Abstract
In the US, nearly 11% of adults were living with diagnosed diabetes in 2017, and significant type 2 diabetes (T2D) disparities are experienced by socioeconomically disadvantaged, racial/ethnic minority populations, including Hispanics. The standard 15-minute primary care visit does not allow for the ongoing self-management support that is needed to meet the complex needs of individuals with diabetes. “Team-based” chronic care delivery is an alternative approach that supplements physician care with contact from allied health personnel in the primary care setting (e.g., medical assistants; MAs) who are specially trained to provide ongoing self-management support or “health coaching.” While rigorous trials have shown MA health coaching to improve diabetes outcomes, less is known about if and how such a model can be integrated within real world, primary care clinic workflows. Medical Assistant Health Coaching for Type 2 Diabetes in Diverse Primary Care Settings – A Pragmatic, Cluster-Randomized Controlled Trial will address this gap. Specifically, this study compares MA health coaching versus usual care in improving diabetes clinical control among N=600 at-risk adults with T2D, and is being conducted at four primary care clinics that are part of two health systems that serve large, ethnically/racially, and socioeconomically diverse populations in Southern California. Electronic medical records are used to identify eligible patients at both health systems, and to examine change in clinical control over one year in the overall sample. Changes in behavioral and psychosocial outcomes are being evaluated by telephone assessment in a subset (n=300) of participants, and rigorous process and cost evaluations will assess potential for sustainability and scalability.
ClinicalTrials.gov: NCT02643797 (registered November 30, 2015: https://clinicaltrials.gov/ct2/show/NCT02643797?term=philis-tsimikas&rank=10)
Trial registration: NCT02643797
Keywords: Type 2 diabetes, health coaching, glycemic control, pragmatic
Introduction
In 2017, 425 million adults worldwide were living with diabetes; this prevalence is projected to increase by nearly 50% to 629 million by the year 2045.1 In the US, nearly 11% of adults were living with diagnosed diabetes in 2017,1 which represented an annual economic burden of $327.2 billion.2 Significant type 2 diabetes (T2D) disparities are experienced by socioeconomically disadvantaged, racial/ethnic minority populations. 3,4 Hispanics/Latinos (hereafter Hispanics), have a 66% greater risk for T2D.5 The 2013–2016 National Health and Nutrition Survey reported a 19.8% total diabetes prevalence in Hispanics, versus 12.4% in non-Hispanic Whites.6 In addition to greater prevalence, Hispanics also exhibit poorer self-management, clinical control, and outcomes once diagnosed with T2D compared to non-Hispanic whites.7–10
In current healthcare models, the standard 15-minute visit in primary care (where most diabetes is managed) does not allow for the ongoing self-management support that is needed to meet the complex medical, educational, behavioral, and psychosocial needs of individuals with diabetes.11 “Team-based” chronic care delivery is an alternative approach that supplements physician care with contact from allied health personnel in the primary care setting (e.g., medical assistants; MAs) who are specially trained to provide ongoing self-management support or “health coaching.” 11,12 While health coaching approaches vary in form, common elements include ensuring understanding of the care plan, reviewing lab results and targets, shared decision-making, and collaborative goal-setting and action-planning for health behavior change.13 Recent reviews have summarized the effectiveness of health coaching for chronic disease outcomes overall,14 and for diabetes specifically,15 including among underserved groups.16,17 Research that has specifically trained nonclinicians (e.g., MAs) as health coaches has found this approach to improve medication adherence and improve HbA1c, lipids, and blood pressure in diverse samples of adults with T2D,18,19,20 with sustained clinical improvements at one year.21
The evidence from these well-controlled studies supports the effectiveness of MA health coaching for T2D. However, less is known about if and how such a model can be integrated within real world, primary care clinic workflows. Medical Assistant Health Coaching (“MAC”) for Type 2 Diabetes in Diverse Primary Care Settings – A Pragmatic, Cluster-Randomized Controlled Trial [National Institutes of Health/National Institute for Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) 5R18DK104250, Philis-Tsimikas/Gallo, Multiple Principal Investigators] was specifically designed to address this gap. Specifically, this study compares MA health coaching versus usual care (UC) in improving diabetes clinical control among at-risk adults with T2D, and is being conducted within primary care clinics of two health systems that serve large, ethnically/racially, and socioeconomically diverse populations in Southern California: Neighborhood Healthcare [a Federally-Qualified Health Center (FQHC) and designated Patient-Centered Medical Home] and Scripps Health (a large, non-profit, private insurance-based health system). The EMR is used to identify eligible patients at both health systems, and to examine change in diabetes clinical control over one year. Changes in patient-reported behavioral and psychosocial outcomes are being evaluated by telephone assessment in a subset (n=300) of MAC and UC participants, and rigorous process and cost evaluations will assess potential for sustainability and scalability.
Study Aims
The primary aim of this trial is to document the effectiveness of a primary care-based, MA health coaching model versus diabetes care as usual (UC) in improving diabetes clinical control as indicated by glycosylated hemoglobin (HbA1c), low-density lipoprotein cholesterol (LDL-c), and systolic blood pressure (SBP) over 12 months among N=600 adults with poorly controlled T2D who are receiving care at Scripps Health or Neighborhood Healthcare. The secondary aims are:
To examine the effectiveness of the MAC intervention versus UC in improving patient-reported behavioral (diabetes self-management) and psychosocial outcomes (quality of life, patient activation) across 12 months (patient-reported outcomes sub-study; n=300).
To document the long-term cost-effectiveness of the MAC intervention versus UC from the health system perspective.
Guided by the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework,22,23 to evaluate success in: (1) Reaching a large, representative segment of the population (reach); (2) Enhancing Chronic Care Model (CCM)24 processes and increasing patient and provider satisfaction through a well-implemented intervention (implementation); and (3) Creating an intervention that can be adopted by and maintained in real-world primary care environments (adoption/maintenance).
Trial Design
This is a cluster-randomized, controlled, single-blind, parallel-groups, superiority trial. Consistent with the pragmatic design and clinic-level randomization, both the MAC and UC conditions are implemented as the standard model of care at the respective clinics. The protocol was developed in accordance with Good Clinical Practice, SPIRIT, and CONSORT 2013 guidelines.
Methods: Participants, Interventions, and Outcomes
Primary Study Settings
To enhance the generalizability of findings and scalability of the intervention, the MAC trial is being conducted in two diverse primary care settings in Southern California: Neighborhood Healthcare and Scripps Health. Neighborhood Healthcare, an FQHC system and designated Patient-Centered Medical Home, serves higher proportions of un- and underinsured patients and underserved ethnic/racial patients (>60%, of whom 80% are Hispanic) relative to Scripps Health. Scripps Health is a large, non-profit, private insurance-based health system comprised of 4 hospitals on 5 campuses, and 20 primary care clinics, which are organized within two integrated medical groups, Scripps Clinic Medical Group and Scripps Coastal Medical Center. As the size of diabetes panels vary widely across the individual Neighborhood Healthcare and Scripps primary care clinics (N<100 to N=1999, Mean=595; data from 2014), the two clinics at each health system with diabetes panels closest to this mean size were selected for the MAC Trial.
Clinic Partnership
During study months one through eight, the investigative team held multiple planning meetings with on-site leadership and providers at each of the four study clinics to provide a study overview, describe implementation aspects, and document staff and provider feedback to refine the approach prior to study kick-off. A primary focus of these meetings was ensuring feasibility of the MAC model in the intervention clinics, and minimizing disruption to the routine/real-world clinic flow. After study kick-off (month nine) site-specific provider updates and meetings have continued on an annual basis. During the annual update meetings, study successes and challenges are shared with site providers, who are in turn encouraged to provide their feedback and observations with the investigative team.
Community Advisory Board
Consistent with community-engaged research principles, the following stakeholders were involved in study planning and have been engaged throughout the study period as Community Advisory Board (CAB) members to optimize implementation and sustainability, and facilitate dissemination efforts: adults with T2D; primary care providers; MAs; Neighborhood Healthcare and Scripps Health administrative leaders; primary care and nutrition researchers; patient care managers; and payer representatives. The CAB was convened in-person during study startup to refine protocol elements. Thereafter, members receive an e-newsletter one to two times per year that includes detailed study updates, and encourages CAB members to send questions and feedback to the investigative team to guide implementation efforts. In Year 5, the CAB will reconvene in-person to review preliminary results, guide dissemination of findings, and discuss the potential for sustaining and scaling the intervention beyond the research period.
Eligibility Criteria
The target population for this trial includes adults who are at least 18 years of age, registered patients of a designated study clinic (i.e. Neighborhood Health Care – Temecula, CA or Escondido, CA clinic; Scripps Health – Carlsbad, CA or Encinitas, CA clinic), and diagnosed with T2D with one or more of the following in the last 90 days: HbA1c ≥ 8.0%, SBP ≥ 140 mmHg; LDL-c ≥ 100 mg/dL. We focus on individuals with an elevated HbA1c, SBP, and/or LDL in the last three months, as these individuals are at greater risk for complications and thus, are in the greatest need of self-management support. Due to the pragmatic nature of this trial, and to promote generalizability of findings, no further exclusion criteria are imposed. All eligible individuals who enroll in the MAC Trial and are willing to provide verbal consent, and complete a brief telephone survey in English or Spanish are considered eligible to participate in the patient-reported outcomes sub-study.
Sample Size
The target sample size for the study is N = 600 participants, allocated equally between health systems and intervention groups (Neighborhood Healthcare: MAC n = 150, UC n = 150; Scripps Health: MAC n = 150, UC n = 150). We project that 50% of individuals enrolled in the MAC Trial will complete the patient-reported outcomes sub-study (Aim 2, n = 300). RMASS225-27 was used to calculate the projected sample size needed for longitudinal data with consideration of attrition to detect clinically meaningful differences between groups for primary outcomes (Aim 1), HbA1c (0.5%), LDL-c (10 mg/dL), and SBP (5 mm/Hg). Effect sizes were estimated using standard deviations from a prior study in a similar population,28 and were d = 0.29 for HbA1c, d = 0.25 for LDL-c, and d = 0.29 for SBP. Additional assumptions included: (1) Alpha 0.05 and power 0.80; (2) missing data rate of 20% between time-points, and (3) a stationary autoregressive structure (lag 1) for the variance-covariance matrix of the repeated measures, using an autocorrelation of 0.50. The following baseline sample sizes are needed to find the predicted intervention effects: 432 for HbA1c, 598 for LDL-c, and 434 for SBP. An effect size of d = 0.50 is considered a clinically significant change in quality of life,29 a primary patient reported outcome in the current trial, and is a meaningful, moderate effect size for all patient reported outcomes. With n = 300, based on estimated 50% response rate, alpha of 0.05, and estimated 20% attrition between time points, power will be 0.82 to detect this effect size, indicating adequate statistical power for Aim 2.
Eligible Patient Identification and Study Enrollment
Patient identification.
An automated Patient Identification report was created by EMR analysts at both health systems to identify patients who have an upcoming appointment scheduled at one of the four study primary care clinic sites and meet study criteria [≥ 18 years of age, T2D diagnosis with at least one of the following qualifying clinical indicator(s) in the last 90 days: HbA1c ≥ 8.0%, LDL-c ≥ 100 mg/dL, SBP ≥ 140 mmHg]. The report is populated daily and includes patient name, medical record number, and contact information, value(s) and date(s) of the qualifying clinical indicator(s), and a clinical “risk score.” As outlined in Table 1, the risk score is derived via an algorithm developed for the MAC intervention, and is used to stratify (and prioritize) potential MAC participants by relative clinical risk.
Table 1.
Clinical Risk Score Algorithm
| Clinical Indicator | Range | Risk Score |
|---|---|---|
| HbA1c (%) | < 8.0 | 0 |
| 8.0–9.9 | 1 | |
| 10–11.9 | 2 | |
| ≥ 12 | 3 | |
| LDL-c (mg/dL) | < 100 | 0 |
| 100–129 | 1 | |
| 130–159 | 2 | |
| 160–189 | 3 | |
| ≥ 190 | 4 | |
| SBP (mmHg) | < 140 | 0 |
| 141–159 | 1 | |
| 160–179 | 2 | |
| ≥ 180 | 3 | |
HbA1c = glycosylated hemoglobin. LDL-c = low-density lipoprotein cholesterol. SBP = systolic blood pressure.
Study enrollment.
At intervention clinics, the MA Health Coach reviews the Patient Identification Report on a daily basis to identify, and conducts a brief EMR review for eligible patients presenting to the intervention clinic that day. When eligible patients have appointments at, or close to the same time, the MA Health Coach prioritizes the patient with the highest clinical risk score. The MA Health Coach then approaches an eligible patient when he/she presents for the scheduled (“index”) visit, and attempts to complete the initial health coaching encounter immediately after the clinical visit; however, if this is not convenient for the patient, he/she is offered a telephone-based appointment for another date and time within one week. If the initial health coaching session occurs same-day, or by phone within 1 week of the index visit, the patient is deemed “enrolled” in the MAC Trial. Conversely, if an initial health coaching session is not conducted same-day, or within 1-week of the index visit, the patient is not considered “enrolled.” Should this patient remain eligible at his/her subsequent primary care visit, he/she will appear on the Patient Identification Report at that time.
For each intervention participant enrolled, a research assistant identifies a patient from the Patient Identification Report who completed an appointment (i.e., index visit) that same business week at the respective UC site and has a comparable clinical risk score. Should more than one UC clinic patient meet these criteria, the patient who completed a primary care visit closest to the date and time of the MAC intervention patient’s index visit will be “enrolled” in the UC group. Because there is no change to care nor are there any required study visits, UC participants’ enrollment is invisible to these individuals; however, the enrollment process serves to include their clinical data in the primary outcome analysis and qualifies them for the patient-reported outcomes sub-study.
Patient-reported outcomes sub-study.
Upon enrollment in the MAC Trial, participants in both groups are mailed a letter informing them that they will receive a phone call within one week inviting them to participate in a survey evaluating their healthcare experience at Scripps Health or Neighborhood Healthcare. The letter informs participants that the survey pertains to their recent visit with their doctor (i.e., the index visit for both arms), and that they will be asked questions about their healthcare and the actions they take to manage their health. The Project Manager’s phone number is included in the recruitment letter, to provide the participant the opportunity to ask questions or opt out before being contacted by a research assistant. Approximately one week after mailing the recruitment letter, research assistants call MAC Trial participants to provide a brief overview of the patient-reported outcomes sub-study and to obtain verbal consent for participation. Individuals who decline participation are asked their reason(s) for declining, if willing to provide. Those who are interested in participating may choose to enroll in the patient-reported outcomes sub-study (and complete the baseline survey) at that time if available, or schedule a more convenient time within 14 days of their MAC Trial “enrollment” date/index visit. Once the baseline survey is completed, participants are considered enrolled in the patient-reported outcomes sub-study, and are contacted to complete the six and 12month telephone follow-up assessments, unless they request to discontinue participation. The overview and verbal consenting process is repeated for each assessment. Individuals who cannot be reached or who decline participation in the patient-reported outcomes sub-study at baseline are not contacted for either of the patient-reported outcomes follow-up assessments.
Consent.
Given the cluster-randomized design, the fact that the MAC intervention poses very low psychological and physical risk and is delivered as part of routine medical care, and because clinical outcomes are collected as part of routine medical care, the reviewing Scripps Health Institutional Review Board (IRB) waived the requirement for individual informed consent for Aim 1/clinical outcomes portion of the study. This approach is supported by literature indicating that in cluster-randomized trials, it is typically not possible, or is incompatible with the aims of the study to obtain individual informed consent.30 To-date, several published, pragmatic, cluster-randomized trials (e.g., 31,32) that have been conducted in large healthcare settings, including primary care clinics, have set the precedence for excluding individual consent and provide ethical guidance on this trial approach.
Patient-reported outcomes sub-study.
When contacted for potential participation in the patient-reported outcomes sub-study, individuals are informed that participation is completely voluntary, that they can skip any question they are not comfortable answering, and that they may discontinue their participation at any time without penalty or jeopardizing their relationship with Scripps Health or Neighborhood Healthcare. The verbal consent process is conducted in the participant’s preferred language (Spanish or English) by a specially-trained research assistant. After verbal consent is provided, the baseline, six or 12-month survey is administered.
Interventions
This two group, parallel design compares the MAC intervention to UC.
Group 1, UC.
Participants at the UC clinics continue to receive evidence-based, standard diabetes care without any modifications. Standard diabetes care includes visits with a primary care provider, certified diabetes educator(s), and group or individual diabetes self-management education (DSME). Per the physician’s discretion, patients may also be provided with education materials, and/or referrals to specialist(s) (e.g., endocrinology, cardiology). The use of the services described as standard diabetes care is dependent on physician and patient initiative.
Group 2, MAC Intervention.
The MAC model, which integrates an MA Health Coach in primary care to provide self-management support to patients with T2D is informed by three complementary theories.
Theoretical framework.
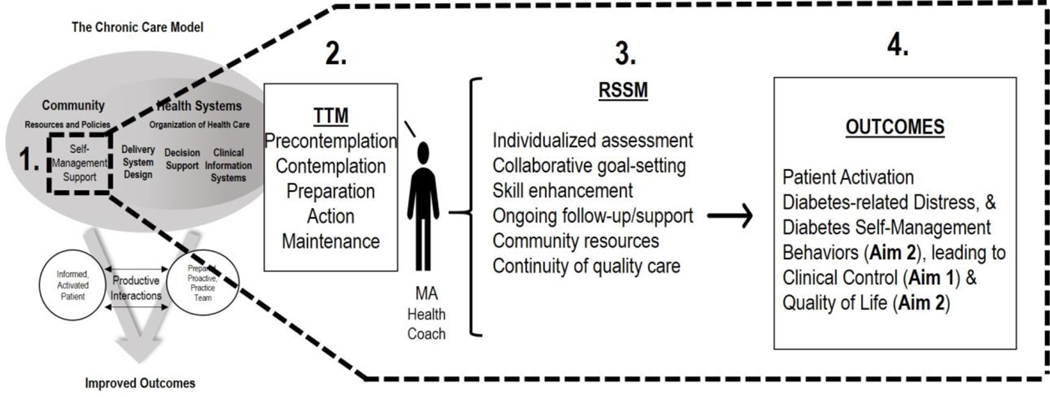
The overarching design of the MAC model is guided by the CCM – i.e., a well-established approach for improving care of chronic conditions like diabetes (see Figure 1).33,34 This framework recognizes that self-management, or the actions an individual takes to manage their own health outside of the traditional healthcare encounter, is central to good outcomes. The care team’s provision of self-management support, via assessment, goal-setting, action planning, problem-solving and follow-up, is an integral component of the CCM.
Figure 1.
Theoretical Mapping of the MAC Intervention
The specific MAC health coaching strategies are derived from two complementary theories that have guided effective chronic disease management interventions. First, the Robert Wood Johnson Foundation Initiative developed the Resources and Support for Self-Management Model (RSSM) for describing six types of resources that facilitate successful self-management of T2D: (1) individualized assessment, (2) collaborative goal-setting, (3) skill enhancement, (4) ongoing follow-up and support, (5) community resources, and (6) continuity of quality care. Second, as research has shown Transtheoretical Model (TTM)-based interventions to effectively enhance RSSM components one through three, the TTM guides the MA Health Coach’s specific intervention strategies. For RSSM components four through six, the MA Health Coach is afforded the flexibility to offer “as needed” follow-up (telephone and/or in-person) self-management support sessions to patients, and is trained to provide necessary referrals (e.g., for mental health or social needs). As depicted in Figure 1, to enhance the self-management support component of the CCM (Box 1), TTM-based strategies (Box 2) are implemented by the MA Health Coach to target RSSM components (Box 3) to promote successful diabetes management, and the hypothesized antecedents and consequences thereof (all Box 4). As outlined in the Process Evaluation Outcomes section, the “Ready, Set, Action” Forms, MAC Encounter Forms, and the Patient Assessment of Chronic Illness Care (PACIC) measure will facilitate a comprehensive evaluation of the proposed theoretical framework.
MAC encounters.
Eligible patients at the MAC clinics are offered health coaching/self-management support for 12 months as a supplement to the standard diabetes care described above. To reduce patient burden and enhance feasibility, the MAC intervention is delivered in conjunction with routine (quarterly, on average) primary care clinic visits and by telephone; no separate clinic visits are scheduled for health coaching. Specifically, as part of existing clinic workflows, all patients are roomed by a clinic MA for their primary care visit. The clinic MA completes a brief clinical intake and vitals assessment during the rooming process, and then exits. The MA Health Coach then enters the exam room with the primary care provider, or prior to the visit depending on the provider’s timing, and is introduced as part of the patient’s “Care Team.” With the patient and provider’s permission, the MA Health Coach remains in the exam room for the duration of the visit to understand the care plan outlined by the provider. Following the index visit, the patient is invited to the MA Health Coach’s office for a brief, one-on-one coaching session (see Initial Encounter section below). Thereafter, the MA Health Coach conducts in-person health coaching sessions with enrolled participants at subsequent clinic visits and telephone-based health coaching between visits as needed (see Follow-Up Encounters section below). MAC clinic patients have the right to refuse any or all intervention components provided as part of their routine medical care.
Initial encounter.
Following the index physician visit, the patient is invited to the MA Health Coach’s office for a brief one-on-one coaching session. The initial MAC encounter is guided by the Ready, Set, Action Form. The encounter commences with a brief, nine item assessment of participants’ engagement in diabetes self-management behaviors (i.e., medication adherence, physical activity, healthful eating, self-monitoring of blood-glucose and blood pressure), knowledge and understanding of diabetes clinical indicators, emotional well-being, and health literacy. Following the assessment, the MA Health Coach provides feedback on each of the behavioral domains and engages the participant in a discussion about potential areas of focus for goalsetting. Information ascertained as part of the assessment about knowledge, emotional well-being, and health literacy is used to tailor the goal-setting process to participant’s individual needs.
The MAC goal-setting process is further tailored to each participant’s unique readiness-to-change. According to the TTM, readiness to change behavior exists on a continuum, ranging from pre-contemplation to action and maintenance, and assessing where an individual lies on this continuum can highlight stage-appropriate intervention techniques. Motivational Interviewing35 is used to explore ambivalence and motivation, elicit “change talk”, and help patients advance through the TTM stages of change. As part of the goal-setting process, the MA Health Coach collaborates with the participant to identify potential strengths/resources to maximize success, problem-solve potential barriers, and ultimately, record a “SMART” (Specific, Measurable, Attainable, Relevant, Time-Bound) goal on the Ready, Set, Action form. If a lack of relevant diabetes knowledge is identified as a barrier, educational visuals may be incorporated into the session. Given the wide range of barriers that are commonly encountered when setting health behavior change goals, particularly in underserved patient populations, each MA Health Coach is equipped with a manual of social services and other resources available to patients within the health system and/or the surrounding community (e.g., social work; discount pharmacy information; DSME, for those whose informational needs exceed content offered in educational visuals). Additional tools available to the MA Health Coach include a Medication Reconciliation Form, for participants endorsing challenges with medication adherence, and a Blood Glucose Monitoring Calendar, for participants to track blood sugars, and/or the impact of health behaviors on blood sugar control. For more information on the health coaching tools presented in italics see Intervention Materials section.
Follow-up encounters.
Following the initial encounter, MAC participants receive ongoing, brief (≤ 30 minute) self-management support from the MA Health Coach after each of their (approximately quarterly) clinic visits for the next 12 months. Enrolled MAC participants appear on the Patient Identification Report with a designation of “Returning” to alert the MA Health Coach of each upcoming appointment. The MA Health Coach attempts to join the return visit and conduct after-visit health coaching; however, similar to the initial encounter, if it is not convenient for the patient to meet after the clinical appointment, the health coach offers a telephone-based health coaching session at a later date. To enhance the continuity of self-management support between clinic visits, the MA Health Coach also conducts telephone follow-up as an adjunct to in-clinic health coaching encounters. These telephone encounters are conducted on a tapered frequency over the course of 12 months – i.e., weekly for 2–4 weeks after the initial encounter, and then monthly thereafter. However, due to the pragmatic nature of the trial, this is offered on a flexible, as-needed basis to tailor dosage and content coverage in order to meet patient-specific needs. The in-clinic and telephone follow-up encounters mirror the process of, and incorporate the same intervention materials as, the initial encounter, but add to this a review of each patient’s clinical and behavioral progress. As part of this review, the MA Health Coach reinforces positive progress, reviews challenges, problem-solves barriers that may have impeded progress, and collaborates with patients to refine their self-management goals.
MAC documentation.
As part of the team-based approach to care, the EMR is used to communicate relevant information between the MA Health Coach and primary care providers. Specifically, a “Mode, Assessment, Plan” progress note is entered by the MA Health Coach following every health coaching encounter to document MAC participants’ progress. The three-part MAP note documents the “Mode” of the health coaching session (in-clinic or telephone), “Assessment” (a review of the patient’s progress in self-management), and “Plan” (the collaborative goal set by the patient and health coach). The MAP note is simple, and intended to include only the information that would be relevant for the patient’s care. The MA Health Coach maintains more detailed notes in the patient’s file folder to assist him/her in tracking progress and conducting follow-up sessions.
Intervention materials.
The MAC intervention materials were created by study investigators with backgrounds in Endocrinology, Clinical Psychology, and Behavioral Medicine; Clinical Psychology and Public Health pre-doctoral trainees; and Master’s level project staff with training in Psychology. Whenever possible, intervention content was adapted from existing evidence-based diabetes intervention materials, including Project Dulce 28 and Health Coaching Principles 36. All intervention materials were made available in English and Spanish, and reviewed and edited for low literacy and cultural relevance. All intervention materials are included in Appendices A-D and described in detail in Table 2.
Table 2.
MAC intervention materials.
| Item | Purpose | Content | Mode and frequency of use |
|---|---|---|---|
| Ready, set, action form | To guide assessment and goal-setting activities. | “Ready” includes assessment of: • Diabetes self-management behaviors (diet, exercise, blood glucose monitoring, medication adherence) via 5 items adapted from the SDSCA [39] • Knowledge of diabetes clinical indicators (1-item created for this study); • Emotional well-being via the 2-item, Diabetes Distress Screener [77]; • Health literacy via the Single-Item Literacy Screener [78]. “Set” is used to explore a participant’s readiness-to- change self-management behaviors that are not currently at target based on the brief assessment. “Act” details the participant’s SMART goal, and potential barriers and strengths/resources that are relevant to achieving the selected goal. |
Used during initial health coaching encounter, as well as during any subsequent (in-clinic or telephone) encounter in which a reassessment is conducted and/ or a new SMART goal is set. |
| Educational visuals | To address informational deficits that are identified as barriers to effective diabetes self-management. | Core education in the following diabetes domains: healthful eating, physical activity, medication adherence, blood glucose monitoring, blood pressure monitoring, clinical targets, and emotional well-being. | Used during clinic-based encounters as needed. While primarily used as a conversational tool, one or more pages may be printed and provided to the participant in handout form at the MA Health Coach’s discretion. |
| Medication reconciliation form | To assess knowledge, use, and barriers related to medications for diabetes and related chronic conditions. | Structured assessment of: • Knowledge of the medication’s intended purpose. • Understanding of how to take the medication (dosage, timing, frequency). • Actual use of the medication. • Barriers to taking the medication as prescribed (e.g., side effects, insurance coverage). |
Used during in-clinic or telephone encounters when medication adherence challenges are reported or suspected. |
| Blood glucose monitoring calendar | To document blood glucose values and increase participants’ awareness of the impact of health behaviors on glucose levels. | Structured in month-at-a-glance format, the calendar includes a red oval on each calendar day where participants are asked to record (fasting or post-prandial) blood glucose values. Each calendar day also includes blank space for participants to transcribe observations of what behaviors might have contributed to the observed value(s). | Provided to the participants during in-clinic encounters, and reviewed during subsequent (in-clink or telephone) health coaching encounters. |
MA - Medical assistant. SDSCA - Summary of diabetes self-care activities.
The “Ready, Set, Action Form” is a three-part tool that combines a nine item behavioral and psychosocial assessment, readiness-to-change scale, and goal-setting guide. Importantly, diabetes self-management behaviors are listed and addressed in order of relative importance on the Ready, Set, Action Form: (1) Medication, (2) Blood glucose and (3) Blood pressure monitoring, (4) Diet, (5) Exercise. For example, if a patient is not taking medications as prescribed (#1) and is also not exercising (#5), the former would be prioritized first for health coaching. Once completed, the “Ready” and “Set” sections are transmitted by the MA Health Coach to the research team on a weekly basis for intervention fidelity tracking purposes (see Intervention Fidelity, below), while the participant is provided with the “Action” portion of the form. Educational visuals include basic information on diabetes medications and clinical indicators, healthful eating, physical activity, blood glucose and blood pressure self-monitoring, and emotional well-being, and are used to complement health coaching when an informational deficit is identified as a barrier in the action planning process. Importantly, because the MAC intervention is a health coaching program (i.e., not DSME), the visuals are not a primary component of the health coaching process but rather are used to provide the foundation needed to engage in effective self-management when needed. For participants with known or suspected difficulty taking their diabetes, cholesterol, and/or blood pressure medications as prescribed, the MA Health Coach utilizes a Medication Reconciliation Form to gain a better understanding of participants’ medication adherence challenges. The information ascertained is used to guide goal-setting related to medication adherence and to empower the participant to communicate with their provider about medication-related concerns. Finally, the Blood Glucose Monitoring Calendar is provided by the MA Health Coach to patients who are working to increase blood glucose monitoring frequency and/or to understand daily blood glucose trends. As a flexible tool, the calendar-based tracking not only guides participants’ identification of associations between health behaviors and glucose levels, but also facilitates review of progress in subsequent health coaching encounters.
MA Health Coach selection, training, and supervision.
One MA was designated at each intervention clinic as the MA Health Coach and was relieved of all other MA responsibilities. The MA Health Coach at each intervention site was specially selected as someone who is a “natural helper” and is interested in career advancement. Interview questions were specifically designed to ensure candidates understood, were interested in, and qualified for the significant transition from (traditional) MA to MA Health Coach.
Once selected, the MA Health Coaches completed a comprehensive, five-day MA Health Coach Training described in Table 3. After this initial training, the MA Health Coaches receive ongoing case consultation from study investigators on a bi-weekly basis, and “refresher” trainings in diabetes and/or motivational interviewing every six to nine months for the duration of the trial. The MA Health Coaches also have an appointed “clinical lead” at each of the intervention clinics with whom they consult about practical, non-study-related issues. At each intervention clinic, the MA Health Coach is located in a central office to facilitate the real-time, in-person coaching when patients present for their clinic visits.
Table 3.
MA Health Coach Training Components
| Training Topic | Duration | Main Content Areas | Instructor(s) |
|---|---|---|---|
| Fundamentals of Diabetes | 8 hours | Clinical and behavioral aspects of T2D | Advanced Practice Nurse and Certified Diabetes Educator |
| Principles of Health Coaching | 16 hours | Action planning, closing the loop, medication reconciliation, setting the agenda | Physician and Health Coaching Researcher |
| Motivational Interviewing | 8 hours | Motivational Interviewing for Behavioral Change | Clinical Psychologist |
| Research Methods | 8 hours | Study Procedures and EMR Documentation | Project Manager |
| EMR = Electronic Medical Records | |||
Intervention Monitoring, Adherence, and Withdrawals
Intervention fidelity.
MAC intervention “dosage”, via the MAC Encounter Form (below), and topic coverage, via the MAC Encounter Form, and “Ready” and “Set” sections of the Ready, Set, Action Form, are the primary indicators of MAC intervention fidelity. Fidelity data are entered into a Research Electronic Data Capture (REDCap) database by centralized research staff; formative feedback is provided to the interventionists regarding adherence to protocols and areas for improvement as needed.
MAC Encounter Form.
For all in-clinic and telephone encounters, the MAC Encounter Form is completed by the MA Health Coach to document: the mode (in-clinic or telephone), duration, purpose(s) (progress review, goal-setting, resource provision and/or other), whether or not a Ready, Set, Action Form was completed (yes/no), and the behavioral focus of the encounter (medications, self-monitoring, healthful eating, and/or physical activity). The MAC Encounter Form is included in Appendix E.
Participant withdrawals.
The intervention is permanently discontinued if the participant dies or is no longer a patient of the study health system. These participants are referred to as administrative withdrawals and do not receive further contact. At any time, a participant may request to no longer receive health coaching, complete patient-reported outcomes assessments, or both. These voluntary withdrawals (and the reasons, whenever possible) are documented by centralized research staff in the REDCap database for tracking purposes. Participants who refuse further coaching are also removed from (the returning portion of) the Patient Identification report, while those who decline further assessment calls are removed from the patient-reported outcomes sub-study call schedule. A participant’s decision to withdraw from either or both study components does not have any bearing on the medical care they receive at their respective health system. Further, consistent with an intent-to-treat analytic approach, available data for administrative and voluntary withdrawals will be extracted and included in outcome analyses.
Concomitant interventions.
The MAC intervention is implemented in real-world primary care settings with no alterations to typical primary care processes, and no restrictions on concomitant interventions received. The MAC intervention is overlaid on top of, and integrated with, usual primary care process at the designated MAC clinics.
Assessment of Outcomes and Other Variables
Details regarding assessment of primary and secondary outcomes, demographic factors, and other variables are shown in Table 4.
Table 4.
Assessment of Outcomes and Other Variables
 |
 |
CCM = Chronic Care Model. EMR = Electronic Medical Record. HbA1c = Glycosylated Hemoglobin. LDL-c = Low-density lipoprotein cholesterol. MA = Medical Assistant. SBP = Systolic blood pressure.
Variables will also be used for cost-effectiveness analyses.
Primary outcomes.
Primary indicators of diabetes control (HbA1c, LDL-c, SBP), collected as part of routine medical care at Scripps Health and Neighborhood Healthcare, will be evaluated individually to address Aim 1. HbA1c reflects an individual’s average blood glucose level over the past two to three months, and is commonly used to diagnose, and monitor treatment and control of diabetes. Typically an HbA1c test is conducted at least twice yearly if patients are at target, and quarterly if an individual is on insulin, undergoing treatment changes, and/or not meeting treatment goals.37 HbA1c is reported as a percentage, with higher levels indicating a greater proportion of glucose in the blood and greater risk of developing diabetes complications. Most adults with diabetes have an HbA1c goal of ≤7%; however, treatment targets are individualized due to factors such as age and risk of hypoglycemia.37 As patients with T2D are at greater risk for cardiovascular disease, monitoring and optimizing lipid and blood pressure control is recommended as part of diabetes medical care. LDL-c is generally assessed annually among adults with T2D (especially those over the age of 40), and more frequently in the context of statin initiation or dosage change.38 Blood pressure measurement is recommended at every routine clinical visit. The blood pressure target for adults with diabetes is 140/90 mmHg, and 130/80 mmHg among those with higher cardiovascular risk. HbA1c, LDL-c, and SBP will be derived from the EMR at the conclusion of the trial (see Electronic Medical Records Abstraction section); no study-specific lab draws or blood pressure assessments are conducted as part of this trial.
Secondary outcomes.
Secondary outcomes collected by telephone at baseline, six, and 12 months include diabetes self-management behaviors (SDSCA39), perceived physical health and quality of life [Patient Reported Outcomes Measurement Information System (PROMIS) Global-10 Health Scale40], patient activation (Patient Activation Measure, PAM41), quality of chronic care (PACIC42), and demographic and health information. Measures were chosen based on psychometric evidence, applicability, brevity, availability in both English and Spanish, and sensitivity to change. For brevity, seven items from the SDSCA were selected to evaluate healthful eating, physical activity, glucose monitoring, and medication intake (pills and/or insulin, as applicable).39 In prior research, the SDSCA has demonstrated associations with other measures of diabetes self-management, adequate test-retest reliability and sensitivity to change,39 and has been translated and validated in Spanish.43 Perceived physical health and quality of life are assessed using the PROMIS Global-10 Health Scale.40 The scale’s 10 items assess physical function, pain, fatigue, emotional distress, social health and general perceptions of health, and are combined to form two subscales: Physical Health and Mental Health. The PROMIS Global-10 Health Scale has evidence of internal consistency, and validity in respect to factor structure and magnitude and direction of association with conceptually relevant constructs.40 Research has shown patient activation, an important process in disease self-management and health outcomes, to play a particularly important role in addressing health and healthcare quality disparities.44,45 In the present study, patient activation is evaluated using the 13-item version of the PAM, which has demonstrated strong psychometric properties, including reliability, and content, construct, and criterion validity.41,46 The PAM was translated into Spanish for a prior study of US and foreign-born Latinos by a bilingual team of translators and was shown to be reliable in both languages.45 The PACIC42 is used to assess the extent to which MAC Trial participants perceive that the care they receive for their diabetes is congruent with the central components of the CCM.24 The PACIC has been shown to have good psychometric properties (i.e., construct validity, reliability, internal consistency, and test-retest reliability) in English 42,47 and Spanish.48
Demographic and other variables.
The following demographic and health information will be derived from the Scripps and Neighborhood Healthcare EMRs to describe the overall sample (N = 600) and for use as possible covariates in outcome analyses: gender, age, race/ethnicity, preferred language, marital status, employment status, and health insurance status. More detailed demographics and health history information (e.g., country of origin, income, education, information about diabetes diagnosis, including whether or not participants have been told by a physician they have diabetes, age at first diagnosis) will be collected via self-report survey among the subset of participants who complete the patient-reported outcomes sub-study (n = 300).
Cost outcomes.
Data from the both health systems’ EMRs will be derived to inform the cost effectiveness analysis. Patient characteristics (e.g. age and gender), time-varying risk factors (HbA1c, SBP, cholesterol, smoking status), and healthcare utilization (primary and specialty care encounters, procedure codes) and costs (estimated using internal accounting systems) will be analyzed to document the cost effectiveness of the 12-month MAC intervention from the health system perspective.
Process outcomes.
The RE-AIM model 22,23 will be used as a framework to evaluate feasibility, acceptability, sustainability, and dissemination and scaling potential of the MAC intervention. To examine intervention Reach, we will compare the number of clinic patients enrolled to those eligible, and also document the proportion of patients who were offered yet declined health coaching. Demographics and other factors available via the EMR will be compared between enrolled vs. not enrolled/declined patients to document differences between these groups. Efficacy of the MAC intervention will be demonstrated via between-group differences in clinical and patient-reported outcomes over the 12-month evaluation period. Semi-structured post-study interviews will be conducted with MA Health Coaches (see Appendix F), and clinic staff and providers to gauge adoption rates, facilitators, and barriers. Topic and content coverage will be tracked using the MAC Encounter and Ready, Set, Action Forms to document implementation. Together, these two forms and the PACIC,42 collected as part of the patient-reported outcomes sub-study, will assess the extent to which the MAC intervention operationalized central elements of the CCM, including patient activation, goal setting, problem solving, and follow-up (theoretical fidelity). Finally, the maintenance potential of the MAC model will be explored via stakeholder interviews and CAB discussions, and informed via clinical and cost-effectiveness results. Findings from this multi-method process evaluation will also be used to guide program revisions prior to dissemination.
Participant Timeline
As shown in Figure 2, the index visit marks the beginning of the 12-month enrollment period for participants in both groups. Within two weeks of that visit, all participants will be contacted and offered enrollment in the patient-reported outcomes sub-study. EMR data will be extracted for 12 months forward for all participants; MAC participants will receive in-clinic and telephone health coaching, and UC participants will receive standard diabetes care over this same period.
Figure 2.
Summary of Participant Timeline
Methods: Assignment of Interventions
Randomization and Blinding
A total of N = 600 participants will be enrolled in the MAC Trial, split equally between conditions and health systems: Scripps Health n = 300 (MAC n = 150, UC n = 150) and Neighborhood Healthcare n = 300 (MAC n = 150, UC n = 150). Consistent with the cluster-randomized controlled trial design, assignment to condition will occur at the clinic level. Two Neighborhood Healthcare sites, matched according to approximate clinic size and patient characteristics, and two similarly matched Scripps Health clinics were selected. One clinic within each health system was randomly allocated to MAC, and one to UC, using a random numbers generator. Research assistants who conduct patient-reported outcome assessments, EMR analysts who are responsible for extracting outcome data, and the study statistician are blinded to study arm.
Methods: Data collection, Management, and Analysis
Data Collection Methods
Electronic medical records abstraction.
The Scripps Health IRB approval provides permission to audit the EMR for patient identification and outcome analysis purposes. Demographic and other (static) data will be extracted from the EMR for each participant upon enrollment, and clinical and health service utilization data will be abstracted for 12 months from each patient’s unique enrollment date. Based on screening guidelines, we anticipate up to five data points may be available for HbA1c (months 0, 3, 6, 9, 12), up to two for LDL-c, and one SBP per clinic visit over the course of 12 months. The EMR query will include all Scripps Health and Neighborhood Healthcare laboratory and ambulatory clinic sites. Given that clinical outcomes are assessed as part of routine medical care versus incentivized research visits, we anticipate a greater rate of missing data than traditional, non-pragmatic trials. However, anticipated missingness was factored into sample size determination, and variables significantly associated with missingness will be incorporated into analyses as auxiliary variables.
The data abstraction report will be developed jointly by study investigators and Scripps and Neighborhood Healthcare EMR analysts. To ensure accuracy and completeness of data extraction, two research staff will verify 10% of the extracted data report against live EMR records to evaluate accuracy and completeness. A third staff member will perform the final validation to compile a list of data report feedback to EMR analysts. If needed, analysts will evaluate data discrepancies and refine extraction procedures to produce an updated accurate report. Research staff will repeat the verification process until all rectifiable data discrepancies are resolved.
Patient-reported outcome assessments.
Patient-reported outcomes are assessed via telephone interviews conducted in the participant’s preferred language, English or Spanish. All interviews are conducted by trained bilingual, bicultural research assistants using a standardized protocol. Participants who enroll in the patient-reported outcomes sub-study and complete the baseline survey are invited for follow-up assessments at six and 12 months following the baseline assessment. Enrolled participants are invited for the 12-month assessment even if they do not complete the six-month survey. Within two weeks of each survey completion, participants are mailed a $25 gift card and a “thank you” card for the participant’s time and effort, for a total of up to $75.
Data Quality Control Procedures
Staff training.
Prior to commencing work with study data, all research staff complete CITI Protection of Human Subjects Training, including Good Clinical Practice and Conflict of Interest certifications, and an NIH Information Privacy and Security training. Additionally, they are trained and certified in standardized study procedures which include questionnaire administration, recruitment procedures, consenting, and REDCap database use. Research assistants review all databases weekly for completeness and accuracy. The number of enrolled participants, follow-up assessment rates, and appropriate coding of patients (e.g., refusals) are verified and confirmed. Research staff indicate their employee identification number with each data entry and verification, and are consulted when discrepancies, errors, or omissions are identified by staff performing quality control checks.
Cohort retention procedures.
Multiple procedures are used to maximize cohort retention and data quality. First, immediately upon enrollment, all participants’ medical records are flagged to denote active enrollment in the MAC trial; at the conclusion of the 12-month follow-up period, the flag is removed. For intervention participants, the EMR flagging (i.e., denoting enrolled patients as “Returning”) serves as an additional notification (secondary to the Patient Identification Report) to alert the MA Health Coach when an enrollee has an upcoming return clinic visit, so that in-person health coaching can be integrated with that visit. Telephone health coaching sessions between clinic visits are also designed to maintain participants’ engagement in the MAC intervention and their routine medical care.
Participants who are also enrolled in the patient-reported outcomes sub-study are mailed an appointment reminder letter approximately one week prior to telephone follow-up assessments (month-six and month-12). Three months before each follow-up assessment, participants are mailed reminder postcards that inform them of the phone number that will be called, the date/time of the assessment (which is mutually agreed upon during the prior assessment), and the study phone number to call if their contact information has changed. In addition, approximately 24 hours before their assessment appointment, participants receive a reminder phone call. Two-month windows are allowed for completion of the follow-up assessments to maximize opportunity to obtain these data.
Statistical Methods
Primary analyses.
Multilevel models using full information maximum likelihood estimation will be conducted to examine changes in HbA1c, LDL-c, and SBP (Aim 1, primary outcomes) and patient reported outcomes (Aim 2) over time. Each outcome will be treated continuously and examined in separate models to evaluate Aims 1 and 2. Analyses will include the between-subjects factor of treatment group (MAC versus UC), the within-subjects factor of time (i.e., assessment occasion), and a cross-level group by time interaction effect, the primary effect of interest in evaluating the trial. If a given interaction is statistically significant, follow-up analyses will follow recommended approaches.49 Assessment of outcomes will adhere to the intention-to-treat principle. The generalizability of the proposed intervention differences will be explored by statistically evaluating whether or not healthcare system (Neighborhood Healthcare, Scripps Health) moderates this effect (i.e. site-by-group-by-time interaction effects). Possible clustering as a function of clinic site will be estimated by examining intraclass correlation coefficients. If significant clustering is identified, these variables will be treated as fixed effects in multilevel models, given the relatively small number of units for these variables.50 Healthcare system will also be accounted for in the multilevel models as fixed effects. We do not predict differential effectiveness of the MAC intervention across gender and ethnic/racial groups and have not powered the trial to test such differences. However, consistent with the requirements for a Phase III clinical trial, we will conduct exploratory analyses that examine differences in effects by sex and race/ethnicity. These supplementary findings will help guide future research and inform intervention dissemination efforts.
Cost-effectiveness analysis.
The cost-effectiveness analysis will document the long-term cost effectiveness of the MAC intervention relative to UC from the health system perspective. Intervention costs will be estimated using a combination of process mapping and time-driven, activity-based costing.51,52 Process mapping elucidates each element involved in the intervention, including clinic resources and staff that are involved in the integration of health coaching into the clinic workflow. Time-driven, activity-based costing is used to assign levels of effort and costs associated with each activity identified in the process mapping. The process mapping and activity estimates will be developed collaboratively by the research and clinic staff.
Intervention costs, along with the observed changes in clinical outcomes (i.e. HbA1c, LDL-c, and SBP from AIM 1), will be used as inputs into a diabetes simulation model designed to evaluate the long-term health outcomes and economic consequences of interventions among patients with T2D.53. The United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model employs an integrated system of parametric equations to estimate the absolute risk of the first occurrence of each of seven diabetes related complications (fatal or non-fatal myocardial infarction, other ischemic heart disease, stroke, heart failure, amputation, renal failure, and eye disease) and death based on patient characteristics (e.g. age and gender) and time-varying risk factors (HbA1c, SBP, cholesterol, smoking status). Data from the UKPDS was used to develop the predictive equations for diabetes-related complications, mortality, as well as progressive time paths for the risk factors, and to assign utilities conditional on disease state. Data from a large, integrated health plan were used to develop U.S. specific costs for diabetes related complications.54
The base case of the cost-effectiveness analysis will assume a health system perspective, a 40-year time horizon, and a 3% discount rate for both Quality of Life Adjusted Years (QALYs) and costs. The base case also assumes that the effects of MAC on clinical outcomes will persist over time. Sensitivity analyses will consider alternatives in which ongoing intervention is required to maintain the observed improvements in clinical outcomes, and in which the observed improvement in HbA1c and other clinical indicators diminishes over time. Sensitivity analyses will be used to investigate influence of the estimated treatment effects and intervention costs, as well as the influence of the time horizon and discount rate. Additional sensitivity analyses will consider second order uncertainty. The UKPSD Outcomes Model provides a full set of equation parameters that were derived from bootstrap samples of the original UKPDS population. These estimates will be used to calculate an incremental cost-effectiveness plane.
Pay for Performance (P4P) Analysis.
As medical practices are accountable to P4P targets (e.g., HbA1c < 8.0%, LDL-c < 100 mg/dL, and SBP < 140) and receive added reimbursement if they achieve these targets, a program that can improve performance on these clinical metrics carries added value to the health system. Thus, we also propose to estimate the financial gains from improving performance on P4P clinical criteria (i.e., where the added P4P financial gains attributable to MAC will be categorized as a quality indicator in the Value equation: value = quality/cost; http://www.nejm.org/doi/full/10.1056/NEJMp1011024?viewType=Print). This information will be presented to health system stakeholders, as the value added represents additional incentive for sustaining and scaling the MAC program beyond the funding period.
Methods: Monitoring
Data Monitoring
Data monitoring procedures were established to ensure ongoing attainment of enrollment and follow-up completion milestones, and to track the fidelity of the MAC intervention over the course of the study. Research assistants track the enrollment of all intervention and control patients at Neighborhood Healthcare and Scripps Health, plot these against the weekly target (N = 5 total, or an average of n = 2.5 per health system), and distribute enrollment graphs to the research team on a biweekly basis. The rates of enrollment into the patient-reported outcomes sub-study are tracked similarly, and compared to projected targets – i.e., 50% of parent trial participants, split roughly equivalently across intervention groups and healthcare systems. Six and 12-month follow-up completion rates for the patient-reported outcome assessments are monitored on a monthly basis and compared to the 80% target rate. Finally, implementation of the MAC intervention at both health systems is tracked using the MAC Encounter and Ready, Set, Action Forms. Process evaluations are conducted every six months to monitor intervention fidelity and provide feedback to interventionists if warranted. All data reports are reviewed by the study’s independent, three-member Data and Safety Monitoring Board, which meets once per year (or more frequently in the event of an adverse event or data concern), as outlined in a Data Safety Monitoring Plan that was approved by the NIH and governing IRB.
Ethics and dissemination
Research ethics approval.
Institutional agreements were established during study start-up to designate Scripps Health as the “reviewing” IRB and San Diego State University as the “relying” IRB. The MAC Trial protocol was approved prior to study-start (initial approval date: 05/06/2015), and undergoes continuing review on an annual basis by the Scripps Health IRB. All required documents (i.e., assessment and intervention materials; patient-reported outcomes sub-study letter, telephone scripts, verbal consent, and assessments) were submitted to the IRB in English and Spanish for approval. Substantive modifications to study protocol and documents, as well as the addition or removal of study staff, are submitted to the IRB as protocol modifications. There have been no protocol changes that would necessitate reporting to the funding agency (i.e., changes that would affect scope of work or fulfillment of study aims).
Declaration of interests.
Conflict of Interest Disclosure Forms are completed by study investigators on an annual basis and are maintained by the Scripps Health IRB. The study investigators have no financial or other competing interests to declare.
Access to data.
As the prime institute on the NIH award, Scripps Health maintains ownership of all study data. As stewards of these data, the study investigators will make de-identified data sets available to interested collaborators pending the publication of primary reports and establishment of appropriate data exchange/use agreements.
Dissemination policy.
The Study Investigators registered this clinical trial, and will ensure that summary results are submitted to ClinicalTrials.gov according to the timelines in the NIH Dissemination Policy. Specifically, within 12 months of completion of the final follow-up assessment, results will be posted on ClinicalTrials.gov to ensure timely communication of findings to the public. Beyond ClinicalTrials.gov, study investigators will work closely with the CAB to determine optimal approaches for disseminating findings to patient, healthcare, payer, and academic communities. Patients, families, and other community members will be reached via media outlets, patient advocacy organizations, and printed information distributed at community settings. The Scripps Health, San Diego State University and Neighborhood Healthcare Public Relations teams will produce and disseminate press releases to media outlets to maximize impact. Healthcare providers and administrators will be reached via organization-based email blasts and live presentations locally and nationally. Widespread dissemination of findings to scientific audiences will occur via national and international presentations and manuscripts. Scientific reports of study findings will follow all CONSORT standards to ensure comparability of trial results to other studies of related interventions.
Discussion
The unprecedented increase in T2D prevalence has taxed the US healthcare system, while reducing quantity and quality of life for millions of adults. Primary drivers of cost in T2D are diabetes-related complications, including neuropathy, nephropathy, retinopathy, coronary artery disease, stroke, and peripheral vascular disease. Optimizing cardiometabolic control, as demonstrated by improving HbA1c, blood pressure, and lipids, can lead to significant reductions in complications and mortality.55–57 However, despite significant advances in treatment over the past decade, diabetes clinical control remains suboptimal.58 Between 2007 and 2010, only 52.5%, 51.1%, and 56.2% of people with diabetes achieved HbA1c, blood pressure, and LDL-c goals, respectively; a mere 18.8% met all three targets.58 In the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), only 8.4% of participants with diabetes met clinical targets for HbA1c, lipid, and blood pressure control. 59 A retrospective EMR analysis of 53,120 patients from 2004 to 2010 documented that single- and dual-goal achievers experienced higher complication and mortality rates than triple-goal achievers.60 As of 2015, diabetes was the 7th leading cause of death in the US.61
To complicate matters, the increasing demand for primary care services (due to the aging adult population, rising rates of diabetes and obesity, etc.), combined with a shortage of new primary care physicians, has contributed to a primary care “depend-capacity” imbalance, and in turn, growing patient panels.62 More than 40% of primary care physicians report not having enough time with their patients, and research has found that 50% of patients leave a medical visit without understanding their providers’ recommendations.11,63 Traditional DSME programs have been shown to improve clinical, quality of life, and healthcare cost outcomes.64 However, many individuals are unable to access this resource due to practical and/or healthcare access barriers65–68. In 2015, a mere 5% of Medicare beneficiaries with newly diagnosed T2D utilized DSME.69 In addition to DSME, ongoing support is required to maintain benefits derived from DSME participation.70 The limitations of current healthcare models, coupled with rising prevalence, disparities, and rates of sub-optimal clinical control in T2D highlight the need for innovative, cost-effective, flexible, and accessible interventions to improve care processes and outcomes for the diverse groups affected by T2D.
Team-based care, or empowering non-clinicians to “share the care,” is posited as a foundational element that improves chronic disease care and outcomes. Training MAs to provide diabetes self-management support as health coaches is consistent with recent proposals to transform primary care through models that optimize application of skills or personnel working at “top of license.” 11,12,71,72 Further, MAs comprise one of the fastest growing and widely available allied health professions, and are often selected for cultural and linguistic concordance with the target patient population.73,74 Many care settings that have shifted responsibilities to MAs and implemented higher MA to physician ratios have demonstrated improved productivity and efficiency.73 There is consistent evidence from well-controlled studies that supports the effectiveness of MA health coaching for T2D. Qualitative studies have highlighted methods for enhancing the success of MA health coaches,75 and have provided evidence of primary care providers’ satisfaction with (and acceptance of) delegating health coaching responsibilities to non-clinician staff.76 Indeed, it has been proposed that MA Health Coaches serve as cultural brokers or liaisons between the patient and provider – enhancing the relevance and benefit of this model for underserved populations.
This study builds upon these findings by evaluating a pragmatic health coaching intervention, delivered by existing, non-licensed personnel in “real world” primary care environments to better meet the complex needs of diverse individuals with diabetes (i.e., insured and uninsured; varied ethnic/racial background and socioeconomic standing). The cluster-randomized, MAC trial is being conducted by a multidisciplinary investigative team with substantial focal expertise, through collaborations with two diverse and representative healthcare settings. Although it would have been preferable to evaluate a greater number of clinics, this was not feasible within the scope (budget, time) of this study. By focusing on a small number of well characterized clinics we can establish intervention effectiveness to support scaling and dissemination across the systems.
The study incorporates several innovative elements, including integrating health coaching into ongoing primary care workflows, training and supporting existing staff to practice at “top-of-license,” and maximizing the use of available health information technology for sample identification, program evaluation, and cross-provider communication. Pragmatic methods are appropriately balanced with rigor to provide a valuable test of effectiveness and cost efficiency, while facilitating the sustainability, scalability, and dissemination of the health coach training and intervention to other healthcare systems across the nation. The MAC program offers a potential solution to the burgeoning primary care demand-capacity imbalance that can be applied in diverse healthcare settings to better address the needs of the growing number of individuals with T2D.
Supplementary Material
Acknowledgements:
We thank the participants, staff, trainees, interventionists, volunteers, and community advisory board members who contributed to the MAC trial.
Funding: The current study was supported by a grant from the National Institutes of Health/ National Institutes of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK R18DK104250; Philis-Tsimikas and Gallo, Multiple Principal Investigators). The funding body had no role in the study design, collection, analysis, or interpretation, or in preparing the manuscript. Ms. Savin is supported by a research and training grant from the National Heart, Lung, and Blood Institute (5T32HL079891-13).
List of abbreviations:
- CAB
Community Advisory Board
- CCM
Chronic Care Model
- CITI
Collaborative Institutional Training Initiative
- DSME
Diabetes Self-Management Education
- EMR
Electronic Medical Record
- FQHC
Federally Qualified Health Center
- HbA1c
Glycosylated Hemoglobin
- HIPAA
Health Insurance Portability and Accountability Act
- IRB
Institutional Review Board
- LDL-c
Low Density Lipoprotein Cholesterol
- MA
Medical Assistant
- MAC
Medical Assistant Health Coaching
- MAP
Mode Assessment Plan
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIH
National Institute of Health
- P4P
Pay for Performance Analysis
- PACIC
Patient Assessment of Chronic Illness Care
- PAM
Patient Activation Measure
- PRO
Participant Reported Outcomes
- PROMIS
Patient Reported Outcomes Measurement Information System Global-10 Health Scale
- QALYs
Quality of Life Adjusted Years
- RE-AIM
Reach, Effectiveness, Adoption, Implementation, and Maintenance
- REDCap
Research Electronic Data Capture
- RSSM
Resources and Support for Self-Management Model
- SBP
Systolic Blood Pressure
- SDSCA
The Summary of Diabetes Self Care Activities Measure
- SMART
Specific, Measurable, Attainable, Relevant, Time-Bound
- T2D
Type 2 Diabetes
- TTM
Transtheoretical Model
- UC
Usual Care
- UKPDS
United Kingdom Prospective Diabetes Study
Footnotes
Trial status:
Recruitment started in March 2016 and the active intervention period concluded in October 2019.
Protocol version 1.0 (05/06/2015).
Declarations:
Ethics approval and consent to participate: All procedures and materials for the current study are approved by the Scripps IRB (#IRB-15–6600), which served as the reviewing IRB, and the San Diego State University (no protocol number), which served as the relying IRB. Written informed consent was waived for Aim 1, and all participants provided verbal consent for Aim 2.
Consent for publication: No individual data will be reported.
Availability of data and materials: de-identified datasets used and/or analyzed during the current study will be made available from the study investigators following completion of study activities, on reasonable request, and with appropriate data use agreements. Materials not included in this protocol will be made available by request to the study investigators following completion of all study activities.
Competing Interests: All authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. http://www.diabetesatlas.org. Published 2017. Accessed Septemeber 30, 2019.
- 2.Dall TM, Yang W, Gillespie K, et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2019;42(9):1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, US Dept of Health and Human Services. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/data/statistics/statisticsreport.html. 2017. [Google Scholar]
- 5.U.S. Dept of Health and Human Services Office Of Minority Health. Diabetes and Hispanic Americans https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63.2014.
- 6.Mendola ND, Chen TC, Gu Q, Eberhardt MS, Saydah S. Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United States, 2013–2016. CHS Data Brief. 2018(319):1–8. [PubMed] [Google Scholar]
- 7.Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic community health study/study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: Final Data for 2015. Natl Vital Stat Rep. 2017;66(6):1–75. [PubMed] [Google Scholar]
- 9.Dominguez K, Penman-Aguilar A, Chang MH, et al. Vital signs: leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States - 2009–2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):469–478. [PMC free article] [PubMed] [Google Scholar]
- 10.Chow E, Foster H, Gonzalez V, McIver L. The disparate impact of diabetes on racial/ethnic minority populations. Clinical Diabetes. 2012;30(3):130–133. [Google Scholar]
- 11.Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5(5):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solberg LI, Klevan DH, Asche SE. Crossing the quality chasm for diabetes care: the power of one physician, his team, and systems thinking. J Am Board Fam Med. 2007;20(3):299–306. [DOI] [PubMed] [Google Scholar]
- 13.Ghorob A Health coaching: teaching patients how to fish. Fam Pract Manag. 2013;20(3):40–42. [PubMed] [Google Scholar]
- 14.Kivela K, Elo S, Kyngas H, Kaariainen M. The effects of health coaching on adult patients with chronic diseases: a systematic review. Patient Educ Couns. 2014;97(2):147–157. [DOI] [PubMed] [Google Scholar]
- 15.Pirbaglou M, Katz J, Motamed M, Pludwinski S, Walker K, Ritvo P. Personal health coaching as a type 2 diabetes mellitus self-management strategy: a systematic review and meta-analysis of randomized controlled trials. Am J Health Promot. 2018;32(7):1613–1626. [DOI] [PubMed] [Google Scholar]
- 16.Ruffin L Health coaching strategy to improve glycemic control in african- american adults with type 2 diabetes: an integrative review. J Natl Black Nurses Assoc. 2017;28(1):54–59. [PubMed] [Google Scholar]
- 17.Dennis SM, Harris M, Lloyd J, Powell Davies G, Faruqi N, Zwar N. Do people with existing chronic conditions benefit from telephone coaching? A rapid review. Aust Health Rev. 2013;37(3):381–388. [DOI] [PubMed] [Google Scholar]
- 18.Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willard-Grace R, Chen EH, Hessler D, et al. Health coaching by medical assistants to improve control of diabetes, hypertension, and hyperlipidemia in low-income patients: a randomized controlled trial. Ann Fam Med. 2015;13(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thom DH, Willard-Grace R, Hessler D, et al. The impact of health coaching on medication adherence in patients with poorly controlled diabetes, hypertension, and/or hyperlipidemia: a randomized controlled trial. J Am Board Fam Med. 2015;28(1):38–45. [DOI] [PubMed] [Google Scholar]
- 21.Sharma AE, Willard-Grace R, Hessler D, Bodenheimer T, Thom DH. What happens after health coaching? Observational study 1 year following a randomized controlled trial. Ann Fam Med. 2016;14(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler RS, Purcell EP, Glasgow RE, Klesges LM, Benkeser RM, Peek CJ. What does it mean to “employ” the RE-AIM model? Eval Health Prof. 2013;36(1):44–66. [DOI] [PubMed] [Google Scholar]
- 24.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64–78. [DOI] [PubMed] [Google Scholar]
- 25.Roy A, Bhaumik DK, Aryal S, Gibbons RD. Sample size determination for hierarchical longitudinal designs with differential attrition rates. Biometrics. 2007;63(3):699–707. [DOI] [PubMed] [Google Scholar]
- 26.Bhaumik DK, Roy A, Aryal S, et al. Sample size determination for studies with repeated continuous outcomes. Psychiatr Ann. 2008;38(12):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between groups. Journal of Educational and Behavioral Statistics. 1999;24:70–93. [Google Scholar]
- 28.Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, Walker C, Gallo LC. Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care. 2011;34(9):1926–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 30.Sim J, Dawson A. Informed consent and cluster-randomized trials. Am J Public Health. 2012;102(3):480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrara A, Hedderson MM, Albright CL, et al. A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. BMC pregnancy and childbirth. 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krist AH, Glenn BA, Glasgow RE, et al. Designing a valid randomized pragmatic primary care implementation trial: the my own health report (MOHR) project. Implement Sci. 2013;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affairs (Millwood). 2001;20(6):64–78. [DOI] [PubMed] [Google Scholar]
- 34.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 35.Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991. 1. Journal of Community & Applied Social Psychology. 1992;2(4):299–300. [Google Scholar]
- 36.University of California at San Francisco Center for Excellence in Primary Care. Health coaching. https://cepc.ucsf.edu/health-coaching. Accessed June 1, 2019.
- 37.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes2020. Diabetes Care. 2020;43(Suppl 1):S66–S76. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111–S134. [DOI] [PubMed] [Google Scholar]
- 39.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. [DOI] [PubMed] [Google Scholar]
- 40.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gugiu PC, Coryn C, Clark R, Kuehn A. Development and evaluation of the short version of the patient assessment of chronic illness care instrument. Chronic Illn. 2009;5(4):268–276. [DOI] [PubMed] [Google Scholar]
- 43.Vincent D, McEwen MM, Pasvogel A. The validity and reliability of a Spanish version of the summary of diabetes self-care activities questionnaire. Nurs Res. 2008;57(2):101–106. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham PJ, Hibbard J, Gibbons CB. Raising low ‘patient activation’ rates among Hispanic immigrants may equal expanded coverage in reducing access disparities. Health Affairs (Millwood). 2011;30(10):1888–1894. [DOI] [PubMed] [Google Scholar]
- 45.Alegria M, Sribney W, Perez D, Laderman M, Keefe K. The role of patient activation on patient-provider communication and quality of care for US and foreign born Latino patients. J Gen Intern Med. 2009;24 Suppl 3:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glasgow RE, Whitesides H, Nelson CC, King DK. Use of the Patient Assessment of Chronic Illness Care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care. 2005;28(11):2655–2661. [DOI] [PubMed] [Google Scholar]
- 48.Aragones A, Schaefer EW, Stevens D, Gourevitch MN, Glasgow RE, Shah NR. Validation of the Spanish translation of the Patient Assessment of Chronic Illness Care (PACIC) survey. Prev Chronic Dis. 2008;5(4):A113. [PMC free article] [PubMed] [Google Scholar]
- 49.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- 50.McNeish D, Wentzel KR. Accommodating small sample sizes in three-level models when the third level is incidental. Multivariate Behav Res. 2017;52(2):200–215. [DOI] [PubMed] [Google Scholar]
- 51.Trebble TM, Hansi N, Hydes T, Smith MA, Baker M. Process mapping the patient journey: an introduction. BMJ. 2010;341:c4078. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan RS, Norton DP. Strategy Mapping, Converting Intangible Assets into Tangible Outcomes. Boston, MA: Harvard Business School Publishing Corporation; 2004. [Google Scholar]
- 53.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–1759. [DOI] [PubMed] [Google Scholar]
- 54.Gilmer TP, O’Connor PJ, Sperl-Hillen JM, et al. Cost-effectiveness of an electronic medical record based clinical decision support system. Health Serv Res. 2012;47(6):2137–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer AJ, Roze S, Valentine WJ, et al. Impact of changes in HbA1c, lipids and blood pressure on long-term outcomes in type 2 diabetes patients: an analysis using the CORE Diabetes Model. Curr Med Res Opin. 2004;20 Suppl 1:S53–58. [DOI] [PubMed] [Google Scholar]
- 56.Eddy DM, Pawlson LG, Schaaf D, et al. The potential effects of HEDIS performance measures on the quality of care. Health Affairs (Millwood). 2008;27(5):1429–1441. [DOI] [PubMed] [Google Scholar]
- 57.Summary of Revisions: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S4–S6. [DOI] [PubMed] [Google Scholar]
- 58.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36(8):2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casagrande SS, Aviles-Santa L, Corsino L, et al. Hemoglobin A1c, Blood Pressure, and Ldl-Cholesterol Control among Hispanic/Latino Adults with Diabetes: Results from the Hispanic Community Health Study/Study of Latinos (Hchs/Sol). Endocr Pract. 2017;23(10):1232–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Q, Liu S, Krousel-Wood M, Shao H, Fonseca V, Shi L. Long-term outcomes associated with triple-goal achievement in patients with type 2 diabetes mellitus (T2DM). Diabetes Res Clin Pract. 2018;140:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. About Underlying Cause of Death 1999–2015. CDC WONDER Database. http://wonder.cdc.gov/ucd-icd10.html. Published 2016. Accessed April 4, 2017.
- 62.Bodenheimer TS, Smith MD. Primary care: proposed solutions to the physician shortage without training more physicians. Health Affairs (Millwood). 2013;32(11):1881–1886. [DOI] [PubMed] [Google Scholar]
- 63.Roter DL, Hall JA. Studies of doctor-patient interaction. Annu Rev Public Health. 1989;10:163–180. [DOI] [PubMed] [Google Scholar]
- 64.Summary of Revisions: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S4–S6. [DOI] [PubMed] [Google Scholar]
- 65.National Center for Chronic Disease Prevention and Health Promotion. Emerging Practices in Diabetes Prevention and Control: Medicaid Coverage for Diabetes Self-Management Educations. 2015. https://www.cdc.gov/diabetes/pdfs/programs/stateandlocal/emerging_practices-dsme.pdf
- 66.American Diabetes Association. 1. Strategies for improving care. Diabetes Care. 2016;39 Suppl 1:S6–12. [DOI] [PubMed] [Google Scholar]
- 67.Horigan G, Davies M, Findlay-White F, Chaney D, Coates V. Reasons why patients referred to diabetes education programmes choose not to attend: a systematic review. Diabet Med. 2017;34(1):14–26. [DOI] [PubMed] [Google Scholar]
- 68.Schwennesen N, Henriksen JE, Willaing I. Patient explanations for non-attendance at type 2 diabetes self-management education: a qualitative study. Scand J Caring Sci. 2016;30(1):187–192. [DOI] [PubMed] [Google Scholar]
- 69.Strawbridge LM, Lloyd JT, Meadow A, Riley GF, Howell BL. Use of medicare’s diabetes self-management training benefit. Health Educ Behav. 2015;42(4):530–538. [DOI] [PubMed] [Google Scholar]
- 70.Norris SL, Lau J, SMith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002; 25(7): 1159–71. [DOI] [PubMed] [Google Scholar]
- 71.Margolius D, Bodenheimer T. Transforming primary care: from past practice to the practice of the future. Health Affairs (Millwood). 2010;29(5):779–784. [DOI] [PubMed] [Google Scholar]
- 72.Ladden MD, Bodenheimer T, Fishman NW, et al. The emerging primary care workforce: preliminary observations from the primary care team: learning from effective ambulatory practices project. Acad Med. 2013;88(12):1830–1834. [DOI] [PubMed] [Google Scholar]
- 73.Bennett HD, Coleman EA, Parry C, Bodenheimer T, Chen EH. Health coaching for patients with chronic illness. Fam Pract Manag. 2010;17(5):24–29. [PubMed] [Google Scholar]
- 74.Nelson K, Pitaro M, Tzellas A, Lum A. Transforming the role of medical assistants in chronic disease management. Health Affairs (Millwood). 2010;29(5):963–965. [DOI] [PubMed] [Google Scholar]
- 75.Willard-Grace R, Najmabadi A, Araujo C, et al. “I don’t see myself as a medical assistant anymore”: Learning to become a health coach, in our own voices. Inquiry in Education. 2013;4(2):http://digitalcommons.nl.edu/ie/vol4/iss2/2. Accessed on January 3, 2014. [Google Scholar]
- 76.Margolius D, Wong J, Goldman ML, Rouse-Iniguez J, Bodenheimer T. Delegating responsibility from clinicians to nonprofessional personnel: the example of hypertension control. J Am Board of Fam Med. 2012;25(2):209–215. [DOI] [PubMed] [Google Scholar]
- 77.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.