Abstract
Background
Convergence insufficiency is a common binocular vision disorder in which the eyes have a strong tendency to drift outward (exophoria) with difficulty turning the eyes inward when reading or doing close work.
Objectives
To assess the comparative effectiveness and relative ranking of non‐surgical interventions for convergence insufficiency through a systematic review and network meta‐analysis (NMA).
Search methods
We searched CENTRAL, MEDLINE, Embase, PubMed and three trials registers up to 20 September 2019.
Selection criteria
We included randomized controlled trials (RCTs) examining any form of non‐surgical intervention versus placebo, no treatment, sham treatment, or other non‐surgical interventions. Participants were children and adults with symptomatic convergence insufficiency.
Data collection and analysis
We followed standard Cochrane methodology. We performed NMAs separately for children and adults.
Main results
We included 12 trials (six in children and six in adults) with a total of 1289 participants. Trials evaluated seven interventions: 1) office‐based vergence/accommodative therapy with home reinforcement; 2) home‐based pencil/target push‐ups; 3) home‐based computer vergence/accommodative therapy; 4) office‐based vergence/accommodative therapy alone; 5) placebo vergence/accommodative therapy or other placebo intervention; 6) prism reading glasses; and 7) placebo reading glasses.
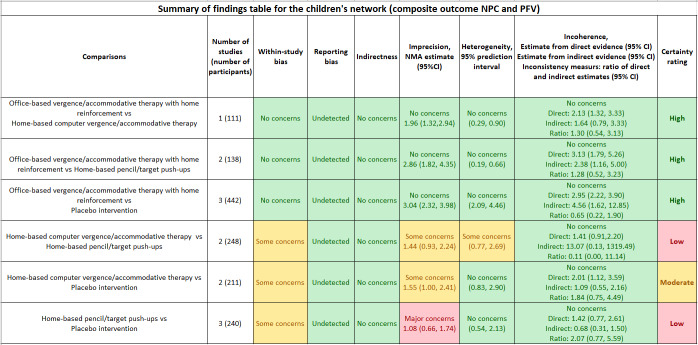
Six RCTs in the pediatric population randomized 968 participants. Of these, the Convergence Insufficiency Treatment Trial (CITT) Investigator Group completed four RCTs with 737 participants. All four CITT RCTs were rated at low risk of bias. Diagnostic criteria and outcome measures were identical or similar among these trials. The four CITT RCTs contributed data to the pediatric NMA, incorporating interventions 1, 2, 3 and 5. When treatment success was defined by a composite outcome requiring both clinical measures of convergence to be normal, and also show a pre‐specified magnitude of improvement, we found high‐certainty evidence that office‐based vergence/accommodative therapy with home reinforcement increases the chance of a successful outcome, compared with home‐based computer vergence/accommodative therapy (risk ratio (RR) 1.96, 95% confidence interval (CI) 1.32 to 2.94), home‐based pencil/target push‐ups (RR 2.86, 95% CI 1.82 to 4.35); and placebo (RR 3.04, 95% CI 2.32 to 3.98). However, there may be no evidence of any treatment difference between home‐based computer vergence/accommodative therapy and home‐based pencil/target push‐ups (RR 1.44, 95% CI 0.93 to 2.24; low‐certainty evidence), or between either of the two home‐based therapies and placebo therapy, for the outcome of treatment success.
When treatment success was defined as the composite convergence and symptom success outcome, we found moderate‐certainty evidence that participants who received office‐based vergence/accommodative therapy with home reinforcement were 5.12 (95% CI 2.01 to 13.07) times more likely to achieve treatment success than those who received placebo therapy. We found low‐certainty evidence that participants who received office‐based vergence/accommodative therapy with home reinforcement might be 4.41 (95% CI 1.26 to 15.38) times more likely to achieve treatment success than those who received home‐based pencil push‐ups, and 4.65 (95% CI 1.23 to 17.54) times more likely than those who received home‐based computer vergence/accommodative therapy. There was no evidence of any treatment difference between home‐based pencil push‐ups and home‐based computer vergence/accommodative therapy, or between either of the two home‐based therapies and placebo therapy.
One RCT evaluated the effectiveness of base‐in prism reading glasses in children. When base‐in prism reading glasses were compared with placebo reading glasses, investigators found no evidence of a difference in the three outcome measures of near point convergence (NPC), positive fusional vergence (PFV), or symptom scores measured by the Convergence Insufficiency Symptom Survey (CISS).
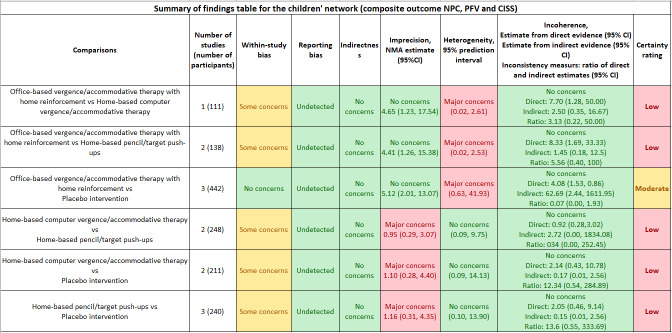
Six RCTs in the adult population randomized 321 participants. We rated only one RCT at low risk of bias. Because not all studies of adults included composite success data, we could not conduct NMAs for treatment success. We thus were limited to comparing the mean difference (MD) between interventions for improving NPC, PFV, and CISS scores individually using data from three RCTs (107 participants; interventions 1, 2, 4 and 5). Compared with placebo treatment, office‐based vergence accommodative therapy was relatively more effective in improving PFV (MD 16.73, 95% CI 6.96 to 26.60), but there was no evidence of a difference for NPC or the CISS score. There was no evidence of difference for any other comparisons for any outcomes. One trial evaluated base‐in prism glasses prescribed for near‐work activities and found that the prism glasses group had fewer symptoms compared with the placebo glasses group at three months (MD ‐8.9, 95% CI ‐11.6 to ‐6.3). The trial found no evidence of a difference with this intervention in NPC or PFV.
No adverse effects related to study treatments were reported for any of the included studies. Excellent adherence was reported for office‐based vergence/accommodative therapy (96.6% or higher) in two trials. Reported adherence with home‐based therapy was less consistent, with one study reporting decreasing adherence over time (weeks 7 to 12) and lower completion rates with home‐based pencil/target push‐ups.
Authors' conclusions
Current research suggests that office‐based vergence/accommodative therapy with home reinforcement is more effective than home‐based pencil/target push‐ups or home‐based computer vergence/accommodative therapy for children. In adults, evidence of the effectiveness of various non‐surgical interventions is less clear.
Plain language summary
How do different treatments for the vision disorder, convergence insufficiency, compare in effectiveness?
Why is this question important? Convergence insufficiency is a common vision disorder in which a person's eyes tend to drift outwards when they try to use their eyes together up close. This can cause eye strain, headaches, blurred and double vision. When reading, people with convergence insufficiency frequently lose their place or have to re‐read text.
There are two main types of treatment for convergence insufficiency: 1) prism‐lensed reading glasses, designed to improve visual comfort, and 2) eye (vision) therapy designed to restore normal visual function and improve visual comfort.
Different types of vision therapy are prescribed for the treatment of convergence insufficiency that aim to improve the affected person’s convergence ability (the ability of eyes to turn inwards). Treatment can be self‐administered at home using only a pencil (pencil push‐ups) or a computer software program (home‐based computer therapy). Alternatively, it can consist of a sequence of activities individually prescribed and monitored by the doctor, administered by trained therapists in an office setting along with practice at home (office‐based therapy with home reinforcement).
We reviewed the evidence from research studies to compare the effectiveness of these different treatments (prism reading glasses, office‐based therapy with home reinforcement, and home‐based treatments), and also to determine whether the treatments are associated with adverse (unwanted) effects.
How did we identify and evaluate the evidence? First, we searched the medical literature for randomized controlled studies (clinical studies where people are randomly put into one of two or more treatment groups). This type of study provides the strongest evidence about the effects of a treatment. We compared the results and summarized the evidence from all the studies. Finally, we assessed how certain the evidence was by considering factors such as the way studies were conducted, the number of people in the studies, and the consistency of findings across studies. Based on our assessments, we categorized the evidence as being of very low‐, low‐, moderate‐, or high‐certainty.
What did we find? We found 12 studies with a total of 1289 people with convergence insufficiency. Six studies were conducted in children aged seven to 18 years, five studies in young adults aged 15 to 40 years, and one study in adults aged 40 years and older. Studies lasted for between six weeks and six months.
Results in children For improving convergence ability, high‐certainty evidence showed that office‐based therapy with home reinforcement is better than placebo, home‐based computer therapy, and home‐based pencil push‐ups.
For improving convergence ability, as well as symptoms reported by children (such as headaches or frequent loss of place when reading), low‐ to moderate‐certainty evidence suggested that office‐based therapy with home reinforcement is better than placebo, home‐based computer therapy, and home‐based pencil push‐ups.
It is not clear (low‐ to moderate‐certainty evidence) whether there is a difference for improving convergence alone, or convergence and symptoms as reported by children, between home‐based computer therapy and home‐based pencil push‐ups, or between these two home‐based treatments and placebo.
One study compared prism reading glasses against placebo reading glasses, and found no evidence of a difference in improvement in convergence or symptoms.
Results in adults Evidence from three studies indicated that office‐based therapy could be more effective than placebo for improving convergence when it was measured one way (‘positive fusional vergence’), but not when measured another way (‘near point convergence’). There was no difference between treatments for changes in symptoms reported by adults.
One study compared glasses with prism lenses against placebo glasses, and found that adults with prism glasses reported fewer symptoms. However, there was no evidence of a difference for improvement in convergence.
Are there any adverse effects from treatment? No study, in children or adults, reported any adverse effects related to study treatments.
What does this mean? High‐certainty evidence indicates that, office‐based therapy with home reinforcement is more effective than home‐based pencil push‐ups, home‐based computer therapy, and placebo for treating convergence insufficiency in children. For adults, the comparative effects of these interventions are less clear.
How‐up‐to date is this review? The evidence in this Cochrane Review is current to September 2019.
Background
Description of the condition
Convergence insufficiency is a common binocular vision disorder in which the eyes have a strong tendency to drift outward (exophoria), with difficulty turning the eyes inward when reading or doing close work. Because the eyes do not converge adequately, this condition may lead to symptoms including eye strain, headaches, double vision, a sense of print moving on the page, frequent loss of place when reading, inability to concentrate, and short attention span. Convergence insufficiency is diagnosed when exophoria is greater at near than at distance, and the patient has both a remote NPC and decreased positive fusional vergence.
There is considerable variability in the reported prevalence of convergence insufficiency. Estimates of prevalence are based on samples of elementary, high school, and university students, with estimates that range from 2.25% to 17.6% (Davis 2016; Hussaindeen 2017; Letourneau 1979; Letourneau 1988; Ma 2018; Porcar 1997; Rouse 1999; Wajuihian 2015). There is a paucity of data regarding whether the prevalence of convergence insufficiency varies by ethnicity, race, age, sex, geographic location, or socioeconomic status.
Description of the intervention
Various non‐surgical treatments are prescribed for treating convergence insufficiency, including base‐in prism reading glasses, home‐based convergence therapy (pencil push‐ups or near target push‐up therapy), home‐based vergence/accommodative therapy with or without computer software, and office‐based vergence/accommodative therapy (Chin 1995; Gallaway 2002; Griffin 2002; Grisham 1998; Hugonnier 1969; Pratt‐Johnson 2001; Press 1997; Scheiman 2002a; Scheiman 2002b; von Noorden 1994; von Noorden 1996). Although surgery and botulinum toxin A injections are potential treatment options for the less common strabismic form of convergence insufficiency, they are rarely used due to the availability of effective non‐surgical interventions. These surgical procedures are not considered in this review.
Base‐in prism reading glasses
There are various methods for determining the amount of prism to prescribe (Scheiman 2019). In a Convergence Insufficiency Treatment Trial (CITT) trial of children nine to 17 years of age (CITT 2005a), and in the Nabovati 2020 study of young adults, the investigators prescribed prism based on Sheard's criterion (Sheard 1930). For convergence insufficiency, this criterion states that the magnitude of the prism should be two‐thirds of the exophoria magnitude minus one‐third the compensatory near positive fusional vergence (PFV), i.e. 2/3 (phoria) minus 1/3 (PFV).
Home‐based convergence therapy: basic (pencil or target push‐ups)
Home‐based convergence therapy is described by Duke‐Elder as follows: "carried out simply by the subject holding a target at arm's length and then gradually bringing it towards the eye, all the time maintaining bifoveal fixation" (Duke‐Elder 1973). This procedure should be carried out several times each day for a few minutes. Placing a target in the background that can be used to monitor physiological diplopia is often recommended (Hugonnier 1969; Press 1997; Scheiman 2002a; Scheiman 2002b; von Noorden 2001). Studies surveying the ophthalmic community have suggested that home‐based convergence exercises are the most commonly prescribed treatment for convergence insufficiency by both optometrists and ophthalmologists (Chin 1995; Scheiman 2002a; Scheiman 2005). However, these surveys were completed more than 15 years ago, and thus preceded the availability of comparative effectiveness data from RCTs.
In three trials (CITT 2005c; CITT 2008, PEDIG 2016), the home‐based convergence procedure (referred to in the trials as pencil or target push‐ups) used a pencil with 20/60 (6/18) Snellen optotypes for the target that was slowly brought closer to the participant's eyes, while the participant attempted to keep the target single and clear. An index card was placed in the background so that physiological diplopia awareness could be used to monitor suppression. Participants were instructed to perform the pencil push‐up procedure 15 minutes per day, five days per week.
Home‐based convergence therapy: more intensive
Some clinicians prescribe home‐based therapy that is more intensive than pencil push‐ups alone (Scheiman 2002a; Scheiman 2002b). Additional home‐based therapy techniques for convergence insufficiency include the use of prism, stereoscopes, and computer software programs designed for vergence/accommodative therapy (Huston 2015; Nehad 2018; Scheiman 2002a; Scheiman 2005; Serna 2011).
In two trials (CITT 2008, PEDIG 2016), participants in the computer vergence/accommodative therapy group were prescribed the aforementioned pencil push‐up procedure, as well as therapy using the Home Therapy System/Computerized Vergence System (HTS/CVS) computer software. Using this therapy program, participants performed fusional vergence and accommodative therapy procedures designed to improve convergence and divergence amplitudes and accommodative ability. Participants were instructed to do pencil push‐ups five minutes per day and use the HTS/CVS software program for 15 minutes per day.
Office‐based vergence/accommodative therapy
Office‐based vergence/accommodative therapy for convergence insufficiency involves a sequence of activities designed to develop normal and efficient visual function. It incorporates purposeful, controlled manipulation of target blur, disparity, and proximity, with the aim of normalizing the accommodative and vergence systems and their mutual interactions (Ciuffreda 2002).
In three CITT trials (CITT 2005b; CITT 2008; CITT‐ART 2019), participants in the outpatient (referred to as "office‐based" in the trials) vergence/accommodative therapy group received weekly 60‐minute in‐office therapy sessions, with additional prescribed procedures to be performed at home for 15 minutes a day, five days per week. At each office‐based therapy session, participants performed four to five procedures with supervision and guidance from a therapist. The therapist followed a treatment protocol from the CITT Manual of Procedures, which detailed the sequence of procedures to be performed, with specifications for length of time to spend, instructions to be provided to participants, goals, and criteria for advancing to a more difficult level or the next therapy procedure. The Aletaha 2018 study included two office‐based therapy groups. The first consisted of therapy using the major amblyoscope twice weekly, with home‐based therapy (pencil push‐ups) prescribed 15 minutes a day, five days per week. Those in the second office‐based treatment group performed the same therapy using the major amblyoscope, supplemented by having the participants wear ‐3.00D lenses and base‐out prism while reading or performing near tasks.
Office‐based placebo therapy
In three CITT trials (CITT 2005b; CITT 2008; CITT‐ART 2019) participants in the office‐based placebo therapy group received placebo therapy during a weekly 60‐minute office visit and were prescribed placebo therapy procedures to be performed at home for 15 minutes per day, five days per week. The placebo procedures were designed to look like real vergence/accommodative therapy yet not stimulate vergence, accommodation, or fine saccadic eye movement skills beyond normal daily visual activities. The therapist also followed a detailed protocol from the CITT Manual of Procedures (accessed at www.optometry.osu.edu/research/CITT/4363.cfm).
How the intervention might work
The two main categories of intervention for convergence insufficiency are base‐in reading glasses and convergence therapy. Convergence therapy can be subdivided into basic home‐based convergence therapy (i.e. pencil or target push‐ups), more intensive home‐based vergence/accommodative therapy (e.g. home‐based computer vergence/accommodative therapy), and office‐based vergence/accommodative therapy, as described above.
Patients with convergence insufficiency are often symptomatic because they have insufficient convergence ability to compensate for their exophoria at near. Base‐in prism reading glasses are believed to work by decreasing the amount of compensatory PFV needed for comfortable vision. While the exact mechanism of how convergence therapy works is unknown, the hypothesis is that the resultant increase in PFV ability relieves the symptoms associated with convergence insufficiency. In addition, individuals with symptomatic convergence insufficiency have abnormal vergence adaptation, which is the mechanism that allows convergence to dissociate from accommodation (Schor 1986). Vergence adaptation has been shown to normalize in convergence insufficiency patients after vergence/accommodative therapy (North 1982; Brautaset 2006).
The three convergence therapies (home‐based pencil or target push‐ups, home‐based computer vergence/accommodative therapy, and office‐based vergence/accommodative therapy) differ in their 1) ability to control/manipulate stimulus parameters; 2) dosage; 3) mode of administration (office or home); 4) use of motor learning theory and patient feedback; and 5) cost.
Controlling/manipulating stimulus parameters
To increase fusional vergence amplitudes a therapy procedure must either maintain accommodation at the plane of regard and increase the vergence demand, or maintain vergence at the plane of regard and increase the stimulus to accommodation (Scheiman 2002b). There are a variety of available instruments and procedures that allow manipulation of the stimulus parameters to create various vergence demands.
The three convergence therapies described above vary significantly in their ability to allow the manipulation of stimulus parameters. With home‐based convergence exercises, the stimulus is typically a small letter on a pencil that is moved closer and closer to the patient's eyes. To maintain single vision, a combination of proximal, accommodative, and fusional vergence is used with the accommodative and convergence systems synchronized. In contrast, office‐based vergence/accommodative therapy uses a wide variety of instrumentation that is designed to improve the dynamics of the fusional vergence and accommodative systems, typically using stimuli that require an accommodative demand different from the vergence demand. Hence, fusional vergence must be used while proximal and accommodative vergence are minimized. Fusional vergence is trained separately and directly, using numerous procedures with varied stimulus parameters. Home‐based convergence exercises using solely a few procedures such as loose prism and computer‐based vergence/accommodative therapy provide an intermediate level of manipulation of the vergence/accommodative relationship, but lack the variety of stimuli available with office‐based vergence/accommodative therapy, as well as therapist feedback.
Dosage
Patients are prescribed procedures to be performed for approximately the same amount of time at home for all three types of convergence therapy. However, those undergoing office‐based treatment, also perform an additional 60 minutes per week of therapy in the doctor's office, resulting in more total therapy time. Total therapy time prescribed tends to be least with home‐based convergence exercises and the most with office‐based vergence/accommodative therapy.
Mode of administration
For office‐based vergence/accommodative therapy, a trained therapist administers the treatment providing the patient with feedback regarding performance, attempting to motivate the patient to make his or her best effort, and increasing the difficulty of the therapy procedures based on the patient's progress. For the two home‐based convergence therapy approaches, close supervision from a trained therapist is not available, although parents are expected to assist younger children with their therapy.
Motor learning principles and patient feedback
Learning is a set of internal processes associated with practice or experience that result in a relatively permanent change in responding (Schmidt 1988). These processes are believed to be a central nervous system phenomenon in which sensory and motor information is organized and integrated (Aikon 1988; Arbib 1981; Lisberger 1988) with an ultimate goal of transferring the motor learning outside of the therapy setting.
For motor learning, numerous variables are considered important determinants. These include the use of feedback, modeling and demonstration, transfer of training, part to whole task practice, variability in practice, and positive reinforcement. Of the three therapy approaches, office‐based vergence/accommodative therapy uses the principles of motor learning and patient feedback most frequently and consistently (Birnbaum 1977; Scheiman 2002b).
Changes in underlying neurophysiology
Alvarez and colleagues have used objective recording of vergence eye movements and functional magnetic resonance imaging (fMRI) as outcome measures in studies of participants with convergence insufficiency who received vergence/accommodative therapy (Alvarez 2010; Alvarez 2014, Alvarez 2019). They found significant changes in functional activity of the vergence neural circuits between pre‐ and post‐therapy measurements. These data suggest that post‐therapy, there is both an increase in recruitment of neurons and better synchronization of metabolic demand from the neurons, specifically in the frontal eye fields and the oculomotor vermis. Another group that used fMRI as an outcome measure found that after therapy the activation in the occipital lobe decreased in spatial extent but increased in the posterior, inferior portion of the occipital lobe (Widmer 2018). These data suggest that disparity processing for vergence may be enhanced following vergence/accommodative therapy for convergence insufficiency.
Why it is important to do this review
While all interventions described in this review are prescribed for patients with convergence insufficiency, there is a lack of consensus among eye care professionals regarding the most effective treatment. One possible reason is that existing trials made pairwise comparisons of one treatment to another, and it is difficult to form a coherent picture of the comparative effectiveness and hierarchy of all interventions using the traditional pairwise meta‐analysis. Because significant differences exist in the time commitment for the patient, number of office visits, cost, and complexity of the intervention, a systematic review and network meta‐analysis (NMA) is the best approach to synthesizing the available evidence and providing useful findings to help clinicians and patients select the most appropriate treatments for symptomatic convergence insufficiency. The current review updates the evidence from an earlier version (Scheiman 2011) and extends the analysis to incorporate NMA.
Objectives
To assess the comparative effectiveness (primary objective) and relative ranking (secondary objective) of non‐surgical interventions for convergence insufficiency, through a systematic review and NMA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in this review.
Types of participants
We included trials in which participants had been treated for convergence insufficiency using non‐surgical interventions. The definition of convergence insufficiency varies considerably from study to study. For this review, we define convergence insufficiency as a condition characterized by a larger exophoria at near than at far, and one or both of the following objective clinical signs:
A receded near point of convergence (NPC) (6 cm or greater) (Hayes 1998; Scheiman 2003);
Insufficient PFV at near (i.e. less than twice the near phoria (Sheard's criterion) or PFV less than 15 ∆) (Sheard 1930; Scheiman 2002b).
We analyzed data for children (< 18 years old) and adults (18 years or older) separately because the effectiveness of the interventions are likely to be different in these two populations. The transitivity assumption for NMA is more likely to hold by analyzing these two populations separately.
Types of interventions
We included trials comparing any form of non‐surgical intervention against placebo, no intervention, a sham intervention, or another type of intervention, for patients with convergence insufficiency.
Based on our knowledge and a preliminary review of literature, possible nodes for NMA included home‐based convergence exercises (pencil/target push‐ups), home‐based computer vergence/accommodative therapy, office‐based vergence/accommodative therapy, base‐in prism reading glasses, base‐in prism glasses combined with progressive addition lenses, and placebo or sham intervention. All these interventions are available for patients and can be used for convergence insufficiency. Therefore, the concept of "joint randomizability" applies to all interventions included in the network (Salanti 2012). Note that some of the interventions might not be connected to the main network for analysis.
Types of outcome measures
Primary outcomes
We introduced 'treatment success at 12 weeks' as a new outcome, during this review's current update. Treatment success is defined in two ways:
Composite convergence outcome: achieved normal and improved NPC and PFV: NPC is normal (< 6 cm) and has improved by ≥ 4 cm; PFV is normal (passing Sheard's criterion and a PFV break > 15 ∆) and has improved ≥ 10 ∆; and
Composite signs (convergence) and symptoms outcome: achieved normal and improved NPC, PFV, and Convergence Insufficiency Symptom Survey (CISS) score: NPC is normal (< 6 cm) and has improved by ≥ 4 cm, PFV is normal (passing Sheard's criterion and a PFV break > 15 ∆) and has improved ≥ 10 ∆, and CISS is normal (< 16) and has improved ≥ 10 points.
We introduced these two outcome measures to this review because the treatment of convergence insufficiency is designed to improve patients’ convergence ability. Clinically, we evaluate this function based on the two clinical convergence measurements that are used to diagnose convergence insufficiency: NPC and PFV. The expectation is that both of these clinical measures should no longer be deficient after successful treatment for convergence insufficiency with active forms of therapy. Because there are no data to indicate that one convergence measure is more important than the other, both measures were used in defining this 'composite convergence outcome.' Evaluating whether a pre‐determined magnitude of improvement occurred for both clinical measures was deemed to be a more robust indication of successful treatment than using either clinical measure by itself. In addition, we incorporated a second outcome measure (composite signs and symptoms) that added patient‐reported symptoms (using the CISS) to the composite convergence outcome of NPC and PFV. These two outcome measures have been reported previously (CITT 2008; CITT‐ART 2019).
When the follow‐up period was shorter than 12 weeks, we analyzed outcome data at the longest follow‐up point. When the follow‐up period was longer than 12 weeks, we analyzed outcome data at the time point closest to 12 weeks.
Secondary outcomes
We considered NPC and PFV measures at near after 12 weeks of intervention (the primary outcomes of earlier versions of this review) to be secondary outcomes.
In addition, we analyzed participant symptoms at 12 weeks, as reported in the included studies. We assessed symptoms whenever trials had used some formal instrument for examining symptoms (Borsting 2003; Rouse 2004; Rouse 2009). One instrument that has been developed and validated for assessing convergence insufficiency symptoms before and after treatment is the CISS Version 15, a 15‐item questionnaire that measures symptoms experienced when reading or doing other close work (Borsting 2003; Rouse 2004; Rouse 2009). The higher the CISS score, the more symptoms experienced by the patient. In differentiating individuals with symptomatic convergence insufficiency from those with normal binocular vision, the CISS was shown to have a sensitivity of 96% and specificity of 88%, when using a cut‐off score of ≥ 16 for children (Borsting 2003; Rouse 2009), and a sensitivity of 98% and specificity of 87%, when using a cut‐off score ≥ 21 for adults (Rouse 2004).
We reported adherence to treatment.
Adverse outcomes
Adverse effects of interest included worsening of diplopia (double vision), worsening of headaches, and convergence spasm.
We summarized the reported adverse effects related to each intervention.
Quality of life data
We planned to describe data on quality of life when available from included trials.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for randomized controlled trials and controlled clinical trials. There were no restrictions to language or year of publication. The electronic databases were last searched on 20 September 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 20 September 2019) (Appendix 1).
MEDLINE Ovid (1946 to 20 September 2019) (Appendix 2).
Embase.com (1947 to 20 September 2019) (Appendix 3).
PubMed (1948 to 20 September 2019) (Appendix 4).
metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com; last searched 6 October 2010 as this database is no longer available) (Appendix 5)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 20 September 2019) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 20 September 2019) (Appendix 7).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We used the Science Citation Index (SCI) to find studies that had cited the reports of included trials. We contacted the primary investigators of identified trials for details of additional trials when needed. We also conducted manual searches of the following journals: Journal of the American Optometric Association (1990 to 2009); Journal of Behavioral Optometry (1990 to 2009); Optometry & Vision Development (1969 to 2009); American Orthoptic Journal (1951 to 2009); Australian Orthoptic Journal (1973 to 2009); and British and Irish Orthoptic Journal (formerly the British Orthoptic Journal) (1954 to 2009).
Data collection and analysis
Selection of studies
At least two review authors independently reviewed the titles and abstracts resulting from the electronic and manual searches according to the eligibility criteria stated above. We classified abstracts as 'definitely exclude,' 'unsure,' or 'definitely include.' We obtained the full text for articles in the 'unsure' and 'definitely include' categories and re‐assessed them for final eligibility. After examining the full text, studies labeled as 'excluded' by both authors were not included in the review and the reasons for exclusion documented. Included studies were assessed further for their methodological quality. We resolved discrepancies through discussion and consensus.
Data extraction and management
At least two review authors independently extracted the data onto paper data collection forms. We resolved discrepancies through discussion. One review author entered all data into Review Manager 5 (RevMan 5) (Review Manager 2014). Data entered were verified by a second author. We extracted the following details from the studies, including those relevant for assessing the transitivity assumption: methods, participants, interventions, outcomes, adverse events, quality of life issues, economic data, and important information not captured otherwise. We requested and received individual participant data on treatment success from PEDIG 2016.
Assessment of risk of bias in included studies
At least two review authors assessed the sources of potential systematic bias in trials according to the first version of the Cochrane risk of bias tool, described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). The following parameters were considered: a) randomization sequence generation; b) allocation concealment; c) masking (blinding) of the primary and secondary outcome assessors; d) completeness of outcome data for the primary and secondary outcomes; and e) selective outcome reporting. Each of the parameters was graded as being at 'low risk of bias,' 'high risk of bias,' or 'unclear risk of bias.' Because of the nature of the intervention, masking of participants and care providers was not possible in all trials, and consequently was not used as a quality parameter in this review.
The version of 'Risk of bias' tool we used did not incorporate an algorithm to derive an overall risk of bias for each trial. In order to use the Confidence in Network Meta‐Analysis (CINeMA) framework developed by Salanti and colleagues for evaluating the confidence in the evidence from the NMAs (Salanti 2014), we judged the overall risk of bias of a trial as high if two more domains (out of a total of six domains) were rated at 'high risk of bias,' unclear if four or more domains were rated at 'unclear risk of bias,' and low if five or more domains were rated at 'low risk of bias.'
Measures of treatment effect
We calculated a summary risk ratio (RR) for dichotomous outcomes and mean difference (MD) between interventions for continuous outcomes. We reported the estimate of effect and associated 95% confidence intervals (CI).
Unit of analysis issues
We conducted a person‐based analysis because convergence insufficiency is a binocular vision disorder. None of the trials included in this review used a cluster or cross‐over design. We included all eligible treatment groups from multi‐arm trials in the NMA without combining any groups.
Dealing with missing data
We contacted the lead investigator of the trial in an attempt to obtain additional information, when necessary. Whenever the authors did not respond within four weeks, we continued the review based on the available information.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity qualitatively by examining the characteristics of each included trial. We assessed statistical heterogeneity quantitatively using the Chi2 test and the I2 values. We pre‐specified that a P value of less than 0.1 from the Chi2 test and an I2 statistic of greater than 50% indicated substantial statistical heterogeneity.
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias if a sufficient number of trials were identified.
Data synthesis
We analyzed two networks of trials. One network comprised the trials with children as participants and the other comprised the trials with adult participants. For each network, we first conducted a pairwise meta‐analysis for every direct comparison using a random‐effects model. We then fitted random‐effects NMA models following the multivariate approach by Chaimani and White (Chaimani 2013; Chaimani 2015; White 2015). We executed these analyses using Stata packages ‘mvmeta,' ‘network,' and ‘network graphs,’ assuming a common heterogeneity across all comparisons in the network. We generated the effect estimates (RR or MD) between any two interventions in the network and used the 'mean rank' value to rank the interventions on all outcomes where NMA was possible. For the children's network, we excluded Nehad 2018 due to data errors discovered in the manuscript. Authors of Nehad 2018 did not respond to our inquiry for clarification.
The indirect comparisons made in the NMA are built on the assumptions of transitivity and coherence (Salanti 2012). The transitivity assumption indicates that the indirect comparison is a valid estimation of the unobserved direction comparison, the validity of which can be conceptually evaluated. The transitivity assumption is likely to hold in our data because the interventions analyzed are all treatments for convergence insufficiency. The coherence assumption implies agreement between direct and indirect estimates, which can be tested statistically. We evaluated the coherence assumption using the loop‐specific approach (Bucher 1997; Veroniki 2013). When statistical incoherence was detected, we first examined the trial characteristics, and then conducted a sensitivity analysis by fitting an incoherence model.
We used STATA 14 (StataCorp LP, College Station, TX) for all analyses. Raw data for all analyses are available on request.
Subgroup analysis and investigation of heterogeneity
We examined potential sources of heterogeneity qualitatively. As described above, to control for known heterogeneity, we analyzed the children's and adults' networks separately.
Sensitivity analysis
We pre‐specified that we would conduct sensitivity analyses to determine the impact of exclusion of studies at higher risk of bias, unpublished studies, and industry‐funded studies. However, because there were so few studies for the NMA, we did not conduct any sensitivity analyses. The incoherence model for NMA did not converge.
Evaluating confidence in the evidence
We used the CINeMA framework by Salanti and colleagues for evaluating the confidence in the evidence from the NMAs (Salanti 2014) for treatment success (using both definitions). This approach covers six confidence domains: within‐study bias (i.e., risk of bias in the included studies), across‐studies bias (i.e., publication and other reporting bias in the included studies), indirectness, imprecision, heterogeneity, and incoherence. CINeMA assigns judgements at three levels (no concerns, some concerns, or major concerns) to each of the six domains. Judgements across the six domains are then summarized to obtain four levels of confidence for each relative treatment effect, corresponding to the usual GRADE approach: very low, low, moderate, or high (Nikolakopoulou 2019). Among the six confidence domains, the domains for within‐study bias and indirectness are based on the contribution made by each study to each estimate of effect on a 0 to 100 percent scale ("percentage contribution matrix"). Judgement on imprecision, heterogeneity, and incoherence relies on defining relative treatment effects that exclude any clinically important differences in outcomes between interventions. For treatment success expressed in RR, we used a margin of equivalence of (0.8 to 1.25) for this purpose. This interval is symmetrical on the natural logarithm scale.
Results
Description of studies
Results of the search
The electronic searches identified 1526 unique records (Figure 1). We screened the records and obtained full‐text reports of 72 records. After assessment we included 16 records reporting on 12 trials that were relevant to this systematic review (Aletaha 2018; Birnbaum 1999; CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019; PEDIG 2016; Momeni Moghaddam 2015; Nabovati 2020; Nehad 2018; Widmer 2018). We excluded 60 studies, see Characteristics of excluded studies for details. A study by Teitelbaum 2009 was included in the previous version of this review, however it has been re‐assessed and is now an excluded study as the participants did not meet the clinical definition of convergence insufficiency. The following four studies are classed as ongoing trials and will be assessed when data becomes available (CTRI/2018/05/013560; DRKS00014187; NCT03593031; U1111‐1194‐7855).
1.
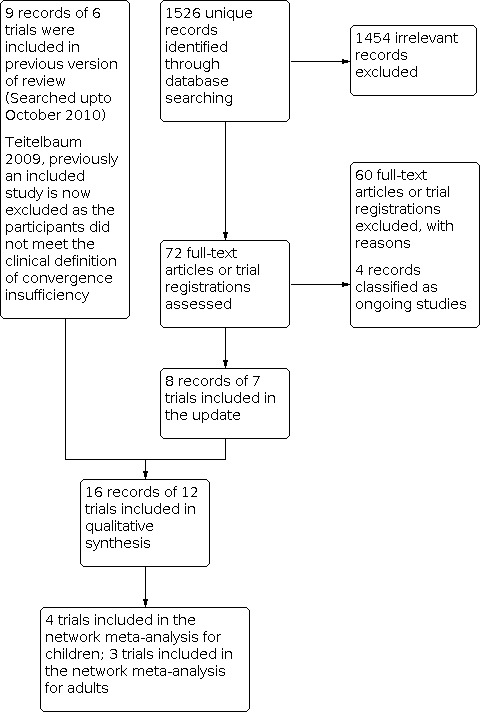
Study flow diagram
Included studies
We have presented the characteristics for each included study in the 'Characteristics of included studies' table.
We included 12 trials with a total of 1289 participants with convergence insufficiency. We excluded Nehad 2018 from analysis due to data errors discovered in the manuscript. We contacted the authors and asked them to clarify the data discrepancies, but they failed to respond. Eight trials were conducted in the United States, three in Iran, and one in Egypt. The trials varied in size, with the smallest enrolling seven participants (Widmer 2018) and the largest enrolling 311 participants (CITT‐ART 2019; one participant was determined ineligible after randomization). Five of the included trials, funded by the National Eye Institute of the US National Institutes of Health, were conducted by the CITT Study Group (CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019). These trials randomized 697 (54%) of participants included in this systematic review. Symptomatic convergence insufficiency was defined consistently across the five CITT trials and the eligibility criteria were also comparable. Six other trials (Aletaha 2018; PEDIG 2016; Momeni Moghaddam 2015; Nabovati 2020; Nehad 2018; Widmer 2018) adopted similar eligibility criteria to the CITT trials. The remaining trial (Birnbaum 1999) enrolled 60 adult male participants from a US Veterans Health Administration Medical Center. We found clinical heterogeneity in several aspects, mainly in the age distribution of trial participants. Six trials were conducted in participants < 18 years of age (CITT 2005a; CITT 2005b; CITT 2008; CITT‐ART 2019; PEDIG 2016; Nehad 2018); five trials were conducted in young adult participants up to 40 years of age (Aletaha 2018; CITT 2005c; Momeni Moghaddam 2015; Nabovati 2020; Widmer 2018); the remaining trial was conducted in adults 40 years or older (Birnbaum 1999). Because of age‐related differences in accommodation, it was important to analyze the findings for children separately from young adults and from presbyopic adults.
Types of interventions
The included trials evaluated a variety of interventions, including passive treatment with base‐in prism reading glasses, and active treatments with office‐based vergence/accommodative therapy, basic home‐based convergence therapy (pencil/target push‐ups), home‐based computer vergence/accommodative therapy plus pencil push‐ups, and placebo or sham procedures. The interventions and comparison interventions are described in detail in the 'Characteristics of included studies' table and Table 1. We kept the same terms that were used in the trials to refer to each intervention.
1. Interventions compared in the included trials.
| Study ID | Home‐based pencil/target push‐ups | Home‐based computer vergence/accommodative therapy | Office‐based vergence/accommodative therapy only |
Office‐based vergence/accommodative therapy with home reinforcement |
Prism reading glasses | Other therapy | Placebo reading glasses | Placebo vergence/accommodative therapy or other placebo intervention | Population |
| Aletaha 2018 | ✓ | ✓ (office‐therapy was amblyoscope only) | Office therapy (amblyoscope only) performed while wearing ‐3D lenses and base‐out prism | Adolescent and adults aged 15 to 35 years | |||||
| Birnbaum 1999 | ✓ | ✓ | ✓ | Male adult ≥ 40 years | |||||
| CITT 2005a | ✓ | ✓ | Children aged 9 to 18 years | ||||||
| CITT 2005b | ✓ | ✓ | ✓ | Children aged 9 to 18 years | |||||
| CITT 2005c | ✓ | ✓ | ✓ | Young adults aged 19 to 30 years | |||||
| CITT 2008 | ✓ | ✓(and pencil push‐ups) | ✓ | ✓ | Children aged 9 to 17 years | ||||
| CITT‐ART 2019 | ✓ | ✓ | Children aged 9 to 14 years | ||||||
| PEDIG 2016 | ✓ | ✓ (and near target push‐ups) | ✓ | Children aged age 9 to 17 years | |||||
| Momeni Moghaddam 2015 | ✓ | ✓ | University students (mean age 21.3 ± 0.9 years) | ||||||
| Nabovati 2020 | ✓ | ✓ | Young adults aged 18‐38 years | ||||||
| Nehad 2018 | ✓ | ✓ | Children < 16 years | ||||||
| Widmer 2018 | ✓ | ✓ | Young adults aged 18 to 30 years |
Aletaha 2018 randomly assigned 84 participants (aged 15 to 35 years) to 6 weeks of home‐based pencil push‐ups, office‐based vision therapy (major amblyoscope only) with home‐based pencil push‐ups, or augmented office‐based vision therapy using both the major amblyoscope and also convergence procedures (‐3.00D lenses and base‐out prism while performing near tasks) with additional home‐based reinforcement. The outcome visit was 12 weeks after the completion of treatment.
Birnbaum 1999 randomly assigned 60 male adults (≥ 40 years; median age 65 years) to receive office‐based vergence/accommodative therapy with supplemental home therapy, home‐based convergence therapy only, or no treatment (control) for 24 weeks with the primary outcome visit after 24 weeks.
CITT 2005a randomly assigned 72 children nine to 18 years of age with symptomatic convergence insufficiency to wear either base‐in prism reading glasses or placebo reading glasses. Participants assigned to base‐in prism reading glasses received glasses that corrected the participants' refractive error, when necessary, and base‐in prism. Participants in the placebo reading glasses group received glasses that corrected their refractive error when necessary, or plano lenses for those who did not require refractive correction. Participants were asked to wear these glasses for all reading and near tasks requiring more than five minutes for the six‐week study duration.
CITT 2005b was considered as a pilot study by the CITT Study Group. In this study, 47 children were randomly assigned to receive a 12‐week program of home‐based pencil push‐ups, office‐based vergence/accommodative therapy, or office‐based placebo therapy, with the primary outcome visit after 12 weeks. These same three treatment modalities were also evaluated in 46 young adults (ages 19 to 30 years) in CITT 2005c.
CITT 2008 randomly assigned 221 children to receive a 12‐week program of home‐based pencil push‐ups, home‐based computer vergence/accommodative therapy plus pencil push‐ups, office‐based vergence/accommodative therapy with home reinforcement, or office‐based placebo therapy, with the primary outcome visit after 12 weeks. The home‐based computer vergence/accommodative therapy plus pencil push‐ups group was considered a more intensive regimen than pencil push‐ups alone. The other three treatment modalities were essentially the same as those in the aforementioned CITT trials.
CITT‐ART 2019 randomly assigned 311 children nine to 14 years of age to receive a 16‐week program of weekly office‐based vergence/accommodative therapy or office‐based placebo therapy, with the primary outcome visit after 16 weeks.
PEDIG 2016 randomized 204 children nine to 18 years of age to receive 12 weeks of either home‐based computer vergence/accommodative therapy, home‐based pencil push‐ups, or home‐based placebo intervention, with the primary outcome visit after 12 weeks.
Momeni Moghaddam 2015 randomly assigned 60 young adults (mean age 21.3 ± 0.9 years) to 8 weeks of home‐based pencil push‐ups or office‐based vergence/accommodative therapy, with the primary outcome visit after 8 weeks.
Nabovati 2020 randomly assigned 64 young adults 18 to 40 years of age to wear either base‐in prism reading glasses or placebo reading glasses for all near work activities lasting more than 15 minutes for 12 weeks, with the primary outcome visit after 12 weeks.
Nehad 2018 randomized 113 children seven to 13 years of age to receive 12 weeks of office‐based vergence/accommodative therapy or office‐based vergence/accommodative therapy with additional home‐based computer vergence/accommodative therapy, with the primary outcome visit after 12 weeks.
Widmer 2018 undertook an fMRI study to investigate changes in brain activation in response to office‐based vergence/accommodative therapy. Seven adult participants (18 to 30 years of age) with convergence insufficiency were randomized to receive either 12 weeks of office‐based vergence/accommodative therapy (n = 4) or placebo therapy (n = 3), with the primary outcome visit after 12 weeks.
Types of outcomes
Eleven of the 12 included trials used consistent outcome measures. The primary outcome for Aletaha 2018, Nabovati 2020, and four of the CITT trials (CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008) was symptom improvement as measured by the CISS V‐15 (Borsting 2003; Rouse 2009;). Aletaha 2018 measured the CISS one week after the six‐week therapy program concluded (primary outcome visit) and subsequently 12 weeks and 24 weeks after discontinuation of therapy. The CISS was evaluated after six weeks in CITT 2005a, and measured after 12 weeks of therapy in the following trials: CITT 2005b; CITT 2005c; CITT 2008; Widmer 2018. Secondary outcome measures of NPC and PFV at near were available in all of these studies. The secondary outcomes for CITT‐ART 2019 were NPC and PFV, evaluated after 16 weeks of therapy. The primary and secondary outcome measures were not explicitly specified in Birnbaum 1999, but the study examined "success" and "failure," defined by the investigators on the basis of the improvement shown with respect to the asthenopia and functional criteria. The primary outcome for PEDIG 2016, Momeni Moghaddam 2015, and Nehad 2018 was a combination of CISS score, NPC, and PFV, measured after either eight weeks (Momeni Moghaddam 2015) or 12 weeks (PEDIG 2016; Nehad 2018) of therapy.
Excluded studies
We excluded 60 reports that initially appeared to be relevant (see reasons in the 'Characteristics of excluded studies' table).
Risk of bias in included studies
We evaluated the risk of bias in each of the 12 included trials, using eight pre‐specified criteria. Three trials (Aletaha 2018; PEDIG 2016; Nabovati 2020) were judged to have high potential for bias in two domains. Ten trials were judged to have unclear risk of bias in two or more domains (Figure 2).
2.

Risk of bias summary.
Allocation
Birnbaum 1999 and Nehad 2018 did not report the procedure used to generate random sequences and whether the intervention allocation was concealed until assigned. Aletaha 2018, Momeni Moghaddam 2015 and Nabovati 2020 did not report whether the intervention allocation was concealed until assigned. When participant assignment involves a non‐random approach, confounding and selection bias may be introduced. Five RCTs (CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019) used a central study website to randomize study participants, and the treatment assignment was concealed to researchers enrolling and allocating participants. PEDIG 2016 and Widmer 2018 also adequately concealed the allocation (personal communication with the review authors).
Blinding
Aletaha 2018 and Nabovati 2020 were judged to have high potential risk of bias because the outcome assessors were not blinded (masked to participant treatment assignment). Birnbaum 1999, Momeni Moghaddam 2015, and Nehad 2018 did not report whether the primary or the secondary outcomes were measured by masked personnel. Inadequate masking may introduce information bias. The other seven trials (CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019; PEDIG 2016; and Widmer 2018) reported that masking was used for the primary and secondary outcomes.
Incomplete outcome data
PEDIG 2016 was judged to have a high potential risk of attrition bias arising from differential loss to follow up among treatment groups with missing outcome visits for 8%, 19%, and 30% of participants in the home‐based computer vergence, home‐based near target push‐up, and home‐based placebo groups, respectively. Aletaha 2018 and Nehad 2018 did not report whether the outcome data were complete. Personal communication of the review authors with the CITT trial statistician revealed that missing data were not imputed in the four CITT trials (CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008).Therefore, available outcome data were used in the analyses. For CITT 2008, 218/221 (99%) completed the outcome visit. In CITT‐ART 2019, data were complete for 97% of the 206 participants in the vergence/accommodative group and 100% of the 104 participants in the placebo group. No participants were lost to follow‐up in Birnbaum 1999. Follow‐up was high in Nabovati 2020 (97% in the prism group and 93% in the placebo group).
Selective reporting
We had insufficient information to assess the risk of selective reporting bias in Aletaha 2018, Birnbaum 1999, Momeni Moghaddam 2015; Nabovati 2020; Nehad 2018; and Widmer 2018. All outcomes described in the study protocol or trial registration of the remaining six trials (CITT 2005a; CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019; PEDIG 2016c) were reported.
Effects of interventions
Children's network
The included trials for children assessed seven interventions, numbered as follows:
office‐based vergence/accommodative therapy with home reinforcement;
home‐based pencil/target push‐ups;
home‐based computer vergence/accommodative therapy;
office‐based vergence/accommodative therapy alone;
placebo vergence/accommodative therapy or other placebo intervention;
prism reading glasses; and
placebo reading glasses.
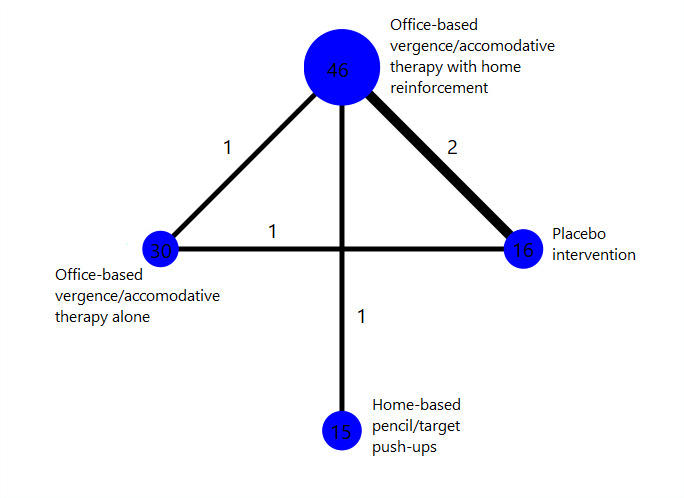
Interventions 1, 2, 3 and 5 formed a network, in which interventions were connected by RCTs comparing at least two of them (Figure 3). Interventions 6 and 7 were disconnected from the network because they were only compared with each other in CITT 2005a but not with other interventions. Interventions 1 and 4 were assessed in Nehad 2018, but we excluded this trial from analysis due to data errors.
3.
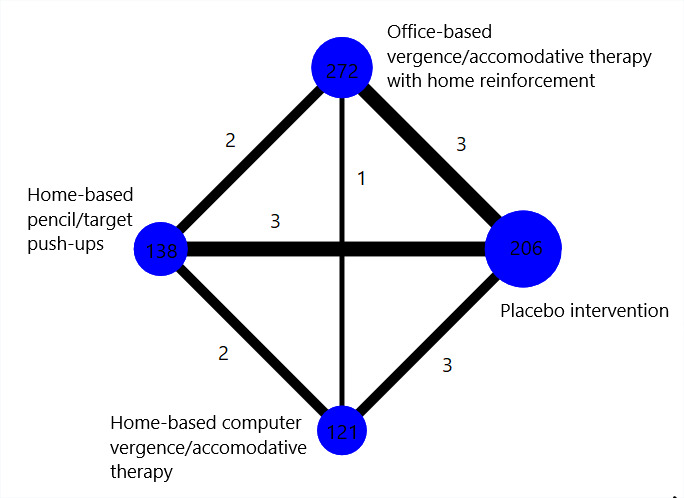
Network graph for the children's network
Number of trials: 4
Number of participants analyzed: 737
Table 2 shows the pairwise meta‐analysis results based on the direct comparisons of two interventions from RCTs, including interventions 6 and 7. There was no evidence of a difference between prism reading glasses and placebo reading glasses for the outcomes of NPC, PFV, or CISS score.
2. Pairwise meta‐analysis or individual study results: children.
| Pairwise comparison | Number of RCTs | Treatment success (normal NPC and PFV, RR with 95% CI); I2 | Treatment success (normal NPC, PFV, and CISS, RR with 95% CI); I2 | Improvement in NPC (MD with 95% CI); I2 | Improvement in PFV (MD with 95% CI); I2 | Improvement in CISS score (MD with 95% CI); I2 |
| Office‐based vergence/accommodative therapy with home reinforcement vs Placebo intervention |
3 | 2.95 (2.23, 3.90); 20.6% | 4.08 (1.53, 0.86); 57.2% | 5.02 (3.14, 6.90); 50.6% | 13.44 (10.97, 15.91); 0.0% | 6.36 (0.30, 12.41); 83.0% |
| Home‐based pencil/target push‐ups vs Office‐based vergence/accommodative therapy with home reinforcement |
2 | 0.32 (0.19, 0.56); ‐ (one trial only for this composite outcome) | 0.12 (0.03, 0.59); ‐ (one trial only for this composite outcome) | ‐3.98 (‐5.85, ‐2.11); 0.0% | ‐13.91 (‐19.23, ‐8.59); 45.5% | ‐12.89 (‐24.10, ‐1.67); 85.4% |
| Home‐based pencil/target push‐ups vs Placebo intervention |
3 | 1.42 (0.77, 2.61); 0.0% | 2.05 (0.46, 9.14); 26.9% | 2.18 (0.43, 3.92); 0.0% | 0.14 (‐3.42, 3.71); 52.0% | ‐1.03 (‐3.88, 1.83); 0.0% |
| Home‐based computer vergence/accommodative therapy vs Home‐based pencil/target push‐ups |
2 | 1.41 (0.91,2.20); ‐ (one trial only for this composite outcome) | 0.92 (0.28,3.02); ‐ (one trial only for this composite outcome) | 1.25 (‐0.40, 2.90); 0.0% | 4.10 (1.63, 6.57); 0.0% | 0.15 (‐2.47, 2.77); 0.0% |
| Home‐based computer vergence/accommodative therapy vs Placebo intervention |
2 | 2.01 (1.12, 3.59); 0.0% | 2.14 (0.43, 10.78); 10.8% | 3.54 (1.78, 5.30); 0.0% | 5.55 (2.84, 8.26); 0.0% | ‐1.15 (‐4.18, 1.88); 0.0% |
| Home‐based computer vergence/accommodative therapy vs Office‐based vergence/accommodative therapy with home reinforcement |
1 | 0.47 (0.30, 0.76); ‐ | 0.13 (0.02, 0.78); ‐ | ‐2.90 (‐4.84, ‐0.96); ‐ | ‐7.70 (‐11.31, ‐4.09); ‐ | ‐8.80 (‐12.34, ‐5.26); ‐ |
| Prism reading glasses vs Placebo reading glasses |
1 | ‐ | ‐ | 2.81 (‐1.67, 7.29); ‐ | ‐0.69 (‐3.96, 2.58); ‐ | 4.26 (‐1.90, 10.42); ‐ |
CI: confidence interval; CISS: Convergence Insufficiency Symptom Survey; MD: mean difference; NPC: near point of convergence; PFV: positive fusional vergence; RCT: randomized controlled trial; RR: risk ratio
Table 3 shows the relative effects of interventions 1, 2, 3, and 5 and the mean ranks based on NMAs of four RCTs (737 participants).
3. Network meta‐analysis results: children.
| Outcome | Mean rank | Interventions | |||
| Treatment success defined as achieving both normal and improved NPC and PFV RR (95% CI) |
1.0 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 2.1 | 1.96 (1.32, 2.94); high‐certainty evidence | Home‐based computer vergence/accommodative therapy | |||
| 3.3 | 2.86 (1.82, 4.35); high‐certainty evidence | 1.44 (0.93, 2.24); low‐certainty evidence | Home‐based pencil/target push‐ups | ||
| 3.6 | 3.04 (2.32, 3.98); high‐certainty evidence | 1.55 (1.00, 2.41); moderate‐certainty evidence | 1.08 (0.66, 1.74); low‐certainty evidence | Placebo intervention | |
| Treatment success defined as achieving normal and improved NPC, PFV, and CISS RR (95% CI) |
1.0 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 3.4 | 4.65 (1.23, 17.54); low‐certainty evidence | Home‐based computer vergence/accommodative therapy | |||
| 3.1 | 4.41 (1.26, 15.38); low‐certainty evidence | 0.95 (0.29, 3.07); low‐certainty evidence | Home‐based pencil/target push‐ups | ||
| 2.5 | 5.12 (2.01, 13.07); moderate‐certainty evidence | 1.10 (0.28, 4.40); low‐certainty evidence | 1.16 (0.31, 4.35); low‐certainty evidence | Placebo intervention | |
| NPC MD (95% CI) |
1.0 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 2.1 | 2.08 (0.11, 4.06) | Home‐based computer vergence/accommodative therapy | |||
| 3.0 | 3.43 (1.46, 5.40) | 1.35 (‐0.57, 3.27) | Home‐based pencil/target push‐ups | ||
| 3.9 | 5.01 (3.56, 6.46) | 2.93 (1.03, 4.83) | 1.58 (‐0.33, 3.49) | Placebo intervention | |
| PFV MD (95% CI) |
1.0 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 2.0 | 8.51 (5.67, 11.35) | Home‐based computer vergence/accommodative therapy | |||
| 3.3 | 13.10 (10.42, 15.78) | 4.58 (2.17, 6.99) | Home‐based pencil/target push‐ups | ||
| 3.7 | 13.78 (11.41, 16.14) | 5.26 (2.72, 7.81) | 0.68 (‐1.67, 3.04) | Placebo intervention | |
| CISS score MD (95% CI) |
1.0 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 3.0 | 8.63 (1.84, 15.42) | Home‐based computer vergence/accommodative therapy | |||
| 3.5 | 9.92 (3.72, 16.12) | 1.29 (‐4.74, 7.32) | Home‐based pencil/target push‐ups | ||
| 2.4 | 6.79 (1.21, 12.36) | ‐1.84 (‐7.95, 4.27) | ‐3.13 (‐8.65, 2.38) | Placebo intervention | |
CI: confidence interval; CISS: Convergence Insufficiency Symptom Survey; MD: mean difference; NPC: near point of convergence; PFV: positive fusional vergence; RR: risk ratio
Values in cells equal improvement (or risk) in column‐defining intervention, minus (or divided by) improvement (or risk) in row‐defining intervention; a MD greater than zero or a RR greater than 1 indicates greater improvement in column‐defining intervention. We used the Confidence in Network Meta‐Analysis (CINeMA) framework for evaluating the certainty of evidence for our primary outcome of 'treatment success' (see Figure 4 and Figure 5).
Treatment success (composite convergence outcome)
Compared with placebo intervention, RR estimates for treatment success, using the composite convergence outcome (defined as achieving a normal and pre‐specified improvement in both NPC and PFV), ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy with home reinforcement (RR 3.04, 95% CI 2.32 to 3.98; high‐certainty evidence); home‐based computer vergence/accommodative therapy (RR 1.55, 95% CI 1.00 to 2.41; moderate‐certainty evidence); and home‐based pencil/target push‐ups (RR 1.08, 95% CI 0.66 to 1.74; low‐certainty evidence). We found high‐certainty evidence that office‐based vergence/accommodative therapy with home reinforcement increases the chance of a successful outcome compared with: home‐based computer vergence/accommodative therapy (RR1.96, 95% CI 1.32 to 2.94); home‐based pencil/target push‐ups (RR 2.86, 95% CI: 1.82 to 4.35); and placebo (RR 3.04, 95% CI: 2.32 to 3.98). There was no evidence of differences between the two home‐based therapies or between the two home‐based therapies and placebo intervention for this outcome (Table 3). The certainty of evidence and any reasons for downgrading for each pair‐wise comparison are available in Table 3 and Figure 4.
4.
Composite convergence outcome: achieved normal and improved NPC and PFV: NPC is normal (<6cm) and has improved by ≥4cm; PFV is normal (passing Sheard's criterion and a PFV break > 15 ∆) and has improved ≥ 10 ∆.
The certainty of evidence was rated 'high' if all domains were of 'no concerns.' The certainty of evidence was rated 'moderate' if two or more domains were of 'some concerns.'
The certainty of evidence was rated 'low' if two or more domains were of 'major concerns' or one domain of 'major concerns' plus one domain of 'some concerns.'
CI: confidence interval.
Treatment success (composite convergence/symptoms outcome)
Compared with placebo intervention, RR estimates for treatment success, using the composite convergence signs and symptoms outcome (defined as achieving normal and improved NPC, PFV, and CISS), ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy with home reinforcement (RR 5.12, 95% CI 2.01 to 13.07; moderate‐certainty evidence); home‐based pencil/target push‐ups (RR 1.16, 95% CI 0.31 to 4.35; low‐certainty evidence); and home‐based computer vergence/accommodative therapy (RR 1.10, 95% CI 0.28 to 4.40; low‐certainty evidence). Office‐based vergence/accommodative therapy with home reinforcement was more effective than the two home‐based therapies and placebo intervention. However, there was no evidence of difference between the two home‐based therapies or between the two home‐based therapies and placebo intervention for this outcome (Table 3). The certainty of evidence and any reasons for downgrading for each pair‐wise comparison are shown in Table 3 and Figure 5.
5.
Composite signs (convergence) and symptoms outcome: achieved normal and improved NPC, PFV, and Convergence Insufficiency Symptom Score (CISS): NPC is normal (<6cm) and has improved by ≥ 4cm, PFV is normal (passing Sheard's criterion and a PFV break > 15∆) and has improved ≥ 10 ∆, and CISS is normal (< 16) and has improved ≥ 10 points.
The certainty of evidence was rated 'high' if all domains were of 'no concerns.' The certainty of evidence was rated 'moderate' if two or more domains were of 'some concerns.'
The certainty of evidence was rated 'low' if two or more domains were of 'major concerns' or one domain of 'major concerns' plus one domain of 'some concerns.'
CI: confidence interval.
Near point of convergence
Compared with placebo intervention, the mean improvements (reduction) in NPC, ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy with home reinforcement (MD 5.01, 95% CI 3.56 to 6.46); home‐based computer vergence/accommodative therapy (MD 2.93, 95% CI 1.03 to 4.83); and home‐based pencil/target push‐ups (MD 1.58, 95% CI ‐0.33 to 3.49) (Table 3).
Positive fusional vergence at near
Compared with placebo intervention, the mean improvements (increase) in PFV, ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy with home reinforcement (MD 13.78, 95% CI 11.41 to 16.14); home‐based computer vergence/accommodative therapy (MD 5.26, 95% CI 2.72 to 7.81); and home‐based pencil/target push‐ups (MD 0.68, 95% CI ‐1.67 to 3.04) (Table 3).
Convergence insufficiency symptom survey
Compared with placebo intervention, the mean improvements (reduction) in CISS score, ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy with home reinforcement (MD 6.79, 95% CI 1.21 to 12.36); home‐based computer vergence/accommodative therapy (MD ‐1.84, 95% CI ‐7.95 to 4.27); and home‐based pencil/target push‐ups (MD ‐3.13, 95% CI ‐8.65 to 2.38) (Table 3).
Adverse effects
No adverse effects related to study treatments were reported for any of the included studies.
Assessment of the transitivity assumption
We found no evidence of statistically significant incoherence for overall treatment success (defined by either of the composite success criteria). However, we found evidence of statistically significant incoherence for the CISS score in two triangle loops. One included interventions 1, 2, and 3, with an incoherence factor of 10.25 (95% CI 1.23 to 19.27), and the other included interventions 1, 3, and 5, with an incoherence factor of 5.47 (95% CI 0.21 to 10.74). Because all trials measured CISS scores using the same instrument and in a consistent way, and because there was no statistically significant incoherence for the other two outcomes, we did not have a good qualitative explanation for the statistical incoherence. We tried to fit an incoherence model, but the model failed to converge due to the limited numbers of trials analyzed.
Adults' network
The eligible trials on adults examined interventions 1, 2, 4, and 5, of the seven interventions enumerated above. These four interventions constructed a network (Figure 6).
6.
Network graph for the adults' network
Number of trials: 3
Number of participants analyzed: 107
Table 4 shows the pairwise meta‐analysis results based on the direct comparison of two interventions from RCTs. Table 5 shows the relative effects of any two interventions and the mean ranks based on NMAs of three RCTs (107 participants).
4. Pairwise meta‐analysis or individual study results: adults.
| Pairwise comparison | Number of RCTs | Improvement in NPC (MD with 95% CI); I2 | Improvement in PFV (MD with 95% CI); I2 | Improvement in CISS score (MD with 95% CI); I2 |
| Office‐based vergence/accommodative therapy alone vs Office‐based vergence/accommodative therapy with home reinforcement |
1 | 0.90 (‐2.03, 3.83); ‐ | ‐3.80 (‐7.53, ‐0.07); ‐ | 0.20 (‐2.84, 3.24); ‐ |
| Office‐based vergence/accommodative therapy with home reinforcement vs Placebo intervention |
2 | 2.43 (‐0.02, 4.87); 0.0% | 16.73 (7.11, 26.36); 56.3% | 3.03 (‐6.33, 12.39); 0.0% |
| Home‐based pencil/target push‐ups vs Placebo intervention |
1 | ‐0.20 (‐6.38, 5.98); ‐ | 4.60 (‐3.66, 12.86); ‐ | ‐1.20 (‐11.13, 8.73); ‐ |
| Home‐based pencil/target push‐ups vs Office‐based vergence/accommodative therapy with home reinforcement |
1 | ‐2.80 (‐8.75, 3.15); ‐ | ‐7.80 (‐17.61, 2.01); ‐ | ‐4.70 (‐13.99, 4.59); ‐ |
| Base‐in prism reading glasses vs Placebo intervention |
1 | 0.4 (‐1.9, 2.7)*; ‐ | 0.0 (‐1.3, 1.2)*; ‐ | ‐8.9 (‐11.6, ‐6.3)*; ‐ |
CI: confidence interval; CISS: Convergence Insufficiency Symptom Survey; MD: mean difference; NPC: near point of convergence; PFV: positive fusional vergence; RCT: randomized controlled trial
*Difference in values measured at a follow up time point (instead of change from baseline)
5. Network meta‐analysis results: adults.
| Outcome | Mean rank | Interventions | |||
| NPC MD (95% CI) |
1.9 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 1.5 | ‐0.90 (2.03, ‐3.83) | Office‐based vergence/accommodative therapy alone | |||
| 3.2 | 2.72 (7.95, ‐2.51) | 3.62 (‐2.38, 9.62) | Home‐based pencil/target push‐ups | ||
| 3.4 | 2.43 (‐0.02, 4.87) | 3.33 (‐0.49, 7.15) | ‐0.29 (‐5.56, 4.98) | Placebo intervention | |
| PFV MD (95% CI) |
1.3 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 2.0 | 3.80 (14.75, ‐7.15) | Office‐based vergence/accommodative therapy alone | |||
| 2.9 | 10.33 (23.65, ‐2.99) | 6.53 (‐10.72, 23.77) | Home‐based pencil/target push‐ups | ||
| 3.8 | 16.73 (6.96, 26.50) | 12.92 (‐1.75, 27.60) | 6.40 (‐6.46, 19.25) | Placebo intervention | |
| CISS score MD (95% CI) |
2.0 | Office‐based vergence/accommodative therapy with home reinforcement | |||
| 1.9 | ‐0.20 (2.84, ‐3.24) | Office‐based vergence/accommodative therapy alone | |||
| 3.3 | 4.49 (13.33, ‐4.36) | 4.69 (‐4.67, 14.04) | Home‐based pencil/target push‐ups | ||
| 2.9 | 3.03 (‐6.33, 12.39) | 3.23 (‐6.61, 13.07) | ‐1.46 (‐10.77, 7.85) | Placebo intervention | |
CI: confidence interval; CISS: Convergence Insufficiency Symptom Survey; MD: mean difference; NPC: near point of convergence; PFV: positive fusional vergence
Values in cells equal improvement in column‐defining intervention, minus improvement in row‐defining intervention; a MD greater than zero indicates greater improvement in column‐defining intervention.
Treatment success (composite convergence outcome)
No trials presented data on this outcome.
Treatment success (composite convergence/symptoms outcome)
No trials presented data on this outcome.
Near point of convergence
Compared with placebo therapy, the mean improvement (reduction) in NPC, ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy alone (MD 3.33, 95% CI ‐0.49 to 7.15); office‐based vergence/accommodative therapy with home reinforcement (MD 2.43, 95% CI ‐0.02 to 4.87); and home‐based pencil/target push‐ups (MD ‐0.29, 95% CI ‐5.56 to 4.98).
Positive fusional vergence at near
Compared with placebo therapy, the mean improvement (increase) in PFV, ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy with home reinforcement (MD 16.73, 95% CI 6.96 to 26.50); office‐based vergence/accommodative therapy alone (MD 12.92, 95% CI ‐1.75 to 27.60); and home‐based pencil/target push‐ups (MD 6.40, 95% CI ‐6.46 to 19.25).
Convergence insufficiency symptom survey
Compared with placebo therapy, the mean improvement (reduction) in CISS score, ordered from the most effective to the least effective therapy, based on the mean ranks, were as follows: office‐based vergence/accommodative therapy alone (MD 3.23, 95% CI ‐6.61 to 13.07); office‐based vergence/accommodative therapy with home reinforcement (MD 3.03, 95% CI ‐6.33 to 12.39); and home‐based pencil/target push‐ups (MD ‐1.46, 95% CI ‐10.77 to 7.85).
One trial (Nabovati 2020), disconnected from the network, evaluated base‐in prism glasses prescribed for near‐work activities and found evidence that the base‐in prism glasses group had less symptoms compared with the placebo glasses group at three months (MD ‐8.9, 95% CI‐11.6 to ‐6.3); no evidence of difference was found in NPC or PFV.
Adverse effects
No adverse effects related to study treatments were reported for any of the included studies.
Assessment of the transitivity assumption
We found no evidence of statistically significant incoherence for any of the outcomes in the adult network.
Adherence with treatment
Treatment adherence is the degree to which study participants comply with the prescribed treatment regimen. The proportion of office therapy visits completed is the typical measure of treatment adherence for office‐based therapy. Determining participant adherence with a home treatment or the home‐based component of an office‐based therapy regimen, however, is more challenging because these estimates are typically based, at least in part, on participant or parental report. Several studies have provided estimates of treatment adherence. In the CITT trial of base‐in prism for children (CITT 2005a), adherence was assessed by asking the child and parent to report the frequency (0%, 25%, 50%, 75%, or 100%) with which the child wore the study glasses for reading or near work. In the base‐in prism group, 90% of the children and 81% of their parents reported that the glasses were worn at least 75% of the prescribed time. For the placebo group, 79% of the children and 79% of the parents reported that the frequency of glasses wear was 75%. The reported percentage of glasses wearing time for the adult participants in the Nabovati study of base‐in prism was 83% and 79% for the prism and placebo groups, respectively (Nabovati 2020). Adherence data for therapy performed in the office can be deduced from the proportion of therapy visits completed compared with visits scheduled. Two of the CITT trials reported these data (CITT 2008; CITT‐ART 2019).
In CITT 2008, adherence with the 12 weekly therapy visits was excellent at 97.6% for the office‐based vergence/accommodative therapy group and 97.2% for the placebo therapy group. There was also excellent adherence for the office‐based therapies in CITT‐ART 2019, with 96.8% and 96.6% of therapy visits completed for the 16 sequential weeks of office‐based vergence/accommodative therapy and placebo therapy, respectively. The CITT trials (CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019) of office‐based vergence/accommodative therapy with a prescribed home therapy component, and the PEDIG CITS trial (PEDIG 2016) of solely home therapy procedures, determined treatment adherence based on therapist opinion, gathered from participant and/or parent interview, or some combination of interview, calendar log, and/or electronic data from those prescribed computer therapy procedures (Table 6). In the four CITT trials in which office‐based vergence/accommodative therapy supplemented with home therapy procedures were evaluated (CITT 2005b; CITT 2005c; CITT 2008; CITT‐ART 2019), the therapist estimated the average proportion of time (0%, 1% to 24%, 25% to 49%, 50% to 74%, 75% to 99%, or 100%) the participant adhered to the home therapy protocol (Table 6). For the most recent trials (CITT 2008; CITT‐ART 2019; PEDIG 2016), study personnel also used electronic data based on computer therapy usage (when computer therapy was a component of the prescribed home therapy) for their estimates of treatment adherence. A substantial decrease in therapy adherence was reported for week 7 through week 12, versus week 1 through week 6, for participants in the CITS home therapy study, particularly in the home‐based pencil push‐up and home‐based placebo groups, who were also less likely to complete the study than those in the home‐based computer group (PEDIG 2016).
6. Home therapy: Proportion of participants with estimated adherence of ≥ 75%.
| Adherence determination | Types of therapy | |||||
| Study | Interview with participant and/or parent (I) Home Log (L) Computer (C)a |
Home‐therapy component of office‐based vergence/accommodative therapy | Home‐therapy component of office‐based placebo therapy | Home‐based pencil/target push‐ups | Home‐based computer vergence/accommodative therapy | Home‐based placebo therapy |
| CITT 2005b | I, L | 73% | 92% | 73% | ‐ | ‐ |
| CITT 2005c | I, L | 50% | 69% | 87% | ‐ | ‐ |
| CITT 2008 | I, L, C | 91% | 87% | 85% | 67% | ‐ |
| CITT‐ART 2019 | I, L, C | 64% | 76% | ‐ | ‐ | ‐ |
| PEDIG 2016bc | I, C | ‐ | ‐ | 49% | 68% | 52% |
a Electronic data from computer therapy prescribed as a component of home therapy
b All three home therapies tested in PEDIG 2016 had a computer component to monitor home usage
c Study personnel estimated proportion of participants with average compliance of > 75% for ≥ 5 days/week for the primary therapy
Economic data
Cost‐analysis data (i.e. separate calculations of monetary and health outcomes and the relative value of the treatment modality measured as the added cost to achieve an incremental health benefit) are not available for any of the included studies. However, the general out‐of‐pocket monetary costs for the different treatments can be described. The monetary cost of office‐based vergence/accommodative therapy is dependent on the therapy‐visit fee and the number of therapy sessions completed. However, because this therapy is a covered service by certain medical insurance companies in the US and some publicly‐funded healthcare systems outside the US, the direct patient cost may be reduced significantly or may be zero in these instances. The cost of home‐based therapy is out‐of‐pocket and determined by the number of follow‐up visits with the provider and the therapy equipment that would need to be purchased to use at home, with software for computer therapy typically having the highest cost. Prism glasses would be covered by vision insurance in the US for individuals having vision insurance, with additional office‐visit fees for associated follow‐up visits with the provider. In publicly‐funded healthcare systems, such as the National Health Service in the United Kingdom, assistance for the cost of glasses for children and other eligible groups is also available.
Discussion
Summary of main results
The objective of this systematic review was to identify and synthesize the available RCT evidence on the effectiveness of non‐surgical treatments for symptomatic convergence insufficiency in children and adults. Seven RCTs that were not available at the time of the last review are included in this updated version.
Summary of main results in children
There were a total of 968 participants in the six RCTs in the pediatric population. The diagnostic criteria for convergence insufficiency and outcome measures were identical in four trials (CITT 2005a; CITT 2005b; CITT 2008; CITT‐ART 2019) and similar in two trials (Nehad 2018; PEDIG 2016). The treatments evaluated were base‐in prism reading glasses and several office‐ and home‐based vergence/accommodative therapy modes of therapy. The CITT Investigator Group completed four of the six RCTs with 651 participants; all four RCTs were rated at low risk of bias (CITT 2005a; CITT 2005b; CITT 2008; CITT‐ART 2019).
We found high‐certainty evidence that office‐based vergence/accommodative therapy with home reinforcement was 3.04 times more effective than placebo therapy, 2.86 times more effective than home based pencil push‐ups, and 1.96 times more effective than home‐based computer vergence/accommodative therapy in achieving treatment success defined by normal and improved clinical measures of convergence. However, there was no evidence of any treatment difference between home‐based pencil push‐ups and home‐based computer vergence/accommodative therapy, or between either of the two home‐based therapies and placebo therapy for treatment success.
We found moderate‐certainty evidence that participants who received office‐based vergence/accommodative therapy with home reinforcement were 5.12 times more likely to achieve treatment success defined by normal and improved clinical measures of convergence and symptoms than those who received placebo therapy. We found low‐certainty evidence that participants who received office‐based vergence/accommodative therapy with home reinforcement were 4.41 times more likely than those received home based pencil push‐ups, and 4.65 times more likely than those who received home‐based computer vergence/accommodative therapy to achieve treatment success. Similar to the aforementioned findings for treatment success using the clinical measures of convergence, there was no evidence of any treatment difference between home‐based pencil push‐ups and home‐based computer vergence/accommodative therapy, or between either of the two home‐based therapies and placebo therapy for this measure of treatment success using symptoms and clinical measures of convergence.
We also analyzed the effectiveness of the interventions for improving the individual clinical measures of the NPC and PFV, as well as symptoms measured by the CISS score. Compared with placebo therapy, there was a statistically significant MD favoring office‐based vergence/accommodative therapy with home reinforcement for improving NPC, PFV, and the CISS score. Compared with placebo, there was also a statistically significant MD favoring home‐based computer vergence/accommodative therapy for improving NPC and PFV. However, for patient symptoms, there was no statistically significant difference. Home‐based pencil push‐up therapy yielded similar results to placebo therapy for all three outcomes (i.e. NPC, PFV, and CISS score). This is an important finding given the frequency in which home‐based pencil push‐up therapy is prescribed as the sole treatment for children with symptomatic convergence insufficiency.
One RCT (CITT 2005a) evaluated the effectiveness of base‐in prism reading glasses for children with symptomatic convergence insufficiency (this comparison is disconnected from the network analyzed). In this RCT, there was no statistically significant difference in symptom improvement as measured by CISS scores or in improvements in the clinical measures of NPC or PFV when the base‐in prism reading glasses group was compared with the placebo reading glasses group.
Summary of main results in adults
We identified six RCTs using non‐surgical interventions for treating convergence insufficiency in adults with a modest total number of participants (n = 321). Only one RCT (CITT 2005c) was rated at low risk of bias. Four RCTs (Birnbaum 1999; Momeni Moghaddam 2015; Aletaha 2018; Nabovati 2020) were graded as having a high risk of bias and the remaining study (Widmer 2018) had only seven participants. Participants were young adults in five of the six clinical trials (CITT 2005c; Momeni Moghaddam 2015; Aletaha 2018; Widmer 2018; Nabovati 2020) whereas the mean age was 63.9 years in the remaining trial (Birnbaum 1999). Another problem is the heterogeneity of the interventions. The treatment protocol for the office‐based therapy interventions varied considerably (CITT 2005c; Momeni Moghaddam 2015; Aletaha 2018; Widmer 2018). Only one study (Nabovati 2020) evaluated the effectiveness of base‐in prism reading glasses. In addition, most of the studies did not include our primary outcome measures using composite success data, which prevented us from conducting an NMA for the primary outcome of treatment success (i.e. meeting the criteria for the composite convergence outcome or for composite clinical signs and symptoms outcome). We were thus limited to comparing mean differences between the interventions for improvements in the NPC, PFV, and symptoms individually.
Compared with placebo treatment, office‐based vergence/accommodative therapy was relatively more effective in improving PFV; the differences were not statistically significantly for the NPC or the CISS score. There was no evidence of differences for any other comparisons for any outcomes.
One trial (Nabovati 2020) evaluated base‐in prism glasses prescribed for near‐work activities and found that the prism glasses group had significantly less symptoms compared with the placebo glasses group at three months; no evidence of difference was found in NPC or PFV.
For both populations, no adverse effects related to study treatments were reported for any of the included studies. Excellent adherence was reported for office‐based vergence/accommodative therapy (96.6% or higher) in three trials; reported adherence with home‐based therapy was less consistent with one study reporting decreasing adherence (weeks 7 to 12) and lower completion rates with home‐based pencil/target push‐ups.
Overall completeness and applicability of evidence
The population of interest were children and adults with symptomatic convergence insufficiency. Due to the differences in the accommodative ability between children and adults, these groups were analyzed separately. The included trials evaluated the most commonly prescribed non‐surgical interventions for convergence insufficiency. The majority or them used the same or similar diagnostic criteria, based on specified thresholds for NPC, PFV, and the CISS score.
For this 2020 update, we introduced 'treatment success at 12 weeks' as a new primary outcome, which was based on achieving normal values together with pre‐specified improvement for NPC and for PFV (composite convergence outcome) and for NPC, PFV and the CISS score (composite signs and symptoms outcome). These data were available for trials included in the children’s network. This composite outcome further enhances the applicability of the evidence, as treatment success is likely to be the most important outcome for patients and clinicians.
All of the included trials evaluated short‐term outcomes (measured at 16 weeks or less). In the case of studies with placebo comparisons, this was due to the ethical and logistical challenges of following a group of symptomatic patients in a placebo group. It is therefore unclear whether the maximum treatment effect that could have been achieved with the various interventions had been reached. One study (CITT 2008) followed all asymptomatic study participants for one year after the 12‐week primary outcome visit, reporting the percentage of participants that remained asymptomatic in each group: 84% (27/32) for office‐based vergence/accommodative therapy with home reinforcement, 67% (10/15) for home‐based pencil/target push‐ups, 80% (8/10) for home‐based computer vergence/accommodative therapy, and 77% (10/13) for placebo therapy.
In terms of the magnitude of the treatment effect and the relative ranking of interventions, office based vergence/accommodative therapy with home reinforcement was more effective than the two home‐based therapies for the majority of outcomes (high‐certainty evidence). This was consistent for both the pair‐wise comparisons and the NMA. There was little evidence that pencil or target push‐ups, which are commonly recommended in routine clinical practice, are an effective treatment for convergence insufficiency and in fact, may be no more effective than placebo therapy.
Despite the availability of further evidence on the effectiveness of interventions for convergence insufficiency in adults for this update, imprecision of NMA estimates and heterogeneity in the relative effects of interventions limited the applicability of the evidence for the adult population. Furthermore, it was not possible to determine the composite measures of treatment success in adults.
Intuitively, one would expect that improving convergence function may have a beneficial effect on reading performance and attention. This was investigated as the primary outcome in one of the included studies (CITT‐ART 2019), which compared office‐based vergence/accommodative therapy to office‐based placebo therapy in children ages 9 to 14 with symptomatic convergence insufficiency. Despite demonstrating clinically significant improvements in convergence function as shown for both the NPC and PFV, office‑based vergence/accommodative therapy was found to be no more effective than placebo therapy for improving reading performance or attention after 16 weeks of treatment. Although improved convergence ability could make reading and school work performed at near more visually comfortable for children with symptomatic convergence insufficiency, the authors concluded that eye care professionals should not suggest that such treatment, on average, will lead to improvements on standardized assessments of reading performance or attention after 16 weeks of treatment.
None of the included trials reported on quality of life or performed a health economic analysis of convergence insufficiency treatments. The monetary cost of office‐based vergence/accommodative therapy is based on the therapy‐visit fee and the number of follow‐up visits. Although the cost of this therapy is covered by some medical insurance companies in the US and in some publicly‐funded healthcare systems outside the US, the cost of the intervention is still a barrier to equitable access to treatment. Eight of the included trials, randomizing 76% of study participants, were conducted in the US and the remaining four studies in upper‐middle‐income (Iran) or lower‐middle‐income (Egypt) countries. None of the trials were conducted in low‐income countries, so the results of this review could be less applicable for these settings.
Ongoing studies
Three of the four ongoing studies are recruiting adults, and the fourth study is recruiting both children and adults. If these studies are completed, they may provide new data about the effectiveness of non‐surgical treatments for convergence insufficiency in adults. One study (NCT03593031) includes outcome measures that have not been reported in the studies that form the basis for this Cochrane Review. In addition, this ongoing study is specifically designed to test potential hypotheses regarding the underlying neural mechanisms of convergence insufficiency that might be improved after successful treatment with vergence/accommodative therapy.
Quality of the evidence
In the children’s network, the overall certainty of evidence was high for all comparisons involving office‐based vergence/accommodative therapy and moderate‐certainty to low‐certainty for comparisons between the two home‐based interventions. For almost all comparisons in the adult network, the certainty of evidence was low (see 'Summary of findings' tables). We downgraded the certainty of evidence primarily due to concerns regarding within study bias, lack of precision in treatment effects, and/or the existence of heterogeneity.
Four trials were included in the children’s network (CITT 2005b; CITT 2008; CITT‐ART 2019 and PEDIG 2016). We excluded Nehad 2018 from this network due to data errors and CITT 2005a tested a disconnected intervention (prism reading glasses) that could not be compared with other interventions in the network. The four CITT trials (CITT 2005a; CITT 2005b; CITT 2008; CITT‐ART 2019) were graded at low risk of bias. PEDIG 2016 was judged to be at high risk of attrition bias and Nehad 2018 was rated as unclear risk across all bias domains. Two of the three trials that formed the adult network (Aletaha 2018; Nabovati 2020) were also judged to have a high risk of bias, which reduced our confidence in the estimates of treatment effect from the adult network.
The clinical homogeneity in study populations, interventions, and outcomes ensured that there were no systematic differences between the available comparisons other than the treatments being compared. The transitivity assumption was met for the primary outcomes in both networks and there were no concerns about indirectness.
Imprecision in the NMA estimates led to some concerns in the children’s network and major concerns for a number of comparisons in the young adult network, since the 95% confidence interval crossed our pre‐defined range of clinical equivalence. Imprecision and heterogeneity were particularly evident for home‐based interventions in the children's network, which reduced the certainty rating. Similarly, for many of the comparisons in the adult network, there was evidence of inconsistency, which could have led to different conclusions on the relative effectiveness of the interventions.
Potential biases in the review process
We took several measures to prevent potential bias in the systematic review process, including having pre‐specified eligibility criteria, performing an extensive literature search, and having two review authors working independently to evaluate eligibility, assess risk of bias, and abstract data. We also contacted trial investigators for additional information.
There is a potential conflict of interest as the lead author of this review (Dr. Mitchell Scheiman) was the Principal Investigator for the four CITT trials and served as Protocol Chair for the PEDIG trial. Two other authors (SAC and MTK) also served as investigators in the CITT trials and in the PEDIG trial. However, none of these investigators participated in data abstraction and risk of bias assessment for the trials in which they were involved.
Agreements and disagreements with other studies or reviews
Findings from our systematic review are consistent with findings from older narrative reviews on the same topic (Cacho 2009; Cooper 2012; Scheiman 2009) as well as a more recent narrative review (Trieu 2018). None of these publications included a meta‐analysis.
Authors' conclusions
Implications for practice.
This systematic review found high‐certainty evidence that children with symptomatic convergence insufficiency who undergo office‐based vergence/accommodative therapy are significantly more likely to achieve treatment success than those who receive placebo therapy, home‐based pencil push‐up treatment, or home‐based computer vergence/accommodative therapy. There was no evidence of difference between home‐based pencil push‐ups and home‐based computer vergence/accommodative therapy, or between either of the two home‐based therapies and placebo therapy, for treatment success in children. Base‐in prism reading glasses were found to be no more effective than placebo reading glasses in children. Adherence issues may be one reason home‐based therapy is not as effective as office‐based therapy, but closer monitoring of adherence to home‐based treatment did not lead to improved success rates. Evidence is less clear for the adult population, although office‐based vergence/accommodative therapy was more effective than home‐based pencil push‐ups for improving the near point of convergence and positive fusional vergence.
Implications for research.
There are ‘unknowns’ related to research on non‐surgical interventions in the area of convergence insufficiency. These include determining answers to the following questions.
Are certain office‐based vergence/accommodative therapy procedures more effective than others for treating convergence insufficiency? Is there an office‐based therapy program that would be equally as effective or perhaps even more effective, but could be administered for a shorter duration or adapted for home therapy?
Are there different home‐based therapy combinations (e.g. computer therapy combined with therapy procedures using loose prisms or free‐space fusion cards rather than pencil push‐ups alone) and/or a modified computer therapy program that would be more effective than the combined computerized therapy and pencil push‐up approach (prescribed in PEDIG 2016), or equally as effective as office‐based therapy?
Is it possible that a telemedicine or virtual format could improve the effectiveness of home‐based therapies?
Could replacing some or all of the office‐based therapy visits with telemedicine or virtual office visits increase access and cost‐effectiveness while maintaining treatment effectiveness?
Is there a better method of prescribing prism, such as based on fixation disparity testing, that might be more effective in reducing symptoms associated with convergence insufficiency?
The effect of successful treatment of symptomatic convergence insufficiency on health‐related quality of life should be investigated.
Because insurance providers often do not pay for office‐based therapy with home reinforcement, it would be helpful to understand whether there are disparities in access to care for particular populations. This would allow healthcare systems to allocate appropriate resources.
What is the cost utility of the various treatment interventions for convergence insufficiency?
There is a need for more studies in adults, given the low‐certainty of evidence for treatment effectiveness.
What's new
| Date | Event | Description |
|---|---|---|
| 8 September 2020 | New citation required and conclusions have changed | Issue 12, 2020: Seven new trials were included (Aletaha 2018; CITT‐ART 2019; Momeni Moghaddam 2015; Nabovati 2020; Nehad 2018; PEDIG 2016; Widmer 2018). |
| 8 September 2020 | New search has been performed | Issue 12, 2020: New review authors added (MTK, JGL, LW); new search performed; network meta‐analysis conducted; certainty of evidence graded. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 3, 2011
| Date | Event | Description |
|---|---|---|
| 19 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the CEV Information Specialist for devising the electronic search strategy for our review. We thank the following peer reviewers for their comments: Gayathri Srinivasan (New England College of Optometry), William Bobier (University of Waterloo), and William Vaughan (consumer). This review was managed by CEV@US and was signed off by Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Ocular Motility Disorders] explode all trees #2 MeSH descriptor: [Convergence, Ocular] explode all trees #3 MeSH descriptor: [Accommodation, Ocular] explode all trees #4 MeSH descriptor: [Vision, Binocular] explode all trees #5 MeSH descriptor: [Exotropia] explode all trees #6 (Convergence near/3 insufficienc*) #7 (Convergence near/3 disorder*) #8 Exodeviation* #9 Heterophoria* #10 Exotropi* #11 Exophori* #12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13 prism* #14 (pencil* near/2 push*) #15 MeSH descriptor: [Orthoptics] explode all trees #16 orthoptic* #17 ((exercise* or therap* or treat*) near/10 home*) #18 ((exercise* or therap* or treat*) near/10 office*) #19 (HBPP or HBCVAT* or OBVAT or OBPT) #20 (convergence insufficienc* near/5 (exercise* or therap* or treat*)) #21 vision therap* #22 lens therap* #23 sterogram* #24 MeSH descriptor: [Eyeglasses] explode all trees #25 (Eyeglass* or spectacle* or eye glass* or glasses) #26 MeSH descriptor: [Exercise Therapy] explode all trees #27 MeSH descriptor: [Therapy, Computer‐Assisted] explode all trees #28 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 #29 #12 and #28
Appendix 2. MEDLINE search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Ocular Motility Disorders/ 13. exp Convergence, Ocular/ 14. exp Accommodation, Ocular/ 15. exp Vision, Binocular/ 16. exp Exotropia/ 17. (Convergence adj3 insufficienc*).tw. 18. (Convergence adj3 disorder*).tw. 19. Exodeviation*.tw. 20. Heterophoria*.tw. 21. Exotropi*.tw. 22. Exophori*.tw. 23. or/12‐22 24. prism*.tw. 25. (pencil* adj2 push*).tw. 26. exp Orthoptics/ 27. orthoptic*.tw. 28. ((exercise* or therap* or treat*) adj10 home*).tw. 29. ((exercise* or therap* or treat*) adj10 office*).tw. 30. (HBPP or HBCVAT* or OBVAT or OBPT).tw. 31. (convergence insufficienc* adj5 (exercise* or therap* or treat*)).tw. 32. vision therap*.tw. 33. lens therap*.tw. 34. sterogram*.tw. 35. exp Eyeglasses/ 36. (Eyeglass* or spectacle* or eye glass* or glasses).tw. 37. exp Exercise Therapy/ 38. exp Therapy, Computer‐Assisted/ 39. or/24‐38 40. 11 and 23 and 39
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'eye movement disorder'/exp #34 'binocular convergence'/exp #35 'accommodation'/exp #36 'binocular vision'/exp #37 'divergent strabismus'/exp #38 (convergence NEAR/3 insufficienc*):ab,ti #39 (convergence NEAR/3 disorder*):ab,ti #40 exodeviation*:ab,ti #41 heterophoria*:ab,ti #42 exotropi*:ab,ti #43 exophori*:ab,ti #44 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 #45 prism*:ab,ti #46 (pencil* NEAR/2 push*):ab,ti #47 'orthoptics'/exp #48 orthoptic*:ab,ti #49 ((exercise* OR therap* OR treat*) NEAR/10 home*):ab,ti #50 ((exercise* OR therap* OR treat*) NEAR/10 office*):ab,ti #51 hbpp:ab,ti OR hbcvat*:ab,ti OR obvat:ab,ti OR obpt:ab,ti #52 convergence:ab,ti AND (insufficienc* NEAR/6 (exercise* OR therap* OR treat*)):ab,ti #53 (vision NEXT/1 therap*):ab,ti #54 (lens NEXT/1 therap*):ab,ti #55 sterogram*:ab,ti #56 'spectacles'/exp #57 eyeglass*:ab,ti OR spectacle*:ab,ti OR 'eye glass':ab,ti OR 'eye glasses':ab,ti OR glasses:ab,ti #58 'kinesiotherapy'/exp #59 'computer assisted therapy'/exp #60 #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 #61 #32 AND #44 AND #60
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (Convergence[tw] AND insufficienc*[tw]) NOT Medline[sb] 3. (Convergence[tw] AND disorder*[tw]) NOT Medline[sb] 4. Exodeviation*[tw] NOT Medline[sb] 5. Heterophoria*[tw] NOT Medline[sb] 6. Exotropi*[tw] NOT Medline[sb] 7. Exophori*[tw] NOT Medline[sb] 8. #2 OR #3 OR #4 OR #5 OR #6 OR #7 9. prism*[tw] NOT Medline[sb] 10. (pencil*[tw] AND push*[tw]) NOT Medline[sb] 11. orthoptic*[tw] NOT Medline[sb] 12. ((exercise*[tw] OR therap*[tw] OR treat*[tw]) AND home*[tw]) NOT Medline[sb] 13. ((exercise*[tw] OR therap*[tw] OR treat*[tw]) AND office*[tw]) NOT Medline[sb] 14. (HBPP[tw] OR HBCVAT*[tw] OR OBVAT[tw] OR OBPT[tw]) NOT Medline[sb] 15. (convergence insufficienc*[tw] AND (exercise*[tw] OR therap*[tw] OR treat*[tw])) NOT Medline[sb] 16. vision therap*[tw] NOT Medline[sb] 17. lens therap*[tw] NOT Medline[sb] 18. sterogram*[tw] NOT Medline[sb] 19. (Eyeglass*[tw] OR spectacle*[tw] OR eye glass*[tw] OR glasses[tw]) NOT Medline[sb] 20. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 21. #1 AND #8 AND #20
Appendix 5. metaRegister of Controlled Trials search strategy
Convergence insufficiency OR Convergence disorder OR exodeviation OR Heterophoria OR Exotropia OR Exophoria
Appendix 6. ClinicalTrials.gov search strategy
Convergence insufficiency OR Convergence disorder OR exodeviation OR Heterophoria OR Exotropia OR Exophoria
Appendix 7. ICTRP search strategy
Convergence insufficiency OR Convergence disorder OR exodeviation OR Heterophoria OR Exotropia OR Exophoria
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aletaha 2018.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 84 (26 assigned to each group) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: assumed 84 (100%) Number of centers: 1 Date of first enrolment: January 2014 Length of treatment: unknown Length of follow‐up: planned: 6 months after completion of treatment; actual: 6 months after completion of treatment Sample size estimation: not reported |
|
| Participants | Country of recruitment: Iran Mean age: 26.8 ± 8.3 (SD) years Sex: 76% were female Key inclusion criteria: participants aged between 15 and 35 years and had symptomatic convergence insufficiency diagnosed using the Convergence Insufficiency Symptom Survey (CISS) ( > 16 points) ; best‑corrected visual acuity 20/25 or better; exophoria at near at least 4 Δ greater than at distance; NPC > 6.0 cm break; insufficient near PFV (failing Sheard’s criterion or PFV ≤ 15 Δ base‑out). Key exclusion criteria: amblyopia (visual acuity worse than 20/30 in each eye); presence of manifest strabismus; history of ocular surgery; any systemic disorder; anisometropia of more than 1.50 diopters of myopia or hyperopia or significant refractive error; nystagmus; usage of medications that may impair accommodation or convergence; ocular surface abnormalities or a history of ocular allergy; or those who had previously been treated for CI. |
|
| Interventions |
Intervention regimen #1:home‑based vision therapy (HBVOT) Participants were trained to perform the pencil push‑up procedure. This procedure is done by holding a pencil with 20/60 size letters at arm’s length in front of the eyes. The patient attempts to keep the letters clear and pencil single while moving the pencil slowly towards his/her nose.";" Participants were instructed to perform the pencil push‑up procedure for 15 min/day for 5 days/week. Intervention regimen #2: office‑based vision orthoptic therapy (OBVOT) OBVOT included 60 min of vision therapy using the major amblyoscope performed twice weekly with additional home vision therapy (pencil push‑ups) prescribed for 15 min/day for 5 days/week (home reinforcement). Intervention regimen #3: augmented office‑based vision therapy (AOBVOT) In‐office therapy using the major amblyoscope & augmented vision therapy procedures having participants view ‐3.00D over‑minus lenses (according to the patient’s refraction) and base‑out prism, while the participant was performing a near task like reading a book.in addition to using the major amblyoscope. This therapy was performed for 60 min twice weekly with additional home reinforcement. |
|
| Outcomes | Primary outcome: symptoms measured using the Convergence Insufficiency Symptom Survey (CISS) (points); near point of convergence (cm); positive fusional vergence (∆) Key secondary outcomes: primary and secondary outcomes were not distinguished No harm was reported |
|
| Notes | Funding sources: no funding Subgroup analyses: none reported Trial registration: NCT03431454 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomly allocated to one of the three groups by using permuted‑block randomization: HBVOT, OBVOT, and AOBVOT |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Primary outcome | High risk | Although the author says this was "single masked", the patients clearly knew that they were in office‐based or home‐based therapy, so they were not masked. The examiners were not masked because the authors state that the therapists did the follow‐up testing. |
| Blinding (performance bias and detection bias) Secondary outcomes | High risk | The examiners were not masked because the authors state that the therapists did the follow‐up testing. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | There is no flow chart showing the number of participants in the study at each of the outcome assessments. None of the tables include the number of participants. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | See above. |
| Selective reporting (reporting bias) | Unclear risk | Objective outcomes including PFV, NPC, exophoria at far and near distances, and stereoacuity at near distance which were presented in the paper were not reported in ClinicalTrial.gov |
Birnbaum 1999.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 60 (21 assigned to office‐based therapy with supplemental home therapy; 20 assigned to home therapy group; and 19 assigned to control group) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 60 (100%) Number of centers: 1 Date of first enrolment: not reported Length of follow‐up: planned: 26 weeks after initiation of treatment; actual: varied Sample size estimation: not reported |
|
| Participants | Country of recruitment: United States Mean age: 63.9 years in the office‐based therapy group, 61.1 in home therapy group, and 62.9 in control group Sex: 100% male Key inclusion criteria: male adults aged 40 years with symptomatic convergence insufficiency; demonstrated asthenopic symptoms; diplopia at near; headaches, eyestrain, or loss of concentration during near work; and failed at least two of the four criteria for convergence insufficiency. Convergnece insufficiency criteria: NPC break worse than 10cm or recovery worse than 15cm; NPC with red lens to diplopia worse than 20cm or recovery worse than 30cm; von Graefe near phoria of 8 ∆ exo or greater; near PFV < 15∆ or recovery measure,< 10 ∆. Key exclusion criteria: patients with systemic neurologic disease; use of psychotropic medications that might influence vergence or accommodation; constant or noncomitant strabismus; visual acuity poorer than 20/40 in either eye, or previous vision therapy. |
|
| Interventions |
Intervention regimen #1: office‐based therapy with supplemental home therapy Patients assigned to this group were scheduled for 24 weekly 45 minute office‐based therapy sessions (some patients discharged earlier, once their treatment was successfully concluded; some patients required somewhat longer treatment periods). The office therapy procedures typically used include series of eye movement procedures and binocular fusion procedures. Procedures were assigned for practice at home to supplement office therapy. Intervention regimen #2: home therapy group Patients were seen for one office visit for instruction on the home therapy procedures. The home therapy procedures include four‐corner oculomotor calisthenic fixations; Brock string; eccentric circles base‐in and base‐out; red‐green lifesaver cards, base‐in and base‐out; and pointer‐straw. Intervention regimen #3: control group Patients were given a handout "Care of Your Eyes" (which was also given to patients in the two treatment groups). This handout provided general information on ocular health, but provided no specific information relative to convergence insufficiency. |
|
| Outcomes | Primary outcome: "Success" criterion met if participant passed both of following criteria: (1) asthenopia criteria (ability to read comfortably for at least 1 hour; no diplopia; and ability to read without headaches, eyestrain, or loss of concentration at least 75% of the time) and (2) At least 3 of the 4 functional criteria (adequate clinical measures for CNP; CNP with red lens, the near phoria, and base‐out vergence at near). Secondary outcome: unclear No harms were reported. |
|
| Notes | Funding sources: none reported Subgroup analyses: none reported Trial registration: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Primary outcome | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Secondary outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There was no loss to follow‐up. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No access to the protocol. |
CITT 2005a.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 72 (36 assigned to base‐in prism reading glasses; 36 assigned to placebo reading glasses) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 65 (90%) (31 of 36 assigned to base‐in prism reading glasses; 34 of 36 assigned to placebo reading glasses) Number of centers: 9 Date of first enrolment: July 21, 2003 Length of follow‐up: planned: 6 weeks after initiation of treatment; actual: 6 weeks after initiation of treatment Sample size estimation: all sample size calculations were performed using PASS 2000 software assuming a two‐sided test with α=0.05 and ß=0.10 (90% power). Preliminary data from CITT 2005b were used to obtain estimates of variability to be used in the calculations. With 32 patients per group, the study would have 90% power to find differences in the mean near point of convergence as small as 3.7 cm. |
|
| Participants | Country of recruitment: United States Mean age: 11.5 ± 2.3 (SD) years in the base‐in prism reading glasses group; 11.0 ± 2.0 (SD) years in the placebo reading glasses group Sex: 63.9% were female in base‐in prism reading glasses group; 47.2% were female in placebo reading glasses group Key inclusion criteria: age 9 to <18 years; best corrected visual acuity of 20/25 or better in both eyes at distance and near; willingness to wear eyeglasses to correct refractive error, if necessary; exophoria at near at least 4∆ greater than at far; insufficient positive fusional convergence at near (fails Sheard's criterion); receded near point of convergence of ≥ 6 cm break; appreciation of at least 500 seconds of arc on the forms part of the Randot Stereotest; Convergence Insufficiency Symptom Survey‐V15 score ≥16; informed consent and willingness to participate in the study and be randomized. Key exclusion criteria: convergence insufficiency previously treated with prism, pencil push ups, or office based vergence/accommodative therapy (no more than 2 months of treatment within the past year); amblyopia; constant strabismus; history of strabismus surgery; anisometropia > 1.50 D (spherical equivalent) difference between eyes; previous refractive surgery; vertical heterophoria greater than 1∆; systemic diseases known to affect accommodation, vergence, and ocular motility such as multiple sclerosis, Grave's thyroid disease, myasthenia gravis, diabetes, and Parkinsons disease; any ocular or systemic medication known to affect accommodation or vergence; monocular accommodative amplitude less than 4 D in either eye as measured by the push up method; manifest or latent nystagmus; attention deficit hyperactivity disorder or learning disability diagnosis by parental report that, in the investigator's opinion, would interfere with treatment. |
|
| Interventions |
Intervention regimen #1: base‐in prism reading glasses Patients in this group received glasses that corrected for the patient’s refractive error, if necessary, and base‐in prism. The amount of prism was based on the minimum amount necessary to meet Sheard’s criterion (prism to be prescribed = 2/3 phoria ‐1/3 compensating fusional vergence) with no less than 1 Δ prescribed. The amount of prism was rounded up to the nearest one‐half prism diopter and split equally between the two eyes if the magnitude exceeded 1 Δ. The patient was asked to wear these glasses for all reading and near tasks requiring more than 5 minutes. Intervention regimen #2: placebo reading glasses Patients in this group received glasses that corrected their refractive error, or plano lenses were prescribed for those who did not require a refractive correction. The patient was asked to wear these glasses for all reading and near tasks requiring more than 5 minutes. |
|
| Outcomes | Primary outcome: Symptoms measured using Convergence Insufficiency Symptom Survey V‐15 after 6 weeks of therapy. Key secondary outcomes: Clinical measures of near point of convergence and positive fusional vergence at near after 6 weeks of wearing prescribed reading glasses . No harms were reported. |
|
| Notes | Funding sources: grants from the Pennsylvania and Ohio Lions. Subgroup analyses: none reported Trial registration: NCT00347581 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The data coordinating center randomly assigned eligible patients with equal probability to either base‐in prism reading glasses or placebo reading glasses. Randomization was accomplished with the study’s web site using a permuted block design stratified by site." |
| Allocation concealment (selection bias) | Low risk | "Allocation to treatment group was achieved using a secure web site. Researchers entered eligibility data on the study website after enrolment; after verification of eligibility, the coordinating center provided the treatment group assignment. (personal communication with the lead investigator)". |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | "Neither the patient nor the examiner performing testing at the outcome examination was aware of the treatment assignment. To prevent potential examiner unmasking by observation of the glasses, the study coordinator placed Tarc 'N Stik reusable adhesive around the edges of the eyeglasses. The edges of the lenses were therefore obscured, making it impossible for the examiner to see the edge thickness of the lenses." |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | "Thirty one of the 36 patients (86%) assigned to receive base‐in prism reading glasses and 34 of the 36 (94%) assigned to placebo reading glasses completed their 6 week outcome examination. There was no statistically significant difference in the percentage loss to follow up between the two treatment groups (P = 0.43)." "Statistical analyses techniques were employed which allowed for incomplete data. No imputation or sensitivity analyses were performed (personal communication with the lead investigator)". |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | See above. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the study protocol were reported. |
CITT 2005b.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 47 (15 assigned to pencil push‐ups; 17 assigned to vergence/accommodative therapy; 15 assigned to placebo vergence/accommodative therapy) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 38 (81%); (11 of 15 assigned to pencil push‐ups; 15 of 17 assigned to vergence/accommodative therapy; 12 of 15 assigned to placebo vergence/accommodative therapy) Number of centers: 6 Date of first enrolment: October 2000 Length of follow‐up: planned: 12 weeks after initiation of treatment; actual: 12 weeks after initiation of treatment Sample size estimation: no formal sample size calculations were performed a priori because one goal of this pilot trial was to estimate the variability of the outcome measure. At the study completion, using the observed variability in the Convergence Insufficiency Symptom Survey, with α=0.05, assuming a 2‐sided test, and assuming the post treatment mean of the most effective treatment group would approximate the mean among patients with normal binocular vision, the mean for the placebo group would decrease 20% from its baseline value, and the mean for the other treatment group would fall in the middle of these two groups, the sample size of 47 yields a power of 92.8%. |
|
| Participants | Country of recruitment: United States Mean age: 11.2 ± 2.2 (SD) years Sex: 57% were female Key inclusion criteria: ages 9 to 18 years inclusive; best‐corrected visual acuity of 20/25 OU at distance and near; willingness to wear eyeglasses or contact lenses to correct refractive error, if necessary; exophoria at near at least 4 Δ greater than at far; insufficient positive fusional convergence (i.e., failing Sheard's criterion or < 15 Δ break on positive fusional vergence testing using a prism bar); receded near point of convergence of greater than or equal to 6 cm break; appreciation of at least 500s of arc on the forms part of the Randot Stereotest; Convergence Insufficiency Symptom Survey‐V13 (original 13‐item version) score > 9; informed consent and willingness to participate in the study and be randomized. Key exclusion criteria: convergence insufficiency previously treated with pencil push‐ups (no more than 2 mo of treatment within the past year); convergence insufficiency previously treated with office‐based vergence/accommodative therapy (no more than 2 mo of treatment within the past year); amblyopia; constant strabismus; history of strabismus surgery; anisometropia > 1.50‐D difference between eyes; prior refractive surgery; vertical heterophoria > 1 Δ; systemic diseases known to affect accommodation, vergence, and ocular motility, such as multiple sclerosis, Graves thyroid disease, myasthenia gravis, diabetes, and Parkinson disease; any ocular or systemic medication known to affect accommodation or vergence; monocular accommodative amplitude < 4 D in either eye as measured by the Donder push‐up method; manifest or latent nystagmus; attention‐deficit/hyperactivity disorder or learning disability diagnosis by parental report; household member or sibling already enrolled in the CITT; any eye care professional, technician, medical student, or optometry student. |
|
| Interventions |
Intervention regimen #1: pencil push‐ups Patients in the pencil push‐ups group were taught a pencil push‐up procedure that included monitoring for suppression. Patients were instructed to hold a pencil at arm’s length directly between their eyes, and an index card, serving as a suppression control, was placed on the wall 6 to 8 feet away. Patients were instructed to look at the very tip of the sharpened pencil and to try and keep the pencil point single while moving it toward their nose. If one of the cards in the background disappeared, patients were instructed to stop moving the pencil and blink their eyes until both cards were present. Patients were told to continue moving the pencil slowly toward their nose until it could no longer be kept single and then to try and get the pencil point back into one. If patients were able to regain single vision, they were asked to continue moving the pencil closer to their nose. If patients could not get the pencil back to one, they were instructed to start the procedure again. Patients were instructed to do three sets of 20 pencil push‐ups per day at home, 5 days per week for 12 weeks, and this treatment required an average of 15 minutes per day. Prior to doing the procedure at home, children had to demonstrate their understanding and ability to perform the procedure according to protocol. Intervention regimen #2: office‐based vergence/accommodative therapy The vergence/accommodative therapy group received therapy administered by a trained therapist during a weekly, 60‐minute office visit, with additional procedures to be performed at home for 15 minutes a day, five times per week for 12 weeks. The therapy procedures are provided in the publication. In addition, treatment procedures were practiced at home. During a typical office‐based treatment session, the patient practiced four to five procedures with constant supervision and guidance from the therapist. There were no diagnostic tests performed during these sessions. The therapist followed a very detailed and specific CITT protocol from the manual of procedures, which described the proper treatment technique, amount of time the technique was to be used, expected performance, and criteria for ending the procedure and advancing to a more difficult level. Intervention regimen #3: placebo office‐based vergence/accommodative therapy Like the vergence/accommodative therapy group, the placebo vergence/accommodative therapy group received therapy administered by a trained therapist during a 60‐minute office visit and was prescribed procedures to be performed at home for 15 minutes, five times per week for 12 weeks. The procedures for placebo vergence/accommodative therapy were designed to simulate real vergence/accommodative therapy procedures without the expectation of affecting vergence, accommodation, or saccadic function. |
|
| Outcomes | Primary outcome: convergence insufficiency symptoms measured using Convergence Insufficiency Symptom Survey V‐15 after 12 weeks of therapy. Key secondary outcomes: near point of convergence; positive fusional vergence at near. No harms were reported. |
|
| Notes | Funding sources: National Eye Insititute, National Insitutes of Health, Bethesda, Maryland, USA. Subgroup analyses: none reported Trial registration: NCT00347945 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The data‐coordinating center for the study, randomly assigned eligible patients with equal probability to either pencil push‐ups, vergence/accommodative therapy, or placebo vergence/accommodative therapy. Randomization was accomplished with the study's Web site using blocks of 6 so that the investigator could not predict the sequence of treatment assignments. To ensure approximately equal numbers of patients in each treatment arm, randomization was performed separately for each site." |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | "At these follow‐up visits, an examiner who was masked to the patient’s treatment group administered the Convergence Insufficiency Symptom Survey V‐15, the cover test, and near point of convergence and positive fusional vergence at near measurements." |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | Completion rate was 88% (15/17) for the office‐based vergence/accommodative therapy group, 80% (12/15) for the office‐based placebo therapy group, and 73% (11/15) for the pencil push‐up group. "The completion rate was not related to treatment assignment (P = .59). Of the nine patients not completing the primary outcome examination, four were lost to follow‐up, two parents decided after randomization that they preferred to have their children treated outside of the study, and three did not complete the outcome examination within the visit window." "There were no statistically significant or clinically relevant differences in demographic or clinical measures at eligibility found between these patients and those who completed the study within the treatment window." "Statistical analyses techniques were employed which allowed for incomplete data. No imputation or sensitivity analyses were performed (personal communication with the lead investigator)". |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | See above. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the study protocol were reported. |
CITT 2005c.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 46 (17 assigned to pencil push‐ups; 15 assigned to vergence/accommodative therapy; 14 assigned to placebo vergence/accommodative therapy) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 40 (87%) (15 of 17 assigned to pencil push‐ups; 12 of 15 assigned to vergence/accommodative therapy; 13 of 14 assigned to placebo vergence/accommodative therapy) Number of centers: 6 Date of first enrolment: November 2000 Length of follow‐up: planned: 12 weeks after initiation of treatment; actual: 12 ± 2 weeks after initiation of treatment Sample size estimation: no formal sample size calculations were performed a priori because one goal of this pilot trial was to estimate the variability of the outcome measure. At the study completion, using the observed variability in the Convergence Insufficiency Symptom Survey, with α=0.05, assuming a 2‐sided test, and assuming the post treatment mean of the most effective treatment group would approximate the mean among patients with normal binocular vision at 12 weeks, the mean for the placebo group would decrease 20% from its baseline value, and the mean for the other treatment group would fall in the middle of these two groups, the sample size of 46 yielded a power of 99.6%. |
|
| Participants | Country of recruitment: United States Mean age: 24.4 ± 3.4 (SD) years in the pencil push‐ups group; 23.7 ± 3.9 (SD) years in the vergence/accommodative therapy group; 25.1 ± 3.5 (SD) years in the placebo vergence/accommodative therapy group Sex: 70.6% were female in the pencil push‐ups group; 73.3% were female in the vergence/accommodative therapy group; 71.4% were female in the placebo vergence/accommodative therapy group Key inclusion criteria: age 19 to 30 years; best corrected visual acuity of 20/25 or better in both eyes at distance and near; willingness to wear eyeglasses or contact lenses to correct refractive error, if necessary; exophoria at near at least 4 Δ greater than at far; insufficient positive fusional convergence at near (i.e., failing Sheard's criterion or a positive fusional vergence measure or less than 15 Δ base‐out break); receded near point of convergence of ≥ 6 cm break; appreciation of at least 500 seconds of arc on the forms part of the Randot Stereotest; Convergence Insufficiency Symptom Survey V‐13 score ≥ 9; informed consent and willingness to participate in the study and be randomized. Key exclusion criteria: convergence insufficiency previously treated with pencil push ups, or office‐based vergence/accommodative therapy (no more than 2 months of treatment within the past year); amblyopia; constant strabismus; history of strabismus surgery; anisometropia > 1.50 D (spherical equivalent) difference between eyes; prior refractive surgery; vertical heterophoria > 1 Δ; systemic diseases known to affect accommodation, vergence, and ocular motility such as multiple sclerosis, Grave's thyroid disease, myasthenia gravis, diabetes, and Parkinsons disease; any ocular or systemic medication known to affect accommodation or vergence; monocular accommodative amplitude less than 4 D in either eye as measured by the push up method; manifest or latent nystagmus; household member already enrolled in the CITT; any eye care professional, ophthalmic technician, medical student, or optometry student. |
|
| Interventions |
Intervention regimen #1: pencil push‐ups Patients in the pencil push‐ups group were taught a pencil push‐up procedure that included monitoring for suppression. Patients were instructed to hold a pencil at arm’s length directly between their eyes, and an index card, serving as a suppression control, was placed on the wall 6 to 8 feet away. Patients were instructed to look at the very tip of the sharpened pencil and to try and keep the pencil point single while moving it toward their nose. If one of the cards in the background disappeared, patients were instructed to stop moving the pencil and blink their eyes until both cards were present. Patients were told to continue moving the pencil slowly toward their nose until it could no longer be kept single and then to try and get the pencil point back into one. If patients were able to regain single vision, they were asked to continue moving the pencil closer to their nose. If patients could not get the pencil back to one, they were instructed to start the procedure again. Patients were instructed to do three sets of 20 pencil push‐ups per day at home, 5 days per week for 12 weeks, and this treatment required an average of 15 minutes per day. Prior to doing the procedure at home, the patient had to demonstrate their understanding and ability to perform the procedure according to protocol. Intervention regimen #2: office‐based vergence/accommodative therapy The vergence/accommodative therapy group received therapy administered by a trained therapist during a weekly, 60‐minute office visit, with additional procedures to be performed at home for 15 minutes a day, five times per week for 12 weeks. The items are listed elsewhere. In addition, treatment procedures were practiced at home. During a typical office‐based treatment session, the patient practiced four to five procedures with constant supervision and guidance from the therapist. There were no diagnostic tests performed during these sessions. The therapist followed a very detailed and specific CITT protocol from the manual of procedures, which described the proper treatment technique, amount of time the technique was to be used, expected performance, and criteria for ending the procedure and advancing to a more difficult level. Intervention regimen #3: placebo office‐based vergence/accommodative therapy Like the vergence/accommodative therapy group, the placebo vergence/accommodative therapy group received therapy administered by a trained therapist during a 60‐minute office visit and was prescribed procedures to be performed at home for 15 minutes, five times per week for 12 weeks. The procedures for placebo vergence/accommodative therapy were designed to simulate real vergence/accommodative therapy procedures without the expectation of affecting vergence, accommodation, or saccadic function. |
|
| Outcomes | Primary outcome: symptoms measured using Convergence Insufficiency Symptom Survey V‐15 after 12 weeks of therapy. Key secondary outcomes: near point of convergence, and positive fusional vergence at near. No harms were reported. |
|
| Notes | Funding sources: National Eye Insititute, National Insitutes of Health, Bethesda, Maryland, USA. Subgroup analyses: none reported Trial registration: NCT00347945 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The data‐coordinating center for the study, randomly assigned eligible patients with equal probability to either pencil push‐ups, vergence/accommodative therapy, or placebo vergence/accommodative therapy. Randomization was accomplished with the study's Web site using blocks of 6 so that the investigator could not predict the sequence of treatment assignments. To ensure approximately equal numbers of patients in each treatment arm, randomization was performed separately for each site." |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | "Examiners were masked to the treatment assignment (personal communication with the lead investigator)." |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | "All results are reported for only those patients with data at the 12‐week visit. Further analyses were performed after imputing outcome values for patients lost to follow‐up. That is, the value at the last available examination was used for each patient who did not complete the study. For 5/6 patients who did not have outcome data at 12 weeks, the only data available were collected at baseline. When differences in statistical analyses were found, the results from analyses with imputed data are also reported." |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | See above. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the study protocol were reported. |
CITT 2008.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 221 (54 assigned to home‐based pencil push‐ups (HBPP); 53 assigned to home‐based computer vergence/accommodative therapy and pencil push‐ups (HBCVAT+); 60 assigned to office‐based vergence/accommodative therapy with home reinforcement (OBVAT); 54 assigned to office‐based placebo therapy with home reinforcement (OBPT)) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 219 (99%) (53 of 54 assigned to HBPP; 52 of 53 assigned HBCVAT+; 59 of 60 assigned to OBVAT; 54 of 54 assigned to OBPT) Number of centers: 9 Date of first enrolment: July 2005 Length of follow‐up: planned: 12 weeks after initiation of treatment; actual: 12 weeks after initiation of treatment Sample size estimation: all sample size calculations were performed using PASS 2000 software and assuming a 2‐sided test with 90% power. For a given outcome measure, the common standard deviation (SD) obtained from the CITT pilot study was used as an estimate of variability. To control for multiple comparisons (4 groups, with 2 compared at a time [6 pair‐wise comparisons]), the α level used for determining sample size was set at 0.0083 (0.05/6). The sample size of 52 children per group was based on the required sample size for the 3 outcome variables and adjusted for a 10% loss to follow‐up. |
|
| Participants | Country of recruitment: United States Mean age: 11.9 ± 2.2 (SD) years in the HBPP group; 11.6 ± 2.3 (SD) years in the HBCVAT+ group; 12.0 ± 2.6 (SD) years in the OBVAT group; 11.8 ± 2.2 (SD) years in the OBPT group Sex: 50% were female in the HBPP group; 58% were female in the HBCVAT+ group; 68% were female in the OBVAT group; 59% were female in the OBPT group Key inclusion criteria: aged 9 to 17 years; exodeviation at near of at least 4 prism diopters greater than at far; receded near point of convergence (NPC) break (≥ 6 cm); insufficient positive fusional vergence at near (i.e., failing Sheard's criterion or < 15 Δ break on PFV); Convergence Insufficiency Symptom Survey (CISS) score ≥ 16; best‐corrected visual acuity of 20/25 or better in both eyes at distance and near; willingness to wear eyeglasses or contact lenses to correct refractive error, if necessary; appreciation of at least 500 seconds of arc on the forms part of the Randot Stereotest. Key exclusion criteria: convergence insufficiency previously treated with pencil push‐up therapy (> 2 weeks of treatment), home‐ or office‐based vergence/accommodative therapy; amblyopia; constant strabismus; history of strabismus surgery; necessary refractive error correction worn for at least 2 weeks prior to eligibility determination; prior refractive surgery; vertical heterophoria > 1 Δ; systemic diseases known to affect accommodation, vergence and ocular motility; accommodative amplitude < 5 D in either eye as measured by the Donders' push‐up method; manifest or latent nystagmus; developmental disability, mental retardation, attention‐deficit/hyperactivity disorder, or a learning disability; family or household member or sibling already enrolled in the CITT; family or household member of an eye care professional, ophthalmic technician, ophthalmology or optometry resident, or optometry student; convergence insufficiency secondary to acquired brain injury or any other neurological disorder. |
|
| Interventions |
Intervention regimen #1: home‐based pencil push‐ups The pencil push‐ups procedure involved using a pencil with 20/60 reduced Snellen letters and a white index card placed in the background to provide a suppression check by using physiological diplopia awareness. The goal of the procedure was to move the pencil to within 2 to 3 cm of the brow, just above the nose on each push‐up while trying to keep the target single and clear. Patients were instructed to perform the pencil push‐ups procedure 15 minutes per day, 5 days per week. They maintained home therapy logs, recording the closest distance that they could maintain fusion after each 5 minutes of therapy. Intervention regimen #2: home‐based computer vergence/accommodative therapy and pencil push‐ups Patients in this group were taught to perform the pencil push‐up procedure as well as procedures on the Home Therapy System/Computerized Vergence System (HTS/CVS) computer software system (Computer Orthoptics, Gold Canyon, Arizona). Using this program, they performed fusional vergence and accommodative therapy procedures, including vergence base‐in, vergence base‐out, autoslide vergence, and jump ductions vergence programs using random‐dot stereopsis targets. The accommodative rock program was used for accommodative therapy. Much like a clinician would do at each follow‐up visit, this computer program automatically modified the therapy program after each session based on the patient’s performance. Patients were instructed to do pencil push‐ups 5 minutes per day, 5 days per week, and the HTS software program for 15 minutes per day, 5 days per week, and to save their data on a disk provided by the study and to bring the disk to each follow‐up visit. Intervention regimen #3: office‐based vergence/accommodative therapy with home reinforcement The OBVAT group received a weekly 60‐minute in‐office therapy visit with additional prescribed procedures to be performed at home for 15 minutes a day, 5 days per week. The therapy procedures are described in detail elsewhere (CITT 2008). At each office‐based therapy session, the patient performed 4 to 5 procedures with constant supervision and guidance from the therapist. There were no diagnostic tests performed during these sessions. The therapist followed a detailed and specific protocol from the CITT manual of procedures (http://optometry.osu.edu/research/CITT/4363.cfm); this document describes each procedure, amount of time procedure was performed, expected performance, and criteria for ending the procedure and advancing to a more difficult level. Intervention regimen #4: office‐based placebo therapy with home reinforcement Patients in the OBPT group received therapy during a weekly 60‐minute office visit and were prescribed procedures to be performed at home for 15 minutes per day, 5 days per week. The placebo therapy program consisted of 16 in‐office therapy procedures and 4 home therapy procedures, which were designed to look like real vergence/accommodative therapy procedures yet not to stimulate vergence, accommodation, or fine saccadic eye movement skills beyond normal daily visual activities. The therapist followed a detailed protocol from the CITT manual of procedures. Five procedures were performed during each office therapy visit and 2 procedures were assigned for home therapy each week. Objectives and goals were established for each placebo procedure to simulate real therapy. For motivational purposes, the therapist told the patient the objective of each procedure before beginning the technique. |
|
| Outcomes | Primary outcome: convergence insufficiency symptoms measured using Convergence Insufficiency Symptom Survey V‐15 after 12 weeks of therapy. The CI symptoms was also measured at baseline, 4 and 8 weeks of therapy. Key secondary outcomes: near point of convergence, and positive fusional vergence at near. The secondary outcomes were measured at baseline, 4, 8 and 12 weeks of therapy. No harms were reported. |
|
| Notes | Funding sources: National Eye Insititute, National Insitutes of Health, Bethesda, Maryland, USA. Subgroup analyses: none reported Trial registration: NCT00338611 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was achieved using a secure web site created and managed by the data coordinating center. The web site generated the patient’s group assignment and assigned the patient a unique study identification number using a pre‐determined list generated by the data coordinating center. The randomization algorithm assigned patients to the four treatment groups with equal probability using a randomized permuted block design so investigators could not predict the sequence of treatment assignments. To ensure approximately equal numbers of patients in each treatment arm, randomization was performed separately for each clinical site. |
| Allocation concealment (selection bias) | Low risk | Access to the list was limited to the programmer and principal investigator of the data coordinating center (personal communication with the lead investigator). |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | The examiners responsible for obtaining the outcome measures were masked to patient treatment assignment. None of the examiners felt that they could identify the patients’ group assignment at the 4 or 8 week masked examinations, and only one examiner felt that he could identify the group assignment at outcome. One third of the examiners responded that their patient was assigned to the OBVAT group, 24% responded that he/she was assigned to HBCVAT+, 21% said their patient was assigned to HBPP, and 21% said their patient was assigned to the OBPT group. Examiners, when asked to guess, were correct in identifying the patient’s group assignment only 34% of the time, which is less than is expected by chance. There was low agreement between the actual group assignment and the examiner’s guess of assigned treatment group (0.11, 95% confidence interval, 0.04 to 0.20). |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | "Statistical analyses techniques were employed which allowed for incomplete data. No imputation or sensitivity analyses were performed (personal communication with the lead investigator)". |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | See above. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the study protocol were reported. |
CITT‐ART 2019.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 311 (206 assigned to 16 weeks of office‐based vergence/accommodative therapy (OBVAT); 104 assigned to 16 weeks of office‐based placebo therapy (OBPT); one participant was determined ineligible after randomization) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 302 (97%) (198 of 206 assigned to OBVAT; 104 of 104 assigned OBPT) Number of centers: 9 Date of first enrolment: September 2014 Length of follow‐up: planned: 16 weeks after initiation of treatment; actual: 16 weeks after initiation of treatment Sample size estimation:The CITT‐ART's pre‐planned sample size of 324 participants (216 in the OBVAT group and 108 in the OBPT group) was chosen to provide sufficient power for the trial's primary aim of determining whether treatment improved reading comprehension. This sample size provided > 95% power with a two‐sided type I error rate of 5% to detect treatment group differences in near point of convergence of ≥ 4 cm, CISS score of ≥ 10 points, positive fusional vergence of ≥ 10 Δ, and vergence facility of ≥ 3 cpm. |
|
| Participants | Country of recruitment: United States Mean age: 10.8 ±1.5 (SD) years in the OBVAT group; 10.9 ± 1.4 (SD) years in the OBPT group Sex: 58% were female in the OBVAT group; 46% were female in the OBPT group Key inclusion criteria: Age 9 to 14 y; Grades 3 to 8; CISS score ≥ 16; Exophoria at near (40 cm) at least 4 Δ greater than at far (4 m); Receded near point of convergence of ≥ 6‐cm break; Insufficient positive fusional vergence at near (40 cm; i.e., failing Sheard's criterion or positive fusional vergence ≤ 15 Δ BO break); Best‐corrected distance (4 m) and near visual acuity (40 cm) of 20/25 or better in each eye; Random‐dot stereopsis appreciation of 500 seconds of arc or better (40 cm); Willing to wear refractive correction for uncorrected refractive errors (based on cycloplegic refraction within prior 6 mo; correction must be worn for at least 2 weeks); Not wearing BI prism or a plus add at near for 2 weeks before study enrolment and for duration of study; The timing of enrolment must allow a participant to be attending school at both the baseline and the 16‐week outcome examination; English is primary language spoken at home, or the child is proficient in English as determined by the school; Parental permission to contact the child's teacher(s) for study purposes; The parent and child understand the protocol and are willing to accept randomization; The parent does not expect the child to start any new ADHD medicine or change the dose of any currently taken ADHD medicine while the child is being treated in the study. Key exclusion criteria: Constant strabismus at distance or near; Esophoria of ≥2Δ at distance; Vertical heterophoria ≥ 2 Δ at distance or near; ≥ 2‐line interocular difference in best‐corrected distance visual acuity; Monocular near point of accommodation > 20 cm (accommodative amplitude < 5 D) as measured by push‐up method; Manifest or latent nystagmus; Word reading subtest score < 80 on the WRAT‐4; KBIT‐2 matrices subtest score < 70; History of strabismus, intraocular, or refractive surgery; CI previously treated with any form of office‐based vergence/accommodative therapy or home‐based vergence therapy (e.g., computerized vergence therapy); CI associated with head trauma or known disease of the brain; Diseases known to affect accommodation, vergence, or ocular motility such as multiple sclerosis, Graves orbitopathy, myasthenia gravis, diabetes mellitus, Parkinson disease; Inability to comprehend and/or perform any study‐related test or procedure; Speech‐language disorder (e.g., stuttering) that would interfere with interpretation of digital recordings of reading tests; Significant hearing loss; Household member enrolled in the present CITT‐ART or treated within the past 6 mo with any form of office‐based vergence/accommodative therapy or home‐based vergence therapy (e.g., computerized vergence therapy); Household member is an eye care professional, ophthalmic technician, ophthalmology or optometry resident, or optometry student. |
|
| Interventions | A 16‐week program of weekly 60‐minute in‐office therapy specific to the assigned therapy (vergence/accommodative or placebo) group was administered by study‐certified optometrists, with four to five therapy procedures administered in the office and 15 minutes of daily home therapy prescribed for 5 days per week. Intervention regimen #1: OBVAT The OBVAT program was divided into 4 phases. Within each phase there are a number of categories such as gross convergence, vergence, and accommodation. Intervention regimen #2: OBPT The OBPT program comprised pre‐determined sequentially administered procedures designed to appear to be genuine therapy techniques but not to stimulate vergence, accommodation, or fine saccadic eye movements beyond normal daily visual activities. |
|
| Outcomes | Primary outcome: The change in the Wechsler Individual Achievement Test‐III (WIAT‐III) reading comprehension score as measured after the completion of 16 weeks of assigned treatment (OBVAT or OBPT) . Key secondary outcomes: change in the near point of convergence from baseline to 16 weeks; change in positive fusional vergence from baseline to 16 weeks; change in the CISS score from baseline to 16 weeks; composite outcome measures. A successful outcome was a score of < 16 on the CISS, a normal NPC (6 cm), and a normal PFV (15 and passing the Sheard’s criterion). Improved was defined as a score of < 16 or a 10‐point decrease in the CISS score, and at least 1 of the following: normal NPC, an improvement in NPC of more than 4 cm, normal PFV, or an increase in PFV of more than 10 Δ. No harms were reported. |
|
| Notes | Funding sources: National Eye Insititute, National Insitutes of Health, Bethesda, Maryland, USA. Subgroup analyses: none reported Trial registration: NCT02207517 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated using a permuted block (sizes of 3, 6, and 9) design stratified by site and parent reported attention‐deficit/hyperactivity disorder status (yes/no) in a 2:1 allocation ratio to office‐based vergence/accommodative therapy or office‐based placebo therapy (hereafter placebo therapy), respectively." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | Protocol‐specified follow‐up visits were conducted by study‐certified optometrists and ophthalmologists masked to participants' treatment group after 4, 8, 12, and 16 weeks of therapy. Examiners were asked if they became unmasked to the participant's treatment group after each examination, and participants were asked upon completion of their 16‐week therapy program whether they thought they had received “real” vergence/accommodative therapy or placebo therapy. None of the examiners felt that they could identify the patients’ group assignment at the 4‐ or 8‐week masked examinations, and only 1 examiner felt that he could identify the group assignment at outcome. |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | "The 16‐week primary outcome visit was completed by 199 (96.6%) of the 206 participants in the vergence/accommodative group and by 100% of the 104 participants in the placebo group. Because only a few participants (n = 7) missed their 16‐week outcome visit, we believe that the probability of bias is low, and thus, an imputation analysis was not conducted." |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | See above. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Momeni Moghaddam 2015.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 60 (30 assigned to home‐based pencil push‐up therapy; 30 assigned to office‐based therapy) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: assumed 60 (100%, no loss to follow up reported and no sample sizes provided in results tables) Number of centers: 1 Date of first enrolment: not reported Length of follow‐up: planned: 8 weeks; actual 8 weeks Sample size estimation: not reported |
|
| Participants | Country of recruitment: Iran Mean age: 21.4 ± 0.9 (SD) years in home‐based pencil push‐up therapy group; 21.2 ± 0.9 (SD) years in office‐based therapy group Sex: 60% were female in both groups Key inclusion criteria: Convergence Insufficiency Symptom Survey (CISS) score of ≥ 21 ; near exodeviation which was at least 4 Δ more than distance; remote NPC (≥ 6 cm); insufficient near PFV (not meeting Sheard's criterion, or break point < 15 Δ ); best corrected monocular VA of 20/25 or better at far and near. Key exclusion criteria: Constant strabismus; amblyopia; history of refractive surgery; vertical phoria of 1 prism diopter or more; presence of manifest or latent nystagmus; presence of eye disease; and/or a history of strabismus surgery. |
|
| Interventions |
Intervention regimen #1: home‐based pencil push‐up therapy (PPT) For home‐based PPT, the participants held a pencil at 50 cm along the midline. They were instructed to position themselves so that when they looked at the tip of the pencil, they were aware of diplopia at far. A target such a clock on the wall behind the pencil was used to control suppression with use of physiological diplopia. Next, the pencil was moved toward their eyes slowly, and the participants were instructed to try to maintain fixation so that the target appeared as a single pencil. When they perceived double vision of the target even with maximum effort, the pencil was moved back slowly until they regained fusion. If suppression occurred and one of the physiologic diplopic images disappeared, the participant was instructed to blink or shake the pencil as an anti‐suppression technique. The subjects performed this exercise at least three times a day for 5 minutes each time. Intervention regimen #2: office‐based vision therapy (OBVT) The office‐based therapy group was given exercises for improvement of vergence amplitude by prism, vergence facility, accommodative amplitude, and facility. These exercises were performed two days per week for 60 minutes. Participants had one minute break for each 5 minutes of therapy |
|
| Outcomes | Primary outcome: symptoms measured using Convergence Insufficiency Symptom Survey (CISS), near point of convergence, positive fusional vergence after 8 weeks of therapy Key secondary outcomes: primary and secondary outcomes were not distinguished No harms were reported |
|
| Notes | Founding sources: Zahedan University of Medical Sciences Subgroup analyses: none reported Trial registration: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization was used to divide subjects into two groups: Home‐based PPT and office‐based therapy groups. Odd‐ numbered subjects were assigned to the home‐based therapy group and, even‐numbered subjects to the office‐based therapy group." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Primary outcome | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Secondary outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants completed follow up. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | See above. |
| Selective reporting (reporting bias) | Unclear risk | No prior trial registration with which to compare. |
Nabovati 2020.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 64 (32 assigned to prism reading glasses; 32 assigned to placebo reading glasses) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 61 (three participants lost to follow up) Number of centers: 1 Date of first enrolment: November 2018 Length of follow‐up: 12 weeks Sample size estimation: not reported |
|
| Participants | Country of recruitment: Iran Mean age: 25.5 ± 5.5 (SD) years (no data presented by treatment group) Sex: 44% were male (no data presented by treatment group) Key inclusion criteria: 18 to40 years old; convergence insufficiency diagnosed as 1) exophoria at near of at least 4 prism diopters greater than at far, 2) receded near point of convergence (NPC) break (≥ 6 cm), 3) insufficient positive fusional vergence (PFV) at near, i.e. failing Sheard's criterion (near PFV less than twice the near exophoria); 4) A normal monocular accommodative amplitude according to Hofstetter's formula: measured monocular amplitude is above the minimum accommodative age‐expected amplitude according to formula: 15 ‐ 0.25 x (age); moderate to severe stages of CI based on the amount of PFV measured at baseline; not a candidate for vision therapy due to financial issues, time limitation, or lack of motivation. Key exclusion criteria: best corrected visual acuity worse than 20/25 in either eye; constant strabismus; latent or manifest nystagmus; previous treatment of CI in the past year; history of strabismus or refractive surgery; history of any intraocular surgery; use of ophthalmic or systemic drugs affecting binocular vision or accommodation; and history of ocular trauma |
|
| Interventions |
Intervention regimen #1: prismatic spectacles Non‐emmetropic participants were instructed to wear the prescribed prism lenses only for near‐work activities lasting more than 15 minutes. If a participant was ametropic and required distance optical correction, two pairs of glasses for near and far were prescribed and the prescribed prism was only applied in the near spectacles. The participant was instructed to use the distance glasses routinely and wear the near spectacles for near‐work activities like reading. Intervention regimen #2: placebo spectacles Non‐emmetropic participants were instructed to wear the prescribed plano lenses only for near‐work activities lasting more than 15 minutes. If a participant was ametropic and required distance optical correction, two similar pairs of glasses were prescribed. One pair was introduced as near glasses and the participant was asked to use them for near‐work activities. |
|
| Outcomes | Primary outcome: Convergence Insufficiency Symptom Survey score Secondary outcomes: near exophoria, near PFV, NPC, vergence facility, monocular accommodative facility, accommodative response, negative relative accommodation, and accommodative convergence/accommodation (AC/A) ratio |
|
| Notes | Funding sources: not reported Subgroup analyses: none reported Trial registration: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The permuted block design approach was applied for the randomisation process using Microsoft Excel software". |
| Allocation concealment (selection bias) | Unclear risk | "Each participant was first randomly assigned to either treatment (prism) or control (placebo) groups by a coordinator outside the optometry examination room." |
| Blinding (performance bias and detection bias) Primary outcome | High risk | "The patients were masked to the group to which they were assigned." "Since it was possible to identify the type of the assigned correction by the experienced examiner, the examiner was not masked to the participant's group assignment at the outcome examination." |
| Blinding (performance bias and detection bias) Secondary outcomes | High risk | "Since it was possible to identify the type of the assigned correction by the experienced examiner, the examiner was not masked to the participant's group assignment at the outcome examination." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | One participant (out of 32) and two participants (out of 32) were lost to follow up in the prism group and placebo group, respectively. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | See above. |
| Selective reporting (reporting bias) | Unclear risk | Although there was a trial registration number with the Iranian Registry of Clinical Trials, we could not retrieve the registration information. All outcomes described in the Methods were reported. |
Nehad 2018.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 113 (numbers assigned to each group were not reported) Unit of randomization: not reported Number analyzed: 102 (90%) (50 in office‐based vision therapy with home therapy system group; 52 in office‐based vision therapy group) Number of centers: 1 Date of first enrolment: May 2013 Length of follow‐up: planned: 12 weeks; actual: 12 weeks Sample size estimation: not reported |
|
| Participants | Country of recruitment: Egypt Mean age: 9.3 ± 1.2 (SD) years in office‐based vision therapy with home therapy system group; 9.1 ± 1.6 (SD) years in office‐based vision therapy group Key inclusion criteria: an age of less than 16 years; exodeviation at near for at least 4 Δ more than at far; a NPC break of ≥ 6 cm and insufficient PFV at near defined as failing Sheard’s criterion [PFV <twice the near phoria] or minimum PFV of ≤ 15 Δ base‐out blur or break; a CI Symptom Survey (CISS) score of ≥ 16 Key exclusion criteria: constant strabismus; past history of cover therapy; past‐history of strabismus surgery; lack of facilities for home computer |
|
| Interventions |
Intervention #1: office‐based vision therapy (OBVT) with home therapy system (HTS) In addition to OBVT, patients, using this program, performed fusional vergence therapy actions including vergence base‐in, vergence base‐out, auto‐slide vergence, and jump ductions vergence programs by means of random dot stereopsis targets. The HTS software program was used for 20 minutes daily for 6 days/week. Intervention #2: office‐based vision therapy (OBVT) Patients had a weekly 60‐minute in‐office therapy. At each office‐based therapy session, the patient completed 4 to 5 procedures, such as the Brock string, binocular accommodative rock – flipper, stereograms, vectograms, tranaglyphs, and stereoscopes under supervision from one of the authors |
|
| Outcomes | Primary outcome: "success" defined by using symptoms measured with the Convergence Insufficiency Symptom Survey, near point of convergence, and positive fusional vergence after 12 weeks of treatment. Key secondary outcomes: none reported No harm was reported. |
|
| Notes | Funding sources: no funding Subgroup analyses: none reported Trial registration: NCT03431454 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Primary outcome | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Secondary outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol available for comparison |
PEDIG 2016.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 204 (75 assigned to home‐based computer vergence; 85 assigned to home‐based near target push‐up; 44 assigned to home‐based placebo) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 169 (83%) (69 of 75 assigned to home‐based computer vergence; 69 of 85 assigned to home‐based near target push‐up; 31 of 44 assigned to home‐based placebo) Number of centers: 30 Date of first enrolment: June 2012 Length of follow‐up: planned: 12 weeks after initiation of treatment; actual: 12 weeks after initiation of treatment Sample size estimation: "The pre‐planned sample size was 595 participants (238 participants in each of the two active treatment groups, 119 participants in the placebo (HB‐P) group) to have 90% power to detect a treatment group difference for each of the two pairwise comparisons, HB‐C versus HB‐PU and HB‐C versus placebo, assuming true population success percentages of 30%, 15% and 10% for the HB‐C group, HB‐PU group, and HB‐P group, respectively, with a type I error rate of 2.5% per comparison (5% overall) including adjustments for 3 planned interim analyses for futility and no more than 10% loss to follow‐up. The assumed success percentages were determined based on the Convergence Insufficiency Treatment Trial (CITT) and clinical expertise. Due to sample size considerations, it was not feasible to compare the HB‐PU group with the HB‐P group as a primary outcome pairwise comparison based on the assumed successful outcome percentages of 15% versus 10%." |
|
| Participants | Country of recruitment: United States Mean age: 12.2 ± 2.4 (SD) years in home‐based computer vergence group; 12.6 ± 2.5 (SD) years in home‐based near target push‐up group; 12.3 ± 2.3 (SD) years in home‐based placebo group Sex: 61% were female in home‐based computer vergence group; 54% were female in home‐based near target push‐up group; 59% were female in home‐based placebo group Key inclusion criteria: age 9 to <18 years; near exophoria 4 Δ or greater than at distance; reduced positive fusional vergence at near (PFV), defined as < 20 Δ mean PFV blur or failing Sheards’ criterion (mean PFV measured less than twice the near phoria magnitude); mean near point of convergence (NPC) of ≥ 6 cm break; symptomatic convergence insufficiency, defined as a Convergence Insufficiency Symptom Survey (CISS) score of ≥ 16 points; best‐corrected visual acuity of 20/25 or better in each eye at distance and near; near random dot stereoacuity of at least 400 seconds of arc Key exclusion criteria: ≥ 2 logMAR line difference in best‐corrected visual acuity between the two eyes; constant or intermittent exotropia at distance; constant exotropia at near; any esotropia at distance or near; distance exophoria > 10 Δ; history of strabismus surgery; anisometropia ≥ 2.00 D in any meridian between the eyes; prior intraocular or refractive surgery; primary vertical heterophoria greater than 1 Δ; diseases known to affect accommodation, vergence, and ocular motility such as multiple sclerosis, Graves orbitopathy, myasthenia gravis, diabetes mellitus, or Parkinson disease; current use of any ocular or systemic medication known to affect accommodation or vergence such as anti‐anxiety agents; near point of accommodation > 20 cm in the right eye; manifest or latent nystagmus evident clinically; history of chronic headaches unrelated to reading activity |
|
| Interventions |
Intervention regimen #1: home‐based computer vergence/accommodative therapy (HB‐C) The HB‐C group was prescribed 15 minutes of active computer vergence/accommodative therapy (CVAT) and 5 minutes of placebo flipper exercises. Intervention regimen #2: home‐based near target push‐up therapy (HB‐PU) The HB‐PU group was prescribed 15 minutes (in full or split into three 5‐minute intervals) of a well‐defined near target push‐up (NTP) procedure and 5 minutes of placebo CVAT. Intervention regimen #3: home‐based placebo treatment (HB‐P) The HB‐P group was prescribed 15 minutes of placebo CVAT and 5 minutes of placebo flipper exercises. |
|
| Outcomes | Primary outcome: successful outcome if meets at 12 weeks success criteria for all of the followings: (1) Convergence Insufficiency Symptom Survey score of < 16 points and improvement of ≥ 9 points since baseline; (2) mean near point of convergence (NPC) break of < 6 cm and a 12‐week to baseline ratio of < 0.763 for mean NPC break; (3) mean positive fusional vergence at near blur of >15 Δ and a 12‐week to baseline ratio of > 1.419 for mean PFV blur. Key secondary outcomes: percentage of participants who met success criteria of the original components of the primary outcome for both vergence measures (NPC and PFV). and percentage classified as "improvers" (12‐week CISS score improvement of ≥9 points since baseline; 12‐week to baseline ratio of > 0.763 for mean NPC break, and 12‐week to baseline ratio of > 1.419 for mean PFV blur. No harms were reported. |
|
| Notes | Funding sources: National Eye Institute, National Institutes of Health, Bethesda, Maryland, USA. Subgroup analyses: none reported Trial registration: NCT01515943 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned (using a permutated block design stratified by site) to 1 of 3 HB treatment groups (HB‐C group, HB‐PU group, and HB‐P group) in a 2:2:1 ratio with a 1 in 5 chance of being randomized to the HB‐P group" |
| Allocation concealment (selection bias) | Low risk | Allocation was adequately concealed (personal communication with the investigator). |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | "Participants were to remain masked to their treatment group until they completed the study." "Placebo CVAT was similar to the active version except there was no specific accommodation program and the procedures were designed not to stimulate or exert any extra demand on the vergence system." |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | "With the exception of the cycloplegic refraction and stereoacuity testing, the CISS and clinical testing were repeated at each follow‐up examination by an examiner who was masked to participants’ treatment group". "Examiners were masked to treatment group for all examinations." |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | Differential loss to follow‐up [6(8%) participants in home‐based computer vergence group, 16(19%) in home‐based near target push‐up, and 13(30%) in home‐based placebo therapy] who were randomized were not included in the final analysis. |
| Incomplete outcome data (attrition bias) Secondary outcomes | High risk | See above. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
Widmer 2018.
| Study characteristics | ||
| Methods | Study design: RCT Number randomized: 7 (4 assigned to office‐based vergence‐accommodative therapy group; 3 assigned to control group) Unit of randomization: individual participant (convergence insufficiency is a binocular vision disorder) Number analyzed: 7 (100%) Number of centers: 1 Date of first enrolment: not reported Length of follow‐up: planned: 12 weeks; actual: 12 weeks Sample size estimation: not reported |
|
| Participants | Country of recruitment: United States Mean age: 26.1 ± 2.5 (SD) years Sex: 86% were female Key inclusion criteria: Participants aged 18 to 30 years; best‐corrected visual acuity of 20/25 or better in each eye at distance and near; exophoria at near ≥ 4 Δ greater than distance; receded near point of convergence of ≥ 6 cm break; reduced positive fusional vergence (less than twice the near phoria OR < 15 Δ base‐out blur, or break if no reported blur); CISS ≥ 21; accommodative amplitude ≥ 5 D; random‐dot stereopsis of at least 500 seconds of arc; cycloplegic refraction within the past 3 months; wearing any needed refractive error correction for at least 2 weeks prior to eligibility; willingness to discontinue any base‐in prism or plus add wear; access to computer with Internet to perform the computerized home therapy procedures; willingness to be randomized into either the active or placebo therapy; presumptive ability to successfully complete fMRI scanning; No previous treatment with vision therapy; not personally or living with an eye‐care professional, ophthalmology/optometry student/resident, ophthalmic technician, or employed in an eye care setting; no household member enrolled in any vergence therapy study or currently completing vision therapy Key exclusion criteria: Amblyopia; constant strabismus; vertical phoria >1 Δ; refractive surgery manifest or latent nystagmus; refractive error (not corrected by contact lenses) beyond the range of the fMRI safe trial lens set; systemic diseases known to affect accommodation, vergence, or ocular motility; current use of any medication known to affect accommodation, vergence, or ocular motility, history of brain injury, neurological disease, or any condition that may be in conflict with obtaining normal fMRI scans; pregnancy; presence of a pacemaker or any metallic implant that might be incompatible with fMRI safety; developmental or learning disability that may interfere with treatment; left‐handed dominance |
|
| Interventions |
Intervention #1: office‐based vergence‐accommodative therapy "Each therapy visit consisted of 55 to 60 minutes of procedures, questions, and homework instructions. Subjects were asked to perform home reinforcement procedures 15 minutes a day, 5 days a week.Office‐based vergence‐accommodative therapy subjects completed therapy designed to stress vergence and accommodative abilities." Intervention #2: office‐based placebo therapy (No‐vergence) "...placebo subjects completed placebo therapy that did not involve vergence or accommodation beyond that involved in normal near tasks" |
|
| Outcomes | Primary outcome: Changes in brain activation (using the blood oxygenation level–dependent signal from fMRI) following office‐based vergence/accommodative therapy versus placebo therapy Key secondary outcomes: Success as defined as meeting all three of the following criteria: Convergence Insufficiency Symptom Survey (CISS) score < 21 points, normal near point of convergence (< 6 cm), and normal positive fusional vergence (> 15 Δ base‐out and at least twice the near phoria) after 12 weeks of therapy. No harms were reported. |
|
| Notes | Funding sources: Beta Sigma Kappa‐College of Optometristsin Vision Development Research Grant; Wright State University Research Initiation Grant; Optometric Educators Incorporated; Ohio Lions Eye Research Foundation Fellowship Program; Center for Cognitive and Behavioral Brian Imaging, Phychology Department, Ohio State University; Home Therapy Solutions, Gold Canyon, Arizona (home reinforcement therapy) Subgroup analyses: none reported Trial registration: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After completion of the baseline functional magnetic resonance imaging scan, each subject was randomized to 12 weeks of weekly office‐based vergence‐accommodative therapy or office‐based placebo therapy." |
| Allocation concealment (selection bias) | Low risk | Allocation was adequately concealed (personal communication with the investigator). |
| Blinding (performance bias and detection bias) Primary outcome | Low risk | "After 12 weeks of therapy, an examiner masked to the subject's assigned treatment group performed the primary outcome vision testing and functional magnetic resonance imaging scans." |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | "After 12 weeks of therapy, an examiner masked to the subject's assigned treatment group performed the primary outcome vision testing and functional magnetic resonance imaging scans." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | No loss to follow up in either arm. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | See above |
| Selective reporting (reporting bias) | Unclear risk | No mention of pre‐specified protocol or registration for verification. |
CITT: Convergence Insufficiency Treatment Trial; HBPP: Home‐based pencil push‐ups; HBCVAT+: Home‐based computer vergence/accommodative therapy and pencil push‐ups; OBVAT: Office‐based vergence/accommodative therapy with home reinforcement; OBPT: Office‐based placebo therapy with home reinforcement; RCT: Randomized controlled trial; SD: Standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Qurainy 1995 | Not in patients with convergence insufficiency |
| Alvarez 2014 | Not a RCT |
| Alvarez 2015 | Not a RCT |
| Barnhardt 2012 | INot an RCT, it is a secondary analysis of symptom data from the CITT 2008 |
| Borsting 2016 | Not a RCT |
| Bremond Gignac 2014 | Not a RCT |
| Cooper 2009 | Not a RCT |
| Daum 1986 | Not a RCT |
| Daum 1987 | Not in patients with convergence insufficiency |
| Dragomir 2001 | Not a RCT |
| Farid 2018 | Not intervention of interest |
| Frantz 1993 | Not a RCT |
| Gaertner 2013 | Not a RCT |
| Gall 1998 | Not a RCT |
| Gallaway 2002 | Not a RCT |
| Gallaway 2017 | Not a RCT |
| Granet 2005 | Not a RCT |
| Grisham 1996 | Unclear how many patients were affected by convergence insufficiency |
| Harele 2006 | Not a RCT |
| Hatt 2013 | Not in patients with convergence insufficiency |
| Horan 2015 | Not a RCT |
| Horwood 2014a | Not in patients with convergence insufficiency |
| Horwood 2014b | Not a RCT |
| Hu 2012 | Not in patients with convergence insufficiency |
| Hussaindeen 2018 | Not in patients with convergence insufficiency |
| ISRCTN77268814 | Not intervention of interest |
| Josephson 2017 | Not the interventions of interest |
| Kapoula 2015 | Not a RCT |
| Kerkhoff 1994 | Not a RCT |
| Kommerell 2002 | Not a RCT |
| Lambert 2013 | Not a RCT |
| Ludlam 1988 | Not in patients with convergence insufficiency |
| McGregor 2014 | Not a RCT |
| Mitchell 2005 | Not a RCT |
| NCT00917982 | Not in patients with convergence insufficiency |
| NCT01435876 | Not a RCT |
| O'Leary 2006 | Not a RCT |
| Pang 2012 | Not in patients with convergence insufficiency |
| Radakovi 2012 | Not a RCT |
| Ramsay 2014 | Not in patients with convergence insufficiency |
| Rawstron 2005 | Not a RCT |
| Rowe 2017 | Not in patients with convergence insufficiency |
| Rucker 2017 | Not a RCT |
| Rutstein 1988 | Not in patients with convergence insufficiency |
| Salus 2018 | Not a RCT |
| Santos 2018 | Not a RCT |
| Scheiman 2017 | Not a RCT |
| Scheiman 2018 | Not a RCT |
| Shah 2016 | Not a RCT |
| Singh 2017 | Not a RCT |
| Singman 2014 | Not a RCT |
| Sreenivasan 2015 | Not a RCT |
| Stavis 2002 | Not a RCT |
| Teitelbaum 2009 | Not in patients with convergence insufficiency |
| Thiagarajan 2013 | Not in patients with convergence insufficiency |
| Thiagarajan 2014 | Not a RCT |
| Wang 2014 | Not a RCT |
| Wang 2017 | Not a RCT |
| Whitecross 2013 | Not a RCT |
| Worrell 1971 | Not a RCT |
RCT: Randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
CTRI/2018/05/013560.
| Study name | A randomized controlled trial to compare the efficacy of pencil push‐up therapy with orthoptic therapy in emmetropic patients of convergence insufficiency |
| Methods | Study type: RCT, parallel group Location: Dehradun, India Number of centers: 1 Duration of study: NR Follow‐up: 6 weeks Protocol: NR |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Treatment groups:
|
| Outcomes | Primary outcome: Near point of convergence: a. Any value less than 7.5 cm will be considered cured b. Improvement of 4 cm or more from the baseline will be considered improved Secondary outcomes: Convergence Insufficiency Symptom Survey score a. Convergence Insufficiency Symptom Survey score less than 16 points will be considered asymptomatic b. Decrease of at least 10 or more points from the baseline will be considered improved |
| Starting date | May, 2018 |
| Contact information | Dr. Anupam; +8475000188; dr.anupamsingh@gmail.com |
| Notes | Funding: all India institute of medical sciences, Rishikesh Conflict of interest: NR |
DRKS00014187.
| Study name | Convergence insufficiency: improvement of the near point of convergence after Institute Freespace Stereogram exercises |
| Methods | Study type: RCT, parallel group Location: Thun, Switzerland Number of centers: NR (multicenter) Duration of study: NR Follow‐up: 4 weeks Protocol: NR |
| Participants | Inclusion criteria:
Exclusion criteria: Strabismus, nystagmus, neurological diseases |
| Interventions | Treatment groups:
|
| Outcomes | Primary outcome Change of the near point of convergence after visual therapy. Secondary outcomes
|
| Starting date | April, 2018 |
| Contact information | Mr. BSc optom Volkhard Schroth; +41629572605; volkhard.schroth@fhnw.ch |
| Notes | Funding: Institutional budget, no external funding (budget of sponsor/PI) Conflict of interest: NR |
NCT03593031.
| Study name | Neural mechanism of vision therapy for patients with convergence insufficiency |
| Methods | Study type: RCT, parallel group Location: New Jersey, United States Number of centers: NR Duration of study: 6 years Follow‐up: 1 year Protocol: NR |
| Participants | Inclusion criteria: 18 to 35 years old Diagnosis of symptomatic convergence insufficiency binocularly normal control Exclusion criteria: History of head trauma any systematic disease that can interfere with vergence or accommodation such as multiple sclerosis |
| Interventions | Treatment groups:
|
| Outcomes | Primary outcome: Disparity vergence response amplitude Secondary outcomes: NR |
| Starting date | April 2014 |
| Contact information | Tara L Alvarez, 9735965272, tara.l.alvarez@njit.edu |
| Notes | Funding: New Jersey Institute of Technology; National Eye Institute, National Institutes of Health, Bethesda, Maryland, USA. Conflict of interest:NR |
U1111‐1194‐7855.
| Study name | Effectiveness of ophthalmic physiotherapy versus home exercises in the convergence insufficiency |
| Methods | Study type: RCT, parallel group Location: Cascavel, Brazil Number of centers: NR Duration of study:NR Follow‐up: NR Protocol:NR |
| Participants | Inclucion criteria:
Exclusion criteria:
|
| Interventions | Treatment groups: 1. Participants of the ophthalmic physiotherapy group will be attended by 6 physiotherapy sessions to the total, being performed twice a week, lasting 40 minutes. 2. Participants of the patient education will receive a manual with home exercises and will be guided to perform them for 6 sessions, twice a week, lasting 40 minutes. |
| Outcomes | Primary outcome: Convergence insufficiency: Questionnaire Convergence Insufficiency Symptom Survey Secondary outcomes: No |
| Starting date | NR |
| Contact information | Marcelo Taglietti; +554532291558; marcelotaglietti@gmail.com |
| Notes | Funding: Faculdade Assis Gurgacz Conflict of interest: NR |
RCT: randomized controlled trial; NR: not reported
Differences between protocol and review
Adherence to treatment is reported as an ad hoc secondary outcome since the 2011 version of this Cochrane Review .
Cochrane methodology regarding assessments of the risk of bias in included studies has been modified. We updated the 'Assessment of risk of bias in included studies' section of our 'Methods' to reflect updated methodological considerations since the 2011 version of the review. We removed "intention‐to‐treat" as a domain for bias in the 2020 update.
In the 2020 update, we used NMA for data synthesis, introduced new primary outcomes, and graded the certainty of evidence, following the CINeMA approach, for the primary outcomes. These changes were needed because pairwise comparisons do not answer the question of the comparative effectiveness of all available interventions. The rationale for introducing new primary outcomes is described under 'Methods.'
We also changed the title from non‐surgical to all interventions because non‐surgical implied that surgery is a viable option.
Contributions of authors
Conceiving the review: MS, MTK, SAC, JGL, TL Designing the review: All Coordinating the review: TL Data collection for the review ‐ Designing search strategies: CEV Trials Search Co‐ordinator ‐ Undertaking electronic searches: CEV Trials Search Co‐ordinator ‐ Undertaking manual searches: MS ‐ Screening search results: MS, MTK, SAC ‐ Organizing retrieval of papers: TL ‐ Screening retrieved papers against inclusion criteria: All ‐ Appraising quality of papers: MS, MTK, SAC, LW, TL ‐ Extracting data from papers: MS, MTK, SAC, LW, TL ‐ Writing to authors of papers for additional information: MS, SAC ‐ Providing additional data about papers: MS ‐ Obtaining and screening data on unpublished studies: MS, TL Data management for the review ‐ Entering data into RevMan: LW, TL Analysis of data: LW Interpretation of data ‐ Providing a methodological perspective: JGL, LW, TL ‐ Providing a clinical perspective: MS, MTK, SAC, JGL ‐ Providing a policy perspective: MS, MTK, SAC, JGL Writing the review: All Providing general advice on the review: All Securing funding for the review: TL Performing previous work that was the foundation of the current study: MS, TL
Sources of support
Internal sources
Johns Hopkins Bloomberg School of Public Health, USA
External sources
Contract N‐01‐EY‐2‐1003 and Grant 1 U01 EY020522‐01, National Eye Institute, National Institutes of Health, USA
Grant EY11756, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
This review update was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Declarations of interest
Dr. Mitchell Scheiman is the Study Chair of the Convergence Insufficiency Treatment Trial (CITT) Study Group. Drs. Susan Cotter and Marjean Kulp also served as investigators for the CITT trials. This group completed five included trials in this review. Dr. Scheiman was the co‐Protocol Chair for PEDIG 2016 study and Drs. Cotter and Kulp were two of the clinical site principal investigators for this trial.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aletaha 2018 {published data only}
- Aletaha M, Daneshvar F, Mosallaei M, Bagheri A, Khalili MR. Comparison of three vision therapy approaches for convergence insufficiency. Journal of Ophthalmic and Vision Research 2018;13(3):307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birnbaum 1999 {published data only}
- Birnbaum MH, Soden R, Cohen AH. Efficacy of vision therapy for convergence insufficiency in an adult male population. Journal of the American Optometric Association 1999;70(4):225-32. [PubMed] [Google Scholar]
CITT 2005a {published and unpublished data}
- Scheiman M, Cotter S, Rouse M, Mitchell GL, Kulp M, Cooper J, et al Convergence Insufficiency Treatment Trial Study Group. Randomised clinical trial of the effectiveness of base-in prism reading glasses versus placebo reading glasses for symptomatic convergence insufficiency in children. British Journal of Ophthalmology 2005;89(10):1318-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
CITT 2005b {published and unpublished data}
- Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, et al Convergence Insufficiency Treatment Trial Study Group. A randomized clinical trial of treatments for convergence insufficiency in children. Archives of Ophthalmology 2005;123(1):14-24. [DOI] [PubMed] [Google Scholar]
CITT 2005c {published and unpublished data}
- Scheiman M, Mitchell GL, Cotter S, Kulp MT, Cooper J, Rouse M, et al. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optometry and Vision Science 2005;82(7):583-95. [DOI] [PubMed] [Google Scholar]
CITT 2008 {published and unpublished data}
- Convergence Insufficiency Treatment Trial Study Group. Long-term effectiveness of treatments for symptomatic convergence insufficiency in children. Optometry and Vision Science 2009;86(9):1096-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convergence Insufficiency Treatment Trial Study Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Archives of Ophthalmology 2008;126(10):1336-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convergence Insufficiency Treatment Trial Study Group. The convergence insufficiency treatment trial: design, methods, and baseline data. Ophthalmic Epidemiology 2008;15(1):24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp M, Mitchell GL, Borsting E, Scheiman M, Cotter S, Rouse M, et al Convergence Insufficiency Treatment Trial Study Group. Effectiveness of placebo therapy for maintaining masking in a clinical trial of vergence/accommodative therapy. Investigative Ophthalmology and Vision Science 2009;50(6):2560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
CITT‐ART 2019 {published data only}
- Convergence Insufficiency Treatment Trial-Attention and Reading Trial Investigator Group. Treatment of symptomatic convergence insufficiency in children enrolled in the Convergence Insufficiency Treatment Trial-Attention and Reading Trial: A Randomized Clinical Trial. Optometry and Vision Science 2019;96(11):825-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Momeni Moghaddam 2015 {published data only}
- Momeni-Moghaddam H, Kundart J, Azimi A, Hassanyani F. The effectiveness of home-based pencil push-up therapy versus office-based therapy for the treatment of symptomatic convergence insufficiency in young adults. Middle East African Journal of Ophthalmology 2015;22(1):97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabovati 2020 {published data only}
- Nabovati P, Kamali M, Mirzajani A, Jafarzadehpur E, Khabazkhoob M. The effect of base-in prism on vision-related symptoms and clinical characteristics of young adults with convergence insufficiency; a placebo-controlled randomised clinical trial. Ophthalmic and Physiological Optics 2020;40(1):8-16. [DOI] [PubMed] [Google Scholar]
Nehad 2018 {published data only}
- Nehad T, Salem T, Elmohamady MN. Combined office-based vergence therapy and home therapy system for convergence insufficiency in Egyptian children. Open Ophthalmology Journal 2018;12:12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
PEDIG 2016 {published data only}
- Pediatric Eye Disease Investigator Group. Home-based therapy for symptomatic convergence insufficiency in children: A randomized clinical trial. Optometry and Vision Science 2016;93(12):1457-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Widmer 2018 {published data only}
- Widmer DE, Oechslin TS, Limbachia C, Kulp MT, Toole AJ, Kashou NH, et al. Post-therapy functional magnetic resonance imaging in adults with symptomatic convergence insufficiency. Optometry and Vision Science 2018;95(6):505-14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Qurainy 1995 {published data only}
- Al-Qurainy IA. Convergence insufficiency and failure of accommodation following midfacial trauma. British Journal of Oral and Maxillofacial Surgery 1995;33(2):71-5. [DOI] [PubMed] [Google Scholar]
Alvarez 2014 {published data only}
- Alvarez T, Jaswal R, Gohel S, Biswal BB. Functional activity significantly correlates to symmetrical vergence peak velocity: An fMRI study of vision therapy. Investigative Ophthalmology and Visual Science 2014;55(13):ARVO E-abstract 2661. [DOI] [PMC free article] [PubMed]
Alvarez 2015 {published data only}
- Alvarez TL. A pilot study of disparity vergence and near dissociated phoria in convergence insufficiency patients before vs. after vergence therapy. Frontiers in Human Neuroscience 2015;27(9):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnhardt 2012 {published data only}
- Barnhardt C, Cotter SA, Mitchell Gl, Scheiman M, Kulp MT. Symptoms in children with convergence insufficiency: before and after treatment. Optometry and Vision Science 2012;89(10):1512‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borsting 2016 {published data only}
- Borsting E, Mitchell GL, Arnold LE, Scheiman M, Chase C, Kulp M, et al Convergence Insufficiency Treatment Trial – Reading Study Group. Behavioral and emotional problems associated with convergence insufficiency in children: An open trial. Journal of Attention Disorders 2016;20(10):836-44. [DOI] [PubMed] [Google Scholar]
Bremond Gignac 2014 {published data only}
- Bremond-Gignac D, Ricard J, Rivalan D, Dhainaut C, Louage P, Kapoula Z. Postural control in children and young adults with vergence insufficiency. Investigative Ophthalmology and Visual Science 2014;55(13):ARVO E-abstract 2575. [Google Scholar]
Cooper 2009 {published data only}
- Cooper J, Feldman J. Reduction of symptoms in binocular anomalies using computerized home therapy-HTS™. Optometry 2009;80(9):481-6. [DOI] [PubMed] [Google Scholar]
Daum 1986 {published data only}
- Daum KM. Characteristics of exodeviations: II. Changes with treatment with orthoptics. American Journal of Optometry and Physiological Optics 1986;63(4):244-51. [PubMed] [Google Scholar]
Daum 1987 {published data only}
- Daum KM, Rutstein RP, Eskridge JB. Efficacy of computerized vergence therapy. American Journal of Optometry and Physiological Optics 1987;64(2):83-9. [DOI] [PubMed] [Google Scholar]
Dragomir 2001 {published data only}
- Dragomir M, Tru L, Chiril D, Stingu C. Orthoptic treatment efficiency in convergence insufficiency treatment. Oftalmologia 2001;53(3):66-9. [PubMed] [Google Scholar]
Farid 2018 {published data only}
- Farid MF, Abdelbaset EA. Surgical outcomes of three different surgical techniques for treatment of convergence insufficiency intermittent exotropia. Eye 2018;32(4):693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frantz 1993 {published data only}
- Frantz KA, Cotter SA, Scharre JE, Coffey B, Harrist R, et al. Pilot study of a vision therapy protocol for intermittent exotropia: Scientific Program abstract from the 1993 American Academy of Optometry Meeting. www.aaopt.org/detail/knowledge-base-article/pilot-study-vision-therapy-protocol-intermittent-exotropia (accessed 08 June 2020).
Gaertner 2013 {published data only}
- Gaertner C, Bucci MP, Ajrezo L, Wiener-Vacher S. Binocular coordination of saccades during reading in children with clinically assessed poor vergence capabilities. Vision Research 2013;87:22-9. [DOI] [PubMed] [Google Scholar]
Gall 1998 {published data only}
- Gall R, Wick B, Bedell H. Vergence facility: establishing clinical utility. Optometry and Vision Science 1998;75(10):731-42. [DOI] [PubMed] [Google Scholar]
Gallaway 2002 {published data only}
- Gallaway M, Scheiman M, Malhotra K. The effectiveness of pencil pushups treatment for convergence insufficiency: A pilot study. Optometry and Vision Science 2002;79(4):265-7. [DOI] [PubMed] [Google Scholar]
Gallaway 2017 {published data only}
- Gallaway M, Scheiman M, Mitchell GL. Vision therapy for post-concussion vision disorders. Optometry and Vision Science 2017;94(1):68-73. [DOI: 10.1097/OPX.0000000000000935] [DOI] [PubMed] [Google Scholar]
Granet 2005 {published data only}
- Granet DB, Gomi CF, Ventura R, Miller-Scholte A. The relationship between convergence insufficiency and ADHD. Strabismus 2005;13(4):163-8. [DOI] [PubMed] [Google Scholar]
Grisham 1996 {published data only}
- Grisham D, Buu T, Lum R, Nguyen BK, Shen K, Truong E, et al. Efficacy of prism prescription by the associated phoria criterion: Scientific Program abstract from the 1996 American Academy of Optometry Meeting. In: www.aaopt.org/detail/knowledge-base-article/efficacy-prism-prescription-associated-phoria-criterion. (accessed 08 June 2020).
Harele 2006 {published data only}
- Harle DE, Evans BJ. Subtle binocular vision anomalies in migraine. Ophthalmic and Physiological Optics 2006;26(6):587-96. [DOI] [PubMed] [Google Scholar]
Hatt 2013 {published data only}
- Hatt SR, Gnanaraj L. Interventions for intermittent exotropia. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD003737. [DOI: 10.1002/14651858.CD003737.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horan 2015 {published data only}
- Horan LA, Ticho BH, Khammar AJ, Allen MS, Shah BA. Is the convergence insufficiency symptom survey specific for convergence insufficiency? A prospective, randomized study. American Orthoptic Journal 2015;65:99‐103. [DOI] [PubMed] [Google Scholar]
Horwood 2014a {published data only}
- Horwood A, Toor S. Clinical test responses to different orthoptic exercise regimes in typical young adults. Ophthalmic and Physiological Optics 2014;34(2):250‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horwood 2014b {published data only}
- Horwood AM, Toor SS, Riddell PM. Change in convergence and accommodation after two weeks of eye exercises in typical young adults. Journal of the American Association for Pediatric Ophthalmology and Strabismus 2014;18(2):162‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2012 {published data only}
- Hu XD, Chen J, Peng XJ, Yan SM, Yang MD. The effect of digital multimedia visual training applied after intermittent exotropia. International Eye Science 2012;12(7):1362‐4. [Google Scholar]
Hussaindeen 2018 {published data only}
- Hussaindeen JR, Shah P, Ramani KK, Ramanujan L. Efficacy of vision therapy in children with learning disability and associated binocular vision anomalies. Journal of Optometry 2018;11(1):40-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN77268814 {published data only}
- ISRCTN77268814. Randomised controlled trial of efficacy of optometric interventions. www.isrctn.com/ISRCTN77268814 (first received 30 September 2005).
Josephson 2017 {published data only}
- Josephson M, Mathias SA. Treatment of intermittent exotropia of the convergence insufficiency type with bupivicaine 0.75%: 5-year experience and outcomes. Journal of the American Association for Pediatric Ophthalmology and Strabismus 2017;21(4):e11. [Google Scholar]
Kapoula 2015 {published data only}
- Kapoula Z, Morize A, Daniel F, Jonqua F, Orssaud C, Bremond-Gignac D. A research based novel method for vergence rehabilitation. Investigative Ophthalmology and Visual Science 2015;56(7):ARVO E-abstract 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerkhoff 1994 {published data only}
- Kerkhoff G, Stogerer E. Recovery of fusional convergence and binocular vision in brain damaged patients after training. Klinische Monatsblatter fur Augenheilkunde 1994;205(2):70-5. [DOI] [PubMed] [Google Scholar]
Kommerell 2002 {published data only}
- Kommerell G, Kromeier M. Prism correction in heterophoria. Ophthalmologe 2002;99(1):3-9. [DOI] [PubMed] [Google Scholar]
Lambert 2013 {published data only}
- Lambert J. Vision therapy and computer orthoptics: Evidence-based approach to use in your practice. American Orthoptic Journal 2013;63(1):32-5. [DOI] [PubMed] [Google Scholar]
Ludlam 1988 {published data only}
- Ludlam WM, Ludlam DE. Effects of prism-induced, accommodative convergence stress on reading comprehension test scores. Journal of the American Optometric Association 1988;59(6):440-5. [PubMed] [Google Scholar]
McGregor 2014 {published data only}
- McGregor ML. Convergence insufficiency and vision therapy. Pediatric Clinics of North America 2014;61(3):621-30. [DOI] [PubMed] [Google Scholar]
Mitchell 2005 {published data only}
- Mitchell GL, Scheiman M. Masking in the base-in prism for convergence insufficiency treatment trial. Clinical Trials 2005;2:S86-7. [Google Scholar]
NCT00917982 {published data only}
- NCT00917982. The effect of vision therapy/orthoptic on motor & sensory status of the 3 to 7 years old strabismic patients. www.clinicaltrials.gov/ct2/show/NCT00917982 (first received 11 June 2009).
NCT01435876 {published data only}
- NCT01435876. Surgery and convergence insufficiency intermittent exotropia. www.clinicaltrials.gov/ct2/show/NCT01435876 (first received 19 September 2011).
O'Leary 2006 {published data only}
- O'Leary CI, Evans BJ. Double-masked randomised placebo-controlled trial of the effect of prismatic corrections on rate of reading and the relationship with symptoms. Ophthalmic and Physiological Optics 2006;26(6):555-65. [DOI] [PubMed] [Google Scholar]
Pang 2012 {published data only}
- Pang Y, Teitelbaum B, Krall J. Factors associated with base-in prism treatment outcomes for convergence insufficiency in symptomatic presbyopes. Clinical and Experimental Optometry 2012;95(2):192‐7. [DOI] [PubMed] [Google Scholar]
Radakovi 2012 {published data only}
- Radaković M, Ivetić V, Naumović N, Čanadanović V, Stankov B. Heterophoria and fusional convergence and divergence in preschool children. Medicinski Glasnik 2012;9(2):293-8. [PubMed] [Google Scholar]
Ramsay 2014 {published data only}
- Ramsay MW, Davidson C, Ljungblad M, Tjärnberg M, Brautaset R, Nilsson M. Can vergence training improve reading in dyslexics? Strabismus 2014;22(4):147-51. [DOI] [PubMed] [Google Scholar]
Rawstron 2005 {published data only}
- Rawstron JA, Burley CD, Elder MJ. A systematic review of the applicability and efficacy of eye exercises. Journal of Pediatric Ophthalmology and Strabismus 2005;42(2):82-8. [DOI] [PubMed] [Google Scholar]
Rowe 2017 {published data only}
- Rowe FJ, Elliott S, Gordon I, Shah A. A review of Cochrane systematic reviews of interventions relevant to orthoptic practice. Strabismus 2017;25(3):101-11. [DOI] [PubMed] [Google Scholar]
Rucker 2017 {published data only}
- Rucker JC, Phillips PH. Efferent vision therapy. Journal of Neuro-Ophthalmology 2017;38(2):230-6. [DOI] [PubMed] [Google Scholar]
Rutstein 1988 {published data only}
- Rutstein RP, Daum KM, Cho M, Eskridge JB. Horizontal and vertical vergence training and its effect on vergences, fixation disparity curves, and prism adaptation: II. Vertical data. American Journal of Optometry and Physiological Optics 1988;65(1):8-13. [DOI] [PubMed] [Google Scholar]
Salus 2018 {published data only}
- NCT03248336. Objective assessment of disparity vergence after treatment of symptomatic CI in children. www.clinicaltrials.gov/ct2/show/record/NCT03248336 (first received 14 August 2017).
Santos 2018 {published data only}
- Santos EM, Yaramothu C, Alvarez TL. Comparison of symmetrical prism adaptation to asymmetrical prism adaptation in those with normal binocular vision. Vision Research 2018;149:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheiman 2017 {published data only}
- Scheiman MM, Talasan H, Mitchell GL, Alvarez TL. Objective assessment of vergence after treatment of concussion-related CI: A pilot study. Optometry and Vision Science 2017;94(1):74-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheiman 2018 {published data only}
- Scheiman MM. 2017 Glenn A. Fry Award Lecture: Establishing an evidence-based literature for vision therapy - A 25-year journey. Optometry and Vision Science 2018;95(8):632-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2016 {published data only}
- Shah K, Portello J, Rosenfield M. Effect of lens presentation on the clinical oculomotor assessment at near: Scientific Program abstract from the 2016 American Academy of Optometry Meeting. In: www.aaopt.org/detail/knowledge-base-article/effect-lens-presentation-clinical-oculomotor-assessment-near. (accessed 8 June 2020).
Singh 2017 {published data only}
- Singh NK, Mani R, Hussaindeen JR. Changes in stimulus and response AC/A ratio with vision therapy in convergence insufficiency. Journal of Optometry 2017;10(3):169-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singman 2014 {published data only}
- Singman EL. Convergence insufficiency associated with migraine: A case series. American Orthoptic Journal 2014;64:112-6. [DOI] [PubMed] [Google Scholar]
Sreenivasan 2015 {published data only}
- Sreenivasan V, Bobier WR. Increased onset of vergence adaptation reduces excessive accommodation during the orthoptic treatment of convergence insufficiency. Vision Research 2015;111(Part A):105-13. [DOI] [PubMed] [Google Scholar]
Stavis 2002 {published data only}
- Stavis M, Murray M, Jenkins P, Wood R, Brenham B, Jass J. Objective improvement from base-in prisms for reading discomfort associated with mini-convergence insufficiency type exophoria in school children. Binocular Vision and Strabismus Quarterly 2002;17(2):135-42. [PubMed] [Google Scholar]
Teitelbaum 2009 {published data only}
- Teitelbaum B, Pang Y, Krall J. Effectiveness of base in prism for presbyopes with convergence insufficiency. Optometry and Vision Science 2009;86(2):153-6. [DOI] [PubMed] [Google Scholar]
Thiagarajan 2013 {published data only}
- Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury. Journal of Rehabilitation Research and Development 2013;50(9):1223-40. [DOI] [PubMed] [Google Scholar]
Thiagarajan 2014 {published data only}
- Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on accommodative responsivity in mild traumatic brain injury. Journal of Rehabilitation Research and Development 2014;51(2):175‐91. [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang B, Wang L, Wang Q, Ren M. Comparison of different surgery procedures for convergence insufficiency-type intermittent exotropia in children. British Journal of Ophthalmology 2014;98(10):1409‐13. [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang J, Ma X, Wu Y, Liao M, Liu L. The effectiveness of disc synoptoscope on patients with abnormal binocular vision: a prospective cohort study. International Ophthalmology 2017;37(5):1139-46. [DOI] [PubMed] [Google Scholar]
Whitecross 2013 {published data only}
- Whitecross S. Vision therapy: Are you kidding me? Problems with current studies. American Orthoptic Journal 2013;63(1):36-40. [DOI] [PubMed] [Google Scholar]
Worrell 1971 {published data only}
- Worrell BE Jr, Hirsch MJ, Morgan MW. An evaluation of prism prescribed by Sheard's criterion. American Journal of Optometry and Archives of American Academy of Optometry 1971;45(5):373-6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2018/05/013560 {published data only}
- CTRI/2018/05/013560. A randomized controlled trial to compare the efficacy of pencil push-up therapy with orthoptic therapy in emmetropic patients of convergence insufficiency. www.ctri.nic.in/Clinicaltrials/showallp.php?mid1=25726&EncHid=&userName=013560 (first received 1 May 2018).
DRKS00014187 {published data only}
- DRKS00014187. Convergence insufficiency: Improvement of the near point of convergence after Institute Freespace Stereogram exercises? www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00014187 (first received 29 March 2018).
NCT03593031 {published data only}
- NCT03593031. Neural mechanism of vision therapy for patients with convergence insufficiency. www.clinicaltrials.gov/ct2/show/NCT03593031 (first received 19 July 2018).
U1111‐1194‐7855 {published data only}
- U1111-1194-7855. Effectiveness of ophthalmic physiotherapy versus home exercises in the convergence insufficiency. www.ensaiosclinicos.gov.br/rg/RBR-8kzvsk/ (first received 29 March 2017).
Additional references
Aikon 1988
- Aikon DL. Memory traces in the brain. New York: Cambridge University Press, 1988. [Google Scholar]
Alvarez 2010
- Alvarez TL, Vicci VR, Alkan Y, Kim EH, Gohel S, Barrett AM, et al. Vision therapy in adults with convergence insufficiency: Clinical and functional magnetic resonance imaging measures. Optometry and Vision Science 2010;87(12):E985-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez 2019
- Alvarez TL, Scheiman M, Santos EM, Morales C, Yaramothu C, d'Antonio-Bertagnolli JV, et al. Clinical and functional imaging changes induced from vision therapy in patients with convergence insufficiency. In: 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Berlin, Germany, 2019:104-9. [DOI] [PubMed]
Arbib 1981
- Arbib MA. Perceptual structures and distributed motor control. Handbook of physiology. Vol. II: Motor control. Bethesda, MD: Brooks BV. American Physiological Society, 1981. [Google Scholar]
Birnbaum 1977
- Birnbaum MH. The role of the trainer in visual training. Journal of American Optometric Association 1977;48(8):1035-9. [PubMed] [Google Scholar]
Borsting 2003
- Borsting EJ, Rouse MW. Validity and reliability of the revised convergence insufficiency symptom survey in children ages 9-18 years. Optometry and Vision Science 2003;80(12):832-8. [DOI] [PubMed] [Google Scholar]
Brautaset 2006
- Brautaset RL, Jennings AJ. Effects of orthoptic treatment on the CA/C and AC/A ratios in convergence insufficiency. Investigative Ophthalmology and Visual Science 2006;47(7):2876-80. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50:683-91. [DOI] [PubMed] [Google Scholar]
Cacho 2009
- Cacho Martinez P, Garcia Munoz A, Ruiz-Cantero MT. Treatment of accommodative and nonstrabismic binocular dysfunctions: a systematic review. Optometry 2009;80(12):702-16. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15:905-50. [Google Scholar]
Chin 1995
- Chin B, Fabish B, Hisaka C, Thal L, Tsuda K. A survey of the treatment of convergence insufficiency. Journal of Behavioral Optometry 1995;6:91-2. [Google Scholar]
CITT Manual of Procedures
- Convergence Insufficiency Treatment Trial Manul of Procedures. www.optometry.osu.edu/research/CITT/4363.cfm.
Ciuffreda 2002
- Ciuffreda KJ. The scientific basis for and efficacy of optometric vision therapy in nonstrabismic accommodative and binocular vision disorders. Optometry 2002;73(12):735-62. [PubMed] [Google Scholar]
Cooper 2012
- Cooper J, Jamal N. Convergence insufficiency-a major review. Optometry 2012;83(4):137-58. [PubMed] [Google Scholar]
Davis 2016
- Davis AL, Harvey EM, Twelker JD, Miller JM, Leonard-Green T, Campus I. Convergence insufficiency, accommodative insufficiency, visual symptoms, and astigmatism in Tohono O'odham Students. Journal of Ophthalmology 2016;2016:6963976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duke‐Elder 1973
- Duke-Elder S, Wybar K. Ocular motility and strabismus. In: System of Ophthalmology. Vol. 6. St. Louis: Mosby, 1973:547-51. [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Griffin 2002
- Griffin JR, Grisham JD. Binocular anomalies: diagnosis and vision therapy. Boston: Butterworth-Heinemann, 2002. [Google Scholar]
Grisham 1998
- Grisham JD. Visual therapy results for convergence insufficiency: a literature review. American Journal of Optometry and Physiological Optics 1988;65(6):448-54. [DOI] [PubMed] [Google Scholar]
Hayes 1998
- Hayes GJ, Cohen BE, Rouse MW, De Land PN. Normative values for the nearpoint of convergence of elementary schoolchildren. Optometry and Vision Science 1998;75(7):506-12. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hugonnier 1969
- Hugonnier R, Clayette-Hugonnier C. Strabismus, heterophoria and ocular motor paralysis. St. Louis: CV Mosby, 1969. [Google Scholar]
Hussaindeen 2017
- Hussaindeen JR, Rakshit A, Singh NK, George R, Swaminathan M, Kapur S, et al. Prevalence of non-strabismic anomalies of binocular vision in Tamil Nadu: report 2 of BAND study. Clinical and Experimental Optometry 2017;100(6):642-8. [DOI] [PubMed] [Google Scholar]
Huston 2015
- Huston PA, Hoover DL. Treatment of symptomatic convergence insufficiency with home-based computerized vergence system therapy in children. Journal of American Association for Pediatric Ophthalmology and Strabismus 2015;19(5):417-21. [DOI] [PubMed] [Google Scholar]
Letourneau 1979
- Letourneau JE, Lapierre N, Lamont A. The relationship between convergence insufficiency and school achievement. American Journal of Optometry and Physiological Optics 1979;56(1):18-22. [DOI] [PubMed] [Google Scholar]
Letourneau 1988
- Letourneau JE, Ducic S. Prevalence of convergence insufficiency among elementary school children. Canadian Journal of Optometry 1988;50:194-7. [Google Scholar]
Lisberger 1988
- Lisberger SG. The neural basis for learning of simple motor skills. Science 1998;242(4879):728-35. [DOI] [PubMed] [Google Scholar]
Ma 2018
- Ma MM, Long W, She Z, Li W, Chen X, Xie L, et al. Convergence insufficiency in Chinese high school students. Clinical and Experimental Optometry 2019;102(2):166-71. [DOI] [PubMed] [Google Scholar]
Nikolakopoulou 2019
- Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. Assessing confidence in the results of network meta-analysis (CINeMA). PLoS Med 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
North 1982
- North RV, Henson DB. Effect of orthoptics upon the ability of patients to adapt to prism-induced heterophoria. American Journal of Optometry and Physiological Optics 1982;59(12):983-6. [DOI] [PubMed] [Google Scholar]
Porcar 1997
- Porcar E, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optometry and Vision Science 1997;74(2):111-3. [DOI] [PubMed] [Google Scholar]
Pratt‐Johnson 2001
- Pratt-Johnson JA, Tillson G. Management of strabismus and amblyopia. New York: Thieme Medical Publishers, 2001. [Google Scholar]
Press 1997
- Press LJ. Applied concepts in vision therapy. St. Lous: Mosby-Year Book, 1997. [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rouse 1999
- Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, et al. Frequency of convergence insufficiency among fifth and sixth graders. Optometry and Vision Science 1999;76(9):643-9. [DOI] [PubMed] [Google Scholar]
Rouse 2004
- Rouse MW, Borsting EJ, Mitchell GL, Scheiman M, Cotter SA, Cooper J, et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic and Physiological Optics 2004;24(5):384-90. [DOI] [PubMed] [Google Scholar]
Rouse 2009
- Rouse M, Borsting E, Mitchell GL, Cotter SA, Kulp M, Scheiman M, et al, Convergence Insufficiency Treatment Trial Investigator Group. Validity of the convergence insufficiency symptom survey: a confirmatory study. Optometry and Vision Science 2009;86(4):357-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(80):97. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PloS One 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheiman 2002a
- Scheiman M, Cooper J, Mitchell GL, De LP, Cotter S, Borsting E, et al. A survey of treatment modalities for convergence insufficiency. Optometry and Vision Science 2002;79(3):151-7. [DOI] [PubMed] [Google Scholar]
Scheiman 2002b
- Scheiman M, Wick B. Clinical management of binocular vision: heterophoric, accommodative and eye movement disorders. Philadelphia: Lippincott, Williams and Wilkins, 2002. [Google Scholar]
Scheiman 2003
- Scheiman M, Gallaway M, Frantz KA, Peters RJ, Hatch S, Cuff M, et al. Near point of convergence: test procedure, target selection and expected findings. Optometry and Vision Science 2003;80(3):214-25. [DOI] [PubMed] [Google Scholar]
Scheiman 2005
- Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, et al. In reply: convergence Insufficiency randomized clinical trial. Archives of Ophthalmology 2005;123(12):1760-1. [DOI] [PubMed] [Google Scholar]
Scheiman 2009
- Scheiman M, Rouse M, Kulp MT, Cotter S, Hertle R, Mitchell GL. Treatment of convergence insufficiency in childhood: a current perspective. Optometry and Vision Science 2009;86(5):420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheiman 2019
- Scheiman M, Wick B. Clinical management of binocular vision: heterophoric, accommodative and eye movement disorders. 5th edition. Philadelphia: Wolters Kluwer, 2019. [Google Scholar]
Schmidt 1988
- Schmidt RA. Motor control and learning: a behavioral emphasis. Champaign, Illinois: Human Kinetics Publishers, Inc, 1988. [Google Scholar]
Schor 1986
- Schor CM. The Glenn A. Fry award lecture: adaptive regulation of accommodative vergence and vergence accommodation. American Journal of Optometry and Physiological Optics 1986;63(8):587-609. [PubMed] [Google Scholar]
Serna 2011
- Serna A, Rogers DL, McGregor ML, Golden RP, Bremer DL, Rogers GL. Treatment of symptomatic convergence insufficiency with a home-based computer orthoptic exercise program. Journal of American Association of Pediatric Ophthalmology and Strabismus 2011;15(2):140-3. [DOI] [PubMed] [Google Scholar]
Sheard 1930
- Sheard C. Zones of ocular comfort. American Journal of Ophthalmology 1930;7:9-25. [Google Scholar]
Trieu 2018
- Trieu LH, Lavrich JB. Current concepts in convergence insufficiency. Current Opinion in Ophthalmology 2018;29(5):401-6. [DOI] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JPT, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42:332-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Noorden 1994
- Noorden GK. Binocular vision and ocular motility: theory and management of strabismus. St. Louis: Mosby-Year Book, 1994. [Google Scholar]
von Noorden 1996
- Noorden GK. Binocular vision and ocular motility: theory and management of strabismus. St. Louis: Mosby-Year Book, 1996. [Google Scholar]
von Noorden 2001
- Noorden GK. Burian-von Noorden's binocular vision and ocular motility: theory and management of strabismus. St. Louis: C.V. Mosby, 2001. [Google Scholar]
Wajuihian 2015
- Wajuihian SO, Hansraj R. Vergence anomalies in a sample of high school students in South Africa. Journal of Optometry 2016;9(4):246-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta-analysis. Stata Journal 2015;15(4):951-85. [Google Scholar]
References to other published versions of this review
Scheiman 2007
- Scheiman M, Gwiazda J, Li T. Non-surgical interventions for convergence insufficiency. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006768. [DOI: 10.1002/14651858.CD006768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheiman 2011
- Scheiman M, Gwiazda J, Li T. Non‐surgical interventions for convergence insufficiency. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD006768. [DOI: 10.1002/14651858.CD006768.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]