Abstract
A systematic review was conducted to identify the range of terminology used in studies to describe maximum walking distance and the exercise testing protocols, and testing modalities used to measure it in patients with intermittent claudication. A secondary aim was to assess the implementation and reporting of the exercise testing protocols. CINAHL, Medline, EMBASE and Cochrane CENTRAL databases were searched. Randomised controlled trials whereby patients with intermittent claudication were randomised to an exercise intervention were included. The terminology used to describe maximal walking distance was recorded, as was the modality and protocol used to measure it. The implementation and reporting quality was also assessed using pre-specified criteria. Sixty-four trials were included in this review. Maximal walking distance was reported using fourteen different terminologies. Twenty-two different treadmill protocols and three different corridor tests were employed to assess maximal walking distance. No single trial satisfied all the implementation and reporting criteria for an exercise testing protocol. Evidence shows that between-study interpretation is difficult given the heterogenous nature of the exercise testing protocols, test endpoints and terminology used to describe maximal walking distance. This is further compounded by poor test reporting and implementation across studies. Comprehensive guidelines need to be provided to enable a standardised approach to exercise testing in patients with intermittent claudication.
Introduction
Peripheral artery disease (PAD) is characterised by atherosclerotic lesions of the arteries in the lower limbs, resulting in a reduction of blood flow [1]. Globally, it is estimated that 236 million people are living with PAD [2]. The classical symptom of PAD is intermittent claudication (IC), characterised by ischaemic muscle pain precipitated by exertion and relieved by rest [3]. IC profoundly decreases walking capacity, physical activity levels, functional ability and leads to poorer quality of life [4]. Supervised exercise therapy is recommended as first line treatment for patients with IC [3, 5], and it is effective for ameliorating symptoms by improving walking capacity and quality of life [6].
To assess change/improvement in functional capacity following an exercise intervention or rehabilitation programme, clinicians or practitioners commonly measure maximal walking distance (MWD). Indeed, this is often the primary outcome to assess the efficacy of treatments in randomised controlled trials (RCT’s) [7, 8]. The measurement of MWD involves a patient walking for as long as possible until they are limited by their ischaemic leg symptoms [9]. However, there are a number of different terms used to describe MWD and it is measured using a variety of protocols [8, 9], making direct comparison between studies challenging and results less reproducible. In addition, regardless of testing protocol, procedures are often poorly implemented [10] and it is not always clear whether patients have reached MWD or if the test was terminated for other reasons. These issues regarding unstandardised exercise testing and reporting of outcomes have also been highlighted in a recent scientific statement from the American Heart Association [7]. Therefore, the aims of this systematic review were to: assess the terminology used to describe MWD, examine the various testing protocols used in randomized controlled trials including exercise interventions, and assess the implementation and reporting of exercise testing protocols using adapted recommendations and guidelines for patients with IC [9–13]. These assessments were only made for randomised controlled trials that included an exercise intervention to ensure that there was sufficient scope, but also to ensure that the review had focus, rather than including an unnecessarily large number of trials.
Methods
Search strategy and inclusion criteria
We included prospective RCTs in patients with IC where MWD was measured via a clearly specified protocol and patients were part of a structured exercise training programme. A structured exercise training programme was defined as one that stated the prescribed frequency, intensity and / or duration. Studies that included patients with critical limb-threatening ischaemia or asymptomatic PAD were excluded. In addition, studies that randomised patients to an exercise or comparator arm following revascularisation were also excluded. Finally, studies that offered all patients the same exercise programme with or without some other intervention (e.g. exercise + drug therapy vs. exercise alone) were excluded. Exclusion of these studies, and non-RCT’s, is in line with a previous review on reporting standards and also ensured that the current review and the number of included studies was manageable [14].
The search was conducted using the following databases: CINAHL, Medline, EMBASE and Cochrane CENTRAL. Terms related to IC, peripheral arterial disease, exercise and MWD (S2 Table). Only full-text articles published from 1995 up to June 2020 in the English language were included. We excluded studies that were published prior to 1995 as the majority of exercise programmes after this date were based on a specific meta-analysis [15] felt that the period of 25 years would provide sufficient information to inform our findings, especially given the increase in exercise-based trials in recent years. In addition, five existing systematic reviews and meta-analyses were used to identify other trials eligible for inclusion [6, 16–19]. Where authors made reference to published protocols these were also consulted.
Data management, selection and collection process
Search results were imported into Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) for duplicate removal and screening. Titles and abstracts were screened by two independent reviewers (SB and SP) and conflicts resolved via consensus. The full texts of any potentially eligible articles were then independently screened against the inclusion criteria by the same two reviewers.
Data extraction was performed equally by five reviewers (SB, AEH, SI, EC and SP) using a standardised Microsoft Excel database form (Microsoft, 2010, Redmond, WA, USA). Extraction was then cross-checked for accuracy by two reviewers (SB and SP). Data extraction included study characteristics (author, year, country), sample size and description, a description of the exercise intervention and comparator conditions, length of follow-up and methods for assessing MWD, including how walking distance was reported, the modality, protocol and whether it was the primary or secondary outcome.
Assessment of walking distance description and implementation
For the purpose of scoring protocols on their reporting standards, for treadmill tests, implementation was evaluated via a modified version of the criteria outlined by Hiatt et al [10]. The original criteria were applied when directly observing treadmill tests and as such, certain criteria have been omitted or modified as they cannot be clearly confirmed via reported methods only. This modified version included information about the testing equipment and protocol, pre-test instructions, and the steps involved in conducting the test (Table 1). A maximum score of 11 was available.
Table 1. Treadmill testing criteria.
| Criteria | Possible Score |
|---|---|
| Testing equipment and protocol | |
| • Clearly states equipment was calibrated | 1 |
| • Cites and correctly implements protocol | 1 |
| Pre-Test | |
| • Clearly states participants were rested prior to the test | 1 |
| • Clearly states participants were in a fasted state and instructed to avoid cigarette smoking and alcohol before the test | 1 |
| • States if a claudication pain scale was used | 1 |
| • Maximum walking distance was explained as the sole termination criteria (excluding safety criteria) | 1 |
| Conducting the test | |
| • Qualification / skill level of the test administrator is documented | 1 |
| • Clearly states that a familiarisation test to the same protocol was used | 1 |
| • Clearly states that the treadmill screen / clock, timer or watch was hidden from the participant | 1 |
| • Clearly states whether handrail support was permitted | 1 |
| • Clearly states whether all (or what % of) participants terminated the test due to maximally tolerated claudication pain | 1 |
| Maximum possible score: 11 | |
One element of the original criteria that could have been reported, ‘failure to have the patient straddle the moving belt at the start of the treadmill’ has not been included. We felt that, based on professional experiences and the balance limitations experienced by those with IC [20], this was not a safe practice and as such this criteria was omitted.
For corridor-based tests, namely the incremental shuttle walk test (ISWT), and six-minute walking test (6MWT) we assessed implementation based on the original study by Singh et al [11] and the guidelines provided by the American Thoracic Society (ATS) respectively [13]. We also considered the original PAD specific 6MWT study conducted by Montgomery et al [12]. For these corridor tests, a pro forma was used that again included information about the testing equipment and protocol, the pre-test instructions, and the steps involved in conducting the test (Table 2). A maximum score of 10 was available for the 6MWT and 13 for the ISWT. For the 6MWT, there is no equipment that requires calibration, whereas for the ISWT, the audio file used for testing should be calibrated. In addition, the 6MWT is not designed to measure claudication limited MWD, whilst the ISWT is.
Table 2. Corridor walk testing criteria (6MWT and ISWT).
| Criteria | Possible Score |
|---|---|
| Testing equipment and protocol | |
| • Clearly states equipment was calibrated * | 1 |
| • Cites protocol or accepted guidelines | 1 |
| Pre-Test | |
| • The corridor length is stated and in line with the cited protocol / guidelines | 1 |
| • Clearly states that participants were instructed not to perform any vigorous exercise 24hrs before the test | 1 |
| • Clearly states participants were rested prior to the test | 1 |
| • Clearly states participants were instructed to avoid cigarette smoking and alcohol before the test | 1 |
| • States if a claudication pain scale was used | 1 |
| • Clearly states that standardised instructions / requirements about the test were given to the participant | 1 |
| • Maximum walking distance was explained as the sole termination criteria (excluding safety criteria) * | 1 |
| Conducting the test | |
| • Qualification / skill level of the test administrator is documented | 1 |
| • Clearly states that a familiarisation test to the same protocol was used | 1 |
| • Clearly states standardised verbal phrases were administered during the test | 1 |
| • Clearly states whether all (or what % of) participants terminated the test due to maximally tolerated claudication pain * | 1 |
| Maximum possible score: 10 (6MWT) or 13 (ISWT) | |
*shuttle walk tests only. 6MWT, six-minute walk test; ISWT, incremental shuttle walk test
For both the treadmill and corridor-based tests, reference to previous studies (including the original studies of Hiatt et al, Singh et al or Montgomery et al) was not sufficient to deem that the same specific methodology had been used and all elements had to be explicitly stated to satisfy our criteria. However, for the 6MWT, reference to the ATS guidelines was deemed sufficient to satisfy our criteria, regardless of whether each element was specifically stated. The rationale for this is that the ATS provide specific practice guidelines [12] whilst other documents simply report outcomes from previous studies. If a study outlined a specific element in its methodology which cited the ATS guidelines, we checked for compliance. If compliance was breached the study was penalised.
Data analysis
Data analysis regarding how walking distance was reported, and the modality and protocol used to measure it, is presented as number and percentage for each combination. In addition, the number and percentage of studies meeting each implementation criteria is presented narratively and graphically.
Results
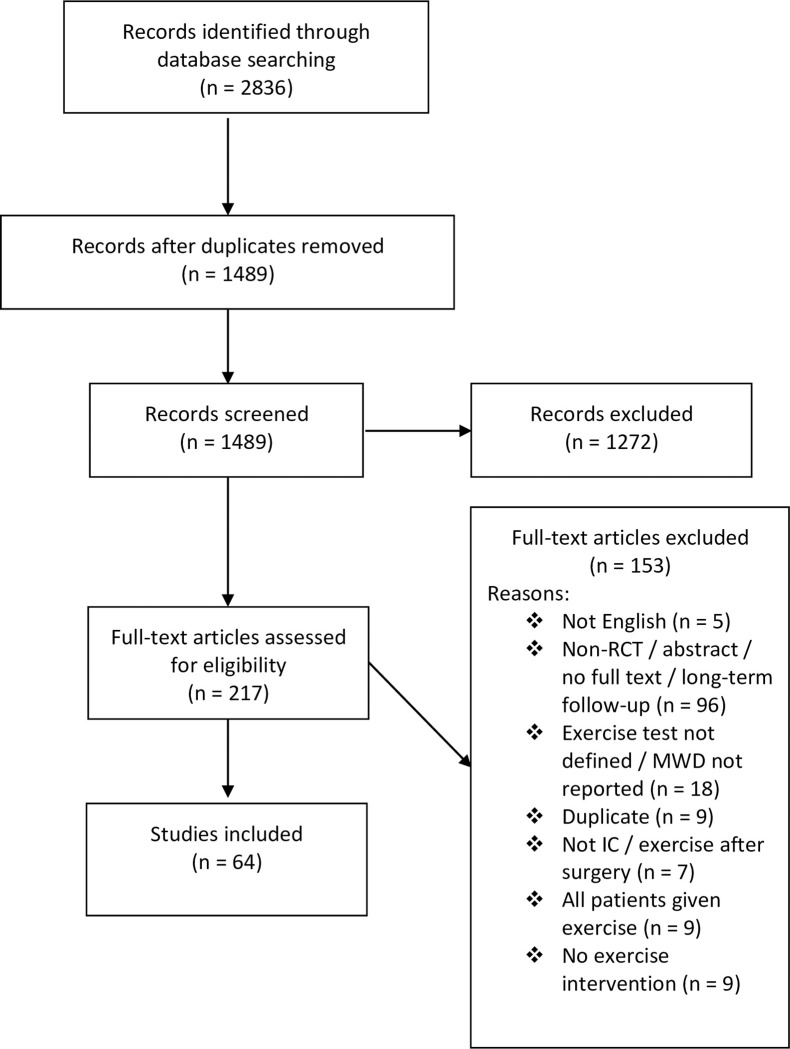
Our search yielded 2836 results. Of these, sixty-four trials, including 3881 patients, met the inclusion criteria and were ultimately included in this review (Fig 1) [21–84]. A list of included trials, including a brief description of the intervention(s) and comparator(s), is provided in S1 Table. MWD was stated as the primary outcome in 55% of trials, though it was not specified in 14%.
Fig 1. PRISMA flow chart for study identification.
MWD, maximal walking distance; IC, intermittent claudication.
Exercise testing protocols and terminology
Of the sixty-four RCT’s that assessed MWD, twenty-nine employed a graded treadmill test [21–24, 27–31, 35–39, 42, 46, 48–52, 56, 57, 63, 68, 74, 77, 82, 83], and twelve a constant work rate treadmill test [26, 34, 41, 47, 53, 66, 67, 69–71, 73, 76]. Three trials conducted both a graded and a constant work rate test [45, 58, 59], fourteen conducted a graded test and a 6MWT [25, 40, 43, 55, 60–62, 64, 65, 72, 75, 78–80], and one employed a constant work rate test and a 6MWT [44]. Of the five trials to only employ a corridor test, two conducted a 6MWT [32, 54], two an incremental shuttle walk (ISWT) [81, 84] and one a modified ISWT [33].
Regarding the exercise testing protocols, forty-six studies that conducted a graded test, eleven different protocols were employed. The most widely adopted protocols were the Gardener-Skinner and Hiatt protocols accounting for 65% and 13%, respectively [85, 86]. From the sixteen trials that adopted a constant work rate test, eleven different protocols were employed, commonly using a speed of 3 km/h at a 10% gradient (four trials, 25%).
The MWD was reported using fourteen different terminologies: peak walking time [21, 22, 38, 42, 51, 63–65, 79], maximal/maximum walking distance [23–25, 27, 33, 34, 41, 46, 55, 70, 71, 73–77, 79–81, 85], absolute claudication distance [26, 35, 44, 52, 61, 62, 66, 67, 69, 71], maximal walking time [28, 29, 36, 37, 39, 40, 47–50, 57, 68, 83], total walking distance [30, 31], total walking capacity [56], time to maximal pain [59, 78], time to exhaustion [82], maximum pain distance [27], absolute walking distance [53], exercise duration [43, 58], maximal claudication pain [60], maximal distance [43], and six minute walk distance [32, 54]. Only two trials documented the level of encouragement given to the patient during treadmill testing. One trial stated encouragement was refrained [34], and the other that it was administered [29].
Implementation and reporting checklist for treadmill testing
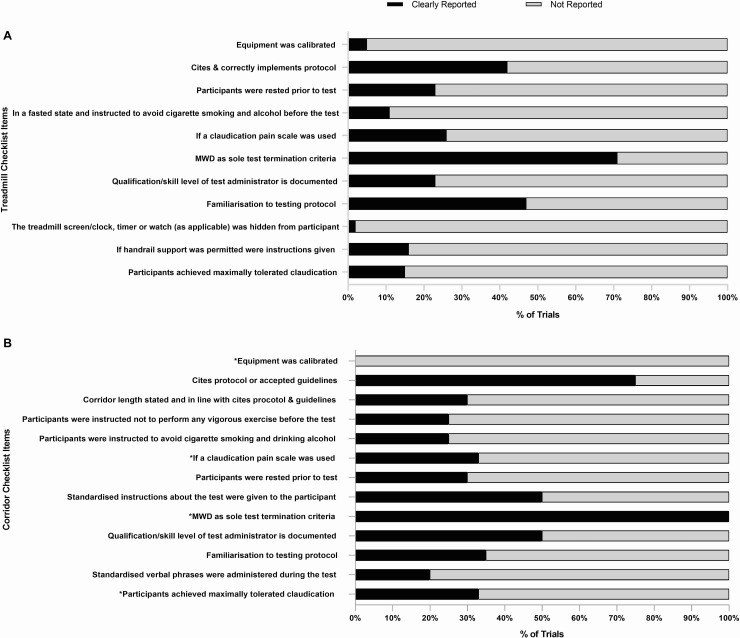
Fig 2 panel A shows the percentage of RCT’s satisfying each of the treadmill testing implementation and reporting criteria as shown in Table 1. Full analysis and rating of the individual trials is shown in S3 Table. No single trial satisfied all the criteria. The MWD was stated as the sole test termination criteria in forty-four trials (71%). The remaining 29% of trials incorporated additional criteria (Table 3) such as: volitional exhaustion or fatigue, or reaching a certain time, distance, pain scale or heart rate based endpoints [23, 28, 29, 34, 37, 41–44, 46, 68, 72–74]. Only nine trials (15%) reported whether all (or what % of) patients terminated the test due to claudication limited MWD.
Fig 2. The percentage of trials that reported the treadmill and corridor testing criteria.
MWD, maximal walking distance.
Table 3. Additional test endpoints adopted in treadmill tests.
| Endpoint |
|---|
| Volitional exhaustion[23, 28, 29, 46, 74] |
| Fatigue and shortness of breath [43] |
| Time limit: 30 min [23, 52], 25 min [29, 46] |
| Distance limit: 207m [41] and 1000m [34] |
| Score of 8 on the CR-10 RPE scale [23] |
| A score of 3 or 4 on the pain scale [68] |
| 80% of heart rate maximum [44] |
| Point of termination [25, 37] |
| Exercise induced factors [42] |
| Strong pain [72] |
| Walking distance in the absence of claudication pain [73] |
Twenty-six trials (42%) cited and correctly implemented the intended protocol. However, nine trials employed the Gardener-Skinner protocol but failed to cite the original publication [21, 43, 55, 59, 63, 68, 72, 78, 79], whilst three trials cited the publication but failed to correctly implement the protocol due to a modification of the speed [23, 40], or incline [80]. As such these variations were counted as three separate graded test protocols. Familiarisation to the treadmill testing protocol was reported in twenty-nine trials (47%). Sixteen trials (26%) reported that a claudication specific pain scale was used during the test, whilst five trials used the CR-10 rating of perceived exertion (Borg) scale to monitor pain [23, 40, 45, 58, 74]. The qualification/skill level of the test administrator was reported in fourteen trials (23%).
Seven trials (11%) clearly stated that participants were in a fasted state and instructed to avoid cigarette smoking and drinking alcohol before the test, whilst fourteen (23%) stated that patients were rested prior to the test. A small number of trials reported that the treadmill was calibrated (5%) and that the treadmill screen/clock, timer or watch (as applicable) was hidden from participants during the test (2%). Finally, ten trials (16%) specified whether handrail support was permitted.
Implementation and reporting checklist for corridor testing
Fig 2 panel B shows the percentage of RCT’s satisfying each of the corridor testing implementation and reporting criteria as shown in Table 2. Full analysis and rating of the individual trials is shown in S4 Table. No single trial satisfied all the criteria. Fifteen trials (75%) cited a previous protocol or accepted guidelines, whilst five (25%) did not. For the 6MWT, eight trials cited Montgomery et al [40, 44, 60–62, 64, 65, 75], four cited the ATS guidelines [25, 43, 79, 80], and one cited Guyatt et al [54]. For the ISWT both trials cited Singh et al [81, 84]. However only six trials (30%) used the correct corridor length in line with the cited protocol or guidelines [25, 40, 43, 64, 79, 80]. The two trials that employed the ISWT reported that the cones were placed ten meters apart [81, 84] and the trial using a modified ISWT reported a 50-metre figure of eight [33]. Seven trials did not state a corridor length for the 6MWT [32, 55, 61–63, 65, 75], whilst four reported varying lengths of 20 meters [78], 22 meters [44], 33 meters [54] and 50 meters [72].
Five trials (25%) reported that patients had abstained from vigorous activity, cigarette smoking and alcohol prior to the test, whilst six (30%) reported that patients were rested prior to the test. Ten trials (50%) implemented standardised instructions/phrases prior to the test, whilst only four (20%) adopted standardised phrases during it. The qualification/skill level of the test administrator and familiarisation to the testing protocol was reported in ten (50%) and seven trials (35%) respectively.
Implementation criteria specific to the ISWT’s showed that no trials reported calibration of the audio recording, one trial (33%) used a claudication pain scale [33] and two trials used the CR-10 rating of perceived exertion (Borg) scale to monitor pain [81, 84]. All trials (100%) reported that claudication limited MWD was the sole test termination criteria, however only one trial reported whether all (or what % of) patients terminated the test due to this [33].
Discussion
Our review aimed to identify the varied terminology used to describe MWD and examine the different protocols used to measure it, in patients with IC. We also aimed to evaluate the implementation and reporting of these testing protocols. Our findings demonstrated substantial heterogeneity in how MWD is reported and measured across RCT’s. Furthermore, the implementation and reporting of the exercise testing protocols was relatively poor.
Maximum walking capacity and exercise testing protocols
In the current review we found fourteen different terminologies used to describe MWD in just sixty-four studies. This lack of standardised terminology may have a direct impact on patient care as a recent Cochrane review [6], which have informed clinical guidelines, only included trials that described maximum walking distance or time as an outcome measure. Although other variants of MWD will have been included in the review, it is possible that studies adopting less standard terminology may have been missed during the screening process, especially when the term maximum or maximal is not used in the descriptor (e.g. total walking distance). Consequently, it is possible that such reviews do not encompass the full evidence base. Future trials should adopt recognised terminology and include the term maximal or maximum when describing MWD.
Our findings have also shown that twenty-two different treadmill protocols were across just sixty-four RCT’s. The heterogenous nature of treadmill testing is a major concern. Graded and constant work rate tests that differ in speed and gradient result in varying relationships between workload and maximal distance/time [87, 88]. As such the choice of testing protocol will directly influence MWD independent of the exercise intervention. Trials should employ an internationally recognised graded exercise test, given it has the highest reliability across differing severities of IC [85, 87]. However, despite the available evidence, only 72% of trials in the current review employed a graded test. As such, almost 30% adopted a suboptimal testing protocol, meaning that direct between-study comparison may be inappropriate. In addition, pooling studies with different, and less reliable testing protocols may impact upon the effect estimate and should be considered in meta-analyses possibly via sensitivity analysis. Indeed, the implications are that incorrect conclusions may be drawn from the analysis and therefore rule out a potentially viable treatment. Of further concern, the inconsistency of treadmill protocols in clinical trials may translate down to general testing in rehabilitation programmes and impact clinical outcomes for patients in ‘usual care’.
A lack of clear guidance for treadmill testing in patients with IC is a likely contributor to the heterogeneity identified. Current guidelines fail to provide adequate details or recommendations for the assessment of MWD [3, 5, 89, 90]. Therefore, as previously recommended [87], guidelines for the assessment of MWD, which advocate for a universal graded test protocol, adopting the reporting criteria outlined in Table 1 are required. This will bring vascular care in line with other clinical specialities such as respiratory medicine, who provide comprehensive testing guidelines [91].
For corridor-based testing the 6MWT was the most commonly employed, usually as a secondary outcome. The 6MWT provides important additional information regarding ambulatory function and it may be a better representation of walking in daily life [8, 92]. However, caution is strongly advised when comparing both the 6MWT and a treadmill test following the same exercise intervention, due to the differing responses [93]. This lack of sensitivity to change may be attributed to the ceiling effect that is evident with the 6MWT [94] and the fact that the test is self-paced, thus reducing standardisation.
Implementation and reporting checklist for treadmill testing
Our review also demonstrated that implementation of treadmill testing in exercise trials is poorly reported with no single trial satisfying all of our criteria. Although claudication limited MWD was stated as the sole test termination criteria in the majority of trials, almost a third incorporated additional endpoints (e.g. 8 out 10 on the CR-10 scale or 3 or 4 on the pain scale). A major concern is that these endpoints will inherently limit a patient’s peak performance and therefore lead to an under or overestimation of their true MWD [9]. The implications of this is that each individual trial could under/overestimate the true effect, which would also be the case for pooled effect estimates. Indeed, in the most recent Cochrane review [6] several studies were included that documented additional test endpoints [22, 28, 46, 74]. Trials should aim to ensure that MWD is the primary test termination point, particularly when the objective is to measure change in walking capacity. Where patients do not terminate due to maximally tolerated claudication but other endpoints, this should also be reported by authors. However, in the current review 85% of trials did not provide this information.
Furthermore, lack of detail regarding familiarisation testing and handrail support use also limits how the individual study results can be interpreted [85]. This lack of standardisation will may lead to measurement variability with data being less reproducible and less sensitive to the exercise intervention [88].
We also found that less than half of the included trials cited and correctly implemented the intended protocol, which inhibits study replication. Furthermore, three trials cited the Gardener-Skinner graded protocol but deviated from it by manipulating the speed and/or incline. A rationale (for reducing the gradient) was provided by one trial, but it was not appropriate given the reduction for severe IC was in reference to a constant work rate test not a graded [87]. Such deviation means that the reliability of the test is unknown and should not be assumed to be comparable to the original protocol [85], which again impacts upon interpretation. This also highlights a limitation of the peer review process, as important issues such incorrect implementation should have been identified and rectified prior to publication.
Implementation and reporting checklist for corridor testing
Encouragingly 75% of trials cited a protocol or accepted guidelines for the methodology of their corridor test. For the 6MWT (seventeen trials), 47% cited Montgomery et al, 24% the ATS guidelines, and 6% Guyatt et al. For the ISWT both trials cited Singh et al [81, 84]. Whilst Montgomery et al is a PAD specific study it does not offer a standardised approach for conducting the 6MWT. In this study, patients were given verbal encouragement every two minutes, however the content and nature of these phrases is unknown. Conversely, the ATS guidelines provide comprehensive standardised verbal phrases which are delivered more frequently (each minute). A concern is that adopting different protocols between trials may impact upon the results given that encouragement effects performance by over 30 meters [95]. This impact is significant as the estimated minimally clinically important difference (MCID) for the 6MWT is 12–34 meters [96]. Interestingly, despite the ATS guidelines being available since 2002, six trials published after this date still chose to cite Montgomery et al [12] or Guyatt et al [95].
In addition to different protocols being followed, there were also differences with regards to the corridor lengths used, ranging from 20 to 50 meters, despite guidelines advocating 30 metres [13]. Varying corridor lengths may impact upon the distance walked, as patients have to turn and change direction more or less frequently [97]. One study has demonstrated that patients with chronic lung disease walked nearly 50 meters further when the corridor length was 30 vs. 10 meters [97]. A dearth of evidence exists in other clinical populations, therefore further studies are required to examine the influence of encouragement and corridor length on 6MWT performance in patients with IC.
The incorrect corridor length was also apparent for the ISWT. Both trials that implemented the ISWT cited the original Singh et al publication [11], but reported that the cones were placed 10 meters apart, when they should be 9 meters apart, allowing for 0.5 meter turning circle at each end [91]. Although this is only a small difference, it has the potential to be significant given it will underestimate patients walking capacity by 1 meter for every shuttle walked. Another limitation of these trials is that they did not perform a familiarisation test. This is very important given that a learning effect of up to 30 meters is apparent with both tests [91].
This review is not without limitations. Firstly, we did not contact study authors for further details regarding the interventions as this was not feasible given the volume of studies included. Secondly, we did not hand search the reference lists of included studies though it is unlikely any trials were overlooked given that four databases were used, and five existing systematic reviews and meta-analyses were consulted. Finally, we excluded certain studies, such as those that included exercise performed after revascularisation. However, this ensured that the review was manageable and as 64 trials were still included in spite of this, it is unlikely that including these studies would have altered our findings.
Conclusion
Evidence shows that between-study interpretation is difficult given the heterogenous nature of the exercise testing protocols, test endpoints and terminology used to describe MWD. We recommend that future trials adopt a standardised approach to exercise testing, implementation and reporting by adopting the minimum reporting criteria outlined in Tables 1 and 2. However, we acknowledge that strict word counts may inhibit this, therefore we recommend two actions; first, for authors to publish a protocol and second, to include this information as S1 to S4 Tables. Importantly, we also strongly recommend that specific guidelines are created to recommend an international, standardised testing procedure to measure MWD using a treadmill in patients with IC, providing step-by-step guidance and standardised reporting terminology.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344(21):1608–21. 10.1056/NEJM200105243442108 [DOI] [PubMed] [Google Scholar]
- 2.Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. The Lancet Global Health. 2019;7(8):e1020–e30. 10.1016/S2214-109X(19)30255-4 [DOI] [PubMed] [Google Scholar]
- 3.Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763–816. 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 4.Gerage AM, Correia MdA, Oliveira PMLd, Palmeira AC, Domingues WJR, Zeratti AE, et al. Physical Activity Levels in Peripheral Artery Disease Patients. Arquivos brasileiros de cardiologia. 2019;113(3):410–6. 10.5935/abc.20190142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE. Peripheral arterial disease: diagnosis and management. Clinical guidance 147. 2012. [Google Scholar]
- 6.Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12(12):Cd000990. 10.1002/14651858.CD000990.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, et al. Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139(4):e10–e33. 10.1161/CIR.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130(1):61–8. 10.1161/CIRCULATIONAHA.114.007002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiatt WR, Rogers RK, Brass EP. The treadmill is a better functional test than the 6-minute walk test in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130(1):69–78. 10.1161/CIRCULATIONAHA.113.007003 [DOI] [PubMed] [Google Scholar]
- 10.Hiatt WR, Cox L, Greenwalt M, Griffin A, Schechter C. Quality of the assessment of primary and secondary endpoints in claudication and critical leg ischemia trials. Vascular medicine (London, England). 2005;10(3):207–13. 10.1191/1358863x05vm628oa [DOI] [PubMed] [Google Scholar]
- 11.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–24. 10.1136/thx.47.12.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery PS, Gardner AW. The Clinical Utility of a Six-Minute Walk Test in Peripheral Arterial Occlusive Disease Patients. Journal of the American Geriatrics Society. 1998;46(6):706–11. 10.1111/j.1532-5415.1998.tb03804.x [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic S. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 14.Tew GA, Brabyn S, Cook L, Peckham E. The Completeness of Intervention Descriptions in Randomised Trials of Supervised Exercise Training in Peripheral Arterial Disease. PLOS ONE. 2016;11(3):e0150869. 10.1371/journal.pone.0150869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain: a meta-analysis. Jama. 1995;274(12):975–80. [PubMed] [Google Scholar]
- 16.Hageman D, Fokkenrood H, Gommans L, van den Houten M, Teijink J. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. The Cochrane database of systematic reviews. 2018;4:CD005263. 10.1002/14651858.CD005263.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmenter BJ, Raymond J, Dinnen P, Singh MAF. A systematic review of randomized controlled trials: walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis. 2011;218(1):1–12. 10.1016/j.atherosclerosis.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 18.Fakhry F, van de Luijtgaarden KM, Bax L, den Hoed PT, Hunink MGM, Rouwet EV, et al. Supervised walking therapy in patients with intermittent claudication. Journal of vascular surgery. 2012;56(4):1132–42. 10.1016/j.jvs.2012.04.046 [DOI] [PubMed] [Google Scholar]
- 19.Vemulapalli S, Dolor RJ, Hasselblad V, Schmit K, Banks A, Heidenfelder B, et al. Supervised vs unsupervised exercise for intermittent claudication: a systematic review and meta-analysis. American Heart Journal. 2015;169(6):924–37. 10.1016/j.ahj.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Gohil RA, Mockford KA, Mazari F, Khan J, Vanicek N, Chetter IC, et al. Balance impairment, physical ability, and its link with disease severity in patients with intermittent claudication. Annals of vascular surgery. 2013;27(1):68–74. 10.1016/j.avsg.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 21.Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, et al. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3months of exercise training. Free radical biology & medicine. 2010;49(6):1138–44. 10.1016/j.freeradbiomed.2010.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker WB, Li Z, Schenkel SS, Chandra M, Busch DR, Englund EK, et al. Effects of exercise training on calf muscle oxygen extraction and blood flow in patients with peripheral artery disease. Journal of applied physiology (Bethesda, Md: 1985). 2017;123(6):1599–609. 10.1152/japplphysiol.00585.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner IKM, Hains SJM, Tranmer J, Brown CA, Zelt DT, Brown PM. Low-Intensity Exercise Training Increases Heart Rate Variability in Patients With Peripheral Artery Disease. Biological research for nursing. 2020;22(1):24–33. 10.1177/1099800419884642 [DOI] [PubMed] [Google Scholar]
- 24.Bronas UG, Treat-Jacobson D, Leon AS. Comparison of the effect of upper body-ergometry aerobic training vs treadmill training on central cardiorespiratory improvement and walking distance in patients with claudication. Journal of vascular surgery. 2011;53(6):1557–64. 10.1016/j.jvs.2011.01.077 [DOI] [PubMed] [Google Scholar]
- 25.Bulińska K, Kropielnicka K, Jasiński T, Wojcieszczyk-Latos J, Pilch U, Dąbrowska G, et al. Nordic pole walking improves walking capacity in patients with intermittent claudication: a randomized controlled trial. Disability and rehabilitation. 2016;38(13):1318–24. 10.3109/09638288.2015.1077398 [DOI] [PubMed] [Google Scholar]
- 26.Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. Eur J Vasc Endovasc Surg. 2004;27(1):17–23. 10.1016/j.ejvs.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 27.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes care. 2011;34(10):2174–9. 10.2337/dc10-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowther RG, Leicht AS, Spinks WL, Sangla K, Quigley F, Golledge J. Effects of a 6-month exercise program pilot study on walking economy, peak physiological characteristics, and walking performance in patients with peripheral arterial disease. 2012;8:225‐32. 10.2147/VHRM.S30056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowther RG, Spinks WL, Leicht AS, Sangla K, Quigley F, Golledge J. Effects of a long-term exercise program on lower limb mobility, physiological responses, walking performance, and physical activity levels in patients with peripheral arterial disease. Journal of vascular surgery. 2008;47(2):303–9. 10.1016/j.jvs.2007.10.038 [DOI] [PubMed] [Google Scholar]
- 30.Grizzo Cucato G, de Moraes Forjaz CL, Kanegusuku H, da Rocha Chehuen M, Riani Costa LA, Wolosker N, et al. Effects of walking and strength training on resting and exercise cardiovascular responses in patients with intermittent claudication. VASA Zeitschrift fur Gefasskrankheiten. 2011;40(5):390–7. 10.1024/0301-1526/a000136 [DOI] [PubMed] [Google Scholar]
- 31.Cucato GG, Chehuen MdR, Costa LAR, Ritti-Dias RM, Wolosker N, Saxton JM, et al. Exercise prescription using the heart of claudication pain onset in patients with intermittent claudication. Clinics (Sao Paulo, Brazil). 2013;68(7):974–8. 10.6061/clinics/2013(07)14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaney CL, Miller MD, Chataway TK, Spark JI. A Randomised Controlled Trial of Supervised Exercise Regimens and their Impact on Walking Performance, Skeletal Muscle Mass and Calpain Activity in Patients with Intermittent Claudication. European Journal of Vascular and Endovascular Surgery. 2014;47(3):304–10. 10.1016/j.ejvs.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 33.Spafford C, Oakley C, Beard JD. Randomized clinical trial comparing Nordic pole walking and a standard home exercise programme in patients with intermittent claudication. The British journal of surgery. 2014;101(7):760–7. 10.1002/bjs.9519 [DOI] [PubMed] [Google Scholar]
- 34.Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Gröger M, et al. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011;217(1):240–8. 10.1016/j.atherosclerosis.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 35.Savage P, Ricci MA, Lynn M, Gardner A, Knight S, Brochu M, et al. Effects of home versus supervised exercise for patients with intermittent claudication. J Cardiopulm Rehabil. 2001;21(3):152–7. 10.1097/00008483-200105000-00006 [DOI] [PubMed] [Google Scholar]
- 36.Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short-term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. Journal of vascular surgery. 2006;44(1):119–27. 10.1016/j.jvs.2006.03.037 [DOI] [PubMed] [Google Scholar]
- 37.Sandercock GR, Hodges LD, Das SK, Brodie DA. The impact of short term supervised and home-based walking programmes on heart rate variability in patients with peripheral arterial disease. Journal of sports science & medicine. 2007;6(4):471–6. [PMC free article] [PubMed] [Google Scholar]
- 38.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48(4):291–300. 10.1177/000331979704800402 [DOI] [PubMed] [Google Scholar]
- 39.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. Journal of vascular surgery. 1997;25(2):312. 10.1016/s0741-5214(97)70352-5 [DOI] [PubMed] [Google Scholar]
- 40.McGuigan MRM, Bronks R, Newton RU, Sharman MJ, Graham JC, Cody DV, et al. Resistance Training in Patients With Peripheral Arterial Disease: Effects on Myosin Isoforms, Fiber Type Distribution, and Capillary Supply to Skeletal Muscle. The Journals of Gerontology: Series A. 2001;56(7):B302–B10. [DOI] [PubMed] [Google Scholar]
- 41.Mazari FAK, Gulati S, Rahman MNA, Lee HLD, Mehta TA, McCollum PT, et al. Early Outcomes From a Randomized, Controlled Trial of Supervised Exercise, Angioplasty, and Combined Therapy in Intermittent Claudication. Annals of Vascular Surgery. 2010;24(1):69–79. 10.1016/j.avsg.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 42.Mays RJ, Hiatt WR, Casserly IP, Rogers RK, Main DS, Kohrt WM, et al. Community-based walking exercise for peripheral artery disease: An exploratory pilot study. Vascular medicine (London, England). 2015;20(4):339–47. 10.1177/1358863X15572725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kropielnicka K, Dziubek W, Bulińska K, Stefańska M, Wojcieszczyk-Latos J, Jasiński R, et al. Influence of the Physical Training on Muscle Function and Walking Distance in Symptomatic Peripheral Arterial Disease in Elderly. BioMed research international. 2018;2018:1937527. 10.1155/2018/1937527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamberti N, Malagoni AM, Ficarra V, Basaglia N, Manfredini R, Zamboni P, et al. Structured Home-Based Exercise Versus Invasive Treatment: A Mission Impossible? A Pilot Randomized Study in Elderly Patients With Intermittent Claudication. Angiology. 2016;67(8):772–80. 10.1177/0003319715618481 [DOI] [PubMed] [Google Scholar]
- 45.Langbein WE, Collins EG, Orebaugh C, Maloney C, Williams KJ, Littooy FN, et al. Increasing exercise tolerance of persons limited by claudication pain using polestriding. J Vasc Surg. 2002;35(5):887–93. 10.1067/mva.2002.123756 [DOI] [PubMed] [Google Scholar]
- 46.Leicht AS, Crowther RG, Golledge J. Influence of peripheral arterial disease and supervised walking on heart rate variability. Journal of vascular surgery. 2011;54(5):1352–9. 10.1016/j.jvs.2011.05.027 [DOI] [PubMed] [Google Scholar]
- 47.Maejima Y, Yasu T, Ueba H, Kobayashi N, Hashimoto S, Kubo N, et al. Exercise after heparin administration new: Therapeutic program for patients with non-option arteriosclerosis obliterans. Circulation Journal. 2005;69(9):1099–104. 10.1253/circj.69.1099 [DOI] [PubMed] [Google Scholar]
- 48.Mika P, Spodaryk K, Cencora A, Mika A. Red blood cell deformability in patients with claudication after pain-free treadmill training. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2006;16(4):335–40. 10.1097/00042752-200607000-00009 [DOI] [PubMed] [Google Scholar]
- 49.Mika P, Wilk B, Mika A, Marchewka A, Nizankowski R. The effect of pain-free treadmill training on fibrinogen, haematocrit, and lipid profile in patients with claudication. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18(5):754–60. [DOI] [PubMed] [Google Scholar]
- 50.Mika P, Konik A, Januszek R, Petriczek T, Mika A, Nowobilski R, et al. Comparison of two treadmill training programs on walking ability and endothelial function in intermittent claudication. International journal of cardiology. 2013;168(2):838–42. 10.1016/j.ijcard.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 51.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125(1):130–9. 10.1161/CIRCULATIONAHA.111.075770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicolaï S, Teijink J, Prins M. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. Journal of vascular surgery. 2010;52:348–55. 10.1016/j.jvs.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 53.Novaković M, Krevel B, Rajkovič U, Vižintin Cuderman T, Janša Trontelj K, Fras Z, et al. Moderate-pain versus pain-free exercise, walking capacity, and cardiovascular health in patients with peripheral artery disease. Journal of vascular surgery. 2019;70(1):148–56. 10.1016/j.jvs.2018.10.109 [DOI] [PubMed] [Google Scholar]
- 54.Parmenter BJ, Raymond J, Dinnen P, Lusby RJ, Fiatarone Singh MA. High-intensity progressive resistance training improves flat-ground walking in older adults with symptomatic peripheral arterial disease. J Am Geriatr Soc. 2013;61(11):1964–70. 10.1111/jgs.12500 [DOI] [PubMed] [Google Scholar]
- 55.Parr BM, Noakes TD, Derman EW. Peripheral arterial disease and intermittent claudication: efficacy of short-term upper body strength training, dynamic exercise training, and advice to exercise at home. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2009;99(11):800–4. [PubMed] [Google Scholar]
- 56.Chehuen M, Cucato GG, Carvalho CRF, Ritti-Dias RM, Wolosker N, Leicht AS, et al. Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: A randomized controlled trial. Journal of science and medicine in sport. 2017;20(10):886–92. 10.1016/j.jsams.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 57.Christman SK. Intervention to slow progression of peripheral arterial disease. Intervention to Slow Progression of Peripheral Arterial Disease. 2003:123 p. [Google Scholar]
- 58.Collins EG, Edwin Langbein W, Orebaugh C, Bammert C, Hanson K, Reda D, et al. PoleStriding exercise and vitamin E for management of peripheral vascular disease. Medicine and science in sports and exercise. 2003;35(3):384–93. 10.1249/01.MSS.0000053658.82687.FF [DOI] [PubMed] [Google Scholar]
- 59.Jones PP, Skinner JS, Smith LK, John FM, Bryant CX. Functional improvements following StairMaster vs. treadmill exercise training for patients with intermittent claudication. Journal of cardiopulmonary rehabilitation. 1996;16(1):47–55. 10.1097/00008483-199601000-00006 [DOI] [PubMed] [Google Scholar]
- 60.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. Journal of the American Geriatrics Society. 2001;49(6):755–62. 10.1046/j.1532-5415.2001.49152.x [DOI] [PubMed] [Google Scholar]
- 61.Gardner AW, Katzel LI, Sorkin JD, Goldberg AP. Effects of long-term exercise rehabilitation on claudication distances in patients with peripheral arterial disease: a randomized controlled trial. Journal of cardiopulmonary rehabilitation. 2002;22(3):192–8. 10.1097/00008483-200205000-00011 [DOI] [PubMed] [Google Scholar]
- 62.Gardner AW, Montgomery PS, Flinn WR, Katzel LI. The effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. Journal of vascular surgery. 2005;42(4):702–9. 10.1016/j.jvs.2005.05.049 [DOI] [PubMed] [Google Scholar]
- 63.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123(5):491–8. 10.1161/CIRCULATIONAHA.110.963066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication: A randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention. 2011;31(4). [Google Scholar]
- 65.Gardner Andrew W, Parker Donald E, Montgomery Polly S, Blevins Steve M. Step‐Monitored Home Exercise Improves Ambulation, Vascular Function, and Inflammation in Symptomatic Patients With Peripheral Artery Disease: A Randomized Controlled Trial. Journal of the American Heart Association.3(5):e001107. 10.1161/JAHA.114.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The constitutive procoagulant and hypofibrinolytic state in patients with intermittent claudication due to infrainguinal disease significantly improves with percutaneous transluminal balloon angioplasty. Journal of vascular surgery. 2006;43(1):40–6. 10.1016/j.jvs.2005.09.013 [DOI] [PubMed] [Google Scholar]
- 67.Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in intermittent claudication: a randomized controlled trial. J Vasc Surg. 2007;45(1):65–70; discussion 10.1016/j.jvs.2006.08.084 [DOI] [PubMed] [Google Scholar]
- 68.Hodges LD, Sandercock GRH, Das SK, Brodie DA. Randomized controlled trial of supervised exercise to evaluate changes in cardiac function in patients with peripheral atherosclerotic disease. Clinical physiology and functional imaging. 2008;28(1):32–7. 10.1111/j.1475-097X.2007.00770.x [DOI] [PubMed] [Google Scholar]
- 69.Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg. 2005;30(2):164–75. 10.1016/j.ejvs.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 70.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PMT, Hunink MGM. Intermittent Claudication: Clinical Effectiveness of Endovascular Revascularization versus Supervised Hospital-based Exercise Training—Randomized Controlled Trial. Radiology. 2009;250(2):586–95. 10.1148/radiol.2501080607 [DOI] [PubMed] [Google Scholar]
- 71.Stewart AH, Smith FC, Baird RN, Lamont PM. Local versus systemic mechanisms underlying supervised exercise training for intermittent claudication. Vascular and endovascular surgery. 2008;42(4):314–20. 10.1177/1538574408314442 [DOI] [PubMed] [Google Scholar]
- 72.Szymczak, Oszkinis G, Majchrzycki M. The Impact of Walking Exercises and Resistance Training upon the Walking Distance in Patients with Chronic Lower Limb Ischaemia. BioMed Research International. 2016;2016:1–8. 10.1155/2016/7515238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tebbutt N, Robinson L, Todhunter J, Jonker L. A plantar flexion device exercise programme for patients with peripheral arterial disease: a randomised prospective feasibility study. Physiotherapy. 2011;97(3):244–9. 10.1016/j.physio.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 74.Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clinical science (London, England: 1979). 2009;117(12):405–13. 10.1042/CS20080688 [DOI] [PubMed] [Google Scholar]
- 75.Tew GA, Humphreys L, Crank H, Hewitt C, Nawaz S, Al-Jundi W, et al. The development and pilot randomised controlled trial of a group education programme for promoting walking in people with intermittent claudication. Vascular medicine (London, England). 2015;20(4):348–57. 10.1177/1358863X15577857 [DOI] [PubMed] [Google Scholar]
- 76.Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise-induced inflammatory response? Eur J Vasc Endovasc Surg. 1997;14(5):344–50. 10.1016/s1078-5884(97)80283-3 [DOI] [PubMed] [Google Scholar]
- 77.Treat-Jacobson D, Bronas UG, Leon AS. Efficacy of arm-ergometry versus treadmill exercise training to improve walking distance in patients with claudication. Vascular medicine (London, England). 2009;14(3):203–13. 10.1177/1358863X08101858 [DOI] [PubMed] [Google Scholar]
- 78.Tsai JC, Chan P, Wang CH, Jeng C, Hsieh MH, Kao PF, et al. The effects of exercise training on walking function and perception of health status in elderly patients with peripheral arterial occlusive disease. Journal of internal medicine. 2002;252(5):448–55. 10.1046/j.1365-2796.2002.01055.x [DOI] [PubMed] [Google Scholar]
- 79.Van Schaardenburgh M, Wohlwend M, Rognmo Ø, Mattsson E. Calf raise exercise increases walking performance in patients with intermittent claudication. Journal of vascular surgery. 2017;65(5):1473–82. 10.1016/j.jvs.2016.12.106 [DOI] [PubMed] [Google Scholar]
- 80.Villemur B, Thoreau V, Evra V, Guinot M, Gailledrat E, Perennou D, et al. Short interval or continuous training programs to improve walking distance for intermittent claudication: Pilot study. Annals of Physical and Rehabilitation Medicine. 2020. 10.1016/j.rehab.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 81.Walker RD, Nawaz S, Wilkinson CH, Saxton JM, Pockley AG, Wood RFM. Influence of upper- and lower-limb exercise training on cardiovascular function and walking distances in patients with intermittent claudication. Journal of Vascular Surgery. 2000;31(4):662–9. 10.1067/mva.2000.104104 [DOI] [PubMed] [Google Scholar]
- 82.Wang E, Hoff J, Loe H, Kaehler N, Helgerud J. Plantar flexion: an effective training for peripheral arterial disease. European journal of applied physiology. 2008;104(4):749–56. 10.1007/s00421-008-0826-3 [DOI] [PubMed] [Google Scholar]
- 83.Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clinical science (London, England: 1979). 2006;111(6):401–9. 10.1042/CS20060151 [DOI] [PubMed] [Google Scholar]
- 84.Zwierska I, Male JS, Saxton JM, Walker RD, Choksy SA, Pockley AG. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: A randomized controlled trial. Journal of Vascular Surgery. 2005;42(6):1122–30. 10.1016/j.jvs.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 85.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23(4):402–8. [PubMed] [Google Scholar]
- 86.Hiatt WR, Nawaz D, Regensteiner JG, Hossack KF. The Evaluation of Exercise Performance in Patients with Peripheral Vascular Disease. Journal of Cardiopulmonary Rehabilitation and Prevention. 1988;8(12):525–32. [Google Scholar]
- 87.Nicolaï SPA, Viechtbauer W, Kruidenier LM, Candel MJJM, Prins MH, Teijink JAW. Reliability of treadmill testing in peripheral arterial disease: A meta-regression analysis. Journal of Vascular Surgery. 2009;50(2):322–9. 10.1016/j.jvs.2009.01.042 [DOI] [PubMed] [Google Scholar]
- 88.Hiatt WR, Rogers RK, Brass EP. In Clinical Trials, Is the 6-Minute Walk Test a Better Functional Test of Interventions for Peripheral Artery Disease Than Treadmill Walking Tests?: The Treadmill Is a Better Functional Test Than the 6-Minute Walk Test in Therapeutic Trials of Patients With Peripheral Artery Disease. Circulation (New York, NY). 2014;130(1):69–78. [DOI] [PubMed] [Google Scholar]
- 89.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery. 2007;45(1):S5–S67. [DOI] [PubMed] [Google Scholar]
- 90.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):1465–508. 10.1016/j.jacc.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 91.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. European Respiratory Journal. 2014;44(6):1428. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 92.Sandberg A, Cider Å, Jivegård L, Nordanstig J, Wittboldt S, Bäck M. Test-retest reliability, agreement, and minimal detectable change in the 6-minute walk test in patients with intermittent claudication. Journal of vascular surgery. 2020;71(1):197–203. 10.1016/j.jvs.2019.02.056 [DOI] [PubMed] [Google Scholar]
- 93.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165–74. 10.1001/jama.2008.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frost AE, Langleben D, Oudiz R, Hill N, Horn E, McLaughlin V, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol. 2005;43(1):36–9. 10.1016/j.vph.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 95.Guyatt GH, Pugsley SO, Sullivan MJ, Thompson PJ, Berman L, Jones NL, et al. Effect of encouragement on walking test performance. Thorax. 1984;39(11):818–22. 10.1136/thx.39.11.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vascular medicine (London, England). 2018;23(4):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beekman E, Mesters I, Hendriks EJ, Klaassen MP, Gosselink R, van Schayck OC, et al. Course length of 30 metres versus 10 metres has a significant influence on six-minute walk distance in patients with COPD: an experimental crossover study. Journal of physiotherapy. 2013;59(3):169–76. 10.1016/S1836-9553(13)70181-4 [DOI] [PubMed] [Google Scholar]