Abstract
Vertebrovertebral arteriovenous fistula is an uncommon vascular disease with no clear management guidelines. It is most commonly caused by iatrogenic injury. We have presented the details of two iatrogenic cases and a review to discuss strategies for endovascular and surgical approaches. From the digital subtraction angiography findings, the vertebrovertebral arteriovenous fistulas were occluded by endovascular coil positioning (patient 1) and surgical ligation of the fistulas (patient 2). Although endovascular approaches are increasing in popularity and considered well-tolerated treatments, open surgical treatment is still reserved for the most complex cases and those not feasible for endovascular treatment.
Keywords: Endovascular surgery, Open surgery, Vertebrovertebral arteriovenous fistula
Vertebrovertebral arteriovenous fistula (VVAVF) is a rare vascular disorder that results in a direct high-flow shunt between the extracranial vertebral artery (VA) or its branches and adjacent veins such as the vertebral venous plexus, venous lakes, or jugular veins. Iatrogenic VVAVF is a unique branch of the disorder that can result from internal jugular vein catheterization or after angiography injury to the vertebral artery,1,2 leading to tinnitus, palpable pulsating masses, neurologic deficits, and, even, ocular symptoms.3,4 Using surgical or endovascular techniques, VVAVFs can be treated by direct fistula closure or occlusion of the VA above and below the fistula. To date, only a few cases of iatrogenic VVAVFs have been reported, with no definitive guidelines on its clinical management.2,3 With the patient's consent for information usage and report, we have described two cases of iatrogenic VVAVF, which were successfully treated with selected coil embolization and open repair, respectively.
Case report
Patient 1
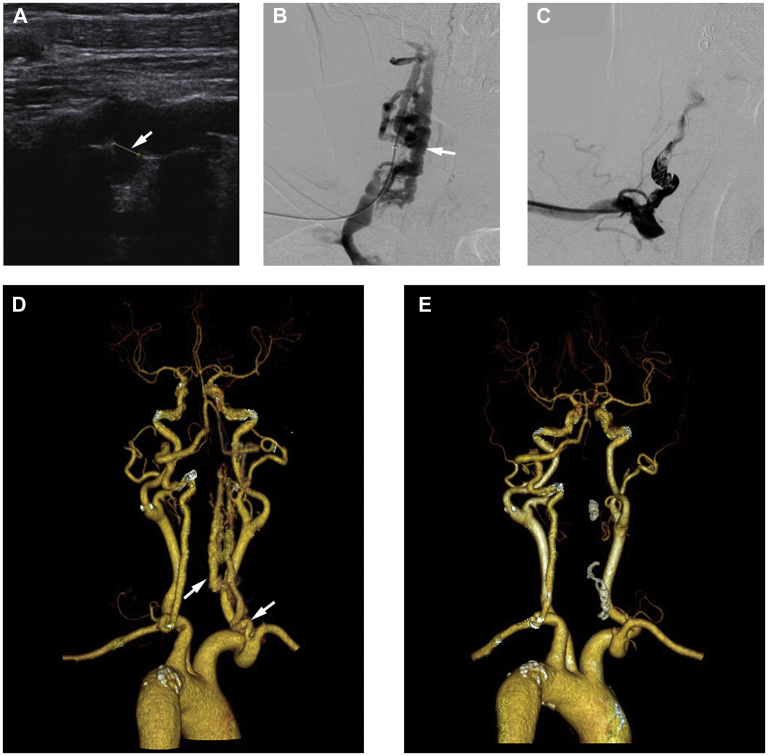
A 63-year-old woman with previous cervical spondylosis and acupuncture treatment was admitted to our department for complaints of right-sided pulsatile tinnitus and objective vertigo of 9 months' duration. The physical examination revealed bruits located at the right mastoid and carotid region. Carotid ultrasonography showed a fistula between the right VA and vein (Fig 1, A), where the pulsatile vein was located behind the VA. Computed tomography angiography (CTA) revealed multiple dilated serpiginous vessels surrounding the right VA at the C4 level. Imaging studies of the right internal jugular vein and epidural venous plexus revealed a V2 segment VVAVF (Fig 1, D). Digital subtraction angiography also showed a dilated extracranial segment between the muscular branches of the VA and a dilated periradicular venous plexus (Fig 1, B). Given that significant tortuosity and narrowing of the arterial lumen were present, which prevented the passage of detachable balloons and stent, an embolization coil approach was chosen. Because the imaging studies showed the dominant left VA supplying the posterior circulation and retrograde filling to the fistula in the right VA, which demonstrated the safety of blocking the right VA. A 6F introducer sheath (Cordis, a Cardinal Health Co, Dublin, Ohio) was placed in the right subclavian artery, and the right VA was catheterized. A microcatheter (Turbo Tracker 18; Boston Scientific, Marlborough, Mass) was placed proximal to the arterial edge of the fistula, and coiling was attempted with six interlocking detachable coils (Cook Medical, Bloomington, Ind), which resulted in complete occlusion of the right VA and fistula (Fig 1, C). The patient tolerated the procedure without neurological deficits or discomfort. Her pulsatile tinnitus had resolved after treatment, and she was discharged the next day after postoperative CTA had revealed the success of the treatment (Fig 1, E).
Fig 1.
A, Carotid ultrasound scan showing a fistula between the right vertebral artery (VA) and vein. B,C, Digital subtraction angiogram revealing multiple dilated serpiginous vessels surrounding the right VA and epidural venous plexus. D,E, Preoperative and postoperative computed tomography angiograms (CTAs) showing the diagnosis and confirming successful treatment.
Patient 2
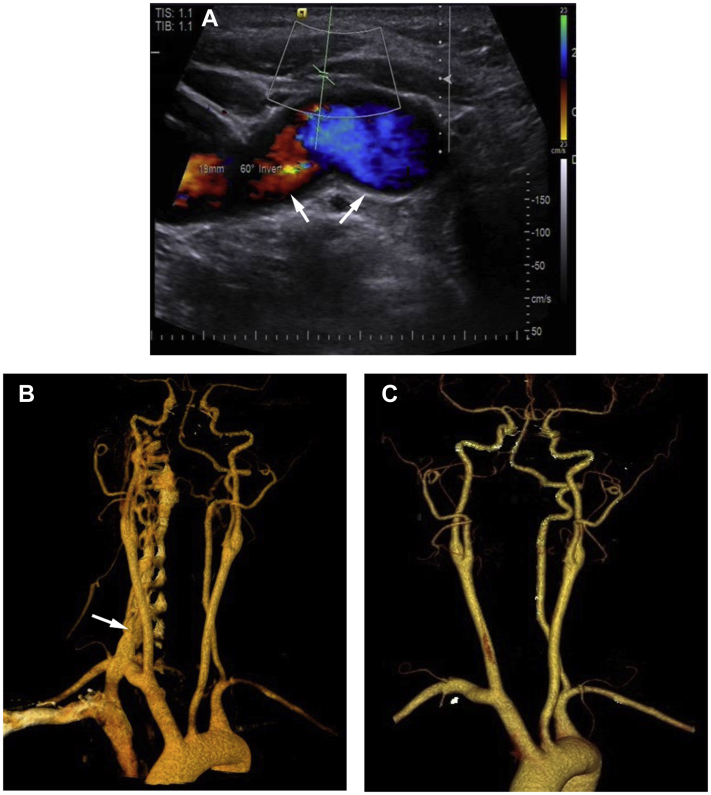
The second patient was a 40-year-old woman with notable history of right internal jugular vein catheterization 5 years previously. She was admitted to our hospital because of right-sided tinnitus, a pulsatile neck mass, and an overlying audible bruit, which was more striking during sleep. On examination, a palpable fremitus and a continuous audible bruit were found over her right neck. Carotid ultrasound scanning showed a fistula between the right VA and vein, and the pulsatile vertebral vein was found located in front of the VA (Fig 2, A). CTA demonstrated multiple dilated vessels surrounding the V1 segment of the right VA and epidural venous plexus (Fig 2, B). A dominant left VA was also found, which supplied the posterior circulation, with retrograde filling to the fistula in the right VA.
Fig 2.
A, Carotid ultrasound scan showing a large fistula between the right vertebral artery (VA) and vein. B, Preoperative computed tomography angiogram (CTA) showing the diagnosis of multiple oversized fistulas with significant tortuosity. C, Follow-up CTA at 3 months after the procedure demonstrating successful treatment.
An endovascular approach was chosen at first. With the patient under local anesthesia, a 5F Roadmaster guiding catheter (Goodman Co, Ltd, Aichi, Japan) was placed in the right VA via the right femoral artery. Angiography showed increased shunt flow, reflux, and enlargement of the fistula. However, during the procedure, we found an oversized venous portion of the fistula, which prevented reliable and stable occlusion. Therefore, the use of detachable balloons was abandoned. We also considered coil embolization. Nonetheless, coil embolization carries the risk of embolus material migration or ischemic complications resulting from the high flow velocity through the VA and fistula. In addition, the evident tortuosity at the beginning of the VA prevented us from choosing endovascular stent therapy. Therefore, open surgery was chosen as the alternative. With the patient under general anesthesia, the right VA was exposed through the supraclavicular incision. Continuous dilated fistulas were found between the VA and vertebral vein. Their complicated structure corroborated our perspective that endovascular therapy was not the most reliable option. Because of the good collateral circulation, right VA occlusion was planned. Ligation of the VA was performed above and below the fistula. The patient's pulsatile tinnitus had immediately resolved after surgery, and she did not experience neurologic deficits. Follow-up CTA was performed 3 months after surgery and confirmed the absence of recurrence of the right cervical VA fistula (Fig 2, C).
Discussion
VVAVFs are rare entities without sufficient clinical data and guidelines.3 Typically, VVAVFs arise from trauma or iatrogenic punctures but can also develop spontaneously owing to a possible genetic disorder.5 A literature review of 280 cases showed that 24% of VVAVFs were secondary to iatrogenic complications. The three most common causes of iatrogenic vertebrovertebral fistulas are central venous catheterization (65%), angiographic catheterization (9%), and C1-C2 pedicle or transarticular screw fixation during cervical surgery (7%).6
Tinnitus and audible bruit are most common symptoms owing to the rapid arteriovenous shunting. The diagnosis will be incidental at routine clinical examinations for some patients, who will remain asymptomatic. Neurologic symptoms of vertigo and diplopia can occur secondary to the vertebral arterial steal phenomena resulting from partial cutoff of the cerebral blood supply.7
Management of VVAVFs, especially the optimal standard treatment, has not yet been defined. Although guidelines are available for treating VA injuries after nonpenetrating cervical trauma,8 guidelines specific to VVAVFs would be important and beneficial given the general lack of experience. Although spontaneous VVAVF occlusion has been reported in pediatric cases,9,10 a delay in treatment for adult patients should not be recommended to the best of our knowledge. A delay in intervention will allow time for the fistula to recruit extra feeding vessels, leading to more difficulties and hinder possible treatment in the future. Therefore, we believe that for patients with symptomatic bruit or existing retrograde, intracranial, or spinal cord venous drainage, treatment is required for symptomatic relief.
Regarding the treatment technique, endovascular therapy has been increasing in popularity as the treatment modality for VVAVFs in recent years.11 Depending on the patient's individual anatomy, endovascular treatment can be constructive or deconstructive. Constructive treatment selectively occludes the fistula, and keeps the parent artery intact.12,13 Several studies have demonstrated that detachable balloons are most appropriate for the occlusion of fistulas because they can be repeatedly inflated and deflated, allowing for precise placement and achievement of optimal fistula occlusion.14,15 However, the balloon could deflate, resulting in recurrence of the fistula. Although covered stent reconstruction offers a therapeutic alternative and has achieved success in reported studies,16,17 it also shares the risks of stent migration, kinking, fracture and fistula recurrence, especially in tortuous vessels.18, 19, 20 Deconstructive treatment supplements constructive treatment, excluding the partial risks of recurrence and complications by blocking the fistula and parent artery containing the fistula. It is considered relatively safe to block the parent artery if the patient has adequate contralateral VA flow, because the ipsilateral VA will compensate and exclusively feed the fistula.
Given that our first patient presented with significant tortuosity, which prevented the passage of detachable balloons, and considering all the factors we have discussed, VA sacrifice with coil embolization was chosen. We also performed adequate preparation and evaluation preoperatively, such as imaging measurements of the bilateral VA diameter, which we believe are important before treatment. For our first patient, we used interlocking detachable coils. Their use was relatively safe in our first patient because the blood supply from the contralateral VA was adequate. However, if the VA that contains the fistula were dominant, covered stent reconstruction might still serve as a good option. Although detachable coils can slow the flow and lower the pressure within the fistula, other embolic agents such as the nonadhesive and well-penetrating Onyx (Medtronic, Dublin, Ireland) can also be used. However, the embolic agents that present with the characteristics of high adhesiveness and early polymerization should be avoided during arteriovenous fistula treatment according to the reported data.21, 22, 23
Although open ligation surgery might seem more invasive and challenging owing to the anatomic position and the artery's course through the cervical vertebrae,3 some selected cases can still be, and should be, treated by surgery, especially when endovascular treatment is not feasible or carries a high risk of failure in patients with complicated pathologic cases.24 In our second patient, the preoperative carotid ultrasound scan showed high flow in the VA fistula, with confirmation by digital subtraction angiography. Although no classification system of velocity for VVAVFs is available, we thought the flow was strong enough to potentially result in the risk of embolization and material migration through the fistula. In addition, multiple arterialized venous channels, evident tortuosity, and inaccessibility of the fistula were present. Thus, we did not choose endovascular therapy. With a dominant left VA supplying the posterior circulation, as demonstrated by the imaging studies, we believed that occlusion of the VA had a relatively low risk of causing significant basilar territory ischemia. Therefore, ligation of the VA was performed above and below the fistula to achieve complete obliteration of the fistula, which can prevent retrograde flow from the contralateral VA, leading to systemic pressurization or fistula recurrence. Although the patient's symptoms resolved, long-term follow-up is necessary to fully assess the eventual outcome.
Conclusions
The clinical strategy is important to surgeons regarding the treatment of iatrogenic VVAVFs. To date, no guidelines are available for the best treatment of VVAVFs. Although the use of endovascular coiling or detachable balloons is considered well-tolerated treatment, open surgical treatment should still be reserved for the most complex cases and those not feasible for endovascular treatment.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Dodson T., Quindlen E., Crowell R., McEnany M.T. Vertebral arteriovenous fistulas following insertion of central monitoring catheters. Surgery. 1980;87:343–346. [PubMed] [Google Scholar]
- 2.Jamieson K.G. Vertebral arteriovenous fistula caused by angiography needle: report of a case. J Neurosurg. 1965;23:620–621. doi: 10.3171/jns.1965.23.6.0620. [DOI] [PubMed] [Google Scholar]
- 3.Edwards M.K., Christenson E.N., Corliss B.M., Polifka A.J., Allen B.R. Vertebral arteriovenous fistula: an unwelcome thrill. Case Rep Emerg Med. 2017;2017:8386459. doi: 10.1155/2017/8386459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felbaum D., Chidambaram S., Mason R.B., Armonda R.A., Liu A.H. Vertebral–venous fistula: an unusual cause for ocular symptoms mimicking a carotid cavernous fistula. Case Rep. 2015;2015 doi: 10.1136/bcr-2015-011796. bcr2015011796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao P., Chen Y., Zhang H., Zhang P., Ling F. Vertebral arteriovenous fistulae (AVF) in neurofibromatosis type 1: a report of two cases. Turk Neurosurg. 2013;23:289–293. doi: 10.5137/1019-5149.JTN.4993-11.0. [DOI] [PubMed] [Google Scholar]
- 6.Aljobeh A., Sorenson T.J., Bortolotti C., Cloft H., Lanzino G. Vertebral arteriovenous fistula: a review article. World Neurosurg. 2019;122:e1388–e1397. doi: 10.1016/j.wneu.2018.11.063. [DOI] [PubMed] [Google Scholar]
- 7.Tse G.H., Patel U.J., Coley S.C., Dyde R.A. Cervical cord decompression following embolisation of a giant cervical vertebro-vertebral arteriovenous fistula. Interv Neuroradiol. 2017;23:399–404. doi: 10.1177/1591019917708569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrigan M.R., Hadley M.N., Dhall S.S., Walters B.C., Aarabi B., Gelb D.E. Management of vertebral artery injuries following non-penetrating cervical trauma. Neurosurgery. 2013;72(Suppl 3):234–243. doi: 10.1227/NEU.0b013e31827765f5. [DOI] [PubMed] [Google Scholar]
- 9.Adams A., Allouni K., Basheer S., Evanson J., Mankad K. Case report: spontaneous resolution of an established iatrogenic vertebral arteriovenous fistula. J Child Neurol. 2013;28:255–258. doi: 10.1177/0883073812441066. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell P., Thomas P., Anderson D. Spontaneous resolution of a non-traumatic vertebro-vertebral arteriovenous fistula in a paediatric patient. J Clin Neurosci. 2018;53:220–222. doi: 10.1016/j.jocn.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Nagashima C., Iwasaki T., Kawanuma S., Sakaguchi A., Kamisasa A., Suzuki K. Traumatic arteriovenous fistula of the vertebral artery with spinal cord symptoms: case report. J Neurosurg. 1977;46:681–687. doi: 10.3171/jns.1977.46.5.0681. [DOI] [PubMed] [Google Scholar]
- 12.Uneda A., Suzuki K., Okubo S., Hirashita K., Yunoki M., Yoshino K. Neurofibromatosis type 1–associated extracranial vertebral artery aneurysm complicated by vertebral arteriovenous fistula after rupture: case report and literature review. World Neurosurg. 2016;96:609.e13–609.e18. doi: 10.1016/j.wneu.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Amdani S.M., Forbes T. Congenital vertebral arteriovenous fistula. Indian J Pediatr. 2018;85:325–326. doi: 10.1007/s12098-017-2466-2. [DOI] [PubMed] [Google Scholar]
- 14.Modi M., Bapuraj J.R., Lal A., Prabhakar S., Khandelwal N. Vertebral arteriovenous fistula presenting as cervical myelopathy: a rapid recovery with balloon embolization. Cardiovasc Intervent Radiol. 2010;33:1253–1256. doi: 10.1007/s00270-009-9708-2. [DOI] [PubMed] [Google Scholar]
- 15.Reddy M., Schöggl A., Saringer W., Reddy B., Matula C. Traumatic arteriovenous fistula of the vertebral artery. Neurol Med Chir (Tokyo) 2002;42:289–292. doi: 10.2176/nmc.42.289. [DOI] [PubMed] [Google Scholar]
- 16.Sancak T., Bilgic S., Ustuner E. Endovascular stent-graft treatment of a traumatic vertebral artery pseudoaneurysm and vertebrojugular fistula. Korean J Radiol. 2008;9(Suppl):S68–S72. doi: 10.3348/kjr.2008.9.s.s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F., Song X., Liu C., Liu B., Zheng Y. Endovascular stent-graft treatment for a traumatic vertebrovertebral arteriovenous fistula with pseudoaneurysm. Ann Vasc Surg. 2014;28 doi: 10.1016/j.avsg.2012.12.013. 489.e11-489.e14. [DOI] [PubMed] [Google Scholar]
- 18.Sadato A., Satow T., Ishii A., Takayama M., Hashimoto N. Large vertebral arteriovenous fistula treated with stent-grafts. Neurol Med Chir (Tokyo) 2003;43:250–254. doi: 10.2176/nmc.43.250. [DOI] [PubMed] [Google Scholar]
- 19.Surber R., Werner G.S., Cohnert T.U., Wahlers T., Figulla H.R. Recurrent vertebral arteriovenous fistula after surgical repair: treatment with a self-expanding stent-graft. J Endovasc Ther. 2003;10:49–53. doi: 10.1177/152660280301000111. [DOI] [PubMed] [Google Scholar]
- 20.Hüttl K., Sebestyén M., Entz L., Molnár A.Á., Nemes B., Bérczi V. Covered stent placement in a traumatically injured vertebral artery. J Vasc Interv Radiol. 2004;2:201–202. doi: 10.1097/01.rvi.0000109406.52762.98. [DOI] [PubMed] [Google Scholar]
- 21.Long X.A., Karuna T., Zhang X., Luo B., Duan C.Z. Onyx 18 embolisation of dural arteriovenous fistula via arterial and venous pathways: preliminary experience and evaluation of the short-term outcomes. Br J Radiol. 2012;85:e395–e403. doi: 10.1259/bjr/25192972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakhloo A.K., Perlow A., Linfante I., Sandhu J.S., Cameron J., Troffkin N. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26:1888–1897. [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z.R., Jiang Z.B., Huang M.S., Zhu K.S., Wang Q., Shan H. Transvenous embolization of cavernous sinus dural arteriovenous fistulas using detachable coils and Glubran 2 acrylic glue via the inferior petrosal sinus approach. Eur Radiol. 2010;20:2939–2947. doi: 10.1007/s00330-010-1857-9. [DOI] [PubMed] [Google Scholar]
- 24.Briganti F., Tedeschi E., Leone G., Marseglia M., Cicala D., Giamundo M. Endovascular treatment of vertebro-vertebral arteriovenous fistula: a report of three cases and literature review. Neuroradiol J. 2013;26:339–346. doi: 10.1177/197140091302600315. [DOI] [PMC free article] [PubMed] [Google Scholar]