Abstract
The incidence of dural venous sinus thrombosis (DVST) and the cerebral venous infarct have not exactly known, but DVST is closely related to the skull fracture around the venous sinus. A 56-year-old man experienced massive watery discharge after hitting on his face by a falling machine. He was alert and no cerebrospinal fluid discharge on admission. Air-density was shown on the jugular fossa in the brain computed tomography. On the 3th day of trauma, he suddenly had dyspnea and loss of consciousness and became comatose. Acute edema on medulla, pons and right cerebellar hemisphere and focal infarct on right medulla were visualized on the brain magnetic resonance imaging. And the sigmoid sinus and the jugular vein were occluded and venous circulation on the right posterior fossa was diminished on the cerebral angiography. Air-density on the sinus may be an indicator into developing venous thrombosis and brainstem venous infarct could be followed by the DVST round the jugular bulb.
Keywords: Sinus thrombosis, intracranial; Brain stem infarction; Skull fracture; Traumatic brain injury
INTRODUCTION
Dural venous sinus thrombosis (DVST) is a rare condition representing roughly less than 1% of all cases of stroke.3,14) DVST is usually idiopathic but may be associated with thrombophilia, pregnancy, chronic inflammation, or trauma.9,18) Venous congestion and hemorrhagic transformation may develop in the brain and lead to cerebral edema with mass effect. These brain lesions of DVST mostly involve the supratentorial area.14) The clinical course of DVST ranges from benign to life threatening depending on the extent of the thrombosis, its location, and the collateral circulation.
DVST often goes unrecognized in the initial evaluation of cases of blunt head trauma. It usually occurs in combination with an overlying skull fracture.5) However, many cases may be missed, in particular if the DVST is small. Here we present a patient who developed DVST and right medullary infarction following blunt head trauma injury and discuss how best to manage such patients.
CASE REPORT
This study was approved by Soonchunhyang University Bucheon Hospital Institutional Review Board (IRB) and informed consent was waived by the IRB (2020-02-017).
A 58-year-old man came to the emergency room with multiple lacerations and swelling on his face after having been struck on the face by falling heavy machinery while welding in a factory. He was alert, and his Glasgow Coma Scale score was 15 on presentation. Blood pressure and respiration were stable. At the time of the trauma, he reported CSF leakage from the nose. No discharge from the nose was seen in the emergency room. Numerous areas of air density were visualized on the basal cisterns, lateral ventricles, and third ventricle. On initial computed tomography (CT), a linear skull base fracture was found in the central skull base and extensive pneumocephalus was found. Furthermore, a tiny air density was detected in the jugular fossa (FIGURE 1A-D). At this time, he had limited lateral gaze of his right eye, but no symptoms was observed associated with lower cranial nerve damage such as hoarseness, difficulty swallowing nor dysarthria. Also, he did not feel any nasal discharge and no further intracranial lesions were noted on brain pre-contrast CT on the day after admission. In addition, CT angiography was performed to verify injury to the petrous carotid artery because of the petrous skull fracture. No arterial injury was found, but a contrast-filling defect was noted in the right jugular fossa and the sigmoid sinus (FIGURE 1E & F). At that time, venous sinus changes were not carefully observed. The patient was transferred to the general ward on the third day after the trauma.
FIGURE 1. Initial pre-contrast brain CT and brain CTA. Initial brain CT of a 58-year-old man after a crushing injury to the face. Numerous areas of air density were noted in almost the entire intracranial cerebrospinal fluid space (A, B). Central skull base and right zygomatic fractures (arrows) and air fluid level in the ethmoid and sphenoid sinus on brain CT with the bone algorithm are shown (C, D). Focal air density (arrow) in the right jugular bulb is shown (D). No arterial abnormalities were found on CTA (E). Retrospectively, no contrast filling was found in the right jugular bulb compared to the left on source images of CTA (F).
CT: computed tomography, CTA: computed tomography angiography.
By the morning of the fourth day after the trauma, he developed mild fever of 37.8°C and dyspnea due to aspiration pneumonia, but no new neurological signs were observed. In the afternoon, the patient suddenly lost consciousness and was comatose, and there was no spontaneous respiration. We performed intubation and cardio-pulmonary resuscitation for 15 minutes, then his blood pressure and pulse were normalized, but breathing did not return, so a ventilator was applied. The patient was semi-comatose with abnormal extension to pain, and the pupils were reactive to light with size 2/2 mm. No newly developed abnormal lesions were found in a brain pre-contrast CT taken 3 hours after respiratory arrest. The air density in the basal cisterns had mostly disappeared, but small air bubbles were still noted in the right jugular fossa (FIGURE 2A-C).
FIGURE 2. After sudden respiratory arrest on the 4th day of the trauma. Images after sudden respiratory arrest on the fourth day after trauma. No definite abnormal density in the brain was found immediately after resuscitation on pre-contrast computed tomography (A, B). Although the air density present in the intracranial cerebrospinal fluid spaces decreased (A), persistent air density was noted in the right jugular bulb (arrow; C).
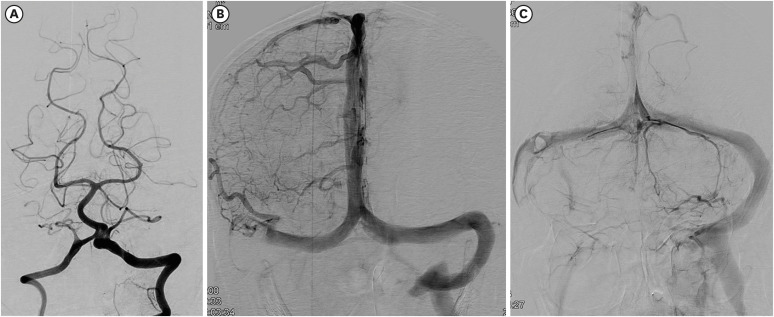
Spontaneous breathing recovered and the patient was weaned off the mechanical ventilator on the second day of intubation. He showed no improvement in consciousness. Brain magnetic resonance imaging (MRI) was performed to evaluate his poor consciousness on the eighth day of respiratory arrest. Ill-defined hyperintense lesions were noted on the right medulla, pons, and some parts of the right cerebellum on T2-weighted images. The lesions mainly showed high signal intensity on diffusion-weighted images with a high apparent diffusion coefficient (ADC) value on ADC map, indicating vasogenic edema. There was an intralesional hemorrhage of dark SI on T2* gradient echo images and small cytotoxic edema in an area of restricted diffusion in the right pons and medulla (FIGURE 3A-H). These findings were compatible with neuroimaging findings suggesting venous infarction induced by venous congestion. We started low-molecular-weight heparin (enoxaparin 6,000 IU every 12 hours, subcutaneously) and continued for approximately 72 hours. Unfortunately, the cerebral hemispheres showed diffuse changes compatible with hypoxic brain injury on MRI taken after 8 days from respiratory arrest (FIGURE 3I-K). The right sigmoid sinus and jugular vein were occluded on magnetic resonance (MR) venography underwent 9 days after respiratory arrest. On 23th days from respiratory arrest, no injury was observed in the vertebrobasilar artery on catheter cerebral angiography (FIGURE 4A). The right petrosal vein and sinus were not traced, and contrast-filling defects on the right sigmoid sinus and jugular vein were noted on cerebral angiography (FIGURE 4B & C). Conservative treatment was provided for pneumonia, and the patient was tracheostomized. Unfortunately, he did not regain consciousness and survived for only 2 years.
FIGURE 3. The 8th day of respiratory arrest. On the eighth day after respiratory arrest, brain magnetic resonance imaging was performed. Localized vasogenic edema was noted in the medulla oblongata, pons, and some parts of the right cerebellum with focal hemorrhaging in the right posterior medulla oblongata (A-H; A, E: T2-weighted images; B, F: diffusion-weighted images; C, G: ADC maps; D, H: T2*gradient images). Subacute stage of global hypoxic-ischemic encephalopathy of edematous swelling in the bilateral caudate nucleus and putamen is shown (I-K; I: T2-fluid inversion recovery image, J: diffusion-weighted image, K: ADC map).
ADC: apparent diffusion coefficient.
FIGURE 4. Cerebral catheter angiogram of the case (23th day of respiratory arrest). No arterial abnormalities were revealed on vertebral angiography (A). However, the right sigmoid sinus and jugular bulb were not visualized (B) in the venous phase on the right carotid angiogram, and the right jugular bulb was obliterated and some of the venous channels on the right posterior fossa were not visualized (C).
DISCUSSION
This report demonstrates the potentially life-threatening complication of DVST in blunt head trauma injury. Although DVST in traumatic brain injury is rare, its actual occurrence may be higher than clinically realized. Before respiratory difficulty occurred in our case, we overlooked venous thrombosis in the jugular fossa. Venous occlusive disease of the brainstem and cerebellum has rarely been reported because of abundant venous collateral drainage in this region.4,13,16)
DVST is often overlooked in cases of blunt head trauma. It usually occurs in combination with an overlying skull fracture.5) The most frequent but least specific symptom of DVST is severe headache, which is present in more than 90% of adult patients.18) Nonspecific symptoms and signs can delay appropriate diagnosis and treatment. The highly variable clinical and radiological findings associated with traumatic DVST can make the condition difficult to diagnose and manage.1,3) The clinical symptoms are typically of acute onset, rapidly evolving to coma and death, but can be subacute or chronic.
In one study, initial pre-contrast CT showed normal findings in 40% of cases (23 of 62 cases) of isolated lateral sinus thrombosis.4) In the retrospective review of CT venography performed in patients for the presence of a fracture near a dural venous sinus or jugular bulb or a high index of clinical suspicion, patients with skull fractures extending to at least one dural venous sinus or jugular bulb had an overall risk for traumatic DVST of 40.7% (57 of 140 patients).5) No DVST occurred without skull fractures. Therefore, CT venography or MRI should be performed only if there is a fracture extending to the dural venous sinus or jugular bulb. Furthermore, in one study intrasinus gas was strongly associated with DVST, present in 75% of cases (18 of 24 cases).17) These researchers reported that the presence of intrasinus gas may suggest considerable dural injury, increasing the risk for sinus thrombosis. In contrast to the study of So et al.,17) in which intrasinus gas disappeared within 1 day, intrasinus gas was visible until 4 days after the trauma in this patient. According to previous studies that used CT venography, associated venous infarction occurred only in patients with occlusive DVST, and the incidence was about 7%. In our case, the air bubble did not change for 4 days after the trauma, possibly causing occlusive venous thrombus, resulting in associated venous infarction. This possible association between persistent intrasinus gas and occlusive venous thrombosis should be confirmed in future studies, but based on our experience, careful observation would be recommended if intrasinus air bubbles do not change over follow-up for more than 1 day.
Although a catastrophic outcome has been reported,6) the overall outcome is relatively good.8) Clinical outcome was good with complete recovery in a case of isolated lateral sinus thrombosis.4) The priority in the acute phase is to stabilize the patient's condition and prevent or reverse cerebral herniation.18) The most obvious treatment option is anticoagulation with heparin to arrest the thrombotic process. However, there is controversy regarding anticoagulant treatment because of the tendency of venous infarcts to become hemorrhagic. In one study, this treatment was applied to more than 80% of cases of sinus thrombosis.8) However, only 7 of 624 cases were related to head trauma. In another study, only 4 (7%) of 57 patients with traumatic DVST developed hemorrhagic venous infarctions in subsequent images.5) In addition, the use of anticoagulation medication to prevent thrombus propagation in trauma is also controversial, and this therapy is often used on a case-by-case basis.2,19) Low-molecular-weight heparin may be an alternative to heparin to reduce the risk for hemorrhage. Chemical and/or mechanical thrombectomy in conjunction with systemic anticoagulation is an alternative strategy for treating patients with progressive deterioration on heparin therapy or those who are moribund on presentation.12) Venous infarction in the cerebellum and brainstem is very rare. On imaging studies, cerebellar infarction can be unilateral or bilateral, and it is frequently hemorrhagic and edematous. Cerebellar involvement likely follows thrombosis of the surface veins of the cerebellum. Venous infarction in the posterior fossa depends on many factors, such as the dominance of the internal jugular vein, venous collateral circulation, and the extent of the occlusion.11) In our case, the lesions on the brainstem and cerebellum may have been caused by hypoxic events due to cardiopulmonary insufficiency. After moderate or severe hypoxic ischemic encephalopathy, abnormal signal intensity is commonly detected in the basal ganglia and thalami, corticospinal tract, white matter, and cortex.7) Hypoxic-ischemic injury involving the brainstem is rarely reported in neonates.19) Reported MRI findings show bilateral columnar T2 high-signal-intensity lesions involving the tegmentum due to selective vulnerability of the tegmentum,15) which were not compatible with our case. In addition, in our case, the lesions showed both vasogenic and cytotoxic edema with intralesional hemorrhage. The lesions were not confined to the arterial territory and showed relatively ill-defined margins. These findings indicate venous infarction rather than hypoxic encephalopathy.10)
Although there was no fracture line on the jugular fossa on CT, air density in the jugular fossa may be a strong indicator of injury and subsequent thrombus formation in the jugular vein. As absolute bed rest was recommended to manage CSF leakage in our case and no anticoagulants were prescribed, the overlooked thrombosis around the jugular bulb propagated with time, progressing to further occlusion in the brainstem and cerebellar veins. This likely led to medullary hemorrhagic infarction and edema. The patient was semi-comatose and hypoxic cerebral encephalopathy was evident, so we did not attempt mechanical thrombectomy because no benefit was expected.
CONCLUSION
Air density in the jugular fossa without a fracture line is a valuable predictor of jugular bulb thrombosis, and CT or MR venography should be performed. Although DVST in the jugular vein and brainstem infarction are very rare, it is very important to maintain a high degree of suspicion and practice proper management to prevent catastrophic neurological outcome.
Footnotes
Funding: This work was supported by the Soonchunhyang University Research Fund.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Beer-Furlan A, de Almeida CC, Noleto G, Paiva W, Ferreira AA, Teixeira MJ. Dural sinus and internal jugular vein thrombosis complicating a blunt head injury in a pediatric patient. Childs Nerv Syst. 2013;29:1231–1234. doi: 10.1007/s00381-013-2184-7. [DOI] [PubMed] [Google Scholar]
- 2.Bishop FS, Finn MA, Samuelson M, Schmidt RH. Endovascular balloon angioplasty for treatment of posttraumatic venous sinus thrombosis. Case report. J Neurosurg. 2009;111:17–21. doi: 10.3171/2009.2.JNS08491. [DOI] [PubMed] [Google Scholar]
- 3.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6:162–170. doi: 10.1016/S1474-4422(07)70029-7. [DOI] [PubMed] [Google Scholar]
- 4.Damak M, Crassard I, Wolff V, Bousser MG. Isolated lateral sinus thrombosis: a series of 62 patients. Stroke. 2009;40:476–481. doi: 10.1161/STROKEAHA.107.509711. [DOI] [PubMed] [Google Scholar]
- 5.Delgado Almandoz JE, Kelly HR, Schaefer PW, Lev MH, Gonzalez RG, Romero JM. Prevalence of traumatic dural venous sinus thrombosis in high-risk acute blunt head trauma patients evaluated with multidetector CT venography. Radiology. 2010;255:570–577. doi: 10.1148/radiol.10091565. [DOI] [PubMed] [Google Scholar]
- 6.Dobbs TD, Barber ZE, Squier WL, Green AL. Cerebral venous sinus thrombosis complicating traumatic head injury. J Clin Neurosci. 2012;19:1058–1059. doi: 10.1016/j.jocn.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169:397–403. doi: 10.1001/jamapediatrics.2014.3269. [DOI] [PubMed] [Google Scholar]
- 8.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 9.Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16:523. doi: 10.1007/s11886-014-0523-2. [DOI] [PubMed] [Google Scholar]
- 10.Forbes KP, Pipe JG, Heiserman JE. Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. AJNR Am J Neuroradiol. 2001;22:450–455. [PMC free article] [PubMed] [Google Scholar]
- 11.Holzmann D, Huisman TA, Linder TE. Lateral dural sinus thrombosis in childhood. Laryngoscope. 1999;109:645–651. doi: 10.1097/00005537-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Ilyas A, Chen CJ, Raper DM, Ding D, Buell T, Mastorakos P, et al. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg. 2017;9:1086–1092. doi: 10.1136/neurintsurg-2016-012938. [DOI] [PubMed] [Google Scholar]
- 13.Krespi Y, Gurol ME, Coban O, Tuncay R, Bahar S. Venous infarction of brainstem and cerebellum. J Neuroimaging. 2001;11:425–431. doi: 10.1111/j.1552-6569.2001.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni GB, Mustare V, Abbas MM. Profile of patients with cerebral venous sinus thrombosis with cerebellar involvement. J Stroke Cerebrovasc Dis. 2014;23:1106–1111. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Quattrocchi CC, Fariello G, Longo D. Brainstem tegmental lesions in neonates with hypoxic-ischemic encephalopathy: Magnetic resonance diagnosis and clinical outcome. World J Radiol. 2016;8:117–123. doi: 10.4329/wjr.v8.i2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhoton AL., Jr The posterior fossa veins. Neurosurgery. 2000;47:S69–S92. doi: 10.1097/00006123-200009001-00012. [DOI] [PubMed] [Google Scholar]
- 17.So TY, Dixon A, Kavnoudias H, Paul E, Maclaurin W. Traumatic dural venous sinus gas predicts a higher likelihood of dural venous sinus thrombosis following blunt head trauma. J Med Imaging Radiat Oncol. 2019;63:311–317. doi: 10.1111/1754-9485.12865. [DOI] [PubMed] [Google Scholar]
- 18.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–1798. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 19.Yokota H, Eguchi T, Nobayashi M, Nishioka T, Nishimura F, Nikaido Y. Persistent intracranial hypertension caused by superior sagittal sinus stenosis following depressed skull fracture. Case report and review of the literature. J Neurosurg. 2006;104:849–852. doi: 10.3171/jns.2006.104.5.849. [DOI] [PubMed] [Google Scholar]