Abstract
Background:
Depression among older adults with cancer is under recognized and under treated. This study characterizes the burden of depression in older adults with gastrointestinal (GI) malignancies prior to chemotherapy and its relationship with geriatric assessment (GA) domains, health-related quality of life (HRQOL), and self-reported healthcare utilization.
Methods:
Patients ≥60 years in GI oncology clinics at UAB were asked to complete a GA entitled the Cancer & Aging Resilience Evaluation (CARE). We examined depression using the Patient-Reported Outcomes Measurement Information System (PROMIS®) Depression four-item short form; moderate/severe depression was defined by a t-score ≥60. Multivariate analysis was used to examine associations between those with and without moderate/severe depression.
Results:
Of 355 included patients, 46 had mild depression (13%) and an additional 46 patients had moderate/severe depression (13%). After adjustment for age, sex, education, cancer type, and cancer stage, those who reported moderate/severe depression had a significantly increased odds of reporting falls (adjusted odds ratio [aOR] 4.01, 95% confidence interval [CI] 1.94-8.26), dependence in lADLs (aOR 7.06, CI 2.91-17.1), dependence in ADLs (aOR 6.23, CI 2.89-13.4), malnutrition (aOR 5.86, CI 2.40-14.3), frailty (aOR 13.7, CI 5.80-32.1), and fatigue (aOR 11.2, CI 3.31-37.6). Moderate/severe depression was also significantly associated with worse physical (aOR 7.58, CI 3.30-17.4) and mental (aOR 26.3, CI 10.1-68.8) HRQOL sub-scores, without significant differences in healthcare utilization.
Conclusions:
More than one out of eight older adults with a GI malignancy reported moderate/severe depression prior to chemotherapy, which was associated with impairments in several GA domains and HRQOL.
Keywords: Depression, Geriatric Oncology, Gastrointestinal Malignancies, Cancer and Aging, Mental Health, Patient-Reported Outcomes
Introduction
Cancer is largely a disease of older adults, with peak incidence around the seventh decade of life and advancing age shown as an important risk factor for most.1 As the population of the United States (US) ages, approximately 70% of all cancers diagnosed by 2030 will occur in older adults.2 Gastrointestinal (GI) malignancies also occur predominantly among older adults, with a median age of diagnosis >65 years.3 As cancer screening and treatments continue to become more effective, they will be accompanied by an increase in the prevalence of cancer survivors into older age, creating a need to understand long-term sequelae of cancer and its treatments in order to provide optimal care throughout the lifetime, including and beyond active treatment for patients and caregivers.
The mental health of older Americans has been identified as a priority by several national public health agendas, including a 1999 Surgeon General report on mental health and the 2005 White House Conference on Aging. The Center for Disease Control’s Healthy Aging Program and Healthy Brain Initiative have focused education and resources on depression in older adults, as it is a central tenet to the mental health of this vulnerable population and has been identified as an important, emerging public health issue with pleiotropic effects that is definitively not a normal part of aging.4,5 National estimates suggest that 20% of the population aged ≥55 years have a mental health concern, with depression being the most prevalent and actively affecting 7.7% in those aged ≥50 years, yet it is widely under recognized and under treated despite highly effective, accessible interventions.4,5 Depression in patients with cancer has a large degree of heterogeneity – cancer type, cancer stage, pre-existing mood disorders, treatment phase, various instruments/scales of measure, and more make it difficult to study the complex interrelationships that develop to impact health (see Figure 1). Even less is known about older adults with depression and cancer, as screening and diagnosis is increasingly complicated in this vulnerable population, which is more likely to report somatic complaints (e.g., gastrointestinal distress, sleep disturbances, fatigue, malaise) rather than an overtly depressed mood, in addition to unknowns regarding barriers to treatment and access to care.6,7
Figure 1. Multifactorial and Resonant Interdependencies on Health in the Aging Cancer Population.
Abbreviation: HRQOL, health-related quality of life
This study aims to quantify the prevalence of depression in older adults with GI malignancies prior to chemotherapy as well as assess associations with demographics, geriatric assessment (GA) domains, health-related quality of life (HRQOL), and healthcare utilization after adjusting for baseline clinical variables. The authors hypothesized that older adults with GI cancers reporting moderate/severe depression would have higher odds of GA domain deficits, worse HRQOL, and more healthcare utilization when compared to those patients who did not demonstrate moderate/severe depression.
Methods
Study Population
This cross-sectional, single-institution study analyzed older adults aged ≥60 with GI malignancies derived from the prospectively enrolled Cancer and Aging Resilience Evaluation (CARE) Registry at the University of Alabama at Birmingham (UAB) when they were initially seen in GI oncology clinics as previously described.8 The UAB institutional review board approved CARE in September 2017 and recruitment began soon thereafter. In order to minimize heterogeneity and eliminate confounding effects, this study examined patients who were chemotherapy naïve.
Depression
Depression was measured using the Patient-Reported Outcomes Measurement Information System (PROMIS®) Depression Short Form 4a v1.0, using Likert responses (Never, Rarely, Sometimes, Often, Always) to four separate statements: “I felt worthless,” “I felt helpless,” “I felt depressed,” and “I felt hopeless” within the past week. Importantly, this instrument omits confounding somatic symptoms and was designed to capture patient-reported outcomes in an efficient, flexible, and precise manner that has been validated in the US on a national level when compared with legacy measures of depression.9–12 Importantly, it has been validated in oncology populations as well as in geriatric populations of varying cognitive function, serving as a valuable longitudinal instrument by demonstrating responsiveness to depression treatment in the outpatient setting.12–15 Depression responses were converted to the appropriate t-score as previously described, with subsequent stratification of severity into no depression (t-score <55), mild depression (t-score 55-59), moderate depression (t-score 60-69), and severe depression (t-score 70+).16
Geriatric Assessment and Covariates
Patients completed a patient-reported GA, based on the Cancer and Aging Research Group GA, as previously described.8,17–19 The CARE survey evaluated various domains, including demographic information, social support and activities (assessed via Medical Outcomes Survey Social Support), number of falls within the prior six months, activities of daily living (ADL), instrumental ADLs (lADLs), performance status (self-rated ECOG), nutrition (assessed via patient-generated subjective global assessment of nutrition), comorbidities (assessed via Older Americans Resources and Services Comorbidity), financial distress (assessed via a single item from the Patient Satisfaction Questionnaire-18), cognitive complaints (assessed via the PROMIS® cognitive function abilities), polypharmacy (assessed via the patient-reported number of daily medications within the past seven days), and mental health (assessed via PROMIS® Anxiety and Depression short form instruments).8,20–23 Frailty was defined using a frailty index approach based on the principles of deficit accumulation.24 HRQOL was assessed via the PROMIS 10-item Global Health, which includes separate mental and physical HRQOL subdomains. Lastly, the CARE survey included the presence of hospitalizations and emergency room visits within the prior year.
Statistical Analyses
Group differences between those with moderate/severe depression and those without moderate/severe (i.e., mild/none) depression were examined using Studenťs t-tests for continuous variables and Chi-square for categorical variables. All tests were 2-sided with an alpha value of <0.05 considered statistically significant. Univariate and multivariable logistic regression analyses were conducted to estimate the association between moderate/severe depression and the previously described variables of interest, with calculation of unadjusted odds ratios, adjusted odds ratios (aOR), and 95% confidence intervals (95% CIs). Multivariable analyses were adjusted for age, sex, education, cancer type, and cancer stage. These variables are especially important to adjust for as some studies have shown a female predilection for depression in older patients as well as a worse clinical course with advancing age.25–27
Results
Over approximately two and a half years, 355 patients >60 years old with a GI malignancy were enrolled in the CARE registry and completed a GA prior to chemotherapy. The mean age was 70 (range 60-96), and patients were predominantly white (74.4%) males (56.3%) with advanced stage disease (lll/IV, 70.1%) (see Table 1). The most common cancer types were colorectal (34.4%), pancreatic (24.8%), and hepatobiliary (16.6%) cancer. The median time interval between cancer diagnosis and study participation was one month (interquartile range one to two months).
Table 1.
Patient Characteristics.
| All | No depression | Depression | p-value | |
|---|---|---|---|---|
| Total Patients | N= 355 | N= 309 | N= 46 | |
| Age, mean (SD) | 70.0 (7.2) | 70.1 (7.0) | 69.9 (8.8) | 0.4331 |
| Range | 60-96 | 60-91 | 60-96 | |
| Sex, n(%) | ||||
| Male | 200 (56.3) | 172 (55.7) | 28 (60.9) | 0.5066 |
| Female | 155 (43.7) | 137 (44.3) | 18 (39.1) | |
| Race, n(%) | ||||
| White | 264 (74.4) | 230 (74.4) | 34 (73.9) | 0.6632 |
| Black | 86 (24.2) | 74 (23.9) | 12 (26.1) | |
| Other | 5 (1.4) | 5 (1.6) | 0 (0.0) | |
| Ethnicity, n(%) | ||||
| Hispanic | 8 (2.3) | 8 (2.6) | 0 (0.0) | 0.2697 |
| Educational Level, n(%) | ||||
| Less than high school | 61 (17.2) | 49 (15.9) | 12 (26.1) | 0.3166 |
| High school graduate | 96 (27.0) | 83 (26.9) | 13 (28.3) | |
| Some college | 66 (18.6) | 57 (18.4) | 9 (19.6) | |
| Associate/Bachelors | 97 (27.3) | 87 (28.2) | 10 (21.7) | |
| Advanced Degree | 35 (9.9) | 33 (10.7) | 2 (4.3) | |
| Employment, n(%) | ||||
| Retired | 215 (60.6) | 192 (62.1) | 23 (50.0) | 0.0019 |
| Disabled | 47 (13.2) | 34 (11.0) | 13 (28.3) | |
| Part-time (<32hr/wk) | 10 (2.8) | 10 (3.2) | 0 (0.0) | |
| Full-time (>32hr/wk) | 38 (10.7) | 37 (12.0) | 1 (2.2) | |
| Other | 45 (12.7) | 36 (11.7) | 9 (19.6) | |
| Marital Status, n(%) | ||||
| Single | 25 (7.0) | 22 (7.1) | 3 (6.5) | 0.0084 |
| Widowed/Divorced | 102 (28.7) | 80 (25.9) | 22 (47.8) | |
| Married | 228 (64.2) | 207 (67.0) | 21 (45.7) | |
| Cancer Type, n(%) | ||||
| Colorectal | 122 (34.4) | 110 (35.6) | 12 (26.1) | 0.1024 |
| Pancreatic | 88 (24.8) | 74 (23.9) | 14 (30.4) | |
| Hepatobiliary | 59 (16.6) | 55 (17.8) | 4 (8.7) | |
| Gastroesophageal | 38 (10.7) | 29 (9.4) | 9 (19.6) | |
| Other (NEC, GIST, Anal) | 48 (13.5) | 41 (13.3) | 7 (15.2) | |
| Cancer Stage, n(%) | ||||
| 0-II | 106 (29.9) | 92 (29.8) | 14 (30.4) | 0.1683 |
| III | 92 (25.9) | 85 (27.5) | 7 (15.2) | |
| IV | 157 (44.2) | 132 (42.7) | 25 (54.3) |
Abbreviations: NEC, neuroendocrine carcinoma; GIST, gastrointestinal stromal tumor.
The majority did not demonstrate depression (n=263, 74%), though 46 patients had mild depression (13%) and an additional 46 patients had moderate/severe depression (13%) (see Figure 2). The overall t-score mean was 48.5 (standard deviation 8.7). Patients with moderate/severe depression did not differ in age, sex, race, ethnicity, education level, cancer type, or cancer stage; however, they were more likely to be disabled and widowed/divorced than those patients without moderate/severe depression (see Table 1).
Figure 2. Stratification of Depression.
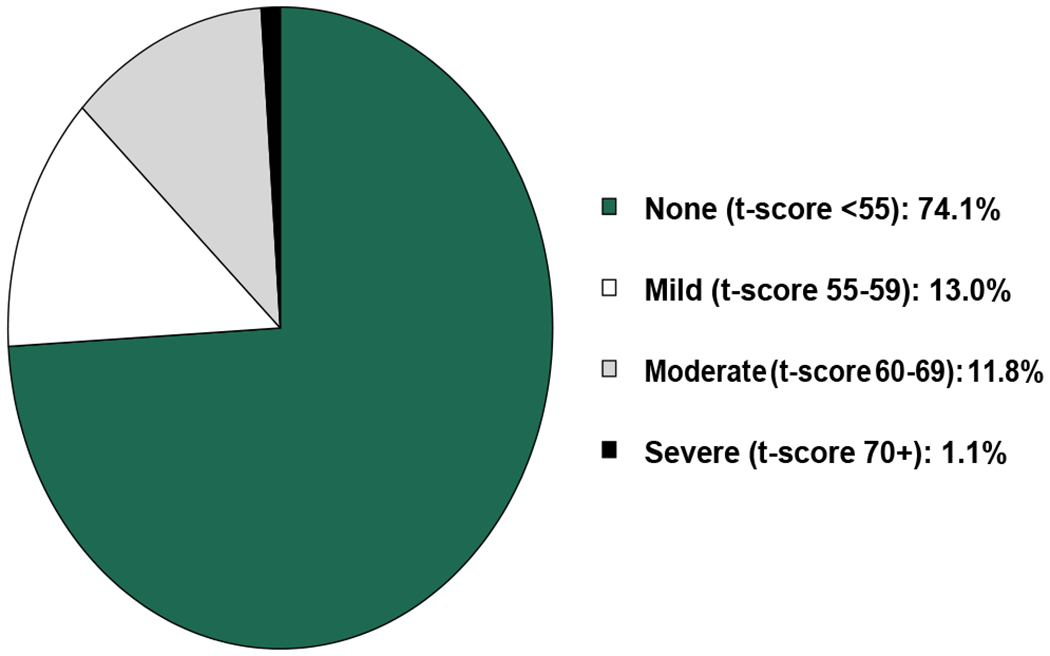
The overall t-score mean was 48.5 (standard deviation 8.7). Out of the 355 patients analyzed, 46 (13%) reported mild depression and 46 (13%) reported moderate to severe depression.
Patients with moderate/severe depression were more likely to report a history of falls (43.5 v 17.2%, p<.001), impaired performance status (60.9 v 29%, p<.001), dependence in IADLs (84.4 v 46.2%, p<.001), dependence in ADLs (44.4 v 13.2%, p<.001), malnutrition (84.1 v 47.4%, p<.001), frailty (82.6 v 27.5%, p<.001), three or more non-cancer comorbidities (71.7 v 49.3%, p<.005), nine or more daily medications (37 v 22.3%, p=.03), financial distress (17.8 v 7.4%, p=.02), limitations in social activities (53.3 v 19.4%, p<.001), moderate/severe cognitive dysfunction (34.8 v 2.3%, p<.001), moderate/severe anxiety (77.3 v 9.9%, p<.001), moderate/severe fatigue (93.3 v 52.1%, p<.001), and moderate/severe pain (75.6 v 40.7%, p<.001) compared to those patients without moderate/severe depression (see Table 2). Those with moderate/severe depression also reported lower mean physical (31.9 v 44.6, p<.001) and mental (35.5 v 49.6, p<.001) HRQOL sub-scores compared to those patients without moderate/severe depression (see Table 2). No differences in reported emergency room visits or hospitalizations within the prior year were detected between those with or without moderate/severe depression.
Table 2.
Differences in Geriatric Assessment Domains, Health-Related Quality of Life, and Prior Healthcare Utilization by Moderate/Severe Depression Status.
| Moderate/Severe Depression | |||
|---|---|---|---|
| No | Yes | p-value | |
| Geriatric Assessment Domains | |||
| ≥1 falls, n (%) | 50 (17.2) | 20 (43.5) | <.0001 |
| Impaired (≥2) performance status, n (%) | 88 (29.0) | 28 (60.9) | <.0001 |
| Reported limitations in walking one block, n (%) | 149 (49.3) | 39 (84.8) | <.0001 |
| Any IADL dependence, n (%) | 138 (46.2) | 38 (84.4) | <.0001 |
| Any ADL dependence, n (%) | 40 (13.2) | 20 (44.4) | <.0001 |
| Moderate/Severe Cognitive Dysfunction, n (%) | 7 (2.3) | 16 (34.8) | <.0001 |
| ≥3 comorbidities other than cancer, n (%) | 149 (49.3) | 33 (71.7) | 0.0046 |
| ≥9 medications daily, n (%) | 67 (22.3) | 17 (37.0) | 0.0302 |
| Limitations in social activities, n (%) | 59 (19.4) | 24 (53.3) | <.0001 |
| Malnutrition, n (%) | 135 (47.4) | 37 (84.1) | <.0001 |
| Moderate/Severe Anxiety, n (%) | 30 (9.9) | 34 (77.3) | <.0001 |
| Vision impairment, n (%) | 64 (21.2) | 24 (53.3) | <.0001 |
| Hearing impairment, n (%) | 77 (25.7) | 19 (41.3) | 0.0274 |
| Moderate/severe Fatigue, n (%) | 160 (52.1) | 42 (93.3) | <.0001 |
| Moderate/severe Pain, n (%) | 124 (40.7) | 34 (75.6) | <.0001 |
| Frail, n (%) | 85 (27.5) | 38 (82.6) | <.0001 |
| Financial Distress, n (%) | 22 (7.4) | 8 (17.8) | 0.0219 |
| Health-Related Quality of Life | |||
| Physical Health Score, mean (SD) | 44.6 (10.1) | 31.9 (9.1) | <.0001 |
| Mental Health Score, mean (SD) | 49.6 (8.4) | 35.5 (8.5) | <.0001 |
| Healthcare Utilization | |||
| Emergency Room visit, n (%) | 164 (54.8) | 31 (70.5) | 0.0510 |
| Hospitalized at least one night, n (%) | 194 (64.0) | 32 (69.6) | 0.4637 |
Upon multivariate logistic regression modeling, after adjustment for age, sex, education, cancer type, and cancer stage, variables other than financial distress continued to demonstrate a significant association with moderate/severe depression status. Those patients who reported moderate/severe depression had a significantly increased odds of reporting a history of falls (aOR 4.01, 95% CI 1.94-8.26), impaired performance status (aOR 3.76, 95% CI 1.88-7.52), dependence in IADLs (aOR 7.06, 95% CI 2.91-17.1), dependence in ADLs (aOR 6.23, 95% CI 2.89-13.4), malnutrition (aOR 5.86, 95% CI 2.40-14.3), frailty (aOR 13.7, 95% CI 5.80-32.1), three or more non- cancer comorbidities (aOR 2.69, 95% CI 1.29-5.60), nine or more daily medications (aOR 2.51, 95% CI 1.21-5.20), limitations in social activities (aOR 4.49, 95% CI 2.20-9.18), moderate/severe cognitive complaints (aOR 42.2, 95% CI 11.2-159), moderate/severe anxiety (aOR 58.9, 95% CI 20.7-167), moderate/severe fatigue (aOR 11.2, 95% CI 3.31-37.6), and moderate/severe pain (aOR 4.73, 95% CI 2.12-10.5) compared to those patients without moderate/severe depression (see Table 3). Those with moderate/severe depression also had significantly increased odds of reporting reduced physical (aOR 7.58, 95% CI 3.30-17.4) and mental (aOR 26.3, 95% CI 10.1- 68.8) HRQOL sub-scores without significant differences in patient-reported hospitalizations or emergency room visits in the past year compared to those patients without moderate/severe depression.
Table 3.
Unadjusted and Adjusted Odds Ratios of Geriatric Assessment Domains, Health-Related Quality of Life, and Healthcare Utilization Based on Moderate/Severe Depression Status.
| Geriatric Assessment | Unadjusted Odds, 95% CI | Adjusted Odds*, 95% CI |
|---|---|---|
| ≥1 Fall | 3.71 (1.92-7.16) | 4.01 (1.94-8.26) |
| Impaired (≥2) ECOG Performance Status | 3.80 (2.00-7.22) | 3.76 (1.88-7.52) |
| Reported Limitations in Walking One Block | 5.72 (2.48-13.2) | 5.18 (2.15-12.4) |
| IADL Dependence | 6.33 (2.74-14.6) | 7.06 (2.91-17.1) |
| ADL Dependence | 5.24 (2.67-10.3) | 6.23 (2.89-13.4) |
| Moderate/Severe Cognitive Dysfunction | 22.4 (8.54-58.7) | 42.2 (11.2-159) |
| ≥3 Comorbidities other than cancer | 2.61 (1.32-5.15) | 2.69 (1.29-5.60) |
| ≥9 Daily Medications | 2.05 (1.06-3.95) | 2.51 (1.21-5.20) |
| Limitations in Social Activities | 4.75 (2.48-9.10) | 4.49 (2.20-9.18) |
| Malnutrition | 5.87 (2.53-13.6) | 5.86 (2.40-14.3) |
| Moderate/Severe Anxiety | 30.8 (13.9-68.6) | 58.9 (20.7-167) |
| Vision Impairment | 4.25 (2.22-8.12) | 5.07 (2.43-10.6) |
| Hearing Impairment | 2.04 (1.07-3.87) | 2.01 (0.97-4.16) |
| Moderate/Severe Fatigue | 12.9 (3.90-42.4) | 11.2 (3.31-37.6) |
| Moderate/Severe Pain | 4.51 (2.20-9.24) | 4.73 (2.12-10.5) |
| Frail | 12.5 (5.61-27.9) | 13.7 (5.80-32.1) |
| Financial Distress | 2.70 (1.12-6.51) | 1.89 (0.66-5.38) |
| Health-Related Quality of Life | ||
| Physical Health Score, <40 | 8.04 (3.70-17.4) | 7.58 (3.30-17.4) |
| Mental Health Score, <40 | 22.3 (9.47-52.3) | 26.3 (10.1-68.8) |
| Healthcare Utilization | ||
| ER Visit | 1.28 (0.66-2.51) | 1.29 (0.61-2.70) |
| Hospitalization | 1.96 (0.99-3.90) | 1.90 (0.90-4.04) |
Adjusted for Age, Sex, Education, Cancer Type, and Cancer Stage.
Discussion
Over one quarter of older adults with a GI malignancy report some level of depression prior to receiving chemotherapy, half of which can be classified as moderate to severe. The prevalence of depression was largely independent of demographic and clinical variables, including cancer stage. In comparison with national baselines, these data suggest that depression in older adults with cancer prior to chemotherapy is approximately two times more prevalent when compared to those without cancer.25 The presence of depression in older adults with a GI malignancy prior to chemotherapy was associated with reported impairments in various GA domains, including reduced performance status, functional status limitations, frailty, malnutrition, polypharmacy, falls, pain, and reduced HRQOL even after adjusting for social and clinical variables (see Figure 1). In this phase prior to chemotherapy, there were no significant differences in prior emergency room visits or hospitalizations.
These results underscore the strong associations between depression and physical health as well as the relationships between depression and other aspects of mental health, including other mood disorders and cognitive function. The three patient-reported measures with the highest adjusted odds ratios (each above 20) in older adults with GI cancers and moderate/severe depression were moderate/severe cognitive dysfunction, moderate/severe anxiety, and worse mental HRQOL compared to those without moderate/severe depression. This reinforces the common clinical practice of screening for depression as a potentially reversible cause of dementia in older adults presenting with cognitive dysfunction and treatment with pharmacotherapy (e.g., selective serotonin reuptake inhibitor) and/or cognitive behavioral therapy. Anxiety often co-exists with depression, consistent with these results, and also has similar treatment strategies of pharmacotherapy (e.g., selective serotonin reuptake inhibitor) and/or cognitive behavioral therapy. Physical comorbidities associated with moderate/severe depression were also reported, though causality was not determined. While it is plausible that physical comorbidities lead to depression, it is also plausible that those with depression experience networked events that lead to physical comorbidities (e.g., those with depression may eat less, have more malnutrition, get placed on additional medications for pain and fatigue, and experience falls and overall frailty leading to a worsened functional status). Practically, there may exist bi-directionality between mental and physical health domains that can easily reinforce and exacerbate each other (see Figure 1), requiring a comprehensive approach in this vulnerable population.
Given concerns that the oncology care team often underestimates patients’ depression, the American Society of Clinical Oncology has put forth guidelines to routinely assess for depression throughout the trajectory of care, stating that a failure to identify it would lead to a worsened quality of life as well as potential morbidity and mortality.28,29 Psychosocial care of patients with cancer is considered to be a patient right, with calls for routine assessment of patient distress and to consider it the sixth vital sign.30 In large meta-analyses of various cancers, depression was shown to predict mortality and continued to do so after adjusting for clinical prognosticators suggesting the possibility of a causal role.31,32 Furthermore, depressive symptoms prior to hospitalization in those with advanced cancer have been linked to a longer length of stay, an effect moderated by the use of anti-depressants.33 Cancer survivors in the US report using medications for depression at almost twice the rate of the general public, underscoring the emotional strains associated with cancer and/or the treatments. 34
Despite a lack of exact numbers, depression in the setting of cancer is understood to have a relatively higher prevalence compared with those without cancer and thought to be multifaceted in nature, including psychosocial elements as well as physical elements such as widespread inflammation and biochemical dysregulation with contributions possibly dependent on phase of treatment.35,36 A study of patients with cancer that used an initial patient-reported screen, then confirmatory phone interview estimated the overall prevalence of depression to be 5-13% with differences between cancer types (7% in those patients with colorectal cancer) and found that almost 75% of patients with depression were not receiving any form of treatment.37 Depression has been shown to begin almost a year prior to cancer diagnosis, peak during the week after diagnosis, then decline though remains higher than patients without cancer up to a decade later.38 A separate meta-analysis found that depression is highest during treatment but may improve with time, estimating the prevalence at 8-24% and underscoring the large amount of heterogeneity.39
In those aged ≥60 years, the prevalence of moderate to severe depression has been estimated at just above 5% with a female predilection, and in those aged >65 years, the prevalence has been estimated to be 3-4% with a continued female predilection and only one-third actively receiving treatment.25,26 As age increases, the overall clinical course of depression worsens as measured by depression at two years from diagnosis, chronic symptoms, time to remission, and the change in depression severity, suggesting the need for highly targeted, structured interventions.27 It has been reported that those older adults exhibiting depression are more likely to decline physically over time in proportion to depression severity, suggesting that mental and physical health are mutually reinforcing domains and that mental health interventions could mitigate physical decline in older adults.40 Alternatively, frailty has been demonstrated to predict incident depression in older adults.41,42 The implications are far reaching, as symptoms of depression in older adults are independently associated with an increase in informal caregiving from loved ones, even after adjustment for other major co-existing conditions, and carry the weight of burdensome time commitments in addition to other societal economic costs.43
There are a limited number of studies evaluating depression in older adults with cancer, but they underscore the need for increased attention. Eight of the most commonly used patient-reported depression instruments lack validation in older adults with cancer and often do not identify symptoms in this highly vulnerable population.44 When older adults actively undergoing cancer treatment underwent depression screening with patient reported legacy instruments, such as the Geriatric Depression Scale Short Form, the Hospital Anxiety and Depression Scale, and the Center for Epidemiological Studies Depression Scale, use of published cut off scores missed 33-83% of depression.45 When identified, depression in older adults with cancer has been reported to have important clinical consequences. A prospective cohort study in France using a geriatrician-based semi-structured interview for diagnosis of depression, including somatic complaints, in multiple cancer types at various stages prior to treatment found an overall prevalence of 28.4%, and that it was associated with inadequate social support, cognitive impairment, polypharmacy, and pain.46 Depression in older females with advanced ovarian cancer has been shown as an independent predictor of severe toxicity from chemotherapy as well as worse overall survival.47 Older females with a recent diagnosis of depression and breast cancer are at a higher risk of receiving non-definitive treatment and having worse overall survival.48 Unfortunately, those aged ≥60 years with cancer and depression, if detected, are less likely to receive treatment for depression than those who are younger.37
However, older adults with advanced cancers and actively receiving treatment had improved levels of depression and overall distress at six months with monthly educational materials, telephone monitoring, and proper referrals.49 Interventions such as these can be extended to patients throughout the US, as there are multidisciplinary, evidence-based community resources that have been developed to mitigate the growing public health concern of older adults with depression, such as PEARLS (Program to Encourage Active Rewarding Lives for Seniors) and Healthy IDEAS (Identifying Depression, Empowering Activities for Seniors).4,5 There are also clinical practice guidelines for treating depression in patients with cancer given the demonstrated efficacy of pharmacologic and psychosocial approaches, though relative and combined efficacy require further investigation.50,51
This study has its limitations. Given its cross-sectional nature in a specific patient population (older adults with GI cancers prior to chemotherapy) and no direct non-cancer control group, no statistical causality can be derived, though national data are available to estimate comparisons. Data regarding prior diagnoses of mood disorders, including depression, was not captured. Furthermore, data regarding active use of pharmacotherapy or psychotherapy was not captured. Additionally, there is no longitudinal follow up to assess how depression changes over time and throughout the course of treatment. These help to define criteria for future studies and next steps. In addition to more routine screening for the presence of depression in older adults with cancer, particular attention should to be given to whether or not the patient is receiving therapy, as almost 75% of those of any age with cancer and depression are not receiving active treatment.37 If not, the patient should begin receiving treatment for depression and continue to be monitored for response throughout various treatment phases per guidelines.29
To our knowledge, this is the first study using the PROMIS® Depression Short Form within a GA to quantify the prevalence of depression in older adults with cancer, specifically with GI malignancies and prior to chemotherapy. Although older adults with cancer and depression is an overall poorly understood clinical demographic, the available data suggest an especially vulnerable population that requires a high degree of suspicion to identify. Depression in older adults with cancer appears to be a prevalent and treatable disease pattern that has adverse associations with multiple other facets of health. Depression deserves dedicated screening and interventions, as outlined in several guidelines. Despite lack of clear evidence and need for additional research in older adults with cancer and depression, there are several resources and interventions that can be used to assist in the management of this population that should be widely adopted in oncology clinics. Given what is known regarding the adverse associations of depression in those with cancer and/or in older adults, it is paramount to be aware of such a widespread yet underappreciated and treatable disease associated with pleiotropic comorbidities in the largest and fastest growing segment of the oncology population.
Acknowledgments
Funding
Supported in part by the National Cancer Institute of the National Institutes of Health (K08CA234225). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Declaration of Competing Interests
The authors have no conflict to disclose.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJCK. Cancer Statistics Review, 1975-2013 - SEER Statistics. SEER Cancer Stat Rev 1975-2013, Natl Cancer Institute; Bethesda, MD. 2015. https://seer.cancer.gov/archive/csr/1975_2016/. Accessed May 10, 2020. [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- 3.Scher KS, Hurria A. Under-Representation of Older Adults in Cancer Registration Trials: Known Problem, Little Progress. J Clin Oncol. 2012;30(17):2036–2038. doi: 10.1200/JCO.2012.41.6727 [DOI] [PubMed] [Google Scholar]
- 4.CDC. Issue Brief No 1: What Do the Data Tell Us? The State of Mental Health and Aging in America. https://www.cdc.gov/aging/pdf/mental_health.pdf. Accessed May 10, 2020.
- 5.CDC. Issue Brief No 2: Addressing Depression in Older Adults: Selected Evidence-Based Programs The State of Mental Health and Aging in America. In: CDC. ; 2014. http://www.gmhfonline.org/gmhf/consumer/depression.html. Accessed May 10, 2020. [Google Scholar]
- 6.Weinberger MI, Bruce ML, Roth AJ, Breitbart W, Nelson CJ. Depression and barriers to mental health care in older cancer patients. Int J Geriatr Psychiatry. 2011. ;26(1 ):21–26. doi: 10.1002/gps.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnuson A, Sattar S, Nightingale G, Saracino R, Skonecki E, Trevino KM. A Practical Guide to Geriatric Syndromes in Older Adults With Cancer: A Focus on Falls, Cognition, Polypharmacy, and Depression. Am Soc Clin Oncol Educ B. 2019;(39):e96–e109. doi: 10.1200/edbk_237641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol. 2020;11(2):270–273. doi: 10.1016/j.jgo.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celia D, Riley W, Stone A, et al. Initial adult health item banks and first wave testing of the patient reported outcomes measurement information system (PROMIS) network. J Clin Epidemiol. 2011. ;63(11 ):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011.Initial [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Celia D. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): Depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi: 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley WT, Pilkonis P, Celia D. Application of the national institutes of health patient-reported outcome measurement information system (PROMIS) to mental health research. J Ment Health Policy Econ. 2011. ;14(4):201–208. [PMC free article] [PubMed] [Google Scholar]
- 12.Schalet BD, Pilkonis PA, Yu L, et al. Clinical Validity of PROMIS Depression, Anxiety, and Anger Across Diverse Clinical Samples. J Clin Epidemiol. 2016;73. doi: 10.1016/J.JCLINEPI.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clover K, Lambert SD, Oldmeadow C, et al. PROMIS depression measures perform similarly to legacy measures relative to a structured diagnostic interview for depression in cancer patients. Qual Life Res. 2018;27(5):1357–1367. doi: 10.1007/s11136-018-1803-x [DOI] [PubMed] [Google Scholar]
- 14.Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC, Lawrence SM. Validation of the Depression Item Bank From the Patient-Reported Outcomes Measurement Information System (PROMIS) in a Three-Month Observational Study. J Psychiatr Res. 2014;56. doi: 10.1016/J.JPSYCHIRES.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin JB, Aebi ME, Smyth KA, et al. Comparing Patient-Reported Outcomes Measure Information System Depression Scale with Legacy Depression Measures in a Community Sample of Older Adults with Varying Levels of Cognitive Functioning. Am J Geriatr Psychiatry. 2015;23(11): 1134–1143. doi: 10.1016/j.jagp.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A Brief Guide to the PROMIS © Depression Instruments. http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Depression_Scoring_Manual_02222017.pdf. Accessed May 10, 2020.
- 17.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol. 2014;5(3):245–251. doi: 10.1016/j.jgo.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 18.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: A feasibility study. Cancer. 2005;104(9): 1998–2005. doi: 10.1002/cncr.21422 [DOI] [PubMed] [Google Scholar]
- 19.Williams GR, Al-Obaidi M, Dai C, et al. Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies. Cancer. September 2020:cncr.33122. doi: 10.1002/cncr.33122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight TG, Deal AM, Dusetzina SB, et al. Financial Toxicity in Adults With Cancer: Adverse Outcomes and Noncompliance. J Oncol Pract. 2018;14(11 ):e665–e673. doi: 10.1200/jop.18.00120 [DOI] [PubMed] [Google Scholar]
- 21.Mir N, MacLennan P, Al-Obaidi M, et al. Patient-reported cognitive complaints in older adults with gastrointestinal malignancies at diagnosis– Results from the Cancer & Aging Resilience Evaluation (CARE) study. J Geriatr Oncol. 2020:4–10. doi: 10.1016/j.jgo.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams GR, Deal AM, Nyrop KA, et al. Geriatric assessment as an aide to understanding falls in older adults with cancer. Support Care Cancer. 2015;23(8):2273–2280. doi: 10.1007/s00520-014-2598-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams GR, Deal AM, Lund JL, et al. Patient-Reported Comorbidity and Survival in Older Adults with Cancer. Oncologist. 2018;23(4):433–439. doi: 10.1634/theoncologist.2017-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. JNCCN J Natl Compr Cancer Netw. 2017;15(7):894–902. doi: 10.6004/jnccn.2017.0122 [DOI] [PubMed] [Google Scholar]
- 25.Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief. 2014;(172):1–8. [PubMed] [Google Scholar]
- 26.Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: The Cache County study. Arch Gen Psychiatry. 2000;57(6):601–607. doi: 10.1001/archpsyc.57.6.601 [DOI] [PubMed] [Google Scholar]
- 27.Schaakxs R, Comijs HC, Lamers F, Kok RM, Beekman ATF, Penninx BWJH. Associations between age and the course of major depressive disorder: a 2-year longitudinal cohort study. The Lancet Psychiatry. 2018;5(7):581–590. doi: 10.1016/S2215-0366(18)30166-4 [DOI] [PubMed] [Google Scholar]
- 28.Passik SD, Dugan W, McDonald MV., Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16(4): 1594–1600. doi: 10.1200/JCO.1998.16.4.1594 [DOI] [PubMed] [Google Scholar]
- 29.Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605–1619. doi: 10.1200/JCO.2013.52.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland J, Watson M, Dunn J. The IPOS new international standard of quality cancer care: Integrating the psychosocial domain into routine care. Psychooncology. 2011. ;20(7):677–680. doi: 10.1002/pon.1978 [DOI] [PubMed] [Google Scholar]
- 31.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561 [DOI] [PubMed] [Google Scholar]
- 32.Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol Med. 2010;40(11): 1797–1810. doi: 10.1017/S0033291709992285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong RL, El-Jawahri A, D’Arpino SM, et al. Use of Antidepressant Medications Moderates the Relationship Between Depressive Symptoms and Hospital Length of Stay in Patients with Advanced Cancer. Oncologist. 2018:theoncologist.2018–0096. doi: 10.1634/theoncologist.2018-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkins NA, Soman A, Lunsford NB, Leadbetter S, Rodriguez JL. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35(1):78–85. doi: 10.1200/JCO.2016.67.7690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith HR. Depression in cancer patients: Pathogenesis, implications and treatment (review). Oncol Lett. 2015;9(4):1509–1514. doi: 10.3892/ol.2015.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361 (April): 1–6. doi: 10.1136/bmj.k1415 [DOI] [PubMed] [Google Scholar]
- 37.Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. The Lancet Psychiatry. 2014;1(5):343–350. doi: 10.1016/S2215-0366(14)70313-X [DOI] [PubMed] [Google Scholar]
- 38.Lu D, Andersson TML, Fall K, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: A nationwide matched cohort study in Sweden. JAMA Oncol. 2016;2(9):1188–1196. doi: 10.1001/jamaoncol.2016.0483 [DOI] [PubMed] [Google Scholar]
- 39.Krebber AMH, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. doi: 10.1002/pon.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penninx BWJH, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJH, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. J Am Med Assoc. 1998;279(21 ):1720–1726. doi: 10.1001/jama.279.21.1720 [DOI] [PubMed] [Google Scholar]
- 41.Jia F, Shi X, Li X, Wang B, Liu F, Cao F. Physical frailty and the risk of major depressive disorder: The Irish Longitudinal Study on Ageing. J Psychiatr Res. 2020;125(44):91–95. doi: 10.1016/j.jpsychires.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 42.Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer. 2019;27(7):2693–2698. doi: 10.1007/s00520-018-4558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langa KM, Valenstein MA, Fendrick AM, Kabeto MU, Vijan S. Extent and Cost of Informal Caregiving for Older Americans with Symptoms of Depression. Am J Psychiatry. 2004;161(5):857–863. doi: 10.1176/appi.ajp.161.5.857 [DOI] [PubMed] [Google Scholar]
- 44.Nelson CJ, Cho C, Berk AR, Holland J, Roth AJ. Are gold standard depression measures appropriate for use in geriatric cancer patients? A systematic evaluation of self-report depression instruments used with geriatric, cancer, and geriatric cancer samples. J Clin Oncol. 2010;28(2):348–356. doi: 10.1200/JCO.2009.23.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saracino RM, Weinberger MI, Roth AJ, Hurria A, Nelson CJ. Assessing depression in a geriatric cancer population. Psychooncology. 2017;26(10):1484–1490. doi: 10.1002/pon.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canoui-Poitrine F, Reinald N, Laurent M, et al. Geriatric assessment findings independently associated with clinical depression in 1092 older patients with cancer: The ElCAPA Cohort Study. Psychooncology. 2016;25(1):104–111. doi: 10.1002/pon.3886 [DOI] [PubMed] [Google Scholar]
- 47.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol. 2005;16(11):1795–1800. doi: 10.1093/annonc/mdi368 [DOI] [PubMed] [Google Scholar]
- 48.Goodwin JS, Zhang DD, Ostir GV. Effect of Depression on Diagnosis, Treatment, and Survival of Older Women with Breast Cancer. J Am Geriatr Soc. 2004;52(1 ):106–111. doi: 10.1111/j.1532-5415.2004.52018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kornblith AB, Dowell JM, Herndon JE, et al. Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer: A Cancer and Leukemia Group B study. Cancer. 2006;107(11):2706–2714. doi: 10.1002/cncr.22296 [DOI] [PubMed] [Google Scholar]
- 50.Li M, Fitzgerald P, Rodin G. Evidence-based treatment of depression in patients with cancer. J Clin Oncol. 2012;30(11):1187–1196. doi: 10.1200/JCO.2011.39.7372 [DOI] [PubMed] [Google Scholar]
- 51.Li M, Kennedy EB, Byrne N, et al. Management of Depression in Patients With Cancer: A Clinical Practice Guideline. J Oncol Pract. 2016;12(8):747–756. doi: 10.1200/JOP.2016.011072 [DOI] [PubMed] [Google Scholar]


