Abstract
Hemophilia A is a hemorrhagic disease due to congenital deficiencies of coagulation factor VIII (FVIII). Studies show that hemophilia patients with anticoagulant deficiency present less severe hemorrhagic phenotypes. We aimed to find a new therapeutic option for hemophilia patients by RNA interference (RNAi) targeting heparin cofactor II (HCII), a critical anticoagulant protein inactivating the thrombin. The optimal small interfering RNA (siRNA) was conjugated to an asialoglycoprotein receptor ligand (N-acetylgalactosamine [GalNAc]-HCII), promoting targeted delivery to the liver. After administration, GalNAc-HCII demonstrated effective, dose-dependent, and persistent HCII inhibition. After 7 days, in normal mice, GalNAc-HCII reduced HCII levels to 25.04% ± 2.56%, 11.65% ± 2.41%, and 6.50% ± 1.73% with 2, 5, and 10 mg/kg GalNAc-HCII, respectively. The hemostatic ability of hemophilia mice in the GalNAc-HCII-treated group significantly improved, with low thrombus formation time in the carotid artery thrombosis models and short bleeding time in the tail-clipping assays. After repeated administration, the prolonged activated partial thromboplastin time (APTT) was reduced. A 30 mg/kg dose did not cause pathological thrombosis. Our study confirmed that GalNAc-HCII therapy is effective for treating hemophilia mice and can be considered a new option for treating hemophilia patients.
Keywords: hemostasis, hemophilia, RNA interference, heparin cofactor II, thrombin
Graphical abstract
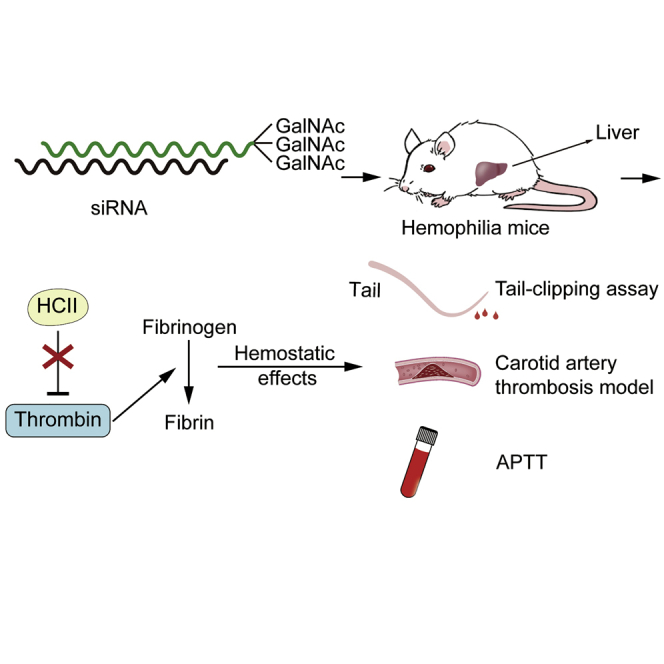
Wen-yi et al. develop a new RNA interference (RNAi) therapy for hemophilia targeting heparin cofactor II (HCII), a critical anticoagulant protein in vivo. The inhibition of HCII can effectively and safely improve the hemostatic ability of hemophilia A mice, and this method provides a new option for hemophilia patients.
Introduction
Hemophilia A (HA) is a congenital hemorrhagic disorder caused by the absence of coagulation factor VIII (FVIII). In more than 90% of cases, the first symptoms develop in childhood, presenting hemorrhage primarily in the skin, joints, and deep tissues. Severe cases may develop hemophiliac arthropathy leading to joint deformities or present intracerebral hemorrhage, causing permanent neurological sequelae and even death.1,2 In the past, for the management of hemophilia, intravenous supplementation of clotting factors is performed two or three times per week, demonstrating low patient compliance, high cost, and production of inhibitory antibodies after long-term treatment.3 Recently, gene therapy has been explored and proved to be a promising therapeutics for hemophilia.4,5 For example, the recombinant FVIII (N8-GP) has a longer half-life, lower immunogenicity, and superior hemostatic efficacy.6 Also, recombinant adeno-associated virus (AAV) delivery of FVIII has shown very promising efficacy in several preclinical and clinical studies.7 As the routine prophylaxis and treatment, a recently developed drug emicizumab, mainly connecting FIX and X for exerting the FVIIIa cofactor function to rebuild the disordered coagulation system, is now widely approved for patients.8,9 Moreover, the inhibition of anticoagulants to correct hemorrhage tendency has exhibited superior effectiveness and specificity as expected, such as antithrombin III (ATIII; fitusiran), tissue factor pathway inhibitor (TFPI; concizumab), activated protein C (APC; KRK α1AT), and protein S (PS; PS small interfering RNA [siRNA]).10, 11, 12, 13 These are attractive, innovative approaches with excellent prospects for clinical applications but having some limitations to their efficacy and safety. For example, to maintain the level of supplementary factors in plasma for successfully preventing hemorrhage, the standard dosing regimen of recombinant FVIII needs to be further determined because of individual differences of FVIII activity.14,15 Although AAV gene therapy is a great success, some significant safety concerns are associated with it, such as the genotoxicity of viral integration and the immune responses against the virus in humans.16,17 Despite a proven clinical application, the efficacy of emicizumab is not indicated in hemophilia B.18 A patient who participated in one of the clinical trials of fitusiran suffered cerebral sinus venous thrombosis after FVIII treatment, resulting in death. Therefore, further risk assessment is necessary to ensure the safety of fitusiran usage.19 The potency of KRK α1AT has been proved in animals, but its side effects on other systems need deeper exploration and evaluation because of the strong anti-inflammatory and cytoprotective roles of APC.20 Similarly, it remains to be observed how PS siRNA influences the complement system for which PS participates in humans’ complement regulation.21 In order to obtain stable therapeutic effects, further research and development are needed for concizumab because the plasma drug concentration varies significantly among patients taking the same dosage.22 It is thus essential to find more effective and safer approaches to meet the extensive need for HA treatment.
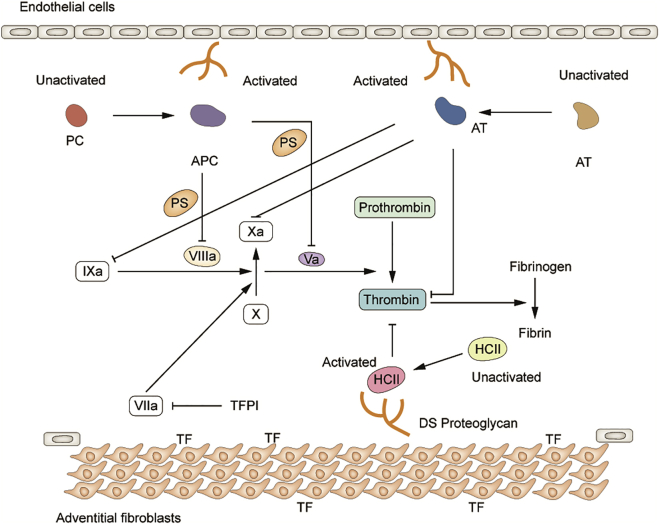
Heparin cofactor II (HCII), containing 480 amino acid residues, is a serine protease inhibitor encoded by the SERPIND1 gene.23 It is secreted by the liver and circulates in the plasma at 1.2 ± 0.4 μmol/L.24 Being one of the homologous proteins of ATIII, HCII plays a vital role in the anticoagulant system with its typical thrombin inhibition mechanism.25 Unlike ATIII with several target proteases (thrombin, FXa, and FIXa), HCII specifically inhibits thrombin and does not affect other coagulants (Figure 1). This is due to different residues at the active site (Arg for ATIII, Leu for HCII), leading to inconsistent binding sites with thrombin.26 Under normal physiological conditions, a third of thrombin is inactivated by HCII at a slow basal rate. Its efficiency to inactivate thrombin is about 1/10 of ATIII.27 However, the activity of HCII can be significantly enhanced by the catalysis of glycosaminoglycans (heparin, heparan sulfate, dermatan sulfate [DS], etc.), which promotes the acidic N terminus of HCII that binds tightly to the anion-binding exosite I of thrombin for rapidly forming the thrombin-HCII complex.28 As a specific catalyst, DS can increase the activity of HCII by more than 1000-fold, much higher than heparin and heparin sulfate. This is primarily due to the different binding sites with HCII.29 DS is synthesized by vascular smooth muscle cells and fibroblasts and generally exists on the blood vessel walls. This indicates that HCII mainly plays a role in connective tissues, while ATIII mostly in plasma. When the vessel wall is damaged, HCII enters it and comes in contact with DS to exert its anticoagulant effect.30 The physiological function of HCII is relatively single. Studies have shown that lack of HCII does not interfere with hematopoiesis and pregnancy and does not cause clinically severe complications or death.31 Besides, many case reports found that individuals with HCII deficiency had a medical history of thrombotic diseases.32,33 Lopaciuk et al.34 have reported a significantly higher prevalence of HCII deficiency in thrombotic patients than in controls (5.7% versus 0.9%). Furthermore, a previous study indicated that compared with wild-type (WT) control mice, HCII depletion mice had significantly shorter thrombus formation time under vascular damage (34 ± 4 versus 60 ± 12 min).35 Therefore, we speculated that the reduction of HCII might promote hemostasis in bleeding disorders, such as hemophilia. This study aimed to explore the effect of RNA interference (RNAi) therapy targeting HCII on the coagulation pathway and confirm a novel way for hemostasis in HA patients.
Figure 1.
A simple description of the coagulation pathway, expounding the inactivation effect of HCII for thrombin with the help of DS
An anti-coagulation pathway was also depicted with anticoagulants, including antithrombin (AT), protein C (PC), and protein S (PS).
Results
In vitro thrombin generation tests (TGTs)
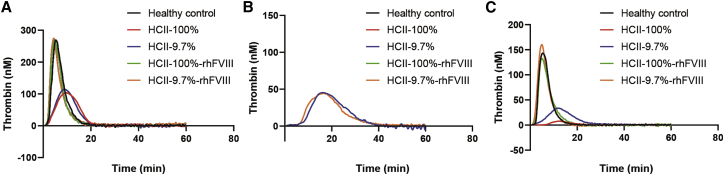
In hemophilia, it is confirmed that the ability of thrombin generation in plasma is associated with the clinical severity of the bleeding phenotype.36 To evaluate the effect of HCII reduction on the hemostatic capacity in hemophilia plasma, we measured the thrombin levels in FVIII-deficient plasma depleted of HCII to 9.7%, including peak thrombin and endogenous thrombin potential (ETP). Because the ability of HCII to inactivate thrombin was determined by DS content with different tissue distributions, we had set up various levels of DS in TGT to assess the potency of decreased HCII for thrombin generation accurately.37 In the absence of DS, results showed that there was no significant difference in thrombin generation between HCII-reduced (9.7%) and HCII normal (100%) groups (peak thrombin, p = 0.435; ETP, p = 0.987) (Figure 2A; Table 1). After adding a large amount of DS (250 μg/mL), thrombin was entirely inhibited in healthy control and HCII normal groups. However, the reduction of HCII promoted the generation of thrombin (Figure 2B; Table1). Then, we tried a lower concentration of DS (25 μg/mL) and found that the thrombin levels in the HCII-decreased group (9.7%) were remarkably higher than that of the HCII normal (100%) group (p = 0.032 for peak thrombin and p < 0.001 for ETP) (Figure 2C; Table 1). Furthermore, it demonstrated an additive thrombin generation response to recombinant human FVIII (rhFVIII) in the background of reduced and not reduced HCII. However, neither the peak thrombin nor ETP in the HCII-diminished group with rhFVIII surpasses healthy control (p = 0.143 and p = 0.769). Thus, the risk for thrombin overproduction, namely, the potential risk of thrombosis, may be low in the HCII-reduced plasma even with the replacement factor (rhFVIII). Also, we demonstrated that the depletion of HCII did not affect other anticoagulants’ activities, such as antithrombin (AT), protein C (PC), and PS, indicating that the alteration of thrombin generation was due to only the reduction in HCII activity (Figure S1). Our results were per the hypothesis that the decreased expression of HCII protein could effectively and safely rebalance the coagulation and anti-coagulation pathways to improve the hemostatic ability in hemophilia plasma.
Figure 2.
Thrombin generation curves in HA plasma depleted of HCII activity to 9.7% in the background of DS with various levels
(A–C) Thrombin generation conducted in the absence of DS (A), with DS at a concentration of 250 (B) or 25 μg/mL (C). Healthy control plasma was generated from the mixture plasma of 15 volunteers. Further control plasma was produced by adding back rhFVIII (1 IU/mL) to recover the thrombin generation to an average level. All measurements were run with 1 pM tissue factor and 4 mM phospholipids. The ordinate represented the alterations of thrombin levels over time.
Table 1.
Parameters of thrombin generation from healthy control and FVIII-deficient plasma (HA plasma) with various levels of DS
| Groups | Lag time (min) | Time to peak (min) | Time to tail (min) | Peak thrombin (nM) | ETP (nM·min) |
|---|---|---|---|---|---|
| DS = 0 μg/mL | |||||
| Healthy control | 2.4 ± 0.1 | 5.5 ± 0.6 | 20.2 ± 0.4 | 278.0 ± 15.7 | 1,636.0 ± 56.8 |
| HCII-100% | 3.2 ± 0.2 | 9.8 ± 0.3 | 21.5 ± 1.8 | 104.8 ± 8.2 | 1,248.0 ± 113.2 |
| HCII-9.7% | 3.1 ± 0.1 | 9.1 ± 0.4 | 21.6 ± 0.8 | 115.3 ± 6.0 | 1,240.0 ± 27.6 |
| HCII-100%-rhFVIII | 2.1 ± 0.2 | 5.0 ± 0.2 | 20.2 ± 0.5 | 271.1 ± 12.9 | 1,554.0 ± 53.86 |
| HCII-9.7%-rhFVIII | 2.0 ± 0.2 | 4.4 ± 0.2 | 24.0 ± 2.2 | 278.3 ± 14.6 | 1,665.0 ± 61.9 |
| DS = 250 μg/mL | |||||
| Healthy control | −1 | −1 | −1 | 0 | 0 |
| HCII = 100% | −1 | −1 | −1 | 0 | 0 |
| HCII = 9.7% | 8.9 ± 0.4 | 16.1 ± 0.9 | 38.5 ± 1.1 | 45.8 ± 5.5 | 792.3 ± 70.8 |
| HCII-100%-rhFVIII | −1 | −1 | −1 | 0 | 0 |
| HCII-9.7%-rhFVIII | 7.6 ± 0.1 | 16.5 ± 0.9 | 41.0 ± 1.5 | 45.0 ± 4.4 | 767.0 ± 115.7 |
| DS = 25 μg/mL | |||||
| Control | 2.8 ± 0.1 | 5.8 ± 0.4 | 18.6 ± 0.7 | 145.1 ± 16.7 | 853.3 ± 99.4 |
| HCII = 100% | 9.4 ± 1.2 | 12.2 ± 0.5 | 26.0 ± 0.6 | 8.2 ± 2.1 | 96.0 ± 6.5 |
| HCII = 9.7% | 5.9 ± 1.1 | 12.0 ± 0.3 | 32.5 ± 2.1 | 34.2 ± 3.1 | 455.4 ± 18.8 |
| HCII-100%-rhFVIII | 2.8 ± 0.6 | 5.2 ± 0.3 | 19.7 ± 0.9 | 134.2 ± 9.4 | 814.1 ± 31.5 |
| HCII-9.7%-rhFVIII | 2.2 ± 0.5 | 5.0 ± 0.5 | 16.8 ± 1.1 | 165.6 ± 8.5 | 884.7 ± 23.8 |
Experiments were carried out in triplicate. Values are mean ± SD. “−1” represents error in curve parameters due to no thrombin generation; HCII = 9.7% or HCII = 100%, HA plasma with reduced or non-reduced HCII activity.
Pharmacokinetics and pharmacodynamics in WT mice
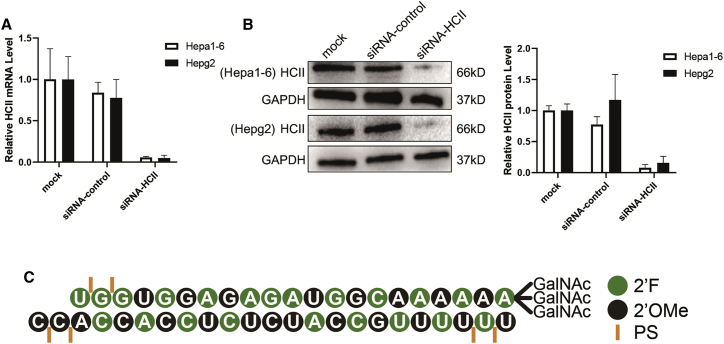
The RNAi therapeutic technology was applied to the present study to inhibit the expression of HCII in vivo. An optimal siRNA targeting the HCII gene was screened out via cellular assays (siRNA-HCII). HCII mRNA levels, consistent with the protein alterations, were significantly reduced in the siRNA-HCII-treated group, about 6.65% ± 1.61% and 6.17% ± 4.50% relative to the siRNA-control-treated group in mouse (Hepa 1–6) or human (Hepg2) cells (Figures 3A and 3B). This siRNA was then conjugated to the GalNAc ligand and was chemically modified for specific delivery to hepatocytes and stable inhibition of HCII in vivo (Figure 3C).
Figure 3.
The reduction effects of siRNA-HCII in cellular assays and the synthesis of GalNAc-modified siRNA-HCII
(A and B) Analysis of mRNA transcript levels (A) and HCII protein levels (B) of HCII gene in Hepa 1–6 and Hepg2 cell lines transfected with siRNA-HCII or a control siRNA (siRNA-control) by Lipofectamine 3000 reagent. The housekeeping gene GAPDH was used to normalize the abundance of HCII mRNA in quantitative real-time PCR or as an internal control to normalize the gray values of HCII protein in western blot. Untreated hepatocytes were evaluated as control (mock). The graph bars represent the mean value ± SD in three independent experiments. (C) Structure diagram of GalNAc-HCII showing the siRNA-HCII conjugated with a GalNAc ligand. Also presented are the modifications of the nucleotides performed in this study, including 2′-O-methyl (2′-OMe) and 2′-deoxy-2′-fluoro (2′-F). Phosphorothioate (PS) linkages are used for the modifications of the backbone.
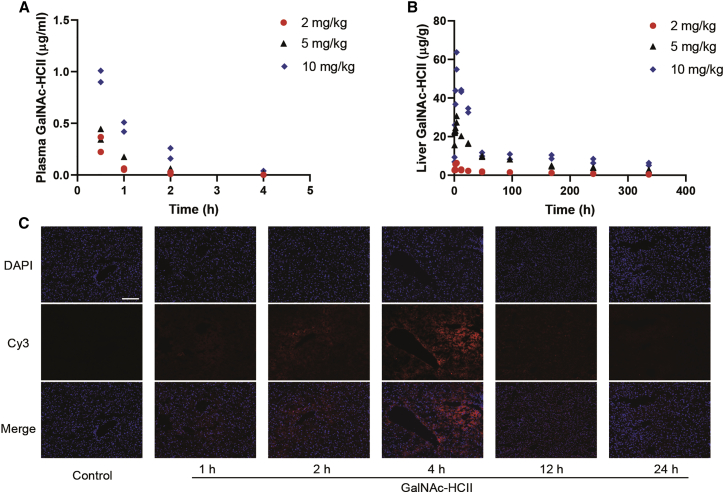
After a single subcutaneous injection, GalNAc-HCII rapidly diffused into the plasma with maximum concentrations that appeared after 30 min, about 0.30, 0.39, and 0.95 μg/mL for dosages of 2, 5, and 10 mg/kg, respectively. Drug levels significantly decreased at 4 h after dosing (Figure 4A). Simultaneously, the levels of GalNAc-HCII in the liver increased promptly, showing the peak concentrations with 6.2, 29.0, and 59.3 μg/g for administrations of 2, 5, and 10 mg/kg, respectively. The drug remained in the liver for a long time, up to 2 weeks (Figure 4B). The concentration of GalNAc-HCII in the liver and plasma was predominantly dependent on the administered dosage. Furthermore, to verify the liver specificity of GalNAc-HCII, we observed the drug levels in other organs, which were relatively low or even below the limit of quantitation (Figures S2A–S2D). In addition, we analyzed frozen sections of the liver after the administration of GalNAc-HCII at 1 mg/kg labeled with cyanine dye 3 (Cy3). Results showed that the drug specifically targeted the liver with utmost fluorescence intensity emerging at 4 h after dosing, and no drug accumulation was observed in other organs (Figure 4C; Figure S2E). Alterations in the fluorescence intensity were consistent with the drug pharmacokinetics curve mentioned above.
Figure 4.
Pharmacokinetics and liver targeting of GalNAc-HCII in WT mice
(A and B) Drug levels of GalNAc-HCII in plasma (A) and liver (B) after a single administration in WT mice at different dosages. Two mice were sacrificed in each dose group at each time point. All detection values were plotted, and those lower than the minimum quantitation were considered 0 μg/mL in plasma or 0 μg/g in the liver. (C) Fluorescence intensity of Cy3-labeled GalNAc-HCII (excited, 550 nm; visualized, 570 nm) in liver at 1, 2, 4, 12, and 24 h after a single administration with a dose of 1 mg/kg. The nucleus of tissue sections is incubated with DAPI (excited, 358 nm; visualized, 461 nm). WT mice treated with PBS were used as control. Scale bar, 50 μm.
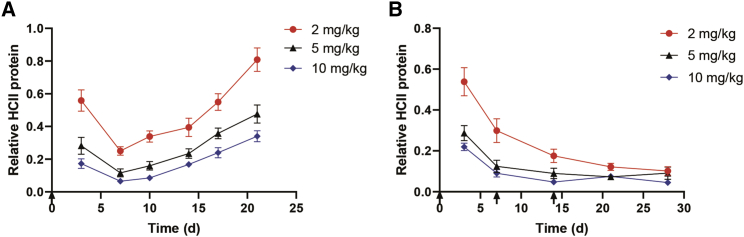
Furthermore, we explored the inhibition efficacy of GalNAc-HCII in WT mice. After a single subcutaneous administration, dose-dependent inhibition of HCII protein levels was observed in the plasma. The greatest inhibitory potency appeared on day 7, with relative HCII protein level reduced to 25.04% ± 2.56%, 11.65% ± 2.41%, and 6.50% ± 1.73% at doses of 2, 5, and 10 mg/kg, respectively. HCII suppression was long-lasting with the maintenance of protein levels less than 50% for 7 and 21 days at dosages of 2 and 5 mg/kg. Moreover, a high dose of 10 mg/kg resulted in maintenance of HCII levels less than 20% for 14 days (Figure 5A). As expected, the inhibition of HCII antigen in plasma was correlated with reductions of plasma HCII activity, liver HCII mRNA, and HCII protein, per the mechanism of RNAi action (Figures S3A–S3C). When dosed repeatedly, GalNAc-HCII induced dose-dependent, persistent suppression, with HCII protein decreased to stable levels (∼20%) after 2 weeks of administration (Figure 5B).
Figure 5.
Pharmacodynamics of GalNAc-HCII in WT mice
(A) Dose-response and maintenance of HCII reduction after a single injection of GalNAc-HCII at different dosages. Plasma HCII levels were detected using an ELISA kit. Relative HCII protein levels at each time point for each mouse were determined by normalizing to HCII levels seen in PBS-treated WT mice. The graph bars represent the group mean (n = 4) ± SD. The black arrow below the vertical axis represented the time of dosing (0 days). (B) Stable maintenance of HCII reduction after repeated dosing of GalNAc-HCII at different dosages. Relative HCII protein levels at each time point for each mouse were determined by normalizing to HCII levels detected in PBS-treated WT mice. The graph bars represent the group mean (n = 4) ± SD. Black arrows below the vertical axis represented the time of dosing (0, 7, and 14 days).
The hemostatic capacity of HA mice
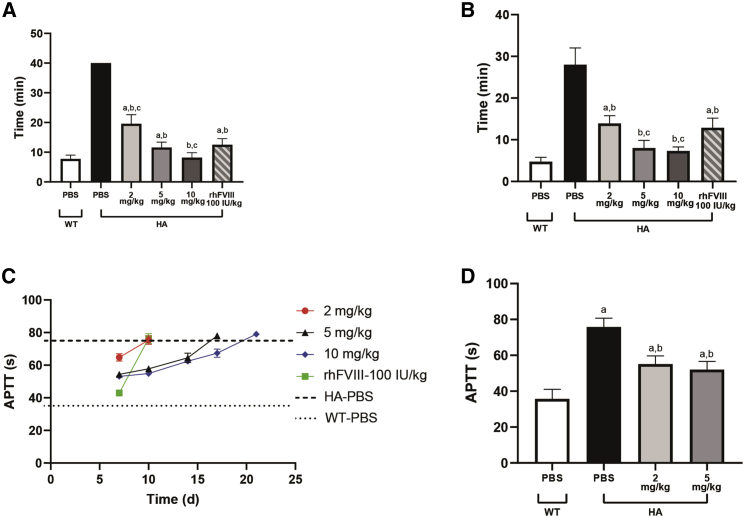
To explore the hemostatic effects of HCII reduction in HA mice, we generated carotid artery thrombosis models to evaluate the capacity of thrombus formation. Stable thrombus formation was observed at the carotid artery after induction by ferric chloride solution (FeCl3) in GalNAc-HCII-treated HA mice (15/15), while no stable clot was observed in the phosphate-buffered saline (PBS)-treated HA group (0/4). The time of thrombus formation was closely correlated to the doses, with approximately 19.6 ± 3.05, 11.6 ± 1.82, and 8.2 ± 1.64 min for 2, 5, and 10 mg/kg, respectively (Figure 6A; Figure S4). Moreover, there was no significant difference in thrombus formation time between WT and HA mice administered GalNAc-HCII at a dosage of 10 mg/kg (p = 0.996). Because factor replacement is an important therapy in hemophilia, we needed to compare the hemostatic potency of GalNAc-HCII and rhFVIII treatments for HA mice. Results showed better hemostatic capacity when dosed with 10 mg/kg in GalNAc-HCII-treated mice compared with rhFVIII supplementary group (p = 0.014), while no difference was observed between HA mice administered a dose of 5 mg/kg and rhFVIII (p = 0.927).
Figure 6.
Hemostatic effects of GalNAc-HCII in HA mice via carotid artery thrombosis models, tail-clipping assays, and measurements of APTT
(A and B) Thrombus formation time of carotid artery in FeCl3-induced thrombosis models (A) and bleeding time of tail artery in tail-clipping assays (B) on HA mice administered with GalNAc-HCII at different dosages (n = 5 for each dosage) or rhFVIII at a concentration of 100 IU/kg (n = 4). WT (n = 4) and HA mice (n = 4) are injected PBS as controls. Bars represent the group mean ± SD. A one-way ANOVA test followed by a multiple comparison test was used. ap < 0.05 versus WT mice with PBS; bp < 0.05 versus HA mice with PBS; cp < 0.05 versus HA mice with rhFVIII. (C) Alterations of APTT over time in HA mice after a single dosing of GalNAc-HCII with different doses or rhFVIII at 100 IU/kg. WT and HA mice with PBS were used as controls. Plasma from two mice was mixed to generate a plasma sample. Six mice were used for each group. Bars represent the group mean ± SD. (D) APTT correction in HA mice after three weekly injections of 2 or 5 mg/kg GalNAc-HCII for 2 weeks (n = 3 for each group). WT (n = 4) and HA mice (n = 4) with PBS were used as controls (35.7 ± 5.3 s for WT mice and 75.8 ± 4.9 s for HA mice). Bars represent the group mean ± SD. A one-way ANOVA test followed by a multiple comparison test was used. ap < 0.05 versus WT mice with PBS; bp < 0.05 versus HA mice with PBS.
We then conducted a tail-clipping assay to evaluate further the effect of HCII depletion on the hemostatic ability in HA mice. HA mice treated with GalNAc-HCII showed significantly shortened bleeding time of the tail artery (15/15), while most HA mice treated with PBS could not stop bleeding within 30 min (3/4). A dose-dependent effect was observed for bleeding time, with approximately 13.9 ± 1.88, 8.0 ± 1.84, and 7.3 ± 0.97 in the GalNAc-HCII-dosed group at 2, 5, and 10 mg/kg, respectively (Figure 6B). When administered 5 or 10 mg/kg, the hemostatic potency of the GalNAc-HCII-dosed group was similar and showed no difference to that of the WT group (p = 0.129 and p = 0.298). As an additional control, four animals were administered 100 IU/kg rhFVIII before tail clipping. Results showed better hemostatic effect when dosed with 5 or 10 mg/kg in GalNAc-HCII-treated mice compared with the animals supplemented with rhFVIII (p = 0.013 and p = 0.004), whereas no difference was observed between groups administered GalNAc-HCII at 2 mg/kg and rhFVIII (p = 0.923).
Activated partial thromboplastin time (APTT) is an important evaluation index of the hemostatic ability in hemophilia. Prolonged APTT was evident in HA mice with PBS due to abnormal coagulation mechanisms. At 7 days after single dosing, mice treated with GalNAc-HCII showed a reduced APTT, with 64.8 ± 2.3, 54.4 ± 0.8, and 53.1 ± 1.4 s at 2, 5, and 10 mg/kg, respectively. Moreover, according to APTT values in plasma with various FVIII levels, the correction effects of APTT in HA mice with GalNAc-HCII corresponded to approximately 6.6% ± 1.6%, 19.7% ± 1.6%, and 22.8% ± 3.3% normal levels of FVIII protein in vivo (Figure S5). Although the correction capacity of APTT was not better than for the rhFVIII supplementary group, the maintenance of APTT was more durable in GalNAc-HCII-dosed mice, with 10 and 14 days for 5 and 10 mg/kg, respectively (Figure 6C). After repeated administration, the APTT was further reduced in the GalNAc-HCII-treated group but did not reach normal levels of WT mice (35.7 ± 5.3 s), even when the HCII inhibition was over 80% (Figure 6D; Figure S6).
Acute toxicity studies
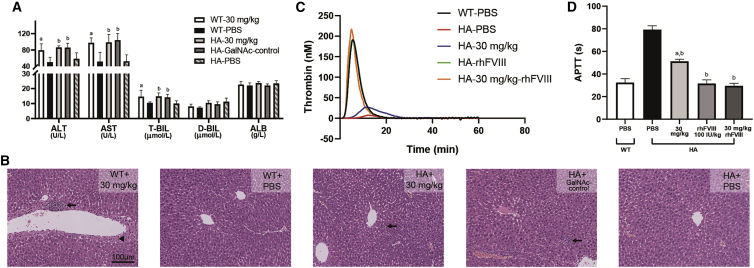
To assess the short-term accumulation effects and acute toxicity of GalNAc-HCII in vivo, we gave repeated injections of 30 mg/kg. Both WT and HA mice well tolerated the drug after 2 weeks of administration, with no abnormal phenotypes and no significant alterations in some hematological indexes, including complete blood count (CBC), renal functions, and myocardial enzymes. Nevertheless, the slight elevation of liver function indexes was observed in all mice treated with GalNAc-HCII or GalNAc-control, involving alanine transaminase (ALT), aspartate aminotransferase (AST), and total bilirubin (T-BIL) (Figure 7A; Figures S7A–S7C). There was no difference in liver functions between GalNAc-HCII- and GalNAc-control-dosed groups. Results of histopathological sections indicated mild liver injury in treated groups, characterized by punctate hepatocyte necrosis with leukocyte infiltration and hepatocyte degeneration in the focal perivascular (Figure 7B). Moreover, no difference of serum HCII levels was observed between HA mice dosed with GalNAc-control and PBS (p = 0.919; Figure S7D). It suggested that the liver damage was related to generalized toxicity from repeated GalNAc-RNAi molecules, but not to the knockdown of HCII. In addition, no death occurred in all groups at this dosage and frequency of administration.
Figure 7.
Potential hepatotoxicity and thrombosis risk for GalNAc-HCII in HA mice
(A) The toxicity effect on liver functions after repeated dosing of GalNAc-HCII or GalNAc-control at a concentration of 30 mg/kg in WT and HA mice (n = 6 for each group), including ALT, AST, T-BIL, D-BIL, and ALB. Bars represent the group mean ± SD. A one-way ANOVA test followed by a multiple comparison test was used. ap < 0.05 versus WT mice with PBS; bp < 0.05 versus HA mice with PBS. (B) H&E staining of liver sections prepared to explore the microscopic appearance of hepatotoxicity in WT and HA mice (n = 6 for each group) dosed with GalNAc-HCII or GalNAc-control at 30 mg/kg for six times. WT (n = 6) and HA mice (n = 6) with PBS were used as controls. The black arrow represented punctate hepatocyte necrosis with leukocyte infiltration, and the black triangle represented hepatocyte degeneration. (C) Thrombin generation curves in HA mice dosed with GalNAc-HCII at 30 mg/kg every 2 days for six times or 100 IU/kg rhFVIII once a day for 3 days after the last dosing of GalNAc-HCII. WT and HA mice with PBS were used as controls. Four mice were used for each group. (D) APTT detections in HA mice after frequent dosing of GalNAc-HCII at 30 mg/kg or rhFVIII at 100 IU/kg. WT and HA mice with PBS were used as controls. Four mice were used for each group. Bars represent the group mean ± SD. A one-way ANOVA test followed by a multiple comparison test was used. ap < 0.05 versus WT mice with PBS; bp < 0.05 versus HA mice with PBS.
Potential risk for thrombosis
Because thrombotic events have been reported in hemophilia patients with fitusiran after FVIII replacement treatment, we need to evaluate the potential risk for thrombosis in HA mice receiving GalNAc-HCII therapy, especially those given hemostatic agents at the same time. After repeated dosing of 30 mg/kg, no edema or paleness was observed in the upper and lower limbs. Results of organs anatomy showed no abnormalities in the size, shape, color, and weight. Then plasma was collected. As expected, results of TGT indicated improved thrombin generation in GalNAc-HCII-dosed mice compared with untreated HA mice. After supplemented FVIII, a more obvious elevation of thrombin levels was observed in HA mice with or without GalNAc-HCII, but neither different from the normal level of WT mice (peak thrombin, p = 0.113 and p = 0.926; ETP, p = 0.792 and p = 1.00) (Figure 7C; Table 2). In accordance with the results above, APTT exhibited a decrease in GalNAc-HCII-injected mice with not reaching the normal value of WT mice. Although significantly corrected with rhFVIII, APTT still does not surpass the normal range of WT mice (Figure 7D).
Table 2.
Parameters of thrombin generation from plasma of WT mice (n = 4) treated with PBS and HA mice (n = 4 for each group) dosed with PBS, GalNAc-HCII (30 mg/kg), or rhFVIII (100 IU/kg)
| Groups | Lag time (min) | Time to peak (min) | Time to tail (min) | Peak thrombin (nM) | ETP (nM·min) |
|---|---|---|---|---|---|
| WT-PBS | 2.7 ± 0.5 | 5.8 ± 0.4 | 19.8 ± 1.8 | 195.8 ± 16.7 | 1125.0 ± 95.0 |
| HA-PBS | 10.2 ± 1.1 | 12.6 ± 0.5 | 30.3 ± 1.7 | 7.9 ± 2.5 | 72.3 ± 15.2 |
| HA-30 mg/kg | 7.3 ± 0.5 | 11.2 ± 0.3 | 26.7 ± 0.8 | 27.9 ± 4.1 | 360.0 ± 38.9 |
| HA-rhFVIII | 2.2 ± 0.2 | 5.5 ± 0.4 | 19.0 ± 1.3 | 192.2 ± 14.2 | 1124.0 ± 112.8 |
| HA-30 mg/kg-rhFVIII | 2.0 ± 0.2 | 4.9 ± 0.4 | 21.5 ± 0.6 | 218.9 ± 15.7 | 1089.0 ± 46.1 |
Values are group mean ± SD.
Discussion
It has been reported that HA patients with anticoagulant deficiency present less severe hemorrhagic phenotypes.38,39 This study suggested that on reducing HCII, an important anticoagulant protein, specifically inactivating thrombin, thrombin generation could be upregulated, and the hemostatic ability of HA mice could be significantly improved. Therefore, targeting HCII may be a potential treatment for HA.
We know that the inactivation rate of thrombin by HCII is regulated explicitly by DS.37 For the absence of DS in commercial FVIII-deficient plasma, the ability of HCII to inactivate thrombin is relatively low, whether it is reduced or not, resulting in higher thrombin generation than that of hemophilia patient plasma in other research works. After adding DS, we found that thrombin levels in the HCII-reduced group with DS at low concentrations were much lower than that of high concentrations for the dose-dependent regulation of DS on HCII. The higher the concentration of DS, the more the thrombin is inactivated by HCII, which means that thrombin will be retained more when HCII is reduced. In addition, even in the presence of DS with high concentrations, the thrombin generation curve was not corrected to the normal level. This is because HCII just inactivates a part of thrombin in vivo even with the maximum activity, and the HCII reduction did not affect the inactivation of thrombin by other anticoagulants. These data also suggested that HCII reduction was relatively safe for avoiding excessive thrombin production to over-activate coagulation. Furthermore, HA mice supplemented with rhFVIII did not obtain the hemostatic capacity equivalent to that of WT mice, whereas suppression of HCII did. Possible reasons are as follows: the rhFVIII mainly affects the production of thrombin in plasma,40 while HCII inhibition primarily affects the injured vessels for DS’s existence. In our study, mice models primarily involving vascular damage resulting in rhFVIII could not prove themselves adequately. This may be one reason why APTT in HA mice with HCII suppression was not corrected to normal, reflecting the coagulation in plasma.41
Heterozygous HCII deficiency has been reported to be associated with thromboembolic events, but some epidemiological studies suggested that heterozygous HCII deficiency is as common in healthy people as in patients with deep vein thrombosis (DVT).42 The first subject with homozygous HCII deficiency had been reported for recurrent thrombosis, which was confirmed to be accompanied by ATIII deficiency.43 A screening study of thromboembolic events in adolescents revealed two patients with HCII Rimini mutation, and they were proved to be complicated with FV Leiden mutation and type I PC deficiency.44 Besides, it was shown that mice with homozygous HCII deficiency presented significantly shorter time of thrombus formation in the carotid artery after endothelial injury. However, no evidence of spontaneous thrombosis was found in mice without induction.35 Accordingly, HCII deficiency may play a limited role as a risk factor for thrombosis in vivo. Patients with HCII deficiency are likely to have a thrombotic tendency when other thrombogenic factors exist simultaneously, such as thrombophilia-related gene defects or obvious risk factors of thrombosis. One possible reason has been mentioned above that the activity of HCII in plasma is relatively low, and only a small part of thrombin is inactivated specifically.27 In addition, this study demonstrated that HCII inhibition mainly affected the hemostasis of damaged vessels with slightly improved thrombin levels and marginally shortened APTT. Even with the addition of rhFVIII, the thrombin generation and APTT in HCII-reduced groups still did not surpass the normal levels in vitro and in vivo. Moreover, pathological thrombosis was not found in the organs of mice after frequent injection of GalNAc-HCII. Therefore, our method is safer for significantly reducing the potential risks of thrombosis than other anticoagulant inhibition therapies.
Our research also has some shortcomings. First, acute hepatotoxicity was found in the GalNAc-HCII-treated group with large doses, which raised some concerns about the safety of this approach in patients with chronic liver diseases, such as fibrosis and cirrhosis. It is essential for us to develop more toxicity-reducing studies in the future to improve the feasibility of this therapy for patients with liver diseases. Second, our protocols are limited to HA. In theory, for HA and hemophilia B, this treatment’s effect should be the same because HCII specifically acts on thrombin with no effect on other coagulation factors.26 However, due to the lack of FIX-deficient mice, this drug’s effects on hemophilia B and even other hemophilia types remain to be elucidated. Finally, some clinical studies have suggested that the concentration of HCII in plasma is negatively correlated to atherosclerosis and restenosis after angioplasty, which is due to the promotion of HCII deficiency to the neointimal formation and vascular remodeling.45, 46, 47, 48 In our study, the period of explorations on pharmacodynamics and toxicity for GalNAc-HCII is relatively short. In addition, there are no hemophilia mice with atherosclerosis currently. So we fail to find apparent alterations of HCII inhibition on vascular intima, which may require further research.
This study aimed to elucidate a novel RNAi therapy for HA targeting HCII. This method has no mention in any of the previous studies. Our results suggest that it is an effective treatment for HA mice. Although there are still some issues to be solved, this method represents a new idea for HA treatment and may provide a better choice for patients in the future.
Materials and methods
We have received informed consent from all subjects. This study was reviewed and approved by the Medical Ethical Committee and the Animal Ethics Committee of Union Hospital, Huazhong University of Science and Technology.
Preparation of plasma samples
Healthy control plasma was obtained from the mixture plasma of 15 healthy volunteers. The FVIII-deficient plasma (Hyphen Biomed, France) was used to simulate HA plasma from patients. The depletion of HCII in vitro was performed by adding HCII neutralizing antibody (Affinity Biologicals, ON, Canada) to the FVIII-deficient plasma in the ratio 1:9, which leads to the activity of HCII reduced to 9.7%, detected by the chromogenic substrate method.49 Activities of ATIII, PC, and PS in human plasma samples were detected on an automatic coagulator (Sysmex, Japan) by corresponding kits according to the manufacturer’s instructions (Siemens Healthcare Diagnostics, Germany). Further control plasma was generated by adding rhFVIII (obtained from Union Hospital) to the FVIII-deficient plasma at 1 IU/mL to recover the thrombin generation to the normal level of healthy control.
The plasma of mice was obtained from the inferior vena cava by adding 3.8% sodium citrate (1:9). All plasma samples were centrifuged at 2,000 × g for 20 min to obtain platelet-poor plasma (PPP) and then stored at −80°C until use. The plasma of HA mice was diluted with WT mice plasma in different ratios to generate HA plasma with various levels of FVIII. APTT and FVIII activity of mixture plasma was measured on the coagulation analyzer (Diagnostica Stago, France).
TGT
TGT was conducted in human and animal plasma samples through an automatic thrombin generation detector (Thrombinoscope, the Netherlands) according to the manufacturer’s protocols.36 Before measuring thrombin generation, DS (Affandi-e, China) was added to human plasma with various concentrations (0, 25, 250 μg/mL). The coagulation pathway was activated by adding tissue factors (1 pM), phospholipids (4 mM), and FluCa kit (containing CaCl2) to the plasma. Then, thrombin was generated to cleavage the fluorescent substrates to release signals for detection.
Cell culture and siRNA transfection
Hepatocyte cell lines (mouse: Hepa 1–6; human: Hepg2), purchased from the cell bank of the Chinese Academy of Sciences, were maintained in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco, Carlsbad, CA, USA). Cell lines were seeded in a six-well plate at a density of 105 cells/well. After 24 h, cells were transfected with an optimal siRNA targeting HCII gene (siRNA-HCII, 5′-UGGUGGAGAGAUGGCAAAAAA-3′, suitable for mouse and human) or a negative control siRNA (siRNA-control, 5′-UUCUCCGAACGUGUCACGU-3′, suitable for mouse and human) (100 pmol) by using Lipofectamine 3000 (Invitrogen, USA). 48 h later, total RNA was extracted using the TRIzol reagent (Takara, Japan). After reverse transcription, quantitative real-time PCR was performed to detect the relative abundance of HCII mRNA normalized to the housekeeping gene GAPDH using a ChamQ SYBR qPCR Master Mix (Vazyme, China).
Besides, western blotting (WB) was conducted to assess protein levels of HCII relative to the internal control (GAPDH). Total cellular lysates (30 μg/lane) were separated on 10% SDS-PAGE gels. The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes. Afterward, the membranes were blocked and incubated overnight with the rabbit anti-mouse or human polyclonal antibodies against HCII (1:1,000) (ABclonal, BSN, USA) or GAPDH (1:1,000) (Antgene, China), followed by incubation with goat anti-rabbit immunoglobulin G (IgG) antibody (1:3,000) for 1 h. The enhanced chemiluminescence (ECL) reagent was used to visualize the target bands in an imaging analyzer (Bio-Rad, CA, USA), and the gray value of each band was quantified by an ImageJ software.
Synthesis of GalNAc-siRNAs
GalNAc-HCII and GalNAc-control were synthesized with the help of the TranSheepBio company (Shanghai, China). In brief, optimal siRNA-HCII and siRNA-control were chemically modified and conjugated to an asialoglycoprotein receptor ligand derived from N-acetylgalactosamine (GalNAc) to promote targeted delivery to the liver in vivo.50,51
Pharmacokinetics in mice
Male mice (C57BL/6, 6–8 weeks old) (GemPharmatech, China) were subcutaneously administered GalNAc-HCII at 2, 5, or 10 mg/kg (10 mL/kg body weight). At 0.5, 1, 2, 4, 12, 24, 48, 96, 168, 240, and 336 h of dosage, two mice were sacrificed at each dose level. Plasma and tissue samples, including liver, heart, spleen, lung, and kidney, were collected to assess for drug levels. Saline perfusion was used for mice before tissue acquisition and after blood collection. GalNAc-HCII levels in plasma and tissues were detected via stem-loop quantitative real-time PCR assays (Vazyme, China).52, 53, 54 The lower limit of detection range, determined in PBS-treated mice, was 0.001 μg/mL in plasma and 0.116, 0.137, 0.156, 0.235, and 0.254 μg/g in liver, heart, spleen, lung, and kidney. All detection values are normalized by subtracting the background values in PBS-treated mice, and those lower than the minimum quantitation were considered 0 μg/mL or 0 μg/g.
Another group of mice was dosed GalNAc-HCII labeled with Cy3 dye (TranSheepBio, China), excited by 550 nm and visualized at 570 nm, at 1 mg/kg. At the different time point of administration, frozen sections of tissue samples were collected and stained with DAPI dye, excited by 358 nm, and visualized at 461 nm. The fluorescence intensity of Cy3 and DAPI was observed to demonstrate the targeting specificity of GalNAc-HCII by a fluorescence microscope (Olympus, Japan).
Pharmacodynamics in WT mice
Male mice (C57BL/6, 6–8 weeks old) were subcutaneously administered PBS or GalNAc-HCII at 2, 5, or 10 mg/kg (10 mL/kg body weight). At 3, 7, 10, 14, 17, and 21 days of dosage, plasma samples from retro-orbital were collected to detect HCII protein levels by an ELISA according to the manufacturer’s protocols (ELISA, Biorbyt, UK). Relative HCII levels at each time point are determined by normalizing to serum HCII concentration detected in PBS-treated mice. A separate cohort of mice was sacrificed at 3 days after dosing. HCII protein levels and activities were measured in plasma, as mentioned previously. Liver tissues were obtained, and the mRNA level of HCII was tested by quantitative real-time PCR. Another batch of mice was repletely treated with GalNAc-HCII at 2, 5, or 10 mg/kg weekly for a total of 3 weeks. At 3, 7, 14, 21, and 28 days of dosage, relative HCII protein levels in plasma were detected using the method mentioned above.52
Hemostatic models in HA mice
Hemophilia mice (HA mice, male, 6–8 weeks old; B6/JGpt-F8em430Cd5d452/Gpt) (GemPharmatech, China) were subcutaneously injected with 2, 5, and 10 mg/kg GalNAc-HCII and PBS, respectively. HA mice, treated with 100 IU/kg rhFVIII 15 min before doing hemostatic models, were used as positive controls and WT mice treated with PBS as negative controls. After 7 days of dosage, carotid artery thrombosis models were induced by FeCl3 and recorded on Laser Doppler Flowmetry (Moor Instruments, UK) in line with the manufacturer’s instructions. In brief, the carotid artery was separated, gently lifted with a silk thread, and then infiltrated with 10% FeCl3 for 1 min, immediately followed by a laser probe detection to alter blood flow.35,55,56 Stable thrombus formation was defined as a reduction in blood flow to less than 20% of the maximum level, and the thrombus formation time was recorded, which was no more than 40 min for those that failed to generate a stable clot. Tail-clipping models were generated by transecting the distal tails at 4 mm with the bleeding time recorded at no more than 30 min.11,13 Besides, at 7, 10, 14, 17 and 21 days after dosing, plasma from the retro-orbital in another batch of mice was collected to detect APTT.
A separate cohort of mice received three weekly injections of 2 or 5 mg/kg GalNAc-HCII or PBS for 2 weeks. On day 21 (7 days after the last dose), plasma was collected to measure APTT and HCII antigen levels by the method described above.52
Acute toxicity studies and potential risk for thrombosis
WT (male, 6–8 weeks old) and HA mice (male, 6–8 weeks old) were administered GalNAc-HCII or GalNAc-control every 2 days at 0 and 30 mg/kg for six times. On the day of the last dosing, HA mice received 100 IU/kg rhFVIII once a day until the termination of the observation (3 days after the last dose). The clinical manifestations, such as weight, diet, activity, limbs, and survival, were observed in administration duration. Plasma was collected to detect thrombin generation, APTT, CBC, and biochemistry indexes, including liver functions (AST, ALT, T-BIL, direct bilirubin [D-BIL], albumin [ALB]), renal function (blood urea nitrogen [BUN], creatinine [CR], uric acid [UA]), and myocardial enzyme (creatine kinase [CK], L-type lactate dehydrogenase [LDH-L]). Anatomy of organs, including heart, liver, spleen, lung, and kidney, was performed to evaluate the size, shape, color, and weight. Furthermore, histopathological sections of tissue samples were obtained to evaluate the microscopic appearance of cells by using a microscope (Olympus, Japan).52,57
Statistical analysis
Data were expressed as group mean ± SD (standard deviation), and comparisons between the groups were analyzed using one-way ANOVA test with GraphPad Prism 8.0 software. The significance level was set at p <0.05.
Acknowledgments
The authors would like to thank the researchers and study participants for their contributions. This work was supported by the Program for HUST Academic Frontier Youth Team (no. 2018QYTD14).
Author contributions
W.L., R.Z., and Z.Z.: design of the study, collection and analysis of data, preparation of figures and tables, and drafting of the manuscript; X.L., H.W., and W.H.: preparation and revision of figures and tables; L.T. and Y.H.: supervision of the study. All authors reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2021.03.022.
Contributor Information
Yu Hu, Email: dr_huyu@126.com.
Liang Tang, Email: lancet.tang@qq.com.
Supplemental information
References
- 1.Peyvandi F., Garagiola I., Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187–197. doi: 10.1016/S0140-6736(15)01123-X. [DOI] [PubMed] [Google Scholar]
- 2.Castaman G., Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019;104:1702–1709. doi: 10.3324/haematol.2019.221093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schep S.J., Schutgens R.E.G., Fischer K., Boes M.L. Review of immune tolerance induction in hemophilia A. Blood Rev. 2018;32:326–338. doi: 10.1016/j.blre.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Weyand A.C., Pipe S.W. New therapies for hemophilia. Blood. 2019;133:389–398. doi: 10.1182/blood-2018-08-872291. [DOI] [PubMed] [Google Scholar]
- 5.Swystun L.L., Lillicrap D. Gene Therapy for Coagulation Disorders. Circ. Res. 2016;118:1443–1452. doi: 10.1161/CIRCRESAHA.115.307015. [DOI] [PubMed] [Google Scholar]
- 6.Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J. Thromb. Haemost. 2015;13(Suppl 1):S176–S179. doi: 10.1111/jth.12929. [DOI] [PubMed] [Google Scholar]
- 7.Pasi K.J., Rangarajan S., Mitchell N., Lester W., Symington E., Madan B., Laffan M., Russell C.B., Li M., Pierce G.F., Wong W.Y. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N. Engl. J. Med. 2020;382:29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 8.Yada K., Nogami K. Novel Insights and New Developments Regarding Coagulation Revealed by Studies of the Anti-Factor IXa (Activated Factor IX)/Factor X Bispecific Antibody, Emicizumab. Arterioscler. Thromb. Vasc. Biol. 2020;40:1148–1154. doi: 10.1161/ATVBAHA.120.312919. [DOI] [PubMed] [Google Scholar]
- 9.Blair H.A. Emicizumab: A Review in Haemophilia A. Drugs. 2019;79:1697–1707. doi: 10.1007/s40265-019-01200-2. [DOI] [PubMed] [Google Scholar]
- 10.Pasi K.J., Rangarajan S., Georgiev P., Mant T., Creagh M.D., Lissitchkov T., Bevan D., Austin S., Hay C.R., Hegemann I. Targeting of Antithrombin in Hemophilia A or B with RNAi Therapy. N. Engl. J. Med. 2017;377:819–828. doi: 10.1056/NEJMoa1616569. [DOI] [PubMed] [Google Scholar]
- 11.Prince R., Bologna L., Manetti M., Melchiorre D., Rosa I., Dewarrat N., Suardi S., Amini P., Fernández J.A., Burnier L. Targeting anticoagulant protein S to improve hemostasis in hemophilia. Blood. 2018;131:1360–1371. doi: 10.1182/blood-2017-09-800326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polderdijk S.G., Adams T.E., Ivanciu L., Camire R.M., Baglin T.P., Huntington J.A. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood. 2017;129:105–113. doi: 10.1182/blood-2016-05-718635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdary P. Inhibition of Tissue Factor Pathway Inhibitor (TFPI) as a Treatment for Haemophilia: Rationale with Focus on Concizumab. Drugs. 2018;78:881–890. doi: 10.1007/s40265-018-0922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins P.W., Björkman S., Fischer K., Blanchette V., Oh M., Schroth P., Fritsch S., Casey K., Spotts G., Ewenstein B.M. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J. Thromb. Haemost. 2010;8:269–275. doi: 10.1111/j.1538-7836.2009.03703.x. [DOI] [PubMed] [Google Scholar]
- 15.Pasi K.J., Fischer K., Ragni M., Nolan B., Perry D.J., Kulkarni R., Ozelo M., Mahlangu J., Shapiro A.D., Baker R.I. Long-term safety and efficacy of extended-interval prophylaxis with recombinant factor IX Fc fusion protein (rFIXFc) in subjects with haemophilia B. Thromb. Haemost. 2017;117:508–518. doi: 10.1160/TH16-05-0398. [DOI] [PubMed] [Google Scholar]
- 16.Chandler R.J., Sands M.S., Venditti C.P. Recombinant Adeno-Associated Viral Integration and Genotoxicity: Insights from Animal Models. Hum. Gene Ther. 2017;28:314–322. doi: 10.1089/hum.2017.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandamme C., Adjali O., Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitazawa T., Esaki K., Tachibana T., Ishii S., Soeda T., Muto A., Kawabe Y., Igawa T., Tsunoda H., Nogami K. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb. Haemost. 2017;117:1348–1357. doi: 10.1160/TH17-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Hemophilia Foundation . 2017. Alnylam Reports Patient Death in Fitusiran Clinical Study.https://www.hemophilia.org/news/alnylam-reports-patient-death-in-fitusiran-clinical-study [Google Scholar]
- 20.Polderdijk S.G.I., Baglin T.P., Huntington J.A. Targeting activated protein C to treat hemophilia. Curr. Opin. Hematol. 2017;24:446–452. doi: 10.1097/MOH.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlbäck B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb. Haemost. 1991;66:49–61. [PubMed] [Google Scholar]
- 22.Shapiro A.D., Angchaisuksiri P., Astermark J., Benson G., Castaman G., Chowdary P., Eichler H., Jiménez-Yuste V., Kavakli K., Matsushita T. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood. 2019;134:1973–1982. doi: 10.1182/blood.2019001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blinder M.A., Marasa J.C., Reynolds C.H., Deaven L.L., Tollefsen D.M. Heparin cofactor II: cDNA sequence, chromosome localization, restriction fragment length polymorphism, and expression in Escherichia coli. Biochemistry. 1988;27:752–759. doi: 10.1021/bi00402a039. [DOI] [PubMed] [Google Scholar]
- 24.Tollefsen D.M., Pestka C.A. Heparin cofactor II activity in patients with disseminated intravascular coagulation and hepatic failure. Blood. 1985;66:769–774. [PubMed] [Google Scholar]
- 25.Gettins P.G. Serpin structure, mechanism, and function. Chem. Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 26.Griffith M.J., Noyes C.M., Tyndall J.A., Church F.C. Structural evidence for leucine at the reactive site of heparin cofactor II. Biochemistry. 1985;24:6777–6782. doi: 10.1021/bi00345a008. [DOI] [PubMed] [Google Scholar]
- 27.Tollefsen D.M., Maimone M.M., McGuire E.A., Peacock M.E. Heparin cofactor II activation by dermatan sulfate. Ann. N Y Acad. Sci. 1989;556:116–122. doi: 10.1111/j.1749-6632.1989.tb22495.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Deerlin V.M., Tollefsen D.M. The N-terminal acidic domain of heparin cofactor II mediates the inhibition of alpha-thrombin in the presence of glycosaminoglycans. J. Biol. Chem. 1991;266:20223–20231. [PubMed] [Google Scholar]
- 29.Blinder M.A., Andersson T.R., Abildgaard U., Tollefsen D.M. Heparin cofactor IIOslo. Mutation of Arg-189 to His decreases the affinity for dermatan sulfate. J. Biol. Chem. 1989;264:5128–5133. [PubMed] [Google Scholar]
- 30.Tollefsen D.M. Heparin cofactor II modulates the response to vascular injury. Arterioscler. Thromb. Vasc. Biol. 2007;27:454–460. doi: 10.1161/01.ATV.0000256471.22437.88. [DOI] [PubMed] [Google Scholar]
- 31.Corral J., Aznar J., Gonzalez-Conejero R., Villa P., Miñano A., Vayá A., Carrell R.W., Huntington J.A., Vicente V. Homozygous deficiency of heparin cofactor II: relevance of P17 glutamate residue in serpins, relationship with conformational diseases, and role in thrombosis. Circulation. 2004;110:1303–1307. doi: 10.1161/01.CIR.0000140763.51679.D9. [DOI] [PubMed] [Google Scholar]
- 32.Tran T.H., Marbet G.A., Duckert F. Association of hereditary heparin co-factor II deficiency with thrombosis. Lancet. 1985;2:413–414. doi: 10.1016/s0140-6736(85)92736-9. [DOI] [PubMed] [Google Scholar]
- 33.Sie P., Dupouy D., Pichon J., Boneu B. Constitutional heparin co-factor II deficiency associated with recurrent thrombosis. Lancet. 1985;2:414–416. doi: 10.1016/s0140-6736(85)92737-0. [DOI] [PubMed] [Google Scholar]
- 34.Lopaciuk S., Bykowska K., Kopeć M. Prevalence of heparin cofactor II deficiency in patients with a history of venous thrombosis. Pol. J. Pharmacol. 1996;48:109–111. [PubMed] [Google Scholar]
- 35.He L., Vicente C.P., Westrick R.J., Eitzman D.T., Tollefsen D.M. Heparin cofactor II inhibits arterial thrombosis after endothelial injury. J. Clin. Invest. 2002;109:213–219. doi: 10.1172/JCI13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dargaud Y., Béguin S., Lienhart A., Al Dieri R., Trzeciak C., Bordet J.C., Hemker H.C., Negrier C. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb. Haemost. 2005;93:475–480. doi: 10.1160/TH04-10-0706. [DOI] [PubMed] [Google Scholar]
- 37.Tollefsen D.M. Vascular dermatan sulfate and heparin cofactor II. Prog. Mol. Biol. Transl. Sci. 2010;93:351–372. doi: 10.1016/S1877-1173(10)93015-9. [DOI] [PubMed] [Google Scholar]
- 38.Huang J.N., Koerper M.A. Factor V deficiency: a concise review. Haemophilia. 2008;14:1164–1169. doi: 10.1111/j.1365-2516.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 39.Spreafico M., Peyvandi F. Combined FV and FVIII deficiency. Haemophilia. 2008;14:1201–1208. doi: 10.1111/j.1365-2516.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 40.Samuelson Bannow B., Recht M., Négrier C., Hermans C., Berntorp E., Eichler H., Mancuso M.E., Klamroth R., O’Hara J., Santagostino E. Factor VIII: Long-established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev. 2019;35:43–50. doi: 10.1016/j.blre.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Favaloro E.J., Kershaw G., Mohammed S., Lippi G. How to Optimize Activated Partial Thromboplastin Time (APTT) Testing: Solutions to Establishing and Verifying Normal Reference Intervals and Assessing APTT Reagents for Sensitivity to Heparin, Lupus Anticoagulant, and Clotting Factors. Semin. Thromb. Hemost. 2019;45:22–35. doi: 10.1055/s-0038-1677018. [DOI] [PubMed] [Google Scholar]
- 42.Tollefsen D.M. Heparin cofactor II deficiency. Arch. Pathol. Lab. Med. 2002;126:1394–1400. doi: 10.5858/2002-126-1394-HCID. [DOI] [PubMed] [Google Scholar]
- 43.Villa P., Aznar J., Vaya A., España F., Ferrando F., Mira Y., Estellés A. Hereditary homozygous heparin cofactor II deficiency and the risk of developing venous thrombosis. Thromb. Haemost. 1999;82:1011–1014. [PubMed] [Google Scholar]
- 44.Bernardi F., Legnani C., Micheletti F., Lunghi B., Ferraresi P., Palareti G., Biagi R., Marchetti G. A heparin cofactor II mutation (HCII Rimini) combined with factor V Leiden or type I protein C deficiency in two unrelated thrombophilic subjects. Thromb. Haemost. 1996;76:505–509. [PubMed] [Google Scholar]
- 45.Aihara K., Azuma H., Takamori N., Kanagawa Y., Akaike M., Fujimura M., Yoshida T., Hashizume S., Kato M., Yamaguchi H. Heparin cofactor II is a novel protective factor against carotid atherosclerosis in elderly individuals. Circulation. 2004;109:2761–2765. doi: 10.1161/01.CIR.0000129968.46095.F3. [DOI] [PubMed] [Google Scholar]
- 46.Takamori N., Azuma H., Kato M., Hashizume S., Aihara K., Akaike M., Tamura K., Matsumoto T. High plasma heparin cofactor II activity is associated with reduced incidence of in-stent restenosis after percutaneous coronary intervention. Circulation. 2004;109:481–486. doi: 10.1161/01.CIR.0000109695.39671.37. [DOI] [PubMed] [Google Scholar]
- 47.Aihara K., Azuma H., Akaike M., Ikeda Y., Sata M., Takamori N., Yagi S., Iwase T., Sumitomo Y., Kawano H. Strain-dependent embryonic lethality and exaggerated vascular remodeling in heparin cofactor II-deficient mice. J. Clin. Invest. 2007;117:1514–1526. doi: 10.1172/JCI27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vicente C.P., He L., Tollefsen D.M. Accelerated atherogenesis and neointima formation in heparin cofactor II deficient mice. Blood. 2007;110:4261–4267. doi: 10.1182/blood-2007-04-086611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai C.W., Zhang G.S. [Heparin cofactor II (HCII) activity and antigen assay and their significance in thrombotic diseases] Zhonghua Xue Ye Xue Za Zhi. 2003;24:452–454. [PubMed] [Google Scholar]
- 50.Haringsma H.J., Li J.J., Soriano F., Kenski D.M., Flanagan W.M., Willingham A.T. mRNA knockdown by single strand RNA is improved by chemical modifications. Nucleic Acids Res. 2012;40:4125–4136. doi: 10.1093/nar/gkr1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair J.K., Willoughby J.L., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 52.Sehgal A., Barros S., Ivanciu L., Cooley B., Qin J., Racie T., Hettinger J., Carioto M., Jiang Y., Brodsky J. An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat. Med. 2015;21:492–497. doi: 10.1038/nm.3847. [DOI] [PubMed] [Google Scholar]
- 53.Hatakeyama H., Wu S.Y., Mangala L.S., Lopez-Berestein G., Sood A.K. Assessment of In Vivo siRNA Delivery in Cancer Mouse Models. Methods Mol. Biol. 2016;1402:189–197. doi: 10.1007/978-1-4939-3378-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T., Barbisin M., Xu N.L., Mahuvakar V.R., Andersen M.R. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vicente C.P., He L., Pavão M.S., Tollefsen D.M. Antithrombotic activity of dermatan sulfate in heparin cofactor II-deficient mice. Blood. 2004;104:3965–3970. doi: 10.1182/blood-2004-02-0598. [DOI] [PubMed] [Google Scholar]
- 56.Gewirtz J., Thornton M.A., Rauova L., Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J. Thromb. Haemost. 2008;6:1160–1166. doi: 10.1111/j.1538-7836.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- 57.Janas M.M., Schlegel M.K., Harbison C.E., Yilmaz V.O., Jiang Y., Parmar R., Zlatev I., Castoreno A., Xu H., Shulga-Morskaya S. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018;9:723. doi: 10.1038/s41467-018-02989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.