Highlights
-
•
Accelerated atherosclerosis is common when SVGs, but not arterial grafts, are used for myocardial revascularization during CABG.
-
•
This review will provide an overview of the available data on the most commonly used conduits in CABG, highlighting the differences in their cellular biology, mechanical, biochemical, and vasoconstrictive properties.
-
•
Clinical and scientific evidence support the use of arterial grafts over venous conduits at the time of CABG. These arterial conduits seem to be more protected toward the development of atherosclerosis. Exploring the molecular and cellular mechanisms, of the various cell populations within these conduits, will help unveil the pathways responsible for these protective effects.
Key Words: coronary artery bypass graft, coronary artery disease, gastroepiploic artery, internal mammary artery, radial artery, saphenous vein graft
Abbreviations and Acronyms: CABG, coronary artery bypass graft; EC, endothelial cell; ECM, extracellular matrix; GEA, gastroepiploic artery; IMA, internal mammary artery; LAD, left anterior descending artery; RA, radial artery; SVG, saphenous vein graft; VSMC, vascular smooth muscle cell
Summary
Coronary artery bypass graft (CABG) is the gold standard for coronary surgical revascularization. Retrospective, prospective, and meta-analysis studies looking into long-term outcomes of using different conduits have pointed to the superiority of arterial grafts over veins and have placed the internal mammary artery as the standard conduit of choice for CABG. The superiority of the internal mammary artery over other conduits could be attributable to its intrinsic characteristics; however, little is known regarding the features that render some conduits atherosclerosis-prone and others atherosclerosis-resistant. Here, an overview is provided of the available data on the most commonly used conduits in CABG (internal mammary artery, saphenous vein, radial artery, gastroepiploic artery), highlighting the differences in their cellular biology, mechanical, biochemical, and vasoconstrictive properties. This information should help in furthering our understanding of the clinical outcomes observed for each of these conduits.
Central Illustration
Data examining patient population from the original FHS (Framingham Heart Study) cohort, in addition to 20 years’ follow-up of their offspring, revealed that the lifetime risk of developing coronary artery disease is 49% in men and 32% in women, for people 40 years of age (1). If left untreated, coronary artery disease can lead to severe complications (ischemia, myocardial infarction, stroke) and death. Reperfusion of the myocardium can be re-established by either percutaneous coronary intervention or coronary artery bypass graft (CABG).
Results from clinical observations, meta-analyses, and morphological studies have demonstrated that accelerated atherosclerosis is common when saphenous vein grafts (SVGs), but not internal mammary artery (IMA) grafts, are used for CABG, and that the utilization of mammary arteries has a better long-term survival advantage over that of using veins (2). Here, we have performed an extensive review of the published reports to identify the rationale behind the observed differences in clinical outcomes based on conduit selection for CABG. In addition, we describe the different cellular and molecular mechanisms that help explain these outcomes.
Conduits for CABG
To establish coronary revascularization, both venous and arterial conduits, have been used with the goal to provide long-term patency. Only 4 conduits proved to be effective: the SVG; the left and right IMAs; the radial artery (RA); and the gastroepiploic artery (GEA). In 2011, the use of arterial grafts for anastomosis to the left anterior descending artery (LAD) was put forward by the American College of Cardiology Foundation/American Heart Association Guidelines for CABG Surgery (2). The left IMA has not always been the graft of choice for the LAD but became the preferred graft for the LAD during the 1980s, because its caliber is a good fit for the LAD diameter. In fact, left IMA to LAD anastomoses have been proven to be patent for years post-operatively, with a high patency rate up to 95% to 98% after 20 years post-CABG, a feature that was attributed to their reduced incidence of atherosclerosis development (<4% of cases) (3). SVGs exhibit lower patency and higher mortality rate compared with those of IMAs. SVG grafts have been shown to occlude (up to 50%) as early as 10 years after implantation (2). The failure of these grafts reached a rate of 30% to 40% after 10 years, due to patients developing SVG intimal hyperplasia (4). In addition to intimal thickening, SVGs can undergo atherosclerosis, with angiographic studies demonstrating an attrition rate of the SVG of 2% from the first to the seventh post-operative year with only 38% to 45% of SVGs remaining patent after 10 years (5).
The RA is a third conduit that can be used in CABG based on the experience of Carpentier et al. (6); a 30% rate of obstruction was observed with RA usage when compared with other grafts. Results from the RAPS (Radial artery Patency Study), the RAPCO (Radial Artery Patency and Clinical Outcome) trial, and others have significantly contributed to placing the RA as a conduit for bypass (7,8). The RAPS revealed the superiority of the RA to SVG, after a follow-up of more than 5 years after surgery (7). Similar results were reported in a meta-analysis of randomized control trials, where a higher rate of patency at 10 years of follow-up and a lower rate of adverse cardiac events in RA when compared with those in SVG were observed (9). The GEA is another arterial graft allowing revascularization and showing patency rates, at 1-, 5-, and 10-year follow-ups, of 91%, 80%, and 62%, respectively; however, it is less commonly used, given that it is more prone to spasm and demands the addition of a small laparotomy for harvesting (10).
It was not until recently that vascular biology started providing a possible explanation to the underlying differences in graft conduit success. These discoveries have quickly extrapolated to influence the surgeon’s rationale behind conduits’ choice. The Central Illustration and Figure 1 summarize the findings highlighting the differences among the most commonly used conduits.
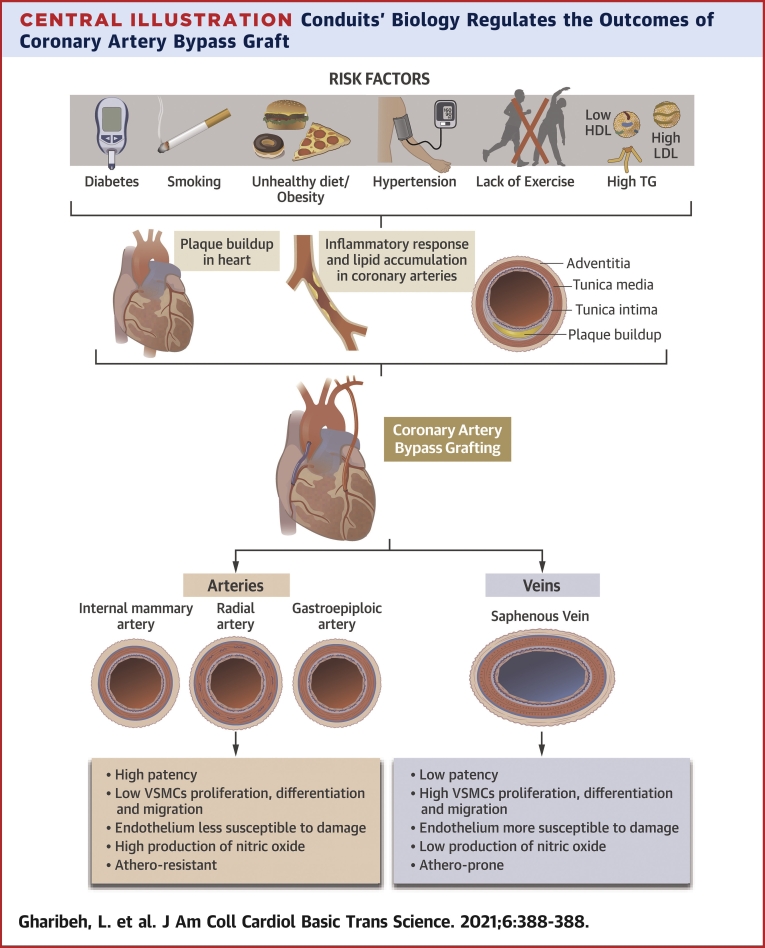
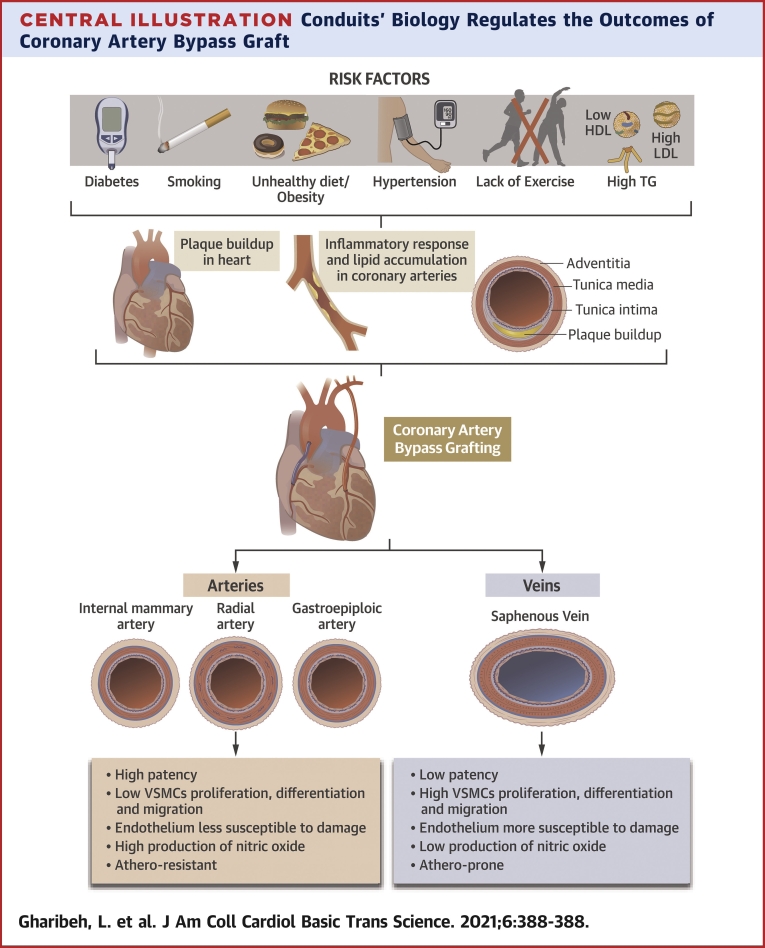
Central Illustration.
Conduits’ Biology Regulates the Outcomes of Coronary Artery Bypass Graft
The biology of the conduits regulates the outcomes of coronary artery bypass graft. HDL = high-density lipoprotein; LDL = low-density lipoprotein; TG = triglycerides; VSMC = vascular smooth muscle cell.
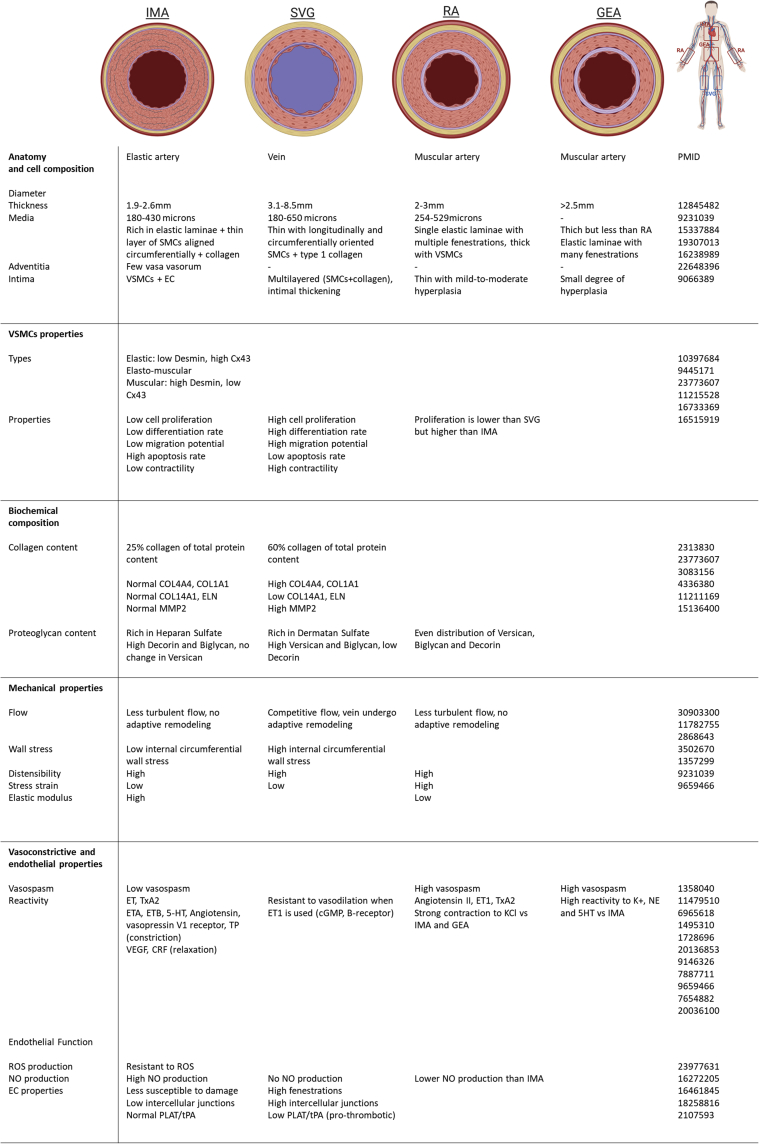
Figure 1.
Summary of Differences of Most Commonly Used Conduits
Comparative summary highlighting the differences among the most commonly used conduits: internal mammary artery (IMA), saphenous vein graft (SVG), radial artery (RA), and gastroepiploic artery (GEA). (Far right) The numbers are PubMed identifiers. The top panel was created using BioRender. cGMP = cyclic guanidine monophosphate; COL = collagen; CRF = corticotropin releasing factor; Cx43 = connexin-43; ECs = endothelial cells; ELN = elastin; ET: endothelin; HT = hydroxytryptamine; MMP = matrix metalloproteinase; NE = norepinephrine; PLAT/tPA = tissue-type plasminogen activator; PMID = PubMed ID; ROS = reactive oxygen species; SMCs = smooth muscle cells; TP = thromboxane-prostanoid; TxA2 = thromboxane A2; VEGF = vascular endothelial growth factor; VSMC = vascular smooth muscle cell.
Anatomy and cellular composition
The success rates of using arteries versus veins lies in their intrinsic differences. The IMA is an elastic artery with a diameter in adults ranging between 1.9 and 2.6 mm and a wall thickness of 180 to 430 μm (11). The media consists of a thin layer of vascular smooth muscle cells (VSMCs) aligned circumferentially, located between the elastic layers, and surrounded by collagen. Its intima consists of some VSMCs and endothelium with some neointima, seen in 50% of cases (11). The SVG has a large diameter ranging between 3.1 and 8.5 mm and a wall thickness from 180 to 650 μm (11). The media and adventitia consist of longitudinally oriented VSMCs, in addition to circumferentially oriented VSMCs in between, all surrounded by type I collagen. Elastic laminae are observed in the adventitia and media, whereas the intima display a multilayered configuration shaped by its VSMCs and collagen. The intima also displays intimal thickening, which is observed usually at the time of implantation occupying <25% of the vein cross-sectional area (11).
The RA has a single-layer elastic lamina with multiple fenestrations. It has a thick tunica media with abundant VSMCs, and a thin intima with mild-to-moderate hyperplasia (12). It has 18.84% of elastic fibers in the tunica media and its wall thickness usually varies between 254 to 529 μm (13). The harvested RA conduit has a length of approximately 18 to 20 cm with 2 to 3 mm in diameter, allowing the surgeon to reach most blocked coronary arteries. When harvested, the GEA has a caliber of >2.5 mm and a length of ∼20 cm. Its medial layer is less thick than that of the RA but is composed of muscular cells and its elastic lamina, despite the presence of many fenestrations, has a smaller degree of hyperplasia (14).
VSMCs properties
Atherosclerosis buildup and graft failure occur when VSMCs of the tunica media migrate to the intima. This migration is facilitated by components of the extracellular matrix (ECM) involving several interactions, in addition to ECM secretion and degradation. Therefore, understanding the VSMC and ECM characteristics of each of the vessels could provide insight into the underlying mechanisms leading to distinct stenosis outcomes. VSMCs composing the various arteries and veins are thought to have different embryonic origins and to exhibit differential intrinsic characteristics. The heterogeneity of the IMA is reflected by the array of phenotypes of the VSMCs cells composing it, ranging from elastic to elastomuscular to muscular (15). Studies have revealed that SMCs composing the muscular medial regions express high desmin/low connexin-43, whereas elastic medial regions express low desmin/high connexin-43. The 2 subpopulations of VSMCs in this region represent differentiated contractile cells with the high connexin-43/low desmin subpopulation reflecting a less contractile and a more synthetic state (15).
Compared with IMAs-derived VSMCs, VSMCs from SVGs are shown to have a higher cell proliferation rate, are more differentiated, and exhibit higher contractility (16). Moreover, SVG-VSMCs have a higher expression of ECM and, therefore, have greater migrating capabilities, and they are more prone to restenosis after CABG (17). VSMC cell death also plays a role in disease progression; apoptosis examined in IMAs and SVGs revealed higher apoptosis rates in IMAs, rendering it more protective toward restenosis than other conduits are (18). Little is known regarding the RA-VSMCs, opening the possibilities for more research in this field. A study revealed that VSMC proliferation in RA is significantly lower than that observed in SVG, whereas it is higher in RA as compared with in IMA (19).
Biochemical composition
The accumulation of connective tissue (collagen and glycosaminoglycan-containing proteoglycans) in vessels is a highlight of atherosclerosis formation. It has been shown that the IMA content of collagen is 25% of its total protein content. In contrast, collagen accounts for almost 60% of total protein in veins, further explaining why increased atherogenesis is observed in these conduits versus in arteries (20). The IMA has been shown to have a predominance of heparan sulfate, reflecting the higher cell density and media thickness in its arterial wall in comparison to the vein (rich in dermatan sulfate). Dermatan sulfate quantity has been reported to be increased in atherosclerotic arteries compared with in lesion-free arteries, hence the higher vulnerability of SVGs to atherosclerosis development (21).
Matrix proteoglycans and metalloproteases involved in the vessel structure are crucial players during matrix degradation, facilitating the VSMC proliferation and migration toward the intima, initiating the process of atherosclerosis. High versican, high biglycan, and low decorin in SVGs were observed when compared with IMAs and RAs, further supporting their predisposition to atherosclerosis (22). Furthermore, a comparative study looking at IMA and SVG conduits from the same patient revealed that, although the same matrix metalloproteinase constituents were present in IMAs and SVGs, matrix metalloproteinase 2 levels and activity were significantly increased in SVGs when compared with in IMAs (23).
Mechanical properties
Flow disturbances in blood vessels can lead to cytoskeletal changes and ultimately, to structural instability. In IMAs, the absence of intimal thickening could be attributable to the less turbulent flow resulting from the similarity in diameter between coronary arteries and IMAs, rendering them more accustomed to systemic pressure. The RA is also preconditioned to high pressure (24). However, this is not the case for SVGs; when placed in high-pressure regions, these veins undergo extensive adaptive remodeling, also known as “arterialization,” to support the new environment (25).
Following the operation, the SVG and the IMA become exposed to the cyclic transmural pressures of the systemic circulation. This exposure leads to VSMC proliferation that is observed in the SVG but not in the IMA (26). However, at the same transmural pressure, IMAs were shown to be more mechanically distensible than SVGs were (11). Compared with IMAs, RAs are shown to develop a higher distensibility, higher stress strain, and lower elastic modulus (27).
Endothelial dysfunction, vasoconstrictive properties, and thrombosis
Vasoconstriction can be triggered by several stimuli including mechanical trauma, nerve stimulation, or vasoconstrictor substances. Arterial grafts are prone to developing spasms with vasospasm developing less frequently in IMA compared with in RA and GEA (28). Endothelial dysfunction is usually characterized by a reduction of bioviability of vasodilators (such as NO) and an increase in the expression of contracting factors. For this reason, endothelial-dependent vasodilation via NO, either with acetylcholine infusion or exercise, is only seen in vessels grafted with IMA. On the other hand, paradoxical vasoconstrictions are observed in vessels grafted with SVGs, where the endothelium fails to respond by producing NO normally (29).
The RA has stronger receptor-mediated contractions than the IMA does. The RA graft is also more reactive to angiotensin II, whereas RA and IMA are similarly responsive to endothelin-1, an endothelium-derived constricting factor (30). In addition, it was shown to develop a higher tension to vasoconstricting agents (KCl, potassium chloride, and norepinephrine) and a higher relaxation to isradipine (Ca2+ channel blocker) when compared with IMA (27). The difference in responses of these arteries is partially attributable to their VSMC content and endothelial cell (EC) function. In fact, although the basal rate of NO production in RA is lower than in IMA, the NO release was shown to be higher in RA than in IMA following stimulation with carbamylcholine, an endothelium-dependent vasodilator. The RA has demonstrated a higher distensibility than the IMA has both at baseline and during stimulation (27).
Endothelial dysfunction can lead to atherosclerotic plaque destabilization and ultimately plaque rupture, a process resulting from an interplay between inflammatory cells and the endothelium, involving proinflammatory mediators and cellular plaque components (31). On the other hand, a healthy endothelium can secrete anti-aggregatory factors such as NO or prostacyclin that can affect platelet function or factors with fibrinolytic or anticoagulatory properties such as tissue plasminogen activator, favoring an antithrombotic milieu (32). The endothelium of IMAs is resistant to ROS and hence less susceptible to damage by external stimuli such as smoking or dyslipidemia. IMAs produce, at the baseline or after external stimulation, high levels of NO, rendering it a conduit with a better patency than the RA when tested in active smokers (33). The IMA endothelium also has fewer intercellular junctions and fenestrations as compared to the SVG endothelium, which may play a role in preventing the infiltration of lipoproteins into the subendothelial space of IMA (34).
Studies have tested whether histamine can induce endothelium-dependent relaxations in RA compared with in SVG and IMA. Following histamine exposure, the RA responded with a contraction of the vessel at all doses, whereas the response in the IMA and SVG was a vessel relaxation at low doses and a vessel contraction at high doses (35). The contractions are mediated by the H1-receptor in all 3 vessels. Endothelial H2-receptor activation leads to an increase in NO release and a relaxation in the SVG and IMA. On the other hand, lack of NO production in the RA, due to minimal endothelial expression of the H2-receptor in this vessel, renders it less susceptible to the relaxation effect (35).
Harvesting the SVG, even under optimized conditions, can cause severe endothelial disruptions with more than 50% of ECs becoming affected. The SVG possesses, at the time of implantation, areas of focal absence of endothelium, platelet, and fibrin deposition with numerous inflammatory cells along the intimal surface of the graft wall (34). To prevent thrombosis, healthy ECs secrete tissue-type plasminogen activator that converts plasminogen to plasmin and degrades fibrin, therefore contributing to the circulatory fibrinolytic system (36). Loss of the endothelial layer due to damage during the harvesting of SVG compromises this natural response. In fact, expression of the tissue-type plasminogen activator was shown to be lower in human SVGs when compared with IMAs (36). After 1 month of implantation, neointimal growth involving VSMCs, proteoglycans and collagen occurs in SVGs as a response to endothelial injury and hemodynamic stress from the de novo arterial pressure (34).
Impact of harvesting techniques in outcomes
Harvesting techniques can have a big influence on the long-term clinical outcomes of different conduits. Vein-graft harvesting can be performed either endoscopically, using an open approach or using a no-touch technique. A comparative study in 2009 looking at 3,000 patients who underwent CABG, who were assigned by the surgeons for an open or endoscopic vein graft harvesting, showed a decreased graft patency and increased rates of adverse clinical outcomes (death, myocardial infarction, or repeat revascularization) in the cases where an endoscopic approach was employed (37). These complications were attributed to vascular damage including traction, adventitial stripping, endothelial damage, and venous compression and were shown to be less pronounced in cases where open vein harvesting was employed when compared with the endoscopic approach. However, a more recent randomized clinical trial in 2019 comparing these 2 techniques revealed no significant differences when it comes to risk of major adverse cardiac events (38).
The no-touch technique is an atraumatic technique to harvest the SVG, providing a superior graft patency that is comparable to that of the IMA. When comparing the endoscopic vein graft harvesting to the no-touch technique, a high graft performance in patients receiving the no-touch SVG is observed, despite their increased incidence of developing wound complications (39). The evidence in the published reports also support a better patency of the no-touch technique when compared with the open SVG approach with results revealing a reduction in vascular damage and preservation of endothelial integrity and the vasa vasorum when the no-touch technique is employed.
When exploring the different techniques used to harvest the RA, a prospective randomized trial, comparing endoscopic RA harvest versus open technique, showed no negative affect in the length of harvested RA, harvest time, conduit quality, occurrence of hand and forearm complications, and wound healing (40). The GEA, on the other hand, can be harvested as skeletonized or pedicled with skeletonization reported to be remarkably superior by preventing vasoconstriction, with the conduit remaining wider and longer (41).
Discussion and Future Perspectives
Functional and structural similarities between the coronary artery and the conduit utilized for grafting determine its adequate functioning and long-term durability. Among these features are its length, wall thickness, luminal diameter, and histological properties (42). Arterial grafts, when compared with SVGs, appear to superiorly answer these demands: 1) their structure has all the elements necessary to sustain systemic arterial pressure; 2) their endothelium secretes more endothelium-derived relaxing factors and more NO, also protecting against platelet aggregation; 3) and they are less prone to VSMC migration and proliferation.
The structural biology of the graft dictates its patency and survival. The incidence of atherosclerosis during follow-up after CABG in arteries (IMA, RA, or GEA) is either completely absent or extremely low compared with that of SVG grafts. The SVG starts its deterioration as a conduit at the time of harvesting; its adventitial layer is often removed during this process and distended, leading to hypoxia in the vessel wall, promoting platelet activation and early thrombosis (43). Following implantation, the SVG becomes exposed to a high arterial pressure that leads to an increase in its luminal diameter and shear stress, damaging the EC layer and promoting vasospasm and reduced graft patency (44). Consequently, graft failure in < 1 year can then occur and is attributable to neointimal hyperplasia caused by elevated arterial pressure on the vein. Proliferation and migration of VSMCs simultaneously occur during this process and, with time, lead to luminal loss and predisposition to atherosclerosis (45). Beyond the 1-year term, graft failure is often attributable to severe atherosclerosis and high occurrence of plaque rupture, aneurysmal dilatation, and thrombosis. These mechanisms occur due to the inability of the SVG to undergo positive remodeling, unlike the IMA, which has a low rate of VSMC proliferation and migration, matrix activation, and cellular apoptosis (46).
Given that all arterial conduits are believed to possess similar biological characteristics, this may infer that they also share similar functional responses. However, functional studies comparing histology, endothelial function, and contractility have demonstrated differences among these arteries. For example, compared with IMA, the RA and GEA are more susceptible to developing spasm during surgical dissection and perioperative procedure (47). This vasospasm can be reversed in vitro by using Ca2+ channel blockers such as isradipine (27). This has been confirmed in clinical series where they showed that the use of Ca2+ channel blockers after CABG, when RA grafts were used, was associated with higher graft patency and lower major adverse cardiovascular events (48). Even though biologically the IMA appears to be the ideal conduit for grafting a coronary artery, there are technical and clinical limitations that sometimes make the use of a second IMA challenging. It is at this time that the use of the RA represents an outstanding alternative, as long as adequate precautions against vasospasm are undertaken in the perioperative period.
Transcriptional analysis of human surgically resected IMAs, SVGs, and aortas have provided some mechanistic explanation to support the superiority of the clinical findings observed when using IMA in CABG. In a study looking at patients who underwent isolated CABG, examination of the right and left IMAs revealed lower transcript expression of proatherosclerotic and proinflammatory genes, when compared with aortic buttons taken from the same patient (49). This protective effect against atherosclerosis in the IMAs does not seem to exist in all arterial branches from the same patient, such as in the ascending aorta (49). Another study looking at the resistance of IMA toward thrombotic occlusion revealed a lower susceptibility of IMAs, compared with SVGs, to neointima formation and thrombosis (36). Clinical and angiographic data, from a 10-year follow-up study by Dimitrova et al. (50), demonstrated 2 significant main findings: 1) a significantly increased patency of both IMAs and RAs over SVGs; and 2) a slower progression of coronary atherosclerosis on the coronary vessels that had been grafted with those arterial conduits versus with veins.
A true understanding of how to link the biology of the conduits to the clinical outcome will help open the way for future and improved therapies. However, our knowledge is still limited to fully understand why the IMAs, when compared with other conduits, are resistant to atherosclerosis development. Therefore, it is important to understand why VSMCs from IMAs can remain for a long period of time in a contractile state and are resistant to proliferation. In addition, understanding why the ECs are more resistant to shear stress is also crucial. To better address these questions, a molecular approach identifying the molecular mechanisms involved in VSMC and EC homeostasis, of each of the conduits, is required. Furthermore, identifying how these cells behave in disease state versus at baseline and how they regulate inflammatory cell responses, lipid uptake, and ECM regulation will reveal the rationale behind the observed clinical outcomes. Once identified, this knowledge can be transferable to potentially develop preventative measures for development of atherosclerosis.
Funding Support and Author Disclosures
Dr. Gharibeh has received a Strategic Endowed Fellowship from the University of Ottawa Heart Institute. Drs. Ouimet and Grau have received funding from a translational research grant from the University of Ottawa, Faculty of Medicine. Dr. Ferrari has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Lloyd-Jones D., Adams R.J., Brown T.M. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Cameron A., Davis K.B., Green G., Schaff H.V. Coronary bypass surgery with internal-thoracic-artery grafts—effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 3.Sims F.H. A comparison of coronary and internal mammary arteries and implications of the results in the etiology of arteriosclerosis. Am Heart J. 1983;105:560–566. doi: 10.1016/0002-8703(83)90478-7. [DOI] [PubMed] [Google Scholar]
- 4.Blaas I., Heinz K., Würtinger P. Vein graft thrombi, a niche for smooth muscle cell colonization—a hypothesis to explain the asymmetry of intimal hyperplasia. J Thromb Haemost. 2016;14:1095–1104. doi: 10.1111/jth.13295. [DOI] [PubMed] [Google Scholar]
- 5.Shelton M.E., Forman M.B., Virmani R., Bajaj A., Stoney W.S., Atkinson J.B. A comparison of morphologic and angiographic findings in long-term internal mammary artery and saphenous vein bypass grafts. J Am Coll Cardiol. 1988;11:297–307. doi: 10.1016/0735-1097(88)90094-0. [DOI] [PubMed] [Google Scholar]
- 6.Carpentier A., Guermonprez J.L., Deloche A., Frechette C., DuBost C. The aorta-to-coronary radial artery bypass graft: a technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–121. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 7.Deb S., Cohen E.A., Singh S.K. for the RAPS Investigators. Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study) J Am Coll Cardiol. 2012;60:28–35. doi: 10.1016/j.jacc.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Hayward P.A.R., Hare D.L., Gordon I., Matalanis G., Buxton B.F. Which arterial conduit? Radial artery versus free right internal thoracic artery: six-year clinical results of a randomized controlled trial. Ann Thorac Surg. 2007;84:493–497. doi: 10.1016/j.athoracsur.2007.03.053. discussion 497. [DOI] [PubMed] [Google Scholar]
- 9.Gaudino M., Benedetto U., Fremes S., for the RADIAL Investigators Association of radial artery graft vs saphenous vein graft with long-term cardiovascular outcomes among patients undergoing coronary artery bypass grafting: a systematic review and meta-analysis. JAMA. 2020;324:179–187. doi: 10.1001/jama.2020.8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suma H., Isomura T., Horii T., Sato T. Late angiographic result of using the right gastroepiploic artery as a graft. J Thorac Cardiovasc Surg. 2000;120:496–498. doi: 10.1067/mtc.2000.108690. [DOI] [PubMed] [Google Scholar]
- 11.Canham P.B., Finlay H.M., Boughner D.R. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc Res. 1997;34:557–567. doi: 10.1016/s0008-6363(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 12.González Santos J.M., López Rodríguez J., Dalmau Sorlí M.J. [Arterial grafts in coronary surgery. Treatment for everyone?] Rev Esp Cardiol. 2005;58:1207–1223. [PubMed] [Google Scholar]
- 13.Appleson T., Hill R.V. Histological comparison of the candidate arteries for bypass grafting of the posterior interventricular artery. Anat Sci Int. 2012;87:150–154. doi: 10.1007/s12565-012-0139-9. [DOI] [PubMed] [Google Scholar]
- 14.van Son J.A., Smedts F.M., Yang C.Q. Morphometric study of the right gastroepiploic and inferior epigastric arteries. Ann Thorac Surg. 1997;63:709–715. doi: 10.1016/s0003-4975(96)01115-0. [DOI] [PubMed] [Google Scholar]
- 15.Ko Y.-S., Yeh H.-I., Haw M. Differential expression of connexin43 and desmin defines two subpopulations of medial smooth muscle cells in the human internal mammary artery. Arterioscler Thromb Vasc Biol. 1999;19:1669–1680. doi: 10.1161/01.atv.19.7.1669. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z., Oemar B.S., Carrel T., Kipfer B., Julmy F., Lüscher T.F. Different proliferative properties of smooth muscle cells of human arterial and venous bypass vessels: role of PDGF receptors, mitogen-activated protein kinase, and cyclin-dependent kinase inhibitors. Circulation. 1998;97:181–187. doi: 10.1161/01.cir.97.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Zhu T., Lan B., Meng L. ECM-related gene expression profile in vascular smooth muscle cells from human saphenous vein and internal thoracic artery. J Cardiothorac Surg. 2013;8:155. doi: 10.1186/1749-8090-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frischknecht K., Greutert H., Weisshaupt C. Different vascular smooth muscle cell apoptosis in the human internal mammary artery and the saphenous vein: implications for bypass graft disease. J Vasc Res. 2006;43:338–346. doi: 10.1159/000093606. [DOI] [PubMed] [Google Scholar]
- 19.Mekontso-Dessap A., Kirsch M., Guignambert C. Vascular-wall remodeling of 3 human bypass vessels: organ culture and smooth muscle cell properties. J Thorac Cardiovasc Surg. 2006;131:651–658. doi: 10.1016/j.jtcvs.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 20.Sisto T., Ylä-Herttuala S., Luoma J., Riekkinen H., Nikkari T. Biochemical composition of human internal mammary artery and saphenous vein. J Vasc Surg. 1990;11:418–422. doi: 10.1067/mva.1990.17248. [DOI] [PubMed] [Google Scholar]
- 21.Ylä-Herttuala S., Sumuvuori H., Karkola K., Möttönen M., Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest. 1986;54:402–407. [PubMed] [Google Scholar]
- 22.Merrilees M.J., Beaumont B., Scott L.J. Comparison of deposits of versican, biglycan and decorin in saphenous vein and internal thoracic, radial and coronary arteries: correlation to patency. Coron Artery Dis. 2001;12:7–16. doi: 10.1097/00019501-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Anstadt M.P., Franga D.L., Portik-Dobos V. Native matrix metalloproteinase characteristics may influence early stenosis of venous versus arterial coronary artery bypass grafting conduits. Chest. 2004;125:1853–1858. doi: 10.1378/chest.125.5.1853. [DOI] [PubMed] [Google Scholar]
- 24.Virk H.U.H., Lakhter V., Ahmed M., O’ Murchu B., Chatterjee S. Radial artery versus saphenous vein grafts in coronary artery bypass surgery: a literature review. Curr Cardiol Rep. 2019;21:36. doi: 10.1007/s11886-019-1112-1. [DOI] [PubMed] [Google Scholar]
- 25.Bashour T.T., Hanna E.S., Mason D.T. Myocardial revascularization with internal mammary artery bypass: an emerging treatment of choice. Am Heart J. 1986;111:143–151. doi: 10.1016/0002-8703(86)90566-1. [DOI] [PubMed] [Google Scholar]
- 26.Predel H.G., Yang Z., von Segesser L., Turina M., Bühler F.R., Lüscher T.F. Implications of pulsatile stretch on growth of saphenous vein and mammary artery smooth muscle. Lancet. 1992;340:878–879. doi: 10.1016/0140-6736(92)93287-w. [DOI] [PubMed] [Google Scholar]
- 27.Chamiot-Clerc P., Copie X., Renaud J.F., Safar M., Girerd X. Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Cardiovasc Res. 1998;37:811–819. doi: 10.1016/s0008-6363(97)00267-8. [DOI] [PubMed] [Google Scholar]
- 28.Acar C., Jebara V.A., Portoghese M. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54:652–659. doi: 10.1016/0003-4975(92)91007-v. discussion 659–60. [DOI] [PubMed] [Google Scholar]
- 29.Nishioka H., Kitamura S., Kameda Y., Taniguchi S., Kawata T., Mizuguchi K. Difference in acetylcholine-induced nitric oxide release of arterial and venous grafts in patients after coronary bypass operations. J Thorac Cardiovasc Surg. 1998;116:454–459. doi: 10.1016/S0022-5223(98)70011-X. [DOI] [PubMed] [Google Scholar]
- 30.He G.-W., Yang C.-Q. Radial artery has higher receptor-mediated contractility but similar endothelial function compared with mammary artery. Ann Thorac Surg. 1997;63:1346–1352. doi: 10.1016/s0003-4975(97)00106-9. [DOI] [PubMed] [Google Scholar]
- 31.Napoli C., de Nigris F., Palinski W. Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 32.McGorisk G.M., Treasure C.B. Endothelial dysfunction in coronary heart disease. Curr Opin Cardiol. 1996;11:341–350. doi: 10.1097/00001573-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Müller-Schweinitzer E., Müller S.E., Reineke D.C. Reactive oxygen species mediate functional differences in human radial and internal thoracic arteries from smokers. J Vasc Surg. 2010;51:438–444. doi: 10.1016/j.jvs.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 34.Otsuka F., Yahagi K., Sakakura K., Virmani R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg. 2013;2:519–526. doi: 10.3978/j.issn.2225-319X.2013.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stähli B.E., Greutert H., Mei S. Absence of histamine-induced nitric oxide release in the human radial artery: implications for vasospasm of coronary artery bypass vessels. Am J Physiol Heart Circ Physiol. 2006;290:H1182–H1189. doi: 10.1152/ajpheart.00280.2005. [DOI] [PubMed] [Google Scholar]
- 36.Payeli S.K., Latini R., Gebhard C. Prothrombotic gene expression profile in vascular smooth muscle cells of human saphenous vein, but not internal mammary artery. Arterioscler Thromb Vasc Biol. 2008;28:705–710. doi: 10.1161/ATVBAHA.107.155333. [DOI] [PubMed] [Google Scholar]
- 37.Lopes R.D., Hafley G.E., Allen K.B. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 2009;361:235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 38.Zenati M.A., Bhatt D.L., Bakaeen F.G., for the REGROUP Trial Investigators Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med. 2019;380:132–141. doi: 10.1056/NEJMoa1812390. [DOI] [PubMed] [Google Scholar]
- 39.Goldman S., Zadina K., Moritz T. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 40.Tamim M., Alexiou C., Al-Hassan D., Al-Faraidy K. Prospective randomized trial of endoscopic vs open radial artery harvest for CABG: clinical outcome, patient satisfaction, and midterm RA graft patency. J Card Surg. 2020;35:2147–2154. doi: 10.1111/jocs.14706. [DOI] [PubMed] [Google Scholar]
- 41.Asai T., Tabata S. Skeletonization of the right gastroepiploic artery using an ultrasonic scalpel. Ann Thorac Surg. 2002;74:1715–1717. doi: 10.1016/s0003-4975(02)03765-7. [DOI] [PubMed] [Google Scholar]
- 42.He G.-W. Springer Science and Business Media; New York, NY: 2006. Arterial Grafting for Coronary Artery Bypass Surgery. [Google Scholar]
- 43.McGeachie J.K., Meagher S., Prendergast F.J. Vein-to-artery grafts: the long-term development of neo-intimal hyperplasia and its relationship to vasa vasorum and sympathetic innervation. Aust N Z J Surg. 1989;59:59–65. doi: 10.1111/j.1445-2197.1989.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 44.Angelini G.D., Passani S.L., Breckenridge I.M., Newby A.C. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987;21:902–907. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- 45.Allaire E., Clowes A.W. Endothelial cell injury in cardiovascular surgery: the intimal hyperplastic response. Ann Thorac Surg. 1997;63:582–591. doi: 10.1016/s0003-4975(96)01045-4. [DOI] [PubMed] [Google Scholar]
- 46.Cameron A.A., Green G.E., Brogno D.A., Thornton J. Internal thoracic artery grafts: 20-year clinical follow-up. J Am Coll Cardiol. 1995;25:188–192. doi: 10.1016/0735-1097(94)00332-k. [DOI] [PubMed] [Google Scholar]
- 47.Uydeş-Doǧan B.S., Nebigil M., S-Aslamaci M.D., Onuk E., Kanzik I., Akar F. The comparison of vascular reactivities of arterial and venous grafts to vasodilators: management of graft spasm. Int J Cardiol. 1996;53:137–145. doi: 10.1016/0167-5273(95)02533-2. [DOI] [PubMed] [Google Scholar]
- 48.Gaudino M., Benedetto U., Fremes S.E., for the RADIAL Investigators Effect of calcium-channel blocker therapy on radial artery grafts after coronary bypass surgery. J Am Coll Cardiol. 2019;73:2299–2306. doi: 10.1016/j.jacc.2019.02.054. [DOI] [PubMed] [Google Scholar]
- 49.Ferrari G., Quackenbush J., Strobeck J. Comparative genome-wide transcriptional analysis of human left and right internal mammary arteries. Genomics. 2014;104:36–44. doi: 10.1016/j.ygeno.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimitrova K.R., Hoffman D.M., Geller C.M., Dincheva G., Ko W., Tranbaugh R.F. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg. 2012;94:475–481. doi: 10.1016/j.athoracsur.2012.04.035. [DOI] [PubMed] [Google Scholar]