Abstract
Epilepsy has garnered increased public health focus because patients who suffer from epilepsy experience pronounced and persistent health and socioeconomic disparities despite treatment and care advances. The epidemiology of epilepsy is diverse in different countries and regions. This nationwide population-based cross-sectional study was conducted to determine the life time prevalence and health related factors of epilepsy for the first time in Iran through a two-phase door-to-door survey method. In phase I, a screening for epilepsy was performed on 68,035 people. Then in phase II, after the neurological evaluation of participants and reviewing medical records, 1130 subjects with epilepsy was confirmed. The life time prevalence of epilepsy was achieved to be 16.6 per 1000 people (95% CI 15.4–17.8) with the average age onset 19.1 ± 21.1 (active prevalence 9.5 per 1000 people). Focal seizure (59.3%), generalized epilepsy (38%) and unknown types of epilepsy (2.7%) were detected among participants. The overall life time prevalence of febrile convulsion was 4.1 per 1000 people. The frequency of attacks per year and per month were 3.0 ± 1.6 and 0.5 ± 0.1, respectively. Age-specific life time prevalence was highest among the age group of 15–19 years old [32.7 per 1000 persons (95% CI 29.1–36.8)] and it was higher in male (53.8%) than female (46.2%) participants. Our results showed that the life time prevalence of epilepsy in Iran is higher than worldwide average.
Subject terms: Medical research, Neurology
Introduction
Epilepsy has garnered increased public health focus because patients who suffer from epilepsy experience pronounced and persistent health and socioeconomic disparities despite treatment and care advances. The epidemiology of epilepsy is diverse in different countries and regions1. The lifetime prevalence of epilepsy varies between 3.5 and 10.7 per 1000 persons in developed countries, and from 0.9 to 74.4 per 1000 persons in Asia, sub-Saharan Africa, and Latin America2,3. Furthermore, epilepsy life time prevalence is higher in rural areas than urban centers4. Vast numbers of risk factors besides miscellaneous methodology are partially at fault of above-mentioned differences in epidemiology of epilepsy in previous researches. In addition, diagnosis of epilepsy is highly dependent on the patient history and in the lack of a precise route, differences in the criteria that were utilized in the surveys intensify the differences in the epidemiological findings.
The epidemiological and clinical features of the disorder are diverse in different races and ethnicities. Although there are various reports on prevalence of epilepsy in different regions, large nationwide survey in the epidemiology of epilepsy has not conducted in Iran. Iran is one of the most influential middle-east-located country in its region with the income level 3 since 1955, so far and little has been discovered about the epidemiological and clinical features of epilepsy in Iranian population. In the light of previous sparse-population studies that were proposed in Iran, life time prevalence of epilepsy was estimated to be circa 50 per 1000 people in a meta-analysis5.
The present study is the first nationwide study which provided updated national and modeled state-specific numbers of active epilepsy cases. Moreover, this survey was proposed in order to clarify and determine the life time prevalence of epilepsy among both sexes, besides the most common risk factors, etiologies, the mean age of onset of epilepsy, the pharmacotherapy approach of Iranian neurologists and the average expenditure paid off by the patients.
Public health practitioners, health care providers, policy makers, epilepsy researchers, and other epilepsy stakeholders, including family members and people with epilepsy, can use these findings to ensure that evidence-based programs meet the complex needs of adults and children with epilepsy and reduce the disparities resulting from it.
Results
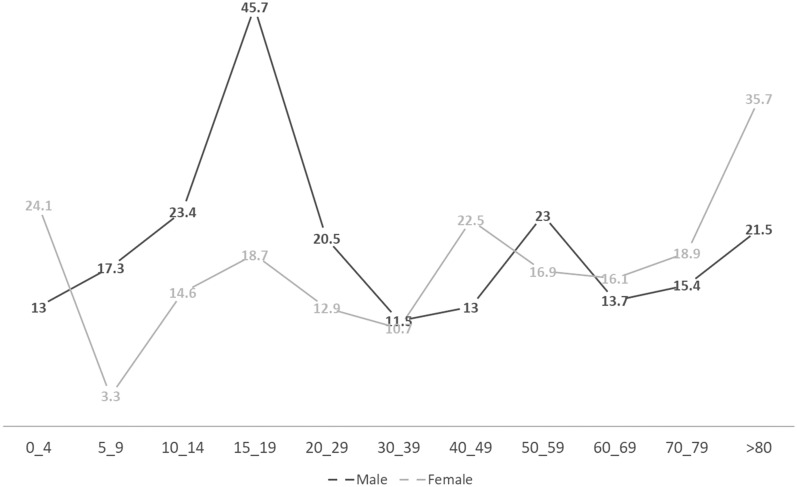
Finally, 68,035 residents of considered areas of the present study were screened in the first phase of the study (Table 1). The mean age of participants was 36.2 ± 19.8 (mean ± SD). The mean years of education was 8.4 ± 7.6 and 50.4% of participants were male. Moreover, 80.2% of persons were from urban areas. Overall, 5.8% of the individuals were positive responders (who answered ‘yes’ to at least one of the epilepsy-related questions of the questionnaire). Our analysis revealed a remarkable correlation between the age and sex of the positive responders as compared with the age (p < 0.0001) and sex (p = 0.038) of the negative responders (who answered ‘no’ to all of the epilepsy-related questions) (Table 2 and Fig. 1).
Table 1.
Demographics of the participants.
| Gender N (%) | |
| Male (34,290) | 50.4% |
| Female (33,745) | 49.6% |
| Age (year) | |
| Mean (SD) | 36.2 ± 19.8 |
| Urban residents (54,564) | 80.2% |
| Rural residents (13,471) | 19.8% |
| Mean years of education (SD) | 8.4 ± 7.6 |
Table 2.
Correlations between sex and age of participants on the life time prevalence.
| Age groups (year) | Male (per 1000 persons) | Female (per 1000 persons) | Total (per 1000 persons) |
|---|---|---|---|
| 0–4 | 13.0 | 24.1 | 18.8 |
| 5–9 | 17.3 | 3.3 | 10.8 |
| 10–14 | 23.4 | 14.6 | 19.2 |
| 15–19 | 45.7 | 18.7 | 32.7 |
| 20–29 | 20.5 | 12.9 | 16.6 |
| 30–39 | 11.5 | 10.7 | 11.1 |
| 40–49 | 13.0 | 22.5 | 17.8 |
| 50–59 | 23.0 | 16.9 | 19.8 |
| 60–69 | 13.7 | 16.1 | 14.9 |
| 70–79 | 15.4 | 18.9 | 17.0 |
| ≥ 80 | 21.5 | 35.7 | 27.1 |
| Life-time prevalence | 17.8 (16.0–19.5 CI 95%) | 15.5 (13.8–17.2 CI 95%) | 16.6 (15.4–17.8 CI 95%) |
Figure 1.
The life time prevalence of epilepsy in age and sex groups.
At second phase positive screened subjects were reviewed by expert neurologists and 1130 patients was confirmed as epileptic according to inclusion criteria in which 608 (53.8%) were male and 522 (46.2%) were female. Accordingly, the life time prevalence of epilepsy was 16.6 per 1000 people (95% CI 15.4–17.8) in Iran and active prevalence was 57.4% (9.5 per 1000 people). In male participants the life time prevalence was 17.8 per 1000 people (95% CI 16.0–19.5). It was calculated 15.5 per 1000 persons (95% CI 13.8–17.2) in female group. There was no difference between male and female in prevalence. The average age of epilepsy onset was 19.1 ± 21.1. The frequency of attack per year and per month were 3.0 ± 1.6 and 0.5 ± 0.1, respectively.
About 67% of patients were symptom free since the last year in which 23% of them was in remission for at least two years. It was revealed that 18.8% of patients suffered from refractory epilepsy (i.e., more than 2 seizures per year). In the last year, 14.2% of patients had one attack. Moreover, we found that 5.8% patients had more than 1 attacks per month.
In addition to that, our data demonstrated that most of the patients (59.3%) suffered from focal epilepsy, in which 42.3% of them complicated to generalized seizure. About 28.3% and 21.7% of patients who suffered from partial seizure, declared that their attacks were manifested by staring and aura, respectively. Generalized epilepsy was found in 38% patients. In approximately 2.7% of patients were of unknown types of epilepsy. The overall life time prevalence of febrile convulsion was 4.1 per 1000 people (4.8 per 1000 in male and 3.4 per 1000 in female patients) (Table 3).
Table 3.
Types of epilepsy.
| Types of epilepsy | Total N(%; CI 95%) | Male (CI 95%) | Female (CI 95%) |
|---|---|---|---|
| Generalized epilepsy | 429 (38%; 36.0–40.0) | 37.9% (35.3–40.7) | 38% (35.0–41.0) |
| Focal epilepsy | 670 (59.3%; 57.3–61.3) | 59% (56.2–61.8) | 59.6% (56.6–62.6) |
| Unknown | 31 (2.7%; 2.0–3.4) | 3.1% (2.2–4.0) | 2.4% (1.4–3.4) |
Age-specific life time prevalence was highest among the age group of 15–19 years old [32.7 per 1000 persons (95% CI 29.1–36.8)] and it was remarkably higher in male participants [45.7 per 1000 persons (95% CI 39.6–50.0)] than female participants (18.7 per 1000 persons (95% CI 17.0–19.7)). In view of aetiology, we determined an underlying aetiology in 50.6% of patients which could be categorised in of the following aetiological groups including trauma (21.9%), stroke (7.6%), infectious diseases (5.7%), brain tumours (5.3%) and others (10.1%). Therefore, trauma including head trauma and neurosurgical complications was determined as the most potential cause.
Circa half of the patients (51.8%) declared that they received antiepileptic drugs (AEDs). In further details, 61.9% of them were on single-drug pharmacotherapy while 38% of the patients were on multiple-drug pharmacotherapy (11.9% of them received more than 3 AEDs) (Table 4).
Table 4.
Epilepsy Pharmacotherapeutics in Iran.
| Received Antiepileptic medication | (585) 51.8% | Single-drug pharmacotherapy | (362) 61.9% | Valproate sodium | 13.6 | 22 |
| Phenytoin | 11.7 | 19 | ||||
| Carbamazepine | 10.5 | 17 | ||||
| Levetiracetam | 9.9 | 16 | ||||
| Lamotrigine | 6.8 | 11 | ||||
| Phenobarbital | 4.3 | 7 | ||||
| Others | 4.9 | 8 | ||||
| Total | 61.9% | 100 | ||||
| Multiple-drug pharmacotherapy | (223) 38.0% | Phenytoin + Phenobarbital | 7.6 | 20 | ||
| Valproate sodium + Carbamazepine | 6.4 | 17 | ||||
| Valproate sodium + Levetiracetam | 6.4 | 17 | ||||
| Others | 14.8 | 39 | ||||
| Total | 38.0% | 100 | ||||
| Not Received Antiepileptic medication | (545) 48.2% |
From the socio-economical aspect, our data disclosed that the out-of-pocket expenses of physician office visit/ ward round and medication were $76 per month (circa $0.71–$284.69 monthly).
Discussion
This nationwide illustrative, population-based survey depicted that the prevalence of epilepsy in Iran in the present study population was 16.6 per 1000 people. The life time prevalence of epilepsy in this study was remarkably lower than other small conducted similar studies in Iran due to the large representative sample size that was used in the present study. Furthermore, our results concluded that the life time prevalence of epilepsy in Iran is superior to that compared with global life time prevalence of epilepsy in 20166, probably due to the high prevalence of traumatic disasters especially road traffic injuries, occupational and the Iran-Iraq war injuries7. In addition, the life time prevalence of epilepsy in Iran was higher than developed countries including USA, Canada, Japan, France, Germany and Israel6. Moreover, in order to more accurate interpret our findings, the author have a tendency to compare our results with other nationwide studies (Table 5). Certainly, our neighbourhood countries particularly Turkey, Afghanistan and Iraq were exemplified to comparison due to our similarities in culture, healthcare system and socioeconomic states. However, lack of nationwide studies in neighbours and totally Middle East made it unachievable. Despite that, the increment in the rate of urbanization and westernization in Iran in the last 90 years, probably enhanced the comparison of Iran status with other western countries. As it was discussed previously, Beghi and colleagues estimated the global prevalence of epilepsy 0.6215% in 20166. Their study mainly focused on idiopathic and conditions secondary to infectious diseases including meningitis, tetanus, malaria, cysticercosis6. Despite their finding, probably due to improvement in the control of infectious diseases in Iran8, our results did not conclude to a remarkable participation of these mechanisms in the life time prevalence of epilepsy in Iran. Nevertheless, the active prevalence of idiopathic epilepsy in Iran may be higher than the global, 2.7% against 0.038%, respectively6. As it was achieved by the present study, the prevalence of all active epilepsy (both idiopathic and secondary) was 9.5 persons per 1000 which is higher than the global prevalence of all active epilepsy in 2016 (6.215 persons per 1000)6. The prevalence of active epilepsy is highly variable between countries due to local distribution of risk factors. This point is exemplified by the paper of Beghi9, in which the prevalence of active epilepsy was compared between low/middle- and high-income countries. He concluded that the prevalence of active epilepsy in the low/middle-income countries was higher than high-income countries (8.75 vs. 5.18 persons per 1000, respectively). Therefore, socioeconomic risk factors may be the major determinant of the prevalence of active epilepsy. This point is clearly evident by Ngugi and colleagues’ paper4. They found not only the higher prevalence of active epilepsy in developing countries than developed countries but also higher prevalence in the rural areas of the developing countries as compared with the urban areas of the same countries.
Table 5.
A comparison of epilepsy life time prevalence in nationwide scale in other countries/regions.
| Study | Study year | Population size | Country/Region | Life-time prevalence (%) | Epilepsy types | Aetiologies |
|---|---|---|---|---|---|---|
| Beghi et al.6 | 2016 | 27,737,043 | Global | 0.6215 | Idiopathic, Secondary | Meningitis, tetanus, malaria, cysticercosis, cystic echinococcosis, preterm birth complications, neonatal encephalopathy, neonatal sepsis, and neonatal haemolytic disease |
| Zack et al.29 | 2015 | 3,470,000 | USA | 1.2 | Active | Not determined |
| Hamer et al.30 | 2009 | 634,566 | Germany | 0.91 | Prevalence of patients receiving antiepileptic medication | Not determined |
| Serrano-Castro31 | 2012 | 1741 | Spain | 1.487 | Partial seizure with/without secondary generalization, generalized tonic–clonic seizures, myoclonic seizures, idiopathic epilepsy, cryptogenic epilepsy | Not determined |
| Giussani et al.32 | 2011 | 912,458 | Italy | 0.79 | Not determined | Not determined |
| Keränen et al.33 | 1989 | 2080 | Eastern Finland | 0.63 | Active epilepsy, secondary to organic causes, generalized seizure, partial seizure, unclassified seizure | Not clarified |
| Joensen34 | 1986 | 43,609 | Faroes, Denmark | 0.78 | Generalized epilepsy including primary (grand mal, petit mal, juvenile myoclonus) and secondary (west syndrome, Lennox-Gastaut syndrome), partial seizure | Not determined |
| Olafsson et al.35 | 1999 | 428 | Rural Iceland | 0.48 | Partial seizure (simple partial, complex partial, partial secondarily generalized), primary generalized seizure (absence, myoclonic with or without other types, major motor seizure alone), other major motor seizures without aura, not classified | Idiopathic, remote symptomatic (cerebrovascular disease, MR/CP, infections, trauma), progressive symptomatic (primary and metastatic neoplasms), degenerative diseases (dementia) |
| Forsgren36 | 1992 | 713 | Northern Sweden | 0.55 | Partial seizure (simple, complex, secondarily generalized), generalized seizure (tonic–clonic, myoclonic, absence, other), unclassifiable | Ischemic and haemorrhagic cerebrovascular disorders, trauma, tumour, infections, pre/perinatal asphyxia, prematurity, chromosomal aberration (Down syndrome, fragile X, (46XX, 13q +)), Rett syndrome, idiopathic |
| Onal et al.37 | 1999 | 2187 | Rural areas of Istanbul, Turkey | 0.8 | Partial, generalized, unclassifiable | Not determined |
| Aziz et al.38 | 1997 | 24,130 | Pakistan | 0.99 | Generalized tonic–clonic, simple partial, complex partial, generalized, absence, tonic and atonic, myoclonic | Idiopathic, past history of meningitis, encephalitis, neonatal jaundice, neonatal convulsions, hypertension, ischemic heart disease |
| Radhakrishnan et al.39 | 2000 | 238,102 | Kerala, South India | 0.49 | Generalized, other | Not determined |
| Al Rajeh et al.40 | 2001 | 23,700 | Saudi Arabia | 0.654 | Partial, generalized | Pre/perinatal encephalopathy, head injury, childhood neurological infection, stroke, febrile |
| Li et al.41 | 1983 | 63,195 | China | 0.44 | Generalized nonconvulsive (akinetic, atonic), Generalized convulsive (grand mal), Partial epilepsy (with or without impairment consciousness, Multiple types | Brain injury, intracranial infection, and cerebrovascular disease |
| Guekht et al.42 | 2010 | 517,624 | Russia | 0.34 | Generalized (Myoclonic,Atonic, Absence, Tonic, Tonic—clonic) Partial seizures (simple, Complex, Partial (simple and/ or complex) evolving to generalized) | Head injuries, cerebrovascular diseases, CNS infection, Pre/perinatal disorders, neurodegenerative disorders, tumours, unknown |
| Osuntokun et al.43 | 1982 | 18,954 | Igbo-Ora, Nigeria | 0.5 | Generalized (Tonic–clonic, Petit mal,Grand mal, Partial (Simple, Complex),Unclassified | Not determined |
| Tekle-Haimanot et al.44 | 1986–1988 | 60,820 | Meskan and Mareko, Ethiopia | 0.52 | Generalized tonic–clonic seizures, Partial, absence, unclassified | Not determined |
| Rwiza et al.45 | 1989 | 18,000 | Ulanga, Tanzania | 1.02 | Partial (Simple, Complex, secondarily generalized), generalized (Absence, Tonic–clonic, Myoclonic, Tonic, Atonic), Unclassifiable | Idiopathic, Febrile convulsion, Unspecified encephalitis, Birth trauma, Cerebral malaria, Meningitis, Head trauma, Cerebrovascular disease, Suspected tumour |
| Birbeck et al.46 | 2000–2001 | 799 | 1.45 | Not determined | Not determined |
From the aspect of age and sex as risk factors that influence the life-time prevalence of epilepsy, we found that the life time prevalence of epilepsy was highest in the young who aged between 15 and 19 years old. Our finding is not exactly in harmony with previous studies. In other words, the life time prevalence of epilepsy was found to be lowest in the early life; nonetheless, we concluded that the life time prevalence of the epilepsy is lowest in the third decade of the life1. In addition to that, as it is depicted by our results, there was an increment trend toward the prevalence of epilepsy from the fourth decade to the sixth decade, then the life time prevalence of epilepsy reduced in the sixth decade of the life. Henceforth, the life time prevalence of epilepsy increased from the age 70 until the end of life. Despite our finding, similar studies in Europe and other industrialised countries revealed a decrement in the life time prevalence of epilepsy in 3rd decade of life and a plateau state thereafter1,10.
Despite the above-mentioned findings in Europe, Weatherburn and colleagues disclosed an increment in the prevalence of epilepsy with age increment in Scotland in the population of older than 14 years old11. Nevertheless, we did not conclude to a remarkable difference among the children, younger, middle and elder adults, as it was reported a twofold higher life time prevalence of epilepsy in children and younger adults as compared with middle aged and elder adult in the middle east12. In this study, the mean age of onset of seizures was 19.1 years old which is in approximately in harmony with previous studies13.
This study is the first study in Iran that accurately assessed the life time prevalence in the age groups. Previous studies usually classified the prevalence of epilepsy by age as upper and lower 20 years old14. The main deficit of previous literature was that they did not provide a detailed life time prevalence estimation classified by age and sex, probably due to their limitation of sources and the size of their sample15–18. Hence, above-mentioned items were considered in the design of present study. In fact, there is a controversy that sex can influence the prevalence of epilepsy19. In the present study, it was revealed that epilepsy is more prevalent among male rather than female. This finding is in concert with previous literature1,20. Interestingly, the life time prevalence of epilepsy was remarkably higher in boys aged between 5 and 9 years than same-aged girls. In addition, men aged 15 to 19 year were more susceptible to epilepsy. However, women aged more than 80 years old showed a higher tendency toward epilepsy than other life period.
As it was not evident by previous literature, this study is distinguished from previous studies in providing of above-mentioned information. As compared with similar studies in the middle east, we also found that epilepsy life time prevalence is overall higher in male than female patients12. This finding may be attributable to the specific social and cultural atmosphere of Middle East, in which women encourage to conceal their diseases in order not to become isolated from the society and to improve their chance of marriage19,20.
In the present study also, we endeavoured to detect the prevalence of epilepsy as considered by types. Most of the Iranian patients in this study were diagnosed to suffer from focal epilepsy. This is interesting when generalized epilepsy is more prevalent in Middle East as it was evident by previous regional-wide studies13. The classification of epilepsy is highly dependent on the complex medical technology, and it may be the cause of differences among different studies.
This study is the first study in Iran that determined the exact pharmacotherapeutics received by patients. Most of the patients that were assessed in this study took a single drug monotherapy as it was revealed by previous studies in the middle east13. Previously, carbamazepine was determined to be the most common-prescribed AEDs, nonetheless, in this study we found that sodium valproate nowadays is the most administered drug. On the one hand, we determined that most of the Iranian patients suffered from focal epilepsy, but on the other, sodium valproate which is preferable drug for generalized seizure was the most common-used drug. As it was disclosed by previous studies, carbamazepine is highly effective for focal seizure therapy21,22.
Aetiologically, we found that trauma exemplified as head trauma and complications of neurological surgery was the most common underlying cause, however, previously, fever, tonic-colonic convulsions and epilepsy were found to be the predominant causes of epilepsy5. Our study was highly successful to determine an aetiology in circa half of the participants, while previous studies which assessed all age groups, concluded to a clear aetiology in 14% to 39% of cases23. To our knowledge, this study is the first study attempted to clarify the out-of-pocket expenses of patients with epilepsy in Iran. It is evident that epilepsy is more prevalent among low-income people24.
Our nationwide illustrative, population-based survey revealed that the life time prevalence of epilepsy in Iran was lower than other small conducted similar small studies in Iran. However, our results showed that the life time prevalence of epilepsy in Iran is higher than average worldwide prevalence of epilepsy. We hope that further investigations would be run to determine more precisely the effect of socio-economic status of patients with epilepsy on their prognosis and disease procedure in Iran.
Methods and materials
Study design
This study was designed as a population-based cross-sectional study. It was conducted from 2018 to 2021 in the both urban and rural regions of all provinces of Iran. The country's population according to last statistics provided by the United Nation data is 83,992,949 in 2020, of which 71.3% live in urban areas and 28.7% in rural areas. Iran is 1 648 195 km2 and consists of 31 provinces. There are more than 7 ethnic groups which approximately all are Caucasian-white.
Sample size
In Previous study that has been conducted in Tehran, epilepsy life time prevalence estimated 10 per 1000 people25. Twenty-five thousand families were selected from 21,049,934 families in country through cluster sampling. In other words, there was 500 clusters of families consisted of 357 urban clusters and 143 rural clusters.
Diagnostic criteria
According to the guidelines for epidemiologic studies on epilepsy26, epilepsy was defined as the condition characterized by recurrent (two or more) epileptic seizures, unprovoked by any immediate identified cause. Multiple seizures occurring in a 24-h period were considered a single event. Single epileptic seizures, and epileptic seizures with an obvious precipitant were excluded. Life time prevalence was a diagnosis of epilepsy (recurrent unprovoked seizures) at some point prior to the prevalence period or date. A prevalent case of active epilepsy was defined as a person with epilepsy who has had at least one seizure in the previous 5 years, regardless of antiepileptic drug treatment.
Seizures were classified in accordance with the international classification of epileptic seizures of ILAE27, based on the clinical history, age at onset, seizure patterns, evolution of the disease, clinical EEG and neuroradiologic examinations. A seizure was considered focal seizure on the basis of clinical evidence of focal onset, regardless of whether it was secondarily generalized. Remission referred to when epilepsy patients who have never been treated with any antiepileptic drugs were free of seizures for two years or more. Treatment gap was the number of people with active epilepsy not on adequate treatment, expressed as a percentage of total number with active epilepsy.
Related aetiology was accepted while there was strong association between cause and effect i.e., hospital recorded documents, localization (imaging and EEG) and/or time relevancy. Car accidents and war victims were well documented subjects in the head trauma category. If such a strong association was not found, the suspected risk factor was ignored. This strategy was followed by each etiological factor including trauma, stroke, infection, brain tumors and others. Self-report alone was not considered as sufficient criteria. Final decision for each case was made by an expert committee.
Steps of the study
The present study used a two-phase door-to-door survey method. In phase I, a screening instrument for epilepsy was administered to subjects who agreed to participate; in phase II, the neurological evaluation of possible epilepsy was performed by expert neurologists among those subjects who screened positive for that condition in phase I.
Phase I: screening
The field workers composed by local health staffs in the survey were given a standard training. The validated screening questionnaire by Placencia et al.28 was used to detect possible cases of epilepsy. The health workers who had been trained and could understand the screening questionnaires translated them into ethnic languages and dialects, if the people surveyed could not understand the screening questionnaires written in Persian. All staffs and interviews were trained and examined preceding the initiation of the study in order to ensure their qualifications required for the current study. The screening procedure was conducted during interview so that investigators had to verify that all subjects understood the questions asked in the questionnaire. Each interview was conducted by a native health staff due to the ethnic languages and dialects. Each adult resident in the house was interviewed. The head of the household, usually the husband, and his wife provided information about each child with age less than 14 years old. If necessary, the patients and their families were required to give a detailed demonstration of the seizure. In case of seizure history of family member(s), details were taken from the cases and also from a reliable eyewitness of the ictal event. The screening was completed when all subjects in a certain area were investigated.
Phase II: Diagnosis and confirmation
Individuals whose responses to the questionnaire suggested they might have epilepsy were then scrutinized by a neurologist. Expert neurologists clinically examined these subjects at their residences, and reviewed relevant investigation records if available. The date of onset of seizure was ascertained as accurately as possible. On the basis of these observations, neurologists made the diagnosis of epilepsy and of other forms of seizure disorder and also identified and excluded the false positives. Most of patients with epilepsy were diagnosed through clinical evaluation and reviewing medical records. Experts from department of neurology together discussed the patients with unascertained diagnosis. As for the case of a disagreement among senior neurologists, the clinical, EEG and imaging data of the patients were discussed together to reach a consensus.
Statistical analysis
Data were analysed through SPSS 18 (Chicago, IL). Chi-square and odd ratio were calculated in order to determine the correlation between epilepsy and demographic factors including sex and age.
Ethics approval
The study was approved by the ethical committee of the Shahid Beheshti University of Medical Sciences (IRB code 1395.479). All participants, patients and interviewers gave written informed consent. Informed consent was given from the parents or legal guardians of participants who were under the codified age. All procedures performed in the present study were in accordance with the ethical standards of the institutional and national research committee of the Shahid Beheshti University of Medical Sciences and the 1964 Helsinki declaration and its further updates.
Author contributions
H.P., A.A.H., K.G. and F.A. participated in the study design, neurologic assessments of participants and supervised the study progression. F.A. also analysed the data. A.A.H. also edited and reviewed the manuscript. A.E., S.H.M., T.D., P.K., P.B. and H.K. contributed in data collection and evaluation of the participants.
Funding
Funding was provided by Shahid Beheshti University of Medical Science (Grant No. 2335_8913).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon C-S, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radhakrishnan K. Challenges in the management of epilepsy in resource-poor countries. Nat. Rev. Neurol. 2009;5(6):323–330. doi: 10.1038/nrneurol.2009.53. [DOI] [PubMed] [Google Scholar]
- 3.Ngugi AK, Bottomley C, Kleinschmidt I, Wagner RG, Kakooza-Mwesige A, Ae-Ngibise K, Owusu-Agyei S, Masanja H, Kamuyu G, Odhiambo R, Chengo E, Sander JW, Newton CR, group S Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12(3):253–263. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia. 2010;51(5):883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayehmiri K, Tavan H, Sayehmiri F, Mohammadi I, Carson KV. Prevalence of epilepsy in Iran: A meta-analysis and systematic review. Iran J. Child Neurol. 2014;8(4):9–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Beghi E, Giussani G, Nichols E, Abd-Allah F, Abdela J, Abdelalim A, Abraha HN, Adib MG, Agrawal S, Alahdab F, Awasthi A, Ayele Y, Barboza MA, Belachew AB, Biadgo B, Bijani A, Bitew H, Carvalho F, Chaiah Y, Daryani A, Do HP, Dubey M, Endries AYY, Eskandarieh S, Faro A, Farzadfar F, Fereshtehnejad S-M, Fernandes E, Fijabi DO, Filip I, Fischer F, Gebre AK, Tsadik AG, Gebremichael TG, Gezae KE, Ghasemi-Kasman M, Weldegwergs KG, Degefa MG, Gnedovskaya EV, Hagos TB, Haj-Mirzaian A, Haj-Mirzaian A, Hassen HY, Hay SI, Jakovljevic M, Kasaeian A, Kassa TD, Khader YS, Khalil I, Khan EA, Khubchandani J, Kisa A, Krohn KJ, Kulkarni C, Nirayo YL, Mackay MT, Majdan M, Majeed A, Manhertz T, Mehndiratta MM, Mekonen T, Meles HG, Mengistu G, Mohammed S, Naghavi M, Mokdad AH, Mustafa G, Irvani SSN, Nguyen LH, Nixon MR, Ogbo FA, Olagunju AT, Olagunju TO, Owolabi MO, Phillips MR, Pinilla-Monsalve GD, Qorbani M, Radfar A, Rafay A, Rahimi-Movaghar V, Reinig N, Sachdev PS, Safari H, Safari S, Safiri S, Sahraian MA, Samy AM, Sarvi S, Sawhney M, Shaikh MA, Sharif M, Singh G, Smith M, Szoeke CEI, Tabarés-Seisdedos R, Temsah M-H, Temsah O, Tortajada-Girbés M, Tran BX, Tsegay AAT, Ullah I, Venketasubramanian N, Westerman R, Winkler AS, Yimer EM, Yonemoto N, Feigin VL, Vos T, Murray CJL. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):357–375. doi: 10.1016/S1474-4422(18)30454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahadorimonfared A, Soori H, Mehrabi Y, Delpisheh A, Esmaili A, Salehi M, Bakhtiyari M. Trends of fatal road traffic injuries in Iran (2004–2011) PLoS ONE. 2013;8(5):e65198. doi: 10.1371/journal.pone.0065198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzaei H, Abdi Z, Ahmadnezhad E, Gohrimehr M, Abdalmaleki E, Alvandi R, Harirchi I. Health status in the Islamic Republic of Iran, Middle East and North Africa countries: Implications for global health. Iran J. Public Health. 2020;49(1):86–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(2):185–191. doi: 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- 10.Forsgren L, Beghi E, Oun A, Sillanpää M. The epidemiology of epilepsy in Europe—A systematic review. Eur. J. Neurol. 2005;12(4):245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 11.Weatherburn CJ, Heath CA, Mercer SW, Guthrie B. Physical and mental health comorbidities of epilepsy: Population-based cross-sectional analysis of 1.5 million people in Scotland. Seizure. 2017;45:125–131. doi: 10.1016/j.seizure.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Benamer HT, Grosset DG. A systematic review of the epidemiology of epilepsy in Arab countries. Epilepsia. 2009;50(10):2301–2304. doi: 10.1111/j.1528-1167.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- 13.Baker GA, Jacoby A, Gorry J, Doughty J, Ellina V. Quality of life of people with epilepsy in Iran, the Gulf, and Near East. Epilepsia. 2005;46(1):132–140. doi: 10.1111/j.0013-9580.2005.20704.x. [DOI] [PubMed] [Google Scholar]
- 14.Etemadifar M, Mirabdolbaghe P. Demographic and clinical characteristics of young epilepsy mortalities in Isfahan. Two Q. South Pediatr. Persian Golf Center Health Res. Boushehr Univ. Med. Sci. 2005;2:160–164. [Google Scholar]
- 15.Rezaei A, Sh S. Survey of starting age and gender of epilepsy and effective parameters on the Sina and Ghaem hospitals patients at 1989 till 1995. Rehabil. Mag. 2000;2:52–57. [Google Scholar]
- 16.Kaheni S, Riyasi H, Rezvani Kharashad M, Sharifzadeh Gh NS. Prevalence of epilepsy in children at primary schools and awareness of teachers about epilepsy at primary schools of Birjand at 2010. Novel Cares Q. Sci. J. Nurs. Midwifery Birjand Univ. Med. Sci. 2011;3:135–142. [Google Scholar]
- 17.Nasehi M, Mahvalati Shamsabadi F, Ghofrani M. Associated factors in response to treatment in children with refractory epilepsy. J. Babol Univ. Med. Sci. 2010;12(4):61–66. [Google Scholar]
- 18.Mohammadi MR, Ghanizadeh A, Davidian H, Mohammadi M, Norouzian M. Prevalence of epilepsy and comorbidity of psychiatric disorders in Iran. Seizure. 2006;15(7):476–482. doi: 10.1016/j.seizure.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. 2009;85(1):31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of epilepsy in the Parsi community of Bombay. Epilepsia. 1988;29(2):111–115. doi: 10.1111/j.1528-1157.1988.tb04405.x. [DOI] [PubMed] [Google Scholar]
- 21.Marson AG, Williamson PR, Clough H, Hutton JL, Chadwick DW, Group OBOTEMT Carbamazepine versus valproate monotherapy for epilepsy: A meta-analysis. Epilepsia. 2002;43(5):505–513. doi: 10.1046/j.1528-1157.2002.20801.x. [DOI] [PubMed] [Google Scholar]
- 22.Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. N. Engl. J. Med. 1992;327(11):765–771. doi: 10.1056/nejm199209103271104. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy—A review. Epilepsy Res. 2009;85(1):31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurman DJ, Kobau R, Luo YH, Helmers SL, Zack MM. Health-care access among adults with epilepsy: The U.S. National Health Interview Survey, 2010 and 2013. Epilepsy Behav. E&B. 2016;55:184–188. doi: 10.1016/j.yebeh.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noorbala AA, Bagheri Yazdi SA, Yasamy MT, Mohammad K. Mental health survey of the adult population in Iran. Br. J. Psychiatry J. Ment. Sci. 2004;184:70–73. doi: 10.1192/bjp.184.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy (1993). Epilepsia 34(4):592–596. 10.1111/j.1528-1157.1993.tb00433.x [DOI] [PubMed]
- 27.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshé SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang Y-H, Zuberi SM. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain J. Neurol. 1992;115(Pt 3):783–794. doi: 10.1093/brain/115.3.783. [DOI] [PubMed] [Google Scholar]
- 29.Zack MM. Kobau R (2017) National and state estimates of the numbers of adults and children with active epilepsy—United States. MMWR Morb. Mortal. Wkly. Rep. 2015;66(31):821–825. doi: 10.15585/mmwr.mm6631a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamer HM, Dodel R, Strzelczyk A, Balzer-Geldsetzer M, Reese J-P, Schöffski O, Graf W, Schwab S, Knake S, Oertel WH, Rosenow F, Kostev K. Prevalence, utilization, and costs of antiepileptic drugs for epilepsy in Germany—A nationwide population-based study in children and adults. J. Neurol. 2012;259(11):2376–2384. doi: 10.1007/s00415-012-6509-3. [DOI] [PubMed] [Google Scholar]
- 31.Serrano-Castro PJ, Mauri-Llerda JA, Hernández-Ramos FJ, Sánchez-Alvarez JC, Parejo-Carbonell B, Quiroga-Subirana P, Vázquez-Gutierrez F, Santos-Lasaosa S, Mendez-Lucena C, Redondo-Verge L, Tejero-Juste C, Morandeira-Rivas C, Sancho-Rieger J, Matías-Guiu J. Adult prevalence of epilepsy in Spain: EPIBERIA, a population-based study. Sci. World J. 2015;2015:602710. doi: 10.1155/2015/602710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giussani G, Cricelli C, Mazzoleni F, Cricelli I, Pasqua A, Pecchioli S, Lapi F, Beghi E. Prevalence and incidence of epilepsy in Italy based on a nationwide database. Neuroepidemiology. 2014;43(3–4):228–232. doi: 10.1159/000368801. [DOI] [PubMed] [Google Scholar]
- 33.Keränen T, Riekkinen PJ, Sillanpaa M. Incidence and prevalence of epilepsy in adults in eastern Finland. Epilepsia. 1989;30(4):413–421. doi: 10.1111/j.1528-1157.1989.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 34.Joensen P. Prevalence, incidence, and classification of epilepsy in the Faroes. Acta Neurol. Scand. 1986;74(2):150–155. doi: 10.1111/j.1600-0404.1986.tb04642.x. [DOI] [PubMed] [Google Scholar]
- 35.Olafsson E, Hauser WA. Prevalence of epilepsy in rural Iceland: A population-based study. Epilepsia. 1999;40(11):1529–1534. doi: 10.1111/j.1528-1157.1999.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 36.Forsgren L. Prevalence of epilepsy in adults in northern Sweden. Epilepsia. 1992;33(3):450–458. doi: 10.1111/j.1528-1157.1992.tb01690.x. [DOI] [PubMed] [Google Scholar]
- 37.Onal AE, Tumerdem Y, Ozturk MK, Gurses C, Baykan B, Gokyigit A, Ozel S. Epilepsy prevalence in a rural area in Istanbul. Seizure. 2002;11(6):397–401. doi: 10.1053/seiz.2001.0665. [DOI] [PubMed] [Google Scholar]
- 38.Aziz H, Güvener A, Akhtar SW, Hasan KZ. Comparative epidemiology of epilepsy in Pakistan and Turkey: Population-based studies using identical protocols. Epilepsia. 1997;38(6):716–722. doi: 10.1111/j.1528-1157.1997.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 39.Radhakrishnan K, Pandian JD, Santhoshkumar T, Thomas SV, Deetha TD, Sarma PS, Jayachandran D, Mohamed E. Prevalence, knowledge, attitude, and practice of epilepsy in Kerala, South India. Epilepsia. 2000;41(8):1027–1035. doi: 10.1111/j.1528-1157.2000.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 40.Al Rajeh S, Awada A, Bademosi O, Ogunniyi A. The prevalence of epilepsy and other seizure disorders in an Arab population: A community-based study. Seizure. 2001;10(6):410–414. doi: 10.1053/seiz.2001.0602. [DOI] [PubMed] [Google Scholar]
- 41.Li SC, Schoenberg BS, Wang CC, Cheng XM, Zhou SS, Bolis CL. Epidemiology of epilepsy in urban areas of the People's Republic of China. Epilepsia. 1985;26(5):391–394. doi: 10.1111/j.1528-1157.1985.tb05669.x. [DOI] [PubMed] [Google Scholar]
- 42.Guekht A, Hauser WA, Milchakova L, Churillin Y, Shpak A, Gusev E. The epidemiology of epilepsy in the Russian Federation. Epilepsy Res. 2010;92(2):209–218. doi: 10.1016/j.eplepsyres.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Osuntokun BO, Adeuja AO, Nottidge VA, Bademosi O, Olumide A, Ige O, Yaria F, Bolis CL, Schoenberg BS. Prevalence of the epilepsies in Nigerian Africans: A community-based study. Epilepsia. 1987;28(3):272–279. doi: 10.1111/j.1528-1157.1987.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 44.Tekle-Haimanot R, Forsgren L, Abebe M, Gebre-Mariam A, Heijbel J, Holmgren G, Ekstedt J. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: A community-based study. Epilepsy Res. 1990;7(3):230–239. doi: 10.1016/0920-1211(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 45.Rwiza HT, Kilonzo GP, Haule J, Matuja WB, Mteza I, Mbena P, Kilima PM, Mwaluko G, Mwang'ombola R, Mwaijande F, et al. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: A community-based study. Epilepsia. 1992;33(6):1051–1056. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 46.Birbeck GL, Kalichi EM. Epilepsy prevalence in rural Zambia: A door-to-door survey. Trop. Med. Int. Health TM & IH. 2004;9(1):92–95. doi: 10.1046/j.1365-3156.2003.01149.x. [DOI] [PubMed] [Google Scholar]