We evaluated the CLSI M44ed3E disk diffusion method compared with the CLSI M27ed4 broth microdilution method for caspofungin and fluconazole and the Etest method for amphotericin B to categorize susceptibility of 347 clinical isolates of Candida auris. Utilizing the zone diameter cutoffs established here, we observed overall categorical agreement between the two methods.
KEYWORDS: Candida auris, disk diffusion, fluconazole, amphotericin B, caspofungin, antifungal susceptibility, antifungal, mycology, susceptibility
ABSTRACT
We evaluated the CLSI M44ed3E disk diffusion method compared with the CLSI M27ed4 broth microdilution method for caspofungin and fluconazole and the Etest method for amphotericin B to categorize susceptibility of 347 clinical isolates of Candida auris. Utilizing the zone diameter cutoffs established here, we observed overall categorical agreement between the two methods. For caspofungin, concordant results were observed for 98% of isolates, with <1% very major and 1% major errors. For fluconazole, concordant results were observed for 91% of isolates, with 1% very major and 8% major errors. For amphotericin B, concordant results were observed for 74% of isolates, with <1% very major errors and 25% major errors. The disk diffusion approach provides an accurate method for determining the susceptibility of C. auris for caspofungin and fluconazole and for identification of at least 75% of amphotericin B-susceptible isolates.
INTRODUCTION
Candida auris is an emerging multidrug-resistant yeast that causes invasive infections with high mortality (1). Within a short time, C. auris has emerged in health care settings in more than 30 countries (2). Transmission of C. auris is aided by its ability to colonize skin and persist on surfaces for weeks (3). The infection is challenging to treat, as resistance to fluconazole is widespread and susceptibility to other azoles, amphotericin B and the echinocandins, is variable (4). There is an additional encumbrance to susceptibility testing in resource-limited countries due to a paucity of facilities able to afford or to perform the testing (5).
Disk diffusion is a cost-effective susceptibility testing method conventionally used in clinical laboratories for bacterial pathogens (6). This method is reproducible, easy to interpret, and the least costly of susceptibility methods. Conversely, the standard broth microdilution method requires expertise and specialized equipment and can be laborious; both of these limitations pose challenges to clinical laboratories, especially in resource-limited countries (7).
The purpose of this study was to evaluate the M44ed3E disk diffusion method to determine the in vitro susceptibility of C. auris to 10 μg amphotericin B, 5 μg caspofungin, and 25 μg fluconazole. For each isolate, the disk diffusion zone diameters were compared to the MICs determined by the M27ed4 broth microdilution method or by Etest for amphotericin B.
MATERIALS AND METHODS
We tested 347 clinical isolates of C. auris from various body sites (Table 1). Isolates were identified by matrix-assisted laser desorption ionization–time of flight (Bruker Daltonics) using a laboratory-developed database (8). Isolates were stored at –80°C in 20% glycerol until used.
TABLE 1.
Candida auris isolate dataa
| Antifungal | Zone diam cutoff, in mm | Susceptible isolates | Indeterminate isolates | Resistant isolates |
|---|---|---|---|---|
| Caspofungin | ≤12 | 315 (91) | 24 (7) | |
| Fluconazole | ≤12 | 48 (14) | 267 (77) | |
| Amphotericin B | ≤17 | 191 (55) | 66b (19) | 66 |
The isolates were tested using the disk diffusion method. Except for zone diameter cutoffs, data are presented as number (%).
With the high number of major errors, this category should be indeterminate rather than resistant.
Reference antifungal susceptibility testing of C. auris for fluconazole and caspofungin was performed according to the broth microdilution method described in CLSI document M27ed4. The MIC endpoints were read visually after 24 h of incubation. Gradient diffusion susceptibility testing for amphotericin B was performed as previously described (4). Disk diffusion testing was performed as described in CLSI document M44ed3E. Briefly, 150-mm-diameter plates containing Mueller-Hinton agar (made in-house) supplemented with 2% glucose and methylene blue were used. A swab dipped in a cell suspension adjusted to 0.5 McFarland standard turbidity was used to inoculate the agar. The plates were subsequently incubated at 35°C and read at 24 h. The zone diameter endpoints were read at 100% growth inhibition with calipers. Amphotericin B (10 μg), caspofungin (5 μg), and fluconazole (25 μg) disks were purchased from Liofilchem, Inc. Isolates were tested in duplicate, and two different lot numbers were used for each antifungal tested. Isolates ATCC 22019 and ATCC 6258 were tested multiple times and always found to be in range, as defined in CLSI M60ed2 (9).
The CDC-developed breakpoints were used for this study: caspofungin resistance, MIC of ≥2 μg/ml; fluconazole resistance, MIC of ≥32 μg/ml; and amphotericin B resistance, MIC of ≥2 (1.5 by Etest) μg/ml (10). The breakpoints were developed based on MIC distribution, known molecular mechanisms of resistance, and pharmacokinetic/pharmacokinetic data based on a mouse model of infection (11). The breakpoints were used to determine categorical agreement between the two methods. Major errors were identified as a classification of resistant by the disk test and susceptible by broth microdilution. Very major errors were identified as a classification of susceptible by the disk diffusion test and resistant by broth microdilution.
RESULTS
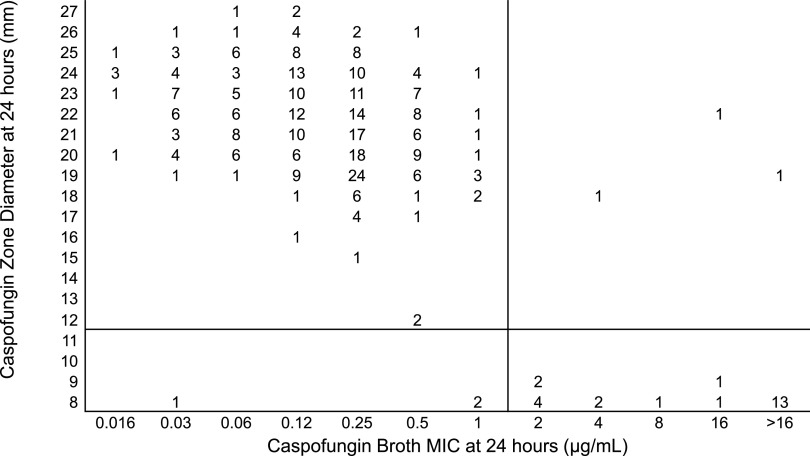
Most isolates had a low MIC value to caspofungin. Figure 1 shows the relationship between the MIC determined using broth microdilution with ≥2 μg/ml as a breakpoint for resistance and zone diameters using 5-μg caspofungin disks. Selection of ≤12 mm as the zone diameter cutoff resulted in 98% categorical agreement between the results obtained by the two methods (339 of 347 isolates had concordant results). With this cutoff, 5 (1%) major and 3 (<1%) very major errors were observed.
FIG 1.
Zones of inhibition for 347 isolates of C. auris around 5-μg caspofungin disks on Mueller-Hinton methylene blue agar plotted against the 24-h MICs determined by the reference broth microdilution method. The numbers inside the graph indicate the number of isolates. The black horizontal line is the suggested breakpoint for the disk diffusion method.
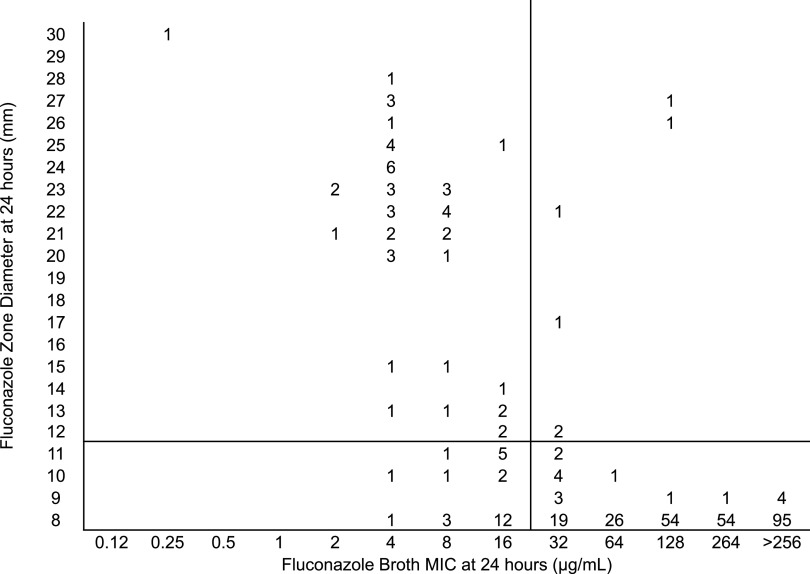
Most of the isolates had a high MIC value to fluconazole. Figure 2 shows the relationship between broth microdilution MICs, with ≥32 μg/ml as the breakpoint for resistance and zone diameters using 25-μg fluconazole disks. Selection of ≤12-mm zone diameter cutoff resulted in 91% categorical agreement between the two methods (315 of 347 isolates were concordant). Using these parameters, 26 (7%) major errors but only 6 (2%) very major errors were observed.
FIG 2.
Zones of inhibition for 347 isolates of C. auris around 25-μg fluconazole disks on Mueller-Hinton methylene blue agar plotted against the 24-h MICs determined by the reference broth microdilution method. The numbers inside the graph indicate the number of isolates. The black horizontal line is the suggested breakpoint for the disk diffusion method.
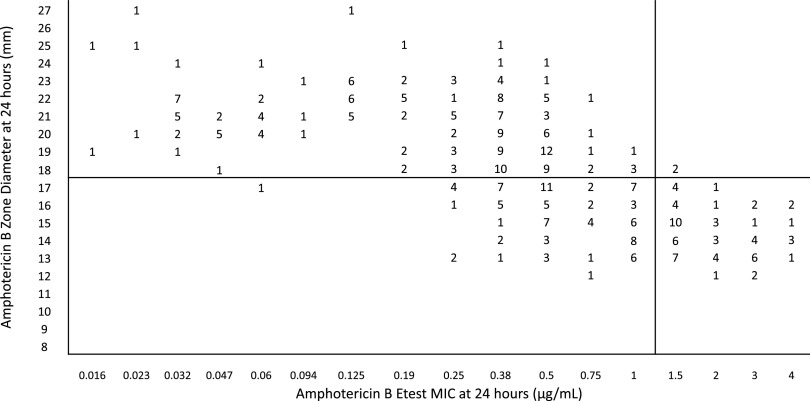
Most of the isolates had a low MIC value to amphotericin B. Figure 3 shows the correlation between MICs using ≥1.5 μg/ml as the breakpoint for resistant isolates and 10-μg amphotericin B disk zone diameters. Selection of a ≤17-mm zone diameter as the cutoff resulted in 74% categorical agreement between the two methods: 257 of 347 isolates had concordant results with 88 (25%) major and 2 (<1%) very major errors observed. When the zone diameter cutoff was changed to ≤15 mm, the categorical agreement was increased to 82% with 45 (13%) major errors, but the very major errors increased to 16 (5%). When the zone diameter cutoff was increased to ≤20 mm, there were no very major errors, but 182 major (52%) errors were observed.
FIG 3.
Zones of inhibition for 347 isolates of C. auris around 10-μg amphotericin B disks on Mueller-Hinton methylene blue agar plotted against the 24-h MICs determined by Etest. The numbers inside the graph indicate the number of isolates. The black horizontal line is the suggested breakpoint for the disk diffusion method. Zone diameters of ≤17 mm must be reported as indeterminate.
DISCUSSION
Although there are no established Candida auris-specific susceptibility breakpoints, the CDC has established tentative breakpoints based on MIC distributions, molecular mechanisms of resistance, and pharmacokinetic and pharmacodynamic values in a mouse model of infection (10). These tentative breakpoints were used here to define zone diameter cutoffs for the disk diffusion method that distinguish between susceptible and resistant isolates for caspofungin and fluconazole and identified 75% of isolates susceptible to amphotericin B.
Selection of a ≥12-mm zone diameter cutoff for fluconazole and caspofungin generated highly concordant results between the broth microdilution and disk diffusion methods, with 91 and 98% categorical agreement, respectively. However, for amphotericin B it was difficult to establish a zone diameter that had an acceptable amount of both major and very major errors, most likely because the difference between resistant and susceptible isolates was within the testing margin of error for broth microdilution. One possible solution to this problem may be to select the largest zone diameter with an acceptable amount of very major errors and define it as the zone of susceptibility without defining a zone of resistance. Selection of a ≤17-mm zone diameter for amphotericin B generated <1% very major errors but 25% major errors. These numbers are unacceptable. However, if we use ≥18 mm as the cutoff for susceptible isolates, with no definition for resistant isolates, the categorical agreement is 99% for the single category. The caveat to this method is that 24% (the major errors) of the susceptible isolates will not be categorized as susceptible. Zone diameters of ≤17 mm must be reported as indeterminate.
Antifungal susceptibility testing is an important tool for guiding antifungal therapy for multidrug-resistant organisms. However, most methods of antifungal susceptibility testing are costly or require acquired expertise. The Clinical and Laboratory Standards Institute has developed a standard for disk diffusion testing of Candida species (12). This method could greatly facilitate testing in resource-limited settings, as disk diffusion has proven to be an economical and reliable option (13). This study expands the application of caspofungin, fluconazole, and amphotericin B disk diffusion tests to include Candida auris. Utilizing the disk diffusion method with Mueller-Hinton agar supplemented with glucose and methylene blue appears to be a useful alternative for determining Candida auris resistance to caspofungin and fluconazole and susceptibility to amphotericin B.
ACKNOWLEDGMENTS
We are grateful to the members of the Mycotic Diseases Branch for their support.
The findings and conclusions in this work are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Forsberg K, Woodworth K, Walters M, Berkow EL, Jackson B, Chiller T, Vallabhaneni S. 2019. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol 57:1–12. doi: 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- 2.Chow NA, Muñoz JF, Gade L, Berkow EL, Li X, Welsh RM, Forsberg K, Lockhart SR, Adam R, Alanio A, Alastruey-Izquierdo A, Althawadi S, Araúz AB, Ben-Ami R, Bharat A, Calvo B, Desnos-Ollivier M, Escandón P, Gardam D, Gunturu R, Heath CH, Kurzai O, Martin R, Litvintseva AP, Cuomo CA. 2020. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 11:e03364-19. doi: 10.1128/mBio.03364-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. 2019. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis 25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sana F, Hussain W, Zaman G, Satti L, Khurshid U, Khadim MT. 2019. Candida auris outbreak report from Pakistan: a success story of infection control in ICUs of a tertiary care hospital. J Hosp Infect 103:108–110. doi: 10.1016/j.jhin.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Strauss M, Zoabi K, Sagas D, Reznik-Gitlitz B, Colodner R. 2020. Evaluation of Bio-Rad discs for antimicrobial susceptibility testing by disc diffusion and the ADAGIO system for the automatic reading and interpretation of results. Eur J Clin Microbiol Infect Dis 39:375–384. doi: 10.1007/s10096-019-03735-4. [DOI] [PubMed] [Google Scholar]
- 7.Nuh A, Ramadan N, Schelenz S, Armstrong-James D. 2020. Comparative evaluation of MIRONAUT-AM and CLSI broth microdilution method for antifungal susceptibility testing of Aspergillus species against four commonly used antifungals. Med Mycol 58:1085–1090. doi: 10.1093/mmy/myaa020. [DOI] [PubMed] [Google Scholar]
- 8.Girard V, Mailler S, Chetry M, Vidal C, Durand G, van Belkum A, Colombo AL, Hagen F, Meis JF, Chowdhary A. 2016. Identification and typing of the emerging pathogen Candida auris by matrix-assisted laser desorption ionisation time of flight mass spectrometry. Mycoses 59:535–538. doi: 10.1111/myc.12519. [DOI] [PubMed] [Google Scholar]
- 9.CLSI. 2020. Performance standards for antifungal susceptibility testing of yeasts, 2nd ed, vol M60ed2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Berkow EL, Lockhart SR, Ostrosky-Zeichner L. 2020. Antifungal susceptibility testing: current approaches. Clin Microbiol Rev 33:e00069-19. doi: 10.1128/CMR.00069-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepak AJ, Zhao M, Berkow EL, Lockhart SR, Andes DR. 2017. Pharmacodynamic optimization for treatment of invasive Candida aurisinfection. Antimicrob Agents Chemother 61:e00791-17. doi: 10.1128/AAC.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. 2018. Method for antifungal disk diffusion susceptibility testing of yeasts, vol M44ed3E. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Sánchez-Calvo JM, de Francisco JL, Torres-Martos E, Alados Arboledas JC, López Prieto MD. 2017. A cost-saving strategy for processing isolated uropathogens in community-acquired urinary tract infections. J Microbiol Methods 139:130–134. doi: 10.1016/j.mimet.2017.05.017. [DOI] [PubMed] [Google Scholar]