Highlights
-
•
Patients with epilepsy undergoing bariatric surgery (or extensive intestinal resection for a different indication) should be followed by a multidisciplinary team including a pharmacist and a neurologist.
-
•
ASM concentrations should be frequently checked before and after surgery.
-
•
Upon discharge, ensure close follow up with a neurologist and pharmacist to monitor ASM levels.
-
•
Extended release formulations should be avoided.
-
•
Pharmacokinetic factors should be considered when selecting and adjusting ASMs.
Keywords: Gastric bypass, Antiseizure medications, Anticonvulsants, Malabsorption, Pharmacokinetics
Abstract
Healthcare professionals are encountering an increasing number of patients who have undergone bariatric surgeries. Antiseizure medications (ASM) have a narrow therapeutic window, and patients with malabsorptive states receiving ASM present a complex situation as the pharmacokinetics of these drugs have only been studied in patients with a normal functioning gastrointestinal tract. Patients with malabsorptive states may have altered pharmacokinetics, and there is limited literature to guide drug selection and dosage adjustment in patients with malabsorptive states. This review highlights pharmacokinetic parameters of common ASM, and considerations when managing patients on them. The effect of pH, lipophilicity, absorption, and metabolism should be taken into account when selecting and managing ASMs in this patient population. Based on these parameters, levetiracetam, and topiramate have fewer issues referable to absorption related to bariatric surgery while oral formulations of phenytoin, carbamazepine, oxcarbamazepine and valproic acid have reduced absorption due to effects of bariatric surgery based on the pharmacokinetic properties of these medications. Extended formulations should be avoided and ASM serum concentrations should be checked before and after surgery. The care of patients with epilepsy who are scheduled to undergo bariatric surgery should be guided by a multidisciplinary team including a pharmacist and a neurologist who should be involved in the adjustment of the ASMs throughout the pre-surgical and post-surgical periods.
Introduction
The prevalence of obesity in the United States is 39.8%, with an annual medical cost of $147 billion in 2008 [1]. One of the treatment options for obesity, particularly for morbid obesity (BMI > 40), is bariatric surgery. The number of bariatric procedures has increased worldwide. In 2017 it was estimated that 228,000 bariatric surgeries were performed in the United Sates [2].
Bariatric procedures and malabsorptive states, in general, may alter the absorption, dissolution, metabolism and bioavailability of antiseizure medications (ASMs). In addition, weight loss associated with these procedures can also lead to changes in the pharmacokinetics of ASMs. These changes may lead to clinically relevant changes of ASMs and can lead to loss of seizure control or toxicity. A population based study found that approximately 30–45% of obese patients (BMI ≥ 30) were receiving central nervous system drugs, antimicrobials, cardiovascular agents or agents for musculoskeletal diseases over a period of 18 months [3]. Furthermore, a study evaluating drugs taken in bariatric surgery patients found that approximately 14% were on ASMs drugs prior to their procedures [4]. Although it is not uncommon for patients on ASMs to undergo bariatric surgery there is very little data to guide the adjustment of antiseizure therapy in these situations [5], [6], [7].
A recent case prompted our interest in evaluating dose adjustments and selection of ASMs in patients with malabsorptive states. This review aims to evaluate pharmacokinetics alterations of common ASMs after various gastric bypass procedures and malabsorptive states.
Patient case
A 38-year-old female with well controlled genetic generalized epilepsy manifested exclusively by generalized tonic-clonic seizures, presented to epilepsy clinic for recommendations involving ASM prior to a Roux-en-Y gastric bypass surgery. Preoperatively she had a weight of 156 kg and a BMI of 58 kg/m2 and her seizures were well controlled, being seizure-free for approximately 8 years. Her ASM included valproic acid delayed release tablets 1500 mg twice a day (BID) and phenytoin extended release capsules 200 mg BID. Since the patient was seizure-free for 8 years, and tolerated phenytoin and valproic acid, she remained on these medications despite known drug interaction [8]. In preparation for the procedure her ASM were changed to immediate release formulations and included: valproic acid capsules 1000 mg three times a day (TID) (19.2 mg/kg/day) and phenytoin immediate release tablets 200 mg BID (2.6 mg/kg/day). Prior to the change in formulations of her medications it was reported her valproic acid concentrations were within the normal range, and her total and free phenytoin concentrations were 12.2 mcg/mL (reference range: 10–20 mcg/mL) and 2.2 mcg/mL (reference range: 1–2 mcg/mL), respectively. Her ASM concentrations after the change and prior to surgery were within the therapeutic ranges and are shown in Table 1. The patient subsequently underwent the surgery and her drug concentrations two weeks after the surgery are also shown in Table 1. Since her free phenytoin concentration was decreasing, her phenytoin was subsequently increased to 250 mg BID. Her repeat total and free phenytoin concentrations (3 weeks later, 5 weeks post-operatively) were 8.5 mcg/mL and 1.4 mcg/mL, respectively. The phenytoin dose was not changed further given the free concentration was deemed therapeutic. The patient’s weight at 6 months post-surgery had decreased to 126.6 kg. The patient followed-up approximately 9 months post-operatively with serum drug concentrations that are shown in Table 1. Due to a decreased valproic acid concentration her valproic acid was increased to 1500 mg TID. Her valproic acid dose was increased further to 1750 mg TID and eventually to 1500 mg four times a day (QID). During her visit 1.5 years after her surgery her BMI was 32.4 and her weight was 87 kg. Her serum ASM concentrations are shown in Table 1. Due to a slightly high free phenytoin level, her dose was decreased to 200 mg BID. She was ultimately maintained on 200 mg BID (4.6 mg/kg/day) of phenytoin and 1500 mg QID (68.9 mg/kg/day) of valproic acid.
Table 1.
Patient case - phenytoin and valproic acid dosing and concentrations.
| Pre-Surgery | 2 weeks Post-Operative | 5 weeks Post-Operative | 9 months Post-Operative | 10 months Post-Operative* | 11 months Post-Operative* | 1 year Post-Operative* | 1.5 years Post-Operative | |
|---|---|---|---|---|---|---|---|---|
| Patient’s weight (kg) | 156 | 148 | 100 | 88 | ||||
| Phenytoin Dose | 200 mg BID | 200 mg BID | 250 mg BID | 250 mg BID | 250 mg BID | 250 mg BID | 250 mg BID | 250 mg BID |
| Total Phenytoin Concentration (mcg/mL) Therapeutic range: 10–20 mcg/mL |
7 | 5.8 | 8.5 | 13.1 | 15.7 | |||
| Free Phenytoin Concentration (mcg/mL) Therapeutic range: 1–2 mcg/mL |
1.5 | 1.1 | 1.4 | 1.6 | 3.1 | |||
| Valproic Acid Dose | 1000 mg TID | 1000 mg TID | 1000 mg TID | 1000 mg TID | 1500 mg TID | 1750 mg TID | 1500 mg QID | 1500 mg QID |
| Total Valproic Acid Concentration (mcg/mL) Therapeutic range: 50–125 mcg/mL) |
83 | 66 | 82 | 26 | 39 | 42 | 54 | 62 |
| Free Valproic Acid Concentration (mcg/mL) Therapeutic range: 5–25 mcg/mL |
9 | 15 | 13 | 4 | 3 | 4 | 5 | 5 |
*No patient weight, phenytoin levels were not available for 10 months, 11 months, and 1 year post-operatively.
Abbreviations: Kg: kilogram, BID: Twice a day, Mcg: microgram, mL: Milliliters, TID: Three times a day.
Types of bariatric surgeries
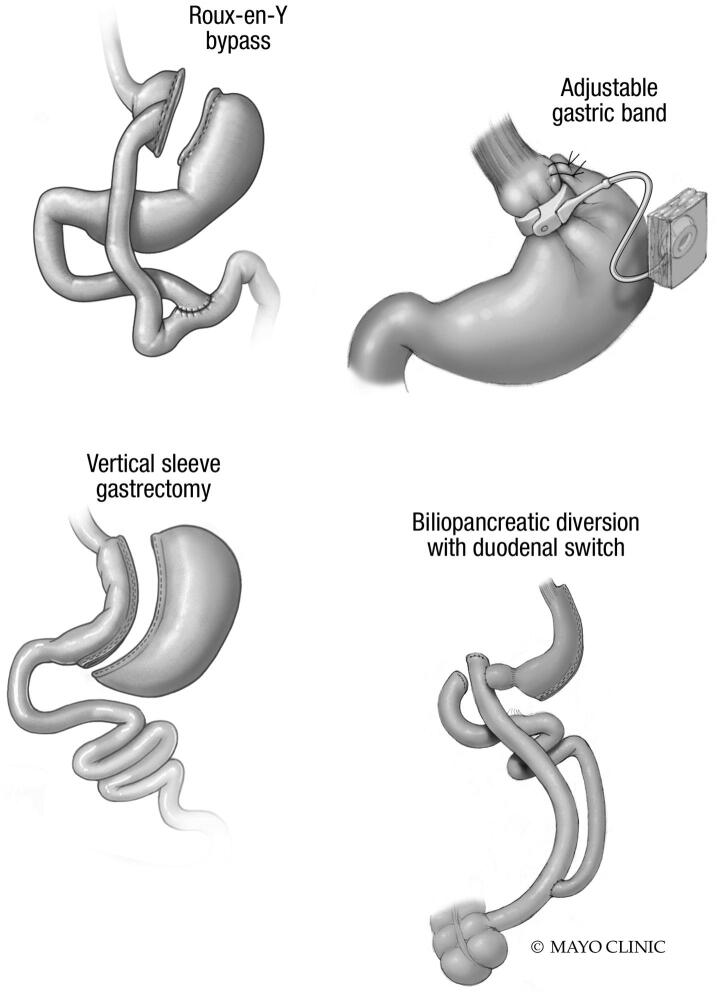
Bariatric surgeries are classified into three categories: restrictive (limiting stomach size or structure), malabsorptive (shortening intestinal length or modifying gut anatomy), and combination of restrictive and malabsorptive. Restrictive procedures include laparoscopic sleeve gastrectomy (LSG; aka the sleeve), laparoscopic adjustable gastric banding (LAGB; aka the band), and vertical banded gastroplasty (VBG), with bowel beyond the stomach remaining intact. The strictly malabsorptive surgery is the jejunoileal bypass (JIB), which isolates the proximal jejunum to distal ileum segment, resulting in bypass of the vast majority of the small intestine. Due to severe metabolic and hepatic complications, JIB is no longer performed as a weight loss surgery, but was prevalent in the 1960 s and 1970 s. Combination surgeries include Roux-en-Y gastric bypass (RYGB; the “gold standard” of weight loss surgery), biliopancreatic diversion (BPD), and biliopancreatic diversion with duodenal switch (BPD/DS). These combined restrictive and malabsorptive surgeries involve stomach resection with a residual gastric pouch (RYGB) or sleeve (BPD) and produce altered intestinal length and anatomy, typically resulting in three intestinal limbs or channels. The alimentary or “Roux” limb allows food passage to the common channel where food comes into contact with pancreatic fluids and bile that enter the common channel via the bypassed biliopancreatic limb. This common channel is typically longer in RYBG compared to BPD, and therefore the latter results in more malabsorption of fats and nutritents [9], [10], [11]. These procedures are illustrated in Fig. 1.
Fig. 1.
Bariatric Procedures. A. Roux-en-Y bypass involves creation of a small gastric pouch from the newly isolated stomach and proximal small bowel that is excluded from gastric transfer. The pouch is anastomosed to the small intestine, forming the Roux limb. Food enters the pouch, moves through Roux limb and then reaches the common channel where pancreatic fluids and bile have entered from the bypassed bilipancreatic limb. B, C. Banding and gastric sleeve are purely restrictive procedures with bowel beyond the stomach remaining intact. D. BPD/DS involves creation of a gastric sleeve along with and resection of the majority of duodenum beyond the most proximal portion to the stomach. The distal segment of small intestine is then connected to the stomach and the bypassed duodenal portion (biliopancreatic limb) anastomosed to the last portion of the small intestine, forming a common channel, often shorter and resulting in greater malabsorption than that with Roux-en-Y. From Roust LR & DiBaise JK. Nutrient deficiencies prior to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2017 Mar;20(2):138–144; used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Post-surgical malabsorptive states
Drug malabsorption also occurs after non-bariatric abdominal surgeries. Extensive surgical resection of small bowel may be necessary because of trauma, mesenteric ischemia, radiation enteritis, malignancy, and Crohn’s disease. While any bowel resection has the potential to alter drug absorption, malabsorption is especially concerning in the context of gastrectomy and short bowel syndrome, defined as less than 200 cm of small bowel in continuity beyond the ligament of Treitz and further classified by the presence or absence of colonic continuity. Notably, absorptive capacity can be regained to some extent with small bowel adaptation that occurs over a period of two years post bowel resection [9].
Various diseases causing intestinal malabsorption can affect the pharmacokinetics of ASMs. A discussion of these diseases is beyond the scope of this review, but some of the concepts discussed here in relation to post-surgical malabsorption may also apply to patients with those disorders.
Pharmacokinetic changes from malabsorptive states
Pharmacokinetic parameters are altered in post-surgical malabsorptive states due to the changes in intestinal anatomy. There is limited data available to guide therapy for patients on ASMs. Therefore, pharmacokinetics parameters should be taken into consideration when selecting and dosing ASM in patients with malabsorptive states. The pharmacokinetics of medications through the GI tract can be divided between the stomach and small intestine. The stomach is responsible for dissolution, while the small intestine is responsible for absorption, transport, and metabolism. The small intestine has a larger surface area and more permeable membranes, leading to increased drug absorption in comparison to the large intestine [12]. Alterations in the GI anatomy can alter the pharmacokinetics at either of these sites [7], [13]. It is important to note that these proposed mechanisms are based on pharmacokinetic modeling, and further investigations are needed in patients with malabsorptive states.
Effect of pH
The intraluminal pH of the stomach is normally acidic with a pH of 1.5 to 3.5 while the pH of the small intestine is 6.6 to 7.5 and increases rapidly beyond proximal duodenum to peak at 7.5 in the distal ileum [14], [15]. After partial or total gastrectomy, as with some bariatric surgeries, the available parietal cells that secrete hydrochloric acid may be reduced or bypassed leading to an increase in gastric pH [13]. This may cause basic drugs to be less soluble and therefore have decreased dissolution. Conversely, acidic drugs may have unchanged or a slight increase in absorption [13], [16]. The acid-base behavior of ASM is multifaceted as some medications have multiple functional groups, and therefore, multiple pKa values. The pKa of common ASM medications are listed in Table 2.
Table 2.
Pharmacokinetic parameters of oral antiseizure drugs.
| Medication | pKa | Lipophilicity (LogP) | Intestinal Metabolism | Active Transport Pumps | Enterohepatic Recirculation | Approximate Bioavailability/Site of Absorption | Impact of Food on Absorption | May be Largely Affected by Malabsorptive Procedures |
|---|---|---|---|---|---|---|---|---|
| Carbamazepine [32], [33], [34], [35], [36] | 16.0, −3.8 | 2.7 | Substrate CYP3A4 (to active metabolite carbamazepine-10,11-epoxide) | – | Yes | 70 – 79% | None with slow release formulation | Yes |
| Felbamate [32], [37], [38] | 14.9 | 0.6 | Substrate of CYP2C19, CYP3A4, CYP2E1 (inactive metabolites) | – | – | 90%, Small intestine | None with tablet. Suspension unknown | Yes |
| Gabapentin [32], [39], [40], [41] | 4.6, 9.9 | −1.9 | – | L amino transport protein | – | 60%, Absorption decreases with increased dose | Slight increase in absorption with food | Yes |
| Lacosamide [32], [42], [43], [44], [45], [46] | 12.5, −1.5 | 0.2 | Substrate of CYP2C9, CYP2C19, CYP3A4 (inactive metabolites) | – | – | 100% | None | Yes |
| Lamotrigine [32], [47], [48] | 14.9, 5.87 | 1.9 | Substrate of UGT1A4 and UGT2B7 | ABCB1, SLC22A1. P-gp | – | 98% | None | Yes |
| Levetiracetam [32], [49], [50], [51] | 16.1, −1.6 | −0.6 | – | – | – | 100% | May delay absorption by 0.5 hours, but extent is not affected | No |
| Oxcarbazepine [32], [52], [53] | 13.2, −4.3 | 1.8 | Substrate of CYP3A4 and UGT to 10-Hydroxy-10, 11-dihydro carbazepine | – | Possible | ≥95% | None | Yes |
| Phenobarbital [32], [54], [55] | 7.14 | 1.4 | Substrate of CYP2C9, CYP2C19, CYP2E1 | – | Yes | ≥95% | Unknown | Yes |
| Phenytoin [24], [32], [56], [57] | 9.5, −9 | 2.3 | Substrate of CYP2C9, CYP2C19 | – | Yes | 80% | Increased absorption/no change with food Caution with tube feeds |
Yes |
| Topiramate [32], [58], [59], [60] | 11, −3.7 | −0.6 | – | – | – | 80–90% | None | No |
| Valproic Acid [32], [61], [62], [63] | 5.14 | 2.5 | UGT1A3, UGT1A4, UGT1A6, UGT1A8, UGT1A9, UGT1A10, UGT2B7, and UGT2B15 Substrate of CYP2C9, CYP2A6, and to a lesser extent by CYP2B6 Metabolite activity unknown |
– | Yes | 90% | None | Yes |
Abbreviations: CYP: Cytochrome, UGT: Urindinediphosphate, glucoronosyltransferases, ABC: ATP-binding cassette protein B1, SLC: solute carrier family 22, member 1 PgP: P-glycopreotein.
Absorption
Most ASM are absorbed in the small intestine due to its large surface area [17]. Depending on anatomy or underlying malabsorptive condition, small intestinal transit time for drugs may be accelerated and therefore lead to decreased absorption. There is limited literature evaluating extended release formulations in patients with intestinal malabsorption, but they are routinely avoided because absorption is expected to be diminished; thus, it is generally recommended to switch to immediate-release formulations to maximize absorption in these patients [13], [17], [18]. Food and nutritional support may also impact medication absorption, and the impact of absorption of ASMs with food is listed in Table 2.
Lipophilicity
Lipophilicity can also affect drug absorption. After RYGB there is a delay and reduction in bile secretion [13], [17]. Highly lipophilic drugs may be more affected because they often depend upon bile acids to enhance solubility. A higher logP indicates a drug is lipophilic, while a lower logP indicates a drug is hydrophilic. In patients with malabsorptive states compared with lipophilic drugs, the impact on altered absorption may be less affected for more hydrophilic drugs (i.e. a drug with lower logP) given it does not need bile acids to facilitate its absorption [16]. An optimal logP for dissolution and passive diffusion across the intestinal membrane is 1–2 [16]. The lipophilicity of common ASM is shown in Table 2.
First pass metabolism
Due to a reduction in the length of the small intestine in malabsorptive state enzymes and carrier proteins may be affected [13]. Before drugs reach the liver and plasma, they may encounter enzymes in the intestinal mucosa, particularly cytochrome (CYP) P450 enzymes. CYP enzymes play a role in drug metabolismand can produce inactive and active metabolites. The majority of CYP enzymes are located in the liver; however some are located in the small intestine, the most prevalent of which is CYP3A4, accounting for 80% of intestinal CYPs [19]. Generally, the proportion of CYP enzymes is higher in the proximal regions of the small intestines, which may be bypassed in bariatric surgery and other malabsorptive states [19]. In addition to CYP enzymes, urindinediphosphate, glucoronosyltransferases (UGT), phenylsulfotrasnferases (PST), and glutathione S-transferases (GST) are present in the small intestine and may also affect metabolism. Decreased exposure to these intestinal enzymes may cause variable pharmacodynamic effects depending on the specific drug: increased drug activity may result from decreased metabolism to inactive metabolites or decreased drug activity may result from increased metabolism to active metabolites. Table 2 indicates drugs that may be susceptible to intestinal metabolism by CYP, UGT, PST, or GST enzymes. A common example of this with ASMs is the inhibition of CYP3A4 by grapefruit juice, leading to increased concentrations of carbamazepine [20]. Of note, there is insufficient understanding about the extent to which these drugs have intestinal metabolism. Also, the GI tract can adapt over time, and changes in drug concentrations for medications with intestinal metabolism may be transient [13].
Drug absorption
ASM can move across the intestinal membrane to the plasma via passive diffusion or active transport. Numerous active transport pumps are found primarily in the jejunum and small intestine [17], [21]. These transporters include organic anion transporting polypeptides (OATP2B1, OATP1A2), monocarboxylic acid transporter 1 (MCT1), and oligopeptide transporter (PEPT1) [21]. If the medication undergoes active transport via these pumps, less drug may reach the plasma when exposure to the pumps is diminished after intestinal resection. ASM with active transport pumps are shown in Table 2.
Additionally, medications may be affected by efflux pumps, such as P-glycopreotein (PgP), breast cancer resistance protein (BCRP) and multi-drug resistance associated protein (MRP2). These efflux pumps are located in the intestinal epithelial cells and they transport drugs from the blood into the intestinal lumen, thereby decreasing the drug plasma concentrations. In patients with malabsorptive anatomy there may be a decrease in these efflux pumps, which might lead to a subsequent increase in plasma concentration of the drug [16].
Enterohepatic recirculation
Lastly, some drugs undergo enterohepatic recirculation. In patients with a functional intestinal tract drugs may re-enter the duodenum for repeated absorption after circulating through the liver. In patients with RYBG this surface area is reduced, potentially leading to a cycle of decreased absorption [16]. Drugs that undergo enterohepatic recirculation are listed in Table 2. In post-surgical malabsorptive states these drugs may have decreased absorption.
Collectively, all these factors may affect concentrations of the medications in patients with malabsorptive anatomy. Predicting drug absorption, dissolution, metabolism, and transport in patients with malabsorptive states is complex and therefore drug concentrations should be closely monitored. The true extent of the clinical impact these pharmacokinetic factors have on drug absorption in malabsorptive states remains unknown, and more studies are warranted.
Pharmacokinetic changes from weight loss
In addition to pharmacokinetic factors that are affected by anatomical changes, there are numerous pharmacokinetic alterations that may occur after bariatric surgery because of the physiological changes related to the resulting weight loss. The volume of distribution (Vd) is the hypothetical distribution of drug into tissues outside the vascular system [22]. Vd can be affected by both adipose tissue and protein binding [17]. As patients lose weight their adipose tissue decreases. The resulting alterations in Vd are dependent on specific drug properties, particularly lipid solubility. Lipid soluble drugs will deposit in the adipose tissue. For these drugs, the concentrations in the plasma will increase as the tissue concentration in the adipose tissue decreases if patients lose weight. The contrary is true for water soluble or hydrophilic ASM, which will stay in the vascular space despite changes in adipose tissue post-surgery [22]. Deposition into adipose tissue is also dependent on protein binding [17]. It is unclear how drug-protein binding is affected after weight loss. Major proteins that bind to drugs include albumin and α-1-acid glycoprotein (AAG). It postulated that AAG may be reduced following weight loss, which would lead to increased free active drug; however, the true extent of this phenomenon is unknown [23].
Practical considerations
Patients with malabsorptive states are at increased risk of altered drug absorption. Given the lack of literature to guide selection of ASMs, pharmacokinetic factors may be considered when selecting and optimizing ASMs in patients with malabsorptive states. ASMs with the following attributes are less likely to be associated with dosage and pharmacokinetic issues following bariatric surgery: acidic properties, lower log P (i.e. more hydrophilic), minimal intestinal metabolism and transportation through efflux pumps, and lack of enterohepatic recirculation. Based on these pharmacokinetic factors the following ASMs show these attributes: levetiracetam and topiramate. Levetiracetam and topiramate both have a low log P, do not undergo intestinal metabolism, enterohepatic recirculation, or transport through active transport pumps. By contrast, the following ASM do not have these properties (basic, higher log P (more lipophilic), intestinal metabolism, transport through transport pumps, and enterohepatic circulation) and are likely to be associated with pharmacokinetic issues following bariatric surgery: phenytoin, carbamazepine, oxcarbazepine, and valproic acid. The extent to which each of these respective pharmacokinetic factors impacts clinical efficacy and toxicity remains unclear. Patient specific factors should always be taken into consideration when selecting and adjusting ASMs. Additionally, delayed-release and extended release formulations are less optimal, and immediate release formulations are generally preferred. Attentive drug monitoring is imperative including frequent concentration checks, particularly in the early post-operative phase.
Very few reports have described the effect of bariatric surgery and other post-surgical malabsorptive states on ASM efficacy. A study compared absorption and elimination of phenytoin in seven patients who had undergone JIB with nine controls. The half-life and absorption of phenytoin was decreased in the patients who had undergone JIB [24]. As previously mentioned, JIB is no longer a procedure of choice for weight control. In addition, there is a case report of undetectable phenytoin concentrations post RYGB despite adequate concentrations before the surgery [25].
Due to the complexities of pharmacokinetic factors and the narrow therapeutic window of ASMs, patients with epilepsy are at increased risk of having recurrent seizures after the gastric bypass surgeries or development of malabsorption states. In addition to the complexities that malabsorptive states have on ASMs, drug interactions (enzyme induction, enzyme inhibition, and competition for protein binding) as seen in patients with a normal functioning GI tract need to be considered. Optimal care for these patients requires a pharmacist and a neurologist working together as part of a multidisciplinary team from before until after the surgery. Close collaboration with the surgeon – to understand the patient’s anatomical changes –is essential. Key considerations to keep in mind listed on Table 3. ASM therapeutic drug monitoring (TDM) plays a critical role in the management of these patients, and ASM concentrations should be monitored frequently. We recommend that these concentrations should be checked weekly for the first 4 weeks, then monthly for the next 3 months if stable. These intervals need to be adjusted if problems with maintaining stable concentration are identified in an individual patient. For patients on stable ASM regimens, concentrations should be collected prior to surgery to determine the therapeutic concentration(s) that have been successful in controlling the seizures, and such concentrations can be used as a target post-operatively. Given the altered protein binding in patients with malabsorptive states, free concentrations of valproic acid and phenytoin should be monitored in addition to total concentrations [26], [27], [28]. If adequate concentrations cannot be obtained or there is concern for particularly poor absorption, intravenous medications can be given in the short term for those ASMs with a parenteral dosage formulation. Parenteral administration may temporarily mitigate some of the post-operative pharmacokinetic changes. Once stable, transition to oral medications can be accomplished with close TDM. After discharge from the hospital close follow-up with a neurologist and additional assistance from the pharmacist to monitor and adjust ASMs is always recommended.
Table 3.
Take home points.
| Take Home Points |
|---|
|
In our index case, the patient had a significantly altered GI tract due to her laparoscopic Roux-en-Y gastric bypass surgery. There are multiple factors that may have contributed to alterations in her serum ASM concentrations. She initially had therapeutic concentrations of phenytoin and valproic acid and her drug formulations were switched from sustained release to immediate release. Subsequently after the surgery she required frequent drug concentration assessment, and her doses of both ASM were increased to maintain therapeutic concentrations despite her weight and BMI being reduced. It also must be noted, enzyme interactions can also affect serum drug concentrations when phenytoin and valproic acid are used in combination. This combination also leads to alterations in protein binding, with valproic acid displacing plasma bound phenytoin [29], [30], [31]. Given the complexity of this drug combination in addition to the pharmacokinetic complexities of malabsorptive states, it is imperative to follow serum drug concentrations when using phenytoin and valproic acid in combination. The challenges of ASM management after bariatric surgery are outlined by our case report.
Conclusion
We describe issues associated with a patient undergoing bariatric surgery while on ASM. As the number of patients undergoing gastric bypass procedures increases, it is imperative to consider the pharmacokinetic changes in patients with post-surgical malabsorptive states. Pharmacokinetic changes should also be anticipated after non-bariatric surgery resulting in malabsorptive anatomy. This is especially important in patients undergoing surgeries involving gastrectomy or those leading to short bowel syndrome. Patients dependent on ASMs for the management of epilepsy present a particularly challenging situation given the narrow therapeutic index of many of these agents. Close attention to the pharmacokinetic properties of ASM and careful monitoring of serum drug concentrations are important in guiding optimal therapy for these patients.
CRediT authorship contribution statement
Caitlin S. Brown: Conceptualization, Methodology, Investigation, Writing - original draft. Alejandro A. Rabinstein: Conceptualization, Methodology, Writing - review & editing. Erin M. Nystrom: Methodology, Investigation, Writing - original draft. Jeffrey W. Britton: Investigation, Writing - review & editing. Tarun D. Singh: Conceptualization, Methodology, Investigation, Writing - original draft.
Declaration of Competing Interest
JWB is a consultant for UCB pharmaceuticals (investigational drug discussion, Rozanolixizumab) and a Co-investigator (unpaid) for: 1) GW Pharma, A double-blind, randomized, placebo-controlled study to investigate the efficacy and safety of cannabidiol (GWP42003-P, CBD) as add-on therapy in patients with tuberous sclerosis complex who experience inadequately-controlled seizures, and 2) Grifols Pharmaceuticals, A Randomized Double Blind Placebo Controlled Study of IVIG in Patients with Voltage Gated Potassium Channel Complex Antibody Associated Autoimmune Epilepsy.
References
- 1.Overweight & Obesity [Internet]. [cited 2019 Sep 16]. Available from: https://www.cdc.gov/obesity/data/adult.html
- 2.Estimate of bariatric surgery numbers 2011-2017 [Internet]. [cited 2019 Sep 16]. Available from: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- 3.Gibbs H., Broom J., Brown J., Reckless L., Noble P., Kumar S. Impact of obesity on drug prescribing in primary care. Br J Gen Pract. 2005;55(519):743–749. [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy A.L., Nelson T., Pettine S., Miller B.F., Hamilton K.L. Medication use following bariatric surgery: Factors associated with early discontinuation. Obes Surg. 2014;24(5):696–704. doi: 10.1007/s11695-013-1131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darwich A.S., Henderson K., Burgin A., Ward N., Whittam J., Ammori B.J. Trends in oral drug bioavailability following bariatric surgery: Examining the variable extent of impact on exposure of different drug classes. Br J Clin Pharmacol. 2012;74(5):774–787. doi: 10.1111/j.1365-2125.2012.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padwal R., Brocks D., Sharma A.M. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41–50. doi: 10.1111/j.1467-789X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 7.Azran C., Wolk O., Zur M., Fine-Shamir N., Shaked G., Czeiger D. Oral drug therapy following bariatric surgery: an overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016;17(11):1050–1066. doi: 10.1111/obr.12434. [DOI] [PubMed] [Google Scholar]
- 8.Brodie M.J., Mintzer S., Pack A.M., Gidal B.E., Vecht C.J., Schmidt D. Enzyme induction with antiepileptic drugs: Cause for concern? Epilepsia. 2013;54(1):11–27. doi: 10.1111/j.1528-1167.2012.03671.x. [DOI] [PubMed] [Google Scholar]
- 9.Pironi L., Sasdelli A.S., Pazzeschi C. Short bowel syndrome (SBS): classification, underlying causes, and global footprint. In: Corrigan M.L., Roberts K., Steiger E., editors. Adult Short Bowel Syndrome: Nutrition, Medical, and Surgical management. Elselvier Academic Press; London: 2018. pp. 17–25. [Google Scholar]
- 10.Mahawar K.K., Sharples A.J. Contribution of malabsorption to weight loss after Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2017;27(8):2194–2206. doi: 10.1007/s11695-017-2762-y. [DOI] [PubMed] [Google Scholar]
- 11.Andari Sawaya R, Jaffe J, Friedenberg L, K. Friedenberg F. Vitamin, Mineral, and Drug Absorption Following Bariatric Surgery. Curr Drug Metab. 2012;13(9):1345–55. [DOI] [PMC free article] [PubMed]
- 12.Le J. Drug Absorption [Internet]. Merck Manual. 2019 [cited 2020 Aug 13]. Available from: https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-absorption#:~:text=However%2C whether a drug is,Drug Absorption %3A Oral Administration).
- 13.Kararli T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 14.Evans D.F., Pye G., Bramley R., Clark A.G., Dyson T.J., Hardcastle J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliano C. Pharmacokinetic Changes Secondary to Roux en Y Gastric Bypass. Adv Pharmacoepidemiol Drug Saf. 2012;01(S1):1–6. [Google Scholar]
- 16.Brocks D.R., Ben-Eltriki M., Gabr R.Q., Padwal R.S. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505–1519. doi: 10.1517/17425255.2012.722757. [DOI] [PubMed] [Google Scholar]
- 17.De Smet J., Van Bocxlaer J., Boussery K. The influence of bypass procedures and other anatomical changes in the gastrointestinal tract on the oral bioavailability of drugs. J Clin Pharmacol. 2013;53(4):361–376. doi: 10.1002/jcph.65. [DOI] [PubMed] [Google Scholar]
- 18.Dressman J.B., Thelen K. Cytochrome P450-mediated metabolism in the human gut wall. J Pharm Pharmacol. 2009;61(5):541–558. doi: 10.1211/jpp/61.05.0002. [DOI] [PubMed] [Google Scholar]
- 19.Garg S.K., Kumar N., Bhargava V.K., Prabhakar S.K. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286–288. doi: 10.1016/S0009-9236(98)90177-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith A., Henriksen B., Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Heal Pharm. 2011;68(23):2241–2247. doi: 10.2146/ajhp100630. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt H.K., Greenblatt D.J. Altered drug disposition following bariatric surgery: A research challenge. Clin Pharmacokinet. 2015;54(6):573–579. doi: 10.1007/s40262-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 22.Yska J.P., van der Linde S., Tapper Véronique.V., Apers J.A., Emous M., Totté E.R. Influence of bariatric surgery on the use and pharmacokinetics of some major drug classes. Obes Surg. 2013;23(6):819–825. doi: 10.1007/s11695-013-0882-6. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy MC, Wade DN. Phenytoin absorption in patients with ileojejunal bypass. Br J Clin Pharmacol. 1979;7(5):515–518. doi: 10.1111/j.1365-2125.1979.tb00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pournaras D.J., Footitt D., Mahon D., Welbourn R. Reduced Phenytoin levels in an epileptic patient following Roux-En-Y gastric bypass for obesity. Obes Surg. 2011;21(5):684–685. doi: 10.1007/s11695-010-0107-1. [DOI] [PubMed] [Google Scholar]
- 25.Riker R.R., Gagnon D.J., Hatton C., May T., Seder D.B., Stokem K. Valproate protein binding is highly variable in ICU patients and not predicted by total serum concentrations: A case series and literature review. Pharmacotherapy. 2017;37(4):500–508. doi: 10.1002/phar.1912. [DOI] [PubMed] [Google Scholar]
- 26.Doré M., San Juan A.E., Frenette A.J., Williamson D. Clinical importance of monitoring unbound valproic acid concentration in patients with hypoalbuminemia. Pharmacotherapy. 2017;37(8):900–907. doi: 10.1002/phar.1965. [DOI] [PubMed] [Google Scholar]
- 27.Rg B., Jh L., Nolte J., Westwood A. Use of total and free anticonvulsant serum levels in clinical practice. Clin Exp Neurol. 1985;21:69–77. [PubMed] [Google Scholar]
- 28.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246–255. doi: 10.1111/j.1365-2125.2005.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodin E.A., DeSousa G., Haidukewych D., Lodhi R., Berchou R.C. Dissociation between free and bound phenytoin levels in presence of valproate sodium. Arch Neurol. 1981;38(4):240–242. doi: 10.1001/archneur.1981.00510040066011. [DOI] [PubMed] [Google Scholar]
- 30.Bruni J., Gallo J.M., Lee P.D.C.S., Perchalski R.J., Wilder B.J. Interactions of valproic acid with phenytoin. Neurology. 1980;30(11):1233–1236. doi: 10.1212/wnl.30.11.1233. [DOI] [PubMed] [Google Scholar]
- 31.Wishart D.S., Feunang Y.D., Marcu A., Guo A.C., Liang K., Vázquez-Fresno R. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novartis. Carbamazepine (tegretol) package insert. East Hanover, New Jersey; 2007.;
- 33.Owen A., Pirmohamed M., Tettey J.N., Morgan P., Chadwick D., Park B.K. Carbamazepine is not a substrate for P-glycoprotein. Br J Clin Pharmacol. 2000;51:345–349. doi: 10.1046/j.1365-2125.2001.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearney T.E. Carbamazepine and Oxcarbazepine. In: Olson K.R., editor. Poisoning & Drug Overdose. The McGraw-Hill Companies; New York, NY: 2012. [Google Scholar]
- 35.Retzow A., Schurer M., Schulz H. Influence of food on the bioavailability of a carbamazepine slow-release formulation. Int J Clin Pharmacol Ther. 1997;35(12):557–560. [PubMed] [Google Scholar]
- 36.Palmer K.J., McTavish D. Felbamate A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in epilepsy. Drugs. 1993;45(6):1041–1065. doi: 10.2165/00003495-199345060-00008. [DOI] [PubMed] [Google Scholar]
- 37.Somerset; NJ: 2011. Felbamate (felbatol) [package insert]. Somerset, NJ: Pharmaceuticals MEDA, 2011. [Google Scholar]
- 38.Mclean M.J. Clinical pharrnacokinetics of gabapentin. Neurology. 1994;44:17–22. [Google Scholar]
- 39.Stewart B., Kugler A., Thompson P., Bockbrader H. A saturable transport mechanism in intenstinal absorption of gabapentin is the underlying cause of lack of proportionality between increase dose and drug levels in plasma. Pharm Res. 1993;10:276–281. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- 40.Pfizer, Inc. Gabapentin (neurotin) package insert. New York, NY; 2009.;
- 41.Cawello W. Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin Pharmacokinet. 2015;54(9):901–914. doi: 10.1007/s40262-015-0276-0. [DOI] [PubMed] [Google Scholar]
- 42.Patsalos P.N., Berry D.J. Pharmacotherapy of the third-generation AEDs: Lacosamide, retigabine and eslicarbazepine acetate. Expert Opin Pharmacother. 2012;13(5):699–715. doi: 10.1517/14656566.2012.667803. [DOI] [PubMed] [Google Scholar]
- 43.Cawello W., Boekens H., Bonn R. Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: Mass balance following intravenous and oral administration. Eur J Drug Metab Pharmacokinet. 2012;37(4):241–248. doi: 10.1007/s13318-012-0093-x. [DOI] [PubMed] [Google Scholar]
- 44.Cawello W., Stockis A., Andreas J.-O., Dimova S. Advances in epilepsy treatment: Lacosamide pharmacokinetic profile. Ann N Y Acad Sci. 2014;1329(1):18–32. doi: 10.1111/nyas.12513. [DOI] [PubMed] [Google Scholar]
- 45.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–82. [DOI] [PMC free article] [PubMed]
- 46.Milosheska D., Lorber B., Vovk T., Kastelic M., Dolžan V., Grabnar I. Pharmacokinetics of lamotrigine and its metabolite N-2-glucuronide: Influence of polymorphism of UDP-glucuronosyltransferases and drug transporters. Br J Clin Pharmacol. 2016;82(2):399–411. doi: 10.1111/bcp.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garnett W.R. Lamotrigine: Pharmacokinetics. J Child Neurol. 1997;12(1_suppl):S10–S15. doi: 10.1177/0883073897012001041. [DOI] [PubMed] [Google Scholar]
- 48.Stockis A., Sargentini-Maier M.L., Otoul C., Connor A., Wilding I., Wray H. Assessment of levetiracetam bioavailability from targeted sites in the human intestine using remotely activated capsules and gamma scintigraphy: Open-label, single-dose, randomized, four-way crossover study in healthy male volunteers. Clin Ther. 2010;32(10):1813–1821. doi: 10.1016/j.clinthera.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 49.De Smedt T., Raedt R., Vonck K., Boon P. Levetiracetam: The profile of a novel anticonvulsant drug-Part I: preclinical data. CNS Drug Rev. 2007;13(1):43–56. doi: 10.1111/j.1527-3458.2007.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright C., Downing J., Mungall D., Khan O., Williams A., Fonkem E. Clinical pharmacology and pharmacokinetics of levetiracetam. Front Neurol. 2013;4 doi: 10.3389/fneur.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.May T.W., Korn-Merker E., Rambeck B. Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet. 2003;42(12):1023–1042. doi: 10.2165/00003088-200342120-00002. [DOI] [PubMed] [Google Scholar]
- 52.Flesch G. Overview of the clinical pharmacokinetics of oxcarbazepine. Clin Drug Investig. 2004;24(4):185–203. doi: 10.2165/00044011-200424040-00001. [DOI] [PubMed] [Google Scholar]
- 53.Redinger R.N., Small D.M. Primate biliary physiology. 8. The effect of phenobarbital upon bile salt synthesis and pool size, biliary lipid secretion, and bile composition. J Clin Invest. 1973;52(1):161–172. doi: 10.1172/JCI107160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patsalos PN, Berry DJ, Bourgeois BFD, Cloyd JC, Glauser TA, Johannessen SI, et al. Antiepileptic drugs - Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239–76. [DOI] [PubMed]
- 55.Melander A., Brante G., Johansson O., Lindberg T., Wahlin-Boll E. Influence of food on absorption of phenytoin in man. Eur J Clin Pharmacol. 1979;15:269–274. doi: 10.1007/BF00618516. [DOI] [PubMed] [Google Scholar]
- 56.Yeung S.CS.A., Ensom M.HH. Phenytoin and enteral feedings: Does evidence support an interaction? Ann Pharmacother. 2000;34(7-8):896–905. doi: 10.1345/aph.19355. [DOI] [PubMed] [Google Scholar]
- 57.Bourgeois B.F.D. Pharmacokinetics and metabolism of topiramate. Drugs of Today. 1999;35(1):43–48. doi: 10.1358/dot.1999.35.1.522947. [DOI] [PubMed] [Google Scholar]
- 58.Perucca E. A pharmacological and clinical review on Topiramate, a new antiepileptic drug. Pharmacol Res. 1997;35(4):241–256. doi: 10.1006/phrs.1997.0124. [DOI] [PubMed] [Google Scholar]
- 59.Single-Dose Pharmacokinetics and Effect of Food on the Bioavailability of Topiramate, A Novel Antiepileptic Drug. J Clin Pharmacol 1996;36(10):884–91. [DOI] [PubMed]
- 60.Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, et al. Valproic acid pathway. Pharmacogenet Genomics. 2013;23(4):236–41. [DOI] [PMC free article] [PubMed]
- 61.Depakore ER (divalproex sodium extended release tablets) [package insert]. North Chicago, IL: Abbott Laboratories. 2003 [Google Scholar]
- 62.Malik M.Y., Jaiswal S., Sharma A., Shukla M., Lal J. Role of enterohepatic recirculation in drug disposition: cooperation and complications. Drug Metab Rev. 2016;48(2):281–327. doi: 10.3109/03602532.2016.1157600. [DOI] [PubMed] [Google Scholar]
- 63.Hamilton R.A., Garnett W.R., Kline B.J., Pellock J.M. Effects of food on valrpoic acid absorption. Am J Hosp Pharm. 1981;38(10):1490–1493. [PubMed] [Google Scholar]