Abstract
Background
Substance use during pregnancy mainly khat chewing (20%) and alcohol drinking (18.1%) are commonly practiced in Ethiopia. However, the effect of using these substances has not been studied nationally yet. Thus, this study was aimed to examine national evidence about the effect of substance use during pregnancy on birth outcome in the country, 2020.
Methods
Primary studies were accessed through Google scholar, HINARI, SCOPUS and PubMed databases. The methodological and evidence quality of the included studies were critically appraised by the modified Newcastle-Ottawa quality assessment tool scale adapted for observational studies. From eligible studies, two authors extracted author/year, study region, study design, sample size and reported effect of antenatal substance use on birth outcome on an excel spreadsheet. During critical appraisal and data extraction, disagreements between the two authors were resolved by the involvement of a third author. The extracted data were then exported to stata version 14. Effect sizes were pooled using the fixed-effects model due to homogenous primary studies (I2 = 0.0%). Presence of publication bias was detected from asymmetry of funnel plot and statistically significant Egger's test (p = 0.000).
Results
In this systematic review and meta-analysis, a total of 5,343 mother-neonate pairs were included from 15 studies. Alcohol, khat, cigarette and narghile were used during pregnancy, and significant adverse birth outcomes attributable to these substances were reported. From the pooled effect of alcohol use, drinking mothers were twice (95%CI: AOR = 2.16; 1.16, 3.17) likely to have newborns with birth defect; 9 times (95% CI: AOR = 9.39; 2.84, 15.94) more prone to own low birth weight neonates; and 1.9 times more prone to deliver preterm neonates (95% CI: AOR = 1.93; 0.52, 3.33) than the nondrinkers. Khat users were 2.4 times (95%CI: AOR = 2.4; 1.11, 5.19) more likely to have congenitally defected neonates; and 3.1 times (95%CI: AOR = 3.19; 1.01, 5.37) more risked to possess low birth weight neonates. Furthermore, antenatal cigarette smokers (95% CI: AOR = 4.36 (1.75, 6.98)) and narghile users (95% CI: AOR = 20.1; 3.94, 103) were at 4 and 20 times more likelihood of having low birth weight neonates as compared to their counterparts.
Conclusion
Prematurity, low birth weight and congenital malformation were the investigated adverse effects of antenatal substance use in Ethiopia. Therefore, the existing public health efforts should be encouraged to help women stop using these substances completely before pregnancy. Moreover, increasing public awareness about the potential negative impacts of substance use during pregnancy on birth outcome would be of greatest importance for comprehensive prevention of the problem.
Keywords: Ethiopia, Birth outcome, Substance use, Pregnancy, Meta-analysis
Ethiopia, Birth outcome, Substance use, Pregnancy, Meta-analysis
1. Introduction
World wise evidence shows the use of different substances among pregnant mothers [1, 2]. The broad variety of these substances includes alcohol, tobacco products, caffeine, illegal drugs, prescription drugs, inhalants and solvents [2]. Globally, the most frequently used substance during pregnancy is tobacco followed by alcohol, cannabis, Khat and narghile [3, 4, 5, 6].
Evidence suggests several worse fetal consequences when the mother smokes tobacco during pregnancy [7]. For example, as of DiFranza et al [8] and Centers for Disease Control and Prevention (CDC) [9], antenatal use of tobacco products is responsible for a yearly estimated range of 32,000–61,000 low birth weight and preterm neonates. Moreover, studies in Brazil [10], China [11] and Turkey [12] showed positive association of tobacco smoking during pregnancy with low birth weight. Further studies about the effect of antenatal tobacco use in the United States of America (USA) [13] and Jordan [14] revealed strong associations of smoking status with preterm birth and congenital malformation.
Increased rates of fetal demise have been reported among women who drink alcohol during pregnancy. For instance, according to the World Health Organization report (WHO) Expert Committee on Drug Dependence (ECDD) critical review result, 1 in 100 babies are estimated to be born with alcohol-related damage [15]. In line to this, studies in Japan [16] and Boston [17] disclosed significant association of alcohol use during pregnancy with increased risk of preterm birth and low birth weight. Moreover, evidence shows neonates born to alcoholic women are prone to develop fetal alcohol effects which include Fetal Alcohol Syndrome (FAS), Alcohol-Related Neurodevelopmental Disorder (ARND) and Alcohol Related Birth Defects (ARBDs) [18, 19, 20, 21]. The effect of alcohol use during pregnancy can extend to the childhood disorders including partial Fetal Alcohol Spectrum Disorder (FASD), hyperactivity, attention problems, learning and memory deficits, problems with social and emotional development [22] and childhood leukemia [23]. These childhood effects may even proceed to the adulthood career and beyond [22].
Khat is highly cultivated and marketed in Ethiopia, mainly in eastern part of the country [24]. According to a systematic review and meta-analysis in Ethiopia, the pooled burden of khat use among pregnant mothers was 20% [25]. Khat chewing during pregnancy is associated with maternal anemia [26], stillbirths [27] and low birth weight [28, 29, 30]. The effect of khat chewing during pregnancy increases with its increased frequency and duration of use [31].
The tradition of water-pipe smoking, also known as argileh, narghileh, narghile, hooka, shisha, goza, or hubble-bubble smoking [32] is practiced among many age groups of all social classes, even during pregnancy, and evidence shows the adversity of its use on birth weight. For example, evidence from the Greater Beirut area [33] and Lebanon [34] showed a strong association between narghile smoking during pregnancy and low birth weight newborns, with newborns of narghile-smoking mothers at greater risk of having low birth weight than those born to nonsmoking mothers. The risk increased among those who started smoking narghiles in the first trimester [32, 33, 34].
Despite the aforementioned adverse impacts, national evidence disclosed considerable prevalence of khat [25] and alcohol use [35] during pregnancy in Ethiopia. Furthermore, there are multiple primary studies [28, 29, 30, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46] showing the delivery of preterm, low birth weight and congenitally malformed neonates among alcohol drinkers, khat chewers, cigarette smokers and narghile users during pregnancy in the country. However, there is no pooled prior evidence regarding the effect of using these substances during pregnancy on birth outcome. Therefore, this study was aimed to estimate the pooled effect of antenatal substance use on neonatal outcomes in Ethiopia. As findings are the first in kind in the country, this study provides clinicians, policy makers and all other concerned bodies with comprehensive up to date information towards developing plans for optimizing birth outcomes through intervening on the use of substance before and during pregnancy among reproductive age group women. Furthermore, the findings will be used as baseline information for further studies.
2. Methods
This systematic review and meta-analysis was conducted based on the methodology of Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist [47]. It was undertaken through systematic synthesis of the eligible pocket studies on the adverse birth outcome of substance use during pregnancy in Ethiopia.
2.1. Search strategy
Five international online databases (PubMed, Scopus, Hinari, Google and Google Scholar) were searched for pocket studies on birth asphyxia in Ethiopia. For accessing relevant data about significance of antenatal substance use on birth outcomes, a comprehensive search was conducted through the aforementioned databases using adapted PICO questions i.e. ‘PEO’ (Population, Exposure, Outcome) format was followed. These questions were developed from the following search key words and/or Medical Subject Headings (MeSH): (a) population (fetus, newborn, neonate, infant) (b) exposure (substance use, khat khewing, alcohol drinking, cigarette/tobacco smoking, Narghile (shisha)) (c) outcome (adverse birth outcome, adverse pregnancy outcome, adverse neonatal outcome, premature birth, low birth weight, perinatal asphyxia, congenital anomaly, congenital malformation, birth defect, hypoxic-ischaemic encephalopathy, postasphyxial encephalopathy, intrauterine asphyxia, intrapartum asphyxia, perinatal asphyxia, perinatal suffocation, neonatal asphyxia, birth asphyxia, postnatal asphyxia, asphyxia neonatorum, suffocation, APGAR score) (d) study design (observational studies), and (e) setting (Ethiopia). These search terms were combined using the “OR” and “AND” Boolean operators. We extended our search from systematic database searching to retrieving reference lists of eligible articles and hand searches for grey literature. Besides, the ‘cited by’ and ‘related articles’ functions of PubMed were considered for further literature searching. Finally, all studies which were in agreement with the review title were retrieved and screened for inclusion criteria. Literature search was conducted from July 21/2020 (start date) until August 29/2020 (end date). The literature search was performed by two independent researchers, with discrepancies resolved by discussion and consensus. A sample of the literature search strategy, PubMed search strategy, developed using a combination of MeSH terms and free texts is presented as a supplementary file (Additional file 1).
2.2. Eligibility criteria
2.2.1. Inclusion criteria
All studies with cross-sectional, cohort and case–control study design were eligible for this meta-analysis. Articles that assessed the effect of antenatal substance use on birth outcome in Ethiopia were considered. Both published and unpublished studies without time restriction were eligible for inclusion.
2.2.2. Exclusion criteria
Studies were excluded due to any of the following reasons: (a) no report on birth outcome of substance use during pregnancy, and (b) case series, case reports, qualitative studies, editorials, correspondence and abstracts.
2.3. Study screening and selection
Searches were downloaded into Endnote version IX and de-duplicated. Then, the screening and selection of studies was conducted in two stages. First, title and abstract screening was conducted followed by full-text reviewing. Through title and abstract screening by two independent researchers, studies that reported the effect of antenatal substance use on birth outcomes were selected for full text review. Then, from full-text reviewing, any article classified as potentially eligible by either reviewer was considered as a full text and screened by both reviewers independently. At times of disagreement where a consensus could not be reached between the researchers, a third researcher reviewed and resolved the disagreements.
2.4. Data extraction
Data from the included studies were extracted using a standardized data abstraction form, developed in excel spreadsheet. For each study, the following data were extracted: (a) identification data (first author's last name and publication year), (b) Study region (c) Study period (d) Study design (e) type of substance used during pregnancy, (e) odds ratio with 95% confidence intervals for the effects of substance use on birth outcome, and (f) total number of sample size included in the study. Two researchers completed data extraction on the aforementioned components of each study and then the extracted data were crosschecked for any discrepancies. Discrepancies were resolved by the involvement of a third reviewer.
2.5. Risk of bias and reliability check
All the included studies were critically appraised and scored for the validity of their results. To appraise the methodological and evidence quality of the included studies, we used the modified Newcastle-Ottawa quality assessment tool scale adapted for observational studies [48]. The tool has 3 sections consisting of 9 questions that measure quality of the study. The first section was for evaluating the methodological plausibility and it was rated from five stars. The second section was for assessing comparability of the included study and it was rated from three stars. The third section was for appraising the statistical analysis and outcome of each study and it was rated from two points. The scoring was done by two reviewers, with discrepancies resolved by discussion and consensus. When disagreements were beyond consensus and discussion between the two researchers, a third reviewer (tie breaker) resolved the disagreements. Finally, the original studies with the scale of ≥6 out of 10 were considered as high quality after reviewing different literature.
2.6. Data synthesis
Both narrative (qualitative) and quantitative approaches were used to summarize the effect sizes of the included studies. Choice of the meta-analysis model was guided by the between studies heterogeneity, which was assessed by Higgin's I2- Statistics [49]. According to Higgins's et al., I2 < 49%, 50–75, and >75% represents low, moderate, and high levels of heterogeneity, respectively. We intended to pool the estimates with fixed-effects models if the level of heterogeneity was <50%. Accordingly, there was a low level of between-studies heterogeneity. Thus, the pooled estimates were calculated with the fixed inverse variance -effects model, which accounts for only between-studies variations [50]. We assessed publication bias by visual inspection of funnel plots, Begg's rank or Egger's regression tests, as appropriate. Stata version 14.0 software was used for the quantitative analyses. A summary list of the effects of antenatal substance use on birth outcomes was reported with their respective odds ratios.
2.7. Ethical consideration
In this study, no study participants' consent or ethical approval was needed because the study was conducted based on data extracted from published studies.
3. Results
3.1. Literature search findings
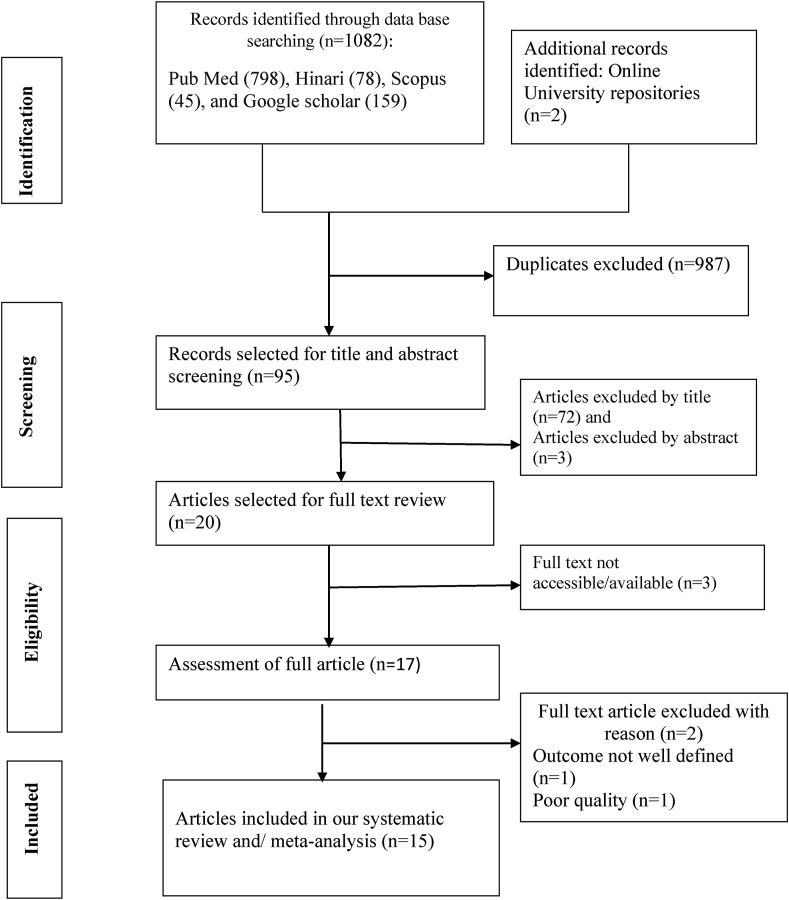
The database search yielded a total of 1082 articles from our exhaustive searching of both published and unpublished sources. From the overall 1082 articles, 1080 of which were obtained through database searching whereas the rest 2 articles were retrieved from Addis Ababa and Haramaya Universities institutional online repositories. Among 1080 articles accessed from database searching, 159 articles were obtained using Google scholar, 798 articles were from PubMed, 78 from Hinari and 45 from SCOPUS. A total of 987 duplicate articles were excluded. The remaining 95 articles were screened for their title and abstract based on which 75 articles were excluded for not being topics of interest because the objective of this study was to include only empirical pocket studies that reported association of substance use during pregnancy with birth outcomes in Ethiopia. Then, the rest 20 articles were considered for the presence of full text, and only 17 of which had full text content. After full text review of the 17 articles, 2 studies were excluded for a) not having clearly defined outcome b) poor quality. Finally, a total of 15 empirical pocket studies [28, 29, 30, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46] were included in this systematic review and meta-analysis (Figure 1).
Figure 1.
PRISMA flow diagram showing the literature search results.
3.2. Characteristics of the included studies
In this systematic review and meta-analysis, 15 studies have been considered from different regions of Ethiopia. In the study, a total of 5,343 neonates were included with sample size ranging from 220 [38] to 472 [46]. Regarding study design, 8 studies [28, 29, 30, 35, 37, 40, 41, 44] employed case control type whereas 7 studies [36, 38, 39, 42, 43, 45, 46] had cross-sectional design. Furthermore, concerning study region, 5 studies [28, 30, 38, 44, 45] were from Oromiya region. Only three studies [28, 29, 30] were conducted after the launch of SDG (2015). Alcohol, khat, cigarette and narghile were the reported substance types. Congenital anomaly, preterm birth and low birth weight were the reported adverse neonatal outcomes of substance use during pregnancy. All the included studies had good score of the critical appraisal as shown by the score of >7 (Table 1).
Table 1.
Characteristics of the included studies.
| Author year | Study region | Study period | Study design | Substance type (exposure) | Sample size | Substance users | Outcome (effect) | AOR (95%CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Tsehay et al (2019) [41] | Amhara | 2017/2018 | Case control | Alcohol | 398 | 256 | Congenital anomaly | 12.7 (3.3, 48.7) | 8 |
| Taye et al. (2018) [40] | Addis Ababa and Amhara | 2015 | Case control | Alcohol | 414 | 67 | Congenital anomaly | 2.394 (1.21, 4.72) | 8 |
| Mekonnen et al. (2020) [44] | Oromiya | 2018/2019 | Case control | Khat | 409 | 52 | Congenital anomaly | 2.4 (1.11, 5.19) | 7 |
| Fentahun Adane and Girma Seyoum (2018) [39] | Amhara | 2017/2018 | cross-sectional | Alcohol | 321 | 167 | Congenital anomaly | 2.02 (1.13, 3.6) | 7 |
| Woday et al (2019) [37] | Amhara | 2017 | Case control | Alcohol | 402 | 178 | Preterm birth | 1.62 (1.06, 2.46) | 8 |
| Bekele et al.(2017) [38] | Oromiya | 2015 | cross-sectional | Substance | 220 | 65 | Preterm birth | 1.89 (1.02, 3.56) | 9 |
| Kelkay et al. (2019) [36] | Tigray | 2018 | cross-sectional | cigarette/alcohol | 325 | 33 | Preterm birth | 3.61 (1.59, 8.23) | 9 |
| Demelash et al. (2015) [28] | Oromiya | 2013 | Case control | Khat | 387 | 48 | Low birth weight | 6.4 (2.42, 17.1) | 8 |
| Aboye et al (2018) [42] | Tigray | 2018 | cross-sectional | Alcohol | 308 | 50 | Low birth weight | 6.4 (1.24, 33.94) | 9 |
| Tsegaye Mehare and Yewbmirt Sharew (2020) [46] | Southern Ethiopia | 2018/2019 | cross-sectional | Cigarette | 472 | 23 | Low birth weight | 4.35 (2.46, 7.69) | 7 |
| Eyasu Alem Lake & Robera Olana Fite (2019) [43] | Southern Ethiopia | 2017/2018 | cross-sectional | Alcohol | 304 | 135 | Low birth weight | 8.111 (2.36, 27.90) | 9 |
| Tesfaye et al (2018) [30] | Oromiya | 2013 | Case control | Khat | 336 | 43 | Low birth weight | 12.4 (2.5, 65.6) | 8 |
| Belete et al 2018 [35] | Tigray | 2016 | Case control | Alcohol | 352 | 66 | Low birth weight | 10.8 (2.34, 19.59) | 7 |
| Emebet Dendir and Negussie Deyessa (2017) [29] | Addis Ababa | 2014 | Case control | Khat Cigarette Narghile |
347 | 55 26 23 |
Low birth weight | 2.83 (1.35, 5.93) 24.24 (2.79, 210.2) 20.1 (3.94, 103) |
9 |
| Getachew et al (2020) [45] | Oromiya | 2018 | cross-sectional | Khat | 348 | 56 | Low APGAR Score | 3.21 (1.26, 8.85) | 7 |
3.3. Meta-analysis
3.3.1. The effect of antenatal substance use on birth defect
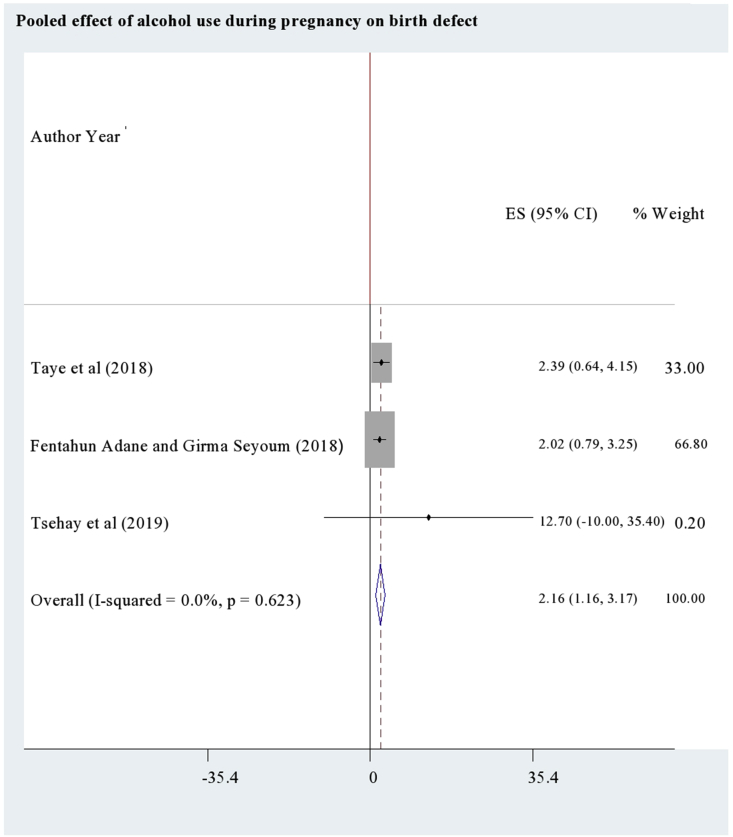
According to a study [44], mothers who used khat during their pregnancy were 2.4 times (95%CI: AOR = 2.4; 1.11, 5.19) more likely to have congenitally malformed neonates as compared to the non users. Regarding the effect of alcohol use, pooling of three studies [37, 38, 39] showed higher odds of birth defect among antenatal alcohol users (95%CI: AOR = 2.16; 1.16, 3.17) than the non users (Figure 2). As the pooled studies were homogenous (I2 = 0.0%), the pooled estimate was resulted from fixed effects analysis.
Figure 2.
The pooled effect of alcohol use during pregnancy on birth defect.
3.3.2. The effect of antenatal substance use on preterm birth
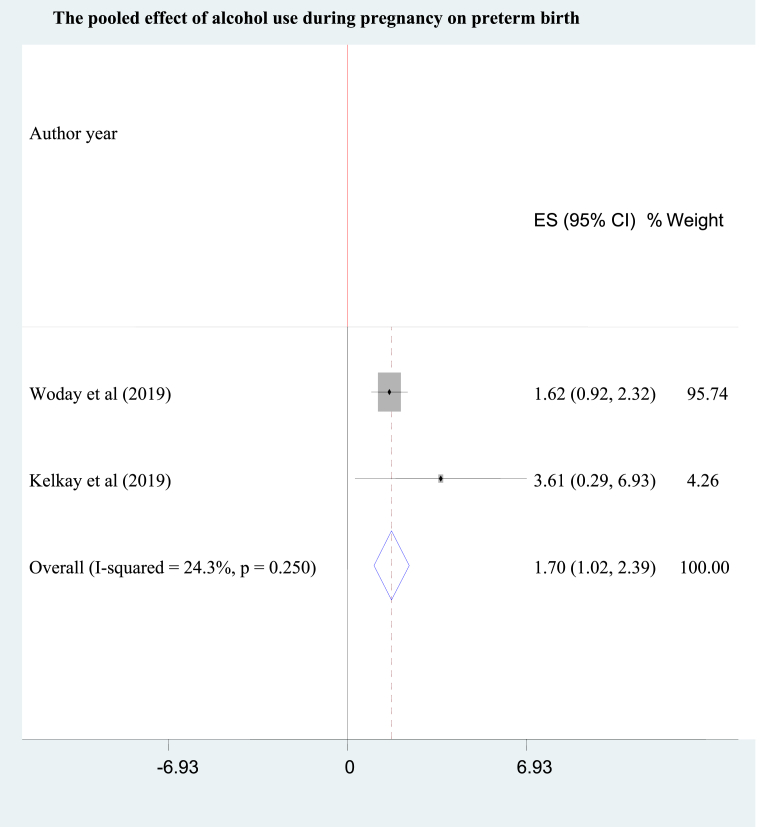
As of a study [38] in South West Ethiopia, antenatal substance users were nearly 2 folds (95% CI: AOR = 1.89: 1.02, 3.56) more likely to deliver preterm babies than the non users. Concerning the effect of alcohol use, pooling of two studies [36, 37] disclosed a 1.7 times (95% CI: AOR = 1.70; 1.02, 2.39) higher likelihood of preterm birth among mothers who drank alcohol than those who abstained from alcohol drinking during pregnancy. Fixed effects mode was used to pool the individual studies because the heterogeneity between the studies was almost negligible (I2 = 24.3%) (Figure 3).
Figure 3.
The pooled effect of alcohol use during pregnancy on preterm birth.
3.3.3. The effect of antenatal substance use on low birth weight
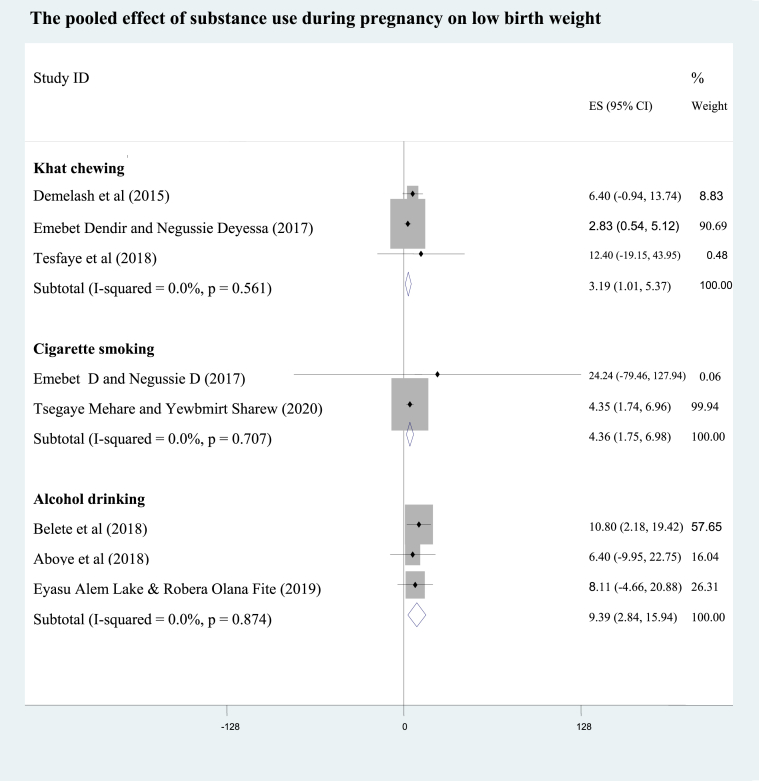
A study at Addis Ababa [29] showed mothers who smoked narghile during pregnancy had a 20 -fold higher odds of low birth weight when compared with the nonsmokers (95% CI: AOR = 20.1; 3.94, 103). From pooling of two studies [29, 46], the odds of low birth weight babies among pregnant mothers who smoked cigarette during pregnancy were 4.4 times higher than the nonsmokers (95%CI: AOR = 4.36; 1.75, 6.98; I2 = 0.0%). Besides, pooling of three studies [35, 42, 43] revealed mothers who used alcohol during their pregnancy were 9 times more likely to deliver low birth weight neonates as compared to the non users (95% CI: AOR = 9.39; 2.84, 15.94; I2 = 0.0%). Regarding the pooled effect of khat use during pregnancy [28, 29, 30], antenatal khat chewers were at 3.2 increased odds of having low birth weight neonates compared with non chewing mothers (95% CI: AOR = 3.19; 1.01, 5.37; I2 = 0.0%) (Figure 4). As the pocket studies were homogenous (I2 = 0.0%), the pooled effects were resulted from fixed effects analysis.
Figure 4.
The pooled effect of khat chewing, alcohol drinking and cigarette smoking during pregnancy on low birth weight.
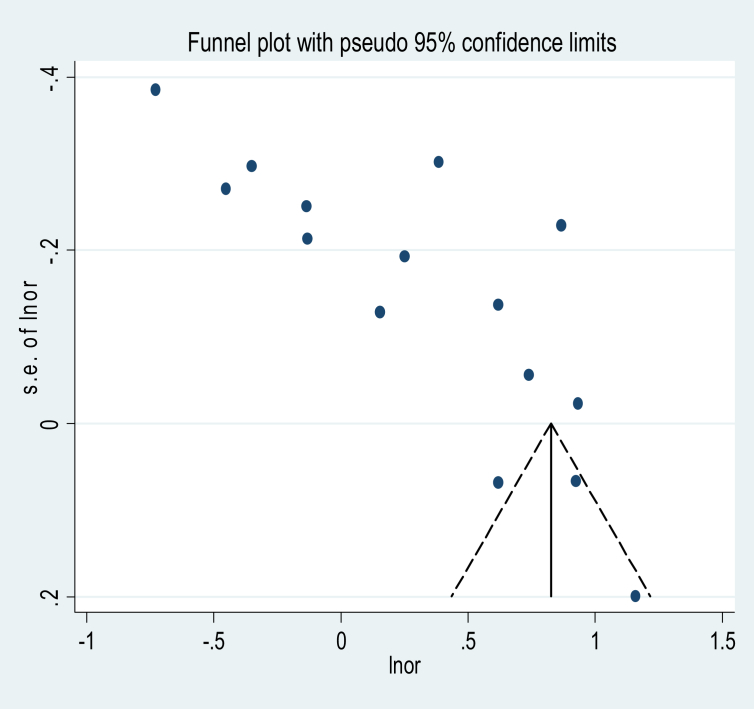
3.3.4. Publication bias
Subjectively, visual inspection of the funnel plot suggests asymmetry (Figure 5). Moreover, the result of Egger's test is a statistically significant objective evidence for the presence of publication bias (p = 0.000) (Table 2).
Figure 5.
Funnel plot.
Table 2.
Egger's test.
| Std_Eff | Coef. | Std. Err. | T | P > t | [95% Conf.Interval] |
|
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Slope | .4782276 | .0651614 | 7.34 | 0.000 | .3393394 | .6171157 |
| Bias | .3034239 | .0650815 | 4.66 | 0.000 | .1647059 | .4421419 |
4. Discussion
Globally, substance use during pregnancy remains a significant public health problem because it can lead to several harmful neonatal outcomes [1, 2, 3]. In this study, synthesis of the available pocket studies about birth outcome of antenatal khat chewing, cigarette smoking, alcohol drinking and narghile smoking showed positive odds of association with low birth weight, preterm birth, congenital malformation and low Apgar score, each of which is discussed in the following sections.
Birth weight is the best and most sensitive indicator of fetal insult to different maternal environmental exposure, thus indicating neonatal mortality and the quality of a country's health care system [46]. Low birth weight can be resulted from a multitude of factors. Among the factors, antenatal use of different substances has been identified to be significant [51]. Besides, the WHO Expert Committee on Drug Dependence (ECDD) critical review result showed that substance use during pregnancy may have different obstetric effects like low birth weight and stillbirths [15]. In this systematic review and meta-analysis, the pooled odds of low birth weight babies among mothers who chewed khat during pregnancy were 3 folds (95%CI: AOR = 3.19; 1.01, 5.37) higher than the non chewers. Consistent with this estimate, studies from Africa and Middle East demonstrate strong association between khat use and low birth weight [52, 53, 54]. Antenatal khat cheweres were also at 3 folds (95%CI: AOR = 3.21; 1.26, 8.85) increased likelihood of having neonates with low APGAR score. Such positive association of antenatal khat use with low birth weight and low APGAR score may be due to the sympathomimetic activity of cathinone, the active ingredient of khat responsible for its vasoconstrictive effects thus complicating pregnancy and birth outcome. The vasoconstrictive effects include maternal tachycardia, preeclampsia, reduced placental blood flow and fetal hypoperfusion, leading to intrauterine fetal hypoxia and restricted fetal growth [55]. Moreover, low birth weight could be due to the decreased maternal food intake (anorexia) which is attributable to the combined effects of gastrointestinal and nervous system changes induced by cathinone [25].
In this study, the odds of low birth weight newborns among mothers who drank alcohol during pregnancy were 9 folds (95% CI: AOR = 9.39; 2.84, 15.94) greater than the non drinkers, which is in agreement with studies in Japan [16] and Boston [17]. Similarly, antenatal alcohol users had 2 -fold higher likelihood of preterm baby than those who abstained from drinking (95% CI: AOR = 1.93; 0.52, 3.33), and it was in line with results from USA [13], Japan [16], Jordan [14] and Turkey [12]. Besides, our study showed significant effect of alcohol use on birth defect where drinkers were 2 times (95%CI: AOR = 2.16; 1.16, 3.17) more likely to possess congenitally defected neonates, and it was congruent with other settings [14, 56, 57, 58, 59, 60]. These adverse effects of alcohol use during pregnancy may be due to the fact that alcohol can disturb fetal development, especially affecting the fetal central nervous system with potentially severe lifelong consequences [5, 6]. Damage can occur in the earliest weeks of pregnancy, even before a woman knows that she is pregnant. Different mechanisms have been offered to explain the teratogenic effects of alcohol on the developing embryo. The mechanisms include the following: (1) Increased oxidative stress; (2) Disturbed glucose, protein, lipid and DNA metabolism; (3) Impaired neurogenesis and increased cellular apoptosis, especially of neural crest cells; (4) Endocrine effect; (5) Effects on gene expression [60, 61, 62, 63].
Most importantly, it was found that antenatal cigarette smoking mothers were 4 times more prone to deliver LBW neonates (95% CI: AOR = 4.36 (1.75, 6.98)) than their counter parts, and this finding accords with findings from studies in Brazil [10], China [11] and Turkey [12]. The LBW effect of smoking could be due to the assertion that when a woman smokes tobacco during pregnancy, her fetus is risked to hypoxia from the following reasons. Firstly, structural and functional integrity of the placenta becomes disrupted by different harmful chemicals within tobacco, thus impairing gas exchange [64]. Secondly, nicotine substrate of tobacco can cross the placenta and harm the fetus through constriction of fetal blood vessels resulting in fetal hypo-perfusion with oxygen and nutrients [65]. Thirdly, carbon monoxide of the tobacco smoke causes feto-maternal hypoxemia due to higher affinity of red blood cells (hemoglobin) to carbon mono oxide than oxygen, thus the red blood cells transport carbon monoxide (fetotoxic) to the fetus. Fourthly, if the mother is a chronic smoker, the tobacco smoke induces maternal pulmonary diseases due to its destructive effects of the respiratory mucosal hair cells and lung macrophages thus causing maternal hypoxemia and respiratory compromise [7, 8, 9, 66, 67]. Besides, smoking during pregnancy has been shown to result in decreased transfer of amino acids across the placenta [68], thereby causing symmetrical fetal growth impairment and LBW.
Antenatal narghile smokers were also 20 times more likely to own low birth weight newborns, and it was consistent with studies in Lebanon [34] and Greater Beirut area [33] where the odds of LBW among narghile smokers were 2.4 and 2.6 times greater than those who did not smoke. The consistency may be because narghile smoke has the same harmful components found in cigarette smoke including carbon monoxide, heavy metals, potentially cancerous tar fractions and nicotine [69, 70]. One of the greatest health hazards associated with narghile smoking is the increase in carboxyhaemoglobin level, which is formed by the binding of carbon monoxide with haemoglobin [71]. Carbon monoxide exposure has been shown to increase the risk of intrauterine growth restriction resulting in LBW [14, 72].
Our study focused on adverse birth outcomes of substance use during pregnancy. However, the effects of antenatal substance use is not limited to only adverse birth outcomes but also increasing the risk of hypertension, diabetic mellitus and other chronic illnesses during childhood career. Moreover, it has physical and mental health implications beyond childhood. Therefore, it is most advisable to help mothers quit using substance before getting pregnant. Moreover, strict comprehensive screening of every pregnant mother should be made at antenatal care clinics for early identification and management of any antenatal substance users.
5. Conclusion
This study gives national estimate on the adverse neonatal effects of khat, tobacco, alcohol and narghile use during pregnancy in Ethiopia. Prematurity, low birth weight and congenital malformation were the investigated adverse effects of antenatal substance use. Therefore, the existing public health efforts should be encouraged to help women cease using these substances completely before pregnancy. Increasing public awareness about the potential negative impacts of substance use during pregnancy on birth outcome would be of greatest importance for comprehensive intervention in the prevention of antenatal substance use. Most importantly, the authors have just reached the following 2 new hypotheses that should be tested by further studies:
-
1.
Using different dosages of a substance during pregnancy may have different effects on adverse birth outcome.
-
2.
Maternal poly-substance use could have additive effects on adverse birth outcome.
5.1. Limitations of the study
Despite strength of the study in synthesizing national evidence about the effect of antenatal substance use on birth outcomes, there are some limitations that need to be considered in future researches. The main limitation was lack of articles from Benishangul Gumuz, Harari, Dire Dawa, Somali, Gambela and Afar regions of Ethiopia. Once more, there were scarce of publications about the low APGAR score effect of substance use during pregnancy in Ethiopia. Moreover, a dose-response relationship between the quantity of substance consumed prenatally and the effect on neonatal outcomes was not addressed. We did not also assess the long-term outcomes of antenatal substance use on childhood growth and neurodevelopment.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
PubMed search strategy.
References
- 1.World Health Organization . 2014. WHO Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. [PubMed] [Google Scholar]
- 2.Forray A. Substance use during pregnancy [version 1; referees: 2 approved] F1000Research. 2016;5(887) [Google Scholar]
- 3.ACOG . 2017. (the American College of Obstetricians and Gynacologists) Tobacco, Alcohol, Drugs, and Pregnancy. [Google Scholar]
- 4.Barakoti R., Ghimire A., Pokharel P.K., Pandey A.R., B D.D. Tobacco use during pregnancy and its associated factors in a mountain district of eastern Nepal. Front. Publ. Health. 2017;5(129) doi: 10.3389/fpubh.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. (Centers for Disease Control and Prevention) 2016. Vital Signs: Alcohol and Pregnancy. [Google Scholar]
- 6.CDC (Centers for Disease Control and Prevention) U.S. Surgeon General; 2005. Advisory on Alcohol Use in Pregnancy: A 2005 Message to Women from the. [Google Scholar]
- 7.Shankaran Impact of maternal substance use during pregnancy on childhood outcome April. Semin. Fetal Neonatal Med. 2013;12(2):143–150. doi: 10.1016/j.siny.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiFranza J.R., Lew R.A. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J. Fam. Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Smoking during pregnancy—United States, 1990-2002. MMWR Morb. Mortal. Wkly. Rep. 2004;53:911–915. [PubMed] [Google Scholar]
- 10.Kataoka Smoking during pregnancy and harm reduction in birth weight: a cross-sectional study. BMC Pregnancy Childbirth. 2018;18:67. doi: 10.1186/s12884-018-1694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S.-H. The effects of maternal smoking exposure during pregnancy on postnatal outcomes: a cross sectional study. J. Chin. Med. Assoc. 2017;80:796e802. doi: 10.1016/j.jcma.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Bolat F. Maternal smoking during pregnancy and eff ects on neonatal anthropometry: a prospective study. Turk. J. Med. Sci. 2012;42(6):999–1005. © TÜBİTAK. E-mail: medsci@tubitak.gov.tr. [Google Scholar]
- 13.Liu B., Xu G., Sun Y., Qiu X., Ryckman K.K., Yu Y. Maternal cigarette smoking before and during pregnancy and the risk of preterm birth: a dose–response analysis of 25 million mother–infant pairs. PLoS Med. 2020;17(8) doi: 10.1371/journal.pmed.1003158. pmed.1003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amasha Hadayat A., Jaradeh Malak S. Effect of Active and Passive smoking during pregnancy on its outcomes. Health Sci. J. 2012;6(2) [Google Scholar]
- 15.Dependence WECoD, World Health Organization . World Health Organization; 2006. WHO Expert Committee on Drug Dependence: Thirty-Fourth Report. Report No.: 9241209429. [Google Scholar]
- 16.Miyake Alcohol consumption during pregnancy and birth outcomes: the kyushu okinawa maternal and child health study. BMC Pregnancy Childbirth. 2014;14:79. doi: 10.1186/1471-2393-14-79. http://www.biomedcentral.com/1471-2393/14/79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marbury The association of alcohol consumption with outcome of pregnancy. Am. J. Publ. Health. 1983;73:1165–1168. doi: 10.2105/ajph.73.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streissguth A.P. Paul H. Brookes Publishing Co.; Baltimore, M.D.: 1997. Fetal Alcohol Syndrome: A Guide for Families and Communities. [Google Scholar]
- 19.Stratton K., Howe C., Battaglia F., editors. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- 20.Spohr H.-L., Steinhausen H.-C., editors. Alcohol, Pregnancy and the Developing Child. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- 21.Abel E.L. Plenum Press; New York, N.Y.: 1998. Fetal Alcohol Abuse Syndrome. [Google Scholar]
- 22.Jacobson J.L., Jacobson S.W. Effects of prenatal alcohol exposure on child development. Alcohol Res. Health. 2002;26(4):282–286. [PMC free article] [PubMed] [Google Scholar]
- 23.Latino-Martel Paule, Chan Doris S.M., Druesne-Pecollo Nathalie, Barrandon Emilie, Hercberg Serge, Norat Teresa. Cancer epidemiology, biomarkers and prevention. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010 May;19(5):1238–1260. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- 24.Alebachew Wubet. Prevalence, associated factors and consequences of substance use among health and medical science students of Haramaya University, eastern Ethiopia, 2018: a cross-sectional study. BMC Psychiatr. 2019;19(1) doi: 10.1186/s12888-019-2340-z. NA. Gale OneFile: Health and Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bifftu Berhanu Boru. Europe PMC; 2020. Prevalence of Khat Chewing during Pregnancy in Ethiopia: a Systematic Review and Meta-Analysis. Preprint from Research Square. [Google Scholar]
- 26.Kedir H., Berhane Y., Worku A. Khat chewing and restrictive dietary behaviors are associated with anemia among pregnant women in high prevalence rural communities in eastern Ethiopia. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0078601. PMID: 24223828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam M.W., al-Shabanah O.A., al-Harbi M.M., al-Gharably N.M. Evaluation of teratogenic potential of khat (Catha edulis Forsk.) in rats. Drug Chem. Toxicol. 1994;17(1):51–68. doi: 10.3109/01480549409064046. PMID: 8168433. [DOI] [PubMed] [Google Scholar]
- 28.Demelash H., Motbainor A., Nigatu D., Gashaw K., Melese A. Risk factors for low birth weight in Bale zone hospitals, South-East Ethiopia: a case-control study. BMC Pregnancy Childbirth. 2015;15:264. doi: 10.1186/s12884-015-0677-y. PMID: 26463177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dendir E., Deyessa N. Substance use and birth weight among mothers attending public hospitals: a case control study. Ethiop. J. Health Dev. 2017;31(1):27–35. [Google Scholar]
- 30.Tesfaye K. 2018. The Effect of Khat Chewing on Low Birthweight in Southern Ethiopia. [Google Scholar]
- 31.Nakajima M., Jebena M.G., Taha M. Correlates of khat use during pregnancy: a cross-sectional study. Addict. Behav. 2017;73:178–184. doi: 10.1016/j.addbeh.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Kiter G., Ucan E.S., Ceylan E., Kilinc O. Water-pipe smoking and pulmonary functions. Respir. Med. 2000;94:891–894. doi: 10.1053/rmed.2000.0859. [DOI] [PubMed] [Google Scholar]
- 33.Tamim H., Yunis K., Chemaitelly H., Alameh M. Nassar A for the national collaborative perinatal neonatal network Beirut, Lebanon. Effect of narghile and cigarette smoking on newborn birthweight. BJOG. 2008;115:91–97. doi: 10.1111/j.1471-0528.2007.01568.x. [DOI] [PubMed] [Google Scholar]
- 34.Nuwayhid I.A., Yamout B., Azar G., Kambris M.A. Narghile (hubble bubble) smoking, low birth weight, and other pregnancy outcomes. Am. J. Epidemiol. 1998;148:375–383. doi: 10.1093/oxfordjournals.aje.a009656. [DOI] [PubMed] [Google Scholar]
- 35.Belete . 2019. The effect of alcohol use during pregnancy on birth outcome in Southern Tigray. Northern Ethiopia. [Google Scholar]
- 36.Kelkay B., Omer A., Teferi Y., Moges Y. Factors associated with singleton preterm birth in Shire Suhul general hospital, northern Ethiopia, 2018. J. Pregnancy. 2019;2019:4629101. doi: 10.1155/2019/4629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woday A., Muluneh M.D., Sherif S. Determinants of preterm birth among mothers who gave birth at public hospitals in the Amhara region, Ethiopia: a case-control study. PloS One. 2019;14(11) doi: 10.1371/journal.pone.0225060. e0225060–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekele I., Demeke T., Dugna K. Prevalence of preterm birth and its associated factors among mothers delivered in jimma university specialized teaching and referral hospital, jimma zone, oromia regional state, South west Ethiopia. J. Women's Health Care. 2017;6:356. [Google Scholar]
- 39.Adane Fentahun, Seyoum Girma. Prevalence and associated factors of birth defects among newborns at referral hospitals in Northwest Ethiopia. Ethiop. J. Health Dev. 2018;32(3) 00-000. [Google Scholar]
- 40.Taye Factors associated with congenital anomalies in Addis Ababa and the Amhara Region, Ethiopia: a case-control study. BMC Pediatr. 2018;18:142. doi: 10.1186/s12887-018-1096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsehay Determinants and seasonality of major structural birth defects among newborns delivered at primary and referral hospital of East and West Gojjam zones, Northwest Ethiopia 2017–2018: case–control study. BMC Res. Notes. 2019;12:495. doi: 10.1186/s13104-019-4541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aboye Prevalence and associated factors of low birth weight in Axum town, Tigray, North Ethiopia. BMC Res. Notes. 2018;11:684. doi: 10.1186/s13104-018-3801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alem Lake Eyasu, Fite Robera Olana. Low birth weight and its associated factors among newborns delivered at wolaita sodo university teaching and referral hospital, southern Ethiopia. Int. J. Pediatr. 2019:2018. doi: 10.1155/2019/4628301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mekonnen Modifiable risk factors of congenital malformations in bale zone hospitals, Southeast Ethiopia: an unmatched casecontrol study. BMC Pregnancy Childbirth. 2020:20–129. doi: 10.1186/s12884-020-2827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Getachew Determinants of low fifth minute apgar score among newborns delivered in jimma university medical center, southwest Ethiopia. Int. J. Pediatr. 2020 doi: 10.1155/2020/9896127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehare Tsegaye, Sharew Yewbmirt. Prevalence and associated factors of low birth weight among term newborns in dilla town, Southern Ethiopia. Int. J. Pediatr. 2020 doi: 10.1155/2020/8394578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller M., D’Addario M., Egger M., Cevallos M., Dekkers O., Mugglin C. Methods to systematically review and meta-analyze observational studies: a systematic scoping review of recommendations. BMC Med. Res. Methodol. 2018;18:44. doi: 10.1186/s12874-018-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 50.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 51.WHO . 2014. World Health Ranking: Ethiopia Low Birth Weight. [Google Scholar]
- 52.WHO . 2014. WHA Global Nutrition Targets 2025: Low Birth Weight Policy Brief. [Google Scholar]
- 53.Khawaja M., Al-Nsour M., Saad G. Khat (Catha edulis) chewing during pregnancy in Yemen: findings from a national population survey. Matern. Child Health J. 2008;12:308–312. doi: 10.1007/s10995-007-0231-2. [DOI] [PubMed] [Google Scholar]
- 54.Hassan N., Gunaid A., Murray Lyon I. Khat (Catha edulis): health aspects of khat chewing. East. Mediterr. Health J. 2007;13:706–718. [PubMed] [Google Scholar]
- 55.Mwenda J.M., Arimi M.M., Kyama M.C. Effects of khat (Catha edulis) consumption on reproductive functions: a review. East Afr. Med. J. 2003;80:318–323. doi: 10.4314/eamj.v80i6.8709. [DOI] [PubMed] [Google Scholar]
- 56.Smid Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin. Obstet. Gynecol. 2019;62(Number 1):168–184. doi: 10.1097/GRF.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornman-Homonoff Joshua, Kuehn Devon, Aros Sofía, Carter Tonia C., Conley Mary R., James Troendle, Cassorla Fernando, James L., Mills Heavy prenatal alcohol exposure and risk of stillbirth and preterm delivery. J. Matern. Fetal Neonatal Med. 2012;25(6):860–863. doi: 10.3109/14767058.2011.587559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patra J., Bakker R., Irving H., Jaddoe V.W.V., Malini S., Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG An Int. J. Obstet. Gynaecol. 2011;118(12):1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foltran Francesca, Gregori Dario, Franchin Laura, Verduci Elvira, Giovannini Marcello. Effect of alcohol consumption in prenatal life, childhood, and adolescence on child development. Nutr. Rev. 2011;69(11):642–659. doi: 10.1111/j.1753-4887.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- 60.Aliyu Muktar H., Lynch O’Neil, Nana Philip N., Alio Amina P., Wilson Roneé E., Marty Phillip J., Zoorob Roger, Hamisu M., Salihu Alcohol consumption during pregnancy and risk of placental abruption and placenta previa. Matern. Child Health J. 2011;15(5):670–676. doi: 10.1007/s10995-010-0615-6. [DOI] [PubMed] [Google Scholar]
- 61.Luczak S.E., Glatt S.J., Wall T.L. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol. Bull. 2006;132:607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- 62.Cartwright M.M., Smith S.M. Increased cell death and reduced neural crest cell numbers in ethanol-exposed embryos: partial basis for the fetal alcohol syndrome phenotype. Alcohol Clin. Exp. Res. 1995;19:378–386. doi: 10.1111/j.1530-0277.1995.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 63.Heaton M.B., Paiva M., Mayer J., Miller R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci. Lett. 2002;334:83–86. doi: 10.1016/s0304-3940(02)01123-0. [DOI] [PubMed] [Google Scholar]
- 64.Kay H.H., Tsoi S., Grindle K., Magness R.R. Markers of oxidative stress in placental villi exposed to ethanol. J. Soc. Gynecol. Invest. 2006;13:118–121. doi: 10.1016/j.jsgi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Zdravkovic T., Genbacev O., McMaster M.T., Fisher S.J. The adverse effects of maternal smoking on the human placenta: a review. Placenta. 2005;26(Suppl A):S81–S86. doi: 10.1016/j.placenta.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Latino-Martel P., Chan D.S., Druesne-Pecollo N., Barrandon E., Hercberg S., Norat T. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Canc. Epidemiol. Biomarkers Prev.: Publ. Am. Assoc. Canc. Res. Cosponsored Am Soc. Prev. Oncol. 2010;19(5):1238–1260. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- 67.Raguz Marjana Jerkovic. Prevalence and effect of cigarette smoking in pregnancy and pregnancy outcomes. EC Gynaecol. 2017;5(2):63–68. [Google Scholar]
- 68.Ingvarsson R.F. The effects of smoking in pregnancy on factors influencing fetal growth. Acta Pædiatrica. 2007;96:383–386. doi: 10.1111/j.1651-2227.2007.00103.x. [DOI] [PubMed] [Google Scholar]
- 69.Pastrakuljic A. Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta. 1999;20:499–512. doi: 10.1053/plac.1999.0418. [DOI] [PubMed] [Google Scholar]
- 70.Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem. Toxicol. 2003;41:143–152. doi: 10.1016/s0278-6915(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 71.Sajid K.M. Carbon monoxide fractions in cigarette and hookah (hubble bubble) smoke. J. Pakistan Med. Assoc. 1993;43:179–182. [PubMed] [Google Scholar]
- 72.Zahran F.M. Carboxyhemoglobin concentrations in smokers of sheesha and cigarettes in Saudi Arabia. Br. Med. J. 1985;291:1768–1770. doi: 10.1136/bmj.291.6511.1768-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PubMed search strategy.
Data Availability Statement
Data included in article/supplementary material/referenced in article.