AcrAB-TolC is a major tripartite multidrug efflux pump conferring resistance to a wide variety of compounds in Gram-negative pathogens. Many AcrB mutants have been constructed through site-directed mutagenesis to probe the mechanism of AcrB function in antibiotic resistance.
KEYWORDS: AcrB substitution, Salmonella, efflux pumps, multidrug resistance
ABSTRACT
AcrAB-TolC is a major tripartite multidrug efflux pump conferring resistance to a wide variety of compounds in Gram-negative pathogens. Many AcrB mutants have been constructed through site-directed mutagenesis to probe the mechanism of AcrB function in antibiotic resistance. However, much less is known about the actual drug resistance-related mutants that naturally occur in clinically isolated pathogens. Here, we report two novel AcrB substitutions, M78I and P319L, in clinically isolated Salmonella strains with high-level ciprofloxacin resistance. Plasmids expressing the detected acrB mutations were constructed and introduced into SL1344 ΔacrB. Antimicrobial susceptibility assays showed that AcrB M78I, AcrB P319L, and AcrB M78I/319L all conferred reduced susceptibilities to multiple substrates, including fluoroquinolones, erythromycin, tetracyclines, bile salts, and dyes. Site-directed mutagenesis and MIC results revealed that the increased hydrophobicity of M78I was one of the reasons the AcrB M78I mutant had lower susceptibility to fluoroquinolones. Fluorescence labeling experiments suggested that the AcrB M78I substitution enhanced the binding of substrates to certain amino acid sites in the efflux pathway (e.g., sites Q89, E673, and F617) and weakened the binding to other amino acids (e.g., S134 and N274). Structural modeling disclosed that the increased flexibility of Leu was favorable for the functional rotation of AcrB compared to the original Pro residue. AcrA 319L makes the functional rotation of AcrB more flexible; this enables substrate efflux more efficiently. In order to understand the mechanism of AcrAB-TolC drug efflux well, the interaction between AcrA and AcrB in the role of the substrate efflux of AcrAB-TolC should be further investigated.
INTRODUCTION
Antibiotic resistance in pathogens has become a major threat to global health, as the effectiveness of all classes of commercial antibiotics is undermined by the development of bacterial drug resistance (1–3). The overexpression of the intrinsic RND family tripartite efflux pump is one of the main mechanisms of multidrug resistance (MDR) in Gram-negative bacteria (3, 4). The AcrAB-TolC multidrug efflux pump from Escherichia coli is one of the best-studied antibiotic efflux pumps to date (2, 5). The structure of each component of the AcrAB-TolC complex has been determined via X-ray crystallography (6–8), and the structure of the tripartite complex has been revealed by single-particle cryo-electron microscopy (CryoEM) (9, 10). In this tripartite efflux pump, AcrB is the main energy transduction and substrate specificity determinant of the entire three-component complex.
AcrB is an asymmetric homotrimer, and each of the three monomers undergoes a drug extrusion cycle through three different conformations, access, binding, and extrusion, which is called the functionally rotating mechanism (11–13). Each AcrB subunit contains an independent drug binding pocket and translocation pathway. Many reports illustrated that AcrB can bind to a wide range of substrates due to its large substrate binding pockets with multiple binding sites and multiple entry pathways (4, 13–15). Several AcrB-substrate cocrystallized structures have revealed that there are two binding pockets in AcrB, the distal binding pocket (DBP) and the proximal binding pocket (PBP) (11, 16). Generally, during the substrate efflux process, high-molecular-mass drugs (HMMDs) such as rifampicin and erythromycin bind to the PBP and loosely to the DBP, while low-molecular-mass drugs (LMMDs) such as minocycline and doxorubicin bind loosely to the PBP and more tightly to the DBP. However, the size of the substrate is not the only determinant of the selectivity and binding of compounds. Many specific residues in AcrB residing in the PBP and DBP have been reported to play a role in substrate recognition, as they interact with drugs identified in the crystal structures (4, 16).
To date, MDR in clinically relevant infections mediated by RND efflux pumps, including AcrAB-TolC, has been documented to be due to the overexpression of the efflux pump and the concomitant increased efflux of antibiotics from the bacterial cell (3, 17, 18). In 2015, a naturally occurring G288D substitution in AcrB of Salmonella was reported to have contributed to resistance to ciprofloxacin, which is a novel mechanism of antimicrobial resistance (19). In that study, the AcrB G288D substitution arose during clinically appropriate ciprofloxacin treatment. Inspired by this research, we believed that the acrB mutation would have occurred in clinical ciprofloxacin-resistant Salmonella strains. In order to confirm this hypothesis, 108 Salmonella isolates showing different ciprofloxacin resistance were selected for acrB mutation detection. In this study, we report two new AcrB substitutions that lead to MDR in Salmonella and analyzed the resistance mechanisms of these two AcrB substitutions.
RESULTS
AcrB substitutions detected in Salmonella strains.
Sequencing and alignment of the acrB gene of 108 Salmonella isolates revealed two acrB mutations, G234A and C956T, which led to Met78Ile (M78I) and Pro319Leu (P319L) substitutions in the AcrB protein, respectively. It is quite interesting that these acrB mutations were detected in only 22 isolates with high-level ciprofloxacin resistance. Twelve of the 22 strains contain a single substitution, AcrB P319L, and 10 of them contain double substitutions in AcrB, M78I and P319L.
Molecular typing of the strains containing the AcrB mutation(s).
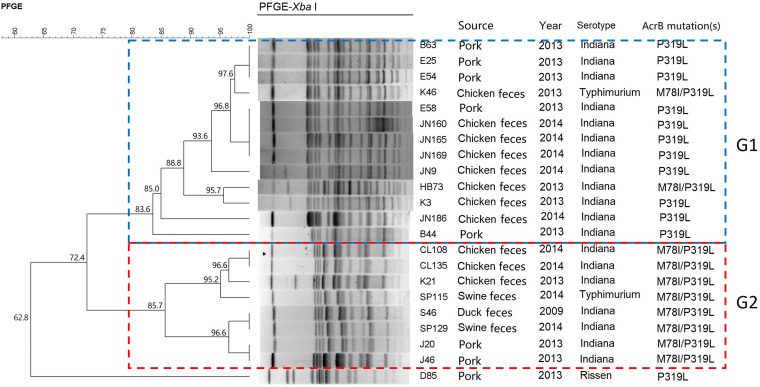
Pulsed-field gel electrophoresis (PFGE) was performed to determine the genetic relationship of the 22 AcrB substitution-carrying Salmonella isolates. With 80% genetic similarity as the cutoff, the 22 Salmonella isolates are grouped into two clusters (named G1 and G2) plus one unique PFGE pattern (strain D85) (Fig. 1). Interestingly, most of the isolates in cluster G1 contain the AcrB P319L substitution, except for strains K46 and HB73, which contain the AcrB M78I/P319L substitutions. All strains belonging to cluster G2 carry M78I/P319L substitutions in AcrB. Identical PFGE patterns were obtained from strains derived from the same source and the same year, for example, three strains isolated from pork in 2013 (strains B63, E25, and E54 in cluster G1) and two strains isolated from chicken in 2014 (strains CL108 and CL135) (Fig. 1). However, identical PFGE patterns are also observed between isolates from different sources and/or different years. For example, strain K46 isolated from chicken in 2013 has the same PFGE pattern as four strains from pork in 2013 (B63, E25, and E54). In addition, strain S46 isolated from duck in 2009 has the same PFGE pattern as strain SP115 isolated from feces of swine in 2014. These results reveal that AcrB M78I- and AcrB M78I/319L-carrying Salmonella isolates show both genetic diversity and original clonality, suggesting that both clonal expansion and horizontal transmission are involved in the dissemination of these acrB mutation-carrying high-level ciprofloxacin-resistant Salmonella strains.
FIG 1.
Results of PFGE typing. According to their PFGE types, 22 Salmonella strains were mainly grouped into two clusters, G1 and G2, which are indicated by the blue dotted frame and the red dotted frame, respectively.
Substitutions M78I and P319L in AcrB increase drug resistance of Salmonella.
In order to investigate the effect of the AcrB M78I and AcrB P319L substitutions on drug resistance, the AcrB wild type (WT) and AcrB mutants (including two single mutants, AcrB M78I and AcrB P319L, and one double mutant, AcrB M78I/P319L) were cloned into plasmid pBAD33. The constructed plasmids were transformed into Salmonella SL1344 ΔacrB (see Table S1 in the supplemental material). A Western blot assay shows that the expression levels of these plasmid-expressed AcrB mutants are similar to that of plasmid-expressed wild-type AcrB (Fig. S2). The MIC values of 11 different drugs and toxic compounds for these constructed strains are shown in Table 1. All the single or double mutants of M78I and P319L cause a 1- or 2-dilution-step increase in the MICs of the tested fluoroquinolones as well as the other drugs, including erythromycin, novobiocin, minocycline, and tetracycline.
TABLE 1.
MIC values for SL1344 ΔacrB harboring AcrB variantsa
| Strain | MIC (μg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LMMDs |
HMMDs |
PACs |
||||||||
| CIP | NAL | ENR | NOR | MIN | TET | ERY | NOV | EtBr | R6G | |
| SL1344 ΔacrB | 0.008 | 4 | 0.015 | 0.03 | 0.5 | 1 | 8 | 8 | 64 | 16 |
| Wild-type AcrB | 0.015 | 8 | 0.03 | 0.06 | 2 | 2 | 256 | 128 | 512 | >1,024 |
| AcrB M78I | 0.06 | 16 | 0.12 | 0.5 | 4 | 4 | 512 | 256 | 512 | >1,024 |
| AcrB P319L | 0.03 | 16 | 0.12 | 0.25 | 4 | 4 | 512 | 128 | 512 | >1,024 |
| AcrB M78I/P319L | 0.06 | 16 | 0.12 | 0.5 | 4 | 4 | 512 | 256 | 512 | >1,024 |
MIC values for the substrates nalidixic acid (NAL), ciprofloxacin (CIP), enrofloxacin (ENR), norfloxacin (NOR), erythromycin (ERY), novobiocin (NOV), minocycline (MIN), tetracycline (TET), ethidium bromide (EtBr), and rhodamine 6G (R6G) are shown. LMMDs, low-molecular-mass drugs; HMMDs, high-molecular-mass drugs; PACs, planar aromatic cations.
Growth kinetics and bile salt tolerance.
In order to know whether the AcrB substitutions change the fitness and adaptability of the cell, the growth curves in LB broth with or without bile salts were measured. The growth curves of the AcrB WT and AcrB mutant strains in LB medium are shown in Fig. 2. The growth rate in the exponential phase for each strain was evaluated (Table S3). Statistical analysis results reveal that the growth ability of the AcrB P319L mutant had no difference from that of the AcrB M78I/P319L mutant (P > 0.5); however, it was significantly lower than those of the AcrB WT and AcrB M78I strains (P < 0.5). There are no significant differences among AcrB WT, AcrB M78I, AcrB M78I/P319L, and an acrB knockout strain bearing the pBAD33 vector. These results indicate that the P319L substitution in AcrB causes a reduction of fitness in Salmonella but that the M78I substitution does not. Comparing the growth of the AcrB M78I/P319L mutant with that of the AcrB P319L mutant, the growth of the AcrB M78I/P319L mutant is recovered. It is possible that M78I is a compensatory mutation that occurs to restore the loss of fitness caused by P319L, and this may be a reason why M78I does not appear as a single mutation in the clinical isolates.
FIG 2.
Growth kinetics of AcrB variants in LB medium (a) and LB medium with 1% bile salts (b). Experiments were carried out on at least three separate occasions, and the mean values and standard deviations are presented.
Tolerance to bile salts is a basic requirement for intestinal bacteria to colonize the intestine of the host. AcrAB-TolC has been shown to play a predominant role in bile salt resistance (5, 12). The growth curves of the AcrB WT and AcrB mutant strains in the presence of 1% bile salts are shown in Fig. 2. Based on the growth rate in log phase and the maximum cell density (Table S4), the AcrB P319L and AcrB M78I/P319L mutants show a significantly increased growth ability compared to the AcrB WT strain. The AcrB M78I/P319L mutant has the highest growth rate (μ = 0.183 h−1) in log phase and the highest maximum cell density (OD600max [maximum optical density at 600 nm] = 0.874), followed by the AcrB P319L mutant (μ = 0.176 h−1 and OD600max = 0.786). The growth ability of the AcrB M78I mutant (μ = 0.145 h−1 and OD600max = 0.662) is similar to that of the AcrB WT strain (μ = 0.150 h−1 and OD600max = 0.672). These results indicate that the occurrence of the P319L or M78I/P319L substitutions in AcrB increases bile salt tolerance.
Increased hydrophobicity of residues at site 78 is responsible for the higher activity of the AcrB M78I mutant.
A comparison of the side chains of Met and Ile revealed that they have similar lengths, and both of them were hydrophobic. However, Ile has significantly higher hydrophobicity than Met. So we hypothesized that the increased hydrophobicity of the M78I mutant is responsible for the increased efflux activity. To confirm our hypothesis, we replaced M78 with 4 amino acids, Ala (M78A), Leu (M78L), Thr (M78T), and Gln (M78Q). Ala and Leu are hydrophobic amino acids, and Thr and Gln are hydrophilic. These amino acids are ranked in order of hydrophobicity as Ile and Leu > Ala >Met > Thr > Gln (20, 21). The antimicrobial susceptibilities of these four mutants to the 11 drugs were tested, and the results are shown in Table 2. As expected, the AcrB M78A and AcrB M78L mutants show 2- to 8-fold-higher resistance to all tested drugs (except for ethidium bromide [EtBr] and rhodamine 6G [R6G]) than the AcrB M78T and AcrB M78Q mutants. However, although the hydrophobicity of Ala is significantly lower than those of Ile and Leu, the AcrB M78I, AcrB M78L, and AcrB M78A mutants have similar MIC values of most drugs, and the AcrB M78A mutant even shows higher resistance to ciprofloxacin, tetracycline, and EtBr than the AcrB M78I and AcrB M78L mutants. Moreover, the AcrB M78T mutant has drug susceptibility similar to that of the AcrB WT strain, while the hydrophobicity of Met is higher than that of Thr. The results suggest that the hydrophobicity of residues at site 78 seems not to be the only determinant of the AcrB drug efflux activity.
TABLE 2.
MIC values for different AcrB mutantsa
| Strain | Hydrophobicity index | MIC (μg/ml) |
% bile salts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMMDs |
HMMDs |
PACs |
||||||||||
| CIP | NAL | ENR | NOR | ERY | NOV | MIN | TET | EtBr | R6G | |||
| AcrB WT | 1.9 | 0.015 | 8 | 0.03 | 0.06 | 256 | 128 | 2 | 2 | 512 | >1,024 | 2.5 |
| AcrB M78I | 4.5 | 0.06 | 16 | 0.12 | 0.5 | 512 | 256 | 4 | 4 | 512 | >1,024 | 2.5 |
| AcrB M78A | 1.8 | 0.12 | 16 | 0.12 | 0.5 | 512 | 256 | 4 | 8 | 1,024 | >1,024 | 5 |
| AcrB M78L | 3.8 | 0.06 | 16 | 0.12 | 0.5 | 512 | 256 | 4 | 8 | 512 | >1,024 | 5 |
| AcrB M78T | −0.7 | 0.03 | 8 | 0.06 | 0.12 | 256 | 64 | 2 | 4 | 512 | >1,024 | 2.5 |
| AcrB M78Q | −3.5 | 0.015 | 4 | 0.03 | 0.06 | 256 | 64 | 2 | 2 | 512 | >1,024 | 2.5 |
MIC values for the substrates nalidixic acid (NAL), ciprofloxacin (CIP), enrofloxacin (ENR), norfloxacin (NOR), erythromycin (ERY), novobiocin (NOV), minocycline (MIN), tetracycline (TET), ethidium bromide (EtBr), and rhodamine 6G (R6G) are shown. LMMDs, low-molecular-mass drugs; HMMDs, high-molecular-mass drugs; PACs, planar aromatic cations.
Site 78 is not in the direct drug efflux pathway.
Husain and Nikaido developed a BODIPY (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionic acid)-FL-maleimide labeling method to probe the substrate translocation pathway in the AcrB transport domain (22). Here, we applied the BODIPY-FL-maleimide labeling assay to confirm if site 78 was located in the substrate translocation pathway of AcrB and if the M78I substitution would affect the binding of substrates to other sites along the translocation pathway. The location of M78 in the AcrB structure is shown in Fig. 3. To probe substrate binding, Cys was introduced at site 78 to create AcrB M78C. In addition, seven sites along the drug translocation pathway (16, 22), including Q89, S134, N274, F617, F664, E673, and R717, were selected, and amino acids at these sites were replaced with Cys in both AcrB WT and AcrB M78I strains (Table S1). The locations of the seven sites in AcrB are shown in Fig. 4: sites F664 and R717 are located in the PBP; sites Q89, S134, N274, and E673 are located in the DBP; and site F617 is located on the switch loop between the PBP and DBP. Two intrinsic cysteines (C493 and C887), which are far from the translocation pathway in AcrB (Fig. 4), are used as the negative controls to confirm that BODIPY-FL-maleimide specifically labels Cys at the substrate binding site. The labeling results are shown in Fig. 5. As expected, AcrB WT has no detectable fluorescence signal. AcrB M78C has very few fluorescence signals, which indicates that site 78 is not located in the substrate translocation pathway, so it does not bind with the substrate directly. Interestingly, the fluorescence intensities of sites S134C and N274C in the AcrB M78I mutant are significantly lower than the fluorescence intensities of sites S134C and N274C in the AcrB WT strain. However, the fluorescence intensities of sites E673C, Q89C, and F617C in the AcrB M78I mutant are significantly higher than the fluorescence intensities of the same sites in the AcrB WT strain. The fluorescence intensities of sites 664C and 717C in both the AcrB M78I and AcrB WT strains are similar.
FIG 3.
Locations of M78 and P319 residues in the AcrB structure. (a) Top view of the structure of the AcrB transport domain. Residues M78 and P319L are shown as red and green spheres, respectively. The four subdomains of the AcrB transport domain are PN1, PN2, PC1, and PC2. M78 is located on PN1, and P319 is located on PN2. (b) Top view of the structure of the AcrB trimer. Each monomer is shown in different conformations, named access (yellow), binding (pale green), and extrusion (magenta).
FIG 4.
Positions of residues in AcrB selected for fluorescent dye labeling. Shown are structures of the AcrB access monomer (left) and zoomed-in transport domain structures (right). (a) View from the vestibule of the AcrB transport domain. Two intrinsic cysteines, C887 and C493, which are located in the transmembrane domain of AcrB, are shown as black spheres. The selected substrate binding sites are shown as colored sticks. (b) View from the cleft of the AcrB transport domain. The PBP and DBP are separated by a red dashed line.
FIG 5.
BODIPY-FL-maleimide labeling results of different sites in the substrate pathway of AcrB WT and AcrB mutants. (a) Coomassie blue-stained gel images. (b) Labeling of AcrB sites 134C, 664C, 673C, 717C, 89C, 274C, and 617C in both AcrB WT and AcrB M78I variants. The intensity of the band indicates the extent of labeling by BODIPY-FL-maleimide. M, protein ladder.
From the above-described experimental results, it can be concluded that site 78 in AcrB is not located in the substrate transportation pathway. The AcrB M78I substitution changes substrate binding with the drug binding sites located in the DBP or on the switch loop of AcrB (Fig. 4). The substrate binding abilities of the residues in the PBP of AcrB are not affected by the AcrB M78I substitution.
Structural analysis of AcrB P319 and L319 with related sites on AcrA.
By visualization of the E. coli AcrABZ-TolC complex structure recently determined using CryoEM (10), we noticed that P319 was located outside the AcrB drug transportation pathway and was very close to AcrA. In order to investigate the potential role of P319 in the AcrB-AcrA interaction, homology modeling of AcrAB of Salmonella was performed by using the AcrAB structure of E. coli (PDB accession number 5O66) as the template. As shown in Fig. 6, AcrB P319 is located on the loop between an α-helix and a β-sheet in the PN2 subregion, and it is very close to sites V367 and R368 of AcrA protomer II (Fig. 6b). The status of P319 in different AcrB conformations (access, binding, and extrusion) is shown in Fig. 7. During the access-binding-extrusion cycle, the side chain of P319 moves from outside to inside AcrB, which results in P319 being closer to residue V367 of AcrA.
FIG 6.
Positional relationship of AcrB P319 with AcrA in the modeling structure of AcrAB of Salmonella. (a) Overall view of the AcrAB complex structures. Structures with different conformations were separated by colors: AcrA protomer I (yellow), AcrA protomer II (green), the AcrB access monomer (blue), the AcrB binding monomer (cyan), and the AcrB extrusion monomer (magenta). (b) Zoomed-in structures of the AcrB transport domain and the AcrA-AcrB interface area. The following subdomains of AcrB transport are shown: PC1 (orange), PC2 (pale green), PN1 (hot pink), and PN2 (marine). Residues AcrB P319 (red), AcrA R368 (yellow), and AcrA V367 (magenta) are shown as sticks.
FIG 7.
Positions of and distances between AcrB P319 or L319 and AcrA site 367 or 368 in different AcrB efflux conformation structures. AcrB access, binding, and extrusion monomers are shown as blue, cyan, and magenta cartoon structures, respectively. The AcrA structure is shown in a green cartoon style. (a) Distance changes between Cγ of AcrB P319 and Cα of AcrA V367 or Cδ of AcrA R368 during the functional rotation of AcrB, shown by magenta and yellow dashes, respectively. The units of the numbers are angstroms. (b) Distance changes between Cγ of AcrB L319 and Cα of AcrA V367 or Cδ of AcrA R368 during the functional rotation of AcrB.
The distances between AcrB P319 or L319 and AcrA V367 or R368 were measured (shown in Fig. 7 and Table S5). Apparently, AcrB L319 is closer to both V367 and R368 of AcrA. The distance between AcrB L319 and AcrA V367 is 0.8 Å to 2 Å shorter than the distance between P319 and AcrA V367, and the distance between L319 and AcrA R368 is 0.4 Å to 1.7 Å shorter than the distance between P319 and AcrA R368. Thus, we hypothesized that the AcrB P319L substitution changed the interaction of AcrB and AcrA, which led to an increasing substrate efflux ability of this tripartite efflux pump.
AcrB L319 enhances the interaction of AcrB with AcrA.
To confirm our hypothesis, V367 and R368 of AcrA were substituted for amino acids with different properties (Table S1). The antimicrobial susceptibility results of the mutants are shown in Table 3. The results show that the AcrB P319L-AcrA mutants have higher (2- to 4-fold) resistance to LMMDs (including all tested fluoroquinolones, minocycline, and tetracycline) and HMMDs (including erythromycin and novobiocin) than AcrA mutants bearing a wild-type AcrB. The impact of AcrA substitutions on resistance to different types of substrates varies. Compared to the AcrA WT strain, the changes of MIC values for both the AcrB WT-AcrA mutant and the AcrB P319L-AcrA mutant against most of the LMMDs are similar. For example, compared to AcrB WT-AcrA WT, the MIC values of the AcrB WT-AcrA V367G, AcrB WT-AcrA V367S, and AcrB WT-AcrA R368A strains against ciprofloxacin increased 2-fold, and the MICs of ciprofloxacin for the AcrB WT-AcrA R368E and AcrB WT-AcrA R368Q strains had no change. Similarly, compared to the AcrB P319L-AcrA WT strain, the MIC values of the AcrB P319L-AcrA V367G, AcrB P319L-AcrA V367S, and AcrB P319L-AcrA R368A strains against ciprofloxacin increased 2-fold as well, and the MICs of ciprofloxacin for the AcrB P319L-AcrA R368E and AcrB P319L-AcrA R368Q strains also had no change. However, this is not the case for the HMMDs and planar aromatic cations (PACs). For example, the MICs of novobiocin, EtBr, and R6G for the AcrB WT-AcrA V367G and AcrB WT-AcrA V367S strains decreased, while the MICs of novobiocin for the AcrB P319L-AcrA V367G and AcrB P319L-AcrA V367S strains increased, and the MICs of EtBr and R6G for the AcrB P319L-AcrA V367G and AcrB P319L-AcrA V367S strains had no change. The results described above indicate that AcrA sites 367 and 368 could interact with AcrB site 319, and the alteration of their interaction could alter the substrate efflux ability of AcrAB-TolC.
TABLE 3.
MIC values for different AcrB and AcrA mutants against different substratesa
| Strain | MIC (μg/ml) |
% bile salts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LMMDs |
HMMDs |
PACs |
|||||||||
| CIP | NAL | ENR | NOR | MIN | TET | ERY | NOV | EB | R6G | ||
| AcrB WT-AcrA WT | 0.015 | 8 | 0.03 | 0.06 | 2 | 2 | 256 | 128 | 512 | >1,024 | 2.5 |
| AcrB WT-AcrA V367G | 0.03 | 4 | 0.06 | 0.12 | 2 | 4 | 256 | 64 | 256 | 256 | 0.625 |
| AcrB WT-AcrA V367S | 0.03 | 8 | 0.06 | 0.12 | 2 | 4 | 128 | 64 | 256 | 256 | 0.625 |
| AcrB WT-AcrA R368A | 0.03 | 4 | 0.015 | 0.12 | 4 | 2 | 256 | 128 | 512 | >1,024 | 2.5 |
| AcrB WT-AcrA R368E | 0.015 | 8 | 0.03 | 0.06 | 2 | 2 | 256 | 128 | 512 | >1,024 | 1.25 |
| AcrB WT-AcrA R368Q | 0.015 | 4 | 0.03 | 0.06 | 2 | 2 | 256 | 128 | 512 | >1,024 | 1.25 |
| AcrB P319L-AcrA WT | 0.03 | 16 | 0.12 | 0.25 | 4 | 4 | 512 | 128 | 512 | >1,024 | 2.5 |
| AcrB P319L-AcrA V367G | 0.06 | 16 | 0.12 | 0.5 | 8 | 4 | 512 | 256 | 512 | >1,024 | 2.5 |
| AcrB P319L-AcrA V367S | 0.06 | 16 | 0.25 | 0.5 | 8 | 8 | 512 | 512 | 512 | >1,024 | 5 |
| AcrB P319L-AcrA R368A | 0.06 | 16 | 0.12 | 0.25 | 4 | 2 | 512 | 256 | 512 | >1,024 | 2.5 |
| AcrB P319L-AcrA R368E | 0.03 | 8 | 0.06 | 0.12 | 2 | 2 | 256 | 128 | 256 | >1,024 | 1.25 |
| AcrB P319L-AcrA R368Q | 0.03 | 8 | 0.06 | 0.06 | 2 | 2 | 256 | 128 | 256 | 1,024 | 1.25 |
MIC values for the substrates nalidixic acid (NAL), ciprofloxacin (CIP), enrofloxacin (ENR), norfloxacin (NOR), erythromycin (ERY), novobiocin (NOV), minocycline (MIN), tetracycline (TET), ethidium bromide (EtBr), and rhodamine 6G (R6G) are shown. LMMDs, low-molecular-mass drugs; HMMDs, high-molecular-mass drugs; PACs, planar aromatic cations. The bold text indicates that the MIC value is different from that of the AcrB WT-AcrA WT strain.
The structures of all AcrA-AcrB mutants were obtained by homology simulation, and the distances between AcrB P319 or L319 and different residues at AcrA site 367 or 368 were analyzed (Fig. S2 and S3). The results show that the distances between AcrB 319L and AcrA site 367 or AcrA site 368 range from 4.0 Å to 9.6 Å and that the distances between AcrB 319L and AcrA site 367 or AcrA site 368 range from 5.2 Å to 10.1 Å. From the above-described results, it can be concluded that the longer and more flexible side chain of Leu than Pro is one of the reasons the AcrB P319L mutant has higher substrate resistance. The residue Leu makes the functional rotation of AcrB more flexible, which enables substrate efflux more efficiently.
Furthermore, an EtBr accumulation assay was conducted for AcrAB mutants to confirm the efflux ability of the mutant pumps (Fig. 8). The rate of accumulation of the AcrA WT-AcrB WT strain is higher than that of the AcrA WT-AcrB P319L strain (Fig. 8a), indicating that the AcrB P319L substitution improves the efflux ability of AcrAB-TolC. For AcrB WT-AcrA mutants, the substitution of AcrA led to a significant difference in the rate of EtBr accumulation (Fig. 8b). However, for AcrB P319L-AcrA mutants, the substitution of AcrA does not lead to a significant difference in the rate of EtBr accumulation (Fig. 8c). These results further confirm our hypothesis that AcrB P319L has a more flexible interaction with AcrA.
FIG 8.
EtBr accumulation of all AcrAB mutants. (a) Accumulation rates of acrAB knockout, AcrA WT-AcrB WT, and AcrA WT-AcrB P319L strains. (b) Accumulation rates of AcrA mutants in an AcrB WT background. (c) Accumulation rates of AcrA mutants in an AcrB P319L background. AU, arbitrary units.
DISCUSSION
AcrAB-TolC is a major tripartite multidrug efflux pump of Escherichia coli and Salmonella, conferring resistance to a wide variety of compounds (1, 23). Many studies have reported that AcrB-mediated resistance was caused by the overproduction of the protein (2, 17, 24–26). However, a naturally occurring mutation in AcrB of Salmonella that alters substrate specificity and drug susceptibility updates our knowledge about the mechanism of antimicrobial resistance (19). Excitingly, acrB single mutation (G234A [AcrB M78I]) and double mutation (G234A and C956T [AcrB M78I/P319L]) were detected in clinically isolated Salmonella strains. Mutations were detected only in isolates with high-level ciprofloxacin resistance. The AcrB M78I single substitution was not detected in isolates in this study. The analysis of the genetic background by PFGE suggests that the occurrence of these two types of AcrB mutations is popular in clinical Salmonella isolates. Antimicrobial susceptibility testing revealed that the AcrB M78I, AcrB P319L, and AcrB M78I/P319L mutants had increased resistance to LMMDs (fluoroquinolones and tetracyclines) and HMMDs (erythromycin and novobiocin) but had little influence on resistance to the PACs (EtBr and R6G). The literature contains many examples of engineered mutations in AcrB in E. coli (4, 27, 28), but before this study, there was no information about the association of the M78I and P319L substitutions of AcrB with antibiotic resistance increases, not even the function of these two sites in AcrB.
The growth ability of AcrB WT and AcrB mutants indicates that the AcrB P319L substitution causes a fitness cost. However, the AcrB M78I/P319L mutant shows a growth ability similar to that of the AcrB WT strain, which suggests that M78I might be a compensatory mutation that appears to ameliorate the fitness cost of P319L. This may explain why no AcrB M78I single substitution was detected. Bile salts are detergent-like biological substances that are synthesized in the liver from cholesterol and stored in the gallbladder. This study revealed that the AcrB P319L and AcrB M78I/P319L mutants were more tolerant to bile salts than the AcrB WT and AcrB M78I strains. These advantages of the AcrB P319L and AcrB M78I/P319L mutants over the AcrB M78I mutant could be another reason why no AcrB M78I single mutant was detected. During infection of the intestinal tract and gallbladder, Salmonella is exposed to bile acids. Thus, the mutants that are more tolerant to bile acid will have more opportunities to survive and spread.
The crystallographic data obtained over time have led to the evolution of our molecular knowledge on substrate efflux mechanisms of AcrB. Now we know that the wide substrate spectrum of AcrB resulted from its multidrug binding pockets, multidrug binding sites, and multidrug transport pathways (3, 4). According to the description of the distal binding pocket (DBP), we hypothesized that AcrB M78 was one of the drug binding sites in DBP and that the decreased drug susceptibilities of the AcrB M78I mutant to HMMDs and LMMDs were caused by the increased hydrophobicity of the amino acid (from Met to Ile). This hypothesis was proven by site-directed mutagenesis and antimicrobial resistance results. However, the MIC results indicated that hydrophobicity was not the only impactful factor. Mutant AcrB M78A showed drug susceptibility similar to that of the AcrB M78I mutant, while Ile has much higher hydrophobicity than Ala. One reason for this result is the small side chain of Ala, which possibly leads to a larger drug binding pocket to let the substrate pass through the pathway more easily (19, 29). In a recent study, the E. coli AcrB G616P variants conferred complete sensitivity to substrates of AcrB (including erythromycin, R6G, EtBr, and novobiocin, etc.) because the bulky side chain of proline prevents movement toward the deep binding pocket due to steric hindrance (29). However, the activity was recovered after the removal of the phenyl group at position 615 (F615A) and position 617 (F617A) for this inactive G616P variant, which implies that the steric property is one of the determinants affecting substrate transportation in AcrB (29). Surprisingly, by fluorescence labeling, it was revealed that site 78 of AcrB was not a drug binding site; the decreased drug susceptibility in the M78I mutant was mediated by altering the drug binding abilities of the binding sites in the AcrB substrate pathway. The Met-to-Ile substitution at position 78 enhanced the binding ability of sites 89, 673, and 617; however, it reduced the binding ability of site 134 and especially site 274, which had no detectable fluorescence at all. All the residues affected are located in the DBP of AcrB. The substrate binding abilities of residues 664 and 717, which are in the PBP, were not affected. AcrB site 78 is adjacent to the DBP, while F664 and R717 are at the entrance of the substrate pathway and are related to substrate uptake. The cocrystal structure of AcrB and the substrate shows that Ser134 and Asn274 in DBP and Phe617 on the switch loop are related to the binding of LMMDs such as minocycline and doxorubicin (29–31). Studies have also shown that Phe664, Glu673, and Arg717 located at the entrance of the proximal binding pocket are associated with the binding of high-molecular-weight drugs such as erythromycin and rifampicin (22, 30, 32).
By analyzing the modeled structure of Salmonella AcrAB, we found that AcrB P319 was close to AcrA V367 and R368. AcrB is an asymmetric homotrimer, and each of the three monomers undergoes a drug extrusion cycle through three different conformations: access, binding, and extrusion (11). Analysis of the distance between AcrB P319 or L319 and AcrA V367 or R368 reveals that the changed drug susceptibilities caused by the AcrB P319L substitution are associated with the changed interaction between AcrA and AcrB. The properties of the 2 amino acids indicate that Leu has a more flexible side chain than Pro. Proline is an α-imino acid that contains a bulky pyrrolidine. However, Leu has a side chain of —CH2-CH(CH3)-CH3, which is obviously more flexible than pyrrolidine. This flexible side chain makes the interaction of AcrA and AcrB smoother and leads to a wider movement range of AcrB during its functional rotation, which thereby results in more efficient substrate efflux. Site-directed mutagenesis and MIC assays of various AcrAB mutants further proved our flexibility theory for the AcrB P319L substitution. The role of the interaction between AcrA and AcrB in drug susceptibility is still unknown. Although changes in the distance between AcrA and AcrB were analyzed based on the static structure of AcrAB and evidence to prove whether the AcrB P319L substitution changes the substrate efflux pathway was not provided in this study, we believe that the flexibility of AcrB P319L is not the only reason leading to MDR of the strain. Another possible reason might be that the change of the interaction between AcrA and AcrB, which is raised by either AcrA or AcrB mutation, causes a rearrangement of the binding sites in the binding pocket of AcrB.
In conclusion, we describe two new types of AcrB substitutions, AcrB P319L and AcrB M78I/P319L, which were popular in clinical isolates of Salmonella that are highly resistant to fluoroquinolones and were proven to cause MDR. Here, we verify that AcrB M78 is not a drug binding site in the DBP; however, the Met-to-Ile substitution alters drug susceptibilities by changing the drug binding abilities of different substrate binding sites. The high hydrophobicity and small steric hindrance of the AcrB residue at position 78 are responsible for the high efflux abilities of AcrAB-TolC, especially for LMMDs. This study also demonstrates that the multidrug susceptibility reduction in the AcrB P319L mutant resulted from the significantly increased flexibility of the side chain of Leu. The P319L substitution may make the functional rotation of AcrB smoother and thereby lead to more efficient substrate efflux. This is the first report about the popularity of two multidrug resistance-related AcrB mutations in clinical Salmonella isolates in China and worldwide. We think that it is necessary to survey the prevalence and distribution of AcrB substitutions in clinical Salmonella isolates, and in order to provide more basis for rational drug design, more detailed structural knowledge about these important mutants needs to be explored in the near future.
MATERIALS AND METHODS
Strains and antimicrobial susceptibility testing.
A total of 108 Salmonella strains isolated from pork and feces of swine, chicken, and duck from Guangdong, Shandong, Hubei, and Henan, China, from 2009 to 2014 were selected for acrB mutation screening. The distribution of ciprofloxacin MICs is shown in Fig. 9. According to the breakpoints of the Clinical and Laboratory Standards Institute (CLSI) (document M100Ed30 [33]), 65 of 108 isolates were nonresistant to ciprofloxacin (ciprofloxacin MIC [MICCIP] < 1 μg/ml). Forty-three isolates were resistant to ciprofloxacin (MICCIP of ≥1 μg/ml, ranging from 1 μg/ml to 64 μg/ml) (Fig. 9). The ciprofloxacin-resistant isolates were further classified into two groups: a low-level ciprofloxacin-resistant group (21 isolates [MICCIP = 1 to 4 μg/ml]) and a high-level ciprofloxacin-resistant group (22 isolates [MICCIP = 8 to 64 μg/ml]) (Fig. 9).
FIG 9.
Distribution of ciprofloxacin MICs for 108 Salmonella isolates. The black dotted line is the ciprofloxacin breakpoint (resistant, MICCIP of ≥1 μg/ml) for Salmonella spp. recommended by the CLSI (Clinical and Laboratory Standards Institute). In CLSI interpretive criteria, an MICCIP of 0.5 μg/ml is defined as an intermediate MIC, so here, we define the isolates with MICCIP values of <1 μg/ml as “nonresistant.” The gray dotted line is the breakpoint of low-level and high-level ciprofloxacin resistance defined in this study.
The MIC values were determined using LB agar plates or by growth in LB broth supplemented with substrates according to the criteria of the CLSI (33). E. coli ATCC 25922 was used as a quality control.
Detection of AcrB mutation.
The acrB gene in each strain was amplified by PCR using the PfuUltra high-fidelity DNA polymerase. The obtained PCR products were submitted to BGI Life Tech Co., Ltd. (Beijing, China), for sequencing, and sequences were aligned with the published acrB sequence of Salmonella enterica serovar Typhimurium SL1344 (GenBank accession number FQ312003.1).
Site-directed mutagenesis.
The plasmid pBAD33acrB or pBAD33acrAB (see Table S1 in the supplemental material) was used for site-directed mutagenesis. Mutations were introduced using the QuikChange mutagenesis kit according to the instructions provided by the manufacturer (Agilent). Plasmid pBAD33 was kindly provided by Yinan Wei’s laboratory (27). Primers used for site-directed mutagenesis are shown in Table S2. The mutations were confirmed by sequencing. Plasmids were transformed into acrB- or acrAB-deficient Salmonella Typhimurium SL1344 cells. The double mutants were created from the single mutant plasmid.
In vitro growth assay.
Growth assays were performed in the presence and absence of 1% bile salts for SL1344 ΔacrB/pBAD33AcrBWT and SL1344 ΔacrB/pBAD33AcrB mutants. Briefly, freshly prepared cultures were inoculated into fresh LB medium with or without 1% bile salts (Sigma) and incubated at 37°C for 8 h or 14 h, respectively. The absorbance (Abs) values were measured at 600 nm every 30 min. Two biological and three technical replicates were performed. The growth rate values in exponential phase were determined by using the equation lnX = lnX0 + μt (where X is the Abs at 600 nm [Abs600] at the end of exponential phase, X0 is the Abs600 at the end of exponential phase, t is the time [hours] of exponential phase, and μ is the growth rate of exponential phase) (34). The statistical significance of differences was evaluated by analysis of variance (ANOVA) using SPSS 17.0 software.
PFGE.
The genetic relatedness of the isolates was determined by pulsed-field gel electrophoresis (PFGE) using a Chef-Mapper system (Bio-Rad Laboratories, Hercules, CA), as previously described (35). Comparison of PFGE patterns was conducted with BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium) using the Dice similarity coefficient.
BODIPY-FL-maleimide labeling.
The fluorescent substrate labeling assay was performed according to a previously described protocol (22, 27). Briefly, 10 ml of cells was grown overnight at 37°C. Cells were collected and washed twice with phosphate buffer. Cell pellets were then resuspended with phosphate buffer. The cell density was adjusted to an OD600 of ∼1.0. Glucose and BODIPY-FL-maleimide (Sigma-Aldrich, St. Louis, MO) were added to concentrations of 0.4% (wt/wt) and 6 mM, respectively. The cells were incubated for 1 h at 30°C and then washed twice with 5 ml phosphate buffer containing 0.4% glucose. AcrB protein purification was conducted as described previously (36). Briefly, the cell pellet was suspended in lysis buffer (30 mM NaPO4, 0.5 M NaCl, 0.5 mM phenylmethanesulfonylfluoride [PMSF]) and sonicated to break the cells. The cell lysate was centrifuged, and the pellet was suspended in extraction buffer containing 30 mM NaPO4, 0.5 M NaCl, 1.5% (wt/vol) Triton X-100, and 0.5 mM PMSF (pH 7.5). The supernatant containing detergent-solubilized AcrB was collected and subjected to metal affinity chromatography. Proteins were eluted by using elution buffer (500 mM imidazole, 30 mM NaPO4, 0.1 M NaCl, 0.03% [wt/vol] DDM [n-dodecyl β-d-maltoside], 0.5 mM PMSF [pH 7.5]). Purified proteins were analyzed using SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The fluorescence image was taken using the Typhoon 9410 phosphorimager/fluorescence imager (GE Life Science, Pittsburgh, PA) at an excitation wavelength of 488 nm. The same gel was then stained using Coomassie blue stain, and the image of the gel was taken again under normal white light.
Protein structure modeling.
The structures of AcrAB of Salmonella and its mutants were predicted by homology modeling with the SWISS-MODEL program. The predictions were based on profile-profile sequence alignments by utilizing the structure of AcrAB (PDB accession number 5O66) (10) as the template. Structural representations were rendered using PyMOL software (PyMOL molecular graphics system; Schrödinger, LLC).
EtBr accumulation.
The EtBr accumulation assay was performed according to established procedures, with slight modification (37). Briefly, bacteria were grown in 15 ml of LB medium until the mid-log phase (OD600 of 0.6 to 0.8). The cells were then centrifuged at 3,000 × g for 15 min and washed once with 50 mM Na-Pi buffer (pH 7.4) under the same conditions. The cells were then resuspended in the same buffer to adjust the OD600 of the cellular suspension to 2.0. One milliliter of the cell suspension was loaded into a cuvette and monitored using a fluorescence spectrometer (LS 550; Perkin-Elmer) at excitation and emission wavelengths of 520 nm and 590 nm, respectively. After exactly 40 s, 1 ml of EtBr was added to a final concentration of 10 μM, and the emission was monitored for an additional 560 s.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National Natural Science Foundation of China (31772792), the National Key Research Program of China (2016YFD0501300), the Guangzhou Science and Technology Project (201607010269), and the National Science Foundation (NSF) (CHE-1709381).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Piddock LJV. 2006. Multidrug-resistance efflux pumps? Not just for resistance. Nat Rev Microbiol 4:629–636. 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 2.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 3.Zwama M, Yamaguchi A. 2018. Molecular mechanisms of AcrB-mediated multidrug export. Res Microbiol 169:372–383. 10.1016/j.resmic.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Zwama M, Yamasaki S, Nakashima R, Sakurai K, Nishino K, Yamaguchi A. 2018. Multiple entry pathways within the efflux transporter AcrB contribute to multidrug recognition. Nat Commun 9:124. 10.1038/s41467-017-02493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin Microbiol Infect 10:12–26. 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 6.Murakami S, Nakashima R, Yamashita E, Yamaguchi A. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587–593. 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 7.Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. 2006. Conformational flexibility in the multidrug efflux system protein AcrA. Structure 14:577–587. 10.1016/j.str.2005.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914–919. 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 9.Du D, Wang Z, James NR, Voss JE, Klimont E, Ohene-Agyei T, Venter H, Chiu W, Luisi BF. 2014. Structure of the AcrAB-TolC multidrug efflux pump. Nature 509:512–515. 10.1038/nature13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Fan G, Hryc CF, Blaza JN, Serysheva II, Schmid MF, Chiu W, Luisi BF, Du D. 2017. An allosteric transport mechanism for the AcrAB-TolC multidrug efflux pump. Elife 6:e24905. 10.7554/eLife.24905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173–179. 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 12.Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. 2006. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 313:1295–1298. 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 13.Sennhauser G, Amstutz P, Briand C, Storchenegger O, Grütter MG. 2007. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol 5:e7. 10.1371/journal.pbio.0050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eicher T, Cha H, Seeger MA, Brandstätter L, El-Delik J, Bohnert JA, Kern WV, Verrey F, Grütter MG, Diederichs K, Pos KM. 2012. Transport of drugs by the multidrug transporter AcrB involves an access and a deep binding pocket that are separated by a switch-loop. Proc Natl Acad Sci U S A 109:5687–5692. 10.1073/pnas.1114944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster S, Vavra M, Kern WV. 2016. Evidence of a substrate-discriminating entrance channel in the lower porter domain of the multidrug resistance efflux pump AcrB. Antimicrob Agents Chemother 60:4315–4323. 10.1128/AAC.00314-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakashima R, Sakurai K, Yamasaki S, Nishino K, Yamaguchi A. 2011. Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature 480:565–569. 10.1038/nature10641. [DOI] [PubMed] [Google Scholar]
- 17.Baucheron S, Tyler S, Boyd D, Mulvey MR, Chaslus-Dancla E, Cloeckaert A. 2004. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium DT104. Antimicrob Agents Chemother 48:3729–3735. 10.1128/AAC.48.10.3729-3735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abouzeed YM, Baucheron S, Cloeckaert A. 2008. ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother 52:2428–2434. 10.1128/AAC.00084-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair JMA, Bavro VN, Ricci V, Modi N, Cacciotto P, Kleinekathöfer U, Ruggerone P, Vargiu AV, Baylay AJ, Smith HE, Brandon Y, Galloway D, Piddock LJV. 2015. AcrB drug-binding pocket substitution confers clinically relevant resistance and altered substrate specificity. Proc Natl Acad Sci U S A 112:3511–3516. 10.1073/pnas.1419939112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyte J, Doolittle RF. 1982. Amino acid scale: hydropathicity. J Mol Biol 157:105–132. 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 21.Biswas KM, DeVido DR, Dorsey JG. 2003. Evaluation of methods for measuring amino acid hydrophobicities and interactions. J Chromatogr A 1000:637–655. 10.1016/s0021-9673(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 22.Husain F, Nikaido H. 2010. Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Mol Microbiol 78:320–330. 10.1111/j.1365-2958.2010.07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJV, Luisi BF. 2018. Multidrug efflux pumps: structure, function and regulation. Nat Rev Microbiol 16:523–539. 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 24.Grimsey EM, Weston N, Ricci V, Stone JW, Piddock LJV. 2020. Overexpression of RamA, which regulates production of the multidrug resistance efflux pump AcrAB-TolC, increases mutation rate and influences drug resistance phenotype. Antimicrob Agents Chemother 64:e02460-19. 10.1128/AAC.02460-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atac N, Kurt-Azap O, Dolapci I, Yesilkaya A, Ergonul O, Gonen M, Can F. 2018. The role of AcrAB-TolC efflux pumps on quinolone resistance of E. coli ST131. Curr Microbiol 75:1661–1666. 10.1007/s00284-018-1577-y. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Yang B, Gu Q, Zhang G, Yang J, Xue F, Shao J, Yi X, Jiang Y. 2017. The role of AcrAB-TolC efflux pump in mediating fluoroquinolone resistance in naturally occurring Salmonella isolates from China. Foodborne Pathog Dis 14:728–734. 10.1089/fpd.2017.2291. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Zhong M, Lu W, Chai Q, Wei Y. 2015. Repressive mutations restore function-loss caused by the disruption of trimerization in Escherichia coli multidrug transporter AcrB. Front Microbiol 6:4. 10.3389/fmicb.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, Lu W, Ye C, Wang Z, Zhong M, Chai Q, Sheetz M, Wei Y. 2013. Role of a conserved residue R780 in Escherichia coli multidrug transporter AcrB. Biochemistry 52:6790–6796. 10.1021/bi400452v. [DOI] [PubMed] [Google Scholar]
- 29.Müller RT, Travers T, Cha H, Phillips JL, Gnanakaran S, Pos KM. 2017. Switch loop flexibility affects substrate transport of the AcrB efflux pump. J Mol Biol 429:3863–3874. 10.1016/j.jmb.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Edward WY, Aires JR, McDermott G, Nikaido H. 2005. A periplasmic drug-binding site of the AcrB multidrug efflux pump: a crystallographic and site-directed mutagenesis study. J Bacteriol 187:6804–6815. 10.1128/JB.187.19.6804-6815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ababou A, Koronakis V. 2016. Structures of gate loop variants of the AcrB drug efflux pump bound by erythromycin substrate. PLoS One 11:e0159154. 10.1371/journal.pone.0159154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drew D, Klepsch MM, Newstead S, Flaig R, De Gier J-W, Iwata S, Beis K. 2008. The structure of the efflux pump AcrB in complex with bile acid. Mol Membr Biol 25:677–682. 10.1080/09687680802552257. [DOI] [PubMed] [Google Scholar]
- 33.CLSI. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI, Wayne, PA. [Google Scholar]
- 34.Dalgaard P, Koutsoumanis K. 2001. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J Microbiol Methods 43:183–196. 10.1016/s0167-7012(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang HX, Song L, Liu J, Zhang XH, Ren YN, Zhang WH, Zhang JY, Liu YH, Webber MA, Ogbolu DO, Zeng ZL, Piddock LJ. 2014. Multiple transmissible genes encoding fluoroquinolone and third-generation cephalosporin resistance co-located in non-typhoidal Salmonella isolated from food-producing animals in China. Int J Antimicrob Agents 43:242–247. 10.1016/j.ijantimicag.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Lu W, Rajapaksha P, Wilkop T, Cai Y, Wei Y. 2018. Comparison of in vitro and in vivo oligomeric states of a wild type and mutant trimeric inner membrane multidrug transporter. Biochem Biophys Rep 16:122–129. 10.1016/j.bbrep.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajapaksha P, Pandeya A, Wei Y. 2020. Probing the dynamics of AcrB through disulfide bond formation. ACS Omega 5:21844–21852. 10.1021/acsomega.0c02921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.