Abstract
Background
Shock wave lithotripsy (SWL) is a widely used method to treat renal and ureteral stone. It fragments stones into smaller pieces that are then able to pass spontaneously down the ureter and into the bladder. Alpha‐blockers may assist in promoting the passage of stone fragments, but their effectiveness remains uncertain.
Objectives
To assess the effects of alpha‐blockers as adjuvant medical expulsive therapy plus usual care compared to placebo and usual care or usual care alone in adults undergoing shock wave lithotripsy for renal or ureteral stones.
Search methods
We performed a comprehensive literature search of the Cochrane Library, the Cochrane Database of Systematic Reviews, MEDLINE, Embase, several clinical trial registries and grey literature for published and unpublished studies irrespective of language. The date of the most recent search was 27 February 2020.
Selection criteria
We included randomized controlled trials of adults undergoing SWL. Participants in the intervention group had to have received an alpha‐blocker as adjuvant medical expulsive therapy plus usual care. For the comparator group, we considered studies in which participants received placebo.
Data collection and analysis
Two review authors independently selected studies for inclusion/exclusion, and performed data abstraction and risk of bias assessment. We conducted meta‐analysis for the identified dichotomous and continuous outcomes using RevManWeb according to Cochrane methods using a random‐effects model. We judged the certainty of evidence on a per outcome basis using GRADE.
Main results
We included 40 studies with 4793 participants randomized to usual care and an alpha‐blocker versus usual care alone. Only four studies were placebo controlled. The mean age of participants was 28.6 to 56.8 years and the mean stone size prior to SWL was 7.1 mm to 13.2 mm. The most widely used alpha‐blocker was tamsulosin; others were silodosin, doxazosin, terazosin and alfuzosin.
Alpha‐blockers may improve clearance of stone fragments after SWL (risk ratio (RR) 1.16, 95% confidence interval (CI) 1.09 to 1.23; I² = 78%; studies = 36; participants = 4084; low certainty evidence). Based on the stone clearance rate of 69.3% observed in the control arm, an alpha‐blocker may increase stone clearance to 80.4%. This corresponds to 111 more (62 more to 159 more) participants per 1000 clearing their stone fragments.
Alpha‐blockers may reduce the need for auxiliary treatments after SWL (RR 0.67, 95% CI 0.45 to 1.00; I² = 16%; studies = 12; participants = 1251; low certainty evidence), but also includes the possibility of no effect. Based on a rate of auxiliary treatments in the usual care arm of 9.7%, alpha‐blockers may reduce the rate to 6.5%. This corresponds 32 fewer (53 fewer to 0 fewer) participants per 1000 undergoing auxiliary treatments.
Alpha‐blockers may reduce major adverse events (RR 0.60, 95% CI 0.46 to 0.80; I² = 0%; studies = 7; participants = 747; low certainty evidence). Major adverse events occurred in 25.8% of participants in the usual care group; alpha‐blockers would reduce this to 15.5%. This corresponds to 103 fewer (139 fewer to 52 fewer) major adverse events per 1000 with alpha‐blocker treatment. None of the reported major adverse events appeared drug‐related; most were emergency room visits or rehospitalizations.
Alpha‐blockers may reduce stone clearance time in days (mean difference (MD) –3.74, 95% CI –5.25 to –2.23; I² = 86%; studies = 14; participants = 1790; low certainty evidence). We found no evidence for the outcome of quality of life.
For those outcomes for which we were able to perform subgroup analyses, we found no evidence of interaction with stone location, stone size or type of alpha‐blocker. We were unable to conduct an analysis by lithotripter type. The results were also largely unchanged when the analyses were limited to placebo controlled studies and those in which participants explicitly only received a single SWL session.
Authors' conclusions
Based on low certainty evidence, adjuvant alpha‐blocker therapy following SWL in addition to usual care may result in improved stone clearance, less need for auxiliary treatments, fewer major adverse events and a reduced stone clearance time compared to usual care alone. We did not find evidence for quality of life. The low certainty of evidence means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Plain language summary
After using shock waves to break up kidney stones, do medicines called alpha‑blockers help to get rid of the stone fragments?
What are kidney stones?
Waste products in the blood can sometimes form crystals that collect inside the kidneys. These can build up over time to form a hard stone‐like lump, called a kidney stone.
Kidney stones can develop in both kidneys especially in people with certain medical conditions or who are taking certain medicines, or if people do not drink enough water or fluids. Stones can cause severe pain, fever and a kidney infection if they block the ureter.
Treatments for kidney stones
Most stones are small enough to pass out in the urine: drinking plenty of water and other fluids will help. Larger kidney stones may be too big to pass out naturally and are usually removed by surgery.
Shock wave lithotripsy is a non‐surgical way to treat stones in the kidney or ureter. High energy sound waves are applied to the outside of the body to break kidney stones into smaller pieces. After shock wave treatment, medicines called alpha‐blockers are sometimes given to help the stone fragments pass out naturally.
Alpha‐blockers work by relaxing muscles and helping to keep blood vessels open. They are usually used to treat high blood pressure and problems with storing and passing urine in men who have an enlarged prostate gland. Alpha‐blockers may relax the muscle in the ureters, which might help to get rid of kidney stones and fragments.
Why we did this Cochrane Review
We wanted to find out how well alpha‐blockers work to help kidney stone fragments pass out in the urine. We also wanted to find out about potential unwanted effects that might be associated with alpha‐blockers.
What did we do?
We searched for studies that looked at giving alpha‐blockers to adults, after shock wave treatment, to clear kidney stone fragments.
We looked for randomized controlled studies, in which the treatments that people received were decided at random, because these studies usually give the most reliable evidence about the effects of a treatment.
Search date: we included evidence published up to 27 February 2020.
What we found
We found 40 studies including 4793 people who had shock wave treatment to break up their kidney stones. Most of the studies were done in Asia; some were in Europe, Africa and South America. Most studies did not report their sources of funding.
The studies compared giving an alpha‐blocker with giving a placebo (dummy) treatment or usual care (could include antibiotics, painkillers and fluids given by mouth or through a drip).
Tamsulosin was the most commonly studied alpha‐blocker; the others were silodosin, doxazosin, terazosin and alfuzosin.
What are the results of our review?
Compared with usual care or a placebo treatment, alpha‐blockers may:
clear kidney stones in more people: in 111 more people for every 1000 people treated (36 studies);
clear stones faster: by nearly four days (14 studies);
reduce the need for extra treatments to clear stones: in 32 fewer people for every 1000 people treated (12 studies); and
cause fewer unwanted effects: affecting 103 fewer people for every 1000 people treated (seven studies).
Most unwanted effects were emergency visits to hospitals, and people going back into hospital for stone related problems. Unwanted effects were more common in people who had usual care or a placebo treatment than in people given alpha‐blockers.
None of the studies looked at people's quality of life (well‐being).
How reliable are these results?
We are uncertain about these results because they were based on studies in which it was unclear how people were chosen to take part; it was unclear if results were reported fully; some results were inconsistent and in some studies the results varied widely. Our results are likely to change if further evidence becomes available.
Conclusions
Giving an alpha‐blocker after shock wave treatment to break up kidney stones might clear the fragments faster, in more people and reduce the need for extra treatments. Alpha‑blockers might cause fewer unwanted effects than usual care or a placebo.
Summary of findings
Summary of findings 1. Alpha‐blocker as adjuvant medical expulsive therapy plus usual care compared to usual care for renal and ureteral stones.
| Alpha‐blocker and usual care compared to usual care for renal and ureteral stones | |||||
| Patient or population: adults with renal and ureteral stones undergoing shock wave lithotripsy Setting: outpatient or inpatient Intervention: alpha‐blocker and usual care Comparison: usual care | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Effect size (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with standard care | Risk difference with alpha‐blocker | ||||
|
Stone clearance assessed by imaging Follow‐up range: 1 week to 3 months |
4084 (36 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | RR 1.16 (1.09 to 1.23) | Moderate risk population | |
| 693 per 1000 | 111 more per 1000 (62 more to 159 more) | ||||
|
Auxiliary treatment Follow‐up range: 1 week to 3 months |
1251 (12 RCTs) | ⊕⊕⊝⊝ Lowa,c,d |
RR 0.67 (0.45 to 1.00) |
Moderate risk population | |
| 97 per 1000 | 32 fewer per 1000 (53 fewer to 0 fewer) |
||||
|
Major adverse events determined by study investigators Follow‐up range: 1 week to 3 months |
747 (7 RCTs) | ⊕⊕⊝⊝ Lowe | RR 0.60 (0.46 to 0.80) | Moderate risk population | |
| 258 per 1000 | 103 fewer per 1000 (139 fewer to 52 fewer) | ||||
| Low risk populationf | |||||
| 138 per 1000 | 55 fewer per 1000 (139 fewer to 34 fewer) |
||||
| Quality of life | Not reported | ||||
|
Stone clearance time measured in days |
1790 (14 studies) | ⊕⊕⊝⊝ Lowa,g,h | N/A | Moderate risk population | |
| Range: 3.61–47.2 days | 3.74 fewer days (5.25 fewer to 2.23 fewer days) |
||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded one level for unclear risk of selection bias, high risk of performance and detection bias, and unclear risk of selective reporting bias. bDowngraded one level due to clinically important, unexplained inconsistency with high I² value. cConcerns over possible publication bias given funnel plot asymmetry contributed to decision to downgraded by two levels overall. dImprecision with wide confidence intervals around absolute effect size estimates that crossed the threshold of 3% clinically relevant absolute risk reduction. eDowngraded two levels for unclear risk of selection bias, high risk of performance and detection bias, and high risk of selective reporting bias for this infrequently reported outcome. fLower, presumably more representative control event rate of 17% obtained by excluding Ahmed 2016. gDowngraded one level given funnel plot asymmetry and serious risk of publication bias. hWe noted a high degree of inconsistency but did not downgrade given its perceived lack of clinical importance.
Background
Description of the condition
Urinary tract stones are the result of a complex cascade of events that involves supersaturation of stone forming salts that precipitate out of solution to form crystals or nuclei. Once formed, these can either flow out and be excreted or they are retained in the kidney where crystals can aggregate and grow to form macroscopic stones that may cause urinary symptoms and obstruction.
Urinary tract stones are a common urologic problem and the worldwide prevalence and incidence is increasing (Chewcharat 2020; Romero 2010). The prevalence has been reported as 16.9% in 1997 in Thailand, 14.8% in 1989 in Turkey and 14% in 2013/2014 in England (Romero 2010; Rukin 2017). In the USA, the prevalence of stone disease has been estimated at 10.6% in men and 7.1% in women in the 2007–2010 National Health and Nutrition Examination Survey (Scales 2012). Proposed modifiable risk factors include maintaining a normal body mass index, drinking an adequate fluids, and eating fruits and vegetables (Ferraro 2017). The cost of this disease is high, with estimates upwards of several billion dollars in 2000 in the USA (Saigal 2005). There are variable costs associated with urinary tract stones based on acute, medical or surgical management options (Canvasser 2017).
Diagnosis
People presenting with clinical suspicion for symptomatic urinary tract stones are evaluated with history and physical exam, followed by imaging studies. The primary imaging modality used depends on the availability of the tools. In an older study of people presenting to an emergency room with a stone, 90% had acute unilateral flank pain, hematuria, and positive imaging by kidney, ureter and bladder (KUB) radiograph (Elton 1993). The European Association of Urology (EAU) now recommends ultrasound as the initial diagnostic imaging tool in people suspected of urinary tract stones due to its safety profile and low cost (Turk 2016). However, imaging beyond ultrasound may be needed to best characterize the stone and its location. Non‐contrast‐enhanced computed tomography (NCCT) is the gold standard diagnostic tool for nephrolithiasis in any location of the urinary tract. It characterizes stone density and determines precise location including defining skin‐to‐stone distance – factors important in determining the best treatment modality (El‐Nahas 2007; Kim 2007; Zarse 2007). NCCT has largely replaced intravenous urography in diagnosing acute urinary tract stones due to its higher diagnostic accuracy (Worster 2002). It also represents the most accurate treatment modality to establish treatment success but has the downside of costs and radiation exposure.
Treatment
Urinary tract stones may pass on their own or require intervention to assist with expulsion. The likelihood of spontaneous passage depends on the size and location of the stone. Smaller stones located more distally in the urinary tract, notably the distal ureter and beyond, have the highest rates of spontaneous passage (Hubner 1993). Segments of the ureter are defined radiographically: proximal from its origin to the upper border of the sacroiliac joint; middle overlying the SI joint; and distal from the lower border of the sacroiliac joint and beyond. Ureteral stones less than 10 mm have the highest incidence of spontaneous expulsion, and the American Urological Association (AUA) recommends observation with trial of passage in people whose pain is well controlled and are free of signs of infection or high grade obstruction (Assimos 2016a; Assimos 2016b). Furthermore, for uncomplicated ureteral colic due to ureteral stones of the distal ureter, these guidelines recommend medical expulsive therapy (MET) with alpha‐blockers (alpha‐adrenoreceptor antagonists) (Assimos 2016a; Assimos 2016b). A panel using GRADE and following the British Medical Journal (BMJ) Rapid Recommendations procedure recommend MET, even in settings when stone size and location has not been established by imaging studies (Vermandere 2018). Supporting evidence for the use of MET as primary treatment for ureteral stones comes from several high‐quality reviews (Campschroer 2018; Hollingsworth 2016). It should be noted that MET is an off‐label indication for alpha‐blockers and their actual value for this indication remains controversial given concerns over the quality of the underlying trials as well as their adverse effects and costs (De Coninck 2019; Pickard 2015). In addition, people with a more complicated presentation, for example those with signs of a systemic infection, as witnessed by fever or an elevated white blood cell count (or both), should undergo immediate urinary drainage by ureteral stent or percutaneous nephrostomy placement.
Renal colic is a likely symptom of acute stone episodes and must be treated accordingly. Pain management is part of the usual treatment regimen for symptomatic stones. The EAU and AUA recommend non‐steroidal anti‐inflammatory drugs (NSAIDs) including metamizole to treat renal colic (Assimos 2016a; Assimos 2016b; Turk 2016). Definitive stone treatment may be offered to patients if spontaneous stone passage is not achieved or sooner intervention is clinically necessary. The typical timeframe for a trial of spontaneous passage ranges from four to six weeks. People with pain uncontrolled with oral analgesics, worsening renal function or sepsis from the urinary tract require surgical management, either definitive management with stone removal or urinary drainage (in the setting of signs of sepsis) (Assimos 2016a; Assimos 2016b; Turk 2016). Two commonly used options for definitive management are ureteroscopy (URS) and shock wave lithotripsy (SWL). An advantage of URS is the greater stone free rate, which has been shown even when stones less than 10 mm are stratified by location in the ureter (Preminger 2007). The higher stone free rate after a single procedure is particularly notable for distal ureteral stones, and thus URS typically is recommended over SWL. Advantages of SWL over URS are decreased complication rates and lower morbidity (Aboumarzouk 2012). The complications of urinary tract infections (UTI), ureteral strictures and ureteral avulsion are similar between SWL and URS, but URS has a higher risk of ureteral perforation (Aboumarzouk 2012). Additional options for definitive treatment of stones include percutaneous nephrolithotomy (PCNL), laparoscopic, open surgical removal or robotic surgical removal.
SWL is a non‐invasive procedure where high energy shock waves are applied to the outside of the body to break up urinary tract stones in the kidney and ureter. The tiny stone fragments can then pass through the urinary system to be excreted. To aid in patient comfort, SWL may be performed under mild sedation, or local or general anesthesia. Fluoroscopy or ultrasound (or both) are used for imaging studies throughout the procedure to localize the stones and monitor treatment progression (Kohrmann 1995). The technique of SWL encompasses several factors to optimize treatment outcomes (Matlaga 2016). Modifiable SWL parameters include the number of shocks, period of shock wave administration, voltage, type of shock wave generator and rate of shock wave delivery. A large skin‐to‐stone distance negatively impact stone fragmentation (Pareek 2005). In addition, focal zones differ considerably by lithotripter type, manufacturer and model, and can greatly impact stone fragmentation effectiveness. Of note, current evidence based guidelines only recommend SWL in people with normal anatomy of the collecting system, normal renal function and the absence of infection. Given its unknown effect on the fetus (especially given the common use of fluoroscopy), SWL is contraindicated in pregnant women (Assimos 2016a; Assimos 2016b; Turk 2016; Turk 2020).
Further possible complications from SWL of renal or ureteral stones are related to incomplete stone fragmentation and renal colic symptoms when fragments cause distention and obstruction of the ureter (Skolarikos 2006). The term steinstrasse refers to when multiple stone fragments or debris line the ureter (Sayed 2001). Steinstrasse occurs in 1% to 4% of SWL cases (Madbouly 2002). This complication can lead to clinically significant obstruction, pain and infection (Sayed 2001). Trauma to the kidneys causes bleeding in the urinary tract when SWL is performed. The shock waves cause small vessels in the kidney to rupture which can lead to hematoma formation (Matlaga 2016).
Description of the intervention
Alpha‐blockers work by relaxing smooth muscle and help keep small blood vessels open. Examples of alpha‐blockers include tamsulosin, alfuzosin, terazosin, naftopidil and silodosin. They are typically used to treat or improve symptoms of high blood pressure and benign prostatic hyperplasia (BPH), and are particularly helpful if a person has both conditions. Because there is a lack of evidence supporting the cardioprotective effects of alpha‐blockers compared to placebo, alpha‐blockers are no longer recommended as first‐line treatment for high blood pressure (Pool 2005). Alpha‐blockers have been shown to improve lower urinary tract symptoms (LUTS), the complex of symptoms associated with BPH (Shapiro 1992). The rationale for the use of alpha‐blockers is that LUTS are at least partly due to bladder outlet obstruction, a process mediated by alpha1 adrenoreceptors in prostatic smooth muscle (Caine 1976).
Alpha‐blockers are available as an adjuvant medical therapy to enhance stone fragment passage after SWL. If fragments do not readily pass after SWL, patients can develop complications including steinstrasse. Urinary tract obstruction, infection and significant pain can develop from incomplete stone passage. The use of SWL as treatment for stones may result in need for repeat or additional procedures to clear all stone fragments. Therefore, we are interested in the use of alpha‐blockers to facilitate stone passage after SWL. Like MET for improvement of spontaneous stone passage, MET after SWL is an off‐label use of the medication in the USA (Campschroer 2018).
Adverse effects of the intervention
The most frequent adverse effects of alpha‐blockers are related to the cardiovascular system. The American Geriatrics Society 2015 recommends avoidance of the alpha‐blockers doxazosin, prazosin and terazosin as antihypertensive medications in elderly people due to the high risk of orthostatic hypotension. Because of the risk of orthostatic hypotension, as well as bradycardia, avoidance of use in people with history of syncope is also recommended (Boehringer 2019). Alpha‐blockers may exacerbate heart failure. Tamsulosin has been reported to cause atrial fibrillation in postmarketing studies (Boehringer 2019). Additionally, those studies have reported adverse effects of palpitations, peripheral edema, tachycardia and cardiac dysrhythmia.
Adverse effects of terazosin on the genitourinary tract have been reported. Erectile dysfunction has been known to occur in 1.2% to 1.6% of men (Abbott Laboratories 2019). Priapism – prolonged and painful erection of the penis – has been reported, but only rarely (Abbott Laboratories 2019). Abnormal ejaculation has been reported with alpha‐blocker use. In men taking tamsulosin, the incidence of abnormal ejaculation is between 8.4% and 18.1% (Boehringer 2019). The abnormal ejaculation was reversible in 76% of men upon discontinuation of the drug (Hofner 1999). Decreased ejaculate volume has been reported in 89.6% of men taking tamsulosin, and anejaculation, the lack of any ejaculation, has been reported in 35.4% of men taking tamsulosin (Hellstrom 2006). Furthermore, alpha‐blockers may worsen incontinence in women with stress or mixed urinary incontinence (Kiruluta 1981; Thien 1978).
How the intervention might work
The rationale for the use of alpha‐blockers as an adjuvant medical therapy for stones is based on the natural history of stones causing contraction of the ureters during passage that may inhibit expulsion. Contractility of the ureters is mediated by alpha‐ and beta‐adrenoreceptors located in the ureteral walls (Park 2007). The ureters contains alpha1D‐ and alpha1A‐adrenoreceptor subtypes and the less prevalent alpha1B‐adrenoreceptor subtype (Itoh 2007; Karabacak 2013; Sigala 2005). The distal ureter contains the highest density of alpha1‐adrenoreceptors, as observed based on the ability of the distal ureter to generate a higher contractile force compared to the proximal ureter (Sasaki 2011).
Adrenergic transmission is mediated by the chemical norepinephrine, which is synthesized within neurons. Norepinephrine activates alpha‐adrenoreceptors and causes stimulation of ureteral activity (Hernández 1992; McLeod 1973). Stimulation of alpha‐adrenoreceptors has been shown to increase contraction of ureteral smooth muscle and promote more frequent peristalsis (Park 2007; Sasaki 2011). Therefore, blockade of alpha‐adrenoreceptors with alpha‐blockers leads to decrease in ureteral contractions (Rose 1974). The decrease in ureteral spasm by alpha‐blockers has the potential benefit of easing spontaneous passage of stones by increasing the rate of expulsion and decreasing pain (Crowley 1990; Laird 1997). It is the alpha‐blockers that have selectivity for alpha1A‐adrenoreceptor subtype, namely alfuzosin, doxazosin, prazosin, tamsulosin, terazosin and silodosin, that have primarily been used for MET.
Pharmacological agents that facilitate ureteral relaxation have the potential to aid in stone expulsion (Sivula 1967). Medications with alpha‐blocking activity help to relax ureteral smooth muscle and could aid in stone passage. Other agents that mediate ureteral relaxation through mechanisms other than alpha‐adrenoreceptors (for example, calcium channel blockers) have been explored in enhancing stone passage, but are outside the scope of this review (Gupta 2014; Pickard 2015).
Why it is important to do this review
Whereas several trials have been conducted to assess the effect of alpha‐blockers in people undergoing SWL for urinary tract stones, there is no consensus as to its effects. Underlying issues relate to clinical differences between trials, such as the type of lithotripter and the definition used for successful stone fragmentation as well as varying methodological quality of these trials. These issues mirror those in the use of alpha‐blockers in people with ureteral colic which were addressed in one Cochrane Review (Campschroer 2018). Campschroer 2018 and another high‐quality review (Hollingsworth 2016) have suggested a possible subgroup effect based on stone size with greater effectiveness in larger stones (5 mm and greater). This appear relevant to our review given that SWL stone fragments can be expected to be smaller (3 mm or less) in size, thereby drawing into question the effectiveness of MET in this setting. Our review will, therefore, address the specific clinical scenario of alpha‐blocker use after SWL. Adjuvant treatment to SWL may provide important benefits for people with residual fragments after SWL. There is potential to accelerate stone passage, thereby leading to less analgesic use, faster recovery and less time away from work. Adjuvant treatment may also reduce costly and invasive secondary treatments. Alpha‐blockers are particularly appealing for MET due to their reported favorable adverse effect profile and low cost. We expect this review to provide important guidance for individual patients, clinicians, guideline developers and policy makers by rigorously assessing the magnitude of both potential desirable and undesirable effects and our confidence in these estimates of effect.
Existing systematic reviews on the use of MET after SWL to date have not applied the same methodological rigor as a Cochrane Review (Lee 2012; Li 2015; Losek 2008; Schuler 2009; Seitz 2009; Skolarikos 2015; Yang 2017; Zheng 2010), where we focus on patient‐centered outcomes by applying the GRADE approach (Guyatt 2008). Our review is structured to address an ongoing knowledge gap on the effectiveness of MET after SWL in clinical practice.
Objectives
To assess the effects of alpha‐blockers as adjuvant medical expulsive therapy plus usual care compared to placebo and usual care or usual care alone in adults undergoing shock wave lithotripsy for renal or ureteral stones.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel group randomized controlled trials (RCTs). We included studies regardless of their publication status or language of publication. We considered that cross‐over trials were unsuitable for this review; also, cluster randomized controlled trials were also not relevant to this review question and therefore not considered. We excluded non‐RCTs and trials using pseudo‐randomization techniques as they are at greater risk of bias.
Types of participants
We included studies of men and non‐pregnant women (ages 18 years or older) of either gender who had undergone SWL for renal and ureteral stones. We included trials irrespective of the lithotripter type used, the number of shock waves applied and the number of sessions performed. We included only studies that use imaging to confirm stone diagnosis. The imaging modality may have been a single test – for example, NCCT – or a combination of tests such as KUB radiograph and ultrasound.
We excluded studies on MET for the primary expulsion of stones. We also excluded studies of people with renal insufficiency (defined as an estimated glomerular filtration rate less than 60 ml/minute/1.73 m²), obstructive uropathy or UTI, as these represent contraindications for SWL.
If we identified studies in which only a subset of participants was relevant to this review, we included such studies if data were available separately for the relevant subset.
Types of interventions
We investigated the following comparisons of experimental intervention versus comparator intervention. Concomitant interventions had to be the same in the experimental and comparator groups to establish fair comparisons.
Experimental interventions
Alpha‐blockers as adjuvant medical expulsive therapy plus usual care.
Comparator interventions
Placebo and usual care, or usual care alone.
Comparisons
Alpha‐blockers as adjuvant medical expulsive therapy plus usual care versus placebo and usual care, or usual care alone.
For the purpose of this review, usual care in the context of SWL for kidney and ureteral stones may have been used in the alpha‐blocker treatment group if the same care was also used in the control group. Usual care may have included oral or intravenous hydration, NSAIDs, pain medication and antibiotics as deemed clinically appropriate. We excluded studies that included antispasmodics, corticosteroids or herbal supplements in the usual care regimens as these could potentially alter the treatment effect; this approach was consistent with that of high‐quality reviews on MET (Hollingsworth 2016). We recognized that this determination may have limited the applicability of our review findings with regard to practice settings in which these adjuvants are commonly used and limit further exploratory analyses as to their role. However, the main objectives of this study were the effects of alpha‐blockers, and inclusion of these adjuvants pose the risk of adding both noise (random error) and bias to the planned analysis.
We anticipated potential variation in the intraoperative management of anesthetic, sedation, pain and antibiotics for people undergoing SWL, but did not consider those factors relevant unless they differed between treatment and control groups.
Types of outcome measures
We did not use the measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Stone clearance (dichotomous outcome).
Auxiliary treatment (dichotomous outcome).
Major adverse events (dichotomous outcome).
Secondary outcomes
Quality of life (continuous outcome).
Stone clearance time (continuous outcome).
Method and timing of outcome measurement
When reviewing outcomes, we considered clinically important differences by predefined thresholds to rate the overall quality of evidence in the 'Summary of findings' table (Jaeschke 1989; Johnston 2013). In the absence of published minimal clinically important differences, we established thresholds with input from our content experts.
Stone clearance
Participants with documented passage of all stones from the kidney and ureter of a given size criterion based on imaging (e.g. KUB radiograph, NCCT) as defined by the investigators.
We assessed this outcome up to 90 days after SWL.
We considered a 5% absolute difference in stone clearance as clinically important.
Auxiliary treatment
Participants requiring unplanned, additional treatments such as URS or stent placement due to failure of stones to pass or to treat secondary complications such ureteral colic or hydronephrosis. We did not consider additional SWL sessions as auxiliary treatment for this analysis.
We assessed this outcome up to 30 days after SWL.
We considered a 3% absolute difference in retreatment rates as clinically important.
Major adverse event
Example: syncope or hypotension requiring hospitalization or unplanned emergency room visit.
We used the Food and Drug Administration (FDA) definition of serious adverse events (FDA 2018).
We assessed this outcome up to 90 days after SWL.
We considered a 1% absolute difference in major adverse events rates as clinically important.
Quality of life
Mean change from baseline or final mean value measured using a validated scale. For example, the RAND 36‐Item Short Form Health Survey (SF‐36) (Ware 1992).
We assessed this outcome up to 90 days after SWL.
We considered a clinically important mean difference (MD) of points on quality of life scores based on the specific scale used.
Stone clearance time
Length of time from onset of treatment to stone clearance (in participants who pass their stone) as measured in days.
We considered an MD of one day as clinically important.
Main outcomes for 'Summary of findings' table
We presented a 'Summary of findings' table that reports on the following outcomes (listed according to priority).
Stone clearance
Auxiliary treatment
Major adverse event
Quality of life
Stone clearance time
Search methods for identification of studies
We performed a comprehensive search with no restrictions on the language of publication or publication status. We reran searches within three months prior to anticipated publication of the review; the latest search date was 27 February 2020.
Electronic searches
We searched the following sources from inception of each database (Appendix 1).
-
Cochrane Library via Wiley:
Cochrane Database of Systematic Reviews (CDSR);
Cochrane Central Register of Controlled Trials (CENTRAL);
Database of Abstracts of Reviews of Effects (DARE);
Health Technology Assessment Database (HTA).
MEDLINE via PubMed (from 1946).
Embase via Elsevier (from 1974).
We also searched the following.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch).
Grey literature repository from the current Grey Literature Report (www.greylit.org).
If detected additional relevant key words during any of the electronic or other searches, we modified the electronic search strategies to incorporate these terms and document the changes.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses and health technology assessment reports. We also contacted study authors of included trials to identify any further studies that we may have missed. We contacted drug/device manufacturers for ongoing or unpublished trials. We did not search abstract proceedings of relevant meetings, specifically those of the AUA, the EAU and the Endourological Society for the last three years (2017 to 2019; no meetings in 2020) separately for unpublished studies since the abstract proceedings for these meetings were included in our electronic searches.
Data collection and analysis
Selection of studies
We used the reference management software EndNote to identify and remove potential duplicate records. Two review authors (MO, RV or NS) independently scanned the abstract, title, or both, of remaining records retrieved, to determine which studies should be assessed further using Covidence software. Two review authors (MO, RV or NS) independently investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any discrepancies through consensus or recourse to a third review author (PD). If resolution was not possible, we designated the study as 'awaiting classification' and we contacted study authors for clarification. We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a dedicated data abstraction form that we pilot tested.
For studies that fulfilled inclusion criteria, two review authors (MO, RV or NS) independently abstracted the following information, which we provided in the Characteristics of included studies table.
Study design.
Study dates (if dates were not available then we reported as such).
Study settings and country.
Type of lithotripter device used and target size for stone fragments.
Participant inclusion and exclusion criteria (i.e. stone size, stone location).
Participant details, baseline demographics (i.e. participant age, stone size, stone location, laterality).
Procedure details (i.e. mean number of shock waves administered, number of session).
Number of participants by study and study arm.
Details of relevant experimental and comparator interventions (i.e. type of alpha‐blocker, dosage, duration of treatment in weeks).
Definitions of relevant outcomes, and method and timing of outcome measurement as well as any relevant subgroups.
Imaging modality used to assess stone clearance (i.e. KUB radiograph, ultrasound, NCCT).
Study funding sources.
Declarations of interest by primary investigators.
We extracted outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for population of a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information.
We resolved any disagreements by discussion, or, if required, by consultation with a third review author (PD).
We provided information, including trial identifier, about potentially relevant ongoing studies in the Characteristics of ongoing studies table.
We attempted to contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximized yield of information by mapping all publications to unique studies and collating all available data. We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Three review authors (MO, RV, NS) independently assessed the risk of bias of each included study. We resolved disagreements by consensus, or by consultation with a third review author (PD).
We assessed risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2017). We assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged risk of bias domains as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We presented a 'Risk of bias' summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel), we considered all outcomes similarly susceptible to performance bias.
For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (investigator or participant assessed) or not susceptible to detection bias (objective).
We defined the following endpoints as investigator assessed outcomes.
Stone clearance.
Major adverse events.
Stone clearance time.
We defined the following endpoint as a participant assessed outcome.
Quality of life.
We defined the following endpoint as an objective outcome.
Auxiliary treatments.
We assessed attrition bias (incomplete outcome data) on an outcome specific basis and presented the judgment for each outcome separately when reporting our findings in the 'Risk of bias' tables.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of the risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs unless studies use different measures to assess the same outcome, in which case we expressed data as standardized mean differences with 95% CIs. We expressed time‐to‐event data as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant and we accounted for the level at which randomization occurred. If we identified trials with more than two intervention groups for inclusion in the review, we handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We obtained missing data from study authors, if feasible, and performed intention to treat (ITT) analyses if data were available; we otherwise performed available case analyses but identified the analysis as such. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis but provided a narrative description of the results of each study.
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I² statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); we interpreted the I² statistic as follows (Deeks 2017).
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess for selective outcome reporting.
If we included 10 studies or more investigating a particular outcome, we used funnel plots to assess small study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. Therefore, we interpreted results carefully.
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes, we used the Mantel‐Haenszel method and for continuous outcomes, we used the inverse variance method. We used Review Manager 5 software to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity and planned to carry out subgroup analyses with investigation of interactions.
Stone location (renal or proximal ureter versus distal ureter).
Stone size (less than 10 mm versus 10 mm or greater).
Specific alpha‐blocker (e.g. terazosin versus doxazosin).
Type of lithotripter (HM3 versus others)
The subgroup analyses by stone location, size and type of alpha‐blocker were based on observations of potential subgroup effects demonstrated in previous studies for the use of MET for ureteral colic (Campschroer 2018; Hollingsworth 2016; Preminger 2007). The subgroup analysis based on type of lithotripter was based on the fact that different shock wave lithotripter devices vary in their effectiveness in stone fragmentation with the HM3 lithotripter (as first generation lithotripter with the largest acoustic energy focal zone) being the most powerful in achieving stone fragmentation (McClain 2013).
In addition, we performed post hoc analyses that were suggested by one of the peer reviewers and were based on a different categorization of stone location. The underlying rationale was that the targeted alpha‐1 receptors are primarily found in the ureteral (not renal pelvis), predominantly in its distal part Campschroer 2018; Hollingsworth 2016, therefore raising the possibility of a reduced effect in renal stones.
Stone location (renal or ureter).
We used the test for subgroup differences in Review Manager 5 to compare subgroup analyses if there were sufficient studies (Review Manager 2014). We limited subgroup analyses to primary outcomes only.
Sensitivity analysis
We performed sensitivity analyses, limited to the primary outcomes, in order to explore the influence of the following factors (when applicable) on effect sizes.
Restricting the analysis by considering risk of bias, by excluding studies at 'high risk' or 'unclear risk'.
Limiting the analysis to studies with a documented single SWL session and studies with multiple SWL sessions that reported outcomes separately by the number of sessions (thereby allowing us to focus on the results of a single session only).
Summary of findings and assessment of the certainty of the evidence
We present the overall certainty of evidence for each outcome according to the GRADE approach, which takes into account five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias), and external validity, such as directness of results (Guyatt 2008). GRADE has good interobserver agreement when used by trained individuals (Mustafa 2013). For each comparison, two review authors (MO, RV or NS) independently rated the certainty of evidence for each outcome as 'high', 'moderate', 'low' or 'very low' using GRADEpro GDT. We resolved any discrepancies by consensus or, if needed, by arbitration by a third review author (PD). For each comparison, we presented a summary of the evidence for the main outcomes in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2017). If meta‐analysis had not been possible, we would have presented the results in a narrative 'Summary of findings' table. We applied a partially conceptualized approach defining a minimally clinically important difference that was based on the published literature or the input of clinical expertise of the coauthors, or both (Hultcrantz 2017). We used GRADE guidance to describe both the certainty of evidence and the magnitude of the effect size (Santesso 2020).
Results
Description of studies
Our comprehensive literature search identified 412 records. We found no applicable records in trials registers or the grey literature repository.
Results of the search
After duplicates were removed, we screened the titles and abstracts of 249 records, and excluded 181 records. We screened 74 full text records (65 studies) and excluded 27 records (25 studies) for the reasons given in the Characteristics of excluded studies table. We included 47 records (40 studies) in the systematic review. There were no ongoing studies that met inclusion criteria. The details of the literature search are shown in the PRISMA flowchart (Figure 1).
1.
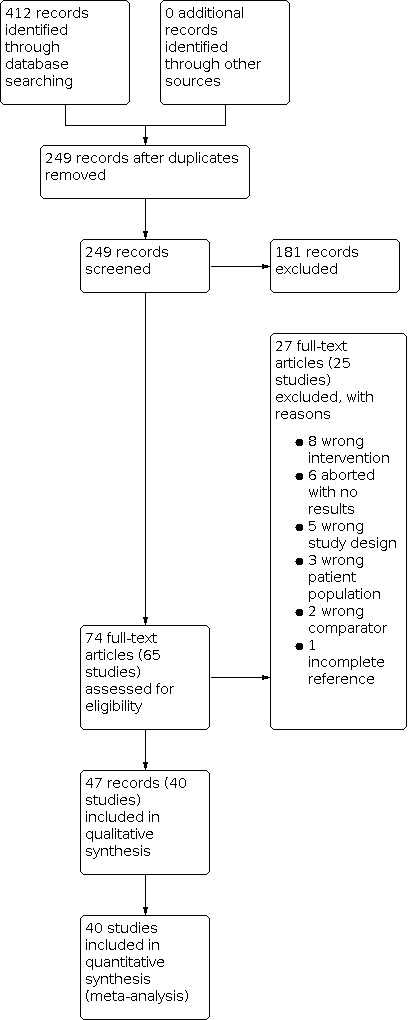
Study flow diagram.
Included studies
We presented details of the included studies in the Characteristics of included studies table, Table 2, and Table 3.
1. Baseline characteristics.
| Study name | Trial period (year to year) | Setting/country | Description of participants | Duration of follow‐up | SWL description (lithotripter; number shocks; power setting) | Number SWL sessions | Intervention(s) and comparator(s) | Stone location (n) | Largest stone size (mm, mean ± SD) |
| Agarwal 2009 | 2006–2007 | Single center/India | Single upper ureteric stone < 15 mm in size | 3 months | Electromagnetic lithotripter Lithostar Multiline; max 3500 shocks; 14.4–15.1 kV | Max 4 | Tamsulosin 0.4 mg + usual care | Upper ureteral | 9.4 ± 1.9 |
| Usual care (NSAIDs, antispasmodics, or tramadol PRN) | 10.4 ± 3 | ||||||||
| Ahmed 2016 | 2013–2016 | Multicenter/Saudi Arabia | Solitary renal stone < 20 mm | 12 weeks | Electromagnetic lithotripter Dornier SII; max 3500 shocks; NR | 1 | Tamsulosin 0.4 mg + usual care | Renal | 12.06 ± 3.82 |
| Usual care (diclofenac potassium 50 mg BID for 2 days, additional doses PRN; drink plenty of fluids) | 12.56 ± 3.97 | ||||||||
| Ateş 2012 | 2009–2010 | Multicenter/Turkey | Renal colic and upper ureteral stones | 2 weeks | Siemens Lithoscope; max 3000 shocks; NR | Max 2 | Doxazosin 4 mg + usual care | Upper ureteral | 9.06 ± 1.45 |
| Usual care (diclofenac sodium PRN; drink fluid to provide urine output ≥ 2 L/day) | 8.3 ± 2.51 | ||||||||
| Baloch 2011 | 2010 | NR/Pakistan | Renal calculi | Max 3 months | NR | 1 | Alfuzosin 10 mg + usual care | Renal | NR |
| Usual care (standard analgesia PRN) | |||||||||
| Bhagat 2007 | 2004–2005 | NR/India | Single radiopaque renal 6–24 mm or ureteral 6–15 mm calculus | 30 days | Dornier Compact S Lithotripter; 1,500 shocks; 14–15 kV | 1 | Tamsulosin 0.4 mg + usual care | 14 calyx/6 pelvis/5 upper renal/4 lower ureteral | NR |
| Usual care (proxyvon [65 mg dextropropoxyphene hydrochloride and 400 mg acetaminophen] daily PRN; minimum 2.5 L fluids) | 12 calyx/9 pelvis/6 upper renal/2 lower ureteral | ||||||||
| Cakıroglu 2013 | 2008–2011 | Multicenter/Turkey | Solitary 6–15 mm ureteral stone | 4 weeks | Storz Medical AG Modulith Slk; 2700–3600 shocks; 6–19 kV | 1 | Tamsulosin 0.4 mg + usual care | NR | 11.4 ± 3.01 |
| Usual care (diclofenac 75 mg IM PRN, pantoprazole 40 mg/day, after discharge drink 2 L water daily) | 10.7 ± 3.2 | ||||||||
| Chau 2015 | NR | NR/China | Renal stones undergoing ESWL up to 3 times | 4 weeks | NR | Max 3 | Tamsulosin 0.4 mg + usual care | Renal | NR |
| Usual care (analgesic not further defined) | |||||||||
| Cho 2013 | 2010–2011 | Single center/South Korea | Participants with radio‐opaque ureter stones 5–10 mm in diameter | Max 42 days | Comed Lithotripsy SDS‐5000; NR; NR | Max 2 | Alfuzosin 10 mg + usual care | 35 upper ureteral/6 lower ureteral | 7.1 ± 1.7 |
| Usual care (loxoprofen sodium 68.1 mg PRN; 2 L fluids daily) | 37 upper ureteral/6 lower ureteral | 7.2 ± 1.8 | |||||||
| De Nunzio 2016 | 2012–2015 | Presumed single center/Italy | Single radiopaque renal stone (0.5–2 cm) | 3 weeks | EDAP integrated Sonolith 4000 plus; max 3500 shocks; NR | 1 | Tamsulosin 0.4 mg + usual care | 2 upper renal/7 mid‐renal/5 lower renal/5 pelvis | 10.28 ± 2.46 |
| Usual care not further defined | 5 upper renal/6 mid‐renal/2 lower renal/9 pelvis | 9.23 ± 2.04 | |||||||
| Silodosin 8 mg/day + usual care | 2 upper renal/4 mid‐renal/9 lower renal/4 pelvis | 10.45 ± 1.73 | |||||||
| Elkoushy 2012 | NR | Presumed single center/Egypt | Single radio‐opaque renal or upper ureteral stones ≤ 2 cm in largest diameter | 3 months or until stone free | Electromagnetic Siemens Lithostar; max 4000 shocks; 14–15 kV | Multiple | Tamsulosin 0.4 mg + usual care | 35 renal (5 upper calyx/3 mid‐calyx/12 lower calyx/15 pelvis)/28 upper ureteral | 12.3 ± 1.8 renal, 9.7 ± 2.6 ureteral |
| Usual care (sodium diclofenac 50 mg oral or 75 mg IM PRN) | 42 renal (4 upper calyx/6 mid‐calyx/14 lower calyx/18 pelvis)/21 upper ureteral | 11.5 ± 2.3 renal, 8.6 ± 1.7 ureteral | |||||||
| Eryildirim 2016 | 2015 | Presumed single center/Turkey | 5 to 10 mm single radio‐opaque upper ureteral stones (above iliac vessels) | 4 weeks | Electromagnetic lithotriptor Compact Sigma; max 4000 shocks; 14–15 kV | 1 | Tamsulosin 0.4 mg + usual care | Upper ureteral | NR |
| Usual care (diclofenac sodium, enteric‐coated tablets 75 mg PRN) | |||||||||
| Falahatkar 2011 | 2008–2009 | Single center/Iran | Renal or ureteral stone 4–20 mm | 12 weeks | Storz lithotriptor; NR; NR | 1 | Tamsulosin 0.4 mg + usual care | 69 renal/9 ureteral | 13.22 ± NR |
| Usual care (ofluxacine 200 mg per 12 hours for 5 days; minimum 2 L fluid daily) | 61 renal/14 ureteral | 12.88 ± NR | |||||||
| Gaafar 2011 | NR | NR/Egypt | Solitary renal pelvic calculi who were successfully fragmented by ESWL | 12 weeks | NR | 1 | Tamsulosin 0.4 mg + usual care | Renal | NR |
| Usual care (diclofenac sodium 75 mg PRN) | |||||||||
| Doxazocin daily for up to 12 weeks + usual care | |||||||||
| H 2012 | NR | NR/China | 5–20 mm ureteric stone in any position | 4 weeks | NR | 1 | Tamsulosin 0.4 mg + usual care | NR | NR |
| Usual care (analgesic not further specified 1 week PRN) | |||||||||
| Hammoud 2014 | 2010–2012 | Single center/Egypt | Males with solitary, radio‐opaque upper urinary tract stones, ≤ 20 mm in the max diameter | 2 weeks after last SWL session | NR | Max 3 | Tamsulosin 0.4 mg + usual care | 35 renal/12 upper ureteral | 13 ± 4.96 |
| Usual care (drink liberal fluids, analgesic not further specified PRN) | 33 renal/10 upper ureteral | 12.3 ± 4.82 | |||||||
| Han 2006 | 2005–2006 | Single center/South Korea | Upper ureteral stone 6–12 mm | 2 weeks | Piezolith‐3000; 3000 shocks; 15 kV | Multiple | Tamsulosin 0.2 mg + usual care | Upper ureteral | NR |
| Usual care (drink 2 L fluid daily) | |||||||||
| Hong 2012 | NR | Single center/Singapore | Upper ureteric or renal stones | 12 weeks | NR | 1 | Alfuzosin XL 10 mg + usual care | 11 renal/8 upper ureteral | NR |
| Usual care only (not further described) | 14 renal/7 upper ureteral | ||||||||
| Itaya 2011 | NR | NR/Japan | Males with ureteral stones | 28 days | NR | 1 | Silodosin 0.8 mg + usual care | Upper ureteral | NR |
| Usual care (pain relieving therapy) | |||||||||
| Janane 2014 | 2008–2012 | Presumed single center/Morocco | Lower ureteral stones undergoing ESWL | 3 months | Storz MODULITH SLX‐F2; NR; NR | 1 | Tamsulosin 0.4 mg + usual care | 30 upper calyx/23 mid‐calyx/79 pelvis/54 lumbar ureteral | 9.2 ± 2.8 |
| Usual care (diclofenac 25 mg TID; minimum 2 L water daily) | 26 upper calyx/19 mid‐calyx/75 pelvis/50 lumbar ureteral | 9.4 ± 3 | |||||||
| Kang 2009 | 2007 | Multicenter/South Korea | ≥ 4 mm ureteral stone with acute pain | 1 week | Compact Delta II, E‐3000, Sonolith Praktis; NR; NR | multiple | Tamsulosin 0.2 mg and diclofenac 100 mg + usual care | 18 renal/50 upper ureteral/47 lower ureteral | NR |
| Usual care only | 19 renal/34 upper ureteral/39 lower ureteral | ||||||||
| Kim 2008 | 2006–2007 | Single center/South Korea | ≤ 10 mm upper and lower ureteral stone | NR | Sonolith Praktis; 2500–3000 shocks; 10–18 kV | 1 | Tamsulosin 0.2 mg and pethidine 50 mg IV (once during SWL) + usual care | 18 upper ureteral/24 lower ureteral | NR |
| Education for hydration and exercise + usual care | 21 upper ureteral/13 lower ureteral | ||||||||
| Kobayashi 2008 | 2005–2006 | Multicenter/Japan | Males with ureteral stones > 4 mm | Until stone clearance | Dornier lithotripter, Stoltz SLX‐MX, Simens modularis variostar; NR; NR | 1 | Tamsulosin 0.2 mg + usual care | 27 proximal ureteral/3 mid‐ureteral/8 distal ureteral | 10.61 ± 4.45 |
| Usual care (diclofenac 50 mg suppository PRN; 2 L water per day) | 23 proximal ureteral/3 mid‐ureteral/8 distal ureteral | 9.85 ± 3.13 | |||||||
| Küpeli 2004 | 2003–2004 | NR/Turkey | Lower ureteral stones within the distal 5 cm of the ureter 3–15 mm in size | 15 days | Siemens Lithostar Plus; 2000–3500 shocks; 18.7 kV | 1 | Tamsulosin 0.4 mg + usual care | NR | 8.6 ± NR |
| Usual care (diclofenac sodium 100 mg/day for 15 days; oral hydration) | 8.6 ± NR | ||||||||
| Lanchon 2017 | 2015 | Single center/France | Participants with urinary stone | 1 month | NR | 1 | Tamsulosin or silodosin + usual care | 44 renal/24 ureteral | 8.4 ± NR |
| Usual care (analgesic PRN) | 31 renal/26 ureteral | 8.2 ± NR | |||||||
| Liu 2009 | NR | NR/China | Single ureteral stone | 2 weeks | NR | 1 | Tamsulosin 0.2 mg + usual care | NR | NR |
| Usual care (hydration, antibiotics, acetaminophen PRN) | |||||||||
| Micali 2007 | 2003–2005 | NR/Italy | Radiopaque or radiolucent ureteral lithiasis | 60 days | Dornier Lithotripter S; NR; NR | 1 | Tamsulosin 0.4 mg; ketoprofene 50 mg BID for 7 days + usual care | Lower ureteral | 10 ± 2.59 |
| Usual care (diclofenac 75 mg IM PRN; 1.5–2 L water daily) | 9.9 ± 1.37 | ||||||||
| Mohamed 2013 | 2010–2012 | Single center/Egypt | Solitary ureteric stone 5–15 mm diameter | 90 days | Electromagnetic lithotripter Dornier SII; max 3000–4000 shocks; 12–15 kV | Multiple | Tamsulosin 0.4 mg + usual care | 25 upper ureteral/14 mid‐ureteral/26 lower ureteral | NR |
| Usual care (furosemide 20 mg every morning; diclofenac sodium 50 mg TID or a 75 mg ampoule PRN; oral fluids) | 31 upper ureteral/13 mid‐ureteral/21 lower ureteral | ||||||||
| Naja 2008 | 2006–2007 | NR/India | Single radiopaque renal stone (5–20 mm) undergoing ESWL | 12 weeks | Electromagnetic lithotripter Siemens Lithostar‐Multiline; max 3500 shocks; 13.4–15.1 kV | Multiple | Tamsulosin 0.4 mg + usual care | 38 renal pelvis/9 superior calyx/4 middle calyx | 12.12 ± 3.59 |
| Usual care (NSAIDs, antispasmodics or tramadol PRN) | 52 renal pelvis/7 superior calyx/6 middle calyx | 13.06 ± 3.49 | |||||||
| Park 2013 | 2011–2013 | NR/Korea | Ages 18–70 years with symptomatic, unilateral and single proximal ureteral stones 6–20 mm in the longest axis | 3 weeks | Sonolith Praktis electroconductive lithotripter EDAP TMS S.A.; NR; NR | 1 | Tamsulosin 0.2 mg + usual care | Proximal ureteral | 9.2 ± NR |
| Usual care (aceclofenac 100 mg PRN; 1.5–2 L water daily) | 9.6 ± NR | ||||||||
| Qadri 2014 | 2010 | Single center/Pakistan | Single radio‐opaque renal stone (0.5–2.0 cm) | 8 weeks | Electromagnetic shock wave generator Storz Medical Modulith SLK; max 4000 shocks; max 70 kV | Multiple | Tamsulosin 0.4 mg + usual care | 36 pelvis/17 lower renal/5 mid‐renal/2 upper renal | 11.2 ± 3.1 |
| Usual care (diclofenac sodium 50 mg BID for 1 day) | 43 pelvis/13 lower renal/3 mid‐renal/1 upper renal | 10.5 ± 2.6 | |||||||
| Rakesh 2015 | NR | NR/India | Inclusion criteria for ESWL | NR | NR | 1 | Tamsulosin (dose NR) + usual care | NR | NR |
| Usual care (not further defined) | |||||||||
| Seungok 2009 | NR | NR/NR | Ureteral stones < 10 mm treated with ESWL | NR | NR | Multiple | Tamsulosin 0.2 mg + usual care | NR | NR |
| Usual care (not further defined) | |||||||||
| Shaikh 2018 | 2013 | Single center/Pakistan | Ages > 18 to < 50 years, single radio‐opaque and size < 20 mm | 8 weeks | NR | 1 | Tamsulosin 0.4 mg + usual care | Renal | 10.4 ± 2.59 |
| Usual care (diclofenac 50 mg BID) | 10.61 ± 3.01 | ||||||||
| Sighinolfi 2010 | 2009 to NR | NR/Italy | Apparent ESWL‐fragmentation of renal stones | NR | NR | 1 | Tamsulosin (dose NR) + usual care | Renal | 9.8 ± 4.2 |
| Usual care (not further defined) | 9.1 ± 2.6 | ||||||||
| Singh 2011a | 2006–2008 | Single center/India | Ages ≥ 18 years with symptomatic, unilateral, solitary lower ureteric calculus 4–12 mm in major axis | 4 weeks | Electromagnetic lithotripter HK–ESWL–VI; max 3000 shocks; 12–15 kV | 1 | Tamsulosin 0.4 mg + usual care | Lower ureteral | NR |
| Usual care (antibiotics and diclofenac PRN during the study period; 2500 mL fluid daily) | |||||||||
| Singh 2011b | 2006–2008 | Single center/India | Ages 18–70 years with symptomatic, unilateral and solitary upper ureteral calculi 6–15 mm in major axis | 3 months | Electromagnetic lithotripter HK–ESWL–VI; max 3000 shocks; 12–15 kV | Max 3 | Tamsulosin 0.4 mg + usual care | Upper ureteral | NR |
| Usual care (diclofenac PRN during the study period; 2500 mL fluid daily) | |||||||||
| Tajari 2009 | 2006–2007 | Single center/Iran | 7–19 mm stone diameter | 3 months | NR | 1 | Tamsulosin 0.4 mg + usual care | Ureteral | NR |
| Usual care (diclofenac 100 mg suppositories daily; diclofenac 25 mg) orally | |||||||||
| Terazosin 2 mg + usual care | |||||||||
| Teleb 2015 | 2012–2014 | NR/Egypt | Participants who underwent successful SWL (fragments < 4 mm) for single renal stone ≤ 2 cm | 4 weeks | NR | 1 | Tamsulosin 0.4 mg + usual care | Renal | NR |
| Usual care (analgesic and anti‐inflammatory) | |||||||||
| Vicentini 2011 | 2006–2009 | Single center/Brazil | Ages > 18 years, radio‐opaque non‐lower pole renal stone (5–20 mm) and ESWL | 30 days | Electromagnetic lithotripter Dornier Compact Delta Lithotriptor; 4000 shocks; 11–14 kV | 1 | Tamsulosin 0.4 mg + usual care | 11 superior calyx/13 mid‐calyx/14 pelvis | NR |
| Usual care (celecoxib 200 mg BID PRN; 3 L liquid daily) | 7 superior calyx/16 mid‐calyx/15 pelvis | ||||||||
| Wang 2008 | 2005–2007 | NR/China | Lower ureteral stones | 2 weeks | NR | NR | Tamsulosin 0.4 mg + usual care | Lower ureteral | 8.6 ± 2.6 |
| Usual care (not further defined) | 8.2 ± 3.1 |
BID: twice daily; ESWL: extracorporeal shock wave lithotripsy; IM: intramuscular; IV: intravenous; max: maximum; n: number; NR: not reported; NSAID: non‐steroidal anti‐inflammatory drug; PRN: on demand; SD: standard deviation; SWL: shock wave lithotripsy; TID: three times daily.
2. Participants in included studies and imaging modality used to assess stone clearance.
| Study name | Intervention(s) and comparator(s) | Screened/eligible (n) | Randomized (n) | Analyzed (n) | Finishing trial (n [%]) | Follow‐up imaging modality |
| Agarwal 2009 | Tamsulosin 0.4 mg | 55/NR | 20 | 20 | 20 (100) | KUB |
| NSAIDs, antispasmodics or tramadol PRN | 20 | 20 | 20 (100) | |||
| Total | 40 | 40 | 40 (100) | |||
| Ahmed 2016 | Tamsulosin 0.4 mg | 326/279 | 135 | 123 | 123 (91.1) | KUB, ultrasound and CT |
| Diclofenac potassium 50 mg BID for 2 days, additional doses PRN; drink plenty of fluids | 136 | 126 | 126 (92.6) | |||
| Total | 271 | 249 | 249 (91.9) | |||
| Ateş 2012 | Doxazosin 4 mg | NR/NR | NR | 35 | 35 (NR) | KUB |
| Diclofenac sodium PRN; drink fluid to provide urine output ≥ 2 L/day | NR | 44 | 44 (NR) | |||
| Total | 90 | 79 | 79 (87.7) | |||
| Baloch 2011 | Alfuzosin 10 mg | NR/NR | 65 | 65 | 65 | NR |
| Standard analgesia on demand | 65 | 65 | 65 | |||
| Total | 130 | 130 | 130 (100) | |||
| Bhagat 2007 | Tamsulosin 0.4 mg | NR/NR | 30 | 29 | 29 | KUB |
| Proxyvon (dextropropoxyphene hydrochloride 65 mg and acetaminophen 400 mg ) daily PRN; minimum 2.5 L fluids | 30 | 29 | 29 | |||
| Total | 60 | 58 | 58 (96.7) | |||
| Cakıroglu 2013 | Tamsulosin 0.4 mg | NR/NR | NR | 59 | 59 | KUB and ultrasound |
| Diclofenac 75 mg IM on demand, pantoprazole 40 mg/day, after discharge drink 2 L water daily | NR | 64 | 64 | |||
| Total | NR | 123 | 123 (NR) | |||
| Chau 2015 | Tamsulosin 0.4 mg | NR/NR | 88 | 88 | 88 | NR |
| Analgesic | 95 | 95 | 95 | |||
| Total | 183 | 183 | 183 (100) | |||
| Cho 2013 | Alfuzosin 10 mg | NR/NR | 45 | 41 | 41 (91.1) | KUB |
| Loxoprofen sodium 68.1 mg PRN; 2 L fluids daily | 45 | 43 | 43 (95.6) | |||
| Total | 90 | 84 | 84 (93.3) | |||
| De Nunzio 2016 | Tamsulosin 0.4 mg | NR/NR | NR | 19 | 19 | Ultrasound and CT |
| No alpha‐blocker | NR | 22 | 22 | |||
| Silodosin 8 mg/day for 21 days | NR | 19 | 19 | |||
| Total | 66 | 60 | 60 (90.1) | |||
| Elkoushy 2012 | Tamsulosin 0.4 mg | NR/NR | 63 | 63 | 63 | KUB and ultrasound |
| Sodium diclofenac 50 mg oral or 75 mg IM PRN | 63 | 63 | 63 | |||
| Total | 126 | 126 | 126 (100) | |||
| Eryildirim 2016 | Tamsulosin 0.4 mg | NR/NR | 40 | 28 | 28 (70) | NR |
| Diclofenac sodium, enteric‐coated tablets 75 mg PRN | 40 | 26 | 26 (65) | |||
| Total | 80 | 54 | 54 (67.5) | |||
| Falahatkar 2011 | Tamsulosin 0.4 mg | NR/NR | 75 | 70 | 70 (93.3) | KUB and ultrasound |
| Ofluxacine 200 mg per 12 hours for 5 days; minimum 2 L fluid daily | 75 | 71 | 71 (94.7) | |||
| Total | 150 | 141 | 141 (94) | |||
| Gaafar 2011 | Tamsulosin 0.4 mg | NR/NR | 50 | NR | NR | NR |
| Diclofenac sodium 75 mg PRN | 50 | NR | NR | |||
| Doxazocin daily for up to 12 weeks | 50 | NR | NR | |||
| Total | 150 | NR | NR | |||
| H 2012 | Tamsulosin 0.4 mg | NR/NR | NR | 8 | NR | NR |
| Analgesic 1 week PRN | NR | 12 | NR | |||
| Total | NR | 20 | NR | |||
| Hammoud 2014 | Tamsulosin 0.4 mg | NR/NR | 47 | 47 | 47 | NR |
| Drink liberal fluids, analgesic PRN | 49 | 49 | 49 | |||
| Total | 96 | 96 | 96 (100) | |||
| Han 2006 | Tamsulosin 0.2 mg | NR/45 | 22 | 22 | 22 | KUB and IVP |
| Drink 2 L fluid daily | 23 | 23 | 23 | |||
| Total | 45 | 45 | 45 (100) | |||
| Hong 2012 | Alfuzosin XL 10 mg | NR/NR | 19 | 19 | NR | NR |
| No alpha‐blocker | 21 | 21 | NR | |||
| Total | 40 | 40 | NR | |||
| Itaya 2011 | Silodosin 0.8 mg | NR/NR | 16 | 16 | NR | NR |
| Pain relieving therapy | 16 | 16 | NR | |||
| Total | 32 | 32 | NR | |||
| Janane 2014 | Tamsulosin 0.4 mg | NR/NR | 186 | 186 | NR | KUB, ultrasound and CT |
| Diclofenac 25 mg TID; minimum 2 L water daily | 170 | 170 | NR | |||
| Total | 356 | 356 | NR | |||
| Kang 2009 | Tamsulosin 0.2 mg and diclofenac 100 mg | NR/247 | 115 | 115 | 115 | NR |
| No alpha‐blocker | 92 | 92 | 92 | |||
| Total | 207 | 207 | 207 (100) | |||
| Kim 2008 | Tamsulosin 0.2 mg and pethidine 50 mg IV (once during SWL) | NR/76 | 42 | 42 | 42 | KUB, IVP or CT |
| Education for hydration and exercise | 34 | 34 | 34 | |||
| Total | 76 | 76 | 76 (100) | |||
| Kobayashi 2008 | Tamsulosin 0.2 mg | NR/NR | 38 | 38 | 38 | KUB and ultrasound |
| Diclofenac 50 mg suppository PRN; 2 L water per day | 34 | 34 | 34 | |||
| Total | 72 | 72 | 72 (100) | |||
| Küpeli 2004 | Tamsulosin 0.4 mg | NR/97 | 24 | 24 | 24 | KUB and CT |
| Diclofenac sodium 100 mg/day for 15 days; oral hydration | 24 | 24 | 24 | |||
| Total | 48 | 48 | 48 (100) | |||
| Lanchon 2017 | Tamsulosin or silodosin | NR/NR | 68 | 68 | NR | NR |
| Analgesic PRN | 57 | 57 | NR | |||
| Total | 125 | 125 | NR | |||
| Liu 2009 | Tamsulosin 0.2 mg | NR/NR | 53 | 53 | NR | NR |
| Conservative therapy PRN, e.g. hydration, antibiotics, acetaminophen | 55 | 55 | NR | |||
| Total | 108 | 108 | NR | |||
| Micali 2007 | Tamsulosin 0.4 mg; ketoprofene 50 mg BID for 7 days | NR/NR | 28 | 28 | 28 | KUB, ultrasound and CT |
| Diclofenac 75 mg IM PRN; 1.5–2 L water daily | 21 | 21 | 21 | |||
| Total | 49 | 49 | 49 (100) | |||
| Mohamed 2013 | Tamsulosin 0.4 mg | 156/156 | 65 | 65 | 65 | KUB and ultrasound |
| Furosemide 20 mg every morning; diclofenac sodium 50 mg TID or a 75 mg ampoule PRN; oral fluids | 65 | 65 | 65 | |||
| Total | 130 | 130 | 130 | |||
| Naja 2008 | Tamsulosin 0.4 mg | NR/NR | 67 | 51 | 51 (76.1) | KUB and ultrasound |
| NSAIDs, antispasmodics or tramadol PRN | 72 | 65 | 65 (90.3) | |||
| Total | 139 | 116 | 116 (83.5) | |||
| Park 2013 | Tamsulosin 0.2 mg | NR/NR | 48 | 44 | 44 (91.7) | KUB and ultrasound |
| Aceclofenac 100 mg PRN; 1.5–2 L water daily | 48 | 44 | 44 (91.7) | |||
| Total | 96 | 88 | 88 (91.7) | |||
| Qadri 2014 | Tamsulosin 0.4 mg | NR/NR | 60 | 60 | 60 | KUB |
| Diclofenac sodium 50 mg BID for 1 day | 60 | 60 | 60 | |||
| Total | 120 | 120 | 120 (100) | |||
| Rakesh 2015 | Tamsulosin (dose NR) | NR/NR | NR | NR | NR | NR |
| No alpha‐blocker | NR | NR | NR | |||
| Total | 120 | 120 | NR | |||
| Seungok 2009 | Tamsulosin 0.2 mg | NR/NR | NR | NR | NR | NR |
| No alpha‐blocker | NR | NR | NR | |||
| Total | 55 | 55 | NR | |||
| Shaikh 2018 | Tamsulosin 0.4 mg | NR/NR | 80 | 80 | 80 | NR |
| Diclofenac 50 mg BID | 80 | 80 | 80 | |||
| Total | 160 | 160 | 160 (100) | |||
| Sighinolfi 2010 | Tamsulosin (dose NR) | NR/NR | 60 | 60 | NR | NR |
| No alpha‐blocker | 69 | 69 | NR | |||
| Total | 129 | 129 | NR | |||
| Singh 2011a | Tamsulosin 0.4 mg | NR/NR | 60 | 60 | 60 (100) | KUB and ultrasound |
| Antibiotics and diclofenac PRN during the study period; 2500 mL fluid daily | 60 | 59 | 59 (98.3) | |||
| Total | 120 | 119 | 119 (99.2) | |||
| Singh 2011b | Tamsulosin 0.4 mg | NR/NR | NR | 59 | 59 (NR) | KUB and ultrasound |
| Diclofenac PRN during the study period; 2500 mL fluid daily | NR | 58 | 58 (NR) | |||
| Total | 120 | 117 | 117 (97.5) | |||
| Tajari 2009 | Tamsulosin 0.4 mg | NR/NR | 80 | 80 | NR | NR |
| Diclofenac 100 mg suppositories daily; diclofenac 25 mg orally | 80 | 80 | NR | |||
| Terazosin 2 mg | 80 | 80 | NR | |||
| Total | 240 | 240 | NR | |||
| Teleb 2015 | Tamsulosin 0.4 mg | NR/NR | 106 | 106 | NR | NR |
| Analgesic and anti‐inflammatory | 106 | 106 | NR | |||
| Total | 212 | 212 | NR | |||
| Vicentini 2011 | Tamsulosin 0.4 mg | NR/NR | 45 | 38 | 38 (84.4) | KUB and ultrasound |
| Celecoxib 200 mg BID PRN; 3 L liquid daily | 46 | 38 | 38 (82.6) | |||
| Total | 91 | 76 | 76 (83.5) | |||
| Wang 2008 | Tamsulosin 0.4 mg | NR/NR | 40 | 40 | 40 | NR |
| Control group | 40 | 40 | 40 | |||
| Total | 80 | 80 | 80 (100) | |||
BID: twice daily; CT: computer tomography; IM: intramuscular; IV: intravenous; IVP: intravenous pyelography; KUB: kidney, ureter, bladder radiograph; n: number; NR: not reported; NSAID: non‐steroidal anti‐inflammatory drug; PRN: on demand; SWL: shock wave lithotripsy; TID: three times daily.
Source of data
We included 26 studies published in full text and 14 as abstract proceedings (Baloch 2011; Chau 2015; Gaafar 2011; H 2012; Hammoud 2014; Hong 2012; Itaya 2011; Lanchon 2017; Liu 2009; Rakesh 2015; Seungok 2009; Sighinolfi 2010; Tajari 2009; Teleb 2015). Most studies were published in English; three were in Korean (Han 2006; Kang 2009; Kim 2008), and one in Chinese (Wang 2008). The Korean studies were translated by a review author (ECH) and we used Google translator for the Chinese study. We attempted to contact all corresponding authors of included trials to obtain additional information on study methodology and results and received replies from only a few. Details of this communication are provided in the notes section of the Characteristics of included studies table.
Study design and settings
We included all parallel RCTs. Only four studies were described as 'double blind' (Bhagat 2007; Elkoushy 2012; Falahatkar 2011; Vicentini 2011). One study was reported as single blind, but it was unclear who was blinded (Cho 2013). It was unclear if blinding was performed in five studies (De Nunzio 2016; Hammoud 2014; Janane 2014; Kang 2009; Wang 2008). The remaining 30 studies were open label.
Studies were performed in both inpatient and outpatient centers. Four studies were in the hospital setting (Ateş 2012; Cakıroglu 2013; Hong 2012; Kobayashi 2008). Eight studies were in an outpatient setting (Cho 2013; Han 2006; Kang 2009; Kim 2008; Mohamed 2013; Park 2013; Singh 2011a; Singh 2011b). Two studies reported they were performed specifically in an SWL center (Falahatkar 2011; Tajari 2009). Most studies were performed in Asia (China, India, Iran, Japan, Korea, Pakistan, Saudi Arabia, Singapore and Turkey), but also in Africa (Egypt and Morocco), Europe (France and Italy) and South America (Brazil). Four trials were multicenter (Ahmed 2016; Ateş 2012; Kang 2009; Kobayashi 2008). The studies were performed from 2003 to 2015.
Participants
We included 4793 randomized participants, of whom 3087 completed the trials. However, six studies did not clearly report the number randomized to each group (Ateş 2012; De Nunzio 2016; H 2012; Rakesh 2015; Seungok 2009; Singh 2011b), and 12 studies did not clearly report the number completing the trial in each group (Gaafar 2011; H 2012; Hong 2012; Itaya 2011; Janane 2014; Lanchon 2017; Liu 2009; Rakesh 2015; Seungok 2009; Sighinolfi 2010; Tajari 2009; Teleb 2015). The mean age of participants was 28.6 years to 56.8 years. Twelve studies did not report participants' age (Baloch 2011; Chau 2015; Gaafar 2011; H 2012; Hong 2012; Itaya 2011; Lanchon 2017; Liu 2009; Rakesh 2015; Seungok 2009; Sighinolfi 2010; Teleb 2015). As reported in Table 2, studies used a variety of lithotripters but no study used the Dornier HM3 device (thereby precluding one of our predefined subgroup analyses).
The mean size of stones prior to SWL was 7.1 mm to 13.2 mm. Twelve studies did not report stone size (Baloch 2011; Chau 2015; Gaafar 2011; H 2012; Hong 2012; Itaya 2011; Lanchon 2017; Liu 2009; Rakesh 2015; Seungok 2009; Sighinolfi 2010; Teleb 2015). The stone location for 12 studies was ureteral (Cakıroglu 2013; Cho 2013; H 2012; Itaya 2011; Kang 2009; Kim 2008; Kobayashi 2008; Liu 2009; Micali 2007; Mohamed 2013; Seungok 2009; Tajari 2009). In 11 it was renal (Ahmed 2016; Baloch 2011; Chau 2015; De Nunzio 2016; Gaafar 2011; Naja 2008; Qadri 2014; Shaikh 2018; Sighinolfi 2010; Teleb 2015; Vicentini 2011). Six studies specified only upper ureteral stones (Agarwal 2009; Ateş 2012; Eryildirim 2016; Han 2006; Park 2013; Singh 2011b). Four studies included only lower ureteral stones (Janane 2014; Küpeli 2004; Singh 2011a; Wang 2008). Three studies included renal and ureteral stones (Bhagat 2007; Falahatkar 2011; Lanchon 2017). An additional three studies included renal and upper ureteral stones (Elkoushy 2012; Hammoud 2014; Hong 2012). One study did not report on stone location (Rakesh 2015).
Interventions, comparators and comparisons
Twenty‐seven of 40 studies used tamsulosin. The dosage of tamsulosin was typically 0.4 mg daily, but seven studies used 0.2 mg daily (Han 2006; Kang 2009; Kim 2008; Kobayashi 2008; Liu 2009; Park 2013; Seungok 2009). Two studies did not report the dosage (Rakesh 2015; Sighinolfi 2010). Three studies compared tamsulosin directly to another alpha‐blocker: to silodosin (De Nunzio 2016), doxazosin (Gaafar 2011), and terazosin (Tajari 2009). One study used either tamsulosin or silodosin (Lanchon 2017). Three studies used alfuzosin (Baloch 2011; Cho 2013; Hong 2012). One study used doxazosin (Ateş 2012) and one used silodosin (Itaya 2011).
The standard therapies for comparators in 23/40 studies included NSAIDs, and diclofenac was the most frequently used. Nineteen of 40 studies included counseling about general fluid intake or to a specific urine output goal (Ahmed 2016; Ateş 2012; Bhagat 2007; Cakıroglu 2013; Cho 2013; Falahatkar 2011; Hammoud 2014; Han 2006; Janane 2014; Kim 2008; Kobayashi 2008; Küpeli 2004; Liu 2009; Micali 2007; Mohamed 2013; Park 2013; Singh 2011a; Singh 2011b; Vicentini 2011). Seven studies used an unspecified analgesia as the comparator (Baloch 2011; Chau 2015; H 2012; Hammoud 2014; Itaya 2011; Lanchon 2017; Teleb 2015). Three studies used narcotic pain medications (Agarwal 2009; Bhagat 2007; Kim 2008). Two studies used acetaminophen as a pain reliever (Bhagat 2007; Liu 2009). Four studies potentially used antibiotics as part of standard therapy (Falahatkar 2011; Liu 2009; Singh 2011a; Singh 2011b). One study used a diuretic in the comparator group (Mohamed 2013). Six studies used no alpha‐blocker as the comparator group and did not provide further details (De Nunzio 2016; Hong 2012; Rakesh 2015; Seungok 2009; Sighinolfi 2010; Wang 2008). The duration of follow‐up ranged from two weeks to 12 weeks or until stone free.
Outcomes
We identified the primary outcome of stone clearance in 36 of 40 studies. We found fewer studies with data on the other primary outcomes auxiliary treatment (12/40), major adverse events (7/40) and the secondary outcome of stone clearance time (14/40). We identified no studies reporting on participants' quality of life.
The modality of follow‐up imaging was frequently not reported (18/40 studies; Table 3). When reported, it was based on KUB alone (5/40), KUB and ultrasound (10/40), or other combinations such as KUB plus intravenous pyelography or computer tomography imaging (7/40).
Funding sources and conflicts of interest
Two studies reported no funding source (Hammoud 2014; Mohamed 2013). One study reported funding from an industry grant (Park 2013). The funding source in the remaining studies was not reported. Twelve studies reported no conflicts of interest (Ahmed 2016; Cakıroglu 2013; Cho 2013; De Nunzio 2016; Eryildirim 2016; Falahatkar 2011; Janane 2014; Mohamed 2013; Shaikh 2018; Sighinolfi 2010; Singh 2011a; Singh 2011b). One study reported an industry grant as a conflict of interest (Park 2013). The remaining studies did not report on conflicts of interest. The funding source and conflict of interest status was not identifiable for one study due to the language of publication (Wang 2008).
Excluded studies
We excluded 25 studies after evaluation of the full text articles. For details, see Characteristics of excluded studies table. Common reason for exclusion were wrong patient population, and wrong intervention or comparison. We also found several trials that had been aborted without any results.
Studies awaiting classification
We identified no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We assessed risk of bias using the following domains as summarized in Figure 2.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Nineteen studies described the method of random sequence generation; the remaining studies did not describe the method of random sequence generation and were judged at unclear risk of bias.
With regard to allocation concealment, one study described the use of alternating group assignment, which we judged as an inappropriate method of allocation concealment (Eryildirim 2016). Only one study reported centralized randomization and was rated as low risk of bias in this domain (Lanchon 2017); all other studies (38/40) did not address the issue of allocation concealment and we rated them at unclear risk of bias.
Blinding
Performance bias
Thirty‐one studies were clearly identifiable as open label studies that did not blind participants or personnel. Only four studies described appropriate methods of blinding and were judged as low risk for performance bias (Bhagat 2007; Elkoushy 2012; Falahatkar 2011; Vicentini 2011). The remaining studies had an unclear risk of bias.
Detection bias
We assessed the risk of detection bias on a per outcome basis.
Stone clearance
This was an investigator assessed outcome that required judgment and, therefore, was viewed as an outcome for which blinding of outcome assessors was important. Twenty‐eight studies were likely to have not blinded outcome assessors and were, therefore, rated as high risk. Only three studies provided assurance that outcome assessors were blinded and were rated as low risk (and informed our predefined sensitivity analyses; see Analysis 6.1; Analysis 6.2; and Analysis 6.3) (Küpeli 2004; Singh 2011b; Vicentini 2011).
6.1. Analysis.

Comparison 6: Alpha‐blockers and usual care versus usual care: risk of bias (sensitivity analysis), Outcome 1: Stone clearance
6.2. Analysis.

Comparison 6: Alpha‐blockers and usual care versus usual care: risk of bias (sensitivity analysis), Outcome 2: Auxiliary treatment
6.3. Analysis.

Comparison 6: Alpha‐blockers and usual care versus usual care: risk of bias (sensitivity analysis), Outcome 3: Major adverse events
Auxiliary treatments
This was an investigator assessed outcome not requiring judgment. Therefore, all 12 studies reporting this outcome were at low risk for detection bias (Agarwal 2009; Ahmed 2016; Ateş 2012; Bhagat 2007; De Nunzio 2016; Elkoushy 2012; Eryildirim 2016; Mohamed 2013; Naja 2008; Qadri 2014; Singh 2011b; Vicentini 2011).
Major adverse events
This was an investigator assessed outcome that required judgment and, therefore, was viewed as an outcome for which blinding of outcome assessors was important. Based on the available information, four of seven studies were rated as high risk of bias (Ahmed 2016; Han 2006; Mohamed 2013; Sighinolfi 2010); one as unclear (De Nunzio 2016) and two as low risk (Bhagat 2007; Vicentini 2011).
Quality of life
We found no trial reporting quality of life.
Stone clearance time (continuous outcome)
This was an investigator assessed outcome that required judgment, and blinding of outcome assessors was therefore perceived as important. Two studies reported appropriate blinding (Singh 2011b; Vicentini 2011), in the three studies blinding status was unclear (Bhagat 2007; De Nunzio 2016; Elkoushy 2012), whereas seven were unlikely to have blinded outcome assessors (Agarwal 2009; Ahmed 2016; Ateş 2012; Eryildirim 2016; Mohamed 2013; Naja 2008; Qadri 2014).
Incomplete outcome data
We assessed the risk of detection bias on a per outcome basis but since judgments were consistent across outcomes, we collapsed reporting into a single column. Two studies were at high risk of bias (for attrition levels of at least 20% in at least one treatment arm) (Ateş 2012; De Nunzio 2016), 14 were at low risk of bias (with attrition levels less than 10% for both treatment arms) (Agarwal 2009; Ahmed 2016; Baloch 2011; Bhagat 2007; Cho 2013; Elkoushy 2012; Falahatkar 2011; Kang 2009; Micali 2007; Mohamed 2013; Park 2013; Qadri 2014; Singh 2011a; Singh 2011b), whereas 20 were at unclear risk of bias (either because attrition rates could not be determined or ranged between 10% and less than 20%).
Selective reporting
This bias was rated on a study level and reflected whether outcome reporting and analyses corresponded with an a priori protocol. We did not find any study protocols to compare; accordingly, all studies were at unclear risk for selective reporting bias.
Other potential sources of bias
Studies differed by the number of SWL sessions that were used, which we identified as potential source of bias. We sought to address this in a sensitivity analysis limited to studies in which participants clearly only underwent a single session (Analysis 7.1; Analysis 7.2; Analysis 7.3), which were only seven studies (Ahmed 2016; De Nunzio 2016; Janane 2014; Kobayashi 2008; Micali 2007; Park 2013; Singh 2011a). Ten studies used more than one session (Agarwal 2009; Ateş 2012; Cakıroglu 2013; Chau 2015; Cho 2013; Elkoushy 2012; Naja 2008; Qadri 2014; Seungok 2009; Singh 2011b). The remainder were at unclear risk of bias since we could not determine the number if SWL sessions.
7.1. Analysis.

Comparison 7: Alpha‐blocker and usual care versus usual care: single SWL session (sensitivity analysis), Outcome 1: Stone clearance
7.2. Analysis.

Comparison 7: Alpha‐blocker and usual care versus usual care: single SWL session (sensitivity analysis), Outcome 2: Auxiliary treatment
7.3. Analysis.

Comparison 7: Alpha‐blocker and usual care versus usual care: single SWL session (sensitivity analysis), Outcome 3: Major adverse events
Effects of interventions
See: Table 1
Alpha‐blockers as adjuvant medical expulsive therapy plus usual care compared to placebo and usual care or usual care alone
Stone clearance
Alpha‐blockers may improve the clearance of stone fragments after SWL (RR 1.16, 95% CI 1.09 to 1.23; I² = 78%; studies = 36; participants = 4084; low certainty evidence; Analysis 1.1; Figure 3). We downgraded the certainty of the evidence for study limitations (mainly due unclear allocation concealment, lack of blinding of participants and outcome assessors and unclear risk of selective reporting bias) and clinically important inconsistency (Table 1). Funnel plot asymmetry contributed to the decision to downgrade by two levels overall (Figure 4). Preplanned subgroup analyses identified no meaningful interactions to explain the observed inconsistency.
1.1. Analysis.

Comparison 1: Alpha‐blocker and usual care versus usual care alone, Outcome 1: Stone clearance
3.

Forest plot: alpha blocker and usual care versus usual care alone for stone clearance.
4.

Funnel plot: stone clearance.
Based on the stone clearance rate of 69.3% observed in the control arm, alpha‐blockers may increase stone clearance to 80.4%. This corresponds to 111 more (62 more to 159 more) per 1000 participants clearing their stone fragments after SWL with an alpha‐blocker versus usual care alone.
Auxiliary treatment
Alpha‐blockers may reduce the need for auxiliary treatments after SWL (RR 0.67, 95% CI 0.45 to 1.00; I² = 16%; studies = 12; participants = 1251; low certainty evidence; Analysis 1.2; Figure 5), but also includes the possibility of no effect. We downgraded the certainty of the evidence for study limitations (mainly due unclear allocation concealment, lack of blinding of participants and unclear risk of selective reporting bias) and clinically important imprecision given that the 95% CI of the absolute effect size estimate crossed an assumed 3% threshold of clinical importance (and included the possibility of no effect) (Table 1). Funnel plot asymmetry contributed to the decision to downgrade by two levels overall (Figure 6).
1.2. Analysis.

Comparison 1: Alpha‐blocker and usual care versus usual care alone, Outcome 2: Auxiliary treatment
5.

Forest plot: alpha blocker and usual care versus usual care alone for auxiliary treatment.
6.

Funnel plot: auxiliary treatment.
Based on a rate of auxiliary treatments in the usual care arm of 9.7%, alpha‐blockers may reduce the rate to 6.5%. This corresponds to 32 fewer (53 fewer to 0 fewer) per 1000 participants undergoing auxiliary treatments. This analysis did not consider additional SWL sessions as a form of auxiliary treatment (although several trials reported its use in this way, i.e. Ahmed 2016; De Nunzio 2016; Micali 2007; and Naja 2008).
Major adverse events
Alpha‐blockers may reduce major adverse events (RR 0.60, 95% CI 0.46 to 0.80; I² = 0%; studies = 7; participants = 747; low certainty evidence; Analysis 1.3; Figure 7); this corresponds to 106 fewer (144 fewer to 53 fewer) major adverse events per 1000. We downgraded the certainty of the evidence twice for study limitations mainly due to unclear allocation concealment, lack of blinding of participants and outcome assessors, and concerns about selective reporting bias in some studies (all without protocols) addressing this outcome (Table 1). There were too few studies to assess for funnel plot asymmetry.
1.3. Analysis.

Comparison 1: Alpha‐blocker and usual care versus usual care alone, Outcome 3: Major adverse events
7.

Forest plot: alpha blocker and usual care versus usual care alone for major adverse events.
Major adverse events occurred in 25.8% of participants in the usual care group; alpha‐blockers would reduce this to 15.5%. This corresponds to 103 fewer (139 fewer to 52 fewer) major adverse events per 1000 with alpha‐blocker treatment compared to usual care alone.
Ahmed 2016 reported much higher rates of major adverse events than any other study for both treatments arms. Removing this study resulted in a lower control event of 13.8%. Using this as control event rate, the observed relative effect size would result in 55 fewer (75 fewer to 28 fewer) major adverse events per 1000 participants compared to usual care.
None of the reported major adverse events were reported to be related to the alpha‐blocker such as syncope or hypotension. Unplanned emergency room visits and rehospitalization for stone related issues were the main contributors.
Quality of life
No study reported quality of life.
Stone clearance time
Alpha‐blockers may reduce the time for stone clearance (MD –3.74, 95% CI –5.25 to –2.23; I² = 86%; studies = 14; participants = 1790; low certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence for study limitations (mainly due unclear allocation concealment, lack of blinding of participants and outcome assessors, and unclear risk of selective reporting bias) and concerns over publication bias given the asymmetric funnel plot (Figure 8). We did not downgrade for inconsistency despite the high I² statistic since this (and the observed imprecision) did not appear clinically relevant to its interpretation.
1.4. Analysis.

Comparison 1: Alpha‐blocker and usual care versus usual care alone, Outcome 4: Stone clearance time
8.

Funnel plot: stone clearance time.
The time to stone clearance varied very considerably between studies with a range from 3.6 days to 47.2 days.
Predefined subgroup analysis by stone location (renal and proximal ureter versus distal ureter)
Stone clearance
The test for subgroup differences did not meet statistical significance (P = 0.10; Analysis 2.1). Differences between results for participants with renal and proximal ureteral stones (RR 1.15, 95% CI 1.06 to 1.25; I² = 72%; studies = 19; participants = 1947) and those with distal ureteral stones (RR 1.40, 95% CI 1.13 to 1.74; I² = 71%; studies = 6; participants = 699) may be attributable to chance.
2.1. Analysis.

Comparison 2: Alpha‐blocker and usual care versus usual care: stone location subgroup (renal and proximal ureter versus distal ureter), Outcome 1: Stone clearance
Auxiliary treatment
The test for subgroup differences did not meet statistical significance (P = 0.51; Analysis 2.2). Differences between results between participants with renal and proximal ureteral stones (RR 0.62, 95% CI 0.40 to 0.98; I² = 4%; studies = 11; participants = 1121) and those with distal ureteral stones (RR 1.00, 95% CI 0.26 to 3.83; studies = 1; participants = 130) may be attributable to chance.
2.2. Analysis.

Comparison 2: Alpha‐blocker and usual care versus usual care: stone location subgroup (renal and proximal ureter versus distal ureter), Outcome 2: Auxiliary treatment
Major adverse events
The test for subgroup differences did not meet statistical significance (P = 0.45; Analysis 2.3). Differences between results for participants with renal and proximal ureteral stones (RR 0.59, 95% CI 0.44 to 0.79; I² = 0%; studies = 6; participants = 617) and those with distal ureteral stones (RR 1.00, 95% CI 0.26 to 3.83; studies = 1; participants = 130) may be attributable to chance.
2.3. Analysis.

Comparison 2: Alpha‐blocker and usual care versus usual care: stone location subgroup (renal and proximal ureter versus distal ureter), Outcome 3: Major adverse events
Post hoc subgroup analysis by stone location (renal versus ureteral)
Stone clearance
The test for subgroup differences did not meet statistical significance (P = 0.09; Analysis 3.1). Differences between results for participants with renal stones (RR 1.13, 95% CI 1.03 to 1.24; I² = 73%; studies = 12; participants = 1483) and those with ureteral stones (RR 1.31, 95% CI 1.14 to 1.51; I² = 69%; studies = 13; participants = 1163) may be attributable to chance.
3.1. Analysis.

Comparison 3: Alpha‐blocker and usual care versus usual care: stone location subgroup (renal versus ureter; post hoc), Outcome 1: Stone clearance
Auxiliary treatment
The test for subgroup differences did not meet statistical significance (P = 0.44; Analysis 3.2). Differences between results between participants with renal stones (RR 0.52, 95% CI 0.25 to 1.08; I² = 4%; studies = 8; participants = 922) and those with ureteral stones (RR 0.74, 95% CI 0.44 to 1.25; I² = 0%; studies = 4; participants = 329) may be attributable to chance.
3.2. Analysis.

Comparison 3: Alpha‐blocker and usual care versus usual care: stone location subgroup (renal versus ureter; post hoc), Outcome 2: Auxiliary treatment
Major adverse events
The test for subgroup differences did not meet statistical significance (P = 0.45; Analysis 3.3). Differences between results for participants with renal and proximal ureteral stones (RR 0.59, 95% CI 0.44 to 0.79; I² = 0%; studies = 6; participants = 617) and those with distal ureteral stones (RR 1.00, 95% CI 0.26 to 3.83; studies = 1; participants = 130) may be attributable to chance.
3.3. Analysis.

Comparison 3: Alpha‐blocker and usual care versus usual care: stone location subgroup (renal versus ureter; post hoc), Outcome 3: Major adverse events
Predefined subgroup analysis by stone size (less than 10 mm versus 10 mm or greater)
Stone clearance
The test for subgroup differences did not meet statistical significance (P = 0.08; Analysis 4.1). Differences between results for participants with stones less than 1 cm in size (RR 1.03, 95% CI 0.96 to 1.11; I² = 0%; studies = 7; participants = 411) and those with stones 1 cm or greater in size (RR 1.24, 95% CI 1.02 to 1.50; I² = 44%; studies = 6; participants = 369) may be attributable to chance.
4.1. Analysis.

Comparison 4: Alpha‐blocker and usual care versus usual care: stone size subgroup, Outcome 1: Stone clearance
Auxiliary treatment
We found no data to perform this subgroup analysis on auxiliary treatment.
Major adverse events
We found no data to perform this subgroup analysis on major adverse events.
Predefined subgroup analysis by specific alpha‐blocker
Stone clearance
The test for subgroup differences was not significant (P = 0.75; Analysis 5.1). Results for participants receiving a different type of alpha‐blocker ranged from RR 1.02 for silodosin (95% CI 0.46 to 2.26; I² = 9%; studies = 2; participants = 54), RR 1.12 for alfuzosin (95% CI 0.89 to 1.40; I² = 68%; studies = 3; participants = 254), RR 1.17 for tamsulosin (95% CI 1.09 to 1.25; I² = 81%; studies = 31; participants = 3465), to RR 3.37 for naftopidil (95% CI 0.50 to 22.69; studies = 1; participants = 27). The differences may be attributable to chance variation.
5.1. Analysis.

Comparison 5: Alpha‐blocker and usual care versus usual care: alpha‐blocker type subgroup, Outcome 1: Stone clearance
Auxiliary treatment
The test for subgroup differences was not significant (P = 0.41; Analysis 5.2). Of 12 included studies, 10 used tamsulosin, one used doxazosin (Ateş 2012) and one used silodosin (De Nunzio 2016).
5.2. Analysis.

Comparison 5: Alpha‐blocker and usual care versus usual care: alpha‐blocker type subgroup, Outcome 2: Auxiliary treatment
Major adverse events
The test for subgroup differences was not significant (P = 0.50; Analysis 5.3). Of seven included studies, six used use tamsulosin, and one used silodosin (De Nunzio 2016).
5.3. Analysis.

Comparison 5: Alpha‐blocker and usual care versus usual care: alpha‐blocker type subgroup, Outcome 3: Major adverse events
Predefined subgroup analysis by type of lithotripter (HM3 versus others)
We were unable to conduct this preplanned subgroup analysis as none of the studies used an HM3 lithotripter.
Sensitivity analyses limited to low risk of bias studies
Stone clearance
When limited to studies in which outcome assessors were blinded (Küpeli 2004; Singh 2011b; Vicentini 2011), alpha‐blockers may improve the clearance of stone fragments after SWL (RR 1.52, 95% CI 0.86 to 2.68; I² = 83%; studies = 3; participants = 237; Analysis 6.1), which were broadly similar to the results of the overall analysis (RR 1.16, 95% CI 1.09 to 1.23; I² = 78%; studies = 36; participants = 4084; Analysis 1.1).
Auxiliary treatment
Blinding of outcome assessors was not relevant to this outcome. When limited to studies in which participants were blinded and this outcome was reported (Bhagat 2007; Elkoushy 2012; Vicentini 2011), alpha‐blockers may reduce the need for auxiliary treatments after SWL (RR 0.18, 95% CI 0.03 to 1.00; I² = 0%; studies = 3; participants = 260; Analysis 6.2), which were similar to the results of the overall analysis (RR 0.67, 95% CI 0.45 to 1.00; I² = 0%; studies = 12; participants = 1251; Analysis 1.2).
Major adverse events
When limited to the single study in which outcome assessors were blinded and this outcome was reported (Vicentini 2011), alpha‐blockers may not effect major adverse events (RR 1.0, 95% CI 0.32 to 3.17; studies = 1; participants = 76; Analysis 6.3), in contrast to the results of overall analysis (RR 0.60, 95% CI 0.46 to 0.80; I² = 0%; studies = 7; participants = 747; Analysis 1.3).
Sensitivity analyses limited to studies with a single shock wave lithotripsy session
Stone clearance
When limited to studies that used a single SWL session (Ahmed 2016; De Nunzio 2016; Janane 2014; Kobayashi 2008; Micali 2007; Park 2013; Singh 2011a), alpha‐blockers may increase stone clearance (RR 1.22, 95% CI 1.04 to 1.42; I² = 71%; studies = 7; participants = 993; Analysis 7.1), similar to the results of the overall analysis (RR 1.16, 95% CI 1.09 to 1.23; I² = 78%; studies = 36; participants = 4084; Analysis 1.1).
Auxiliary treatment
When limited to studies that employed a single SWL session (Ahmed 2016; De Nunzio 2016), alpha‐blockers may reduce auxiliary treatments after SWL (RR 0.81, 95% CI 0.54 to 1.23; I² = 25%; studies = 2; participants = 309; Analysis 7.2), similar to the results of the overall analysis (RR 0.67, 95% CI 0.46 to 0.80; participants = 1251; studies = 12; I² = 0%; Analysis 1.2).
Major adverse events
When limited to studies that employed a single SWL session (Ahmed 2016; De Nunzio 2016), alpha‐blockers may reduce major adverse events (RR 0.62, 95% CI 0.45 to 0.85; I² = 0%; studies = 2; participants = 309; Analysis 7.3), similar to the results of the overall analysis (RR 0.60, 95% CI 0.46 to 0.80; I² = 0%; studies = 7; participants = 747; Analysis 1.3).
Discussion
Summary of main results
Based on the findings of this systematic review, alpha‐blockers may improve stone clearance, reduce the need for auxiliary treatments and reduce major adverse events. We found no evidence to suggest that stone clearance might differ by stone location (renal and proximal ureter versus distal ureter) or stone size (less than 1 cm versus 1 cm or greater). Stone clearance does not appear to vary by type of alpha‐blocker. We were unable to perform subgroup analyses for the outcomes of auxiliary treatments and major adverse events. We were unable to compare results by type of lithotripter. Results of sensitivity analyses limited to studies in which participants were blinded and those in which participants clearly received only one SWL session did not substantially differ from the main analysis for any of the three primary outcomes of this review.
Overall completeness and applicability of evidence
This systematic review included 40 RCTs from a variety of different geographic areas enrolling participants with stones of different locations, stone sizes and using different types of alpha‐blockers. The shock wave lithotripter participants were treated with the most commonly used device currently found in clinical practice. Therefore, results of this review appear largely applicable to people with renal or ureteral stones for the outcomes for which we could find relevant evidence.
We included the outcome of major adverse events to capture potential drug‐related adverse effects such as episodes of syncope or symptomatic hypotension but found none. This was unexpected given that this review accounts for an aggregate number of over 2000 participants receiving an alpha‐blocker. Based on a subgroup analysis of higher quality studies in a Cochrane Review on the role of alpha‐blockers as MET in ureteral colic (Campschroer 2018), we expected to find approximately three more major adverse events per 1000 as drug‐related adverse effects. Failure to find any such events may relate to the mostly poor study quality of trials included in this review, which did not systematically query participants for drug‐related adverse events.
We would also like to emphasize that the outcome of time to stone clearance should not be misinterpreted as a time‐to‐event outcome. Instead, it is a continuous outcome that reflects the time to stone clearance of those participants that passed their stone fragments.
The decision by guideline panels whether to recommend alpha‐blockers after SWL or not likely hinges on the perceived trade‐off of desirable and undesirable effects of this drug class. This review would suggest that the rate of major adverse events favor the use of alpha‐blockers whereas minor adverse events were not captured. The findings of Campschroer 2018 with regard to the adverse effect profile of this drug class as primary treatment for ureteral colic would suggest the treatment burden to be low. Current AUA guidelines make a 'moderate recommendation' for the use of alpha‐blockers after SWL (Assimos 2016a). The EAU guidelines stating that alpha‐blockers after SWL for ureteral or renal stones may expedite expulsion, increase stone free rates and reduce analgesic requirements mention alpha‐blockers but fail to make an explicit recommendation (Turk 2020). National Institute for Health and Care Excellence (NICE) guidelines indicate that clinicians should consider adjunctive therapy for adults having SWL for ureteral stones less than 10 mm (NICE 2019).
Quality of the evidence
Findings of this review are based on a large number of relatively small trials that lacked published protocols, were not transparently reported and had important methodological limitations. None of the included trials had a registered protocol, which is noteworthy and very concerning. Therefore, we consistently downgraded the certainty of evidence (assessed on a per outcome basis) for several issues. Allocation concealment was frequently unclear, and participants and outcome assessors were commonly not blinded. In addition, loss to follow‐up rates were often unclear and the number participants who received more than one SWL session and the number of such sessions was unclear.
In addition, several analyses were marked by high degrees of unexplained, clinically relevant inconsistency, highly suspected publication bias, or a combination of these. Finally, CIs around pooled effect size estimates for the outcomes of auxiliary treatment and major adverse events were often wide, which prompted us to downgrade the certainty of evidence further for some outcomes.
Potential biases in the review process
All aspects of this review were governed by an a priori protocol and conducted based on rigorous Cochrane guidance. However, a few aspects could have potentially introduced bias beyond the issues already described.
Although we conducted a comprehensive literature search of multiple electronic databases of published and unpublished studies as well as trial registries, it is possible that some studies were missed, in particular small, negative studies in non‐English languages. Failure to include such studies (if they exist) would have biased studies results towards a larger effect size. Concern over publication bias prompted us to lower our confidence in the estimates of effect for the need for auxiliary treatment and major adverse events. It may also be helpful to note that at least six studies in the Excluded studies section were aborted for unclear reasons, which have been due to negative findings.
We were unable to fully assess eligibility of a study originating from China, for which we could not find a qualified translator. Its results are included based on the English language abstract. We also contacted study authors for additional methodological or clinical data but rarely received informative responses.
We revised our threshold for a clinically important effect size for auxiliary treatments from originally 5% in the protocol (Oestreich 2019) to 3% after realizing that relatively small differences in rates likely resulted in large perceived disutilities by patients. We fully acknowledge that different thresholds may lead to different interpretations and would like to emphasize the importance of transparency (Hultcrantz 2017).
Results of this study were limited to RCTs in accordance with the published protocol. This was motivated not only by Cochrane guidance, but also prior knowledge of a large body of RCTs based on published systematic reviews. Although it is possible that evidence from well‐designed non‐RCTs may have provided higher quality evidence for select outcomes, this appears unlikely.
Agreements and disagreements with other studies or reviews
Several systematic reviews have been performed to address the same clinical question, which will be reviewed in chronological order.
Losek 2008 reported a narrative review focused on tamsulosin. It included, but did not pool, five trials. The certainty of evidence was not assessed. It found that tamsulosin may promote the passage of renal stones after SWL but that for ureteral stones was inconclusive.
Schuler 2009 identified four trials of different adjunctive agents (including two trials of tamsulosin). It did not assess the certainty of evidence. Its findings suggested improved stone clearance with MET, in particular for stones greater than 10 mm.
In the most rigorous and comprehensive systematic review to date of primary MET and after SWL that included both alpha‐blockers and calcium channel blockers. Seitz 2009 found an RR of stone clearance of 1.29 (95% CI 1.16 to 1.43) with alpha‐blocker use based on 13 trials with 1007 participants. It assessed study quality using the Jadad scale, which is no longer considered appropriate today but the certainty of evidence was otherwise not qualified.
Zheng 2010 reported a systematic review focused on tamsulosin and the upper urinary tract. It assessed study quality using the Jadad scale but did not provide the certainty of the evidence. Stone clearance was similarly improved.
Zhu 2010 considered all alpha‐blockers but only included seven trials excluding those with unclear methods and those that included spasmolytics. They reported an absolute risk difference favoring alpha‐blockers of 16% (95% CI 5% to 27%). Based on an assessment using the Cochrane 'Risk of bias' tool, it found the methodological quality of the included studies to be adequate.
Lee 2012 focused on the role of tamsulosin as MET both as primary adjuvant treatment and after SWL (as did Seitz 2009), but limited to Korean patients only. It used the Jadad scale and did not provide the certainty of the evidence rating. The results favored the use of tamsulosin over no MET.
Li 2015 included 23 trials for any alpha‐blocker. It used the Jadad scale and did not rate the certainty of evidence. Alpha‐blockers improved stone clearance and reduced pain. Dizziness, anejaculation and headache were increased.
Skolarikos 2015 included calcium channel blockers, alpha‐blockers and other adjuvant agents. The authors did not report any assessment of study quality. They found improved stone expulsion rates with alpha‐blockers, which appeared independent of the type of alpha‐blocker, the stone diameter or the stone location.
Yang 2017 reported the latest systematic review that included a meta‐analysis but did not characterize the quality of evidence on a per outcome basis. The findings of the network meta‐analysis supported a positive impact of alpha‐blockers over no adjuvant therapy and favored doxazosin over tamsulosin over terazosin. In contrast, subgroup analyses conducted in this review were not suggestive of any interaction of the effect on stone clearance and the type of alpha‐blockers.
In summary, a large number of existing systematic reviews support a positive effect of alpha‐blockers on stone clearance as well as other outcomes. What distinguishes our review from prior reviews was the certainty of evidence rating on a per outcomes basis. The certainty of evidence was low for all three primary outcomes of this review, which means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. This qualifier appears relevant in the context of the ongoing controversy about the effect of alpha‐blockers also as part of the primary management approach for ureteral colic (Campschroer 2018). Whereas some doubt their value (De Coninck 2019), others see its main benefit in people with larger ureteral stones of 5 mm or greater in size (Dahm 2018; Hollingsworth 2016). These systematic reviews included several large randomized trials of much higher methodological rigor than any of the studies included here. Stone fragments resulting from SWL should be in the 1 mm to 3 mm size range; if these were primary ureteral stones and the result from systematic reviews of primary MET for ureteral colic were directly applicable, the benefit would be questionable.
Authors' conclusions
Implications for practice.
Findings of this review indicate that there may be a benefit of medical expulsive therapy across the three predefined outcomes considered the most important: namely, stone clearance, auxiliary treatment and major adverse events. The certainty of evidence was consistently low. This means that our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. We did not eligible information on quality of life.
Implications for research.
The low certainty of evidence resulting from trials of low methodological rigor with poor reporting quality of small sample size and without stratification for important prognostic variables such as stone size and location implies a need for better trials as have been conducted for the use of alpha‐blockers for ureteral colic (Pickard 2015; Ye 2018). Future trials need to meet higher methodological standards which includes an a priori registered protocol (Roberts 2015). In addition, quality of life outcomes should be assessed.
History
Protocol first published: Issue 8, 2019 Review first published: Issue 11, 2020
Notes
We have based parts of the Methods section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We thank Robert Lane as Managing Editor and the Cochrane Urology Editorial Teams based in Minneapolis (MN), USA and Wonju, South Korea for their help and support. We also thank the peer referees (Roger Sur, Vernon Pais, Matthew Bultitude, Emmanuel Effa, Mariano Sebastian Gonzalez and Thijs Campschroer) for the invaluable input. We thank our contact editor Juan Franco as well the Nicole Skoetz and Eve Tomlison from the Cancer Network for their constructive feedback.
Appendices
Appendix 1. Search strategy
| Database | Search terms |
| MEDLINE (via PubMed) |
|
| Embase (via Elsevier) |
|
| Cochrane Library |
|
| ClinicalTrials.gov and ICTRP |
|
Data and analyses
Comparison 1. Alpha‐blocker and usual care versus usual care alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Stone clearance | 36 | 4084 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.09, 1.23] |
| 1.2 Auxiliary treatment | 12 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.00] |
| 1.3 Major adverse events | 7 | 747 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.80] |
| 1.4 Stone clearance time | 14 | 1790 | Mean Difference (IV, Random, 95% CI) | ‐3.74 [‐5.25, ‐2.23] |
Comparison 2. Alpha‐blocker and usual care versus usual care: stone location subgroup (renal and proximal ureter versus distal ureter).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Stone clearance | 24 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.11, 1.33] |
| 2.1.1 Renal and upper ureteral | 19 | 1947 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.25] |
| 2.1.2 Lower ureteral | 6 | 699 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.13, 1.74] |
| 2.2 Auxiliary treatment | 12 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.00] |
| 2.2.1 Renal and upper ureteral | 11 | 1121 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.40, 0.98] |
| 2.2.2 Lower ureteral | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.26, 3.83] |
| 2.3 Major adverse events | 7 | 747 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.80] |
| 2.3.1 Renal and upper ureteral | 6 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.44, 0.79] |
| 2.3.2 Lower ureteral | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.26, 3.83] |
Comparison 3. Alpha‐blocker and usual care versus usual care: stone location subgroup (renal versus ureter; post hoc).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Stone clearance | 24 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.11, 1.33] |
| 3.1.1 Renal | 12 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.03, 1.24] |
| 3.1.2 Ureter | 13 | 1163 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.14, 1.51] |
| 3.2 Auxiliary treatment | 12 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.00] |
| 3.2.1 Renal | 8 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.08] |
| 3.2.2 Ureteral | 4 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.25] |
| 3.3 Major adverse events | 7 | 747 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.80] |
| 3.3.1 Renal | 6 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.44, 0.79] |
| 3.3.2 Ureteral | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.26, 3.83] |
Comparison 4. Alpha‐blocker and usual care versus usual care: stone size subgroup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Stone clearance | 7 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.00, 1.21] |
| 4.1.1 < 10 mm | 7 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 4.1.2 ≥ 10 mm | 6 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.02, 1.50] |
Comparison 5. Alpha‐blocker and usual care versus usual care: alpha‐blocker type subgroup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Stone clearance | 35 | 3999 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.09, 1.23] |
| 5.1.1 Tamsulosin | 31 | 3465 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.09, 1.25] |
| 5.1.2 Terazosin | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.26] |
| 5.1.3 Silodosin | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.46, 2.26] |
| 5.1.4 Alfuzosin | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.89, 1.40] |
| 5.1.5 Naftopidil | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 3.37 [0.50, 22.69] |
| 5.1.6 Doxazosin | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.00, 1.41] |
| 5.2 Auxiliary treatment | 12 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.00] |
| 5.2.1 Tamsulosin | 10 | 1112 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.46, 1.08] |
| 5.2.2 Doxazosin | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.06, 1.21] |
| 5.2.3 Silodosin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.08, 41.65] |
| 5.3 Major adverse events | 7 | 747 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.80] |
| 5.3.1 Tamsulosin | 6 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.45, 0.80] |
| 5.3.2 Silodosin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.08, 41.65] |
Comparison 6. Alpha‐blockers and usual care versus usual care: risk of bias (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Stone clearance | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.86, 2.69] |
| 6.2 Auxiliary treatment | 3 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.00] |
| 6.3 Major adverse events | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.32, 3.17] |
Comparison 7. Alpha‐blocker and usual care versus usual care: single SWL session (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Stone clearance | 7 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.04, 1.42] |
| 7.2 Auxiliary treatment | 2 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.54, 1.23] |
| 7.3 Major adverse events | 2 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2009.
| Study characteristics | ||
| Methods |
Study design: open label RCT Number of centers/setting: single center/not reported Country: India Dates of the study: June 2006 to December 2007 |
|
| Participants |
Total number of participants randomized: 40
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with single upper ureteric stone < 15 mm in size electing SWL Exclusion criteria: extremes of ages; serum creatinine > 2.0 mg/dL; concomitant stones in ipsilateral kidney; radiolucent stones; history of previous unsuccessful SWL; active UTI; diabetes mellitus; concomitant treatment with calcium channel blockers, alpha‐blockers, corticosteroids or a combination; previous pyeloureteral surgery; severe vertebral malformation; morbid obesity; pregnancy; aortic or renal artery aneurysm (or both); uncorrected coagulopathy; people on ureteral stent |
|
| Interventions |
Treatment group:
Control group: standard care only. SWL:
|
|
| Outcomes |
Primary: success rate
Secondary: sessions required for clearance, days required for clearance, pain intensity, incidence of steinstrasse, need for auxiliary procedures Subgroups: stone size, number of SWL sessions |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry – Drs Agarwal and Mandal Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…divided into 2 groups…using random number table." Comment: appropriate method of random sequence generation used. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "…placebo control was not possible in our study…" Comment: participants and personnel not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Quote: "…placebo control was not possible in our study…" Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Quote: "…placebo control was not possible in our study…" Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed follow‐up with no dropout." Comment: all randomized participants appeared to have been included in all analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | 9/20 participants in tamsulosin + usual care group and 8/20 participants in usual care alone group underwent second SWL session; 3/20 participants in tamsulosin + usual care group and 5/20 participants in usual care alone group underwent third SWL session. |
Ahmed 2016.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: multicenter/not reported Country: Saudi Arabia Dates of the study: March 2013 to January 2016 |
|
| Participants |
Total number of participants randomized: 271
Age (mean, years):
Sex (M/F):
Stone location: renal Stone size (mean, mm):
Inclusion criteria: adults with solitary renal stone < 20 mm size who underwent a single session SWL Exclusion criteria: pregnant women; people with severe vertebral malformation, morbid obesity, UTI, renal impairment, uncorrected coagulation disorder, urinary obstruction distal to the stone or with severe hydronephrosis were not allowed to SWL for stone kidney; people with lower pole stones, mal‐rotated kidneys, ureteral stents and medical conditions that impeded the usage of study medications and concomitant use of calcium channel blockers or alpha‐blockers |
|
| Interventions |
Treatment group:
Control group: standard care only SWL:
|
|
| Outcomes |
Primary: stone free rate
Secondary: time to stone clearance, number pain episodes, analgesia dosage, adverse events, rehospitalization, secondary interventions Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry – Dr Ahmed Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "coin toss method." Comment: appropriate method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were stratified by stone size and randomized in block of two and each center had its own list to keep the groups closely balanced." Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "out of 271 randomized patients, 249 (123 in TG [treatment group] and 126 in CG [control group] where compliant with the study medications and completed the required investigations and follow‐up, and were included in data analysis." Comment: small proportion (< 10%) of randomized participants excluded from analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Quote: "single session SWL." Comment: single SWL session. |
Ateş 2012.
| Study characteristics | ||
| Methods |
Study design: multicenter, prospective RCT Number of centers/setting: multicenter/hospital Country: Turkey Dates of the study: April 2009 to October 2010 |
|
| Participants |
Total number of participants randomized: 90
Age (mean, years):
Sex (M/F):
Stone location: upper ureteral Stone size (mean, mm):
Inclusion criteria: people with renal colic, who were admitted to the emergency rooms or urology clinics and whose KUB graphs revealed radio‐opaque upper ureteral stones Exclusion criteria: abnormal renal anatomy and function, use of medications that may have led to stone formation, pregnancy or suspicion of pregnancy, distal obstruction, history of previous urinary stone surgery, hydronephrosis grade > 1, presence of coagulopathy, active UTI, history of hypersensitivity for doxazosin, serum creatinine level > 2 mg/dL, existence of > 1 ureteral stone, hypotension and pain that could not be controlled with an analgesic |
|
| Interventions |
Treatment group:
Control group: standard care only SWL:
|
|
| Outcomes | Time to stone passage, number SWL sessions, stone free/failure, need for analgesics, need for additional procedure, steinstrasse
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry – Dr Ateş Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized into two groups according to a computer‐based randomization schedule." Comment: appropriate method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "initially, a total of 90 patients were planned to be enrolled in the study, but eventually 79 patients completed the study." Comment: 11 randomized participants with unclear group assignment not included in outcome analyses. With 35 (alpha‐blocker) and 44 (control arm) participants included, there was major concern that attrition primarily affected the intervention arm. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "if, during the follow‐up visit after the first session, the stone was not influenced or the stone was fragmented into pieces equal to or larger than 6 mm, a second session of SWL was performed three days after the first procedure." Comment: participants received > 1 SWL session. |
Baloch 2011.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: Pakistan Dates of the study: February to August 2010 |
|
| Participants |
Total number of participants randomized: 130
Age: not reported Sex (M/F): not reported Stone location: renal Stone size: not reported Inclusion criteria: people who underwent ESWL for renal calculi Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: standard analgesia PRN SWL: not reported |
|
| Outcomes | Stone clearance
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: unable to find contact information Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description of placebo use; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Group A (Alfuzosin group) had a clearance rate of 86.2% (56 out of 65) versus 66.2% (43 out of 65) in Group B (Control group) with p value = 0.01." Comment: reporting suggests that all randomized participants were included analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Bhagat 2007.
| Study characteristics | ||
| Methods |
Study design: placebo controlled, double blind, prospective RCT Number of centers/setting: not reported Country: India Dates of the study: September 2004 to July 2005 |
|
| Participants |
Total number of participants randomized: 60
Age (mean, years):
Sex (M/F):
Stone location:
Stone size: not reported Inclusion criteria: people who were to receive SWL for a single radiopaque renal 6–24 mm or ureteral 6–15 mm calculus Exclusion criteria: recent open or endoscopic surgical intervention, radiolucent calculus, past unsuccessful SWL, renal impairment (creatinine > 1.5 mg/dL), inadequate kidney function, recurrent calciuria, UTI, receiving calcium channel blockers or alpha1‐blockers (or both), congenital urinary anomalies, history of pyeloureteral surgery and children |
|
| Interventions |
Treatment group:
Control group: placebo SWL:
|
|
| Outcomes |
Primary: number stone clearance
Secondary: median analgesic dose Subgroups: stone size |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Drs Devasia and Kekre Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomization was performed with even distribution, using computer‐generated numbers." Comment: appropriate method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled, randomized, double‐blind study": "the patients were randomized into either placebo or study group." Comment: participants likely blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: blinding of outcome assessors not addressed. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: blinding of outcome assessors not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "of the 60 patients randomized 58 completed the study, and one from each group discontinued medication and was excluded from analysis." Comment: low (< 10%) loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Cakıroglu 2013.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: single center/hospital Country: Turkey Dates of the study: June 2008 to July 2011 |
|
| Participants |
Total number of participants randomized: 123
Age (mean, years):
Sex (M/F):
Stone location: ureteral Stone size (mean, mm):
Inclusion criteria: people with a solitary ureteral stone ≥ 6 mm up to 15 mm and located in the upper, middle or lower ureter underwent SWL Exclusion criteria: ages ≤ 18 years; weight < 50 kg or > 100 kg; severe skeletal malformation; pregnancy; aortic or renal artery aneurysm, or both; history of drug or alcohol abuse; long‐term use of drugs such as antidepressants, histamine blockers and anxiolytics; allergy study medications; concomitant treatment with calcium antagonists or alpha1‐blocker, or both; concomitant renal stones; previous unsuccessful attempts at SWL; elevated serum creatinine (> 2 mg/dL); UTI; diabetes; peptic ulcers; history of spontaneous stone expulsion; hypotension; coagulopathy; urinary congenital anomalies; or previous nephroureteral surgery |
|
| Interventions |
Treatment group:
Control group: standard medical therapy alone SWL:
|
|
| Outcomes | Not reported Subgroups: stone location |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Drs Cakiroglu and Sinanoglu: Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using the coin toss method." Comment: method of sequence generation described and appropriate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the data for the 123 patients who completed follow‐up without dropping out met the criteria." Comment: description implied that number randomized was greater than the number included in analysis; no further details provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Comment: Table 1 refers to > 1 SWL per participant. |
Chau 2015.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: China Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 183
Age: not reported Sex (M/F): not reported Stone location: renal Stone size: not reported Inclusion criteria: people with renal stones undergoing ESWL up to 3 times Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: analgesic only SWL:number of sessions: max 3 |
|
| Outcomes | Stone clearance rate
Subgroups: number of ESWL sessions |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: 7 July 2019 – general inquiry to Dr Chau Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "93 patients, 51 patients, and 39 patients completed one, two and three ESWL respectively." Comment: multiple SWL sessions. |
Cho 2013.
| Study characteristics | ||
| Methods |
Study design: prospective, single blind RCT Number of centers/setting: single center/outpatient Country: Korea Dates of the study: June 2010 to July 2011 |
|
| Participants |
Total number of participants randomized: 90
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with radio‐opaque ureter stones of 5–10 mm in diameter Exclusion criteria: radiolucent stones, paper‐thin cortex, non‐functional kidney, previous genitourinary tract surgery, elevated serum creatinine (> 1.5 mg/dL), severe obesity, pregnancy, concurrent alpha‐blocker/calcium channel blocker/steroid/frusemide usage, aortic or renal artery aneurysm, or contraindications to alpha‐blocker treatment |
|
| Interventions |
Treatment group:
Control group: standard care only SWL:
|
|
| Outcomes | Stone free rate, time of stone expulsion, pain severity, adverse events
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Dr Tag Keun Yoo Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "single blind clinical trial." Comment: use of placebo not described; unclear if participants blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Quote: "single blind clinical trial." Comment: blinding of outcome assessors not addressed; unclear if blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Quote: "single blind clinical trial." Comment: blinding of outcome assessors not addressed; unclear if blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 90 patients completed the study. Four patients in group 1 and two patients in group 2 dropped out owing to migration or discontinuation of medications over the last a follow‐up." Comment: small proportion of randomized participants (< 10%) excluded from analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "The number of ESWL sessions was 1.34±0.65 and 1.41±0.85 in groups 1 and 2, respectively (p=0.33)." Comment: > 1 SWL session was administered. |
De Nunzio 2016.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: presumed single center/not reported Country: Italy Dates of the study: January 2012 to March 2015 |
|
| Participants |
Total number of participants randomized: 66
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: consecutive series of people undergoing SLW for a single radiopaque renal stone (0.5–2 cm) Exclusion criteria: people with congenital or acquired urinary anomalies, severe vertebral malformation, renal impairment, hydronephrosis, ureteral stent, UTI, previous SLW, ureterolithotripsy or recent open/endoscopic surgical intervention; receiving calcium channel blocker or alpha‐blocker and corticosteroids |
|
| Interventions |
Treatment group:
Control group: placebo Comparator group: silodosin 8 mg/day for 21 days SWL:
|
|
| Outcomes | Pain (6, 12, 24 hours after SWL), stone free rate, adverse events
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Dr De Nunzio Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by closed envelopes." Comment: likely appropriate of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "by closed envelopes." Comment: not described as opaque and numbered; unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "those receiving placebo group C." Comment: no other information to support adequate blinding except mention of placebo. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: unclear whether outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: unclear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "overall 66 patients were enrolled but six were excluded…" Comment: 6/66 participants excluded; unclear what group allocation was. Risk of bias deemed high. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Comment: published study wording suggests single SWL only. |
Elkoushy 2012.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: presumed single center/not reported Country: Egypt Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 126
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: single radio‐opaque renal or upper ureteral stones ≤ 2 cm in largest diameter Exclusion criteria: ages < 18 years; multiple stones; radiolucent stones; stones > 2 cm in largest diameter; previous SWL failure; history of spontaneous stone expulsion; UTI; distal obstruction; congenital renal or ureteral anomalies; serum creatinine ≥ 2 mg/dL; uncorrectable bleeding disorders; hypotension; morbid obesity or pregnancy; concomitant use of calcium channel blockers, alpha‐blockers or corticosteroids |
|
| Interventions |
Treatment group:
Control group: placebo SWL:
|
|
| Outcomes |
Primary: stone free rate and the factors that might affect it
Secondary: time required for stone clearance, pain frequency and intensity, incidence of steinstrasse and need for auxiliary procedures Subgroups: stone location |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Dr El Koushy Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random number generator and assisted by computer program." Comment: appropriate method of random sequence generation was used. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized placebo‐controlled study"; "received 0.4 mg tamsulosin or placebo once daily." Comment: participants and personnel likely blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: blinding of outcome assessors not addressed. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: blinding of outcome assessors not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all the patients completed the follow‐up schedule." Comment: all randomized participants appeared to have been included in analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "the GT patients required fewer SWL sessions to become stone free." Comment: > 1 SWL session; unclear how many participants received how many SWL sessions. |
Eryildirim 2016.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: presumed single center/not reported Country: Turkey Dates of the study: January 2015 to December 2015 |
|
| Participants |
Total number of participants randomized: 80
Age (mean, years):
Sex (M/F): not reported Stone location: upper ureteral Stone size: not reported Inclusion criteria: > 18 years, treated with SWL for 5–10 mm single radio‐opaque upper ureteral stones (above iliac vessels) Exclusion criteria: people with multiple stones, previous stone related procedures, obstruction, stent placement, auxiliary procedures, congenital anomalies, active UTI, pregnancy or renal insufficiency |
|
| Interventions |
Treatment group:
Control group: standard care only SWL:
|
|
| Outcomes |
Primary: HRQoL
Secondary: stone free rate, analgesic requirement, number renal colic attacks and emergency room visits Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Dr Eryildirim Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a simple method by generating a random digit (0–60) was used." Comment: appropriate method of random sequence generation was used. |
| Allocation concealment (selection bias) | High risk | Quote: "even numbers were used for cases undergoing SWL without MET and odd numbers were used for cases undergoing SWL procedure followed by MET initiation." Comment: allocation not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: no blinding of outcome assessors described; likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: no blinding of outcome assessors described; likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "following the exclusion of cases requiring DJ stent placement (a total of 12 cases; seven cases in group 1 and five cases in group 2)." Comment: 10–19% of randomized participants not included in analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Falahatkar 2011.
| Study characteristics | ||
| Methods |
Study design: placebo controlled, double blind RCT Number of centers/setting: single center/ESWL center Country: Iran Dates of the study: February 2008 to September 2009 |
|
| Participants |
Total number of participants randomized: 150
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with renal or ureteral stone 4–20 mm referred to ESWL center Exclusion criteria: recent open or endoscopic surgical intervention, radiolucent calculus, elevated serum creatinine (> 1.5 mg/dL), UTI, high grade hydronephrosis, peptic ulcer, concomitant treatment with calcium antagonists or alpha‐blockers (or both), hypotension, coagulopathy, urinary congenital anomalies, severe skeletal malformation, severe obesity, pregnancy, aortic or renal artery aneurysm, and if they were children |
|
| Interventions |
Treatment group:
Control group: placebo SWL:
|
|
| Outcomes | Stone clearance rate, time to stone passage
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: 7 July 2019 – general inquiry to Dr Khosropanah Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled, randomized, double‐blind clinical trial"; "and control group received a placebo tablet once a day." Comment: participants likely blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: no blinding of outcome assessors described; unclear blinding status. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: no blinding of outcome assessors described; unclear blinding status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "nine patients were excluded from analysis due to discontinued drug consumption (two patients in placebo group and in three patients in case group) and the migration of patients (two patients in placebo group and two patients in case group)." Comment: < 10% per group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Gaafar 2011.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported Country: Egypt Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 150
Age: not reported Sex (M/F): not reported Stone location: renal Stone size: not reported Inclusion criteria: solitary renal pelvic calculi who were successfully fragmented by ESWL Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: standard care only Comparator group: doxazocin daily for up to 12 weeks SWL: not reported |
|
| Outcomes | Time to complete clearance of stone fragments, number of analgesic doses, pain intensity
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
H 2012.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: China Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: not reported Age: not reported Sex (M/F): not reported Stone location: ureteric, any position Stone size: not reported Inclusion criteria: people with ureteric stone in any position with the size limited to 5–20 mm Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: standard care only SWL: not reported |
|
| Outcomes |
Primary: stone clearance rate
Secondary: pain control Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated random number." Comment: appropriate method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Hammoud 2014.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: single center/not reported Country: Egypt Dates of the study: January 2010 to October 2012 |
|
| Participants |
Total number of participants randomized: 96
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: males with solitary, radio‐opaque upper urinary tract stones, ≤ 20 mm in the maximum diameter Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: control SWL:
|
|
| Outcomes |
Primary: stone clearance and analgesic dose
Secondary: clearance rate, time to clearance Subgroups: stone location (renal, upper ureteric), size (5–10 mm, 11–20 mm), timing (2, 4, 6, 8 weeks) |
|
| Funding sources | None | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Han 2006.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized RCT Number of centers/setting: single center/outpatient Country: South Korea Dates of the study: March 2005 to May 2005 |
|
| Participants |
Total number of participants randomized: 45
Age (mean, years):
Sex (M/F):
Stone location: upper ureteral Stone size: not reported Inclusion criteria: upper ureteral stone ≥ 6 mm and ≤ 12 mm Exclusion criteria: UTI, radiolucent stone, severe hydronephrosis, pregnancy, hypotension, serum creatinine > 2 mg/dL, use of calcium channel blocker, previous history of urolithiasis, history of URS or SWL, multiple stone, severe obesity |
|
| Interventions |
Treatment group:
Control group: caroverine (Spamon) 20 mg; 3 times daily SWL:
|
|
| Outcomes | Stone clearance, analgesics use after SWL, drug adverse events
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: Korean Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Unclear risk | . |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Hong 2012.
| Study characteristics | ||
| Methods |
Study design: prospective, non‐placebo controlled RCT Number of centers/setting: single center/hospital Country: Singapore Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 40
Age: not reported Sex (M/F): not reported Stone location:
Stone size: not reported Inclusion criteria: people with upper ureteric or renal stones undergoing ESWL Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: control SWL: not reported |
|
| Outcomes | Stone free rate, pain
Subgroups: stone location (renal, upper ureteral) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "prospective randomized non‐placebo‐controlled trial." Comment: open label study; participants not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Itaya 2011.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported Country: Japan Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 51
Age: not reported Sex (M/F): all male Stone location:
Stone size: not reported Inclusion criteria: males with ureteral stones who underwent SWL Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: control Comparator group: naftopidil 75 mg/day SWL: not reported |
|
| Outcomes | Stone clearance, stone free rate
Subgroups: stone location (upper ureteral) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Janane 2014.
| Study characteristics | ||
| Methods |
Study design: placebo controlled RCT Number of centers/setting: presumed single center/not reported Country: Morocco Dates of the study: January 2008 to December 2012 |
|
| Participants |
Total number of participants randomized: 356
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with lower ureteral stones undergoing ESWL Exclusion criteria: UTI, multiple stones, severe hydronephrosis, solitary kidney, congenital urinary anomalies or previous ureteral surgery, severe obesity, pregnancy, lactation or previous treatment with alpha‐blockers |
|
| Interventions |
Treatment group:
Control group: placebo SWL:
|
|
| Outcomes | Stone free rate, stone expulsion time, ureteral colic
Subgroups: stone size |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "simple random allocation." Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "simple random allocation." Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "placebo‐controlled medical treatment." Comment: no additional information provided to support that participants were adequately blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: blinding of outcome assessors not addressed. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: blinding of outcome assessors not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Quote: "after single ESWL session…" Comment: single SWL session. |
Kang 2009.
| Study characteristics | ||
| Methods |
Study design: prospective, multicenter RCT Number of centers/setting: multicenter/outpatients Country: South Korea Dates of the study: July 2007 to December 2007 |
|
| Participants |
Total number of participants randomized: 207
Age (mean, years):
Sex (M/F):
Stone location:
Stone size: not reported Inclusion criteria: people with ≥ 4 mm renal or ureteral stone with acute pain Exclusion criteria: non‐functioning kidney, severe pain which is not relieved by conservative treatment, multiple stones, severe hydronephrosis, pregnancy, serum creatinine ≥ 2.5 mg/dL, history of ureteral operation, ureteral stricture and ureteral stent placement |
|
| Interventions |
Treatment group:
Control group: diclofenac 100 mg once per day for 1 week SWL:
|
|
| Outcomes | Stone clearance rate, pain change after treatment, distance of stone migration (if stone was not passed), stone clearance rate according to SWL machines
Subgroups: expulsion rate according to SWL machines |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: Korean Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective randomized study." |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | No information given. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias (additional SWL sessions) | Unclear risk | Comment: number of SWL sessions unclear. |
Kim 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Number of centers/setting: single center/outpatients Country: South Korea Dates of the study: June 2006 to July 2007 |
|
| Participants |
Total number of participants randomized: 76
Age (mean, years):
Sex (M/F):
Stone location:
Stone size: not reported Inclusion criteria: ≤ 10 mm upper and lower ureteral stone Exclusion criteria: severe hydronephrosis, history of ureteral stent, multiple stones, radiolucent stone, taken drugs such as calcium channel blocker which might affect ureteral smooth muscle contraction |
|
| Interventions |
Treatment group:
Control group: pethidine 50 mg (once during SWL) SWL:
|
|
| Outcomes | Stone clearance, analgesics use after SWL, frequency of SWL until stone passage, drug adverse events
Subgroups: stone location |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: Korean Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo use described; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Kobayashi 2008.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: 3/hospital Country: Japan Dates of the study: July 2005 to April 2006 |
|
| Participants |
Total number of participants randomized: 72
Age (mean, years):
Sex (M/F): all male Stone location:
Stone size (mean, mm):
Inclusion criteria: males with ureteral stones > 4 mm who underwent ESWL Exclusion criteria: UTI; severe hydronephrosis; multiple stones; diabetes; ulcer disease; non‐functioning kidney; morbid obesity; treatment with choreito, calcium antagonists or alpha1‐blockers |
|
| Interventions |
Treatment group:
Control group: no medications SWL:
|
|
| Outcomes |
Primary: stone clearance: absence of all stones on KUB or the presence of clinically insignificant asymptomatic residual fragments < 3 mm in diameter
Secondary: stone free rate Subgroups: stone size |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Quote: "all patients had a single ESWL session." Comment: single SWL session. |
Küpeli 2004.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: Turkey Dates of the study: February 2003 to March 2004 |
|
| Participants |
Total number of participants randomized: 48
Age (mean, years):
Sex (M/F): not reported Stone location: not reported Stone size (mean, mm):
Inclusion criteria: lower ureteral stones within the distal 5 cm of the ureter 3–15 mm in size Exclusion criteria: signs and symptoms of UTI, pregnancy, severely impacted stones, multiple stones, non‐opaque stones, severe hydronephrosis, hepatic dysfunction, nonfunctioning kidney, treatment with calcium antagonists, and morbid obesity |
|
| Interventions |
Treatment group:
Control group: conventional treatment SWL:
|
|
| Outcomes |
Primary: stone free rate
Secondary: adverse effects at 15‐day follow‐up Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using the coin method." Comment: appropriate method of random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Low risk | Quote: "patient follow‐up examinations were performed by two of us who were unaware of the treatment received." Comment: outcome assessors appeared to have been blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Low risk | Quote: "patient follow‐up examinations were performed by two of us who were unaware of the treatment received." Comment: outcome assessors appeared to have been adequately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "all patients would not [who were not] stone free at this follow‐up examination were excluded from the study protocol…" Comment: unable to assess what proportion of randomized participants were included in outcome analyses; concern about selective exclusion of participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether receipt of additional SWL treatment was within study period or later. |
Lanchon 2017.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: single center/not reported Country: France Dates of the study: April 2015 to December 2015 |
|
| Participants |
Total number of participants randomized: 125
Age: not reported Sex (M/F): not reported Stone location:
Stone size (mean, mm):
Inclusion criteria: people with urinary stone undergoing ESWL Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: control SWL:
|
|
| Outcomes |
Primary: success rate
Secondary: analgesic consumption time, time to stone expulsion, need for additional procedure or hospitalization Subgroups: stone location (renal, ureteral) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "centrally randomized." Comment: appropriate method of random sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "centrally randomized"; "blocks of 10 with a ratio of 2:1." Comment: allocation likely concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Liu 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Number of centers/setting: not reported Country: China Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 108
Age: not reported Sex (M/F): not reported Stone location: ureteral Stone size: not reported Inclusion criteria: single ureteral stone Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: conservative therapy only SWL: not reported |
|
| Outcomes |
Primary: primary stone clearance
Secondary: number and intensity postdischarge pain Subgroups: stone size (≥ 10 mm, < 10 mm), stone location (proximal, distal ureteral) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: unlikely that outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: unlikely that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Micali 2007.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported Country: Italy Dates of the study: January 2003 to March 2005 |
|
| Participants |
Total number of participants randomized: 113
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with radiopaque or radiolucent ureteral lithiasis selected for ESWL treatment Exclusion criteria: signs and symptoms of UTI, pregnancy, multiple stones, severe hydronephrosis, hypotension, gastric ulcer disease, obesity, history of spontaneous stone expulsion, previous ureteral surgery |
|
| Interventions |
Treatment group:
Control group: standard care only (lower ureteral stones) Comparator group: nifedipine 30 mg/day orally for 14 days Control 2 group: standard care only (mid‐ureteral stones) SWL:
|
|
| Outcomes | Stone free condition: complete absence of any stone or the presence of residual fragments < 3 mm in diameter
Subgroups: stone location (upper, middle, lower ureteral), follow‐up stage (1, 2 months) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomization not specified but confirmed by authors; method of random sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not addressed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: no mention of placebo; outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: no mention of placebo; outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the patients completed the study." Comment: based on reported denominators, all randomized participants appear to have been included in analyses of all reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Quote: "all patients underwent a single session of ESWL." Comment: single SWL session; additional treatments accounted for as auxiliary treatments. |
Mohamed 2013.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: single center/outpatient Country: Egypt Dates of the study: July 2010 to May 2012 |
|
| Participants |
Total number of participants randomized: 130
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm): not reported Inclusion criteria: solitary ureteric stone 5–15 mm diameter Exclusion criteria: aged < 15 years, pregnancy, uncontrolled UTI, multiple ureteric stones, presence of ureteric stricture distal to stone, previous unsuccessful ESWL, concomitant use of calcium‐channel blockers or alpha‐blockers, uncorrected coagulation profile, severe vertebral malformation, morbid obesity, severe cardiopulmonary disorders, elevated serum creatinine (> 2 mg/dL), high grade hydronephrosis, diabetes mellitus, bladder outlet obstruction, neuropathic bladder, gastric ulcer disease (to avoid exacerbation of ulcer disease by analgesics) |
|
| Interventions |
Treatment group:
Control group: standard medical therapy alone SWL:
|
|
| Outcomes |
Primary: stone clearance, failure, clinically significant residual fragments, stone free, complications, auxiliary procedures
Secondary: expulsion time, median number of ESWL sessions, steinstrasse, cumulative diclofenac dose Subgroups: stone location, stone size |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the 130 and 30 patients were randomized into two equal groups using a computer program." Comment: appropriate method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: no mention of placebo; outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: no mention of placebo; outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: based on flow diagram, all randomized participants included in analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: several sessions; unclear how many participants received how many SWL sessions. |
Naja 2008.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported Country: India Dates of the study: 2006–2007 |
|
| Participants |
Total number of participants randomized: 139
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with a single radiopaque renal stone (5–20 mm) undergoing ESWL Exclusion criteria: age extremes; creatinine > 2.0 mg/dL; distal obstruction; lower caliceal or radiolucent stones; previous unsuccessful ESWL; diabetes mellitus; concomitant use of calcium channel blockers, alpha1‐blockers or corticosteroids; previous pyeloureteral surgery; severe vertebral malformation; morbid obesity; pregnancy; aortic/renal artery aneurysm; coagulopathy; presence of a ureteral stent |
|
| Interventions |
Treatment group:
Control group: control SWL:
|
|
| Outcomes |
Primary: success rate
Secondary: sessions and days required for clearance, pain intensity, incidence of steinstrasse and need for auxiliary procedures Subgroups: stone location, stone size |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numbers." Comment: appropriate method of random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label randomized non‐placebo‐controlled study." Comment: participants not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "of the 139 randomized patients (67 in group 1 and 72 and group 2) the data 16 patients from group 1 and 7 from group to where not included in the final analysis for various reason…" Comment: large proportion of randomized participants (especially in group 1) not included in analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "all patients underwent ESWL every three weeks…" Comment: several sessions; unclear how many participants received how many SWL sessions. |
Park 2013.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported/outpatient Country: Korea Dates of the study: March 2011 to February 2013 |
|
| Participants |
Total number of participants randomized: 96
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: ages 18–70 years with symptomatic, unilateral and single proximal ureteral stones 6–20 mm in longest axis confirmed on x‐ray KUB radiography and kidney NCCT Exclusion criteria: active UTI; severe hydronephrosis; pregnancy; inadequate renal function (serum creatinine > 2.0 mg/dL); concomitant treatment with alpha‐blockers, calcium channel blockers or steroids; hypotension; multiple urinary stones; morbid obesity (BMI > 30 kg/m²); stone on non‐functioning kidney; previous failed ESWL; previous urinary tract surgery; uncorrected urinary tract obstruction |
|
| Interventions |
Treatment group:
Control group: no medication SWL:
|
|
| Outcomes |
Primary: stone free rate
Secondary: time until stone clearance, pain intensity, analgesic requirement, incidence of complications Subgroups: not reported |
|
| Funding sources | Grant from Astellas Pharma Korea (06‐2008‐2480) | |
| Declarations of interest | Grant from Astellas Pharma Korea (06‐2008‐2480) | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization." Comment: adequate method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "concealed in a sealed envelope until the day of ESWL." Comment: not described as opaque and numbered; unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label." Comment: open label study; participants not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: outcome assessors likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: outcome assessors likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "for patients in the treatment group and four and the control group dropped out of the trial own to withdrawal of consent (n=3) or loss of follow‐up for an unknown reason (n=5)." Comment: 4/48 and 4/48 randomized participants not included in analyses of reported outcomes; proportion < 10%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Quote: "patients who would not stone free after three weeks…were successfully treated with… repeated ESWL (n=8)." Comment: reporting suggests that participants received only 1 SWL session during trial. |
Qadri 2014.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: single center/not reported Country: Pakistan Dates of the study: July 2010 to December 2010 |
|
| Participants |
Total number of participants randomized: 120
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (mean, mm):
Inclusion criteria: people with single radio‐opaque renal stone (0.5–2.0 cm) Exclusion criteria: age extremes (18–60 years), recent open or endoscopic surgical intervention, presence of ureteral stent, radiolucent calculus, past unsuccessful ESWL, renal impairment (serum creatinine level above normal range), UTI, receiving calcium channel blocker or alpha‐blocker and corticosteroids, congenital urinary anomalies, severe vertebral malformation |
|
| Interventions |
Treatment group:
Control group: control SWL:
|
|
| Outcomes | Stone clearance rate, time to stone clearance (in weeks), mean intensity of pain, incidence of steinstrasse formation and incidence of auxiliary procedure required
Subgroups: stone location, stone size, gender, age |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by envelope method to either standard therapy or alpha blocker." Comment: appropriate method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "randomized non‐placebo‐controlled study." Comment: open label study; participants not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "none of them 120 patients included in the study dropped out and all were followed till the end of the study." Comment: no attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "the subsequent sessions of SWL needed were given after every two weeks." Comment: multiple SWL sessions; unclear how many per group. |
Rakesh 2015.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported Country: India Dates of the study: not reported |
|
| Participants |
Total number of participantsrandomized: 120
Age: not reported Sex (M/F): not reported Stone location: not reported Stone size: not reported Inclusion criteria: inclusion criteria for ESWL Exclusion criteria: exclusion criteria for ESWL |
|
| Interventions |
Treatment group:
Control group: no adjuvant therapy SWL: not reported |
|
| Outcomes | Stone fragmentation, stone clearance, postprocedure analgesic requirement and final success of ESWL procedure
Subgroups: stone location (lower, non‐lower calyx), stone size, stone density (CT Hounsfield units) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Seungok 2009.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: not reported Country: not reported Dates of the study: not reported |
|
| Participants |
Total number of participants randomized: 55
Age: not reported Sex (M/F): not reported Stone location: not reported Stone size: not reported Inclusion criteria: people with ureteral stones < 10 mm treated with ESWL Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: no medications SWL:number of sessions: multiple; repeated until complete stone clearance |
|
| Outcomes | Number of episodes of ureteral colic, expulsion rates of stones after ESWL, mean number of sessions of ESWL until complete expulsions of stones
Subgroups: stone location (upper, lower ureteral) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Comment: multiple SWL sessions reported with mean number varying by group; unclear how many participants received how many SWL sessions. |
Shaikh 2018.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Number of centers/setting: single center/not reported Country: Pakistan Dates of the study: January 2013 to July 2013 |
|
| Participants |
Total number of participants randomized: 160
Age (mean, years):
Sex (M/F): not reported Stone location: renal Stone size (mean, mm):
Inclusion criteria: ages > 18 and < 50 years, single radio‐opaque and stone size < 20 mm Exclusion criteria: pregnancy, uncontrolled coagulopathy, severe hydronephrosis, ipsilateral lower ureter stone, multiple or bilateral stone, solitary kidney, renal insufficiency, stone with UTI |
|
| Interventions |
Treatment group:
Control group: control SWL: not reported |
|
| Outcomes | Stone clearance, pain intensity, steinstrasse formation
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized into groups buy lottery methods; equal slips was made and kept in and one box and patients were asked to take one slip." Comment: adequate method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Sighinolfi 2010.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: Italy Dates of the study: January 2009 to not reported |
|
| Participants |
Total number of participants randomized: 129
Age: not reported Sex (M/F): not reported Stone location: renal Stone size (mean, mm):
Inclusion criteria: people with an apparent ESWL‐fragmentation of renal stones Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: no adjunctive MET SWL: not reported |
|
| Outcomes | Effectiveness quotient, stone free rate, stone‐expulsion, renal colic with hospitalization
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Singh 2011a.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: single center/outpatient Country: India Dates of the study: January 2006 to June 2008 |
|
| Participants |
Total number of participants randomized: 120
Age (mean, years):
Sex (M/F):
Stone location: lower ureter Stone size: not reported Inclusion criteria: consecutive patients ages > 18 years with symptomatic, unilateral, solitary lower ureteric calculus confirmed by abdominal x‐ray and sonography KUB 4–12 mm in major axis Exclusion criteria: active UTI, fever, acute renal failure, chronic renal failure, history of urinary tract surgery or endoscopic treatment, uncorrected distal obstruction, severe hydronephrosis, pregnancy, concomitant treatment with alpha‐blockers, calcium channel blockers, steroids, morbid obesity, history of previous failed ESWL |
|
| Interventions |
Treatment group:
Control group: control SWL:
|
|
| Outcomes | Doses of analgesic required, stone free rate, clearance time, any complications
Subgroups: stone size (4–7 mm, 8–12 mm) |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label study." Comment: participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one patient from control group was withdrawn from the study due to severe colic and underwent ureteroscopy." Comment: low loss‐to‐follow‐up rate. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Low risk | Quote: "single session of ESWL." Comment: 1 SWL session was administered. |
Singh 2011b.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: single center/outpatient Country: India Dates of the study: January 2006 to June 2008 |
|
| Participants |
Total number of participants randomized: 120
Age (mean, years):
Sex (M/F):
Stone location: upper ureteral Stone size: not reported Inclusion criteria: ages 18–70 years with symptomatic, unilateral and solitary upper ureteral calculi proved on x‐ray KUB and ultrasound of the kidney, 6–15 mm in major axis. Upper ureter defined as part of the ureter between pelvi‐ureteral junction and sacroiliac joint Exclusion criteria: active UTI; fever; acute renal failure; chronic renal failure; history of urinary tract surgery or endoscopic treatment; uncorrected distal obstruction; severe hydronephrosis; pregnancy; concomitant treatment with alpha‐blockers, calcium channel blockers or steroids; morbid obesity (BMI > 30 kg/m²); history of previous failed ESWL |
|
| Interventions |
Treatment group:
Control group: control SWL:
|
|
| Outcomes |
Primary: success rate
Secondary: clearance time, sessions required for clearance, pain intensity, incidence of steinstrasse Subgroups: stone size (6–10 mm, 11–15 mm), gender |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done by sealed envelope technique." Comment: appropriate method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was done by sealed envelope technique." Comment: not described as opaque and numbered; unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of placebo; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: blinding not relevant to this outcome. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Low risk | Quote: "… All the patients were evaluated by the doctor who was blinded to the treatment given." Comment: outcome assessor reported to have been blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Low risk | Quote: "… All the patients were evaluated by the doctor who was blinded to the treatment given." Comment: outcome assessor reported to have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all but 3/120 randomized participants not included in analyses of reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | High risk | Quote: "the patient was termed as shockwave lithotripsy failure when incomplete or no fragmentation was found after three sessions." Comment: up to 3 sessions; unclear how many participants received how many SWL sessions. |
Tajari 2009.
| Study characteristics | ||
| Methods |
Study design: double blind RCT Number of centers/setting: single center/lithotripsy center Country: Iran Dates of the study: October 2006 to October 2007 |
|
| Participants |
Total number of participants randomized: 240
Age (mean, years):
Sex (M/F): not reported Stone location: ureteral Stone size: not reported Inclusion criteria: people with 7–19 mm stone diameter Exclusion criteria: opium addiction |
|
| Interventions |
Treatment group:
Control group: standard care Comparator group: terazosin 2 mg SWL: not reported |
|
| Outcomes | Time of stone passage, severity of pain, frequency analgesic use, course of disability
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block balance randomize." Comment: computer generated random sequence generation assumed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description of placebo use; participants likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Teleb 2015.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: Egypt Dates of the study: March 2012 to April 2014 |
|
| Participants |
Total number of participants randomized: 212
Age: not reported Sex (M/F): not reported Stone location: renal Stone size: not reported Inclusion criteria: people who underwent successful SWL (fragments < 4 mm) for single renal stone ≤ 2 cm Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: control SWL: not reported |
|
| Outcomes | Rate and timing of stone free state achievement, need for additional analgesia, occurrence of any complications
Subgroups: stone size (≤ 1 cm, 1–2 cm) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: abstract only Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "group 1 was assigned to take an analgesic and anti‐inflammatory only… and group 2 for which daily tamsulosin 0.4 mg was added." Comment: participants and personnel likely not blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | High risk | Comment: investigators likely not blinded in this open label study. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | High risk | Comment: investigators likely not blinded in this open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess what proportion of randomized participants were included in outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unclear whether > 1 SWL session was administered. |
Vicentini 2011.
| Study characteristics | ||
| Methods |
Study design: prospective, double blind, placebo controlled RCT Number of centers/setting: single center/not reported Country: Brazil Dates of the study: October 2006 to December 2009 |
|
| Participants |
Total number of participants randomized: 136
Age (mean, years):
Sex (M/F):
Stone location:
Stone size (median (range), mm):
Inclusion criteria: ages > 18 years, a radiopaque non‐lower pole renal stone (5–20 mm), and ESWL Exclusion criteria: radiolucent stones, lower pole renal stones, presence of a ureteral stent, use of alpha‐blockers or calcium channel blockers, UTI, coagulopathy, pregnancy, urinary congenital anomalies, aortic or renal artery aneurism (or both), high grade hydronephrosis |
|
| Interventions |
Treatment group:
Control group: placebo Comparator group: nifedipine 20 mg/day retard SWL:
|
|
| Outcomes |
Primary: success rate
Secondary: pain intensity, speed of fragment elimination Subgroups: stone size (5–9 mm, 10–20 mm) |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using a computer a random number generator." Comment: appropriate method of random sequence generation used. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "all the capsules were identical and were given in identical boxes randomly named K, R, or S with thirty capsules in each box. Neither the researcher nor the patience knew the meaning of the letters." Comment: unclear whether allocation was concealed (and concealment was maintained). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all the capsules were identical"; "the meaning of the letters was revealed only after the statistical analysis, keeping the double‐blind character of the study." Comment: participants likely blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; not susceptible (auxiliary treatments) | Low risk | Comment: outcome judged not susceptible to detection bias. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Low risk | Quote: "neither the researcher (F.C.V) nor the patients knew the meaning of the letter. Only the main researcher (F.C.V) was the outcome assessor." Comment: outcome assessor likely blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Low risk | Quote: "neither the researcher (F.C.V) nor the patients knew the meaning of the letter. Only the main researcher (F.C.V) was the outcome assessor." Comment: outcome assessor likely blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 38/45 and 38/46 of randomized participants were included in the analyses of reported outcomes. Comment: attrition rates as high as 10–19%; rated as 'unclear.' |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: no indication of > 1 SWL session per participant. |
Wang 2008.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Number of centers/setting: not reported Country: China Dates of the study: 2005–2007 |
|
| Participants |
Total number of participants randomized: 80
Age (mean, years):
Sex (M/F):
Stone location: lower ureteral Stone size (mean, mm):
Inclusion criteria: people with lower ureteral stones undergoing ESWL Exclusion criteria: not reported |
|
| Interventions |
Treatment group:
Control group: not reported SWL: not reported |
|
| Outcomes | Stone free rate, renal colic relapse, occurrence of any complications
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Full study text in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: appropriate method of random sequence generation used. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear whether participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (stone clearance, time to stone clearance) | Unclear risk | Comment: unclear whether outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) Investigator assessed; susceptible (major adverse events) | Unclear risk | Comment: unclear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to assess. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. |
| Other bias (additional SWL sessions) | Unclear risk | Comment: unable to assess. |
BMI: body mass index; CT: computer tomography; EQ‐5D: EuroQol‐5D; ESWL: extracorporeal shock wave lithotripsy; F: female; HRQoL: health related quality of life; IV: intravenous; IVP: intravenous pyelography; KUB: kidney, ureter, bladder radiograph; M: male; MET: medical expulsive therapy; max: maximum; min: minute; N/A: not applicable; NCCT: non‐contrast‐enhanced computed tomography; NSAID: non‐steroidal anti‐inflammatory drug; PRN: on demand; RCT: randomized controlled trial; SD: standard deviation; SWL: shock wave lithotripsy; URS: ureteroscopy; UTI: urinary tract infection; VAS: visual analog scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| 2nd ESD "Experts in Stone Disease" Conference | Wrong study design (no applicable RCTs) |
| Choi 2008 | Wrong comparator (trospium chloride 5 mg orally twice daily) |
| Georgiev 2011 | Wrong study design (not truly randomized, participants assigned treatment based on order of enrollment) |
| Gravas 2007 | Wrong study design (not truly randomized, performed based on last digit of hospital code number) |
| Gravina 2005 | Wrong intervention (tamsulosin and methylprednisolone 16 mg twice daily for 15 days) |
| Hirasawa 2012 | Wrong intervention (urapidil, a mixed alpha1‐blocker and 5‐HT1A receptor agonist) |
| Hussein 2010 | Wrong study design (not truly randomized, every third eligible patient selected for inclusion) |
| Lee 2008 | Wrong intervention (alfuzosin or tamsulosin with trospium chloride) |
| Mehrabi 2016 | Wrong intervention (tamsulosin and Lithotrex B, an herbal drug) |
| Moursy 2010 | Wrong patient population (unilateral steinstrasse) |
| NCT00209131 | Aborted with no results |
| NCT00409227 | Aborted with no results |
| NCT00454402 | Aborted with no results |
| NCT00478998 | Wrong comparator (ureteral stents) |
| NCT01010048 | Aborted with no results |
| NCT01215708 | Aborted with no results |
| NCT01560091 | Aborted with no results |
| Pirzada 2011 | Wrong intervention (standard treatment included drotaverine hydrochloride, an antispasmodic) |
| Resim 2005 | Wrong patient population (patients with steinstrasse) |
| Shahat 2015 | Wrong patient population (pediatrics) |
| Wang 2009 | Wrong study design (not truly randomized, participants assigned to study arm based on outpatient urologist) |
| Wang 2010 | Wrong intervention (standard treatment included 5 g paishi granules, a Chinese herbal medicine, 3 times daily) |
| Zaytoun 2012 | Wrong intervention (standard treatment included phloroglucinol 240 mg/day) |
| Zhang 2015 | Incomplete reference |
| Zordani 2013 | Wrong intervention (Aporfina, Renalit Colic Combi) |
RCT: randomized controlled trial.
Differences between protocol and review
We did not search abstract proceedings of relevant meetings separately, since they were included in the electronic searches.
Based on post hoc input from our clinical experts (who were part of this author team), we revised the threshold for a clinically important difference for auxiliary treatments to 3% (30 in 1000) instead of 5% (50 per 1000) as defined in the protocol. This was based on increasing recognition that this was a very important outcome to patients (reflecting to the need to undergo additional treatment, often requiring anesthesia) and that relatively small increases were associated with large disutility ratings.
We added further post hoc subgroup analyses grouping stone location as renal versus ureter. This was informed by comments by one of the reviewers about the importance of such analyses, which resonated with us.
We renamed the outcome 'time to stone clearance' that invokes a time‐to‐event outcome to 'stone clearance time' to better reflect its characteristic as a continuous outcome that refers only to those participants that actually did pass their stone.
Cluster‐randomized controlled trials were not considered eligible to this review question but this has not been specified in the published protocol. We have revised the methods to make this clearer.
Contributions of authors
MO: performed data abstraction, risk of bias assessment, analysis and drafted the review.
RV: performed data abstraction, risk of bias assessment and critical input (clinical and methodological) to the review.
NS: performed data abstraction, risk of bias assessment and critical input (clinical and methodological) to the review.
EH: performed data abstraction, risk of bias assessment, critical input (clinical and methodological) to the review; GRADE ratings.
GK: developed search strategy and completed searches.
AK: assisted with analysis.
CS: provided clinical input.
PD: conceptualized review and oversaw all aspects of its completion, developed GRADE 'Summary of findings' table, addressed reviewers' and editors' feedback, finalized the review.
Sources of support
Internal sources
-
Department of Urology, University of Minnesota, Minneapolis, MN, USA
Salary support for PD
-
Minneapolis Veterans Administration Health Care System, Minneapolis, MN, USA
Support to Cochrane Urology Group (Library services)
External sources
-
No external support, USA
No external support received
Declarations of interest
MO: none.
RV: none.
NS: none.
EH: none.
GK: none.
AK: none.
CS: none.
PD: none.
New
References
References to studies included in this review
Agarwal 2009 {published data only}
- Agarwal MM, Naja V, Singh SK, Mavuduru R, Mete UK, Kumar S, et al. Is there an adjunctive role of tamsulosin to extracorporeal shockwave lithotripsy for upper ureteric stones: results of an open label randomized nonplacebo controlled study. Urology 2009;74(5):989-92. [DOI] [PubMed] [Google Scholar]
Ahmed 2016 {published data only}
- Ahmed AF, Shalaby E, El-Feky M, Kotb A, Elsotohi E, El-Kholy M, et al. Role of tamsulosin therapy after extracorporeal shockwave lithotripsy for renal stones: randomized controlled trial. Urologia Internationalis 2016;97(3):266-72. [DOI] [PubMed] [Google Scholar]
Ateş 2012 {published data only}
- Ateş F, Erylıdırım B, Öztürk MI, Turan T, Gürbüz C, Ekinci MO, et al. Does the use of doxazosin influence the success of SWL in the treatment of upper ureteral stones? A multicenter, prospective and randomized study. Urological Research 2012;40(5):537-42. [DOI] [PubMed] [Google Scholar]
- Ates F, Eryildirim B, Iozturk M, Turan T, Gurbuz C, Ekinci MO, et al. Does the use of alpha blockers influence the success of SWL in the treatment of upper ureteral stones? A multicenter, prospective and randomized study. European Urology 2011;10(7):515. [Google Scholar]
Baloch 2011 {published data only}
- Baloch H, El Khalid S. Efficacy of Alfuzosin (alpha blocker) after extra corporeal shockwave lithotripsy for passage of renal calculi. European Urology 2011;10(7):479. [Google Scholar]
Bhagat 2007 {published data only}
- Bhagat SK, Chacko NK, Kekre NS, Gopalakrishnan G, Antonisamy B, Devasia A. Is there a role for tamsulosin in shock wave lithotripsy for renal and ureteral calculi? Journal of Urology 2007;177(6):2185-8. [DOI] [PubMed] [Google Scholar]
Cakıroglu 2013 {published data only}
- Cakıroglu B, Sinanoglu O, Uraz M. The effect of tamsulosin on pain and clearance according to ureteral stone location after shock wave lithotripsy. Current Therapeutic Research, Clinical and Experimental 2013;74:33-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chau 2015 {published data only}
- Chau H, Chan HC, Li TB, Cheung MH, Lam KM, So HS. Prospective randomized controlled trial for patient with renal stone undergoing extracorporeal shockwave lithotripsy (ESWL) using tamsulosin as adjuvant medical explusive therapy: are there any added benefits? BJU International 2015;115(S1):9-10. [Google Scholar]
Cho 2013 {published data only}
- Cho HJ, Shin SC, Seo DY, Min DS, Cho JM, Kang JY, et al. Efficacy of alfuzosin after shock wave lithotripsy for the treatment of ureteral calculi. Korean Journal of Urology 2013;54(2):106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Nunzio 2016 {published data only}
- De Nunzio C, Brassetti A, Bellangino M, Lombardo R, Presicce F, Voglino O, et al. Concomitant tamsulosin and silodosin treatment is not associated with a better clinical outcome or an increased stone free rate in patients treated with ESWL: a randomized placebo controlled study. European Urology Supplements 2016;15(3):e458. [Google Scholar]
- De Nunzio C, Brassetti A, Bellangino M, Trucchi A, Petta S, Presicce F, et al. Tamsulosin or silodosin adjuvant treatment is ineffective in improving shockwave lithotripsy outcome: a short-term follow-up randomized, placebo-controlled study. Journal of Endourology 2016;30(7):817-21. [DOI] [PubMed] [Google Scholar]
Elkoushy 2012 {published data only}
- Elkoushy M A. Adjuvant alpha adrenergic blockers: are they equally efficient for renal and upper ureteral calculi disintegrated by shock wave lithotripsy? African Journal of Urology 2012;18(1):24-8. [Google Scholar]
- Elkoushy MA. Adjuvant alpha adrenergic blockers for renal and upper ureteral calculi disintegrated by shock wave lithotripsy. Journal of Endourology 2010;24(S1):A103-4. [Google Scholar]
Eryildirim 2016 {published data only}
- Eryildirim B, Sahin C, Tuncer M, Sabuncu K, Tarhan F, Sarica K. Medical expulsive therapy following shock wave lithotripsy in ureteral calculi: an effective approach for the improvement of health-related quality of life. Urologia Internationalis 2016;97(3):260-5. [DOI] [PubMed] [Google Scholar]
- Eryildirim B, Sahin C, Tuncer M, Sabuncu K, Tarhan F, Sarica K. Medical expulsive therapy following SWL in ureteral calculi: an effective approach for the improvement of health-related quality of life. European Urology Supplements 2017;16(3):e66. [DOI] [PubMed] [Google Scholar]
Falahatkar 2011 {published data only}
- Falahatkar S, Khosropanah I, Vajary AD, Bateni ZH, Khosropanah D, Allahkhah A. Is there a role for tamsulosin after shock wave lithotripsy in the treatment of renal and ureteral calculi? Journal of Endourology 2011;25(3):495-8. [DOI] [PubMed] [Google Scholar]
Gaafar 2011 {published data only}
- Gaafar SE, Motawee O, Marzouk ME, Foda MK, Zahran AM. Role of alpha-adrenergic blockers in enhancing clearance of stone fragments after ESWL of renal calculi. European Urology 2011;10(7):479-80. [Google Scholar]
H 2012 {published data only}
- H C, Yui L. Prospective randomized controlled trial for patients with ureteric stones undergoing extracorporeal shockwave lithotripsy (ESWL) using tamsulosin as adjuvant medical explusive therapy: are there any added benefits? International Journal of Urology 2012;19(S1):172. [Google Scholar]
Hammoud 2014 {published data only}
- Hammoud KM, Hindawy W, Galal M, Sadek S. Does tamsulosin improve the results of SWL for upper urinary tract stones? Journal of Urology 2014;191(4 Suppl 1):e204. [Google Scholar]
Han 2006 {published data only}
- Han MC, Jeong WS, Shim BS. Additive expulsion effect of tamsulosin after shock wave lithotripsy for upper ureteral stones. Korean Journal of Urology 2006;47(8):813-7. [Google Scholar]
Hong 2012 {published data only}
- Hong SK, Tan YK, Chong YL. A pilot study on the use of alfuzosin as medical expulsive therapy after extracorporeal shockwave lithotripsy. Journal of Endourology 2012;26(S1):A107. [Google Scholar]
Itaya 2011 {published data only}
- Itaya N, Sugata A, Hayashi K, Hara H, Tamubo M, Sisido T, et al. Efficacy of an alpha1-blocker in expulsive therapy of ureteral stone after shock wave lithotripsy. Journal of Endourology 2011;25(S1):A248. [Google Scholar]
Janane 2014 {published data only}
- Janane A, Hamdoun A, Hajji F, Dakkak Y, Ghadouane M, Ameur A, et al. Usefulness of adjunctive alpha1-adrenergic antagonists after single extracorporeal shock wave lithotripsy session in ureteral stone expulsion. Canadian Urological Association Journal 2014;8(1-2):E8-E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2009 {published data only}
- Kang DI, Cho WY, Kim TH, Chung JM, Park J, Yoon JH, Lee SD. Effect of tamsulosin 0.2 mg on the short-term treatment of urinary stones: multicenter, prospective, randomized study. Korean Journal of Urology 2009;50(6):586-90. [Google Scholar]
Kim 2008 {published data only}
- Kim TH, Oh SY, Moon YT. The effect of tamsulosin on expulsion of ureteral stones after extracorporeal shock wave lithotripsy. Korean Journal of Urology 2008;49(12):1100-4. [Google Scholar]
Kobayashi 2008 {published data only}
- Kobayashi M, Naya Y, Kino M, Awa Y, Nagata M, Suzuki H, et al. Low dose tamsulosin for stone expulsion after extracorporeal shock wave lithotripsy: efficacy in Japanese male patients with ureteral stone. International Journal of Urology 2008;15(6):495-8. [DOI] [PubMed] [Google Scholar]
Küpeli 2004 {published data only}
- Küpeli B, Irkilata L, Gürocak S, Tunç L, Kiraç M, Karaoglan U, et al. Does tamsulosin enhance lower ureteral stone clearance with or without shock wave lithotripsy? Urology 2004;64(6):1111-5. [DOI] [PubMed] [Google Scholar]
Lanchon 2017 {published data only}
- Lanchon C, Ronna M, Descotes JL, Rambeaud JJ, Fiard G, Thuillier C, et al. Adjuvant alpha blockers to extracorporeal shock wave lithotripsy: a randomized controlled trial. European Urology Supplements 2017;16(3):e65. [Google Scholar]
Liu 2009 {published data only}
- Liu Y, Zhuang S, Ma L. Low-dose tamsulosin improves clinical effect of single extracorporeal shock wave lithotripsy for ureteral calculi. Urology 2009;74(4 Suppl 1):S136. [Google Scholar]
Micali 2007 {published data only}
- Micali S, Grande M, Sighinolfi MC, De Stefani S, Bianchi G. Efficacy of expulsive therapy using nifedipine or tamsulosin, both associated with ketoprofene, after shock wave lithotripsy of ureteral stones. Urological Research 2007;35(3):133-7. [DOI] [PubMed] [Google Scholar]
Mohamed 2013 {published data only}
- Mohamed HI. The efficacy of tamsulosin therapy after extracorporeal shock-wave lithotripsy for ureteric calculi: a prospective randomised, controlled study. Arab Journal of Urology 2013;11(4):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naja 2008 {published data only}
- Naja V, Agarwal MM, Mandal AK, Singh SK, Mavuduru R, Kumar S, et al. Tamsulosin facilitates earlier clearance of stone fragments and reduces pain after shockwave lithotripsy for renal calculi: results from an open-label randomized study. Urology 2008;72(5):1006-11. [DOI] [PubMed] [Google Scholar]
Park 2013 {published data only}
- Park YH, Lee HE, Park JY, Lee SB, Kim HH. A prospective randomized controlled trial of the efficacy of tamsulosin after extracorporeal shock wave lithotripsy for a single proximal ureteral stone. Korean Journal of Urology 2013;54(8):527-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Park JY, Ahn H, Kim HH. A prospective randomized controlled trial of the efficacy of tamsulosin after extracorporeal shock wave lithotripsy for a single proximal ureteral stone. Journal of Endourology 2013;27(S1):A263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qadri 2014 {published data only}
- Qadri SS, Khalid SE, Mahmud SM. Effects and outcome of tamsulosin more than just stone clearance after extracorporeal shock wave lithotripsy for renal calculi. Journal of the Pakistan Medical Association 2014;64(6):644-8. [PubMed] [Google Scholar]
Rakesh 2015 {published data only}
- Rakesh S, Subrata MK, Ranjan DK, Supriya B, Ranjit DK. Is adjuvant therapy helpful for successful outcome in patients undergoing ESWL? Indian Journal of Urology 2015;31(S1):S81. [Google Scholar]
Seungok 2009 {published data only}
- Seungok Y, Jeongki L, Tae YJ, Yun BK. The effect of tamsulosin on expulsion of ureteral stones after extracorporeal shock wave lithotripsy. Journal of Endourology 2009;23(Suppl 1):A169. [Google Scholar]
Shaikh 2018 {published data only}
- Shaikh AA, Patujo YH, Shaikh AB, Shaikh NA, Jalbani MH. Comparison of efficacy with and without tamsulosin as medical adjuvant therapy after extracorporeal shockwave lithotripsy in renal stone. Rawal Medical Journal 2018;43(3):471-4. [Google Scholar]
Sighinolfi 2010 {published data only}
- Sighinolfi MC, Micali S, Mofferdin A, Pini G, Isgro G, De Stefani S, et al. Efficacy of tamsulosin treatment after extracorporeal shock wave lithotripsy of stones located in the kidney: a prospective and randomized study on 129 patients. Journal of Endourology 2010;24(Suppl 1):A106. [Google Scholar]
Singh 2011a {published data only}
- Singh SK, Pawar DS, Griwan MS. Tamsulosin as an expulsive therapy for lower ureteric calculus after extra corporeal shock wave lithotripsy: a randomized controlled study. Nephro-Urology Monthly 2011;3(1):62-8. [Google Scholar]
Singh 2011b {published data only}
- Singh SK, Pawar DS, Griwan MS, Indora JM, Sharma S. Role of tamsulosin in clearance of upper ureteral calculi after extracorporeal shock wave lithotripsy: a randomized controlled trial. Urology Journal 2011;8(1):14-20. [PubMed] [Google Scholar]
Tajari 2009 {published data only}
- Tajari H, Pedram P, Keshtkar A, Ghazi Moghadam B, Amidi A. The comparison between efficacy of tamsulosin and terazosin in alphaadrenergic blockers after ESWL (a double blind clinical trial). Urology 2009;74(4):S340-1. [Google Scholar]
- Tajari H, Pedram PM, Keshtkar AA, Ghazi Moghadam B, Amidi A. The comparison between efficacy of tamsulosin and terazosinin alpha-adrenergic blockers after ESWL (a double blind clinical trial). Journal of Endourology 2009;23(11):A33-4. [Google Scholar]
Teleb 2015 {published data only}
- Teleb M, Dawod T, Salem E, Ragab A, Shabana W, Fawzi A, et al. The efficacy of tamsulosin in stone clearance after shock wave lithotripsy for renal calculi. European Urology Supplements 2015;14(2):e99. [Google Scholar]
Vicentini 2011 {published data only}
- Vicentini F, Mazzucchi E, Brito A, Neto EC, Danilovic A, Ebaid G, et al. Adjuvant use of tamsulosin and nifedipine after extracorporeal shock waves lithotripsy in patients with kidney stones – a prospective, double-blind and randomized study. Journal of Urology 2011;184(4 Suppl 1):e898. [DOI] [PubMed] [Google Scholar]
- Vicentini FC, Mazzucchi E, Brito AH, Chedid Neto EA, Danilovic A, Srougi M. Adjuvant tamsulosin or nifedipine after extracorporeal shock wave lithotripsy for renal stones: a double blind, randomized, placebo-controlled trial. Urology 2011;78(5):1016-21. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang HJ, Liu K, Ji ZG, Li HZ. Application of α1-adrenergic antagonist with extracorporeal shock wave lithotripsy for lower ureteral stone. Acta Academiae Medicinae Sinicae 2008;30(4):506-8. [PubMed] [Google Scholar]
References to studies excluded from this review
2nd ESD "Experts in Stone Disease" Conference {published data only}
- N/A. 2nd ESD "Experts in Stone Disease" Conference. Urolithiasis 2014;42(6):not provided. [Google Scholar]
Choi 2008 {published data only}
- Choi NY, Ahn SH, Han JH, Jang IH. The effect of tamsulosin and nifedipine on expulsion of ureteral stones after extracorporeal shock wave lithotripsy. Korean Journal of Urology 2008;49(2):150-4. [Google Scholar]
Georgiev 2011 {published data only}
- Georgiev MI, Ormanov DI, Vassilev VD, Dimitrov PD, Mladenov VD, Popov EP, et al. Efficacy of tamsulosin oral controlled absorption system after extracorporeal shock wave lithotripsy to treat urolithiasis. Urology 2011;78(5):1023-6. [DOI] [PubMed] [Google Scholar]
Gravas 2007 {published data only}
- Gravas S, Tzortzis V, Karatzas A, Oeconomou A, Melekos MD. The use of tamsulozin as adjunctive treatment after ESWL in patients with distal ureteral stone: do we really need it? Results from a randomised study. Urological Research 2007;35(5):231-5. [DOI] [PubMed] [Google Scholar]
Gravina 2005 {published data only}
- Gravina GL, Costa AM, Ronchi P, Galatioto GP, Angelucci A, Castellani D, et al. Tamsulosin treatment increases clinical success rate of single extracorporeal shock wave lithotripsy of renal stones. Urology 2005;66(1):24-8. [DOI] [PubMed] [Google Scholar]
Hirasawa 2012 {published data only}
- Hirasawa Y, Ide H, Ito Y, Hoshino K, Sato A, Hongo H, et al. Improvement of expulsion of upper ureteral stones by adjuvant use of urapidil after extracorporeal shock wave lithotripsy – a prospective randomized placebo controlled stud. Journal of Urology 2012;187(4):e908-9. [Google Scholar]
Hussein 2010 {published data only}
- Hussein MM. Does tamsulosin increase stone clearance after shockwave lithotripsy of renal stones? A prospective, randomized controlled study. Scandinavian Journal of Urology and Nephrology 2010;44(1):27-31. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee KS, Seo YJ, Kim KH. The effectiveness of alfuzosin and tamsulosin on expulsion of lower ureteral stones after extracorporeal shock wave lithotripsy (ESWL). Journal of Endourology / Endourological Society 2008;22(Suppl):A155-6. [Google Scholar]
Mehrabi 2016 {published data only}
- Mehrabi S. Comparison of tamsulosin and Lithorex B on renal colic and the expulsion of urinary stones after extracorporeal shock wave lithotripsy (ESWL). Journal of Endourology 2016;30:A175-6. [Google Scholar]
Moursy 2010 {published data only}
- Moursy E, Gamal W, Abuzeid A. Tamsulosin as an expulsive therapy for steinstrasse after extracorporeal shock wave lithotripsy: a randomized controlled study. Journal of Urology 2014;191(4 Suppl 1):e276. [DOI] [PubMed] [Google Scholar]
- Moursy E, Gamal WM, Abuzeid A. Tamsulosin as an expulsive therapy for steinstrasse after extracorporeal shock wave lithotripsy: a randomized controlled study. Scandinavian Journal of Urology and Nephrology 2010;44(5):315-9. [DOI] [PubMed] [Google Scholar]
NCT00209131 {published data only}
- NCT00209131. Efficacy of flomax to improve stone passage following shock wave lithotripsy. clinicaltrials.gov/ct2/show/NCT00209131 (first received 21 September 2005).
NCT00409227 {published data only}
- NCT00409227. Does treatment with alfuzosin increase success rates of (SWL) shock wave lithotripsy. clinicaltrials.gov/ct2/show/NCT00409227 (first received 8 December 2006).
NCT00454402 {published data only}
- NCT00454402. ALF-STONE: alfuzosin in uretheric stones. clinicaltrials.gov/ct2/show/NCT00454402 (first received 7 March 2007).
NCT00478998 {published data only}
- NCT00478998. Comparing patients after ESWL treated with ureteral stents versus expulsion therapy with tamsulosin. clinicaltrials.gov/ct2/show/NCT00478998 (first received 25 May 2007).
NCT01010048 {published data only}
- NCT01010048. Compare the therapeutic effect treated with tamsulosin and progesterone after ESWL (extra corporeal shock wave lithotripsy) in urinary calculus. clinicaltrials.gov/ct2/show/NCT01010048 (first received 9 November 2009).
NCT01215708 {published data only}
- NCT01215708. Effects of the use of adjuvant drugs after extracorporeal shockwave lithotripsy (ESWL) in renal calculus. clinicaltrials.gov/ct2/show/NCT01215708 (first received 6 October 2010).
NCT01560091 {published data only}
- NCT01560091. Differential effect of silodosin versus tamsulosin on stone clearance after extra-corporeal shock wave lithotripsy. clinicaltrials.gov/ct2/show/NCT01560091 (first received 17 February 2012).
Pirzada 2011 {published data only}
- Pirzada AJ, Anwar A, Javed A, Memon I, Mohammad A. Role of alpha-1 blocker in expulsion of stone fragments after extracorporeal shock wave lithotripsy for renal stones. Journal of Ayub Medical College, Abbottabad 2011;23(2):125-9. [PubMed] [Google Scholar]
Resim 2005 {published data only}
- Resim S, Ekerbicer HC, Ciftci A. Role of tamsulosin in treatment of patients with steinstrasse developing after extracorporeal shock wave lithotripsy. Urology 2005;66(5):945-8. [DOI] [PubMed] [Google Scholar]
Shahat 2015 {published data only}
- Shahat A, Elderwy A, Safwat A, Badawy A, Abdelsalam Y, Sayed M, et al. Is tamsulosin effective after shockwave lithotripsy for pediatric renal stones? a randomized controlled study. Journal of Urology 2015;193(4 Suppl 1):e467. [DOI] [PubMed] [Google Scholar]
- Shahat A, Elderwy A, Safwat AS, Abdelkawi IF, Reda A, Abdelsalam Y, et al. Is tamsulosin effective after shock wave lithotripsy for pediatric renal stones? A randomized, controlled study. Journal of Urology 2016;195(4 Pt 2):1284-8. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang CJ, Huang SW, Chang CH. Adjunctive medical therapy with an alpha-1A-specific blocker after shock wave lithotripsy of lower ureteral stones. Urologia Internationalis 2009;82(2):166-9. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang H, Liu K, Ji Z, Li H. Effect of alpha1-adrenergic antagonists on lower ureteral stones with extracorporeal shock wave lithotripsy. Asian Journal of Surgery 2010;33(1):37-41. [DOI] [PubMed] [Google Scholar]
Zaytoun 2012 {published data only}
- Zaytoun OM, Yakoubi R, Zahran AR, Fouda K, Marzouk E, Gaafar S, et al. Tamsulosin and doxazosin as adjunctive therapy following shock-wave lithotripsy of renal calculi: randomized controlled trial. Urological Research 2012;40(4):327-32. [DOI] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang ZG, Xi XL, Bing JY. The observation of the efficacy of tamsulosin combined with furosemide as adjuvant expulsive therapy of ureteral calculi after ESWL. Unavailable/unable to identify 2015;18(15):110-1.
Zordani 2013 {published data only}
- Zordani A, Rosa M, Mofferdin A, Sighinolfi MC, Martorana E, Micali S, et al. Prospective comparison between tamsulosin and renalit combi colic as a treatment of reno-ureteral calculi expulsion after extracorporeal shock wave lithotripsy (ESWL). Unavailable/unable to find 2013;27:A19-A20. [Google Scholar]
Additional references
Abbott Laboratories 2019
- Abbott Laboratories. Product information: HYTRIN(R) oral tablets, terazosin HCl oral tablets. www.accessdata.fda.gov/drugsatfda_docs/label/2009/019057s022lbl.pdf (accessed prior to 26 June 2019).
Aboumarzouk 2012
- Aboumarzouk OM, Kata SG, Keeley FX, McClinton S, Nabi G. Extracorporeal shock wave lithotripsy (ESWL) versus ureteroscopic management for ureteric calculi. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD006029. [DOI: 10.1002/14651858.CD006029.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Geriatrics Society 2015
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society 2015;63(11):2227-46. [DOI] [PubMed] [Google Scholar]
Assimos 2016a
- Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American Urological Association/Endourological Society guideline, part I. Journal of Urology 2016;196(4):1153-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Assimos 2016b
- Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American Urological Association/Endourological Society guideline, part II. Journal of Urology 2016;196(4):1161-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boehringer 2019
- Boehringer Ingelheim Pharmaceuticals, Incorporated. Product Information: Flomax(R) oral capsules, tamsulosin HCl oral capsules. www.accessdata.fda.gov/drugsatfda_docs/label/2005/020579s016lbl.pdf (accessed prior to 26 June 2019).
Caine 1976
- Caine M, Pfau A, Perlberg S. The use of alpha-adrenergic blockers in benign prostatic obstruction. British Journal of Urology 1976;48(4):255-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Campschroer 2018
- Campschroer T, Zhu X, Vernooij RW, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD008509. [DOI: 10.1002/14651858.CD008509.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canvasser 2017
- Canvasser NE, Alken P, Lipkin M, Nakada SY, Sodha HS, Tepeler A, et al. The economics of stone disease. World Journal of Urology 2017;35(9):1321-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chewcharat 2020
- Chewcharat A, Curhan G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis 2020;N/A:N/A. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 30 April 2020. Melbourne, Australia: Veritas Health Innovation, N/A. Available at covidence.org.
Crowley 1990
- Crowley AR, Byrne JC, Vaughan ED Jr, Marion DN. The effect of acute obstruction on ureteral function. Journal of Urology 1990;143(3):596-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dahm 2018
- Dahm P, Hollingsworth JM. Medical expulsive therapy for ureteral stones – stone age medicine. JAMA Internal Medicine 2018;178(8):1058-9. [DOI] [PubMed] [Google Scholar]
De Coninck 2019
- De Coninck V, Antonelli J, Chew B, Patterson JM, Skolarikos A, Bultitude M. Medical expulsive therapy for urinary stones: future trends and knowledge gaps. European Urology 2019;76(5):658-66. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
El‐Nahas 2007
- El-Nahas AR, El-Assmy AM, Mansour O, Sheir KZ. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. European Urology 2007;51(6):1688-93; discussion 1693-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Elton 1993
- Elton TJ, Roth CS, Berquist TH, Silverstein MD. A clinical prediction rule for the diagnosis of ureteral calculi in emergency departments. Journal of General Internal Medicine 1993;8(2):57-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
EndNote [Computer program]
- EndNote. Version 7.5. Philadelphia (PA): Clarivate Analytics, 2016.
FDA 2018
- US Food and Drug Administration. CFR – Code of Federal Regulations Title 21. Sec. 312.32 IND Safety Reporting. Part 312. Subpart B – Investigational New Drug Application (IND). Subchapter D – Drugs for Human Use. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed prior to 26 June 2019).
Ferraro 2017
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. Journal of Urology 2017;198(4):858-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 30 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), N/A. Available at gradepro.org.
Gupta 2014
- Gupta A, Aboumarzouk OM, Jefferies MT, Kynaston HG, Datta S. Calcium channel blockers as medical expulsive therapy for ureteric stones. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD011162. [DOI: 10.1002/14651858.CD011162] [DOI] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed.) 2008;336(7651):995-8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Hellstrom 2006
- Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. Journal of Urology 2006;176(4 Pt 1):1529-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hernández 1992
- Hernández M, Prieto D, Simonsen U, Rivera L, Barahona MV, García-Sacristán A. Noradrenaline modulates smooth muscle activity of the isolated intravesical ureter of the pig through different types of adrenoceptors. British Journal of Pharmacology 1992;107(4):924-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hofner 1999
- Hofner K, Claes H, De Reijke TM, Folkestad B, Speakman MJ. Tamsulosin 0.4 mg once daily: effect on sexual function in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. European Urology 1999;36(4):335-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hollingsworth 2016
- Hollingsworth JM, Canales BK, Rogers MA, Sukumar S, Yan P, Kuntz GM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ (Clinical Research Ed.) 2016;355:i6112. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hubner 1993
- Hubner WA, Irby P, Stoller ML. Natural history and current concepts for the treatment of small ureteral calculi. European Urology 1993;24(2):172-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Itoh 2007
- Itoh Y, Kojima Y, Yasui T, Tozawa K, Sasaki S, Kohri K. Examination of alpha 1 adrenoceptor subtypes in the human ureter. International Journal of Urology 2007;14(8):749-53. [DOI] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schunemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses – Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013;11:109. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karabacak 2013
- Karabacak OR, Yilmazer D, Ozturk U, Sener NC, Saltas H, Karabacak Y, et al. The presence and distribution of alpha adrenergic receptors in human renal pelvis and calyces. Urolithiasis 2013;41(5):385-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim SC, Burns EK, Lingeman JE, Paterson RF, McAteer JA, Williams JC Jr. Cystine calculi: correlation of CT-visible structure, CT number, and stone morphology with fragmentation by shock wave lithotripsy. Urological Research 2007;35(6):319-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kiruluta 1981
- Kiruluta GH, Mercer AR, Winsor GM. Prazosin as cause of urinary incontinence. Urology 1981;18(6):618-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kohrmann 1995
- Kohrmann KU, Rassweiler JJ, Manning M, Mohr G, Henkel TO, Junemann KP, et al. The clinical introduction of a third generation lithotriptor: Modulith SL 20. Journal of Urology 1995;153(5):1379-83. [PubMed] [Google Scholar]
Laird 1997
- Laird JM, Roza C, Cervero F. Effects of artificial calculosis on rat ureter motility: peripheral contribution to the pain of ureteric colic. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 1997;272(5):R1409-16. [DOI] [PubMed] [Google Scholar]
Lee 2012
- Lee JK, Jeong CW, Jeong SJ, Hong SK, Byun SS, Lee SE. Impact of tamsulosin on ureter stone expulsion in Korean patients: a meta-analysis of randomized controlled studies. Korean Journal of Urology 2012;53(10):699-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2015
- Li M, Wang Z, Yang J, Guo X, Wang T, Wang S, et al. Adjunctive medical therapy with alpha-blocker after extracorporeal shock wave lithotripsy of renal and ureteral stones: a meta-analysis. PloS One 2015;10(4):e0122497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Losek 2008
- Losek RL, Mauro LS. Efficacy of tamsulosin with extracorporeal shock wave lithotripsy for passage of renal and ureteral calculi. Annals of Pharmacotherapy 2008;42(5):692-7. [DOI] [PubMed] [Google Scholar]
Madbouly 2002
- Madbouly K, Sheir KZ, Elsobky E, Eraky I, Kenawy M. Risk factors for the formation of a steinstrasse after extracorporeal shock wave lithotripsy: a statistical model. Journal of Urology 2002;167(3):1239-42. [PMID: ] [PubMed] [Google Scholar]
Matlaga 2016
- Matlaga BR, Krambeck AE, Lingeman JE. Surgical management of upper urinary tract calculi. In: Wein AJ, Kavoussi LR, Partin AE, Peters CA, editors(s). Campbell-Walsh Urology. 11th edition. Vol. 54. Philadelphia (PA): Elsevier, 2016:1260-90.e7. [Google Scholar]
McClain 2013
- McClain PD, Lange JN, Assimos DG. Optimizing shock wave lithotripsy: a comprehensive review. Reviews in Urology 2013;15(2):49-60. [PMC free article] [PubMed] [Google Scholar]
McLeod 1973
- McLeod DG, Reynolds DG, Swan KG. Adrenergic mechanisms in the canine ureter. American Journal of Physiology ‒ Legacy Content 1973;224(5):1054-8. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42; quiz 742 e1-5. [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. NICE guideline – renal and ureteric stones: assessment and management. BJU International 2019;123(2):220-32. [PubMed] [Google Scholar]
Pareek 2005
- Pareek G, Hedican SP, Lee FT Jr, Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology 2005;66(5):941-4. [DOI] [PubMed] [Google Scholar]
Park 2007
- Park HK, Choi EY, Jeong BC, Kim HH, Kim BK. Localizations and expressions of α-1A, α-1B and α-1D adrenoceptors in human ureter. Urological Research 2007;35(6):325-9. [DOI] [PubMed] [Google Scholar]
Pickard 2015
- Pickard R, Starr K, MacLennan G, Lam T, Thomas R, Burr J, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet 2015;386(9991):341-9. [DOI] [PubMed] [Google Scholar]
Pool 2005
- Pool, JL. α-Adrenoceptor blockers for management of hypertension in the elderly. In: Prisant LM, editors(s). Hypertension in the Elderly (Clinical Hypertension and Vascular Diseases). Totowa (NJ): Humana Press, 2005. [Google Scholar]
Preminger 2007
- Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck C, Gallucci M, et al. 2007 Guideline for the management of ureteral calculi. Journal of Urology 2007;178(6):2418-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI] [PubMed] [Google Scholar]
Romero 2010
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Reviews in Urology 2010;12(2-3):e86-96. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Rose 1974
- Rose JG, Gillenwater JY. The effect of adrenergic and cholinergic agents and their blockers upon ureteral activity. Investigative Urology 1974;11(6):439-51. [PMID: ] [PubMed] [Google Scholar]
Rukin 2017
- Rukin NJ, Siddiqui ZA, Chedgy EC, Somani BK. Trends in upper tract stone disease in England: evidence from the Hospital Episodes Statistics database. Urologia Internationalis 2017;98(4):391-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Saigal 2005
- Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney International 2005;68(4):1808-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al, GRADE Working Group. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Sasaki 2011
- Sasaki S, Tomiyama Y, Kobayashi S, Kojima Y, Kubota Y, Kohri K. Characterization of alpha1-adrenoceptor subtypes mediating contraction in human isolated ureters. Urology 2011;77(3):762.e13-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sayed 2001
- Sayed MA, el-Taher AM, Aboul-Ella HA, Shaker SE. Steinstrasse after extracorporeal shockwave lithotripsy: aetiology, prevention and management. BJU International 2001;88(7):675-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scales 2012
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. European Urology 2012;62(1):160-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuler 2009
- Schuler TD, Shahani R, Honey RJ, Pace KT. Medical expulsive therapy as an adjunct to improve shockwave lithotripsy outcomes: a systematic review and meta-analysis. Journal of Endourology 2009;23(3):387-93. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. Chapter 11: Completing 'Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Seitz 2009
- Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? European Urology 2009;56(3):455-71. [DOI] [PubMed] [Google Scholar]
Shapiro 1992
- Shapiro E, Hartanto V, Lepor H. The response to alpha blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate 1992;21(4):297-307. [DOI] [PubMed] [Google Scholar]
Sigala 2005
- Sigala S, Dellabella M, Milanese G, Fornari S, Faccoli S, Palazzolo F, et al. Evidence for the presence of α1 adrenoceptor subtypes in the human ureter. Neurourology and Urodynamics 2005;24(2):142-8. [DOI] [PubMed] [Google Scholar]
Sivula 1967
- Sivula A, Lehtonen T. Spontaneous passage of artificial concretions applied in the rabbit ureter. Scandinavian Journal of Urology and Nephrology 1967;1(3):259-63. [DOI] [PubMed] [Google Scholar]
Skolarikos 2006
- Skolarikos A, Alivizatos G, La Rosette J. Extracorporeal shock wave lithotripsy 25 years later: complications and their prevention. European Urology 2006;50(5):990-81. [DOI] [PubMed] [Google Scholar]
Skolarikos 2015
- Skolarikos A, Grivas N, Kallidonis P, Mourmouris P, Rountos T, Fiamegos A, et al. The efficacy of medical expulsive therapy (MET) in improving stone-free rate and stone expulsion time, after extracorporeal shock wave lithotripsy (SWL) for upper urinary stones: a systematic review and meta-analysis. Urology 2015;86(6):1057-64. [DOI] [PubMed] [Google Scholar]
Thien 1978
- Thien T, Delaere KP, Debruyne FM, Koene RA. Urinary incontinence caused by prazosin. British Medical Journal 1978;1(6113):622-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turk 2016
- Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. European Urology 2016;69(3):468-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Turk 2020
- Turk C, Neisius A, Petrik A, Seitz C, Skolarikos A. EAU urolithiasis guidelines, 2020. uroweb.org/guideline/urolithiasis (accessed prior to 19 August 2020).
Vermandere 2018
- Vermandere M, Kuijpers T, Burgers JS, Kunnamo I, Lieshout J, Wallace E, et al. alpha-Blockers for uncomplicated ureteric stones: a clinical practice guideline. BJU International 2018;122(6):924-31. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):483-73. [PubMed]
Worster 2002
- Worster A, Preyra I, Weaver B, Haines T. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Annals of Emergency Medicine 2002;40(3):280-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2017
- Yang TX, Liao BH, Chen YT, Li H, He Q, Liu QY, et al. A network meta-analysis on the beneficial effect of medical expulsive therapy after extracorporeal shock wave lithotripsy. Scientific Reports 2017;7(1):14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2018
- Ye Z, Zeng G, Yang H, Tang K, Zhang X, Li H, et al. Efficacy and safety of tamsulosin in medical expulsive therapy for distal ureteral stones with renal colic: a multicenter, randomized, double-blind, placebo-controlled trial. European Urology 2018;73(3):385-91. [DOI] [PubMed] [Google Scholar]
Zarse 2007
- Zarse CA, Hameed TA, Jackson ME, Pishchalnikov YA, Lingeman JE, McAteer JA, et al. CT visible internal stone structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urological Research 2007;35(4):201-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2010
- Zheng S, Liu LR, Yuan HC, Wei Q. Tamsulosin as adjunctive treatment after shockwave lithotripsy in patients with upper urinary tract stones: a systematic review and meta-analysis. Scandinavian Journal of Urology and Nephrology 2010;44(6):425-32. [DOI] [PubMed] [Google Scholar]
Zhu 2010
- Zhu Y, Duijvesz D, Rovers MM, Lock TM. Alpha-blockers to assist stone clearance after extracorporeal shock wave lithotripsy: a meta-analysis. BJU International 2010;106(2):256-61. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Oestreich 2019
- Oestreich MC, Sathianathen NJ, Hwang EC, Vernooij RW, Kuntz GM, Scales CD, et al. Alpha-blockers after shock wave lithotripsy for renal or ureteral stones in adults. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013393. [DOI: 10.1002/14651858.CD013393] [DOI] [PMC free article] [PubMed] [Google Scholar]


