Abstract
Background
Human milk alone may provide inadequate amounts of protein to meet the growth requirements of preterm infants because of restrictions in the amount of fluid they can tolerate. It has become common practice to feed preterm infants with breast milk fortified with protein and other nutrients but there is debate about the optimal concentration of protein in commercially available fortifiers.
Objectives
To compare the effects of different protein concentrations in human milk fortifier, fed to preterm infants, on growth and neurodevelopment.
Search methods
We used the standard search strategy of Cochrane Neonatal to search CENTRAL (2019, Issue 8), Ovid MEDLINE and CINAHL on 15 August 2019. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included all published and unpublished randomised, quasi‐randomised and cluster‐randomised trials comparing two different concentrations of protein in human milk fortifier.
We included preterm infants (less than 37 weeks' gestational age). Participants may have been exclusively fed human milk or have been supplemented with formula.
The concentration of protein was classified as low (< 1g protein/100 mL expressed breast milk (EBM)), moderate (≥ 1g to < 1.4g protein/100 mL EBM) or high (≥ 1.4g protein/100 mL EBM). We excluded trials that compared two protein concentrations that fell within the same category.
Data collection and analysis
We undertook data collection and analyses using the standard methods of Cochrane Neonatal. Two review authors independently evaluated trials. Primary outcomes included growth, neurodevelopmental outcome and mortality. Data were synthesised using risk ratios (RR), risk differences and mean differences (MD), with 95% confidence intervals (CI). We used the GRADE approach to assess the certainty of the evidence.
Main results
We identified nine trials involving 861 infants. There is one trial awaiting classification, and nine ongoing trials. The trials were mostly conducted in infants born < 32 weeks' gestational age or < 1500 g birthweight, or both. All used a fortifier derived from bovine milk. Two trials fed infants exclusively with mother's own milk, three trials gave supplementary feeds with donor human milk and four trials supplemented with preterm infant formula. Overall, trials were small but generally at low or unclear risk of bias.
High versus moderate protein concentration of human milk fortifier
There was moderate certainty evidence that a high protein concentration likely increased in‐hospital weight gain compared to moderate concentration of human milk fortifier (MD 0.66 g/kg/day, 95% CI 0.51 to 0.82; trials = 6, participants = 606). The evidence was very uncertain about the effect of high versus moderate protein concentration on length gain (MD 0.01 cm/week, 95% CI –0.01 to 0.03; trials = 5, participants = 547; very low certainty evidence) and head circumference gain (MD 0.00 cm/week, 95% CI –0.01 to 0.02; trials = 5, participants = 549; very low certainty evidence).
Only one trial reported neonatal mortality, with no deaths in either group (participants = 45).
Moderate versus low protein concentration of human milk fortifier
A moderate versus low protein concentration fortifier may increase weight gain (MD 2.08 g/kg/day, 95% CI 0.38 to 3.77; trials = 2, participants = 176; very low certainty evidence) with little to no effect on head circumference gain (MD 0.13 cm/week, 95% CI 0.00 to 0.26; I² = 85%; trials = 3, participants = 217; very low certainty evidence), but the evidence is very uncertain. There was low certainty evidence that a moderate protein concentration may increase length gain (MD 0.09 cm/week, 95% CI 0.05 to 0.14; trials = 3, participants = 217).
Only one trial reported mortality and found no difference between groups (RR 0.48, 95% CI 0.05 to 5.17; participants = 112).
No trials reported long term growth or neurodevelopmental outcomes including cerebral palsy and developmental delay.
Authors' conclusions
Feeding preterm infants with a human milk fortifier containing high amounts of protein (≥ 1.4g/100 mL EBM) compared with a fortifier containing moderate protein concentration (≥ 1 g to < 1.4 g/100 mL EBM) results in small increases in weight gain during the neonatal admission. There may also be small increases in weight and length gain when infants are fed a fortifier containing moderate versus low protein concentration (< 1 g protein/100 mL EBM). The certainty of this evidence is very low to moderate; therefore, results may change when the findings of ongoing studies are available. There is insufficient evidence to assess the impact of protein concentration on adverse effects or long term outcomes such as neurodevelopment. Further trials are needed to determine whether modest increases in weight gain observed with higher protein concentration fortifiers are associated with benefits or harms to long term growth and neurodevelopment.
Plain language summary
Comparison of different protein concentrations of human milk fortifier for promoting growth and neurological development in preterm infants
Review question
Among preterm infants, does the amount of protein used to fortify breast milk feeds result in any difference in growth and neurodevelopmental (development of the brain to improve performance or functioning (e.g. intelligence, reading ability, social skills, memory, attention or focus skills) outcomes?
Background
Breast milk is the best form of nutrition for preterm infants. However, as preterm infants often have difficulties tolerating large amounts of milk, they may not get the recommended amounts of protein from breast milk alone. It has become common clinical practice to fortify breast milk for preterm infants with additional nutrients with a product known as human milk fortifier. Over time the amount (concentration) of protein used in human milk fortifiers has increased, but there is debate about what the optimal protein concentration of human milk fortifier is.
Study characteristics
This review included nine trials involving 861 infants. Six trials compared a high versus moderate concentration of protein in the human milk fortifier, and three trials compared a moderate versus low concentration of protein. Our main outcomes were growth (e.g. weight, length, head circumference), neurodevelopment and death. Reporting was incomplete for all outcomes; most were at low or unclear risk of bias. The search is up to date as of August 2019.
Key results
Feeding preterm infants with a human milk fortifier that contains a high protein concentration versus a moderate protein concentration resulted in small increases in weight gain but not length gain or head growth during hospital admission after birth. There were small increases in weight gain and length gain in infants fed a human milk fortifier that contained moderate concentrations of protein compared with low concentrations. There was no clear effect of protein concentration on infant death during the initial hospitalisation. There were only limited data on other health outcomes, and this evidence suggests the amount of protein in human milk fortifier does not affect the risk of infections or feeding or bowel problems. There were no available trial data about infant growth after hospital discharge, or long term development.
Conclusions
Although there was some evidence that use of human milk fortifiers with a high or moderate protein concentration are associated with small increases in weight gain during hospital stay, there are no data about the impact on growth after the hospital admission or on developmental outcomes. Further well designed trials are needed to determine whether the amount of protein in human milk fortifiers is associated with benefits or harms in the longer term.
Certainty of the evidence
The certainty of this evidence was very low to moderate due to inconsistent results reported by some trials and potential bias related to the way some trials were conducted.
Summary of findings
Background
Description of the condition
Human milk is the optimal nutrition for preterm infants because of its immunological properties, ease of digestion and absorption, and it has been associated with more favourable neurodevelopmental outcomes when compared with infant formula (AAP 2012). However, as preterm infants are often unable to tolerate large fluid volumes initially, feeding these infants with human milk alone (in usual volumes) may not provide adequate amounts of protein, energy and minerals to meet the high needs for growth (Arslanoglu 2019). Infants with insufficient nutrient intake are at increased risk of postnatal growth faltering and impaired neurodevelopment, which is particularly a problem for extremely low birth weight (< 1000 g) infants (Kumar 2017). To address the nutritional needs of preterm infants, it has become a common clinical practice to supplement or fortify breast milk with additional nutrients using commercially available human milk fortifier (Arslanoglu 2019; Brown 2020).
Description of the intervention
Protein is one of the main constituents of human milk fortifiers. Preterm infants require higher levels of protein intake as they grow rapidly (in line with fetal growth trajectories in the last trimester) and have higher nitrogen excretion in urine and faeces and via the skin than term infants (Hay 2010). However, protein needs cannot be considered in isolation since adequate energy must be supplied in order for the protein to be used for anabolic growth. Protein intake and protein energy ratio (grams of protein per 100 kcal) are the main determinants of growth in preterm infants (Koletzko 2014). Despite the routine use of commercial human milk fortifiers, there is evidence that many preterm infants do not meet their needs for protein intake and accrue substantial protein deficits during their hospital admission (Hay 2016; Moro 2015; Radmacher 2017). For this reason, clinical guidelines have changed to recommend an increased protein intake that is based on weight and gestation, ranging between 3.5 g/kg/day and 4.5 g/kg/day (ESPGHAN 2010; Koletzko 2014; Ziegler 2011), particularly in extremely preterm (less than 28 weeks' gestation) or extremely low birth weight infants where the recommended intake is 4 g/kg/day to 4.5 g/kg/day (ESPGHAN 2010). However, evidence about the impact of high intakes of protein on infant health outcomes is lacking. For example, one Cochrane Review of different protein concentrations in infant formula found a protein intake ≥ 3g/kg/day and < 4 g/kg/day was associated with improved weight gain in formula fed low birth weight infants, but there was insufficient evidence to evaluate the impact of formula containing ≥ 4g/kg/day protein or greater protein (Fenton 2020).
The protein in fortifiers is usually derived from bovine (cow) milk, predominantly whey protein, with some fortifiers using hydrolysed protein. The use of hydrolysed protein in formula may increase gastric emptying (Mihatsch 2002), and calcium absorption rate (Picaud 2001), without inducing the risk of feeding intolerance or necrotising enterocolitis (NEC) (Ng 2019). However, it is not known if these effects also apply to hydrolysed protein fortifier. More recently, fortifiers derived from human milk have become available, which allows for a diet that is exclusively human milk based. Bovine protein is thought to induce inflammation (Abdelhamid 2013), increase gut permeability (Taylor 2009), and may even cause death of intestinal cells (Penn 2012).
Regardless of the source and type of proteins used in fortifiers, there is considerable clinical practice variation in how and when fortifiers are administered. The usual regimen of a standard amount of fortifier added to human milk fails to account for individual and temporal variations in the protein concentration in maternal milk (Gidrewicz 2014), and the differing requirements of preterm infants of different gestational ages and weight (ESPGHAN 2010). Individualised regimens are now recommended by some groups (Arslanoglu 2019; ESPGHAN 2010), where either the amount of fortifier is titrated against blood urea nitrogen concentration or adjusted according to the level of nutrients in mother's milk. In addition, several trials have evaluated the timing of fortification, with evidence that starting fortification early rather than delaying is well tolerated but does not improve short term growth of infants (Godden 2019). These different fortification strategies (Fabrizio 2019), and timing of commencement of fortification (Thanigainathan 2019), are the focus of other Cochrane Reviews and consequently, are not addressed in this review.
How the intervention might work
Protein is an essential component of all cells in the body. Protein provides the amino acids required for adequate growth, particularly lean tissue and maturation of multiple organ systems. Growth failure is commonly reported in very low birth weight (< 1500 g) infants and this is thought to be related to inadequate protein intake (Arslanoglu 2019; Kumar 2017). Related Cochrane Reviews have shown that, when compared with unsupplemented human milk, both protein (Amissah 2018), and multi‐nutrient fortifier (Brown 2020), resulted in short term weight gain (protein: mean difference (MD) 3.82 g/kg/day, 95% confidence interval (CI) 2.94 to 4.70; multi‐nutrient fortifier: 1.76 g/kg/day, 95% CI 1.30 to 2.22).
However, increasing protein intake has potential adverse effects. These include metabolic acidosis and high serum levels of protein metabolites, such as urea and ammonia (Goldman 1969; Senterre 1983), high levels of some amino acids (Avery 1967), and increased risk of sepsis (Moltu 2013). However, results from these earlier studies may be due to the poor quality of protein (Hay 2010), and research has shown that adverse effects may be due to other nutritional components (e.g. minerals) (Rochow 2011). Amissah's Cochrane Review showed increased blood urea levels in the protein supplements groups (MD 0.95 mmol/L, 95% CI 0.81 to 1.00) (Amissah 2018). However, these remained within the normal range and may reflect adequate rather than excess protein intake. Nevertheless, there may be risks of feed intolerance and NEC associated with increased protein intake (Amissah 2018), therefore, the benefits of increased protein intake must be balanced with any potential adverse effects.
Why it is important to do this review
There remains considerable debate regarding the optimum protein concentration of fortifiers (Bertino 2017). When human milk fortifier was first introduced, the common practice was to add less than 1 g of protein per 100 mL of human milk due to safety and tolerance concerns. Since then, there has been some evidence of more favourable developmental outcomes with higher protein (with adequate energy intakes) (Coviello 2018; Stephens 2009). The concentration of protein added into human milk has gradually increased over time in many neonatal units, from around 1 g/100 mL of expressed breast milk (EBM), to greater than 1.4 g/100 mL of EBM. However, the upper limit of protein intake, where no further benefit is conferred or an adverse effect may occur (or both), has not been determined. There has been no systematic review assessing the effect of different levels of protein fortification of human milk on growth and safety in preterm infants. Although there is another review comparing human milk‐derived versus bovine milk‐derived human milk fortifier for mortality prevention and subsequent growth and neurodevelopment of preterm infants (Premkumar 2019), we will undertake subgroup analyses specifically comparing the effect of proteins derived from these different sources.
Objectives
To compare the effects of different protein concentrations in human milk fortifier, fed to preterm infants, on growth and neurodevelopment.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised trials, quasi‐randomised trials and cluster‐randomised trials comparing two or more different concentrations of protein in human milk fortifier.
Types of participants
Preterm infants (less than 37 weeks' gestational age) fed any human milk with added human milk fortifier. Infants may also be receiving parenteral nutrition when beginning fortification. Participants may have been exclusively fed human milk or be supplemented with formula.
Types of interventions
The intervention should have compared two or more different concentrations of protein added as a human milk fortifier to EBM. This may have included comparison of two different fortifier products or comparison of the same base fortifier product but with a protein supplement added to increase protein concentration. The fortifier could have been bovine (or other animal) milk based or human milk based and could have included other nutrients including fat and vitamins and minerals. We classified the protein concentrations of the fortifier as:
low: less than 1 g protein/100 mL EBM;
moderate: 1 g to less than 1.4 g protein/100 mL EBM; or
high: 1.4 g, or greater, protein/100 mL EBM.
We excluded trials that compared two different protein concentrations that both fell within the same category (low, moderate or high). Infants in trial comparison groups should have received similar care.
Types of outcome measures
Primary outcomes
-
Growth as assessed at birth (or as defined by study author) to discharge from hospital, at 12 months or 18 months (or both) or beyond corrected age expressed in absolute terms or relative to intrauterine or postnatal growth standard for the following:
weight gain (g/kg/day or g/day);
length gain (cm/week);
head circumference gain (cm/week);
measures of body composition (lean/fat mass);
proportion of infants who were small for gestational age (SGA) (less than 10th percentile of intrauterine growth standards or post‐term growth charts as defined by study author).
-
Neurodevelopmentaloutcomes
-
Neurodevelopmental disability at 18 months' corrected age or greater defined as a neurological abnormality including any one of the following:
cerebral palsy on clinical examination;
developmental delay more than two standard deviations (SD) below population mean on a standardised test of development;
blindness (visual acuity less than 6/60);
deafness (any hearing impairment requiring amplification) at any time after term corrected.
-
Mortality.
Secondary outcomes
-
Safety measures, as reported by study authors, including:
blood urea nitrogen (mmol/L);
plasma amino acid levels (µmol/L);
plasma pH levels;
incidence of NEC (Bell's Stage II or greater);
sepsis (as confirmed by blood culture).
-
Tolerance as assessed by:
episodes of interruption of feeds;
days to reach full enteral feeds (enteral intake 150 mL/kg/day or greater) or as defined by study author.
Length of hospital stay (days).
Search methods for identification of studies
We used the criteria and standard methods of Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register; neonatal.cochrane.org/resources-review-authors). We searched for errata or retractions for included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 8) in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 15 August 2019); and CINAHL (1981 to 15 August 2019). We included the search strategies for each database in Appendix 1. We applied no language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched The World Health Organization's International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the US National Library of Medicine's ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not found through the Cochrane CENTRAL search (www.isrctn.com/).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
We used standard methods of Cochrane Neonatal to extract study information (Higgins 2011a).
Selection of studies
One review author (CG) screened titles and abstracts of all records and a second review author (split between JM, CC, AR) independently reviewed the titles and abstracts. One review author (CG) read the full texts and assessed each article for eligibility for inclusion based on the prespecified review criteria and another review author checked them (of JM, CC and AR). We resolved any disagreements by discussion, or when necessary with a third review author.
Data extraction and management
Two review authors (CG, JM) independently extracted data using a form based on the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC 2017). Where there review authors were authors of included studies (Miller 2012; Reid 2018), another review author performed data extraction (CG).
We extracted the following characteristics from each included study:
administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; presence of vested interest; details of other relevant papers cited;
study: study design; type, duration and completeness of follow‐up (e.g. greater than 80%); country and location of study; informed consent; ethics approval;
participants: sex, birth weight, gestational age, number;
interventions: initiation, dose and duration of administration;
outcomes as mentioned above under Types of outcome measures.
We resolved any disagreements by discussion. We collected information about ongoing studies identified by our search, when available, detailing the primary author, research question(s), methods and outcome measures, together with an estimate of the reporting date.
If the data reported were insufficient or incomplete, we contacted study investigators/authors for clarification. We used Cochrane's statistical software for data entry (Review Manager 2014). We replaced any standard error (SE) of the mean by the corresponding SD.
Assessment of risk of bias in included studies
Two review authors (CG, JM) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2011b), for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or with a third review author. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager 5 (Review Manager 2014). We analysed treatment effects for categorical data using risk ratio (RR) and risk difference (RD). We calculated mean differences (MDs) between treatment groups where outcomes were measured in the same way for continuous data. Where outcomes were measured differently, we reported data as standardised mean differences (SMD). Where trials reported continuous data as median and interquartile range (IQR), we summarised data narratively in the results section. We report 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
All included trials were individually randomised, thus the unit of analysis was the participating infant. All infants were considered only once in the analysis. If future updates of this review include cluster randomised trials we will analyse them using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or from a study with a similar population as described in Section 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dealing with missing data
Where outcome data were missing, we contacted the study investigators. We did not impute any missing data.
Assessment of heterogeneity
We examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. The degree of heterogeneity was graded as: less than 50%, not serious, low heterogeneity; 50% to 75%: serious, substantial heterogeneity; more than 75%: very serious, considerable heterogeneity. Where we detected serious/very serious statistical heterogeneity (I² greater than 50%), we explored the possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments).
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes and reported outcomes, where study protocols were available, we compared these to full publications to determine the likelihood of reporting bias. We were unable to formally assess publication bias by generating funnel plots due to insufficient numbers of studies reporting the same outcome (fewer than 10).
Data synthesis
We performed meta‐analysis using Review Manager 5 (Review Manager 2014). We used a fixed‐effect model to combine data where it was reasonable to assume that studies were estimating the same underlying treatment effect. We used a random‐effects model to combine data where there was substantial level of heterogeneity. Where there was evidence of clinical heterogeneity, we attempted to explain this based on the different study characteristics and planned to perform subgroup analyses where there was sufficient data on subgroups.
Certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook to assess the certainty of evidence of the primary outcomes (Schünemann 2013).
Two review authors (CG, JM) independently assessed the certainty of the evidence for each of the primary outcomes. We considered evidence from randomised controlled trials (RCTs) as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create 'Summary of findings' tables to report the certainty of the evidence for the primary outcomes.
Subgroup analysis and investigation of heterogeneity
We planned to explore high statistical heterogeneity in the outcomes by visually inspecting the forest plots and by removing the outlying studies in the sensitivity analysis (Higgins 2011a). Where statistical heterogeneity was significant, we interpreted the results of the meta‐analyses accordingly; and downgraded the certainty of evidence in the 'Summary of findings' tables, according to the GRADE recommendations.
We planned to consider the following groups for subgroup analysis of the primary outcomes:
less mature infants (defined as less than 1250 g birth weight or less than 28 weeks' gestation);
source of protein (bovine, human, other animal);
use of hydrolysed protein;
the energy content of the two human milk fortifiers compared (isocaloric or non‐isocaloric).
Sensitivity analysis
We planned to conduct sensitivity analyses for primary outcomes to determine if the findings were affected by inclusion of only those trials considered to have used adequate methodology with a low risk of bias (selection and performance bias). We performed a sensitivity analysis for high versus moderate protein comparison including three trials (Maas 2017; Miller 2012; Reid 2018). We were unable to perform a sensitivity analysis for the moderate versus low protein comparison due to only one trial that met the standard to be included in such an analysis (Dogra 2017).
Results
Description of studies
Results of the search
See Figure 1 for the study flow diagram.
1.
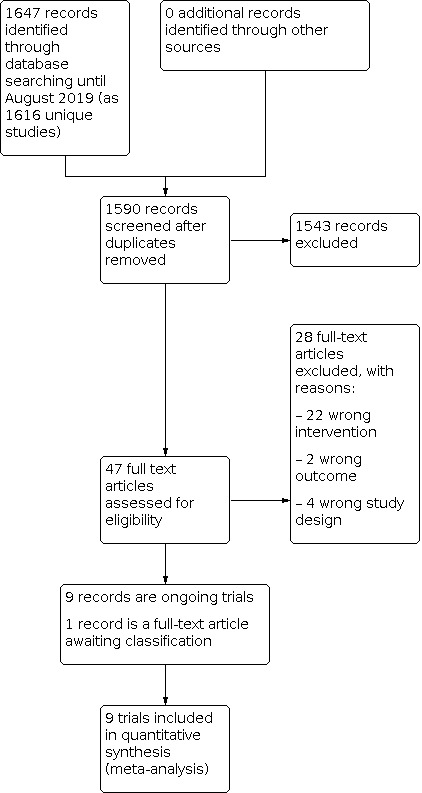
Study flow diagram.
We included nine trials (see Characteristics of included studies table), and excluded 28 full text reports (see Characteristics of excluded studies table).
There is one study awaiting classification due to lack of detail about the protein concentration of the fortifiers compared (see Characteristics of studies awaiting classification table). We identified nine ongoing trials (see Characteristics of ongoing studies table).
Included studies
The nine included trials were conducted in different countries/regions including USA, Europe, Australia and India (Dogra 2017; Kim 2015; Maas 2017; Miller 2012; Moya 2012; Porcelli 2000; Reid 2018; Rigo 2017; Sankaran 1996). In general, the included trials that were undertaken in the late 1990s compared low and moderate protein concentrations, whereas the more recent trials compared moderate to high protein concentrations, reflecting a shift in practice to higher protein fortification.
Participants
In total, 861 preterm infants participated in the included trials, most of whom were born at less than 32 weeks' gestation or with a birth weight less than 1500 g, or both, were appropriate weight for gestational age, and their mothers intended to provide breast milk during the study period. We excluded preterm infants with a congenital or chromosomal abnormality.
Intervention
Six trials compared moderate (≥ 1 g, < 1.4 g/100 mL EBM) versus high (≥ 1.4 g/100 mL EBM) protein concentrations (Kim 2015; Maas 2017; Miller 2012; Moya 2012; Reid 2018; Rigo 2017).
Three trials compared low (< 1 g/100 mL EBM) versus moderate protein concentrations ( ≥ 1 g, < 1.4 g/100 mL EBM) (Dogra 2017; Porcelli 2000; Sankaran 1996).
Human milk fortifiers used in these studies were mostly commercially available and all were bovine milk based, either in liquid or powder form. All fortifiers had additional nutrients, with varying amounts of fat, vitamins, minerals and trace elements. Three trials manipulated the concentration of other macronutrients to ensure the fortifiers were strictly isocaloric (Miller 2012; Reid 2018; Rigo 2017).
Where there were insufficient volumes of mother's own milk to meet enteral feeding requirements, three trials supplemented infants with donor human milk (Kim 2015; Moya 2012; Rigo 2017), and four trials supplemented with preterm infant formula (Dogra 2017; Maas 2017; Miller 2012; Reid 2018). Only two trials fed infants with mother's own milk exclusively (Porcelli 2000; Sankaran 1996).
Outcomes
All trials reported growth parameters until the end of the intervention, which was either the end of study period, or until hospital discharge or 40 weeks' postmenstrual age. The most common time period reported for growth was from when the infant achieved 80 mL/kg/day to 100 mL/kg/day feeding until hospital discharge or estimated delivery date. Other commonly reported outcomes included blood biochemistry (blood urea nitrogen and pH), adverse events (mortality, incidence of NEC and sepsis). No study reported neurodevelopmental outcomes of these infants.
Excluded studies
We excluded 28 studies (Arco 2002; Atchley 2019; Berseth 2004; Biasini 2012; Brumberg 2010; Coscia 2018; Costa‐Orvay 2011; Davies 1975; Ditzenberger 2013; ISRCTN27916681; Kanmaz 2013; Liang 2018; Loui 2004; Lucas 1996; McLeod 2016; Moltu 2013; Morlacchi 2016; Moro 1991; Moyer‐Mileur 1992; Polberger 1989; Quan 2020; Schanler 2018; Strommen 2017; Tan 2008; Thoene 2014; Thoene 2016; Thomaz 2014; Tracy 2015).
See Characteristics of excluded studies for details of exclusion.
Studies awaiting classification
We identified one study as potentially eligible for inclusion (Kashaki 2018). The study administered human milk fortifier without mixing with EBM, instead, as in‐between feeds four to five times a day. Both groups received human milk fortifiers, and the intervention group received an additional protein supplement of 0.6 g/kg/day to 0.8 g/kg/day mixed with EBM to achieve a higher level of intake. We are unable to determine the protein concentration consumed by the infants as grams per 100 mL fortified EBM, and are awaiting a response from the authors to clarify the protein dosage. See Characteristics of studies awaiting classification table.
Ongoing studies
We identified nine ongoing trials. See Characteristics of ongoing studies table.
Risk of bias in included studies
See 'Risk of bias' summary (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven trials used computer generated randomisation schedules and were considered low risk of bias (Dogra 2017; Kim 2015; Maas 2017; Miller 2012; Reid 2018; Rigo 2017; Sankaran 1996); the remaining two trials provided insufficient information about randomisation, and were at unclear risk of bias (Moya 2012; Porcelli 2000).
Five trials were at low risk of bias for allocation concealment (Dogra 2017; Kim 2015; Maas 2017; Miller 2012; Reid 2018). Four trials were at unclear due to insufficient information (Moya 2012; Porcelli 2000; Rigo 2017; Sankaran 1996).
Blinding
Six trials were at low risk of performance and detection bias, as there were adequate methods described for blinding or personnel and outcome assessors (Dogra 2017; Maas 2017; Miller 2012; Reid 2018; Rigo 2017), or a clear rationale for use of single blinding (Porcelli 2000).
Moya 2012 reported that outcome assessors were blinded but it was unclear if carers were blinded; therefore, the trial was rated at unclear risk of bias.
Sankaran 1996 did not provide sufficient information for assessment regarding blinding and was at unclear risk of bias.
Kim 2015 reported the trial was unblinded and was, therefore, at high risk of bias.
Incomplete outcome data
Six trials were at low risk of attrition bias, as all infants were accounted for, and there was similar attrition rate between the comparison groups (Dogra 2017; Kim 2015; Maas 2017; Miller 2012; Reid 2018; Rigo 2017).
Three trials were at unclear risk of attrition bias due to insufficient information provided (Moya 2012; Porcelli 2000; Sankaran 1996). In Sankaran 1996, results from 41 infants (out of 60 infants originally enrolled) were available, whereas Porcelli 2000 reported most outcomes on a per protocol analysis. Moya 2012 reported significant attrition (27% overall, 23.6% for moderate protein group and 31.1% for high protein group) as the trial progressed, classifying the reasons as either 'fortifier related' or 'non‐fortifier related' but with no further details.
Selective reporting
There was insufficient evidence to judge whether there was reporting bias as we did not have access to trial protocols. As a result, all trials were considered unclear risk of reporting bias (Dogra 2017; Kim 2015; Maas 2017; Miller 2012; Moya 2012; Porcelli 2000; Reid 2018; Rigo 2017; Sankaran 1996).
Other potential sources of bias
Five trials were sponsored by the formula industry (Kim 2015; Moya 2012; Porcelli 2000; Rigo 2017; Sankaran 1996). For all these trials, there was no further information reported regarding the role of the sponsor in trial design and conduct, or data analysis and interpretation, or preparation and approval of the manuscripts; therefore, they were at unclear risk of bias. The remaining four trials specified that the human milk fortifier manufacturer donated products for trial but were not involved in any other way and were, therefore, at low risk of bias (Dogra 2017; Maas 2017; Miller 2012; Reid 2018).
Effects of interventions
Summary of findings 1. High compared to moderate protein concentration of human milk fortifier for promoting growth and neurological development in preterm infants.
| High compared to moderate protein concentration of human milk fortifier for promoting growth and neurological development in preterm infants | ||||||
|
Patient or population: promoting growth and neurological development in preterm infants Setting: neonatal intensive care units in Australia, Belgium, France, Germany, Italy, Switzerland, UK, USA Intervention: addition of human milk fortifier Comparison: high (≥ 1.4 g protein/100 mL EBM) vs moderate (≥ 1 g to < 1.4 g protein/100 mL EBM) protein concentration of human milk fortifier | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with moderate protein concentration of human milk fortifier | Risk with high protein concentration of human milk fortifier | |||||
| Weight gain (g/kg/day) until the end of the intervention | Mean weight gain was 16.51 g/kg/day | MD 0.66 g/kg/day higher (0.51 higher to 0.82 higher) | — | 606 (6 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Length gain (cm/week) until the end of the intervention | Mean length gain was 1.11 cm/week | MD 0.01 cm/week higher (0.01 lower to 0.03 higher) | — | 547 (5 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | — |
| Head circumference gain (cm/week) until the end of the intervention | Mean head circumference gain was 1.00 cm/week | MD 0 cm/week higher (0.01 lower to 0.02 higher) | — | 549 (5 RCTs) | ⊕⊝⊝⊝ Very lowc,d | — |
| Cerebral palsy at ≥ 18 months | — | — | — | (0 studies) | — | None of the included studies reported cerebral palsy. |
| Developmental delay at ≥ 18 months | — | — | — | (0 studies) | — | None of the included studies reported developmental delay. |
| Mortality during intervention period | Preterm infants | Not estimable | 45 (1 study) | — | — | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EBM: expressed breast milk; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRisk of bias: three studies were rated low risk of bias overall, two studies provided insufficient details of methodology for assessment and one study was rated high risk of bias for blinding; therefore, the overall evidence was downgraded one level. bInconsistency: evidence was downgraded one level because of substantial heterogeneity (≥ 50%, < 75%). cImprecision: evidence was downgraded two levels due confidence intervals crossing 0, which included the possibility of no beneficial effect and potential harmful effect of the treatment. dRisk of bias: two were rated low risk of bias overall, two studies provided insufficient details of methodology for assessment, and one study was rated high risk of bias for blinding; therefore, the overall evidence was downgraded by one level.
Summary of findings 2. Moderate compared to low protein concentration of human milk fortifier for promoting growth and neurological development in preterm infants.
| Moderate compared to low protein concentration of human milk fortifier for promoting growth and neurological development in preterm infants | ||||||
|
Patient or population: preterm infants Setting: neonatal intensive care units in Canada, India, USA Intervention: addition of human milk fortifier Comparison: moderate (≥ 1 g to < 1.4 g protein/100 mL EBM) versus low (< 1 g protein/100 mL EBM) protein concentration of human milk fortifier | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low protein concentration of human milk fortifier | Risk with moderate protein concentration of human milk fortifier | |||||
| Weight gain (g/kg/day) until the end of the intervention | Mean weight gain was 12.86 g/kg/day | MD 2.08 g/kg/day higher (0.38 higher to 3.77 higher) | — | 176 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | — |
| Length gain (cm/week) until the end of the intervention | Mean length gain was 0.77 cm/week | MD 0.09 cm/week higher (0.05 higher to 0.14 higher) | — | 217 (3 RCTs) | ⊕⊕⊝⊝ Lowd,e | — |
| Head circumference gain (cm/week) until the end of the intervention | Mean head circumference gain was 0.71 cm/week | MD 0.13 cm/week higher (0 to 0.26 higher) | — | 217 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,d,e,f | — |
| Cerebral palsy at ≥ 18 months | — | — | — | (0 studies) | — | None of the included studies reported cerebral palsy. |
| Developmental delay at ≥ 18 months | — | — | — | (0 studies) | — | None of the included studies reported developmental delay. |
| Mortality during the intervention period | Preterm infants | RR 0.48 (0.05 to 5.17) | 112 (1 study) | — | — | |
| 36 per 1000 | 17 per 1000 (2 to 188) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EBM: expressed breast milk; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRisk of bias: one study was rated low risk of bias overall and the other study provided insufficient details of methodology for assessment; therefore, the overall evidence was downgraded one level. bInconsistency: the evidence was downgraded two levels due to substantial heterogeneity (≥ 75%). cImprecision: the evidence was downgraded one level due to wide confidence intervals (10 times difference between lower and upper confidence interval), which includes a wide range of possible beneficial effects. dImprecision: the evidence was downgraded one level as the total sample size across RCTs was fewer than 400. eRisk of bias: one study was rated low risk of bias overall and the other two studies provided insufficient details of methodology for assessment; therefore, the overall evidence was downgraded one level. fImprecision: the evidence was downgraded two levels due to wide confidence intervals that crosses 0, which includes a possibility of no beneficial effect of the treatment.
High (≥ 1.4 g/100 mL EBM) versus moderate (≥ 1 g, < 1.4 g/100 mL EBM) protein concentration
Primary outcomes
1. Growth outcomes
1.1 Weight gain
Six trials including 606 infants reported data for weight gain (Kim 2015; Maas 2017; Miller 2012; Moya 2012; Reid 2018; Rigo 2017). Meta‐analysis showed there was a higher rate of weight gain in infants fed high protein fortifier compared with moderate protein fortifier (MD 0.66 g/kg/day, 95% CI 0.51 to 0.82; I² = 21%; moderate certainty evidence; Analysis 1.1; Figure 3).
1.1. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 1: Weight gain (g/kg/day)
3.

Forest plot of comparison: 1 High versus moderate protein concentration of human milk fortifier, outcome: 1.1 Weight gain (g/kg/day).
1.2 Length gain
Five trials including 547 infants reported length gain (Kim 2015; Miller 2012; Moya 2012; Reid 2018; Rigo 2017). Meta‐analysis showed no difference in length gain between infants fed a high protein fortifier compared with a moderate protein fortifier (MD 0.01 cm/week, 95% CI –0.01 to 0.03; I² = 53%; very low certainty evidence; Analysis 1.2; Figure 4).
1.2. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 2: Length gain (cm/week)
4.

Forest plot of comparison: 1 High versus moderate protein concentration of human milk fortifier, outcome: 1.2 Length gain (cm/week).
1.3 Head circumference gain
Five trials including 549 infants contributed data (Kim 2015; Miller 2012; Moya 2012; Reid 2018; Rigo 2017). Meta‐analysis showed there was no effect of protein concentration on head circumference gain (MD 0 cm/week, 95% CI –0.01 to 0.02; I² = 0%; very low certainty evidence; Analysis 1.3; Figure 5).
1.3. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 3: Head circumference gain (cm/week)
5.

Forest plot of comparison: 1 High versus moderate protein concentration of human milk fortifier, outcome: 1.3 Head circumference gain (cm/week).
1.4 Measures of body composition
No trials reported body composition data.
1.5 Proportion of infants who were small for gestational age
Miller 2012 reported the proportion of infants classified SGA at the end of study period, based on weight, length and head circumference measurements (where available). For weight, 15/35 (43%) infants in the high and 17/35 (49%) in the moderate protein group were SGA (P = 0.84). For length, 21/49 (43%) infants in the high and 31/63 (49%) in the moderate protein group were SGA (P = 0.047). For head circumference, 8/19 (42%) infants in the high and 11/22 (50%) infants in the moderate protein group were SGA (P = 0.66).
2. Neurodevelopmental outcomes
No trials reported on neurodevelopmental outcomes including cerebral palsy, developmental delay, blindness and deafness.
3. Mortality
Maas 2017 reported no deaths in either the high or moderate protein groups.
Four other trials did not specifically report death, but all infants were accounted for in the trial follow‐up period (Kim 2015; Miller 2012; Reid 2018; Rigo 2017).
Secondary outcomes
1. Safety measures
1.1 Blood urea nitrogen
Three trials including 230 infants monitored blood urea nitrogen regularly during the study period (Kim 2015; Miller 2012; Reid 2018). The meta‐analysis was based on the last measurement taken before the trial concluded and showed that infants fed a high protein fortifier had a higher blood urea nitrogen concentration compared with infants fed a moderate protein fortifier (MD 1.25 mmol/L, 95% CI 1.19 to 1.32; I² = 0%; Analysis 1.6).
1.6. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 6: Blood urea nitrogen (mmol/L)
1.2 Plasma amino acid levels
No trials reported plasma amino acid levels.
1.3 Plasma pH levels
One study reported no difference in plasma pH levels between groups (mean 7.35, SD 0.03 for high protein group versus 7.35, SD 0.04 for moderate protein groups; P = 0.8) (Miller 2012).
1.4 Incidence of necrotising enterocolitis
Four trials including 326 infants reported on NEC (Kim 2015; Maas 2017; Miller 2012; Reid 2018). Meta‐analysis showed there was no difference in the risk of NEC between infants fed a higher versus lower protein fortifier (RR 0.78, 95% CI 0.27 to 2.27; I² = 0%; Analysis 1.8).
1.8. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 8: Incidence of necrotising enterocolitis
1.5 Sepsis
Four trials including 326 infants reported on sepsis (Kim 2015; Maas 2017; Miller 2012; Reid 2018). Meta‐analysis showed there was no difference in the risk of sepsis between infants fed a higher versus lower protein fortifier (RR 1.18, 95% CI 0.51 to 2.73; I² = 0%; Analysis 1.9).
1.9. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 9: Sepsis
2. Tolerance
2.1 Episodes of interruption of feeds
One trial reported a significantly higher rate of feed interruptions in the high protein group (n = 11, 35%) compared with moderate protein (n = 6, 20%) (P = 0.01) (Reid 2018).
2.2 Days to reach full enteral feed
Two trials including 162 infants reported days to reach full enteral feed (Miller 2012; Reid 2018). Meta‐analysis showed there was no difference in the number of days to reach full enteral feeds between infants fed a higher versus moderate protein fortifier (MD 0.26 days, 95% CI –1.78 to 2.31; I² = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 11: Days to reach full enteral feeds
3. Length of hospital stay
One trial reported length of hospital stay and found no difference between groups (mean: 77 days (SD 21) in high protein group versus 73 days (SD 16) in moderate protein group; P = 0.06) (Miller 2012).
High (≥ 1.4 g/100 mL EBM) versus low (< 1 g/100 mL EBM) protein concentration
No trials compared a high versus low protein concentration fortifier.
Moderate (≥ 1 g, < 1.4 g/100 mL EBM) versus low (< 1 g/100 mL EBM) protein concentration
Primary outcomes
1. Growth outcomes
1.1 Weight gain
Two trials including 176 infants reported weight gain (Dogra 2017; Porcelli 2000). Meta‐analysis showed there was a higher rate of weight gain in infants fed a moderate protein fortifier compared with low protein fortifier (MD 2.08 g/kg/day, 95% CI 0.38 to 3.77; I² = 91%; very low certainty evidence; Analysis 2.1; Figure 6).
2.1. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 1: Weight gain (g/kg/day)
6.

Forest plot of comparison: 2 Moderate versus low protein concentration of human milk fortifier, outcome: 2.1 Weight gain (g/kg/day).
1.2 Length gain
Three trials involving 217 infants reported length gain (Dogra 2017; Porcelli 2000; Sankaran 1996). Meta‐analysis showed there was a higher rate of length gain in infants fed a moderate protein fortifier compared with low protein fortifier (MD 0.09 cm/week, 95% CI 0.05 to 0.14; I² = 0%; low certainty evidence; Analysis 2.2; Figure 7).
2.2. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 2: Length gain (cm/week)
7.

Forest plot of comparison: 2 Moderate versus low protein concentration of human milk fortifier, outcome: 2.2 Length gain (cm/week).
1.3 Head circumference gain
Three trials including 217 infants reported head circumference gain (Dogra 2017; Porcelli 2000; Sankaran 1996). Meta‐analysis showed there was no difference in head circumference gain in infants fed a moderate protein fortifier versus low protein fortifier (MD 0.13 cm/week, 95% CI 0 to 0.26; I² = 85%; very low certainty evidence; Analysis 2.3; Figure 8).
2.3. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 3: Head circumference gain (cm/week)
8.

Forest plot of comparison: 2 Moderate versus low protein concentration of human milk fortifier, outcome: 2.3 Head circumference gain (cm/week).
1.4 Measures of body composition
No trials reported data on body composition.
1.5 Proportion of infants who were small for gestational age
No trials reported data on SGA for weight, length or head circumference.
2. Neurodevelopmental outcomes
No trials reported on neurodevelopmental outcomes including cerebral palsy, developmental delay, blindness and deafness.
3. Mortality
One trial reported neonatal death, with no difference observed between groups (1/57 in moderate protein group versus 2/55 in low protein group; P = 1.00) (Dogra 2017).
Sankaran 1996 did not report death specifically, but all infants were accounted for in the trial follow‐up period.
Secondary outcomes
1. Safety measures
1.1 Blood urea nitrogen
Two trials including 165 infants reported blood urea nitrogen measurements either at the end of study (Porcelli 2000), or at hospital discharge (Dogra 2017). Meta‐analyses showed there was no difference in blood urea concentrations between infants fed a moderate protein fortifier versus low protein fortifier (MD 0.44 mmol/L, 95% CI –0.16 to 1.03; I² = 97%; Analysis 2.5).
2.5. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 5: Blood urea nitrogen (mmol/L)
1.2 Plasma amino acid levels
No trials reported data for plasma amino acid levels.
1.3 Plasma pH levels
No trials reported data for plasma pH levels.
1.4 Incidence of necrotising enterocolitis
One trial reported on NEC, and found no difference between groups (1/57 in moderate protein group versus 1/55 in low protein group; P = 1.00) (Dogra 2017).
1.5 Sepsis
One trial reported on sepsis, and found no differences between groups (1/57 in moderate protein group versus 2/55 in low protein group; P = 0.68) (Dogra 2017).
2. Tolerance
2.1 Episodes of interruption of feeds
No trials reported episodes of interruption of feeds.
2.2 Days to reach full enteral feed
One trial reported median days to reach full enteral feed, with no difference between groups (median 9 days (IQR 5 to 11); n = 57 in moderate protein group versus 8 days (IQR 5 to 11); n = 55 in low protein group; P = 0.37) (Dogra 2017).
3. Length of hospital stay
One trial reported length of hospital stay, with no difference between groups (median 29.2 days (IQR 22 to 45); n = 57 in moderate protein group versus 29.5 days (IQR 20 to 43); n = 55 in low protein group; P = 0.89) (Dogra 2017).
Subgroup analysis – birth weight < 1000 g
One trial reported growth outcomes among infants with a birth weight less than 1000 g fed a high versus moderate protein concentration fortifier; therefore, meta‐analyses could not be performed (Rigo 2017). Rigo 2017 showed no significant difference between the two groups in length gain (1.07 cm/week (SD 0.52); n = 19 in high protein group versus 1.27 cm/week (SD 0.52); n = 21 in low protein group; P = 0.563) or head circumference gain (1.04 cm/week (SD 0.34); n = 19 in high protein group versus 0.94 cm/week (SD 0.28); n = 21 in low protein group; P = 0.223).
Subgroup analysis – energy content
Three trials compared fortifiers that were designed to be strictly isocaloric (e.g. the same energy content in both groups) (Miller 2012; Reid 2018; Rigo 2017). However, in other trials, there were relatively small differences in the energy content of the two human milk fortifiers compared, especially when the fortifier was further diluted with EBM. Therefore, we also considered trials to be isocaloric when there was less than a 5% difference in the total energy content between comparison groups when the fortifiers were mixed with EBM (assuming 85 kcal/100 mL unless otherwise stated by the study authors). Based on this, seven trials compared fortifiers that were classified as isocaloric (Dogra 2017; Maas 2017; Miller 2012; Moya 2012; Porcelli 2000; Reid 2018; Rigo 2017), and two were non‐isocaloric (Kim 2015; Sankaran 1996).
High versus moderate protein concentration
In this comparison, five trials compared fortifiers that were isocaloric (Maas 2017; Miller 2012; Moya 2012; Reid 2018; Rigo 2017), and one compared non‐isocaloric fortifiers (Kim 2015). Meta‐analyses showed a significant subgroup effect for the outcome of length gain (Chi² = 4.14, degree of freedom (df) = 1, P = 0.04, I² = 75.8%; Analysis 3.2; Figure 9). In the subgroup of trials that compared isocaloric fortifiers, meta‐analysis showed there was a higher rate of length gain in infants fed a high versus moderate protein fortifier (MD 0.05 cm/week, 95% CI 0.01 to 0.09; I² = 30%; studies = 4; participants = 418; Analysis 3.2; Figure 9), whereas there was no difference in length gain in the one trial that compared fortifiers that were non‐isocaloric (MD 0.00 cm/week, 95% CI –0.02 to 0.02; participants = 129). There were no subgroup differences by energy content for the outcomes of weight gain (Analysis 3.1) or head circumference gain (Analysis 3.3).
3.2. Analysis.

Comparison 3: Subgroup analysis – calorie content, Outcome 2: Length gain (cm/week) – high vs moderate
9.

Forest plot of comparison: 4 Subgroup analysis – calorie content, outcome: 4.2 Length gain (cm/week) – high versus moderate.
3.1. Analysis.

Comparison 3: Subgroup analysis – calorie content, Outcome 1: Weight gain (g/kg/day) – high vs moderate
3.3. Analysis.

Comparison 3: Subgroup analysis – calorie content, Outcome 3: Head circumference gain (cm/week) – high vs moderate
Moderate versus low protein concentration
In this comparison, two trials reporting on weight gain compared fortifiers that were isocaloric, thus subgroup analyses could not be performed (Analysis 3.4) (Dogra 2017; Porcelli 2000). There were no subgroup differences by energy content for the outcomes length gain (Analysis 3.5), or head circumference gain (Analysis 3.6).
3.4. Analysis.

Comparison 3: Subgroup analysis – calorie content, Outcome 4: Weight gain (g/kg/day) – moderate vs low
3.5. Analysis.

Comparison 3: Subgroup analysis – calorie content, Outcome 5: Length gain (cm/week) – moderate vs low
3.6. Analysis.

Comparison 3: Subgroup analysis – calorie content, Outcome 6: Head circumference gain (cm/week) – moderate vs low
Subgroup analysis – use of hydrolysed protein
We were unable to perform subgroup analysis using hydrolysed protein due to the lack of trials that used intact protein in both groups. Four trials compared fortifiers that both used hydrolysed protein, either extensively or partially hydrolysed (Maas 2017; Miller 2012; Reid 2018; Rigo 2017). Two trials specifically compared an intact and a hydrolysed protein human milk fortifier, making it difficult to separate out the effects of protein concentration from use of hydrolysed protein (Kim 2015; Moya 2012). Three studies did not report sufficient detail about the use of hydrolysed or intact protein (Dogra 2017; Porcelli 2000; Sankaran 1996).
Subgroup analysis – source of protein
We were unable to perform subgroup analysis by the source of protein as all fortifiers in the included trials were derived from bovine milk.
Sensitivity analysis
For the comparison of high versus moderate protein concentration, three trials were at low risk of bias and included in the sensitivity analysis (Maas 2017; Miller 2012; Reid 2018). All three reported weight gain, and meta‐analyses showed no difference between groups (MD 0.09 g/kg/day, 95% CI –0.52 to 0.70; I² = 0%; participants = 200; Analysis 4.1). Two of these trials reported length gain and head circumference gain, and meta‐analyses showed no difference in either outcome between comparison groups (length gain: MD 0.04 cm/week, 95% CI –0.01 to 0.08; I² = 38%; participants = 152; Analysis 4.2; head circumference gain: MD –0.01 cm/week, 95% CI –0.05 to 0.04; I² = 0%; participants = 152; Analysis 4.3) (Miller 2012; Reid 2018).
4.1. Analysis.

Comparison 4: Sensitivity analysis, Outcome 1: Weight gain (g/kg/day) – high vs moderate
4.2. Analysis.

Comparison 4: Sensitivity analysis, Outcome 2: Length gain (cm/week) high vs moderate
4.3. Analysis.

Comparison 4: Sensitivity analysis, Outcome 3: Head circumference gain (cm/week) high vs moderate
We were unable to perform sensitivity analysis for trials that compared the effect of moderate versus low protein concentration as there was only one trial considered low risk of bias for allocation concealment and randomisation (Dogra 2017).
Discussion
Summary of main results
We included nine trials involving 861 preterm infants. The available evidence suggests there is a small but statistically significant increase in weight gain among preterm infants fed a high protein fortifier when compared with a moderate protein fortifier. There was no overall effect on length gain; however, in the subgroup analysed on energy content, there were subgroup differences, with a significant increase in length gain identified only in trials that compared fortifiers with the same or similar energy content. Small increases in weight gain and length gain were also observed in infants fed a moderate protein fortifier compared with a low protein fortifier. The level of protein in the fortifier had no effect on head circumference gain.
No trials reported on measures of quality of growth (e.g. body composition), growth after hospital discharge or long term neurodevelopmental outcomes, and there were insufficient data to examine any effect on neonatal mortality.
Blood urea nitrogen concentrations were generally higher in infants fed a high or moderate protein concentration fortifier but all reported blood urea nitrogen values were within the normal range. There was insufficient evidence to examine the effect of protein concentration on other biochemical outcomes, including plasma pH and plasma amino acid concentrations. There was no evidence of an effect of protein concentration on any other clinical outcomes including sepsis, days to reach full enteral feeds, feed intolerance or NEC, or on length of stay of the initial neonatal admission.
Overall completeness and applicability of evidence
While the meta‐analyses showed statistically significant effects that suggested a higher protein fortifier is associated with increased growth rates, these findings should be interpreted and applied with caution as the effect sizes are small. Based on moderate certainty evidence, the use of a high protein fortifier rather than a moderate protein fortifier, over a 12‐week admission to the neonatal unit, would result in 55 g/kg difference in weight gain. The effects were greater in the moderate to low protein comparison, namely 175 g/kg difference in weight gain and 1.08 cm/kg difference in length gain; however, the certainty of the evidence for this comparison was low to very low. In addition, the relevance of the moderate versus low comparison to contemporary clinical practice is unclear as manufacturers of commercially available fortifiers have tended to increase their protein concentration in recent years.
For some growth outcomes, the meta‐analysis showed moderate to high levels of heterogeneity. Due to the small number of trials overall, there was limited ability to explore possible sources of heterogeneity in sensitivity and subgroup analyses. Nevertheless, for the high versus moderate protein comparison, the small improvements in weight gain seen overall were not present in meta‐analysis of the trials rated at low risk of bias, suggesting differences in trial design may be contributing to heterogeneity. Sensitivity analyses could not be undertaken for the moderate to low protein fortifier comparisons as only one trial was rated at low risk of bias. However, data reported in Porcelli 2000 were based on a per protocol analysis, which may explain the greater effect size observed and contribute to heterogeneity.
Participants in the included trials were broadly similar, mostly preterm infants born less than 32 weeks or of low birth weight, or both. We found no major differences in the subgroup analyses undertaken to explore the effect of different energy content. The exception was length gain, where a small but significant increase in length gain was observed in trials that compared isocaloric high versus moderate protein fortifiers, suggesting that additional energy may contribute to the higher length gain in infants fed a high protein fortifier. However, this should be interpreted with caution, as there was high heterogeneity in this subgroup, and very few trials included in this review compared a non‐isocaloric intervention. In addition, the clinical relevance of modest differences in length gain is unclear. Subgroup analyses based on source of protein (human versus bovine) and use of hydrolysed protein could not be undertaken.
It is possible that the high heterogeneity seen in the moderate versus low protein comparison may reflect different supplementary feeding practices, as some of the trials gave infants complementary feeds with infant formula whereas in others infants were exclusively fed mother's own milk. Trials in this comparison were also conducted almost two decades apart, therefore it is possible that other changes in clinical practice such as the introduction of probiotics into feeding regimens may also have contributed to heterogeneity. Four trials specifically excluded infants given probiotics or other interventions known to influence growth (e.g. steroids), or both (Kim 2015; Moya 2012; Porcelli 2000; Rigo 2017), whereas the extent of these practices was unclear in the remaining trials.
We found no trials that compared high protein versus low protein concentration. This is unlikely to be addressed in new trials, as current clinical practice has shifted towards higher protein fortification due to concerns about growth. This is reflected in the trials included in this review, where in general, trials comparing moderate to low protein concentrations fortifiers were the oldest, whereas more recent studies published since the 2010s compared higher to moderate concentrations. Notably, only one trial was undertaken in a low/middle income setting (Dogra 2017), thus the review findings may not be generalisable to clinical practice outside of high income settings where the burden of preterm birth lies.
The clinical significance of the small increases in growth rates during the neonatal admission is not clear, as none of the trials reported on long term growth or development of the preterm infants.
Quality of the evidence
The overall certainty of evidence of primary outcomes was moderate, low or very low (Table 1; Table 2). The methodological approach was mostly well described, and the very low certainty of evidence was mainly driven by considerable levels of heterogeneity and imprecision due to wide CI or CI that included zero, which includes an indicator of potential harmful effects. The certainty of evidence was further downgraded if the number of total participants included for the outcome was fewer than 400. We did not grade the overall certainty of evidence of secondary outcomes due to small numbers of trials and infrequent reporting of these outcomes.
Potential biases in the review process
Potential bias during study screening and selection and data extraction was minimised by at least two review authors working independently. However, there was an insufficient number of studies to assess reporting bias by generating funnel plots. As manufacturers of the fortifiers sponsored many of the trials in this review, it is possible that trials that did not find favourable results for one particular concentration of fortifier have not been published. We attempted to minimise the risk of publication bias by searching reference lists and clinical trial registries to identify trials that have not yet been published. However, it is possible that trials that did not report statistically significant differences have not been published and therefore not included in this review.
We classified trials into the low, moderate or high protein groups based on the protein concentration added to EBM. We did this to overcome difficulties ascertaining protein intake specifically from the fortifier, as trials often only report total protein intake based on the fortifier concentration plus an assumed protein concentration of EBM. This is problematic as there can be significant variation in the protein concentration of breast milk between mothers and by postnatal age (Gidrewicz 2014). Therefore, to minimise bias associated with misclassification of exposure to added protein, we compared the protein concentration of the fortifier as reported by the manufacturer (often reported as numbers of packs per volume of human milk).
The protein concentrations classified as low, moderate and high were designed to capture changes in fortification practices over time. When the review was first prepared in 2008, we considered fortifiers with less than 1 g of added protein per 100 mL EBM to be low and anything above this was considered high protein concentration, reflecting updated clinical guidelines recommending increased protein intake (e.g. ESPGHAN 2010). However, in more recently, several human milk fortifiers containing 1.4 g to 1.8 g added protein per 100 mL EBM have been developed and tested (e.g. see Maas 2017; Reid 2018; Rigo 2017). To permit examination of the impact of more recent changes in practice, in this version of the review we created another category by separating the high concentration group into two groups (moderate and high). Therefore, the classifications reflect a pragmatic approach and are not based on specific cut‐offs postulated to be associated with benefits or harms.
Agreements and disagreements with other studies or reviews
We found one systematic review and meta‐analysis that compared growth outcomes of preterm infants fed with human milk fortifier containing higher‐than‐standard versus standard (0.7 g to 1.1 g added protein/100 mL EBM) concentrations of protein (Liu 2015). The review included five studies (both trials and observational studies) involving 352 preterm infants and found improved weight gain (MD 1.77 g/kg/day, 95% CI 0.81 to 2.73; P = 0.0003; I² = 29%), length gain (MD 0.21 cm/week, 95% CI 0.12 to 0.29; P < 0.0001; I² = 0%) and head circumference gain (MD 0.19 cm/week, 95% CI 0.07 to 0.31; P = 0.002; I² = 56%) in infants fed a higher protein concentration fortifier. The reported effect sizes were generally higher than those reported in our review. This may reflect differences in the categorisation of fortifier concentration, with the review by Liu and colleagues grouping moderate and high protein concentration fortifiers in the same category. In addition, the "standard" fortifier category in that review included concentrations of protein that would now be considered low and thus their findings may have less relevance to current clinical practice.
The findings of our review are broadly consistent with other Cochrane Reviews examining the effects of protein for promoting growth in preterm infants, with short‐term weight gain reported in meta‐analyses of infants fed with higher versus lower protein concentration infant formula (Fenton 2020) or multi‐nutrient fortified versus unfortified human milk (Brown 2020), and among those fed with human milk supplemented with additional protein (Amissah 2018).
Authors' conclusions
Implications for practice.
Very low to moderate certainty evidence suggests feeding preterm infants with human milk fortifier containing higher protein concentration results in small increases in weight gain, and may increase length gain during the neonatal admission. The concentration of protein in fortifiers was not associated with any clear differences in head growth, or neonatal complications such as feed intolerance and sepsis, although few trials reported these outcomes. There is insufficient evidence about the possible effect of protein concentration on adverse outcomes and long term growth or neurological development.
Implications for research.
There is considerable uncertainty about the relevance of small increases in short term growth among preterm infants for longer term growth and developmental outcomes. Although there did not appear to be any adverse effects associated with a higher protein concentration fortifier, existing trials were underpowered to assess the effects of protein concentration on important outcomes including necrotising enterocolitis. As fortifier is routinely used in clinical practice in many neonatal units, further trials are warranted to determine whether higher protein concentration fortifier is indeed beneficial for longer term health outcomes. The nine ongoing trials identified during the review process may include up to 1038 preterm infants from both low and high income settings. Some trials have already concluded active enrolment. The majority of these ongoing trials are designed to determine the effect of different protein concentrations on short term growth outcomes, with some planned follow‐up to assess neurodevelopmental outcomes at 24 or 40 months' corrected age. Future trials should be powered to assess long term growth, neurodevelopmental and cardiometabolic outcomes. These trials should also be conducted outside of high income settings.
History
Protocol first published: Issue 2, 2008 Review first published: Issue 11, 2020
| Date | Event | Description |
|---|---|---|
| 8 November 2019 | Amended | Protocol update |
Acknowledgements
We would like to thank members of Cochrane Neonatal, including: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐ordinating Editor and Bill McGuire, Co‐ordinating Editor, who provided editorial and administrative support, as well as Carol Friesen, Information Specialist, who designed the literature searches.
We thank Bill McGuire, Eugene Dempsey and Tanis Fenton who peer reviewed and provided feedback on this review.
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
We created the randomised controlled trial filters using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2011a). The Cochrane Neonatal Information Specialist created and tested neonatal filters.
Cochrane CENTRAL via CRS Web
Date searched: 15 August 2019 Terms: 1 MESH DESCRIPTOR Milk, Human EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Milk Ejection EXPLODE ALL AND CENTRAL:TARGET 3 MESH DESCRIPTOR Breast Milk Expression EXPLODE ALL AND CENTRAL:TARGET 4 ((human or breast* or mother* or expressed or maternal or donor*) and milk*) AND CENTRAL:TARGET 5 breastmilk* AND CENTRAL:TARGET 6 #1 OR #2 OR #3 OR #4 OR #5 7 MESH DESCRIPTOR Dietary Proteins EXPLODE ALL AND CENTRAL:TARGET 8 protein* AND CENTRAL:TARGET 9 #8 OR #7 10 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 11 infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 12 #11 OR #10 AND CENTRAL:TARGET 13 #6 AND #9 AND #12
MEDLINE via Ovid
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R). Date ranges: 1946 to 15 August 2019 Terms: 1. exp Milk, Human/ 2. exp Milk Ejection/ 3. exp Breast Milk Expression/ 4. ((human or breast* or mother* or expressed or maternal or donor*) and milk*).mp. 5. breastmilk*.mp. 6. 1 or 2 or 3 or 4 or 5 7. exp Dietary Proteins/ 8. protein*.mp. 9. 7 or 8 10. exp infant, newborn/ 11. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab. 12. 10 or 11 13. randomized controlled trial.pt. 14. controlled clinical trial.pt. 15. randomized.ab. 16. placebo.ab. 17. drug therapy.fs. 18. randomly.ab. 19. trial.ab. 20. groups.ab. 21. or/13‐20 22. exp animals/ not humans.sh. 23. 21 not 22 24. 12 and 23 25. 6 and 9 and 24
MEDLINE via PubMed
Date ranges: 1 August 2018 to 15 August 2019 Terms: ((("Milk, Human"[Mesh] OR "Milk Ejection"[Mesh] OR "Breast Milk Expression"[Mesh] OR ((human OR breast* OR mother* OR expressed OR maternal OR donor*) AND milk*) OR breastmilk*))) AND ("Dietary Proteins"[Mesh] OR protein*)) AND (((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR neonat*[TIAB]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))) Filters activated: Publication date from 2018/08/01
CINAHL via EBSCOhost
Date ranges: 1981 to 15 August 2019 Terms: (((human OR breast* OR mother* OR expressed OR maternal OR donor*) AND milk*) OR breastmilk* OR “milk ejection”) AND protein* AND (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
ISRCTN.com
Search terms: milk AND Interventions: Protein* AND Participant age range: Neonate
Appendix 2. Risk of bias
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (less than 20% missing data);
high risk (20% missing data or greater); or
unclear risk.
6. Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could have put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could have put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through sensitivity analyses.
Data and analyses
Comparison 1. High versus moderate protein concentration of human milk fortifier.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Weight gain (g/kg/day) | 6 | 606 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [0.51, 0.82] |
| 1.2 Length gain (cm/week) | 5 | 547 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 1.3 Head circumference gain (cm/week) | 5 | 549 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 1.4 Proportion of infants who were small for gestational age | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.57, 1.76] |
| 1.5 Mortality | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.6 Blood urea nitrogen (mmol/L) | 3 | 230 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [1.19, 1.32] |
| 1.7 Plasma pH levels | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 1.8 Incidence of necrotising enterocolitis | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.27, 2.27] |
| 1.9 Sepsis | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.51, 2.73] |
| 1.10 Episodes of interruption of feeds | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.73, 4.04] |
| 1.11 Days to reach full enteral feeds | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐1.78, 2.31] |
| 1.12 Length of hospital stay (days) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 4.00 [‐3.71, 11.71] |
1.4. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 4: Proportion of infants who were small for gestational age
1.5. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 5: Mortality
1.7. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 7: Plasma pH levels
1.10. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 10: Episodes of interruption of feeds
1.12. Analysis.

Comparison 1: High versus moderate protein concentration of human milk fortifier, Outcome 12: Length of hospital stay (days)
Comparison 2. Moderate versus low protein concentration of human milk fortifier.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Weight gain (g/kg/day) | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 2.08 [0.38, 3.77] |
| 2.2 Length gain (cm/week) | 3 | 217 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.05, 0.14] |
| 2.3 Head circumference gain (cm/week) | 3 | 217 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.00, 0.26] |
| 2.4 Mortality | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.17] |
| 2.5 Blood urea nitrogen (mmol/L) | 2 | 165 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.16, 1.03] |
| 2.6 Incidence of necrotising enterocolitis | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 15.05] |
| 2.7 Sepsis | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.17] |
| 2.8 Length of hospital stay (days) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐7.13, 1.13] |
2.4. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 4: Mortality
2.6. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 6: Incidence of necrotising enterocolitis
2.7. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 7: Sepsis
2.8. Analysis.

Comparison 2: Moderate versus low protein concentration of human milk fortifier, Outcome 8: Length of hospital stay (days)
Comparison 3. Subgroup analysis – calorie content.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Weight gain (g/kg/day) – high vs moderate | 6 | 606 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [0.51, 0.82] |
| 3.1.1 Isocaloric | 5 | 477 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.19, 0.83] |
| 3.1.2 Non‐isocaloric | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.54, 0.86] |
| 3.2 Length gain (cm/week) – high vs moderate | 5 | 547 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 3.2.1 Isocaloric | 4 | 418 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 3.2.2 Non‐isocaloric | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 3.3 Head circumference gain (cm/week) – high vs moderate | 5 | 549 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 3.3.1 Isocaloric | 4 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.03, 0.04] |
| 3.3.2 Non‐isocaloric | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 3.4 Weight gain (g/kg/day) – moderate vs low | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [2.10, 2.94] |
| 3.4.1 Isocaloric | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [2.10, 2.94] |
| 3.4.2 Non‐isocaloric | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.5 Length gain (cm/week) – moderate vs low | 3 | 217 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.05, 0.14] |
| 3.5.1 Isocaloric | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.05, 0.14] |
| 3.5.2 Non‐isocaloric | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.89, 2.49] |
| 3.6 Head circumference gain (cm/week) – moderate vs low | 3 | 217 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.10, 0.18] |
| 3.6.1 Isocaloric | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.10, 0.18] |
| 3.6.2 Non‐isocaloric | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.86, 2.46] |
Comparison 4. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Weight gain (g/kg/day) – high vs moderate | 3 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.52, 0.70] |
| 4.2 Length gain (cm/week) high vs moderate | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.08] |
| 4.3 Head circumference gain (cm/week) high vs moderate | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dogra 2017.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: neonatal intensive care unit of a tertiary hospital in northern India Timing: October 2012 to April 2014 |
|
| Interventions |
Intervention: bovine based human milk fortifier containing 1 g hydrolysed protein added to 100 mL EBM (n = 57) Control: bovine based human milk fortifier containing 0.4 g protein (not reported whether hydrolysed) added to 100 mL EBM (n = 55) Calorie content: isocaloric, providing 17.2 kcal (intervention) vs 13 kcal (control) energy added to 100 mL EBM (102.2 kcal vs 98 kcal in fortified EBM, 4% difference) Start of intervention: when infants tolerating feed volume of 100 mL/kg/day. Mean age at study entry: 6.7 days (SD 4.7) in intervention group and 6.49 days (SD 4.6) in control group End of intervention: when infants tolerating feed volume of 180 mL/kg/day |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Conflict of interest: none declared Source of funding: Nestec, Switzerland donated the human milk fortifier. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence numbers with a block size of 4 were generated by an independent researcher. |
| Allocation concealment (selection bias) | Low risk | The sequence was kept in serially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treating neonatologist, research personnel and families of the enrolled infants were unaware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treating neonatologist and research personnel were unaware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were accounted for, analyses were performed for all available infants at each time point. Attrition rate was 5% for intervention group and 8.3% for control group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | Trial product was donated by manufacturer, who was not further involved in the trial. |
Kim 2015.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multi‐centre, stratification by infant sex and birth weight | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: 14 neonatal intensive care units from across the USA Study date: not reported |
|
| Interventions |
Intervention: bovine based liquid human milk fortifier containing 1.66 g extensively hydrolysed protein added to 100 mL EBM (n = 66) Control: bovine based human milk fortifier containing 1 g intact protein added to 100 mL EBM (n = 63) Calorie content: non‐isocaloric, providing 30 kcal (intervention) vs 14 kcal (control) energy added to 100 mL EBM (110 kcal vs 99 kcal in fortified EBM, 11% difference) Start of intervention: within 72 hours after the infant had reached an intake of ≥ 100 mL/kg/day of human milk Mean age at study day 1: 12.8 days (SE 0.6) in intervention group and 12.3 days (SE 0.7) in control group End of intervention: 29 days or hospital discharge |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Conflict of interest: JHK is on the speakers' bureaus for Abbott Nutrition, Mead Johnson Nutrition, Nestle Nutrition, Nutricia and Medela. JHK and RS are on the medical advisory board for Medela. Source of funding: Abbott Nutrition funded the study. Note that the human milk fortifier used in the intervention group was liquid; therefore, the added protein concentration was converted to gram per 100 mL of fortified EBM in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules were computer generated using a (quote:) "pseudorandom permuted blocks algorithm". A separate computer generated randomisation schedule was produced for twins to ensure that eligible twins were both assigned to the same product. The randomisation block was stratified by infant birth weight (700–1000 g and 1000–1500 g) and sex. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes contained the treatment group assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for, and reported by intention to treat, the attrition rate was 12% in the intervention group and 12.5% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Trial was sponsored by manufacturer, the role of whom was not described further. |
Maas 2017.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: neonatology department at Tuebingen University Children's Hospital, Germany Timing: October 2012 to October 2014 |
|
| Interventions |
Intervention: bovine based human milk fortifier containing 1.8 g hydrolysed protein added to 100 mL EBM using either standard fortification (n = 15) or individual fortification (n = 15) regimen (only the results from the standard fortification group were included in this review) Control: bovine based human milk fortifier containing 1 g hydrolysed protein added to 100 mL EBM (n = 30) Calorie content: isocaloric, providing 22 kcal (intervention) versus 18 kcal (control) energy added to 100 mL EBM (107 kcal vs 103 kcal in fortified EBM, 4% difference) Start of intervention: not stated other than "intervention continued according to allocation from randomization" Median age at study entry: 7 days (IQR 6–10) in intervention group and 7 days (IQR 6–7) in control group End of intervention: until definite discharge planning (< 1 week before discharge) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Note: some outcomes (head circumference gain, lower leg length gain) were reported for the higher protein group overall without reporting results for the standard fortification group separately. The trial authors were contacted to request separate data but no response has been received to date. |
|
| Notes |
Conflict of interest: ARF received speaker or consultant honoraria from Nestla, Milupa and Hipp, and received grants from Nestle for educational seminars. Source of funding: Nestle Nutrition, Frankfurt Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated randomisation scheme was produced by an independent statistician to assign the infants to intervention groups in a 2:1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using sequentially numbered, sealed, opaque envelopes. Siblings of multiple birth were randomised individually. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Carers and parents were blinded to the type of study fortifier in all groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Carers and parents were blinded to the type of study fortifier in all groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants were excluded or lost to follow‐up. All infants were included in the primary outcome analyses. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | Trial product was donated by manufacturer, who was not further involved in the trial. |
Miller 2012.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multi‐centre, with stratification by gestational age and infant sex | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: 2 neonatal intensive care units in Adelaide, Australia Study date: October 2006 to June 2008 |
|
| Interventions |
Intervention: bovine based human milk fortifier containing 1.4 g extensively hydrolysed protein added to 100 mL EBM (n = 43) Control: bovine based human milk fortifier containing 1 g extensively hydrolysed protein added to 100 mL EBM (n = 49) Calorie content: isocaloric, providing 17 kcal energy added to 100 mL EBM Start of intervention: when infant's enteral intake reached 80 mL/kg/day Median age at trial entry: 13 days (IQR 10–16) in intervention group and 13 days (IQR 10–18) in control group End of intervention: at discharge or the expected date of delivery |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Conflict of interest: MM and RAG have conducted clinical trials funded by the formula industry. MM serves on advisory boards for Nesle, Fonterra, Nutricia and Danone. RAG serves on scientific advisory boards for Fonterra. Source of funding: Grant‐in‐Aid from Denis Harwood. Nestle Nutrition, Switzerland donated the human milk fortifiers. Unpublished data regarding weight gain as g/kg/day were sought and used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was developed by an independent researcher using a computer random number generator to select random permuted blocks of 4. Stratification was by gestational age (< 28 and 28–32 weeks) and infant sex. Multiple births were randomly assigned to the same group according to the sex and gestational age of the first born infants. |
| Allocation concealment (selection bias) | Low risk | The tins of fortifier were sequentially numbered by an independent staff member according to the allocation sequence. The study fortifiers were supplied as a powder in 100 g tins and were identical in appearance, packaging and rate of mixing. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research personnel, outcome assessors and analysts were unaware of the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research personnel, outcome assessors and analysts were unaware of the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were accounted for, the attrition rate was similar in the 2 groups (only 1 infant in each group discontinued intervention, but their data were included). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | Trial product was donated by manufacturer, who was not further involved in the trial. |
Moya 2012.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multi‐centre, stratification by infant sex and birth weight | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: multi‐centre (14 centres) but no further details Study date: not reported |
|
| Interventions |
Intervention: bovine based liquid human milk fortifier containing 1.6 g hydrolysed protein added to 100 mL EBM (n = 74) Control: bovine based human milk fortifier containing 1 g intact protein added to 100 mL EBM (n = 72) Calorie content: isocaloric, providing total of 81 kcal (intervention) vs 78 kcal per 100 mL fortified EBM (4% difference) Start of intervention: start time point not described in methods – other than day of commencement noted as study day 0 Mean age at day 1: 18.4 days (SE 0.9) in intervention group and 17.8 days (SE 0.9) in control group End of intervention: 28 days, or hospital discharge, or termination of fortified breast milk feeding |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Conflict of interest: CLB and KRW are employees of Mead Johnson. Source of funding: Mead Johnson supported the study. Note to the protein concentration: there was insufficient information from the text to determine the protein concentration of the two human milk fortifiers compared, only stating the intervention fortifier providing 20% more protein than the control fortifier, when mixed with EBM. We searched for secondary papers related to this manuscript and found the same description, where the following additional information was available: Intervention (liquid, 4 vials per 100 mL EBM): containing 1.8 g protein per 4 vials Control (powder, 4 sachet per 100 mL EBM): containing 1.1 g protein per 4 sachets Note that the human milk fortifier used in the intervention group was liquid; therefore, the added protein concentration was converted to gram per 100 mL of fortified EBM in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information, attrition rate was 31.1% in intervention group and 23.6% in control group, based on 2 reasons as (quote:) "fortifier related" and "not fortifier related". |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Trial was sponsored by manufacturer, the role of whom was not described further. |
Porcelli 2000.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multi‐centre | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: neonatal intensive care units at 8 hospitals across USA Study date: not reported |
|
| Interventions |
Intervention: bovine based human milk fortifier containing 1.0 g protein added to 100 mL EBM (n = 35) Control: bovine based human milk fortifier containing 0.7 g protein added to 100 mL EBM (n = 29) Calorie content: isocaloric, 13 kcal (intervention) versus 14 kcal (control) energy added to 100 mL EBM (98 kcal vs 99 kcal in fortified EBM, 1% difference) Start of intervention: not stated other than "when human milk fortification was introduced" Mean age at trial entry: 21.2 days (SE 1.5) in intervention group and 17.7 days (SE 1.3) in control group End of intervention: when fully weaned from the assigned fortifier and receiving only unsupplemented human milk |
|
| Outcomes |
Primary outcome: outcomes were not specified as primary or secondary, but study was powdered to detect difference in weight gain. Outcomes included:
|
|
| Notes |
Conflict of interest: not reported Source of funding: Wyeth Nutritionals International, USA supported the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided – study only described as (quote:) "… prospective, randomized controlled …" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators blinded to study group, carers unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded to study group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data reported in this trial were based on a per protocol analysis, only select reported safety outcomes were based on intention to treat analysis. The overall attrition rate was 28.9% (25.5% for intervention, 32.5% for control). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Trial was sponsored by manufacturer, the role of whom was not described further. |
Reid 2018.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre, with stratification by infant sex and gestational age | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: neonatology department Women's and Children's Hospital, Adelaide, Australia Timing: February 2012 to May 2013 |
|
| Interventions |
Intervention: bovine based human milk fortifier containing 1.8 g extensively hydrolysed protein added to 100 mL EBM (n = 31) Control: bovine based human milk fortifier containing 1 g extensively hydrolysed protein added to 100 mL EBM (n = 29) Calorie content: isocaloric, 21 kcal energy added to 100 mL EBM Start of intervention: within 1 or 2 days after randomisation Mean age at study entry: 8.9 days (SD 3.2) in intervention group and 9.0 days (SD 2.5) in control group End of intervention: until removal of the nasogastric tube or estimated date of delivery |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Conflict of interest: MM serves on scientific advisory boards for Fonterra and Nestle. Source of funding: a Women's and Children's Hospital Foundation Grant funded the research. Nestle Nutrition and Nutricia donated human milk fortifiers and supplements used in this study. Unpublished data regarding weight gain as g/kg/day were sought and used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent researcher created the randomisation schedule using a computer generated variable block design of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Upon consent, infants were randomised by telephoning an independent researcher who held the randomisation schedule and assigned a unique study identification number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, clinicians, outcome assessors and data analysts were blinded to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians, outcome assessors and data analysts were blinded to randomisation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were accounted for in the analyses, 2 infants in each group discontinued intervention but their data were included. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | Trial product was donated by manufacturer, who was not further involved in the trial. |
Rigo 2017.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multi‐centre, stratification was by centre, sex and birth weight | |
| Participants | Inclusion criteria
Exclusion criteria
Setting: neonatal intensive care unit at 11 metropolitan hospitals in France, Belgium, Germany and Switzerland and Italy Timing: April 2011 to March 2014 |
|
| Interventions |
Intervention: bovine based human milk fortifier containing 1.42 g hydrolysed protein added to 100 mL EBM (n = 76) Control: bovine based human milk fortifier containing 1 g hydrolysed protein added to 100 mL EBM (n = 74) Calorie content: isocaloric, providing 84.5 kcal energy per 100 mL fortified human milk Start of intervention: infants tolerating > 100 mL/kg/day of human milk for > 24 hours Median age at study day 1: 16 days (IQR 13–20) in intervention group and 17 days (IQR 13–23) in control group End of intervention: minimum of 21 days, or until discharged/medical decision to stop fortification |
|
| Outcomes | Outcomes were not specified as primary or secondary
|
|
| Notes |
Conflict of interest: authors either received research funding from Nestle Nutrition or were affiliated with Nestle Nutrition. Source of funding: Nestle Nutrition funded the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers was used to allocate group assignment. Minimisation algorithm with allocation ratio 1:1 and second best probability of 15% was used. |
| Allocation concealment (selection bias) | Unclear risk | Reported as (quote:) "Group coding was used with 2 non speaking codes per group; fortifier packaging was coded accordingly but otherwise identical in appearance". Lack of clarity, uncertain of what the quote above means. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel (both site and sponsor based) and participants (infants' families) were blind to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel (both site and sponsor based) were blind to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for in flow diagram, attrition rate similar between groups (only 1 infant in the intervention group and 2 infants in the intervention group were excluded due to violation of the exclusion criteria). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Trial was sponsored by manufacturer, the role of whom was not described further. |
Sankaran 1996.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multi‐centre | |
| Participants |
Inclusion criteria
Exclusion criteria
Setting: 2 neonatal intensive care units in Canada Study date: not reported |
|
| Interventions |
Intervention: bovine based liquid human milk fortifier containing 1.09 g protein added to 100 mL EBM (n = 29) Control: bovine based human milk fortifier containing 0.7 g protein added to 100 mL EBM (n = 31) Calorie content: non‐isocaloric, 73.5 kcal (intervention) vs 80.6 kcal (control) energy per 100 mL fortified EBM (9% difference) Start of intervention: tolerating full feeds of 120 mL/kg/day preterm milk after 3 consecutive days Mean age at trial entry: 20.1 days (SE 2.4) in intervention group and 24.5 days (SE 3.1) in control group End of intervention: when the infants reached 2 kg bodyweight or gained 35 g/day averaged over 10 days |
|
| Outcomes | Outcomes were not specified as primary or secondary
|
|
| Notes |
Conflict of interest: none declared Source of funding: Abbott Laboratories and Mead Johnson Canada partially funded the study. Note regarding the protein concentration: there was insufficient information regarding the protein concentration of the 2 human milk fortifier as g/100 mL EBM and following calculations were done to determine the protein concentration. Note: although confirmation was sought from the authors, no response has been received to date. Intervention (liquid, mixed with EBM in 1:1 ratio): liquid human milk fortifier containing 2.7 g protein per 124 mL as provided in the text, equal to 1.09 g/50 mL when mixed with 50 mL EBM, therefore the protein derived from the fortifier was 1.09 g/(50 + 50 mL). The final fortified EBM containing 2.6 g protein per 136 mL, half of the volume was derived from the liquid human milk fortifier which contained 1.48 g protein, therefore the protein concentration of EBM was 1.12 g/68 mL = 1.65 g/100 mL Control (powder, mixed with EBM as 4 packs/100 mL EBM): the final fortified EBM containing 2.9 g protein per 124 mL as provided in the text, and the protein from EBM was 2.05 g (based on 1.65 g/100 mL and 124 mL), therefore the protein derived from the fortifier was 0.85 g/124 mL = 0.7 g/100 mL. Note that the human milk fortifier used in the intervention group was liquid, therefore the added protein concentration was converted to gram per 100 mL of fortified EBM in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial was designed to determine the efficacy of the product, no intention to treat data reported. Attrition were described (41/60 infants' data were used for analysis) but unclear which group the infants originally enrolled in. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Trial was sponsored by manufacturer, the role of whom was not described further. |
EBM: expressed breast milk; GI: gastrointestinal; IQR: interquartile range; n: number of participants; SD: standard deviation; SE: standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arco 2002 | Wrong intervention – compared fortified human milk with formula. |
| Atchley 2019 | Wrong intervention – intervention included both parenteral and enteral nutrition. |
| Berseth 2004 | Wrong intervention – both fortifiers fell into the same category (≥ 1 g, < 1.4 g protein /100 mL EBM). |
| Biasini 2012 | Wrong intervention – compared 2 different fortification strategies (adjustable vs standard). |
| Brumberg 2010 | Wrong intervention – compared human milk fortified with either medium chain triglycerides or protein. |
| Coscia 2018 | Wrong intervention – both intervention groups followed an adjustable fortification regimen that led to similar level of protein intake. |
| Costa‐Orvay 2011 | Wrong intervention – infant formula based study. |
| Davies 1975 | Wrong intervention – compared human milk with non‐specified milk with varying level of proteins. (There was insufficient information in the conference proceeding, but considering the time of publication and the way the data were presented, it is likely cow's milk vs human milk comparison; unfortunately this could not be confirmed.) |
| Ditzenberger 2013 | Wrong intervention – used targeted fortification regimen. |
| ISRCTN27916681 | Wrong study design – observational cohort study. |
| Kanmaz 2013 | Wrong intervention – higher protein content was achieved by adding more fortifier. |
| Liang 2018 | Wrong study design – quality improvement study. |
| Loui 2004 | Wrong intervention – fortifiers compared fell into the same category (< 1 g protein/100 mL EBM). |
| Lucas 1996 | Wrong intervention – compared human milk fortified with either protein or fats and carbohydrates. |
| McLeod 2016 | Wrong intervention – compared different fortification strategies. |
| Moltu 2013 | Wrong intervention – intervention included both parenteral and enteral nutrition. |
| Morlacchi 2016 | Wrong intervention – compared different fortification strategies. |
| Moro 1991 | Wrong intervention – fortifiers compared were the same level (1 g/100 mL EBM). |
| Moyer‐Mileur 1992 | Wrong outcomes – only bone mineral contents were reported. We have written to authors enquiring if other outcome data were available; however, we have received no response. |
| Polberger 1989 | Wrong intervention – compared unfortified human milk with milk fortified with either protein or fat. |
| Quan 2020 | Wrong intervention – compared different fortification strategies (individualised vs standard). |
| Schanler 2018 | Wrong intervention – fortifiers compared were in the same category (≥ 1.4 g/100 mL EBM). |
| Strommen 2017 | Wrong intervention – intervention included both parenteral and enteral nutrition. |
| Tan 2008 | Wrong intervention – intervention was about parenteral nutrition. |
| Thoene 2014 | Wrong study design – retrospective study. |
| Thoene 2016 | Wrong study design – retrospective study. |
| Thomaz 2014 | Wrong outcomes – only serum phenylalanine concentrations were reported. We have written to authors enquiring if other outcome data were available; however, we have received no response. |
| Tracy 2015 | Wrong intervention – compared effect of early vs late fortification. |
EBM: expressed breast milk.
Characteristics of studies awaiting classification [ordered by study ID]
Kashaki 2018.
| Methods | Randomised trial |
| Participants | Infants weighing < 1000 g |
| Interventions | Infants in both groups received human milk fortifier, additional protein supplements were given to infants in intervention group |
| Outcomes | Growth (weight, height and head circumference), blood urea nitrogen |
| Notes | We have written to authors for clarification of the dosage of protein used, but no response received as yet. |
Characteristics of ongoing studies [ordered by study ID]
IRCT2016041927474N1.
| Study name | Study of varying protein supplement effect on the growth criteria and maturation of immune system in very low birth weight infants |
| Methods | Randomised, double blind trial |
| Participants | Preterm infants born < 1500 g and gestational age < 32 weeks |
| Interventions | Added protein supplements to breast milk at 2 different dosages |
| Outcomes | Head circumference |
| Starting date | 21 December 2015 |
| Contact information | Dr Majid Hamidi (M.hamidi@skums.ac.ir) |
| Notes |
IRCT20180923041095N1.
| Study name | Comparing usual protein and rich protein regimens on growth among premature neonates weighed less than 1500 gram: a double blind randomized clinical trial |
| Methods | Randomised, double blind trial |
| Participants | Preterm infants with birth weight < 1500 g |
| Interventions | Protein supplements added to breast milk at 2 different dosages |
| Outcomes | Head circumference |
| Starting date | 19 February 2017 |
| Contact information | Maral Ghasemzadeh (maral.ghasemzade1984@gmail.com) |
| Notes |
ISRCTN96620855.
| Study name | NutriBrain: a controlled study to investigate the effect of a food product on brain development in very early born infants |
| Methods | Randomised double blind controlled trial |
| Participants | Very early born infants (24–30 weeks' gestation) |
| Interventions | Control (maltodextrin casein and whey protein hydrosates) vs intervention (probiotics, prebiotics and free amino acids) |
| Outcomes | Fractional anisotropy in the posterior limb of the internal capsule |
| Starting date | 1 March 2015 |
| Contact information | Dr Manon Benders (m.benders@umcutrecht.nl) |
| Notes |
NCT03001479.
| Study name | Noninferioriy trial of liquid human milk fortifier hydrolyzed protein versus liquid HMF with supplemental liquid protein |
| Methods | Randomised clinical trial |
| Participants | Preterm infants < 32 weeks' gestation and birth weight < 1500 g |
| Interventions | Extensively hydrolysed liquid human milk fortifier vs liquid human milk fortifier with supplemental liquid protein |
| Outcomes | Weight gain, length and head circumference |
| Starting date | 23 December 2016 |
| Contact information | Dr Catherine Cibulskis (ccibulsk@slu.edu) |
| Notes |
NCT03191617.
| Study name | Nutrition of premature infants with human breast milk fortifier (EFORT‐LM) |
| Methods | Randomised trial |
| Participants | Preterm infants ≤ 31 weeks' gestation and birth weight ≤ 1250 g |
| Interventions | Liquid human milk fortifier with higher protein and long‐chain polyunsaturated fatty acids content vs powder human milk fortifier with less protein and no long‐chain polyunsaturated fatty acids |
| Outcomes | Weight and linear growth |
| Starting date | September 2015 |
| Contact information | Dr Jose L Tapia (jlta@med.puc.cl) |
| Notes |
NCT03315221.
| Study name | Renoir: a randomized, double‐blind, controlled trial to evaluate the effects of a new human milk fortifier on growth and tolerance in preterm infants |
| Methods | Randomised, double blind controlled trial |
| Participants | Preterm infants < 32 weeks' gestation and birth weight < 1500 g |
| Interventions | Human milk fortifier with added lipids and human milk fortifier without lipids |
| Outcomes | Weight gain velocity |
| Starting date | 8 March 2018 |
| Contact information | Jan van der Mooren (jan.vandermooren@danone.com) |
| Notes |
NCT03586102.
| Study name | Effect of increased enteral protein on body composition of preterm infants |
| Methods | Randomised trial |
| Participants | Preterm infants of 25–28 weeks' gestation |
| Interventions | Added protein supplements to breast milk at 2 different dosages |
| Outcomes | Body composition |
| Starting date | 23 August 2018 |
| Contact information | Dr Ariel A Salas (asalas@peds.uab.edu) |
| Notes |
NCT03797157.
| Study name | Human milk fortification in extremely preterm infants (N‐forte) |
| Methods | Randomised trial |
| Participants | Preterm infants of 22–27 weeks' gestation |
| Interventions | A bovine milk based human milk fortifier vs a human milk based human milk fortifier |
| Outcomes | Incidence of necrotising enterocolitis, sepsis and mortality |
| Starting date | 1 January 2019 |
| Contact information | Dr Thomas R Abrahamsson (thomas.abrahamsson@liu.se) |
| Notes |
Paria 2016.
| Study name | A clinical trial to study the effect of enriching expressed human milk with additional protein on growth of very low birth weight newborns |
| Methods | Randomised clinical trial |
| Participants | Preterm infants with birth weight of 800–1500 g |
| Interventions | Fortified human breast milk plus additional protein vs fortified human breast milk plus additional glucose |
| Outcomes | Weight, length and head circumference gain |
| Starting date | 5 March 2012 |
| Contact information | Dr Anshuman Paria (dr.anshuparia@gmail.com) |
| Notes | We have written to the author to ask if there has been any full text published, but no response received to date. |
Differences between protocol and review
We made the following changes to the published protocol (Miller 2008).
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see 'How CENTRAL is created'; www.cochranelibrary.com/central/central-creation). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs from ClinicalTrials.gov or the World Health Organization's International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/), as records from both platforms are added to CENTRAL on a monthly basis (see 'How CENTRAL is created'; www.cochranelibrary.com/central/central-creation). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
For the 2019 update, we developed a new search strategy, which we ran without date limits (Appendix 1).
In the protocol (Miller 2008), we stated that the review would include interventions 'comparing two or more different levels of protein concentration in human milk fortifier', which requires the two human milk fortifiers compared to have different protein concentration per se. However, during the study selection process, there were several trials that gave the two comparison groups the same commercially available human milk fortifier as a base, and then altered the protein concentration in the intervention group with an additional protein supplement. These interventions did not fit in the original description of eligible studies in the protocol. However, as the trials aimed to examine the effect of varying the protein concentration of human milk fortifier, the study selection criteria have been amended to include these studies (e.g. the trials by Reid 2018, and the ongoing trial by Paria 2016).
Contributions of authors
The methods section of this review was based on a standard template used by Cochrane Neonatal.
All review authors conceptualised the protocol.
JM wrote the initial protocol; other authors edited and approved the final version of the protocol.
CG was responsible for screening titles, data extraction, quality assessment and writing the first draft of the review.
JM, CTC and AR contributed to screening and data extraction.
JM undertook quality appraisal and data entry into Review Manager 5.
All authors edited and approved the final version of the review.
Sources of support
Internal sources
Carmel Collins is supported by a National Health and Medical Research Council (NHMRC) Translating Research into Practice Fellowship (APP1132596), Australia
South Australian Health and Medical Research Institute, Australia
Chang Gao is supported by a PhD Scholarship from the University of Adelaide, Australia
Nutrition for Mother and Child Centre of Research Excellence, Australia
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
CG received an Adelaide Graduate Research Scholarship from the University of Adelaide to support her PhD.
JM is an author of the following included studies: Miller 2012; Reid 2018.
CTC is an author of the following included studies: Miller 2012; Reid 2018. CTC is supported by a National Health and Medical Research Council (NHMRC) Translating Research into Practice Fellowship (APP1132596). The views expressed in this article are solely the responsibility of the authors and do not reflect the views of the NHMRC.
JM and CTC declared the following conflicts of interest: Nestle Nutrition donated half of the human milk fortifier, and Nutricia donated Polyjoule and Protifar, used in a randomised controlled trial (RCT) of increased protein concentration human milk fortifier (Reid 2018). Study product for a separate RCT of human milk fortifier was manufactured and donated by Nestle Product Technology Centre, Konolfingen, Switzerland (Miller 2012). The sponsors had no role (in either Miller 2012 or Reid 2018), in the design of the study, in the collection, analyses or interpretation of data, in writing of the manuscript, or the decision to publish the results. Data extraction of these two studies (Miller 2012; Reid 2018) was completed by CG.
AR has no interests to declare.
Core editorial and administrative support for this review was provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor had no input on the content of the review or the editorial process. The review authors did not receive any direct funding from The Gerber Foundation (Sources of support).
New
References
References to studies included in this review
Dogra 2017 {published data only}
- Dogra S, Thakur A, Garg P, Kler N. Effect of differential enteral protein on growth and neurodevelopment in infants < 1500g: a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2017;64(5):e126-32. [DOI: 10.1097/MPG.0000000000001451] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim JH, Chan G, Schanler R, Groh-Wargo S, Bloom B, Dimmit R, et al. Growth and tolerance of preterm infants fed a new extensively hydrolyzed liquid human milk fortifier. Journal of Pediatric Gastroenterology and Nutrition 2015;61(6):665-71. [DOI: 10.1097/MPG.0000000000001010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maas 2017 {published data only}
- Maas C, Mathes M, Bleeker C, Vek J, Bernhard W, Wiechers C, et al. Effect of increased enteral protein intake on growth in human milk-fed preterm infants: a randomized clinical trial. JAMA Pediatrics 2017;171(1):16-22. [DOI: 10.1001/jamapediatrics.2016.2681] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 2012 {published and unpublished data}
- Miller J, Makrides M, Gibson RA, McPhee AJ, Stanford TE, Morris S, et al. Effect of increasing protein content of human milk fortifier on growth in preterm infants born at <31 wk gestation: a randomized controlled trial. American Journal of Clinical Nutrition 2012;95(3):648-55. [DOI: 10.3945/ajcn.111.026351] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moya 2012 {published data only}
- Moya F, Sisk PM, Walsh KR, Berseth CL. A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics 2012;130(4):e928-35. [DOI: 10.1542/peds.2011-3120] [PMID: ] [DOI] [PubMed] [Google Scholar]
Porcelli 2000 {published data only}
- Porcelli P, Schanler R, Greer F, Chan G, Gross S, Mehta N, et al. Growth in human milk-fed very low birth weight infant receiving a new human milk fortifier. Annals of Nutrition and Metabolism 2000;44(1):2-10. [DOI: 10.1159/000012814] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reid 2018 {published and unpublished data}
- Reid J, Makrides M, McPhee AJ, Stark MJ, Miller J, Collins CT. The effect of increasing the protein content of human milk fortifier to 1.8g/100ml on growth in preterm infants: a randomised controlled trial. Nutrients 2018;10(5):634. [DOI: 10.3390/nu10050634] [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigo 2017 {published data only}
- RIgo J, Hascoet JM, Billeaud C, Picaud JC, Mosca F, Rubio A, et al. Growth and nutritional biomarkers of preterm infants fed a new powdered human milk fortifier: a randomized trial. Journal of Pediatric Gastroenterology and Nutrition 2017;65(4):e83-93. [DOI: 10.1097/MPG.0000000000001686] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankaran 1996 {published data only}
- Sankaran K, Papageorgiou A, Ninan A, Sankaran R. A randomized, controlled evaluation of two commercially available human breast milk fortifiers in healthy preterm neonates. Journal of the American Dietetic Association 1996;96(11):1145-9. [DOI: 10.1016/s0002-8223(96)00295-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arco 2002 {published data only}
- Arco A, Gitto E, Sacco F, Barberi I, Mondello I, Nicolo A, et al. Growth parameters in very-low-birth-weight infants feeding human milk fortified and formulas with different protein concentration: multicentric study. Journal of Pediatric Gastroenterology and Nutrition 2002;34:488. [Google Scholar]
Atchley 2019 {published data only}
- Atchley CB, Cloud A, Thompson D, Blunt MH, Satnes KJ, Szyld E, et al. Enhanced protein diet for preterm infants: a prospective, randomized, double-blind, controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2019;69(2):218-23. [DOI: 10.1097/MPG.0000000000002376] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berseth 2004 {published data only}
- Berseth CL, Van Aerde JE, Gross S, Stolz SI, Harris CL, Hansen JW. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics 2004;114(6):e699-706. [DOI: 10.1542/peds.2004-0911] [PMID: ] [DOI] [PubMed] [Google Scholar]
Biasini 2012 {published data only}
- Biasini A, Marvulli L, Neri E, China M, Stella M, Monti F. Growth and neurological outcome in ELBW preterms fed with human milk and extra-protein supplementation as routine practice: do we need further evidence? Journal of Maternal-Fetal and Neonatal Medicine 2012;25(4):72-4. [DOI: 10.3109/14767058.2012.715032] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brumberg 2010 {published data only}
- Brumberg HL, Kowalski L, Troxell-Dorgan A, Gettner P, Konstantino M, Poulsen JF, et al. Randomized trial of enteral protein and energy supplementation in infants less than or equal to 1250g at birth. Journal of Perinatology 2010;30:517-21. [DOI: 10.1038/jp.2010.10] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coscia 2018 {published data only}
- Coscia A, Bertino E, Tonetto P, Peila C, Cresi F, Arslanoglu S, et al. Nutritional adequacy of a novel human milk fortifier from donkey milk in feeding preterm infants: study protocol of a randomized controlled clinical trial. Nutrition Journal 2018;17(1):6. [DOI: 10.1186/s12937-017-0308-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa‐Orvay 2011 {published data only}
- Costa-Orvay HA, Figueras-Aloy J, Romera G, Closa-Monasterolo R, Carbonell-Estrany X. The effects of varying protein and energy intakes on the growth and body composition of very low birth weight infants. Nutrition Journal 2011;10:140. [DOI: 10.1186/1475-2891-10-140] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 1975 {published data only}
- Davies DP. Proceedings: is human milk the best food for preterm infants? Archives of Disease in Childhood 1975;50:330. [DOI: 10.1136/adc.50.4.330-b] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ditzenberger 2013 {published data only}
- Ditzenberger GR, Wallen LD, Phelan L, Escoe S, Collins SD. Supplemental protein and postnatal growth of very low birth weight infants: a randomized trial. Journal of Neonatal-Perinatal Medicine 2013;6(4):285-94. [DOI: 10.3233/NPM-1371213] [PMID: ] [DOI] [PubMed] [Google Scholar]
ISRCTN27916681 {published data only}
- ISRCTN27916681. Effect on body and brain development of feeding very premature babies with breast milk containing different supplements. www.isrctn.com/ISRCTN27916681 (first received 10 January 2015).
Kanmaz 2013 {published data only}
- Kanmaz HG, Mutlu B, Erdeve O, Canpolat FE, Oguz SS, Uras N, et al. Does enteral protein intake affect renal glomerular and tubular functions in very low birth weight infants? Clinical Nephrology 2013;80(5):355-60. [DOI: 10.5414/CN107727] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liang 2018 {published data only}
- Liang A, Calma E, Judkins A, Truong G, Kadri M, Deming D, et al. Does choice of human milk fortifiers affect feeding tolerance and nutrition in premature infants? Journal of Investigative Medicine 2018;66:176. [Google Scholar]
Loui 2004 {published data only}
- Loui A, Raab A, Wagner M, Weigel H, Gruters-Kieslich A, Bratter P, et al. Nutrition of very low birth weight infants fed human milk with or without supplemental trace elements: a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2004;39:346-53. [DOI: 10.1097/00005176-200410000-00009] [EMBASE: 15448423] [DOI] [PubMed] [Google Scholar]
Lucas 1996 {published data only}
- Lucas A, Fewtrell MS, Morley R, Lucas PJ, Baker BA, Lister G, et al. Randomized outcome trial of human milk fortification and developmental outcome in preterm infants. American Journal of Clinical Nutrition 1996;64(2):142-51. [DOI: 10.1093/ajcn/64.2.142] [PMID: ] [DOI] [PubMed] [Google Scholar]
McLeod 2016 {published data only}
- McLeod G, Sherriff J, Hartmann PE, Nathan E, Geddes D, Simmer K. Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: a randomized controlled trial. British Journal of Nutrition 2016;115(3):431-9. [DOI: 10.1017/S0007114515004614] [PMID: 26627899] [DOI] [PubMed] [Google Scholar]
Moltu 2013 {published data only}
- Moltu SJ, Strommen K, Blakstad EW, Almaas AN, Westerberg AC, Braekke K, et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbance and septicemia – a randomized controlled trial. Clinical Nutrition 2013;32(2):207-12. [DOI: 10.1016/j.clnu.2012.09.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morlacchi 2016 {published data only}
- Morlacchi L, Mallardi D, Gianni ML, Roggero AP, Amato O, Piemontese P, et al. Is targeted fortification of human breast milk an optimal nutrition strategy for preterm infants? An interventional study. Journal of Translational Medicine 2016;14(1):195. [DOI: 10.1186/s12967-016-0957-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moro 1991 {published data only}
- Moro GE, Minoli I, Fulconis F, Clementi M, Raiha NC. Growth and metabolic responses in low-birth-weight infants fed human milk fortified with human milk protein or with a bovine milk protein preparation. Journal of Pediatric Gastroenterology and Nutrition 1991;13(2):150-4. [DOI: 10.1097/00005176-199108000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moyer‐Mileur 1992 {published data only}
- Moyer-Mileur L, Chan GM, Gill G. Evaluation of liquid or powdered fortification of human milk on growth and bone mineralization status of preterm infants. Journal of Pediatric Gastroenterology and Nutrition 1992;15(4):370-4. [DOI: 10.1097/00005176-199211000-00002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Polberger 1989 {published data only}
- Polberger SK, Axelsson IA, Raiha NC. Growth of very low birth weight infants on varying amounts of human milk protein. Pediatric Research 1989;25(4):414-9. [DOI: 10.1203/00006450-198904000-00022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quan 2020 {published data only}
- Quan M, Wang D, Gou L, Sun Z, Ma J, Zhang L, et al. Individualized human milk fortification to improve the growth of hospitalized preterm infants. Nutrition in Clinical Practice 2020;35(4):680-8. [DOI: 10.1002/ncp.10366] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schanler 2018 {published data only}
- Schanler RJ, Groh-Wargo SL, Barrett-Reis B, White RD, Ahmad KA, Oliver J, et al. Improved outcomes in preterm infants fed a nonacidified liquid human milk fortifier: a prospective randomized clinical trial. Journal of Pediatrics 2018;202:31-7. [DOI: 10.1016/j.jpeds.2018.07.005] [PMID: 30195561] [DOI] [PubMed] [Google Scholar]
Strommen 2017 {published data only}
- Strommen K, Haag A, Moltu SJ, Veierod MB, Blakstad EW, Nakstad B, et al. Enhanced nutrient supply to very low birth weight infants is associated with higher blood amino acid concentrations and improved growth. Clinical Nutrition 2017;18:16-22. [DOI: 10.1016/j.clnesp.2017.01.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 2008 {published data only}
- Tan MJ, Cooke RW. Improving head growth in very preterm infants – a randomised controlled trial I: neonatal outcomes. Archives of Disease in Childhood Fetal and Neonatal edition 2008;93(5):F337-41. [DOI: 10.1136/adc.2007.124230] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thoene 2014 {published data only}
- Thoene M, Hanson C, Lyden E, Dugick L, Ruybal L, Anderson-Berry A. Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients 2014;6(1):261-75. [DOI: 10.3390/nu6010261] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoene 2016 {published data only}
- Thoene M, Lyden E, Wishaar K, Elliot E, Wu R, White K, et al. Comparison of a powdered, acidified liquid and non-acidified liquid human milk fortifier on clinical outcomes in premature infants. Nutrients 2016;8(8):451. [DOI: 10.3390/nu8080451] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomaz 2014 {published data only}
- Thomaz DM, Serafin PO, Palhares DB, Tavares LV, Grance TR. Serum phenylalanine in preterm newborns fed different diets of human milk. Jornal de Pediatria 2014;90(5):518-22. [DOI: 10.1016/j.jped.2014.02.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tracy 2015 {published data only}
- Tracy M, Green R, Weintraub A. Early liquid protein supplementation of human milk in very low birth weight infants. Pediatric Academic Societies Annual Meeting; 2015 Apr 25-28; San Diego (CA).
References to studies awaiting assessment
Kashaki 2018 {published data only}
- Kashaki M, Mazouri A, Bordbar A, Saboute M, Behnamfar Z, Talebi A. Effect of protein supplementation on the growth of infants weighing less than 1000 grams hospitalised on the neonatal intensive care unit of Akbar Abadi Hospital in Tehran, Iran (2015–2016). Iranian Journal of Neonatology 2018;9(3):49-56. [DOI: 10.22038/ijn.2018.27558.1368] [DOI] [Google Scholar]
References to ongoing studies
IRCT2016041927474N1 {published data only}
- IRCT2016041927474N1. Study of varying protein supplement effect on the growth criteria and maturation of immune system in very low birth weight infants. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016041927474N1 (first received 21 May 2016).
IRCT20180923041095N1 {published data only}
- IRCT20180923041095N1. Comparing usual protein and rich protein regimens on growth among premature neonates weighed less than 1500 gram: a double blind randomized clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180923041095N1 (first received 11 November 2018).
ISRCTN96620855 {published data only}
- ISRCTN96620855. NutriBrain: a controlled study to investigate the effect of a food product on brain development in very early born infants. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN96620855 (first received 10 October 2017).
NCT03001479 {published data only}
- NCT03001479. Noninferiority trial of liquid human milk fortifier (HMF) hydrolyzed protein versus liquid HMF with supplemental liquid protein. clinicaltrials.gov/show/NCT03001479 (first received 23 December 2016).
NCT03191617 {published data only}
- NCT03191617. Nutrition of premature infants with human breastmilk fortifier (EFORT-LM). clinicaltrials.gov/ct2/show/NCT03191617 (first received 19 June 2017).
NCT03315221 {published data only}
- NCT03315221. Renoir: a randomised, double-blind, controlled trial to evaluate the effects of a new human milk fortifier on growth and tolerance in preterm infants. clinicaltrials.gov/show/NCT03315221 (first received 20 October 2017).
NCT03586102 {published data only}
- NCT03586102. Effect of increased enteral protein on body composition of preterm infants. clinicaltrials.gov/show/nct03586102 (first received 13 July 2018).
NCT03797157 {published data only}
- NCT03797157. Human milk fortification in extremely preterm infants (N-forte). clinicaltrials.gov/show/nct03797157 (first received 9 January 2019).
Paria 2016 {published data only}
- Paria A, Sardar S, Sur A. A clinical trial to study the effect of enriching expressed human milk with additional protein on growth of very low birth weight newborns. European Journal of Pediatrics 2016;175:1614. [Google Scholar]
Additional references
AAP 2012
- American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129(3):e827-41. [DOI: 10.1542/peds.2011-3552] [PMID: ] [DOI] [PubMed] [Google Scholar]
Abdelhamid 2013
- Abdelhamid AE, Chuang SL, Hates P, Fell JM. Evolution of in vitro cow's milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotising enterocolitis. Journal of Pediatric Gastroenterology and Nutrition 2013;56(1):5-11. [DOI: 10.1097/MPG.0b013e31826ee9ec] [PMID: ] [DOI] [PubMed] [Google Scholar]
Amissah 2018
- Amissah EA, Brown J, Harding JE. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD000433. [DOI: 10.1002/14651858.CD000433.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arslanoglu 2019
- Arslanoglu S, Boquien CY, King C, Lamireau D, Tonetto P, Barnett D, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) working group on human milk fortification. Frontier in Pediatrics 2019;7:76. [DOI: 10.3389/fped.2019.00076] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Avery 1967
- Avery M, Clow C, Menkes J, Ramos A, Scriver C, Stern L, et al. Transient tyrosinemia of the newborn: dietary and clinical aspects. Pediatrics 1967;39(3):378-84. [PMID: ] [PubMed] [Google Scholar]
Bertino 2017
- Bertino E, Giribaldi M, Cester EA, Coscia A, Trapani BM, Peila C, et al. New human milk fortifiers for the preterm infant. Journal of Pediatric and Neonatal Individualized Medicine 2017;6(1):e060124. [DOI: 10.7363/060124] [DOI] [Google Scholar]
Brown 2020
- Brown JV, Lin L, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD000343. [DOI: 10.1002/14651858.CD000343.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC resources for review authors. epoc.cochrane.org/resources/epoc-specific-resources-review-authors (accessed prior to 11 November 2019).
Coviello 2018
- Coviello C, Keunen K, Kersbergen KJ, Groenendaal F, Leemans A, Peels B, et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatric Research 2018;83(1):102-10. [DOI: 10.1038/pr.2017.227] [PMID: ] [DOI] [PubMed] [Google Scholar]
ESPGHAN 2010
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pedatric, Gastroenterology and Nutrition 2010;50(1):85-91. [DOI: 10.1097/MPG.0b013e3181adaee0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fabrizio 2019
- Fabrizio V, Trzaski JM, Brownell EA, Esposito P, Lainwala S, Lussier MM, et al. Targeted or adjustable versus standard diet fortification for growth and development in very low birth weight infants receiving human milk. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013465. [DOI: 10.1002/14651858.CD013465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenton 2020
- Fenton TR, Al-Wassia H, Premji SS, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD003959. [DOI: 10.1002/14651858.CD003959.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gidrewicz 2014
- Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Godden 2019
- Godden B, Collins CT, Hiditch C, McLeod G, Keir A. Does early compared to late fortification of human milk for preterm infants improve clinical outcomes? Journal of Pediatric and Child Health 2019;55(7):867-72. [DOI: 10.1111/jpc.14499] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldman 1969
- Goldman H, Freudenthal R, Holland B, Karelitz S. Clinical effects of two different levels of protein intake on low-birth-weight infants. Journal of Pediatrics 1969;74(6):881-9. [DOI: 10.1016/s0022-3476(69)80222-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 11 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Hay 2010
- Hay WW, Thureen P. Protein for preterm infants: how much is needed? How much is enough? How much is too much? Pediatrics and Neonatology 2010;51(4):198-207. [DOI: 10.1016/S1875-9572(10)60039-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hay 2016
- Hay WW, Ziegler EE. Growth failure among preterm infants due to insufficient protein is not innocuous and must be prevented. Journal of Perinatology 2016;36(7):500-2. [DOI: 10.1038/jp.2016.85] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Koletzko 2014
- Koletzko B, Poindexter B, Uauy R. Recommended nutrient intake levels for stable, fully enteral fed very low birth weight infants. World Review of Nutrition and Dietetics 2014;110:297-9. [DOI: 10.1159/000360195] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2017
- Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing nutrition in preterm low birth weight infants – consensus summary. Frontier in Nutrition 2017;4:20. [DOI: 10.3389/fnut.2017.00020] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2015
- Liu TT, Dang D, Lv XM, Wang TF, Du JF, Hui W. Human milk fortifier with high versus standard protein content for promoting growth of preterm infants: a meta-analysis. Journal of International Medical Research 2015;43:278-89. [DOI: 10.1177/0300060515579115] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mihatsch 2002
- Mihatsch W, Franz A, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002;110(6):1199-203. [DOI: 10.1542/peds.110.6.1199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moro 2015
- Moro GE, Arslanoglu S, Bertino E, Corvagila L, Montirosso R, Picaud JC. XII. Human milk in feeding premature infants: consensus statement. Journal of Pediatric Gastroenterology and Nutrition 2015;61:S16-9. [DOI: 10.1097/01.mpg.0000471460.08792.4d] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ng 2019
- Ng DH, Klassen JR, Embleton ND, McGuire W. Protein hydrolysate versus standard formula for preterm infants. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD012412. [DOI: 10.1002/14651858.CD012412.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penn 2012
- Penn AH, Altshuler AE, Small JW, Taylor SF, Dobkins KR, Schmid-Schobein GW. Digested formula but not digested fresh human milk causes death of intestinal cells in vitro: implications for necrotizing enterocolitis. Pediatric Research 2012;72(6):560-7. [DOI: 10.1038/pr.2012.125] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Picaud 2001
- Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, et al. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2001;32(5):555-61. [DOI: 10.1097/00005176-200105000-00012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Premkumar 2019
- Premkumar M, Pammi M, Suresh G. Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013145. [DOI: 10.1002/14651858.CD013145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radmacher 2017
- Radmacher PG, Admkin DH. Fortification of human milk for preterm infants. Seminars in Fetal & Neonatal Medicine 2017;22(1):30-5. [DOI: 10.1016/j.siny.2016.08.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rochow 2011
- Rochow N, Jochum F, Redich A, Korinekova Z, Linnemann K, Weitmann K, et al. Fortification of breast milk in VLBW infants: metabolic acidosis is linked to the consumption of fortifier and alters weight gain and bone mineralization. Clinical Nutrition 2011;30(1):99-105. [DOI: 10.1016/j.clnu.2010.07.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Senterre 1983
- Senterre J, Voyer M, Putet G, Rigo J. Nitrogen, fat and mineral balance studies in preterm infants fed bank human milk, a human milk formula, or a low birth-weight infant formula. In: Baum D, editors(s). Human Milk Processing, Fractionation, and the Nutrition of the Low Birth-Weight Baby. Vol. 3. New York (NY): Raven Press, 1983:102-11. [Google Scholar]
Stephens 2009
- Stephens BE, Walden RV, Gargus RA, Tucker R, McKinley L, Mance M, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009;123(5):1337-43. [DOI: 10.1542/peds.2008-0211] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2009
- Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: mother's versus formula. Breastfeeding Medicine 2009;4(1):11-5. [DOI: 10.1089/bfm.2008.0114] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thanigainathan 2019
- Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013392. [DOI: 10.1002/14651858.CD013392] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 2011
- Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Annals of Nutrition & Metabolism 2011;58(Suppl 1):8-18. [DOI: 10.1159/000323381] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Miller 2008
- Miller J, Makrides M, Collins CT. High versus standard protein content of human milk fortifier for promoting growth and neurological development in preterm infants. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD007090. [DOI: 10.1002/14651858.CD007090] [DOI] [PMC free article] [PubMed] [Google Scholar]


