Abstract
Introduction
We report a case of post COVID-19 Sino-orbital Mucormycosis infection caused by Rhizopus oryzae and its management.
Presentation of case
The patient was diagnosed with COVID-19 and treated according to the persisting protocols. Following recovery, on the 18th day, the patient developed chemosis and pain in the left eye. A diagnosis of mucormycosis was established after Magnetic Resonance Imaging (MRI) and Functional Endoscopic Sinus Surgery (FESS). Initially, conservative management with intravenous (IV) Fluconazole & Amphotericin B was done and later on with surgical debridement. The patient recovered with minimal residual deformity.
Discussion
Mucormycosis generally develops secondary to immunosuppression or debilitating diseases. In Head and Neck cases, the mold usually gains entry through the respiratory tract involving the nose and sinuses, with possible further progression into the orbital and intracranial structures. Hence, an early diagnosis and intervention is required for a good prognosis, decreasing the morbidity. This can be achieved on the basis of clinical picture and direct smears.
Conclusion
Research needs to be carried out in COVID-19 patients for better prevention and management of opportunistic infections in order to reduce its incidence and morbidity. Prophylactic treatment protocols need to be established, along with rational use of corticosteroids.
Keywords: Covid-19, Mucormycosis, Amphotericin B, Fungal infection, Opportunistic infection, Case report
Highlights
-
•
Sino-orbital Mucormycosis post COVID-19
-
•
Opportunistic infections in COVID-19
-
•
Immuno-suppressants need to be more judicious
-
•
Early diagnosis and management reduces morbidity and mortality.
1. Introduction
The outbreak of coronavirus disease (COVID-19) has spread rapidly on a global scale [1,2]. Despite great efforts, there is no definitive treatment of the disease. However, prevention and symptomatic management are the best options.
Secondary infections are a well-described phenomenon in influenza, SARS, MERS, and other respiratory viral illnesses. But super-infections and co-infections in COVID-19 pneumonia are still under exploration [3]. Secondary infections are reportedly common in hospitalized, severely ill Covid-19 patients, encompassing between 10 and 30% of cases, fungal being 10 times more common [3]. As the nature of the disease is still not completely unveiled, it can't be confirmed if it's a complication of the disease or its management.
Drugs like Corticosteroids i.e., Methylprednisolone and Dexamethasone are believed to modulate inflammation mediated lung injury and thereby reduce progression of respiratory failure [4] in Covid-19. There side effects include increased secondary infections, immune modulation, manifestation of latent diabetes mellitus, dizziness, weight gain, mood changes, insomnia and muscle weakness [5].
Mucormycosis is amongst the most fulminant form of Zygormycosis caused by Mucorales species of the phylum Zygomycota [6] described as a potentially lethal infection occurring mostly in immunocompromised hosts, particularly in those with diabetes mellitus, leukemia and lymphoma [7].
The incidence rate of mucormycosis globally varies from 0.005 to 1.7 per million population [8]. Whereas, in Indian population its prevalence is 0.14 per 1000, which is about 80 times higher than developed countries [9]. The fatality rate of mucormycosis is 46% globally [10]. However, factors like intracranial or orbital involvement, irreversible immune suppression increases fatality to as high as 50% to 80% [11]. A high suspicion for this disease must be considered in patients who are immunocompromised. Tissue necrosis, a hallmark of mucormycosis is often a late sign [10].
Mucormycosis is difficult to diagnosis which affects outcome and results in poor prognosis. Early diagnosis and treatment is essential. Delay of a week often doubles the 30-day mortality from 35% to 66%. Despite early aggressive combined surgical and medical therapy, the prognosis of mucormycosis is poor [10].
2. Presentation of case
A 38-year-old male was admitted to our Hospital, a tertiary care center with a history of fever for 4 days, on 2nd September 2020. He presented with a high grade fever, body ache, cough and shortness of breath. Nasopharyngeal swab was sent for RT-PCR which came positive and a diagnosis of COVID-19 was confirmed. The patient had no history of diabetes or any other debilitating conditions and no relevant family history. On admission, the derranged investigations were: Neutrophil count 83.1% (35–66%), Lymphocyte count 9.5% (24–44%), Fasting blood sugar (FBS) 98 mg/dL (70-110 mg/dL), Post-prandial blood sugar (PPBS) 146 mg/dL (110-140 mg/dL), HbA1c 6.3% (<6%), Serum Interleukin-6 37.93 pg/mL (<6.4 pg/mL), CRP 17.84 mg/L (0–6 mg/L), D-Dimer 460 ng/mL (0–500 ng/mL).
He was monitored in the Intensive Care Unit for 5 days and was started on Inj.Remdesivir IV with a loading dose of 200 mg, followed by 100 mg daily for 11 days. Methylprednisolone was given by IV infusion, 80 mg/day in 240 mL saline at 10 mL/h for 18 days. Also Inj.Dexamethasone 4 mg twice daily was given for 12 days as a part of COVID-19 management. Post-treatment FBS 125 mg/dL, PPBS 352 mg/dL and HbA1c 12.3%.
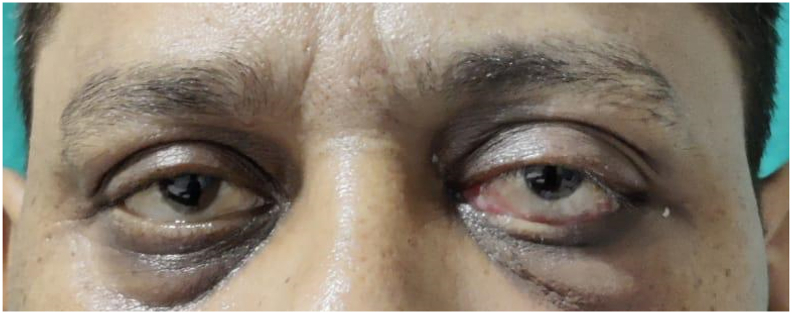
After 18 days, the patient complaint of swelling and pain in the left eye. He was referred to the Department of Head and Neck Oncology for the same. On clinically examination, there was malaise, proptosis, chemosis, periorbital cellulitis and restricted medial gaze. Visual acuity was 6/6 with partial opthalmoplegia and no nasal discharge was seen (Fig. 1). The work has been reported in line with the SCARE 2020 criteria [12].
Fig. 1.
Preoperative photograph showing left eye exopthalmous and chemosis.
2.1. Management and follow-up
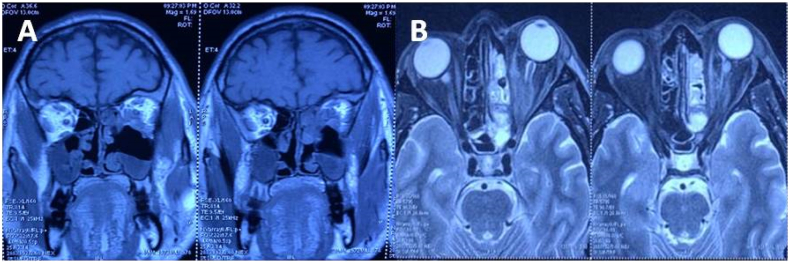
MRI brain & orbit was done showing an ill-defined heterogenous soft tissue signal intensity (hypointense on T1W-imaging), polyploidal mucosal thickening involving left maxillary and ethmoid sinuses was seen. A breach was seen in the posterior portion of left lamina paprycea with altered signal intensity involving conal and extra-conal infero-medial portion of the left orbit. There was displacement of adjacent medial and inferior rectus. Retrobulbar soft tissue fat stranding and edema with resultant displacement of left eye ball anteriorly leading to proptosis was seen. It was also found to be closely abutting the left optic nerve (Fig. 2).
Fig. 2.
(A) Coronal Section of MRI T1 weighted image and (B) Axial Section of MRI T2 weighted image showing extension of the lesion.
FESS was done for right & left maxillary sinus and left ethmoid sinus. Crusting was noted over posterior aspect of inferior turbinate, septum and conchae. The sinuses were debrided and the specimen obtained was sent for culture sensitivity and histopathology. Inj.Piptaz 4.5 g-8hourly, Inj.Metronidazole 500 mg-8hourly and Inj.Fluconazole 200 mg-12hourly was started postoperatively. On histopathology examination, aseptate broad based hyphae and gram positive bacilli were seen and hence Mucormycosis suspected. The medical regime changed to Inj.Amphotericin B 300 mg/day, eyedrops Tobramycin BD and Nepalact TDS. The swelling and chemosis reduced slightly, but no improvement in extraocular movements was seen after 3 days of treatment.
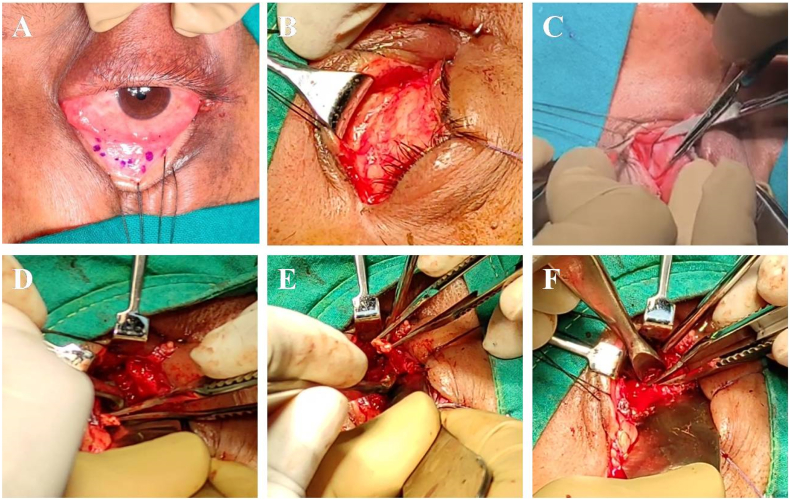
Surgical debridement was planned under general anesthesia. Lateral canthotomy and inferior cantholysis was done. Inferior fornix incision was taken, septum incised and orbit was approached along the medial wall. Inferior and medial rectus muscles were tagged and intraconal space was entered. Debridement and Amphotericin B lavage (1 mg/mL) was done (Fig. 3, Fig. 4). Closure was done with 6-0 resorbable suture. Monocef 1 g–12hourly was added to the pre-existing regime.
Fig. 3.
(A) Inferior fornix incision marked and retraction sutures taken. (B) Inferior fornix incision taken, lateral canthotomy and inferior cantholysis done. (C) Dissection done in preseptal plane (D) Septum incised and orbit approached along the medial wall. Inferior and medial rectus muscles tagged. (E) Intraconal space entered between the two muscles. (F) Debridement of Intraconal space.
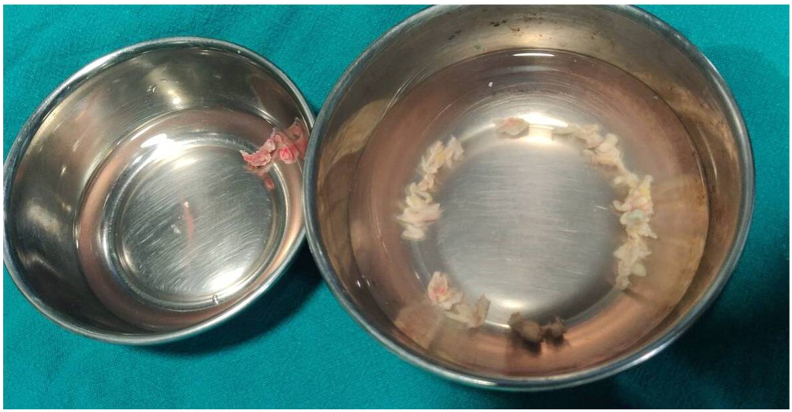
Fig. 4.
Necrotic tissue removed during debridement.
Post-operative MRI orbit showed mucosal thickening of the paranasal sinuses. The left medial and inferior rectus appear mildly bulky. No obvious residual lesion seen.
Total hospitalization for the patient was 38 days. On discharge, Inj.Amphotericin B 300 mg/day 18 days, followed by Tab.Fluconazole 200 mg was prescribed.
On a 7 day follow up, chemosis was present and the eye movements were sluggish on medial and lateral movement. On a 2 month follow up, minimal residual deformity was seen along with normal eye movements as compared to the other eye. FBS, PPBS and HbA1c levels dropped to 100 mg/dL, 124 mg/dL and 7.4% respectively. Patient was satisfied with treatment and outcome.
2.2. Microbiology
Microbiological studies were performed on tissue biopsies, inoculated on Sabouraud's agar, incubated at 30 °C and the sample was examined microscopically. Fungal growth was studied macroscopically at 37 °C and 45 °C after staining with lactofuchsin. After 4 days, colonies of Rhizopus oryzae were seen growing on the media. Special stains for fungal hyphae: PAS and GMS were positive.
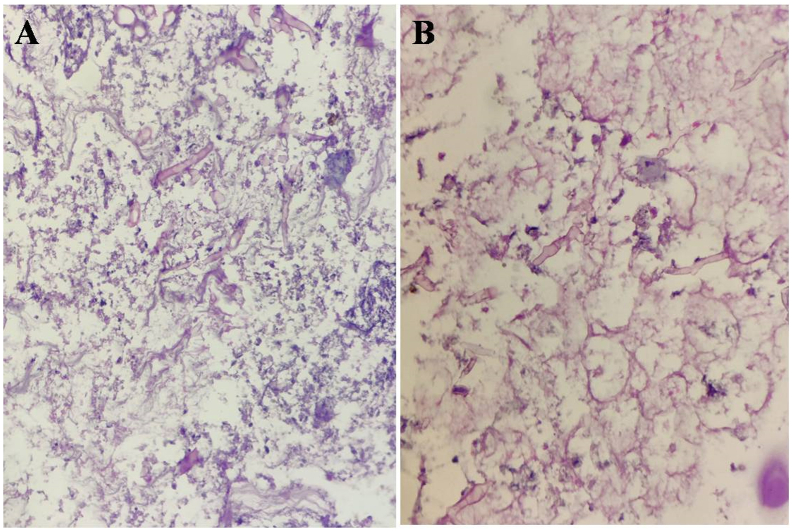
Aseptate, branching broad based fungal hyphae, areas of necrosis along with epitheloid cell granulomas comprising of epitheloid cells, multinucleated giant cells and chronic inflammatory cell infiltrate were seen (Fig. 5).
Fig. 5.
Lactofuchsin stained section showing typical aseptate, branching broad based fungal hyphae, areas of necrosis, epitheloid cell granulomas, multinucleated giant cells and chronic inflammatory cell infiltrate. (A) 100× (B) 10×.
3. Discussion
The nomenclature of Mucormycosis is suggested by anatomic site localization rather than by mycologic classification. For the head and neck region, they can be classified into isolated nasal, rhino-orbital or rhino-orbital-cerebral Mucormycosis. Other accepted forms are pulmonary, disseminated, cutaneous, gastrointestinal and miscellaneous [13]. Fungi of the genus Rhizopus account for the majority of clinical isolates.
Mucoraceae are ubiquitous saprophytic fungi and are common inhabitants of decaying matter also found in bread, soil, air, dust and hospital ward rooms [[13], [14], [15]]. Seasonal variance could theoretically be related to use of air conditioners. The organisms are potent in the temperate climates [7].
The most common risk factors being diabetes mellitus, immunosuppressive therapy, leukemias, neutropenias [7]. Patients with neutrophil dysfunction, hematopoetic stem cell transplantation, diabetic ketoacidosis, iron-overload and HIV/AIDs are some identifiable risk factors.
The mold usually gains entry into the host through the respiratory tract and exhibits a remarkable affinity for arteries and grows along internal elastic lamina causing thrombosis and infarction [16,17]. The progression of the disease from nose and sinuses is either direct or through vascular occlusion. Intracranial involvement also occurs by invasion through superior orbital fissure, ophthalmic vessels, cribriform plate [18], carotid artery or possibly via a perineural route [19].
Waiting for cultures is impractical and may lead to delay in initiation of treatment. If clear clinical picture of mucormycosis exists, positive direct smears may be sufficient for initiating treatment [7].
Diagnosis is classically dependent on clinical features, pathological findings and imaging plays an important role in defining the extent of involvement [11]. Early establishement of the diagnosis and prompt surgical intervention aids in controlling the extent and severity of the disease.
The primary guideline for treating the disease is to correct the underlying cause, but this cannot be achieved in patients dependent on high dose steroid therapy like in COVID-19. The two mainstays of treatment are medical treatment with Amphotericin B and surgical debridement. Hyperbaric oxygen therapy and local treatment with Amphotericin B are adjunctive modalities. Amphotericin B is a fungistatic agent rather than fungicidal, which leads to longer treatment duration.
Prognosis is dependent on multiple factors and early initiation of treatment is an important element. Once the diagnosis is confirmed, conservative management is started for the patient. Orbital exenteration remains the most difficult decision in rhino-orbital cases, due to concerns about disability and disfigurement. Although, exenteration is the last resort but can be life-saving at the price of mutilating procedure.
4. Conclusion
The question still prevails about the cause and origin of enhanced prevalence in post COVID-19 patients. Research is needed for better prevention and management of opportunistic infections in COVID-19 patients. The use of prophylactic treatment protocols for the same needs to be assessed and guidelines need to established in order to reduce morbidity. Moreover, use of Immuno-suppressants should be more judicious along with continuous monitoring.
Sources of funding
No source of funding.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr. Aastha Maini: Analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. Gaurav Tomar: Analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. Deepak Khanna: Analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. Yogesh Kini: Analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. Hardik Mehta: Analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. V. Bhagyasree: Analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Research registration (for case reports detailing a new surgical technique or new equipment/technology): NOT APPLICABLE.
Guarantor
Dr. Aastha Maini.
Dr. Gaurav Tomar.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
None.
References
- 1.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020 Sep;188:109819. doi: 10.1016/j.envres.2020.109819. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7293495/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- 3.Superinfections and coinfections in COVID-19 MedPage Today. https://www.medpagetoday.com/infectiousdisease/covid19/86192.
- 4.RECOVERY Collaborative Group, Horby P., Lim W.S. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021 Feb;384(8):693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Methylprednisolone for patients with COVID-19 severe acute respiratory syndrome - full text view. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04323592
- 6.Sugar A.M. Mucormycosis. Clin. Infect. Dis. 1992;14:S126–S129. doi: 10.1093/clinids/14.supplement_1.s126. https://www.jstor.org/stable/4456403 Available from: [DOI] [PubMed] [Google Scholar]
- 7.Talmi Y.P., Goldschmied-Reouven A., Bakon M., Barshack I., Wolf M., Horowitz Z. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol. Head Neck Surg. 2002 Jul 1;127(1):22–31. doi: 10.1067/mhn.2002.1265874. Available from. [DOI] [PubMed] [Google Scholar]
- 8.Jeong W., Keighley C., Wolfe R. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Chander J., Kaur M., Singla N., Punia R., Singhal S., Attri A., Guarro J. Mucormycosis: battle with the deadly enemy over a five-year period in India. J. Fungi. 2018;4(2):46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutsch P.G., Whittaker J., Prasad S. Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina. 2019;55:1–14. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Safi M., Ang M.J., Patel P., Silkiss R.Z. Rhino-orbital-cerebral mucormycosis (ROCM) and associated cerebritis treated with adjuvant retrobulbar amphotericin B. Am. J. Ophthalmol. Case Rep. 2020;19:100771. doi: 10.1016/j.ajoc.2020.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolekar J.S. Rhinocerebral mucormycosis: a retrospective study. Indian J. Otolaryngol. Head Neck Surg. 2015;67(1):93–96. doi: 10.1007/s12070-014-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camara-Lemarroy C.R., González-Moreno E.I., Rodríguez-Gutiérrez R., Rendón-Ramírez E.J., Ayala-Cortés A.S., Fraga-Hernández M.L., García-Labastida L., Galarza-Delgado D.Á. Clinical features and outcome of mucormycosis. Interdiscip. Perspect. Infect. Dis. 2014;2014:562610. doi: 10.1155/2014/562610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S., Goyal R., Kaore N.M. Rhino-orbital-cerebral mucormycosis: battle with the deadly enemy. Indian J. Otolaryngol. Head Neck Surg. 2020;72(1):104–111. doi: 10.1007/s12070-019-01774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groote C.A. Rhinocerebral phycomycosis. Arch. Otolaryngol. 1970 Sep 1;92(3):288–292. doi: 10.1001/archotol.1970.04310030078019. https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/603163 [DOI] [PubMed] [Google Scholar]
- 18.Bawankar P., Lahane S., Pathak P., Gonde P., Singh A. Central retinal artery occlusion as the presenting manifestation of invasive rhino-orbital-cerebral mucormycosis. Taiwan J. Ophthalmol. 2020;10(1):62–65. doi: 10.4103/tjo.tjo_72_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsi K., Itgampalli R.K., Vittal R., Kumar A. Perineural spread of rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. Ann. Indian Acad. Neurol. 2013 Jul 1;16(3):414. doi: 10.4103/0972-2327.116921. https://www.annalsofian.org/article.asp?issn=0972-2327;year=2013;volume=16;issue=3;spage=414;epage=417;aulast=Parsi;type=0 [DOI] [PMC free article] [PubMed] [Google Scholar]