Abstract
An enriched environment is widely used to improve domestic animals’ welfare and promote their natural behaviors. Music can reduce abnormal behavior in humans, nonhuman primates, and rodents. However, little is known about the effects of music on pigs. This study aims to explore the effects of repeated music stimulation on the behavior, physiology, and immunity of growing pigs. A total of 72 hybrid piglets (Large White × Duroc × Minpig) were randomly divided into three groups, including music (Mozart K.448, 60 to 70 dB), noise (recorded mechanical noise, 80 to 85 dB), and control (natural background sound, <40 dB), and 6 h sound stimulation was given per day (1000 to 1600 hours) from 40 to 100 d of age. The behavioral activities of the pigs were observed during the music stimulation, and their serum cortisol, salivary cortisol, and serum immune indices were also measured. Compared with the control group, the music group and noise group increased activity but decreased lying of pigs (P < 0.05). A significant increase in tail-wagging, playing, and exploring behaviors of pigs was found in the music group (P < 0.05), and the noise significantly increased the aggressive behavior of the pigs (P < 0.05). Tail-wagging, playing, exploring, manipulating, and aggressive behaviors decreased over time. Short-term (8 d) music stimulus had a lower cortisol level than that of the noise and control groups (P < 0.05), whereas long-term (60 d) music stimulus increased immunoglobulin G (IgG), interleukin-2 (IL-2), and interferon-gamma (IFN-γ) levels (P < 0.05) and decreased interleukin-4 (IL-4) level (P < 0.05). Long-term noise stimulus significantly reduced the level of IgG (P < 0.05) but did not affect the level of IL-2, IL-4, and IFN-γ levels (P > 0.05). In conclusion, short-term music stimulus (8 d) reduced the stress response, whereas long-term music stimulus (60 d) enhanced the immune responses. In addition, the noise increased the aggressive behavior, and long-term noise reduced the immunity of the growing pigs.
Keywords: behavior, growing pig, immunity, music, noise, stress
Introduction
Environmental enrichment helps animals expressing natural behaviors, prevents or reduces abnormal behaviors, and improves biological functions (Newberry, 1995). Due to the low cost and the ease of administration of music to domestic animals, there is a growing interest in using music therapy techniques to improve the health of domestic and captive animals (Alworth and Buerkle, 2013). In recent years, several studies have reported the impact of music on improving animal health. For instance, zebrafish (Danio rerio) exposed to the musical environment was less anxious in the novel tank test, decreasing peripheral levels of pro-inflammatory cytokines (Barcellos et al., 2018). The sows housed in individual cages or collective stalls had lower respiratory frequency values and smaller expressions of stereotypes when exposed to the musical environment (Backus et al., 2017). Short-term classical music stimulus reduced the aggressive behavior of nonpregnant and non-lactating Arabi ewes (Meshabaz et al., 2017).
As an environmental enrichment, music plays an important role in the development of animals. Early auditory enrichment with music from postnatal 14 d enhanced N-methyl-d-aspartate receptor subunit and N-methyl-d-aspartate receptor 2B (NR2B) subunit protein expression in the auditory cortex of rats. Furthermore, auditory enrichment with music starting from postnatal 28 or 56 d did not influence NR2B expression in the auditory cortex of rats (Xu et al., 2009). Repeated music exposure during the perinatal period influences brain derived neurotrophic factor (BDNF)/tyrosine kinase receptor B (TrkB) signaling and its intracellular signaling pathway targets, including TrkB and 3-phosphoinositide-dependent protein kinase-1, induce improved learning and memory functions of mice (Chikahisa et al., 2006). Early music stimulation can affect the neuroplasticity of mice (Chikahisa et al., 2006; Xu et al., 2009). The music positively affected the emotional state of racehorses. The influence on heart rate variability parameters was noticeable after the first month of featuring the music and increased in the second and third months (Stachurska et al., 2015).
The enriched environment positively affects animal immune response and specific antibody responses, antibody production (Arranz et al., 2010; Matur et al., 2016; Luo et al., 2020), and physiological stress responses (Meijer et al., 2007). A previous study has reported that a musical environment can change the heterophil-to-lymphocyte ratio and reduce the stress of chicks (Dávila et al., 2011). Short-term classical music can reduce saliva cortisol levels of dogs (Bowman et al., 2015). However, the effect of repeated music stimulation on the immune function of growing pigs still remains unclear. According to Brouček (2014), continuous noise has a significant negative impact on animal health. It was reported that weaned piglets avoided continuous high-intensity sounds, which indicates that the pigs abhor continuous noise stimulation (Talling et al., 1998). According to Talling et al. (1996, 1998), pigs can be adapted to the constant background noise (85 dB). Therefore, it is necessary to investigate the effects of long-term repeated noise stimulation on the behavior and immunity of growing pigs. In this study, we evaluated both short-term and long-term effects of stimulation of music and noise on behavioral, physiological, and immune responses of the growing pigs.
Materials and Methods
Ethics statement
The experimental protocol was approved by The Animal Ethics Committee of Northeast Agricultural University (Project number: 31972606). The sampling procedures were followed in accordance with the Guidelines on Ethical Treatment of Experimental Animals (2006) (No. 398) by the Ministry of Science and Technology, China.
Animals and management
A total of 72 hybrid piglets (Large White × Durac × Minpig) of 40 d of age (±3 d) with similar body weight (8.09 ± 2.10 kg) were obtained from the Lanxi animal experiment farm in Heilongjiang, China, which were given iron supplements (Yuanda Animal Health Products Co., Ltd., Jinan, China) at 3 d, fed creep feed (Wellhope Animal Husbandry Co., Ltd., Harbin, China) from the 14 d, castrated at 21 d, and weaned at 30 ± 3.0 d. Each individual was tagged with a distinct number for individual identification, and different colors were used to differentiate between male and female piglets. All piglets were assigned to 12 equal-sized pens (6 pigs per pen, including 3 male and 3 female piglets; Figure 1). All piglets were given ad libitum access to water and artificially fed at 0800 h and 1700 hours daily (the average feed intake before the 28 d of the test was 0.87 kg/d/pig, and the average feed intake before the 60 d of the test was 1.28 kg/d/pig); the feed supplied to the piglets was 11.87 MJ/kg in energy; and the crude protein, crude fiber, and lysine were 16.5%, 5%, and 1.2%, respectively. The room temperature and humidity were maintained at 20 to 25 °C and 60% to 75%, respectively. Lighting was given between 0800 and 2000 hours at 20 lx level. The health of piglets was routinely inspected every day.
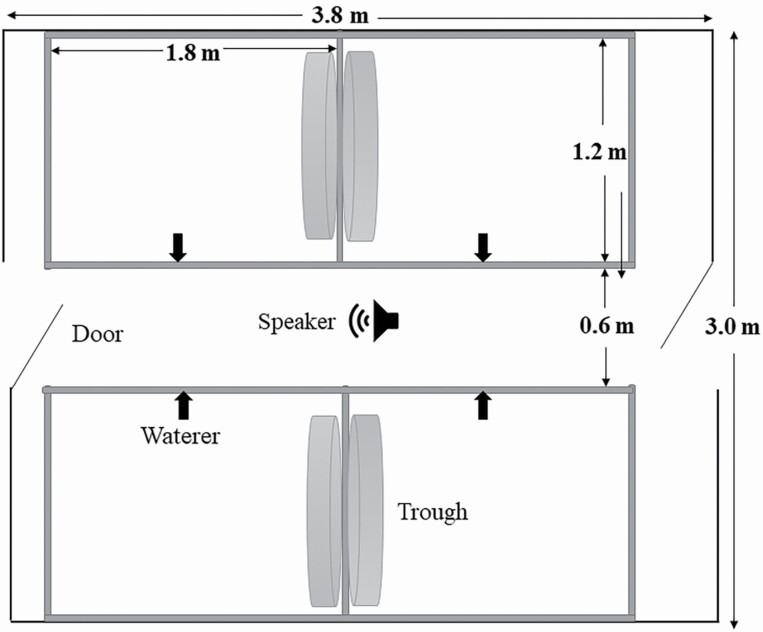
Figure 1.
Overlooking plan of the pen. The area of the housing is 3.8 m length × 3.0 m width, the pens are connected in pairs, each size is 1.8 m length × 1.2 m width, and the aisle is 0.6 m width. Each pen is equipped with a waterer and a trough. Speakers with right and left channels on the ceiling.
Experimental design
The animals were housed in three separate rooms (3 × 3.8 m2 of each room). A total of 72 piglets were randomly allocated to three experimental groups, including music, noise, and control groups. Each group had four replicate pens with a size of 1.8 m length × 1.2 m width × 1 m height (Figure 1) equipped with a waterer, a trough, and a slatted floor. There were six piglets (three males and three females) per pen with a stocking density of 0.36 m2/pig. After entering the pens, the piglets were acclimatized for 5 d. Mozart sonatas K.448 was applied to the music group. The noise sound recordings from the original housing background sounds (mechanical noise such as a fan) were used to create the sound for the noise group. The control group was used as a blank control (without any music and mechanical noise). The music sound intensity was set at 60 to 70 dB. The intensity of the noise group was set at 80 to 85 dB, and the background sound of the control group was below 40 dB. The music and noise groups were equipped with a speaker with the right and left channels on the ceiling. From 1000 to 1600 hours, music and noise were played in a regular loop for 6 h every day. The experiment started from 40 to 100 d of age, and the trial period is 60 d.
Measurement
Behavioral observation
For behavioral observation, infrared ray network cameras (Hangzhou Hikvision Digital Technology Co. Ltd., Hangzhou, China) were fixed on the ceiling for a panoramic view. Two pigs were randomly selected from each pen and marked with a crayon on the back as the focal animals (n = 8). Continuous sampling was used to observe state behaviors, which were recorded as percentages (%). The event behaviors (playing, tail-wagging, exploring, manipulative, and aggressive behaviors) were observed by instantaneous observation at 20 s intervals with the one-zero sampling method. All the occurrences of those abovementioned behaviors during the observation were recorded as frequency (n). The behavioral categories and definitions are presented in Table 1. The behavioral observation was conducted from 1000 to 1600 hours at 7, 14, 27, 42, and 56 d during the experiment. The experiment was conducted in the field by one of the experimenters, and behavioral observation was conducted by another experimenter. So, the observer scoring the behavior was blinded to the treatment groups.
Table 1.
Behavioral categories and definitions (Bolhuis et al., 2005)
| Behavior | Definition |
|---|---|
| State behavior | |
| Active behavior | |
| Standing | Bodyweight supported by four legs and pig remaining still |
| Walking | The pig’s legs move back and forth in a slow, rhythmic, symmetrical motion |
| Lying | |
| Lateral lying | Lying with all the members extended laterally in one side |
| Ventral lying | Lying with the belly facing the ground with all the limbs under the body |
| Event behavior | |
| Playing behavior | Jumping and turning around, running fast at a short distance, and suddenly lie down; focus animal close to pen mate, sniffing and lightly rooting; puts its front legs on the pen mates’ front body or its back |
| Tail-wagging | Moving the tail to the left or right when walking or standing |
| Exploring behavior | Sniffing or nosing the floor; biting or rooting the pen or through |
| Manipulative behavior | Rubbing belly of a pen mate with up and down movements of the snout; nibbling, sucking, or chewing an ear or the tail of a pen mate |
| Aggressive behavior | Ramming or pushing pen mate with head, with biting; mutual pushing or ramming or lifting pen mate |
Salivary and serum cortisol detection
Saliva collection was performed on the 8, 29, and 57 d. One sample was collected from each pen during the sound stimulation between 1400 and 1500 hours. The saliva was collected using degreasing cotton tied to a long rubber band. The cotton was chewed until it was completely wet and placed into a 5-mL tube. Subsequently, the tube was packed in a refrigerator, and all samples were frozen at −20 °C before enzyme-linked immunosorbent assay (ELISA) analysis. One pig in each pen was randomly selected to slaughter at 0800 hours at 4, 28, and 60 d. Before slaughtering, the pigs fasted for 24 h. The pigs were stunned with 85 V for 15 s, and the blood samples were collected. The blood samples were centrifuged at 3,000 rpm for 10 min, and the prepared serum was stored at −20 °C for ELISA analysis. The levels of cortisol in saliva and serum were measured using commercially available ELISA kits (Nos. H094-1-1 and H094-1-2; Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. Briefly, the capture antibodies to the cortisol were coated to the wells of 96 ELISA plates (Corning, USA). The samples were incubated with the solution and then washed five times to remove the nonspecific binding. Then, the detecting antibodies crosslinking with horseradish peroxidase were incubated in the wells. Absorbance at a wavelength of 450 nm was measured using an ELISA reader (TECAN GENios, Austria). A standard curve was prepared with the concentration and absorbance of standards to produce a linear equation to quantify experimental samples.
Serum immune-related factors analysis
The levels of interleukin-2 (IL-2), interleukin-4 (IL-4), interferon-γ (IFN-γ), and immunoglobulin G (IgG) in the serum of different groups were measured using commercially available ELISA kits (Nos. H003-1, H005-1, H025-1, and H106-1; Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. The intra-assay coefficient of variation was less than 10%, and the inter-assay coefficient of variation was below 12%. The experimental procedures were the same as above.
Statistical analysis
Statistical analyses were carried out using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Before statistical analysis, all data were tested for normal distribution with the one-sample Kolmogorov–Smirnov test. The data of lateral lying and manipulative behaviors were sin transformed into a normal distribution. The data were analyzed by repeated analysis of variance (ANOVA) to determine differences among subjects in all behavioral observation time (30 h) based on groups. Duncan multiple comparison tests were used to compare the mean values.
Data of cortisol and immune indices were analyzed by two-way factorial ANOVA, and none of the data showed a non-normal distribution. The equation of the model was as follows:
; where Yij = value observed for characteristics analyzed, μ = overall average, Ai = effect of treatment, Bj = effect of time, and Ai × Bj = interaction between treatment and time. If the interaction effects were significant, the data analyzed by the general linear model would be univariate, Ai and Bj reduced to the main effects, and one-factor ANOVA analysis was used. The results are presented as the mean ± SEM. P < 0.05 was considered a statistically significant difference.
Results
Effects of music and noise on the behavior of growing pigs
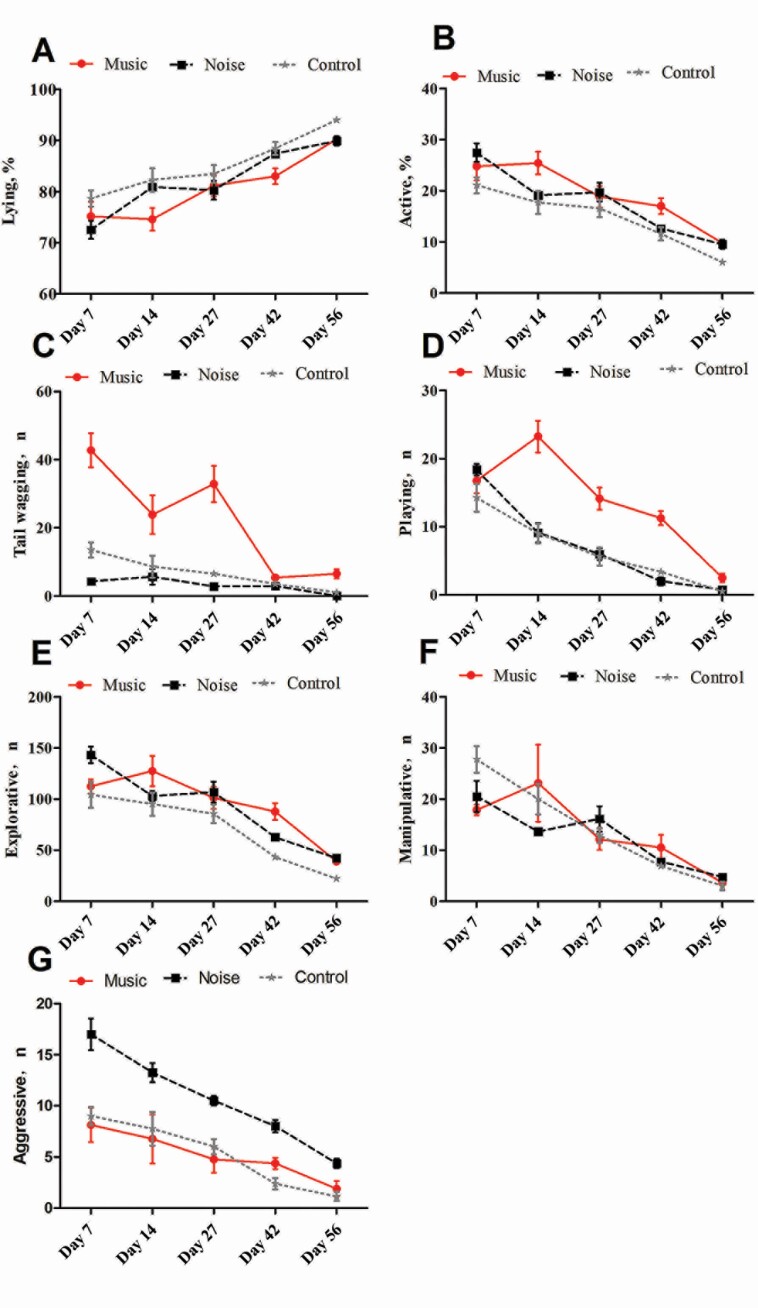
The influence of music and noise stimuli on behavior is presented in Table 2, and the behavioral responses over time are shown in Figure 2 (the detailed data are presented in Supplementary Table S1). Lying behavior in the music and noise groups was significantly lower than that in the control group (P < 0.001), while standing (P < 0.05) and walking behaviors (P < 0.05) of the music and noise groups were significantly higher than those in the control group. However, there was no significant difference in ventral lying (P > 0.05) and lateral lying behaviors (P > 0.05) among the groups. Lying behaviors in the music, noise, and control groups were increased and active behaviors were decreased over time (P < 0.05). The frequency of tail-wagging in the music group was significantly higher than that in the noise and control groups (P < 0.001). The number of tail-wagging in all groups decreased over time. In the music group, tail-wagging on the 42 and 56 d was significantly lower than that at 7, 14, and 27 d (P < 0.05). The playing behavior in the music group was significantly higher than that in the noise and control groups (P < 0.001), and playing behavior was gradually decreased over time in all groups. There was no significant difference in manipulative behavior between different groups (P > 0.05), but the manipulative behavior decreased with time. The pigs in the music and noise groups showed more exploring behavior than the control group (P < 0.001), and the exploring behavior in the music and noise groups was significantly higher than that in the control group at 42 and 56 d (P < 0.05). The aggressive behavior in the noise group was significantly higher than that in the control and music groups (P < 0.001), and the aggressive behavior decreased with time in all groups.
Table 2.
Effects of music and noise stimuli on the behavior of piglets
| Music | Noise | Control | F 2,21 | P-value | |
|---|---|---|---|---|---|
| State behavior, % | |||||
| Lying | 80.83b ± 0.76 | 82.21b ± 0.37 | 85.18a ± 0.65 | 13.06 | <0.001 |
| Ventral lying | 47.10 ± 1.41 | 48.13 ± 0.92 | 49.43 ± 1.19 | 0.97 | 0.396 |
| Lateral lying | 33.74 ± 1.29 | 34.08 ± 0.96 | 35.87 ± 1.09 | 0.23 | 0.799 |
| Active | 19.17a ± 0.76 | 17.79a ± 0.37 | 14.82b ± 0.65 | 13.06 | <0.001 |
| Standing | 14.68a ± 0.56 | 13.81a ± 0.73 | 12.27b ± 0.56 | 6.52 | 0.006 |
| Walking | 4.49a ± 0.24 | 3.42b ± 0.20 | 2.55c ± 0.16 | 23.45 | <0.001 |
| Event behavior, n | |||||
| Tail-wagging | 111.38a ± 22.72 | 15.50b ± 2.82 | 33.13b ± 4.25 | 14.41 | <0.001 |
| Playing behavior | 67.88a ± 4.63 | 36.25b ± 2.01 | 32.75b ± 1.81 | 39.07 | <0.001 |
| Manipulative behavior | 64.75 ± 7.00 | 62.75 ± 2.20 | 70.50 ± 5.25 | 0.25 | 0.784 |
| Exploring behavior | 467.63a ± 16.35 | 457.38a ± 9.45 | 350.13b ± 11.06 | 26.54 | <0.001 |
| Aggressive behavior | 25.88b ± 3.66 | 53.13a ± 1.99 | 26.25b ± 2.27 | 32.57 | <0.001 |
a,bDifferent superscripts indicate a significant difference in different groups at P <0.05.
Figure 2.
Behavioral responses under stimulation of music and noise environments of piglets. The x-axis is the time of behavior observation, and the y-axis is the percentage of time spent on behavior(A & B) or the frequency of the behavior (C–G).
Effects of music and noise on cortisol levels in growing pigs
The level of serum cortisol in the music group was significantly lower than that in both noise and the control groups at 4 d (Table 3, P < 0.05). At 28 d, the levels of serum cortisol in the music and the noise groups were significantly lower than that in the control group (P < 0.05). At 60 d, there was no difference in serum cortisol levels among the groups (P > 0.05). Moreover, the interaction effects between time and treatment significantly affected the serum cortisol level (P = 0.001). Salivary cortisol level significantly decreased over time (P < 0.001), and the salivary cortisol level of the music group was significantly lower than that of the noise and control groups at 8 d (P < 0.05). However, at 29 and 57 d, the salivary cortisol level was not different among the groups (P > 0.05).
Table 3.
Effects of music and noise stimuli on the cortisol level of growing pigs
| Day 4 | Day 28 | Day 60 | ||
|---|---|---|---|---|
| Serum cortisol, ng/L | Music | 34.60b ± 0.78 | 34.52b ± 0.72 | 37.22 ± 2.48 |
| Noise | 44.04ax ± 2.17 | 35.47by ± 0.78 | 37.03y ± 1.39 | |
| Control | 43.95ax ± 1.34 | 42.68ax ± 0.58 | 35.62y ± 1.29 | |
|
P
treatment = 0.001, F2,34 = 8.13; Pday = 0.008, F2,34 = 5.61; Ptreatment × day = 0.001, F2,34 = 5.61 |
||||
| Day 8 | Day 29 | Day 57 | ||
| Salivary cortisol, ng/L | Music | 39.05bx ± 0.27 | 31.43y ± 2.56 | 26.63y ± 1.01 |
| Noise | 45.01ax ± 0.77 | 33.94y ± 1.83 | 25.50z ± 1.42 | |
| Control | 46.36ax ± 2.07 | 36.53y ± 0.65 | 27.56z ± 0.88 | |
|
P
treatment = 0.003, F2,34 = 7.04; Pday< 0.001, F2,34 = 101.80; Ptreatment × day = 0.149, F2,34 = 1.85 |
a– cDifferent superscripts indicate a significant difference in different groups at P < 0.05. x–zDifferent superscripts indicate a significant difference between different time points at P < 0.05.
Effect of music and noise on immune indexes of growing pigs
The effects of music and noise stimuli on the immune reaction of growing pigs are presented in Table 4 (P < 0.001). At 4 d, there was no significant difference in the IL-2 level among the groups (P > 0.05). At 28 and 60 d, the IL-2 level in the music group was significantly higher than that in the noise and the control groups (P < 0.05). The interaction effect of time and treatment showed a significant difference in the IL-2 level (P < 0.001). The IL-4 level of the music group decreased with time (P < 0.05), while there was no significant difference in the IL-4 level between the noise and control groups (P > 0.05). At 28 d, the level of IL-4 in the noise group was significantly higher than that in the control and music groups (P < 0.05). At 60 d, the IL-4 level in the music group was significantly lower than that in the noise and the control groups (P < 0.05). Besides, the interaction effect of time and treatment had a significant difference in the level of IL-4 (P = 0.013). The level of IFN-γ in the music group was significantly higher than that in the noise and control groups at 28 and 60 d (P < 0.05), which was not different among groups at 4 d (P > 0.05). In the music group, the level of IFN-γ at 60 d was significantly higher than that at 4 and 28 d (P < 0.05). At 4 and 28 d, the IgG level of the noise group was significantly higher than that of the music and control groups (P < 0.05). At 60 d, the IgG level of the music group was significantly higher than that of the noise and control groups (P < 0.05). The IgG level of music and control groups increased significantly with time (P < 0.05), while the IgG level of noise group decreased significantly with time (P < 0.05), and the interaction effect of time and treatment had a significant effect on the IgG level (P < 0.001).
Table 4.
Effects of music and noise on immune indexes of growing pigs
| Treatment | Day | |||
|---|---|---|---|---|
| Day 4 | Day 28 | Day 60 | ||
| IL-2, ng/L | Music | 156.40z ± 2.44 | 170.28ay ± 3.31 | 186.82ax ± 1.20 |
| Noise | 158.42 ± 2.29 | 155.36b ± 3.10 | 163.60c ± 1.51 | |
| Control | 159.39y ± 2.27 | 159.99by ± 0.74 | 169.29bx ± 1.21 | |
|
P
treatment
< 0.001, F2,34 = 27.73; Pday< 0.001, F2,34 = 51.38; Ptreatment × day< 0.001, F2,34 = 11.77 |
||||
| IL-4, ng/L | Music | 33.96x ± 1.28 | 31.75bx ± 1.12 | 25.11by ± 1.69 |
| Noise | 35.52 ± 1.02 | 34.63a ± 0.85 | 33.12a ± 1.48 | |
| Control | 32.11 ± 0.71 | 30.43b ± 0.65 | 31.65a ± 1.25 | |
|
P
treatment = 0.002, F2,34 = 7.63; Pday= 0.003, F2,34 = 7.01; Ptreatment × day = 0.013, F2,34 = 3.74 |
||||
| IFN-γ, ng/L | Music | 92.81y ± 2.05 | 96.03ay ± 2.43 | 103.98ax ± 1.80 |
| Noise | 88.78 ± 4.92 | 89.04b ± 2.33 | 88.08b ±2 .39 | |
| Control | 87.76 ± 0.95 | 85.87b ± 1.27 | 86.78b ± 1.43 | |
|
P
treatment
< 0.001, F2,34 = 3.74; Pday = 0.180, F2,34 = 1.81; Ptreatment × day = 0.065, F2,34 = 2.46 |
||||
| IgG, ug/mL | Music | 502.77by ± 4.66 | 472.15bz ± 2.62 | 567.32ax ± 2.48 |
| Noise | 574.41ax ± 2.15 | 511.43az ± 2.92 | 545.46by ± 1.39 | |
| Control | 501.01by ± 2.46 | 479.08by ± 0.71 | 528.02bx ± 1.29 | |
|
P
treatment = 0.001, F2,34 = 2.10; Pday< 0.001, F2,34 = 41.59; Ptreatment × day< 0.001, F2,34 = 11.40 |
||||
a–cDifferent superscripts indicate a significant difference in different groups at P < 0.05. x,– zDifferent superscripts indicate a significant difference between different time points at P < 0.05.
Discussion
Effects of music and noise on the behavior of growing pigs
One measure of welfare in captive animals is that animals can successfully cope with the environment. This ability can influence species-specific behaviors and behavioral variability. On the other hand, it avoids the presence of aggressive and abnormal behaviors (Protopopova, 2016). Our study found that music and noise stimuli reduced the lying behavior but increased standing and walking behaviors of growing pigs, which is consistent with the findings reported by Talling et al. (1996), Wilson et al. (2011), and Meshabaz et al. (2017), who found that animals exposed to music or noise environment were active than the quiet environment. In addition, the lying time gradually increased as the music and noise stimulation progressed. The previous studies observed that lying time increased with the age of animals (Ekkel et al., 2003; Nasirahmadi et al., 2017).
Tail movement is an indicator of the positive emotions of animals (Reefmann et al., 2009; Reimert et al., 2013). This study found that the frequency of tail-wagging was higher in the music group than that in the other two groups, and it was not significantly reduced until the 42 d of musical stimulation, suggesting that the effect of music on emotional regulation in growing pigs can last for 27 d. Playing behavior disappears when animals are under fitness challenge, and it is accompanied by a pleasurable emotional experience (Held and Špinka, 2011; Mintline et al., 2013). Our study observed that the music group increased tail-wagging and playing behavior, which indicated the positive emotional state induced by the music stimulus. We found that playing behavior in the music group at 56 d was significantly reduced, indicating that the positive effects of music declined significantly over time. This may be because pigs become less sensitive to music stimulation with age (Bolhuis et al., 2005; O′Connor et al., 2010).
In this study, the music and noise groups did not significantly affect the manipulative behaviors of pigs, while Bolhuis et al. (2005) found that straw substrate decreases manipulative oral behaviors directed at conspecifics, which indicates that the effect of music on manipulative behavior is not as good as the physical enriched environmental stimuli. However, the results showed that the manipulative behavior decreased over time, which may be due to the attenuation of the weaning-associated stress response (Turpin et al., 2017). Our study presented that the music and noise groups showed more exploring behavior than the control group. Some studies on pigs have demonstrated that restrictive feeding increased the occurrence of rooting behavior, that is, appetitive foraging (Beattie and O′Connellt, 2002; Stern and Andresen, 2003). Our study indicates that the music and noise stimuli may increase the expression of motives for exploring behaviors such as sniffing and rooting of pigs in restrictive feeding conditions.
In terms of aggressive behavior, the present study indicated that the noise group increased the aggression of growing pigs, which is consistent with a previous study, in which 80 dB level of noise increased the degree of mutual attack of pigs (Parker et al., 2010). A previous study reported that long-term noise stimulation would cause negative emotions and cognitive deficits in animals (Cheng et al., 2011). Aggression can be elicited by discomfort, irritability, annoyance, or frustration in pigs (Ruis et al., 2002). The music group increased positive behaviors and reduced aggressive behavior of pigs in the current study. The decrease in aggressive behavior may be offset by increased tail-wagging, exploring, and playing behaviors (Bolhuis et al., 2005; Chaloupková et al., 2007).
It was also found that the frequency of event behaviors gradually decreased over time, and this tendency may indicate the adaptability of pigs to sound stimulation. On the other hand, the increase in the body size of pigs affected the shared area of the pen and then restrained the possibility of their behavioral expression (Leme et al., 2013). Due to the weight gain of pigs, the sharing space is relatively reduced, increasing the lying behavior of pigs (Vermeer et al., 2014). Although the piglets can be adapted to repeated music stimulation and reduce the level of behavioral expression, the music stimuli can still increase the behavioral expression related to positive emotions of growing pigs, and the positive effect can last for 42 d or even longer.
Effects of music and noise on physiological stress of growing pigs
Increased activity of the hypothalamic–pituitary–adrenal (HPA) axis indicates a physiological response to stressors, and the measurement of cortisol is an essential indicator of stress (Cook et al., 1997). This study indicated that the pigs of the music group (8 d of music stimulation) had lower cortisol levels than the pigs of the control group. The results are consistent with the previous studies (Ventura et al., 2012; Bowman et al., 2017). The group exposed to short-term noise had higher cortisol levels than the other groups, which was consistent with previous reports, in which animals exposed to a noisy environment activated the responses of the HPA axis and increased cortisol levels (Eggermont, 2014; Zahra et al., 2018). The cortisol level in the music group was lower than the control group, indicating that the short-time exposure to music stimulation was efficient to relieve the stress on piglets. It had been confirmed that the synthesis and release of cortisol increased during acute stress and decreased when stress levels are low. In this study, the regrouped weaning piglets in the pen were in the station of acute stress. The cortisol level was relatively high in this situation; therefore, after exposure to music for 7 d, the pigs had lower cortisol levels than pigs not exposed to music. However, there was no significant difference in saliva cortisol levels in different groups at 28 d, whereas the serum cortisol level in the control group was higher than that in the noise and music groups at 28 d. It was found that the noise was less stressful with prolonged exposure to the noise, and it could be speculated that the effect of noise on cortisol concentration is time-sensitive. Although Parrott et al. (1989) demonstrated good consistency between cortisol concentrations in pig serum and saliva, the saliva cortisol is noninvasively sampled, whereas the serum cortisol was sampled in an invasive way, which might cause acute stress to the animals by blood sampling. The previous studies found that compared with other stimuli, such as the high concentration of ammonia and low-intensity light, noise stimulus of 85 dB induced a low degree of release of adrenocorticotropic hormone and cortisol in pigs (O′Connor et al., 2010; Parker et al., 2010). According to the survey results of the intensive system of growing pigs in Germany, the mean equivalent noise level in pen was 70.2 ± 5.2 dB, while the noise (85 dB) does not reduce the welfare and health of the pigs but affected some behavioral expressions (Wegner et al., 2019), which is consistent with our results. Therefore, pigs can easily adapt to acute or short-term high sound pressure levels (Otten et al., 2004; Kanitz et al., 2005), while repeated exposure to noise stimulus does not cause an enduring stress response in the pigs. So, the strong physiological stress response cannot be induced by the noise (<85 dB). This study found that on the 4 and 8 d, the serum and salivary cortisol levels in the music group were lower than the noise and control groups, but there was no difference on the 57 and 60 d among groups, indicating that the role of music in relieving stress is noncontinuous and noneffective over the long term. It seems that the effects of music stimuli on pigs are attenuation and adaptation (Bowman et al., 2015).
Effects of music and noise on the immunity of growing pigs
Music can boost the immune system and enhance well-being (Fancourt et al., 2014). Koyama et al. (2009) found that music can enhance the activity of the T lymphocytes and promote the secretion of cytokines, such as IL-1, IL-2, and IFN-γ, thereby improving the body’s immunity. Lu et al. (2010) showed that long-term music stimulation reduced serum IL-4 levels in asthmatic rats. Zhang et al. (2013) found that long-term music therapy (21 d) increased serum IgG level and caused liver depression and spleen deficiency in rats. The results of this study indicated that short-term music stimulation did not affect serum cytokines, whereas long-term music stimulation increased the levels of IL-2, IFN-γ, and IgG and decreased the level of IL-4 in serum, which highlights that long-term music stimulation promotes the level of positive immune regulation in growing pigs. It is supposed that music stimulation promoted Th1 cell response by increasing the level of IL-2 and IFN-γ and inhibited Th2 cell responses by reducing the level of IL-4, thus led to the improvement of T cell-mediated immune function. Also, long-term repeated noise stimulation did not significantly affect the levels of cytokines (IL-2, IFN-γ, and IL-4) in serum but reduced the level of IgG in this study. IgG is the main Ig produced by natural infection and active immunization of animals. It is the body’s main anti-infection antibody. Previous studies have found that when pregnant mice were repeatedly exposed to the noise environment of 85 to 95 dB, their offspring’s thymus weight became smaller and serum IgG levels were reduced (Kight and Swaddle, 2011), which are consistent with this study, indicating that long-term repeated noise stimulation reduces the ability to produce and secrete antibodies of growing pigs.
Conclusions
In summary, the music stimulus increased the activity, tail-wagging, and playing behavior of growing pigs. Some general behaviors, including active, tail-wagging, playing, manipulative, exploring, and aggression, decreased with age and trial process of piglets. Short-term music stimulation helped to reduce stress and long-term music enhanced immunity. Long-term noise stimulation resulted in more aggressive behavior and less playing and tail-wagging behaviors of growing pigs. It also induced the physiological stress via higher cortisol levels and reduced the immune horizontally by secreting the lower serum IgG. The effects of music stimulus on pigs associated with the behavioral, emotional, and neuroendocrine regulatory mechanisms will continue to be investigated in the future.
Supplementary Material
Acknowledgments
We would like to greatly thank members of the Animal Behavior and Welfare Laboratory in the College of Animal Science and Technology. This study was supported by the Natural Science Foundation of China Grant (31972606) and the Earmarked Fund for China Agriculture Research System Grant (CARS-35B). We are very glad and thankful that this paper’s abstract was accepted and a virtual poster (No. PSV-7) was presented at the 2020 ASAS-CSAS-WSASAS Virtual Annual Meeting and Trade Show.
Glossary
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- HPA
hypothalamic–pituitary–adrenal
- IFN-γ
interferon-γ
- IgG
immunoglobulin G
- IL-2
interleukin-2
- IL-4
interleukin-4
- NR2B
N-methyl-d-aspartate receptor 2B
- TrkB
tyrosine kinase receptor B
Conflict of interest statement
The authors did not provide a conflict of interest statement.
Literature Cited
- Alworth, L. C., and Buerkle S. C.. . 2013. The effects of music on animal physiology, behavior and welfare. Lab Anim. (NY). 42:54–61. doi: 10.1038/laban.162 [DOI] [PubMed] [Google Scholar]
- Arranz, L., De Castro N. M., Baeza I., Maté I., Viveros M. P., and De la Fuente M.. . 2010. Environmental enrichment improves age-related immune system impairment: long-term exposure since adulthood increases life span in mice. Rejuvenation Res. 13:415–428. doi: 10.1089/rej.2009.0989 [DOI] [PubMed] [Google Scholar]
- Backus, B. L., Sutherland M. A., and Brooks T. A.. . 2017. Relationship between environmental enrichment and the response to novelty in laboratory-housed pigs. J Am. Assoc. Lab. Anim. 37:215–225. doi: 10.138/gov.29256368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos, H. H. A., Koakoski G., Chaulet F., Kirsten K. S., Kreutz L. C., Kalueff A. V., and Barcellos L. J. G.. . 2018. The effects of auditory enrichment on zebrafish behavior and physiology. PeerJ 6:e5162. doi: 10.7717/peerj.5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie, V. E., and O′Connell N. E.. . 2002. Relationship between rooting behaviour and foraging in growing pigs. Anim. Welf. 11:295–303. doi: 10.3758/BF03192837 [DOI] [Google Scholar]
- Bolhuis, J. E., Schouten W. G. P., Schrama J. W., and Wiegant V. M.. . 2005. Behavioural development of pigs with different coping characteristics in barren and substrate-enriched housing conditions. Appl. Anim. Behav. Sci. 93:213–228. doi: 10.1016/j.applanim.2005.01.006 [DOI] [Google Scholar]
- Bowman, A., Dowell F., and Evans N. P.. . 2017. The effect of different genres of music on the stress levels of kennelled dogs. Physiol. Behav. 171:207–215. doi: 10.1016/j.physbeh.2017.01.024 [DOI] [PubMed] [Google Scholar]
- Bowman, A., Spca S., Dowell F. J., and Evans N. P.. . 2015. ‘Four Seasons’ in an animal rescue centre; classical music reduces environmental stress in kennelled dogs. Physiol. Behav. 143:70–82. doi: 10.1016/j.physbeh.2015.02.035 [DOI] [PubMed] [Google Scholar]
- Brouček, J. 2014. Effect of noise on performance, stress, and behaviour of animals. Slovak J. Anim. Sci. 47:111–123. [Google Scholar]
- Chaloupková, H., Illmann G., Bartos L., and Spinka M.. . 2007. The effect of preweaning housing on the play and agonistic behavior of domestic pigs. Appl. Anim. Behav. Sci. 103:25–34. doi: 10.1016/j.applanim.2006.04.020 [DOI] [Google Scholar]
- Cheng, L., Wang S. H., Chen Q. C., and Liao X. M.. . 2011. Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol. Behav. 104:981–988. doi: 10.1016/j.physbeh.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Chikahisa, S., Sei H., Morishima M., Sano A., Kitaoka K., Nakaya Y., and Morita Y.. . 2006. Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav. Brain Res. 169:312–319. doi: 10.1016/j.bbr.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Cook, N. J., Schaefer A. L., Lepage P., and Jones S. D. M.. . 1997. Radioimmunoassay for cortisol in pig saliva and serum. J. Agr. Food Chem. 45:398–399. doi: 10.1021/jf960619d [DOI] [Google Scholar]
- Dávila, S. G., Campo J. L., Gil M. G., Prieto M. T., and Torres O.. . 2011. Effects of auditory and physical enrichment on 3 measurements of fear and stress (tonic immobility duration, heterophil to lymphocyte ratio, and fluctuating asymmetry) in several breeds of layer chicks. Poult. Sci. 90:2459–2466. doi: 10.3382/ps.2011-01595 [DOI] [PubMed] [Google Scholar]
- Eggermont, J. J. 2014. Noise and the brain: experience dependent developmental and adult plasticity. Chapter 11-Noise in the brain. Academic Press, Elsevier; p. 5–8. [Google Scholar]
- Ekkel, D., Spoolder H. A. M., Hulsegge I., and Hopster H.. . 2003. Lying characteristics as determinants for space requirements in pigs. Appl. Anim. Behav. Sci. 80:19–30. doi: 10.1016/S0168-1591(02)00154-5 [DOI] [Google Scholar]
- Fancourt, D., Ockelford A., and Belai A.. . 2014. The psychoneuroimmunological effects of music: a systematic review and a new model. Brain. Behav. Immun. 36:15–26. doi: 10.1016/j.bbi.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Held, S., and Špinka M.. . 2011. Animal play and animal welfare. Anim. Behav. 81:891–899. doi: 10.1016/j.anbehav.2011.01.007 [DOI] [Google Scholar]
- Kanitz, E., Otten W., and Tuchscherer M.. . 2005. Central and peripheral effects of repeated noise stress on hypothalamic–pituitary–adrenocortical axis in pigs. Livest. Prod. Sci. 94: 213–224. doi: 10.1016/j.livprodsci.2004.12.002 [DOI] [Google Scholar]
- Kight, C. R., and Swaddle J. P.. . 2011. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14:1052–1061. doi: 10.1111/j.1461-0248.2011.01664.x [DOI] [PubMed] [Google Scholar]
- Koyama, M., Wachi M., Utsuyama M., Bittman B., Hirokawa K., and Kitagawa M.. . 2009. Recreational music-making modulates immunological responses and mood states in older adults. J. Med. Dent. Sci. 56:79–90. PMID: 20099470. [PubMed] [Google Scholar]
- Leme, T. M. D. C., Titto E. A. L., Titto C. G., Pereira A. M. F., and Neto M.. . 2013. Influence of stocking density on weight gain and behavior of feedlot lambs. Small Rumin. Res. 115:1–6. doi: 10.1016/j.smallrumres.2013.07.010 [DOI] [Google Scholar]
- Lu, Y., Liu M., Shi S., Jiang H., Yang L., Liu X., Zhang Q., and Pan F.. . 2010. Effects of stress in early life on immune functions in rats with asthma and the effects of music therapy. J. Asthma 47:526–531. doi: 10.3109/02770901003801964 [DOI] [PubMed] [Google Scholar]
- Luo, L., Jansen C. A., Bolhuis J. E., Arts J. A. J., Kemp B., and Parmentier H. K.. . 2020. Early and later life environmental enrichment affect specific antibody responses and blood leukocyte subpopulations in pigs. Physiol. Behav. 217:112799. doi: 10.1016/j.physbeh.2020.112799 [DOI] [PubMed] [Google Scholar]
- Matur, E., Akyazi İ., Eraslan E., Ergul Ekiz E., Eseceli H., Keten M., Metiner K., and Aktaran Bala D.. . 2016. The effects of environmental enrichment and transport stress on the weights of lymphoid organs, cell-mediated immune response, heterophil functions and antibody production in laying hens. Anim. Sci. J. 87:284–292. doi: 10.1111/asj.12411 [DOI] [PubMed] [Google Scholar]
- Meijer, M. K., Sommer R., Spruijt B. M., van Zutphen L. F., and Baumans V.. . 2007. Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Lab. Anim. 41:161–173. doi: 10.1258/002367707780378168 [DOI] [PubMed] [Google Scholar]
- Meshabaz, R., Hussein N. J., Mersham M. A. M., and Mhamed M. S. S.. . 2017. Effect of using two music types on non-pregnant non-lactating Arabi ewes behaviour as a tool for welfare improvement. Sci. J. Univ. Zakho. 4:301–306. doi: 10.25271/2017.5.4.412 [DOI] [Google Scholar]
- Mintline, E. M., M., Stewart, Rogers A. R., Cox N. R., Verkerke G. A., Stookey J. M., Webster J. R., and Tucker C. B.. . 2013. Play behavior as an indicator of animal welfare: disbudding in dairy calves. Appl. Anim. Behav. Sci. 144:22–30. doi: 10.1016/j.applanim.2012.12.008 [DOI] [Google Scholar]
- Nasirahmadi, A., Edwards S. A., Matheson S. M., and Sturm B.. . 2017. Using automated image analysis in pig behavioural research: assessment of the influence of enrichment substrate provision on lying behaviour. Appl. Anim. Behav. Sci. 196:30–35. doi: 10.1016/j.applanim.2017.06.015 [DOI] [Google Scholar]
- Newberry, R. C. 1995. Environmental enrichment: increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 44:229–243. doi: 10.1016/0168-1591(95)00616-z [DOI] [Google Scholar]
- O′Connor, E. A., Parker M. O., McLeman M. A., Demmers T. G., Lowe J. C., Cui L., Davey E. L., Owen R. C., Wathes C. M., and Abeyesinghe S. M.. . 2010. The impact of chronic environmental stressors on growing pigs, Sus scrofa (Part 1): stress physiology, production and play behaviour. Animal 4:1899–1909. doi: 10.1017/S1751731110001072 [DOI] [PubMed] [Google Scholar]
- Otten, W., Kanitz E., Puppe B., Tuchscherer M., Brüssow K. P., Nurnberg G., and Stabenow B.. . 2004. Acute and long term effects of chronic intermittent noise stress on hypothalamic-pituitary-adrenocortical and sympathoadrenomedullary axis in pigs. Anim. Sci. J. 78:271–283. doi: 10.1017/S1357729800054060 [DOI] [Google Scholar]
- Parker, M. O., O′Connor E. A., McLeman M. A., Demmers T. G., Lowe J. C., Owen R. C., Davey E. L., Wathes C. M., and Abeyesinghe S. M.. . 2010. The impact of chronic environmental stressors on growing pigs, Sus scrofa (Part 2): social behaviour. Animal 4:1910–1921. doi: 10.1017/S1751731110001084 [DOI] [PubMed] [Google Scholar]
- Parrott, R., Misson B. H., and Baldwin B. A.. . 1989. Salivary cortisol in pigs following adrenocorticotrophic hormone stimulation: comparison with plasma levels. Br. Vet. J. 145:362–366. doi: 10.1016/0007-1935(89)90034-1 [DOI] [PubMed] [Google Scholar]
- Protopopova, A. 2016. Effects of sheltering on physiology, immune function, behavior, and the welfare of dogs. Physiol. Behav. 159:95–103. doi: 10.1016/j.physbeh.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Reefmann, N., Kaszàs F. B., Wechsler B., and Gygax L.. . 2009. Ear and tail postures as indicators of emotional valence in sheep. Appl. Anim. Behav. Sci. 118:199–207. doi: 10.1016/j.applanim.2009.02.013 [DOI] [Google Scholar]
- Reimert, I., Bolhuis J. E., Kemp B., and Rodenburg T. B.. . 2013. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 109:42–50. doi: 10.1016/j.physbeh.2012.11.002 [DOI] [PubMed] [Google Scholar]
- Ruis, M. A. W., te Brake J. H. A., Engel B., Buist W. G., Blokhuis H. J., and Koolhaas J. M.. . 2002. Implications of coping characteristics and social status for welfare and production of paired growing gilts. Appl. Anim. Behav. Sci. 75:207–231. doi: 10.1016/S0168-1591(01)00191-5 [DOI] [Google Scholar]
- Stachurska, A., Janczarek I., Wilk I., and Kedzierski W.. . 2015. Does music influence emotional state in race horses. J. Equine Vet. Sci. 35:650–656. doi: 10.1016/j.jevs.2015.06.008 [DOI] [Google Scholar]
- Stern, S., and Andresen N.. . 2003. Performance, site preferences, foraging and excretory behaviour in relation to feed allowance of growing pigs on pasture. Livest. Prod. Sci. 79: 257–265. doi: 10.1016/S0301-6226(02)00171-9 [DOI] [Google Scholar]
- Talling, J., Lines J. A., Wathes C., and Waran N. K.. . 1998. The acoustic environment of the domestic pig. J. Agr. Eng. Res. 71:1–12. doi: 10.1006/jaer.1998.0291 [DOI] [Google Scholar]
- Talling, J., Waran N. K., Wathes C., and Lines J. A.. . 1996. Behavioural and physiological responses of pigs to sound. Appl. Anim. Behav. Sci. 48:187–201. doi: 10.1016/0168-1591(96)01029-5 [DOI] [Google Scholar]
- Turpin, D. L., Langendijk P., Plush K., and Pluske J. R.. . 2017. Intermittent suckling with or without co-mingling of non-littermate piglets before weaning improves piglet performance in the immediate post-weaning period when compared with conventional weaning. J. Anim. Sci. Biotechnol. 8:14. doi: 10.1186/s40104-017-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura, T., Gomes M. C., and Carreira T.. . 2012. Cortisol and anxiety response to a relaxing intervention on pregnant women awaiting amniocentesis. Psychoneuroendocrinology 37:148–156. doi: 10.1016/j.psyneuen.2011.05.016 [DOI] [PubMed] [Google Scholar]
- Vermeer, H. M., De Greef K. H., and Houwers W.. . 2014. Space allowance and pen size affect welfare indicators and performance of growing pigs under Comfort Class conditions. Livest. Sci. 159:79–86. doi: 10.1016/j.livsci.2013.10.021 [DOI] [Google Scholar]
- Wegner, B., Spiekermeierb I., Nienhoff H., Große-Kleimannc J., Rohnc K., Meyerd H., Plated H., Gerhardye H., Kreienbrockc L., Beilagef E., . et al. 2019. Status quo analysis of noise levels in pig fattening units in Germany. Livest. Sci. 230:103847. doi: 10.1016/j.livsci.2019.103847 [DOI] [Google Scholar]
- Wilson, M. E., Phillips C. J. C., Lisle A. T., Anderson S. T., Bryden W. L., and Cawdell-Smith A. J.. . 2011. Effect of music on the behavioural and physiological responses of stabled weanlings. J. Equine Vet. Sci. 31:321–322. doi: 10.1016/j.jevs.2011.03.157 [DOI] [Google Scholar]
- Xu, J., Yu L., Cai R., Zhang J., and Sun X.. . 2009. Early auditory enrichment with music enhances auditory discrimination learning and alters NR2B protein expression in rat auditory cortex. Behav. Brain Res. 196:49–54. doi: 10.1016/j.bbr.2008.07.018 [DOI] [PubMed] [Google Scholar]
- Zahra, J., Kolb B. E., and Mohajerani M. H.. . 2018. Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice. Exp. Neurol. 308:1–12. doi: 10.1016/j.expneurol.2018.06.011 [DOI] [PubMed] [Google Scholar]
- Zhang, S. Y., Peng G. Y., Gu L. G., Li Z. M., and Yin S. J.. . 2013. Effect and mechanisms of Gong-tone music on the immunological function in rats with Liver (Gan)-qi depression and Spleen (Pi)-qi deficiency syndrome in rats. Chin. J. Integr. Med. 19: 212–216. doi: 10.1007/s11655-011-0946-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.