Abstract
Iliac branch endograft devices offer an elegant solution to preserve perfusion to the internal iliac artery when treating aortoiliac aneurysms; however, they are difficult to perform when bilateral access is not available owing to aortoiliac anatomy or previous endovascular aortic aneurysm repair. We present a technique to perform iliac branch endograft deployment from ipsilateral access in a patient with a prior EVAR endovascular aortic aneurysm repair, obviating the need for a difficult up-and-over access.
Keywords: Iliac branch device, Iliac aneurysm, Ipsilateral deployment
Iliac aneurysms can complicate distal seal in standard endovascular aneurysm repair (EVAR). The development of iliac branched endograft (IBE) devices has allowed for the safe treatment of iliac aneurysms while maintaining perfusion to the internal iliac arteries.1, 2, 3, 4 However, the treatment of bilateral iliac aneurysms in patients with a previous EVAR and loss of distal seal resulting in type Ib endoleaks is complicated by the raised bifurcation from the previous endograft. Here, we describe a technique for ipsilateral IBE deployment to avoid the challenges of navigating a raised aortic bifurcation.
Case report
A 79-year-old man with a history of EVAR using the Endurant device (Medtronic, Minneapolis, Minn) for ruptured abdominal aortic aneurysm in 2016 was referred to our service for treatment of aneurysm sac enlargement and bilateral type Ib endoleaks (Fig 1). The patient agreed to repair with bilateral IBE devices delivered from each ipsilateral groin and signed the institutional consent form with permission to publish images and clinical details from his procedure.
Fig 1.
Preoperative imaging. Preoperative computed tomography angiography scan demonstrated interval 2 cm aneurysm sac enlargement with a good proximal seal (A), no evidence of type II endoleak (B), and poor distal apposition of the iliac limbs within aneurysmal common iliac arteries bilaterally (C).
After obtaining bilateral percutaneous access, an 18F Dryseal sheath (W. L. Gore & Associates, Flagstaff, Ariz) was placed on the right. An arteriogram with an ipsilateral oblique projection, given the posterolateral oriented hypogastric origin, was used to identify the origin of the right internal iliac artery, and a 23- × 12-mm bell-bottom iliac limb was deployed into the prior EVAR stent graft to allow adequate seal of the IBE, ensuring that the proximal graft did not extend above the aortic endograft bifurcation. A 23- × 14.5- × 10-cm Gore IBE device (W. L. Gore & Associates) was then advanced and was noted to be biasing toward the lesser curvature of the iliac, pinning it against the origin of the right internal iliac artery. To avoid trapping the iliac branch along this lesser curvature, the IBE device was, therefore, deployed with the contralateral gate facing laterally, away from the internal iliac artery.
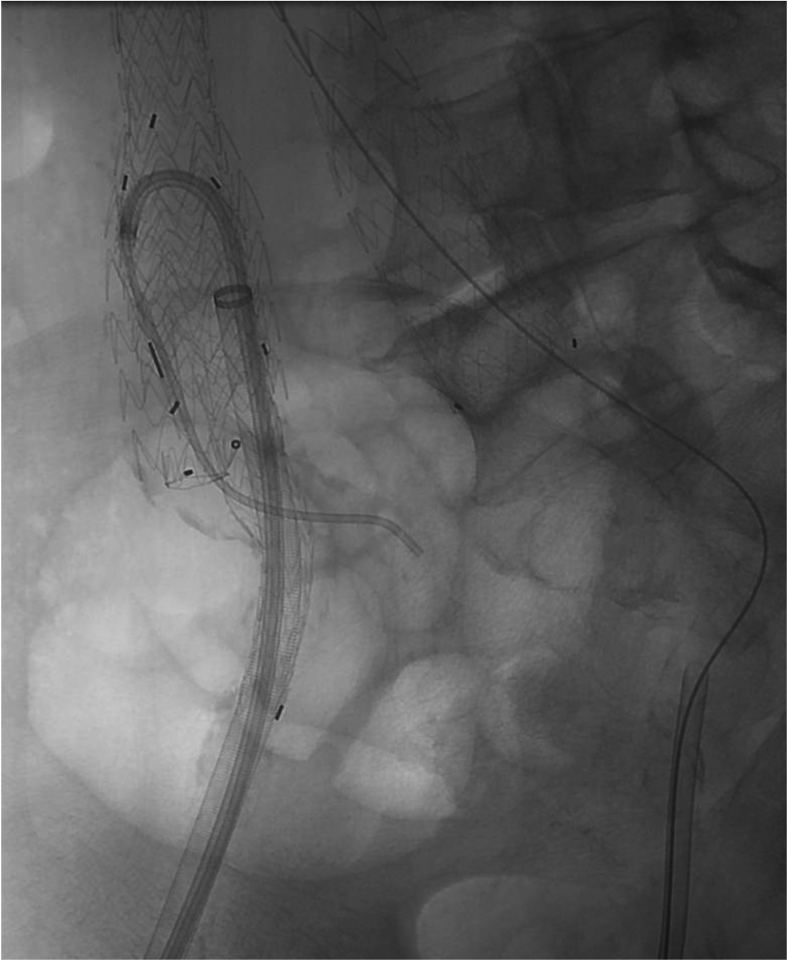
The 18F sheath was carefully readvanced above the flow divider of the IBE, and a steerable sheath (Destino Twist; Oscor Inc, Palm Harbor, Fla) was advanced in a buddy fashion and directed down the contralateral limb to the hypogastric orifice, which was cannulated using a glide wire and glide catheter (Fig 2).5 Arteriography confirmed correct cannulation, and the catheter was advanced over the wire into distal branches of the internal iliac artery before the glide wire was exchanged for an Amplatz wire. The steerable sheath was then removed, and a Lunderquist wire and Coda balloon were advanced in a buddy fashion and inflated in the distal bell-bottom graft. Using the Coda balloon as a backboard, an 8F Pinnacle Destination sheath (Terumo Interventional Systems, Somerset, NJ) was advanced over the IBE bifurcation to the distal end of the contralateral limb (Fig 3).
Fig 2.
Selection of the right internal iliac artery. After deployment of the iliac branch endograft (IBE) with the internal iliac gate facing laterally to provide room for cannulation, the 18F sheath is advanced into the main body of the IBE. A steerable sheath is then used to navigate over the IBE flow divider and allow cannulation of the internal iliac artery using a glide wire and glide catheter.
Fig 3.
Advancing the sheath over the flow divider of the iliac branch endograft (IBE) using the Coda balloon. With the Coda balloon acting as a backboard, the 8F sheath is advanced over the IBE flow divider into the internal iliac artery to allow deployment of the internal iliac limb.
An 8- × 79-mm VBX balloon-expandable stent was then advanced into the cannulated right internal iliac artery and deployed. The proximal portion of the graft was flared with a 12 × 2 balloon (Fig 4), and a completion arteriogram demonstrated seal in the internal iliac artery with patent side branches and no endoleak.
Fig 4.
Proximal flaring of the internal iliac limb. Demonstrated is the 18F sheath within the right external iliac artery (black arrow), the Coda balloon placed in a buddy fashion within the proximal main body of the iliac branch endograft (IBE) to provide support for device tracking over the IBE bifurcation (red arrow), and a 12 mm × 2 cm balloon (white arrow) used to flair the proximal VBX stent within the internal iliac artery (gold arrow).
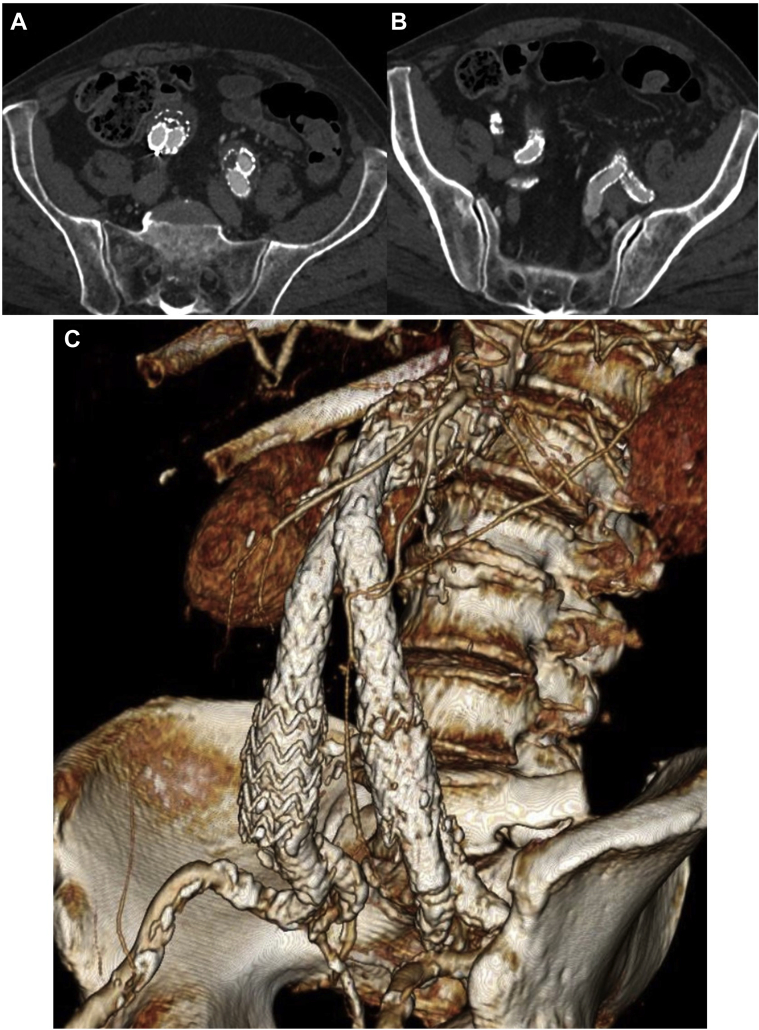
All overlap and seal zones were then ballooned with a Coda balloon, except in the region of the VBX to avoid stent collapse. We then completed the identical procedure on the left side. On completion imaging we observed excellent stent apposition and preservation of blood flow into both internal iliac arteries. At 18 months follow-up, the prior EVAR and bilateral IBEs were widely patent with no evidence of endoleak seen on computed tomography scan (Fig 5).
Fig 5.
Follow-up imaging. At 18 months follow-up, the prior endovascular aortic aneurysm repair (EVAR) and bilateral iliac branch endograft (IBE) devices are widely patent on computed tomography angiography (A and B) with no endoleaks seen. A 3-dimensional reconstructed image (C), with a projection of left anterior oblique 51° and caudal 13°, demonstrates the final appearance.
Discussion
The success of IBE in maintaining perfusion to the internal iliac arteries while treating iliac aneurysms or type Ib endoleaks after EVAR has been demonstrated by multiple studies.1, 2, 3 As a result of the mounting evidence of pelvic ischemic complications and the quality of life implications for patients suffering from buttock claudication or erectile dysfunction after hypogastric artery embolization, maintaining internal iliac artery perfusion after EVAR is becoming increasingly common.6, 7, 8 In patients with a previous EVAR, however, maintaining internal iliac perfusion with an IBE is technically challenging given the new anatomic constraints created by the endograft. Tracking a 12F sheath over the flow divider in these instances is challenging and requires either downward tension on the main body of the endograft, risking graft migration, or the use of alternative access (ie, brachial access) or various up-and-over techniques, such as the push-and-pull technique previously described.9, 10, 11
Although these techniques avoid placing downward tension on the flow divider, they are associated with other possible complications, especially in the setting of brachial access, such as brachial sheath hematomas, median nerve injury, and upper extremity ischemic complications from distal embolization, brachial artery thrombosis, or dissection. Previous studies have demonstrated minor complication (pseudoaneurysm or hematoma) rates of 2% to 14% and major complication (distal embolization; brachial artery thrombosis, dissection, or fistula formation) rates of 1.9% to 10.0% associated with brachial access.12, 13, 14 In addition to access site complications, upper extremity access is also associated with an increased risk of stroke from wire manipulation within the aortic arch.15
The technique described in this report avoids the risks associated with upper extremity access or navigation of large sheaths over the raised bifurcation of a previously placed EVAR, allowing for the treatment of common iliac aneurysms through an entirely ipsilateral approach and preventing undue caudally directed forces on the graft bifurcation with subsequent potential migration. In addition, this approach can also be used in cases of contralateral iliac occlusive disease or others in which bilateral femoral access is not an option.
There are several important anatomic constraints that limit the ipsilateral IBE technique. There must be enough length between the iliac bifurcation and the EVAR limb such that the IBE can be deployed without trapping the internal iliac gate. Additionally, the distal common iliac or EVAR limb must be wide enough to accommodate a steerable 6F sheath used to cannulate the internal iliac artery. In our case, we used a bell-bottom limb to provide sufficient space, although this technique is not required. The Gore IBE requires a minimum 17-mm common iliac diameter, which can accommodate a 6.5F Destino Twist (Oscor Inc) based on the curve bend of 9 mm, which corresponds with an approximate 15-mm length of the flexed portion of the sheath. Given these measurements, this technique is not possible with the Cook (Cook Medical, Bloomington, Ind) device owing to the 15-mm proximal diameter.
Conclusions
As the use of IBE devices continues to expand beyond the treatment of common iliac aneurysms to include more complicated pathology (type Ib endoleak after EVAR) or patient anatomy (contralateral iliac occlusive disease), the development of alternative, safe delivery techniques is necessary. Ipsilateral IBE deployment, as described here, can be safely done in complex situations to avoid complications associated with upper extremity access or navigating previously placed endografts.
From the Western Vascular Society
Footnotes
Author conflict of interest: B.W.S. is the co-founder of Aortica Corporation, acquired in November 2019 by Terumo Corporation. There are no other disclosures to report.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Maldonado T.S., Mosquera N.J., Lin P., Bellosta R., Barfield M., Moussa A. Gore iliac branch endoprosthesis for treatment of bilateral common iliac artery aneurysms. J Vasc Surg. 2018;68:100–108.e3. doi: 10.1016/j.jvs.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Schneider D.B., Matsumura J.S., Lee J.T., Peterson B.G., Chaer R.A., Oderich G.S. Prospective, multicenter study of endovascular repair of aortoiliac and iliac aneurysms using the Gore Iliac Branch Endoprosthesis. J Vasc Surg. 2017;66:775–785. doi: 10.1016/j.jvs.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Itoga N.K., Fujimura N., Hayashi K., Obara H., Shimizu H., Lee J.T. Outcomes of endovascular repair of aortoiliac aneurysms and analyses of anatomic suitability for internal iliac artery preserving devices in japanese patients. Circ J. 2017;81:682–688. doi: 10.1253/circj.CJ-16-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce B.J., Varu V.N., Glocker R., Novak Z., Jordan W.D., Lee J.T. Anatomic suitability of aortoiliac aneurysms for next generation branched systems. Ann Vasc Surg. 2015;29:69–75. doi: 10.1016/j.avsg.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Derubertis B.G., Quinones-Baldrich W.J., Greenberg J.I., Jimenez J.C., Lee J.T. Results of a double-barrel technique with commercially available devices for hypogastric preservation during aortoilac endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2012;56:1252–1259. doi: 10.1016/j.jvs.2012.04.070. [DOI] [PubMed] [Google Scholar]
- 6.Mansour W., Capoccia L., Sirignano P., Montelione N., Pranteda C., Formiconi M. Clinical and functional impact of hypogastric artery exclusion during EVAR. Vasc Endovascular Surg. 2016;50:484–490. doi: 10.1177/1538574416665968. [DOI] [PubMed] [Google Scholar]
- 7.Jean-Baptiste E., Brizzi S., Bartoli M.A., Sadaghianloo N., Baque J., Magnan P.E. Pelvic ischemia and quality of life scores after interventional occlusion of the hypogastric artery in patients undergoing endovascular aortic aneurysm repair. J Vasc Surg. 2014;60:40–49. doi: 10.1016/j.jvs.2014.01.039. e1. [DOI] [PubMed] [Google Scholar]
- 8.Kouvelos G.N., Katsargyris A., Antoniou G.A., Oikonomou K., Verhoeven E.L.G. Outcome after interruption or preservation of internal iliac artery flow during endovascular repair of abdominal aorto-iliac aneurysms. Eur J Vasc Endovasc Surg. 2016;52:621–634. doi: 10.1016/j.ejvs.2016.07.081. [DOI] [PubMed] [Google Scholar]
- 9.Dawson D.L., Sandri G., Tenorio E., Oderich G.S. Up-and-Over Technique for implantation of iliac branch devices after prior aortic endograft repair. J Endovasc Ther. 2018;25:21–27. doi: 10.1177/1526602817747283. [DOI] [PubMed] [Google Scholar]
- 10.Tenorio E.R., Oderich G.S., Sandri G.A., Karkkainen J.M., Kalra M., DeMartino R.R. Outcomes of an iliac branch endoprosthesis using an “up-and-over” technique for endovascular repair of failed bifurcated grafts. J Vasc Surg. 2019;70:497–508.e1. doi: 10.1016/j.jvs.2018.10.098. [DOI] [PubMed] [Google Scholar]
- 11.Wang S.K., Miladore J.N., Yee E.J., Liao J.L., Donde N.N., Motaganahalli R.L. Combined transbrachial and transfemoral strategy to deploy an iliac branch endoprosthesis in the setting of a pre-existing endovascular aortic aneurysm repair. J Vasc Surg Cases Innov Tech. 2019;5:305–309. doi: 10.1016/j.jvscit.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz R.W., Tanga C.F., Herrmann J.W. Treatment of peripheral arterial disease via percutaneous brachial artery access. J Vasc Surg. 2017;66:461–465. doi: 10.1016/j.jvs.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 13.Treitl K.M., König C., Reiser M.F., Treitl M. Complications of transbrachial arterial access for peripheral endovascular interventions. J Endovasc Ther. 2015;22:63–70. doi: 10.1177/1526602814564363. [DOI] [PubMed] [Google Scholar]
- 14.Madden N.J., Calligaro K.D., Zheng H., Troutman D.A., Dougherty M.J. Outcomes of brachial artery access for endovascular interventions. Ann Vasc Surg. 2019;56:81–86. doi: 10.1016/j.avsg.2018.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Benson R.A., Matthews D., Loftus V., Nicholson G., Tropman D., Loftus I.M. Cerebral embolization during endovascular infrarenal, juxtarenal, and suprarenal aortic aneurysm repair, high-risk maneuvers, and associated neurologic outcomes. J Vasc Surg. 2018;68:1374–1381. doi: 10.1016/j.jvs.2018.01.041. [DOI] [PubMed] [Google Scholar]