Drug combination therapy is an interesting approach to increase the success of drug repurposing for neglected diseases. Thus, the objective of this work was to evaluate binary and ternary therapies composed of itraconazole, ezetimibe, and miltefosine for the treatment of visceral leishmaniasis.
KEYWORDS: Leishmania infantum, azoles, ezetimibe, miltefosine, drug repurposing, combined therapy
ABSTRACT
Drug combination therapy is an interesting approach to increase the success of drug repurposing for neglected diseases. Thus, the objective of this work was to evaluate binary and ternary therapies composed of itraconazole, ezetimibe, and miltefosine for the treatment of visceral leishmaniasis. Intracellular Leishmania infantum amastigotes were incubated with the drugs alone or in combination for 72 h. For in vivo experiments, we tested long-course (21 days, once per day) and short-course (5 days, twice per day) treatments for the binary combination of itraconazole and ezetimibe. For the ternary therapy including miltefosine, we adopted the short-course treatment and varied the vehicle. None of the combinations were toxic to macrophages. The binary combination of itraconazole plus ezetimibe and the ternary combination of itraconazole, ezetimibe, and miltefosine had synergistic effects in intracellular amastigotes, for some of the proportions evaluated. Although the in vivo long-course therapy had been more effective than the short-course protocol, it showed hepatic toxicity signs. Ezetimibe has been proven to be able to reduce the parasite burden alone or in combination. Both suspensions of the ternary combination were active, but when the drugs were suspended in the commercial Ora-Plus formulation instead of purified water, the parasite burden was reduced by 98% in the liver and spleen. Altogether, the results demonstrate for the first time the activity of ezetimibe in a viscerotropic species of Leishmania and indicate that ternary treatment composed of miltefosine, itraconazole, and ezetimibe at low doses is a promising therapeutic alternative for the treatment of visceral leishmaniasis.
INTRODUCTION
Visceral leishmaniasis (VL), also known as kala-azar, is a neglected tropical disease (NTD) caused by obligatory intracellular protozoan parasites of the genus Leishmania, which are usually transmitted by sandflies of the subfamily Phlebotominae (1). The main causative species of VL are Leishmania donovani in Asia and Africa and Leishmania infantum in the Mediterranean Basin, the Middle East, Central Asia, and South and Central America (2–5). Most cases (∼90%) of VL are reported in three endemic foci, (i) India, (ii) East Africa, and (iii) Brazil, with 20,000 to 40,000 deaths annually (2).
VL is characterized by prolonged fever, splenomegaly, hepatomegaly, pancytopenia, progressive anemia, and weight loss. If untreated, VL is uniformly fatal (2). Since the 1940s, treatment for leishmaniasis has included the pentavalent antimonials N-methylglucamine antimoniate (meglumine antimoniate) and sodium stibogluconate (3). These drugs have many side effects, including cardiac, hepatic, pancreatic, and renal toxicities, and should be used with caution under clinical and laboratory monitoring (4). In cases of treatment failure or resistance to first-line therapy, other drugs such as pentamidine, amphotericin B, and paromomycin are used, even though they have significant toxic effects (3). In Brazil, meglumine antimoniate is the first-choice drug for the treatment of VL, except in certain medical conditions, when the use of amphotericin B is recommended, especially in its liposomal formulation (5).
Miltefosine (MT), an alkylphosphocholine initially developed as an antineoplastic agent, was repurposed for the treatment of leishmaniasis and served as the basis of the VL elimination program in India, Nepal, and Bangladesh. It is the only oral drug in clinical use. However, after more than a decade of use, its efficacy has declined in India and Nepal (3). Long-term follow-up data suggested that failure rates may reach 10% at 6 months and 20% at 12 months, which is considered an alarming signal for the VL elimination campaign (6). Resistant cases have also been reported (7).
Therefore, other therapy strategies have been tested. In vitro and in vivo studies have evaluated the potential of drug combinations using classical or alternative leishmanicidal drugs (8, 9). Clinical trials have also been carried out using this therapeutic approach, particularly for the treatment of HIV/VL coinfection (10). Diro and colleagues showed that the combination of liposomal amphotericin B (AmBisome) and miltefosine was more effective than liposomal amphotericin B monotherapy in HIV/VL-coinfected patients in Ethiopia (11). Another interesting approach for treating VL is drug repurposing, which is the process through which one finds new indications for approved drugs for which safety in humans has already been established (12). Azole antifungal drugs such as itraconazole (ITR), ketoconazole, and posaconazole inhibit sterol biosynthesis by acting on sterol C14 demethylase and have leishmanicidal activity (13). Clinical trials revealed that treatment of localized cutaneous leishmaniasis (CL) with oral ketoconazole in combination with intralesional sodium stibogluconate was more effective than each drug alone (14). However, some clinical studies have shown that monotherapy with azoles such as ketoconazole and itraconazole may not be effective for the treatment of CL and VL (15, 16).
In another report, our group demonstrated the leishmanicidal activity of ezetimibe (EZ), a drug used in the treatment of hypercholesterolemia (17). We have shown that ezetimibe is active in a murine model of cutaneous leishmaniasis and prevents sterol biosynthesis in Leishmania amazonensis. In addition, the combination of ezetimibe with azoles (ketoconazole and miconazole) showed a synergistic effect in vitro and increased its in vivo activity (17). Thus, here, we investigated the efficacy of binary and ternary drug combinations composed of miltefosine, itraconazole, and ezetimibe in Leishmania infantum-infected mice. We carried out new drug combinations using binary (ezetimibe plus itraconazole) and ternary (ezetimibe plus itraconazole and miltefosine) therapeutic combinations for VL treatment. Our results showed for the first time the activity of ezetimibe against the viscerotropic species of Old and New World infections, L. infantum.
RESULTS
In vitro cytotoxicity of drug combinations.
Prior to assessing the leishmanicidal activity of the drugs against intracellular amastigotes, we determined the nontoxic concentrations for BALB/c resident peritoneal macrophages (see Fig. S1 in the supplemental material). Itraconazole (ITR), miltefosine (MT), and ezetimibe (EZ) showed no toxicity against macrophages until 3, 2, and 30 μM, respectively, the highest concentrations evaluated. None of the combinations showed toxic effects on macrophages (Fig. S2). We next tested the same concentrations and proportions to evaluate the antileishmanial activity in intracellular amastigotes.
In vitro effect of the combination of itraconazole, ezetimibe, and miltefosine.
First, the 50% inhibitory concentration (IC50) for each drug was determined in L. infantum-infected BALB/c resident peritoneal macrophages, as shown in Tables 1 and 2. The IC50 values of itraconazole, ezetimibe, and miltefosine were 0.89 (0.73 to 1.08), 11.46 (10.11 to 12.98), and 0.63 (0.37 to 1.08) μM, respectively. The combination of itraconazole plus ezetimibe was performed by incubating the drugs alone and in combination, with serial dilutions according to the ratios described above. In Table 2, we can observe the combination of itraconazole plus ezetimibe, which has an average fractional inhibitory concentration index (FICI) of 0.56, indicating an additive interaction. However, at 3:2 and 1:4 ratios, the calculated FICI showed values of 0.39 and 0.47, respectively, demonstrating a synergistic effect with these combinations. Figure 1 represents an isobologram with the IC50 values of the drugs alone or combined. The untreated control cultures showed a percentage of infected macrophages above 90%, with about 12 amastigotes per macrophage (Fig. S3).
TABLE 1.
IC50, FICI50, and ΣFICI50 of the combination of itraconazole and ezetimibe against L. infantum intracellular amastigotesa
| ITR/EZ ratio | Mean IC50 (μM) (SD) |
FICI |
||||
|---|---|---|---|---|---|---|
| ITR | EZ | ITR | EZ | ΣFICI | ||
| 5:0 | 0.89 (0.73–1.08) | |||||
| 4:1 | 0.53 (0.45–0.63) | 1.34 (1.12–1.59) | 0.59 | 0.11 | 0.7 | |
| 3:2 | 0.30 (0.13–0.69) | 0.80 (0.71–0.89) | 0.33 | 0.06 | 0.39 | |
| 2:3 | 0.28 (0.16–0.48) | 4.19 (2.4–7.33) | 0.31 | 0.36 | 0.67 | 0.56 |
| 1:4 | 0.15 (0.10–0.22) | 3.63 (3.10–4.25) | 0.16 | 0.31 | 0.47 | |
| 0:5 | 11.46 (10.11–12.98) | |||||
IC50, concentration that inhibits 50% of parasite growth (followed by the standard deviation [SD]); ITR, itraconazole; EZ, ezetimibe. According to published guidelines, a fractional inhibitory concentration index (FICI) of 0.5 to 4.0 is indicative of an additive effect.
TABLE 2.
IC50, FICI50, and ΣFICI50 of the combination of itraconazole, ezetimibe, and miltefosine against L. infantum intracellular amastigotesa
| ITR/EZ/MT ratio | Mean IC50 (μM) (SEM) |
FICI |
||||||
|---|---|---|---|---|---|---|---|---|
| ITR | EZ | MT | ITR | EZ | MT | ΣFICI | ||
| 5:0:0 | 0.89 (0.73–1.08) | |||||||
| 4:1:1 | 0.23 (0.20–0.25) | 0.57 (0.52–0.63) | 0.038 (0.034–0.04) | 0.25 | 0.049 | 0.060 | 0.36 | |
| 3:2:2 | 0.07 (0.05–0.11) | 0.51 (0.33–0.77) | 0.034 (0.022–0.05) | 0.078 | 0.044 | 0.053 | 0.17 | |
| 2:3:3 | 0.32 (0.24–0.41) | 4.81 (3.71–6.24) | 0.37 (0.28–0.48) | 0.35 | 0.42 | 0.58 | 1.35 | 0.81 |
| 1:4:4 | 0.029 (0.023–0.037) | 1.17 (0.92–1.50) | 0.07 (0.06–0.1) | 0.032 | 0.1 | 0.11 | 0.24 | |
| 0:5:0 | 11.46 (10.1–12.9) | |||||||
| 4:1:4 | 0.83 (0.74–0.93) | 2.08 (1.87–2.32) | 0.55 (0.49–0.62) | 0.93 | 0.18 | 0.87 | 1.98 | |
| 3:2:3 | 0.18 (0.15–0.22) | 1.25 (1.03–1.50) | 0.12 (0.10–0.15) | 0.20 | 0.11 | 0.19 | 0.5 | |
| 2:3:2 | 0.46 (0.37–0.57) | 6.93 (5.60–8.57) | 0.3 (0.24–0.38) | 0.51 | 0.60 | 0.47 | 1.58 | |
| 1:4:1 | 0.062 (0.05–0.078) | 2.51 (2.0–3.15) | 0.04 (0.03–0.05) | 0.069 | 0.22 | 0.063 | 0.35 | |
| 0:0:5 | 0.63 (0.37–1.08) | |||||||
IC50, concentration that inhibits 50% of parasite growth (followed by the standard error of the mean [SEM]). According to published guidelines, a fractional inhibitory concentration index (FICI) of 0.5 to 4.0 is indicative of an additive effect.
FIG 1.

Isobologram analysis of the effects of the combination of itraconazole and ezetimibe at different ratios on intracellular L. infantum amastigotes. Infected macrophages were incubated with itraconazole and ezetimibe at different concentrations in serial dilutions for 72 h at 37°C. Each plotted point in the isobologram is the IC50 of the drug alone or in combination. The straight dashed line links the IC50s of the drugs alone. The experiments were performed in triplicate (n = 3). Data are presented as the means ± standard errors of the means (SEM) from three independent experiments.
In Table 2, the ternary combination had an average FICI of 0.81, characterizing an additive effect. However, the 4:1:1, 3:2:2, 1:4:4, 3:2:3, and 1:4:1 ratios resulted in specific FICI values equal to 0.36, 0.17, 0.24, 0.5, and 0.35, respectively, which represents a synergistic effect in these combinations. In Fig. 2 and Fig. S4 (isobologram), we can observe the strong antileishmanial effect of the ternary combination at the 3:2:2, 1:4:4, and 1:4:1 ratios. Treated macrophages exhibited a normal morphology compared to that of untreated cells and complete elimination of the parasites with the highest concentrations of the drugs (Fig. 2).
FIG 2.
Micrographs of L. infantum-infected macrophages treated with the ternary combination. Peritoneal macrophages infected with L. infantum were incubated in the absence or presence of the drugs for 72 h. (A) Control; (B) itraconazole plus ezetimibe and miltefosine at a ratio of 3:2:2 (1.8, 12, and 0.8 μM); (C) itraconazole plus ezetimibe and miltefosine at a ratio of 1:4:4 (0.6, 24, and 1.6 μM); (D) itraconazole plus ezetimibe and miltefosine at a ratio of 1:4:1 (0.6, 32, and 0.4 μM).
Effect of the combination of itraconazole plus ezetimibe on the parasite burden in the liver and spleen of L. infantum-infected mice.
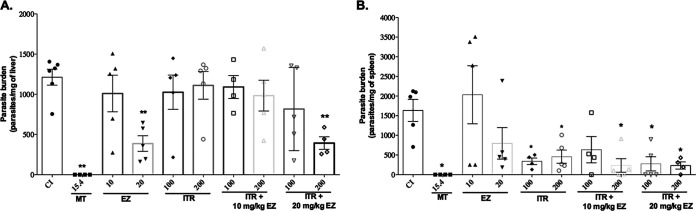
To evaluate the antileishmanial activity of the drugs in vivo, we initially used protocol 1, similar to a protocol previously reported by our group (17). The results in Fig. 3A and B show that the parasite load was decreased in the liver with treatment with MT at 15.4 mg/kg of body weight, EZ at 20 mg/kg, and the combination of ITR at 200 mg/kg plus EZ at 20 mg/kg. In the spleen, we observed a decrease in the parasite load with treatment with MT at 15.4 mg/kg, ITR at 100 or 200 mg/kg, and the combinations of ITR and EZ. A toxicological evaluation was also carried out, as shown in Fig. 4. We observed increases in the enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and in total bilirubin (BILT) in treated animals compared to those in infected control (CI) animals. Thus, although a reduction of the parasite burden was observed, the toxicological results demonstrated hepatotoxicity, with increases of the hepatic enzymes ALT and AST, raising the need for alteration of the treatment regimen.
FIG 3.
Parasite burden in mouse liver and spleen after treatment with itraconazole (ITR) plus ezetimibe (EZ). BALB/c mice were infected with 1 × 108 L. infantum promastigotes, and 7 days later, they were treated once per day for 21 days. After this period, the animals were euthanized to evaluate the parasite burden by a limiting dilution assay (LDA). The results are presented as the means ± standard errors for each group. (A) Parasitic load in the liver; (B) parasitic load in the spleen. MT, miltefosine. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (by Student’s t test compared to the infected control [CI] group).
FIG 4.
Toxicity of treatment with the combination of itraconazole (ITR) plus ezetimibe (EZ). BALB/c mice were infected with 1 × 108 promastigotes of L. infantum, and after 7 days, they were treated with the drugs once a day for 21 days. After this period, the animals were euthanized, and blood was collected by cardiac puncture for biochemical measurements. The results are presented as the means ± standard errors for each group. (A) Urea (URE); (B) cholesterol (CHO); (C) albumin (ALB); (D) aspartate aminotransferase (AST); (E) alanine aminotransferase (ALT); (F) total bilirubin (BILT). CNI, uninfected control; MT, miltefosine. *, P < 0.05; **, P < 0.01 (by Student’s t test compared to the infected control [CI] group).
Effect of the combination of itraconazole plus ezetimibe and miltefosine on the parasite burden in the liver and spleen using the Ora-Plus formulation.
When the drugs were suspended just in purified water, the parasite burden was decreased mainly in the spleen, with a significant difference in the treatments with 3.85 mg/kg or 7.7 mg/kg miltefosine and 10 mg/kg or 20 mg/kg ezetimibe. The ternary combination of EZ, ITR, and MT at 10 mg/kg, 100 mg/kg, and 3.85 mg/kg and at 20 mg/kg, 200 mg/kg, and 3.85 mg/kg decreased parasite burdens. Figure S6 shows that an increased level of albumin was detected in the serum of infected animals (CI) compared to that in the serum of uninfected animals (noninfected control [CNI]). No treatment regimens were toxic to mice.
Poorly water-soluble drugs often exhibit dissolution rate limitations and low bioavailability. Thus, we used the Ora-Plus vehicle for drug suspension in an attempt to improve the potency and efficacy of the ternary therapy. In Fig. 5A and B, we noted a decrease in the liver and spleen parasite loads. The combination of EZ and ITR, as well as the ternary combination, using the Ora-Plus formulation caused a significant reduction in hepatic and splenic parasite loads compared with that using just water to suspend the drugs. For example, treatment with the combination of 200 mg/kg ITR, 20 mg/kg EZ, and 3.85 mg/kg MT reduced the parasite burden in both the liver and spleen by 98%. The toxicological evaluation shown in Fig. 6 revealed that all doses used were nontoxic to mice.
FIG 5.
Effect of ternary therapy using the Ora-Plus formulation on the parasite burden in the liver and spleen. BALB/c mice were infected with 1 × 108 promastigotes of L. infantum, and after 7 days, they were treated with the drugs twice per day for 5 days. At 28 days postinfection, the animals were euthanized for parasite load evaluation. The results are presented as the means ± standard errors for each group. (A) Parasite burden in the liver; (B) parasite burden in the spleen. EZ, ezetimibe; ITR, itraconazole. *, P > 0.05; **, P > 0.01; ***, P < 0.001 (by Student’s t test compared to the infected control [CI] group). ##, P > 0.01 (by Student’s t test compared to 3.85 mg/kg miltefosine [MT]).
FIG 6.
Toxicity of the ternary therapy combination using the Ora-Plus formulation. BALB/c mice were infected with 1 × 108 promastigotes of L. infantum, and after 7 days, they were treated with the drugs twice per day for 5 days. At 28 days postinfection, the animals were euthanized, and blood was collected by cardiac puncture for biochemical measurements. The results are presented as the means ± standard errors for each group. (A) Urea (URE); (B) albumin (ALB); (C) aspartate aminotransferase (AST); (D) alanine aminotransferase (ALT); (E) cholesterol (CHO); (F) total bilirubin (BILT). CNI, uninfected control; MT, miltefosine; EZ, ezetimibe; ITR, itraconazole. *, P > 0.05; **, P > 0.01; ***, P < 0.001 (by Student’s t test compared to the infected control [CI] group).
DISCUSSION
Different approaches have been proposed to identify and optimize new drug candidates against leishmaniasis, including combination therapies, new formulations for drugs currently in use, and drug repurposing (18, 19). Oral combinations have been used with success to treat different infectious diseases such as malaria and tuberculosis, and this approach may be interesting for the treatment of visceral leishmaniasis. This strategy has the advantage of reducing the risk of resistance development in parasites, as drugs with different molecular targets can be used.
Thus, the present work aimed to study the potency and efficacy of ternary therapy using the drugs itraconazole, ezetimibe, and miltefosine to treat L. infantum-infected mice.
The inhibition of C14 demethylase (CYP51) by azoles causes the depletion of ergosterol and the accumulation of 14-methyl sterols, resulting in the inhibition of protozoan parasite growth (20, 21). de Macedo-Silva et al. showed that the combination of the C14 demethylase inhibitors itraconazole and posaconazole exhibited a synergistic effect against L. amazonensis (20).
Our group has shown that the combination of ketoconazole and ezetimibe, a Niemann-Pick C1-like 1 (NPC1L1) inhibitor, has activity in vitro and in vivo against L. amazonensis (17). Here, the combination of ezetimibe with itraconazole showed an overall additive effect in vitro (Fig. 1 and Table 1), with some combinations showing synergy in intracellular amastigotes of L. infantum. The approach used to determine each drug’s proportion in the combination was based on the serial dilution midpoint, as used for the drug interaction of tamoxifen and miltefosine in L. amazonensis (22). The antileishmanial activity of the itraconazole-ezetimibe combination may be related to total sterol inhibition, as itraconazole is a classical ergosterol inhibitor, and our group demonstrated that ezetimibe also inhibits sterol biosynthesis in L. amazonensis (17, 20, 23). Binary combinations using miltefosine and one azole have already been shown to be effective. Animals treated with fluconazole (50 mg/kg for 5 days) plus miltefosine (5 mg/kg for 5 days) showed enhancement in antileishmanial efficacy compared to individual drugs (24). Animals treated with the combination of ketoconazole (50 mg/kg for 5 days) plus miltefosine (5 mg/kg for 5 days) showed more efficacy in a visceral model than those treated with ketoconazole and miltefosine separately (25). Thus, we decided to include this reference drug in our study to form a ternary therapy. The ternary association of itraconazole with ezetimibe and miltefosine showed a similar effect in vitro, with some points of synergy (Table 2). It is essential to highlight that even additive effects in drug associations can represent an advantage to patients, as reduced doses of each drug can prevent toxicity and side effects, achieving the same outcome and decreasing the probability of induction of resistance (26, 27).
For in vivo studies, we used two distinct protocols. First, we tested a longer treatment (21 days), with only one administration per day. Second, we opted for a shorter therapy (5 days) with two doses per day, as proposed by Katsuno et al. (28). Although the longer treatment with the binary combination of itraconazole and ezetimibe had been more effective than each drug in reducing the parasite burden in the spleen at the highest dose, it also showed signs of liver toxicity. Ezetimibe is considered a safe drug, but there may be cases of hepatotoxicity (29, 30). Cases of hepatotoxicity have also been reported using ergosterol biosynthesis inhibitors (ketoconazole, fluconazole, and itraconazole) (31). Treatment with ketoconazole and itraconazole for 28 days demonstrated hepatotoxicity, increasing ALT and AST levels (32). Thus, for the ternary therapy, we adopted the shorter protocol. In this case, we tested two forms of drug suspensions: purified water only (see Fig. S5 in the supplemental material) or a formulation commercially developed to suspend powdered drugs, Ora-Plus. In both assays, the ternary therapy reduced the parasite burden. However, when we used Ora-Plus for preparing the suspension, the results were more impressive (Fig. 5), reaching 98% parasite suppression and suggesting that better homogenization of the drugs can reflect better absorption and efficacy of the ternary treatment (33–36). These findings indicate that using a suitable vehicle that results in a stable formulation may influence the treatment’s efficacy (37). Ora-Plus consists of a synergistic blend of suspending agents with a high degree of colloidal activity. The suspending agents form a structured gel-like matrix that suspends particles and retards settling. Some studies describe the stability of different drugs with the use of Ora-Plus, including resuspended ketoconazole (38, 39).
Here, we show the activity of ezetimibe in L. infantum in vitro and in infected mice for the first time. Moreover, our results suggest that a short-course ternary treatment composed of low doses of miltefosine, itraconazole, and ezetimibe is a promising therapeutic alternative for the treatment of visceral leishmaniasis. Further efforts are necessary to evaluate if the mechanism of action of ezetimibe in this model involves sterol metabolism, as seen previously in L. amazonensis.
MATERIALS AND METHODS
Parasites.
The Leishmania infantum (MHOM/MA/67/ITMAP-263) strain used in this study is a well-established strain that was kindly provided by the Institute of Molecular and Cellular Biology, Porto University, Portugal. This strain was used in the in vitro and in vivo experiments. Promastigotes of L. infantum were cultivated at 26°C in Schneider’s insect medium (Sigma-Aldrich Corp., St. Louis, MO, USA) supplemented with 20% fetal bovine serum (SFB), streptomycin (100 μg/ml), and penicillin (100 U/ml). Parasites were maintained by serial passaging twice per week until the fifth passage.
Drugs.
Ezetimibe (EZ) (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) was provided by Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Itraconazole (ITR) and miltefosine (MT) were provided by Sigma-Aldrich Corp. (St. Louis, MO, USA). Stocks of the drugs were dissolved at 10 mM in dimethyl sulfoxide (DMSO) or phosphate-buffered saline (PBS) and stored at −20°C.
Cytotoxicity assay.
Peritoneal macrophages from BALB/c mice were seeded at 1 × 106 cells/ml in 200 μl of supplemented RPMI 1640 into 96-well plates at 37°C in 5% CO2 for 4 h for adherence. Next, the plates were gently washed two times with PBS (150 mM NaCl, 20 mM phosphate buffer [pH 7.2]) to remove nonadherent cells and treated with ITR (0 to 3 μM), EZ (0 to 30 μM), or MT (0 to 2 μM) alone or in combination. No treatment of macrophages represented the control. For combination experiments, a modified fixed-ratio method was used (22, 40, 41). Briefly, the previously determined 50% effective concentration (EC50) value of each drug was used to choose the concentration of the individual drugs in the fixed-ratio associations. The solutions were prepared in proportions of 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5 of drugs (ITR to EZ), where each proportion was diluted (base 2) four times to calculate the 50% cytotoxic concentration (CC50). The 5:0 ratio corresponds to the highest concentration of ITR (3 μM), and the 0:5 ratio corresponds to the highest concentration of ezetimibe (30 μM). For the ternary combinations (ITR-EZ-MT), the proportions used were 4:1:1, 3:2:2, 2:3:3, 1:4:4, 4:1:4, 3:2:3, 2:3:2, 1:4:1, and 0:0:5. The 0:0:5 ratio corresponds to the maximal concentration of miltefosine alone (2 μM). As described above, each proportion was diluted (base 2) four times. Cytotoxicity experiments in uninfected macrophages were also performed for the combination of itraconazole plus ezetimibe at proportions of 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5. The ternary combination (itraconazole plus ezetimibe and miltefosine) was further evaluated at proportions of 4:1:1, 3:2:2, 2:3:3, 1:4:4, 4:1:4, 3:2:3, 2:3:2, and 1:4:1. The exact concentration values, according to the proportions, are shown in Table 3. Here, because the drugs have different potencies, we worked with proportions, where “5” represents the highest concentration of the drug and “1” represents the lowest concentration. Each proportion was diluted (base 2) four times to calculate the CC50.
TABLE 3.
Concentrations used for drug combinations
| Proportiona | Concn (μM) |
||
|---|---|---|---|
| Itraconazole | Ezetimibe | Miltefosine | |
| 5 | 3 | 30 | 2 |
| 4 | 2.4 | 24 | 1.6 |
| 3 | 1.8 | 18 | 1.2 |
| 2 | 1.2 | 12 | 0.8 |
| 1 | 0.6 | 6 | 0.4 |
“5” represents the highest concentration of the drug, and “1” represents the lowest concentration.
Next, the cells were incubated with drugs for 72 h, and a resazurin solution (50 μM) was added for an additional 4 h. Subsequently, fluorescence was measured using wavelengths of 560 nm for excitation and 590 nm for detection (Molecular Devices, San Jose, CA, USA) (42, 43). The results are expressed as the percentage of viable cells compared to that for the control cells treated with the highest DMSO dose used to dissolve the drugs. The CC50 was calculated using GraphPad Prism 6.0 software.
Antiamastigote activity.
Murine peritoneal macrophages were collected and plated at a density of 1 × 106 cells/ml into 24-well plates (Costar, Corning, NY) containing glass coverslips and supplemented with RPMI 1640. Promastigotes of L. infantum (5 × 106 cells/ml) at the stationary phase were added to adherent cells and incubated for 4 h at 37°C in 5% CO2. Next, the plates were washed with warm PBS, and the infected macrophages were incubated with ITR, EZ, or MT alone or in combination at 37°C for 72 h. The drugs were prepared in proportions as described above for the cytotoxicity assay. After incubation, the glass coverslips were stained using the Instant Prov hematological dye system (Newprov, Curitiba, Brazil), and the infection rate was determined by counting under a light microscope. The infection rate was calculated using the following formula: % infected macrophages × number of amastigotes/number of total macrophages. The 50% inhibitory concentration (IC50) was calculated by nonlinear regression using GraphPad Prism 6.0 software. Each point was tested in triplicate with three biological replicates. The fractional inhibitory concentration index (FICI) for the analysis of synergy was calculated as follows: ΣFICI = (IC50 of drug A in combination/IC50 of drug A alone) + (IC50 of drug B in combination/IC50 of drug B alone) + (IC50 of drug C in combination/IC50 of drug C alone). The interpretation of the FICI, according to published guidelines, is synergy at a FICI of ≤0.5, antagonism at a FICI of >4.0, and “no interaction” or an additive effect at a FICI of 0.5 to 4.0 (44).
Experimental animals and ethics statement.
Female BALB/c mice (6 to 8 weeks old) were obtained from the Institute of Science and Technology in Biomodels (ICTB-FIOCRUZ). Mice were housed at 5 mice per cage and maintained under standard environmental conditions. All of the animal procedures used in this study were performed according to the recommendations of the Ethics Committee for Animal Use of the Institute Oswaldo Cruz (CEUA-FIOCRUZ) (license number L-026/2015).
Mouse infection and treatment.
Mouse infection and treatment were performed using two protocols. In protocol 1, BALB/c mice were infected intraperitoneally with 1.0 × 108 stationary-phase L. infantum promastigotes. After 7 days of infection, animals were treated by the oral route daily for 21 days, according to the dosages described below (17). In protocol 2, BALB/c mice were infected as described above, and after 7 days, they were treated by the oral route for 5 days with a 12-h interval, according to the dosages described below (9, 28). In both protocols, at 28 days postinfection, the animals were euthanized; the spleen and liver were aseptically removed, weighed, and homogenized in supplemented Schneider’s medium; and the parasite load was estimated by a limiting dilution assay (LDA) (9, 43, 45). The animals were treated with different doses of ITR, EZ, or MT alone or in combination. The dosages of miltefosine, ezetimibe, and itraconazole were chosen based on our and others’ previous studies (9, 17, 24, 46). Parasite load and toxicological analyses were performed. Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol (CHO), urea (URE), creatinine (CREA), and total bilirubin (BILT) were measured enzymatically by the Program of Technological Development in Tools for Heath (PDTIS-FIOCRUZ).
Therapeutic schemes.
Two therapeutic schemes were used. First, using protocol 1, drugs were suspended in MilliQ water (water suspension), and mice were treated by the oral route daily for 21 days with MT (15.4 mg/kg) alone and EZ (10 mg/kg or 20 mg/kg) or ITR (100 mg/kg or 200 mg/kg) alone or in combination by the oral route. Animals were separated into groups, including control noninfected and nontreated (group 0), control infected and treated with the vehicle only (MilliQ water) (group 1), and groups corresponding to infected mice, as follows: MT at 15.4 mg/kg (group 2), EZ at 10 mg/kg (group 3), EZ at 20 mg/kg (group 4), ITR at 100 mg/kg (group 5), ITR at 200 mg/kg (group 6), EZ at 10 mg/kg plus ITR at 100 mg/kg (group 7), EZ at 10 mg/kg plus ITR at 200 mg/kg (group 8), EZ at 20 mg/kg plus ITR at 100 mg/kg (group 9), and EZ at 20 mg/kg plus ITR at 200 mg/kg (group 10).
In the second scheme, using protocol 2, two oral formulations were evaluated. Drugs were suspended in MilliQ water or added to an oral formulation for drug suspension named Ora-Plus (Perrigo, Minneapolis, MN, USA), as indicated in each case. Ora-Plus is a vehicle used for the extemporaneous compounding of oral suspensions. According to the data sheet, Ora-Plus contains purified water, microcrystalline cellulose, sodium carboxymethylcellulose, xanthan gum, and carrageenan as suspending agents; calcium sulfate, sodium phosphate, and citric acid as buffering agents; simethicone as an antifoaming agent; and potassium sorbate and methylparaben as preservatives. Mice were treated twice a day for 5 days with EZ (10 mg/kg or 20 mg/kg), ITR (100 mg/kg or 200 mg/kg), and MT (3.85 mg/kg or 7.7 mg/kg) alone or in combination by the oral route. Animals were divided into groups, including control noninfected and nontreated (group 0), control infected and treated with the vehicle only (group 1), and groups corresponding to infected mice, as follows: MT at 3.85 mg/kg (group 2), MT at 7.7 mg/kg (group 3), EZ at 10 mg/kg (group 4), EZ at 20 mg/kg (group 5), ITR at 100 mg/kg (group 6), ITR at 200 mg/kg (group 7), EZ at 10 mg/kg plus ITR at 100 mg/kg (group 8), EZ at 20 mg/kg plus ITR at 100 mg/kg (group 9), EZ at 10 mg/kg plus ITR at 200 mg/kg (group 10), EZ at 20 mg/kg plus ITR at 200 mg/kg (group 11), MT at 3.85 mg/kg plus EZ at 10 mg/kg and ITR at 100 mg/kg (group 12), and MT at 3.85 mg/kg plus EZ at 20 mg/kg and ITR at 200 mg/kg (group 13). Each group was composed of at least five mice, and the experiment was repeated two times independently.
Statistical analysis.
The results are expressed as the means ± standard errors of the means (SEM). The differences among groups were analyzed by using GraphPad Prism 6.0 software. The data were analyzed by nonparametric Student’s t test with Wilcoxon-Mann-Whitney analysis to calculate the significant differences, and P values of ≤0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grants E-26/010.101087/2018, E-26/202.348/2017, and E-26/202.918/2018.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D. 2016. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis 10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burza S, Croft SL, Boelaert M. 2018. Leishmaniasis. Lancet 392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarty J, Sundar S. 2019. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother 20:1251–1265. doi: 10.1080/14656566.2019.1609940. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, Andrade CA. 2011. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop 118:87–96. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 5.de Carvalho IPSF, Peixoto HM, Romero GAS, de Oliveira MRF. 2019. Treatment for human visceral leishmaniasis: a cost‐effectiveness analysis for Brazil. Trop Med Int Health 24:1064–1077. doi: 10.1111/tmi.13284. [DOI] [PubMed] [Google Scholar]
- 6.Georgiadou SP, Makaritsis KP, Dalekos GN. 2015. Leishmaniasis revisited: current aspects on epidemiology, diagnosis and treatment. J Transl Int Med 3:43–50. doi: 10.1515/jtim-2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deep DK, Singh R, Bhandari V, Verma A, Sharma V, Wajid S, Sundar S, Ramesh V, Dujardin JC, Salotra P. 2017. Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress. PLoS Negl Trop Dis 11:e0005641. doi: 10.1371/journal.pntd.0005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seifert K, Munday J, Syeda T, Croft SL. 2011. In vitro interactions between sitamaquine and amphotericin B, sodium stibogluconate, miltefosine, paromomycin and pentamidine against Leishmania donovani. J Antimicrob Chemother 66:850–854. doi: 10.1093/jac/dkq542. [DOI] [PubMed] [Google Scholar]
- 9.Rebello KM, Andrade-Neto VV, Gomes CRB, de Souza MVN, Branquinha MH, Santos ALS, Torres-Santos EC, d’Avila-Levy CM. 2019. Miltefosine-lopinavir combination therapy against Leishmania infantum infection: in vitro and in vivo approaches. Front Cell Infect Microbiol 9:229. doi: 10.3389/fcimb.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahajan R, Das P, Isaakidis P, Sunyoto T, Sagili KD, Lima MA, Mitra G, Kumar D, Pandey K, Van Geertruyden J-P, Boelaert M, Burza S. 2015. Combination treatment for visceral leishmaniasis patients coinfected with human immunodeficiency virus in India. Clin Infect Dis 61:1255–1262. doi: 10.1093/cid/civ530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diro E, Blesson S, Edwards T, Ritmeijer K, Fikre H, Admassu H, Kibret A, Ellis SJ, Bardonneau C, Zijlstra EE, Soipei P, Mutinda B, Omollo R, Kimutai R, Omwalo G, Wasunna M, Tadesse F, Alves F, Strub-Wourgaft N, Hailu A, Alexander N, Alvar J. 2019. A randomized trial of AmBisome monotherapy and AmBisome and miltefosine combination to treat visceral leishmaniasis in HIV co-infected patients in Ethiopia. PLoS Negl Trop Dis 13:e0006988. doi: 10.1371/journal.pntd.0006988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser M, Mäser P, Tadoori LP, Ioset J-R, Brun R. 2015. Antiprotozoal activity profiling of approved drugs: a starting point toward drug repositioning. PLoS One 10:e0135556. doi: 10.1371/journal.pone.0135556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ. 2003. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol Biochem Parasitol 126:129–142. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 14.El-Sayed M, Anwar AE. 2010. Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: a comparative study. J Eur Acad Dermatol Venereol 24:335–340. doi: 10.1111/j.1468-3083.2009.03417.x. [DOI] [PubMed] [Google Scholar]
- 15.Rashid JR, Wasunna KM, Gachihi GS, Nyakundi PM, Mbugua J, Kirigi G. 1994. The efficacy and safety of ketoconazole in visceral leishmaniasis. East Afr Med J 71:392–395. [PubMed] [Google Scholar]
- 16.Nassiri-Kashani M, Firooz A, Khamesipour A, Mojtahed F, Nilforoushzadeh M, Hejazi H, Bouzari N, Dowlati Y. 2005. A randomized, double-blind, placebo-controlled clinical trial of itraconazole in the treatment of cutaneous leishmaniasis. J Eur Acad Dermatol Venereol 19:80–83. doi: 10.1111/j.1468-3083.2004.01133.x. [DOI] [PubMed] [Google Scholar]
- 17.Andrade-Neto VV, Cunha-Júnior EF, do Canto-Cavalheiro MM, Atella GC, Fernandes TDA, Costa PRR, Torres-Santos EC. 2016. Antileishmanial activity of ezetimibe: inhibition of sterol biosynthesis, in vitro synergy with azoles, and efficacy in experimental cutaneous leishmaniasis. Antimicrob Agents Chemother 60:6844–6852. doi: 10.1128/AAC.01545-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joice AC, Yang S, Farahat AA, Meeds H, Feng M, Li J, Boykin DW, Wang MZ, Werbovetz KA. 2018. Antileishmanial efficacy and pharmacokinetics of DB766-azole combinations. Antimicrob Agents Chemother 62:e01129-17. doi: 10.1128/AAC.01129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade-Neto VV, Cunha-Junior EF, Dos Santos Faioes V, Pereira TM, Silva RL, Leon LL, Torres-Santos EC. 2018. Leishmaniasis treatment: update of possibilities for drug repurposing. Front Biosci (Landmark Ed) 23:967–996. doi: 10.2741/4629. [DOI] [PubMed] [Google Scholar]
- 20.de Macedo-Silva ST, Urbina JA, de Souza W, Rodrigues JCF. 2013. In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis. PLoS One 8:e83247. doi: 10.1371/journal.pone.0083247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepesheva GI, Friggeri L, Waterman MR. 2018. CYP51 as drug targets for fungi and protozoan parasites: past, present and future. Parasitology 145:1820–1836. doi: 10.1017/S0031182018000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinconi CT, Reimão JQ, Coelho AC, Uliana SRB. 2016. Efficacy of tamoxifen and miltefosine combined therapy for cutaneous leishmaniasis in the murine model of infection with Leishmania amazonensis. J Antimicrob Chemother 71:1314–1322. doi: 10.1093/jac/dkv495. [DOI] [PubMed] [Google Scholar]
- 23.Hart DT, Lauwers WJ, Willemsens G, Bossche HV, Opperdoes FR. 1989. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol Biochem Parasitol 33:123–134. doi: 10.1016/0166-6851(89)90026-1. [DOI] [PubMed] [Google Scholar]
- 24.Shakya N, Sane SA, Gupta S. 2011. Antileishmanial efficacy of fluconazole and miltefosine in combination with an immunomodulator—picroliv. Parasitol Res 108:793–800. doi: 10.1007/s00436-010-2230-2. [DOI] [PubMed] [Google Scholar]
- 25.Shakya N, Sane SA, Vishwakarma P, Bajpai P, Gupta S. 2011. Improved treatment of visceral leishmaniasis (kala-azar) by using combination of ketoconazole, miltefosine with an immunomodulator—Picroliv. Acta Trop 119:188–193. doi: 10.1016/j.actatropica.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Trinconi CT, Reimão JQ, Yokoyama-Yasunaka JKU, Miguel DC, Uliana SRB. 2014. Combination therapy with tamoxifen and amphotericin B in experimental cutaneous leishmaniasis. Antimicrob Agents Chemother 58:2608–2613. doi: 10.1128/AAC.01315-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun W, Sanderson PE, Zheng W. 2016. Drug combination therapy increases successful drug repositioning. Drug Discov Today 21:1189–1195. doi: 10.1016/j.drudis.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsuno K, Burrows JN, Duncan K, Hooft van Huijsduijnen R, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby BT. 2015. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 14:751–758. doi: 10.1038/nrd4683. [DOI] [PubMed] [Google Scholar]
- 29.Crespo-Leiro MG, Paniagua MJ, Marzoa R, Grille Z, Naya C, Flores X, Rodriguez JA, Mosquera V, Franco R, Castro-Beiras A. 2008. The efficacy and safety of ezetimibe for treatment of dyslipidemia after heart transplantation. Transplant Proc 40:3060–3062. doi: 10.1016/j.transproceed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Bhardwaj SS, Chalasani N. 2007. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis 11:597–613. doi: 10.1016/j.cld.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoza S, Moyo I, Ncube D. 2017. Comparative hepatotoxicity of fluconazole, ketoconazole, itraconazole, terbinafine, and griseofulvin in rats. J Toxicol 2017:6746989. doi: 10.1155/2017/6746989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. 2017. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 16:149–165. doi: 10.1080/14740338.2017.1270264. [DOI] [PubMed] [Google Scholar]
- 33.Stegemann S, Leveiller F, Franchi D, de Jong H, Lindén H. 2007. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci 31:249–261. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 34.Bandyopadhyay S, Katare OP, Singh B. 2012. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf B Biointerfaces 100:50–61. doi: 10.1016/j.colsurfb.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Dixit RP, Nagarsenker MS. 2008. Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci 35:183–192. doi: 10.1016/j.ejps.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Helin-Tanninen M, Autio K, Keski-Rahkonen P, Naaranlahti T, Järvinen K. 2012. Comparison of six different suspension vehicles in compounding of oral extemporaneous nifedipine suspension for paediatric patients. Eur J Hosp Pharm 19:432–437. doi: 10.1136/ejhpharm-2012-000159. [DOI] [Google Scholar]
- 37.Savjani KT, Gajjar AK, Savjani JK. 2012. Drug solubility: importance and enhancement techniques. ISRN Pharm 2012:195727. doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis PO, Cluck DB, Huffman JD, Ogle AP, Brown SD. 2017. Stability of a pyrimethamine suspension compounded from bulk powder. Am J Health Syst Pharm 74:2060–2064. doi: 10.2146/ajhp160551. [DOI] [PubMed] [Google Scholar]
- 39.Allen LV, Erickson MA. 1996. Stability of ketoconazole, metolazone, metronidazole, procainamide hydrochloride, and spironolactone in extemporaneously compounded oral liquids. Am J Health Syst Pharm 53:2073–2078. doi: 10.1093/ajhp/53.17.2073. [DOI] [PubMed] [Google Scholar]
- 40.Fivelman QL, Adagu IS, Warhurst DC. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother 48:4097–4102. doi: 10.1128/AAC.48.11.4097-4102.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrickx S, Van den Kerkhof M, Mabille D, Cos P, Delputte P, Maes L, Caljon G. 2017. Combined treatment of miltefosine and paromomycin delays the onset of experimental drug resistance in Leishmania infantum. PLoS Negl Trop Dis 11:e0005620. doi: 10.1371/journal.pntd.0005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulshrestha A, Bhandari V, Mukhopadhyay R, Ramesh V, Sundar S, Maes L, Dujardin JC, Roy S, Salotra P. 2013. Validation of a simple resazurin-based promastigote assay for the routine monitoring of miltefosine susceptibility in clinical isolates of Leishmania donovani. Parasitol Res 112:825–828. doi: 10.1007/s00436-012-3212-3. [DOI] [PubMed] [Google Scholar]
- 43.Cunha-Júnior EF, Andrade-Neto VV, Lima ML, da Costa-Silva TA, Galisteo Junior AJ, Abengózar MA, Barbas C, Rivas L, Almeida-Amaral EE, Tempone AG, Torres-Santos EC. 2017. Cyclobenzaprine raises ROS levels in Leishmania infantum and reduces parasite burden in infected mice. PLoS Negl Trop Dis 11:e0005281. doi: 10.1371/journal.pntd.0005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 45.Buffet PA, Sulahian A, Garin YJ, Nassar N, Derouin F. 1995. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother 39:2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangneux JP, Dullin M, Sulahian A, Garin YJ, Derouin F. 1999. Experimental evaluation of second-line oral treatments of visceral leishmaniasis caused by Leishmania infantum. Antimicrob Agents Chemother 43:172–174. doi: 10.1128/AAC.43.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.