants the use of antidepressants in the community and in nursing homes is depression (Bourgeois 2012; Wong 2016).
According to the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM‐V) classification, a major depressive disorder is defined as a period of at least two weeks during which there is either depressed mood or loss of interest or pleasure in nearly all activities, along with at least four of the following symptoms: changes in appetite, weight, sleep, or psychomotor activity; decreased energy; feelings of worthlessness or guilt; difficulty concentrating or thinking or making decisions; or having recurrent thoughts of death or suicidal ideation or making suicide plans or attempts (APA 2013). These symptoms must persist for most of the day, nearly every day, for at least two consecutive weeks. The episode must be accompanied by clinically significant distress or impairment in social, occupational, or other important areas of functioning. A major depressive episode is a period of two weeks or longer that is characterised by symptoms of a major depressive disorder (APA 2013). Severity of the major depressive episode (mild, moderate, severe) must be based on the number of criterion symptoms, the severity of those symptoms, and the extent to which usual social and working activity is limited, and must not rely simply on counting the number of symptoms (APA 2013).
Diagnosing depression can be difficult due to these broad and subjective diagnostic criteria and lack of a reliable and valid test for depression in primary care that takes into account the cause of depression.
An untreated depressive episode typically lasts about four to six months on average (NICE 2009; Solomon 1997). The prognosis for a first depression is rather positive; approximately 50% to 60% of people who become depressed will have only a single episode of depression in their lifetime, approximately 35% to 40% will have one or more recurrences in the next 15 years, and approximately 15% will develop chronic depression (Eaton 2008; Mattisson 2007; Moffit 2010). Therefore the National Institute for Health and Care Excellence (NICE) recommends that treatment should focus not only on improving symptoms of the acute episode but also on preventing relapse (return of symptoms of the original depressive episode) and recurrence (development of a subsequent episode) (NICE 2009).
Treatment of depressive disorder consists of three distinct phases: acute therapy, continuation phase, and maintenance treatment (APA 2010). Acute therapy is the treatment phase that is focused on treating the patient; it typically lasts 6 to 12 weeks. The continuation phase of treatment phase occurs during the first six months after improvement and aims to prevent relapse of the original depressive episode. Maintenance treatment is provided to prevent recurrence of a new depressive episode after remission (Frank 1991).
Acute treatment
Antidepressants have been shown to be efficacious in adults, compared to placebo, for acute treatment of major depressive disorder in the short term, although the effect is small (Cipriani 2018). In a previous Cochrane Review (Arroll 2009), authors found that for depression in primary care, between seven and eight people needed to be treated with an SSRI, and between seven and 16 people with a TCA, for one person to experience improvement in depression due to antidepressant use (i.e. number needed to treat for an additional beneficial outcome (NNTB)). A review of 131 randomised controlled trials (RCTs) revealed that SSRIs significantly reduced scores on the Hamilton Depression Rating Scale (HDRS) at completion of treatment, but this was below the threshold of clinical significance (Jakobsen 2017). However, criticism about lack of evidence for effectiveness of antidepressants in acute treatment of mild to moderate depressive disorders is increasing due to important limitations of the available evidence base (Hengartner 2017; McCormak 2018; Munkholm 2019). Evidence is insufficient to support or contest the efficacy of antidepressant medication for subthreshold and mild depressive disorder (Cameron 2014).
Continuation and maintenance treatment
Evidence suggests that continuation of antidepressant treatment is effective, as it reduces risk of relapse and recurrence by 50% to 70% (Geddes 2003; Glue 2010; Kaymaz 2008), although none of the trials on which this conclusion was based measured withdrawal effects (Cohen 2019; Hengartner 2020; Recalt 2019), and almost all trials were conducted in secondary care and probably are not representative of primary care patients (Aroll 2017). Evidence for treatment durations longer than nine months is relatively limited, with lack of long‐term trials in which participants were randomised six months after remission to determine the effects of treatment after this time (Kaymaz 2008). A previous Cochrane Review on continuation and maintenance trials for older people with depressive disorders found that the long‐term benefits and harms of continuing antidepressant medication for prevention or recurrence of depression are not clear, and no firm treatment recommendations can be made (Wilkinson 2016). Moreover, evidence showed no association between relapse rates and duration of treatment (Geddes 2003), and there was no evidence to justify the defined clinical distinction between continuation treatment (six to nine months) and maintenance treatment (two years or longer; Kaymaz 2008). Another Cochrane Review of trials in people with persistent depressive disorder found that it is uncertain whether continued or maintained pharmacotherapy (or both) with antidepressant agents is a robust treatment for preventing relapse and recurrence due to moderate or high risk of bias (Machmutow 2019).
Guidelines have underlined the importance of giving antidepressants for a sufficient period of time. The duration of continuation treatment recommended in depression guidelines varies from four months to 12 months after remission (APA 2010; CANMAT 2016; Declercq 2017; NHG 2012; NICE 2009; WHO 2017). Continuation treatment should be continued for at least two years after remission for those at high risk of relapse (APA 2010; CANMAT 2016; Declercq 2017; NHG 2012; NICE 2009; WHO 2017), which is defined as having two or more episodes, residual symptoms, or severe previous episodes (Geddes 2003). However, these time points were based on the findings of Geddes 2003, in spite of the fact that there was no association between relapse rate and duration of treatment. People with depression on long‐term maintenance treatment should be regularly re‐evaluated. Due to lack of evidence for the optimal duration of continuation and maintenance treatment, most guidelines are based on expert opinion.
Anxiety disorders
After depression, anxiety disorders (e.g. generalised anxiety disorder, panic disorders, any other anxiety disorder) are the most frequent indications for which antidepressants are prescribed (Wong 2016). Both cognitive‐behavioural therapy (CBT) and antidepressants are first‐line options for treatment of anxiety disorders with proven effectiveness (Batelaan 2017; NICE 2011). Antidepressant treatment duration of up to one year results in lower relapse rates among responders compared with treatment discontinuation in anxiety disorders, irrespective of the type of anxiety disorder (Batelaan 2017). However, withdrawal confounding bias may lead to overestimation of the effects of antidepressants (as many symptoms of withdrawal overlap with domains on an anxiety score), and long‐term trials are scarce. International guidelines are therefore consensus‐based and advise continuation of treatment for variable durations (6 to 24 months; Baldwin 2014; NICE 2011).
Long‐term antidepressant use
Long‐term antidepressant prescription is driving much of the rise in antidepressant use (Brett 2017; Kjosavik 2016; Mars 2017). In The Netherlands, approximately 30% of people taking antidepressants take them longer than one year (Meijer 2004). In the UK, nearly half of antidepressant users (8% of the total population) have been taking them longer than two years (Johnson 2012), and in the USA, half of antidepressant users (7% of the total population) have been taking them longer than five years (Mojtabi 2014). In Australia, the average duration of treatment with antidepressants is about four years (Kjosavik 2016). High antidepressant use has also been reported in people living in nursing homes. About 40% of Belgian nursing home residents take antidepressants daily (Bourgeois 2012), with similar rates seen in other European countries (Janus 2016), as well as in the USA (Karkare 2011).
Use of antidepressants that are initially appropriate but are not discontinued after the treatment period can lead to long‐term unnecessary medication. A recent trial described inappropriate duration of antidepressants, for example, longer than needed, as “legacy prescribing” (Mangin 2018).
Trials suggest that the motives and barriers of patients and physicians in continuing or discontinuing antidepressants are numerous and complex (Bosman 2016; Maund 2018). A recent review synthesised 49 barriers and facilitators in nine themes related to patient perspectives: psychological and physical capabilities, perception of antidepressants, fears of relapse and/or recurrence, intrinsic motivators and goals, the physician as a navigator to maintenance or discontinuation, perceived cause of depression, aspects of information that support decision‐making, significant others (a help or a hindrance), and support from other health professionals (Maund 2018). A key barrier was the patient's belief that the physician was responsible for initiating discussions about discontinuation. Other identified barriers are related to belief in a ‘chemical imbalance’, availability of supportive non‐pharmacological guidance during discontinuation, personal circumstances of the patient, and underlying considerations and knowledge of patients and physicians (Eveleigh 2019; Bosman 2016). Patients continued to use antidepressants because of fear of relapse ‐ a perceived biological cause for depressive symptoms ‐ and their experience of improved functioning. It was also easier for physicians to prescribe antidepressants for depression as they are more accessible than psychological treatment. In one open, single‐arm trial with older patients from nursing homes, resistance to discontinuing antidepressants largely came from the relatives, not the carers, of older individuals (Lindstrom 2007).
Discontinuing antidepressant treatment can be complicated by the potential for patients to experience withdrawal symptoms. Withdrawal symptoms refer to physical and psychological symptoms that occur with stopping, missing, or reducing doses (even with gradual tapering) of any antidepressant (APA 2013). Symptoms generally begin two to four days after abrupt discontinuation of antidepressants that had been taken continuously for at least one month (APA 2013). Late onset or longer persistence, lasting weeks to months, has also occurred (Davies 2019). Antidepressant withdrawal reactions are widespread. A recent review suggests incidence rates from 27% to 86%, with an average incidence of 56%, and with up to half of those experiencing withdrawal symptoms having severe reactions (Davies 2019).
Withdrawal symptoms often are very similar to symptoms of relapse or recurrence and sometimes may be misleading and misdiagnosed as depressive recurrence, leading to unnecessary continuation of antidepressant use. If the same or a similar drug is restarted, withdrawal symptoms will usually resolve fully within one to three days, and subsequently the antidepressant can be withdrawn more cautiously (Haddad 2007). Withdrawal symptoms can be distinguished from relapse of the original disorder by their rapid onset after antidepressants are stopped. Whereas relapse is uncommon in the first weeks after stopping, rapid response after the original antidepressant is reintroduced and the presence of somatic and psychological withdrawal symptoms are different from the original depressive or anxiety disorder (Haddad 2007; Horowitz 2019). However, many withdrawal variations after discontinuation are possible including late onset of symptoms (sometimes after several months), making misdiagnosis of withdrawal possible. Additionally, common withdrawal reactions can involve low mood or anxiety symptoms. As antidepressants have been widely prescribed for depression and anxiety disorders, it is a challenge to distinguish withdrawal from relapse of the original disorder (Davies 2019). The risk of withdrawal symptoms is higher with antidepressants with a shorter half‐life (the time taken for blood concentration to be reduced by 50%) when high doses have been prescribed and in cases where rapid tapering occurs (APA 2013). This risk also appears to be higher for those who have taken antidepressants for eight weeks or longer (APA 2013). It is important to note that the majority of relapse prevention discontinuation trials did not distinguish relapse from symptoms of withdrawal after discontinuation; this is a confounder that makes study findings difficult to interpret.
Description of the intervention
Guidelines recommend discontinuation of antidepressant drugs after successful treatment (NICE 2009). Abrupt discontinuation of antidepressants may lead to temporary withdrawal symptoms.
A common strategy is to taper (gradually reduce the dose) antidepressants over weeks to reduce the risk of withdrawal symptoms (NICE 2009). NICE recommends gradually reducing the antidepressant dose every one to two weeks over a four‐week period, although some people may require longer treatment periods, particularly with drugs with a shorter half‐life (such as paroxetine and venlafaxine; NICE 2009). Evidence is lacking regarding strategies for discontinuation of antidepressants, and the optimal method of stopping antidepressants is currently unknown; however slow enough tapering can make withdrawal symptoms tolerable (Horowitz 2019).
Furthermore, the Royal College of Psychiatry in the UK recommended that use of antidepressants should always include discussion with the patient and with family/carers (as appropriate) about potential levels of benefit and harm, including withdrawal (RCP 2019).
Psychological interventions in combination with discontinuation of antidepressants could support the process of discontinuation.
CBT focuses on the cognitive content of negative thinking and on learning a repertoire of coping skills appropriate to target thoughts, beliefs, or problem areas (NICE 2009). Patients work with a therapist, either face to face or via telecommunication technologies (online therapy), and CBT can be delivered individually or in groups. A meta‐analysis on the sequential integration of pharmacotherapy and psychotherapy in major depressive disorders suggests that participants receiving CBT after antidepressant discontinuation were significantly less likely to relapse compared to those receiving continued pharmacotherapy (Guidi 2016). A recent review found that risk of relapse or recurrence was lower with CBT plus tapering compared to clinical management plus tapering after two years in two trials (Maund 2019).
Mindfulness‐based cognitive therapy (MBCT) is a group‐based clinical intervention programme designed to reduce relapse or recurrence of major depressive disorders by means of systematic training in mindfulness meditation combined with cognitive‐behavioral methods (Piet 2011). MBCT is a manualised, group‐based skills training programme intended to teach people skills to deal with their negative feelings and thoughts as a part of their lives by becoming aware of negative cognitive patterns (Piet 2011). A meta‐analysis of six trials showed that MBCT suggests that the 12‐month relapse/recurrence risk compared with usual care or placebo, with a relative risk reduction of 43%, in a prespecified subgroup of patients with a history of three or more episodes of depression (Piet 2011). A recent systematic review about the effectiveness of interventions to manage antidepressant discontinuation found that in two trials, relapse or recurrence rates were similar for MBCT in combination with tapering and for maintenance antidepressants (Maund 2019).
Low‐intensity psychological or psychosocial interventions may use simple approaches that are less complex to undertake than CBT or MBCT. Contact with people is generally briefer than in other forms of therapy and can be delivered by paraprofessionals or peer supporters via non‐traditional methods such as telephone or the Internet. The literature provides no clear definition of low‐intensity psychological or psychosocial intervention for depression or anxiety (NICE 2009; Rodgers 2012). The NICE guidelines reflect evidence on three main forms of low‐intensity psychosocial interventions for acute treatment of depression: individual, guided self‐help based on the principles of CBT, and computerised CBT and a structured group physical activity programme (NICE 2009). However, low‐intensity psychological or psychosocial interventions can also be used for relapse prevention. One trial suggested that the combination of a self‐help intervention with a self‐help book and weekly guidance in primary care could prevent relapse of depression in participants with recurrent depressive episodes (Biesheuvel 2015). Online psychosocial interventions could offer online modules and information to patients and practitioners to support antidepressant discontinuation (ISRCTN15036829).
Other interventions such as antidepressant discontinuation can be supported by minimal intervention. Minimal interventions were defined as simple interventions applicable to large groups of people, for example, an advisory letter or a meeting at which people with long‐term antidepressant use are advised to stop taking the drug (Voshaar 2006). A minimal intervention can also be a guided primary care clinician review of the medication and the condition (Johnson 2012), or it can comprise a letter to the clinician with a recommendation to discontinue antidepressant with tapering advice (Eveleigh 2017). This was based on previous evidence when a letter with discontinuation advice was sent to long‐term benzodiazepine users in family practice, followed by a follow‐up consultation (Gorgels 2005).
These findings suggest that cognitive therapy and MBCT in combination with discontinuation of antidepressants are potential alternatives to antidepressants for preventing relapse or recurrence. However, CBT and MBCT are resource intensive, and access to these psychological interventions is often limited. Low‐intensity psychological or psychosocial interventions have the potential to reach more people.
Pharmacological treatment with benzodiazepines is suggested by some experts as short‐term treatment to counteract insomnia associated with withdrawal (Haddad 2007; NHG 2018). However, benzodiazepines may contribute to risk of dependence, especially in older people, and prescribing long‐term benzodiazepines as a substitute for unnecessary antidepressant treatment is not appropriate (Pottie 2018; Wilson 2010). Tapering can also be facilitated by a changed pharmacological form (e.g. liquid paroxetine or fluoxetine) (Wilson 2015). Some antidepressants are available in tapering strips by which each strip contains a slightly lower dose on each consecutive day (Groot 2013).
How the intervention might work
Discontinuation of antidepressants may decrease risk of adverse events and risk of drug‐drug interactions and may minimise the number of medicines, whilst making no difference in depressive and anxiety symptoms. However, discontinuation might cause withdrawal symptoms and recurrence or worsening of the original depressive or anxiety symptoms, which contributes to unsuccessful discontinuation.
The exact therapeutic mechanism of antidepressants is not known (Pringle 2011). Most antidepressants seem to increase concentrations of monoamine neurotransmitters in the synaptic cleft (Berton 2006). The effect of most antidepressants fully develops after some weeks, indicating that neurophysiological changes in brain tissue (e.g. changes in sensitivity and frequency of receptors) occurring in the presence of a constant level of active ingredients are necessary for improvement in depressive symptoms (Machmutow 2019). Others have suggested a non‐physiological mechanism for this change: the placebo effect (Kirsch 2019). Depending on the active ingredient, antidepressants can have mood‐enhancing, anxiolytic, or sedative effects and are able to increase or decrease inner drive (Machmutow 2019). The rationale for continuing antidepressant treatment after clinical recovery is that it will sustain the regulation of monoamine activity (Wilkinson 2016). However, suggesting that a single biochemical deficiency is the cause of depression and that antidepressants work by correcting chemical deficiency is not correct.
Various explanations have been proposed for the mechanism of withdrawal symptoms seen when antidepressants are stopped (Fava 2015; Horowitz 2019). Daily drug treatment activates receptors, which in turn can affect the availability of several neurotransmitters that can lead to many downstream physiological consequences. When drug treatment abruptly stops, the homeostatic equilibrium is disturbed and the body’s adaptive changes take time to re‐calibrate, resulting in a period of possible withdrawal symptoms (Fava 2015; Horowitz 2019). The neurobiological mechanism of tapering is based on the rationale that biological systems will have more time to adapt to reductions in available ligand, thus reducing the intensity of withdrawal symptoms (Fava 2015).
Additional non‐pharmacological treatments can support discontinuation of antidepressants. CBT approaches focus on dysfunctional thoughts, feelings, and behaviours and learning skills (NICE 2009). MBCT was specifically developed to reduce relapse and recurrence in depression (Piet 2011; Segal 2002). However, the exact mechanisms of preventing relapse and recurrence of psychological interventions remain unclear (Beshai 2011).
Symptoms of discontinuation of antidepressants could be treated by short treatment with benzodiazepines. Benzodiazepines act by binding at, and enhancing the effect of, gamma‐aminobutyric acid (GABA) receptors (Ma 2019). Enhancement of the effects of GABA on this receptor results in sedative, anxiolytic, hypnotic, and muscle relaxant effects, thus reducing withdrawal symptoms. A changed pharmacological form enables a more precise and graduated taper (Wilson 2015).
It is important that practitioners share decision‐making about discontinuation strategies with patients and with their relatives for successful antidepressant discontinuation.
Why it is important to do this review
Antidepressant use can be accompanied by minor adverse events as well as by serious adverse events. Although recommendations have underlined the importance of giving antidepressants for a sufficient length of time, concern about overuse of antidepressants (i.e. longer than recommended) for depression and for a growing number of other conditions is on the rise (Kjosavik 2016; Wong 2017). Reviews suggest that 30% to 50% of long‐term antidepressant prescriptions had no evidence‐based indication supporting their use, and that users could try to stop treatment (Ambresin 2015; Cruickshank 2008; Piek 2010).
The effectiveness of interventions aimed at discontinuation of long‐term antidepressant use is unknown. Most antidepressant guidelines recommend a slow taper approach over several weeks (APA 2010; CANMAT 2016; NICE 2009). NICE recommendations for stopping antidepressants include gradually reducing the dose, usually over a four‐week period (NICE 2009). A non‐systematic review found that slower tapering over months leads to fewer withdrawal symptoms (Horowitz 2019). However, most of the evidence for discontinuing more gradually comes from observational cohort studies and case reports. Overall, strong evidence indicating whether long‐term antidepressants can be discontinued effectively and safely is lacking.
Therefore we performed a systematic review of approaches to discontinuation among participants using antidepressants for longer periods than recommended, defined as use over six months or longer.
A systematic review of discontinuation trials on long‐term antidepressants will assist clinicians and patients in shared decision‐making about an evidence‐based choice for appropriate antidepressant prescribing and will have an impact on guidelines for management of depressive and anxiety disorders.
Objectives
To assess the effectiveness and safety of approaches for discontinuation versus continuation of long‐term antidepressant use for depressive and anxiety disorders in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), published and unpublished trials, open‐label and double‐blinded trials, as well as cluster‐RCTs. We included both placebo‐controlled and non‐placebo‐controlled trials. We excluded cross‐over trials.
Types of participants
Participant characteristics
Trial participants, aged 18 years and older, using long‐term antidepressants prescribed for depressive or anxiety disorder. Long‐term is defined as use of any antidepressant treatment for at least six months. Diagnosis of depressive and anxiety disorders was defined by trial authors. We included trials exploring any of the following classes of antidepressants.
Selective serotonin reuptake inhibitors (SSRIs).
Serotonin‐noradrenaline antidepressants (SNRIs).
Noradrenaline reuptake inhibitors (NARIs).
Tricyclic antidepressants (TCAs).
Noradrenaline‐dopamine reuptake inhibitors (NDRIs).
Monoamine oxidase inhibitors (MAOIs).
-
Other antidepressants.
Melatonergic antidepressants.
Noradrenergic and specific serotonergic antidepressants (NASSAs).
Serotonin antagonist and reuptake inhibitors.
Multi‐modal serotonin reuptake inhibitors and receptor blockers.
St. John's Wort (Hypericum perforatum) and other natural products.
We did not place restrictions on the dose of antidepressant treatment.
In this review, we used the term 'depression' to refer to the DSM‐V diagnosis of major depressive disorder.
Settings
We included trials conducted in a range of settings (e.g. primary care, outpatient psychiatric hospital, nursing home).
Exclusion criteria
We excluded trials that included participants with or with a history of bipolar disorder, obsessive‐compulsive disorder, or psychosis.
We excluded discontinuation trials in the context of electroconvulsive therapy or hospital admission.
We excluded discontinuation trials if participants were not treated in line with the recommended duration of treatment. Therefore, we excluded discontinuation trials if participants had received less than six months of antidepressant treatment.
Types of interventions
We included trials that compared discontinuation to continued antidepressant use or usual care.
Experimental Intervention
We defined discontinuation of antidepressants as one or more of the following interventions.
Abrupt discontinuation: abruptly discontinuing an antidepressant using placebo or no medication.
Tapering: gradually reducing the dose until complete discontinuation of antidepressant use by using placebo or no medication.
Combined intervention (high‐intensity intervention): one or more high‐intensity psychological interventions combined with discontinuation of antidepressants, either abruptly or by tapering. Psychological interventions must have been based on a scientific theory, with at least one contact between therapist and participant, either face to face or via telecommunication technologies. The intervention must have considered the personal needs of participants or a group of participants. We considered CBT, MBCT, behaviour therapy, interpersonal therapy (IPT), behaviour modification/therapy, or any other psychologically oriented interventions.
Combined intervention (low‐intensity psychological or psychosocial intervention): one or more psychosocial interventions combined with discontinuation of antidepressants, either abruptly or by tapering. Psychosocial interventions are simple approaches that are less complex to undertake than formal high‐intensity psychological interventions; contact with people is generally briefer than with other forms of therapy and can be delivered by para‐professionals or peer supporters by non‐traditional methods such as telephone or the Internet. We considered individual guided self‐help based on the principles of CBT, computerised CBT, a structured group physical activity programme, or any other psychosocial interventions.
Discontinuation: attained abruptly or by tapering with a minimal intervention, such as giving simple advice in the form of a letter to the clinician or long‐term antidepressant user or presenting a meeting to a large group of people.
Discontinuation: attained abruptly or by tapering with pharmacological treatment to counteract antidepressant withdrawal symptoms, for example, benzodiazepines as short‐term treatment to support discontinuation and to counteract insomnia associated with discontinuation or tapering, or with a changed pharmacological form (e.g. liquid paroxetine or fluoxetine).
Comparator intervention
Continuation of antidepressant use
Usual care or treatment as usual
Co‐interventions of any kind of non‐pharmacological treatments for discontinuation in intervention and control groups are allowed.
Types of outcome measures
We included trials that met the above inclusion criteria regardless of whether they reported on the following outcomes.
We used the definitions of diagnosis, response, relapse, and recurrence as provided by trial authors. Appendix 1 lists abbreviations for the measuring instruments used in this review.
Primary outcomes
-
Successful discontinuation rate: the proportion (%) of participants who successfully stopped antidepressants at the end of the trial. We defined successful discontinuation rate as:
no antidepressant use;
absence of depressive or anxiety symptoms or diagnosis, or both; and
no dropout before the end of the trial
-
Relapse rate: the proportion (%) of participants who did not successfully stop antidepressants at the end of the trial due to relapse or recurrence of depressive and/or anxiety symptoms and/or diagnosis. We defined relapse rate as:
relapse of depressive and/or anxiety symptoms and/or diagnosis after continuation;
relapse of antidepressant use after discontinuation due to the presence of depressive and/or anxiety symptoms and/or diagnosis;
relapse of depressive and/or anxiety symptoms after discontinuation; or
dropout from the trial due to relapse to depressive and/or anxiety symptoms and/or diagnosis. We used the definition of relapse as defined by study authors
Presence of withdrawal symptoms (measured by Discontinuation‐Emergent Signs and Symptoms (DESS) Scale (Rosenbaum 1988), symptoms assessment form, Unified Parkinson Disease Rating Scale, or any relevant instrument)
Any adverse events attributable to continuation of antidepressant use
Secondary outcomes
Depressive symptoms and anxiety symptoms as measured on a scale (measured by the Hamilton Depression Rating Scale (HDRS; Hamilton 1960), the Montgomery‐Åsberg Depression Rating Scale (MADRS; Montgomery 1997), the Beck Depression Inventory (BDI; Beck 1961), the Inventory of Depressive Symptomatology (IDS; Rush 2000), the Patient Health Questionnaire (PHQ; Spitzer 1999), the Beck Anxiety Inventory (BAI; Beck 1993), the Hamilton Anxiety Scale (HAM‐A) (Hamilton 1959; Maier 1988), the General Anxiety Disorder Scale (GAD‐7; Spitzer 2006), the Panic and Agoraphobia Scale (PAS; Bandelow 1992), or any other instrument)
Time to relapse after randomisation (measured in weeks)
Quality of life of participants (measured by Short Form 36 (SF‐36; Ware 1992), Short Form 12 (SF‐12; Gandek 1998), or any other instrument)
Social and occupational functioning (measured by Global Assessment of Function Scale (GAF; APA 2000), Occupational Functioning Scale (OFS; Hannula 2006), or any other instrument)
Severity of a patient's illness as assessed by a health professional (clinical impression measured by Clinical Global Impression of Change Scale (CGI‐C; Busner 2007), or any other instrument)
Timing of outcome assessment
We analysed effects of discontinuation over the short term (trials with follow‐up of four weeks or less), over the titration period used in the trial, over the medium term (trials with follow‐up from four weeks to six months), and over the long term (trials with follow‐up of six months or longer after discontinuation).
Hierarchy of outcome measures
We considered outcome measurements at the pre‐defined endpoint of the trial.
If trials used different outcome measures, we included data as per the following rules: in cases of available data from both observer‐rated scales and self‐report questionnaires, we prioritised data from observer‐rated scales. We used DSM‐V definitions for depressive disorder and anxiety disorder (APA 2013), relapse and recurrence definitions of Frank 1991, the Hamilton Rating Scale for Depression (HAM‐D) for depressive symptoms, and the HAM‐A scale for anxiety symptoms. In cases of several outcome measures of the same hierarchy level used in one trial, we selected the outcome measure most frequently used across all trials. In cases of several outcome measures at the same hierarchy level and the same availability across trials, we randomly selected the outcome measure. If trials did not report the HAM‐D or the HAM‐A, when applicable, we selected the outcome measure used most frequently across all trials.
Search methods for identification of studies
Electronic searches
Information Specialists with Cochrane and the Centre for Reviews and Dissemination (CRD), University of York, conducted searches of the following bibliographic databases using relevant subject headings (controlled vocabularies) and search syntax, as appropriate to each resource. The date of the latest search was 21 April 2020.
CCMD Specialised Register (CCMD‐CTR) (all available years) (Appendix 2).
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 4), in the Cochrane Library (searched 21 April 2020) (Appendix 3).
Ovid MEDLINE databases (1946 to January 16, 2020).
Ovid Embase (1974 to 2020 January 16).
Ovid PsycINFO (all years to January Week 1 2020).
We did not apply any restrictions on date, language, or publication status to the searches.
We searched international trials registries via the World Health Organization trials portal (ICTRP) and ClinicalTrials.gov to identify unpublished or ongoing trials.
Searching other resources
Grey literature
We searched the grey literature for dissertations and theses.
Open Grey (www.opengrey.eu/).
ProQuest Dissertations & Theses Global (www.proquest.com/).
DART‐Europe E‐theses Portal (www.dart-europe.eu/).
Reference lists
We checked the reference lists of all included trials and relevant systematic reviews to identify additional trials missed during the original electronic searches (e.g. unpublished citations, in‐press citations).
Correspondence
We contacted trial authors and subject experts for information on unpublished or ongoing trials, or to request additional trial data.
Data collection and analysis
Selection of studies
Two review authors (EVL, MD) independently screened titles and abstracts for inclusion of all potential trials identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text trial reports and publications, and two review authors (EVL, MD) independently screened the full text, identified trials for inclusion, and identified and recorded reasons for exclusion of ineligible trials. We resolved any disagreements through discussion, or, if required, we consulted a third review author (MVD). We identified and excluded duplicate records, and we collated multiple reports that relate to the same trial, so that each trial rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), along with Characteristics of included studies and Characteristics of excluded studies tables.
Data extraction and management
We used a data collection form that we had piloted on at least one trial in the review, to extract trial characteristics and outcome data. Two review authors (EVL, LR) independently extracted trial characteristics and outcome data from included trials. We extracted the following trial characteristics.
Methods: trial design, total duration of trial, details of any 'run‐in' period, number of trial centres and locations, trial settings, withdrawals, and dates of trials.
Participants: number, mean age, age range, gender, severity of condition, duration of antidepressant treatment before trial, comorbidity, setting (primary care versus outpatient psychiatric hospital versus nursing home), diagnostic criteria, inclusion criteria, and exclusion criteria.
Interventions: intervention inclusive mode of discontinuation (gradually or abruptly), comparison, and concomitant treatment (psychological treatment, medications).
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
We noted in the Characteristics of included studies table if trials did not report outcome data in a usable way. We resolved disagreements by reaching consensus or by involving a third review author (MVD). One review author (EVL) transferred data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data had been entered correctly by comparing data presented in the systematic review with data provided in trial reports. A second review author (LR) spot‐checked trial characteristics for accuracy against the trial report.
Main planned comparisons
Abrupt discontinuation versus continued antidepressant use or usual care
Tapering versus continued antidepressant use or usual care
Combined intervention (discontinuation with high‐intensity psychological treatment) versus continued antidepressant use or usual care
Combined intervention (discontinuation with low‐intensity psychological intervention or psychosocial treatment) versus continued antidepressant use or usual care
Combined intervention (discontinuation with minimal intervention) versus continued antidepressant use or usual care
Discontinuation with pharmacological treatment versus continued antidepressant use or usual care
Co‐interventions of any kind of non‐pharmacological treatment for discontinuation in intervention and control groups were allowed.
A single overall comparison of discontinuation versus continuation was not planned because we anticipated the interventions would be too heterogeneous to allow an overall estimate that is meaningful and reliable.
Assessment of risk of bias in included studies
Two review authors (EVL, LR) independently assessed risk of bias for each trial using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by consultation with another review author (MVD). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We assessed each potential source of bias as high, low, or unclear and provided a supporting quote from the trial report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different trials for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a participant‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with trial authors, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for trials that contributed to that outcome.
Measures of treatment effect
Dichotomous data
We analysed dichotomous data as proportions and expressed pooled estimates as odds ratios (ORs) with 95% confidence intervals (95% CIs).
Continuous data
For continuous data, we entered data from various scales, questionnaires, and other clinical measures. We analysed continuous data as means and expressed the pooled estimate as a mean difference (MD) if trials used the same scale, or as a standardised mean difference (SMD) if trials used different scales to measure the same outcome, along with a standard deviation (SD). We calculated a 95% CI for each estimate. We analysed ‘time to relapse’ as hazard ratios when data were provided as time to event. We extracted the reported HR or calculated the estimated HR from information presented in the trial report (Tierney 2007).
Unit of analysis issues
Participants in RCTs are the unit of analysis.
Cluster‐randomised trials
We planned to incorporate results from cluster‐RCTs into the review using generic inverse variance methods (Deeks 2017). With cluster‐RCTs, it is important to ensure that data were analysed with consideration of their clustered nature. We extracted the intra‐class correlation coefficient (ICC) for each trial. We adjusted for the clustering effect by dividing the clusters by a 'design effect'. We calculated this by using the number of participants or the mean size per cluster and the ICC. If ICCs were not reported, we requested them from trial authors. If these data were not available, we planned to use estimates from similar trials to 'correct' data for clustering when this has not been done (Deeks 2017).
Trials with multiple treatment groups
For trials with two or more active treatment arms, we undertook the following approach according to whether the outcome was continuous or dichotomous. For a continuous outcome, we pooled means, SDs, and the number of participants for each active treatment group across treatment arms as a function of the number of participants in each arm for comparison against the control group (Deeks 2017). For a dichotomous outcome, we combined active treatment groups into a single arm to compare against the control group (in terms of numbers of people with events and sample sizes) or to split the control group equally (Deeks 2017). This means that we divided out the number of participants in the shared intervention group evenly among comparisons. For dichotomous outcomes, we divided up the number of events and the total number of patients. For continuous outcomes, we divided up only the total number of participants and left means and standard deviations unchanged.
Dealing with missing data
When possible, we carried out an intention‐to‐treat (ITT) analysis for all outcomes. We contacted investigators or trial sponsors to verify key trial characteristics and to obtain missing numerical outcome data when possible (e.g. when a trial is identified as abstract only). We documented all correspondence with trial authors and reported in the review which trial authors responded. We calculated the proportion lost to follow‐up for each group and reported this. We did not make any assumptions about loss to follow‐up for dichotomous or continuous data. We conducted a complete‐case analysis and included in the analysis only participants with a recorded outcome.
Assessment of heterogeneity
We assessed heterogeneity in two ways. First, we explored the presence of heterogeneity at face value by comparing population groups, interventions, or outcomes across trials. In the case of clear face value heterogeneity, we did not pool the results. We performed meta‐analysis only when trials were sufficiently homogeneous in terms of participants, interventions, and outcomes. If there was no obvious clinical heterogeneity, we used statistical tests such as the Chi² test and the I² statistic to determine the presence and level of statistical heterogeneity for each outcome (Higgins 2003). We interpreted the I² statistic, accompanied by a statistically significant Chi² test, as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity (Deeks 2017).
Assessment of reporting biases
To minimise risk of publication bias, we performed a comprehensive search of multiple databases, including searching for unpublished trials. We investigated potential publication bias by using a funnel plot when at least 10 trials met the inclusion criteria of the review.
Data synthesis
We used a random‐effects model because we assumed that the included studies would not be sufficiently similar and would show considerable clinical and methodological heterogeneity (Deeks 2017).
When a meta‐analysis was not possible (e.g. owing to insufficient data, due to substantial heterogeneity), we described the results for individual trials separately. We discussed the heterogeneity of included trials and the external validity of the review in the section Overall completeness and applicability of evidence. We used hazard ratios (HRs) to compare the outcome time to relapse between trials. We pooled the log (HRs) for time‐to‐event data using a random‐effects model and the generic inverse variance method in Review Manager 5 (RevMan 2014). When data for HR were not available, and when providing follow‐up was the same for each treatment group, we calculated relative risk and used relative risk for relapse to compare time to relapse. We calculated the primary outcomes (i.e. successful discontinuation rate, relapse rate, withdrawal symptoms, and adverse events due to antidepressant use) as the proportions of all participants included in both intervention groups (ITT), and as the proportions of all participants with the intention to attempt discontinuation of unnecessary antidepressants (per‐protocol analysis).
Subgroup analysis and investigation of heterogeneity
We investigated heterogeneity by conducting pre‐specified subgroup analyses, and we compared subgroups using the formal test for subgroup differences for primary outcomes.
Age group: younger than 65 years versus 65 years and older. Harms of antidepressants and higher risk for drug‐drug interactions are more common in older people with often existing polypharmacy and comorbidities due to changed pharmacokinetics and pharmacodynamics.
Setting: primary care versus outpatient specialist clinics. Primary care can comprise consultation in primary care or in a nursing home/aged care facility. The setting could influence the effect due to severity of disease treated with antidepressants in outpatient specialist clinics.
Indication of antidepressant drugs: anxiety versus depression. The original indication could influence the success of discontinuation due to differences in clinical presentation and management of anxiety and depression.
Types of antidepressants: TCA versus SSRI. The type of antidepressant could influence the effect due to higher risk of adverse events with TCAs because of their anticholinergic properties. TCAs have comparable effectiveness to SSRIs with a less favourable risk–benefit ratio (NICE 2009).
Duration of antidepressant treatment: one year or longer versus less than one year. The recommended duration of continuation of treatment after remission varies from 4 months to 12 months in depression guidelines. Duration of use could influence the success of discontinuation due to dependence on medication (Kendrick 2015).
Sensitivity analysis
We carried out sensitivity analysis to assess the impact on the effect estimate of trials with high risk of bias. We tested the impact of including trials assessed as having high risk of bias by removing trials with at least one high risk rating in the 'Risk of bias' assessment. We performed additional sensitivity analyses to test the impact of industry sponsorship by excluding trials with industry sponsorship or with an unclear source of funding because drug companies whose primary interest may not be in discontinuation. We performed an additional sensitivity analysis to test the impact of a long tapering scheme as current guidelines recommend tapering over 4 weeks and more.
Summary of findings and assessment of the certainty of the evidence
We included a ’Summary of findings’ table, prepared using GRADEpro GDT, for seven outcomes.
Successful discontinuation rate.
Relapse rate.
Withdrawal symptoms.
Adverse events due to antidepressant use.
Depressive and anxiety symptoms
Quality of life.
Social and occupational functioning.
Three review authors (EVL, MVD, TC) used the GRADE approach independently to assess evidence certainty for all outcomes. We assessed evidence as high, moderate, low, or very low certainty, depending on the seriousness of concern about risk of bias, imprecision, inconsistency, indirectness, and publication bias. We presented the comparisons 'abrupt discontinuation versus continuation', 'discontinuation by tapering versus continuation', 'discontinuation combined with support from high‐intensity psychological interventions versus continuation', and 'discontinuation with a minimal intervention compared to usual care' in the 'Summary of findings' tables. For each outcome in the 'Summary of findings’ table, we presented a summary of available data, the magnitude of effect size, and the certainty of evidence. We justified all decisions to downgrade the certainty of evidence in the footnotes of the 'Summary of findings' table.
We prioritised outcomes over the medium term (from four weeks to six months) and over the long term (follow‐up six months or longer).
Results
Description of studies
Results of the search
In May 2019 and January 2020 an Information Specialist conducted searches of the CCMD‐CTR, MEDLINE, Embase, PsycINFO, CENTRAL, trial registries, and several sources of grey literature and by checking reference lists of other relevant systematic reviews, retrieving a total of 10,148 records. The search of CENTRAL was also updated and appended, April 2020. After removal of duplicates, we retained 7320 records. Two review authors (EVL, MD) independently screened all 7320 records by title and abstract and excluded 7173 records, as they did not meet the inclusion criteria. Two review authors (EVL, MD) independently checked each of the 147 full‐text articles for eligibility. We excluded 78 full‐text articles, leaving 33 studies reported in 64 references and 2 ongoing studies (Duffy 2019; ISRCTN12417565). Three studies are awaiting assessment (Gunn 2020; Mangin 2015; Molenaar 2016); we have contacted the study authors to obtain further data. The reasons for excluding 78 records are listed under Characteristics of excluded studies.
See Figure 1.
1.
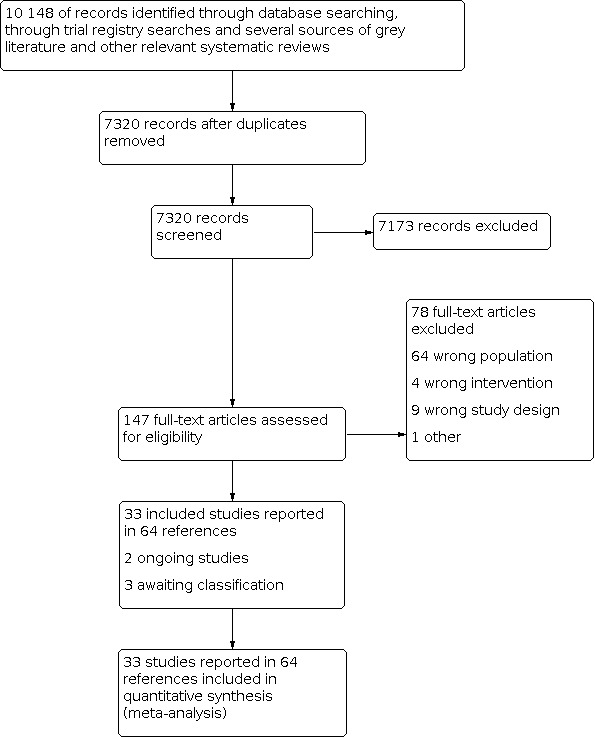
Study flow diagram.
Included studies
We included 33 studies described in 64 publications involving 4995 participants (see Characteristics of included studies).
Kane 1982 included participants with bipolar disease and with unipolar disease, but we included only the study arms with participants with unipolar depression who were treated with antidepressants (or discontinued the antidepressant).
We included three three‐arm studies. The "shared" treatment group was divided evenly into two smaller groups. These studies were split for the purposes of analysis as marked with footnotes (Bockting 2018; Khan 2014; Segal 2010).
Three studies included two different treatment arms before randomisation; each treatment arm was randomised separately, but results were reported in a single publication. These studies were split for the purposes of analysis as marked with footnotes (Derubeis 2019; Peterson 2010; Stewart 1997).
In three included studies, responders in the antidepressant treatment group entered a subsequent study (responders to antidepressant treatment in Keller 2007 and Mavissakalian 1999 entered, respectively, Kocsis 2007 and Mavissakalian 2001). Rickels 2010 included results of the first trial and the subsequent trial. Rickels 2010 was split for the purposes of analysis as marked with footnotes.
Design
Thirty‐two studies were RCTs, and one was a cluster‐RCT (Eveleigh 2018).
Twenty‐six studies included a placebo arm (Bialos 1982; Cook 1986; Gelenberg 2003; Gilaberte 2001; Kane 1982; Keller 1998; Keller 2007; Khan 2014; Klysner 2002; Kocsis 1996; Kocsis 2007; Kornstein 2006; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Segal 2010; Stewart 1997; Terra 1998; Wilson 2003). None of these studies used an active placebo tablet. In eight studies, participants discontinued (or tapered) the antidepressant and the antidepressant was not replaced by placebo (Bockting 2018; Derubeis 2019; Eveleigh 2018; Huijbers 2016; Kuyken 2008; Kuyken 2015; Segal 2010; Streim 2012). All studies compared discontinuation with continuation of antidepressants, except one study that compared discontinuation with usual care (Eveleigh 2018).
Eleven studies were published in 2000 or earlier. We identified one unpublished study for which data were obtained from clinicaltrials.gov and a conference abstract (Streim 2012).
Twenty‐six studies included multiple treatment phases before randomisation to antidepressants or to placebo ("multi‐phase studies"). Participants meeting response criteria at the end of the treatment phase were allowed to enter the subsequent phase: four studies used a two‐phase method (Kane 1982; Khan 2014; Mavissakalian 1999; Rickels 2010), 21 employed a three‐phase method (Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Keller 1998; Klysner 2002; Kocsis 1996; Kocsis 2007; Kornstein 2006; Kupfer 1992; Mavissakalian 2001; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Segal 2010; Stewart 1997; Terra 1998; Wilson 2003), and one used a four‐phase method (Keller 2007).
Discontinuation of antidepressant occurred during "continuation" treatment to prevent relapse (within six months of stabilisation) or during "maintenance" treatment to prevent recurrence (six months or longer after stabilisation).
13 studies discontinued during "continuation" treatment (Gelenberg 2003; Keller 1998; Klysner 2002; Kocsis 1996; Kornstein 2006; Mavissakalian 1999; Montgomery 1988; Montgomery 2004; Rickels 2010; Rouillon 2000; Segal 2010; Terra 1998; Wilson 2003), and 16 studies discontinued at the start of or during maintenance treatment (Cook 1986; Derubeis 2019; Gilaberte 2001; Kane 1982; Keller 2007; Kocsis 2007; Kupfer 1992; Kuyken 2008; Kuyken 2015; Mavissakalian 2001; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Stewart 1997; Streim 2012).
In one study, discontinuation took place during continuation or maintenance treatment (Bockting 2018).
In four studies, duration of treatment after stabilisation was not specified (Bialos 1982; Eveleigh 2018; Huijbers 2016; Khan 2014). One of the four studies randomised participants after 24 weeks of treatment were completed and did not use remission criteria to enter randomisation (Khan 2014).
Aims of studies
The objective of this review was to assess the effectiveness and safety of approaches for discontinuation versus continuation of long‐term antidepressant use.
Three types of studies were included. All types investigated discontinuation versus continuation of long‐term antidepressants, but they described initially different study aims (four studies with multiple treatment arms had two different aims).
"Relapse prevention" discontinuation studies (26 studies): the initial aim of these studies was to examine how continuation of long‐term antidepressant could prevent relapse. In this study, highly selected participants were given an antidepressant for six months or longer and then were divided into two groups: responders whose symptoms improved and non‐responders. Participants not able to tolerate the antidepressant, non‐adherent participants, and participants not willing to continue for any other reason were excluded from the trial. Responders were randomised to remain on antidepressants or to discontinue the antidepressant by replacing the antidepressant with a placebo (Bialos 1982; Cook 1986; Derubeis 2019; Gilaberte 2001; Kane 1982; Keller 1998; Keller 2007; Khan 2014; Klysner 2002; Kocsis 1996; Kocsis 2007; Kornstein 2006; Mavissakalian 1999; Mavissakalian 2001; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Segal 2010; Stewart 1997; Streim 2012; Terra 1998; Wilson 2003).
Studies investigating the effectiveness of an intervention designed to facilitate discontinuation of long‐term antidepressants (five studies): a letter to the general practitioner (GP) with a recommendation to discontinue and with tapering advice (Eveleigh 2018), preventive cognitive therapy (PCT) with tapering (Bockting 2018), and mindfulness‐based cognitive therapy (MBCT) with tapering (Kuyken 2008; Kuyken 2015; Segal 2010). In these studies, participants and clinicians discussed discontinuation and potential withdrawal symptoms.
Studies with a psychological co‐intervention (six studies) (Bockting 2018; Derubeis 2019; Gelenberg 2003; Huijbers 2016; Kupfer 1992; Peterson 2010): the aim of these studies was to investigate whether adding/continuing an antidepressant treatment with psychotherapy enhances protection against recurrence compared to providing psychotherapy alone.
Setting
Study authors described 21 studies as multi‐centre studies (Bialos 1982; Bockting 2018; Cook 1986; Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Huijbers 2016; Keller 1998; Keller 2007; Khan 2014; Kocsis 2007; Kornstein 2006; Kuyken 2015; Montgomery 1988; Montgomery 2004; Perahia 2009; Rapaport 2001; Rouillon 2000; Segal 2010; Terra 1998; Wilson 2003). All studies included participants who were living in the community, except one study that included 20% hospitalised participants (Rouillon 2000). Of the 33 included studies, 25 were conducted in a psychiatric outpatient setting. Four studies were conducted in general practice/primary care settings (Eveleigh 2018; Kuyken 2008; Kuyken 2015; Wilson 2003), one only in nursing homes (Streim 2012), and two in a combination of a general practice setting and a specialist mental healthcare service (Bockting 2018; Rickels 2010). The setting was not described in one study (Kupfer 1992). Nineteen studies were conducted in the USA (Bialos 1982; Cook 1986; Derubeis 2019; Gelenberg 2003; Kane 1982; Keller 1998; Keller 2007; Khan 2014; Kocsis 1996; Kocsis 2007; Kornstein 2006; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Peterson 2010; Rapaport 2001; Rickels 2010; Stewart 1997; Streim 2012), 11 in Europe (Bockting 2018; Eveleigh 2018; Gilaberte 2001; Huijbers 2016, Klysner 2002; Kuyken 2008; Kuyken 2015; Montgomery 1988; Rouillon 2000; Terra 1998; Wilson 2003), one in Canada (Segal 2010), and two in the USA and Europe (Montgomery 2004; Perahia 2009).
Sample size
Sample size varied from 11 in Mavissakalian 2001 to 336 participants in Kocsis 2007 (average, 151). The mean age of participants ranged from 36 years in Mavissakalian 1999 to 76 years in Wilson 2003. Most studies included adults between 18 and 65 years of age. Four studies included only participants 65 years of age and older (Cook 1986; Klysner 2002; Streim 2012; Wilson 2003), and one study included participants between 18 and 81 years old (Kornstein 2006). All studies included more female than male participants, except one study that included only males (Cook 1986).
Participants
All studies had to meet the main inclusion criterion that participants received long‐term antidepressants (for at least 24 weeks) for depressive or anxiety disorder.
In prevention relapse discontinuation trials, only a subgroup of antidepressant users who responded to antidepressant treatment were randomised. People who had not improved, who were not able to tolerate the antidepressant, or who were non‐adherent or not willing to continue for any other reason were discontinued from the pre‐randomisation phase. Randomised participants represent a small fraction of participants in the pre‐randomisation phase. See numbers of participants and numbers of randomised participants in Characteristics of included studies.
Indication for antidepressant
Depression (28 studies)
Streim 2012 included older adults receiving antidepressant treatment for a single episode of depression and in full remission for at least six months. In two studies, most participants had no or one previous episode of depression: Klysner 2002 included 96.7% of participants with no or one previous episode and 3.3% with two previous episodes. Wilson 2003 included 72.5% of participants in remission from a first depression.
Twenty‐two studies included participants with the main diagnosis of recurrent major depressive disorder (Bockting 2018; Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Huijbers 2016; Kane 1982; Keller 1998; Keller 2007; Kocsis 1996; Kocsis 2007; Kornstein 2006; Kupfer 1992; Kuyken 2008; Kuyken 2015; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rouillon 2000; Segal 2010; Stewart 1997; Terra 1998). Of these 22 studies, one study also included participants with dysthymia superimposed by depression, chronic depression (duration longer than three years), or incomplete inter‐episode recovery (Peterson 2010); one included participants with recurrent major depression with history of incomplete inter‐episode recovery and dysthymia superimposed by depression or chronic depression (≥ 2 years' duration) (Gelenberg 2003); one included participants with dysthymia with or without current major depression or major depression‐chronic subtype (Kocsis 1996); and one included participants with chronic atypical definite or probable depression, defined as meeting Columbia University Criteria for diagnosis of major depression, dysthymia, or both (Stewart 1997).
Bialos 1982 included participants receiving antidepressant treatment for at least six months with a history of depression (mean number or range not described). Two studies included participants with a mix of single and recurrent depression (Cook 1986; Khan 2014). Cook 1986 reported a range from zero to "too many to count" previous depressive episodes. One study with two treatment arms did not describe the number of previous episodes (Khan 2014).
Anxiety (4 studies)
Four studies included participants with anxiety disorder: three included participants with panic disorder with agoraphobia (Mavissakalian 1999; Mavissakalian 2001), or with or without agoraphobia (Rapaport 2001), and one included participants with generalised anxiety disorder (Rickels 2010).
Depression and/or anxiety (1 study)
One study included participants with long‐term inappropriate antidepressant use (≥ 36 weeks) according to the Dutch depression and anxiety guidelines and excluded participants with appropriate long‐term antidepressant use (e.g. history of recurrent depression ≥ 3 episodes) and/or a recurrent psychiatric disorder with two or more relapses after antidepressant discontinuation (Eveleigh 2018). Lifetime psychiatric diagnoses of participants were as follows: 51% depression, 18% panic disorder or agoraphobia, 24% generalised anxiety disorder, and 25% social phobia. About 30% of participants did not have a lifetime psychiatric diagnosis (Eveleigh 2018).
Antidepressant treatment
Duration
Eight studies (with no pre‐randomisation phase) included participants with long‐term antidepressant treatment (Bialos 1982; Bockting 2018; Cook 1986; Eveleigh 2018; Huijbers 2016; Kuyken 2008; Kuyken 2015; Streim 2012). These studies included participants receiving antidepressant treatment for 24 weeks or longer (Bialos 1982; Bockting 2018; Huijbers 2016), 36 weeks or longer (Eveleigh 2018), or 52 weeks or longer (Cook 1986); two studies included participants receiving maintenance treatment in line with the British National Formulary (BNF) (Kuyken 2008; Kuyken 2015). One study included participants currently using antidepressant medication who had been in remission from a first episode of depression for six months or longer (Streim 2012).
In the multi‐phase RCTs, antidepressant duration before randomisation varied widely, at 24 weeks (Kane 1982; Khan 2014; Klysner 2002; Kornstein 2006; Mavissakalian 1999; Montgomery 1988; Montgomery 2004; Rickels 2010; Rouillon 2000; Terra 1998; Wilson 2003), 26 weeks (Kocsis 1996), 28 weeks (Gelenberg 2003; Keller 1998; Perahia 2009; Segal 2010), 30 weeks (Stewart 1997), 32 weeks (Gilaberte 2001), 34 weeks (Kocsis 2007), 36 weeks (Peterson 2010), 48 weeks (Rickels 2010), 62 weeks (Rapaport 2001), 76 weeks (Mavissakalian 2001), and 86 weeks (Keller 2007). The duration of the acute treatment in three studies was prolonged by two weeks or longer if patients did not meet the criteria for response (Kocsis 1996; Perahia 2009; Wilson 2003).
In one study, acute and continuation treatment lasted until participants met the criteria for response and/or remission (Derubeis 2019); mean duration of treatment before randomisation was 80 weeks, but mean duration of acute treatment was not described. In one study, duration of antidepressant treatment (continuation and maintenance treatment) was 173 weeks, and duration of acute treatment was not clearly reported (Kupfer 1992).
Type
Trials examined effects of discontinuation of several antidepressants including sertraline (Keller 1998; Rapaport 2001; Wilson 2003); citalopram (Klysner 2002); fluoxetine (Gilaberte 2001; Montgomery 1988; Peterson 2010); fluvoxamine (Terra 1998); venlafaxine IR (Montgomery 2004); venlafaxine ER (Keller 2007; Kocsis 2007; Rickels 2010), desvenlafaxine (Khan 2014); amitriptyline (Bialos 1982); imipramine (Kane 1982; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Stewart 1997); desipramine (Kocsis 1996); phenelzine (Stewart 1997); milnacipran (Rouillon 2000); nefazodone (Gelenberg 2003); duloxetine (Perahia 2009); TCA (Cook 1986); one of four different SSRIs for acute treatment and escitalopram for continuation treatment (Kornstein 2006); SSRI, SNRI, TCA, atypical antidepressant class, MAO inhibitor, or more than one (Bockting 2018); treatment algorithm with one of four different classes of antidepressants (sertraline, venlafaxine, two others not reported) (Derubeis 2019); SSRI, SNRI, TCA, or other antidepressant except MAO inhibitor (Eveleigh 2018); SSRI, TCA, or other (Huijbers 2016); SSRI, TCA, or combination Kuyken 2008); and a treatment algorithm with citalopram, sertraline, venlafaxine, or mirtazapine (Segal 2010). Two studies did not describe the type of antidepressant (Kuyken 2015; Streim 2012). One study used an antidepressant treatment algorithm that could involve up to four different classes of antidepressants as well as additional medication (Derubeis 2019). Detailed information on the four different antidepressant classes (only venlafaxine and sertraline are reported) and how medication was adjusted is not provided.
The dose of antidepressant given at the start of the trial varied within and between studies. However, the dose of antidepressant was not systematically reported in the included studies (see Characteristics of included studies).
Severity of depressive disorder/anxiety disorder at the moment of randomisation
Most studies required that participants were in remission to enter the discontinuation trial and reported remission criteria. Two studies did not report remission criteria (Bialos 1982; Khan 2014). Bialos 1982 included participants experiencing mild to moderate depressive symptoms. In one study (Khan 2014), participants were required to complete the open‐label 24‐week treatment; however the mean Quick Inventory of Depressive Symptomatology Self‐Report (QIDS‐SR16; self‐administered rating scale that assesses 16 items associated with depression) showed that most participants had fewer depressive symptoms after the open‐label phase than at the moment before open‐label treatment (Khan 2014). Five of the 33 studies included participants in partial remission.
The definition of response/remission varied widely between studies and involved specific scores on scales, clinical judgement, guidelines, or a mix of criteria.
Scale measurements
-
Hamilton Rating Scale for Depression (HAM‐D), also called the Hamilton Depression Rating Scale (HDRS): 11 studies
Current score ≤ 10 on HAM‐D (Bockting 2018); 50% reduction in HAM‐D total score from acute phase baseline achieved at end of acute phase and then maintained at end of continuation phase and HAM‐D total score < 16 at end of continuation phase (Gelenberg 2003); HAM‐D score ≤ 12 and ≥ 50% decrease from acute phase baseline and HAM‐D score ≤ 7 for the intake episode of major depressive disorder (MDD) (Keller 2007); HAM‐D score ≤ 12 and ≥ 50% decrease from acute baseline at end of phase 1 and HAM‐D score ≤ 7 at end of phase 2 (Kocsis 2007); HAM‐D score < 8 (Kuyken 2008; Kuyken 2015); HAM‐D score < 12 (Montgomery 1988); HAM‐D score ≤ 7 for three consecutive weeks (Peterson 2010); HAM‐D score ≤ 12 on Day 56 of acute treatment with ≤ 2 HAM‐D scores > 10 and no CGI‐S ≥ 4 between Months 2 and 6 of continuation treatment and HAM‐D score ≤ 8 (Rouillon 2000); HAM‐D score ≤ 7 during continuation treatment (Segal 2010); or 50% reduction in baseline HAM‐D by Week 8 and HAM‐D score ≤ 10 had to be maintained for four weeks (Wilson 2003)
-
Other scales: 4 studies
MADRS score ≤ 11 (Klysner 2002); MADRS score ≤ 12 (Kornstein 2006); Clinical Global Impression of Severity Scale (CGI‐S) score of 1 or 2 (Stewart 1997); or Inventory of Depressive Symptomatology, Clinician Rated (IDS‐C) score ≤ 11 (Huijbers 2016)
-
Combination of two scales: 6 studies
Four consecutive weeks of Longitudinal Internal Follow‐up Evaluation (LIFE) Problem Symptom Rating Scale score ≤ 2 and HAM‐D score ≤ 8 for four consecutive weeks and six consecutive months following remission without relapse (Derubeis 2019); Full remission/response (HAM‐D score ≤ 7 and CGI‐I score of 1 or 2) or satisfactory therapeutic response (reduction of 50% in HAM‐D plus HAM‐D ≤ 15 and CGI‐I ≤ 2 and CGI‐S ≤ 3) (Keller 1998); HAM‐D ≤ 7 and Raskin score ≤ 5 for three weeks (Kupfer 1992; Montgomery 2004); MADRS score < 10 and CGI‐S score of 1 or 2 at Week 6 and MADRS score < 12 for all measures and CGI‐S score of 1 or 2 at 24 weeks (Terra 1998); or HAM‐D score < 7 and GAS score > 70 on three consecutive bi‐weekly ratings (Kocsis 1996)
Two studies used only clinical judgement: Cook 1986 used antidepressant without evidence of recurrence of depressive symptoms warranting a change in therapy; Kane 1982 used "euthymia" with no criteria specified.
One study used recommendations from guidelines: no indication for long‐term antidepressant treatment in line with the Dutch guidelines for depression and anxiety (Eveleigh 2018).
Two studies used a combination of criteria and scale measurements: no longer met diagnostic criteria for major depression by using DSM‐III criteria and HAM‐D score ≤ 8 and CGI‐S score ≤ 2 (Gilaberte 2001); or HAM‐D score ≤ 9, CGI‐S score ≤ 2, and did not meet DSM‐IV criteria for major depressive episode (Perahia 2009).
Partial remission
Five of the 33 included studies also included participants in partial remission: response criteria for partial remission were IDS‐C score > 11 (Huijbers 2016); ≥ 50% reduction from baseline HAM‐D score, with HAM‐D score ranging from 7 to 12, and GAS score ≥ 60 on three successive ratings (Kocsis 1996); HAM‐D score ≥ 8 (Kuyken 2008); HAM‐D score ≥ 8 (Kuyken 2015); or HAM‐D score ≤ 7 with occasional elevated scores between 8 and 14 and followed by a score ≤ 7 (Segal 2010).
Anxiety disorders
Four of the 33 included studies included only participants with anxiety disorders. Criteria for remission: CGI‐I score ≤ 2 (Rickels 2010); ≥ 50% improvement in the composite index of end‐state from pre‐treatment scores or a cut‐off score signifying mild to absent symptoms simultaneously on six or all of these measures of end‐state at both 16‐week and 24‐week assessments (Mavissakalian 1999); maintained in stable remission after 52 weeks of imipramine treatment (Mavissakalian 2001); or CGI‐I score of 1 or 2 at Week 52 compared to baseline for acute treatment (Rapaport 2001).
Intervention
Trials evaluated several regimens for antidepressant discontinuation including abrupt discontinuation, discontinuation by tapering, discontinuation by tapering combined with high‐intensity psychological treatment, and discontinuation by tapering combined with a minimum intervention. None of the studies included discontinuation with changed pharmacological form (e.g. fluoxetine or paroxetine liquid) or a low‐intensity psychological intervention.
Abrupt discontinuation without psychological support (or psychological support as co‐intervention)
The antidepressant is abruptly discontinued and is replaced by placebo. In all, 13 studies compared abrupt discontinuation to continuation of long‐term antidepressant treatment. Abrupt discontinuation also includes eight studies without description of the tapering scheme (Gilaberte 2001; Kane 1982; Montgomery 1988; Peterson 2010; Rouillon 2000; Streim 2012; Terra 1998; Wilson 2003).
Of the 13 studies, 11 compared abrupt discontinuation of antidepressant without psychological support to continuation of antidepressant. Included antidepressants were fluoxetine (Gilaberte 2001; Montgomery 1988; Peterson 2010), imipramine (Kane 1982), desvenlafaxine (Khan 2014), citalopram (Klysner 2002), escitalopram (Kornstein 2006), sertraline (Rapaport 2001: Wilson 2003), milnacipran (Rouillon 2000), and fluvoxamine (Terra 1998). Streim 2012 did not describe the antidepressant. One study did not replace the antidepressant with a placebo tablet (Streim 2012).
Two studies (of the 13) investigated abrupt discontinuation versus continuation of antidepressant with psychological treatment (before or after randomisation) in both groups (co‐intervention) (Gelenberg 2003; Peterson 2010).
Gelenberg 2003 compared abrupt discontinuation to nefazodone continuation. Participants in both treatment groups received a cognitive‐behavioural analysis system of psychotherapy (CBASP) during acute and continuation treatment before randomisation (frequency varied from two times per week to monthly). No CBASP sessions occurred in both groups during the discontinuation trial. Results for placebo versus nefazodone were published and included all participants regardless of whether they received psychotherapy or nefazodone alone. The distribution of CBASP or not over placebo and nefazodone treatment arms in the discontinuation trial was balanced (66% with CBASP in both groups, 34% with no CBASP). Study authors described that prior concomitant psychotherapy during acute/continuation treatment, although enhancing the initial response, was not associated with lower recurrence rates (author conclusions not supported by data).
Peterson 2010 compared abrupt discontinuation to continuation of fluoxetine with CBT in both groups. All participants received 12 weekly and seven bi‐weekly CBT sessions during continuation treatment delivered by psychologists. CBT was modified to address residual symptoms and to enhance coping skills. The therapy used for this study was designed specifically for remitted depressed patients, who are at high risk for relapse and recurrence. CBT sessions ‐ 7 bi‐weekly and 16 monthly sessions ‐ were maintained in both groups during the discontinuation trial.
Detailed information is provided under Characteristics of included studies.
Discontinuation by "tapering" without psychological support (or psychological support as co‐intervention)
The antidepressant is discontinued by "tapering" over one week or longer in 18 studies. Four of the 18 did not replace the antidepressant with a placebo tablet (Bockting 2018; Derubeis 2019; Huijbers 2016; Kupfer 1992).
Fourteen studies investigated antidepressant discontinuation by "tapering" to continuation of antidepressant without psychological support.
Antidepressant and tapering schemes included the following: amitriptyline over three weeks (Bialos 1982); SSRI (≥80 %); other: SNRI, TCA, atypical antidepressant (AD), MAO inhibitor, or more than one over four weeks (Bockting 2018); TCA (despiramine, amitriptyline, doxepine, imipramine) over four or eight weeks (Cook 1986); sertraline (or venlafaxine and two others not reported) over four weeks or longer (left to clinicians’ discretion; Derubeis 2019); SSRI, TCA, or other over five weeks (Huijbers 2016); sertraline over four weeks (Keller 1998); venlafaxine ER over four weeks (Keller 2007); venlafaxine over one week (Peterson 2010); desvenlafaxine (Khan 2014); desipramine over four weeks (Kocsis 1996); venlafaxine ER over four weeks (Kocsis 2007); imipramine over three weeks (Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001); venlafaxine IR over two weeks (Montgomery 2004); duloxetine over four weeks (Perahia 2009); venlafaxine over one to three weeks (Rickels 2010); citalopram (or sertraline if not tolerating) and if needed in combination with venlafaxine (or mirtazapine if not tolerating) over four weeks (Segal 2010); imipramine over two weeks (Stewart 1997); and phenelzine over two weeks (Stewart 1997).
Four studies compared discontinuation by "tapering" to continuation of antidepressant with a high‐intensity psychological intervention in both treatment groups (co‐intervention). Psychological treatment included PCT, CBT, MBCT, and IPT.
Bockting 2018 compared tapering to continuation of long‐term antidepressant. All participants received preventive cognitive treatment (PCT). PCT included eight weekly group or individual sessions. Study authors described that the main components of the PCT intervention were identification and evaluation of dysfunctional attitudes and schemas that activate positive affect and emotions; enhancement of memories of positive experiences; and formulation of prevention strategies. GPs and psychiatrists were advised to taper antidepressants over a period of four weeks. The GP or the psychiatrist and participants received a letter with instructions to guide tapering and a tapering schedule per type of drug. Most (60%) individuals tapered antidepressants over six months. Study authors reported that a time frame of four weeks was not considered feasible for many participants. Study participants were allowed to restart antidepressants at any time. Participants in the tapering group were also monitored on their progress via telephone by an independent researcher.
Derubeis 2019 compared tapering over four weeks or longer (left to clinicians’ discretion) to continuation of long‐term antidepressant. Participants in both treatment groups received cognitive‐behavioural therapy (CBT) during acute and continuation treatment before randomisation. CBT ended before entry into the discontinuation trial. CBT comprised 50‐minute sessions two times per week for at least the first two weeks during acute treatment, at least weekly thereafter, and then at least monthly during the continuation phase or longer if needed.
Huijbers 2016 compared MBCT followed by tapering over five weeks starting after the seventh session of MBCT to continuation of long‐term antidepressants and MBCT. Tapering was supervised by a psychiatrist and followed a protocol for medication tapering with a minimum of three and a maximum of 12 consultations during the tapering period. Study researchers recommended to fully discontinue the antidepressant before the six‐month follow‐up assessment (i.e. within six months after baseline and within approximately three to four months after the last MBCT session). MBCT intervention consisted of eight weekly group sessions. Groups were mixed, comprising participants from both treatment groups, as well as participants not included in the trial. CBT techniques included education, monitoring and scheduling of activities, identification of negative automatic thoughts, and a relapse prevention plan. MBCT included formal meditation exercises.
Kupfer 1992 compared tapering over three weeks versus imipramine continuation with IPT for all participants before randomisation. Participants received IPT (from weekly to monthly) during acute treatment and continuation treatment. At the end of the 17‐week continuation treatment, responders were randomised to monthly IPT sessions or to IPT discontinuation combined with antidepressant treatment for 156 weeks in both groups. Remitters (with and without IPT) were re‐randomised to antidepressant or to placebo for 104 weeks and continued psychotherapy consistent with their previous treatment.
For detailed information, see Characteristics of included studies.
Discontinuation ("taper") combined with support from high‐intensity psychological therapy
Four studies compared "tapering" combined with high‐intensity psychological interventions. Psychological interventions included PCT and MBCT.
Bockting 2018 compared antidepressant "tapering" over four weeks (without placebo) combined with PCT versus continuation of antidepressant.
Three studies compared "tapering" combined with MBCT versus continuation of long‐term antidepressant (Kuyken 2008; Kuyken 2015; Segal 2010).
The antidepressant and tapering regimen included a not specified antidepressant and tapering over a period determined by physicians and participants (Kuyken 2008; Kuyken 2015), along with tapering of citalopram over four weeks (Segal 2010).
MBCT was slightly different between the three studies. Detailed information is provided under Characteristics of included studies.
Discontinuation in combination with support from low‐intensity psychological intervention or psychosocial support
No studies used low‐intensity psychological intervention or psychosocial support to support discontinuation.
Discontinuation in combination with a minimal intervention
One study investigated tapering with a recommendation to review the antidepressant to GP versus usual care (Eveleigh 2018). A participant‐specific letter was sent to the GP, stating that the participant did not meet the criteria for a depressive or anxiety disorder in the past six months and providing a recommendation to discontinue the antidepressant through a gradual tapering scheme (Eveleigh 2018). Tapering advice from a psychiatrist and a general practitioner from the research team was based on the results of a questionnaire the patient had completed. The GP invited the participant to discuss the recommendation. GP and participants were allowed to not comply with the recommendation to discontinue: 15% of antidepressant users consented to participate, and the recommendation to discontinue was rejected by 34 of 70 participants in the intervention group. The control group continued usual care without tapering advice. The antidepressant included mainly SSRI (> 80%), SNRI, TCA, or atypical antidepressant. Duration of tapering (one step per two weeks with a maximum of four steps) was slow and was based primarily on dosage and half‐life of different antidepressants, but the study did not report the mean duration for tapering.
Discontinuation with changed pharmacological form (e.g. fluoxetine or paroxetine liquid) or with additional pharmacological support
No included studies used a changed pharmacological form or additional pharmacological support.
Study duration
Study duration varied widely from 4 weeks to 156 weeks.
One study with medium‐term follow‐up (4 weeks to 24 weeks): four weeks (Khan 2014).
32 studies with long‐term follow‐up (24 weeks or longer): 24 weeks (Bialos 1982; Rickels 2010; Stewart 1997), 28 weeks (Cook 1986; Rapaport 2001), 48 weeks (Gilaberte 2001; Klysner 2002), 52 weeks (Eveleigh 2018; Gelenberg 2003; Keller 2007; Kocsis 2007; Kornstein 2006; Mavissakalian 1999; Mavissakalian 2001; Montgomery 1988; Montgomery 2004; Perahia 2009; Rouillon 2000; Streim 2012; Terra 1998), 64 weeks (Kuyken 2008), 65 weeks (Huijbers 2016), 76 weeks (Keller 1998; Segal 2010), 80 weeks (Peterson 2010), 100 weeks (Wilson 2003), 104 weeks (Bockting 2018; Kane 1982; Kocsis 1996; Kupfer 1992; Kuyken 2015), and 156 weeks (Derubeis 2019).
No studies had short‐term follow‐up (< 4 weeks).
Outcome measures
All studies addressed at least one of our primary outcomes.
Succesful antidepressant discontinuation rate
This included five studies (Bockting 2018; Eveleigh 2018; Huijbers 2016; Kuyken 2008; Kuyken 2015), but sufficient information for both groups was provided by only two studies (Eveleigh 2018; Huijbers 2016).
Relapse (as defined by study authors)
This included 31 studies. All studies except two addressed relapse (Khan 2014; Streim 2012). All studies except two reported relapse using hazard ratios (Cook 1986; Kane 1982).
We used the relapse rate defined by the authors of included studies. The definition of relapse varied widely between studies and involved measurement of a specific score on clinical scales, meeting DSM criteria for MDD, clinical judgement, or a combination of these (see Characteristics of included studies).
Five studies used only scale measurements: LIFE Rating Scale score of 5 or 6 for two consecutive weeks at any time after the first eight weeks and for three consecutive weeks during the first eight weeks (Derubeis 2019), MADRS score ≥ 22 confirmed after three to seven days (Klysner 2002), HAM‐D score > 18 (Montgomery 1988), CGI‐S score ≥ 4 (Montgomery 2004), and two consecutive weeks of a CGI rating ≥ 3 (Stewart 1997).
Two studies used only clinical judgement: appearance of a depressive episode as decided upon by the patient and by the blinded research clinician (Bialos 1982), as well as investigator’s clinical assessment that change in pharmacological treatment was indicated (Cook 1986).
Six studies used only DSM or research diagnostic criteria (RDC) for diagnosis of major depression: DSM‐IV criteria were assessed with a structured clinical interview for DSM‐IV Axis I Disorders (SCID‐I), including retrospective parts and information from monthly ratings on the Inventory of Depressive Symptomatology–Self‐Report (IDS‐SR) (Bockting 2018). RDC for major depressive disorder and symptoms persisted for a week after evaluation, and RDC for a minor depressive disorder persisted for four successive weeks (Kane 1982). An episode meeting DSM–IV criteria for major depression using SCID‐I was reported in Huijbers 2016Kuyken 2008 and Kuyken 2015; DSM‐V criteria for depressive or anxiety disorder were assessed with the Composite International Diagnostic Interview (CIDI) 3.0 (Eveleigh 2018).
Seven studies used a combination of DSM or RDC for major depression and scale measurements: HAM‐D score ≥ 16 plus major depression (diagnosed on two consecutive visits via DSM‐IV) (Gelenberg 2003); meeting DSM‐III criteria for major depression with HAM‐D score ≥ 18 and CGI‐S score ≥ 4, or both of these, for at least two weeks (Gilaberte 2001); DSM‐III criteria for major depression for at least three weeks, CGI‐S score ≥ 4 (at least moderate severity), CGI‐I score ≥ 3 (minimally improved or less), and increase in HAM‐D score ≥ 4 points higher than baseline, in total, for at least four weeks (Keller 1998); meeting criteria for major depression and ≥ 15 on HAM‐D scale and confirmed one week later (Peterson 2010); meeting DSM‐IV criteria for GAD using SCID‐I with HAM‐A score ≥ 16, CGI‐S score ≥ 4, CGI‐I score of 6 or 7 compared with baseline value and present for two successive visits, with two weeks between, with the last visit conducted at least three weeks after taper completion (Rickels 2010); HAM‐D score ≥ 16 assessed twice for at least two weeks and meeting criteria for major depression measured with SCID‐I (Segal 2010); HAM‐D score ≥ 13 and meeting DSM–III criteria for major depression (Wilson 2003).
Six studies used a combination of scale measurements and clinical judgement: HAM‐D score > 12 and GAS score < 60 on three successive ratings over a period of four weeks, or at least one rating meeting these criteria, and urgent need for alternative treatment for a depressive syndrome (Kocsis 1996); MADRS score ≥ 22 or withdrawal from the study due to insufficient treatment response based on judgement of the principal investigator (Kornstein 2006); HAM‐D score ≥ 15 and Raskin score ≥ 7 on two occasions within seven days and confirmed by clinical evaluation of senior psychiatrist (Kupfer 1992); withdrawn from the study with End‐State Functioning (ESF) score ≤ 4 signifying ≥ 33% decline in ESF (definition of "worsening") and insistent request for therapeutic action with worsening still present at a repeated confirmatory assessment two weeks later (Mavissakalian 1999; Mavissakalian 2001); reappearance of depressive symptoms in the opinion of the investigator ‐ at least five symptoms in the DSM‐III criteria for diagnosis of major depression at two assessments by investigator eight days later, with attempted or completed suicide also considered as a recurrence (Terra 1998).
Four studies used combinations of different relapse definitions: HAM‐D > 12 and HAM‐D < 50% lower than acute phase baseline at two consecutive visits or at the last valid visit before discontinuation and meeting DSM‐IV criteria for major depression as judged by the investigator (Keller 2007; Kocsis 2007); meeting DSM‐III criteria with HAM‐D score ≥ 18 and with the need to treat recurrence (Rouillon 2000); and CGI‐I score ≥ 3 at three consecutive visits at two‐week intervals, meeting DSM‐III criteria for panic disorder by the third visit, and reporting more full symptom panic attacks during previous four weeks than during the last four weeks of open‐label treatment (Rapaport 2001).
One study considered relapse if participants met any of the following criteria: CGI‐S score ≥ 4 and meeting DSM‐IV criteria for major depression (as assessed by the Mini International Neuropsychiatric Interview (MINI) depression module) for at least two weeks; three consecutive visits that met re‐emergence criteria, or 10 total re‐emergence visits or discontinued the study with the reason "lack of efficacy"; significant re‐emergence of depressive symptoms defined as CGI‐S score ≥ 4 but not meeting DSM‐IV criteria for major depression as assessed by the MINI depression module (Perahia 2009).
Withdrawal symptoms
This was measured in three studies, but only one reported data on withdrawal symptoms (Khan 2014).
One study reported adjusted mean DESS total score over the first two weeks after discontinuation and the proportion of participants with withdrawal syndrome based on DESS (Khan 2014). Experiencing a withdrawal syndrome was defined as an increase ≥ 4 in DESS score. Eveleigh 2018 used the DESS scale but did not analyse the data. Bialos 1982 did not report use of the scale.
Adverse events
A total of 21 studies reported serious adverse events and/or adverse events: Cook 1986; Bockting 2018; Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Keller 1998; Keller 2007; Khan 2014; Klysner 2002; Kornstein 2006; Kocsis 2007; Kuyken 2008; Kuyken 2015; Montgomery 2004; Perahia 2009; Rapaport 2001; Rickels 2010; Rouillon 2000; Streim 2012; Terra 1998; Wilson 2003.
Bialos 1982 did not report the number of (serious) adverse events but reported only the insomnia factor and the autonomic anticholinergic factor of the Treatment‐Emergent Symptoms Scale in the tapering group but not in the continuation group.
Depressive symptoms
A total of 17 studies used 10 different scales to measure depressive symptoms: HAM‐D (Bialos 1982; Cook 1986; Gilaberte 2001; Keller 1998; Keller 2007; Kocsis 2007; Kornstein 2006; Kuyken 2008; Kuyken 2015; Montgomery 2004; Peterson 2010; Perahia 2009; Stewart 1997) and/or HAM‐D subscale depressed mood item 1 score (Montgomery 2004; Perahia 2009), MADRS (Cook 1986; Keller 1998; Kornstein 2006; Montgomery 2004; Terra 1998), Center for Epidemiological Studies Depression Scale (CESD) (Eveleigh 2018), Caroll Depression Scale (Cook 1986), QIDS‐SR16 (Khan 2014), IDS‐SR (Keller 2007; Kocsis 2007), Cornell Dysthymia Scale (Cook 1986; Keller 1998), Geriatric Depression Scale (GDS) (Streim 2012), BDI (Keller 1998; Kuyken 2008; Kuyken 2015; Peterson 2010), and Symptom Questionnaire (SQ) depression (Peterson 2010).
Five other studies measured depressive symptoms by using the HAM‐D scale but did not report data for each treatment group (Gelenberg 2003; Mavissakalian 1999; Mavissakalian 2001; Segal 2010; Stewart 1997).
Anxiety symptoms
Eight studies used nine different scales: HAM‐A (Keller 2007; Kocsis 2007; Rapaport 2001; Rickels 2010); anxiety/somatisation subscale of the HAM‐D scale (Perahia 2009); IDS‐SR anxiety (Keller 2007; Kocsis 2007); BAI and SQ anxiety scores (Peterson 2010); Covi Anxiety Scale (Terra 1998); Panic and Agoraphobia Scale (PAS) (Eveleigh 2018); Hospital Anxiety and Depression Scale (HADS), anxiety factor (Rickels 2010); and Panic Disorder Severity Scale (PDSS) (Rapaport 2001). Rapaport 2001 also reported frequency and panic attacks per week.
Quality of life
Nine studies used 18 different scales: Short Form (SF)‐36 subscales of social functioning, role limitations due to emotional problems and to physical health problems (Keller 1998; Keller 2007; Kocsis 2007); SF‐36 mental and physical component summary scores (Keller 2007; Kocsis 2007; Perahia 2009); SF‐36 subscales of bodily pain, general health, and vitality (Keller 2007; Kocsis 2007); Quality of Life Enjoyment and Satisfaction Questionnaire (Q‐LES‐Q) (Keller 2007; Kocsis 2007); quality‐adjusted life‐year (QALY) with EuroQoL Group Quality of Life Questionnaire based on 5 dimensions (EQ‐5D) (Bockting 2018; Eveleigh 2018); World Health Organization Cross‐Cultural Comparisons of Quality of Life (WHO QoL‐BREF) subscales of the physical, psychological, and social relationships domain (Huijbers 2016; Kuyken 2008; Kuyken 2015); WHO QoL‐BREF subscales for overall perception Q1, Q2, and environmental domain (Huijbers 2016; Kuyken 2015); health‐related quality of life with EQ‐5D (Kuyken 2015); and Life Enjoyment and Satisfaction Scale (LES‐S) (Keller 2007; Kocsis 2007).
One study reported on the General Health Questionnaire but data were insufficient for interpretation of results (Rickels 2010),
Social and occupational functioning
Five studies used five different scales: Social Adjustment Scale Self‐Report (SAR‐SR) (Bialos 1982; Keller 1998; Keller 2007; Kocsis 2007), LIFE subject assessment, interviewer assessment, and satisfaction assessment (Keller 1998), and Sheehan Disability Scale (SDS) (Perahia 2009). One study reported also on interference with daily activity (Perahia 2009).
One study reported SDS details but provided insufficient data for interpretation of the result (Rickels 2010), Two studies measured social functioning by using SAR‐SR total score but did not report the data (Bialos 1982Kocsis 1996).
Severity of illness
In all, 11 studies used five different scales: CGI‐S (Gilaberte 2001; Terra 1998; Keller 1998; Keller 2007; Kornstein 2006; Kocsis 2007; Montgomery 2004; Peterson 2010; Perahia 2009; Rapaport 2001; Rickels 2010), CGI‐I (Gilaberte 2001; Keller 1998; Kornstein 2006; Peterson 2010; Rapaport 2001; Rickels 2010); Patient Global Impressions of improvement and Symptom Questionnaire Somatic Subscale (SQ‐SS) (Perahia 2009); SQ, hostility subscale (Peterson 2010).
Suicide, suicide attempt, or suicidal ideation
Eight studies reported suicide, suicide attempt, or suicidal ideation as a criterion of relapse or as a (serious) adverse event (Bockting 2018; Keller 2007; Khan 2014; Kocsis 2007; Kornstein 2006; Perahia 2009; Rouillon 2000; Terra 1998). Most studies excluded participants at high risk for suicide.
Funding
Seventeen studies were supported by a grant from the pharmaceutical industry (Bialos 1982; Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Keller 1998; Keller 2007; Khan 2014; Klysner 2002; Kocsis 1996; Kocsis 2007; Kornstein 2006; Montgomery 2004; Perahia 2009; Rapaport 2001; Rickels 2010; Rouillon 2000; Wilson 2003). Two of the 17 studies also reported non‐commercial sponsorship (Derubeis 2019; Kocsis 1996). Twelve studies reported only non‐commercial sponsorship (Bockting 2018; Eveleigh 2018; Huijbers 2016; Kane 1982; Kupfer 1992; Kuyken 2008; Kuyken 2015; Mavissakalian 1999; Mavissakalian 2001; Segal 2010; Stewart 1997; Streim 2012). The source of funding was not described in four studies (Cook 1986; Montgomery 1988; Peterson 2010; Terra 1998).
Exclusion criteria
Most studies excluded participants at high risk for suicide, drug abuse, anxiety disorders, and other Axis I or Axis II disorders (see Characteristics of included studies).
Excluded studies
See Characteristics of excluded studies.
We excluded 78 studies from the review (see Characteristics of excluded studies). The most common reason for exclusion was that participants had received antidepressant treatment for less than six months (e.g. Dobson 2008, Walker 2000). Other studies did not report total treatment duration before randomisation (e.g. Alexopoulos 2000; Coppen 1978). In some studies, participants received antidepressant treatment of varied duration, and not all participants met our inclusion criterion of antidepressant treatment for at least six months (e.g. Georgotas 1989Hochstrasser 2001). Duration of antidepressant treatment in Davidson 2008 is described as six months; however the mean duration of antidepressant treatment for participants was 136 days. Some studies were follow‐up extension studies without randomisation (e.g. Duboff 1993; Weissman 1976). Other studies examined effects of psychological treatment without discontinuation of antidepressants versus usual care (e.g. Bockting 2005). One study was withdrawn (NCT00878748), with no reason reported. Kane 1982 examined effects of imipramine plus lithium versus imipramine versus lithium versus placebo for relapse prevention in patients receiving antidepressants for 24 weeks, prescribed for unipolar depressive disorder. We excluded the lithium treatment arm. One study examined effects of discontinuation of antidepressants supported by mindfulness‐based cognitive therapy compared to discontinuation supported by monitoring alone but included no control continuation group (Wentink 2019).
Risk of bias in included studies
Two review authors (EVL, LR) independently assessed each of the 33 included studies for risk of bias across the six domains, using the Cochrane ’Risk of bias’ assessment tool (Higgins 2017), as described in the Methods (Assessment of risk of bias in included studies). We contacted all study authors if possible (contact email available) and requested detailed information to assess the risk of bias, but we have not received responses yet, except for one. One study author has responded but could not remember the details (the study was conducted 30 years ago).
We have reported these assessments in the ’Risk of bias’ table associated with each study, as well as in the ’Risk of bias’ summary. We judged all studies to be at high risk of bias in at least one domain. The most common high‐risk domain was reporting bias, followed by attrition bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We judged 12 studies to be at low risk of bias for this domain. All clearly described the method of sequence generation used (Bockting 2018; Derubeis 2019; Eveleigh 2018; Huijbers 2016; Keller 2007; Kocsis 2007; Kuyken 2008; Kuyken 2015; Mavissakalian 1999; Mavissakalian 2001; Segal 2010; Wilson 2003). For example, Bockting 2018 used automated permuted block randomisation via a computer random number generator.
We judged 21 studies as having unclear risk as they did not mention or provided insufficient information on the method of sequence generation used (Bialos 1982; Cook 1986; Gelenberg 2003; Gilaberte 2001; Kane 1982; Keller 1998; Khan 2014; Klysner 2002; Kocsis 1996; Kornstein 2006; Kupfer 1992; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Stewart 1997; Streim 2012; Terra 1998).
Allocation concealment
Allocation concealment was described in sufficient detail to assess risk of bias as low in only 11 studies (Bockting 2018; Eveleigh 2018; Huijbers 2016; Keller 2007; Kocsis 2007; Kuyken 2008; Kuyken 2015; Mavissakalian 1999; Mavissakalian 2001; Segal 2010; Wilson 2003). For example, an independent hospital pharmacist was responsible for allocation concealment in Mavissakalian 1999.
Risk of allocation concealment bias was unclear in 22 studies as the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement (Bialos 1982; Cook 1986; Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Kane 1982; Keller 1998; Khan 2014; Klysner 2002; Kocsis 1996; Kornstein 2006; Kupfer 1992; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Stewart 1997; Streim 2012; Terra 1998).
Blinding
Performance bias (blinding of participants and personnel)
We judged 11 studies as having low risk of performance bias. These studies explicitly described the technique used in blinding (Bialos 1982; Cook 1986; Keller 1998; Keller 2007; Kocsis 2007; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Perahia 2009; Stewart 1997; Wilson 2003).
We judged 14 studies as having unclear risk of bias; these studies did not specify whether participants and personnel were blinded or how they were blinded (Gelenberg 2003; Gilaberte 2001; Kane 1982; Khan 2014; Klysner 2002; Kocsis 1996; Kornstein 2006; Montgomery 1988; Montgomery 2004; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Terra 1998).
We assessed eight studies including psychotherapy or minimal intervention as having high risk of performance bias (Bockting 2018; Derubeis 2019; Eveleigh 2018; Huijbers 2016; Kuyken 2008; Kuyken 2015; Segal 2010; Streim 2012). In these eight studies, participants and/or clinicians were not blinded and were aware of the treatment, and the discontinued antidepressant was not replaced by a placebo. One of the eight studies was judged as having high risk of bias, as the antidepressant was stopped and was not replaced by a placebo (Streim 2012).
Detection bias (blinding of outcome assessment)
We deemed 12 studies to be at low risk of detection bias as they provided a clear description of the method of blinding of outcome assessors (Bialos 1982; Bockting 2018; Cook 1986; Derubeis 2019; Eveleigh 2018; Gelenberg 2003; Kupfer 1992; Kuyken 2008; Kuyken 2015; Segal 2010; Stewart 1997; Wilson 2003).
We assessed 19 studies as being at unclear risk; study authors did not specify whether outcome assessors were blinded or how they were blinded (Gilaberte 2001; Kane 1982; Keller 1998; Keller 2007; Khan 2014; Klysner 2002; Kocsis 2007; Kornstein 2006; Mavissakalian 1999; Mavissakalian 2001; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Streim 2012; Terra 1998).
We assessed two studies as having high of bias; in one study, authors reported possible bias due to absence of independent raters (Kocsis 1996); in the other study, authors reported that it was impossible to keep research assistants at different sites masked to intervention group because they were involved in the practical organisation of the trial (Huijbers 2016).
Incomplete outcome data
We judged eight studies to be at low risk of attrition bias because there were no missing data, all study dropouts were reported, or the number of dropout participants was low (< 20%) and was similar across treatment groups (Bockting 2018; Cook 1986; Khan 2014; Kocsis 1996; Kupfer 1992; Kuyken 2015; Peterson 2010; Wilson 2003).
We assessed 20 studies to be at high risk as a large proportion of data were missing and/or there was an imbalance in the number of missing participants across treatment groups (Bialos 1982; Derubeis 2019; Eveleigh 2018; Gelenberg 2003; Gilaberte 2001; Huijbers 2016; Keller 1998; Keller 2007; Klysner 2002; Kocsis 2007; Kornstein 2006; Mavissakalian 1999; Mavissakalian 2001; Montgomery 2004; Perahia 2009; Rapaport 2001; Rickels 2010; Rouillon 2000; Segal 2010; Streim 2012).
We judged four studies to be at unclear risk as they did not report the reasons for dropout but dropout numbers were similar between groups or the number of dropouts was not clear from the text (Kane 1982; Montgomery 1988; Stewart 1997; Terra 1998). We judged one study as having unclear risk as the authors used last observation carried forward (LOCF) as the imputation method for missing data for secondary outcomes, and we considered this to be an inappropriate method in this context (see discussion) (Kuyken 2008).
Selective reporting
Antidepressants have been associated with withdrawal symptoms since 1960 for TCAs and MAOs (Nilsen 2012), and since the 1990s for SSRIs and SNRIs (Fava 2015; Fava 2018b). In all, 32 studies (97%) failed to include withdrawal symptoms, although this is a fundamental outcome in drug discontinuation trials. Therefore we judged not measuring withdrawal symptoms as introducing a large source of bias. A total of 21 studies measured adverse events of antidepressants, but this outcome may be biased because it may include withdrawal symptoms as these studies did not distinguish withdrawal symptoms from adverse events of antidepressants. None of the studies made adjustments for withdrawal symptoms.
The primary outcomes successful discontinuation rate, relapse, and adverse events were not reported in 31 (94%), 2 (6%), and 12 (36%) trials. The secondary outcomes depressive symptoms, anxiety symptoms, quality of life, social functioning, and severity of illness were not clearly reported in 16 (48%), 25 (76%), 24 (73%), 28 (85%), and 22 (67%) trials. Data on suicide, suicide attempt, or suicidal ideation were not reported in 25 (76%) trials. In addition, several studies did not report all outcomes described in the methods section, or they reported data incompletely.
While following recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions, and in considering all relevant outcomes for a study‐level judgement (Higgins 2017), we judged all studies except one to be at high risk of reporting bias (Khan 2014).
Other potential sources of bias
We judged the only cluster‐RCT ‐ Eveleigh 2018 ‐ to be at low risk for cluster bias (Higgins 2017).
We judged 20 studies receiving support from the pharmaceutical industry or reporting an unclear source of funding to be at unclear risk for industry sponsorship bias (Catalogue of Bias 2019) (Bialos 1982; Cook 1986; Derubeis 2019; Gelenberg 2003; Gilaberte 2001; Keller 1998; Keller 2007; Klysner 2002; Kocsis 1996; Kocsis 2007; Kornstein 2006; Montgomery 1988; Montgomery 2004; Perahia 2009; Peterson 2010; Rapaport 2001; Rickels 2010; Rouillon 2000; Terra 1998; Wilson 2003). In one study, editorial and medical writing support was provided by a medical writer funded by the sponsor of the trial (Khan 2014); therefore we judged this study to be at high risk of other bias. We judged 12 studies with only non‐commercial sponsorship to be at low risk for industry sponsorship (Bockting 2018; Eveleigh 2018; Huijbers 2016; Kane 1982; Kupfer 1992; Kuyken 2008; Kuyken 2015; Mavissakalian 1999; Mavissakalian 2001; Segal 2010; Stewart 1997; Streim 2012).
We judged no studies to be at risk for baseline imbalance.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The following comparisons are included.
Discontinuation abrupt versus continuation of long‐term antidepressants (without psychological support or with psychological support as co‐intervention) (Table 1).
Discontinuation by "tapering" versus continuation of long‐term antidepressants (without psychological support or with psychological support as co‐intervention) (Table 2).
Discontinuation combined with high‐intensity psychological intervention versus continuation of long‐term antidepressants (Table 3).
Discontinuation with a minimal intervention versus usual care (Table 4).
None of the included studies compared discontinuation combined with low‐intensity psychological intervention or psychosocial support or discontinuation with changed pharmacological form (e.g. fluoxetine or paroxetine liquid) to continuation of long‐term antidepressants.
For each outcome of each comparison, we reported the prioritised outcome. We summarised studies with a non‐prioritised outcome measure in a narrative synthesis.
For the outcomes discontinuation and relapse, we first pooled together studies with and without co‐intervention. We then pooled studies with and without co‐intervention separately because psychological support may lead to higher risk of successful antidepressant discontinuation and lower risk of relapse.
For studies with prioritised and non‐prioritised outcome measures, we summarised the non‐prioritised outcome measures in Table 6.
2. Other reported outcomes (with non‐prioritised outcome measures), not included in meta‐analysis.
| Outcome | Study ID | Intervention | Number intervention | Control group | Number control | Study authors' conclusions about differences between groups |
| Comparison 1: abrupt discontinuation vs continuation of long‐term antidepressants | ||||||
| 3. Withdrawal symptoms | ||||||
| Adjusted mean DESS total score over first 2 weeks of the discontinuation period | Khan 2014 | 5.3 (SE 0.52) | 146 | 4.1 (SE 0.72) | 36 | no conclusions made by study authors |
| 5. Depressive symptoms | ||||||
| BDI, mean change | Peterson 2010 | ‐0.47 (SD 0.48) | 16 | 1.5 ( SD 1.33) | 14 | study authors reported no differences between groups |
| BDI, mean change | Peterson 2010 | 2.38 (SD 4.64) | 11 | 0.43 (SD 0.82) | 11 | study authors reported no differences between groups |
| MADRS, mean change | Kornstein 2006 | ‐0.3 (SD 3) | 65 | 0.1 (SD 5.8) | 73 | study authors reported no differences between groups |
| SQ Depression, mean change | Peterson 2010 | 0.56 (SD 0.28) | 16 | 0.15 (SD 0.08) | 14 | study authors reported no differences between groups |
| SQ Depression, mean change | Peterson 2010; | 2.67 (SD 1.32) | 11 | 0.14 (SD 0.1) | 11 | study authors reported no differences between groups |
| 6. Anxiety symptoms | ||||||
| PDSS, mean change | Rapaport 2001 | 2.27 (SD 4.44) | 89 | 1.51 (SD 4.3) | 92 | study authors reported no differences between groups |
| Frequency of full panic attacks, mean change | Rapaport 2001 | 1.14 (SD 5.59) | 89 | 0.75 (SD 3.59) | 92 | study authors reported no differences between groups |
| 10. Global severity of illness | ||||||
| CGI‐I, mean change | Kornstein 2006 | ‐0.1 (SD 0.3) | 65 | 0 (SD 0.6) | 73 | study authors reported no differences between groups |
| CGI‐I, mean change | Peterson 2010 | 0.32 (SD 0.29) | 16 | 0.18 (SD 0.27) | 14 | study authors reported no differences between groups |
| CGI‐I, mean change | Peterson 2010; | 0.33 (SD 0.22) | 11 | 0.35 (SD 0.15) | 11 | study authors reported no differences between groups |
| Endpoint CGI‐I rating | Rapaport 2001 | 0.9 (SD 1.41) | 89 | 0.34 (SD 1.03) | 92 | study authors reported a modest statistically significant advantage in favour of continuation of antidepressant |
| Patient Global Impression, mean change | Peterson 2010 | 0.33 (SD 0.12) | 16 | 0.08 (SD 0.31) | 14 | study authors reported no differences between groups |
| CGI‐I, mean change | Peterson 2010 | 0.33 (SD 0.22) | 11 | 0.29 (SD 0.26) | 11 | study authors reported no differences between groups |
| 11. Other reported outcomes | ||||||
| Symptom Questionnaire Somatic Subscale, mean change | Peterson 2010 | ‐0.01 (SD 0.32) | 16 | 0.28 (SD 0.54) | 14 | study authors reported no differences between groups |
| Symptom Questionnaire Somatic Subscale, mean change | Peterson 2010 | 1.19 (SD 1.01) | 11 | 0.61 (SD 0.45) | 11 | study authors reported no differences between groups |
| SQ Hostility, mean change | Peterson 2010 | 0.51 (SD 0.77) | 16 | 0.77 (SD 0.62) | 14 | study authors reported no differences between groups |
| SQ Hostility, mean change | Peterson 2010 | 1.69 (SD 1.18) | 11 | 0.34 (SD 0.59) | 11 | study authors reported no differences between groups |
| Beck Hopelessness Scale, mean change | Peterson 2010 | ‐0.83 (SD 1.06) | 16 | ‐0.17 (SD 0.23) | 14 | study authors reported no differences between groups |
| Beck Hopelessness Scale, mean change | Peterson 2010 | 1.07 (SD 1.02) | 11 | 0.48 (SD 0.20) | 11 | study authors reported no differences between groups |
| Cognitive function assessed by Mini‐Mental State Examination, mean endpoint score | Streim 2012 | 24.9 (SD 4) | 13 | 23.1 (SD 4.4) | 23 | no conclusions made by study authors |
| Comparison 2: discontinuation by tapering vs continuation of long‐term antidepressants | ||||||
| 3. Withdrawal symptoms | ||||||
| Adjusted mean DESS total score for first 2 weeks of the discontinuation period | Khan 2014 | 4.8 (SE 0.54) | 139 | 4.1 (SE 0.72) | 36 | no conclusions made by study authors |
| 5. Depressive symptoms | ||||||
| BDI, mean endpoint score | Keller 1998 | 13.9 (SD 10.7) | 84 | 9 (SD 9.9) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| MADRS, mean endpoint score | Keller 1998 | 15 (SD 12.5) | 84 | 7.9 (SD 9.6) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| MADRS, mean endpoint score | Cook 1986 | 12.8 (SD 10.1) | 9 | 4.8 (SD 4.5) | 6 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Cornell Dysthymic Rating Scale, mean endpoint score | Keller 1998 | 25.6 (SD 18.4) | 84 | 14.3 (SD 14.9) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Carroll Depression Scale, mean endpoint score | Cook 1986 | 17.6 (SD 10.6) | 9 | 10.7 (SD 7.2) | 6 | study authors reported no differences between groups |
| IDS‐SR, mean endpoint score | Keller 2007 | 18.7 (SE 1.7) | 40 | 11.9 (SE 1.7) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| IDS‐SR, mean endpoint score | Kocsis 2007 | 18.6 (SE 1.2) | 129 | 15.5 (SE 1.2) | 129 | study authors reported no differences between groups |
| HAM‐D subscale score, depressed mood item 1 score, mean endpoint score | Montgomery 2004 | 1.5 (SE 0.11) | 107 | 0.9 (SE 0.11) | 108 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| HAM‐D subscale score, depressed mood item 1 score, mean change | Perahia 2009 | 0.67 (SE 0.1) | 142 | 0.27 (SE 0.09) | 145 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| HAM‐D subscale score, core factor, mean change | Perahia 2009 | 1.74 (SE 0.24) | 142 | 0.75 (SE 0.22) | 145 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| HAM‐D subscale score, Maier, mean change | Perahia 2009 | 2.25 (SE 0.31) | 142 | 0.91 (SE 0.29) | 145 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| HAM‐D subscale score, retardation, mean change | Perahia 2009 | 1.49 (SE 0.22) | 142 | 0.59 (SE 0.20) | 145 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| HAM‐D subscale score, sleep, mean change | Perahia 2009 | 0.71 (SE 0.13) | 142 | 0.13 (SE 0.12) | 145 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| MADRS, mean endpoint score | Montgomery 2004 | 15.1 (SE 1.14) | 107 | 9.5 (SE 1.01) | 106 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| 6. Anxiety symptoms | ||||||
| Hospital Anxiety and Depression Scale (HADS), anxiety factor, mean endpoint score | Rickels 2010 | 9.29 (SE 0.44) | 54 | 7.43 (SE 0.38) | 82 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Hospital Anxiety and Depression Scale (HADS), anxiety factor, mean endpoint score | Rickels 2010 | 8.42 (SE 1.05) | 34 | 5.8 (SE 1.28) | 25 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| IDS‐SR, anxiety/arousal, mean endpoint score | Keller 2007 | 6.9 (SE 0.6) | 40 | 4.1 (SE 0.6) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| IDS‐SR, anxiety/arousal, mean endpoint score | Kocsis 2007 | 6.2 (SE 0.5) | 129 | 5.3 (SE 0.5) | 129 | study authors reported no differences between groups |
| 8. Quality of life | ||||||
| GHQ, adjusted endpoint score | Rickels 2010 | 28.17 (SD 8.41) | 54 | 23.73 (6.26) | 82 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| LES‐S, mean endpoint score | Keller 2007 | 55.2 (SE 3.4) | 40 | 66.1 (SE 2.9) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Q‐LES‐Q, mean endpoint score | Keller 2007 | 66.9 (SE 2.2) | 40 | 74.7 (SE 2.1) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| SF‐36, role functioning, physical mean endpoint score | Keller 2007 | 65.8 (SE 5.2) | 40 | 74.2 (SE 4.9) | 43 | study authors reported no differences between groups |
| SF‐36, bodily pain, mean endpoint score | Keller 2007 | 71.2 (SE 3.4) | 40 | 77.4 (SE 3.2) | 43 | study authors reported no differences between groups |
| SF‐36, general health, mean endpoint score | Keller 2007 | 65.9 (SE 2.5) | 40 | 73.0 (SE 2.3) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| SF‐36, vitality, mean endpoint score | Keller 2007 | 47.3 (SE 3.3) | 40 | 57.9 (SE 3.1) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| SF‐36, physical component summary, mean endpoint score | Keller 2007 | 51.9 (SE 1.1) | 40 | 51.8 (SE 1.1) | 43 | study authors reported no differences between groups |
| SF‐36, mental component summary, mean endpoint score | Keller 2007 | 39.2 (SE 1.9) | 40 | 47.6 (SE 1.8) | 43 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| LES‐S, mean endpoint score | Kocsis 2007 | 58.1 (SE 2.2) | 129 | 62.9 (SE 2.1) | 129 | study authors reported no differences between groups |
| Q‐LES‐Q, mean endpoint score | Kocsis 2007 | 67.3 (SE 1.3) | 129 | 72.3 (SE 1.3) | 129 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| SF‐36, role functioning, physical mean endpoint score | Kocsis 2007 | 69.0 (SE 3.8) | 129 | 76.2 (SE 3.8) | 129 | study authors reported no differences between groups |
| SF‐36, bodily pain, mean endpoint score | Kocsis 2007 | 74.6 (SE 2.2) | 129 | 75.1 (SE 2.1) | 129 | study authors reported no differences between groups |
| SF‐36, general health, mean endpoint score | Kocsis 2007 | 71.0 (SE 1.6) | 129 | 72.7 (SE 1.5) | 129 | study authors reported no differences between groups |
| SF‐36, vitality, mean endpoint score | Kocsis 2007 | 48.4 (SE 2.2) | 129 | 51.3 (SE 2.1) | 129 | study authors reported no differences between groups |
| SF‐36, physical component summary, mean endpoint score | Kocsis 2007 | 53.3 (SE 0.8) | 129 | 52.6 (SE 0.8) | 129 | study authors reported no differences between groups |
| SF‐36, mental component summary, mean endpoint score | Kocsis 2007 | 40.5 (SE 1.3) | 129 | 44.5 (SE 1.3) | 129 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| WHO QoL‐Q1, overall perception of quality of life, 3 months, mean endpoint score | Huijbers 2016 | 3.4 (SD 0.9) | 93 | 3.6 (SD 0.8) | 86 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL‐Q1, overall perception of quality of life, 15 months, mean endpoint score | Huijbers 2016 | 3.6 (SD 0.8) | 83 | 3.7 (SD 0.9) | 68 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life, 3 months, mean endpoint score | Huijbers 2016 | 3.3 (SD 1.0) | 92 | 3.3 (SD 1.0) | 85 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life, 15 months, mean endpoint score | Huijbers 2016 | 3.4 (SD 1.0) | 83 | 3.4 (SD 1.1) | 68 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, physical domain, 3 months, mean endpoint score | Huijbers 2016 | 24.4 (SD 4.5) | 93 | 24.6 (SD 4.7) | 86 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, physical domain, 15 months, mean endpoint score | Huijbers 2016 | 25.4 (SD 4.9) | 83 | 25.6 (SD 4.5) | 67 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, psychological domain, 3 months, mean endpoint score | Huijbers 2016 | 18.8 (SD 3.7) | 93 | 19.9 (SD 3.6) | 86 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, psychological domain, 15 months, mean endpoint score | Huijbers 2016 | 19.7 (SD 3.6) | 82 | 20.0 (SD 3.8) | 68 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, social domain, 3 months, mean endpoint score | Huijbers 2016 | 10.1 (SD 2.1) | 93 | 10.0 (SD 2.2) | 86 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, social domain, 15 months, mean endpoint score | Huijbers 2016 | 10.5 (SD 2.3) | 83 | 10.1 (SD 2.2) | 68 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, environmental domain, 3 months, mean endpoint score | Huijbers 2016 | 30.7 (SD 4.9) | 93 | 31.6 (SD 4.5) | 86 | study with psychological co‐intervention; study authors reported no differences between groups |
| WHO QoL, environmental domain, 15 months, mean endpoint score | Huijbers 2016 | 30.5 (SD 4.2) | 83 | 31.9 (SD 4.0) | 68 | study with psychological co‐intervention; study authors reported no differences between groups |
| 9. Social and occupational functioning | ||||||
| LIFE, subject assessment score, mean endpoint score | Keller 1998 | 3.08 (SD 1.17) | 84 | 2.27 (SD 1.23) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| LIFE, interviewer assessment score, mean endpoint score | Keller 1998 | 3.00 (SD 1.11) | 84 | 2.23 (SD 1.14) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| LIFE, satisfaction assessment score, mean endpoint score | Keller 1998 | 2.99 (SD 1.06) | 84 | 2.24 (SD 1.08) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Interference with daily activities | Perahia 2009 | 2.81 (SE 1.82) | 142 | 3.16 (SE 1.74) | 145 | study authors reported no differences between groups |
| 10. Global severity of illness | ||||||
| CGI‐I, mean endpoint score | Keller 1998 | 2.7 (SD 1.5) | 84 | 1.9 (SD 1.3) | 77 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| CGI‐I, mean endpoint score | Rickels 2010 | 3.29 (SE 0.19) | 54 | 1.96 (SE 0.12) | 82 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| CGI‐I, mean endpoint score | Rickels 2010 | 2.78 (SE0.32) | 34 | 2.20 (SE 0.41) | 15 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Patient Global Impressions of improvement, endpoint score | Perahia 2009 | 2.34 (SE 0.11) | 142 | 1.72 (SE 0.11) | 145 | study authors reported a statistically significant advantage in favour of continuation of antidepressant |
| Symptom Questionnaire Somatic Subscale (SQ‐SS), mean change | Perahia 2009 | 0.81 (SE 0.4) | 142 | 0.79 (SD 0.39) | 145 | study authors reported no differences between groups |
| 11. Other reported outcomes | ||||||
| VAS score, mean change | Perahia 2009 | 4.57 (SD 1.86) | 142 | 3.92 (SE 1.78) | 145 | study authors reported no differences between groups |
| Headache, mean change | Perahia 2009 | 2.80 (SE 1.80) | 142 | 4.77 (SE 1.72) | 145 | study authors reported no differences between groups |
| Back pain, mean change | Perahia 2009 | 3.40 (SE 1.72) | 142 | 1.77 (SE 1.65) | 145 | study authors reported no differences between groups |
| Schoulder pain, mean change | Perahia 2009 | 3.02 (SE 1.62) | 142 | 0.51 (SE 1.55) | 145 | study authors reported no differences between groups |
| Pain while awake, mean change | Perahia 2009 | 4.69 (SE 2.19) | 142 | 3.64 (SE 2.10) | 145 | study authors reported no differences between groups |
| Comparison 3: discontinuation with psychological support vs continuation of long‐term antidepressants | ||||||
| 1. Successful discontinuation rate | ||||||
| Mean number of days on antidepressant treatment | Kuyken 2008 | 266.5 (SD 167.7) | 61 | 411.4 (SD 91.8) | 62 | study authors reported a statistically significant difference in favour of discontinuation group |
| 2. Relapse rate | ||||||
| Total number of relapses/recurrences, mean | Kuyken 2008 | 1.45 (95% CI 1.21 to 1.69) | 61 | 1.57 (95% CI 1.32 to 1.81) | 62 | study authors reported no differences between groups |
| Duration of relapses/recurrences (period of time in months that a person met SCID criteria), mean | Kuyken 2008 | 3.36 (95% CI 2.2 to 4.5) | 61 | 3.0 (95% CI 2.1 to 3.9) | 62 | study authors reported no differences between groups |
| Severity of relapses/recurrences (scale range between 1 and 4: mild, moderate, severe without psychotic feature, severe with psychotic features), mean | Kuyken 2008 | 1.79 (95% CI 1.56 to 2.02) | 61 | 1.72 (95% CI 1.48 to 1.95) | 62 | study authors reported no differences between groups |
| 5. Depressive symptoms | ||||||
| HAM‐D, mean score at 1 month | Kuyken 2015 | 6.3 (SD 5.6) | 186 | 7.4 (SD 6.3) | 183 | study authors reported no differences between groups |
| HAM‐D, mean score at 9 months | Kuyken 2015 | 6 (SD 5.5) | 177 | 5.6 (SD 6.4) | 175 | study authors reported no differences between groups |
| HAM‐D, mean score at 12 months | Kuyken 2015 | 5.7 (SD 5.7) | 184 | 4.7(SD 5.2) | 181 | study authors reported no differences between groups |
| HAM‐D, mean score at 18 months | Kuyken 2015 | 5.7 (SD 5.7) | 184 | 5.3 (SD 6.1) | 174 | study authors reported no differences between groups |
| BDI, mean score at 1 month | Kuyken 2015 | 9.9 (SD 9.7) | 174 | 13.9 (SD 10.9) | 174 | study authors reported no differences between groups |
| BDI, mean score at 9 months | Kuyken 2015 | 11 (SD 10.5) | 151 | 10.5 (SD 9.7) | 142 | study authors reported no differences between groups |
| BDI, mean score at 12 months | Kuyken 2015 | 10.7 (SD 10) | 167 | 11.3 (SD 9.2) | 157 | study authors reported no differences between groups |
| BDI, mean score at 18 months | Kuyken 2015 | 11.7 (SD 10.6) | 142 | 11.3 (SD 10.7) | 149 | study authors reported no differences between groups |
| BDI, mean score at 24 months | Kuyken 2015 | 11.6 (SD 10.9) | 169 | 11.9 (SD 10.7) | 167 | study authors reported no differences between groups |
| HAM‐D, mean score at 1 month | Kuyken 2008 | 5.83 (95% CI 4.49 to 7.3) | 59 | 7.75 (95% CI 5.86 to 9.34) | 59 | study authors reported a statistically significant difference in favour of discontinuation group |
| BDI, mean score at 1 month | Kuyken 2008 | 13.12 (95% CI 10.27 to 15.97) | 59 | 17.47 (95% CI 14.31 to 20.62) | 58 | study authors reported a statistically significant difference in favour of discontinuation group |
| BDI, mean score at 15 months | Kuyken 2008 | 12.61 (95% CI 9.96 to 15.26) | 59 | 17.02 (95% CI 13.16 to 20.87) | 58 | study authors reported a statistically significant difference in favour of discontinuation group |
| 8. Quality of life | ||||||
| WHO QoL‐Q1, overall perception at MBCT + 1 month, mean score | Kuyken 2015 | 3.8 (SD 0.8) | 174 | 3.8 (SD 0.9) | 173 | study authors reported no differences between groups |
| WHO QoL‐Q1, overall perception at 9 months, mean score | Kuyken 2015 | 3.7 (SD 0.9) | 151 | 3.9 (SD 0.8) | 141 | study authors reported no differences between groups |
| WHO QoL‐Q1, overall perception at 12 months, mean score | Kuyken 2015 | 3.7 (SD 0.9) | 166 | 3.9 (SD 0.9) | 157 | study authors reported no differences between groups |
| WHO QoL‐Q1, overall perception at 18 months, mean score | Kuyken 2015 | 3.7 (SD 0.9) | 141 | 3.9 (SD 0.9) | 149 | study authors reported no differences between groups |
| WHO QoL‐Q1, overall perception at 24 months, mean score | Kuyken 2015 | 3.7 (SD 0.9) | 169 | 3.8 (SD 1) | 167 | study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life at MBCT + 1 month, mean score | Kuyken 2015 | 3.1 (SD 1) | 174 | 3.2 (SD 1) | 173 | study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life at 9 months, mean score | Kuyken 2015 | 3.1 (SD 1.1) | 151 | 3.2 (SD 1) | 141 | study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life at 12 months, mean score | Kuyken 2015 | 3.2 (SD 1.1) | 166 | 3.3 (SD 1.0) | 157 | study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life at 18 months, mean score | Kuyken 2015 | 3.2 (SD 1.0) | 141 | 3.3 (SD 1.1) | 149 | study authors reported no differences between groups |
| WHO QoL‐Q2, overall perception of quality of life at 24 months, mean score | Kuyken 2015 | 3.1 (SD 1.0) | 169 | 3.2 (SD 1) | 167 | study authors reported no differences between groups |
| WHO QoL, physical health domain (e.g. How satisfied are you with your sleep?) at MBCT + 1 month, mean score | Kuyken 2015 | 14.3 (SD 3.3) | 174 | 14.3 (SD 3.0) | 173 | study authors reported no differences between groups |
| WHO QoL, physical health domain at 9 months, mean score | Kuyken 2015 | 14.2 (SD 3.3) | 151 | 14.8 (SD 3.2) | 141 | study authors reported no differences between groups |
| WHO QoL, physical health domain at 12 months, mean score | Kuyken 2015 | 14.1 (SD 3.4) | 166 | 14.7 (SD 3.3) | 157 | study authors reported no differences between groups |
| WHO QoL, physical health domain at 18 months, mean score | Kuyken 2015 | 13.9 (SD 3.5) | 141 | 14.7 (SD 3.3) | 149 | study authors reported no differences between groups |
| WHO QoL, psychological domain (e.g. How much do you enjoy life?) at MBCT + 1 month, mean score | Kuyken 2015 | 13.4 (SD 2.6) | 174 | 12.6 (SD 2.8) | 173 | study authors reported no differences between groups |
| WHO QoL, psychological domain at 9 months, mean score | Kuyken 2015 | 13.3 (SD 3) | 151 | 13.4 (SD 2.7) | 141 | study authors reported no differences between groups |
| WHO QoL, psychological domain at 12 months, mean score | Kuyken 2015 | 13.3 (SD 2.9) | 166 | 13.3 (SD 2.7) | 157 | study authors reported no differences between groups |
| WHO QoL, psychological domain at 18 months, mean score | Kuyken 2015 | 12.9 (SD 2.8) | 141 | 13.3 (SD 3) | 149 | study authors reported no differences between groups |
| WHO QoL, social relationships domain (e.g. How satisfied are you with your personal relationship?) at MBCT + 1 month, mean score | Kuyken 2015 | 13.8 (SD 2.9) | 174 | 13.3 (SD 3.4) | 173 | study authors reported no differences between groups |
| WHO QoL, social relationships domain at 9 months, mean score | Kuyken 2015 | 13.7 (SD 3.4) | 151 | 14 (SD 3.4) | 141 | study authors reported no differences between groups |
| WHO QoL, social relationships domain at 12 months, mean score | Kuyken 2015 | 13.9 (SD 3.5) | 166 | 14.2 (SD 3.3) | 157 | study authors reported no differences between groups |
| WHO QoL, social relationships domain at 18 months, mean score | Kuyken 2015 | 14 (SD 3.4) | 141 | 14.2 (SD 3.4) | 148 | study authors reported no differences between groups |
| WHO QoL, social relationships domain at 24 months, mean score | Kuyken 2015 | 13.7 (3.3) | 169 | 13.9 (SD 3.5) | 167 | study authors reported no differences between groups |
| WHO QoL, environment domain (e.g. How satisfied are you with your access to health services?) at MBCT + 1 month, mean score | Kuyken 2015 | 15.2 (SD 2.4) | 174 | 15.3 (SD 2.5) | 173 | study authors reported no differences between groups |
| WHO QoL, environment domain at 9 months, mean score | Kuyken 2015 | 15.4 (SD 2.6) | 151 | 15.7 (SD 2.3) | 141 | study authors reported no differences between groups |
| WHO QoL, environment domain at 12 months, mean score | Kuyken 2015 | 15.2 (SD 2.6) | 166 | 15.6 (SD 2.6) | 157 | study authors reported no differences between groups |
| WHO QoL, environment domain at 18 months, mean score | Kuyken 2015 | 15.3 (SD 2.6) | 141 | 15.7 (SD 2.6) | 149 | study authors reported no differences between groups |
| WHO QoL, environment domain at 24 months, mean score | Kuyken 2015 | 14.9 (SD 2.6) | 169 | 15.7 (SD 2.7) | 167 | study authors reported no differences between groups |
| EQ‐5D, tariff at MBCT + 1 month, mean score | Kuyken 2015 | 0.727 (SD 0.295) | 174 | 0.760 (SD 0.226) | 173 | study authors reported no differences between groups |
| EQ‐5D, tariff at 9 months, mean score | Kuyken 2015 | 0.735 (SD 0.256) | 151 | 0.733 (SD 0.234) | 142 | study authors reported no differences between groups |
| EQ‐5D, tariff at 12 months, mean score | Kuyken 2015 | 0.721 (SD 0.293) | 167 | 0.764 (SD 0.248) | 156 | study authors reported no differences between groups |
| EQ‐5D, tariff at 18 months, mean score | Kuyken 2015 | 0.723 (SD 0.282) | 142 | 0.768 (SD 0.243) | 149 | study authors reported no differences between groups |
| EQ‐5D, tariff at 24 months, mean score | Kuyken 2015 | 0.715 (SD 0.310) | 169 | 0.757 (SD 0.266) | 166 | study authors reported no differences between groups |
| WHO QoL‐BREF, psychological domain at MBCT at 1 month, mean score | Kuyken 2015 | 24.08 (95% CI 22.62 to 25.53) | 60 | 22.86 (95% CI 21.34 to 24.39) | 59 | study authors reported a statistically significant difference in favour of discontinuation group |
| WHO QoL‐BREF, psychological domain at 1 month, mean score | Kuyken 2015 | 18.88 (95% CI 17.88 to 19.89) | 60 | 17.47 (95% CI 16.24 to 18.70) | 59 | study authors reported a statistically significant difference in favour of discontinuation group |
| WHO QoL‐BREF, social domain at 1 month, mean score | Kuyken 2015 | 10.09 (95% CI 9.55 to 10.64) | 60 | 9.07 (95% CI 8.37 to 9.77) | 59 | study authors reported no differences between groups |
| 11. Other reported outcomes | ||||||
| Medical comorbidity (measured by medical symptom list) at 12 months, mean score | Kuyken 2015 | 21 (SD 14) | 167 | 19.3 (SD 13.7) | 156 | study authors reported no differences between groups |
| Medical comorbidity (measured by medical symptom list) at 24 months, mean score | Kuyken 2015 | 22.2 (SD 14.6) | 169 | 21.7 (SD 16.3) | 167 | study authors reported no differences between groups |
| Psychiatric comorbidity (number of comorbid diagnoses) at 12 months, mean score | Kuyken 2015 | 0.1 (SD 0.3) | 196 | 0.1 (SD 0.4) | 169 | study authors reported no differences between groups |
| Psychiatric comorbidity (number of comorbid diagnoses) at 24 months, mean score | Kuyken 2015 | 0.3 (SD 0.7) | 183 | 0.3 (SD 0.6) | 183 | study authors reported no differences between groups |
| Psychiatric comorbidity (number of comorbid diagnoses) at 15 months, mean score | Kuyken 2008 | 0.34 (SD 0.64) | NR | 0.7 (SD 1.01) | NR | study authors reported a statistically significant difference in favour of discontinuation group (N = 114; number for each group not reported) |
| Subjective distress (rated by patients on a scale of 1 (the least distressing episode of depression I have ever experienced) to 100 (the most distressing episode of depression I have ever experienced)) | Kuyken 2008 | 59.65 (95% CI 51.82 to 67.18) | 61 | 62.56 (95% CI 56.16 to 68.96) | 62 | study authors reported a non‐statistically significant difference |
| Comparison 4: discontinuation with minimal intervention vs usual care | ||||||
| 11. Other reported outcomes | ||||||
| Penn State Worry Questionnaire (PSWQ) (frequency and severity of symptoms of worrying) at endpoint, mean score | Eveleigh 2018 | 42 (SD 14.3) | 50 | 39.3 (SD 12.7) | 54 | no conclusions made by study authors |
| Fear of Negative Evaluation Scale (FNES) for assessing expectations and distress associated with negative evaluations by others | Eveleigh 2018 | 10.3 (SD 11.0) | 54 | 8.4 (SD 10.1) | 56 | no conclusions made by study authors |
BDI: Beck Depression Inventory.
CGI‐I: Clinical Global Impression of Improvement Scale.
DESS: Discontinuation‐Emergent Signs and Symptoms Scale.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
GHQ: General Health Questionnaire.
HADS: Hospital Anxiety and Depression Scale.
HAM‐D: Hamilton Rating Scale for Depression.
IDS‐SR: Inventory of Depressive Symptomatology–Self‐Report.
LES‐S: Life Enjoyment and Satisfaction Scale.
LIFE: Longitudinal Internal Follow‐up Evaluation.
MADRS: Montgomery‐Åsberg Depression Rating Scale.
MBCT: mindfulness‐based cognitive therapy.
PDSS: Panic Disorder Severity Scale.
Q‐LES‐Q: Quality of Life Enjoyment and Satisfaction Questionnaire.
SCID: scheduled clinical interview for DSM‐IV.
SD: standard deviation.
SE: standard error.
SF‐36: Short Form 36.
SQ: Symptom Questionnaire.
SQ‐SS: Symptom Questionnaire Somatic Subscale.
VAS: visual analog scale.
WHO QoL: World Health Organization Quality of Life.
WHO QoL‐BREF: World Health Organization Cross‐Cultural Comparisons of Quality of Life.
Comparison 1. Discontinuation abrupt versus continuation of long‐term antidepressants (without psychological support or with psychological support as co‐intervention)
Thirteen studies (N = 1780) compared abrupt discontinuation with continuation of antidepressant (Gelenberg 2003; Gilaberte 2001Kane 1982; Khan 2014; Klysner 2002; Kornstein 2006; Montgomery 1988Peterson 2010; Rapaport 2001Rouillon 2000; Streim 2012; Terra 1998; Wilson 2003); 2 of the 13 studies included psychological support as a co‐intervention (CBT in Peterson 2010; CBASP in Gelenberg 2003).
Twelve studies included antidepressants for depressive disorder, and one study included antidepressants for panic disorder with or without agoraphobia (Rapaport 2001).
Included studies compared abrupt discontinuation with continuation of antidepressant treatment: eight studies used SSRI (Gilaberte 2001; Klysner 2002; Kornstein 2006; Montgomery 1988; Peterson 2010; Rapaport 2001; Terra 1998; Wilson 2003), one study TCA (Kane 1982), one study an atypical antidepressive (Gelenberg 2003), two studies SNRI (Khan 2014; Rouillon 2000), and one study a non‐specified type of antidepressant (Streim 2012).
For characteristics of studies, see Table 7.
3. Studies with abrupt discontinuation, included in comparison 1.
| Study ID | Design | Antidepressant duration before randomisation | Age | Main disorder | Study duration | Number | Intervention 1 | Intervention 2 | Co‐intervention |
| Gelenberg 2003 | 3‐phase RCT | 28 weeks | 18 to 75 years | diagnosis of chronic depression (2 years' duration), concurrent MDD superimposed on an antecedent dysthymic disorder (double depression), recurrent depression with incomplete inter‐episode recovery ≥ 2 years’ duration | 52 weeks | 160 | discontinuation ‐ replaced by placebo | nefazodone continuation | CBASP |
| Gilaberte 2001 | 2‐phase RCT | 32 weeks | 18 to 65 years | ≥ 1 previous major depressive episode in the last 5 years | 48 weeks | 140 | discontinuation ‐ replaced by placebo | fluoxetine continuation | |
| Kane 1982 | 2‐phase RCT | 6 months | 18 and 65 years | diagnosis of recurrent unipolar major depressive disorder (≥ 2 episodes of depression or mania in the previous 7 years and euthymic for 6 months before study entry | 104 weeks | 11 | discontinuation ‐ replaced by placebo | imipramine continuation | |
| Khan 2014 | 2‐phase RCT | 24 weeks | ≥ 18 years | single or recurrent MDD | 4 weeks | 184 | discontinuation ‐ replaced by placebo | desvenlafaxine continuation | |
| Klysner 2002 | 3‐phase RCT | 24 weeks | ≥ 65 years | diagnosis of MDD not longer than 12 months | 48 weeks | 121 | discontinuation ‐ replaced by placebo | citalopram continuation | |
| Kornstein 2006 | 3‐phase RCT | 24 weeks | 18 to 81 years | depressive episode ≥ 4 weeks’ duration and ≥ 2 major depressive episodes before the index episode | 52 weeks | 139 | discontinuation ‐ replaced by placebo | escitalopram continuation | |
| Montgomery 1988 | 3‐phase RCT | 24 weeks | not reported | diagnosis of MDD and ≥ 1 major episode in last 5 years with interval | 53 weeks | 220 | discontinuation ‐ replaced by placebo | fluoxetine continuation | |
| Peterson 2010 | 3‐phase RCT | 36 weeks | 18 to 65 years | history of MDD and history of ≥ 3 major depressive episodes (with the prior episode no longer than 2.5 years before onset of the current episode) or current episode as chronic, or both MDD and dysthymia | 80 weeks | 32 | discontinuation ‐ replaced by placebo | fluoxetine continuation | |
| Peterson 2010; | 4‐phase RCT | 36 weeks | 18 to 65 years | history of MDD and history of ≥ 3 major depressive episodes (with the prior episode no longer than 2.5 years before onset of the current episode) or current episode chronic, or both MDD and dysthymia | 80 weeks | 23 | discontinuation ‐ replaced by placebo | fluoxetine continuation | CBT |
| Rapaport 2001 | 3‐phase RCT | 52 or 62 weeks | 18 years and older | diagnosis of panic disorder with or without agoraphobia | 28 weeks | 183 | discontinuation ‐ replaced by placebo | sertraline continuation | |
| Rouillon 2000 | 2‐phase RCT | 24 weeks | 18 to 70 years | history of MDD; current major depressive episode without psychotic symptoms | 52 weeks | 214 | discontinuation ‐ replaced by placebo | milnacipran continuation | |
| Streim 2012 | RCT | not reported | ≥ 65 years | single episode of depression, in full remission for ≥ 6 months | 52 weeks | 36 | discontinuation ‐ no placebo | antidepressant (type not reported) continuation | |
| Terra 1998 | 3‐phase RCT | 24 weeks | 18 to 70 years | MDD and history of ≥ 2 episodes in previous 5 years | 52 weeks | 204 | discontinuation ‐ replaced by placebo | fluvoxamine continuation | |
| Wilson 2003 | 3‐phase RCT | 24 to 28 weeks | ≥ 65 years | MDD | 100 weeks | 113 | discontinuation ‐ replaced by placebo | sertraline continuation |
CBASP: cognitive‐behavioural analysis system of psychotherapy.
CBT: cognitive‐behavioural therapy.
MDD: major depressive disorder.
RCT: randomised controlled trial.
1.1 Successful discontinuation rate
None of the studies reported successful discontinuation rate as an outcome.
1.2 Relapse (as defined by study authors)
See Analysis 1.1.
1.1. Analysis.

Comparison 1: Abrupt discontinuation versus continuation of antidepressants, Outcome 1: Relapse (as defined by study authors)
Eleven studies contributed data for this outcome (Gelenberg 2003; Gilaberte 2001; Kane 1982; Klysner 2002; Kornstein 2006; Montgomery 1988; Peterson 2010; Rapaport 2001; Rouillon 2000; Terra 1998; Wilson 2003). All studies measured relapse of depression except one, which reported relapse of panic disorder in participants with panic disorder (Rapaport 2001). Relapse was based on the criteria defined by study authors, which varied widely between studies. Most studies were based on clinical scale scores that include domains for depressed mood, insomnia, agitation, gastrointestinal somatic symptoms, anxiety, general somatic symptoms, and sexual dysfunction ‐ all of which are symptoms of withdrawal. None of these studies measured separately symptoms of withdrawal nor attempted to make adjustments for them.
1.2.1 All studies (with and without psychological support)
See Analysis 1.1.
Pooling was possible for 11 studies (Gelenberg 2003; Gilaberte 2001; Kane 1982; Klysner 2002; Kornstein 2006; Montgomery 1988; Peterson 2010; Rapaport 2001; Rouillon 2000; Terra 1998; Wilson 2003). Of the 11 studies, all but one reported relapse as time‐to‐event using hazard ratios (HRs). Kane 1982 did not report data for HRs; we calculated relative risk and used relative risk for relapse to compare time to relapse.
A total of 775 (49.8%) participants were included in the pooled discontinuation group, and 780 (50.2%) in the pooled continuation group. Abrupt discontinuation increased the risk of relapse (as defined by study authors) (HR 1.97, 95% confidence interval (CI) 1.56 to 2.50; 1555 participants, 11 studies) compared to discontinuation, without specific assessment of withdrawal symptoms (Analysis 1.1).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, and withdrawal confounding bias) and by one level for indirectness (majority of participants had recurrent depression, and one study included panic disorder). We did not downgrade for attrition bias as censoring for survival data is unlikely to introduce bias.
1.2.2 Studies without psychological support
See Analysis 1.1.
Ten studies without co‐intervention were suitable for pooling. In nine studies with long‐term follow‐up (24 weeks or longer), relapse is reported as time‐to‐event using HRs (Gilaberte 2001; Klysner 2002; Kornstein 2006; Montgomery 1988; Peterson 2010; Rapaport 2001; Rouillon 2000; Terra 1998; Wilson 2003). Kane 1982 did not report data for HR; we calculated relative risk and used relative risk for relapse to compare time to relapse.
In all, 680 (49.5%) participants were included in the pooled discontinuation group, and 693 (50.5%) in the pooled continuation group. Abrupt discontinuation increased the risk of relapse (as defined by study authors) (HR 2.09, 95% CI 1.59 to 2.74; 1373 participants, 10 studies) compared to discontinuation, without specific assessment of withdrawal symptoms (Analysis 1.1).
One of the pooled studies (n = 181) investigated the effect of antidepressant continuation among participants with anxiety disorder and reported the rate of depression as adverse events (Rapaport 2001). Study authors reported no difference in depression between the discontinuation group (9/89; 10.1%) and the continuation group (9/92; 9.8%) (P = 1) (Rapaport 2001).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, and withdrawal confounding bias) and by one level for indirectness (majority of participants had recurrent depression, and one study included panic disorder). We did not downgrade for attrition bias as censoring for survival data is unlikely to introduce bias.
1.2.3 Studies with psychological support as co‐intervention
See Analysis 1.1.
We considered two studies with long‐term follow‐up and psychotherapy as co‐intervention suitable for pooling (Gelenberg 2003; Peterson 2010). In all 95 (52.2%) participants were included in the pooled discontinuation group, and 87 (47.8%) in the pooled continuation group. There was no difference in time to relapse between discontinuation versus continuation with psychotherapy as co‐intervention in both groups (HR 1.48, 95% CI 0.93 to 2.34; 182 participants, 2 studies). None of these studies performed specific assessment of withdrawal symptoms.
We assessed the certainty of the outcome as very low, downgraded by one level for risk of bias (poor description of randomisation process and blinding, and withdrawal confounding bias), by one level for imprecision (small number of studies, small number of participants, and wide 95% confidence interval, which includes the null effect of no difference), and by one level for indirectness (studies included participants with recurrent depressive disorder). We did not downgrade for attrition bias as censoring for survival data is unlikely to introduce bias.
1.3 Withdrawal symptoms
One study provided data for this outcome by using the Discontinuation‐Emergent Signs and Symptoms Scale (Khan 2014), a 43‐item observer‐administered assessment of withdrawal symptoms (DESS). Study authors reported the proportion of participants with a withdrawal syndrome based on DESS. Experiencing a withdrawal syndrome was defined as an increase of four or more in DESS score (regardless of severity of withdrawal symptoms) between baseline and the first two weeks of the double‐blind phase.
Khan 2014 compared venlafaxine abrupt discontinuation (n = 146) to venlafaxine continuation (n = 36; control group split to allow multiple‐arm comparison) in participants during a four‐week trial. In all, 31 of the 146 participants in the discontinuation group experienced a withdrawal syndrome, along with 4 of the 36 participants in the continuation group. Study authors reported there were no statistically significant differences between groups in the proportions of participants with withdrawal syndrome based on DESS scores (abrupt discontinuation 21.2%; continuation 11.1%; P ≥ 0.06).
We assessed the certainty of evidence for this outcome as very low, downgraded by one level due to imprecision (single study included a small number of participants), by one level for risk of bias (randomisation and blinding were poorly described, severity of withdrawal symptoms was not scored in the outcome, and the period for observing withdrawal symptoms was relatively short), and by one level for indirectness (study included participants with single or recurrent depressive disorder but did not report numbers of participants with single or recurrent disorder nor previous numbers of episodes).
1.3.1 Types of withdrawal symptoms
The most commonly reported DESS symptoms on the DESS scale (by 10% or more participants) in the discontinuation group were irritability, trouble sleeping and/or insomnia, nervousness, anxiety, sudden worsening of mood, sudden outbursts of anger, bouts of crying or tearfulness, agitation, feeling unreal or detached, confusion or trouble concentrating, forgetfulness or problems with memory, mood swings, sweating more than usual, muscle tension or stiffness, muscle aches or pain, restless feelings in legs, muscle cramps, nose running, nausea, stomach bloating, diarrhoea, increased dreaming, fatigue or tiredness, and dizziness.
This study reported the proportions of people experiencing each DESS symptom as mild, moderate, or severe. Study authors reported that most DESS symptoms were rated mild or moderate in severity. The DESS items most commonly reported to be severe were trouble sleeping/insomnia (4.1% of participants in the discontinuation group, and 4.2% in the antidepressant group) and nervousness/anxiety (4.1% in the discontinuation group, and 1.4% in the antidepressant group). This study did not provide detailed information on differences in severity between groups.
1.4 Adverse events
See Analysis 1.2.
1.2. Analysis.

Comparison 1: Abrupt discontinuation versus continuation of antidepressants, Outcome 2: Adverse events
Ten studies contributed data on adverse events (Gelenberg 2003; Gilaberte 2001; Khan 2014; Klysner 2002; Kornstein 2006; Rapaport 2001; Rouillon 2000; Streim 2012; Terra 1998; Wilson 2003). It is important to note that adverse events may include withdrawal symptoms, as these studies did not distinguish between adverse events and withdrawal symptoms.
1.4.1 Pooled studies
Seven studies measured adverse events and were considered suitable for pooling (Gilaberte 2001; Khan 2014; Klysner 2002; Kornstein 2006; Rapaport 2001; Rouillon 2000; Streim 2012).
One study reported the incidence of adverse events (Gilaberte 2001), another study treatment‐emergent adverse events (Rapaport 2001), and another study measurements of taper/post‐therapy‐emergent adverse events (Khan 2014). In all, three studies reported the incidence of serious adverse events (Klysner 2002; Kornstein 2006; Rouillon 2000). Streim 2012 reported the incidence of adverse events (excluding serious adverse events).
A total of 554 (54.7%) participants were included in the discontinuation group, and 458 (45.3%) in the continuation group. In the pooled discontinuation group, more (152) adverse events were reported (including withdrawal symptoms) compared to 98 in the continuation group, but there were no differences in adverse events between discontinuation and continuation of antidepressants (odds ratio (OR) 1.11, 95% CI 0.62 to 1.99; 1012 participants, 7 studies; I² = 37%).
Streim 2012 assessed also the numbers of participants experiencing one or more serious adverse events in the discontinuation group (4/13; 30.7%) and in the continuation group (8/23; 34.8%) but provided no data on differences between groups.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for risk of bias (poor description of randomisation and blinding), high risk of attrition bias, and withdrawal confounding bias (withdrawal symptoms may be misdiagnosed as adverse events), by one level for imprecision (wide 95% confidence interval, which includes the null effect of no difference), and by one level for indirectness (three studies reported only serious adverse events).
1.4.2 Studies with other outcome measures
We could not pool data from three studies because they used different outcomes (Gelenberg 2003; Terra 1998; Wilson 2003). These three studies reported data for adverse events that were serious enough to cause discontinuation, precluding their inclusion in the above analysis.
In one study (n = 160), 4 of 84 (4.8%) participants in the discontinuation group (with or without CBT) and 4 of 76 (5.3%) participants in the continuation group (with or without CBASP) experienced adverse events serious enough to discontinue the trial, but data on differences between groups were not reported (Gelenberg 2003).
Wilson 2003 (n = 113) reported that 2 of 57 (3.5%) participants in remission of a first depression in the discontinuation group experienced adverse events that necessitated discontinuation compared to 2 of 56 (3.6%) participants treated with antidepressants. Study authors did not report data on differences between groups.
Terra 1998 (n = 204) reported that two participants in the antidepressant group (n = 110) withdrew during the trial due to adverse events, along with zero participants in the discontinuation group (n = 94).
1.4.3 Types of adverse events
The most common adverse events (≥ 10%) among antidepressant discontinuers included headache, asthenia, malaise, depression, pain, abdominal pain, flu‐like symptoms, infection, back pain, dyspepsia, insomnia, upper airway infection, anxiety, libido decreased, nausea, dysmenorrhoea (only in women), psychiatric disorder, dizziness, and malaise.
The most common adverse events amongst participants who continued their antidepressant included headache, asthenia, pain, flu‐like symptoms, infection, back pain, nausea, traumatic injury, dyspepsia, somnolence and insomnia, upper airway infection, anxiety, decreased libido, nausea, abdominal pain, weight loss, psychiatric disorder, dizziness, and fatigue.
1.5 Depressive symptoms
See Analysis 1.3.
1.3. Analysis.

Comparison 1: Abrupt discontinuation versus continuation of antidepressants, Outcome 3: Depressive symptoms
Seven studies contributed data on depressive symptoms, but investigators used different outcome scales (Gilaberte 2001; Gelenberg 2003; Khan 2014; Kornstein 2006; Peterson 2010; Streim 2012; Terra 1998).
1.5.1 Pooled studies
Three studies used HAM‐D to assess depressive symptoms and were considered suitable for pooling (Gilaberte 2001; Kornstein 2006; Peterson 2010). One study measured mean HAM‐D total score at endpoint (Gilaberte 2001), and two studies used HAM‐D mean change score at end of treatment compared to baseline (Kornstein 2006; Peterson 2010). The HAM‐D scale contains domains for depressed mood, insomnia, agitation, gastrointestinal somatic symptoms, anxiety, general somatic symptoms, and sexual dysfunction, all of which are symptoms of withdrawal. None of the studies measured withdrawal symptoms separately or made adjustments for withdrawal symptoms.
A total of 162 (49%) participants were included in the pooled discontinuation group, and 168 (51%) participants in the pooled antidepressant group. No difference in the severity of depressive symptoms was measured by the HAM‐D scale between participants in the discontinuation group and those in the continuation group (mean difference (MD) 0.44, 95% CI ‐1.12 to 2.00; 330 participants, 3 studies; I² = 85%; random‐effects model).
Two studies seemed to contribute to statistical heterogeneity (Gilaberte 2001; Peterson 2010). Removing these two studies from the analyses reduced statistical heterogeneity to 0%. This did not change the direction of effect but slightly increased the effect size (MD 0.64, 95% CI ‐0.27 to 1.54), favouring continuation of long‐term antidepressant.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for risk of bias (poor description of randomisation and blinding, attrition bias, and withdrawal confounding bias), by one level for imprecision (wide 95% confidence interval, which includes the null effect of no differences between treatments), and by one level for indirectness (participants with recurrent disorder). We did not downgrade for inconsistency as heterogeneity has been explained.
1.5.2 Studies that could not be pooled
Gelenberg 2003 (n = 160) used the 24‐item HAM D (HAM D‐24) at 52 weeks but reported means according to recurrence/no recurrence status within the discontinuation group (n = 84) and the antidepressant group (n = 76).
1.5.3 Depressive symptoms measured with other scales
We could not pool data from three studies because they used different outcome measures.
Terra 1998 used mean MADRS total score at endpoint (52 weeks) of the study to assess depressive symptoms. Mean MADRS score was 12.5 (standard deviation (SD) 11.5) in the discontinuation group (n = 94) and 7.8 (SD 9.7) in the continuation group (n = 109). Study authors concluded that fluvoxamine continuation was more effective than discontinuation (P < 0.001).
Streim 2012 measured depressive symptoms by using the GDS for people in remission of a single episode of late‐life depression. Mean GDS score at endpoint was 5.03 (SD 1.15) in the discontinuation group (n = 13) and 5.11 (SD 0.86) in the continuation group (n = 23). Study authors concluded that there were no differences in GDS score. Their conclusion was not supported by data on differences.
Khan 2014 reported mean (self‐rated) Quick Inventory of Depressive Symptomatology Self‐Report 16 total scores (QIDS‐SR16) at the end of the trial: 6.5 (SD 4.7) in the abrupt discontinuation group (n = 146) and 6.7 (SD 4.8) in the continuation group (n = 36) (LCOF analysis). Study authors concluded that there were no clinically significant changes in QIDS‐SR16 scores. Their conclusion was not supported by data on differences.
1.6 Anxiety symptoms
Three studies contributed data on anxiety symptoms (Peterson 2010; Rapaport 2001; Terra 1998).
We could not pool data because they were clinically too heterogeneous (different outcome measures, different outcome scales). Only one study (n = 183) investigated anxiety symptoms by using HAM‐A, our prioritised outcome measure (Rapaport 2001). Mean change from baseline to 28 weeks on the HAM‐A Scale for participants with panic disorder was 3.95 (SD 7.35) in the discontinuation group (n = 89) and 1.65 (SD 7.01) in the antidepressant group (n = 92). Study authors reported no differences between groups (P = 0.154; P value obtained from (ANCOVA) model, with treatment, site, and baseline values as effects). It is important to note that HAM‐A contains domain symptoms of psychic anxiety and somatic anxiety, all of which are symptoms of withdrawal. This study did not measure withdrawal symptoms separately nor make adjustments based on these.
1.6.1 Anxiety symptoms measured with other scales
Two other studies measured anxiety by using different scales.
One study with two different treatment arms (discontinuation versus continuation in participants with (n = 22) and without (n = 30) CBT) measured anxiety symptoms by using mean change and effect size (Cohen's d) for the self‐rated mean Beck Anxiety Inventory (BAI) score and the Symptom Questionnaire (SQ) anxiety score (Peterson 2010). Study authors concluded that there were no significant differences in change scores on BAI nor on SQ Anxiety between groups (BAI ‐0.31 (SD 0.15) discontinuation (n = 16) and 0.4 (SD 0.64) antidepressant (n = 14) in treatment arms without CBT, and 0.66 (SD 2.04) discontinuation (n = 11) and ‐0.34 (SD 0.65) antidepressant (n = 11) in treatment arms with CBT; and SQ Anxiety 1.53 (SD 1.10) discontinuation (n = 16) and 0.02 (SD 0.07) antidepressant (n = 14) in treatment arms without CBT and 2.05 (SD 1.32) discontinuation (n = 11) and 0.79 (SD 0.47) antidepressant (n = 11) in treatment arms with CBT).
Terra 1998 (n = 204) used mean COVI Anxiety Scale total score (with LOCF) to assess anxiety in people with recurrent major depression. Participants in the discontinuation group (n = 94) had a higher endpoint score (5.8 (SD 3.0)) on the COVI Anxiety Scale than participants in the continuation group (n = 109) (4.7 (SD 2.4); P = 0.003), favouring continuation of long‐term antidepressants. A weakness of the COVI Anxiety Scale is that anxiety and other symptoms of withdrawal may register on the COVI Anxiety Scale.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for imprecision (no pooling and small number of participants), by one level for risk of bias (confounding withdrawal bias, poor description of randomisation and blinding), and by one level for indirectness (only one of the three studies included participants with anxiety disorder (panic disorder)).
1.7 Quality of life
No study measured quality of life.
1.8 Social and occupational functioning
No study measured social and occupational functioning.
1.9 Severity of illness
See Analysis 1.4.
1.4. Analysis.

Comparison 1: Abrupt discontinuation versus continuation of antidepressants, Outcome 4: Severity of illness
Five studies reported severity of illness as an outcome (Gilaberte 2001; Kornstein 2006; Peterson 2010; Rapaport 2001; Terra 1998). Withdrawal symptoms including psychological symptoms may impair the severity of illness. None of the studies measured withdrawal symptoms nor made adjustments based on withdrawal symptoms.
1.9.1 Pooled studies
Two studies reported the CGI‐S total score (Gilaberte 2001; Kornstein 2006), and three studies reported mean CGI‐S change (Peterson 2010; Rapaport 2001; Terra 1998). CGI‐S is a clinician‐rated scale that measures illness severity from 1 to 7. The six studies were considered suitable for pooling.
A total of 345 (48.3%) participants were included in the pooled discontinuation group and 369 (51.7%) in the pooled antidepressant group. Participants in the discontinuation group had higher severity score rated on the CGI‐S compared to those in the continuation group (MD 0.31, 95% CI 0.13 to 0.49), favouring antidepressant continuation (714 participants, 5 studies; I² = 65%; random‐effects model).
Two studies caused statistical heterogeneity (Kornstein 2006; Peterson 2010); removing them from the analyses reduced statistical heterogeneity to 0%. This did not change the direction of the effect but reduced its magnitude (MD 0.44, 95% CI 0.30 to 0.57).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, attrition bias, and withdrawal confounding bias; CGI‐S rating of the clinician may be influenced by withdrawal symptoms, which include psychological symptoms and may worsen participants' condition) and by one level for indirectness (nearly all studies included participants with recurrent depression, and one study panic disorder).
1.10 Suicide, suicide attempt, or suicidal ideation
We found four studies reporting on suicide or suicide attempt. We could not pool data due to heterogeneity of outcome measures. We therefore summarised evidence in a narrative synthesis.
Terra 1998 (n = 204) reported suicide score on the MADRS. Participants in discontinuation groups reported significantly higher scores (0.6 (SD 1) discontinuation (n = 109) versus 0.3 (SD 0.8) antidepressant (n = 94)) on the MADRS suicide scale (P = 0.009).
Khan 2014 (n = 184) reported that one participant (1/148; number in control group split) in the abrupt discontinuation group reported suicidal ideation on the Columbia Suicide Severity Rating Scale during the discontinuation phase. The trial reported no suicidal behaviours.
Kornstein 2006 (n = 139) reported one suicide attempt one month after the last reported dose of blinded placebo in a participant with a history of suicide attempt in the discontinuation group (n = 66), and this was assessed by the investigator as 'possibly related' to study medication. However, "possibly related" may be misleading, as suicidal ideas and attempts were well recognised as withdrawal effects (Valuck 2009). No suicide attempts were reported in the antidepressant group (n = 73).
Rouillon 2000 (n = 214) reported two participants with two suicide attempts in each group.
Comparison 2. Discontinuation by "tapering" versus continuation of long‐term antidepressants (without psychological support or with psychological support as co‐intervention)
Eighteen studies (n = 2442) compared discontinuation by "tapering" to continuation of antidepressant (Bialos 1982; Bockting 2018; Cook 1986; Derubeis 2019; Huijbers 2016; Keller 1998; Keller 2007; Khan 2014; Kocsis 1996; Kocsis 2007; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Montgomery 2004; Perahia 2009; Rickels 2010; Segal 2010; Stewart 1997). Four of the 18 studies included psychological support as co‐intervention (PCT in Bockting 2018 MBCT in Huijbers 2016 CBT before randomisation in Derubeis 2019, and IPT in Kupfer 1992).
Fifteen studies included antidepressants for depressive disorders, and three studies antidepressants for anxiety disorders (Mavissakalian 1999; Mavissakalian 2001; Rickels 2010).
Included studies compared discontinuation by "tapering" with continuation of antidepressant treatment: seven studies used TCA (Bialos 1982; Cook 1986; Kocsis 1996; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Stewart 1997), one study SSRI (Keller 1998), six studies SNRI (Keller 2007; Khan 2014; Kocsis 2007; Montgomery 2004; Perahia 2009; Rickels 2010), one study MAO inhibitor (Stewart 1997), and four studies a mix of different types of antidepressants (Bockting 2018; Derubeis 2019; Segal 2010; Huijbers 2016). Duration of "tapering" was one week or longer. Most studies tapered over four weeks or less.
See characteristics of studies with discontinuation by "tapering" in Table 8.
4. Studies with tapering, included in comparison 2.
| Study ID | Study design | Duration of AD treatment before randomisation | Inclusion criteria age | Main disorder | Duration of trial | Number | Intervention 1 | Duration of tapering | Intervention 2 | Co‐intervention |
| Bialos 1982 | RCT | 3.7 years (0.5 to 8) | NR | history of MDD | 24 weeks | 19 | taper to placebo | 3 weeks | continuation ‐ amitriptyline | |
| Bockting 2018 | RCT | ≥ 24 weeks | NR | in remission of MDD > 2 months and ≤ 2 years for ≥ 2 previous depressive episodes in the past 5 years | 104 weeks | 146 (intervention group split to allow multiple‐arm comparisons) | taper to "no placebo" | 4 weeks or longer (and within 24 weeks) | continuation ‐ different classes | PCT |
| Cook 1986 | RCT | 12 to 192 months | NR | MDD and treated with a TCA for a year without evidence of reoccurrence of depressive symptoms warranting a change in therapy | 28 weeks | 18 | taper to placebo | 4 or 8 weeks | continuation ‐ TCA | |
| Derubeis 2019 | 2‐phase RCT | mean 80.3 (40) weeks | ≥ 18 years | MDD either chronic (episode duration ≥ 2 years) or recurrent (with an episode in the past 3 years if only the second episode) | 156 weeks | 137 | taper to "no placebo" | 4 weeks or longer if clinically indicated (not specified) | continuation ‐ different classes | |
| Derubeis 2019 | 2‐phase RCT | mean 80.3 (40) weeks | ≥ 18 years | MDD either chronic (episode duration ≥ 2 years) or recurrent (with an episode in the past 3 years if only the second episode) | 156 weeks | 155 | taper to "no placebo" | 4 weeks or longer if clinically indicated (not specified) | continuation ‐ different classes | CBT prerandomisation |
| Huijbers 2016 | RCT | ≥ 24 weeks | ≥ 18 years | history of ≥ 3 depressive episodes and in full or partial remission | 65 weeks | 249 | taper to "no placebo" | 5 weeks | continuation ‐ different classes | MBCT |
| Keller 1998 | 3‐phase RCT | 28 weeks | NR | chronic MDD of 2 years' duration or dysthymic disorder with concurrent diagnosis of MDD (double depression) | 76 weeks | 161 | taper to placebo | 4 weeks | continuation ‐ sertraline | |
| Keller 2007 | 4‐phase RCT | 86 weeks | ≥ 18 years | recurrent depression: history of ≥ 3 episodes of major depression | 52 weeks | 83 | taper to placebo | 4 weeks | continuation ‐ venlafaxine ER | |
| Khan 2014 | 2‐phase RCT | 24 weeks | ≥ 18 years | single or recurrent MDD | 4 weeks | 176 (control group split to allow multiple‐arm comparisons) | taper to placebo | 1 week | continuation ‐ desvenlafaxine | |
| Kocsis 1996 | 2‐phase RCT | 26 to 28 weeks | NR | pure dysthymia, double depression, chronic major depression | 104 weeks | 53 | tapering to placebo | 4 weeks | continuation ‐ desipramine | |
| Kocsis 2007 | 3‐phase RCT | 34 weeks | ≥18 years | recurrent depression (DSM‐IV criteria): history of ≥ 3 episodes of major depression | 52 weeks | 336 | tapering to placebo | 4 weeks | continuation ‐ venlafaxine | |
| Kupfer 1992 | RCT | 3 years | 21 to 65 years | ≥ 3 episodes unipolar depression | 104 weeks | 20 | tapering to placebo | 3 weeks | continuation ‐ imipramine | IPT |
| Mavissakalian 1999 | 2‐phase RCT | 24 weeks | NR | panic disorder with agoraphobia | 52 weeks | 56 | tapering to placebo | 3 weeks | continuation ‐ imipramine | |
| Mavissakalian 2001 | 3‐phase RCT | 76 weeks | NR | panic disorder with agoraphobia | 52 weeks | 11 | tapering to placebo | 3 weeks | continuation ‐ imipramine | |
| Montgomery 2004 | 3‐phase RCT | 24 weeks | ≥ 18 years | recurrent major depression (≥ 1 previous episode in the last 5 years) | 2 weeks | 235 | tapering to placebo | 2 weeks | continuation ‐ venlafaxine IR | |
| Perahia 2009 | 3‐phase RCT | 28 to 34 weeks | ≥ 18 years | recurrent major depression (≥ 3 episodes of depressive disorder) | 52 weeks | 288 | tapering to placebo | 4 weeks | continuation ‐ duloxetine | |
| Rickels 2010 | 2‐phase RCT | 24 weeks | ≥ 18 years | generalised anxiety disorder | 24 weeks | 136 | tapering to placebo | 4 weeks | continuation ‐ venlafaxine | |
| Rickels 2010 | 3‐phase RCT | 48 weeks | ≥ 18 years | generalised anxiety disorder | 24 weeks | 59 | tapering to placebo | 4 weeks | continuation ‐ venlafaxine | |
| Segal 2010; | 3‐phase RCT | 28 weeks | between 18 and 65 years | MDD in remission and ≥ 2 previous episodes | 76 weeks | 44 (control group split to allow multiple‐arm comparisons) | tapering to placebo | 4 weeks | continuation ‐ different types | |
| Stewart 1997 | 3‐phase RCT | 30 weeks | NR | MDD, dysthymia, or both ≥ 2 years and definite or probable atypical depression | 24 weeks | 32 | tapering to placebo | 2 weeks | continuation ‐ imipramine | |
| Stewart 1997 | 3‐phase RCT | 30 weeks | NR | MDD, dysthymia, or both ≥ 2 years and definite or probable atypical depression | 24 weeks | 28 | tapering to placebo | 2 weeks | continuation ‐ phenelzine |
AD: antidepressant.
CBT: cognitive‐behavioural therapy.
IPT: interpersonal therapy.
NR: not reported.
MBCT: mindfulness‐based cognitive therapy.
MDD: major depressive disorder.
PCT: preventive cognitive therapy.
RCT: randomised controlled trial.
2.1 Successful discontinuation rate
2.1.1 All studies
Two studies with psychological support as co‐intervention reported antidepressant discontinuation as an outcome (Bockting 2018; Huijbers 2016). Neither of the studies assessed withdrawal symptoms; therefore withdrawal symptoms could be misclassified, and this may result in a restart of antidepressants due to intolerable withdrawal symptoms.
We could not pool data because data were insufficient.
Huijbers 2016 (n = 249) assessed adherence to the allocated antidepressant (discontinuation of antidepressant or continuation of antidepressant) regimen with MBCT in both treatment groups (Huijbers 2016). Participants in the discontinuation group were recommended by psychiatrists to withdraw their antidepressant gradually over a period of five weeks, starting after the seventh session of MBCT.
In the discontinuation group (n = 128), 68 of 128 (53%) discontinued antidepressant within six months after baseline and 2 of 128 (2%) additional participants discontinued after six months compared to 14 of 121 (12%) in the continuation group who discontinued antidepressant during the 15‐month follow‐up. Study authors reported a significant difference in discontinuation, favouring the discontinuation group (Van Leeuwen 2020c [pers comm]): 17 of 128 (13%) in the discontinuation group reduced the dose of antidepressant and 17 of 121 (14%) in the antidepressant group. Study authors reported no difference in adherence to the allocated intervention (chi² = 3.30; P = 0.07).
Bockting 2018 (n = 146) assessed successful tapering among participants with recurrent depression with PCT in both treatment groups. This study reported that 17 of 42 (40%) participants in the discontinuation group discontinued the antidepressant after six months and 8 of 42 participants (19%) were able to reduce the dose by a minimum of 50%. This study did not report the antidepressant discontinuation rate for the control group. We contacted the study author to request information; these authors responded to our mail, but we have not yet received the data.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for imprecision (no meta‐analysis), by one level for indirectness (studies included participants with recurrent depression in remission), and by one level for risk of bias (incomplete outcome reporting, withdrawal confounding bias). We did not downgrade for risk of performance bias because the outcome is unlikely to be influenced by lack of blinding (objective outcome).
2.1.2 Studies without psychological support as co‐intervention
No studies reported a successful discontinuation rate.
2.1.3 Studies with psychological support as co‐intervention
See Section 2.1.2.
2.2 Relapse rate (as defined by study authors)
See Analysis 2.1.
2.1. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 1: Relapse (as defined by study authors)
Seventeen studies reported relapse rate as an outcome (Bialos 1982; Bockting 2018; Cook 1986; Derubeis 2019; Huijbers 2016; Keller 1998; Keller 2007; Kocsis 1996; Kocsis 2007; Kupfer 1992; Mavissakalian 1999; Mavissakalian 2001; Montgomery 2004; Perahia 2009; Rickels 2010; Segal 2010; Stewart 1997). All but one ‐ Cook 1986 ‐ reported time to relapse by using hazard ratios. None of these studies measured withdrawal symptoms separately nor made adjustments for withdrawal.
2.2.1 All studies
2.2.1.1 Pooled studies
Sixteen studies with and without co‐intervention were pooled together. In all, 1025 (48.3%) participants were included in the discontinuation group and 1095 (51.7%) participants in the continuation group.
Participants discontinuing antidepressants had higher risk of relapse (as defined by study authors) compared to those in the continuation group (Hazard Ratio 2.59, 95% CI 2.07 to 3.25; participants = 2120; studies = 16) without specific assessment of withdrawal symptoms. We used the most strict definition for relapse if more than one definition for relapse was provided.
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, withdrawal confounding bias) and by one level for indirectness (three studies included participants with panic disorder; the other studies included recurrent depression or chronic depression). We did not downgrade for attrition bias, as censoring for survival data is unlikely to introduce bias. Because we identified more than 10 RCTs, we generated funnel plots to test for reporting bias. These funnel plots showed low risk of publication bias.
2.2.1.2 Studies that could not be pooled
Segal 2010 reported relapse (as defined by study authors) survival data for stable and unstable remitters but did not report data for the discontinuation group compared to the continuation group. For stable remitters (all HAM‐D scores ≥ 7 during remission), there were no differences in relapse of absence of withdrawal symptoms measured after antidepressants were tapered (relapse rates: 50% discontinuation group, 59% antidepressant group; HR not reported; P = 0.49). Among unstable remitters (one or more with HAM‐D score ≥ 7 during remission), participants in the discontinuation group showed higher risk of relapse compared with those in the continuation group (relapse rates: discontinuation group 71%, 27% antidepressant; HR 4.16, 95% CI 1.12 to 14.3; P = 0.03).
2.2.2 Studies with no co‐intervention
2.2.2.1 Pooled studies
We considered 13 studies without psychological support as co‐intervention suitable for pooling (Bialos 1982; Cook 1986; Derubeis 2019; Keller 1998; Keller 2007; Kocsis 1996; Kocsis 2007; Mavissakalian 1999; Mavissakalian 2001; Montgomery 2004; Perahia 2009; Rickels 2010; Stewart 1997).
Participants discontinuing antidepressants (n = 776) had higher risk of relapse (as defined by study authors) compared to those in the continuation group (n = 770) (Hazard Ratio 2.97, 95% CI 2.24 to 3.93; participants = 1546; studies = 13) without specific assessment of withdrawal symptoms.
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, withdrawal confounding bias) and by one level for indirectness (3 studies included participants with panic disorder; the other studies included participants with recurrent depression or chronic depression). We did not downgrade for attrition bias, as censoring for survival data is unlikely to introduce bias. Because we identified more than 10 RCTs, we generated funnel plots to test for reporting bias. These funnel plot showed low risk of publication bias.
2.2.2.2 Studies that could not be pooled
Segal 2010 could not be pooled; see Section 2.2.1.
2.2.3 Studies with co‐intervention
In 4 of the 17 studies, participants received a psychological co‐intervention (Bockting 2018; Derubeis 2019; Huijbers 2016; Kupfer 1992). We considered these four studies with psychological co‐intervention suitable for pooling. We found higher risk of relapse (as defined by study authors) in the discontinuation group compared to continuation of antidepressant with psychological treatment as co‐intervention (HR 1.90, 95% CI 1.42 to 2.53; 570 participants, 4 studies). None of these studies assessed withdrawal symptoms.
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (performance bias due to no placebo in the discontinuation group, detection bias due to lack of blinding of assessors in the study of greatest weight in the analysis, and risk of withdrawal confounding bias) and by one level for indirectness (studies included participants with recurrent depression). We did not downgrade for attrition bias, as censoring for survival data is unlikely to introduce bias.
2.3 Withdrawal symptoms
See Analysis 2.2.
2.2. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 2: Adverse events
Two studies reported on withdrawal symptoms (Bialos 1982; Khan 2014). We could not pool these studies due to different outcome measures and insufficient data.
Khan 2014 compared venlafaxine tapering (n = 139) over one week to venlafaxine continuation (n = 37; control group split to allow comparison of multiple arms) over four weeks. Study authors reported the proportion of participants with withdrawal syndrome based on DESS. Experiencing a withdrawal syndrome was defined as an increase in DESS score of 4 or more (regardless of the severity of symptoms) during the first two weeks of the double‐blind phase. Data show no differences in the proportions of participants with withdrawal syndrome based on DESS scores between the discontinuation group (30/139; 21.6%) and the continuation group (4/36; 11.1%) (P ≥ 0.06).
We assessed the certainty of evidence for this outcome as very low, downgraded by one level due to imprecision (single study with small number of participants), by one level for risk of bias (poor description of randomisation and blinding; severity of withdrawal symptoms was not scored for the outcome and withdrawal symptoms were monitored for a relatively short period) and by one level for indirectness (study included participants with single or recurrent depressive disorder but did not report numbers of participants with single and recurrent disorders).
2.3.1 Types of withdrawal symptoms
The most commonly reported symptoms on the DESS scale (by ≥ 10% of participants) in the discontinuation group were irritability, trouble sleeping and/or insomnia, nervousness, anxiety, sudden worsening of mood, sudden outbursts of anger, bouts of crying or tearfulness, agitation, feeling unreal or detached, confusion or trouble concentrating, forgetfulness or problems with memory, mood swings, sweating more than usual, muscle tension or stiffness, unsteady gait or inco‐ordination, muscle aches or pains, restless feelings in legs, muscle cramps, nose running, nausea, increased dreaming, fatigue or tiredness, and dizziness (Khan 2014).
This study reported the proportions of people experiencing each DESS symptom at mild, moderate, and severe levels. Study authors reported that most DESS symptoms were rated as mild or moderate in severity. However, we calculated that the sum of participants who rated a withdrawal symptom as "severe" was lower in the discontinuation group (17.6%) than in the continuation group (42%). The DESS item most commonly reported as severe was trouble sleeping/insomnia (2.2% of participants in the discontinuation group and 4.2% in the continuation group). This study did not provide detailed information on differences in severity between groups.
2.3.2 Other studies measuring withdrawal symptoms
Bialos 1982 reported that 8 of 10 (80%) participants in the discontinuation group experienced a mild withdrawal syndrome within the first two weeks consisting of "irritability, dream and sleep disturbance, and restless[ness] but not accompanied by psychological craving for the drug". Supporting data were not provided.
One study measured potential withdrawal symptoms as adverse events by using a patient‐completed withdrawal checklist but did not report data on withdrawal symptoms (Rickels 2010).
2.4 Adverse events
See Analysis 2.3.
2.3. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 3: Depressive symptoms
Ten studies contributed data on serious adverse events and/or adverse events (Bockting 2018; Cook 1986; Derubeis 2019; Keller 1998; Keller 2007; Khan 2014; Kocsis 2007; Montgomery 2004; Perahia 2009; Rickels 2010). One study reported only the insomnia factor and the autonomic anticholinergic factor of the Treatment‐Emergent Symptoms Scale (Bialos 1982). These studies did not distinguish between adverse events and withdrawal symptoms.
2.4.1 Pooled studies
We could pool the findings of seven studies (Derubeis 2019; Keller 1998; Keller 2007; Khan 2014; Kocsis 2007; Montgomery 2004; Perahia 2009).
Three studies reported the number of participants with at least one or more (treatment‐emergent) adverse events (Keller 1998; Montgomery 2004; Perahia 2009), one study reported measurements of taper/post‐therapy‐emergent adverse events (Khan 2014), two studies reported the number of participants with one or more serious adverse events (some participants experienced one or more events but did not report the number of serious adverse events) (Kocsis 2007; Keller 2007), and one study reported the number of serious adverse events (Derubeis 2019).
A total of 786 (53.1%) participants were included in the pooled discontinuation group and 693 (46.9%) in the pooled antidepressant group. The pooled discontinuation group showed 337 (42.9%) adverse events (including withdrawal symptoms) compared to 290 (41.8%) in the pooled continuation group. The difference between discontinuation and continuation groups was not significant (odds ratio (OR) 1.06, 95% CI 0.82 to 1.38; 1479 participants, 7 studies; I² = 0%).
Three of the pooled studies measured only serious adverse events (Derubeis 2019; Keller 2007; Kocsis 2007). Sensitivity analysis excluding these three studies did not substantially alter the meta‐analysis result (OR 1.00, 95% CI 0.73 to 1.37). In one study, the antidepressant was not replaced by placebo (Derubeis 2019). When this study was removed from the meta‐analysis, the overall results did not change (OR 1.04, 95% CI 0.77 to 1.41).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, high risk of attrition bias, confounding withdrawal bias; withdrawal symptoms may be misdiagnosed as adverse events) and by one level for indirectness (three studies reported only serious adverse events).
2.4.2 Types of adverse events
The most common adverse events (≥ 10%) in the discontinuation group included headache, insomnia, dizziness, fatigue, dyspepsia, pain, asthenia, diarrhoea, upper respiratory infection, nausea, dizziness, vasodilatation, dry mouth, insomnia, sweating, nausea, nervousness, and paraesthesia.
The most common adverse events (≥ 10%) in the antidepressant group included headache, insomnia, sexual dysfunction, sweating increased, weight gain, diarrhoea, dry mouth, nausea, dizziness, fatigue, dyspepsia, upper respiratory infection, dizziness, accidental injury, abnormal ejaculation/orgasm, abnormal dreams, asthenia, libido decreased, accidental injury, lightheadedness, flu syndrome, infection, asthenia, and rhinitis.
2.4.3 Studies that could not be pooled
We could not pool data from three studies due to insufficient data and use of different outcome measures.
Rickels 2010 reported no differences in measurement of adverse events between antidepressant and discontinuation groups. There were no new adverse events compared to the treatment period before randomisation. No data were provided to support this conclusion. Study authors reported adverse events data for events serious enough for participants to drop out of the study, but these data were not reported for each treatment group.
Cook 1986 (n = 18) reported no differences in adverse events between discontinuation and antidepressant groups. No data were provided to support this author conclusion.
Bockting 2018 (n = 146) recorded serious adverse events and reported one suicide attempt in the tapering group; one participant in the continuation with PCT group died. Study authors reported no indication that these were related to the intervention. No detailed information on other adverse events was reported.
2.4.4 Adverse events measured on other scales
One study reported only the insomnia factor and the autonomic anticholinergic factor of the Treatment‐Emergent Symptoms Scale (for the tapering group, not for the control group) (Bialos 1982). Study authors did not report data on differences between groups and did not offer any conclusions.
2.5 Depressive symptoms
See Analysis 2.3.
Twelve studies reported on depressive symptoms (Bialos 1982; Cook 1986; Keller 1998; Keller 2007; Khan 2014; Kocsis 2007; Mavissakalian 1999; Mavissakalian 2001; Montgomery 2004; Perahia 2009; Segal 2010; Stewart 1997). Low mood is a common withdrawal symptom, but none of these studies measured withdrawal symptoms nor made adjustments for withdrawal symptoms.
2.5.1 Pooled studies
Six studies reported depressive symptoms as measured by our prioritised outcome measure ‐ HAM‐D (Cook 1986; Keller 1998; Keller 2007; Kocsis 2007; Montgomery 2004; Perahia 2009). Five studies reported HAM‐D endpoint data (Cook 1986; Keller 1998; Keller 2007; Kocsis 2007; Montgomery 2004). Two of the five studies reported only HAM‐D values expressed as least squares means (adjustments for study site, depression type, probability of recurrence, and maintenance baseline value) with standard error (Keller 2007; Kocsis 2007). One study reported least squares means (analysis of variance model with investigator, treatment, and baseline) for HAM‐D change with standard errors (Perahia 2009).
We considered the six studies suitable for pooling.
In all, 511 (50.2%) participants were included in the pooled discontinuation group and 506 (49.8%) in the pooled continuation group. In the pooled discontinuation group, participants reported significantly higher scores on the HAM‐D scale compared to those in the antidepressant group (MD 3.50, 95% CI 2.31 to 4.68; 1017 participants, 6 studies; I² = 29%).
One study caused some statistical heterogeneity (Kocsis 2007); removing this study from the analysis reduced statistical heterogeneity to 0%. This did not change the direction of the effect but slightly increased the magnitude of the effect (MD 3.86, 95% CI 2.79 to 4.93).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and blinding, attrition bias, and confounding withdrawal bias; low mood as withdrawal symptom may be misdiagnosed as depressive symptoms with scores from the HAM‐D scale or other clinical scales) and by one level for indirectness (five of six studies included recurrent or chronic depression).
2.5.2 Studies that could not be pooled
We could not pool data from five studies due to insufficient data.
Segal 2010 measured relapse by using the HAM‐D scale but did not report data.
Bialos 1982 measured depressive symptoms by using the HAM‐D scale. Study authors found that the HAM‐D score was significantly higher at endpoint in comparison to the baseline value in the discontinuation group (n = 10) compared to the continuation group (n = 7); however no data were provided to support this conclusion made by the study authors.
Mavissakalian 1999 and Mavissakalian 2001 measured depressive symptoms by using HAM‐D scores of participants experiencing relapse but did not report data separately for each treatment group.
Stewart 1997 reported HAM‐D scores for remitted participants and for participants experiencing relapse but did not report data for each treatment.
2.5.3 Depressive symptoms measured on other scales
We could not pool data from one study due to use of different scales to assess depressive symptoms.
Khan 2014 reported depressive symptoms as measured by QIDS‐SR total scores (with LCOF) at endpoint. Mean endpoint scores at four weeks were 6.2 (SD 4.5) in the taper group (n = 139) and 6.7 (SD 4.8) in the continuation group (n = 36), with no differences between groups.
2.6 Anxiety symptoms
See Analysis 2.4.
2.4. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 4: Anxiety symptoms
Four studies contributed data on anxiety symptoms (Keller 2007; Kocsis 2007; Perahia 2009; Rickels 2010).
2.6.1 Pooled studies
Three studies provided data on the Hamilton Anxiety Scale (HAM‐A) (Keller 2007; Kocsis 2007; Rickels 2010). Two studies reported least squares means with SE (adjustments for study site, depression type, probability of recurrence, and maintenance baseline value) (Keller 2007; Kocsis 2007). One study measured mean HAM‐A for repeated continuous measures during the time of the study (Rickels 2010). HAM‐A scales contain symptoms from domains of psychic anxiety and somatic anxiety, all of which are symptoms of withdrawal. These studies did not measure withdrawal symptoms nor make adjustments for withdrawal symptoms.
A total of 257 (48.9%) participants were included in the pooled discontinuation group and 269 (51.1%) in the pooled continuation group.
Participants in the pooled discontinuation group (n = 257) showed higher scores on the HAM‐A scale compared to those in the pooled antidepressant group (n = 269) (MD 3.53, 95% CI 1.92 to 5.14; 526 participants, 3 studies; I² = 53%; higher score signifies more anxiety symptoms).
One study caused statistical heterogeneity (Kocsis 2007); removing this study from the analysis reduced statistical heterogeneity to 0%. This did not change the direction but increased the magnitude of the effect (MD 4.39, 95% CI 3.08 to 5.71).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of randomisation and binding, attrition bias, and confounding withdrawal bias) and by one level for indirectness (two studies included participants with generalised anxiety disorder; one study included recurrent depression).
2.6.2 Anxiety symptoms measured by other scales
We could not pool data from one study due to use of different measurement scales.
Perahia 2009 used the anxiety/somatisation subscale of the Hamilton Depression Scale to assess anxiety symptoms. The least squares (analysis of variance model with investigator, treatment, and baseline) HAM‐D 17 anxiety subscale mean change was an increase of 1.54 (SD 2.6) in the discontinuation group (n = 142) and a smaller increase of 0.46 (SD 2.4) in the continuation group (n = 145) (increase in mean change score signifies worsening). Study authors reported a significant difference between groups (P ≤ 0.001), favouring the continuation group.
2.7 Quality of life
See Analysis 2.5.
2.5. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 5: Quality of life
Seven studies reported on quality of life (Bockting 2018; Keller 1998; Keller 2007; Kocsis 2007; Huijbers 2016; Perahia 2009; Rickels 2010). Withdrawal symptoms may impair work, socialising, and emotional well‐being (social functioning and emotional role functioning) because of disturbing physical and emotional symptoms. These studies did not measure withdrawal symptoms nor make adjustments for withdrawal symptoms.
2.7.1 Pooled studies
Three studies reported quality of life by using the self‐rated SF‐36 and reported data on the three subscales (social functioning, role limitations due to emotional problems, role limitations due to physical health problems) (Keller 1998; Keller 2007; Kocsis 2007). Two studies reported the endpoint score for three subscales by using least squares mean scores (SE) (adjustments for study site, depression type, probability of recurrence, and maintenance baseline value) (Keller 2007; Kocsis 2007), and one study by using mean endpoint scores (Keller 1998).
A total of 253 participants were included in the pooled discontinuation group and 249 in the pooled continuation group.
There were no differences between groups on the subscale quality of life (QoL) physical health problems (MD ‐2.08, 95% CI ‐5.66 to 1.49; 502 participants, 3 studies; I² = 0%). Participants in the discontinuation group reported lower scores on the QoL social functioning subscale (MD ‐6.44, 95% CI ‐12.10 to ‐0.77; 502 participants, 3 studies; I² = 43%; a decrease in score indicates worsening, favouring continuation of antidepressants) and on the QoL emotional functioning subscale (MD ‐18.81, 95% CI ‐26.66 to ‐10.97; 502 participants, 3 studies; I² = 0%; lower scores indicate impairment of functioning, favouring continuation of antidepressant) compared to participants in the antidepressant group.
One study caused statistical heterogeneity in the social functioning subscale (Kocsis 2007); removing this study from the analysis reduced statistical heterogeneity to 0%. This did not change the direction of the effect but increased the magnitude of the effect (OR ‐9.80, 95% CI‐15.72 to ‐3.88).
We assessed the certainty of evidence for the three QoL subdomains as very low, downgraded by one level for risk of bias (poor description of blinding outcome assessors, attrition bias, and withdrawal confounding bias; withdrawal symptoms may impair QoL measures) and by one level for indirectness (studies included participants with recurrent depression or chronic/double depression) and imprecision (wide 95% CI).
2.7.2 Quality of life measured by other scales
We could not pool data from four other studies due to use of different outcome scales.
Perahia 2009 reported a difference in the SF‐36 mental component summary mean change score, favouring continuation of antidepressant: ‐5.74 (SE 1.2) in the discontinuation group (n = 142) and ‐1.11 (SE 1.11) in the continuation group (n = 146) (P = 0.002) and no difference in the SF‐36 physical component summary score: 0.33 (SE 0.76) in the discontinuation group and ‐0.45 (SE 0.7) in the continuation group (P = 0.415).
Huijbers 2016 reported no differences between groups on six scales (World Health Organization (WHO) Quality of Life overall perception, Q1 and Q2, and WHO Quality of Life emotional, physical, psychological, and environmental domains) after 15 months of follow‐up (see Table 6).
Rickels 2010 reported QoL as measured by the General Health Questionnaire (GHQ), but information was insufficient to permit interpretation of the results.
Bockting 2018 reported quality‐adjusted life‐years (QALYs) over 104 weeks. Mean QALYs over 24 months were 1.59 (range 0.64 to 1.94) for the discontinuation with PCT group (n = 42) and 1.62 (range 0.95 to 1.95) for the continuation and PCT group (n = 104). Study authors did not report the difference between the two groups.
2.8 Social and occupational functioning
See Analysis 2.6.
2.6. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 6: Social and occupational functioning
Seven studies reported on social and occupational functioning (Bialos 1982; Keller 1998; Keller 2007; Kocsis 1996; Kocsis 2007; Perahia 2009; Rickels 2010). Withdrawal symptoms may influence social and occupational functioning due to disturbing physical and emotional symptoms. The included studies did not measure withdrawal symptoms nor make adjustments for withdrawal symptoms.
2.8.1 Pooled studies
Three studies reported social functioning as measured by the Social Adjustments‐Self‐Report total score (SAS‐SR) (Keller 1998; Keller 2007; Kocsis 2007). Keller 2007 and Kocsis 2007 reported SAS‐SR by using least squares mean scores, and Keller 1998 reported mean scores (adjusted for study site, depression type, probability of recurrence, and maintenance baseline value).
In all, 253 (50.4%) participants were included in the pooled discontinuation group and 249 (49.6%) in the pooled antidepressant group.
In the pooled discontinuation group, participants had higher total scores on the SAS‐SR compared to participants in the pooled antidepressant group, favouring continuation of antidepressants (MD 0.19, 95% CI 0.11 to 0.28; 502 participants, 3 studies; I² = 0%; lower scores indicate less impairment).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of blinding of outcome assessors, attrition bias, and withdrawal confounding bias) and by one level for indirectness (studies included participants with recurrent or chronic/double depression).
2.8.2 Studies that could not be pooled
Bialos 1982 measured social functioning by using SAS‐SR total score but did not report data.
Kocsis 1996 reported measurements of SAS‐SR but did not report data at endpoint.
2.8.3 Social and occupational functioning as measured by other scales
We could not pool data from two studies using other scales to assess social and occupational functioning.
Perahia 2009 reported social functioning as least squares mean changes in global functioning as measured on the Sheehan Disability Scale. Participants in the discontinuation group (n = 142) reported a higher score for Sheehan Disability Scale global functioning compared to those in the antidepressant group (n = 145) (mean change (SE) 2.06 (0.77) in the discontinuation group and ‐0.05 (0.71) in the antidepressant group; P = 0.029; increase in score signifies worsening).
Rickels 2010 reported the adjusted Sheehan Disability Scale but provided insufficient information to permit interpretation of the data.
2.9 Severity of illness
See Analysis 2.7.
2.7. Analysis.

Comparison 2: Discontinuation by tapering versus continuation, Outcome 7: Severity of illness
Six studies contributed data for severity of illness (Keller 1998; Keller 2007; Kocsis 2007; Montgomery 2004; Perahia 2009; Rickels 2010). Withdrawal symptoms including psychological symptoms may impair the severity of illness. None of these studies measured withdrawal symptoms nor made adjustments based on withdrawal symptoms.
2.9.1 Pooled studies
The six studies reported severity of illness by measurements of Clinical Global Impressions‐Severity (CGI‐S), as rated by clinicians (Keller 1998; Keller 2007; Kocsis 2007; Montgomery 2004; Perahia 2009; Rickels 2010).
Keller 2007 and Kocsis 2007 reported least squares mean endpoint data but no raw data. Perahia 2009 reported least squares mean changes. One study reported PGI‐S mean scores for repeated continuous measures over the time of the trial (Rickels 2010).
A total of 590 (49.7%) participants were included in the pooled discontinuation group and 597 (50.3%) in the pooled antidepressant group.
In the pooled discontinuation group, participants had higher scores on CGI‐S compared to those in the pooled antidepressant group, favouring the antidepressant group (lower score indicates less impairment) (MD 0.61, 95% CI 0.44 to 0.79; 1187 participants, 6 studies; I² = 29%).
We assessed the certainty of evidence for this outcome as very low, downgraded by two levels for risk of bias (poor description of blinding of outcome assessors, attrition bias, and withdrawal confounding bias) and by one level for indirectness (studies included participants with recurrent or chronic/double depression).
2.10 Suicide, suicide attempt, or suicidal ideation
Four studies reported data on suicide. We could not pool these data due to heterogeneity of outcomes measures. We summarised evidence in a narrative synthesis.
Kocsis 2007 (n = 336) reported that one participant in the continuation group experienced suicidal thoughts; study authors considered this not related to treatment. However, "possibly related" may be misleading, as suicidal ideas and attempts were well recognised as withdrawal effects (Valuck 2009). Suicidal ideations were also reported by one participant in the discontinuation group. No suicide attempts during the trial were reported.
Keller 2007 (n = 83) reported that one (1/40) participant in the discontinuation group experienced suicidal ideation; study authors considered this possibly related to study treatment. No other suicide‐related events in the continuation group or in the discontinuation group were reported.
Perahia 2009 (n = 288) reported no suicide attempts.
Comparison 3. Discontinuation with high‐intensity psychological support versus continuation of long‐term antidepressants (or usual care)
Four studies (n = 730) compared discontinuation by tapering with psychological support to continuation of antidepressants (Bockting 2018; Kuyken 2008; Kuyken 2015; Segal 2010). All four studies included participants with recurrent depression in remission. Three studies used a mix of different types of antidepressants (Bockting 2018; Kuyken 2008; Segal 2010), and one study did not report the type of antidepressant (Kuyken 2015).
Psychological interventions included MBCT (Kuyken 2008; Kuyken 2015; Segal 2010), as well as PCT (Bockting 2018). The tapering scheme consisted of four weeks (Segal 2010); a taper regimen determined by physicians and participants (Kuyken 2008; Kuyken 2015); or four weeks or longer (left to clinicians’ discretion) (Bockting 2018), but mean duration was not reported.
See "Characteristics of studies with high‐intensity psychological intervention" (Table 9).
5. Studies with high‐intensity psychological interventions, included in comparison 3.
| Study ID | Study design | Duration of antidepressant treatment | Main disorder | Study duration | Total number randomised participants | Intervention 1 | Intervention 2 | Tapering scheme |
| Bockting 2018 | RCT | ≥ 24 weeks | Recurrent MDD (≥ 2 previous episodes in the last 5 years) in remission | 104 weeks | 143 (control group split to allow multiple‐arm comparisons) | 8‐weekly PCT sessions and tapering (no placebo) | Continuation | 4 weeks or longer and before 6 months (left to clinicians’ discretion) |
| Kuyken 2008 | RCT | ≥ 6 months | Recurrent MDD (≥ 3 previous episodes of depression) in full or partial remission | 64 weeks | 123 | 8‐weekly MBCT sessions and tapering (no placebo), with follow‐up sessions | Continuation | regimen determined by participants and GP |
| Kuyken 2015 | RCT | in "maintenance" treatment | Recurrent MDD (≥ 3 previous episodes) in full or partial remission | 104 weeks | 424 | 8‐weekly MBCT sessions and tapering (no placebo), with follow‐up sessions | Continuation | regimen determined by participants and GP |
| Segal 2010 | RCT | 28 weeks | Recurrent MDD (≥ 3 previous episodes) in remission | 76 weeks | 40 (control group split to allow multiple‐arm comparisons) | 8‐weekly MBCT sessions and tapering (no placebo), with follow‐up sessions | Continuation | 4 weeks |
GP: general practitioner.
MBCT: mindfulness‐based cognitive therapy.
MDD: major depressive disorder.
PCT: preventive cognitive therapy.
RCT: randomised controlled trial.
3.1 Successful discontinuation rate
Three studies reported on discontinuation rate in the discontinuation group. None of these studies assessed withdrawal symptoms.
We could not pool the data because studies reported insufficient information on discontinuation rate in the continuation group. We emailed the study authors to request data on discontinuation rate in the continuation group; study authors responded to our email, but we have not received data.
We have therefore summarised the evidence in a narrative synthesis.
In the discontinuation group (n = 316), discontinuation with MBCT or PCT varied: 40% (17/43) after six months (Bockting 2018), 75% (46/61) after six months (Kuyken 2008), and 124/212 (59%) after 24 months (Kuyken 2015). In Kuyken 2015, 13.7% (29/212), and in Bockting 2018, 19% (8/43) of participants in the discontinuation group reduced their dose by a minimum of 50% (intention‐to‐treat (ITT) analysis).
Bockting 2018 reported that 34 of 85 (split to allow multiple‐arm comparisons: 17/43) (40%) participants in the discontinuation with PCT group followed the discontinuation advice and discontinued their antidepressant after six months and 16 of 85 (split 8/43) (19%) were able to reduce the dose by a minimum of 50%. In all, 38 of 100 participants in the control group adhered to antidepressant treatment (maintaining ≥ 20 mg antidepressant) after six months. In this trial, included participants were interested in receiving psychological therapy and were advised to completely taper their antidepressant over four weeks, or this was extended if preferred. We contacted study authors to request information on antidepressant discontinuation rate in the control group. These authors responded to our request, but we have not yet received data.
Kuyken 2008 reported that at the end of the six‐month window for tapering, 46 of 61 (75%) participants in the MBCT arm of the trial discontinued their medication. In the further six‐month follow‐up period, rates of antidepressant usage between the two groups continued to be highly significantly different (P < 0.0001), although no data on antidepressant use in both groups were provided. Ten (16%) participants in the continuation group withdrew from the trial due to discontinuation of antidepressants. We have requested detailed data. Study authors responded to our mail that they could not extract these data because they defined relapse and adherence in different ways (Van Leeuwen 2020a [pers comm]).
Kuyken 2015 reported that 124 of 212 (59%) discontinued the antidepressant and 29 of 212 (13.7%) reduced the dose by a minimum of 50%. In all, 162 of 212 (76%) participants in the continuation group remained on the therapeutic dose and 50 (24%) did not remain on the therapeutic dose. Data on discontinuation rate in the continuation group were not reported. We requested these data. Study authors responded to our mail that they could not extract the data because they defined relapse and adherence in different ways.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for bias (withdrawal confounding bias) and by one level for imprecision (no pooling due to insufficient information) and indirectness (studies included participants with recurrent depression in remission). We did not downgrade for lack of blinding (objective outcome).
3.2 Relapse rate (as defined by study authors)
See Analysis 3.1.
3.1. Analysis.

Comparison 3: Discontinuation with high‐intensity psychological interventions versus continuation, Outcome 1: Relapse (as defined by study authors)
Four studies comparing discontinuation with psychological support provided data on relapse rate (Bockting 2018; Kuyken 2008; Kuyken 2015; Segal 2010).
These four studies reported risk of relapse as time‐to‐relapse data (survival data). We used the log hazard ratio to compare survival curves of the different studies. Relapse was based on the criteria defined by study authors. None of these studies distinguished symptoms of relapse from symptoms of withdrawal nor made adjustments for withdrawal symptoms.
3.2.1 Pooled studies
Pooling was possible for only three studies (Bockting 2018; Kuyken 2008; Kuyken 2015). A total of 316 (45.8%) participants were included in the pooled discontinuation group and 374 (54.2%) in the pooled continuation group.
There was no difference in risk of relapse (as defined by study authors) between participants discontinuing antidepressants with support from MBCT or PCT compared to those continuing antidepressants (HR 0.89, 95% CI 0.66 to 1.19; 690 participants, 3 studies).
We assessed the certainty of evidence for this outcome as low, downgraded by one level for bias (risk of withdrawal confounding bias; studies did not use very slow tapering regimens or low doses) and by one level for indirectness (studies included participants with recurrent depression in remission). We did not downgrade for imprecision (95% confidence interval includes the null effect; however imprecision is less important, as the effect seems to be of patient importance). We did not downgrade for attrition bias (censoring for survival data is unlikely to introduce bias) or lack of blinding (blinding was not possible but outcome assessors were masked).
3.2.2 Studies that could not be pooled
Pooling was not possible because insufficient data were reported.
Segal 2010 (n = 54; split 40) reported only hazard ratios for time to relapse related to status of stable (49% of participants) or unstable (51% of participants) remitters during remission before randomisation. However, there was no difference between discontinuation with MBCT and continuation of antidepressants in stable remitters (62% for MBCT and discontinuation, 59% for continuation of antidepressant; P = 0.77) and unstable remitters (28% for MBCT and discontinuation, 27% for continuation of antidepressant; HR 1.07, 95% CI 0.25 to 4.49; P = 0.93).
3.3 Withdrawal symptoms
No studies assessed this outcome.
3.4 Adverse events
Three studies contributed data on adverse events (Bockting 2018; Kuyken 2008; Kuyken 2015). None of these studies distinguished between adverse events and withdrawal symptoms nor made adjustments for withdrawal symptoms.
We could not pool data because insufficient data were reported.
Kuyken 2015 (n = 424) reported three non‐fatal and two fatal serious adverse events (deaths) in each treatment group; these were considered probably not related to the intervention or the trial.
Kuyken 2008 (n = 123) reported that no serious adverse events were recorded through the oversight of the Trial Steering Committee.
Bockting 2018 (n= 185; split 143) reported that suspected serious adverse events were measured and were reported to the multi‐centre ethics committee, but study authors reported only suicide data. They reported two suicide attempts (one in the PCT with tapering of antidepressants group, and one in the antidepressants only group). Study authors reported no indication that these were related to the interventions. They reported no deaths in both groups.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for risk of bias (withdrawal confounding bias; adverse events may include withdrawal symptoms), by one level for imprecision (no meta‐analysis possible; low numbers of events and insufficient data supported study authors' conclusions), and by one level for indirectness (studies measured only serious adverse events). We did not downgrade for performance bias (blinding is not possible due to the nature of the intervention, but outcome assessors were blinded).
3.5 Depressive symptoms
See Analysis 3.2.
3.2. Analysis.

Comparison 3: Discontinuation with high‐intensity psychological interventions versus continuation, Outcome 2: Depressive symptoms
Two studies with long‐term follow‐up provided data on severity of depressive symptoms by using the HAM‐D scale at the end of the study (Kuyken 2008; Kuyken 2015). Neither of these studies measured withdrawal symptoms nor made adjustments for withdrawal symptoms.
In all, 242 (50%) participants were included in the pooled discontinuation group and 242 (50%) in the pooled continuation group.
Data show no difference in depressive symptom severity on the HAM‐D scale between antidepressant discontinuation with MBCT and continuation of antidepressant (MD ‐0.42, 95% CI ‐1.82 to 0.98; 484 participants, 2 studies; I² = 28%).
We assessed the certainty of evidence for this outcome as low, downgraded by one level due to risk of bias (withdrawal confounding bias) and by one level due to indirectness (studies included recurrent depressive disorder). We did not downgrade for imprecision (95% confidence interval includes the null effect; however imprecision is less important, as the effect seems to be of patient importance) nor risk of performance bias (blinding was not possible due to the nature of the intervention, but outcome assessors were masked to treatment allocation).
3.6 Anxiety symptoms
No studies measured this outcome.
3.7 Quality of life
See Analysis 3.3.
3.3. Analysis.

Comparison 3: Discontinuation with high‐intensity psychological interventions versus continuation, Outcome 3: Quality of life
Two studies with long‐term follow‐up comparing discontinuation with MBCT to antidepressant continuation provided data on quality of life (Kuyken 2008; Kuyken 2015). These studies measured physical, psychological, and social relations domains of quality of life by using the WHO QoL‐BREF for participants with recurrent depression. Neither of these studies measured withdrawal symptoms nor made adjustments for withdrawal symptoms.
The two studies were considered suitable for pooling for meta‐analysis.
In all, 229 (50.3%) participants were included in the pooled discontinuation group and 226 (49.7%) in the pooled continuation group.
Data show no differences between discontinuation and continuation in the physical domain (MD ‐0.22, 95% CI ‐2.16 to 1.73; 455 participants, 2 studies; I² = 65%; random‐effects model), the psychological domain (MD 0.37, 95% CI ‐0.75 to 1.49; 455 participants, 2 studies; I² = 46%; random‐effects model), or the social relations domain (MD 0.05, 95% CI ‐0.56 to 0.66; 455 participants, 2 studies; I² = 9%; random‐effects model) after long‐term follow‐up.
We assessed the certainty of evidence for quality of life as very low, downgraded by one level due to risk of bias (withdrawal confounding bias, attrition bias, performance bias; outcome assessed with self‐report questionnaire and likely to be influenced by lack of blinding of participants), by one level due to imprecision (wide 95% confidence interval includes the null effect), and by one level due to indirectness (studies included participants with recurrent depressive disorder).
3.8 Social and occupational functioning
No studies measured this outcome.
3.9 Severity of illness
No studies measured this outcome.
3.10 Suicide, suicide attempt, or suicidal ideation
One study provided data on suicide.
Bockting 2018 (n = 185; split 143) reported two suicide attempts (one in the discontinuation with PCT group, and one in the continuation group) during the 104‐week follow‐up. Study authors reported that these events were not related to the interventions.
Comparison 4. Discontinuation with a minimal intervention versus usual care
One study compared discontinuation (taper) of antidepressant with low‐intensity intervention to usual care (Eveleigh 2018). The intervention included a patient‐specific letter to the GP with a recommendation to discontinue the antidepressant and tapering advice (Eveleigh 2018). The control group continued usual care without letter nor tapering advice. The study used different types of antidepressants prescribed for a lifetime depressive or anxiety disorder (30% had no lifetime psychiatric disorder).
The study did not make adjustments for withdrawal symptoms.
4.1 Successful discontinuation rate
This study assessed successful discontinuation defined as no antidepressant use during the preceding six months and the absence of a depressive or anxiety disorder during the one‐year trial.
In the tapering advice group, 4 of 70 (6%; 95% CI 2 to 14) participants successfully stopped their antidepressant compared to 6 of 76 (8%; 95% CI 4 to 16) participants in the usual care group who spontaneously discontinued after one year (Eveleigh 2018). Study authors concluded that the intervention was not effective (P = 0.6). They also reported that of the 70 participants in the tapering group, 34 (49%) did not comply with the advice to stop their antidepressant medication. Of the 36 (51%) participants who agreed to try, only 4 (6%) successfully stopped the antidepressant. Furthermore, 6642 long‐term antidepressant users were identified, of whom 37% were deemed eligible by their GP.
We assessed the certainty of evidence for this outcome as low, downgraded by one level for imprecision (single study with a small number of participants) and by one level for risk of bias (withdrawal confounding bias; withdrawal symptoms may be misclassified as relapse and this may lead to restart of the antidepressant). We did not downgrade for performance bias (objective outcome not likely to be influenced by lack of blinding), attrition bias (unknown outcome was classified as not discontinued antidepressant), and indirectness (balanced mix of participants in primary care with depressive and anxiety disorders).
4.2 Relapse rate
Eveleigh 2018 measured relapse of depressive or anxiety disorder with the CIDI 3.0, a psychiatric interview done by interviewers.
In the tapering advice group, 18 of 70 (26%) participants relapsed compared to 10 of 76 (13%) in the usual care group (Eveleigh 2018). Study authors concluded that there was a "marginally significant higher" relapse rate (defined by study authors) in the tapering advice group (P = 0.05) compared to the usual care group. Of participants who continued their antidepressant, there was a non‐statistically significant higher relapse rate in the tapering advice group versus the usual care group (25% versus 11%; P = 0.07). Therefore study authors concluded that this difference was not associated with antidepressant discontinuation.
We assessed the certainty of evidence for this outcome as low, downgraded by one level for withdrawal confounding bias (study measured DESS symptoms but did not analyse data) and by one level for imprecision (single study had a small number of participants). We did not downgrade for risk of performance bias (blinding was not feasible, but the interviewer was blinded in the cluster‐randomised trial with practice as the unit) and attrition bias (participants who dropped out were classified as failures).
4.3 Withdrawal symptoms
Eveleigh 2018 measured the prevalence and severity of antidepressant withdrawal symptoms using the Discontinuation‐Emergent Signs and Symptoms (DESS) Scale as the outcome; however this was not analysed (Van Leeuwen 2020b [pers comm]).
4.4 Adverse events
Adverse events were not measured.
4.5 Depressive symptoms
Eveleigh 2018 measured the severity of depressive symptoms by using the CESD scale. Study authors reported a higher mean CESD score in the tapering group (n = 51) (mean CESD total endpoint score 13.7 (SD 8.9)) compared to the usual care group (n = 55) (mean CESD total endpoint score 12.6 (SD 7.9)) at the end of the trial but no differences between groups (P = 0.51) (higher CESD score means increased intense symptom severity) (Van Leeuwen 2020b [pers comm]).
We assessed the certainty of evidence for this outcome as low, downgraded by one level due to risk of bias (withdrawal confounding bias, attrition bias) and by one level due to imprecision (a single study).
4.6 Anxiety symptoms
Eveleigh 2018 measured the severity of illness in patients with panic disorder by using the Panic and Agoraphobic Scale (PAS). Study authors reported a higher mean PAS score in the tapering group (n = 50) (mean PAS 4.1 (SD 7.2)) compared to the usual care group (n = 51) (mean PAS 3.6 (SD 7.1)) at the end of the study but no differences between groups (P = 0.71) (higher PAS scores indicate greater severity) (Van Leeuwen 2020b [pers comm]).
We assessed the certainty of evidence for this outcome as low, downgraded by one level due to risk of bias (withdrawal confounding bias, attrition bias) and by one level due to imprecision (a single study).
4.7 Time to relapse
Time to relapse was not measured.
4.8 Quality of life
Eveleigh 2018 measured quality of life by calculating quality‐adjusted life‐years (QALYs) by using the EuroQoL Group Quality of Life Questionnaire based on 5 dimensions (EQ‐5D). Participants completed the EuroQol (EQ‐5D) health status questionnaire at baseline and at three, six, nine, and twelve months. There was a high dropout rate in returning all three monthly self‐report questionnaires: 32 of 70 (45%) participants in the discontinuation group and 33 of 76 (43%) did not have complete data. Missing data were handled by the multiple imputation method.
Participants in the tapering advice (n = 70) group had a mean of 0.70 QALYs (SD 0.25), and in the usual care group (n = 76) a mean of 0.72 QALYs (SD 0.26). There was no difference in quality of life between discontinuation with tailored recommendation and usual care (MD (with multiple imputation for missing values) ‐0.02, 95% CI ‐0.05 to 0.10; higher scores indicates better quality of life). Study authors reported 0.07 as the minimally important difference for the EQ‐5D.
We assessed the certainty of evidence for this outcome as very low, downgraded by one level for imprecision (single study with a small number of participants) and by two levels for risk of bias (withdrawal confounding bias, large number of missing values, risk of performance bias).
4.9 Social and occupational functioning
Social and occupational functioning was not measured.
4.10 Severity of illness
Severity of illness was not measured.
4.11 Suicide, suicide attempt, or suicidal ideation
This study did not provide data on suicidality.
Comparison 5. Discontinuation with psychosocial interventions versus continuation of long‐term antidepressants versus continuation of long‐term antidepressants
None of the included studies compared discontinuation with changed pharmacological form versus continuation of antidepressants.
Comparison 6. Discontinuation (with changed pharmacological form) versus continuation of antidepressants
None of the included studies compared discontinuation with changed pharmacological form versus continuation of antidepressants.
Subgroup analysis
Discontinuation abrupt versus continuation of long‐term antidepressants
See Analysis 4.1.
4.1. Analysis.

Comparison 4: Subgroup age, abrupt discontinuation versus continuation, Outcome 1: Relapse (HR)
For the outcome relapse, the test for subgroup differences for age was not statistically significant (age; P = 0.69; I² = 0%). This suggests that there is no difference between participants based on age (younger than 65 years versus 65 years and older). Despite inclusion of 10 studies in the meta‐analysis, we were unable to perform subgroup analysis for setting, disorder, type of antidepressant, or duration of antidepressant treatment due to unevenly distributed covariates (primary care (1 study), anxiety disorder (1 study), SNRI (1 study), atypical antidepressant (1 study), TCA (1 study), and antidepressant duration of 52 weeks or longer (1 study)).
We were unable to perform subgroup analysis for the outcome withdrawal symptoms and for adverse events due to lack of pooling.
Discontinuation by "tapering" versus continuation of long‐term antidepressants
See Analysis 5.1Analysis 6.1 and Analysis 7.1.
5.1. Analysis.

Comparison 5: Subgroup indication, tapering versus continuation, Outcome 1: Relapse (HR)
6.1. Analysis.

Comparison 6: Subgroup type of antidepressant, tapering versus continuation, Outcome 1: Relapse
7.1. Analysis.

Comparison 7: Subgroup duration of antidepressant, tapering versus continuation, Outcome 1: Relapse
For the outcome relapse, the test for subgroup differences was significant only for the indication of antidepressant (P = 0.02; I² = 81.7%). The estimated effect for anxiety disorder was HR 7.06 (95% CI 3.22 to 15.52) and for depressive disorder HR 2.62 (95% CI 2.01 to 3.42). The remaining tests were not statistically significant (type of antidepressant (TCA versus SNRI): P = 0.48 (no SSRI subgroup due to only 1 study), I² = 0%; duration of antidepressant (shorter than 52 weeks versus 52 weeks or longer: P = 0.36; I² = 0%). We were unable to analyse subgroups on the basis of age and setting.
We did not perform subgroup analysis for the other comparisons (discontinuation with high‐intensity psychological intervention or minimal intervention) due to the small number of included studies.
Sensitivity analysis
For each outcome in each comparison, we planned to exclude studies with high risk to compare these results with results of the analysis including all studies. However, we could not perform sensitivity analyses excluding studies at high risk of bias. All included studies had high risk for at least one risk of bias domain. We performed additional sensitivity analysis to test the impact of industry sponsorship by excluding trials with industry sponsorship or an unclear source of funding and to test the impact of a slow tapering regimen.
We did not perform sensitivity analyses for studies with a co‐intervention, for studies with a high‐intensity psychological intervention, or for studies with minimal intervention due to the small number of studies.
Discontinuation abrupt versus continuation of long‐term antidepressants
For the outcome relapse, of the 10 studies, nine reported industry sponsorship or failed to report the source of funding. Sensitivity analyses excluding these nine studies reduced substantially the magnitude of the treatment effect, and the confidence interval included the null effect (HR 1.20, 95% CI 0.84 to 1.71). For the outcome adverse events, one included study had non‐commercial sponsorship or an unknown source of funding. Sensitivity analyses excluding six studies (with funding from industry or an unknown source of funding) made confidence intervals substantially wider but did not change the direction of the effect (OR 1.14, 95% CI 0.09 to 13.97).
We could not perform sensitivity analysis for depressive symptoms or severity of illness because all included studies had non‐commercial sponsorship or failed to report the source of funding.
Discontinuation by "tapering" versus continuation of long‐term antidepressants
For the outcome relapse, 9 of the 13 studies without co‐intervention received support from the pharmaceutical industry or failed to describe the source of funding. Sensitivity analyses excluding these 9 studies did not change the direction of the effect but increased the magnitude of the treatment effect and made the confidence interval wider (HR 3.36, 95% CI 1.58 to 7.16). We could not perform sensitivity analysis for adverse events, depressive symptoms, anxiety symptoms, quality of life, social functioning, or severity of illness because all studies had non‐commercial sponsorship or failed to report the source of funding.
We could not perform sensitivity analysis to assess the potential impact of a tapering regimen of four weeks or less because none of the studies (without co‐intervention) reported a tapering regimen longer than four weeks.
Discussion
Summary of main results
In this review, we included 33 randomised controlled trials (RCTs) with 4995 participants prescribed antidepressants long term (24 weeks or longer). We identified two ongoing studies and three studies awaiting assessment.
Of our four primary outcomes of interest, 2 studies addressed successful discontinuation rate, 31 addressed relapse, 1 withdrawal symptoms, and 21 adverse events.
The findings and certainty of evidence assessment corresponding to each of four comparisons (i.e. abrupt discontinuation, "tapered" discontinuation, discontinuation with psychological therapy support, and discontinuation with minimal intervention) are described in the 'Summary of findings' tables (see Table 1, Table 2, Table 3, and Table 4).
None of the studies compared discontinuation with low‐intensity psychological interventions or a changed pharmacological form to continuation of antidepressants.
Abrupt discontinuation versus continuation of long‐term antidepressants
See Table 1.
Thirteen studies (1780 participants) compared abrupt discontinuation to continuation of long‐term antidepressants. Two of these studies included a psychological co‐intervention (cognitive‐behavioural therapy (CBT) and cognitive‐behavioural analysis system of psychotherapy (CBASP)) in both treatment groups.
Very low‐certainty evidence suggests that abrupt discontinuation of long‐term antidepressants may increase relapse (10 studies) compared to continuation of antidepressant, but none of the studies distinguished between relapse and symptoms of withdrawal. The predefined subgroup analysis based on age did not show a difference in relapse. We could not perform subgroup analyses based on setting, disorder, type of antidepressant, or duration of antidepressant use prior to randomisation. There is insufficient evidence of its effect on adverse events (7 studies; very low certainty evidence) and depressive symptoms (3 studies). Evidence about the effects of discontinuation on withdrawal symptoms (1 study) is very uncertain. Compared to continuation of antidepressants, abrupt discontinuation may increase severity of illness (5 studies; very low‐certainty evidence). Pooling data on anxiety symptoms was not possible due to heterogeneity in measures (3 studies; very low‐certainty evidence). None of the eligible studies in this comparison reported the antidepressant discontinuation rate or either of the patient‐centred outcomes quality of life and social and occupational functioning.
There is insufficient evidence of the effect of abrupt discontinuation with psychological support as a co‐intervention on relapse compared to continuation of antidepressants (2 studies; very low‐certainty evidence).
Discontinuation by "tapering" versus continuation of long‐term antidepressants
See Table 2.
Eighteen studies (2442 participants) compared discontinuation by "tapering" (one week or longer) to continuation of long‐term antidepressants. Four of the studies included a psychological co‐intervention (preventive cognitive therapy (PCT), mindfulness‐based cognitive therapy (MBCT), CBT, or interpersonal therapy (IPT)) in both treatment groups. Most tapering regimens lasted four weeks or less.
Very low‐certainty evidence suggests that discontinuation by "tapering" of long‐term antidepressants may increase risk of relapse compared to continuation of antidepressants (13 studies with no co‐intervention; very low‐certainty evidence). None of the studies distinguished relapse from symptoms of withdrawal. One subgroup analysis suggested that discontinuation of antidepressants prescribed for anxiety disorder leads to more relapse than when prescribed for depressive disorders. Subgroup analyses based on type of antidepressant and duration of antidepressant did not show a difference. Subgroup analyses based on age and setting was not possible. Low study numbers prohibited sensitivity analysis based on tapering regimens longer than four weeks.
Compared to continuation, very low‐certainty evidence suggests that "tapering" of long‐term antidepressants may have little or no effect on withdrawal symptoms (1 study) or adverse events (7 studies) but may increase severity of depressive symptoms (6 studies) and anxiety symptoms (3 studies). "Tapering" may reduce social and occupational functioning (3 studies; very low‐certainty evidence) as well as the quality of life subdomain social functioning (3 studies; very low‐certainty evidence) and the subdomain emotional functioning (3 studies; very low‐certainty evidence). There is insufficient evidence of its effect on the quality of life subdomain physical health problems (3 studies; very low‐certainty evidence) and may increase the severity of illness (6 studies; very low‐certainty evidence) compared to continuation of antidepressants.
Insufficient data pooling of results for the discontinuation outcome was not possible for the two studies in which “tapering” with a psychological support as co‐intervention was investigated. Discontinuation rates in the discontinuation group varied: 55% in the discontinuation group and 12% in the continuation group after 15 months in one study; and 40% in the discontinuation group after 6 months in the other study (2 studies; very low‐certainty evidence). Very low‐certainty evidence suggests that "tapering" with a psychological co‐intervention may increase the risk of relapse compared to continuation of antidepressants (4 studies).
Discontinuation with high‐intensity psychological interventions
See Table 3.
Four studies (730 participants) comparing "tapering" with high‐intensity psychological support to continuation of long‐term antidepressants were eligible for inclusion. Psychological support included MBCT (3 studies) or PCT (1 study). Duration of tapering was four weeks (1 study) or four weeks or longer (left to clinician's discretion) (2 studies), or a regimen was determined by participants and clinician (but mean duration was not reported) (1 study).
Again, pooling was not possible for discontinuation rate due to insufficient reporting of data. Discontinuation rates in the discontinuation group varied widely: 40%, 59%, and 75% (3 studies; very low‐certainty evidence).
Discontinuation by "tapering" with PCT or MBCT may result in little or no difference in relapse (3 studies; low‐certainty evidence), but none of the studies distinguished relapse from symptoms of withdrawal. "Tapering" with PCT and MBCT may make little or no difference in severity of depressive symptoms (2 studies; low‐certainty evidence) and may have little or no effect on the quality of life subdomains psychological or physical or social functioning (2 studies; very low‐certainty evidence) compared to continuation of long‐term antidepressants. Pooling was not possible for adverse events due to insufficient information (3 studies; very low‐certainty evidence). None of the eligible studies in this comparison category reported withdrawal symptoms, anxiety symptoms, social and occupational functioning, or severity of illness.
Discontinuation with minimal intervention to usual care
See Table 4.
One eligible study (n = 146 participants) compared a letter to patients’ general practitioner (GP) with a recommendation to discontinue long‐term inappropriate antidepressant use by "tapering" versus usual care (without advice). Discontinuation with a letter to the GP may make little or no difference in the discontinuation rate (1 study; low‐certainty evidence). The discontinuation rate was low, with 6% of participants discontinuing compared to 8% in the usual care group. Discontinuation with a letter to the GP may make little or no difference in relapse (1 study; low‐certainty evidence), depressive symptoms (1 study; low‐certainty evidence), and anxiety (1 study; low‐certainty evidence) and may have little or no effect on quality of life (1 study; very low‐certainty evidence) compared to usual care. Withdrawal symptoms were measured after tapering of antidepressants but were not analysed. The study did not report adverse events, social and occupational functioning, or severity of illness.
Overall completeness and applicability of evidence
Evidence for discontinuation of long‐term antidepressant use lacks completeness and has low applicability.
The most important aspect regarding applicability of evidence is the finding that trials did not distinguish relapse from symptoms of withdrawal. Withdrawal reactions are common in clinical practice. A recent review suggested incidence rates between 27% and 86%, with an average incidence of 56%, reduced to 50.7% when restricted to RCTs (6 studies), and excluding observational studies and online surveys (Davies 2019). Davies 2019 found withdrawal symptoms are often severe and may last from a few weeks to several months or even longer. Withdrawal symptoms may be misdiagnosed as relapse, known as "withdrawal confounding bias". This occurs when relapse criteria rely on clinical scale scores that include domains for depressed mood, insomnia, agitation, gastrointestinal somatic symptoms, anxiety, general somatic symptoms, and/or sexual dysfunction ‐ all potential symptoms of withdrawal. It is surprising that withdrawal symptoms, well known since the 1960s for tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) (Nielsen 2012), and since the 1990s for selective serotonin reuptake inhibitors (SSRIs) and serotonin‐noradrenaline antidepressants (SNRIs) (Fava 2015; Fava 2018b), were not systematically reported in the included studies. Withdrawal symptoms were measured in only one study. Nor did any of the included studies make adjustments for withdrawal symptoms. Consequently, it is unclear to what degree misclassified withdrawal symptoms contributed to "relapse" rates. Research suggests this could pertain to most relapses (El‐Mallakh 2012; Greenhouse 1991; Hengartner 2020; Recalt 2019; Rosenbaum 1988). Moreover, withdrawal symptoms may have an effect on almost every outcome including adverse events, quality of life, social functioning, severity of illness, and anxiety and depression scores. For example, low mood and other withdrawal symptoms may register on the Hamilton Rating Scale for Depression (HAM‐D) ‐ the prioritised measure for depressive symptoms ‐ and may result in people falsely allocated to having "severe" depressive symptoms. Furthermore, physical and emotional symptoms of withdrawal may impair work, socialising, and emotional well‐being, thereby affecting scores on quality of life and functioning scales.
Another limitation to the applicability of discontinuation evidence relates to the approach used, specifically, tapering versus rapid discontinuation. Thirteen (39%) studies discontinued the antidepressant abruptly. Nearly all tapering studies used a tapering schedule of four weeks or less. First, in our review, we did not compare abrupt discontinuation with "tapering" per se. It is therefore not possible for our review to draw conclusions about the best way to taper antidepressants. However, one included study found that most participants (60%) tapered antidepressants over six months, suggesting that a time frame of four weeks is not feasible for many long‐term users of antidepressants (Bockting 2018). Second, recommendations about tapering regimens have changed over time. The method of discontinuation (either abrupt or tapering) in our included studies contrasts with the updated guidelines for discontinuation of antidepressants. Recently, the National Institute for Health and Care Excellence (NICE) has updated antidepressant guidelines to reflect severity and length of withdrawal symptoms related to "rapid" tapering (Iacobucci 2019). The recommended duration of tapering has been revised to four weeks or longer, particularly for drugs with a shorter half‐life (such as paroxetine and venlafaxine) (NICE 2009). Also, the UK Royal College of Psychiatrists guidelines now recommend ‘months of tapering’ (UK Royal College of Psychiatrists 2020). Recently, it has been recognised that very low doses of antidepressants have significant effects on target receptors, suggesting that pharmacologically informed rational tapering of antidepressants requires reduction to very low doses before stopping (perhaps 1/40th of regular therapeutic doses) (Horowitz 2019). In summary, these results suggest the potential benefits of hyperbolic tapering, where the dose initially is tapered quickly, then more slowly, to very small doses to doses lower than therapeutic minimums before complete discontinuation. This approach may be of particular benefit for those unable to tolerate withdrawal symptoms. As all studies captured in our review used commercially available formulations of antidepressants, no study included a slow tapering regimen with very low doses over some months or a changed pharmacological form such as liquid or tapering strips (to allow low doses of antidepressants). The effect of this is that abrupt changes in effect on target receptors occur when the dose is reduced to zero, meaning that many of the regimens in the tapering studies are very similar to abrupt cessation, perhaps explaining the lack of success in discontinuation and the apparent ‘relapse’ rate.
Applicability of the evidence in clinical practice is limited because most studies recruited participants from specialist outpatient mental healthcare settings. A recent review of observational studies found worse long‐term outcomes for patients with depression who are managed in specialist settings compared to those managed in primary care (5 studies) (Ormel 2020). Many depressive episodes (50% to 80%) treated in primary care are self‐limiting within three to twelve months (6 studies) (Ormel 2020). Most of the studies in our review included participants in remission from severe depression with a history of more than two previous episodes. Overall, the prognosis of a first episode of depression is positive, with 50% to 60% achieving stable recovery, 35% to 40% experiencing at least one recurrence in the next 15 years, and 15% developing chronic depression (Eaton 2008; Mattisson 2007; Moffit 2010). Our review therefore cannot be generalised to patients seen in primary care with low risk of relapse. Discontinuation may be more appropriate and effective in such a population. Furthermore, most included studies had stringent inclusion and exclusion criteria (e.g. excluding other psychiatric conditions such as anxiety, suicidal ideation, somatic comorbidity, co‐medication, older people), which limits the generalisability of our findings. A clear distinction between depression and anxiety is often difficult in clinical practice (MaGPIe Research Group 2003). Studies in people aged 65 years and older are sparse (3 studies), indicating a gap in the evidence. Furthermore, most studies included in our review had a limited duration of antidepressant treatment (between six and twelve months) before discontinuation. In contrast, the mean duration of antidepressant treatment is longer than two years in the UK (Johnson 2012: Petty 2006), four years in Australia (Kjosavik 2016), and longer than five years in the United States (Mojtabi 2014; Pratt 2011). A Canadian primary care study (n = 8119) found that antidepressants were prescribed longer than needed (15 months or longer, with a mean duration of 4.8 years) for 46% of patients (Mangin 2018).
The four studies that included people prescribed antidepressants for anxiety differed in terms of study characteristics, type of anxiety disorder, and intervention. Therefore, we were not able to draw conclusions about whether it is effective and safe to discontinue long‐term antidepressant use for patients with anxiety disorders.
As described in the Background section of this review, there are other methods to support discontinuation that were not captured here. This review illustrates that initiation of high‐intensity interventions such as PCT and MBCT may support discontinuation in patients with recurrent depression (very‐low certainty of evidence); however these psychological interventions are resource‐intensive, and access is often limited. Studies investigating less resource‐intensive psychosocial interventions such as psycho‐education, psychologically informed digital support for GPs and patients, or mental health support delivered by trained nurses or social workers to facilitate discontinuation have the potential to generate findings that reach more people, yet none were identified. A minimal intervention such as a letter with tapering advice sent to the GP was investigated in only one trial.
Limited information on the majority of our primary and secondary outcomes restricted investigation of the benefits and harms of discontinuing long‐term antidepressant use. Few studies had a focus on de‐prescribing long‐term antidepressants. This review included successful discontinuation rate as one of the primary outcomes. Overall, data on discontinuation either were not reported or were insufficiently reported. Nearly all studies were designed to examine how continuation of long‐term antidepressants could avoid relapse and recurrence rather than discontinuation success. Only two studies provided sufficient information to assess successful discontinuation rate, yet its relevance as an outcome in terms of redressing increasing use of antidepressants in clinical practice is important. Systematic research into possible benefits and harms related to antidepressant discontinuation has been a low priority for both researchers and the pharmaceutical industry (Hengartner 2019). Therefore, we have provided little detail on whether studies assessed systematically beneficial and harmful effects of withdrawal. Additionally, a minority of the studies assessed patients’ preferences for outcomes (Eiring 2015): nine studies quality of life, and five of the 33 studies social and occupational functioning (e.g. daily activities, social activities, return to work). Consequently, the effect of discontinuation on person‐centred outcomes is unknown.
In clinical practice, shared decision‐making between clinician and patient would guide decisions about ceasing long‐term antidepressant use and choosing the best approach to tapering. Patients would be monitored and withdrawal symptoms seen as a sign to taper more slowly. One study included a tapering schedule determined by clinician and patient together but poorly described the discontinuation and tapering process.
Another issue that limits applicability of the evidence is lack of consistency. There was significant variability across studies in terms of participant characteristics; antidepressant type, dosage, and duration; inclusion criteria (severity of depression, remission criteria); exclusion criteria; interventions; duration of tapering; and outcomes, making it difficult to interpret the pooled result. Use of different definitions of relapse, evaluated at different time points, compounds the difficulty and further limits generalisability.
We investigated the effectiveness and safety of approaches for discontinuation versus continuation of long‐term antidepressants prescribed for depression and anxiety; however, discontinuation of long‐term antidepressants could be considered in other contexts, for example, prescribing in the context of medicalising "appropriate misery" due to adverse life events (Johnson 2017; Pilgrim 1999), and indeed prescribing in the absence of an indication (Ulfarson 2003), or for patients with frailty, polypharmacy, or multi‐morbidity. Such scenarios are not included in our review, limiting its completeness. However, these situations provide important opportunities to assess the benefits and harms of long‐term antidepressant use and shared decision‐making (Reeve 2017).
Additionally no studies have systematically reported other medication use (e.g. chronic psychoactive medication) and psychotherapy at baseline or at trial initiation, and few have examined other psychiatric disorders or non‐psychiatric disorders (if not excluded). Given that such factors may influence the benefits and harms of long‐term antidepressant use, this limits the applicability of review results to clinical practice.
Quality of the evidence
We found the certainty of evidence for primary and secondary outcomes to be very low to low due to limitations in study design, indirectness, and imprecision. Certainty of evidence for all comparisons is described in the 'Summary of findings' tables (see Table 1, Table 2, Table 3, and Table 4).
Limitations in study design or execution
All studies had methodological limitations and were judged to be at unclear or high risk of bias in at least one domain. In more than half of the studies, information on random sequence generation or allocation concealment, or both, was insufficient, although we contacted all study authors for detailed information if possible (if contact email was available). Eight studies were assessed as having high risk of performance bias due to lack of blinding of participants and/or research staff. We assessed two studies as having high risk of detection bias due to lack of blinding in outcome assessment. Overall, blinding was poorly described. More than half of the studies were assessed as having high risk for attrition bias due to missing data, high dropout or unbalanced dropouts. Thirty‐two studies were assessed as having high risk of bias due to selective reporting. Failure to measure withdrawal symptoms was assessed as a considerable source of bias. We assessed 20 studies to be at unclear risk and one study at high risk for sponsorship bias. The level of evidence was downgraded by one or two levels, respectively.
Inconsistency of results
We have not downgraded the evidence for any comparison for inconsistency.
Indirectness of evidence
Twenty‐five included of the 33 studies focused on participants with recurrent depression. Some studies included dysthymia, chronic depression, or double depression. This is a specific subpopulation of patients taking long‐term antidepressants in the community; therefore the generalisability of our results is limited to those with multiple episodes of depression. Because of this, we downgraded the evidence by one level for indirectness.
Imprecision
For most outcomes, we did not downgrade for imprecision, as the sample was sufficiently large to detect a statistically significant difference between treatment groups. However, for some outcomes, the effect estimate of the pooled studies had a wide 95% confidence interval, which included the null effect (i.e. no difference between treatments). Therefore, we downgraded the evidence by one level. Because pooling was not possible for some outcomes, the potential benefit of a meta‐analysis to produce a more precise effect estimate could not be realised; therefore we reduced the evidence by one level for imprecision. We included only one study with discontinuation combined with a minimal intervention; therefore we downgraded the evidence for all outcomes by one level for imprecision.
Publication bias
We conducted a comprehensive search for published and unpublished studies, thereby reducing the risk of publication bias. For those primary outcomes for which we had more than 10 studies, a funnel plot showed low risk of publication bias.
Potential biases in the review process
Our review process had several limitations.
We used a comprehensive search strategy in multiple databases with no restrictions on language. We also contacted experts in the field. We identified one unpublished study. We may have missed other relevant studies, especially those with a negative result. Five studies that are ongoing or awaiting classification have not been included and may be a source of potential bias.
Two review authors independently selected studies for inclusion, extracted data, and assessed risk of bias. A third review author acted as arbiter to minimise risk of bias in the review process. In addition, we contacted several study authors for additional data but received useful data from only three authors.
It is important to note that adverse events may include withdrawal symptoms, as the studies did not distinguish between adverse events and withdrawal symptoms.
We prioritised the HAM‐D to measure depressive symptoms. However, this scale has been criticised because of its problems in differentiating between withdrawal and depressive symptoms (Fava 2015: Haddad 2007). Relapse, one of our primary outcomes, was based on the criteria defined by study authors and may be a source of potential bias for several reasons. Withdrawal symptoms may be misdiagnosed as relapse when relapse is assessed with the HAM‐D scale (see above, Overall completeness and applicability of evidence) (Fava 2015: Haddad 2007). Definitions of remission and relapse differed widely between studies, and this could introduce bias. Many study authors used one continuous scale measuring depressive symptoms (e.g. Hamilton Depression Rating Scale (HDRS), Montgomery‐Åsberg Depression Rating Scale (MADRS)) to assess relapse, then dichotomised the outcome as a threshold value (e.g. HAM‐D score > 17). Dichotomisation is a potential concern and has been criticised by several authors (Altman 2006: Jakobsen 2017: Kirsch 2007: Munkholm 2019). Information is lost when continuous data are transformed to dichotomous data and the data can be influenced by data distribution and the cut‐off point selected (Jakobsen 2017). All things considered, this is a major limitation of our review and may be a source of potential bias; therefore, our findings for relapse should be interpreted with caution.
For all outcomes, we included the outcome measurement at the end of each study (as defined in our protocol). As study endpoints differed, we pooled studies with different follow‐up durations. It is unclear if this difference in study duration impacted the conclusions (however, all except one study had a follow‐up period of six months or longer).
Long‐term psychoactive drug use and psychotherapy, other psychiatric disorders (if not excluded), and baseline antidepressant type or dose were not systematically reported and may be a source of potential bias. Consequently, the effect of these on outcomes is unknown.
Many studies did not systematically assess the harmful effects of antidepressants separately or did not report a safety composite endpoint, and this may be a source of potential bias (Warren 2020). Moreover, few studies reported data on suicide, and none of the study authors assessed suicide, suicide attempt, or suicidal ideation related to the medication. The conclusion that suicide was "possibly related" or "not related" may be misleading, as suicidal ideation and suicide attempts were well recognised as withdrawal effects (Valuck 2009); therefore the data on suicide may be a source of potential bias.
None of the included studies used an active placebo that mimics side effects of antidepressants (e.g. drugs with anticholinergic actions, such as atropine, mimic TCA side effects). Given that withdrawal symptoms are common, participants may be aware if randomised to the discontinuation group, thereby compromising blinding and introducing bias (Moncrieff 2004). Studies without active placebo are considered to be at unclear or high risk of performance bias (Munkholm 2019). Only a few studies reported blinding integrity. We did not use this criterion to assess performance and detection bias, and this could introduce bias. We considered studies to be at low risk of performance and detection bias if they provide a clear description of the blinding procedure.
Other potential biases include attrition bias and industry sponsorship bias. We considered studies to be at high risk of attrition bias if dropout rate was greater than 20%, or if dropout was imbalanced between treatment groups and/or reasons for dropouts were not reported. The last observation carried forward (LOCF) method was considered inappropriate for imputation, as it may lead to bias and overestimation (Woolley 2009). However, for studies that used the LOCF method for secondary outcomes, when dropout was low and balanced, we did not consider the study to be at high risk of attrition bias. We considered no other imputation method as inappropriate. Overall, the dropout rate in these studies was high. A previous Cochrane Review reported that sponsorship of drug studies by the manufacturing company leads to more favourable efficacy results and conclusions than sponsorship by other sources (Lundh 2017). This issue was discussed with the Cochrane Review group, which advised us to assess studies with industry sponsorship as having unclear risk of other bias and to perform post‐defined sensitivity analysis for industry sponsorship. However, we were not able to perform the sensitivity analysis for all outcomes and comparisons due to few studies with low risk of industry sponsorship.
Sensitivity analyses excluding studies at high risk of bias could not be performed because all studies were assessed as having high risk in at least one risk of bias domain. Due to the small number of studies involving psychological therapy or minimal intervention to support discontinuation, we were unable to undertake all planned subgroup and sensitivity analyses.
We pooled studies with and without co‐intervention separately for the primary outcomes successful discontinuation rate and relapse because psychological support may increase the risk on discontinuation and reduce the risk of relapse. However, we pooled studies with and without psychological support for our secondary outcomes, and this may be a source of potential bias. We also performed overall pooling for the primary outcomes successful discontinuation rate and relapse to be consistent in our analysis.
One review author (TK) was involved in two ongoing trials (Duffy 2019; ISRCTN12417565). None of the other review authors was involved in any of the included trials.
Agreements and disagreements with other studies or reviews
One previous systematic review investigated interventions to discontinue antidepressant use compared to an alternative discontinuation procedure, usual care, or clinical management but did not focus on long‐term antidepressant use (Maund 2019). Maund 2019 included observational studies and excluded placebo‐controlled trials. The discontinuation rate in the discontinuation intervention group was reported in eight studies and varied widely, from 6% to 95%. Two studies showed a very high discontinuation rate (87%) for CBT plus tapering compared to clinical management plus tapering (95%) (Fava 1994; Fava 1998a), and with no difference in relapse rates between groups after 20 weeks. They found no difference in recurrence or quality of life between MBCT and tapering versus maintenance antidepressants treatment (2 studies). Based on one RCT ‐ Eveleigh 2018 ‐ and one uncontrolled trial ‐ Johnson 2012, they concluded that prompting GPs to review participants on long‐term antidepressants was not an effective intervention to discontinue antidepressants (Maund 2019). This finding is consistent with the findings of our review. Maund 2019 found higher risk of withdrawal symptoms with abrupt discontinuation compared with tapering in two studies (one RCT and one retrospective cohort study), but we could not confirm this finding. In the RCT, there was lower risk of taper‐emergent adverse events with one week taper compared to abrupt discontinuation of desvenlafaxine but no difference in the risk of withdrawal syndrome (Khan 2014). The retrospective cohort study found that withdrawal syndrome occurred significantly less frequently in patients who discontinued gradually compared with patients who discontinued abruptly, and most patients could discontinue paroxetine with a taper of 5 mg every two or four weeks (Himei 2006). These results are consistent with the findings of a previous review reporting consensus about tapering antidepressants slowly over a period of weeks or months to avoid withdrawal symptoms (Wilson 2015).
A recent non‐systematic review showed that short tapers of between two and four weeks have minimal benefit over abrupt discontinuation, and that short tapering regimens such as these often are not tolerated by patients (Horowitz 2019). Review authors found four studies ‐ Groot 2013; Himei 2006; Murata 2010; van Geffen 2005 ‐ showing that tapering over several months results in fewer withdrawal symptoms (Horowitz 2019). In one study (n = 74), participants who tapered their SSRI dose over a period of up to four months had significantly fewer withdrawal symptoms compared to patients who discontinued abruptly (van Geffen 2005). In another study (n = 56) with paroxetine, participants who tapered their paroxetine over an average duration of 38.6 weeks (range 2 to 197 weeks), titrated to the individual, had a 6.1% incidence of withdrawal syndrome, compared with 78.2% for abrupt discontinuation (Murata 2010). Groot 2018 used tapering strips, allowing reductions in the antidepressant dose to small fractions of the minimum therapeutic dose. In this study, which included participants with median antidepressant use of two to five years, 71% of the 895 participants (97% of whom had experienced some withdrawal previously) discontinued their medication over a median of 56 days, with 68% still off their antidepressant after one to five years (Groot 2018Groot 2020). In contrast, randomised studies with a tapering period up to 14 days showed either no or minimal reduction in withdrawal symptom severity compared with abrupt discontinuation (Baldwin 2006; Bogetto 2002; Montgomery 2004a). In addition, review authors recommended hyperbolic antidepressant tapering regimens based on the relationship between SSRI dose and the serotonin transporter occupancy and on findings that hyperbolically reducing doses of SSRI reduces their effect on the transporter in a linear manner (Horowitz 2019). However, we are unable to compare our findings to these, as our review did not compare abrupt discontinuation to discontinuation by "tapering", and none of the studies used slow dose reduction over several months or liquid formulations or tapering strips.
An individual‐patient data meta‐analysis found evidence that MBCT compared to continuation of antidepressant treatment was associated with a reduction in the risk of relapse or recurrence, with a hazard ratio (HR) of 0.77 (95% confidence interval (CI) 0.66 to 0.98) over 60 weeks (4 studies) (Kuyken 2016). Our review could not confirm this significant effect at 60 weeks, possibly because we pooled studies with their original longer duration (e.g. longer than 60 weeks) (as defined in our protocol) and included a later published study (Bockting 2018).
Although we excluded studies with participants without depression and/or anxiety, we did identify two RCTs that assessed discontinuation of antidepressants among older people in nursing homes without a documented history of depression or anxiety (Bergh 2012; Ulfarson 2003). It is interesting that both studies concluded that discontinuation of long‐term antidepressants is feasible if patients are carefully monitored and are given the option of restarting if needed.
A previous review that did not specifically focus on long‐term use found that continuation of antidepressant treatment for anxiety disorders resulted in lower relapse rates among responders compared with treatment discontinuation (28 studies) (Batelaan 2017). However, as is the case for our review, withdrawal confounding bias could have resulted in overestimation of the effectiveness of antidepressants.
We identified one RCT that compared tapering with CBT to tapering without CBT for people with a remitted anxiety disorder. The antidepressant discontinuation rate was low: 37% of participants discontinued their antidepressant over 16 months with no difference between groups, and only 28% discontinued their antidepressant without recurrence (Scholten 2018). Our review included only four studies of participants with anxiety and therefore is unable to provide a clear conclusion on discontinuation in anxiety disorders.
Our review has been unable to demonstrate that the relapse rate in primary care is lower than in secondary care. An unpublished primary care placebo‐controlled discontinuation trial of maintenance SSRI treatment in New Zealand ‐ one of our ongoing studies ‐ found a recurrence rate of 23% in the taper arm over 18 months and 10% in the continuation arm, and 6% had to restart because of intolerable withdrawal symptoms despite tapering (Mangin 2015). Study authors concluded that the absolute benefit of SSRIs in preventing depression recurrence is much smaller than was previously estimated, with a number needed to treat for an additional beneficial outcome (NNTB) of 8 (18 months). We classified this study as ongoing due to incomplete data. We requested data (personal communication) but did not receive data prior to publication of this review.
In the STAR‐D trial, the largest naturalistic study of antidepressants so far conducted, the remission rate at one year for all participants taking antidepressants was 2.7% (108/4000), and 92.9% relapsed or dropped out (Pigott 2010). Although the validity of observational studies is limited and people with recurrent depression were over‐represented, the STAR‐D trial raises questions about the long‐term effects of antidepressants and suggests overestimation of their effects (Pigott 2015).
Guidelines recommend six months of "continuation" treatment after a first depression and "maintenance" treatment for two years or longer for people at high risk of relapse. These time points were based on the findings of the Geddes 2003 review, in spite of the fact that there was no association between relapse rate and duration of treatment in this review. It remains unclear how long antidepressant treatment has to be maintained after remission, as we did not find any difference between subgroups on the basis of total duration of antidepressant use, and this is consistent with the findings of other systematic reviews. Another systematic review found no evidence for treatment of a single episode for six to nine months, or for more than two episodes for longer than two years (Kaymaz 2008). In sum, the categories of treatment for one episode for six to nine months, or for more than two episodes for longer than two years, were based on consensus and were introduced into guidelines without evidence.
Authors' conclusions
Implications for practice.
We present five key messages.
Based on available evidence, it is not possible to confidently determine the effectiveness and safety of the approaches to discontinuing antidepressant medication. Relapse rather than discontinuation success was the primary outcome for the vast majority of studies eligible for inclusion in this review. In addition, most studies involved people with recurrent depression. Finally, the lack of distinction between withdrawal symptoms and relapse in the studies reviewed limits interpretation about the effectiveness and safety of approaches for stopping versus continuing long‐term antidepressants.
Most tapering regimens in the included studies were limited to four weeks or less, which makes them very similar to rapid discontinuation regimens. This may explain why we did not find a difference between abrupt discontinuation and (rapid) tapering. Clinicians should be aware of the distinction between withdrawal symptoms and relapse when reducing the dose of antidepressants, particularly in the early phases of reduction. Withdrawal symptoms are an indication to taper more slowly and do not imply that the underlying disorder has re‐emerged. However, a slow tapering regimen with very low doses over months or discontinuation with a changed pharmaceutical form (such as liquids or tapering strips) to allow low doses was not included in our review.
Available evidence is insufficient to underpin conclusions for the important group of people taking antidepressants in the community (i.e. those with only one or no prior episode of depression, those aged 65 years and older, or those taking antidepressants for anxiety).
As we did not find any differences between subgroups on the basis of total duration of antidepressant use in the comparison tapering versus continuation, and as we could not perform subgroup analyses based on duration for the other comparisons, it is unclear how long antidepressant treatment has to be maintained after remission. Guidelines recommend six months of "continuation" treatment after a first depression and "maintenance" treatment for two years or longer for people at high risk of relapse. However, these recommendations are based on consensus and were introduced into guidelines without evidence.
Several features distinguish withdrawal symptoms from relapse or recurrence, such as timing of onset (within days rather than weeks), a rapid response to reintroduction of the antidepressant, and the presence of somatic and psychological symptoms that are different from the original illness (e.g. shock‐like sensations, dizziness, pronounced insomnia). Yet, it remains difficult to distinguish relapse from withdrawal with certainty. Therefore, it can be helpful to measure patient progress with, for example, the Discontinuation‐Emergent Signs and Symptoms (DESS) Scale, during the discontinuation process. When the patient's DESS score returns to baseline, further reduction is appropriate. A simpler method may be to ask patients to compare the new symptoms they are experiencing versus the symptoms of the condition for which they were originally treated.
Implications for research.
We present six key messages for future research.
To prevent withdrawal confounding bias, future discontinuation trials should distinguish carefully between relapse and withdrawal symptoms. We suggest measuring withdrawal symptoms at the same time that relapse is measured and excluding people who have significant withdrawal symptoms from the category of relapse. Tapering should be very slow and tailored according to patient tolerability. Future research should assess (1) the incidence of withdrawal symptoms in patients tapering antidepressants, (2) identification of risk factors to better predict withdrawal symptoms, and (3) the relative advantages of different dose reduction regimens (Horowitz 2019Ruhe 2019).
Despite the growing de‐prescribing movement (Reeve 2017), only a few studies focused on de‐prescribing long‐term antidepressants have been undertaken. Systematic research into possible benefits and harms related to antidepressant discontinuation has been a low priority for both researchers and the pharmaceutical industry (Hengartner 2019). Future trials should determine the potential benefits and safety of de‐prescribing and should explore shared decision‐making. Future studies should systematically address key outcomes important for de‐prescribing, such as successful discontinuation rate, withdrawal symptoms, and adverse events (including rare events such as suicide). Furthermore, future studies should undertake long‐term follow‐up to evaluate person‐centred outcomes such as quality of life, including return to work and daily and social activities.
Future trials should include a wider representation of patient populations, for example, those with one or no prior episodes of depression, those with anxiety disorders, and older people including those who take multiple medications (polypharmacy) and those who are frail. Trials investigating people who have been treated with antidepressants for many years are also needed. Research suggests that most antidepressants are prescribed by GPs (Johnson 2017), reinforcing the urgency for more research in the primary care setting, particularly for people with low risk of relapse and those for whom there is uncertainty about the benefit of antidepressant treatment.
Psychological interventions such as CBT or MBCT may support antidepressant discontinuation and reduce the risk of relapse in people with recurrent depression. Other interventions based on less resource‐intensive and more accessible psychological approaches can reach more people and should be investigated (e.g. psycho‐education and good clinical management; telephone‐delivered counselling; online support; support from other health professionals such as nurses and social workers).
Systematic reviews have reported that barriers to and facilitators of discontinuing antidepressants are numerous and complex (Maund 2018: Scholten 2020). Further research to investigate the perspectives and attitudes of GPs, psychiatrists, other healthcare professionals (e.g. pharmacists, nurses, psychotherapists, social workers) and long‐term users would help build a more complete understanding of the issues and how they could be addressed.
Our review did not evaluate different discontinuation support strategies (e.g. studies that compare discontinuation with psychological support to discontinuation guided by practitioners). Further work is needed to synthesise the findings of available studies comparing different discontinuation strategies for people who discontinue their antidepressant.
History
Protocol first published: Issue 2, 2020 Review first published: Issue 4, 2021
Acknowledgements
We thank Mark Jones, biostatistician at the Institute for Evidence‐Based Healthcare, Bond University, Australia, for assistance with pooling and interpretation of hazard ratios.
We thank Peter Lucassen, GP and researcher, and Renier Akkermans, statistician, at Radboud University, Nijmegem, The Netherlands, for extracting the missing data of the Eveleigh 2018 study.
We thank Marloes Huijbers, psychologist and researcher at Radboud University, Nijmegem, The Netherlands, for extracting the missing data of the Huijbers 2016 study.
We thank the editorial team of Cochrane Common Mental Disorders (CCMD) for providing guidance. We developed the search strategies with Sarah Dawson, the CCMD Information Specialist.
The review authors and the CCMD Editorial Team are grateful to the peer reviewers for their time and comments, including Nuala Livingstone, Nick Meader, Andrew Moriarty, and Karen Morley. We would also like to thank Dolores Matthews from the copy edit team.
CRG funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of CCMD.
Disclaimer: the views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. Abbreviations
| BAI | Beck Anxiety Inventory |
| BDI | Beck Depression Inventory |
| BSI | Brief Symptom Inventory |
| CESD | Centre for Epidemiological Studies Depression Scale |
| CGI | Clinical Global Impression |
| IDS | Inventory of Depressive Symptomatology |
| DESS | Discontinuation‐Emergent Signs and Symptoms Scale |
| DSM‐V | Diagnostic and Statistical Manual of Mental Disorders |
| GAD‐7 | General Anxiety Disorder 7‐item |
| GAFS | Global Assessment of Functioning Score |
| HAM‐A | Hamilton Anxiety Scale |
| HDRS | Hamilton Depression Rating Scale |
| LSAS | Liebowitz Social Anxiety Scale |
| MADRS | Montgomery‐Åsberg Depression Rating Scale |
| NNTB | Number needed to treat for an additional beneficial outcome |
| NNTH | Number needed to treat for an additional harmful outcome |
| OFS | Occupational Functioning Scale |
| PAS | Panic and Agoraphobia Scale |
| PHQ | Patient Health Questionnaire |
| QALY | Quality‐Adjusted Life‐Years |
| SF‐12 | Short Form 36‐Item Health Survey |
| SF‐36 | Short Form 12‐item Health Survey |
| SSRI | Selective Serotonin Reuptake Inhibitors |
| TCA | Tricyclic Agent |
Appendix 2. Cochrane Common Mental Disorders' Controlled Trials Register (CCMD‐CTR)
Cochrane Common Mental Disorders (CCMD) maintains two archived clinical trials registers at its editorial base in York, UK: a references register and a studies‐based register. The CCMDCTR‐References Register contains over 40,000 reports of RCTs in depression, anxiety and neurosis. Approximately 50% of these references have been tagged to individual coded trials. The coded trials are held in the CCMDCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary; (please contact the CCMD Information Specialists for further details). Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to 2016), Embase (1974 to 2016) and PsycINFO (1967 to 2016); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registers via the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCMD's generic search strategies (used to identify RCTs) can be found on the Group's website, (cmd.cochrane.org/specialised-register), with an example of the core MEDLINE search (used to inform the register) listed below. The Group’s Specialised Register has fallen out‐of‐date with the Editorial Group’s move from Bristol to York in the summer of 2016.
Core search strategy used to inform the Cochrane Common Mental Disorders Group's Specialised Register: OVID MEDLINE (to June 2016) A weekly search alert based on condition + randomised controlled trial (RCT) filter only.
1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
At the time, records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were also tagged to the appropriate study record. Similar weekly search alerts were conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource. A quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL) was conducted c/o the Cochrane Register of Studies Online (CRSO).
************************************************************************************************
For this review, CCMD's information specialist searched the CCMD‐CTR (studies and references register) using the following terms:
CCMDCTR Studies and References Register (c/o Cochrane Register of studies (CRS‐Web)) (current to 14‐June‐2016)
#1 ((deprescrib* or "de prescrib*" or deprescrip* or "de prescrip*" or cease or cessation* or discontinu* or dropout or "drop out" or interrupt or interruption* or interrupting or taper* or reduce or "drug holiday" or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (antidepress* or anti‐depress* or ADM or mADM or psychotropic* or SSRI* or SNRI* or MAOI* or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA* or ((serotonin or monoamine oxidase or MAO) adj2 inhibitor*))) AND INSEGMENT
#2 (deprescription* or "drug withdrawal" or "inappropriate prescribing" or "treatment termination" or "withholding treatment"):EH,EMT,KW,MH AND INREGISTER
#3 (deprescrib* or "de prescrib*" or deprescrip* or "de prescrip*") AND INREGISTER
#4 "stop using" or "stop taking" or "stopping treatment" AND INREGISTER
#5 ((cease or cessation* or discontinu* or dropout or "drop out" or interrupt or interruption* or interrupting or taper* or reduce or "drug holiday" or stop or stopping or withdraw* or withhold* or terminat*)):EH,EMT,MH,KW AND INREGISTER
#6 ((long term or longterm) adj3 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*)) AND INREGISTER
#7 (#2 OR #3 OR #4 OR #5 OR #6)
#8 (("antidepress* agent*" or "antidepress* drug*" or "neurotransmitter uptake inhibitor*" or "monoamine oxidase inhibitor*" or "noradrenalin uptake inhibitor*" or "dopamine receptor affecting agent*" or "dopamine uptake inhibitor*" or "serotonin receptor affecting agent*" or "serotonin reuptake inhibitor*" or "serotonin uptake inhibitor*" or "serotonin norepinephrine reuptake inhibitor*" or "serotonin and noradrenaline reuptake Inhibitor*" or "adrenergic receptor affecting agent*" or "adrenergic uptake inhibitor*")):EH,EMT,MH,KW,KY AND INREGISTER
#9 ((psychotropic* or antidepress* or anti depress* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or MAOI* or ((serotonin or monoamine oxidase or MAO) adj2 inhibit*) or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA*)):EH,EMT,KW,KY,MH,TI AND INREGISTER
#10 ((Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or Dosulepin* or Dothiepin or Doxepin* or Duloxetine or "DVS 233" or Enilospirone or Eptapirone or Escitalopram or Etoperidone or Femoxetine or Fenelzine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine)):EH,EMT,KW,KY,MH,TI AND INREGISTER
#11 ((Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or “Lu AA21004” or Vortioxetine or “Lu AA24530” or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*)):EH,EMT,KW,KY,MH,TI AND INREGISTER
#12 ((Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Pertofrane or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or “5 Hydroxytryptophan” or “5 HT” or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Vortioxetine or Zalospirone or Zimeldine)):EH,EMT,KW,KY,MH,TI AND INREGISTER
#13 MESH DESCRIPTOR Antidepressive Agents EXPLODE ALL AND INREGISTER
#14 MESH DESCRIPTOR Neurotransmitter Uptake Inhibitors EXPLODE ALL AND INREGISTER
#15 MESH DESCRIPTOR Monoamine Oxidase Inhibitors EXPLODE ALL AND INREGISTER
#16 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 (#16 AND #7)
#18 (#17 OR #1)
***************************
Appendix 3. Other database searches
An information specialist conducted additional searches of the following databases, tailored to this review, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource. Searches were initially conducted in May 2019 and updated January 2020. The search of CENTRAL on the Cochrane Library was also updated in April 2020.
Cochrane Central Register of Controlled Trials (CENTRAL) via Wiley http://onlinelibrary.wiley.com/ Issue 5 of 12, May 2019; Issue 1 of 12, January 2020; Issue 4 of 12, April 2020 #1 ((deprescrib* or de next prescrib* or deprescrip* or de next prescrip* or cease or cessation* or discontinu* or dropout or drop next out or interrupt or interruption* or interrupting or taper* or reduce or drug next holiday or stop* next taking or stop* next using or stopping or withdraw* or withhold* or terminat*) near/4 (antidepress* or anti‐depress* or anti next depress* or ADM or mADM or psychotropic* or SSRI* or SNRI* or MAOI* or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA*)):ti,ab,kw #2 ((deprescrib* or de next prescrib* or deprescrip* or de next prescrip* or cease or cessation* or discontinu* or dropout or drop next out or interrupt or interruption* or interrupting or taper* or reduce or drug next holiday or stop* next taking or stop* next using or stopping or withdraw* or withhold* or terminat*) near/4 ((serotonin or monoamine next oxidase or MAO) near/2 inhibitor*)):ti,ab,kw #3 (#1 or #2) #4 MeSH descriptor: [Deprescriptions] this term only #5 MeSH descriptor: [Inappropriate Prescribing] this term only #6 MeSH descriptor: [Withholding Treatment] this term only #7 (deprescrib* or de next prescrib* or deprescrip* or de next prescrip*):ti,ab,kw #8 (stop next using or stop next taking or stopping next treatment):ti,ab,kw #9 (cease or cessation* or discontinu* or dropout or drop next out or interrupt or interruption* or interrupting or taper* or reduce or drug next holiday or stop or stopping or withdraw* or withhold* or terminat*):ti,kw #10 ((long next term or longterm) near/3 (cease or cessation* or discontinu* or dropout or drop next out or interrupt or interruption* or interrupting or taper* or reduce or drug next holiday or stop or stopping or withdraw* or withhold* or terminat*)):ab #11 {OR #4‐#10} #12 MeSH descriptor: [Antidepressive Agents] explode all trees #13 MeSH descriptor: [Neurotransmitter Uptake Inhibitors] explode all trees #14 MeSH descriptor: [Monoamine Oxidase Inhibitors] explode all trees #15 (psychotropic* or antidepress* or anti‐depress* or anti next depress* or noradrenerg* or antiadrenergic or anti next adrenergic or SSRI* or SNRI* or MAOI* or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA*):ti,kw #16 ((serotonin or norepinephrine or noradrenaline or nor next epinephrine or nor next adrenaline or neurotransmitt* or dopamine*) near/3 (uptake or reuptake or re next uptake)):ti,kw #17 ((serotonin or monoamine next oxidase or MAO) near/2 inhibit*):ti,kw #18 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or Dosulepin* or Dothiepin or Doxepin*or Duloxetine or “DVS 233” or Enilospirone or Eptapirone or Escitalopram or Etoperidone or Femoxetine or Fenelzine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine):ti,kw #19 (Harmaline or Harmine or Hyperforin or Hypericum or John* next Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*):ti,kw #20 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Pertofrane or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or “5 Hydroxytryptophan” or “5 HT” or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Vortioxetine or Zalospirone or Zimeldine):ti,kw #21 {OR #12‐#20} #22 (#11 and #21) #23 (#3 or #22) #24 MeSH descriptor: [Depression] this term only and with qualifier(s): [drug therapy ‐ DT] #25 MeSH descriptor: [Depressive Disorder] this term only and with qualifier(s): [drug therapy ‐ DT] #26 MeSH descriptor: [Depressive Disorder, Major] this term only and with qualifier(s): [drug therapy ‐ DT] #27 (#24 or #25 or #26) #28 (#11 and #27) #29 (#23 or #28) #30 (smoking or tobacco or nicotine or alcohol or substance):ti #31 (#29 not #30) #32 (smoking next cessation):ab,kw #33 (#31 not #32) in Trials In April 2020, the search of CENTRAL was appended to include the following terms:
#34 (continuation or maintenance):ti,ab,kw #35 ((continu* or maintain*) near/2 (antidepress* or drug* or therap* or treat* or medicat*)):ti,ab,kw #36 (remission or remitte* or responde* or recover*):ti,ab,kw #37 (#34 or #35 or #36) #38 (relaps* or recurr* or reoccurr* or re‐occurr* or reemerg* or re‐emerg* or ((new or repeat) next episode*)):ti,ab,kw #39 (#37 and #38 and #21) #40 (#39 not #33) #41 (#40 NOT (#30 OR #32)) *************************** MEDLINE(R) ALL via Ovid http://ovidsp.ovid.com/ 1946 to May 14, 2019 and January 16, 2020 Searched on: 15th May 2019 and 17th January 2020 1 ((deprescrib* or de prescrib* or deprescrip* or de prescrip* or cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (antidepress* or anti‐depress* or ADM or mADM or psychotropic* or SSRI* or SNRI* or MAOI* or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA* or ((serotonin or monoamine oxidase or MAO) adj2 inhibitor*))).ti,ab,kf. 2 deprescriptions/ 3 Inappropriate Prescribing/ 4 Withholding Treatment/ 5 (deprescrib* or de prescrib* or deprescrip* or de prescrip*).ti,ab,kf. 6 (stop using or stop taking or stopping treatment).ti,ab,kf. 7 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*).ti,kf,hw. 8 ((long term or longterm) adj3 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*)).ab. 9 (2 or 3 or 4 or 5 or 6 or 7 or 8) 10 exp Antidepressive Agents/ (144356) 11 exp Neurotransmitter Uptake Inhibitors/ 12 exp Monoamine Oxidase Inhibitors/ 13 (psychotropic* or antidepress* or anti depress* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or MAOI* or ((serotonin or monoamine oxidase or MAO) adj2 inhibit*) or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA*).ti,kf,hw. 14 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or Dosulepin* or Dothiepin or Doxepin* or Duloxetine or DVS 233 or Enilospirone or Eptapirone or Escitalopram or Etoperidone or Femoxetine or Fenelzine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine).ti,kf,hw. 15 (Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or Lu AA21004 or Vortioxetine or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*).ti,kf,hw. 16 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Pertofrane or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5 Hydroxytryptophan or 5 HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Vortioxetine or Zalospirone or Zimeldine).ti,kf,hw. 17 or/10‐16 18 9 and 17 19 (1 or 18) 20 controlled clinical trial.pt. 21 randomised controlled trial.pt. 22 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf. 23 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kf. 24 placebo*.ab,ti,kf. 25 trial.ab,ti,kf. 26 groups.ab. 27 (control* and (trial or study or group*) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,hw. 28 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf. 29 double‐blind method/ or random allocation/ or single‐blind method/ 30 or/20‐29 31 exp animals/ not humans.sh. 32 (30 not 31) 33 (19 and 32) 34 *Depression/ 35 *Depressive Disorder/ 36 *Depressive Disorder, Major/ 37 drug therapy.fs. 38 (34 or 35 or 36) 39 (37 and 38) 40 (9 and 32 and 39) 41 (33 or 40) 42 (smoking or tobacco or nicotine or alcohol or substance).ti. or smoking cessation.ab,kf,hw. 43 (41 not 42)
*************************** Embase via Ovid http://ovidsp.ovid.com/ 1974 to 2019 May 14 and 2020 January 16 Searched on: 15th May 2019 and and 17th January 2020 1 deprescription/ 2 inappropriate prescribing/ 3 treatment withdrawal/ 4 drug withdrawal/ 5 (deprescrib* or de prescrib* or deprescrip* or de prescrip*).ti,ab,kw. 6 (stop using or stop taking or stopping treatment).ti,ab,kw. 7 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*).ti,kw,hw. 8 ((long term or longterm) adj3 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*)).ab. 9 (1 or 2 or 3 or 4 or 5 or 6 or 7 or 8) 10 antidepressant agent/ 11 exp *antidepressant agent/ 12 *neurotransmitter uptake inhibitor/ 13 exp *monoamine oxidase inhibitor/ 14 *noradrenalin uptake inhibitor/ 15 *dopamine receptor affecting agent/ 16 *dopamine uptake inhibitor/ 17 *serotonin receptor affecting agent/ 18 *adrenergic receptor affecting agent/ 19 (psychotropic* or antidepress* or anti depress* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or MAOI* or ((serotonin or monoamine oxidase or MAO) adj2 inhibit*) or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA*).ti,kw. 20 or/10‐19 21 (9 and 20) 22 ((deprescrib* or de prescrib* or deprescrip* or de prescrip* or cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (antidepress* or anti‐depress* or ADM or mADM or psychotropic* or SSRI* or SNRI* or MAOI* or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA* or ((serotonin or monoamine oxidase or MAO) adj2 inhibitor*))).ti,ab,kw. 23 ((deprescrib* or de prescrib* or deprescrip* or de prescrip* or cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or Dosulepin* or Dothiepin or Doxepin* or Duloxetine or DVS 233 or Enilospirone or Eptapirone or Escitalopram or Etoperidone or Femoxetine or Fenelzine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine)).ti,ab,kw. 24 ((deprescrib* or de prescrib* or deprescrip* or de prescrip* or cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or Lu AA21004 or Vortioxetine or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*)).ti,ab,kw. 25 ((deprescrib* or de prescrib* or deprescrip* or de prescrip* or cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Pertofrane or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5 Hydroxytryptophan or 5 HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Vortioxetine or Zalospirone or Zimeldine)).ti,ab,kw. 26 or/22‐25 27 (21 or 26) 28 randomised controlled trial/ 29 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. 30 *clinical trial/ 31 trial.ti. 32 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. 33 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,kw. 34 randomization.de. 35 or/28‐34 36 ((animal or nonhuman) not (human and (animal or nonhuman))).de. 37 (35 not 36) 38 (27 and 37) 39 (smoking or tobacco or nicotine or alcohol or substance).ti. or smoking cessation.ab,kw,hw. 40 (38 not 39) *************************** PsycINFO via Ovid http://ovidsp.ovid.com/ 1806 to May Week 1 2019 and January Week 1 2020 Searched: 15 May 2019 and and 17th January 2020 1 ((deprescrib* or de prescrib* or deprescrip* or de prescrip* or cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or (stop* adj (taking or using)) or stopping or withdraw* or withhold* or terminat*) adj4 (antidepress* or anti‐depress* or ADM or mADM or psychotropic* or SSRI* or SNRI* or MAOI* or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA* or ((serotonin or monoamine oxidase or MAO) adj2 inhibitor*))).ti,ab,id. 2 treatment termination/ 3 treatment dropouts/ 4 drug withdrawal/ 5 (deprescrib* or de prescrib* or deprescrip* or de prescrip*).ti,ab,id. 6 (stop using or stop taking or stopping treatment).ti,ab,id. 7 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*).ti,id,hw. 8 ((long term or longterm) adj3 (cease or cessation* or discontinu* or dropout or drop out or interrupt or interruption* or interrupting or taper* or reduce or drug holiday or stop or stopping or withdraw* or withhold* or terminat*)).ab. 9 (2 or 3 or 4 or 5 or 6 or 7 or 8) 10 exp antidepressant drugs/ 11 exp neurotransmitter uptake inhibitors/ 12 exp monoamine oxidase inhibitors/ 13 exp tricyclic antidepressant drugs/ 14 exp serotonin reuptake inhibitors/ or exp serotonin norepinephrine reuptake inhibitors/ 15 psychopharmacology/ or neuropsychopharmacology/ 16 "3340".cc. 17 (psychotropic* or antidepress* or anti depress* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or MAOI* or ((serotonin or monoamine oxidase or MAO) adj2 inhibit*) or TCA* or tricyclic* or NARI or NARIs or NDIR* or SARI or SARIs or NaSSA*).ti,id,hw. 18 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or Dosulepin* or Dothiepin or Doxepin* or Duloxetine or DVS 233 or Enilospirone or Eptapirone or Escitalopram or Etoperidone or Femoxetine or Fenelzine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine).ti,id,hw. 19 (Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or Lu AA21004 or Vortioxetine or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*).ti,id,hw. 20 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Pertofrane or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5 Hydroxytryptophan or 5 HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Vortioxetine or Zalospirone or Zimeldine).ti,id,hw. 21 or/10‐20 22 (9 and 21) 23 (1 or 22) 24 clinical trials.sh. 25 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. 26 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,id. 27 (control* and (trial or study or group*) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. 28 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. 29 trial.ti,ab,id. 30 placebo.ti,ab,id,hw. 31 treatment outcome.md. 32 treatment effectiveness evaluation.sh. 33 mental health program evaluation.sh. 34 or/24‐33 35 (23 and 34) 36 (smoking or tobacco or nicotine or alcohol or substance).ti. or smoking cessation.ab,id,hw. 37 (35 not 36) *************************** Grey literature searches
Theses Databases Proquest Dissertations & Theses A&I via Proquest Inception to 21st January 2020 S1 ((TI,AB,IF,SU((single OR double OR triple OR treble) NEAR/2 (blind* OR mask* OR dummy)) OR TI,AB,IF,SU(control* NEAR/3 (trial OR study OR group*)) OR TI,AB,IF,SU(random* OR RCT OR placebo* OR trial*)) AND ((TI,AB,IF,SU(deprescrib* OR de‐prescrib* OR deprescrip* OR de‐prescrip*) OR TI,AB,IF,SU(cease OR cessation* OR discontinu* OR dropout OR drop‐out) OR TI,AB,IF,SU(interrupt OR interruption* OR interrupting OR taper*) OR TI,AB,IF,SU(reduce OR drug‐holiday OR stop* PRE/0 taking OR stop PRE/0 using) OR TI,AB,IF,SU(stopping OR withdraw* OR withhold* OR terminat*)) AND (TI,AB,IF,SU(antidepress* OR anti‐depress*) OR TI,AB,IF,SU(psychotropic* OR SSRI* OR SNRI* OR MAOI*) OR TI,AB,IF,SU(serotonin NEAR/2 inhibitor*) OR TI,AB,IF,SU(monoamine PRE/0 oxidase NEAR/2 inhibitor*) OR TI,AB,IF,SU(MAO NEAR/2 inhibitor*)))) NOT (TI(mouse OR mice OR rat OR rats OR trout) OR TI(smoking OR tobacco OR nicotine OR alcohol OR substance) OR TI,AB((smoking OR smoker*) PRE/0 cessation)) DART‐Europe E‐theses Portal http://www.dart‐europe.eu/ Searched on: 21st January 2020 Keywords = antidepress* AND (discontinu* OR taper* OR withdraw* OR deprescri*) Results rowsed for relevance Trial Registers ClinicalTrials.gov https://clinicaltrials.gov/ Searched on: 17th January 2020 1. (antidepressant OR antidepressive) AND (discontinue OR discontinuation OR discontinued OR discontinues OR discontinuing) | Depression OR depressive 2. (antidepressant OR antidepressive) AND (taper OR tapers OR tapered OR tapering) | Depression OR depressive 3. (antidepressant OR antidepressive) AND (withdraw OR withdraws OR withdrawn OR withdrawal OR withdrawing) | Depression OR depressive 4. (antidepressant OR antidepressive) AND (discontinue OR discontinuation OR discontinued OR discontinues OR discontinuing) | anxiety OR phobia OR panic 5. (antidepressant OR antidepressive) AND (taper OR tapers OR tapered OR tapering) | anxiety OR phobia OR panic 6. (antidepressant OR antidepressive) AND (withdraw OR withdraws OR withdrawn OR withdrawal OR withdrawing) | anxiety OR phobia OR panic 7. (antidepressant OR antidepressive) AND (deprescribe OR deprescribed OR deprescribing OR de‐prescribe OR de‐prescribed OR de‐prescribing) 8. (antidepressant OR antidepressive) AND (deprescription OR deprescriptions OR de‐prescription OR de‐prescriptions) WHO International Clinical Trials Registry Platform (ICTRP) https://apps.who.int/trialsearch/ Searched on: 17th January 2020 1. Advanced search Recruitment status: ALL Condition field: Depression OR depressive Intervention field: (discontinue OR discontinuation OR discontinued OR discontinues OR discontinuing) (no synonyms) 2. Advanced search Recruitment status: ALL Condition field: Depression OR depressive Intervention field: (taper OR tapers OR tapered OR tapering) 3. Advanced search Recruitment status: ALL Condition field: Depression OR depressive Intervention field: (withdraw OR withdraws OR withdrawn OR withdrawal OR withdrawing) 4. Advanced search Recruitment status: ALL Condition field: Depression OR depressive Intervention field: (deprescribe OR deprescribed OR deprescribing OR de‐prescribe OR de‐prescribed OR de‐prescribing) 5. Advanced search Recruitment status: ALL Condition field: Depression OR depressive Intervention field: (deprescription OR deprescriptions OR de‐prescription OR de‐prescriptions) 6. Advanced search Recruitment status: ALL Condition field: anxiety OR phobia OR panic Intervention field: discontinue OR discontinuation OR discontinued OR discontinues OR discontinuing (no synonyms) 7. Advanced search Recruitment status: ALL Condition field: anxiety OR phobia OR panic Intervention field: taper OR tapers OR tapered OR tapering 8. Advanced search Recruitment status: ALL Condition field: anxiety OR phobia OR panic Intervention field: (withdraw OR withdraws OR withdrawn OR withdrawal OR withdrawing) 9. Advanced search Recruitment status: ALL Condition field: anxiety OR phobia OR panic Intervention field: (deprescribe OR deprescribed OR deprescribing OR de‐prescribe OR de‐prescribed OR de‐prescribing) 10. Advanced search Recruitment status: ALL Condition field: anxiety OR phobia OR panic Intervention field: (deprescription OR deprescriptions OR de‐prescription OR de‐prescriptions)
***************************
Data and analyses
Comparison 1. Abrupt discontinuation versus continuation of antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Relapse (as defined by study authors) | 11 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 All studies | 11 | 1555 | Hazard Ratio (IV, Random, 95% CI) | 1.97 [1.56, 2.50] |
| 1.1.2 No co‐intervention follow‐up > 24 weeks | 10 | 1373 | Hazard Ratio (IV, Random, 95% CI) | 2.09 [1.59, 2.74] |
| 1.1.3 With co‐intervention follow‐up > 24 weeks | 2 | 182 | Hazard Ratio (IV, Random, 95% CI) | 1.48 [0.93, 2.34] |
| 1.2 Adverse events | 7 | 1012 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.62, 1.99] |
| 1.3 Depressive symptoms | 3 | 330 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.12, 2.00] |
| 1.3.1 HAM‐D total score endpoint | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 3.40 [0.42, 6.38] |
| 1.3.2 HAM‐D mean change | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐1.54, 1.32] |
| 1.4 Severity of illness | 5 | 714 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.13, 0.49] |
| 1.4.1 CGI‐S total score | 2 | 343 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.27, 0.93] |
| 1.4.2 CGI‐S mean change | 3 | 371 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.05, 0.43] |
Comparison 2. Discontinuation by tapering versus continuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Relapse (as defined by study authors) | 16 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 All studies | 16 | 2120 | Hazard Ratio (IV, Random, 95% CI) | 2.59 [2.07, 3.25] |
| 2.1.2 No co‐intervention long‐term follow‐up (≥ 24 weeks) | 13 | 1546 | Hazard Ratio (IV, Random, 95% CI) | 2.97 [2.24, 3.93] |
| 2.1.3 With co‐intervention | 4 | 570 | Hazard Ratio (IV, Random, 95% CI) | 1.90 [1.42, 2.53] |
| 2.2 Adverse events | 7 | 1479 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.82, 1.38] |
| 2.3 Depressive symptoms | 6 | 1017 | Mean Difference (IV, Random, 95% CI) | 3.50 [2.31, 4.68] |
| 2.3.1 HAM‐D endpoint (≥ 24 weeks) | 5 | 730 | Mean Difference (IV, Random, 95% CI) | 3.83 [2.20, 5.46] |
| 2.3.2 HAM‐D mean changes (≥ 24 weeks) | 1 | 287 | Mean Difference (IV, Random, 95% CI) | 2.96 [1.43, 4.49] |
| 2.4 Anxiety symptoms | 3 | 526 | Mean Difference (IV, Random, 95% CI) | 3.53 [1.92, 5.14] |
| 2.5 Quality of life | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Physical health functioning | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐5.66, 1.49] |
| 2.5.2 Social functioning | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐6.44 [‐12.10, ‐0.77] |
| 2.5.3 Emotional functioning | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐18.81 [‐26.66, ‐10.97] |
| 2.6 Social and occupational functioning | 3 | 502 | Mean Difference (IV, Random, 95% CI) | 0.19 [0.11, 0.28] |
| 2.7 Severity of illness | 6 | 1187 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.44, 0.79] |
| 2.7.1 CGI‐S total score | 5 | 900 | Mean Difference (IV, Random, 95% CI) | 0.63 [0.40, 0.85] |
| 2.7.2 CGI‐S mean change | 1 | 287 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.32, 0.88] |
Comparison 3. Discontinuation with high‐intensity psychological interventions versus continuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Relapse (as defined by study authors) | 3 | 690 | Hazard Ratio (IV, Random, 95% CI) | 0.89 [0.66, 1.19] |
| 3.2 Depressive symptoms | 2 | 484 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.82, 0.98] |
| 3.2.1 HAM‐D endpoint | 2 | 484 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.82, 0.98] |
| 3.3 Quality of life | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 Quality of life, physical health domain, long‐term follow‐up | 2 | 455 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐2.16, 1.73] |
| 3.3.2 Quality of life, psychological health domain, long‐term follow‐up | 2 | 455 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.75, 1.49] |
| 3.3.3 Quality of life, social relationships domain, long‐term follow‐up | 2 | 455 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.56, 0.66] |
Comparison 4. Subgroup age, abrupt discontinuation versus continuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Relapse (HR) | 10 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 No co‐intervention follow‐up > 24 weeks | 10 | Hazard Ratio (IV, Random, 95% CI) | 2.09 [1.59, 2.74] | |
| 4.1.2 Subgroup age < 65 years | 3 | Hazard Ratio (IV, Random, 95% CI) | 1.72 [0.97, 3.05] | |
| 4.1.3 Subgroup age ≥ 65 years | 2 | Hazard Ratio (IV, Random, 95% CI) | 2.09 [0.95, 4.60] |
Comparison 5. Subgroup indication, tapering versus continuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Relapse (HR) | 13 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 No co‐intervention long‐term follow‐up (≥ 24 weeks) | 13 | Hazard Ratio (IV, Random, 95% CI) | 2.97 [2.24, 3.93] | |
| 5.1.2 Subgroup Indication ‐ depressive disorder | 10 | Hazard Ratio (IV, Random, 95% CI) | 2.62 [2.01, 3.42] | |
| 5.1.3 Subgroup Indication‐ anxiety disorder | 3 | Hazard Ratio (IV, Random, 95% CI) | 7.06 [3.22, 15.52] |
Comparison 6. Subgroup type of antidepressant, tapering versus continuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Relapse | 11 | Odds Ratio (IV, Random, 95% CI) | 3.04 [2.12, 4.34] | |
| 6.1.1 Subgroup type of AD ‐ TCA | 6 | Odds Ratio (IV, Random, 95% CI) | 3.69 [1.67, 8.13] | |
| 6.1.2 Subgroup type of AD ‐ SNRI | 5 | Odds Ratio (IV, Random, 95% CI) | 2.69 [1.85, 3.91] |
Comparison 7. Subgroup duration of antidepressant, tapering versus continuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Relapse | 11 | Odds Ratio (IV, Random, 95% CI) | 2.98 [2.19, 4.06] | |
| 7.1.1 Subgroup duration of antidepressant: ≥ 52 weeks | 3 | Odds Ratio (IV, Random, 95% CI) | 4.32 [1.97, 9.48] | |
| 7.1.2 Subgroup duration of antidepressant < 52 weeks | 8 | Odds Ratio (IV, Random, 95% CI) | 2.91 [2.06, 4.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bialos 1982.
| Study characteristics | ||
| Methods |
Design: RCT Prerandomisation phase: no Duration post randomisation: 24 weeks Aim: "what would happen to these patients taking tricyclic antidepressants for more than 24 months if the medication were discontinued under placebo‐controlled, double‐blind conditions" |
|
| Participants |
Country: USA
Setting: community adults (outpatients from mental hygiene clinic of West Haven Veterans Administration Medical Center)
Type of AD: amitriptyline Duration of antidepressant treatment prerandomisation: 3.7 years (0.5 to 8) Duration of antidepressant treatment post stabilisation: not reported Total number of randomised participants: 19 (10 placebo, 7 antidepressant) (2 discontinued, but no further information) Primary diagnosis: major depression (research diagnostic criteria) Severity of depressive symptoms at randomisation, mean (SD) HDRS 14.1 (not reported), placebo 15.6 (8.6), antidepressant not reported Raskin 6.6 (not reported), placebo 6.8 (1.7), antidepressant not reported Note: "although clinically stable, they experienced mild to moderate continuing depressive symptoms and anticholinergic side effects. Residual depressive symptoms may either be characteristic of these patients (dysthymia) or secondary to their being at the lower end of the recommended therapeutic range of amitriptyline dosage and plasma levels" Average age of onset of depressive disorder: 44.5 years (range 24 to 60 years) Comorbidity: anxiety neurosis 47%, alcohol problems 12%, 24% alcohol abuse in the past. Only 4 patients were free of medical illness Common problems were diabetes (n = 5; 29%), arthritis (n = 4; 24%), hypertension (n = 3; 18%), and gastrointestinal disorders (n = 2; 12%) Gender distribution (F): 3 (17%) of the completers Mean age, years (SD): 57.3 years (not reported), range 41 to 71 years Definition of remission: not described Inclusion criteria Subjects were considered clinically stable with a history of major depressive disorder and duration of long‐term antidepressant greater than 24 months Exclusion criteria Not reported |
|
| Interventions |
Intervention 1: placebo Tapering scheme: tapering in 3 weekly decrements to placebo Intervention 2: continuation of the same dose of amitriptyline. The night‐time daily dose was 138 mg (range 50 to 250 mg) Co‐intervention: in both groups allowed: individual or group psychosocial treatment, unchanged throughout the study |
|
| Outcomes | Completion of the 6‐month protocol or the appearance of a depressive episode as decided upon by the patient and the research clinician
Depressive symptoms measured by Hamilton total score and the 6 individual items (depression; early, middle, and late insomnia; retardation, diurnal variation), Raskin assessed by research clinician Weissman Social Adjustment Scale and Profile of Mood States at baseline, assessed by participants Adverse events measured by treatment‐emergent symptoms (TESs) (insomnia, autonomic anticholinergic) Assessed every 2 weeks for the first month, then every 4 weeks up to 6 months |
|
| Notes |
Funding: not described; medication was provided by pharmaceutical industry COI: not described Note: all subjects were considered clinically stable and had been taking amitriptyline for an average of 3.7 years (range 0.5 to 8 years) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not enough information provided. Quote: "the subjects were randomly divided into two groups, active medication and placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information provided Quote: "identical placebo tablets" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: study is described as double‐blinded, and blinding appeared to be maintained Quote: "double‐blind conditions"; "a final evaluation was completed, after which the code was broken to determine whether the patient was taking active medication or placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: study is described as double‐blinded, and blinding appeared to be maintained Quote: "the research clinician, who was blind to the patient's medication status" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2 participants (10.5%) withdrew before completion of study. Not clear what group they were randomised to. Analysis was done on 17 completing study rather than on 19 randomised Quote: "nineteen patients started the study, but 2 stopped within the first 3 weeks. One discontinued medication on his own and the other developed a serious medical illness"; "the entire group (N = 17) was ...." |
| Selective reporting (reporting bias) | High risk | Comment: protocol not available. Hamilton and Raskin scores reported only for total (N = 17) participants and placebo (N = 10) participants. Not reported for active (N = 7) participants. Outcomes measured by Weissman's Social Adjustment Scale and Profile of Mood States were not reported. Adverse events were measured and reported. Study authors described withdrawal symptoms among participants who discontinued the antidepressant but reported no data Quote: "at baseline and end point there were no statistically significant differences on any scale scores for subjects receiving active medication..." |
| Other bias | Unclear risk | Comment: funding not described |
Bockting 2018.
| Study characteristics | ||
| Methods |
Design: multi‐centre single‐blind 3‐arm randomised controlled trial Prerandomisation phase: no Duration post randomisation: 104 weeks Aim: to compare the effectiveness of antidepressants alone with preventive cognitive therapy (PCT) while tapering off antidepressants and with PCT added to antidepressants in prevention of relapse and recurrence |
|
| Participants |
Country: Netherlands Setting: community adults recruited via general practitioners (GPs), pharmacists, secondary mental health care and media, or other means Type of antidepressant: SSRI (≥ 80%), other: SNRI, TCA, atypical AD, MAOI, or more than 1 Duration of antidepressant treatment prerandomisation: at least 24 weeks Duration of antidepressant treatment post stabilisation: in remission for at least 8 weeks and no longer than 104 weeks Primary diagnosis: recurrent major depressive disorder Total number of randomised participants: 289 (85 in PCT + discontinuation, 100 antidepressant alone, 104 antidepressants, and PCT) → discontinuation group was split to allow multiple‐arm comparison in the analysis: 43 → 143 participants for comparison PCT + discontinuation compared to AD and 146 participants for comparison PCT and tapering vs PCT and AD continuation Number of previous depressive episodes, median (IQR): 5 (3 to 6) PCT + discontinuation, 4 (3 to 6) antidepressants, 5 (3 to 6) antidepressant + PCT Psychological or psychotherapeutic treatment at randomisation: 16/85 (19%) PCT + discontinuation, 18/99 (18%) antidepressant, 21/104 (20%) antidepressant + PCT Severity of depressive symptoms at randomisation (HDRS score), mean (SD): 3.6 (3.0) PCT + discontinuation; 3.8 (3.1) antidepressant; 3.6 (3.1) PCT + antidepressant Gender distribution (F): 62% PCT + discontinuation, 64% antidepressant, 69% PCT + antidepressant Mean age, years (SD): 47.7 (11.1) PCT + discontinuation, 47.2 (10.5) antidepressant, 47.0 (9.3) PCT + antidepressant Inclusion criteria ‐ ≥ 2 previous depressive episodes (DSM‐IV‐criteria) in the past 5 years ‐ in remission (according to DSM‐IV criteria) for > 2 months and ≤ 2 years ‐ recovery had to have been achieved with acute antidepressant treatment ‐ remitted on antidepressant treatment and use of antidepressant (delivered in primary or secondary care) ≥ 6 months Definition of response/remission: current score ≤ 10 on HDRS 17 Exclusion criteria ‐ current mania or hypomania ‐ history of bipolar disorder ‐ any history of psychosis, including major depressive episode with psychotic features ‐ current alcohol or drug abuse ‐ predominant anxiety disorder ‐ receiving psychological treatment more than twice a month ‐ diagnosis of organic brain damage |
|
| Interventions |
Intervention 1: preventive cognitive therapy (PCT) combined with tapering antidepressants (no placebo) Description: PCT, based on a treatment manual, comprised 8 weekly group or individual sessions delivered by therapists. GPs and psychiatrists were advised to taper antidepressants over a period of 4 weeks. The GP or psychiatrist and participant received a letter with instructions to guide tapering and a tapering schedule per type of drug. Participants were asked for an intention to taper antidepressants and were allowed to restart antidepressants at any time, which was monitored. Doses of antidepressants were assessed in all participants with the Trimbos and Institute for Medical Technology Assessment questionnaire on costs associated with psychiatric illness. Participants in the tapering group were also monitored on their progress via telephone by an independent researcher. For all antidepressants, equivalent doses in mg of fluoxetine were computed Providers: therapists were psychologists fully trained in cognitive‐behavioural therapy who received an additional 16 hours of training specific to this study Integrity of delivery: to maintain treatment integrity, therapists followed a PCT manual and were supervised by a fully trained cognitive‐behavioural therapist or a licensed psychologist Adherence to PCT (i.e. completing ≥ 5 sessions): 90% Tapering scheme: 4 weeks; GP and psychiatrists received a tapering schedule per type of drug. Most (60%) individuals tapered antidepressants with their doctors over 6 months, indicating that a time frame of 4 weeks was not considered feasible for many individuals Intervention 2: continuation of antidepressant treatment Description: GPs and psychiatrists were advised to continue guidance and prescription of antidepressants at minimally required adequate doses or higher (≥ 20 mg fluoxetine equivalent), as recommended by guidelines. Participants were encouraged to use antidepressants as prescribed, and GPs and psychiatrists were encouraged to prescribe therapeutic doses and to discuss problems with adherence Intervention 3: antidepressant treatment and preventive cognitive therapy (PCT) Description: GPs and psychiatrists were advised to continue guidance and prescription of antidepressants at minimally required adequate doses or higher (≥ 20 mg fluoxetine equivalent), as recommended by guidelines. Participants were encouraged to use antidepressants as prescribed Co‐intervention: 18 in the antidepressants alone group, 16 in the PCT with tapering of antidepressants group, 21 in the PCT and antidepressants group received additional psychological or psychotherapeutic treatment. Two participants in each group received inpatient treatment |
|
| Outcomes |
Primary ‐ time to recurrence ‐ remission after 3, 9, 15, and 24 months using DSM‐IV‐TR criteria assessed with SCID‐I, including retrospective parts and information from monthly ratings on the Inventory of Depressive Symptomatology–Self‐Report (IDS‐SR) Definition of recurrence/relapse: DSM‐IV criteria assessed with SCID‐I, including retrospective parts and information from monthly ratings on the IDS‐SR Secondary ‐ number of major depressive episodes (assessed with SCID‐I), severity of the last major depressive episode (assessed with SCID‐I), and level of residual symptoms (assessed with HDRS), median duration of recurrence ‐ cost‐effectiveness and quality‐adjusted life‐years (EQ‐5D every 3 months) ‐ suspected serious adverse events |
|
| Notes |
Funding: The Netherlands Association for Health Research and Development (ZONMW) and The Netherlands Organisation for Scientific Research Conflicts of interest: first author is a member of the scientific advisory board of the National Insure Institute, for which she receives an honorarium, although this role has no direct relation to this study. CLHB has presented keynote addresses at conferences such as the European Psychiatry Association and the European Conference Association, for which she sometimes receives an honorarium. She has presented clinical training workshops, some of which include a fee. CLHB receives royalties from her books and co‐edited books, and she developed PCT on the basis of the cognitive model of AT Beck. One co‐author has received fees from several pharmaceutical companies and grants from The Netherlands Organisation for Health Research and Development and the European Union. Provider of study drugs is not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was performed by using a computer random number generator Quote: "...the automated permuted‐block randomisation using a computer‐generated random numbers with a predefined allocation ratio of 10:10:8 to PCT and antidepressants, antidepressants alone and PCT while tapering off antidepressants" |
| Allocation concealment (selection bias) | Low risk | Comment: an independent research assistant was responsible for allocation (central allocation) Quote: "an independent research assistant masked to the randomisation sequence entered the stratification characteristics and implemented the automated permuted‐block randomisation..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: study is described as single‐blind; participants and physicians were not blinded, and the outcome is likely to be influenced by lack of blinding Quote: "participants and physicians were aware of treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comments: study is described as single‐blind and it is unlikely that blinding could have been broken Quote: "trained assessors masked to treatment allocation did all subsequent follow‐up assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: rates and reasons for dropouts were described in the flow chart. Dropouts were low and were similar in both groups (3/85 discontinuation, 4/100 antidepressant). Primary analysis was done by intention‐to‐treatment principle Quote: "primary analyses were done by intention to treat"; "we also did two per‐protocol analyses"; "in 43 (23%) of 189 participants assigned to PCT, the intervention was not started and follow‐up data were not available because the travel time was too long to visit the PCT group or the group sessions were planned at a time they could not attend ... In addition to these 43 participants, 16 in the PCT groups and 21 in the antidepressants alone group dropped out for other reasons" |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is available, and all of the study’s pre‐specified (primary and secondary) outcomes have been reported in the pre‐specified way. Suspected serious adverse events were recorded and were reported to the ethics committee, although they were not mentioned in the report. Withdrawal symptoms were not an outcome |
| Other bias | Low risk | Comment: none |
Cook 1986.
| Study characteristics | ||
| Methods |
Design: double‐blind randomised placebo‐controlled trial Prerandomisation phase: no Duration post randomisation: 28 weeks Aim: to evaluate the rate of relapse following discontinuation of antidepressant therapy in elderly depressives |
|
| Participants |
Country: USA Setting: community adults (outpatients from Iowa City VA Geriatric Psychiatry Clinic) Type of antidepressant: TCA (desipramine, amitriptyline, doxepine, imipramine) Duration of antidepressant treatment prerandomisation: from 12 months to 192 months Duration of antidepressant treatment post stabilisation: from 12 months to 96 months Total number of randomised participants: 18 (9 placebo, 6 antidepressant) Primary diagnosis: major depression disorder with at least 1 previous episode Number of previous depressive episodes: from 1 to "too many to count" placebo, from 1 to "too many to count" antidepressants Severity of depressive symptoms at randomisation (HDRS score), mean (SD): 5.1 (3.9) placebo, 4.8 (3.5) antidepressant (at week 4, before discontinuation) Gender distribution (F): 0% Mean age, years: 63.2 Inclusion criteria ‐ chart diagnosis of major depressive disorder (Research Diagnostic Criteria) ‐ had been treated with a TCA for a year or longer without evidence of recurrence of depressive symptoms warranting a change in therapy ‐ patients interviewed using SADS‐L and only those who met Research Diagnostic Criteria for unipolar depression were included Definition of remission: use of AD without evidence of recurrence of depressive symptoms warranting a change in therapy Exclusion criteria ‐ not reported |
|
| Interventions |
Intervention 1: tapering to placebo Tapering scheme: gradually over either 4 (4 persons) or 8 (5 persons) weeks. Intervention 2: continuation of the same dose of active medication |
|
| Outcomes | Recurrence, monthly measured or until recurrence Definition of relapse/recurrence: investigator’s clinical assessment that change in pharmacological treatment was indicated Change from baseline in depressive symptoms, measured by Hamilton Depression Rating Scale (HDRS), Montgomery Asberg Depression Rating Scale (MADRS), Carroll Depression Scale (Carroll) Adverse events, measured by Treatment‐Emergent Symptoms Scale (TESS) |
|
| Notes |
Funding: not described COI: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to judge Quote: “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and key study personnel ensured; unlikely that blinding could have been broken Quote: “the study was conducted under double‐ blind conditions”; " ... identical appearing placebo or active medication..."; "...reoccurrence was assessed by the psychiatrist (B.L.C.) blind to whether the patient was on placebo or active medication" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment ensured; unlikely that blinding could have been broken Quote: "reoccurrence was defined as the blind investigator’s clinical assessment that change in pharmacologic treatment was indicated" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3/18 withdrew before randomisation but reasons clearly stated |
| Selective reporting (reporting bias) | High risk | Comment: study protocol not available, although given the age of this study, protocols might not have been standard practice at that time. Study authors report that they assessed participants on the Treatment‐Emergent Symptoms Scale, yet adverse events were reported incompletely. Withdrawal symptoms were not an outcome |
| Other bias | Unclear risk | Source of funding not reported |
Derubeis 2019.
| Study characteristics | ||
| Methods |
Design: multi‐phase randomised controlled trial Prerandomisation phases Phase 1a: acute treatment with antidepressants (and any of the augmenting or adjunctive agents) with and without cognitive‐behavioural therapy (CBT) lasted until the patient met the criteria for remission (at least 4 consecutive weeks of minimal symptoms) (up to 19 months of treatment was allowed for remission). Results of phase 1 were reported in Hollon 2016 Phase 1b: continuation treatment with antidepressants (and any of the augmenting or adjunctive agents) with and without CBT lasted until the patient met the criteria for recovery (another 26 consecutive weeks without relapse) (up to 42 months for recovery was allowed) Duration post randomisation: 156 weeks Aim: to determine the effects of combining CBT with antidepressants and antidepressants alone for prevention of depressive recurrence when antidepressants were withdrawn or maintained after recovery in patients with major depressive disorder |
|
| Participants |
Country: USA Setting: community adults (3 outpatient research clinics) Type of AD: 4 different classes of antidepressants (sertraline, venlafaxine, 2 others not reported) Duration of antidepressant treatment before randomisation: up to 42 months, mean 80.3 (40.0) weeks (reported for the combination and antidepressants alone study arms; data not separately reported for each study arm) + mean (SD) number of CBT sessions in the combination group during phase 1 was 33.3 (22.8) Duration of antidepressant treatment post stabilisation: minimum 30 weeks Number of participants: participants with CBT: 227 randomised to phase 1, 170 responded to phase 1, 155 randomised to RCT; participants without CBT: 225 randomised to phase 1, 148 responded to phase 1, 137 randomised to RCT Total number of randomised participants: 155 participants with CBT (70 discontinuation, 85 antidepressant), 137 participants without CBT (69 discontinuation, 68 antidepressant) Primary diagnosis ‐ in participants with CBT: chronic major depressive disorder: 35% discontinuation, 31% antidepressant; recurrent depression: 84% discontinuation, 89% antidepressant ‐ in participants without CBT: chronic major depressive disorder: 34% discontinuation, 50% antidepressant; recurrent depression: 88% discontinuation, 82% antidepressant previous episodes Severity of depressive symptoms at randomisation (HDRS), mean (SD): participants with PCT: discontinuation 5.4 (3.9), antidepressant 5.8 (4.0); participants without PCT: discontinuation 6.0 (4.4), antidepressant 5.4 (4.0) Gender distribution (F): participants with PCT: discontinuation 59%, continuation 56%; participants without PCT: discontinuation 62%, continuation 37% Mean age, years (SD): participants with PCT: discontinuation 43.9 (11.8), antidepressant 45.6 (13.0); participants without PCT: discontinuation 43.9 (11.8), antidepressant 45.6 (13.0) Inclusion criteria ‐ DSM‐IV major depressive disorder (MDD) either chronic (episode duration ≥ 2 years) or recurrent (with an episode in the past 3 years if only the second episode) ‐ 17‐item Hamilton Rating Scale for Depression (HDRS) score ≥ 14 ‐ ≥ 18 years of age at entry to phase 1 ‐ English speaking ‐ willing and able to provide informed consent Patients were previously randomised in phase 1 of the clinical trial, which compared patients who received antidepressant treatment with patients who received antidepressant treatment in combination with PCT. Patients from phase 1 were eligible to participate in phase 2 if they met the criteria for recovery before the maximum allowable time (3.5 years) Response/remission criteria: 4 consecutive weeks of LIFE Problem Symptom Rating (PSR) Scale values ≤ 2 and HDRS scores ≤ 8 for 4 consecutive weeks (with partial remission defined as LIFE PSR values ≤ 3 and HDRS scores ≤ 12 after month 12 only) Recovery criteria: 6 consecutive months following remission without relapse (2 weeks of elevated LIFE PSR scores ≥ 4 and HDRS scores ≥ 14) Exclusion criteria ‐ history of bipolar disorder or non‐affective psychosis ‐ substance dependence in the past 3 months ‐ DSM‐IV Axis I disorders requiring non‐protocol treatment ‐ DSM‐IV Axis II disorders poorly suited to study treatments (antisocial, borderline, and schizotypal) ‐ suicide risk requiring immediate hospitalisation ‐ medical condition precluding the use of study medications (including pregnancy) ‐ current medications that induce depression ‐ mandated treatment or compensation issues |
|
| Interventions |
Intervention 1: discontinuation of antidepressant (without placebo) and discontinuation of CBT Tapering scheme: 4‐week period or longer if clinically indicated Intervention 2: continuation of antidepressant (adjustment or augmentation of the medication regimen was permitted) and discontinuation of CBT Co‐intervention: participants in both treatment groups received CBT sessions 2x weekly for at least the first 2 weeks, at least weekly thereafter during acute treatment, and then at least monthly during continuation phase and delivered by therapist. Therapists were free to vary the frequency to meet the needs of patients. Sessions were based on a treatment manual for CT of depression augmented when indicated for participants with comorbid Axis II disorders. CBT ended before entry to the discontinuation trial |
|
| Outcomes |
Primary outcomes ‐ estimates of the recurrence of depression. Recurrence of depression was measured by the Longitudinal Interval Follow‐up Evaluation (LIFE) (every 4 weeks for the first 12 weeks and every 12 weeks thereafter); the LIFE tool measures psychiatric status rating on a scale of 1 to 6, with score of 5 or 6 indicating the patient met DSM‐IV symptom criteria for MDD that week Definition of relapse/recurrence ‐ 3 consecutive weeks with LIFE rating of 5 or 6 during the first 8 weeks ‐ LIFE rating of 5 or 6 for 2 consecutive weeks at any time after the first 8 weeks Secondary outcome Serious adverse events Results were separated for participants with and without PCT |
|
| Notes |
Funding: study was supported by 3 grants from the National Institute of Mental Health; 2 pharmaceutical companies provided sertraline and venlafaxine for the trial COI: 2 study authors received grants from the pharmaceutical industry Note: a principle‐based algorithm was implemented that could involve up to 4 different classes of ADMs and any of the augmenting or adjunctive agents commonly used in clinical practice. Study authors did not report the 4 different classes nor the augmenting agents |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a random component is used Quote: "random assignment to treatment was implemented using an adaptive (urn) randomisation procedure"; "adaptive randomisation algorithms for phase 2 assignment were..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: medication withdrawn was not accompanied by the use of placebo. Due to the nature of the trial (monotherapy vs combination therapy), blinding would not be possible for patients and providers Quote: "because medication withdrawal was not accompanied by the use of placebo, phase 1 assignments were not blinded for patients or pharmacotherapists" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment ensured; likely that blinding was successful Quote: "interviewers who were blinded to the patients’ treatment conditions assessed patient status using the LIFE ..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout rates different across groups (28.5% discontinuation group, 35.5% antidepressant group); reasons for dropout not accounted for |
| Selective reporting (reporting bias) | High risk | Comment: study protocol is available; predefined outcomes were not reported (types of serious adverse events). Other adverse events and withdrawal symptoms were not an outcome |
| Other bias | Unclear risk | Comment: grant from pharmaceutical industry |
Eveleigh 2018.
| Study characteristics | ||
| Methods |
Design: cluster‐randomised controlled trial Prerandomisation phase: no Duration post randomisation: 52 weeks Unit of clustering: general practice Aim: to assess the effectiveness of a tailored recommendation to withdraw antidepressant treatment |
|
| Participants |
Country: Netherlands Setting: outpatients from 45 general practices Type of AD: SSRI, SNRI, TCA, or other antidepressant except MAOI Duration of antidepressant treatment prerandomisation: ≥ 9 months; median duration, years (range): 8.0 (1 to 48) discontinuation, 9.5 (1 to 56) usual care Duration of antidepressant treatment post stabilisation: not described Total numbers of randomised participants: 146 (70 discontinuation, 76 usual care) Primary diagnosis ‐ discontinuation: any lifetime psychiatric diagnosis 76%, depression 56%, panic disorder or agoraphobia 19%, generalised anxiety disorder 31%, social phobia 23% ‐ usual care: any lifetime psychiatric diagnosis 63%, depression 46%, panic disorder or agoraphobia 17%, generalised anxiety disorder 17%, social phobia 26% Median duration of antidepressant use at inclusion, years (range): 8.0 (1 to 48) discontinuation; 9.5 (1 to 56) usual care Gender distribution (male): discontinuation 71%, usual care 68% Mean age, years (SD): discontinuation 56 (12.9), usual care 56 (14.3) Severity of depressive symptoms at randomisation: not described Inclusion criteria ‐ long‐term antidepressant use (≥ 9 months); all antidepressants were included, except MAOI ‐ written informed consent Definition of remission: no indication for long‐term antidepressant treatment in line with Dutch guidelines Exclusion criteria ‐ current treatment in a psychiatric inpatient or outpatient clinic ‐ appropriate use of long‐term antidepressants according to the Dutch guidelines for depressive and anxiety disorders (i.e. a history of recurrent depression (≥ 3 episodes) and/or a recurrent psychiatric disorder with ≥ 2 relapses after antidepressant discontinuation) ‐ history of psychosis, bipolar disorder, or obsessive‐compulsive disorder ‐ current diagnosis of substance use disorder, excluding tobacco, because of the necessity for specialised treatment ‐ non‐psychiatric indication for long‐term antidepressant usage (e.g. neuropathic pain) ‐ hearing impairment and/or insufficient understanding of the Dutch language Age was not an exclusion criterion |
|
| Interventions |
Intervention 1: a patient‐specific letter was sent to the GP with the recommendation to discontinue the antidepressant without placebo. Information on antidepressant tapering and the withdrawal syndrome was provided. The GP invited the patient to discuss the recommendations. No treatment restrictions were imposed in case of a relapse or onset of a new psychiatric disorder after discontinuation. A return slip was included to ascertain the patient’s intention to comply with the recommendation Tapering scheme: a gradual tapering programme was recommended Intervention 2: GPs were unaware which patients participated in this study and continued usual care: no restrictions on GPs to deliver care or to refer to specialised mental health care, including continuation or discontinuation of psychotropic drugs Comparison: discontinuation with psychosocial interventions vs continuation of antidepressant (or usual care) |
|
| Outcomes |
Primary outcome ‐ proportion of participants who successfully discontinued their long‐term antidepressant use after 1 year defined as no antidepressant use during preceding 6 months and absence of a depressive or anxiety disorder during 1‐year follow‐up, as assessed by the CIDI Secondary outcomes ‐ severity of global distress and global psychopathology and depressive symptoms, assessed by the Brief Symptom Inventory (BSI‐53) sum score and the Centre for Epidemiological Studies Depression Scale (CESD) at 3, 6, 9, and 12 months' follow‐up ‐ somatic comorbidity measured by TiC‐P ‐ quality of life at 3, 6, 9, and 12 months' follow‐up (reported in Eveleigh 2014) ‐ costs at baseline, 3, 6, 9, and 12 months' follow‐up (reported in Eveleigh 2014) |
|
| Notes |
Funding: study supported by Netherlands Organization for Health Research and Development (ZonMW) grant COI: study authors declare that no competing interests exist Other: low participation rate: 15% of participants deemed eligible by the GP; consented to participate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comments: randomisation was done by picking an envelope Quote: "random assignment was ensured by picking a sealed envelope with intervention or control group" |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes were used to conceal allocation Quote: "random assignment was ensured by picking a sealed envelope with intervention or control group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding or incomplete blinding; outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment ensured; unlikely that blinding could have been broken Quote: "interviewers who conducted the baseline and follow‐up interviews as well as the psychiatrist and general practitioner who judged the indication of maintenance treatment will remain blinded throughout the trial" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout was higher in discontinuation group (n = 20/70; 28%) compared to continuation group (n = 10/76; 13%). Intention to comply was much lower in discontinuation group (34/70) compared to continuation group (74/76) |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is available. Most predefined primary and secondary outcomes were reported in a predefined way. Withdrawal symptoms were measured but were not reported. No adverse events was not an outcome. No CESD; BSI‐53 results reported only at 3 months' follow‐up ‐ not at end of study |
| Other bias | Low risk | Bias for cluster‐RCT Recruitment bias: low risk Comment: participants were recruited before clusters had been randomised Quote: “to prevent contamination between intervention and control groups, a cluster randomisation was performed with the general practice as the unit of clustering. Random assignment was executed after patient recruitment was concluded per practice; a practice was either an intervention practice or a control practice” Baseline imbalance: low risk Comment: there was baseline comparability of clusters from the data presented Quote: "patient characteristics were well balanced at randomisation; any differences were not statistically significant" Incorrect analysis: low risk Comment: study authors used intra‐class correlation (ICC) adjustment for clustering in the analysis Quote: "because our trial is cluster randomised, calculations to determine the minimum number of general practices is stricter than in a non‐clustered trial. To account for this, we used an ICC of 0.05" Loss of clusters: unclear risk Comment: insufficient information to permit judgement |
Gelenberg 2003.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 1: acute treatment with nefazodone alone or nefazodone in combination with cognitive‐behavioral analysis system of psychotherapy (CBASP); sessions twice weekly throughout first 4 weeks and weekly for last 8 weeks; additional twice‐weekly sessions were permitted until Week 8 if a patient was having difficulty mastering the therapy's social problem‐solving approach (12 weeks) (reported in Keller 2000) Phase 2: continuation treatment with nefazodone alone (16 weeks) or continuation treatment in combination with CBASP; sessions were tapered over to every other week for the first 8 weeks and monthly for the subsequent 8 weeks of continuation treatment Duration post randomisation: 52 weeks Aim: to determine the efficacy and safety of nefazodone maintenance treatment for preventing recurrence in chronic depression |
|
| Participants |
Country: USA Setting: 12 academic centres Type of antidepressant: nefazodone Duration of antidepressant treatment prerandomisation: 28 weeks Duration of antidepressant treatment post stabilisation: 16 weeks Total numbers of participants: 681 and 269 responded to either nefazodone alone or combined treatment; 165 maintained response at end of phase 2 and entered RCT Total numbers of randomised participants: 160 (84 discontinuation, 76 continuation) Primary diagnosis ‐ placebo: chronic depression 28.6%, double depression 42.9%, recurrent depression with incomplete inter‐episode recovery 28.6% ‐ nefazodone: chronic depression 34.2%, double depression 36.8%, recurrent depression with incomplete inter‐episode recovery 29% Severity of depressive symptoms at randomisation (HAM‐D 24), score (SD): 5.6 (4.0) placebo, 5.9 (4.4) nefazodone Duration of current MDD, years (SD): 7.3 (7.5) placebo, 8.4 (10.8) nefazodone Duration of current dysthymia, years (SD): 23.3 (14.3) placebo, 23.5 (15.9) nefazodone Gender distribution (female): 65.5% placebo, 69.7% nefazodone Mean age, years (SD): 44.1 (8.4) placebo, 44.4 (11.1) nefazodone Prior treatment (phases 1 and 2): ‐ placebo group: 28/84 (33.5%) nefazodone, 56/84 (66.7%) nefazodone + CBASP ‐ nefazodone group: 26/76 (34.2%) nefazodone, 50/76 (65.8%) nefazodone + CBASP Inclusion criteria ‐ 18 to 75 years ‐ patients who had responded to nefazodone or continuation treatment in combination with CBASP during a 12‐week acute treatment study and who maintained their response over 16 weeks of continuation treatment ‐ diagnosis of chronic MDD (≥ 2 years’ duration), concurrent MDD superimposed on an antecedent dysthymic disorder (“double depression”), or recurrent MDD with incomplete inter‐episode recovery ≥ 2 years’ duration (DSM‐IV criteria) ‐ ≥ 20 on the HAM‐D 24 both at screening and at baseline after a 2‐week drug‐free period at entry of phase 1 Definition of response: 50% reduction in HAM‐D 24 total score from acute phase baseline achieved at end of acute phase, then maintained at the end of the continuation phase; HAM‐D 24 total score < 16 at end of continuation phase Exclusion criteria ‐ history of seizures, abnormal ECG, stroke, severe head trauma, psychotic symptoms, schizophrenia, bipolar, eating disorders not remitted for 1 year, OCD, dementia ‐ high risk for suicide, antisocial, schizotypal, or severe borderline personality disorders ‐ diagnosis within past 6 months of panic, GAD, PTSD, social phobia, or substance abuse/dependence ‐ did not respond to 3 previous AD trials (of 2 different classes), psychotherapy, or ECT during last 3 years ‐ serious, unstable, concurrent medical conditions ‐ women of childbearing age with inadequate contraception |
|
| Interventions |
Intervention 1: placebo Intervention 2: nefazodone (300 to 600 mg/d twice daily based on response and tolerability) Tapering scheme: abruptly with no tapering and with identical inactive tablets Co‐intervention ‐ participants could not receive anxiolytics, sedatives, hypnotics, or other pharmacological or behavioural sleep aids during any phase of the study ‐ medication visits in both groups: "the treating physician followed a guideline manual for clinical management (e.g. review of symptoms, side effects, illnesses, and concomitant medications) that proscribed any formal psychotherapeutic interventions (e.g. giving suggestions regarding coping with stressful life events). Medication visits were limited to 15‐20 min" ‐ no CBASP sessions during the trial |
|
| Outcomes | Relapse/recurrence every 4 weeks for 52 weeks Definition of relapse/recurrence ‐ HAM‐D 24 ≥ 16 plus MDD (DSM‐IV criteria diagnosed on 2 consecutive visits) ‐ time to recurrence of MDD, in days, every 4 weeks for 52 weeks ‐ HAM‐D 24 mean ‐ dropout any ‐ dropout due to adverse events ‐ treatment‐emergent adverse events |
|
| Notes |
Funding: study supported by grants from pharmaceutical companies COI: not reported; several trial authors (included first author) report grants from several pharmaceutical companies in Keller 2000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not enough information to judge Quote: "two separate randomisation schedules were used: one for patients receiving nefazodone monotherapy during the continuation phase and the second for patients receiving combined nefazodone and CBASP during the continuation phase. Within each schedule, patients were randomised in a 1:1 ratio to nefazodone or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information on blinding of personnel to permit judgement Quote: "...patients assigned to placebo received apparently identical, inactive tablets. Patients were switched abruptly to placebo with no downward taper..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment ensured; unlikely that blinding could have been broken Quote: "HAM‐D, which was rated by trained, independent evaluators blind to treatment assignment"; " ...a blinded review of symptom exacerbations by a consensus committee of research clinicians..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: although reasons for withdrawal are stated, the withdrawal rate is much higher in the placebo group (61.9%) compared to the nefazodone group (38.2%). Dropout rates not reported separately for participants who had responded to nefazodone alone or in combination with psychotherapy. ITT analysis on 160/165 patients entered into the RCT with at least 1 post‐baseline assessment Quote: "the distribution of patients discontinuing was unequal between the two treatment groups, with 29 of 76 (38.2%) nefazodone treated patients compared with 52 of 84 (61.9%) placebo treated patients discontinuing before the end of the study" |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is not available; information on adverse events was reported. Withdrawal symptoms were not an outcome. None of our secondary outcomes were measured |
| Other bias | Unclear risk | Comment: grant from pharmaceutical industry |
Gilaberte 2001.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 0: washout phase (1 week) Phase 1: acute treatment with fluoxetine 20 mg of 40 mg (8 weeks) Phase 2: continuation treatment with fluoxetine 20 mg of 40 mg (24 weeks) Duration post randomisation: 48 weeks Aim: to evaluate the efficacy and safety of fluoxetine 20 mg/d for relapse prevention and for extending the time free of symptoms among patients with recurrent unipolar major depression |
|
| Participants |
Country: Spain Setting: community adults (10 centres) Type of antidepressant: fluoxetine 20 mg Duration antidepressant treatment prerandomisation: 32 weeks Duration antidepressant treatment post stabilisation: 24 weeks Primary diagnosis: recurrent depression Number of participants: 253 entered phase 1, 145 achieved recovery at end of phase 2 Total number of randomised participants: 140 (70 placebo, 70 fluoxetine) Severity of depressive symptoms at randomisation (HAM‐D 17) (SD): 3.1 (2.7) placebo, 2.8 (2.0) fluoxetine Number of previous episodes: 2.6 (1.5) placebo, 2.3 (1.2) antidepressant Gender (female %): 78.6 in both groups Age, years: 43.8 placebo, 44.4 fluoxetine Inclusion criteria ‐ 18 to 65 years ‐ ≥ 1 previous major depressive episode in the last 5 years (DSM‐III‐R criteria) ‐ ≥ 18 on the HAM‐D 17 and ≥ 4 on the CGI Severity Scale in the index episode of depression ‐ received no pharmacological treatment during index depressive episode ‐ responded to acute treatment and remained in response during continuation period Definition of response/remission: no longer met the diagnostic criteria for major depression per DSM‐III‐R, and had HAM‐D 17 scores ≤ 8 and CGI severity scores ≤ 2) Exclusion criteria ‐ patients with other Axis I diagnoses ‐ organic mental disorders ‐ history of drug abuse or severe physical illness ‐ at risk for suicide ‐ pregnant or breast‐feeding women and women of childbearing potential not using adequate contraceptive measures ‐ resistant to pharmacological treatment during previous depressive episodes |
|
| Interventions |
Intervention 1: placebo Intervention 2: fluoxetine 20 mg per day Tapering scheme: not reported |
|
| Outcomes | Recurrence Definition of relapse/recurrence: meeting DSM‐III‐R criteria for major depression and HAM‐D 17 score ≥ 18 and CGI‐S score ≥ 4, or both of these, for at least 2 weeks HAM‐D 17, CGI‐S, CGI‐I, and Hamilton Rating Scale for Anxiety Treatment‐emergent adverse events, reasons for discontinuations, changes in blood pressure, ECG, weight Adverse events were collected by non‐probing inquiry and were recorded without regard to causality |
|
| Notes |
Funding: study was supported by a grant from pharmaceutical companies COI: not reported; first author is a member of European Product Team Physicians of the pharmaceutical company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement Quote: " ...patients were evaluated monthly by psychiatrists for recurrence of depression..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: much higher dropout rate in discontinuation group (41/70) than in antidepressant group (21/70) |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; outcomes were not clearly pre‐specified, nor were the time points at which they were to be measured. Adverse events were reported. Withdrawal symptoms were not an outcome. None of our secondary outcomes were reported |
| Other bias | Unclear risk | Comment: grant from pharmaceutical industry |
Huijbers 2016.
| Study characteristics | ||
| Methods |
Design: parallel 2‐group non‐inferiority randomised trial Prerandomisation phases: no Duration post randomisation: 65 weeks (15 months) Aim: to investigate whether mindfulness‐based cognitive therapy (MBCT) with discontinuation of long‐term antidepressant treatment is non‐inferior to MBCT + long‐term antidepressant treatment |
|
| Participants |
Country: Netherlands Setting: community adults (outpatients from 12 secondary and tertiary psychiatric outpatient clinics, referred by mental healthcare professionals or recruited by advertisements in the media (television, magazines, and newspapers)) Type of AD: SSRI, TCA, other Duration of antidepressant treatment prerandomisation: at least 24 weeks (mean duration not reported) Duration of antidepressant treatment post stabilisation: not reported Primary diagnosis: recurrent depression Number of previous episodes (SD): 5.9 (5.3) discontinuation, 5.6 (4.1) antidepressant Total number of randomised participants: 249 (128 discontinuation, 121 antidepressant) Severity of depressive symptoms at randomisation (IDS‐C), mean (SD): 12.6 (9.6) discontinuation, 12.6 (10.5) antidepressant Number of participants in full remission (IDS‐C ≤ 11), n: 70/128 (55%) discontinuation, 63/121 (52%) antidepressant Number of participants in partial remission (IDS‐C > 11), n: 58/128 (45%) discontinuation, 58/121 (48%) antidepressant Gender distribution (F): 72% intervention, 63% control Mean age, year (SD): 50.7 (10.6) discontinuation, 49.9 (10.5) antidepressant Inclusion criteria ‐ history ≥ 3 depressive episodes (DSM‐IV criteria) ‐ in full or partial remission ‐ currently treated with antidepressants for at least 6 months ‐ 18 years of age or older ‐ Dutch speaking Definition of response/remission: not currently meeting DSM‐IV criteria for major depressive disorder ‐ full remission (IDS‐C ≤ 11) ‐ partial remission (IDS‐C > 11) Exclusion criteria ‐ bipolar disorder ‐ any primary psychotic disorder ‐ clinically relevant neurological/somatic illness ‐ current alcohol or drug dependency ‐ high dosage of benzodiazepines (42 mg lorazepam equivalents daily) ‐ recent electroconvulsive therapy ‐ previous MBCT and/or extensive meditation experience ‐ current psychological treatment with frequency more than once per 3 weeks ‐ inability to complete interviews and self‐report questionnaires |
|
| Interventions |
Intervention 1: MBCT followed by guided discontinuation of antidepressants (without placebo) Description: MBCT was largely based on the protocol by Segal, Williams, & Teasdale with some adaptations. The intervention consisted of 8 weekly sessions of 2.5 hours and 1 day of silent practice between the sixth and seventh sessions. MBCT included formal meditation exercises such as the body scan, sitting meditation, walking meditation, and mindful movement, as well as informal exercises such as bringing present‐moment awareness to everyday activities. Cognitive–behavioural techniques included education, monitoring and scheduling of activities, identification of negative automatic thoughts, and devising a relapse prevention plan. Participants were encouraged to practice meditation at home for about an hour a day using CDs Delivery: treatment was delivered in groups of 8 to 12 participants at 12 different centres, with a total of 19 teachers and 111 MBCT courses. Groups were mixed, comprising patients from both treatment groups, as well as patients not included in the trial Treatment duration: 8 consecutive weeks Tapering scheme: recommendation to withdraw over a period of 5 weeks, starting after the seventh session of MBCT. A protocol for medication tapering was developed for this study by 2 experts in pharmacological treatment of major depressive disorder. For discontinuation, we recommended a minimum of 3 and a maximum of 12 consultations during the follow‐up period Providers: withdrawal of AD supervised by psychiatrists Adherence to the study protocol was defined as attending 4 or more MBCT sessions, as in previous studies, and having fully discontinued antidepressant before the 6‐month follow‐up assessment (i.e. within 6 months after baseline and within approximately 3 to 4 months after the last MBCT session) Integrity of delivery: videotapes of MBCT sessions were available for 15 teachers. Two tapes per teacher were randomly selected. Teacher competency was examined by 2 independent expert raters, using Mindfulness‐Based Interventions: Teaching Assessment Criteria Intervention 2: MBCT and continuation of antidepressants Description: MBCT as in Intervention 1 For continuation of antidepressant, a minimum of 1 consultation with a psychiatrist was recommended. Psychiatrists were instructed to maintain or reinstate an adequate dose of antidepressants, and recommendations to manage side effects were provided Compliance: adherence to the study protocol was defined as attending 4 or more MBCT sessions and using a therapeutic dose of antidepressant at each follow‐up contact during the observed time period (using last observation carried forward for participants who did not complete all assessments) |
|
| Outcomes |
Primary outcomes ‐ relapse/recurrence, assessed with SCID‐I every 3 months ‐ definition of relapse, measured with SCID‐I Secondary outcomes ‐ time to relapse/recurrence, calculated in weeks from the start of the study until the start of the first relapse/recurrence ‐ severity of (residual) depressive symptoms, measured with the Dutch version of the IDS‐C at every assessment during 15 months ‐ quality of life, assessed at baseline and at 3 and 15 months using the 26‐item self‐report WHO QoL short version |
|
| Notes | Participants with strong treatment preference for MBCT A parallel RCT was comparing MBCT + continuation of antidepressant versus antidepressant alone for patients wanting to hold on to their medication Funding: study was an independent study supported by ZonMW, The Netherlands Organization for Health Research and Development (ZonMW) grant COI: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comments: randomisation was done by using a computer random number generator Quote: "randomisation was performed using a website‐based application, developed specifically for this study by an independent statistician, with a minimisation procedure ... stratified for..." |
| Allocation concealment (selection bias) | Low risk | Comments: central allocation Quote: “one to one allocation performed online” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: not double‐blinded; results likely to be affected Quote: "unblinding of patients and research assistants could not be avoided" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comments: no blinding of outcome assessment; results likely to be affected. Quote: "the research assistants conducting the assessments could not be masked to treatment group since they were also involved in the practical organisation of the trial"; "it was impossible to keep the research assistants at the different sites masked to group..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: lost to follow‐up 28% in intervention and 31% in control Quote: "patients whose follow‐up data were unavailable or who did not experience a relapse/recurrence before the end of the follow‐up period were treated as censored observations ..." |
| Selective reporting (reporting bias) | High risk | Comment: study protocol is available. Depressive symptoms reported only at 3 months ‐ not at other pre‐specified time points. Adverse events and withdrawal symptoms should be reported as this is fundamental in a drug discontinuation trial |
| Other bias | Low risk | None |
Kane 1982.
| Study characteristics | ||
| Methods |
Design: multi‐phase, randomised, placebo‐controlled trial Prerandomisation phase: phase 1: open continuation treatment with imipramine flexible dose and 150 mg in last 6 weeks (24 weeks) Duration post randomisation: 104 weeks Aim: to compare lithium carbonate, imipramine, lithium carbonate plus imipramine, and placebo for relapse prevention in patients with unipolar disease. Only treatment arms without lithium were relevant for this review (as the review is looking at antidepressant discontinuation; lithium is not an antidepressant) |
|
| Participants |
Country: USA Setting: depression clinic Type of AD: imipramine Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 24 weeks Number of participants: not reported for phase 1 Total numbers of randomised participants: 11 (imipramine 5, placebo 6) Primary diagnosis: recurrent unipolar major depressive disorder (study arm bipolar disorders not relevant) Number of previous episodes: mean 7.2 (SD 6.2), 70% ≥ 4 episodes, 22.6% 3 episodes, 7.4% 2 episodes Age at first depressive episode: 21.3 placebo, 36.7 imipramine Gender distribution (female): 63% of total sample male (with bipolar treatment study arm); gender not reported by treatment group Mean age, years (SD): 53.2 imipramine (SD not reported), 38.5 placebo (SD not reported) Severity of depressive symptoms at randomisation: not reported Inclusion criteria ‐ met Research Diagnostic Criteria (RDC) for recurrent unipolar major depressive disorder or for bipolar depression with hypomania (bipolar II illness) ‐ had experienced ≥ 2 episodes of depression or mania in the previous 7 years ‐ had been euthymic for 6 months before entry into the study ‐ between the ages of 18 and 65 years ‐ free of coexisting medical illnesses that might complicate the use of lithium carbonate or imipramine ‐ signed a consent form Definition of response/remission: "euthymia"; no criteria specified Exclusion criteria ‐ not clearly defined |
|
| Interventions |
Intervention 1: imipramine Intervention 2: placebo Imipramine: dosage at 100 to 150 mg/d, according to clinical judgement. Tapering scheme: not reported Each treatment group received lithium placebo (placebo tablets cancel each other out) |
|
| Outcomes |
Primary outcomes ‐ relapse ‐ definition of relapse/recurrence: RDC for major depressive disorder and symptoms persisted for a week after evaluation; RDC criteria for minor depressive disorder persisted for 4 successive weeks and were removed (also RDC criteria for mania, even if symptoms had not persisted for a week) Secondary outcome ‐ mania, hypomania, and dropouts due to euthymia |
|
| Notes |
Funding: supported in part by a grant from the Goldman Foundation (non‐commercial foundation) COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ”patients were randomly assigned” Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “double‐blind.....patients were given treatment by a physician blind to the regimen” Comment: does not state how patients were blinded; insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: although reasons for dropping out are reported, study authors do not explicitly state the numbers and reasons by treatment group (e.g. depressive relapse outcome); only 5 patients in placebo group, whereas 6 were randomised |
| Selective reporting (reporting bias) | High risk | Comment: study report fails to include results for adverse events and withdrawal symptoms that would be expected to have been reported for a discontinuation study. None of our secondary outcomes were reported |
| Other bias | Low risk | Comment: study appears to be free from other sources of bias |
Keller 1998.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind placebo‐controlled trial Prerandomisation phases Phase 1: acute treatment with sertraline (12 weeks) Phase 2: continuation treatment with sertraline (16 weeks) Duration post randomisation: 76 weeks Aim: to determine if maintenance therapy with sertraline hydrochloride can prevent recurrence of depression in the high‐risk group of patients experiencing chronic major depression or major depression with antecedent dysthymic disorder |
|
| Participants |
Country: USA Setting: 10 academic centres and 2 clinical research centres Type of AD: sertraline Duration of antidepressant treatment prerandomisation: 28 weeks Duration of antidepressant post stabilisation: 16 weeks Number of participants in phase 1: 426; 209 entered continuation phase, 169 response at end of continuation treatment Total number of randomised participants: 161 (77 sertraline, 84 placebo) Primary diagnosis ‐ chronic major depression (at least 2 years' duration): 43% placebo, 52% antidepressant ‐ double depression (dysthymic disorder with concurrent diagnosis of major depression): 57% placebo, 48% antidepressant Number of previous episodes: 1.9 (2.1) placebo, 1.8 (2.2) sertraline Severity of depressive symptoms at randomisation (HAMD‐24), mean (SD): 6.3 (3.7) placebo, 5.5 (4.2) sertraline History of comorbidities ‐ anxiety disorder ‐ placebo: panic disorder 5%, social phobia 13%, GAD 2%, any anxiety disorders 22% ‐ antidepressant: panic disorder 16%, social phobia 10%, GAD 3%, any anxiety disorders 30% ‐ history of alcohol abuse: 24% placebo, 36% antidepressant ‐ history of substance abuse: 30% placebo, 43% antidepressant ‐ comorbid Axis II personality disorder: 51% placebo, 44% antidepressant Gender distribution (F): 69% placebo, 62% antidepressant Mean age, years (SD): 42.4 (9.7) placebo, 40.8 (9.0) antidepressant Inclusion criteria ‐ SCID‐I chronic MDD of 2 years' duration or dysthymic disorder with concurrent diagnosis of MDD (double depression) (DSM‐III criteria) ‐ patients entered continuation phase if full remission/response or satisfactory therapeutic response to acute treatment ‐ patients were eligible to enter the RCT if they had sustained at least a satisfactory antidepressant response to sertraline through the end of continuation therapy Defintion of response/remission: full remission/response (HAM‐D score ≤ 7 and CGI improvement 1 or 2) or satisfactory therapeutic response (reduction of 50% in HAM‐D total score plus HAM‐D ≤ 15 and CGI improvement ≤ 2 and CGI severity ≤ 3) Exclusion criteria ‐ not reported |
|
| Interventions |
Intervention 1: placebo Tapering scheme: tapered sertraline over 4 weeks; 50 mg/week reduction Intervention 2: sertraline hydrochloride (at a flexible daily dose of 50 to 200 mg) |
|
| Outcomes |
Primary ‐ time to recurrence of a major depressive episode Definition of recurrence: (1) DSM criteria for major depression for at least 3 weeks, (2) CGI severity score ≥ 4 (at least moderate severity), (3) CGI improvement score ≥ 3 (minimally improved or less), and (4) increase in HAM‐D score to a score ≥ 4 points higher than the maintenance phase baseline and within 1 week (total duration of clinical worsening criteria of at least 4 weeks) to meet all criteria and judged as recurrence of major depression by an investigator ‐ double‐blind titration to maximum dose of 200 mg per day was used for participants meeting recurrence criteria; no change in study medication was needed ‐ experiencing exacerbation but did not meet criteria Secondary ‐ time to re‐emergence of clinically significant depression: blinded review of HAM‐D, GCI, and overall clinical picture of all patients who discontinued the study prematurely. Agreement among 6 (75%) of 8 senior investigators was required for a patient to be categorised as having met this clinical endpoint (less stringent post hoc endpoint) ‐ time to re‐emergence of first symptoms of depression: blinded review of HAM‐D, GCI, and overall clinical picture of all patients who discontinued the study prematurely. Agreement among 6 (75%) of 8 senior investigators was required for a patient to be categorised as having met this clinical endpoint (less stringent post hoc endpoint) ‐ HAM‐D 24, CGI severity and improvement scale (every 2 weeks during first 12 weeks, then monthly) ‐ MADRS, Cornell Dysthymia Scale, Beck Depression Inventory (monthly) ‐ quality of life ‐ psychosocial functioning: SAS‐SR, SF‐36, LIFE (reported in Kocsis 2002) |
|
| Notes |
Funding: study is supported by a grant from the pharmaceutical industry COI: main study author is a consultant from the pharmaceutical company, has received grants, and serves on the advisory board. Co‐authors received grants from the pharmaceutical industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: trial described as double‐blind Quote: “to maintain blinding, this group of patients continued (as a parallel but non‐randomised group) to receive imipramine during subsequent continuation and maintenance phases… the integrity of the study’s double‐blind component was not compromised” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high dropouts and imbalance across treatment groups (placebo group 60/84 (71%) and sertraline group 42/77 (54.5%)) |
| Selective reporting (reporting bias) | High risk | Comment: study protocol not available; adverse events and withdrawal symptoms not an outcome of the study |
| Other bias | Unclear risk | Comment: grant received from the pharmaceutical industry |
Keller 2007.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 1: acute treatment with venlafaxine ER (75 mg to 300 mg/d) (10 weeks) Phase 2: continuation treatment with venlafaxine ER (24 weeks) Phase 3: maintenance treatment with venlafaxine ER (52 weeks) (reported in Kocsis 2007); those who responded to venlafaxine entered this study and were randomised to venlafaxine or to placebo Duration post randomisation: 52 weeks Aim: to report second‐year results from the 2‐year maintenance phase of a long‐term study to evaluate the efficacy and safety of venlafaxine extended release (ER) in preventing recurrence of depression |
|
| Participants |
Country: USA Setting: 29 clinical sites Type of antidepressant: venlafaxine 100% Duration of antidepressant treatment prerandomisation: 86 weeks Duration of antidepressant treatment post stabilisation: 76 weeks Number of participants: 821 entered phase 1, 530 entered phase 2, 409 completed phase 2 in remission or improved response, 336 entered phase 3, 136 completed phase 3 Total number of randomised participants: 83 (40 placebo, 43 AD); third arm (48 continued placebo) (not relevant for this review) Primary diagnosis: recurrent depression Number of previous episodes: not reported Severity of depressive symptoms at randomisation (HAM‐D 17 score), mean (SD): 4.1 (3.7) placebo, 4.8 (2.6) antidepressant Gender distribution (F): 70% placebo, 60% antidepressant Mean age, mean, years: 42.8 placebo, 44.8 antidepressant Inclusion criteria ‐ 18 years or older ‐ DSM‐IV diagnostic criteria for MDD confirmed by structured diagnostic interview at entry of phase 1. Experienced depressive symptoms for ≥ 1 month before the start of phase 1. ‐ met the following criteria for recurrent depression: history ≥ 3 episodes of major depression, with ≥ 2 episodes in the past 5 years (including the current episode), and an interval ≥ 2 months between the end of the previous episode and the beginning of the current episode ‐ had a response (HAM‐D 17 total score ≤ 12 and ≥ 50% decrease from acute phase baseline) or a remission (HAM‐D 17 score ≤ 7) of the intake episode of MDD at the end of maintenance phase 3. Patients achieving a response (therapeutic response, defined as a HAM‐D 17 total score ≤ 12 and ≥ 50% decrease from baseline, or remission, defined as HAM‐D 17 score ≤ 7) during the acute phase 1 were eligible to enter the 6‐month continuation phase 2. Patients who continued to demonstrate a response at the end of the continuation phase 2 entered maintenance phase 3. Patients continuing to respond at the end of maintenance phase 3 were eligible to enter the RCT Definition of response/remission: response: HAM‐D 17 total score ≤ 12 and ≥ 50% decrease from acute phase baseline, remission (HAM‐D 17 score ≤ 7) of the intake episode of MDD Exclusion criteria ‐ patients for whom an adequate trial of fluoxetine, venlafaxine, or venlafaxine ER had failed during the current episode of MDD, or who had treatment‐resistant depression (for whom ≥ 3 previous adequate trials of ≥ 2 classes of antidepressant medication, ECT, or 2 adequate trials of psychotherapy in the past 3 years had failed) were not eligible to participate. Patients with known hypersensitivity to venlafaxine or fluoxetine were excluded, as were those with histories or presence of any of the following: clinically significant hepatic, cardiovascular, renal, or other serious medical disease that might compromise the study; seizure disorder other than a single childhood febrile seizure; bipolar disorder; OCD; eating disorder (if not remitted for ≥ 5 years); drug or alcohol dependence or abuse within 6 months before screening; current postpartum depression; any psychotic disorder, including psychotic depression; significant Axis II disorders; or any organic mental disorder. Patients were not eligible to participate if they met DSM‐IV criteria for a primary diagnosis of panic disorder, OCD, GAD, social phobia, or PTSD. Patients were excluded if the investigator judged them to be at risk for suicide to such a degree that precautions against suicide were required, or if they had clinically significant abnormalities on pre‐study physical examination, ECG, or laboratory tests; had diagnoses of cancer in the past 3 years (excluding squamous or basal cell carcinoma) and/or had active neoplastic disease; or were women of childbearing age who were pregnant, breastfeeding, or not using a medically acceptable method of birth control |
|
| Interventions |
Intervention 1: placebo Tapering scheme: 4‐week taper period Intervention 2: venlafaxine ER 75 to 300 mg/d with dose increases allowed to optimise treatment response |
|
| Outcomes |
Primary outcomes ‐ time to recurrence of major depression Definition of remission Primary definition HAM‐D 17 total scores > 12 and HAM‐D 17 reduction from acute phase baseline that was not more than 50% at 2 consecutive visits or at the last valid visit before discontinuation. Participants also had to meet DSM‐IV criteria for MDD and had to be judged by the investigator to have had a recurrence Secondary definition Participants who, at 1 visit, had HAM‐D 17 scores > 12 and HAM‐D 17 reductions from acute phase baseline that were not more than 50% but did not meet the primary definition of recurrence and were reviewed by a committee of experienced psychiatrists, which assessed whether each of these patients experienced recurrence after a review of blinded clinical data. This clinical definition of recurrence therefore included all patients who met the primary definition and patients whom the committee determined had experienced recurrence Secondary outcomes ‐ Hamilton Rating scale for Depression (HAM‐D), Inventory for Depressive Symptomatology Self‐Report (monthly), Rothschild Scale for Antidepressant Tachyphylaxis (monthly), 3‐monthly Hamilton Rating Scale for Anxiety (HAM‐A), Clinical Global Impressions‐Severity of Illness (CGI‐S) Scale (monthly), Longitudinal Internal Follow‐up Evaluation ‐ quality of life measured 3‐monthly with 36‐item Short Form Health Survey (SF‐36), Quality of life Enjoyment and Satisfication Questionnaire (Q‐LES‐Q), Life Enjoyment Scale‐Short version ‐ Social Adjustment Scale Self‐Report (SAS‐SR) ‐ adverse events monitored via reports of adverse events, vital sign measurements (supine pulse and standing and supine blood pressure), and laboratory evaluations. Standard 12‐lead electrocardiography was performed at screening for all patients at least 50 years of age and those for whom the investigator considered this medically indicated. Comprehensive physical examinations were performed at screening. Discontinuation to adverse events ‐ health service utilisation questionnaire |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry COI: study authors are employees of the drug company or have received grants from pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer random number generator Quote: "...were randomly assigned in a double blind fashion in a 1:1 ratio to receive either venlafaxine or placebo..." For each phase of the study, randomisation records were stratified by site and were generated using a block size of 4. A central randomisation scheme was implemented using Quintiles' IVR system |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation Quote: "after a site deemed a patient eligible to enrol/continue in the study, they contacted the IVR system, which ascertained the site where the patient was located and then the patient was allocated to the next available treatment assignment (i.e. next sequence number) in the randomisation schedule for that site" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: described as double‐blind (in Kocsis 2007); unlikely that blinding could have been broken Quote: "patients and investigators remained blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not enough information to permit a judgement Quote: "HAM‐D 17 ratings were performed by individuals who had been trained and certified. Abstracts of the data, including mood ratings and clinical notes from the case report forms were presented to the recurrence review committee: a committee of experienced psychiatrist who assessed whether each of these patient experienced recurrence after a review of the blinded clinical data" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout rates were higher in the placebo group (25/40) than in the venlafaxine group (12/43) |
| Selective reporting (reporting bias) | High risk | Comment: adverse events reported; withdrawal symptoms not an outcome |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Khan 2014.
| Study characteristics | ||
| Methods |
Design: 2‐phase double blind randomised 3‐arm controlled interruption trial Prerandomisation phase Phase 1: treatment with desvenlafaxine 50 mg (24 weeks) Duration post randomisation: 4 weeks Aim: to evaluate the tolerability of a tapering regimen compared with abrupt discontinuation and with continuation of long‐term desvenlafaxine treatment for major depression |
|
| Participants |
Country: USA Setting: 38 clinical research centres Type of AD: desvenlafaxine Duration of antidepressant prerandomisation (weeks): 24 Duration of antidepressant treatment post stabilisation: not specified Total number of participants: 480 enrolled in phase 1, 362 completed phase 1 Total number of randomised participants: 361 (148 abrupt discontinuation, 140 tapering, 73 antidepressant) → control group was split to allow multiple‐treatment comparison: 36 and 37 → 184 participants in the comparison abrupt discontinuation vs continuation (148 abrupt discontinuation, 36 antidepressant) and 176 participants in the comparison tapering vs continuation (140 taper and 37 antidepressants) Primary diagnosis: single or recurrent depression Severity of depressive symptoms at randomisation (QIDS‐SR16), mean (SD): 6.3 (4.5) abrupt, 6.3 (4.3) taper, 6.8 (4.4) antidepressant Gender distribution (F): 67.6% abrupt discontinuation, 73.6% taper, 67.1% antidepressant Mean age, years (SD): 47.8 (13.7) abrupt discontinuation, 47.9 (11.2) taper, 46.7 (11.3) antidepressant Inclusion criteria ‐ ≥18 years ‐ primary diagnosis of single or recurrent MDD without psychotic features (DSM‐IV criteria), assessed by MINI ‐ depressive symptoms ≥ 30 days before screening visit of phase 1 and HAM‐D 17 ≥ 14 at baseline of phase 1 ‐ participants who completed the 24‐week open‐label treatment of phase 1 were enrolled in the discontinuation trial Exclusion criteria ‐ current primary diagnosis of anxiety disorder ‐ significant risk of suicide based on response to question 4 or 5 on the C‐SSRS at screening or baseline ‐ current psychoactive substance abuse or dependence ‐ unstable hepatic, renal, pulmonary, cardiovascular (including uncontrolled hypertension, unstable angina, or recent myocardial infarction); ophthalmologic or neurologic disorder ‐ other clinically important medical disease (including uncontrolled diabetes) |
|
| Interventions |
Intervention 1: placebo Tapering scheme: abrupt discontinuation of venlafaxine Intervention 3: gradually tapering of venlafaxine to placebo; tapering scheme: desvenlafaxine 25 mg/d for 1 week followed by placebo for 3 weeks. Intervention 2: continuation of desvenlafaxine 50 mg/d |
|
| Outcomes |
Primary ‐ withdrawal symptoms measured with Discontinuation‐Emergent Signs and Symptoms (DESS), during the first 2 weeks Secondary ‐ severity of withdrawal symptoms, measured with Discontinuation Symptoms Severity Index (DSSI), an exploratory scale rating DESS items’ severity and relationship to discontinuation, and DESS score at the end of Weeks 3 and 4 of the double‐blind phase to determine if symptoms were present or worsened ‐ adverse events: incidence and timing of taper/post‐therapy‐emergent adverse events (TPAEs), which are adverse events that started or increased in severity during the double‐blind phase, and rate of study discontinuation due to treatment‐emergent adverse events (adverse events that emerged between first administration of the open‐label study medication and 14 days or fewer after last administration of double‐blind treatment) during the double‐blind phase ‐ proportion of participants with withdrawal syndrome (increase in DESS ≥ 4 during double‐blind baseline) ‐ depressive symptoms measured by Quick Inventory of Depressive Symptomatology Self‐Report (QIDS‐SR16) ‐ DESS and DSSI were administered at the end of the OL period (DB baseline) and at DB Weeks 1 through 4 |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry COI: editorial assistance and medical writing were provided by KMD and were funded by the company that funded the trial; 4 of the 6 study authors were employees of the pharmaceutical company that funded the trial; 1 of the 6 study authors was a former employee, and the head study author is the medical director of a pharmaceutical company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not enough information to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not enough information to permit a judgement Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not enough information to permit a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout rates low, balanced; missing data were handled using the last observation carried forward approach |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available, and all of the study’s pre‐specified (primary and secondary) outcomes have been reported (DSSI and QIDS‐SR in a related paper Ninan 2015) |
| Other bias | High risk | Comment: grant from the pharmaceutical industry; medical writer paid by the pharmaceutical company that funded the trial |
Klysner 2002.
| Study characteristics | ||
| Methods |
Design: multi‐phase randomised placebo‐controlled trial Prerandomisation phase: Phase 1: acute treatment with sertraline 20 to 40 mg/d (8 weeks) Phase 2: continuation treatment with citalopram 20 to 40 mg/d (16 weeks) Duration post randomisation: 48 weeks Aim: to compare the prophylactic efficacy of citalopram and placebo in elderly patients; to evaluate long‐term tolerability of citalopram |
|
| Participants |
Country: Denmark Setting: psychiatric research centre Type of AD: citalopram Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 8 weeks Number of participants: 230 entered phase 1, 172 completed phase 1, 172 entered phase 2, 121 completed phase 2 Total numbers of randomised participants: 121 (61 placebo, 60 continuation) Primary diagnosis: unipolar major depression Number of previous episodes 0 previous episodes: 51 (85.0%) discontinuation, 52 (85.3%) continuation 1 previous episodes: 8 (13.3%) discontinuation, 6 (9.8%) continuation 2 previous episodes: 1 (1.7%) discontinuation, 3 (4.9%) continuation Gender distribution (female): placebo 72%, citalopram 82% Mean age, years (SD): discontinuation 75 (range 66 to 87) years, continuation 64 (range 65 to 87) years Severity of depressive symptoms at randomisation: MADRS ≤ 11 at baseline maintenance Inclusion criteria ‐ outpatients; ≥ 65 years of age with unipolar major depression (DSM‐IV) and MADRS ≥ 22 Definition of remission: MADRS ≤ 11 Exclusion criteria ‐ index episode > 12 months' duration ‐ history of schizophrenia, mania, hypomania, epilepsy, drug or alcohol misuse ‐ severe somatic disorders ‐ received fluoxetine within 5 weeks or other antidepressants within 3 days of the start of the study ‐ received lithium, carbamazepine, or valproate within 2 weeks of the study ‐ received ECT within 8 weeks of the study or sumatriptan or anticoagulants at the study start ‐ score of 55 on MADRS item 10 (suicidality) |
|
| Interventions |
Intervention 1: placebo: identical looking tablets Tapering scheme: abrupt discontinuation Intervention 2: citalopram: same dose as continuous phase (20, 30, or 40 mg/d) No concomitant psychotropic medication was allowed, except for benzodiazepines and other hypnotics, the dose of which was to remain unchanged after Week 8 of phase 2. Treatment with benzodiazepines and other hypnotics could not be started during Periods II or III, except in case of relapse/recurrence, if the investigator felt that intervention was needed before relapse (phase 2) or recurrence (phase 3) was confirmed |
|
| Outcomes |
Primary outcome ‐ recurrence of depressive episode Definition of relapse/recurrence: MADRS total score ≥ 22, confirmed after 3 to 7 days Secondary outcome ‐ adverse events |
|
| Notes |
Funding: funded by pharmaceutical industry COI: study author (MA) works for pharmaceutical company that funded the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised on a 1:1 basis, using a block size of ten” Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: information insufficient to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: large numbers of dropouts in placebo group and antidepressant group (55/61 (90%) in placebo group and 37/60 (62%) in antidepressant group); only 6/61 placebo patients completed the trial compared to 23/60 citalopram patients |
| Selective reporting (reporting bias) | High risk | Comment: protocol is not available; reports include all expected outcomes. Adverse events were reported. Withdrawal symptoms were not an outcome. None of our secondary outcomes were reported |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Kocsis 1996.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 1: acute treatment with desipramine 20 mg to 200 mg (10 to 12 weeks) Phase 2: continuation treatment with desipramine same dose (16 weeks) Post randomisation duration: 104 weeks Aim: to compare desipramine hydrochloride and placebo for maintenance therapy of remitted patients with chronic depression |
|
| Participants |
Country: USA Setting: community adults (selected from responders to advertisements for chronic depression and patients seeking treatment for depression at a psychiatric outpatient clinic) Type of AD: desipramine Duration of antidepressant prerandomisation: 26 to 28 weeks Duration of antidepressant after stabilisation: 16 weeks Number of participants: 129 entering phase 1, 105 completed phase 1, 66 entered phase 2, 54 completed phase 2 Total number of randomised participants: 53 (25 placebo, 28 desipramine) Primary diagnosis ‐ pure dysthymia ‐ double depression ‐ chronic major depression (number of participants not reported for RCT) Severity of depressive symptoms at randomisation (HMDR), mean (SD): not reported for each group at randomisation; full remission reported for 40 participants; partial remission reported for 10 participants Gender distribution (F): presented by type of depression rather than by treatment group: 51% pure dysthymia, 61% double depression, 64% chronic major depression at baseline of phase 1 Mean age: presented by type of depression rather than by treatment group: 37 (10) pure dysthymia, 37 (9) double depression, 36 (11) chronic major depression at baseline of phase 1 Inclusion criteria ‐ outpatients who met DSM‐III‐R diagnostic criteria for "pure" dysthymia, dysthymia with current major depression ("double depression"), or chronic major depression with full/partial remission after desipramine treatment (10 to 12 weeks acute and 16 weeks continuation phases) Definition remission Full remission: HAM‐D scores < 7 and GAS scores > 70 on 3 consecutive biweekly ratings Partial remission: ≥ 50% reduction from baseline HAM‐D score, HAM‐D score ranging from 7 to 12, and GAS ≥ 60 on 3 successive ratings Exclusion criteria ‐ diagnosis of schizophrenia ‐ current substance abuse or dependence ‐ history of mania or definite hypomania ‐ any severe or chronic medical illness or medical contraindication to desipramine |
|
| Interventions |
Intervention 1: identical placebo at the same dose equivalent Tapering scheme: tapered by approximately 25% a week over a month, then received identical placebo for the same dose equivalence for the next 100 weeks or until relapse Intervention 2: continuation of desipramine at the same dose Tapering scheme: tapered by approximately 25% per week over the month Co‐intervention: stable long‐term psychotherapy during the study is allowed |
|
| Outcomes | ‐ relapse Definition of relapse: HAM‐D > 12 and GAS < 60 on 3 successive ratings over 4 weeks, or at least 1 rating meeting these criteria and an urgent need for alternative treatment for a depressive syndrome ‐ time to relapse |
|
| Notes |
Funding: study was supported by a grant from the National Institute of Mental Health and the pharmaceutical industry; matching placebo was provided by the pharmaceutical industry COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement Quote: “double‐blind”; “plasma drug level was reviewed by a non‐blind observer who was not involved in the treatment. The nonblind observer gave instructions or dummy instructions for dosage adjustments” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: possible bias due to absence of independent raters is reported by study authors Quote: "ratings were done by study clinicians who may have been able to guess the maintenance treatment based on side effects" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 50/53 patients completed the study; number of dropouts balanced across both groups – 2/25 placebo and 1/28 desipramine |
| Selective reporting (reporting bias) | High risk | Comment: study protocol is not available (given age of study, might not have been a necessity then). Study does not measure safety outcomes (adverse events and withdrawal symptoms). Withdrawal symptoms were not an outcome. None of our secondary outcomes were reported. |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Kocsis 2007.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 1: acute treatment with venlafaxine ER (75 mg to 300 mg/d) (10 weeks) Phase 2: continuation treatment with venlafaxine ER (75 mg to 300 mg/d) (24 weeks) Duration post randomisation: 52 weeks; those who respond to venlafaxine were randomised in a second year of treatment (venlafaxine or placebo), as reported in Keller 2007 Aim: to test the long‐term efficacy and safety of venlafaxine extended release (ER) in preventing recurrence in patients with major depression |
|
| Participants |
Country: USA Setting: 29 clinical sites Type of AD: venlafaxine ER Duration of antidepressant treatment prerandomisation: 34 weeks Duration of antidepressant treatment post stabilisation: 24 weeks Number of participants: 821 entered phase 1, 530 entered phase 2, 409 completed phase 2 in remission or improved response Total number of randomised participants: 336 (172 placebo, 164 venlafaxine ER) Primary diagnosis: recurrent depression Number of previous episodes: at least 3; mean not reported Severity of depressive symptoms at randomisation (HAM‐D 17 score), mean (SD): 4.9 (3.5) placebo, 4.3 (3.3) venlafaxine ER Number of previous episodes: not reported Gender distribution (F): 67% placebo, 69% venlafaxine ER Mean age, mean, years: 42.6 placebo, 42.0 venlafaxine ER Inclusion criteria ‐ ≥ 18 years ‐ recurrent depression (DSM‐IV criteria): history ≥ 3 episodes of major depression, of which ≥ 2 episodes occurred in the past 5 years (including the current episode of phase 1) and ≥ 12 months between the end of the previous episode and the beginning of the current episode of phase 1 ‐ ≥ 20 HAM‐D 17 at screening and ≥ 18 at randomisation 1 week later for phase 1 and experienced depressive symptoms ≥ 1 month before the start of phase 1 of the study ‐Patients achieving a therapeutic response during the acute phase 1 were eligible to enter the 6‐month continuation phase 2. Patients who continued to demonstrate a therapeutic response at the end of the continuation phase 2 were eligible to enter RCT Definition of response/remission ‐ response: HAM‐D 17 total score ≤ 12 and ≥ 50% decrease from acute baseline ‐ remission: HAM‐D 17 score ≤ 7 Exclusion criteria ‐ patients who had failed an adequate trial of fluoxetine, venlafaxine, or venlafaxine ER during the current episode of major depression and those who were treatment resistant (i.e. had failed [1] ≥ 3 previous adequate trials of at least 2 classes of antidepressant medication, or [2] electroconvulsive therapy, or [3] 2 adequate trials of psychotherapy for mood disorder in the past 3 years) were not eligible to participate. Patients with known hypersensitivity to venlafaxine or fluoxetine were excluded, as were those with a history or presence of any of the following: clinically significant hepatic, cardiovascular, renal, or other serious medical disease that might compromise the study; seizure disorder other than a single childhood febrile seizure; bipolar disorder; OCD; eating disorder (if not remitted for 5 years); drug or alcohol dependence or abuse within 6 months before screening; any psychotic disorder, including psychotic depression; current postpartum depression; significant Axis II disorders; or any mental disorder due to a substance or medical condition. Patients were not eligible to participate if they met DSM‐IV criteria for a primary diagnosis of panic disorder, OCD, GAD, social phobia, or PTSD. Patients were excluded if the investigator judged them to be at risk for suicide to such a degree that required precautions against suicide; had clinically significant abnormalities on pre‐study physical examination, electrocardiogram (ECG), or laboratory tests; had a diagnosis of cancer in the past 3 years (excluding squamous or basal cell carcinoma) and/or had active neoplastic disease; or were women of childbearing age who were pregnant, breastfeeding, or not using a medically acceptable method of birth control. Use of any of the following was prohibited: any investigational drug, antipsychotic drug, fluoxetine, or monoamine oxidase inhibitor (MAOI) within 30 days, or ECT within 3 months of randomisation; any antidepressant, other than fluoxetine or an MAOI, within 14 days of randomisation; any anxiolytic, sedative‐hypnotic drug (except chloral hydrate or zaleplon), sumatriptan (and similar agents), or any other psychotropic drug or substance within 7 days of randomisation; or any non‐psychopharmacological drug with psychotropic effects within 7 days of randomisation, unless a stable dose of the drug had been maintained for ≥ 1 month before randomisation |
|
| Interventions |
Intervention 1: placebo Tapering scheme: a single down‐titration kit, which tapered the dose of venlafaxine ER over 4 weeks, was dispensed at the start of the maintenance phase Intervention 2: venlafaxine ER (75 mg/d to 300 mg/d). The dose received at the end of the continuation phase was maintained during the maintenance phase, with dose increases allowed to optimise treatment response |
|
| Outcomes |
Primary outcomes ‐ time to recurrence Definition of relapse/recurrence Primary definition HAM‐D 17 score > 12, with HAM‐D 17 score that was less than 50% lower than the acute phase baseline at 2 consecutive visits or at the last valid visit before patient’s discontinuation, and meeting DSM‐IV criteria for major depressive disorder as judged by a senior investigator Secondary definition ("clinical definition") Having 1 visit with a HAM‐D 17 score > 12, with a difference in HAM‐D 17 score from acute phase baseline of not more than 50%, and not meeting the primary definition of recurrence, HAM‐D 17 ratings were performed by individuals who had been trained and certified. Abstracts of the data, including mood ratings and clinical notes from case report forms, were presented to the recurrence review committee ‐ a committee of experienced psychiatrists who assessed whether each of these patients experienced recurrence after a review of the blinded clinical data Secondary outcomes ‐ depressive symptoms, measured with Clinical Global Impressions‐Severity of Illness (CGI‐S) scale (monthly), Inventory for Depressive Symptomatology Self‐Report (IDS‐SR) (monthly), Rothschild Scale for Antidepressant Tachyphylaxis (monthly), 3‐monthly Hamilton Rating Scale for Anxiety (HAM‐A), and Longitudinal Internal Follow‐up Evaluation ‐ quality of life measured 3‐monthly with 36‐item Short Form Health Survey (SF‐36), Quality of Life Enjoyment and Satisfication Questionnaire (Q‐LES‐Q), Life Enjoyment Scale‐Short version, Social Adjustment Scale Self‐Report (SAS‐SR) ‐ safety, monitored by reports of adverse events, vital sign measurements (supine pulse, standing and supine blood pressure), and laboratory evaluations |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry COI: study authors were employees of the pharmaceutical company or received grants from the pharmaceutical industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer random number generator Quote: "...were randomly assigned in a double blind fashion in a 1:1 ratio to receive either venlafaxine or placebo..."; "for each phase of the study, the randomisation records were stratified by site and were generated using a block size of four. A central randomisation scheme was implemented using Quintiles' IVR system" |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation Quote: "after a site deemed a patient eligible to enrol/continue in the study, they contacted the IVR system, which ascertained the site where the patient was located and then the patient was allocated to the next available treatment assignment (i.e. next sequence number) in the randomisation schedule for that site" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and key study personnel ensured; unlikely that blinding could have been broken Quote: "both patients and investigators remained blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not enough information to permit a judgement Quote: "HAM‐D 17 ratings were performed by individuals who had been trained and certified. Abstracts of the data, including mood ratings and clinical notes from the case report forms were presented to the recurrence review committee: a committee of experienced psychiatrist who assessed whether each of these patient experienced recurrence after a review of the blinded clinical data" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high rates of dropout in both groups (98/135 placebo and 66/132 venlafaxine) and higher in placebo group Quote: “approximately 33 placebo patients, all of whom were enrolled on or before March 24, 2002, inadvertently received down‐titration kits at more than 1 post taper period visit (when they should have received placebo kits). Therefore, an efficacy evaluable population was defined, which excluded all patients directly affected by the kit dispensing error as well as all patients who were enrolled into maintenance treatment during the same period. Thus, the efficacy evaluable population included all patients in the intent‐to‐treat population who were enrolled into maintenance treatment after March 24, 2002, and was the primary population of interest for all efficacy analyses (venlafaxine ER, N = 129; placebo, N = 129)” |
| Selective reporting (reporting bias) | High risk | Comment: adverse events reported; withdrawal symptoms not an outcome |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Kornstein 2006.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised controlled trial Prerandomisation phase Phase 1: acute treatment with 1 of 4 SSRIs (fluoxetine, sertraline, paroxetine, citalopram) (8 weeks) Phase 2: continuation treatment with escitalopram 10 to 20 mg (16 weeks) Duration post randomisation: 52 weeks Aim: to examine the efficacy of maintenance escitalopram treatment in preventing depression recurrence in patients who responded to acute SSRI therapy |
|
| Participants |
Country: USA Setting: 28 centres Type of AD: escitalopram Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 16 weeks Number of participants: 515 entered phase 1, 386 completed phase 1, and 259 responded to phase 1; 234 entered phase 2, 164 completed phase 2, and 139 responded to phase 2 Total numbers of randomised participants: 139 (66 placebo, 73 antidepressant) Primary diagnosis: recurrent major depressive disorder Number of previous episodes, mean (SD): 5.8 (6.0) placebo, 4.7 (3.1) antidepressant Gender distribution (female): placebo 78.8%, escitalopram 79.5% Mean age, years (SD): placebo 43.7 (12.4), escitalopram 42.0 (11.3) Severity of depressive symptoms at randomisation MADRS (mean (SD)): placebo 4.9 (3.6), escitalopram 4.7 (4.0) HAM‐D (mean (SD)): placebo 5.2 (3.8), escitalopram 5.2 (4.0) CGI‐I (mean (SD)): placebo 1.2 (0.4), escitalopram 1.2 (0.5) CGI‐S (mean (SD)): placebo 1.6 (0.7), escitalopram 1.5 (0.6) Inclusion criteria ‐ DSM‐IV criteria for a current major depressive episode ≥ 4 weeks’ duration and ≥ 2 major depressive episodes before the index episode, with 1 of the episodes resolving within the previous 5 years. A MADRS total score ≥ 22 and ≥ 2 on item 1 of the HAM‐D were also required, both at screening and at baseline. Enrolled patients had normal or clinically insignificant findings on physical examination, laboratory tests, and 12‐lead ECGs at the screening visit ‐ responded to acute open‐label treatment and maintained response criteria at the end of the continuation treatment Definition of response/remission: MADRS score ≤ 12 Exclusion criteria ‐ DSM‐IV criteria for bipolar disorder, schizophrenia, or any psychotic disorder ‐ OCD ‐ mental retardation ‐ any pervasive developmental or cognitive disorder ‐ diagnosis of any Axis I disorder other than MDD (including dysthymic disorder) ‐ history of any psychotic disorder ‐ exhibited any psychotic features ‐ significant personality disorder ‐ history of substance abuse or dependence (other than nicotine) in the previous 6 months per DSM‐IV criteria ‐ presenting suicide risk ‐ ≥ 5 on MADRS item 10 (suicidality) ‐ required concomitant psychotropic medication (other than zolpidem for sleep) ‐ women who were pregnant or nursing and were required to practise a reliable method of birth control |
|
| Interventions |
Intervention 1: placebo Tapering scheme: abrupt, without down‐tapering Intervention 2: escitalopram 10 to 20 mg/d; same dose that was administered at the end of the continuation period |
|
| Outcomes |
Primary outcome ‐ time to recurrence Definition of relapse: MADRS score ≥ 22 or withdrawal from the study due to insufficient treatment response based on the judgement of the principal investigator Secondary outcomes ‐ depressive symptoms ‐ global severity of illness using HAM‐ D 24, CGI‐I, and CGI‐S ‐ adverse events (treatment‐emergent adverse events and serious adverse events) |
|
| Notes |
Funding: supported by a grant from the pharmaceutical industry (Forest Research Institute is an affiliate of the pharmaceutical industry) COI: principal study author has received research support and honoraria from the pharmaceutical industry and has served on the advisory board including the funding company. Other co‐authors are employees of the funding company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned on a 1:1 ratio” Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: information insufficient to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high dropout in placebo group (45%) and 31% in antidepressant group; 18% of placebo patients (12/66) completed the trial compared to 51% of escitalopram patients (37/73) |
| Selective reporting (reporting bias) | High risk | Comment: protocol is not available; reports include all expected outcomes, and adverse events were reported. Withdrawal symptoms were not an outcome |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Kupfer 1992.
| Study characteristics | ||
| Methods |
Design: multi‐phase randomised controlled trial Prerandomisation phase Phase 1: short‐term treatment with interpersonal psychotherapy (IPT) and imipramine (duration not clearly reported) Phase 2: continuation treatment with IPT and imipramine (17 weeks) Phase 3: maintenance treatment with imipramine with or without IPT (156 weeks) Duration post randomisation: 104 weeks Aim: to determine whether maintaining antidepressant medication at the dosage used to treat the acute episode beyond 3 years would continue to provide a significant prophylactic effect compared with medication discontinuation after the 3 years of effective maintenance treatment |
|
| Participants |
Country: USA Setting: not reported Type of AD: imipramine Duration of antidepressant treatment prerandomisation: 173 weeks + short‐term treatment (duration not clearly reported) Duration of antidepressant treatment post stabilisation: 173 weeks Number of randomised participants: 230 entered study, 157 entered phase 2, 128 completed and responded to phase 2, 128 entered phase 3, 106 completed and responded to phase 3, 28 completed phase 3 and remained in remission Total numbers of randomised participants: 20 (9 placebo, 11 antidepressant) Primary diagnosis: recurrent depression Number of previous episodes, mean (SD): 6.4 (4.4) placebo, 6.5 (3.1) antidepressant Gender distribution (male): 50% placebo, 37.5% antidepressant Mean age, years (SD): 44.3 (9.9) placebo, 35.6 (8.2) antidepressant Severity of depressive symptoms at randomisation (SD) HDRS‐17: 3.7 (1.5) placebo, 3.0 (2.1) antidepressant HDRS‐25: 5.1 (1.6) placebo, 3.9 (2.0) antidepressant Inclusion criteria ‐ 21 to 65 years of age ‐ ≥ 3 episodes unipolar depression, immediately preceding episode ‐ < 2.5 years before onset of present episode ‐ ≥ 10 weeks remission (RDC criteria) between index episode and immediately preceding episode ‐ HDRS ≥ 15 and Raskin ≥ 7 at entry to short‐term treatment Definition of response/remission ‐ HDRS ≤ 7 and Raskin ≤ 5 for 3 weeks Exclusion criteria ‐ any other Axis I diagnosis except GAD or panic disorder ‐ antisocial or borderline personality disorder ‐ any condition considered to be incompatible with imipramine therapy |
|
| Interventions |
Intervention 1 ‐ placebo Tapering scheme: reduction of 33% a week for first 3 weeks; varied slightly depending on the number of pills the patient was taking at random assignment Intervention 2 ‐ active imipramine: target dose 200 mg, at the same dose patient had been taking at the end of the 3‐year maintenance treatment Co‐interventions ‐ maintenance IPT (methods by Klerman) if patient received it during the first 3 phases of the study (13 participants). The purpose of IPT was to improve social adjustment and thus to provide additional protection against future episodes. Study authors elected IPT because of pronounced deficits in social adjustment that they had observed, even among patients who remained well for 2 years ‐ all participants received the same acute treatment consisting of a combination of imipramine and IPT. IPT treatment sessions were scheduled weekly for 12 weeks, then biweekly for 8 weeks, then monthly. If patients met the criteria for remission (at whatever point in this short‐term treatment regimen), they continued to receive combined treatment for an additional 17 weeks. IPT was conducted according to the methods described by Klerman et al. Participants met with their primary therapist for 45 to 50 minutes of IPT, after which they were joined by the physician member of the treatment team that was responsible for the pharmacotherapy. During the 17‐week continuation treatment phase (phase 2), patients continued to see their psychotherapist but with decreasing frequency in preparation for maintenance treatment. Toward the end of the 17‐week continuation treatment phase, therapists and patients discussed the possibility that psychotherapy and active medication would be discontinued, and a review of psychotherapeutic accomplishments in the short term and in continuation treatment phases was carried out. In phase 3 of the trial; participants were randomised to monthly IPT sessions or to discontinuation of IPT sessions. In the discontinuation trial, patients were randomised to receive continuation pharmacotherapy or placebo and were informed that they would continue to receive psychotherapy or monthly medication clinic visits consistent with their previous maintenance treatment phase ‐ medication clinics monthly (7 participants) |
|
| Outcomes |
Primary outcome ‐ recurrence of major depression Definition of relapse/recurrence ‐ HDRS ≥ 15 and Raskin ≥ 7 on 2 occasions within 7 days by an independent clinical evaluator and confirmed by clinical evaluation of senior psychiatrist ‐ survival time |
|
| Notes |
Funding: study supported by a grant from the National Institute of Mental Health COI: not reported Of the 20 patients, 13 continued to receive psychotherapy once a month, and the remaining 7 attended medication clinic NO significant difference was evident in the number of individuals receiving psychotherapy and active medication vs psychotherapy (n = 6) vs psychotherapy and placebo (n = 7) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned” Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind, both the patients and members of their treatment team remained blind to whether they were receiving active medication or placebo, only the research pharmacist and one physician, who had no direct care patient responsibilities, were aware of patients’ actual treatment assignments” Comment: blinding of participants and personnel ensured; unlikely that blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the patient was seen by an independent senior psychiatrist not affiliated with the study and who was blind to the patient’s maintenance treatment assignment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all 20 patients accounted for. 1 in the active medication group did not complete the trial; reason given was non‐compliance with medication |
| Selective reporting (reporting bias) | High risk | Comment: the study report fails to include results for adverse events and withdrawal symptoms that would be expected to have been reported for such a study |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Kuyken 2008.
| Study characteristics | ||
| Methods |
Design: randomised controlled trial Prerandomisation phase: no Duration post randomisation: 64 weeks Aim: to examine whether MBCT provides an alternative approach to maintenance ADM in preventing depressive relapse/recurrence |
|
| Participants |
Country: UK Setting: community adults (primary care practices across a range of urban and rural locations; primary care physicians then screened the list of selected patients and wrote letters to potential participants describing the study, enclosing the study information sheet, and stating that unless they decided to opt out, they would be contacted by a member of the study team) Type of antidepressant: SSRI (58%), TCA (22%), or combination (20%) Duration of antidepressant treatment prerandomisation: 6 months or longer of maintenance treatment; mean time 340 days Duration of antidepressant treatment post stabilisation: not described Primary diagnosis: recurrent depression Number of previous episodes, median: discontinuation 6, antidepressant 6; with more than 10 episodes: 38% discontinuation, 31% continuation Total numbers of randomised participants: 123 (61 discontinuation, 62 antidepressant) Severity of depressive symptoms at randomisation, HDRS score, mean (SD): 5.62 (4.3) discontinuation, 5.76 (4.69) antidepressant In full remission (HDRS < 8), n (%): discontinuation 42 (69%), 41 (66%) antidepressant In partial remission (HDRS ≥ 8), n (%): discontinuation 19 (31%), 21 (34%) antidepressant Gender distribution (male): 77% discontinuation, 76% antidepressant Mean age, years (SD): 48.95 (10.55) discontinuation, 49.37 (11.84) antidepressant Inclusion criteria ‐ ≥ 18 years ‐ recurrent depression (history of ≥ 3 previous episodes) (DSM–IV criteria) ‐ treated with a therapeutic dose of maintenance antidepressant treatment in line with the British National Formulary ≥ previous 6 months ‐ currently in full or partial remission from the most recent episode of depression Definition of remission ‐ full remission: asymptomatic: HDRS < 8, partial remission: HDRS ≥ 8 Exclusion criteria ‐ comorbid diagnoses of current substance dependence ‐ organic brain damage ‐ current/past psychosis ‐ bipolar disorder ‐ persistent antisocial behavior ‐ persistent self‐injury requiring clinical management/therapy ‐ unable to engage with MBCT for physical, practical, or other reasons (e.g. very disabling physical problem, unable to comprehend materials) ‐ formal concurrent psychotherapy |
|
| Interventions |
Intervention: mindfulness‐based cognitive therapy (MBCT) and support to taper/discontinue antidepressant (no placebo) Tapering scheme: tapering/discontinuation regimens were determined by physicians and patients. Patients and physicians were initially prompted to begin to discuss a tapering/discontinuation regimen after 4 to 5 weeks of MBCT. At the end of MBCT, patients were reminded to ensure a tapering/discontinuation regimen was in place MBCT ‐ mindfulness‐based cognitive therapy was delivered in primary care settings with MBCT groups of 9 to 15 patients following the treatment protocol (Segal, Williams, & Teasdale, 2002) ‐ 2‐hour sessions over 8 consecutive weeks, followed by 4 follow‐up sessions in the following year ‐ session content included guided mindfulness practices (i.e. body scan, sitting meditation, yoga); inquiry into patients’ experience of these practices; review of weekly homework (i.e. 40 minutes of mindfulness practice per day and generalisation of session learning); and teaching/discussion of cognitive–behavioural skills ‐ 5 groups were instructed by trained clinical psychologist or an occupational therapist (both therapists had undergone a training programme taught and supervised by one of the developers of MBCT (John D. Teasdale), had the experience of running at least 2 supervised pilot groups, and had an ongoing personal mindfulness practice). An independent check on therapist competency was established before therapists progressed to running trial groups: an experienced MBCT therapist independent of the trial rated at least 2 videotapes of MBCT therapy sessions and made an overall judgement as to whether the therapists were competent ‐ an adequate dose of MBCT was defined as participation in at least 4 of the 8 MBCT group sessions ‐ patients were supported in tapering and discontinuing their ADM by their primary care physician. Tapering/discontinuation regimens were determined by physicians and patients, although the research team asked that patients consider tapering/discontinuing their medication as soon following MBCT as they deemed appropriate and within 6 months of the MBCT group ending. This allowed (1) tapering to be conducted at a pace determined by physicians and patients and (2) a substantial window to the study’s end when patients had discontinued m‐ADM to monitor primary and secondary outcomes. The study team provided guideline information to physicians and patients about typical tapering/discontinuation regimens and possible withdrawal effects. If at any time the study team became aware of difficulties with medication tapering/ discontinuation, the MBCT therapist first contacted the patient to understand the difficulty, and then whenever appropriate encouraged the patient together with his or her physician to review the tapering/discontinuation regimen Compliance: all trial groups were videotaped with digital cameras for therapist supervision, checks on therapist competence, and checks on treatment adherence Control: maintenance antidepressant treatment ‐ changes in medication sometimes occurred during the maintenance treatment stage, but physicians and participants were asked to ensure the dose remained within therapeutic limits ‐ participants were monitored and treated by their physicians in primary care settings. During the maintenance phase, physicians were asked to manage antidepressant treatment in line with standard clinical practice ‐ primary care physicians were asked to meet with participants regularly to review their medication treatment ‐ protocol adherence was defined as continuing to take m‐ADM at a therapeutic maintenance dose for the duration of the trial ‐ compliance: medication adherence was monitored through patients’ self‐report at follow‐ups every 3 months, practice databases, and the Morisky Medication Adherence Scale (MMAS). If there were ongoing problems with adherence, these were addressed on a case‐by‐case basis with the goal of encouraging patients to continue taking a therapeutic dose of m‐ADM for the duration of the follow‐up period. Protocol adherence was defined as continuing to take antidepressants at a therapeutic maintenance dose for the duration of the trial |
|
| Outcomes |
Primary outcomes ‐ relapse/recurrence; retrospectively assessed the 3‐month period between assessments every 3 months Definition of relapse/recurrence ‐ an episode meeting DSM–IV criteria for major depressive disorder using structured clinical interview for DSM‐IV Axis I disorders (SCID‐I) Secondary outcomes ‐ severity of relapse/recurrence: using DSM–IV specifiers: “mild,” “moderate,” “severe without psychotic features,” and “severe with psychotic features” (scale range 1 to 4) ‐ duration of relapse/recurrence: period of time in months that a person met SCID‐I criteria ‐ associated distress of relapse/recurrence, which was rated by patients on a 1 to 100‐point scale ranging from 0 (the least distressing episode of depression I have ever experienced) to 100 (the most distressing episode of depression I have ever experienced) ‐ residual depressive symptoms: observer‐rated interviewer‐administered 17‐item version of the HDRS and the 21‐item self‐report BDI ‐ psychiatric comorbidity; comorbid diagnoses identified at intake were reassessed at study end using relevant SCID‐I modules ‐ quality of life: the 26‐item, self‐report, short version of the WHO Quality of Life instrument (WHO QoL‐BREF) ‐ economic evaluation |
|
| Notes |
Funding: study was supported by a grant from the UK Medical Research Council COI: no financial or other conflicts of interest Pilot trial of Kuyken 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation by using computer random number generator Quote: "block randomisation (block size 4) to the two groups was performed by an independent statistician using computer‐generated quasi‐random numbers" |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation concealment Quote: "block randomisation to the two groups was performed by an independent statistician using computer‐generated quasi‐random numbers” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and prescribing physician was not possible due to the nature of the intervention Quote: "if problems were identified at any assessment point, these were resolved through dialogue between a member of the research team not blind to treatment condition, the prescribing physician, and the patient, but we ensured that the research officer conducting follow‐ups remained blind to treatment condition" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors are described as blinded to treatment allocation Quote: "patients were assessed by research staff blind to treatment allocation at intake and then again every 3 months up to 15 months post randomisation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: low missing outcome data equal across groups (9/61 MBCT group and 10/62 antidepressant group) and reasons reported; inappropriate method used to impute missing data for secondary outcome (see discussion) Quote: "the analysis was performed according to the principle of intention to treat (ITT; i.e. all patients according to and included in random allocation)”; "for the primary survival outcome analyses, drop out/missing data were handled by censoring"; "for the small subset of cases with missing data on secondary outcomes, we used last variable carried forward to impute missing data“ |
| Selective reporting (reporting bias) | High risk | Comment: study protocol available; majority of predefined primary and secondary outcomes reported. Study reported no adverse events but insufficient information about methods to judge. Other adverse events and withdrawal symptoms were not reported Quote: "no adverse events were recorded through the oversight of the Trial Steering Committee" |
| Other bias | Low risk | No other bias |
Kuyken 2015.
| Study characteristics | ||
| Methods |
Design: multi‐centre single‐blind randomised controlled trial Prerandomisation phases: no Duration post randomisation: 104 weeks Aim: to determine whether MBCT with support to taper or discontinue antidepressant treatment (MBCT‐TS) was superior to maintenance antidepressants for prevention of depressive relapse or recurrence over 24 months |
|
| Participants |
Country: UK Setting: community adults (from 4 general practices) Type of antidepressant: not described Treatment of antidepressant prerandomisation: on a therapeutic maintenance dose of maintenance treatment; mean not reported Treatment of antidepressant post stabilisation: on a therapeutic maintenance dose of maintenance treatment; mean not reported Primary diagnosis: recurrent depression Previous major depressive episodes: < 6 episodes: 57% discontinuation, 50% antidepressant; ≥ 6 episodes: 43% discontinuation, 50% antidepressant Total numbers of randomised participants: 424 (212 discontinuation, 212 antidepressant) Severity of depressive symptoms at randomisation GRID‐HAM‐D, n (SD): 4.8 (4.3) discontinuation; 4.6 (4.3) antidepressant Asymptomatic at randomisation (GRID‐HAM‐D < 8): 77% discontinuation, 76% antidepressant Symptomatic at randomisation (GRID‐HAM‐D ≥ 8): 23% discontinuation, 24% antidepressant Gender distribution (male): 71% discontinuation, 82% antidepressant Mean age, years (SD): 50 (12) discontinuation, 49 (13) antidepressant Inclusion criteria ‐ diagnosis of recurrent major depressive disorder (DSM‐IV criteria) in full or partial remission ‐ ≥ 3 previous major depressive episodes ‐ ≥ 18 years of age ‐ on a therapeutic dose of maintenance antidepressant drugs in line with BNF and NICE guidance Definition of remission ‐ full remission: GRID‐HAM‐D < 8, partial remission: GRID‐HAM‐D ≥ 8 Exclusion criteria ‐ current major depressive episode ‐ comorbid diagnosis of current substance misuse ‐ organic brain damage ‐ current or past psychosis, including bipolar disorder ‐ persistent antisocial behaviour ‐ persistent self‐injury needing clinical management or therapy ‐ formal concurrent psychotherapy |
|
| Interventions |
Intervention: mindfulness‐based cognitive therapy (MBCT) with support to taper or discontinue antidepressant treatment (MBCT‐TS) (no placebo) Tapering: support to taper or discontinue maintenance antidepressants. Patients received support to support to taper or discontinue their antidepressant treatment both from the MBCT therapist and their GP. The study team provided guideline information to GPs and patients about typical tapering or discontinuation regimens and possible withdrawal effects. The guidelines recommended that patients begin a tapering regimen after 4 to 5 weeks of treatment; however, GPs and patients determined the tapering or discontinuation regimen. Letters signed by the chief investigator and the trial GP were sent to patients’ GPs and were copied to the patient, prompting the GP to have a discussion with the patient about a suitable tapering or discontinuation regimen after 4 to 5 weeks of MBCT‐TS group sessions. At the end of the 8 MBCT‐TS sessions, another letter was sent to remind the GP to ensure a tapering or discontinuation regimen was in place MBCT ‐ based on the protocol of Segal, Williams, and Teasdale ‐ a fully manualised psychosocial intervention with the treatment rationale for each session ‐ an 8‐week, group‐based programme (12 to 15 patients per group) designed to teach skills that prevent depressive relapse/recurrence ‐ consists of eight 2.25‐hour group sessions, normally over consecutive weeks, with 4 refresher sessions approximately every 3 months for the following year ‐ MBCT therapists are mental health professionals with extensive training in MBCT Compliance: during the trial, a rater assessed 2 sessions from each of the 21 MBCT‐TS courses using the MBCT‐Adherence Scale (AS), which indicated that the MBCT teaching was provided at required competency or adherence levels and above Intervention 2: maintenance antidepressants. Patients in the maintenance antidepressant group received support from their GP to maintain a therapeutic level of antidepressant medication in line with BNF and NICE guidelines for the 2‐year follow‐up period |
|
| Outcomes |
Primary outcomes ‐ time to relapse/recurrence of depression according to the depression module of the Structured Clinical Interview for DSM–IV Axis I disorders (SCID‐I) at 5 separate intervals Definition of relapse or recurrence ‐ an episode meeting DSM‐IV criteria for a major depressive episode assessed by SCID‐I Secondary outcomes ‐ number of depression‐free days based on episode duration as assessed by the SCID‐I ‐ residual depressive symptoms assessed by the GRID‐HAM‐D and 21‐item self‐report BDI ‐ psychiatric and medical comorbidity using relevant SCID‐I modules and medical comorbidity using the Medical Symptom Checklist ‐ quality of life using the WHO Quality of Life instrument (WHO QoL‐BREF) and health‐related quality of life using the EQ‐5D‐3L (3‐level version) ‐ cost‐effectiveness Assessments for all outcomes at baseline, 1 month after the end of the 8‐week MBCT programme (or equivalent time in the maintenance antidepressant group), which varied between 12 and 24 weeks post randomisation, and at 9, 12, 18, and 24 months post randomisation |
|
| Notes |
Funding: study supported by a grant from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme and the NIHR Collaboration for Leadership in Applied Health Research and Care South West Peninsula COI: WK and AE are co‐directors of the Mindfulness Network Community Interest Company and teach nationally and internationally on MBCT. The other study authors declare no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence generation by computer random number generator Quote: "participants were randomly assigned to either MBCT‐TS or maintenance antidepressants (in a 1:1 ratio) with a computer‐generated random number sequence with stratification by centre and symptomatic status" |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation was used to conceal allocation Quote: "allocation was undertaken using a password‐protected website maintained by the Peninsula Clinical Trials Unit, independent of the trial. The trial administrator informed participants of the outcome of randomisation via a letter; research assessors remained masked to treatment allocation for the duration of the follow‐up period" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and prescribing physician was not possible due to the nature of the intervention. Some outcomes are likely to be influenced by lack of blinding Quote: "in view of the nature of the interventions, patients and clinicians were aware of treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment ensured; unlikely that the blinding could have been broken Quote: "research assessors remained masked to treatment allocation for the duration of the follow‐up period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropouts equal between groups and reported at each follow‐up stage of the study. 10% of participants were censored at their last follow‐up for the primary outcome |
| Selective reporting (reporting bias) | High risk | Comment: study protocol is available; all predefined primary and secondary outcomes were reported in pre‐specified way; only serious adverse events were monitored; other adverse events and withdrawal symptoms were not reported |
| Other bias | Low risk | None |
Mavissakalian 1999.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 0: single‐blind placebo run‐in (2 weeks) Phase 1: acute open‐label treatment with imipramine (24 weeks) Duration post randomisation: 52 weeks Aim: to assess the 12‐month cumulative risk of relapse specifically due to discontinuation of imipramine and to test the hypothesis that maintenance treatment with imipramine protects patients with panic disorder and agoraphobia from such reversals |
|
| Participants |
Country: USA Setting: community adults (outpatients from Phobia and Anxiety Disorders Clinic, Ohio State University Medical Center, Columbus; either trough clinical referrals or in response to media coverage or advertisement) Type of AD: imipramine Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 0 weeks Number of participants: 110 entered phase 1, 59 were in stable remission after phase 1 Total number of randomised participants: 56 (27 placebo, 29 antidepressant) Primary diagnosis: panic disorder with agoraphobia Severity of anxiety symptoms at randomisation, mean (SD) GAS: 1.37 (0.49) placebo, 1.59 (0.5) antidepressant Self‐rating severity: placebo 0.81 (0.92), 1.1 (0.82) antidepressant 17‐HRDS: placebo 3.85 (3.5), 3.9 (2.88) antidepressant Comorbidity: 1 or 2 concurrent anxiety disorders (%): 37% placebo, 27.6% antidepressant Gender distribution (F): 20% placebo, 18% antidepressant Mean age, years (SD): 37.89 (9.92) placebo, 34.28 (8.23) antidepressant Inclusion criteria ‐ patients who met operationalised response criteria at both 16‐ and 24‐week assessments after 24 weeks of imipramine were entered into phase 2 of the trial ‐ meeting SCI DSM‐III‐R diagnostic criteria for panic disorder with agoraphobia ≥ moderate severity and 3 months’ duration and experiencing active recurrent panic attacks at the time of initial evaluation ‐ free of psychotropic drug use for 14 days before treatment began, except patients who were unable to stop using benzodiazepines initially (and have to taper off gradually, starting at Week 4 and discontinued by Week 16 assessment) to qualify for randomisation to the maintenance study. 3 patients in each group began treatment while taking benzodiazepines Definition of response/remission: ≥ 50% improvement from pretreatment scores or a cutoff score signifying mild to absent symptoms simultaneously on 6 or all of these measures Exclusion criteria ‐ evidence of organic mental disorders ‐ psychotic, bipolar, and OCD ‐ primary or current major depression with melancholia ‐ suicidal intention or score ≥ 18 on HDRS‐17 ‐ PTSD somatisation disorder, severe personality disorders (borderline, schizotypal), and substance abuse disorder (current or in remission for < 6 months). In addition, patients had to be in good general health, have no contraindications for the use of imipramine because of illness or because of treatment necessary for the illness, and had to demonstrate compliance during an initial 2‐week single‐blind placebo run‐in |
|
| Interventions |
Intervention 1: placebo (4 identical looking tablets) Tapering scheme: 25% decrements in dose each week so that dose of 0 mg was reached on the 22nd day of randomisation. Identical‐looking tablets daily at bedtime Intervention 2: imipramine at same dose (10 mg, 25 mg, 50 mg, or 75 mg), identical looking tablets daily at bedtime. Target dose of 2.25 mg/kg per day Co‐intervention: no additional psychological or psychiatric treatments, but if participants had problems, additional visits for supportive intervention were provided (brief 1‐ or 2‐session, crisis‐type intervention) |
|
| Outcomes | ‐ relapse: based on composite index of End‐State (ESF range 0 to 7) derived from 7 measures
‐ definition of worsening: ESF ≤ 4 signified at least a 33% decline in ESF Defnition of relapse ‐ at which time patients exited the study, also required that worsening be accompanied by insistent requests for therapeutic action and/or that worsening still be present in a repeated confirmatory assessment 2 weeks later ‐ survival analysis for worsening ‐ survival time without relapse, worsening, exiting (months) ‐ number of panic attacks (DSM‐III definition of panic attacks, 2‐week diary, monthly) ‐ depressive symptoms assessed by 17‐HDRS |
|
| Notes |
COI: not reported Funding: supported by grant from the National Institute of Mental Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random sequence generation by computer random number generator Quote: “hospital pharmacist, who used computer numbers to randomly assign patients to same‐dose imipramine continuation or placebo substitution” |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation Quote: "treatment condition was known only by the hospital pharmacist, who used computer numbers"; “hospital pharmacist, who used computer numbers to randomly assign patients to same‐dose imipramine continuation or placebo substitution” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and key study personnel ensured; unlikely that the blinding could have been broken Quote: "dated packets prepared by the hospital pharmacist, each containing four identical looking tablets composed of placebo or 10, 25, 50, or 75 mg of imipramine hydrochloride to be taken at bedtime"; "treatment condition was only known by the hospital pharmacist" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement Quote: "same clinical psychologist administered all diagnostic and clinical assessments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 62% of placebo and 31% of imipramine patients did not complete |
| Selective reporting (reporting bias) | High risk | Comment: adverse events and withdrawal symptoms are not reported and are fundamental outcomes in drug discontinuation studies. Panic attack/HAM‐D 17 measures reported only for participants who had relapse after discontinuation of antidepressant |
| Other bias | Low risk | None |
Mavissakalian 2001.
| Study characteristics | ||
| Methods |
Design: double‐blind placebo‐controlled RCT (i.e. second‐year double‐blind extension of Mavissakalian 1999 RCT) Prerandomisation phases Phase 0: single‐blind placebo run‐in (2 weeks) Phase 1: acute open‐label treatment with imipramine (24 weeks) Phase 2: continuation treatment with imipramine (52 weeks) (results reported in Mavissakalian 1999); remitters entered this study and were randomised to imipramine or to placebo Duration post randomisation: 52 weeks Aim: to further explore the protective effects of long‐term maintenance imipramine therapy for panic disorder in patients who survived, in stable remission, in the 1‐year maintenance discontinuation study |
|
| Participants |
Country: USA Setting: community adults (outpatients from Phobia and Anxiety Disorders Clinic, clinical referrals, or in response to media coverage or advertisement) Type of antidepressant: imipramine Duration of antidepressant treatment prerandomisation: 76 weeks Duration of antidepressant post stabilisation: 52 weeks Number of participants: 110 entered phase 1, 59 in stable remission after phase 1, 56 entered phase 2, 30 completed in stable remission phase 2 Total number of randomised participants: 11 (7 placebo, 4 imipramine); 7 continued placebo and were not relevant for this review Primary diagnosis: panic disorder with agoraphobia Severity of anxiety symptoms at randomisation, mean (SD) GAS: 1.53 (0.51); self‐rating severity: 1.03 (0.89); 17‐HRDS: 4.07 (3.23) Comorbidities (n): 1 or 2 concurrent anxiety disorders 26.7% Mean age, years (SD): 35.47 (8.3) Gender distribution: not reported Inclusion criteria ‐ meeting SCID‐I DSM‐III‐R diagnostic criteria for panic disorder with agoraphobia of at least moderate severity and 3 months’ duration; patients must have been experiencing active, recurrent panic attacks at the time of initial evaluation ‐ fear of panicking or losing control must have been the primary motivation for escape or avoidance behaviours ‐ those who met operationalised response criteria at both 16‐ and 24‐week assessments after 24 weeks of imipramine were entered into phase 2 of the trial. Patients who survived, in stable remission, the first 12 months of maintenance/discontinuation were entered into the second‐year extension phase (Mavissakalian 2001). Only those patients on imipramine were re‐randomised Definition of response: either ≤ 50% improvement from pretreatment scores or a cut‐off score signifying mild to absent symptoms simultaneously on 6 or all of these measures (ESF score > 6) Exclusion criteria ‐ evidence of organic mental disorders ‐ psychotic, bipolar, and obsessive‐compulsive disorders ‐ primary or current major depression with melancholia ‐ suicidal intention or score ≥ 18 on HDRS‐17, PTSD, somatisation disorder, severe personality disorder (borderline, schizotypal), and substance abuse disorder (current or in remission for < 6 months). In addition, patients had to be in good general health, had to have no contraindications for the use of imipramine because of illness or because of treatment necessary for the illness, and had to demonstrate compliance during an initial, 2‐week, single‐blind placebo run‐in |
|
| Interventions |
Intervention 1: placebo (4 identical looking tablets) Tapering scheme: 25% decrements in dose each week so that dose of 0 mg was reached on the 22nd day of randomisation Intervention 2: same dose of imipramine (2.25 mg/kg/d) Co‐intervention: no additional psychological or psychiatric treatments, but if participants had problems, additional visits for supportive intervention were provided (brief 1 or 2 sessions, crisis‐type intervention) |
|
| Outcomes | Relapse: based on composite index of End‐State Functioning (ESF, range 0 to 7) derived from 7 measures
Definition of worsening: ESF ≤ 4 signified at least a 33% decline in ESF and defined worsening Defnition of relapse: at which time patients exited the study, also required that worsening be accompanied by insistent requests for therapeutic action and/or that worsening still be present in a repeated confirmatory assessment 2 weeks later Survival time without relapse, worsening, exiting in months ‐ number of panic attacks (DSM‐III definition of panic attacks, 2‐week diary, monthly) ‐ depressive symptoms assessed by 17‐HDRS |
|
| Notes |
COI: not reported Funding: supported by a grant from the National Institute of Mental Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation by computer random number generator Quote: “hospital pharmacist, who used computer numbers to randomly assign patients to same‐dose imipramine continuation or placebo substitution”; “those on imipramine will be randomly assigned to placebo substitution or imipramine continuation” |
| Allocation concealment (selection bias) | Low risk | Comment: pharmacist controlled randomisation Quote: “drug condition was known only by the hospital pharmacist ... reassigned following a 2:1 ratio of those on imipramine to placebo substitution or imipramine continuation"; “identical looking tablets” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and key study personnel ensured; unlikely that blinding could have been broken Quote: "double‐blind"; "4 identical looking tablets daily..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 3/7 placebo and 1/4 imipramine patients did not complete the study |
| Selective reporting (reporting bias) | High risk | Comment: withdrawal symptoms and adverse events are not reported; they are fundamental outcomes in drug discontinuation studies |
| Other bias | Low risk | None |
Montgomery 1988.
| Study characteristics | ||
| Methods |
Design: multi‐centre multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phase Phase 1: acute treatment with fluoxetine 40 to 80 mg (6 weeks) Phase 2: continuation treatment with a stabilised dose of 40 mg at 24 weeks (18 weeks) Duration post randomisation: 52 weeks Aim: to test the efficacy of fluoxetine in preventing recurrence of new episodes among patients with major unipolar recurrent depression |
|
| Participants |
Country: France Setting: 5 centres Type of AD: fluoxetine Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 18 weeks Number of participants: 456 entered phase 1, 254 responded to phase 1 Total numbers of randomised participants: 220 (112 placebo, 108 fluoxetine) Primary diagnosis: major unipolar recurrent depression Number of previous episodes last 5 years, mean (SD): 3.6 (3.1) placebo, 4.0 (4.8) antidepressant Gender distribution (male): not reported Mean age, years (SD): not reported Severity of depressive symptoms at randomisation: HDRS not reported Inclusion criteria ‐ MDD (DSM‐III), score > 18 HDRS, ≥ 1 major episode in last 5 years with interval ≥ 6 months between end of previous and start of present episode ‐ response in the first 6 weeks of acute treatment; remained in remission during continuation treatment ‐ definition of response: HDRS score < 12 Exclusion criteria ‐ history of manic episodes or received lithium during last 5 years |
|
| Interventions |
Intervention 1: placebo Tapering scheme: abrupt probably ("results consistent with suggestion withdrawn abruptly") Intervention 2: fluoxetine 40 mg/d No concomitant psychotropic medication was permitted, with the exception of benzodiazepines |
|
| Outcomes |
Primary outcome ‐ recurrence of depression ‐ definition of relapse: HAM‐D > 18 Secondary outcome ‐ none |
|
| Notes |
Funding: not reported COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned” Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: information insufficient to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 38/220 dropouts (20/112 placebo, 18/108 antidepressant) but reasons not stated according to group |
| Selective reporting (reporting bias) | High risk | Comment: study report fails to include results for adverse events and withdrawal symptoms that would be expected to have been reported for such a study. None of our secondary outcomes were reported |
| Other bias | Unclear risk | Comment: source of funding not reported |
Montgomery 2004.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phase Phase 0: single‐blind placebo lead (4 to 10 days) Phase 1: open‐label treatment with venlafaxine IR 100 to 200 mg (8 weeks) Phase 2: continuation treatment with venlafaxine IR 100 to 200 mg (16 weeks) Duration post randomisation: 52 weeks Aim: to investigate the efficiency of venlafaxine for prevention of recurrence of depression among patients who have responded to treatment |
|
| Participants |
Country: USA and Europe (countries not described) Setting: community adults (outpatients from psychiatric clinics) Type of antidepressant: venlafaxine immediate release (IR) Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 16 weeks Number of participants: 496 entered phase 1, 286 completed open treatment Total number of randomised participants: 235 (placebo 123, venlafaxine 112) Primary diagnosis: recurrent major depression Number of previous episodes: 2 or 3 episodes: 72% placebo, 71% antidepressant; 4 or 5 episodes: 25% placebo, 23% antidepressant Severity of depressive symptoms at randomisation HAM‐D 21 total score, mean (SE): 4.9 (3.7) placebo, 4.5 (3.4) venlafaxine IR MADRS total score, mean (SE): 5.2 (4.8) placebo, 4.3 (3.5) venlafaxine IR Gender distribution (F): 67% placebo, 71% venlafaxine Mean age, years (SD): 43.5 (11.2) placebo, 43.8 (11) venlafaxine Inclusion criteria ‐ ≥ 18 years ‐ meet diagnosis of recurrent major depression (≥ 1 previous episode in the last 5 years with a symptom‐free period > 6 months between episodes) (DSM‐III criteria) ‐ responders to acute open‐label treatment and remained relapse‐free during continuation treatment with venlafaxine Definition of response and remission: HAM‐D 21 score ≤ 12 on Day 56 of acute treatment; no more than 2 HAM‐D 21 scores > 10; and no CGI‐S ≥ 4 between Months 2 and 6 during continuation treatment Exclusion criteria ‐ history of drug and alcohol dependence within 2 years of the start of open treatment ‐ recent myocardial infarction ‐ history of hepatic or renal disease ‐ seizure disorder ‐ psychotic disorder ‐ bipolar disorder ‐ concomitant psychiatric diagnosis meeting DSM‐III criteria ‐ pregnant and breastfeeding women |
|
| Interventions |
Intervention 1: placebo Intervention 2: venlafaxine IR 100 to 200 mg per day Tapering scheme: tapering over 2 weeks Concomitant treatment ‐ psychotropic medication not permitted with the exception of chloral hydrate in USA and short‐acting benzodiazepines in Europe ‐ those with established psychotherapy or counselling were allowed to enter open treatment, but initiation or change in intensity of either modality was not permitted |
|
| Outcomes |
Primary outcomes ‐ number of participants with major depression (cumulative probability of recurrence) Definition of relapse/recurrence: CGI‐S ≥ 4 (moderate to severe depression) ‐ time to recurrence Secondary outcomes ‐ time to discontinuation in patients who withdrew from the study because of lack of efficacy ‐ time to recurrence excluding those who discontinued during the first 28 days of the RCT ‐ depressive symptoms and global severity of illness using mean HAM‐D 21, total HAM‐D, MADRS total, CGI‐S scores Safety: assessed by physical examinations, vital signs, ECGs, clinical laboratory tests, monitoring of adverse events, and patient reports throughout the study |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry COI: not reported by study authors; 4 of 5 study authors were employees in the pharmaceutical industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement Quote: "patients were randomly assigned to venlafaxine or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind but information insufficient to permit judgement Quote: "...double blind...placebo substitution..."; "randomly assigned to either venlafaxine or receive placebo under double blind conditions..."; "those assigned to the placebo group had their venlafaxine dose tapered off in a blinded fashion..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout rates were higher in the placebo group (76%) than in the venlafaxine group (50%); LOCF analysis |
| Selective reporting (reporting bias) | High risk | Comment: no protocol available; relevant outcomes measured; adverse events reported; no withdrawal symptoms |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Perahia 2009.
| Study characteristics | ||
| Methods |
Design: multi‐centre multi‐phase randomised placebo‐controlled trial Prerandomisation Phase 1: acute open‐label treatment with duloxetine 60 to 120 mg per day (4 to 10 weeks) Phase 2: open‐label continuation treatment with duloxetine at the same dose (24 weeks) Duration post randomisation: 52 weeks Aim: to assess the efficacy of duloxetine 60 to 120 mg once daily for prevention of depressive recurrence among outpatients with recurrent major depressive disorder |
|
| Participants |
Country: 5 European countries (France, Germany, Italy, Russia, Sweden) and USA Setting: community adults (43 study centres) Type of AD: duloxetine 60 to 120 mg per day Duration of antidepressant treatment prerandomisation: between 28 and 34 weeks Duration of antidepressant treatment post stabilsation: 24 weeks Number of participants: 514 entered phase 1, 413 responded and entered phase 2, 288 maintained response at end of phase 2 Total number of randomised participants: 288 (142 placebo, 146 antidepressant) Primary diagnosis: recurrent depression Number of previous episodes, mean (SD): 4.0 (1.5) placebo, 4.4 (2.3) antidepressant Severity of depressive symptoms at randomisation, mean (SD) HAM‐D 17 score at baseline: 4.49 (2.51) placebo, 4.12 (2.52) antidepressant CGI‐S: 1.46 (0.50) placebo, 1.49 (0.52) antidepressant Gender distribution (F): 74.6% discontinuation, 68.5% antidepressant Mean age, years (SD): 48.0 (12.3) placebo, 47.1 (12.8) antidepressant Inclusion criteria ‐ ≥ 18 years of age ‐ recurrent MDD (DSM‐IV criteria), confirmed with the MINI ‐ ≥ 3 episodes of depressive disorder (including the presenting episode at entry of phase 1) within past 5 years and in remission between these 3 episodes ‐ had to have been stable and off antidepressant medication ≥ 2 months before onset of the presenting episode ‐ responded to 10 weeks of acute treatment and remained in remission during 24 weeks of continuation treatment Definition of response/remission: HAM‐D 17 total score ≤ 9, CGI‐S scale score ≤ 2, did not meet DSM‐IV criteria for major depressive episode Exclusion criteria ‐ current and primary Axis I disorder other than MDD, including but not limited to dysthymia ‐ previous diagnosis of bipolar disorder, schizophrenia, or other psychotic disorders ‐ any anxiety disorder as a primary diagnosis within the past year ‐ Axis II disorder that in the judgement of the investigator would interfere with compliance with the study protocol ‐ DSM‐IV–defined history of substance abuse or dependence within the past year, excluding nicotine and caffeine ‐ positive urine drug screen for any substances of abuse, including benzodiazepines ‐ taking any excluded medications (which included most centrally acting medications such as antidepressants and antipsychotics) within 7 days before visit 2 ‐ treatment with a monoamine oxidase inhibitor within 14 days before study onset ‐ treatment with fluoxetine within 30 days before study onset ‐ patients who had a prior treatment history with duloxetine, who were judged to be at serious suicide risk, or who had a serious medical illness likely to require hospitalisations and/or the use of prohibited medications were also excluded, as were women who were breastfeeding or pregnant |
|
| Interventions |
Intervention 1: placebo Tapering scheme: duloxetine treatment gradually tapered down over 4‐week period Intervention 2: duloxetine at the same dose per day to which patients had previously responded |
|
| Outcomes |
Primary outcomes ‐ time to depressive recurrence Defintion of relapse/recurrence ‐ (1) CGI‐S ≥ 4 and meeting DSM‐IV criteria for MDD (as assessed by the MINI depression module) for at least 2 weeks, or (2) 3 consecutive visits that met re‐emergence criteria or 10 total (on a total up to 16 visits) re‐emergence visits, or (3) discontinued the study due to lack of efficacy Definition of significant re‐emergence of depressive symptoms ‐ CGI‐S ≥ 4 but not meeting DSM‐IV criteria for depression as assessed by the MINI depression module (= re‐emergence criteria) If re‐emergence criteria were met, patient had weekly re‐emergence visits until re‐emergence criteria were no longer met or the patient met criteria for recurrence. If a patient had 3 consecutive weekly re‐emergence visits or a total of 10 re‐emergence visits (of a total of up to 16 visits) throughout the maintenance phase, the patient was considered to have had a depressive recurrence and was discontinued from the study Secondary outcomes ‐ HAM‐D 17 total score and subscales ‐ CGI‐S ‐ Patient Global Impressions of Improvement (PGI‐I) scale ‐ Symptom Questionnaire Somatic Subscale (SQ‐SS) ‐ visual analog scale (VAS) for pain ‐ health outcome and quality of life, assessed by SF‐36, Sheehan Disability Scale, Resouce Utilisation and Hospitalisation Modules ‐ time to worsening: time from random assignment to first visit during the maintenance phase at which the patient met worsening criteria (> 50% increase from maintenance phase baseline on HAM‐D 17 and CGI‐S score ≥ 3) was assessed, as was loss of response (HAM‐D 17 total score > 9 and CGI‐S score > 2 at any time during the double‐blind maintenance phase) ‐ time to worsening (worsening criteria defined as > 50% increase from maintenance phase baseline on HAM‐D 17 and CGI‐S score > 2 at any time) ‐ safety assessed by spontaneously reported adverse events, vital signs and weight, sexual function measured by Arizona Sexual Experience Scale (ASEX), blood chemistry, haematology tests |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry COI: 5 study authors (including head author) were employees of pharmaceutical company that funded the trial; 1 study author received grants from the pharmaceutical industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement Quote: "patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and key study personnel ensured; likely that blinding could have been successful Quote: "blinded randomisation was employed in our study so that neither investigators nor their patients were aware of the exact visits at which randomisation would occur" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high rate of missing data (69/142 (48%) placebo group, 50/146 (34%) duloxetine group); although reasons reported, still high dropout |
| Selective reporting (reporting bias) | High risk | Comment: adverse events were reported; withdrawal symptoms were not measured |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Peterson 2010.
| Study characteristics | ||
| Methods |
Design: double‐blind randomised placebo‐controlled trial Prerandomisation phase Phase 1: open‐label acute treatment with fluoxetine 20 mg/d (8 weeks) Phase 2: open‐label continuation treatment (fluoxetine increase to fixed dose of 40 mg/d) with and without CBT (12 weekly sessions, then 7 biweekly) (28 weeks) Duration post randomisation: 80 weeks Aim: to evaluate the effectiveness of CBT and fluoxetine and fluoxetine alone in preventing recurrence of depressive disorder during maintenance treatment for patients with remitted MDD |
|
| Participants |
Country: USA Setting: outpatients from Depression Clinical and Research Program (DCRP) of Massachusetts General Hospital 1992‐1998 Type of antidepressant: fluoxetine Duration of antidepressant treatment prerandomisation: 36 weeks Duration of AD treatment post stabilisation: 28 weeks Number of participants: 391 entered phase 1, 132 entered phase 2 (66 entered fluoxetine study arm and 66 fluoxetine and CBT arm) Total number of randomised participants: 55 (antidepressant only 15, placebo only 17, CBT and placebo 12, CBT and antidepressant 11) Primary diagnosis ‐ recurrent depression (history of 3 or more episodes) ‐ chronic depression (onset of continuous depressive symptoms > 36 months before the study) ‐ history of poor inter‐episode recovery ‐ both MDD and dysthymia Number of previous episodes: fluoxetine 4.2 (± 5.6), placebo 4.2 (± 5.6), CBT placebo 8.6 (± 15.1), CBT and fluoxetine 2.3 (± 1.5) Severity of depressive symptoms: HAM‐D 17, mean (SD): fluoxetine 5.5 (2.1), placebo 4.3 (3.7), CBT and placebo 2.8 (2.5), CBT and fluoxetine 5.4 (4.5) Mean age, years (SD): fluoxetine 43.5 (8.8), placebo 43.2 (9.8), CBT and placebo 42.9 (9.3), CBT and fluoxetine 45.1 (8.1) Female: fluoxetine 47%, placebo 53%, CBT and placebo 70%, CBT and fluoxetine 67% Inclusion criteria ‐ participants were drug‐free outpatients who met criteria for MDD, as diagnosed with the Structured Clinical Interview for DSM‐III‐R ‐ initial HAM‐D 17 score of 16 ‐ 18 to 65 years of age ‐ required to meet at least 1 of the following criteria: history of ≥ 3 major depressive episodes, with the prior episode no more than 2.5 years before onset of the current episode; diagnosis of current episode as chronic (onset of continuous depressive symptoms > 36 months before the study); history of poor inter‐episode recovery; or both MDD and dysthymia Definition of remission: HAMD‐17 ≤ 7 for 3 consecutive weeks Exclusion criteria ‐ pregnant women and women of childbearing potential who were not using a medically accepted means of contraception ‐ women of childbearing potential taking a birth control pill, or women who were currently lactating ‐ patients with serious risk of suicide, seizure disorder history, major unstable medical illness, history of multiple adverse drug reactions, or allergy to the study drugs ‐ antisocial personality disorder, or a DSM‐III‐R comorbid diagnosis of Axis I pathology other than anxiety disorders ‐ patients currently using non‐study‐related psychotropic drugs or exhibiting evidence of hypothyroidism Patients were excluded if their depression failed to respond in the past to a trial of (1) a higher dose of fluoxetine (60 to 80 mg/d), (2) the combination of fluoxetine and desipramine, or (3) the combination of fluoxetine and lithium. Finally, patients were excluded if they failed to respond during the course of their current major depressive episode to at least 1 adequate antidepressant trial, defined as 6 weeks or more of treatment with 150 mg of imipramine (or its tricyclic equivalent) or 60 mg of phenelzine (or its monoamine oxidase inhibitor equivalent) |
|
| Interventions |
Intervention 1: placebo Intervention 2: fluoxetine 40 mg/d Intervention 3: CBT and placebo Intervention 4: CBT and fluoxetine 40 mg/d Tapering scheme: not described Medication: psychopharmacologists followed a standard protocol for medication management visits and were instructed not to engage in cognitive or behavioural interventions CBT Description: cognitive therapy was conducted by highly trained doctoral‐level psychologists according to a treatment manual adapted from Beck et al. (1979) and Mercier and Leahy (1992). Each session followed a conventional cognitive therapy format, which includes symptom check, agenda setting, homework review, cognitive and behavioural exercises for specific problem areas, and assignment of new homework. CBT was modified to address residual symptoms specifically and to enhance patient coping skills. The therapy used for this study was designed specifically to target symptoms and issues common to remitted depressed patients, who are at high risk for relapse and recurrence. Three content domains are emphasised when working with remitted depressed patients. The first is recovery, which involves working to resolve any residual symptoms that are present after clinical remission. Such residual symptoms are common and include irritability, neurovegetative disturbances, and hopelessness. The second content area is re‐entry, which entails working to improve a patient’s functioning in key life roles such as student, family member, spouse, and employee. An acute depressive episode typically results in lowered levels of functioning in 1 or more of these areas, thus the gap between current and optimal psychosocial functioning may be significant. One common target of this content area is avoidant behaviour, which is often activated by a patient to maintain tentative short‐term stability but in turn prevents return to premorbid levels of functioning. The third content area, risk, involves focusing on maladaptive cognitive and behavioural patterns that contribute to heightened relapse rates. Such patterns include lack of assertiveness and self‐care, as well as perfectionism and unrealistic self‐expectations Frequency: the structure of therapy for the maintenance phase of this protocol (an 80‐week period) consisted of 7 biweekly, 50‐minute sessions followed by 16 monthly, 50‐minute sessions Integrity of delivery and compliance: no independent ratings of treatment quality and fidelity |
|
| Outcomes | ‐ depressive recurrence Definition of relapse: meeting criteria for MDD and ≥ 15 in the HAM‐D 17; confirmed 1 week later with another clinician blind to treatment status Depressive symptoms and improvement: HAM‐D 17, CGI‐S, CGI‐I, 92‐item Symptom Questionnaire (SQ), Beck Hopelessness Scale (BHS), Beck Depression Inventory (BDI), Patient Global Impression of Improvement (PGI‐I), Beck Anxiety Inventory (BAI), monthly |
|
| Notes |
Funding: not reported COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement Quote: “patients were randomised” |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement Quote: "another clinician blind to treatment status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low withdrawals post randomisation (1 CBT and placebo group, 1 medication only group, 1 placebo only group); reasons stated |
| Selective reporting (reporting bias) | High risk | Comment: neither withdrawal symptoms nor adverse events were reported. |
| Other bias | Unclear risk | Source of funding was not reported |
Rapaport 2001.
| Study characteristics | ||
| Methods |
Design: multi‐centre multi‐phase double‐blind randomised placebo‐controlled study Prerandomisation phases Phase 0: 2‐week placebo run‐in Phase 1: 3 double‐blind acute treatments with sertraline 50 to 200 mg/d vs placebo (10 weeks) Phase 2: open‐label continuation treatment with sertraline at the same dose (52 weeks) Duration post randomisation: 28 weeks Aim: to investigate long‐term efficacy, prevention of relapse, and safety of sertraline for treatment of panic disorder |
|
| Participants |
Country: USA Setting: community adults (outpatients from 31 clinical centres) Type of AD: sertraline Duration of antidepressant prerandomisation: 62 weeks Duration of antidepressant post stabilisation: 52 weeks Number of participants: 555 entered phase 1, 426 completed, 396 entered phase 2, 217 completed and responded to phase 2 Total number of randomised participants: 183 (placebo 90, sertraline 93) Primary diagnosis: panic disorder with or without agoraphobia Anxiety severity symptoms at randomisation (SD) PDSS score: 2.48 (2.6) placebo, 2.10 (2.0) antidepressant CGI severity: 1.73 (0.8) placebo, 1.71 (0.7) antidepressant HAM‐A: 5.97 (4.4) placebo, 6.04 (4.6) antidepressant Gender distribution (F): 62.2% placebo, 65.6% sertraline Mean age, years (SD): 40.30 (11.4) placebo, 41.00 (10.8) sertraline Inclusion criteria ‐ 18 years of age or older ‐ SCID‐I‐confirmed DSM‐III diagnosis of panic disorder with or without agoraphobia ‐ 4 or more panic attacks during the 4 weeks before screening, 3 or more panic attacks during a 2‐week placebo washout period, and HAM‐A total score ≥ 18 at baseline of phase 1 ‐ after 1 year, patients who met responder criteria were randomised to 28 weeks of double‐blind treatment with either sertraline or placebo Defintion of response/remission: CGI‐Improvement score of 1 (‘very much improved’) or 2 (‘much improved’) at Week 52 compared to baseline in the acute studies Exclusion criteria ‐ HDRS‐21 total score ≥ 18 at baseline of phase 1 ‐ current diagnosis of major depression, bipolar disorder, organic mental disorder, schizophrenic disorder; alcohol or substance abuse in the previous 6 months ‐ current principal diagnosis of dysthymia, obsessive‐compulsive disorder, any other anxiety disorder (besides panic disorder), or any personality disorder ‐ use of concomitant psychotropic medication (or a positive urine drug screen) ‐ previous treatment with sertraline ‐ females who are pregnant, nursing, or not practising a medically accepted form of birth control |
|
| Interventions |
Intervention 1: placebo Tapering scheme: "discontinuation was immediate" Intervention 2: sertraline treatment 50 to 200 mg/d, with daily dose adjusted by 50‐mg increments on a weekly basis determined by clinical response and tolerability |
|
| Outcomes |
Primary outcomes ‐ relapse Definition of relapse (1) CGI‐I ≥ 3 (reflecting, at best, minimal improvement from baseline in acute studies) at 3 consecutive visits at 2‐week intervals (additional visits were scheduled, if required) (2) meeting criteria for DSM‐III‐R panic disorder by the third visit; and (3) reporting more full symptom panic attacks during previous 4 weeks than during the last 4 weeks of open‐label treatment ‐ discontinuation due to insufficient clinical response, clinician rated. Any subject is discontinued from the double‐blind portion of the study by the investigator because of insufficient clinical response; this includes patients who meet the strict definition of relapse ‐ exacerbation of panic disorder symptoms Definition of exacerbation: 2 consecutive visits where there is: (1) CGI‐I ≥ 4 (no change or worsening from baseline in acute studies); (2) a 2‐point increase in CGI‐Improvement score (e.g. from 1, ‘very much improved’, to a minimum of 3, ‘minimally improved’); or (3) a score on at least 1 subscale of the PDSS that increased to 3 (‘severe’ or ‘definite’ discomfort) ‐ adverse events, observed and volunteered (rating instrument was not used) |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry COI: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement Quote: "double‐blind treatment"; "during double‐blind treatment, both groups were permitted to have their daily dose adjusted by 50‐mg increments on a weekly basis determined by clinical response and tolerability" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: higher dropout rate in placebo group (50.6%) than in sertraline group (31.5%); last observation carried forward ('LCOF') analyses |
| Selective reporting (reporting bias) | High risk | Comment: study protocol not available; adverse events reported; no withdrawal symptoms measured |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Rickels 2010.
| Study characteristics | ||
| Methods |
Design: single‐centre multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 1: for all participants: open‐label treatment with venlafaxine extended release (ER) (70 to 225 mg) (24 weeks) Phase 2: for participants with 12 months' treatment before randomisation: open‐label treatment with venlafaxine extended release (ER) (70 to 225 mg) (24 weeks) and continuation treatment with venlafaxine extended release (ER) (70 to 225 mg) (24 weeks) Duration post randomisation: 24 weeks Aim: to examine the long‐term efficacy of venlafaxine XR in patients with chronic GAD who responded therapeutically to 6‐month or 12‐month venlafaxine XR treatment |
|
| Participants |
Country: USA Setting: community adults (recruited by research psychiatrists in 4 primary care practices, recruited by media advertising, or were treated in our central clinic at University of Pennsylvania) Type of antidepressant: venlafaxine ER (70 to 225 mg) Duration of antidepressant treatment prerandomisation AD: 24 weeks or 48 weeks Duration of antidepressant treatment post stabilisation: 0 weeks or 24 weeks Primary diagnosis: generalised anxiety disorder Number of participants: 286 entered phase 1, 158 completed phase 1, 136 entered phase 2 Total number of randomised participants: 136 participants with 6 months' antidepressant before randomisation (54 placebo, 82 antidepressant) and 49 participants with 12 months' before randomisation (15 venlafaxine ER, 34 placebo) Severity of anxiety symptoms at randomisation (HAM‐A), mean (SD): participants with 6 months' antidepressant before randomisation: 4.17 (3.10); participants with 12 months antidepressant before randomisation: 3.89 (3.43) Severity of depression symptoms at randomisation (HAM‐D), mean (SD): participants with 6 months' antidepressant before randomisation: 3.71 (3.08); participants with 12 months' antidepressant before randomisation: 3.17 (2.45) Gender distribution (F): participants with 6 months' antidepressant before randomisation: 62.5%; participants with 12 months' antidepressant before randomisation: 59.3% Mean age, years (SD): participants with 6 months' antidepressant before randomisation: 49.8 (15.8); participants with 12 months' antidepressant before randomisation: 59.3 (15.0) Inclusion criteria ‐ ≥ 18 years of age ‐ meeting the criteria for generalised anxiety disorder (determined by the SCID‐I for DSM‐IV criteria) ‐ sufficient symptoms to require anxiolytic drug therapy, including HAM‐A ≥ 20 at screen and CGI‐S ≥ 4 at baseline ‐ responders to 6 months' open‐label treatment (at least moderately improved (CGI‐I score ≤ 2) from baseline) or 6 months' open‐label treatment and open‐label and continuation treatment with venlafaxine extended release (ER) (70 to 225 mg) (24 weeks) Definition of response: CGI‐I score ≤ 2 Exclusion criteria ‐ eating disorder such as bulimia and anorexia, substance abuse, or dependence during the past 6 months ‐ any current anxiety spectrum DSM‐IV diagnosis other than GAD ‐ an episode of MDD in the previous 6 months or depressive symptoms at study intake (HAM‐D score ≥ 18) ‐ current or past history of dementia, bipolar disorder, schizophrenia, or other psychotic disorders ‐ positive urine drug screens for amphetamines, cocaine, phencyclidine hydrochloride, methadone hydrochloride, or barbiturates were immediate exclusion criteria, as were uncontrolled medical conditions and hypersensitivity to venlafaxine, as well as pregnancy, breastfeeding, or a positive urine pregnancy test |
|
| Interventions |
Intervention 1: placebo Tapering scheme: tapering over 1 to 3 weeks. Study dosage was reduced by 75 mg weekly, with reduction to 37.5 mg during the last week. Depending on the patient’s study drug daily dose, this taper could last from 1 to 3 weeks. Gradual tapering was facilitated with a patient diary Intervention 2: venlafaxine 75 to 225 mg per day Results were separately reported for participants with 6 months' antidepressants before randomisation and for participants with 12 months' antidepressants before randomisation |
|
| Outcomes |
Primary outcomes ‐ relapse Definition of relapse: meeting symptom criteria for a Structured Clinical Interview for DSM‐IV GAD diagnosis with HAM‐A score ≥ 16, a CGI, CGI‐S score ≥ 4 (moderate or higher), and CGI‐I score of 6 or 7 (worse or very much worse) compared with baseline of the double‐blind relapse phase, and present for 2 successive visits, spaced 2 weeks apart, with the last visit conducted at least 3 weeks after taper completion ‐ remission Defintion of remission: CGI of 1 or HAM‐A ≤ 7 Secondary outcomes ‐ HAM‐A, CGI‐S, CGI‐I, patient‐rated Hospital Anxiety and Depression Scale (biweekly for the first 8 weeks and monthly thereafter) ‐ HAM‐D, Sheehan Disability Scale, and quality of life assessed by General Health Questionnaire ‐ adverse events were assessed at each visit by an open‐ended approach, which was facilitated by the use of a physician‐completed medication problem checklist. Adverse events were rated by severity, duration, and association with study medication (probably related, possibly related, or non‐related) ‐ potential withdrawal symptoms were assessed at selective time points using a patient‐completed withdrawal checklist, which was based on a checklist developed by Fava et al |
|
| Notes |
Funding: study was supported by a grant from the pharmaceutical industry. Pharmaceutical company provided all study medication COI: 2 study authors (including principal author) received grants from the pharmaceutical company that provided study medication for this trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement Quote: "responders to 6 months of open‐label venlafaxine XR treatment were randomised to double‐blind treatment in a 60:40 ratio of drug to placebo"; "a stratified randomisation was used, including level of secondary depressive symptoms at intake and improvement status after 6 months of venlafaxine XR therapy“ |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: study described as double‐blind trial; however information insufficient to permit judgement Quote: "double‐blind treatment in a 60:40 ratio of drug to placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout rates high and not balanced across groups: 14/54 (25%) dropouts in placebo group and 18/82 (21%) dropouts in antidepressant group. Reasons for dropout reported |
| Selective reporting (reporting bias) | High risk | Comment: study protocol not available; adverse events reported but incompletely (not all adverse events for each treatment group reported); no information on withdrawal symptoms reported |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Rouillon 2000.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised placebo‐controlled trial Prerandomisation phases Phase 0: placebo washout period 4 to 7 days Phase 1: open‐label treatment with milnacipran 2 mg bid (6 weeks) Phase 2: open continuation treatment with milnacipran (18 weeks) Duration post randomisation: 52 weeks Aim: to compare the efficacy and assess the tolerability of milnacipran 50 mg bid to placebo for prevention of recurrence in depressed patients who had responded to acute treatment and had remained in remission after a 4‐month continuation phase |
|
| Participants |
Country: France Setting: 104 centres Type of AD: milnacipran Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilsation: 18 weeks Number of participants: 500 entered phase 1, 323 entered phase 2, 227 completed phase 2 and recovered Total number of randomised participants: 214 (104 milnacipram, 110 placebo) Primary diagnosis: recurrent depressive disorder Number of previous episodes (SD): placebo group 2.7 (2.3); milnacipran group 3.1 (2.3) Severity of depressive symptoms at randomisation (HDRS), mean (SD): 4.58 (2.42) placebo, 4.77 (2.99) antidepressant Gender distribution (F): placebo 68.2%, milnacipran 66.3% Mean age, years (SD): placebo 44.6 (10), milnacipran 46.1 (10.2) years Inclusion criteria ‐ 18 to 70 years of age ‐ history of MDD ‐ current major depressive episode without psychotic symptoms (DSM‐III‐R) ‐ HDRS‐21 ≥ 18 at baseline of phase 0 For entry into maintenance phase: HDRS ≤ 8, improvement or disappearance of initial symptoms, very much improved/much improved on CGI‐I Definition of remission: HDRS ≤ 8 Exclusion criteria Mania, hypomania, dysthymia, depression secondary to schizophrenia, schizoaffective disorder, alcoholism or drug addiction, suicidal intent, treatment‐resistant depression, cardiac rhythm disorders requiring antiarrhythmic treatment, kidney failure, past history of epilepsy, past history of serious allergic reaction or toxic reaction to a drug, life‐threatening disorders, abnormalities in clinical chemistry, haematology or urinalysis, pregnancy, lack of effective birth control or breastfeeding |
|
| Interventions |
Intervention 1: placebo Tapering scheme: not described Intervention 2: milnacipran 50 mg twice a day |
|
| Outcomes | Recurrence, every 2 months Defintion of recurrence: return to diagnostic criteria required for entry into the trial (depression according to DSM‐III‐R, ≥ 18 on HDRS with need to treat recurrence) Adverse events, every 2 months Quality of life, measured by DIP |
|
| Notes |
Funding: study supported by a grant from the pharmaceutical industry COI: not reported Mean number of previous major depressive episodes significantly lower in placebo group (2.7 (2.3)) than in milnacipran group (3.1 (2.3)); P < 0.05 Number of hospitalised patients at the moment of inclusion: 19.2% in antidepressant group and 20.7% in placebo group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 25% (28/110) placebo and 18% (20/104) milnacipran patients discontinued for reasons other than relapse; reasons not stated |
| Selective reporting (reporting bias) | High risk | Comment: adverse events were reported incompletely (not separated for each treatment group; no numbers for increased sweating); withdrawal symptoms were not measured |
| Other bias | Unclear risk | Comment: grant from the pharmaceutical industry |
Segal 2010.
| Study characteristics | ||
| Methods |
Design: multi‐centre randomised controlled trial Prerandomisation phases Phase 1: acute treatment with citalopram (or if not tolerating sertraline) and if needed in combination with venlafaxine (if not tolerating mirtazapine) (8 weeks) Phase 2: continuation treatment with antidepressant treatment (20 weeks) Duration after randomisation: 76 weeks (18 months) Aim: to compare rates of relapse in depressed patients in remission receiving MBCT against taper to placebo and clinical management vs maintenance antidepressant pharmacotherapy, the current standard of care |
|
| Participants |
Country: Canada Setting: community adults (outpatients from the Centre for Addiction and Mental Health, Toronto, Ontario, Canada, and St Joseph’s Healthcare, Hamilton, Ontario, recruited through clinical referrals, physician outreach, and media announcements describing the Mood Disorders Clinics) Type of AD: 2‐step, standardised monotherapy algorithm: citalopram, or if not tolerating: sertraline; 17% of participants in combination with venlafaxine (step 2), or if not tolerating mirtazapine Duration of antidepressant treatment prerandomisation: 28 weeks Duration of antidepressant treatment post stabilsation: 20 weeks Number of participants: 160 entered phase 1, 94 responded to phase 1, 84 entered trial Total number of randomised participants: 84 (28 antidepressant, 26 MBCT + discontinuation, 30 placebo) → control antidepressant group was split to allow multiple‐arm comparison: 40 participants for comparison MBCT vs continuation (26 MBCT and 14 antidepressant) and 44 participants in the comparison placebo vs antidepressant (30 placebo and 14 antidepressant) Primary diagnosis: recurrent depression Number of previous episodes (SD): 4.5 (2.2) MBCT and discontinuation, 4.8 (2.1) placebo, 4.9 (2.6) antidepressant Severity of depressive symptoms at randomisation (HDRS), mean (SD) HDRS score at randomisation: MBCT and discontinuation 3.0 (2.8), placebo 3.3 (3.0), antidepressant 2.0 (2.3) Stable remitters in acute phase: 8/26 MBCT and discontinuation, 16/30 placebo, 17/28 antidepressant Unstable remitters in acute phase: 18/26 MBCT and discontinuation, 14/30 placebo, 11/28 antidepressant Any Axis I comorbidity: MBCT and discontinuation 35%, placebo 27%, antidepressant 39% Any Axis II comorbidity: MBCT and discontinuation 58%, placebo 37%, antidepressant 18% Gender distribution (F): 50% MBCT and discontinuation, 67% placebo, 71% antidepressant Mean age, years (SD): 44.8 (9.4) MBCT discontinuation, 41.9 (11.6) placebo, 45.8 (11.4) antidepressant Inclusion criteria ‐ responders to antidepressant open‐label acute treatment with continued response during continuation phase ‐ ≥ 2 previous episodes of MDD (to ensure that those randomised would have a minimum of 3 past episodes) ‐ diagnosis of MDD (DSM‐IV criteria) and score ≥ 16 on HDRS at entry to phase 1 ‐ between 18 and 65 years of age ‐ English speaking ‐ ability to provide informed consent Definition of response: HDRS score ≤ 7 during continuation treatment, unstable remitters achieved the same HDRS threshold but had occasional elevated scores across this interval; their score subsequent to an elevation was ≤ 7 and the range of elevated scores fell between 8 and 14 Exclusion criteria ‐ current diagnosis of bipolar disorder, substance abuse disorder, schizophrenia, or borderline or antisocial personality disorder ‐ trial of ECT within the past 6 months ‐ depression secondary to concurrent medical disorder ‐ current or planned pregnancy within the 6 months of acute phase treatment ‐ current practice of meditation more than once per week or yoga more than twice per week |
|
| Interventions |
Intervention 1: medication taper and mindfulness‐based cognitive therapy (MBCT) (no placebo) MBCT delivered according to the protocol described by Segal et al. Patients attended 8 weekly group meetings of 2 hours’ duration and a retreat day held between sessions 6 and 7. In addition, an optional, monthly, 1‐hour mindfulness meditation class was offered throughout the maintenance phase. This is accomplished through daily homework exercises. Prescription of additional medication for sleep complaints or anxiety symptoms was also permitted during this period (zopiclone and benzodiazepines) Tapering scheme: medication tapered gradually during a 4‐week period via placebo substitution and reduced pill count, respectively, at the recommended rate for the specific medication to minimise the risk of withdrawal syndrome. Prescription of additional medication for sleep complaints or anxiety symptoms was permitted during this period (e.g. zopiclone, benzodiazepines) Intervention 2: medication taper to placebo with clinical management Tapering scheme: medication tapered gradually during a 4‐week period via placebo substitution and reduced pill count, respectively, at the recommended rate for the specific medication to minimise the risk of withdrawal syndrome. Prescription of additional medication for sleep complaints or anxiety symptoms was permitted during this period (e.g. zopiclone, benzodiazepines) Intervention 3: antidepressant treatment Pharmacotherapy sessions were 20 minutes long and emphasised both medication management (education, dosage adjustment, dosage scheduling, and side effects) and clinical management (discussion of functionality, support, and limited advice). Psychotherapeutic strategies, especially cognitive‐behavioural therapy techniques, were prohibited |
|
| Outcomes | ‐ time to relapse/recurrence Definition of relapse: ≥ 16 HDRS‐17 assessed twice for at least 2 weeks, then criteria for MDD measured with SCID‐I, depressive symptoms with HDRS‐17 |
|
| Notes |
COI: not reported Funding: study was funded by a grant from the National Institute of Mental Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random sequence generation by computer‐generated random numbers Quote: "block randomisation, with a block size of 4, was performed at CAMH by an independent statistician using computer‐generated quasi‐random numbers" |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes were used to conceal allocation Quote: "details of group assignment were contained in sealed envelopes that were opened by the statistician and communicated to the coordinator once a patient was deemed suitable for study entry" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: incomplete blinding; outcome is likely to be influenced by lack of blinding Quote: "study psychiatrists were blind to treatment assignment, whereas once patients in MBCT completed their taper, they no longer took any pills" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment ensured; unlikely that the blinding could have been broken Quote: "patients were assessed by clinical evaluators blind to treatment allocation at randomisation, biweekly for the first 8 weeks, monthly for the next 3 months, and bimonthly" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: large quantity of missing outcome data and imbalance between groups: 25% in antidepressant group, 19% in MBCT and discontinuation group. Reasons for dropout not reported |
| Selective reporting (reporting bias) | High risk | Comments: study does not measure adverse events and withdrawal symptoms, which are fundamental outcomes in drug discontinuation trials |
| Other bias | Low risk | None |
Stewart 1997.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind parallel 2‐arm randomised placebo‐controlled trial Prerandomisation phases Phase 1: acute treatment with imipramine or phenelzine (6 weeks) Phase 2: continuation trial with imipramine or phenelzine (24 weeks) Duration post randomisation: 24 weeks Aim: to assess the efficacy of phenelzine and imipramine for patients with chronic atypical depression |
|
| Participants |
Country: USA Setting: community adults (outpatients from Depression Evaluation Service, an outpatient research clinic of the New York State Psychiatric Institute) Type of AD: imipramine or phenelzine Duration of antidepressant treatment prerandomisation: 30 weeks Duration of antidepressant treatment post stabilisation: 24 weeks Total number of randomised participants: imipramine comparison: 32 (15 placebo, 17 imipramine); phenelzine comparison: 28 (15 placebo and 13 phenelzine) Primary diagnosis: chronic atypical depression ‐ imipramine comparison: major depression (63%), dysthymia (18%) or both (18% currently dysthymic and history of major depression; 28% with depression superimposed on dysthymia) at least 2 years ‐ definite (67%) or probable (30%) atypical depression (phenelzine comparison: major depression (63%), dysthymia (18%), or both (18% currently dysthymic and history of major depression; 28% with depression superimposed on dysthymia) at least 2 years ‐ definite (67%) or probable (30%) atypical depression Severity of depressive symptoms at randomisation: CGI‐I 1 or 2 relative to depressed baseline state Mean duration of current depression, months (SD): imipramine 176 (142), phenelzine 284 (169) Percentage of adult life depressed (SD): imipramine 56% (23), phenelzine 73 (21) Age at onset, years (SD): imipramine 17 (13), phenelzine 11 (8) Gender distribution (F): imipramine 66%, phenelzine 46% Mean age, years (SD): imipramine 38 (7, range 27 to 55), phenelzine 39 (10, range 23 to 58) Comorbidity, n (%) ‐ imipramine: panic disorder 7 (12), GAD 3 (5), social phobia 18 (30), OCD 4 (7), eating disorder 6 (10), past history alcohol abuse/dependence 7 (28), past history substance abuse 9 (15) ‐ phenelzine: panic disorder 7 (12), GAD 3 (5), social phobia 18 (30), OCD 4 (7), eating disorder 6 (10), past history alcohol abuse/dependence 7 (28), past history substance abuse 9 (15) Inclusion criteria ‐ depressive symptoms ≥ 2 years at entry to phase 1 ‐ responders to acute trial of imipramine or phenelzine; maintained remission ≥ 6 months ‐ diagnosis of major depression, dysthymia, or both (DSM‐III criteria) and definite or probable atypical depression (Columbia University criteria) Definition of response: CGI‐S 1 or 2 Exclusion criteria ‐ not reported |
|
| Interventions |
Study arm 1 Intervention 1: placebo Intervention 2: imipramine, continuation dose maintained from acute phase Study arm 2 Intervention 1: placebo Intervention 2: phenelzine, continuation dose maintained from acute phase Tapering scheme: tapering over 2 weeks |
|
| Outcomes | ‐ recurrence Definition of recurrence: 2 consecutive weeks of CGI rating ≥ 3 (minimally improved, unchanged, or various categories of worsening compared with pretreatment baseline) ‐ survival analysis ‐ time to recurrence |
|
| Notes |
COI: not reported Funding: study supported by a grant from the National Institute of Mental Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and personnel; blinding unlikely to have been broken Quote: "after randomisation and for the remainder of the study, patients and doctors were blind to treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessors (doctors); blinding unlikely to have been broken Quote: "at each visit, the physician completed a Hamilton depression scale and CGI" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: although dropouts are low (2), reasons are not reported |
| Selective reporting (reporting bias) | High risk | Comment: adverse events and withdrawal symptoms are not reported; they are a fundamental outcome in drug discontinuation studies |
| Other bias | Low risk | None |
Streim 2012.
| Study characteristics | ||
| Methods |
Design: partially randomised controlled trial (only randomised arms were relevant for the review) Prerandomisation phases: no Duration post randomisation: 52 weeks Aim: to assess benefits and adverse effects of antidepressant drug continuation vs discontinuation among older people in nursing homes receiving maintenance treatment for a single episode of depression |
|
| Participants |
Country: USA Setting: nursing homes and assisted living facilities residents Type of AD: not described Duration of antidepressant treatment prerandomisation: not reported Duration of antidepressant treatment post stabilisation: at least 24 weeks Total number of randomised participants: 36 (23 antidepressant, 13 no antidepressant) Primary diagnosis: single episode of depression Severity of depressive symptoms at randomisation (Geriatric Depression Scale (GDS) Score from 0 to 30 for severe depression symptoms) at randomisation (SD): 4.6 (2.9) no antidepressant, 4.3 (3.1) antidepressant Gender distribution (F): 76.9% no antidepressant, 78.3% antidepressant Age: between 60 and 95 years; mean not described Inclusion criteria ‐ ≥ 65 years of age ‐ current use of antidepressant medication ‐ in full remission from first episode of depression for ≥ 6 months ‐ long‐term care or assisted living facility residents Exclusion criteria ‐ bedridden and severe cognitive impairment |
|
| Interventions |
Intervention 1: discontinuation antidepressant (no antidepressant medication) Intervention 2: continuation of antidepressants Tapering scheme: not reported |
|
| Outcomes |
Primary outcomes ‐ change in depressive symptoms (presence and severity) measured by Geriatric Depression Scale (GDS) score, assessed at 52 weeks (scores range from 0 (no depression symptoms) to 30 (severe depression symptoms)) ‐ number of falls experienced by participants over 12 months of surveillance Secondary outcomes ‐ cognitive function measured by Mini Mental State Examination (MMSE), assessed at Month 12. Scores range from 0 (severe cognitive impairment) to 30 (intact cognitive function) |
|
| Notes |
Funding: supported by a grant from the National Institute for Mental Health COI: not reported Note: this was an unpublished study; study results were reported in the protocol and in a conference paper. The study author stated in his email that this study was never published as a paper, as the sample size was considered insufficient to detect a significant difference in primary outcome measures in intent‐to‐treat analyses (Van Leeuwen 2019 [pers comm]) Note: no relapse outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo used; not double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropouts not reported |
| Selective reporting (reporting bias) | High risk | Comment: adverse events and withdrawal symptoms were not an outcome; mean difference was not reported by treatment groups |
| Other bias | Low risk | None |
Terra 1998.
| Study characteristics | ||
| Methods |
Design: multi‐phase, multi‐centre, randomised controlled trial Prerandomisation phase Phase 1: acute treatment with fluvoxamine (6 weeks) Phase 2: continuation treatment with fluvoxamine (18 weeks) Duration post randomisation: 52 weeks Aim: to test the efficacy of fluvoxamine in reducing the risk of new episodes of depression |
|
| Participants |
Country: France Setting: 63 centres Type of AD: fluvoxamine Duration of antidepressant treatment prerandomisation: 24 weeks Duration of antidepressant treatment post stabilisation: 18 weeks Number of participants: 436 entered phase 1, 283 responded to phase 1 treatment, 204 maintained remission trough continuation phase 2 Total numbers of randomised participants: 204 (110 fluvoxamine, 94 placebo) Primary diagnosis: recurrent major depressive disorder Number of previous episodes, mean (SD): 3.5 (1.5) placebo, 3.5 (1.4) Gender distribution (female): placebo 78%, fluvoxamine 70% Mean age, years (SD): placebo 45 (11.4) years, fluvoxamine 44.5 (10.7) years Severity of depressive symptoms at randomisation: not reported Inclusion criteria ‐ 18 to 70 years ‐ moderate/severe episode of MDD (DSM‐III R) at entry to phase 1 ‐ no psychotic feature ‐ MADRS ≥ 25 ‐ history of ≥ 2 episodes in previous 5 years separated by symptom‐free interval ≥ 6 months ‐ "good responders" at Week 6 of acute treatment and sustained response during 18‐week open continuation treatment phase Definition of response: response to acute treatment: at Week 6, total score < 10 on MADRS and score of 1 or 2 on CGI‐S; response to continuation treatment: score < 12 for all assessments and no score higher than 1 or 2 on the CGI Severity of Illness Scale Exclusion criteria ‐ pregnant, lactating, childbearing potential and not taking adequate contraceptive measures or wishing to become pregnant during the period of the study ‐ concomitant clinically unstable disease ‐ epilepsy or history of convulsions ‐ bipolar disorder ‐ history of schizophrenia or manic episodes ‐ known hypersensitivity to fluvoxamine or previous unsuccessful treatment with fluvoxamine ‐ chronic alcoholism ‐ ECT within previous 2 weeks, requiring treatment with any non‐psychotropic drug that might interfere with the pharmacokinetics of fluvoxamine |
|
| Interventions |
Intervention 1: placebo Tapering scheme: not reported Intervention 2: fluvoxamine 100 mg once a day Benzodiazepines and hypnotics for severe anxiety and insomnia were permitted during open acute and continuation treatment, provided treatment had been initiated more than 3 months before the start of the study |
|
| Outcomes |
Primary outcome ‐ recurrence Definition of recurrence: reappearance of at least 5 symptoms in DSM‐III‐R criteria for major depression (2 assessments by investigator 8 days apart). Attempted or completed suicide also considered a recurrence Secondary outcome ‐ time to recurrence, depressive symptoms using MADRS score and HAM‐D score ‐ anxiety symptoms using Covi Anxiety Scale ‐ global severity of illness using CGI‐S ‐ unwanted signs and symptoms |
|
| Notes |
Funding: not reported COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned” Comment: information insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: information insufficient to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: reasons for withdrawals in phase 1 (n = 98) and in phase 2 (n = 60) Number of discontinuation was not described in the text and reason was not reported (110 fluvoxamine patients – 109 presented in Table 2); last observation carried forward analysis for MADRS, MADRS suicide score, CGI‐S, and Covi Anxiety Scale |
| Selective reporting (reporting bias) | High risk | Comment: no protocol available; withdrawal symptoms not an outcome; incomplete information on adverse events reported |
| Other bias | Unclear risk | Source of funding not reported |
Wilson 2003.
| Study characteristics | ||
| Methods |
Design: multi‐phase double‐blind randomised controlled trial Prerandomisation phase Phase 1: acute treatment with sertraline 50 to 200 mg (8 weeks) Phase 2: continuation treatment with sertraline 50 to 200 mg (16 to 20 weeks) Duration post randomisation: 100 weeks Aim: to examine the efficacy of sertraline in preventing recurrence of depression among older people living in the community |
|
| Participants |
Country: UK Setting: community older people from a community survey conducted at the same time as the trial; 20 general practices and 4 old age psychiatry teams Type of AD: sertraline Duration of antidepressant treatment prerandomisation: 24 to 28 weeks Duration of antidepressant treatment post stabilisation: 16 to 20 weeks Number of participants: 254 entered treatment phases 1 and 2; 113 completed phase 2 Total numbers of randomised participants: 113 (57 placebo, 56 antidepressant) Primary diagnosis: major depressive disorder First episode of depression: 73.6% placebo, 71.4% antidepressant Gender distribution (male): placebo 66%, sertraline 34% Mean age, years (SD): placebo 76.8 (7.0), sertraline 76.6 (6.6) Severity of depressive symptoms at randomisation, mean (SD) ‐ HDRS score: placebo 20.3 (3.3), sertraline 20.7 (3.7) ‐ MADRS score: placebo 26.0 (5.4), sertraline 26.48 (6.5) Inclusion criteria ‐ ≥ 65 years of age ‐ psychiatric diagnoses: Geriatric Mental State AGECAT depression ≥ level 3, DSM–III–R diagnoses of major depressive disorder, HDRS 17‐item score ≥ 18 at entry to phase 1 Definition of response: 50% reduction in baseline HDRS by Week 8 and HDRS score ≤ 10 had to be maintained for 4 weeks Exclusion criteria ‐ MMSE score ≤ 11 ‐ severe and unstable physical illness ‐ clinically significant alcohol misuse ‐ significant suicidal or delusional experiences ‐ concomitant drug treatment, including other psychotropic drugs, warfarin, and anticonvulsants |
|
| Interventions |
Intervention 1: placebo Tapering scheme: not described Intervention 2: sertraline. All participants were maintained on their final therapeutic dosage during the randomised, controlled phase of the study, with the exception of those treated with 200 mg. In the latter cases, the maintenance dosage was decreased from 200 mg to 150 mg |
|
| Outcomes |
Primary outcome ‐ recurrence Definition of recurrence: HDRS score ≥13 as well as meeting DSM–III–R criteria for major depressive disorder as determined by a trained psychiatrist Secondary outcome: none reported |
|
| Notes |
Funding: study supported by a grant from the pharmaceutical industry COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer random number generator was used Quote: “a computer‐generated randomisation list was provided by Pfizer Ltd. The list was stratified by dosage and used to produce numbered containers for the identical capsules of either sertraline or placebo” |
| Allocation concealment (selection bias) | Low risk | Comment: independent trialist was responsible for allocation concealment Quote: “a computer‐generated randomisation list was provided by Pfizer Ltd. A company independent of the sponsor and trialist was responsible for packaging the trial drugs and randomisation. The list was stratified by dosage and used to produce numbered containers for the identical capsules of either sertraline or placebo. Codes were maintained in opaque, sealed envelopes. They were broken on trial completion, after locking the study database. External research auditors maintained the security of the codes” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind described; unlikely that the code could have been broken Quote: “double‐blind"; "identical capsules"; "codes were maintained in opaque, sealed envelopes"; "they were broken on trial completion, after locking the study database”; "external research auditors maintained the security of the codes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding to outcome assessment ensured; unlikely that blinding could have been broken Quote: “research staff conducted follow‐up assessments. External research auditors maintained the security of the codes, and verified data collection and cleaning" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All missing data accounted for and reasons for dropouts reported |
| Selective reporting (reporting bias) | High risk | Comment: the study report fails to include results for adverse events and withdrawal symptoms that would be expected to have been reported for such a study |
| Other bias | Unclear risk | Comment: a grant from the pharmaceutical industry |
AD: antidepressant.
AE: adverse event.
AGECAT: Automated Geriatric Examination for Computer‐Assisted Taxonomy.
BAI: Beck Anxiety Inventory.
BDI: Beck Depression Inventory.
BHS: Beck Hopelessness Scale.
bid: twice a day.
BNF: British National Formulary.
CBASP: Cognitive‐Behavioural Analysis System of Psychotherapy.
CBT: cognitive‐behavioural therapy.
CESD: Center for Epidemiological Studies Depression Scale.
CGI: Clinical Global Impressions Scale.
CGI‐I: Clinical Global Impressions‐Improvement Scale.
CGI‐S: Clinical Global Impressions‐Severity Scale.
CIDI: Composite International Diagnostic Interview.
DESS: Discontinuation‐Emergent Signs and Symptoms Scale.
DSSI: Discontinuation Symptoms Severity Index.
DIP: disability and impact profile.
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition.
DSM‐IV (‐TR): Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision.
ECG: electrocardiogram.
ECT: electroconvulsive therapy.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
ESF: End‐State Functioning.
GAD: generalised anxiety disorder.
GAS: Goal Attainment Scale.
GDS: Geriatric Depression Scale.
GP: general practitioner.
GRID: GRID–Hamilton Rating Scale for Depression.
HAM‐A: Hamilton Anxiety Rating Scale.
HAM‐D or HDRS: Hamilton Depression Rating Scale.
IDS‐C: Inventory for Depressive Symptomatology‐Clinician.
IDS‐SR: Inventory of Depressive Symptomatology–Self‐Report.
IPT: interpersonal therapy.
IGR: interquartile range.
IPT: Interpersonal psychotherapy.
LIFE: Longitudinal Internal Follow‐up Evaluation.
MADRS: Montgomery–Åsberg Depression Rating Scale.
MAOI: monoamine oxidase inhibitor.
MBCT: mindfulness‐based cognitive therapy.
MDD: major depressive disorder.
MDE: major depressive episode.
MINI: mini international neuropsychiatric interview.
MMSE: Mini‐Mental State Examination.
NICE: National Institute of Clinical Excellence.
OCD: obsessive‐compulsive disorder.
PCT: preventive cognitive therapy.
PDSS: Panic Disorder Severity Scale.
PGI‐I: Patient Global Impressions of Improvement Scale.
PTSD: posttraumatic stress disorder.
QIDS: Quick Inventory of Depressive Symptomatology.
Q‐LES‐Q: Quality of Life Enjoyment and Satisfaction Questionnaire.
RCT: randomised controlled trial.
RDC: Research Diagnostic Criteria.
SAR‐SR: Social Adjustment Scale‐Self‐Report.
SCID‐I: Structured Clinical Interview for DSM‐IV Axis I Disorders.
SD: standard deviation.
SE: standard error.
SF‐36: Short‐Form Health Survey 36.
SNRI: serotonin and norepinephrine reuptake inhibitor.
SQ‐SS: Symptom Questionnaire Somatic Subscale.
SSRI: selective serotonin reuptake inhibitor.
TCA: tricyclic antidepressant.
TESS: Treatment‐Emergent Symptoms Scale.
TiC‐P: Treatment Inventory of Costs in Patients.
WHO QoL: World Health Organization Quality of Life.
WHO QoL‐BREF: World Health Organization Cross‐Cultural Comparisons of Quality of Life.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexopoulos 2000 | Participants did not meet the criteria for long‐term antidepressants (16 weeks of continuation treatment; duration of acute treatment not reported; total duration < 6 months) |
| Allgulander 2006 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Baldwin 2006 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Baldwin 2012 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Bockting 2005 | Intervention is not discontinuation of antidepressant treatment |
| Bockting 2006 | Intervention is not discontinuation of antidepressant treatment |
| Bockting 2008 | Study is not an RCT |
| Bockting 2009 | Wrong study design (long‐term follow‐up study) |
| Boulenger 2012 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Caillard 2003 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Cohen 2006 | Study was not a randomised discontinuation trial |
| Coppen 1978 | Participants did not meet the criteria for long‐term antidepressants (duration of antidepressant treatment not described) |
| Croft 2002 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Curtis 1993 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Davidson 2001 | Participants did not meet the criteria for long‐term antidepressants prescribed for depressive or anxiety disorder |
| Davidson 2005 | Participants did not meet the criteria for long‐term antidepressants prescribed for depressive or anxiety disorder |
| Davidson 2005a | Participants did not meet the criteria for long‐term antidepressants; there is no control intervention |
| Davidson 2008 | Participants did not meet the criteria for long‐term antidepressants (mean duration of antidepressant treatment 136 days) |
| Dinan 2001 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Dobson 2008 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Doogan 1992 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Duboff 1993 | Wrong study design (long‐term follow‐up study) |
| Durgam 2019 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Fava 1994 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Fava 1998 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Fava 2002 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Ferguson 2007 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Georgotas 1989 | Not all participants met the criteria for long‐term antidepressants (23 to 25 weeks of antidepressant treatment (< 6 months)) |
| GlaxoSmithKline 1994 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| GlaxoSmithKline 1999 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Glen 1984 | Wrong study design |
| Godfrin 2010 | Participants did not meet the criteria for long‐term antidepressant treatment (participants without antidepressant treatment) |
| Goodwin 2009 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Goodwin 2013 | Wrong study design |
| Gorwood 2007 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Harrison 1986 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Hochstrasser 2001 | Not all participants met the criteria for long‐term antidepressants (6 to 9 weeks of acute treatment and 16 weeks of continuation treatment < 6 months) |
| Kamijima 2005 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Kamijima 2006 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Klerman 1974 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Lepine 2004 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Licht 2013 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Liebowitz 1999 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Liebowitz 2010 | Interventon was not discontinuation of antidepressants (quetiapine) |
| Lustman 2006 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Lyketsos 2011 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Markowitz 2000 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| McGrath 2006 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Michelson 1999 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Montgomery 1993 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Montgomery 1993a | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Montgomery 2005 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| NCT00878748 | Study withdrawn |
| OADIG 1993 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Rapoport 2010 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Reimherr 1998 | Participants did not meet the criteria for long‐term antidepressants (randomisation after 12 or 14 weeks of open‐label treatment) |
| Reynolds 1992 | Study is not a controlled study |
| Reynolds 1999 | Not everyone met the criteria for long‐term antidepressant treatment (acute treatment mean 11.2 (SD 7.8) weeks; 16 weeks of continuation treatment) |
| Robinson 1991 | Participants did not meet the criteria for long‐term antidepressants (duration 6 to 13 weeks of acute treatment and 16 weeks of continuation treatment < 6 months) |
| Rosenbaum 1998 | Not all participants met the criteria for long‐term antidepressants (duration < 6 months) |
| Rosenthal 2013 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Sackeim 2001 | Study is excluded due to patients having electroconvulsive treatment before randomisation |
| Schmidt 2002 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Shawyer 2012 | Participants did not meet the criteria for long‐term antidepressants (participants without antidepressant treatment) |
| Shimazu 2011 | Intervention is not discontinuation of antidepressant treatment |
| Shimodera 2012 | Intervention is not discontinuation of antidepressant treatment |
| Stein 1996 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Stein 2012 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Stewart 1998 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Stocchi 2003 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Teasdale 2000 | Participants did not meet the criteria for long‐term antidepressants (off antidepressant treatment) |
| Thase 2001 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Tint 2008 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Walker 2000 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Weissman 1976 | Wrong study design (follow‐up study) |
| Wentink 2019 | No comparator control intervention |
| Wetherell 2013 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
| Zajecka 1998 | Participants did not meet the criteria for long‐term antidepressants (duration < 6 months) |
RCT: randomised controlled trial. SD: standard deviation.
Characteristics of studies awaiting classification [ordered by study ID]
Gunn 2020.
| Methods | Randomised controlled trial |
| Participants | Participants taking antidepressants for 12 months or longer. Mild or no symptoms of depression as assessed by the PHQ9 (< 10). No depressive episodes in last 12 months or history of recurrent MDD; stable on ADs; low risk of self‐harm |
| Interventions | Intervention 1: personalised tapering schedule with the support of an online de‐prescribing tool, which will monitor progress, and of GP Intervention 2: usual care plus attention control through signposting to an educational fact sheet about antidepressants |
| Outcomes | ‐ unknown |
| Notes | Trial duration: 5 years Recruitment starts in 2021. Study will end in June 2024 Contact information: cath.kaylorhughes@unimelb.edu.au |
Mangin 2015.
| Methods | Randomised controlled trial |
| Participants | Primary care‐treated patients currently taking fluoxetine for maintenance to prevent recurrence of depression |
| Interventions | Intervention 1: discontinuation by taper to placebo Intervention 2: continuation of maintenance SSRI Trial duration: 18 months |
| Outcomes | ‐ occurrence of moderately severe depression over 18 months ‐ mood ‐ quality of life ‐ overall psychological distress/symptoms ‐ social and occupational functioning |
| Notes | Last participants enrolled 15/02/2012 Results were presented at North American Primary Care Research Group (NAPCRG) 43rd Annual Meeting; Oct 27, 2015; Cancun, Mexico Contact information: mangind@mcmaster.ca (we requested data but have not received response) |
Molenaar 2016.
| Methods | Randomised controlled trial |
| Participants | Pregnant women with gestational age less than 16 weeks who use SSRIs without clinically relevant depressive symptoms |
| Interventions | Intervention 1: preventive cognitive therapy with gradual, guided discontinuation of SSRIs under medical management Intervention 2: continuation of SSRI |
| Outcomes | ‐ cumulative incidence of relapse or recurrence of maternal depressive disorder during pregnancy and up to 3 months postpartum ‐ child outcome (neonatal outcomes and psychomotor and behavioural outcomes up to 24 months' postpartum) ‐ healthcare costs |
| Notes | Unclear if participants meet the criteria for long‐term antidepressants (duration < 6 months) Miminum duration of antidepressant treatment not reported in the protocol Paper is accepted |
AD: antidepressant.
GP: general practitioner.
MDD: major depressive disorder.
PHQ9: Patient Health Questionnaire‐9.
SSRI: selective serotonin reuptake inhibitor.
Characteristics of ongoing studies [ordered by study ID]
Duffy 2019.
| Study name | A randomised controlled trial assessing the use of citalopram, sertraline, fluoxetine, and mirtazapine in preventing relapse in primary care patients who are taking long‐term maintenance antidepressants (ANTLER: aNTidepressants to prevent reLapse in dEpRession): study protocol for a randomised controlled trial |
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants between 18 and 74 years; have had at least 2 episodes of depression; and have been taking antidepressants for 9 months or longer, and are currently taking citalopram 20 mg, sertraline 100 mg, fluoxetine 20 mg, or mirtazapine 30 mg, but are well enough to consider stopping their medication |
| Interventions | Intervention 1: placebo Intervention 2: long‐term maintenance Trial duration: 52 weeks |
| Outcomes | ‐ time in weeks to the beginning of the first episode of depression ‐ depressive and anxiety symptoms ‐ adverse effects ‐ withdrawal symptoms ‐ emotional processing tasks ‐ quality of life ‐ resources and costs |
| Starting date | The trial began to recruit participants in March 2017; recruitment was ongoing until the end of February 2019 |
| Contact information | larisa.duffy@ucl.ac.uk |
| Notes | Expected end of data collection: February 2020; paper is submitted |
ISRCTN12417565.
| Study name | REDUCE (Reviewing long‐term antidepressant use by careful monitoring in everyday practice) Internet and telephone support to people coming off long‐term antidepressants: protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Participants taking antidepressants longer than 1 year for a first episode of depression or longer than 2 years for repeated episodes of depression who are no longer depressed and want to try to taper off their antidepressant use |
| Interventions | Intervention 1: provision of ‘ADvisor’ Internet programmes to general practitioners or nurse practitioners and to patients designed to support antidepressant withdrawal, plus 3 patient telephone calls from a psychological well‐being practitioner Intervention 2: usual care Trial duration: 52 weeks |
| Outcomes | ‐ depressive symptoms at 6 months ‐ depressive symptoms at other follow‐up time points ‐ anxiety ‐ discontinuation of antidepressants ‐ social functioning ‐ well‐being ‐ enablement ‐ quality of life ‐ satisfaction ‐ use of health services for costs |
| Starting date | Recruitment of practices began on 1 December 2019 |
| Contact information | a.r.kendrick@southampton.ac.uk |
| Notes | Approximate date when recruitment of patients will be completed is 30 June 2021. End of the study is defined as the date of the last follow‐up visit of the last patient (expected to occur 12 months after the last patient is recruited) |
Differences between protocol and review
Although in our protocol we indicated that we would exclude discontinuation trials in the context of hospital admission, we have included Rouillon 2000, in which 20% of participants were hospitalised at study inclusion.
In the original protocol, we planned to include antidepressants prescribed for depressive or anxiety disorder. However, we have also included a study in which 30% of participants did not have a lifetime psychiatric diagnosis (Eveleigh 2018).
We conducted an additional post‐hoc sensitivity analysis for industry sponsorship bias because drug companies whose primary interest may not be in discontinuation. We performed an additional sensitivity analysis to test the impact of a long tapering scheme as current guidelines recommend tapering over 4 weeks and more.
We included an additional comparison and SoF table, as one study provided data for the comparison 'discontinuation with other intervention (minimal intervention) versus continuation (usual care)' (Eveleigh 2018). The intervention of this study met the definition of 'minimal intervention (Voshaar 2006) and therefore we added a new type of intervention.
We provided 'Summary of findings' tables for the four comparisons with available data.
In the original protocol, we planned to analyse (1) number of relapses and (2) time to relapse. In the included studies, authors reported relapse as time to events by using a hazard ratio (time‐to‐event data). Time‐to‐event outcomes are most appropriately analysed using hazard ratios (HRs), which take into account the number and timing of events and the time until last follow‐up for each patient who has not experienced an event (i.e. has been censored) (Tierney 2007). Therefore, we adjusted our analysis plan to include time‐to‐event analysis.
We planned to apply an adjustment in the analysis of data from cluster‐randomised trials. However, in the comparison 'discontinuation with other intervention versus usual care', only one cluster‐randomised trial provided data (Eveleigh 2018); therefore, we did not adjust the data with the intra‐cluster correlation coefficient (ICC).
Due to the small number of included studies, not all defined subgroup and sensitivity analyses could be performed.
We did not carry out an ITT analysis for all outcomes as planned in the protocol. Most studies reported outcomes for all participants, but if data were not available for all participants, we included only participants with a recorded outcome. The analysis for our primary outcome, discontinuation rate, is reported as ITT.
Other research authors participated in the screening of searches than were planned in the protocol.
Maria Donald, Tony Kendrick, and Mark Horowitz joined the review author team in 2020.
The title of the review was changed from 'Discontinuation versus continuation of long‐term antidepressant use for depressive and anxiety disorders" to "Approaches for discontinuation versus continuation of long‐term antidepressant use for depressive and anxiety disorders" after discussions with the Cochrane editorial team.
Although we indicated in our protocol feasibility and safety as objectives, we changed this into effectiveness and safety in the review after discussion with the Cochrane editorial team to make explicitly clear that the focus of this review is to determine whether long‐term antidepressants can be discontinued effectively and safely.
Contributions of authors
EVL: conceived and designed the protocol. Lead author for the review; searched for and selected trials; obtained copies of trial reports and correspondence; extracted data and conducted 'risk of bias' assessments; entered data into RevMan and into GRADEpro; conducted analysis, interpretation of data analyses, and grading; drafted the review.
MVD: conceived and designed the protocol, selected included studies, served as arbiter in the 'risk of bias' assessment, interpreted data analyses and grading, contributed to the text, edited text.
MH: interpreted data analyses and grading, contributed to the background and text.
TK: interpreted data analyses and grading, contributed to the text.
MD: searched for and selected trials, contributed to the text, edited text.
ADS: contributed to the background of the protocol, contributed to the text.
LR: contributed to the methods of the protocol, extracted data, conducted 'risk of bias' assessment, entered data into RevMan, contributed to the text.
TC: conceived and designed the protocol, served as arbiter in selection of included studies, contributed to 'risk of bias' table, interpreted data analyses and grading, contributed to the text.
Sources of support
Internal sources
Ghent University, Belgium
External sources
-
National Institute for Health Research (NIHR), UK
LR work on this review is supported by NIHR Cochrane Infrastructure funding to the Common Mental Disorders Cochrane Review Group.
Declarations of interest
EVL: none known.
MVD: none known.
MH: none known.
TK: is lead researcher on a grant from the NIHR PGfAR programme for the REviewing long term anti‐Depressant Use by Careful monitoring in Everyday practice (REDUCE) programme to determine the feasibility, effectiveness, safety and cost‐effectiveness of Internet and telephone support for discontinuing inappropriate long‐term antidepressant use. TK is also an unpaid member of the national guideline committee updating the NICE clinical guideline on depression, 2015‐2021.
MD: none known.
ADS: none known.
LR: none known.
TC: none known.
New
References
References to studies included in this review
Bialos 1982 {published data only}
- Bialos D, Giller E, Jatlow P, Docherty J, Harkness L. Recurrence of depression after discontinuation of long-term amitriptyline treatment. American Journal of Psychiatry 1982;139(3):325-9. [DOI] [PubMed] [Google Scholar]
- Giller E Jr, Bialos D, Harkness L, Jatlow P, Waldo M. Long-term amitriptyline in chronic depression. Hillside Journal of Clinical Psychiatry 1985;7(1):16-33. [PubMed] [Google Scholar]
Bockting 2018 {published data only}
- Bockting CLH, Klein NS, Elgersma HJ, Rijsbergen GD, Slofstra C, Ormel J, et al. Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised controlled trial. Lancet Psychiatry 2018;5(5):401-10. [DOI] [PubMed] [Google Scholar]
- Klein NS, Rijsbergen GD, ten Doesschate MC, Hollon SD, Burger H, Bockting CLH. Beliefs about the causes of depression and recovery and their impact on adherence, dosage, and successful tapering of antidepressants. Depression and Anxiety 2017;34(3):227-35. [DOI] [PubMed] [Google Scholar]
- Klein NS, Wijnen BFM, Lokkerbol J, Buskens E, Elgersma HJ, Rijsbergen GD, et al. Cost-effectiveness, cost-utility and the budget impact of antidepressants versus preventive cognitive therapy with or without tapering of antidepressants. British Journal of Psychiatry Open 2019;5(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 1986 {published data only}
- Cook BL, Helms PM, Smith RE, Tsai M. Unipolar depression in the elderly. Reoccurrence on discontinuation of tricyclic antidepressants. Journal of Affective Disorders 1986;10(2):91-4. [DOI] [PubMed] [Google Scholar]
Derubeis 2019 {published data only}
- DeRubeis RJ, Zajecka J, Shelton RC, Amsterdam JD, Fawcett J, Xu C, et al. Prevention of recurrence after recovery from a major depressive episode with antidepressant medication lone or in combination with cognitive behavioral therapy: a phase 2 randomized clinical trial. JAMA Psychiatry 2020;77(3):237-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Fawcett J, Amsterdam JD, Shelton RC, Zajecka J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomised clinical trial. JAMA Psychiatry 2014;71(10):1157-64. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O'Reardon JP, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Archives of General Psychiatry 2005;62(4):417-22. [DOI] [PubMed] [Google Scholar]
- NCT00057577. Prevention of Recurrence in Depression With Drugs and CT. https://clinicaltrials.gov/ct2/show/results/NCT00057577 (first received 8 April 2003).
Eveleigh 2018 {published data only}
- Eveleigh R, Grutters J, Muskens E, Oude Voshaar R, Weel C, Speckens A, et al. Cost-utility analysis of a treatment advice to discontinue inappropriate long-term antidepressant use in primary care. Family Practice 2014;31(5):578–84. [DOI] [PubMed] [Google Scholar]
- Eveleigh R, Muskens E, Lucassen P, Verhaak P, Spijker J, Weel C, et al. Withdrawal of unnecessary antidepressant medication: a randomised controlled trial in primary care. British Journal of General Practice Open 2018;1(4):bjgpopen17X101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskens E, Eveleigh R, Lucassen P, Weel C, Spijker J, Verhaak P, et al. Prescribing ANtiDepressants Appropriately (PANDA): a cluster randomized controlled trial in primary care. BMC Family Practice 2013;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelenberg 2003 {published data only}
- Gelenberg AJ, Trivedi MH, Rush AJ, Thase ME, Howland R, Klein DN, et al. Randomized, placebo-controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biological Psychiatry 2003;54(8):806-17. [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine 2000;342(20):1462-70. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Rush AJ, Markowitz JC, Borian FE, Dunner DL, Koran LM, et al. Continuation treatment of chronic depression: a comparison of nefazodone, cognitive behavioral analysis system of psychotherapy, and their combination. Psychopharmacology Bulletin 2003;37(4):73-87. [PubMed] [Google Scholar]
Gilaberte 2001 {published data only}
- Gilaberte I, Montejo AL, De La Gandara J, Perez-Sola V, Bernardo M, Massana J, et al. Fluoxetine in the prevention of depressive recurrences: a double-blind study. Journal of Clinical Psychopharmacology 2001;21(4):417-24. [DOI] [PubMed] [Google Scholar]
Huijbers 2016 {published data only}
- Huijbers MJ, Spinhoven P, Spijker J, Ruhe HG, Schaik DJ, Oppen P, et al. Discontinuation of antidepressant medication after mindfulness-based cognitive therapy for recurrent depression: randomised controlled non-inferiority trial. British Journal of Psychiatry 2016;208(4):366-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers MJ. Data supplement to Huijbers et al. Discontinuation of antidepressant medication after mindfulness-based cognitive therapy for recurrent depression: randomised controlled non-inferiority trial. British Journal of Psychiatry 2016;doi: 10.1192/bjp.bp.115.168971. [DOI] [PMC free article] [PubMed]
Kane 1982 {published data only}
- Kane JM, Quitkin FM, Rikfin A, Ramos-Lorenzi JR, Nayak DD, Howard A. Lithium carbonate and imipramine in the prophylaxis of unipolar and bipolar II illness. Archives of General Psychiatry 1982;39:1065-9. [DOI] [PubMed] [Google Scholar]
Keller 1998 {published data only}
- Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, et al. Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA 1998;280(19):1665-72. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Schatzberg A, Rush AJ, Klein DN, Howland R, Gniwesch L, et al. Psychosocial outcomes following long-term, double-blind treatment of chronic depression with sertraline vs placebo. Archives of General Psychiatry 2002;59:723-8. [DOI] [PubMed] [Google Scholar]
Keller 2007 {published data only}
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, et al. The prevention of recurrent episodes of depression with venlafaxine for two years (PREVENT) study: outcomes from the 2-year and combined maintenance phases. Journal of Clinical Psychiatry 2007;68(8):1246-56. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Kocsis JH, Ahmed S, et al. Assessing the efficacy of 2 years of maintenance treatment with venlafaxine extended release 75-225 mg/day in patients with recurrent major depression: a secondary analysis of data from the PREVENT study. International Clinical Psychopharmacology 2008;23(6):357-63. [DOI] [PMC free article] [PubMed]
- Kornstein SG, Pedersen RD, Holland PJ, et al. Influence of sex and menopausal status on response, remission, and recurrence in patients with recurrent major depressive disorder treated with venlafaxine extended release or fluoxetine: analysis of data from the PREVENT study. Journal of Clinical Psychiatry 2014;75(1):62-8. [DOI] [PubMed]
- Watanabe K, Thase M, Kikuchi T, Tsuboi T, Asami Y, Pappadopulos E, et al. Long-term function and psychosocial outcomes with venlafaxine extended release 75-225 mg/day versus placebo in the PREVENT study. International Clinical Psychopharmacology 2017;32(5):271-80. [DOI] [PubMed]
Khan 2014 {published data only}
- Khan A, Musgnung J, Ramey T, Messig M, Buckley G, Ninan PT. Abrupt discontinuation compared with a 1-week taper regimen in depressed outpatients treated for 24 weeks with desvenlafaxine 50 mg/d. Journal of Clinical Psychopharmacology 2014;34(3):365-8. [DOI] [PubMed] [Google Scholar]
- Khan A. Study comparing discontinuation symptoms of DVS SR in subjects with major depressive disorder (MDD). ClinicalTrials.gov Published 26 Jan 2010; updated 28 Feb 2012 (accessed 23 Feb 2020).
- Ninan PT, Musgnung J, Messig M, Buckley G, Guico-Pabia CJ, Ramey TS. Incidence and timing of taper/post therapy-emergent adverse events following discontinuation of desvenlafaxine 50 mg in patients with major depressive disorder. Primary Care Companion CNS Disorders 2015;17(1):10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klysner 2002 {published data only}
- Klysner R, Bent-Hansen J, Hansen HL, Lunde M, Pleidrup E, Poulsen DL, et al. Efficacy of citalopram in the prevention of recurrent depression in elderly patients: placebo-controlled study of maintenance therapy. British Journal of Psychiatry 2002;181:29-35. [DOI] [PubMed] [Google Scholar]
Kocsis 1996 {published data only}
- Kocsis JH, Friedman RA, Markowitz JC, Leon AC, Miller NL, Gniwesch L, et al. Maintenance therapy for chronic depression: a controlled clinical trial of desipramine. Archives of General Psychiatry 1996;53(9):769-76. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Friedman RA, Markowitz JC, Miller N, Gniwesch L, Bram J. Stability of remission during tricyclic antidepressant continuation therapy for dysthymia. Psychopharmacology Bulletin 1995;31(2):213-6. [PubMed] [Google Scholar]
- Miller NL, Kocsis JH, Leon AC, Portera L, Dauber S, Markowitz JC. Maintenance desipramine for dysthymia: a placebo-controlled study. Journal of Affective Disorders 2001;64(2-3):231. [DOI] [PubMed] [Google Scholar]
Kocsis 2007 {published data only}
- Dunlop BW, Li T, Kornstein SG, et al. Correlation between patient and clinician assessments of depression severity in the PREVENT study. Psychiatry Research 2013;177:177-83. [DOI] [PMC free article] [PubMed]
- Gelenberg AJ, Dunner DL, Rothschild AJ, et al. Sexual functioning in patients with recurrent major depressive disorder enrolled in the PREVENT study. Journal of Nervous and Mental Diseases 2013;201(4):266-73. [DOI] [PubMed]
- Kocsis JH, Thase ME, Trivedi MH, Shelton RC, Kornstein SG, Nemeroff CB, et al. Prevention of recurrent episodes of depression with venlafaxine ER in a 1-year maintenance phase from the PREVENT study. Journal of Clinical Psychiatry 2007;68:1014-23. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Pedersen RD, Holland PJ, Nemeroff CB, Rothschild AJ, Thase ME, et al. Influence of sex and menopausal status on response, remission, and recurrence in patients with recurrent major depressive disorder treated with venlafaxine extended release or fluoxetine: analysis of data from the PREVENT study. Journal of Clinical Psychiatry 2014;75(1):62-8. [DOI] [PubMed] [Google Scholar]
- Scapicchio P. Beyond remission. Prevention of recurrences in long-term treatment of depression. First data from the PREVENT study. Giornale Italiano di Psicopatologia / Italian Journal of Psychopathology 2008;14(1):80-7. [Google Scholar]
- Thase ME, Gelenberg A, Kornstein SG, Kocsis JH, Trivedi MH, Ninan P, et al. Comparing venlafaxine extended release and fluoxetine for preventing the recurrence of major depression: results from the PREVENT study. Journal of Psychiatry Research 2011;45:412-20. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Dunner DL, Kornstein SG, Thase ME, Zajecka JM, Rothschild AJ, et al. Psychosocial outcomes in patients with recurrent major depressive disorder during 2 years of maintenance treatment with venlafaxine extended release. Journal of Affective Disorders 2010;126(3):420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kornstein 2006 {published data only}
- Kornstein SG, Bose A, Li D, Saikali KG, Ghandi C. Escitalopram maintenance treatment for prevention of recurrent depression: a randomised, placebo-controlled trial. Journal of Clincial Psychiatry 2006;67:1767-75. [DOI] [PubMed] [Google Scholar]
Kupfer 1992 {published data only}
- Frank E, Kupfer DJ, Perel JM, Cornes C, Jarret DB, Mallinger AG, et al. Three-year outcomes for maintenance therapies in recurrent depression. Archives of General Psychiatry 1990;47(12):1093-9. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Perel JM, Cornes C, Mallinger AG, Thase ME, et al. Five-year outcomes for maintenance therapies in recurrent depression. Archives of General Psychiatry 1992;49:767-73. [DOI] [PubMed] [Google Scholar]
Kuyken 2008 {published data only}
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting & Clinical Psychology 2008;76(6):966-78. [DOI] [PubMed] [Google Scholar]
Kuyken 2015 {published data only}
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet 2015;386(9988):63-73. [DOI] [PubMed] [Google Scholar]
Mavissakalian 1999 {published data only}
- Mavissakalian MR, Perel JM, Talbott-Green M, Sloan C. Gauging the effectiveness of extended imipramine treatment for panic disorder with agoraphobia. Biological Psychiatry 1998;43:848–54. [DOI] [PubMed] [Google Scholar]
- Mavissakalian MR, Perel JM. 2nd year maintenance and discontinuation of imipramine in panic disorder with agoraphobia. Annals of Clinical Psychiatry 2001;13(2):63-7. [DOI] [PubMed] [Google Scholar]
- Mavissakalian MR, Perel JM. Long-term maintenance and discontinuation of imipramine therapy in panic disorder with agoraphobia. Archives of General Psychiatry 1999;56(9):821-7. [DOI] [PubMed] [Google Scholar]
Mavissakalian 2001 {published data only}
- Mavissakalian MR, Perel JM, Talbott-Green M, Sloan C. Gauging the effectiveness of extended imipramine treatment for panic disorder with agoraphobia. Biological Psychiatry 1998;43:848–54. [DOI] [PubMed] [Google Scholar]
- Mavissakalian MR, Perel JM. 2nd year maintenance and discontinuation of imipramine in panic disorder with agoraphobia. Annals of Clinical Psychiatry 2001;13(2):63-7. [DOI] [PubMed] [Google Scholar]
- Mavissakalian MR, Perel JM. Long-term maintenance and discontinuation of imipramine therapy in panic disorder with agoraphobia. Archives of General Psychiatry 1999;56(9):821-7. [DOI] [PubMed] [Google Scholar]
Montgomery 1988 {published data only}
- Montgomery SA, Dufour H, Brion S, Gailledreau J, Laquille X, Ferrey G, et al. The prophylactic efficacy of fluoxetine in unipolar depression. British Journal of Psychiatry 1988;153(3):69-76. [PubMed] [Google Scholar]
Montgomery 2004 {published data only}
- Montgomery SA, Entsuah R, Hackett D, Kunz NR, Rudolph RL. Venlafaxine versus placebo in the preventive treatment of recurrent major depression. Journal of Clinical Psychiatry 2004;65(3):328-36. [DOI] [PubMed] [Google Scholar]
Perahia 2009 {published data only}
- Kelin K, Berk M, Spann M, Sagman D, Raskin J, Walker D, et al. Duloxetine 60 mg/day for the prevention of depressive recurrences: post hoc analyses from a recurrence prevention study. International Journal of Clinical Practice 2010;64(6):719-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Montejo AL, Perahia DG, Spann ME, Wang F, Walker DJ, Yang CR, et al. Sexual function during long-term duloxetine treatment in patients with recurrent major depressive disorder. Journal of Sexual Medicine 2011;8(3):773-82. [DOI] [PubMed] [Google Scholar]
- Perahia DGS, Maina G, Thase ME, Spann ME, Wang F, Walker DJ, et al. Duloxetine in the prevention of depressive recurrences: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2009;70(5):706-16. [DOI] [PubMed] [Google Scholar]
Peterson 2010 {published data only}
- Petersen TJ, Pava JA, Buchin J, Matthews JD, Papakostas GI, Nierenberg AA, et al. The role of cognitive-behavioral therapy and fluoxetine in prevention of recurrence of major depressive disorder. Cognitive Therapy and Research 2010;34(1):13-23. [Google Scholar]
Rapaport 2001 {published data only}
- Rapaport MH, Wolkow R, Rubin A, Hackett E, Pollack M, Ota KY. Sertraline treatment of panic disorder: results of a long-term study. Acta Psychiatrica Scandinavica 2001;104(4):289-98. [DOI] [PubMed] [Google Scholar]
Rickels 2010 {published data only}
- Rickels K, Etemad B, Khalid-Khan S, Lohoff FW, Rynn MA, Gallop RJ. Time to relapse after 6 and 12 months' treatment of generalized anxiety disorder with venlafaxine extended release. Archives of General Psychiatry 2010;67(12):1274-81. [DOI] [PubMed] [Google Scholar]
Rouillon 2000 {published data only}
- Rouillon F, Berdeux G, Bisserbe JC, Warner B, Mesbah M, Smadja C, et al. Prevention of recurrent depressive episodes with milnacipran: consequences on quality of life. Journal of Affective Disorders 2000;58:171-80. [DOI] [PubMed] [Google Scholar]
- Rouillon F, Warner B, Pezous N, Bisserbe JC, Milnacipran Recurrence Prevention Group. Milnacipran efficacy in the prevention of recurrent depression: a 12-month placebo-controlled study. International Clinical Psychopharmacology 2000;15:133-40. [DOI] [PubMed] [Google Scholar]
Segal 2010 {published data only}
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry 2010;67(12):1256-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1997 {published data only}
- Stewart JW, Tricamo E, McGrath PJ, Quitkin FM. Prophylactic efficacy of phenelzine and imipramine in chronic atypical depression: likelihood of recurrence on discontinuation after 6 months' remission. American Journal of Psychiatry 1997;154(1):31-6. [DOI] [PubMed] [Google Scholar]
Streim 2012 {published data only}
- NCT00076622. Medication Treatment for Depression in Nursing Home Residents. https://clinicaltrials.gov/ct2/show/results/NCT00076622.
- Streim JE, Di Filippo S, Ten Have T, Mavandadi S, Weintraub D, Oslin D. Antidepressant discontinuation associated with cognitive decline in older adult residents of long-term care facilities. American Journal of Geriatric Psychiatry 2012;1):S147-8. [Google Scholar]
Terra 1998 {published data only}
- Terra JL, Montgomery SA. Fluvoxamine prevents recurrence of depression: results of a long-term, double-blind, placebo-controlled study. International Clincial Psychopharmacology 1998;13:55-62. [PubMed] [Google Scholar]
Wilson 2003 {published data only}
- Wilson KCM, Mottram PG, Ashworth L, Abou-Saleh MT. Older community residents with depression: long-term treatment with sertraline. British Journal of Psychiatry 2003;182:492-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexopoulos 2000 {published data only}
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, et al. Executive dysfunction and long-term outcomes of geriatric depression. Archives of General Psychiatry 2000;57(3):285-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Allgulander 2006 {published data only}
- Allgulander C, Florea I, Huusom AKT. Prevention of relapse in generalized anxiety disorder by escitalopram treatment. International Journal of Neuropsychopharmacology 2006;9(5):495-505. [DOI] [PubMed] [Google Scholar]
Baldwin 2006 {published data only}
- Baldwin DS, Huusom AK, Maehlum E. Escitalopram and paroxetine in the treatment of generalised anxiety disorder: randomised, placebo-controlled, double-blind study. British Journal of Psychiatry 2006;189:264-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Baldwin 2012 {published data only}
- Baldwin DS, Loft H, Florea I. Lu AA21004, a multimodal psychotropic agent, in the prevention of relapse in adult patients with generalized anxiety disorder. International Clinical Psychopharmacology 2012;27(4):197-207. [DOI] [PubMed] [Google Scholar]
Bockting 2005 {published data only}
- Bockting CL, Schene AH, Spinhoven P, Koeter MW, Wouters LF, Huyser J, et al. Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2005;73(4):647-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bockting 2006 {published data only}
- Bockting CL, Spinhoven P, Koeter MW, Wouters LF, Visser I, Schene AH. Differential predictors of response to preventive cognitive therapy in recurrent depression: a 2-year prospective study. Psychotherapy and Psychosomatics 2006;75(4):229-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bockting 2008 {published data only}
- Bockting CL, ten Doesschate MC, Spijker J, Spinhoven P, Koeter MW, Schene AH. Continuation and maintenance use of antidepressants in recurrent depression. Psychotherapy and Psychosomatics 2008;77(1):17-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bockting 2009 {published data only}
- Bockting CL, Spinhoven P, Wouters LF, Koeter MW, Schene AH. Long-term effects of preventive cognitive therapy in recurrent depression: a 5.5-year follow-up study. Journal of Clinical Psychiatry 2009;70(12):1621-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boulenger 2012 {published data only}
- Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. Journal of Psychopharmacology 2012;26(11):1408-16. [DOI] [PubMed] [Google Scholar]
Caillard 2003 {published data only}
- Caillard VF, Boyer P, Hotton JM, Lepine JP, Troy S, Bisserbe JC. Prophylactic treatment of recurrent depression: a randomized controlled trial of sertraline in remitted patients. In: 156th Annual Meeting of the American Psychiatric Association. San Francisco, CA, 2003, May 17-22:NR426.
Cohen 2006 {published data only}
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport J, Viguera A, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;5:499-507. [DOI] [PubMed] [Google Scholar]
Coppen 1978 {published data only}
- Coppen A, Ghose K, Montgomery S, Rama Rao VA, Bailey J, Jorgensen A. Continuation therapy with amitriptyline in depression. British Journal of Psychiatry 1978;133:28-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Croft 2002 {published data only}
- Croft H, Houser TL, Jamerson BD, Leadbetter R, Bolden-Watson C, Donahue R, et al. Effect on body weight of bupropion sustained-release in patients with major depression treated for 52 weeks. Clinical Therapeutics 2002;24(4):662-72. [DOI] [PubMed] [Google Scholar]
Curtis 1993 {published data only}
- Curtis GC, Massana J, Udina C, Ayuso JL, Cassano GB, Perugi G. Maintenance drug therapy of panic disorder. Journal of Psychiatric Research 1993;27 Suppl 1:127-42. [DOI] [PubMed] [Google Scholar]
Davidson 2001 {published data only}
- Davidson J, Pearlstein T, Londborg P, Brady KT, Rothbaum Bn Bell J, Maddock R, et al. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: results of a 28-week double-blind, placebo-controlled study. American Journal of Psychiatry 2001;158:1974-81. [DOI] [PubMed] [Google Scholar]
Davidson 2005 {published data only}
- Davidson JR, Connor KM, Hertzberg MA, Weisler RH, Wilson WH, Payne VM. Maintenance therapy with fluoxetine in post traumatic stress disorder: a placebo-controlled discontinuation study. Journal of Clinical Psychopharmacology 2005;25:166-9. [DOI] [PubMed] [Google Scholar]
Davidson 2005a {published data only}
- Davidson JR, Bose A, Wang Q. Safety and efficacy of escitalopram in the long-term treatment of generalised anxiety disorder. Journal of Clinical Psychiatry 2005;66:1441-6. [DOI] [PubMed] [Google Scholar]
Davidson 2008 {published data only}
- Davidson JRT, Wittchen HU, Llorca PM, Erickson J, Detke M, Ball SG, et al. Duloxetine treatment for relapse prevention in adults with generalized anxiety disorder: a double-blind placebo-controlled trial. European Neuropsychopharmacology 2008;18(9):673-81. [DOI] [PubMed] [Google Scholar]
Dinan 2001 {published data only}
- Dinan TG. Efficacy and safety of weekly treatment with enteric-coated fluoxetine in patients with major depressive disorder. Journal of Clinical Psychiatry 2001;62 Suppl 22:48‐52. [PubMed] [Google Scholar]
Dobson 2008 {published data only}
- Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. Journal of Consulting and Clinical Psychology 2008;76(3):468-77. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doogan 1992 {published data only}
- Doogan DP, Caillard V. Sertraline in the prevention of depression. British Journal of Psychiatry 1992;160:217-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Duboff 1993 {published data only}
- Duboff E A. Long-term treatment of major depressive disorder with paroxetine. Journal of Clinical Psychopharmacology 1993;13(6 Suppl 2):28S‐33S. [DOI] [PubMed] [Google Scholar]
Durgam 2019 {published data only}
- Durgam S, Chen C, Migliore R, Prakash C, Thase ME. Relapse prevention with levomilnacipran ER in adults with major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. Depression & Anxiety 2019;36(3):225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fava 1994 {published data only}
- Fava GA, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. American Journal of Psychiatry 1994;151(9):1295-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fava 1998 {published data only}
- Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Archives of General Psychiatry 1998;55(9):816-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fava 2002 {published data only}
- Fava M, Schmidt ME, Zhang S, Gonzales J, Raute NJ, Judge R. Treatment approaches to major depressive disorder relapse. Part 2: reinitiation of antidepressant treatment. Psychotherapy & Psychosomatics 2002;71(4):195-9. [DOI] [PubMed] [Google Scholar]
Ferguson 2007 {published data only}
- Ferguson JM, Khan A, Mangano R, Entsuah R, Tzanis E. Relapse prevention of panic disorder in adult outpatient responders to treatment with venlafaxine extended release. Journal of Clinical Psychiatry 2007;68(1):58-68. [DOI] [PubMed] [Google Scholar]
Georgotas 1989 {published data only}
- Georgotas A, McCue RE, Cooper TB. A placebo-controlled comparison of nortriptyline and phenelzine in maintenance therapy of elderly depressed patients. Archives of General Psychiatry 1989;46(9):783-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 1994 {published data only}
- GlaxoSmithKline. A Double-Blind, Placebo-Controlled Continuation of Study 29060/120 to Assess the Long Term Safety and Efficacy of Paroxetine in the Treatment of Panic Disorder and Its Role in the Prevention of Relapse/Recurrence. GSK - clinical study register; www.gsk-clinicalstudyregister.com. 1994.
GlaxoSmithKline 1999 {published data only}
- GlaxoSmithKline. A Multicenter Placebo-Controlled Study of WELLBUTRIN (Bupropion Hydrochloride) Sustained Release (SR) for the Prevention of Relapse/Recurrence in Subjects Whose Depression Responded to Treatment With WELLBUTRIN SR. GSK - clinical study register; www.gsk-clinicalstudyregister.com. 1999.
Glen 1984 {published data only}
- Glen AI, Johnson AL, Shepherd M. Continuation therapy with lithium and amitriptyline in unipolar depressive illness: a randomized, double-blind, controlled trial. Psychological Medicine 1984;14(1):37-50. [DOI] [PubMed] [Google Scholar]
Godfrin 2010 {published data only}
- Godfrin KA, Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: a randomized controlled study. Behaviour Research and Therapy 2010;48(8):738-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goodwin 2009 {published data only}
- Goodwin GM, Emsley R, Rembry S, Rouillon F, Agomelatine Study Group. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2009;70(8):1128-37. [DOI] [PubMed] [Google Scholar]
Goodwin 2013 {published data only}
- Goodwin GM, Boyer P, Emsley R, Rouillon F, Bodinat C. Is it time to shift to better characterization of patients in trials assessing novel antidepressants? An example of two relapse prevention studies with agomelatine. International Clinical Psychopharmacology 2013;28(1):20-8. [DOI] [PubMed] [Google Scholar]
Gorwood 2007 {published data only}
- Gorwood P, Weiller E, Lemming O, Katona C. Escitalopram prevents relapse in older patients with major depressive disorder. American Journal of Geriatric Psychiatry 2007;15(7):581-93. [DOI] [PubMed] [Google Scholar]
Harrison 1986 {published data only}
- Harrison W, Rabkin J, Stewart JW, McGrath PJ, Tricamo E, Quitkin F. Phenelzine for chronic depression: a study of continuation treatment. Journal of Clinical Psychiatry 1986;47(7):346-9. [PMID: ] [PubMed] [Google Scholar]
Hochstrasser 2001 {published data only}
- Hochstrasser B, Isaksen PM, Koponen H, Lauritzen L, Mahnert FA, Rouillon F, et al. Prophylactic effect of citalopram in unipolar, recurrent depression: placebo-controlled study of maintenance therapy. British Journal of Psychiatry 2001;178:304-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kamijima 2005 {published data only}
- Kamijima K, Kuboki T, Kumano H, Burt T, Cohen G, Arano I, et al. A placebo-controlled, randomized withdrawal study of sertraline for panic disorder in Japan. International Clinical Psychopharmacology 2005;20(5):265-73. [DOI] [PubMed] [Google Scholar]
Kamijima 2006 {published data only}
- Kamijima K, Burt T, Cohen G, Arano I, Hamasaki T. A placebo-controlled, randomized withdrawal study of sertraline for major depressive disorder in Japan. International Clinical Psychopharmacology 2006;21(1):1-9. [DOI] [PubMed] [Google Scholar]
Klerman 1974 {published data only}
- Klerman GL, Dimascio A, Weissman M, Prusoff B, Paykel ES. Treatment of depression by drugs and psychotherapy. American Journal of Psychiatry 1974;131(2):186-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lepine 2004 {published data only}
- Lepine JP, Caillard V, Bisserbe JC, Troy S, Hotton JM, Boyer P. A randomized, placebo-controlled trial of sertraline for prophylactic treatment of highly recurrent major depressive disorder. American Journal of Psychiatry 2004;161(5):836-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Licht 2013 {published data only}
- Licht RW. Is it possible to evaluate true prophylactic efficacy of antidepressants in severely ill patients with recurrent depression? Lessons from a placebo-controlled trial. The Fifth Trial of the Danish University Antidepressant Group (DUAG-5). Journal of Affective Disorders 2013;148(2‐3):286-90. [DOI] [PubMed] [Google Scholar]
Liebowitz 1999 {published data only}
- Liebowitz MR, Heimberg RG, Schneier FR, Hope DA, Davies S, Holt CS, et al. Cognitive-behavioral group therapy versus phenelzine in social phobia: long term outcome. Depression and Anxiety 1999;10(3):89‐98. [PubMed] [Google Scholar]
Liebowitz 2010 {published data only}
- Liebowitz M, Lam RW, Lepola U, Datto C, Sweitzer D, Eriksson H. Efficacy and tolerability of extended release quetiapine fumarate monotherapy as maintenance treatment of major depressive disorder: a randomized, placebo-controlled trial. Depression and Anxiety 2010;27(10):964-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lustman 2006 {published data only}
Lyketsos 2011 {published data only}
- Lyketsos CG, Weiller E, Katona C, Gorwood P. Are old-old patients with major depression more likely to relapse than young-old patients during continuation treatment with escitalopram? BMC Geriatrics 2011;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Markowitz 2000 {published data only}
- Markowitz JS, DeVane CL, Liston HL, Montgomery SA. An assessment of selective serotonin reuptake inhibitor discontinuation symptoms with citalopram. International Clinical Psychopharmacology 2000;15(6):329-33. [DOI] [PubMed] [Google Scholar]
McGrath 2006 {published data only}
Michelson 1999 {published data only}
- Michelson D, Pollack M, Lydiard RB, Tamura R, Tepner R, Tollefson G. Continuing treatment of panic disorder after acute response: randomised, placebo-controlled trial with fluoxetine. British Journal of Psychiatry 1999;174:213-8. [DOI] [PubMed] [Google Scholar]
Montgomery 1993 {published data only}
- Montgomery SA, Rasmussen JG, Tanghoj P. A 24-week study of 20 mg citalopram, 40 mg citalopram, and placebo in the prevention of relapse of major depression. International Clinical Psychopharmacology 1993;8(3):181-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Montgomery 1993a {published data only}
- Montgomery SA, Dunbar G. Paroxetine is better than placebo in relapse prevention and the prophylaxis of recurrent depression. International Clinical Psychopharmacology 1993;8(3):189-95. [DOI] [PubMed] [Google Scholar]
Montgomery 2005 {published data only}
- Montgomery SA, Nil R, Durr-Pal N, Loft H, Boulenger JP. A 24-week randomized, double-blind, placebo-controlled study of escitalopram for the prevention of generalized social anxiety disorder. Journal of Clinical Psychiatry 2005;66(10):1270-8. [DOI] [PubMed] [Google Scholar]
NCT00878748 {published data only}
- NCT00878748. Study Evaluating Effexor XR in Chinese Subjects With Major Depressive Disorder. https://clinicaltrials.gov/show/NCT00878748 (data entered 9 April 2009).
OADIG 1993 {published data only}
- Old Age Depression Interest Group. How long should the elderly take antidepressants? A double-blind placebo-controlled study of continuation/prophylaxis therapy with dothiepin. British Journal of Psychiatry 1993;162:175-82. [PMID: ] [PubMed] [Google Scholar]
Rapoport 2010 {published data only}
- Rapoport MJ, Mitchell RA, McCullagh S, Herrmann N, Chan F, Kiss A, et al. A randomized controlled trial of antidepressant continuation for major depression following traumatic brain injury. Journal of Clinical Psychiatry 2010;71(9):1125-30. [DOI] [PubMed] [Google Scholar]
Reimherr 1998 {published data only}
- Reimherr FW, Amsterdam JD, Quitkin FM, Rosenbaum JF, Fava M, Zajecka J, et al. Optimal length of continuation therapy in depression: a prospective assessment during long-term fluoxetine treatment. American Journal of Psychiatry 1998;155:1247-53. [DOI] [PubMed] [Google Scholar]
Reynolds 1992 {published data only}
- Reynolds CF III, Frank E, Perel JM, Imber SD, Cornes C, Morycz RK, et al. Combined pharmacotherapy and psychotherapy in the acute and continuation treatment of elderly patients with recurrent major depression: a preliminary report. American Journal of Psychiatry 1992;149(12):1687-92. [DOI] [PubMed] [Google Scholar]
Reynolds 1999 {published data only}
- Reynolds CF, Frank E, Perel JM, Imber SD, Cornes C, Mileer MD, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281(1):39-45. [DOI] [PubMed] [Google Scholar]
Robinson 1991 {published data only}
- Robinson DS, Lerfald SC, Bennett B, Laux D, Devereaux E, Kayser A, et al. Continuation and maintenance treatment of major depression with the monoamine oxidase inhibitor phenelzine: a double-blind placebo-controlled discontinuation study. Psychopharmacology Bulletin 1991;27(1):31-9. [PubMed] [Google Scholar]
Rosenbaum 1998 {published data only}
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biological Psychiatry 1998;44(2):77-87. [DOI] [PubMed] [Google Scholar]
Rosenthal 2013 {published data only}
- Rosenthal JZ, Boyer P, Vialet C, Hwang E, Tourian KA. Efficacy and safety of desvenlafaxine 50 mg/d for prevention of relapse in major depressive disorder: a randomized controlled trial. Journal of Clinical Psychiatry 2013;74(2):158-66. [DOI] [PubMed] [Google Scholar]
Sackeim 2001 {published data only}
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 2001;285(10):1299-307. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2002 {published data only}
- Schmidt NB, Wollaway-Bickel K, Trakowski JH, Santiago HT, Vasey M. Antidepressant discontinuation in the context of cognitive behavioral treatment for panic disorder. Behaviour Research & Therapy 2002;40(1):67-73. [DOI] [PubMed] [Google Scholar]
Shawyer 2012 {published data only}
- Shawyer F, Meadows GN, Judd F, Martin PR, Segal Z, Piterman L. The DARE study of relapse prevention in depression: design for a phase 1/2 translational randomised controlled trial involving mindfulness-based cognitive therapy and supported self monitoring. BMC Psychiatry 2012;12:3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimazu 2011 {published data only}
- Shimazu K, Shimodera S, Mino Y, Nishida A, Kamimura N, Sawada K, et al. Family psychoeducation for major depression: randomised controlled trial. British Journal of Psychiatry 2011;198(5):385-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shimodera 2012 {published data only}
- Shimodera S, Furukawa TA, Mino Y, Shimazu K, Nishida A, Inoue S. Cost-effectiveness of family psychoeducation to prevent relapse in major depression: results from a randomized controlled trial. BMC Psychiatry 2012;12:40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 1996 {published data only}
- Stein MB, Chartier MJ, Hazen AL, Kroft CD, Chale RA, Cote D, et al. Paroxetine in the treatment of generalized social phobia: open-label treatment and double-blind placebo-controlled discontinuation. Journal of Clinical Psychopharmacology 1996;16(3):218-22. [DOI] [PubMed] [Google Scholar]
Stein 2012 {published data only}
- Stein DJ, Ahokas A, Albarran C, Olivier V, Allgulander C. Agomelatine prevents relapse in generalized anxiety disorder: a 6-month randomized, double-blind, placebo-controlled discontinuation study. Journal of Clinical Psychiatry 2012;73(7):1002-8. [DOI] [PubMed] [Google Scholar]
Stewart 1998 {published data only}
- Stewart JW, Quitkin FM, McGrath PJ, Amsterdam J, Fava M, Fawcett J, et al. Use of pattern analysis to predict differential relapse of remitted patients with major depression during 1 year of treatment with fluoxetine or placebo. Archives of General Psychiatry 1998;55(4):334-43. [DOI] [PubMed] [Google Scholar]
Stocchi 2003 {published data only}
- Stocchi F, Nordera G, Jokinen RH, Lepola UM, Hewett K, Bryson H, et al. Efficacy and tolerability of paroxetine for the long-term treatment of generalized anxiety disorder. Journal of Clinical Psychiatry 2003;64(3):250-8. [DOI] [PubMed] [Google Scholar]
Teasdale 2000 {published data only}
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology 2000;68(4):615-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thase 2001 {published data only}
- Thase ME, Nierenberg AA, Keller MB, Panagides J. Efficacy of mirtazapine for prevention of depressive relapse: a placebo-controlled double-blind trial of recently remitted high-risk patients. Journal of Clinical Psychiatry 2001;62(10):782-8. [DOI] [PubMed] [Google Scholar]
Tint 2008 {published data only}
- Tint A, Haddad PM, Anderson IM. The effect of rate of antidepressant tapering on the incidence of discontinuation symptoms: a randomised study. Journal of Psychopharmacology (Oxford, England) 2008;22(3):330-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Walker JR, Van Ameringen MA, Swinson R, Bowen RC, Chokka PR, Goldner E, et al. Prevention of relapse in generalized social phobia: results of a 24-week study in responders to 20 weeks of sertraline treatment. Journal of Clinical Psychopharmacology 2000;20(6):636-44. [DOI] [PubMed] [Google Scholar]
Weissman 1976 {published data only}
- Weissman MM, Kasl SV, Klerman GL. Follow-up of depressed women after maintenance treatment. American Journal of Psychiatry 1976;133(7):757-60. [DOI] [PubMed] [Google Scholar]
Wentink 2019 {published data only}
- Wentink C, Huijbers MJ, Lucassen P, Kramers C, Akkermans R, Adang E, et al. Discontinuation of antidepressant medication in primary care supported by monitoring plus mindfulness-based cognitive therapy versus monitoring alone: design and protocol of a cluster randomized controlled trial. BMC Family Practice 2019;20(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetherell 2013 {published data only}
- Wetherell JL, Petkus AJ, White KS, Nguyen H, Kornblith S, Andreescu C, et al. Antidepressant treatment plus cognitive behavioral therapy for generalized anxiety disorder in older adults. American Journal of Psychiatry 2013;170(7):782-9. [DOI] [PMC free article] [PubMed]
Zajecka 1998 {published data only}
- Zajecka J, Fawcett J, Amsterdam J, Quitkin F, Reimherr F, Rosenbaum J, et al. Safety of abrupt discontinuation of fluoxetine: a randomized, placebo-controlled study. Journal of Clinical Psychopharmacology 1998;18(3):193-7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gunn 2020 {unpublished data only}
Mangin 2015 {published data only}
- Mangin D, Dowson C, Mulder R, Wells E, Toop L, Dowell T. The effectiveness of maintenanceSSRI treatment in primary care depression to prevent recurrence: multicentre double blinded placebo controlled RCT. In: Proceedings of the 2015 North American Primary Care Research Group (NAPCRG) 43rd Annual Meeting. 2015.
Molenaar 2016 {published data only}
- Molenaar NM, Brouwer ME, Bockting CL, Bonsel GJ, Veere CN, Torij HW, et al. Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry 2016;16:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Duffy 2019 {published data only}
- Duffy L, Bacon F, Clarke CS, Donkor Y, Freemantle N, Gilbody S, et al. A randomised controlled trial assessing the use of citalopram, sertraline, fluoxetine and mirtazapine in preventing relapse in primary care patients who are taking long-term maintenance antidepressants (ANTLER: ANTidepressants to prevent reLapse in dEpRession): study protocol for a randomised controlled trial. Trials 2019;20:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN12417565 {published data only}
- Kendrick T, Geraghty AWA, Bowers H, Stuart B, Leydon G, May C, et al. REDUCE (reviewing long-term antidepressant use by careful monitoring in everyday practice) Internet and telephone support to people coming off long-term antidepressants): protocol for a randomised controlled trial. Trials 2020;21:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Altman 2006
- Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambresin 2015
- Ambresin G, Palmer V, Densley K, Dowrick C, Gilchrist G, Gunn JM. What factors influence long-term antidepressant use in primary care? Findings from the Australian diamond cohort study. Journal of Affective Disorders 2015;176:125–32. [DOI] [PubMed] [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2010
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. dsm.psychiatryonline.org/doi/full/10.1176/appi.books.9780890425596.dsm04 (accessed 8 April 2019).
APA 2013
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-V). Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Aroll 2017
- Arroll B, Moir F, Kendrick T. Effective management of depression in primary care: a review of the literature. British Journal of General Practice 2017;1(2):bjgpopen17X101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arroll 2009
- Arroll B, Elley CR, Fishman T, Goodyear‐Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007954. [DOI: 10.1002/14651858.CD007954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 2014
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, Den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology 2014;28(5):403-39. [DOI] [PubMed] [Google Scholar]
Bandelow 1992
- Bandelow B. Assessing the efficacy of treatments for panic disorder and agoraphobia. II. The Panic and Agoraphobia Scale. International Clinical Psychopharmacology 1992;10(2):73-81. [DOI] [PubMed] [Google Scholar]
Batelaan 2017
- Batelaan NM, Bosman RC, Muntingh A, Scholten WD, Huijbregts JM, Van Balkom AJ. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ 2017;358:j3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
BCFI/CBIP 2018
- Belgian Center for Pharmacotherapeutical Information (BCFI/CBIP). Belgian National Formulary [Gecommentarieerd Geneesmiddelenrepertorium 2018]. www.bcfi.be/nl/chapters/11?frag=7997 (accessed 8 April 2019).
Beck 1961
- Beck AT, Ward CH. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [DOI] [PubMed] [Google Scholar]
Beck 1993
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio: TX: Psychological Corporation, 1993. [Google Scholar]
Bergh 2012
- Bergh S, Selbæk G, Engedal K. Discontinuation of antidepressants in people with dementia and neuropsychiatric symptoms (DESEP study): double blind, randomised, parallel group, placebo controlled trial. BMJ 2012;344:e1566. [DOI] [PubMed] [Google Scholar]
Berton 2006
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Reviews Neuroscience 2006;7(2):137-51. [DOI] [PubMed] [Google Scholar]
Beshai 2011
- Beshai S, Dobson KS, Bockting CL, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clinical Psychology Review 2011;31(8):1349-60. [DOI] [PubMed] [Google Scholar]
Bet 2013
- Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ. Side effects of antidepressants during long-term use in a naturalistic setting. European Neuropsychopharmacology 2013;23(11):1443-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Biesheuvel 2015
- Biesheuvel-Leliefeld KE, Kok GD, Bockting CL, Cuijpers P, Hollon SD, Van Marwijk HW, et al. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: meta-analysis and meta-regression. Journal of Affective Disorders 2015;174:400-10. [DOI] [PubMed] [Google Scholar]
Bogetto 2002
- Bogetto F, Bellino S, Revello RB, Patria L. Discontinuation syndrome in dysthymic patients treated with selective serotonin reuptake inhibitors: a clinical investigation. CNS drugs 2002;16(4):273-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bourgeois 2012
- Bourgeois J, Elseviers M, Van Bortel L, Petrovic M, Vander Stichele R. The use of antidepressants in Belgian nursing homes: focus on indications and dosages in the Phebe study. Drugs & Aging 2012;29:759–69. [DOI] [PubMed] [Google Scholar]
Brett 2017
- Brett J, Karanges EA, Daniels B, Buckley NA, Schneider C, Nassir A, et al. Psychotropic medication use in Australia, 2007 to 2015: changes in annual incidence, prevalence and treatment exposure. Australian and New Zealand Journal of Psychiatry 2017;51:990–9. [DOI] [PubMed] [Google Scholar]
Busner 2007
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry 2007;4:28-37. [PMC free article] [PubMed] [Google Scholar]
Cameron 2014
- Cameron IM, Reid IC, MacGillivray SA. Efficacy and tolerability of antidepressants for sub-threshold depression and for mild major depressive disorder. Journal of Affective Disorders 2014;166:44-58. [DOI] [PubMed] [Google Scholar]
CANMAT 2016
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: pharmacological treatments. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 2016;61:540–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cartwright 2016
- Cartwright C, Gibson K, Read J, Cowan O, Dehar T. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Preference and Adherence 2016;10:1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catalogue of Bias 2019
- Catalogue of Bias Collaboration, Holman B, Bero L, Mintzes B. Industry sponsorship bias. https://catalogofbias.org/biases/industry-sponsorship-bias/ (accessed 20 May 2020).
Cipriani 2018
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391(10128):1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2019
- Cohen D, Recalt A. Discontinuing psychotropic drugs from participants in randomized controlled trials: a systematic review. Psychotherapy and Psychosomatics 2019;88(2):96-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cruickshank 2008
- Cruickshank G, Macgillivray S, Bruce D, Mather A, Matthews K, Williams B. Cross-sectional survey of patients in receipt of long-term repeat prescriptions for antidepressant drugs in primary care. Mental Health in Family Medicine 2008;5(2):105-9. [PMC free article] [PubMed] [Google Scholar]
Davies 2019
- Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addictive Behaviors 2019;97:111-21. [10.1016/j.addbeh.2018.08.027] [DOI] [PubMed] [Google Scholar]
Declercq 2017
- Declercq T, Habraken H, Van den Ameele H, Callens J, De Lepeleire J, Cloetens H, Domus Medical Flemish College of General Practitioners. Depressive Disorder in Adults. www.domusmedica.be/richtlijnen/depressie-bij-volwassenen (accessed 8 April 2019).
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook (accessed 8 April 2019).
DHSC 2018
- Department of Health and Social Care (DHSC). Hansard: Prescriptions Drugs – Written Question – 128871. www.parliament.uk/business/publications/written-questions-answers-statements/written-question/Commons/2018-07-11/163180/ (accessed 8 April 2019).
Eaton 2008
- Eaton WW, Shao H, Nestadt G, Lee BH, Bienvenu OJ, Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry 2008;65(5):513-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eiring 2015
- Eiring Ø, Landmark BF, Aas E, Salkeld G, Nylenna M, Nytrøen K. What matters to patients? A systematic review of preferences for medication-associated outcomes in mental disorders. BMJ Open 2015;5(4):e007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Mallakh 2012
- El-Mallakh RS, Briscoe B. Studies of long-term use of antidepressants: how should the data from them be interpreted? CNS Drugs 2012;26(2):97-109. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eveleigh 2017
- Eveleigh R, Muskens E, Lucassen P, Verhaak P, Spijker J, Van Weel C, et al. Withdrawal of unnecessary antidepressant medication: a randomised controlled trial in primary care. British Journal of General Practice Open 2017;1(4):bjgpopen17X101265. [DOI: 10.3399/bjgpopen17X101265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eveleigh 2019
- Eveleigh R, Speckens A, Weel C, Oude Voshaar R, Lucassen P. Patients' attitudes to discontinuing not-indicated long-term antidepressant use: barriers and facilitators. Therapeutic Advances in Psychopharmacology 2019;9:2045125319872344. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fava 1998a
- Fava GA, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. American Journal of Psychiatry 1998;155(10):1443-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fava 2015
- Fava GA, Gatti A, Belaise C, Guidi J, Offidani E. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychotherapy and Psychosomatics 2015;84:72-81. [DOI] [PubMed] [Google Scholar]
Fava 2018b
- Fava GA, Benasi G, Lucente M, Offidani E, Cosci F, Guidi J. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychotherapy and Psychosomatics 2018;87(4):195-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frank 1991
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder remission, recovery, relapse, and recurrence. Archives of General Psychiatry 1991;48(9):851-5. [DOI] [PubMed] [Google Scholar]
Gandek 1998
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. Journal of Clinical Epidemiology 1998;51:1171-8. [DOI] [PubMed] [Google Scholar]
Geddes 2003
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361:653–61. [DOI] [PubMed] [Google Scholar]
Glue 2010
- Glue P, Donovan MR, Kolluri S, Emir B. Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Australian and New Zealand Journal of Psychiatry 2010;44:697–705. [DOI] [PubMed] [Google Scholar]
Gorgels 2005
- Gorgels WJ, Oude Voshaar RC, Mol AJ, Van de Lisdonk EH, Van Balkom AJ, Van den Hoogen HJ, et al. Discontinuation of long-term benzodiazepine use by sending a letter to users in family practice: a prospective controlled intervention study. Drug Alcohol Dependence 2005;78(1):49-56. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 8 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Greenhouse 1991
- Greenhouse JB, Stangl D, Kupfer DJ, Prien RF. Methodologic issues in maintenance therapy clinical trials. Archives of General Psychiatry 1991;48(4):313-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Groot 2013
- Groot P. Tapering strips for paroxetine and venlafaxine. Tijdschrift Psychiatrie 2013;55:789–94. [PubMed] [Google Scholar]
Groot 2018
- Groot PC, Van Os J. Antidepressant tapering strips to help people come off medication more safely. Psychosis 2018;10:142-5. [Google Scholar]
Groot 2020
- Groot PC, Van Os J. Outcome of antidepressant drug discontinuation with tapering strips after 1–5 years. Therapeutic Advances in Psychopharmacology 2020;10:1-8. [10.1177/2045125320954609] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guidi 2016
- Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. American Journal of Psychiatry 2016;73(2):128-37. [DOI] [PubMed] [Google Scholar]
Gusmão 2013
- Gusmão R, Quintão S, McDaid D, Arensman E, Van Audenhove C, Coffey C, et al. Antidepressant utilization and suicide in Europe: an ecological multi-national study. PLoS ONE 2013;8(6):e66455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddad 2007
- Haddad PM, Anderson IM. Recognising and managing antidepressant discontinuation symptoms. Advances in Psychiatric Treatment 2007;13:447-57. [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Health Psychology 1959;32(5):50-5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannula 2006
- Hannula JA, Lahtela K, Järvikoski A, Salminen JK, Mäkelä P. Occupational Functioning Scale (OFS) – an instrument for assessment of work ability in psychiatric disorders. Nordic Journal of Psychiatry 2006;60(5):372-8. [DOI] [PubMed] [Google Scholar]
Harmer 2017
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017;4(5):409-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hengartner 2017
- Hengartner MP. Methodological flaws, conflicts of interest, and scientific fallacies: implications for the evaluation of antidepressants' efficacy and harm. Frontiers in Psychiatry 2017;8:275. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hengartner 2019
- Hengartner MP, Davies J, Read J. Antidepressant withdrawal - the tide is finally turning. Epidemiology and Psychiatric Sciences 2019;29:e52. [PMID: ] [DOI] [PMC free article] [PubMed]
Hengartner 2020
- Hengartner MP. How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding. Therapeutic Advances in Psychopharmacology 2020;10:2045125320921694. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook (accessed 8 April 2019).
Himei 2006
- Himei A, Okamura T. Discontinuation syndrome associated with paroxetine in depressed patients: a retrospective analysis of factors involved in the occurrence of the syndrome. CNS Drugs 2006;20(8):665-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Horowitz 2019
- Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry 2019;6:538-46. [DOI] [PubMed] [Google Scholar]
Iacobucci 2019
- Iacobucci G. NICE updates: antidepressant guidelines to reflect severity and length of withdrawal symptoms. BMJ (Clinical research ed.) 2019;367:l6103. [PMID: ] [DOI] [PubMed] [Google Scholar]
ISRCTN15036829
- ISRCTN15036829. REDUCE Programme WS4: REviewing long term anti-DEpressant Use by Careful monitoring in Everyday practice. www.isrctn.com/ISRCTN15036829 (accessed 20 June 2019).
Jakobsen 2017
- Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Moller K, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis. BMC Psychiatry 2017;17(1):58. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janus 2016
- Janus S, Van Manen J, IJzerman M, Zuidema S. Psychotropic drug prescriptions in Western European nursing homes. International Psychogeriatrics / IPA 2016;28(11):1775-90. [DOI] [PubMed] [Google Scholar]
Johnson 2012
- Johnson CF, Macdonald HJ, Atkinson P, Buchanan AI, Downes N, Dougall N. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. British Journal of General Practice 2012;62:e773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2017
- Johnson CF, Williams B, MacGillivray SA, Dougall NJ, Maxwell M. 'Doing the right thing': factors influencing GP prescribing of antidepressants and prescribed doses. BMC Family Practice 2017;18(1):72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karkare 2011
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. American Journal of Geriatric Pharmacotherapy 2011;9(2):109–19. [DOI] [PubMed] [Google Scholar]
Kaymaz 2008
- Kaymaz N, Van Os J, Loonen AJ, Nolen WA. Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation: a meta-analysis of placebo-controlled randomized trials. Journal of Clinical Psychiatry 2008;69:1423–36. [DOI] [PubMed] [Google Scholar]
Kendrick 2015
- Kendrick T. Long-term antidepressant treatment: time for a review? Prescriber 2015;26(19):7-8. [Google Scholar]
Kirsch 2007
- Kirsch I, Moncrieff J. Clinical trials and the response rate illusion. Contemporary Clinical Trials 2007;28(4):348-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kirsch 2019
- Kirsch I. Placebo effect in the treatment of depression and anxiety. Frontiers in Psychiatry 2019;10:407. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjosavik 2016
- Kjosavik SR, Gillam MH, Roughead EE. Average duration of treatment with antidepressants among concession card holders in Australia. Journal of Psychiatric Practice 2016;50(12):1180-5. [DOI] [PubMed] [Google Scholar]
Kok 2017
- Kok RM, Reynolds CF. Management of depression in older adults: a review. JAMA 2017;317(20):2114-22. [DOI] [PubMed] [Google Scholar]
Kuyken 2016
- Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, et al. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry 2016;73(6):565-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. The Cochrane Database of Systematic Reviews 2017;2:MR000033. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2019
- Ma A, Thompson W, Polemiti E, Hussain S, Magwood O, Welch V, et al. Deprescribing of chronic benzodiazepine receptor agonists for insomnia in adults. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD013371. [DOI: 10.1002/14651858.CD013371] [DOI] [Google Scholar]
Machmutow 2019
- Machmutow K, Meister R, Jansen A, Kriston L, Watzke B, Härter MC, et al. Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012855. [DOI: 10.1002/14651858.CD012855.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MaGPIe Research Group 2003
- MaGPIe Research Group. The nature and prevalence of psychological problems in New Zealand primary healthcare: a report on Mental Health and General Practice Investigation (MaGPIe). New Zealand Medical Journal 2003;116(1171):U379. [PubMed] [Google Scholar]
Maier 1988
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders 1988;14(1):61-8. [DOI] [PubMed] [Google Scholar]
Mangin 2018
- Mangin D, Lawson J, Cuppage J, Shaw E, Ivanyi K, Davis A, et al. Legacy drug prescribing patterns in primary care. Annals of Family Medicine 2018;16(6):515-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mars 2017
- Mars B, Heron J, Kessler D, Davies NM, Martin RM, Thomas KH, et al. Influences on antidepressant prescribing trends in the UK: 1995-2011. Social Psychiatry and Psychiatric Epidemiology 2017;52(2):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattisson 2007
- Mattisson C, Bogren M, Horstmann V, Munk-Jorgensen P, Nettelbladt P. The long-term course of depressive disorders in the Lundby Study. Psychological Medicine 2007;37(6):883-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maund 2018
Maund 2019
- Maund E, Stuart B, Moore M, Dowrick C, Geraghty A, Dawson A, et al. Managing antidepressant discontinuation: a systematic review. Annals of Family Medicine 2019;17(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormak 2018
- McCormack J, Korownyk C. Effectiveness of antidepressants. BMJ 2018;360:k1073. [DOI] [PubMed] [Google Scholar]
Meijer 2004
- Meijer WE, Heerdink ER, Leufkens HG, Herings RM, Egberts AC, Nolen WA. Incidence and determinants of long-term use of antidepressants. European Journal of Clinical Pharmacology 2004;60:57–61. [DOI] [PubMed] [Google Scholar]
Moffit 2010
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine 2009;40:899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mojtabi 2014
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. Journal of Clinical Psychiatry 2014;75:169-77. [DOI] [PubMed] [Google Scholar]
Moncrieff 2004
- Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD003012. [DOI: 10.1002/14651858.CD003012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1997
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Montgomery 2004a
- Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. International Clinical Psychopharmacology 2004;19:271-80. [DOI] [PubMed] [Google Scholar]
Munkholm 2019
- Munkholm K, Paludan-Müller AS, Boesen K. Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta-analysis. BMJ Open 2019;9:e024886. [DOI: 10.1136/bmjopen-2018-024886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murata 2010
- Murata Y, Kobayashi D, Imuta N, Haraguchi K, Ieiri I, Nishimura R, et al. Effects of the serotonin 1A, 2A, 2C, 3A, and 3B and serotonin transporter gene polymorphisms on the occurrence of paroxetine discontinuation syndrome. Journal of Clinical Psychopharmacology 2010;30(1):11-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
NHG 2012
- Van Weel-Baumgarten EM, Van Gelderen MG, Grundmeijer HG, Licht-Strunk E, Van Marwijk HW, Van Rijswijk HC, et al. The NHG guideline depression (second revision of the NHG guideline depressive disorder). Huisarts en Wetenschap 2012;55:252-9. [Google Scholar]
NHG 2018
- Dutch College of General Practitioners (NHG). Deprescribing SSRI and TCA. www.nhg.org/sites/default/files/content/nhg_org/uploads/201809_multidisciplinair_document_afbouwen_ssris_en_snris.pdf (accessed 8 April 2019).
NICE 2009
- National Institute for Health and Care Excellence (NICE). Depression in adults: recognition and management. [CG90]. www.nice.org.uk/guidance/cg90 (accessed 1 February 2021).
NICE 2011
- National Institute for Health and Care Excellence (NICE). Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults: management in primary, secondary and community care. [CG113]. www.nice.org.uk/guidance/cg113 (accessed 8 April 2019).
Nielsen 2012
- Nielsen M, Hansen EH, Gotzsche PC. What is the difference between dependence and withdrawal reactions? A comparison of benzodiazepines and selective serotonin re-uptake inhibitors. Addiction (Abingdon, England) 2012;107(5):900-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
OECD 2017
- Organisation of Economic Co-operation and Development (OECD). Antidepressant drugs consumption. dx.doi.org/10.1787/health_glance-2017-graph181-en (accessed 8 April 2019).
Ormel 2020
Petty 2006
- Petty DR, House A, Knapp P, Raynor T, Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age and Ageing 2006;35(5):523-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Piek 2010
- Piek E, Van der Meer K, Nolen WA. Guideline recommendations for long-term treatment of depression with antidepressants in primary care: a critical review. European Journal of General Practice 2010;16(2):106–12. [DOI] [PubMed] [Google Scholar]
Piet 2011
- Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clinical Psychology Review 2011;31(6):1032-40. [DOI] [PubMed] [Google Scholar]
Pigott 2010
- Pigott HE, Leventhal AM, Alter GS, Boren JJ. Efficacy and effectiveness of antidepressants: current status of research. Psychotherapy and Psychosomatics 2010;79(5):267-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pigott 2015
- Pigott HE. The STAR*D trial: it is time to reexamine the clinical beliefs that guide the treatment of major depression. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 2015;60(1):9-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilgrim 1999
- Pilgrim D, Bental RP. The medicalisation of misery: a critical realist analysis of the concept of depression. Journal of Mental Health 1999;8(3):26-7. [Google Scholar]
Pottie 2018
- Pottie K, Thompson W, Davies S, Grenier J, Sadowski CA, Welch V, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Canadian Family Physician 2018;64(5):339–51. [PMC free article] [PubMed] [Google Scholar]
Pratt 2011
- Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. NCHS Data Brief 2011;76:1-8. [PMID: ] [PubMed] [Google Scholar]
Pratt 2018
- Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011–2014. www.cdc.gov/nchs/data/databriefs/db283.pdf (accessed 8 April 2019).
Pringle 2011
- Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Progress in Neuro-psychopharmacology & Biological Psychiatry 2011;35(7):1586-92. [DOI] [PubMed] [Google Scholar]
RCP 2019
- Royal College of Psychiatrists 2019. Position statement on antidepressants and depression. https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/position-statements/ps04_19---antidepressants-and-depression.pdf?sfvrsn=ddea9473_5 (accessed 25 Septembre 2020).
Recalt 2019
- Recalt AM, Cohen D. Withdrawal confounding in randomized controlled trials of antipsychotic, antidepressant, and stimulant drugs, 2000-2017. Psychotherapy and Psychosomatics 2019;88(2):105-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reeve 2017
- Reeve E, Thompson W, Farrell B. Deprescribing: a narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. European Journal of Internal Medicine 2017;38:3-11. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RIZIV 2014
- RIZIV. Infospot. www.riziv.fgov.be/SiteCollectionDocuments/infospot-2014-02-nl.pdf (accessed 8 April 2019).
Rodgers 2012
- Rodgers M, Asaria M, Walker S, McMillan D, Lucock M, Harden M, et al. The clinical effectiveness and cost-effectiveness of low-intensity psychological interventions for the secondary prevention of relapse after depression: a systematic review. Health Technology Assessment 2012;16(28):1-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbaum 1988
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biological Psychiatry 1988;44:77-87. [DOI] [PubMed] [Google Scholar]
Ruhe 2019
- Ruhe HG, Horikx A, Avendonk MJP, Woutersen-Koch H. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry 2019;6:561-2. [DOI] [PubMed] [Google Scholar]
Rush 2000
- Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): clinician (IDS‐C) and self‐report (IDS‐SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research 2000;9(2):45-9. [Google Scholar]
Scholten 2018
- Scholten WD, Batelaan NM, Oppen P, Smit JH, Hoogendoorn AW, Megen HJGM, et al. The efficacy of a group CBT relapse prevention program for remitted anxiety disorder patients who discontinue antidepressant medication: a randomized controlled trial.. Psychotherapy and Psychosomatics 2018;87(4):240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholten 2020
- Scholten W, Batelaan N, Van Balkom A. Barriers to discontinuing antidepressants in patients with depressive and anxiety disorders: a review of the literature and clinical recommendations. Therapeutic Advances in Psychopharmacology. 2020;10:2045125320933404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segal 2002
- Segal ZV, Williams JM, Teasdale JD. Mindfulness‐based cognitive therapy for depression: a new approach to preventing relapse. New York (NY): Guilford, 2002. [Google Scholar]
Solomon 1997
- Solomon DA, Keller MB, Leon AC, Mueller TI, Shea MT, Warshaw M, et al. Recovery from major depression: a 10-year prospective follow-up across multiple episodes. Archives of General Psychiatry 1997;54(11):1001–6. [DOI] [PubMed] [Google Scholar]
Spitzer 1999
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282(18):1737-44. [DOI] [PubMed] [Google Scholar]
Spitzer 2006
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine 2006;166(10):1092-7. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK Royal College of Psychiatrists 2020
- UK Royal College of Psychiatrists. Stopping antidepressants. https://www.rcpsych.ac.uk/docs/default-source/mental-health/treatments-and-wellbeing/print-outs/stopping-antidepressant-printable.pdf?sfvrsn=2c9a63e0_2 (accessed 22 November 2020).
Ulfarson 2003
- Ulfvarson J, Adami J, Wredling R, Kjellman B, Reilly M, Bahr CV. Controlled withdrawal of selective serotonin inhibitor drugs in elderly patients in nursing homes with no indication of depression. European Journal Clinical Pharmacology 2003;59:735–40. [DOI] [PubMed] [Google Scholar]
Valuck 2009
- Valuck RJ, Orton HD, Libby AM. Antidepressant discontinuation and risk of suicide attempt: a retrospective, nested case-control study. Journal of Clinical Psychiatry 2009;70(8):1069-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Geffen 2005
- Geffen EC, Hugtenburg JG, Heerdink ER, Hulten RP, Egberts AC. Discontinuation symptoms in users of selective serotonin reuptake inhibitors in clinical practice: tapering versus abrupt discontinuation. European Journal of Clinical Pharmacology 2005;61(4):303-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Leeuwen 2019 [pers comm]
- Van Leeuwen E. Study results. Email to: J. Streim 17 June 2019.
Van Leeuwen 2020a [pers comm]
- Van Leeuwen E. Study results. Email to: W. Kuyken 14 September 2020.
Van Leeuwen 2020b [pers comm]
- Van Leeuwen E. Results RCT. Email to: P Lucassen 23 June 2020.
Van Leeuwen 2020c [pers comm]
- Van Leeuwen E. Study results. Email to: M Huijbers 7 September 2020.
Voshaar 2006
- Voshaar R, Couvée J, Van Balkom A, Mulder P, Zitman F. Strategies for discontinuing long-term benzodiazepine use: meta-analysis. British Journal of Psychiatry 2006;189(3):213-20. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30:473-83. [PubMed] [Google Scholar]
Warren 2020
- Warren JB. The trouble with antidepressants: why the evidence overplays benefits and underplays risks - an essay by John B Warren. British Medical Journal 2020;370:m3200. [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization (WHO). Fact Sheets Depression. www.who.int/news-room/fact-sheets/detail/depression (accessed 8 April 2019).
Wilkinson 2016
- Wilkinson P, Izmeth Z. Continuation and maintenance treatments for depression in older people. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD006727. [DOI: 10.1002/14651858.CD006727.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2010
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology 2010;24(11):1577-601. [DOI] [PubMed] [Google Scholar]
Wilson 2015
- Wilson E, Lader M. A review of the management of antidepressant discontinuation symptoms. Therapeutic Advances in Psychopharmacology 2015;5(6):357-68. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2016
- Wong J, Motulsky A, Eguale T, Buckeridge DL, Abrahamowicz M, Tamblyn R. Treatment indications for antidepressants prescribed in primary care in Quebec, Canada, 2006-2015. JAMA 2016;315(20):2230-2. [DOI] [PubMed] [Google Scholar]
Wong 2017
- Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. British Medical Journal 2017;356:j603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolley 2009
- Woolley SB, Cardoni AA, Goethe JW. Last-observation-carried-forward imputation method in clinical efficacy trials: review of 352 antidepressant studies. Pharmacotherapy 2009;29(12):1408-16. [PMID: ] [DOI] [PubMed] [Google Scholar]


