Abstract
Ipsilateral hemiparesis is a rare and challenging sign in clinical neurological practice. Although the etiology of this manifestation is poorly understood, recent studies have attempted to probe the pathomechanism of this sign with advanced radiological techniques. Additional knowledge about the lesion and unraveling the pathomechanisms causing neurological impairments are important to predict the prognosis and clinical course and to aid in rehabilitation. Therefore, we present a case of a patient with a traumatic subdural hematoma on the left hemisphere and left spastic hemiparesis. Using diffusion tensor imaging (DTI), we concluded that the right corticospinal tract injury caused by compression of the cerebral peduncle accounted for the ipsilateral hemiparesis, also known as Kernohan's notch phenomenon. Thus, this case report highlights the usefulness of the newer radiological techniques, such as DTI, to identify the pathomechanisms of neurological presentations.
Keywords: Corticospinal tract, Diffusion tensor imaging, Hemiplegia, Neurology, Traumatic subdural hematoma
INTRODUCTION
In a standard neurological examination, the anatomical localization of the lesion allows neurologists to decide on the anatomical focus of radiological imaging and its management. Traumatic brain injury (TBI) has a different pathomechanism from strokes with respect to treatment and prognosis, but previous neuroanatomical studies have reported that it shares the basics of anatomical localization. Most output from the primary motor cortex descends through the internal capsule to the brainstem and spinal cord as the corticospinal tract (CST); most of them cross in the medulla to the contralateral side. Therefore, CST injuries usually result in contralateral hemiparesis. Previously reported cases of ipsilateral hemiparesis were caused by various pathomechanisms, such as cortical reorganization in the unaffected hemisphere after the previous stroke,2,5) Kernohan's notch phenomenon,1,3) and anatomical variants.1) Recently, we encountered a case of ipsilateral hemiparesis that developed after TBI. Herein, we report the clinical features and imaging findings of this case. This study was approved by the Institutional Review Board of Kwangju Christian Hospital (KCHIRB-RE-2019-09-003), which waived the requirement for informed consent owing to the retrospective nature of this study.
CASE REPORT
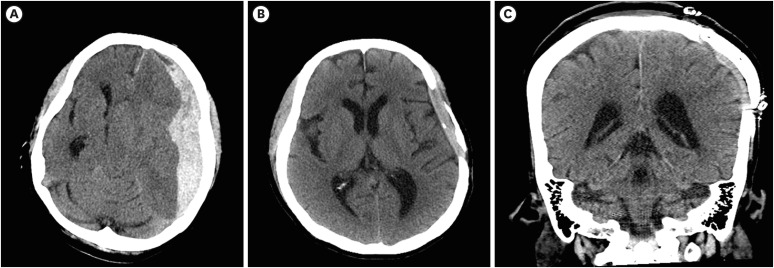
A 47-year-old woman with no history of brain lesion experienced a blunt trauma to her head. A brain computed tomography scan established a diagnosis of traumatic subdural hematoma (SDH) in the left frontotemporal lobe with midline shifting (FIGURE 1). The patient's mental state was comatose, and the Glasgow Coma Scale score at presentation was 3/15. She underwent an emergency surgery for removal of the hematoma with craniectomy to reduce increased intracranial pressure caused by cerebral edema and hematoma. However, in the intensive care unit after surgery, she unexpectedly developed left hemiplegia, despite the SDH being in the left frontotemporal lobe.
FIGURE 1. (A) Brain CT showing a subdural hematoma in the left fronto-temporo-parietal lobe with midline shifting at the time of injury. Subfalcine and downward transtentorial herniation scans. (B) Absorbed brain hematoma without hydrocephalus 2 years after the accident. (C) Coronal brain CT after cranioplasty.
CT: computed tomography.
She had been under continuous rehabilitation therapy in the local inpatient clinic for the last 2 years; she presented to our hospital and was admitted for intensive rehabilitation treatment. In order to evaluate hemiplegia, on the manual muscle test, the patient's muscle strength was classified as Medical Research Council grade 2 in the left upper and lower extremities with normal strength on the right side. On the Fugl-Meyer Assessment, she scored 17 points for the upper extremities and 34 points for the lower extremities.
Deep tendon reflexes were increased on the left side, and spasticity was evaluated as Modified Ashworth Scale (MAS) score 3. On neurological examination, she had a Korean Mini-Mental State Examination score of 30, and a Loewenstein Occupational Therapy Cognitive Assessment score of 111 out of 114; these scores indicated no prominent deterioration in the left hemisphere functions, such as memory, calculation, executive function, and language.
Because the ipsilateral symptoms did not correlate with the brain lesion, we considered 2 possibilities that could explain her clinical symptoms. The first was a missed diagnosis, such as diffuse axonal injury caused by countercoup or ischemic infarct followed by cerebral edema with midline shifting (Kernohan's notch phenomenon), and the second was an uncrossed CST.
To investigate the pathomechanism of the ipsilateral hemiplegia, conventional magnetic resonance imaging (MRI), including diffusion tensor imaging (DTI), was performed 3 years after the accident using a 3.0T MAGNETOM Skyra (Siemens AG, Medical Solutions, Erlangen, Germany). Fibers passing through 2 regions of interest at the upper and middle pons were selected as the CSTs, with a fractional anisotropy (FA) threshold >0.2 and an angle threshold <60°. Both CSTs originating from the cerebral cortex, including the primary motor cortex, descended to the medullary pyramid through the normal CST pathway. FA and apparent diffusion coefficient (ADC) values were obtained in the pons.
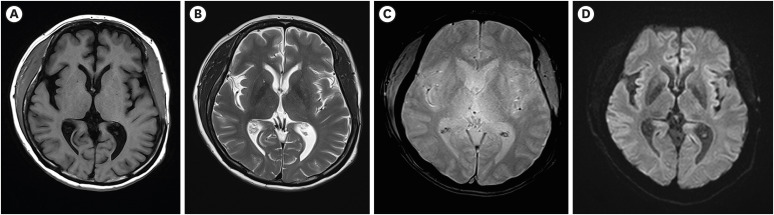
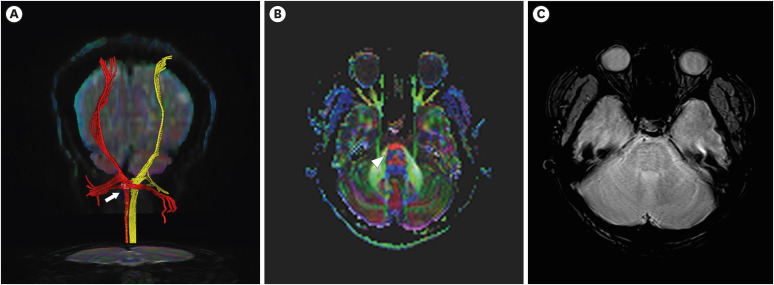
There was no pathological evidence that correlated with left hemiplegia, such as diffuse axonal injury or leukomalacia changes in conventional MRI (FIGURE 2). However, the right CST was sharply narrowed at the lower pons level. In contrast, the left CST was relatively well preserved within normal limits despite the huge hematoma that had caused midline shifting (FIGURE 3). Moreover, DTI revealed that the FA values of the right CST decreased and the ADC values of the right hemisphere increased when compared with those of the left hemisphere (TABLE 1).
FIGURE 2. Brain magnetic resonance imaging revealing no diffuse axonal injury or leukomalacia lesions in the white matter in both hemispheres. (A) Axial T1-weighted image. (B) Axial T2-weighted image. (C) Axial gradient-echo image. (D) Axial diffusion-weighted image.
FIGURE 3. (A) Diffusion tensor imaging revealing sharp narrowing of the right CSTs (yellow) at the lower pons level comparing with those of left CSTs (red) Arrow indicates narrowed CSTs on right side. (B) Decreased FA value in the right lower pons and faded blue color (arrowhead) in the right lower pons level on color coded FA map. (C) Axial gradient-echo image.
CST: corticospinal tract, FA: fractional anisotropy.
TABLE 1. Comparison of values for FA and ADC obtained in the pons.
| Brain structure | Right hemisphere | Left hemisphere | ||
|---|---|---|---|---|
| FA | ADC | FA | ADC | |
| Pons | 308.1 | 808.8 | 400.3 | 761.1 |
FA, fractional anisotropy, ADC, apparent diffusion coefficient.
To rule out the presence of uncrossed CST, motor evoked potentials (MEPs) were checked. However, magnetic stimulation of the hand area of the left cerebral motor cortex did not produce MEPs in the left thenar muscles.
To obtain somatosensory evoked potential (SEP), following electrical stimulation of the left median nerve, P9, P13, N19, and P23 were recorded bilaterally in the frontal region, and cortical responses were localized in the right central and parietal regions, which were contralateral to the side of stimulation. Right median nerve stimulation evoked a cortical response in the left frontal and parietal lobes. Electrodiagnostic examination was performed to rule out peripheral nerve lesions, including radiculopathy or brachial and/or lumbosacral plexopathy; the results revealed mild grade bilateral carpal tunnel syndrome on both sides.
DISCUSSION
This report describes the case of a patient who had left SDH and immediately underwent an emergency operation. However, she showed unexplained ipsilateral hemiparesis. A class I recommendation exists for MRI when there are persistent unexplained neurologicalfindings.4) To evaluate a possible missed diagnosis to explain the ipsilateral hemiplegia, a conventional MRI was performed; however, there was no pathological evidence in the conventional MRI correlated with the left hemiplegia, such as diffuse axonal injury or leukomalacia changes. Moen et al.7) revealed that only early MRI shows edematous and hemorrhagic lesions, and that lesions in fluid-attenuated inversion recovery images and ADC maps disappear by 3 months and by 21 months for susceptibility-weighted images. We assumed that the interval of 3 years between the trauma and MRI scan might be the reason for the unremarkable findings on conventional MRI.
It is crucial to identify and understand the lesion and the pathomechanism causing the ipsilateral hemiparesis to predict prognosis, clinical course, and to set goals of rehabilitation with the patient and family members. For more than 100 years, physicians have recognized that lesions in supratentorial structures cause contralateral hemiparesis. Far from this clear and simple principle, ipsilateral hemiparesis is not common and may allow physicians to falsely localize lesions through physical examination. The exact pathological mechanism of ipsilateral hemiparesis remains unknown, and various hypotheses have been proposed recently.
Carrasco-Moro et al.1) conducted a thorough review of 75 cases of ipsilateral hemiparesis in the pre-MRI era (before 1980) and presented a list of most probable pathophysiological mechanisms; this was followed by another study wherein cases of ipsilateral hemiparesis in the MRI era (1990–2019) were examined. They reported a lack of anatomical decussation of the CST, diaschisis, Kernohan's notch phenomenon, and blood supply impairment to the contralateral hemisphere as the major phenomena induced by the primary hematoma. They also pointed out that among the various hypotheses, Kernohan's notch phenomenon is largely supported by modern neuroimaging and electrophysiological examinations. The American neuropathologist, James W. Kernohan (1896–1981), identified the evidence of mechanical injury at the cerebral peduncle caused by its compression. Later, injury of the contralateral CST caused by the cerebral peduncle was named Kernohan's notch phenomenon. In our case, DTI revealed that the patient's left tracts were relatively spared, but the right CST revealed sharp narrowing at the lower pons level, which resulted in ipsilateral hemiplegia. Kernohan's notch phenomenon seems to be relevant to this finding.
DTI is an advanced technique based on DWI that enables the characterization of water diffusion directionality in 3D space. DTI can quantify diffusion using a variety of parameters, the most common of which are FA and ADC.6) Increases in ADC and/or decreases in FA typically are interpreted to reflect the decreased microstructural integrity of white matter in a variety of disorders such as strokes, TBI, multiple sclerosis and neurodegenerative disease.9)
We investigated the patient with thorough neurological examination and performed further evaluations, such as SEP, MEP, and electrodiagnostic exam, to investigate other differential diagnoses. The patient had left hemiparesis with a sensory deficit in the left limbs. Her left deep tendon reflexes increased with spasticity, which was evaluated as MAS 3. On electrodiagnostic examination, carpal tunnel syndrome was identified, without other abnormalities, which explains the patient's hemiparesis. The results of the SEP study indicated that the primary cortical response N20 appeared exclusively in the contralateral central and parietal areas after left median nerve stimulation. Since the SEP response by electrical stimulation is believed to be mediated by the dorsal column-medial lemniscal pathway, the present finding suggests that the dorsal column-medial lemniscal pathway mainly projects to the contralateral primary somatosensory cerebral cortex. These findings from SEP align with the fact that no anatomical variant, at least in terms of the dorsal column-medial lemniscal pathway, was observed.
Another theory to account for ipsilateral hemiparesis is cortical reorganization in the unaffected hemisphere after the previous stroke.2,5) In a study by Inatomi et al.,2) 14 patients with ipsilateral hemiplegia were studied among 8,360 ischemic stroke cases. The authors concluded that 13 of the 14 patients had a past history of stroke, contralateral to the recent one, resulting in post-stroke compensation in the motor system. Indeed, increased recruitment of ipsilateral corticospinal projections after the damage is one of the mechanisms for functional recovery after stroke.8) However, in our case, the patient had no previous stroke or underlying central nervous system disease.
In addition, the reason for preservation of motor function of the right arm and leg despite the initial extensive lesion on the left hemisphere remains unanswered. From a clinical perspective, the reason seems to be an immediate craniectomy, which reduced intracranial pressure and minimized subsequent secondary injury on the left side.
However, the limitations of this study should be considered. First, further imaging studies at the time of incidence were not obtained owing to the lack of family support and her economic status. Secondly, we assume transtentorial and uncal herniation must have caused Kernohan's notch phenomenon, but coronal reconstruction images were not obtained to assess for herniation.
CONCLUSION
We could identify and localize the causative lesion for unexplained ipsilateral hemiplegia using DTI. A class I recommendation exists for MRI when there are persistent unexplained neurological findings. However, DTI does not belong to the guideline. Using DTI could be a useful and assistive radiological tool to reveal the functional status of the brain.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Carrasco-Moro R, Castro-Dufourny I, Martínez-San Millán JS, Cabañes-Martínez L, Pascual JM. Ipsilateral hemiparesis: the forgotten history of this paradoxical neurological sign. Neurosurg Focus. 2019;47:E7. doi: 10.3171/2019.6.FOCUS19337. [DOI] [PubMed] [Google Scholar]
- 2.Inatomi Y, Nakajima M, Yonehara T, Ando Y. Ipsilateral hemiparesis in ischemic stroke patients. Acta Neurol Scand. 2017;136:31–40. doi: 10.1111/ane.12690. [DOI] [PubMed] [Google Scholar]
- 3.Jang SH, Seo YS. Ipsilateral hemiparesis following epidural hematoma in a patient with traumatic brain injury. Ann Rehabil Med. 2019;43:352–354. doi: 10.5535/arm.2019.43.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kara A, Celik SE, Dalbayrak S, Yilmaz M, Akansel G, Tireli G. Magnetic resonance imaging finding in severe head injury patients with normal computerized tomography. Turk Neurosurg. 2008;18:1–9. [PubMed] [Google Scholar]
- 5.Kim TH, Shin CS, Lee KR, Lee SH, Choi SM, Park MS, et al. Ipsilateral Hemiparesis caused by a internal capsule infarct after a previous stroke on the opposite side. J Korean Neurol Assoc. 2006;24:468–471. [Google Scholar]
- 6.Lee AL. Advanced imaging of traumatic brain injury. Korean J Neurotrauma. 2020;16:3–17. doi: 10.13004/kjnt.2020.16.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moen KG, Skandsen T, Folvik M, Brezova V, Kvistad KA, Rydland J, et al. A longitudinal MRI study of traumatic axonal injury in patients with moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83:1193–1200. doi: 10.1136/jnnp-2012-302644. [DOI] [PubMed] [Google Scholar]
- 8.Muellbacher W, Artner C, Mamoli B. The role of the intact hemisphere in recovery of midline muscles after recent monohemispheric stroke. J Neurol. 1999;246:250–256. doi: 10.1007/s004150050343. [DOI] [PubMed] [Google Scholar]
- 9.Schweitzer AD, Niogi SN, Whitlow CT, Tsiouris AJ. Traumatic brain injury: imaging patterns and complications. Radiographics. 2019;39:1571–1595. doi: 10.1148/rg.2019190076. [DOI] [PubMed] [Google Scholar]