Hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) are biocides used for cleaning and debriding chronic wound infections, which often harbor drug-resistant bacteria. Here, we evaluated the in vitro activity of H2O2 and HOCl against 27 isolates of eight bacterial species involved in wound infections.
KEYWORDS: H2O2, HOCl, biofilm, e-scaffold, MIC, MBIC, MBBC, resistance
ABSTRACT
Hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) are biocides used for cleaning and debriding chronic wound infections, which often harbor drug-resistant bacteria. Here, we evaluated the in vitro activity of H2O2 and HOCl against 27 isolates of eight bacterial species involved in wound infections. Minimum inhibitory concentrations (MICs) and minimum biofilm bactericidal concentrations (MBBCs) were measured. Compared to their respective MICs, MBBCs of isolates exposed to H2O2 were 16- to 1,024-fold higher, and those exposed to HOCl were 2- to 4-fold higher. We evaluated the selection of resistance after exposure of Staphylococcus aureus and Pseudomonas aeruginosa biofilms to 10 iterations of electrochemically generated HOCl or H2O2 delivered using electrochemical scaffolds (e-scaffolds), observing no decrease in antibiofilm effects with serial exposure to e-scaffold-generated H2O2 or HOCl. Twenty-four-hour exposure to H2O2-generating e-scaffolds consistently decreased the number of CFU of S. aureus and P. aeruginosa biofilms by ∼5.0 log10 and ∼4.78 log10 through 10 iterations of exposure, respectively. Four-hour exposure to HOCl-generating e-scaffolds consistently decreased the number of CFU of S. aureus biofilms by ∼4.9 log10, and 1-h exposure to HOCl-generating e-scaffolds consistently decreased the number of CFU of P. aeruginosa biofilms by ∼1.57 log10. These results suggest that HOCl has similar activity against planktonic and biofilm bacteria whereas the activity of H2O2 is less against biofilm than planktonic bacteria, and that repeat exposure to either biocide, generated electrochemically under the experimental conditions studied, does not lessen antibiofilm effects.
INTRODUCTION
Chronic wound infections caused by antibiotic-resistant bacteria are a challenge in clinical practice. In the United States, costs associated with treating chronic wound infections exceed $10 billion yearly (1). Such infections are commonly associated with the presence of biofilms in wound beds, making them especially difficult to treat, since many antimicrobial agents are poorly active against bacterial biofilms (2). Biofilms in wound beds can hamper wound healing by impairing movement of keratinocytes/fibroblasts or decreasing angiogenesis, for example (3–5). Antiseptics and topical disinfectants used for cleaning and debriding chronic wound infections include chlorhexidine, povidone-iodine, sodium hypochlorite, hypochlorous acid (HOCl), peracetic acid, quaternary ammonium compounds, and hydrogen peroxide (H2O2), to name a few (6, 7). Biofilms in wound beds may hinder the optimal efficacy of these biocides. Among the various biocides, H2O2 and HOCl are generated as part of natural cellular inflammatory responses and are noteworthy for their inherent potential properties in eliminating biofilms in wound beds and stimulating wound healing (8–11). Increased migration and differentiation of keratinocytes and fibroblasts have been reported in the presence of H2O2 and HOCl (12, 13). A limitation in the application of H2O2 and HOCl to wounds is, however, that they are rapidly oxidized/reduced in wound environments, losing activity over time. Therefore, the continuous generation and delivery of H2O2 and HOCl to wound beds to reduce biofilms could be considered for ideal antibacterial effects.
Few studies have assessed the antibiofilm activity of H2O2 and HOCl in terms of minimum biofilm inhibitory concentrations (MBICs) and minimum biofilm bactericidal concentrations (MBBCs) against biofilms formed by numerous/broad-spectrum clinically important pathogens. Since wound infections often involve biofilms in wound beds, antibiofilm activity is an important consideration. Previously, we described H2O2- and HOCl-generating electrochemical scaffolds (e-scaffolds) as prototypes of devices being developed to treat wound infections (13, 14).
Here, we studied the susceptibility of selected clinically relevant Gram-positive and -negative bacteria by determining MICs, MBICs, and MBBCs of H2O2 and HOCl. The literature has contrasting reports regarding the selection of H2O2 and HOCl resistance (15–17), so we also assessed whether there would be a decrease in antibiofilm activity with serial application of H2O2 or HOCl. Specifically, we tested S. aureus and P. aeruginosa biofilms with multiple exposures to H2O2- and HOCl-generating e-scaffolds.
RESULTS
Susceptibility of planktonic bacteria to hydrogen peroxide and hypochlorous acid.
The 27 bacterial isolates studied had mean H2O2 MICs ranging from 0.20 to 3.19 mM (Table 1). Gram-positive and -negative bacteria showed wide H2O2 MIC ranges. P. aeruginosa PA14 had a mean H2O2 MIC of 3.19 mM, whereas its isogenic mutant strain lacking the catalase genes katA and katB, P. aeruginosa PA14 ΔkatAB, had a mean H2O2 MIC of 0.20 mM, ∼16-fold lower than the parent strain.
TABLE 1.
Susceptibility of bacterial isolates (planktonic and biofilm forms) to H2O2 and HOCl
| Bacteria | Isolate designation | Isolate characteristics | Value (means ± SD, in mM) fora: |
|||||
|---|---|---|---|---|---|---|---|---|
| H2O2 |
HOCl |
|||||||
| MIC | MBIC | MBBC | MIC | MBIC | MBBC | |||
| S. aureus | USA100 | Clinical isolate, resistant to methicillin | 0.40 ± 0.00 | 0.40 ± 0.00 | 85 ± 29 | 1.65 ± 0.57 | 1.32 ± 0.57 | 1.32 ± 0.57 |
| S. aureus | USA200 | Clinical isolate, resistant to methicillin | 0.27 ± 0.11 | 0.40 ± 0.00 | 85 ± 29 | 1.65 ± 0.57 | 0.99 ± 0.00 | 1.32 ± 0.57 |
| S. aureus | USA300 | Clinical isolate, resistant to methicillin | 0.40 ± 0.00 | 0.66 ± 0.23 | 68 ± 29 | 1.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 |
| S. aureus | IDRL-6169 | Prosthetic hip isolate; resistant to methicillin and mupirocin | 0.40 ± 0.00 | 0.66 ± 0.23 | 51 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 |
| S. aureus | Xen 30 | Clinical isolate; resistant to methicillin | 0.66 ± 0.23 | 0.53 ± 0.23 | 119 ± 78 | 1.32 ± 0.57 | 1.32 ± 0.57 | 1.32 ± 0.57 |
| S. aureus | IDRL-4284 | Clinical isolate; resistant to methicillin | 0.66 ± 0.23 | 0.66 ± 0.23 | 170 ± 59 | 1.99 ± 0.00 | 1.32 ± 0.57 | 0.99 ± 0.00 |
| S. epidermidis | ATCC 35984 | Catheter sepsis isolate; resistant to methicillin | 0.53 ± 0.23 | 0.53 ± 0.23 | 170 ± 59 | 1.65 ± 0.57 | 1.32 ± 0.58 | 1.65 ± 0.57 |
| S. epidermidis | IDRL-6461 | Prosthetic knee infection isolate; susceptible to methicillin | 0.53 ± 0.23 | 0.66 ± 0.23 | 136 ± 59 | 1.32 ± 0.57 | 0.99 ± 0.00 | 0.99 ± 0.00 |
| S. epidermidis | Xen 43 | Catheter isolate; susceptible to methicillin | 0.40 ± 0.00 | 1.06 ± 0.46 | 102 ± 0.00 | 1.32 ± 0.57 | 1.32 ± 0.58 | 1.32 ± 0.57 |
| E. faecalis | ATCC 29212 | Urine isolate | 3.19 ± 0.00 | 1.86 ± 1.22 | 136 ± 59 | 0.66 ± 0.29 | 1.32 ± 0.57 | 1.65 ± 0.57 |
| E. faecalis | IDRL-8618 | Prosthetic hip infection isolate | 0.53 ± 0.23 | 1.33 ± 0.46 | 102 ± 0.00 | 0.50 ± 0.00 | 1.32 ± 0.57 | 1.99 ± 0.00 |
| E. faecalis | IDRL-7107 | Prosthetic knee infection isolate | 3.19 ± 0.00 | 4.25 ± 1.84 | 170 ± 59 | 0.50 ± 0.00 | 1.99 ± 0.00 | 2.32 ± 1.52 |
| E. faecium | IDRL-11790 | Abscess isolate; resistant to vancomycin and penicillin, and susceptible to linezolid | 0.80 ± 0.00 | 0.80 ± 0.69 | 55 ± 45 | 0.99 ± 0.00 | 0.82 ± 0.28 | 1.32 ± 0.57 |
| E. coli | IDRL-10366 | blaKPC-positive isolate; resistant to ceftolozane-tazobactam, imipenem, meropenem, ertapenem, ceftriaxone, and cefepime | 1.33 ± 0.46 | 0.66 ± 0.23 | 170 ± 59 | 0.99 ± 0.00 | 1.32 ± 0.57 | 1.32 ± 0.57 |
| E. coli | IDRL-7029 | Prosthetic hip infection isolate | 1.59 ± 0.00 | 1.86 ± 1.22 | 340 ± 118 | 0.99 ± 0.00 | 1.32 ± 0.57 | 3.31 ± 1.15 |
| E. coli | IDRL-6199 | Prosthetic knee infection isolate | 2.13 ± 0.92 | 1.59 ± 0.00 | 408 ± 0.00 | 0.99 ± 0.00 | 1.65 ± 0.57 | 3.31 ± 1.15 |
| E. coli | IDRL-8110 | Blood isolate | 2.66 ± 0.92 | 3.72 ± 2.44 | 340 ± 118 | 0.99 ± 0.00 | 1.65 ± 0.57 | 3.97 ± 0.00 |
| P. aeruginosa | IDRL-7262 | Prosthetic hip infection isolate | 0.66 ± 0.23 | 170 ± 58.89 | 408 ± 0.00 | 0.99 ± 0.00 | 1.65 ± 0.57 | 1.65 ± 0.57 |
| P. aeruginosa | Xen 5 | Blood isolate | 2.13 ± 0.92 | 170 ± 58.89 | 612 ± 353 | 0.99 ± 0.00 | >3.97 | >3.97 |
| P. aeruginosa | PAO1, ATCC 47085 | Wound isolate; type strain | 2.66 ± 0.92 | 153 ± 88.33 | 680 ± 236 | 0.99 ± 0.00 | 1.65 ± 0.57 | 1.99 ± 0.00 |
| P. aeruginosa | PA14 | Wild-type laboratory strain | 3.19 ± 0.00 | 85 ± 29.44 | 408 ± 0.00 | 0.99 ± 0.00 | 1.65 ± 0.57 | 1.65 ± 0.57 |
| P. aeruginosa | PA14 ΔkatAB | katA and katB double knockout of PA14 | 0.20 ± 0.00 | 3.72 ± 2.43 | 51 ± 0.00 | 0.99 ± 0.00 | 1.32 ± 0.57 | 1.65 ± 0.57 |
| P. aeruginosa | IDRL-11442 | Groin isolate; resistant to piperacillin-tazobactam, cefepime, ceftazidime, meropenem, aztreonam, ciprofloxacin, levofloxacin; susceptible to colistin | 0.60 ± 0.34 | 51 ± 0.00 | 170 ± 59 | 0.99 ± 0.00 | 1.65 ± 0.57 | 1.32 ± 0.57 |
| A. baumannii | ATCC 17978 | Meningitis isolate | 0.80 ± 0.00 | 2.13 ± 0.92 | 85 ± 29 | 0.83 ± 0.29 | 0.99 ± 0.00 | 1.32 ± 0.57 |
| A. baumannii | ATCC BAA-1605 | Sputum isolate; resistant to ceftazidime, gentamicin, ticarcillin, piperacillin, aztreonam, cefepime, ciprofloxacin, imipenem and meropenem | 0.80 ± 0.00 | 2.12 ± 0.92 | 68 ± 29 | 0.83 ± 0.29 | 1.32 ± 0.57 | 0.83 ± 0.29 |
| A. baumannii | ARLG-1268 | Wound isolate; resistant to amikacin, ampicillin, cefepime, ceftazidime, ciprofloxacin and tobramycin | 1.06 ± 0.46 | 2.66 ± 0.92 | 102 ± 0.00 | 0.66 ± 0.29 | 0.66 ± 0.29 | 0.66 ± 0.29 |
| K. pneumoniae | IDRL-10377 | blaKPC-positive isolate; resistant to ceftolozane-tazobactam, imipenem, meropenem, ertapenem, ceftriaxone and cefepime | 0.40 ± 0.00 | 2.12 ± 0.92 | 102 ± 0.00 | 0.99 ± 0.00 | 0.66 ± 0.29 | 0.99 ± 0.00 |
Susceptibility data values (i.e., MIC, MBIC, and MBBC) are represented as means ± SD (n = 3). All experiments were performed in triplicates. S. aureus USA100, USA200, and USA300 strains were provided by Henry Chambers III (University of California, San Francisco). Xen 30, Xen 43, and Xen 5 strains were provided by Caliper Life Sciences. P. aeruginosa PAO1, PA14, and PA14 ΔkatAB strains were provided by Daniel Hassett (University of Cincinnati). A. baumannii ARLG-1268 was provided by the Antibacterial Resistance Leadership Group of the National Institutes of Health.
The 27 bacterial isolates studied had mean HOCl MIC values ranging from 0.5 to 1.99 mM (Table 1), a tighter range than H2O2 and below concentrations considered toxic to mammalian cells (∼15.12 mM) (10). As with H2O2, both Gram-positive and -negative bacteria showed similar HOCl MIC ranges.
Susceptibility of bacterial biofilms to hydrogen peroxide and hypochlorous acid. (i) Biofilm inhibitory concentrations.
As shown from Table 1, mean H2O2 MBICs ranged from 0.40 to 170 mM. For most tested Gram-positive bacteria, H2O2 MBICs were similar or slightly higher than corresponding MIC values. For Gram-negative bacteria, except for P. aeruginosa, H2O2 MBICs were also similar/slightly higher than their MICs. Almost all P. aeruginosa isolates, except P. aeruginosa PA14 ΔkatAB, had 128- to 256-fold MBICs compared to MICs.
All bacteria studied had mean HOCl MBICs similar to or slightly higher than their respective MICs; an exception was P. aeruginosa IDRL-7543, which had a mean HOCl MBIC of ≥3.97 mM, markedly higher than its MIC (0.99 mM).
(ii) Biofilm bactericidal concentrations.
Mean H2O2 MBBC values ranged from 51 to 680 mM, 32- to 512-fold higher than their MIC or MBIC. P. aeruginosa isolates had the highest H2O2 MBBC values of the bacteria studied. Almost all P. aeruginosa isolates had an overall 256- to 512-fold higher H2O2 MBBC than MIC and MBIC.
Mean HOCl MBBCs ranged from 0.66 to ≥3.97 mM, the same or slightly higher than the respective MICs and MBICs. HOCl MBBCs for the Gram-positive bacteria studied were generally similar to their MICs and MBICs, whereas for Gram-negative bacteria, HOCl MBBCs were generally higher than their MICs and MBICs.
Measurement of susceptibility following repeated exposure of S. aureus and P. aeruginosa biofilms to H2O2 and HOCl generated by e-scaffolds.
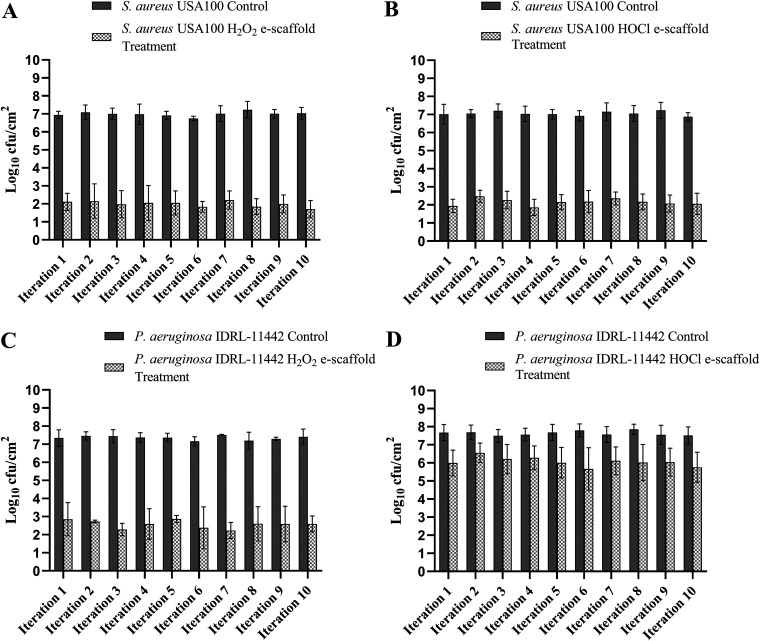
Figure 1 shows the results of repeated e-scaffold treatment of S. aureus USA100 and P. aeruginosa IDRL-11442 biofilms. Based on our prior e-scaffold studies, ∼45 mM H2O2 is typically generated over a 24-h treatment period and ∼22 mM HOCl over a 4-h treatment period (13, 14). When S. aureus USA100 biofilms were exposed to an H2O2-producing e-scaffold for 24 h, a mean reduction of ∼5.00 log10 CFU/cm2 was observed compared to controls over 10 iterations (Fig. 1A). When the same biofilms were exposed to an HOCl-generating e-scaffold treatment for 4 h, a mean reduction of ∼4.90 log10 CFU/cm2 compared to controls was observed over 10 iterations (Fig. 1B). The degree of effect was maintained with repeated e-scaffold treatment, with reductions in CFU counts remaining consistent between iterations for both e-scaffold types.
FIG 1.
Repeated treatment with e-scaffolds demonstrating no decrease in effect over 10 sequential iterations. (A) Staphylococcus aureus USA100 biofilms exposed to an H2O2 generating e-scaffold. (B) S. aureus USA100 biofilms exposed to an HOCl-generating e-scaffold. (C) Pseudomonas aeruginosa IDRL-11442 biofilms exposed to an H2O2 generating e-scaffold. (D) P. aeruginosa IDRL-11442 biofilms exposed to an HOCl-generating e-scaffold. Data are expressed as means ± SD (n = 3). All the experiments were performed in triplicate.
When P. aeruginosa biofilms were exposed to an H2O2-producing e-scaffold for 24 h, a mean reduction of ∼4.78 log10 CFU/cm2 was observed compared to controls over 10 iterations (Fig. 1C). When the same P. aeruginosa biofilms were exposed to an HOCl-generating e-scaffold treatment for 1 h, a mean reduction of ∼1.57 log10 CFU/cm2 was observed compared to controls over 10 iterations (Fig. 1D). P. aeruginosa biofilms were exposed to the HOCl-producing e-scaffold for only 1 h, since longer exposure times resulted in biofilm eradication (data not shown). Reductions in CFU counts of P. aeruginosa biofilm were consistent over 10 iterations.
We did not observe the emergence of resistance with exposure of S. aureus or P. aeruginosa biofilms to H2O2- or HOCl-producing e-scaffolds over 10 iterations. We measured the susceptibilities of planktonic and biofilm forms of S. aureus USA100 and P. aeruginosa IDRL-11442 after 10 iterations of exposure to both H2O2- and HOCl-producing e-scaffolds. As evident from Table 2, there was no significant difference in the mean MIC, MBIC, or MBBC before and after e-scaffold exposure, suggesting that repeated exposure to H2O2 or HOCl as delivered herein does not affect susceptibility to H2O2 or HOCl.
TABLE 2.
Planktonic and biofilm susceptibilities of Staphylococcus aureus USA100 and Pseudomonas aeruginosa IDRL-11442 before and after repeated e-scaffold exposure for 10 sequential iterations
| Bacteria | Value (means ± SD, in mM) fora: |
|||||
|---|---|---|---|---|---|---|
| H2O2 |
HOCl |
|||||
| MIC (planktonic) | MBIC (biofilm) | MBBC (biofilm) | MIC (planktonic) | MBIC (biofilm) | MBBC (biofilm) | |
| S. aureus USA100 before e-scaffold exposure | 0.40 ± 0.00 | 0.40 ± 0.00 | 85 ± 29 | 1.65 ± 0.57 | 1.32 ± 0.57 | 1.32 ± 0.57 |
| S. aureus USA100 after e-scaffold exposure for 10 sequential iterations | 0.66 ± 0.23 | 0.66 ± 0.23 | 68 ± 29 | 1.99 ± 0.00 | 1.65 ± 0.57 | 1.65 ± 0.57 |
| P. aeruginosa IDRL-11442 before e-scaffold exposure | 0.60 ± 0.34 | 51 ± 0.00 | 170 ± 59 | 0.99 ± 0.00 | 1.32 ± 0.57 | 0.99 ± 0.00 |
| P. aeruginosa IDRL-11442 after e-scaffold exposure for 10 sequential iterations | 0.66 ± 0.23 | 85 ± 29 | 204 ± 0.00 | 1.32 ± 0.57 | 0.99 ± 0.00 | 1.65 ± 0.57 |
Susceptibility data values (i.e., MIC, MBIC, and MBBC) are represented as means ± SD (n = 3). All experiments were performed in triplicate.
DISCUSSION
We assessed planktonic and biofilm susceptibilities to H2O2 and HOCl of 27 bacterial isolates. Mean H2O2 MICs ranged from 0.20 to 3.19 mM. Low concentrations of H2O2 disrupt cell membranes, oxidize DNA, and destabilize enzymes and proteins (16). Moreover, H2O2 is rapidly oxidized to a hydroxyl radical (·OH), which promotes oxidative stress (17). In other studies, H2O2 MIC values have been reported to range between 0.40 and 14 mM, similar to those observed here (18, 19). In prior studies, variable susceptibility to H2O2 has been reported and tolerance to H2O2 described as more strain than species specific (19). The exact mechanism of action of HOCl is not fully understood. HOCl is a highly active oxidizing agent that disrupts cellular activities of proteins and oxidative phosphorylation and inhibits DNA synthesis (16). In one study, the HOCl MIC of bacterial isolates was >0.025% (∼3.78 mM), similar to what was found with P. aeruginosa IDRL-7543 (20). Mazzola et al. described a MIC range of HOCl against various bacterial species of 0.02 to 0.06% (3 to 9 mM) (21) and that these values were dependent on the pH of the HOCl solution. Thus, HOCl MICs may depend on factors such as pH; at pH 4.0 to 7.0, HOCl was most active (21). At pH >7.5, HOCl is no longer the active moiety in solution, as free chlorine speciation becomes dominated by OCl−.
Prior work has shown that biofilms have reduced susceptibilities to H2O2 and HOCl compared to the same isolates grown planktonically (22, 23). Compared to their planktonic forms, biofilms are more evolved and complex. Bacteria in biofilms grow slowly and have overall reduced metabolic activity. They are also encased in an extracellular polymeric substance matrix comprised of DNA, proteins, and polysaccharides, which protects them from adverse environmental conditions and confers mechanical and biochemical protection against biocides and antibiotics (24, 25). Additionally, the interior of biofilms has a lower pH than the surface, alongside less oxygen and water availability, which may render biocides ineffective (26). The MBICs of H2O2 were not higher than MICs for most bacterial isolates used in this study. The exception was P. aeruginosa, which showed 128- to 256-fold higher H2O2 MBIC than MIC values. Overall, Gram-negative bacteria had relatively higher MBBCs for H2O2 than Gram-positive bacteria. Escherichia coli and P. aeruginosa isolates studied were found to have the most tolerance to H2O2 when grown as biofilms. Perumal et al. found similar results (18); they performed MIC and MBBC assays to evaluate the activity of various disinfectants against Gram-negative bacteria, observing that bacterial biofilms were 266-fold less susceptible to H2O2 than bacteria in the planktonic state. The addition of another acidic agent (e.g., peracetic acid or 2-furoic acid) in combination with H2O2 improved the susceptibility of bacteria to these agents when exposed for short time intervals. In several other studies, bacterial biofilms were exposed to H2O2 for short durations as part of surface contact treatments, with varying results (15, 27). For example, in one study, among different disinfectants used, only H2O2 and sodium hypochlorite removed both S. aureus and P. aeruginosa biofilm matrix and bacterial viable mass (28). It is expected that over a 24-h period, H2O2 will be oxidized into other reactive oxygen species (ROS), including hydroxyl radical and singlet oxygen species, and undergo autocatalytic degradation to oxygen and water. Bacterial cells embedded in outer layers of biofilms can produce free radical scavenger molecules that destroy some ROS during early stages of interactions, which occur when biofilm cells are presented with H2O2 (29). Prior studies have revealed that H2O2 cannot effectively penetrate mature biofilms with outer surface biofilm layers decomposing H2O2 and abrogating its effective diffusion into interior layers (30). The effective diffusion coefficients of solute molecules like H2O2 and HOCl are reduced in biofilm environments compared to water (31). Expression of new genes and their resulting products has been hypothesized to play a prominent role in reduced susceptibility of biofilms toward biocides (32). In addition, bacterial cells present in biofilm layers can produce a plethora of enzymes, including catalases, peroxidases, glutathione reductase, and superoxide dismutase (16), which can break down H2O2, HOCl, and antibiotics. A common enzyme produced by bacteria to destroy H2O2 is catalase. The degradation of H2O2 due to catalase production could be a reason we observed high MBBC values with H2O2 exposure. Among the isolates studied here, E. coli and P. aeruginosa are known to have strong SOS response signaling pathways when challenged with sublethal concentrations of H2O2. Work done by Elkin et al. demonstrates a protective role of catalase genes katA and katB in P. aeruginosa mutant strains (in planktonic and biofilm forms) when exposed to sublethal concentrations of H2O2 (33). The authors conclude that KatA catalase is important for conferring resistance to H2O2, especially at high concentrations, whereas KatB catalase helps confer resistance when initial levels of H2O2 are sublethal. In our study, P. aeruginosa PA14 ΔkatAB had a 16-fold lower MBIC value than its parent wild-type isolate, P. aeruginosa PA14. This supports the idea that catalase produced by P. aeruginosa has a protective role against H2O2 by degrading it. E. coli isolates have distinct stress response elements when exposed to H2O2; induction of SoxR and OxyR regulons is mainly responsible for providing resistance to H2O2 (34). The high MBBC values observed here may be attributed, at least in part, to the activation of enzymes connected to oxidative stress response signaling. H2O2 has a higher probability of being degraded in the presence of bacterial enzymes than other biocides. Therefore, it is our view that to ideally use H2O2 as an antibiofilm agent, a high working concentration of H2O2 along with a long surface contact time are likely to be needed.
The mean MICs of HOCl against the bacteria studied ranged from 0.50 to 1.99 mM. In contrast to H2O2, we did not observe large variations in MIC, MBIC, or MBBC ranges. The mechanism of action of HOCl is incompletely defined, and how bacterial molecular stress mechanisms respond to it are also poorly understood. It has been proposed that the transport of free chlorine into biofilms is a significant factor in imparting resistance (35). In work done by Castillo et al., HOCl was used as oral rinses to remove dental plaque (36). HOCl was a more effective antibacterial agent than chlorhexidine and reduced bacterial viability of different periodontopathic bacteria found in biofilms. The authors suggested that HOCl can oxidize taurine, an amino acid, promoting the formation of chlorine-taurine complexes that have antibacterial activity. In another study, 0.018% HOCl (2.72 mM) removed lipopolysaccharides found in Porphyromonas gingivalis biofilms. The authors suggested that HOCl forms chlorohydrins, which attack acyl chains in unsaturated fatty acids, causing cell membrane damage along with cytolysis (37). HOCl has been found to interact with sulfur-containing amino acids, aromatic amino acids, nitrogen-containing compounds, and lipids (38). Various ATP-independent HOCl-sensing chaperones, like Hsp33, RidA, CnoX, etc., have been found to be activated as part of the immediate counter-response to HOCl, especially in Gram-negative bacteria.
In previous work, we evaluated e-scaffold antibiofilm activity against biofilms of S. aureus, P. aeruginosa, and A. baumannii (13, 14, 39). In these studies, we observed time-dependent increases in antibiofilm activity with >4-log10 biofilm reductions after 24 h of treatment for biofilms exposed to H2O2-producing e-scaffolds and complete eradication of biofilms when exposed to HOCl-producing e-scaffold for 4 h. We also found that treatments were not toxic to host tissue (13, 14). Given the prolonged exposure to biocides associated with e-scaffolds, there might be concerns about selection for resistance to H2O2 or HOCl. Here, we show that H2O2- and HOCl-generating e-scaffolds maintain activity against S. aureus and P. aeruginosa biofilms after 10 iterations of exposure under the conditions studied. Until now, there have been few studies of biocide resistance in planktonic bacteria over several generations of exposure. Ikai et al. evaluated antibacterial activity of hydroxyl radicals generated by the photolysis of H2O2 (40), examining repeated biocide exposure over 40 continuous generations in selected bacterial pathogens and reporting no evidence of selection of biocide resistance.
Our e-scaffold system continuously produces small amounts of H2O2 or HOCl, below concentrations that are toxic to tissue. We produced ∼45 mM H2O2 in 24 h of continuous treatment and ∼22 mM HOCl in 4 h (13, 14). By continuously producing small amounts of these biocides, we achieved an ∼5-log10 reduction in the number of CFU of S. aureus USA100 despite a mean H2O2 MBBC of 85 mM. The continuous production of H2O2 and HOCl likely can overwhelm oxidative stress response systems in bacterial biofilms to the point where that they cannot respond.
A limitation of this study is that we performed the biocide resistance experiments on biofilms formed for short durations. This does not fully represent the chronic wound infection environment, which frequently harbors mature biofilms. Furthermore, susceptibility testing was done on biofilms on pegged lids whose material composition is different from that of biofilms used for resistance iteration testing and also may not represent the actual situation found in wound infections. Additionally, we only tested two bacterial strains commonly found in wounds to evaluate the potential emergence of resistance. Another limitation is that we grew subsequent iterations of biofilms from two/three colonies of bacteria, which were exposed to e-scaffold treatment. This reduces the probability of selecting a mutation in the next iteration. Bacteria also were grown without selective pressure (in broth culture and then used to establish biofilms for 24 h). Finally, selective evolution of biocide resistance depends on the initial number of cells before treatment (41), and we did not study large population sizes.
In conclusion, our data suggest that HOCl has similar activity against planktonic and biofilm bacteria, whereas H2O2 is substantially less active against biofilm than planktonic bacteria. We did not observe the emergence of antibiofilm resistance with repeated exposure to either H2O2- or HOCl-producing e-scaffolds under the conditions studied.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
The 27 isolates studied are listed in Table 1. Isolates were removed from −80°C freezer stocks and streaked onto sheep blood agar plates.
Susceptibility of planktonic bacterial isolates to hydrogen peroxide or hypochlorous acid.
H2O2 and HOCl MICs for S. aureus, Staphylococcus epidermidis, Enterococcus faecalis, Enterococcus faecium, E. coli, P. aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae were determined by a modified broth microdilution protocol described in the Clinical and Laboratory Standards Institute (CLSI) guidelines (42). Overnight-grown bacterial colonies were used to inoculate 5 ml of tryptic soy broth (TSB; catalog no. 211825; BD Company, Sparks, MD) and cultures grown to a 0.5 McFarland standard. A 30% (wt/wt) stock solution of H2O2 (H1009; Sigma-Aldrich) was diluted to a 5% working solution in cation-adjusted Mueller-Hinton broth (CAMHB) for susceptibility assays. This was serially diluted to concentrations ranging from 1.632 to 0.19 mM so that each well contained 50 μl of H2O2, and then 50 μl of CAMHB (212322; BD Company, Sparks, MD) containing ∼5 × 105 CFU of bacteria was added to wells of U-bottom 96-well plates (non-tissue culture treated; 35117; Corning Incorporated, Corning, NY). A stock solution of 0.0525% (∼7.94 mM) HOCl (Aquaox, Loxahatchee, FL) was diluted in CAMHB to create testing concentrations ranging from 3.97 to 0.062 mM, and the addition of bacteria was done as described above. Plates were incubated at 37°C for 18 to 20 h and MICs recorded as the wells with the lowest concentration of H2O2 or HOCl with no turbidity. All experiments were performed in triplicate, with data represented as means ± standard deviations (SD).
Susceptibility of bacterial biofilms to hydrogen peroxide or hypochlorous acid.
Minimum biofilm inhibitory concentrations (MBICs) and minimum biofilm bactericidal concentrations (MBBCs) of H2O2 and HOCl against bacteria were determined using a pegged-lid microtiter plate assay (43). Briefly, 150 μl of bacterial suspension in TSB (standardized to a 0.5 McFarland) was added to wells of flat-bottom 96-well plates (243656; Thermo Scientific, Roskilde, Denmark) and covered with 96-pegged lid (445497; Nunc-TSP; Thermo Scientific). Plates were incubated for 6 h at 37°C on an orbital shaker (120 rpm). Pegged lids were rinsed with 1× phosphate-buffered saline (PBS; 10× PBS buffer; AM9625; Invitrogen) and transferred to a microplate containing serial dilutions of H2O2 (1.632 to 0.19 mM) or HOCl (3.97 to 0.062 mM) in CAMHB. Plates were incubated for 24 h at 37°C without shaking. MBICs were recorded as the lowest concentration of biocide showing no visible bacterial growth. Next, the pegged lids were washed in PBS and transferred to recovery microtiter plates containing 200 μl of CAMHB per well and incubated at 37°C for an additional 24 h. MBBCs were recorded as the wells with the lowest concentration of H2O2 or HOCl with no turbidity. All experiments were performed in triplicate, with data represented as means ± SD.
Repeated exposure of methicillin-resistant S. aureus (MRSA) and P. aeruginosa biofilms to H2O2 and HOCl generated by e-scaffolds to assess decrease in activity with repetitive exposure.
For these experiments, we used H2O2- and HOCl-generating e-scaffolds made of carbon fabric designed and assembled as in our previous study (14). e-scaffolds electrochemically reduce dissolved oxygen to H2O2 when polarized at −0.6 VAg/AgCl or produce HOCl when polarized at +1.5 VAg/AgCl (13, 14). We evaluated changes in activity using polarized e-scaffolds against S. aureus USA100 and P. aeruginosa IDRL-11442. Biofilms were grown in vitro in 6-well plates for 24 h at 37°C and then exposed to H2O2-generating e-scaffolds for 24 h (for both S. aureus and P. aeruginosa). For HOCl-generating e-scaffold treatment, S. aureus biofilms were exposed for 4 h and P. aeruginosa for 1 h at room temperature (initial inoculum, ∼1 × 104 CFU [CFU/well]). Controls were biofilms exposed to nonpolarized e-scaffolds. After e-scaffold treatment, biofilms were removed and quantified and results reported as log10 CFU/cm2, as previously described (39). After the first treatment, two to three colonies recovered from quantitative culture were used to prepare a new biofilm, which was again exposed to treatment for the same time; this was repeated for 10 iterations. At the end of 10 iterations of exposure, we again determined the MIC, MBIC, and MBBC of the two studied bacterial isolates. All experiments were performed in triplicate, with data represented as means ± SD.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (award number R01 AI091594). R.P. reports grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics Limited, and Shionogi.
We acknowledge Melissa Karau and Suzannah Schmidt-Malan for critical review of the manuscript. We thank Henry Chambers III (University of California, San Francisco) for providing S. aureus USA100, USA200, and USA300, Caliper Life Sciences for providing S. aureus Xen 30, S. epidermidis Xen 43, and P. aeruginosa Xen 5, Daniel Hassett (University of Cincinnati) for providing P. aeruginosa PAO1, PA14, and the PA14 ΔkatAB mutant, and the Antibacterial Resistance Leadership Group for providing A. baumannii ARLG-1268.
R.P. is a consultant for Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, and Qvella; monies are paid to the Mayo Clinic. In addition, R.P. has patents on Bordetella pertussis/parapertussis PCR, a device/method for sonication with royalties paid by Samsung to the Mayo Clinic, and an antibiofilm substance. R.P. receives travel reimbursement from ASM and IDSA, an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. H.B. holds a patent (US20180207301A1), “Electrochemical reduction or prevention of infections,” which refers to the electrochemical scaffold described here.
REFERENCES
- 1.Bumpus K, Maier MA. 2013. The ABC’s of wound care. Curr Cardiol Rep 15:346. doi: 10.1007/s11886-013-0346-6. [DOI] [PubMed] [Google Scholar]
- 2.Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 3.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranoski S, Ayello EA. 2008. Wound care essentials: practice principles. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 5.Eming SA, Krieg T, Davidson JM. 2007. Inflammation in wound repair: molecular and cellular mechanisms. J Investig Dermatol 127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 6.Lipsky BA, Hoey C. 2009. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 49:1541–1549. doi: 10.1086/644732. [DOI] [PubMed] [Google Scholar]
- 7.Atiyeh BS, Dibo SA, Hayek SN. 2009. Wound cleansing, topical antiseptics and wound healing. Int Wound J 6:420–430. doi: 10.1111/j.1742-481X.2009.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreml S, Landthaler M, Schäferling M, Babilas P. 2011. A new star on the H2O2rizon of wound healing? Exp Dermatol 20:229–231. doi: 10.1111/j.1600-0625.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong DG, Bohn G, Glat P, Kavros SJ, Kirsner R, Snyder R, Tettelbach W. 2015. Expert recommendations for the use of hypochlorous solution: science and clinical application. Ostomy Wound Manage 61:S2–S19. [PubMed] [Google Scholar]
- 10.Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, Hwong W, Barati E, Belisle B, Celeri C, Robson MC. 2007. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds 6:e5. [PMC free article] [PubMed] [Google Scholar]
- 11.Rojkind M, Dominguez-Rosales JA, Nieto N, Greenwel P. 2002. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci 59:1872–1891. doi: 10.1007/pl00012511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. 2014. Hypochlorous acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 26:342–350. [PubMed] [Google Scholar]
- 13.Kiamco MM, Zmuda HM, Mohamed A, Call DR, Raval YS, Patel R, Beyenal H. 2019. Hypochlorous-acid-generating electrochemical scaffold for treatment of wound biofilms. Sci Rep 9:2683. doi: 10.1038/s41598-019-38968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultana ST, Atci E, Babauta JT, Falghoush AM, Snekvik KR, Call DR, Beyenal H. 2015. Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Sci Rep 5:14908. doi: 10.1038/srep14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaieb K, Zmantar T, Souiden Y, Mahdouani K, Bakhrouf A. 2011. XTT assay for evaluating the effect of alcohols, hydrogen peroxide and benzalkonium chloride on biofilm formation of Staphylococcus epidermidis. Microb Pathog 50:1–5. doi: 10.1016/j.micpath.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montagna MT, Triggiano F, Barbuti G, Bartolomeo N, De Giglio O, Diella G, Lopuzzo M, Rutigliano S, Serio G, Caggiano G. 2019. Study on the in vitro activity of five disinfectants against nosocomial bacteria. IJERPH 16:1895. doi: 10.3390/ijerph16111895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perumal PK, Wand ME, Sutton JM, Bock LJ. 2014. Evaluation of the effectiveness of hydrogen-peroxide-based disinfectants on biofilms formed by Gram-negative pathogens. J Hosp Infect 87:227–233. doi: 10.1016/j.jhin.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Aarestrup FM, Hasman H. 2004. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Vet Microbiol 100:83–89. doi: 10.1016/j.vetmic.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, Sleiman J, Johani K, Vickery K. 2018. Hypochlorous acid versus povidone-iodine containing irrigants: which antiseptic is more effective for breast implant pocket irrigation? Aesthet Surg J 38:723–727. doi: 10.1093/asj/sjx213. [DOI] [PubMed] [Google Scholar]
- 21.Mazzola PG, Jozala AF, Novaes LCDL, Moriel P, Penna TCV. 2009. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz J Pharm Sci 45:241–248. doi: 10.1590/S1984-82502009000200008. [DOI] [Google Scholar]
- 22.Bardouniotis E, Huddleston W, Ceri H, Olson ME. 2001. Characterization of biofilm growth and biocide susceptibility testing of Mycobacterium phlei using the MBEC assay system. FEMS Microbiol Lett 203:263–267. doi: 10.1016/S0378-1097(01)00364-0. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues D, Cerca N, Teixeira P, Oliveira R, Ceri H, Azeredo J. 2011. Listeria monocytogenes and Salmonella enterica enteritidis biofilms susceptibility to different disinfectants and stress-response and virulence gene expression of surviving cells. Microb Drug Resist 17:181–189. doi: 10.1089/mdr.2010.0183. [DOI] [PubMed] [Google Scholar]
- 24.Mah T-FC, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 25.O'Toole GA, Stewart PS. 2005. Biofilms strike back. Nat Biotechnol 23:1378–1379. doi: 10.1038/nbt1105-1378. [DOI] [PubMed] [Google Scholar]
- 26.de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock RE. 2013. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 27.DeQueiroz GA, Day DF. 2007. Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces. J Appl Microbiol 103:794–802. doi: 10.1111/j.1365-2672.2007.03299.x. [DOI] [PubMed] [Google Scholar]
- 28.Tote K, Horemans T, Berghe DV, Maes L, Cos P. 2010. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 76:3135–3142. doi: 10.1128/AEM.02095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun HS, Kim Y, Oh S, Jeon WM, Frank JF, Kim SH. 2012. Susceptibility of Listeria monocytogenes biofilms and planktonic cultures to hydrogen peroxide in food processing environments. Biosci Biotechnol Biochem 76:2008–2013. doi: 10.1271/bbb.120238. [DOI] [PubMed] [Google Scholar]
- 30.Stewart PS, Roe F, Rayner J, Elkins JG, Lewandowski Z, Ochsner UA, Hassett DJ. 2000. Effect of catalase on hydrogen peroxide penetration into Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 66:836–838. doi: 10.1128/aem.66.2.836-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart PS. 1998. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng 59:261–272. doi:. [DOI] [PubMed] [Google Scholar]
- 32.Cochran WL, McFeters GA, Stewart PS. 2000. Reduced susceptibility of thin Pseudomonas aeruginosa biofilms to hydrogen peroxide and monochloramine. J Appl Microbiol 88:22–30. doi: 10.1046/j.1365-2672.2000.00825.x. [DOI] [PubMed] [Google Scholar]
- 33.Elkins JG, Hassett DJ, Stewart PS, Schweizer HP, McDermott TR. 1999. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl Environ Microbiol 65:4594–4600. doi: 10.1128/AEM.65.10.4594-4600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechevallier MW, Cawthon CD, Lee RG. 1988. Inactivation of biofilm bacteria. Appl Environ Microbiol 54:2492–2499. doi: 10.1128/AEM.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo DM, Castillo Y, Delgadillo NA, Neuta Y, Jola J, Calderón JL, Lafaurie GI. 2015. Viability and effects on bacterial proteins by oral rinses with hypochlorous acid as active ingredient. Braz Dent J 26:519–524. doi: 10.1590/0103-6440201300388. [DOI] [PubMed] [Google Scholar]
- 37.Chen C-J, Chen C-C, Ding S-J. 2016. Effectiveness of hypochlorous acid to reduce the biofilms on titanium alloy surfaces in vitro. Int J Mol Sci 17:1161. doi: 10.3390/ijms17071161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Cruz Nizer WS, Inkovskiy V, Overhage J. 2020. Surviving reactive chlorine stress: responses of Gram-negative bacteria to hypochlorous acid. Microorganisms 8:1220. doi: 10.3390/microorganisms8081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raval YS, Mohamed A, Zmuda HM, Patel R, Beyenal H. 2019. Hydrogen-peroxide-generating electrochemical scaffold eradicates methicillin-resistant Staphylococcus aureus biofilms. Glob Chall 3:1800101. doi: 10.1002/gch2.201800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikai H, Odashima Y, Kanno T, Nakamura K, Shirato M, Sasaki K, Niwano Y. 2013. In vitro evaluation of the risk of inducing bacterial resistance to disinfection treatment with photolysis of hydrogen peroxide. PLoS One 8:e81316. doi: 10.1371/journal.pone.0081316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boles BR, Thoendel M, Singh PK. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc Natl Acad Sci U S A 101:16630–16635. doi: 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CLSI. 2015. Methods for dilution–antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed. CLSI, Wayne, PA. [Google Scholar]
- 43.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776. doi: 10.1128/JCM.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]