Natural killer (NK) cells are innate lymphoid cells (ILCs) that eliminate virally infected and cancerous cells. NK cells directly induce target cell death and release cytokines and chemokines that activate the adaptive arm of the immune system.1
NK cells target cells that lose the expression of major histocompatibility complex class I (MHC-I) and are regulated by the balance of cell surface receptors, which are either stimulatory or repressive.2 Engagement of inhibitory NK cell receptors, such as killer-cell immunoglobulin-like receptors (KIRs), by cognate MHC-I ligands, activates Src homology 2 domain (SH2)-containing protein tyrosine phosphatases (SHP-1/2), suppressing NK cell activity.3–5
Harnessing NK cells for immunotherapy is attractive due to their innate ability to recognize cancer cells, their relatively low toxicity, and their ability to initiate robust adaptive immunity.6 NK cell lines can thus be used for “off the shelf” adoptive cell transfer therapies, incentivizing the generation of superior molecularly engineered NK cells.7,8
Since most genetic engineering approaches for immunotherapy focus on modulation of cell surface molecules, intracellular inhibitory signaling cascades may still constrain immune responses. Therefore, we focused on modulating the intracellular inhibitory checkpoint molecule SHP-1 in NK cells.
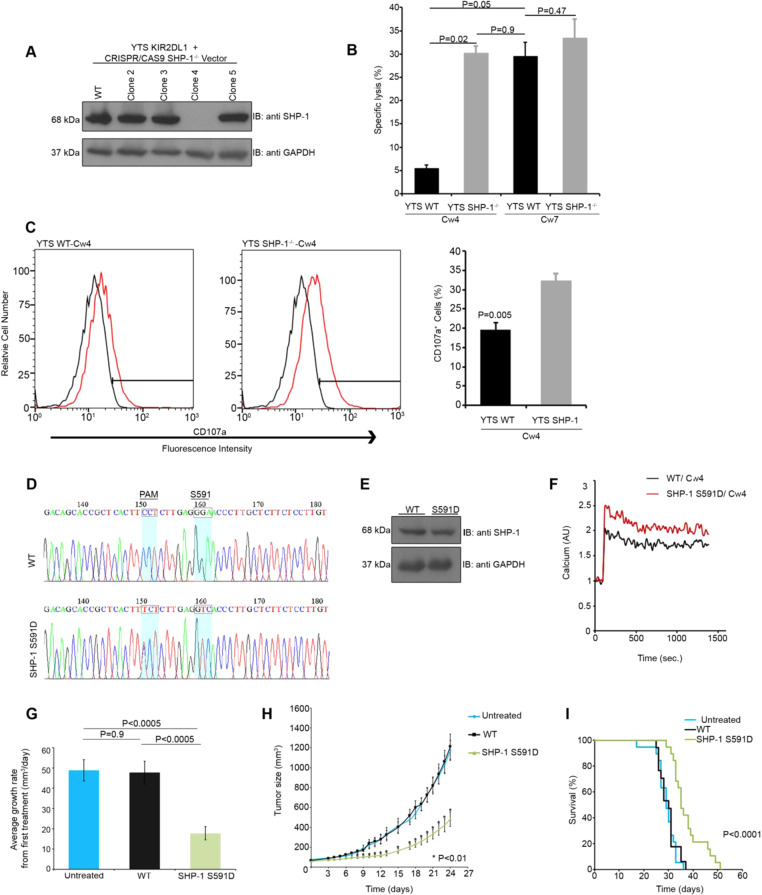
SHP-1-deficient (SHP-1−/−) NK cells overexpressing the inhibitory KIR2DL1 receptor (YTS-2DL1) were generated by targeting the PTPN6 locus using CRISPR/CAS9 gene editing.5 After clonal limiting dilution, we generated YTS-2DL1 SHP-1−/− NK cells (Fig. 1a). Next, we tested the cytotoxicity of YTS-2DL1 SHP-1−/− cells (Fig. 1b). NK cells were incubated with repressive 721.221 cancer cells overexpressing the cognate ligand for KIR2DL1 (Cw4-HLA) or with stimulatory 721.221 target cells expressing an irrelevant HLA molecule (Cw7-HLA). Strikingly, YTS-2DL1 SHP-1−/− cells eliminated Cw4 tumor targets to a significantly higher degree than wt YTS-2DL1 cells (wt 5.5 ± 0.7%; SHP-1−/− 30 ± 1.5%, P = 0.0007). A modest increase in killing of Cw7-carrying cells was seen for YTS-2DL1 SHP-1−/− cells compared with wt YTS-2DL1 cells (wt 30 ± 3%; SHP-1−/− 34 ± 4%, P = 0.47). Since the threshold for activation against targets expressing irrelevant HLA molecules is extremely low, inhibition of SHP-1 in NK cells may be particularly relevant for tumors with high expression of ligands for inhibitory NK cell receptors that recruit SHP-1 (such as KIRs, NKG2A, and PD-1). We therefore focused on repressive Cw4 targets for the remainder of the study. Indeed, YTS-2DL1 SHP-1−/− cells also showed superior degranulation compared to wt YTS-2DL1 cells (Fig. 1c).
Fig. 1.
Modulation of SHP-1 activity enhances NK cell effector function. a YTS-2DL1 cells were transfected with pSpCas9(BB)-2A-GFP (PX458) and guide RNAs directed against the second exon of SHP-1, and subjected to limiting dilution. Clones were then screened for SHP-1 expression. Clone 4 was selected. b wt YTS-2DL1 or YTS-2DL1 SHP-1−/− cells were incubated with [35S]Met labeled 721-Cw4 or 721-Cw7 target cells at a ratio of 10:1 for 5 h at 37 °C. The specific lysis of target cells was subsequently measured (quantification of different independent experiments is shown, n = 3, mean±SEM). c wt YTS-2DL1 or YTS-2DL1 SHP-1−/− cells were incubated with mCherry expressing 721-Cw4 target cells for 2 h at 37 °C and analyzed for degranulation via FACS (P = 0.005, quantification on the right of independent experiments, n = 4, mean ± SEM). d Sequencing of CRISPR/CAS9 induced knock in of the SHP-1 S591D mutation in YTS-2DL1 cells. Highlighted codons indicate S591 and PAM mutations relative to the wt sample. e YTS-2DL1 wt or YTS SHP-1 S591D cells were lysed and immunoblotted for SHP-1. f YTS-2DL1 wt or SHP-1 S591D cells were loaded with calcium- sensitive Fluo-3-AM, and analyzed for basal intracellular calcium levels for 1 minute. The NK cells were then mixed with 721-Cw4 target cells and incubated at 37°C and analyzed by spectrofluorometry. g, h Tumor growth rate in NOD-Rag1nullIL2rgnull (NRG) mice. NOD-Rag1nullIL2rgnull (NRG) mice were subcutaneously injected with 3 × 106 721-Cw4 expressing tumor cells. Mice were administered either 3*106 wt YTS-2DL1 or YTS-2DL1 SHP-1 S591D cells by intratumor injections every 3 days. Tumor volumes were calculated as described in the Methods. i Kaplan–Meier curve displaying survival of untreated mice, or mice injected with wt or SHP-1 S591D NK cells. The results represent three independent experiments. For untreated, wt, and SHP-1 S591D injected groups, n = 19, 17, and 19 mice, respectively
We next asked whether modification of SHP-1 catalytic activity may have repercussions for antitumor NK cell activity in vivo. Modification of SHP-1 on the C-terminal tail in different cells previously showed contrasting effects.9,10 Therefore, we generated YTS-2DL1 cells expressing a mutant form of SHP-1 at serine 591 (YTS-2DL1 SHP-1 S591D, Fig. 1d). SHP-1 S591D was expressed at similar levels to wt SHP-1 in YTS-2DL1 cells (Fig. 1e). We first validated the activation threshold of wt SHP-1- and SHP-1 S591D-expressing NK cells via intracellular calcium flux analysis against repressive Cw4-HLA-expressing targets (Fig. 1f). Indeed, NK cells expressing SHP-1 S591D demonstrated significantly higher intracellular calcium flux than wt SHP-1-expressing cells.
Finally, the effect of SHP-1 inactivation was demonstrated in vivo. To this end, NOD-Rag1nullIL2rgnull (NRG)-immunodeficient mice were subcutaneously engrafted with 721-Cw4-HLA cells and treated with either wt YTS-2DL1 or YTS-2DL1 SHP-1 S591D cells. Mice injected with SHP-1 S591D cells demonstrated significantly decreased tumor growth rates and sizes (484 mm3 ± 73 mm3 compared to 1212 mm3 ± 126 mm3 for wt NK cell-treated mice at day 24) compared with those injected with wt NK cells (P < 0.0005 and P < 0.01, respectively; Fig. 1g, h), which showed no significant difference from untreated mice (P = 0.9). Furthermore, mice injected with SHP-1 S591D cells demonstrated longer survival than those injected with wt NK cells (P < 0.0001 via the log-rank test, Fig. 1i).
In summary, we inactivated the activity of the SHP-1 checkpoint to increase NK cell anti-tumor function. Lowering immune cell activation thresholds by modulating intracellular signaling molecules may therefore synergize with and benefit current immunotherapeutic approaches.
Supplementary information
Acknowledgements
This research was funded by Israel Science Foundation grant no. 747/13, grant no. 3-10151 from the Chief Scientist Office of the Ministry of Health, and a Taubenblatt Family Foundation Bio-Medicine excellence grant.
Author contributions
M.B.S. and A.B.S. designed the research. A.B.S., G.B., and B.S. performed the experiments. ABS, GB, BS, and MBS analyzed the data. M.B.S. and A.B.S. wrote the paper.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-0443-6) contains supplementary material.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stebbins CC, et al. Vav1 Dephosphorylation by the Tyrosine Phosphatase SHP-1 as a Mechanism for Inhibition of Cellular Cytotoxicity. Mol. Cell. Biol. 2003;23:6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matalon O, et al. Dephosphorylation of the adaptor LAT and phospholipase C-γ by SHP-1 inhibits natural killer cell cytotoxicity. Sci. Signal. 2016;9:ra54. doi: 10.1126/scisignal.aad6182. [DOI] [PubMed] [Google Scholar]
- 5.Matalon O, et al. Actin retrograde flow controls natural killer cell response by regulating the conformation state of SHP-1. EMBO J. 2018;37:e96264. doi: 10.15252/embj.201696264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Shmuel A, Biber G, Barda-Saad M. Unleashing Natural Killer Cells in the Tumor Microenvironment–The Next Generation of Immunotherapy? Front. Immunol. 2020;11:275. doi: 10.3389/fimmu.2020.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr. Opin. Immunol. 2018;51:146–153. doi: 10.1016/j.coi.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jochems C, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–86373. doi: 10.18632/oncotarget.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RY, Gaits F, Ragab A, Ragab-Thomas JM, Chap H. Tyrosine phosphorylation of an SH2-containing protein tyrosine phosphatase is coupled to platelet thrombin receptor via a pertussis toxin-sensitive heterotrimeric G-protein. EMBO J. 1995;14:2519–2526. doi: 10.1002/j.1460-2075.1995.tb07249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Kruhlak MJ, Hao J-J, Shaw S. Rapid T cell receptor-mediated SHP-1 S591 phosphorylation regulates SHP-1 cellular localization and phosphatase activity. J. Leukoc. Biol. 2007;82:742–751. doi: 10.1189/jlb.1206736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.