Abstract
Obsessive compulsive disorder (OCD) is associated with disruption of sensorimotor gating, which may contribute to difficulties inhibiting intrusive thoughts and compulsive rituals. Neural mechanisms underlying these disturbances are unclear; however, striatal dopamine is implicated in regulation of sensorimotor gating and OCD pathophysiology. The goal of this study was to examine the relationships between sensorimotor gating, compulsive behavior, and striatal dopamine receptor levels in Sapap3 knockout mice (KOs), a widely used preclinical model system for OCD research. We found a trend for disruption of sensorimotor gating in Sapap3-KOs using the translational measure prepulse inhibition (PPI); however, there was significant heterogeneity in both PPI and compulsive grooming in KOs. Disruption of PPI was significantly correlated with a more severe compulsive phenotype. In addition, PPI disruption and compulsive grooming severity were associated with reduced dopamine D1 and D2/3 receptor density in the nucleus accumbens core (NAcC). Compulsive grooming progressively worsened in Sapap3-KOs tested longitudinally, but PPI disruption was first detected in high-grooming KOs at 7 months of age. Through detailed characterization of individual differences in OCD-relevant behavioral and neurochemical measures, our findings suggest that NAcC dopamine receptor changes may be involved in disruption of sensorimotor gating and compulsive behavior relevant to OCD.
Subject terms: Neuroscience, Diseases of the nervous system, Sensorimotor processing, Obsessive compulsive disorder
Introduction
Obsessive compulsive disorder (OCD) is a severe neuropsychiatric disorder affecting 1–3% of the population1–3. Intrusive thoughts (obsessions) and uncontrollable actions (compulsions) are the core clinical features of OCD. Subjects with OCD also show disturbances in sensorimotor gating4–8, a filtering process that impacts the content and organization of what is consciously perceived by an individual. It has been proposed that sensorimotor gating deficits in OCD may facilitate intrusive thoughts and impair inhibitory control over compulsive actions, although these relationships have not been clearly demonstrated in patients.
Underlying mechanisms responsible for disruption of sensorimotor gating in OCD are unclear; however, disturbances in striatal dopamine have been implicated in both the regulation of sensorimotor gating and manifestation of compulsive behavior. In rodent models sensorimotor gating is disrupted by increased dopamine neurotransmission following systemic administration of dopamine agonists/releasers9, 10 or site specific manipulations in dorsal or ventral striatum11–13. Dopamine system disturbances have also been described in the striatum in patients with OCD14–18 using in vivo molecular imaging with single photon emission computerized tomography (SPECT) and positron emission tomography (PET). Binding of striatal dopamine D1 and D2/3 receptors is reduced in OCD patients14–16, 19, and dopamine transporter binding is increased17, 20. It has been proposed that these alterations may represent compensatory changes in response to elevated dopamine tone, consistent with evidence that transdiagnostic compulsive behaviors are associated with elevated striatal dopamine. Specifically, increased cue-evoked striatal dopamine release has been observed via PET imaging in cocaine users, subjects with binge eating disorder, and patients with Parkinson’s disease who develop compulsive behavior following dopamine agonist treatment21–24. There is also evidence that drugs that increase dopamine signalling can exacerbate compulsive behavior in OCD patients25. Further supporting the role of elevated dopamine in the manifestation of OCD symptoms, dopamine antagonists can be useful as an adjunct therapy in OCD patients that don’t show clinical improvement following treatment with first-line selective serotonin reuptake inhibitors (SSRIs)26. Together, these results suggest a potential relationship between alterations in striatal dopamine signalling and disruption of both sensorimotor gating and compulsive behavior in OCD.
Preclinical model systems can be used to gain mechanistic insight into the relationship between dopamine signalling, impaired sensorimotor gating, and other OCD-relevant behaviors using a straightforward translational paradigm: prepulse inhibition (PPI) of the acoustic startle response27. For example, studies in mouse models suggest that elevated striatal dopamine and disruption of dorsal striatum function may have relevance to disruption of PPI in Tourette Syndrome28, 29. Although prior studies have demonstrated PPI deficits in pharmacologic models of relevance to OCD30, 31, sensorimotor gating deficits and their relationship to compulsive behaviors have not been characterized in transgenic mouse models. Here, we examined PPI in Sapap3 knockout mice (KOs), a widely used model system for OCD research32–34 that displays compulsive grooming, anxiety32, and cognitive changes in tasks examining reversal learning35, 36 and habit learning37, 38. Sapap3-KOs also show hyperactivity in the dorsal and central striatum (CS)32–34, 39 that would be predicted to influence PPI; in contrast, the ventral striatum has not been thoroughly characterized in this model system40. The goal of this study was therefore to probe the relationship between PPI, striatal dopamine receptor levels, and compulsive behavior in Sapap3-KOs using behavioral testing and ex vivo autoradiography.
Results
Disruption of PPI is associated with compulsive grooming phenotype in Sapap3-KOs
PPI was examined in male Sapap3-KO and wild-type (WT) control mice at ~ 8 months of age, when approximately half of KOs (10/18) showed lesions resulting from compulsive grooming. Analysis of average %PPI revealed a trend for differences between genotypes (Fig. 1A; unpaired Welch’s T-test: p = 0.10, t27.28 = 1.70), with both groups showing similar increased PPI with higher prepulse intensities (Figure S1A; main effect prepulse, F(2,56) = 87.4, p < 0.0001). However, visual inspection of the data and an F test to compare variance between the groups demonstrated more variability in KOs compared to WTs (F(17,11) = 3.3, p = 0.0499), prompting us to determine whether this heterogeneity within KOs mapped onto other behavioral measures. As expected, Sapap3-KOs groomed significantly more than WTs (Fig. 1B; Mann–Whitney test: WT: M = 38.5, KO: M = 628, p < 0.0001). In addition, we determined that a subgroup of Sapap3-KOs with skin lesions (KO-L, n = 10, unfilled symbols Fig. 1B) showed elevated grooming relative to KOs without lesions (KO-NL, n = 8, filled symbols), supporting the idea that lesions result from elevated grooming. However, KO-NL also groomed significantly more than WTs (Bonferroni post-hoc test, p = 0.012; also see Figure S3C-E for data from an independent cohort).
Figure 1.
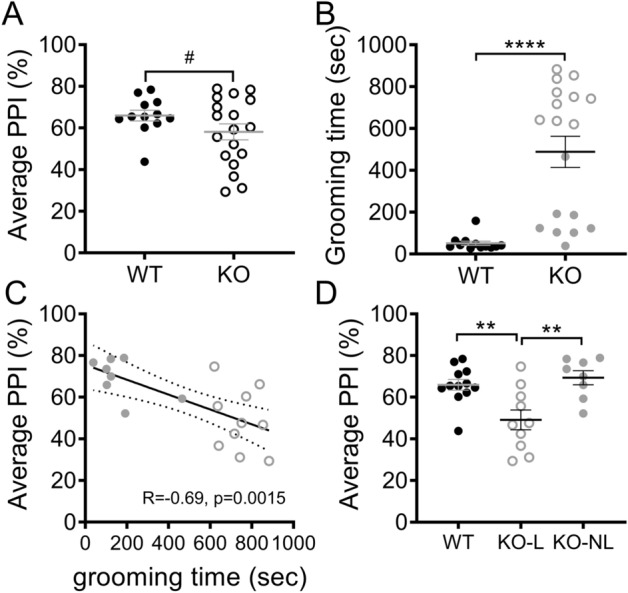
Disruption of PPI is associated with compulsive grooming phenotype in Sapap3-KOs. (A) PPI (average % across 3 prepulse intensities tested) did not differ between Sapap3-KO and WT mice. (B) Time spent grooming was significantly elevated in Sapap3-KO relative to WT, as previously described. Unfilled grey symbols denote KO-L, filled grey symbols denote KO-NL. (C) Average PPI was significantly negatively correlated with time spent grooming, suggesting disruption of PPI in Sapap3-KOs with the most severe compulsive grooming phenotype. (D) When Sapap3-KOs were separated based on the presence (KO-L) or absence (KO-NL) of lesions resulting from compulsive grooming, significant disruption of PPI in KO-L was revealed relative to WT and KO-NL (Bonferroni post-hoc tests: WT vs KO-L, p = 0.006; WT vs KO-NL, p > 0.99, KO-L vs KO-NL p = 0.003). In panel A, # indicates results of unpaired Welch’s T test, in panel B * indicates results of Mann–Whitney test, in panel D * indicate results of Bonferroni post-hoc test. **** p < 0.0001; ** p < 0.01; # p = 0.10. n = 12 WT, 18 KO (10 KO-L, 8 KO-NL). PPI prepulse inhibition, KO knockout, KO-L KOs with lesions, KO-NL KOs without lesions, WT wild-type.
Given the significant heterogeneity in compulsive grooming phenotype in Sapap3-KOs, the relationship between PPI and grooming levels was assessed to determine whether PPI deficits were observed in mice with more severe compulsive grooming. In Sapap3-KOs, average %PPI was significantly negatively correlated with time spent grooming (Fig. 1C; KO: R = − 0.69, p = 0.0015; WT: R = − 0.10, p = 0.75), and was selectively impaired in KO-L relative to WTs and KO-NL (Fig. 1D; main effect of group, F(2,27) = 8.5, p = 0.0013). Further examination of this heterogeneity using unbiased k-means clustering of grooming and PPI data showed that KO mice could be grouped into three clusters that largely corresponded with lesion status (Figure S2). Interestingly, cluster 1 had elevated grooming and impaired PPI (all KO-L), cluster 2 had elevated grooming but intact PPI (all but one KO-L) and cluster 3 was unimpaired for both behaviors (all KO-NL).
Importantly, startle amplitude during blocks 2 and 3, which is used to calculate %PPI, did not differ between groups (main effect of group, p = 0.43; Figure S1B).
Individual differences in compulsive grooming and disruption of PPI in Sapap3-KOs are associated with changes in striatal dopamine receptor binding density
To determine whether heterogeneous disruption of PPI and grooming severity in Sapap3-KOs were associated with changes in striatal dopamine receptor density, ex vivo autoradiography was used to measure binding of D1 and D2/3 in CS and ventral striatum (nucleus accumbens core; NAcC) of animals described in Fig. 1 (see Fig. 2A for representative binding and regions of interest). Receptor densities were then correlated with behavioral measures in Sapap3-KOs. D2/3 receptor density was significantly different between the genotypes (Fig. 2B; main effect genotype, F(1,25) = 4.6, p = 0.042), and there was a trend for interaction with striatal region of interest suggesting that this effect may be more prominent in NAcC (genotype × region interaction, p = 0.097). Reduced D2/3 density in KOs was also associated with individual differences in compulsive grooming and disruption of PPI. Again this effect was selective to NAcC, with reduction of D2/3 binding associated with both disruption of PPI (Fig. 2C, R = 0.59, p = 0.017) and increased grooming (Fig. 2D, R = − 0.68, p = 0.004). In contrast, D2/3 density in CS showed no association with these OCD-relevant behaviors (Fig. 2E,F; PPI: p = 0.46, R = 0.20; grooming: p = 0.72, R = − 0.01).
Figure 2.
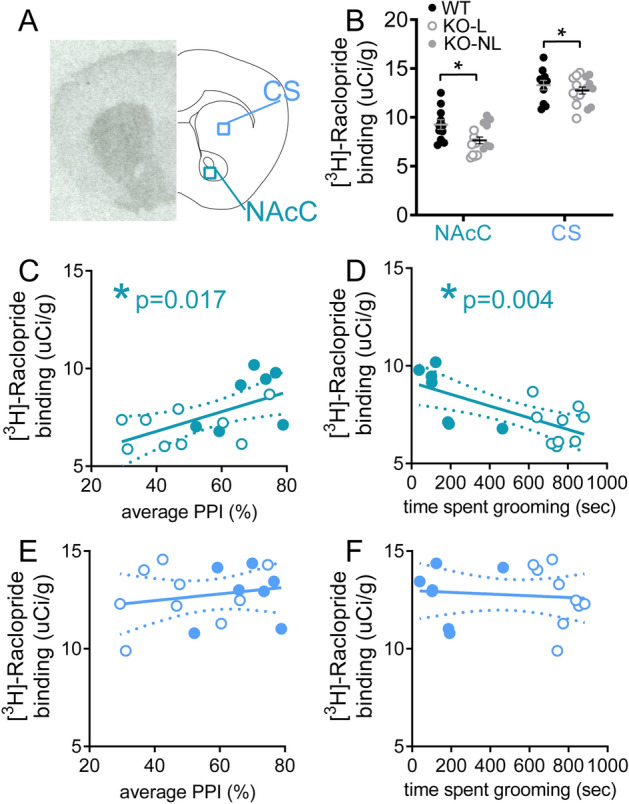
Reduced dopamine D2/3 receptor density in NAcC is associated with severity of OCD-relevant phenotypes in Sapap3-KO mice. (A) Autoradiography was performed in striatal tissue to quantify density of D2/3 receptors in NAcC and CS using [3H]-Raclopride. (Left) Representative autoradiograph for total binding. (Right) Schematic of coronal brain section with regions of interest used for analysis. (B) D2/3 was significantly reduced in Sapap3-KO. Reduced D2/3 binding in NAcC was associated with lower %PPI (C) and increased severity of compulsive grooming (D) in Sapap3-KOs. D2/3 density in CS showed no association with %PPI (E) or grooming (F) in Sapap3-KOs. In panel B, * indicates significant difference between WT and KOs (p < 0.05), in panels C-D * indicates significant correlation (p < 0.05). In panels B-F, unfilled KO symbol indicates KO-L, filled KO symbol indicates KO-NL. n = 11 WT, 16 KO. PPI prepulse inhibition, CS central striatum, NAcC Nucleus accumbens core, KO Sapap3 knockout, KO-L KOs with lesions, KO-NL KOs without lesions, WT wild-type.
D1 receptor density was not significantly different between genotypes (Fig. 3A shows representative binding and regions of interest; Fig. 3B, p = 0.74); however, reduced D1 receptor density was associated with increased severity of OCD-relevant behaviors in Sapap3-KO mice. Specifically, in the NAcC, disruption of PPI was significantly correlated with reduced D1 density (Fig. 3C, NAcC: R = 0.54, p = 0.021), and there was a trend for correlation between increased grooming and reduced D1 density (Fig. 3D, NAcC: R = − 0.43, p = 0.074). In CS, there was also a trend for correlation between disruption of PPI and reduced D1 density (Fig. 3E, CS: R = 0.40, p = 0.096), but D1 density was not associated with individual differences in grooming severity (Fig. 3F, R = − 0.35, p = 0.16).
Figure 3.
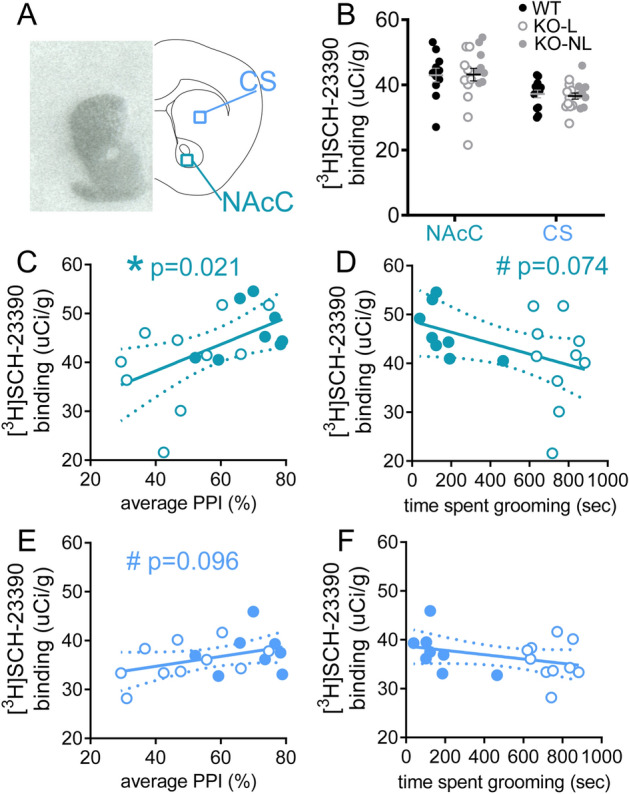
Reduced dopamine D1 receptor density in NAcC is associated with severity of disruption of PPI in Sapap3-KO mice. (A) Autoradiography was performed in striatal tissue to quantify density of D1 receptors in CS and NAcC using [3H]-SCH-23390. Representative autoradiograph for total binding is shown (left) and schematic of coronal brain section (right). (B) D1 density was unchanged in Sapap3-KOs. In Sapap3-KOs, reduced D1 in NAcC was significantly correlated with reduced PPI (C) and showed a trend association with severity of compulsive grooming (D). D1 density in CS showed a trend correlation with PPI (E) and no association with compulsive grooming in Sapap3-KOs (F). * indicates significant correlation, # indicates trend correlation (0.05 < p < 0.10). In panels (B–F), unfilled KO symbol indicates KO-L, filled KO symbol indicates KO-NL. n = 13 WT, 18 KO. PPI prepulse inhibition, CS central striatum, NAcC Nucleus accumbens core, KO Sapap3 knockout, KO-L KOs with lesions, KO-NL KOs without lesions, WT wild-type.
Dopamine transporter binding density is increased in striatum of Sapap3-KO mice
Striatal dopamine system changes were also examined by measuring binding density for the dopamine transporter (DAT) using ex vivo autoradiography (Fig. 4A shows representative binding and regions of interest). DAT density was increased in Sapap3-KOs compared to WT littermates (Fig. 4B, main effect of genotype, F(1,29) = 4.8; p = 0.037). There was a trend for genotype x striatal subregion interaction (p = 0.094), suggesting that this effect may be more prominent in CS. In contrast to striatal dopamine receptor densities, DAT binding density in NAcC and CS was not correlated with changes in PPI or grooming in Sapap3-KOs (NAcC: Fig. 4C,D, PPI p = 0.36, grooming p = 0.52; CS: Fig. 4E,F; PPI p = 0.71, grooming p = 0.62).
Figure 4.
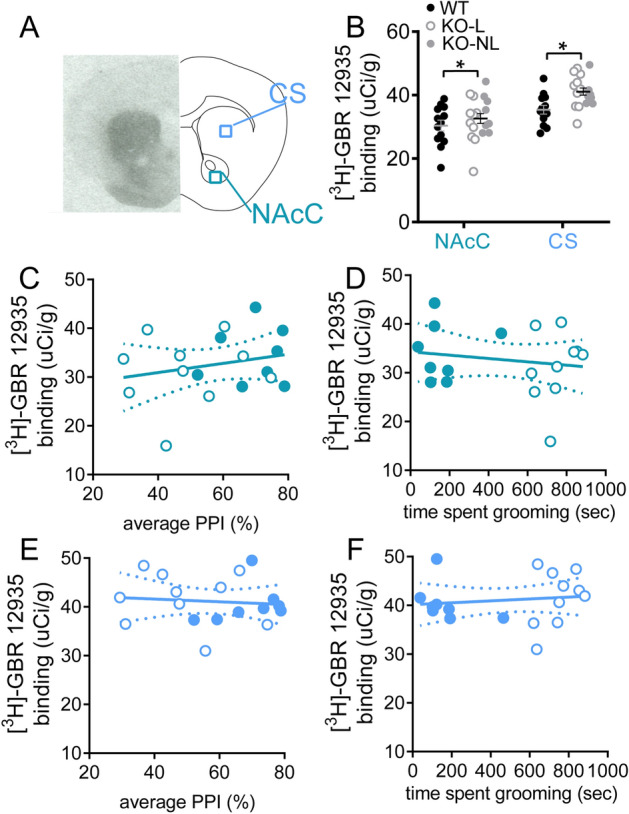
DAT binding in striatum is increased in Sapap3-KOs. (A) Autoradiography was performed in striatal tissue to quantify density of DAT in CS and NAcC using [3H]-GBR-12935. Representative autoradiograph for total binding is shown in the left panel, with schematic of coronal brain section on the right. (B) DAT density was elevated in Sapap3-KOs. DAT binding in NACc was not correlated with disruption of PPI (C) or grooming severity (D) in Sapap3-KOs. DAT binding in CS showed no association with PPI (E) or grooming (F) in Sapap3-KOs. In panel (B), * indicates significant difference between WT and KO (p < 0.05). In panels (B–F), unfilled KO symbol indicates KO-L, filled KO symbol indicates KO-NL. n = 13 WT, 18 KO. PPI prepulse inhibition, CS central striatum, NAcC Nucleus accumbens core, DAT dopamine transporter, KO Sapap3 knockout, KO-L KOs with lesions, KO-NL KOs without lesions, WT wild-type.
Comparison of the developmental progression of compulsive grooming, lesions, and PPI impairment
It is hypothesized that impaired sensorimotor gating in OCD may contribute to disruption of inhibitory control over compulsive actions. To test whether disruption of PPI in Sapap3-KOs might precede development of the compulsive grooming phenotype, and thereby contribute to the onset of compulsive behavior, PPI and grooming were both assessed monthly from 2 months (before the development of lesions) until 8 months of age (when approximately half of KOs have lesions) in male and female Sapap3-KOs and WT littermates. Time spent grooming progressively increased with age (Fig. 5A, genotype × age interaction: F(6,270) = 15.8, p < 0.0001, trajectories of individual animals are shown in Figure S3A), with significantly elevated grooming detected at 4 months in Sapap3-KOs (Bonferroni post-hoc tests). The number of Sapap3-KOs with lesions resulting from severe compulsive grooming increased with age (Figure S3C-E; 6 months: n = 5; 7 months: n = 9; 8 months: n = 12). Note, in other cohorts tested weekly in our laboratory at an earlier age, a modest elevation in grooming was detected relative to WT littermates as early as 7 weeks of age (Figure S4; 39); however, this small effect-size difference was not detectable during the less frequent (monthly) testing performed in the current study.
Figure 5.
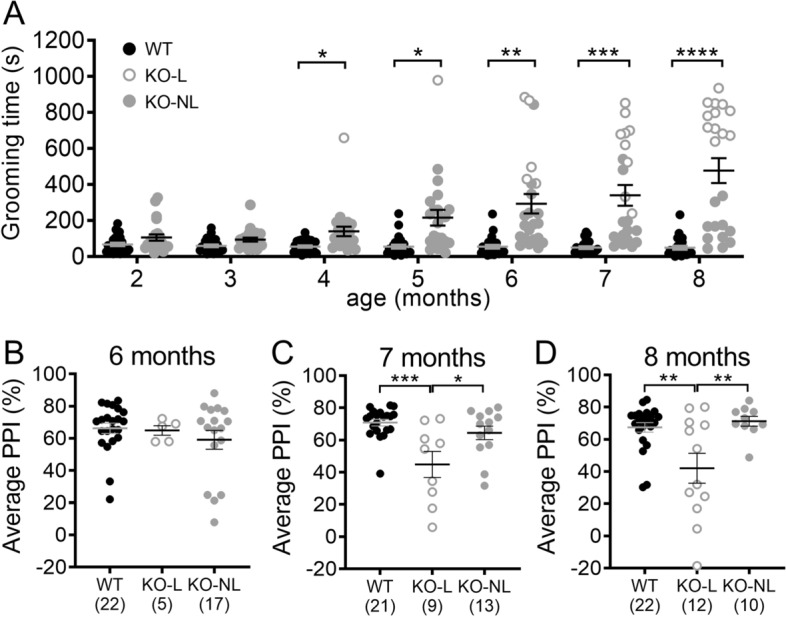
Comparison of the developmental progression of excessive grooming, lesions, and PPI impairment. (A) Longitudinal progression of compulsive grooming phenotype in Sapap3-KOs from 2–8 months of age. Unfilled grey symbols are KO-L; grey filled symbols are KO-NL. Grooming was significantly increased in Sapap3-KOs relative to WT from 4 months of age. (B–D) PPI was compared between groups at 6–8 months of age when startle did not significantly differ between genotypes (see Figure S5B). Although there are no group differences at 6 months of age, PPI disruption emerges in KO-L at 7 months of age. * indicates significant Bonferroni post-hoc test comparing KO to WT (panel A) or KO-L to comparison group (panel B–D). **** p < 0.0001, ** p < 0.01, * p < 0.05. n = 24 WT, 23 KO (panel A). KO Sapap3 knockout, KO-L KOs with lesions, KO-NL KOs without lesions, WT wild-type, PPI prepulse inhibition.
When comparing PPI between WTs and KOs across development (not taking grooming severity into account), there was a trend towards reduced PPI in KOs (Figure S5A, p = 0.095), but no interaction with age (p = 0.93) (note: PPI increased with age consistent with previous reports41, 42; main effect of age: F(6,270) = 29.2, p < 0.0001). However, analysis of startle amplitude revealed age-dependent genotype differences (Figure S5B, genotype x age interaction F(6,270) = 3.8, p = 0.001). Whereas startle amplitude decreased in WT mice as they aged (consistent with other studies in mice41, 42), KOs had relatively stable startle amplitude over time. Since group differences in startle amplitude confound interpretation of PPI, we therefore focused analysis on 6–8 months of age when startle amplitude did not differ between the groups (Figure S5C-E). PPI was compared between KO-L, KO-NL and WT to account for effects of compulsive grooming phenotype. This demonstrated that PPI was not significantly different between groups at 6 months (Fig. 5B, F(2,41) = 0.7, p = 0.49); however, KO-L showed reduced PPI relative to the other two groups at 7 and 8 months (Fig. 5C,D, 7 months: F(2,40) = 9.2, p = 0.0005; 8 months: F(2,41) = 7.8, p = 0.0013). PPI was significantly correlated with grooming at 8 months of age (R = − 0.59, p = 0.003), but not at 6–7 months of age (Figure S6); unbiased clustering replicated the presence of 3 sub-types within Sapap3-KOs at 8 months of age (Figure S7). At earlier ages during longitudinal testing, these clusters differed in levels of grooming (clusters 1 and 2 groomed more than cluster 3), but not in PPI (data not shown).
Discussion
Here, we demonstrate that Sapap3-KOs show impaired sensorimotor gating as measured by disruption of PPI, but found that these deficits only manifested in a subset of mice that had developed a severe compulsive grooming phenotype. Autoradiography revealed that disruption of PPI was associated with reduced D1 and D2/3 receptor binding in the NAcC. In addition, severity of compulsive grooming was associated with reduced D2/3 binding in NAcC. Finally, DAT binding was elevated in KOs independent of the striatal region examined. Our findings are consistent with PET/SPECT imaging in patients with OCD, and may reflect compensatory responses to striatal hyperdopaminergic tone that are more prominent in the NAcC. Together, these observations suggest that alterations in dopamine signalling within the NAcC may contribute to OCD-relevant behavioral disturbances in Sapap3-KOs, and that longer exposure to altered dopamine signalling may be necessary to impact PPI relative to grooming.
The observed association between severity of compulsive grooming and disruption of PPI in the Sapap3-KO model could reflect that one of these behaviors plays a causal role in the presentation of the other, or that a common neural mechanism(s) contributes to the development or manifestation of both behaviors. Our findings suggest that if a causal relationship is present, compulsive behavior influences disruption of PPI, and not the other way around. This conclusion is based on earlier onset of the severe grooming phenotype, as well as observation of PPI impairment in only a subset of high-grooming mice. A clear relationship between PPI and other OCD symptoms has not been established in previous clinical studies. Early studies in medicated patients demonstrated that severity of PPI impairment was significantly associated with severity of OCD symptoms as measured by the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)7, 8. Although these associations have not been replicated in more recent studies examining unmedicated OCD patients4, 6, reduced PPI in patients with tics was noted in one of these samples, supporting the hypothesis that disruption of PPI may be associated with lack of inhibitory control over repetitive actions4, 5. Future studies seeking to examine the relationship between OCD symptoms and other behavioral measures will likely benefit from a large sample that reflects the broad range of patient severity (and subtypes) present across the diagnosis of OCD, similar to our current examination of PPI in Sapap3-KOs displaying a range of severity of compulsive behavior.
Interestingly, we recently demonstrated that disruption of cognitive task is not associated with severity of compulsive grooming in Sapap3-KOs36, and these findings have been replicated by other laboratories35,43. Others have also recently demonstrated aberrant habit learning in Sapap3-KOs, and have begun to examine potential relationships between compulsive grooming and flexible cognition in this context37, 38. In the first study, increased habitual behavior in Sapap3-KOs (insensitivity to outcome devaluation) was not directly compared to compulsive grooming38. However, mice were 7–12 weeks old at the start of habit training, suggesting that a severe compulsive grooming phenotype was not present based on the results of our study. In the second study, which used different testing conditions that specifically promoted habitual behavior in WT mice, Sapap3-KOs showed more goal-directed behavior (sensitive to outcome devaluation). Although analysis of the relationship between habitual behavior and compulsive grooming was underpowered, there was a trend for a bias towards habitual behavior in low grooming Sapap3-KOs and flexible goal-directed behavior in high grooming Sapap3-KOs37. These studies of cognitive flexibility largely support the idea that OCD-relevant cognitive changes and compulsive grooming are distinct behavioral domains in Sapap3-KOs, whereas our current findings suggest that compulsive grooming and disruption of PPI in Sapap3-KOs may be related. The Sapap3-KO model therefore serves as a useful system for further dissection of the contributions of overlapping versus distinct circuits associated with different OCD-relevant behavioral domains. This is an important question given that distinct patterns of activity in striatum and prefrontal cortex are associated with symptoms vs cognitive deficits44. Better understanding of these behavior-specific neural changes may thus be valuable for guiding the development of more effective treatments.
Striatal dopamine signalling is implicated in both the pathophysiology of OCD and regulation of PPI. Our autoradiography results are consistent with PET/SPECT imaging studies demonstrating reduced D2/3 and D1 binding in striatum of OCD patients15, 16, 19. Compulsive behavior and disruption of PPI are both typically associated with a hyperdopaminergic state in striatum13, 21–25, 45. Given this, it is plausible that the observed correlations in Sapap3-KOs between severity of these OCD-relevant phenotypes and reduced dopamine receptor density may reflect compensatory downregulation of receptors in response to increased dopamine tone. This has been proposed as a likely mechanism for decreased striatal dopamine receptor binding observed using PET imaging in OCD patients16, and is supported by our observation of increased DAT binding density in Sapap3-KOs which may reflect increased dopaminergic innervation/release (Fig. 4B). Interestingly, our prior study assessing the concentration of dopamine and its metabolites in ex vivo striatum using high performance liquid chromatography (HPLC) did not provide evidence of hyperdopaminergic tone in Sapap3-KO mice46. This suggests that measurement of behavior-associated dopamine release in vivo may be necessary to identify the changes in striatal dopamine suggested by our data. This possibility is in line with clinical studies demonstrating that cues associated with compulsive behavior are necessary to detect increased striatal dopamine in disorders associated with compulsive behavior21–24.
To our knowledge, these studies are the first to thoroughly investigate the longitudinal progressive development of the compulsive grooming phenotype in Sapap3-KOs, and raise the important question of whether there are distinct neural changes that lead to low vs high grooming KOs. Recently it has been reported that Sapap3-KO mice do not show age-dependent changes in grooming phenotype in a study using a cross-sectional design in a group of ~ 2–5 month old Sapap3-KO mice47. In contrast, our within-subjects data tracking grooming from 2–8 months of age suggests that distinct trajectories for development of compulsive behavior exist among individual Sapap3-KOs. We show that grooming progressively escalates to a point where it continues despite the presence of skin lesions in a subset of KOs, and that mice with lesions show significantly elevated grooming relative to non-lesioned KO mice (Fig. 1D and S3C-E). Interestingly, we also show that there is evidence of increased grooming in KOs at an early age (7–8 weeks), almost 2 months prior to the first emergence of lesions. This early increase in grooming could reflect a premorbid phenotype with a different neural substrate, compared to the later emerging compulsive grooming behavior which persists despite negative consequences (e.g. development of lesions32). However, we cannot rule out negative consequences of this early change in grooming that we have not measured (e.g. changes in sleeping and social behavior). Importantly, this behavioral change in early adulthood may provide insight regarding neural mechanisms early in pathophysiological development that could be targeted by preventive interventions.
Given the significant heterogeneity in compulsive grooming severity in Sapap3-KOs, observed both within animals (across time) and between animals at a given age, it will be important to report grooming severity in future research detailing the molecular, cellular, and circuit disturbances present in this model. Careful examination of these individual differences may help to understand neural mechanisms associated with resilience vs susceptibility to the development of compulsive behavior. Although this is outside the scope of the current studies, earlier stage and longitudinal measurements of neural changes (e.g. in vivo striatal dopamine measures and ex vivo dopamine receptor levels) associated with the emergence of the compulsive grooming phenotype will provide important insight about underlying pathophysiological mechanisms.
There are several limitations of these studies that will be important to address in future research. First, although there is some evidence that rare Sapap3 variants play a role in OCD48, Sapap3 is not a leading candidate for OCD genetic risk (though note that current genome wide association studies in OCD are underpowered49). However, our lab recently demonstrated decreased Sapap3 gene expression in orbitofrontal cortex and striatum in post mortem tissue from human subjects with OCD, supporting a potential role for Sapap3 in OCD pathophysiology50. In further support of its use for OCD research, the Sapap3-KO mouse has abnormalities in OCD-relevant behavioral constructs (compulsive behavior, anxiety, executive dysfunction, and now disruption of sensorimotor gating), and exhibits therapeutic response to treatments that can be effective in OCD patients including repeated fluoxetine administration and deep brain stimulation32, 51. Nonetheless, further examination of the relationship between striatal dopamine measures, compulsive behavior, and sensorimotor gating is warranted in other OCD-relevant model systems and human subjects with OCD to better understand the relevance of our findings to OCD pathophysiology. Another limitation is that these studies rely on correlative evidence to link striatal dopamine receptor changes with severity of OCD-relevant behaviors. We propose that our observations provide indirect evidence that increased striatal dopamine may be a common mechanism for generation of distinct OCD-relevant behaviors. We believe these findings warrant further causal mechanistic examination of dopaminergic regulation of behavioral disturbances relevant to OCD, which is beyond the scope of the current studies.
These studies are the first to demonstrate an association between the development of OCD-relevant compulsive grooming and disruption of PPI in a transgenic mouse model. Through ex vivo neurochemical analysis of brain tissue from mice that have undergone behavioral testing, we have identified a potential role of reduced NAcC dopamine receptor density in the manifestation of these maladaptive behaviors. By leveraging heterogeneity in the penetrance and progression of OCD-relevant behaviors in this preclinical model system, we have highlighted associations that may be relevant to neural changes occurring in distinct subsets of patients with OCD and related disorders.
Methods
Animals
Male and female Sapap3-KO and WT littermates were bred from heterozygous x heterozygous breeding pairs on a C57/BL6 background, and were derived from a colony initially established at MIT by Dr Guoping Feng32. Mice were housed in groups of 2–5 same-sex mice in individually ventilated cages, with ad libitum access to standard chow and drinking water, in 12:12 light/dark cycle. All behavioral testing was conducted in the light cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, and all methods were carried out in compliance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. This study was also carried out in compliance with the ARRIVE guidelines.
Prepulse inhibition testing
PPI was tested using SR-LAB startle response system (San Diego Instruments, San Diego, CA) with methods similar to those previously described9, 27, 52, to examine the effects of three prepulse intensities (3, 6 and 12 dB above background noise levels) on the magnitude of acoustic startle reflex elicited by 120 dB pulse of noise. All mice were tested in “startle only” session (10 × 120 dB pulse alone trials) prior to first PPI session for habituation to testing procedures. Following standard protocols, startle blocks 2 and 3 were used to calculate PPI, and startle blocks 2/3 were compared to startle blocks 1 and 4 to assess startle habituation27. PPI is calculated as follows:
Because these studies examined mice at an age where poor hearing could potentially impact PPI, mice were excluded from analysis if they exhibited very weak startle response to pulse alone trials relative to measurements made in the absence of a startling pulse (“no stimulus” trials, Figure S1D). The following criteria were used to exclude mice with weak startle reflex likely reflecting poor hearing: (average of “pulse alone” trial amplitudes in blocks 2&3) < (3 × average of “no stimulus” trial amplitudes).
Grooming testing
Prior to first grooming test, mice were habituated to acrylic testing chambers (8 × 8 × 12 inches) for 2 consecutive days (20 min/session). Mice were then videotaped for a 20 min session, and grooming was manually scored offline by trained experimenters blind to genotype and PPI values. Grooming time is calculated as the sum of time spent grooming the face, grooming the body, and scratching.
Lesion severity scoring
A trained experimenter blind to genotype and mouse behavioral scores rated each animal for evidence of skin lesions on the day of PPI testing. Mice that had regions on the face or body where skin was raw, inflamed, or bloody were classified in the “lesion” group. In line with institutional IACUC protocols, mice classified with lesions were treated using topical antibiotic as needed (Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc Opthalmic Ointment; Akorn, Lake Forest, IL), with the exclusion of the day of and prior to behavioral testing.
Autoradiography
Fresh frozen brains were obtained from mice assessed in PPI and grooming tests, and processed according to standard protocols to detect dopamine D1 and D2/3 receptor and DAT binding using specific radioligands10, 53, 54. Mice were sacrificed by cervical dislocation followed by rapid decapitation. Brains were then extracted, frozen immediately on dry ice, and stored at − 80 °C. 20 μm sections were cut on a cryostat (Lecia, Wetzlar, Germany) and stored on slides (Fisherbrand, Pittsburgh, PA) at − 80 °C until autoradiography experiments were performed.
Slides for total binding (TB) included tissue from 2 animals from different experimental groups, with 4 striatal sections collected every 200 μm. Slides for non-specific binding (NSB) included tissue from 4 animals with 2 sections collected every 400 μm. Position of subjects on the slides was counter-balanced across groups to account for fluid distribution during radioisotope incubation, and all slides for a given assay were incubated on the same day with the same binding solutions. Slides were thawed for 1 h before preincubation in buffer (except for DAT, no preincubation). Following preincubation, slides were allowed to completely airdry for > 1 h before incubation in radioligand solutions. The following radioisotopes were used: [3H]-SCH-23390 for D1 binding (2 nM), [3H]-Raclopride for D2/3 binding (5 nM), [3H]-GBR-12935 for DAT binding (2 nM) (Perkin Elmer, Boston, MA). To terminate radioligand incubation, slides were dipped and then washed in ice cold buffer (3 × 5 min for D1 and D2/3, and 4 × 30 min DAT). Following washes slides were dipped in ice cold dH2O and allowed to completely airdry before they were opposed to BioMax MR film (Kodak, Rochester, NY) with 3H standards (American Radiolabeled Chemicals, Inc., St Louis, MO). More details regarding reagents and conditions used for specific autoradiography experiments are included in Table S1; these were based on published protocols53, 54. MR films were developed using MINI-MED 90 X ray film development system (AFP Manufacturing, Peachtree City, GA). Developed films were then scanned at 2400dpi (Perfection V550 Photo scanner, Epson, Suwa, Japan) and luminosity was measured using Photoshop (Adobe, San Jose, CA) bilaterally in each region of interest on TB and NSB slides. These values were converted to radioactivity (uCi/g) using a standard curve, and average NSB radioactivity was subtracted from average TB to give specific binding (SB) used for analysis. Figure S8 shows representative images for both TB and NSB for each assay.
Cohort details
Experiment 1: Comparison of PPI and compulsive grooming severity
Male mice (n = 14 WT, 18 KO) were tested at ~ 8 months of age. Mice were assessed in the grooming test and PPI paradigm, and lesion severity was recorded. 2 WTs were excluded from PPI analysis due to poor hearing, leaving 12 WTs.
Experiment 2: Autoradiography
Brains from animals used in experiment 1 were used for autoradiography.
Experiment 3: Longitudinal characterization
From 2 months of age (before Sapap3-KOs show lesions) until 8 months, grooming was tested monthly, followed ~ 3 days later by PPI testing [n = 24 WT (12 male), 23 KO (10 males)]. Analysis of behavioral data separated by sex demonstrated no evidence for sex differences (Figure S9). Sexes were therefore combined for all analyses presented. Prior to the first test (but at no other time-points), mice were habituated for each paradigm as described above. Animals with %PPI > 2 standard deviations (SD) outside group average for that age were kept in the repeated measures longitudinal analysis (across 7 timepoints) but were excluded from analysis at 6–8 months of age as appropriate [n excluded: WT: 6 months = 2, 7 months = 3, 8 months = 2; KO-NL: 6 months = 1, 7 months = 1, 8 months = 1 (same mouse across all points); KO-L: none at any month].
Experiment 4: Early grooming phenotype
To examine grooming early in development, mice [n = 17 WT (9 males), 17 KO (9 males)] were acclimatized twice to grooming chambers between postnatal day (PND) 18–20. Mice were weaned at PND21, tested the following day, and then tested weekly until week 8. Data for week 8 of this experiment has been published elsewhere39, and is reproduced with permission (Figure S4).
Statistical analysis
Welch’s t-tests were used for analyses of data where the groups had unequal variance (e.g. PPI when KOs were analysed as one heterogeneous group, Fig. 1A). Nonparametric t-tests were used for analyses of data that were not normally distributed (e.g. grooming when KOs were analysed as one heterogeneous population, Fig. 1B). Repeated measures (RM) ANOVAs were used to compare the effects of group or genotype across time and different prepulse intensities; however, analysis of group differences examined average PPI across the three prepulse intensities. Geisser-Greenhouse correction was used for RM ANOVA of longitudinal studies. Pearson’s R was used to assess correlations between different measurements. T-tests, correlations, and 1 or 2-way ANOVAs were performed using GraphPad Prism (La Jolla, CA; version 9.0.0). Unbiased clustering was performed in SPSS (IBM, Armonk, NY) using kmeans clustering function on z-scored grooming and PPI data in KO mice. Mice were excluded from analysis if PPI values were more than 2 SDs outside their group mean or PPI data indicated poor hearing (as defined above). Graphs show mean ± SEM for bar graphs and best-fit linear regression ± 95% confidence intervals.
Supplementary Information
Acknowledgements
We would like to thank Ms Leela Ekambarapu and Mr Vinayak Banerjee for assistance with grooming assessments, Dr David Volk for use of his laboratory’s radioactive standards, and Dr Bianca Jupp for advice regarding autoradiography protocols. These studies were supported by BRAINS R01MH104255, McKnight Neuroscience Scholar Award, MQ Fellows Award, Burroughs Wellcome Fund CAMS, and Klingenstein Fellowship in the Neurosciences to S.E.A.
Author contributions
E.E.M. and S.E.A. conceptualized the studies and wrote the manuscript, E.E.M, A.Y.W. and A.S.W. performed behavioral experiments, E.E.M. and L.M.S. performed autoradiography experiments. All authors contributed to and approved the final manuscript.
Data availability
The data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88769-5.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koran LM. Quality of life in obsessive-compulsive disorder. Psychiatr. Clin. North Am. 2000;23:509–517. doi: 10.1016/S0193-953X(05)70177-5. [DOI] [PubMed] [Google Scholar]
- 3.Koran LM, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am. J. Psychiatry. 2007;164:5–53. [PubMed] [Google Scholar]
- 4.Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Prepulse inhibition deficits in obsessive-compulsive disorder are more pronounced in females. Neuropsychopharmacology. 2016;41:2963–2964. doi: 10.1038/npp.2015.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman SA, et al. Prepulse inhibition deficits only in females with obsessive-compulsive disorder. Depress. Anxiety. 2016;33:238–246. doi: 10.1002/da.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol. Psychiat. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol. Psychiat. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 9.Manning EE, van den Buuse M. BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Front. Cell. Neurosci. 2013;7:92. doi: 10.3389/fncel.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavez C, Gogos A, Jones ME, van den Buuse M. Psychotropic drug-induced locomotor hyperactivity and prepulse inhibition regulation in male and female aromatase knockout (ArKO) mice: role of dopamine D1 and D2 receptors and dopamine transporters. Psychopharmacology. 2009;206:267–279. doi: 10.1007/s00213-009-1604-6. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues S, Salum C, Ferreira TL. Dorsal striatum D1-expressing neurons are involved with sensorimotor gating on prepulse inhibition test. J. Psychopharmacol. 2017;31:505–513. doi: 10.1177/0269881116686879. [DOI] [PubMed] [Google Scholar]
- 12.Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res. 1996;722:168–176. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow NR, Braff DL, Masten VL, Geyer MA. Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology. 1990;101:414–420. doi: 10.1007/BF02244063. [DOI] [PubMed] [Google Scholar]
- 14.Perani D, et al. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 15.Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol. Psychiat. 2004;55:1041–1045. doi: 10.1016/j.biopsych.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Denys D, et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2013;23:1423–1431. doi: 10.1016/j.euroneuro.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 17.van der Wee NJ, et al. Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]{beta}-CIT SPECT. Am. J. Psychiatry. 2004;161:2201–2206. doi: 10.1176/appi.ajp.161.12.2201. [DOI] [PubMed] [Google Scholar]
- 18.Kim CH, et al. Dopamine transporter density in the basal ganglia in obsessive-compulsive disorder, measured with [123I]IPT SPECT before and after treatment with serotonin reuptake inhibitors. Neuropsychobiology. 2007;55:156–162. doi: 10.1159/000106474. [DOI] [PubMed] [Google Scholar]
- 19.Olver JS, et al. Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J. Affect. Disord. 2009;114:321–326. doi: 10.1016/j.jad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, et al. Dopamine transporter density of basal ganglia assessed with [123I]IPT SPET in obsessive-compulsive disorder. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1637–1643. doi: 10.1007/s00259-003-1245-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang GJ, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong DF, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, et al. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Sullivan SS, et al. Cue-induced striatal dopamine release in Parkinson's disease-associated impulsive-compulsive behaviours. Brain. 2011;134:969–978. doi: 10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- 25.Jhanda S, Singla N, Grover S. Methylphenidate-induced obsessive-compulsive symptoms: A case report and review of literature. J. Pediatr. Neurosci. 2016;11:316–318. doi: 10.4103/1817-1745.199461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keuneman RJ, Pokos V, Weerasundera R, Castle DJ. Antipsychotic treatment in obsessive-compulsive disorder: A literature review. Aust. N. Z. J. Psychiatry. 2005;39:336–343. doi: 10.1080/j.1440-1614.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 27.Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr. Protoc. Neurosci. 2003;8:17. doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- 28.Baldan Ramsey LC, Xu M, Wood N, Pittenger C. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldan LC, et al. Histidine decarboxylase deficiency causes tourette syndrome: Parallel findings in humans and mice. Neuron. 2014;81:77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanahan NA, et al. Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol. Psychiat. 2009;65:401–408. doi: 10.1016/j.biopsych.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanahan NA, Velez LP, Masten VL, Dulawa SC. Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol. Psychiat. 2011;70:1039–1048. doi: 10.1016/j.biopsych.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ade KK, et al. Increased metabotropic glutamate receptor 5 signaling underlies obsessive-compulsive disorder-like behavioral and striatal circuit abnormalities in mice. Biol. Psychiat. 2016;80:522–533. doi: 10.1016/j.biopsych.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Boom BJG, Mooij AH, Miseviciute I, Denys D, Willuhn I. Behavioral flexibility in a mouse model for obsessive-compulsive disorder: Impaired Pavlovian reversal learning in SAPAP3 mutants. Genes Brain Behav. 2019;18:e12557. doi: 10.1111/gbb.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning EE, Dombrovski AY, Torregrossa MM, Ahmari SE. Impaired instrumental reversal learning is associated with increased medial prefrontal cortex activity in Sapap3 knockout mouse model of compulsive behavior. Neuropsychopharmacology. 2019;44:1494–1504. doi: 10.1038/s41386-018-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehmer I, Feenstra M, Willuhn I, Denys D. Instrumental learning in a mouse model for obsessive-compulsive disorder: Impaired habit formation in Sapap3 mutants. Neurobiol. Learn. Mem. 2020;168:107162. doi: 10.1016/j.nlm.2020.107162. [DOI] [PubMed] [Google Scholar]
- 38.Hadjas LC, Luscher C, Simmler LD. Aberrant habit formation in the Sapap3-knockout mouse model of obsessive-compulsive disorder. Sci. Rep. 2019;9:12061. doi: 10.1038/s41598-019-48637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbit VL, Manning EE, Gittis AH, Ahmari SE. Strengthened inputs from secondary motor cortex to striatum in a mouse model of compulsive behavior. J. Neurosci. 2019;39:2965–2975. doi: 10.1523/JNEUROSCI.1728-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu P, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc. Natl. Acad. Sci. U.S.A. 2013;110:10759–10764. doi: 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain. 2016;9:11. doi: 10.1186/s13041-016-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young JW, Wallace CK, Geyer MA, Risbrough VB. Age-associated improvements in cross-modal prepulse inhibition in mice. Behav. Neurosci. 2010;124:133–140. doi: 10.1037/a0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benzina, N., N’Diaye, K., Pelissolo, A., Mallet, L. & Burguière, E. A cross-species assessment of behavioral flexibility in compulsive disorders. Communications Biologyhttps://www.nature.com/articles/s42003-020-01611-y (2021). [DOI] [PMC free article] [PubMed]
- 44.Manning EE, Ahmari SE. How can preclinical mouse models be used to gain insight into prefrontal cortex dysfunction in obsessive-compulsive disorder? Brain Neurosci. Adv. 2018;2:2398212818783896. doi: 10.1177/2398212818783896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doherty JM, et al. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–2656. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- 46.Wood J, LaPalombara Z, Ahmari SE. Monoamine abnormalities in the SAPAP3 knockout model of obsessive-compulsive disorder-related behaviour. Philos. Trans. R. .Soc. Lond. B Biol. Sci. 2018 doi: 10.1098/rstb.2017.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadjas LC, et al. Projection-specific deficits in synaptic transmission in adult Sapap3-knockout mice. Neuropsychopharmacology. 2020 doi: 10.1038/s41386-020-0747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuchner S, et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol. Psychiatry. 2009;14:6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Obsessive Compulsive Disorder Foundation Genetics Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry. 2018;23:1181–1188. doi: 10.1038/mp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piantadosi SC, Chamberlain BL, Glausier JR, Lewis DA, Ahmari SE. Lower excitatory synaptic gene expression in orbitofrontal cortex and striatum in an initial study of subjects with obsessive compulsive disorder. Mol. Psychiatry. 2019 doi: 10.1038/s41380-019-0431-3. [DOI] [PubMed] [Google Scholar]
- 51.Pinhal CM, et al. Differential effects of deep brain stimulation of the internal capsule and the striatum on excessive grooming in Sapap3 mutant mice. Biol. Psychiat. 2018;84:917–925. doi: 10.1016/j.biopsych.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Ahmari SE, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jupp B, et al. Dopaminergic and GABA-ergic markers of impulsivity in rats: Evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur. J. Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- 54.Mennicken F, Savasta M, Peretti-Renucci R, Feuerstein C. Autoradiographic localization of dopamine uptake sites in the rat brain with 3H-GBR 12935. J. Neural Transm. Gen. Sect. 1992;87:1–14. doi: 10.1007/BF01253106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author upon request.


