Highlights
Recent advances of various bottom-up approaches for constructing nanostructured semiconductor thermoelectric materials with different dimensions are reviewed.
The relationships between the nanostructures and the key electronic and thermal transport parameters contributing to ZT are discussed.
The challenges of the bottom-up strategies and suggestions for future development toward thermoelectric applications are provided.
Keywords: Thermoelectric, Nanostructures, Bottom-up, Synthesis, Nanomaterials
Abstract
The recent advancements in thermoelectric materials are largely credited to two factors, namely established physical theories and advanced materials engineering methods. The developments in the physical theories have come a long way from the “phonon glass electron crystal” paradigm to the more recent band convergence and nanostructuring, which consequently results in drastic improvement in the thermoelectric figure of merit value. On the other hand, the progresses in materials fabrication methods and processing technologies have enabled the discovery of new physical mechanisms, hence further facilitating the emergence of high-performance thermoelectric materials. In recent years, many comprehensive review articles are focused on various aspects of thermoelectrics ranging from thermoelectric materials, physical mechanisms and materials process techniques in particular with emphasis on solid state reactions. While bottom-up approaches to obtain thermoelectric materials have widely been employed in thermoelectrics, comprehensive reviews on summarizing such methods are still rare. In this review, we will outline a variety of bottom-up strategies for preparing high-performance thermoelectric materials. In addition, state-of-art, challenges and future opportunities in this domain will be commented.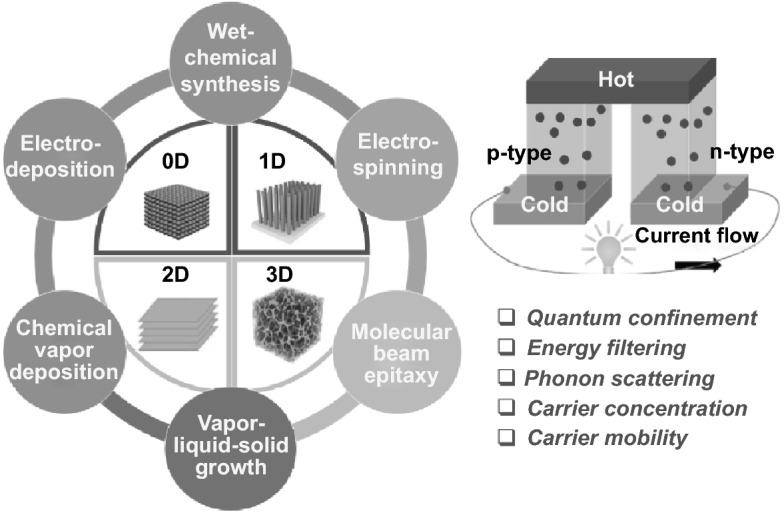
Introduction
The ever-increasing demand on electricity has driven the expansion of electricity supply sources, such as solar energy, nuclear power and photovoltaics. All these electricity sources are alternatives to the conventional fossil fuels. However, all these power generation approaches cannot address more than 60% of energy loss worldwide as waste heat. The ubiquity of low-grade waste heat (< 200 °C) in modern electronic devices is an opportunity in terms of energy recovery. In a bid to utilize the waste heat, thermoelectrics becomes is a viable option as it converts a thermal gradient into electrical energy in the solid state. This type of conversion from thermal heat to electricity possesses a lot of advantages, such as silent mode, no noise and less pollution, which has also triggered the development of various niche applications, including radioisotope thermoelectric (TE) generators (TEGs) for the space exploration, for example, National Aeronautics and Space Administration’s Voyager 1 and 2. However, all these applications put forward strict requirements for energy conversion efficiency, and traditionally, high-performance inorganic semiconducting materials, such as bulk Bi2Te3, SnSe, and PbTe [1–6], have been extensively studied and fabricated into the TEGs.
The efficiency of TE materials can be expressed by dimensionless figure of merit (ZT = σS2T/κ), where S, σ, T, and κ represent Seebeck coefficient, electrical conductivity, absolute temperature, and thermal conductivity, respectively. In order to enhance ZT, a large S, a high σ, and a lower κ are preferred, but these parameters are inter-dependent on each other. For instance, while the S has an inverse dependency on the carrier concentration (n), the σ is proportional to the n. To optimize the power factor (PF = σS2) and ZT, the n needs to be optimized either via doping or defect engineering to be around 1019 cm−3 level [7, 8]. However, the introduced dopants can deteriorate the charge carrier mobility, thus hindering the magnitude of the optimal power factor [9, 10]. A popular and effective strategy for enhancing the PF is via the electronic band-engineering [11–16]. In the electronic band-engineering, either the band-convergence or the effective mass manipulation can be explored to increase the S or σ, respectively [13, 17, 18]. In addition to the PF, the κ is also an important parameter that affects the ZT value. The κ is composed of electrical thermal conductivity (κe) and lattice thermal conductivity (κL). The κe value is predominantly affected by the σ through the charge carrier concentration and the κL is controlled by the introduced impurities through various phonon scattering mechanisms. The close inter-correlation between the S, σ, and κ makes it highly challenging to achieve a high ZT value.
Another useful approach to improve ZT is to prepare nanostructured semiconducting materials. The advantages of nanostructuring TE materials offer a pathway to positively decouple the correlation between the S, σ, and κ. The quantum confinement effects associating with these nanostructures help to alter the electronic density-of-states, and therefore improve the PF [19]. In addition, another noteworthy feature of nanostructuring is that it is able to decrease the κL through respective phonon scattering effects. For instance, one approach to reduce the κL is to minimize the phonon relaxation time through the introduction of phonon scattering sources such as point defects, dislocations and interfaces [20].
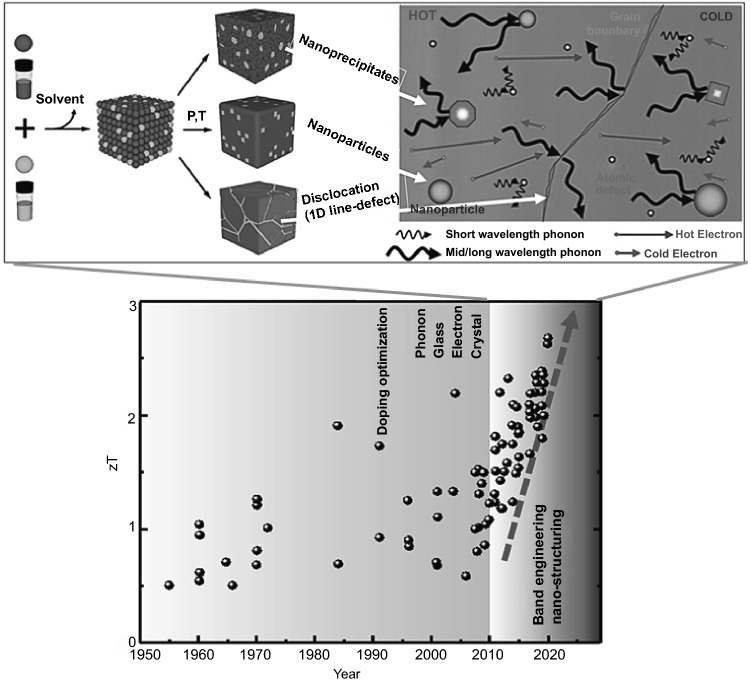
Traditionally, a popular method to prepare nanostructured TE materials is to make use of a top-down approach, including ball milling, exfoliation, annealing, and so on. However, most of these processes are energy-consuming. Additionally, the precise control of the particle size, size-distribution and shape remains challenging. In order to address these issues, bottom-up approaches is sought after. The primary advantage of bottom-up approach lies in its versatility in designing nanostructured materials, which is favorable for phonon scattering (i.e., lower thermal conductivity). Approaches such as liquid-phase synthesis, vapor–liquid–solid (VLS) growth, solution–liquid–solid (SLS) growth, chemical vapor deposition (CVD), electrochemical deposition, electrospinning, and so on have provided a wide variety of nanoscaled architectures, offering many advantages over some traditional methods in controlling phase purity, crystallinity, density and dimensions of nanostructures. For nanomaterial-based thermoelectric devices, the nanoscaled particles can be assembled at a macroscopic scale and homogeneously distributed, either in thin film or in bulk materials. The final thermoelectric properties will very much depend on not only the individual nanoparticles, but also the manner they assemble. For instance, by controlling annealing temperature and/or pressure, various nano-sized defects can be engineered, as shown in Fig. 1. Consequently, by engineering multiscale defects such as point defect (nanoparticle), nano-precipitates, and line dislocations, it is possible to scatter phonons of a wide-range of wavelengths, resulting in very low κL. Another advantage of bottom-up approaches is that they generally do not require a substrate to support the growth, therefore enabling higher throughput than traditional methods such as physical vapor deposition. This paper will cover the recent studies of using various nanostructures to tune the TE performance through bottom-up engineering strategies, which have, however, not extensively been attracted attention in existing reviews on the nanostructured TE materials [8, 21–26].
Fig. 1.
Illustrations showing the bottom-up assembly to synthesize nanoscaled thermoelectric materials and the advantageous effects brought about by multi-wavelength phonon scattering due to the various shape, and size defects brought about by these bottom-up approaches. Adapted with permission from Ref. [22, 27]
Benefits of Nanostructuring
Understanding the inter-correlation between different TE parameters that make up the ZT can be a useful guide to design nanostructured TE materials. Through extensive studies on the electron and phonon transport and electron–phonon couplings, multiple strategies have been investigated and applied for the enhancement of the TE performance. In this section, these strategies including size effect, mean free path and quantum effect, energy filtering effect at grain boundary, phonon scattering effect by nanoparticles as well as other related effects, will be summarized in details, embodying the latest understanding and effective manipulation of the interplay among carrier, phonon, lattice, interface and electronic states in TE nanomaterials.
Nanostructuring Effect in Thermoelectrics
Conceptually, in order to obtain a high ZT value, both the S and σ must be large, while the κ must be minimized so as to maximize the power output at a high temperature difference. Traditionally, there are two main design principles in searching for bulk TE materials with high ZT values. The first approach is the “phonon glass electron crystal” approach, and the other approach is the nanostructuring of TE materials. To achieve the phonon glass electron crystal, materials with complex structures are in general preferred [11, 28–31]. Recently, TE materials with exceptionally high ZT values of above 2 have been reported owing to the advance of various materials development approaches, including band engineering, defect optimization and nanostructuring. Nanostructuring is a promising way to improve the TE performance by means of reduction of the characteristic length of the phonons mean-free paths [8, 32–34]. It is generally accepted that the mean-free paths for phonons are much longer than that of electrons, and therefore by judiciously tuning the nanostructure size to the same order as the phonon mean-free paths, it is possible to selectively scatter phonons and not electrons, resulting in a lower κL while maintaining a high σ [35, 36].
The classical nanostructuring effects concern the scattering-limited mean free paths and the confinement-induced variation in the electronic dispersion relation, respectively. For instance, heat is carried by phonons with a wide, momentum and energy temperature-dependent spectrum, prohibiting the kL by limiting the phonon mean-free path over a broad temperature range therefore requiring all-scale hierarchical nanostructuring and microstructuring [37]. Under the circumstance of the classical size effect, the lowest possible kL is defined as the amorphous limit, when the phonon mean-free path gets as small as the interatomic spacing and heat is carried by the random-walking Einstein mode, just like in amorphous materials. The minimum lattice thermal conductivity kmin can be expressed as [38]:
| 1 |
where the is the cutoff frequency, is the number of atoms per unit volume, ħ is the Planck’s constant h divided by 2π, and is the sound velocity vector.
Theoretical and experimental reports show that the high density of nanoscaled grains can induce the effective phonon scattering of over 60% and remarkably reduce the κ [39]. In addition, due to the low dimension of nanostructures, the phonon confinement effects must be considered. For instance, the Umklapp scattering process in superlattice is different from those of bulk materials. The so-called mini-Umklapp arises due to the periodically alternating layers of a material that has a large superlattice constant, resulting in a mini-Brillouin zone, and hence a lower κ [40].
Quantum Effect
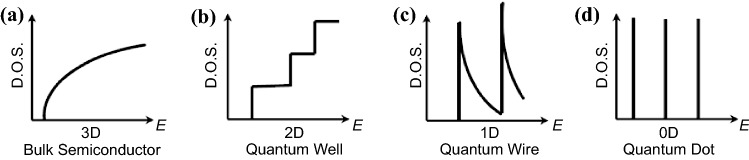
Low dimensional materials/nanostructures are defined by a characteristic dimension in order comparable to the de Broglie wavelength of charge carriers. As a result, the degree of freedom of the carrier motion is restricted by the dimension of the nanostructures. Consequently, the electronic transport behavior is drastically altered, resulting in the so-called quantum size effect [41]. Figure 2 illustrates the electronic band structure behavior of various low dimensional materials as compared with traditional three-dimensional (3D) bulk materials. Compared with 3D materials, all low dimensional materials have a very sharp feature in the density of states, especially for one-dimensional (1D) and zero-dimensional (0D) materials. These features are very beneficial to enhance the S and thus the PF [42, 43]. Therefore, this quantum size effect underlies the paradigm of TE nanostructuring [44–46].
Fig. 2.
Illustrations showing the electronic density-of-states of bulk and low dimensional materials. a Bulk semiconductor. b 2D materials. c 1-D materials (i.e., nanowires (NWs), nanorods). d Quantum dots. Adapted with permission from Ref. [47]
To understand the origin of the S enhancement in low dimensional materials, it is helpful to look into the Mott equation shown below:
| 2 |
The first and second terms inside the square bracket represent the contribution to the S due to the carrier concentration modulation and carrier mobility modulation, respectively. In most TE studies, the second term (carrier mobility modulation) is ignored because of the low energy dependence of mobility (i.e., r = − 0.5 for the acoustic phonon scattering). The density of states vs the energy profile shown in Fig. 2 is directly proportional to the first term in Eq. 2, . Therefore, a higher slope in the density of states vs the energy will result in a higher S. Theoretically, the enhancement in the S in low dimensional materials has been predicted in several literatures for 2D superlattice as well as 1D NWs [48–52]. More recently, numerous experimental enhancements on the S in low dimensional materials have been reported, such as Pb1−xEuxTe/PbTe multiple quantum wells and PbTe1−xSex/PbTe quantum dot superlattice [53, 54].
In addition to low dimensional materials, the concept of enhancing the slope of density of states vs the energy has been successfully applied using resonant doping in bulk 3D materials. Most notably, elements Tl and In have been shown to be a resonant dopant for PbTe and GeTe, respectively [12, 55, 56]. These observation suggests that physical intuition derived from the studies of low dimensional materials can also be applied to a bulk materials system.
Energy Filtering Effect
The first concept of energy filtering was introduced by Ioffe in 1959 [57] and further investigated by Rowe and Min in 1995 [58]. They studied the effect of different barriers on the σ and the S by the relaxation-time approximation method, indicating that the flow of minority charge carriers should become obstructed by the high-energy barriers, thereby suppressing the bipolar effects. This presented obvious reduction of the σ by promoting transport of the primary charge carriers. Nanostructures can also be used to enhance the PF via the energy filtering. The presence of nano-sized precipitates can act as a filter for carriers with low energy, therefore increasing the S and hence the PF. An illustration of the energy filtering mechanism is shown in Fig. 3. It is noteworthy that the overall benefit of the energy filtering is debatable, even to date. On one hand, by filtering out the low energy carriers, the S can be improved. On the other hand, the fewer number in carriers leads to a decrease in the σ. More recently, the manifestation of the energy filtering was elucidated in terms of the scattering exponent (r). The change in r from − 0.5 for the acoustic phonon scattering to near 0 due to the energy filtering has been shown to be greatly beneficial for enhancement in TE performance [59].
Fig. 3.
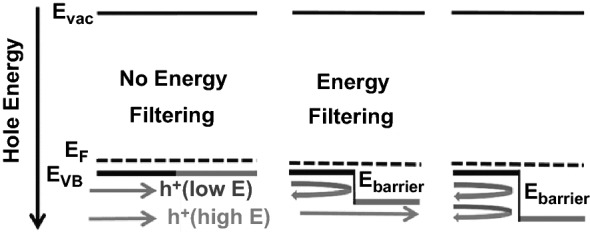
An illustration of the energy filtering effect. In the middle image, carriers (holes) with high energy (red-arrow) travel unobstructed through the energy barrier while carriers with lower energy (blue) are blocked. Adapted with permission from Ref. [60]. (Color figure online)
Phonon Scattering Effect by Nanoparticles
In low dimensional or nanostructured TE materials, in addition to the phonon–phonon scattering as the dominant effect, the phonon scattering effect due to nanostructures cannot be negligible. Recently, it is generally accepted that the nanoparticles or nanocrystals can be easily in-situ in bulk materials to obtain the lower κ [8]. Here, the phonon relaxation time of nanoparticle scattering () is given by the Mathiessen-type interpolation between the short- and long-wavelength scattering regimes [61].
| 3 |
| 4 |
| 5 |
The parameter ρ is the density of a nanoparticle/nanocrystal, R is the radius of a nanoparticle/nanocrystal, D is the density of the matrix, and ΔD is the difference in densities between a nanoparticle/nanocrystal and matrix. Kim et al. observed an apparent reduction in the κ by almost a factor of 2 below the limit of alloy when embedding the ErAs nanoparticles into In0.53Ga0.47As [62]. According to the simulation of the κ of Si–Ge nanocomposite [63], SiGe nanoparticle-in-alloys [61], an observable decrease in the κ was reported. In previous studies, Kundu et al. noted that the decrease in the κ of nanoparticles materials of matrix depended on the relative atomic mass difference between the nanoparticle and matrix, which is consistent with Eq. (3) [64, 65]. Similar reduction of the κ was also observed in various TE materials with nanostructuring such as PbTe [66, 67], PbS [68, 69], and SnTe [70], thus improving the ultimate TE performance.
Bottom-up Nanostructuring of Thermoelectrics
To date, the most popular method to prepare thermoelectrics is to use conventional solid-state sintering that involves ball milling and/or spark plasma sintering (SPS), which is energy-consuming and lack of mechanisms to precisely control the size, shape and surface chemistry. In comparison with the top-down nanostructuring or nanopatterning such as electron beam lithography [71], the bottom-up approaches are relatively cost-effective and also offer advantages in controlling phase purity, crystallinity, density and dimensions. Nanostructured or nanocomposite thermoelectric materials can help enhance ZT via increasing power factor through modulation doping, decreasing thermal conductivity via phonon scattering [72], increasing S by modulating the density of states of carriers, and energy filtering which results in simultaneous increase in S and σ [73]. In addition, nanomaterials may help to enhance mechanical properties via precipitation hardening which pins dislocations from moving [74]. However, the difficulties in designing nanostructured thermoelectrics lie in strong inter-correlation between materials transport properties, which demand careful adjustment of carrier densities. Therefore, tuning the size, shape, composition and phase is important in addition to distribution and orientation of these nanostructures. This is to ensure the coherence and band alignment between different phases in nanostructured TE materials, and modulate the effect of defects (dislocations, point defects, and stacking faults) and surface roughness on the thermoelectric properties such as thermal conductivity.
In terms of materials processing, vacuum-based thin film deposition methods are the only mature technologies to date that can reliably produce nanostructured materials and accurately control the composition or stoichiometry. In thin film thermoelectrics, some of the highest ever reported power factor have been reported [75]. Nevertheless, the main drawback from such vacuum deposition is its high cost and low productivity. In addition, it cannot be so easily scaled up to obtain bulk or thick films, and even not to mention the various issues in accurately measuring the thermoelectric properties, especially S and κ. During these measurements, the heat from hot side not only transfers to cold side, but also to the substrate underneath the film, which makes data interpretation extremely challenging. In comparison, nanostructuring via solution-based processes is very attractive because it does not require substrate (often made of expensive single crystal) to support the growth and can be readily scaled up. The solution-based bottom-up processes also provide convenience for the device fabrication in terms of size, shape, flexibility and conformability, which is promising for application in wearable thermoelectric energy harvest systems.
In this section, the recent progress on various bottom-up approaches towards the preparation of nanostructured thermoelectric materials with different dimensions will be summarized, their structure–property relationships as well as mechanisms for performance enhancement will also be discussed.
0D Nanoparticles and Nanoinclusion
Colloidal Synthesis
At present, vapor-phase approaches are generally found not suitable for the synthesis of high-quality nanocrystals due to existing limitations found in instruments and precursors [76–78]. In contrast, the liquid-phase colloidal synthesis of monodisperse semiconductor nanocrystals can offer a convenient route towards low-cost and scalable low-dimensional TE materials. In addition, the optoelectronic properties of nanocrystals can be tuned via synthesis, engineering surface of nanocrystals and control of the size down to sub-10 nm range. This opens up the possibility to explore properties of TE materials with strong quantum-confine effect [79]. The use of capping ligands and surfactants facilitates the dispersion of colloidal semiconductor nanocrystals in solvents. The shapes and sizes of nanocrystals can be easily tailored by making use of the kinetic control over the nucleation and growth processes with the assistance of organic ligands. The colloidal semiconductor nanocrystals exhibit attractive TE features owing to the low dimensionality of the materials. On one hand, abundant grain boundaries are able to scatter the phonons to reduce the κ. On the other hand, the quantum confinement effect brings about increased density of states near the Fermi level, giving rise to an enhanced S. Moreover, the stable colloidal suspensions are highly solution-processable, making them particularly attractive for ultrahigh throughput device manufacturing such as spin-coating, inkjet printing and roll-to-roll casting [80, 81].
Despite the above advantages, the major obstacle of exploiting solution-processed nanocrystals for high-performance TE devices is that organic ligands are insulating in nature, hindering charge transfer between nanocrystals. Furthermore, the large interface of nanocrystals adversely affects the charge transfer processes, and thus leads the σ of nanocrystal films to inevitably low. Therefore, the key to improve the TE performance of nanocrystals is to find an effective way to remove the organic ligands on the surface of the nanocrystals after synthesis, and properly engineer electronic coupling at the interface of nanocrystals, while maintaining the nanocrystal features such as the quantum confinement effect and interfaces.
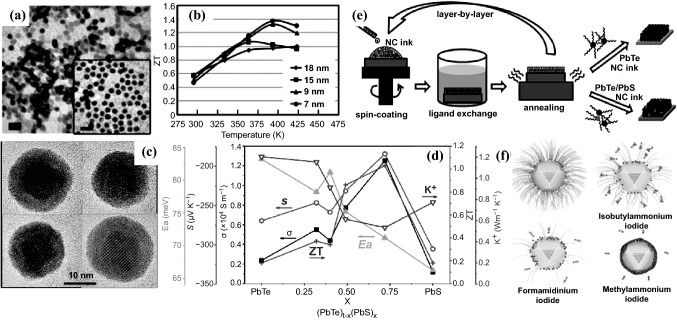
In 2008, Wang et al. prepared colloidal PbSe nanocrystals with sizes from ∼4.3 to ∼8.6 nm by reacting lead oleate with tri-n-octylphosphine selenide in squalane in the presence of oleic acid capping ligands [82]. Oleic acid at the nanocrystal surface was then stripped by hydrazine, which reduced the interparticle spacing from ∼1.1 down to 0.4 nm and resulted in a greatly improved σ. In 2013, Yang et al. synthesized PbSe colloidal quantum dots (CQD) with different sizes using conductive metal chalcogenide complexes SnS2–N2H4 to replace the organic ligands (Fig. 4a) [83]. It was found that the films of smaller QDs have a larger S, indicating the presence of stronger quantum confinement, and lower σ and κ values. The prepared PbSe(SnS2) QD films displayed enhanced ZT from 0.5 at room temperature to 1.0–1.37 at 400 K (Fig. 4b). In 2016, Ding et al. reported a convenient approach to fabricate spin-coated thin films with colloidal lead chalcogenide nanocrystals using a two-step interface engineering treatment (Fig. 4e): (1) the ligand exchange process was performed on the PbTe or PbTe/PbS layer by treatment with ethylenediamine, and (2) the post-treated layer was then annealed at different temperatures for proper coupling of the nanocrystals [84]. Notably, the nanocrystal thin films were found to exhibit have a higher S (400–460 μV K−1) than that of bulk PbTe, which is believed to be ascribed to the effect of the quantum confinement of the nanocrystals. The S of all the PbTe nanocrystal thin films was observed to decrease with increasing annealing temperature, likely due to the weakened quantum confinement effect of the fused nanocrystals. It was also found that ligand replacement with ethylenediamine could assist in necking between nanocrystals, resulting in an increased σ. The fabricated PbTe/PbS nanocrystal thin films exhibited a high ZT value of ≈ 0.30 at 405 K after thermal annealing at 400 °C.
Fig. 4.
a TEM image of colloidal PbSe QDs after metal chalcogenide complex treatment with a diameter of 9 nm. b ZT of the prepared PbSe(SnS2) QD films versus temperature. c HRTEM graphs of a few (PbTe)0.25@(PbS)0.75 core–shell nanoparticles. d σ, S, porosity-corrected κ, and ZT at 710 K and activation energy for electrical transport in the low-temperature range (Ea), as a function of the PbS concentration in the core–shell (PbTe)1−x (PbS)x nanoparticles. e Synthesis of colloidal nanocrystal thin films by layer-by-layer method and strategy for optimizing TE performance. f Schematic of oleic acid ligand exchange using different iodide salts. Adapted with permission from Refs. [83–86]
The replacement of short-chain organic ligands to modify colloidal nanocrystals leads to strong inter-particle electronic coupling and thus promotes efficient charge transport in colloidal nanocrystals films. In addition to offering excellent surface defect passivation, the use of appropriate halides in engineering the electronic coupling in nanocrystal films would be also crucial for efficient charge transport. In 2019, Nugraha et al. reported n-type TE iodide-capped PbS CQD film which allows for the fabrication of highly efficient TEG devices [85]. The counter-ions in iodide salts were found to play a critical role in facilitating ligand removal and charge transport in CQD films (Fig. 4f). Methylammonium iodide (MAI) could bring about efficient charge transport in the QD films which was resulted from the complete removal of oleic acid ligands and excellent passivation of surface defects. An impressive improvement in the σ of 100%, exceeding 12 S cm−1, was obtained for the MAI-treated CQD films, leading to a promising n-type PF of up to 24 Μw m−1 K−2 at relatively low temperatures (< 360 K), which was significantly improved compared to previously reported n-type lead chalcogenide CQD films (< 1 μW m−1 K−2).
In 2013, Ibanez et al.reported a one-pot two-step colloidal synthetic route to prepare PbTe@PbS core–shell structured nanoparticles with narrow size distributions and exceptional composition control (Fig. 4c, d) [86]. As-synthesized PbTe@PbS nanoparticles served as the building blocks for bottom-up production of PbTe–PbS nanocomposites. Interestingly, a doping-like effect was observed when PbTe and PbS were mixed at the nanometer scale. In such PbTe–PbS nanocomposites, synergistic nanocrystal doping effects resulted in up to tenfold increase in the σ compared to pure PbTe and PbS nanomaterials alone. Without intentionally doping of any of the two phases, (PbTe)0.28(PbS)0.72 reached σ up to 1.2 × 104 S m−1. At the same time, the acoustic impedance mismatch between PbTe and PbS phases and a partial phase alloying collectively provided PbTe–PbS nanocomposites with a significantly reduced κ (down to 0.53 W m−1 k−1). As a result, a high TE ZT of ∼1.1 was obtained at 710 K.
Hydrothermal/Solvothermal Synthesis
Hydrothermal synthesis involves the growth of crystals with different sizes at the submicron to nanometer scale. This is usually achieved via chemical reactions in an aqueous medium, at elevated temperature and high pressure. The successful synthesis of the nanostructures is highly dependent on the precise control over the internal reaction conditions, such as reaction time, pressure, pH value, reagent concentration, and presence of organic additives or templates, as well as external reaction environment including microwave or conventional heating. The solvothermal method can be employed to have more control over the size, shape, reactivity, and phase of the nanostructures in organic solvents than in water. The viscosity and polarity of solvent can influence the transport behavior and solubility of the reagents in the liquid medium, and hence the properties of nanostructured product. Although there are many reports on the preparation of semiconductor nanocrystals using hydrothermal/solvothermal methods [87–90], these approaches generally require high temperature and pressure as well as prolonged reaction periods, which significantly hinder applications for large-scale synthesis.
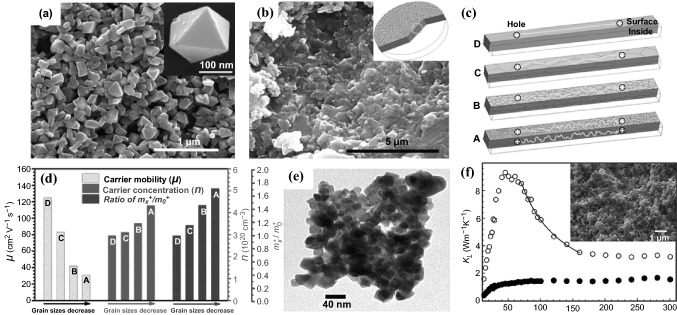
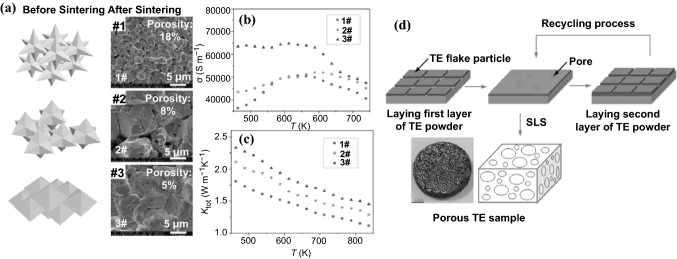
In 2016, Li et al. reported a facile, rapid, environmentally-green and high-yield microwave hydrothermal method for preparing SnTe nanoparticles with controlled sizes from micro-scale to nano-scale [91]. The reaction rates can be controlled by adjusting the concentration of reagents, resulting in SnTe nanoparticles with sizes ranging from 165 to 8.2 nm. After the SPS treatment, an ultra-low κ of 0.60 W m−1 K−1 at 800 K, being only 11.8% of the reference sample, was obtained using the 165 nm nanoparticles (Fig. 5a) owing to the enhanced phonon scattering effect introduced by refined grains, grain boundaries and point defects in the sintered dense materials. This sintered materials (Fig. 5b) exhibited a relatively higher S (58–90 μV K−1, 323–800 K) and much a higher ZT value (about 0.49 at 803 K) compared to pure SnTe bulk material, which can be ascribed to the enhanced phonon scattering and the intensified energy filtering effect. The size effect was also found to have some influences on the σ. The electronic transport mechanism (Fig. 5c) was proposed and the hole mobility, carrier concentration and the effective mass (mx*/m0*) as function of the decreased grain sizes were measured by the corresponding carrier mobility test as shown in Fig. 5d. Evidently, the hole mobility reduced from 125 to 31 cm2 V−1 s−1 while the carrier concentration increased when the grain sizes gradually decreased to 165 nm with the increasing crystal defects. It is noteworthy that the decrease in mobility is higher than the increase in carrier concentration, which results in a lower σ. In 2017, Tang et al. reported the synthesis of tetradecahedron Cu2S microcrystals with the size of 1–7 μm via the hydrothermal method, which could achieve a ZT value of 0.38 at 573 K after the SPS process [92]. Datta et al. also developed phase-pure FeSb2 nanocrystals with an average size of 40 nm (Fig. 5e) through an ethanol-mediated, low-temperature solvothermal process [93]. Without a template or capping chemical used in the process, these nanocrystals grew in their inherent orthorhombic symmetry. Due to significant grain boundary phonon scattering, the densified FeSb2 nanocomposite showed a drastic reduction in κL compared to the bulk material prepared by a solid-state synthesis process (Fig. 5f).
Fig. 5.
a SEM image of the SnTe nanoparticles synthesized by microwave hydrothermal method. b SEM image of the dense specimen from the 165 nm nanoparticles. c Proposed electrical transporting mechanism of the dense samples sintered from the 165 nm, 550 nm nanoparticles (NPs), 8.2 μm microparticles (MPs), and mechanically alloyed (MA) powder. d Hole mobility, carrier concentration and the ratio of mx*/m0* as function of the decreased grain sizes. e TEM image of 40 nm FeSb2 nanocrystals prepared by solvothermal method. f κL of the densified FeSb2 nanocrystals (solid circle) and bulk FeSb2 (open circle). The line of 1/T dependence (solid line) is indicative of phonon–phonon scattering. The inset shows an SEM image of a fractured surface of the densified FeSb2 specimen. Adapted with permission from Refs. [91–93]
Electrodeposition
In addition to the above described methods, electrodeposition is another unique process known to make nanoparticles with a controlled size and morphology [94, 95], offering the benefits of fast speed, simplicity, low-cost and avoidance of use of binders. Moreover, the electrochemical deposition of TE materials enables the easy fabrication of thin TE film although this method still suffers from drawbacks, such as the existence of impurity in as-deposited thin films and poor crystallization. Therefore, it is imperative to precisely control the composition and crystallographic structure of nanoparticles to achieve an effective electrodeposition process.
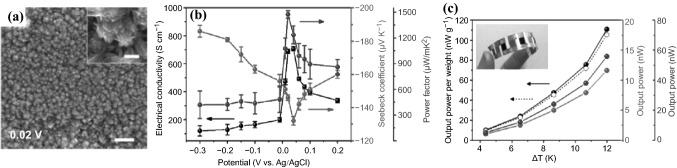
In 2016, Na et al. reported a method for preparing highly conductive n-type Bi2Te3 nanocrystal films on a flexible substrate using electrodeposition [96]. The growth of the Bi2Te3 crystals was precisely controlled by adjusting the electrochemical deposition potential, which was critical to modulate the size and preferential orientation of the crystal growth along the (110) direction, and thus to improve the TE properties of the fabricated flexible TE generator (FTEG) (Fig. 6b). A Bi2Te3 nanocrystal film prepared under a potential of 0.02 V (Fig. 6a) showed a high σ (691 S cm−1) with a maximum PF of 1473 μW m−1 K−2, which is the highest among the Bi2Te3 films prepared by the electrodeposition methods. Integrating it with an n-type Bi2Te3 FTEG, a prototype of a p-n-type flexible TEG (pn-FTEG) was prepared using a p-type polymer, poly(3,4-ethylenedioxythiophene)s. The pn-FTEG (5-couples) generated an output voltage of 5 mV at ΔT = 12 K with a high output power of 105 nW g−1 (Fig. 6c). Very recently, Zhao et al. also prepared micrometer-thick Bi2Te3 nanocrystal films using the electrochemical deposition process [97]. The optimum PF of as-grown Bi2Te3 films was achieved by shortening the period of the electrochemical deposition and introducing a photon-based rapid annealing process for the material post-crystallization. Compared with single crystalline or vacuum deposited Bi2Te3 films, the electronic transportations of the electrochemically deposited Bi2Te3 are more influenced by the carrier scatterings by the grain boundaries and lattice defect. In 2019, Nguyen et al. reported the synthesis of the gold nano-particles-bismuth telluride composites using the electrochemical co-deposition, and significant improvement in TE properties was achieved [98]. The composite with 5 wt% of 5 nm-diameter gold nanoparticles showed a highest absolute S of ∼380 μV K−1, a low κ of ∼0.5 W m−1 K−1 and a high ZT of ∼0.62 at room temperature.
Fig. 6.
a SEM image of electrodeposited Bi2Te3 nanocrystals at a potential of 0.02 V (scale bar: 500 nm). b Corresponding σ (black circle), S (red circle), and PF (blue circle) of the flexible TE generators (FTEGs) with Bi2Te3 films deposited at different potentials (V vs. Ag/AgCl). c Output power per weight of 1-couple (filled black circle) and 5-couples (open black circle) and output power of 1-couple (red circle) and 5-couples (blue circle) of the pn-FTEGs over a temperature gradient (ΔT). Adapted with permission from Ref. [96]. (Color figure online)
0D-Nanoinclusion
The enhancement in TE properties of nanostructures by lowering the κ is often off-set by the concurrent deterioration of the σ. As such, selectively lowering the κ without compromising with the σ remains a challenge. One possible way is to embed nanoparticles (particularly metal nanoparticles) with controlled sizes into a bulk matrix to increase ZT values. The advantage of incorporating nanoparticles into TE materials is able to reduce the κL due to the interfaces scattering of heat-carrying phonons, and it simultaneously enhances the S via the electron energy filtering effect caused by the scattering of electrons on the band bending at the interfaces between nanoinclusions and the semiconductor host. The enhanced S could compensate for the reduction in the σ to some extent, thus maintaining the PF at the similar level. The nanoinclusion composite structures have been generally prepared by the ball milling of metal (Ag, Au, Cu, Zn) or ceramic (ZrO2, SiC) nanoparticles with TE raw materials as the matrix phase [99–106]. However, such top-down processes for fabricating the low-dimensional structures are relatively expensive and time-consuming, probably not feasible for large-scale synthesis. Furthermore, enhancement in TE performance may be limited by the inhomogeneous distribution and aggregation of nanoparticles within the matrix.
In 2014, Sun and co-workers demonstrated the first bottom-up preparation of textured n-type Bi2Te2.7Se0.3 thin films with content-adjustable Pt nanoinclusions by the pulsed laser deposition [107]. Addition of Pt nanoinclusions resulted in a higher in-plane PF based on the simultaneous increase in both the σ and absolute S. The PF of the optimized nanocomposite thin film reached 3.51 × 10–3 W m−1 K−2 at room temperature, which is a more than 20% enhancement as compared to the single phase Bi2Te2.7 Se0.3 thin film.
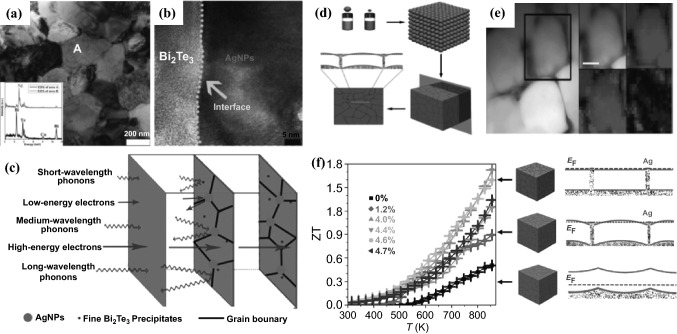
In 2015, Zhang et al. developed a facile and robust bottom-up chemical route to synthesize silver nanoparticles (AgNPs)-dispersed Bi2Te3 composites with a hierarchical two-phased heterostructure, in which the Bi2Te3 nanopowder was prepared by the surfactant-mediated hydrothermal method and AgNPs (60 nm) were obtained by using polyol reduction of silver nitrate, respectively, followed by the ultrasonic dispersion treatment and the SPS process [108]. The results clearly demonstrated that uniformly-dispersed AgNPs could lead to (1) growth-suppression of Bi2Te3 grains, (2) the introduction of nanoscale precipitates, and (3) the formation of new interfaces with Bi2Te3 matrix, leading to a hierarchical two-phased hetero-structure (Fig. 7a, b), which caused the intense scattering of phonons with multiscale mean free paths (Fig. 7c) and therefore significantly reduced the κL. Meanwhile the improved PF is maintained because of the Ag’s low-energy electron filtering and superior electrical transport. A maximum ZT value of 0.77 was obtained at 475 K from the bulk Bi2Te3 dispersed with 2.0 vol% AgNPs, which was significantly enhanced by 304% compared with that of the pristine bulk Bi2Te3.
Fig. 7.
a Low magnification TEM image of the bulk Bi2Te3 nanocomposite with 5.0 vol% AgNPs nanocomposites. b HRTEM image showing the newly-built interface between the Bi2 Te3 matrix and AgNPs. c Schematic drawing of a hierarchical two-phased heterostructure, showing the strong scattering of phonons with multiscale mean free paths along with the energy filtering effect. d Bottom-up assembly process to produce PbS–Ag TE nanocomposites from the assembly of PbS (blue) and Ag (red) NCs, and the corresponding band alignment of the resulting nanocomposite. e HRTEM and elemental EDX mapping of the PbS–Ag 4.4 mol% nanocomposite. f Figure of merit and schematic representation of the electron energy band alignment in PbS–Ag nanocomposite. Adapted with permission from Refs. [108, 109]. (Color figure online)
In 2016, Ibanez reported the preparation of consolidated yet nanostructured TE materials based on a straightforward and versatile strategy involving bottom-up assembly of colloidal nanocrystals (Fig. 7d) [109]. PbS–Ag nanocomposites were prepared by mixing cubic PbS nanocrystals (ca. 11 nm) with spherical Ag nanocrystals (ca. 3 nm), followed by the removal of solvent through evaporation. Annealing was then performed to remove residual organic compounds, after which the resultant powdered nanocrystal blend was hot-pressed into pellets. PbS-Ag nanocomposites showed a highly homogeneous distribution of Ag nanodomains at the interfaces of PbS grains, as evidenced by high-resolution transmission electron microscope (HRTEM) (Fig. 7e). Ag nanodomains in the nanocomposites not only blocked phonon propagation, but also supplied electrons to the PbS host semiconductor and reduced the energy barriers between PbS crystal domains (Fig. 7f). The resultant nanocomposites therefore exhibited a reduced κ and a higher charge carrier concentration and mobility compared to pure PbS nanomaterial. The σ of the composites can reach up to 660 S cm−1 with a Ag concentration of above 4 mol%. The simultaneous combination of an outstanding σ, a relatively large S, and a reduced κ contributed to a ZT up to 1.7 at 850 K.
Apart from the highly conductive metal nanoinclusions [110, 111], Lim et.al. has recently introduced nonmetal (Te or Se) nanodomains into a silver selenide matrix through solution blending of Ag2Se nanoparticles with Te or Se nanorods before powder consolidation [112]. Different from the injection of conductive metal nanoinclusions that can lead to both an enhanced σ and a lower S, the injection of a reduced concentration of charge carriers into a doped semiconductor could cause a band bending that promotes electron filtering. Interfacial energy filtering effects resulted in remarkable improvement in the S being recorded for nanocomposite with 5 wt% Te nanoinclusion without significantly compromising with the σ. This nanocomposite displayed an improved average ZT value of 0.84 in the temperature range of 300–400 K, higher than most of previously reported bulk Ag2Se.
1D Nanowires/Nanofibers/Nanotubes
The ZT values of TE materials can be improved by introducing 1D nanostructures by (1) increasing PF through the quantum confinement and/or energy filtering effect, or (2) reducing the κL via the enhanced phonon scattering [80, 113, 114]. The reduction in dimensionality from 3D bulks to 1D results in an enhancement in the electronic density of states at the energy band edges and thus causes an increase in the PF. In this section, we will introduce the bottom-up methods for preparation of semiconductor NWs and their structure–property relationships will be summarized.
Solution-phase Synthesis
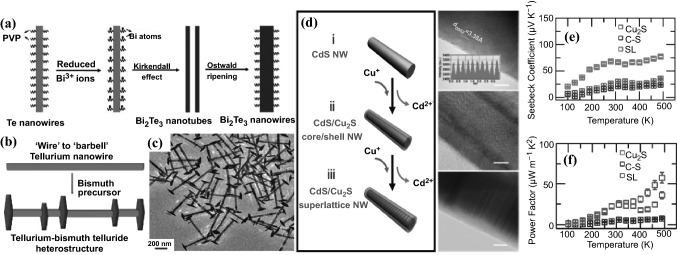
Over the past decade, solution phase template-directed synthesis has been widely employed for the preparation of 1D metal chalcogenide NWs. In 2011 Wang et al. first reported a synthetic method for the controlled formation of ultrathin Te NWs using polyvinylpyrrolidone (PVP) surfactant. The as-prepared Te NWs served as sacrificial templates to facilitate the synthesis of highly uniform Bi2Te3 NWs with a diameter of 15–17 nm and a length of tens of micrometers. The reaction was performed in triethylene glycol solution by adding Bi precursor and hydrazine to Te NWs at 200 °C and atmospheric pressure [115]. The formation of Bi2Te3 NWs was demonstrated to be a result of the Kirkendall effect and Ostwald ripening (Fig. 8a). Using a similar method, Zhang et al. also prepared n-type ultrathin Bi2Te3 NWs with an average diameter of 8 nm in ethylene glycol solution with a high yield up to 93% [116]. The as-prepared Bi2Te3 NWs were then compressed to bulk pellets via the SPS process and was found to exhibit a high ZT value of 0.96 at 380 K, attributing to the increased phonon scattering taking place at NW boundaries and hence resulting in a notable decrease in the κ. Following this method, different kinds of binary telluride NWs with small diameters, including PbTe [117], CdTe [118], Cu2Te [119], and Ag2Te NWs [117], have been prepared via a solution phase synthesis. Solution phase synthesis can therefore be potentially translatable for low-cost, large-scale synthesis of materials for TE applications.
Fig. 8.
a Schematic illustrations of the formation mechanism of the Bi2Te3 NWs via a template-assisted solution phase process. b Schematic of tellurium NW formed in the first step and tellurium–bismuth telluride heterostructure after adding bismuth precursor in the second step. c TEM image of the Te–Bi2Te3 heterostructured NWs. d Schematic diagram and corresponding TEM images (scale bar: 5 nm) of phase evolution from pristine CdS NW to CdS/Cu2S core–shell NW, and eventually to CdS/Cu2S superlattice NW. Temperature dependence of e thermopower and f power factor of Cu2S nanowire, CdS/Cu2S core–shell nanowires (C–S) and CdS/Cu2S superlattice nanowires (SL). Adapted with permission from Refs. [115, 120, 121]
Compared with binary alloys, ternary alloys exhibit greater potential in tuning the band gap, elemental composition, charge-carrier density and conductivity, making them more promising for TE materials. In 2016, Zhou et al. reported a general synthesis approach to prepare ultrathin ternary metal chalcogenide NWs [122] involving the synthesis of ultrathin 6 nm diameter Te NWs, which served as precursors to fabricate TexSe1−x NWs with tunable aspect ratios. The as-prepared TexSe1−x NWs could then be converted into a series of ternary alloyed metal-TexSe1−x NWs by injecting appropriate metal precursors under specific conditions. A series of ultrathin ternary alloyed NWs, including Bi2TexSe3−x, Ag2TexSe1−x, Cu1.75TexSe1−x, CdTexSe1−x, and PbTexSe1−x were synthesized and characterized for the first time, with controllable Te/Se ratios. Among these, Bi2Te2.7Se0.3 NW-based bulk material exhibited outstanding TE performance with a high PF of 1023 μW m−1 K−2 and a ZT of 0.75 at 320 K.
In 2012, Wu’s group demonstrated a design principle to prepare new categories of telluride-based TE NW heterostructures through solution-phase reactions [120]. The catalyst-free synthesis started with the preparation of Te NWs, followed by the growth of Bi2Te3 nanoplates on the Te NW tips and bodies, yielding Te–Bi2Te3 “barbell” NW heterostructures with a narrow diameter (~ 36 nm) and length distribution as well as a rough control over the density of the hexagonal Bi2Te3 nanoplates by varying the reaction conditions (Fig. 8b, c). The hot-pressed nanostructured bulk pellets of the Te–Bi2Te3 heterostructure showed a largely enhanced S (up to 608 μV K−1 at 300 K) and a greatly reduced κ (0.365 W m−1 K−1 at 300 K) due to the energy filtering effect occurring at the grain–grain interfaces and the phonon scattering at the NW–NW, NW–plate, and plate–plate interfaces. In the follow-up study, these “barbell”-like Te–Bi2Te3 NWs were further converted to other telluride-based compositional modulated NW heterostructures such as PbTe–Bi2Te3 and Ag2Te–Bi2Te3, which displayed a high ZT of 1.2 (at 620 K) and 0.41 (at 400 K), respectively [123, 124].
A low-cost solution process, the strain induced selective phase segregation technique, to produce superlattice nanostructures, was reported by Tang, et al. in a CdS/Cu2S system. Due to the energy filtering effect, the superlattice NWs exhibited an improved S without sacrificing the σ [121]. The distinct interface formation energy at different CdS facets and the self-regulated strain energy relaxation at the CdS–Cu2S interface facilitated the conversion of CdS NW into CdS/Cu2S core–shell structures (Fig. 8d). Their S can be significantly enhanced (Fig. 8e) by the energy filtering effect which was favored by the junction formed at the CdS–Cu2S interface, while the σ of the superlattice NWs was not greatly compromised, leading to greatly enhanced power factor at temperature higher than 400 K (Fig. 8f).
Vapor–liquid–solid Growth
The vapor–liquid–solid method (VLS) is widely used to grow 1D structures, such as NWs [125, 126]. The synthetic procedures typically start with the deposition of metal catalyst on a substrate, which is then converted to liquid alloy droplets by adsorbing the precursor vapor component at a high temperature (Fig. 9a). Crystal growth through direct gas phase adsorption onto a solid surface is typically very slow. The VLS method therefore circumvents this by introducing a catalytic liquid alloy phase which can rapidly adsorb a vapor to a supersaturation level, allowing crystal growth to occur from nucleated seeds at the liquid–solid interface. The bottom-up growth of semiconductor NWs by the VLS method is able to precisely control their size, morphology, growth density, spatial distribution, composition as well as element doping.
Fig. 9.
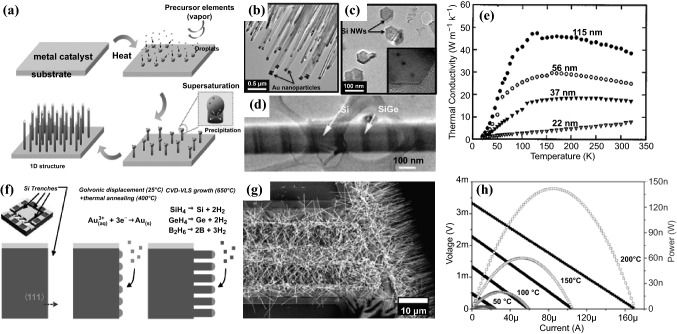
a Schematic diagram of the synthesis of the NWs by VLS growth method. b TEM image of Si NW arrays showing gold nanoparticles on the tip. c Cross-section TEM image of silicon NWs showing hexagonal shape. d TEM image of Si/SiGe superlattice NWs. e Measured thermal conductivity of different diameter Si NWs. f Schematic steps for CVD-VLS growth of boron-doped SiGe NWs and integration in μTEGs. g Top-view SEM image of SiGe NWs selectively grown in Si exposed parts. h Current–voltage and current-power curves obtained from SiGe NWs based μTEGs with three trenches for different hot plate temperatures. Adapted with permission from Refs. [127–129]
In an early work, Li et al. fabricated VLS-grown individual single crystalline intrinsic Si NWs with diameters of 22, 37, 56, and 115 nm [127]. The size effects on the κ of these individual NWs were studied (Fig. 9e). The κ observed was lower than the bulk value, and the strong diameter dependence of the κ in NWs was ascribed to the increased phonon-boundary scattering and possible phonon spectrum modification. Using the hybrid pulsed laser ablation and the VLS growth process, the same group also prepared single crystalline Si/SiGe superlattice NWs (Fig. 9d) with diameters of 58 and 83 nm [128]. Compared with the pure Si NWs in which alloy scattering is suggested to be the dominant phonon scattering mechanism for the thermal transport, this study demonstrated that the NW boundary scattering played a role in reducing the κ. In 2011 and 2012, Kim and Park et al. synthesized VLS-grown rough Si and Si0.96Ge0.04 NWs with various surface roughness and diameters with the assistance of different catalysts [130, 131]. It was found that the surface roughness affected the κ more significantly than the diameter of the NWs. Theoretical analysis reveals that the surface roughness scattering affects mid-wavelength phonons, whereas the phonon boundary scattering affects long-wavelength phonons and the alloy scattering affects short-wavelength phonons.
The characteristics of CVD-VLS process enables the convenient creation of microscale TE devices with control over photonic, electronic, and thermal properties. In 2013, Davila et al. first fabricated dense arrays of well-oriented and size-controlled Si NWs (Fig. 9b, c) obtained from the CVD-VLS process and implemented them into microfabricated structures to make a planar unileg TE microgenerator (uTEGs) [132, 133]. The average diameter and the length of the Si NWs are 100 nm and 10 μm, respectively. The resulting TEG can generate power densities of 1.44 mW cm−2 and 9 μW cm−2 under temperature differences of 300 and 27 K, respectively. In 2017, Hill et al. synthesized VLS-grown uniform, linear, and degenerately boron- and phosphorous-doped Si NW superlattices with abrupt transitions between p-type, intrinsic, and n-type segments [134]. Recently, Noyan et al. reported the bottom–up growth of SiGe NW arrays by means of CVD-VLS and their monolithic integration into TE microgenerators (Fig. 9f–h) [129]. Densely aligned boron-doped SiGe NWs with a diameter of 64 ± 11 nm, a length of 10 μm, 30% Ge content, and doping of ~ 1020 cm−3 were grown simultaneously and integrated via the gold-catalyzed CVD-VLS approach in devices with different numbers of micro-trenches. A three-trench single thermocouple placed on a 200 °C heat source could achieve a maximum power of 142 nW which is equivalent to a power density of 7.1 μW cm−2, demonstrating the great potential of the as-prepared material for energy harvesting from waste heat.
Template-Assisted Electrodeposition Method
Template-assisted electrodeposition is the most convenient method for synthesizing NWs with controlled stoichiometry, size, morphology and crystallinity [135]. Moreover, it is also cost-effective and scalable for applications. For conventional TE materials based on chalcogenide semiconductors, electrodeposition methods are widely employed for creating high-aspect-ratio NWs using hard templates with enormous 1D nanochannels. The two most common templates to obtain NWs by electrodeposition are anodic aluminum oxide (AAO) and polycarbonate (PC) membranes with different pore sizes (ranging from tens of nanometers to hundreds of nanometers) and template thickness. The AAO membranes are typically removed by chemical dissolution, while the PC membranes can be removed by either chemical dissolution or heat treatment in the air. The electrodeposition process can be conducted at three different modes: constant potential, current density and pulsed electrodeposition, among which the pulsed electrodeposition can provide more uniform growth and higher crystallinity of NWs [136].
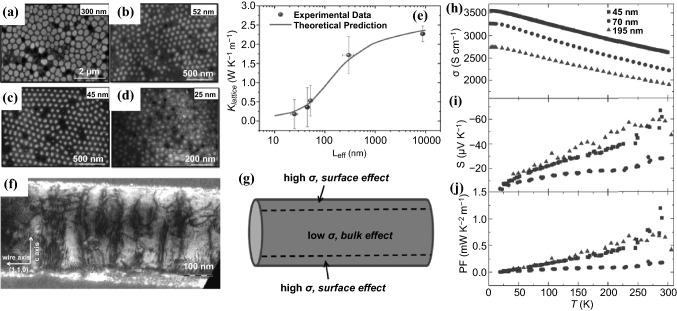
It is well known that Bi2Te3 can behave as an n-type or p-type semiconductor depending on its stoichiometry. The Bi-rich composition shows a p-type semiconductor with a positive S, while the Te-rich stoichiometric ratio is an n-type semiconductor with a negative S. During the past decade, tremendous efforts have been made to prepare Bi-Te NWs with tunable compositions, morphologies and crystallographic structures using the template-assisted electrodeposition method [137–144]. In 2017, Proenca and co-workers studied the effect of deposition applied potential on the morphology, stoichiometry and crystallinity of Bi-Te NWs using the AAO template [138]. The morphology and the Te% content was found to be highly dependent on the deposition potential. X-ray diffraction measurements revealed that there was strong relationship between the material's crystallinity and the deposition potential, being monocrystalline at very low potentials, but almost completely amorphous at high potentials due to high growth rates. In the same year, Rojo et al. reported electrodeposited Bi2Te3 NWs with 300, 52, 45, and 25 nm diameters using the AAO template (Fig. 10a–d), and in-depth study of how the κ of Bi2Te3 NWs was affected when reducing its diameter from an experimental and theoretical point of view [145]. The κ was observed to decrease more than 70% (from 1.78 ± 0.46 to 0.52 ± 0.35 W K−1 m−1) when the diameter of the NW was reduced one order of magnitude (from 300 to 25 nm) (Fig. 10e). An increment of the phonon scattering is believed to be responsible for the reduction in the NWs’ κ. As mentioned by the Kinetic–Collective model (KCM) [146], the reduction in the κ is mainly caused by the alteration of the mean free path of the acoustic phonons due to the size confinement. Reeves et al. fabricated electrodeposited sub-10 nm Bi2Te3 NW arrays using novel silica-coated AAO templates for the pore confinement [137]. The obtained sub-10 nm NWs displayed a greatly increased electrical-to-thermal conductivity ratio as the pore diameter decreased.
Fig. 10.
SEM images of a 300 nm, b 52 nm, c 45 nm and d 25 nm average diameter Bi2Te3 NWs. e κL of the NWs versus diameters. f TEM image of a Bi2Te3−ySey ternary NW. g Schematic of the NW showing the contribution of the surface states with a high σ, whereas bulk part yields a low electrical conductivity and a high S. Temperature-dependent h electrical conductivity, i Seebeck coefficient and j power factor of Bi2Te3−ySey ternary NWs of three different diameters (45, 70 and 195 nm). Adapted with permission from Refs. [137, 147]
Furthermore, it is well-known that Bi2Te3 can switch its n-type or p-type semiconductor response if it is doped with Se and Sb, respectively. Various ternary NWs based on Sb-doped Bi-Te [136, 148–155] and Se-doped Bi-Te NWs [147, 149, 156, 157] have also been developed using the electrodeposition method. In 2013, Baßler et al. published p- and n-type single-crystalline NWs of bismuth antimony telluride and bismuth telluride selenide grown by the template-based millisecond pulsed electrochemical deposition in self-ordered Al2O3 membrane templates [149]. The as-grown NWs with a diameter of 80 and 200 nm were annealed in helium and tellurium atmosphere to reduce the crystal defects which led to higher TE performance. The PFs of the obtained Bi38Te55Se7 and Bi15Sb29Te56 NWs reached 2820 and 1750 μW K−2 m−1, respectively, at room temperature, which are significantly higher compared to thin films as a result of the higher σ of the 1D structure. In 2015, Li et al. reported the pulse-deposited of Bi-Sb–Te NWs which displayed more homogeneous element distribution and higher crystallinity compared to direct current (DC)-deposited NWs [136]. The ZT of the pulse-deposited single Bi0.5Sb1.5Te3 NWs reached as high as 1.14 at 330 K, which is approximately 54% higher than that of the DC-deposited ones. Kumar et al.also prepared single-crystalline, ternary n-type Bi-Te-Se NWs (Fig. 10f) with different nominal diameters of 45, 70, and 195 nm by electrodeposition in a nanostructured Al2O3 matrix [147]. The transport properties of individual NWs were measured, yielding the largest σ (2620 S cm−1 at room temperature) for the smallest NW (Fig. 10h). This behavior of the σ might be attributed to the highly conductive surface states (Fig. 10g). Compared to bulk materials, relatively lower S (up to -60 μV K−1 at room temperature) was obtained for these NWs due to the existence of metallic surface states (Fig. 10h). The highest PF (0.8 mW K−2 m−1) was achieved with the 195 nm NWs (Fig. 10i), which is suppressed compared to bulk values but higher than those of thin films.
Electrospinning
Electrospinning is a simple and versatile technique for producing continuous nanofibers from polymers and ceramics under a high electric field with controllable morphology, diameter, composition and orientation [158, 159]. In some early work, TE properties of oxide materials have been significantly improved when their bulk 3D dimensions are reduced to 1D nanoscale by the sol–gel based electrospinning. In 2010, Yin et al. fabricated nanocrystalline Ca3Co4O9 nanofibers with diameters around 350 nm using the sol–gel electrospinning process, which were consolidated into bulk ceramics by the SPS process [160]. The nanofiber-sintered ceramic with a much smaller grain size exhibited simultaneously enhanced S, σ and thermal resistivity, resulting in 55% enhancement in ZT (around 0.40 at 975 K). In the same year, Ma et al. also reported TE nanocrystalline electrospun NaCo2O4 nanofibers with a grain size of as small as 10 nm [161], and Xu et al. produced TE La0.95Sr0.05CoO3 nanofibers with a diameter of ∼35 nm by electrospinning with a greatly enhanced S of 650 μV K−1 at room temperature [162].
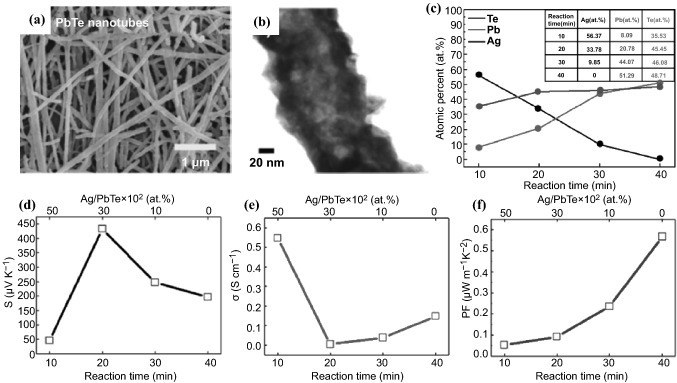
Metal chalcongenide semiconductor nanofibers have also been synthesized using the electropinning technique combined with electrochemical reactions. In 2018, Park et al. first reported the large scale fabrication of a few millimeter-long lead telluride (PbTe) hollow nanofibers employing a three-step sequential process involving electrospinning, then electrodeposition and finally, cationic exchange reaction [163]. Electrodeposition of Te onto as-prepared electrospun Ag nanofibers possessing an ultra-long aspect ratio of 10,000 afforded silver telluride nanotubes, which then underwent a cationic exchange reaction in Pb(NO3)2 solution to obtain polycrystalline PbTe nanotubes with 100 nm average diameter and 20 nm wall thickness (Fig. 11a, b). The Ag-to-Pb ratio in the AgxTey–PbTe nanocomposites could be easily tuned during the cationic exchange reaction (Fig. 11c), which rendered good control over the TE properties of resulting 1D hollow nanofibers (Fig. 11d–f). The content of Ag ion led to the enhancement of TE properties in the AgxTey–PbTe 1D nanocomposite mats, which showed the highest value of S of 433 μV K−1 at 300 K when the remained Ag content was 30%. Zhang et al. also fabricated PbTe hollow nanofiber mats through combining electrospinning, followed by galvanic displacement reactions using electrospun cobalt nanofibers as the sacrificial material [164]. Through tuning the diameter of the sacrificial cobalt nanofibers as well as the electrolyte concentrations in the galvanic displacement reactions, PbTe hollow nanofibers with various dimensions, surface morphologies and compositions were synthesized, demonstrating that both the quantum confinement and surface scattering effects displayed an additional degree of control over the TE properties within such polycrystalline tubular nanostructures.
Fig. 11.
a SEM and b TEM images of PbTe nanotubes fabricated via combined electrospinning, electrodeposition and cationic exchange reaction. c Stoichiometry ratio of different cation exchange reaction time from Ag2Te to PbTe. Different reaction time of d Seebeck coefficient, e electrical conductivity and f power factor of PbTe, included 10–50% of Ag content in the 1D AgxTey–PbTe composite nanotubes. Adapted with permission from Ref. [163]
2D Nanoflake/Nanosheet/Nanoplate
Since the discovery of graphene through the mechanical exfoliation by scotch tape in 2004 [165], various structures of 2D materials have been designed and fabricated to facilitate its physical and electronic properties because of its unique and advantageous structural features. Unlike bulk materials, 2D structure can easily tune its charge concentration, carrier transition through its network or different layers although some parameters, especially the size and shape of 2D materials, are rather difficult to be controlled [166–168]. In order to prepare various 2D structures based on semiconducting materials, various top-down methods have been designed including exfoliation, ball milling [169, 170]. This section will provide an overview of various bottom-up approaches developed recently for preparation of semiconducting 2D nanomaterials for TE applications, including vacuum-based techniques and wet-chemical synthesis. The vacuum-based deposition methods are limited to fabrication of thin films with highest-level control of crystal quality and composition. Although high power factors can be achieved using these approaches, the main drawback lies in its high cost and low productivity. In comparison, wet-chemical synthesis is more cost-effective for massive production of both thin films and bulk materials. More importantly, wet-chemical synthesis offers easy control over size, shape and composition of the 2D materials and it is also convenient to introduce dopants in the synthetic process to fine-tune and optimize the thermoelectric properties of the prepared 2D materials.
Chemical Vapor Deposition
2D layered IV–VI chalcogenides have attracted great attention for the electronic applications due to their band gaps that can facilitate the carrier movement in the 2D network. As a most representative example, SnSe has been widely studied for solar cell and optoelectronic devices [171, 172]. Owing to its layered structure, the κL of SnSe could be reduced significantly to as low as 0.2–0.3 W m1 K−1 at 800 K [173]. Meantime, SnSe has a semiconducting energy gap of ≈ 0.86 eV and in general a very low σ (10–5 to 0.1 S cm−1). Therefore, CVD has been used to introduce extra elements to enhance the carrier concentration so as to improve its σ [174–176]. However, doping certain elements to SnSe via vapor deposition is rarely studied because of its extreme growth process conditions.
Recently, Gao et al. reported a facile CVD approach to grow and dope SnSe nanoflakes, and fabricate the nanostructured thin films (Fig. 12) [177]. The nanostructured structure belongs to the Pnma space group with a layered structure along a-axis, enabling the CVD growth-based nanostructure favorable towards one direction. This is of great interest for TE performance as it potentially moves the carrier towards one particular direction. The CVD growth of SnSe nanoflakes was conducted using 99.999% SnSe powder on the Si wafer with 300 nm thick silicon oxide in the temperature range of 750 to 550 K. Figure 12c, d show the SnSe nanoflakes formed on the surface of the substrate, illustrating that the uniform of nanoflakes were generated during the process.
Fig. 12.
a SnSe structure and CVD growth. b PF of SnSe and Ag doped SnSe nanoflakes. c SnSe nanoflakes grown in the high-temperature area on substrate. d nanostructured thin films formed in the low temperature area on substrate. Adapted with permission from Ref. [177]
Upon obtaining the nanoflakes, Gao’s group prepared SnSe1.13 and SnSe0.75 aiming to study how the ratio difference affects the TE performance [177]. Se deficiency in SnSe0.75 contributed to a much higher hole density with a lower hole mobility when compared with SnSe1.13, showing poorer TE performance. In the case of SnSe1.13, the obtained highest σ and the S were about 2.5 S cm−1 and 300 µV K−1, making the PF at the level of 0.16 µW cm−1 K−2 (Fig. 12b), which is about 5% of the PF of SnSe single crystals. The low PF value could be due to the low carrier mobility and carrier concentration. The same group also doped Ag atoms into SnSe at different ratios and observed that a doping level at about 1% could significantly improve the σ and S. The highest σ and S could be up to 4 S cm−1 and 370 µV K−1 respectively, making the PF to be improved to 0.66 µW cm−1 K−2, four times of the value of undoped sample. Besides, the synthesized SnSe samples could possess a low κ, which could further improve the ZT value.
Molecular Beam Epitaxy
Molecular beam epitaxy (MBE) is widely used to generate 2D layer nanostructures [178–180] on the solid layer of materials, such as silicon [181, 182]. In this area, the preparation of a 2D layer of semiconducting metals or alloys becomes very attractive. Mori et al. fabricated Mg2Sn (111) thin film on a sapphire c-plane using pure Mg and Sn elements under MBE condition [183]. Below 250 °C, polycrystalline films with an axial preferred orientation were obtained. However, with the increase of the temperature, the loss of Mg element in the thin film made the film towards Sn element rich, which could tune the electronic properties of the Mg2Sn thin film. Many other types of semiconducting materials have also been widely studied, including PbTe (111) thin films [184], Bi2Se3/In2Se3 superlattices [185–187]. However, the preparation of 2D semiconducting materials using the MBE approach for TE applications is limited. Cecchi et al. obtained epitaxial Sb2+xTe3 alloys using MBE to obtain the highest σ, S, and PF at 1810 S cm−1, 118 µV K−1 and 2.52 mW m−1 K−2, respectively. Hu et al. reported a nanoporous (00l)-oriented Bi2Te3 nanoplate film made by MBE. In their experiment, pure Bi and Te element were placed in SiO2/Si substrates with an oxidized layer of 600 nm. Upon controlling temperature, annealing and hydrothermal process, nanoporous Bi2Te3 nanoplates was obtained. HRTEM was used to identify the nanopores in the nanoplates. It was observed that the nanoplates film was changed from intersected to tiling, weakening the carrier scattering along the plane and the carrier mobility. The increased carrier mobility thus could increase the σ. The carrier concentration of nanoplate films varied with the annealing time also. When the carrier concentration dropped to about 1020 cm−3, the S increased to 187.3 mV K−1. This trade-off phenomenon was commonly observed between the σ and the S. In addition, ethylene glycol was used to treat different Bi2Te3 nanoplates and it was observed that the boundary density of the pores is the dominant factor to affect the TE performance rather than the total area of the pores because of the boundary density affecting the carrier scattering.
Wet Chemistry Method
CVD and MBE could be used to synthesize various semiconducting nanostructure, but some limitations still remain, such as high energy consumption and difficult to control the size of nanostructures. Compared to CVD and MBE, the solution-based chemical synthesis possesses great gains, such as low cost, low energy consumption and easy scaling-up. More importantly, chemical synthesis is able to control size, morphology, composition of the 2D materials more easily by regulating delicate reaction parameters such as temperature, concentration catalyst and dispersants. In this section, the chemical synthesis including hydrothermal, solvothermal and solution chemical synthesis for the TE materials will be summarized in details.
Hydrothermal Method
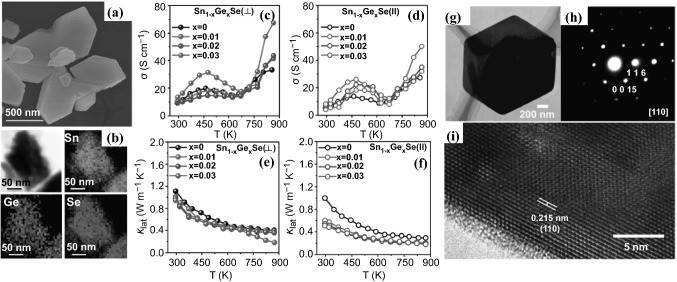
Aqueous solution-based hydrothermal synthesis has been widely used for the synthesis of 2D type transition metal dichalcogenides [188–190]. Hydrothermal synthesis of 2D materials can provide different structure and morphology, affording a new pathway to change the carrier mobility and concentration, making the synthesized 2D materials as a good candidate for TE applications. Recently, Chen et al. reviewed the hydrothermal method for preparation of various SnSe structures with different dopants for TE studies [191]. Biswas et al. reported 2D nanoplates of Ge-doped SnSe synthesized by the hydrothermal approach together with the SPS process [192]. SnCl2·2H2O and GeI4, NaOH and Se powder were placed in a Teflon-lined stainless steel autoclave at 130 °C for 36 h to afford the Sn1−xGexSe (x = 1–3 mol%) nanoplates (Fig. 13a, b) with a very high yield. Unlike CVD and MBE, this simple synthesis method could be potentially scalable. The as-prepared sample was then densified by the SPS process at 450 °C to afford the sample for TE performance evaluation. The Ge was doped to the SnSe 2D structure to enhance the carrier concentration in the crystalline structure. TEM revealed that the range of lateral dimension of SnSe nanoplates was within 0.5 to 1.0 μm. HRTEM also found that lattice spacing between two apparent planes was estimated to be 3.07 Å, indicating a set of planes (011) favorable in the structure. The TE performance of the synthesized 2D SnSe nanoplates was evaluated from both directions of || (parallel) and ⊥ (perpendicular), and their σ and κL are summarized in Fig. 13c–f. Figure 13c, d show that both parallel and perpendicular directions possess the similar σ and the trend is also similar starting from semiconducting type to metallic type. The introduction of Ge element enhanced the carrier concentration which significantly improved the σ. The S was also evaluated for both parallel and perpendicular directions. The S increased in positive correlation with temperature, and reached to a maximum value from 550 to 650 K, consistent with solution-processed SnSe samples. A highest PF was obtained at ∼5.10 mW cm−1 K−2 at 3 mol% Ge in SnSe at 873 K. It was observed that synergistic interaction of lattice anharmonicity, point defects, nanoscale grains, and precipitates reduced the κL (Fig. 13e, f) in both parallel and perpendicular directions, which assisted in the enhancement of ZT up to ∼2.1 at 873 K.
Fig. 13.
a FESEM image of Sn0.97Ge0.03Se nanoplates. b STEM image of Sn0.97Ge0.03Se nanoplates along with EDAX color mapping for Sn, Ge, and Se. Temperature-dependent c, d electrical conductivity and e, f lattice thermal conductivity of Sn1−xGexSe measured parallel (open symbols) and perpendicular (solid symbols) to the SPS pressing direction, respectively. g TEM image, h SAED pattern and i HRTEM image of a single Sb2Te3 nanoplate. Adapted with permission from Refs. [192, 193]
Zhang et al. reported a facile and rapid synthesis method of Sb2Te3 hexagonal nanoplate using hydrothermal treatment in the absence of organic solvent or additive [193]. In the synthesis of this nanoplate, SbCl3 and tartaric acid, NH3⋅H2O solution, K2TeO3, and N2H4⋅H2O were placed in an autoclave, and the reaction temperature was controlled at 180 °C for 5 h, followed by filtration to obtain the Sb2Te3 nanoplates. Figure 13g shows a HRTEM image of a typical single Sb2Te3 hexagonal nanoplate. As seen in Fig. 13h, the Sb2Te3 nanoplate is a well-crystallized single crystal. Figure 13i displays a well-resolved 2D lattice fringe with a plane spacing of 0.22 nm, in accordance with the lattice planes of (110) in rhombohedral Sb2Te3 nanoplates. The hydrothermal reaction conditions to obtain the Sb2Te3 hexagonal nanoplate are very critical and it requires to be well-controlled to avoid the formation of nanoparticle or NW. Using the home-made setup, the S of Sb2Te3 nanoplate was evaluated to be 125 mV K−1 as p-type semiconductor using a home-made setup, which is higher than these of other type nanocrystals, such as Sb2Te3 nanoparticles and nanorods. Due to the anti-site effect derived from Sb atom on the Te lattice sites, the S is approximately 1.6 times of that value of undoped bulk crystals at 79 mV K−1, which is attributed to the energy filtering due to the nano-boundaries created by the extra atom throughout the doping process.
Solvothermal Method
As one of the best TE materials, antimony telluride (Sb2Te3) has been well investigated as a p-type semiconductor [30, 194]. However, the study of how the shape and size changes in 2D type Sb2Te3 nanomaterials is limited. Lee et al. reported a solvothermal approach to prepare the Sb2Te3 nanoplates. SbCl3, K2TeO3 and PVP were dissolved in diethylene glycol, followed by the addition NaOH aqueous solution. Upon the solvothermal process in a Teflon-lined stainless-steel autoclave at 230 °C for 24 h, the corresponding nanoplates were obtained. SEM analysis showed the edge lengths and thicknesses of the nanoplates are 3–5 μm and ∼100 nm respectively. Sequentially, the TE performance was evaluated in comparison with the sample prepared by the ball milling. Interestingly, the κ of the Sb2Te3 nanoplates is much lower than that of samples obtained from the ball milling process. Such a low κ could be attributed to the grain boundary generated between nanoplates. The S is in the range of 300–350 μV K−1, about two times higher than those of Sb2Te3 prepared by the melting method (102–144 μV K−1). The improvement in the S can arise from the energy filtering effects caused by the boundaries between Sb2Te3 nanoplates.
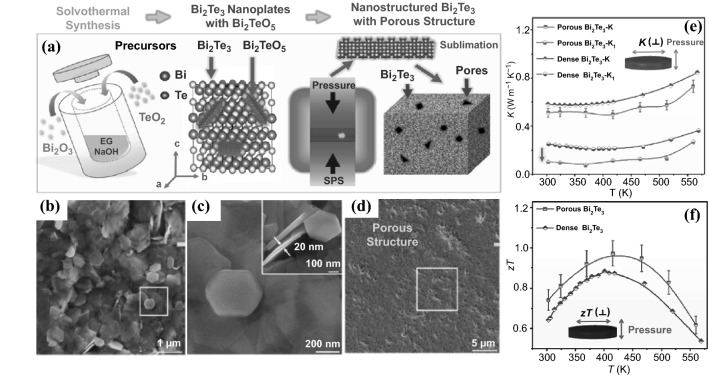
As another important class of semiconducting materials, 2D Bi2Te3 nanostructures have attracted great attention to enhance the TE performance. Takashiri et al. used Bi2O3 and TeO2 under basic conditions via a solvothermal process to obtain the Bi2Te3 nanoplate, followed by electrical deposition and annealing to afford the electrodeposited layers [195, 196]. Annealing at 250 °C helped reduce the boundaries between nanoplates, and further enhanced the σ and S by about 200% and 50%, respectively. Thermal annealing improved the crystallinity of electrodeposited layers, which decreased the number of defects (carrier concentrations) and the number of boundaries (increasing mobility). The structure variation arising from the post treatment could significantly improve the TE performance through tuning the carrier concentration and mobility. Chen et al. reported a solvothermal method to introduce high porosity in Bi2Te3 hexagonal plates and decrease the overall κ as well as the κL [197]. Through reduction of the κ, the TE performance could be enhanced significantly when the PF remained unchanged. In the synthesis of Bi2Te3 nanoplates (Fig. 14a), Bi2O3 and TeO2 were placed in an autoclave in the presence of NaOH and ethylene glycol at 210 °C for 24 h. The Bi2Te3 nanoplates were obtained through filtration. Then the obtained Bi2Te3 nanoplates were subjected to the SPS process and sublimation to obtain the sample for the structure characterization. SEM analysis showed the Bi2Te3 nanoplate has the thickness of 20 nm (Fig. 14b, c) and can induce a high density of grain boundaries in the pellet after the sintering. As shown in Fig. 14d, the sintering process could introduce the density of pores in the structure and the pore size is at a size of ∼400 nm in the matrix. Sequentially, the σ and S were measured in comparison with the dense sample. It was found that the similar value was obtained between the dense sample in other literature and the as-prepared porous structure, leading to a similar magnitude of the PF. The porous structure in the matrix could significantly reduce the κL to less than 0.1 W m−1 K−1 (Fig. 14e) due to the phonon gas theory. Owing to the overall reduction in the κ, the ZT value was improved dramatically up to 0.97 at 420 K (Fig. 14f), the highest values reported for pure n-type Bi2Te3 semiconductors.
Fig. 14.
a Illustration of the solvothermal synthesis of Bi2Te3 nanoplates followed by SPS to fabricate a porous nanostructured Bi2Te3 pellet. SEM images of b, c the as-synthesized Bi2Te3 nanoplates and d the as-sintered nanostructured porous Bi2Te3. Temperature dependence of e κ and κL, f ZT of the as-sintered nanostructured porous Bi2Te3 pellet compared with dense nanostructured Bi2Te3. Adapted with permission from Ref. [197]
Zou et al. reported a microwave-assisted solvothermal synthesis method to obtain Bi2Te3−xSex nanoplates which could enhance the TE performance [198]. In the synthesis of Bi2Te3−xSex nanoplates, Bi(NO3)3·5H2O, Na2TeO3 and Na2SeO3 were placed in reaction vessel in the presence of ethylene glycol and NaOH at 230 °C for 5 min. During the synthesis, various loadings of Se were doped in the samples to substitute the Te element to study its impact on the TE performance. The obtained Bi2Te3−xSex nanoplates were subjected to the SPS process to make the pellets for the TE measurement. It was found that the loading of Se element doesn’t seem to have huge impact on the overall performance, leading to the PF in the range of 1.5–1.9 mW m−1 K−2. The texture fraction in the nanostructure materials is smaller than that of the bulk polycrystalline counterpart, and can help reduce the phonon scattering, leading to the reduction in the κ. A lowest κ of 0.69 W m−1 K−1 value was achieved in the Bi2Te2.7Se0.3 pellet, which is much lower than the value between 1.5 and 2 W m−1 K−1 of their bulk counterpart materials. Such reduction is attributed to the nanostructure grains and boundaries in the pellet made by the Bi2Te3−xSex nanoplates. There are mainly two factors promoting the reduction of the κ namely the Umklapp phonon–phonon scattering by the inherently strong anharmonicity and the wide frequency phonon scatterings caused by the multi-scattering pathways. The same group also performed the theoretical studies on the thermal transport and phonon transport to understand the rationale behind the κ reduction, revealing that the complex carrier scatterings helped suppress the bipolar effect and weakened the dependence of transport properties on the carrier movement.
Solution-Phase Synthesis
Hydrothermal and solvothermal approaches have been used to grow 2D type inorganic semiconducting materials. These two methods usually require the reaction to be performed at high temperature (e.g., from 150 to 300 °C) in a sealed stainless vessel. At this reaction condition, the inorganic species in general can grow in a specific direction to form the 2D type of materials. As another approach, solution chemical synthesis can be used to prepare 2D semiconducting materials with a lot of benefits, including tunable reaction conditions and easy scale-up. Cabot and co-workers reported a chemical synthesis method using tin selenide molecular precursor under solution process to prepare 2D SnSe nanoplate for TE studies [199]. In the synthesis of 2D SnSe nanoplate, tin chloride selenium dioxide, tri-n-octylphosphine and oleyamine were premixed in a very short of time, followed by the addition of oleic acid to produce dentritic SnSe nanostructures. Then the SnSe precursor was injected into a reaction flask preheated at 420 °C for decomposition to prepare the SnSe nanostructures. TEM and SEM characterization of SnSe nanostructure showed that as-prepared nanoplates had the size of 4 ± 1 μm and a thickness of 90 ± 20 nm and the crystal followed the [100] crystal direction oriented along the pressure axis by XRD analysis. TE property study revealed that both cross-plane and in-plane afforded very similar σ of 15–30 S cm−1. A slightly higher S of 400 µV K−1 was obtained in the cross-plane direction, which could be attributed to the energy filtering introduced by the grain boundaries, specifically the preferential scattering of the low-energy carriers at the plate interfaces. A very low κ was obtained in both directions, significantly improving the ZT value of the SnSe nanoplates. The low κ could be arisen from the low κL because of the phonon scattering.
Biswas et al. reported a chemical synthesis to prepare several n‑type ultrathin layers of Bi doped SnSe nanosheets [200, 201]. In the synthesis of SnSe nanosheets, SnCl4·5H2O and SeO2 were taken in a mixture of oleylamine and 1,10-phenanthroline. The reaction was performed at 200 °C under N2 followed by filtration to afford the nanostructured materials at the gram scale. Bi precursor was used in the reaction to dope the nanosheet to afford Sn0.94Bi0.06Se. Powder X-ray diffraction analysis shows the 2D SbSe nanosheet has the lattice parameters of a = 11.50 Å, b = 4.15 Å, and c = 4.45 Å and the indirect band gap was measured to be ∼0.88 eV. Field emission scanning electron microscope (FESEM) showed that the Bi doped SnSe nanosheets possessed a thickness of 1.2–3 nm. Such a dimension is critical to create the nanoscale grain boundaries and point defects across the interphase between Se-Se layers. Such boundaries are effective for the phonon scattering and improvement of TE performance. Based on the Hall Effect measurement, the introduction of Bi element in the SnSe could significantly enhance the carrier concentration, leading the σ up to 12.5 S cm−1 at 750 °C for the cross-plane direction. Negative S was observed for both cross- and in-plane directions. The highest PF of 100 μW m−1 K−2 was obtained for Sn0.94Bi0.06Se nanosheets, much greater than that of SnSe. More interestingly, the nanoboundaries in the nanosheet could facilitate the phonon scattering, leading to a low κL of ∼0.3 W m−1 K−1. This low κ could contribute significantly to the ZT enhancement to 0.21 at about 700 °C for Bi doped SnSe nanosheets. Similarly, Hu et al. reported a chemical synthesis method using SnCl2⋅2H2O and Se in the basic solution to prepare SnSe 2D nanosheets [202]. In their work, extremely low κ of 0.09 W m−1 K−1 was obtained, demonstrating the thin film made from 2D nanosheet could be used for low-grade waste heat recovery.
Zou et al. reported a chemical method to dope Te element into the SnSe nanosheets to enhance the TE performance [203]. In the synthesis of SnSe nanosheets, SnCl2⋅2H2O, Na2SeO3, and Na2TeO3 were added into the NaOH solution in ethylene glycol. After the stirring, the reaction was heated at 230 °C for 10 min to obtain the Te doped SeSe nanoplates. SEM and TEM analysis shows the nanoplates have the average lateral size and thickness of about 10 µm and 200 nm, respectively. With the increasing Te element in the SnSe nanoplates, the S dropped from 365 to about 341 µV K−1. Due to the trade-off relationship between the σ and S. The σ improved doubly from 50 to about 100 S cm−1. The Te element in the alloy could reduce the band gap and the hole concentration, resulting in a stronger bipolar effect, and thus increase in the σ and reduction in the S.
Another noteworthy point is that with increasing Te element into the alloy, the point defects were introduced, which enhanced the phonon scattering and led the κ to be reduced by about 30% overall. In this case, the average ZT of 0.58 was obtained. Gregory et al. reported a similar method to synthesize SnSe nanoplates using water as solvent without using any surfactant [204]. The PF is eightfold higher than that of material made using citric acid as a structure-directing agent. As another type of important semiconducting materials, 2D Bi2Te3 nanostructure is also well-studied, especially in 1D type. Hyeon et al. reported a chemical method to prepare n-type of ultrathin Bi2Te3 nanoplates with the improved TE performance [205]. In the synthesis, bismuth neodecanoate and tri-n-octylphosphine-tellurium were used as Bi and Te sources in polyamine, respectively. 1-Dodecanethiol was used with the proper concentration to form the Bi2Te3 nanoplates. The thickness of the nanoplates was about 1 nm. Sequentially the as-prepared nanoplates were sintered into pellets for the TE performance measurement. The S was in the range of − 130 to − 160 μV K−1. It was noteworthy that the electron concentrations and mobility varied at different sintering temperatures possibly because of the interphase effect, which facilitated the carrier scattering and defects by the organic residue from the synthesis. Meantime, the κ was observed to be reduced significantly (lowest at about 0.4 W m−1 K−1). This phenomenon was also observed in other 2D materials and this can be attributed to the scattering of carriers and boundaries between the 2D materials.
3D Nanostructure
One simple approach to improve TE property in bulk materials is to induce the formation of porosity. Although the traditional approach to TE materials was to create bulk samples with very little remaining porosity (> 99% relative density), many recent studies have shown that either random or template-assisted uniform porosity can be beneficial for TE performance when properly optimized [21, 206]. Reported studies have shown that the sizes of the pores in TE materials and their distribution pattern play critical role in TE properties. To maintain sufficient σ, these structures generally must be sintered to at least 80% theoretical density, effectively limiting the porosity.
Random Porous Structure
Randomly porous structures are created by deliberately partially densifying a material to generate a distribution of pores throughout the structure. Randomly porous structures are easily synthesized but the effects of nano-structuring on the κ (and in some cases, the S) are much weaker due to the lack of morphological control.
In 2018, Xu et al. synthesized PbS nanocrystals with different shapes and consolidated them into highly porous and well crystalline monoliths using the SPS process (Fig. 15a) [207]. It was found the relative density and TE performance of the porous PbS monoliths could be tuned simultaneously (Fig. 15b, c). The as-obtained porous monolith with a large grain size has low relative mass density (82%) and high porosity, and also exhibits a high σ, a low κ, and hence an excellent ZT (1.06 at 838 K). The origin of high ZT was studied by DFT calculations, indicating that high ZT can be attributed to enhanced scattering of phonons caused by the porous structures.
Fig. 15.
a Schematic illustration (left) and SEM images (right) of the as-sintered porous monoliths from 1# (hexapods), 2# (less-protruding hexapods) and 3# (octahedra) PbS nanocrystals. Temperature dependence of b σ and c κ of the three sintered TE monoliths. d Schematic diagram of selective laser sintering 3D printing process to prepare porous TE monoliths. Adapted with permission from Refs. [207, 208]
In 2019, Shi et al. employed a 3D printing technique (Fig. 15d) to fabricate porous bismuth antimony telluride (Bi0.5Sb1.5Te3, BST) and its TE properties were studied [208]. The porous TE samples were fabricated with the selective laser sintering method using 100 mesh BST powder. Energy density of the laser light that scans across the thin layer of TE powder was carefully controlled to ensure that TE powder was only partially melted to form the desired porous TE material. The laser sintering process of BST particles led to the formation of many micrometer- and nanometer-sized random pores, accounting for the reduction of the κ. The minimum κ of the porous BST samples was measured to be 0.27 W m−1 K−1 at 54 ℃. The reduction in the κ is attributed to the boundaries and defects formed during the selective laser sintering process as well as the porous structures of the materials. The ZT of the porous samples was found to have a maximum value of about 1.29 at 327 K, higher than that of the BST bulk material.
Template-Assisted Uniform Porous Structure
In 2017, Hong et al. reported the preparation of a periodic 3D nanostructured TE monolith, with an approximate pore size of 140 nm, by electrochemical deposition of a Bi–Sb–Te ternary alloy into a highly ordered, interstitial porous network in an epoxy template predefined by advanced lithography (Fig. 16a) [209]. The electroplating conditions for the 3D nanoconfined geometry was optimized to facilitated the uniform and dense filling of Bi1.5Sb0.5Te3 into the template over a large area of 625 mm2. Most nanostructured TE materials suffer from undesirable degradation of the σ. In contrast, the 3D nanostructures, however, are able to maintain electron transport properties because the sizes of the TE struts in a structural unit cell are sufficiently larger (140 nm) than the mean free path of electrons (~ 40 to 60 nm). It is suggested that the extrinsic phonon scattering at the interfaces of the nanostructures without changing electrical transport is responsible for the selective reduction of the κ while the S and σ are almost intact (Fig. 16b). The 3D nanostructure successfully resulted in a decreased κ from 1.14 to 0.89 W m−1 K−1, at 350 K while maintaining a σ of 644.5 S cm−1 and a κ of 144 mV K−1 (Fig. 16c, d). The 3D nanostructured Bi1.5Sb0.5Te3 film showed an improved ZT value of 0.56 at 400 K, which was approximately 50% higher than the value for an ordinary Bi1.5Sb0.5Te3 film. In 2018, the same group also reported the deposition of nanoscaled ZnO film on the 3D nanostructured epoxy template via the atomic layer deposition [210]. In this work, the suppressed κ of the 3D ZnO film is ∼3.6 W m−1 K−1 at 333 K, which is ∼38 times lower than that of the blanket ZnO film.
Fig. 16.
a Schematic illustration of procedures for the fabrication of a freestanding and transferrable 3D nanostructured TE film. b Structural unit cell of a 3D nanostructured Bi1.5Sb0.5Te3 film illustrating the concept of increasing phonon boundary scattering without altering electrical transport. Comparison of c electrical conductivity and d thermal conductivity of the 3D nanostructured and ordinaryBi1.5Sb0.5Te3 films as a function of temperature. Adapted with permission from Ref. [209]
Nanoscale Doping in Bottom-up Process
For semiconductor thermoelectric materials, the introduction of small quantities of impurity (element doping) during the bottom-up preparation can not only tailor the carrier concentration and/or mobility to optimize the σ, but also induce point defects (vacancies or self-interstitials) and adjust the microstructures (e.g., phase separation, formation of nanoscaled precipitates or ultra-fine grains) to reduce the κL [211–220]. Although the carrier concentration in a material can be modulated using defect-engineering, or stoichiometry control during synthesis, reaching the optimized carrier concentration (typically 1019 cm−3) often require additional extrinsic doping. Intuitively, this 1019 carrier concentration is equivalent to about 1–30 electronically active sites in a 10 nm particle. Schematic illustrating doping mechanisms in nanostructures is shown in Fig. 17. The most commonly used strategy in modulating the carrier concentration at the nanoparticle level is the incorporation of dopants at the initial reaction solution [221]. In this approach, the dopants and host precursors are homogeneously mixed before the nucleation, resulting in uniform doping. However, not all dopants can be incorporated using this method. The coordinating ligands and proper control of reaction conditions are critical to enable high doping efficiency of these extrinsic elements rather than just a secondary phase or nanocomposite. In addition, surfactants, which can be used to modulate surface energies, can either inhibit or promote the incorporation of external dopant species. Generally, the addition of any type of impurities will affect the growth kinetics and thermodynamics of these nanostructures. Therefore, understanding the solubility and kinetics of the dopants and the host is very important to control the optimal amount of dopant that can be incorporated into these materials.
Fig. 17.
Scheme of possible strategies to tune doping in nanoparticle-based solution-processed nanomaterials: a extrinsic dopants during synthesis; b stoichiometry control; c post-synthesis atomic substitution; d surface chemistry; e dopant additives during consolidation; f modulation doping. Adapted with permission from Ref. [22]
Alternatively, dopant ions can be introduced within the preformed nanoparticles. Ion exchange processes can be employed in this case, which is akin to ion-implantation in semiconductor processing. Although this is a more expensive strategy, a non-equilibrium thermodynamic doping process can be achieved, thus making it possible to introduce a large amount of dopant beyond the solubility limit allowed by thermodynamics [222]. Besides introducing impurities, a common strategy to tune carrier concentration in nanoscale level (especially in 2D structures) is by applying gate-voltage [223]. In essence, gate voltage not only provides carrier concentration modulation, but can also be used to alter the carrier scattering mechanism, resulting in multi-fold enhancements of thermoelectric properties by enhancing electrical conductivity and reducing thermal conductivity.
Conclusion and Outlook
In this review paper, we provided a comprehensive review on the state-of-the-art strategies and bottom-up approaches that had been employed for constructing nanostructured semiconductor TE materials with different dimensions, focusing on the relationships between the structures and the key electronic and thermal transport parameters contributing to ZT, such as the S enhancement due to the energy filtering or quantum confinement effects, and the κ reduction inherently due to the phonon scattering. Table 1 summarizes the TE properties of various nanostructured materials prepared via bottom-up approaches. The vapor-based processes are expected to play an important role in preparing high-precision materials for theoretical mechanism studies and/or fabrication of micron-scale TE devices, while the solution-based preparation methods with controllable size, morphology and surface chemistry offer a convenient route towards inexpensive and scalable low-dimensional TE materials. However, the practical adoption of those liquid-based processes towards high quality nanomaterials still remains a challenge. Scale-up production of these nanostructured materials inevitably requires automated processes with nearly 100% yields, high nanoparticle concentrations in solution, low cost and environmentally-benign precursors, solvents, reductants and surfactants, as well as the recycling of solvents and possible side-products. Moreover, the consolidated conditions which may cause significant atomic and interface redistribution should also be investigated and optimized to assemble the nanostructured materials with a maximum retention of their electronic and phononic characteristics. Deep fundamental understanding of the electronic and thermal transport properties of these nanostructured materials will be highly valuable for further development of advanced, practical and high-performance TE devices. In addition, through developments in low-dimensional materials, some promising concepts such as nano-porous structures have been applied and have seen some early successes in traditional 3D bulk materials. Remarkably, it has been shown that it is in fact possible for porous structures to be 3D-printed through selective laser sintering, which results in an enhanced ZT. Moving forward, to realize widespread commercial interest, several key factors need to be taken into account in general: low-cost and scalable processing methods, earth-abundant and non-toxic materials to ensure sustainability, easy device integration (i.e. ensuring adequate performance on the device level). Therefore, any breakthrough in any of the aforementioned aspects will undoubtedly be an important step forward towards making TE devices more competitive.
Table 1.
Summary of properties of TE materials prepared via various bottom-up methods
| Process type | TE Materials | Mechanism on property improvement | σ (S cm−1) | S (μV K−1) | κ (Wm−1 K−1) | PF (μW m−1 K−2) | ZTmax | Temp. (K) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 0D nanoparticles & nanoinclusion | |||||||||
| Colloidal synthesis | PbSe quantum dot films | Quantum confinement, phonon scattering | ~ 240 | ~ 425 | ~ 1.15 | – | 1.37 | 400 | [83] |
| PbTe/PbS nanocrystal films | Quantum confinement, phonon scattering | ~ 21 | ~ 490 | ~ 0.675 | ~ 450 | 0.3 | 405 | [84] | |
| PbTe@PbS core–shell nanoparticle pellets | Phonon scattering | 125 | − 185 | 0.53 | – | 1.03 | 710 | [86] | |
| Hydrothermal synthesis | SnTe nanocrystal pellets | Phonon scattering energy filtering | 600 | 90 | 0.60 | 350 | 0.49 | 803 | [91] |
| Cu2S microcrystal pellets | Phonon scattering | 100 | 120 | 0.2 | – | 0.38 | 573 | [92] | |
| Electrodeposition process | Bi2Te3 nanocrystals on PEDOT films | Phonon scattering | 691 | − 146 | – | 1473 | – | 298 | [96] |
| Gold nano-particles-BiTe composite pellets | Phonon scattering | 71 | − 380 | 0.5 | – | 0.62 | 298 | [98] | |
| Nanoinclusion | Pt nanoparticles in Bi2Te2.7Se0.3thin films | Energy filtering, phonon scattering | 720 | 220 | – | 3510 | – | 298 | [107] |
| Ag nanoparticles in Bi2Te3 nanopowder pellets | Energy filtering, phonon scattering | 525 | − 140 | 0.58 | – | 0.77 | 475 | [108] | |
| Ag nanocrystals in PbS nanocrystal pellets | Energy filtering, phonon scattering | 660 | − 200 | 0.8 | > 1000 | 1.7 | 850 | [109] | |
| 1D Nanowires/nanofibers/nanotubes | |||||||||
| Solution-phase synthesis | Bi2Te3 NW pellets | Phonon scattering | 430 | − 240 | 1.0 | 2520 | 0.96 | 380 | [116] |
| PbTe NW pellets | Phonon scattering | 95 | 295 | 0.9 | 850 | 0.33 | 350 | [117] | |
| Bi2Te2.7Se0.3 NW pellets | Phonon scattering | 465 | − 160 | 0.46 | 1023 | 0.75 | 320 | [122] | |
| Te–Bi2Te3 NW pellets | Energy filtering, phonon scattering | 5.244 | 588 | 0.309 | ~ 300 | 0.236 | 400 | [120] | |
| PbTe–Bi2Te3 NW pellets | Energy filtering, phonon scattering | 100 | − 310 | 0.51 | ~ 950 | 1.2 | 620 | [124] | |
| Ag2Te–Bi2Te3 NW pellets | Energy filtering, phonon scattering | 28 | 275 | 0.2 | 210 | 0.41 | 400 | [123] | |
| Template-assisted electrodeposition method | Bi0.5Sb1.5Te3 NW membranes | Phonon scattering | 78 | 138 | – | 153 | – | 298 | [143] |
| Bi38Te55Se7 NW thin films | Phonon scattering | 2200 | − 115 | – | 2820 | – | 298 | [149] | |
| Bi15Sb29Te56 NW thin films | Phonon scattering | 720 | 156 | – | 1750 | – | 298 | [149] | |
| Electrospinning | PbTe nanotube mats | Quantum confinement, phonon scattering | 0.148 | 196 | – | 0.567 | – | 298 | [163] |
| 2D Nanoflake/nanosheet /nanoplate | |||||||||
| Chemical vapor deposition | Ag-doped SnSe nanoflake thin films | Phonon scattering | 5 | 370 | – | 66 | – | 300 | [177] |
| Molecular beam epitaxy | Sb2.31Te3 thin films | Phonon scattering | 1810 | 118 | – | 2520 | – | 298 | [224] |
| Hydrothermal method | Ge-doped SnSe nanoplate pellets | Phonon scattering | 70 | 275 | 0.18 | 510 | 2.1 | 873 | [192] |
| Solvothermal method | Bi2Te3 nanoplate films | Phonon scattering | 122 | − 103 | – | 128 | – | 298 | [196] |
| Bi2Te3 nanoplate pellets | Phonon scattering | 600 | − 137 | 0.1 | 1057 | 0.97 | 420 | [197] | |
| Bi2Te3 nanosheet pellets | Phonon scattering | 735 | − 180 | 1.2 | 2400 | 0.69 | 333 | [225] | |
| Bi2Te2.7Se0.3 nanoplate pellets | Phonon scattering | 480 | − 198 | 0.72 | 1875 | 1.23 | 480 | [198] | |
| Solution-phase synthesis | SnSe nanoplate pellets | Phonon scattering | 31 | 320 | 0.55 | 375 | 1.05 | 805 | [226] |
| Sn0.94Bi0.06Se nanosheet pellets | Phonon scattering | 12.5 | − 285 | 0.3 | 100 | 0.21 | 719 | [201] | |
| SnSe nanoplate pellet | Phonon scattering | 35 | 340 | – | 400 | – | 550 | [204] | |
| 3D porous nanostructure | |||||||||
| Random porous structure | PbS nanocrystal monolith | Phonon scattering | ~ 400 | ~ 187 | 0.56 | 1375 | 1.06 | 838 | [207] |
| Bi0.5Sb1.5Te3 powder monolith | Phonon scattering | 403 | 173 | 0.27 | 1100 | 1.29 | 327 | [208] | |
| Nanoporous SnSe pellets | Phonon scattering | ~ 40 | ~ 325 | 0.24 | 506 | 1.7 | 823 | [217] | |
| Template-assisted uniform porous structure | Bi1.5Sb0.5Te3 film | Phonon scattering | 644.5 | 144 | 0.89 | 1250 | 0.56 | 400 | [209] |
| ZnO film | Phonon scattering | ~ 45 | ~ 253 | ~ 3.6 | 290 | 0.072 | 693 | [210] | |
| Nanoscale doping | |||||||||
| Hydrothermal method | Pb, Cd-doped poly-crystalline SnSe | Phonon scattering, carrier concentration | ~ 85 | ~ 285 | 0.23 | 750 | 1.9 | 873 | [215] |
| Hydrothermal method | Pb, S-doped poly-crystalline SnSe | Phonon scattering, carrier concentration | ~ 38 | ~ 325 | 0.13 | 418 | 1.85 | 873 | [218] |
| Hydrothermal method | Se quantum dot/Sn 0.99Pb0.01Se nanocomposite | Phonon scattering, energy filtering | ~ 31 | ~ 420 | 0.245 | 560 | 2.0 | 873 | [214] |
| Solvothermal method | Cd-doped poly-crystalline SnSe | Phonon scattering, carrier concentration | 79 | 295 | 0.33 | 690 | 1.7 | 823 | [227] |
| Solvothermal method | Cu-doped poly-crystalline SnSe | Phonon scattering, carrier mobility | 56 | 316 | 0.32 | 557 | 1.41 | 823 | [218] |
Acknowledgement
The authors acknowledge support from A*STAR’s Science and Engineering Research Council, PHAROS program on Hybrid Thermoelectrics for Ambient Applications (Grant Nos.: 1527200019, 1527200020 and 1527200021) and Agritech program on Sustainable Hybrid Lighting System for Controlled Environment Agriculture: A19D9a0096.
Footnotes
Qiang Zhu and Suxi Wang have contributed equally to this work.
References
- 1.Zhai J, Wang T, Wang H, Su W, Wang X, et al. Strategies for optimizing the thermoelectricity of PbTe alloys. Chin. Phys. B. 2018;27(4):047306. doi: 10.1088/1674-1056/27/4/047306/meta. [DOI] [Google Scholar]
- 2.Mamur H, Bhuiyan MRA, Korkmaz F, Nil M. A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew. Sust. Energ. Rev. 2018;82:4159–4169. doi: 10.1016/j.rser.2017.10.112. [DOI] [Google Scholar]
- 3.Zheng Y, Xie H, Zhang Q, Suwardi A, Cheng X, et al. Unraveling the critical role of melt-spinning atmosphere in enhancing the thermoelectric performance of p-type Bi0.52Sb1.48Te3 alloys. ACS Appl. Mater. Interfaces. 2020;12(32):36186–36195. doi: 10.1021/acsami.0c09656. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Cai S, Hua X, Chen H, Liang Q, et al. High thermoelectric performance in polycrystalline SnSe via dual-doping with Ag/Na and nanostructuring with Ag8SnSe6. Adv. Energy Mater. 2019;9(2):1803072. doi: 10.1002/aenm.201803072. [DOI] [Google Scholar]
- 5.Luo ZZ, Zhang X, Hua X, Tan G, Bailey TP, et al. High thermoelectric performance in supersaturated solid solutions and nanostructured n-type PbTe–GeTe. Adv. Funct. Mater. 2018;28(31):1801617. doi: 10.1002/adfm.201801617. [DOI] [Google Scholar]
- 6.Luo Y, Zheng Y, Luo Z, Hao S, Du C, et al. N-type SnSe2 oriented-nanoplate-based pellets for high thermoelectric performance. Adv. Energy Mater. 2018;8(8):1702167. doi: 10.1002/aenm.201702167. [DOI] [Google Scholar]
- 7.Tan G, Zhao L-D, Kanatzidis MG. Rationally designing high-performance bulk thermoelectric materials. Chem. Rev. 2016;116(19):12123–12149. doi: 10.1021/acs.chemrev.6b00255. [DOI] [PubMed] [Google Scholar]
- 8.Mao J, Liu Z, Zhou J, Zhu H, Zhang Q, et al. Advances in thermoelectrics. Adv. Phys. 2018;67(2):69–147. doi: 10.1080/00018732.2018.1551715. [DOI] [Google Scholar]
- 9.Repaka DVM, Suwardi A, Kumar P. New paradigm for efficient thermoelectrics. Energy Saving Coating Mater. 2020 doi: 10.1016/B978-0-12-822103-7.00008-X. [DOI] [Google Scholar]
- 10.Shi X-L, Zou J, Chen Z-G. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020;120(15):7399–7515. doi: 10.1021/acs.chemrev.0c00026. [DOI] [PubMed] [Google Scholar]
- 11.Toberer ES, Zevalkink A, Snyder GJ. Phonon engineering through crystal chemistry. J. Mater. Chem. 2011;21(40):15843–15852. doi: 10.1039/C1JM11754H. [DOI] [Google Scholar]
- 12.Heremans JP, Jovovic V, Toberer ES, Saramat A, Kurosaki K, et al. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science. 2008;321(5888):554–557. doi: 10.1126/science.1159725. [DOI] [PubMed] [Google Scholar]
- 13.Pei Y, Shi X, LaLonde A, Wang H, Chen L, et al. Convergence of electronic bands for high performance bulk thermoelectrics. Nature. 2011;473(7345):66–69. doi: 10.1038/nature09996. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L-D, Lo S-H, Zhang Y, Sun H, Tan G, et al. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature. 2014;508(7496):373–377. doi: 10.1038/nature13184. [DOI] [PubMed] [Google Scholar]
- 15.Recatala-Gomez J, Suwardi A, Nandhakumar I, Abutaha A, Hippalgaonkar K. Toward accelerated thermoelectric materials and process discovery. ACS Appl. Energy Mater. 2020;3(3):2240–2257. doi: 10.1021/acsaem.9b02222. [DOI] [Google Scholar]
- 16.Suwardi A, Cao J, Hu L, Wei F, Wu J, et al. Tailoring the phase transition temperature to achieve high-performance cubic GeTe-based thermoelectrics. J. Mater. Chem. A. 2020;8(36):18880–18890. doi: 10.1039/D0TA06013E. [DOI] [Google Scholar]
- 17.Pei Y, LaLonde AD, Wang H, Snyder GJ. Low effective mass leading to high thermoelectric performance. Energy Environ. Sci. 2012;5(7):7963–7969. doi: 10.1039/C2EE21536E. [DOI] [Google Scholar]
- 18.Suwardi A, Bash D, Ng HK, Gomez JR, Repaka DM, et al. Inertial effective mass as an effective descriptor for thermoelectrics via data-driven evaluation. J. Mater. Chem. A. 2019;7(41):23762–23769. doi: 10.1039/C9TA05967A. [DOI] [Google Scholar]
- 19.M. Dresselhaus, Y. Lin, G. Dresselhaus, X. Sun, Z. Zhang et al., Advances in 1D and 2D thermoelectric materials. In Eighteenth International Conference on Thermoelectrics Proceedings, ICT'99 (Cat No 99TH8407). 92–99 (1999). https://doi.org/10.1109/ICT.1999.843342
- 20.Wu Y, Chen Z, Nan P, Xiong F, Lin S, et al. Lattice strain advances thermoelectrics. Joule. 2019;3(5):1276–1288. doi: 10.1016/j.joule.2019.02.008. [DOI] [Google Scholar]
- 21.Novak TG, Kim K, Jeon S. 2D and 3D nanostructuring strategies for thermoelectric materials. Nanoscale. 2019;11(42):19684–19699. doi: 10.1039/C9NR07406F. [DOI] [PubMed] [Google Scholar]
- 22.Ortega S, Ibáñez M, Liu Y, Zhang Y, Kovalenko MV, et al. Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 2017;46(12):3510–3528. doi: 10.1039/C6CS00567E. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Feng T, Li Z, Zheng W, Wu Y. Large-scale, solution-synthesized nanostructured composites for thermoelectric applications. Adv. Mater. 2018;30(48):1801904. doi: 10.1002/adma.201801904. [DOI] [PubMed] [Google Scholar]
- 24.Alam H, Ramakrishna S. A review on the enhancement of figure of merit from bulk to nano-thermoelectric materials. Nano Energy. 2013;2(2):190–212. doi: 10.1016/j.nanoen.2012.10.005. [DOI] [Google Scholar]
- 25.Goldsmid HJ. Introduction to Thermoelectricity. Berlin: Springer; 2010. [Google Scholar]
- 26.Mao J, Liu W, Ren Z. Carrier distribution in multi-band materials and its effect on thermoelectric properties. J. Materiomics. 2016;2(2):203–211. doi: 10.1016/j.jmat.2016.03.001. [DOI] [Google Scholar]
- 27.Chen Z-G, Han G, Yang L, Cheng L, Zou J. Nanostructured thermoelectric materials: current research and future challenge. Prog. Nat. Sci. Mater. Int. 2012;22(6):535–549. doi: 10.1016/j.pnsc.2012.11.011. [DOI] [Google Scholar]
- 28.Lin S, Li W, Bu Z, Gao B, Li J, et al. Thermoelectric properties of Ag9GaS6 with ultralow lattice thermal conductivity. Mater. Today Phys. 2018;6:60–67. doi: 10.1016/j.mtphys.2018.09.001. [DOI] [Google Scholar]
- 29.Li W, Lin S, Weiss M, Chen Z, Li J, et al. Crystal structure induced ultralow lattice thermal conductivity in thermoelectric Ag9AlSe6. Adv. Energy Mater. 2018;8(18):1800030. doi: 10.1002/aenm.201800030. [DOI] [Google Scholar]
- 30.Snyder GJ, Toberer ES. Complex Thermoelectric Materials. Singapore: World Scientific; 2011. pp. 101–110. [DOI] [PubMed] [Google Scholar]
- 31.Suwardi A, Hu L, Wang X, Tan XY, Repaka DVM, et al. Origin of high thermoelectric performance in earth-abundant phosphide–tetrahedrite. ACS Appl. Mater. Interfaces. 2020;12(8):9150–9157. doi: 10.1021/acsami.9b17269. [DOI] [PubMed] [Google Scholar]
- 32.Chen G. Nanoscale Energy Transport and Conversion: A Parallel Treatment of Electrons, Molecules, Phonons, and Photons. Oxford: Oxford University Press; 2005. [Google Scholar]
- 33.Lundstrom M, Guo J. Nanoscale Transistors: Device Physics, Modeling and Simulation. Berlin: Springer; 2006. [Google Scholar]
- 34.Liu T-H, Zhou J, Li M, Ding Z, Song Q, et al. Electron mean-free-path filtering in dirac material for improved thermoelectric performance. PNAS. 2018;115(5):879–884. doi: 10.1073/pnas.1715477115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J-H, Grossman J, Reed J, Galli G. Lattice thermal conductivity of nanoporous Si: molecular dynamics study. Appl. Phys. Lett. 2007;91(22):223110. doi: 10.1063/1.2817739. [DOI] [Google Scholar]
- 36.Chung JD, Kaviany M. Effects of phonon pore scattering and pore randomness on effective conductivity of porous silicon. Int. J. Heat. Mass Transf. 2000;43(4):521–538. doi: 10.1016/S0017-9310(99)00165-9. [DOI] [Google Scholar]
- 37.Biswas K, He J, Blum ID, Wu C-I, Hogan TP, et al. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature. 2012;489(7416):414–418. doi: 10.1038/nature11439. [DOI] [PubMed] [Google Scholar]
- 38.Cahill DG, Watson SK, Pohl RO. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B. 1992;46(10):6131. doi: 10.1103/PhysRevB.46.6131. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Carrete J, Zhang Z, Qu Y, Shen X, et al. Strong enhancement of phonon scattering through nanoscale grains in lead sulfide thermoelectrics. NPG Asia Mater. 2014;6(6):e108–e108. doi: 10.1038/am.2014.39. [DOI] [Google Scholar]
- 40.Ren SY, Dow JD. Thermal conductivity of superlattices. Phys. Rev. B. 1982;25(6):3750. doi: 10.1103/PhysRevB.25.3750. [DOI] [Google Scholar]
- 41.Mao J, Liu Z, Ren Z. Size effect in thermoelectric materials. NPJ Quantum Mater. 2016;1(1):1–9. doi: 10.1038/npjquantmats.2016.28. [DOI] [Google Scholar]
- 42.Hicks L, Dresselhaus MS. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B. 1993;47(19):12727. doi: 10.1103/PhysRevB.47.12727. [DOI] [PubMed] [Google Scholar]
- 43.Hicks L, Dresselhaus MS. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B. 1993;47(24):16631. doi: 10.1103/PhysRevB.47.16631. [DOI] [PubMed] [Google Scholar]
- 44.Pichanusakorn P, Bandaru P. Nanostructured thermoelectrics. Mater. Sci. Eng. R Rep. 2010;67(2–4):19–63. doi: 10.1016/j.mser.2009.10.001. [DOI] [Google Scholar]
- 45.Vineis CJ, Shakouri A, Majumdar A, Kanatzidis MG. Nanostructured thermoelectrics: Big efficiency gains from small features. Adv. Mater. 2010;22(36):3970–3980. doi: 10.1002/adma.201000839. [DOI] [PubMed] [Google Scholar]
- 46.Li J-F, Liu W-S, Zhao L-D, Zhou M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010;2(4):152–158. doi: 10.1038/asiamat.2010.138. [DOI] [Google Scholar]
- 47.Dresselhaus MS, Chen G, Tang MY, Yang R, Lee H, et al. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007;19(8):1043–1053. doi: 10.1002/adma.200600527. [DOI] [Google Scholar]
- 48.Hicks L, Harman T, Sun X, Dresselhaus M. Experimental study of the effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B. 1996;53(16):R10493. doi: 10.1103/PhysRevB.53.R10493. [DOI] [PubMed] [Google Scholar]
- 49.Rogacheva E, Tavrina T, Nashchekina O, Grigorov S, Nasedkin K, et al. Quantum size effects in pbse quantum wells. Appl. Phys. Lett. 2002;80(15):2690–2692. doi: 10.1063/1.1469677. [DOI] [Google Scholar]
- 50.Ohta H, Kim S, Mune Y, Mizoguchi T, Nomura K, et al. Giant thermoelectric seebeck coefficient of a two-dimensional electron gas in SrTiO3. Nat. Mater. 2007;6(2):129–134. doi: 10.1038/nmat1821. [DOI] [PubMed] [Google Scholar]
- 51.Boukai AI, Bunimovich Y, Tahir-Kheli J, Yu J-K, Goddard Iii WA, et al. Silicon nanowires as efficient thermoelectric materials. Nature. 2008;451(7175):168–171. doi: 10.1038/nature06458. [DOI] [PubMed] [Google Scholar]
- 52.Wu PM, Gooth J, Zianni X, Svensson SF, Gluschke JGR, et al. Large thermoelectric power factor enhancement observed in inas nanowires. Nano Lett. 2013;13(9):4080–4086. doi: 10.1021/nl401501j. [DOI] [PubMed] [Google Scholar]
- 53.Harman T, Spears D, Manfra M. High thermoelectric figures of merit in pbte quantum wells. J. Electron. Mater. 1996;25(7):1121–1127. doi: 10.1007/BF02659913. [DOI] [Google Scholar]
- 54.Harman T, Taylor P, Spears D, Walsh M. Thermoelectric quantum-dot superlattices with high ZT. J. Electron Mater. 2000;29(1):L1–L2. doi: 10.1007/s11664-000-0117-1. [DOI] [Google Scholar]
- 55.Wu L, Li X, Wang S, Zhang T, Yang J, et al. Resonant level-induced high thermoelectric response in indium-doped GeTe. NPG Asia Mater. 2017;9(1):e343. doi: 10.1038/am.2016.203. [DOI] [Google Scholar]
- 56.Suwardi A, Cao J, Zhao Y, Wu J, Chien SW, et al. Achieving high thermoelectric quality factor towards high figure of merit in GeTe. Mater. Today Phys. 2020 doi: 10.1016/j.mtphys.2020.100239. [DOI] [Google Scholar]
- 57.Ioffe AF, Stil'Bans L, Iordanishvili E, Stavitskaya T, Gelbtuch A, et al. Semiconductor thermoelements and thermoelectric cooling. Phys. Today. 1959;12(5):42. doi: 10.1063/1.3060810. [DOI] [Google Scholar]
- 58.Rowe D, Min G. Multiple potential barriers as a possible mechanism to increase the Seebeck coefficient and electrical power factor. AIP Conf. Proc. 1994;316(1):339–342. doi: 10.1063/1.46827. [DOI] [Google Scholar]
- 59.Li C, Ma S, Wei P, Zhu W, Nie X, et al. Magnetism-induced huge enhancement of the room-temperature thermoelectric and cooling performance of p-type BiSbTe alloys. Energy Environ. Sci. 2020;13(2):535–544. doi: 10.1039/C9EE03446C. [DOI] [Google Scholar]
- 60.Liang Z, Boland MJ, Butrouna K, Strachan DR, Graham KR. Increased power factors of organic–inorganic nanocomposite thermoelectric materials and the role of energy filtering. J. Mater. Chem. A. 2017;5(30):15891–15900. doi: 10.1039/C7TA02307C. [DOI] [Google Scholar]
- 61.Mingo N, Hauser D, Kobayashi N, Plissonnier M, Shakouri A. “Nanoparticle-in-alloy” approach to efficient thermoelectrics: Silicides in SiGe. Nano Lett. 2009;9(2):711–715. doi: 10.1021/nl8031982. [DOI] [PubMed] [Google Scholar]
- 62.Kim W, Zide J, Gossard A, Klenov D, Stemmer S, et al. Thermal conductivity reduction and thermoelectric figure of merit increase by embedding nanoparticles in crystalline semiconductors. Phys. Rev. Lett. 2006;96(4):045901. doi: 10.1103/PhysRevLett.96.045901. [DOI] [PubMed] [Google Scholar]
- 63.Jeng M-S, Yang R, Song D, Chen G. Modeling the thermal conductivity and phonon transport in nanoparticle composites using monte carlo simulation. J. Heat Transf. 2008;130(4):042410. doi: 10.1115/1.2818765. [DOI] [Google Scholar]
- 64.Kundu A, Mingo N, Broido D, Stewart D. Role of light and heavy embedded nanoparticles on the thermal conductivity of SiGe alloys. Phys. Rev. B. 2011;84(12):125426. doi: 10.1103/PhysRevB.84.125426. [DOI] [Google Scholar]
- 65.Zhang H, Minnich AJ. The best nanoparticle size distribution for minimum thermal conductivity. Sci. Rep. 2015;5:8995. doi: 10.1038/srep08995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He J, Sootsman JR, Girard SN, Zheng J-C, Wen J, et al. On the origin of increased phonon scattering in nanostructured pbte based thermoelectric materials. J. Am. Chem. Soc. 2010;132(25):8669–8675. doi: 10.1021/ja1010948. [DOI] [PubMed] [Google Scholar]
- 67.Zhao LD, Wu HJ, Hao SQ, Wu CI, Zhou XY, et al. All-scale hierarchical thermoelectrics: MgTe in PbTe facilitates valence band convergence and suppresses bipolar thermal transport for high performance. Energy Environ. Sci. 2013;6(11):3346–3355. doi: 10.1039/C3EE42187B. [DOI] [Google Scholar]
- 68.Zhao L-D, He J, Hao S, Wu C-I, Hogan TP, et al. Raising the thermoelectric performance of p-type PbS with endotaxial nanostructuring and valence-band offset engineering using cds and ZnS. J. Am. Chem. Soc. 2012;134(39):16327–16336. doi: 10.1021/ja306527n. [DOI] [PubMed] [Google Scholar]
- 69.Zhao L-D, He J, Wu C-I, Hogan TP, Zhou X, et al. Thermoelectrics with earth abundant elements: High performance p-type PbS nanostructured with SrSand CaS. J. Am. Chem. Soc. 2012;134(18):7902–7912. doi: 10.1021/ja301772w. [DOI] [PubMed] [Google Scholar]
- 70.Zhao L-D, Zhang X, Wu H, Tan G, Pei Y, et al. Enhanced thermoelectric properties in the counter-doped SnTe system with strained endotaxial SrTe. J. Am. Chem. Soc. 2016;138(7):2366–2373. doi: 10.1021/jacs.5b13276. [DOI] [PubMed] [Google Scholar]
- 71.Recatala-Gomez J, Ng HK, Kumar P, Suwardi A, Zheng M, et al. Thermoelectric properties of substoichiometric electron beam patterned bismuth sulfide. ACS Appl. Mater. Interfaces. 2020;12(30):33647–33655. doi: 10.1021/acsami.0c06829. [DOI] [PubMed] [Google Scholar]
- 72.Tan LP, Sun T, Fan S, Ng LY, Suwardi A, et al. Facile synthesis of Cu7Te4 nanorods and the enhanced thermoelectric properties of Cu7Te4–Bi0.4Sb1.6Te3 nanocomposites. Nano Energy. 2013;2(1):4–11. doi: 10.1016/j.nanoen.2012.07.004. [DOI] [Google Scholar]
- 73.Wang X, Suwardi A, Zheng Y, Zhou H, Chien SW, et al. Enhanced thermoelectric performance of nanocrystalline indium tin oxide pellets by modulating the density and nanoporosity via spark plasma sintering. ACS Appl. Nano Mater. 2020;3(10):10156–10165. doi: 10.1021/acsanm.0c02113. [DOI] [Google Scholar]
- 74.Suwardi A, Lim SH, Zheng Y, Wang X, Chien SW, et al. Effective enhancement of thermoelectric and mechanical properties of germanium telluride via rhenium-doping. J. Mater. Chem. C. 2020;8(47):16940–16948. doi: 10.1039/D0TC04903D. [DOI] [Google Scholar]
- 75.Venkatasubramanian R, Siivola E, Colpitts T, O’quinn B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature. 2001;413(6856):597–602. doi: 10.1038/35098012. [DOI] [PubMed] [Google Scholar]
- 76.Chang H-T, Wang C-C, Hsu J-C, Hung M-T, Li P-W, et al. High quality multifold Ge/Si/Ge composite quantum dots for thermoelectric materials. Appl. Phys. Lett. 2013;102(10):101902. doi: 10.1063/1.4794943. [DOI] [Google Scholar]
- 77.Chang H-T, Wang S-Y, Lee S-W. Designer ge/si composite quantum dots with enhanced thermoelectric properties. Nanoscale. 2014;6(7):3593–3598. doi: 10.1039/C3NR06335F. [DOI] [PubMed] [Google Scholar]
- 78.Nadtochiy A, Kuryliuk V, Strelchuk V, Korotchenkov O, Li P-W, et al. Enhancing the seebeck effect in Ge/Si through the combination of interfacial design features. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-52654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang J, Waclawik ER. Colloidal semiconductor nanocrystals: controlled synthesis and surface chemistry in organic media. RSC Adv. 2014;4(45):23505–23527. doi: 10.1039/C4RA02684E. [DOI] [Google Scholar]
- 80.Dasgupta NP, Sun J, Liu C, Brittman S, Andrews SC, et al. 25th anniversary article: semiconductor nanowires–synthesis, characterization, and applications. Adv. Mater. 2014;26(14):2137–2184. doi: 10.1002/adma.201305929. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Suwardi A, Lim SL, Wei F, Xu J. Transparent flexible thin-film p–n junction thermoelectric module. NPJ Flex. Electron. 2020;4(1):1–9. doi: 10.1038/s41528-020-00082-9. [DOI] [Google Scholar]
- 82.Wang RY, Feser JP, Lee J-S, Talapin DV, Segalman R, et al. Enhanced thermopower in PbSe nanocrystal quantum dot superlattices. Nano Lett. 2008;8(8):2283–2288. doi: 10.1021/nl8009704. [DOI] [PubMed] [Google Scholar]
- 83.Yang D, Lu C, Yin H, Herman IP. Thermoelectric performance of PbSe quantum dot films. Nanoscale. 2013;5(16):7290–7296. doi: 10.1039/C3NR01875J. [DOI] [PubMed] [Google Scholar]
- 84.Ding D, Wang D, Zhao M, Lv J, Jiang H, et al. Interface engineering in solution-processed nanocrystal thin films for improved thermoelectric performance. Adv. Mater. 2017;29(1):1603444. doi: 10.1002/adma.201603444. [DOI] [PubMed] [Google Scholar]
- 85.Nugraha MI, Kim H, Sun B, Desai S, de Arquer FPG, et al. Highly passivated n-type colloidal quantum dots for solution-processed thermoelectric generators with large output voltage. Adv. Energy Mater. 2019;9(28):1901244. doi: 10.1002/aenm.201901244. [DOI] [Google Scholar]
- 86.Ibáñez M, Zamani R, Gorsse S, Fan J, Ortega S, et al. Core–shell nanoparticles as building blocks for the bottom-up production of functional nanocomposites: PbTe–PbS thermoelectric properties. ACS Nano. 2013;7(3):2573–2586. doi: 10.1021/nn305971v. [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Zhang G, Fan S, Li Y. Hydrothermal synthesis of PbSe, PbTe semiconductor nanocrystals. J. Phys. Chem. Solids. 2001;62(11):1957–1960. doi: 10.1016/S0022-3697(01)00035-X. [DOI] [Google Scholar]
- 88.Zhang H, Wang L-P, Xiong H, Hu L, Yang B, et al. Hydrothermal synthesis for high-quality CdTe nanocrystals. Adv. Mater. 2003;15(20):1712–1715. doi: 10.1002/adma.200305653. [DOI] [Google Scholar]
- 89.Wang Q, Pan D, Jiang S, Ji X, An L, et al. A solvothermal route to size-and shape-controlled CdSe and CdTe nanocrystals. J. Crystal Growth. 2006;286(1):83–90. doi: 10.1016/j.jcrysgro.2005.05.083. [DOI] [Google Scholar]
- 90.Wang Q, Pan D, Jiang S, Ji X, An L, et al. Luminescent CdSe and CdSe/CdS core-shell nanocrystals synthesized via a combination of solvothermal and two-phase thermal routes. J. Lumin. 2006;118(1):91–98. doi: 10.1016/j.jlumin.2005.06.010. [DOI] [Google Scholar]
- 91.Li Z, Chen Y, Li J-F, Chen H, Wang L, et al. Systhesizing snte nanocrystals leading to thermoelectric performance enhancement via an ultra-fast microwave hydrothermal method. Nano Energy. 2016;28:78–86. doi: 10.1016/j.nanoen.2016.08.008. [DOI] [Google Scholar]
- 92.Tang Y-Q, Ge Z-H, Feng J. Synthesis and thermoelectric properties of copper sulfides via solution phase methods and spark plasma sintering. Curr. Comput. Aided Drug Des. 2017;7(5):141. doi: 10.3390/cryst7050141. [DOI] [Google Scholar]
- 93.Datta A, Nolas GS. Synthesis and characterization of nanocrystalline FeSb2 for thermoelectric applications. Eur. J. Inorg. Chem. 2012;2012(1):55–58. doi: 10.1002/ejic.201100864. [DOI] [Google Scholar]
- 94.Shao Z, Zhu W, Li Z, Yang Q, Wang G. One-step fabrication of cds nanoparticle-sensitized TiO2 nanotube arrays via electrodeposition. J. Phys. Chem. C. 2012;116(3):2438–2442. doi: 10.1021/jp2078117. [DOI] [Google Scholar]
- 95.Baeck S, Jaramillo T, Stucky G, McFarland E. Controlled electrodeposition of nanoparticulate tungsten oxide. Nano Lett. 2002;2(8):831–834. doi: 10.1021/nl025587p. [DOI] [Google Scholar]
- 96.Na J, Kim Y, Park T, Park C, Kim E. Preparation of bismuth telluride films with high thermoelectric power factor. ACS Appl. Mater. Interfaces. 2016;8(47):32392–32400. doi: 10.1021/acsami.6b10188. [DOI] [PubMed] [Google Scholar]
- 97.Zhao D, Chen J, Ren Z, Chen J, Song Q, et al. Thermoelectric transport and magnetoresistance of electrochemical deposited Bi2Te3 films at micrometer thickness. Ceram. Int. 2020;46(3):3339–3344. doi: 10.1016/j.ceramint.2019.10.043. [DOI] [Google Scholar]
- 98.Nguyen TH, Enju J, Ono T. Enhancement of thermoelectric properties of bismuth telluride composite with gold nano-particles inclusions using electrochemical co-deposition. J. Electrochem. Soc. 2019;166(12):D508. doi: 10.1149/2.1011912jes. [DOI] [Google Scholar]
- 99.Wang S, Li H, Lu R, Zheng G, Tang X. Metal nanoparticle decorated n-type Bi2Te3-based materials with enhanced thermoelectric performances. Nanotechnology. 2013;24(28):285702. doi: 10.1088/0957-4484/24/28/285702. [DOI] [PubMed] [Google Scholar]
- 100.Biswas K, He J, Blum ID, Wu C-I, Hogan TP, et al. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature. 2012;490(7421):570–570. doi: 10.1038/nature11645. [DOI] [PubMed] [Google Scholar]
- 101.Jung D, Kurosaki K, Seino S, Ishimaru M, Sato K, et al. Thermoelectric properties of au nanoparticle-supported Sb1.6Bi0.4Te3 synthesized by a γ-ray irradiation method. Phys. Status Solidi B. 2014;251(1):162–167. doi: 10.1002/pssb.201349226. [DOI] [Google Scholar]
- 102.Fan S, Zhao J, Yan Q, Ma J, Hng HH. Influence of nanoinclusions on thermoelectric properties of n-type Bi2Te3 nanocomposites. J. Electron. Mater. 2011;40(5):1018–1023. doi: 10.1007/s11664-010-1487-7. [DOI] [Google Scholar]
- 103.He Z, Stiewe C, Platzek D, Karpinski G, Müller E, et al. Effect of ceramic dispersion on thermoelectric properties of nano-ZrO2∕ CoSb3 composites. J. Appl. Phys. 2007;101(4):043707. doi: 10.1063/1.2561628. [DOI] [Google Scholar]
- 104.Zhou X, Wang G, Zhang L, Chi H, Su X, et al. Enhanced thermoelectric properties of Ba-filled skutterudites by grain size reduction and Ag nanoparticle inclusion. J. Mater. Chem. 2012;22(7):2958–2964. doi: 10.1039/C2JM15010G. [DOI] [Google Scholar]
- 105.Li J, Tan Q, Li JF, Liu DW, Li F, et al. BiSbTe-based nanocomposites with high zt: the effect of sic nanodispersion on thermoelectric properties. Adv. Funct. Mater. 2013;23(35):4317–4323. doi: 10.1002/adfm.201300146. [DOI] [Google Scholar]
- 106.Gupta S, Agarwal DC, Sivaiah B, Amrithpandian S, Asokan K, et al. Enhancement in thermoelectric properties due to Ag nanoparticles incorporated in Bi2Te3 matrix. Beilstein J. Nanotechnol. 2019;10(1):634–643. doi: 10.3762/bjnano.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun T, Samani MK, Khosravian N, Ang KM, Yan Q, et al. Enhanced thermoelectric properties of n-type Bi2Te2.7Se0.3 thin films through the introduction of Pt nanoinclusions by pulsed laser deposition. Nano Energy. 2014;8:223–230. doi: 10.1016/j.nanoen.2014.06.011. [DOI] [Google Scholar]
- 108.Zhang Q, Ai X, Wang L, Chang Y, Luo W, et al. Improved thermoelectric performance of silver nanoparticles-dispersed Bi2Te3 composites deriving from hierarchical two-phased heterostructure. Adv. Funct. Mater. 2015;25(6):966–976. doi: 10.1002/adfm.201402663. [DOI] [Google Scholar]
- 109.Ibánez M, Luo Z, Genc A, Piveteau L, Ortega S, et al. High-performance thermoelectric nanocomposites from nanocrystal building blocks. Nat. Commun. 2016;7(1):1–7. doi: 10.1038/ncomms10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang H, Wong E, Zhao T, Lee JD, Xin HL, et al. Charge transport modulation in PbSe nanocrystal solids by AuxAg1–x nanoparticle doping. ACS Nano. 2018;12(9):9091–9100. doi: 10.1021/acsnano.8b03112. [DOI] [PubMed] [Google Scholar]
- 111.Tarachand A, Mukherjee B, Saxena M, Kuo Y-K, Okram GS, et al. Ag-nanoinclusion-induced enhanced thermoelectric properties of Ag2S. ACS Appl. Energy Mater. 2019;2(9):6383–6394. doi: 10.1021/acsaem.9b01016. [DOI] [Google Scholar]
- 112.Lim KH, Wong KW, Liu Y, Zhang Y, Cadavid D, et al. Critical role of nanoinclusions in silver selenide nanocomposites as a promising room temperature thermoelectric material. J. Mater. Chem. C. 2019;7(9):2646–2652. doi: 10.1039/C9TC00163H. [DOI] [Google Scholar]
- 113.Chen R, Lee J, Lee W, Li D. Thermoelectrics of nanowires. Chem. Rev. 2019;119(15):9260–9302. doi: 10.1021/acs.chemrev.8b00627. [DOI] [PubMed] [Google Scholar]
- 114.Qi Y, Wang Z, Zhang M, Yang F, Wang X. Thermoelectric devices based on one-dimensional nanostructures. J. Mater. Chem. A. 2013;1(20):6110–6124. doi: 10.1039/C3TA01594G. [DOI] [Google Scholar]
- 115.Wang K, Liang H-W, Yao W-T, Yu S-H. Templating synthesis of uniform Bi2Te3 nanowires with high aspect ratio in triethylene glycol (TEG) and their thermoelectric performance. J. Mater. Chem. 2011;21(38):15057–15062. doi: 10.1039/C1JM12384J. [DOI] [Google Scholar]
- 116.Zhang G, Kirk B, Jauregui LA, Yang H, Xu X, et al. Rational synthesis of ultrathin n-type Bi2Te3 nanowires with enhanced thermoelectric properties. Nano Lett. 2012;12(1):56–60. doi: 10.1021/nl202935k. [DOI] [PubMed] [Google Scholar]
- 117.Finefrock SW, Zhang G, Bahk J-H, Fang H, Yang H, et al. Structure and thermoelectric properties of spark plasma sintered ultrathin pbte nanowires. Nano Lett. 2014;14(6):3466–3473. doi: 10.1021/nl500997w. [DOI] [PubMed] [Google Scholar]
- 118.Liang H-W, Liu S, Wu Q-S, Yu S-H. An efficient templating approach for synthesis of highly uniform cdte and PbTe nanowires. Inorg. Chem. 2009;48(11):4927–4933. doi: 10.1021/ic900245w. [DOI] [PubMed] [Google Scholar]
- 119.Wang K, Yang Y, Liang H-W, Liu J-W, Yu S-H. First sub-kilogram-scale synthesis of high quality ultrathin tellurium nanowires. Mater. Horizons. 2014;1(3):338–343. doi: 10.1039/C4MH00004H. [DOI] [Google Scholar]
- 120.Zhang G, Fang H, Yang H, Jauregui LA, Chen YP, et al. Design principle of telluride-based nanowire heterostructures for potential thermoelectric applications. Nano Lett. 2012;12(7):3627–3633. doi: 10.1021/nl301327d. [DOI] [PubMed] [Google Scholar]
- 121.Xiong Z, Cai Y, Ren X, Cao B, Liu J, et al. Solution-processed CdS/Cu2S superlattice nanowire with enhanced thermoelectric property. ACS Appl. Mater. Interfaces. 2017;9(38):32424–32429. doi: 10.1021/acsami.7b09346. [DOI] [PubMed] [Google Scholar]
- 122.Zhou C, Dun C, Wang K, Zhang X, Shi Z, et al. General method of synthesis ultrathin ternary metal chalcogenide nanowires for potential thermoelectric applications. Nano Energy. 2016;30:709–716. doi: 10.1016/j.nanoen.2016.10.043. [DOI] [Google Scholar]
- 123.Fang H, Yang H, Wu Y. Thermoelectric properties of silver telluride–bismuth telluride nanowire heterostructure synthesized by site-selective conversion. Chem. Mater. 2014;26(10):3322–3327. doi: 10.1021/cm501188c. [DOI] [Google Scholar]
- 124.Fang H, Feng T, Yang H, Ruan X, Wu Y. Synthesis and thermoelectric properties of compositional-modulated lead telluride–bismuth telluride nanowire heterostructures. Nano Lett. 2013;13(5):2058–2063. doi: 10.1021/nl400319u. [DOI] [PubMed] [Google Scholar]
- 125.H.-J. Choi, in Vapor–liquid–solid Growth of Semiconductor Nanowires. ed. by (Springer; 2012), pp. 1–36. 10.1007/978-3-642-22480-5_1
- 126.Kolasinski KW. Catalytic growth of nanowires: vapor–liquid–solid, vapor–solid–solid, solution–liquid–solid and solid–liquid–solid growth. Curr. Opin. Solid State Mater. Sci. 2006;10(3–4):182–191. doi: 10.1016/j.cossms.2007.03.002. [DOI] [Google Scholar]
- 127.Li D, Wu Y, Kim P, Shi L, Yang P, et al. Thermal conductivity of individual silicon nanowires. Appl. Phys. Lett. 2003;83(14):2934–2936. doi: 10.1063/1.1616981. [DOI] [Google Scholar]
- 128.Li D, Wu Y, Fan R, Yang P, Majumdar A. Thermal conductivity of Si/SiGe superlattice nanowires. Appl. Phys. Lett. 2003;83(15):3186–3188. doi: 10.1063/1.1619221. [DOI] [Google Scholar]
- 129.Noyan ID, Gadea G, Salleras M, Pacios M, Calaza C, et al. SiGe nanowire arrays based thermoelectric microgenerator. Nano Energy. 2019;57:492–499. doi: 10.1016/j.nanoen.2018.12.050. [DOI] [Google Scholar]
- 130.Park Y-H, Kim J, Kim H, Kim I, Lee K-Y, et al. Thermal conductivity of VLS-grown rough si nanowires with various surface roughnesses and diameters. Appl. Phys. A. 2011;104(1):7. doi: 10.1007/s00339-011-6474-1. [DOI] [Google Scholar]
- 131.Kim H, Park Y-H, Kim I, Kim J, Choi H-J, et al. Effect of surface roughness on thermal conductivity of VLS-grown rough Si1−xGex nanowires. Appl. Phys. A. 2011;104(1):23–28. doi: 10.1007/s00339-011-6475-0. [DOI] [Google Scholar]
- 132.Dávila D, Tarancón A, Calaza C, Salleras M, Fernández-Regúlez M, et al. Improved thermal behavior of multiple linked arrays of silicon nanowires integrated into planar thermoelectric microgenerators. J. Electron. Mater. 2013;42(7):1918–1925. doi: 10.1007/s11664-013-2470-x. [DOI] [Google Scholar]
- 133.Dávila D, Huber R, Hierold C. Bottom-up silicon nanowire-based thermoelectric microgenerators. J. Phys. Conf. Series. 2015;660(1):012101. doi: 10.1088/1742-6596/660/1/012101. [DOI] [Google Scholar]
- 134.Hill DJ, Teitsworth TS, Kim S, Christesen JD, Cahoon JF. Encoding highly nonequilibrium boron concentrations and abrupt morphology in p-type/n-type silicon nanowire superlattices. ACS Appl. Mater. Interfaces. 2017;9(42):37105–37111. doi: 10.1021/acsami.7b08162. [DOI] [PubMed] [Google Scholar]
- 135.F. Nasirpouri, in Template Electrodeposition of Nanowires Arrays. ed. by (Springer; 2017), pp. 187–259. 10.1007/978-3-319-44920-3_5
- 136.Li L, Xu S, Li G. Enhancement of thermoelectric properties in Bi–Sb–Te alloy nanowires by pulsed electrodeposition. Energy Technol. 2015;3(8):825–829. doi: 10.1002/ente.201500071. [DOI] [Google Scholar]
- 137.Reeves RD, Crosser LA, Chester GE, Hill JJ. Thermoelectric property enhancement via pore confinement in template grown bismuth telluride nanowire arrays. Nanotechnology. 2017;28(50):505401. doi: 10.1088/1361-6528/aa9733. [DOI] [PubMed] [Google Scholar]
- 138.Proenca M, Rosmaninho M, Resende P, Sousa C, Ventura J, et al. Tailoring bi-te based nanomaterials by electrodeposition: Morphology and crystalline structure. Mater. Des. 2017;118:168–174. doi: 10.1016/j.matdes.2017.01.020. [DOI] [Google Scholar]
- 139.Lee J, Farhangfar S, Lee J, Cagnon L, Scholz R, et al. Tuning the crystallinity of thermoelectric Bi2Te3 nanowire arrays grown by pulsed electrodeposition. Nanotechnology. 2008;19(36):365701. doi: 10.1088/0957-4484/19/36/365701. [DOI] [PubMed] [Google Scholar]
- 140.Martín J, Manzano CV, Caballero-Calero O, Martín-González M. High-aspect-ratio and highly ordered 15-nm porous alumina templates. ACS Appl. Mater. Interfaces. 2013;5(1):72–79. doi: 10.1021/am3020718. [DOI] [PubMed] [Google Scholar]
- 141.Krieg J, Giraud R, Funke H, Dufouleur J, Escoffier W, et al. Magnetotransport measurements on Bi2Te3 nanowires electrodeposited in etched ion-track membranes. J. Phys. Chem. Solids. 2019;128:360–366. doi: 10.1016/j.jpcs.2018.02.002. [DOI] [Google Scholar]
- 142.Kojda D, Mitdank R, Mogilatenko A, Töllner W, Wang Z, et al. The effect of a distinct diameter variation on the thermoelectric properties of individual Bi0.39Te0.61 nanowires. Semicond. Sci. Technol. 2014;29(12):124006. doi: 10.1088/0268-1242/29/12/124006. [DOI] [Google Scholar]
- 143.Muñoz Rojo M, Grauby S, Rampnoux J-M, Caballero-Calero O, Martín-González M, et al. Fabrication of Bi2Te3 nanowire arrays and thermal conductivity measurement by 3ω-scanning thermal microscopy. J. Appl. Phys. 2013;113(5):054308. doi: 10.1063/1.4790363. [DOI] [Google Scholar]
- 144.Mavrokefalos A, Moore AL, Pettes MT, Shi L, Wang W, et al. Thermoelectric and structural characterizations of individual electrodeposited bismuth telluride nanowires. J. Appl. Phys. 2009;105(10):104318. doi: 10.1063/1.3133145. [DOI] [Google Scholar]
- 145.Rojo MM, Abad B, Manzano CV, Torres P, Cartoixà X, et al. Thermal conductivity of Bi2Te3 nanowires: how size affects phonon scattering. Nanoscale. 2017;9(20):6741–6747. doi: 10.1039/C7NR02173A. [DOI] [PubMed] [Google Scholar]
- 146.De Tomas C, Cantarero A, Lopeandia A, Alvarez F. From kinetic to collective behavior in thermal transport on semiconductors and semiconductor nanostructures. J. Appl. Phys. 2014;115(16):164314. doi: 10.1063/1.4871672. [DOI] [Google Scholar]
- 147.Kumar P, Pfeffer M, Peranio N, Eibl O, Bäßler S, et al. Ternary, single-crystalline Bi2(Te, Se)3 nanowires grown by electrodeposition. Acta Mater. 2017;125:238–245. doi: 10.1016/j.actamat.2016.11.057. [DOI] [Google Scholar]
- 148.Li L, Jin C, Xu S, Yang J, Du H, et al. Thermal conductivity of a single Bi0.5Sb1.5Te3 single-crystalline nanowire. Nanotechnology. 2014;25(41):415704. doi: 10.1088/0957-4484/25/41/415704. [DOI] [PubMed] [Google Scholar]
- 149.Bäßler S, Böhnert T, Gooth J, Schumacher C, Pippel E, et al. Thermoelectric power factor of ternary single-crystalline Sb2Te3-and Bi2Te3-based nanowires. Nanotechnology. 2013;24(49):495402. doi: 10.1088/0957-4484/24/49/495402. [DOI] [PubMed] [Google Scholar]
- 150.Kuo C-G, Hsieh Y-T, Yang C-F, Huang C-H, Yen C-Y. Growth of anodic aluminum oxide templates and the application in fabrication of the BiSbTe-based thermoelectric nanowires. Int. J. Photoenergy. 2014 doi: 10.1155/2014/978184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hasan M, Gautam D, Enright R. Electrodeposition of textured Bi27Sb28Te45 nanowires with enhanced electrical conductivity. Mater. Chem. Phys. 2016;173:438–445. doi: 10.1016/j.matchemphys.2016.02.035. [DOI] [Google Scholar]
- 152.Danine A, Schoenleber J, Ghanbaja J, Montaigne F, Boulanger C, et al. Microstructure and thermoelectric properties of p-type bismuth antimony telluride nanowires synthetized by template electrodeposition in polycarbonate membranes. Electrochim. Acta. 2018;279:258–268. doi: 10.1016/j.electacta.2018.05.071. [DOI] [Google Scholar]
- 153.Datta A, Sangle A, Hardingham N, Cooper C, Kraan M, et al. Structure and thermoelectric properties of Bi2−xSbxTe3 nanowires grown in flexible nanoporous polycarbonate templates. Materials. 2017;10(5):553. doi: 10.3390/ma10050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu J, Shen J, Shi H, Liang Y, Qu Z, et al. Preparation and characterisation of single-crystalline structure Sb/Bi2Te3 superlattice nanowires. Micro Nano Lett. 2016;11(11):738–740. doi: 10.1049/mnl.2015.0457. [DOI] [Google Scholar]
- 155.Yoo B, Xiao F, Bozhilov KN, Herman J, Ryan MA, et al. Electrodeposition of thermoelectric superlattice nanowires. Adv. Mater. 2007;19(2):296–299. doi: 10.1002/adma.200600606. [DOI] [Google Scholar]
- 156.Limmer SJ, Yelton WG, Siegal MP, Lensch-Falk JL, Pillars J, et al. Electrochemical deposition of Bi2(Te, Se)3 nanowire arrays on Si. J. Electrochem. Soc. 2012;159(4):D235. doi: 10.1149/2.084204jes. [DOI] [Google Scholar]
- 157.Li X, Cai K, Yu D, Wang Y. Electrodeposition and characterization of thermoelectric Bi2Te2Se/Te multilayer nanowire arrays. Superlattices Microstruct. 2011;50(5):557–562. doi: 10.1016/j.spmi.2011.09.001. [DOI] [Google Scholar]
- 158.Li D, McCann JT, Xia Y, Marquez M. Electrospinning: A simple and versatile technique for producing ceramic nanofibers and nanotubes. J. Am. Ceram. Soc. 2006;89(6):1861–1869. doi: 10.1111/j.1551-2916.2006.00989.x. [DOI] [Google Scholar]
- 159.Wang S-X, Yap CC, He J, Chen C, Wong SY, et al. Electrospinning: A facile technique for fabricating functional nanofibers for environmental applications. Nanotechnol. Rev. 2016;5(1):51–73. doi: 10.1515/ntrev-2015-0065. [DOI] [Google Scholar]
- 160.Yin T, Liu D, Ou Y, Ma F, Xie S, et al. Nanocrystalline thermoelectric Ca3Co4O9 ceramics by sol−gel based electrospinning and spark plasma sintering. J. Phys. Chem. C. 2010;114(21):10061–10065. doi: 10.1021/jp1024872. [DOI] [Google Scholar]
- 161.Ma F, Ou Y, Yang Y, Liu Y, Xie S, et al. Nanocrystalline structure and thermoelectric properties of electrospun NaCo2O4 nanofibers. J. Phys. Chem. C. 2010;114(50):22038–22043. doi: 10.1021/jp107488k. [DOI] [Google Scholar]
- 162.Xu W, Shi Y, Hadim H. The fabrication of thermoelectric La0.95Sr0.05CoO3 nanofibers and Seebeck coefficient measurement. Nanotechnology. 2010;21(39):395303. doi: 10.1088/0957-4484/21/39/395303. [DOI] [PubMed] [Google Scholar]
- 163.Park K-R, Cho H-B, Song Y, Kim S, Kwon Y-T, et al. Large-scale synthesis of lead telluride (PbTe) nanotube-based nanocomposites with tunable morphology, crystallinity and thermoelectric properties. Appl. Surf. Sci. 2018;436:785–790. doi: 10.1016/j.apsusc.2017.12.102. [DOI] [Google Scholar]
- 164.Zhang M, Park S-D, Kim J, Nalbandian M, Kim S, et al. Synthesis and thermoelectric characterization of lead telluride hollow nanofibers. Front. Chem. 2018;6:436. doi: 10.3389/fchem.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 166.Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012;7(11):699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 167.Zhang X, Xie Y. Recent advances in free-standing two-dimensional crystals with atomic thickness: design, assembly and transfer strategies. Chem. Soc. Rev. 2013;42(21):8187–8199. doi: 10.1039/C3CS60138B. [DOI] [PubMed] [Google Scholar]
- 168.Xu M, Liang T, Shi M, Chen H. Graphene-like two-dimensional materials. Chem. Rev. 2013;113(5):3766–3798. doi: 10.1021/cr300263a. [DOI] [PubMed] [Google Scholar]
- 169.Zhang H, Zeng W, Pan C, Feng L, Ou M, et al. SnTe@ MnO2-SP nanosheet–based intelligent nanoplatform for second near-infrared light–mediated cancer theranostics. Adv. Funct. Mater. 2019;29(37):1903791. doi: 10.1002/adfm.201903791. [DOI] [Google Scholar]
- 170.Oh JY, Lee JH, Han SW, Chae SS, Bae EJ, et al. Chemically exfoliated transition metal dichalcogenide nanosheet-based wearable thermoelectric generators. Energy Environ. Sci. 2016;9(5):1696–1705. doi: 10.1039/C5EE03813H. [DOI] [Google Scholar]
- 171.Huang Y, Li L, Lin Y-H, Nan C-W. Liquid exfoliation few-layer snse nanosheets with tunable band gap. J. Phys. Chem. C. 2017;121(32):17530–17537. doi: 10.1021/acs.jpcc.7b06096. [DOI] [Google Scholar]
- 172.Baumgardner WJ, Choi JJ, Lim Y-F, Hanrath T. Snse nanocrystals: synthesis, structure, optical properties, and surface chemistry. J. Am. Chem. Soc. 2010;132(28):9519–9521. doi: 10.1021/ja1013745. [DOI] [PubMed] [Google Scholar]
- 173.Chen YX, Ge ZH, Yin M, Feng D, Huang XQ, et al. Understanding of the extremely low thermal conductivity in high-performance polycrystalline snse through potassium doping. Adv. Funct. Mater. 2016;26(37):6836–6845. doi: 10.1002/adfm.201602652. [DOI] [Google Scholar]
- 174.Ahn J-H, Lee M-J, Heo H, Sung JH, Kim K, et al. Deterministic two-dimensional polymorphism growth of hexagonal n-type SnS2 and orthorhombic p-type SnS crystals. Nano Lett. 2015;15(6):3703–3708. doi: 10.1021/acs.nanolett.5b00079. [DOI] [PubMed] [Google Scholar]
- 175.Ma X-H, Cho K-H, Sung Y-M. Growth mechanism of vertically aligned SnSe nanosheets via physical vapour deposition. CrystEngComm. 2014;16(23):5080–5086. doi: 10.1039/C4CE00213J. [DOI] [Google Scholar]
- 176.Tian Z, Guo C, Zhao M, Li R, Xue J. Two-dimensional sns: A phosphorene analogue with strong in-plane electronic anisotropy. ACS Nano. 2017;11(2):2219–2226. doi: 10.1021/acsnano.6b08704. [DOI] [PubMed] [Google Scholar]
- 177.Liu S, Sun N, Liu M, Sucharitakul S, Gao XP. Nanostructured snse: Synthesis, doping, and thermoelectric properties. J. Appl. Phys. 2018;123(11):115109. doi: 10.1063/1.5018860. [DOI] [Google Scholar]
- 178.Chen H-A, Sun H, Wu C-R, Wang Y-X, Lee P-H, et al. Single-crystal antimonene films prepared by molecular beam epitaxy: Selective growth and contact resistance reduction of the 2D material heterostructure. ACS Appl. Mater. Interfaces. 2018;10(17):15058–15064. doi: 10.1021/acsami.8b02394. [DOI] [PubMed] [Google Scholar]
- 179.Fortin-Deschênes M, Waller O, Mentes T, Locatelli A, Mukherjee S, et al. Synthesis of antimonene on germanium. Nano Lett. 2017;17(8):4970–4975. doi: 10.1021/acs.nanolett.7b02111. [DOI] [PubMed] [Google Scholar]
- 180.Wu X, Shao Y, Liu H, Feng Z, Wang YL, et al. Epitaxial growth and air-stability of monolayer antimonene on PdTe2. Adv. Mater. 2017;29(11):1605407. doi: 10.1002/adma.201605407. [DOI] [PubMed] [Google Scholar]
- 181.Nagao T, Doi T, Sekiguchi T, Hasegawa S. Epitaxial growth of single-crystal ultrathin films of bismuth on Si (111) Jpn. J. Appl. Phys. 2000;39(7S):4567. doi: 10.1143/JJAP.39.4567. [DOI] [Google Scholar]
- 182.Nagao T, Sadowski J, Saito M, Yaginuma S, Fujikawa Y, et al. Nanofilm allotrope and phase transformation of ultrathin bi film on Si (111)−7×7. Phys. Rev. Lett. 2004;93(10):105501. doi: 10.1103/PhysRevLett.93.105501. [DOI] [PubMed] [Google Scholar]
- 183.Aizawa T, Ohkubo I, Lima MS, Sakurai T, Mori T. Fabrication of Mg2Sn (111) film by molecular beam epitaxy. J. Vac. Sci. Technol. A: Vac. Surfaces Films. 2019;37(6):061513. doi: 10.1116/1.5122844. [DOI] [Google Scholar]
- 184.Ye Z, Cui S, Shu T, Ma S, Liu Y, et al. Electronic band structure of epitaxial pbte (111) thin films observed by angle-resolved photoemission spectroscopy. Phys. Rev. B. 2017;95(16):165203. doi: 10.1103/PhysRevB.95.165203. [DOI] [Google Scholar]
- 185.Ren W, Li H, Gao L, Li Y, Zhang Z, et al. Epitaxial growth and thermal-conductivity limit of single-crystalline Bi2Se3/In2Se3 superlattices on mica. Nano Res. 2017;10(1):247–254. doi: 10.1007/s12274-016-1282-8. [DOI] [Google Scholar]
- 186.Chen M-W, Kim H, Ovchinnikov D, Kuc A, Heine T, et al. Large-grain mbe-grown gase on gaas with a mexican hat-like valence band dispersion. NPJ 2D Mater. Appl. 2018;2(1):1–7. doi: 10.1038/s41699-017-0047-x. [DOI] [Google Scholar]
- 187.Rice A, Kawasaki J, Verma N, Pennachio D, Schultz B, et al. Structural and electronic properties of molecular beam epitaxially grown Ni1+xTiSn films. J. Crystal Growth. 2017;467:71–76. doi: 10.1016/j.jcrysgro.2017.03.015. [DOI] [Google Scholar]
- 188.Chen X, Fan R. Low-temperature hydrothermal synthesis of transition metal dichalcogenides. Chem. Mater. 2001;13(3):802–805. doi: 10.1021/cm000517+. [DOI] [Google Scholar]
- 189.Zhang X, Ma G, Wang J. Hydrothermal synthesis of two-dimensional MoS2 and its applications. Tungsten. 2019;1(1):59–79. doi: 10.1007/s42864-019-00014-9. [DOI] [Google Scholar]
- 190.Alli U, Hettiarachchi SJ, Kellici S. Chemical functionalisation of 2D materials by batch and continuous hydrothermal flow synthesis. Chem. Eur. J. 2020;26(29):6447–6460. doi: 10.1002/chem.202000383. [DOI] [PubMed] [Google Scholar]
- 191.Shi XL, Tao X, Zou J, Chen ZG. High-performance thermoelectric SnSe: aqueous synthesis, innovations, and challenges. Adv. Sci. 2020;7(7):1902923. doi: 10.1002/advs.201902923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chandra S, Biswas K. Realization of high thermoelectric figure of merit in solution synthesized 2D SnSe nanoplates via Ge alloying. J. Am. Chem. Soc. 2019;141(15):6141–6145. doi: 10.1021/jacs.9b01396. [DOI] [PubMed] [Google Scholar]
- 193.Shi W, Zhou L, Song S, Yang J, Zhang H. Hydrothermal synthesis and thermoelectric transport properties of impurity-free antimony telluride hexagonal nanoplates. Adv. Mater. 2008;20(10):1892–1897. doi: 10.1002/adma.200702003. [DOI] [Google Scholar]
- 194.Park JG, Lee YH. High thermoelectric performance of Bi-Te alloy: defect engineering strategy. Curr. Appl. Phys. 2016;16(9):1202–1215. doi: 10.1016/j.cap.2016.03.028. [DOI] [Google Scholar]
- 195.Hosokawa Y, Tomita K, Takashiri M. Growth of single-crystalline Bi2Te3 hexagonal nanoplates with and without single nanopores during temperature-controlled solvothermal synthesis. Sci. Rep. 2019;9(1):10790. doi: 10.1038/s41598-019-47356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mori R, Mayuzumi Y, Yamaguchi M, Kobayashi A, Seki Y, et al. Improved thermoelectric properties of solvothermally synthesized Bi2Te3 nanoplate films with homogeneous interconnections using Bi2Te3 electrodeposited layers. J. Alloys Compd. 2020;818:152901. doi: 10.1016/j.jallcom.2019.152901. [DOI] [Google Scholar]
- 197.Wang Y, Liu W-D, Gao H, Wang L-J, Li M, et al. High porosity in nanostructured n-type Bi2Te3 obtaining ultralow lattice thermal conductivity. ACS Appl. Mater. Interfaces. 2019;11(34):31237–31244. doi: 10.1021/acsami.9b12079. [DOI] [PubMed] [Google Scholar]
- 198.Hong M, Chasapis TC, Chen Z-G, Yang L, Kanatzidis MG, et al. N-type Bi2Te3–xSex nanoplates with enhanced thermoelectric efficiency driven by wide-frequency phonon scatterings and synergistic carrier scatterings. ACS Nano. 2016;10(4):4719–4727. doi: 10.1021/acsnano.6b01156. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Y, Liu Y, Xing C, Zhang T, Li M, et al. Tin selenide molecular precursor for the solution processing of thermoelectric materials and devices. ACS Appl. Mater. Interfaces. 2020;12(24):27104–27111. doi: 10.1021/acsami.0c04331. [DOI] [PubMed] [Google Scholar]
- 200.Saha S, Banik A, Biswas K. Few-layer nanosheets of n-type SnSe2. Chem. Eur. J. 2016;22(44):15634–15638. doi: 10.1002/chem.201604161. [DOI] [PubMed] [Google Scholar]
- 201.Chandra S, Banik A, Biswas K. N-type ultrathin few-layer nanosheets of bi-doped SnSe: synthesis and thermoelectric properties. ACS Energy Lett. 2018;3(5):1153–1158. doi: 10.1021/acsenergylett.8b00399. [DOI] [Google Scholar]
- 202.Rongione NA, Li M, Wu H, Nguyen HD, Kang JS, et al. High-performance solution-processable flexible SnSe nanosheet films for lower grade waste heat recovery. Adv. Electron. Mater. 2019;5(3):1800774. doi: 10.1002/aelm.201800774. [DOI] [Google Scholar]
- 203.Hong M, Chen Z-G, Yang L, Chasapis TC, Kang SD, et al. Enhancing the thermoelectric performance of SnSe1−xTex nanoplates through band engineering. J. Mater. Chem. A. 2017;5(21):10713–10721. doi: 10.1039/C7TA02677C. [DOI] [Google Scholar]
- 204.Han G, Popuri SR, Greer HF, Bos JWG, Zhou W, et al. Facile surfactant-free synthesis of p-type SnSe nanoplates with exceptional thermoelectric power factors. Angew. Chem. Int. Ed. 2016;55(22):6433–6437. doi: 10.1002/anie.201601420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Son JS, Choi MK, Han M-K, Park K, Kim J-Y, et al. N-type nanostructured thermoelectric materials prepared from chemically synthesized ultrathin Bi2Te3 nanoplates. Nano Lett. 2012;12(2):640–647. doi: 10.1021/nl203389x. [DOI] [PubMed] [Google Scholar]
- 206.Martín-González M, Caballero-Calero O, Díaz-Chao P. Nanoengineering thermoelectrics for 21st century: Energy harvesting and other trends in the field. Renew. Sust. Energ. Rev. 2013;24:288–305. doi: 10.1016/j.rser.2013.03.008. [DOI] [Google Scholar]
- 207.Xu B, Feng T, Li Z, Pantelides ST, Wu Y. Constructing highly porous thermoelectric monoliths with high-performance and improved portability from solution-synthesized shape-controlled nanocrystals. Nano Lett. 2018;18(6):4034–4039. doi: 10.1021/acs.nanolett.8b01691. [DOI] [PubMed] [Google Scholar]
- 208.Shi J, Chen H, Jia S, Wang W. 3d printing fabrication of porous bismuth antimony telluride and study of the thermoelectric properties. J. Manuf. Process. 2019;37:370–375. doi: 10.1016/j.jmapro.2018.11.001. [DOI] [Google Scholar]
- 209.Hong S, Park J, Jeon SG, Kim K, Park SH, et al. Monolithic Bi1.5Sb0.5Te3 ternary alloys with a periodic 3D nanostructure for enhancing thermoelectric performance. J. Mater. Chem. C. 2017;5(35):8974–8980. doi: 10.1039/C7TC02717F. [DOI] [Google Scholar]
- 210.Kim K, Park J, Hong S, Park SH, Jeon SG, et al. Anomalous thermoelectricity of pure ZnO from 3D continuous ultrathin nanoshell structures. Nanoscale. 2018;10(6):3046–3052. doi: 10.1039/C7NR08167G. [DOI] [PubMed] [Google Scholar]
- 211.Tang G, Wei W, Zhang J, Li Y, Wang X, et al. Realizing high figure of merit in phase-separated polycrystalline Sn1–xPbxSe. J. Am. Chem. Soc. 2016;138(41):13647–13654. doi: 10.1021/jacs.6b07010. [DOI] [PubMed] [Google Scholar]
- 212.Tang G, Liu J, Zhang J, Li D, Rara KH, et al. Realizing high thermoelectric performance below phase transition temperature in polycrystalline snse via lattice anharmonicity strengthening and strain engineering. ACS Appl. Mater. Interfaces. 2018;10(36):30558–30565. doi: 10.1021/acsami.8b10056. [DOI] [PubMed] [Google Scholar]
- 213.Gong Y, Chang C, Wei W, Liu J, Xiong W, et al. Extremely low thermal conductivity and enhanced thermoelectric performance of polycrystalline SnSe by Cu doping. Scripta Mater. 2018;147:74–78. doi: 10.1016/j.scriptamat.2017.12.035. [DOI] [Google Scholar]
- 214.Xu R, Huang L, Zhang J, Li D, Liu J, et al. Nanostructured snse integrated with se quantum dots with ultrahigh power factor and thermoelectric performance from magnetic field-assisted hydrothermal synthesis. J. Mater. Chem. A. 2019;7(26):15757–15765. doi: 10.1039/C9TA03967H. [DOI] [Google Scholar]
- 215.Li S, Lou X, Li X, Zhang J, Li D, et al. Realization of high thermoelectric performance in polycrystalline tin selenide through schottky vacancies and endotaxial nanostructuring. Chem. Mater. 2020;32(22):9761–9770. doi: 10.1021/acs.chemmater.0c03657. [DOI] [Google Scholar]
- 216.Shi X, Chen Z-G, Liu W, Yang L, Hong M, et al. Achieving high figure of merit in p-type polycrystalline Sn0.98Se via self-doping and anisotropy-strengthening. Energy Storage Mater. 2018;10:130–138. doi: 10.1016/j.ensm.2017.08.014. [DOI] [Google Scholar]
- 217.Shi X, Wu A, Liu W, Moshwan R, Wang Y, et al. Polycrystalline snse with extraordinary thermoelectric property via nanoporous design. ACS Nano. 2018;12(11):11417–11425. doi: 10.1021/acsnano.8b06387. [DOI] [PubMed] [Google Scholar]
- 218.Shi X, Zheng K, Hong M, Liu W, Moshwan R, et al. Boosting the thermoelectric performance of p-type heavily Cu-doped polycrystalline SnSe via inducing intensive crystal imperfections and defect phonon scattering. Chem. Sci. 2018;9(37):7376–7389. doi: 10.1039/C8SC02397B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shi XL, Liu WD, Wu AY, Nguyen VT, Gao H, et al. Optimization of sodium hydroxide for securing high thermoelectric performance in polycrystalline Sn1−xSe via anisotropy and vacancy synergy. InfoMat. 2019;2(6):1201–1215. doi: 10.1002/inf2.12057. [DOI] [Google Scholar]
- 220.Zheng Y, Shi X-L, Yuan H, Lu S, Qu X, et al. A synergy of strain loading and laser radiation in determining the high-performing electrical transports in the single Cu-doped SnSe microbelt. Mater. Today Phys. 2020 doi: 10.1016/j.mtphys.2020.100198. [DOI] [Google Scholar]
- 221.Liu Y, García G, Ortega S, Cadavid D, Palacios P, et al. Solution-based synthesis and processing of Sn-and Bi-doped Cu3SbSe4 nanocrystals, nanomaterials and ring-shaped thermoelectric generators. J. Mater. Chem. A. 2017;5(6):2592–2602. doi: 10.1039/C6TA08467B. [DOI] [Google Scholar]
- 222.De Trizio L, Manna L. Forging colloidal nanostructures via cation exchange reactions. Chem. Rev. 2016;116(18):10852–10887. doi: 10.1021/acs.chemrev.5b00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang F, Wu J, Suwardi A, Zhao Y, Liang B, et al. Gate-tunable polar optical phonon to piezoelectric scattering in few-layer Bi2O2Se for high-performance thermoelectrics. Adv. Mater. 2020 doi: 10.1002/adma.202004786. [DOI] [PubMed] [Google Scholar]
- 224.Cecchi S, Dragoni D, Kriegner D, Tisbi E, Zallo E, et al. Interplay between structural and thermoelectric properties in epitaxial Sb2+xTe3 alloys. Adv. Funct. Mater. 2019;29(2):1805184. doi: 10.1002/adfm.201805184. [DOI] [Google Scholar]
- 225.Bao D, Chen J, Yu Y, Liu W, Huang L, et al. Texture-dependent thermoelectric properties of nano-structured Bi2Te3. Chem. Eng. J. 2020;388:124295. doi: 10.1016/j.cej.2020.124295. [DOI] [Google Scholar]
- 226.Zhang Y, Liu Y, Xing C, Zhang T, Li M, et al. Tin selenide molecular precursor for the solution-processing of thermoelectric materials and devices. ACS Appl. Mater. Interfaces. 2020;12(24):27104–27111. doi: 10.1021/acsami.0c04331. [DOI] [PubMed] [Google Scholar]
- 227.Shi X, Wu A, Feng T, Zheng K, Liu W, et al. High thermoelectric performance in p-type polycrystalline Cd-doped SnSe achieved by a combination of cation vacancies and localized lattice engineering. Adv. Energy Mater. 2019;9(11):1803242. doi: 10.1002/aenm.201803242. [DOI] [Google Scholar]