Abstract
Rationale and Objectives
There are limited data on pretreatment imaging features that can predict response to neoadjuvant chemotherapy (NAC). To extract volumetric pretreatment MRI radiomics features and assess corresponding associations with breast cancer molecular subtypes, pathological complete response (pCR), and residual cancer burden (RCB) in patients treated with NAC.
Materials and Methods
In this IRB-approved study, clinical and pretreatment MRI data from patients with biopsy-proven breast cancer who received NAC between 9/2009–7/2016 were retrospectively analyzed. Tumors were manually identified and semi-automatically segmented on first post-contrast images. Morphological and three-dimensional (3D) textural features were computed, including unfiltered and filtered image data, with spatial scaling factors (SSF) of 2, 4, and 6 mm. Wilcoxon rank-sum tests and area under the receiver operating characteristic curve (AUC-ROC) were used for statistical analysis.
Results
259 patients with unilateral breast cancer, including 73 (28.2%) HER2+, 112 (43.2%) luminal, and 74 (28.6%) triple negative breast cancers (TNBC), were included. There was a significant difference in the median volume (p=0.008), median longest axial tumor diameter (p=0.009), and median longest volumetric diameter (p=0.01) among tumor subtypes. There was also a significant difference in minimum signal intensity and entropy among the tumor subtypes with SSF=4 mm (p=0.009 and p=0.02 respectively) and SSF=6 mm (p=0.007 and p<0.001 respectively). Additionally, sphericity (p=0.04) in HER2+ tumors and entropy with SSF=2, 4, 6 mm (p=0.004, 0.02, 0.047 respectively) in luminal tumors were significantly associated with pCR. Multiple features demonstrated significant association (p<0.05) with pCR in TNBC and with RCB in luminal tumors and TNBC, with standard deviation of intensity with SSF=6 mm achieving the highest AUC (AUC=0.734) for pCR in TNBC.
Conclusion
MRI radiomics features are associated with different molecular subtypes of breast cancer, pCR, and RCB. These features may be non-invasive imaging biomarkers to identify cancer subtype and predict response to NAC.
Keywords: Radiomics, Breast, Neoadjuvant Chemotherapy, Pathological Complete Response, Residual Cancer Burden
Introduction
Neoadjuvant chemotherapy (NAC) is the standard of care for the treatment of larger human epidermal growth factor receptor 2 (HER2+), triple negative breast cancer (TNBC), and estrogen receptor positive (ER+) tumors with aggressive biological features. NAC allows for increased resectability of locally advanced breast cancer and inflammatory breast cancer, improved feasibility and cosmetic outcome of breast conserving surgery, and decreased morbidity and extent of axillary surgery in women with significant nodal disease (1). Response to NAC is associated with long-term outcomes including disease-free survival and overall survival (2–4) and is used for determination of additional post-surgical adjuvant therapies including systemic therapy and radiation.
Pathological complete response (pCR) has commonly been used as a primary endpoint in studies assessing NAC, although there is variability in the definition of pCR related to presence or absence of nodal disease, and residual in situ disease (5). Residual cancer burden (RCB) categorizes extent of remaining cancer in the breast and lymph nodes (5). RCB incorporates the tumor bed area, cancer cellularity, percentage in situ cancer, number of positive nodes, and diameter of largest lymph node metastasis (5). RCB ranges from 0-III with 0 indicating a complete response (pCR) and III indicating extensive residual tumor burden (5) and is a better biomarker of long-term outcomes compared to a dichotomous endpoint of pCR versus any residual disease (5, 6).
Response to NAC is frequently assessed using breast magnetic resonance imaging (MRI). However, there are increasing studies that indicate pretreatment MRI features can predict response to NAC (7–25) with the potential to help tailor treatment based on the likelihood of response. Studies have suggested that diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) values (25), tumor oxygenation assessment via blood oxygenation level-dependent (BOLD)-MRI (24), and MRI parametric response maps are associated with response to NAC. However, these findings have not been replicated in larger studies. In the recent past, radiomics, quantitative features from image data (23), have been used in studies to predict response to NAC. In particular, studies have reported several textural features, which are variations in gray-level intensities within an image (23), to be associated with pCR in patients undergoing NAC (7–22). However, these studies have overall been limited by the small number of patients, use of two-dimensional (2D) data only, and lack of correlation with RCB. The purpose of our study is to extract volumetric pretreatment MRI radiomics features and assess corresponding associations with breast cancer molecular subtypes, pCR, and RCB in patients treated with NAC.
Materials and Methods
Study Subjects
In this institutional review board-approved retrospective study, the need for informed consent was waived. We performed a review of our medical record and imaging database for female unilateral stage I-III breast cancer patients who underwent NAC and surgery at our institution between 9/1/2009 and 7/31/2016 and had clinical, imaging, and pathology data available. Inclusion criteria involved patients with biopsy-proven unilateral breast cancer who underwent NAC, had documented information regarding estrogen receptor, progesterone receptor, and HER2 status, had a breast MRI with intravenous contrast, and subsequently had surgical resection of the tumor with staging of the axillary nodes and documented surgical histopathology. Tumor histology and receptor information were based on core needle biopsy pathology. Patients without documented clinical information to define molecular subtype of breast cancer (n=5), with significant artifacts on the MRI images preventing assessment of the tumor (n=15), and those with first available breast MRI performed after the initiation of the chemotherapy date (n=7) were excluded (Figure 1). Chemotherapy regimens and dosing are described in Appendix 1.
Figure 1:
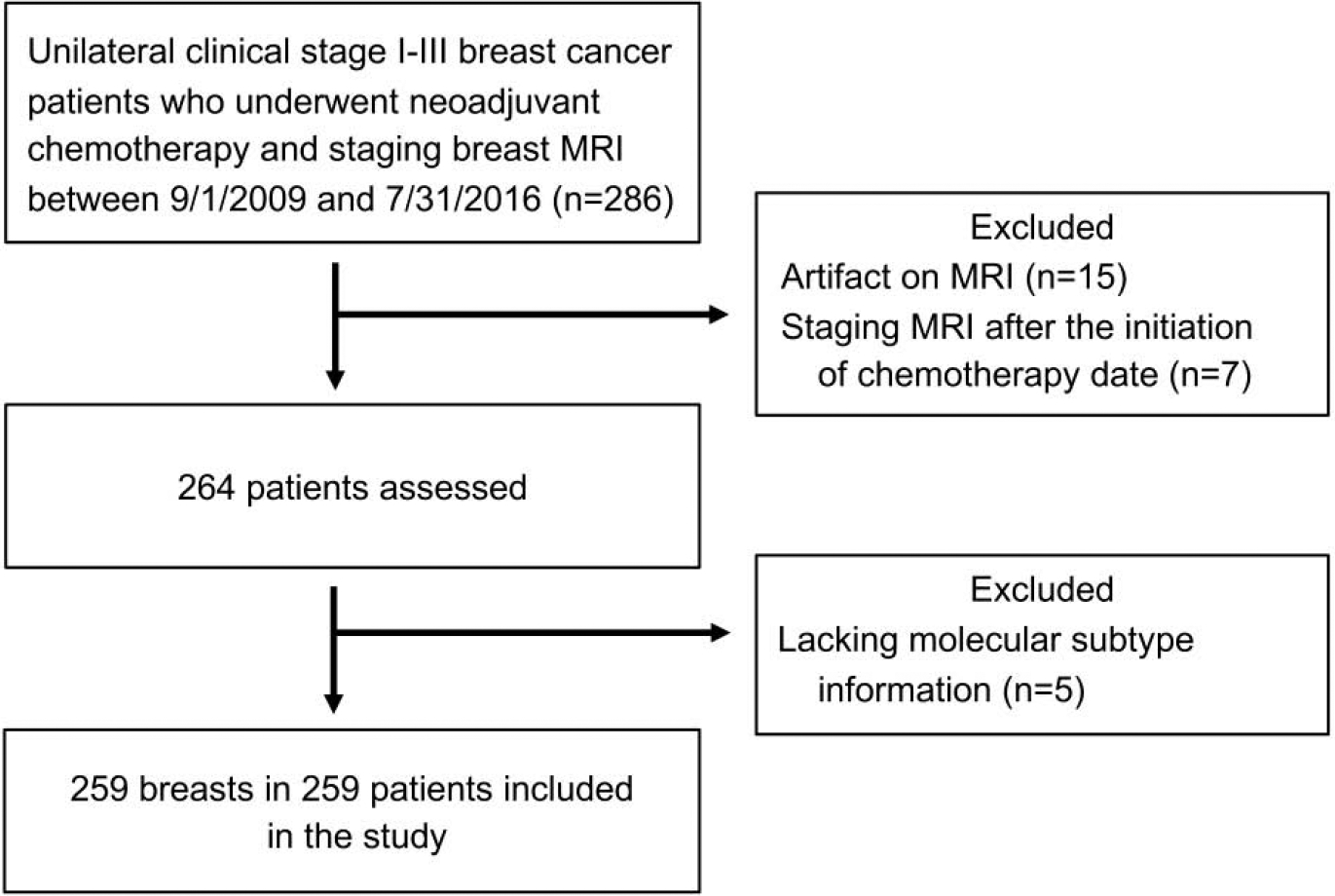
Flowchart shows the process for selecting our study population.
Imaging Technique
MRI exams were performed on GE 1.5T HDxt, MRI scanners (General Electric Healthcare, Milwaukee, WI) using a dedicated breast receiver coil array. MRI exams included a T2 axial acquisition, with and without fat suppression pre-contrast and three post-contrast T1 series, and a diffusion-weighted imaging (DWI) series. Importantly, the T1 series were acquired with a three-dimensional (3D) T1-weighted fat suppressed gradient echo sequence in the axial or sagittal direction using the following parameters: Flip angle=10°; TR=4.5–7.8ms; TE=2.2–3.4ms. Axial acquisitions were obtained with slice thickness=1.4–2.6mm, acquisition matrix=288–416 rows × 256–416 columns, and reconstruction matrix=512×512voxels. Sagittal acquisitions were done with slice thickness=3–3.2mm, acquisition matrix=256×192, and reconstruction matrix=256×256 voxels. Gadobutrol (Gadovist; Bayer Healthcare, Berlin, Germany) was used as contrast at a dose of 0.1 mmol/kg, power-injected intravenously at 2.0 mL/s.
Radiomics Assessment
Using post-contrast subtracted images, pretreated tumors were manually identified and semi-automatically segmented on first axial post-contrast fat-suppressed T1-weighted images by a fellowship-trained, breast radiologist (SC) using a custom-built MATLAB-based software tool (The MathWorks, Inc., Natick, MA). Using the axial slice with the largest tumor diameter and the corresponding reformatted sagittal and coronal planes, the radiologist placed a seed on the most conspicuous area of the tumor volume and validated the success of a segmented two-dimensional (2D) region of interest for each plane obtained via an active contours image segmentation algorithm (26). For multifocal or multicentric cancers, the seeds were placed in the area of the tumor with the greatest diameter. These 2D segmentations were then used to automatically perform 3D segmentation using the same active contours algorithm. 3D segmentations were then manually reviewed and boundaries were adjusted as needed to accurately delineate the tumor in 3D.
Once the segmented volumes were obtained, morphological and 3D textural features were computed. Morphological features included tumor volume, longest axial and volumetric diameters, and sphericity. The longest axial and volumetric diameters were obtained by extracting the major axis and principal axis of a fitted ellipse and ellipsoid, respectively. The sphericity was computed as the ratio between the surface area of the segmented tumor volume over the surface area of a perfect sphere that has the same tumor volume. Sphericity values range then between 0 and 1, with 1 indicating the tumor is a perfect sphere. 3D textural features were then extracted from the unfiltered image data and also from filtered (Laplacian of Gaussian) image data. Filtered images enabled the accentuation of different feature sizes by using spatial scaling factors (SSFs) of 2 (fine features), 4 (medium-sized features), and 6 mm (coarse features) (Figure 2). Textural features extracted from the segmented volumes included signal intensity (minimum, median, maximum, mean, and standard deviation), entropy, skewness, and kurtosis.
Figure 2:
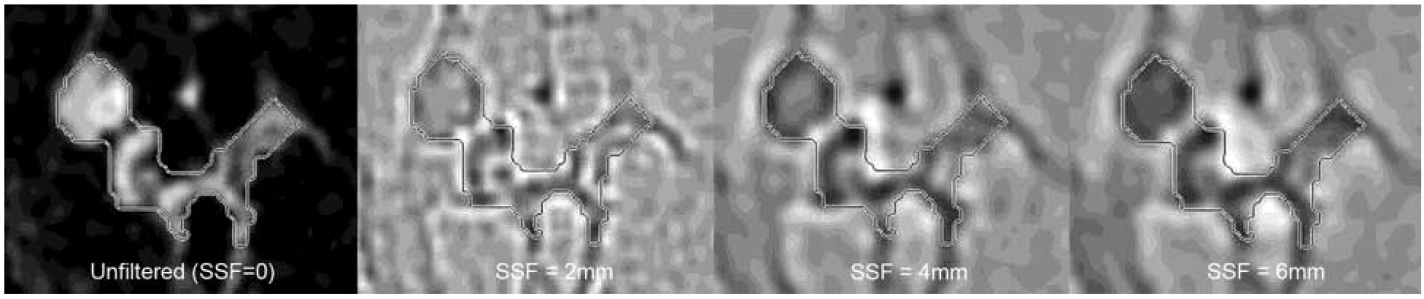
Textural analysis of a segmented breast tumor on first post-contrast T1-weighted image, demonstrating unfiltered image and image appearance after application of filters with spatial scaling factor (SSF) of 2 mm (fine features), 4 mm (medium-sized features), and 6 mm (coarse features).
Clinical and Histopathological Data Collection
Clinico-pathological data including age, clinical T and N category, clinical TNM stage, tumor histology, grade, receptor status, Ki-67, chemotherapy treatment, and pathological complete response were obtained from the medical record. RCB was calculated from the surgical pathology report (5). Using the St. Gallen criteria (27), we divided the breast cancers into three subgroups: luminal, HER2+, and triple negative breast cancer (TNBC). We explored subdivision of the luminal tumors into luminal A and luminal B but were not able to define luminal subtype for all due to some missing data on Ki-67; thus, our primary analysis combined all luminal tumors. pCR was defined as no residual invasive cancer in the breast and/or ipsilateral axillary nodes removed during surgery. RCB was defined using established criteria (5) from 0, indicating a complete response, to RCB III, indicating extensive residual disease.
Statistical Analysis
The association of MRI features with biologic subtype was assessed by Kruskal-Wallis tests. Where this omnibus test was significant, pairwise comparisons between subtypes were performed using Wilcoxon rank-sum tests. The outcomes of pCR and RCB class were analyzed separately for each biologic subtype. The association of MRI features with pCR were analyzed using Wilcoxon rank-sum tests and receiver-operating characteristic (ROC) analysis. The area under the curve (AUC) values were reported with 95% confidence intervals estimated using the DeLong error method. Multivariable logistic regression was performed using the best subset selection method based on the score criterion (28). The association of MRI measures with RCB class was assessed using Spearman rank correlation coefficients. Analysis was performed using SAS (Version 9.4). P-values < 0.05 were considered statistically significant. Given the exploratory nature of this study and the modest sample size, particularly within each biologic subtype, no correction for multiple comparisons was performed.
Results
259 patients (median age 50, range 24–84 years) were included in our study. Of these, 73 (28.2%) were HER2+, 112 (43.2%) were luminal, and 74 (28.6%) were TNBC tumors. Patient and clinical characteristics are summarized in Table 1. Luminal tumors included 68 luminal B, 15 luminal A, and 29 with unknown luminal subtype. The HER2+ tumors included 43 ER−/HER2+ and 30 ER+/HER2+. On MRI prior to NAC, the median tumor volume was 6309.8 mm3 (range 290.6–103474.5 mm3), median longest axial tumor diameter was 32.8 mm (range 8.8–110.5 mm), and median longest volumetric diameter was 32.8 mm (range 14.2–106.1 mm). Among the 259 tumors, 74 (28.6%) achieved pCR. pCR was highest in HER2+ (39/73=53.4%), followed by TNBC (27/74=36.5%), and luminal (8/112=7.1%) tumors. Further subdivided, 67.4% (29/43) ER−/HER2+ tumors achieved pCR versus 33.3% (10/30) ER+/HER2+, while among the luminal tumors, pCR was 11.8% for luminal B, 0% for luminal A, and 0% for luminal unknown. All of the luminal unknown tumors were grade I or II. RCB index was available in 178 of 259 (68.7%) patients. Of these 178 tumors, there were 74 (41.6%) RCB-0, 18 (10.1%) RCB-I, 48 (27%) RCB-II, and 38 (21.3%) RCB-III (Table 1).
Table 1.
Characteristics of Patients and Breast Cancers in Our Study
| Total (N=259) | HER2+ (N=73) | Luminal (N=112) | TNBC (N=74) | p value | |
|---|---|---|---|---|---|
| Age | 0.33 | ||||
| Median (Range) | 50 (24–84) | 49 (26–84) | 49 (32–79) | 51 (24–77) | |
| Histology | 0.001 | ||||
| Ductal | 225 (86.9%) | 71 (97.3%) | 92 (82.1%) | 62 (83.8%) | |
| Lobular | 12 (4.6%) | 0 (0.0%) | 11 (9.8%) | 1 (1.4%) | |
| Mixed Ductal & Lobular | 20 (7.7%) | 2 (2.7%) | 9 (8.0%) | 9 (12.2%) | |
| Other | 2 (0.8%) | 0 (0.0%) | 0 (0.0%) | 2 (2.7%) | |
| Biopsy Tumor Grade | <0.001 | ||||
| 1 (Well differentiated) | 8 (3.1%) | 1 (1.4%) | 7 (6.3%) | 0 (0.0%) | |
| 2 (Moderately differentiated) | 98 (38.1%) | 26 (36.6%) | 57 (50.9%) | 15 (20.3%) | |
| 3 (Poorly differentiated) | 151 (58.8%) | 44 (62.0%) | 48 (42.9%) | 59 (79.7%) | |
| Not Done | 2 | 2 | 0 | 0 | |
| Clinical T Category | 0.04 | ||||
| cT1 | 15 (5.8%) | 6 (8.2%) | 2 (1.8%) | 7 (9.5%) | |
| cT2 | 147 (56.8%) | 42 (57.5%) | 59 (52.7%) | 46 (62.2%) | |
| cT3 | 71 (27.4%) | 15 (20.5%) | 40 (35.7%) | 16 (21.6%) | |
| cT4 | 26 (10.0%) | 10 (13.7%) | 11 (9.8%) | 5 (6.8%) | |
| Clinical N Category | 0.14 | ||||
| cN0 | 97 (37.5%) | 24 (32.9%) | 40 (35.7%) | 33 (44.6%) | |
| cN1 | 134 (51.7%) | 42 (57.5%) | 61 (54.5%) | 31 (41.9%) | |
| cN2 | 18 (6.9%) | 3 (4.1%) | 10 (8.9%) | 5 (6.8%) | |
| cN3 | 10 (3.9%) | 4 (5.5%) | 1 (0.9%) | 5 (6.8%) | |
| Clinical Stage | 0.27 | ||||
| Stage I | 7 (2.7%) | 2 (2.7%) | 1 (0.9%) | 4 (5.4%) | |
| Stage II | 160 (61.8%) | 46 (63.0%) | 66 (58.9%) | 48 (64.9%) | |
| Stage III | 92 (35.5%) | 25 (34.2%) | 45 (40.2%) | 22 (29.7%) | |
| pCR | <0.001 | ||||
| No | 185 (71.4%) | 34 (46.6%) | 104 (92.9%) | 47 (63.5%) | |
| Yes | 74 (28.6%) | 39 (53.4%) | 8 (7.1%) | 27 (36.5%) | |
| RCB Class | <0.001 | ||||
| Missing | 81 | 13 | 46 | 22 | |
| 0 | 74 (41.6%) | 39 (65.0%) | 8 (12.1%) | 27 (51.9%) | |
| I | 18 (10.1%) | 7 (11.7%) | 6 (9.1%) | 5 (9.6%) | |
| II | 48 (27.0%) | 10 (16.7%) | 26 (39.4%) | 12 (23.1%) | |
| III | 38 (21.3%) | 4 (6.7%) | 26 (39.4%) | 8 (15.4%) |
IDC=invasive ductal carcinoma, ILC=invasive lobular carcinoma, HER2+=human epidermal growth factor receptor 2+, TNBC=triple negative breast cancer, pCR=pathological complete response, RCB=residual cancer burden
Association with Tumor Subtype
When comparing MRI features between HER2+, luminal, and TNBC tumor subtypes, there was a significant difference in the median volume (p=0.008), medial longest axial tumor diameter (p=0.009), and median longest volumetric diameter (p=0.01) by tumor subtypes (Table 2). In pairwise comparisons, HER2+ tumors had significantly lower volume (p=0.003 and p=0.02) compared to luminal and TNBC, respectively. Additionally, HER2+ had significantly smaller longest axial diameter (p=0.002) and longest volumetric diameter (p=0.004) than luminal tumors.
Table 2.
Radiomics features demonstrating a significant difference between molecular subtypes of breast cancer on pretreatment MRI in patients undergoing neoadjuvant chemotherapy
| Radiomics feature | HER2+ (n=73) Median (range) | Luminal (n=112) Median (range) | TNBC (n=74) Median (range) | P value |
|---|---|---|---|---|
| Volume (mm3) | 4297.46 (368.84, 84519.84) | 7684.77 (290.55, 103474.46) | 7483.03 (783.75, 100355.29) | 0.008 |
| Longest axial diameter (mm) | 27.1 (9.24, 99.54) | 34.89 (8.79, 110.54) | 33.49 (14.04, 100.25) | 0.009 |
| Longest volumetric diameter (mm) | 30.5 (16.32, 93.63) | 36.48 (14.16, 106.07) | 33.02 (16.69, 93.29) | 0.01 |
| Minimum signal int ensity (SSF=4 mm) | −0.21 (−0.76, −0.08) | −0.25 (−0.62, −0.09) | −0.26 (−0.62, −0.12) | 0.009 |
| Entropy (SSF=4 mm) | 2.37 (0.92–2.95) | 2.47 (1.19–4.09) | 2.50 (0.88, 3.26) | 0.02 |
| Minimum signal int ensity (SSF=6 mm) | −0.19 (−0.62, −0.07) | −0.23 (−0.59, −0.09) | −0.25 (−0.54, −0.12) | 0.007 |
| Entropy (SSF=6 mm) | 1.77 (0.51, 2.59) | 2.00 (0.56, 4.22) | 2.09 (0.84, 3.08) | <0.001 |
HER2+=human epidermal growth factor receptor 2+, TNBC=triple negative breast cancer, SSF=spatial scaling factor
For textural features, there was a significant difference in minimum signal intensity and entropy at SSF=4 mm (p=0.009 and p=0.02 respectively) and SSF=6 mm (p=0.007 and p<0.001 respectively) among the subtypes (Table 2). In pairwise comparisons, HER2+ tumors had significantly higher minimal signal intensity at SSF=4 mm (p=0.01 and p=0.005) and SSF=6 mm (p=0.008 and p=0.004) and lower entropy at SSF=6 mm (p=0.006 and p<0.001) compared to luminal and TNBC, respectively. Additionally, HER2+ had significantly lower entropy at SSF=4 mm compared to TNBC tumors (p=0.007). No significant differences were identified between luminal and TNBC tumor subtypes.
Association with pCR by Tumor Subtype
Assessment of radiomics features for pCR by tumor subtype showed sphericity was significantly higher (p=0.04) for HER2+ tumors that achieved pCR compared to those that did not, and entropy (SSF=2 mm, 4 mm, 6 mm) was significantly higher (p=0.004, 0.02, 0.047 respectively) for luminal tumors that achieved pCR than those that did not. The remaining features including morphological features (volume, longest axial, and volumetric diameter) and textural features (signal intensity and standard deviation, skewness, and kurtosis) showed no significant difference (p>0.05) between tumors that achieved pCR and those that did not for HER2+ and luminal tumors.
For unfiltered data (SSF=0 mm), TNBC tumors with pCR had significantly lower entropy (p=0.049), lower maximum signal intensity (p=0.03), and lower standard deviation of signal intensity (p=0.009) (Table 3). For filtered image data with SSF=2 mm, TNBC tumors that achieved pCR demonstrated significantly higher mean signal intensity (p=0.009), higher median signal intensity (p=0.03), lower maximum signal intensity (p=0.02), higher minimum signal intensity (p=0.01), and lower standard deviation of signal intensity (p=0.004) (Table 3). At SSF=4 mm, TNBC tumors with pCR had a significantly higher mean intensity (p=0.02), higher minimum signal intensity (p=0.007) and lower standard deviation of intensity (p<0.001) than those without pCR (Table 3). At SSF=6 mm, TNBC tumors with pCR had a significantly higher minimum signal intensity (p=0.004) and lower standard deviation of intensity than tumors that did not have pCR (p<0.001) (Table 3).
Table 3.
Radiomics features demonstrating a significant difference between triple negative breast cancers (TNBC) with and without pathological complete response (pCR)
| Radiomics feature | No pCR (n=47) Median (range) | pCR (n=27) Median (range) | p value | AUC (CI) |
|---|---|---|---|---|
| Entropy (SSF=0 mm) | 7.05 (6.03, 7.63) | 6.88 (3.39, 7.27) | 0.049 | 0.64 (0.51–0.77) |
| Mean signal intensity (SSF= 2 mm) | −0.01 (−0.03, −0.00) | 0.00 (−0.03, −0.00) | 0.009 | 0.68 (0.55–0.82) |
| Mean signal intensity (SSF= 4 mm) | −0.022 (−0.11, −0.01) | −0.017 (−0.13, −0.01) | 0.02 | 0.67 (0.53–0.80) |
| Median signal intensity (SSF= 2 mm) | −0.01 (−0.03, −0.00) | 0.00 (−0.04, −0.00) | 0.03 | 0.66 (0.52–0.79) |
| Maximum signal intensity (SSF= 0 mm) | 2156 (1048, 3674) | 1820 (1053, 2869) | 0.03 | 0.65 (0.51–0.79) |
| Maximum signal intensity (SSF= 2 mm) | 0.11 (0.04, 0.22) | 0.07 (0.03, 0.16) | 0.02 | 0.66 (0.53–0.79) |
| Minimum signal intensity (SSF= 2 mm) | −0.14 (−0.46, −0.05) | −0.11 (−0.26, −0.04) | 0.01 | 0.67 (0.55–0.8) |
| Minimum signal intensity (SSF= 4 mm) | −0.29 (−0.62, −0.12) | −0.22 (−0.45, −0.13) | 0.007 | 0.69 (0.57–0.82) |
| Minimum signal intensity (SSF= 6 mm) | −0.27 (−0.54, −0.12) | −0.20 (−0.43, −0.13) | 0.004 | 0.7 (0.57–0.83) |
| Standard deviation of intensity (SSF=0 mm) | 273.85 (136.75, 426.75) | 225.16 (108.92, 350.09) | 0.009 | 0.68 (0.55–0.82) |
| Standard deviation of intensity (SSF=2 mm) | 0.02 (0.01, 0.05) | 0.01 (0.01, 0.04) | 0.004 | 0.70 (0.57–0.84) |
| Standard deviation of intensity (SSF=4 mm) | 0.05 (0.03, 0.13) | 0.04 (0.02, 0.09) | <0.001 | 0.73 (0.61–0.85) |
| Standard deviation of intensity (SSF=6 mm) | 0.05 (0.03, 0.13) | 0.04 (0.02, 0.12) | <0.001 | 0.73 (0.61–0.86) |
SSF=spatial scaling factor, AUC=area under the curve, CI=confidence interval
For radiomics features that were significantly different between TNBC tumors with and without pCR, AUC calculations demonstrated that standard deviation of intensity at SSF= 6 mm achieved the highest AUC=0.734 (Confidence Interval: 0.611–0.856) (Table 3 and Figure 3). A multivariable model for predicting pCR in TNBC using longest volumetric diameter and standard deviation of intensity, kurtosis, and maximum signal intensity at SSF=6 mm had an AUC=0.811 (Confidence Interval: 0.713–0.908) (Table 3 and Figure 3).
Figure 3:
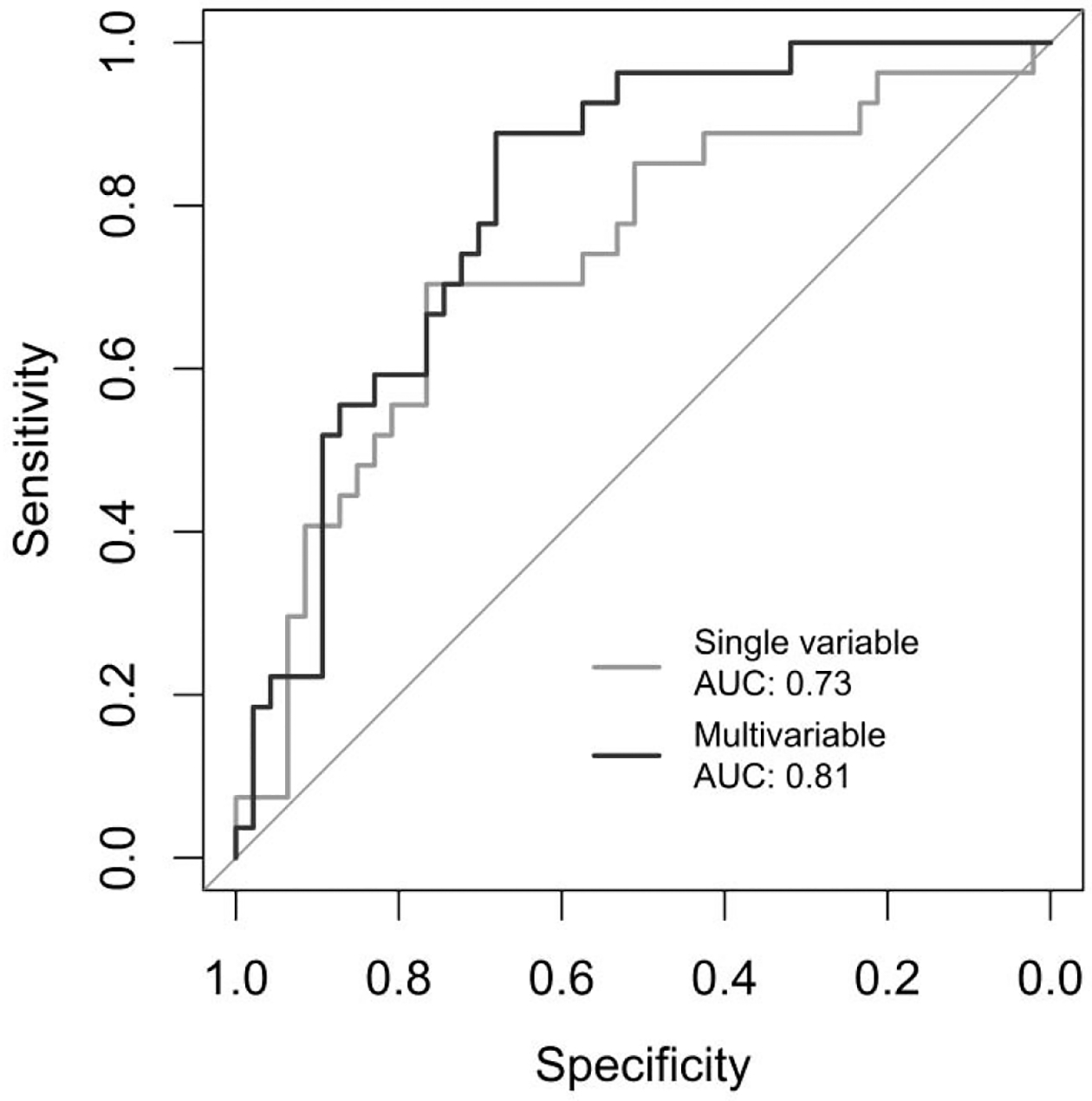
Receiver operative characteristic (ROC) curves for predicting pathological complete response (pCR) in triple negative breast cancer (TNBC) using a single variable (standard deviation of intensity at spatial scaling factor (SSF)= 6 mm) and a multivariable model using the longest volumetric diameter and standard deviation of intensity, kurtosis, and maximum signal intensity (SSF=6 mm).
Association with RCB by Tumor Subtype
Correlation of radiomics features with RCB by tumor subtype demonstrated significant correlations (p<0.05) between RCB index and sphericity, skewness, and maximum signal intensity in luminal tumors (Table 4). Additionally, significant correlations (p<0.05) were seen between RCB class and mean signal intensity (SSF=2 mm), maximum signal intensity (SSF=2, 4 mm), minimum signal intensity (SSF=0, 2, 4, 6 mm), and standard deviation of intensity (SSF=2, 4, 6 mm) for TNBC (Table 4 and Figure 4). This correlation was the highest for minimum signal intensity and standard deviation of intensity at SSF=2 mm, with a Spearman’s rank correlation of −0.36 and 0.36 respectively (Table 4 and Figure 4). No significant correlations between radiomics features and RCB class were noted for HER2+ breast cancers.
Table 4.
Radiomics features that significantly correlate with residual cancer burden (RCB) in luminal and triple negative breast cancers (TNBC)
| Tumor subtype | Radiomics feature | Spearman’s rank correlation coefficient |
|---|---|---|
| Luminal | Sphericity | −0.27 |
| Skewness (SSF=0 mm) | −0.28 | |
| Maximum signal intensity (SSF=4 mm) | 0.26 | |
| TNBC | Minimum signal intensity (SSF=0 mm) | −0.30 |
| Mean signal intensity (SSF=2 mm) | −0.28 | |
| Standard deviation of intensity (SSF=2 mm) | 0.36 | |
| Maximum signal intensity (SSF=2 mm) | 0.34 | |
| Minimum signal intensity (SSF=2 mm) | −0.36 | |
| Standard deviation of intensity (SSF=4 mm) | 0.32 | |
| Maximum signal intensity (SSF=4 mm) | 0.30 | |
| Minimum signal intensity (SSF=4 mm) | −0.34 | |
| Standard deviation of intensity (SSF=6 mm) | 0.29 | |
| Minimum signal intensity (SSF=6 mm) | −0.34 |
Figure 4a:
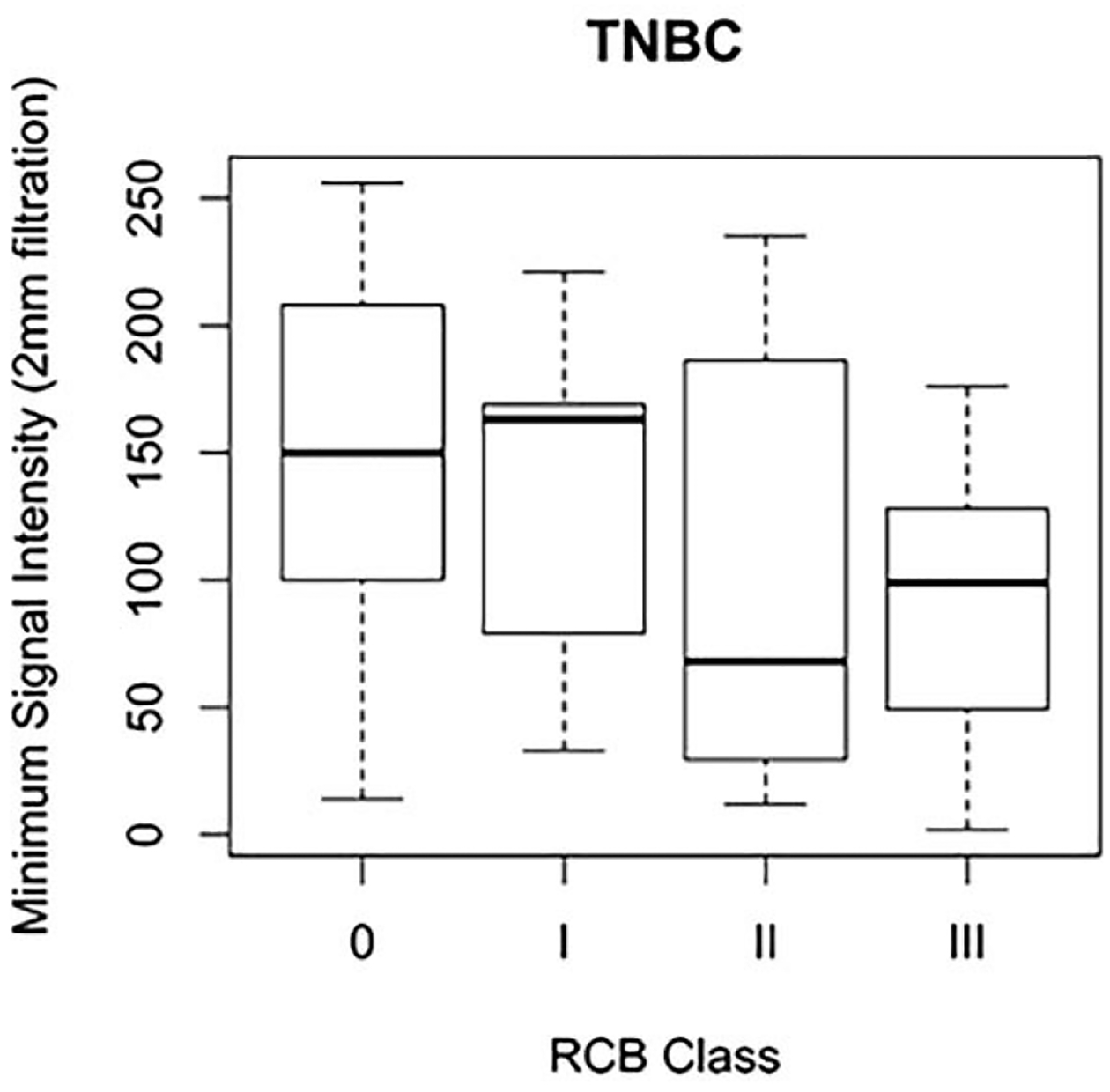
Box and whisker plot demonstrating minimum signal intensity at spatial scaling factor (SSF) = 2 mm by residual cancer burden (RCB) class for triple negative breast cancers (TNBC).
Discussion
Our study suggests that radiomics features on pretreatment breast MRI may distinguish breast cancer subtype and are associated with chemotherapy response as measured by pCR and RCB within tumor subtype. In particular, our study shows that HER2+ tumors have significantly higher minimum signal intensity, and lower entropy than luminal tumors and TNBC. Higher sphericity in HER2+ tumors, and higher entropy in luminal tumors pre-treatment are significantly associated with pCR after NAC. In TNBC, lower entropy, lower maximum signal intensity and standard deviation of intensity, and higher mean, median and minimum intensity are significantly associated with achieving pCR. Additionally, there are significant correlations between RCB and sphericity, skewness, and maximum signal intensity in luminal tumors and between mean signal intensity, maximum signal intensity, minimum signal intensity, and standard deviation of intensity in TNBC.
Breast cancer aggressiveness is thought to have a correlation with intra-tumor heterogeneity (29–31), which may not be completely assessed on histopathological tissue samples obtained from needle biopsies depending on the size and number of samples obtained for breast cancer diagnosis and tumor size. Imaging, on the other hand, is a non-invasive method of assessing the whole-tumor and can allow for assessment of entire tumor heterogeneity (23). Quantitative analysis of the entire tumor on imaging, including analysis reflecting heterogeneity of tumor and surrounding microenvironment, can be performed with radiomics, which extracts quantitative features from image data. Textural features are radiomics features that analyze the variations in gray-level intensities within an image, leading to discovery of image patterns not discernible by a human observer (23).
Pathological complete response and RCB, metrics that assess tumor response to NAC in breast cancer patients, have been shown to be associated with long-term outcomes including disease-free survival and overall survival (2–6) and this information is used to recommend additional therapy post-surgery. Identifying patients who will not respond to NAC could allow treatment to be tailored for optimal outcomes. There is growing literature on the potential role of radiomics in predicting response to NAC (7–22). However, these studies have been limited overall by the number of patients assessed and lack of 3D data and RCB assessment. Our study is unique in that we evaluated 3D volumetric tumor radiomics for RCB prediction along with tumor subtype and pCR prediction based on pretreatment MRI.
In our study, HER2+ tumors had lower entropy than luminal tumors and TNBC. Prior radiomics studies have shown differences in textural features such as entropy (32) between HER2+ tumors and other molecular subtypes of cancers. Entropy is the measure of texture irregularity or heterogeneity (33) and has been shown to be associated with lymphocyte infiltration (33). There are extensive data on the role of the immune system in the prognosis of HER2+ breast cancer, including response to HER2-directed therapy (34). Specifically, increasing levels of tumor infiltrating lymphocytes (TILs) in HER2+ tumors are associated with higher pCR rates and longer disease-free survival in patients treated with NAC (35). It is feasible that entropy may be a radiomics signature of the immunological infiltrate in HER2+ breast cancers and further research to evaluate this is warranted. Other studies have shown that radiomics correlate with luminal A and B tumors but not with HER2+ tumors (36). There is a need for further study to assess radiomics features that can distinguish the molecular subtype of cancer.
Prior studies assessing radiomics on pretreatment MRI have shown several different features to be associated with pCR. In our study, HER2+ tumors that achieved pCR had significantly higher sphericity. A study by Michishita et al. predicting pCR by tumor morphology on pretreatment MRI showed that round and oval tumors are more likely to achieve pCR (37). Although tumor shape was assessed by human readers and not quantified in this study like our study, these findings do suggest a potential relationship between tumor morphology on pretreatment MRI and pCR in patients undergoing NAC. Higher entropy in luminal tumors at pretreatment MRI was associated with pCR in our study. Although we did not assess changes in entropy over multiple scans in our study, a prior study has shown that a reduction in entropy on T2-weighted images between the pretreatment MRI and MRI performed after 2–3 cycles of chemotherapy correlates with pCR and RCB (13). Understanding changes in radiomics features over time during the patient’s treatment course may provide additional information about imaging markers predictive of response to NAC.
For TNBC, lower entropy, lower maximum signal intensity and standard deviation of intensity, and higher mean, median, and minimum intensity were significantly associated with pCR in our study. Our multivariable model for predicting pCR in TNBC achieved an AUC of 0.81, which is similar to the AUCs of 0.79–0.96 described for predicting pCR in TNBC using radiomics (19). However, it must be noted that our multivariable model may be overfit given the small number of patients (n=27) with TNBC who achieved pCR in our study, and this would ideally be assessed in a larger sample size. Nonetheless, these exploratory results suggest that radiomics have the potential to predict pCR in patients with TNBC, which is being increasingly treated with neoadjuvant chemotherapy in clinical practice (38).
Our study is unique in that we also evaluated radiomics for predicting RCB index. We found significant correlations between various radiomics features and RCB in luminal tumors and TNBC (Table 4). There is limited data in the literature evaluating RCB and radiomics on pretreatment MRIs. Thibault et al. demonstrated in a study of 38 patients that changes in radiomics features on MRI over the course of the treatment were associated with RCB and pCR (8). Similarly, Henderson et al. showed in a study of 88 patients that changes in entropy between pretreatment and early treatment MRI correlate with RCB and pCR. In contrast to these studies, we had a larger number of patients (n=178) for RCB analysis and we showed significant correlations between RCB and various features by tumor subtype on the pretreatment MRI alone. Although assessing changes in radiomics between MRIs over the treatment course may provide additional information, our study shows the potential of pretreatment MRI radiomics in predicting response to treatment as measured by RCB, which has been shown to be associated with long-term survival outcomes (5, 6).
Our study has several limitations, including its retrospective, single-institution design and the assessment of radiomics on single post-contrast series. Due to incomplete Ki-67 and genomic data, not all luminal tumors could be classified as luminal A or B, thus limiting our ability to examine differences within the luminal group. RCB index was missing for 31% of patients. Given the large number of comparisons performed without multiple comparisons corrections, some statistically significant findings may be false positives and these results should be considered exploratory. However, because of our modest sample size, particularly when considered separately by tumor subtype, it is also the case that some analyses had low power to detect differences. For example, only 8/112 luminal tumors achieved pCR; thus, clinically important differences may not have been detectable with this sample size. Finally, our ability to perform multivariable analysis was also limited by sample size, and although we developed a multivariable model for pCR among TNBC patients, that model was only based on 27 patients who achieved pCR and should be validated in an external sample. Lastly, artifacts from biopsy clips were not excluded from volumetric segmentation of tumors, and this may have affected tumor volumes. Despite these limitations, our study generated multiple compelling findings which strongly suggest that radiomics has the potential to provide meaningful pretreatment data in breast cancer patients undergoing NAC and warrants further study.
In summary, our study demonstrates that various breast tumor radiomics features observed on pretreatment MRI of women undergoing NAC are associated with molecular subtype and response to NAC, including pCR and RCB. Among the subtypes of breast cancer, TNBC has the most radiomics features associated with pathological response. In this era of personalized medicine, information from radiomics may help individualize treatment selection and timing of surgical and systemic interventions in breast cancer patients. Future studies with a larger number of patients are needed to provide additional information about the role of radiomics in patients undergoing NAC.
Figure 4b:
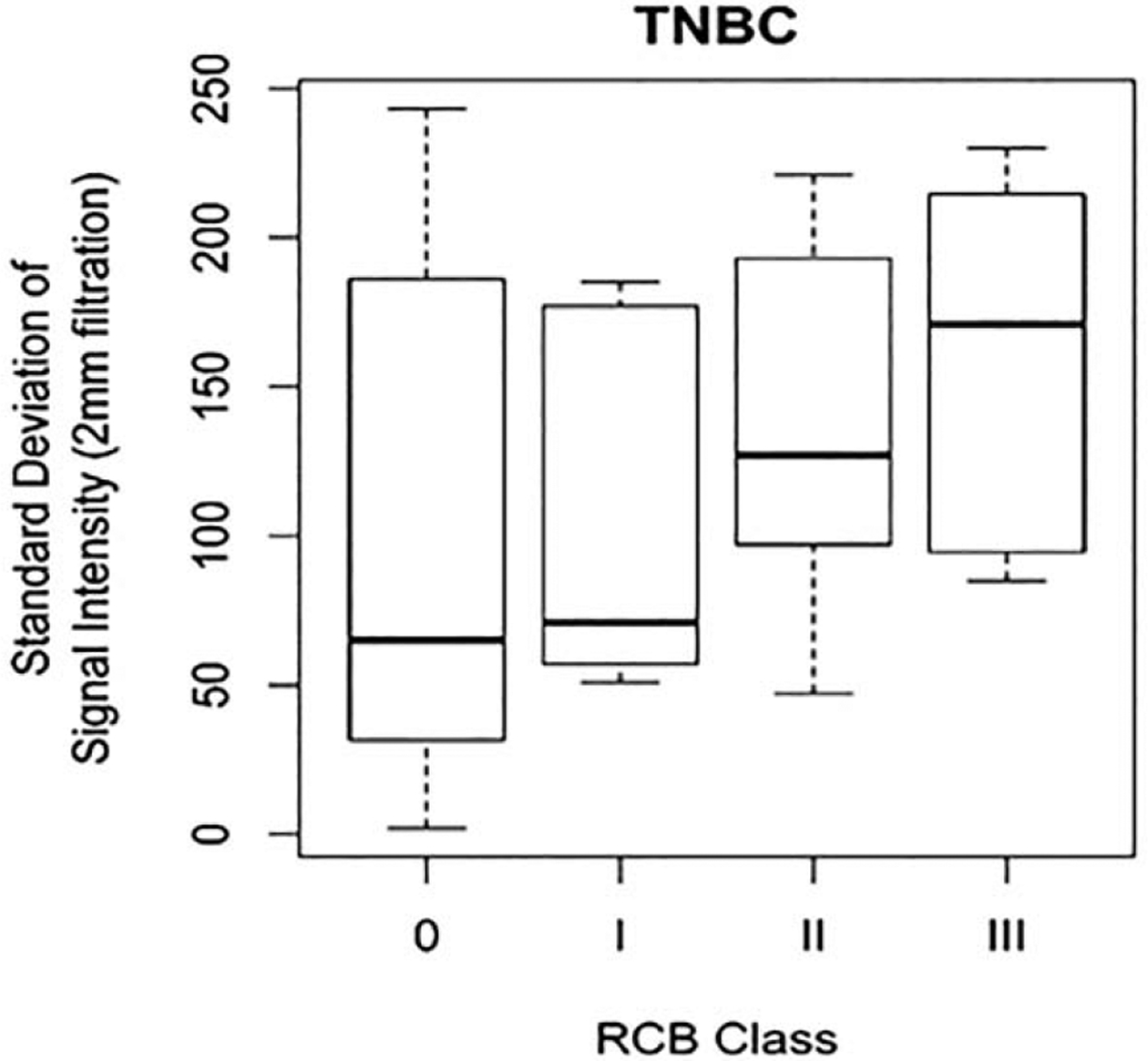
Box and whisker plot demonstrating standard deviation of signal intensity at spatial scaling factor (SSF) = 2 mm by residual cancer burden (RCB) class for triple negative breast cancers (TNBC).
Acknowledgement
Research reported in this article was supported by the National Cancer Institute of the National Institutes of Health under Award Number P50 CA116201 (Mayo Clinic Breast Cancer Specialized Program of Research Excellence) awarded to SC and MG.
Abbreviations:
- MRI
Magnetic Resonance Imaging
- NAC
Neoadjuvant Chemotherapy
- HER2+
Human Epidermal Growth Factor Receptor 2 Positive
- TNBC
Triple Negative Breast Cancer
- ER+
Estrogen Receptor Positive
- SSF
Spatial Scaling Factor
- pCR
Pathological Complete Response
- RCB
Residual Cancer Burden
- AUC
Area Under the Curve
- ROC
Receiver Operating Characteristic
Appendix 1
Patients with HER2-negative breast cancer received one of the following chemotherapy regimens: TC (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 × 4 cycles); AC (doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 × 4 cycles); TaxAC consisting of an anthracycline, cyclophosphamide, and a taxane (most commonly doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 × 4 cycles followed or preceded by 12 weekly doses of paclitaxel 80 mg/m2); TaxAC with carboplatin (most commonly every 3 week carboplatin at AUC 6 in combination with weekly paclitaxel 80 mg/m2); or, CMF (cyclophosphamide 600 mg/m2 + methotrexate 40 mg/m2 + fluorouracil 600 mg/m2 × 6 cycles). Some of the anthracycline-containing regimens (TaxAC or AC) included fluorouracil 500 mg/m2 × 4 cycles. Patients with HER2-positive breast cancer received one of the following regimens: TCH (docetaxel 75 mg/m2 + carboplatin AUC 6 + trastuzumab 8 mg/m2 loading dose and then 6 mg/m2 thereafter × 6 cycles); AC-TH (doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 × 4 cycles followed or preceded by 12 weekly doses of paclitaxel 80 mg/m2 with trastuzumab 4 mg/m2 loading dose and then 2 mg/m2 thereafter); TH (12 weekly doses of paclitaxel 80 mg/m2 with trastuzumab 4 mg/m2 loading dose and then 2 mg/m2). Some patients received dual HER2-targeted therapy in which pertuzumab (840 mg loading dose and then 420 mg thereafter) was administered sequentially with trastuzumab. Some patients, regardless of HER2 status, participated in a clinical trial and received an investigational agent(s), most commonly in addition to standard systemic therapy with TaxAC or AC-TH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Dr. Goetz reports consulting fees from Lilly, Biovica, Novartis, Sermonix, Context Pharmaceuticals, Pfizer, and Biotheranostics (all dollars directed to institution), and grants from Pfizer, Lilly, and Sermonix.
Dr. Boughey reports research funding from Lilly to the institution.
Dr. Choudhery/Mayo Clinic have a research and development agreement with Imago Systems.
References
- 1.Holmes D, Colfry A, Czerniecki B, Dickson-Witmer D, Francisco Espinel C, Feldman E, Gallagher K, Greenup R, Herrmann V, Kuerer H, Malik M, Manahan E, O’Neill J, Patel M, Sebastian M, Wheeler A, Kass R. Performance and Practice Guideline for the Use of Neoadjuvant Systemic Therapy in the Management of Breast Cancer. Ann Surg Oncol 2015;22(10):3184–3190. doi: 10.1245/s10434-015-4753-3 [DOI] [PubMed] [Google Scholar]
- 2.Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, Valero V. Ten-Year Outcomes of Patients With Breast Cancer With Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA Oncol 2016;2(4):508–516. doi: 10.1001/jamaoncol.2015.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007(2):CD005002. doi: 10.1002/14651858.CD005002.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Jr., Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 5.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 6.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, Walls A, Bousamra A, Ramineni M, Sinn B, Hunt K, Buchholz TA, Valero V, Buzdar AU, Yang W, Brewster AM, Moulder S, Pusztai L, Hatzis C, Hortobagyi GN. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol 2017;35(10):1049–1060. doi: 10.1200/JCO.2015.63.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan M, Wu G, Cheng H, Zhang J, Shao G, Li L. Radiomics analysis of DCE-MRI for prediction of response to neoadjuvant chemotherapy in breast cancer patients. Eur J Radiol 2017;94:140–147. doi: 10.1016/j.ejrad.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 8.Thibault G, Tudorica A, Afzal A, Chui SY, Naik A, Troxell ML, Kemmer KA, Oh KY, Roy N, Jafarian N, Holtorf ML, Huang W, Song X. DCE-MRI Texture Features for Early Prediction of Breast Cancer Therapy Response. Tomography 2017;3(1):23–32. doi: 10.18383/j.tom.2016.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machireddy A, Thibault G, Tudorica A, Afzal A, Mishal M, Kemmer K, Naik A, Troxell M, Goranson E, Oh K, Roy N, Jafarian N, Holtorf M, Huang W, Song X. Early Prediction of Breast Cancer Therapy Response using Multiresolution Fractal Analysis of DCE-MRI Parametric Maps. Tomography 2019;5(1):90–98. doi: 10.18383/j.tom.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Gong G, Cui Y, Li R. Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. J Magn Reson Imaging 2016;44(5):1107–1115. doi: 10.1002/jmri.25279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamming’s F, Ueno Y, Ferre R, Kao E, Jannot AS, Chong J, Omeroglu A, Mesurolle B, Reinhold C, Gallix B. Features from Computerized Texture Analysis of Breast Cancers at Pretreatment MR Imaging Are Associated with Response to Neoadjuvant Chemotherapy. Radiology 2018;286(2):412–420. doi: 10.1148/radiol.2017170143 [DOI] [PubMed] [Google Scholar]
- 12.Teruel JR, Heldahl MG, Goa PE, Pickles M, Lundgren S, Bathen TF, Gibbs P. Dynamic contrast-enhanced MRI texture analysis for pretreatment prediction of clinical and pathological response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. NMR Biomed 2014;27(8):887–896. doi: 10.1002/nbm.3132 [DOI] [PubMed] [Google Scholar]
- 13.Henderson S, Purdie C, Michie C, Evans A, Lerski R, Johnston M, Vinnicombe S, Thompson AM. Interim heterogeneity changes measured using entropy texture features on T2-weighted MRI at 3.0 T are associated with pathological response to neoadjuvant chemotherapy in primary breast cancer. Eur Radiol 2017;27(11):4602–4611. doi: 10.1007/s00330-017-4850-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eun NL, Kang D, Son EJ, Park JS, Youk JH, Kim JA, Gweon HM. Texture Analysis with 3.0-T MRI for Association of Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiology 2020;294(1):31–41. doi: 10.1148/radiol.2019182718 [DOI] [PubMed] [Google Scholar]
- 15.Drukker K, Edwards A, Doyle C, Papaioannou J, Kulkarni K, Giger ML. Breast MRI radiomics for the pretreatment prediction of response to neoadjuvant chemotherapy in node-positive breast cancer patients. J Med Imaging (Bellingham) 2019;6(3):034502. doi: 10.1117/1.JMI.6.3.034502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghaei F, Tan M, Hollingsworth AB, Qian W, Liu H, Zheng B. Computer-aided breast MR image feature analysis for prediction of tumor response to chemotherapy. Med Phys 2015;42(11):6520–6528. doi: 10.1118/1.4933198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghaei F, Tan M, Hollingsworth AB, Zheng B. Applying a new quantitative global breast MRI feature analysis scheme to assess tumor response to chemotherapy. J Magn Reson Imaging 2016;44(5):1099–1106. doi: 10.1002/jmri.25276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braman N, Prasanna P, Whitney J, Singh S, Beig N, Etesami M, Bates DDB, Gallagher K, Bloch BN, Vulchi M, Turk P, Bera K, Abraham J, Sikov WM, Somlo G, Harris LN, Gilmore H, Plecha D, Varadan V, Madabhushi A. Association of Peritumoral Radiomics With Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)-Positive Breast Cancer. JAMA Netw Open 2019;2(4):e192561. doi: 10.1001/jamanetworkopen.2019.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Li Z, Qu J, Zhang R, Zhou X, Li L, Sun K, Tang Z, Jiang H, Li H, Xiong Q, Ding Y, Zhao X, Wang K, Liu Z, Tian J. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin Cancer Res 2019;25(12):3538–3547. doi: 10.1158/1078-0432.CCR-18-3190 [DOI] [PubMed] [Google Scholar]
- 20.Braman NM, Etesami M, Prasanna P, Dubchuk C, Gilmore H, Tiwari P, Plecha D, Madabhushi A. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 2017;19(1):57. doi: 10.1186/s13058-017-0846-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh J, Selmi M, Charles-Edwards G, Glendenning J, Ganeshan B, Verma H, Mansi J, Harries M, Tutt A, Goh V. Changes in primary breast cancer heterogeneity may augment midtreatment MR imaging assessment of response to neoadjuvant chemotherapy. Radiology 2014;272(1):100–112. doi: 10.1148/radiol.14130569 [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Gibbs P, Pickles M, Turnbull L. Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging 2013;38(1):89–101. doi: 10.1002/jmri.23971 [DOI] [PubMed] [Google Scholar]
- 23.Chitalia RD, Kontos D. Role of texture analysis in breast MRI as a cancer biomarker: A review. J Magn Reson Imaging 2019;49(4):927–938. doi: 10.1002/jmri.26556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang L, Weatherall PT, McColl RW, Tripathy D, Mason RP. Blood oxygenation level-dependent (BOLD) contrast magnetic resonance imaging (MRI) for prediction of breast cancer chemotherapy response: a pilot study. J Magn Reson Imaging 2013;37(5):1083–1092. doi: 10.1002/jmri.23891 [DOI] [PubMed] [Google Scholar]
- 25.Li X, Abramson RG, Arlinghaus LR, Kang H, Chakravarthy AB, Abramson VG, Farley J, Mayer IA, Kelley MC, Meszoely IM, Means-Powell J, Grau AM, Sanders M, Yankeelov TE. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol 2015;50(4):195–204. doi: 10.1097/RLI.0000000000000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan Tony FV, Luminita A. Active contours without edges. IEEE Transactions on Image Processing, 2001;Volume 10 (Issue 2):pp. 266–277. [DOI] [PubMed] [Google Scholar]
- 27.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel m. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furnival GM, and Wilson RW Regression by Leaps and Bounds. Technometrics 1974;16:499–511. [Google Scholar]
- 29.Kim JH, Ko ES, Lim Y, Lee KS, Han BK, Ko EY, Hahn SY, Nam SJ. Breast Cancer Heterogeneity: MR Imaging Texture Analysis and Survival Outcomes. Radiology 2017;282(3):665–675. doi: 10.1148/radiol.2016160261 [DOI] [PubMed] [Google Scholar]
- 30.Koren S, Bentires-Alj M. Breast Tumor Heterogeneity: Source of Fitness, Hurdle for Therapy. Mol Cell 2015;60(4):537–546. doi: 10.1016/j.molcel.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 31.Januskeviciene I, Petrikaite V. Heterogeneity of breast cancer: The importance of interaction between different tumor cell populations. Life Sci 2019;239:117009. doi: 10.1016/j.lfs.2019.117009 [DOI] [PubMed] [Google Scholar]
- 32.Li H, Zhu Y, Burnside ES, Huang E, Drukker K, Hoadley KA, Fan C, Conzen SD, Zuley M, Net JM, Sutton E, Whitman GJ, Morris E, Perou CM, Ji Y, Giger ML. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2016;2. doi: 10.1038/npjbcancer.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko ES, Kim JH, Lim Y, Han BK, Cho EY, Nam SJ. Assessment of Invasive Breast Cancer Heterogeneity Using Whole-Tumor Magnetic Resonance Imaging Texture Analysis: Correlations With Detailed Pathological Findings. Medicine (Baltimore) 2016;95(3):e2453. doi: 10.1097/MD.0000000000002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 2014;15(2):e58–68. doi: 10.1016/S1470-2045(13)70477-7 [DOI] [PubMed] [Google Scholar]
- 35.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kummel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 36.Grimm LJ, Zhang J, Mazurowski MA. Computational approach to radiogenomics of breast cancer: Luminal A and luminal B molecular subtypes are associated with imaging features on routine breast MRI extracted using computer vision algorithms. J Magn Reson Imaging 2015;42(4):902–907. doi: 10.1002/jmri.24879 [DOI] [PubMed] [Google Scholar]
- 37.Michishita S, Kim SJ, Shimazu K, Sota Y, Naoi Y, Maruyama N, Kagara N, Shimoda M, Shimomura A, Noguchi S. Prediction of pathological complete response to neoadjuvant chemotherapy by magnetic resonance imaging in breast cancer patients. Breast 2015;24(2):159–165. doi: 10.1016/j.breast.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 38.Bagegni NA, Tao Y, Ademuyiwa FO. Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer: A report from the National Cancer Database. PLoS One 2019;14(9):e0222358. doi: 10.1371/journal.pone.0222358 [DOI] [PMC free article] [PubMed] [Google Scholar]


