The performance of Xpert MTB/RIF using bronchoalveolar lavage fluid (BAL) for the diagnosis of pulmonary tuberculosis (PTB) remains unclear. Therefore, a systematic review/meta-analysis was conducted.
KEYWORDS: Xpert MTB/RIF, bronchoalveolar lavage fluid, pulmonary tuberculosis, meta-analysis, systematic review
ABSTRACT
The performance of Xpert MTB/RIF using bronchoalveolar lavage fluid (BAL) for the diagnosis of pulmonary tuberculosis (PTB) remains unclear. Therefore, a systematic review/meta-analysis was conducted. Studies published before 31 December 2019 were retrieved from the PubMed, Embase, and Web of Science databases using the keywords “pulmonary tuberculosis,” “Xpert MTB/RIF,” and “BAL.” Two independent evaluators extracted the data and assessed the bias risk of the included studies. A random-effects model was used to calculate the overall sensitivity, specificity, positive and negative likelihood ratios (PLR and NLR, respectively), diagnostic odds ratio (DOR), and the area under the curve (AUC), as well as the respective 95% confidence intervals (CIs). Nineteen trials involving 3,019 participants met the inclusion criteria. Compared to the culture method, the pooled sensitivity, specificity, PLR, NLR, DOR, and the AUC with 95% CIs of Xpert MTB/RIF were 0.87 (0.84 to 0.90), 0.92 (0.91 to 0.93), 10.21 (5.78 to 18.02), 0.16 (0.12 to 0.22), 78.95 (38.59 to 161.53), and 0.9467 (0.9462 to 0.9472), respectively. Relative to the composite reference standard, the observed values were 0.69 (0.65 to 0.72), 0.98 (0.98 to 0.99), 37.50 (18.59 to 75.62), 0.30 (0.21 to 0.43), 171.98 (80.82 to 365.96), and 0.9691 (0.9683 to 0.9699), respectively. All subgroups, except children, showed high sensitivity and specificity. In conclusion, the use of Xpert MTB/RIF in the context of BAL samples has a high diagnostic performance for PTB (except for children) and may serve as an alternative rapid diagnostic tool.
INTRODUCTION
The World Health Organization (WHO) has identified tuberculosis as 1 of the top 10 leading causes of death worldwide (1). The early diagnosis of pulmonary tuberculosis (PTB) is essential to reduce the spread, morbidity, mortality, and escalating costs associated with advanced disease (2). Traditional diagnostic tools, including sputum acid-fast bacillus (AFB) smear and Mycobacterium culture, are fraught with challenges. Mycobacterium tuberculosis culture based on solid media takes up to 4 to 8 weeks, and the sensitivity of the traditional AFB smear can be as low as 20% (3, 4). Additionally, although the M. tuberculosis liquid culture method is faster, it still takes 2 to 4 weeks (4). Moreover, tuberculosis culture methods to support the diagnosis of PTB are not widely available in high-burden settings (5).
In 2011, the WHO recommended the Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA) for the diagnosis of PTB and the detection of rifampicin resistance. The Xpert MTB/RIF assay is an automated, single-cartridge-based nucleic acid amplification test that is able to simultaneously detect M. tuberculosis and rifampicin resistance within 2 to 3 h. Currently, Xpert MTB/RIF often uses sputum samples to diagnose PTB. Remarkably, a small clinical validation study of the Xpert MTB/RIF test using 107 clinical sputum samples from suspected tuberculosis cases in Vietnam detected 29/29 (100%) smear-positive culture-positive cases and 33/39 (84.6%) or 38/53 (71.7%) smear-negative culture-positive cases, as determined by growth on solid medium or on both solid and liquid media, respectively (6). Additionally, another large sample study showed that among culture-positive patients, the Xpert MTB/RIF test identified 551 of 561 patients with smear-positive tuberculosis (98.2%) and 124 of 171 with smear-negative tuberculosis (72.5%) (7), suggesting that the sensitivity of the Xpert MTB/RIF test for patients with smear-negative tuberculosis is lower. However, there is a considerable proportion of PTB patients with a negative sputum test, or sputum-scarce PTB, in the clinical practice. Interestingly, fiberoptic bronchoscopy was suggested as a helpful approach for the diagnosis of sputum smear-negative and sputum-scarce PTB, once it provides high-quality biological samples such as bronchoalveolar lavage fluid (BAL) (8). Some evidence has indicated that the performance of the Xpert MTB/RIF method using BAL is superior to that using sputum samples for the diagnosis of PTB, especially for patients with sputum smear-negative PTB (9). However, a systematic review of the Xpert MTB/RIF method using BAL for the diagnosis of PTB was never performed. Thus, the purpose of the present meta-analysis was to clarify the value of the Xpert MTB/RIF test using BAL for the diagnosis of PTB.
METHODS
Trial search.
Two researchers independently searched all studies published in the PubMed, EMBASE, and Web of Science databases using the key terms “pulmonary tuberculosis,” “Xpert MTB/RIF,” and “bronchoalveolar lavage fluid;” all studies published until 31 December 2019 were considered. Two evaluators independently screened the literature, extracted the data, and assessed the bias risk of the included studies. When the opinions of the two researchers did not match, a third researcher was consulted.
Inclusion and exclusion criteria.
The inclusion and exclusion criteria for the studies were determined prior to data extraction. The inclusion criteria were as follows: (i) the subjects were suspected PTB patients; (ii) the diagnosis method was the Xpert MTB/RIF assay; (iii) the samples used were BAL; (iv) the reference tests used were M. tuberculosis cultures (tuberculosis culture based on solid or liquid media) or the composite reference standard (PTB was defined as a positive culture or clinically diagnosed based on the clinical symptoms of the patients, including cough for more than 2 weeks, fever, or weight loss, pneumonia that did not improve using antibiotics, or contact with an adult who had tuberculosis); and (v) the available data were used for calculating the sensitivity, specificity, and likelihood ratios. The exclusion criteria included the following: (i) republished literature;( ii) letters, abstracts, or conference abstracts; (iii) the use of non-Xpert MTB/RIF methods for the detection of tuberculosis; (iv) incomplete data; and (v) unrelated literature. Based on the above criteria, the researchers used EndNote X7 software (Thomson Corporation, Stamford, CT) to remove duplicated studies.
Data extraction and methodological quality evaluation.
The literature selection and data extraction were carried out by two independent researchers. Any inconsistencies were solved through discussion. The extracted data included the following: first author; publication year; age; country; study design; reference test (M. tuberculosis culture method); the preanalytic procedure of the BAL samples (using concentrated BAL and/or digested BAL); sputum test (smear-positive; smear-negative or sputum scarce); the number of samples; test results involving true positives, false positives, false negatives, and true negatives; and additional relevant items for bias risk assessment. The risk of bias in each study was evaluated independently by two researchers using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (10). A “yes” (low degree of bias risk or good applicability), “no” (high degree of bias risk or poor applicability), or “unclear” (lack of relevant information or uncertainty of bias risk) designation was attributed to each item to define the quality of each study.
Statistical analysis.
The Stata 15.1 (StataCorp, College Station, TX), Meta-Disc 1.4 (Clinical Biostatistics Unit, Madrid, Spain), and Review Manager 5.2 (Cochrane Collaboration, Oxford, UK) software tools were used for this meta-analysis. The overall sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence interval (CI) and the area under the curve (AUC) in the context of the Xpert MTB/RIF test were calculated. Heterogeneity was evaluated using I2 statistics. The I2 was interpreted as follows: 0% to 25%, no significant heterogeneity; 26 to 50%, low heterogeneity; 51% to 75%, moderate heterogeneity; and >75%, high heterogeneity. Spearman correlation was employed to determine the presence of a threshold effect (a strong correlation indicated a threshold effect). Meta-regression and subgroup analyses were conducted using six independent variables, including age (children or adults), country (developed or developing country), study design (retrospective or prospective), sputum test (smear positive or smear negative/sputum scarce), culture method of the reference test (liquid culture or other culture methods) and the preanalytic procedure of BAL (yes or no). Using DOR as the dependent variable and the reciprocal of the square root of the effective sample size as the independent variable, a Deeks funnel plot was generated to evaluate the publication bias. P values lower than 0.05 were considered statistically significant.
RESULTS
Literature screening and inclusion.
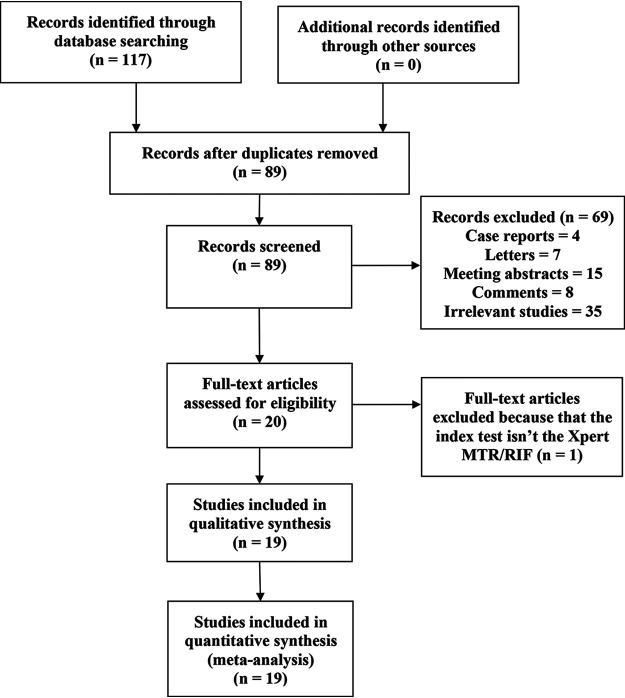
A total of 117 documents were obtained from the initial examination. After removal of duplicates and irrelevant records, 19 studies met the inclusion criteria (Fig. 1).
FIG 1.
Literature search and screening process.
Characteristics and quality assessment of the included studies.
Altogether, 3,019 samples from the 19 studies were included in this meta-analysis (11–29). There were 7 prospective studies and 12 retrospective studies. The M. tuberculosis culture method was used as the reference standard in 18 studies, whereas the clinical composite diagnosis method was used as the reference standard in 9 studies. Among the studies, 16 involved adults and the other 3 focused on children. The characteristics of the included trials and participants are summarized in Table 1.
TABLE 1.
Characteristics of the included studies and their respective participants
| Study no. | Authors (reference) | Yr | Country | Age category | Sputum smear | Study design | Reference standard | Preanalytic procedure | Sample size | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Theron et al. (9) | 2013 | South Africa | Adults | Negative | Prospective | Culture (liquid) | Yes | 152 | 25 | 5 | 2 | 120 |
| 2 | Barnard et al. (11) | 2015 | South Africa | Adults | Negative | Retrospective | Culture (liquid) | No | 112 | 36 | 9 | 3 | 64 |
| CRSa | 112 | 39 | 6 | 5 | 62 | ||||||||
| 3 | Feliciano et al. (12) | 2019 | Brazil | Adults | Positive | Retrospective | Culture (liquid) | Unclear | 348 | 19 | 8 | 1 | 320 |
| CRS | 348 | 22 | 5 | 1 | 320 | ||||||||
| 4 | Gowda et al. (13) | 2018 | India | Adults | Negative | Prospective | Culture (liquid) | No | 56 | 13 | 11 | 3 | 29 |
| CRS | 60 | 24 | 0 | 28 | 8 | ||||||||
| 5 | Khalia and Butt (14) | 2014 | Pakistan | Adults | Negative | Prospective | Culture (liquid) | Unclear | 93 | 79 | 2 | 7 | 5 |
| 6 | Kilaru et al. (15) | 2019 | India | Adults | Negative | Prospective | Culture (liquid) | No | 56 | 9 | 22 | 1 | 24 |
| 7 | To et al. (16) | 2018 | Hong Kong | Adults | Positive | Prospective | Culture (uncertain) | Unclear | 223 | 32 | 7 | 6 | 178 |
| CRS | 223 | 36 | 3 | 9 | 175 | ||||||||
| 8 | Lee et al. (17) | 2018 | Korea | Adults | Negative | Retrospective | Culture (both) | No | 132 | 31 | 0 | 7 | 94 |
| 9 | Lu et al. (18) | 2018 | China | Adults | Positive | Retrospective | Culture (both) | Unclear | 238 | 49 | 2 | 9 | 178 |
| CRS | 238 | 62 | 2 | 23 | 151 | ||||||||
| 10 | Mok et al. (19) | 2016 | Singapore | Adults | Negative | Retrospective | Culture (liquid) | Unclear | 158 | 30 | 0 | 14 | 112 |
| 11 | Le Palud et al. (20) | 2014 | France | Adults | Negative | Retrospective | Culture (liquid) | Yes | 162 | 16 | 2 | 4 | 140 |
| CRS | 162 | 18 | 0 | 12 | 132 | ||||||||
| 12 | Pan et al. (21) | 2018 | China | Adults | Positive | Prospective | Culture (liquid) | Yes | 190 | 64 | 18 | 13 | 95 |
| CRS | 190 | 80 | 2 | 51 | 57 | ||||||||
| 13 | Saini et al. (22) | 2018 | India | Children | Positive | Prospective | Culture (liquid) | Unclear | 41 | 9 | 15 | 2 | 15 |
| 14 | Silva et al. (23) | 2018 | Brazil | Adults | Positive | Retrospective | Culture (solid) | Unclear | 199 | 19 | 9 | 0 | 171 |
| 15 | Ullah et al. (24) | 2017 | Pakistan | Adults | Positive | Prospective | Culture (uncertain) | Yes | 88 | 32 | 6 | 8 | 42 |
| 16 | Walters et al. (25) | 2013 | South Africa | Children | Positive | Prospective | Culture (liquid) | No | 14 | 7 | 2 | 2 | 3 |
| 17 | Xu et al. (26) | 2019 | China | Adults | Positive | Prospective | Culture (uncertain) | Yes | 182 | 75 | 3 | 2 | 102 |
| 18 | Yin et al. (27) | 2014 | China | Children | Positive | Prospective | CRS | Unclear | 251 | 44 | 0 | 39 | 168 |
| 19 | Jo et al. (28) | 2016 | Korea | Adults | Positive | Retrospective | Culture (uncertain) | Unclear | 320 | 59 | 47 | 5 | 209 |
| CRS | 320 | 104 | 2 | 26 | 188 |
CRS, composite reference standard.
Figure 2 shows the quality and applicability evaluations of the 19 included studies. With respect to patient selection, 14 studies had a low risk of bias, whereas the remaining 5 were rated as “unclear” owing to unclear recruitment procedures. Regarding the inclusion of patients in the applicability analysis, 12 studies were rated as “of high concern” because their patient populations were assessed in a tertiary hospital center rather than the local community or primary hospital, and 7 studies were defined as “of unknown concern.” Concerning index testing and reference standards, all bias risks in the studies as well as their applicability were rated as “of low concern.”
FIG 2.
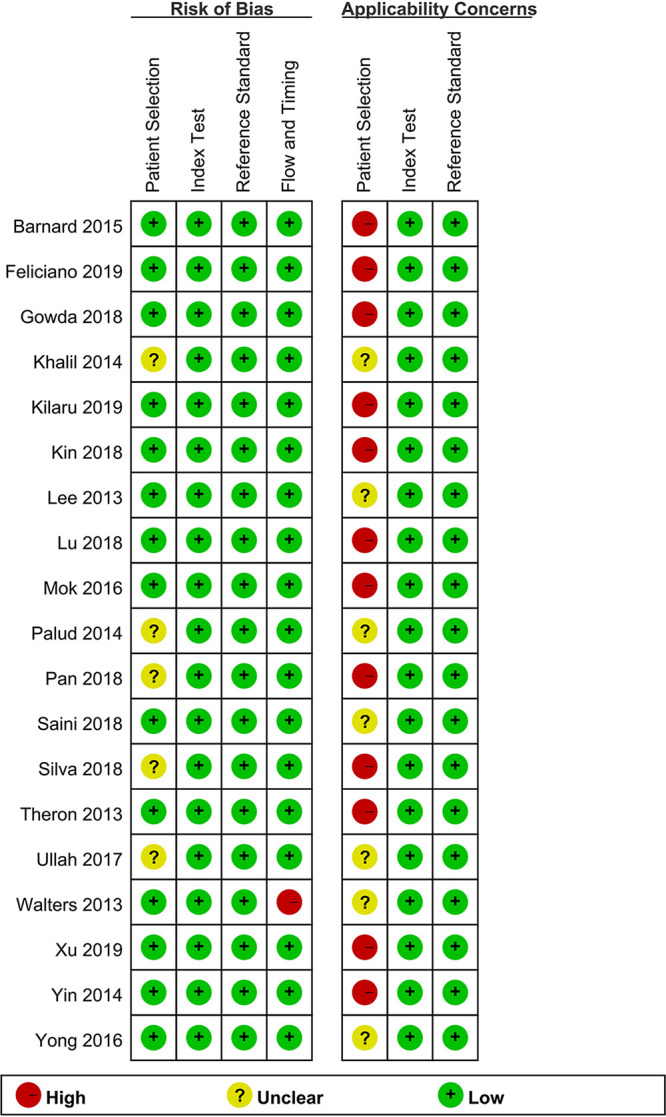
Bias risks and applicability concerns.
Meta-analysis results of Xpert MTB/RIF.
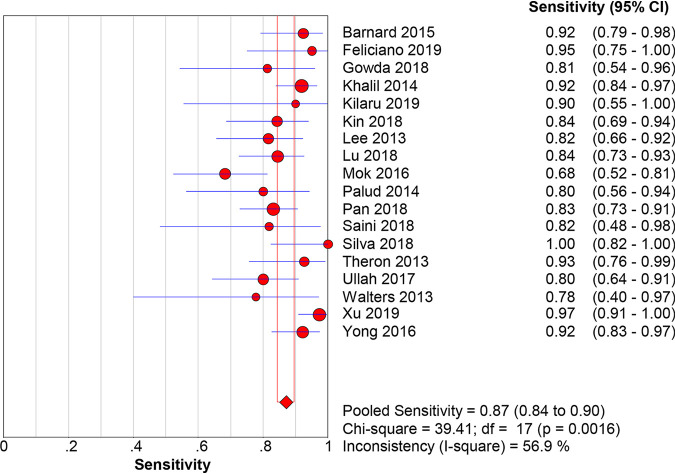
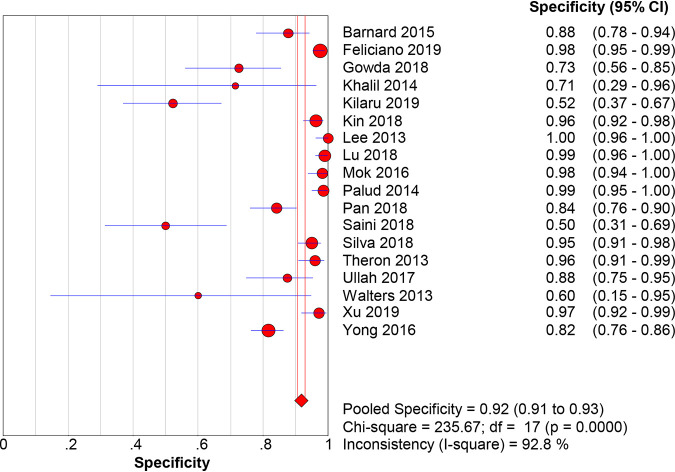
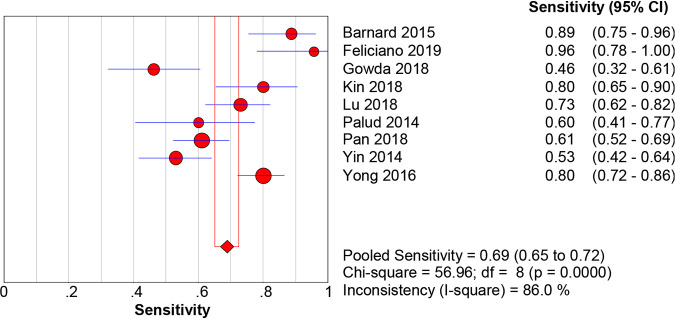
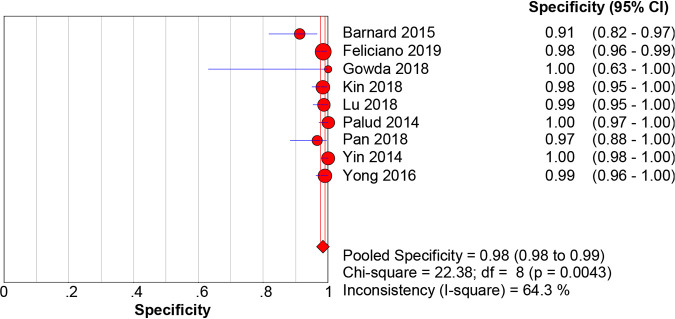
When M. tuberculosis culture was used as the gold standard for diagnosis, the pooled sensitivity, specificity, PLR, NLR, DOR, and AUC with 95% CIs of Xpert MTB/RIF using BAL for the diagnosis of PTB were 0.87 (0.84 to 0.90), 0.92 (0.91 to 0.93), 10.21 (5.78 to 18.02), 0.16 (0.12 to 0.22), 78.95 (38.59 to 161.53), and 0.9467 (0.9462 to 0.9472), respectively (Fig. 3 and 4; Fig. S1). When the composite reference standard (CRS) was the reference method, the pooled sensitivity, specificity, PLR, NLR, DOR, and AUC with 95% CIs of Xpert MTB/RIF using BAL for the diagnosis of M. tuberculosis were 0.69 (0.65 to 0.72), 0.98 (0.98 to 0.99), 37.50 (18.59 to 75.62), 0.30 (0.21 to 0.43), 171.98 (80.82 to 365.96), and 0.9691 (0.968 to 0.9699), respectively (Fig. 5 and 6; Fig. S2). Of note, there was a significant heterogeneity (I2 > 75%) in the pooled sensitivity and specificity among the studies.
FIG 3.
The pooled sensitivity of Xpert MTB/RIF using bronchoalveolar lavage fluid for the diagnosis of PTB (versus the culture method). The diamond represents the pooled sensitivity; lines represent the respective 95% confidence intervals (CI).
FIG 4.
The pooled specificity of Xpert MTB/RIF using bronchoalveolar lavage fluid for the diagnosis of PTB (versus the culture method). The diamond represents the pooled sensitivity; lines represent the respective 95% CI.
FIG 5.
The pooled sensitivity of Xpert MTB/RIF using bronchoalveolar lavage fluid for the diagnosis of PTB (versus the composite reference standard method). The diamond represents the pooled sensitivity; lines represent the respective 95% CI.
FIG 6.
The pooled specificity of Xpert MTB/RIF using bronchoalveolar lavage fluid for the diagnosis of PTB (versus the composite reference standard). The diamond represents the pooled sensitivity; lines represent the respective 95% CI.
Meta-regression and subgroup analyses of the sensitivity and specificity.
The meta-regression analyses showed that the sensitivity values were consistent between subgroups based on different countries, age, study designs, sputum test, culture method of the reference test, and preanalytic procedure (P > 0.05). The specificity values were also consistent between subgroups based on different countries, study designs, sputum test, culture method of the reference test, and preanalytic procedure (P > 0.05); however the specificity (0.92 [0.90 to 0.95]) in adults was higher than that (0.51 [0.35 to 0.68]) in children (P < 0.05) (Table 2). Of note, subgroup analyses revealed that the sensitivity and specificity of the Xpert MTB/RIF test using BAL for sputum smear-negative patients with PTB were 0.86 (0.80 to 0.92) and 0.93 (0.86 to 1.00), respectively (Table 2). Together, our data showed slight fluctuations in the sensitivity and specificity between subgroups; however, all subgroups showed high sensitivity and specificity except for children (Table 2).
TABLE 2.
Sensitivity and specificity subgroup analyses: different regions, economic statuses, study designs, sputum smear statuses, and ages
| Parameter (no. of studies) | Sensitivity (95% CI) | P | Specificity (95% CI) | P |
|---|---|---|---|---|
| Age | ||||
| Adult (16) | 0.88 (0.84–0.92) | >0.05 | 0.92 (0.90–0.95) | <0.05 |
| Children (2) | 0.81 (0.60–1.00) | 0.51 (0.35–0.68) | ||
| Country | ||||
| Developed countries (5) | 0.83 (0.75–0.90) | >0.05 | 0.97 (0.94–1.00) | >0.05 |
| Developing countries (13) | 0.90 (0.86–0.93) | 0.89 (0.81–0.96) | ||
| Study design | ||||
| Retrospective studies (8) | 0.86 (0.79–0.92) | >0.05 | 0.96 (0.92–1.00) | >0.05 |
| Prospective studies (10) | 0.89 (0.85–0.94) | 0.88 (0.78–0.98) | ||
| Sputum test | ||||
| Sputum smear negative/sputum scarce (8) | 0.86 (0.80–0.92) | >0.05 | 0.93 (0.86–1.00) | >0.05 |
| Sputum smear positive (10) | 0.89 (0.85–0.94) | 0.92 (0.85–0.99) | ||
| Culture method | ||||
| Liquid or both media (14) | 0.87 (0.82–0.91) | >0.05 | 0.91 (0.85–0.98) | >0.05 |
| Solid media or uncertain (4) | 0.91 (0.85–0.97) | 0.95 (0.88–1.00) | ||
| Preanalytic procedure | ||||
| Yes (5) | 0.88 (0.82–0.95) | >0.05 | 0.95 (0.89–1.00) | >0.05 |
| No or uncertain (13) | 0.88 (0.83–0.92) | 0.91 (0.84–0.98) |
Threshold analysis.
When M. tuberculosis culture and CRS were each considered the reference standard for diagnosis, the Spearman correlation coefficients were −0.011 and 0.243, respectively (P > 0.05). No threshold effects were observed.
Publication bias.
No publication bias was found as per the Deeks funnel plot with a P value of 0.1 (Fig. S3).
DISCUSSION
At present, M. tuberculosis culture methods and sputum smears are often used in the laboratory diagnosis of PTB (29–31). However, diagnosis using sputum smear is not a good option for PTB. In addition, the specificity of the sputum smear is limited because acid-fast staining is unable to distinguish between M. tuberculosis and non-M. tuberculosis specimens (31, 32). M. tuberculosis culture is generally considered the gold standard for the diagnosis of tuberculosis, but this method tends to be time-consuming (report period, 2 to 8 weeks) (31, 32), which is less than ideal for the diagnosis of PTB.
As a fast and automatic molecular testing tool, Xpert MTB/RIF has been reported to display potential value using sputum samples for the diagnosis of PTB. This said, in clinical practice (29–33), using BAL samples may be necessary and valuable in the context of sputum smear-negative or sputum-scarce patients. However, the performance of Xpert MTB/RIF in the context of the diagnosis of PTB using BAL samples remains unclear.
According to the results of this meta-analysis, the use of Xpert MTB/RIF for the diagnosis of PTB using BAL samples had higher sensitivity and specificity. The high AUCs (>0.9) indicated that the Xpert MTB/RIF method using BAL has a high diagnostic value in PTB. However, the PLRs (>10) and NLR (>0.1) revealed that the advantage of Xpert MTB/RIF was in terms of confirmation rather than of exclusion. Therefore, a negative result in the context of the Xpert MTB/RIF method cannot completely rule out M. tuberculosis infection and should be interpreted with caution.
In this study, neither threshold effects nor publication bias was observed. However, there was substantial heterogeneity in the sensitivity and specificity among studies. Of note, heterogeneity can affect the accuracy of meta-analysis results, impacting their validity. Importantly, most of the individual studies revealed higher diagnostic performance based on the overall results of sensitivity and specificity.
According to the results of our meta-regression, the sensitivities and the specificities of Xpert MTB/RIF were consistent between subgroups based on different countries, study designs, sputum smear results, culture method of the reference test, and preanalytic procedure; however, the specificity in adults was higher than that in children.
Emphatically, the subgroup analysis revealed that the sensitivity and specificity of the Xpert MTB/RIF method using BAL has a high diagnostic value for smear-negative patients with PTB. Meanwhile, low specificity was observed for diagnosis in children. However, this conclusion may not be completely reliable owing to the small number of included studies and of their high heterogeneity. Data from a previous meta-analysis reported that the positive rate of detection from sputum smears in children is low, ranging from 0.5% in children 0 to 4 years old to 14% in children 5 to 14 years old (34). However, Xpert MTB/RIF was reported to have increased clinical value for the rapid and accurate diagnosis of PTB in children when fiber-optic bronchoscopy was recommended based on a chest radiograph (35).
In clinical practice, Xpert MTB/RIF using BAL has obvious advantages. For sputum smear-negative patients with clinical radiologic features of active PTB, the Xpert MTB/RIF assay using BAL displays high sensitivity (13). Moreover, Xpert MTB/RIF using BAL could achieve an early diagnosis of PTB, contributing to early patient treatment (32).
However, Xpert MTB/RIF relies on the availability of complex instruments and has a high cost; moreover, the collection of BAL requires invasive procedures, especially in children with poor compliance. Therefore, its clinical application is limited to hospitals. At present, the tool is mainly used in tertiary hospitals. Although we included 19 studies and conducted a large sample meta-analysis, this review still has the following limitations: (i) the methodological quality of the included studies was not high owing to constraints on the level of study design; (ii) some subgroups, such as children, were included only in a small number of studies; (iii) substantial heterogeneity with an unknown source was observed; and (iv) this study did not include unpublished literature. Therefore, systematic research should be continued in the future.
In conclusion, this systematic review and meta-analysis showed that the Xpert MTB/RIF test using BAL has a high diagnostic performance for PTB and may serve as an alternative rapid diagnostic tool, especially for suspected PTB patients with a negative sputum test or sputum-scarce PTB. However, for children, the specificity of this method is low.
Supplementary Material
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
This project is supported by Shanghai Pudong New Area Science and Technology Development Fund (PKJ2020-Y12).
Study design, C.-T.Z., H.-C.L., Y.-Q.P., and Y.-L.G.; literature search, data collection, and analysis, H.-C.L., C.-T.Z., Y.-L.G., D.-F.L., and X.-Y.Z.; manuscript writing and editing, H.-C.L., C.-T.Z., Y.-Q.P., and Y.-L.G.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, Aledort JE, Hillborne L, Rafael ME, Girosi F, Dye C. 2006. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 444:49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 3.Pfyffer GE, Wittwer F. 2012. Incubation time of mycobacterial cultures: how long is long enough to issue a final negative report to the clinician? J Clin Microbiol 50:4188–4189. doi: 10.1128/JCM.02283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie SR, Harrison AC, Vaughan RH, Calder L, Morris AJ. 2007. New recommendations for duration of respiratory isolation based on time to detect Mycobacterium tuberculosis in liquid culture. Eur Respir J 30:501–507. doi: 10.1183/09031936.00131406. [DOI] [PubMed] [Google Scholar]
- 5.Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Detjen AK, Steingart KR, Mandalakas AM. 2020. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev 8:CD013359. doi: 10.1002/14651858.CD013359.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-López EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan A, Sharma SK. 2008. Fibre optic bronchoscopy in the diagnosis of sputum smear-negative pulmonary tuberculosis: current status. Indian J Chest Dis Allied Sci 50:67–78. [PubMed] [Google Scholar]
- 9.Theron G, Peter J, Meldau R, Khalfey H, Gina P, Matinyena B, Lenders L, Calligaro G, Allwood B, Symons G, Govender U, Setshedi M, Dheda K. 2013. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax 68:1043–1051. doi: 10.1136/thoraxjnl-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group. 2011. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 11.Barnard DA, Irusen EM, Bruwer JW, Plekker D, Whitelaw AC, Deetlefs JD, Koegelenberg CF. 2015. The utility of Xpert MTB/RIF performed on bronchial washings obtained in patients with suspected pulmonary tuberculosis in a high prevalence setting. BMC Pulm Med 15:103. doi: 10.1186/s12890-015-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feliciano CS, Menon LJB, Anselmo LMP, Dippenaar A, Warren RM, Silva WA, Jr, Bollela VR. 2019. Xpert MTB/RIF performance to diagnose tuberculosis and rifampicin resistance in a reference centre in southern Brazil. ERJ Open Res 5:00043-2019. doi: 10.1183/23120541.00043-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gowda N, Ray A, Soneja M, Khanna A, Sinha S. 2018. Evaluation of Xpert® Mycobacterium tuberculosis/rifampin in sputum-smear negative and sputum-scarce patients with pulmonary tuberculosis using bronchoalveolar lavage fluid. Lung India 35:295–300. doi: 10.4103/lungindia.lungindia_412_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalil KF, Butt T. 2015. Diagnostic yield of bronchoalveolar lavage gene Xpert in smear-negative and sputum-scarce pulmonary tuberculosis. J Coll Physicians Surg Pak 25:115–118. doi: 10.2015/JCPSP.115118. [DOI] [PubMed] [Google Scholar]
- 15.Kilaru SC, Chenimilla NP, Syed U, Momin K, Kilaru H, Patil E, Nerurkar V. 2019. Role of Xpert MTB/RIF in bronchoalveolar lavage fluid of sputum-scarce, suspected pulmonary TB patients. J Clin Tuberc Other Mycobact Dis 14:7–11. doi: 10.1016/j.jctube.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To KW, Kam KM, Chan DPC, Yip WH, Chan KP, Lo R, Ng S, Ngai J, Lee SS. 2018. Utility of GeneXpert in analysis of bronchoalveolar lavage samples from patients with suspected tuberculosis in an intermediate-burden setting. J Infect 77:296–301. doi: 10.1016/j.jinf.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Lee HY, Seong MW, Park SS, Hwang SS, Lee J, Park YS, Lee CH, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ. 2013. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuber Lung Dis 17:917–921. doi: 10.5588/ijtld.12.0885. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Zhu Y, Shen N, Tian L, Sun Z. 2018. Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. Int J Infect Dis 71:14–19. doi: 10.1016/j.ijid.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Mok Y, Tan TY, Tay TR, Wong HS, Tiew PY, Kam JW, Siao C. 2016. Do we need transbronchial lung biopsy if we have bronchoalveolar lavage Xpert® MTB/RIF? Int J Tuber Lung Dis 20:619–624. doi: 10.5588/ijtld.15.0463. [DOI] [PubMed] [Google Scholar]
- 20.Le Palud P, Cattoir V, Malbruny B, Magnier R, Campbell K, Oulkhouir Y, Zalcman G, Bergot E. 2014. Retrospective observational study of diagnostic accuracy of the Xpert® MTB/RIF assay on fiberoptic bronchoscopy sampling for early diagnosis of smear-negative or sputum-scarce patients with suspected tuberculosis. BMC Pulm Med 14:137. doi: 10.1186/1471-2466-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan X, Yang S, Deighton MA, Qu Y, Hong L, Su F. 2018. A comprehensive evaluation of Xpert MTB/RIF assay with bronchoalveolar lavage fluid as a single test or combined with conventional assays for diagnosis of pulmonary tuberculosis in China: a two-center prospective study. Front Microbiol 9:444. doi: 10.3389/fmicb.2018.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini I, Mukherjee A, Gautam H, Singla M, Jat KR, Lodha R, Singh UB, Kabra SK. 2018. Diagnostic yield of Xpert MTB/RIF in bronchoalveolar lavage in children with probable pulmonary tuberculosis. Indian Pediatr 55:1062–1065. doi: 10.1007/s13312-018-1443-9. [DOI] [PubMed] [Google Scholar]
- 23.Silva TMD, Soares VM, Ramos MG, Santos AD. 2019. Accuracy of a rapid molecular test for tuberculosis in sputum samples, bronchoalveolar lavage fluid, and tracheal aspirate obtained from patients with suspected pulmonary tuberculosis at a tertiary referral hospital. J Bras Pneumol 45:e20170451. doi: 10.1590/1806-3713/e20170451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullah I, Javaid A, Masud H, Ali M, Basit A, Ahmad W, Younis F, Yasmin R, Khan A, Jabbar A, Husain M, Butt ZA. 2017. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear-negative pulmonary suspects using Xpert MTB/RIF. J Med Microbiol 66:412–418. doi: 10.1099/jmm.0.000449. [DOI] [PubMed] [Google Scholar]
- 25.Walters E, Goussard P, Bosch C, Hesseling AC, Gie RP. 2014. GeneXpert MTB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: a pilot study. Pediatr Pulmonol 49:1133–1137. doi: 10.1002/ppul.22970. [DOI] [PubMed] [Google Scholar]
- 26.Xu P, Tang P, Song H, Zhao J, Chen H, Xue J, Zhai Y, Pang Y, Wu M. 2019. The incremental value of bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in a high-burden urban setting. J Infect 79:24–29. doi: 10.1016/j.jinf.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Yin QQ, Jiao WW, Han R, Jiao AX, Sun L, Tian JL, Ma YY, Rao XC, Shen C, Li QJ, Shen AD. 2014. Rapid diagnosis of childhood pulmonary tuberculosis by Xpert MTB/RIF assay using bronchoalveolar lavage fluid. Biomed Res Int 2014:1–6. doi: 10.1155/2014/310194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo YS, Park J, Lee JK, Heo EY, Chung HS, Kim DK. 2016. Discordance between MTB/RIF and real-time tuberculosis-specific polymerase chain reaction assay in bronchial washing specimen and its clinical implications. PLoS One 11:e164923. doi: 10.1371/journal.pone.0164923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guenaoui K, Harir N, Ouardi A, Zeggai S, Sellam F, Bekri F, Cherif Touil S. 2016. Use of GeneXpert Mycobacterium tuberculosis/rifampicin for rapid detection of rifampicin resistant Mycobacterium tuberculosis strains of clinically suspected multi-drug resistance tuberculosis cases. Ann Transl Med 4:168. doi: 10.21037/atm.2016.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, Drobniewski F, Lalvani A. 2007. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 11:1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 31.Nicol MP. 2013. Xpert MTB/RIF: monitoring response to tuberculosis treatment. Lancet Respir Med 1:427–428. doi: 10.1016/S2213-2600(13)70133-4. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich SO, Rachow A, Saathoff E, Singh K, Mangu CD, Dawson R, Phillips PP, Venter A, Bateson A, Boehme CC, Heinrich N, Hunt RD, Boeree MJ, Zumla A, McHugh TD, Gillespie SH, Diacon AH, Hoelscher M. 2013. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Me 1:462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 33.Rie AV, Page-Shipp L, Scott L, Sanne I, Stevens W. 2010. Xpert® MTB/RIF for point-of-care diagnosis of TB in high-HIV burden, resource-limited countries: hype or hope? Expert Rev Mol Diagn 10:937–946. doi: 10.1586/erm.10.67. [DOI] [PubMed] [Google Scholar]
- 34.Kunkel A, Abel Zur Wiesch P, Nathavitharana RR, Marx FM, Jenkins HE, Cohen T. 2016. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infect Dis 16:282. doi: 10.1186/s12879-016-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L, Qi X, Liu F, Wu X, Yin Q, Guo Y, Xu B, Jiao A, Guo Y, Jiao W, Shen C, Xiao J, Shen A. 2019. A test for more accurate diagnosis of pulmonary tuberculosis. Pediatrics 144:e20190262. doi: 10.1542/peds.2019-0262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.