Isavuconazole (ISA) is an azole antifungal used in the treatment of invasive aspergillosis and mucormycosis. Patients with mild or moderate hepatic impairment have lower clearance (CL) than the healthy population.
KEYWORDS: isavuconazole, physiologically based pharmacokinetic model, modeling, simulation, hepatic impairment
ABSTRACT
Isavuconazole (ISA) is an azole antifungal used in the treatment of invasive aspergillosis and mucormycosis. Patients with mild or moderate hepatic impairment have lower clearance (CL) than the healthy population. Currently, there are no data on ISA in patients with severe hepatic impairment (Child-Pugh class C). The purposes of this study were to build a physiologically based pharmacokinetic (PBPK) model to describe the pharmacokinetics (PK) of intravenous ISA and to predict changes in ISA disposition in different patient populations and in patients with hepatic impairment so as to guide personalized dosing. By incorporating the systemic and drug-specific parameters of ISA, the model was initially developed in a healthy population and was validated with 10 independent PK profiles obtained from healthy subjects and from patients with normal liver function. The results showed satisfactory predictive capacity; most of the relative predictive errors were within ±30% for the area under the concentration-time curve (AUC) and the maximum concentration of the drug in serum (Cmax). The observed concentration-time profiles of ISA in plasma were well described by the model-predicted profiles. The model adequately predicted the reduced CL of ISA in patients with mild or moderate hepatic impairment. Furthermore, the model predicted a decrease in CL of about 60% in patients with severe hepatic impairment. Therefore, we recommend reducing the dose by 50% in patients with severe hepatic impairment. The model also predicted differences in the PK of ISA between Caucasian and Asian populations, with a Chinese/Caucasian CL ratio of 0.67. The PBPK model of ISA that was developed provides a reasonable approach for optimizing the dosage regimen in different ethnic populations and in patients with severe hepatic impairment.
INTRODUCTION
Isavuconazole (ISA), an azole antifungal used in the treatment of invasive aspergillosis and mucormycosis, was approved in 2015 by the U.S. Food and Drug Administration (FDA) and the Europe Medicines Agency (EMA) (1, 2). In comparison with other triazoles, such as itraconazole or voriconazole, the prodrug of ISA, isavuconazonium sulfate, has high solubility, and solubilizing agents such as cyclodextrin, which are nephrotoxic, are not used in the intravenous (i.v.) formulation (3, 4). Its long half-life allows ISA to be given once daily (5). The absolute bioavailability of oral ISA is reported to be 98%, which makes the intravenous and oral formulations of ISA interchangeable (6). ISA has broad-spectrum coverage and can be used for prophylaxis or treatment of fungal infections caused by yeasts and molds, including fungi of the order Mucorales (4, 7, 8). It is a moderate cytochrome P450 3A (CYP3A) inhibitor with relatively weak drug-drug interaction potential (9, 10) and hence can used in different patient populations without drug interaction concerns.
The pharmacokinetics (PK) of intravenous ISA have been extensively studied in healthy volunteers (3, 11–13), in patients with hepatic impairment (14) and renal impairment (15), in acute myeloid leukemia (AML) patients with neutropenia (16), and in solid-organ transplantation (SOT) recipients (17). Several population pharmacokinetic (pop PK) models have been used to evaluate the effects of various demographic and clinical factors on the disposition of ISA (18–22). Both PK and pop PK studies have shown that mild or moderate hepatic impairment decreases the clearance (CL) of ISA from that for healthy subjects (14, 19). However, there are no reports on the impact of severe hepatic impairment (Child-Pugh class C) on ISA pharmacokinetics. The Child-Pugh score is determined by scoring five clinical measures of liver disease. A score of 1, 2, or 3 (from least to most severe) is given to each measure. All scores are added, and the sum is the Child-Pugh score. Class C has total scores of 10 to 15, indicating the most severe liver disease (23). In addition, the CL of ISA has been reported to be higher in Caucasians than in Asians (18).
Considering the complexity of patients in the clinic, it is necessary to understand various factors that can alter the PK of drugs in different patient populations. The physiologically based pharmacokinetic (PBPK) model is a novel approach that may provide some insight into how demographic and clinical factors can alter the PK profile of ISA in different patient populations (24–27). A PBPK analysis that incorporates the drug properties, physiology, and anatomy of human beings uses modeling and simulation to mechanistically describe the disposition of a drug in the body (27, 28). The effects of physiological and biochemical changes in different healthy populations and in patients with different pathological conditions can be quantitatively predicted using PBPK modeling. The results of PBPK predictions are used to support clinical studies and to make dosing recommendations for patients in product labeling (24, 29–31). The aim of the current study was to develop a PBPK model that could predict the PK behavior of ISA in patients with fungal infections, especially those with severe liver disease, and to provide an approach to guide the rational use of ISA in clinical practice.
RESULTS
Model development and validation in healthy subjects.
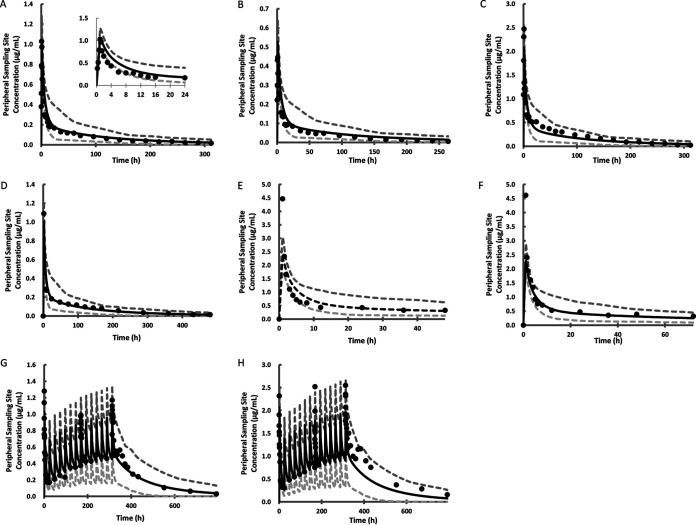
All parameters used to develop the PBPK model of ISA are listed in Table 1. Tissue-to-plasma partition coefficients (Kp) in 12 organs or tissues exceeded 1, with the highest predicted Kp values in adipose tissue, brain, and liver. Figure 1A shows the predicted PK profile for 50 mg ISA given intravenously over a duration of 1 h. A visual predictive check (VPC) showed that the predicted concentration-time points overlapped the observed points (12). The relative predictive errors, expressed as percentages (PE%), of the area under the concentration-time curve (AUC) and the maximum concentration of the drug in serum (Cmax) were 27.6% and 18.2%, respectively (Fig. 2). Seven data sets from healthy subjects (11, 12, 14, 15), including five single-dose regimens and two multiple-dose regimens, were used for verification. The doses ranged from 50 mg to 200 mg (Table 2). The results are shown in Fig. 1B to H. The multiple-dose studies lasted 14 days, and the Cmax and AUC on days 1, 8, and 14 were reported. PE% of Cmax and AUC were between –30% and +30% for all the validation sets, with the three exceptions for AUC and Cmax shown in Fig. 2.
TABLE 1.
Parameters used to develop the PBPK model of ISA
| Parametera | Input value | Prediction method or reference |
|---|---|---|
| Physicochemical and blood binding data | ||
| MW (g/mol) | 437.47 | 39 |
| Compound type | Ampholyte | 39 |
| pKa 1 | 12.59 | 39 |
| pKa 2 | 2.32 | 39 |
| Log Po:w | 4.14 | 39 |
| B/P | 0.55 | 3 |
| fu in plasma | 0.01 | 12, 34 |
| Distribution model | Full PBPK | |
| Vss (liters/kg) | 5.20 | Method 3 |
| Tissue-to-plasma partition coefficients (Kp) | Method 3 | |
| Adipose tissue | 14.64 | |
| Bone | 1.584 | |
| Brain | 8.778 | |
| Gut | 3.254 | |
| Heart | 2.609 | |
| Kidney | 2.707 | |
| Liver | 4.182 | |
| Lung | 1.438 | |
| Muscle | 1.462 | |
| Skin | 2.236 | |
| Spleen | 3.172 | |
| Pancreas | 2.086 | |
| Kp scalar | 1 | |
| Elimination model | Enzyme kinetics | |
| CLint (μl/min/pmol) of CYP3A4 | 0.609 | Retrograde model |
| fumic | 1 | Retrograde model |
| CLint (bile) (μl/min/106 cells) | 5.011 | 3 |
| CLR (liters/h) | 0.016 | 15 |
B/P, blood-to-plasma partition coefficient; fu, unbound drug fraction; log Po:w, logarithm of the octanol-to-water partition coefficient; MW, molecular weight; pKa, negative logarithm of the acid dissociation constant; CLint, in vitro intrinsic clearance; CYP, cytochrome P450; fumic, fraction of unbound drug in the in vitro microsomal incubation; CLint (bile), biliary clearance; CLR, renal clearance. Vss, volume of distribution at steady state, predicted by method 3 (membrane potential model).
FIG 1.
Predicted and observed concentration-time profiles following intravenous administration of ISA to healthy volunteers. (A) Plot of the developed model with a single dose of 50 mg intravenous ISA compared with the observed data from reference 12. (Inset) The plot for the first 24 hours. (B and C) Validation plots with a single dose of 100 mg (B) or 200 mg (C) intravenous ISA compared with the observed data from reference 12. (D) Validation plot with a single dose of 100 mg intravenous ISA compared with the observed data from reference 14. (E and F) Validation plots with a single dose of 200 mg intravenous ISA compared with the observed data in study part 1 (n = 8) (E) and study part 2 (n = 9) (F) from reference 15. (G) Validation plot with a daily dose of 50 mg intravenous ISA for 14 days (with a loading dose of 100 mg on day 1) compared with the observed data from reference 11. (H) Validation plot with a daily dose of 100 mg intravenous ISA for 14 days (with a loading dose of 200 mg on day 1) compared with the observed data from reference 11. In each graph, the middle (black) curve represents the simulated mean concentration-time profile, circles represent observed data, and the lower and upper (gray) curves show the simulated 5th and 95th percentiles, respectively.
FIG 2.
Comparison of observed with predicted PK parameters in each dosing regimen for healthy volunteers or patients (11, 12, 14, 16, 17). (A) Peak concentration of the drug in plasma (Cmax); (B) area under the concentration-time curve (AUC). SD, single dose; MD, multiple doses; PE%, relative predictive error, calculated as (Vpred – Vobs)/Vobs × 100%, where Vpred and Vobs are the simulated and observed mean Cmax or AUC values for each clinical trial. Each circle represents the observed value versus the predicted value. Solid lines represent a PE% of 0; left and right dashed lines represent PE% of +50% and –50%, respectively.
TABLE 2.
Clinical studies used in PBPK model development and verification
| Study type and no. | Subjects | Dose regimen (dose [mg], infusion time)b | No. of subjects (% female) | Age range (yr) | Reference |
|---|---|---|---|---|---|
| Single dose in healthy volunteers | |||||
| 1a | 6 White | 50, 1 h | 6 (0) | 21–45 | 12 |
| 2c | 6 White | 100, 1 h | 6 (0) | 21–45 | 12 |
| 3c | 6 White | 200, 1 h | 6 (0) | 21–45 | 12 |
| 4c | 16 White | 100, 2 h | 16 (25.0) | 42–56 | 14 |
| 5c | 8 White, 1 other | 200, 1 h | 9 (44.4) | 19–64 | 15 |
| 6c | 6 White, 2 Black | 200, 1 h | 8 (37.5) | 34–57 | 15 |
| Multiple doses in healthy volunteers | |||||
| 1c | 6 White | 100 q.d. × 1, 50 q.d. × 14, 1 h | 6 (0) | 21–45 | 11 |
| 2c | 6 White | 200 q.d. × 1, 100 q.d. × 14, 1 h | 6 (0) | 21–45 | 11 |
| Multiple doses in patients with normal hepatic function | |||||
| 1c | 11 White | 400, 200, 200 on day 1 (4 h), 200 b.i.d. on day 2 (2 h), 200 q.d. × 5 (2 h) | 11 (36.4) | 32–57 | 16 |
| 2c | 12 White | 800, 400, 400 on day 1 (4 h), 400 b.i.d. on day 2 (2 h), 400 q.d. × 5 (2 h) | 12 (16.7) | 24–62 | 16 |
| 3c | 26 White | 200 t.i.d. × 2, 200 q.d. × 4, 1 h | 26 (38) | 21–73 | 17 |
| Single dose in patients with hepatic impairment | |||||
| 1c,d | 12 White | 100, 2 h | 12 (25) | 45–59 | 14 |
| 2c,e | 12 White | 100, 2 h | 12 (25) | 45–59 | 14 |
Model development.
q.d., once a day; b.i.d., twice a day; t.i.d., three times a day.
Model verification.
Patients with mild hepatic impairment.
Patients with moderate hepatic impairment.
Prediction of ISA PK in patients without hepatic impairment.
Figure 3A to C shows three model validation plots for patients without hepatic impairment. The observed PK profiles were extracted from clinical studies conducted on AML patients with low-dose and high-dose regimens and on SOT patients with a standard dosing regimen (16, 17). Multiple doses were administered to the patients. Most of the observed data fell within the interval between the 5th and 95th percentiles. The predictive performance was satisfactory; the PE% of Cmax varied −11.02%, 15.83%, and −2.13%, while the PE% of the AUC varied −0.97%, 9.15%, and 8.33%, respectively (Fig. 2).
FIG 3.
Predicted and observed concentration-time profiles following intravenous ISA administration to patients. (A) Validation plot with multiple doses of ISA (400 mg, 200 mg, and 200 mg every 8 h [q8h] on day 1, 200 mg twice a day [b.i.d.] on day 2, 200 mg once a day [q.d.] for 5 days) compared with the data observed for AML patients from reference 16. (B) Validation plot with multiple doses of ISA (800 mg, 400 mg, and 400 mg q8h on day 1, 400 mg b.i.d. on day 2, and 400 mg q.d. for 5 days) compared with the data observed for AML patients from reference 16. (C) Validation plot with multiple doses of ISA (200 mg b.i.d. for 2 days, 200 mg q.d. for 4 days) compared with the data observed for SOT recipients from reference 17. (D and E) Validation plots with a single dose of 100 mg intravenous ISA compared with data observed for patients with mild (D) or moderate (E) hepatic impairment from reference 14. In each graph, the middle (black) curve represents the simulated mean concentration-time profile, circles represent observed data, and the lower and upper curves (gray) show the simulated 5th and 95th percentiles, respectively.
Prediction of ISA PK in patients with hepatic impairment.
The model was able to predict ISA PK in patients with various stages of hepatic impairment (Fig. 3D and E). The AUC from 0 to 480 h (AUC0–480) values after a single dose of 100 mg of ISA given by infusion over 2 h were 34.2, 41, and 56.9 μg/ml·h in healthy subjects and patients with mild or moderate hepatic impairment, respectively (Fig. 2). The relative predictive errors for Cmax in patients with mild or moderate hepatic impairment were −7.14% and −9.52%, respectively, and those for AUC were −27.30% and −15.83%, respectively (Fig. 2). No clinical ISA PK data are available for patients with severe hepatic impairment (14). Considering the predictive accuracy, we simulated the PK profile in patients with severe hepatic impairment and compared it to that in healthy volunteers. The results are shown in Fig. 4. The AUC0-480 and Cmax for healthy volunteers were 34.2 μg/ml·h and 1.01 μg/ml, while those for patients with severe hepatic impairment were 59.7 μg/ml·h and 0.63 μg/ml, respectively. The predicted CL at steady state in the virtual healthy population was 3.2 liters/h, and that in patients with severe hepatic impairment was 1.28 liters/h. CL decreased about 60% in patients with severe hepatic impairment from levels for the healthy population. Figure 5 shows the contribution of each elimination route to total CL after intravenous administration of ISA in healthy volunteers and in patients with hepatic impairment. The contribution of hepatic CL decreased with the severity of hepatic impairment, while the proportion of biliary CL increased to compensate for the reduction in metabolism. Hepatic CL in patients with severe hepatic impairment accounted for 56.4% of total CL, while the contribution of biliary CL increased to 42.9%.
FIG 4.
Comparison of PK profiles after a single dose of 100 mg intravenous ISA in healthy volunteers and patients with severe hepatic impairment. The demographic details of the subjects were matched with those from reference 14.
FIG 5.
Comparison of the contributions of different elimination routes to total clearance after a single dose of 100 mg intravenous ISA in healthy volunteers and patients with hepatic impairment. CYP, cytochrome P450. The demographic details of the subjects were matched with those from reference 14.
Prediction of ISA PK differs between Caucasian and Chinese populations.
With the model that was built, the PK of ISA in a healthy Chinese population after an infusion of 50 mg medication over 1 h was simulated. The demographic information was adjusted with that of a Caucasian population. The predicted CL ratio is 0.67 (CL is 3.69 liters/h for Caucasians and 2.49 liters/h for Chinese individuals) (Fig. 6).
FIG 6.

Comparison of PK profiles after the administration of a single dose of 100 mg intravenous ISA to healthy Caucasian and Chinese volunteers. The demographic details of the subjects were matched with those from reference 18. AUC0–inf, area under the concentration-time curve from 0 h to infinity; Cmax, peak plasma concentration; CL, clearance.
DISCUSSION
PBPK model-based predictions and simulations are particularly useful due to their abilities to provide new insight on the distribution, metabolism, and elimination of drugs in the absence of actual clinical data. To our knowledge, no PBPK model of ISA has been reported yet. First, we developed a robust PBPK model to predict the PK of intravenous ISA, and we validated it successfully by using data from seven clinical trials conducted on healthy volunteers in which a single dose or multiple doses of ISA were given across a dose range of 50 mg to 200 mg. The contributions of different elimination routes, including hepatic CL, biliary CL, and renal CL, were incorporated into the model. The distribution of the drug in 12 important organs or tissues was observed. Second, the PK of ISA in patients with normal hepatic function was simulated using the model that was built. The predicted PK profiles aligned well with the observed data after multiple doses of ISA in AML patients with neutropenia and in SOT recipients. The PE% of AUC and Cmax fell within ±30% of the corresponding PK parameters obtained from the clinical trials. Patients with mild or moderate hepatic impairment have lower CL of ISA, and this was verified with the model, with PE% within ±20%. The CL of ISA in patients with severe hepatic impairment was predicted to decrease about 60% from that in healthy volunteers, which has not been reported clinically. Therefore, it is reasonable to reduce the dose by half in patients with severe hepatic impairment.
The volume of distribution (V) of ISA was reported to be between 304 and 542 liters (11, 12, 14, 15), which indicated that the drug was distributed widely in the body. The average V was calculated to be 440 liters, or 5.67 liters/kg. With the full PBPK model built in the Simcyp simulator, V was predicted by using method 3 (the membrane potential model) (32–34). The predicted V was 5.20 liters/kg, which is similar to the reported value. The membrane potential model involves equilibrium of the unbound, un-ionized fraction of the drug between the intracellular and extracellular water at steady state, the binding of the drug to proteins within the extracellular water and to lipids present within the intracellular compartment, and a driving or limiting force of negative potential difference across the membrane for the passage of ionized drug into the polarized cells. The large Kp values in 12 organs or tissues, especially in adipose tissue, brain, and liver, explained the large V of 440 liters for ISA. An animal study using quantitative whole-body autoradiography showed that the highest Cmax values were observed in the bile and the liver. A species-specific distribution may explain this difference (35). According to a mass balance study conducted with 14C-labeled isavuconazonium sulfate in healthy volunteers, the ratios of Cmax and AUC radioactivity in the blood and plasma were 0.53 and 0.56, respectively, implying limited association of ISA with the erythrocytes. Therefore, the whole-blood-to-plasma partition coefficient (B/P) was set to be 0.55 in the model (3).
For the elimination component of the model, the enzyme kinetic parameter, the intrinsic hepatic clearance (CLint) of ISA metabolized by CYP3A4 (36), was predicted by the retrograde model (32). The CL reported in the literature ranged from 2.73 to 5.03 liters/h (11, 12, 14, 15), with a mean of 3.6 liters/h. A phase I trial performed with healthy adults to investigate the effect of renal function on the ISA PK showed that the amount of unchanged ISA excreted in the urine was 0.44% of the total dose. The mass balance study showed ISA to be eliminated via bile, accounting for approximately 15% of the dose. A mean of 91.6% of radioactivity was recovered in the urine and feces over a 600-h period after the oral dose (3). Considering the long half-life of ISA (mean value, 144 h) and a bioavailability of 98% from oral capsules (37), we assumed that all the leftover dose was metabolized by CYP3A4. Therefore, the proportions of ISA cleared via hepatic metabolism, the biliary pathway, and renal metabolism were predicted to be 85.8%, 13.6%, and 0.5%, respectively (Fig. 5). The values for intrinsic CL via CYP3A4 and bile were 0.609 μl/min/pmol and 5.01 μl/min/106 cells (Table 1).
PK predictions based on mathematical models can guide clinical medication dosage regimens for special populations, such as patients with renal or hepatic impairment (14). For ISA, with a renal clearance (CLR) of 0.016 liters/h, renal function is not expected to affect PK, and this was reverified by the model (data not shown) and the observed data (15). Our study focused mainly on the effect of the hepatic function on the disposition of ISA. Key features of patients with hepatic impairment are reduced hepatic CYP expression, decreased liver size, reduced protein binding by both albumin and α-1 acid glycoprotein, and altered blood flows, which have been modeled in the Simcyp population-based simulator. The mean CYP3A4 abundance and liver volume in healthy individuals and patients with severe hepatic impairment are 137 and 31 pmol/mg of protein and 1.651 and 1.007 liters, respectively. CLint is proportional to liver weight and enzyme abundance, which may decrease significantly based on in vitro-in vivo extrapolation (IVIVE) methods. The average concentration of albumin in male patients with severe hepatic impairment is 29.69 g/liter, which is much lower than that in healthy male subjects (50.34 g/liter). Blood flow through the hepatic artery is expressed as a percentage of cardiac output. In male patients with severe hepatic impairment, the value is 12.45%, higher than that for healthy male subjects (6.5%). The factors affecting hepatic CL (CLh) include hepatic blood flow (Q), CLint, and blood protein binding. CLh is predicted by the following equation in Simcyp:
where fu is the unbound fraction of the drug.
The prediction of AUC in healthy subjects and in patients with mild or moderate hepatic impairment is similar to the observed data (Fig. 2) (14). CL at steady state in healthy subjects and in patients with mild or moderate hepatic impairment was 3.20, 2.68, and 1.64 liters/h, respectively. CL was reduced by 16.3% and 48.8% in patients with mild or moderate hepatic disease, respectively. In the clinical trial, CL decreased by 47.6% and 23.5% in subjects with moderate hepatic impairment after intravenous and oral administration of ISA (14). Considering interpatient variability, the difference between the prediction and the observed CL is acceptable. From the clinical perspective, it is meaningful to understand the effect of severe hepatic impairment on the PK of ISA. With the model that was built, we predicted that CL decreased by 60% from that for healthy subjects (Fig. 4). The contribution of each elimination route to the total CL is shown in Fig. 5. The reduced proportion of CL due to hepatic impairment is compensated for by the biliary pathway. The contribution of biliary CL increased from 13.6% in healthy subjects to 42.9% in patients with severe hepatic impairment, which should alert the clinician to pay more attention to patients suffering from both reduced hepatic function and bile duct obstruction.
Pop PK analysis estimated CL in Asians to be approximately 36% lower than that in the Caucasian population (18). Asians have higher exposures than Caucasians due to lower CL of ISA. The differences in the PK of ISA between Caucasian and Asian populations can also be predicted adequately with the model (Fig. 6). CYP3A4 is the predominant enzyme responsible for the metabolism of ISA, accounting for about 85% of CL of the medication. In the model, enzyme abundances in healthy Caucasian and Chinese populations are 137 pmol/mg protein and 120 pmol/mg protein, respectively. Caucasians, on average, have larger liver volumes than Chinese people (1.65 liters versus 1.40 liters). IVIVE methods using enzyme-specific microsomal metabolism parameters and intersystem extrapolation factor (ISEF)-based estimates were used to extrapolate recombinant in vitro enzyme activities to in vivo CLint (38). Therefore, the higher CLint due to the larger liver volume and higher enzyme abundance in Caucasians may result in their higher total CL.
There are two alternate elimination models that can also predict the PK behavior of ISA in different populations. With in vivo intravenous clearance (CLiv) of 3.6 liters/h and CLR of 0.016 liter/h, or hepatic metabolic CL of 33.43 μl/min/106 cells (whole-organ metabolic CL pane in Simcyp simulator) and CLR of 0.016 liter/h, predicted PK profiles fit well with the observed profiles. However, due to the lack of biliary CL and enzyme kinetic parameters in the two models above, their abilities to extrapolate to special populations are limited.
ISA is given as a prodrug of isavuconazonium sulfate. No clinical data on the PK of isavuconazonium sulfate are available. In vivo, isavuconazonium sulfate is catalyzed to ISA rapidly and almost completely (99%) by esterases in plasma (3, 11). Therefore, we neglect the transient duration of hydrolysis in the model. The present study has some limitations. First, it is not appropriate to use PK data in subjects with AML to validate the predictive performance of the PBPK model in patients without hepatic impairment. Although subjects with AML were excluded if they demonstrated hepatic dysfunction (i.e., total bilirubin >3 times the upper limit of normal (ULN), an alanine aminotransferase or aspartate aminotransferase level >5 times the ULN) in the literature, the detailed information for each patient was not clear and explicit. Second, CYP3A4 is the main enzyme contributing to the metabolism of ISA, with a minor contribution from CYP3A5 (9, 39). Currently, we do not know the relative contributions of the two enzymes to the metabolism of ISA. CYP3A5 is not incorporated into the model that was built. More in vitro enzyme kinetic information is needed to improve the model. Finally, there is no clinical trial of ISA in patients with severe hepatic impairment; it is necessary to conduct a further PK study of ISA in this specific patient population in order to validate the predictability of the current PBPK model completely.
In conclusion, a PBPK model of intravenous ISA was established and validated by using data from 13 clinical trials. The dosing regimen covered a single dose and multiple doses over the dose range of 50 to 800 mg. Most of the observed concentrations fell within the 5th- to 95th-percentile interval of the predicted concentrations in plasma. Most of the PE% of AUC and Cmax were within ±30%. The model predicted a decrease in CL of about 60% in patients with severe hepatic impairment. Therefore, reduction of the dose by half is recommended for this special population. The model that was built had the capacity to predict PK behavior in patients without or with hepatic impairment and thus can be used to guide individualized dosage regimens in clinical practice. Further research will be done to predict the drug-drug interaction by incorporating the inhibitory constant into the model.
MATERIALS AND METHODS
Clinical pharmacokinetic studies and software.
PubMed was extensively searched for clinical PK studies of intravenous ISA with the search query ((("Pharmacokinetics"[Mesh]) OR (Drug Kinetics)) OR (Kinetics, Drug)) AND (((((("isavuconazole" [Supplementary Concept]) OR (BAL 8557)) OR (BAL8557)) OR (BAL-8557)) OR (isavuconazonium sulfate)) OR (Cresemba)). Thirteen clinical studies were used in the PBPK model for development and verification as shown in Table 2. WebPlotDigitizer (version 3.8) was used to obtain the concentration-time data from the articles. ISA PBPK model development and simulations were conducted using the Simcyp population-based simulator (version 17, release 1; Certara, Sheffield, UK).
PBPK model development and validation in healthy subjects.
Physicochemical and blood binding data for ISA, with parameters such as molecular weight, pKa, and B/P, were derived from the published papers (12, 35; https://www.drugbank.ca/drugs/DB11633). A full PBPK model was used in the distribution component due to the large volume of distribution (V) of ISA (24). Tissue-to-plasma partition coefficients (Kp) were predicted and used to calculate V by method 3 (membrane potential model) (32). The enzyme kinetic model was used in the elimination component. PK parameters that were not found in the literature were estimated by using the retrograde model, which was based on information about CLiv, the hepatic uptake factor, the elimination pathways, and blood protein binding. All parameters used to develop the PBPK model of ISA are listed in Table 1.
By incorporating the systemic and drug-specific parameters of ISA mentioned above, the PBPK model was initially developed using a data set obtained from healthy subjects who received 50 mg intravenous ISA (12). The simulated parameters and PK profile were compared with the observed clinical results. Seven naive PK data sets for healthy subjects were used for model validation (11, 12, 14, 15). One hundred virtual subjects divided into 10 equal groups were created for all PBPK simulations, and the anthropometric characteristics (i.e., age, gender) were matched with the published data. When the duration of the study exceeded 100 h, the sampling interval was set as 0.5 h; otherwise, default minimal samples of 200 were used.
To increase the predictive accuracy of Cmax at early time points after the infusion, the built-in modified Levitt arm “arterial” (LAA) model was used. Peripheral-site concentrations were compared with the observed values (40).
Prediction of ISA in patients without hepatic impairment.
The PBPK model that was built was applied to predict the PK of ISA in patients without hepatic impairment. The PK profile and parameters of AML patients and solid-organ transplant recipients were used for model validation (16, 17). Dosage regimens, such as dose, interval, and duration, were matched with those in the literature.
Prediction of ISA in patients with hepatic impairment.
The PBPK model that was developed was extrapolated to patients with hepatic impairment at different stages. PK profiles of ISA were predicted for patients with mild or moderate hepatic impairment who received 100 mg i.v. ISA, and the results were compared with the observed results (14). However, there is no clinical study of ISA in patients with severe hepatic impairment (Child-Pugh class C). Simulation of the PK profile was conducted in patients with severe hepatic impairment, and CL, AUC, and Cmax were compared with the observed data obtained from the healthy population.
Model evaluation.
The predicted PK profiles were compared with the observed data by VPC. The predictability of the built model was evaluated by comparing the predicted with the observed concentration-time profiles, and the 5th-to-95th-percentile intervals were computed to show the overall variability. To intuitively understand the results of model evaluation, relative predictive errors (PE%) were calculated for each parameter by the following equation:
where Vpred and Vobs stand for the predicted and observed parameters of ISA, respectively. Taking account of the clinical variation, a maximum PE% of ±50% was considered acceptable.
Data availability.
All data included in this study are available upon request to the corresponding author.
ACKNOWLEDGMENTS
We thank the Certara company for providing us with Simcyp simulator, an academic license, and necessary training.
This study was sponsored by the Fujian provincial health technology project (grant 2020CXB014) and Joint Funds for the innovation of science and technology, Fujian province (grant 2019Y9095).
There is no conflict of interest to declare.
REFERENCES
- 1.Ananda-Rajah MR, Kontoyiannis D. 2015. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol 10:693–708. doi: 10.2217/fmb.15.34. [DOI] [PubMed] [Google Scholar]
- 2.Desai AV, Kovanda LL, Hope WW, Andes D, Mouton JW, Kowalski DL, Townsend RW, Mujais S, Bonate PL. 2017. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother 61:e01034-17. doi: 10.1128/AAC.01034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend R, Kato K, Hale C, Kowalski D, Lademacher C, Yamazaki T, Akhtar S, Desai A. 2018. Two phase 1, open-label, mass balance studies to determine the pharmacokinetics of 14C-labeled isavuconazonium sulfate in healthy male volunteers. Clin Pharmacol Drug Dev 7:207–216. doi: 10.1002/cpdd.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miceli MH, Kauffman CA. 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 5.Chitasombat MN, Kontoyiannis DP. 2015. The 'cephalosporin era' of triazole therapy: isavuconazole, a welcomed newcomer for the treatment of invasive fungal infections. Expert Opin Pharmacother 16:1543–1558. doi: 10.1517/14656566.2015.1057500. [DOI] [PubMed] [Google Scholar]
- 6.Natesan SK, Chandrasekar PH. 2016. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist 9:291–300. doi: 10.2147/IDR.S102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis J, Ledoux MP, Nivoix Y, Herbrecht R. 2018. Isavuconazole: a new broad-spectrum azole. Part 1: in vitro activity. J Mycol Med 28:8–14. doi: 10.1016/j.mycmed.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Warrilow AGS, Parker JE, Price CL, Rolley NJ, Nes WD, Kelly DE, Kelly SL. 2019. Isavuconazole and voriconazole inhibition of sterol 14α-demethylases (CYP51) from Aspergillus fumigatus and Homo sapiens. Int J Antimicrob Agents 54:449–455. doi: 10.1016/j.ijantimicag.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Townsend R, Dietz A, Hale C, Akhtar S, Kowalski D, Lademacher C, Lasseter K, Pearlman H, Rammelsberg D, Schmitt-Hoffmann A, Yamazaki T, Desai A. 2017. Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev 6:44–53. doi: 10.1002/cpdd.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groll AH, Desai A, Han D, Howieson C, Kato K, Akhtar S, Kowalski D, Lademacher C, Lewis W, Pearlman H, Mandarino D, Yamazaki T, Townsend R. 2017. Pharmacokinetic assessment of drug-drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev 6:76–85. doi: 10.1002/cpdd.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:286–293. doi: 10.1128/AAC.50.1.286-293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt-Hoffmann A, Desai A, Kowalski D, Pearlman H, Yamazaki T, Townsend R. 2016. Isavuconazole absorption following oral administration in healthy subjects is comparable to intravenous dosing, and is not affected by food, or drugs that alter stomach pH. Int J Clin Pharmacol Ther 54:572–580. doi: 10.5414/CP202434. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt-Hoffmann A, Roos B, Spickermann J, Heep M, Peterfaí E, Edwards DJ, Stoeckel K. 2009. Effect of mild and moderate liver disease on the pharmacokinetics of isavuconazole after intravenous and oral administration of a single dose of the prodrug BAL8557. Antimicrob Agents Chemother 53:4885–4890. doi: 10.1128/AAC.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend RW, Akhtar S, Alcorn H, Berg JK, Kowalski DL, Mujais S, Desai AV. 2017. Phase I trial to investigate the effect of renal impairment on isavuconazole pharmacokinetics. Eur J Clin Pharmacol 73:669–678. doi: 10.1007/s00228-017-2213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. 2015. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother 59:2078–2085. doi: 10.1128/AAC.04569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Clancy CJ, Rivosecchi RM, Zhao W, Shields RK, Marini RV, Venkataramanan R, Nguyen MH. 2018. Pharmacokinetics of intravenous isavuconazole in solid-organ transplant recipients. Antimicrob Agents Chemother 62:e01643-18. doi: 10.1128/AAC.01643-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai A, Kovanda L, Kowalski D, Lu Q, Townsend R, Bonate PL. 2016. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to Aspergillus and other filamentous fungi. Antimicrob Agents Chemother 60:5483–5491. doi: 10.1128/AAC.02819-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai A, Schmitt-Hoffmann AH, Mujais S, Townsend R. 2016. Population pharmacokinetics of isavuconazole in subjects with mild or moderate hepatic impairment. Antimicrob Agents Chemother 60:3025–3031. doi: 10.1128/AAC.02942-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, Venkataramanan R, Rivosecchi RM, Tang C, Marini RV, Shields RK, Clancy CJ, Nguyen MH. 2019. Population pharmacokinetics of intravenous isavuconazole in solid-organ transplant recipients. Antimicrob Agents Chemother 64:e01728-19. doi: 10.1128/AAC.01728-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai AV, Han D, Kowalski DL, Lademacher C, Pearlman H, Yamazaki T. 2019. No dose adjustment for isavuconazole based on age or sex. Antimicrob Agents Chemother 63:e02629-18. doi: 10.1128/AAC.02629-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovanda LL, Desai AV, Lu Q, Townsend RW, Akhtar S, Bonate P, Hope WW. 2016. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL study). Antimicrob Agents Chemother 60:4568–4576. doi: 10.1128/AAC.00514-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. 1973. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Zhang H, Miah MK, Caritis SN, Venkataramanan R. 2020. Physiologically based pharmacokinetic approach can successfully predict pharmacokinetics of citalopram in different patient populations. J Clin Pharmacol 60:477–488. doi: 10.1002/jcph.1541. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang X, Lu C. 2016. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B 6:430–440. doi: 10.1016/j.apsb.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. 2015. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 43:1823–1837. doi: 10.1124/dmd.115.065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smits A, De Cock P, Vermeulen A, Allegaert K. 2019. Physiologically based pharmacokinetic (PBPK) modeling and simulation in neonatal drug development: how clinicians can contribute. Expert Opin Drug Metab Toxicol 15:25–34. doi: 10.1080/17425255.2019.1558205. [DOI] [PubMed] [Google Scholar]
- 28.Zhao P. 2017. Report from the EMA workshop on qualification and reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. CPT Pharmacometrics Syst Pharmacol 6:71–72. doi: 10.1002/psp4.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watt KM, Cohen-Wolkowiez M, Barrett JS, Sevestre M, Zhao P, Brouwer KLR, Edginton AN. 2018. Physiologically based pharmacokinetic approach to determine dosing on extracorporeal life support: fluconazole in children on ECMO. CPT Pharmacometrics Syst Pharmacol 7:629–637. doi: 10.1002/psp4.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmanshenn C, Scherholz M, Androulakis IP. 2016. Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine. J Pharmacokinet Pharmacodyn 43:481–504. doi: 10.1007/s10928-016-9492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsousi N, Desmeules JA, Rudaz S, Daali Y. 2017. Usefulness of PBPK modeling in incorporation of clinical conditions in personalized medicine. J Pharm Sci 106:2380–2391. doi: 10.1016/j.xphs.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Michelet R, Van Bocxlaer J, Allegaert K, Vermeulen A. 2018. The use of PBPK modeling across the pediatric age range using propofol as a case. J Pharmacokinet Pharmacodyn 45:765–785. doi: 10.1007/s10928-018-9607-8. [DOI] [PubMed] [Google Scholar]
- 33.Rodgers T, Leahy D, Rowland M. 2005. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci 94:1259–1276. doi: 10.1002/jps.20322. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers T, Rowland M. 2006. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95:1238–1257. doi: 10.1002/jps.20502. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt-Hoffmann AH, Kato K, Townsend R, Potchoiba MJ, Hope WW, Andes D, Spickermann J, Schneidkraut MJ. 2017. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 61:e01292-17. doi: 10.1128/AAC.01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellmann R, Smuszkiewicz P. 2017. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection 45:737–779. doi: 10.1007/s15010-017-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy MW, Moriyama B, Petraitiene R, Walsh TJ, Petraitis V. 2018. Clinical pharmacokinetics and pharmacodynamics of isavuconazole. Clin Pharmacokinet 57:1483–1491. doi: 10.1007/s40262-018-0673-2. [DOI] [PubMed] [Google Scholar]
- 38.Proctor NJ, Tucker GT, Rostami-Hodjegan A. 2004. Predicting drug clearance from recombinantly expressed CYPs: intersystem extrapolation factors. Xenobiotica 34:151–178. doi: 10.1080/00498250310001646353. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki T, Desai A, Han D, Kato K, Kowalski D, Akhtar S, Lademacher C, Kovanda L, Townsend R. 2017. Pharmacokinetic Interaction between isavuconazole and a fixed-dose combination of lopinavir 400 mg/ritonavir 100 mg in healthy subjects. Clin Pharmacol Drug Dev 6:93–101. doi: 10.1002/cpdd.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musther H, Gill KL, Chetty M, Rostami-Hodjegan A, Rowland M, Jamei M. 2015. Are physiologically based pharmacokinetic models reporting the right Cmax? Central venous versus peripheral sampling site. AAPS J 17:1268–1279. doi: 10.1208/s12248-015-9796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request to the corresponding author.