Abstract
OBJECTIVE
To determine the impact of tumor characteristics and treatment approach on (1) local recurrence, (2) scoliosis development and (3) patient-reported quality of life in children with sarcoma of the chest wall.
SUMMARY BACKGROUND DATA
Children with chest wall sarcoma require multimodal therapy including chemotherapy, surgery and/or radiation. Despite aggressive therapy which places them at risk for functional impairment and scoliosis, these patients are also at significant risk for local recurrence.
METHODS
A multi-institutional review of 175 children (median age 13 years) with chest wall sarcoma treated at seventeen Pediatric Surgical Oncology Research Collaborative institutions between 2008–2017 was performed. Patient-reported quality of life was assessed prospectively using PROMIS surveys.
RESULTS
The most common diagnoses were Ewing sarcoma (67%) and osteosarcoma (9%). Surgical resection was performed in 85% and radiation in 55%. A median of 2 ribs were resected (IQR=1–3), and number of ribs resected did not correlate with margin status (p=0.36). Local recurrence occurred in 23% and margin status was the only predictive factor (HR 2.24, p=0.039). With a median follow-up of 5 years, 13% developed scoliosis (median Cobb angle 26) and 5% required corrective spine surgery. Scoliosis was associated with posterior rib resection (HR 8.43; p=0.003) and increased number of ribs resected (HR 1.78; p=0.02). Overall, patient-reported quality of life is not impaired following chest wall tumor resection.
CONCLUSIONS
Local recurrence occurs in one-quarter of children with chest wall sarcoma and is independent of tumor type. Scoliosis occurs in 13% of patients, but patient-reported quality of life is excellent.
Mini Abstract
This study is a multi-institutional review of local recurrence and functional outcomes for children with chest wall sarcoma. Local recurrence occurs in 23% of children and is independent of tumor type but correlates with margin status. Scoliosis occurs in 13% of patients, but patient-reported quality of life is excellent.
1. INTRODUCTION
Chest wall tumors are rare in children, and account for less than 2% of solid tumors in pediatric patients (1,2). The majority of primary tumors are malignancies of mesenchymal origin, with approximately 45% soft tissue sarcoma histology and 55% cartilaginous or bony origin (2). Ewing sarcoma is the most common malignant histology followed by osteosarcoma, rhabdomyosarcoma and other soft tissue sarcomas (3–7).
Multimodal treatment of pediatric chest wall tumors includes a combination of chemotherapy, surgery and/or radiation (3,4,7). Each treatment modality has its own potential short and long-term morbidities. Complications of surgical resection and reconstruction can include scoliosis, cosmetic disfigurement, chronic pain and activity restriction due to inadequate thoracic protection (6–10). Adverse effects of radiation can include pulmonary or cardiac dysfunction, functional impairment, secondary malignancy, and chest wall hypoplasia, especially when exposed in the prepubertal age group (10–12). The late effects of chemotherapy in children include increased risk of developing cardiac, endocrine, or musculoskeletal conditions, as well as second malignancies (13). Although prognosis has been historically poor, advances in multimodal treatment strategies have greatly improved survival for these patients (7,14,15).
Because of the importance of obtaining negative microscopic margins, extensive resection, including removal of the rib above and below the tumor, is often required (3). The functional effects of these extensive resections and reconstructions are incompletely understood.
Reconstructive techniques must take into account the effect on chest wall stability and function, as well as thoracic protection (1,7). There have been single-institution descriptions of patients who underwent chest wall resection and reconstruction (6,7), but multi- institutional data is needed to better assess local recurrence, risk of scoliosis and patient- reported quality of life. This study represents a multi-institutional collaborative effort to evaluate these oncologic and functional outcomes.
2. METHODS
2.1. Patient Data
Patients from 17 hospitals participating in the Pediatric Surgical Oncology Research Collaborative (PSORC) were included in the study. PSORC is a multi-institutional consortium of North American pediatric surgeons dedicated to advancing the surgical care of children with cancer. Central Institutional Review Board (IRB) approval was obtained at Cincinnati Children’s Hospital Medical Center (IRB#2018–7707) and each institution obtained IRB approval, either individually or through reliance on the central IRB. Study data were collected and managed using REDCapTM electronic data capture tools hosted at Cincinnati Children’s Hospital Medical Center. Patients were included in the study if they were 1–20 years old at the time of diagnosis between 2008–2017. The study included patients with malignant tumors of the chest wall, including Ewing sarcoma, osteosarcoma, rhabdomyosarcoma and non-rhabdomyomatous soft tissue sarcoma. The chest wall was defined to include rib, sternum, cartilage and the surrounding soft tissues. Pleural-based tumors were included only if they required resection of the chest wall for removal. Scoliosis diagnosis was designated by each institution. Cobb angle was reported and is defined as the angle between the lines parallel to the endplate of the superior and inferior end vertebrae (16). Retrospective data collected included patient age, tumor characteristics, treatment modalities, oncologic outcomes, survival and scoliosis presence and outcomes (Supplemental Digital Content 1, http://links.lww.com/SLA/C703).
2.2. Quality of Life Survey
Patient-Reported Outcomes Measurement Information System (PROMIS) surveys were utilized to measure patient reported outcomes. Only patients and families who spoke English (all sites), Spanish (all sites) and French (Centre Hospitalier Universitaire Sainte-Justine only) were included. Patients who qualified for the prospective arm of the study were contacted via phone, electronic communication and/or postal mailing by the local site investigators with an IRB-approved standardized script. Consent was exempt for the retrospective chart review but was obtained for parents of children less than 18 years, and from patients older than 18 years for the prospective arm of the study.
Patients who were older than 8 years, but younger than 18 years, were assessed using the PROMIS Pediatric Profile 37, version 2 (Supplemental Digital Content 2, http://links.lww.com/SLA/C704). For patients who were over 18 years at the time of survey distribution, the PROMIS 43 Profile, version 2.1 was utilized (Supplemental Digital Content 3, http://links.lww.com/SLA/C705). Surveys were completed electronically in REDCapTM or by paper surveys returned to the institutional investigators. For the PROMIS 37, higher scores indicate better outcomes for physical function and peer relationships, and worse outcomes for anxiety, depressive symptoms, fatigue and pain interference. For the PROMIS 43, higher scores indicate better outcomes for physical function, and worse outcomes for participation in social roles and activities, anxiety, depression, fatigue, pain interference and sleep disturbance. Patients with T scores within one-half standard deviation of the mean were considered normal, one-half to one standard deviation were considered trending better/worse, and more than one standard deviation from the mean was considered better/worse compared to the reference population.
2.3. Statistical Analysis
Results are reported as proportions for categorical variables and medians with interquartile ranges for continuous variables. Rate estimates for recurrence free survival (RFS) with the event of local recurrence were obtained via the Kaplan-Meier method and differences between the treatment regimens were assessed using the log-rank test. Multivariable logistic regression was conducted in SAS v9.4 to obtain odds ratio estimates and 95% confidence intervals, with p<0.05 indicating statistical significance. Multivariate Cox regression models were fit to obtain hazard ratio estimates and their 95% confidence intervals, also conducted in SAS v9.4 with p<0.05 indicating statistical significance.
To score the PROMIS surveys, item response theory was used to convert raw scores into T scores, using the appropriate version-specific guidelines. The HealthMeasures scoring service was used to calculate T scores (https://www.assessmentcenter.net/ac_scoringservice). A T score of 50 indicates the reference population, and the standard deviation is 10.
3. RESULTS
3.1. Patient characteristics
One hundred seventy-five patients from 17 institutions were included in the retrospective study. The median age at diagnosis was 13 years (IQR 9–16). Sixty-seven percent of patients had Ewing sarcoma and 9% had osteosarcoma. 51% of tumors were on the left side, 48% were located posteriorly and 50% were on ribs 4–8. Of note, thirty-eight patients had multifocal tumors (Table 1). With a median follow-up of 5 years (IQR 2.6–7.3), 71% (124/175) of patients are alive (with or without evidence of disease) including 75% with Ewing sarcoma, 60% with osteosarcoma and 66% with other tumors.
Table 1:
Patient Demographics and Tumor Characteristics
| N | % | |
|---|---|---|
| Tumor histology | ||
| Ewing sarcoma | 118 | 67% |
| Other | 28 | 16% |
| Osteosarcoma | 15 | 9% |
| Rhabdomyosarcoma | 13 | 7% |
| Tumor laterality | ||
| Left | 90 | 51% |
| Right | 83 | 47% |
| Location of tumor on rib | ||
| Anterior | 65 | 37% |
| Middle | 27 | 15% |
| Posterior | 84 | 48% |
| Rib number | ||
| 1–3 | 49 | 23% |
| 4–8 | 106 | 50% |
| 9–12 | 57 | 27% |
3.2. Local control technique
The majority of patients received multimodal therapy including surgery, chemotherapy and radiation therapy in 42%; surgery and chemotherapy in 35%; and chemotherapy and radiation therapy in 13%. Overall, 85% of patients underwent surgical resection and 55% received radiation therapy. Surgery was utilized in 88% of patients with Ewing sarcoma and 93% with osteosarcoma, while radiation was utilized in 58% of patients with Ewing sarcoma and 20% with osteosarcoma.
For those who received radiation therapy, the timing was preoperative in 9% (9/96) and postoperative in 69% (66/96), with three patients receiving both pre- and postoperative therapy (3%). Eighteen percent (17/96) had definitive radiation therapy without surgery. The median radiation dose was 50.4 Gy (IQR 41.3–56.0).
For those who underwent surgery, 79% of procedures occurred after neoadjuvant chemotherapy (117/149). A median of 2 ribs (IQR 1–3) were resected (completely or partially) and 31% (41/131) had at least one rib completely resected. For Ewing sarcoma a median of 3 ribs were resected (IQR 1–3) and for osteosarcoma 1.5 ribs (IQR 1–3). Sixty-five percent (93/142) had an R0 resection, 33% (47/142) had an R1 resection and only 1% (2/142) had an R2 resection. An R0 resection was achieved in 63% (24/38) of those who had one rib resected, 61% (17/28) with two ribs resected, and 74% (50/68) with three or more ribs resected (p=0.36). Chest wall reconstruction was performed at the time of resection in 69% (103/149) of patients, including 89% of patients with 2 or more ribs resected. Reconstruction techniques included synthetic mesh alone (47/103, 46%), methyl methacrylate (without or with mesh) (26/103, 25%), biologic mesh alone (11/103, 11%) and muscle flap alone (8/103, 8%).
3.3. Local recurrence
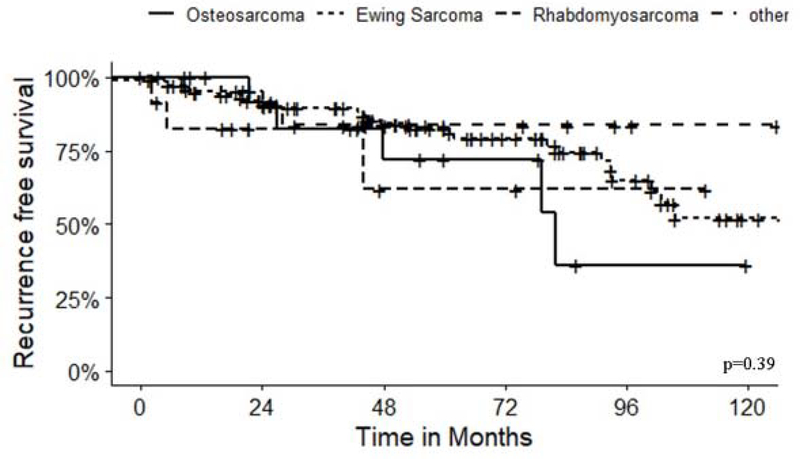
Local recurrence occurred in 23% (41/172) of patients at a median of 1.6 years (IQR=1.1–2.4) after diagnosis, including 24% of patients with Ewing sarcoma and 40% with osteosarcoma. Tumor histology did not correlate with local recurrence (Figure 1) (p=0.31). In a multivariable Cox regression analysis, margin status was the only factor predictive of recurrence (HR 2.24, p=0.039) (Table 2). Additional chest surgery performed after the initial tumor resection was performed in 24% of patients, including additional oncologic surgery in 17%, additional reconstruction in 5% and reoperation for bleeding or infection in 4% of patients. Six of the additional reconstruction procedures were concurrent with additional oncologic resection (5/6) or procedure for complication (1/6).
Figure 1:
Recurrence-free survival for all patients stratified by diagnosis, p=0.39.
Table 2:
Factors predictive of local recurrence in Cox regression analysis.
| Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Ewing Sarcoma | 0.4 | 0.15–1.1 | 0.075 |
| Age <6 years old | Ref | ||
| Ages 6–11 years old | 3.64 | 0.45–29.67 | 0.229 |
| Ages 12–15 years old | 2.22 | 0.26–18.68 | 0.462 |
| Ages >15 years old | 5.24 | 0.64–42.97 | 0.123 |
| Positive margins | 2.24 | 1.04–4.79 | 0.039 |
| Posterior rib tumor | 0.64 | 0.29–1.41 | 0.268 |
| 1 rib resected | 2.44 | 0.25–23.86 | 0.444 |
| 2 ribs resected | 2.73 | 0.27–27.19 | 0.392 |
| ≥ 3 ribs resected | 1.71 | 0.16–17.73 | 0.653 |
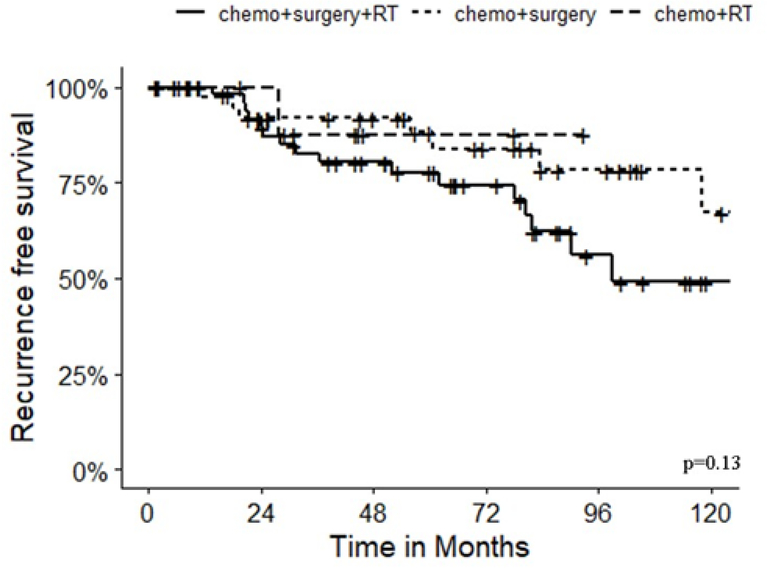
In the subset of patients with Ewing sarcoma, local 5-year recurrence free survival (RFS) was 78% with chemotherapy, surgery and radiation; 88% with chemotherapy and surgery; and 88% with chemotherapy and radiation (p=0.13) (Figure 2). Importantly, microscopic margins were positive in 42% (22/53) of patients who had radiation in addition to chemotherapy and surgery but only 22% (10/46) who had chemotherapy and surgery alone (p=0.11) suggesting that radiation was typically added for patients who had incomplete resection. However, for patients with a positive microscopic margin, local recurrence occurred in 32% (7/22) who had adjuvant radiation therapy compared to 40% (4/10) without radiation therapy. The location of the tumor (p=0.41), number of ribs resected (p=0.9) and radiation therapy (p=0.61) were not associated with recurrence (Table 3).
Figure 2:
Recurrence-free survival among patients with Ewing sarcoma stratified by treatment approach, p=0.13
Table 3:
Cox regression analysis of factors associated with local recurrence for those with Ewing sarcoma
| Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Tumor location | 0.361 | 0.033–3.986 | 0.41 |
| Number of ribs resected | 1.060 | 0.422–2.662 | 0.9 |
| Radiation therapy | 1.886 | 0.169–21.094 | 0.61 |
3.4. Scoliosis
With a median follow-up of 5 years (IQR=2.6–7.3), 13% (23/175) of patients developed scoliosis with a median Cobb angle of 26 (IQR=16–34). All of these patients had undergone surgery and 52% (12/23) also had radiation therapy. Initial reconstruction techniques in patients who developed scoliosis included synthetic mesh in 39%, methyl methacrylate (with or without mesh) in 26%, and biologic mesh with or without muscle flap in 13%. Nine patients required corrective spine surgery which represents 5% of all patients and 39% of those with scoliosis. The median Cobb angle of patients requiring surgery was 37 (IQR 29.8–43). For those who required corrective spine surgery, four (4/9, 44.4%) had an additional chest wall surgery, three for additional oncologic resection and one for removal of an infected mesh. Scoliosis was associated with posterior rib location (OR=5.42, p=0.003) and increasing number of ribs resected (OR=1.5, p=0.03). There was no significant difference in scoliosis development for age, tumor histology, rib number, radiation therapy, or reconstruction material (Table 4).
Table 4:
Multivariate regression analysis for risk of scoliosis
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Age (year) | 0.947 | 0.861–1.042 | 0.27 |
| Tumor histology | 0.877 | 0.283–2.719 | 0.82 |
| Ribs 1–3 | 0.322 | 0.057–1.827 | 0.2 |
| Ribs 4–8 | 0.735 | 0.15–3.593 | 0.7 |
| Ribs 9–12 | 0.401 | 0.080–2006 | 0.27 |
| Tumor location (posterior vs anterior/middle) | 5.423 | 1.75–16.79 | 0.003 |
| Number of ribs resected | 1.54 | 1.04–2.29 | 0.03 |
| Radiation therapy | 1.063 | 0.379–2.979 | 0.91 |
3.5. Patient reported outcomes
Thirty-six patients from 14 institutions completed prospective quality of life (PROMIS) surveys, with 25 (69.4%) under the age of 18 years completing the PROMIS 37 Profile and 11 (30.6%) over the age of 18 years completing the PROMIS 43 Profile. Overall, the median age of the patient at the time of survey completion was 17.5 years old (IQR=14–22), with a median of 5.5 years (IQR=4.1–9) between diagnosis and survey completion. Nine patients with scoliosis completed the surveys.
Overall, in comparison to the reference population, patients had equivalent or trending better physical function, depressive symptoms, fatigue, pain interference and social interactions to the reference population. For the adult patients, anxiety (T score 63.9) and social interaction (T score 59.2) were around one standard deviation worse than in the reference population (Table 5). On a scale of 0–10 (0=no pain, 10=worst pain), when asked for average pain score over the last seven days, those less than 18 years old reported a median pain rating of 1 (IQR=0–3), and for those greater than 18 years a rating of 1 (IQR=0–2).
Table 5:
Patient reported-quality of life metric T-scoresa
| PROMIS 37 (Pediatric) | PROMIS 43 (Adult) | Combined | |
|---|---|---|---|
| Physical Function | 58.4 (trending better) | 51.3 (equivalent) | 55.3 (equivalent) |
| Anxiety | 55 (equivalent) | 63.9 (worse) | 58.3 (trending worse) |
| Depression | 45.7 (equivalent) | 55.2 (equivalent) | 47.5 (equivalent) |
| Fatigue | 48.3 (equivalent) | 55.1 (equivalent) | 49.7 (equivalent) |
| Pain | 41.4 (trending better) | 41.1 (trending better) | 41.1 (equivalent) |
| Sleep | n/a | 57.2 (trending worse) | n/a |
| Social | 50.3 (equivalent) | 59.2 (trending worse) | n/a |
T score: 4–55: equivalent; 40–44 or 56–60 trending toward better/worse; <40 or >60 better/worse
4. DISCUSSION
In this multi-institutional review of children treated for chest wall sarcoma, local recurrence occurred in one-quarter of patients despite multimodal therapy. The majority of patients had Ewing sarcoma, followed by osteosarcoma and rhabdomyosarcoma and there was no difference in local failure by diagnosis. Scoliosis occurred in 13% of patients. Posterior tumor location and increasing number of ribs was associated with a greater risk of scoliosis. Overall, patient reported outcomes are excellent, although patients over 18 years old report increased concerns about anxiety and social interactions.
This is the largest study of pediatric patients with chest wall tumors. The overall distribution of tumors and treatment approach in this study are compatible with data from previous single- institution series. Ewing sarcoma accounted for 41–54% and osteosarcoma 11–36% of chest wall tumors in prior studies, in line with our findings of 68% and 9% respectively (6,7).
Upfront resection is rarely indicated for chest wall tumors, and actively discouraged for Ewing sarcoma, so management typically begins with biopsy followed by metastatic workup and induction chemotherapy (NCT01231906) (4). Ewing sarcoma is generally a chemo- and radio-sensitive tumor, so preoperative chemotherapy can facilitate a less morbid surgical resection. Radiation therapy can be avoided for many patients who undergo an R0 resection and is often given as adjuvant therapy for patients with positive margins; and can be used as definitive local control for patients in whom surgical resection is not safe or feasible.
Osteosarcoma, in contrast, is not radiosensitive, and survival is dependent on complete surgical excision. The utilization of surgery and radiation in our study are reflective of these treatment paradigms. Surgery was utilized in 88% of patients with Ewing sarcoma and 93% with osteosarcoma while radiation was administered in 58% and 20% respectively.
Surgical principles in chest wall surgery include removal of all involved ribs and soft tissue with negative microscopic margins. While this has traditionally involved resection of one rib above and below the lesion, including full thickness musculoskeletal chest wall and underlying pleura, more conservative procedures have also yielded good results. At a minimum, resection of all intercostal muscle above and below the involved rib, along with the periosteum of the adjacent rib is required. Partial circumference rib resection may also be performed on the adjacent ribs. In our study, the median number of ribs resected was 2, and there was no difference in margin status based on number of ribs resected. Margin status, however, was predictive of local recurrence in multivariable Cox regression analysis.
In addition to margin status, there are other factors which may impact local failure-free survival including tumor-size and location, patient age, and utilization of radiotherapy (6,7). We focused on patients with Ewing sarcoma, the largest patient population in our study, to investigate determinants of local failure in a relatively homogenous population. Rates of local recurrence in these patients have been reported to range from 30–55% (6,7). Seitz et al evaluated patients with intrathoracic and chest wall sarcomas and found that the event-free survival of those with chest wall sarcomas was 62%, compared to 38% for those with intrathoracic sarcomas (p=0.008) (17). For patients with Ewing sarcoma of the chest wall, Shamberger et al found an increase in adverse events for tumors greater than 8 cm, and for increasing age (18). They hypothesized that the increased survival with smaller tumors and in younger patients may be related to the increased likelihood of negative surgical margins, as well as altered tumor biology in younger patients (17). Twenty-four percent of patients with Ewing sarcoma in our study had local recurrence and half of those patients died. There was no correlation between tumor location or number of ribs resected, and the risk of local recurrence. These findings would suggest that resection of adjacent ribs can be avoided as long as negative margins can be achieved with a more conservative approach. We also found no difference in local recurrence between patients who had local control with surgery, radiation or both. Local recurrence was only 14% for patients who had R0 resection and no radiotherapy, which was not significantly different from R0 patients who received radiation, and consistent with other studies (15,18,19) supporting the avoidance of radiation for patients with complete surgical excision. There was, however, a significant difference between patients with an R0 resection regardless of radiation, compared to those with an R1 or R2 resection regardless of radiation; further emphasizing the importance of an R0 resection.
Scoliosis was diagnosed in 13% of patients, however less than half of those patients required corrective spinal surgery. Posterior rib location and an increasing number of ribs resected were associated with development of scoliosis. Notably, age, tumor type, tumor rib number, radiation therapy and reconstruction material were not associated with scoliosis development. Our findings generally correlate with prior reports from single institutions, although those findings have varied significantly, with reported scoliosis rates from 11–67% (6–10).
Saltsman et al, evaluated 76 patients over 30 years and although they found patients who needed corrective spinal surgery for scoliosis (32%) were more likely to be younger (<13 years old) at chest wall resection, they did not find any factor significantly associated with scoliosis development (6). Lopez et al, evaluated 44 patients over 15 years and found that tumor location in the posterior rib was associated with scoliosis (7). Scalabre et al, in a multicenter study of 40 patients over 21 years, found scoliosis development was correlated with rapid growth period (<6 years or between 12–15 years old), resection of greater than three ribs and posterior location (10). Interiano et al, described a single center cohort of 367 patients with sarcomas of all locations evaluated over 38 years and found chest radiation (RR 1.8, p<0.005) and higher number of ribs resected (RR 2.64, p<0.0001) increased the risk of scoliosis development when included in the treatment regimen. This study also evaluated self-reported outcomes and found that compared to those without scoliosis, patients described increased functional impairment and cancer related pain, although they had similar mental health parameters (8).
Results of this multi-institutional study may facilitate counselling of patients undergoing therapy for chest wall sarcomas and inform providers about the need for close follow-up for those at the highest risk of developing scoliosis. In determining the factors associated with scoliosis development and functional outcomes, the broad nature of this study accounts for the heterogeneity of chest wall sarcoma treatment, including variations by tumor type and treating institution. Patients with a posteriorly located tumor, or who require rib resection, should be referred early to a specialist in scoliosis for surveillance and treatment if indicated. Scoliosis following chest wall resection has been shown to be convex toward the resection side suggesting a paralytic etiology and thus the possibility of preventative measures including spinal arthrodesis and superficial splints (5,8–10).
Patient-reported quality of life findings were overall very reassuring in this study. On key domains of physical function, depression, fatigue, pain, and sleep, survivors reported function well within one standard deviation of the reference population. However, our study indicates potential increased anxiety and impaired social roles and peer relationships for survivors who are now in adulthood. Psychosocial sequelae of surviving cancer, especially for adolescents, is vital to evaluate in long term survivorship clinics. Adolescent survivors of pediatric cancer are at a high risk for anxiety and posttraumatic stress syndrome, although large studies are lacking in this field (17–19). In addition, survivors of childhood cancer can have challenges in social realms, including, friendships, general social interactions, intimate relationships, educational achievement and future employment (20). Similar to findings from our study, other reports have demonstrated an intersection between psychosocial distress and social difficulties (21,22), which should be discussed with survivors.
Although this represents the largest reported multi-institutional study, it remains retrospective in nature and subject to data availability. Additionally, since Ewing sarcoma represents the vast majority of patients with chest wall sarcoma, it is difficult to draw conclusions on oncology and functional outcomes in the subset of patients with more rare tumors. The majority of patients were alive during the study period and thus eligible to return the PROMIS survey. The relatively limited number of survey participants may have been due to the failure of multiple institutions (representing a large number of cases) to participate in the prospective QOL assessment; difficulties contacting patients, especially for those who were now adults; and patient access and ease of use with REDCapTM for survey completion.
5. CONCLUSION
Sarcomas of the chest wall present unique challenges and are most often treated with multimodal therapy. The majority of patients had surgery, and the number of ribs resected did not significantly affect margin status, suggesting that resection of adjacent ribs might safely be avoided in patients who can achieve negative margins with more conservative surgery. For patients with Ewing sarcoma, good outcomes with chemotherapy and R0 surgical resection suggest that radiation therapy can often be avoided in this situation. Scoliosis occurs in a minority of patients undergoing chest wall resection and is associated with posterior location and increased number of ribs resected. While patient-reported outcomes are excellent, further work is needed to better understand contributors to functional outcomes and quality of life.
Supplementary Material
References
- 1.Soyer T, Karnak İ, Ciftci AO, Şenocak ME, Tanyel FC, Büyükpamukçu N. The results of surgical treatment of chest wall tumors in childhood. Ped Surgery Int. Springer- Verlag; 2005. Nov 19;22(2):135–9. [DOI] [PubMed] [Google Scholar]
- 2.Incarbone M, Pastorino U. Surgical treatment of chest wall tumors. World J Surg. Springer-Verlag; 2001. Feb;25(2):218–30. [DOI] [PubMed] [Google Scholar]
- 3.La Quaglia MP. Chest wall tumors in childhood and adolescence. Semin Pediatr Surg. 2008. Aug;17(3):173–80. [DOI] [PubMed] [Google Scholar]
- 4.Dang NC, Siegel SE, Phillipds JD. Malignant Chest Wall Tumors in Children and Young Adults. J Pediatr Surg. 1999. Dec;34(12):1773–8. [DOI] [PubMed] [Google Scholar]
- 5.Sandler G, Hayes-Jordan A. Chest wall reconstruction after tumor resection. Semin Pediatr Surg. Elsevier Inc; 2018. Jun 1;27(3):200–6. [DOI] [PubMed] [Google Scholar]
- 6.Saltsman JA, Danzer E, Hammond WJ, Rhee D, Berhe S, Monteagudo J, et al. Survival and Scoliosis Following Resection of Chest Wall Tumors in Children and Adolescents. Annals of Surgery. 2019. Jul;:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez C, Correa A, Vaporciyan A, Austin M, Rice D, Hayes-Jordan A. Outcomes of chest wall resections in pediatric sarcoma patients. J Pediatr Surg. Elsevier B.V; 2017. Jan 1;52(1):109–14. [DOI] [PubMed] [Google Scholar]
- 8.Interiano RB, Kaste SC, Li C, Srivastava DK, Rao BN, Warner WC, et al. Associations between treatment, scoliosis, pulmonary function, and physical performance in long-term survivors of sarcoma. Journal of Cancer Survivorship; 2017. Sep 12;:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glotzbecker MP, Gold M, Puder M, Hresko MT. Scoliosis after chest wall resection. J Child Orthop. 2013. Oct;7(4):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scalabre A, Parot R, Hameury F, Cunin V, Jouve J-L, Chotel F. Prognostic Risk Factors for the Development of Scoliosis After Chest Wall Resection for Malignant Tumors in Children. The Journal of Bone and Joint Surgery-American Volume. 2014. Jan;96(2):e10–1–7. [DOI] [PubMed] [Google Scholar]
- 11.Lucas JT, Fernandez-Pineda I, Tinkle CL, Bishop MW, Kaste SC, Heda R, et al. Late toxicity and outcomes following radiation therapy for chest wall sarcomas in pediatric patients. Pract Radiat Oncol. 2017. Dec;7(6):411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-Induced Lung Injury . CHEST. Elsevier Inc; 2019. Jul 1;156(1):150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study . Lancet Oncol. 2020. Mar;21(3):421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes-Jordan A, Stoner JA, Anderson JR, Rodeberg D, Weiner G, Meyer WH, et al. The impact of surgical excision in chest wall rhabdomyosarcoma: a report from the children’s oncology group. J Pediatr Surg. 2008. May;43(5):831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedetti B Local Control in Ewing Sarcoma of the Chest Wall: Results of the EURO- EWING 99 Trial. Annals Of Surgical Oncology. Springer US; 2015. Jun 23;22(9):2853–9. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Kim HS, Moon ES, Yoon C-S, Chung T-S, Song H-T, et al. Scoliosis Imaging: What Radiologists Should Know. RadioGraphics. 2010. Nov;30(7):1823–42. [DOI] [PubMed] [Google Scholar]
- 17.Seitz G, Urla C, Sparber Sauer M, Schuck A, Vokuhl C, Blank B, et al. Treatment and outcome of patients with thoracic tumors of the Ewing sarcoma family: A report from the Cooperative Weichteilsarkom Studiengruppe CWS< 81, < 86, < 91, < 96, and < 2002P trials. Pediatric Blood & Cancer. 2019. May 16;66(S3):173–7. [DOI] [PubMed] [Google Scholar]
- 18.Shamberger RC, LaQuaglia MP, Krailo MD, Miser JS, Pritchard DJ, Gebhardt MC, et al. Ewing sarcoma of the ribResults of an intergroup study with analysis of outcome by timing of resection. J Thorac Cardiovasc Surg. 2000. Jun;119(6):1154–61. [DOI] [PubMed] [Google Scholar]
- 19.Denbo JW, Shannon Orr W, Wu Y, Wu J, Billups CA, Navid F, et al. Timing of Surgery and the Role of Adjuvant Radiotherapy in Ewing Sarcoma of the Chest Wall: A Single-institution Experience. Annals Of Surgical Oncology. 2012. Jul 3;19(12):3809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, et al. Social Outcomes in the Childhood Cancer Survivor Study Cohort. Journal of Clinical Oncology. 2009. May 10;27(14):2390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeltzer LK, Lu Q, Leisenring W, Tsao JCI, Recklitis C, Armstrong G, et al. Psychosocial Outcomes and Health-Related Quality of Life in Adult Childhood Cancer Survivors: A Report from the Childhood Cancer Survivor Study. Cancer Epidemiology Biomarkers & Prevention. 2008. Feb 4;17(2):435–46. [DOI] [PubMed] [Google Scholar]
- 22.Zebrack BJ, Zevon MA, Turk N, Nagarajan R, Whitton J, Robison LL, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: A report from the childhood cancer survivor study. Pediatric Blood & Cancer. 2007;49(1):47–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.