Abstract
SARS-CoV-2, the cause of COVID-19, infected over 100 million people globally by February 2021. Reverse zoonotic transmission of SARS-CoV-2 from humans to other species has been documented in pet cats and dogs, big cats and gorillas in zoos, and farmed mink. As SARS-CoV-2 is closely related to known bat viruses, assessment of the potential risk of transmission of the virus from humans to bats, and its subsequent impacts on conservation and public health, is warranted. A qualitative risk assessment was conducted by a multi-disciplinary group to assess this risk in bats in the Australian context, with the aim of informing risk management strategies for human activities involving interactions with bats. The overall risk of SARS-CoV-2 establishing in an Australian bat population was assessed to be Low, however with a High level of uncertainty. The outcome of the assessment indicates that, for the Australian situation where the prevalence of COVID-19 in humans is very low, it is reasonable for research and rehabilitation of bats to continue, provided additional biosecurity measures are applied. Risk assessment is challenging for an emerging disease where information is lacking and the situation is changing rapidly; assessments should be revised if human prevalence or other important factors change significantly. The framework developed here, based on established animal disease risk assessment approaches adapted to assess reverse zoonotic transmission, has potential application to a range of wildlife species and situations.
Keywords: SARS-CoV-2, COVID-19, Wildlife, Disease risk assessment, Zoonoses, Reverse zoonosis, Bats, One health
Graphical abstract
1. Introduction
SARS-CoV-2 is a human betacoronavirus closely related to known bat coronaviruses, particularly those found in bats from the Rhinolophus genus [[1], [2], [3]]. The progenitor of SARS-CoV-2 likely exists or existed in bats; however, the chain of transmission to humans and the possible involvement of an intermediate host are unknown [4,5]. There are over 80 species of bats in nine families in Australia [6], and coronavirus surveillance has been conducted in around 40 of these [7]. While alpha- and betacoronaviruses have been found in Australian bats [[7], [8], [9]] there have been no detections to date of Sarbecoviruses or Merbecoviruses (SARS-CoV-1, MERS-CoV, SARS-CoV-2 and related viruses) in bats or other wildlife in Australia. However, serological evidence of exposure to a coronavirus antigenically related to SARS-CoV-1 has been found in multiple bat species [[8], [9], [10]].
The COVID-19 pandemic is caused by infection with the SARS-CoV-2 virus. As of 7 February 2021 there were over 100 million human cases of COVID-19 reported globally, including 28,848 in Australia [11]. Human-to-human transmission is primarily via respiratory droplets in close contact situations and aerosol transmission under particular conditions [12]. SARS-CoV-2 may also be spread via fomites [12,13], with studies under controlled laboratory conditions showing virus can remain viable on surfaces for hours to days, depending on the material [14].
There are a small number of reports of human-to-animal transmission of SARS-CoV-2 in pet cats and dogs [15] and in gorillas, tigers, lions and other felines in zoos in the USA, Europe and South Africa [[16], [17], [18], [19], [20]]. Initial human-to-animal transmission has resulted in sustained outbreaks in farmed mink in Europe and North America [[21], [22], [23]], with likely mink-to-human transmission reported in the Netherlands [24]. Infection has also been identified in wild mink in the USA [25]. Experimental infection of Egyptian fruit bats (Rousettus aegyptiacus) resulted in a transient subclinical respiratory infection with oral and faecal shedding, and virus detection in an in-contact bat [26]. Big brown bats (Eptesicus fuscus), a common North American species, showed no evidence of SARS-CoV-2 infection after experimental infection [27].
Given the probable source of SARS-CoV-2 or its progenitor is a bat species, the potential risk of reverse zoonotic transmission from humans to bats and the subsequent negative impacts on conservation and public health have been recognised globally. This risk has been assessed for North American bats [28,29] and bats in the UK [30], for bats globally by the IUCN SSG Bat Specialist Group [[31], [32], [33]], and for wildlife more broadly [34]. In Australia, the considerably lower prevalence of COVID-19 and the different nature of human-bat interactions highlighted the need for an assessment specific to this biogeographical context.
A working group of government and non-government stakeholders established by Wildlife Health Australia (WHA) developed interim biosecurity guidance for those interacting with bats, and agreed that a formal risk assessment should be conducted [35]. A small multi-disciplinary subgroup conducted a qualitative rapid risk assessment of transmission of SARS-CoV-2 from humans to bats in Australia, using a collective group approach. The purpose of the assessment was primarily to inform risk management strategies for human activities involving interactions with bats. Our approach may also have application for different scenarios in other countries.
The assessment described here reflects the knowledge and situation current at the time (July–August 2020). Given the dynamic situation with COVID-19, it is likely that the assessment will need to be revised over time; however, the risk analysis framework developed and the documented rationale will allow the process to be undertaken rapidly when new information becomes available or the situation changes.
2. Methods
The assessment was conducted by a group of six assessors with expertise in bat ecology, veterinary epidemiology/disease ecology, emerging diseases, virology, immunology, wildlife health and disease risk assessment. It was undertaken from May to August 2020 using data and information available at that time. The risk was assessed collectively, using a combination of available scientific literature and expert opinion of the group members. Input on specific details was sought from outside the group when needed. An initial draft was sent to three reviewers with expertise in disease risk assessment and decision science, and the final draft was reviewed by the broader WHA working group.
A qualitative risk assessment approach was chosen due to the lack of quantitative information on the majority of relevant parameters (e.g. number of human-bat interactions, level of susceptibility of bats to SARS-CoV-2 infection, viral shedding characteristics, etc.) and for consistency with Australian Government risk assessments. The process was based on that used for import risk analysis [36], with the addition of elements from published animal disease risk assessments [37,38], as outlined below.
The risk question was defined as: “What is the risk of SARS-CoV-2 establishing in an Australian bat population as a result of human-to-bat transmission?”. The hazard was defined as SARS-CoV-2. The risk period was defined as 6–12 months from the commencement of the assessment.
The risk assessment process was based on the principle of “Risk = Likelihood x Consequence”, conducted according to published methods and Australian and UK guidelines, as follows:
Table 1.
Scoring system for assessing likelihood (adapted from [39]).
| Description | Definition |
|---|---|
| Negligible | The event would almost certainly not occur |
| Extremely low | The event would be extremely unlikely to occur |
| Very low | The event would be very unlikely to occur |
| Low | The event would be unlikely to occur |
| Moderate | The event would be likely to occur |
| High | The event would be very likely to occur |
Table 2.
Matrix for combining introduction and exposure/establishment (adapted from [38]).
| Introduction |
|||||||
|---|---|---|---|---|---|---|---|
| Negligible | Extremely low | Very low | Low | Moderate | High | ||
| Exposure & establishment | Negligible | N | N | N | N | N | N |
| Extremely low | N | EL | EL | EL | EL | EL | |
| Very low | N | EL | VL | VL | VL | VL | |
| Low | N | EL | VL | L | L | L | |
| Moderate | N | EL | VL | L | M | M | |
| High | N | EL | VL | L | M | H | |
Table 3.
Scoring system for assessing consequences (adapted from [40]).
| Description | Definition |
|---|---|
| Insignificant | No detectable conservation or welfare effects |
| Very minor | Local short-term population loss, no significant ecosystem effect; OR mild animal welfare effects |
| Minor | Some localised, reversible ecosystem impact; OR mild animal welfare effects |
| Moderate | Measurable long-term damage to populations and/or ecosystem, but little spread, no extinction; OR more significant animal welfare effects |
| High | Long-term irreversible ecosystem change, spreading beyond local area; OR significant animal welfare effects |
| Catastrophic | Widespread, long-term population loss affecting several species OR extinction of a species, serious ecosystem effects; OR severe animal welfare effects |
Table 4.
Matrix for combining likelihood and consequences (adapted from [36]).
| Consequences |
|||||||
|---|---|---|---|---|---|---|---|
| Insignificant | Very minor | Minor | Moderate | High | Catastrophic | ||
| Likelihood | High | N | VL | L | M | H | E |
| Moderate | N | VL | L | M | H | E | |
| Low | N | N | VL | L | M | H | |
| Very low | N | N | N | VL | L | M | |
| Extremely low | N | N | N | N | VL | L | |
| Negligible | N | N | N | N | N | VL | |
N = Negligible risk; VL = Very low risk; L = Low risk; M = Moderate risk; H = High risk; E = Extreme risk.
The level of uncertainty of each score was rated using the scale defined in Table 5, and the uncertainty scores were combined by using the highest score for any of the steps [38]. Where information was lacking, or where considerable variation in risk components was expected, a precautionary approach to scoring was taken that tended towards a higher likelihood and a higher uncertainty rating.
Table 5.
Rating scale for level of uncertainty.
| Description | Definition |
|---|---|
| Low | Strong level of confidence in the assessment. Scientific evidence and/or previous experience of similar situations is available. |
| Medium | Moderate level of confidence in the assessment. Some scientific evidence and/or previous experience of somewhat similar situations is available. |
| High | Limited level of confidence in the assessment. Scientific evidence and previous experience is lacking; high degree of variation across the scenarios considered; high potential for variability in the outcomes. |
The scope was restricted to people with occupational interaction with bats (specifically bat carers/rehabilitators, researchers and ecological consultants) and recreational visitors to caves. Veterinary staff were not specifically included; however, much of the assessment is also relevant to those working in a veterinary capacity. While members of the public also interact with bats, this is generally only a single instance of short-lived contact. ‘Interaction’ includes physical contact, being in close proximity, and indirect contact via surfaces or fomites. The assessment was conducted assuming a baseline level of risk mitigation measures, i.e. standard biosecurity practices in use prior to the COVID-19 pandemic. An infected person was assumed to be shedding viable virus. For the purpose of this risk assessment, we considered flying-foxes (Pteropus spp.) separately from the other Australian bat species, due to differences in the nature of interactions with humans between these groups. The assessment assumes that SARS-CoV-2 is not already present in bats in Australia.
3. Results
3.1. Likelihood assessment
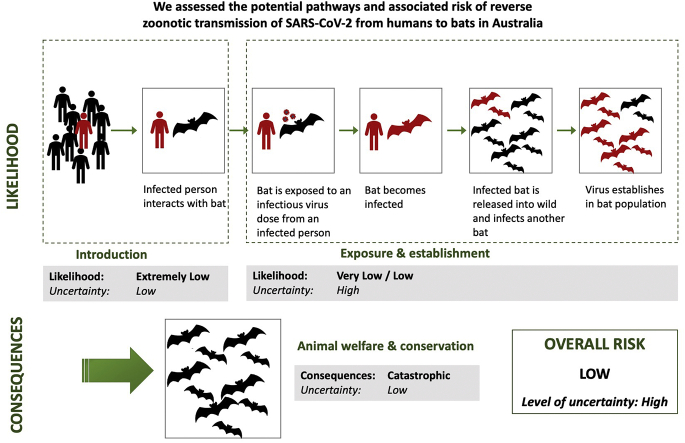
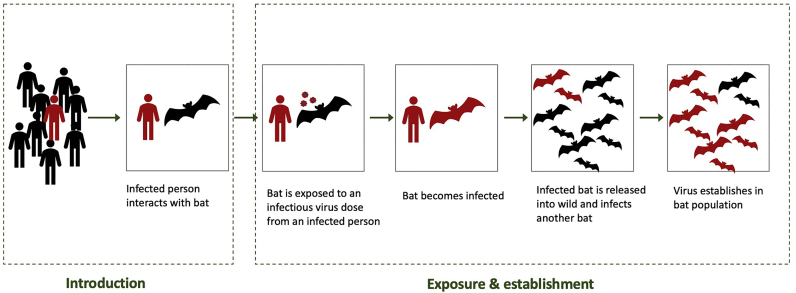
The introduction pathway for SARS-CoV-2 into Australian bat populations was identified as the interaction between an infected person and a bat within Australia (Fig. 1). Other possible pathways were identified, such as migration of an infected bat into Australia, but were not considered of sufficient likelihood to assess further.
Fig. 1.
Steps in the likelihood assessment.
3.1.1. Introduction assessment
In assessing the likelihood that the hazard (SARS-CoV-2) is introduced into the immediate physical environment of an Australian bat, the primary consideration was the very low prevalence of COVID-19 in the human population in Australia at the time of the assessment and the low number of people interacting with bats. As of 19 August (at the completion of the assessment), there were 23,993 confirmed cases of SARS-CoV-2 infection in Australia, of which 7434 were active, and 5,440,495 tests had been conducted [41]. This was around the peak of transmission experienced in Australia, so it represented a worst-case scenario to date. On 16 August, the rate of infection of locally acquired cases was 73.3 per 100,000 population, and for the preceding two weeks was 14.8 per 100,000 population [42]. The likelihood of introduction was assessed as Extremely Low, with an uncertainty score of Low (Table 6).
Table 6.
Likelihood and uncertainty scores for introduction.
| Introduction pathway | Likelihood | Uncertainty |
|---|---|---|
| Persona infected with SARS-CoV-2 interacts with a bat(s) | Extremely Low | Low |
Scope limited to a person with occupational interaction with bats or a person visiting a cave for recreation.
3.1.2. Exposure and establishment assessment
The likelihood of exposure of susceptible individual bats and establishment in a wild bat population was considered to be different for flying-foxes (Pteropus spp.) and the other bat species due to factors such as susceptibility to SARS-CoV-2 infection, numbers in care, field capture methods, and ecological characteristics such as colony size and social interactions. Similarly, the nature of interactions with bats and biosecurity practices differ between categories of people (bat rehabilitators, researchers and ecological consultants, recreational visitors to caves). For each pathway, the component steps were considered, as described below. The likelihood of exposure and establishment was assessed as Low for the pathway involving a bat rehabilitator and flying-fox, and Very Low for all other pathways; the uncertainty score was High for all pathways (Table 7).
Table 7.
Likelihood and uncertainty scores for exposure/establishment.
| Exposure & establishment pathway | Likelihood | Uncertainty |
|---|---|---|
| SARS-CoV-2 establishes in bat population as a result of initial transmission from: | ||
| Bat carer to a flying-fox | Low | High |
| Bat carer to other bat species | Very low | High |
| Researcher/consultant to a flying-fox | Very low | High |
| Researcher/consultant to other bat species | Very low | High |
| Person visiting a cave to other bat species | Very low | High |
3.1.2.1. Bat is exposed to an infectious dose of SARS-CoV-2 by direct or indirect contact with an infected person
3.1.2.1.1. Bat rehabilitators
In Australia, wildlife rehabilitators rescue and care for sick, injured and orphaned wildlife with the aim of rehabilitation and release back into the wild. Bat rehabilitators are generally volunteers, and bats may be kept in the rehabilitator's home, in an outdoor enclosure, or in some cases in an established rehabilitation centre. The number of bats in care across Australia is not centrally recorded, but is likely to be in the thousands per year [43]. Biosecurity practices vary significantly among rehabilitators; for this assessment, a basic level of biosecurity was assumed. The exposure likelihood was scored higher for flying-foxes than other bat species, as significantly more flying-foxes come into care and they are generally held in care for a longer time (J. Mclean, Tolga Bat Hospital, personal communication).
3.1.2.1.2. Researchers and consultants
Biosecurity for researchers was assumed to range from a medium to high level. A higher level of personal protective equipment (PPE) is generally used with flying-foxes than other bat species, and blowing on smaller bats (e.g. to encourage movement or to release their hold) is common practice (J. Welbergen, unpublished observation). Compared with rehabilitators, the average contact between researchers and bats is considerably shorter, less frequent, not as physically close, and usually outdoors.
3.1.2.1.3. Cave visitors
Caves are an enclosed environment with shared airspace and limited ventilation, some caves have very large colonies of bats, and high numbers of general public including tourists visit some caves. However, most of the ‘show’ caves open to the public do not have bats, or where bats are present they tend to roost high up in the cave (N. White, Australian Speleological Federation, personal communication), and there are codes of practice for recreational and scientific use of caves to avoid disturbance of bats [44]. Close encounters with bats may occur, but only infrequently and usually only for advanced club cavers (N. White, personal communication). There is a high level of uncertainty due to variability in cave visitation and conditions, seasonal movements of bats, and the unknown potential for viral particles to aerosolise inside caves.
3.1.2.2. Bat becomes infected with SARS-CoV-2
The susceptibility of Australian bat species to SARS-CoV-2 is unknown. The following factors contributed to the assessment: a) the premise that bats are likely the source of the progenitor of SARS-CoV-2; b) the potential for immunity due to cross-protection from existing coronaviruses in the Australian bat population; and c) the available scientific information on molecular analysis of the virus, and evidence that at least one bat species can be experimentally infected with SARS-CoV-2 [26].
3.1.2.3. Infected bat is: A) released; and B) transmits SARS-CoV-2 to another bat in the wild after release; and C) there is ongoing transmission sufficient for virus to establish in population
Only a proportion of rescued bats recover sufficiently in care to be released (e.g. [43]). As bats are usually in care for at least 1–2 weeks, some would likely complete the infectious period while in captivity [26]. If infected, it was considered unlikely that bats would display clinical signs, given the evident evolutionary relationship between bats and coronaviruses [2], and the results from initial experimental infections with SARS-CoV-2 in bats [26]. In the research context, almost every bat captured is released after short term handling and the infectious period would therefore occur in the wild. When compared to bats in rehabilitation that have recently recovered from illness or injury, bats captured for research are also more likely to be healthy and immunocompetent.
Due to the colonial nature of Australian mainland flying-fox species, an infected individual would likely expose other flying-foxes to the virus. For other bat species there are significant species and seasonal differences in roosting behaviour. The highest risk will be for colonial species roosting in high densities in caves. Based on current epidemiological knowledge of other bat coronaviruses and the known circulation of betacoronaviruses in Australian bats [[7], [8], [9]], the virus would likely establish in the bat population.
3.1.3. Likelihood of occurrence (introduction, exposure and establishment)
For all pathways, the combination of introduction and exposure/establishment scores resulted in an Extremely Low estimated likelihood of SARS-CoV-2 establishing in an Australian bat population as a result of human-to-bat transmission, with a High level of uncertainty.
3.2. Consequence assessment
Establishment of SARS-CoV-2 in an Australian bat population would result in conservation and animal welfare, public health, economic and social impacts. Based on the expertise in our group, only impacts for conservation and animal welfare were scored (Table 8). While the other categories were not formally assessed, they are also discussed below and were considered to have a lower consequence than conservation/animal welfare, so that the overall risk estimate is valid within our precautionary approach.
Table 8.
Consequence and uncertainty scores (conservation & animal welfare).
| Disease scenario | Consequence | Uncertainty |
|---|---|---|
| SARS-CoV-2 established in a flying-fox population | CATASTROPHIC | LOW |
| SARS-CoV-2 established in other bat population | CATASTROPHIC | LOW |
3.2.1. Conservation/animal welfare
As explained above, it was considered unlikely that SARS-CoV-2 would cause disease in bats. The conservation and welfare consequences would therefore be secondary impacts due to human reactions to bats rather than primary impacts of the disease itself. A backlash against bats due to COVID-19 has already been reported in Australia and overseas [[45], [46], [47], [48], [49]]. Based on experience with other bat viruses, such as Australian bat lyssavirus and Hendra virus, likely human reactions include increased calls for dispersal and destruction of flying-fox roosts, culling of bats, and an escalation of anti-bat sentiment leading to cruelty, harassment and illegal killing [50,51]. As well as the severe animal welfare impacts, these types of actions would add to existing threats to species conservation and could conceivably lead to extinction of some species, particularly the endangered spectacled flying-fox (Pteropus conspicillatus). Serious ecosystem effects are likely to result from the extinction or population loss of flying-fox species, due to their role as long-distance pollinators and seed dispersers [52]. Erosion of the perceived biodiversity value of bats could result in loss of current protections for some threatened bat species. The conservation and animal welfare consequences were therefore rated as Catastrophic, with a Low level of uncertainty (Table 8).
3.2.2. Public health
If SARS-CoV-2 became established in bats it could potentially spill back to humans. Given the highly mobile nature of bats, and the low likelihood of SARS-CoV-2 infection significantly compromising their health, establishment of a reservoir in Australian bats could result in sporadic human cases or clusters, leading to ongoing public health demands. Any future attempts to eradicate the virus would be complicated by an established wildlife reservoir.
3.2.3. Economic
Economic consequences - calibrated against other major events such as the pandemic as a whole, or Australia's recent bushfires, etc. - may not be as severe as the public health and conservation consequences. These could include costs associated with the public health response, loss of overseas tourism, restriction of travel and closed borders, reputational risk for Australia and potential trade impacts. At a more local level, there may be economic consequences associated with roost management, disruption of living arrangements of residents near roosts, and cost of risk communication by public health agencies, councils, etc.
3.2.4. Social
Public amenity impacts will vary between regions, being highest in urban and city settings where flying-foxes roost and forage, and particularly for houses near bat colonies, i.e. a severe impact on individual people in specific areas. Impacts could include loss of access to parks and outdoor areas, anxiety due to proximity of flying-foxes, loss of large trees due to roost management, and the implications of contamination of areas with bat faeces. For other bat species, a much lower proportion of people would be affected.
3.3. Overall risk estimation
The final estimation of the risk of SARS-CoV-2 establishing in an Australian bat population is Low, with a High level of uncertainty associated with the estimate.
4. Discussion
Assessments of COVID-19 risk to wildlife differ from many other disease risk assessments in that they consider the possible transmission of a disease from humans to wildlife, i.e. a ‘reverse zoonosis’. We chose a rapid qualitative risk assessment approach and developed a framework based on established animal disease risk assessment processes, adapted to meet the needs of the situation and the Australian context, which differed from that on other continents [28,[30], [31], [32]].
Based on the situation and information available at the time of the assessment, the overall risk of human-to-bat transmission of SARS- CoV-2 in Australia was assessed to be Low. A UK risk assessment [30] similarly found the overall risk to be low, while the US risk assessment [28] found a “non-negligible” risk, and the IUCN SSG Bat Specialist Group [[31], [32], [33]] found a “credible” risk of human-to-bat transmission of SARS- CoV-2. While similarities exist, it is difficult to directly compare our outcome with overseas assessments due to differences in approach and scope. For example, the UK study [30] assessed the ecological and environmental consequences of infection in bats to be low, based on the low likelihood of clinical disease associated with infection. In contrast, we considered negative human reactions to bats in rating the conservation/animal welfare consequences as Catastrophic. The US risk assessment [28] used a quantitative approach with expert elicitation, and focussed on likelihood and effect of risk management actions, rather than a formal assessment of consequences. The scope of that assessment was specifically for little brown bats (Myotis lucifugus) as a surrogate species during the active bat season in temperate areas of North America. We assessed how the risk of SARS-CoV-2 transmission from people to bats varied between flying-foxes and other species, as a result of differences in their ecology and interactions with people. While our scope was broader, we acknowledged through the uncertainty ratings that the risk will not be the same across all bat populations in Australia, due to factors such as species susceptibility, location, ecology, behaviour, and frequency of contact with people.
In our assessment, areas with high levels of uncertainty included bat-related factors such as susceptibility of Australian bat species within both suborders to SARS-CoV-2 infection and variation across bat populations, and human-related factors such as variability in activities and biosecurity practices of people interacting with bats, and geographic variation in human COVID-19 prevalence. To manage the human-related factors, we advise that individuals assess their personal risk according to their own particular circumstances, considering factors such as the number and nature of bat interactions and COVID-19 prevalence in their area. In the US risk assessment [28], critical uncertainties identified in the estimation of risk included the probability that a person working with bats is shedding virus, the replication of SARS-CoV-2 in bat tissue, and virus transmission within bat populations. The previous detection of various alpha- and betacoronaviruses in multiple Australian bat species [[7], [8], [9]] forms the basis for our greater certainty with respect to likely ongoing transmission in this assessment. The key factor influencing the outcome of our risk assessment was the very low prevalence of COVID-19 in the Australian human population at the time of the assessment (and subsequently into the defined risk period), which resulted in an Extremely low likelihood of introduction. The assessment will thus need to be revisited if the prevalence changes significantly.
The outcome of the assessment indicates that for situations where the human COVID-19 prevalence is very low, it is reasonable for research and rehabilitation of bats to continue; however, additional biosecurity measures over and above routine protocols should be applied. These measures are outlined for the Australian context in WHA's guidelines [35]. Similar measures are outlined in the IUCN SSC Bat Specialist Group recommendations [[31], [32], [33]], and for wildlife more broadly in guidelines developed by the IUCN Wildlife Health Specialist Group and the World Organisation for Animal Health (OIE) [53]. Broadly speaking, the same precautions recommended for prevention of human-to-human transmission should be applied to interactions with bats, including not interacting if the person is a COVID-19 case or a close contact of a case, practicing good hygiene, and wearing a face mask where it is not possible to maintain physical distancing between the person and the bat. PPE is recommended along with dedicated clothing and regular cleaning of linen, equipment, enclosures and surfaces. Additional precautions recommended for rehabilitators include—where possible without compromising animal welfare—reducing the number of bats in care in individual homes, minimising the number of people interacting with bats in care, and avoiding unnecessary contact with the bats. If individuals assess their own risk as higher than outlined in this assessment, higher level precautions are recommended and, where feasible, it may be appropriate to restrict, postpone or cancel activities until the risk is reduced.
Risk assessment is challenging for an emerging disease where information is lacking and the situation is changing rapidly. This was a rapid assessment using the best available information at the time, and reassessment will be needed as the COVID-19 situation changes and we learn more about the disease. As well as the direct outcomes of the assessment, we have provided a framework for risk assessments of other zoonotic diseases, in particular for potential transmission of disease from humans to wildlife.
Conflict statement
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the valuable input and review by Jenny Mclean, Nicholas White, Matthew Mo, Andrea Reiss, Terry Walshe, Sandra Parsons, the COVID-19 & Bats Working Group, and the WHA Bat Health Focus Group. AJP was supported by an ARC DECRA fellowship (DE190100710). JAW was supported, in part, by an ARC Discovery Grant (DP170104272) and an ARC Linkage Grant (LP160100439). Funding for the national wildlife health program is provided by the Australian Government Department of Agriculture, Water and the Environment and state and territory governments.
References
- 1.Zhou H., Chen X., Hu T., Li J., Song H., Liu Y., Wang P., Liu D., Yang J., Holmes E.C. A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr. Biol. 2020;30(11):2196–2203. doi: 10.1016/j.cub.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latinne A., Hu B., Olival K.J., Zhu G., Zhang L., Li H., Chmura A.A., Field H.E., Zambrana-Torrelio C., Epstein J.H., Li B., Zhang W., Wang L.-F., Shi Z.-L., Daszak P. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020;11(1):4235. doi: 10.1038/s41467-020-17687-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong K.N., Reardon T.B., J.S. M . Austr. Bat. Soc. 2020. A current taxonomic list of Australian Chiroptera.http://ausbats.org.au/species-list Version 2020-06-09. [Google Scholar]
- 7.Peel A.J., Field H.E., Aravena M.R., Edson D., McCallum H., Plowright R.K., Prada D. Coronaviruses and Australian bats: a review in the midst of a pandemic. Austr. J. Zool. 2020;67(6):346–360. [Google Scholar]
- 8.Prada D., Boyd V., Baker M.L., O’Dea M., Jackson B. Viral diversity of microbats within the south west botanical province of western Australia. Viruses. 2019;11(12):1157. doi: 10.3390/v11121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith C., De Jong C., Meers J., Henning J., Wang L.-F., Field H. Coronavirus infection and diversity in bats in the Australasian region. Ecohealth. 2016;13(1):72–82. doi: 10.1007/s10393-016-1116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boardman W.S.J., Baker M.L., Boyd V., Crameri G., Peck G.R., Reardon T., Smith I.G., Caraguel C.G.B., Prowse T.A.A. Serological evidence of exposure to a coronavirus antigenically related to severe acute respiratory syndrome virus (SARS-CoV-1) in the Grey-headed flying fox (Pteropus poliocephalus) Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13908. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Coronavirus disease (COVID-2019) weekly epidemiological update - 9 February 2021. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 12.World Health Organization Coronavirus disease (COVID-19): How is it transmitted? 2020. https://www.who.int/news-room/q-a-detail/q-a-how-is-covid-19-transmitted
- 13.Australian Government Department of Health . Vol. 44. 2020. COVID-19, Australia: Epidemiology Report 19: Fortnightly reporting period ending 21 June 2020, Communicable Diseases Intelligence. [DOI] [PubMed] [Google Scholar]
- 14.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leroy E.M., Ar Gouilh M., Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;10:100133. doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAloose D., Laverack M., Wang L., Killian M.L., Caserta L.C., Yuan F., Mitchell P.K., Queen K., Mauldin M.R., Cronk B.D. From people to Panthera: natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. mBio. 2020;11(5) doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Agriculture Confirmation of COVID-19 in a Snow Leopard at a Kentucky Zoo. 2020. https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2020/sa-12/ky-snow-leopard-covid
- 18.World Organisation for Animal Health Information received on 12/08/2020 from Dr Bothle Michael Modisane, Chief Director , Department of Agriculture, Forestry and Fisheries, Animal Production and Health, PRETORIA, South Africa. 2020. https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=35399
- 19.World Organisation for Animal Health COVID-19 Portal, Events in Animals. 2020. https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/events-in-animals/
- 20.U.S. Department of Agriculture Confirmation of COVID-19 in Gorillas at a California Zoo. 2021. https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-01/ca-gorillas-sars-cov-2
- 21.World Health Organization SARS-CoV-2 mink-associated variant strain – Denmark, Disease Outbreak News, 6 November 2020. 2020. https://www.who.int/csr/don/06-november-2020-mink-associated-sars-cov2-denmark/en/
- 22.Cahan E. COVID-19 hits US mink farms after ripping through Europe. Science. 2020;80(10.1126) [Google Scholar]
- 23.Oreshkova N., Molenaar R.J., Vreman S., Harders F., Munnink B.B.O., Hakze-van der Honing R.W., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance. 2020;25(23):2001005. doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munnink B.B.O., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., Van Der Spek A., Tolsma P., Rietveld A., Brouwer M. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2020;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLiberto T. Coronavirus Disease 2019 Update (536): Animal, USA (Utah) Wild Mink, First Case, ProMed, International Society for Infectious Diseases. 2020. https://promedmail.org/promed-post/?id=8015608
- 26.Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., Höper D., Mettenleiter T.C., Balkema-Buschmann A., Harder T. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1(5):e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall J.S., Knowles S., Nashold S.W., Ip H.S., Leon A.E., Rocke T., Keller S., Carossino M., Balasuriya U., Hofmeister E. Experimental challenge of a North American bat species, big brown bat (Eptesicus fuscus), with SARS-CoV-2. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13949. [DOI] [PubMed] [Google Scholar]
- 28.Runge M.C., Grant E.H.C., Coleman J.T., Reichard J.D., Gibbs S.E., Cryan P.M., Olival K.J., Walsh D.P., Blehert D.S., Hopkins M.C. 2020. Assessing the risks posed by SARS-CoV-2 in and via North American bats—Decision framing and rapid risk assessment, US Geological Survey. [DOI] [Google Scholar]
- 29.Olival K.J., Cryan P.M., Amman B.R., Baric R.S., Blehert D.S., Brook C.E., Calisher C.H., Castle K.T., Coleman J.T., Daszak P. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: a case study of bats. PLoS Pathog. 2020;16(9) doi: 10.1371/journal.ppat.1008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Common S.M., Shadbolt T., Walsh K., Sainsbury A.W. The risk from SARS-CoV-2 to bat species in England and mitigation options for conservation field workers. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuñez G., Cunningham A., Fils E., Frick W., Islam M., Jolliffe J., Kading R., Kepel A., Kingston T., Leopardi S., Medellín R., Mendenhall I., Parsons S., Racey P., Russo D., Shapiro J., Vicente-Santos A., Víquez-R L., Dinh T. Vu. MAP: Minimize, Assess, Protect. Living Document Version 1.0 Released 19th June 2020. 2020. IUCN SSC Bat Specialist Group (BSG) Recommended Strategy for Researchers to Reduce the Risk of Transmission of SARS-CoV-2 from Humans to Bats.https://www.iucnbsg.org/uploads/6/5/0/9/6509077/map_recommendations_for_researchers_v._1.0_final.pdf [Google Scholar]
- 32.Jolliffe T., Kepel A., Kingston T., Mclean J., Parsons S., Russo D., Shapiro J., Worledge L. MAP: Minimize, Assess, Protect. Living Document Version 1.1 Released 15th July 2020. 2020. IUCN SSC Bat Specialist Group (BSG) recommendations to reduce the risk of transmission of SARS-CoV-2 from humans to bats in bat rescue and rehabilitation centers.https://www.iucnbsg.org/uploads/6/5/0/9/6509077/recommendations_rehab_draft1.pdf [Google Scholar]
- 33.Estrada L.B.G.M., Kading R., Kingston T., Mandl I., Medellin R., Parsons S., Russo D., Shapiro J.T., Pool F.T., Sánchez I.T., Vicente-Santos A., Worledge L. MAP: Minimize, Assess, Protect. 2020. IUCN SSC Bat Specialist Group (BSG) recommendations to reduce the risk of transmission of SARS-CoV-2 from humans to bats by cavers.https://www.iucnbsg.org/uploads/6/5/0/9/6509077/map_recommendations_for_cavers_22_aug_2020.pdf [Google Scholar]
- 34.Delahay R., de la Fuente J., Smith G., Sharun K., Snary E., Giron L.F., Nziza J., Fooks A., Brookes S., Lean F. Assessing the Risks of SARS-CoV-2 in Wildlife. One Health Outlook. 2021;3 doi: 10.1186/s42522-021-00039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildlife Health Australia COVID-19 and Australian bats – information for bat carers and others interacting with bats. 2020. https://www.wildlifehealthaustralia.com.au/ProgramsProjects/BatHealthFocusGroup.aspx#COVIDBats
- 36.Australian Government Department of Agriculture and Water Resources . 2016. Biosecurity Import Risk Analysis Guidelines 2016: managing biosecurity risks for imports into Australia. [Google Scholar]
- 37.Dufour B., Plee L., Moutou F., Boisseleau D., Chartier C., Lancelot R., Saergerman C., Thebault A., Hattenberger A.-M., Toma B. A qualitative risk assessment methodology for scientific expert panels. Rev. sci. tech. Off. int. Epiz. 2011;30(3):673–681. doi: 10.20506/rst.30.3.2063. [DOI] [PubMed] [Google Scholar]
- 38.Roche S., Costard S., Meers J., Field H., Breed A. Assessing the risk of Nipah virus establishment in Australian flying-foxes. Epidemiol. Infect. 2015;143(10):2213–2226. doi: 10.1017/S0950268813003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Australian Government Department of Agriculture and Water Resources . 2017. Draft pest risk analysis for brown marmorated stink bug (Halyomorpha halys) [Google Scholar]
- 40.CABI . Centre for Environment, Fisheries and Aquaculture Science, Centre for Ecology and Hydrology, Central Science Laboratory, Imperial College London and the University of Greenwich; 2005. UK Non-native Organism Risk Assessment Scheme - User Manual version 3.3. [Google Scholar]
- 41.Australian Government Department of Health Coronavirus (COVID-19) Current Situation and Case Numbers. 2020. https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/coronavirus-covid-19-current-situation-and-case-numbers
- 42.Australian Government Department of Health, COVID-19 . 2020. Australia: Epidemiology Report 23: Fortnightly reporting period ending 16 August 2020, Communicable Diseases Intelligence 44. [DOI] [PubMed] [Google Scholar]
- 43.Mo M., Roache M., Haering R., Kwok A. Using wildlife carer records to identify patterns in flying-fox rescues: a case study in new South Wales, Australia. Pac. Conserv. Biol. 2020;27:61–69. [Google Scholar]
- 44.Australian Speleological Federation ASF Code of Ethics. 2014. https://www.caves.org.au/administration/codes-and-standards/send/8-codes-and-standards/7-code-of-ethics
- 45.Hobbs E.C., Reid T.J. Animals and SARS-CoV-2: species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lentini P., Peel A., Field H., W. J No, Aussie bats won't give you COVID-19. We rely on them more than you think, The Conversation. 2020. https://theconversation.com/no-aussie-bats-wont-give-you-covid-19-we-rely-on-them-more-than-you-think-137168
- 47.National Resource Defence Council Experts Urge People All Over the World to Stop Killing Bats out of Fears of Coronavirus. 2020. https://www.nrdc.org/stories/experts-urge-people-all-over-world-stop-killing-bats-out-fears-coronavirus
- 48.Rocha R., Aziz S., Brook C., Carvalho W., Cooper-Bohannon R. Bat conservation and zoonotic disease risk: a research agenda to prevent misguided persecution in the aftermath of COVID-19. Anim. Conserv. 2020 doi: 10.1111/acv.12636. [DOI] [Google Scholar]
- 49.Sasse D.B., Gramza A.R. Influence of the COVID-19 pandemic on public attitudes toward bats in Arkansas and implications for bat management. Hum. Dimens. Wildl. 2021;26(1):90–93. [Google Scholar]
- 50.Degeling C., Kerridge I. Hendra in the news: public policy meets public morality in times of zoonotic uncertainty. Soc. Sci. Med. 2013;82:156–163. doi: 10.1016/j.socscimed.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiriet D. Flying fox conservation laws, policies and practices in Australia: a case study in conserving unpopular species. Austr. J. Nat. Res. Law Policy. 2010;13(2):161. [Google Scholar]
- 52.Kunz T.H., de Torrez E.B., Bauer D., Lobova T., Fleming T.H. Ecosystem services provided by bats. Europe. 2011;31:32. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- 53.IUCN Wildlife Health Specialist Group, World Organisation for Animal Health, Guidelines for Working with Free-Ranging Wild Mammals in the Era of the COVID-19 Pandemic. 2020. http://www.iucn-whsg.org/COVID-19GuidelinesForWildlifeResearchers