Stenotrophomonas maltophilia is an emerging cause of serious infections with high associated mortality in immunocompromised patients. Treatment of S. maltophilia infections is complicated by intrinsic resistance to many antimicrobials, including carbapenems, aminoglycosides, and some cephalosporins.
KEYWORDS: Stenotrophomonas, antibiotic resistance, antimicrobial agents, blood culture, bloodstream infections, diagnostics, Gram-negative bacteria, immunocompromised hosts, opportunistic infections, susceptibility testing
ABSTRACT
Stenotrophomonas maltophilia is an emerging cause of serious infections with high associated mortality in immunocompromised patients. Treatment of S. maltophilia infections is complicated by intrinsic resistance to many antimicrobials, including carbapenems, aminoglycosides, and some cephalosporins. Despite this, >90% of isolates are susceptible to trimethoprim-sulfamethoxazole (SXT), which is the front-line therapy for this organism. Side effects of SXT include bone marrow suppression, which precludes its use for many neutropenic patients. In this population, levofloxacin (LEV), minocycline (MIN), ceftazidime (CAZ), ciprofloxacin (CIP), and tigecycline (TIG) are used as alternative therapies, all of which require testing to inform susceptibilities. The reference standard method for testing S. maltophilia is broth microdilution (BMD), but very few clinical laboratories perform reference BMD. Furthermore, interpretive criteria are not available for CIP or TIG for S. maltophilia, although generic pharmacokinetic/pharmacodynamic (PK/PD) MIC breakpoints are available for these drugs. We assessed performance of disk and gradient diffusion tests relative to BMD for 109 contemporary isolates of S. maltophilia. Categorical agreement values for SXT, LEV, and MIN disk diffusion were 93%, 89%, and 95%, respectively. Categorical agreement values for SXT, LEV, MIN, and CAZ gradient strips were 98%, 85%, 93%, and 71%, respectively, by Etest (bioMerieux) and 98%, 83%, 99%, and 73% by the MIC test strip (MTS; Liofilchem). CIP and TGC, two clinically valuable alternatives to SXT, did not demonstrate promising disk-to-MIC correlates using CLSI document M100-ED30 (Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Testing, M100-ED30, 2020) P. aeruginosa or PK/PD breakpoints. Manual commercial tests perform well for S. maltophilia, with the exception of tests for LEV and CAZ, for which high error rates were observed.
INTRODUCTION
Stenotrophomonas maltophilia is a Gram-negative, opportunistic, multidrug-resistant bacterium that can cause severe nosocomial infections in immunocompromised hosts (1). Infections caused by S. maltophilia can be associated with high mortality rates (∼70%), particularly in patients who are critically ill, are receiving broad-spectrum antimicrobial therapy, have hematological malignancies, or have prolonged neutropenia (2, 3). Limited therapeutic options are available for the treatment of S. maltophilia, due to intrinsic resistance of this organism to penicillins, most cephalosporins and β-lactam combination agents, carbapenems, and aminoglycosides (4). Trimethoprim-sulfamethoxazole (SXT) is the front-line treatment for S. maltophilia infections, and >90% of isolates remain susceptible to this antimicrobial (5). However, SXT is associated with myelosuppression, markedly limiting its use in neutropenic patients, particularly those with hematological malignancies. In the United States, cancer centers rely on alternative therapeutic options such as minocycline (MIN), levofloxacin (LEV), ciprofloxacin (CIP), ceftazidime (CAZ), or tigecycline (TGC) to treat neutropenic patients with S. maltophilia infections (4).
In 2009, the U.S. Food and Drug Administration (FDA) discontinued the clearance of antimicrobial susceptibility testing (AST) devices using Clinical and Laboratory Standards Institute (CLSI) breakpoints. As there are no FDA-recognized S. maltophilia breakpoints, FDA clearance of commercial AST devices for this organism is no longer possible. Thus, laboratories in the United States perform AST on S. maltophilia isolates using commercial systems that were cleared by the FDA prior to 2009 (6) or off-label for devices cleared by FDA after 2009. Manual methods, such as disk diffusion or gradient diffusion, are also used, although these too have not been evaluated by the FDA since 2008. Consequently, there are limited data available on the accuracy of these methods for testing contemporary isolates of S. maltophilia. Susceptibility rates for S. maltophilia have changed substantially since 2008. Isolates collected at U.S. centers between 2002 and 2005, directly prior to CLSI breakpoints being established and the last FDA clearances of devices with CLSI breakpoints, were more often susceptible to CAZ (49.1%) and LEV (86.5%) than contemporary isolates. The ability of devices to detect emerging resistance in S. maltophilia is unknown. Similarly, the degree to which disk tests correlate with MIC values obtained by broth microdilution (BMD) have not been assessed since the CLSI first established disk breakpoints in 2007. One survey of susceptibility data for S. maltophilia in Los Angeles County demonstrated that laboratory-reported SXT susceptibility rates ranged from 64 to 100% across institutions which performed commercial AST (7). It is unclear if this represents inaccuracies in AST results or true resistance, but testing issues are likely, as no studies have documented such high SXT resistance rates by reference methods as were seen in that study.
Recent data published on U.S. isolates of S. maltophilia (2014 to 2018) highlight the need for reliable and accurate testing to inform therapeutic choices for S. maltophilia. Only 26.8% of isolates were susceptible to CAZ, 75.8% to LEV, 99.5% to MIN, and 94.6% to SXT (8). AST results for S. maltophilia can be interpreted using CLSI-defined MIC breakpoints for CAZ, LEV, MIN, SXT, ticarcillin-clavulanic acid, and chloramphenicol. CLSI also publishes disk breakpoints, but only for SXT, MIN, and LEV. These were established in 2007 (9). In 2020, CLSI further defined investigational MIC and disk breakpoints for cefiderocol (10). In contrast to CLSI, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) defines MIC and disk breakpoints for SXT alone and the FDA does not recognize any clinical breakpoints for S. maltophilia. For some agents used to treat S. maltophilia (e.g., CIP and TGC), there are no clinical breakpoints. To aid with this type of situation, EUCAST has defined a variety of organism-agnostic pharmacokinetic/pharmacodynamic (PK/PD) breakpoints, including for CIP and TGC. PK/PD breakpoints relate expected antimicrobial exposure at a given dose to the MIC, using PK/PD indices (e.g., area under the concentration curve to MIC ratio for CIP) that relate to patient response for other organisms (11). These PK/PD breakpoints can be used when there are no species-specific breakpoints for a given antimicrobial agent, as a loose interpretation of susceptibility and resistance, but are only available as MIC interpretations, as disk correlates must be established for each species tested.
In this study, we aimed to address the gap in contemporary AST data for S. maltophilia by assessing the accuracy of disk and gradient diffusion methods compared to reference BMD against contemporary bloodstream isolates of S. maltophilia, recovered between 2015 to 2019, for CAZ, LEV, MIN, and SXT. In addition, disk-to-MIC correlates were evaluated for CIP and TGC against EUCAST PK/PD breakpoints.
RESULTS
MIC distributions.
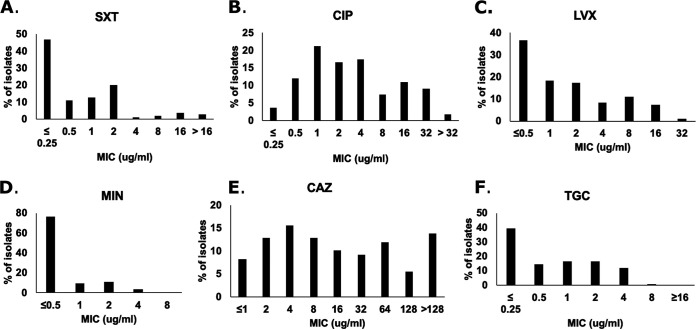
A total of 110 S. maltophilia bloodstream isolates were tested. One isolate was eliminated due to lack of growth in multiple attempts of BMD. Thus, 109 isolates were tested against six antimicrobials, including SXT, LEV, MIN, CAZ, CIP, and TGC. Breakpoints applied to the various agents are listed in Table 1. The most active compound tested against the isolates was MIN (MIC50/MIC90, ≤0.5/2 μg/ml [100% susceptible]), followed by SXT (MIC50/MIC90, 0.5/2 μg/ml [90% susceptible]), LEV (MIC50/MIC90, 1/8 μg/ml [72% susceptible]), and CAZ (MIC50/MIC90, 16/≥128 μg/ml [50% susceptible]), when using CLSI breakpoints (Fig. 1). The MIC50/MIC90 values for the agents without CLSI breakpoints were as follows: CIP, 2/32 μg/ml, and TGC, 1/4 μg/ml (Fig. 1). When using EUCAST PK/PD breakpoints, susceptibility rates for CIP and TGC were 5% and 54%, respectively. CIP PK/PD breakpoints are substantially lower than LEV breakpoints (Table 1), and when LEV was evaluated using the PK/PD breakpoints, 37% of isolates were considered susceptible.
TABLE 1.
MIC and disk breakpoints used in this studya
| Antimicrobial (breakpoint source) | MIC breakpoint (μg/ml) |
Disk breakpoint (mm) |
||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| SXT (CLSI document M100 S30) | ≤2 | ≥4 | ≥16 | 11–15 | ≤10 | |
| LEV (CLSI document M100 S30) | ≤2 | 4 | ≥8 | ≥17 | 14–16 | ≤13 |
| LEV P. aeruginosa (CLSI document M100 S30) | ≤1 | 2 | ≥4 | |||
| MIN (CLSI document M100 S30) | ≤4 | 8 | ≥16 | ≥19 | 15–18 | ≤14 |
| CAZ (CLSI document M100 S30) | ≤8 | 16 | ≥32 | |||
| CIP (EUCAST PK/PD) | ≤0.25 | >0.5 | ||||
| CIP P. aeruginosa (CLSI document M100 S30) | ≤0.5 | 1 | ≥2 | |||
| LEV (EUCAST PK/PD) | ≤0.5 | >1 | ||||
| TGC (EUCAST PK/PD) | ≤0.5 | >0.5 | ||||
S, susceptible; I, intermediate; R, resistant.
FIG 1.
MIC distribution of the six most common antimicrobials against S. maltophilia, determined by broth microdilution.
Performance of disk diffusion.
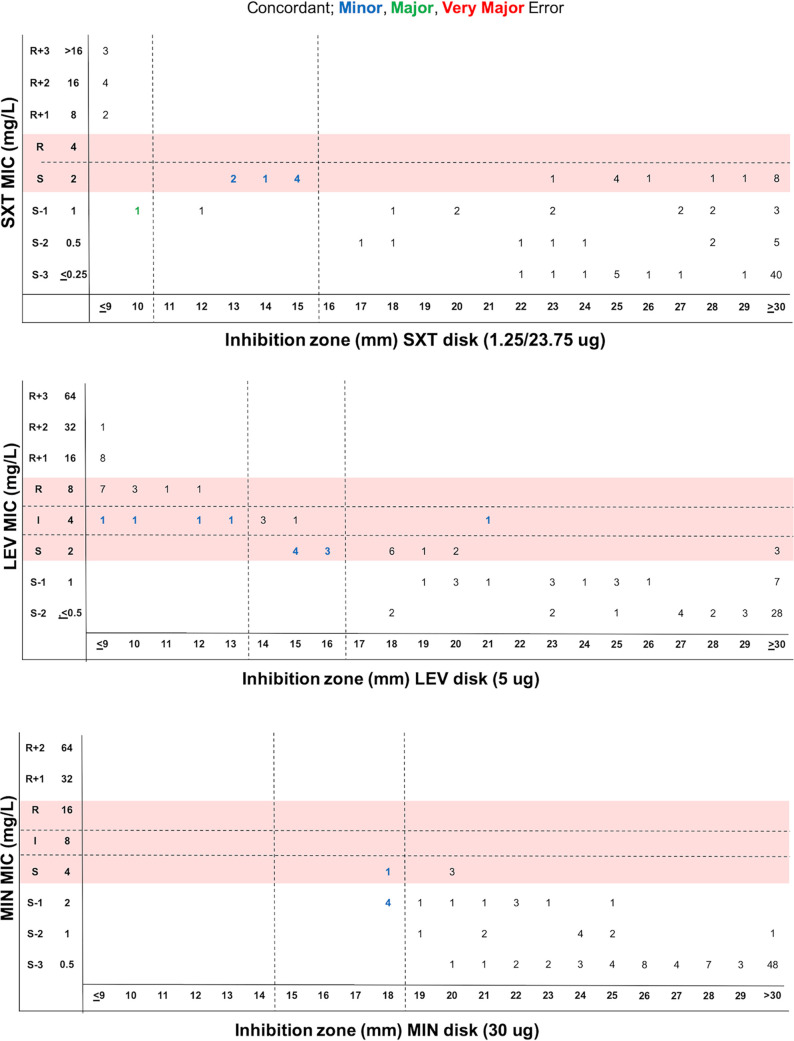
The agreement between disk diffusion and BMD was assessed using methods described in CLSI document M23 (12). Figure 2 shows the performance of the SXT, LEV, and MIN disk diffusion tests compared to the BMD MICs. Of note, CAZ disk was not evaluated, as there are no CLSI disk breakpoints for this drug. SXT, LEV, and MIN susceptibility testing by disk diffusion was associated with categorical agreement (CA) values of 93%, 89%, and 95%, respectively (Table 2). Initial testing for SXT revealed four major errors (MEs), 3 of which were resolved upon repeat testing, yielding 1 ME, 8 minor errors (MIs), and no very major errors (VMEs) (Table 2). MIs were largely due to the disk diffusion yielding an intermediate result when BMD categorized them as susceptible (n = 7) (Table 2). All 7 isolates had an MIC value of 2 μg/ml, which abuts the susceptible breakpoint of ≤2 μg/ml. One isolate was intermediate by disk diffusion but resistant by BMD.
FIG 2.
Scattergram distribution of S. maltophilia isolates according to SXT, LEV, and MIN MIC values determined by BMD and disk diffusion zones following existing CLSI document M100 MIC and zone diameter breakpoints (red shading).
TABLE 2.
Overall performance of disk diffusion compared to BMD for 109 S. maltophilia bloodstream isolatesa
| Antimicrobial, breakpoint source | No./total (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall CA | VME |
ME |
MI |
||||||||
| Total | ≥R + 1 or ≥I + 2 | R + S or I ±1 | Total | ≤S − 1 or ≤I − 2 | S + R or I ±1 | Total | ≥R + 1 or ≥I + 2 | S + R or I ±1 | ≤S − 1 or ≤I − 2 | ||
| SXT, S. maltophilia M100 | 101/109 (93) | 0/10 (0) | 0/9 (0) | 0/23 (0) | 1/100 (1) | 0/77 (0) | 1/23 (4) | 8/109 (7) | 0/9 (0) | 7/23 (30) | 1/77 (1) |
| LEV, S.maltophilia M100 | 97/109 (89) | 0/22 (0) | 0/9 (0) | 0/40 (0) | 0/78 (0) | 0/60 (0) | 0/40 (0) | 12/109 (11) | 0/9 (0) | 12/40 (30) | 0/60 (0) |
| MIN, S. maltophilia M100 | 104/109 (95) | 0/0 (0) | 0/0 (0) | 0/4 (0) | 0/109 (0) | 0/105 (0) | 0/4 (0) | 5/109 (5) | 0/0 (0) | 1/4 (25) | 4/105 (4) |
| CAZ, * S. maltophilia M100 | 83/109 (76) | 4/44 (9) | 2/34 (6) | 2/35 (6) | 7/54 (13) | 5/40 (13) | 2/35 (6) | 15/109 (14) | 0/34 (0) | 14/35 (40) | 1/40 (3) |
| LEV, * P. aeruginosa M100 | 91/109 (83) | 3/31 (10) | 0/31 (0) | 3/78 (4) | 0/60 (0) | 0/31 (0) | 0/78 (0) | 15/109 (14) | 0/21 (0) | 15/78 (19) | 0/10 (0) |
| LEV,* EUCAST PK/PD | 96/109 (88) | 10/69 (14) | 3/40 (8) | ||||||||
| CIP,* EUCAST PK/PD | 86/109 (79) | 22/105 (21) | 1/4 (25) | ||||||||
| CIP, * P. aeruginosa M100 | 78/109 (72) | 15/69 (22) | 6/69 (9) | 9/40 (23) | 1/40 (3) | 0/69 (0) | 1/40 (3) | 15/109 (14) | 0/51 (0) | 14/40 (35) | 1/18 (6) |
| TGC, * EUCAST PK/PD | 79/109 (73) | 3/50 (6) | 27/59 (46) | ||||||||
SXT, MIN, and LEV evaluations are based on existing CLSI M100 breakpoints for S. maltophilia. CAZ, LEV, CIP, and TGC evaluations indicated by an asterisk (*) are based on disk-to-MIC correlates and zone diameter breakpoints generated by dBETS software using either M100 S. maltophilia MIC breakpoints, CLSI P. aeruginosa MIC breakpoints, or EUCAST PK/PD breakpoints as indicated. Categorical agreement (CA), very major errors (VMEs), major errors (MEs), and minor errors (MIs) calculated. Errors rates for antimicrobials with no intermediate MIC category parsed as follows: ≥R + 1, MIC greater than or equal to 1 doubling dilution of the resistant breakpoint; R + S, MIC at susceptible or resistant breakpoint; and ≤S − 1, MIC less than or equal to 1 doubling dilution of the susceptible breakpoint. Errors rates for antimicrobials with intermediate MIC category parsed as follows: ≥I + 2, MIC greater than or equal to 2 doubling dilutions of the intermediate breakpoint; I ± 1, MIC plus or minus 1 doubling dilution of the intermediate breakpoint; and ≤I − 2, MIC less than or equal to 2 doubling dilutions of the intermediate breakpoint.
Initial testing for LEV revealed 1 VME and 1 ME, both of which were resolved in repeat testing, and 12 MIs (Table 2). Seven of the MIs were for isolates that tested intermediate by disk diffusion but susceptible by BMD, two were due to an intermediate BMD result and a susceptible disk diffusion result, and three were due to an intermediate BMD result and resistant disk diffusion result. Initial testing for MIN revealed 1 ME, which was resolved in repeat testing, 5 MIs, and no VMEs (Table 2). All of MIs were intermediate by disk diffusion but susceptible by BMD.
MIC-to-disk correlates.
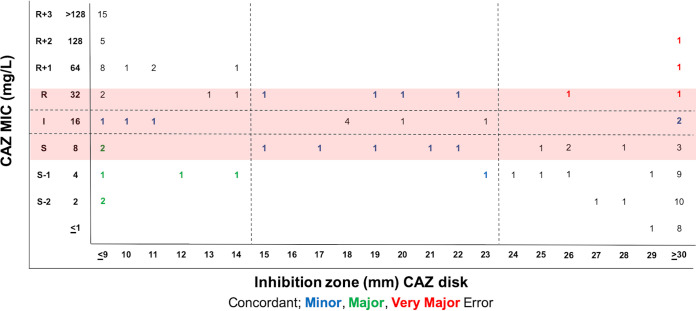
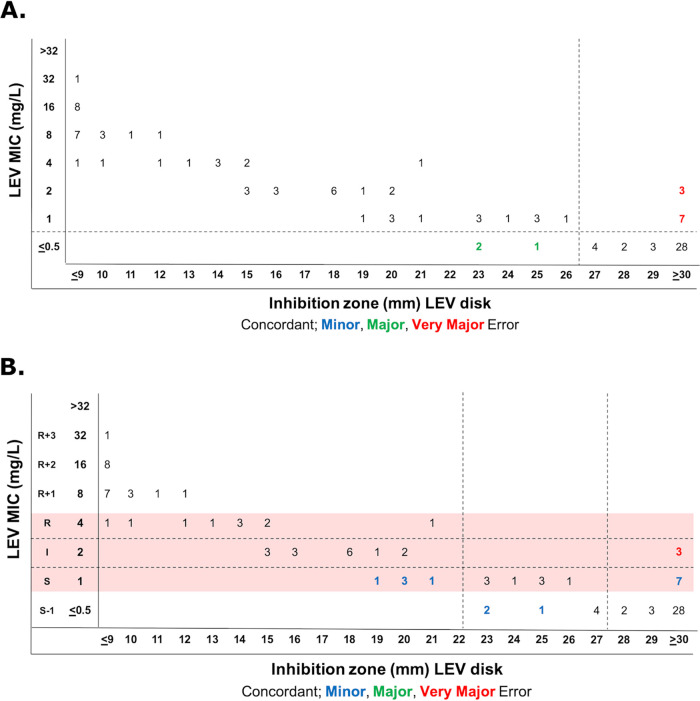
The scattergrams of MIC-to-disk data show that existing SXT, MIN, and LEV disk breakpoints are a good fit (Fig. 2; Table 2). S. maltophilia CLSI breakpoints are based on the 2007 Pseudomonas aeruginosa breakpoints, which have since been updated by the FDA, CLSI, and EUCAST. There are, however, no CLSI disk breakpoints for CAZ, CIP, or TGC, and the LEV breakpoints likely need revision to be more consistent with PK/PD data. Thus, we evaluated disk-to-MIC correlates using CAZ MIC breakpoints from CLSI document M100 (susceptible, ≤8 μg/ml), EUCAST PK/PD breakpoints for TGC (susceptible, ≤0.5 μg/ml), and EUCAST PK/PD breakpoints or CLSI document M100 P. aeruginosa breakpoints for CIP (susceptible, ≤0.25 or ≤0.5 or μg/ml) and LEV (susceptible, ≤0.5 or ≤1 μg/ml). Best-fit disk breakpoints were generated using the open-access diffusion Breakpoint Estimation Testing Software (dBETS) with the error rate-bound method. CAZ disk breakpoints defined by dBETS were ≥24 mm for susceptibility and ≤14 mm for resistance (Fig. 3). These breakpoints yielded an overall CA of 76% (83/109) with 4 VMEs, 7 MEs, and 15 MIs. Of these, 2 VMEs (6%), 2 MEs (6%), and 14 MIs (40%) were within 1 doubling dilution of the intermediate MIC breakpoint (Fig. 3). The LEV disk susceptibility breakpoint that best fit to the EUCAST PK/PD breakpoint was ≥27 mm and yielded a CA of 88% (96/109) with 10 VMEs (14%) and 3 MEs (8%) (Fig. 4A). LEV disk breakpoints that best fit to CLSI P. aeruginosa MIC breakpoints were ≥28 mm for susceptibility and ≤22 mm for resistance (Fig. 4B). These yielded a CA of 83% (91/109) with 3 VMEs (4%), 0 MEs, and 15 MIs (19%). All LEV MIs were for isolates with an MIC within 1 doubling dilution of the intermediate MIC breakpoint (Fig. 4B). Therefore, disk diffusion had acceptable performance for LEV with CLSI P. aeruginosa MIC breakpoints, but acceptable disk correlates could not be determined (outlined in Table S1 in the supplemental material) for CAZ or for LEV with EUCAST PK/PD breakpoints (outlined in Table 1).
FIG 3.
Estimation of CAZ disk diffusion breakpoints for S. maltophilia with dBETS software using error rate-bound method using CAZ MIC breakpoints from CLSI document M100 (red shading). Shown is the distribution of 109 isolates according to MIC and zone diameter values, with errors indicated.
FIG 4.
Estimation of LEV disk diffusion breakpoints for S. maltophilia with dBETS software using the error rate-bound method using EUCAST PK/PD breakpoints (A) or CLSI document M100 P. aeruginosa breakpoints (red shading) (B). Shown is the distribution of 109 isolates according to MIC and zone diameter values, with errors indicated.
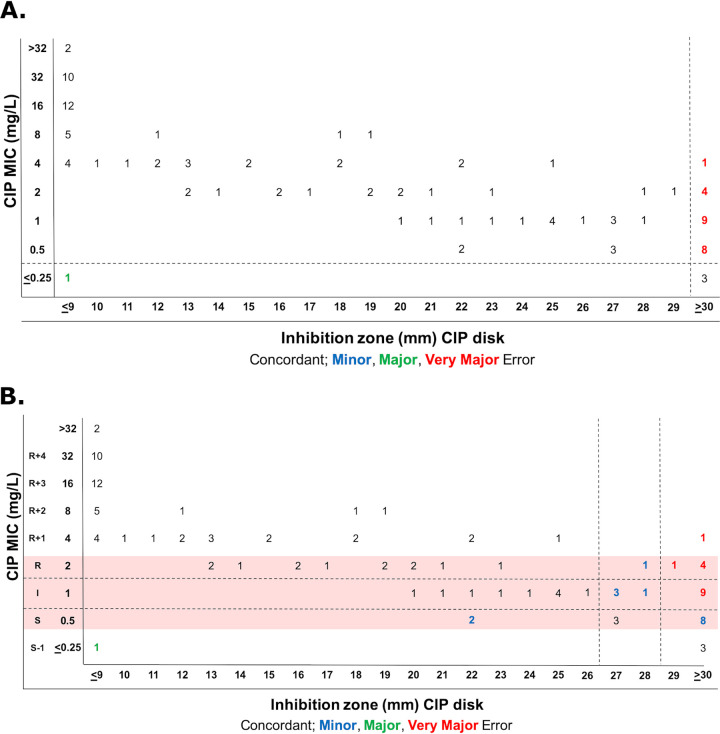
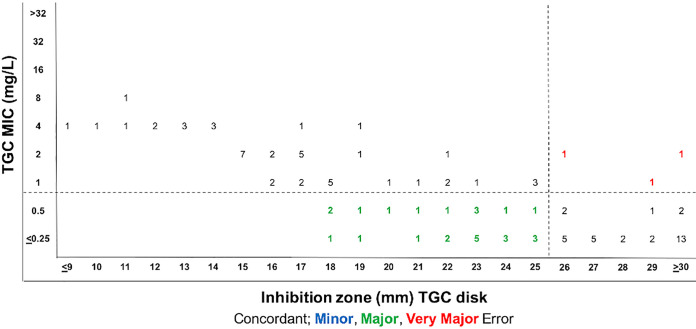
The CIP disk susceptibility breakpoint that best fit to the EUCAST PK/PD breakpoint was ≥30 mm and yielded a CA of 79% (86/109) with 22 VMEs (21%) and 1 ME (25%) (Fig. 5A). CIP disk breakpoints that best fit to CLSI P. aeruginosa MIC breakpoints were ≥29 mm for susceptibility and ≤26 mm for resistance (Fig. 5B). These breakpoints yielded an overall CA of 72% (78/109) with 15 VMEs, 1 ME, and 15 MIs. Of these, 9 VMEs (23%), 1 ME (3%), and 14 MIs (35%) fell within the 1-doubling-dilution range of the intermediate breakpoint (Fig. 5B). TGC disk breakpoints that best fit to EUCAST PK/PD breakpoints were ≥26 mm for susceptibility and ≤25 mm for resistance (Fig. 6). These breakpoints yielded a CA of 73% (79/109) with 3 VMEs (6%) and 27 MEs (46%). Therefore, disk diffusion did not have acceptable performance for CIP and TGC by the error rate-bound method (Table S1).
FIG 5.
Estimation of CIP disk diffusion breakpoints for S. maltophilia with dBETS software using the error rate-bound method using EUCAST PK/PD breakpoints (A) or CLSI document M100 P. aeruginosa breakpoints (red shading) (B). Shown is the distribution of 109 isolates according to MIC and zone diameter values, with errors indicated.
FIG 6.
Estimation of TGC disk diffusion breakpoints for S. maltophilia with dBETS software using the error rate-bound method using EUCAST PK/PD breakpoints. Shown is the distribution of 109 isolates according to MIC and zone diameter values, with errors indicated.
Gradient strip performance.
The performances of two brands of gradient strips were evaluated against BMD (Table 3). CLSI document M100 breakpoints were used for SXT, MIN, LEV, and CAZ, while EUCAST PK/PD breakpoints were used for CIP and TGC (Table 1). Etest performance met overall acceptance criteria for SXT, MIN, and LEV (Table 3). Overall values for CA with Etest for SXT, MIN, LEV, and CAZ were 98%, 93%, 85%, and 71%, respectively (Table 3). Etest for SXT yielded 1 VME within the acceptable error range for an isolate with an MIC at the breakpoint (4 μg/ml) by BMD. All SXT MEs were resolved with repeat testing. Etest for LEV yielded 0 VMEs, 17 MIs, and 5 MEs, 3 of which were resolved upon repeat testing. One ME was within 1 doubling dilution of the intermediate MIC breakpoint (Table 3). The majority of the MIs (15/17) were within 1 doubling dilution of the intermediate breakpoint, while 2 had MICs lower than 2 doubling dilutions of the intermediate breakpoint, yielding results that were close to the acceptance range (3%) (Table 3). The LEV Etest yielded a more resistant result for 16 of the 17 MIs, calling 9 isolates as intermediate when they had a BMD at the susceptible breakpoint (2 μg/ml) (Table S2). Seven of the MIs were called resistant by the LEV Etest when they had a BMD MIC at the intermediate breakpoint (4 μg/ml) (Table 3).
TABLE 3.
Overall performance of Etest and MTS compared to BMD for 109 S. maltophilia bloodstream isolatesa
| Antimicrobial | Isolate group | n | No. (%) with indicated value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Etest |
MTS |
|||||||||
| CA | VME | ME | MI | CA | VME | ME | MI | |||
| SXT | Overall | 109 | 107 (98) | 1 (9) | 0 (0) | 0 (0) | 107 (98) | 1 (9) | 0 (0) | 109 (0.9) |
| ≥R + 1 | 9 | 0 | NA | 0 | 0 | NA | 0 | |||
| R + S | 23 | 1 (4) | 0 | 0 | 1 (4) | 0 | 1 (4) | |||
| ≤S − 1 | 77 | NA | 0 | 0 | NA | 0 | 0 | |||
| LEV | Overall | 109 | 93 (85) | 4 (9) | 4 (7) | 17 (16) | 90 (83) | 1 (5) | 0 (0) | 16 (15) |
| ≥R + 2 | 9 | 0 | NA | 0 | 0 | NA | 0 | |||
| I ± 1 | 40 | 0 | 1 (3) | 15 (38) | 1 (3) | 0 | 14 (35) | |||
| ≤I − 2 | 60 | NA | 1 (2) | 2 (3) | NA | 0 | 2 (3) | |||
| MIN | Overall | 101 (93) | 0 (0) | 0 (0) | 0 (0) | 108 (99) | 0/0 (0) | 0 (0) | 0 (0) | |
| ≥R + 2 | 0 | 0 | NA | 0 | 0 | NA | 0 | |||
| I ± 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| ≤I − 2 | 105 | NA | 0 | 8 (8) | NA | 0 | 1 (1) | |||
| CAZ | Overall | 109 | 77 (71) | 13 (30) | 3 (6) | 16 (15) | 90 (73) | 4 (9) | 7 (13) | 19 (17) |
| ≥R + 2 | 34 | 1 (3) | NA | 0 | 1 (3) | 0 | 2 (6) | |||
| I ± 1 | 35 | 3 (9) | 1 (3) | 14 (40) | 3 (9) | 1 (3) | 14 (40) | |||
| ≤I − 2 | 40 | NA | 3 (8) | 1 (3) | NA | 6 (15) | 3 (8) | |||
| CIP (PK/PD) | 109 | 90 (83) | 0 (0) | 2 (50) | 17 (15) | 86 (76) | 2 (2) | 0 (0) | 21 (19) | |
| TGC (PK/PD) | 109 | 60 (55) | 1 (2) | 48 (80) | 65 (60) | 5 (10) | 30 (65) | |||
CLSI breakpoints (M100) and EUCAST PK/PD breakpoints used to interpret MIC results as indicated. Categorical agreement (CA), very major errors (VMEs), major errors (MEs), and minor errors (MIs) were calculated. Errors rates for antimicrobials with no intermediate MIC category parsed as follows: ≥R + 1, MIC greater than or equal to 1 doubling dilution of the resistant breakpoint; R + S, MIC at susceptible or resistant breakpoint; and ≤S − 1, MIC less than or equal to 1 doubling dilution of the susceptible breakpoint. Errors rates for antimicrobials with intermediate MIC category parsed as follows: ≥I + 2, MIC greater than or equal to 2 doubling dilutions of the intermediate breakpoint; I ± 1, MIC plus or minus 1 doubling dilution of the intermediate breakpoint; and ≤I − 2, MIC less than or equal to 2 doubling dilutions of the intermediate breakpoint. NA, not applicable.
Initial testing for MIN yielded 1 ME, which was resolved with repeat testing, 0 VME, and 8 MIs. All MI were intermediate by Etest but susceptible by BMD (Table S2). The CAZ Etest strip yielded 4 VMEs, 4 MEs, and 15 MIs, none of which resolved on repeat testing. Of these, 3 VMEs (9%), 1 ME (3%), and 14 MIs (40%) were within 1 doubling dilution of the intermediate breakpoint (Table 3). Three MEs were isolates with an MIC lower than 1 doubling dilution of the intermediate breakpoint (Table 3).
The MIC test strip (MTS; Liofilchem, Roseto degli Abruzzi, Italy) performance met the acceptance criteria for SXT, LEV, and MIN (Table 3). Values for CA with MTS for SXT, MIN, LEV, and CAZ were 98%, 99%, 83%, and 73%, respectively. Initial testing with SXT yielded 1 ME, which resolved with repeat testing, and 1 VME and 1 MI, which were both within 1 doubling dilution of the intermediate breakpoint MIC (error rates of 4%) (Table 3). The MIN MTS yielded 1 MI and no VMEs or MEs. The LEV MTS yielded 1 VME, 16 MIs, and 0 MEs (Table 3). Thirteen of the LEV MIs were within 1 doubling dilution of the intermediate breakpoint MIC. Eight MIs were susceptible by BMD and intermediate by MTS, 3 were resistant by BMD and intermediate by MTS, 2 were intermediate by BMD and resistant by MTS, and 3 were intermediate by BMD and susceptible by MTS (Table S2). Twelve out of 16 MIs had MICs within essential agreement between BMD and MTS.
CAZ MTS did not have an acceptable performance (73% CA) and yielded 4 VMEs, 7 MEs, and 19 MIs (Table 3). Six of the MEs were MICs lower than 1 doubling dilution from the intermediate breakpoint (15% error rate), which fell outside the acceptable performance criteria (Table 3).
DISCUSSION
Evaluating S. maltophilia for antimicrobial susceptibility is challenging, due to an absence of tests and breakpoints for clinically valuable agents. Clinical microbiology laboratories typically rely on commercial methods, including disk and gradient diffusion, and generally test only SXT, LEV, and CAZ. These agents have CLSI breakpoints and are recommended for routine testing by CLSI (13). However, SENTRY studies have reported LEV and CAZ resistances as high as 22% and 63%, respectively (4), leaving clinicians few therapeutic options to treat severe infections, particularly in immunocompromised patients. The most active agent against our cohort of S. maltophilia bloodstream isolates was MIN, with 100% of the isolates categorized as susceptible, followed by SXT and LEV according to the CLSI document M100 breakpoints (Table 1; Fig. 1). Thus, clinical laboratories should strongly consider testing for MIN. Previous studies have also reported high rates of susceptibility of S. maltophilia isolates to MIN across geographic regions (8).
Few data exist regarding the performance of AST for S. maltophilia. The FDA has not recognized any breakpoints for this organism, meaning that no commercial AST devices can achieve FDA clearance for S. maltophilia. Disk diffusion is one testing option, but disk breakpoints were established in 2007. The present study confirms that these breakpoints remain relevant for contemporary S. maltophilia (Table 2). Similarly, both brands of gradient strips (Etest and MTS) had acceptable performance for SXT, MIN, and LEV (Table 3; Table S2). Thus, any of these manual testing methods could be used reliably for all three agents. Laboratories should be cognizant of MIs when using disk diffusion for SXT and LEV or gradient strips for LEV. The majority of the MIs occur at or near the breakpoints, so laboratories may consider confirming results for isolates with MICs or disk results that abut the breakpoint.
In contrast, no test performed well for CAZ. The data presented here confirm that CAZ disk diffusion should not be performed, and Etest and MTS also do not yield reliable results. Additionally, 50% or 37% of the contemporary isolates in our study were susceptible to CAZ using CLSI or EUCAST PK/PD breakpoints, respectively. Given the even lower susceptibility rates observed in the recent SENTRY study (i.e., 23%), testing of this agent may no longer be relevant since it does not have high efficacy against S. maltophilia.
CIP and TGC, two agents that are used clinically for S. maltophilia, did not demonstrate promising disk-to-MIC correlates using CLSI document M100 P. aeruginosa or PK/PD breakpoints (Fig. 5 and 6). Of note, CLSI, EUCAST, and FDA have updated LEV and CIP breakpoints for Enterobacterales and Pseudomonas aeruginosa, based predominantly on PK/PD data (10, 14, 15). S. maltophilia breakpoints, which were originally based on those of P. aeruginosa, were not updated at this time. As such, it is probable that the more appropriate breakpoint to use for the fluoroquinolones is a PK/PD one (as listed in Table 1). Disk correlates for LEV and a P. aeruginosa breakpoint of ≤1 μg/ml were evaluated and provide an alternative option for assessing susceptibility.
The limitations of this study are that we involved only one testing center, and one brand of disks and Mueller-Hinton agar (MHA) were used. The large number of off-scale MICs was a major limitation that prevented assessment of essential agreement. However, the CA is a more reliable indication of accuracy for our study and provides more clinically valuable information overall for S. maltophilia, since the precise MIC value is not generally used to inform dosing regimens for this organism. Additionally, the EUCAST SXT breakpoint is very low (<0.001 μg/ml), which makes it impossible to categorize isolates with BMD MICs of <670.25 μg/ml as susceptible or resistant, as the breakpoint is off-scale. Lastly, we did not evaluate the performance of eravacycline and cefiderocol against S. maltophilia in our study.
In summary, we show that disk diffusion and gradient strips perform reasonably well with the CLSI breakpoints for MIN, SXT, and LEV, which were the most active agents against our S. maltophilia isolates. CIP and TGC are viable alternative agents, though the disk-to-MIC correlates were unreliable.
MATERIALS AND METHODS
Bacterial strains.
A total of 110 isolates of S. maltophilia were recovered from clinical blood cultures spanning 2015 to 2019 with the following susceptibility patterns included: 10 isolates resistant to SXT, ∼30% resistant to CAZ, and ∼20% resistant to LEV, to cover a wider range of MICs. Isolates were selected based on results of testing by the clinical laboratories at the time of their isolation. The majority of isolates came from MD Anderson Cancer Center, followed by University of California, Los Angeles, and Children’s Hospital of Los Angeles. A total of 109 isolates were included in this study, with 1 discarded due to the lack of growth on AST media. Prior to testing, frozen isolates were subcultured twice, and fresh isolates were subcultured once, on tryptic soy agar with 5% sheep blood (BAP; BD, Sparks, MD). Quality control (QC) strains tested with each run included Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853.
Antimicrobial susceptibility testing.
Each isolate was tested in a single replicate concurrently by BMD and disk diffusion at University of Texas Health Science Center (UTHealth). Etest and MIC test strip (MTS) were concurrently run at MD Anderson Cancer Center. A suspension equivalent to that of a 0.5 McFarland standard was prepared using 3 to 5 isolated colonies from a single plate. Testing by BMD, disk diffusion, and gradient strip assays was performed according to CLSI standards and manufacturers’ instructions, using custom reference BMD panels prepared by Accelerate Diagnostics following CLSI protocols and disks from BD and gradient strips from bioMerieux (Etest; Durham, NC) and Liofilchem (MTS; Roseto degli Abruzzi, Italy) placed on Mueller-Hinton agar (MHA; BD) (10). BMD panels and plates with disks or strips were incubated in ambient air at 35°C ± 2°C and read after 20 to 24 h of incubation.
Data analysis.
Disk and gradient diffusion results were compared to BMD results as the gold standard. Categorical agreement (CA), major errors (ME), very major errors (VME), and minor errors (MIs) were evaluated using the error rate-bound method and criteria listed in Table S1. CA was defined as the agreement of interpretive results between the method under evaluation and BMD using CLSI or EUCAST breakpoints. Discrepancies between the method under evaluation and BMD were categorized as follows: VME, false-susceptible result for an isolate resistant by BMD; ME, false-resistant result for an isolate susceptible by BMD; and MI, a discrepancy between the test and reference methods involving an intermediate result. All CLSI and EUCAST PK/PD breakpoints that were used to categorize isolates are listed in Table 1.
Disk diffusion breakpoint estimates for CAZ, CIP, and TGC were generated using disk-to-MIC correlates on the diffusion Breakpoint Estimation Testing Software (dBETS; version 1.5) (16). The ideal error rate criteria (outlined in Table S1) when using CLSI document M100 breakpoints to determine VME, ME, and MI within 1 doubling dilution of the intermediate breakpoint were <10%, <10%, and <40%, respectively, and the criteria outside 1 doubling dilution of the intermediate breakpoint were <2%, <2%, and <5%. The standard error rate criteria when using EUCAST PK/PD breakpoints to determine VME, ME, and MI were <3%, <3%, and ≤10%.
Discrepancy testing.
Isolates with a VME or ME by any method were retested in parallel using BMD, disk diffusion, Etest, and MTS, along with an equal number of concordant isolates and the single isolate with growth failure. QC strains tested with each run included Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. The isolate that failed to grow during repeat testing was excluded from analysis. CA, VME, ME, and MI were calculated after repeat testing. If an error persisted after repeat testing, it was included in the final calculations. If an error resolved after repeat testing, it was not counted as an error and the initial result was disregarded. If a concordant isolate produced an error after repeat testing, it was included in the final calculations.
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis 6:S34–S46. 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garazi M, Singer C, Tai J, Ginocchio CC. 2012. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect 81:114–118. 10.1016/j.jhin.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Cho S-Y, Lee D-G, Choi S-M, Park C, Chun H-S, Park Y-J, Choi J-K, Lee H-J, Park SH, Choi J-H, Yoo J-H. 2015. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis 15:69. 10.1186/s12879-015-0801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y-T, Lin C-Y, Chen Y-H, Hsueh P-R. 2015. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 6:893. 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safdar A, Rolston KV. 2007. Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis 45:1602–1609. 10.1086/522998. [DOI] [PubMed] [Google Scholar]
- 6.Humphries RM, Hindler JA. 2016. Emerging resistance, new antimicrobial agents…but no tests! The challenge of antimicrobial susceptibility testing in the current us regulatory landscape. Clin Infect Dis 63:83–88. 10.1093/cid/ciw201. [DOI] [PubMed] [Google Scholar]
- 7.Humphries R, Mendez J, Miller LG, Miner A, Fernandes P, Richter S, Franco R, Felix-Mendez J, Agrawal S, Sinkowitz J, Sinkowitz D, Hershey C, Zhou N, Eliopulos A, Bhaurla S, Mendez A, Marquez P, Niakan S, Terashita D, Schwartz B, McKinnell JA. 2017. The regional antibiogram is an important public health tool to improve empiric antibiotic selection, Stenotrophomonas maltophilia as a case example. Open Forum Infect Dis 4(Suppl 1):S258. 10.1093/ofid/ofx163.563. [DOI] [Google Scholar]
- 8.Flamm RK, Shortridge D, Castanheira M, Sader HS, Pfaller MA. 2019. In vitro activity of minocycline against U.S. isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus species complex, Stenotrophomonas maltophilia, and Burkholderia cepacia complex: results from the SENTRY antimicrobial surveillance program, 2014 to 2018. Antimicrob Agents Chemother 63:e01154-19. 10.1128/AAC.01154-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing. M100 S17. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing: CLSI M100 edition 30. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Mouton JW, Brown DFJ, Apfalter P, Cantón R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy C-J, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2018. Development of in vitro susceptibility testing criteria and quality control parameters, M23, 5th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.CLSI. 2020. January 2020 meeting, methods development and standardization working group report. Clinical and Laboratory Standards Institute, Tempe, Arizona.
- 14.Chantell C, Humphries RM, Lewis J. 2019. Fluoroquinolone breakpoints for Enterobacteriaceae and Pseudomonas aeruginosa. CLSI rationale document MR02. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.FDA. 2020. Antibacterial susceptibility test interpretive criteria. https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria.
- 16.DePalma G, Turnidge J, Craig BA. 2017. Determination of disk diffusion susceptibility testing interpretive criteria using model-based analysis: development and implementation. Diagn Microbiol Infect Dis 87:143–149. 10.1016/j.diagmicrobio.2016.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.