Dalbavancin is gaining interest in the treatment of complex osteoarticular (OA) infections. We aimed to conduct a population pharmacokinetic analysis of dalbavancin in a prospective cohort of adult patients with OA infections caused by Gram-positive organisms and to identify optimal dosing regimens for long-term treatment.
KEYWORDS: dalbavancin, osteomyelitis, prosthetic joint infections, MRSA, Monte Carlo simulation
ABSTRACT
Dalbavancin is gaining interest in the treatment of complex osteoarticular (OA) infections. We aimed to conduct a population pharmacokinetic analysis of dalbavancin in a prospective cohort of adult patients with OA infections caused by Gram-positive organisms and to identify optimal dosing regimens for long-term treatment. Nonlinear mixed-effects modeling was performed with Monolix. Monte Carlo simulations were performed with six dalbavancin regimens (1,500 mg at day 1; 1,000 mg at day 1 plus 500 mg at day 8; 1,500 mg at days 1 and 8; and 1,500 mg at days 1 and 8 plus 500, 1,000, or 1,500 mg at day 36) to assess the probability of target attainment (PTA) of three pharmacodynamic targets of area under the concentration-time curve for the free, unbound fraction of a drug at 24 h/MIC (fAUC24h/MIC) against Staphylococcus aureus (>27.1, 53.3, and 111.1). The cumulative fraction of response (CFR) was calculated against the MIC distribution of both methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA). Desirable PTAs and CFRs were ≥90%. Fifteen patients provided 120 plasma concentrations. Most (73.3%) had prosthetic joint infections. The clinical cure rate was 87%. A two-compartment model with linear elimination well described the data. No covariate was retained in the final model. Pharmacokinetic dalbavancin estimates were 0.106 liter/h for total body clearance (CL) and 36.4 liter for volume of distribution at steady state(Vss). The tested dosing regimens granted desirable CFRs against S. aureus at the most effective pharmacokinetic/pharmacodynamic (PK/PD) target for a period ranging 3 to 9 weeks. Giving a regimen of two 1,500-mg doses of dalbavancin 1 week apart may ensure efficacy against both MSSA and MRSA up to 5 weeks in patients with OA infections. Clinical assessment at that time may allow for considering whether an additional dose should be administered for prolonging effective treatment.
INTRODUCTION
Osteoarticular (OA) infections, including osteomyelitis, orthopedic implant-related infections, and septic arthritis, are among the most difficult-to-treat infectious diseases (1, 2). Gram-positive microorganisms are the most common causative pathogens of these infections, with Staphylococcus aureus and coagulase-negative staphylococci being the most frequent ones, followed by streptococci and enterococci (1, 2).
The most challenging clinical issues in the treatment of OA infections are related to the relevant incidence of resistant strains among Staphylococcus spp. and Enterococcus spp. (1, 2), the limited penetration of antibiotics into bone and joint structures (3), and the need of long-term antibiotic treatment for achieving definitive microbiological eradication of bacteria both in planktonic and in biofilm-embedded status (4).
Vancomycin, teicoplanin, daptomycin, fosfomycin, and/or linezolid, alone or associated with rifampin or beta-lactams, are usually the preferred antibiotics for treating OA infections caused by methicillin-resistant staphylococci (5, 6). Treatment usually lasts for at least 6 weeks, but duration of more than 8 weeks may sometimes be necessary.
Dalbavancin is a long-acting lipoglycopeptide antibiotic active against most infections caused by Gram-positive organisms, including multidrug-resistant strains. It is currently approved for the treatment of skin and skin structure infections at the intravenous dosage of 1,500 mg as a single infusion or of 1,000 mg followed by 500 mg 1 week apart. This long-acting lipoglycopeptide may be promising for long-term treatment of MDR infections caused by Gram-positive organisms in relation to both its strong bactericidal activity against different resistance patterns and its pharmacokinetic properties. The dalbavancin clinical breakpoints against S. aureus are currently set at 0.125 and 0.25 mg/liter by the EUCAST and the U.S. Committee on Antimicrobial Susceptibility Testing (USCAST), respectively. The susceptibility rates at these interpretative breakpoints are 99.6 and 100% for methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) strains, respectively. The MIC90 of dalbavancin against S. aureus is 0.06 mg/liter, and 99.9% of strains are inhibited at a concentration of 0.12 mg/liter (7, 8). Bactericidal activity against S. aureus is attained at a total area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC ratio) of 214 to 331 (9). Dalbavancin has extensive plasma protein binding, a very long elimination half-life of approximately 11 days (10, 11), a good tolerability profile (12), and a good bone penetration ratio (13). Consistently, dalbavancin could represent an interesting potential option for the treatment of staphylococcal OA infections. Overall, clinical experiences of dalbavancin use in the treatment of staphylococcal OA infections are quite limited nowadays, and the administered dosing regimens are quite different.
The aim of this study was to conduct a population pharmacokinetic analysis among patients receiving dalbavancin as rescue therapy for OA infections and to perform Monte Carlo simulations for identifying dosing regimens that may ensure effective concentrations of dalbavancin for long-term treatment of staphylococcal OA infections.
RESULTS
Patient characteristics.
A total of 15 patients were enrolled. Patient demographic and clinical characteristics are summarized in Table 1. Median (range, minimum to maximum) age, weight, estimated glomerular filtration rate (eGFR), and serum albumin were 60 (21 to 81) years, 71 (53 to 90) kg, 98.0 (44 to 142) ml/min/1.73 m2, and 3.9 (2.7 to 4.8) g/liter, respectively. Most of the patients had prosthetic joint infections (11/15; 73.3%). MRSA and MRSE accounted for the majority of microbiological isolates (12/15; 80%). Patients’ clinical data and treatment outcomes are reported in Table 2. Most patients (14/15; 93.3%) underwent surgical procedures. Clinical cure was achieved at the end of treatment in 13/15 (86.7%) patients and was confirmed in 12/15 (80%) at 3- and 6-month follow-up. No dalbavancin-related adverse events were reported.
TABLE 1.
Characteristics of the study population (n = 15)
| Characteristica | Value |
|---|---|
| Age (median [range] [yrs]) | 60 (51–72) |
| Gender (no. male/no. female [%]) | 8/7 (53.3/46.7) |
| Body wt (median [range] [kg]) | 71 (66.5–82.5) |
| BMI (median [range] [kg/m]) | 26.7 (25.0–27.5) |
| eGFR (median [range] [ml/min/1.73 m2]) | 98.0 (91.0–105.0) |
| Albumin (median [range] [g/liter]) | 3.9 (3.6–4.3) |
| Type of infection (no. [%]) | |
| Prosthetic joint infection | 11 (73.3) |
| Implant-associated osteomyelitis | 2 (13.3) |
| Spondylodiscitis | 1 (6.7) |
| Septic arthritis | 1 (6.7) |
| Microbiological isolates (no. [%]) | |
| Methicillin-resistant Staphylococcus epidermidis | 7 (46.7) |
| Methicillin-resistant Staphylococcus aureus | 5 (33.3) |
| Staphylococcus hominis | 2 (13.3) |
| Enterococcus faecalis | 1 (6.7) |
| Dalbavancin plasma concentrations (median [range] [mg/liter]) | |
| Peak concentrations at 2 h | 84.8 (72.2–105.3) |
| Trough concentrations at 168 h | 19.5 (15.4–26.7) |
| Peak concentrations at 170 h | 106.0 (91.4–154.0) |
| Treatment outcome (end of treatment) (no. [%]) | |
| Cured | 13 (86.7) |
| Failed | 2 (13.3) |
BMI, body mass index; eGFR, estimated glomerular filtration rate calculated by means of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
TABLE 2.
Patient-specific data and treatment outcomes for 15 patients on dalbavancin rescue therapy (1,500 mg at day 1 and day 8)a
| Patient (n = 15) | Organism | Site of infection | Surgical procedure | Previous antibiotic treatment | Clinical cure reached (end of treatment) | Clinical cure reached (3-mo follow-up) | Clinical cure reached (6-mo follow-up) | AE |
|---|---|---|---|---|---|---|---|---|
| 1 | MRSE | Hip | Spacer removal | Teicoplanin | Yes | Yes | Yes | None |
| 2 | MRSA | Knee | Spacer revision | Teicoplanin | Yes | Yes | Yes | None |
| 3 | MRSE | Ankle | Surgical debridement | Co-trimoxazole, teicoplanin, levofloxacin | Yes | Yes | Yes | None |
| 4 | S. hominis | Ankle | Surgical debridement | Teicoplanin | Yes | Yes | Yes | None |
| 5 | MRSA | Ankle | Surgical debridement | Daptomycin | Yes | Yes | Yes | None |
| 6 | MRSE | Knee | Spacer revision | Daptomycin, linezolid | No | No | No | None |
| 7 | MRSA | Knee | Spacer revision | Teicoplanin | Yes | Yes | Yes | None |
| 8 | MRSA | Spine | None | Teicoplanin | Yes | No | No | None |
| 9 | E. faecalis | Knee | Spacer revision | Teicoplanin, daptomycin | Yes | Yes | Yes | None |
| 10 | S. hominis | Knee | Surgical debridement | Daptomycin, teicoplanin | Yes | Yes | Yes | None |
| 11 | MRSE | Ankle | Surgical debridement | Daptomycin, teicoplanin | Yes | Yes | Yes | None |
| 12 | MRSE | Knee | Spacer revision | Daptomycin, teicoplanin | Yes | Yes | Yes | None |
| 13 | MRSE | Knee | Spacer revision | Daptomycin, teicoplanin | No | No | No | None |
| 14 | MRSE | Ankle | Surgical debridement | Teicoplanin | Yes | Yes | Yes | None |
| 15 | MRSA | Ankle | Surgical debridement | Vancomycin | Yes | Yes | Yes | None |
AE, adverse events; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis.
Population pharmacokinetic modeling.
A total of 120 dalbavancin plasma concentrations were included in the population pharmacokinetic model. Median (interquartile range [IQR]) dalbavancin concentrations at the end of infusion were 84.8 mg/liter (72.2 to 105.3 mg/liter) after the first dose and 106.0 mg/liter (91.4 to 154.0 mg/liter) after the second one, whereas the median trough level at the end of the first week was 19.5 mg/liter (15.4 to 26.7 mg/liter).
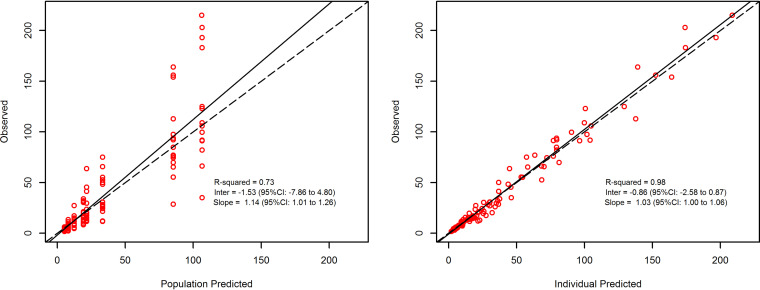
Dalbavancin pharmacokinetics was best described by a two-compartment model (comparative objective function values/Bayesian information criteria [OFV/BIC] were 875.2/890.4, 745.7/778.2, and 741.8/789.6 for the one-, two-, and three-compartment models, respectively). The linear regression of the observed versus model-predicted concentrations showed a good fit for both the population (r2 = 0.73) and the individual estimates (r2 = 0.98) (Fig. 1).
FIG 1.
Diagnostic plot for the final population pharmacokinetic model. Shown are observed versus population-predicted concentrations (left) and individual-predicted concentrations (right) in plasma. Solid lines refer to linear regression between observed and predicted concentrations. Dashed lines are the identity lines between observed and predicted concentrations.
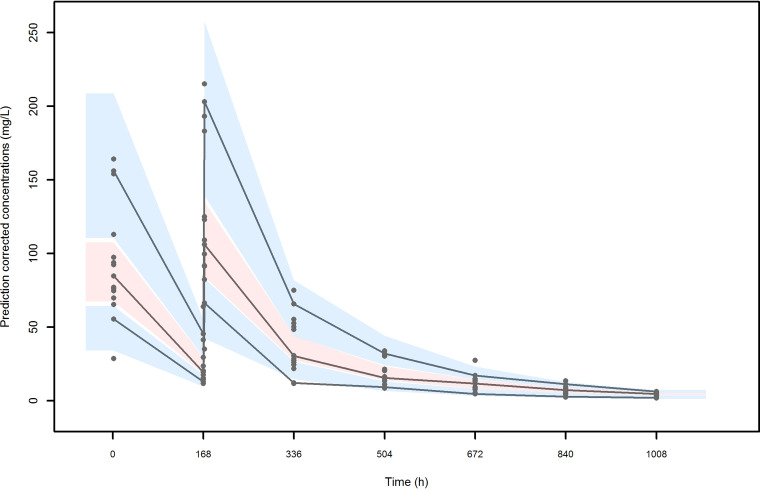
No significant covariate was identified to be added at the basic model. The parameter estimates of the final model are summarized in Table 3. All estimates were accurate with relative standard errors (RSE) less than 30%, except that for the intercompartmental clearance (Q) and the interindividual variability of Q. The results of bootstrap medians and 95% confidence intervals were consistent and confirmed the reliability of the parameter and the random-effect estimates. The visual predicted check (VPC) plot (Fig. 2) showed a good predictive performance of the model. The residuals were symmetrical around zero (P = 0.077 at the symmetry test for normalized prediction distribution error [NPDE]) and normally distributed (P = 0.444 at the Shapiro-Wilk test for NPDE).
TABLE 3.
Estimates of the final population pharmacokinetic model and bootstrap results based on 1,000 simulations
| Parameterc | Final model |
Bootstrap results |
||
|---|---|---|---|---|
| Estimate | % RSE | Median | 5th to 95th percentiles | |
| Fixed effects | ||||
| CL (liter/h) | 0.106 | 9.33 | 0.104 | 0.1–0.110 |
| V1 (liter) | 17.40 | 12.0 | 17.16 | 16.52–17.96 |
| Q (liter/h) | 0.103 | 37.4 | 0.093 | 0.05–0.162 |
| V2 (liter) | 15.10 | 15.8 | 15.04 | 14.07–16.80 |
| t1/2 (days) | 11.29a | 11.68a | ||
| Vss (liter) | 36.40a | 35.88a | ||
| Random effects (CV [%]) | ||||
| IIVCL | 36.21 | 20.7 | 36.42 | 33.05–37.34 |
| IIVV1 | 44.27 | 22.4 | 40.29 | 35.14–47.51 |
| IIVQ | 130.46 | 33.7 | 121.96 | 83.38–174.85 |
| IIVV2 | 62.34 | 22.2 | 69.16 | 55.53–76.33 |
| IIVt1/2 | 54.37b | 53.53b | ||
| IIVVss | 50.59b | 48.61b | ||
| Residual variability | ||||
| b (proportional)d | 0.18 | 7.76 | 0.18 | 0.17-0.19 |
Calculated from individual parameter values with microconstants,
Calculated as (standard deviation/mean) × 100.
% RSE, relative standard error of the estimate; CV, coefficient of variation; CL, total body clearance; V1, central volume of distribution; Q, intercompartmental clearance; V2, peripheral volume of distribution; Vss, volume of distribution at steady-state; t1/2, terminal half-life; IIV, interindividual variability (associated with CL [IIVCL], with V1 [IIVV1], with Q [IIVQ], with V2 [IIVV2], with t1/2 [IIVt1/2], and with Vss [IIVVss]).
Proportional residual error model.
FIG 2.
Visual predictive check (VPC) of dalbavancin plasma concentration versus time for the final model. The continuous lines indicate the 10th, 50th, and 90th percentiles for observed data, while the shaded gray areas represent 90% prediction intervals from the corresponding percentiles calculated from simulated data.
Monte Carlo simulation for evaluation of dosing regimens.
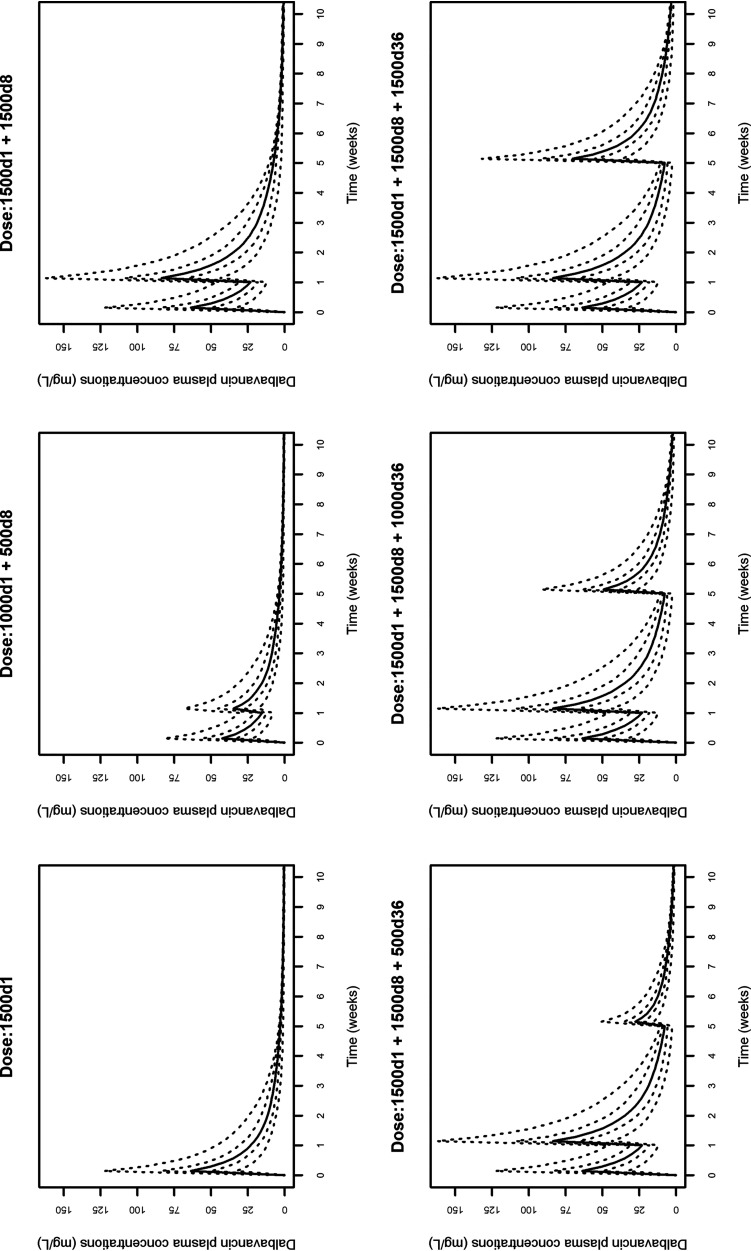
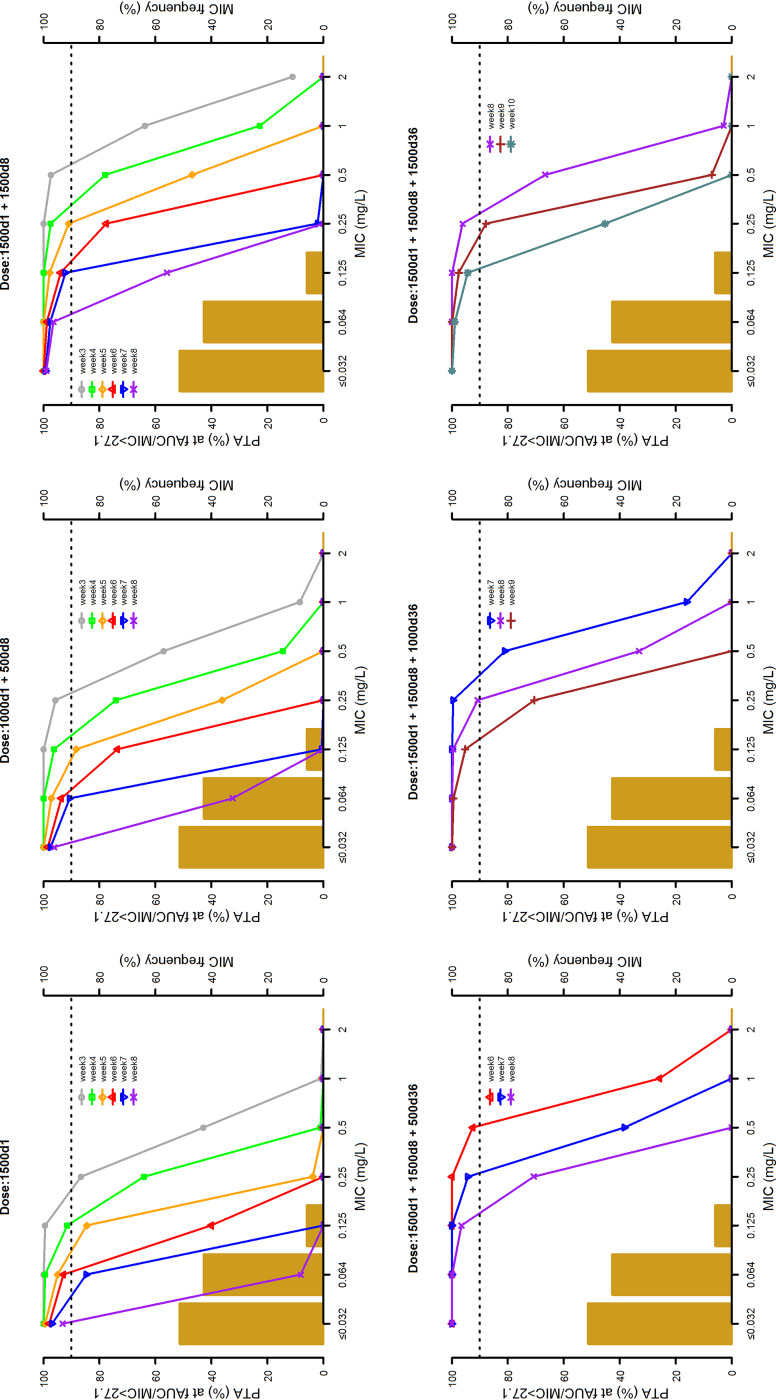
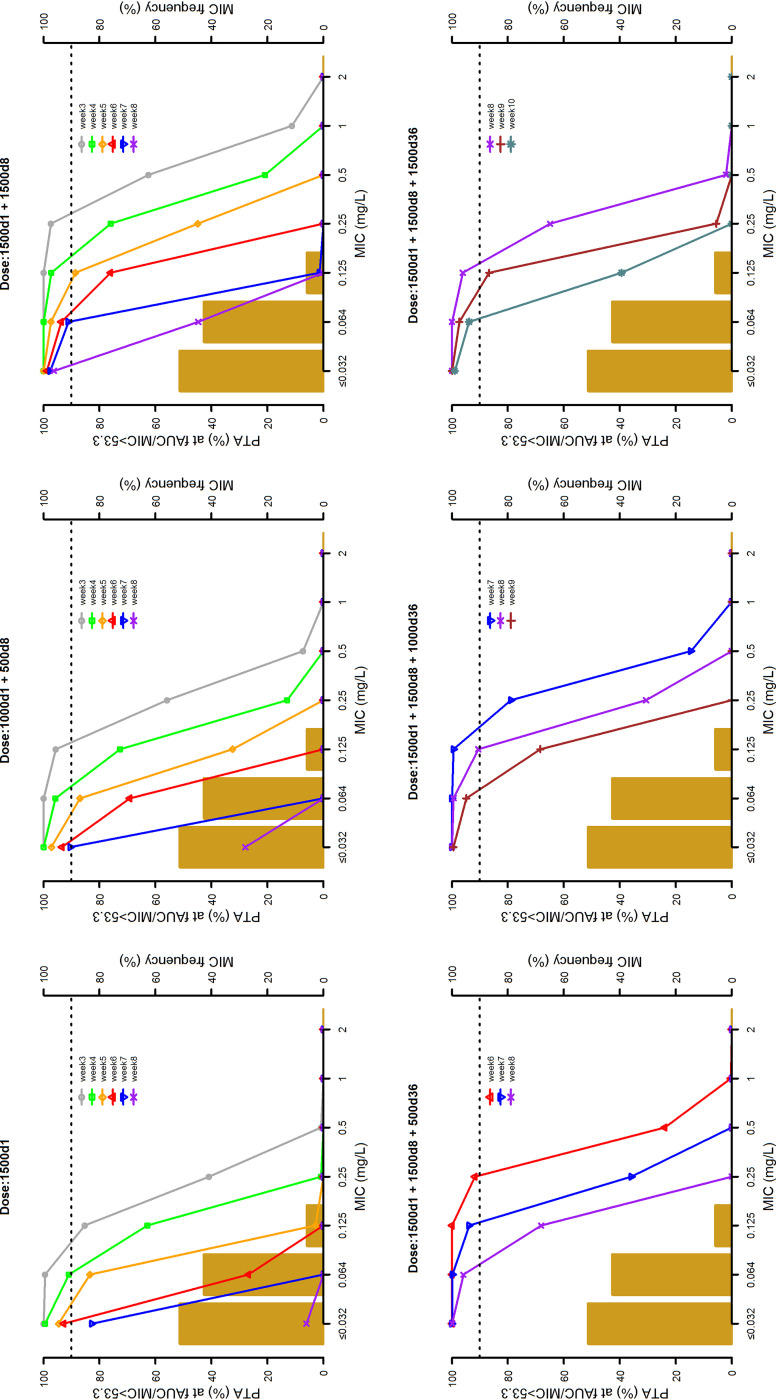
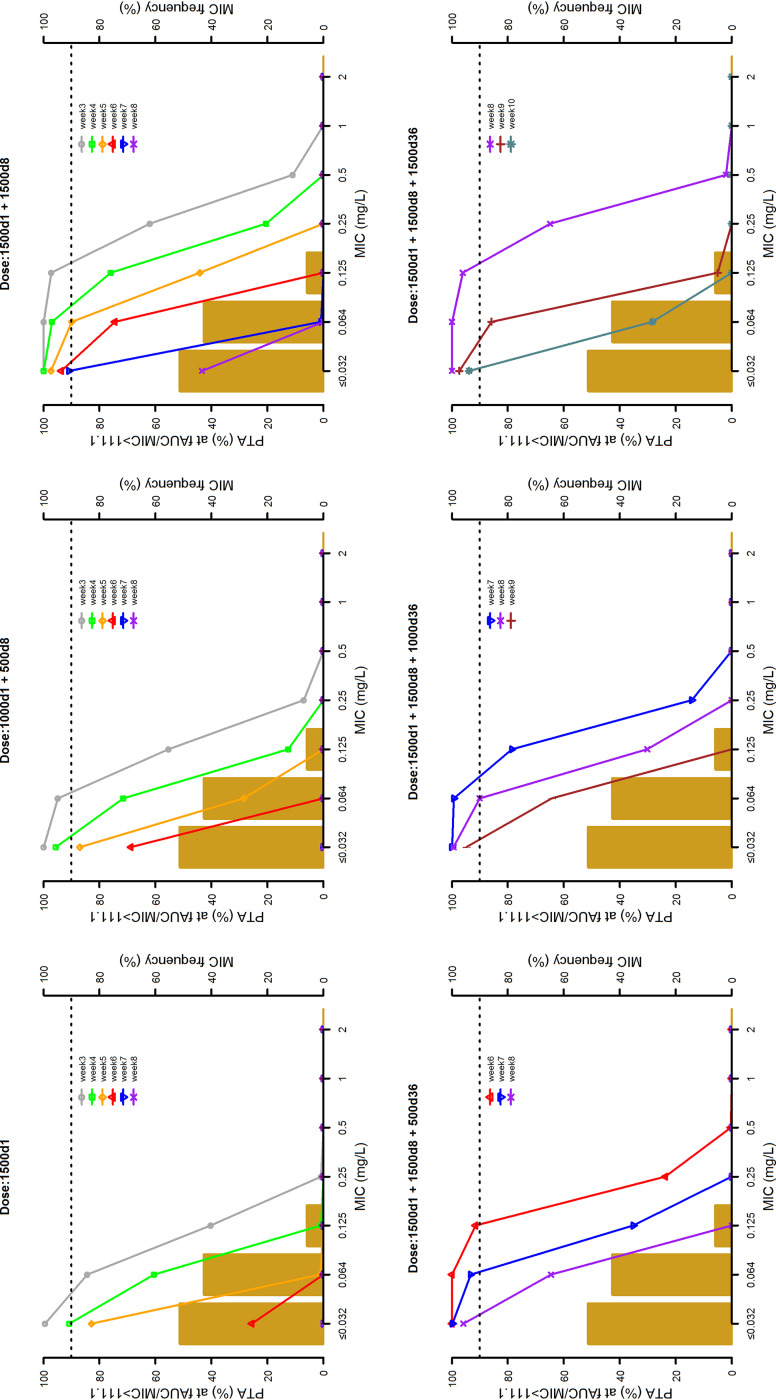
The 10,000 Monte Carlo simulations of dalbavancin concentration-time profiles conducted with the six dosing regimens are depicted in Fig. 3. The probabilities of target attainment (PTAs) at selected weeks of an AUC for the free, unbound fraction of a drug at 24 h/MIC (fAUC24h/MIC) of >27.1, >53.3, and >111.1 tested with the six dalbavancin dosing regimens against the MIC distribution frequencies of a large collection of S. aureus strains yielded from bone and joint (14) are shown in Fig. 4 to 6, respectively. Focusing at the EUCAST and the USCAST clinical breakpoints (0.125 mg/liter and 0.25 mg/liter, respectively), the six dosing regimens influenced the duration of the different desirable pharmacokinetic/pharmacodynamic (PK/PD) targets in a dose-dependent fashion. The two licensed dosing regimens were shown to ensure desirable PTAs up to a maximum of 4 weeks only at the PK/PD targets of fAUC24h/MIC > 27.1 and/or >53.3. The regimen of two 1,500-mg doses of dalbavancin 1 week apart (days 1 and 8) granted an extension of desirable PTAs up to 5 to 7 weeks at the PK/PD target of fAUC24h/MIC > 27.1, up to 3 to 4 weeks at that of >53.3, and up to 3 weeks at that of >111.1 (but only for the EUCAST clinical breakpoint). An additional dose of 500, 1,000, or 1,500 mg administered at the end of week 5 (day 36) after the regimen of two 1,500-mg doses 1 week apart (days 1 and 8) was helpful in prolonging the duration of desirable PTAs in a dose-dependent fashion. The 500-mg additional dose granted a prolongation of desirable PTAs up to 7 to 8 weeks at the PK/PD target of fAUC24h/MIC > 27.1, up to 6 to 7 weeks at that of >53.3, and up to 6 weeks at that of >111.1 (but only for the EUCAST clinical breakpoint). The 1,000-mg additional dose granted a prolongation of desirable PTAs up to 8 to 9 weeks at the PK/PD target of fAUC24h/MIC > 27.1 and up to 8 weeks at that of >53.3 (but only for the EUCAST clinical breakpoint). The 1,500-mg additional dose granted a prolongation of desirable PTAs up to 10 weeks at the PK/PD target of fAUC24h/MIC > 27.1 and up to 8 weeks at that of >111.1 (but both only for the EUCAST clinical breakpoint).
FIG 3.
Simulated dalbavancin plasma concentration versus time profiles of six different dosing regimens (1,500 mg at day 1; 1,000 mg at day 1 and 500 mg at day 8; 1,500 mg at days 1 and 8; 1,500 mg at day 1, 1,500 mg at day 8, and 500 mg at day 36; 1,500 mg at day 1, 1,500 mg at day 8, and 1,000 mg at day 36; and 1,500 mg at day 1, 1,500 mg at day 8, and 1,500 mg at day 36). The solid line is the simulated median concentration. The dashed lines are the 5th, 25th, 75th, and 95th percentiles of simulated concentrations.
FIG 4.
Probabilities of target attainment (PTAs) of achieving a fAUC24h/MIC value of 27.1 with six different dosing regimens (1,500 mg at day 1; 1,000 mg at day 1 and 500 mg at day 8; 1,500 mg at days 1 and 8; 1,500 mg at day 1, 1,500 mg at day 8, and 500 mg at day 36; 1,500 mg at day 1, 1,500 mg at day 8, and 1,000 mg at day 36; and 1,500 mg at day 1, 1,500 mg at day 8, and 1,500 mg at day 36). The histograms are the MIC distribution frequencies of 801 Staphylococcus aureus isolates yielded from bone and joint infections in 78 European and U.S. hospitals between 2011 and 2016 (14). The dotted lines identify desirable PTA (≥90%).
FIG 5.
Probabilities of target attainment (PTAs) of achieving a fAUC24h/MIC value of 53.3 with six different dosing regimens (1,500 mg at day 1; 1,000 mg at day 1 and 500 mg at day 8; 1,500 mg at days 1 and 8; 1,500 mg at day 1, 1,500 mg at day 8, and 500 mg at day 36; 1,500 mg at day 1, 1,500 mg at day 8, and 1,000 mg at day 36; and 1,500 mg at day 1, 1,500 mg at day 8, and 1,500 mg at day 36). The histograms are the MIC distribution frequencies of 801 Staphylococcus aureus isolates yielded from bone and joint infections in 78 European and U.S. hospitals between 2011 and 2016 (14). The dotted lines identify desirable PTA (≥90%).
FIG 6.
Probabilities of target attainment (PTAs) of achieving a fAUC24h/MIC value of 111.1 with six different dosing regimens (1,500 mg at day 1; 1,000 mg at day 1 and 500 mg at day 8; 1,500 mg at days 1 and 8; 1,500 mg at day 1, 1,500 mg at day 8, and 500 mg at day 36; 1,500 mg at day 1, 1,500 mg at day 8, and 1,000 mg at day 36; and 1,500 mg at day 1, 1,500 mg at day 8. and 1,500 mg at day 36). The histograms are the MIC distribution frequencies of 801 Staphylococcus aureus isolates yielded from bone and joint infections in 78 European and U.S. hospitals between 2011 and 2016 (14). The dotted lines identify desirable PTA (≥90%).
The cumulative fraction of response (CFRs) achievable with the six tested dosing regimens against MSSA and MRSA at the three PK/PD targets are summarized in Table 4. When focusing at the most effective PK/PD target of fAUC24h/MIC > 111.1, desirable PTAs were granted up to 3 weeks with the two licensed dosing regimens and up to 5 weeks with the regimen of two 1,500-mg doses 1 week apart (days 1 and 8). Administration of an additional dose of 500 mg, 1,000 mg, or 1,500 mg at the end of week 5 (day 36) after the regimen of two 1,500-mg doses 1 week apart (days 1 and 8) prolonged the duration of desirable PTAs up to 7, 8, or 9 weeks, respectively.
TABLE 4.
Cumulative fractions of response of four dalbavancin dosing regimens against MIC distribution of 801 Staphylococcus aureus clinical isolates causing bone and joint infections in U.S. and European hospitals (14) at the three PK/PD targetsa
| Dalbavancin regimen (mg) at day (d) | CFRs of MSSA isolates (n = 534) at: |
CFRs of MRSA isolates (n = 267) at: |
||||
|---|---|---|---|---|---|---|
| fAUC24h/MIC > 27.1 | fAUC24h/MIC > 53.3 | fAUC24h/MIC > 111.1 | fAUC24h/MIC > 27.1 | fAUC24h/MIC > 53.3 | fAUC24h/MIC > 111.1 | |
| 1,500 d1 + 1,500 d8 | ||||||
| At d21 | 100 | 100 | 99.74 | 100 | 100 | 99.82 |
| At d28 | 100 | 99.74 | 97.29 | 100 | 99.82 | 97.14 |
| At d35 | 99.77 | 98.17 | 90.51 | 99.86 | 98.12 | 90.00 |
| At d42 | 99.60 | 95.29 | 80.31 | 99.08 | 95.09 | 79.22 |
| At d49 | 98.09 | 91.04 | 47.97 | 98.09 | 90.04 | 45.26 |
| At d56 | 95.49 | 69.19 | 22.74 | 95.19 | 67.33 | 21.44 |
| 1,500 d1 + 1,500 d8 + 500 d36 | ||||||
| At d42 | 100 | 100 | 99.43 | 100 | 100 | 99.46 |
| At d49 | 100 | 99.47 | 93.36 | 100 | 99.51 | 92.79 |
| At d56 | 99.67 | 96.34 | 77.32 | 99.74 | 96.09 | 75.93 |
| 1,500 d1 + 1,500 d8 + 1,000 d36 | ||||||
| At d49 | 100 | 99.87 | 98.40 | 100 | 99.96 | 98.32 |
| At d56 | 100 | 99.12 | 91.53 | 99.97 | 99.13 | 90.86 |
| At d63 | 99.43 | 95.73 | 76.67 | 99.48 | 95.47 | 75.30 |
| 1,500 d1 + 1,500 d8 + 1,500 d36 | ||||||
| At d56 | 100 | 100 | 98.00 | 100 | 100 | 97.97 |
| At d63 | 100 | 99.68 | 96.25 | 100 | 99.75 | 95.97 |
| At d70 | 99.17 | 93.43 | 61.1 | 99.19 | 92.93 | 58.93 |
CFR, cumulative fraction of response; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; d, day. Numbers in bold identify desirable CFRs ≥90%.
DISCUSSION
In this study, we assessed the population pharmacokinetics of dalbavancin in a prospective cohort of patients with OA infections. Our aim was to assess the PTAs and the CFRs achievable against MRSA and MSSA with six dalbavancin dosing regimens that could be adopted for treating OA infections.
The population pharmacokinetic model accounted for 73% of the interindividual variability of dalbavancin concentration over time. The model provided highly reliable estimates of PK parameters, as confirmed by the high relationship between individual predicted and observed concentrations (r2 = 0.98), the low RSE of PK parameters, and the close median value of the bootstrap estimates to the population values. The fact that our model estimated a population dalbavancin total body clearance (CL) that was approximately 2-fold higher than observed in healthy volunteers (15) and in other population pharmacokinetic analyses conducted in phase II/III clinical trials of patients with skin and soft tissue infections (16, 17) may be due to the limited sample size of the study. Likewise, eGFR did not result associated with drug CL, probably as a consequence of the lack of patients with impaired renal function in our cohort.
The clinical cure rate of dalbavancin rescue treatment was quite high in our study population (≥80%) either at end of treatment or at 3- to 6-month follow-up, and no patient suffered from adverse drug reactions. This is in agreement with the findings of two recent studies that assessed the efficacy and tolerability of dalbavancin in bone and joint infections as well as implant-associated infections. In a Spanish multicentric retrospective study, patients were treated with different schemes (from 1 dose of 500 mg up to 4 doses of 1,500 mg biweekly). Cure rates were around 90% when considering the composite endpoints of clinical success and improvement (18). Dalbavancin was well tolerated, even after more than 2 doses. Similarly, in another recent multicentric retrospective study, including 31 American patients with osteomyelitis who received a median of 3 dalbavancin doses (ranging from 1 to 14), 90.3% of patients (28/31) achieved clinical success, and none had adverse events (19).
The efficacy of dalbavancin in OA infections may be related to the good bone distribution (13.1%) (13) and its valid activity, even against bacteria embedded in biofilms (20, 21). The dosing schedule that we adopted in our study (two 1,500-mg doses 1 week apart on days 1 and 8) was based on the findings of the study of Dunne et al. (13) carried out in healthy volunteers undergoing prosthetic implantation in which they showed that drug levels measured in bone were very similar to the calculated simultaneous free drug concentrations in serum.
Monte Carlo simulations showed that, when focusing at the most effective PK/PD target (fAUC24h/MIC > 111.1), this dosing regimen might grant, in our patient population, very high CFR (≥90%) against both MRSA and MSSA for a period of time that is 1.7-fold longer than the one granted by each of the two licensed dosing regimens (5 versus 3 weeks). This duration may be furtherly extended up to 7, 8, or 9 weeks by administering, at day 36, an additional dose of 500, 1,000, or 1,500 mg, respectively.
This approach may offer a great opportunity, from the clinical standpoint, in avoiding long-term hospital admission in patients who are treated with dalbavancin for OA infections. Of note in this very challenging period in which COVID-19 is spreading is that having the possibility of performing outpatient parenteral antimicrobial therapy (OPAT) with dalbavancin could represent an added value. However, considering that a recent in vitro study showed that emergence of dalbavancin resistance and cross-resistance could occur in MRSA even at therapeutic concentrations (22), we believe that clinical reevaluation during treatment and at follow-up remains central.
We acknowledge some limitations of our study. The small sample size of our cohort, including only patients with normal renal function, prevented us from establishing any association between eGFR and dalbavancin clearance, different from what was observed in previous studies. We recognize that simulations had to be based on free plasma concentrations as a surrogate marker of bone exposure due to the absence of direct measurements of drug concentrations in bone. However, the prospective design and the reliability of the pharmacokinetic estimates are strengths of our work.
In conclusion, this pilot study suggests that a regimen of two 1,500-mg doses of dalbavancin 1 week apart may ensure efficacy against both MSSA and MRSA for up to 5 weeks in patients with OA infections. Clinical assessment at that time may allow for considering whether an additional dose should be administered for prolonging effective treatment. Prospective studies are warranted for confirming that these higher dalbavancin doses may be effective and safe.
MATERIALS AND METHODS
Study design.
This prospective study was carried out between March 2018 and December 2019 at the IRCCS S. Orsola Teaching Hospital, Bologna, Italy. Adult patients with documented OA infections caused by Gram-positive organisms who failed primary antimicrobial treatment were eligible for inclusion in the study and were treated with dalbavancin as rescue therapy according to the scheme proposed by Dunne et al. (13) (two doses of 1,500 mg 1 week apart on day 1 and day 8 infused over 2 h). The study was approved by the local ethics committee (registration number 80/2020/Oss/AOUBo). Signed informed consent was obtained from each patient before enrollment. Patients underwent serial blood sampling at end of the first infusion on day 1, before dosing and at the end of the second infusion on day 8, and then once weekly up to 6 weeks (on days 14, 21, 28, 35, and 42). Clinical evaluation was carried out at the start of therapy, during treatment, at the end of therapy, and at 3- and 6-month follow-up.
Demographic and clinical data recorded at patient enrollment were age, gender, weight, height, body surface area (BSA), type and site of infection, microbiological isolates, prior antibiotic treatment, and number and type of prior surgical interventions. Serum creatinine, albumin, and C-reactive protein (C-RP) were measured on each day of blood sampling for pharmacokinetic (PK) assessment. The estimated glomerular filtration rate (eGFR) was calculated by means of the chronic kidney disease epidemiology formula (23). Eventual adverse events were verified during and at the end of treatment. Clinical outcome was assessed at the end of therapy and at 3- and 6-month follow-up. A patient was defined as cured in the case of positive clinical response (resolution of signs and symptoms of infection, coupled with C-RP normalization and no need for either additional antibiotic therapy or surgical reintervention) or as failed in the case of absence of clinical response.
Dalbavancin plasma concentrations were measured by means of a liquid chromatography-tandem mass spectrometry analytic method as previously described (24). The intra- and interday coefficients of variation of the quality controls were 0.09 to 0.14% and 4.8 to 14.2%, respectively. The lower limit of quantification was 0.5 mg/liter.
Population pharmacokinetic model building.
Plasma concentration-time profiles of dalbavancin were analyzed by means of nonlinear mixed-effects modeling using the stochastic approximation expectation maximization (SAEM) algorithm implemented within the Monolix software (version 2019R1; Lixofit, Antony, France).
The population pharmacokinetic model was developed in two steps. A basic model without covariates was developed by comparing one-, two-, and three-compartment models with linear elimination. All individual parameters were considered to be log-normally distributed. Exponential random effects were assumed to describe between-subject variability. Correlations between random effects were tested in the variance-covariance matrix and implemented into the structural model accordingly. Several error models (constant, proportional, or combined error model) were tested for describing the residual variability. The most appropriate model was selected on the basis of the following criteria: smaller value of the objective function values (OFV) and Bayesian information criteria (BIC), adequacy of the goodness-of-fit plots, and low relative standard errors (RSE) of the estimated PK parameters.
From the basic model, the effect of the following nine covariates on dalbavancin PK parameters was evaluated: age, gender, weight, height, BSA, serum albumin, serum creatinine, C-RP, and eGFR. The parameter-covariate relationships were modeled as additive or proportional shifts from the reference category for binary covariates, whereas the effect of continuous covariates was modeled using a power function. The covariate model was built using a stepwise procedure with forward inclusion and backward elimination. A covariate was included in the model according to the results of the likelihood ratio test (LRT) and if a reduction of the BIC and a decrease of the interpatient variability of the fixed-effect parameters were obtained.
Model evaluation.
Evaluation of the final model was based on the following goodness-of-fit plots: observation versus individual and population predictions, usual residual-based plots (individual-weighted residuals and population-weighted residuals), and visual predicted check (VPC). The VPC plot shows the time course of the 10th, 50th, and 90th percentiles of observed data and the corresponding 90% prediction intervals calculated from 1,000 Monte Carlo samples. Model performance was assessed also by means of the normalized prediction distribution error (NPDE). The 95% confidence interval of each parameter in the final model was simulated from 1,000 nonparametric bootstraps based on resampling by means of the Rsmlx (R speaks Monolix) package of R.
Monte Carlo simulations for evaluation of dosing regimens.
Monte Carlo simulations based on the final pharmacokinetic model were performed with six dalbavancin dosing regimens (1,500 mg at day 1, 1,000 mg at day 1 plus 500 mg at day 8, 1,500 mg at days 1 and 8, 1,500 mg at days 1 and 8 plus 500 mg at day 36, 1,500 mg at days 1 and 8 plus 1,000 mg at day 36, and 1,500 mg at days 1 and 8 plus 1,500 mg at day 36) by means of Simulx2020.R1 to generate 10,000 dalbavancin pharmacokinetic profiles.
The probability of target attainment (PTA) of three incremental PK/PD targets of fAUC24h/MIC (>27.1, >53.3, and >111.1) against S. aureus (25) was calculated at the end of week 3 and of each subsequent week for predicting duration of efficacy in the long-term period. Dalbavancin AUC24hs at days 21, 28, 35, 42, 49, 56, 63, and 70 were calculated by integrating, over 24 h, the drug concentrations simulated in the central compartment. Dalbavancin is 93% plasma protein bound, and the bone/plasma ratio is 13% (13). In the study of Dunne et al., the calculated free drug concentrations of dalbavancin in serum were very similar to the drug levels measured in bone, and as a result, the bone concentrations were expected to be free and available for antimicrobial activity (13). Consistent with this, we considered the fAUC24h dalbavancin in serum (7%) (10) as being available for antimicrobial activity in bone as well.
The cumulative fraction of response (CFR) for each dalbavancin regimen was assessed by testing the PTA against the MIC distribution of a collection of S. aureus strains yielded from bone and joint infections in 78 European and U.S. hospitals between 2011 and 2016 (14). PTA and CFR were defined as desirable when ≥90% (26).
Statistical analysis.
Data were summarized as medians with 25th to 75th percentiles in the descriptive statistics. All statistical analysis and plotting were performed with R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
ACKNOWLEDGMENTS
This study was carried out as part of our routine work.
F.P. participated in speaker bureaus for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfizer, and Sanofi Aventis and in advisory boards for Angelini, Basilea Pharmaceutica, Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, Novartis, Pfizer, and Thermo Fisher. P.V. has served as a consultant for bioMérieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and received payment for serving on the speaker’s bureaus for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, and Pfizer. All other authors have no conflicts of interest to declare.
REFERENCES
- 1.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeed K, Esposito S, Ascione T, Bassetti M, Bonnet E, Carnelutti A, Chan M, Lye DC, Cortes N, Dryden M, Fernando S, Gottlieb T, Gould I, Hijazi K, Madonia S, Pagliano P, Pottinger PS, Segreti J, Spera AM, International Society of Antimicrobial Chemotherapy (ISAC) Bone and Skin & Soft Tissue Infections Working Group . 2019. Hot topics on vertebral osteomyelitis from the International Society of Antimicrobial Chemotherapy. Int J Antimicrob Agents 54:125–133. 10.1016/j.ijantimicag.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. 2019. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis 81:128–136. 10.1016/j.ijid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Hola V, Imbert C, Kirketerp-Moller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C, ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli . 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21(Suppl 1):S1–S25. 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, III, Petermann GW, Osmon DR, Infectious Diseases Society of America . 2015. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 6.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America . 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 7.Lopez S, Hackbarth C, Romano G, Trias J, Jabes D, Goldstein BP. 2005. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J Antimicrob Chemother 55(Suppl 2):ii21-4. 10.1093/jac/dki007. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF. 2007. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 51:1150–1154. 10.1128/AAC.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowker KE, Noel AR, MacGowan AP. 2006. Pharmacodynamics of dalbavancin studied in an in vitro pharmacokinetic system. J Antimicrob Chemother 58:802–805. 10.1093/jac/dkl311. [DOI] [PubMed] [Google Scholar]
- 10.Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagace-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA. 2010. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs 70:859–886. 10.2165/11534440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Guskey MT, Tsuji BT. 2010. A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin. Pharmacotherapy 30:80–94. 10.1592/phco.30.1.80. [DOI] [PubMed] [Google Scholar]
- 12.Dunne MW, Zhou M, Darpo B. 2015. A thorough QT study with dalbavancin: a novel lipoglycopeptide antibiotic for the treatment of acute bacterial skin and skin-structure infections. Int J Antimicrob Agents 45:393–398. 10.1016/j.ijantimicag.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. 2015. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 59:1849–1855. 10.1128/AAC.04550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Flamm RK, Castanheira M, Sader HS, Mendes RE. 2018. Dalbavancin in-vitro activity obtained against Gram-positive clinical isolates causing bone and joint infections in US and European hospitals (2011–2016). Int J Antimicrob Agents 51:608–611. 10.1016/j.ijantimicag.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Dorr MB, Jabes D, Cavaleri M, Dowell J, Mosconi G, Malabarba A, White RJ, Henkel TJ. 2005. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J Antimicrob Chemother 55(Suppl 2):ii25–30. 10.1093/jac/dki008. [DOI] [PubMed] [Google Scholar]
- 16.Carrothers TJ, Chittenden JT, Critchley I. 2020. Dalbavancin population pharmacokinetic modeling and target attainment analysis. Clin Pharmacol Drug Dev 9:21–31. 10.1002/cpdd.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckwalter M, Dowell JA. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol 45:1279–1287. 10.1177/0091270005280378. [DOI] [PubMed] [Google Scholar]
- 18.Morata L, Cobo J, Fernandez-Sampedro M, Guisado Vasco P, Ruano E, Lora-Tamayo J, Sanchez Somolinos M, Gonzalez Ruano P, Rico Nieto A, Arnaiz A, Estebanez Munoz M, Jimenez-Mejias ME, Lozano Serrano AB, Munez E, Rodriguez-Pardo D, Argelich R, Arroyo A, Barbero JM, Cuadra F, Del Arco A, Del Toro MD, Guio L, Jimenez-Beatty D, Lois N, Martin O, Martinez Alvarez RM, Martinez-Marcos FJ, Porras L, Ramirez M, Vergas Garcia J, Soriano A. 2019. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother 63:e02280-18. 10.1128/AAC.02280-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almangour TA, Perry GK, Terriff CM, Alhifany AA, Kaye KS. 2019. Dalbavancin for the management of gram-positive osteomyelitis: effectiveness and potential utility. Diagn Microbiol Infect Dis 93:213–218. 10.1016/j.diagmicrobio.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Neudorfer K, Schmidt-Malan SM, Patel R. 2018. Dalbavancin is active in vitro against biofilms formed by dalbavancin-susceptible enterococci. Diagn Microbiol Infect Dis 90:58–63. 10.1016/j.diagmicrobio.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez J, Greenwood-Quaintance KE, Patel R. 2016. In vitro activity of dalbavancin against biofilms of staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis 85:449–451. 10.1016/j.diagmicrobio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Werth BJ, Ashford N, Penewit K, Waalkes A, Holmes EA, Ross DH, Shen T, Hines KM, Salipante SJ, Xu L. 2020. Dalbavancin exposure in vitro selects for dalbavancin-nonsusceptible and vancomycin-intermediate strains of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 10.1016/j.cmi.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI, for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alebic-Kolbah T, Demers R, Cojocaru L. 2011. Dalbavancin: quantification in human plasma and urine by a new improved high performance liquid chromatography-tandem mass spectrometry method. J Chromatogr B Analyt Technol Biomed Life Sci 879:2632–2641. 10.1016/j.jchromb.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Lepak A, Marchillo K, VanHecker J, Andes D. 2015. Impact of glycopeptide resistance in Staphylococcus aureus on the dalbavancin in vivo pharmacodynamic target. Antimicrob Agents Chemother 59:7833–7836. 10.1128/AAC.01717-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masterton RG, Kuti JL, Turner PJ, Nicolau DP. 2005. The OPTAMA programme: utilizing MYSTIC (2002) to predict critical pharmacodynamic target attainment against nosocomial pathogens in Europe. J Antimicrob Chemother 55:71–77. 10.1093/jac/dkh511. [DOI] [PubMed] [Google Scholar]