Bacillus anthracis and Yersinia pestis, the causative pathogens for anthrax and plague, respectively, along with Burkholderia mallei and Burkholderia pseudomallei are potential bioterrorism threats. Tebipenem pivoxil hydrobromide (TBP HBr) (formerly SPR994) is an orally available prodrug of tebipenem, a carbapenem with activity versus multidrug-resistant (MDR) Gram-negative pathogens, including quinolone-resistant and extended-spectrum-β-lactamase-producing Enterobacterales.
KEYWORDS: anthrax, biothreat, carbapenems, plague, tebipenem pivoxil hydrobromide
ABSTRACT
Bacillus anthracis and Yersinia pestis, the causative pathogens for anthrax and plague, respectively, along with Burkholderia mallei and Burkholderia pseudomallei are potential bioterrorism threats. Tebipenem pivoxil hydrobromide (TBP HBr) (formerly SPR994) is an orally available prodrug of tebipenem, a carbapenem with activity versus multidrug-resistant (MDR) Gram-negative pathogens, including quinolone-resistant and extended-spectrum-β-lactamase-producing Enterobacterales. We evaluated the in vitro activity and in vivo efficacy of tebipenem against biothreat pathogens. Tebipenem was active in vitro against 30-strain diversity sets of B. anthracis, Y. pestis, B. mallei, and B. pseudomallei with MIC values of 0.001 to 0.008 μg/ml for B. anthracis, ≤0.0005 to 0.03 μg/ml for Y. pestis, 0.25 to 1 μg/ml for B. mallei, and 1 to 4 μg/ml for B. pseudomallei. In a B. anthracis murine model, all control animals died within 52 h postchallenge. The survival rates in the groups treated with tebipenem were 75% and 73% when dosed at 12 h and 24 h postchallenge, respectively. The survival rates in the positive-control groups treated with ciprofloxacin were 75% when dosed at 12 h and 25% when dosed at 24 h postchallenge. Survival rates were significantly (P = 0.0009) higher in tebipenem groups treated at 12 h and 24 h postchallenge and in the ciprofloxacin group at 12 h postchallenge than in the vehicle control group. For Y. pestis, survival rates for all animals in the tebipenem and ciprofloxacin groups were significantly (P < 0.0001) higher than for the vehicle control group. These results support the further development of tebipenem for treating biothreat pathogens.
INTRODUCTION
With political and economic unrest in many parts of the world, the threat of bioterrorism has increased in recent years (1, 2). Potential bioterrorism agents are classified by the Centers for Disease Control and Prevention (CDC) into three categories based on the feasibility of being disseminated in the general population where they can cause severe disease with excess mortality (3, 4). Two of the most significant bacterial bioterrorism threat agents are Bacillus anthracis and Yersinia pestis, which are the causative pathogens for anthrax and plague, respectively, and considered high-priority category A agents by the CDC (4–6). Others, such as Burkholderia mallei and Burkholderia pseudomallei, which cause glanders and melioidosis, also are of concern (CDC category B bioterrorism agents) because of high mortality (7). The potential for encountering antibiotic-resistant biothreat pathogens in the community or on the battlefield highlights the need for new oral antibiotics that are effective for prophylaxis and treatment regimens (8–12). With the limited treatment options currently available, new treatments are needed that can be used safely across a general population at risk.
One of the most valuable and effective classes of antibiotics is the β-lactam antibiotics, which include the carbapenem subclass. Compared to other β-lactams, carbapenems possess the broadest spectrum of activity and have superior β-lactamase stability. In recent times, they have often been used as antibiotics of last resort, particularly for the treatment of infections caused by antibiotic-resistant, Gram-negative pathogens. Despite their obvious clinical utility, there are currently no oral carbapenems approved for adult patients. However, an oral carbapenem candidate, tebipenem pivoxil hydrobromide (TBP HBr) (formerly SPR994), is in advanced clinical development by Spero Therapeutics Inc. as an alternative to intravenous (i.v.) antibiotic therapy for complicated urinary tract infection (cUTI) (13).
Tebipenem HBr is the hydrobromide salt of the orally available pivoxil prodrug of tebipenem. Upon oral administration, the hydrobromide salt rapidly dissociates upon dissolution, and the pivoxil prodrug is rapidly absorbed through the gastrointestinal tract. The prodrug has been shown to rapidly convert to the pharmacologically active moiety tebipenem in enterocytes of the gastrointestinal tract by intestinal esterases (14), with the prodrug being undetectable in systemic circulation after dosing. Tebipenem possesses broad antibacterial activity against multidrug-resistant (MDR), Gram-negative pathogens, including quinolone-resistant and extended-spectrum-β-lactamase (ESBL)-producing Enterobacterales (15–19). Tebipenem demonstrated in vivo efficacy in murine models of soft tissue, pulmonary, and urinary tract infections (20–22). This study evaluated the in vitro activity and/or in vivo efficacy of tebipenem against select CDC category A (B. anthracis and Y. pestis) and category B (B. mallei and B. pseudomallei) biothreat pathogens.
RESULTS
In vitro activity.
Tebipenem HBr displayed antibacterial activity in vitro against B. anthracis, Y. pestis, B. mallei, and B. pseudomallei. The ranges of MIC values for tebipenem were lower than those for comparator antibiotics, and MIC90 values for tebipenem were similar to or lower than those for comparator antibiotics (Table 1). The comparator antibiotics used were ciprofloxacin for B. anthracis and Y. pestis, azithromycin for B. mallei, and ceftazidime for B. pseudomallei. Tebipenem was active in vitro against 30-strain panels of B. anthracis, Y. pestis, B. mallei, and B. pseudomallei, with a range of MIC values of 0.001 to 0.008 μg/ml for B. anthracis, ≤0.0005 to 0.03 μg/ml for Y. pestis, 0.25 to 1 μg/ml for B. mallei, and 1 to 4 μg/ml for B. pseudomallei. These MICs are equivalent to or lower than previously reported MIC values for the comparator carbapenems imipenem and/or meropenem (23–26). In a study of tebipenem against a ciprofloxacin-resistant strain of B. anthracis (BA Ames Cipr), the MICs against BA Ames Cipr were 0.008 μg/ml for tebipenem and 4 μg/ml for ciprofloxacin, while comparator antibiotic MICs were 0.03 μg/ml for meropenem, 0.03 μg/ml for imipenem, 0.5 μg/ml for levofloxacin, 0.06 μg/ml for doxycycline, and 0.12 μg/ml for tetracycline. Tebipenem was found to be inactive against most of the 29 Francisella tularensis strains tested, with an observed MIC50 of 16 μg/ml and an MIC90 of >64 μg/ml.
TABLE 1.
MICs for tebipenem and comparators against biothreat pathogens
| Pathogen and drug | Value (μg/ml) |
||
|---|---|---|---|
| MIC50 | MIC90 | Range | |
| Bacillus anthracis (n = 30) | |||
| Tebipenem | 0.004 | 0.008 | 0.001 to 0.008 |
| Ciprofloxacin | 0.03 | 0.03 | 0.015 to 0.06 |
| Yersinia pestis (n = 29) | |||
| Tebipenem | 0.03 | 0.03 | ≤0.0005 to 0.03 |
| Ciprofloxacin | 0.008 | 0.015 | ≤0.004 to 0.25 |
| Burkholderia mallei (n = 30) | |||
| Tebipenem | 0.5 | 1.0 | 0.25 to 1 |
| Azithromycin | 0.5 | 0.5 | 0.12 to 1 |
| Burkholderia pseudomallei (n = 29) | |||
| Tebipenem | 2 | 2 | 1 to 4 |
| Ceftazidime | 1 | 2 | 0.5 to 64 |
| Francisella tularensis (n = 29) | |||
| Tebipenem | 16 | >64 | 0.5 to >64 |
| Ciprofloxacin | 0.008 | 0.015 | ≤0.004 to 0.5 |
In vivo efficacy.
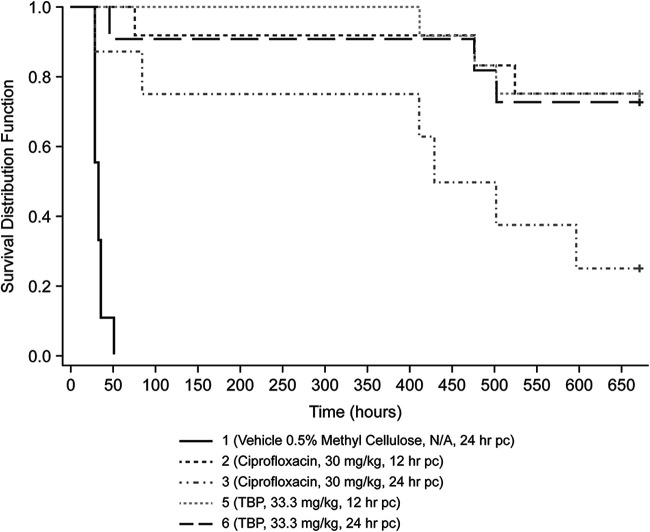
In the murine B. anthracis study featuring an aerosol route of pathogen administration, a 100-fold multiple of the 50% lethal dose (LD50) (∼2.54 × 106 CFU) was targeted, but the actual mean dose was 228× LD50 (5.78 × 106 CFU); more than twice the target challenge dose was delivered across 7 groups by aerosol exposure. Survival rates at end of the study for groups receiving tebipenem HBr were 75% and 73% when administered at 12 h and 24 h postchallenge, respectively (Fig. 1). Groups treated with the positive-control antibiotic ciprofloxacin had end-of-study survival rates of 75% and 25% when dosed at 12 h and 24 h postchallenge, respectively. Survival rates were significantly higher (P = 0.0009) in groups treated with tebipenem HBr at 12 h and 24 h postchallenge and in the group treated with ciprofloxacin at 12 h postchallenge than in the vehicle control group (Table 2). The median time to death was 31.7 h in the vehicle control-treated group. The median times to death were significantly increased (P < 0.0001) in the tebipenem HBr-treated groups dosed at 12 h and 24 h postchallenge. The median times to death in groups treated with ciprofloxacin were also significantly increased when dosed at 12 h (P < 0.0001) and 24 h (P = 0.0115) postchallenge compared to the vehicle control group (Table 2). A trend for better survival was observed after the end of treatment (EOT) with tebipenem HBr than with ciprofloxacin in the 24-h treatment groups. Of note, the dose administered to the vehicle group was >200× LD50, which exceeded the target exposure dose of 100× LD50, which may have accounted for the earlier-than-predicted deaths in the vehicle-treated group (Table 3).
FIG 1.
Kaplan-Meier analysis of mouse survival up to 28 days after challenge with Bacillus anthracis Ames and treatment with tebipenem (TBP) or ciprofloxacin, as indicated. N/A, not applicable; pc, postchallenge.
TABLE 2.
Survival rates and median times to death (Kaplan-Meier analysis) for treatment groups with B. anthracis Ames
| Group | Treatment | Time of treatment initiation postchallenge (h) | No. of surviving mice/no. of mice in group | Survival proportion at 28 days (95% CI) | Survival P valuea | Median time to death (h) (95% CI)c | P valued |
|---|---|---|---|---|---|---|---|
| 1 | Vehicle (0.5% methylcellulose) | 24 | 0/9 | 0.00 (0.00, 0.34) | 31.76 (27.6, 35.7) | ||
| 2 | Ciprofloxacin | 12 | 9/12 | 0.75 (0.43, 0.95) | 0.0009b | — (476.2, —) | <0.0001e |
| 3 | Ciprofloxacin | 24 | 2/8 | 0.25 (0.03, 0.65) | 0.0933 | 465.5 (27.7, —) | 0.0115e |
| 4 | Tebipenem HBr | 12 | 9/12 | 0.75 (0.43, 0.95) | 0.0009b | — (476.2, —) | <0.0001e |
| 5 | Tebipenem HBr | 24 | 8/11 | 0.73 (0.39, 0.94) | 0.0009b | — (476.2, —) | <0.0001e |
P values for one-sided Boschloo’s test comparing the survival rate of the treated group to that of the vehicle group. The overall error level of the six tests was controlled at 5% using the Bonferroni-Holm multiple-comparison procedure.
The survival rate for the treated group is significantly higher than that for the vehicle group.
—, the Kaplan-Meier median time to death or confidence bound(s) could not be estimated due to the large number of surviving animals.
P values for a one-sided log rank test comparing the time to death/overall survival of the treated group to that of the vehicle group. The overall error level of the six tests was controlled at 5% using the Bonferroni-Holm multiple-comparison procedure.
Times to death for the treated groups were significantly greater than that for the vehicle group.
TABLE 3.
Bacillus anthracis Ames LD50s per treatment group
| Group (treatment) | No. of animals/group | Dose (mg/kg)/time of treatment initiation postchallenge (h)a | Mean LD50 ± SD |
|---|---|---|---|
| 1 (vehicle) | 12 | NA/24 | 233 ± 38.0 |
| 2 (ciprofloxacin) | 12 | 30/12 | 278 ± 13.7 |
| 3 (ciprofloxacin) | 12 | 30/24 | 199 ± 0.2 |
| 4 (tebipenem HBr) | 12 | 33.3/12 | 239 ± 55.1 |
| 5 (tebipenem HBr) | 12 | 33.3/24 | 244 ± 22.4 |
NA, not applicable.
Forty-nine samples were included in the analysis for bacterial load reduction, including 8 vehicle-treated mice, 19 ciprofloxacin-treated mice, and 22 tebipenem-treated mice. For all tissues tested (lung, liver, and spleen), the mean bacterial burden was significantly (P < 0.001) decreased in mice treated with ciprofloxacin at 12 h postchallenge and tebipenem at 12 h and 24 h postchallenge compared to mice treated with the vehicle (Table 4). In addition, the mean bacterial burdens for all tissues tested from mice treated with ciprofloxacin (12 and 24 h postchallenge) or tebipenem (12 and 24 h postchallenge) were significantly (P < 0.0001) lower than that of vehicle-treated mice for heat-shocked samples (see Table S1 in the supplemental material).
TABLE 4.
Mean bacterial burdens in liver, lung, and spleen following treatment with ciprofloxacin and tebipenem in a B. anthracis murine infection model
| Treatment | Time (h)a | Liver |
Lung |
Spleen |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of animals | Mean bacterial burden (CFU/g) (95% CI)b | P valuec | No. of animals | Mean bacterial burden (CFU/g) (95% CI)b | P valuec | No. of animals | Mean bacterial burden (CFU/g) (95% CI)b | P valuec | ||
| Vehicle | 24 | 8 | 2.19E+07 (1.46E+07, 3.27E+07) | 8 | 2.86E+08 (1.36E+08, 5.99E+08) | 8 | 1.63E+08 (4.98E+07, 5.34E+08) | |||
| Ciprofloxacin | 12 | 12 | 1.75E+01 (2.50E−01, 1.23E+03) | <0.0001 | 12 | 4.97E+04 (3.20E+03, 7.71E+05) | <0.0001 | 12 | 2.64E+01 (2.05E−01, 3.40E+03) | <0.0001 |
| Ciprofloxacin | 24 | 7 | 1.24E+05 (6.61E+01, 2.32E+08) | 0.348 | 8 | 2.61E+06 (1.17E+05, 5.84E+07) | 0.048 | 8 | 6.82E+04 (2.73E+01, 1.71E+08) | 0.108 |
| Tebipenem | 12 | 12 | 1.74E+01 (2.51E−01, 1.21E+03) | <0.0001 | 12 | 5.01E+04 (3.90E+03, 6.44E+05) | <0.0001 | 12 | 4.58E+01 (5.21E−01, 4.03E+03) | 0.0001 |
| Tebipenem | 24 | 10 | 3.37E+01 (1.67E−01, 6.77E+03) | 0.0003 | 11 | 5.85E+04 (4.34E+03, 7.90E+05) | <0.0001 | 10 | 3.46E+01 (1.56E−01, 7.65E+03) | 0.0002 |
Time postchallenge.
95% CI, 95% confidence interval.
One-sided t test comparing the mean bacterial burden of the treated group to that of the vehicle group. The overall error level of the four tests was controlled at 5% using Dunnett’s multiple-comparison procedure.
Plasma samples were taken from animals in the B. anthracis infection study to assess exposure to tebipenem following oral administration every 8 h (q8h). Samples were taken both 0.25, 0.5, 1, 2, 4, and 8 h after a single dose and following 14 days of dosing. The shape of the plasma concentration-time curves (Fig. 2) showed an apparent monophasic decline after both a single administration and multiple administrations. The pharmacokinetic (PK) parameters were within 2-fold when comparing the day 1 and 14 profiles, suggesting no repeat administration effects on systemic exposure for tebipenem. Tebipenem HBr exhibited time-dependent pharmacodynamics that were best described by the ratio of the free drug area under the concentration-time curve from 0 to 24 h (fAUC0–24)/MIC corrected for the length of the dosing interval (fAUC0–24/MIC · 1/tau) (27).
FIG 2.
Composite concentration-time curve for tebipenem in mouse plasma on day 1 (group 8) and day 14 (group 9) for Bacillus anthracis Ames-challenged mice.
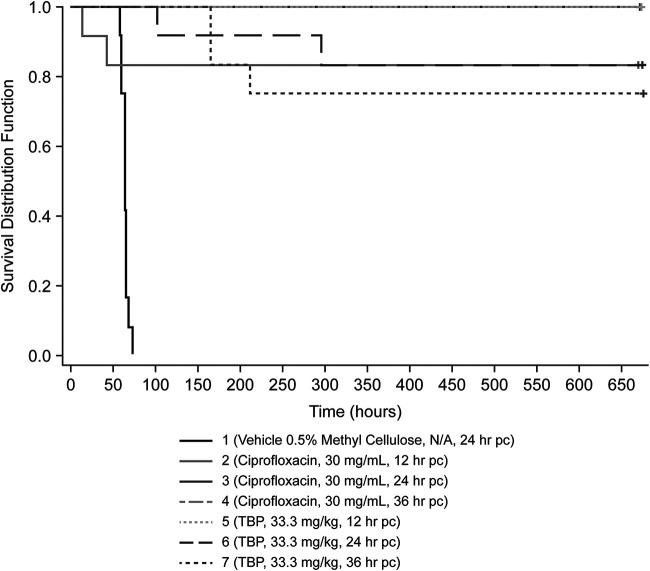
In the murine model of pneumonic Y. pestis infection using a targeted inhaled dose of 1.50 × 105 CFU/animal, groups treated with tebipenem HBr and the positive-control antibiotic ciprofloxacin had significantly (P < 0.0001) higher survival rates than the vehicle control group when dosed at 12 h, 24 h, and 36 h postchallenge (Table 5 and Fig. 3). Median times to death for groups treated with tebipenem HBr or ciprofloxacin were significantly (P < 0.0001) greater than for the vehicle group (Table 5).
TABLE 5.
Survival rates and median times to death (Kaplan-Meier analysis) for treatment groups with Y. pestis CO92
| Group | Treatment | Time of treatment initiation postchallenge (h) | No. of mice that survived/no. of mice in group | Survival proportion (95% CI) | P value for survivala | Median time to death (h) (95% CI)c | P valued |
|---|---|---|---|---|---|---|---|
| 1 | Vehicle (0.5% methylcellulose) | 24 | 0/12 | 0.00 (0.00, 0.26) | 63.6 (59.4, 64.7) | ||
| 2 | Ciprofloxacin | 12 | 10/12 | 0.83 (0.52, 0.98) | <0.0001b | — (43, —) | <0.0001e |
| 3 | Ciprofloxacin | 24 | 12/12 | 1.00 (0.74, 1.00) | <0.0001b | — (—) | <0.0001e |
| 4 | Ciprofloxacin | 36 | 12/12 | 1.00 (0.74, 1.00) | <0.0001b | — (—) | <0.0001e |
| 5 | Tebipenem HBr | 12 | 12/12 | 1.00 (0.74, 1.00) | <0.0001b | — (—) | <0.0001e |
| 6 | Tebipenem HBr | 24 | 10/12 | 0.83 (0.52, 0.98) | <0.0001b | — (296, —) | <0.0001e |
| 7 | Tebipenem HBr | 36 | 9/12 | 0.75 (0.43, 0.95) | <0.0001b | — (166.0, —) | <0.0001e |
P value for one-sided Boschloo’s test comparing the survival rate of the treated group to that of the vehicle group. The overall error level of the six tests was controlled at 5% using the Bonferroni-Holm multiple-comparison procedure.
The survival rate for the treated group is significantly higher than that for the vehicle group.
—, the Kaplan-Meier median time to death or confidence bound(s) could not be estimated due to the large number of surviving animals.
P value for a one-sided log rank test comparing the time to death/overall survival of the treated group to that of the vehicle group. The overall error level of the six tests was controlled at 5% using the Bonferroni-Holm multiple-comparison procedure.
The times to death for the treated group were significantly higher than that for the vehicle group.
FIG 3.
Kaplan-Meier analysis of mouse survival up to 28 days after challenge with Yersinia pestis CO92 and treatment with tebipenem (TBP) or ciprofloxacin, as indicated. pc, postchallenge.
All tissue samples (lung, liver, and spleen) from animals in the vehicle control group were positive for Y. pestis. A single Y. pestis colony was observed in the spleen sample from an animal in the ciprofloxacin group. Tissue samples from groups treated with tebipenem HBr and ciprofloxacin were negative with the exception noted above. For all tissues, the mean bacterial burdens for all treatment groups were significantly (P < 0.0001) lower than that of the vehicle group (Table 6).
TABLE 6.
Mean bacterial burdens in liver, lung, and spleen following treatment with ciprofloxacin and tebipenem HBr in a Y. pestis murine infection model
| Treatment | Time (h)a | Liver |
Lung |
Spleen |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of animals | Mean bacterial burden (CFU/g) (95% CI)b | P valuec | No. of animals | Mean bacterial burden (CFU/g) (95% CI)b | P valuec | No. of animals | Mean bacterial burden (CFU/g) (95% CI)b | P valuec | ||
| Vehicle | 24 | 12 | 3.83E+08 (7.88E+07, 1.86E+09) | 12 | 3.96E+07 (1.38E+07, 1.13E+08) | 12 | 5.04E+07 (1.27E+07, 2.00E+08) | |||
| Ciprofloxacin | 12 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 | 12 | 1.58E+00 (5.75E−01, 4.36E+00) | <0.0001 |
| Ciprofloxacin | 24 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 |
| Ciprofloxacin | 36 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 |
| Tebipenem | 12 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 |
| Tebipenem | 24 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 |
| Tebipenem | 36 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 | 12 | 0 | <0.0001 |
Time postchallenge.
95% CI, 95% confidence interval.
One-sided t test comparing the mean bacterial burden of the treated group to that of the vehicle group. The overall error level of the four tests was controlled at 5% using Dunnett’s multiple-comparison procedure.
Plasma samples were taken from animals in the Y. pestis infection study to assess exposure to tebipenem following q8h oral administration. Samples were taken both 0.25, 0.5, 1, 2, 4, and 8 h after a single dose and following 14 days of dosing. The shape of the plasma concentration-time curves (Fig. 4) showed a monophasic decline after a single administration and multiple administrations. Both the maximum concentration of drug in serum (Cmax) and AUC values of tebipenem were similar between both groups, with a <21% difference, suggesting no repeat dose effect on the PK of tebipenem. Tebipenem HBr exhibited time-dependent pharmacodynamics that were best described by the fAUC0–24/MIC ratio corrected for the length of the dosing interval (fAUC0–24/MIC · 1/tau) (27).
FIG 4.
Composite concentration-time curve for tebipenem in mouse plasma on day 1 (group 8) and day 14 (group 9) for Yersinia pestis CO92-challenged mice.
DISCUSSION
The results demonstrate that the in vitro activity of tebipenem was superior to that of ciprofloxacin against B. anthracis and 10-fold higher than that of ciprofloxacin against Y. pestis, with tebipenem MIC90 values of 0.008 and 0.03 μg/ml, respectively, compared to ciprofloxacin MIC90 values of 0.06 and 0.003 μg/ml, respectively. Tebipenem MIC90 values were 1 and 2 μg/ml for B. mallei and B. pseudomallei, respectively, compared to 0.5 μg/ml for azithromycin and 2 μg/ml for ceftazidime, and it has been suggested that based on the efficacy of intravenous carbapenems, tebipenem may be effective to treat these infections (26). However, based on an evaluation of isolates from cUTI patients, our tentative breakpoint for tebipenem is 0.12 μg/ml; therefore, additional in vitro modeling based on pharmacokinetic data would be required before assessing efficacy in murine models. The expected uniform lethality observed in the vehicle control-treated groups with the indicated targeted challenge doses and delivery route confirm the utility of the model employed in the current study. The efficacy observed with the positive-control antibiotics used in the in vivo studies confirms that the models used were valid for evaluating in vivo efficacy. The late deaths observed following the cessation of ciprofloxacin in the B. anthracis challenge model are not unexpected considering the high spore concentration observed in the lungs of the vehicle control animals at 24 h postchallenge (28). Heine et al. note that the success or failure of treatment correlates with the spore concentration in the lung. Greater than 104 CFU/g in lung tissue is likely a sufficient number of spores in which infection is maintained, and without an extended ciprofloxacin treatment course, posttreatment mortality is likely (29). The in vivo studies demonstrated the significant treatment effects with oral tebipenem HBr, suggesting potent activity for postexposure prophylaxis and treatment against B. anthracis and Y. pestis.
The potential use of anthrax and plague as biothreats could have catastrophic effects on the general population, with widespread morbidity and mortality (5). Inhalational anthrax carries the most serious complications of the biothreat agents and a mortality rate of 90% or higher (30–34). Although B. anthracis and Y. pestis are treatable with penicillins, fluoroquinolones, and tetracyclines, increased rates of natural resistance have been reported (35–38). Furthermore, antibiotic treatment for the recommended 60 days for anthrax could result in the selection of resistant isolates (35, 37). Equally important is the risk of bioengineered resistance that would exacerbate the management of a mass biothreat exposure (39, 40).
Considering the in vitro and in vivo results obtained against B. anthracis and Y. pestis, it is suggested that the oral carbapenem tebipenem HBr represents a viable alternative to penicillins, fluoroquinolones, tetracyclines, and other antibiotics for postexposure prophylaxis and treatment for isolates possessing natural or engineered resistance to any or all of these current therapeutic options. The reported efficacy of imipenem and meropenem against these pathogens further supports the potential utility of tebipenem as an oral carbapenem option for these intravenous drugs (25, 41). For the treatment of anthrax, tebipenem offers a potential new bactericidal partner antibiotic to a protein synthesis inhibitor in the combination regimen required to treat this disease (40). While in vitro studies indicate the potency of tebipenem HBr against B. mallei and B. pseudomallei, additional in vitro modeling (e.g., hollow-fiber modeling) is needed to interrogate translation to in vivo efficacy. In conclusion, these in vitro and in vivo results support the continued development of oral tebipenem HBr for postexposure prophylaxis and treatment of the class A biothreat pathogens B. anthracis and Y. pestis.
MATERIALS AND METHODS
Biosafety.
All protocol testing was performed within a biosafety level 3 (BSL3) laboratory in facilities that are currently registered and licensed by the appropriate federal and state authorities to conduct select-agent research at the respective institutions.
In vitro study.
All in vitro MIC panels were performed by the Bacterial Therapeutics Core at USAMRIID. MICs were determined by the microdilution method in 96-well plates according to Clinical and Laboratory Standards Institute (CLSI) (formerly NCCLS) methods (41, 42). Strains were selected from a USAMRIID global collection to provide strain diversity to ensure an accurate representation of the pathogens. MIC assays were run using cation-adjusted Mueller-Hinton broth (CAMHB) except for F. tularensis strains, where CAMHB with freshly prepared 4% (vol/vol) IsoVitaleX was used as the test medium. The stock solution of tebipenem was diluted to 256 μg/ml in CAMHB by transferring 100 μl of a 5.12-mg/ml solution to 1.9 ml of CAMHB. For testing versus B. anthracis, 50 μl of a 0.128-μg/ml tebipenem solution was transferred to the first well, producing a concentration range of 0.064 μg/ml through 0.0003 μg/ml. Ciprofloxacin, ceftazidime, and azithromycin were formulated into 5.12-mg/ml stock solutions according to CLSI guidelines (43) and stored at −25°C until use. For testing versus Y. pestis, B. mallei, B. pseudomallei, and F. tularensis, 50 μl of a 256-μg/ml tebipenem solution was transferred to the first well of a 96-well plate, and 2-fold dilutions were made through 12 wells, producing a final concentration range of 64 μg/ml through 0.03 μg/ml.
Bacterial inocula were prepared by suspending colonies in CAMHB from 18 to 24 h (B. anthracis, B. mallei, and B. pseudomallei) or 42 to 48 h (Y. pestis and F. tularensis) from plates that were incubated at 35°C. Sheep blood agar (SBA) plates were used for B. anthracis and Y. pestis, and chocolate agar plates were used for F. tularensis, B. mallei, and B. pseudomallei. Suspended cultures were diluted with CAMHB to a final bacterial cell density of 105 CFU/ml. To each well of the 96-well plate, 50 μl of dilutions was added to the wells in 2-fold dilutions. MICs were determined visually at 18 to 24 h (B. anthracis) or 42 to 48 h (Y. pestis). Thirty strains, representing the genetic and geographic diversity of each bacterial species, were used in these studies. Quality control of antibiotic stocks was established by using Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 29213. Strain information for the bacterial isolates used for all in vitro testing can be found in Table S2 in the supplemental material.
The MIC study against the ciprofloxacin-resistant strain of B. anthracis was performed at the Institute for Therapeutic Innovation, Department of Medicine, University of Florida Research and Academic Center at Lake Nona, Orlando, FL, according to CLSI standards. Bacterial inocula were prepared by suspension of B. anthracis colonies in CAMHB from 18- to 24-h-old sheep blood agar plate cultures that were incubated at 35°C. Suspended colonies were each diluted with CAMHB to a bacterial cell density of 106 CFU/ml adjusted based on the optical density at 600 nm (OD600). The conversion factor for B. anthracis was 3.82 × 107 CFU/ml/OD. To each well of the 96-well plates, 50 μl of the adjusted dilution was added for a final inoculum of approximately 5 × 104 CFU/well. MICs were determined by the microdilution method in 96-well plates according to CLSI methods. Antibiotics were serially diluted 2-fold in 50 μl of CAMHB. The antibiotic ranges were 8 to 0.004 μg/ml for tebipenem and other comparator carbapenems and 16 to 0.008 μg/ml for other comparator antibiotics, based on a final well volume of 100 μl after inoculation. MIC plates were incubated at 35°C. MICs were determined visually at 18 h.
Quality control of antibiotic stocks was established using E. coli ATCC 25922 and S. aureus ATCC 29213 inocula prepared as described above from 18- to 24-h-old cultures on SBA plates. Conversion factors were 6.83 × 108 CFU/ml/OD for E. coli and 2.07 × 1010 CFU/ml/OD for S. aureus.
A 2.5-mg/ml tebipenem stock was made in sterile water for injection. It was frozen as 50-μl stocks at −70°C. A 50-μl thawed stock was brought to 1 ml with fresh sterile CAMHB and further diluted 1:4 with CAHMB before final addition/dilution in the 96-well antibiotic testing plates. The comparator antibiotics meropenem, imipenem, ciprofloxacin, levofloxacin, doxycycline, and tetracycline were all purchased from USP; made into 2.5- or 5-mg/ml stocks according to CLSI guidelines, and stored at −70°C until thawed in CAHMB for addition to and dilution in the 96-well plates.
In vivo studies.
(i) Animals. All in vivo studies were performed at Battelle Memorial Institute. Pathogen-free, female BALB/c mice (Charles River Laboratories, Frederick, MD), weighing approximately 20 g, were used throughout the studies. Animals were allowed access to food and water ad libitum and housed in groups. All procedures were performed in accordance with protocols approved by the Battelle Institutional Animal Care and Use Committee and the Department of Defense Animal Care and Use Review Office (ACURO). The protocols met or exceeded the standards of the American Association for the Accreditation of Laboratory Animal Care (AAALAC), the U.S. Department of Health and Human Services, and all local and federal animal welfare laws.
(ii) Preparation of the B. anthracis strain. B. anthracis Ames (tebipenem MIC = 0.008 μg/ml) spores were produced and characterized by Battelle Memorial Institute (Columbus, OH). The Ames strain has been utilized for the evaluation and subsequent FDA approval of multiple countermeasures for the treatment of anthrax based on data obtained from well-characterized animal models of disease. Briefly, working stocks of B. anthracis spores produced via a validated production process are evaluated for percent encapsulation, purity, refractility, viable spore count, potency in an in vivo potency assay, and endotoxin content. Additionally, all spore lots are characterized via the inhalation method to define a spray factor for each spore lot. All spore lots must pass predefined acceptance criteria prior to use. For challenge, B. anthracis spores were obtained from a stock and centrifuged at 12,000 × g (2°C to 8°C) for 15 min to pellet the spores. The pellet was then suspended in sterile water stored at 2°C to 8°C. This procedure was repeated 4 times. The prepared and plated dilutions of the washed spore suspension were used to determine the concentration of the prepared spores. Tween was added to dilute the spore suspension to the target concentration, and the spores were enumerated. Aliquots of the final spore suspension for each challenge run, a prerun, and an extra were prepared based on the results from the enumeration of the washed spore suspension to achieve the calculated volume and target concentration of spores for aerosol challenge. Aerosol concentrations of B. anthracis were quantified by determination of CFU (44–46).
(iii) B. anthracis aerosol exposure. On study day 1, mice were placed into a nose-only exposure tube on a CH Technologies Inc. exposure system tower inside a class III biosafety cabinet system. This approach provided the ability to simultaneously challenge multiple mice with a homogeneous, small-particle aerosol. Mice were challenged with a target dose of 100× LD50 (approximately 2.539 × 106 CFU) of B. anthracis (Ames strain) by controlling the aerosol concentration based on historical spray factor information and the time of exposure. The time of exposure was approximately 15 min. The inhaled dose was determined for each challenge run using Guyton’s formula (47), the group mean body weight of mice in each run, and the concentration of B. anthracis in the impinger samples plated for CFU.
(iv) Preparation of the Y. pestis strain. Y. pestis CO92 (tebipenem MIC = 0.03 μg/ml) challenge material was prepared on the day of aerosol challenge. Strain CO92 is a prototype strain for medical countermeasure evaluations, including both vaccines and antibiotics, and was the strain used in support of the approval of ciprofloxacin and levofloxacin for the treatment pneumonic plague (48, 49). Pigmented CFU from a fresh Congo red agar plate culture were inoculated into heart infusion broth, and the broth culture was incubated overnight to saturation. The bacterial cells were collected, washed by centrifugation, and suspended in buffer to a target nebulizer concentration. To achieve a target dose of 1.50 × 105 CFU/animal (target of 75× to 100× LD50 based on historical Battelle data), the stock was suspended in phosphate-buffered saline plus 0.01% gelatin with 9.7% α-α-trehalose (BSGT). Residual challenge material was stored in a freezer set to maintain at less than −70°C until appropriately disposed of. The prepared challenge material (prenebulizer and postnebulizer) and aerosol impinger samples obtained from each challenge run were analyzed by spread plate enumeration. Challenge material was characterized for phenotype and purity. For phenotype, the percentage of 102-kb pigmentation (pgm+) colonies was determined by plating dilutions of the aerosol challenge material on Congo red agar plates, counting the number of pgm+ colonies after incubation at 28°C ± 2°C for 24 to 96 h, and dividing by the total number of colonies. A minimum of 100 colonies was examined for the pgm+ phenotype. For purity, samples from the aerosol challenge material were tested for purity by streaking for isolation on solid media, with the first set of each medium type being incubated at 28°C ± 2°C, the second set of each medium type being incubated at 37°C ± 2°C, and the third set of each medium type being incubated at room temperature (approximately 23°C ± 2°C). The plates were incubated and observed to test for the presence of any slow-growing microbial contaminants (46).
(v) Y. pestis aerosol exposure. All animals were exposed to aerosolized Y. pestis using a nose-only inhalation exposure system (CH Technologies Inc.) similar to that described above for B. anthracis aerosol exposure. A targeted inhaled dose of 1.50 × 105 CFU/animal (target of 75× to 100× LD50 based on historical Battelle data) was delivered by controlling the aerosol concentration based on historical spray factor information and the time of exposure. The time of exposure was approximately 10 min. The inhaled dose was determined for each challenge run using Guyton’s formula (47), the group mean body weight of mice in each run, and the concentration of Y. pestis in the impinger samples plated for CFU.
(vi) Drug preparation. Tebipenem HBr (manufactured by Asymchem) was received in powder form under refrigeration and stored at 2°C to 8°C. Tebipenem HBr was prepared as a suspension in 0.5% methylcellulose daily. Once prepared, the aliquot was kept at room temperature and stirred at 200 rpm or less continuously between and throughout use for the 24-h stability period. Methylcellulose was received in powder form at room temperature and stored at room temperature. A single solution of 0.5% methylcellulose was prepared for use during the entirety of the study and stored at 2°C to 8°C. Ciprofloxacin for injection (2 mg/ml) was purchased ready-to-use (Hospira) and stored at room temperature.
(vii) Study treatments. Tebipenem was administered orally at a dose of 33.3 mg/kg of body weight every 8 h for 14 days starting 12 to 24 h after the initial B. anthracis challenge. The positive comparator was ciprofloxacin at 30 mg/kg, administered intraperitoneally (i.p.) every 12 h for 14 days, starting 12 to 24 h after challenge. The MIC of ciprofloxacin tested against B. anthracis was 0.03 μg/ml, and that of tebipenem was 0.008 μg/ml. A vehicle group of animals received 0.5% methylcellulose orally every 8 h for 14 days starting at 24 h postchallenge. Two cohorts of 12 mice each received tebipenem HBr or ciprofloxacin, and one cohort of 12 mice received the vehicle.
For Y. pestis, tebipenem HBr was administered orally at a dose of 33.3 mg/kg every 8 h for 14 days starting at 12, 24, or 36 h postchallenge in 3 separate cohorts of 12 animals. Ciprofloxacin at 30 mg/kg was administered i.p. every 12 h for 14 days, starting at 12, 24, or 36 h postchallenge, in 3 separate cohorts of 12 animals. In the vehicle group, 0.5% methylcellulose was administered orally to 12 animals every 8 h for 14 days starting at 24 h postchallenge.
(viii) Assessment of efficacy. Observations for moribundity and mortality were performed at least twice daily during the prechallenge period except on the day of receipt when animals were observed only once. Mice were observed a minimum of three times daily at least 6 h apart postchallenge through study day 14. If all mice were normal at any time during the 14-day postchallenge period, observations were performed twice daily thereafter. Mice were observed at least twice daily after day 14 except on the day of termination when animals were observed only once.
Animals were euthanized on day 29 unless they had previously died. During necropsy, the liver, lung, and spleen were aseptically harvested from animals in the efficacy cohort to evaluate bacterial load reduction. Tissues were collected, homogenized, vortexed, diluted, and cultured by spread plate enumeration to determine the quantity of B. anthracis and Y. pestis bacteria present in each tissue sample. For B. anthracis, duplicate tissue samples were prepared, and one sample was vortexed, diluted, plated in triplicate on tryptic soy agar (TSA), and incubated for colony formation. The second duplicate tissue sample was heat shocked, vortexed, diluted, plated in triplicate on TSA, and incubated for colony formation.
(ix) Bioanalyses of plasma samples.
After induction of deep anesthesia, a target volume of approximately 1.0 ml of blood was collected into a K2-EDTA tube, processed to plasma, sterile filtered, and stored in a freezer set to maintain at less than −70°C until analysis. Plasma samples were analyzed to determine tebipenem concentrations using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with a lower limit of quantitation of 0.01 μg/ml, with a range of 0.01 to 1.0 μg/ml. The accuracy and precision (percent coefficient of variation [%CV]) were <15%. Extraction of the plasma samples, calibration standards, quality controls, and matrix blanks was accomplished by adding 600 μl of acetonitrile to 25 μl of the respective samples, followed by vortexing and centrifugation. The supernatants from the two lowest-dose (8.3 and 16.7 mg/kg) and the two highest-dose (33.3 and 66.6 mg/kg) samples were diluted 1:100 and 1:150, respectively, in order to achieve values within the linear range of the standard curve. The supernatant was then added to a glass vial containing water at a 1:4 ratio prior to analysis by LC-MS/MS.
Study analysis.
The proportion of surviving animals and 95% confidence intervals (CIs) were calculated for each group. One-sided Boschloo’s tests were used to compare survival proportions between each of the six treated groups and the vehicle group. The Bonferroni-Holm multiple-comparison procedure was used to control the overall type I error rate of the six tests at 5%. The log rank test was used to test for significant differences in time to death between each of the six treated groups and the vehicle group. Kaplan-Meier curves were plotted for each group to represent survival and time-to-death data visually.
For quantitative assessment of bacterial loads in blood and tissues, geometric means and 95% confidence intervals were calculated for blood and each tissue type by group in the efficacy cohort. Analysis of variance (ANOVA) models were fitted to the log base 10-transformed bacterial loads to evaluate the effects of treatment on tissue bacterial loads. Values reported as 0 were replaced with values of 1 prior to the log transformation since the logarithm of 0 is undefined. The mean bacterial load for each of the treatment groups was compared to that for the vehicle group within the model for each tissue type. Dunnett’s multiple-comparison procedure was used to control the overall type I error rate of the six tests per tissue at 5%. Statistical analyses were performed using SAS (version 9.4 or later), StatXact (version 11.0 or later; Cytel), or R (version 3.5.1 or later).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the editorial assistance of Richard S. Perry in the development of the manuscript, which was supported by Spero Therapeutics, Cambridge, MA, and Andrew J. Phipps, Tunnell Government Services, Bethesda, MD, contractor supporting the Biomedical Advanced Research and Development Authority (BARDA), for his expertise and assistance with all aspects of the in vivo study design.
All authors performed data analysis and interpretation as well as manuscript review and approval.
N.P.C., M.J.P., and A.J. are paid employees of Spero Therapeutics, Cambridge, MA. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense.
This project has been funded in whole or in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract number HHSO100201800015C. This work was supported in part by funding provided by the Joint Science and Technology Office for Chemical and Biological Defense (JSTO-CBD) of the Defense Threat Reduction Agency (DTRA) under plan number CB3848 to S.D.Z. The JSTO-CBD of the DTRA funded mouse efficacy studies (OTA 18-06-16/TRE18-16-005), and BARDA (HHSO100201800015C) funded the cost of Spero Therapeutics employees to oversee the biodefense development of tebipenem. The funding agencies had no involvement in any aspect of the study, including design, data collection, analysis, interpretation, or composition of the manuscript.
REFERENCES
- 1.Rai B, Kaur J. 2013. Evidence-based forensic dentistry, p 149–152. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 2.Weiss S, Yitzhaki S, Shapira SC. 2015. Lessons to be learned from recent biosafety incidents in the United States. Isr Med Assoc J 17:269–273. [PubMed] [Google Scholar]
- 3.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. 2002. Public health assessment of potential biological terrorism agents. Emerg Infect Dis 8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2018. Bioterrorism agents/diseases. Centers for Disease Control and Prevention, Atlanta, GA. https://emergency.cdc.gov/agent/agentlist-category.asp. [Google Scholar]
- 5.Adalja AA, Toner E, Inglesby TV. 2015. Clinical management of potential bioterrorism-related conditions. N Engl J Med 372:954–962. doi: 10.1056/NEJMra1409755. [DOI] [PubMed] [Google Scholar]
- 6.Pillai SK, Huang E, Guarnizo JT, Hoyle JD, Katharios-Lanwermeyer S, Turski TK, Bower WA, Hendricks KA, Meaney-Delman D. 2015. Antimicrobial treatment for systemic anthrax: analysis of cases from 1945 to 2014 identified through a systematic literature review. Health Secur 13:355–364. doi: 10.1089/hs.2015.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng AC, Dance DAB, Currie BJ. 2005. Bioterrorism, glanders and melioidosis. Euro Surveill 10:pii=528. 10.2807/esm.10.03.00528-en. [DOI] [PubMed] [Google Scholar]
- 8.Schweizer HP. 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7:1389–1399. doi: 10.2217/fmb.12.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatcher CL, Muruato LA, Torres AG. 2015. Recent advances in Burkholderia mallei and B. pseudomallei research. Curr Trop Med Rep 2:62–69. doi: 10.1007/s40475-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, Layton M, McDade J, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Schoch-Spana M, Tonat K. 2000. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 11.Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. 1999. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 281:1735–1745. doi: 10.1001/jama.281.18.1735. [DOI] [PubMed] [Google Scholar]
- 12.Steed DB, Liu J, Wasbrough E, Miller L, Halasohoris S, Miller J, Somerville B, Hershfield JR, Romesberg FE. 2015. Origins of Yersinia pestis sensitivity to the arylomycin antibiotics and the inhibition of type I signal peptidase. Antimicrob Agents Chemother 59:3887–3898. doi: 10.1128/AAC.00181-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain A, Utley L, Parr TR, Zabawa T, Pucci MJ. 2018. Tebipenem, the first oral carbapenem antibiotic. Expert Rev Anti Infect Ther 16:513–522. doi: 10.1080/14787210.2018.1496821. [DOI] [PubMed] [Google Scholar]
- 14.Kato K, Shirasaka Y, Kuraoka E, Kikuchi A, Iguchi M, Suzuki H, Shibasaki S, Kurosawa T, Tamai I. 2010. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharm 7:1747–1756. doi: 10.1021/mp100130b. [DOI] [PubMed] [Google Scholar]
- 15.Citron DM, Tyrrell KL, Rubio A, Goldstein EJC. 2018. In vitro activity of tebipenem (SPR859), tebipenem-pivoxil (SPR994) and meropenem against a broad spectrum of anaerobic bacteria, abstr SUN-559. Abstr ASM Microbe 2018.
- 16.Lacasse E, Brouillette E, Larose A, Parr TR, Jr, Rubio A, Malouin F. 2019. In vitro activity of tebipenem (SPR859) against penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob Agents Chemother 63:e02181-18. doi: 10.1128/AAC.02181-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes RE, Rhomberg PR, Huynh H, Cotroneo N, Rubio A, Flamm RK. 2018. Antimicrobial activity of tebipenem (SPR859) against a global challenge set, abstr SUN-558. Abstr ASM Microbe 2018. [DOI] [PMC free article] [PubMed]
- 18.Mendes RE, Rhomberg PR, Watters A, Cotroneo N, Rubio A, Flamm RK. 2018. Antimicrobial activity assessment of tebipenem (SPR859) against an isolate collection causing urinary tract infections, abstr SUN-560. Abstr ASM Microbe 2018.
- 19.Zou Y, Cotroneo N, Rubio A. 2018. In vitro bactericidal activity and post-antibiotic effect of tebipenem (SPR859) against susceptible and extended-spectrum beta-lactamase producing Enterobacteriaceae as compared to levofloxacin (LVX) and meropenem (MEM), abstr SUN-561. Abstr ASM Microbe 2018.
- 20.Heang K, Grosser L, Farrington K, Controneo N, Jain A, Cacarro L, Corbett D, Rubio A. 2018. In vivo characterization of tebipenem-pivoxil (SPR994) in a murine ascending Escherichia coli urinary tract infection model, abstr SUN-565. Abstr ASM Microbe 2018.
- 21.Grosser L, Heang K, Teague J, Jain A, Warn P, Corbett D, Rubio A. 2018. In vivo efficacy of tebipenem-pivoxil (SPR994) in an acute murine thigh infection caused by Escherichia coli and Klebsiella pneumoniae, abstr SUN-566. Abstr ASM Microbe 2018.
- 22.Teague J, Corbet D, Burgess E, Williams J, Evenden P, Daws G, Vaccaro L, Jain A, Warn P, Rubio A. 2018. In vivo efficacy of tebipenem-pivoxil (SPR994) in neutropenic murine lung models of Gram-negative bacterial infection, abstr SUN-567. Abstr ASM Microbe 2018.
- 23.Heine HS, England MJ, Waag DM, Byrne WR. 2001. In vitro antibiotic susceptibilities of Burkholderia mallei (causative agent of glanders) determined by broth microdilution and E-test. Antimicrob Agents Chemother 45:2119–2121. doi: 10.1128/AAC.45.7.2119-2121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine HS, Hershfield J, Marchand C, Miller L, Halasohoris S, Purcell BK, Worsham PL. 2015. In vitro antibiotic susceptibilities of Yersinia pestis determined by broth microdilution following CLSI methods. Antimicrob Agents Chemother 59:1919–1921. doi: 10.1128/AAC.04548-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heine HS, Shadomy SV, Boyer AE, Chuvala L, Riggins R, Kesterson A, Myrick J, Craig J, Candela MG, Barr JR, Hendricks K, Bower WA, Walke H, Drusano GL. 2017. Evaluation of combination drug therapy for treatment of antibiotic-resistant inhalation anthrax in a murine model. Antimicrob Agents Chemother 61:e00788-17. doi: 10.1128/AAC.00788-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seenama C, Tiengrim S, Thamlikitkul V. 2013. In vitro activity of tebipenem against Burkholderia pseudomallei. Int J Antimicrob Agents 42:375. doi: 10.1016/j.ijantimicag.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 27.McEntee L, Johnson A, Farrington N, Unsworth J, Dane A, Jain A, Cotroneo N, Critchley I, Melnick D, Parr T, Ambrose PG, Das S, Hope W. 2019. Pharmacodynamics of tebipenem: new options for oral treatment of multidrug-resistant Gram-negative infections. Antimicrob Agents Chemother 63:e00603-19. doi: 10.1128/AAC.00603-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heine HS, Bassett J, Miller L, Bassett A, Ivins BE, Lehoux D, Arhin FF, Parr TR, Jr, Moeck G. 2008. Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax. Antimicrob Agents Chemother 52:3350–3357. doi: 10.1128/AAC.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heine HS, Bassett J, Miller L, Hartings J, Ivins B, Pitt ML, Fritz D, Norris S, Byrne WR. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob Agents Chemother 51:1373–1379. doi: 10.1128/AAC.01050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel AK. 2015. Anthrax: a disease of biowarfare and public health importance. World J Clin Cases 3:20–33. doi: 10.12998/wjcc.v3.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 32.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh W-J, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL, National Anthrax Epidemiologic Investigation Team. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis 8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.König R. 2013. Anthrax as a biothreat and our current understanding of this disease. J Immunol Clin Res 1:1002. [Google Scholar]
- 34.Sweeney DA, Hicks CW, Cui X, Li Y, Eichacker PQ. 2011. Anthrax infection. Am J Respir Crit Care Med 184:1333–1341. doi: 10.1164/rccm.201102-0209CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Athamna A, Athamna M, Abu-Rashed N, Medlej B, Bast DJ, Rubinstein E. 2004. Selection of Bacillus anthracis isolates resistant to antibiotics. J Antimicrob Chemother 54:424–428. doi: 10.1093/jac/dkh258. [DOI] [PubMed] [Google Scholar]
- 36.Brook I, Elliott TB, Pryor HI, II, Sautter TE, Gnade BT, Thakar JH, Knudson GB. 2001. In vitro resistance of Bacillus anthracis Sterne to doxycycline, macrolides and quinolones. Int J Antimicrob Agents 18:559–562. doi: 10.1016/S0924-8579(01)00464-2. [DOI] [PubMed] [Google Scholar]
- 37.Brouillard JE, Terriff CM, Tofan A, Garrison MW. 2006. Antibiotic selection and resistance issues with fluoroquinolones and doxycycline against bioterrorism agents. Pharmacotherapy 26:3–14. doi: 10.1592/phco.2006.26.1.3. [DOI] [PubMed] [Google Scholar]
- 38.Gargis AS, Cherney B, Conley AB, McLaughlin HB, Sue D. 2019. Rapid detection of genetic engineering, structural variation, and antimicrobial resistance markers in bacterial biothreat pathogens by nanopore sequencing. Sci Rep 9:13501. doi: 10.1038/s41598-019-49700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler RA, Shafazand S. 2011. Anthrax bioterrorism: prevention, diagnosis and management. J Bioterror Biodef 2:107. doi: 10.4172/2157-2526.1000107. [DOI] [Google Scholar]
- 40.Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, Rubinstein E, Holty J-EC, Messonnier NE, Smith TL, Pesik N, Treadwell TA, Bower WA, Workgroup on Anthrax Clinical Guidelines. 2014. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 20:e130687. doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louie A, Vanscoy B, Liu W, Kulawy R, Brown D, Heine HS, Drusano GL. 2011. Comparative efficacies of candidate antibiotics against Yersinia pestis in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 55:2623–2628. doi: 10.1128/AAC.01374-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed. CLSI document M100-ED28. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed. CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 44.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 45.Comer J, Ray B, Henning L, Stark G, Barnewall R, Mott J, Meister G. 2012. Characterizing a therapeutic model of inhalational anthrax using an increase in body temperature as a trigger for treatment. Clin Vaccine Immunol 19:1517–1525. doi: 10.1128/CVI.00292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henning LN, Comer JE, Stark GV, Ray BD, Tordoff KP, Knostman KAB, Meister GT. 2012. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin Vaccine Immunol 19:1765–1775. doi: 10.1128/CVI.00288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steenbergen J, Tanaka SK, Miller LL, Halasohoris SA, Hershfield JR. 2017. In vitro and in vivo activity of omadacycline against two biothreat pathogens, Bacillus anthracis and Yersinia pestis. Antimicrob Agents Chemother 61:e02434-16. doi: 10.1128/AAC.02434-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guyton AC. 1947. Measurement of the respiratory volumes of laboratory animals. Am J Physiol 150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- 49.Hewitt JA, Lanning LL, Campbell JL. 2020. The African green monkey model of pneumonic plague and US Food and Drug Administration approval of antimicrobials under the Animal Rule. Clin Infect Dis 70(Suppl 1):S51–S59. doi: 10.1093/cid/ciz1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.