Abstract
Background
Venous thromboembolism (VTE), although rare, is a major cause of maternal mortality and morbidity. Some women are at increased risk of VTE during pregnancy and the early postnatal period (e.g. caesarean section, family history of VTE, or thrombophilia), and so prophylaxis may be considered. As some methods of prophylaxis carry risks of adverse effects, and risk of VTE is often low, benefits of thromboprophylaxis may be outweighed by harms.
Objectives
To assess the effects of thromboprophylaxis during pregnancy and the early postnatal period on the risk of venous thromboembolic disease and adverse effects in women at increased risk of VTE.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (18 October 2019). In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (18 October 2019).
Selection criteria
Randomised trials comparing one method of thromboprophylaxis with placebo or no treatment, or two (or more) methods of thromboprophylaxis.
Data collection and analysis
At least two review authors assessed trial eligibility, extracted data, assessed risk of bias, and judged certainty of evidence for selected critical outcomes (using GRADE). We conducted fixed‐effect meta‐analysis and reported data (all dichotomous) as summary risk ratios (RRs) with 95% confidence intervals (CIs).
Main results
Twenty‐nine trials (involving 3839 women), overall at moderate to high risk of bias were included. Trials were conducted across the antenatal, peripartum and postnatal periods, with most in high‐income countries. Interventions included types and regimens of heparin (low molecular weight heparin (LMWH) and unfractionated heparin (UFH)), hydroxyethyl starch (HES), and compression stockings or devices. Data were limited due to a small number of trials in comparisons and/or few or no events reported. All critical outcomes (assessed for comparisons of heparin versus no treatment/placebo, and LMWH versus UFH) were considered to have very low‐certainty evidence, downgraded mainly for study limitations and imprecise effect estimates. Maternal death was not reported in most studies.
Antenatal (± postnatal) prophylaxis
For the primary outcomes symptomatic thromboembolic events pulmonary embolism (PE) and/or deep vein thrombosis (DVT), and the critical outcome of adverse effects sufficient to stop treatment, the evidence was very uncertain.
Symptomatic thromboembolic events:
‐ heparin versus no treatment/placebo (RR 0.39; 95% CI 0.08 to 1.98; 4 trials, 476 women; very low‐certainty evidence);
‐ LMWH versus UFH (RR 0.47; 95% CI 0.09 to 2.49; 4 trials, 404 women; very low‐certainty evidence);
Symptomatic PE:
‐ heparin versus no treatment/placebo (RR 0.33; 95% CI 0.02 to 7.14; 3 trials, 187 women; very low‐certainty evidence);
‐ LMWH versus UFH (no events; 3 trials, 287 women);
Symptomatic DVT:
‐ heparin versus no treatment/placebo (RR 0.33; 95% CI 0.04 to 3.10; 4 trials, 227 women; very low‐certainty evidence);
‐ LMWH versus UFH (no events; 3 trials, 287 women);
Adverse effects sufficient to stop treatment:
‐ heparin versus no treatment/placebo (RR 0.49; 95% CI 0.05 to 5.31; 1 trial, 139 women; very low‐certainty evidence);
‐ LMWH versus UFH (RR 0.07; 95% CI 0.01 to 0.54; 2 trials, 226 women; very low‐certainty evidence).
Peripartum/postnatal prophylaxis
Vaginal or caesarean birth
When UFH and no treatment were compared, the effects on symptomatic thromboembolic events (RR 0.16; 95% CI 0.02 to 1.36; 1 trial, 210 women; very low‐certainty evidence), symptomatic PE (RR 0.16; 95% CI 0.01 to 3.34; 1 trial, 210 women; very low‐certainty evidence), and symptomatic DVT (RR 0.27; 95% CI 0.03 to 2.55; 1 trial, 210 women; very low‐certainty evidence) were very uncertain. Maternal death and adverse effects sufficient to stop treatment were not reported.
Caesarean birth
Symptomatic thromboembolic events:
‐ heparin versus no treatment/placebo (RR 1.30; 95% CI 0.39 to 4.27; 4 trials, 840 women; very low‐certainty evidence);
‐ LMWH versus UFH (RR 0.33; 95% CI 0.01 to 7.99; 3 trials, 217 women; very low‐certainty evidence);
Symptomatic PE:
‐ heparin versus no treatment/placebo (RR 1.10; 95% CI 0.25 to 4.87; 4 trials, 840 women; very low‐certainty evidence);
‐ LMWH versus UFH (no events; 3 trials, 217 women);
Symptomatic DVT:
‐ heparin versus no treatment/placebo (RR 1.30; 95% CI 0.24 to 6.94; 5 trials, 1140 women; very low‐certainty evidence); LMWH versus UFH (RR 0.33; 95% CI 0.01 to 7.99; 3 trials, 217 women; very low‐certainty evidence);
Maternal death:
‐ heparin versus placebo (no events, 1 trial, 300 women);
Adverse effects sufficient to stop treatment:
‐ heparin versus placebo (no events; 1 trial, 140 women).
Postnatal prophylaxis
No events were reported for LMWH versus no treatment/placebo for: symptomatic thromboembolic events, symptomatic PE and symptomatic DVT (all 2 trials, 58 women), or maternal death (1 trial, 24 women). Adverse effects sufficient to stop treatment were not reported.
We were unable to conduct subgroup analyses due to lack of data.
Sensitivity analysis including the nine studies at low risk of bias did not impact overall findings.
Authors' conclusions
The evidence is very uncertain about benefits and harms of VTE thromboprophylaxis in women during pregnancy and the early postnatal period at increased risk of VTE. Further high‐quality very large‐scale randomised trials are needed to determine effects of currently used treatments in women with different VTE risk factors. As sufficiently large definitive trials are unlikely to be funded, secondary data analyses based on high‐quality registry data are important.
Keywords: Female; Humans; Pregnancy; Anticoagulants; Anticoagulants/therapeutic use; Bias; Cesarean Section; Heparin; Heparin/therapeutic use; Heparin, Low-Molecular-Weight; Heparin, Low-Molecular-Weight/therapeutic use; Pregnancy Complications, Hematologic; Pregnancy Complications, Hematologic/prevention & control; Puerperal Disorders; Puerperal Disorders/prevention & control; Randomized Controlled Trials as Topic; Venous Thrombosis; Venous Thrombosis/prevention & control
Plain language summary
Preventing venous thromboembolism in women during pregnancy, childbirth and after birth
We set out to determine from randomised controlled trials the benefits and harms of treatments during pregnancy, childbirth, and after birth to prevent deep vein clots in women who are at increased risk.
What is the issue?
A blood clot can form in a deep vein, usually in the legs. This is known as deep vein thrombosis (DVT). If part of the clot breaks off it can be carried in the blood to the lungs and block blood vessels there. This is called a pulmonary embolism (PE), and can cause death, although this is rare. Together these are known as venous thromboembolism (VTE) disease. A women's clotting system is more active during pregnancy to protect her from excessive bleeding during birth. Some women are at a higher risk of VTE during pregnancy and around the time of childbirth including women with previous VTE, thrombophilia (a condition which makes people more likely to develop clots) and following a caesarean birth.
Why is this important?
Women at increased risk of VTE during pregnancy and in the six weeks following childbirth are commonly given treatments to prevent blood clots. Treatments vary due to lack of clear guidelines. The treatments to prevent VTE include heparin type drugs, aspirin and the wearing of compression stockings to improve blood flow in the legs. Some of the treatments can potentially harm women, for example, by increasing blood loss after childbirth or interfering with wound healing.
What evidence did we find?
This is an update of a Cochrane Review published in 2014. We searched for new evidence in October 2019. Twenty‐nine randomised controlled studies, involving 3839 women, are now included. The studies were published from 1975 to 2016 and were mainly carried out in high‐income countries. They included women at increased risk of VTE who were pregnant, in childbirth, and after the birth. Treatments assessed included different types and doses of heparin (of low molecular weight heparin and unfractionated heparin), and compression stockings or devices. No deaths occurred. The reported findings were supported by very low‐certainty evidence.
Starting treatment during pregnancy (with or without treatment after childbirth): we looked at the occurrence of symptomatic VTE and adverse effects that caused women to stop treatment. Any benefits of heparin were unclear when compared with no treatment or a placebo (assessed in up to four studies with 476 women). Similarly, for different types of heparin (assessed in up to four studies with 404 women), different doses of low molecular weight heparin (in one study with 144 women), and for compression stockings compared with no stockings (in one study with 44 women).
For treatment during and following vaginal or caesarean birth: we are very uncertain about the effects of heparin when compared with no treatment on the occurrence of symptomatic VTE (assessed in one study with 210 women). This study did not report on adverse effects that led women to stop treatment.
For treatment during and following caesarean birth: we are very uncertain about the effects of heparin compared with no treatment or a placebo (assessed in up to five studies with 1140 women). The studies looked at different types or doses of heparin, and compression devices compared with bed rest (in one study of 49 women). No adverse effects stopping treatment were reported.
Looking at treatment following vaginal or caesarean birth: no symptomatic VTEs were reported in women receiving either heparin or no treatment or placebo in two studies (58 women). No study reported on adverse effects leading to women stopping treatment.
What does this mean?
We are very uncertain if the benefits of treatments used to prevent deep vein clots in high‐risk women during pregnancy and around the time of childbirth outweigh any harms. Small numbers of studies were included in the comparisons with a range of outcomes measured and low numbers of events. Some studies had design limitations and definitions of blood clotting risk factors and outcomes were not always clear. More, large, high‐quality studies are needed.
Summary of findings
Summary of findings 1. Antenatal (± postnatal) prophylaxis: heparin (LMWH or UFH) versus no treatment/placebo.
| Antenatal (± postnatal) prophylaxis: heparin (LMWH or UFH) versus no treatment/placebo for venous thromboembolic disease | ||||||
|
Population: pregnant women at increased risk of VTE during pregnancy and the early postnatal period Settings: UK (2 trials), Australia and Sweden (1 trial), Canada and USA (1 trial), Australia, the Netherlands and Sweden (1 trial) Intervention: heparin (LMWH (4 trials) or UFH (1 trial)) Comparison: no treatment (3 trials) or placebo (2 trials) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment/placebo | Risk with heparin | |||||
| Maternal death | Not reported | |||||
| Symptomatic thromboembolic events (follow‐up: 6 weeks postpartum |
Study population | RR 0.39 (0.08 to 1.98) | 476 (4 trials) |
⊕⊝⊝⊝ very low 1, 2, 3 | 1 trial reported no events. | |
| 17 per 1000 | 7 per 1000 (1 to 34) | |||||
| Symptomatic PE (follow‐up: 6 weeks postpartum) |
Study population | RR 0.33 (0.02 to 7.14) | 187 (3 trials) |
⊕⊝⊝⊝ very low 1, 2, 3, 4 | 2 trials reported no events. | |
| 11 per 1000 | 4 per 1000 (0 to 77) | |||||
| Symptomatic DVT (follow‐up: 6 weeks postpartum) |
Study population | RR 0.33 (0.04 to 3.10) | 227 (4 trials) |
⊕⊝⊝⊝ very low 1, 3, 4 | 2 trials reported no events. | |
| 18 per 1000 | 6 per 1000 (1 to 55) | |||||
| Adverse effects sufficient to stop treatment | Study population | RR 0.49 (0.05 to 5.31) | 139 (1 trial) |
⊕⊝⊝⊝
very low 1, 3, 4 |
3 events: heparin (LMWH) 1 event (bleeding from placental praevia); no treatment 2 events (both stomach complaints) | |
| 29 per 1000 | 14 per 1000 (1 to 154) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; LMWH: low molecular weight heparin; PE: pulmonary embolism; RR: Risk Ratio; UFH: unfractionated heparin; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Design limitations (‐1): unclear risk of selective reporting bias; not downgraded for lack of blinding as unlikely to have influenced objective outcome
2 Imprecision (‐2): few events and wide confidence intervals crossing the line of no effect
3 Indirectness (‐1): women had specific risk factors for VTE during pregnancy and the postpartum period which varied across the trials, and risk factors also varied across women within the trials, limiting applicability of results to all pregnant women and women in the early postnatal period at increased risk of VTE
4 Imprecision (‐2): few events and small sample size
Summary of findings 2. Antenatal (± postnatal) prophylaxis: LMWH versus UFH.
| Antenatal (± postnatal)prophylaxis: LMWH versus UFH for venous thromboembolic disease | ||||||
|
Population: pregnant women at increased risk of VTE during pregnancy and the early postnatal period Settings: Finland (1 trial), USA (3 trials) Intervention: LMWH Comparison: UFH | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Maternal death | Not reported | |||||
| Symptomatic thromboembolic events (Follow‐up: during or immediately following delivery or 6‐8 weeks postpartum) |
Study population | RR 0.47 (0.09 to 2.49) | 404 (4 trials) |
⊕⊝⊝⊝ very low 1, 2, 3 | 3 trials reported no events. | |
| 20 per 1000 | 9 per 1000 (2 to 50) | |||||
| Symptomatic PE (Follow‐up: during or immediately following delivery or 6‐8 weeks postpartum) |
Study population | NA | 287 (3 trials) |
⊕⊝⊝⊝ very low 1, 4, 5 |
No events | |
| NA | NA | |||||
| Symptomatic DVT (Follow‐up: during or immediately following delivery or 6‐8 weeks postpartum) |
Study population | NA | 287 (3 trials) |
⊕⊝⊝⊝ very low 1, 4, 5 |
No events | |
| NA | NA | |||||
| Adverse effects sufficient to stop treatment | Study population | RR 0.07 (0.01 to 0.54) | 226 (2 trials) |
⊕⊝⊝⊝ very low 1,6, 7 | 13 events in UFH group: 1 stopped due to an allergic reaction, 1 due to mild anaemia with no confirmed bleeding and 11 due to excess bruising/allergic rashes (these 11 stopped switched to LMWH (dalteparin) and the adverse effects resolved) | |
| 113 per 1000 | 8 per 1000 (1 to 61) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; LMWH: low molecular weight heparin; NA: not applicable; PE: pulmonary embolism; RR: Risk Ratio; UFH: unfractionated heparin; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Design limitations (‐1): unclear risk of selection, attrition and selective reporting bias; not downgraded for lack of blinding as inadequate blinding unlikely to have influenced objective outcome
2 Imprecision (‐2): few events and wide confidence interval crossing line of no effect
3 Indirectness (‐1): not clear if the events were symptomatic, described as "recurrent thrombosis"; further women had specific risk factors, limiting applicability of results to all pregnant women and women in the early postnatal period at increased risk of VTE
4 Imprecision (‐2): no events and small sample size
5 Indirectness (‐1): women had specific risk factors, limiting applicability of results to all pregnant women and women in the early postnatal period at increased risk of VTE
6 Few events and small sample size
7 Indirectness (‐1): risk factors for VTE poorly described in 1 of the trials (with most weight in the meta‐analysis)
Summary of findings 3. Peripartum/postnatal prophylaxis: UFH versus no treatment.
| Peripartum/postnatal prophylaxis: UFH versus no treatment for venous thromboembolic disease | ||||||
|
Population: women with varicose veins before birth, having a caesarean (elective or emergency) or vaginal birth Settings: Israel (1 RCT) Intervention: UFH Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no UFH | Risk with UFH | |||||
| Maternal death | Not reported | |||||
| Symptomatic thromboembolic events (follow‐up: 6 weeks postpartum) |
Study population | RR 0.16 (0.02 to 1.36) | 210 (1 trial) | ⊕⊝⊝⊝ very low 1, 2, 3 | ||
| 53 per 1000 | 0 per 1000 (1 to 72) |
|||||
| Symptomatic PE (follow‐up: 6 weeks postpartum) |
Study population | RR 0.16 (0.01 to 3.34) | 210 (1 trial) | ⊕⊝⊝⊝ very low 1, 2, 3 | ||
| 21 per 1000 | 0 per 1000 (0 to 71) |
|||||
| Symptomatic DVT (follow‐up: 6 weeks postpartum) |
Study population | RR 0.27 (0.03 to 2.55) | 210 (1 trial) | ⊕⊝⊝⊝ very low 1, 2, 3 | ||
| 32 per 1000 | 0 per 1000 (1 to 81) | |||||
| Adverse effects sufficient to stop treatment | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; RR: Risk Ratio; UFH: unfractionated heparin; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Design limitations (‐1): unclear risk of all sources of bias other than attrition (low risk); not downgraded for lack of blinding as objective outcome
2 Imprecision (‐2): wide confidence intervals crossing line of no effect, few events, and small sample size
3 Indirectness (‐1): specific risk factors for VTE of included women limits applicability of findings to all women at increased risk of VTE intrapartum and in the early postnatal period
Summary of findings 4. Peripartum/postnatal prophylaxis (caesarean): heparin (LMWH or UFH) versus no treatment/placebo.
| Peripartum/postnatal prophylaxis (caesarean): heparin (LMWH or UFH) versus no treatment/placebo for venous thromboembolic disease | ||||||
|
Population: women giving birth by elective or emergency caesarean (elective only (1 trial), emergency or elective (4 trials)) Settings: Australia (1 trial), Saudi Arabia (1 trial), Switzerland (1 trial), UK (2 trials) Intervention: heparin (LMWH (3 RCTs), UFH (2 RCTs)) Comparison: no treatment (1 trial) or placebo (4 trials) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no heparin | Risk with heparin | |||||
| Maternal death (timing of assessment unclear) |
Study population | NA | 300 (1 trial) |
⊕⊝⊝⊝ very low 1, 2 |
No events | |
| NA | NA | |||||
| Symptomatic thromboembolic events (timing of assessment unclear, within 6 weeks postpartum) |
Study population | RR 1.30 (0.39 to 4.27) | 840 (4 trials) |
⊕⊝⊝⊝ very low 3, 4 | ||
| 9 per 1000 | 0 per 1000 (4 to 29) |
|||||
| Symptomatic PE (timing of assessment unclear, within 6 weeks postpartum) |
Study population | RR 1.10 (0.25, 4.87) | 840 (4 trials) | ⊕⊝⊝⊝ very low 3, 4 | ||
| 7 per 1000 | 0 per 1000 (2 to 33) | |||||
| Symptomatic DVT (timing of assessment unclear, within 6 weeks postpartum) |
Study population | 1.30 (0.24, 6.94) | 1140 (5 trials) | ⊕⊝⊝⊝ very low 3, 4 | ||
| 3 per 1000 | 0 per 1000 (1 to 22) | |||||
| Adverse effects sufficient to stop treatment | Study population | NA | 140 (1 trial) |
⊕⊝⊝⊝ very low 2, 5 |
No events | |
| NA | NA | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RCT: randomised controlled trial; RR: Risk Ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Design limitations (‐2) one trial at unclear risk of all sources of bias
2 Imprecision (‐2): no events and small sample size
3 Design limitations (‐2): most trials at unclear risk of selection bias, all trials at unclear risk of selective reporting; not downgraded for lack of blinding as objective outcome
4 Imprecision (‐1): wide confidence intervals crossing line of no effect
5 Design limitations (‐1): unclear risk of selective reporting bias
Summary of findings 5. Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH.
| Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH for venous thromboembolic disease | ||||||
|
Population: women giving birth by elective or emergency caesarean (elective or emergency (1 trial), elective cesarean only (1 trial), elective/emergency unclear (1 trial)) Settings: German (2 trials); UK (1 trial) Intervention: LMWH Comparison: UFH | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Maternal death | Not reported | |||||
| Symptomatic thromboembolic events (timing of assessment unclear, within 6 weeks postpartum) |
Study population | RR 0.33 (0.01 to 7.99) | 217 (3 trials) | ⊕⊝⊝⊝ very low1, 2 | All the events were symptomatic DVT | |
| 9 per 1000 | 0 per 1000 2 to 75) | |||||
| Symptomatic PE (timing of assessment unclear, within 6 weeks postpartum) |
Study population | NA | 217 (3 trials) | ⊕⊝⊝⊝ very low 1, 3 |
No events | |
| NA | NA | |||||
| Symptomatic DVT (timing of assessment unclear, within 6 weeks postpartum) |
Study population | RR 0.33 (0.01 to 7.99) | 217 (3 trials) | ⊕⊝⊝⊝ very low 1, 2 | ||
| 9 per 1000 | 0 per 1000 2 to 75) | |||||
| Adverse effects sufficient to stop treatment | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; LMWH: low molecular weight heparin; NA: not applicable; PE: pulmonary embolism; RR: Risk Ratio; UFH: unfractionated heparin. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Design limitations (‐2): all trials at unclear risk of selection bias, selective reporting and other bias; not downgraded for lack of blinding as objective outcome
2 Imprecision (‐2): wide confidence intervals crossing line of no effect, few events and small sample size
3 Imprecision (‐2): no events and small sample size
Summary of findings 6. Postnatal prophylaxis: LMWH versus no treatment/placebo.
| Postnatal prophylaxis: LMWH versus no treatment/placebofor venous thromboembolic disease | ||||||
|
Population: women at increased risk of VTE in the early postpartum period Settings: multi‐country, Canada and USA (2 trials) Intervention: LMWH Comparison: no treatment (1 trial) or placebo (1 trial) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with no heparin treatment or placebo | Assumed risk with LMWH | |||||
| Maternal death | Study population | NA | 24 (1 trial) | ⊕⊝⊝⊝ very low 1, 2 | No events Maternal death only reported as: “no… other unexpected serious adverse events related to the intervention during follow‐up” |
|
| NA | NA | |||||
| Symptomatic thromboembolic events (follow‐up: 10‐90 days postpartum) |
Study population | NA | 58 (2 trials) | ⊕⊝⊝⊝ very low 2, 3 | No events | |
| NA | NA | |||||
| Symptomatic PE (follow‐up: 10‐90 days postpartum) |
Study population | NA | 58 (2 trials) | ⊕⊝⊝⊝ very low 2, 3 | No events | |
| NA | NA | |||||
| Symptomatic DVT (follow‐up: 10‐90 days postpartum) |
Study population | NA | 58 (2 trials) |
⊕⊝⊝⊝ very low 2, 3 | No events | |
| NA | NA | |||||
| Adverse effects sufficient to stop treatment | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: Risk Ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Design limitations (‐1): unclear risk of selective reporting and other bias
2 Imprecision (‐2): no events and small sample size
3 Design limitations (‐1): unclear risk of selective reporting and other bias; not downgraded for lack of blinding as objective outcome
Background
Description of the condition
Venous thromboembolic disease in pregnancy and the early postnatal period
Venous thromboembolism (VTE) is a condition where the blood clots inappropriately, and which may lead to considerable morbidity and even death. The term VTE encompasses a continuum, including both deep vein thrombosis (DVT) (the formation of clots in the deep veins of the body ‐ predominately in the legs), and pulmonary embolism (PE) (which occurs when a clot in a deep vein breaks free and is carried to the arteries of the lungs) (Di Nisio 2017; Goldhaber 2012). Two of the most common initial symptoms of DVT are pain and swelling in an extremity (such as the lower leg), while symptoms and signs of PE include dyspnoea (shortness of breath), tachypnoea (rapid breathing), chest pain and haemoptysis (coughing up blood). Severe cases of PE can include signs of cyanosis (blue discolouration, particularly of the lips and fingers), and may result in collapse and sudden death (ACOG 2018Di Nisio 2017; Abbasi 2014 ; Greer 2012; Knight 2008 ; Jacobsen 2008; James 2006). Approximately 75% to 80% of cases of pregnancy‐associated VTE are caused by DVT, and 20% to 25% of cases are caused by PE (Blanco‐Molina 2010; James 2006; Simpson 2001).
Pregnancy is associated with a number of physiological and anatomic changes that can increase the risk of VTE (ACOG 2018; Antony 2017).
Description of the intervention
Thromboprophylaxis
The intervention assessed in this review covers VTE thromboprophylaxis (measures taken in order to prevent thrombosis), including pharmacological agents and non pharmacological methods. Despite evidence correlating risk factors and the occurrence of pregnancy‐related VTE being imprecise (ACOG 2018; Okoroh 2012), there is broad agreement that women should be assessed for VTE risk preconception, and again during pregnancy, in order to guide VTE thromboprophylaxis (Friedman 2016; NHMRC 2009).
Globally, guidelines (for example of the American College of Obstetricians and Gynecologists (ACOG) (ACOG 2018), the American College of Chest Physicians (ACCP) (Bates 2012), National Institute for Health and Care Excellence (NICE 2018) and the Royal College of Obstetricians and Gynaecologists (RCOG) (RCOG 2015)), are unanimous in their recommendations that all women undergo a documented assessment of risk factors for VTE in early pregnancy or prepregnancy, and women judged to be at high risk of VTE be offered thromboprophylaxis, where benefit is likely to outweigh potential harms. However, the available guidelines do not reach consensus regarding groups of women at higher risk of VTE, and which type of thromboprophylaxis should be offered. They also differ in the treatment options advised for particular groups of women at increased risk of VTE, including timing of interventions (starting points and lengths of treatments). Pregnancy‐specific guidelines for thromboprophylaxis in COVID‐19 have been published, though are lacking high‐certainty evidence to inform clinical practice (D'Souza 2020).
ACCP and ACOG guidelines advise antenatal and postpartum pharmacologic prophylaxis for a small group of particularly high‐risk women ‐ those women with prior events, and/or thrombophilias. For women undergoing caesarean birth, ACOG supports universal perioperative mechanical prophylaxis and ACCP recommends pharmacologic prophylaxis based on risk factors. In comparison, RCOG recommends pharmacologic prophylaxis to a much larger proportion of women based on common risk factors (ACOG 2018 ; Friedman 2016).
Pharmacological agents that have been used to prevent thrombosis around pregnancy include:
unfractionated heparin (UFH) or low molecular weight heparin (LMWH);
aspirin, a platelet aggregation inhibitor;
hydroxyethyl starch (HES), a nonionic starch derivative;
fondaparinux, a selective inhibitor of activated Factor X;
danaparoid, a heparinoid.
Non‐pharmacological methods used include:
graduated compression stockings;
intermittent pneumatic compression;
early mobilisation;
surveillance.
How the intervention might work
Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period
Thrombin has a key role in haemostasis and thrombosis, and thus anticoagulant strategies focus on either inhibiting thrombin or its generation. UFH, LMWH and coumarin derivatives (such as warfarin) prevent the generation of thrombin through a variety of mechanisms (Ansell 2004). Heparins (such as UFH and LMWH) exert their anticoagulant activity by activating antithrombin, which subsequently inhibits thrombin (and Factor Xa). Coumarin derivatives (such as warfarin) however, produce their anticoagulant effect by interfering with the cyclic conversion of vitamin K (which is required as a co‐factor for the 'carboxylation' of vitamin K‐dependent proteins, which include a number of coagulation factors); by blocking this process, the coagulation factors that are produced have no/little biological activity. Selective inhibitors of activated Factor Xa (such as fondaparinux), exert their antithrombotic activity by neutralisation of Factor Xa, which interrupts the blood coagulation cascade, inhibiting thrombin formation and thrombus development (Ansell 2004).
Non‐pharmacological methods, such as graduated compression stockings or intermittent pneumatic compression may work through their ability to reduce venous stasis and blood stagnation by promoting venous blood flow through external compression (NHMRC 2009).
Despite previously established benefits of thromboprophylaxis for VTE in non‐pregnant patient groups, evidence on the effects, and cost‐effectiveness of thromboprophylaxis is scant and unclear (Ellis‐Kahana 2020; Friedman 2016; Palmerola 2015). There is ongoing debate about whether potential benefits outweigh potential harms in some groups of high‐risk women (ACOG 2018), and whether they should be routinely screened for treatment. Routine screening of all pregnant women to identify women with thrombophilia, for example, has not been recommended (Okoroh 2012), and antenatal prophylaxis for all women with known thrombophilia remains controversial (Brenner 2003; de Jong 2014; Friedman 2016; Middeldorp 2003; Okoroh 2012; Wu 2005).
Pharmacological prophylaxis may cause adverse effects that could be sufficiently severe to outweigh the benefits of thromboprophylaxis. Heparin does not cross the placenta and is believed to be safe for the fetus, and therefore, has generally been used for antenatal therapy. However, it can result in adverse effects for the mother (Nelson‐Piercy 1997); there is a risk of thrombocytopenia (low numbers of platelets), bleeding and allergic reactions and symptomatic osteoporosis (loss of bone density, leading to fractures) in the longer term. When used after caesarean section, heparin may increase the frequency of bleeding and wound complications. Originally, UFH was used, but this now appears to have been largely superseded (at least for use in pregnancy and postnatally) by LMWH. The advantages of LMWH over UFH include a longer half‐life (allowing once‐ or twice‐daily subcutaneous dosing), high bioavailability, and predictable anticoagulant response; avoiding the need for dose adjustment, or laboratory monitoring for most women. In addition, LMWHs are understood to have a lower risk of adverse effects such as osteoporosis, and thrombocytopenia (Bauersachs 2009). Warfarin is known to cause congenital anomalies (Hall 1980) and has, therefore, rarely been used in the first trimester or in the last few weeks of pregnancy (Bauersachs 2009). Both heparin and warfarin have been used for postnatal thromboprophylaxis, as they are regarded to be safe for mothers who are breastfeeding (Bauersachs 2009; Letsky 1997; Orme 1977).
Low‐dose (e.g. 60 mg to 150 mg) aspirin has been widely used in pregnancy in an attempt to prevent the development of pre‐eclampsia (Roberge 2017; Rolnik 2017; ). Aspirin is usually well‐tolerated and has few adverse effects, and its use for thromboprophylaxis in orthopaedic surgery (PEP Trial 2000) suggests that it may have a role to play in the prevention of VTE in pregnancy (Bauersachs 2009). HES has been used for thromboprophylaxis in the past however, it is not commonly prescribed due to concerns about its association with increased risk of anaphylaxis (Paull 1987).
Why it is important to do this review
This review updates a previously published Cochrane Review on interventions for the prophylaxis of VTE in pregnancy and the early postnatal period (Bain 2014), which was an update of an earlier version (Tooher 2010). Both previous versions of this review concluded that there was insufficient evidence on which to base recommendations for thromboprophylaxis during pregnancy and the early postnatal period, and that large scale randomised controlled trials of currently used interventions should be conducted.
Thromboembolic disease, although rare, is a major cause of maternal mortality and morbidity; hence methods of prophylaxis are often used for women at risk. Many methods of prophylaxis carry a risk of adverse effects, and as the risk of VTE is low, it is possible that any benefits of thromboprophylaxis may be outweighed by harm. Current guidelines for clinical practice are based largely on expert opinion, rather than high‐certainty evidence from randomised trials, and guidelines differ in the thromboprophylaxis measures they recommend for women who are pregnant, in birth, or have recently given birth.
The current version of the Cochrane Review on prophylaxis for VTE in pregnancy and the early postnatal period (Bain 2014) searched up to 27 November 2013 and included 19 randomised controlled trials; with 16 trials involving 2529 women contributing data to the review. Additional trials on prophylaxis for VTE have since been performed, which provide new data on the effects of relevant interventions.
Objectives
To assess the effects of thromboprophylaxis during pregnancy and the early postnatal period in women at increased risk of venous thromboembolism (VTE) on the risk of venous thromboembolic disease and adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing any intervention that may prevent venous thromboembolism (VTE) versus placebo or no treatment, or two or more interventions for the prevention of VTE. We excluded quasi‐randomised trials and cross‐over trials, however planned to include cluster‐randomised trials. We included studies reported only as abstracts where it was possible to extract relevant data from the text. When this was not possible, we included these as awaiting assessment studies, pending further publication of their results.
Types of participants
Women who were pregnant or had given birth in the previous six weeks, at increased risk of VTE, were included. Women at increased risk were those having/following a caesarean section, with an acquired or inherited thrombophilia, and/or other risk factors for VTE. Women with artificial heart valves were excluded.
This is one of a series of Cochrane Reviews assessing the effects of interventions to prevent VTE in women at increased risk of VTE. Thromboprophylaxis has been widely used to prevent miscarriage in women with recurrent pregnancy loss. One Cochrane Review examines effects of aspirin and/or heparin for women with unexplained recurrent miscarriage, with or without thrombophilia (de Jong 2014). Another evaluates the effects of aspirin or heparin, or both, for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent miscarriage (Hamulyák 2020). A further Cochrane Review assesses the effects of antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction (Dodd 2013). To avoid duplication, we therefore have not included studies focused on assessing the effects of aspirin and/or heparin or both on the prevention of miscarriage, or the effects of antithrombotic therapy in women considered at risk of placental dysfunction (not otherwise considered to be at increased risk of VTE).
Types of interventions
Any thromboprophylaxis measure (i.e. intervention that may reduce risk of VTE) was eligible, including the following.
1. Pharmacological interventions:
unfractionated heparin (UFH);
low molecular weight heparin (LMWH);
aspirin;
warfarin;
hydroxyethyl starch (HES);
other.
2. Non‐pharmacological interventions:
graduated compression stockings;
intermitted pneumatic compression (intermittent compression of the calves during surgery);
early mobilisation;
surveillance (screening for asymptomatic thromboembolic events to prevent symptomatic deep venous thrombosis (DVT) or pulmonary embolism (PE).
Types of outcome measures
Outcomes were all dichotomous and were measured at the end of the intervention or follow‐up period, as reported by the individual studies.
Primary outcomes
1. Maternal death. 2. Symptomatic thromboembolic events. 3. Symptomatic PE. 4. Symptomatic DVT.
Secondary outcomes
5. Asymptomatic thromboembolic events (detected by screening). 6. Blood transfusion. 7. Bleeding episodes. 8. Serious wound complications (wound infection requiring antibiotics, dehiscence, resuturing). 9. Adverse effects sufficient to stop treatment (undesired harmful effect resulting from the intervention considered serious enough to stop treatment, all author reports). 10. Adverse effects not sufficient to stop treatment (undesired harmful effect resulting from the intervention that was not considered serious enough to stop treatment, all author reports). 11. Symptomatic osteoporosis*. 12. Fetal loss < 20 weeks**. 13. Fetal loss ≥ 20 weeks**. 14. Thrombocytopenia*. 15. Fetal anomalies**.
* Mostly applicable for studies involving use of antenatal heparin.
** Mostly applicable for studies involving use of antenatal heparin or aspirin.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (18 October 2019). We updated this search on 17 February 2021 and added the results to Studies awaiting classification.
The Register is a database containing over 26,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (this includes a Cochrane centralised search feed from WHO International Clinical Trials Registry Platform (ICTRP) ;
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies, Excluded studies, Studies awaiting classification, Ongoing studies).
Searching other resources
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (18 October 2019). We updated the search of ClinicalTrials.gov on 17 February 2021 and added the results to Studies awaiting classification (see Appendix 1 for search methods used).
Data collection and analysis
For methods used in the previous version of this review, see Bain 2014.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search plus the ongoing, awaiting assessment and relevant excluded studies in the previous version of this review.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
At least two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion and where necessary, by involving a third review author.
Data extraction and management
We designed a form to extract data (based on the data extraction template of the Cochrane Pregnancy and Childbirth Group). For eligible studies, two review authors extracted the data using the agreed form. We resolved any discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study, the methods, if any, used to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at a low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study and for each outcome or class of outcomes,the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or was supplied by the trial authors, we included missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. where there was no missing data or where reasons for missing data were balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias (checking for reporting bias)
We described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference, if outcomes for which data were combined from trials in meta‐analysis, were measured in the same way by the included trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. We would have adjusted their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intra cluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we had used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We would have acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We considered cross‐over designs inappropriate for this research question.
Multiple‐armed trials
We combined relevant groups in the multi‐arm trials or included relevant arms as separate comparisons, to create appropriate single pair‐wise comparisons for inclusion in the review analyses, thereby avoiding unit of analysis errors.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
In future updates of this review, if we include any studies where women were recruited preconception, for outcomes relating to pregnancy, we plan to take a pragmatic approach and include in the denominators only those women known to have become pregnant.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial where an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in meta‐analyses we plan to investigate reporting biases (such as publication bias) using funnel plots. We plan to assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data, as it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Had there been clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We would have treated the random‐effects summary as the average range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not considered clinically meaningful, we would not have combined trials.
If we had used random‐effects analyses, we would have presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
We summarised results for 13 intervention comparisons under the following four main headings, based on the intervention time points antenatal ± postnatal, intrapartum + postnatal, postnatal, and the distinction between type of delivery in the intrapartum and postnatal interventions assessed:
antenatal (± postnatal) prophylaxis;
peripartum prophylaxis (vaginal birth or caesarean);
peripartum/postpartum prophylaxis (caesarean);
postnatal prophylaxis.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses based on:
risk factors for VTE (i.e. previous VTE versus family history of VTE versus inherited or acquired thrombophilia versus emergency or elective caesarean section, with or without other risk factors versus other risk factors).
We planned to restrict subgroup analyses to the primary review outcomes. We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value. However, we were unable to conduct subgroup analyses in this update due to lack of data. We will include these analyses in future versions of the review if the necessary data become available. There was no heterogeneity observed.
Sensitivity analysis
We carried out sensitivity analyses to explore the effects of trial quality by omitting trials rated 'high' or 'unclear' risk of selection bias (allocation concealment and sequence generation) or attrition bias, restricting these analyses to the primary outcomes. We were able to conduct these analyses for three of the13 review comparisons, and three of the five primary review outcomes (symptomatic thromboembolic events, symptomatic PE, and symptomatic DVT), using data from nine trials.
Summary of findings and assessment of the certainty of the evidence
For this update we assessed the certainty of the evidence where possible (data allowed) using the GRADE approach as outlined in the GRADE handbook, including the main comparisons (all comparisons of heparin (LMWH or UFH) versus no treatment/placebo, and LMHW versus UFH) and the following five outcomes, selected due to their importance (potential to change practise):
maternal death;
symptomatic thromboembolic events;
symptomatic PE;
symptomatic DVT;
adverse effects sufficient to stop treatment.
The GRADEpro Guideline Development Tool was used to import data from Review Manager 5.4 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
In the previous version of the review (Bain 2014), 19 trials (32 reports) were included, 23 studies were excluded, eight studies (13 reports) were assessed as ongoing, and two reports were classified awaiting further classification. Updated searches of the Cochrane Pregnancy and Childbirth’s Trials Register on 18 October 2019 identified 39 new records; additional searching found five new records. For this update we assessed these new records, plus the two awaiting classification study records and 13 ongoing study records from Bain 2014.
We included 10 new trials (Algahtani 2015; de Vries 2012; Heller 2016; Reddick 2014; Rodger 2014; Rodger 2015; Rodger 2016; Salim 2016; Stephenson 2016; van Hoorn 2016), and excluded 10 studies (Aina 2006; Alalaf 2015; de Jong 2015; Guven 2014; Langer 2013; Laskin 2007; Milic 2018; Rodger 2017; Samantha 2013; Schleussner 2015). Seven studies are listed as ongoing (Dargaud 2018; Heller 2016b; NCT00225108; NCT00878826; NCT01019655; NCT01828697; NCT04153760); and two studies (Dittmer 1991; Nagornaya 2012) remain awaiting classification. See Figure 1.
1.
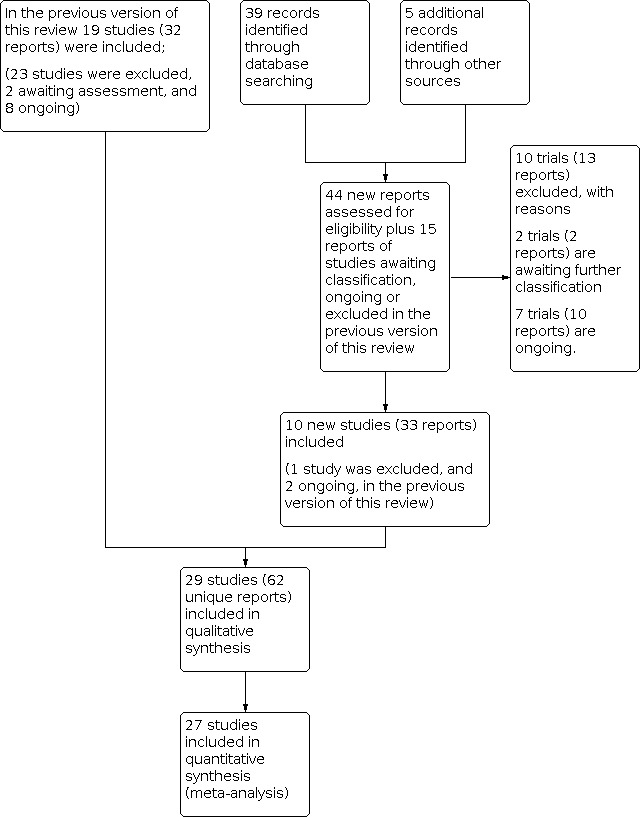
Study flow diagram
We updated the search in February 2021 and identified 10 trial reports. Two of these are additional reports of an ongoing study (NCT01828697), one is an additional report of Gris 2011, and six trials (seven reports) are awaiting further classification (Abdolvand 2019; Ganer 2020; Movahedi 2020; NCT02856295; NCT04305756; NCT04635839.
In summary, the current review update includes: a total of 29 studies (66 reports or 62 unique reports (Gates 2004a and Gates 2004b have three references in common and Rodger 2015; Rodger 2016 have one reference in common)); 32 excluded studies (38 reports); seven ongoing studies (13 reports); and eight studies (nine reports) in awaiting classification.
Included studies
A total of 29 trials (involving 3839 women) (Algahtani 2015; Burrows 2001; Casele 2006; Cornette 2002; Cruz 2011; De Veciana 2001; de Vries 2012; Ellison 2001; Gates 2004a; Gates 2004b; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Hill 1988; Howell 1983; Krauss 1994; O'Riordan 2008; Pettila 1999; Reddick 2014; Rodger 2014; Rodger 2015; Rodger 2016; Salim 2016; Segal 1975; Stephenson 2016; van Hoorn 2016; Welti 1981) are included in this review update. Two of the trials (Cornette 2002; O'Riordan 2008) reported no relevant outcome data, and thus 27 studies contribute to quantitative analyses. All of the included studies were individually‐randomised trials. Three of the trials each had three arms (Ellison 2001; Gibson 1998; Heilmann 2007).
de Vries 2012 and van Hoorn 2016 are both part of the 'FRUIT RCT' ‐ with van Hoorn 2016 recruiting women with antiphospholipid antibodies.
Of the 27 trials contributing data for the review analysis, 11 assessed antenatal (± postnatal)prophylaxis, with five assessing heparin (low molecular weight heparin (LMWH) or unfractionated heparin (UFH)) versus no treatment or placebo (de Vries 2012; Gates 2004a; Howell 1983; Rodger 2014; van Hoorn 2016); four assessing LMWH versus UFH (Casele 2006; De Veciana 2001; Hamersley 1998; Pettila 1999), one assessing adjusted‐dose versus fixed‐dose LMWH (Salim 2016), and one assessing compression stockings versus none (Heller 2016). Fourteen trials assessed peripartum/postnatalprophylaxis, with one trial assessing UFH versus no treatment, in women having a vaginal or caesarean birth (Segal 1975); and the remaining 13 trials assessing interventions in women having a caesarean birth; including five assessing heparin (LMWH or UFH) versus no treatment or placebo (Algahtani 2015; Burrows 2001; Gates 2004b; Hill 1988; Welti 1981), one assessing hydroxyethyl starch (HES) versus UFH (Heilmann 1991), three assessing LMWH versus UFH (Gibson 1998; Heilmann 2007; Krauss 1994), one assessing five‐ versus 10‐day LMWH (Cruz 2011), one assessing weight‐based versus fixed‐dose LMWH (Stephenson 2016), one assessing LMWH versus LMWH (different types) (Ellison 2001), and one assessing compression devices versus bed rest (Reddick 2014). The remaining two trials (Rodger 2015 and Rodger 2016), assessed postnatal prophylaxis, comparing LMWH with no treatment or placebo. Therefore, 13 comparisons of thromboprophylaxis in pregnancy and the early postnatal period (first six weeks after birth), were included in this review.
The included trials have been published over four decades ‐ from 1975 to 2016.
For further details, see Characteristics of included studies and Table 7.
1. Interventions and studies in each comparison.
| Antenatal (± postnatal)prophylaxis (n = 11 RCTs) |
|
Comparison 1 ‐ Heparin (LMWH or UFH) versus no treatment or placebo de Vries 2012; Gates 2004a; Howell 1983; Rodger 2014; van Hoorn 2016 |
|
Comparison 2 ‐ LMWH versus UFH Casele 2006; De Veciana 2001; Hamersley 1998; Pettila 1999 |
|
Comparison 3 ‐ Adjusted‐ versus fixed‐dose LMWH Salim 2016 |
|
Comparison 4 ‐ Compression stockings versus none Heller 2016 |
| Peripartum/postnatalprophylaxis (n = 14 RCTs) |
| Vaginal birth or caesarean |
|
Comparison 5 ‐ UFH versus no heparin treatment Segal 1975 |
| Caesarean |
|
Comparison 6 ‐ Heparin (LMWH or UFH) versus no treatment or placebo Algahtani 2015; Burrows 2001; Gates 2004b; Hill 1988; Welti 1981 |
|
Comparison 7 ‐ HES versus UFH Heilmann 1991 |
|
Comparison 8 ‐ LMWH versus UFH Gibson 1998*; Heilmann 2007*; Krauss 1994 |
|
Comparison 9 ‐ 5‐day versus 10‐day LMWH Cruz 2011 |
|
Comparison 10 ‐ Weight‐based versus fixed‐dose LMWH Stephenson 2016 |
|
Comparison 11 ‐ LMWH versus LMWH (different types) Ellison 2001* |
|
Comparison 12 ‐ Compression devices versus bed rest Reddick 2014 |
| Postnatal prophylaxis (n = 2 RCTs) |
| Comparison 13 ‐ LMWH versus no treatment or placebo Rodger 2015; Rodger 2016 |
*Trial had three arms. Ellison 2001 assessed three different types of heparin, and we included all three groups in the review analysis as pair‐wise comparisons; Gibson 1998 included two separate LMWH groups and an UFH group, and for this trial we combined the two LMWH groups in the review meta‐analysis. Heilmann 2007 included two randomised treatment groups, and a non‐randomised control (no treatment group), so for this trial we included only the two treatment groups.
Two additional studies are included in the review (Cornette 2002; O'Riordan 2008), both of which assessed intrapartum (+ postnatal) prophylaxis (pharmacologic), although are not included in the review comparisons as they contributed no outcomes for analysis.
Abbreviations: RCT: randomised controlled trial; LMWH: low molecular weight heparin; UF: unfractionated heparin
Settings
Almost all of the 29 trials were conducted in high‐income countries. Six were conducted in the UK (Ellison 2001; Gates 2004a; Gates 2004b; Gibson 1998; Hill 1988; Howell 1983), and six in the USA (Casele 2006; De Veciana 2001; Hamersley 1998; Heller 2016; Reddick 2014; Stephenson 2004). Of the remaining 17 trials, three were conducted in Germany (Heilmann 1991; Heilmann 2007; Krauss 1994), two in Israel (Salim 2016; Segal 1975), and one each in Australia (Burrows 2001), Saudi Arabia (Algahtani 2015), Spain (Cruz 2011), Ireland (O'Riordan 2008), Finland (Pettila 1999), and Switzerland (Welti 1981). Five trials were performed in more than one country: de Vries 2012 (Australia, Sweden, the Netherlands); Rodger 2014 (Australia, Canada, France, UK, USA); van Hoorn 2016 (Australia, Sweden); and Rodger 2015 and Rodger 2016 (both Canada and USA). The study setting for Cornette 2002 was unclear (though the authors reported that they were from Belgium).
Participants
All participants in the trials were women who were pregnant, giving birth or who had given birth in the previous six weeks and were judged to be at increased risk of venous thromboembolism (VTE). The number of women randomised varied widely across the trials, however most trials were relatively small. Gates 2004a and Gibson 1998 were the two smallest trials, randomising 16 and 17 women, respectively. The two trials evaluating postnatal prophylaxis randomised only 25 women (Rodger 2015) and 37 women (Rodger 2016). Cruz 2011, which randomised 646 women, and Welti 1981, which randomised 580 women, were the largest trials.
Characteristics of the included women are summarised below, including age, personal history of VTE, thrombophilia, pre‐eclampsia, obesity, and caesarean type (elective versus emergency, relevant for the trials assessing prophylaxis in women scheduled for, undergoing, or after caesarean birth). For further details see Table 8.
2. Participant characteristics.
| Study |
Personal history of VTE (all/none/mixed NR) |
Known acquired or inherited thrombophilia (all/none/mixed/NR) |
Obesity (BMI ≥ 30 kg/m2) (all/none/mixed/NR) |
Advanced age (e.g. ≥ 35 years) (all/none/mixed/NR) |
Pre‐eclampsia (all/none/mixed/NR) |
If post caesarean prophylaxis (emergency/elective/mixed/NR) |
| Algahtani 2015 | None | None | None | None | None | Mixed |
| Burrows 2001 | None | NR (though "Need of therapy with an anticoagulant" was listed as an exclusion) | Mixed | Mixed | Mixed | Mixed |
| Casele 2006 | NR (only reports that "Any patient who was a candidate for low‐dose thromboprophylaxis" were included) | NR (see left) | NR | Mixed | NR | NA |
| Cornette 2002* | NR | None (women with known coagulation or bleeding disorders were excluded) | NR | NR | None (based on discussion, and apparent exclusion of women with pre‐eclampsia) | Elective |
| Cruz 2011 | NR (only reports that "women... who had not required prophylaxis or treatment with any type of LMWH during pregnancy (low risk of VTE during pregnancy)" were included) | NR (see left) | Mixed | Mixed | Mixed (based on baseline characteristics reported ‐ only reported pregnancy‐induced hypertension) | Mixed |
| De Veciana 2001 | Mixed | Mixed | Mixed | NR | NR | NA |
| de Vries 2012 | None | All | Mixed | Mixed | None | NA |
| Ellison 2001 | NR | NR | Mixed | Mixed | Mixed | Mixed |
| Gates 2004a | All | Mixed | NR/mixed (only reports booking weight ≥ 80 kg) | Mixed | NR | NA |
| Gates 2004b | None | Mixed | NR/mixed (only reports booking weight ≥ 80 kg) | Mixed | Mixed | Mixed |
| Gibson 1998 | NR (women "undergoing caesarean section were recruited to the study if this procedure was performed as an emergency... or if they had 1 or more of the other defined risk factors for thromboembolic disease"; "Additional risk factors include advanced maternal age, high parity, obesity, pre‐eclampsia and prolonged bed rest before delivery"; however, no baseline characteristics detailed). | NR | NR (see left) | NR (see left) | NR (see left) | Mixed |
| Hamersley 1998 | NR | All | NR | NR | NR | NA |
| Heilmann 1991 | Mixed (1 woman in HES group) | NR | Mixed | Mixed | NR | NR |
| Heilmann 2007 | NR | All | NR/mixed/none (1 woman in LMWH group had BMI > 26 kg/m2) | Mixed | NR | Elective |
| Heller 2016 | NR | NR | Mixed | Mixed | NR | NA |
| Hill 1988 | None | None (women with coagulation disorders were excluded) | NR | NR | NR/none (women with pregnancy‐induced hypertension were excluded) | Elective |
| Howell 1983 | All | NR | NR | Mixed | NR | NA |
| Krauss 1994 | NR | NR | NR | Mixed | NR | NR |
| O'Riordan 2008* | NR | NR | NR | NR | NR | NR |
| Pettila 1999 | Mixed | Mixed | Mixed | Mixed | NR/none? (reported as an outcome) | NA |
| Reddick 2014 | None | None | Mixed | Mixed | None | Elective |
| Rodger 2014 | Mixed | All | Mixed | Mixed | NR/none? (reported as an outcome) | NA |
| Rodger 2015 | NR | Mixed | Mixed | NR | Mixed | Mixed (vaginal births also included) |
| Rodger 2016 | NR | Mixed | Mixed | NR | Mixed | Mixed |
| Salim 2016 | Mixed | All | Mixed | Mixed | None | NA |
| Segal 1975 | Mixed | NR | NR | NR | NR | Mixed (vaginal births also included) |
| Stephenson 2016 | None | NR | All | Mixed | Mixed | Mixed |
| van Hoorn 2016 | None | All | Mixed | Mixed | Mixed | NA |
| Welti 1981 | NR | NR | NR | Mixed | NR | Mixed |
Abbreviations: BMI: body mass index;HES: hydroxyethyl starch; LMWH: low molecular weight heparin; NR: not reported; NA: not applicable; VTE: venous thromboembolism
*No outcomes included in the review analyses.
Age
Nineteen of the 29 trials (Burrows 2001; Casele 2006; Cruz 2011; de Vries 2012; Ellison 2001; Gates 2004a; Gates 2004b; Heilmann 1991; Heilmann 2007; Heller 2016; Howell 1983; Krauss 1994; Pettila 1999; Reddick 2014; Rodger 2014; Salim 2016; Stephenson 2016; van Hoorn 2016; Welti 1981), included a mix of women of advanced age (≥ 35 years) and younger women. In one trial (Algahtani 2015), no women older than 35 years were included. In the remaining nine trials, due to limited information provided, age ranges of women were unclear (Cornette 2002; De Veciana 2001; Hamersley 1998; Hamersley 1998; Hill 1988; O'Riordan 2008; Rodger 2015; Rodger 2016; Segal 1975).
Personal history of venous thromboembolism (VTE
In six of the included trials, a mix of women with and without a personal history of VTE were included (De Veciana 2001; Heilmann 1991; Pettila 1999; Rodger 2014; Salim 2016; Segal 1975). In eight trials none of the women had a personal history of VTE (Algahtani 2015; Burrows 2001; de Vries 2012; Gates 2004b; Hill 1988; Reddick 2014; Stephenson 2016; van Hoorn 2016), and in two trials all women had a personal history of VTE (Gates 2004a; Howell 1983). In the remaining 13 trials, personal history of VTE was not reported (Casele 2006; Cornette 2002; Cruz 2011; Ellison 2001; Gibson 1998; Hamersley 1998; Heilmann 2007; Heller 2016; Krauss 1994; O'Riordan 2008; Rodger 2015; Rodger 2016; Welti 1981).
Thrombophilia (acquired or inherited)
In six trials all the women included had acquired or inherited thrombophilia (de Vries 2012; Hamersley 1998; Heilmann 2007; Rodger 2014; Salim 2016; van Hoorn 2016). In four trials none of the included women had thrombophilia (Algahtani 2015; Cornette 2002; Hill 1988; Reddick 2014). Six trials included women with and without thrombophilia (De Veciana 2001; Gates 2004a; Gates 2004b; Pettila 1999; Rodger 2014; Rodger 2015). However, participant thrombophilia status was poorly reported. Authors of 10 trials provided insufficient information to determine thrombophilia status (Ellison 2001; Gibson 1998; Heilmann 1991; Heller 2016; Howell 1983; Krauss 1994; O'Riordan 2008; Segal 1975; Stephenson 2016; Welti 1981). In the remaining three trials, whilst this was not clearly reported, information provided suggests that two (Burrows 2001; Cruz 2011) included no women with thrombophilia, and one (Casele 2006) included some women with thrombophilia.
Pre‐eclampsia
Pre‐eclampsia status of women in the included trials was also poorly reported. In five of the 29 trials, authors reported that no women had pre‐eclampsia (Algahtani 2015; Cornette 2002; de Vries 2012; Reddick 2014; Salim 2016). In two trials (Pettila 1999; Rodger 2014) pre‐eclampsia was reported as a study outcome (thus it may be assumed that no women had pre‐eclampsia at baseline). In the Hill 1988 trial, it is likely that none of the women had pre‐eclampsia (although this is not stated), as women with pregnancy‐induced hypertension were not eligible. In eight trials it is clear that women with and without pre‐eclampsia were included (Burrows 2001; Cruz 2011; Ellison 2001; Gates 2004b; Rodger 2015; Rodger 2016; Stephenson 2016; van Hoorn 2016). In the remaining 13 trials the pre‐eclampsia status of women was unclear due to insufficient information (Casele 2006; De Veciana 2001; Gates 2004a; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Howell 1983; Krauss 1994; O'Riordan 2008; Segal 1975; Welti 1981).
Obesity
One trial (Algahtani 2015) included no obese women and one trial (Stephenson 2016) included only obese women. In 14 trials a mix of obese women, and women of other weight categories were included (Burrows 2001; Cruz 2011; De Veciana 2001; de Vries 2012; Ellison 2001; Heilmann 1991; Heller 2016; Pettila 1999; Reddick 2014; Rodger 2014; Rodger 2015; Rodger 2016; Salim 2016; van Hoorn 2016). In the remaining 13 trials (Casele 2006; Cornette 2002; Gates 2004a; Gates 2004b; Gibson 1998; Hamersley 1998; Heilmann 2007; Hill 1988; Howell 1983; Krauss 1994; O'Riordan 2008; Segal 1975;Welti 1981), the weight status of women was unclear.
Emergency versus elective caesarean
Seventeen of the 29 trials, assessed thromboprophylaxis in women having a caesarean (Algahtani 2015; Burrows 2001; Cornette 2002; Cruz 2011; Ellison 2001; Gates 2004b; Heilmann 1991; Heilmann 2007; Hill 1988; Krauss 1994; O'Riordan 2008; Reddick 2014; Rodger 2015; Rodger 2016; Segal 1975; Stephenson 2016; Welti 1981). Four trials included women undergoing elective caesarean birth only (Cornette 2002; Heilmann 2007; Hill 1988; Reddick 2014). In one trial (Heilmann 1991), the status of included caesarean section births (emergency/elective) was unclear. In the remaining 12 trials, women scheduled for, undergoing, or who had an emergency or elective caesarean surgery were included.
Interventions
Eleven of the 29 trials assessed antenatal (± postnatal) thromboprophylaxis, with 10 assessing pharmacological interventions (Casele 2006; De Veciana 2001; de Vries 2012; Gates 2004a; Hamersley 1998; Howell 1983; Pettila 1999; Rodger 2014; Salim 2016; van Hoorn 2016), and one assessing a mechanical intervention (compression stockings) (Heller 2016). Sixteen trials assessed peripartum/postnatal thromboprophylaxis (Algahtani 2015; Burrows 2001; Cornette 2002; Cruz 2011; Ellison 2001; Gates 2004b; Gibson 1998; Heilmann 1991; Heilmann 2007; Hill 1988; Krauss 1994; O'Riordan 2008; Reddick 2014; Salim 2016; Stephenson 2016; Welti 1981). All except one (Salim 2016), were in women having a caesarean birth, and all but one (Reddick 2014), assessed pharmacological interventions. The remaining two trials assessed postnatal thromboprophylaxis (Rodger 2015; Rodger 2016), and pharmacological interventions (following vaginal or caesarean birth).
The interventions assessed by each trial are briefly described below.
Antenatal (± postnatal) prophylaxis
Heparin (LMWH or UFH) versus no treatment or placebo (Comparison 1).
de Vries 2012 assessed once‐daily weight‐adjusted LMWH (dalteparin (fragmin) 5000 international units (IU) subcutaneously starting between six and 12 weeks' gestation up to the onset of labour; combined with 80 mg oral aspirin daily), starting before 12 weeks' gestation, and continued until 36 weeks' gestation (Australian participants received 100 mg aspirin; Swedish participants received 75 mg aspirin), compared with antenatal aspirin daily (80 mg orally, from 12 to 26 weeks' gestation). All women received weight‐adjusted LMWH (dalteparin) in the early postpartum period.
Gates 2004a assessed once‐daily subcutaneous 40 mg enoxaparin (LMWH) from antenatal recruitment until a maximum of six weeks after birth, versus once‐daily subcutaneous placebo (same timing).
Howell 1983 assessed subcutaneous antenatal UFH throughout pregnancy (calcium, 10,000 IU twice daily, commencing shortly after the first antenatal visit) and UFH for six weeks postpartum (8000 IU twice daily) compared with no antenatal heparin and the same postpartum UFH treatment.
Rodger 2014 assessed antepartum LMWH (dalteparin 5000 IU once daily by subcutaneous self‐injection from the day of randomisation until 20 weeks' gestation followed by 5000 IU twice daily from 20 weeks until at least 37 weeks' gestation), compared with placebo. All participants received postpartum dalteparin (5000 IU daily) by subcutaneous self‐injection starting six to 28 hours after birth until day 42.
van Hoorn 2016 assessed the same intervention as the de Vries 2012 trial in a different population.
LMWH versus UFH (Comparison 2)
Casele 2006 assessed self‐administered enoxaparin sodium (30 mg twice daily, starting from before 24 weeks up to 28 weeks' gestation, then 40 mg twice daily until birth), versus heparin sodium (7500 units twice daily, starting before 24 weeks until 28 weeks, then 10,000 units twice daily until birth). All women received adjusted‐dose coumadin for six to eight weeks after birth.
De Veciana 2001 assessed dalteparin (initial dosing 2500 IU (5000 IU if > 70 kg) subcutaneously once daily then increased to a maximum of 10,000 IU/day to maintain alpha‐Factor Xa levels at 0.1 to 0.3 IU/mL), compared with UFH (women were dosed with the standard 5000 IU (8000 IU if > 68 kg) subcutaneously twice daily) (intervention start time not reported).
Hamersley 1998 dose‐adjusted heparin (adjusted to maintain an anti‐Xa (heparin assay) level between 0.03 to 0.05 U/mL) versus UFH, with women in both groups also prescribed daily aspirin (81 mg) (intervention not further specified in the conference abstract) (intervention start time and duration not reported).
Pettila 1999 assessed subcutaneous antenatal plus postnatal dalteparin (Fragmin, once daily starting at 20 weeks' gestation, with a starting dose of 5000 IU (women weighing < 85 kg) or 7500 IU (women weighing ≥ 85 kg), dose adjusted based on anti‐Xa measurements; during birth, 2500 IU dalteparin was administered 18 hours after the previous dose if the woman had not yet given birth; if she gave birth within 18 hours, 5000 IU was given 24 hours after the previous injection; the daily dose postpartum, for six weeks, was 2500 IU lower than during the third trimester; and two weeks after birth, if anti‐Xa was < 0.20, the dose was increased by 2500 IU), versus subcutaneous UFH (7500 IU, adjusted according to the activated partial thromboplastin time (APTT) target values, twice daily starting at 20 weeks' gestation and for six weeks postpartum; at the time of birth, and on the first day postpartum 7500 IU UFH was given at 12‐hour intervals, and then according to APTT target values).
Adjusted‐dose versus fixed‐dose LMWH (Comparison 3)
Salim 2016 assessed antepartum enoxaparin according to the results of anti‐FXa levels (initial dose of 40 mg, increased by fractions of 20 mg according to anti‐FXa level with targeted prophylactic level 0.2 IU/mL or more 3.5 to 4 hours post‐injection) versus a fixed antenatal daily dose of enoxaparin (40 mg daily, by subcutaneous self‐injection) regardless of the results of anti‐factor Xa, until birth. All women were prescribed enoxaparin (40 mg once daily by subcutaneous injection) from day one until day 42 after birth.
Compression stockings versus none (Comparison 4)
Heller 2016 assessed advice to wear 20 to 30 mm Hg maternity pantyhose versus no such advice (intervention timing unclear, although authors report that women were visited three times, between eight to 20 weeks, and 32 ± 4 weeks before the birth, plus at eight weeks postpartum±2 weeks).
Peripartum/postnatal prophylaxis
Vaginal birth or caesarean
UFH versus no treatment (Comparison 5)
Segal 1975 assessed subcutaneous UFH (50 mg (5000 IU) every 12 hours for four to five days after birth, about two‐thirds of women having a vaginal birth had the first dose in active labour and a third after birth; for women having a caesarean section UFH was given two hours before surgery, at the end of surgery, and at 12‐hour intervals; for women having an emergency caesarean, the initial dose was immediately following the decision), versus standard care/no treatment.
Caesarean
Heparin (LMWH or UFH) versus no treatment or placebo (Comparison 6)
Algahtani 2015 assessed tinzaparin (4500 IU subcutaneously once daily), starting from 12 to 24 hours after caesarean section continuing for two weeks, compared with placebo. All women in this trial also received non‐pharmacological prophylaxis using graduated compression stockings.
Burrows 2001 assessed dalteparin (2500 IU once daily) versus placebo (saline) once daily for four to five days, with the interventions starting four to 24 hours after caesarean section.
Gates 2004b assessed once‐daily self‐injected subcutaneous 40 mg enoxaparin in 1 mL versus once‐daily self‐injected subcutaneous placebo (normal saline 1 mL). Treatment with the study drug began within 12 hours of the caesarean section, and its duration was determined by the attending clinician.
Hill 1988 assessed UFH 1000 units, one hour before caesarean, then twice daily for five days versus placebo (saline) one hour before caesarean, then twice daily for five days.
Welti 1981 assessed twice‐daily subcutaneous 5000 IU heparin (UFH) with physiotherapy versus physiotherapy without heparin (intervention timing unclear).
HES versus UFH (Comparison 7)
Heilmann 1991 assessed HES 6%, 3 x 500 mL (first 500 mL administered during the caesarean, second in the evening of the day of the operation, third in the evening of the first postoperative day), versus UFH 5000 IU (first dose two hours after the operation followed by every eight hours for seven days).
LMWH versus UFH (Comparison 8)
Gibson 1998** assessed enoxaparin 20 mg once daily versus enoxaparin 40 mg once daily versus UFH 7500 IU every 12 hours, with each of the interventions starting after the caesarean section (duration of intervention unclear).
Heilmann 2007** assessed dalteparin 5000 IU/daily for seven days post operatively, with the first dose six hours following caesarean section and then at 24‐hourly intervals versus calciparin 2 x 5000 IU daily, with the first dose six hours following caesarean section, (and then at eight‐hour intervals) versus no pharmacological prophylaxis but compression stockings according to the guidelines during hospital days (third group not randomly assigned and excluded in this review).
Krauss 1994 assessed fragmin (2500 to 5000 anti‐Xa units) once daily versus 5000 units UFH (Liquemin) + 500 mL dextran 60 administered two to three times daily with treatment continuing 10 days after surgery (when the intervention commenced is unclear).
Five‐day versus 10‐day LMWH (Comparison 9)
Cruz 2011 assessed bemiparin (3500 IU once daily) starting at ≥ eight hours following caesarean for five days versus 10 days.
Weight‐based versus fixed‐dose LMWH (Comparison 10)
Stephenson 2016 assessed 0.5 mg/kg enoxaparin every 12 hours (dose not capped and rounded to the nearest 5 mg unit) versus 40 mg enoxaparin daily, with the enoxaparin starting between eight and 12 hours after caesarean birth and given as a subcutaneous injection in the abdomen.
LMWH versus LMWH (different types) (Comparison 11)
Ellison 2001** evaluated dalteparin (5000 IU) versus enoxaparin (4000 IU) versus tinzaparin (50 IU/kg based on booking weight), all administered once daily, starting from six hours following caesarean section, and continuing for five days.
(O'Riordan 2008)* daily enoxaparin (40 mg) versus daily tinzaparin (4500 units) following caesarean section.
Compression devices versus bed rest (Comparison 12)
Reddick 2014: assessed intermittent pneumatic compression during caesarean birth (Aircast Venaflow Calf Cuff—DJO, LLC, Vista, CA, placed on their lower extremities, starting one hour before surgery and continuing ≥ 30 minutes following), versus bed rest beginning one hour before the start of surgery.
Timing of LMWH
Cornette 2002* evaluated LMWH (0.3 mL nadroparin calcium) 12 hours before versus 12 hours after elective caesarean birth.
Postnatal prophylaxis
LMWH versus no treatment or placebo (Comparison 13)
Rodger 2015 assessed daily prophylactic dalteparin (5000 IU subcutaneous injections starting approximately 36 hours following delivery of the placenta, continued for three weeks), versus placebo.
Rodger 2016 assessed the same intervention as Rodger 2015, however the dalteparin was administered for longer, following delivery of the placenta (for 10 days), and LMWH was compared with no treatment.
*Trial contributed no data for analysis.
**Trial included more than two arms.
For further details see Characteristics of included studies.
Multi‐arm trials
We combined relevant groups in the multi‐arm trials or included relevant arms as separate comparisons, to create appropriate single pair‐wise comparisons for inclusion in the review analyses, thereby avoiding unit of analysis errors; specifically:
Ellison 2001: three arms assessing different types of intrapartum (+ postnatal) LMWH treatment (dalteparin, enoxaparin, tinzaparin) in women undergoing caesarean: we included all three arms in three pair‐wise comparisons as dalteparin versus enoxaparin, dalteparin versus tinzaparin, and enoxaparin versus tinzaparin (Analysis 11).
Gibson 1998: three arms of intrapartum (+ postnatal) caesarean prophylaxis (LMWH enoxaparin (20 mg daily) versus LMWH enoxaparin (40 mg daily) versus UFH (7500 IU every 12 hours)): we combined the two enoxaparin groups and included this trial in Comparison 8 as LMWH versus UFH.
Heilmann 2007: three arms; two randomised (LMWH dalteparin (5000 IU/daily for seven days post operatively) and UFH (calciparin 2 x 5000 IU daily)), and one not randomised, a control group (a control group received no pharmacological prophylaxis but compression stockings according to guidelines). We included the two randomised groups only, as a pair‐wise comparison of intrapartum (+ postnatal) prophylaxis LMWH versus UFH in Analysis 8.
Outcomes
Primary outcomes: maternal death was reported by three trials (Algahtani 2015; Cruz 2011; Rodger 2015) in three separate comparisons. Symptomatic thromboembolic events were reported by 23 trials in 11 of the 13 review comparisons, with between one and four trials in each. Symptomatic PE was reported by 20 trials in 11 comparisons with between one and four trials in each. Symptomatic DVT was reported by 23 trials in 12 comparisons, with between one and five trials in each.
Each of our secondary outcomes was reported by at least one/some of the trials, although most in very few comparisons with only one or two trials in each.
Sources of trial funding
Thirteen of the 29 included trials reported funding sources: Pharmacia and Upjohn (provided the dalteparin and saline placebo medication, no other intellectual or financial support) (Burrows 2001); Laboratorios Fcos. ROVI, SA (Cruz 2011); two‐year investigator grant by Pfizer (de Vries 2012); National Health Service Executive South East Region Research and Development and a donation to the National Perinatal Epidemiology Unit by Rhone‐Poulenc Rorer (Gates 2004a); donation by Rhone‐Poulenc Rorer (Gates 2004b); Leo Laboratories and the South East Thames Regional Health Authority (grant LORS No 77/19) (Hill 1988); Helsinki University Central Hospital, and Pharmacia and Upjohn (Pettila 1999); Hammond Research Fund, Duke University School of Medicine (Reddick 2014); Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada, drugs supplied by Pharmacia and Upjohn (Rodger 2014); National Institutes of Health Research grant (# NIH 1R34HL107725–01) and Canadian Institutes of Health Research grant (#MOP 106641), Dr. Rodger supported by a Heart and Stroke Foundation Career Investigator Award and a University of Ottawa, Faculty of Medicine Chair in Venous Thrombosis and Thrombophilia, Dr. Kahn supported by a National Research Scholar award from the Fonds de recherche santé Québec (Rodger 2015 and Rodger 2016); Long Beach Memorial Medical Center Foundation (Stephenson 2016); single two‐year investigator grant period 2000–2001 by Pfizer, formerly Pharmacia, grant number 524E‐CVD‐9101‐0001 (Pharmacia was not the sponsor of the study), and a follow‐up study of the trial received a one‐year investigator grant period in 2014 by Pfizer (van Hoorn 2016). In four of these trials (Gates 2004a; Gates 2004b; Pettila 1999; van Hoorn 2016) industry was a source of trial support.
The remaining 16 trials did not report sources of trial funding.
Trial authors’ declarations of interest
In 21 of the included trials authors did not report declarations of interest (Algahtani 2015; Burrows 2001; Casele 2006; Cornette 2002; Cruz 2011; De Veciana 2001; Ellison 2001; Gates 2004a; Gates 2004b; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Hill 1988; Howell 1983; Krauss 1994; O'Riordan 2008; Pettila 1999; Rodger 2016; Segal 1975; Welti 1981). Two trials reported potential conflicts of interest: Heller 2016 declared relevant interests on the part of one author, J. Heller: Consultant/advisory board for BMS/Pfizer; Rodger 2014 declared relevant interests for one author, A.M. Clement: honoraria for educational activities from Leo Pharma, Sanofi, and Bayer. The authors of these studies reported no other relevant potential conflict of interest. Trial authors of the remaining six included trials declared no conflicts of interest (de Vries 2012; Reddick 2014; Rodger 2015; Salim 2016; Stephenson 2016; van Hoorn 2016).
Excluded studies
We excluded 32 studies. Eight were excluded as they were not randomised controlled trials (Alalaf 2015; Blomback 1998; Kutteh 1996a; Kutteh 1996b; Noble 2005; Pyregov 2012; Ratiu 2009; Samantha 2013) (some of these studies were also not eligible based on their study population). Aina 2006 was excluded as it was a protocol reporting a terminated trial.
Eighteen studies were excluded as their primary focus was prevention of recurrent miscarriage (Badawy 2008; Brenner 2005; Dendrinos 2007; de Jong 2015; Farquharson 2002; Giancotti 2012; Guven 2014; Kamin 2008; Kaandorp 2010; Langer 2013; Laskin 2007; Rai 1997; Rodger 2017;Schleussner 2015; Stephenson 2004; Thaler 2004; Tulppala 1997; Visser 2011). Two trials were excluded as they assessed the secondary prevention of placental vascular complications in women with severe pre‐eclampsia or placental abruption and specifically excluded women at increased risk of VTE (Gris 2010; Gris 2011). Three trials were excluded as they did not include pregnant women at increased risk of VTE (Harenberg 1993; Milic 2018; Rey 2009).
For further details, see Characteristics of excluded studies.
Risk of bias in included studies
For most of the trials a number of the 'Risk of bias' items were judged 'unclear' due to insufficient methodological detail. Overall, the trials were judged at moderate to high risk of bias. See Figure 2 and Figure 3.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of the randomisation sequence was considered adequate in 12 trials (Casele 2006; de Vries 2012; Gates 2004b; Gates 2004a; Pettila 1999; Reddick 2014; Rodger 2014; Rodger 2015; Rodger 2016; Salim 2016; Stephenson 2016; van Hoorn 2016) and unclear in 17 trials (Algahtani 2015; Burrows 2001; Cornette 2002; Cruz 2011; De Veciana 2001; Ellison 2001; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Hill 1988; Howell 1983; Krauss 1994; O'Riordan 2008; Segal 1975; Welti 1981). Adequate methods of sequence generation included use of a random number table (Casele 2006; Rodger 2015; Rodger 2016); use of a central telephone randomisation service (Gates 2004b; Gates 2004a); and use of a computer‐generated list (Pettila 1999).
Methods of allocation concealment were judged as adequate in 10 trials, and included use of pre‐prepared treatment packs dispensed by hospital pharmacy departments (Burrows 2001; Gates 2004b; Gates 2004a; Hill 1988; Rodger 2014), randomisation performed by an independent centre or study coordinator with randomisation codes concealed from the investigators (de Vries 2012; Rodger 2015; Rodger 2016; van Hoorn 2016), and sealed opaque envelopes (Pettila 1999). For the remaining 19 studies, the risk of selection bias due to inadequate concealment of allocation was judged to be unclear (Algahtani 2015; Casele 2006; Cornette 2002; Cruz 2011; De Veciana 2001; Ellison 2001; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Howell 1983; Krauss 1994; O'Riordan 2008; Reddick 2014; Salim 2016; Segal 1975; Stephenson 2016; Welti 1981).
Blinding
Twenty‐two of the 29 trials were assessed to be at high risk of performance bias, as they did not report adequate attempts to blind participants and study personnel, and adequate blinding was considered unfeasible due to the type of interventions assessed (Casele 2006; Cornette 2002; Cruz 2011; De Veciana 2001; de Vries 2012; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Howell 1983; Krauss 1994; O'Riordan 2008; Pettila 1999; Reddick 2014; Rodger 2014; Rodger 2016; Salim 2016; Segal 1975; Stephenson 2016; van Hoorn 2016; Welti 1981). Four trials reported adequate methods used to blind women and study personnel, and were judged at low risk of performance bias (Burrows 2001; Gates 2004a; Gates 2004b; Rodger 2015). Performance bias was judged unclear for the remaining three trials as while blinding of women and clinicians may have been feasible, there was insufficient information provided (Algahtani 2015; Ellison 2001; Hill 1988 ).
Few trials reported any information relating to blinding of outcome assessment, and 22 of the trials were assessed unclear risk of detection bias due to inadequate information provided (Algahtani 2015; Casele 2006; Cornette 2002; Cruz 2011; De Veciana 2001; de Vries 2012; Ellison 2001; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Hill 1988; Howell 1983; Krauss 1994; O'Riordan 2008; Pettila 1999; Reddick 2014; Salim 2016; Segal 1975; Stephenson 2016; Welti 1981). One trial (van Hoorn 2016) was judged high risk of detection bias as the authors reported that outcome assessment was not blinded. Six trials were judged at low risk of detection bias as they reported that outcome assessment was blinded (Burrows 2001; Gates 2004a; Gates 2004b; Rodger 2014; Rodger 2015; Rodger 2016).
Therefore, only four of the trials reported adequate blinding of women as well as study personnel and outcome assessors (all with the use of a placebo control), and were judged at low risk of performance and detection bias (Burrows 2001; Gates 2004a; Gates 2004b; Rodger 2015).
Incomplete outcome data
Twenty of the 29 trials were judged at a low risk of attrition bias, with very few or no losses to follow‐up or exclusions post‐randomisation reported (Burrows 2001; Cornette 2002; de Vries 2012; Ellison 2001; Gates 2004b; Gates 2004a; Heilmann 1991; Heilmann 2007; Hill 1988; Krauss 1994; Pettila 1999; Reddick 2014; Rodger 2014; Rodger 2015; Rodger 2016; Salim 2016; Segal 1975; Stephenson 2016; van Hoorn 2016; Welti 1981). While two of these studies appeared to have no losses to follow‐up (Segal 1975; Welti 1981), they both reported very little methodological detail.
The remaining nine trials were assessed at unclear risk of attrition bias (Algahtani 2015; Casele 2006; Cruz 2011; De Veciana 2001; Gibson 1998; Hamersley 1998; Heller 2016; Howell 1983; O'Riordan 2008). Of these, six did not specify whether any losses or exclusions occurred (Algahtani 2015; Cruz 2011; De Veciana 2001; Gibson 1998; Hamersley 1998; O'Riordan 2008). In the Howell 1983 trial, the number of exclusions varied between the tables in the original paper, but it was possible from the text to establish the outcomes for all randomised women. In Casele 2006, 22 of 120 (18%) women were lost to follow‐up; however, data were available for some outcomes. As a result, all women were accounted for in some analyses, but not for the main study outcome (bone mass of the proximal femur), and denominators were not always clear. The Heller 2016 trial was assessed at unclear risk of attrition bias despite the authors reporting that "A total of 44 patients enrolled and completed the study", as this was in a conference abstract, the only report for this study.
Selective reporting
We judged all except one trial (Heilmann 2007) at unclear risk of selective reporting bias. In most cases, the reason for our assessment of unclear was the absence of a trial protocol. However, for Rodger 2014, the reason was multiple trial registrations (including on a website), and Rodger 2016 was assessed as unclear risk due to limited detail provided in the available study protocol. We assessed Rodger 2015 to be at unclear risk of selective reporting due to limited details in the trial registration available, no full protocol available, and results for clinical outcomes reported incompletely in text.
Heilmann 2007, was judged to be at a high risk of reporting bias, as for a number of clinical outcomes, the data were incompletely reported; for example, groups quote: "showed no differences in the blood loss...and thrombocytopenia or osteopenia".
Other potential sources of bias
Thirteen of the trials were judged at a low risk of other potential bias, with no other obvious sources of bias identified (Burrows 2001; Casele 2006; Cornette 2002; de Vries 2012; Ellison 2001; Gates 2004b; Gates 2004a; Pettila 1999; Reddick 2014; Rodger 2014; Rodger 2016; Salim 2016; van Hoorn 2016).
The remaining 16 trials were judged at an unclear risk of other potential bias, largely due to a lack of methodological detail provided in the trial reports (Algahtani 2015; Cruz 2011; De Veciana 2001; Gibson 1998; Hamersley 1998; Heilmann 1991; Heilmann 2007; Heller 2016; Hill 1988; Howell 1983; Krauss 1994; O'Riordan 2008; Rodger 2015; Segal 1975; Stephenson 2016; Welti 1981).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Comparison 1: Antenatal (±postnatal) prophylaxis: heparin (LMWH or UFH) versus no treatment or placebo
Five trials (de Vries 2012; Gates 2004a; Howell 1983; Rodger 2014; van Hoorn 2016) involving 519 women were included. See Table 1.
Primary outcomes
No trial reported on maternal death.
The effects of heparin (LMWH or UFH) versus no treatment or placebo on symptomatic thromboembolic events (risk ratio (RR) 0.39; 95% confidence interval (CI) 0.08 to 1.98; 4 trials, 476 women; Analysis 1.1), symptomatic PE (RR 0.33; 95% CI 0.02 to 7.14; 3 trials, 187 women; Analysis 1.2) and symptomatic DVT (RR 0.33; 95% CI 0.04 to 3.10; 4 trials, 227 women; Analysis 1.3) were very uncertain ‐ all very low‐certainty evidence.
1.1. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 1: Symptomatic thromboembolic events
1.2. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 2: Symptomatic pulmonary embolism
1.3. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
Blood transfusion
No blood transfusions were reported (1 trial, 16 women; Analysis 1.4).
1.4. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 4: Blood transfusion
Bleeding episodes
Bleeding episodes were variably reported across four trials (Analysis 1.5). Effects of LMWH versus no treatment or placebo were very uncertain for placental abruption (RR 1.00; 95% CI 0.31 to 3.20; 3 trials, 463 women), peripartum haemorrhage (RR 0.65; 95% CI 0.24 to 1.79; 1 trial, 289 women); non‐major/minor bleeding (a possible increase with LMWH was observed) (RR 2.12; 95% CI 1.15 to 3.93; 1 trial, 284 women); and major bleeding (RR 1.48; 95% CI 0.25 to 8.72; 1 trial, 284 women). Effects of UFH versus no treatment or placebo were very uncertain for antenatal vaginal bleeding (RR 1.00; 95% CI 0.16 to 6.42; 1 trial, 40 women) and postpartum haemorrhage (RR 3.00; 95% CI 0.13 to 69.52; 1 trial, 40 women).
1.5. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 5: Bleeding episodes
Serious wound complications
There were no serious wound complications (1 trial, 16 women; Analysis 1.6).
1.6. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 6: Serious wound complications
Adverse effects sufficient to stop treatment
The effect of heparin versus no treatment or placebo on adverse effects sufficient to stop treatment was very uncertain (RR 0.49; 95% CI 0.05 to 5.31; 1 trial, 139 women; very low‐certainty evidence; Analysis 1.7). There were 3 events: heparin (LMWH) 1 event (bleeding from placental praevia); no treatment 2 events (both stomach complaints).
1.7. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 7: Adverse effects sufficient to stop treatment
Adverse effects not sufficient to stop treatment
Adverse effects not sufficient to stop treatment were variably reported across four trials. The effects of heparin versus no treatment or placebo were very uncertain for skin/allergic reactions (a possible increase with heparin was observed) (RR 5.11; 95% CI 2.00 to 13.08; 4 trials, 476 women), raised liver enzymes (a possible increase with heparin was observed) (RR 22.53; 95% CI 1.34 to 378.78; 1 trial, 289 women), haematoma (RR 3.98; 95% CI 0.46 to 34.23; 2 trials, 171 women), superficial thrombophlebitis (RR 0.33; 95% CI 0.01 to 7.93; 1 trial, 139 women) and 'other' adverse effects (including transient ischaemic attack, severe allergic reaction) (RR 0.98; 95% CI 0.06 to 15.51; 1 trial, 289 women) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 8: Adverse effects not sufficient to stop treatment
Gates 2004a reported that "Few women reported side effects"; Howell 1983 reported “Minor side‐effects from heparin prophylaxis included bruising at the injection site (reduced by good injection technique), epistaxis and the inconvenience of giving twice‐daily injections"; and de Vries 2012 reported "70 women in the LMWH‐aspirin group had to convert to LMWH prescription".
Symptomatic osteoporosis
The effect of heparin versus no treatment or placebo on thrombocytopenia was very uncertain (RR 3.00; 95% CI 0.13 to 69.52; 4 trials, 479 women; Analysis 1.9).
1.9. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 9: Symptomatic osteoporosis
Fetal loss
The effect of heparin versus no treatment or placebo on fetal loss was very uncertain (Analysis 1.10): where gestation was unclear (RR 1.16; 95% CI 0.54 to 2.51; 2 trials, 329 women), at less than 20 weeks' gestation (RR 2.18; 95% CI 0.50 to 9.41; 2 trials, 171 women), and at least 20 weeks' gestation (RR 0.33; 95% CI 0.04 to 3.12; 2 trials, 166 women).
1.10. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 10: Fetal loss
Thrombocytopenia
The effect of heparin versus no treatment or placebo on thrombocytopenia was very uncertain (RR 3.00; 95% CI 0.14 to 64.26; 5 trials, 511 women; Analysis 1.11).
1.11. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 11: Thrombocytopenia
Fetal anomalies
The effect of heparin versus no treatment or placebo on fetal anomalies was very uncertain (RR 2.94; 95% CI 0.61 to 14.32; 1 trial, 289 women; Analysis 1.12).
1.12. Analysis.

Comparison 1: Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 12: Fetal anomalies
Secondary outcomes not reported
Asymptomatic thromboembolic events.
Comparison 2: Antenatal (±postnatal) prophylaxis: LMWH versus UFH
Four trials (Casele 2006; De Veciana 2001; Hamersley 1998; Pettila 1999) involving 404 women were included. See Table 2.
Primary outcomes
No trial reported on maternal death.
The effect of LMWH versus UFH on symptomatic thromboembolic events was very uncertain (RR 0.47; 95% CI 0.09 to 2.49; 4 trials, 404 women; very low‐certainty evidence; Analysis 2.1). There were no cases of symptomatic PE (3 trials, 287 women; Analysis 2.2), or symptomatic DVT (3 trials, 287 women; Analysis 2.3) in the LWMH or UFH groups.
2.1. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 1: Symptomatic thromboembolic events
2.2. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 2: Symptomatic pulmonary embolism
2.3. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
Blood transfusion
The effect of LMWH versus UFH on blood transfusions was very uncertain (RR 0.22; 95% CI 0.01 to 4.47; 1 trial, 105 women; Analysis 2.4).
2.4. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 4: Blood transfusion
Bleeding episodes
Bleeding episodes were variably reported across three trials (Analysis 2.5). Effects of LMWH versus no treatment or placebo were very uncertain for bruises greater than 2.5 cm (a possible reduction with LMWH was observed) (RR 0.18; 95% CI 0.09 to 0.36; 1 trial, 121 women), bleeding at birth (RR 3.80; 95% CI 0.44 to 32.99; 1 trial, 117 women), and 'bleeding complications' (a possible reduction with LMWH was observed) (RR 0.28; 95% CI 0.15 to 0.53; 1 trial, 105 women).
2.5. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 5: Bleeding episodes
Serious wound complications
No trial reported this outcome.
Adverse effects sufficient to stop treatment
LMWH compared with UFH may reduce adverse effects sufficient to stop treatment but this evidence was very uncertain (RR 0.07; 95% CI 0.01 to 0.54; 2 trials, 226 women; very low‐certainty evidence; Analysis 2.6). There were 13 events in UFH group: 1 stopped due to an allergic reaction, 1 due to mild anaemia with no confirmed bleeding and 11 due to excess bruising/allergic rashes (these 11 stopped switched to LMWH (dalteparin) and the adverse effects resolved).
2.6. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 6: Adverse effects sufficient to stop treatment
Adverse effects not sufficient to stop treatment
The effect of LMWH versus UFH on adverse effects (injection burning) not sufficient to stop treatment was very uncertain (RR 0.79; 95% CI 0.53 to 1.18; 1 trial, 121 women; Analysis 2.7).
2.7. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 7: Adverse effects not sufficient to stop treatment
Symptomatic osteoporosis
The effect of LMWH versus UFH on symptomatic osteoporosis was very uncertain (RR 0.43; 95% CI 0.06 to 2.98; 2 trials, 188 women; Analysis 2.8).
2.8. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 8: Symptomatic osteoporosis
Fetal loss
The effect of LMWH versus UFH on fetal loss was very uncertain (Analysis 2.9): where gestation was unclear (RR 0.61; 95% CI 0.21 to 1.77; 2 trials, 222 women) and at less than 20 weeks' gestation (RR 0.38; 95% CI 0.14 to 1.00; 1 trial, 121 women).
2.9. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 9: Fetal loss
Thrombocytopenia
The effect of LMWH versus UFH on thrombocytopenia was very uncertain (RR 0.18; CI 0.01 to 3.64; 95%; 3 trials, 287 women; Analysis 2.10).
2.10. Analysis.

Comparison 2: Antenatal (± postnatal) prophylaxis: LMWH versus UFH, Outcome 10: Thrombocytopenia
Secondary outcomes not reported
Asymptomatic thromboembolic events; fetal anomalies.
Comparison 3: Antenatal (±postnatal) prophylaxis: adjusted‐ versus fixed‐dose LMWH
One trial (Salim 2016) involving 144 women was included.
Primary outcomes
A single trial did not report on maternal death, and reported no events in either the adjusted‐dose or fixed‐dose groups for symptomatic thromboembolic events (Analysis 3.1), symptomatic PE (Analysis 3.2) or symptomatic DVT (Analysis 3.3); 140 women.
3.1. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 1: Symptomatic thromboembolic events
3.2. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 2: Symptomatic pulmonary embolism
3.3. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
Asymptomatic thromboembolic events
There were no asymptomatic thromboembolic events in the adjusted‐dose or fixed‐dose LMWH groups (1 trial, 140 women; Analysis 3.4).
3.4. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 4: Asymptomatic thromboembolic events
Bleeding episodes
The trial reported variations of bleeding episodes (Analysis 3.5); the effects of adjusted‐dose versus fixed‐dose LMWH on placental abruption (RR 0.22; 95% CI 0.03 to 1.95; 1 trial, 140 women), postpartum haemorrhage (RR 0.08; 95% CI 0.00 to 1.44; 1 trial, 140 women) and 'bleeding side effects' (no events) were very uncertain.
3.5. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 5: Bleeding episodes
Adverse effects sufficient to stop treatment
The effect of adjusted‐dose versus fixed‐dose LMWH on adverse effects sufficient to stop treatment (skin allergy) was very uncertain (RR 0.35, 95% CI 0.01 to 8.50; 1 trial, 144 women; Analysis 3.6).
3.6. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 6: Adverse effects sufficient to stop treatment
Adverse effects not sufficient to stop treatment
The effect of adjusted‐dose versus fixed‐dose LMWH on adverse effects not sufficient to stop treatment (skin allergy) was very uncertain (RR 0.30, 95% CI 0.01 to 7.19; 1 trial, 140 women; Analysis 3.7).
3.7. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 7: Adverse effects not sufficient to stop treatment
Fetal loss
The effects of adjusted‐dose versus fixed‐dose LMWH on fetal loss at less than 20 weeks' gestation (RR 4.47; 95% CI 0.22 to 91.38; 1 trial, 140 women), and at least 20 weeks' gestation (RR 2.68; 95% CI 0.11 to 64.68; 1 trial, 140 women) were very uncertain (Analysis 3.8).
3.8. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 8: Fetal loss
Thrombocytopenia
There were no cases of thrombocytopenia in the adjusted‐dose or fixed‐dose LMWH groups (1 trial, 140 women; Analysis 3.9).
3.9. Analysis.

Comparison 3: Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH, Outcome 9: Thrombocytopenia
Secondary outcomes not reported
Blood transfusion; serious wound complications; symptomatic osteoporosis; fetal anomalies.
Comparison 4: Antenatal (±postnatal) prophylaxis: compression stockings versus none
One trial (Heller 2016) involving 44 women was included.
Primary outcomes
The trial did not report on maternal death, symptomatic thromboembolic events or symptomatic VTE. There were no cases of symptomatic DVT in the compression stockings or no compression stockings groups (1 trial, 44 women Analysis 4.1).
4.1. Analysis.

Comparison 4: Antenatal (± postnatal) prophylaxis: Compression stockings versus none, Outcome 1: Symptomatic deep vein thrombosis
Secondary outcomes
No cases of adverse effects sufficient to stop treatment were reported (1 trial, 44 women; Analysis 4.2).
4.2. Analysis.

Comparison 4: Antenatal (± postnatal) prophylaxis: Compression stockings versus none, Outcome 2: Adverse effects not sufficient to stop treatment
Secondary outcomes not reported
Asymptomatic thromboembolic events; blood transfusion; bleeding episodes; serious wound complications; adverse effects sufficient to stop treatment; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 5: Peripartum prophylaxis: UFH versus no treatment
One trial (Segal 1975) involving 210 women was included. See Table 3.
Primary outcomes
The included trial (Segal 1975) did not report on maternal death. The effects of UFH versus no heparin on symptomatic thromboembolic events (RR 0.16; 95% CI 0.02 to 1.36; 1 trial, 210 women; Analysis 5.1), symptomatic PE (RR 0.16; 95% CI 0.01 to 3.34; 1 trial, 210 women; Analysis 5.2), and symptomatic DVT (RR 0.27; 95% CI 0.03 to 2.55; 1 trial; 210 women; Analysis 5.3) were very uncertain ‐ all very low‐certainty evidence.
5.1. Analysis.

Comparison 5: Peripartum/postnatal prophylaxis: UFH versus no treatment, Outcome 1: Symptomatic thromboembolic events
5.2. Analysis.

Comparison 5: Peripartum/postnatal prophylaxis: UFH versus no treatment, Outcome 2: Symptomatic pulmonary embolism
5.3. Analysis.

Comparison 5: Peripartum/postnatal prophylaxis: UFH versus no treatment, Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
Secondary outcomes not reported
Asymptomatic thromboembolic events; blood transfusion; bleeding episodes; serious wound complications; adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 6: Peripartum/postnatal prophylaxis (caesarean): heparin (LMWH or UFH) versus no treatment or placebo
Five trials (Algahtani 2015; Burrows 2001; Gates 2004b; Hill 1988; Welti 1981) involving 1147 women were included. See Table 4.
Primary outcomes
There were no cases of maternal death (1 trial, 300 women; Analysis 6.1). The effects of heparin versus no treatment or placebo on symptomatic thromboembolic events (RR 1.30; 95% CI 0.39 to 4.27; 4 trials, 840 women; Analysis 6.2), symptomatic PE (RR 1.10; 95% CI 0.25 to 4.87; 4 trials, 840 women; Analysis 6.3), and symptomatic DVT (RR 1.30; 95% CI 0.24 to 6.94; 5 trials, 1140 women; Analysis 6.4) were very uncertain ‐ all very low‐certainty evidence.
6.1. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 1: Maternal death
6.2. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 2: Symptomatic thromboembolic events
6.3. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 3: Symptomatic pulmonary embolism
6.4. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 4: Symptomatic deep vein thrombosis
Secondary outcomes
Blood transfusion
The effect of heparin versus no treatment or placebo on blood transfusion was very uncertain (RR 0.24; 95% CI 0.03 to 2.13; 3 trials, 266 women; Analysis 6.5).
6.5. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 5: Blood transfusion
Bleeding episodes
Bleeding episodes were variously reported across four trials. There were no cases of major bleeding (1 trial, 76 women) or major bruising (1 trial, 76 women). The effects of heparin versus no treatment or placebo were very uncertain for: bleeding complications (a possible increase with heparin was observed) (RR 5.03; 95% CI 2.49 to 10.18; 2 trials, 714 women), bleeding/bruising at discharge (RR 6.17; 95% CI 0.76 to 49.96; 1 trial, 140 women), blood loss less than 500 mL (RR 1.50; 95% CI 0.63 to 3.59; 1 trial, 50 women), blood loss 500 mL to 1000 mL (RR 0.81; 95% CI 0.50 to 1.31; 1 trial, 50 women), blood loss 1000 mL to 1500 mL (RR 0.50; 95% CI 0.05 to 5.17; 1 trial, 50 women), and blood loss 1500 mL to 2000 mL (RR 2.00; 95% CI 0.19 to 20.67; 1 trial, 50 women; Analysis 6.6).
6.6. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 6: Bleeding episodes (variously defined)
Serious wound complications
Serious wound complications were variably reported in three trials; no major wound disruptions were reported in the heparin and no treatment or placebo groups (2 trials, 126 women); the effect of heparin versus no treatment or placebo on wound infection was very uncertain (RR 2.30; 95% CI 0.34 to 15.53; 2 trials, 216 women; Analysis 6.7).
6.7. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 7: Serious wound complications
Adverse effects sufficient to stop treatment
There were no cases of adverse effects sufficient to stop treatment (1 trial, 140 women; Analysis 6.8).
6.8. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 8: Adverse effects sufficient to stop treatment
Adverse effects not sufficient to stop treatment
There were no cases of adverse effects insufficient to stop treatment (1 trial, 76 women; Analysis 6.9).
6.9. Analysis.

Comparison 6: Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo, Outcome 9: Adverse effects not sufficient to stop treatment
Secondary outcomes not reported
Asymptomatic thromboembolic events; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 7: Peripartum/postnatal prophylaxis (caesarean): HES versus UFH
One trial (Heilmann 1991) involving 207 women was included.
Primary outcomes
The trial did not report on maternal death, symptomatic thromboembolic events, symptomatic PE or symptomatic DVT.
Secondary outcomes
Asymptomatic thromboembolic events
The effect of HES versus UFH on asymptomatic thromboembolic events was very uncertain (RR 0.76; 95% CI 0.27 to 2.11; 1 trial, 207 women; Analysis 7.1).
7.1. Analysis.

Comparison 7: Peripartum/postnatal prophylaxis (caesarean): HES versus UFH, Outcome 1: Asymptomatic thromboembolic events
Blood transfusion
The effect of HES versus UFH on blood transfusions was very uncertain (RR 0.50; 95% CI 0.05 to 5.48; 1 trial, 207 women Analysis 7.2).
7.2. Analysis.

Comparison 7: Peripartum/postnatal prophylaxis (caesarean): HES versus UFH, Outcome 2: Blood transfusion
Bleeding episodes
The effect of HES versus UFH on bleeding episodes was very uncertain (RR 0.40; 95% CI 0.08 to 2.03; 1 trial, 207 women; Analysis 7.3).
7.3. Analysis.

Comparison 7: Peripartum/postnatal prophylaxis (caesarean): HES versus UFH, Outcome 3: Bleeding episodes
Wound complications
The effect of HES versus UFH on wound complications was very uncertain (RR 0.67; 95% CI 0.25 to 1.82; 1 trial, 207 women; Analysis 7.4).
7.4. Analysis.

Comparison 7: Peripartum/postnatal prophylaxis (caesarean): HES versus UFH, Outcome 4: Serious wound complications
Secondary outcomes not reported
Asymptomatic thromboembolic events; adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH
Three trials (Gibson 1998; Heilmann 2007; Krauss 1994) involving 217 women were included. See Table 5.
Primary outcomes
No trials reported on maternal death. The effects of LMWH versus UFH on symptomatic thromboembolic events (RR 0.33; 95% CI 0.01 to 7.99; 3 trials, 217 women; Analysis 8.1) and symptomatic DVT (RR 0.33; 95% CI 0.01 to 7.99; 3 trials, 217 women; Analysis 8.3), were very uncertain ‐ both very low‐certainty evidence. There were no cases of symptomatic PE (3 trials, 217 women; Analysis 8.2).
8.1. Analysis.

Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH, Outcome 1: Symptomatic thromboembolic events
8.3. Analysis.

Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH, Outcome 3: Symptomatic deep vein thrombosis
8.2. Analysis.

Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH, Outcome 2: Symptomatic pulmonary embolism
Secondary outcomes
Bleeding episodes
There were no cases of bleeding episodes (variously defined) in the LMWH and UFH groups: 'haemorrhagic event' (1 trial, 17 women), major bleeding (1 trial, 100 women), and post surgical haemorrhage (1 trial, 100 women) (Analysis 8.4).
8.4. Analysis.

Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH, Outcome 4: Bleeding episodes
Adverse effects not sufficient to stop treatment
There were no adverse effects not sufficient to stop treatment (1 trial, 100 women; Analysis 8.5).
8.5. Analysis.

Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH, Outcome 5: Adverse effects not sufficient to stop treatment
Thrombocytopenia
There were no cases of thrombocytopenia (1 trial, 100 women; Analysis 8.6). Another trial (Heilmann 2007) reported that "The clinical outcome showed no differences in... the different prophylaxis groups and thrombocytopenia."
8.6. Analysis.

Comparison 8: Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH, Outcome 6: Thrombocytopenia
Secondary outcomes not reported
Asymptomatic thromboembolic events; blood transfusions; adverse effects sufficient to stop treatment; symptomatic osteoporosis; fetal loss; fetal anomalies.
Comparison 9: Peripartum/postnatal prophylaxis (caesarean): five‐day versus 10‐day LMWH
One trial (Cruz 2011) involving 646 women was included.
Primary outcomes
There were no maternal deaths (1 trial, 646 women; Analysis 9.1) or cases of symptomatic DVT (1 trial, 646 women; Analysis 9.4) in the five‐day and 10‐day LMWH groups. The effects of five‐day versus 10‐day LMWH on symptomatic thromboembolic events (RR 0.36; 95% CI 0.01 to 8.78; 1 trial, 646 women; Analysis 9.2) and symptomatic PE (RR 0.36; 95% CI 0.01 to 8.78; 1 trial, 646 women; Analysis 9.3) were very uncertain.
9.1. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 1: Maternal death
9.4. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 4: Symptomatic deep vein thrombosis
9.2. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 2: Symptomatic thromboembolic events
9.3. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 3: Symptomatic pulmonary embolism
Secondary outcomes
Bleeding episodes
There were no bleeding episodes (1 trial, 646 women; Analysis 9.5).
9.5. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 5: Bleeding episodes
Serious wound complications
The effects of five‐day versus 10‐day LMWH on post caesarean infection (RR 1.13; 95% CI 0.63 to 2.05; 1 trial, 646 women) and post caesarean seroma (RR 1.14; 95% CI 0.59 to 2.23; 1 trial. 646 women) were very uncertain (Analysis 9.6).
9.6. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 6: Serious wound complications
Thrombocytopenia
There were no cases of thrombocytopenia (1 trial, 646 women; Analysis 9.7).
9.7. Analysis.

Comparison 9: Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH, Outcome 7: Thrombocytopenia
Secondary outcomes not reported
Asymptomatic thromboembolic events; blood transfusions; adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment; symptomatic osteoporosis; fetal losses; fetal anomalies.
Comparison 10: Peripartum/postnatal prophylaxis (caesarean): weight‐based versus fixed‐dose LMWH
One trial (Stephenson 2016) involving 90 women was included.
Primary outcomes
Maternal death was not reported. There were no cases of symptomatic thromboembolic events (1 trial, 84 women; Analysis 10.1), symptomatic PE (1 trial, 84 women; Analysis 10.2) or symptomatic DVT (no events; 1 trial, 84 women; Analysis 10.3) in the weight‐based and fixed‐dose LMWH groups.
10.1. Analysis.

Comparison 10: Peripartum/postnatal prophylaxis (caesarean): Weight‐based versus fixed‐dose LMWH, Outcome 1: Symptomatic thromboembolic events
10.2. Analysis.

Comparison 10: Peripartum/postnatal prophylaxis (caesarean): Weight‐based versus fixed‐dose LMWH, Outcome 2: Symptomatic pulmonary embolism
10.3. Analysis.

Comparison 10: Peripartum/postnatal prophylaxis (caesarean): Weight‐based versus fixed‐dose LMWH, Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
Blood transfusion
No blood transfusions were reported (1 trial, 84 women; Analysis 10.4).
10.4. Analysis.

Comparison 10: Peripartum/postnatal prophylaxis (caesarean): Weight‐based versus fixed‐dose LMWH, Outcome 4: Blood transfusion
Adverse effects not sufficient to stop treatment
Stephenson 2016 reported narratively in text on one "possible case of heparin‐induced skin necrosis" in the weight‐based LMWH group.
Serious wound complications
The effects of weight‐based versus fixed‐dose LMWH on wound infection (RR 0.20; 95% CI 0.01 to 4.04; 1 trial, 84 women) and wound haematoma (RR 0.33; 95% CI 0.04 to 3.08; 1 trial, 84 women) were very uncertain (Analysis 10.5). There were no cases of wound dehiscence or reoperation (1 trial, 84 women) (Analysis 10.5).
10.5. Analysis.

Comparison 10: Peripartum/postnatal prophylaxis (caesarean): Weight‐based versus fixed‐dose LMWH, Outcome 5: Serious wound complications
Secondary outcomes not reported
Asymptomatic thromboembolic events; bleeding episodes; adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 11: Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types)
One trial (Ellison 2001) involving 30 women was included.
Primary outcomes
Maternal death was not reported. There were no cases of symptomatic thromboembolic events, symptomatic PE or symptomatic DVT in comparisons of dalteparin versus enoxaparin, dalteparin versus tinzaparin, and enoxaparin versus tinzaparin (1 trial, 20 women in each comparison for each outcome; Analysis 11.1; Analysis 11.2; Analysis 11.3).
11.1. Analysis.

Comparison 11: Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types), Outcome 1: Symptomatic thromboembolic events
11.2. Analysis.

Comparison 11: Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types), Outcome 2: Symptomatic pulmonary embolism
11.3. Analysis.

Comparison 11: Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types), Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
Bleeding episodes
There were no bleeding episodes (excessive bruising) in comparisons of dalteparin versus enoxaparin, dalteparin versus tinzaparin, and enoxaparin versus tinzaparin (1 trial, 20 women in each comparison; Analysis 11.4).
11.4. Analysis.

Comparison 11: Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types), Outcome 4: Bleeding episodes (excessive bruising)
Adverse effects not sufficient to stop treatment
There were no adverse effects (not sufficient to stop treatment (skin reactions)) in comparisons of dalteparin versus enoxaparin, dalteparin versus tinzaparin, and enoxaparin versus tinzaparin (1 trial, 20 women in each comparison; Analysis 11.5).
11.5. Analysis.

Comparison 11: Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types), Outcome 5: Adverse effects not sufficient to stop treatment
Secondary outcomes not reported
Asymptomatic thromboembolic events; blood transfusion; serious wound complications; adverse effects sufficient to stop treatment; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 12: Peripartum/postnatal prophylaxis (caesarean): compression devices versus bed rest
One trial (Reddick 2014) involving 50 women was included.
Primary outcomes
Maternal death was not reported. There were no cases of symptomatic thromboembolic events (1 trial, 49 women; Analysis 12.1), symptomatic PE (1 trial, 49 women; Analysis 12.2) or symptomatic DVT (1 trial, 49 women; Analysis 12.3) in the compression devices and bed rest groups.
12.1. Analysis.

Comparison 12: Peripartum/postnatal prophylaxis (caesarean): Compression devices versus bed rest, Outcome 1: Symptomatic thromboembolic events
12.2. Analysis.

Comparison 12: Peripartum/postnatal prophylaxis (caesarean): Compression devices versus bed rest, Outcome 2: Symptomatic pulmonary embolism
12.3. Analysis.

Comparison 12: Peripartum/postnatal prophylaxis (caesarean): Compression devices versus bed rest, Outcome 3: Symptomatic deep vein thrombosis
Secondary outcomes
No blood transfusions were reported (1 trial, 49 women; Analysis 12.4).
12.4. Analysis.

Comparison 12: Peripartum/postnatal prophylaxis (caesarean): Compression devices versus bed rest, Outcome 4: Blood transfusion
Secondary outcomes not reported
Asymptomatic thromboembolic events; bleeding episodes; serious wound complications; adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment; symptomatic osteoporosis; fetal loss; thrombocytopenia; fetal anomalies.
Comparison 13: Postnatal prophylaxis: LMWH versus no treatment or placebo
Two trials (Rodger 2015; Rodger 2016) involving 62 women, were included. See Table 6.
Primary outcomes
There were no cases of maternal death (1 trial, 24 women; Analysis 13.1), symptomatic thromboembolic events (2 trials, 58 women; Analysis 13.2), symptomatic PE (2 trials, 58 women; Analysis 13.3), or symptomatic DVT (2 trials, 58 women; Analysis 13.4) in the LMWH and no treatment or placebo groups.
13.1. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 1: Maternal death
13.2. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 2: Symptomatic thromboembolic events
13.3. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 3: Symptomatic pulmonary embolism
13.4. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 4: Symptomatic deep vein thrombosis
Secondary outcomes
Assymptomatic thromboembolic events
There were no asymptomatic thromboembolic events (2 trials, 58 women; Analysis 13.5).
13.5. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 5: Asymptomatic thromboembolic events
Bleeding episodes
Bleeding episodes were variably reported by the two trials. The effects of LMWH versus no treatment or placebo on major bleeding (RR 3.53; 95% CI 0.15 to 81.11; 2 trials, 59 women), clinically relevant bleeding events (RR 5.88; 95% CI 0.30‐114.28; 1 trial, 35 women), and minor bleeding events (RR 3.53; 95% CI 0.15 to 81.11; 1 trial, 35 women) were very uncertain (Analysis 13.6).
13.6. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 6: Bleeding episodes
Adverse effects not sufficient to stop treatment
The effect of LMWH versus placebo or no treatment on adverse effects not sufficient to stop treatment was very uncertain (RR 3.53; 95% CI 0.15 to 81.11; 2 trials, 59 women; Analysis 13.7).
13.7. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 7: Adverse effects not sufficient to stop treatment
Thrombocytopenia
There were no cases of thrombocytopenia (1 trial, 24 women; Analysis 13.8).
13.8. Analysis.

Comparison 13: Postnatal prophylaxis: LMWH versus no treatment/placebo, Outcome 8: Thrombocytopenia
Secondary outcomes not reported
Blood transfusion; serious wound complications; adverse effects sufficient to stop treatment; symptomatic osteoporosis; fetal loss; fetal anomalies.
Sensitivity analysis
Nine high‐quality trials (judged to be at low risk of both selection bias and attrition bias) (de Vries 2012; Gates 2004a; Gates 2004b; Pettila 1999; Rodger 2014; Rodger 2015; Rodger 2016; Salim 2016; van Hoorn 2016) were identified for sensitivity analyses. Sensitivity analyses for Comparisons 3 (Salim 2016) and 13 (Rodger 2015; Rodger 2016) were not replicated below; as trials for inclusion were the same as in the main analyses.
Comparison 1: Antental (± postnatal) prophylaxis: heparin (LMWH or UFH) versus no treatment or placebo
For the outcomes symptomatic thromboembolic events (Analysis 14.1) and symptomatic PE (Analysis 14.2), sensitivity analyses included the same trials (and thus results) as the main analyses. In the sensitivity analysis the effect of heparin versus no treatment or placebo on symptomatic DVT was very uncertain (RR 0.33; 95% CI 0.01 to 7.93; 3 trials, 187 women), as in the main analysis.
14.1. Analysis.

Comparison 14: Sensitivity analysis: antenatal (± postnatal) prophylaxis with heparin versus no treatment/placebo, Outcome 1: Symptomatic thromboembolic events
14.2. Analysis.

Comparison 14: Sensitivity analysis: antenatal (± postnatal) prophylaxis with heparin versus no treatment/placebo, Outcome 2: Symptomatic pulmonary embolism
Comparison 2: Antental (± postnatal) prophylaxis: LMWH versus UFH
On sensitivity analysis, there were no symptomatic thromboembolic events, symptomatic PE or DVT reported (1 trial, 105 women) (Analysis 15.1; Analysis 15.2; Analysis 15.3). Unlike the main analysis for symptomatic thromboembolic events (where the effect was very uncertain), the sensitivity analysis included no cases (1 trial, 105 women) (Analysis 15.1).
15.1. Analysis.

Comparison 15: Sensitivity analysis: antenatal (± postnatal) prophylaxis with LMWH versus UFH, Outcome 1: Symptomatic thromboembolic events
15.2. Analysis.

Comparison 15: Sensitivity analysis: antenatal (± postnatal) prophylaxis with LMWH versus UFH, Outcome 2: Symptomatic pulmonary embolism
15.3. Analysis.

Comparison 15: Sensitivity analysis: antenatal (± postnatal) prophylaxis with LMWH versus UFH, Outcome 3: Symptomatic deep vein thrombosis
Comparison 6: Peripartum/postnatal prophylaxis (caesarean): heparin (LMWH) versus no treatment or placebo
As in the main analyses, sensitivity analyses suggested the effects of heparin versus no treatment or placebo on symptomatic thromboembolic events and PE were very uncertain (both RR 3.09; 95% CI 0.13 to 74.51; 1 trial, 134 women; Analysis 16.1; Analysis 16.2). Unlike in the main analysis for symptomatic DVT (where the effect was very uncertain), the sensitivity analysis included no cases (1 trial, 134 women) (Analysis 16.3).
16.1. Analysis.

Comparison 16: Sensitivity analysis: peripartum/postnatal prophylaxis (caesarean) with LMWH versus no treatment/placebo, Outcome 1: Symptomatic thromboembolic events
16.2. Analysis.

Comparison 16: Sensitivity analysis: peripartum/postnatal prophylaxis (caesarean) with LMWH versus no treatment/placebo, Outcome 2: Symptomatic pulmonary embolism
16.3. Analysis.

Comparison 16: Sensitivity analysis: peripartum/postnatal prophylaxis (caesarean) with LMWH versus no treatment/placebo, Outcome 3: Symptomatic deep vein thrombosis
Discussion
Summary of main results
In this updated review, we included 29 randomised controlled trials comparing effects of various methods of thromboprophylaxis in women who were pregnant or who had recently given birth and were at increased risk of venous thromboembolism (VTE). The included trials contributed data across 13 comparisons.
Antenatal (± postnatal) prophylaxis: effects were very uncertain (very low‐certainty evidence, where assessed; or could not be determined due to no events) across all comparisons that reported symptomatic thromboembolic events, symptomatic pulmonary embolism (PE), symptomatic deep vein thrombosis (DVT) and/or adverse effects sufficient to stop treatment (heparin versus no treatment/placebo (maximum of four trials, 476 women); low molecular weight heparin (LMWH) versus unfractionated heparin (UFH) (maximum of 4 trials, 404 women); adjusted‐dose versus fixed‐dose LMWH (maximum of one trial, 144 women); compression stockings versus none (maximum one trial, 44 women). Maternal death was not reported.
Peripartum/postnatal prophylaxis: forvaginal birth or caesarean birth: effects were very uncertain in the comparison of UFH versus no treatment for symptomatic thromboembolic events, symptomatic PE and symptomatic DVT (one trial, 210 women). Maternal death and adverse effects sufficient to stop treatment were not reported.
For caesarean birth: effects were very uncertain (very low‐certainty evidence where assessed; or could not be determined due to no events) across all comparisons that reported maternal death, symptomatic thromboembolic events, symptomatic PE, symptomatic DVT, adverse effects sufficient to stop treatment (heparin versus no treatment/placebo (maximum of 5 trials, 1140 women); LMWH versus UFH (3 trials, 217 women); five‐day versus 10‐day LMWH (one trial, 646 women); weight‐based versus fixed‐dose LMWH (1 trial, 84 women); comparisons of different types of LMWH (1 trial; 30 women); compression devices versus bed rest (1 trial, 49 women).
Postnatal prophylaxis: there were no events in the comparison of LMWH versus no treatment/placebo for maternal death, symptomatic thromboembolic events, symptomatic PE and symptomatic DVT (maximum of 2 trials, 58 women). Adverse effects sufficient to stop treatment were not reported.
Subgroup analyses were unable to be conducted due to lack of data, and sensitivity analyses did not impact the main findings.
Overall completeness and applicability of evidence
The evidence to assess the effects of thromboprophylaxis during pregnancy and the early postnatal period in women at increased risk of VTE on the risk of venous thromboembolic disease and adverse effects is very incomplete. While maternal deaths were largely not reported (only three trials reported this outcome and observed no deaths), we cannot assume that none occurred. Due to differences in the interventions assessed by the trials, and/or difference in the types of included participants and their risk factors (when reported), we were able to combine only a few trials limiting ability to detect differences between interventions. Between one and five trials were included in the 13 review comparisons, with eight of these comparisons including single trials. In general the sample sizes of the trials were small. The three largest trials recruited 646 women (Cruz 2011), 580 women (Welti 1981), and 292 women (Rodger 2014). Sample sizes of this order are generally inadequate to detect differences in the incidences of rare outcomes such as thromboembolic events, or death from such events, particularly when two active treatments are compared.
The important secondary review outcome 'adverse effects sufficient to stop treatment' was only reported by five trials included within four comparisons. There was a paucity of data reported by the included trials for all other secondary outcomes (e.g. asymptomatic thromboembolic events were reported by only four trials across three comparisons). Unclear and different definitions used in the measurement of some of the secondary outcomes (e.g. bleeding episodes; adverse effects not sufficient to stop treatment) made comparing effects of trials difficult, and prevented synthesising individual effect measures in analyses. It is likely that trial authors had differing definitions of ‘serious”.
While the trials conducted to date (and included in this review), have assessed a large number of thromboprophylaxis comparisons, and this update included interventions not identified in the previous version of the review (Bain 2014), gaps remain. The full range of commonly‐used interventions has not been assessed. For example, none of the included trials assessed intermittent pneumatic compression, early mobilisation or surveillance. Some of the older trials assessed thromboprophylaxis methods which are no longer used (such as hydroxyethyl starch (HES) (Paull 1987)), or are not used as frequently in current thromboprophylactic practice (such as the use of UFH rather than LMWH).
The focus of this review was on determining effects of different methods to prevent VTE in pregnancy and the early postpartum period, in women at increased risk of VTE due to a variety of risk factors; further evidence on the use of heparin and other thromboprophylactic drugs on the prevention of miscarriage and other pregnancy outcomes in specific groups of women at high risk of adverse pregnancy outcomes are examined in related Cochrane Reviews (see de Jong 2014; Dodd 2013; Hamulyák 2020).
Quality of the evidence
Overall, study‐level risk of bias was moderate to high (Figure 2; Figure 3); while attrition bias was considered mostly low risk, there was high or unclear risk of reporting bias in over 80% of trials (due mostly to the absence of protocols and trials published as abstracts only), and over half of the trials were judged at unclear or high risk of selection bias. Most of the included studies had difficulties with adequate blinding of women and personnel due to the interventions they assessed, and in the majority of the trials it was unclear whether outcome assessors were blinded (though absence of blinding for these objective outcomes did not impact our ratings of the certainty of the evidence). The few potential differences in particular secondary outcomes reported in this review were largely derived from small trials which were not considered to be of high methodological quality; there is a strong possibility that they may be caused by bias or chance.
The certainty of evidence (assessed using the GRADE approach) assigned to all critical outcomes reported in 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6) was very low certainty. Reasons for downgrading included design limitations and considerable imprecision (usually due to low event rates and small numbers of trials per outcome). Although, in a few instances, evidence was also downgraded for indirectness (concerns about applicability of results included vague definition of outcomes (e.g. lack of clarity about whether thromboembolic events were symptomatic), poorly defined VTE risk factors of women in the included trials, and specificity and variation in VTE risk factors of women in the included trials).
Potential biases in the review process
The evidence for this review has been derived from trials identified through a detailed search process. It is possible (but unlikely) that additional trials assessing prophylaxis for VTE in pregnancy have been published but not identified. It is also possible that other trials have been conducted but not published. Should such trials be identified we will include them in future updates of this review.
We attempted to reduce bias wherever possible by having at least two review authors independently working on trial selection, data extraction, quality and evidence‐certainty assessments.
Agreements and disagreements with other studies or reviews
A narrative review (Andrew 2020) has described the latest evidence on postpartum VTE prevention in women with modest risk factors, such as those with mild thrombophilias, and transient situational risk factors around labour and birth, including caesareans. Congruent with our review, the authors highlight uncertainty surrounding the effects of postpartum thromboprophylaxis in this group of women, and call for the collection of robust prospective data on VTE risk factors and effects of postpartum thromboprophylaxis.
Three related Cochrane Reviews have assessed the effects of the use of thromboprophylaxis (including heparin) in pregnant women. The de Jong 2014 review (conducted prior to the inclusion of GRADE certainty assessments), assessed effects of anticoagulant agents (including aspirin and heparin) in women with a history of at least two unexplained miscarriages, with or without inherited thrombophilia (primary outcome live birth). Consistent with our review, no clear beneficial effects of anticoagulants in trials considered at low risk of bias were found ‐ in the review's comparison of LMWH versus no treatment, no differences between groups were found in individual trials for pregnancy complications, bleeding or thromboembolic events.
The Hamulyák 2020 review, assessed the effects of aspirin and/or heparin for improving pregnancy outcomes in women with persistent (on two separate occasions) antiphospholipid antibodies, either lupus anticoagulant, anticardiolipin or aβ2‐glycoprotein‐I antibodies or a combination, and recurrent pregnancy loss (two or more, which did not have to be consecutive).The review demonstrated low‐certainty evidence that heparin plus aspirin versus aspirin alone may increase the number of live births and reduce the risk of pregnancy loss. Similar to our review, the authors were very uncertain if heparin (plus aspirin versus aspirin alone) has any effect on adverse effects (bleeding) in the mother; no cases of thrombocytopenia, allergic reactions, venous or arterial thromboembolism or congenital malformations were reported (Hamulyák 2020).
Trials assessing the effects of antithrombotic therapy for improving maternal or infant health outcomes specifically in women considered at risk of placental dysfunction were included in the Dodd 2013 review, which focused on outcomes largely different to those included in this review. While the review (also conducted prior to the inclusion of GRADE certainty assessments), demonstrated potential benefits of the use of heparin versus no treatment ‐ with reductions in perinatal mortality, preterm birth and infant birthweight below the 10th centile for gestational age observed ‐ similar to our review, a lack of reliable information regarding clinically relevant, serious adverse infant health outcomes was identified (Dodd 2013).
Authors' conclusions
Implications for practice.
Current evidence is very uncertain regarding the effects of thromboprophylaxis during pregnancy and the early postnatal period on the risk of venous thromboembolic disease and adverse effects in women at increased risk of venous thromboembolism (VTE).
Effects were very uncertain (or not estimable due to no events) across all antenatal, intrapartum and postnatal comparisons that reported critical outcomes: symptomatic thromboembolic events, symptomatic pulmonary embolism (PE), symptomatic deep vein thrombosis (DVT) and/or adverse effects sufficient to stop treatment. Evidence for these critical outcomes was assessed as very low‐certainty due mainly to trial design limitations and considerable imprecision (usually due to low event rates and small numbers of trials and participants per outcome). No maternal deaths were reported.
Implications for research.
There is a need for certain evidence from rigorously conducted large‐scale randomised controlled trials assessing the effects of methods of thromboprophylaxis on rare outcomes such as thromboembolic events. However, the low number of eligible women, and the need to include very large numbers of women in each trial arm to show a difference in incidence of rare outcomes makes conducting trials of antenatal thromboprophylaxis extremely challenging. To achieve an adequate sample size, a trial needs to be conducted in a very large number of centres, in a range of countries, and involve much international collaboration. Acquiring funding for such trials is difficult, and highly unlikely. Rigorous observational studies based on registry data, for example the Riete Registry, the worlds largest database on patients with VTE, are more feasible, and are therefore warranted to address the gaps in evidence about the safety and effectiveness of VTE prophylaxis for high‐risk women during pregnancy and the early postnatal period.
Future studies need to address currently used treatments. Standardised reporting of a comprehensive range of critical outcomes would facilitate combining effect estimates from individual trials in meta‐analyses.
Feedback
Cundiff, July 2007,
Summary
The guidelines for anticoagulation during pregnancy and post partum by the American College of Chest Physicians [1] and the Royal College of Obstetricians and Gynaecologists[ 2] are arguably the standard for care in the USA and UK, respectively. Despite the lack of evidence from randomised trials, these opinion‐based guidelines recommend anticoagulants in many instances, and they can be referenced in medico‐legal cases.
This review appropriately concludes that anticoagulant thromboprophylaxis during pregnancy is not supported by evidence that it is safe and effective. Since anticoagulation carries risks of bleeding, osteoporosis, and fetal deformity, the appropriate implication for practice would be that thromboprophylaxis with anticoagulants should not be used outside of a randomised trial. The implications for research should state that any randomised trial of anticoagulation conducted in pregnant women should be placebo‐controlled.
1. Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126(3 Suppl):627S‐644. 2. Royal College of Obstetricians and Gynaecologists (RCOG). Thromboprophylaxis during pregnancy, labour and after vaginal delivery. London (UK): Royal College of Obstetricians and Gynaecologists; 2004 (Guideline no. 37).
(Summary of comment from David K Cundiff, July 2007)
Reply
Thanks for these comments. We accept that there remains a need for further randomised trials looking at thromboprophylaxis in pregnant women; as the lack of blinding in previous studies has meant that results are difficult to interpret ideally trials should be placebo‐controlled although the use of placebo may not always be practicable or ethical. We acknowledge that anticoagulation carries risk of bleeding, and several related Cochrane Reviews provide evidence of this. However, reviews which examine thromboprophylaxis in non‐pregnant groups at risk of thromboembolism may not be relevant during pregnancy, as the physiological mechanisms controlling blood coagulation are altered, and the risks of thromboembolic disease and side effects may be different.
In this review, we did not have sufficient evidence from trials to assess the harms and benefits associated with the use of anticoagulants, or with different types of anticoagulant. In the absence of evidence from trials, guidelines based on a range of evidence have been used to underpin clinical practice. While we do not believe it is appropriate for this review to make recommendations about what such guidelines should say, we note under Implications for research, that if all pregnant women being considered for thromboprophylaxis were entered into randomised trials (with appropriate consent) this would help to obtain the needed evidence about safety and effectiveness as quickly as possible.
Contributors
Reply to feedback prepared by Rebecca Tooher and Therese Dowswell.
What's new
| Date | Event | Description |
|---|---|---|
| 18 October 2019 | New search has been performed | Search updated and 10 new trials included. We have updated the methods in line with the standard methods used by Cochrane Pregnancy and Childbirth, including the use of GRADE to assess the certainty of the body of evidence. We updated the search on 18th February 2021 and identified 10 trial reports. Two of these are additional reports of an ongoing study (NCT01828697), one is an additional report of Gris 2011, and six trials (seven reports) are awaiting further classification to be assessed at the next update (Abdolvand 2019; Ganer 2020; Movahedi 2020; NCT02856295; NCT04305756; NCT04635839). |
| 18 October 2019 | New citation required but conclusions have not changed | No changes to conclusions. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 27 November 2013 | New search has been performed | Review updated. Three new authors contributed to this update. |
| 27 November 2013 | New citation required but conclusions have not changed | Search updated. Four new trials have been included (Cruz 2011; De Veciana 2001; Hamersley 1998; O'Riordan 2008); two of which were awaiting classification in the previous version of the review. Seven studies have been excluded (Gris 2010; Gris 2011; Harenberg 1993; Kamin 2008; Pyregov 2012; Ratiu 2009; Visser 2011) (two trials were awaiting classification in the previous version of the review, and one was previously included (Harenberg 1993)). Six new trials have been classified as ongoing. Two studies remain awaiting classification. The main conclusions are unaltered. |
| 26 June 2009 | New search has been performed | Search updated. Data from seven new trials have been included (Casele 2006; Gates 2004b; Gates 2004a; Heilmann 2007; Krauss 1994; Segal 1975; Welti 1981) (including two trials that were ongoing in the previous version of the review). Eleven new studies considered for inclusion have been excluded, and two new trials are still ongoing. One trial which was previously included has now been excluded (Rai 1997). While there is now more evidence on some of the review's outcomes, the main conclusions remain unaltered. The authors have replied to Feedback received from David Cundiff. |
| 26 June 2009 | New citation required but conclusions have not changed | New authors prepared this update. |
| 3 January 2008 | Amended | Converted to new review format. |
| 12 November 2007 | Feedback has been incorporated | Feedback from David Cundiff added. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), and our Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Briony Cutts; Rohan D'Souza, University of Toronto, Canada.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS), or the Department of Health and Social Care.
We acknowledge the support from the Cochrane Pregnancy and Childbirth editorial team in Liverpool.
We thank Agnes Wilson, Rebecca Tooher, Simon Gates and Lucy‐Jane Davis for their contributions as authors to previous versions of this review (Bain 2014), and Therese Dowswell for her contribution as an author to the earlier version of this review (Tooher 2010).
We thank Dr CG Taylor for additional information and data for the Hill 1988 trial, and Prof R Burrows for additional information about the Burrows 2001 trial. Thanks also to Penny Berry for translating the two trials published in German.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
thromboembolism AND pregnan*
thromboembolism AND postpartum
thromboembolism AND c(a)eserean
thromboembolic AND pregnan*
thromboembolic AND postpartum
thromboembolic AND c(a)eserean
anticoagulant AND pregnan*
anticoagulant AND postpartum
anticoagulant AND c(a)esarean
DVT AND pregnan*
DVT AND postpartum
DVT AND c(a)esarean
Data and analyses
Comparison 1. Antenatal (± postnatal) prophylaxis: Heparin (LMWH or UFH) versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Symptomatic thromboembolic events | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.98] |
| 1.1.1 LMWH | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.98] |
| 1.2 Symptomatic pulmonary embolism | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 1.2.1 LMWH | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 1.3 Symptomatic deep vein thrombosis | 4 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| 1.3.1 LMWH | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.93] |
| 1.3.2 UFH | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 1.4 Blood transfusion | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.1 LMWH | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Bleeding episodes | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 LMWH: placental abruption | 3 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.31, 3.20] |
| 1.5.2 LMWH: peripartum haemorrhage | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.24, 1.79] |
| 1.5.3 LMWH non‐major/minor bleeding | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.15, 3.93] |
| 1.5.4 LMWH: major bleeding | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.25, 8.72] |
| 1.5.5 UFH: antenatal vaginal bleeding | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.16, 6.42] |
| 1.5.6 UFH: postpartum haemorrhage | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.52] |
| 1.6 Serious wound complications | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.6.1 LMWH | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 Adverse effects sufficient to stop treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 LMWH | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.31] |
| 1.8 Adverse effects not sufficient to stop treatment | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 LMWH: skin/allergic reactions | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.11 [2.00, 13.08] |
| 1.8.2 LMWH: raised liver enzymes | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 22.53 [1.34, 378.78] |
| 1.8.3 LMWH: haematoma | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.98 [0.46, 34.23] |
| 1.8.4 Superficial thrombophlebitis | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.93] |
| 1.8.5 LMWH: other | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.51] |
| 1.9 Symptomatic osteoporosis | 4 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.52] |
| 1.9.1 LMWH | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.9.2 UFH | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.52] |
| 1.10 Fetal loss | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 LMWH or UFH: gestation unclear | 2 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.51] |
| 1.10.2 LMWH: < 20 weeks | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.50, 9.41] |
| 1.10.3 LMWH: ≥ 20 weeks | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.12] |
| 1.11 Thrombocytopenia | 5 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.14, 64.26] |
| 1.11.1 LMWH | 4 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.14, 64.26] |
| 1.11.2 UFH | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.12 Fetal anomalies | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 LMWH | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.60, 14.32] |
Comparison 2. Antenatal (± postnatal) prophylaxis: LMWH versus UFH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Symptomatic thromboembolic events | 4 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.49] |
| 2.2 Symptomatic pulmonary embolism | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.3 Symptomatic deep vein thrombosis | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Blood transfusion | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.47] |
| 2.5 Bleeding episodes | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Bruises > 1 inch | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.09, 0.36] |
| 2.5.2 Bleeding at birth | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.80 [0.44, 32.99] |
| 2.5.3 Bleeding complications | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.53] |
| 2.6 Adverse effects sufficient to stop treatment | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.54] |
| 2.7 Adverse effects not sufficient to stop treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Injection burning | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.18] |
| 2.8 Symptomatic osteoporosis | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.98] |
| 2.9 Fetal loss | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.9.1 Gestation unclear | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.21, 1.77] |
| 2.9.2 < 20 weeks | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.14, 1.00] |
| 2.10 Thrombocytopenia | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.64] |
Comparison 3. Antenatal (±postnatal) prophylaxis: Adjusted‐dose versus fixed‐dose LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Symptomatic thromboembolic events | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.2 Symptomatic pulmonary embolism | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Symptomatic deep vein thrombosis | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Asymptomatic thromboembolic events | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Bleeding episodes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 Placental abruption | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.95] |
| 3.5.2 Postpartum haemorrhage | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.44] |
| 3.5.3 Side effects: bleeding | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.6 Adverse effects sufficient to stop treatment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.50] |
| 3.7 Adverse effects not sufficient to stop treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 Skin allergy | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.19] |
| 3.8 Fetal loss | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.8.1 < 20 weeks | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [0.22, 91.38] |
| 3.8.2 ≥ 20 weeks | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.11, 64.68] |
| 3.9 Thrombocytopenia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 4. Antenatal (± postnatal) prophylaxis: Compression stockings versus none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Symptomatic deep vein thrombosis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.2 Adverse effects not sufficient to stop treatment | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 5. Peripartum/postnatal prophylaxis: UFH versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Symptomatic thromboembolic events | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.36] |
| 5.2 Symptomatic pulmonary embolism | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.34] |
| 5.3 Symptomatic deep vein thrombosis | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.55] |
Comparison 6. Peripartum/postnatal prophylaxis (caesarean): Heparin (LMWH or UFH) versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Maternal death | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.2 Symptomatic thromboembolic events | 4 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.39, 4.27] |
| 6.2.1 LMWH | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.31, 28.03] |
| 6.2.2 UFH | 2 | 630 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.19, 3.76] |
| 6.3 Symptomatic pulmonary embolism | 4 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.25, 4.87] |
| 6.3.1 LMWH | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 74.51] |
| 6.3.2 UFH | 2 | 630 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.48] |
| 6.4 Symptomatic deep vein thrombosis | 5 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.24, 6.94] |
| 6.4.1 LMWH | 3 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.17, 11.55] |
| 6.4.2 UFH | 2 | 630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.07, 18.02] |
| 6.5 Blood transfusion | 3 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.13] |
| 6.5.1 LMWH | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.54] |
| 6.5.2 UFH | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.97] |
| 6.6 Bleeding episodes (variously defined) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.6.1 Major bleeding | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.6.2 Major bruising | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.6.3 Bleeding complications | 2 | 714 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [2.49, 10.18] |
| 6.6.4 Bleeding/bruising reported at discharge | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.17 [0.76, 49.96] |
| 6.6.5 Blood loss < 500 mL | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.63, 3.59] |
| 6.6.6 Blood loss 500‐1000 mL | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 6.6.7 Blood loss 1000‐1500 mL | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.17] |
| 6.6.8 Blood loss 1500‐2000 mL | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.19, 20.67] |
| 6.7 Serious wound complications | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.7.1 Major wound disruption | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.7.2 Wound infection | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.53] |
| 6.8 Adverse effects sufficient to stop treatment | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.9 Adverse effects not sufficient to stop treatment | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 7. Peripartum/postnatal prophylaxis (caesarean): HES versus UFH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Asymptomatic thromboembolic events | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.11] |
| 7.2 Blood transfusion | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.48] |
| 7.3 Bleeding episodes | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.03] |
| 7.4 Serious wound complications | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.82] |
Comparison 8. Peripartum/postnatal prophylaxis (caesarean): LMWH versus UFH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Symptomatic thromboembolic events | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 8.2 Symptomatic pulmonary embolism | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.3 Symptomatic deep vein thrombosis | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 8.4 Bleeding episodes | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.4.1 "haemorrhagic event" | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.4.2 Major bleeding | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.4.3 Post surgical haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.5 Adverse effects not sufficient to stop treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.6 Thrombocytopenia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 9. Peripartum/postnatal prophylaxis (caesarean): 5‐day versus 10‐day LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Maternal death | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.2 Symptomatic thromboembolic events | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.78] |
| 9.3 Symptomatic pulmonary embolism | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.78] |
| 9.4 Symptomatic deep vein thrombosis | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.5 Bleeding episodes | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.6 Serious wound complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.6.1 Post caesarean infection | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.05] |
| 9.6.2 Post caesarean seroma | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.59, 2.23] |
| 9.7 Thrombocytopenia | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 10. Peripartum/postnatal prophylaxis (caesarean): Weight‐based versus fixed‐dose LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Symptomatic thromboembolic events | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.2 Symptomatic pulmonary embolism | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.3 Symptomatic deep vein thrombosis | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.4 Blood transfusion | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.5 Serious wound complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.5.1 Wound dehiscence or reoperation | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.5.2 Wound infection | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.04] |
| 10.5.3 Wound haematoma | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.08] |
Comparison 11. Peripartum/postnatal prophylaxis (caesarean): LMWH versus LMWH (different types).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Symptomatic thromboembolic events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1.1 Dalteparin versus enoxaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.1.2 Dalteparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.1.3 Enoxaparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.2 Symptomatic pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.2.1 Dalteparin versus enoxaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.2.2 Dalteparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.2.3 Enoxaparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.3 Symptomatic deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.3.1 Dalteparin versus enoxaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.3.2 Dalteparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.3.3 Enoxaparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.4 Bleeding episodes (excessive bruising) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.4.1 Dalteparin versus enoxaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.4.2 Dalteparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.4.3 Enoxaparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.5 Adverse effects not sufficient to stop treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.5.1 Dalteparin versus enoxaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.5.2 Dalteparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.5.3 Enoxaparin versus tinzaparin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 12. Peripartum/postnatal prophylaxis (caesarean): Compression devices versus bed rest.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Symptomatic thromboembolic events | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.2 Symptomatic pulmonary embolism | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.3 Symptomatic deep vein thrombosis | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.4 Blood transfusion | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 13. Postnatal prophylaxis: LMWH versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Maternal death | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.2 Symptomatic thromboembolic events | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.3 Symptomatic pulmonary embolism | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.4 Symptomatic deep vein thrombosis | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.5 Asymptomatic thromboembolic events | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.6 Bleeding episodes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.6.1 Major bleeding event | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.15, 81.11] |
| 13.6.2 Clinically relevant bleeding event | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.88 [0.30, 114.28] |
| 13.6.3 Minor bleeding event | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.15, 81.11] |
| 13.7 Adverse effects not sufficient to stop treatment | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.15, 81.11] |
| 13.8 Thrombocytopenia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 14. Sensitivity analysis: antenatal (± postnatal) prophylaxis with heparin versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Symptomatic thromboembolic events | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.98] |
| 14.2 Symptomatic pulmonary embolism | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 14.3 Symptomatic deep vein thrombosis | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.93] |
14.3. Analysis.

Comparison 14: Sensitivity analysis: antenatal (± postnatal) prophylaxis with heparin versus no treatment/placebo, Outcome 3: Symptomatic deep vein thrombosis
Comparison 15. Sensitivity analysis: antenatal (± postnatal) prophylaxis with LMWH versus UFH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Symptomatic thromboembolic events | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 15.2 Symptomatic pulmonary embolism | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 15.3 Symptomatic deep vein thrombosis | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 16. Sensitivity analysis: peripartum/postnatal prophylaxis (caesarean) with LMWH versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Symptomatic thromboembolic events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1.1 LMWH | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 74.51] |
| 16.2 Symptomatic pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.2.1 LMWH | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 74.51] |
| 16.3 Symptomatic deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.3.1 LMWH | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Algahtani 2015.
| Study characteristics | ||
| Methods | RCT: NCT01321788 | |
| Participants | 300 women were randomised Setting: Riyadh, Saudi Arabia. Dates of the study: not reported. Inclusion criteria: women aged 18 to 35 years, undergoing caesarean section. Exclusion criteria: not reported in abstracts, but detailed in trial registration as being at high risk for thromboembolism (any 1 of the following): aged > 35 years, obese, parity > 4, gross varicose veins, current infection, pre‐eclampsia, immobility prior to surgery, major current disease (including heart of lung disease, cancer, inflammatory bowel disease and nephrotic syndrome), extended major pelvic or abdominal surgery, with a family history of VTE, or a history of superficial phlebitis); more than 36 hours since birth, with need for anticoagulation (women with confirmed thrombophilia, with paralysis of lower limbs, with a personal history of VTE, with antiphospholipid antibody syndrome, or mechanical heart valves), and no contraindications to heparin therapy. |
|
| Interventions |
Group 1 (n = 100): LMWH (tinzaparin), 4500 IU subcutaneously once daily, starting from 12 to 24 hours after caesarean section, for 2 weeks. Group 2 (n = 200): placebo, once daily, timing as above. All women: received non‐pharmacological prophylaxis using graduated compression stockings. |
|
| Outcomes |
Review outcomes reported: maternal death; symptomatic DVT. Other outcomes reported: none |
|
| Notes | Information extracted from conference abstracts. Number randomised somewhat unclear, as there appears to be an error in the abstract, which reports that 200 women consented, and also that there were 100 and 200 women in the intervention and comparison groups, respectively. Unable to find a contact email address for author to clarify. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized into two groups..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to determine. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to determine (despite reported use of a placebo). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to determine. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to determine. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Additionally, "minor and major bleeding events" reported as an outcome of interest in abstract methods, but results not reported for this outcome; trial registration also specifies PE and thrombocytopenia as outcomes of interest, and these outcomes are not reported in the abstracts reporting this study. |
| Other bias | Unclear risk | Insufficient information to determine. |
Burrows 2001.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | 76 women were randomised. Setting: tertiary obstetric centre in Australia. Study dates: June to November 1999. Inclusion criteria: women who had undergone an elective or emergency caesarean section. Exclusion criteria: history of bleeding disorder; need for anticoagulant therapy; history of thrombotic event; heparin sensitivity; recent GI haemorrhage or peptic ulcer; hepatic encephalopathy; renal dysfunction requiring dialysis; uncontrolled hypertension; refusal to give informed consent; insufficient English to provide consent. |
|
| Interventions |
Group 1 (n = 39): LMWH (dalteparin), 2500 IU once daily.The injections, 4 or 5, depending on hospital stay, were given either in the thigh or abdomen, depending on women's preference and the site rotated each day. Enough syringes for 5 days of treatment were provided. Group 2 (n = 37): matching placebo (saline) once daily for 4 to 5 days. Interventions started 4 to 24 hours after caesarean section, and continued for 4 to 5 days. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; blood transfusion; bleeding episodes (reports major bleeding: 20 g/L fall in Hb, the need for a blood transfusion, a retroperitoneal, intraocular or intracranial bleed; reports major bruising); serious wound complications (reports major wound disruption requiring surgical repair and wound disruption); adverse effects not sufficient to stop treatment (reports major reaction) Other outcomes reported: fever post‐operation; antibiotics post‐operation; wound infection; minor wound disruption. |
|
| Notes | This study was a pilot protocol to inform an intended national level multi‐centre RCT. Funding sources: quote: "Pharmacia and Upjohn kindly provided the dalteparin and saline placebo medication but no other intellectual or financial support for this study." Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not detailed. |
| Allocation concealment (selection bias) | Low risk | Described as "each pack contained pre‐filled syringes containing either dalteparin or matching placebo". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was described as double‐blind, with the use of an identical placebo; quote "each pack contained pre‐filled syringes containing either dalteparin or matching placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above ‐ not explicitly stated but considered probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All primary analyses were based on group allocation at randomisation (intention‐to‐treat). No losses to follow‐up after randomisation. Follow‐up to 6 weeks was achieved in all women who were recruited. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | More women in the placebo arm had general anaesthesia, but otherwise the 2 groups had similar characteristics at randomisation. No other obvious risk of bias identified. |
Casele 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 120 women were randomised. Setting: 9 centres in the USA. Study dates: September 1998 to December 2005. Inclusion criteria: women who were candidates for low‐dose thromboprophylaxis for the duration of their pregnancy, aged 18 years or more, who could begin therapy < 24 weeks of gestation. Exclusion criteria: women with contraindication to anticoagulation. |
|
| Interventions |
Group 1 (n = 61): LMWH (enoxaparin sodium). Self‐administered subcutaneous 30 mg twice daily from enrolment (women < 24 weeks' GA) until 28 weeks of gestation, then 40 mg twice daily until birth. Group 2 (n = 59): UFH (heparin sodium). Self‐administered subcutaneous 7500 units twice daily until 28 weeks, then 10,000 units twice daily until birth. Baseline bone density test for women in both groups. All women received adjusted‐dose coumadin for 6 to 8 weeks after birth. All women were asked to take antenatal vitamins and were asked to take calcium supplements (500 mg) daily from enrolment until birth. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events (reports recurrent thrombosis); bleeding episodes (reports bleeding at birth); symptomatic osteoporosis (reports clinically significant bone loss in total femur ≥ 10%); fetal loss (reports spontaneous abortion). Other outcomes reported: other measures of mean bone density (reports bone mineral density change at femoral neck and total proximal femur); gestational age at birth; birthweight. |
|
| Notes | The power calculation was based on detecting bone mass changes, the original sample estimate required was 240. The original power calculation had suggested 240 women would be required to detect meaningful changes in loss of bone mass between groups. However, interim analysis suggested that the sample size required would be 1628 and the study was terminated after 120 women had been recruited over 7 years, and thus the study was terminated before the intended sample size had been reached. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table with each site stratified into blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned, and considered unlikely considering the interventions assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was reported that the radiologists carrying out the bone assessments were blind to group allocation; it is unclear as to whether this was successfully achieved. Not mentioned for other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some discrepancies in the numbers enrolled and outcomes in the 2 published reports. The main study paper used for outcome data in this review. 120 women randomised. 98 women completed the study (18% attrition), but of the 22 women who were lost to follow‐up (11 women had spontaneous abortions, 7 women were non‐compliant, 2 women had allergic reactions, 2 women switched to therapeutic anticoagulation), some data were available for some outcomes. It appeared that all women were accounted for in some of the analysis but not for the main study outcome. There were some missing data for main outcomes (bone mass) and denominators were not always clear. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
Cornette 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 44 women were randomised. Setting: authors from Belgium (no further details provided). Study dates: not reported. Inclusion criteria: women with full‐term singleton pregnancies admitted for elective caesarean section. Exclusion criteria: women with known bleeding or coagulation disorders. |
|
| Interventions |
Group 1 (n = 22): preoperative, 0.3 mL nadroparin calcium (LMWH), administered 12 hours before elective caesarean birth. Group 2 (n = 22): postoperative, 0.3 mL (2850 IU) nadroparin calcium, administered 12 hours after elective caesarean birth. All women received the same fluid regimen before, during and after surgery. Women were allowed to drink freely 6 hours after surgery. It was not clear whether participants received any further doses of LMWH after initial dose. |
|
| Outcomes |
Review outcomes reported: none. Other outcomes reported: Hb and haematocrit concentrations 12 hours before and 48 hours after surgery (as a substitute for blood loss). |
|
| Notes | No outcomes included in the review analysis as outcomes were not relevant to the review. The power calculation was based on changes in Hb levels. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, quote: "randomly divided in two groups". |
| Allocation concealment (selection bias) | Unclear risk | As above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up apparent. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting; furthermore, no relevant outcome data were reported. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Cruz 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 646 women were randomised. Setting: San Cecilio University Hospital, Granada, Spain. Study dates: 1‐year period (not further specified). Inclusion criteria: women who had undergone a caesarean section who had not required prophylaxis or treatment with any type of LMWH during pregnancy (low risk of VTE during pregnancy), with absence of allergy to heparin or derivatives. Exclusion criteria: women who did not fulfil the duration of proposed prevention were excluded. |
|
| Interventions |
Group 1 (n = 311): 5 day bemiparin regimen (3500 IU once daily) as post‐caesarean section prophylaxis ≥ 8 hours following caesarean. Group 2 (n = 335): 10 day bemiparin regimen (3500 IU once daily) as post‐caesarean section prophylaxis ≥ 8 hours following caesarean. |
|
| Outcomes | Review outcomes reported: maternal death; symptomatic thromboembolic events; symptomatic PE; symptomatic DVT (all up to 3 months following caesarean); bleeding episodes; thrombocytopenia; post‐caesarean infection and seroma. Other outcomes reported: variables assessed as possible risk factors for a thromboembolic event included hypertension; diabetes; type of caesarean section; type of anaesthesia; week of birth; preterm birth; placental abruption; intrauterine growth restriction. Post‐caesarean section risk factors that were measured included anaemia; infection; seroma and hypertension. |
|
| Notes | Funding sources: research grant from the Laboratorios Fcos. ROVI, SA. Authors state that the results reported in the manuscript were not influenced by the funding received. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: women were assigned "in a randomly systematic way”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: women were assigned "in a randomly systematic way”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No detail of blinding and considered unfeasible for the participants and personnel in view of the intervention (i.e. 5 days versus 10 days of prophylaxis). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail of any losses to follow‐up or exclusions post‐randomisation. The uneven group numbers suggest that there may have been post‐randomisation exclusions (quote "96 women who underwent a caesarean section were excluded because they did not fulfil the exclusion criteria"), and this may have been possible considering that "women who did not fulfil the duration of proposed prevention were excluded". |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting; further important outcomes such as adverse effects and bleeding episodes were not reported. |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
De Veciana 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 121 women were randomised. Setting: authors from the USA. Dates of the study: not reported. Inclusion criteria: not reported; though as per results presented, women with prophylactic anticoagulation indicators were included: antiphospholipid syndrome, a history of DVT/embolus, protein C/protein S deficiency, Factor V Leiden mutation, and obesity. Exclusion criteria: women with renal/liver disease, bleeding diathesis, pork/heparin sensitivity. |
|
| Interventions |
Group 1 (n = 61): dalteparin (LMWH): initial dosing was 2500 IU (5000 IU if > 70 kg) subcutaneously once daily; increased to a maximum of 10,000 IU/day to maintain alpha‐Factor Xa levels at 0.1 to 0.3 IU/mL Group 2 (n = 60): UFH: dosed with the standard 5000 U (8000 U if > 68 kg) subcutaneously twice daily. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes (reports bruises > 2.5 cm); adverse effects sufficient to stop treatment (reports switched from UFH to LMWH due to excess bruising/allergic rashes); adverse effects not sufficient to stop treatment (reports injection burning); thrombocytopenia. Other outcomes reported: gestation at birth; fetal loss (reports stillbirths < 20 weeks); anaesthesia complications. |
|
| Notes | Published as abstract only. Timing of intervention not specified further than "during pregnancy". Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prospective randomized controlled trial”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Prospective randomized controlled trial”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not detailed, however considered unfeasible in view of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up or exclusions detailed, however insufficient detail to confidently assess attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess selective reporting. |
| Other bias | Unclear risk | Maternal demographics and anticoagulation indicators "were similar". Insufficient information to assess other potential sources of bias. |
de Vries 2012.
| Study characteristics | ||
| Methods | RCT: ISRCTN87325378 | |
| Participants | 139 women were randomised. Setting: 13 centres (all university hospitals in the Netherlands, 2 in Australia and 1 in Sweden, and 6 non‐university/teaching hospitals in the Netherlands). Study dates: December 2000 to December 2009. Inclusion criteria: < 12 weeks' gestation; aged > 18 years; with a history of uteroplacental insufficiency and birth < 34 weeks' gestation (hypertensive disorders of pregnancy (pre‐eclampsia or HELLP syndrome or eclampsia); and/or a SGA infant); with a thrombophilic disorder (1 or more of: protein C deficiency; protein S deficiency; activated protein C resistance; factor V Leiden mutation (heterozygous); prothrombin gene G20210A mutation (heterozygous)). If antiphospholipid antibodies (lupus anticoagulant and/or cardiolipin IgG, and/or cardiolipin IgM antibodies) were also present, women were randomised into a separate study (see van Hoorn 2016). Exclusion criteria: 1 or more of: antithrombin deficiency; homozygosity for factor V Leiden and prothrombin G20210A mutation; diabetes mellitus; known malignancy; known peptic ulceration; severe renal or hepatic insufficiency; history of VTE; haemorrhagic diathesis; idiopathic thrombocytopenia; earlier participation in the FRUIT trial; LMWH use in an earlier pregnancy. |
|
| Interventions |
Group 1 (n = 70): LMWH (dalteparin (Fragmin)) 5000 IU subcutaneously daily commenced between 6 and 12 weeks' gestation, and continued until the onset of labour; together with 80 mg oral aspirin daily, commenced before 12 weeks' gestation, and continued until 36 weeks' gestation (Australian participants received aspirin 100 mg; Swedish participants received aspirin 75 mg). Daily dose of LMWH adjusted for body weight: participants below 50 kg received dalteparin 2500 IU, and those above received 80 kg 7500 IU; further adjusted as the pregnancy proceeded and postpartum. Women received instructions for self‐injection; for women with local irritation, dalteparin was changed to enoxaparin (and if irritation persisted, nadroparin). Group 2 (n = 69): women received 80 mg oral aspirin daily, as above. All women: after birth, all women received LMWH for 6 weeks. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic DVT; bleeding episodes (reports placental abruption); adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment (reports skin reaction: pain, itching, swelling, allergy; haematoma; need to convert LMWH prescription; superficial thrombophlebitis); symptomatic osteoporosis (reports complaints suggestive of osteoporosis); fetal loss (reports spontaneous abortion < 16 weeks; and fetal death > 16 weeks) thrombocytopenia. Other outcomes reported: recurrent hypertensive disorders of pregnancy (any of: pre‐eclampsia, HELLP syndrome and/or eclampsia) before 34 weeks' gestation, and until term; SGA; preterm birth; duration of maternal and neonatal admissions; pre‐eclampsia; HELLP syndrome; eclampsia; termination of pregnancy; gestational age at birth; birthweight; gestational age at hypertensive disorder diagnosis; medications during pregnancy (antihypertensive; magnesium sulphate; corticosteroids); increase in pregnancy duration; measures of fetal growth and uterine and umbilical arterial Doppler. |
|
| Notes | Funding sources: quote: "The study was supported by a single 2‐year investigator grant period 2000–2001 by Pfizer, formerly Pharmacia grant number 524E‐CVD‐9101‐0001, annual Dutch investigators meetings, a single grant to support a midwife to recruit Australian subjects, and support for a local meeting in Sweden in 2004. Pharmacia was not the sponsor of the study." Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer to select random permuted blocks of four, stratifying by hospital and presence/absence of chronic hypertension." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by an independent center… Block length and randomization codes were concealed from the investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither study personnel nor participants were blinded to treatment assignment, as placebo injections were not considered to be ethically acceptable during pregnancy." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 70 women in intervention group, 3 stopped LMWH (bleeding from placenta praevia (1) and inconvenience (2)), all analysed; and of 69 in comparison group, 2 stopped aspirin due to stomach complaints; all analysed. See additional protocol deviations below. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Ellison 2001.
| Study characteristics | ||
| Methods | RCT (3 arms) | |
| Participants | 30 women were randomised. Setting: authors from Glasgow, UK (not further specified). Study dates: not reported. Inclusion criteria: women undergoing caesarean section, with an additional risk factor for thromboembolism (including: obesity, immobility, maternal age older than 35 years, parity > 4, labour > 4 hours, gross varicose veins, current infection, pre‐eclampsia, major current illness, caesarean section performed as an emergency). Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 10): dalteparin (LMWH) 5000 IU once daily. Group 2 (n = 10): enoxaparin (LMWH) 4000 IU once daily. Group 3 2 (n = 10): tinzaparin (LMWH) 50 IU/kg (based on booking weight) once daily. Drugs started from 6 hours following caesarean section and continued for 5 days. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events (reports "thrombotic... events"); symptomatic PE; symptomatic DVT; bleeding episodes (reports "haemorrhagic events"... "excessive bruising"); adverse effects not sufficient to stop treatment (reports "skin reactions"). Other outcomes reported: women were followed up for 1 day to examine laboratory haemostatic parameters (plasma anti‐factor Xa, plasma thrombin‐antithrombin (TAT) complex, plasma tissue factor pathway inhibitor (TFPI)). |
|
| Notes | Funding sources: not reported. Declarations of interest: not reported. Trial included in the review analysis as 3 pair‐wise comparisons of LMWH: dalteparin vs enoxaparin; dalteparin vs tinzaparin; and enoxaparin vs tinzaparin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "simple randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "single blind"; no further detail provided regarding how blinding was achieved, or exactly who was blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women seem to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Gates 2004a.
| Study characteristics | ||
| Methods | RCT ('trial 1'). | |
| Participants | 16 women were randomised. Setting: 23 hospitals in theUK (women were recruited in only 11 hospitals). Study dates: April 1998 to February 2000 Inclusion criteria: pregnant women with clinical uncertainty that antenatal thromboprophylaxis was indicated (women with a history of ≥ 1 previous thromboembolic event, women with a known congenital thrombophilia, or women with other accepted risk factors for which clinicians would consider the use of antenatal heparin (all 16 women recruited had a previous thromboembolic event)); no gestational age limit on recruitment. Exclusion criteria: women with a known allergy to heparin. |
|
| Interventions |
Group 1 (n = 8): self‐administered once‐daily subcutaneous 40 mg enoxaparin (LMWH) in 1 mL from antenatal recruitment until a maximum of 6 weeks after birth. Group 2 (n = 8): self‐administered once‐daily subcutaneous placebo (normal saline 1 mL) from antenatal recruitment until 6 a maximum of weeks after birth. All trial drugs were packaged identically in packs that contained 7 prefilled syringes, which was enough for 1 week. Drugs were stored in the hospital pharmacy, and at each antenatal visit, women who were taking part in the study were given enough packs of the study drug to last until their next visit. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; blood transfusion; serious wound complications; adverse events not sufficient to stop treatment (reports "allergic reaction"); symptomatic osteoporosis (reports osteoporotic symptomatic fracture); thrombocytopenia. Other outcomes reported: primary (process) outcome for this pilot was the number of women recruited; also reported bleeding complications, number of hospital admissions, surgical procedures, number of infants admitted to the NICU and number of infants with major bleeding disorders to be secondary outcomes of interest, however no data were reported for these outcomes. |
|
| Notes | Pilot study. After birth some clinicians elected to discontinue study drugs and 3 women in both groups were given heparin postnatally. Funding sources: quote: "Supported by the National Health Service Executive South East Region Research and Development (trial 1), and both trials were supported by a donation to the National Perinatal Epidemiology Unit by Rhone‐Poulenc Rorer." "Study drugs: Rhone‐Poulenc Rorer/Aventis Pharma Ltd, Kent, UK." Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors report that a central telephone randomisation service was used. |
| Allocation concealment (selection bias) | Low risk | A central telephone randomisation service based at the study office was used. Quote: "Each woman was allocated a unique study number that was recorded on the woman’s prescription chart. For the first few women who were recruited, the number corresponded to a numbered treatment pack that contained enough study drug for the treatment of a woman throughout pregnancy and for 6 weeks after birth. Subsequently, pharmacists at each participating hospital were provided with 2 large bins of study drug (labelled A and B): 1 bin containing LMWH, and the other placebo, together with a list of the study numbers that corresponded to each allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical packaging of trial drugs. Women, clinical staff and investigators were all described as blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above ‐ blinding of all involved in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low recruitment to pilot study. All 16 women randomised were followed up until 6 months after birth. No attrition. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Gates 2004b.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial ('trial 2') | |
| Participants | 141 women were randomised. Setting: 23 hospitals in the UK (women were recruited in only 8 hospitals). Study dates: November 1998 to June 2000. Inclusion criteria: women undergoing caesarean section where there was clinical uncertainty that thromboprophylaxis was indicated. Exclusion criteria: women with a known allergy to heparin. |
|
| Interventions |
Group 1 (n = 70): once‐daily self‐injected subcutaneous 40 mg enoxaparin (LMWH) in 1 mL. Group 2 (n = 71): once‐daily self‐injected subcutaneous placebo (normal saline 1 mL). Treatment with the study drug began within 12 hours of the caesarean section, and its duration was determined by the attending clinician. All trial drugs were packaged identically in packs that contained 14 prefilled syringes. The drug was given by once‐daily subcutaneous injection, from study entry for a maximum of 14 days. All other clinical treatment, including other forms of thromboprophylaxis during or after the caesarean section (such as stockings or inflatable boots), was left to the discretion of the responsible clinician. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; blood transfusion; bleeding episodes (reports "bleeding/bruising reported at discharge"); serious wound complications (reports wound infections); adverse events sufficient to stop treatment (reports "allergic reactions that were sufficient to stop treatment"). Other outcomes reported: main process outcome for this pilot was the number of women recruited; also reports hospital admissions not for thromboembolic disease; in methods indicates that duration of hospital stay, and number of surgical procedures in 6 months after birth to be secondary outcomes of interest, however no data reported for these outcomes. Data collection at hospital discharge following birth and at 6 months postpartum. |
|
| Notes | Pilot study. Funding sources: quote: "both trials were supported by a donation to the National Perinatal Epidemiology Unit by Rhone‐Poulenc Rorer." "Study drugs: Rhoˆ ne‐Poulenc Rorer/Aventis Pharma Ltd, Kent, UK." Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | External randomisation, with a "a pre‐randomized sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "The packs that were supplied to participating hospitals were numbered in a prerandomized sequence. Hospitals were instructed to use the packs in numeric order, which automatically would ensure random allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants, care givers, and investigators were blind to the allocation. An identical placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition < 5%. 141 women randomised, data at discharge for 140, and at 6 months, follow‐up for 132. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Gibson 1998.
| Study characteristics | ||
| Methods | RCT (3 arms). | |
| Participants | 17 women were randomised. Setting: authors from Glasgow, UK. Study dates: not reported. Inclusion criteria: women undergoing a caesarean section; either an emergency caesarean section or with other risk factors for VTE. Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 6): LMWH (enoxaparin) 20 mg once daily. Group 2 (n = 5): LMWH (enoxaparin) 40 mg once daily. Group 3 (n = 6): UFH 7500 IU every 12 hours. Intervention started after caesarean section, duration of intervention not stated. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes (reports "haemorrhagic event"). Other outcomes reported: anti‐Xa activity. |
|
| Notes | 3‐way randomisation (UFH/20 mg enoxaparin/40 mg enoxaparin). 2 enoxaparin groups combined for inclusion in the review analysis. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "women were randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up stated; no detail regarding exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting; furthermore data for many relevant clinical outcomes was not reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
Hamersley 1998.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | 61 women were randomised. Setting: authors from the USA. Study dates: not reported. Inclusion criteria: pregnant women with an underlying diagnosis of either antiphospholipid syndrome, protein S or C deficiency, or idiopathic thrombophilia. Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 32): LMWH Group 2 (n = 29): UFH. For all women, the dose was adjusted to maintain an anti‐Xa (heparin assay) level between 0.03 to 0.05 U/mL. A daily aspirin (81 mg) was also prescribed. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; thrombocytopenia. Other outcomes reported: epidural related complications; physician estimates of blood loss, post‐birth haematocrit. |
|
| Notes | Published as an abstract only; no further information describing intervention (including when in the antepartum period the intervention commenced) provided; authors contacted, with no response. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "patients...were randomized...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote "patients...were randomized...”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unfeasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not detailed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
Heilmann 1991.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 207 women were randomised. Setting: authors from Germany (setting not further specified). Study dates: not stated. Inclusion criteria: women undergoing caesarean section. Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 103): HES 6%, 3 x 500 mL; first 500 mL during the caesarean section (the first 500 mL), second in the evening of the day of the caesarean, third in the evening of the first postoperative day. Group 2 (n = 104): UFH 5000 IU 2 hours after the operation and every 8 hours for 7 days. The treatment was given by injection, either in the outer thigh or upper arm. |
|
| Outcomes |
Review outcomes reported: asymptomatic thromboembolic events (DVT); blood transfusion; bleeding episodes; serious wound complications. Other outcomes reported: a number of laboratory measurements were also taken (relating to blood clotting factors). |
|
| Notes | Information obtained from a translation of the manuscript. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised but no further detail on generation of the randomisation sequence provided. |
| Allocation concealment (selection bias) | Unclear risk | The women were divided into 2 groups (no further detail). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
Heilmann 2007.
| Study characteristics | ||
| Methods | RCT (3 arms, 1 not randomised). | |
| Participants | 100 women were randomised. Setting: authors from Germany (setting not further specified). Study dates: not reported. Inclusion criteria: women with uncomplicated pregnancy; following elective caesarean section (for breech presentation or maternal/fetal indications at birth). Quote: "The indication for prophylaxis was the previous diagnosis of a heterozygote factor V‐Leiden‐mutation.” Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 50): LMWH (dalteparin 5000 IU/daily for 7 days post operatively, with the first dose 6 hours following caesarean section and then at 24‐hour intervals). Group 2 (n = 50): UFH (calciparin 2 x 5000 IU daily, with the first dose 6 hours following caesarean section, and then at 8‐hour intervals). Comparison (n = 50, not randomly assigned) Received no pharmacological prophylaxis but compressions stockings according to the guidelines of RCOG (Controls) during hospital days. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes (reports "bleeding complications (blood loss > 500 mL or reoperation)"); adverse effects sufficient to stop treatment (reports "allergy"); thrombocytopenia. Other outcomes reported: osteopenia; a number of outcomes relating to the rheological properties of blood were also assessed. |
|
| Notes | 50 additional matched controls were assessed in the manuscript. We included data for the 2 treatment groups only in this review. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were allocated to the treatment group by randomization". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned, considered unfeasible in view of the interventions being assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up apparent. |
| Selective reporting (reporting bias) | High risk | Clinical outcome data were incompletely reported, with statements such as "The clinical outcome showed no differences in the blood loss for the different prophylaxis groups and thrombocytopenia or Osteopenia.". |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
Heller 2016.
| Study characteristics | ||
| Methods | RCT: NCT01793194 | |
| Participants | 44 women were randomised. Setting: authors were from the USA (no further details) Study dates: not reported. Inclusion criteria: pregnant women; quote: "Due to the small pilot sample size, ethnicity was limited to Caucasians and African Americans" (unclear if this was an inclusion criterion). Protocol suggests that women with and without varicose veins were included and women age 18‐45 years were eligible. Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 21): compression stockings (20 to 30 mm Hg maternity pantyhose), protocol suggests that women were instructed to wear the stockings daily. Group 2 (n = 23): no compression stockings. All women: timing of interventions unclear, however women were visited 3 times (between 8 to 20 weeks and 32 ± 4 weeks before the birth, and 8 weeks postpartum ± 2 weeks). |
|
| Outcomes |
Review outcomes reported: symptomatic DVT; adverse effects no sufficient to stop treatment (thrombophlebitis). Other outcomes reported: VCSS (Venous Clinical Severity Score); stocking adherence; venous oedema; SF‐36 measures (including pain component); thrombophlebitis; superficial axial venin reflux. |
|
| Notes | Information extracted from conference abstract. Funding sources: not reported. Declarations of interest: Quote: " J. Heller: Consultant/advisory board for BMS/Pfizer, and research grant, principal investigator, collaborator, and consultant for Sigvaris; J. Canner: Nothing to disclose; Y. W. Lum: Nothing to disclose; K. Tsuchiya: Nothing to disclose." DVT not clearly reported as symptomatic |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were entered in a consecutive randomized fashion." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to determine. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to determine. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to determine; though abstract suggests no losses: quote: "A total of 44 patients enrolled and completed the study". |
| Selective reporting (reporting bias) | Unclear risk | Abstract reporting the study describes results of the study, however provides no data. |
| Other bias | Unclear risk | Insufficient information to determine. |
Hill 1988.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 50 women were randomised. Setting: authors from the UK (not further specified). Study dates: not reported. Inclusion criteria: women undergoing elective caesarean section. Exclusion criteria: placenta praevia, multiple pregnancy, pregnancy‐induced hypertension, APH, a history off thromboembolic disease, coagulation disorders or peptic ulceration. |
|
| Interventions |
Group 1 (n = 25): UFH 1000 units, 1 hour before caesarean, then twice daily for 5 days. Group 2 (n = 25): saline, 1 hour before caesarean, then twice daily for 5 days. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic DVT; symptomatic PE; blood transfusion; serious wound complications; adverse effects not sufficient to stop treatment (reports injection "discomfort"). Other outcomes reported: blood loss; Hb values; abnormalities of plasma clotting factors; defects of platelet function. |
|
| Notes | Funding sources: Quote: "We acknowledge the support of Leo Laboratories and the South East Thames Regional Health Authority (grant LORS No 77/19)." Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | Randomisation by pharmacist not involved in trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | While saline was administered to the control group by the same regimen, no information was provided on how blinding was attempted, and it is unlikely considering the interventions assessed that all personnel and participants were blind to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assessed confidently as high or low risk without access to a trial protocol. |
| Other bias | Unclear risk | Insufficient information to assess. |
Howell 1983.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 40 women were randomised. Setting: 1 centre in UK. Study dates: not reported. Inclusion criteria: women with a history of thromboembolism treated with anticoagulants for ≥ 6 weeks. Recruitment at time of referral to clinic (8 to 37 weeks' gestational age). Exclusion criteria: not reported. |
|
| Interventions |
Group 1 (n = 20): subcutaneous calcium heparin antenatally throughout pregnancy (10,000 IU twice daily, started after the first routine antenatal booking) and for 6 weeks postpartum (8000 IU twice daily). Group 2 (n = 20): calcium heparin for 6 weeks postpartum only. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; bleeding episodes (antenatal vaginal bleeding; postpartum haemorrhage); symptomatic osteopenia (reports "severe debilitating bone demineralization"); fetal loss (reports "complete abortion"). Other outcomes reported: "other fetal or neonatal losses"; threatened abortion; blood loss after birth; preterm labour; admission to special care baby unit and indications; birthweight; gestational age; placental weight. |
|
| Notes | Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "The allocation was randomized". |
| Allocation concealment (selection bias) | Unclear risk | Described as "by opening sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants refused any treatment either antenatally or postnatally once the trial had been explained to them. They were not included in the overall analysis, but none developed thromboembolism either before or after birth. 1 participant initially allocated to the control group developed a DVT at 28 weeks and was subsequently treated by intravenous, followed by subcutaneous, heparin. She was omitted from any further analyses. Data could be re‐included for the review. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
Krauss 1994.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 100 women were randomised. Setting: University Hospital, Gottinghen, Germany. Study dates: not reported. Inclusion criteria: women undergoing caesarean section. Exclusion criteria: known heparin allergy, GI ulcers, severe kidney, liver or pancreatic disease or previous cerebral haemorrhage, severe hypertension (BP > 180/120), haemorrhagic diathesis. |
|
| Interventions |
Group 1 (n = 50): LMWH (fragmin) once daily 2500 to 5000 anti‐Xa units. Group 2 (n = 50): 2 to 3 times daily 5000 units UFH (Liquemin) + 500 mL Dextran 60 during caesarean section. Treatment for 10 days after surgery. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes (reports "post surgical haemorrhage"); thrombocytopenia. Other outcomes reported: coagulation parameters (including anti‐Xa activity; anti‐thrombin III; partial thromboplastin time; thrombin time); haematological parameters and liver enzymes. |
|
| Notes | Data extraction from translation notes. Original paper in German. An additional 30 women undergoing tocolysis were randomised to the intervention and comparison groups; data regarding adverse effects (irritation at site of injection; lasting pain at site of injection; secondary bleeding at site of injection; headaches; minor dizziness) were reported for 75 women in the intervention group and 75 women in the comparison group, and thus we could not include these data. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned; considered unfeasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
O'Riordan 2008.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 20 women were randomised. Setting: authors from Ireland. Dates of the study: not reported. Inclusion criteria: women following caesarean section. Exclusion criteria: none detailed. |
|
| Interventions |
Group 1: enoxaparin 40 mg once daily subcutaneously. Group 2: tinzaparin 4500 units once daily subcutaneously. All women: the first dose of LMWH was administered 4 to 6 hours following the caesarean section. |
|
| Outcomes |
Review outcomes reported: none. Other outcomes reported: venous blood samples were taken for: APTT, Factor Xa, Factor II, vWF, platelet count, volume and granularity. |
|
| Notes | Published as abstract only. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "The patients were randomised”. |
| Allocation concealment (selection bias) | Unclear risk | Quote "The patients were randomised”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No detail provided; considered unfeasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to determine risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | "There was no significant difference in characteristics (including BMI) between the two groups.” Insufficient information to assess other potential sources of bias. |
Pettila 1999.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 107 women were randomised. Setting: 8 centres in Finland. Study dates: February 1994 to February 1997. Inclusion criteria: 18 years or older, week 0 to 19 of gestation, any of: (a) previous PE or VTE above knee before current pregnancy; (b) PE or VTE during current pregnancy; (c) previous VTE below knee in association with protein C or protein S deficiency, activated protein C resistance, pregnancy or contraceptive pills. Exclusion criteria: any of the following: surgical procedure within 1 week, surgical procedure in central nervous system, eye or ear within 1 month, intracerebral bleeding within 1 year, platelet count < 100 X 10E9/L twice, hypertension over 150/100 mmHg, S‐creatinine over 155 μmol/L, liver disease, septic endocarditis, known hypersensitivity to heparin, known positive HIV or hepatitis B or C, unsuitable for venous sampling, antithrombin deficiency, artifical hear value, placenta praevia or ablation detected, participation in another study, weight < 45 kg before pregnancy, use of acetylsalicylic acid of any strength. |
|
| Interventions |
Group 1 (n = 51): subcutaneous LMWH dalteparin (fragmin) once daily (starting dose 5000 IU (women weighing < 85 kg) or 7500 IU (women weighing ≥ 85 kg), dose adjusted based on anti‐Xa measurements). During birth, 2500 IU dalteparin was administered 18 hours after the previous dose if the woman had not yet delivered; if she delivered within 18 hours, 5000 IU was given, 24 hours after the previous injection. The daily dose postpartum was 2500 IU lower than during the third trimester; and 2 weeks after birth, if anti‐Xa was < 0.20, the dose was increased by 2500 IU. Group 2 (n = 56): subcutaneous UFH (7500 IU, adjusted according to APTT target values) twice daily. At the time of birth, and on the first day postpartum 7500 IU UFH was given at 12 hour intervals, and then according to APTT target values. All women: treatment started before week 20 of gestation and continued for 6 weeks after birth. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; blood transfusion; bleeding episodes (reports injection‐site haemato mas, bleeding during birth, other bleeding); adverse events sufficient to stop treatment; symptomatic osteoporosis; fetal loss (reports spontaneous abortion); thrombocytopenia. Other outcomes reported: lumbosacral compression fractures, fetal outcomes, laboratory values (Hb, platelet count, serum creatinine, serum alanine aminotransferase); birth bleeding (mL); caesarean section; birthweight; Apgar score. |
|
| Notes | Funding sources: supported by research funds from Helsinki University Central Hospital and a grant from Pharmacia and Upjohn. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation quote: "by means of a computer generated procedure". |
| Allocation concealment (selection bias) | Low risk | Quote: "Immediately after the inclusion and exclusion criteria were met and informed consent was obtained, a closed envelope was opened"; the randomisation list was kept outside the centres. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open design (not feasible). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 randomised participants (1 from each group) received no prophylactic treatment before discontinuation of the study because of withdrawal of consent and were excluded from the analysis. Thus 105 participants (50 from the dalteparin and 55 from the heparin group) were included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Reddick 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 50 women were randomised. Setting: Duke Obstetrics outpatient clinics or the Duke University Hospital Birthing Center, USA. Study dates: April 2009 to March 2010. Inclusion criteria: scheduled (non‐labour) caesarean at term (≥ 37 weeks' gestation); no history of thrombophilia; no history of or current venous or arterial thrombosis; aged ≥ 18 years; BMI 18.5 to 35; English literate. Exclusion criteria: hypertension (chronic and pregnancy related); diabetes (insulin‐dependent, type II and gestational); maternal cardiac disease (valvular heart disease, congenital heart disease, cardiomyopathy); substance use (including tobacco, alcohol, and illicit substances); family history of thrombophilia; multiple gestation; sickle cell disease; and lupus/connective tissue disorder. |
|
| Interventions |
Group 1 (n = 25): intermittent pneumatic compression during caesarean birth. Women had compression devices (Aircast Venaflow Calf Cuff—DJO, LLC, Vista, CA) placed on their lower extremities.The intervention started 1 hour before the start of surgery and continued or ≥ 30 minutes following completion of the procedure. Group 2 (n = 25): women received no compression treatment during the surgery and were placed on bed rest beginning 1 hour before the start of surgery. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; blood transfusion; bleeding: estimated blood loss at birth (mL). Other outcomes reported: gestational age; birthweight; 5‐minute Apgar score; operative time; estimated blood loss; use of regional anaesthesia; serum markers of fibrinolysis (tPA, uPA, TAT complex, PAI‐1, PAI‐2). |
|
| Notes | Funding sources: Quote: "This study was funded by Charles B. Hammond Research Fund, Duke University School of Medicine, Durham, NC, and DJO, LLC, Vista, CA." "The authors thank DJO, LLC (Vista, CA) for providing equipment to complete the study." Declarations of interest: "None of the authors reports conflict." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization sequence." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50 women randomised, 25 to each group; 1 woman randomised to intervention group withdrew, and thus 24 and 25 women analysed in intervention and control groups, respectively. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Rodger 2014.
| Study characteristics | ||
| Methods | RCT (NCT00967382, ISRCTN87441504) | |
| Participants | 292 women were randomised. Setting: initiated in 36 tertiary care centres in Canada, Australia, USA, UK and France; however conducted in 26 of these centres as 10 centres were unable to implement the trial protocol or get referrals to screen potentially eligible participants. Study dates: 28 February 2000 to 14 September 2012. Inclusion criteria: pregnant, confirmed thrombophilia (fact V Leiden; prothrombin gene mutation, or 2 abnormal tests and no normal tests for protein C deficiency, protein S deficiency, or antithrombin deficiency; or anti‐phospholipid antibody confirmed by 2 positive tests) and with raised risk of placenta‐mediated pregnancy complications or VTE (previous pre‐eclampsia, previous unexplained SGA infant, previous major placental abruption, previous pregnancy loss, 1 or more of: previous provoked proximal VTE, previous calf vein thrombosis, previous superficial phlebitis, 1st degree relative with history of PE or DVT treatment with anticoagulants). Exclusion criteria: ≥ 21 weeks or more gestational age at the time of randomisation, contraindication to heparin treatment (history of heparin‐induced thrombocytopenia; placental count < 100,000 x 10^6/L; history of osteoporosis or steroid use; actively bleeding; documented peptic ulcer within 6 weeks; heparin, bisulphite or fish allergy; severe hypertension; severe hepatic failure; serum creatinine > 80 umol/L and 24‐hour clearance < 30 mL/minute), geographically inaccessible, needed anticoagulant therapy as judged by the local investigator (women with recurrent pregnancy loss and antiphospholipid antibody syndrome; women with unprovoked proximal VTE whose PE or DVT was treated with anticoagulants (> 1 month heparin or warfarin), or IVC interruption; women with mechanical heart valves, women on long‐term anticoagulants before pregnancy), had already previously participated in the study, or were younger than the legal lower age limit to provide consent according to country‐specific regulations. |
|
| Interventions |
Group 1 (n = 148): LMWH: dalteparin 5000 IU once daily by subcutaneous self‐injection from the day of randomisation until 20 weeks of gestation, followed by 5000 IU twice daily from 20 weeks until at least 37 weeks gestational age. Group 2 (n = 144): placebo: during the initial 26 months of the study, the control group received matching, identically supplied and formulated placebo in prefilled syringes. Owing to poor recruitment (only 19 in 26 months), on June 25, 2002, the trial steering committee changed the study design to an open‐label trial comparing antepartum open‐label dalteparin with no antepartum dalteparin control. All participants: postpartum dalteparin (5000 IU once daily, by subcutaneous self‐injection); day 1 (started 6–28 hours after birth), to 42. |
|
| Outcomes |
Review outcomes reported: symptomatic VTE; bleeding episodes (minor peripartum haemorrhage, major peripartum haemorrhage, major bleeding, minor bleeding, estimated blood loss at birth, placental abruption); symptomatic osteoporosis (including osteoporotic fracture, and bone mineral density measured at 6 weeks postpartum); adverse events not sufficient to stop treatment (including left parieto‐occipital transient Ischaemic attack at 27 weeks with non thrombocytopenia, severe allergic reaction defined as lip/tongue swelling after first dose of postpartum dalteparin, allergic type skin reaction noted antepartum, allergic type skin reaction noted postpartum, raised levels of liver enzymes defined as 2 times normal values of aspartate aminotransferase or alanine transaminase); neonatal death (in infant born prematurely); thrombocytopenia; fetal loss < 20 weeks (pregnancy loss any); fetal anomalies. Other outcomes reported: composite outcome that included any of the following events: objectively documented symptomatic major VTE (DVT proximal to the calf trifurcation, PE, or sudden maternal death); severe early onset (< 32 weeks) pre‐eclampsia; birth of SGA infant; preterm birth; and symptomatic fracture. |
|
| Notes | Funding sources: Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada. Drugs supplied by Pharmacia and Upjohn. Declarations of interest: 1 author (Clement, AM) reported receiving honoraria for educational activities from Leo Pharma, Sanofi, and Bayer. The other authors declared no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer‐generated randomisation schedule was stratified by country and gestational age at randomization day (<8 weeks, 8‐12 weeks, and 12‐20 weeks) and had a permuted block design (block sizes 4 and 8)". |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation system used to conceal allocation. At the time of the assignment, site pharmacists or other delegates received a randomisation number and treatment allocation from the central web randomisation system (by fax and/or email). The site pharmacist or delegate then dispenses the study drug to the local coordinator who taught the study participant how to administer the assigned treatment and explained the trial procedures to be followed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and study personnel were not masked but outcome adjudicators were masked to treatment assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All suspected primary and secondary outcome events were adjudicated independently and blindly by at least two physicians in a panel that included experts in obstetrics and thrombosis". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/148 and 1/144 women assigned to the intervention and control groups respectively were excluded from analyses due to ineligibility. Therefore 146 women in the intervention group, and 143 women in the control group were included in 'on treatment' analysis, which was reported for the safety outcomes. 26 women crossed over within 10 days of randomisation: 12 from dalteparin to no dalteparin, and 14 from no dalteparin to dalteparin. |
| Selective reporting (reporting bias) | Unclear risk | Multiple trial registrations, including study web site. |
| Other bias | Low risk | Aspirin was used slightly more by women in the control group than women in the intervention group, and analgesic was used slightly more frequently by women in the intervention group than in the control group during the study. However, data presented show that neither of these differences were statistically significant. |
Rodger 2015.
| Study characteristics | ||
| Methods | RCT: NCT01274637 | |
| Participants | 25 women were randomised. Setting: 6 centres in Canada and the USA. Study dates: May 2011 to March 2012. Inclusion criteria: women at higher VTE risk due to known low risk thrombophilia (heterozygous factor V Leiden or prothrombin gene variant or protein C deficiency or protein S deficiency) or immobilisation (90% of waking hours in bed, of a week or more at any point in the antepartum period); or with any 2 of the following: postpartum infection; postpartum haemorrhage (> 1000 mL); pre‐pregnancy BMI > 25 kg/m², emergency caesarean birth; smoking > 5 cigarettes per day prior to pregnancy; pre‐eclampsia; IUGR. Exclusion criteria: < 6 hours or > 36 hours since birth; need for anticoagulation; LMWH started antenatally, contraindication to heparin, received a dose of heparin or LMWH since birth; below the age of legal majority; prior trial participation; no informed consent. |
|
| Interventions |
Group 1 (n = 14): LMWH. Women received daily prophylactic dalteparin (5000 IU), by subcutaneous injection for 3 weeks following birth, starting approximately 36 hours after delivery of the placenta. Group 2 (n = 11): placebo. Women received daily placebo, by subcutaneous injections, for 3 weeks following birth, starting approximately 36 hours after delivery of the placenta. |
|
| Outcomes | Review outcomes reported: maternal death; symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; asymptomatic thromboembolic events (detected by screening); bleeding episodes (reports major bleeding events); adverse effects not sufficient to stop treatment (reports unexpected serious adverse events related to the interventions); thrombocytopenia. | |
| Notes | Funding sources: Quote: "National Institutes of Health Research grant # NIH 1R34HL107725–01 and Canadian Institutes of Health Research grant #MOP 106641. Dr. Rodger is supported by a Heart and Stroke Foundation Career Investigator Award and a University of Ottawa, Faculty of Medicine Chair in Venous Thrombosis and Thrombophilia. Dr. Kahn is supported by a National Research Scholar award from the Fonds de recherche santé Québec. The funders played no role in the design, conduct, analysis or interpretation for the pilot trial." Declarations of interest: "None declared." Pilot trial to determine feasibility. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was in permuted blocks of eight, prepared by the trial statistician using random number tables and stratified by centre." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible consenting women were randomised via central web randomisation by a study coordinator within 36 h after delivery of the placenta." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "They were equally and blindly allocated to the treatment group (prophylactic‐dose dalteparin 5,000 IU) or matching placebo saline, administered subcutaneously once daily for 21 days;" and see quote below. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome adjudicators were blinded to the treatment allocation, as were the participants, care providers and all trial personnel." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/14 woman in intervention group excluded from analyses; all 11 women in placebo group included in analyses. |
| Selective reporting (reporting bias) | Unclear risk | While trial registration available, limited detail, and no full protocol available. Results for clinical outcomes reported incompletely in text. |
| Other bias | Unclear risk | No data provided on key characteristics of the 2 groups of women compared, and therefore it is not possible to confidently assess other bias. |
Rodger 2016.
| Study characteristics | ||
| Methods | RCT: NCT01274637 | |
| Participants | 37 women were randomised. Setting: 6 Canadian teaching hospitals and 2 teaching hospitals in the USA. Study dates: November 2012 to November 2013 (follow‐up to February 2014). Inclusion criteria: women at higher VTE risk due to known low‐risk thrombophilia or immobilisation; or with any 2 of the following: postpartum infection; postpartum haemorrhage; pre‐pregnancy BMI > 25 kg/m²; emergency caesarean birth; smoking > 5 cigarettes per day prior to pregnancy; pre‐eclampsia; infant birthweight < 3rd percentile. Exclusion criteria: women < 6 hours or > 36 hours since birth; need for anticoagulation; contraindication to heparin; received > 1 dose of heparin or LMWH since birth; below the age of legal majority; prior trial participation; no informed consent. |
|
| Interventions |
Group 1 (n = 16):LMWH. Women received daily prophylactic dalteparin 5000 IU subcutaneous injections for 10 days following birth, starting 36 hours after delivery of the placenta. Group 2 (n = 21): no treatment. Women received no treatment for 10 days following birth, starting 36 hours after delivery of the placenta. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; asymptomatic thromboembolic events; bleeding episodes (reports: major bleeding event (> 4 g/dL drop in Hb with excessive vaginal blood loss in early postpartum period); reports 'clinically relevant bleeding events'; reports 'minor bleeding events'); serious wound complications (serious adverse event ‐ wound dehiscence with wound haematoma). Other outcomes reported: number of participants randomised per centre per month; other indicators of feasibility (proportion of referred participants who meet eligibility criteria (> 20%); proportion of eligible participants who provide consent (> 30%); withdrawals/losses to follow‐up (< 10%); and level of compliance with study drug (> 60%). |
|
| Notes | Funding sources: Quote: "National Institutes of Health research grant # NIH 1R34HL107725‐01 and Canadian Institutes of Health Research grant # MOP 106641. Dr. Rodger is supported by a Heart and Stroke Foundation Career Investigator Award and a University of Ottawa, Faculty of Medicine Chair in Venous Thrombosis and Thrombophilia. Dr. Kahn is supported by a National Research Scholar award from the Fonds de recherche santé Québec. The funders played no role in the design, conduct, analysis or interpretation for the pilot trial." Declarations of interest: not reported. Pilot trial to determine feasibility. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was in permuted blocks of eight, prepared by the trial statistician using random number tables and stratified by centre." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible consenting women were randomized via central web randomization by a study coordinator within 36 h after delivery of the placenta." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial with a no treatment arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome adjudicators were blinded to the treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 16 women in intervention group, all analysed; of 21 women in intervention group, 19 analysed (1 withdrew and 1 lost to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | While trial registration available, limited detail, and no full protocol available. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Salim 2016.
| Study characteristics | ||
| Methods | RCT: NCT01068795 | |
| Participants | 144 women were randomised. Setting: 1 university teaching hospital and 3 specialised community clinics in Israel. Study dates: October 2009 to January 2015. Inclusion criteria: singleton pregnancies at ≤ 14 weeks' gestation, prior placenta‐mediated pregnancy complications or a lower leg thrombotic event (documental calf vein thrombosis), and testing positive for any thrombophilia (homozygous or heterozygous for prothrombin‐20210A or factor V Leiden mutations; homozygous for the mutation of cytosine to thymine at nucleotide 677 in the gene encoding methylenetetrahydrofolate reductase accompanied with homocysteine elevated serum levels; and tested positive for anti‐thrombin III, protein C and S deficiencies, lupus anticoagulant, anti‐cardiolipin IgG and/or IgM and anti‐β2 glycoprotein IgG and/or IgM) (prior placenta‐mediated pregnancy complications included: prior severe pre‐eclampsia; prior birth of SGA infant; with placenta related antepartum signs; prior placental abruption; or prior unexplained pregnancy loss (3 losses < 13 weeks, 2 losses 14‐22 weeks, any loss > 23 weeks)). Women were categorised as having low‐risk thrombophilia (factor V Leiden heterozygous; prothrombin gene mutation heterozygous; protein C deficiency; protein S deficiency; anti‐phospholipid antibody) or high‐risk thrombophilia (antithrombin III deficiency; homozygous for factor V Leiden; homozygous for prothrombin mutation; combined thrombophilias). Exclusion criteria: women who had previous pregnancy complications that could be attributed to multiple gestations; having fetuses with major congenital anomalies or chromosomal abnormalities, fetal infection, or hydrops fetalis; women with pre‐gestational diabetes; or women who required therapeutic dosage of LMWH or had a contraindication to LMWH therapy. |
|
| Interventions |
Group 1 (n = 74): LMWH adjusted dose: starting around 14 weeks GA, adjusted dose of antepartum enoxaparin according to the results of anti‐FXa levels until birth. The initial dose was 40 mg and increased by fractions of 20 mg, according to anti‐FXa level. The targeted prophylactic level was 0.2 IU/mL or more 3.5 to 4 hours post‐injection. Quote: "All women had levels of anti‐FXa examined approximately every 8–10 weeks… A blood sample was taken from all women 3.5‐4 h after the injection of enoxaparin at the central laboratory. Subsequently, the results were computerised and enoxaparin dose was then adjusted in the next visit among women in the adjusted‐dose group. Women attended follow‐up visits every 3–4 weeks." Group 2 (n = 70): LMWH fixed dose. starting around 14 weeks GA, maintained fixed dose of 40 mg enoxaparin, once daily by subcutaneous self‐injection, until birth regardless of the results of anti‐factor Xa. Women in both groups with antiphospholipid antibodies were given low dose aspirin in addition to enoxaparin. Postpartum, all women were prescribed enoxaparin 40 mg once daily by subcutaneous injection from day 1 until day 42. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes (reports placental abruption; postpartum haemorrhage; and side effects ‐ bleeding); adverse effects sufficient to stop treatment; adverse effects not sufficient to stop treatment (reports skin allergy); fetal loss (reports spontaneous abortion, assumed to be < 23 weeks; and reports intrauterine fetal death > 23 weeks); thrombocytopenia. Other outcomes reported: composite outcome (1 or more of the following events: any pregnancy loss, pre‐eclampsia, SGA, placental abruption, and VTE); components of the composite outcome; gestational age at birth; preterm birth; mode of birth; birthweight; Apgar score < 7 at 5 minutes; cord pH < 7,1; and anti‐FXa levels (and < 0.2 IU/mL); maternal placental vascular lesions; fetal placental vascular lesions. |
|
| Notes | Funding sources: not reported. Declarations of interest: "None declared." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed using a computer randomisation sequence generation program." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation sequence results were kept in the delivery ward in a closed study box. The site investigator enrolled participants after confirming eligibility. The sequence was concealed until intervention was assigned (and after obtaining a signed informed consent)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Women and providers were not masked to the treatment arm." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | For secondary analyses: "Placentas were examined by the same pathologist who was blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 74 women in intervention group, all included in intention to treat analyses; of 70 women in control group 4 excluded from intention to treat analyses (2 lost to follow‐up, and 2 discontinued intervention – 1 due to side effects, and 1 due to ineligibility). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Segal 1975.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 210 women were randomised. Setting: Jerusalem, Israel. Study dates: 1973. Inclusion criteria: women identified with varicose veins before birth. Exclusion criteria: a history of thrombosis, and thus treatment with heparin. |
|
| Interventions |
Group 1 (n = 116): UFH 50 mg (5000 IU) subcutaneous UFH every 12 hours for 4 to 5 days after birth (time of initial dose varied, for those having a vaginal birth about two‐thirds had the first dose in active labour (2 to 3 cm) and a third after giving birth; for women having a caesarean section UFH was given 2 hours before the caesarean, at the end of the caesarean, and at 12‐hour intervals; for women having an emergency caesarean, the initial dose was immediately following the decision). Group 2 (n = 94): care in the comparison group was not described, there did not seem to be a placebo (routine care/no heparin). |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT. Other outcomes reported: superficial venous thrombosis. |
|
| Notes | Very little information on methods was provided. There appeared to be a baseline imbalance between groups with 16/94 in the control group having a caesarean section versus 6/116 in the intervention group. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote, "divided at random”. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. No placebo use reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear. There did not seem to be any placebo, but it was stated that the outcome assessors were blind to group allocation, quote "the daily clinical evaluation for signs of deep or superficial thrombosis was done by two of us without knowing the mentioned distribution". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women followed up. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | There seemed to be some baseline imbalance between groups with 16/94 in the control group having a caesarean section versus 6/116 in the intervention group. Insufficient information to assess other potential sources of bias. |
Stephenson 2016.
| Study characteristics | ||
| Methods | RCT: NCT02070237 | |
| Participants | 90 women were randomised. Setting: Memorial Care Center for Women at Miller Children’s Hospital, Long Beach Memorial Medical Center, Long Beach, California, USA. Study dates: August 2013 to February 2014. Inclusion criteria: BMI ≥ 35 at admission for birth, undergone a caesarean section (elective or performed during labour) within the previous 12 hours, and scheduled to receive enoxaparin thromboprophylaxis. Exclusion criteria: previous VTE, already receiving anticoagulation of any type (including LMWH or UFH), allergy to enoxaparin, renal impairment (creatinine > 1.2), or contraindications to treatment with enoxaparin, such as active bleeding or thrombocytopenia (platelets < 150). |
|
| Interventions |
Group 1 (n = 45): weight‐based LMWH: twice daily dose of 0.5 mg/kg enoxaparin every 12 hours (dose not capped and rounded to the nearest 5 mg unit), by subcutaneous injection (in abdomen). Group 2 (n = 45): fixed‐dose LMWH: once daily dose of 40 mg, by subcutaneous injection (in abdomen). All women: enoxaparin was started between 8 and 12 hours after the caesarean, and continued until hospital discharge at which time it was discontinued. A peak anti‐Xa activity level was drawn 3.5 to 4 hours after the third dose of each regimen.The result was not available to the care team. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; blood transfusion (reports bleeding events requiring transfusion); bleeding episodes (reports bleeding events requiring reoperation); serious wound complications (reports caesarean wound dehiscence or reoperation; caesarean wound infection; caesarean wound haematoma); adverse events not sufficient to stop treatment (reports heparin‐induced skin necrosis). Other outcomes reported: achievement of the desired prophylactic anti‐Xa level of 0.2 to 0.6 IU/,L; mean anti‐Xa levels; highest peak anti‐Xa levels; anti‐Xa levels reaching therapeutic or supra prophylactic levels of 0.6 to 1.0 IU/mL; |
|
| Notes | Funding sources: "funded by a grant from the Long Beach Memorial Medical Center Foundation." Declarations of interest: "The authors declare no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomized… using computer‐generated block sizes of 20." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Assumed that blinding not possible due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 women randomised, 45 to each group. 3 women withdrew from each group, thus 42 were analysed in each group. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | No other obvious risk of bias identified. |
van Hoorn 2016.
| Study characteristics | ||
| Methods | RCT: ISRCTN87325378 | |
| Participants | 32 women were randomised. Setting: 13 hospital centres in (5 university and 6 non‐university/teaching hospitals in the Netherlands, 2 hospitals in Australia, and 1 hospital in Sweden. Study dates: December 2000 to December 2009. Inclusion criteria: < 12 weeks' gestation, aged ≥ 18 years, history of uteroplacental insufficiency and birth < 34 weeks' gestation (hypertensive disorders of pregnancy (including pre‐eclampsia, HELLP syndrome, eclampsia) and/or SGA infant), and antiphospholipid antibodies (anticardiolipin antibodies present and/or lupus anticoagulant present) [women without antiphospholipid antibodies were included in de Vries 2012) Exclusion criteria: women with 1 or more antithrombin deficiency, homozygosity for factor V Leiden and prothrombin G20210A mutations, diabetes mellitus, known malignancy, known peptic ulceration, severe renal or hepatic insufficiency, history of VTE, haemorrhagic diathesis, idiopathic thrombocytopenia, earlier participation in the FRUIT trial, or LMWH use in earlier pregnancy. |
|
| Interventions |
Group 1 (n = 16): LMWH and aspirin: once‐daily dalteparin (5000 IU, by subcutaneous self‐administration), starting at 6‐12 weeks' gestation until the onset of labour or prior to the caesarean section, plus once‐daily aspirin (80 mg, oral), stating at < 12 weeks and continued to 36 weeks' gestation. The daily dose of dalteparin was adjusted for body weight: women < 50 kg received dalteparin 2500 IU, those > 80 kg 7500 IU. Dalteparin injections started at 6 to 12 weeks' gestation, and stopped at the onset of labour or prior to caesarean section. Aspirin was commenced at < 12 weeks' gestation and stopped at 36 weeks' gestation (women in Australia were treated with aspirin 100 mg daily as the standard dose). In women who developed local skin reactions, treatment with dalteparin was altered to enoxaparin and, if the reaction was persistent, to nadroparin. Group 2 (n = 16): aspirin alone: daily aspirin (80 mg, orally), commencing at < 12 weeks' gestation, until 36 weeks' gestation (women in Australia were treated with aspirin 100 mg daily as the standard dose). All women: educated on self‐injection, and after birth weight‐adjusted dalteparin (for 6 weeks) was prescribed. |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes (reports placental abruption); adverse effects not sufficient to stop treatment (reports skin reactions, pain, itching, swelling, allergy; reports need for an alternate LMWH; reports haematoma); fetal loss (reports spontaneous miscarriage < 16 weeks; reports fetal death > 16 weeks (miscarriage excluded)); thrombocytopenia. Other outcomes reported: hypertensive disorders of pregnancy (pre‐eclampsia and/or eclampsia and/or HELLP syndrome) onset before 34 weeks' gestation and irrespective of gestational age; SGA; miscarriage; preterm birth; length of maternal and neonatal admission; pre‐eclampsia; eclampsia; HELLP syndrome; termination of pregnancy; gestational age at miscarriage; gestational age at birth; birthweight. |
|
| Notes | Funding sources: Quote: "The study was supported by a single 2‐year investigator grant period 2000–2001 by Pfizer, formerly Pharmacia, grant number 524E‐CVD‐9101‐0001, annual Dutch investigators meetings, a single grant to support a midwife to recruit Australian women, and support for a local meeting in Sweden in 2004. Pharmacia was not the sponsor of the study. A follow‐up study of the trial received a 1‐year investigator grant period in 2014 by Pfizer." Declarations of interest: Quote: "All authors have declared that they have no conflicts of interest." "After the completion of recruitment to the inheritable thrombophilia group of the trial, and given the slow inclusion rate of women with acquired thrombophilia, an interim analysis was performed after delivery of 29 women. The Data Monitoring committee advised cessation of recruitment since accrual was slow and the incidence… of early onset HD (3.4%) was far lower than expected (60%), and the decision was taken by the trial management group to halt the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer to select random permuted blocks of four, with stratification for hospital and presence/absence of chronic hypertension." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was carried out by an independent centre." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither study personnel nor participants were blinded to treatment assignment, as placebo injections during pregnancy were at that time not considered to be ethically acceptable." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The adjudication of the study endpoints was not blinded, but was performed by the chief researcher (JdeV) using absolute values of blood pressure, proteinuria and appropriate laboratory tests in the adjudication, after an initial local assessment at each centre." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Welti 1981.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 580 women were randomised Setting: not clear, authors from Switzerland. Study dates: not reported. Inclusion criteria: women undergoing surgery for gynaecological indications (with 580 women undergoing caesarean section (both emergency and elective)). |
|
| Interventions |
Group 1 (n = 272): physiotherapy and twice‐daily subcutaneous 5000 IU heparin (UFH). Group 2 (n = 308): physiotherapy alone (no heparin). |
|
| Outcomes |
Review outcomes reported: symptomatic thromboembolic events; symptomatic PE; symptomatic DVT; bleeding episodes. Other outcomes: unclear (not translated) |
|
| Notes | Data extraction from translation notes and tables in the paper (original paper in French). Limited description of interventionand other (non review) outcomes. No information on timing of the intervention. Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | The study was conducted quote: "selon le principle de la randomisation fermee". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely considering the interventions assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ no detail of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appeared that all women were followed up. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol to confidently assess selective reporting. |
| Other bias | Unclear risk | Insufficient information to assess other potential sources of bias. |
Abbreviations: APH: antepartum haemorrhage; APTT: activated partial thromboplastin time; BMI: body mass index; BP: blood pressure; DVT: deep vein thrombosis; FRUIT: Fractionated heparin in pregnant women with a history of uteroplacental insufficiency and thrombophilia (randomised trial); HELLP: Hemolysis, Elevated Liver enzyme levels, and low platelet levels; GA: gestational age; GI: gastrointestinal; Hb: haemoglobin; HES: hydroxyethyl starch; IgG: immunoglobulin g; IgM: immunoglobulin M; IU: international units; IUGR: intrauterine growth retardation; LMWH: low molecular weight heparin; NICU: neonatal intensive care unit; PAI: plasminogen activator inhibitor; PE: pulmonary embolism; RCT: randomised controlled trial; SGA: small‐for‐gestational age; tPA: tissue plasminogen activator (abbreviate; UFH: unfractionated heparin; VTE: venous thromboembolism; vWF: von Willebrand factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aina 2006 | Trial terminated (principal investigator assigned to a different hospital; difficulty recruiting participants). |
| Alalaf 2015 | Excluded based on study design: sequential, not random, assignment. |
| Badawy 2008 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Blomback 1998 | Excluded based on study design: not a randomised trial. |
| Brenner 2005 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| de Jong 2015 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Dendrinos 2007 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Farquharson 2002 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Giancotti 2012 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Gris 2010 | Excluded based on types of participants: women with abruptio placentae; women at high risk of VTE (e.g. who had a previous DVT or antiphospholipid antibodies) explicitly excluded. |
| Gris 2011 | Excluded based on types of participants: women with pre‐eclampsia; women at high risk of VTE (e.g. who had a previous DVT or antiphospholipid antibodies) explicitly excluded. |
| Guven 2014 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Harenberg 1993 | Excluded based on types of participants: healthy pregnant women; not women at high risk of VTE. |
| Kaandorp 2010 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Kamin 2008 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Kutteh 1996a | Excluded based on study design: not a randomised trial; the first 25 women were allocated to 1 arm, and the next 25 to other arm; and types of participants: women with recurrent miscarriage/pregnancy loss. |
| Kutteh 1996b | Excluded based on study design: allocation was by alternation, not by random assignment: and types of participants: women with recurrent miscarriage/pregnancy loss. |
| Langer 2013 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Laskin 2007 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Milic 2018 | Excluded based on types of participants: women were at low risk of VTE. |
| Noble 2005 | Excluded based on study design: not a randomised trial; and types of participants: women with recurrent miscarriage/pregnancy loss. |
| Pyregov 2012 | Excluded based on study design: not a randomised trial; trial was quasi‐randomised (allocation based on day of the week). |
| Rai 1997 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Ratiu 2009 | Excluded based on study design; not a randomised trial; and types of participants: women with acute stage proximal DVT in pregnancy. |
| Rey 2009 | Excluded based on types of participants: women with a serious adverse event in a previous pregnancy (e.g. miscarriage); women at high risk of VTE (e.g. with known thrombophilia or who had a previous thromboembolic event) were specifically excluded. |
| Rodger 2017 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Samantha 2013 | Excluded based on study design: not a randomised trial. |
| Schleussner 2015 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Stephenson 2004 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Thaler 2004 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Tulppala 1997 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
| Visser 2011 | Excluded based on types of participants: women with recurrent miscarriage/pregnancy loss. |
Abbreviations: DVT: deep venous thrombosis; VTE: venous thromboembolism.
Characteristics of studies awaiting classification [ordered by study ID]
Abdolvand 2019.
| Methods | Randomised controlled trial. |
| Participants | 220 patients older than 18 years undergoing major obstetric‐gynecological surgeries (e.g. total abdominal hysterectomy, total vaginal hysterectomy, cesarean section, cesarean hysterectomy with or without colporrhaphy) with risk factors for VTE (prolonged immobility, obesity or previous history of VTE). |
| Interventions | LHMW types: PDxane versus Clexane. |
| Outcomes | DVT, PE, bleeding, adverse effects (including injection site reactions, confusion, and hematuria). |
| Notes | This study was identified in the updated search (18 Feb 2021) and will be incorporated into this review at the next update. |
Dittmer 1991.
| Methods | Randomised controlled trial. |
| Participants | 100 women undergoing caesarean section. |
| Interventions | LMWH versus UFH. |
| Outcomes | DVT, allergic reactions, bleeding. |
| Notes | Reported as abstract only and includes 30 pregnant women with premature labour (at "low risk" to develop a DVT), and 100 women undergoing gynaecological surgery; awaiting full publication or a response from the author regarding data for pregnant women undergoing caesarean section only. |
Ganer 2020.
| Methods | Randomised controlled trial. |
| Participants | 256 women aged 18–54 years, who underwent cesarean delivery during the study period, with one or more risk factors for thromboembolism. |
| Interventions | Pedometer around the wrist starting 24 hours after delivery, for 48 hours and personalised feedback versus pedometer around the wrist starting 24 hours after delivery and standard care. |
| Outcomes | Serious wound complications. |
| Notes | This study was identified in the updated search (18 Feb 2021) and will be incorporated into this review at the next update. |
Movahedi 2020.
| Methods | Randomised controlled trial. |
| Participants | 31 pregnant women with mechanical heart valves at their first trimester (0‐14 weeks) of pregnancy. |
| Interventions | LMWH versus UFH. |
| Outcomes | Fetal loss. |
| Notes | This study was identified in the updated search (18 Feb 2021) and will be incorporated into this review at the next update. |
Nagornaya 2012.
| Methods | Unclear. The abstract states in the methods that the "study was prospective and randomized" but this design was not clear. |
| Participants | Quote: "500 pregnant women were examined in 39‐40 weeks of pregnancy...97 patients were examined after caesarean section." |
| Interventions | Thromboprophylaxis was conducted with bemiparin‐sodium (with the dose dependent on the woman's weight and risk). |
| Outcomes | Risk factors for VTE; quote: "thrombohaemorrhagic complications during 6 months of follow‐up". |
| Notes | Reported as abstract only. Unclear if this truly was a randomised trial, as the results report on risk factors in a cohort of women. Have attempted to contact trial authors; will await contact or full publication. |
NCT02856295.
| Methods | Randomised controlled trial. |
| Participants | 116 postpartum women (estimated to be enrolled, not yet recruiting) |
| Interventions | Weight‐based versus fixed‐dose LMWH. |
| Outcomes | Symptomatic thromboembolic events, bleeding. |
| Notes | This study was identified in the updated search (18 Feb 2021) and will be incorporated into this review at the next update. |
NCT04305756.
| Methods | Randomised controlled trial. |
| Participants | Post‐caesarian delivery women. |
| Interventions | Weight‐based versus fixed‐dose LMWH. |
| Outcomes | Symptomatic thromboembolic events, wound complications. |
| Notes | This study was identified in the updated search (18 Feb 2021) and will be incorporated into this review at the next update. |
NCT04635839.
| Methods | Randomised controlled trial. |
| Participants | 46 hospitalised antepartum women (estimated enrolment, still recruiting). |
| Interventions | Gestational age‐based versus standard dose UFH. |
| Outcomes | Symptomatic thromboembolic events, blood transfusion, bleeding. |
| Notes | This study was identified in the updated search (18 Feb 2021) and will be incorporated into this review at the next update. |
Abbreviations: DVT: deep vein thrombosis; LMWH: low molecular weight heparin; UFH: unfractionated heparin; VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
Dargaud 2018.
| Study name | NCT03659708: Pregnancy and Risk of Venous Thromboembolism (PRESCOT) |
| Methods | Randomised controlled trial (4 arms) |
| Participants | Pregnant women 18 to 50 years at high risk of VTE (personal history of VTE and/or known thrombophilia). |
| Interventions | Intervention (n = 300), 3 groups informed by Lyon‐VTE score management. The Lyon score classifies patients into 3 risk categories that directs the preventive LMWH prescription. Group 1: women with a score strictly less than 3 (moderate thrombotic risk) will receive no LMWH in ante‐partum; Group 2: women with a score between 3 and 5 (high thrombotic risk) will receive a preventive dose of LMWH, introduced in the third trimester (from the beginning of the 7th month); Group 3: women with a score greater than or equal to 6 (very high thrombotic risk) will receive LMWH at a preventive dose throughout the antepartum. Women in all the 3 intervention groups will also receive: an elasto‐compression prescription, and exercise advice (daily physical activity recommended throughout pregnancy (except obstetric contraindication); and systematic preventive LMWH treatment postpartum for 6 weeks (not further specified in trial protocol). Control (n = 300): management according to relevant study centre guidelines (e.g. ACCP) |
| Outcomes | Primary: cost‐utility ratio Secondary: venous thromboembolism; bleeding complications; quality of life of women (EQ‐5D‐3L), all collected until 12 months after birth |
| Starting date | November 2018 |
| Contact information | Yesim Dargaud (Principal Investigator), Hôpital Cardiologique L. Pradel Bron, France, 69677 email: ydargaud@univ‐lyon1.fr |
| Notes | Last updated posted: September 2018 |
Heller 2016b.
| Study name | Compression stocking use in multiparous women during pregnancy & its effects on chronic venous insufficiency |
| Methods | Randomised controlled trial |
| Participants | Unclear other than: aged 18‐45 years and between 8 to 20 weeks' gestation (although the title of the included abstract reporting this protocol suggests multiparous women). |
| Interventions | Intervention group: compression stockings (20–30 mmg Hg maternity pantyhose); women were "requested to use the knee high stockings until they reach the second trimester and then switch to the pantyhose for the remainder of the pregnancy". Control group: no compression stockings |
| Outcomes | Loosely defined in the objective statement: Objective "Primary 1) To quantify and compare the incidence of symptoms of venous insufficiency in pregnant women between the treatment and control groups. 2) To quantify and compare the incidence of varicose veins between participants randomized to the compression stocking use group and those randomized to the no compression stocking use group. Secondary: 1) To quantify and compare the incidence of superficial thrombophlebitis and DVT". |
| Starting date | |
| Contact information | Unclear. No information provided other than "J Heller Institution JHVC, Baltimore, MD" |
| Notes |
NCT00225108.
| Study name | NCT00225108: Study of LMWH in high‐risk postpartum women following caesarean section. |
| Methods | Randomised controlled trial. |
| Participants | Women at moderate to high risk for VTE following caesarean section. Estimated enrolment: 134 women. |
| Interventions | Intervention: LMWH (4500 IU tinzaparin sodium). Control: placebo once daily for 3 to 7 days postpartum. |
| Outcomes | Event rate of DVT (asymptomatic) on day of hospital discharge. Secondary outcomes symptomatic DVT and PE; death, major and minor bleeding at 6 weeks postpartum. |
| Starting date | 2002. |
| Contact information | Marc Rodger, Ottawa Hospital, Ottawa, Ontario, Canada. |
| Notes |
NCT00878826.
| Study name | NCT00878826: Prophylactic enoxaparin dosing forpPrevention of venous thromboembolism in pregnancy. |
| Methods | Randomised controlled trial (open‐label). Estimated enrolment: 64 women. |
| Participants | Women > 18 years, where prophylaxis against VTE in pregnancy is warranted (according to the American College of Obstetrics and Gynecology Practice Bulletin 2000); history of idiopathic thrombosis; history of thrombosis related to pregnancy or oral contraceptive use; history of thrombosis accompanied by an underlying thrombophilia (other than homozygous for the factor V Leiden mutation, heterozygous for both the factor V Leiden and the prothrombin G20210A mutation or AT‐II deficiency; known thrombophilia (expect those listed above, with a history of adverse pregnancy outcome). Exclusions: need for therapeutic level anticoagulation as determined by physician; renal disease; weight > 90 kg; allergy to enoxaparin. |
| Interventions | Intervention: enoxaparin 40 mg once daily. Active control: enoxaparin 1 mg/kg daily. Active control 2: enoxaparin current dose as prescribed from first prenatal visit. |
| Outcomes | Proportion of women in each arm who have anti‐XA levels within appropriate range; correlation of anti‐XA levels with renal function; adverse outcomes (bleeding events, thromboembolic events, side effects, tolerability). |
| Starting date | May 2009. |
| Contact information | Deirdre Judith Lyell, Standord University. |
| Notes |
NCT01019655.
| Study name | NCT01019655: Heparin for pregnant women with thrombophilia. |
| Methods | Randomised controlled trial (open‐label). |
| Participants | Pregnant women with thrombophilia. Estimated enrolment: 300 women. |
| Interventions | Intervention: nadroparin calcium 0.3 mL daily during pregnancy and 6 weeks postpartum. Control: no intervention other than usual care at the study site. |
| Outcomes | Primary outcome: composite endpoint: pregnancy‐associated VTE; miscarriage; pre‐eclampsia; intrauterine growth restriction. |
| Starting date | January 2010. |
| Contact information | Dr Clemens B Tempfer, University of Vienna, Austria. |
| Notes |
NCT01828697.
| Study name | NCT01828697: Comparison of low and intermediate dose low‐molecular‐weight heparin to prevent recurrent venous thromboembolisms in pregnancy. |
| Methods | Randomised controlled trial (open‐label). |
| Participants | Women 18 years or older; < 14 weeks' gestational age; previously objectively confirmed VTE (unprovoked, in the presence of use of oral contraceptives or oestrogen/progestogen, or related to pregnancy or the postpartum period, or minor risk factors). Exclusions: previous VTE related to a major provoking risk factor or indication for treatment with therapeutic dose anticoagulant therapy, or contraindications. Estimated enrolment: 1000 women. |
| Interventions | Intervention: low‐dose LMWH. Comparator: intermediate dose LMWH. |
| Outcomes | Symptomatic DVT; symptomatic PE; major bleeding; composite of major bleeding and clinically relevant non‐major bleeding; early postpartum haemorrhage; late postpartum haemorrhage; blood transfusion < 24 hours postpartum and < 6 weeks after birth; mortality; minor bleeding; skin complications; easy bruising; necessity to switch to other LMWH; thrombocytopenia; congenital anomalies or birth defects. |
| Starting date | April 2013. |
| Contact information | Dr S Middeldorp, Acadmic Medical Centre, Amsterdam. |
| Notes | Estimated study completion date: December 2020 |
NCT04153760.
| Study name | NCT04153760: Pilot PARTUM Trial: Postpartum Aspirin to Reduce Thromboembolism Undue Morbidity (PARTUM) |
| Methods | Randomised controlled trial (multicentre, placebo‐controlled, trial to determine feasibility) |
| Participants | Women with 1 (or more) specified first order criterion or 2 (or more) specified second order criteria. First order criteria: known inherited thrombophilia diagnosed prior to enrolment; and immobilisation (90% of waking hours spent in bed) for ≥ 7 days anytime during the antepartum period. Second order criteria; postpartum infection; postpartum haemorrhage (> 1000 mL of blood loss, regardless of delivery mode); pre‐pregnancy BMI ≥30kg/m2; emergency or unplanned caesarean; smoking ≥ 5 cigarettes/day before pregnancy; pre‐eclampsia; current pregnancy ending in stillbirth (pregnancy loss > 20 weeks' gestation; SGA infant; and previous history of superficial vein thrombosis. A woman is eligible if they have multiple criteria met, at the discretion of the local investigator. Exclusions: > 48 hours since delivery; received more than 2 doses of LMWH since birth; need for postpartum LMWH prophylaxis or systemic anticoagulation as judged by local investigator; need for postpartum aspirin as judged by the local investigator; contraindication to aspirin; < 18 years of age; unable or refused consent. Estimated enrolment: 336 women |
| Interventions | Intervention: aspirin 81 mg daily for 6 weeks post‐randomisation (postpartum) Comparator: placebo daily for 6 weeks post‐randomisation (postpartum) |
| Outcomes | Primary outcome: recruitment rate (at 6 months). Secondary outcomes: consent rate (at 6 months); withdrawals/loss to follow‐up (at 6 months); level of compliance with study drug (at 6 months); time required to obtain site institutional approvals (timeframe 24 months); VTE events (up to 6 months postpartum); bleeding events(up to 6 months postpartum) |
| Starting date | January 2020 (planned, not yet recruiting) |
| Contact information | Dr L Skeith, University of Calgary, Canada. email: laskeith@ucalgary.ca |
| Notes | First posted: November 6, 2019. Estimated completion date: December 2021 |
Abbreviations: AT‐II: antithrombin II; BMI: body mass index; DVT: deep vein thrombosis; IU: international units; LMWH: low molecular weight heparin; PE: pulmonary embolism; SGA: small for gestational age; VTE: venus thromboembolism.
Differences between protocol and review
2020 update of this review
We have updated the methods in line with those in the standard template used by Cochrane Pregnancy and Childbirth.
We have used the GRADE approach to assess the certainty of the body of evidence and we have included 'Summary of findings' tables.
We have added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
We previously excluded trials specifically focused on the role of heparin for pregnant women with known thrombophilias to prevent adverse pregnancy outcomes, as this was the focus of a related Cochrane Review (Walker 2003); however, in this update we have removed known thrombophilias from our exclusion criteria, as the relevant review has not since been updated.
2014 update of this review
We updated the background and the methods section, including 'Risk of bias' assessment.
We clarified that we would include studies reported only as abstracts in analyses where it was possible to extract relevant data from the text.
Contributions of authors
For this updated review, Judith Gomersall (JG) and Emily Shepherd (ES) applied the selection criteria, extracted data for included studies, assessed risk of bias, carried out GRADE assessments and prepared SoF tables. All three authors (Philippa Middleton, JG and ES) contributed to the drafting and editing of this update.
Sources of support
Internal sources
SAHMRI Women and Kids, South Australian Health and Medical Research Institute (SAHMRI), Adelaide, Australia
External sources
No sources of support supplied
Declarations of interest
Judith Gomersall: none known.
Philippa Middleton: none known.
Emily Shepherd: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Algahtani 2015 {published data only}
- Algahtani F, AL Dohami H, Abo-Harbesh S, Gader A, Aleem A. Thromboembolism prophylaxis after cesarean section (PRO-CS) trial. Thrombosis Research 2012;130(Suppl 1):S197. [Google Scholar]
- Algahtani F, Gader A, Aleem A, Al Dohami H, Garbesh S. Thromboembolism prophylaxis after cesarean section (pro-cs) trial. Haematologica 2013;98:749-50. [Google Scholar]
- Algahtani FH, Al-Dohami H, Abo-Harbesh S, Al-Gader A, Aleem A. Thromboembolism prophylaxis after cesarean section (PRO-CS) trial. Thrombosis Research 2015;135(Suppl 1):S71. [Google Scholar]
- NCT01321788. Venous Thromboembolism Prophylaxis Post Cesarean Section (PROCS). https://clinicaltrials.gov/ct2/show/NCT01321788 (first received 24 March 2011).
Burrows 2001 {published data only}
- Burrows RF, Gan ET, Gallus AS, Wallace EM, Burrows EA. A randomised, double blind placebo controlled trial of low molecular weight heparin as prophylaxis in preventing venous thromboembolic events after caesarean section: a pilot study. British Journal of Obstetrics and Gynaecology 2001;108:835-9. [DOI] [PubMed] [Google Scholar]
Casele 2006 {published data only}
- Casele H, Haney E, James A, Rosene-Montella K, Carson M. Bone density changes in women receiving thromboprophylaxis in pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S14. [DOI] [PubMed] [Google Scholar]
- Casele H, Haney EI, James A, Rosene-Montella K, Carson M. Bone density changes in women who receive thromboprophylaxis in pregnancy. American Journal of Obstetrics and Gynecology 2006;195:1109-13. [DOI] [PubMed] [Google Scholar]
- Casele H. Thrombosis prophylaxis in pregnancy. www.enh.org (accessed 14 June 2005).
Cornette 2002 {published data only}
- Cornette J, Jacquemyn Y, Vercauteren M, Buytaert P. A randomised trial to compare the effect of pre- or postoperative nandroparin on blood loss during elective caesarean section. Phlebology 2002;17:67-9. [Google Scholar]
Cruz 2011 {published data only}
- Cruz M, Fernández-Alonso A, Garrigosa L, Cano A, Carretero P, Vizcaino A, et al. Postcesarean thromboprophylaxis with two different regimens of bemiparin [Article ID 548327]. Obstetrics and Gynecology International 2011 Dec 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
De Veciana 2001 {published data only}
- De Veciana M, Trail P, Dattel B, Slotnick RN, Abuhamad A. Dalteparin versus unfractionated heparin for prophylactic anticoagulation during pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6):S182. [Google Scholar]
de Vries 2012 {published data only}ISRCTN87325378
- Abheiden C, Van Hoorn ME, Hague WM, Kostense PJ, Pampus MG, Vries J. Does low-molecular-weight heparin influence fetal growth or uterine and umbilical arterial doppler in women with a history of early-onset uteroplacental insufficiency and an inheritable thrombophilia? Secondary randomised controlled trial results. BJOG: an international journal of obstetrics and gynaecology 2016;123(5):797-805. [DOI] [PubMed] [Google Scholar]
- ISRCTN87325378. Low molecular weight heparin (Fragmin (R)) in pregnant women with a history of uteroplacental insufficiency and thrombophilia, a randomized trial (FRUIT-study). http://www.isrctn.com/ISRCTN87325378 (first received 1 November 2005).
- NTR337. Low molecular weight heparin (Fragmin (R)) in pregnant women with a history of uteroplacental insufficiency and thrombophilia, a randomized trial (FRUIT-study). Https://www.trialregister.nl/trial/299 (first received 2005).
- Vries J, Pampus MG, Hague WM, Bezemer PD, Joosten JH. Fractionated heparin in pregnant women with a history of uteroplacental insufficiency and thrombophilia, a randomized trial (The FRUIT Study). Reproductive Sciences 2011;18(3 Suppl 1):69A. [Google Scholar]
- Vries JI, Hague WM, Van Pampus MG, for the FRUIT-RCT Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT: a reply to a rebuttal. Journal of Thrombosis & Haemostasis 2012;10(6):1196. [DOI] [PubMed] [Google Scholar]
- Vries JI, Pampus MG, Hague WM, Bezemer PD, Joosten JH and on behalf of FRUIT Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT. Journal of Thrombosis and Haemostasis 2012;10:64-72. [DOI] [PubMed] [Google Scholar]
Ellison 2001 {published data only}
- Ellison J, Thomson AJ, Conkie JA, McCall F, Walker ID, Greer IA. Thromboprophylaxis following caesarean section. A comparison of the antithrombotic properties of three low molecular weight heparins - dalteparin, enoxaparin and tinzaparin. Thrombosis and Haemostasis 2001;86:1374-8. [PubMed] [Google Scholar]
- Ellison J, Thomson AJ, Walker ID, Greer IA. Comparisons of the anti-factor XA activities of three commonly used low molecular weight heparins following caesarean section. Scottish Medical Journal 2000;45(3):94-5. [Google Scholar]
- Ellison J, Thomson AJ, Walker ID, Greer IA. Thromboprophylaxis following caesarean section: comparison of the anti-factor Xa activities of dalteparin, enoxaparin and tinzaparin. British Journal of Obstetrics and Gynaecology 2000;107:823. [Google Scholar]
- Ellison J, Thomson AJ, Walker ID, Greer IA. Thromboprophylaxis following caesarean section: comparison of the anti-factor Xa activities of three commonly used low molecular weight heparins. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S39-S40. [Google Scholar]
Gates 2004a {published data only}
- Brocklehurst P, Thromboprophylaxis in Pregnancy Advisory Group. Thromboprophylaxis in pregnancy trials: apple, plum and peach. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):53. [Google Scholar]
- Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low molecular weight heparin. American Journal of Obstetrics and Gynecology 2004;191:1296-303. [DOI] [PubMed] [Google Scholar]
- Gates S, Brocklehurst P, Davis LJ. Antenatal thromboprophylaxis using low molecular weight heparin (enoxaparin) for women at risk of thromboembolic disease: multicentre placebo controlled randomised trial (APPLE) and systematic review. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S44. [Google Scholar]
- National Perinatal Epidemiology Unit. The APPLE Study. www.npeu.ox.ac.uk/trials/apple.html (first received 12 January 2001).
Gates 2004b {published data only}
- Brocklehurst P, Gates S. Randomised controlled trial (PEACH) and meta-analysis of thromboprophylaxis using low-molecular weight heparin (enoxaparin) after caesarean section. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S52. [Google Scholar]
- Brocklehurst P, Thromboprophylaxis in Pregnancy Advisory Group. Thromboprophylaxis in pregnancy trials: apple, plum and peach. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):53. [Google Scholar]
- Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low molecular weight heparin. American Journal of Obstetrics and Gynecology 2004;191:1296-303. [DOI] [PubMed] [Google Scholar]
- National Perinatal Epidemiology Unit. The PEACH Study. www.npeu.ox.ac.uk/trials/peach.html (first received 12 January 2001).
Gibson 1998 {published data only}
- Gibson JL, Ekevall K, Walker I, Greer IA. Puerperal thromboprophylaxis: comparison of the anti-Xa activity of enoxaparin and unfractionated heparin. British Journal of Obstetrics and Gynaecology 1998;105:795-7. [DOI] [PubMed] [Google Scholar]
Hamersley 1998 {published data only}
- Hamersley S, Landy H. Low molecular weight heparin is associated with less peripartum blood loss than unfractionated heparin [abstract]. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S66. [Google Scholar]
Heilmann 1991 {published data only}
- Heilman L, Heitz R, Koch FU, Ose C. Perioperative thrombosis prophylaxis at the time of caesarean section: results of a randomised prospective comparative study with 6% hydroxyethyl starch 0.62 and low dose heparin [Die perioperative Thromboseprophylaxe beim Kaiserschnitt: Ergebnisse einer randomisierten prospektiven Vergleichsuntersuchung mit 6% Hydroxyathylstarke 0,62 und Low-dose-Heparin]. Zeitschrift fur Geburtshilfe und Perinatologie 1991;195:10-5. [PubMed] [Google Scholar]
Heilmann 2007 {published data only}
- Heilmann L, Rath W, Pollow K, Bick RL. The rheological changes after cesarean section: the influence of low molecular weight or unfractionated heparin on the rheological properties of blood. Clinical Hemorheology and Microcirculation 2007;37:211-8. [PubMed] [Google Scholar]
Heller 2016 {published data only}
- Heller J, Canner J, Wei Lum Y, Tsuchiya K. Compression stockings during pregnancy: essential or superfluous? A pilot study. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2016;4(1):148. [Google Scholar]
- NCT01793194. Preventing the development of venous insufficiency in pregnant women through use of compression stockings: a randomized pilot study. http://www.clinicaltrials.gov/ct2/show/NCT01793194 (first received 21 May 2013).
Hill 1988 {published and unpublished data}
- Hill NC, Hill JG, Sargent JM, Taylor CG, Bush PV. Effect of low dose heparin on blood loss at caesarean section. BMJ 1988;296:505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NC, Hill JG, Sargent JM, Taylor CG, Bush PV. The effect of low dose heparin on blood loss at caesarean section. In: 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23-28; Brazil. 1988. [DOI] [PMC free article] [PubMed]
Howell 1983 {published data only}
- Howell R, Fidler J, Letsky E, Swiet M. The risks of antenatal subcutaneous heparin prophylaxis: a controlled trial. British Journal of Obstetrics and Gynaecology 1983;90:1124-8. [DOI] [PubMed] [Google Scholar]
Krauss 1994 {published data only}
- Krauss T, Rath W, Dittmer U, Kuhn W. Use of LMW heparin (Fragmin) for the prevention of thromboembolism in obstetrics [Thromboembolieprophylaxe mit niedermolekularen Heparin (Fragmin)in der Geburtshilfe]. Zeitschrift fur Geburtshilfe und Perinatologie 1994;198:120-5. [PubMed] [Google Scholar]
O'Riordan 2008 {published data only}
- O'Riordan MN, Horgan R, Arya A, Quinn S, Gaolebale PA, Sarkar RK, et al. Pharmokinetics of low molecular weight heparins in the postpartum period. In: Irish Perinatal Society Annual Meetings; 2008 April 11-12; Cork, Ireland. 2008:10.
Pettila 1999 {published data only}
- Pettila V, Kaaja R, Leinonen P, Ekblad U, Kataja M, Ikkala E. Thromboprophylaxis with low molecular weight heparin (dalteparin) in pregnancy. Thrombosis Research 1999;96:275-82. [DOI] [PubMed] [Google Scholar]
- Pettila V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thrombosis and Haemostasis 2002;87(2):182-6. [PubMed] [Google Scholar]
Reddick 2014 {published data only}
- Reddick KL, Smrtka MP, Grotegut CA, James AH, Brancazio LR, Swamy GK. The effects of intermittent pneumatic compression during cesarean delivery on fibrinolysis. American Journal of Perinatology 2014;31(9):735-40. [DOI] [PubMed] [Google Scholar]
Rodger 2014 {published data only}ISRCTN87441504
- Abou-Nassar K, Kovacs MJ, Kahn SR, Wells P, Doucette S, Ramsay T, et al. The effect of dalteparin on coagulation activation during pregnancy in women with thrombophilia. A randomized trial. Thrombosis and Haemostasis 2007;98(1):163-71. [PubMed] [Google Scholar]
- Abou-Nassar K, Rodger M, Kovacs MJ, Doucette S, Tim R, Kahn S, et al. The effect of dalteparin on coagulation activation during pregnancy in women with thrombophilia: a randomised trial. Blood 2006;108(11 Pt 1):262. [PubMed] [Google Scholar]
- NCT00967382. Thrombophilia in pregnancy prophylaxis study (TIPPS). http://www.clinicaltrials.gov/ct2/show/NCT00967382 (first received 30 January 2013).
- Rodger M, Hague WM, Kingdom J, Kahn SR, Karovitch A, Wells PS, et al. The Thrombophilia in Pregnancy Prophylaxis Study (TIPPS): a multi-national randomized trial of dalteparin vs. no dalteparin to prevent pregnancy complications in pregnant thrombophilic women. Journal of Thrombosis and Haemostasis 2013;11(Suppl 2):78. [Google Scholar]
- Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet 2014;384:1673-83. [DOI] [PubMed] [Google Scholar]
- Rodger MA, Kahn S, Cranney A, Hodson A, Kovacs M, Clement AM, et al. Prophylactic low molecular weight heparin (LMWH) during pregnancy is not associated with a decrease in bone mineral density (BMD) [abstract]. Hypertension in Pregnancy 2006;25(Suppl 1):8. [Google Scholar]
- Rodger MA, Kahn SR, Cranney A, Hodsman A, Kovacs MJ, Clement AM, et al. Long-term dalteparin in pregnancy not associated with a decrease in bone mineral density: substudy of a randomized controlled trial. Journal of Thrombosis and Haemostasis 2007;5:1600-6. [DOI] [PubMed] [Google Scholar]
- Rodger MA, Lazo-Langner A, Kahn S, Kovacs M, Robinson S, Blostein M, et al. Prophylactic low molecular weight heparin (LMWH) during pregnancy is not associated with a decrease in bone mineral density (BMD). The TIPPS (Thrombophilia in Pregnancy Prophylaxis Study) BMD substudy. Blood 2005;106(11):Abstract No 548. [Google Scholar]
- Rodgers M. Thrombophilia in pregnancy prophylaxis study (TIPPS). Ottawa Health Research Institute (http://www.ohri.ca) (accessed 17 July 2002).
Rodger 2015 {published data only}
- NCT01274637. PROSPER: Postpartum prophylaxis for PE randomized control trial pilot. http://clinicaltrials.gov/show/NCT01274637 (first received 15 February 2011).
- Rodger MA, Phillips P, Kahn SR, James AH, Konkle BA. Low-molecular-weight heparin to prevent postpartum venous thromboembolism. A pilot randomised placebo-controlled trial. Thrombosis and Haemostasis 2015;113(1):212-6. [DOI] [PubMed] [Google Scholar]
Rodger 2016 {published data only}
- NCT01274637. PROSPER: Postpartum prophylaxis for PE randomized control trial pilot. http://clinicaltrials.gov/show/NCT01274637 (first received 15 February 2011).
- Rodger MA, Phillips P, Kahn SR, Bates S, McDonald S, Khurana R, et al. Low molecular weight heparin to prevent postpartum venous thromboembolism. A pilot study to assess the feasibility of a randomized open-label trial. Thrombosis Research 2016;142:17-20. [DOI] [PubMed] [Google Scholar]
Salim 2016 {published data only}
- Garmi G, Okopnik M, Zuarez-Easton S, Zafran N, Romano S, Salim R. Placental findings following adjusting enoxaparin dosage according to anti-fxa levels in thrombophilic women. A randomized controlled trial. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl 1):S457. [Google Scholar]
- Garmi G, Zafran N, Okopnik M, Gavish I, Romano S, Salim R. Placental pathological findings following adjusting enoxaparin dosage in thrombophilic women: secondary analysis of a randomized controlled trial. Thrombosis and Haemostasis 2019;119(1):87-91. [DOI] [PubMed] [Google Scholar]
- NCT01068795. Dose adjusting enoxaparin thromboprophylaxis dosage according to anti-factor Xa plasma levels improve pregnancy outcome. http://clinicaltrials.gov/show/NCT01068795 (first received 18 June 2013).
- Salim R, Nachum Z, Gavish I, Romano S, Braverman M, Garmi G. Adjusting enoxaparin dosage according to anti-FXa levels and pregnancy outcome in thrombophilic women. A randomised controlled trial. Thrombosis and Haemostasis 2016;116(4):687-95. [DOI] [PubMed] [Google Scholar]
Segal 1975 {published data only}
- Segal S, Sadovsky E, Weinstein D, Polishuk WZ. Prevention of postpartum venous thrombosis with low doses of heparin. European Journal of Obstetrics and Gynecology and Reproductive Biology 1975;5:273-6. [DOI] [PubMed] [Google Scholar]
Stephenson 2016 {published data only}
- NCT02070237. Comparing anti-XA levels in post-cesarean patients undergoing enoxaparin thromboprophylaxis. https://clinicaltrials.gov/ct2/show/NCT02070237 (first received 25 February 2014).
- Stephenson M, Serra A, Neeper J, Caballero D, McNulty J. Comparing anti-Xa levels in women with body mass index >/=35 post cesarean delivery undergoing enoxaparin thromboprophylaxis with weight-based dosing twice daily versus fixed dose 40 mg daily: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S18. [Google Scholar]
- Stephenson ML, Serra AE, Neeper JM, Caballero DC, McNulty J. A randomized controlled trial of differing doses of postcesarean enoxaparin thromboprophylaxis in obese women. Journal of Perinatology 2016;36(2):95-9. [DOI] [PubMed] [Google Scholar]
van Hoorn 2016 {published data only}ISRCTN87325378
- Hoorn M, Hague WM, Pampus M, Bezemer D, de Vries J for the FRUIT investigators. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: the FRUIT-RCT. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;197:168-73. [DOI] [PubMed] [Google Scholar]
Welti 1981 {published data only}
- Welti H. Prevention of thromboembolism by physiotherapy with and without low dose heparin in gynecology and obstetrics. Results of a controlled, randomized multicenter study [Prophylaxie thrombo-embolique par physiotherapie avec et sans heparine a faibles doses en gynecologie-obstetrique]. Revue Medicale de la Suisse Romande 1981;101(11):925-34. [PubMed] [Google Scholar]
References to studies excluded from this review
Aina 2006 {published data only}
- Aina A, NCT00356434. Study of patient compliance and comfort using sequential compression devices and foot pumps for DVT prevention. https://clinicaltrials.gov/ct2/show/results/NCT00356434 (first received 26 July 2006).
Alalaf 2015 {published data only}
- Alalaf A, NCT01588171. Bemiparin versus enoxaparin as thromboprophylaxis following vaginal and abdominal deliveries: a prospective clinical trial. https://clinicaltrials.gov/ct2/show/NCT01588171 (first received 30 April 2012). [DOI] [PMC free article] [PubMed]
- Alalaf SK, Jawad RK, Muhammad PR, Ali MS, Al Tawil NG. Bemiparin versus enoxaparin as thromboprophylaxis following vaginal and abdominal deliveries: a prospective clinical trial. BMC Pregnancy & Childbirth 2015;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badawy 2008 {published data only}
- Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I. Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. Journal of Obstetrics and Gynaecology 2008;28(3):280-4. [DOI] [PubMed] [Google Scholar]
Blomback 1998 {published data only}
- Blomback M, Bremme K, Hellgren M, Lindberg H. A pharmacokinetic study of dalteparin (Fragmin) during late pregnancy. Blood Coagulation and Fibrinolysis 1998;9:343-50. [DOI] [PubMed] [Google Scholar]
Brenner 2005 {published data only}
- Brenner B, Bar J, Ellis M, Yarom I, Yohai D, Samueloff A, et al. Effects of enoxaparin on late pregnancy complications and neonatal outcome in women with recurrent pregnancy loss and thrombophilia: results from the live-enox study. Fertility and Sterility 2005;84(3):770-3. [DOI] [PubMed] [Google Scholar]
- Brenner B, Hoffman R, Carp H, Dulitsky M, Samueloff A, Yohai D, et al. Enoxaparin treatment improves the gestational outcome of pregnant women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study [abstract]. Blood 2003;102(11):16a. [Google Scholar]
- Brenner B, Hoffman R, Carp H, Dulitsky M, Samueloff A, Yohal D, et al. Efficacy and safety of two doses of enoxaparin in pregnant women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study [abstract]. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):OC084. [DOI] [PubMed] [Google Scholar]
- Brenner B, Hoffman R, Carp H, Dulitsky M, Younis J, LIVE-ENOX investigators. Efficacy and safety of two doses of enoxaparin in women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study. Journal of Thrombosis and Haemostasis 2005;3:227-9. [DOI] [PubMed] [Google Scholar]
- Brenner B, for the LIVE-ENOX Investigators. Efficacy and safety of two doses of enoxaparin in pregnant women with thrombophilia and recurrent pregnancy loss. The LIVE-ENOX study [abstract]. Blood 2002;100(11 Pt 1):702a. [DOI] [PubMed] [Google Scholar]
de Jong 2015 {published data only}
- Jong PG, Quenby S, Bloemenkamp KW, Braams-Lisman BA, Bruin JP, Coomarasamy A, et al. ALIFE2 study: low-molecular-weight heparin for women with recurrent miscarriage and inherited thrombophilia - study protocol for a randomized controlled trial. Trials 2015;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dendrinos 2007 {published data only}
- Dendrinos S, Kalogirou I, Makrakis E, Theodoridis T, Mahmound EA, Christopoulou-Cokkinou V, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clinical and Experimental Obstetrics and Gynecology 2007;34(3):143-5. [PubMed] [Google Scholar]
Farquharson 2002 {published data only}
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstetrics and Gynecology 2002;100(3):408-13. [DOI] [PubMed] [Google Scholar]
Giancotti 2012 {published data only}
- Chistolini A, Torelli F, Giancotti A, Pignoloni P, Muto B, Cosimo C, et al. Recurrent fetal loss: prospective evaluation of the efficacy of three different thromboprophylaxis regimens: aspirin versus low molecular weight heparin versus low molecular weight heparin plus aspirin [abstract]. Hematology Journal 2006;91(Suppl 1):146. [Google Scholar]
- Giancotti A, Torre RL, Spagnuolo A, D'Ambrosio V, Cerekja A, Piazze J, et al. Efficacy of three different antithrombotic regimens on pregnancy outcome in pregnant women affected by recurrent pregnancy loss. Journal of Maternal-Fetal and Neonatal Medicine 2012;25(7):1191-4. [DOI] [PubMed] [Google Scholar]
Gris 2010 {published data only}
- Gris JC, Chauleur C, Faillie JL, Baer G, Mares P, Fabbro-Peray P, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae: the pilot randomised controlled NOH-AP trial. Thrombosis and Haemostasis 2010;104(4):771-9. [DOI] [PubMed] [Google Scholar]
Gris 2011 {published data only}
- Gris JC, Chauleur C, Mares P, Nouvellon E, Mercier E, Bouvier S, et al. Enoxaparin for the secondary prevention of placental complications in women with previous severe pre-eclampsia: the pilot randomised controlled NOH-PE study. Journal of Thrombosis and Haemostasis : JTH 2011;9(Suppl 2):753. [Google Scholar]
- Gris JC, Chauleur C, Molinari N, Mares P, Fabbro-Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia: the pilot randomised controlled NOH-PE trial. Thrombosis and Haemostasis 2011;106(6):1053-61. [DOI] [PubMed] [Google Scholar]
Guven 2014 {published data only}
- Guven D, Bakay K, Kocak I, Ozdemir A. Effectiveness of bemiparin sodium for preventing pregnancy loss in patients with MTHFR C677T mutation and habitual abortus. Open Journal of Obstetrics and Gynecology 2014;4:930-4. [Google Scholar]
Harenberg 1993 {published data only}
- Harenberg J, Schneider D, Heilmann L, Wolf H. Lack of anti-factor Xa activity in umbilical cord vein samples after subcutaneous administration of heparin or low molecular mass heparin in pregnant women. Haemostasis 1993;23:314-20. [DOI] [PubMed] [Google Scholar]
Kaandorp 2010 {published data only}
- Kaandorp SP, Goddijn M, Post JA, Hutten BA, Verhoeve HR, Hamulyák K, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. New England Journal of Medicine 2010;362(17):1586-96. [DOI] [PubMed] [Google Scholar]
- Middeldorp S. Aspirin and/or low molecular weight heparin for women with unexplained recurrent miscarriage and/or intra-uterine fetal death. Netherlands Trial Register (http://www.trialregister.nl) (first received 1 November 2005).
Kamin 2008 {published data only}
- Kamin G, Rogenhofer N, Pildner v Steinberg S, Neuhoffer A, Seeger S, et al. Therapy with dalteparin for habitual abortion - presentation of the ETHiG II-Studie [Therapie mit Dalteparin bei habitueller Abortneigung - Vorstellung der ETHiG II-Studie]. Geburtshilfe und Frauenheilkunde 2008;68:S51. [Google Scholar]
- Rogenhofer N, Markoff A, Wagner A, Klein H-G, Petroff D, Schleussner E, et al. Lessons from the EThIGII trial: Proper putative benefit assessment of low-molecular-weight heparin treatment in M2/ANXA5 haplotype carriers. Clinical and Applied Thrombosis/Hemostasis 2017;23(1):27-33. [DOI] [PubMed] [Google Scholar]
- Schleussner E, Bohlmann M, Kamin G, Rogenhofer N, Seeger S, Toth B. Low molecular weight heparin for the prevention of habitual abortion - introduction of the multicentre study EThIG2 and discussion of the current data [Niedermolekulares heparin zur pravention habitueller aborte - vorstellung der multizentrischen EThIG2-studie und diskussion der aktuellen datenlage]. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S220. [Google Scholar]
- Schleussner E, Kamin G, Seeliger G, Rogenhofer N, Toth B. Low-molecular-weight heparin in recurrent pregnancy loss-Results of the ETHIG II study. Thrombosis Research 2013;131(Suppl 1):S73. [Google Scholar]
Kutteh 1996a {published data only}
- Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody-associated recurrent pregnancy loss with lower dose heparin and aspirin. American Journal of Reproductive Immunology 1996;35:402-7. [DOI] [PubMed] [Google Scholar]
Kutteh 1996b {published data only}
- Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. American Journal of Obstetrics and Gynecology 1996;174(5):1584-9. [DOI] [PubMed] [Google Scholar]
Langer 2013 {published data only}
- Langer E, Fiedler A, Schleusner E, Schlembach D. Placental volume in women under treatment with recurrent miscarriages. Ultraschall in der Medizin, Supplement 2013;34(Suppl 1):WS_SL6_05. [Google Scholar]
Laskin 2007 {published data only}
- Laskin CA, NCT00564174. A randomized controlled trial comparing low molecular weight heparin and aspirin to aspirin alone in women with unexplained recurrent pregnancy loss. https://clinicaltrials.gov/ct2/show/NCT00564174 (first received 27 November 2007).
- Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. Journal of Rheumatology 2009;36(2):279-87. [DOI] [PubMed] [Google Scholar]
Milic 2018 {published data only}
- Milic D, Zivic S, Bogdanovic D, Jovanovic M. Compression stockings in the prevention of venous disorders in pregnancy. Phlebology 2018;33(10):709. [Google Scholar]
Noble 2005 {published data only}
- Noble LS, Kutteh WS, Lashey N, Franklin RD, Herrada J. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low-molecular-weight heparin versus unfractionated heparin. Fertility and Sterility 2005;83(3):684-90. [DOI] [PubMed] [Google Scholar]
Pyregov 2012 {published data only}
- Pyregov A, Shifman E, Shestakova O, Puchko T, Baranov I. Influence of enoxaparine on serum endotoxin concentration in puerpera after abdominal delivery. British Journal of Anaesthesia 2012;108:ii212-3. [Google Scholar]
Rai 1997 {published data only}
- Cohen H. Randomized trial of aspirin versus aspirin and heparin in pregnant women with the antiphospholipid syndrome. Annales de Medicine Interne 1996;147 Suppl 1:44. [PubMed] [Google Scholar]
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997;314:253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratiu 2009 {published data only}
- Ratiu A, Motoc A, Pascut D, Crisan DC, Anca T, Pascut M. Compression and walking compared with bed rest in the treatment of proximal deep venous thrombosis during pregnancy. Revista Medico-Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi 2009;113(3):795-8. [PubMed] [Google Scholar]
Rey 2009 {published data only}
- Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7(1):58-64. [DOI] [PubMed] [Google Scholar]
- Rey E. Dalteparin in prevention of recurrence of severe obstetrical complications in women without thrombophilia. Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S46. [Google Scholar]
Rodger 2017 {published data only}
- Rodger M, NCT03100123. A pilot study assessing the feasibility of a randomized controlled trial evaluating aspirin versus low-molecular-weight heparin (lmwh) and aspirin in women with antiphospholipid syndrome and pregnancy loss. https://clinicaltrials.gov/ct2/show/NCT03100123 (first received 4 April 2017).
Samantha 2013 {published data only}
- Samantha P, Barillari G, Venturelli U, Turello M. Thromboprophylaxis with two different dosages of lmwh (calcic nadroparin) in pregnant women with thrombophilia: a single center experience. Journal of Thrombosis and Haemostasis 2013;11:96-7. [Google Scholar]
Schleussner 2015 {published data only}
- Schleussner E, Kamin G, Seliger G, Rogenhofer N, Ebner S, Toth B, et al. Low-molecular-weight heparin for women with unexplained recurrent pregnancy loss: a multicenter trial with a minimization randomization scheme. Annals of Internal Medicine 2015;162(9):601-9. [DOI] [PubMed] [Google Scholar]
Stephenson 2004 {published data only}
- Stephenson MD, Ballem PJ, Tsang P, Purkiss S, Ensworth S, Houlihan E, et al. Treatment of antiphospholipid antibody syndrome (aps) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. Journal of Obstetrics and Gynaecology Canada 2004;26(8):729-34. [DOI] [PubMed] [Google Scholar]
Thaler 2004 {published data only}
- Thaler I, Brenner B. Efficacy of enoxaparin for improving pregnancy outcomes and uteroplacental blood flow in women with thrombophilia and recurrent pregnancy loss [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S7. [Google Scholar]
Tulppala 1997 {published data only}
- Tulppala M, Marttunen M, Soderstrom-Anttila V, Foudila T, Ailus K, Palosuo T, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect of prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(7):1567-72. [DOI] [PubMed] [Google Scholar]
Visser 2011 {published data only}
- Visser J, Ulander V, Bloemenkamp K, Kaaja R. A randomised controlled multicenter study: the effect of enoxaparin and/or aspirin on prevention of recurrent miscarriage in women with or without thrombophilia, HABENOX-study. International Journal of Gynecology and Obstetrics 2009;107(Suppl 2):S371. [Google Scholar]
- Visser J, Ulander VM, Helmerhorst FM, Lampinen K, Morin-Papunen L, Bloemenkamp KW, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia - HABENOX*: a randomised multicentre trial. Thrombosis and Haemostasis 2011;105(2):295-301. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdolvand 2019 {published data only}
- Abdolvand M, Aleyasin A, Javadi MR, Solduzian M, Hosseini SH, Ziaei Z, et al. Comparison of efficacy and safety of two different enoxaparin products in prevention of venous thromboembolism following major obstetric-gynecological surgeries: an open-label randomized clinical trial. Iranian Journal of Pharmaceutical Research 2019;18(4):2172-9. [CENTRAL: CN-02074323] [EMBASE: 2003440151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dittmer 1991 {published data only}
- Dittmer U, Rath W, Schrader J, Zuchner C, Scheler F, Kuhn W. Prevention of deep vein thrombosis (DVT) in pregnancy, after caesarean section and after gynaecological abdominal surgery. A comparison between low molecular weight heparin (LMW) and standard heparin (UFH) [abstract]. Annals of Haematology 1991;62 Suppl 1:A40. [Google Scholar]
Ganer 2020 {published data only}
- Ganer Herman H, Kleiner I, Tairy D, Gonen N, Ben Zvi M, Kovo M, et al. Effect of digital step counter feedback on mobility after cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2020;135(6):1345-52. [CENTRAL: CN-02131345] [EMBASE: 631910090] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Herman HG, Kleiner I, Tairy D, Gonen N, Ben ZM, Kovo M, et al. 623: improving post-Cesarean mobility with personalized feedback using digital step counters - a randomized controlled trial. American Journal of Obstetrics and Gynecology 2020;222(1):S397-8. [CENTRAL: CN-02075395] [EMBASE: 2004455439] [Google Scholar]
Movahedi 2020 {published data only}
- Movahedi M, Motamedi M, Sajjadieh A, Bahrami P, Saeedi M. Pregnancy outcome in women with mechanical prosthetic heart valves at their first trimester of pregnancy treated with unfractionated heparin (UFH) or enoxaparin: a randomized clinical trial. Journal of Cardiovascular and Thoracic Research 2020;12(3):209-13. [CENTRAL: CN-02194966] [EMBASE: 632980316] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagornaya 2012 {published data only}
- Nagornaya V, Gonta R, Nickolay K, Pokhilchenko M. The risk of venous thromboembolism and its prevention in obstetrics. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S796. [Google Scholar]
NCT02856295 {published data only}
- NCT02856295. anti10a levels in women treated with LMWH in the postpartum period. Https://clinicaltrials.gov/show/NCT02856295 (first received 2016 August 04). [CENTRAL: CN-01592274]
NCT04305756 {published data only}
- NCT04305756. Impact of prophylactic low-molecular weight heparin dosing on clotting parameters following cesarean delivery. Https://clinicaltrials.gov/show/NCT04305756 (first received 2020 March 12). [CENTRAL: CN-02089003]
NCT04635839 {published data only}
- NCT04635839. Heparin Prophylaxis Dosing for antepartum hospitalizations (HEPDOSE). Https://clinicaltrials.gov/show/NCT04635839 (first received 2020 Nov 19). [CENTRAL: CN-02205733]
References to ongoing studies
Dargaud 2018 {published data only}
- Dargaud Y, NCT03659708. Prospective multicentre randomized clinical trial on the management of pregnancies with high risk of venous thrombosis. https://clinicaltrials.gov/ct2/show/NCT03659708 (first received 6 September 2018).
Heller 2016b {published data only}
- Heller J. Compression stocking use in multiparous women during pregnancy & its effects on chronic venous insufficiency. Phlebology 2016;31(2 Suppl):42-3. [Google Scholar]
NCT00225108 {published data only}
- NCT00225108. The STOP CLOT pilot study: study of low molecular weight heparin in high risk postpartum women following cesarean section (ongoing trial). http://clinicaltrials.gov/show/NCT00225108 (first received 21 March 2006).
NCT00878826 {published data only}
- NCT00878826. Prophylactic enoxaparin dosing for prevention of venous thromboembolism in pregnancy. http://clinicaltrials.gov/ct2/show/record/NCT00878826 (first received 31 March 2013).
NCT01019655 {published data only}
- NCT01019655. Heparin for pregnant with thrombophilia. http://clinicaltrials.gov/show/NCT01019655 (first received 29 July 2012).
NCT01828697 {published data only}
- Bistervels I, Bleker S, Middeldorp S, Buchmuller A, Decousus H, Chauleur C, et al. Induced delivery and neuraxial anesthesia in pregnant women using thromboprophylaxis: data from the highlow study. Research and Practice in Thrombosis and Haemostasis 2017;1:41-2. [Google Scholar]
- Bistervels I, Buchmüller A, Bleker S, Chauleur C, Ni Ainle F, Donnelly J, et al. Neuraxial anesthesia in pregnant women using thrombosis prophylaxis: data from the Highlow study. Thrombosis Research 2017;Conference: 7th International Symposium on Women's Health Issues in Thrombosis and Haemostasis. Spain. 151(Suppl 1):S131-2. [Google Scholar]
- Bistervels I, Buchmuller A, Ni Ainle F, Bleker S, Chauleur C, Donnelly J, et al. Management of anticoagulant therapy around delivery: results from the ongoing highlow study. Research and Practice in Thrombosis and Haemostasis 2019;3:866-7. [CENTRAL: CN-01978640] [EMBASE: 628813887] [Google Scholar]
- Bleker SM, Buchmuller A, Chauleur C, Ainle FN, Donnelly J, Verhamme P, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy: Rationale and design of the Highlow study, a randomised trial of two doses. Thrombosis Research 2016;144:62-8. [DOI] [PubMed] [Google Scholar]
- Bleker SM, Ingrid BM, Buchmuller A, Chauleur C, Ainle FN, Donnelly J, et al. Interim report of the highlow study: a randomized controlled trial comparing two doses of low molecular weight heparin for the prevention of pregnancy-associated recurrent venous thromboembolism. Blood 2016;128(22):1444. [Google Scholar]
- EUCTR2012-001505-24-ES. Highlow study: prevention of recurrent deep vein thrombosis and lung embolism in pregnant women [Highlow study Low-molecular-weight heparin to prevent recurrent VTE in pregnancy: a randomized controlled trial of two doses - Highlow study]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-001505-24-ES (first received 2018). [CENTRAL: CN-01905819]
- NCT01828697. Low-molecular-weight heparin to prevent recurrent VTE in pregnancy: a randomized controlled trial of two doses. http://clinicaltrials.gov/ct2/show/NCT01828697 (first received 21 May 2013).
NCT04153760 {published data only}
- NCT04153760. Pilot PARTUM Trial: Postpartum Aspirin to reduce thromboembolism undue morbidity (PARTUM). https://clinicaltrials.gov/ct2/show/NCT04153760 (first received 19 August 2020).
Additional references
Abbasi 2014
- Abbasi N, Balayla J, Laporta DP, Kezouh A, Abenhaim HA. Trends, risk factors and mortality among women with venous thromboembolism during labour and delivery: a population based study of 8 million births. Archives of Gynecology and Obstetrics 2014;289(2):275-84. [DOI] [PubMed] [Google Scholar]
ACOG 2018
- American College of Obstetricians and Gynecologists. Thromboembolism in pregnancy. ACOG Practice Bulletin No. 196. Obstetrics and Gynecology 2018;132:e1-17. [DOI] [PubMed] [Google Scholar]
Andrew 2020
- Andrew L, Ni Ainle F, Blondon M, Rodger M, Skeith L. Preventing postpartum venous thromboembolism: a call to action to reduce undue maternal morbidity and mortality. Thrombosis Research 2020;193:190-7. [DOI] [PubMed] [Google Scholar]
Ansell 2004
- Ansell J, Bergqvist D. Current options in the prevention of thromboembolic disease. Drugs 2004;64(Suppl 1):1-5. [DOI] [PubMed] [Google Scholar]
Antony 2017
- Antony KM, Racusin DA, Aagaard K, Dildy GA. III Maternal physiology. In: Gabbe SG, Niebyl JR, Simpson JO, Landon MB, Galan HL, Jauniaux ER et al, editors(s). Obstetrics: Normal and Problem Pregnancies. 7th edition. Philadelphia: Elsevier, 2017. [Google Scholar]
Bates 2012
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy,and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed:American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):e691S–e6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauersachs 2009
- Bauersachs RM. Treatment of venous thromboembolism during pregnancy. Thrombosis Research 2009;123(Suppl 2):S45-S50. [DOI] [PubMed] [Google Scholar]
Blanco‐Molina 2010
- Blanco-Molina A, Rota L, Di Micco P, Brenner B, Tujilo-Santos J, Ruiz-Gamietea A, et al. Venous thromboembolism during pregnancy, postpartum or during contraceptive use. RIETE Investigators. Thombosis and Haemostasis 2010;103:306-11. [DOI] [PubMed] [Google Scholar]
Brenner 2003
- Brenner B. Antithrombotic prophylaxis for women with thrombophilia and pregnancy complications--Yes. Journal of Thrombosis and Haemostasis 2003;1(10):2070-2. [DOI] [PubMed] [Google Scholar]
D'Souza 2020
- D'Souza R, Malhamé I, Teshler L, Acharya G, Hunt BJ, McLintock C. A critical review of the pathophysiology of thrombotic complications and clinical practice recommendations for thromboprophylaxis in pregnant patients with COVID-19. Acta Obstetricia et Gynecologica Scandinavica 2020;Jul 17 [Epub ahead of print]. [DOI: 10.1111/aogs.13962] [DOI] [PMC free article] [PubMed]
de Jong 2014
- Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD004734. [DOI: 10.1002/14651858.CD004734] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Nisio 2017
- Di Nisio M, Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2017;388(10063):3060-73. [DOI] [PubMed] [Google Scholar]
Dodd 2013
- Dodd JM, McLeod A, Windrim RC, Kingdom J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD006780. [DOI: 10.1002/14651858.CD006780] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis‐Kahana 2020
- Ellis-Kahana J, Sparks A, Gimovsky A, James A, Ahmadzia H. Developing a model for predicting venous thromboembolism in obese pregnant women in a national study. Thrombosis Research 2020;191:42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2016
- Friedman A, Ananth C. Obstetrical venous thromboembolism: Epidemiology and strategies for prophylaxis. Seminars in Perinatology 2016;40:81-6. [DOI] [PubMed] [Google Scholar]
Goldhaber 2012
- Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012;379:1835-46. [DOI] [PubMed] [Google Scholar]
Greer 2012
- Greer IA. Thrombosis in pregnancy: updates in diagnosis and management. Hematologic Diseases in Pregnancy 2012;2012:203-5. [DOI] [PubMed] [Google Scholar]
Hall 1980
- Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. American Journal of Medicine 1980;68:122-40. [DOI] [PubMed] [Google Scholar]
Hamulyák 2020
- Hamulyák EN, Scheres LJ, Marijnen MC, Goddijn M, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD012852. [DOI: 10.1002/14651858.CD012852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jacobsen 2008
- Jacobsen A, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium - a register-based case-control study. American Journal of Obstetrics andGynecology 2008;198(233):e1-7. [DOI] [PubMed] [Google Scholar]
James 2006
- James AH, Jamison MG, Brancazio LR. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. American Journal of Obstetrics and Gynecology 2006;194:1311–5. [DOI] [PubMed] [Google Scholar]
Knight 2008
- Knight M. Antenatal pulmonary emboism: risk factors, management and outcomes. BJOG: an international journal of obstetrics and gynaecology 2008;115:453-61. [DOI] [PubMed] [Google Scholar]
Letsky 1997
- Letsky EA. Peripartum prophylaxis of thromboembolism. Bailliere's Clinical Obstetrics and Gynaecology 1997;11:523-43. [DOI] [PubMed] [Google Scholar]
Middeldorp 2003
- Middeldorp S. Antithrombotic prophylaxis for women with thrombophilia and pregnancy complications--No. Journal of Thrombosis and Haemostasis 2003;1(10):2073-4. [DOI] [PubMed] [Google Scholar]
Nelson‐Piercy 1997
- Nelson-Piercy C. Hazards of heparin: allergy, heparin-induced thrombocytopenia and osteoporosis. Bailliere's Clinical Obstetrics and Gynaecology 1997;11:489-509. [DOI] [PubMed] [Google Scholar]
NHMRC 2009
- National Health and Medical Research Council. Clinical Practice Guideline for the Prevention of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Australian Hospitals. Melbourne: National Health and Medical Research Council, 2009. [DOI] [PubMed] [Google Scholar]
NICE 2018
- NICE 2018. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism (NG89). www.nice.org.uk/guidance/ng89 2018. [PubMed]
Okoroh 2012
- Okoroh EN, Azonobi IC, Grosse SD, Grant AM, Atrash HK, James AH. Prevention of venous thromboembolism in pregnancy: a review of guidelines, 2000–2011. Journal of Women's Health 2012;21(6):611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orme 1977
- Orme ML, Lewis PJ, Swiet M, Serlin MJ, Sibeon R, Baty JD, et al. May mothers given warfarin breast-feed their infants? British Medical Journal 1977;1(6076):1564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmerola 2015
- Palmerola KL, D' Alton ME, Brock CO, Friedman AM. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG: an international journal of obstetrics and gynaecology 2016;123(13):2157-62. [DOI] [PubMed] [Google Scholar]
Paull 1987
- Paull J. A prospective study of dextran-induced anaphylactoid reactions in 5745 patients. Anaesthesia and Intensive Care 1987;15:163-7. [DOI] [PubMed] [Google Scholar]
PEP Trial 2000
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin. Lancet 2000;355:1295-302. [PubMed] [Google Scholar]
RCOG 2015
- The Royal College of Obstetricians and Gynaecologists. Reducing the risk of thrombosis and embolism during pregnancy and puerperium. Green-top guideline. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg37a/ 2015:1-40.
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014.
Roberge 2017
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. American Journal of Obstetrics & Gynecology 2017;February:110-120. [DOI] [PubMed] [Google Scholar]
Rolnik 2017
- Rolnik DL, Wright D, Poon LC, O' Gorman N, Syngelak A, Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. New England Journal of Medicine 2017;177(7):613-22. [DOI] [PubMed] [Google Scholar]
Simpson 2001
- Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. British Journal of Obstetrics and Gynaecology 2001;108(1):56-60. [DOI] [PubMed] [Google Scholar]
Walker 2003
- Walker MC, Ferguson FE, Allen VM. Heparin for pregnant women with acquired or inherited thrombophilias. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD003580. [DOI: 10.1002/14651858.CD003580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2005
- Wu O, Robertson L, Twaddle S, Lowe G, Clark P, Walker I, et al. Screening for thrombophilia in high-risk situations: a meta-analysis and cost-effectiveness analysis. British Journal of Haematology 2005;131(1):80-90. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bain 2014
- Bain E, Wilson A, Tooher R, Gates S, Davis LJ, Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD001689. [DOI: 10.1002/14651858.CD001689.pub3] [DOI] [PubMed] [Google Scholar]
Gates 2002a
- Gates S, Brocklehurst P, Davis LJ. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD001689. [DOI: 10.1002/14651858.CD001689] [DOI] [PubMed] [Google Scholar]
Tooher 2010
- Tooher R, Gates S, Dowswell T, Davis LJ. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD001689. [DOI: 10.1002/14651858.CD001689.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


