Abstract
Objective:
To evaluate whether extremely low gestational age neonates (ELGANs) randomized to erythropoietin have better or worse kidney-related outcomes during hospitalization and at 22–26 months corrected gestational age (cGA) compared with those randomized to placebo.
Study design:
We performed an ancillary study to a multicenter double-blind, placebo-controlled randomized clinical trial of erythropoietin in ELGANs.
Results:
The prevalence of severe (stage 2 or 3) acute kidney injury (AKI) was 18.2%. We did not find a statistically significant difference between those randomized to erythropoietin vs. placebo for in-hospital primary (severe AKI) or secondary outcomes (any AKI and serum creatinine [SCr]/ cystatin C values at days 0, 7, 9 and 14). At 22–26 months cGA, 16% of the cohort had an estimated glomerular filtration rate (eGFR) <90 ml/min/1.73m2, 35.8% had urine albumin/creatinine ratio (ACR) > 30 mg/g, 23% had a systolic blood pressure (SBP) >95th percentile for age, and 40% had a diastolic blood pressure (DBP) >95th percentile for age. SBP >90th percentile occurred less often among recipients of erythropoietin (p<0.04). This association remained even after controlling for gestational age, site and sibship (adjusted OR=0.6 [95% CI=0.39–0.92]). We did not find statistically significant differences between treatment groups in eGFR, ACR, rates of SBP >95th percentile or DBP >90th or >95th percentiles.
Conclusions:
ELGANs have high rates of in-hospital AKI and kidney-related problems at 22–26 months cGA. Recombinant erythropoietin (rhEpo) may protect ELGANs against long-term elevated SBP, but does not appear to protect from AKI, low eGFR, albuminuria or elevated DBP at 22–26 months cGA.
Keywords: Acute Kidney Injury, Acute Renal Failure, Proteinuria, Hypertension, Chronic kidney disease
Extremely low gestational age neonates (ELGANs – born <28 weeks gestation) who graduate from the neonatal intensive care unit (NICU) often have organ dysfunction due to organ underdevelopment and/or organ damage during their initial hospitalization. David Barker is credited with the observation in 1997, that many “adult” diseases have their origins in fetal life.1, 2 Evidence for this “fetal programming” exists for premature infants that go on to develop obesity,3 hypertension,2 insulin resistance,4 coronary artery disease,5 and chronic kidney disease (CKD)6 later in life. A meta-analysis by White7 showed that low birthweight infants (<2500 grams) have an ~80% increased odds of albuminuria, 80% increased odds of a sustained low glomerular filtration rate, and an approximately 60% increased odds of dialysis dependent CKD in later life compared than their term counterparts. The incidence of CKD in ELGANs may be higher than what is reported in the White meta-analysis, as the number of nephrons is lower in more premature neonates. In ELGANS is very common in the NICU. We r described the acute kidney injury (AKI) prevalence rates in a cohort of 923 ELGANs enrolled in a randomized trial of recombinant erythropoietin (rhEpo) entitled the Preterm Epo Neuroprotection Trial (PENUT)8 351/923 (38.0%) had at least one episode of stage 1 or higher AKI, and 168/923 (18.2%) had at least one episode of severe AKI anytime during the hospitalization.8
Erythropoietin is best known for its hematopoietic effects; however, it also has tissue protective effects in clinical models and human studies across several organ systems.9 Epo receptors are present on glomerular, mesangial and tubular epithelial kidney cells.10 Animal studies of ischemia-reperfusion injury and sepsis-induced AKI show that rhEpo preserves kidney function, protects renal proximal tubular cells by decreasing apoptosis, and decreases pro-inflammatory cytokine expression in the renal cortex.11–15 These effects are independent of changes in renal hemodynamics.13 Song et al demonstrated a reduction in AKI in a small clinical trial of 71 adults who underwent elective coronary artery bypass graft surgery and were randomized to rhEpo (300 units/kg intravenous x 1) vs. placebo.16 Long-term outcomes from this cohort reported by Oh et al showed a reduction in all-cause mortality (p<0.03) and a reduction in the composite of all-cause mortality and kidney failure (p<0.01) in those randomized to rhEpo.17 In contrast, a randomized clinical study of 606 adults with traumatic brain injury randomized to 40,000 units rhEpo IV vs. placebo found no reno-protective effect of rhEpo.18
In order to determine whether or not rhEpo improves the short and long-term kidney outcomes in ELGANs, we performed an ancillary study of PENUT, a multi-center randomized clinical trial which randomized ELGANs to receive high dose rhEpo or placebo. Our primary hypothesis was that ELGANs randomized to rhEpo would have a lower rate of in-hospital severe AKI, and lower rates of CKD, albuminuria and elevated blood pressure at 22–26 months corrected gestational age (cGA).
Methods
The PENUT trial is a randomized, placebo-controlled, double-blind clinical trial of rhEpo in ELGANs performed across 19 academic centers and comprised of 30 NICUs across 13 states in the United States from December 2013 - September 2016. PENUT screened 3366 neonates, of whom 941 were enrolled in the study. The reasons for non-enrollment have been described in detail elsewhere. 19, 20 The inclusion criteria included: 1) inborn patients born between 24 – 0/7 and 27– 6/7 weeks gestation in participating NICUs, 2) less than 24 hours of age, 3) parental informed consent obtained, and 4) available arterial or venous access. Exclusion criteria included: 1) Major life-threatening anomalies (brain, cardiac and chromosomal anomalies) 2) hematologic crises such as disseminated intravascular coagulation or hemolysis due to blood group incompatibility, 3) hematocrit >65%, 4) hydrops fetalis and 5) known congenital infection.
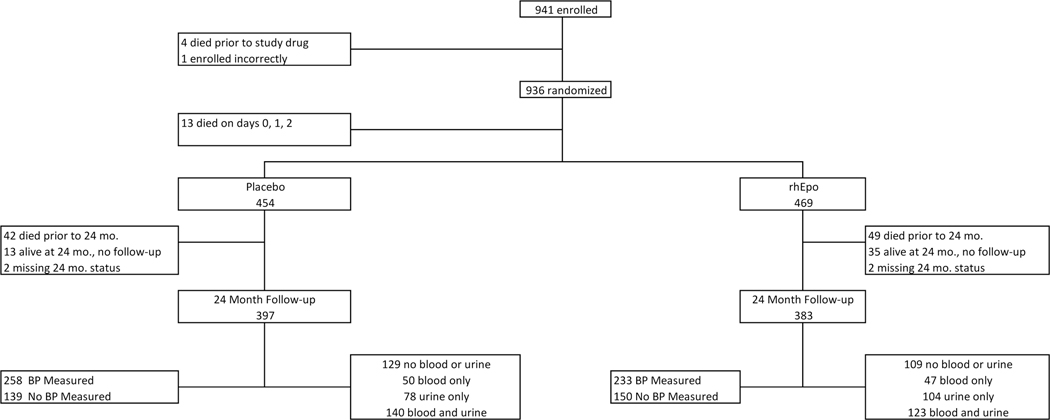
Of the 941 subjects enrolled in the study, we excluded 18 neonates (4 who died prior to receiving study drug, 1 who was enrolled incorrectly, and 13 who died on days 0, 1 or 2) because we were unable to ascertain whether these neonates had AKI, given that it takes days for serum creatinine (SCr) to rise after an event and maternal SCr values affect neonatal SCr in the first postnatal days.21, 22 Therefore, the final sample of ELGANs for the short-term outcomes includes the 923 subjects who received rhEpo (N = 469) or placebo (N = 454) and were alive on day 3 (Figure 1; available at www.jpeds.com).
Figure 1 (online only):
941 subjects were enrolled in the PENUT study. Of the 941, 18 were excluded from this study as 4 died prior to study drug, 1 was enrolled incorrectly and 13 died on days 0,1, 2 and we could not assign any kidney related outcomes. Therefore, the final sample of participants for short-term outcomes in REPaIReD were the 923 who received study drug and were alive on day 3. Of the 923, 454 received placebo and 469 received rhEpo. At the 24 month follow-up, 780 infants were evaluated (397 in placebo and 383 in rhEpo) groups. The number who had blood/urine collected at the 24 month visit are described in the figure and in text.
For the 22–26 months cGA time-point, 383/420 (91.2%) participants who were alive at 2 years and received rhEpo returned for follow up (49 died prior to follow-up time point, 35 were lost to follow-up, and 2 were missing 24 month data). Urine, blood and blood pressure measurements were not a mandatory part of the primary protocol but were encouraged by site personnel to families. Figure 1 shows the breakdown of data ascertainment. Of the 383 subjects who received rhEPO and returned for a follow up visit, 123/383 (32.1%) had both blood and urine collected, 47/383 (12.7%) had blood only, 104/383 (27.2%) had urine only, and 109 / 383 (28.5%) had neither blood nor urine collected for analysis. Of the 383 subjects who returned for follow-up, 233/383 (60.8%) had blood pressure measured and 150/383 (39.2%) did not.
Alternatively, 397/412 (96.4%) participants who received placebo returned for follow up (42 died prior to 24 months, 13 were lost to follow-up, 2 were missing a 24 month status). Of the 397 who came to follow-up visit, 140/397 (35.3%) had both blood and urine collected, 50/397 (12.6%) had blood only, 78/397 (19.6%) had urine only, and 129/397 (32.5%) had neither blood nor urine collected. Of the 397 subjects who came for a follow-up visit, 258/397 (65.0%) had blood pressure measured and 139/397 (35.0%) did not.
The University of Washington Institutional Review Board (IRB) approved this collaborative study, and each center received approval from their respective IRBs.
Timeline of Clinical Trial
The design and primary efficacy/safety outcomes have been published elsewhere.19, 20 In brief, after informed consent, participants were randomized to rhEpo vs placebo within 24 hours of birth. Randomization allocation was 1:1, with patients stratified by gestational age category, multiple births, and study site. Sample size was determined by the primary study to be able to detect a difference in the primary outcome (death or neurologic disability at 22–26 months cGA). Subjects received rhEpo at a dose of 1000 units/kg or placebo intravenously every 48 hours for a total of six doses; thereafter, participants received either 400 U/kg/dose rhEpo subcutaneously or sham injections 3 times a week until they reached 32–6/7 weeks cGA. Study personnel and families were still blinded to randomization groups at the follow up visit.
AKI Definitions and Time Frames of Assessments for AKI using Clinical SCr data
We used the SCr-based Kidney Disease Improving Global Outcomes criteria to define neonatal AKI using clinically measured SCr values.23 Each NICU measured SCr according to their institutional guidelines using the local laboratory methodology available (11 Jaffe, 8 enzymatic). Our a priori primary short-term outcome was severe AKI any time during the hospitalization. Severe AKI is defined as reaching stage 2 or higher AKI as previously described in other multi-center neonatal,24 and pediatric25 AKI studies whereby the neonates had to have a ≥ 200% SCr rise from baseline anytime during the NICU hospitalization. The baseline SCr is defined as the lowest previous value measured (not including any values measured on the day of birth or on the day after birth). The earliest baseline SCr value used to define AKI in this study is on postnatal day 2, as day of birth is denoted as day 0. We chose to exclude the SCr measured on the day of birth or the day after birth because these values represent maternal SCr which plateau over the next 36–48 hours in ELGANs.22 Thus, in our study, it is not until day 3 when a rise in SCr from baseline can be detected. The 23/923 (2.5%) neonates that did not have any SCr values were classified as having no AKI.
For the secondary short-term outcomes we evaluated AKI stages at different time points as follows: AKI was classified into early (days 3–7), middle (days 8–14) and late (days 15-discharge or 44 weeks cGA, whichever comes first) as we have previously reported.8 For these analyses, we included those patients who were alive at the beginning of each time frame such that we report on 923 infants during the first week, 891 in the middle time frame (due to 32 deaths between days 3–7), and 875 in the late time frame (due to 48 deaths between days 3–14). We define any AKI as the highest AKI stage during the entire hospital stay.
Assessment of short-term kidney function using SCr and serum cystatin C at specific pre-determined time points
Using convenience blood samples drawn at time points determined by the primary study (postnatal days 0, 7, 9 and 14), we analyzed SCr (measured at a core laboratory in Seattle, Washington) using the two-point method with the Vitros 4600 (Ortho Clinical Diagnostic; Raritan, NJ). Cystatin C concentrations were analyzed using the same blood samples at the same core laboratory using particle-enhanced immunonephelometry with the BN ProSpec System (Simiens Helathineers; Tarrytown, NY). These analyses allow us to evaluate kidney function at standardized time points, with samples measured using the same methodology on the same postnatal days for the majority of infants. We report both SCr and cystatin C values as absolute measures and changes in the values over time.
Kidney-related measurements at the 22–26 month cGA visit
We defined estimated glomerular filtration rate (eGFR) according to the SCr & Cystatin CKiD equation where eGFR (ml/min per 1.73 m2 ) = 41.6[ht (cm)/Scr(mg/dL)]^0.443 * [1.8/cystatin C (mg/L)]^0.47926..26 Urine was collected as a bag specimen or with a cotton ball in the diaper. We defined albuminuria as an albumin/creatinine ratio (ACR) >30 mg albumin/g creatinine, which has been shown to be a surrogate outcome of CKD progression in children.27 Although there is very little data on normative values of ACR in the United States, a study from the Netherlands reports ACR in 1288 toddlers at around the age of 24 months (median=14 mg/g; IQR of 8–25.6; 5th percentile=4.3 and 95th percentile=89.3). This study found that 23.4% of subjects had a urine ACR >30 mg/g.28
Blood pressure was measured with a Briggs Mabic Healthcare Manual Sphygmomanometer (Des Moines, IA) with blood pressure cuff appropriate for patient size, whereby the inflatable bladder width had to be at least 40% of the child’s mid–upper arm circumference and the length between 80–100% of the mid–upper arm circumference. Standardization of procedures and personnel training was done across all sites. After the child was in a calm state, 2 manual blood pressure measurements at least 5 minutes apart were taken. The lowest systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. We report the lowest SBP and DBP of the two, and describe the population’s values and percentages which exceed the 90th and 95th percentiles for age and sex-related norms according to the 2017 Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents.29 For purposes of analysis in the regression models, we focus only on the 90th percentile values.
Statistical Analyses
Baseline characteristics, AKI status, core SCr values, serum cystatin C values, and 2 year kidney-related outcomes were examined by treatment arm. The 2 year kidney-related outcomes were compared between groups using both the categorical outcomes (eGFR <90 ml/min/1.73 m2, urine ACR >30 mg/g, SBP >90th percentile, SBP >95th percentile, DBP >90th percentile and DBP >95th percentile) as well as the continuous values. Linear and logistic models were used to test for trends using generalized estimating equations (GEE) with clustering by sibship.30 These models were used to determine the association between treatment arm and kidney-related binary outcomes (severe AKI, abnormal eGFR, albuminuria, SBP > 90th percentile and DBP > 90th percentile). We performed a GEE model controlling for sibship whereby we evaluated the interaction term for each demographics x treatment arm to understand if demographic variables were disproportionate in those who had blood pressure ascertained vs. missing. We performed a sensitivity analysis to determine if an alternative approach to reporting BP (using the average, instead of the lowest BP) would have led to differences in treatment effect. Data management and analysis were conducted using R version 5.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Short-term Kidney Outcomes
Of the 923 neonates included in the short term analysis, 51.9% were male, the average birthweight was 801 grams, and most (91.6%) received prenatal steroids. Maternal race was largely white (65%), African-American (26%), and other (9%), and 21% self-identified as Hispanic. Demographic and delivery room intervention differences and maternal characteristics by treatment arm are shown in Table I. The treatment groups were well matched for demographic characteristics and protective perinatal therapies.
Table 1:
Demographic charcateristics by treatment arm
| Treatment Arm | ||||
|---|---|---|---|---|
| All | Placebo | Epo | ||
| n | 923 | 454 | 469 | p-value |
| Male, n (%) | 479 (51.9%) | 228 (50.2%) | 251 (53.5%) | 0.35 |
| Gestational age, n (%) | 0.07 | |||
| 24 weeks | 227 (24.6%) | 117 (25.8%) | 110 (23.5%) | |
| 25 weeks | 242 (26.2%) | 122 (26.9%) | 120 (25.6%) | |
| 26 weeks | 220 (23.8%) | 117 (25.8%) | 103 (22.0%) | |
| 27 weeks | 234 (25.4%) | 98 (21.6%) | 136 (29.0%) | |
| Birth weight (g), mean (sd) | 801.1 (187.9) | 793.2 (181.9) | 808.8 (193.4) | 0.21 |
| Birth length (cm), mean (sd) | 32.9 (2.9) | 32.8 (2.7) | 33 (3.1) | 0.47 |
| Size for Gestational Age | 0.55 | |||
| Large, n (%) | 104 (11.3%) | 50 (11.0%) | 54 (11.5%) | |
| Average, n (%) | 739 (80.1%) | 360 (79.3%) | 379 (80.8%) | |
| Small, n (%) | 80 (8.7%) | 44 (9.7%) | 36 (7.7%) | |
| Apgar 1 min, median (IQR) | 4 (2, 6) | 4 (2, 6) | 4 (2, 6) | 0.14 |
| Apgar 5 min, median (IQR) | 7 (5, 8) | 7 (5, 8) | 7 (5, 8) | 0.74 |
| OFC (cm), mean (sd) | 23.1 (1.9) | 23.1 (1.9) | 23.1 (1.9) | 0.71 |
| Number of festuses, median (IQR) | 1 (1, 2) | 1.3 (0.5) | 1.3 (0.6) | 0.67 |
| Prenatal steroids, n (%) | 831 (91.6%) | 407 (91.5%) | 424 (91.8%) | 0.84 |
| 1 dose, n (%) | 174 (20.9%) | 76 (18.7%) | 98 (23.1%) | 0.25 |
| 2 doses, n (%) | 575 (69.2%) | 289 (71.0%) | 286 (67.5%) | |
| 3 doses, n (%) | 74 (8.9%) | 39 (9.6%) | 35 (8.3%) | |
| Delivery room resuscitation, n (%) | ||||
| Any | 896 (97.1%) | 446 (98.2%) | 450 (95.9%) | 0.09 |
| Oxygen | 738 (80.0%) | 365 (80.4%) | 373 (79.5%) | 0.81 |
| Positive pressure | 797 (86.3%) | 403 (88.8%) | 394 (84.0%) | 0.04 |
| Intubation | 748 (81.0%) | 374 (82.4%) | 374 (79.7%) | 0.35 |
| Surfactant | 480 (52.0%) | 240 (52.9%) | 240 (51.2%) | 0.65 |
| Chest compression | 72 (7.8%) | 37 (8.1%) | 35 (7.5%) | 0.79 |
| Resuscitation drugs | 32 (3.5%) | 14 (3.1%) | 18 (3.8%) | 0.66 |
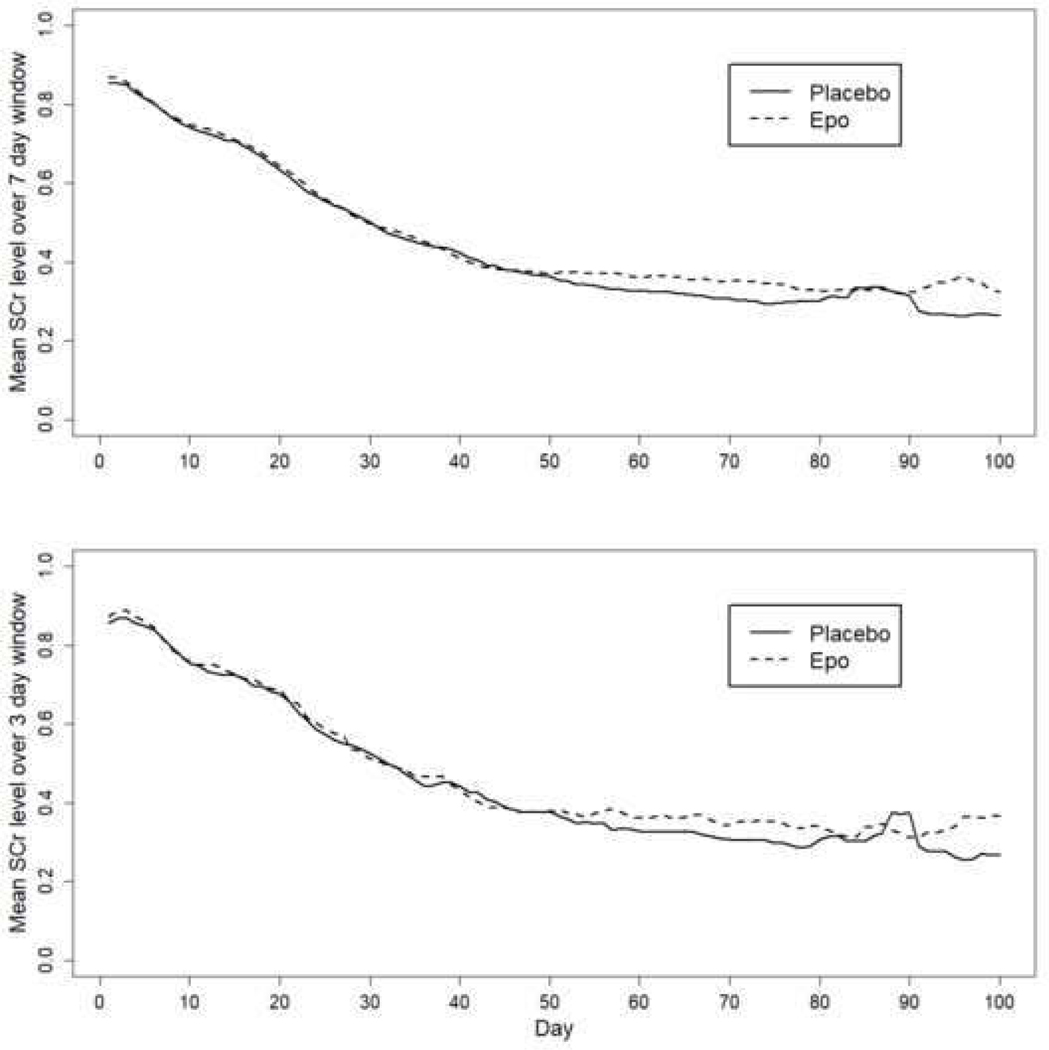
The prevalence rates for AKI in the entire cohort and by treatment arm are shown in Table 2. For the entire cohort, 351/923 (38.0%, CI = 34.8% - 41.3%) had at least one episode of stage 1 or higher AKI, and 168/923 (18.2%, CI = 15.7% - 20.7%) had at least one episode of severe AKI anytime during the hospitalization. The rates of our primary outcome (severe AKI at any time) did not differ in those who received rhEpo vs. placebo. No statistically significant differences were seen in the rates of early, middle or late AKI between treatment groups. No statistically significant differences were seen in the trends of clinically measured mean SCr over time between groups over a 7-day and a 3-day window (Figure 2, A and B).
Table 2:
AKI status by treatment arm
| Treatment Arm | ||||
|---|---|---|---|---|
| All | Placebo | Epo | p-values | |
| n | 923 | 454 | 469 | |
| AKI Max Anytime, n (%) | 0.62 | |||
| Stage 0 | 572 (62%) | 274 (60.4%) | 298 (63.5%) | |
| Stage 1 | 183 (19.8%) | 105 (23.1%) | 78 (16.6%) | |
| Stage 2 | 108 (11.7%) | 47 (10.4%) | 61 (13%) | |
| Stage 3 | 60 (6.5%) | 28 (6.2%) | 32 (6.8%) | |
| Severe AKI max anytime, n (%) | 0.20 | |||
| Yes (stage 2 or 3) | 168 (18.2%) | 75 (16.5%) | 93 (19.8%) | |
| No (stage 0 or 1) | 755 (81.8%) | 379 (83.5%) | 376 (80.2%) | |
| AKI Timing (Max SCr) | ||||
| Early (days 3–7), n (%) | 0.76 | |||
| Stage 0 | 811 (87.9%) | 400 (88.1%) | 411 (87.6%) | |
| Stage 1 | 92 (10%) | 45 (9.9%) | 47 (10%) | |
| Stage 2 | 11 (1.2%) | 6 (1.3%) | 5 (1.1%) | |
| Stage 3 | 9 (1%) | 3 (0.7%) | 6 (1.3%) | |
| Middle (days 8–14), n (%) | 0.91 | |||
| Stage 0 | 749 (81.1%) | 368 (81.1%) | 381 (81.2%) | |
| Stage 1 | 90 (9.8%) | 49 (10.8%) | 41 (8.7%) | |
| Stage 2 | 41 (4.4%) | 19 (4.2%) | 22 (4.7%) | |
| Stage 3 | 11 (1.2%) | 2 (0.4%) | 9 (1.9%) | |
| Missing - due to deaths prior to day 8 | 32 (3.5%) | 16 (3.5%) | 16 (3.4%) | |
| Late (days 15 – discharge or 44 weeks), n (%) | 0.56 | |||
| Stage 0 | 626 (67.8%) | 306 (67.4%) | 320 (68.2%) | |
| Stage 1 | 117 (12.7%) | 64 (14.1%) | 53 (11.3%) | |
| Stage 2 | 84 (9.1%) | 37 (8.1%) | 47 (10%) | |
| Stage 3 | 48 (5.2%) | 25 (5.5%) | 23 (4.9%) | |
| Missing - due to deaths prior to day 15 | 48 (5.2%) | 22 (4.8%) | 26 (5.5%) | |
Children who died on days 0, 1, 2 are excluded from this analysis.
Lab data from days 0, 1 are not included in the AKI calculation.
Children may qualify as severe AKI on day 2 via elevated SCr, but not by SCr ratio to baseline.
Figure 2a and 2b:
Mean creatinine levels over a rolling 7-day and 3-day window by treatment arm over time.
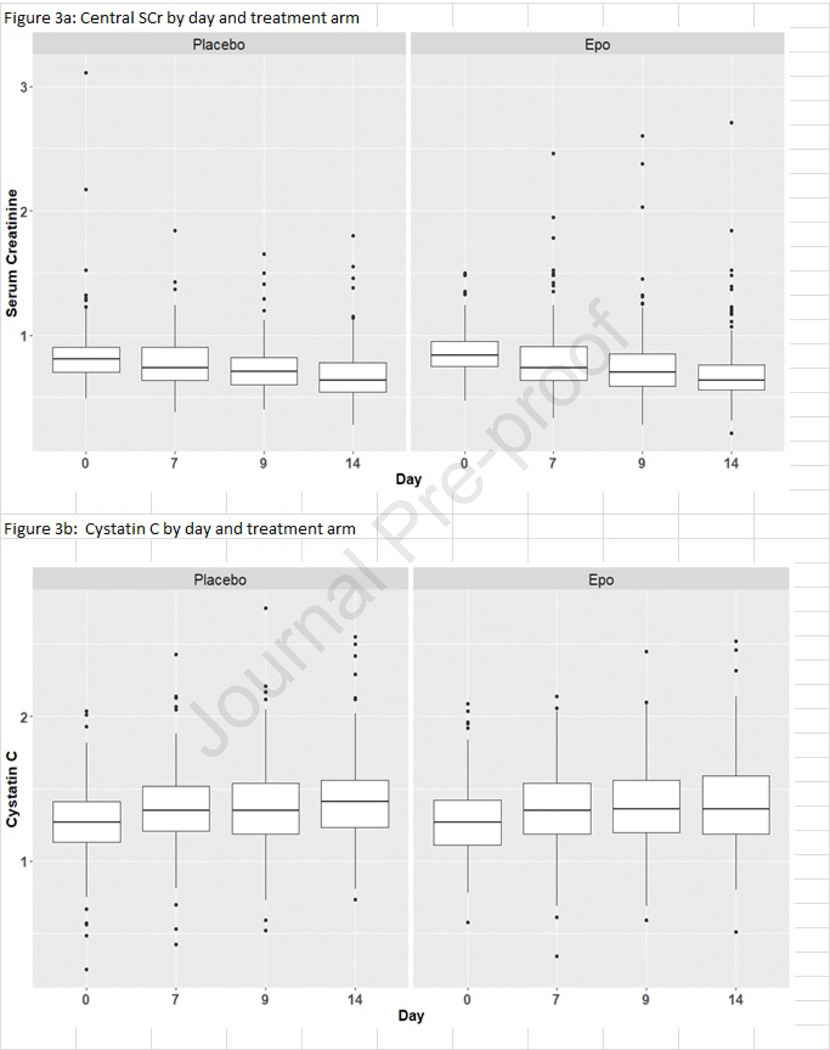
The SCr and the cystatin C values measured at the core laboratory on postnatal days 0, 7, 9 and 14 are reported by treatment arm in Table 3 (available at www.jpeds.com) and depicted in Figure 3, A and B (available at www.jpeds.com), respectively. There was no meaningful difference in either SCr or cystatin C values or changes over time by treatment group. Using the GEE models, we found no differences by treatment arm for early, middle, or late AKI and no differences for severe AKI (Table 4; available at www.jpeds.com).
Table 3:
Central SCr and Cystatin C values by treatment arm
| Treatment Arm | ||||
|---|---|---|---|---|
| All | Placebo | Epo | p-values | |
| n | 624 | 309 | 315 | |
| SCr | mean (sd) [n] | |||
| Day 0 | 0.85 (0.20) [551] | 0.83 (0.22) [273] | 0.86 (0.17) [278] | 0.17 |
| Day 7 | 0.79 (0.23) [514] | 0.79 (0.20) [253] | 0.79 (0.25) [261] | 0.99 |
| Day 9 | 0.74 (0.23) [506] | 0.73 (0.19) [251] | 0.74 (0.26) [255] | 0.62 |
| Day 14 | 0.68 (0.23) [457] | 0.68 (0.21) [232] | 0.69 (0.26) [225] | 0.49 |
| Max | 0.92 (0.25) [621] | 0.91 (0.25) [308] | 0.92 (0.25) [313] | 0.50 |
| Change Day 0 to 7 | −0.06 (0.28) [473] | −0.05 (0.30) [233] | −0.08 (0.26) [240] | 0.28 |
| Change Day 0 to 9 | −0.11 (0.27) [450] | −0.09 (0.26) [223] | −0.12 (0.28) [227] | 0.23 |
| Change Day 0 to 14 | −0.17 (0.28) [430] | −0.16 (0.30) [216] | −0.18 (0.26) [214] | 0.47 |
| Change Day 7 to 9 | −0.05 (0.15) [441] | −0.06 (0.15) [218] | −0.04 (0.14) [223] | 0.14 |
| Change Day 7 to 14 | −0.10 (0.21) [413] | −0.11 (0.21) [210] | −0.09 (0.20) [203] | 0.37 |
| Cystatin C | ||||
| Day 0 | 1.27 (0.23) [537] | 1.27 (0.23) [269] | 1.28 (0.23) [268] | 0.79 |
| Day 7 | 1.38 (0.43) [505] | 1.37 (0.28) [247] | 1.39 (0.53) [258] | 0.66 |
| Day 9 | 1.41 (0.56) [502] | 1.38 (0.28) [248] | 1.44 (0.73) [254] | 0.17 |
| Day 14 | 1.42 (0.29) [454] | 1.42 (0.29) [232] | 1.41 (0.30) [222] | 0.54 |
| Max | 1.54 (0.51) [621] | 1.52 (0.29) [308] | 1.56 (0.66) [313] | 0.36 |
| Change Day 0 to 7 | 0.10 (0.44) [452] | 0.09 (0.28) [223] | 0.11 (0.55) [229] | 0.62 |
| Change Day 0 to 9 | 0.13 (0.59) [433] | 0.10 (0.27) [216] | 0.16 (0.79) [217] | 0.29 |
| Change Day 0 to 14 | 0.13 (0.31) [414] | 0.16 (0.31) [212] | 0.11 (0.31) [202] | 0.11 |
| Change Day 7 to 9 | 0.02 (0.24) [431] | 0.01 (0.25) [210] | 0.03 (0.24) [221] | 0.24 |
| Change Day 7 to 14 | 0.03 (0.49) [402] | 0.04 (0.31) [204] | 0.01 (0.62) [198] | 0.42 |
Figure 3a and 3b (online only):
Core Laboratory SCr and cystatin C (median and IQR) measurements on postnatal days 0, 7, 9, 14 by treatment arm.
Table 4:
GEE model estimates for Severe AKI ~ treatment arm
| AKI groups | OR (95% CI) | |
|---|---|---|
| Early AKI | ||
| NO vs 1/2/3 | 0.93 (0.62, 1.41) | |
| NO or 1 vs 2/3 | 0.79 (0.32, 1.95) | |
| Middle AKI | ||
| NO vs 1/2/3 | 0.99 (0.69, 1.44) | |
| NO or 1 vs 2/3 | 0.67 (0.38, 1.20) | |
| Late AKI | ||
| NO vs 1/2/3 | 1.04 (0.76, 1.42) | |
| NO or 1 vs 2/3 | 0.86 (0.58, 1.25) | |
| Anytime AKI | ||
| NO vs 1/2/3 | 1.11 (0.83, 1.48) | |
| NO or 1 vs 2/3 | 0.76 (0.54, 1.07) |
GEE models accouting for GA, sex, site and sibship clustering.
Estimates shown only for treatment arm - Epo vs Placebo
Kidney-related outcomes at 22–26 month cGA
Table 5 shows the rates of kidney-related outcomes at 22–26 month cGA by treatment arm. In participants who had eGFR available, 54/336 (16.2%) had an eGFR <90 mL/min/1.73m2. In participants who had urine available, 155/435 (35.6%) had a urine ACR >30 mg/g. Evaluation of 24 month cGA outcomes by treatment group shows that the rates of eGFR <90 mL/min/1.73m2 were 16.2% and 15.9% for the placebo and rhEpo groups respectively (p=NS). The rates of urine ACR ratio >30 mg/g were 36.8% and 34.5% for the placebo and rhEpo groups respectively (p=NS).
Table 5:
24-month kidney related outcomes by treatment arm
| Placebo | Epo | p-value | |
|---|---|---|---|
| N = 454 | N = 469 | ||
| eGFR value available, n (%) | 179 (39.4%) | 157 (33.5%) | 0.09 |
| Median mL/min/1.73m2 (IQR) | 101.3 (93.8, 113.8) | 102.7 (96.6, 112.7) | 0.93 |
| <90 mL/min/1.73m2, n (%) | 29 (16.2%) | 25 (15.9%) | 0.95 |
| Alb/Creat ratio available, n (%) | 212 (46.7%) | 223 (47.5%) | 0.81 |
| Median mg/g (IQR) | 21.8 (13.4, 37.7) | 22.0 (14.3, 38.2) | 0.78 |
| ≥30 mg/g, n (%) | 78 (36.8%) | 77 (34.5%) | 0.63 |
| eGFR and Alb/Creat ratio available, n (%) | 131 (28.9%) | 114 (24.3%) | 0.15 |
| eGFR≥90 and/or Alb/Creat<30, n (%) | 125 (95.4%) | 104 (91.2%) | 0.19 |
| eGFR<90 and Alb/Creat≥30, n (%) | 6 (4.6%) | 10 (8.8%) | |
| Hypertension value available, n (%) | 258 (56.8%) | 233 (49.7%) | 0.044 |
| Systolic Blood Pressure (SBP) | |||
| SBP < 90th percentile, n (%) | 163 (63.2%) | 168 (72.1%) | |
| SBP 90–94th percentile, n (%) | 29 (11.2%) | 19 (8.1%) | 0.1 |
| SBP >=95th percentile, n (%) | 66 (25.6%) | 46 (19.7%) | |
| Median SBP (IQR) mmHg | 98.5 (90, 106.75) | 97 (90, 104) | 0.21 |
| Diastolic Blood Pressure (DBP) | |||
| DBP < 90th percentile, n (%) | 117 (45.3%) | 112 (48.1%) | |
| DBP 90–94th %ile, n (%) | 30 (11.6%) | 33 (14.1%) | 0.44 |
| DBP >=95th %ile, n (%) | 111 (43.0%) | 88 (37.6%) | |
| Median DBP (IQR) mmHg | 58.0 (52.0, 65.8) | 58.0 (50.0, 66.0) | 0.49 |
NOTE: Hypertension defined as SBP >90th percentile for age and sex.
Of the participants who had blood pressure measured, 160/491 (32.6%) had a SBP >90th percentile, and 112/491 (22.8%) had a SBP above the 95th percentile for age. Evaluation of DBP showed that 262/491 (53.4%) had a DBP >90th percentile, and 199/491 (40.5%) had a DBP above the 95th percentile for age. Those randomized to Epo were less likely to have SBP >90th percentile than those randomized to placebo (65/258 [27.9] vs. 95/258 [36.8%]; p<0.04). We did not find any differences in the rates of SBP >95th percentile, DBP >90th or 95th percentiles. Eight participants were on anti-hypertensive medications at 24-months (5-Amlodipine, 3-Other). Of these 8 subjects, 5 were noted as having elevated SBP and DBP, 2 were normotensive, and one did not have a blood pressure measured at the 24 month follow-up visit.
Of the 191 participants who returned to follow-up and had blood, urine, SBP and DBP measurements, 47/191 (24.6%) had no abnormalities, 67/191 (35.1%) had 1 abnormality, 54/191 (28.3%) had 2 abnormalities, 21/191 (11.0%) had 3 abnormalities, and 2/191 (1.0%) had all 4 abnormalities.
Table 6 (available at www.jpeds.com) shows that of the patients who survived the NICU stay, there was a statistically significant difference in the “lost to follow-up” rate between those who were randomized to placebo vs. rhEPO (13/412 [3.2%] vs. 35/420 [8.3%]; P < .01). However, we did not find statistically significant differences in the rates of blood, urine, and blood pressure ascertainment by treatment arm for those who survived the NICU stay.
Table 6:
24-month follow-up availability by treatment arm
| Placebo | Epo | ||
|---|---|---|---|
| Number/ Total (%) | Number/ Total (%) | p-value | |
| Died prior to 24-months | 42/ 454 (9.3%) | 49/ 469 (10.4%) | 0.55 |
| Alive at 24 months | 412 / 454 (90.7%) | 420 / 469 (89.6%) | |
| Alive at 24 months, no follow-up | 13 / 412 (3.2%) | 35 / 420 (8.3%) | 0.01 |
| 24 month follow-up conducted | 397 / 412 (96.4%) | 383 / 420 (91.2%) | |
| Blood and urine | 140 / 397 (35.3%) | 123 / 383 (32.1%) | 0.1 |
| blood only | 50 / 397 (12.6%) | 47 / 383 (12.3%) | |
| urine only | 78 / 397 (19.6%) | 104 / 383 (27.2%) | |
| neither blood or urine | 129 / 397 (32.5%) | 109 / 383 (28.5%) | |
| Blood Pressure measured | 258/394 (65.5%) | 234/383 (61.1%) | 0.24 |
| Weight kg, mean (sd) | 11.5 (1.8) | 11.2 (1.8) | 0.48 |
| Height cm, mean (sd) | 84.7 (4.7) | 85.0 (4.2) | 0.08 |
Table 7 (available at www.jpeds.com), provides data on the demographics by treatment arm for survivors who had blood pressure ascertained vs. missing in order to determine whether disproportionate differences in the rates of blood pressure ascertainment by treatment arm exist. Sex was the only demographic characteristic that reached a statistically significant level of p<0.05, and prenatal steroids almost reached the level of statistical significance (p=0.06).
Table 7:
Demographics of survivors by blood pressure availability by treatment arm
| Missing Blood Pressure | Available Blood Pressure | ||||
|---|---|---|---|---|---|
| Placebo | rhEpo | Placebo | rhEpo | interaction | |
| n | N = 139 | N = 150 | N = 258 | N = 233 | p-value |
| Male, n (%) | 62 (44.6%) | 86 (57.3%) | 134 (51.9%) | 116 (49.8%) | 0.048 |
| Gestational Age, n (%) | 0.28 | ||||
| 24 weeks | 42 (30.2%) | 36 (24.0%) | 53 (20.5%) | 49 (21.0%) | |
| 25 weeks | 30 (21.6%) | 40 (26.7%) | 76 (29.5%) | 51 (21.9%) | |
| 26 weeks | 39 (28.1%) | 33 (22.0%) | 67 (26.0%) | 49 (21.0%) | |
| 27 weeks | 28 (20.1%) | 41 (27.3%) | 62 (24.0%) | 84 (36.1%) | |
| Birth weight (g), mean (sd) | 795.1 (170.1) | 815.9 (180.8) | 807.1 (187.6) | 835.1 (196.7) | 0.85 |
| Birth length (cm), mean (sd) | 33.1 (2.5) | 33 (3.1) | 32.9 (2.8) | 33.3 (3.1) | 0.25 |
| Size for Gestational Age, n (%) | 0.57 | ||||
| Large | 16 (11.5%) | 22 (14.7%) | 30 (11.6%) | 23 (9.9%) | |
| Average | 113 (81.3%) | 120 (80.0%) | 202 (78.3%) | 193 (82.8%) | |
| Small | 10 (7.2%) | 8 (5.3%) | 26 (10.1%) | 17 (7.3%) | |
| Apgar 1 min, median (IQR) | 4 (3, 5) | 4 (1.5, 6) | 4 (2, 6) | 4 (2, 6) | 0.82 |
| Apgar 5 min, median (IQR) | 7 (5, 8) | 7 (5, 8) | 7 (5, 8) | 7 (6, 8) | 0.09 |
| OFC (cm), mean (sd) | 23 (1.5) | 23.1 (1.9) | 23.2 (2.1) | 23.3 (1.9) | 0.92 |
| Number of fetuses, mean (sd) | 1.3 (0.5) | 1.3 (0.6) | 1.3 (0.5) | 1.3 (0.6) | 0.95 |
| Prenatal steroids, n (%) | 123 (89.8%) | 140 (94.6%) | 238 (94.1%) | 209 (90.9%) | 0.06 |
| 1 dose, n (%) | 21 (17.1%) | 28 (20.0%) | 44 (18.5%) | 54 (25.8%) | 0.08 |
| 2 doses, n (%) | 94 (76.4%) | 93 (66.4%) | 165 (69.3%) | 138 (66.0%) | |
| 3 doses, n (%) | 8 (6.5%) | 17 (12.1%) | 27 (11.3%) | 14 (6.7%) | |
| Delivery room resuscitation, n (%) | |||||
| Any | 136 (97.8%) | 145 (96.7%) | 253 (98.1%) | 221 (94.8%) | 0.41 |
| Oxygen | 103 (74.1%) | 122 (81.3%) | 219 (84.9%) | 193 (82.8%) | 0.13 |
| Positive pressure | 118 (84.9%) | 127 (84.7%) | 238 (92.2%) | 199 (85.4%) | 0.12 |
| Intubation | 110 (79.1%) | 119 (79.3%) | 217 (84.1%) | 180 (77.3%) | 0.23 |
| Surfactant | 72 (51.8%) | 72 (48.0%) | 136 (52.7%) | 117 (50.2%) | 0.88 |
| Chest compression | 7 (5.0%) | 8 (5.3%) | 23 (8.9%) | 14 (6.0%) | 0.44 |
| Resuscitation drugs | 4 (2.9%) | 5 (3.3%) | 7 (2.7%) | 6 (2.6%) | 0.81 |
Table 8 (available at www.jpeds.com) shows the GEE models for each of the kidney related metrics expressed as continuous and categorical variables at 24 months cGA. After controlling for site, gestational age, and accounting for sibship clustering, participants who were randomized to rhEpo had lower odds of high SBP (adjusted OR=0.60 [95% CI = 0.39 – 0.92]). We did not find statistically significant differences between treatment groups in low eGFR, ACR, or high DBP.
Table 8:
GEE regression estimates for treatment arm ~ CKD
| continuous outcomes | β (95% CI) |
|---|---|
| eGFR | 0.27 (−3.08, 3.61) |
| ACR | −0.41 (−5.49, 4.67) |
| SBP | −1.58 (−3.72, 0.57) |
| DBP | −0.69 (−2.49, 1.11) |
| binary outcomes | OR (95% CI) |
| eGFR<90 | 0.95 (0.52, 1.77) |
| ACR>=30 | 0.90 (0.59, 1.36) |
| SBP > 90th percentile | 0.60 (0.39, 0.92) |
| DBP > 90th percentile | 0.90 (0.61, 1.33) |
Note: Each estimate represents epo vs placebo for the given outcome variable after adjusting for site, GA while accounting for potential sibship clustering.
We performed a sensitivity analysis to determine if our findings on the treatment effect of rhEpo and SBP would have changed if we chose to report BP as the average between two values, instead of the lowest of two blood pressure readings. Of those with at least 1 BP measurement in the placebo arm, 134/258 (60.1%) had 2 measurements. Of those with at least 1 BP measurement in the rhEpo arm, 155/233 (57.5%) had 2 measurements. Table 9 (available at www.jpeds.com) compares these BP measures by treatment arm for the 2 approaches. Compared with the lowest BP approach, using the average BP approach increases the rate of SBP > 90th percentile from 95/258 (36.8%) to 110/258 ( 42.6%) in the placebo arm and from 65/233 (27.8%) to 76/233 (32.6%) in the rhEpo arm. The GEE models for the independent odds of SBP > 90th by treatment groups were almost identical [(0.60 (0.39, 0,92) in the lowest BP approach vs. 0.59 (0.39, 0,89) in the average BP approach].
Table 9 (online only):
Sensitivity analysis of differences in BP if using lowest BP vs. average BP
| BP using lowest value | BP using average value | |||||
|---|---|---|---|---|---|---|
| placebo | rhEpo | p-value | placebo* | rhEpo** | p-value | |
| Hypertension value available, n (% | 258 (56.8%) | 233 (49.7%) | 0.044 | 258 (56.8%) | 233 (49.7%) | 0.044 |
| Systolic Blood Pressure (SBP) | ||||||
| SBP < 90th percentile, n (%) | 163 (63.2%) | 168 (72.1%) | 148 (57.4%) | 157 (67.4%) | ||
| SBP 90–94th percentile, n (%) | 29 (11.2%) | 19 (8.1%) | 0.1 | 33 (12.8%) | 21 (9.0%) | 0.06 |
| SBP >=95th percentile, n (%) | 66 (25.6%) | 46 (19.7%) | 77 (29.8%) | 55 (23.6%) | ||
| Median SBP (IQR) mmHg | 98.5 (90, 106.75) | 97 (90, 104) | 0.21 | 98.75 (92, 107) | 96 (90, 104) | 0.23 |
| Diastolic Blood Pressure (DBP) | ||||||
| DBP < 90th percentile, n (%) | 117 (45.3%) | 112 (48.1%) | 102 (39.5%) | 97 (41.6%) | ||
| DBP 90–94th %ile, n (%) | 30 (11.6%) | 33 (14.1%) | 0.44 | 27 (10.5%) | 32 (13.7%) | 0.36 |
| DBP >=95th %ile, n (%) | 111 (43.0%) | 88 (37.6%) | 129 (50.0%) | 104 (44.6%) | ||
| Median DBP (IQR) mmHg | 58.0 (52.0, 65.8) | 58.0 (50.0, 66.0) | 0.49 | 60.0 (53.1, 67.5) | 59.5 (52.0, 65.0) | 0.29 |
155/258 (60.1%) in placebo had more than one BP.
134/233 (57.5%) in rhEPO group had more than one BP.
Discussion
In this ancillary study of a multi-center double-blind, randomized clinical trial we found that participants randomized to rhEpo had lower independent adjusted odds of high SBP at 22–26 month cGA compared with those randomized to placebo. We did not observe any short term kidney-related benefit by treatment for severe AKI, any AKI, or differences in SCr and cystatin C values during the first 2 postnatal weeks. We did not find differences in eGFR, urine ACR, or DBP by treatment arm at 22–26 months cGA.
There are a few possible explanations for the findings of lower rates of high SBP in patients randomized to rhEpo. Interestingly, although there was a statistically significant independent difference in the categorical variable of SBP, there were no differences in SBP when evaluated as a continuous variable. Erythropoietin is made in the kidney and its presence has a role in normal kidney development. Thus, it is possible that high doses of rhEPO during the first postnatal weeks alter the kidney and vascular architectures such that the rates of long-term hypertension are improved. Alternatively, it is possible our finding of lower rates of high SBP in rhEpo group may be due to selection bias created, in context of a high number of participants in whom a blood pressure was not measured. Indeed, we found statistically significant differences, with a disproportionate number of subjects who were male (p=0.05) and who received prenatal steroids (p=0.06) in those with missing blood pressure measurements. Studies in other cohorts will be needed to validate or disprove this finding.
This study lends insight into the short and long-term kidney outcomes in ELGANs using contemporary definitions of neonatal AKI and CKD. The overall prevalence of AKI in this cohort is similar to other studies in premature neonates.24, 31 We provide 2-year kidney-related data collected prospectively in a large multi-center cohort of ELGANs who survive NICU stay. We found that compared with the general 2 year old population, the participants had very high rates of abnormal kidney-related outcomes. Of the 191 participants who returned to follow-up and had blood, urine, SBP and DBP measured, 47/191 (24.6%) had no abnormalities, and 144/191 (75.4%) had at least one kidney-related abnormality. Specifically, 67/191 (35.1%) had 1 kidney-related abnormality, 54/191 (28.3%) had 2 abnormalities, 21/191 (11.0%) had 3 abnormalities, and 2/191 (1.0%) had all 4 abnormalities. Recognizing that the methodology we used to assess kidney-related outcomes may be limited, these data speak to the significant risk of kidney disease in this population.
The strengths of this study are the size of the cohort, the robust number of SCr measurements available, and the study design (double-blinded, randomized, placebo-controlled trial). It has high generalizability given the multi-center enrollment. Despite these strengths, we acknowledge the following limitations. First, not all neonates had SCr captured every day during the hospitalization; therefore, it is possible that the true AKI rate could be higher. Second, we acknowledge that although we performed study-related measurements that were optimized for a one time visit, the methods to capture kidney-related outcomes (eGFR, spot urine ACR, and one-time manual blood pressure measurements) are not gold-standard methods to assess kidney-related outcomes. Furthermore, we acknowledge that the cutoff value of urine ACR we used (>30 mg/g), which is a surrogate for CKD in pediatric and adult populations, may not be applicable to this population. However, even when compared with a recent study of healthy 2 year-olds in the Netherlands,28 the median ACR (21 vs. 14 mg/g) and the rate of ACR >30 (36% vs. 24%) are both higher in our cohort. We also acknowledge that a large number of patients did not have kidney-related metrics measured at the 2 year cGA time point.
In conclusion, this analysis shows that ELGANs who receive rhEPO in the first postnatal weeks have lower rates of high SBP at two years of age. We did not find any evidence that rhEPO improves the rates of AKI or kidney-related outcomes at around 2 years cGA, except that a higher proportion of those randomized to placebo had SBP >90th percentile. This study also confirms that the kidney-related short and long-term events are very common in ELGANs. Studies that use gold-standard measurements, studies that evaluate interventions to limit or prevent these outcomes, and evaluation of the most cost-effective methods for screening this high risk population are greatly needed. In the meantime, neonatologists and pediatricians must discuss with families the risk of CKD in ELGANs.
Acknowledgements
We thank Lynn Dill, RN and Emily Pao for their assistance in coordinating the REPaIReD study, and to Dana Pass for preparation of the manuscript, none of whom have any reportable relations to industry, funding sources, or conflicts of interest. We thank the additional primary investigators, co-investigators, clinicians, research personnel, study team, and families who participated in the PENUT study.
Recombinant Erythropoietin for Protection of Infant Renal Disease (REPaIReD) Study is an NIH NIDDK funded (R01 DK103608) ancillary study designed to look at kidney outcome in patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (PENUT) which is an NIH NINDS funded (U01 NS077953, U01 NS077955) trial. The clinicaltrials.gov identifier is NCT01378273. Funding sources for this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. D.A. is consultant for Baxter, CHF Solutions Inc, and Medtronic; receives external grant funding for research and education for projects not related to this report from Baxter, CHF Solutions Inc, Medtronic, AKI Foundation, and National Institutes of Health. S.G. reports personal fees from and a position as a consultant to CHF Solutions Inc, Renibus, ExThera, Reata, and Medtronic Inc; receives grant funding from and serves as a consultant and on a Speaker’s Bureau for Baxter Healthcare, Inc; receives grant funding and serves as a consultant for BioPorto, Inc; and serves on a Speaker’s Bureau for Fresenius Medical Corporation. R.S. receives grant funding for studies not related to this project from NHLBI and PCORI.
List of Abbreviations:
- AKI
acute kidney injury
- ACR
albumin/creatinine ratio
- cGA
corrected gestational age
- CKD
chronic kidney disease
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- ELGANs
extremely low gestational age neonates
- GEE
generalized estimating equations
- NICU
neonatal intensive care unit
- PENUT
Preterm Epo Neuroprotection Trial
- rhEpo
recombinant human erythropoietin
- SBP
systolic blood pressure
- SCr
serum creatinine
Appendix
List of additional members of the PENUT Trial Consortium
PENUT Primary Investigators
Bryan A. Comstock1, Rajan Wadhawan2; Dennis E. Mayock1, Sherry E. Courtney3; Tonya Robinson4; Kaashif A. Ahmad5; Ellen Bendel-Stenzel6; Mariana Baserga7; Edmund F. LaGamma8; L. Corbin Downey9; Raghavendra Rao10; Nancy Fahim10; Andrea Lampland11; Ivan D. Frantz, III12; Janine Y. Khan13; Michael Weiss14; Maureen M. Gilmore15; Robin Ohls16; Nishant Srinivasan17; Jorge E. Perez18; Victor McKay19; Phuong T. Vu1; and the PENUT Trial Consortium
PENUT Co-Investigators
Billy Thomas3, Nahed Elhassan3, Sarah Mulkey3, Philip Dydynski4, Vivek K. Vijayamadhavan5, Neil Mulrooney6, Bradley Yoder7, Jordan S. Kase8, Jennifer Check9, Semsa Gogcu9, Erin Osterholm10, Sara Ramel10, Catherine Bendel10, Cheryl Gale10, Thomas George10, Michael Georgieff10, Tate Gisslen10, Sixto Guiang III10, Anne Hall10, Dana Johnson10, Katie Pfister10, Heather Podgorski10, Kari Roberts10, Erin Stepka10, Melissa Engel10, Heidi Kamrath10, Johannah Scheurer10, Angela Hanson10, Katherine Satrom10, Susan Pfister10, Ann Simones10, Erin Plummer10, Elizabeth Zorn10, Camilia R. Martin12, Deirdre O’Reilly12, Nicolas Porta13, Catalina Baza cliu14, Jonathan Williams14, Dhanashree Rajderkar14, Frances Northington15, Raul Chavez Valdez15, Sandra Beauman16, Patel Saurabhkumar17, Magaly Diaz-Barbosa18, Arturo Serize18, Jorge Jordan18
PENUT Research Coordinators
Debbie Ott1, Ariana Franco Mora1, Pamela Hedrick1, Vicki Flynn1, Amy Silvia2, Bailey Clopp2, John B. Feltner2, Isabella Esposito2, Stephanie Hauge2, Samantha Nikirk2, Andrea Purnell3, Emilie Loy3, Natalie Sikes3, Melanie Mason3, Jana McConnell3, Tiffany Brown3, Henry Harrison3, Denise Pearson3, Tammy Drake3, Jocelyn Wright3, Debra Walden3, Annette Guy3, Jennifer Nason4, Morgan Talbot4, Kristen Lee4, Sarah Penny4, Terri Boles4, Melanie Drummond5, Katy Kohlleppel5, Charmaine Kathen5, Brian Kaletka6, 11, Shania Gonzales6, 11, Cathy Worwa6, 11, Molly Fisher, 11, Tyler Richter6, 11, Alexander Ginder6, 11, Brixen Reich7, Carrie Rau7, Manndi Loertscher7, Laura Bledsoe7, Kandace McGrath7, Kimberlee Weaver Lewis7, Jill Burnett7, Susan Schaefer7, Karie Bird7, Clare Giblin8, Rita Daly8, Kristi Lanier9, Kelly Warden9, Jenna Wassenaar10, Jensina Ericksen10, Bridget Davern10, Mary Pat Osborne10, Brittany Gregorich10, Neha Talele12, Evelyn Obregon12, Tiglath Ziyeh12, Molly Clarke12, Rachel E Wegner12, Palak Patel12, Molly Schau13, Annamarie Russow13, Kelly Curry14, Susan Sinnamon14, Lisa Barnhart14, Charlamaine Parkinson15, Sandra Beauman16, Mary Hanson16, Elizabeth Kuan16, Conra Backstrom Lacy16, Edshelee M. Galvis18, Susana Bombino18, Denise Martinez19, Suzi Bell19, Corrie Long19
PENUT Follow-Up Personnel
Cathy Longa2, Michael Westerveld2, Stacy McConkey2, Anne Hay1, Niranjana Natarajan1, Shari Gaudette3, Sarah Cobb3, Gregory Sharp3, Elizabeth Schumacher4, Leslie Schuschke4, Charlotte Frey5, Mario Fierro5, Lois Gilmore6, Pamela Lundequam6, Ronald Hoekstra6, Anastasia Ketko6, Nina Perdue6, Sean Cunningham7, Kelly Stout7, Becky Hall7, Galina Morshedzadeh7, Betsy Ostrander7, Sarah Winter7, Lauren Cox8, Jordan S. Kase8, Matthew A. Rainaldi8, Sarah Hensley9; Melissa Morris9, Dia Roberts9, Semsa Gogcu9, Melissa Tuttle9; Christopher Boys10, Solveig Hultgren10, Elizabeth I. Pierpont10, Nancy Fahim10, Tom George10, Erin Osterholm10, Michael Georgieff10, Kelly E. King10, Katherine Bataglia11, Cathy Neis11, Mark Bergeron11, Cristina Miller11, Cara Accomando12, Jennifer Anne Gavin12, Elizabeth Maczek12, Susan Marakovitz12, Aimee Knorr12, Vincent C. Smith12, Jane E. Stewart12, Marie Weissbourd13, Raye-Ann deRegnier13, Nana Matoba13, Shelly C. Heaton12, Erika M. Cascio12, Janet Brady14, Suman Ghosh14, Jessica Ditto15, Mary Leppert15, Jean Lowe16, Janell Fuller16, Tara DuPont16, Robin Ohls16, Pamela Kloska17, Saurabh Patel17, Lauren Carbonell18, Anna Maria Patino-Fernandez18 Carmen de Lerma18, Susana Bombino18, Arturo Serize18, Kelly McDonough18, Maiana De Cortada18, Lacy Chavis19, Jane Shannon19
University of Washington Data Coordinating Center
Bryan A. Comstock1, Mark A. Konodi1, Christopher Nefcy1, Phuong T. Vu1
PENUT Follow-Up Committee
Karl C. K. Kuban20, Jean R. Lowe16, T. Michael O’Shea21
Radiology Committee
Manjiri Dighe1, Todd Richards1, Dennis W. W. Shaw1, Colin Studholme1, Christopher M. Traudt1
PENUT Executive Committee
Roberta Ballard22, Bryan A. Comstock1, Adam Hartman23, Scott Janis23, Dennis E. Mayock1, T. Robin Ohls16, Michael O’Shea21
DSMB
Ronnie Guillet 24, M. Bethany Ball25, Hannah Glass22, Ben Saville26, Michael Schreiber27
1. University of Washington (Seattle, Washington)
2. AdventHealth for Children, (Orlando, Florida)
3.University of Arkansas for Medical Sciences (Little Rock, Arkansas)
4. University of Louisville, (Louisville, Kentucky)
5. Methodist Children’s Hospital (San Antonio, Texas)
6. Children’s Hospital and Clinics of Minnesota (Minneapolis, MN)
7. University of Utah (Salt Lake City, Utah)
8. Maria Fareri Children’s Hospital at Westchester Medical Center (Valhalla, New York)
9. Wake Forest School of Medicine (Winston-Salem, North Carolina)
10. University of Minnesota Masonic Children’s Hospital (Minneapolis, Minnesota)
11. Children’s Minnesota (St. Paul, MN)
12. Beth Israel Deaconess Medical Center (Boston, Massachusetts)
13. Prentice Women’s Hospital (Chicago, Illinois)
14. University of Florida (Gainesville, Florida)
15. Johns Hopkins University (Baltimore, Maryland)
16. University of New Mexico (Albuquerque, New Mexico)
17. Children’s Hospital of the University of Illinois (Chicago, Illinois)
18. South Miami Hospital (South Miami, Florida)
19. Johns Hopkins All Children’s Hospital (St. Petersburg, Florida)
20. Boston University Medical Center (Boston, Massachusetts)
21. University of North Carolina School of Medicine (Chapel Hill, North Carolina)
22. University of California San Francisco School of Medicine (San Francisco, California)
23. National Institute of Neurological Disorders and Stroke
24. University of Rochester Medical Center (Rochester, NY)
25. Stanford University and Lucile Packard Children’s Hospital (Palo Alto, California)
26. Vanderbilt University Medical Center (Nashville, TN)
27. University of Chicago (Chicago, IL)
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barker DJ, Martyn CN. The fetal origins of hypertension. Advances in nephrology from the Necker Hospital. 1997;26:65–72. [PubMed] [Google Scholar]
- [2].Barker DJ, Osmond C. Low birth weight and hypertension. Bmj. 1988;297:134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91:1660–6. [DOI] [PubMed] [Google Scholar]
- [4].Fall CH, Barker DJ. The fetal origins of coronary heart disease and non-insulin dependent diabetes in India. Indian pediatrics. 1997;34:5–8. [PubMed] [Google Scholar]
- [5].Barker DJ. The fetal origins of coronary heart disease. Acta paediatrica. 1997;422:78–82. [DOI] [PubMed] [Google Scholar]
- [6].Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131:1168–79. [DOI] [PubMed] [Google Scholar]
- [7].White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–61. [DOI] [PubMed] [Google Scholar]
- [8].Askenazi DJ, Heagerty PJ, Schmicker RH, Griffin R, Brophy P, Juul SE, et al. Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nekoui A, Blaise G. Erythropoietin and Nonhematopoietic Effects. Am J Med Sci. 2017;353:76–81. [DOI] [PubMed] [Google Scholar]
- [10].Cantarelli C, Angeletti A, Cravedi P. Erythropoietin, a multifaceted protein with innate and adaptive immune modulatory activity. Am J Transplant. 2019;19:2407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moore E, Bellomo R. Erythropoietin (EPO) in acute kidney injury. Annals of intensive care. 2011;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–24. [DOI] [PubMed] [Google Scholar]
- [13].Solling C, Christensen AT, Krag S, Frokiaer J, Wogensen L, Krog J, et al. Erythropoietin administration is associated with short-term improvement in glomerular filtration rate after ischemia-reperfusion injury. Acta anaesthesiologica Scandinavica. 2011;55:185–95. [DOI] [PubMed] [Google Scholar]
- [14].Souza DS, Spencer DM, Salles TS, Salomao MA, Payen E, Beuzard Y, et al. Death switch for gene therapy: application to erythropoietin transgene expression. Braz J Med Biol Res. 2010;43:634–44. [DOI] [PubMed] [Google Scholar]
- [15].Coldewey SM, Khan AI, Kapoor A, Collino M, Rogazzo M, Brines M, et al. Erythropoietin attenuates acute kidney dysfunction in murine experimental sepsis by activation of the beta-common receptor. Kidney Int. 2013;84:482–90. [DOI] [PubMed] [Google Scholar]
- [16].Song YR, Lee T, You SJ, Chin HJ, Chae DW, Lim C, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. 2009;30:253–60. [DOI] [PubMed] [Google Scholar]
- [17].Oh SW, Chin HJ, Chae DW, Na KY. Erythropoietin improves long-term outcomes in patients with acute kidney injury after coronary artery bypass grafting. J Korean Med Sci. 2012;27:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Skrifvars MB, Moore E, Martensson J, Bailey M, French C, Presneill J, et al. Erythropoietin in traumatic brain injury associated acute kidney injury: A randomized controlled trial. Acta Anaesthesiol Scand. 2019;63:200–7. [DOI] [PubMed] [Google Scholar]
- [19].Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol. 2015;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N Engl J Med. 2020;382:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Go H, Momoi N, Kashiwabara N, Haneda K, Chishiki M, Imamura T, et al. Neonatal and maternal serum creatinine levels during the early postnatal period in preterm and term infants. PLoS One. 2018;13:e0196721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thayyil S, Sheik S, Kempley ST, Sinha A. A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants. J Perinatol. 2008;28:226–9. [DOI] [PubMed] [Google Scholar]
- [23].Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017;376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fuhrman DY, Schneider MF, Dell KM, Blydt-Hansen TD, Mak R, Saland JM, et al. Albuminuria, Proteinuria, and Renal Disease Progression in Children with CKD. Clin J Am Soc Nephrol. 2017;12:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gracchi V, van den Belt SM, Kupers LK, Corpeleijn E, de Zeeuw D, Heerspink HJ. Prevalence and distribution of (micro)albuminuria in toddlers. Nephrol Dial Transplant. 2016;31:1686–92. [DOI] [PubMed] [Google Scholar]
- [29].Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140. [DOI] [PubMed] [Google Scholar]
- [30].Diggle PJ, Heagerty P, Liang KY, Heagerty PJ and Zeger S,. Analysis of longitudinal data. : Oxford University Press; 2002. [Google Scholar]
- [31].Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]