Abstract
Aims
Persons living with HIV (PLWH) have increased cardiovascular mortality, which may in part be due to differences in the management of acute coronary syndromes (ACS). The purpose of this study was to compare the in-hospital and post-discharge management and outcomes of ACS among persons with and without HIV.
Methods and results
This was a retrospective cohort study using data from Symphony Health, a data warehouse. All patients admitted between 1 January 2014 and 31 December 2016 with ACS were identified by International Classification of Diseases billing codes. Multivariate logistic regression models were used to examine in-hospital, 30-day and 12-month event rates between groups. A total of 1 125 126 individuals were included, 6612 (0.59%) with HIV. Persons living with HIV were younger (57.4 ± 10.5 vs. 67.4 ± 12.9 years, P< 0.0001) and had more medical comorbidities. Acute coronary syndrome type did not differ significantly with HIV status. Persons living with HIV were less likely to undergo coronary angiography (35.2% vs. 37.2%, adjusted OR 0.87, 95% CI 0.83–0.92, P < 0.0001), and those with both HIV and STEMI underwent fewer drug-eluting stents (60.1% vs. 68.5%, adjusted OR 0.81, 95% CI 0.68–0.96, P = 0.016). Persons living with HIV had higher adjusted rates of inpatient mortality (OR 1.29, 95% CI 1.15–1.44; P < 0.0001), 30-day readmission (OR 1.18, 95% CI 1.09–1.27; P < 0.0001) and 12-month mortality (OR 1.32, 95% CI 1.22–1.44; P < 0.0001). Twelve months following discharge, PLWH filled cardiac medications at lower rates.
Conclusion
In a contemporary cohort of persons hospitalized for ACS, PLWH received less guideline-supported interventional and medical therapies and had worse clinical outcomes. Strategies to optimize care are warranted in this unique population.
Keywords: HIV, Acute coronary syndrome, Outcomes
Introduction
Though the introduction of highly active antiretroviral therapy (HAART) has led to a reduction in HIV-specific mortality,1 persons living with HIV (PLWH) still have increased overall mortality compared to the general population.2–4 Much of this excess mortality and morbidity is driven by cardiovascular disease (CVD), with CVD now representing an increasing proportion of overall mortality in this population over the past decade.5 A recent meta-analysis of over 790 000 PLWH and 3.5 million person-years of follow-up reported that HIV-associated cardiovascular disease has tripled over the past 2 decades and accounts for 2.6 million disability-adjusted life-years worldwide.6 The underlying mechanism for HIV-associated CVD is thought to be multifactorial, related to a complex interplay of traditional CVD risk factors, side effects from HAART, and chronic inflammation from HIV infection itself.7,33,34 The CVD burden in PLWH is expected to increase dramatically as the current generation ages into their sixth and later decades of life.8 Given these expected demographic changes, identifying areas in which CVD outcomes can be improved is of paramount importance.
One such area is the management of acute coronary syndrome (ACS) in PLWH. While studies from older practice periods demonstrated overall lower rates of coronary interventions and increased mortality following ACS in PLWH,9,10 more recent studies have conflicted on whether these disparities persist.11,12 Prior studies have also suggested that PLWH are at risk of long-term complications of ACS, though few studies have described post-discharge management and outcomes.13–15 Most national-level studies based in the United States have utilized the National Inpatient Sample, a large all-payer inpatient healthcare database that does not include data on subsequent post-discharge follow-up or prescription data.16 Overall, it remains unclear what differences in clinical presentation, inpatient management, or post-discharge care persist in PLWH with ACS.
Thus, the purpose of this study was to examine the contemporary inpatient and post-discharge management and outcomes of ACS in HIV using longitudinal claims and pharmacy data from a nationwide data warehouse. We hypothesized that PLWH hospitalized for ACS were less likely to receive percutaneous coronary interventions and cardiac medications and more likely to have greater short- and long-term mortality, as compared with uninfected individuals.
Methods
This was a retrospective, observational cohort study using the Symphony Health nationwide data warehouse. This data set includes > 220 million patients, links inpatient insurance claims, electronic medical records, and outpatient pharmacy claims, and includes Health Insurance Portability and Accountability Act (HIPAA) compliant patient-level data from the entire United States and from all payer types (including commercial, Medicare, and Medicaid). These data allow patients to be tracked over time across multiple healthcare providers and settings, and they have been utilized in other large-scale studies examining national patterns of practice and clinical outcomes.17,18 As compared to the national databases used in prior large-scale studies,12,16 this database includes patient data from both inpatient stays and post-discharge care, such as the need for hospital readmission and medication utilization. It also allows for longitudinal follow-up to evaluate for out-of-hospital survival.
Study inclusion criteria were patients ≥18 years of age who had any hospital admission between 1 January 2014 and 31 December 2016 for ACS. Hospital admissions were identified by the International Classification of Diseases Ninth and Tenth (ICD-9-CM and ICD-10-CM) codes for ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina (UA) (Supplementary material online, Table S1). If a subject had multiple hospitalizations during the designated study period, a hospitalization was chosen at random as the index event. To be included, subjects also had to have at least one recorded healthcare encounter, including another hospital admission, outpatient encounter, or prescription filled, in the 12-month period following the index hospitalization for ACS. HIV infection status was defined by ICD-9-CM or ICD-10-CM diagnostic coding (Supplementary material online, Table S1). HIV disease-specific information such as CD4 counts and viral load were not available, and so patient AIDS status was not included. Age and sex were reported for all patients. Racial/ethnic data were only available for 240 189 (21.3%) of the entire group and so was not included in multivariate analysis. Comorbidities were collected using the Elixhauser Comorbidity Index. In addition, specific billing codes were used to identify tobacco and other substance use, whereas history of coronary artery disease was defined by hospitalization for ACS in the 12-month period prior to the index hospitalization. Claims codes were also used to identify inpatient procedures and complications during the index hospitalization. All patient-level records were linked via a HIPAA-compliant, de-identified unique patient ID.
The data were verified as meeting the deidentified standard under the HIPAA privacy rule Expert Determination §164.514(b)1 by Scheuren Ruffner Consultants with a very low statistical risk of re-identification, and the certificate is on file at Symphony Health. Therefore, the study was not considered to be human subject research and the Institutional Review Board (IRB) review was not required. The Beth Israel Deaconess Medical Center and University of California San Francisco IRB agreed with this determination. The study authors had full access to all the data in this study and take responsibility for its integrity and analysis. The primary endpoints were inpatient and 12-month mortality. Secondary endpoints included inpatient cardiovascular procedures performed, inpatient complications, 30-day readmission rates, and rate of cardiovascular prescription filling at 12 months following hospital discharge. Inpatient cardiovascular procedures included: cardiac catheterization; percutaneous coronary intervention (PCI) either a bare-metal stent, drug-eluting stent, or balloon angioplasty alone; stress testing; and transthoracic echocardiography. Inpatient complications included: major bleeding; acute kidney injury; acute heart failure; and stroke. Twelve months post-discharge prescription filling was defined by a dispense date at 12 months following the index hospitalization, with medication filling defined as cumulative time up to the dispense date. Categories of cardiovascular medications assessed included P2Y12 inhibitors, beta-blockers, statin medications, nitrates, anticoagulants, Angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and diuretics. Aspirin use was not included due to its availability over-the-counter. Prescription filling of antiretroviral (ARV) medications for HIV and Hepatitis C infection was also assessed. See Supplementary material online, Table S2 for a comprehensive list of medications included. The data underlying this article are available in the article and in its online supplementary material.
Statistical analysis
Continuous variables were summarized by mean and standard deviation and compared by two-sample t-test between groups with and without HIV. Categorical variables were summarized by count and percentage and compared using χ2 test. Multivariable logistic regression models were used to estimate adjusted odds ratios (ORs) for the primary and secondary outcomes. Receipt of inpatient procedures was adjusted for age, sex, medical comorbidities, and ACS sub-type in order to account for differences in the initial presentation of patients. In order to address potential confounding from type II myocardial infarction events, a stratified analysis limited to only those who had undergone coronary angiography and separately for those who presented with STEMI was then performed. Inpatient and post-discharge outcomes were adjusted for age, gender, medical comorbidities, and ACS sub-type. Patients were then stratified into only those who had undergone coronary angiography and then into those who underwent coronary angiography and stenting in order to better examine the effects of coronary interventions on outcomes. A two-sided P-value < 0.05 considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Inc., Cary, NC, USA).
Results
A total of 1 125 126 individuals were included in this study, of whom 6612 (0.59%) had HIV. Table 1 shows the baseline demographic characteristics. The PLWH group was significantly younger (57.4 ± 10.5 vs. 67.4 ± 12.9 years, P < 0.0001) and were less often women (28.81% vs. 40.29%, P < 0.0001). They also had a significantly greater burden of medical comorbidities at admission, including diabetes mellitus, peripheral vascular disease, chronic pulmonary disease, liver disease, renal disease, and hepatitis C infection (P < 0.05 for all comparisons). Persons living with HIV also had higher rates of pre-existing coronary artery disease (4.69% vs. 3.56%, P < 0.001) along with higher rates of smoking, alcohol, and substance abuse (P < 0.05 for all comparisons). Most patients in the cohort were admitted with NSTEMI (52.72%), followed by unstable angina (33.00%) and STEMI (14.28%), with no significant differences between the HIV infected and uninfected groups (P > 0.05) (Table 1).
Table 1.
Baseline demographic characteristics by HIV status
| HIV+ (n = 6612) | HIV− (n = 1 118 514) | P-value | |
|---|---|---|---|
| Age, years (±SD) | 57.4 ± 10.5 | 67.4 ± 12.9 | <0.0001 |
| Sex | |||
| Men | 4707 (71.19%) | 667 910 (59.71%) | <0.0001 |
| Women | 1905 (28.81%) | 450 604 (40.29%) | |
| Race/ethnicity | |||
| White | 474 (7.17%) | 159 429 (14.25%) | <0.0001 |
| African American | 464 (7.02%) | 27 520 (2.46%) | |
| Hispanic | 544 (8.23%) | 42 247 (3.78%) | |
| Other | 108 (1.63%) | 9403 (0.84%) | |
| Unknown | 5022 (75.95%) | 879 915 (78.67%) | |
| Comorbidities | |||
| Alcohol abuse | 1304 (19.72%) | 63 087 (5.64%) | <0.0001 |
| Drug abuse | 2229 (33.71%) | 77 281 (6.91%) | <0.0001 |
| Tobacco use | 4133 (62.51%) | 478 460 (42.78%) | <0.0001 |
| Coronary artery disease | 310 (4.69%) | 39 862 (3.56%) | <0.001 |
| Obesity | 1,719 (26.00%) | 291 032 (26.02%) | 0.9685 |
| Hypertension | 5774 (87.33%) | 970 711 (86.79%) | 0.1958 |
| Diabetes | 3355 (50.74%) | 524 640 (46.91%) | <0.0001 |
| Peripheral vascular disease | 2281 (34.5%) | 344 987 (30.84%) | <0.0001 |
| Chronic pulmonary disease | 3612 (54.63%) | 463 514 (41.44%) | <0.0001 |
| Congestive heart failure | 3556 (53.78%) | 540 535 (48.33%) | <0.0001 |
| Valvular disease | 2087 (31.56%) | 323 236 (28.90%) | <0.0001 |
| Liver disease | 2405 (36.37%) | 135 748 (12.14%) | <0.0001 |
| Renal disease | 2356 (35.63%) | 286 096 (25.58%) | <0.0001 |
| History of hepatitis C | 1163 (17.59%) | 14 956 (1.34%) | <0.0001 |
| ACS sub-type | |||
| STEMI | 933 (14.11%) | 159 710 (14.28%) | 0.4218 |
| NSTEMI | 3447 (52.13%) | 589 753 (52.73%) | |
| Unstable angina | 2232 (33.76%) | 369 051 (32.99%) |
During the index hospitalization, inpatient procedures were compared between the HIV infected and uninfected groups and adjusted for age, gender, medical comorbidities, and ACS sub-type. Persons living with HIV were less likely to receive invasive coronary procedures (defined as receiving any left heart catheterization, balloon angioplasty, bare-metal stent, or drug-eluting stent) (35.31% vs. 37.22%, adjusted OR 0.87, 95% CI 0.83–0.92, P < 0.0001). Other inpatient procedures are summarized (Table 2).
Table 2.
Inpatient procedures by HIV status
| HIV+ (n =6612) | HIV− (n = 1 118 514) | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|
| P-value | OR (95% CI) | P-value | |||
| Invasive coronary procedures | 2335 (35.31%) | 416 346 (37.22%) | 0.0014 | 0.87 (0.83–0.92) | <0.0001 |
| Left heart catheterization | 930 (39.82%b) | 166 724 (40.04%b) | 0.8489 | 0.94 (0.86–1.03) | 0.1922 |
| Balloon angioplasty | 120 (5.14%b) | 17 583 (4.22%b) | 0.0303 | 1.16 (0.96–1.4) | 0.1250 |
| Bare metal stent | 218 (9.34%b) | 32 222 (7.74%b) | 0.0059 | 1.12 (0.97–1.29) | 0.137 |
| Drug-eluting stent | 1074 (46%b) | 201 848 (48.48%b) | 0.0169 | 0.98 (0.9–1.07) | 0.713 |
| Right heart catheterization | 155 (2.34%) | 23 578 (2.11%) | 0.1832 | ||
| IABP | 105 (1.59%) | 20 440 (1.83%) | 0.1533 | ||
| Impella | 17 (0.26%) | 3314 (0.3%) | 0.6497 | ||
| ECMO | 4 (0.06%) | 482 (0.04%) | 0.3774 | ||
| ICD | 13 (0.2%) | 3499 (0.31%) | 0.0966 | ||
| Temporary pacemaker | 17 (0.26%) | 5201 (0.46%) | 0.0107 | ||
| Permanent pacemaker | 13 (0.2%) | 5363 (0.48%) | 0.0003 | ||
| Nuclear stress | 29 (0.44%) | 3358 (0.3%) | 0.054 | 1.33 (0.91–1.92) | 0.138 |
| Stress echo/ETT | 15 (0.23%) | 1860 (0.17%) | 0.2236 | 1.12 (0.67–1.88) | 0.6591 |
| Transthoracic echocardiography | 347 (5.25%) | 41 803 (3.74%) | <0.0001 | 1.37 (1.23–1.52) | <0.0001 |
Adjusted for age, sex, medical comorbidities, ACS sub-type.
Fraction of patients undergoing invasive coronary procedures.
Invasive coronary procedures refer to the group including left heart catheterization alone, balloon angioplasty, bare metal stent, drug-eluting stent
IABP, intra-aortic balloon pump. ECMO, extra-corporeal membrane oxygenation; ETT, exercise treadmill testing.
A separate analysis was performed on subjects who presented with STEMI (160 643 or 14.28% of the total cohort). In this subgroup, patients with and without HIV underwent invasive coronary procedures at similar rates [59.38% vs. 59.67%, adjusted OR 0.93 (95% CI 0.81–1.07), P = 0.29].
Among the STEMI cohort undergoing angiography, PLWH were less likely to receive drug-eluting stents [60.11% vs. 68.54%, adjusted OR 0.81 (95% CI 0.68–0.96), P = 0.016], with trends towards increased balloon angioplasty alone [7.4% vs. 6.93%, adjusted OR 1.03 (95% CI 0.75–1.42), P = 0.86] and bare metal stent placement [17.51% vs. 14.14%, adjusted OR 1.11 (95% CI 0.89–1.39), P = 0.36] (Supplementary material online, Table S3).
In the overall group, persons living with and without HIV had similar rates of inpatient and 12-month mortality. However, after adjusting for demographic factors, medical comorbidities, ACS sub-type, and inpatient procedures, PLWH had statistically significantly greater all-cause inpatient mortality (adjusted OR 1.29, 95% CI 1.15–1.44, P < 0.0001) and 12-month mortality [adjusted OR 1.32 (95% CI 1.22–1.44), P < 0.0001]. They also had an increased risk of 30-day readmission [adjusted OR 1.18 (95% CI 1.09–1.27), P < 0.0001) (Table 3). Conversely, this group had fewer in-hospital cardiovascular and adverse events, including acute heart failure, stroke, or bleeding, as compared with those without HIV (Table 3). These differences were attenuated after restricting to only those patients who underwent coronary angiography (Supplementary material online, Table S4) or stenting (Supplementary material online, Table S5).
Table 3.
Inpatient and post-discharge outcomes by HIV status
| HIV+ (n = 6612) | HIV− (n = 1 118 514) | Unadjusted |
Adjusted |
||
|---|---|---|---|---|---|
| P-value | OR (95% CI) | P-value | |||
| Inpatient mortality | 372 (5.63%) | 59 935 (5.36%) | 0.3378 | 1.29 (1.15–1.44) | <0.0001 |
| Acute heart failure | 1302 (19.69%) | 259 695 (23.22%) | <0.0001 | 0.79 (0.74–0.85) | <0.0001 |
| Acute kidney injury | 1073 (16.23%) | 175 755 (15.71%) | 0.2493 | 1.02 (0.95–1.1) | 0.5842 |
| Major bleed | 175 (2.65%) | 39 242 (3.51%) | 0.0001 | 0.76 (0.66–0.89) | 0.0005 |
| Stroke | 160 (2.42%) | 33 993 (3.04%) | 0.0028 | 0.99 (0.85–1.16) | 0.9062 |
| 12 month mortality | 724 (10.95%) | 114 933 (10.28%) | 0.0739 | 1.32 (1.22–1.44) | <.0001 |
| 30 day readmission | 869 (13.14%) | 99 718 (8.92%) | <0.0001 | 1.18 (1.09–1.27) | <.0001 |
Adjusted for age, sex, medical comorbidities, ACS sub-type, inpatient procedures undergone.
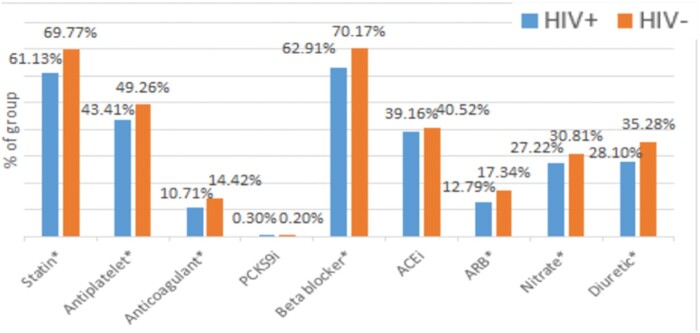
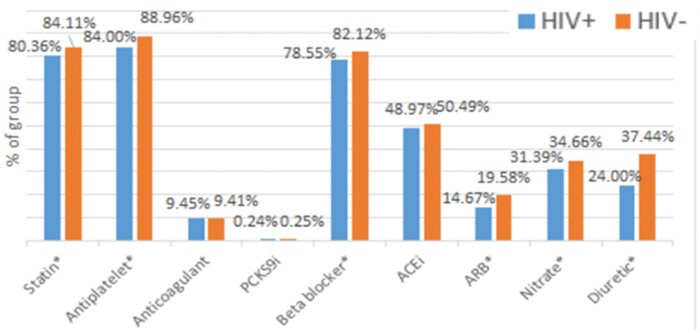
In the 12-month period following hospital discharge, 813 591 patients (74.36%) of the total study cohort filled any prescription, of whom 4893 (0.6%) had HIV. Persons living with HIV filled core cardiac medications at lower rates compared to uninfected individuals. This includes rates of use of statins (66.77% vs. 73.68%, P < 0.0001), beta-blockers (67.91% vs. 73.91%, P < 0.0001), nitrates (31.78% vs. 35.9%, P < 0.0001), and antiplatelet agents (46.76% vs. 51.77%, P < 0.0001) (Figure 1). The percentage of patients receiving at least one prescription for these medications increased in both groups over time from hospital discharge to 12 months follow-up (Supplementary material online, Table S6A–D). After restricting to patients who underwent coronary angiography during their index hospitalization, post-discharge filling rates of statins (80.36% vs. 84.11% P = 0.0033), antiplatelet agents (84.00% vs. 88.96%, P < 0.0001), beta-blockers (78.55% vs. 82.12%, P = 0.0076), ARBs (14.67% vs. 19.58%, P = 0.0004), and nitrates (31.39% vs. 34.66%, P = 0.049) were increased in both groups compared to the entire cohort. However, PLWH continued to fill prescriptions for statins, antiplatelet agents, beta-blockers, ARBs, and nitrates at lower rates compared to those without HIV, though the difference in filling rates between the two groups was reduced in this restricted population as compared to the entire cohort (Figure 2).
Figure 1.
Outpatient pharmacy prescription filling data at 12 months following discharge showing reduced filling of cardiac medications among HIV-infected adults. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; PCSK9i, Proprotein Convertase Subtilisin/Kexin Type 9 inhibitor.
Figure 2.
Outpatient pharmacy prescription filling data at 12 months following discharge showing reduced filling of cardiac medications among HIV-infected adults after restricting to only those who underwent coronary stenting during index hospitalization. ACEi, angiotensin-converting enzyme inhibitor. ARB, angiotensin receptor blocker; PCSK9i, Proprotein Convertase Subtilisin/Kexin Type 9 inhibitor.
Discussion
In this nationwide retrospective cohort of patients hospitalized with acute coronary syndrome between 2014 and 2016, individuals with HIV infection underwent fewer inpatient coronary interventions and suffered higher inpatient and long-term mortality as well as hospital readmission. Persons living with HIV presenting with ACS were less likely to undergo invasive coronary procedures during hospital admission or to fill prescriptions for cardiovascular medications including statins, antiplatelet agents and beta-blockers, which are all Class I indications for therapy per AHA/ACC guidelines following ACS.19,37 This is the largest and most contemporary study to date that describes both the inpatient and post-discharge management and outcomes of ACS in PLWH, including data on prescription filling after discharge. Our findings are strengthened by the large sample size, representative population across the US and different insurance payers, a contemporary study period reflective of present-day practice, and the ability to track patients longitudinally and to link pharmacy claims to inpatient stays. In this study, there were several important differences in the initial presentation and management of PLWH with ACS that may account for their worsened outcomes. As compared to those without HIV infection, PLWH presenting with ACS were significantly younger yet carried a markedly increased burden of medical comorbidities. These findings are consistent with prior studies examining ACS and HIV.13,14 These results further support the conclusion that these patients have a distinct clinical presentation as compared to those without HIV. Their inpatient management also differed significantly from those without HIV. PLWH presenting with ACS had significantly reduced odds of being referred for any invasive coronary procedure (Table 2), even after adjusting for demographics, medical comorbidities, or ACS sub-type. This is a notable difference in practice that may have multiple explanations. One reason is that PLWH may have presented more often with type 2 myocardial infarction (MI) (demand-related) for which angiography and coronary stenting would not have been indicated. In PLWH, approximately half of MIs are categorized as type 2 due to sepsis, bacteraemia, and drug use in the Center for AIDS Research Network of Integrated Clinical System (CNICS) cohort.20,21 We considered multiple approaches to reduce the impact of confounding from type 2 MI in our study, including propensity matching subjects with and without HIV, which was limited by marked differences in these groups’ demographics and medical comorbidities (Table 1). Given our limited ability to distinguish type 2 MI in this retrospective and observational study, we chose to address this confounder through stratified analysis into a separate STEMI-only subgroup. Persons living with HIV presenting with STEMI were referred for invasive procedures at similar rates but were less likely to undergo drug-eluting stent placement with trends towards more bare-metal stents or balloon angioplasty alone (Supplementary material online, Table S3). While type 2 MI or other confounding STEMI presenters could still be present in this subgroup, we also must consider the possibility that PLWH with true STEMI are not receiving appropriate drug-eluting stent placement. This is a concerning finding, as multiple prior studies have demonstrated the safety and efficacy of drug-eluting stents in patients with HIV.15,22–27 We next evaluated inpatient outcomes and found increased in-hospital mortality in PLWH, though this difference was reduced after restricting the analysis to only those who underwent coronary angiography or stenting (Supplementary material online, Tables S4 and S5). There are several potential implications of this finding. One is that PLWH benefitted from PCI and stenting as found in previous studies,11,24–26 and that lower rates of these interventions contributed to worsened outcomes. It is also possible that restricting our analysis to only those who underwent angiography helped to remove confounding among individuals with type 2 MI.
In addition to these inpatient endpoints, we also found that PLWH with ACS had increased hospital readmission, 12-month mortality and reduced use of cardiovascular medications after discharge. Our findings are consistent with prior studies looking at longitudinal outcomes in HIV,13,14 suggesting that PLWH living in the US continue to have poorer long-term outcomes following ACS even in contemporary practice. One potential explanation for these worsened outcomes may be differences in medical therapy following discharge. We demonstrated that medications that are known to improve outcomes after ACS in the general population, including antiplatelet agents, beta-blockers, and statins, were less likely to be filled among PLWH. It is noteworthy that only 48.93% of PLWH (Supplementary material online, Table S6D) were filling prescriptions for HAART at 12 months following discharge. However, medication non-adherence is a well-studied barrier to care in PLWH, with underlying reasons including medical literacy and poor integration into healthcare systems,28–30,35,36 and it is likely that patient non-adherence was present as well. In this study, disparities inequality of care and outcomes persisted not just during the index hospitalization, but up to a year following hospital discharge in PLWH.
There are several limitations to this study. Though this was a large study cohort, there only 6,612 (0.59%) patients had HIV, suggesting that small absolute differences in our measured outcomes could be statistically but not necessarily clinically significant. In addition, though many medical comorbidities were evaluated, there was a marked difference in age and medical comorbidities between the HIV-infected and uninfected groups, and thus unmeasured confounding may still account for some of the study findings. The dataset also did not include CD4 counts or HIV viral loads, nor did it include the specific results of cardiac imaging and angiographic findings, which have previously been associated with poor outcomes after ACS.31 Prior studies have suggested that AIDS status may drive reduced coronary procedures among PLWH presenting with ACS,37 but we could not perform a similar analysis in this study. This cohort also had limited data on racial/ethnic and socioeconomic status of the subjects, and so we could not account for these factors in multivariate analysis. This cohort was also limited to the US, and so its results cannot necessarily be extrapolated to other settings where HIV infection is endemic. The dataset used could not differentiate lower rates of prescription vs. lower rates of adherence to prescriptions. Finally, a notably low rate of ARV prescription filling was seen in this population, which could in part drive differences in clinical outcome. Regardless, this study is one of the largest and most recent analyses to date examining contemporary inpatient management of PLWH hospitalized for ACS, including short- and long-term outcomes as well as discharge medications.
In conclusion, in this large, national study of PLWH hospitalized for ACS between 2014 and 2016, HIV infection was associated with fewer coronary interventions and increased short- and long-term death. In addition, PLWH with ACS experienced increased hospital readmissions and lower use of optimal medical therapy. Targeted interventions and care strategies to improve the outcomes of HIV-infected individuals hospitalized with ACS are needed. Further investigation with prospective data collection is needed to evaluate and identify areas for improvement in the patient, provider, and systemic factors in the management of ACS in HIV.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Funding
National Institutes of Health (Award number K2AI112393-06) awarded to P.H.
Conflict of interest: none declared.
Data availability
The data underlying this article are available in the article and in the online supplementary material.
Supplementary Material
References
- 1. Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT. et al. HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- 2. Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M. et al. Swiss HIV Cohort Study. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet 2003;362:877–878. [DOI] [PubMed] [Google Scholar]
- 3. Lohse N, Hansen A-BE, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT. et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med 2007;146:87–95. [DOI] [PubMed] [Google Scholar]
- 4. Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC. et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 2008;300:51–59. [DOI] [PubMed] [Google Scholar]
- 5. Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K. et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999-2013. Am J Cardiol 2016;117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S. et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsue PY, Waters DD.. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol 2019;16:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A. et al. ATHENA Observational Cohort. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015;15:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearce D, Ani C, Espinosa-Silva Y, Clark R, Fatima K, Rahman M. et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol 2012;110:1078–1084. [DOI] [PubMed] [Google Scholar]
- 10. Smilowitz NR, Gupta N, Guo Y, Coppola JT, Bangalore S.. Influence of human immunodeficiency virus seropositive status on the in-hospital management and outcomes of patients presenting with acute myocardial infarction. J Invasive Cardiol 2016;28:403–409. [PubMed] [Google Scholar]
- 11. Singh V, Mendirichaga R, Savani GT, Rodriguez AP, Dabas N, Munagala A. et al. Coronary revascularization for acute myocardial infarction in the HIV population. J Interv Cardiol 2017;30:405–414. [DOI] [PubMed] [Google Scholar]
- 12. Ogunbayo G, Ha L, Ahmad Q, Misumida N, Okwechime R, Elbadawi A. et al. Treatment bias in management of HIV patients admitted for acute myocardial infarction: does it still exist? J Gen Intern Med 2020;35:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H. et al. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation 2013;127:1767–1774. [DOI] [PubMed] [Google Scholar]
- 14. Jeon C, Lau C, Kendall CE, Burchell AN, Bayoumi AM, Loutfy M. et al. Mortality and health service use following acute myocardial infarction among persons with HIV: a population-based study. AIDS Res Hum Retroviruses 2017;33:1214–1219. [DOI] [PubMed] [Google Scholar]
- 15. Boccara F, Mary-Krause M, Teiger E, Lang S, Lim P, Wahbi K, et al. Prognosis of Acute Coronary Syndrome in HIV-infected patients (PACS) Investigators. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J 2011;32:41–50. [DOI] [PubMed] [Google Scholar]
- 16.HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp (12 February 2020). [Google Scholar]
- 17. Hess GP, Natarajan P, Faridi KF, Fievitz A, Valsdottir L, Yeh RW.. PCSK9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation 2017;136:2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin H, Ketcham JD, Rosenquist JN, Simon KI.. Financial distress and use of mental health care: evidence from antidepressant prescription claims. Econ Lett 2013;121:449–453. [Google Scholar]
- 19. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014;64:e138–e228. [DOI] [PubMed] [Google Scholar]
- 20. Crane HM, Paramsothy P, Drozd DR, Nance RM, Delaney JA, Heckbert SR, et al. Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort. Types of myocardial infarction among HIV-infected individuals in the United States. JAMA Cardiol 2017;2:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feinstein MJ, Nance RM, Delaney JA, Heckbert SR, Budoff MJ, Drozd DR. et al. Mortality following myocardial infarction among HIV-infected persons: the Center for AIDS Research Network Of Integrated Clinical Systems (CNICS). BMC Med 2019;17:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matetzky S, Domingo M, Kar S, Noc M, Shah PK, Kaul S. et al. Acute myocardial infarction in human immunodeficiency virus‐infected patients. Arch Intern Med 2003;163:457–460. [DOI] [PubMed] [Google Scholar]
- 23. Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A. et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation 2004;109:316–319. [DOI] [PubMed] [Google Scholar]
- 24. Boccara F, Teiger E, Cohen A, Ederhy S, Janower S, Odi G. et al. Percutaneous coronary intervention in HIV infected patients: immediate results and long term prognosis. BMJ Heart 2005;92:543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren X, Trilesskaya M, Kwan DM, Nguyen K, Shaw RE, Hui PY.. Comparison of outcomes using bare metal versus drug-eluting stents in coronary artery disease patients with and without human immunodeficiency virus infection. Am J Cardiol 2009;104:216–222. [DOI] [PubMed] [Google Scholar]
- 26. Peyracchia M, Verardi R, Rubin SR, Abu-Assi E, Montrucchio C, Perl L. et al. In-hospital and long-term outcomes of HIV-positive patients undergoing PCI according to kind of stent: a meta-analysis. J Cardiovasc Med (Hagerstown) 2019;20:321–326. [DOI] [PubMed] [Google Scholar]
- 27. Metzger NL, Momary KM.. A patient with HIV and tuberculosis with diminished clopidogrel response. Int J STD AIDS 2014;25:532–534. [DOI] [PubMed] [Google Scholar]
- 28. Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA; HIV Research Network. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr 2012;60:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bozzette SA, Berry SH, Duan N, Frankel MR, Leibowitz AA, Lefkowitz D. et al. The care of HIV-infected adults in the United States. HIV cost and services utilization study consortium. N Engl J Med 1998;339:1897–1904. [DOI] [PubMed] [Google Scholar]
- 30. Baillargeon JG, Giordano TP, Harzke AJ, Baillargeon G, Rich JD, Paar DP.. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public Health Rep 2010;125:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D’Ascenzo F, Cerrato E, Appleton D, Moretti C, Calcagno A, Abouzaki N. et al. Percutaneous coronary intervention and surgical revascularization in HIV Database (PHD) Study Investigators. Prognostic indicators for recurrent thrombotic events in HIV- infected patients with acute coronary syndromes: use of registry data from 12 sites in Europe, South Africa and the United States. Thromb Res 2014;134:558–564. [DOI] [PubMed] [Google Scholar]
- 32. Meredith C, Lin L, Navar A, Okeke N, Naggie S, Douglas P.. Lower likelihood of cardiac procedures after acute coronary syndrome in patients with human immunodeficiency virus/acquired immunodeficiency syndrome. Medicine 2018;97:e9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sidney S, Quesenberry CP, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS. et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol 2016;1:594–599. [DOI] [PubMed] [Google Scholar]
- 34. Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC. et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc 2012;1:jah3–e000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roe MT, Peterson ED, Newby LK, Chen AY, Pollack CV Jr, Brindis RG. et al. The influence of risk status on guideline adherence for patients with non-ST-segment elevation acute coronary syndromes. Am Heart J 2006;151:1205–1213. [DOI] [PubMed] [Google Scholar]
- 36. McAlister FA, Oreopoulos A, Norris CM, Graham MM, Tsuyuki RT, Knudtson M. et al. Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Exploring the treatment-risk paradox in coronary disease. Arch Intern Med 2007;167:1019–1025. [DOI] [PubMed] [Google Scholar]
- 37. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, ESC Scientific Document Group et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in the online supplementary material.