Abstract
Background
The prevalence of type 2 diabetes is increased in individuals with mental disorders. Much of the burden of disease falls on the populations of low‐ and middle‐income countries (LMICs).
Objectives
To assess the effects of pharmacological, behaviour change, and organisational interventions versus active and non‐active comparators in the prevention or delay of type 2 diabetes among people with mental illness in LMICs.
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trials Register, CENTRAL, MEDLINE, Embase and six other databases, as well as three international trials registries. We also searched conference proceedings and checked the reference lists of relevant systematic reviews. Searches are current up to 20 February 2020.
Selection criteria
Randomized controlled trials (RCTs) of pharmacological, behavioural or organisational interventions targeting the prevention or delay of type 2 diabetes in adults with mental disorders in LMICs.
Data collection and analysis
Pairs of review authors working independently performed data extraction and risk of bias assessments. We conducted meta‐analyses using random‐effects models.
Main results
One hospital‐based RCT with 150 participants (99 participants with schizophrenia) addressed our review's primary outcome of prevention or delay of type 2 diabetes onset. Low‐certainty evidence from this study did not show a difference between atypical and typical antipsychotics in the development of diabetes at six weeks (risk ratio (RR) 0.46, 95% confidence interval (CI) 0.03 to 7.05) (among a total 99 participants with schizophrenia, 68 were in atypical and 31 were in typical antipsychotic groups; 55 participants without mental illness were not considered in the analysis).
An additional 29 RCTs with 2481 participants assessed one or more of the review's secondary outcomes. All studies were conducted in hospital settings and reported on pharmacological interventions. One study, which we could not include in our meta‐analysis, included an intervention with pharmacological and behaviour change components. We identified no studies of organisational interventions.
Low‐ to moderate‐certainty evidence suggests there may be no difference between the use of atypical and typical antipsychotics for the outcomes of drop‐outs from care (RR 1.31, 95% CI 0.63 to 2.69; two studies with 144 participants), and fasting blood glucose levels (mean difference (MD) 0.05 lower, 95% CI 0.10 to 0.00; two studies with 211 participants). Participants who receive typical antipsychotics may have a lower body mass index (BMI) at follow‐up than participants who receive atypical antipsychotics (MD 0.57, 95% CI 0.33 to 0.81; two studies with 141 participants; moderate certainty of evidence), and may have lower total cholesterol levels eight weeks after starting treatment (MD 0.35, 95% CI 0.27 to 0.43; one study with 112 participants).
There was moderate certainty evidence suggesting no difference between the use of metformin and placebo for the outcomes of drop‐outs from care (RR 1.22, 95% CI 0.09 to 16.35; three studies with 158 participants). There was moderate‐to‐high certainty evidence of no difference between metformin and placebo for fasting blood glucose levels (endpoint data: MD ‐0.35, 95% CI ‐0.60 to ‐0.11; change from baseline data: MD 0.01, 95% CI ‐0.21 to 0.22; five studies with 264 participants). There was high certainty evidence that BMI was lower for participants receiving metformin compared with those receiving a placebo (MD ‐1.37, 95% CI ‐2.04 to ‐0.70; five studies with 264 participants; high certainty of evidence). There was no difference between metformin and placebo for the outcomes of waist circumference, blood pressure and cholesterol levels.
Low‐certainty evidence from one study (48 participants) suggests there may be no difference between the use of melatonin and placebo for the outcome of drop‐outs from care (RR 1.00, 95% CI 0.38 to 2.66). Fasting blood glucose is probably reduced more in participants treated with melatonin compared with placebo (endpoint data: MD ‐0.17, 95% CI ‐0.35 to 0.01; change from baseline data: MD ‐0.24, 95% CI ‐0.39 to ‐0.09; three studies with 202 participants, moderate‐certainty evidence). There was no difference between melatonin and placebo for the outcomes of waist circumference, blood pressure and cholesterol levels.
Very low‐certainty evidence from one study (25 participants) suggests that drop‐outs may be higher in participants treated with a tricyclic antidepressant (TCA) compared with those receiving a selective serotonin reuptake inhibitor (SSRI) (RR 0.34, 95% CI 0.11 to 1.01). It is uncertain if there is no difference in fasting blood glucose levels between these groups (MD ‐0.39, 95% CI ‐0.88 to 0.10; three studies with 141 participants, moderate‐certainty evidence). It is uncertain if there is no difference in BMI and depression between the TCA and SSRI antidepressant groups.
Authors' conclusions
Only one study reported data on our primary outcome of interest, providing low‐certainty evidence that there may be no difference in risk between atypical and typical antipsychotics for the outcome of developing type 2 diabetes. We are therefore not able to draw conclusions on the prevention of type 2 diabetes in people with mental disorders in LMICs.
For studies reporting on secondary outcomes, there was evidence of risk of bias in the results. There is a need for further studies with participants from LMICs with mental disorders, particularly on behaviour change and on organisational interventions targeting prevention of type 2 diabetes in these populations.
Plain language summary
Preventing type 2 diabetes in adults with mental health conditions in low‐ and middle‐income countries
How did we identify and evaluate the evidence?
We searched the medical literature to review the evidence on the effects of pharmacological (drug), behavioural (behaviour change) and organisational (delivery of health care) interventions for the prevention of type 2 diabetes among people with mental disorders in low‐ and middle‐income countries (LMICs). Type 2 diabetes is a serious health condition that may develop when the body can no longer properly use a hormone called insulin. There are many reasons why a person may develop type 2 diabetes, including being overweight, having high blood pressure, not getting enough exercise, having a family history of the disease, and several other possible risk factors.
We included randomized controlled trials (RCTs) published up to our search date, 20 February 2020.
Why is this important?
People with mental health conditions such as schizophrenia, bipolar disorder, and major depressive disorder are more likely to develop type 2 diabetes than the general population. Many people suffering from mental health conditions, who are at an increased risk of developing diabetes, live in LMICs. Treating diabetes in this population poses challenges to healthcare systems. Preventing the development of diabetes is therefore important to people with mental health conditions and to healthcare systems in LMICs.
What did we find?
Among adults with mental health conditions, we identified only one study that assessed our primary outcome, prevention of type 2 diabetes. This hospital‐based study with 150 participants (99 participants with schizophrenia) found low‐certainty evidence of no difference in risk between the use of older antipsychotic medications (typical antipsychotics) and newer antipsychotic medications (atypical antipsychotics) for the outcome of developing type 2 diabetes.
We included an additional 29 studies with 2481 participants assessing one or more of our secondary outcomes. All studies were conducted in hospital settings and tested pharmacological interventions. No study examined organisational interventions. Only one study evaluated an intervention aimed at changing people's behaviour, but it also included a pharmacological intervention.
For the outcome of study drop‐outs (how many people drop out of a study before it ends), we did not find evidence of a difference when participants were treated with atypical antipsychotics, compared with those who were treated with typical antipsychotics. This was also the case in studies comparing treatment with metformin (a medication used to treat diabetes) with placebo (pretend treatment), and those comparing treatment with melatonin (a hormone that regulates sleep) with placebo. Very low‐certainty evidence from one study suggests that drop‐outs may be higher among participants treated with a tricyclic antidepressant, compared with participants treated with selective serotonin reuptake inhibitors (another type of antidepressant).
We did not find evidence of a difference in fasting blood glucose levels in comparisons between atypical and typical antipsychotics, metformin and placebo, or tricyclic antidepressants and selective serotonin reuptake inhibitors. We did find that fasting blood glucose levels are likely to be lower in participants treated with melatonin, compared to those given placebo.
Body mass index was lower for participants receiving metformin compared with those receiving a placebo, and for participants who received typical antipsychotics, compared with those who received atypical antipsychotics.
Cholesterol levels were lower in participants who received typical antipsychotics, compared with those who received atypical antipsychotics.
We did not find evidence of a difference in waist circumference or blood pressure for any of the intervention groups of the included studies.
Certainty of the evidence
The only study assessing prevention of type 2 diabetes provided low‐certainty evidence. The certainty of evidence was reduced because the study was small, and several important aspects of it were at high risk of bias. The other studies reporting secondary outcomes generally provided moderate‐ to high‐certainty evidence for these outcomes.
Conclusions
For people with mental health conditions in LMICs, we do not know what is the best way to prevent type 2 diabetes. Only one of the included trials provided low‐certainty evidence on diabetes prevention. Further research should focus not just on pharmacological interventions, but should also include behaviour change and organisational interventions, to learn whether such interventions can be effective and appropriate in LMIC settings.
Summary of findings
Background
Mental disorders increasingly add to the global disease burden. They are one of the leading causes of disability worldwide, accounting for almost one‐quarter of all years lived with a disability (Murray 2012; Vos 2012) and result in significantly increased mortality (Correll 2017; Lawrence 2010; Mitchell 2013; Scott 2009). Studies have repeatedly reported a 10 to 20 year mortality gap for people with mental illness, and despite an overall improvement in life expectancy in recent years, the absolute mortality gap between people with and without mental illness is widening (Chesney 2014; Hayes 2017; Olfson 2015; Saha 2007). Studies from low‐ and middle‐Income countries (LMIC) show a similar pattern of increased mortality but with an even greater reduction in life expectancy than in high‐income countries (Dube 1984; Fekadu 2015; Kurihara 2011; Mogga 2006). However, only 0.5% to 2% of national health budgets are allocated for the prevention and treatment of mental disorders in LMICs (Stubbs 2017). Mental illness remains a major health challenge in these countries (Rathod 2017).
A considerable proportion of the increased morbidity and mortality experienced by people with mental disorders is driven by comorbid physical illnesses (Firth 2019; Hayes 2017), not just by the mental illness. The vast majority of deaths (around 80%) are due to preventable physical illnesses, most commonly cardiovascular, metabolic and respiratory diseases, as well as infections (Correll 2017; Crump 2013; Firth 2019; Laursen 2011). Mental and physical disorders have a complex and bidirectional relationship. A higher prevalence of comorbid physical health conditions (e.g. diabetes and cardiovascular disease) and poorer management of these illnesses, contribute to health inequalities in people with mental illness (Vancampfort 2016a; Ward 2015). People with severe mental illness (e.g. schizophrenia and bipolar disorder) have a particularly high risk of developing conditions such as diabetes and cardiovascular disease for reasons associated with the underlying mental disorder. These include health risk behaviours such as physical inactivity, smoking, and poor diet (Vancampfort 2017) and treatments that increase cardio‐metabolic risks and mortality (Liu 2017). Conversely, common mental disorders (e.g. depression and anxiety) are more common in people with these physical health conditions (Das‐Munshi 2007).
Globally, noncommunicable chronic diseases such as diabetes are a major cause of morbidity and mortality (contributing to 60% of all deaths) (Miranda 2008), including in LMICs (Lopez 2006). Diabetes is a serious lifelong condition. It is a major global health challenge, with increasing prevalence worldwide, and showing a particularly sharp rise in prevalence in LMICs (Stubbs 2016). A study using data from nationally representative surveys in 28 LMICs showed an overall diabetes prevalence of 8.8% (95% confidence interval (CI) 8.2% to 9.5%) (Manne‐Goehler 2019). Another study showed that across 29 LMICs the prevalence of diabetes among persons age 25 years or older was 7.5% (95% CI 7.1% to 8%) (Seiglie 2020).
Diabetes is strongly associated with mental illness (Vancampfort 2015a). For example, around 13% of people with severe mental illness (Ward 2015) and 9% of people with major depressive disorder (Vancampfort 2015b) are estimated to have diabetes, compared to an estimated 8.5% of the general population globally (WHO 2016) and compared to 6% in the UK (Reilly 2015). A systematic review and meta‐analysis found that odds of having type 2 diabetes were 1.85 times higher among people with severe mental illness than among matched controls (Vancampfort 2016a). People with schizophrenia and bipolar disorder seem to be particularly at risk of developing type 2 diabetes (Pillinger 2017; Stubbs 2015; Vancampfort 2013; Vancampfort 2015b).
There is also good evidence of an association between diabetes and common mental disorders (Das‐Munshi 2007; Moulton 2015; Vancampfort 2016b). People with diabetes have a two‐ to three‐fold increased prevalence of depressive (Ali 2006; Anderson 2001) and anxiety disorders (Grigsby 2002), although this relationship is likely to be bidirectional (Golden 2008). A systematic review of 48 studies showed that the prevalence of depression among people with diabetes is higher in LMICs than in high‐income countries. Across all studies conducted in LMICs, 35.7% of people with diabetes were found to suffer from depression (Mendenhall 2014).
Multiple complex mechanisms are known to contribute to the association between diabetes mellitus and severe mental illness including genetic, environmental, disease‐specific factors, and treatment‐specific factors (Holt 2015). However, the principles of managing diabetes mellitus in people with severe mental illness are similar to those for the general population and should follow currently established treatment algorithms (Holt 2015). Several interventions have been found to be effective for prevention of type 2 diabetes in the general population (Merlotti 2014; White 2016). Prevention of diabetes in people with mental illness is also important. However, due to a complex combination of psychological, social, and financial barriers, generic interventions to prevent diabetes may not be suitable for people with mental disorders (Chwastiak 2015). Some of the additional barriers faced by people with mental illness, not addressed by generic interventions, include social stigma, poor access to medical care (Bradford 2008), fragmentation and lack of coordination between medical and psychiatric treatment in the healthcare systems of many countries (Druss 2010), and "diagnostic overshadowing," where physical health problems are overlooked by health professional in the presence of mental illness (Liu 2017). These difficulties compound the challenges of managing side effects of psychotropic medication and the higher prevalence of health risk behaviours. This is more challenging in LMICs due to limited resources and facilities (Manne‐Goehler 2019).
Several abnormal clinical and metabolic findings (e.g. hypertension, hyperglycaemia, dyslipidaemia, overweight) are predictive of diabetes and other metabolic syndromes. It is therefore essential to also consider these cardio‐metabolic risk factors in patients in this vulnerable group, monitoring blood pressure, blood glucose level, lipid profile, body mass index (BMI) and waist circumference (De Hert 2009) when considering diabetes prevention.
Description of the condition
Ninety percent of people with diabetes have type 2 diabetes, a metabolic disorder that usually results secondary to insulin resistance (IR). It is commonly seen in individuals with obesity and is associated with disturbances in carbohydrate, fat, and protein metabolism. While pancreatic β‐cells initially respond to IR by increasing insulin secretion, the cells eventually fail to keep up with demand resulting in relative insulin deficiency, consequently leading to hyperglycaemia (elevated levels of plasma glucose) (Weir 2020). Prolonged hyperglycaemia may lead to microvascular complications (Andany 2019) including retinopathy (disease of the retina which results in impairment or loss of vision), nephropathy (renal impairment), neuropathy (an abnormal and usually degenerative state of parts of the nervous system) and macrovascular, including coronary artery, cerebrovascular and peripheral artery complications.
The ‘epidemic’ of diabetes seen over recent decades has been attributed to changes in demographics and lifestyle globally (e.g. increased life expectancy, sedentary behaviours, and consumption of high fat and carbohydrate diets) (Miranda 2008). LMIC populations have experienced especially rapid changes, with which health policy and services have failed to keep pace (Popkin 2002). According to the American Diabetes Association (ADA), the risk of developing diabetes increases with age, obesity, lack of physical activity, dyslipidemia (abnormal amount of lipids in the blood), and hypertension (ADA 2017), all of which have been adversely affected by these changes.
Description of the intervention
Prevention of diabetes includes activities targeted at reducing the frequency or level of causal risk factors for development of diabetes (WHO 1994). Diabetes prevention or delay may be achieved with pharmacological, behaviour change, and organisational interventions. Pharmacological interventions aimed at prevention of diabetes in people with mental disorder include diabetes medication, weight loss medication, a combination of diabetes and weight loss medication, diabetes preventive medication and antipsychotic switching. Behaviour change interventions may target health risk behaviours, and may include patient education programmes, psychological interventions (e.g. cognitive behavioural therapy, counselling or motivational interviewing), self‐monitoring (including telehealth, internet‐based interventions, and other communication technologies) or multicomponent interventions (e.g. self‐management programmes that combine education and behavioural approaches) (Taylor 2017). Organisational interventions may include interventions that aim to improve the delivery of care, such as educating health professionals, care planning, or collaborative models of care (Druss 2010).
It may be that there are particular pharmacological, behaviour change and lifestyle, or organisational interventions that would be more applicable to LMICs as the availability of pharmacological interventions, resources and organisational structures in LMICs are different from those in high‐income countries. For instance, some drugs may not yet be available in LMICs; psychological behaviour change interventions might not be feasible due to lack of trained personnel; or there may not be any collaborative models of care in the health system (Koyanagi 2017). In addition, LMICs are not homogenous and the availability of interventions within healthcare systems may differ among countries due to variability in health care resources and organisational structures (Mate 2013).
How the intervention might work
Pharmacological interventions
There are several modes of action for medication in preventing diabetes. Diabetes medication helps regulate carbohydrate and fat metabolism, by increasing insulin sensitivity and reducing the amount of glucose produced and released by the liver. Weight loss medication or anti‐obesity drugs usually act on the gastrointestinal tract by reducing absorption of dietary fat, stimulate energy expenditure and decrease fat storage, or decrease appetite. Diabetes combination medications allow patients to switch between treatments, depending on clinical response. Switching to or adding an atypical antipsychotic associated with fewer metabolic side effects is hypothesised to alleviate weight gain and metabolic abnormalities caused by commonly used antipsychotics such as olanzapine and clozapine. Other medications may work by enhancing lipid profile and metabolic function and regulating or increasing insulin sensitivity (Taylor 2017).
Behaviour change interventions
These interventions target health risk behaviours using educational, psychological, and behavioural approaches, or combinations of these. For diabetes, there has been a focus on self‐management interventions using behaviour change techniques (McBain 2016), influenced by theories of health behaviour change, including social cognitive theory (Bandura 1986), the theory of reasoned action and planned behaviour (Ajzen 1991), self regulation theory (Leventhal 1984) and the transtheoretical model (Prochaska 1997). All of these theories identify concepts that predict health behaviour (and that may be targeted by behaviour change interventions), with a primary focus on beliefs, attitudes, and expectations. For example, a diabetes self‐management intervention based on social cognitive theory (Bandura 1986) may seek to reduce carbohydrate intake by increasing diet‐related self‐efficacy.
Organisational interventions
Organisational capacity building and training programmes may increase the efficacy and communication skills of mental health or diabetes professionals or other health workers and health services to support prevention of diabetes for people with mental illness (Liu 2017).
Why it is important to do this review
To date, a limited number of non‐Cochrane systematic reviews have investigated the effectiveness of interventions to prevent diabetes for people with mental illness (McGinty 2016; Taylor 2017). These reviews, mostly including data from high‐income countries, have reported that diabetes can be prevented or its onset delayed. A comprehensive review by McGinty and colleagues included 33 studies of interventions for diabetes mellitus in people with severe mental illness. It found no high‐certainty evidence for the effectiveness of any interventions; the best available evidence suggested a potential beneficial effect of metformin on glucose control (McGinty 2016). The review by Taylor and colleagues, which also focused on people with severe mental illness, included 54 randomized controlled trials (RCTs) among which only a few were from LMICs (Brazil, India, China, Iran, Venezuela) (Taylor 2017). The authors found some evidence for the effectiveness of pharmacological and non‐pharmacological interventions in improving glycaemic outcomes, but no subgroup analyses were conducted by country income level.
Other non‐Cochrane reviews have investigated the effect of pharmacological (Maayan 2010; Mizuno 2014), behavioural (Bruins 2014; Caemmerer 2012; Fernández‐San‐Martín 2014), or both pharmacological and behavioural interventions (Faulkner 2007) on glycaemic measurements in people with severe mental illness. They have also reported that these interventions may be effective, but again have focused only on people with severe mental illness or those taking antipsychotic medication, and identified very few studies in LMICs. Moreover, these studies considered glycaemic effects only as a secondary outcome.
It is important to assess the evidence for diabetes prevention in people with mental disorders, specifically for LMICs. Not only is the prevalence of diabetes and comorbid mental illness high in LMICs, but we cannot be certain that interventions shown to be effective in high‐income countries work in the same way in LMICs. Populations may differ in terms of demographics and living conditions, affecting risk of diabetes, and healthcare systems may operate differently, potentially influencing availability, feasibility and effectiveness of interventions. In addition, it is important to consider other cardio‐metabolic risk factor measures, e.g. blood pressure, fasting blood glucose level, serum cholesterol level, BMI and waist circumference for this population.
A review of the effectiveness of interventions designed to prevent diabetes in people with any mental disorder, focused on LMICs, is therefore needed to inform practice and future research for this population.
Objectives
To assess the effects of pharmacological, behaviour change, and organisational interventions versus active and non‐active comparators in the prevention or delay of type 2 diabetes among people with mental illness in LMICs.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs evaluating any interventions to prevent type 2 diabetes in people with any mental disorder in LMICs. LMICs were defined according to the Development Assistance Committee (DAC) list of all countries and territories eligible to receive official development assistance (ODA) (DAC 2017).
Types of participants
We included studies of adults aged 18 years and over, with any mental disorder and without diabetes. Studies that did not explicitly screen for and exclude diabetes at baseline were not included. Mental illness diagnoses were to be established using World Health Organization (WHO) International Classification of Diseases (ICD) criteria for mental and behavioural disorders (ICD‐10, F20‐29 and F30‐31, F 32.3, F33.3) (WHO 1992) and/or the Diagnostic and Statistical Manual of Mental Disorders (DSM) (DSM‐III, APA 1980; DSM‐III‐R, APA 1987; DSM IV, APA 2000; DSM V, APA 2013) or measures based on these. We defined severe mental illness as schizophrenia or other psychotic disorders, bipolar disorder, and depression with psychotic features. Common mental disorders included depression, generalised anxiety disorder (GAD), panic disorder, phobias, social anxiety disorder, obsessive‐compulsive disorder (OCD), and post‐traumatic stress disorder (PTSD) (NICE 2011). Other mental disorders such as personality disorder and somatoform disorders were also included in this review.
Where study populations were mixed (i.e. including people with and without mental disorder), we included studies only if people with mental disorders constituted the predominant population (more than 50%), or if separate outcome data were provided for participants with mental disorders.
To be consistent with changes in the classification of, and diagnostic criteria for diabetes mellitus over the years, studies had to use (and explicitly state) established standard criteria for the diagnosis of type 2 diabetes, valid at the time of the trial commencing (e.g. ADA 1999; ADA 2008; ADA 2017; WHO 1999; WHO 2006).
Types of interventions
Experimental intervention
The review included any pharmacological, behaviour change (targeting health risk behaviours), or organisational interventions targeting the prevention of diabetes in people with any mental disorder in LMICs.
Pharmacological interventions included diabetes medication (e.g. metformin, pioglitazone); weight loss medication (e.g. amantadine, orlistat, sibutramine); combinations of weight loss and diabetes drugs (e.g. amantadine with metformin and zonisamide; metformin with amantadine and zonisamide; metformin with sibutramine); antipsychotic switching (e.g. changing to aripiprazole, quetiapine, or ziprasidone); drugs that can prevent or improve metabolic side effects during antipsychotic treatments (melatonin); antidepressants (e.g fluoxetine, imipramine) or other medications.
The review included any behaviour change interventions aiming to prevent or delay the onset of diabetes, such as patient education programmes, psychological interventions (e.g. cognitive behavioural therapy or counselling, or motivational interviewing), self‐monitoring (including telehealth, internet‐based interventions and other communication technologies) and multicomponent interventions (e.g. self‐management programmes that combine education and behavioural approaches).
Organisational interventions included those that aim to improve the delivery of care, such as educating health professionals, care planning, or collaborative models of care. Examples include relevant training of any health professionals working with people with mental illness, nonspecific health worker interventions, community mental health teams, mass media‐delivered interventions, family interventions, physical health care monitoring, and statutory mental health services interventions.
Comparator intervention
For pharmacological interventions, comparator interventions included no treatment (including trials employing wait‐list conditions), treatment as usual, drug placebo, or an alternative type of medication for diabetes prevention.
For behaviour change and organisational interventions we included the following comparators: usual care or treatment (including pharmacological treatment), attention or other psychological placebo control, or any alternative behaviour change or organisational intervention (as described above under experimental interventions).
Types of outcome measures
Primary outcomes
Our primary outcome is prevention of diabetes, measured as a difference between study arms in the number of participants who developed type 2 diabetes during the study period. A clinical diagnosis of diabetes may be confirmed in the presence of symptoms by various parameters such as HbA1c, fasting blood sugar, random blood sugar or, in unclear cases, 2‐hour plasma glucose following an oral glucose tolerance test (OGTT). We accepted diagnoses made using any of these parameters using cut‐offs consistent with those current at the time of the study, as described in national and international guidance such as WHO (e.g. WHO 2006), National Institute of Health and Care Excellence (NICE) (e.g. NICE 2015), Diabetes UK (e.g. Diabetes UK 2018), American Diabetes Association (e.g. ADA 2017). Current cut‐offs are as follows: HbA1c ≥ 48 mmol/mol, a fasting blood glucose ≥ 7 mmol/L or a random blood glucose ≥ 11.1 mmol/L; and for OGTT 2‐hour glucose ≥ 11.1 mmol/L (ADA 2017). Conversion to prediabetes was not included as part of this outcome.
As the primary adverse outcome, we report drop‐out from care: the number of participants who dropped out of treatment for any reason after randomization.
Secondary outcomes
Fasting blood glucose (mmol/L or mg/dL)
BMI
Waist circumference (cm or inch)
Blood pressure (diastolic and systolic in mmHg)
Total cholesterol (mmol/L or mg/dL)
Depression and anxiety measured by a validated scale, e.g. Patient Health Questionnaire (PHQ 9) (Kroenke 2001), Generalised Anxiety Disorder assessment (GAD‐7) (Spitzer 2006)
Health related quality of life (evaluated with a validated generic or disease‐specific instrument (Wee 2006), e.g. the 36‐Item Short Form Health Survey (SF‐36) (McHorney 1993) or other validated scale). We considered language‐ and culture‐adapted instruments, where these were available
All‐cause mortality, defined as death from any cause
Search methods for identification of studies
Electronic searches
We searched the following electronic databases (20 February 2020) using a comprehensive list of keywords and subject headings related to diabetes, mental disorders, LMICs, RCTs and systematic reviews (Appendix 1). The search strategies were informed by the review of Taylor and colleagues (Taylor 2017), the Cochrane highly sensitive search strategies for identifying RCTs (Lefebvre 2011), and the Academic Unit of Health Economics (AUHE) Information Specialist's LMIC geographic strategies (AUHE 2018).
CINAHL (EBSCO) (1981 to 20 February 2020)
Cochrane Central Register of Controlled Trials (Issue 2, February 2020)
Cochrane Database of Systematic Reviews (Issue 2, February 2020)
Embase Classic + Embase (Ovid) (1947 to 19 February 2020)
Global Health (Ovid) (1910 to week 8, 2020)
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS; Latin American and Caribbean Health Sciences Literature) (all available years)
Ovid MEDLINE (1946 onwards), MEDLINE In‐Process & Other Non‐Indexed Citations, MEDLINE Epub Ahead of Print
PsycINFO (Ovid) (1806 to February, week 2, 2020)
PubMed (US National Library of Medicine) (1946 to 20 February 2020)
PakMedNet (medical journals of Pakistan) (all available years)
We did not apply any limits on date, language, or publication status to the searches.
An information specialist with the Cochrane Common Mental Disorders Group ran a broad search of the Cochrane Common Mental Disorders Controlled Trials Register, using only terms for outcomes (Appendix 2).
In keeping with the Methodological Expectations of Cochrane Intervention Reviews (MECIR) conduct standards, we ran a search for retractions and errata once the included studies were selected.
Searching other resources
Grey literature
We searched the following sources of grey literature.
Conference Proceedings Citation Index ‐ Science (Clarivate Analytics Web of Science) (1990 to the search date)
ProQuest Dissertations & Theses Global
Unpublished studies
We searched the following international trial registries to identify ongoing or unpublished studies (all available years).
ISRCTN registry (Springer Nature)
ClinicalTrials.gov (US National Institutes of Health)
International Clinical Trials Registry Platform (WHO)
Reference lists
We checked the reference lists of relevant systematic reviews to identify additional studies.
Data collection and analysis
Selection of studies
We uploaded citations and available abstracts of the search results into Covidence (Covidence 2017) and screened records for potential eligibility in two stages. The first stage involved screening titles and abstracts to exclude studies that did not meet the inclusion criteria, carried out independently by pairs of review authors (from among EU, NS, MPM, SP, FA, ZAA and RA). We resolved discrepancies through discussion. Where we could not reach agreement, we consulted a third review author (NS). In the second stage, we retrieved the full texts of potentially eligible studies and independently assessed them for eligibility. This was again carried out by two review authors (from among EU, NS, MPM, FA, SP and ZAA). We resolved discrepancies by consulting a third review author (NS). We sought any missing data that could help to assess eligibility by contacting the corresponding authors. We created a PRISMA flow diagram to show the process of trial selection (Liberati 2009). For studies excluded during this stage, we recorded a reason for exclusion. For included studies, we linked multiple reports from the same study.
Data extraction and management
For trials that fulfilled our inclusion criteria, review authors extracted data in duplicate (EU, MPM, SP, NT, ZAA, FA). We resolved any discrepancies by discussion, or, if required, consulted a third review author (NS, RA).
To provide information for assessment of the certainty of the evidence and for evidence synthesis, we extracted the following data, where available.
Study population (including participant inclusion and exclusion criteria)
Country
Setting (primary care, community, secondary care, mental health care)
Study design (single or multicentre RCT)
Number of intervention groups
-
Intervention:
For pharmacological interventions: class of drug, dose, frequency, and duration.
For behaviour change and organisational interventions: a description of the intervention (including process, target group, e.g. patients or healthcare professionals, and presence of other concurrent interventions), theory (informing intervention design), target (including strategies, applications, and components), context of intervention (i.e. primary health facility), provider and mode of delivery (phone, face‐to‐face, group, online), intensity (length, frequency, and number of contacts), duration (period of time over which contacts delivered), details about group leader (demographics, training, professional status, etc.).
Behaviour change techniques: we planned to categorise interventions and identify behaviour change techniques using the ‘template for intervention description and replication’ (TIDieR) checklist (Hoffmann 2014; Hoffmann 2017).
Comparison intervention(s).
Outcome data and information on measures for our primary and secondary outcomes.
We noted in the 'Characteristics of included studies' table if the study authors did not report outcome data in a usable way. Where included trials reported outcome data in insufficient detail to include in a meta‐analysis, for instance, reporting means without confidence intervals (CIs) or standard deviations (SDs), we contacted the study authors to request more information.
Assessment of risk of bias in included studies
We assessed the risk of bias of included randomized trials using the Cochrane 'Risk of bias' tool (Higgins 2011a). Two review authors (from among EU, MPM, SP, NT, ZAA and FA) independently assessed the following items.
Sequence generation (i.e. if allocation sequence was adequately generated)
Allocation sequence concealment (i.e. if allocation was adequately concealed)
Blinding (i.e. if knowledge of the allocated interventions was adequately prevented during the study)
Incomplete outcome data (i.e. if incomplete outcome data was adequately addressed)
Selective outcome reporting (i.e. whether reports of the study are free of suggestion of selective outcome reporting)
Other potential sources of bias (i.e. whether the study is apparently free of other problems that could lead to a high risk of bias e.g. baseline imbalances, evidence of carry‐over in cross‐over trials, comparability of groups in cluster trials)
We judged each potential source of bias as high, low or unclear and provided a supporting quotation from the study report together with a justification for our judgment in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. Differences in assessment were resolved by discussion or consultation with a third review author (NS, EU). Allocation concealment was used as a marker of trial risk of bias for the purposes of undertaking sensitivity analyses.
Measures of treatment effect
For continuous data, we calculated the mean difference (MD) with 95% CIs. Where trials reported the same outcome using different outcome measures, we used standardised mean difference (SMD). For binary outcomes, we calculated a standard estimation of the risk ratio (RR) with a 95% CI.
Unit of analysis issues
We took into account the level at which randomization occurred, with respect to cross‐over trials, cluster RCTs, and multiple observations for the same outcome.
We planned to reanalyse cluster‐RCTs that had not appropriately adjusted for potential clustering of participants in their analyses by inflating the variance of the intervention effects by the design effect. We would have obtained estimates of the intracluster correlation coefficient (ICC) in order to estimate the design effect, through contact with authors, or by imputing them using either estimates from other included trials that reported ICCs or using external estimates from empirical research (e.g. Bell 2013).
In the case of multiple intervention groups, we analysed each intervention group separately against the control group and the sample size for the control group was divided proportionately across each intervention group. Where results were reported at multiple time points in the studies, we analysed each outcome at predefined periods of follow‐up in separate meta‐analyses. We grouped data by time‐point.
If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single pairwise comparison or appropriately reduced the sample size so that the same participants did not contribute data to the meta‐analysis more than once (i.e. splitting the 'shared' group into two or more groups), although we acknowledge this will not account for correlation arising from the same set of participants being in multiple comparisons (Higgins 2011a) .
Dealing with missing data
We carefully evaluated important numerical data such as screened and randomly assigned participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up, withdrawals), and critically appraised issues concerning missing data.
We analysed data primarily using the ITT principle. In the protocol, we mentioned that if the included studies did not provide enough detail to allow an ITT analysis, and where included trials did not report means and SDs for outcomes, we planned to request data from study authors. If we did not receive the necessary information from trial authors, we planned to impute these values (Higgins 2011a; Higgins 2011b), and investigate the impact of imputation on meta‐analyses by performing sensitivity analyses. However, in the review, requesting further data from study authors or imputing data were not required.
Assessment of heterogeneity
We assessed clinical heterogeneity through the description of the setting, baseline measures, and the intervention approach used in each study. In the case of obvious clinical heterogeneity, we did not pool the data, and summarised results narratively instead.
We assessed statistical heterogeneity using the Chi2 test and the I2 statistic. The Chi2 test was considered statistically significant if P ≤ 0.10. If heterogeneity existed between studies (I2 ≥ 50%) for the primary outcome, we planned to explore the reasons, following guidance in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). This chapter suggests the following guidance for interpretation of the I2 statistic:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
When interpreting the I2 statistic, we planned to take into account the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. the P value from the Chi2 test, or a CI for I2). However, in the review, this was not required.
Assessment of reporting biases
If more than 10 studies that investigated a particular outcome were identified for inclusion in this review, we planned to use funnel plots to assess publication biases. We also planned quantitative analysis of publication bias, using the Egger test.
Data synthesis
We combined data from individual trials in meta‐analysis if the interventions, outcomes, and patient groups were sufficiently similar (determined by consensus among review authors). Data were not pooled for meta‐analysis if we detected a high degree of clinical heterogeneity among the studies. Where data were pooled, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses, based on characteristics of the population or intervention that might influence the primary outcome. However, as we identified only one study that assessed the primary outcome, we conducted no subgroup analyses.
Age (65 years and over): this may influence the risk of diabetes and effectiveness of the intervention.
Sex: this may influence the risk of diabetes and effectiveness of the intervention.
Type of mental disorder (severe mental illness versus other mental disorder): people with severe mental illness have additional risk factors for diabetes e.g. side effects of antipsychotic medication).
Prospective identification of diabetes using a robust approach to diagnosis e.g. HbA1c or fasting blood sugar, versus studies using retrospective records, random blood glucose testing, or both.
Intervention duration (less than three months versus three months or more): length of the intervention may influence outcomes.
Duration of follow‐up (less than three months versus three months or more): this is likely to influence detection of outcomes.
Sensitivity analysis
For outcomes where two or more studies were available to include in a meta‐analysis, we performed sensitivity analyses to explore the influence of the following factors (where applicable) on effect sizes:
effect of risk of bias: excluding studies that did not report allocation concealment (we acknowledge that we might have missed some studies where allocation concealment may have been used but not reported);
effect of large trials: excluding large trials to establish the extent to which they dominate the results;
effect of data imputation: excluding trials where missing data have been imputed.
Unplanned sensitivity analysis: we identified two studies (Wu 2006; Wu 2008b) reporting much lower standard deviations than other studies in our review and their effect estimates were extreme outliers in the meta‐analyses. After contacting authors, we were unable to confirm the validity of these data therefore we investigated the impact of removing these studies from our meta‐analyses.
Summary of findings and assessment of the certainty of the evidence
We prepared 'Summary of findings' tables to summarise key findings of this review. We reported the outcomes (including adverse outcomes) and presented standardised effect size estimates and 95% CIs, using the GRADE approach to assess the overall certainty (quality) of the evidence supporting each outcome. GRADE criteria take into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. We used GRADEproGDT to create our 'Summary of findings' tables (GRADEpro 2015), and followed standard methods as described in Chapter 11 of the Cochrane Handbook to prepare our 'Summary of findings' tables (Schünemann 2017). For each of our main comparisons, the following outcomes (measured at the latest time point) were included.
Diabetes diagnosis
Drop‐out from treatment
Fasting blood glucose level
BMI
Health‐related quality of life
All‐cause mortality
The definitive list of comparisons to be included in the ‘Summary of findings’ tables was agreed among review authors once the categories of interventions were known, guided by clinical relevance. This is because the range of interventions to be included was broad, and at the protocol stage, we were not certain which interventions would be identified by the review.
We created 'Summary of findings' tables after we entered data into RevMan (Review Manager 2014), had written up our results, and conducted the 'Risk of bias' assessment. However, the 'Summary of findings' tables were created before writing our discussion, abstract, and conclusions, to allow the opportunity to consider the impact of the risk of bias in the studies contributing to each outcome upon the mean treatment effect, and our confidence in these findings.
Results
Description of studies
Results of the search
We conducted initial searches up to April 2019 and updated our searches on 20 February 2020. We identified 7703 unique records, of which we excluded 7598 after screening titles and abstracts. Full‐text reports of 105 references were obtained and screened against our eligibility criteria. We excluded 55 articles (Figure 1). The most common reason for exclusions was that the study was not conducted in a LMIC (n = 48). This was often not apparent from reading the abstracts. Four studies were ongoing and 10 were awaiting classification. In total, we included 30 studies in the qualitative synthesis. Fifteen of these studies contributed data to meta‐analyses. We contacted the authors of 21 articles to clarify details on risk of bias of their studies and received responses from three authors. Full details of the study flow are given in a PRISMA flow diagram in Figure 1.
1.
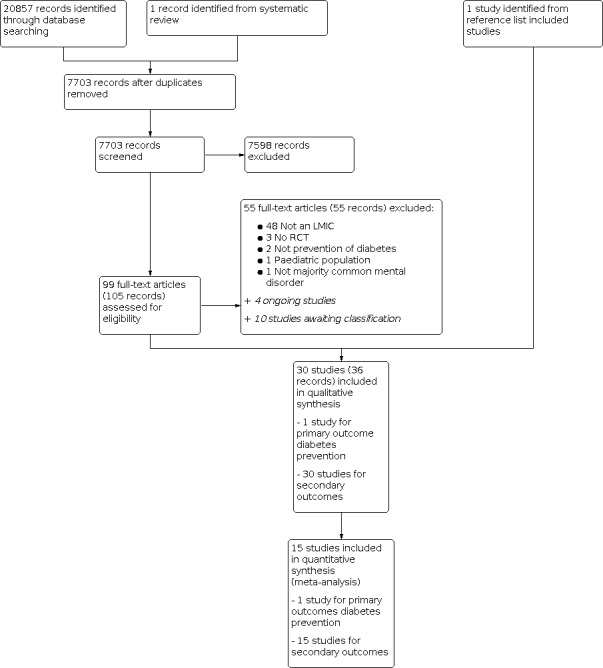
Study flow diagram.
Included studies
We included 30 studies, only one of which (Saddichha 2008) investigated the primary review question, prevention of type 2 diabetes (Characteristics of included studies).
Design
All included studies were parallel‐group RCTs. Two studies were multi‐centre studies (Baptista 2007; Ou 2013) and one was conducted across multiple countries (Tohen 2011).
Sample sizes
For the primary outcome, one study (Saddichha 2008) included 150 participants (99 participants with schizophrenia). An additional 29 studies assessed one or more of the secondary outcomes of the review (2481 participants), giving a total of 30 RCTs with 2631 participants. The number of total participants in any single study ranged from 18 to 260.
Setting
Eleven studies were conducted in China, eight were from Iran, four were from Venezuela, three were from India, two were from South Africa and one study was from Brazil. For one multi‐country study, only data from China were extracted, as other countries in the study were high‐income countries. Participant recruitment and conduct of all of these studies occurred in hospital settings.
Participants
Mean ages ranged from 25 years (Wu 2008a) to 68 years (Chen 2017). One study included only female participants (Moosa 2003), while all others included both men and women. The majority of studies (n = 24) included participants with a diagnosis of schizophrenia or a related psychotic disorder. Four studies included participants with a diagnosis of depression, and one study included participants with depression and bipolar disorder.
Interventions
Studies used a range of pharmacological interventions. Some of these interventions were aimed at treating mental disorders, but the study investigated their potential beneficial or adverse effects on risk factors for the development of diabetes. These include typical and atypical antipsychotic medication for the treatment of psychotic disorders such as schizophrenia, and antidepressants for the treatment of depressive symptoms and disorders. Other interventions were directly aimed at reducing the risk of diabetes, and increased blood glucose levels with metformin, melatonin, and various other medications and supplements.
We identified one study that included a pharmacological and a behaviour change intervention (Wu 2008b). We found no studies of organisational interventions.
Outcomes
Prevention of diabetes was reported in only one study (Saddichha 2008). However, all 30 included studies reported fasting blood glucose levels as a proxy for risk of developing diabetes, in addition to several of the other planned secondary outcomes.
Length of follow‐up was between four weeks (Agnihotri 2013) and 54 weeks (Emsley 2005), with the majority of studies reporting outcomes up to 12 or 14 weeks.
We combined outcome data from 14 studies in meta‐analyses (Agahi 2017; Baptista 2006; Baptista 2007; Carrizo 2009; Emsley 2005; Ghaeli 2004; Modabbernia 2014; Moosa 2003; Saddichha 2008; Salehi 2009; Wang 2012; Wu 2006; Wu 2008a; Wu 2008b). For other studies, it was not possible to combine outcome data in this way due to the variation in intervention and comparator groups used. These data are summarised narratively instead. Meta‐analysis was performed for the following secondary outcomes: fasting blood glucose, BMI, waist circumference, blood pressure, cholesterol and drop outs. No data were available for depression, anxiety, quality of life, or all‐cause mortality (details are summarised under ‘Effects of interventions').
Excluded studies
We excluded 55 studies. Among the excluded studies, 48 were not conducted in LMICs, three were not RCTs, two studies included interventions that were not aimed at preventing diabetes as a primary or secondary objective, one was on a paediatric population, and one did not have a majority of participants with a mental disorder. The numbers of excluded studies and all reasons for exclusion are shown in Figure 1.
Risk of bias in included studies
Risk of bias of included studies is summarised visually in Figure 2 (by domain) and Figure 3 (by study and by domain).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of selection bias was unclear for 20 studies because these studies did not report the information required to assess the 'random sequence generation' and/or 'allocation concealment' bias domains. Eight studies were judged to be at low risk of selection bias for both domains, while one study was judged to be at high risk of bias because it appeared randomization took place between the treatment arms but not between treatment and control arms (Saddichha 2008).
Blinding
Risk of performance bias due to inadequate blinding of participants and/or personnel was unclear or high for 17 studies and low for the remaining 12 studies.
For 19 studies, the risk of detection bias was unclear or high due to incomplete information reported in the paper, as well as inadequate or lack of blinding of outcome assessors. For 10 studies, the risk of bias was low.
Incomplete outcome data
The risk of attrition bias was high for eight studies. For some studies, there were substantial differences between study arms in drop‐out rates and reasons for drop‐out were not reported. In other studies, participants who stopped their involvement in the study were retrospectively excluded, and/or no baseline data were provided. For another eight studies, the risk of attrition bias was unclear, e.g. because information about reasons for drop‐out was not reported.
Selective reporting
For the majority of studies (23 of 30), the risk of reporting bias was unclear because no link to a protocol or online trial registration was provided, or because the protocol was registered online only retrospectively (Akkasheh 2016; Ghaderi 2019; Wu 2008b; Zhao 2015).
Other potential sources of bias
Other potential sources of bias included involvement of the pharmaceutical industry in the funding with potential interference, writing‐up, and monitoring of the trial, no reporting of baseline participant characteristics, indications of failed randomization with unbalanced groups, adjusting of medication throughout the trial, exclusion of unsuitable or unmotivated participants, and unclear study rationale.
For one study, data presented at baseline and in a table raised questions due to identical estimates and 95% CIs for different groups and time points (Characteristics of included studies). The authors did not respond to our request for information (Wu 2008b).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Atypical compared with typical antipsychotic medications for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries.
| Atypical compared with typical antipsychotic medications for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries | ||||||
| Patient or population: Adults with mental disorders in low‐ and middle‐income countries Setting: Hospitals in China, India and South Africa Intervention: Atypical antipsychotic medication Comparison: Typical antipsychotic medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with typical antipsychotic | Risk with atypical antipsychotic | |||||
| Diabetes (ADA criteria) (6 weeks) | Study population | RR 0.50 (0.03 to 7.73) | 93 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 32 per 1,000 | 16 per 1,000 (1 to 249) | |||||
| Drop‐outs (6 to 54 weeks) | Study population | RR 1.31 (0.63 to 2.69) | 144 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | ||
| 148 per 1,000 | 194 per 1,000 (93 to 399) | |||||
| Fasting blood glucose (6 to 8 weeks) | Mean fasting blood glucose was 4.90 to 4.91 mmol/L (normal level) | MD 0.05 lower (0.10 lower to 0.00 lower) | ‐ | 211 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | |
| BMI (8 to 54 weeks) | Mean BMI was 21.2 to 24.6 kg/m2 (healthy weight range) | MD 0.57 higher (0.33 higher to 0.81 higher) | ‐ | 141 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADA: American Diabetes Association; BMI: Body mass index; CI: Confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1One small trial; estimate with wide confidence interval crossing 1. Downgraded two levels for imprecision. 2One trial with all domains at unclear or high risk of bias, including potential conflict of interest. Downgraded one level for risk of bias. 3Two trials with confidence interval crossing 1. Downgraded one level for imprecision. 4For one trial, there were problems with randomization and for the other there was no blinding of participants or investigators. Although blood glucose is an objectively measured outcome, results may still have been influenced by knowledge of the intervention allocation. Downgraded one level for risk of bias. 5One trial with all domains at unclear or high risk of bias; the other trial without blinding. Downgraded one level for risk of bias.
Summary of findings 2. Metformin compared with placebo for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries.
| Metformin compared with placebo for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries | ||||||
| Patient or population: Adults with mental disorders in low‐ and middle‐income countries Setting: Hospitals in China and Venezuela Intervention: Metformin Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with metformin | |||||
| Diabetes | No studies identified | |||||
| Drop‐outs (12 to 14 weeks) | Study population | RR 1.22 (0.09 to 16.35) | 158 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 49 per 1,000 | 11 more per 1,000 (44 fewer to 749 more) | |||||
| Fasting blood glucose (12 to 14 weeks) (endpoint data) | Mean fasting blood glucose was 4.40 to 4.71 mmol/L (normal level) | MD 0.35 lower (0.60 lower to 0.11 lower) | ‐ | 173 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | |
| Fasting blood glucose (12 to 14 weeks) (change from baseline data) | ‐ | MD 0.01 higher (0.21 lower to 0.22 higher) | 91 (2 RCTs) | ⊕⊕⊕⊕ HIGH 4 | ||
| BMI (12 to 14 weeks) | Mean BMI was 23.5 to 25.3 (normal to slightly overweight) | MD 1.37 lower (2.04 lower to 0.7 lower) | ‐ | 264 (5 RCTs) | ⊕⊕⊕⊕ HIGH 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: Body mass index; CI: Confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Some unclear risk of bias domains and unbalanced drop‐out in one study, but this is not a concern for this outcome. 2Three relatively small studies with few drop‐outs, which means the estimate has a wide confidence interval. Downgraded one level for imprecision. 3One study shows positive result for metformin while other four studies show no difference. This affects the magnitude and precision of the pooled estimate. Downgraded one level for imprecision. 4One study shows evidence of attrition bias, but removing this result would not substantially change the pooled estimate.
Summary of findings 3. Melatonin compared with placebo for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries.
| Melatonin compared with placebo for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries | ||||||
| Patient or population: Adults with mental disorders in low‐ and middle‐income countries Setting: Hospitals in China and Iran Intervention: Melatonin Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with melatonin | |||||
| Diabetes | No studies identified | |||||
| Drop‐outs (8 weeks) | Study population | RR 1.00 (0.38 to 2.66) | 48 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 250 per 1,000 | 250 per 1,000 (95 to 665) | |||||
| Fasting blood glucose (8 to 12 weeks) (endpoint data) | Mean fasting blood glucose was 4.9 to 5.0 mmol/L (normal level) | MD 0.17 lower (0.35 lower to 0.01 higher) | ‐ | 102 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | |
| Fasting blood glucose (8 to 12 weeks) (change from baseline data) | ‐ | MD 0.24 lower (0.39 lower to 0.09 lower) | ‐ | 100 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | |
| BMI (8 to 12 weeks) | Mean BMI was 25.1 to 25.2 (slightly overweight) | MD 0.22 lower (2.58 lower to 2.14 higher) | ‐ | 202 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: Body mass index; CI: Confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1One small trial with an equal number of events in both groups. Downgraded two levels for very serious imprecision; estimate includes benefits and harms. 2One trial with mostly low risk of bias. The trial with more weight in the analysis has five unclear risk of bias domains. Downgraded one level for risk of bias. 3One study with unclear risk of bias in all but one domains; downgraded one level. 4One study suggests BMI is increased in the melatonin compared to placebo group. Another study suggests the opposite effect. A third finds no difference. Many bias domains unclear across studies. Downgraded one level for inconsistency, one level for imprecision, and one level for risk of bias.
Summary of findings 4. SSRI antidepressants compared with TCA for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries.
| SSRI antidepressants compared with TCA for preventing type 2 diabetes in adults with mental disorders in low‐ and middle‐income countries | ||||||
| Patient or population: Adults with mental disorders in low‐ and middle‐income countries Setting: Hospitals in Iran and South Africa Intervention: SSRI Comparison: TCA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with TCA | Risk with SSRI | |||||
| Diabetes | No studies identified. | |||||
| Drop‐outs (12 weeks) | Study population | RR 0.34 (0.11 to 1.01) | 25 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 636 per 1,000 | 216 per 1,000 (70 to 643) | |||||
| Fasting blood glucose (8 to 12 weeks) | Mean fasting blood glucose was 4.4 to 5.1 mmol/L (normal level) | MD 0.39 lower (0.88 lower to 0.10 higher) | ‐ | 141 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | |
| BMI (12 weeks) | Mean BMI was 25.2 (slightly overweight) | MD 0.7 higher (1.1 lower to 2.5 higher) | ‐ | 18 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: Body mass index; CI: Confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: Risk ratio; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1One small trial; unclear allocation concealment, no blinding, incomplete outcome data. Downgraded two levels for risk of bias. 2One small trial; drop‐out appears to differ between groups but wide confidence interval. Downgraded one level for imprecision. 3Unclear randomization and allocation concealment in 3/3 trials; high risk of attrition bias in 3/3 trials. Downgraded one level for risk of bias. 4One small trial; demonstrating any difference in BMI is likely to require a larger sample size. Downgraded one level for imprecision.
Only one study reported on diabetes prevention (Saddichha 2008). The other 29 included studies assessed only secondary outcomes. All 30 included studies compared a pharmacological intervention with a placebo, behavioural intervention, and/or another pharmacological intervention, for patients with mental disorders.
For analysis of the outcomes, we categorised these 30 studies into seven groups.
Atypical versus typical antipsychotic medication (Emsley 2005; Saddichha 2008; Wu 2006)
Metformin versus placebo; in all trials both the metformin and placebo arms additionally received antipsychotic medication (Baptista 2006; Baptista 2009; Carrizo 2009; Wang 2012; Wu 2008a; Wu 2008b).
Melatonin versus placebo (Agahi 2017; Modabbernia 2014)
SSRI versus tricyclic antidepressants (TCA) for the treatment of depressive symptoms (Ghaeli 2004; Moosa 2003; Salehi 2009)
Atypical versus atypical antipsychotic medications (Chen 2017; Hu 2013; Li 2009; Ou 2013; Zhang 2012; Zhang 2014)
Miscellaneous interventions versus placebo, including supplements and vitamins, antidepressant medications, and antipsychotic medications (Agnihotri 2013; Akkasheh 2016; Baptista 2009; Assunção 2006; Fadai 2014; Ghaderi 2019; Narula 2010; Tohen 2011; Zhao 2015)
Behavioural intervention versus placebo and metformin (Wu 2008b)
Atypical antipsychotics included aripiprazole, quetiapine, clozapine, olanzapine, ziprasidone, paliperidone, and risperidone. Typical antipsychotics included haloperidol and sulpride.
Meta‐analyses could only be conducted for the first four categories of studies because studies of atypical versus atypical medications could not be compared directly. Studies of miscellaneous interventions could not be compared due to heterogeneity in the interventions and target populations (patients with depressive disorder or schizophrenia).
Sensitivity analyses could only be performed for low risk of bias studies comparing metformin to placebo because no data were available for other outcomes and comparisons. Forest plots were not constructed due to the small number of studies for each comparison.
1. Atypical versus typical antipsychotics
This comparison includes medication for the treatment of psychotic symptoms in patients with schizophrenia or schizoaffective disorder.
Outcome 1.1 Diabetes prevention
One study of 99 participants with schizophrenia showed there may be no difference in the prevention of diabetes between participants with atypical versus typical antipsychotics six weeks after starting treatment (RR 0.46, 95% CI 0.03 to 7.05; 99 participants; 1 study; low‐certainty evidence) (Analysis 1.1) (among total 99 participants with schizophrenia, 68 were in atypical and 31 were in typical antipsychotics group; 55 participants without mental disorder were not considered in the analysis).
1.1. Analysis.

Comparison 1: Atypical versus typical antipsychotic, Outcome 1: diabetes (ADA criteria) (6 weeks)
Outcome 1.2 Drop‐outs
Two studies were included, but only one reported any drop‐outs. The analysis shows there may be no difference in drop‐out rates between study arms 54 weeks after baseline (RR 1.31, 95% CI 0.63 to 2.69; 144 participants; 2 studies; low‐certainty evidence) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Atypical versus typical antipsychotic, Outcome 2: drop‐outs (6‐54 weeks)
Outcome 1.3 Fasting blood glucose
Two studies reported on fasting blood glucose levels. The analysis shows there is probably no difference between patients taking atypical versus typical antipsychotics six and eight weeks after starting treatment (MD ‐0.05, 95% CI ‐0.10 to ‐0.00; 211 participants; 2 studies; moderate‐certainty evidence) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Atypical versus typical antipsychotic, Outcome 3: fasting blood glucose (6‐8 weeks)
Unplanned sensitivity analysis
Given our concerns about the data presented by Wu 2006 (very small SDs), we investigated the impact of these data on the outcomes by removing the study from the analysis, in an unplanned sensitivity analysis. Removing this study shows no difference between patients taking atypical versus typical antipsychotics (MD 0.01, 95% CI ‐0.35 to 0.37; 99 participants; 1 study) (Analysis 7.1).
7.1. Analysis.

Comparison 7: SENSITIVITY‐ Wu2006‐ Atypical versus typical antipsychotic, Outcome 1: fasting blood glucose (6‐8 weeks)
Outcome 1.4 BMI
Participants who received typical antipsychotics probably had a lower BMI at follow‐up than participants who received atypical antipsychotics (MD 0.57, 95% CI 0.33 to 0.81; 141 participants; 2 studies; moderate certainty‐evidence) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Atypical versus typical antipsychotic, Outcome 4: BMI (8‐54 weeks)
Unplanned sensitivity analysis
Given our concerns about the data presented by Wu 2006 (very small SDs), we investigated the impact of these data on the outcomes by removing the study from the analysis, in an unplanned sensitivity analysis. Removing this study shows no difference between patients taking atypical versus typical antipsychotics (MD ‐1.13, 95% CI ‐5.65 to 3.39; 29 participants; 1 study) (Analysis 7.2).
7.2. Analysis.

Comparison 7: SENSITIVITY‐ Wu2006‐ Atypical versus typical antipsychotic, Outcome 2: BMI (52 weeks)
Outcome 1.5 Total cholesterol
Results indicated that total cholesterol eight weeks after starting treatment was probably lower for participants who received typical rather than atypical antipsychotics (MD 0.35, 95% CI 0.27 to 0.43; 112 participants; 1 study) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Atypical versus typical antipsychotic, Outcome 5: total cholesterol (8 weeks)
No evidence was available for the outcomes of waist circumference, blood pressure, depression, anxiety, quality of life, and all‐cause mortality.
2. Metformin versus placebo
This comparison includes studies of patients with schizophrenia or related disorders comparing metformin, an anti‐diabetic medication, to a placebo. In all studies, both groups also received antipsychotic medication, usually olanzapine or clozapine (atypical antipsychotics). The dose of metformin varied across different studies between 750 mg to 2250 mg per day. Studies reported outcomes at 12 to 14 weeks follow‐up.
Outcome 2.1 Drop‐outs
We found there may be no difference in drop‐outs between participants receiving metformin and those receiving placebo (RR 1.22, 95% CI 0.09 to 16.35; 158 participants; 3 studies; moderate‐certainty evidence) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Metformin versus placebo, Outcome 1: drop‐outs (12‐14 weeks)
Unplanned sensitivity analysis
Given our concerns about the data presented by Wu 2008b (Risk of bias in included studies), we investigated the impact of these data on the outcomes by removing the study from the analysis, in an unplanned sensitivity analysis. Removing this study mostly affected the precision of the analysis (RR 2.78, 95% CI 0.08 to 95.87; 94 participants; 2 studies) (Analysis 6.1).
6.1. Analysis.

Comparison 6: SENSITIVITY ‐ Wu2008a ‐ metformin vs placebo, Outcome 1: drop‐outs (12‐14 weeks)
Outcome 2.2 Fasting blood glucose
Five studies reported fasting blood glucose (264 participants). Three studies reported endpoint data and two reported change from baseline data. The use of different measures meant we could not combine these estimates.
There was no difference in fasting blood glucose between participants in the metformin and placebo groups for endpoint data (MD ‐0.35, 95% CI ‐0.60 to ‐0.11; 173 participants; 3 studies; moderate‐certainty evidence) and change from baseline data (MD 0.01, 95% CI ‐0.21 to 0.22; 91 participants; 2 studies; high‐certainty evidence) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Metformin versus placebo, Outcome 2: fasting blood glucose (12‐14 weeks)
Planned sensitivity analysis
A sensitivity analysis of high certainty studies, removing Baptista 2006 from the analysis of endpoint data, did not substantially alter the results (MD‐0.37, 95% CI ‐0.68 to ‐0.07; 136 participants; 2 studies) (Analysis 5.1).
5.1. Analysis.

Comparison 5: SENSITIVITY ‐ high quality ‐ metformin vs placebo, Outcome 1: fasting blood glucose (12‐14 weeks)
Unplanned sensitivity analysis
We removed Wu 2008b from the analysis because of concerns about the primary data. This study was also an outlier, with lower fasting blood glucose levels in the metformin group than in the placebo group at 12 weeks (weighting 19.9%). Without this study, the effect size estimate for endpoint data changed and the CI narrowed (MD‐0.19, 95% CI ‐0.46 to 0.09; 109 participants; 2 studies) (Analysis 6.2). Heterogeneity as indicated by I2 declined from 89% to 0%.
6.2. Analysis.

Comparison 6: SENSITIVITY ‐ Wu2008a ‐ metformin vs placebo, Outcome 2: fasting blood glucose (12‐14 weeks)
Outcome 2.3 BMI
Endpoint and change from baseline data were included in one meta‐analysis for this outcome, as all studies used the same measure of BMI. BMI was lower for participants receiving metformin compared with those receiving a placebo (MD ‐1.37, 95% CI ‐2.04 to ‐0.70; 264 participants; 5 studies; high‐certainty evidence) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Metformin versus placebo, Outcome 3: BMI (12‐14 weeks)
Planned sensitivity analysis
Findings were similar in a sensitivity analysis including high certainty studies (MD ‐1.46, 95% CI ‐2.15 to ‐0.77, I2 = 77%; 227 participants; 4 studies) (Analysis 5.2).
5.2. Analysis.

Comparison 5: SENSITIVITY ‐ high quality ‐ metformin vs placebo, Outcome 2: BMI (12‐14 weeks)
Unplanned sensitivity analysis
Removing Wu 2008b from the analysis reduced the magnitude and precision of the effect (MD ‐1.13, 95% CI ‐1.86 to ‐0.40; 200 participants; 4 studies) (Analysis 6.3).
6.3. Analysis.

Comparison 6: SENSITIVITY ‐ Wu2008a ‐ metformin vs placebo, Outcome 3: BMI (12‐14 weeks)
Outcome 2.4 Waist circumference
The estimates for endpoint (MD‐0.30, 95% CI ‐6.26, 5.66; 101 participants; 2 studies) and change from baseline (MD ‐0.93, 95% CI ‐1.21 to ‐0.64; 91 participants; 2 studies) data did not show a difference between metformin compared with placebo for waist circumference.
Unplanned sensitivity analysis
Statistical heterogeneity was high (I2 = 96% and I2 = 89%), because two of the four studies, with the same first author, showed relatively precise estimates that strongly favoured metformin (Wu 2008a; Wu 2008b). The other studies showed a lesser difference or no difference. Removing Wu 2008b from the analysis meant only one study with endpoint data was included for this outcome (Baptista 2006), which did not show a difference between metformin and placebo in terms of waist circumference (MD 3.40, 95% CI ‐1.99 to 8.79; 37 participants; 1 study) (Analysis 6.4).
6.4. Analysis.

Comparison 6: SENSITIVITY ‐ Wu2008a ‐ metformin vs placebo, Outcome 4: waist circumference (12‐14 weeks)
Outcome 2.5 Systolic blood pressure
Systolic blood pressure was reported by one study (Carrizo 2009) and showed no difference between metformin and placebo (MD ‐2.50, 95% CI ‐9.09 to 4.09; 54 participants; 1 study) (Analysis 2.5).
2.5. Analysis.

Comparison 2: Metformin versus placebo, Outcome 5: systolic blood pressure (14 weeks)
Outcome 2.6 Diastolic blood pressure
Diastolic blood pressure was reported by one study (Carrizo 2009) and showed no difference between metformin versus placebo group (MD 1.20, 95% CI ‐3.55 to 5.95; 54 participants; 1 study) (Analysis 2.6).
2.6. Analysis.

Comparison 2: Metformin versus placebo, Outcome 6: diastolic blood pressure (14 weeks)
Outcome 2.7 Total cholesterol
Meta‐analysis based on two studies showed no difference between metformin and placebo in total cholesterol (MD ‐13.06, 95% CI ‐35.89 to 9.76; 99 participants; 2 studies) (Analysis 2.7).
2.7. Analysis.

Comparison 2: Metformin versus placebo, Outcome 7: total cholesterol (12‐14 weeks)
No data were available for diabetes prevention, depression, anxiety, quality of life, and mortality.
3. Melatonin versus placebo
Three studies included the hormone melatonin versus a placebo in patients with psychotic symptoms and schizophrenia. The dose of melatonin used was 3 mg per day. Outcomes were reported at 8 to 12 weeks.
Outcome 3.1 Drop‐outs
Based on findings from one study, there may be no difference in drop‐out rates between melatonin and placebo (RR 1.00, 95% CI 0.38 to 2.66; 48 participants; 1 study; low‐certainty evidence) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 1: drop‐outs (8 weeks)
Outcome 3.2 Fasting blood glucose
Fasting blood glucose was probably reduced more at endpoint in the melatonin compared with the placebo group when considering endpoint data (MD ‐0.17, 95% CI ‐0.35 to 0.01; 102 participants; 2 studies; moderate‐certainty evidence) and change from baseline data (MD ‐0.24, 95% CI ‐0.39 to ‐0.09); 100 participants; 1 study; moderate‐certainty evidence) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 2: fasting blood glucose (8‐12 weeks)
Outcome 3.3 BMI
Three studies reported BMI, it was uncertain if there was any difference between patients with melatonin versus those in the placebo group (MD ‐0.22, 95% CI ‐2.58 to 2.14; 202 participants; 3 studies; very low‐certainty evidence) (Analysis 3.3). Statistical heterogeneity was high (I2 = 93%) due to one study showing a positive effect and one showing a negative effect of melatonin on BMI, compared with placebo.
3.3. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 3: BMI (8‐12 weeks)
Outcome 3.4 Waist circumference
Two studies reported waist circumference. Analyses showed there may be no difference between patients receiving melatonin versus those in the placebo group for endpoint data (MD 0.68, 95% CI 0.47 to 1.83; 36 participants; 1 study). Analyses showed there may be a benefit of placebo versus melatonin for change from baseline data (MD 1.19, 95% CI 0.29 to 2.09; 100 participants; 1 study) (Analysis 3.4).
3.4. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 4: Waist circumference (8 weeks)
Outcome 3.5 Systolic blood pressure
There may be no difference in systolic blood pressure between melatonin versus placebo in the combined results of two studies (MD ‐1.31, 95% CI ‐6.46 to 3.84; 134 participants; 2 studies) (Analysis 3.5). However, these results showed a high level of statistical heterogeneity (I2 = 86%). In one study, systolic blood pressure at endpoint was higher in the melatonin compared with the placebo group, and in the other study systolic blood pressure reduced in the melatonin group over the course of the study and increased in the placebo group.
3.5. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 5: systolic blood pressure (8 weeks)
Outcome 3.6 Diastolic blood pressure
Two studies reported on diastolic blood pressure, showing there may be no difference between melatonin and placebo (MD ‐1.05, 95% CI ‐1.60 to ‐0.50; 134 participants; 2 studies) (Analysis 3.6).
3.6. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 6: diastolic blood pressure (8 weeks)
Outcome 3.7 Total cholesterol
Results from two studies (134 participants) indicated there may be no difference in total cholesterol between patients receiving melatonin and placebo using endpoint data (MD ‐0.11, 95% CI ‐0.27 to 0.05; 36 participants; 1 study) and change from baseline data (MD 0.02, 95% CI ‐0.19 to 0.23; 100 participants; 1 study) (Analysis 3.7).
3.7. Analysis.

Comparison 3: Melatonin versus placebo, Outcome 7: total cholesterol (8 weeks)
No evidence was available for diabetes prevention, depression, anxiety, quality of life, and all‐cause mortality.
4. SSRI versus TCA
This is a comparison of fluoxetine, an SSRI antidepressant, and imipramine, a TCA, with three studies contributing data. The dose varied across different studies between 20 mg to 40 mg per day for fluoxetine and between 50 mg to 200 mg per day for imipramine. Outcomes were reported at 8 to 12 weeks.
Outcome 4.1 Drop‐outs
One study reported a higher percentage of drop‐out in the TCA group compared with the SSRI group (RR 0.34, 95% CI 0.11 to 1.01; 25 participants; 1 study; very low‐certainty evidence) (Analysis 4.1).
4.1. Analysis.

Comparison 4: SSRI versus TCA, Outcome 1: drop‐outs (12 weeks)
Outcome 4.2 Fasting blood glucose
There was probably no difference in fasting blood glucose between participants who received an SSRI and a TCA (MD ‐0.39 lower, 95% CI ‐0.88 to 0.10; 141 participants; 3 studies; moderate‐certainty evidence) (Analysis 4.2).
4.2. Analysis.

Comparison 4: SSRI versus TCA, Outcome 2: fasting blood glucose (8‐12 weeks)
Outcome 4.3 BMI
Results from one study indicated that there may be no difference in BMI between SSRI and TCA (MD 0.70, 95% CI ‐1.10 to 2.50; 18 participants; 1 study; very low‐certainty evidence) (Analysis 4.3).
4.3. Analysis.

Comparison 4: SSRI versus TCA, Outcome 3: BMI (12 weeks)
Outcome 4.4 Depression
Results from one study showed there may be no difference in depression symptoms between SSRI and TCA (MD 0.30, 95% CI ‐0.59 to 1.19; 18 participants; 1 study) (Analysis 4.4).
4.4. Analysis.

Comparison 4: SSRI versus TCA, Outcome 4: depression (12 weeks)
No evidence was available for diabetes prevention, waist circumference, blood pressure, cholesterol, anxiety, quality of life, and all‐cause mortality.
5. Atypical versus atypical antipsychotics
Six studies with 594 participants in total compared participants receiving different types of atypical antipsychotic medications. Results for five studies reporting outcome data at endpoint (between six and 52 weeks) are shown in Table 5.
1. Findings of studies comparing atypical antipsychotics.
| Reference | Comparison | Follow‐up | Outcome | mean (SD) group 1 | number of drop‐outs group 1 |
n/N group1 |
mean (SD) group 2 | number of drop‐outs group 2 |
n/N group2 |
| Chen 2017 | ziprasidone (n = 19) versus olanzapine (n = 19) | 12 weeks | fasting blood glucose (mmol/L) | 5.59 (1.02) | 19/38 | 7.28 (2.22) | 19/38 | ||
| cholesterol (mmol/L) | 1.28 (0.2) | 2.24 (0.31) | |||||||
| Ou 2013 | ziprasidone (n = 130) versus olanzapine (n = 130) | 6 weeks | drop‐outs | 11 | 130/260 | 19 | 130/260 | ||
| fasting blood glucose (mmol/L) | 4.40 (0.50) | 4.94 (0.50) | |||||||
| cholesterol (mmol/L) | 4.06 (0.74) | 4.51 (0.80) | |||||||
| BMI (kg/m2) | 20.87 (3.34) | 22.28 (2.93) | |||||||
| systolic blood pressure (mm Hg) | 117.43 (13.13) | 117.97 (10.50) | |||||||
| diastolic blood pressure (mm Hg) | 75.93 (7.90) | 76.30 (5.65) | |||||||
| Hu 2013 | paliperidone (n = 33) versus olanzapine (n = 23) | 12 weeks | drop‐outs | 7 | 33/56 | 17 | 23/56 | ||
| fasting blood glucose (mmol/L) | 5.21 (0.6) | 5.19 (0.6) | |||||||
| BMI (kg/m2) | 22.17 (3.31) | 23.17 (4.06) | |||||||
| waist circumference (cm) | 80.3 (12.47) | 82.63 (9.49) | |||||||
| Zhang 2014 | aripiprazole (n = 50) versus olanzapine (n = 50) | 8 weeks | drop‐outs | 5 | 50/100 | 5 | 50/100 | ||
| fasting blood glucose (mmol/L) | 5.49 (1.51) | 5.49 (1.51) | |||||||
| cholesterol (mmol/L) | 4.11 (1.04) | 4.72 (1.12) | |||||||
| Zhang 2012 | aripriprazole (n = 71) versus ziprasidone (n = 69) | 52 weeks | drop‐outs | 19 | 71/140 | 14 | 69/140 | ||
| fasting blood glucose (mmol/L) | 5.2 | 4.6 | |||||||
| cholesterol (mmol/L) | 5.1 | 4.7 | |||||||
| BMI (kg/m2) | 24.5 (5.9 | 20.3 (5.2) | |||||||
| waist circumference (cm) | 71.6 (17.6) | 70.3 (16.7) |
SD: standard deviation; n: number of participants; N: total number of participants; BMI: Body mass index.
Comparisons within studies included ziprasidone, olanzapine, paliperidone, aripiprazole, and ziprasidone. Studies reported on drop‐outs, fasting blood glucose, cholesterol, BMI, waist circumference, and systolic and diastolic blood pressure.
Individual studies generally reported more favourable results in terms of blood glucose level and lipid metabolism (BMI, cholesterol, waist circumference) from ziprasidone than olanzapine or other antipsychotics (Chen 2017; Ou 2013; Zhang 2012).
6. Miscellaneous drugs
Ten studies with 699 participants in total were included under the miscellaneous group. Results of these studies reporting outcome data at endpoint (four to 12 weeks) are shown in Table 6.
2. Findings of studies comparing miscellanious interventions.
| Reference | Comparison | Follow‐up | Outcome | mean (SD) group 1 | number of drop‐outs group 1 |
n/N group1 |
mean (SD) group 2 | number of drop‐outs group 2 |
n/N group 2 |
mean (SD) group 3) | number of drop‐outs group 3 |
n/N group 3 |
| Agnihotri 2013 | Withania somnifera (n = 12) versus placebo (n = 13) | 4 weeks | fasting blood glucose (mmol/L) | 5.14 ( 0.33) | 12/25 | 5.82 ( 0.46) |
13/25 | |||||
| Akkasheh 2016 | probiotic supplements (n = 20) versus placebo (n = 20) | 8 weeks | drop‐outs | 3 | 20/40 | 2 | 20/40 | |||||
| fasting blood glucose (mmol/L) |
5.54 (0.97) | 4.96 (0.42) | ||||||||||
| cholesterol (mmol/L) | 9.58 (1.88) | 9.98 (1.72) | ||||||||||
| BMI (kg/m2) | 27.5 (5.9) | 26.5 (3.9) | ||||||||||
| Assunção 2006 | nizatidine (n = 27) versus placebo (n = 27) | 12 weeks | fasting blood glucose (mmol/L) | 4.84 (0.81) | 27/54 | 4.70 (0.98) | 27/54 | |||||
| cholesterol (mmol/L) | 11.04 (2.56) | 10.02 (1.89) | ||||||||||
| Baptista 2009 | rosiglitazone (n = 14) versus placebo (n = 15) | 12 weeks | fasting blood glucose (mmol/L) | 4.48 (0.77) | 14/29 | 4.38 (0.48) | 15/29 | |||||
| cholesterol (mmol/L) | 9.99 (1.26) | 11.18 (1.45) | ||||||||||
| BMI (kg/m2) | 26.9 (4.2) | 26.3 (2.9) | ||||||||||
| waist circumference (cm) | 91.5 (11.6) | 90.5 (8.6) | ||||||||||
| Fadai 2014 | saffron aqueous extract (n = 20) versus crocin (n = 20) versus placebo (n = 21) | 12 weeks | drop‐outs | 2 | 20/61 | 2 | 20/61 | 1 | 21/61 | |||
| fasting blood glucose (mmol/L) | 5.58 (0.39) | 5.44 (0.38) | 6.03 (0.56) | |||||||||
| cholesterol (mmol/L) | 9.36 (1.35) | 9.94 (1.91) | 10.9 (1.91) | |||||||||
| waist circumference (cm) | 92.1 (7.4) | 91.9 (8.6) | 98.4 (8.4) | |||||||||
| blood pressure (mm Hg) | 116.5 (5.6) | 109.7 (6.8) | 115.9 (9) | |||||||||
| Ghaderi 2019 | vitamin D and probiotic supplements (n = 30) versus placebo (n = 30) | 12 weeks | drop‐outs | 4 | 30/60 | 4 | 30/60 | |||||
| fasting blood glucose (mmol/L) | 4.89 (0.62) | 5.17 (0.48) | ||||||||||
| cholesterol (mmol/L) | 8.99 (2.04) | 9.92 (1.99) | ||||||||||
| BMI (kg/m2) | 23.2 (2.7) | 24.5 (3.7) | ||||||||||
| Narula 2010 | topiramate (n = 33) versus placebo (n = 34) | 12 weeks | fasting blood glucose (mmol/L) | 4.35 (0.37) | 33/67 | 4.92 (0.67) | 34/67 | |||||
| BMI (kg/m2) | 20.1 (4) | 22.55 (4.11) | ||||||||||
| diastolic blood pressure (mm Hg) | 77.94 (4.8) | 81.41 (6.2) | ||||||||||
| systolic blood pressure (mm Hg) | 177.88 (7) | 122.5 (7.71) | ||||||||||
| Tohen 2011 | olanzapine (n = 140) versus placebo (n = 70) | 6 weeks | drop‐outs | 35 | 140/210 | 35/70 | 35 | 70/210 | ||||
| fasting blood glucose (mmol/L) | 5.05 (0.53) | 5.03 (0.61) | ||||||||||
| cholesterol (mmol/L) | 4.47 (0.95) | 4.56 (0.94) | ||||||||||
| diastolic blood pressure (mm Hg) | 72.72 (8.09) | 72.17 (7.36) | ||||||||||
| systolic blood pressure (mm Hg) | 109.84 (11.41) | 109.76 (11.59) | ||||||||||
| Zhao 2015 | aripiprazole (n = 56) versus placebo (n = 57) | 6 weeks | drop‐outs | 2 | 56/113 | 4 | 57/113 | |||||
| fasting blood glucose (mmol/L) | 4.75 (0.8) | 4.95 (0.87) | ||||||||||
| cholesterol (mmol/L) | 3.32 (0.92) | 4.54 (1.11) | ||||||||||
| BMI (kg/m2) | 23.27 (2.63) | 24.8 (3.92) | ||||||||||
| Sepehrmanesh 2016 | Vitamin D (n = 20) versus placebo (n = 20) | 8 weeks | drop‐outs | 2 | 20/40 | 2 | 20/40 | |||||
| fasting blood glucose(mmol/L) | 4.69 (0.48) | 5.02 (0.56) | ||||||||||
| cholesterol (mmol/L) | 4.69 (0.48) | 5.02 (0.56) | ||||||||||
| BMI (kg/m2) | 26.0 (5.1) | 27.3 (3.5) | ||||||||||
| Depression score, Beak Depression Inventory (BDI) | 17.2 (10.6) | 25.2 (9.9) |
SD: standard deviation; n: number of participants; N: total number of participants; BMI: Body mass index.
Comparisons within the studies included Withania somnifera (an herbal medicine) versus placebo in participants with schizophrenia (Agnihotri 2013); probiotic versus placebo in participants with depression (Akkasheh 2016); rosiglitazone versus placebo in participants receiving olanzapine (Baptista 2009); nizatidine versus placebo in participants receiving olanzapine (Assunção 2006); saffron versus placebo in participants with schizophrenia, receiving olanzapine (Fadai 2014); vitamin D and probiotic versus placebo in participants with schizophrenia (Ghaderi 2019); topiramate versus placebo in participants with schizophrenia, receiving olanzapine (Narula 2010); olanzapine versus placebo in participants with depression (Tohen 2011); aripiprazole versus placebo in participants with schizophrenia, on risperidone (Zhao 2015); and vitamin D versus placebo in participants with major depressive disorder (Sepehrmanesh 2015).
A statistically significant reduction was observed for fasting blood glucose for Withania somnifera (Agnihotri 2013); however, the full results were not available. Significant decreases in fasting blood glucose level and cholesterol were reported for vitamin D plus probiotic supplements (Ghaderi 2019) and topiramate (Narula 2010) compared with placebo. Other studies did not report any significant differences in other assessed outcomes.
7. Behavioural intervention
One study with 128 participants included four study arms: lifestyle intervention plus medication (metformin); medication (metformin); lifestyle intervention plus placebo; and placebo (Wu 2008b). Medication versus placebo results are included in Analysis 2. At the 12 week endpoint, the mean fasting blood sugar levels were lowest in the lifestyle plus metformin and lifestyle plus placebo groups (88.3, 95% CI 86.5 to 90.1 for both groups), and in the metformin group (84.7, 95% CI 82.9 to 86.5). They were highest in the placebo group (93.7, 95% CI 91.9 to 95.5).
Discussion
Summary of main results
We included 30 RCTs with 2631 participants from hospital settings in LMICs including Brazil, China, India, Iran, South Africa and Venezuela. All studies were of pharmacological interventions; one included a pharmacological and a behavioural intervention (Wu 2008b). We did not identify any studies evaluating organisational interventions. Only one study reported on our primary outcome, prevention or delay of diabetes. All studies reported blood glucose level as a proxy for risk of developing diabetes.
Atypical versus typical antipsychotics
Three studies were included in this group, with 256 participants with schizophrenia. Only one study reported there may be no difference between atypical and typical antipsychotics in prevention of diabetes (99 participants with schizophrenia, low‐certainty evidence). There maybe no difference between atypical and typical antipsychotics in drop‐outs (two studies including 144 participants, low‐certainty evidence), and fasting blood glucose levels (two studies including 211 participants, moderate‐certainty evidence). However, participants receiving typical antipsychotics showed more favourable results for BMI in terms of reduction or smaller increase (two studies including 141 participants, moderate‐certainty evidence) and lower cholesterol level (one study with 112 participants). No data were available for waist circumference, blood pressure, depression, anxiety, quality of life, and all‐cause mortality.
Metformin versus placebo
Five studies were included in this group with 264 participants. There may be no difference in drop‐outs (moderate‐certainty evidence), fasting blood glucose (moderate‐certainty evidence), waist circumference, blood pressure, and cholesterol between participants receiving metformin and placebo. For participants receiving metformin rather than placebo, BMI was lower at the trial endpoint or further reduced (high‐certainty evidence). Evidence for fasting glucose and waist circumference was highly inconsistent and unclear. There were no data available for effectiveness in diabetes prevention, depression, anxiety, quality of life, and all‐cause mortality.
Melatonin versus placebo
In three studies with 202 participants, there may be no differences in drop‐outs (low‐certainty evidence), BMI (very low‐certainty evidence), blood pressure, and cholesterol level, between participants receiving melatonin and placebo. Moderate‐certainty evidence showed that fasting blood glucose was probably lower or reduced more for participants receiving melatonin, compared to those receiving placebo. Two studies reporting on waist circumference found either no difference or a greater reduction for placebo, compared with melatonin. Evidence for BMI and systolic blood pressure was highly inconsistent. No data were available for prevention of diabetes, depression, anxiety, quality of life, and all‐cause mortality.
SSRIs versus TCA
Three studies with 141 participants found there may be no differences between participants receiving SSRI and TCA in fasting blood glucose levels (moderate‐certainty evidence), BMI (very low‐certainty evidence), and depression. Drop‐outs were higher for participants receiving TCA, rather than SSRIs, but the evidence was judged to be of very low certainty. No data were available for prevention of diabetes, waist circumference, blood pressure, cholesterol, anxiety, quality of life, and all‐cause mortality.
Other comparisons
Results from 15 studies could not be included in meta‐analyses. These studies could not be compared directly due to heterogeneity in the intervention groups.
Overall completeness and applicability of evidence
Studies did not explicitly state that the aim of the evaluated intervention was to prevent type 2 diabetes, although many included relevant outcomes such as fasting blood glucose levels. Only one study included diagnosis of diabetes as an outcome (Saddichha 2008). Fasting blood glucose is a short‐term proxy for risk of diabetes, but a raised blood glucose level does not necessarily equate to development of diabetes in the future. Likewise, measures such as total cholesterol level and BMI may indicate a reduced or increased risk of diabetes, but these changes were often measured in a short timeframe and cannot be seen as a reliable indicator of diabetes prevention. The majority of the evidence identified in this review therefore does not necessarily apply to settings in which the aim is to prevent diabetes.
We did not examine pre‐diabetes as an outcome, and we did not examine the subset of study populations at a higher risk of diabetes at baseline.
The evidence we identified was based on studies conducted in six countries (Brazil, China, India, Iran, South Africa and Venezuela) in hospital settings. This may not be representative of the global population of people with mental disorders.
A further limitation is that all studies we identified were conducted in hospital settings. This may not be an appropriate setting to provide or evaluate diabetes prevention interventions for people with mental health conditions, many of whom are unlikely to frequently visit a hospital to receive mental healthcare. Most studies included participants with schizophrenia, while common mental disorders, which are more prevalent worldwide, were less frequently studied. There were also gaps in the evidence for behavioural and organisational interventions, with studies mostly focussed on antipsychotics, antidepressants, and melatonin.
Finally, interventions may be most effective for people who are highly motivated to prevent diabetes, and these may have been more likely to participate in the included studies. For example, one study used willingness to lose weight as a criteria for participation (Baptista 2009). This may mean that the evidence we found overestimates the effectiveness of interventions for diabetes prevention for the general population.
Quality of the evidence
The certainty of the evidence ranged from very low to high. For the primary outcome of diabetes prevention, one study contributed low‐certainty evidence. We downgraded this evidence for imprecision. For other outcomes, evidence was downgraded for imprecision, high risk of bias, and inconsistency of results.
Risk of bias domains were frequently judged ‘unclear’ because the information required was not reported. For example, only three studies provided a link to a prospectively published protocol or trial registration. For some studies, a risk of attrition bias was identified, and not all studies were double‐blinded. Several studies were funded by the pharmaceutical industry, and in some cases it appeared the funder was involved in the research.
We had concerns about the data for one study and could not reach authors to seek clarification (Wu 2008b). We explored the impact of this study in unplanned sensitivity analyses. Comparing metformin to placebo, this study showed a more favourable profile for metformin than other studies for fasting blood glucose, BMI, and waist circumference.
We could not investigate the impact of reporting bias due to the limited number of studies available for each comparison.
Potential biases in the review process
We applied a comprehensive search strategy to identify all available evidence. However, it is possible that relevant studies were not identified. Successful searching and screening of studies relies on the identification of keywords in the titles, and abstracts of manuscripts. Although included studies measured a diabetes‐related outcome such as fasting blood glucose, the studies were rarely described as an evaluation of interventions aimed at preventing type 2 diabetes and only one study reported on diabetes prevention.
We identified an additional study through checking of systematic review reference lists, which was not found through database searches, possibly because the focus of the study was on weight gain rather than prevention of diabetes (Wu 2008b). Although we searched global and regional databases as well as grey literature, we may also have missed literature not published in English, or published in databases or on websites not covered by our search. We had planned to include data from multi‐country studies if authors would supply data for LMICs separately. However, these data were obtained for only one study. For ten studies, data could not be obtained (Characteristics of studies awaiting classification).
During the selection of studies we realised that, instead of assessing a clinical diagnosis of diabetes, studies frequently measured fasting glucose levels. We added this to our outcomes after registering the protocol, because we felt it provided important evidence on the efficacy of included interventions (Differences between protocol and review).
Although we only included RCTs in this review, to increase the internal validity of our findings, some bias inherent to the RCT design is likely to remain. For example, the trials are likely to represent a selective population, which may mean that results cannot easily be translated to the wider population of people with mental health disorders.
Despite contacting authors of primary studies to obtain missing information, we were not successful in obtaining all information. For risk of bias assessments in particular, this meant we frequently had to rate a domain as 'unclear.' This could mean our appraisal of the evidence is skewed towards more positive or more negative ratings. The review was not inclusive to pregnant women with mental health disorders.
Our review grouped all LMICs together, while in reality these countries represent diverse populations and settings which differ in their demographics, wealth levels, healthcare systems, and determinants and prevalence of diabetes in the population.
Agreements and disagreements with other studies or reviews
We are not aware of similar reviews including studies only from LMICs. As with the evidence base identified in our review, evidence from high‐income countries is mostly focussed on weight‐loss outcomes, rather than diabetes prevention.
Atypical versus typical antipsychotics
Our evidence mostly indicated no difference between atypical and typical antipsychotics for markers of diabetes prevention, except for more favourable impacts for typical antipsychotics on BMI and cholesterol levels. The wider literature emphasises the increased risk of diabetes for atypical antipsychotics and focuses on the difference between types of atypical antipsychotics (see 'Other comparisons') rather than comparing atypical to typical antipsychotics.
Metformin versus placebo
While we did not find strong evidence to suggest that metformin could prevent the increased risk of diabetes caused by antipsychotic medications, a clinical trial from Taiwan reported favourable results of metformin compared with a placebo for fasting blood glucose and related measures (Chen 2008).
Melatonin versus placebo
From our findings, we cannot draw conclusions on the potential effects of melatonin on prevention of diabetes among people with severe mental illness. In a narrative review of global literature, the potential for melatonin to be used for diabetes prevention in people with schizophrenia was also said to be unclear (Morera‐Fumero 2013).
SSRIs versus TCA
In our review, evidence comparing SSRI and TCA antidepressants was largely absent or inconclusive. A review of case‐control studies and cohort studies found that the risk of developing diabetes was increased among people who were prescribed an SSRI or a TCA to treat depression, without any indication of a difference in effect estimates between the two (Yoon 2013). A review of randomized and non‐randomized studies concluded that successful treatment of depression, measured by a decrease in the severity of depression symptoms, could improve glycaemic control in patients who have comorbid diabetes (Roopan 2017). The authors suggested that treatment with a TCA would require closer glycaemic monitoring than treatment with an SSRI, but no meta‐analysis was performed to verify this claim.
Other comparisons
A large systematic review of 100 RCTs, including 25,952 participants, reported on metabolic side effects of antipsychotics. Olanzapine and clozapine were found to have the greatest detrimental impact on metabolism, while profiles of aripiprazole, brexpiprazole, cariprazine, lurasidone, and ziprasidone were more benign (Pillinger 2020). Our narrative synthesis of studies suggested more favourable results for blood glucose level and lipid metabolism (BMI, cholesterol, waist circumference) from ziprasidone than olanzapine or other antipsychotics in individual studies.
A systematic review of studies evaluating interventions to improve glycaemic control in adults with severe mental illness found that non‐pharmacological interventions, including behaviour change interventions, were effective in lowering blood glucose (Taylor 2017). A systematic review of four US studies concluded that diabetes education which incorporates elements of diet and exercise could reduce fasting blood glucose (Cimo 2012). We found only one study, which included a behavioural intervention in people with mental illness in an LMIC setting.
Authors' conclusions
Implications for practice.
The increasing global burden of mental illness and comorbid diabetes has not yet produced a strong evidence base for the prevention of type 2 diabetes in people with mental illness in low‐ and middle‐income countries (LMICs). Even though we know from high‐income countries that pharmacological and behavioural interventions can be effective, evidence from LMICs is lacking in quantity, breadth, and quality. This affects our confidence in the certainty of the evidence). It therefore remains unclear how best to prevent type 2 diabetes in this population, and we are unsure whether interventions shown to be effective in high‐income countries are applicable and suitable for LMIC settings.
Implications for research.
Our Cochrane Review has identified several gaps in the evidence for this topic. Evidence on interventions to prevent diabetes in people with mental disorders is mostly limited to high‐income countries. Most low‐income countries are not represented in this review mainly due to the absence of primary studies. Given the large burden of disease of mental illness and comorbid diabetes worldwide, well‐conducted randomized controlled trials (RCTs) in LMICs should be a priority for future research.
Where evidence from LMIC settings is available, it tends to report blood glucose levels as a proxy of risk of developing diabetes rather than including diabetes itself as an outcome. Although an increased blood glucose level may lead to the development of diabetes in the future, longer‐term studies are needed to determine whether pharmacological and other interventions can delay or prevent the onset of diabetes. Future studies should also target higher risk groups (those in a pre‐diabetic state and with other additional risk factors) as this subset of the population may benefit more than those who have normal glycaemic regulation.
The studies we identified mostly evaluated antipsychotics, anti‐diabetic medication, antidepressants, and various supplements and vitamins. Only one study included a behaviour change intervention (Wu 2008b) and none tested organisational interventions. Apart from evidence on the effectiveness of behaviour change and organisational interventions, it is important to consider whether such interventions would be appropriate and feasible for people in LMICs. For behaviour change interventions in particular, this may include a focus on a patient‐centered rather than paternalistic approach to implementation.
In addition to addressing these gaps in the evidence and further developing the evidence base from LMICs, future research is needed to establish the acceptability and suitability of interventions in LMICs. We used drop‐out from treatment as an indicator of acceptability, but other elements of acceptability, such as cost‐effectiveness and required resources, cultural appropriateness, and availability of healthcare professionals to deliver the interventions, should be considered.
We chose the cut off <3 months vs > 3 months for the treatment/intervention duration because we felt <3 months is a good reflection of short‐term effects, while >3 months would indicate medium and longer term effects. However, further research should consider collecting and reporting data using different cut off points.
Our review was not designed to directly compare the effectiveness of multiple treatments from different clinical trials. A future network meta‐analysis, when more studies are available, would make these comparisons possible, and could, for example, show which antipsychotic medication is the least likely to raise blood glucose levels, and in turn the least likely to lead to the development of type 2 diabetes. While the risk of diabetes due to anti‐psychotic drugs has been recognised, there is still no clear information on the dose‐response, age‐response or time to diabetes for different antipsychotics. Although we were not able to study these interactions in this review, future subgroup analyses or meta‐regression analyses may start to answer questions on dose‐response and age‐response relationships, and longer term effectiveness of diabetes prevention measures in this population.
History
Protocol first published: Issue 3, 2019 Review first published: Issue 2, 2021
Acknowledgements
The study was supported in part by the National Institute of Health Research (NIHR) using Official Development Assistance (ODA) funding (Grant: 17/63/130: NIHR Global Health Research Group: Improving Outcomes in Mental and Physical Multimorbidity and Developing Research Capacity (IMPACT) in South Asia at the University of York).
The following people translated and retrieved full‐text papers. Thanks to their help, we were able to include several studies published in Chinese and Farsi.
Chi Yan Bonnie Cheung, BSc.
Mengyao Zeng, MPH. PhD candidate at NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Public Health School, Fudan University.
Yen‐Chin Chen, RN, PhD. Assistant Head Nurse and Clinical Assistant Professor, Department of Nursing, College of Medicine, National Cheng Kung University and Hospital, Taiwan.
Dr Zoya Ashabi, dentist.
We are grateful to the authors of included and excluded trials who responded to our request for additional information.
We are also grateful for the advice and support received from the editorial team of the Cochrane Common Mental Disorders (CCMD) Group.
The review authors and the CCMD Editorial Team, are grateful to the peer reviewers for their time and comments including: Elena Kostova, Marianna Purgato, Akilesh Ramasamy and Carolina Severiche Mena. They would also like to thank copy editor, Hacsi Horvath.
CRG Funding Acknowledgement The NIHR is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
Electronic Databases
CINAHL (EBSCO) 1981‐ present
Cochrane Central Register of Controlled Trials (Wiley) Issue 2 of 12, February 2020
Cochrane Database of Systematic Reviews (Wiley) Issue 2 of 12, February 2020
Embase Classic+Embase (Ovid) 1947 to 2020 February 19
Global Health (Ovid) 1910 to 2020 Week 08
LILACs (BIREME / PAHO / WHO), all available dates
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to February 19, 2020
PakMediNet (PakCyber) all available dates
PsycINFO (Ovid) 1806 to February Week 2 2020
PubMed (NLM) 1946 – present
All sources were searched 4–8 April 2019 and updated 20–28 February 2020
N.B. In the protocol we also listed the Indian Medlars database(http://indmed.nic.in/), but unfortunately at the time of searching, this database was unavailable.
**************************************************
CINAHL (EBSCO) (1981 onwards)
S55 S53 NOT S54 (2,024)
S54 ( (MH "Child") OR (MH "Adolescence+") OR (MH "Minors (Legal)") ) NOT (MH "Adult+") (389,497)
S53 S49 OR S52 (2,113)
S52 S51 not S44 (292)
S51 S4 AND S12 AND S25 AND S50 (297)
S50 TI ( systematic w2 (review* or synthesis) or "evidence synthesis" or overview* or "umbrella review*" or meta‐analys* or "meta analysis" ) OR AB ( "Search filter*" or "search strateg*" or "literature search*" ) (111,302)
S49 S4 AND S12 AND S25 AND S48 (1,950)
S48 S47 NOT S46 (629,508)
S47 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 (658,140)
S46 S44 NOT S45 (166,381)
S45 (MH "Human") (2,030,419)
S44 S41 OR S42 OR S43 (189,269)
S43 TI animal model* (2,854)
S42 (MH "Animal Studies") (110,913)
S41 (MH "Animals+") (86,130)
S40 AB cluster W3 RCT (323)
S39 (MH "Crossover Design") OR (MH "Comparative Studies") (256,439)
S38 AB control W5 group (99,588)
S37 PT randomized controlled trial (86,198)
S36 (MH "Placebos") (11,533)
S35 (MH "Sample Size") AND AB ( assigned OR allocated OR control ) (3,772)
S34 TI trial (99,553)
S33 AB random* (282,413)
S32 TI randomized or randomised (119,911)
S31 (MH "Cluster Sample") (4,047)
S30 (MH "Pretest‐Posttest Design") (39,659)
S29 (MH "Random Assignment") (57,538)
S28 (MH "Single‐Blind Studies") (13,130)
S27 (MH "Double‐Blind Studies") (43,482)
S26 (MH "Randomized Controlled Trials") (90,656)
S25 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 (1,288,983)
S24 TI ( Montserrat not (Spain or Espana) ) OR AB ( Montserrat not (Spain or Espana) ) OR AF ( Montserrat not (Spain or Espana) ) (30)
S23 TI ( Georgia not (Atlanta or US or USA) ) OR AB ( Georgia not (Atlanta or US or USA) ) OR AF ( Georgia not (Atlanta or US or USA) ) (9,734)
S22 (MH "Armenia") OR (MH "Azerbaijan") OR (MH "Georgia (Republic)") OR (MH "Gibraltar") OR (MH "Mediterranean Islands") OR (MH "Europe, Eastern+") (23,420)
S21 (MH "Bermuda") OR (MH "Pacific Islands+") OR (MH "Indian Ocean Islands+") (33,114)
S20 (MH "Asia, Central+") OR (MH "Asia, Southeastern+") OR (MH "Asia, Western+") OR (MH "China") OR (MH "Hong Kong") OR (MH "Macao") OR (MH "Mongolia") OR (MH "North Korea") OR (MH "South Korea") OR (MH "Taiwan") (217,393)
S19 (MH "Africa+") OR (MH "South America+") OR (MH "Mexico") OR (MH "Central America+") OR (MH "West Indies+") (145,767)
S18 TX ( Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda* ) OR TX ( Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sl ... (369,670)
S17 TX ( Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guiana* or Guyana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian# not "American Indian#") or Indonesia* or Iran* or Iraq* or Israel* ) OR TX ( Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao ... (582,141)
S16 TX ( (Africa* not "African American*") or (Asia* not "Asian American*") ) OR TX ( Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burma or Burmese or Burundi* ) OR TX ( Cambodia* or Cameroon* ... (603,873)
S15 TI ( (country or countries or nation# or economy or economies or world ) N3 ( Developing or "under developed" or underdeveloped or "less* developed" ) ) OR AB ( (country or countries or nation# or economy or economies or world ) N3 ( Developing or "under developed" or underdeveloped or "less* developed" ) ) OR TI ( (country or countries or nation# or economy or economies) N3 ("low* income*" or "middle income*" or "low* middle" or LIC or LICs or underserved or "under served" or deprived or poor* ... (26,774)
S14 TI( LMIC or LMICs or "transition* countr*") or AB( LMIC or LMICs or "transition* countr*") (1,794)
S13 (MH "Developing Countries") OR (MH "Low and Middle Income Countries") (18,517)
S12 S5 or S6 or S7 or S8 or S9 or S10 or S11 (1,462,645)
S11 TX ( ("substance abuse" or "substance use" or "drug abuse" or "drug use" or personality) N2 disorder#). ) OR TX ( sleep# N2 (disorder# or syndrome#) ) (70,976)
S10 TX ( trichotillomani* or OCD or obsess*‐compulsi* or GAD or "stress reaction#" or "acute stress" or neuros#s or neurotic ) OR TX ( "stress syndrome#" or "distress syndrome#" or "pain disorder#" or dementia or alzheimer# or epilepsy ) (116,271)
S9 TX ( (stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous system or eating) N disorder# ) OR TX ( (stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous system or eating) N illness* ) OR TX ( (stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous sy ... (1,114)
S8 TX ( affective* N (disorder# or disease# or illness* or symptom#) ) OR TX ( PTSD or "psychological trauma" or psychotrauma* or "combat disorder#" or "war disorder#" ) OR TX ( (bipolar or behavio#ral or obsessive or compulsive or panic or mood or delusional) N (disorder# or illness* or disease#) ) (14,588)
S7 TX ( movement N5 (disorder or disorders) ) OR TX ( somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or medically unexplained ) OR TX ( (dissociative N3 (disorder* or reaction*)) or dissociation ) (23,333)
S6 TX ( mental or mentally or psychiatr* or psycho* or depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS ) OR TX ( schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc* ) OR TX tardiv* N dyskine* (1,167,525)
S5 (MH "Behavioral and Mental Disorders+") OR (MH "Epilepsy+") (755,365)
S4 S1 OR S2 OR S3 (217,642)
S3 (MH "Hemoglobin A, Glycosylated") OR (MH "Glucose Tolerance Test") OR (MH "Diabetes Mellitus+") (152,334)
S2 TX ( noninsulin*‐depend* or non‐insulin*‐depend* or noninsulin*depend* or non‐insulin*depend* ) OR TX ( "fasting glucose" or "plasma glucose" or "glucose tolerance test*" ) OR TX glyc#emic N2 control* (36,145)
S1 TI ( Diabet* or IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D ) OR AB ( Diabet* or IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D ) (177,375)
**************************************************
Cochrane Central Register of Controlled Trials (Wiley) (Issue 2 of 12, February 2020)
#1 MeSH descriptor: [Diabetes Mellitus] explode all trees
#2 MeSH descriptor: [Glycated Hemoglobin A] explode all trees
#3 MeSH descriptor: [Glucose Tolerance Test] this term only
#4 ("fasting glucose" or "plasma glucose" or "oral glucose tolerance test" or (glyc*emic near/2 control*)):ti,ab,kw
#5 (HbA1c or A1C or A1c or Hb1c or (glycated or glycosylated) NEXT h*emoglobin):ti,ab,kw
#6 diabet*:ti,ab,kw
#7 ("noninsulin‐dependent*" or "non‐insulin‐dependent*" or "noninsulin dependent*" or "non‐insulin dependent*"):ti,ab,kw
#8 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D):ti,ab,kw
#9 {or #1‐#8}
#10 MeSH descriptor: [Mental Disorders] explode all trees
#11 MeSH descriptor: [Behavioral Symptoms] explode all trees
#12 MeSH descriptor: [Epilepsy] explode all trees
#13 (mental or mentally or psychiatr* or psycho* or depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS):ti,ab,kw
#14 (schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc*):ti,ab,kw
#15 (tardiv* NEXT dyskine*):ti,ab,kw
#16 (movement near/5 (disorder or disorders)):ti,ab,kw
#17 (somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or medically unexplained):ti,ab,kw
#18 ((dissociative near/3 (disorder* or reaction*)) or dissociation):ti,ab,kw
#19 (affective* near/1 (disorder* or disease* or illness* or symptom*)):ti,ab,kw
#20 (PTSD or "psychological trauma" or psychotrauma* or "combat disorder*" or "war disorder*"):ti,ab,kw
#21 (post‐trauma* or posttrauma) near/3 (stress* or disorder*)
#22 ((stress or cognitive or cognition or personality or "impulse control" or mood or paranoid or psychotic or neurologic* or nervous or "nervous system" or eating) near/1 (disorder* or illness* or disease*)):ti,ab,kw
#23 ((bipolar or behavioral or behavioural or obsessive or compulsive or panic or mood or delusional) near/1 (disorder* or illness* or disease*)):ti,ab,kw
#24 (trichotillomani* or OCD or "obsess*‐compulsi*" or GAD or "stress reaction*" or "acute stress" or neurosis or neuroses or neurotic):ti,ab,kw
#25 ("stress syndrome*" or "distress syndrome*" or "pain disorder*" or dementia or alzheimer* or epilepsy):ti,ab,kw
#26 (("substance abuse" or "substance use" or "drug abuse" or "drug use" or personality) near/2 disorder*):ti,ab,kw
#27 (sleep* near/2 (disorder* or syndrome*)):ti,ab,kw
#28 {or #10‐#27}
#29 MeSH descriptor: [Developing Countries] explode all trees
#30 ((LIC or "low* income*") near/3 (countr* or nation* or economy or economies)):ti,ab,kw
#31 (middle near/3 (countr* or nation* or economy or economies)):ti,ab,kw
#32 (LMIC or LMICs or "transition* countr*" or "third world"):ti,ab,kw
#33 ((Developing or "under developed" or underdeveloped or "less* developed") near/3 (country or countries or nation? or economy or economies or world)):ti,ab,kw
#34 ((underserved or "under served" or deprived or poor*) near/3 (country or countries or nation? or economy or economies)):ti,ab,kw
#35 ((Africa* not "African American*") or (Asia* not "Asian American*")):ti,ab,kw
#36 (Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burundi* or Burma or Burmese):ti,ab,kw
#37 (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*):ti,ab,kw
#38 (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guiana* or Guyana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian? not "American Indian?") or Indonesia* or Iran* or Iraq* or Israel*):ti,ab,kw
#39 (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Leban* or Lesotho or Liberia* or Libya* or Lithuania*):ti,ab,kw
#40 (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Maurit* or Mayotte* or Melanesia* or Mexic* or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocc* or Mozambique* or Myanmar*):ti,ab,kw
#41 (Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*):ti,ab,kw
#42 (Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Eswantini* or Syria*):ti,ab,kw
#43 (Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga* or Trinidad or Tunisia* or Turk* or Tuvalu* or Uganda* or Ukrain* or "United Arab" or Uruguay* or Uzbek* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or Wallis or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*) .ti,ab,kw
#44 MeSH descriptor: [Africa] explode all trees
#45 MeSH descriptor: [Middle East] explode all trees
#46 MeSH descriptor: [West Indies] explode all trees
#47 MeSH descriptor: [South America] explode all trees
#48 MeSH descriptor: [Atlantic Islands] explode all trees
#49 MeSH descriptor: [West Indies] explode all trees
#50 MeSH descriptor: [Central America] explode all trees
#51 MeSH descriptor: [Mexico] this term only
#52 MeSH descriptor: [Asia, Central] explode all trees
#53 MeSH descriptor: [Asia, Southeastern] explode all trees
#54 MeSH descriptor: [Asia, Western] explode all trees
#55 MeSH descriptor: [Asia] this term only
#56 MeSH descriptor: [Far East] this term only
#57 MeSH descriptor: [China] explode all trees
#58 MeSH descriptor: [Korea] explode all trees
#59 MeSH descriptor: [Taiwan] this term only
#60 MeSH descriptor: [Pacific Islands] this term only
#61 MeSH descriptor: [Melanesia] explode all trees
#62 MeSH descriptor: [Micronesia] explode all trees
#63 MeSH descriptor: [Polynesia] explode all trees
#64 MeSH descriptor: [Europe, Eastern] explode all trees
#65 MeSH descriptor: [Mediterranean Islands] explode all trees
#66 MeSH descriptor: [Gibraltar] explode all trees
#67 (Montserrat not (Spain or Espana)):ti,ab,kw
#68 Georgia:ti,ab,kw not (Atlanta or US or USA or America*):ti,ab,kw
#69 {or #29‐#68}
#70 #9 and #28 and #69
Cochrane Database of Systematic Reviews (Wiley) (Issue 2 of 12, February 2020)
Same strategy as Cochrane Central Register of Controlled Trials (listed above)
**************************************************
Embase Classic+Embase (Ovid) (1947 to 2020 February 19)
1 diabet*.tw,kw. (962654)
2 non insulin dependent diabetes mellitus/ (247861)
3 exp glucose tolerance test/ (65531)
4 exp glycosylated hemoglobin/ (122054)
5 (noninsulin*‐depend* or non‐insulin*‐depend* or noninsulin*depend* or non‐insulin*depend*).tw,kw. (14900)
6 (fasting glucose or plasma glucose or glucose tolerance test* or (glyc?emic adj2 control*)).tw,kw. (147367)
7 (HbA1c or A1C or A1c or Hb1c or ((glycated or glycosylated) adj h?emoglobin?)).tw,kw. (101570)
8 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D).tw,kw. (89033)
9 or/1‐8 (1071930)
10 exp diabetes insipidus/ (16645)
11 diabet* insipidus.tw,kw. (12538)
12 10 or 11 (18221)
13 9 not 12 [DIABETES] (1058139)
14 exp mental disease/ (2281274)
15 (mental or mentally or psychiatr* or psycho*).tw,kw. (1461167)
16 (depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS).tw,kw. (782231)
17 (schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc*).tw,kw. (201585)
18 (tardiv* adj dyskine*).tw,kw. (5672)
19 (movement adj5 (disorder or disorders)).tw,kw. (32741)
20 (somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or medically unexplained).tw,kw. (20483)
21 ((dissociative adj3 (disorder* or reaction*)) or dissociation).tw,kw. (120663)
22 (affective* adj (disorder? or disease? or illness* or symptom?)).tw,kw. (27438)
23 (PTSD or psychological trauma or combat disorder? or war disorder?).tw,kw. (33483)
24 ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).tw,kw. (41388)
25 ((stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous system or eating) adj (disorder? or illness* or disease?)).tw,kw. (220502)
26 ((bipolar or behavio?ral or obsessive or compulsive or panic or mood or delusional) adj (disorder? or illness* or disease?)).tw,kw. (104538)
27 (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).tw,kw. (77078)
28 (stress syndrome? or distress syndrome? or pain disorder? or dementia or alzheimer? or epilepsy).tw,kw. (506131)
29 ((substance abuse or "substance use" or drug abuse or "drug use") adj2 disorder?).tw,kw. (21782)
30 (personality adj2 disorder?).tw,kw. (27771)
31 (sleep? adj2 (disorder? or syndrome?)).tw,kw. (43865)
32 or/14‐31 [ALL MENTAL DISORDERS] (3671008)
33 developing country/ or low income country/ or middle income country/ (103906)
34 (low* income* adj3 (countr* or nation* or economy or economies)).tw,kw. (9305)
35 (middle income* adj3 (countr* or nation* or economy or economies)).tw,kw. (20672)
36 (low* middle adj3 (countr* or nation* or economy or economies)).tw,kw. (2420)
37 (LMIC or LMICs).tw,kw. (5426)
38 ((LIC or LICs) adj3 (countr* or nation* or economy or economies)).tw,kw. (280)
39 "transition* countr*".tw,kw. (414)
40 ((underserved or "under served" or deprived or poor*) adj3 (country or countries or nation? or economy or economies)).tw,kw. (6404)
41 ((Developing or "under developed" or underdeveloped or "less* developed" or "third world") adj3 (country or countries or nation? or economy or economies)).tw,kw. (88317)
42 ((Developing or "under developed" or underdeveloped or "less* developed") adj2 world).tw,kw. (11216)
43 ((Africa* not "African American*") or (Asia* not "Asian American*")).ti,ab,in,ad,kw. (527073)
44 (Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burundi*).ti,ab,in,ad,kw. (859975)
45 (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*).ti,ab,in,ad,kw. (2803440)
46 (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guyana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian? not "American Indian?") or Indonesia* or Iran* or Iraq* or Israel*).ti,ab,in,ad,kw. (2304242)
47 (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Lebanon or Lebanese or Lesotho or Liberia* or Libya* or Lithuania*).ti,ab,in,ad,kw. (758313)
48 (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Mauritania* or Mauritius or Mayotte* or Melanesia* or Mexico or Mexican? or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocco or Moroccan? or Mozambique* or Myanmar*).ti,ab,in,ad,kw. (443758)
49 (Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Peruvian* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*).ti,ab,in,ad,kw. (1180087)
50 (Samoa* or "Sao Toms*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Syria*).ti,ab,in,ad,kw. (506925)
51 (Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga or Trinidad or Tunisia* or Turkey or Turkish or Turkmenistan* or "Turks and Caicos" or Tuvalu* or Uganda* or Ukrain* or "United Arab Emirates" or Uruguay* or Uzbekistan* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or "Wallis and Futuna" or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*).ti,ab,in,ad,kw. (1095602)
52 exp Africa/ (345974)
53 caribbean islands/ or "anguilla (country)"/ or "antigua and barbuda"/ or aruba/ or bahamas/ or barbados/ or caribbean netherlands/ or cayman islands/ or cuba/ or dominica/ or dominican republic/ or grenada/ or haiti/ or jamaica/ or netherlands antilles/ or "saint kitts and nevis"/ or saint lucia/ or "saint vincent and the grenadines"/ or "trinidad and tobago"/ or "turks and caicos islands"/ or "virgin islands (british)"/ or "virgin islands (u.s.)"/ (24984)
54 bermuda/ or "falkland islands (malvinas)"/ or saint helena/ or "sao tome and principe"/ (645)
55 exp "South and Central America"/ (239918)
56 exp Mexico/ (48120)
57 asia/ or kazakhstan/ or kyrgyzstan/ or exp middle east/ or exp south asia/ or tajikistan/ or turkmenistan/ or uzbekistan/ or far east/ or exp china/ or korea/ or mongolia/ or philippines/ or exp southeast asia/ or taiwan/ (868588)
58 exp pacific islands/ (49526)
59 exp indian ocean/ (5624)
60 exp Eastern Europe/ or gibraltar/ or malta/ (237378)
61 Georgia.ti,ab. not "georgia (u.s.)"/ (11290)
62 (Montserrat not (Spain or Espana)).ti,ab. (133)
63 or/33‐62 [LMICs based on ODA DAC flows 2003 ‐ 2020] (9363913)
64 exp randomized controlled trial/ (594543)
65 exp double‐blind procedure/ (172315)
66 exp single‐blind procedure/ (38045)
67 exp crossover‐procedure/ (62550)
68 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. (240142)
69 placebo/ (357031)
70 placebo*.tw. (308687)
71 randomization/ (86159)
72 trial.ti. (299892)
73 clinical trial*.tw. (513726)
74 controlled clinical trial/ (463280)
75 or/64‐74 [RCT or CCT] (1643852)
76 exp animals/ not exp humans/ (5375974)
77 exp nonhuman/ not exp human/ (4556888)
78 exp experimental animal/ (692422)
79 exp veterinary medicine/ (47187)
80 animal experiment/ (2490388)
81 or/76‐80 [Animal studies] (7613254)
82 75 not 81 [Final RCT search] (1531638)
83 13 and 32 and 63 and 82 [Diabetes + Mental Illness + LMICs + RCTs] (2007)
84 13 and 32 and 63 (30736)
85 limit 84 to (meta analysis or "systematic review") (705)
86 limit 84 to "reviews (maximizes specificity)" (575)
87 85 or 86 [Diabetes + Mental Illness + LMICs + Systematic Reviews] (817)
88 83 or 87 [Diabetes + Mental Illness + LMICs + SR or RCTs] (2691)
89 exp juvenile/ not exp adult/ (2494930)
90 88 not 89 [Child‐only studies removed] (2594)
**************************************************
Global Health (Ovid) (1910 to 2020 Week 08)
1 diabet*.tw,id. (134991)
2 exp diabetes mellitus/ (61136)
3 haemoglobin a1/ (2986)
4 glucose tolerance test/ (2037)
5 (fasting glucose or plasma glucose or glucose tolerance test* or (glyc?emic adj2 control*)).tw,id. (27312)
6 (HbA1c or A1C or A1c or Hb1c or ((glycated or glycosylated) adj h?emoglobin?)).tw,id. (10866)
7 (noninsulin*‐depend* or non‐insulin*‐depend* or noninsulin*depend* or non‐insulin*depend*).tw,id. (2035)
8 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D).tw,id. (9786)
9 or/1‐8 (147060)
10 diabetes insipidus/ (134)
11 diabet* insipidus.tw,id. (344)
12 10 or 11 (344)
13 9 not 12 [DIABETES] (146716)
14 exp mental disorders/ (70494)
15 epilepsy/ (5151)
16 (mental or mentally or psychiatr* or psycho* or depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS).tw,id. (179666)
17 (schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc*).tw,id. (13368)
18 (tardiv* adj dyskine*).tw,id. (92)
19 (movement adj5 (disorder or disorders)).tw,id. (685)
20 (somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or medically unexplained).tw,id. (1346)
21 ((dissociative adj3 (disorder* or reaction*)) or dissociation).tw,id. (4331)
22 (affective* adj (disorder? or disease? or illness* or symptom?)).tw,id. (1901)
23 (PTSD or post‐trauma* or posttrauma* or combat disorder? or war disorder?).tw,id. (3749)
24 ((stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous system or eating) adj (disorder? or illness* or disease?)).tw,id. (28731)
25 ((bipolar or behavio?ral or obsessive or compulsive or panic or mood or delusional) adj (disorder? or illness* or disease?)).tw,id. (4360)
26 (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).tw,id. (3491)
27 (stress syndrome? or distress syndrome? or pain disorder? or dementia or alzheimer? or epilepsy).tw,id. (26915)
28 or/14‐27 [ALL MENTAL DISORDERS] (227901)
29 exp developing countries/ (971866)
30 exp least developed countries/ (124904)
31 exp pacific islands/ (14821)
32 exp west asia/ (127771)
33 exp south east asia/ (104555)
34 albania/ or bahamas/ or belarus/ or bosnia‐hercegovina/ or bulgaria/ or exp china/ or croatia/ or czech republic/ or czechoslovakia/ or estonia/ or gibraltar/ or hungary/ or israel/ or kosovo/ or latvia/ or lithuania/ (222635)
35 malta/ or moldova/ or montenegro/ or "republic of macedonia"/ or netherlands antilles/ or poland/ or exp russia/ or serbia/ or "serbia and montenegro"/ or slovenia/ or "turks and caicos islands"/ or ukraine/ or ussr/ or yugoslavia/ (68286)
36 (low* income* adj3 (countr* or nation* or economy or economies)).tw,id. (4204)
37 (middle income* adj3 (countr* or nation* or economy or economies)).tw,id. (9132)
38 (low* middle adj3 (countr* or nation* or economy or economies)).tw,id. (758)
39 (LMIC or LMICs).tw,id. (1993)
40 ((LIC or LICs) adj3 (countr* or nation* or economy or economies)).tw,id. (94)
41 "transition* countr*".tw,id. (207)
42 ((underserved or "under served" or deprived or poor*) adj3 (country or countries or nation? or economy or economies)).tw,id. (3126)
43 ((Developing or "under developed" or underdeveloped or "less* developed" or "third world") adj3 (country or countries or nation? or economy or economies)).tw,id. (980941)
44 ((Developing or "under developed" or underdeveloped or "less* developed") adj2 world).tw,id. (4265)
45 ((Africa* not "African American*") or (Asia* not "Asian American*")).ti,ab,in,gl. (317873)
46 (Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burma or Burmese or Burundi*).ti,ab,in,gl. (244028)
47 (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*).ti,ab,in,gl. (502797)
48 (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guiana* or Guyana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian? not "American Indian?") or Indonesia* or Iran* or Iraq* or Israel*).ti,ab,in,gl. (518060)
49 (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Lebanon or Lebanese or Lesotho or Liberia* or Libya* or Lithuania*).ti,ab,in,gl. (119951)
50 (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Mauritania* or Mauritius or Mayotte* or Melanesia* or Mexico or Mexican? or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocco or Moroccan? or Mozambique* or Myanmar*).ti,ab,in,gl. (117136)
51 (Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*).ti,ab,in,gl. (224570)
52 (Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Eswantini or Syria*).ti,ab,in,gl. (105009)
53 (Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga* or Trinidad or Tunisia* or Turkey or Turkish or Turkmenistan* or "Turks and Caicos" or Tuvalu* or Uganda* or Ukrain* or "United Arab Emirates" or Uruguay* or Uzbek* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or "Wallis and Futuna" or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*).ti,ab,in,gl. (231647)
54 Georgia.ti,ab. not (exp georgia/ or exp "south georgia and the south sandwich islands"/) (1422)
55 (Montserrat not (Spain or Espana)).ti,ab,gl. (78)
56 or/29‐55 [LMICs based on ODA DAC flows 2003 ‐ 2020] (1934914)
57 randomized controlled trials/ (38498)
58 clinical trials/ (22567)
59 placebos/ (2490)
60 trial.ti. (33503)
61 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. (27659)
62 placebo*.tw. (36362)
63 (random* not (random sampl* or random digit* or random effect* or random survey or random regression)).tw. (189898)
64 or/57‐63 [RCT] (223042)
65 systematic reviews/ (29028)
66 meta‐analysis/ (26440)
67 (((systematic or evidence) adj3 review?) or evidence synthes?s).ti. (25389)
68 (medline or pubmed or "literature search*" or "search strateg*" or "search filter").ab. (36432)
69 or/65‐68 [Systematic Reviews] (58095)
70 64 or 69 (268756)
71 (rat or rats or mice or mouse).ti. (217473)
72 70 not 71 [RCTs or Systematic reviews Animal studies removed] (252216)
73 9 and 28 and 56 and 72 [Diabetes + Mental Health + LMICs + RCTs or SRs] (429)
**************************************************
LILACs (BIREME / PAHO / WHO) (all available dates)
http://pesquisa.bvsalud.org/ searched using the following strings in the TW (textword) fields
(tw:(diabet*)) AND (tw:(mental* or psych* or depress* or anxi* or phobia* or schizo* or somatoform* or dementia or alzheimer* or epilep* or PTSD or OCD or CBT or neuros* or neurotic or bipolar or mood or stress or behavioral or behavioural or personality))
Results were
‐ Limited to LILACs database
‐ Limited to Type of Study selections: Controlled clinical trial, Systematic reviews, Health technology assessment
‐ 132 records found
**************************************************
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily (1946 to February 19, 2020)
1 diabet*.tw,kf. (617899)
2 exp Diabetes mellitus/ (416391)
3 Glucose Tolerance Test/ (34478)
4 Glycated Hemoglobin A/ (34099)
5 (noninsulin*‐depend* or non‐insulin*‐depend* or noninsulin*depend* or non‐insulin*depend*).tw,kf. (12285)
6 (fasting glucose or plasma glucose or glucose tolerance test* or (glyc?emic adj2 control*)).tw,kf. (94608)
7 (HbA1c or A1C or A1c or Hb1c or ((glycated or glycosylated) adj h?emoglobin?)).tw,kf. (52667)
8 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D).tw,kf. (51521)
9 or/1‐8 (717246)
10 exp Diabetes Insipidus/ (7820)
11 diabet* insipidus.tw,kf. (8772)
12 10 or 11 (10779)
13 9 not 12 [DIABETES] (707992)
14 exp Mental Disorders/ (1214121)
15 exp Behavioral Symptoms/ (359225)
16 exp Epilepsy/ (109435)
17 (mental or mentally or psychiatr* or psycho* or depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS).tw,kf. (1414264)
18 (schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc*).tw,kf. (145120)
19 (tardiv* adj dyskine*).tw,kf. (4321)
20 (movement adj5 (disorder or disorders)).tw,kf. (18663)
21 (somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or medically unexplained).tw,kf. (13793)
22 ((dissociative adj3 (disorder* or reaction*)) or dissociation).tw,kf. (109114)
23 (affective* adj (disorder? or disease? or illness* or symptom?)).tw,kf. (19168)
24 (PTSD or psychological trauma or psychotrauma* or combat disorder? or war disorder?).tw,kf. (25269)
25 ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).tw,kf. (33148)
26 ((stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous system or eating) adj (disorder? or illness* or disease?)).tw,kf. (154901)
27 ((bipolar or behavio?ral or obsessive or compulsive or panic or mood or delusional) adj (disorder? or illness* or disease?)).tw,kf. (68956)
28 (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).tw,kf. (53670)
29 (stress syndrome? or distress syndrome? or pain disorder? or dementia or alzheimer? or epilepsy).tw,kf. (341613)
30 ((substance abuse or "substance use" or drug abuse or "drug use") adj2 disorder?).tw,kf. (16508)
31 (personality adj2 disorder?).tw,kf. (20045)
32 (sleep? adj2 (disorder? or syndrome?)).tw,kf. (25842)
33 or/14‐32 [ALL MENTAL DISORDERS] (2639725)
34 Developing Countries/ (73781)
35 (low* income* adj3 (countr* or nation* or economy or economies)).tw,kf. (7326)
36 (middle income* adj3 (countr* or nation* or economy or economies)).tw,kf. (17467)
37 (low* middle adj3 (countr* or nation* or economy or economies)).tw,kf. (1743)
38 (LMIC or LMICs).tw,kf. (4349)
39 ((LIC or LICs) adj3 (countr* or nation* or economy or economies)).tw,kf. (184)
40 "transition* countr*".tw,kf. (314)
41 ((underserved or "under served" or deprived or poor*) adj3 (country or countries or nation? or economy or economies)).tw,kf. (5129)
42 ((Developing or "under developed" or underdeveloped or "less* developed" or "third world") adj3 (country or countries or nation? or economy or economies)).tw,kf. (94111)
43 ((Developing or "under developed" or underdeveloped or "less* developed") adj2 world).tw,kf. (8849)
44 ((Africa* not "African American*") or (Asia* not "Asian American*")).ti,ab,in,kf. (381012)
45 (Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burma or Burmese or Burundi*).ti,ab,in,kf. (538487)
46 (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*).ti,ab,in,kf. (1989112)
47 (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guiana* or Guyana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian? not "American Indian?") or Indonesia* or Iran* or Iraq* or Israel*).ti,ab,in,kf. (1463663)
48 (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Lebanon or Lebanese or Lesotho or Liberia* or Libya* or Lithuania*).ti,ab,in,kf. (551861)
49 (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Mauritania* or Mauritius or Mayotte* or Melanesia* or Mexico or Mexican? or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocco or Moroccan? or Mozambique* or Myanmar*).ti,ab,in,kf. (298191)
50 (Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*).ti,ab,in,kf. (572140)
51 (Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Eswantini or Syria*).ti,ab,in,kf. (340632)
52 (Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga* or Trinidad or Tunisia* or Turkey or Turkish or Turkmenistan* or "Turks and Caicos" or Tuvalu* or Uganda* or Ukrain* or "United Arab Emirates" or Uruguay* or Uzbek* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or "Wallis and Futuna" or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*).ti,ab,in,kf. (712952)
53 exp Africa/ (259330)
54 west indies/ or "antigua and barbuda"/ or bahamas/ or barbados/ or "british virgin islands"/ or cuba/ or dominica/ or dominican republic/ or grenada/ or haiti/ or jamaica/ or saint lucia/ or "saint vincent and the grenadines"/ or "saint kitts and nevis"/ or "trinidad and tobago"/ (19090)
55 central america/ or costa rica/ or el salvador/ or guatemala/ or honduras/ or nicaragua/ or exp panama/ or mexico/ (51149)
56 exp south america/ (156507)
57 exp Atlantic Islands/ (1561)
58 asia/ or exp asia, central/ or exp asia, southeastern/ or exp asia, western/ or far east/ or exp china/ or exp korea/ or Taiwan/ (636769)
59 exp Middle East/ (130228)
60 pacific islands/ or exp melanesia/ or micronesia/ or palau/ or polynesia/ or exp samoa/ or tonga/ (13509)
61 exp Europe, Eastern/ or Cyprus/ or Malta/ or Gibraltar/ (178986)
62 Georgia.ti,ab. not Georgia/ (5791)
63 (Montserrat not (Spain or Espana)).ti,ab. (120)
64 or/34‐63 [LMICs based on ODA DAC flows 2003 ‐ 2020] (6294338)
65 randomized controlled trial.pt. (500092)
66 controlled clinical trial.pt. (93530)
67 randomized.ab. (469307)
68 placebo.ab. (204842)
69 clinical trials as topic.sh. (190093)
70 randomly.ab. (327039)
71 trial.ti. (212852)
72 65 or 66 or 67 or 68 or 69 or 70 or 71 (1267933)
73 exp animals/ not humans.sh. (4670180)
74 72 not 73 [RCTs ‐ Cochrane RCT2 Precision Maximising] (1166197)
75 13 and 33 and 64 and 74 [Diabetes + Mental Illness + LMICs + RCTs] (1075)
76 13 and 33 and 64 (14496)
77 limit 76 to systematic reviews (263)
78 75 or 77 [Diabetes + Mental Illness + LMICs + RCTs or Systematic Reviews] (1295)
79 (exp Child/ or Adolescent/ or exp Infant/) not exp Adult/ (1849636)
80 78 not 79 [Final Search child‐only studies removed] (1259)
**************************************************
PakMediNet (PakCyber) (all available dates)
The ‘Simple’ search interface was used as the Advanced search did not function when the search was being developed in April 2019 or February 2020. We conducted 108 short searches, each comprising three terms (one from each column below) for example the first search string was Diabetes Mental Trial, the last search string was Diabetes Personality Synthesis. The database ‘ANDed’ the three terms together. Most searches resulted in 0 records retrieved, but over 108 searches 27 unique studies were identified.
| 1st word | 2nd word | 3rd word |
| Diabetes | Mental, Mentally, Psychiatric, Depression, Depressed, Depressive, Anxiety, Anxious, Phobia, Schizophrenia, Somatoform, Dementia, Alzheimer, Epilepsy, Neurosis, Neuroses, Neurotic, Psychosis, Psychoses, Psychotic, Psychological, Bipolar, Mood, Stress, Behavioral, Behavioural, Personality | Trial Trials Review Synthesis |
**************************************************
PsycINFO (Ovid) (1806 to February Week 2 2020)
1 diabet*.tw,id. (30939)
2 exp Diabetes mellitus/ (8188)
3 Hemoglobin/ (705)
4 *glucose/ or *glucose metabolism/ or blood sugar/ (4713)
5 (fasting glucose or plasma glucose or glucose tolerance test* or (glyc?emic adj2 control*)).tw,id. (3925)
6 (HbA1c or A1C or A1c or Hb1c or ((glycated or glycosylated) adj h?emoglobin?)).tw,id. (2990)
7 (noninsulin*‐depend* or non‐insulin*‐depend* or noninsulin*depend* or non‐insulin*depend*).tw,id. (285)
8 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D).tw,id. (2159)
9 or/1‐8 (36183)
10 exp Diabetes Insipidus/ (173)
11 diabet* insipidus.tw,id. (302)
12 10 or 11 (341)
13 9 not 12 [DIABETES] (35846)
14 exp mental disorders/ (839617)
15 exp behavior disorders/ (81946)
16 exp epilepsy/ (27166)
17 (mental or mentally or psychiatr* or psycho* or depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS).tw,id. (1659030)
18 (schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc*).tw,id. (130807)
19 (tardiv* adj dyskine*).tw,id. (2964)
20 (movement adj5 (disorder or disorders)).tw,id. (6872)
21 (somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or medically unexplained).tw,id. (16663)
22 ((dissociative adj3 (disorder* or reaction*)) or dissociation).tw,id. (20238)
23 (affective* adj (disorder? or disease? or illness* or symptom?)).tw,id. (20305)
24 (PTSD or post‐trauma* or posttrauma* or combat disorder? or war disorder?).tw,id. (50990)
25 ((stress or cognitive or cognition or personality or impulse control or mood or paranoid or psychotic or neurologic* or nervous or nervous system or eating) adj (disorder? or illness* or disease?)).tw,id. (135073)
26 ((bipolar or behavio?ral or obsessive or compulsive or panic or mood or delusional) adj (disorder? or illness* or disease?)).tw,id. (72391)
27 (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).tw,id. (59174)
28 (stress syndrome? or distress syndrome? or pain disorder? or dementia or alzheimer? or epilepsy).tw,id. (133228)
29 or/14‐28 [ALL MENTAL DISORDERS] (2083627)
30 developing countries/ (5398)
31 (low* income* adj3 (countr* or nation* or economy or economies)).tw,id. (1582)
32 (middle income* adj3 (countr* or nation* or economy or economies)).tw,id. (3429)
33 (low* middle adj3 (countr* or nation* or economy or economies)).tw,id. (355)
34 (LMIC or LMICs).tw,id. (823)
35 ((LIC or LICs) adj3 (countr* or nation* or economy or economies)).tw,id. (25)
36 "transition* countr*".tw,id. (138)
37 ((underserved or "under served" or deprived or poor*) adj3 (country or countries or nation? or economy or economies)).tw,id. (1253)
38 ((Developing or "under developed" or underdeveloped or "less* developed" or "third world") adj3 (country or countries or nation? or economy or economies)).tw,id. (11129)
39 ((Developing or "under developed" or underdeveloped or "less* developed") adj2 world).tw,id. (1636)
40 ((Africa* not "African American*") or (Asia* not "Asian American*")).ti,ab,in,lo,id. (71727)
41 (Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burma or Burmese or Burundi*).ti,ab,in,lo,id. (83121)
42 (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*).ti,ab,in,lo,id. (174350)
43 (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guiana* or Guyana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian? not "American Indian?") or Indonesia* or Iran* or Iraq* or Israel*).ti,ab,in,lo,id. (228357)
44 (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Lebanon or Lebanese or Lesotho or Liberia* or Libya* or Lithuania*).ti,ab,in,lo,id. (60339)
45 (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Mauritania* or Mauritius or Mayotte* or Melanesia* or Mexico or Mexican? or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocco or Moroccan? or Mozambique* or Myanmar*).ti,ab,in,lo,id. (63650)
46 (Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*).ti,ab,in,lo,id. (92033)
47 (Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Eswantini* or Syria*).ti,ab,in,lo,id. (47781)
48 (Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga* or Trinidad or Tunisia* or Turkey or Turkish or Turkmenistan* or "Turks and Caicos" or Tuvalu* or Uganda* or Ukrain* or "United Arab Emirates" or Uruguay* or Uzbek* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or "Wallis and Futuna" or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*).ti,ab,in,lo,id. (97550)
49 (Montserrat not (Spain or Espana)).ti,ab,in,lo,id. (48)
50 Georgia.ti,ab,in,lo. not (Atlanta or US or USA).in,lo,id. (6857)
51 or/30‐50 [LMICs] (758816)
52 exp clinical trials/ or experimental design/ (22857)
53 exp treatment effectiveness evaluation/ (24358)
54 exp mental health program evaluation/ (2098)
55 exp random sampling/ (834)
56 randomi*.tw. (84174)
57 (clinic* adj4 trial*).tw. (36685)
58 (random* adj5 (assign* or allocat* or assort*)).tw. (43862)
59 (crossover or cross‐over).tw. (10106)
60 ((singl* or doubl* or tripl* or trebl*) adj (blind* or mask*)).tw. (25795)
61 exp placebo/ (5484)
62 placebo*.tw. (40059)
63 or/52‐62 [Trials] (193158)
64 13 and 29 and 51 and 63 [Diabetes + Mental disorders + LMICs + Trials] (289)
65 13 and 29 and 51 (3555)
66 limit 65 to ("0830 systematic review" or 1200 meta analysis or 1300 metasynthesis) (103)
67 64 or 66 (368)
68 ((child* or adolescen*) not adult*).ag. (498966)
69 67 not 68 [RCTs or Systematic Reviews ‐ Diabetes + Mental disorders + LMICs ‐ child‐only studies removed] (361)
**************************************************
PubMed (NLM) (1946 – present)
Search Query Items found
#7 Search (#5 and #6) 96
#6 Search ((((pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb])))) 3556748
#5 Search (#1 and #4) 500
#4 Search (#2 or #3) 1118026
#3 Search ((Namibia*[Title/Abstract] OR Nauru*[Title/Abstract] OR Niue*[Title/Abstract] OR Nepal*[Title/Abstract] OR "Netherlands Antilles*"[Title/Abstract] OR "Dutch Antilles"[Title/Abstract] OR "New Caledonia*"[Title/Abstract] OR Nicaragua*[Title/Abstract] OR Niger[Title/Abstract] OR Nigeria*[Title/Abstract] OR Oman*[Title/Abstract] OR Pakistan*[Title/Abstract] OR Palau*[Title/Abstract] OR Palestin*[Title/Abstract] OR Panama[Title/Abstract] OR "Papua New Guinea*"[Title/Abstract] OR Paraguay[Title/Abstract] OR Peru* OR[Title/Abstract] OR Philippines*[Title/Abstract] OR Pilipin*[Title/Abstract] OR Filipin*[Title/Abstract] OR Poland[Title/Abstract] OR Polish[Title/Abstract] OR Polynesia*[Title/Abstract] OR Qatar*[Title/Abstract] OR Romania*[Title/Abstract] OR Russia*[Title/Abstract] OR Rwanda*) AND Title/Abstract)) OR (Samoa*[Title/Abstract] OR "Sao Tome*"[Title/Abstract] OR Principe*[Title/Abstract] OR Saudi[Title/Abstract] OR Senegal*[Title/Abstract] OR Serbia*[Title/Abstract] OR Seychelles[Title/Abstract] OR "Sierra Leone"[Title/Abstract] OR Singapor*[Title/Abstract] OR Slovak*[Title/Abstract] OR Sloven*[Title/Abstract] OR "Solomon Islands"[Title/Abstract] OR Somalia*[Title/Abstract] OR "Sri Lanka*"[Title/Abstract] OR "S* Kitts[Title/Abstract] AND Nevis"[Title/Abstract] OR "S* Lucia"[Title/Abstract] OR "S* Helena"[Title/Abstract] OR "S* Vincent[Title/Abstract] AND the Grenadines"[Title/Abstract] OR "South America*"[Title/Abstract] OR Sudan*[Title/Abstract] OR Suriname*[Title/Abstract] OR Swaziland*[Title/Abstract] OR Eswantini*[Title/Abstract] OR Syria*[Title/Abstract])) OR (Taiwan* OR Taipei* OR Tajikistan* OR Tanzania* OR Thai* OR Timor* OR Tobago OR Togo OR Tokelau OR Tonga* OR Trinidad OR Tunisia* OR Turkey OR Turkish OR Turkmenistan* OR "Turks and Caicos" OR Tuvalu* OR Uganda* OR Ukrain* OR "United Arab Emirates" OR Uruguay* OR Uzbek* OR Vanuatu* OR Venezuela* OR Vietnam* OR "Virgin Is*" OR "Wallis and Futuna" OR Futuna OR "West Bank" OR Gaza OR Yemen* OR Zambia* OR Zimbabw*))))) 892332
#2 Search ("Developing Countr*"[Title/Abstract] OR "developing nation*"[Title/Abstract] OR "developing econom*"[Title/Abstract] OR "developing world"[Title/Abstract] OR "third world"[Title/Abstract] OR LMIC[Title/Abstract] OR LMICs[Title/Abstract])) OR ((Africa* NOT "African American*") AND Title/Abstract OR (Asia* NOT "Asian American*") AND Title/Abstract)) OR (Afghanistan*[Title/Abstract] OR Albania*[Title/Abstract] OR Algeria*[Title/Abstract] OR Angola*[Title/Abstract] OR Anguilla*[Title/Abstract] OR Antigua[Title/Abstract] OR Barbuda[Title/Abstract] OR Argentin*[Title/Abstract] OR Armenia*[Title/Abstract] OR Aruba*[Title/Abstract] OR Azerbaijan*[Title/Abstract] OR Bahamas[Title/Abstract] OR Bahrain*[Title/Abstract] OR Bangladesh*[Title/Abstract] OR Barbados[Title/Abstract] OR Belarus*[Title/Abstract] OR Belize*[Title/Abstract] OR Benin*[Title/Abstract] OR Bermuda*[Title/Abstract] OR Bhutan*[Title/Abstract] OR Bolivia*[Title/Abstract] OR Bosnia*[Title/Abstract] OR Herzegovina[Title/Abstract] OR Borneo[Title/Abstract] OR Botswana*[Title/Abstract] OR Brazil*[Title/Abstract] OR Brunei*[Title/Abstract] OR Bulgaria*[Title/Abstract] OR "Burkina Faso"[Title/Abstract] OR Burundi* OR [Title/Abstract] OR Burma OR [Title/Abstract] OR Burmese OR [Title/Abstract])) OR ((Cambodia*[Title/Abstract] OR Cameroon*[Title/Abstract] OR "Cape Verde*"[Title/Abstract] OR "Cabo Verde*"[Title/Abstract] OR Caribbean*[Title/Abstract] OR "Cayman Is*"[Title/Abstract] OR Chad[Title/Abstract] OR Chile*[Title/Abstract] OR China[Title/Abstract] OR Chinese[Title/Abstract] OR (Colombia* NOT "British Colombia*") AND Title/Abstract OR Comoros[Title/Abstract] OR Congo[Title/Abstract] OR "Cook Island*"[Title/Abstract] OR "Costa Rica*"[Title/Abstract] OR "ivory coast"[Title/Abstract] OR "cote d'ivoire"[Title/Abstract] OR Croat*[Title/Abstract] OR Cuba*[Title/Abstract] OR Cyprus[Title/Abstract] OR Cypriot*[Title/Abstract] OR Czech*[Title/Abstract] OR Djibouti*[Title/Abstract] OR Dominica*))) AND (Ecuador*[Title/Abstract] OR Egypt*[Title/Abstract] OR "El Salvador"[Title/Abstract] OR Eritrea*[Title/Abstract] OR Estonia*[Title/Abstract] OR Ethiopia*[Title/Abstract] OR "Falklands Is*"[Title/Abstract] OR Fiji*[Title/Abstract] OR Gabon*[Title/Abstract] OR Gambia*[Title/Abstract] OR Ghana*[Title/Abstract] OR Gibralta*[Title/Abstract] OR Grenada*[Title/Abstract] OR Guatemala*[Title/Abstract] OR Guinea*[Title/Abstract] OR Guiana*[Title/Abstract] OR Guyana*[Title/Abstract] OR Haiti*[Title/Abstract] OR Hondura*[Title/Abstract] OR "Hong Kong*"[Title/Abstract] OR Hungary[Title/Abstract] OR Hungarian*[Title/Abstract] OR India[Title/Abstract] OR (Indian* NOT "American Indian*") AND Title/Abstract OR Indonesia*[Title/Abstract] OR Iran*[Title/Abstract] OR Iraq*[Title/Abstract] OR Israel*[Title/Abstract])) OR (Jamaica*[Title/Abstract] OR Jordan*[Title/Abstract] OR Kazakhstan*[Title/Abstract] OR Kenya*[Title/Abstract] OR Kiribati*[Title/Abstract] OR Korea*[Title/Abstract] OR DPRK[Title/Abstract] OR Kosovo*[Title/Abstract] OR Kuwait*[Title/Abstract] OR Kyrgyz*[Title/Abstract] OR "Lao PDR"[Title/Abstract] OR "Lao People*"[Title/Abstract] OR Laos[Title/Abstract] OR Laotian[Title/Abstract] OR Latvia*[Title/Abstract] OR Lebanon[Title/Abstract] OR Lebanese[Title/Abstract] OR Lesotho[Title/Abstract] OR Liberia*[Title/Abstract] OR Libya*[Title/Abstract] OR Lithuania*[Title/Abstract])) OR (Macao*[Title/Abstract] OR Macau[Title/Abstract] OR Macedonia*[Title/Abstract] OR Madagasca*[Title/Abstract] OR Malawi*[Title/Abstract] OR Malaysia*[Title/Abstract] OR Maldives[Title/Abstract] OR Mali[Title/Abstract] OR Malta[Title/Abstract] OR Maltese[Title/Abstract] OR "Marshall Islands"[Title/Abstract] OR Mauritania*[Title/Abstract] OR Mauritius[Title/Abstract] OR Mayotte*[Title/Abstract] OR Melanesia*[Title/Abstract] OR Mexico[Title/Abstract] OR Mexican?[Title/Abstract] OR Micronesia*[Title/Abstract] OR Moldova*[Title/Abstract] OR Mongolia*[Title/Abstract] OR Montenegro*[Title/Abstract] OR Morocco[Title/Abstract] OR Moroccan?[Title/Abstract] OR Mozambique*[Title/Abstract] OR Myanmar*[Title/Abstract])))) 251095
#1 Search (((Diabet*) AND (mental[Title/Abstract] OR psychiatric[Title/Abstract] OR psychological[Title/Abstract] OR depression[Title/Abstract] OR anxiety[Title/Abstract] OR phobia*[Title/Abstract] OR schizophrenia[Title/Abstract] OR psychosis[Title/Abstract] OR psychotic[Title/Abstract] OR psychoses[Title/Abstract] OR bipolar[Title/Abstract] OR somatoform[Title/Abstract] OR stress[Title/Abstract] OR mood*[Title/Abstract] OR behavioral[Title/Abstract] OR neuroses[Title/Abstract] OR neurosis[Title/Abstract] OR personality[Title/Abstract] OR Dementia[Title/Abstract] OR Alzheimer*[Title/Abstract] OR OCD[Title/Abstract] OR CBT[Title/Abstract] OR panic[Title/Abstract])))) AND (trial[Title/Abstract] OR RCT[Title/Abstract] OR randomized[Title/Abstract] OR placebo[Title/Abstract] OR blind[Title/Abstract] OR blinding[Title/Abstract] OR review[Title/Abstract] OR meta‐analysis[Title/Abstract]))) 14122
**************************************************
Grey Literature
Databases:
Conference Proceedings Citation Index‐ Science (Clarivate Analytics Web of Science) 1990‐present
ProQuest Dissertations & Theses A&I 1743 – present
Trials Registers
ClinicalTrials.gov (U.S. NIH)
International Clinical Trials Registry Platform (WHO)
ISRCTN registry (Springer Nature)
Conference Proceedings Citation Index‐ Science (Clarivate Analytics Web of Science) (1990‐present)
# 32 70 #30 not #31
# 31 173,692 TI=(rat or rats or mice or mouse)
# 30 70 #3 and #13 and #26 and #29
# 29 424,539 #27 or #28
# 28 127,812 TI= (Literature review* or systematic near/2 review* or synthesis or meta‐analys* or "meta analysis") OR TS= ("Search filter*" or "search strateg*" or "literature search*" or medline or pubmed)
# 27 302,186 TS=("clinical trial*" or "controlled trial*" or random* or placebo* or "single blind*" or "double blind" or crossover)
# 26 3,746,723 #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14
# 25 2,439 TOPIC: (Georgia not (Atlanta or US or USA)) OR TOPIC: (Montserrat not (Spain or Espana))
# 24 380,478 TS=(Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga* or Trinidad or Tunisia* or Turkey or Turkish or Turkmenistan* or "Turks and Caicos" or Tuvalu* or Uganda* or Ukrain* or "United Arab Emirates" or Uruguay* or Uzbek* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or "Wallis and Futuna" or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*) OR AD=(Taiwan* or Taipei* or Tajikistan* or Tanzania* or Thai* or Timor* or Tobago or Togo or Tokelau or Tonga or Trinidad or Tunisia* or Turkey or Turkish or Turkmenistan* or "Turks and Caicos" or Tuvalu* or Uganda* or Ukrain* or "United Arab Emirates" or Uruguay* or Uzbekistan* or Vanuatu* or Venezuela* or Vietnam* or "Virgin Is*" or "Wallis and Futuna" or Futuna or "West Bank" or Gaza or Yemen* or Zambia* or Zimbabw*)
# 23 184,769 TS=(Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Eswantini* or Syria*) OR AD=(Samoa* or "Sao Tome*" or Principe* or Saudi or Senegal* or Serbia* or Seychelles or "Sierra Leone" or Singapor* or Slovak* or Sloven* or "Solomon Islands" or Somalia* or "Sri Lanka*" or "S* Kitts and Nevis" or "S* Lucia" or "S* Helena" or "S* Vincent and the Grenadines" or "South America*" or Sudan* or Suriname* or Swaziland* or Syria*)
# 22 537,045 TS=(Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*) OR AD=(Namibia* or Nauru* or Niue* or Nepal* or "Netherlands Antilles*" or "Dutch Antilles" or "New Caledonia*" or Nicaragua* or Niger or Nigeria* or Oman* or Pakistan* or Palau* or Palestin* or Panama or "Papua New Guinea*" or Paraguay or Peru* or Philippines* or Pilipin* or Filipin* or Poland or Polish or Polynesia* or Qatar* or Romania* or Russia* or Rwanda*)
# 21 196,984 TOPIC: (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Mauritania* or Mauritius or Mayotte* or Melanesia* or Mexico or Mexican$ or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocco or Moroccan$ or Mozambique* or Myanmar*) OR ADDRESS: (Macao* or Macau or Macedonia* or Madagasca* or Malawi* or Malaysia* or Maldives or Mali or Malta or Maltese or "Marshall Islands" or Mauritania* or Mauritius or Mayotte* or Melanesia* or Mexico or Mexican$ or Micronesia* or Moldova* or Mongolia* or Montenegro* or Morocco or Moroccan$ or Mozambique* or Myanmar*)
# 20 315,716 TOPIC: (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Lebanon or Lebanese or Lesotho or Liberia* or Libya* or Lithuania*) OR ADDRESS: (Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or DPRK or Kosovo* or Kuwait* or Kyrgyz* or "Lao PDR" or "Lao People*" or Laos or Laotian or Latvia* or Lebanon or Lebanese or Lesotho or Liberia* or Libya* or Lithuania*)
# 19 673,360 TOPIC: (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guyana* or Guiana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian$ not "American Indian$") or Indonesia* or Iran* or Iraq* or Israel*) OR ADDRESS: (Ecuador* or Egypt* or "El Salvador" or Eritrea* or Estonia* or Ethiopia* or "Falklands Is*" or Fiji* or Gabon* or Gambia* or Ghana* or Gibralta* or Grenada* or Guatemala* or Guinea* or Guyana* or Guiana* or Haiti* or Hondura* or "Hong Kong*" or Hungary or Hungarian* or India or (Indian$ not "American Indian$") or Indonesia* or Iran* or Iraq* or Israel*)
# 18 1,404,605 TOPIC: (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*) OR ADDRESS: (Cambodia* or Cameroon* or "Cape Verde*" or "Cabo Verde*" or Caribbean* or "Cayman Is*" or Chad or Chile* or China or Chinese or (Colombia* not "British Colombia*") or Comoros or Congo or "Cook Island*" or "Costa Rica*" or "ivory coast" or "cote d'ivoire" or Croat* or Cuba* or Cyprus or Cypriot* or Czech* or Djibouti* or Dominica*)
# 17 268,351 TS=(Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burma or Burmese or Burundi*) OR AD=(Afghanistan* or Albania* or Algeria* or Angola* or Anguilla* or Antigua or Barbuda or Argentin* or Armenia* or Aruba* or Azerbaijan* or Bahamas or Bahrain* or Bangladesh* or Barbados or Belarus* or Belize* or Benin* or Bermuda* or Bhutan* or Bolivia* or Bosnia* or Herzegovina or Borneo or Botswana* or Brazil* or Brunei* or Bulgaria* or "Burkina Faso" or Burma or Burmese or Burundi*)
# 16 85,221 TOPIC: ((Developing or "under developed" or underdeveloped or "less* developed") NEAR/2 world) OR TOPIC: (((Africa* not "African American*") or (Asia* not "Asian American*"))) OR ADDRESS: (((Africa* not "African American*") or (Asia* not "Asian American*")))
# 15 21,822 TOPIC: (LMIC or LMICs or "transition* countr*") OR TOPIC: ((underserved or "under served" or deprived or poor*) NEAR/3 (country or countries or nation* or economy or economies)) OR TOPIC: ((Developing or "under developed" or underdeveloped or "less* developed" or "third world") NEAR/3 (country or countries or nation$ or economy or economies))
# 14 960 TOPIC: ("low* income*" NEAR/3 (countr* or nation* or economy or economies)) OR TOPIC: ("middle* income*" NEAR/3 (countr* or nation* or economy or economies)) OR TOPIC: ("low* middle" NEAR/3 (countr* or nation* or economy or economies))
# 13 226,623 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
# 12 5,964 TS=(sleep$ NEAR/2 (disorder$ or syndrome$))
# 11 2,046 TS=(("substance abuse" or "substance use" or "drug abuse" or "drug use" or personality) NEAR/2 disorder*)
# 10 52,621 TS=( neurosis or neuroses or neurotic or "stress syndrome*" or "distress syndrome*" or "pain disorder*" or dementia or alzheimer$ or epilepsy)
# 9 3,781 TS=(trichotillomani* OR OCD or "obsess* compulsi*" or GAD or "stress reaction*" or "acute stress")
# 8 12,041 TS=((bipolar or behavio$ral or obsessive or compulsive or panic or mood or delusional) NEAR/1 (disorder$ or illness* or disease$))
# 7 13,271 TS=((stress or cognitive or cognition or personality or "impulse control" or mood or paranoid or psychotic or neurologic* or nervous or "nervous system" or eating) NEAR/1 (disorder$ or illness* or disease$))
# 6 2,445 TS=((post‐trauma* or posttrauma*) NEAR/3 (stress* or disorder$))
# 5 18,936 TS=(somatoform or somatiz* or somatis* or hysteri* or briquet or multisomat* or multi somat* or MUPs or "medically unexplained" or (dissociative NEAR/3 (disorder* or reaction*)) or dissociation or (affective* NEAR/1 (disorder$ or disease$ or illness* or symptom$)) or PTSD or "psychological trauma" or psychotrauma* or combat disorder$ or war disorder$)
# 4 143,429 TS=(mental or mentally or psychiatr* or psycho* or depressi* or depressed or MDD or anxi* or phobia or phobic or agoraphobi* or dysthymi* or ADNOS or schizo* or hebephrenic* or oligophreni* or akathisi* or acathisi* or neuroleptic‐induc* or (tardiv* NEAR/1 dyskine*) or (movement NEAR/5 (disorder or disorders)))
# 3 92,636 #1 NOT #2
# 2 376 TOPIC: ("diabet* insipidus")
# 1 93,012 TOPIC: (diabet* or "noninsulin*‐depend*" or "non‐insulin*‐depend*" or "noninsulin*depend*" or "non‐insulin*depend*") OR TOPIC: ("fasting glucose" or "plasma glucose" or "glucose tolerance test* "or (glyc$emic NEAR/2 control*)) OR TOPIC: (HbA1c or A1C or A1c or Hb1c or ((glycated or glycosylated) NEAR/1 h$emoglobin*)) OR TOPIC: (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D)
**************************************************
ProQuest Dissertations & Theses A&I 1743 – pres Set Search Results
S11 s9 and s10 134
S10 noft(DIABETES OR Diabetic) AND noft(trial OR RCT OR randomized OR randomised OR randomly OR placebo OR blinding OR review OR meta‐analysis) AND noft(mental OR psychiatric OR psychological OR depression OR anxiety OR phobia OR schizophrenia OR psychosis OR psychotic OR psychoses OR bipolar OR somatoform OR stress OR mood OR behavioral OR neuroses OR personality OR Dementia OR alzheimers) 882
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 919,956
S8 noft("St Lucia" OR "St Helena" OR "St Vincent " OR "Saint Lucia" OR "Saint Helena" OR "Saint Vincent" OR "South America*" OR Sudan OR Sudanese OR Suriname Or Surinamese OR Swaziland OR Swazi OR Swazis OR Syria or Syrian OR Syrians OR Taiwan* OR Taipei* OR Tajikistan* OR Tanzania* OR Thai* OR Timor* OR Tobago OR Togo OR Tokelau OR Tonga OR Trinidad OR Tunisia* OR Turkey OR Turkish OR Turkmenistan* OR "Turks and Caicos" OR Tuvalu* OR Uganda* OR Ukrain* OR "United Arab Emirates" OR Uruguay* OR Uzbekistan* OR Vanuatu* OR Venezuela* OR Vietnam* OR "Virgin Is” OR “Virgin Islands" OR "Wallis and Futuna" OR Futuna OR "West Bank" OR Gaza OR Yemen OR Yemeni OR Zambia* OR Zimbabw*) 68,453
S7 noft(Namibia* OR Nauru* OR Niue* OR Nepal* OR "Netherlands Antilles*" OR "Dutch Antilles" OR "New Caledonia*" OR Nicaragua* OR Niger OR Nigeria* OR Oman* OR Pakistan* OR Palau* OR Palestin* OR Panama OR "Papua New Guinea*" OR Paraguay OR Peru OR Peruvian* OR Philippines OR Pilipin* OR Filipin* OR Poland OR Polish OR Polynesia* OR Qatar* OR Romania* OR Russia* OR Rwanda* OR Samoa* OR "Sao Tome*" OR Principe* OR Saudi OR Senegal* OR Serbia* OR Seychelles OR "Sierra Leone" OR Singapor* OR Slovak* OR Sloven* OR "Solomon Islands" OR Somalia* OR "Sri Lanka*" OR "S* Kitts and Nevis") 90,163
S6 noft(Indonesia* OR Iran* OR Iraq* OR Israel* OR Jamaica* OR Jordan* OR Kazakhstan* OR Kenya* OR Kiribati* OR Korea* OR DPRK OR Kosovo* OR Kuwait* OR Kyrgyz* OR "Lao PDR" OR "Lao People*" OR Laos OR Laotian OR Latvia* OR Lebanon OR Lebanese OR Lesotho OR Liberia* OR Libya* OR Lithuania*) OR noft(Macao* OR Macau OR Macedonia* OR Madagasca* OR Malawi* OR Malaysia* OR Maldives OR Mali OR Malta OR Maltese OR "Marshall Islands" OR Mauritania* OR Mauritius OR Mayotte* OR Melanesia* OR Mexico OR Mexican? OR Micronesia* OR Moldova* OR Mongolia* OR Montenegro* OR Morocco OR Moroccan? OR Mozambique* OR Myanmar*) 137,923
S5 noft(Ecuador OR Ecuadorian or Ecuardorians OR Egypt OR Egyptian OR Egyptians OR "El Salvador" OR Eritrea OR Eritrean OR Eritreans OR Estonia OR Estonian OR Estonians OR Ethiopia OR Ethiopian OR Ethiopians OR "Falklands Islands" OR Fiji OR Fijian OR Fijians OR Gabon OR Gabones OR Gambia OR Gambian OR Ghambians OR Ghana OR Ghanian OR Ghanians OR Gibralta OR Gibraltarian OR Gibraltarians OR Grenada OR Grenadian OR Grenadians OR Guatemala IR Guatemalan or Guatemalans OR Guinea OR Guinean or Guineans OR Guiana or Guianese OR Guyana OR Guyanese OR Haiti OR Haitian OR Haitians OR Hondura OR Honduran OR Hondurans OR "Hong Kong" OR Hungary OR Hungarian OR Hungarians OR India OR (Indian NOT "American Indian") OR (Indians NOT "American Indians") ) 127,032
S4 noft(Cambodia* OR Cameroon* OR "Cape Verde*" OR "Cabo Verde*" OR Caribbean* OR "Cayman Is*" OR Chad OR Chile* OR China OR Chinese OR (Colombia* NOT "British Colombia*") OR Comoros OR Congo OR "Cook Island*" OR "Costa Rica*" OR "ivory coast" OR "cote d'ivoire" OR Croat* OR Cuba* OR Cyprus OR Cypriot* OR Czech* OR Djibouti* OR Dominica*) 512,917
S3 noft(Afghanistan OR Afghanistani OR Afghanistanis OR Albania OR Albanian OR Albanians OR Algeria OR Algerian OR Algerians OR Angola OR Angolan OR Angolans OR Anguilla OR Anguillan OR Anguillans OR Antigua OR Antiguan OR Antiguans OR Barbuda OR Barbudan OR Barbudans OR Argentina OR Argentinian OR Argentinians OR Armenia OR Armenian OR Armenians OR Aruba OR Aruban OR Arubans OR Azerbaijan OR Azerbaijani OR Azerbaijanis OR Bahamas OR Bahamain OR Bahamians OR Bahrain OR Bahrani OR Bahranis OR Bangladesh OR Bangladeshi OR Bangladeshis OR Barbados OR Barbaidan OR Barbadians OR Belarus OR Belarusian OR Belarusian OR Belize OR Belizean OR Belizeans OR Benin OR Bermuda OR Bermudian OR Bermudians OR Bhutan OR Bhutanese OR Bolivia OR Bolivian OR Bolivians OR Bosnia OR Bosnian OR Bosnians OR Herzegovina OR Borneo OR Dayak OR Botswana OR Botswanan OR Batswana OR Brazil OR Brazilian OR Brazilians OR Brunei OR Bruneian OR Bruneians OR Bulgaria OR Bulgarian OR Bulgarians OR "Burkina Faso" OR Burkinabé OR Burundi OR Burundian OR Burundians) 36,877
S2 noft((Africa OR African OR Africans) NOT ("African American" OR "African Americans")) OR noft((Asia OR Asian OR Asian) NOT ("Asian American" OR "Asian Americans")) 110,205
S1 noft(("Developing Country" OR ("Developing Countries" OR "developing nation" OR "developing nations" OR "developing economy” OR "developing economies” OR "developing world" OR "third world" OR LMIC OR LMICs) OR ((low OR middle) NEAR/1 "income country") OR ((low OR middle) NEAR/1 "income countries")) ) 27,574
**************************************************
ClinicalTrials.gov (U.S. NIH)
1. The advanced search interface was searched on 08/04/2019 and 28/02/2020 for All Studies. Search strings were entered into the ‘Condition’ and ‘Other terms’ fields as indicated:
Condition or disease: Mental OR Depression OR Psychosis OR anxiety OR dementia OR behavioral OR schizophrenia OR Alzheimers
Other terms: diabetes OR diabetic
2. The Age Group was then limited to: Adult, Older Adult, and the search was run
3. The search results were limited to LMIC countries by selecting the ‘By Topic’ tab, then selecting the Locations –Alphabetical link. Trial records from ODA‐DAC countries (2003‐2020) were downloaded.
184 results found in total
**************************************************
International Clinical Trials Registry Platform (WHO)
The ‘Advanced Search’ interface was searched on 08/04/2019 and 28/02/2020. Search strings were entered into the ‘Intervention’ and ‘Condition’ fields as indicated:
Intervention: diabet* OR prediabetes OR pre‐diabetes OR obesity OR weight OR glucose OR hemoglobin OR haemoglobin
AND
Condition Mental* OR Depressi* OR Psych* OR anxiety OR dementia OR behavioral OR schizophrenia OR Alzheimer*
A limit to LMICs was not applied, it was felt more efficient to screen the relatively small set of results for LMIC Trials to include, rather than conduct multiple searches limiting each to a different LMIC name.
129 results found in total
**************************************************
ISRCTN registry (Springer Nature)
The Advanced Search Mode was searched on 08/04/2019 and 28/02/2020.
We searched for the textword (diabetic OR diabetes) in the Advanced Search Mode, and limited the results to ‘Condition Category: Mental and Behavioural Disorders’. All records were downloaded into an Excel file. Records of trials which did not recruit from an LMIC were removed from the spreadsheet by checking the ‘Country of Recruitment’ column.
141 “LMIC” results found in the downloaded spreadsheet.
**************************************************
Appendix 2. Cochrane Specialized Register
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
The CCMD Group maintains an archived specialised register of RCTs: the CCMD Controlled Trials Register (CCMDCTR). This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies‐based register with more than 50% of reference records tagged to around 12,500 individually PICO‐coded study records. Reports of studies for inclusion in the register were collated from (weekly) generic searches of key bibliographic databases to June 2016, which included: MEDLINE (1950 onwards), Embase (1974 onwards), PsycINFO (1967 onwards), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of studies were also sourced from international trials registries, drug companies, handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) are on the Group's website.
The rationale of maintaining a comprehensive specialised register was reviewed when the editorial group moved from the University of Bristol to the University of York in June 2016. At this time, the Group decided to archive the CCMDCTR and return to searching the medical and psychological literature directly, on a review‐by‐review basis.
The CCMDCTR (studies and references register) was searched (all years to 14 June 2016) for this review, using the following terms based on outcomes (only):
(diabet* or “glucose tolerance test*” or “glycated Hemoglobin A” or “glycated haemoglobin A” or “noninsulin* depend*” or “non insulin* depend*” or "non insulindepend*" or non?insulin?depend* or “fasting glucose” or “plasma glucose” or ((glycemic or glycaemic) adj2 control*) or HbA1c or A1C or A1c or Hb1c or ((glycated or glycosylated) adj (hemoglobin* or haemoglobin*)) or IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D) AND INREGISTER
**************************************************
Data and analyses
Comparison 1. Atypical versus typical antipsychotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 diabetes (ADA criteria) (6 weeks) | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.03, 7.05] |
| 1.2 drop‐outs (6‐54 weeks) | 2 | 144 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.63, 2.69] |
| 1.3 fasting blood glucose (6‐8 weeks) | 2 | 211 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.10, ‐0.00] |
| 1.4 BMI (8‐54 weeks) | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.33, 0.81] |
| 1.5 total cholesterol (8 weeks) | 1 | 112 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.27, 0.43] |
Comparison 2. Metformin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 drop‐outs (12‐14 weeks) | 3 | 158 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.09, 16.35] |
| 2.2 fasting blood glucose (12‐14 weeks) | 5 | 264 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.46, 0.08] |
| 2.2.1 Endpoint | 3 | 173 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.60, ‐0.11] |
| 2.2.2 Change from baseline | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.21, 0.22] |
| 2.3 BMI (12‐14 weeks) | 5 | 264 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.04, ‐0.70] |
| 2.3.1 Endpoint | 3 | 173 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.38, ‐1.12] |
| 2.3.2 Change from baseline | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.17, ‐0.23] |
| 2.4 waist circumference (12‐14 weeks) | 4 | 192 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.93, 0.04] |
| 2.4.1 Endpoint | 2 | 101 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐6.26, 5.66] |
| 2.4.2 Change from baseline | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.21, ‐0.64] |
| 2.5 systolic blood pressure (14 weeks) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐9.09, 4.09] |
| 2.6 diastolic blood pressure (14 weeks) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐3.55, 5.95] |
| 2.7 total cholesterol (12‐14 weeks) | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.93, 0.26] |
2.4. Analysis.

Comparison 2: Metformin versus placebo, Outcome 4: waist circumference (12‐14 weeks)
Comparison 3. Melatonin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 drop‐outs (8 weeks) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.38, 2.66] |
| 3.2 fasting blood glucose (8‐12 weeks) | 3 | 202 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.32, ‐0.10] |
| 3.2.1 Endpoint | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.35, 0.01] |
| 3.2.2 Change from baseline | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.39, ‐0.09] |
| 3.3 BMI (8‐12 weeks) | 3 | 202 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐2.58, 2.14] |
| 3.3.1 Endpoint | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐2.40, ‐0.64] |
| 3.3.2 Change from baseline | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 1.47 [0.45, 2.49] |
| 3.4 Waist circumference (8 weeks) | 2 | 136 | Mean Difference (IV, Random, 95% CI) | 0.68 [‐0.47, 1.83] |
| 3.4.1 Endpoint | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐1.26, 1.26] |
| 3.4.2 Change from baseline | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 1.19 [0.29, 2.09] |
| 3.5 systolic blood pressure (8 weeks) | 2 | 136 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐6.46, 3.84] |
| 3.5.1 Endpoint | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 1.00 [0.07, 1.93] |
| 3.5.2 Change from baseline | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐8.14, ‐0.46] |
| 3.6 diastolic blood pressure (8 weeks) | 2 | 136 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.60, ‐0.50] |
| 3.6.1 Endpoint | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.56, ‐0.44] |
| 3.6.2 Change from baseline | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐5.33, 0.53] |
| 3.7 total cholesterol (8 weeks) | 2 | 136 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.06] |
| 3.7.1 Endpoint | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.27, 0.05] |
| 3.7.2 Change from baseline | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.19, 0.23] |
Comparison 4. SSRI versus TCA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 drop‐outs (12 weeks) | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.01] |
| 4.2 fasting blood glucose (8‐12 weeks) | 3 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.88, 0.10] |
| 4.3 BMI (12 weeks) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.10, 2.50] |
| 4.4 depression (12 weeks) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.59, 1.19] |
Comparison 5. SENSITIVITY ‐ high quality ‐ metformin vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 fasting blood glucose (12‐14 weeks) | 2 | 136 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.68, ‐0.07] |
| 5.2 BMI (12‐14 weeks) | 4 | 227 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐2.15, ‐0.77] |
| 5.2.1 Change from baseline | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.17, ‐0.23] |
| 5.2.2 Endpoint | 2 | 136 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.29, ‐1.49] |
Comparison 6. SENSITIVITY ‐ Wu2008a ‐ metformin vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 drop‐outs (12‐14 weeks) | 2 | 94 | Risk Ratio (IV, Random, 95% CI) | 2.78 [0.08, 95.87] |
| 6.2 fasting blood glucose (12‐14 weeks) | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.46, 0.09] |
| 6.2.1 Endpoint | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.46, 0.09] |
| 6.3 BMI (12‐14 weeks) | 4 | 200 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.86, ‐0.40] |
| 6.3.1 Endpoint | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.48, 0.82] |
| 6.3.2 Change from baseline | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.17, ‐0.23] |
| 6.4 waist circumference (12‐14 weeks) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐1.99, 8.79] |
| 6.4.1 Endpoint | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐1.99, 8.79] |
Comparison 7. SENSITIVITY‐ Wu2006‐ Atypical versus typical antipsychotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 fasting blood glucose (6‐8 weeks) | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.35, 0.37] |
| 7.2 BMI (52 weeks) | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐5.65, 3.39] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agahi 2017.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group Type of RCT: Double blind How were participants recruited: Patents who were referred as out patients or hospitalised in Karegarnejad Hospitalin Kashan (Iran) |
|
| Participants |
Baseline characteristics Melatonin
Placebo
Overall
Inclusion criteria: Age 18 to 64 years, outpatients or hospitalised in one hospital between 2014 to 2015, recently treated with second generation antipsychotics Exclusion criteria: Hypertension,diabetes,lipid disorders, thyroid dysfunction, liver diseases, history of melatonin allergy, pregnancy, lactation and history of drug dependence or abuse in the last 6 months Pretreatment: Type and dosage of antipsychotic use in each group not reported. Type of mental disorder not reported |
|
| Interventions |
Intervention characteristics Melatonin
Placebo
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Waist circumference
Diastolic blood pressure
Systolic blood pressure
Notes: Unclear about unit of measure |
|
| Identification |
Sponsorship source: Authors state no funding has been received Country: Iran Setting: Hospital; outpatients or hospitalised Comments: ‐ Authors name: Mansour Agahi Institution: Department of Psychiatry, Kashan University of Medical Sciences, Kashan, Iran Email: chpharmacy@gmail.com Address: Department of Psychiatry, Kashan University of Medical Sciences, Kashan, Iran. |
|
| Notes | Type of mental disorder not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to two groups by blocked randomization procedures." Judgement comment: No information on how blocks of patients were allocated to groups |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: The study was double blind controlled clinical trial. Even though double blinding is mentioned in methods but no details have been provided how this was achieved, who were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: No information on outcome assessors. No details have been provided who were the assessors taking the anthropometric measurements at the baseline or at follow ups and if they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Unclear how many patients were recruited and whether any were lost to follow up. Study reported as 100 participants with complete outcome data after 8 weeks for all participants. No drop‐out reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No reference made to protocol. |
| Other sources of bias | Unclear risk | Judgement comment: Type and dosage of antipsychotic use in each group not reported. |
Agnihotri 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were patients recruited: Outpatient clinic Type of RCT: Double blind |
|
| Participants |
Baseline characteristics Withania somnifera
Placebo
Overall
Inclusion criteria: Schizophrenia patients receiving second‐generation antipsychotics for 6 months or more, having serum triglycerides more than 150 mg/dl, high‐density lipoprotein (HDL) cholesterol less than 40 mg/dl in men and less than 50 mg/dl in women, fasting blood glucose (FBG) level more than 100 mg/dl, aged above 18 years were included Exclusion criteria: Patients suffering from other psychiatric/systemic illnesses, receiving concurrent medicines, pregnant/lactating women were excluded Pretreatment: Reported to be 'comparable', no further information. |
|
| Interventions |
Intervention characteristics Withania somnifera
Placebo
|
|
| Outcomes |
Fasting blood glucose
|
|
| Identification |
Sponsorship source: Indian Council of Medical ResearchPharmanza Country: India Setting: Psychiatry outpatient department of tertiary care teaching hospital Comments: ‐ Authors name: Dr Smita Sontakke Institution: Government Medical College Nagpur Email: smitaavanti@yahoo.co.in Address: Department of Pharmacology, Government Medical College, Nagpur |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: "Selected patients were randomly allocated by SAS systems of Windows". It is possible to generate a randomization schedule using SAS but not clear how this was done. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Paper states 'double blind' but no information on how blinding was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The author responded that the outcome assessor nutritionist was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only data for participants who completed the follow‐up assessment is shown. 2 participants in the placebo group and 3 in the intervention group dropped out. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No protocol referenced. |
| Other sources of bias | Low risk | Judgement comment: No other sources of bias identified. |
Akkasheh 2016.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Referred from hospital Type of RCT: Double blind |
|
| Participants |
Baseline characteristics Probiotic supplements
Placebo
Overall
Inclusion criteria: Diagnosis of major depressive disorder, score of 15 or greater on HAM‐D Exclusion criteria: < 20 years or > 55 years; a history of coronary infarction, again pectoris, pregnancy or lactation, or substance abuse; and taking dietary supplements or probiotic supplements during the previous 2 months. Pretreatment: Fasting glucose at baseline 89.4 mg/dL placebo versus 102.3 mg/dL in control group. No statistically significant difference in other variables. |
|
| Interventions |
Intervention characteristics probiotic supplements
placebo
|
|
| Outcomes |
fasting plasma glucose
Total cholesterol
depression score
BMI
drop‐out
|
|
| Identification |
Sponsorship source: The study was supported by a grant (no. 9344) from the vice chancellor for research, Kashan University of Medical Sciences, Iran Country: Iran Setting: hospital Comments: ‐ Authors name: Zatollah Asemi Institution: Kashan University of Medical Sciences, Kashan, Iran Email: asemi_r@yahoo.com Address: Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran Contact numbers of Corresponding Author: ‐ |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was performed by the use of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Body weight and height were determined in an overnight fasting state, with minimal clothing and without shoes, by the use of a digital scale (Seca, Hamburg, Germany) by a trained nutritionist at a psychiatry clinic at the beginning of the study and at the end point." Judgement comment: Suggests concealed allocation, but unclear whether nutritionist who performed allocation was involved in study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Double‐blinded, placebo looked identical to the treatment. Randomization and allocation were concealed from the researchers and participants until the main analyses were completed. But there is information regarding the blinding and who were actually blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Anthropometric assessments were performed by a trained nutritionist at a psychiatry clinic at the beginning of the study and at the end point. But it is not clear whether it’s the same nutritionist who was mentioned already above or different. On further contact, the author responded that outcome assessor was the nutritionist who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Similar numbers in both arms lost to follow‐up and withdrawn for personal reasons. Unlikely to be related to treatment. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Within‐group comparisons (endpoint vs. baseline) were done based on the paired‐samples t test." Judgement comment: All outcomes described in protocol but this was registered retrospectively. No baseline values for depression presented; unclear why given that methods state endpoint‐baseline comparisons. |
| Other sources of bias | Unclear risk | Quote: "All capsules were provided by Tak Gen Zist Pharmaceutical Company (Tehran, Iran)" Judgement comment: Protocol states pharmaceutical company TAK GENE ZIST is the funding source of the study. |
Assunção 2006.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Hospital outpatient clinic Type of RCT: Double blind |
|
| Participants |
Baseline characteristics Nizatidine
Placebo
Overall
Inclusion criteria: Outpatient participants (male or female, 18 to 65 years of age) met DSM‐IV 29 diagnostic criteria for schizophrenia,schizoaffective disorder, or schizophreniform disorder. Patients had to be in treatment with olanzapine (5 to 20 mg/day) for no less than 2 months and no more than 6 months, had a record of their body weight when initiated on olanzapine, had gained ≥ 5% since initiated the treatment with olanzapine, and had a total BPRS score < 45. Exclusion criteria: Patients were excluded if they had any known physical illness that could affect body weight and composition, were currently participating in a formal weight loss program, or had a body mass index (BMI) ≥ 40 kg/m2 or weight ≥ 114 kg. Patients with a diagnosis of diabetes mellitus could be enrolled provided their condition was under control and if they were in treatment for DM for at least 6 months. Pretreatment: No significant baseline group differences |
|
| Interventions |
Intervention characteristics Nizatidine
Placebo
|
|
| Outcomes |
Total cholesterol
Glycaemia
|
|
| Identification |
Sponsorship source: This clinical trial was sponsored by Eli Lilly do Brasil Ltda Country: Brazil Setting: outpatient Comments: ‐ Authors name: Sheila Seleri Marques Assunção Institution: Bristol Meyers USA Email: lima_Mauricio_silva_de@lilly.com Address: Eli Lilly do Brasil Avenida Morumbi, 8264 ‐ Brooklin 04703‐002 São Paulo, SP, Brasil |
|
| Notes | Original paper: Assunção SS, Ruschel SI, Rosa LC, Campos JA, Alves MJ, BraccoOL, Lima MS. Weight gain management in patients withschizophrenia during treatment with olanzapine in association with nizatidine. Rev Bras Psiquiatr. 2006;28(4):270‐6. Outcomes N=9 dropouts mentioned in text of article. Not clear how many from each group. Outcome 'N' number cannot be arrived at. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: No information |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: It is a placebo controlled trial and double blinded. The text of the article does not talk about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Authors report study is double blind, but unclear how blinding was achieved. Low risk with participants given that placebo and treatment probably looked similar, but unclear who provided medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Unclear who performed outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: No drop‐out rates reported by study arm. N = 9/54 in total. Unclear how many participants were in each arm at follow‐up. No information regarding reasons for drop‐out. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No reference to protocol. |
| Other sources of bias | High risk | Judgement comment: Trial sponsored by pharmaceutical company and 3 authors worked for the company. |
Baptista 2006.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Recruited from hospital Type of RCT: Double Blind |
|
| Participants |
Baseline characteristics Metformin + olanzapine
Placebo + olanzapine
Overall
Inclusion criteria: Patients with severe schizophrenia or related disorders who have been stabilized for more than 5 years with conventional antipsychotic drugs. Exclusion criteria: Chronic disease, hormone replacement therapy Pretreatment: Authors state neither group significantly differed from the other on any variable at baseline, but no table provided. |
|
| Interventions |
Intervention characteristics Metformin + olanzapine
Placebo + olanzapine
|
|
| Outcomes |
Basal glucose
BMI
Total cholesterol
Waist circumference
Drop‐out
|
|
| Identification |
Sponsorship source: This study was supported by Grant M‐783‐03‐07 from CDCH‐T‐ULA (Consejo de Desarrollo Científico, Humanístico y Tecnológico, Universidad de Los Andes, Mérida, Venezuela); by Fundación Polar (Caracas, Venezuela); and by a NARSDA Young Investigator Award to Serge Beaulieu, Quebec, Canada. Country: Venezuela Setting: Hospital Comments: ‐ Authors name: Dr Trino Baptista Institution: Los Andes University Medical School Email: trinbap@yahoo.com Address: PO Box 93, Mérida, 5101‐A, Venezuela |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐based random allocation of patients" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: The study does not describe of any method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Unclear how blinding was achieved; not reported whether those involved in randomization were involved in providing treatments or in contact with patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Not reported who outcome assessors were and whether blinding was achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Low rates of drop‐out, similar in both groups (1/20 and 2/20). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: There is no protocol made available but the outcomes prespecified in the Methods section were addressed in the study. |
| Other sources of bias | High risk | Judgement comment: The study does not provide patient demographics or baseline characteristics for each treatment group. There is also no explicit mention of exclusion criteria or method of patient recruitment. The study received 'support' from Fundacion Polar, a food and beverage company. |
Baptista 2007.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Recruitment was achieved through treating psychiatrists who informed their patients about the risk of excessive BWG and metabolic dysfunction during olanzapine administration. Type of RCT: Multi‐centre double blind |
|
| Participants | Baseline characteristics Metformin
Placebo
Overall
Inclusion criteria: Patients had to be older than 18 years, free of hormone replacement and any chronic disease besides the mental disorder and with a normal physical and laboratory tests (liver, kidney, thyroid tests and fasting glucose) before starting olanzapine. Exclusion criteria: Not reported Pretreatment: No notable differences. Unclear how many of the 8 drop‐outs were in intervention and placebo groups. |
|
| Interventions |
Intervention characteristics Metformin
Placebo
|
|
| Outcomes |
Fasting glucose
BMI
Total cholesterol
|
|
| Identification |
Sponsorship source: This study was supported by FONACIT, Caracas, Venezuela, Grant 2005‐000‐384 and Eli Lilly Laboratories, Caracas, Venezuela Country: Venezuela Setting: Inpatients and outpatients at psychiatric institutes and hospitals Comments: ‐ Authors name: Trino Baptista Institution: Department of Physiology, Los Andes University Medical School Email: trinbap@yahoo.com Address: Department of Physiology, Los Andes University Medical School, PO Box 93, Mérida, 5101‐A, Venezuela |
|
| Notes | Population A high variability was observed in the magnitude of BWG (kg) under olanzapine before the trial: mean: 3.3;SD: 4.8; median: 1.7;mode: 1.6; range: 0–24 kg (0–36.4%of baseline). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned through a computer‐based program to either metformin (Biotech, Caracas, Venezuela; 850–2550 mg day) or identical placebo pills." Judgement comment: Unclear whether outpatients and inpatients were randomized together or two separate sequences were generated. Difference between two groups pertaining to OP/IP not specified. Computer‐based program |
| Allocation concealment (selection bias) | Low risk | Quote: "Any dosage adjustment was done by the study coordinator (TB) who was the only team's member to know the individual treatment." Judgement comment: Computer program used. Presumably by study coordinator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each outpatient was given a 2‐week treatment supply and had a weekly phone contact with a specific team researcher to control for side effects. Any dosage adjustment was done by the study coordinator (TB) who was the only team's member to know the individual treatment. Metformin was started at the lower dose (850 mg), and at week 4 the full metformin or placebo" Judgement comment: Blinding for participants and personnel is clear. Clarity regarding whether side effect reporting led to unblinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Blinding of assessors would have been compromised by side effect reporting. Does not mention who the assessors were. Low risk because the outcomes are observer reported without judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: 8 drop‐outs, but not reported in which group or why these participants did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Approved protocol not referenced |
| Other sources of bias | High risk | Judgement comment: Placebo group seemed to have higher levels of glucose at baseline, meaning there is more potential for it to be reduced. Also slightly higher cholesterol levels. Study partly sponsored by pharmaceutical company. |
Baptista 2009.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Recruitment was conducted among those participants willing to lose BW or prevent excessive BWG after an educational program of healthy life style. Voluntary participation was requested and a written informed consent was obtained from each patient and from the medical director of the institution. Type of RCT: Open |
|
| Participants |
Baseline characteristics Rosiglitazone
Placebo
Overall
Inclusion criteria: Psychiatric inpatients with schizophrenia who switched to olanzapine 8 months before the study, older than 18, free of hormone replacement and any chronic disease besides the mental disorder and normal physical and laboratory tests. Patients had to be willing to lose weight or prevent excessive weight after an educational healthy lifestyle program. Exclusion criteria: ‐ Pretreatment: Insulin levels seem higher at baseline in rosiglitazone (5.1) than placebo (2.9) group. Men in the placebo group were older (55) than in rosiglitazone group (41). |
|
| Interventions |
Intervention characteristics Rosiglitazone
Placebo
|
|
| Outcomes |
Glucose
BMI
Total cholesterol
Waist circumference
|
|
| Identification |
Sponsorship source: This study was supported by FONACIT, Caracas, Venezuela, Grant2005‐000‐384 Country: Venezuela Setting: inpatient psychiatric institute Comments: N=30 at baseline but N=1 left study; as treated analysis and no baseline information. Authors name: Prof. T. Baptista, MD, PhD Institution: Los Andes University Medical School, Venezuela Email: trinbap@yahoo.com Address: Department of Physiology, Los Andes University Medical School, P.O. Box 93, Mérida 5101‐A, Venezuela |
|
| Notes | Interventions Recruitment was conducted among those participants willing to lose BW or prevent excessive BWG after an educational program of healthy life style. Recommendations for healthy food and physical exercise to control BWG were provided at the beginning of the study. All participants engaged in healthy food and monitored physical exercise programs but their effects could not be quantified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned through a computer‐based program to either rosiglitazone (Avandia, Glaxo‐Smith‐Kline, Caracas, Venezuela) or placebo." Judgement comment: Further details could have given clarity on stratification, etc., |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Medication and placebo were administered as a single daily dose by nurses trained in research and the number of pills was weekly quantified." Judgement comment: Not specifically mentioned whether nurses were aware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Rosiglitazone was started at the lower dose (4 mg), and at week 4 the full rosiglitazone (8 mg) or placebo dose was achieved and maintained thereafter in all subjects. The rosiglitazone schedule encompassed the usual dose range in clinical practice. Since rosiglitazone was devoid of significant side effects, no dose adjustment was necessary." Judgement comment: Blinding of participants is not mentioned clearly. Blinding of assessment personnel is not mentioned clearly. Risk of bias due to changing doses. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Each subject ’ s blood pressure, BW and body mass index (BMI = weight in kg / height squared) were measured at baseline, weeks 6 and 12." Judgement comment: Outcomes are observer reported without judgement, even though assessment personnel are not identified as nurses, psychiatrists or social work professionals. Blinding has not been specifically mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One subject in the rosiglitazone group left the study in the first week of treatment due to a change in residence. Hence, it was not possible to conduct either an “ intention to treat ” or a “ last observation carried forward ” analysis." Judgement comment: As‐treated analysis but unlikely to bias results as only one participant dropped‐out due to change in residence. For the rest, outcome data is completely reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No protocol referenced, although it is mentioned that a protocol was written and approved. |
| Other sources of bias | High risk | Judgement comment: Placebo group had higher cholesterol levels at baseline, and higher psychiatric symptoms measured by BPRS. |
Carrizo 2009.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Patients were invited at the outpatient centre Type of RCT: Double‐blind |
|
| Participants | Baseline characteristics Clozapine + metformin
Clozapine + placebo
Overall
Inclusion criteria: Inclusion criteria were to be under clozapine treatment for at least 3 consecutive months; to be older than 18 years, free of hormone replacement and have normal physical and laboratory tests (kidney, thyroid and liver). Exclusion criteria: Elevated serum glucose and lipid levels were not considered as exclusion criteria. No participants had symptoms of type 2 diabetes. Pretreatment: No significant differences were observed between the groups at baseline in any variable. |
|
| Interventions |
Intervention characteristics Clozapine + metformin
Clozapine + placebo
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Waist circumference
Diastolic blood pressure
Systolic blood pressure
Drop‐outs
|
|
| Identification |
Sponsorship source: This study was supported by FONACIT, Caracas, Venezuela, Grant 2005‐000‐384. (The study Thanked to Valmorca Laboratories, Mérida, Venezuela, for providing the placebo pills.) Country: Venezuela Setting: Outpatient centre (Center for the Attention for Schizophrenia Outpatients and their Families) Comments: ‐ Authors name: Trino Baptista Institution: Department of Physiology, Los Andes University Medical School, Email: trinbap@yahoo.com Address: Department of Physiology, Los Andes University Medical School, P.O. Box 93, Mérida, 5101‐A, Venezuela |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: patients were randomly assigned by a computer programme to either MET or placebo. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: Trino Baptista (TB) was the study coordinator. Only the study coordinator knew the treatment assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Only the study coordinator (TB) knew the treatment assignment. TB was in coordination with the treating psychiatrists of participants, who controlled their clozapine and other psychotropic drug doses and who periodically informed him. TB according to the intolerances adjusted the MET or placebo dose. But there are no more details on the blinding, that who was/were blinded in this study. Only the study coordinator (TB) knew the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: Before starting the study, each patient was evaluated by a psychiatrist trained in clinical research and also later assessed the mental state for the follow up visits. "The same psychiatrist assessed the mental state in every visit (baseline, weeks 7 and 14) and maintained contacts by phone with patients and caregivers every two weeks." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop out 7 in metformin group, 0 in placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: The study protocol is not provided. but in the Methods section, the outcomes were pre‐specified and later addressed. |
| Other sources of bias | Low risk | Quote: "Role of the funding source. No intervention in the study accomplishment, results analysis and manuscript preparation." |
Chen 2017.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: In this study, randomized grouping was used with admission time as the compatibility factor, and every fourth patient (as determined by time of admission) were set as an interval for randomized grouping. As a result, 38 cases of elderly patients with first‐episode schizophrenia was randomly divided into the ziprasidone treatment group (study group) and olanzapine treatment group (control group), with 19 cases in each group. Type of RCT: Single blind (patient blind) parallel controlled trial design |
|
| Participants |
Baseline characteristics Ziprasidone (treatment)
Olanzapine (control)
Overall
Inclusion criteria: Age ≥ 60 years old, 2) met the diagnostic criteria for schizophrenia according to the ICD‐10 classification of mental and behavioral disorders,with a PANSS total score≥60 points, 3) no issues taking ziprasidone or olanzapine, and 4) informed consent was provided by patients’ legal guardians Exclusion criteria: 1) having diabetes mellitus, hyperlipidaemia, or severe liver and renal insufficiency, 2) presence of organic neurological disease such as epilepsy, intracranial infection, brain tumour as determined by MRI or EEG examination, 3) presence of systemic diseases such as systemic lupus erythematosus (SLE), and schizophrenia‐like behavior caused by drug intoxication Pretreatment: No significant statistical difference between two groups |
|
| Interventions |
Intervention characteristics Ziprasidone (treatment)
Olanzapine (control)
|
|
| Outcomes |
Fasting blood sugar
Cholesterol
|
|
| Identification |
Sponsorship source: Zhejiang Province Huzhou 3rd People’s Hospital Public Welfare Application Research project (project # 2016GYB03) Country: China Setting: The Third People’s Hospital of Huzhou, Huzhou, Zhejiang Province, China Comments: ‐ Authors name: Jing Chen, Xingen Pan, Mincai Qian, and Shoukai Yang Institution: Department of Geriatric Psychiatry, The Third People’s Hospital of Huzhou Email: E‐Mail: 781703956@qq.com Address: Department of Geriatric Psychiatry, The Third People’s Hospital of Huzhou, Huzhou,Zhejiang Province, China. Postcode: 313000. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized grouping was used with admission time as the compatibility factor, and every fourth patient (as determined by time of admission) were set as an interval for randomized grouping". |
| Allocation concealment (selection bias) | Unclear risk | Paper did not mention who randomized the patients into two groups and how the allocation was determined. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Single blind (patient blind) parallel controlled trial design was used". Participants were blinded but insufficient information to determine how blinding was tested, guaranteed or maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The paper did not mention who participated in the outcome measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report no drop‐out; data available for all participants. |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol. |
| Other sources of bias | Low risk | No further sources of bias identified. |
Emsley 2005.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Not reported Type of RCT: Blind to investigator |
|
| Participants |
Baseline characteristics Quetiapine
Haloperidol
Overall
Inclusion criteria: 18 to 65 years old, diagnosis of schizophrenia or schizoaffective disorder, psychiatric condition judged to be clinically stable, tardive dyskinesia Exclusion criteria: Another Axis I DSM‐IV diagnosis, significant or unstable general medical condition, and currently receiving clozapine. Pretreatment: Authors report baseline characteristics were similar, but quetiapine group had a higher baseline body weight and slightly higher BMI, and significantly higher prolactin (25.4 vs 15.2). |
|
| Interventions |
Intervention characteristics Quetiapine
Haloperidol
|
|
| Outcomes |
BMI
Drop‐outs
|
|
| Identification |
Sponsorship source: The study was supported in part by the Medical Research Council of South Africa and the University of Stellenbosch. Trial medication was provided by Astra Zeneca. Country: South Africa Setting: inpatient and outpatient Comments: ‐ Authors name: Dr Robin Emsley Institution: University of Stellenbosch Email: rae@sun.ac.za Address: Department of Psychiatry, Faculty of Health Sciences, University of Stellenbosch, PO Box 19063, Tygerberg 7505, Cape Town, South Africa. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were then randomized to receive either quetiapine or haloperidol for a 52‐wk treatment period." Judgement comment: No information |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "investigator‐blinded," Judgement comment: Insufficient information to make judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Not addressed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More participants dropped out in the quetiapine group compared with the haloperidol group, and 7 drop‐outs in the quetiapine group were due to worsening of psychosis, compared with 4 in the haloperidol group. It is possible that any beneficial effects of quetiapine are therefore overestimated. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The University of Stellenbosch Ethics Committee approved the study protocol," Judgement comment: There is a study protocol mentioned but is not referenced or said how to access it. All pre‐specified primary outcomes in the methods section were addressed. |
| Other sources of bias | High risk | Judgement comment: Authors report conflicts of interest; funding and other benefits received from pharmaceutical companies, including company providing medication for this study. Investigator adjusted medication throughout the trial; there is a risk this was influenced by his knowledge of allocation to study arms. |
Fadai 2014.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Not reported Type of RCT: Triple blind |
|
| Participants |
Baseline characteristics Saffron aqueous extract
Placebo
Crocin
Overall
Inclusion criteria: male adult inpatients, 18 to 65 years old, met DSM‐IV‐TR criteria for schizophrenia, clinically stable without signs of acute phase, no history of olanzapine treatment. Exclusion criteria: Patients with acute general medical problems, coronary heart disease, metabolic syndrome, neurological disease, substance use problems (except for nicotine).Treatment with anti‐platelet medications or herbal medicines. Intellectual disability. Weight loss diets. Developing moderate to severe side effects. Change in treatment regimen based on the attending psychiatrist’s opinion. Consent withdrawal Pretreatment: No notable differences. |
|
| Interventions |
Intervention characteristics Saffron aqueous extract
Placebo
Crocin
|
|
| Outcomes |
Fasting blood sugar
Cholesterol
Waist circumference
Blood pressure
Drop‐outs
|
|
| Identification |
Sponsorship source: This study was supported by the Research Council of University of Social Welfare and Rehabilitation Sciences Country: Iran Setting: inpatient ward of psychiatric hospital Comments: ‐ Authors name: S Zhara Bathale Institution: David Geffen School of Medicine Email: bathai_z@modares.ac.ir Address: David Geffen School of Medicine, UCLA, Los Angeles, CA 90024, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer generated codes used |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed using sequentially numbered, opaque, sealed envelopes (SNOSE); the coding was only revealed after analysis of the results." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Participants and personnel were blinded until after analysis of results. The study states that participants are blinded but not specifically who else was. There was insufficient information to determine how blinding was tested, guaranteed or maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Authors state this is a 'triple blinded' study, but no information on who performed outcome assessments or how they were blinded. There is only mention of resident psychiatrists assessing side effects. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Drop‐out was minimal and similar numbers across groups (N=5 across three groups). Reasons for incomplete outcome data are stated in figure 1. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Trial registration reported; outcomes specified in protocol same as those reported. |
| Other sources of bias | Low risk | Judgement comment: No other sources of bias. |
Ghaderi 2019.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Study participants were approached at the psychiatry clinic Type of RCT: Randomized, double‐blind, placebo‐controlled trial |
|
| Participants |
Baseline characteristics Vitamin D + probiotic supplements
Placebo
Overall
Inclusion criteria: Any participant diagnosed with schizophrenia using DSM‐IV‐TR criteria with disease duration of at least two years, PANSS score of 55 or greater, treated with chlorpromazine (300 to 1000 mg/day, except clozapine) and agents anticholinergic (Trihexyphenidyl, 4 to 8 mg/day) during the last 6 months and aged 25 to 65 years old were included in the study. Exclusion criteria: Any participant with mental retardation (Intelligent Quotient of < 70), substance or alcohol addiction (except caffeine or nicotine) within the last 6 months of screening,a score of ≥14 on a 17‐item Hamilton Depression Rating Scale or a score of ≥ 4 on PANSS (depression item), anyone under treatment with lithium, carbamazepine, sodium valproic acid, with existing chronic and acute medical illness, with lactation or pregnancy, the use of antidepressants including MAO, TCA, SSRI in the last 6 months were excluded from this trials. Pretreatment: There was no significant difference between the two arms in terms of height, age, weight and BMI at baseline |
|
| Interventions |
Intervention characteristics Vitamin D + probiotic supplements
Placebo
|
|
| Outcomes |
Fasting plasma glucose
BMI
Cholesterol (Total cholesterol)
Drop‐outs
|
|
| Identification |
Sponsorship source: The research grant provided by Research Deputy of Kashan University ofMedical Sciences (KAUMS). Country: Iran Setting: Psychiatry clinic Comments: ‐ Authors name: Zatollah Asemi Institution: 8 Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences Email: asemi_r@yahoo.com Address: Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, I.R, Iran |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random numbers were used by an instructed staff to randomize study participants at the psychiatry clinic". |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not clearly mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "placebo which were capsules in the similar shape and packaging as vitamin D and probiotic" Judgement comment: No reference to how blinding was done or who provided the medication. The only mention to blinding is that the study is "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: The study addresses all of the primary outcomes prespecified in the Methods section and in trial that is registered. The drop‐outs were included with the reasons addressed. The drop‐out numbers (N = 4 of each group) is minimal and for similar reasons. The study includes all participants in the analysis, including those that dropped out, using intention‐to‐treat methods. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: trial was retrospectively registered. |
| Other sources of bias | Low risk | Quote: "The research grant provided by Research Deputy of Kashan University of Medical Sciences (KAUMS)." Judgement comment: we did not find any other source of bias |
Ghaeli 2004.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Unclear Type of RCT: Double blind |
|
| Participants |
Baseline characteristics Fluoxetine
Imipramine
Overall
Inclusion criteria: Nondiabetic adults with major depressive disorder. Exclusion criteria: Diabetes mellitus, history of heart disease, pregnant women, ECT received up to 6 months before. Pretreatment: Unclear whether there were any differences at baseline. |
|
| Interventions |
Intervention characteristics Fluoxetine
Imipramine
|
|
| Outcomes |
Fasting blood glucose
|
|
| Identification |
Sponsorship source: Not reported Country: Iran Setting: Mental health hospital Authors name: Padideh Ghaeli Institution: Email: mmppg@yahoo.com Address: Psychiatric Research Center, Roozbeh Mental Health Hospital, South Kargar Avenue, Tehran, 13185‐1741, Iran. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Article states this is a double blind trial, but no further information reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors, nor on who assessors were. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17 out of 60 patients entered the study but either dropped out or did not meet inclusion criteria. Reasons for drop‐out not reported, but may be related to side effects from treatments. Analysis as treated. |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol |
| Other sources of bias | High risk | No sponsorship or conflicts of interest reported. Variable dose of medication; potential for influence on outcomes if those providing medication were related to the study. Unclear whether study criteria were applied before or after the randomization. |
Hu 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Hospital Type of RCT: Prospective, randomized, 12‐week, open‐label,flexible‐dose, parallel‐group study |
|
| Participants |
Baseline characteristics Paliperidone ER
olanzapine
Overall
Inclusion criteria: Inpatients, aged 18 to 45 years, fulfilled diagnostic criteria for schizophrenia, body mass index (BMI) 18 to 30 kg/m2, and they must either not have received any antipsychotic treatments in the past or they must not have been taking an antipsychotic medication for at least 3 months were included Exclusion criteria: Treatment with paliperidone, olanzapine, clozapine, or antidepressant within 1 month; current treatment with insulin or oral antidiabetic agents, or a prior diagnosis of diabetes or hyperlipidaemia; current substance abuse; a current medical condition that may affect glucose/lipid metabolism; and pregnancy Pretreatment: The groups did not differ significantly on any characteristic (p>0.05). |
|
| Interventions |
Intervention characteristics Paliperidone ER
Olanzapine
|
|
| Outcomes |
Fasting glucose
BMI
Waist circumference
Drop‐out
|
|
| Identification |
Sponsorship source: This work is partly supported by a grant fromShanghai Commission of Science and Technology (No. 10440710200). Country: China Setting: Hospital (First Affiliated Hospital, College of Medicine, Zhejiang University, and Kangci Hospital of Jiaxing.) Comments: ‐ Authors name: Shaohua Hu, Mingrong Yao Institution: Department of Mental Health, First Affiliated Hospital, Zhejiang University School of Medicine, Email: xuyi61@yahoo.com.cn Address: Department of Mental Health, First Affiliated Hospital,Zhejiang University School of Medicine, Hangzhou 310003, China |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "List of random sequence assignments for each drug was drawn up by a computer at the beginning of the study". "80 participants were randomly assigned to treatment sequentially, with an equal probability of receiving paliperidone ER or olanzapine, 40 participants in each group. " |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "a prospective, randomized, 12‐week, open‐label, flexible‐dose, parallel‐group study". Judgement's comment: No blinding was done. Measures of blood glucose less likely to be influenced than measure of waist circumference. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was done. Measures of blood glucose less likely to be influenced than measure of waist circumference and drop‐outs. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/40 patients in paliperidone group and 17/40 in olanzapine group discontinued treatment. Outcomes only reported for patients who completed treatment (as treated analysis). Some drop‐outs were due to adverse effects (more in olanzapine arm) or poor response. It is likely that adverse effects of olanzapine are underestimated. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not referenced. |
| Other sources of bias | Unclear risk | Quote: "we were unable to perform an intent‐to‐treat analyses because all patients who dropped out failed to complete 4 weeks of treatment, and therefore the missing data could not be addressed appropriately using the Last Observation Carried Forward (LOCF)". Judgement's comment: Only baseline characteristics for those who completed the study are reported. No reporting of conflicts of interest. |
Li 2009.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Hospital Type of RCT: Unclear |
|
| Participants |
Baseline characteristics Aripiprazole
Risperidone
Clozapine
Inclusion criteria: Met diagnostic criteria for schizophrenia (Chinese criteria), not received antipsychotics for > 3 months or discontinued, PANSS ≥ 60. Exclusion criteria: Endocrine disease, malnutrition, diabetes, or other mental disorders, allergic to one of three drugs, refusal to participate. Pretreatment: No statistically significant differences. |
|
| Interventions |
Intervention characteristics Aripriprazole
Risperidone
Clozapine
|
|
| Outcomes |
Fasting glucose
BMI
|
|
| Identification |
Sponsorship source: ‐ Country: China Setting: Hospital Comments: ‐ Authors name: Yi‐Chen Li Institution: ‐ Email: ‐ Address: ‐ |
|
| Notes | Translated from Chinese. Information missing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbered balls drawn randomly from cloth bag. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how allocation would be concealed. Randmoisation was performed by the researcher. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11‐12% drop out in each group. Adverse effects in risperidone (N=1) and clozapine (n = 2) groups. Other reasons for not including in analyses were: drop out, patient stopped drug, and poor efficacy of drug. No baseline characteristics presented for drop‐outs or complete baseline sample. |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol. |
| Other sources of bias | Unclear risk | No further sources of bias identified. Unclear who funded this study; could be pharmaceutical company. |
Modabbernia 2014.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Academic psychiatric hospital Type of RCT: Double‐blind |
|
| Participants |
Baseline characteristics Melatonin + olanzapine
Placebo + olanzapine
Overall
Inclusion criteria: Age 18 to 65 years, first episode schizophrenia (DSM‐IV‐TR), ability to take medicine orally, eligible for starting olanzapine Exclusion criteria: Married women who are at reproductive age, history of taking olanzapine in the recent 3 months, history of allergy or intolerance to olanzapine, history of significant head trauma (causing loss of consciousness more than 5 minutes or neurological or cognitive sequels). Liver, kidney, cerebrovascular or cardiovascular disease, diabetes, metabolic syndrome, cancer. Using antiepileptic (other than benzodiazepines for sleep), antihypertensive, anticoagulant, anti‐platelet drugs, using inhibitors or stimulants of hepatic isoenzymes that metabolize melatonin or olanzapine (e.g. omeprazole, rifampin, fluvoxamine, ciprofloxacin, carbamazepine, modafinil), delirium, need for administration of other antipsychotics. Substance abuse Pretreatment: Placebo group had slightly lower PANSS score, lower fasting glucose, lower cholesterol at baseline. |
|
| Interventions |
Intervention characteristics Melatonin + olanzapine
Placebo + olanzapine
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Waist circumference
Diastolic blood pressure
Systolic blood pressure
Drop‐outs
|
|
| Identification |
Sponsorship source: Grant from Guilan University of Medical Sciences to Prof MJ Modabbernia (grant number 9277). Country: Iran Setting: Academic psychiatric hospital Comments: SEM is reported for outcomes instead of SD Authors name: Amirhossein Modabbernia Institution: Department of Psychiatry, Shafa Hospital, Guilan University of Medical Sciences, Rasht, Iran Email: amirh899@gmail.com Address: 15 Khordad Ave, Rasht 41939‐55599, Iran |
|
| Notes | Trial registration: https://clinicaltrials.gov/ct2/show/NCT01593774Publication: J Psychiatr Res. 2014 Jun;53:133‐40. doi: 10.1016/j.jpsychires.2014.02.013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Random number generator used. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: Treatment allocation was conceal from the study participants and physicians who rated them using sequentially numbered and opaque envelops. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Participants, physicians, and statistician were blinded. The study states that those blinded to allocation include: patients, their family members, the evaluator, statistician and those responsible for administering the intervention. There was insufficient information to determine how blinding was tested. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: clinical evaluators were blinded. The study states that treatment allocation and clinical evaluation were done by different individuals. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Numbers do not match: in section 3.1 7 patients in each group were said to have dropped out, out of a total of 24, but the data is for N=18 participants per group. Reasons for drop‐out do not reveal obvious differences in reasons related to treatment. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Protocol available and outcomes match those reported. |
| Other sources of bias | Low risk | No other sources of bias identified. |
Moosa 2003.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: 28 outpatients and 10 healthy volunteers were recruited Type of RCT: Open label trial |
|
| Participants |
Baseline characteristics Imipramine
Fluoxetine
Overall
Inclusion criteria: female < 50 years of age. Diagnosed with major depressive disorder HAM D > 17. Exclusion criteria: History of a serious physical illness, psychotic symptoms, psychoactive substance abuse or dependency, comorbid illness that required pharmacotherapy, or presence of an eating disorder. Pretreatment: Baseline mean fasting glucose ‐ significant differences between Imipramine, Fluoxetine and controls (5.9mMol/L or 106.2mg% : 4.9mMol/L or 88.2mg%: 4.1mMol/L or 73.8mg%) |
|
| Interventions |
Intervention characteristics Imipramine
Fluoxetine
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Drop‐outs
Depression score (Ham‐D, Hamilton rating scale for depression,)
|
|
| Identification |
Sponsorship source: The funds for this study were provided by the University Research Committee andthe Department of Psychiatry, University of Witwatersrand, South Africa. Country: South Africa Setting: Outpatient clinic at the Chris Hani Baragwanath Hospital, a tertiary level institution situated in Soweto, South Africa Comments: ‐ Authors name: Mahomed Y.H. Moosa, Institution: Department of Psychiatry, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Email: moosamy@medicine.wits.ac.za. Address: FCPsych, P.O. Box 4581, Johannesburg 2000, South Africa |
|
| Notes | Dr Sharad Philip on 16/08/2019 00:13 Outcomes Figures have been converted from mMol/L to mg. Conversion factor is multiplication by 18.02. Drop‐outs have not been included in analysis of baseline characteristics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to receive either imipramine (dose 150 mg/d) or fluoxetine (dose 20 mg/d)." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: open label RCT. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | On further contact, the author responded that the weight and hip measurements was done by a nurse who was blinded to the treatment and for the biochemical measures the lab technician who was blinded to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "During the study, some of the drugs’ side effects such as headache, nausea, and gastric irritation caused uneven numbers of patients to drop out of each group (imipramine n = 7, fluoxetine n = 3)." |
| Selective reporting (reporting bias) | Unclear risk | No protocol is referenced. |
| Other sources of bias | High risk | Judgement comment: Differences in baseline fasting glucose between the three groups is sizable. |
Narula 2010.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Both inpatients and outpatients meeting inclusion criteria attending tertiary Psychiatric hospital. Type of RCT: Double blind |
|
| Participants | Baseline characteristics Olanzapine + placebo
Olanzapine + topiramate
Overall
Inclusion criteria: 18 to 65 years old, first episode, drug naive patients with schizophrenia (ICD‐10), inpatient and outpatient. Exclusion criteria: History of other neuropsychiatric illness, taking SSRIs, mood stabilizers or another drug which could affect weight, substance abuse diagnosis in last 3 months, or another significant medical disorder. Pregnant and lactating women and women of childbearing age not using adequate contraception were excluded. Pretreatment: No significant differences |
|
| Interventions |
Intervention characteristics Olanzapine + placebo
Olanzapine + topiramate
|
|
| Outcomes |
Fasting blood glucose
BMI
Diastolic blood pressure
Systolic blood pressure
|
|
| Identification |
Sponsorship source: None reported Country: India Setting: Psychiatric clinic at tertiary care hopsital Comments: ‐ Authors name: Preeta Kaur Narula Institution: Lady Hardinge Medical college and associated SSK hospitals Email: docpreeta@yahoo.com Address: 13/42, Rajouri Garden, New Delhi 110027, India |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Seventy‐two patients were randomly assigned to the two groups." Judgement comment: No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Topiramate was initially started at a dose of 50 mg/day and after 1 week of therapy, increased to 100 mg/day and maintained on the same dose throughout the study period." Judgement comment: No information regarding those delivering the intervention. Unmasking of blinding probable due to high likelihood of topiramate causing adverse drug reaction. ADR such as worsening of psychosis would cause concern as there were 3 Drop‐outs. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: No information on who outcome assessors were or how blinding would be achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Seventy‐two patients were randomly assigned to the two groups. How‐ ever, 34 patients (10 inpatients, and 24 outpatients) in the olanzapine group and 33 patients (8 inpatients, and 25 outpatients) in the topiramate group were included in the analysis. In the olanzapine group, one patient was lost to follow up and one was non compliant with treatment. In the topiramate group, two patients were lost to follow up and one patient withdrew from the study due to personal reasons." Judgement comment: 5 patients dropped out after randomization; relatively low drop out but unclear from which groups. It seems that a patient who was non‐compliant with treatment was not analysed (per protocol analysis). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No protocol available to compare reported outcome measures. |
| Other sources of bias | Low risk | Judgement comment: No other sources of bias identified. |
Ou 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Inpatients or outpatients attending the three study centers. Type of RCT: Open label, multicentric |
|
| Participants | Baseline characteristics Olanzapine
Ziprasidone
Overall
Inclusion criteria: age 18 to 45 years, diagnosis of schizophrenia (DSM‐IV), duration of illness < 1 year, duration of antipsychotic treatment < 2 weeks, PANSS total score ≥ 60; CGI‐S ≥ 4. Exclusion criteria: Special diet or exercise program for weight loss, female patients planning to be pregnant or pregnant or lactating, psychiatric diagnosis other than schizophrenia, heart disease, epilepsy, hepatic or renal diseases, diabetes, aplastic anemia, systemic lupus erythematosus, or asthma Pretreatment: No notable differences at baseline. |
|
| Interventions |
Intervention characteristics Olanzapine
Ziprasidone
|
|
| Outcomes |
Fasting plasma glucose
BMI
Cholesterol
Diastolic blood pressure
Systolic blood pressure
Drop‐outs
|
|
| Identification |
Sponsorship source: This study was supported by the Pfizer Inc., the National Natural Science Foundation of China (Grant No. 30971052 to RRW), and National R&D Special Program for Health Professions(Grant No. 201002003 to JPZ). Country: China Setting: Two hospitals and one health centre Comments: ‐ Authors name: Ren‐Rong Wu Institution: Institute of Mental Health of the Second Xiangya Hospital, Central South University Email: wurenrong2005@yahoo.com Address: Institute of Mental Health of the Second Xiangya Hospital, Central South University, 139 Middle Renmin Road, Changsha 410011 Hunan, People’s Republic of China |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomization but not how this was done. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: No information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was a multicenter, 6‐week, open‐label, parallelgroup, randomized, trial". No blinding, even though it would have been possible to at least blind the patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The PANSS and CGI‐S were administered at baseline and week 6 (or endpoint). One investigator per treatment center had completed the rater training and especially responsible for evaluating all the scales;" No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out was higher in olanzapine than ziprasidone arm. Missing data was imputed with 'last observation carried forward', which may have led to data biased towards more favourable estimates. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No protocol reference available. |
| Other sources of bias | High risk | Conflict of interest: study received funding from pharmaceutical industry and trial was monitored by the same company (Pfizer Inc). |
Saddichha 2008.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: All consecutive inpatients diagnosed with first episode schizophrenia Type of RCT: Randomized, double‐blind, controlled prospective study |
|
| Participants |
Baseline characteristics Risperidone
haloperidol
olanzapine
control
Overall
Inclusion criteria: Consenting inpatients. Diagnosed with DSM IV schizophrenia. Controls ‐ consecutively seen accompanying persons of patients attending the institute Exclusion criteria: Patients with other psychiatric comorbidity or history of severe physical illness. Alcohol and substance abuse or dependence. History of pre‐existing diabetes or hypertension or family history of hypertension or DM. Pretreatment: There were no significant differences between the groups in age, gender and fasting blood sugar (FBS).However, a significant difference in 2 hour post‐prandial blood sugar (PPBS) was noted between the control group and the treatment group, which persisted only for males |
|
| Interventions |
Intervention characteristics Risperidone
Haloperidol
Olanzapine
Control
|
|
| Outcomes |
Fasting blood sugar
Diabetes ‐ ADA criteria
Diabetes ‐ WHO criteria (FBS)
Drop‐outs
|
|
| Identification |
Sponsorship source: Not mentioned Country: India Setting: Central Institute of Psychiatry, Ranchi, India which is a referral psychiatric institute Comments: ‐ Authors name: S Saddichha Institution: National Tobacco Control Programme, WHO India, Bhubaneshwar Email: saddichha@gmail.com Address: 790, Govind Prasad, Mahabir Nagar Road 2, Cuttack Road, Bhubaneswar 751012, India. |
|
| Notes | Dr Sharad Philip on 11/08/2019 05:47 Population Details not given in the text of the article or tables about O, R and H groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "All consecutive patients with a DSM‐IV diagnosis of schizophrenia, in the Central Institute of Psychiatry, Ranchi, India". "Patients included in the study were randomized to receive risperidone, olanzapine or haloperidol. " Judgement comment: no sequence generation was reported. It appears randomizations may have taken place between antipsychotics but not vs control arm. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not mentioned. Decision of O vs R vs H was in routine care. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: the study was double blind, however, no detailed information on how they were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All assessments were performed by a single investigator blind to the diagnosis and medication prescribed and all investigations were carried out in the same laboratory." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs unclear. |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol. |
| Other sources of bias | Low risk | No other sources of bias identified. |
Salehi 2009.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Randomly selected patients with major depressive disorder referred to the researcher's private clinic at Arak Medical Center and Shahid Hashemi Arak Neurology and Psychiatric Clinic in 2005. Type of RCT: Unclear |
|
| Participants |
Baseline characteristics Fluoxetine
Imipramine
Overall
Inclusion criteria: Diagnosis of major depression (DSM‐IV‐TR), lack of diabetes of family history, no use of effective blood glucose medication, no history of heart, kidney and liver disease, age between 20 to 50 years old,no prohibition to use Flouxetin and Imipramine, not taking antidepressants or electroshock therapy in the last 3 months. Exclusion criteria: Drug sensitivity, patient discontinuation, patient's reluctance and unwillingness to continue treatment, hyperglycemia, non‐response, change of medication, and pregnancy. Pretreatment: No significant differences observed. Similar proportion of participants with acute versus chronic depression in both groups. Similar baseline glucose values. |
|
| Interventions |
Intervention characteristics Fluoxetine
Imipramine
|
|
| Outcomes |
Fasting blood sugar
|
|
| Identification |
Sponsorship source: Not reported Country: Iran Setting: Arak Medical Center Comments: ‐ Authors name: Dr. Bahman Salehi Institution: Arak Medical University Email: basalehi@yahoo.com Address: Arak Medical University, Arak, Iran |
|
| Notes | Translated from Persian. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: There is insufficient information regarding how randomization was achieved although abstract and methods say trial was randomized. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: There is no mention of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: There is no mention of blinding of personnel or participants in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: There is mention of blinding of outcome assessment in the study but testing was done by a technician. Blinding is unlikely to influence the outcome of fasting blood sugar as an objective outcome. It therefore was not likely that a lack of blinding influenced the outcome measurement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: No drop‐outs reported; discontinuation or reluctance to continue are reasons for exclusion. . Non‐response to medication is mentioned as a reason for exclusion, as is patient discontinuation. Inclusion/ exclusion criteria appear to have been applied retrospectively to remove 'unsuitable' participants from the study. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: There is no reference to a protocol or trial registration. |
| Other sources of bias | High risk | Judgement comment: ‐ The study does not clarify what the "specific dietary recommendations" were. |
Sepehrmanesh 2015.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Randomly selected patients with major depressive disorder from hospital of Kashan University of Medical Sciences, Kashan, Iran, between October 2014 to December 2014. Type of RCT: Randomized, double‐blind, placebo‐controlled clinical trial |
|
| Participants |
Baseline characteristics Vitamin D
Placebo
Overall
Inclusion criteria: Age 18 to 65 years, diagnosed with MDD based on DSM IV, who had a score of 15 or greater on the 17‐item HRSD. Exclusion criteria: History of coronary infarction, angina pectoris, stroke, or renal stone disease, pregnant or lactating women, smokers, those with liver problems or substance abuse, or those having non‐normal creatinine concentrations or taking dietary supplements during the last 2 months. Pretreatment: No statistically significant differences at baseline. |
|
| Interventions |
Intervention characteristics Vitamin D
Placebo
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Depression
Drop‐outs
|
|
| Identification |
Sponsorship source: The study was supported by grant from the Kashan University of Medical Sciences Country: Iran Setting: Research Center for Biochemistry and Nutrition in Metabolic Diseases Comments: ‐ Authors name: Dr. Bahman Salehi Institution: Kashan University of Medical Sciences Email: asemi_r@yahoo.com Address: Kashan, Iran |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was conducted by the use of computer‐ generated random numbers. Randomization were concealed from the researcher and participants until the main analyses were completed. |
| Allocation concealment (selection bias) | Low risk | Randomization and allocation were concealed from the researcher and participants until the main analyses were completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blinded and used identical placebo. The appearance of the placebo capsules, including color, shape, size, and packaging, was identical to that of the vitamin D capsules. Vitamin D and placebo capsules were in the same form of packaging, and neither the patients nor the investigators were aware of the content of the package until the end of the data analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not explicitly stated but given use of placebo it seems reasonable to assume that outcome assessors would have been blinded. A trained nutritionist at the psychiatry clinic obtained anthropometric measurements at pre‐ and postintervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop out 2/20 in both groups; reasons for drop‐out not reported (and confusingly worded as 'exclusions') but not likely to substantially impact on results. |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol or trial registration. BDI was said to be the primary outcome but results have not been presented as such. |
| Other sources of bias | Low risk | Vitamin D and placebo capsules were provided by Zahravi Pharmaceutical Company and Barij Essence Pharmaceutical Company, respectively. Though medication was provided by pharmaceutical companies but no pharmaceutical funding reported. |
Tohen 2011.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Patients were recruited in Japan, China, Taiwan, South Korea and the USA in both in‐patient and out‐patient settings. Type of RCT: Double blind for 6 weeks, followed by 18 weeks open label |
|
| Participants |
Baseline characteristics Placebo
Olanzapine
Overall
Inclusion criteria: Meet diagnostic criteria for major depressive episode and bipolar I disorder (DSM‐IV‐TR), HAM‐D score >=18, age 18 to 64 years, informed consent, tested negative for pregnancy if female, using contraception if of childbearing age, not breastfeeding, birth control use for male patients, at least 1 manic or mixed episode within the past 6 years, YMRS total score <=8. Exclusion criteria: Investigator site personnel, Lilly employee, previously withdrawn or completed this study or another study investigating olanzapine, received drug treatment in past 30 days with drug that has not received regulatory approval, participated in another trial or drug research including olanzapine within 1 month, used olanzapine and treatment withdrawn because of side effects, bipolar depression considered to be treatment‐resistant to olanzapine or olanazapine+SSRI, experiencing current episode of bipolar depression > 90 days in duration, pregnant or nursing, serious unstable illness, history or diagnosis of diabetes mellitus, prolactin level > 200 ng/mL (full list of exclusion criteria available upon request) Pretreatment: No significant differences observed. Similar baseline severity of illness. |
|
| Interventions |
Intervention characteristics Placebo
Olanzapine
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Waist circumference
Diastolic blood pressure
Systolic blood pressure
Drop out
MADRS
|
|
| Identification |
Sponsorship source: This study was funded by Eli Lilly and Company Country: Patients were recruited in Japan, China, Taiwan, Korea and the USA (outcomes are shown for China) Setting: 64 study centers; inpatient and outpatient Comments: ‐ Authors name: Mauricio Tohen Institution: University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA Email: Email: tohen@uthscsa.edu Address: UT Health Science Center, Division of Mood and Anxiety Disorders, 7526 Louis Pasteu |
|
| Notes | Noortje Uphoff on 22/08/2019 18:29 Population Population characteristics for China only. Data from Taiwan not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment to treatment groups was determined by a computer‐generated random sequence using an interactive voice response system." Judgement comment: Computer‐generated sequence used. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: Minimal number of Lilly personnel saw randomization table and these people were not involved in administering medication or monitoring patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Double‐blinded. Participant discontinued from study if unblinded. System in place for emergency unblinding in case of adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Reasons for drop‐out were monitored (data not available by country but adverse events similar in the two overall groups). Drop‐out rates were similar between groups; 23% in placebo and 25% in olanzapine group. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Protocol available and outcomes match those reported in study report. One additional outcome collected and reasons for deviation from protocol are discussed. |
| Other sources of bias | High risk | Judgement comment: Study funded by pharmaceutical company Eli Lilly and Company and study authors are either employees of the company and/or stockholders or received grants from the same company. |
Wang 2012.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Participants were recruited from the schizophrenia outpatient clinic of the psychiatry department of two hospitals Type of RCT: Double blind, placebo controlled randomized study |
|
| Participants |
Baseline characteristics Melatonin
placebo
Overall
Inclusion criteria: Patients aged 18 to 60 years with a first psychotic episode of schizophrenia. Participants gained more than 7% of their predrug body weight within the first year of treatment with a targeted antipsychotic agent – clozapine, olanzapine, risperidone,or sulpiride; have relatively stable improvement (the total score of Positiveand Negative Symptom Scale [PANSS]≤60); taking only one antipsychotic agent, whose dose had not changed by more than 25% over the past 3 months. Exclusion criteria: Patient with liver or renal dysfunction, cardiovascular disease,diabetes mellitus, arthritis, pulmonary or neurological disease; pregnant or lactating. Patients were also excluded if they had received a psychiatric diagnosis other than schizophrenia. Pretreatment: No significant baseline group differences |
|
| Interventions |
Intervention characteristics Metformin
Placebo
|
|
| Outcomes |
Fasting blood sugar
BMI
Drop‐out
|
|
| Identification |
Sponsorship source: This article was supported by research grant from the Scientific Research Fund ofLiaoning Science and Technology Agency, China (No. 2011225020). Country: China Setting: Outpatients, schizophrenia clinic, mental health institute Comments: ‐ Authors name: Man Wang Institution: Department of Psychiatry, the First Hospital of China Medical University, Email: wangmancmu@yahoo.com.cn Address: Department of Psychiatry, the First Hospital of China Medical University, 155# Nanjing North Road, Shenyang 110001, Liaoning, PR China |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The participants were randomly assigned to 12 weeks of antipsychotics with either metformin (1000 mg/d) or placebo." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Metformin treatments were administered in a double‐blind placebo‐controlled fashion. For the first three days, participants took 250 mg of metformin or placebo twice a day, one before lunch and one before dinner. Afterwards, 500 mg of metformin or placebo was given twice a day for rest of the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: No information on outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: attrition was reported; 4 in metformin group and 2 in placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No protocol referenced. |
| Other sources of bias | Unclear risk | Judgement comment: the study did not evaluate the patients'diet and exercise, so we did not know if there were some different diet and exercise between the two groups. |
Wu 2006.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: From Institute of Mental Health in a hospital. Type of RCT: Appears to be open label. |
|
| Participants |
Baseline characteristics Clozapine
Olanzapine
Risperidone
Sulpiride
Overall
Inclusion criteria: age 18 to 45 years, first psychotic episode of schizophrenia (DSM IV), no other antipsychotics or drugs before treatment. Exclusion criteria: Pregnant or lactating women, mental retardation, diabetes, dyslipidemia, cardiovascular disease, hypertension. Pretreatment: Similar baseline characteristics |
|
| Interventions |
Intervention characteristics Clozapine
Olanzapine
Risperidone
Sulpiride
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Waist circumference
Diastolic blood pressure
Systolic blood pressure
|
|
| Identification |
Sponsorship source: Supported by National Key Technologies R&D Program in the 10th
5‐year‐plan grant 2004BA720A22 (Dr. Zhao) from the Ministry of Science and Technology of the People’s Republic of China. Country: China Setting: hospital Comments: Authors name: Ren‐Rong Wu, MD Institution: Institute of Mental Health of the Second Xiangya Hospital, Central South University, Changsha, China. Email: wurenrong2005@yahoo.com.cn Address: Institute of Mental Health of the Second Xiangya Hospital, Central South University, 139 Renmin Middle Road, Changsha 410011, Hunan, China. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized (without any restriction or stratification) through a computer‐generated table to one of the 4 treatments in blocks of 8 to ensure approximately equal numbers of participants in the 4 treatments. |
| Allocation concealment (selection bias) | Low risk | To ensure concealment of the randomization, it was conducted independently of the investigators by a research pharmacist at a separate facility. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study let the participants and their family numbers know which antipsychotic drugs they received and the investigators were not blinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Altogether, 120 patients entered the trial. Of these, 8 patients (3 clozapine patients, 1 olanzapine patient, 2 risperidone patients, and 2 sulpiride patients; 5 women and 3 men) dropped out because they were in hospital less than 8 weeks, and they were excluded for lack of glucose levels at points during the study." |
| Selective reporting (reporting bias) | Unclear risk | No protocol referenced. |
| Other sources of bias | Low risk | No other bias identified. |
Wu 2008a.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were patients recruited: The participants were recruited from the Mental Health Institute of the Second Xiangya Hospital, Central South University, P.R.China. Type of RCT: Double blind |
|
| Participants |
Baseline characteristics Olanzapine plus metformin
Olanzapine plus placebo
Overall
Inclusion criteria: Hospitalized at mental health institute, age 18 to 50 years, first psychotic episode of schizophrenia (DSM IV). Exclusion criteria: Not used any antipsychotics or recreational drugs for at least 3 months before enrollment, pregnant or lactating women and patients with mental retardation, addictive disorder, specific systemic diseases, or other medical conditions such as diabetes mellitus, dyslipidemia, cardiovascular diseases, and hypertension. Pretreatment: No statistically significant differences at baseline. |
|
| Interventions |
Intervention characteristics Olanzapine plus metformin
Olanzapine plus placebo
|
|
| Outcomes |
BMI
Waist circumference
Fasting glucose
|
|
| Identification |
Sponsorship source: Supported by National Key Technologies R&D Program in the 10th5‐year‐plan grant 2004BA720A22 (Dr. Zhao) from the Ministry of Science and Technology of the People’s Republic of China. Country: China Setting: Mental Health Institute Comments: ‐ Authors name: Jing‐Ping Zhao Institution: Institute of Mental Health of the Second Xiangya Hospital Email: zhaojingpinghunan@yahoo.com.cn Address: Institute of Mental Health of the Second Xiangya Hospital, Central South University, 139 Renmin Middle Road, Changsha 410011, Hunan, China |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned (without any restriction or stratification) through a computer‐generated table to one of the two treatments (olanzapine plus metformin or olanzapine plus placebo) in blocks of four to ensure approximately equal numbers of participants within the two treatment" Judgement comment: Computer generated sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure concealment of the randomization, which was conducted independently of the investigators by a research phar‐ macist at a separate facility, medication was provided in coded containers containing the identical appearing pills of metformin or placebo supplied by the manufacturer." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Double blinded study. No further information, but given that a placebo was used, it can be assumed that participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The research nurses who performed physical exams were blind to the patients’ treatment assignment." Judgement comment: Its not mentioned clearly whether the SAPS, SANS and treatment Emergent Symptom was applied by whom and whether that assessor was blinded or not. Probably blinded because placebo was used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Forty patients entered the trial. Three withdrew from the study within the first 4 weeks because of lack of re‐ sponse (one placebo patient and two metformin patients; one man and two women). This resulted in 37 (92.7%) patients who were included in the study." Judgement comment: 3 participants excluded from study because they dropped out. Authors mention analysis for completers was similar to ITT analysis. Unclear whether any other participants dropped out during the study. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No reference to protocol or trial registration. |
| Other sources of bias | Low risk | Judgement comment: No other sources of bias identified. Authors report no conflicts of interest. |
Wu 2008b.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were patients recruited: From outpatient clinical of Mental Health Institute between 2004 and 2006. Type of RCT: Double blind |
|
| Participants |
Baseline characteristics Lifestyle intervention + metformin
Metformin
Lifestyle intervention + placebo
Placebo
Overall
Inclusion criteria: Age 18 to 45 years, first psychotic episode of schizophrenia (DSM‐IV), weight gain over 10% in the year after medication started, weight and antipsychotic treatment history available, stable improvement PANSS, taking only one antipsychotic agent with no major dose change in last 3 months. Exclusion criteria: Liver or renal dysfunction, cardiovascular disease, diabetes, pregnant or lactating, other conditions limiting ability to perform lifestyle modifications, psychiatric diagnosis other than schizophrenia or history of substance abuse. Pretreatment: No statistically significant differences at baseline. |
|
| Interventions |
Intervention characteristics Lifestyle intervention + Metformin
Description of the intervention: The lifestyle interventions included psychoeducational, dietary, and exercise programs. Target group was adult patients with schizophrenia. informing intervention design: For the dietary intervention, the American Heart Association (AHA) step 2 diet was prescribed. provider and Mode of delivery: participants maintained 3‐day food records before each follow‐up visit. The dietitian reviewed the food records, compared them with the prescribed AHA diet, and discussed with each patient concerns about adherence to the AHA diet. For the first week of the study, exercise sessions were directed by an exercise physiologist. Participants performed endurance exercise (walking or jogging) on a treadmill 7 times a week for 30 minutes at each session. Exercise was designed to attain 70% of heart rate reserve [0.7(maximum heart rate –resting heart rate)resting heart rate]. Heart rate reserve was estimated from the maximum and resting heart rates observed at baseline maximal aerobic capacity (V˙ O2max) for each individual. After the first week, exercise was home‐based without supervision by investigators. Therapists and patients collaboratively developed individual programs of gradual assignment of exercise. A range of different types of exercise and various levels of intensity was offered, varying from light exercise (increase walking and decrease sedentary activities, particularly time spent watching television or sleeping) to moderate exercise for at least 30 minutes per day. Common moderate‐to‐vigorous physical exercise included walking at a moderate or very strenuous intensity, bicycling, resistance training, skiing, jogging, ball games, and lifestyle activities, such as chopping wood or clear. Adherence: Branching treadmill tests were performed under supervision at each follow‐up visit. Good exercise adherence was defined as an increased V˙ O2max of at least 1.5 mLkg−1.min−1 at 4, 8, and 12 weeks compared with baseline. between 50% and 57% had V˙ O2max results that demonstrated adherence to exercise. The participants’ adherence to metformin treatment for each visit was defined as taking more than 80% of the study drug dosage prescribed for that interval. If a participant was nonadherent, both patient and caregiver were counseled on the importance of taking the prescribed amount of study medication. Participants and their caregivers were instructed to keep records of their exercise activity and heart rate. This information was also used to estimate adherence. Demographics, training, professional status of the investigators was not clearly mentioned. Metformin
Lifestyle intervention + Placebo
Placebo
|
|
| Outcomes |
Fasting blood sugar
BMI
Waist circumference
Drop‐out
|
|
| Identification |
Sponsorship source: National Key Technologies R&D Program in the 10th 5‐year‐plan grant 2004BA720A22 from the Ministry of Science and Technology of the People’s Republic of China. Country: China Setting: Mental Health Institute Comments: ‐ Authors name: Ren‐Rong Wu Institution: Mental Health Institute of the Second Xiangya Hospital Email: wurenrong2005@yahoo.com.cn Address: Mental Health Institute of the Second Xiangya Hospital, Central South University, Mail Code: 410011,139 Renmin Middle Rd, Changsha, Hunan, China |
|
| Notes | Only metformin versus placebo data included in meta‐analyses. Results of other study arms described narratively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized through a computer‐generated table" Judgement comment: Random sequence generation by computer. |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignment was determined after completing all screening assessments and being accepted into the study. To ensure concealment of the treatment assignment, randomization was conducted independently of the investigators by a research pharmacist" Judgement comment: allocation concealed from investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Metformin treatments were administered in a double‐blind placebocontrolled fashion." Judgement comment: Participants were blinded to medication/placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: As placebo was used, outcome assessors were blinded to placebo/medication but possibly not to behavioural intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: No obvious difference in drop‐out between study arms. LOCF used for missing data; this may cause bias but unlikely due to low drop‐out rates. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Trial registered retrospectively. |
| Other sources of bias | Unclear risk | Judgement comment: Even though only participants who had gained more than 10% body weight on medication were included, patients were on average not overweight and mostly fitted within a very narrow BMI range of 24‐25 at baseline. We also had some queries about the data presented in Table 2, because several estimates and confidence intervals were identical for different groups and timepoints. The authors were contacted for more information twice in three weeks, but we received no response. |
Zhang 2012.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Participants were recruited from outpatient and 254 patients entered the trial. Type of RCT: Open‐label study |
|
| Participants |
Baseline characteristics Paliperidone extended‐release (ER)
Aripiprazole
Ziprasidone
Overall
Inclusion criteria: Participants between the ages of 18 and 65 years with first‐episode of schizophrenia. Exclusion criteria: Current substance abuse; Diabetes mellitus; Thyroid diseasePregnancy; Significant medical illness including severe cardiovascular, hepatic or renal disease; Unstable psychiatric illness. Patients treated with medications known to affect metabolism. Pretreatment: There was no significant difference between groups |
|
| Interventions |
Intervention characteristics Paliperidone extended‐release (ER)
Aripiprazole
Ziprasidone
|
|
| Outcomes |
Fasting blood sugar
BMI
Cholesterol
Waist circumference
Diastolic blood pressure
Systolic blood pressure
Drop‐out
|
|
| Identification |
Sponsorship source: Nothing mentioned Country: China Setting: Outpatient clinic of the Fourth People’s Hospital of Chengdu Comments: Standard deviation was not reported for Fasting plasma glucose, HbA1C, Choesterol. Authors name: Yinbo Zhang and Guangzhi Dai Institution: Chengdu Mental Health Center, FourthPeople’s Hospital, Email: boyinzhang@163.com Address: Yinbo Zhang, Chengdu Mental Health Center, FourthPeople’s Hospital, Chengdu 610035, China. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Firstly, each medication was labeled with number: aripiprazole as 1; ziprasidone as 2 and paliperidone as 3. Each patient identifying with first‐episode schizophrenia would get a card with number 1–3 randomly from a nurse working in outpatient." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Open label study; no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is an open‐label study despite of randomization." Judgement comment: Open label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: I infer low risk of bias due to lack of blinding of outcome assessors with regard to anthropometric measurements and laboratory measures. These are all hard outcomes obtained by standardised procedures and are replicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: Baseline characteristics of 51 drop‐outs have not been reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No protocol is referenced. |
| Other sources of bias | Unclear risk | Quote: "Despite partial reimbursement of medication expenditure from Human Resources and Social Security Bureau, the rest part of self‐paying was also a burden for some families. In China, the reimbursement of medication expenditure in outpatient from Human Resources and Social Security Bureau was different according to registered permanent residence of patients. Patients of registered permanent residence in cities could get 90% and higher reimbursement of medication expenditure, whereas patients of registered permanent residence in countries could only get about 50% reimbursement of medication expenditure. But patients without paid medical insurance fee regularly (monthly) have to pay the medication without any reimbursement. Therefore, country patients and self‐paying patients were the most likely to discontinue medication." Judgement comment: Patient may discontinue medicine due to high cost. |
Zhang 2014.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: From patients receiving treatment at hospital in 2012‐2014. Type of RCT: Randomized trial |
|
| Participants |
Baseline characteristics Olanzapine
Quetiapine
Aripriprazole
Overall
Inclusion criteria: Based on the inclusion criteria, eligible participants: (a) met the diagnostic criteria for schizophrenia based on the 3rd edition of the Chinese Classification of Mental Disorders (CCMD‐3); (b) has a total score of >60 on the Positive and Negative Syndrome Scale (PANSS); (c) had never been treated with antipsychotic medication; (d) had a duration of illness ≤ 5 years; (e) were 17 to 60 years of age; (f) had normal functioning heart, liver, and kidneys; and (g) provided written informed consent (or the guardian provided written informed consent) to participate in the study. Exclusion criteria: Individuals were excluded if they: (a) had a mental disorder induced by endocrine dysfunction (e.g., thyroid dysfunction); (b) were pregnant or breast feeding; (c) has a history of alcohol or drug dependence; or (d) took medications that could influence metabolism including glucocorticoids, diuretics, or contraceptives. Pretreatment: There were no statistically differences between groups with respect to gender, proportion of inpatients, age, duration of illness, or severity of illness (measured by the PANSS score). 47 inpatients had a higher PANSS baseline score than the 103 outpatients, as expected but no statistically significant difference between baseline PANSS scores of inpatients across the three treatment groups or across the outpatients of the three groups. |
|
| Interventions |
Intervention characteristics Olanzapine
Quetiapine
Aripriprazole
|
|
| Outcomes |
Fasting blood glucose
Cholesterol
Drop‐outs
|
|
| Identification |
Sponsorship source: The study was not funded by any agency Country: China Setting: Zhangilagang Kangle Hospital Comments: Authors name: Shufen Zhang Institution: Kangle Hospital Email: zxcscompany@163.com Address: Kangle Hospital, Zhangilagang City, Jiangsu Province, China |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Those enrolled were randomly assigned a study group by using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on how participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study mentions that the assessors for clinical assessment were blinded to patients' treatment status. There is no mention of blinding of assessors of biochemical outcomes but these are more objective and these outcome measurements are not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: The study did not include data of participants that dropped out in the baseline characteristics. There is also no mention of intention‐to‐treat analysis. Each group started with either 55 or 54 in the group after random allocation and in both baseline and after 8‐week follow up, N = 50 for each of the three groups. Drop‐out was 5 in each of the three treatment groups with similar reasons for dropping out. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Prespecified outcomes mentioned in the Methods section are addressed in the study. There is no reference to protocol in the study. |
| Other sources of bias | Unclear risk | Judgement comment: The study was not funded by any agency. During the study, benzodiazepines and anticholinergics were used if considered necessary by clinicians. |
Zhao 2015.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group How were participants recruited: Outpatients and hospitalised patients from Henan Mental Hospital were approached between October 2012‐May 2014. Type of RCT: Open |
|
| Participants |
Baseline characteristics Aripiprazole (+ risperidone)
Control (risperidone only)
Overall
Inclusion criteria: 18 to 45 years old, diagnosis of schizophrenia or schizoaffective disorder, stable psychiatric condition, stable on risperidone for at least 8 weeks, elevated serum prolactin level associated with risperidone treatment, willingness to use birth control during study. Exclusion criteria: No informed consent, renal disease, history of immunosuppression, chemotherapy, pregnancy/ breastfeeding, history of adverse reactions to aripiprazole or history of tardive dyskinesia or neuroleptic malignant syndrome, other conditions that could affect serum prolactin levels, taking medications known to affect glucose tolerance, following special diet or exercise program for weight loss. Pretreatment: No notable differences. Smoking only reported for control group. |
|
| Interventions |
Intervention characteristics Aripiprazole (+ risperidone)
Control (risperidone only)
|
|
| Outcomes |
Plasma glucose
BMI
Total cholesterol
Drop‐outs
|
|
| Identification |
Sponsorship source: Funding for this study was provided by the National Natural Science Foundation of China (30971058 to X‐QS, 81071090, 81371472 to L‐XL) and the Natural Science Foundation of Henan (102300413208, 112300413226 to L‐XL) Country: China Setting: Psychiatric hospital Comments: ‐ Authors name: Jingyuan Zhao Institution: Henan Mental Hospital Email: Lvx928@126.com Address: Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang MedicalUniversity, Xinxiang, China |
|
| Notes | Noortje Uphoff on 05/08/2019 20:16 Included Access to protocol: http://www.chictr.org.cn/showproj.aspx?proj=10830 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: No information. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: No information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Currently, clinical trial registration is virtually not required for theses randomized, open‐label, comparative clinical trials in China, and the trial is allowed to be performed after approval by the institutional ethics review committee." Judgement comment: No blinding even though a placebo could have been used. Bias is likely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 'Assays' were conducted by analysts who were blinded, but this is likely to have been the case only for the prolactin outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Drop out was small, but 4 dropped out due to adverse events although this is not explained further. BMI reported in graph only. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: In comparison with retrospectively registered protocol, shows some items such as concomitant medical records, liver function, menstrual cycle and sexual functioning, which have not been mentioned in this article. They may not have significant implications on the outcome that we are assessing. Key outcomes mentioned in protocol match reported results. |
| Other sources of bias | Low risk | Judgement comment: No other sources of bias identified. Unusual study design: comparison of two antipsychotic medications versus one; rationale unclear. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amrami‐Weizman 2013 | Not conducted in an LMIC |
| Basu 2009 | Not conducted in an LMIC |
| Bauer 2006 | Not conducted in an LMIC |
| Breier 2005 | Not conducted in an LMIC |
| Chang 2008 | Not conducted in an LMIC |
| Chen 2013 | Not conducted in an LMIC |
| Chiu 2006 | Not conducted in an LMIC |
| Chrzanowski 2006 | Not conducted in an LMIC |
| DeHert 2011 | Not conducted in an LMIC |
| Detke 2014 | Paediatric population |
| Galynker 2005 | Not conducted in an LMIC |
| Graham 2006 | Not conducted in an LMIC |
| Grilo 2013 | Not conducted in an LMIC |
| Holka Pokorska 2015 | Not conducted in an LMIC |
| Janssen‐Cilag 2007 | Not conducted in an LMIC |
| Jarskog 2013 | Not conducted in an LMIC |
| Kahl 2016 | Not conducted in an LMIC |
| Kerling 2015 | Not conducted in an LMIC |
| Khambaty 2015 | Not conducted in an LMIC |
| Khambaty 2018 | Not conducted in an LMIC |
| Kilzieh 2007 | Not conducted in an LMIC |
| Kim 2006 | Not conducted in an LMIC |
| Krivoy 2017 | Not conducted in an LMIC |
| Lambert 2011 | Not conducted in an LMIC |
| Lin 2010 | Not conducted in an LMIC |
| Lovell 2014 | Not conducted in an LMIC |
| McIntyre 2011 | Not conducted in an LMIC |
| Meltzer 2011 | Not conducted in an LMIC |
| Nam 2004 | Not conducted in an LMIC |
| Newcomer 2009 | Not conducted in an LMIC |
| Patino 2015 | Not conducted in an LMIC |
| Peuskens 2007 | Not conducted in an LMIC |
| Robinson 2015 | Not conducted in an LMIC |
| Scheffler 2018 | Not an RCT |
| Siskind 2018 | Not conducted in an LMIC |
| Smith 2013 | Not conducted in an LMIC |
| Smith 2018 | Not conducted in an LMIC |
| Stroup 2011 | Not conducted in an LMIC |
| Strous 2007 | Not conducted in an LMIC |
| Sulaiman 2009 | Not prevention of diabetes |
| Suppes 2013 | Not conducted in an LMIC |
| Tek 2013 | Not conducted in an LMIC |
| Weisler 2011 | Not conducted in an LMIC |
| Yarborough 2013 | Not conducted in an LMIC |
| Zajecka 2002 | Not conducted in an LMIC |
| Zheng 2014 | Not prevention of diabetes |
LMIC: Low‐ and middle‐income countries; RCT: randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Asemi 2014.
| Methods | RCT |
| Participants | Major depressive disorder, 20 to 50 years old |
| Interventions | Vitamin D supplements and placebo |
| Outcomes | Fasting blood glucose, serum insulin, hs‐CRP, insulin resistance, lipid profiles |
| Notes | Trial registration available. No author response. |
Chang 2014.
| Methods | RCT |
| Participants | Patients with bipolar I or II disorder who experienced antipsychotic‐induced weight gain. |
| Interventions | Continuation of antipsychotics or switching to aripiprazole |
| Outcomes | Body weight, BMI, fasting glucose, cholesterol, triglyceride level. |
| Notes | Abstract only. No author response. |
Djokic 2017.
| Methods | RCT |
| Participants | Patients diagnosed with schizophrenia |
| Interventions | Nutriose‐glucomannan‐antioxidant complex + olanzapine, control group unclear. |
| Outcomes | BMI, waist circumference, blood pressure, fasting glucose, triglycerides, cholesterol. |
| Notes | Conference abstract. No full‐text. |
Ganguli 2011.
| Methods | RCT |
| Participants | Patients with schizophrenia plus overweight/ obesity |
| Interventions | Behaviour therapy for weight loss, usual care, or behavioural treatment for enhancing social skills. |
| Outcomes | Weight. Unclear whether other outcomes were included. |
| Notes | Conference abstract. No full‐text. |
Mondal 2014.
| Methods | RCT |
| Participants | Patients with schizophrenia |
| Interventions | Olanzapine and olanzapine + metformin |
| Outcomes | Body weight, waist circumference, fasting glucose, insulin and insulin resistance, blood pressure and lipid profile, Scale for the Assessment of Positive Symptoms (SAPS) and Scale for the Assessment of Negative Symptoms (SANS) |
| Notes | Conference abstract. No full‐text. |
Ni 2014.
| Methods | RCT |
| Participants | Patients with schizophrenia |
| Interventions | Clozapine and aripiprazole |
| Outcomes | BMI, waist circumference, blood glucose, blood lipid changes |
| Notes | Manuscript received through interlending library request not readable. |
Talaei 2009.
| Methods | RCT |
| Participants | Patients with schizophrenia at the start of hospitalisation |
| Interventions | Various doses of topiramate and placebo |
| Outcomes | Body weight, BMI, waist and wrist diameter |
| Notes | Conference abstract. No full‐text. |
Tessier 2010.
| Methods | RCT |
| Participants | Patients with schizophrenia |
| Interventions | Paliperidone and olanzapine |
| Outcomes | Triglycerides, body weight, lipids, HOMA‐IR, beta‐cell function, insulinogenic index, PANSS, adverse events. |
| Notes | Conference abstract. No full‐text. |
Wu 2012.
| Methods | RCT |
| Participants | Patients with first‐episode schizophrenia |
| Interventions | Placebo, metformin, metformin + lifestyle intervention, lifestyle intervention |
| Outcomes | BMI, waist circumference, insulin level, insulin resistance index |
| Notes | Conference abstract. No full‐text. |
Yao 2014.
| Methods | Potential RCT; randomization unclear |
| Participants | Patients with schizophrenia |
| Interventions | Risperiodone and Clozapine |
| Outcomes | Weight, height, waist circumference, hip circumference, fasting blood sugar, total cholesterol, triglycerides, lipoprotein, apolipoprotein, diabetes. |
| Notes | Paper received from interlending library request; bottom half of pages missing. No author response. |
RCT: randomized controlled trial; BMI: Body mass index
Characteristics of ongoing studies [ordered by study ID]
Ostadmohammadi 2018.
| Study name | Clinical trial of investigation of efficacy of metformin on the body mass index of patients under treatment with selective serotonin re‐uptake inhibitors drugs referred to psychiatry clinics of rasht. |
| Methods | Double blind RCT |
| Participants | 18 to 60 year old patients with schizophrenia. |
| Interventions | Probiotic oral capsules vs probiotic and selenium placebo capsules. |
| Outcomes | Clinical status, metabolic profiles. |
| Starting date | 17 September 2018 |
| Contact information | ostadmohammadi‐vr@kaums.ac.ir |
| Notes |
Saidpour 2019.
| Study name | Effects of sumac powder capsule (Rhus coriaria L.) with restricted calorie diet on anthropometric indices, body composition, level of inflammatory biomarkers, oxidative stress, appetite hormones, glycemic indices, lipid profile and depression in obese or overweight women with depression. |
| Methods | Double‐blind RCT |
| Participants | Overweight or obese individuals with moderate or mild depression. |
| Interventions | Weight loss diet plus sumac capsules vs weight loss diet and placebo capsules. |
| Outcomes | Weight; body mass index; waist circumference; hip circumference; waist to hip; body fat percentage; visceral fat; fat free mass; serum level of hs‐CRP; TNF‐α; IL‐6; malondialdehyde; leptin; neuropeptideY; triglyceride; total cholesterol; low density lipoprotein‐C; high density lipoprotein‐C; glucose; insulin; Insulin resistance |
| Starting date | 1 June 2019 |
| Contact information | a.saidpour@sbmu.ac.ir |
| Notes |
Shokrgozar 2019.
| Study name | Clinical trial of investigation of efficacy of metformin on the body mass index of patients under treatment with selective serotonin re‐uptake inhibitors drugs referred to psychiatry clinics of Rasht. |
| Methods | RCT |
| Participants | Patients with anxiety and depression. |
| Interventions | Metformin plus usual treatment (for depression/ anxiety) vs usual treatment. |
| Outcomes | Changes in BMI and anthropometric indices. |
| Starting date | 6 July 2019 |
| Contact information | dr_shokrgozar@gums.ac.ir |
| Notes |
Sulejmanpasic 2019.
| Study name | Adjunctive treatment of aripiprazole to olanzapine for weight reduction in patients with schizophrenia. |
| Methods | Double blind RCT |
| Participants | Patients with schizophrenia. |
| Interventions | Aripiprazole plus olanzapine versus olanzapine. |
| Outcomes | BMI, psychiatric symptoms. |
| Starting date | Unclear |
| Contact information | ‐ |
| Notes | Abstract only. No contact information. |
RCT: randomized controlled trial; BMI: Body mass index
Differences between protocol and review
During data extraction, it became clear that most studies measured changes in blood glucose level in response to medication, instead of our primary outcome, prevention of diabetes. To ensure that this review included all relevant evidence, we added blood glucose levels as a secondary outcome. This outcome was also added to the 'Summary of findings' tables.
Blood pressure was stated as one outcome, but we report systolic and diastolic blood pressure separately. For cholesterol, we report measures of total cholesterol only.
In the protocol, included time points were not clearly specified. We included the last time point for each study; for most studies the last time point was between six and 14 weeks after the start of the study.
In the protocol, we stated in one paragraph that no meta‐analyses would be performed if obvious clinical heterogeneity were present. In another paragraph, we stated that "data were not pooled for meta‐analysis if we detected a high degree of heterogeneity (I2 > 75%)." When starting data analyses, we therefore did not perform meta‐analyses of studies with highly variable intervention groups. However, for comparisons with high statistical heterogeneity as indicated by the I2 statistic, we found it more valuable to perform meta‐analyses and explore reasons for heterogeneity, rather than not performing meta‐analyses of these comparisons.
We performed additional sensitivity analyses, which were not planned and described in the protocol, to investigate the impact of results from two studies, which were outliers in some analyses and for which we had concerns about the validity of the primary data (Wu 2006; Wu 2008b).
Contributions of authors
Drafting of protocol: MPM, JW, JT, RA, NT, ZA, BS, RC, NS
Search strategy: JW, RC, NS
Study selection and data extraction: MPM, EU, SP, NT, FA, RAA, NS
Statistical analyses: EU, MPM
Drafting of review: MPM, EU, FA, SP, JW, NT, RAA, ZAA, BS, RC, NS
Sources of support
Internal sources
-
The University of York, UK
MPM, EU, RC, JT
-
Hull York Medical School, UK
NS, NT
-
The ARK Foundation, Dhaka, Bangladesh
AZ
-
The University of Leeds, UK
JW and RA
-
Kings College, London, UK
BS
External sources
-
National Institute of Health Research, UK
The development of this protocol was supported in part by the National Institute of Health Research (NIHR) using Official Development Assistance (ODA) funding (Grant: 17/63/130: NIHR Global Health Research Group: Improving Outcomes in Mental and Physical Multimorbidity and Developing Research Capacity (IMPACT) in South Asia at the University of York).
-
National Institute of Health Research Collaboration for Applied Health Research, UK
MPM
Declarations of interest
MP Mishu: no conflicts of interest E Uphoff: no conflicts of interest F Aslam: no conflicts of interest S Philip: no conflicts of interest J Wright: no conflicts of interest N Tirbhowan: no conflicts of interest RA Ajjan: no conflicts of interest ZA Azdi: no conflicts of interest B Stubbs: no conflicts of interest R Churchill: leads and has responsibility for Cochrane Common Mental Disorders, which has supported parts of the review process and is largely funded by a grant from the National Institute of Health and Research (NIHR) in the UK. N Siddiqi: declares a research grant: NIHR Global Health Research Group IMPACT in South Asia, which partly supported work on this review. However, the funder had no role in influencing the research question or the write‐up of findings.
New
References
References to studies included in this review
Agahi 2017 {published data only}
- Agahi M, Akasheh N, Ahmadvand A, Akbari H, Izapanah F. Effect of melatonin in reducing second-generation antipsychotic metabolic effects: a double blind controlled clinical trial. Diabetes & Metabolic Syndrome 2017;12(1):9-15. [DOI: 10.1016/j.dsx.2017.08.004] [DOI] [PubMed] [Google Scholar]
Agnihotri 2013 {published data only}
- Agnihotri AP, Sontakke SD, Thawani VR, Anand S, Goswami VSS. Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian Journal of Pharmacology 2013;45(4):417-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akkasheh 2016 {published data only}
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 2016;32(3):315-20. [DOI: 10.1016/j.nut.2015.09.003] [DOI] [PubMed] [Google Scholar]
Assunção 2006 {published data only}
- Assunção SSM, Ruschel SI, Rosa LCR, Campos JAO, O Alves MJO, Bracco OL, et al. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. Revista Brasileira de Psiquiatria 2006;28(4):270-76. [DOI: 10.1590/S1516-44462007000100025] [DOI] [PubMed] [Google Scholar]
Baptista 2006 {published data only}
- Baptista T, Martinez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Canadian Journal of Psychiatry 2006;51(3):192-6. [DOI] [PubMed] [Google Scholar]
Baptista 2007 {published data only}
- Baptista T, Rangel N, Fernandez V, Carrizo E, El Fakih Y, Uzcategui E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophrenia Research 2007;93(1-3):99-108. [DOI] [PubMed] [Google Scholar]
Baptista 2009 {published data only}
- Baptista T, Rangel N, El Fakih Y, Uzcategui E, Galeazzi T, Beaulieu S, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled, 12-week trial. Pharmacopsychiatry 2009;42(1):14-9. [DOI: 10.1055/s-0028-1085438] [DOI] [PubMed] [Google Scholar]
Carrizo 2009 {published data only}
- Carrizo E, Fernandez V, Connell L, Sandia I, Prieto D, Mogollon J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophrenia Research 2009;113(1):19-26. [DOI: 10.1016/j.schres.2009.05.007] [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen J, Pan X, Qian M, Yang S. Efficacy and metabolic influence on blood-glucose and serum lipid of ziprasidone in the treatment of elderly patients with first-episode schizophrenia. Shanghai Archives of Psychiatry 2017;29(2):104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emsley 2005 {published data only}
- Emsley R, Turner HJ, Schronen J, Botha K, Smit R, Oosthuizen PP. Effects of quetiapine and haloperidol on body mass index and glycaemic control: a long-term, randomized, controlled trial. International Journal of Neuropsychopharmcology 2005;8(2):175-82. [DOI] [PubMed] [Google Scholar]
Fadai 2014 {published data only}
- Fadai F, Mousavi B, Ashtari Z, Ali beigi N, Farhang S, Hashempour S, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: a randomized triple blind placebo controlled study. Pharmacopsychiatry 2014;47(4-5):156-61. [DOI: 10.1055/s-0034-1382001] [DOI] [PubMed] [Google Scholar]
Ghaderi 2019 {published data only}
- Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 2019;19(1):77. [DOI: 10.1186/s12888-019-2059-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi A. Clinical trial of the effect of combined probiotic and vitamin D supplementation compared with the placebo on clinical symptom and metabolic profiles in schizophrenia patients. en.irct.ir/trial/25842 (accessed 12 May 2020).
Ghaeli 2004 {published data only}
- Ghaeli P, Kamkar MZ, Mesbahi M, Khoydaki SD, Shahsavand E, Sadeghi M. Comparing the effects of 8-week treatment with fluoxetine and imipramine on fasting blood glucose in patients with major depression disorder. Iranian Journal of Diabetes & Lipid Disorders 2004;3(2):E8-8. [DOI] [PubMed] [Google Scholar]
- Ghaeli P, Shahsavand E, Mesbahi M, Avarsaji K. Comparing the effects of 8-week treatment with fluoxetine and imipramine on fasting blood glucose of patients with major depressive disorder. Journal of Clinical Psychopharmacology 2004;24(4):386-8. [DOI] [PubMed] [Google Scholar]
- Ghaeli P, Shahsavand E, Mesbahi M, Kamkar MZ, Sadeghi M, Dashti-Khavidaki S. Comparing the effects of 8-week treatment with fluoxetine and imipramine on fasting blood glucose of patients with major depressive disorder. In: XII World Congress of Psychiatry, Yokohama, Japan, August. 2002:24-9. [DOI] [PubMed]
Hu 2013 {published data only}
- Hu S, Yao M, Peterson BS, Xu D, Hu J, Tang J, et al. A randomized, 12-week study of the effects of extended-release paliperidone (paliperidone ER) and olanzapine on metabolic profile, weight, insulin resistance, and beta-cell function in schizophrenic patients. Psychopharmacology (Berl) 2013;230(1):3-13. [DOI: 10.1007/s00213-013-3073-1] [DOI] [PubMed] [Google Scholar]
- Hu S, Yao M, Xu D, Peterson B, Cao L, Xu Y. Effects of paliperidone extended-release tablets (paliperidone er) and olanzapine on metabolic profile, weight, insulin resistance and beta-cell function in schizophrenic patients: a randomized 12-week study. European Psychiatry. Conference: 21st European Congress of Psychiatry, EPA 2013;28:suppl 1. [DOI] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li YC, Zhong BL, Ma J, Gong CP, Xu Y, Zhou XL, et al. Effects of 6-month aripiprazle, risperidone or clozapine treatment on glucose and lipid metabolism and body weight in patients with schizophrenia. Chinese Mental Health Journal 2009;23(8):569-74. [Google Scholar]
Modabbernia 2014 {published data only}
- Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, et al. Melatonin for prevention of metabolic side effects of olanzapine. Journal of Psychiatric Research 2014;53:133-40. [DOI] [PubMed] [Google Scholar]
Moosa 2003 {published data only}
- Moosa MY, Panz VR, Jeenah FY, Joffe BI. African women with depression: the effect of imipramine and fluoxetine on body mass index and leptin secretion. Journal of Clinical Psychopharmacoly 2003;23(6):549-52. [DOI] [PubMed] [Google Scholar]
Narula 2010 {published data only}
- Narula PK, Rehan HS, Unni KE, Gupta N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophrenia Research 2010;118(1-3):218-23. [DOI: 10.1016/j.schres.2010.02.001] [DOI] [PubMed] [Google Scholar]
Ou 2013 {published data only}
- Ou JJ, Xu Y, Chen HH, Fan X, Gao K, Wang J, Guo XF, et al. Comparison of metabolic effects of ziprasidone versus olanzapine treatment in patients with first-episode schizophrenia. Psychopharmacology (Berl) 2013;225(3):627-35. [DOI: 10.1007/s00213-012-2850-6] [DOI] [PubMed] [Google Scholar]
Saddichha 2008 {published data only}
- Saddichha S, Manjunatha N, Ameen S, Akhtar S. Diabetes and schizophrenia: effect of disease or drug? Results from a randomized, double-blind, controlled prospective study in first-episode schizophrenia. Acta Psychiatrica Scandinavica 2008;117(5):342-7. [DOI: 10.1111/j.1600-0447.2008.01158.x] [DOI] [PubMed] [Google Scholar]
Salehi 2009 {published data only}
- Salehi B, Sanjani FG. Comparison of the effect of fluoxetine and imipramine on fasting blood sugar of patients with major depressive disorders, four and eight weeks after treatment. Scientific Journal of Kurdistan University of Medical Sciences 2009;14(2):45-51. [Google Scholar]
- Salehi B. Comparison of the effect on 8 weeks treatment with fluoxetine and imipramine on fasting blood sugar of major depressive patient. European Psychiatry 2009;1:S509. [Google Scholar]
Sepehrmanesh 2015 {published data only}
- Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, et al. Vitamin D supplementation affects the Beck Depression Inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. Journal of Nutrition 2015;146(2):243–8. [DOI] [PubMed] [Google Scholar]
Tohen 2011 {published data only}
- Tohen M, McDonnell DP, Case M, Kanba S, Ha K, Fang Y, et al. A randomized, double-blind, placebo-controlled study of olanzapine in patients with bipolar depression. Bipolar Disorders 2011;1:100. [DOI: 10.1111/j.1399-5618.2011.00912.x] [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang M, Tong JH, Zhu G, Liang GM, Yan HF, Wang XZ. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophrenia Research 2012;138(1):54-7. [DOI: 10.1016/j.schres.2012.02.021] [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu RR, Zhao JP, Liu ZN, Zhai JG, Guo XF, Guo WB, et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology (Berl) 2006;186(4):572-8. [DOI] [PubMed] [Google Scholar]
- Wu RR, Zhao JP, Zhai JG, Guo XF, Guo WB. Sex difference in effects of typical and atypical antipsychotics on glucose-insulin homeostatic and lipid metabolism in first-episode schizophrenia. Journal of Clinical Psychopharmacology 2007;27(4):374-9. [DOI: 10.1097/JCP.0b013e3180cac8db] [DOI] [PubMed] [Google Scholar]
Wu 2008a {published data only}
- Wu RR, Zhao JP, Guo XF, He FQ, Fang MS, Guo WB, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. American Journal of Psychiatry 2008;165(3):352-8. [DOI: 10.1176/appi.ajp.2007.07010079] [DOI] [PubMed] [Google Scholar]
Wu 2008b {published data only}
- Wu RR, Zhao JP, Jin H, Shao P, Fang M-S, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008;299(2):185-93. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang Y, Dai G. Efficacy and metabolic influence of paliperidone ER, aripiprazole and ziprasidone to patients with first-episode schizophrenia through 52 weeks follow-up in China. Human Psychopharmacology 2012;27(6):605-14. [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang S, Lan G. Prospective 8-week trial on the effect of olanzapine, quetiapine, and aripiprazole on blood glucose and lipids among individuals with first-onset schizophrenia. Shanghai Archives of Psychiatry 2014;26(6):339-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2015 {published data only}
- Zhao J, Song X, Ai X, Gu X, Huang G, Li X, et al. Adjunctive aripiprazole treatment for risperidone-induced hyperprolactinemia: an 8-week randomized, open-label, comparative clinical trial. PLoS ONE 2015;10(10):e0139717. [DOI: 10.1371/journal.pone.0139717] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Amrami‐Weizman 2013 {published data only}
- Amrami-Weizman A, Maayan R, Gil-Ad I, Pashinian A, Fuchs C, Kotler M, et al. The effect of reboxetine co-administration with olanzapine on metabolic and endocrine profile in schizophrenia patients. Psychopharmacology (Berl) 2013;230(1):23-7. [DOI: 10.1007/s00213-013-3199-1] [DOI] [PubMed] [Google Scholar]
Basu 2009 {published data only}
- Basu R, Thimmaiah TG, Chawla JM, Schlicht P, Fagiolini A, Brar JS, et al. Changes in metabolic syndrome parameters in patients with schizoaffective disorder who participated in a randomized, placebo-controlled trial of topiramate. Asian Journal of Psychiatry 2009;2(3):106-11. [DOI: 10.1016/j.ajp.2009.08.006] [DOI] [PubMed] [Google Scholar]
Bauer 2006 {published data only}
- Bauer C, Fischer A, Keller U. Effect of sibutramine and of cognitive-behavioural weight loss therapy in obesity and subclinical binge eating disorder. Diabetes, Obesity & Metabolism 2006;8(3):289-95. [DOI: 10.1111/j.1463-1326.2005.00504.x] [DOI] [PubMed] [Google Scholar]
Breier 2005 {published data only}
- Breier A, Berg PH, Thakore JH, Naber D, Gattaz WF, Cavazzoni P, et al. Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. American Journal of Psychiatry 2005;162(10):1879-87. [DOI: 10.1176/appi.ajp.162.10.1879] [DOI] [PubMed] [Google Scholar]
Chang 2008 {published data only}
- Chang JS, Ahn YM, Park HJ, Lee KY, Kim SH, Kang UG, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2008;69(5):720-31. [DOI: 10.4088/JCP.v69n0505] [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen CH, Huang MC, Kao CF, Lin SK, Kuo PH, Chi CC, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2013;74(5):e424-30. [DOI: 10.4088/JCP.12m08186] [DOI] [PubMed] [Google Scholar]
Chiu 2006 {published data only}
- Chiu CC, Chen KP, Liu HC, Lu ML. The early effect of olanzapine and risperidone on insulin secretion in atypical-naive schizophrenic patients. Journal of Clinical Psychopharmacology 2006;26(5):504-7. [DOI] [PubMed] [Google Scholar]
Chrzanowski 2006 {published data only}
- Chrzanowski WK, Marcus RN, Torbeys A, Nyilas M, McQuade RD. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology (Berl) 2006;189(2):259-66. [DOI] [PubMed] [Google Scholar]
DeHert 2011 {published data only}
- De Hert M, Mittoux A, He Y, Peuskens J. Metabolic parameters in the short- and long-term treatment of schizophrenia with sertindole or risperidone. European Archives of Psychiatry and Clinical Neuroscience 2011;261(4):231-39. [DOI: 10.1007/s00406-010-0142-x] [DOI] [PubMed] [Google Scholar]
Detke 2014 {published data only}
- Detke HC, DelBello MP, Landry J, Dittmann RW. A 52-week study of olanzapine with a randomized behavioral weight intervention in adolescents with schizophrenia or bipolar I disorder. European Neuropsychopharmacology 2014;suppl:S720. [DOI] [PubMed] [Google Scholar]
Galynker 2005 {published data only}
- Galynker I. Six month trial of lamotrigine vs. sodium valproate for treatment of mixed mania. clinicaltrials.gov/show/nct00206778 (accessed 28 May 2020).
Graham 2006 {published data only}
- Graham KA. Double blind placebo controlled investigation of amantadine for retarding weight gain in first episode adlt psychotic subjects beginning therapy with olanzapine. clinicaltrials.gov/show/nct00287352 (accessed 2 February 2020).
Grilo 2013 {published data only}
- Grilo CM, White MA. Orlistat with behavioral weight loss for obesity with versus without binge eating disorder: randomized placebo-controlled trial at a community mental health center serving educationally and economically disadvantaged Latino/as. Behaviour Research and Therapy 2013;51(3):167-75. [DOI: 10.1016/j.brat.2013.01.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holka Pokorska 2015 {published data only}
- Holka-Pokorska J, Radzio R, Jarema M, Wichniak A. The stabilizing effect of dehydroepiandrosterone on clinical parameters of metabolic syndrome in patients with schizophrenia treated with olanzapine: a randomized, double-blind trial. Psychiatria Polska 2015;49(2):363-76. [DOI] [PubMed] [Google Scholar]
Janssen‐Cilag 2007 {published data only}
- Janssen-Cilag International. A 6 month study to compare the metabolic effects of paliperidone ER and olanzapine in patients with schizophrenia. clinicaltrials.gov/ct2/show/NCT00645099 (accessed 28 May 2020).
Jarskog 2013 {published data only}
- Jarskog LF, Hamer RM, Catellier DJ, Stewart DD, LaVange L, Ray N, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. American Journal of Psychiatry 2013;170(9):1032-40. [DOI: 10.1176/appi.ajp.2013.12010127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahl 2016 {published data only}
- Kahl KG, Kerling A, Tegtbur U, Gutzlaff E, Herrmann J, Borchert L, et al. Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: a randomized pilot study. Journal of Affective Disorders 2016;192:91-7. [DOI: 10.1016/j.jad.2015.12.015] [DOI] [PubMed] [Google Scholar]
Kerling 2015 {published data only}
- Kerling A, Tegtbur U, Gutzlaff E, Kuck M, Borchert L, Ates Z, et al. Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. Journal of Affective Disorders 2015;177:1-6. [DOI: 10.1016/j.jad.2015.01.006] [DOI] [PubMed] [Google Scholar]
Khambaty 2015 {published data only}
- Khambaty T. Depression treatment and diabetes risk: a 9-year follow-up study of the IMPACT trial [thesis]. Ann Arbor (US): Purdue University, 2015. [Google Scholar]
Khambaty 2018 {published data only}
- Khambaty T, Callahan CM, Stewart JC. Effect of collaborative depression treatment on risk for diabetes: a 9-year follow-up of the IMPACT randomized controlled trial. PLoS ONE 2018;13(8):e0200248. [DOI: 10.1371/journal.pone.0200248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilzieh 2007 {published data only}
- Kilzieh N. Melatonin metabolism abnormality in patients with schizophrenia or schizoaffective disorder treated with olanzapine and melatonin dose finding for the correction of the metabolic abnormality. clinicaltrials.gov/show/nct00512070 (accessed 23 January 2020).
Kim 2006 {published data only}
- Kim JH, Yim SJ, Nam JH. A 12-week, randomized, open-label, parallel-group trial of topiramate in limiting weight gain during olanzapine treatment in patients with schizophrenia. Schizophrenia Research 2006;82(1):115-17. [DOI: 10.1016/j.schres.2005.10.001] [DOI] [PubMed] [Google Scholar]
Krivoy 2017 {published data only}
- Krivoy A, Onn R, Vilner Y, Hochman E, Weizman S, Paz A, et al. Vitamin D supplementation in chronic schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled clinical trial. EBioMedicine 2017;26:138-45. [DOI: 10.1016/j.ebiom.2017.11.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 2011 {published data only}
- Lambert G. A randomised trial investigating the cardiovascular effects of agomelatine and escitalopram in patients with major depressive disorder. clinicaltrials.gov/ct2/show/NCT01483053 (accessed 28 May 2020).
Lin 2010 {published data only}
- Lin CH, Kuo CC, Chou LS, Chen YH, Chen CC, Huang KH, et al. A randomized, double-blind comparison of risperidone versus low-dose risperidone plus low-dose haloperidol in treating schizophrenia. Journal of Clinical Psychopharmacology 2010;30(5):518-25. [DOI: 10.1097/JCP.0b013e3181f28dff] [DOI] [PubMed] [Google Scholar]
Lovell 2014 {published data only}
- Lovell K, Wearden A, Bradshaw T, Tomenson B, Pedley R, Davies LM, et al. An exploratory randomized controlled study of a healthy living intervention in early intervention services for psychosis: the INTERvention to encourage ACTivity, improve diet, and reduce weight gain (INTERACT) study. Journal of Clinical Psychiatry 2014;75(5):498-505. [DOI: 10.4088/JCP.13m08503] [DOI] [PubMed] [Google Scholar]
McIntyre 2011 {published data only}
- McIntyre RS, McElroy SL, Eudicone JM, Forbes RA, Carlson B, Baker RA. A 52-week, double-blind evaluation of the metabolic effects of aripiprazole and lithium in bipolar I disorder. Primary Care Companion to CNS Disorders 2011;13(6):PCC.11m01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltzer 2011 {published data only}
- Meltzer HY, Bonaccorso S, Bobo WV, Chen Y, Jayathilake K. A 12-month randomized, open-label study of the metabolic effects of olanzapine and risperidone in psychotic patients: influence of valproic acid augmentation. Journal of Clinical Psychiatry 2011;72(12):1602-10. [DOI: 10.4088/JCP.10m05997] [DOI] [PubMed] [Google Scholar]
Nam 2004 {published data only}
- Nam CW, Yang BH, Lee JN. The influences of risperidone and clozapine on body weight and glucose level in patients with chronic schizophrenia: comparison study with haloperidol. Korean Journal of Biological Psychiatry 2004;11(2):127-35. [Google Scholar]
Newcomer 2009 {published data only}
- Newcomer JW, Ratner RE, Eriksson JW, Emsley R, Meulien D, Miller F, et al. A 24-week, multicenter, open-label, randomized study to compare changes in glucose metabolism in patients with schizophrenia receiving treatment with olanzapine, quetiapine, or risperidone. Journal of Clinical Psychiatry 2009;70(4):487-99. [DOI: 10.4088/JCP.08m04132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patino 2015 {published data only}
- Patino LR, Rummelhoff R, Blom T, Welge J, Schroeck D, Adler CM, et al. A double-blind placebo-controlled study of exenatide for the treatment of weight gain associated with olanzapine in overweight or obese adults with bipolar disorder, major depressive disorder, schizophrenia or schizoaffective disorder. Biological Psychiatry 2015;Conference: 70th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP 2015 Toronto, ON Canada:132S. [Google Scholar]
Peuskens 2007 {published data only}
- Peuskens J, De Hert M, Mortimer A. Metabolic control in patients with schizophrenia treated with amisulpride or olanzapine. International Clinical Psychopharmacology 2007;22(3):145-52. [DOI: 10.1097/YIC.0b013e3280148c29] [DOI] [PubMed] [Google Scholar]
Robinson 2015 {published data only}
- Robinson DG, Gellego JA, Petrides JM, Hassoun Y, Zhang JP, Lopez L, et al. A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophrenia Bulletin 2015;41(6):1227-36. [DOI: 10.1093/schbul/sbv125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheffler 2018 {published data only}
- Scheffler F, Killian S, Chiliza B, Asmal L, Phahladira L, du Plessis S, et al. Effects of cannabis use on body mass, fasting glucose and lipids during the first 12 months of treatment in schizophrenia spectrum disorders. Schizophrenia Research 2018;199:90-95. [DOI: 10.1016/j.schres.2018.02.050] [DOI] [PubMed] [Google Scholar]
Siskind 2018 {published data only}
- Siskind D, Friend N, Russell A, McGrath JJ, Lim C, Patterson S, et al. CoMET: a protocol for a randomised controlled trial of co-commencement of METformin as an adjunctive treatment to attenuate weight gain and metabolic syndrome in patients with schizophrenia newly commenced on clozapine. BMJ Open 2018;8(3):e021000. [DOI: 10.1136/bmjopen-2017-021000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith RC, Jin H, Li C, Bark N, Shekhar A, Dwivedi S, et al. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophrenia Research 2013;143(1):18-24. [DOI: 10.1016/j.schres.2012.10.023] [DOI] [PubMed] [Google Scholar]
Smith 2018 {published data only}
- Smith RC, Maayan L, Wu R, Youssef M, Jing Z, Sershen H, et al. Betahistine effects on weight-related measures in patients treated with antipsychotic medications: a double-blind placebo-controlled study. Psychopharmacology (Berl) 2018;235(12):3545-58. [DOI: 10.1007/s00213-018-5079-1] [DOI] [PubMed] [Google Scholar]
Stroup 2011 {published data only}
- Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). American Journal of Psychiatry 2011;168(9):947-56. [DOI: 10.1176/appi.ajp.2011.10111609] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strous 2007 {published data only}
- Strous RD, Stryjer R, Maayan R, Gal G, Viglin D, Katz E, et al. Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: a randomized, double-blind placebo controlled trial. Psychoneuroendocrinology 2007;32(2):96-105. [DOI] [PubMed] [Google Scholar]
Sulaiman 2009 {published data only}
- Sulaiman AH. Safety and efficacy of aripiprazole and ziprasidone among schizophrenic patients with metabolic syndrome. clinicaltrials.gov/ct2/show/NCT01714011 (accessed 28 May 2020).
Suppes 2013 {published data only}
- Suppes T, Calabrese JR, Silva R, Kroger H, Cocchiaro J, Pikalov A, et al. Lurasidone adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: A randomized, double-blind, placebo-controlled study (prevail 3). Neuropsychopharmacology 2013;38:S533-4. [DOI: 10.1038/npp.2013.281] [DOI] [Google Scholar]
Tek 2013 {published data only}
- Tek C, Guloksuz S, Srihari VH, Reutenauer EL. Investigating the safety and efficacy of naltrexone for anti-psychotic induced weight gain in severe mental illness: study protocol of a double-blind, randomized, placebo-controlled trial. BMC psychiatry 2013;13:176. [DOI: 10.1186/1471-244X-13-176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisler 2011 {published data only}
- Weisler RH, Nolen WA, Neijber A, Hellqvist A, Paulsson B, trial 144 study investigators. Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder. Journal of Clinical Psychiatry 2011;72(11):1452-64. [DOI: 10.4088/JCP.11m06878] [DOI] [PubMed] [Google Scholar]
Yarborough 2013 {published data only}
- Yarborough BJH, Leo MC, Stumbo S, Perrin NA, Green CA. STRIDE: a randomized trial of a lifestyle intervention to promote weight loss among individuals taking antipsychotic medications. BMC Psychiatry 2013;13(1):238. [DOI: 10.1186/1471-244X-13-238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zajecka 2002 {published data only}
- Zajecka JM, Weisler R, Sachs G, Swann AC, Wozniak P, Sommerville KW. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. Journal of Clinical Psychiatry 2002;63(12):1148-55. [DOI] [PubMed] [Google Scholar]
Zheng 2014 {published data only}
- Zheng ZH, Yang SQ. Metformin combined with mirtazapine for treatment of anorexia nervosa with dyspepsia: Clinical effect and impact on serum levels of norepinephrine, 5-hydroxy tryptamine and dopamine. [Chinese]. World Chinese Journal of Digestology 2014;22(24):3699-3704. [DOI: 10.11569/wcjd.v22.i24.3699] [DOI] [Google Scholar]
References to studies awaiting assessment
Asemi 2014 {published data only}
- Asemi Z. Effects of vitamin D supplementation on insulin resistance and inflammatory factor in patients with depression. ww.irct.ir/trial/6059 (accessed 1 June 2020).
Chang 2014 {published data only}
- Chang JS, Ha TH, Jung HY, Ha K. Differential changes in metabolic profile of bipolar patients following switching to aripiprazole. International Journal of Neuropsychopharmacology 2014;1:53. [Google Scholar]
Djokic 2017 {published data only}
- Djokic GM, Djordjevic V, Agic A, Rankovic A, Vojvodic P, Djukic S. The effects of nutriose-glucomannan-antioxidant complex on the parameters of the metabolic syndrome in the schizophrenia patients treated with olanzapine. European Neuropsychopharmacology 2017;27(suppl 4):S893-S894. [Google Scholar]
Ganguli 2011 {published data only}
- Ganguli R, Brar JS. Behavioural intervention for weight loss in schizophrenia: an RCT with active controls. Indian Journal of Psychiatry 2011;53(suppl 1):S44. [Google Scholar]
Mondal 2014 {published data only}
- Mondal H, Paul S, Guha P. Role of metformin versus topiramate in preventing olanzapine associated weight gain and metabolic syndrome. Indian Journal of Pharmacology 2014;1:S21. [Google Scholar]
Ni 2014 {published data only}
- Ni FR. Effect of clozapine and aripiprazole on metabolic syndrome in patients with schizophrenia. Practical Pharmacy and Clinical Remedies 2014;17(3):283-85. [Google Scholar]
Talaei 2009 {published data only}
- Talaei A. Effects of topiramate in prevention of obesity in schizophrenic patients treated by olanzapine. European Psychiatry 2009;1:S1026. [Google Scholar]
Tessier 2010 {published data only}
- Tessier C, Hoeben D, Korcsog P, Niehaus DJH, Aadamsoo K, Ucok A, et al. A prospective randomized controlled trial of paliperidone ER versus oral olanzapine in patients with schizophrenia. European Neuropsychopharmacology 2010;3:S479. [Google Scholar]
Wu 2012 {published data only}
- Wu RR, Jin H, Gao K, Shao P, Chan PK, Ou JJO, et al. Metformin for treatment of atypical antipsychotic-induced weight gain and endocrinological side effects in patients with first episode schizophrenia: Results from randomized, double blind, placebo-controlled study. Schizophrenia Research 2012;1:S77. [Google Scholar]
Yao 2014 {published data only}
- Yao LL. Effects of risperidone and clozapine on blood glucose and lipid metabolism in patients with schizophrenia. Practical Pharmacy and Clinical Remedies 2014;17(3):292-95. [Google Scholar]
References to ongoing studies
Ostadmohammadi 2018 {published data only}
- Ostadmohammadi V. The effects of combined probiotic and selenium supplementation on clinical status and metabolic profiles in patients with schizophrenia. www.irct.ir/trial/35044 (accessed 12 May 2020).
Saidpour 2019 {published data only}
- Saidpour A. Effects of sumac powder capsule (Rhus coriaria L.) with restricted calorie diet on anthropometric indices, body composition, level of inflammatory biomarkers, oxidative stress, appetite hormones, glycemic indices, lipid profile and depression in obese or overweight women with depression. en.irct.ir/trial/36888 (accessed 12 May 2020).
Shokrgozar 2019 {published data only}
- Shokrgozar S. Clinical trial of investigation of efficacy of metformin on the body mass index of patients under treatment with selective serotonin reuptake inhibitors drugs referred to psychiatry clinics of Rasht. www.irct.ir/trial/40317 (accessed 12 May 2020).
Sulejmanpasic 2019 {published data only}
- Sulejmanpasic G, Bise S, Pepic F, Toskic A. P.105 Adjunctive treatment of aripiprazole to olanzapine for weight reduction in patients with schizophrenia. European Neuropsychopharmacology 2019;29(Supplement 6):S90. [Google Scholar]
Additional references
ADA 1999
- American Diabetes Association. The expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22:S5-19. [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2008;31 (Suppl 1):S12-54. [DOI] [PubMed] [Google Scholar]
ADA 2017
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017;40(Suppl 1):S11-S24. [DOI] [PubMed] [Google Scholar]
Ajzen 1991
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50:179-211. [Google Scholar]
Ali 2006
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetic Medicine 2006;23(11):1165-73. [DOI] [PubMed] [Google Scholar]
Andany 2019
- Adeva-Andany MM, Funcasta-Calderón R, Fernández-Fernández C, Ameneiros-Rodríguez E, Domínguez-Montero A. Subclinical vascular disease in patients with diabetes is associated with insulin resistance. Diabetes Metabolic Syndrome 2019;13(3):2198-2206. [DOI] [PubMed] [Google Scholar]
Anderson 2001
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24(6):1069-78. [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd revised edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
AUHE 2018
- Academic Unit of Health Economics. LMICs Geographic Search Strategy; DAC List of ODA Recipients 2003-2020. medhealth.leeds.ac.uk/download/4274/lmics_geographic_search_strategy_aid_recipients_2003-2020 (accessed prior to 18 September 2018).
Bandura 1986
- Bandura A, National Institute of Mental Health. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall, 1986. [Google Scholar]
Bell 2013
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psychooncology 2013;22(8):1738-47. [DOI] [PubMed] [Google Scholar]
Bradford 2008
- Bradford DW, Kim MM, Braxton LE, Marx CE, Butterfield M, Elbogen EB. Access to medical care among persons with psychotic and major affective disorders. Psychiatric Services 2008;59:847-52. [DOI] [PubMed] [Google Scholar]
Bruins 2014
- Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLOS One 2014;9(12):e112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caemmerer 2012
- Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophrenia Research 2012;140(1):159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008
- Chen CH, Chiu CC, Huang MC, Wu TH, Liu HC, Lu ML. Metformin for metabolic dysregulation in schizophrenic patients treated with olanzapine. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2008;32(4):925-31. [DOI] [PubMed] [Google Scholar]
Chesney 2014
- Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 2014;13(2):153-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chwastiak 2015
- Chwastiak LA, Freudenreich O, Tek C, McKibbin C, Han J, McCarron R, et al. Clinical management of comorbid diabetes and psychotic disorders. Lancet Psychiatry 2015;2(5):465-76. [DOI] [PubMed] [Google Scholar]
Cimo 2012
- Cimo A, Stergiopoulos E, Cheng C, Bonato S, Dewa CS. Effective lifestyle interventions to improve type II diabetes self-management for those with schizophrenia or schizoaffective disorder: a systematic review. BMC Psychiatry 2012;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correll 2017
- Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, 2017. www.covidence.org.
Crump 2013
- Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. American Journal of Psychiatry 2013;170(3):324-33. [DOI] [PubMed] [Google Scholar]
DAC 2017
- Development Assistance Committee (DAC). DAC List of ODA Recipients. www.oecd.org/dac/financing-sustainable-development/development-finance-standards/daclist.htm (accessed 1 June 2020).
Das‐Munshi 2007
- Das-Munshi J, Stewart R, Ismail K, Bebbington PE, Jenkins R, Prince MJ. Diabetes, common mental disorders, and disability: findings from the UK National Psychiatric Morbidity Survey. Psychosomatic Medicine 2007;69(6):543-50. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
De Hert 2009
- De Hert M, Schreurs V, Vancampfort D, Van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry 2009;8(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diabetes UK 2018
- Diabetes UK. Diagnostic criteria for diabetes. www.diabetes.org.uk (accessed August 2018).
Druss 2010
- Druss BG, Bornemann TH. Improving health and health care for persons with serious mental illness: the window for US federal policy change. JAMA 2010;303:1972-73. [DOI] [PubMed] [Google Scholar]
Dube 1984
- Dube KC, Kumar N, Dube S. Long-term course and outcome of the Agra cases in the international pilot-study of schizophrenia. Acta Psychiatrica Scandinavica 1984;70(2):170-79. [DOI] [PubMed] [Google Scholar]
Faulkner 2007
- Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Schizophrenia Bulletin 2007;33(3):654-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fekadu 2015
- Fekadu A, Medhin G, Kebede D, Alem A, Cleare AJ, Prince M, et al. Excess mortality in severe mental illness: 10-year population-based cohort study in rural Ethiopia. British Journal of Psychiatry 2015;206(4):289-96. [DOI] [PubMed] [Google Scholar]
Fernández‐San‐Martín 2014
- Fernández-San-Martín MI, Martín-López LM, Masa-Font R, Olona-Tabueña N, Roman Y, Martin-Royo J, et al. The effectiveness of lifestyle interventions to reduce cardiovascular risk in patients with severe mental disorders: meta-analysis of intervention studies. Community Mental Health Journal 2014;50(1):81-95. [DOI] [PubMed] [Google Scholar]
Firth 2019
- Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6(8):675-712. [DOI] [PubMed] [Google Scholar]
Golden 2008
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner Pamela J, Diez Roux AV, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299(28):2751-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADE Working Group, McMaster University GRADEpro GDT. Version accessed 13 March 2020. Hamilton (ON): GRADE Working Group, McMaster University, 2015. Available at gradepro.org.
Grigsby 2002
- Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes. Journal of Psychosomatic Research 2002;53:1053-60. [DOI] [PubMed] [Google Scholar]
Hayes 2017
- Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000-2014. British Journal of Psychiatry 2017;211:175-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated 2017). The Cochrane Collaboration, 2017. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated 2017). The Cochrane Collaboration, 2017. Available from handbook.cochrane.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hoffmann 2017
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:2998. [DOI] [PubMed] [Google Scholar]
Holt 2015
- Holt R, Mitchell A. Diabetes mellitus and severe mental illness: mechanisms and clinical implications. Nat Rev Endocrinol 2015;11:79–89. [DOI] [PubMed] [Google Scholar]
Koyanagi 2017
- Koyanagi A, Oh H, Stubbs B, Haro JM, DeVylder JE. Epidemiology of depression with psychotic experiences and its association with chronic physical conditions in 47 low- and middle-income countries. Psychological Medicine 2017;47(3):531-42. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16:606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurihara 2011
- Kurihara T, Kato M, Reverger R, Tirta IG. Seventeen-year clinical outcome of schizophrenia in Bali. European Psychiatry 2011;26(5):333-38. [DOI] [PubMed] [Google Scholar]
Laursen 2011
- Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophrenia Research 2011;131(1-3):101-4. [DOI] [PubMed] [Google Scholar]
Lawrence 2010
- Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Canadian Journal of Psychiatry 2010;55(12):752-60. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, editor(s). Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Leventhal 1984
- Leventhal H. A perceptual-motor theory of emotion. Advances in Experimental Social Psychology 1984;17:117-82. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2017
- Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017;16(1):30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367(9524):1747-57. [DOI] [PubMed] [Google Scholar]
Maayan 2010
- Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology 2010;35:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manne‐Goehler 2019
- Manne-Goehler J, Geldsetzer P, Agoudavi K, Andall-Brereton G, Aryal K K, Bicaba BW et al. Health system performance for people with diabetes in 28 low- and middle-income countries: A cross-sectional study of nationally representative surveys. PLoS Med 2019;16(3):e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mate 2013
- Mate KS, Sifrim ZK, Chalkidou K, Cluzeau F, Cutler D, Kimball M, et al. Improving health system quality in low- and middle-income countries that are expanding health coverage: a framework for insurance. International Journal for Quality in Health Care 2013;25(5):497-504. [DOI] [PubMed] [Google Scholar]
McBain 2016
- McBain H, Mulligan K, Haddad M, Flood C, Jones J, Simpson A. Self management interventions for type 2 diabetes in adult people with severe mental illness. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD011361. [DOI: 10.1002/14651858.CD011361.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGinty 2016
- McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: a comprehensive review. Schizophrenia Bulletin 2016;42(1):96-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 1993;31:247-63. [DOI] [PubMed] [Google Scholar]
Mendenhall 2014
- Mendenhall E, Norris S, Shidhaye R, Prabhakaran D. Depression and type 2 diabetes in low- and middle-income countries: a systematic review. Diabetes research and clinical practice 2014;103(2):276-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merlotti 2014
- Merlotti C, Morabito A, Pontiroli, AE. Prevention of type 2 diabetes: a systematic review and meta-analysis of different intervention strategies. Diabetes, Obesity and Metabolism 2014;16:719-27. [DOI] [PubMed] [Google Scholar]
Miranda 2008
- Miranda JJ, Kinra S, Casas JP, Davey Smith G, Ebrahim S. Non-communicable diseases in low- and middle-income countries: context, determinants and health policy. Tropical Medicine and International Health 2008;13(10):1225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2013
- Mitchell AJ, Vancampfort D, Sweers K, Van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders - a systematic review and meta-analysis. Schizophrenia Bulletin 2013;39(2):306-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mizuno 2014
- Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophrenia Bulletin 2014;40(6):1385-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mogga 2006
- Mogga S, Prince M, Alem A, Kebede D, Stewart R, Glozier N, et al. Outcome of major depression in Ethiopia: population-based study. British Journal of Psychiatry 2006;189:241-46. [DOI] [PubMed] [Google Scholar]
Morera‐Fumero 2013
- Morera-Fumero AL, Abreu-Gonzalez P. Role of melatonin in schizophrenia. International Journal of Molecular Sciences 2013;14(5):9037-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulton 2015
- Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes & Endocrinology 2015;3(6):461-71. [DOI] [PubMed] [Google Scholar]
Murray 2012
- Murray C, Vos T, Lozano R, Naghavi M, Flaxman A, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence (NICE). Common mental health problems: identification and pathways to care, Clinical guideline 123. www.nice.org.uk/guidance/cg123 (accessed 14 August 2018).
NICE 2015
- National Institute for Health and Care Excellence (NICE). Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28 (accessed August 2018).
Olfson 2015
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72(12):1172-81. [DOI] [PubMed] [Google Scholar]
Pillinger 2017
- Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 2017;74(3):261-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pillinger 2020
- Pillinger T, McCutcheon R, Vano L, Mizuno Y, Arumuham A, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. The Lancet Psychiatry 2020;7(1):64-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Popkin 2002
- Popkin BM. The shift in stages of the nutrition transition in the developing world differs from past experiences. Public Health Nutrition 2002;5:205–14. [DOI] [PubMed] [Google Scholar]
Prochaska 1997
- Prochaska JO, Redding CA, Evers KE. The transtheoretical model of behavior change. American Journal of Health Promotion 1997;12:38-48. [DOI] [PubMed] [Google Scholar]
Rathod 2017
- Rathod S, Pinninti N, Irfan M, Gorczynski P, Rathod P, Gega L, et al. Mental health service provision in low- and middle-Income countries. Health Services Insights 2017;10:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reilly 2015
- Reilly S, Olier I, Planner C, Doran T, Reeves D, Ashcroft DM, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open 2015;5(12):e009010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roopan 2017
- Roopan S, Larsen E. Use of antidepressants in patients with depression and comorbid diabetes mellitus: A systematic review. Acta Neuropsychiatrica 2017;29(3):127-39. [DOI] [PubMed] [Google Scholar]
Saha 2007
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry 2007;64(10):1123-31. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Scott 2009
- Scott KM, Von Korff M, Alonso J, Angermeyer MC, Bromet E, Fayyad J, et al. Mental-physical co-morbidity and its relationship with disability: results from the World Mental Health Surveys. Psychological Medicine 2009;39(1):33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seiglie 2020
- Seiglie J A, Marcus M E, Ebert C, Prodromidis N, Geldsetzer P, Theilmann M. Diabetes Prevalence and Its Relationship With Education, Wealth, and BMI in 29 Low- and Middle-Income Countries. Diabetes Care 2020;43(4):767-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 2006
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine 2006;166(10):1092-97. [DOI] [PubMed] [Google Scholar]
Stubbs 2015
- Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta‐analysis. Acta Psychiatrica Scandinavica 2015;132(2):144-57. [DOI] [PubMed] [Google Scholar]
Stubbs 2016
- Stubbs B, Koyanagi A, Veronese N, Vancampfort D, Solmi M, Gaughran F, et al. Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low- and middle-income countries. BMC Medicine 2016;14(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubbs 2017
- Stubbs B, Koyanagi A, Schuch F, Firth J, Rosenbaum S, Gaughran F, et al. Physical activity levels and psychosis: a mediation analysis of factors influencing physical activity target achievement among 204,186 people across 46 low- and middle-income countries. Schizophrenia Bulletin 2017;43:536-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2017
- Taylor J, Stubbs B, Hewitt C, Ajjan RA, Alderson SL, Gilbody S, et al. The effectiveness of pharmacological and non-pharmacological interventions for improving glycaemic control in adults with severe mental illness: a systematic review and meta-analysis. PLOS One 2017;12(1):e0168549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vancampfort 2013
- Vancampfort D, Wampers M, Mitchell AJ, Correll CU, De Hert A, Probst M, et al. A meta-analysis of cardio-metabolic abnormalities in drug naive, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry 2013;12(3):240-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vancampfort 2015a
- Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, et al. Prevalence and predictors of type 2 diabetes mellitus in people with bipolar disorder: a systematic review and meta-analysis. The Journal of Clinical Psychiatry 2015;76(11):1490-99. [DOI] [PubMed] [Google Scholar]
Vancampfort 2015b
- Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, et al. Type 2 diabetes in patients with major depressive disorder: a meta-analysis of prevalence estimates and predictors. Depression and Anxiety 2015;32(10):763-73. [DOI] [PubMed] [Google Scholar]
Vancampfort 2016a
- Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry 2016;15(2):166-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vancampfort 2016b
- Vancampfort D, Rosenbaum S, Ward PB, Steel Z, Lederman O, Lamwaka AV, et al. Type 2 diabetes among people with posttraumatic stress disorder: systematic review and meta-analysis. Psychosomatic Medicine 2016;78:465-73. [DOI] [PubMed] [Google Scholar]
Vancampfort 2017
- Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 2017;16:308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2015
- Ward M, Druss B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry 2015;2(5):431-51. [DOI] [PubMed] [Google Scholar]
Wee 2006
- Wee HL, Tan CE, Goh SY, Li SC. Usefulness of the Audit of Diabetes-Dependent Quality-of-Life (ADDQoL) Questionnaire in patients with diabetes in a multi-ethnic Asian country. PharmacoEconomics 2006;24:673-82. [DOI] [PubMed] [Google Scholar]
Weir 2020
- Weir G C, Geglia J, Weir S B. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. The Lancet Diabetes & Endocrinology 2020;8(3):249-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2016
- White M. Population approaches to prevention of type 2 diabetes. PLOS Medicine 2016;13:e1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: WHO, 1992. [Google Scholar]
WHO 1994
- WHO study group. Prevention of Diabetes Mellitus. WHO Technical report series 1994;844:7-8. [PubMed]
WHO 1999
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: diagnosis and classification of diabetes 1999. www.who.int/iris/handle/10665/66040 (accessed October 2018):1-59.
WHO 2006
- WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ (accessed August 2018).
WHO 2016
- WHO. Global Report on Diabetes. apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1 (accessed August 2018).
Yoon 2013
- Yoon JM, Cho EG, Lee HK, Park SM. Antidepressant use and diabetes mellitus risk: a meta-analysis. Korean Journal of Family Medicine 2013;34(4):228-40. [DOI] [PMC free article] [PubMed] [Google Scholar]


