Abstract
In this study, in vitro inhibition effects of (E)-1-(4-aminophenyl)-3-(aryl) prop-2-en-1-one (4-amino-chalcones) derivatives (3a–o) on acetylcholinesterase (AChE) enzyme and human erythrocyte carbonic anhydrase I and II isoenzymes (hCA I- II) were investigated. And also, the biological activities of 4-amino-chalcone derivatives against enzymes which names are acetylcholinesterase (PDB ID: 1OCE), human Carbonic Anhydrase I (PDB ID: 2CAB), human carbonic anhydrase II (PDB ID: 3DC3), were compared. After the results obtained, ADME/T analysis was performed in order to use 4-amino-chalcone derivatives as a drug in the future. Effective inhibitors of carbonic anhydrase I and II isozymes (hCAI and II) and acetylcholinesterase (AChE) enzymes with Ki values in the range of 2.55 ± 0.35–11.75 ± 3.57 nM for hCA I, 4.31 ± 0.78–17.55 ± 5.86 nM for hCA II and 96.01 ± 25.34–1411.41 ± 32.88 nM for AChE, respectively, were the 4-amino-chalcone derivatives (3a–o) molecules.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-021-00094-x.
Keywords: 4-Amino-chalcones, Carbonic anhydrase, Acetylcholinesterase, Molecular docking
Introduction
Acetylcholine is a neurotransmitter substance secreted from the synapses of autonomic ganglia and the ends of nerve strands laying the skeletal muscle. Acetylcholinesterase (AChE; EC 3.1.1.7) is a nonspecific enzyme that hydrolyzes lipotropic acetylcholine in the tissues, free or combined with phospholipids.In other words, acetylcholinesterase functions around by removing the chemicals that accumulate in front of the nerve end and removing them from there. Thus, a possible malfunction in nerve conduction does not occur (Göcer et al. 2017; Ökten et al. 2020; Çakmak et al. 2020).
One of the most important causes of Alzheimer’s disease is the decrease in the amount of acetylcholine in the brain (Göcer et al. 2017).
AChE inhibitors (Donepezil, Rivastigmin) are widely used for Alzheimer's patients to live as high quality (Göcer et al. 2013, 2015). However, such drugs with inhibitory effects cause side effects such as gastrointestinal disorders and hepatotoxicity. For this reason, AChE inhibitors, which are both effective and safe, as well as especially natural, have become more and more important in recent times (Gülçin et al. 2020; Timur et al. 2019).
Carbonic anhydrase inhibitors are among the most potent drugs that reduce the intraocular pressure used in the treatment of eye diseases. These have been recently used for reducing intraocular pressure, topical and is used systemically. Acetazolamide, a systemic inhibitor of carbonic anhydrase, was first used in 1954 by Becker, Grant, Trotter, Breinin and Gürtz to reduce intraocular pressure in glucose disorder (Becker 2020; Kim et al. 2019; Supuran 2019). In addition, acetazolamide is the most used inhibitor of the carbonic anhydrase enzyme. Recently, there has been a search for inhibitors for hCA isoenzymes in structures other than sulfonamides. Inhibitor development and synthesis studies have been increased, especially for use in drug design development.
Recently, in many studies, the importance and place of theoretical studies has increased. Experimental studies conducted in these studies are guiding (Tüzün et al. 2018, 2020; Bilgiçli et al. 2020a, b; Douche et al. 2020). Theoretical studies are preferred because they are very fast, and their cost is cheap. Among the theoretical methods, the most common and fastest molecular docking method was used to compare the biological activities of 4-amino-chalcone derivatives (3a–o) against enzymes (Taslimi et al. 2020). These enzymes are acetylcholinesterase (AChE) (PDB ID: 1OCE) (Cheung et al. 2013), human Carbonic Anhydrase I (hCA I) (PDB ID: 2CAB) (Alterio et al. 2010), human carbonic anhydrase II (hCA II) (PDB ID: 3DC3) (Ivanova et al. 2015). ADME/T (Absorption, distribution, metabolism, excretion, and toxicity) analysis was then performed to investigate the drug availability of 4-amino-chalcone derivatives (3a–o). The parameters obtained as a result of this ADME/T analysis theoretically examined the biological effects and reactions of this molecule in human metabolism.
Whether hCA I and II isoenzymes and AChE, these three enzyme systems are of great importance for human health. Changes in the level of hCA in human erythrocytes are associated with many metabolic disorders such as diabetes, edema and hypertension (Bayindir et al. 2019). AChE enzyme, on the other hand, they are the enzymes responsible for the emergence of many neurological diseases, including Alzheimer's (Güzel et al. 2019).
In the scope of our study, the inhibition properties of 4-amino-chalcone derivatives (3a–o) were investigated by using them as inhibitors. Depending on the results obtained, these molecules or their derivatives are used in drug designs in terms of usability in the treatment of many diseases. We anticipate that it will shed light and make significant contributions in the field of pharmacology.
Results and discussion
Chemistry
4-Amino-chalcone derivatives (3a–o) were resynthesized from the Claisen-Schmidtcondensation of the benzaldehyde derivatives (1a–o) with 4-aminoacetophenone (2) according to the published procedure (Koçyiğit et al. 2018; Gürdere et al. 2020). (Scheme 1, Table 1). All spectroscopic data are in good agreement with the published data.
Scheme 1.

Reagents and conditions: (i) NaOH, C2H5OH, 0 °C, 3 h, (ii) SnCl2·2H2O, C2H5OH, reflux, 2 h
Table 1.
Chemical structures of compounds
Biochemical studies
Inhibition of metabolic enzymes was investigated, and their results were reported as follows.
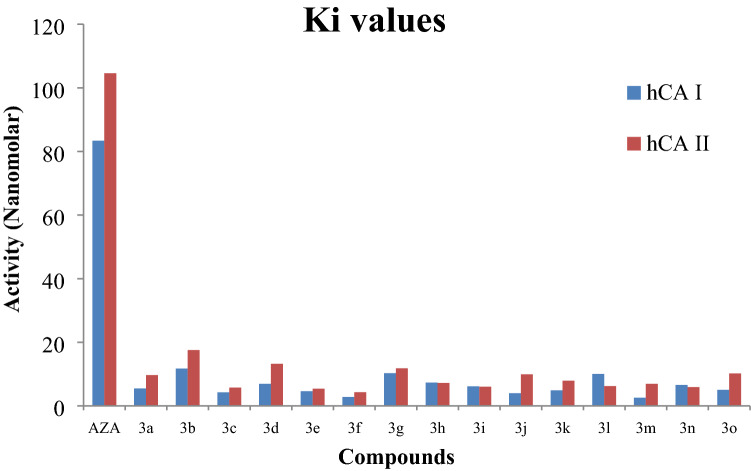
In this study, the compounds 3a–o were screened against the AChE enzyme due to significant reports on AD of phenolic natural or synthetic compounds. IC50 and Ki values of the reference drug Tacrin were 1143.31 nM (IC50) and 858.85 ± 12.11 nM (Ki) towards AChE, as shown in Table 2. The compounds inhibited the AChE enzyme in nanomolar concentration in the range of Ki values of 96.01 ± 25.34–1411.41 ± 302.88 nM and with IC50 values of 179.71—1048.41 nM. The compounds 3a and 3d were found potent AChE inhibitors with the Ki values of 96.01 ± 25.34 nM and 156.45 ± 66.48 nM, respectively, while the compound was the least inhibiting compound with the highest Ki value of 1411.41 ± 302.88 nM. On the other hand, the compound 3e was also considered as one of the potent inhibitors with the lowest IC50 value of 179.71 nM against AChE (Figs. 1, 2). The Table 2 showed that the Ki values of reference drug AZA were 83.90 ± 19.71 nM and 104.60 ± 27.60 nM, whereas the IC50 values of AZA were 97.30 nM and 115.50 nM towards hCA I and II, respectively. Ki values of 3a–o were calculated as 2.55 ± 0.35–11.75 ± 3.57 nM for hCA I, 4.31 ± 0.78–17.55 ± 5.86 nM for hCA II.
Table 2.
Inhibition results of novel 4-Amino-chalcone derivatives (3a-o) on carbonic anhydrase and acetylcholinesterase
| Compounds | IC50 (nM) | Ki (nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| hCA I | r2 | hCA II | r2 | AChE | r2 | hCA I | hCA II | AChE | |
| 3a | 8.35 | 0.9717 | 9.11 | 0.9810 | 261.91 | 0.9639 | 5.46 ± 1.32 | 9.68 ± 2.51 | 156.45 ± 66.48 |
| 3b | 8.15 | 0.9658 | 9.01 | 0.9569 | 408.61 | 0.9817 | 11.75 ± 3.57 | 17.55 ± 5.86 | 259.33 ± 35.95 |
| 3c | 5.87 | 0.9580 | 6.86 | 0.9973 | 700.01 | 0.9872 | 4.26 ± 0.85 | 5.76 ± 0.91 | 514.47 ± 95.66 |
| 3d | 7.01 | 0.9960 | 9.36 | 0.9724 | 222.47 | 0.9848 | 6.94 ± 1.83 | 13.21 ± 4.07 | 96.01 ± 25.34 |
| 3e | 4.35 | 0.9575 | 5.02 | 0.9953 | 382.45 | 0.9811 | 4.62 ± 0.82 | 5.38 ± 1.23 | 474.15 ± 70.66 |
| 3f | 3.82 | 0.9797 | 4.84 | 0.9784 | 179.71 | 0.9668 | 2.81 ± 0.18 | 4.31 ± 0.78 | 159.62 ± 68.17 |
| 3g | 8.25 | 0.9639 | 10.51 | 0.9528 | 1048.41 | 0.9689 | 10.26 ± 1.47 | 11.80 ± 3.95 | 456.80 ± 114.41 |
| 3h | 7.37 | 0.9917 | 8.55 | 0.9768 | 548.26 | 0.9905 | 7.31 ± 2.98 | 7.22 ± 2.26 | 218.63 ± 59.47 |
| 3i | 5.37 | 0.9991 | 6.66 | 0.9896 | 758.21 | 0.9490 | 6.14 ± 1.42 | 6.04 ± 1.52 | 1411.41 ± 302.88 |
| 3j | 6.35 | 0.9839 | 6.93 | 0.9683 | 898.83 | 0.9691 | 3.97 ± 0.91 | 9.93 ± 2.51 | 361.63 ± 73.80 |
| 3k | 4.91 | 0.9965 | 7.29 | 0.9543 | 327.21 | 0.9753 | 4.87 ± 0.83 | 7.91 ± 1.88 | 299.77 ± 47.33 |
| 3l | 6.61 | 0.9660 | 8.88 | 0.9703 | 564.33 | 0.9692 | 10.04 ± 1.80 | 6.23 ± 2.20 | 337.48 ± 99.43 |
| 3m | 3.66 | 0.9722 | 5.82 | 0.9773 | 595.36 | 0.9908 | 2.55 ± 0.35 | 6.94 ± 1.55 | 495.94 ± 72.81 |
| 3n | 5.54 | 0.9847 | 7.45 | 0.9832 | 756.55 | 0.9933 | 6.56 ± 1.96 | 5.91 ± 1.01 | 619.81 ± 85.46 |
| 3o | 7.45 | 0.9865 | 8.46 | 0.9425 | 488.02 | 0.9518 | 5.04 ± 1.55 | 10.18 ± 0.85 | 285.20 ± 86.92 |
| AZA | 97.304 | 0.9889 | 115.50 | 0.9719 | – | – | 83.390 ± 19.71 | 104.60 ± 27.60 | – |
| Tacrine | – | – | – | – | 1143.312 | 0.9948 | – | – | 858.85 ± 12.11 |
Fig. 1.
Ki values of novel 4-Amino-chalcone derivatives (3a–o) on hCA I and hCA II
Fig. 2.
Ki values of novel 4-Amino-chalcone derivatives (3a–o) on AChE enzymes
Glaucoma with high intraocular pressure is one of the most important eye diseases. It causes blindness with a rate of 15–20%. hCA II in the eye retina is the primary cause of intraocular pressure formation. The most important method to eliminate this disease is to inhibit hCA II activity. For this purpose, acetazolamide and heteroaromatic sulfonamides have been used as inhibitors for many years (Ellman et al. 1961; Lineweaver and Burk 1934; Verpoorte et al. 1967).
Acetyl CoA is a product formed as a metabolic product of pyruvate occurring in glycolysis. Alzheimer's disease occurs as a result of decreased neurotransmitters in the brain. The most decreasing neurotransmitter in this disease is widespread dementia and neurodegenerative disease. Alzheimer's disease function of memory.
It was found as disorder. The decrease in the level of acetylcholine in the brain is the biggest biochemical factor of this disease. There is no cure for this disease. The treatments applied today are aimed at eliminating the symptoms of this disease. There is no treatment method that eliminates it. AChE inhibitors such as Rivastigmine and Donepezil are generally used for this purpose (Kocyigit et al. 2017; Güzel et al. 2019; Ökten et al. 2019).
The presence of some unwanted negative physiological effects of these drugs in patients has made the discovery of new inhibitors important.
Molecular docking results
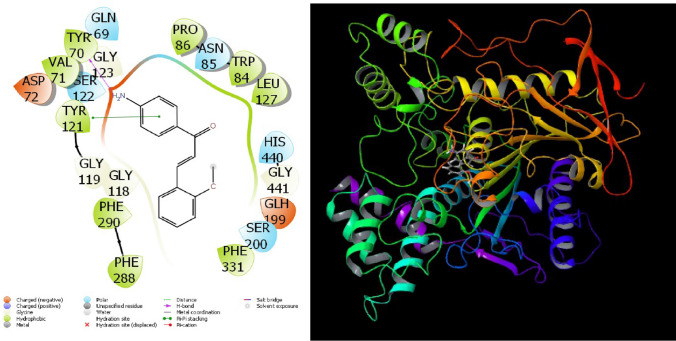
Molecular docking calculations were made to compare the biological activities of 4-amino-chalcone derivatives enzymes against proteins. Molecular docking calculation results of 4-amino-chalcone derivatives appear to be in great agreement with the experimental results (Aktas et al. 2020a, b; Gedikli et al. 2021). In the docking calculations of 4-amino-chalcone derivatives, numerical values of calculated many parameters as a result of docking calculations of molecules and enzymes support this situation. The most important factor affecting the numerical values of the parameters calculated from the interaction of amino-chalcone derivatives with enzymes is the interaction (Tüzün et al. 2021). Many chemical interactions occur between amino-chalcone derivatives with enzymes. As these chemical interactions increase, the biological activities of 4-amino-chalcone derivatives appear to increase. These interactions have many interactions such as hydrogen bonds, polar and hydrophobic interactions, π-π and halogen bonds (Sayin and Karakas 2017, 2018a; b; Sayin and Üngördü 2018, 2019; Üngördü and Sayin 2019; Jayarajan et al. 2020). As a result of calculations, the interactions of molecules with enzymes are given in Figs. 3, 4, and 5. In picture 3, in the interactions between the proteins of the AChE enzyme and molecule 3d, a hydrogen bond is formed between the oxygen in the methoxy group attached to the phenyl group in molecule 3d and the TRP 5 protein. Again, pi-pi interactions occur between the phenyl group in molecule 3d and HIE 64. There is a Pi-cation interaction between the aniline ring and the LYS 170 protein. On the other hand, in the interactions between the proteins of the hCA I enzyme and the molecule 3 m, there is a pi–pi interaction between the aniline ring and the TRY 121 protein. There is a hydrogen bond interaction between the amine group in the molecule and the TRY 70 protein. However, in the interactions between the proteins of the hCA II enzyme and molecule 3f, there is a hydrogen bond between the amino group in the molecule and the HIE protein. Supplementary data for all other interactions is given in Figure S29–S72.
Fig. 3.
Representation of interactions of molecules 3m and hCA I enzymes
Fig. 4.
Representation of interactions of molecules 3d and AChE enzymes
Fig. 5.
Representation of interactions of molecules 3f and hCA II enzymes
Molecular docking calculations were performed against enzymes of 4-amino-chalcone derivatives (3a–o) and biological activities of molecules were compared as a result of calculations. The molecular docking parameters obtained as a result of this comparison are given in Table 3 and S1.
Table 3.
Interaction value of studied molecule with enzymes
| Molecules | Docking score | ||
|---|---|---|---|
| AChE | hCA I | hCA II | |
| 3a | − 5.22 | − 4.37 | − 4.43 |
| 3b | − 6.00 | − 4.10 | − 4.47 |
| 3c | − 3.94 | − 4.28 | − 4.70 |
| 3d | − 6.55 | − 4.38 | − 3.30 |
| 3e | − 6.26 | − 3.79 | − 5.40 |
| 3f | − 3.89 | − 4.49 | − 4.55 |
| 3g | − 6.30 | − 3.88 | − 4.78 |
| 3h | − 5.98 | − 4.03 | − 4.80 |
| 3i | − 5.80 | − 3.83 | − 4.99 |
| 3j | − 6.00 | − 3.46 | − 4.71 |
| 3k | − 6.46 | − 3.80 | − 4.74 |
| 3l | − 5.64 | − 4.22 | − 4.70 |
| 3m | − 6.07 | − 3.46 | − 5.65 |
| 3n | − 6.35 | − 4.14 | − 4.55 |
| 3o | − 5.64 | − 3.80 | − 4.94 |
| AZA | – | − 3.98 | − 5.19 |
| TAC | − 6.06 | – | – |
The first parameter among parameters obtained to compare the biological activities of 4-amino-chalcone derivatives (3a–o) is the docking scooter. this parameter is the most important parameter to compare the biological activities of molecules. The molecular biological activity of the 4-amino-chalcone derivatives (3a–o) with the numerical value of the docking score parameter is the most negative. Another parameter among the parameters obtained is the Glide hbond parameter, whose numerical value gives information about the number of hydrogen bonds formed during interactions. Glide hbond parameter is affected by the atoms forming the hydrogen bond and the geometry of the hydrogen bonds. Another important parameter, that is Glide ligand efficiency parameter, is a numerical value used to sort the effectiveness of 4-amino-chalcone derivatives (3a–o) (Genc Bilgicli et al. 2020; Bilgiçli et al. 2020a, b). After examining the interactions of 4-amino-chalcone derivatives (3a–o) with enzymes, the effects and responses of these derivatives on human metabolism are theoretically investigated in order to study their drug availability properties. For this, ADME/T analysis is required for 4-amino-chalcone derivatives (3a-o).
Each parameter obtained is as important as others to explain the drug properties of 4-amino-chalcone derivatives (3a–o). Because they each examined the effect or response of different organs or tissues. In particular, it should be known very well that one of the most important methods used without experiment to explain the effects and responses of 4-amino-chalcone derivatives (3a–o) in human metabolism is molecular docking calculations. By comparing the numerical values of these effects and reactions, it is possible to design more effective and active molecules (Taslimi et al. 2020).
As a result of the ADME/T analysis, many parameters were obtained. Some of these parameters are given in Table 4. All remaining parameters are given in table S1. The first parameter among the obtained parameters is Solute Molecular Weight, which tells how much the molecular weight of the molecule should be for human metabolism. Another parameter is Solute Hydrophobic SASA, which is the Hydrophobic component of the SASA (saturated carbon and attached hydrogen). Another parameter is QPlogHERG, which is predicted IC50 value for blockage of HERG K channels. Another parameter is QPPCaco, which is Predicted apparent Caco-2 cell permeability in nm/s. Caco-2 cells are a model for the gut-Blood barrier. QikProp predictions are for non-active transport. the numerical value of this parameter should be < 25 poor, > 500 great. Another parameter is QPlogBB, which is Predicted brain/Blood partition coefficient. Another parameter is QPPMDCK, which is Predicted apparent MDCK cell permeability in nm/sec. MDCK cells are considered to be a good mimic for the Blood brain barrier. QikProp predictions are for non-active transport. the numerical value of this parameter should be < 25 poor, > 500 great. Two parameters shown among the most important parameters obtained as a result of ADME/T analysis are RuleOfFive (Lipinski 2004; Lipinski et al. 1997) and RuleOfThree (Jorgensen and Duffy 2002). These parameters are more important than other parameters. When the numerical conditions of this parameter are not met, the molecule is said to not be a definite drug. RuleOfFive is Lipinski Rule of 5 and RuleOfThree is Jorgensen Rule of 3. RuleOfFive is number of violations of Lipinski’s rule of five. The rules are: mol_MW < 500, QPlogPo/w < 5, donorHB ≤ 5, accptHB ≤ 10. Compounds that satisfy these rules are considered drug like (The “five” refers to the limits, which are multiples of 5.). RuleOfThree is number of violations of Jorgensen’s rule of three. The three rules are: QPlogS > -5.7, QP PCaco > 22 nm / s, # Primary Metabolites < 7. Compounds with fewer violations of these rules are more likely to be orally available (Çetiner et al. 2021).
Table 4.
ADME properties of molecules
| Reference range | Solute molecular weight | QPlogHERG | QPPCaco (nm/s) | QPPMDCK (nm/s) | RuleOfFive | RuleOfThree |
|---|---|---|---|---|---|---|
| 130–725 | (concern below − 5) | a < 25 is poor and a > 500 is great | a < 25 is poor and a > 500 is great | Maximum is 4 | Maximum is 3 | |
| 3a | 237 | − 5.56 | 971 | 479 | 0 | 0 |
| 3b | 237 | − 5.64 | 970 | 479 | 0 | 0 |
| 3c | 237 | − 5.63 | 970 | 479 | 0 | 0 |
| 3d | 253 | − 5.67 | 971 | 479 | 0 | 0 |
| 3e | 253 | − 5.57 | 970 | 479 | 0 | 0 |
| 3f | 253 | − 5.55 | 970 | 479 | 0 | 0 |
| 3g | 258 | − 5.6 | 971 | 1050 | 0 | 0 |
| 3h | 258 | − 5.63 | 970 | 1180 | 0 | 0 |
| 3i | 258 | − 5.62 | 970 | 1181 | 0 | 0 |
| 3j | 302 | − 5.62 | 971 | 1130 | 0 | 0 |
| 3k | 302 | − 5.66 | 970 | 1269 | 0 | 0 |
| 3l | 302 | − 5.65 | 970 | 1269 | 0 | 0 |
| 3m | 229 | − 5.32 | 969 | 884 | 0 | 0 |
| 3n | 213 | − 5.09 | 968 | 478 | 0 | 0 |
| 3o | 223 | − 5.68 | 971 | 479 | 0 | 0 |
Many ADME/T parameters were calculated in the calculations. Each of the ADME/T parameters found gives information about a different property of the molecules. Molecules are desirably in the range of 130–725 g/mol. The molecular weight of the molecules is in the range of 237–258. Another parameter for molecules to become drugs is QPPCaco, which is expected to be less than 500. Of all the molecules, there are 970 units. This situation shows that the intestinal absorption of the molecules is difficult. Another parameter is QPPMDCK, the numerical value of this parameter is expected to be less than 500. but the chi of 3g, 3h, 3i, 3j, 3k, 3l, and 3m molecules is greater than 500. These drugs are not suitable for the brain.
Conclusions
Biological activities of 4-amino-chalcone derivatives (3a–o) against enzymes were compared. Afterwards, ADME/T analysis of this molecule was done, and it was theoretically investigated in the future. As a result of molecular docking and ADME/T calculations, the biological activities of molecules were compared against my enzyme. There is a great agreement between the experimental results and the theoretical results. However, the most important reason for some differences is that the theoretical calculations are made in an isolated environment. The results showed that 4-amino-chalcone derivatives (3a–o) do not have any side effects for human metabolism in the future. However, it was a good guide for future in vivo and in vitro studies. 4-Amino-chalcone derivatives (3a–o) had potent effects inhibiting CA enzymes such as AZA. AZA, a well-known CA receptor, is a positive regulation agent, such as topiramate, zonisamide, which methazolamide, and has been approved for epilepsy treatment and epileptic disorder.
Experimental
Measurements
General
4-Amino-chalcone derivatives (3a–o) were resynthesized according to published procedures (Scheme 1) (Kocyigit et al. 2018; Gürdere et al. 2020).
Enzymes studies
Determination of the effects of new compounds on acetylcholinesterase enzyme
The effect of 4-amino-chalcone derivatives (3a–o) used in the study on the enzyme acetylcholinesterase was investigated. In this study, Acetylcholinesterase method was used. Based on the data obtained, the inhibition types were determined by calculating the IC50 and Ki values. The principle of the method; As mentioned in the previous sections, AChE is used for hydrolysis of acetylcholine.
It is responsible for the formation of thiocoline and acetate, which are catalysis decomposition products. 5-thio-2-nitrobenzoic acid, a yellow compound, is formed as a result of the interaction of DTNB, which is used during inhibition studies, with thiocoline, one of the disintegration products. The color intensity of the colored compound formed is measured at 412 nm (Ellman et al. 1961). The absorbance of the sample and blind cuvettes at 412 nm at baseline and at the 5th minute is measured.
Determination of the effects of new compounds on carbonic anhydrase
Esterase activity method was used to measure the carbonic anhydrase enzyme activity. The method is based on the fact that CA has esterase activity. The principle of the method; p-nitrophenylasetate of the carbonic anhydrase enzyme used as a substrate. It is hydrolysis to p-nitrophenol or p-nitrophenol to give absorption at 348 nm.
In this method, both p-nitrophenol and p-nitrophenolate at 348 nm show the same absorbance. Therefore, phenol or phenolate formation does not affect the measurement during the reaction (Lineweaver and Burk 1934; Verpoorte et al. 1967; Kocyigit et al. 2017; Ökten et al. 2019). Since 348 nm p nitro phenyl acetate gives little absorption, it blindly.
It is used. In the measurements, an activity determination procedure was applied by mixing the reaction mixture using 3 mL quartz cuvettes.
Molecular docking method
Molecules docking is the most common method used to compare the biological activities of 4-amino-chalcone derivatives against enzymes, and there are many parameters obtained by this method (Ojha et al. 2020; Koçyiğit et al. 2020; Gezegen et al. 2020). To compare the biological activities of molecules, the numerical value of obtained many parameters that provide important information about molecules [32], is used. These parameters are used to compare the biological activities of molecules. The calculations made for this comparison consist of many stages. First, each molecule is optimized with the Gauss software program (Frisch et al. 2009) to prepare 4-amino-chalcone derivatives for calculations. Maestro Molecular modeling platform (version 12.2) by Schrödinger, LLC (Schrodinger 2019) was used for all subsequent docking calculations.
In the next process, it is the preparation of the studied enzymes that are human acetylcholinesterase (PDB ID: 4M0E), human Carbonic Anhydrase I (PDB ID: 3LXE), human carbonic anhydrase II (PDB ID: 5AML) in this study. The protein preparation module (Schrödinger Release 2019b; Friesner et al. 2006) was used to prepare the calculations for enzymes. With this module, the water molecules in the proteins of the enzymes were removed and then the active sites of the enzymes were determined. All proteins in this active site are given freedom of movement, because in this way the proteins interact more easily with the 4-amino-chalcone derivatives. In the next step, the LigPrep module (Schrödinger Release 2019a; Sastry et al. 2013) was used to prepare 4-amino-chalcone derivatives. In the next step, calculations were made with the Glide ligand docking module (Du et al. 2020) to interact with enzyme proteins with 4-amino-chalcone derivatives. All calculations for molecular docking calculations were made using the OPLS3e method. Finally, a detailed analysis of ADME / T analysis (absorption, distribution, metabolism, excretion and toxicity) was performed for 4-amino-chalcone derivatives to be drug molecules. Many parameters were calculated using the Qik-prop module (Schrödinger Release, 2020-1) of the Maestro Molecular modeling platform.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work is supported by the Scientific Research Project Fund of Sivas Cumhuriyet University under the project number RGD-020 and ECZ-079. This research was made possible by TUBITAK ULAKBIM, High Performance and Grid Computing Center (TR-Grid e-Infrastructure).
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meliha Burcu Gürdere, Email: burcugurdere@gmail.com.
Burak Tüzün, Email: theburaktuzun@yahoo.com.
References
- Aktaş A, Tüzün B, Aslan R, Sayin K, Ataseven H (2020a) New anti-viral drugs for the treatment of COVID-19 instead of favipiravir. J Biomol Struct Dynamics 1–11 [DOI] [PMC free article] [PubMed]
- Aktas A, Tuzun B, Taskin AH, Sayin K, Ataseven H. How do arbidol and its analogs inhibit the SARS-CoV-2? Bratisl Lek Listy. 2020;121(10):705–711. doi: 10.4149/BLL_2020_115. [DOI] [PubMed] [Google Scholar]
- Alterio V, Monti SM, Truppo E, Pedone C, Supuran CT, De Simone G. The first example of a significant active site conformational rearrangement in a carbonic anhydrase-inhibitor adduct: the carbonic anhydrase I–topiramate complex. Org Biomol Chem. 2010;8(15):3528–3533. doi: 10.1039/b926832d. [DOI] [PubMed] [Google Scholar]
- Bayindir S, Caglayan C, Karaman M, Gülcin İ. The green synthesis and molecular docking of novel N-substituted rhodanines as effective inhibitors for carbonic anhydrase and acetylcholinesterase enzymes. Bioorg Chem. 2019;90:103096. doi: 10.1016/j.bioorg.2019.103096. [DOI] [PubMed] [Google Scholar]
- Becker HM. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer. 2020;122(2):157–167. doi: 10.1038/s41416-019-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgiçli HG, Bilgiçli AT, Günsel A, Tüzün B, Ergön D, Yarasir MN, Zengin M. Turn-on fluorescent probe for Zn2+ ions based on thiazolidine derivative. Appl Organomet Chem. 2020;34(6):e5624. [Google Scholar]
- Bilgiçli HG, Ergön D, Taslimi P, Tüzün B, Kuru İA, Zengin M, Gülçin İ (2020) Novel propanolamine derivatives attached to 2-metoxifenol moiety: synthesis, characterization, biological properties, and molecular docking studies. Bioorganic Chem 103969 [DOI] [PubMed]
- Çakmak O, Ökten S, Alımlı D, Ersanlı CC, Taslimi P, Koçyiğit ÜM. Novel piperazine and morpholine substituted quinolines: selective synthesis through activation of 3, 6, 8-tribromoquinoline, characterization and their some metabolic enzymes inhibition potentials. J Mol Struct. 2020;1220:128666. [Google Scholar]
- Çetiner E, Sayin K, Tüzün B, Ataseven H. Could Boron-Containing compounds (BCCs) be effective against SARS-CoV-2 as anti-viral agent? Bratisl Lek Listy. 2021 doi: 10.4149/BLL_2021_044. [DOI] [PubMed] [Google Scholar]
- Cheung J, Gary EN, Shiomi K, Rosenberry TL. Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med Chem Lett. 2013;4(11):1091–1096. doi: 10.1021/ml400304w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douche D, Elmsellem H, Guo L, Hafez B, Tüzün B, El Louzi A, et al. (2020) Anti-corrosion performance of 8-hydroxyquinoline derivatives for mild steel in acidic medium: gravimetric, electrochemical, DFT and molecular dynamics simulation investigations. J Mol Liquids 113042
- Du Q, Qian Y, Yao X, Xue W. Elucidating the tight-binding mechanism of two oral anticoagulants to factor Xa by using induced-fit docking and molecular dynamics simulation. J Biomol Struct Dynamics. 2020;38(2):625–633. doi: 10.1080/07391102.2019.1583605. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, et al. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J Med Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, BKoino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision D.01. Gaussian Inc, Wallingford CT
- Gedikli MA, Tüzün B, Aktaş A, Sayin K, Ataseven H, (2021) Do clarithromycin, azithromycin and their analogues effective in the treatment of COVID19? Bratislavske lekarske listy 122 just accepted. 10.4149/BLL_2021_15 [DOI] [PubMed]
- Genc Bilgicli H, Taslimi P, Akyuz B, Tuzun B, Gulcin I. Synthesis, characterization, biological evaluation, and molecular docking studies of some piperonyl-based 4-thiazolidinone derivatives. Arch Pharm. 2020;353(1):1900304. doi: 10.1002/ardp.201900304. [DOI] [PubMed] [Google Scholar]
- Gezegen H, Gürdere MB, Dinçer A, Özbek O, Koçyiğit ÜM, Taslimi P, et al (2020) Synthesis, molecular docking, and biological activities of new cyanopyridine derivatives containing phenylurea. Arch Pharm e2000334 [DOI] [PubMed]
- Göçer H, Akıncıoğlu A, Öztaşkın N, Göksu S, Gülçin İ. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine-R elated compounds. Arch Pharm. 2013;346(11):783–792. doi: 10.1002/ardp.201300228. [DOI] [PubMed] [Google Scholar]
- Göçer H, Akincioğlu A, Göksu S, Gülçin İ, Supuran CT. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem. 2015;30(2):316–320. doi: 10.3109/14756366.2014.928704. [DOI] [PubMed] [Google Scholar]
- Göcer H, Akıncıoğlu A, Göksu S, Gülçin İ. Carbonic anhydrase inhibitory properties of phenolic sulfonamides derived from dopamine related compounds. Arab J Chem. 2017;10(3):398–402. [Google Scholar]
- Gülçin İ, Gören AC, Taslimi P, Alwasel SH, Kılıc O, Bursal E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal Agric Biotechnol. 2020;23:101441. [Google Scholar]
- Gürdere MB, Aydın A, yencilek B, Ertürk F, özbek O, Erkan S, et al. (2020) Synthesis, antiproliferative, cell cytotoxicity activity, DNA binding features and molecular d ocking study of novel enamine derivatives. Chem Biodiversity [DOI] [PubMed]
- Güzel E, Koçyiğit ÜM, Arslan BS, Ataş M, Taslimi P, Gökalp F, et al. Aminopyrazole-substituted metallophthalocyanines: preparation, aggregation behavior, and investigation of metabolic enzymes inhibition properties. Arch Pharm. 2019;352(2):1800292. doi: 10.1002/ardp.201800292. [DOI] [PubMed] [Google Scholar]
- Ivanova J, Leitans J, Tanc M, Kazaks A, Zalubovskis R, Supuran CT, Tars K. X-ray crystallography-promoted drug design of carbonic anhydrase inhibitors. Chem Commun. 2015;51(33):7108–7111. doi: 10.1039/c5cc01854d. [DOI] [PubMed] [Google Scholar]
- Jayarajan R, Satheeshkumar R, Kottha T, Subbaramanian S, Sayin K, Vasuki G. Water mediated synthesis of 6-amino-5-cyano-2-oxo-N-(pyridin-2-yl)-4-(p-tolyl)-2H-[1,2’-bipyridine]-3-carboxamide and 6-amino-5-cyano-4-(4-fluorophenyl)-2-oxo-N-(pyridin-2-yl)-2H-[1,2’-bipyridine]-3-carboxamide—an experimental and computational studies with non-linear optical (NLO) and molecular docking analyses. Spectrochim Acta Part A Mol Biomol Spectrosc. 2020;229:117861. doi: 10.1016/j.saa.2019.117861. [DOI] [PubMed] [Google Scholar]
- Jorgensen WL, Duffy EM. Prediction of drug solubility from structure. Adv Drug Deliv Rev. 2002;54(3):355–366. doi: 10.1016/s0169-409x(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Kim S, Yun J, Kang DY, Park HJ, Jo EJ, Jung JW, et al. Carbonic anhydrase inhibitor-induced Stevens-Johnson syndrome/toxic epidermal necrolysis leads to extensive cutaneous involvement. J Allergy Clin Immunol. 2019;7(8):2851. doi: 10.1016/j.jaip.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Kocyigit UM, Taşkıran AŞ, Taslimi P, Yokuş A, Temel Y, Gulçin İ. Inhibitory effects of oxytocin and oxytocin receptor antagonist atosiban on the activities of carbonic anhydrase and acetylcholinesterase enzymes in the liver and kidney tissues of rats. J Biochem Mol Toxicol. 2017;31(11):e21972. doi: 10.1002/jbt.21972. [DOI] [PubMed] [Google Scholar]
- Kocyigit UM, Budak Y, Gürdere MB, Ertürk F, Yencilek B, Taslimi P, et al. Synthesis of chalcone-imide derivatives and investigation of their anticancer and antimicrobial activities, carbonic anhydrase and acetylcholinesterase enzymes inhibition profiles. Arch Physiol Biochem. 2018;124(1):61–68. doi: 10.1080/13813455.2017.1360914. [DOI] [PubMed] [Google Scholar]
- Koçyiğit ÜM, Taslimi P, Tüzün B, Yakan H, Muğlu H, Güzel E (2020) 1, 2, 3-Triazole substituted phthalocyanine metal complexes as potential inhibitors for anticholinesterase and antidiabetic enzymes with molecular docking studies. J Biomol Struct Dynamics 1–11 [DOI] [PubMed]
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56(3):658–666. [Google Scholar]
- Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Ojha LK, Tüzün B, Bhawsar J. Experimental and theoretical study of effect of allium sativum extracts as corrosion inhibitor on mild steel in 1 M HCl medium. J Bio- Tribo-Corrosion. 2020;6(2):1–10. [Google Scholar]
- Ökten S, Ekiz M, Tutar A, Koçyiğit ÜM, Bütün B, Topçu G, Gülçin İ. SAR evaluation of disubstituted tacrine analogues as promising cholinesterase and carbonic anhydrase inhibitors. Indian J Pharm Educ Res. 2019;53:268–275. [Google Scholar]
- Ökten S, Aydın A, Koçyiğit ÜM, Çakmak O, Erkan S, Andac CA, et al. Quinoline-based promising anticancer and antibacterial agents, and some metabolic enzyme inhibitors. Arch Pharm. 2020;353(9):2000086. doi: 10.1002/ardp.202000086. [DOI] [PubMed] [Google Scholar]
- Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27(3):221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Sayin K, Karakas D. Determination of structural, spectral, electronic and biological properties of tosufloxacin boron complexes and investigation of substituent effect. J Mol Struct. 2017;1146:191–197. [Google Scholar]
- Sayin K, Karakas D. Investigation of structural, electronic properties and docking calculations of some boron complexes with norfloxacin: a computational research. Spectrochim Acta Part A, Mol Biomol Spectrosc. 2018;202:276–283. doi: 10.1016/j.saa.2018.05.055. [DOI] [PubMed] [Google Scholar]
- Sayin K, Karakas D. Quantum chemical investigation of levofloxacin-boron complexes: a computational approach. J Mol Struct. 2018;1158:57–65. [Google Scholar]
- Sayin K, Üngördü A. Investigation of anticancer properties of caffeinated complexes via computational chemistry methods. Spectrochim Acta Part A, Mol Biomol Spectrosc. 2018;193:147–155. doi: 10.1016/j.saa.2017.12.013. [DOI] [PubMed] [Google Scholar]
- Sayin K, Üngördü A. Investigations of structural, spectral and electronic properties of enrofloxacin and boron complexes via quantum chemical calculation and molecular docking. Spectrochim Acta Part A, Mol Biomol Spectrosc. 2019;220:117102. doi: 10.1016/j.saa.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Schrodinger L (2019) Small-molecule drug discovery suite 2019-4
- Schrödinger Release 2019-4: LigPrep, Schrödinger, LLC, New York, 2019a
- Schrödinger Release 2019-4: Protein preparation wizard; Epik, Schrödinger, LLC, New York, NY, 2016; Impact, Schrödinger, LLC, New York, NY, 2016; Prime, Schrödinger, LLC, New York, 2019b
- Schrödinger Release 2020-1: QikProp, Schrödinger, LLC, New York, 2020
- Supuran CT (2019) Carbonic anhydrase inhibitor—no donor hybrids and their pharmacological applications. In Therapeutic application of nitric oxide in cancer and inflammatory disorders (pp. 229–242). Academic Press
- Taslimi P, Erden Y, Mamedov S, Zeynalova L, Ladokhina N, Tas R, et al. (2020) The biological activities, molecular docking studies, and anticancer effects of 1-arylsuphonylpyrazole derivatives. J Biomol Struct Dynamics 1–20 [DOI] [PubMed]
- Timur İ, Kocyigit ÜM, Dastan T, Sandal S, Ceribası AO, Taslimi P, et al. In vitro cytotoxic and in vivo antitumoral activities of some aminomethyl derivatives of 2, 4-dihydro-3H-1, 2, 4-triazole-3-thiones—Evaluation of their acetylcholinesterase and carbonic anhydrase enzymes inhibition profiles. J Biochem Mol Toxicol. 2019;33(1):e22239. doi: 10.1002/jbt.22239. [DOI] [PubMed] [Google Scholar]
- Tüzün B, Bhawsar J (2021) Quantum chemical study of thiaozole derivatives as corrosion inhibitors based on density functional theory, Arabian J Chem 102927
- Tüzün B, Saripinar E. Molecular docking and 4D-QSAR model of methanone derivatives by electron conformational-genetic algorithm method. J Iran Chem Soc. 2020;17(5):985–1000. [Google Scholar]
- Tüzün B, Çağlar Yavuz S, Sarıpınar E. 4D-QSAR analysis and pharmacophore modeling: propoxy methylphenyl oxasiazole derivatives by electron conformatitional-genetic algorithm method. J Phys Theoret Chem. 2018;14(2):149–164. [Google Scholar]
- Üngördü A, Sayin K. Quantum chemical calculations on sparfloxacin and boron complexes. Chem Phys Lett. 2019;733:136677. [Google Scholar]
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem. 1967;242(18):4221–4229. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.