Triazole resistance in Aspergillus fumigatus is an increasing worldwide problem that causes major challenges in the management of aspergillosis. New antifungal drugs are needed, with novel targets, that are effective in triazole-resistant infection.
KEYWORDS: olorofim, Aspergillus fumigatus, antifungal susceptibility, azole resistance, genetic diversity, China
ABSTRACT
Triazole resistance in Aspergillus fumigatus is an increasing worldwide problem that causes major challenges in the management of aspergillosis. New antifungal drugs are needed, with novel targets, that are effective in triazole-resistant infection. In this study, we retrospectively evaluated the potency of the novel drug olorofim compared to contemporary antifungal agents against 111 clinical A. fumigatus isolates collected from Huashan Hospital, Shanghai, China, using EUCAST methodology, and we reviewed the literature on triazole-resistant A. fumigatus (TRAF) published between 1966 and 2020 in China. Olorofim was active in vitro against all tested A. fumigatus isolates, with a MIC90 of 0.031 mg/liter (range, 0.008 to 0.062 mg/liter). For 4 triazole-resistant A. fumigatus isolates, the olorofim MIC ranged between 0.016 and 0.062 mg/liter. The reported rates of TRAF in China are 2.5 to 5.56% for clinical isolates and 0 to 1.4% for environmental isolates. TR34/L98H/S297T/F495I is the predominant resistance mechanism, followed by TR34/L98H. Non-TR-mediated TRAF isolates, mostly harboring a cyp51A single point mutation, showed greater genetic diversity than TR-mediated resistant isolates. Resistance due to TR34/L98H and TR34/L98H/S297T/F495I mutations among TRAF isolates might have evolved from separate local isolates in China. Continuous isolation of TRAF in China underscores the need for systematic resistance surveillance as well as the need for novel drug targets, such as olorofim.
INTRODUCTION
Invasive aspergillosis (IA) in immunocompromised patients results in substantial morbidity and mortality (1). More than 40 Aspergillus species have been reported as causative agents of IA. Aspergillus fumigatus is the most common etiological agent of invasive and chronic pulmonary aspergillosis (1). Two classes of antifungal agents are licensed for the primary therapy of IA, namely, the triazoles and the polyene amphotericin B. Currently, triazole antifungals are recommended as the first choice for prophylaxis and treatment of aspergillosis (1). However, since the first report of triazole resistance in 1997 (2), many centers/hospitals around the world have reported resistance. Furthermore, voriconazole-resistant IA was found to be associated with treatment failure and excess mortality, which threatens the current treatment strategy for this pathogen (3, 4).
The most common mechanism of triazole resistance is associated with mutations in the cyp51A gene, which encodes the protein targeted by the triazoles (5). Apparently, the mutant allele has spread throughout the A. fumigatus population and, thus, has been reported worldwide from patients as well as from the environment. In addition, several point mutations, such as G54, G138, and M220, intervene with the docking of azole drugs to CYP51A protein and render an azole-resistant phenotype (3). Rates of azole resistance in A. fumigatus vary extensively among countries and centers worldwide (6–9), and in many countries the presence and frequency of azole resistance remain unknown. Multiple factors contribute to the observed variation in resistance frequency, including sample size, method of resistance detection, and geographical differences (10). The overall azole resistance rates ranged from 0 to 27.8% in different surveys (11–13). Since the spread of antifungal drug resistance has shown no signs of diminishing and new resistance mechanisms continue to emerge (14), understanding the genetic variability and relationship among resistant isolates from various parts of the world is of major importance. Azole resistance surveillance programs are scarce, and in China data on the prevalence of azole-resistant A. fumigatus are very limited. A few Chinese reports on triazole resistance in A. fumigatus are available, although most reports are from restricted geographic areas and include only a modest number of isolates (7, 13, 15–19). Furthermore, the genetic relationship and variability of azole-resistant isolates of A. fumigatus in China remain unclear.
The clinical development of new antifungal drug classes is critical to overcoming current and future challenges in the management of Aspergillus diseases. Olorofim (formerly F901318), a leading representative of a novel class of drug belonging to orotomides, is an antifungal drug in clinical development that demonstrates excellent potency against a broad range of dimorphic and filamentous fungi, and it targets an important enzyme for pyrimidine biosynthesis, dihydroorotate dehydrogenase (20). The drug has in vitro activity against Aspergillus species and other difficult-to-treat molds, including Scedosporium and Lomentospora species, but lacks activity against Candida, Cryptococcus, and Mucorales species due to differences in drug target affinity (20–22). For Aspergillus species specifically, Buil et al. demonstrated in vitro activity against azole wild-type (WT) isolates as well as azole-resistant cyp51A mutant A. fumigatus isolates, also including a limited number of other Aspergillus species originating from the Netherlands (20).
We aimed to evaluate the potency of olorofim against a large set of clinical A. fumigatus isolates collected from China and compare the activity with that of contemporary antifungal agents. We further reviewed the prevalence of azole resistance and underlying cyp51A mutations in clinical and environmental A. fumigatus isolates in China.
RESULTS
The in vitro activities of olorofim and comparator agents against 111 clinical A. fumigatus isolates from China are shown in Table 1. The ofolorofim MICs ranged between 0.008 and 0.062 mg/liter, which were, in general, lower than the MICs of the azoles and amphotericin B. Compared with echinocandins, olorofim showed MICs (MIC90, 0.031 mg/liter; modal MIC, 0.031 mg/liter; n = 70) similar to those of anidulafungin (90% minimum effective concentration [MEC90], 0.031 mg/liter; modal MEC, 0.016 mg/liter; n = 67), slightly higher than those of micafungin (MEC90, 0.016 mg/liter; modal MEC, 0.008 mg/liter; n = 61), and significantly lower than those of caspofungin (MEC90, 0.5 mg/liter; modal MEC, 0.25 mg/liter; n = 67). Posaconazole (modal MICs, 0.062 mg/liter; n = 70) exhibited the lowest modal MICs of the azoles in this study, followed by itraconazole (0.25 mg/liter; n = 53), voriconazole (0.5 mg/liter; n = 85), and isavuconazole (0.5 mg/liter; n = 83). Amphotericin B had relatively higher modal MICs (0.5 mg/liter; n = 97).
TABLE 1.
MIC/MEC ranges and geometric means, modal MIC/MEC, and distribution of MIC/MEC of 111 clinical A. fumigatus isolates from China for 9 antifungal agentsa
| Antifungal agent | MIC/MEC range (mg/liter) | Geometric mean (mg/liter) | MIC/MEC50 (mg/liter) | MIC/MEC90 (mg/liter) | No. of isolates with MIC/MEC of: |
No. (%) of resistant strains | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.002 | 0.004 | 0.008 | 0.016 | 0.031 | 0.062 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ||||||
| Olorofim | 0.008–0.062 | 0.025 | 0.031 | 0.031 | 3 | 34 | 70 | 4 | |||||||||||
| Itraconazole | 0.125–16 | 0.373 | 0.25 | 0.5 | 7 | 53 | 47 | 4 | 4 (3.60) | ||||||||||
| Voriconazole | 0.25–8 | 0.500 | 0.5 | 1 | 14 | 85 | 10 | 2* | 0 | ||||||||||
| Posaconazole | 0.031–1 | 0.078 | 0.062 | 0.125 | 7 | 70 | 29 | 3* | 2 | 2 (1.80) | |||||||||
| Isavuconazole | 0.25–16 | 0.574 | 0.5 | 1 | 6 | 83 | 19 | 2* | 1 | 3 (2.70) | |||||||||
| Amphotericin B | 0.125–1 | 0.529 | 0.5 | 1 | 1 | 1 | 97 | 12 | |||||||||||
| Anidulafungin | 0.016–0.062 | 0.021 | 0.016 | 0.031 | 67 | 43 | 1 | ||||||||||||
| Caspofungin | 0.062–0.5 | 0.271 | 0.25 | 0.5 | 3 | 11 | 67 | 30 | |||||||||||
| Micafungin | 0.002–0.062 | 0.009 | 0.008 | 0.016 | 1 | 14 | 61 | 30 | 2 | 3 | |||||||||
For modal MIC/MEC, values in boldface indicate the most frequent MIC/MEC, underlined values indicate the resistant isolates, and values with an asterisk indicate the strains in the area of technical uncertainty (ATU). MICs are shown for amphotericin B, itraconazole, voriconazole, posaconazole, isavuconazole, and olorofim; MECs are shown for anidulafungin, caspofungin, and micafungin.
In vitro activities of olorofim and comparator agents against 4 TRAF isolates are shown in Table 2. Four TRAF isolates were highly resistant to itraconazole (MIC, >16 mg/liter), and two isolates were in the area of technical uncertainty (ATU) of voriconazole (MIC, 2 mg/liter), posaconazole (MIC, 0.25 mg/liter), and isavuconazole (MIC, 2 mg/liter). Isolate 247-34 was resistant to both posaconazole (MIC, 1 mg/liter) and isavuconazole (MIC > 16 mg/liter), and isolate 247-32 was resistant to posaconazole (MIC, 1 mg/liter). A resistance mutation was detected in cyp51A of two isolates, G54V in isolate 247-32 and TR34/L98H/S297T/F495I in isolate 247-34 (Table 2).
TABLE 2.
MIC/MEC values and cyp51A gene mutation type of four azole-resistant A. fumigatus isolates detected in this studya
| Isolate no. | MIC/MEC (mg/liter) |
Mutation in cyp51A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Olo | Itra | Vori | Posa | Isa | AmB | Anid | Cas | Mica | ||
| 247-11 | 0.062 | >16 | 2* | 0.25* | 2* | 0.5 | 0.031 | 0.25 | 0.008 | WT |
| 247-20 | 0.031 | >16 | 2* | 0.25* | 2* | 0.5 | 0.016 | 0.25 | 0.008 | WT |
| 247-32 | 0.016 | >16 | 0.5 | 1 | 0.5 | 0.5 | 0.016 | 0.25 | 0.008 | G54V |
| 247-34 | 0.031 | >16 | 0.5 | 1 | >16 | 0.5 | 0.016 | 0.5 | 0.008 | TR34/L98H/S297T/F495I |
Abbreviations: amphotericin B, AmB; itraconazole, Itra; voriconazole, Vori; posaconazole, Posa; isavuconazole, Isa; olorofim, Olo; anidulafungin, Anid; caspofungin, Cas; micafungin, Mica. MICs are shown for amphotericin B, itraconazole, voriconazole, posaconazole, isavuconazole, olorofim; MECs are shown for anidulafungin, caspofungin, micafungin. Asterisks indicate values in the area of technical uncertainty.
Among the four TRAF isolates detected in this study, MIC values of olorofim (range, 0.016 to 0.062 mg/liter) were in the same range as those observed for the azole WT isolates. The lowest olorofim MIC was seen in isolate 247-32 with G54V, and the highest MIC was 0.062 mg/liter, for azole-resistant A. fumigatus isolates with the WT cyp51A gene.
Microsatellite typing.
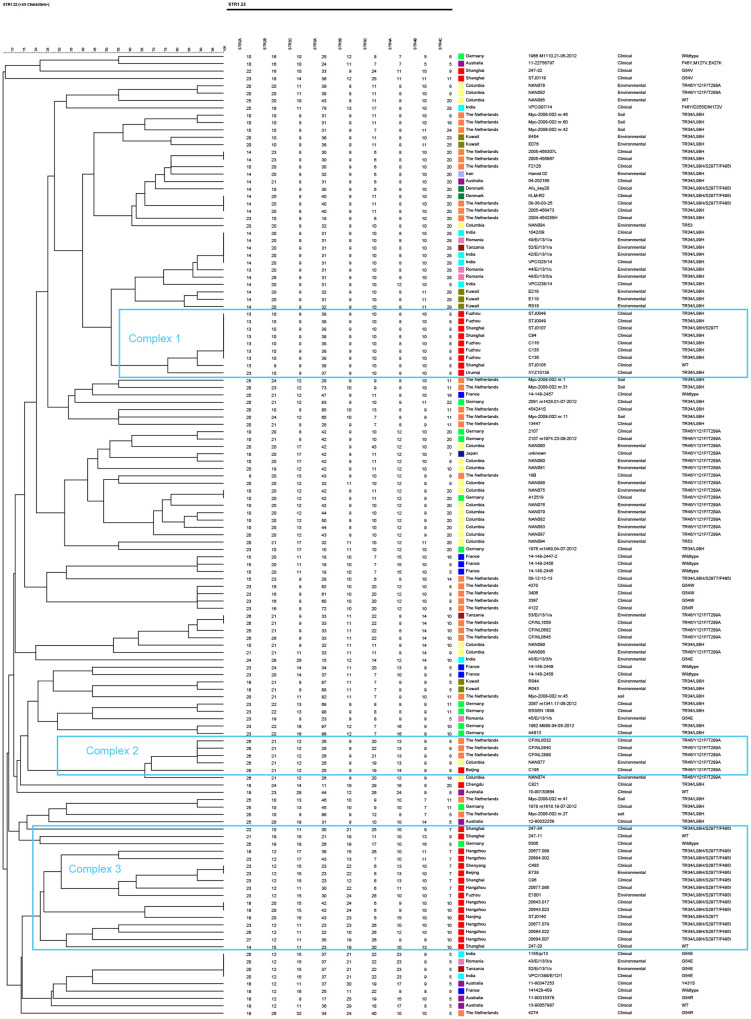
The genetic polymorphism of TRAF isolates from China and outside China was studied using short tandem repeat (STR) typing (Fig. 1). Multiple distinct clusters can be identified based on microsatellite markers. STR typing of 29 Chinese TRAF isolates revealed 21 distinct genotypes distributed among environmental and clinical isolates that represented a major complex of the TRAF isolates disseminating all around the world.
FIG 1.
Genotypic relationship of Chinese azole-resistant A. fumigatus isolates (clinical, n = 27; environmental, n = 2) with isolates from Columbia (environmental, n = 19), Denmark (clinical, n = 2), France (clinical, n = 7), Germany (clinical, n = 12), India (clinical, n = 6; environmental, n = 2), Iran (clinical, n = 1), Japan (clinical, n = 1), Kuwait (environmental, n = 7), the Netherlands (clinical, n = 21; environmental, n = 9), Romania (environmental, n = 5), Tanzania (environmental, n = 3), and Australia (clinical, n = 7).
Three microsatellite complexes (MCs) among the 21 cyp51A mutant genotypes of Chinese TRAF were recognizable, representing three distinct complexes of TRAF (Fig. 1). Seven isolates with TR34/L98H in complex 1 were clonal and shared all nine loci except for two isolates, with one difference in one repeat at a single locus (2B) and the other at three loci (2A, 2B, and 3A). Thirteen isolates with mutation TR34/L98H/S297T/F495I in complex 3 were highly polymorphic and different from the isolates with the same mutation from the Netherlands and Denmark, which clustered in complex 1. Among 13 polymorphic genotypes observed in TR34/L98H/S297T/F495I isolates, an identical allelic profile was observed in a clinical (isolate C485) and an environmental isolate (isolate E739). One isolate with TR46/Y121F/T289A from Beijing was clustered in a complex group with isolates harboring TR46/Y121F/T289A from the Netherlands and Columbia.
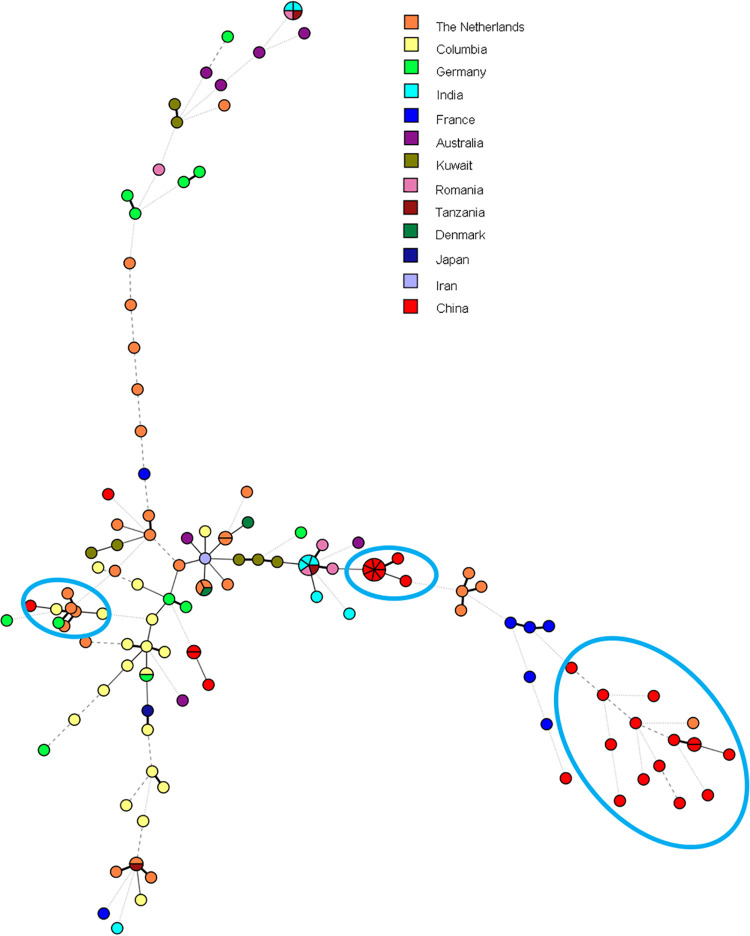
The genotypic relationships among Chinese and global isolates were also inferred from the minimum spanning tree (Fig. 2). High genetic variability was observed among A. fumigatus isolates, which was not associated with the country and continent of origin. TRAF in China showed divergence in genetic variability as well.
FIG 2.
Minimum spanning tree of 131 azole-resistant A. fumigatus isolates based on all nine microsatellite markers of STR typing.
Literature review.
Our literature review resulted in 8 publications reporting azole resistance in A. fumigatus in China (7, 13, 15–18, 23, 24). TRAF was first reported in 2004 involving two isolates with single resistance point mutations (Table 3). Resistance involving TRs was first reported in 2011 and has since been the dominant resistance variant in China. Azole resistance rates in A. fumigatus isolates ranged from 2.5% to 5.56% for clinical isolates and 0 to 1.4% for environmental isolates, and the rate of azole resistance in A. fumigatus isolates in the current study was 3.6% (four of 111 isolates), which was within this range. Origin, source, and antifungal susceptibility profiles of TR-mediated azole-resistant A. fumigatus isolates in China from 2004 to 2019 are summarized in Table 4.
TABLE 3.
Overview of TRAF isolates harboring mutations in the cyp51A gene from clinical and environmental sources in China, 2004 to 2019
| Yr published | Resistance mechanism | Source | Resistance rate [no. of resistant isolates/no. of isolates tested (%)] | Antifungal susceptibility testing methods | Reference or source |
|---|---|---|---|---|---|
| 2020 | G54V (n = 1); TR34/L98H/S297T/F495 (n = 1); WT (n = 2) | Clinical | 4/111 (3.60) | EUCAST 9.3.1 | Current study |
| 2017 | M220I (n = 1); TR34/L98H (n = 2); WT (n = 1) | Clinical | 4/126 (3.17) | EUCAST 9.1 | Zhang et al. (23) |
| 2017 | TR34/L98H (n = 3); TR34/L98H/S297T (n = 2); G54V (n = 1); WT (n = 1) | Clinical | 7/159 (4.40) | CLSI M38-A2 | Deng et al. (7) |
| 2017 | TR46/Y121F/T289A (n = 2); TR34/L98H/S297T/F495I (n = 1) | Environmental | 3/144 (2.08) | CLSI M38-A2 | Ren et al. (24) |
| 2016 | TR34/L98H (n = 5); TR34/L98H/S297T/F495I (n = 2); TR46/Y121F/T289A (n = 1) | Clinical | 8/317 (2.5) | EUCAST 9.3 | Chen et al. (15) |
| TR34/L98H/S297T/F495I (n = 2) | Environmental | 2/144 (1.4) | |||
| 2015 | TR34/L98H/S297T/F495I (n = 2); G432A (n = 1); TR34/L98H (n = 1) | Clinical | 4/72 (5.56) | EUCAST 9.1 | Liu et al. (16) |
| 2014 | Environmental | 0/51 (0.00) | CLSI M38-A2 | Wang et al. (18) | |
| 2011 | TR34/L98H/S297T/F495I (n = 8); WT (n = 2) | Clinical | 24 (above ECVa); 10 (ITR or VORI, >2 μg/ml) | CLSI M38-A2 | Lockhart et al. (13) |
| 2004 | M220I (n = 1); G54R (n = 3) | Clinical | 4/6b | NCCLS M38-A | Chen et al. (17) |
Epidemiological cutoff values (ECV) are 1 μg/ml for itraconazole and voriconazole and 0.25 μg/ml for posaconazole. The total number of strains tested is not reported.
Six clinical strains isolated from the same patient.
TABLE 4.
Origin, source, and antifungal susceptibility of TR-mediated azole-resistant A. fumigatus isolates in China originating from the literature, 2004 to 2020a
| Resistance mechanism | Strain ID no. | Region | Source | MIC, mg/liter |
||
|---|---|---|---|---|---|---|
| Itra | Vori | Posa | ||||
| TR34/L98H/S297T/F495I (n = 16) | 247-34 | Shanghai | Clinical | >16 | 0.5 | 1 |
| 51 | Zhejiang | Environmental | 8–16 | 1 | 0.5 | |
| C96 | Shanghai | Clinical | >16 | 1 | 0.5 | |
| C485 | Shenyang | Clinical | >16 | 2 | 1 | |
| E739 | Beijing | Environmental | >16 | 2 | 0.5 | |
| E1001 | Fuzhou | Environmental | >16 | 1 | 0.5 | |
| SHJT42b | Fuzhou | Clinical | 16 | 2 | 0.5 | |
| NJ21-76 | Nanjing | Clinical | 16 | 0.25 | 0.5 | |
| 20643.017 | Hangzhou | Clinical | 16 | 2 | 2 | |
| 20643.023 | Hangzhou | Clinical | 16 | 2 | 2 | |
| 20677.079 | Hangzhou | Clinical | 16 | 1 | 1 | |
| 20677.086 | Hangzhou | Clinical | 16 | 2 | 2 | |
| 20677.089 | Hangzhou | Clinical | 16 | 4 | 2 | |
| 20684.002 | Hangzhou | Clinical | 16 | 2 | 2 | |
| 20684.007 | Hangzhou | Clinical | 16 | 2 | 2 | |
| 20684.022 | Hangzhou | Clinical | 16 | 2 | 1 | |
| TR34/L98H n = 11 | AF.44 | Nanjing | Clinical | >8 | 4 | 0.5 |
| AF.98 | Nanjing | Clinical | >8 | 2 | 0.25 | |
| STJ0048 | Fuzhou | Clinical | >16 | 1 | 1 | |
| STJ0049 | Fuzhou | Clinical | >16 | 1 | 1 | |
| XJ138 | Urumqi | Clinical | 16 | 2 | 0.5 | |
| C94 | Shanghai | Clinical | ≥16 | 2 | 1 | |
| C116 | Fuzhou | Clinical | ≥16 | 4 | 0.5 | |
| C135 | Fuzhou | Clinical | ≥16 | 2 | 0.5 | |
| C136 | Fuzhou | Clinical | ≥16 | 2 | 0.5 | |
| C821 | Chengdu | Clinical | ≥16 | 4 | 1 | |
| SHJT40 | Shanghai | Clinical | 16 | 1 | 0.5 | |
| TR34/L98H/S297T n = 2 | STJ0107 | Shanghai | Clinical | >16 | 0.5 | 1 |
| STJ0140 | Nanjing | Clinical | >16 | 0.5 | 1 | |
| TR46/Y121F/T289A n = 3 | 15 | Zhejiang | Environmental | 0.5 | 8–16 | 0.25 |
| 44 | Zhejiang | Environmental | 0.5 | 8–16 | 0.25 | |
| C195 | Beijing | Clinical | 1 | ≥16 | 0.5 | |
DISCUSSION
In this study, we show that olorofim exhibits potent in vitro activity against 111 clinical A. fumigatus isolates, including TRAF from China. For the determination of wild-type upper limits (WT-UL) of visual values of A. fumigatus susceptibility to olorofim, we followed the 0.25 mg/liter value, as proposed by Jørgensen et al. (25). Olorofim MICs were low against 111 A. fumigatus isolates (modal MIC, 0.031 mg/liter; MIC range, 0.008 to 0.062 mg/liter), indicating that all MICs were within the range of the WT population. The observed MIC ranges are similar to those reported in previous reports from other geographic areas (19–21). The potency of olorofim was superior to that of triazoles and amphotericin B and comparable to those of three echinocandins tested. No substantial implications of the specific azole resistance mechanism for the activity of olorofim were demonstrated.
In an itraconazole-resistant A. fumigatus isolate with a G54V mutation, obtained from a patient undergoing high-dose itraconazole therapy, olorofim was 5- to 6-fold more potent than voriconazole and posaconazole, respectively. Furthermore, in an isolate harboring TR34/L98H/S297T/F495I, olorofim was 4-, 5-, and 9-fold more potent than voriconazole, posaconazole, and isavuconazole, respectively. Olorofim was also more active than voriconazole and isavuconazole against the two other TRAF isolates with WT cyp51A genes. These findings confirm previous reports (20, 22, 26) and indicate that triazole resistance does not affect olorofim activity, as olorofim MICs of these isolates are within the olorofim WT population (25).
The rate of azole resistance in A. fumigatus isolates in China (2.5% to 5.56%) is around the lowest border compared to the high prevalence in Europe, including the United Kingdom (6.6 to 27.8%), the Netherlands (3.1 to 4.6%), and Germany (3.2%) (27–30) The first report on the occurrence of TRAF isolates originated from China during 2008 to 2009 from the ARTEMIS global sentinel surveillance program, which demonstrated the TR34/L98H/S297T mechanism in 27.5% (8/29) of A. fumigatus isolates (13) (Table 3). Our study, reviewing Chinese TRAF isolates from 2004 to 2019, confirmed that TR34/L98H/S297T/F495I (n = 16) was the predominant resistance mechanism in 34.78% of the China TRAF isolates, followed by TR34/L98H (n = 11), TR46/Y121F/T289A (n = 3), G54R (n = 3), G54V (n = 2), TR34/L98H/S297T (n = 2), M220I (n = 2), G432A (n = 1), and nonsynonymous mutations (n = 6).
The geographic origin of the TRAF isolates appeared to concentrate in eastern and southeastern areas (Table 4). All isolates harboring TR34/L98H-related mutations exhibited high-level resistance to itraconazole (MIC, 8 to 16 mg/liter) and intermediate susceptibility or resistance to posaconazole and voriconazole, except for two TR34/L98H/S297T isolates, which had lower voriconazole MICs. In total, three voriconazole-resistant isolates harboring TR46/Y121F/T289A were identified so far, two from the environment and one from a patient.
As shown by microsatellite genotyping, STR typing of the Chinese TRAF isolates demonstrated two major clusters. Seven isolates with the TR34/L98H mutant type in China showed no genetic variability, suggesting a single and recent origin for these resistant isolates. Similarly, Abdolrasouli et al. (31) have described a similar structure in the TR-mediated azole-resistant A. fumigatus population in India. However, these observations contrast with the heterogeneity that was observed in environmental and clinical isolates in the Netherlands (32). The total of 13 Chinese isolates with TR34/L98H/S297T/F495I emerged from only one branch, notably an identical allelic profile with TR34/L98H/S297T/F495I, present in clinical and environmental A. fumigatus isolates from China, suggesting an environmental origin of this major resistance mechanism. The two groupings suggested that these isolates have different evolutionary sources than the major TR34/L98H complex. Our study confirmed that resistance due to TR34/L98H mutation among A. fumigatus isolates evolved from separate local isolates (33).
Our study was limited by the relatively small number of clinical A. fumigatus isolates included and the uneven geographic distribution in China. There are currently no azole resistance surveillance programs in China and many other countries, which would allow for more systematic collection and analysis of clinical A. fumigatus isolates. Furthermore, routine MIC testing is not performed in most clinical microbiology laboratories, which further complicates setting up such surveillance networks.
In conclusion, olorofim displays potent in vitro activity against A. fumigatus originating from China, including TRAF isolates. Further studies are needed to evaluate the in vivo efficacy of olorofim for the treatment of IA.
The need for novel targets is underscored by the increasing reports of TRAF both in patients and the environment. Despite multiple reports of TRAF in China, there is a need for systematic resistance surveillance to increase our understanding of resistance epidemiology and to guide antifungal treatment recommendations.
MATERIALS AND METHODS
Aspergillus isolates and species identification.
A total of 111 clinical A. fumigatus isolates were collected from Huashan Hospital, Fudan University, from 2012 to 2017 in Shanghai, China. The isolates were identified based on morphological features and sequence analysis of the partial β-tubulin gene (benA) sequences (7). The primers used are listed in Table S1 in the supplemental material. Isolate information and GenBank accession numbers for the generated benA sequences are listed in Table S2.
Antifungal susceptibility testing.
In vitro antifungal susceptibility testing of the 111 isolates was performed according to the EUCAST definitive document (E.DEF 9.3.1). Olorofim was provided by F2G, Ltd. (Manchester, UK). Comparator antifungal agents, including amphotericin B, itraconazole, voriconazole, posaconazole, isavuconazole, anidulafungin, caspofungin, and micafungin, were purchased from Sigma-Aldrich (MO, USA). The testing ranges for olorofim, voriconazole, and micafungin were 0.008 to 8 mg/liter, 0.002 to 0.2 mg/liter, and 0.004 to 0.4 mg/liter, respectively. The ranges for amphotericin B, itraconazole, posaconazole, isavuconazole, anidulafungin, and caspofungin were 0.016 to 16 mg/liter. For olorofim, endpoints were determined after 48 h of incubation at 100% inhibition compared with the growth control.
Resistant isolates were defined according to the EUCAST breakpoints (version 10.0). There are no clinical breakpoints available for echinocandins and olorofim. Candida parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as the quality control strains.
cyp51A gene sequencing.
Non-WT A. fumigatus isolates were selected for detection of cyp51A mutations. Genomic DNA was extracted as previously described (34), and full sequences of the cyp51A gene together with the promoter region were amplified and sequenced (35) (the primers used are listed in Table S1). The promoter and full sequence of cyp51A were aligned with the WT A. fumigatus strain (GenBank accession no. AF338659) using MAFFT version 7 (36). Tandem repeats (TR) in the gene promoter and mutations in the open reading frame were characterized after sequence alignment.
Genotyping of A. fumigatus isolates.
Four azole-resistant A. fumigatus isolates were subjected to microsatellite typing, as previously described (37). Nine STR loci (STR Af2A, 2B, 2C, 3A, 3B, 3C, 4A, 4B, and 4C) were amplified in three separated multiplex PCRs. Each of the multiplex PCRs contained three different STRs. The fragments obtained were mixed with formamide and analyzed with GeneScan 500 LIZ on a 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA). The repeat numbers of the nine markers of all isolates were analyzed using Peak Scanner software 2 (Thermo Fisher, CA, USA).
Genetic analysis of microsatellite genotypes.
To understand the genetic relationship of the azole-resistant A. fumigatus isolates in China to the global collection, a total of 29 Chinese azole-resistant A. fumigatus isolates (27 clinical and 2 environmental) and 102 azole-resistant A. fumigatus isolates collected globally were included by literature searching in PubMed. The twenty-nine Chinese azole-resistant A. fumigatus isolates included 25 isolates from the literature (7, 13, 15) and 4 isolates from the current study. The 102 azole-resistant A. fumigatus isolates were selected from the literature (6, 19, 38–46) as representative of different genotypes and geographic areas worldwide. The composite genotype for each of the 131 A. fumigatus isolates was identified based on alleles at all nine microsatellite loci. The genotype markers were then used to identify genetic relationships among isolates. Dendrograms were generated by the unweighted pair group method using average linkages implemented in BioNumerics 7.6 (bioMérieux). A minimum spanning tree was also calculated in BioNumerics 7.6 using advanced cluster analysis. Results of these analyses were used to infer the potential source(s) of the triazole-resistant clinical and environmental A. fumigatus isolates in China.
Literature review.
A literature searching was carried out in databases including Pubmed/Medline, Scopus, Web of Science, Embase, and China National Knowledge Infrastructure (CNKI; https://www.cnki.net/). The English and Chinese language (CNKI database) literature between 1966 and 2020 was reviewed using search terms “China,” “Chinese,” “Aspergillus fumigatus,” “genetic diversity,” “short tandem repeats,” “STR,” “antifungal susceptibility,” “azole resistance,” and “fungicide resistance.”
Data availability.
Accession numbers of 111 clinical strains in this study are listed in Table S2.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge funding from the Suzhou Health and Family Planning Commission (LCZX201728) and Suzhou National New & Hi-Tech Industrial Development Zone (2017Z008) to Shuwen Deng and partial funding by an international joint project from the National Natural Science Foundation of China (81720108026). Huilin Su acknowledges financial support from the China Postdoctoral Science Foundation (2019M663276).
All authors listed made substantial, direct, and intellectual contributions to the work and approved the final manuscript for publication.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Patterson TF, Thompson GR, III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:433–442. doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denning DW, Venkateswarlu K, Oakley KL, Anderson MJ, Manning NJ, Stevens DA, Warnock DW, Kelly SL. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 41:1364–1368. doi: 10.1128/AAC.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhary A, Sharma C, Hagen F, Meis JF. 2014. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol 9:697–711. doi: 10.2217/fmb.14.27. [DOI] [PubMed] [Google Scholar]
- 4.Lestrade PP, Bentvelsen RG, Schauwvlieghe A, Schalekamp S, van der Velden W, Kuiper EJ, van Paassen J, van der Hoven B, van der Lee HA, Melchers WJG, de Haan AF, van der Hoeven HL, Rijnders BJA, van der Beek MT, Verweij PE. 2019. Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clin Infect Dis 68:1463–1471. doi: 10.1093/cid/ciy859. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden JW, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badali H, Vaezi A, Haghani I, Yazdanparast SA, Hedayati MT, Mousavi B, Ansari S, Hagen F, Meis JF, Chowdhary A. 2013. Environmental study of azole-resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses 56:659–663. doi: 10.1111/myc.12089. [DOI] [PubMed] [Google Scholar]
- 7.Deng S, Zhang L, Ji Y, Verweij PE, Tsui KM, Hagen F, Houbraken J, Meis JF, Abliz P, Wang X, Zhao J, Liao W. 2017. Triazole phenotypes and genotypic characterization of clinical Aspergillus fumigatus isolates in China. Emerg Microbes Infect 6:e109. doi: 10.1038/emi.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen KL, Mellado E, Lass-Flörl C, Rodriguez-Tudela JL, Johansen HK, Arendrup MC. 2010. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother 54:4545–4549. doi: 10.1128/AAC.00692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 9:e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeulen E, Lagrou K, Verweij PE. 2013. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 26:493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara D, Watanabe A, Kamei K. 2016. Sensitisation of an azole-resistant aspergillus fumigatus strain containing the Cyp51A-related mutation by deleting the SrbA gene. Sci Rep 6:38833. doi: 10.1038/srep38833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhary A, Sharma C, Meis JF. 2017. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S436–S444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Snelders E, Zwaan BJ, Schoustra SE, Meis JF, van Dijk K, Hagen F, van der Beek MT, Kampinga GA, Zoll J, Melchers WJG, Verweij PE, Debets AJM. 2017. A novel environmental azole resistance mutation in aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio 8:e00791-17. doi: 10.1128/mBio.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Lu Z, Zhao J, Zou Z, Gong Y, Qu F, Bao Z, Qiu G, Song M, Zhang Q, Liu L, Hu M, Han X, Tian S, Zhao J, Chen F, Zhang C, Sun Y, Verweij PE, Huang L, Han L. 2016. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother 60:5878–5884. doi: 10.1128/AAC.01005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Zeng R, Zhang L, Li D, Lv G, Shen Y, Zheng H, Zhang Q, Zhao J, Zheng N, Liu W. 2015. Multiple cyp51A-based mechanisms identified in azole-resistant isolates of Aspergillus fumigatus from China. Antimicrob Agents Chemother 59:4321–4325. doi: 10.1128/AAC.00003-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Li H, Li R, Bu D, Wan Z. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother 55:31–37. doi: 10.1093/jac/dkh507. [DOI] [PubMed] [Google Scholar]
- 18.Wang DY, Gricourt M, Arné P, Thierry S, Seguin D, Chermette R, Huang WY, Dannaoui E, Botterel F, Guillot J. 2014. Mutations in the Cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus isolated from avian farms in France and China. Poult Sci 93:12–15. doi: 10.3382/ps.2013-03541. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Li Z, Han X, Tian S, Zhao J, Chen F, Su X, Zhao J, Zou Z, Gong Y, Qu F, Qiu G, Wang S, Jia X, Lu Z, Hu M, Huang L, Verweij PE, Han L. 2018. Elevated MIC values of imidazole drugs against Aspergillus fumigatus isolates with TR(34)/L98H/S297T/F495I mutation. Antimicrob Agents Chemother 62:e01549-17. doi: 10.1128/AAC.01549-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buil JB, Rijs A, Meis JF, Birch M, Law D, Melchers WJG, Verweij PE. 2017. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 72:2548–2552. doi: 10.1093/jac/dkx177. [DOI] [PubMed] [Google Scholar]
- 21.Wiederhold NP, Law D, Birch M. 2017. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 72:1977–1980. doi: 10.1093/jac/dkx065. [DOI] [PubMed] [Google Scholar]
- 22.Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, Du Pré S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A 113:12809–12814. doi: 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Feng CL, Chen F, He Q, Su X, Shi Y. 2017. Triazole resistance in Aspergillus fumigatus clinical isolates obtained in Nanjing, China. Chin Med J 130:665–668. doi: 10.4103/0366-6999.201609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren J, Jin X, Zhang Q, Zheng Y, Lin D, Yu Y. 2017. Fungicides induced triazole-resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J Hazard Mater 326:54–60. doi: 10.1016/j.jhazmat.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen KM, Astvad KMT, Hare RK, Arendrup MC. 2018. EUCAST determination of olorofim (F901318) susceptibility of mold species, method validation, and MICs. Antimicrob Agents Chemother 62:e00487-18. doi: 10.1128/AAC.00487-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirchhoff L, Dittmer S, Buer J, Rath PM, Steinmann J. 2020. In vitro activity of olorofim (F901318) against fungi of the genus, Scedosporium and Rasamsonia as well as against Lomentospora prolificans, Exophiala dermatitidis and azole-resistant Aspergillus fumigatus. Int J Antimicrob Agents 56:106105. doi: 10.1016/j.ijantimicag.2020.106105. [DOI] [PubMed] [Google Scholar]
- 27.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother 65:2116–2118. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 30.Bader O, Weig M, Reichard U, Lugert R, Kuhns M, Christner M, Held J, Peter S, Schumacher U, Buchheidt D, Tintelnot K, Groß U, MykoLabNet-D Partners . 2013. cyp51A-based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob Agents Chemother 57:3513–3517. doi: 10.1128/AAC.00167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, Meis JF, Armstrong-James D, Fisher MC. 2015. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. mBio 6:e00536. doi: 10.1128/mBio.00536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klaassen CH, Gibbons JG, Fedorova ND, Meis JF, Rokas A. 2012. Evidence for genetic differentiation and variable recombination rates among Dutch populations of the opportunistic human pathogen Aspergillus fumigatus. Mol Ecol 21:57–70. doi: 10.1111/j.1365-294X.2011.05364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashu EE, Hagen F, Chowdhary A, Meis JF, Xu J. 2017. Global population genetic analysis of Aspergillus fumigatus. mSphere 2:e00019-17. doi: 10.1128/mSphere.00019-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballard E, Zoll J, Melchers WJG, Brown AJP, Warris A, Verweij PE. 2019. Raw genome sequence data for 13 isogenic Aspergillus fumigatus strains isolated over a 2 year period from a patient with chronic granulomatous disease. Data Brief 25:104021. doi: 10.1016/j.dib.2019.104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Özmerdiven GE, Ak S, Ener B, Ağca H, Cilo BD, Tunca B, Akalın H. 2015. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J Infect Chemother 21:581–586. doi: 10.1016/j.jiac.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Valk HA, Meis JF, Curfs IM, Muehlethaler K, Mouton JW, Klaassen CH. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol 43:4112–4120. doi: 10.1128/JCM.43.8.4112-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. 2012. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS One 7:e52871. doi: 10.1371/journal.pone.0052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma C, Hagen F, Moroti R, Meis JF, Chowdhary A. 2015. Triazole-resistant Aspergillus fumigatus harbouring G54 mutation: is it de novo or environmentally acquired? J Glob Antimicrob Resist 3:69–74. doi: 10.1016/j.jgar.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Lavergne RA, Morio F, Favennec L, Dominique S, Meis JF, Gargala G, Verweij PE, Le Pape P. 2015. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother 59:4331–4335. doi: 10.1128/AAC.00127-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagiwara D, Takahashi H, Fujimoto M, Sugahara M, Misawa Y, Gonoi T, Itoyama S, Watanabe A, Kamei K. 2016. Multi-azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother 22:577–579. doi: 10.1016/j.jiac.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez-Moreno C, Lavergne RA, Hagen F, Morio F, Meis JF, Le Pape P. 2017. Azole-resistant Aspergillus fumigatus harboring TR(34)/L98H, TR(46)/Y121F/T289A and TR(53) mutations related to flower fields in Colombia. Sci Rep 7:45631. doi: 10.1038/srep45631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2015. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol 6:428. doi: 10.3389/fmicb.2015.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad S, Khan Z, Hagen F, Meis JF. 2014. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ Res 133:20–26. doi: 10.1016/j.envres.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession numbers of 111 clinical strains in this study are listed in Table S2.