Abstract
Although weight loss suggests poor prognosis of COPD, only a few studies have examined total energy expenditure (TEE) or physical activity level (PAL) using the doubly labelled water (DLW) method. We evaluated TEE and PAL using the DLW method together with a triaxial accelerometer to elucidate the relationships between TEE, PAL and clinical parameters leading to a practical means of monitoring COPD physical status.
This study evaluated 50- to 79-year-old male patients with mild to very severe COPD (n=28) or at risk for COPD (n=8). TEE, activity energy expenditure for 2 weeks and basal metabolic rate were measured by DLW, an accelerometer and indirect calorimetry, respectively. All patients underwent pulmonary function, chest-computed tomography, 6-min walk test, body composition and grip strength tests. Relationships between indices of energy expenditure and clinical parameters were analysed. Bland–Altman analysis was used to examine the agreement of TEE and PAL between the DLW method and the accelerometer.
TEE and PAL using DLW in the total population were 2273±445 kcal·day−1 and 1.80±0.20, respectively. TEE by DLW correlated well with that from the accelerometer and grip strength (p<0.0001), and PAL by DLW correlated well with that from the accelerometer (p<0.0001), grip strength and 6-min walk distance (p<0.001) among various clinical parameters. However, the accelerometer underestimated TEE (215±241 kcal·day−1) and PAL (0.18±0.16), with proportional biases in both indices.
TEE and PAL can be estimated by accelerometer in patients with COPD if systematic errors and relevant clinical factors such as muscle strength and exercise capacity are accounted for.
Short abstract
Although total energy expenditure and physical activity level in COPD patients were underestimated by an accelerometer, they can be predicted using indices of the accelerometer and clinical parameters such as the 6-min walk distance and grip strength. https://bit.ly/2OYbuaI
Introduction
COPD is characterised by airflow limitation, with percentage forced expiratory volume in 1 s (FEV1) used as an index of the disease severity [1]. However, the introduction of the body mass index (BMI), airflow obstruction, dyspnoea, exercise capacity (BODE) index demonstrated that weight loss, dyspnoea and reduced exercise capacity should be considered simultaneously when trying to predict the COPD prognosis in addition to the obstructive disorder [2]. Furthermore, it has been reported that a low physical activity level (PAL) determined by a multisensory armband is the best predictor of poor COPD prognosis among all clinical parameters, which include percentage FEV1, 6-min walk distance (6 MWD), BMI, the modified Medical Research Council (mMRC) dyspnoea scale and the BODE index [3]. Recent investigations have highlighted the importance of providing adequate nutrition and exercise in order to maintain ideal body weight, muscle volume and strength, and physical activity, which can lead to improvement in the quality of life (QOL) and prognosis in COPD [4–7]. However, only a few studies have investigated total energy expenditure (TEE) in COPD using the standard doubly labelled water (DLW) method [8, 9]. Although prior studies that used the DLW method have suggested that patients with severe and very severe COPD tended to have an increased basal metabolic rate (BMR) and decreased activity energy expenditure (AEE) [10, 11], the characteristics of energy expenditure remain unclear in patients with mild to moderate COPD, who need to be prevented from developing physical inactivity and malnutrition. Although estimations of PAL using a pedometer or an accelerometer have shown a decrease in the PAL in accordance with the severity of airflow limitation [12, 13], it was reported that AEE determined by an accelerometer tended to underestimate values as compared to the DLW method [14], and that TEE calculated using the AEE and the predicted BMR was possibly underestimated in COPD patients.
Therefore, the present study attempted to accurately measure TEE and PAL in patients with mild to moderate COPD using the DLW method and indirect calorimetry in conjunction with an accelerometer, in order to elucidate the relationships between the TEE and PAL and clinical COPD parameters, which included pulmonary function, muscle volume and strength and exercise capacity. In addition, we tried to clarify the differences in the TEE and PAL assessments in COPD between the DLW method and the accelerometer, in order to evaluate the practical usefulness of the accelerometer.
Methods
Study population
This study enrolled patients with COPD (n=28) and those at risk of COPD (n=9). All patients were consecutive outpatients seen at the Saitama Medical University Hospital (Saitama, Japan) between June 2017 and February 2018, and who met the following criteria: male, age 50–79 years, presence of COPD (Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, 2, 3, 4) or at risk of COPD (GOLD 0). COPD was diagnosed in accordance with the GOLD 2020 guideline [1]. All GOLD 0 patients had chronic respiratory symptoms including cough, sputum or dyspnoea on exertion and a ≥10-pack-year smoking history in the absence of airflow limitation (FEV1/forced vital capacity ratio ≥0.7) after inhalation of bronchodilators. Exclusion criteria are described in the supplementary material. After one GOLD 0 patient discontinued the study due to acute bronchitis, a total of 36 patients were included in the analysis. This study was approved by the institutional review board of Saitama Medical University Hospital (no. 16-003-1), Keio University (protocol no. 2015-03) and National Institutes of Biomedical Innovation, Health and Nutrition (protocol no. 29). Written informed consent was obtained from each patient.
Pulmonary function tests and 6-min walk test
Pulmonary function tests were performed using a FUDAC-7 instrument (Fukuda Denshi, Tokyo, Japan). Spirometry parameters, lung volume subdivisions and the diffusing capacity of the lung for carbon monoxide (DLCO) were measured in all patients. As indices of respiratory muscle strength, maximal expiratory and inspiratory pressure were measured using a spirometer (Autospiro AS-507; Minato Medical Science, Osaka, Japan). The predicted pulmonary function values were calculated according to the Japanese Respiratory Society guidelines [15]. The 6-min walk test (6MWT) was performed by experienced technicians in accordance with the American Thoracic Society guidelines, except for duplication [16]. The following data were collected in all patients: baseline oxygen saturation measured by pulse oximetry (SpO2) and heart rate (HR), lowest SpO2 and highest HR during the test. Dyspnoea and leg fatigue were assessed using a modified Borg scale from 0 to 10 and the 6-min walk distance (6MWD) was measured at the end of the test.
Questionnaires and chest computed tomography analysis
At the beginning of the study, severity of dyspnoea was estimated using the mMRC dyspnoea scale, while the disease-related QOL was evaluated using the COPD Assessment Test score. A chest computed tomography (CT) scanner (Somatom Emotion 16; Siemens Healthcare, Erlangen, Germany) and a Synapse Vincent volume analyser (Fujifilm Medical, Tokyo, Japan) were used for this study [17]. The conditions of the chest CT analysis are presented in the supplementary material.
Study schedule
This study was conducted in the hospital during two scheduled visits that occurred over 13–15 days. For both visits, patients arrived in a fasted state after overnight fasting. At visit 1, height, body weight and other baseline information was obtained. BMR was measured by indirect calorimetry while body composition was measured by bioelectrical impedance analysis. After taking DLW at visit 1, the physical activity was then measured using a triaxial accelerometer for 13–15 days. The 6MWT was performed within a month, while chest CT scans and pulmonary function tests were performed within 3 months before and after the study period. All of the examinations were performed under stable conditions.
Weight, body composition and grip strength
The methods for obtaining the weight, body composition and grip strength are shown in the supplementary material.
Measurement of TEE by the DLW method
TEE was measured by the DLW method (modified two-point approach) as reported previously [18]. At visit 1, an oral dose of 0.1 g 2H2O and 2.0 g H218O (Taiyo Nippon Sanso, Tokyo, Japan) per kg of estimated total body water was given to each patient. The specific details are described in the supplementary material.
Measurement and prediction of BMR
BMR was measured by indirect calorimetry (BMRI) at visit 1 (Quark RMR; COSMED, Rome, Italy) [19], while BMRG was predicted using the Ganpule equation [20, 21]. The detail is described in the supplementary material.
Evaluation of PAL by the DLW method and an accelerometer
PALDLW was defined as follows:
In addition, physical activity was evaluated using a triaxial accelerometer (Active Style Pro, HJA-750C; Omron Healthcare, Kyoto, Japan), which was developed to classify locomotive and nonlocomotive activities through the use of a ratio of unfiltered and filtered synthetic acceleration combined with a gravity-removal physical activity classification algorithm that was utilised for determining an accurate estimation of the AEE of nonlocomotive activities [22]. Metabolic equivalents (METs) were estimated by applying different equations for different types of activities. This analysis used the 60-s-epoch data. Periods with >60 min of consecutive nonwear time were classified as nonwear time, while a valid day was defined as ≥600 min per day of wear time. Patients wore the accelerometer on their waist for 13–15 days, except during bathing and sleep. AEE is reasonably measured by the device, which also provides data regarding steps per day, sedentary (<1.5 METs) time and light (1.5 to <3.0 METs), moderate (3.0 to <6.0 METs) and vigorous (≥6.0 METs) walking and daily activity times. In this study, PALACC (PAL estimated by an accelerometer) was calculated as follows:
(10/9 was added to the equation for the correction of diet-induced thermogenesis (DIT)).
Statistical analysis
Data are presented as mean±sd. Values were compared between the DLW and the accelerometer using the paired t-test. Multiple comparisons were performed using the Tukey–Kramer test. Univariate associations were analysed using Pearson's correlation coefficient. Multiple regression analysis was performed to predict TEEDLW and PALDLW. The Bland–Altman plot was used to evaluate the agreement of TEE and PAL between DLW and accelerometer methods, and of BMR between indirect calorimetry and the Ganpule equation. p-values <0.05 were considered significant. All data were analysed using JMP version 14 software (SAS Institute, Cary, NC, USA).
Results
Characteristics of the patients
As shown in table 1, the total population primarily consisted of patients with mild to moderate COPD (20 out of 36), with a mean FEV1 of 69.4% predicted.
TABLE 1.
Patient characteristics
| All | GOLD 0 | COPD (GOLD 1–4) | GOLD 0 versus COPD | |
| Subjects | 36 | 8 | 28 (1 n=6, 2 n=14, 3 n=6, 4 n=2) | |
| Age years | 70.3±5.8 | 70.3±7.1 | 70.3±5.5 | ns |
| mMRC | 0.9±1.0 | 0.4±0.5 | 1.1±1.0 | ns |
| CAT score | 10.1±6.1 | 9.6±4.0 | 10.2±6.7 | ns |
| BMI kg·m−2 | 21.9±3.2 | 21.2±3.7 | 22.1±3.2 | ns |
| Fat mass % | 23.3±5.0 | 24.6±3.4 | 22.9±5.3 | ns |
| FFMI kg·m−2 | 16.7±2.3 | 15.9±2.7 | 16.9±2.2 | ns |
| SMI kg·m−2 | 9.1±1.0 | 8.8±1.2 | 9.2±0.9 | ns |
| Grip strength kg | 33.9±7.0 | 29.6±6.1 | 35.1±6.8 | p<0.05 |
| PEmax % | 72.6±18.7 | 71.8±22.1 | 72.8±18.0 | ns |
| PImax % | 87.6±29.3 | 89.8±15.7 | 87.0±32.3 | ns |
| Vital capacity % | 95.3±16.2 | 98.9±12.0 | 94.2±17.2 | ns |
| FEV1 % pred | 69.4±24.4 | 94.6±10.7 | 62.1±22.3 | p<0.001 |
| FEV1/FVC % | 55.4±17.4 | 78.5±7.8 | 48.8±13.2 | p<0.0001 |
| Residual volume % | 117.1±35.4 | 99.4±17.2 | 122.2±37.7 | ns |
| DLCO/VA % | 74.0±27.4 | 83.1±19.4 | 71.5±29.0 | ns |
| LAA % | 13.9±13.6 | 9.4±8.6 | 15.1±14.6 | ns |
| 6MWD m | 435±95 | 442±23 | 433±107 | ns |
| ΔSpO2 % | 7.3±4.6 | 4.4±2.4 | 8.1±4.7 | p<0.05 |
| ΔHR beats·min−1 | 38.2±15.2 | 31.5±7.2 | 40.1±16.4 | ns |
| Dyspnoea | 2.2±2.2 | 1.1±1.4 | 2.5±2.4 | ns |
| Leg fatigue | 0.7±1.3 | 0.8±1.2 | 0.7±1.3 | ns |
Data are presented as n or mean±sd, unless otherwise stated. GOLD: Global Initiative for Chronic Obstructive Lung Disease; mMRC: modified Medical Research Council dyspnoea scale; CAT: COPD Assessment Test; BMI: body mass index; FFMI: fat-free mass index; SMI: skeletal muscle mass index; PEmax: maximum expiratory pressure; PImax: maximum inspiratory pressure; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; VA: alveolar volume; LAA: low attenuation area; 6MWD: 6-min walk distance; SpO2: percutaneous oxygen saturation; ΔHR: difference in heart rate; ns: nonsignificant.
Energy expenditure and physical activity in accordance with the severity of airflow limitation
BMI was lower in GOLD grades 3 and 4 versus GOLD 2 (table 2). There were no differences in TEEDLW, PALDLW and BMRI among patients at risk of COPD or with mild to very severe COPD. For the accelerometer, there was no difference in any of the parameters except for a decrease in the moderate walking time in GOLD grades 3 and 4 versus GOLD grade 1.
TABLE 2.
Energy expenditure and physical activity
| All (GOLD 0–4) | GOLD 0 | GOLD 1 | GOLD 2 | GOLD 3, 4 | COPD (GOLD 1–4) | Multiple comparison | |
| Subjects | 36 | 8 | 6 | 14 | 8 | 28 | |
| Age years | 70.3±5.8 | 70.3±7.1 | 68.8±8.9 | 71.4±4.4 | 69.6±4.5 | 70.3±5.5 | ns |
| BMI kg·m−2 | 21.9±3.2 | 21.2±3.7 | 21.8±1.3 | 23.5±3.0 | 19.7±3.2# | 22.1±3.2 | p<0.05# |
| BMRI kcal·day−1 | 1262±180 | 1229±234 | 1297±173 | 1316±158 | 1172±148 | 1271±165 | ns |
| BMRG kcal·day−1 | 1272±145 | 1254±201 | 1278±124 | 1309±133 | 1223±125 | 1278±129 | ns |
| BMRI/BMRG | 0.99±0.07 | 0.98±0.08 | 1.01±0.06 | 1.01±0.07 | 0.96±0.07 | 0.99±0.07 | ns |
| TEEDLW kcal·day−1 | 2273±445 | 2240±629 | 2496±435 | 2378±322 | 1956±296 | 2283±393 | ns |
| TEEACC kcal·day−1 | 2058±315 | 1982±353 | 2168±292 | 2146±308 | 1897±269 | 2080±307 | ns |
| TEEDLW−TEEACC kcal·day−1 | 215±241 | 258±324 | 327±299 | 232±188 | 59±120 | 203±218 | ns |
| PALDLW | 1.80±0.20 | 1.80±0.22 | 1.92±0.16 | 1.81±0.19 | 1.67±0.21 | 1.80±0.20 | ns |
| PALACC | 1.61±0.14 | 1.58±0.12 | 1.70±0.13 | 1.64±0.13 | 1.55±0.14 | 1.63±0.14 | ns |
| PALDLW−PALACC | 0.18±0.16 | 0.22±0.16 | 0.22±0.16 | 0.18±0.18 | 0.12±0.09 | 0.17±0.16 | ns |
| AEEACC kcal·day−1 | 580±178 | 529±156 | 674±180 | 623±181 | 484±162 | 594±184 | ns |
| Steps per day | 5978±3209 | 5808±3171 | 7778±3590 | 5874±2116 | 5079±4482 | 6055±3273 | ns |
| Sedentary min | 506±137 | 456±112 | 456±112 | 472±155 | 580±125 | 499±144 | ns |
| Light walking min | 38.8±27.6 | 30.0±18.5 | 40.3±22.5 | 35.7±20.8 | 51.9±44.6 | 41.3±29.5 | ns |
| Moderate walking min | 28.4±23.5 | 30.6±21.4 | 44.5±29.1 | 30.3±22.0 | 10.6±14.1* | 27.7±24.3 | p<0.05* |
| Vigorous walking min | 0.08±0.28 | 0.13±0.35 | 0.17±0.41 | 0.07±0.27 | 0 | 0.07±0.26 | ns |
| Light daily activity min | 261±83 | 243±84 | 301±83 | 267±80 | 237±87 | 266±83 | ns |
| Moderate daily activity min | 19.9±17.9 | 16.9±14.6 | 25.5±14.2 | 25.6±22.4 | 8.8±7.9 | 20.8±18.8 | ns |
Data are presented as n or mean±sd, unless otherwise stated. GOLD: Global Initiative for Chronic Obstructive Lung Disease; BMI: body mass index; BMR: basal metabolic rate; BMRI: BMR measured by indirect calorimetry; BMRG: BMR predicted using the Ganpule equation; TEE: total energy expenditure; DLW: doubly labelled water; ACC: accelerometer; PAL: physical activity level; AEE: activity energy expenditure; ns: nonsignificant. *: p<0.05 versus GOLD 1; #: p<0.05 versus GOLD 2.
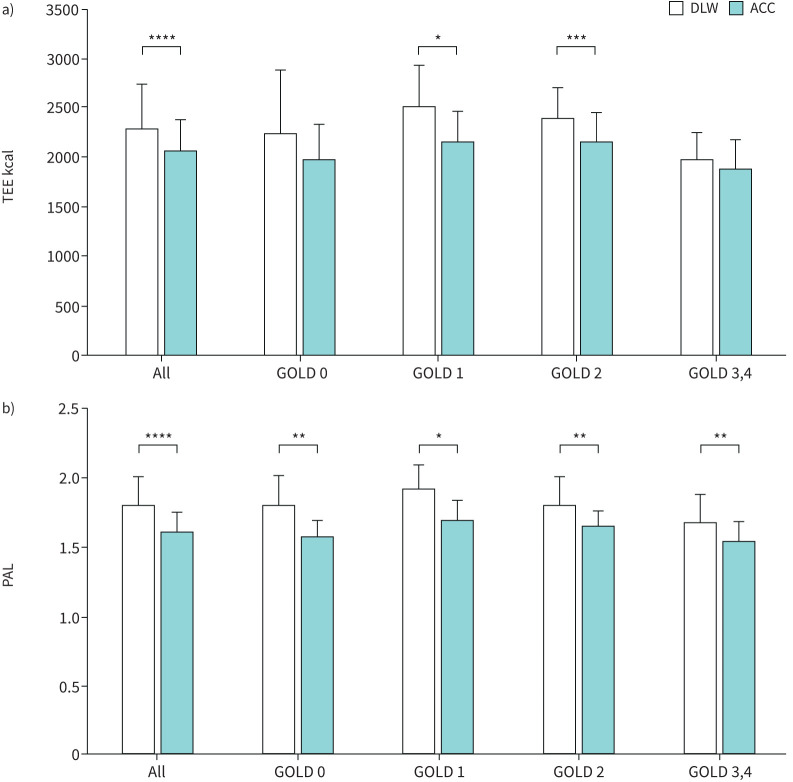
There were significant differences in the TEE between GOLD 1 and 2 observed for the DLW and the accelerometer (figure 1a). For PAL, there were significant differences observed for all comparisons between the DLW and the accelerometer (figure 1b).
FIGURE 1.
Differences in a) total energy expenditure (TEE) and b) physical activity level (PAL) between the doubly labelled water (DLW) method and measurement by accelerometer (ACC). Significant differences in TEE (all, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1 and GOLD 2) and PAL (all, GOLD 0, GOLD 1, GOLD 2 and GOLD 3,4) were observed between the two methods. *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001.
Validation of TEEACC and PALACC in comparison with TEEDLW and PALDLW, and of BMRG to BMRI using Bland–Altman plots
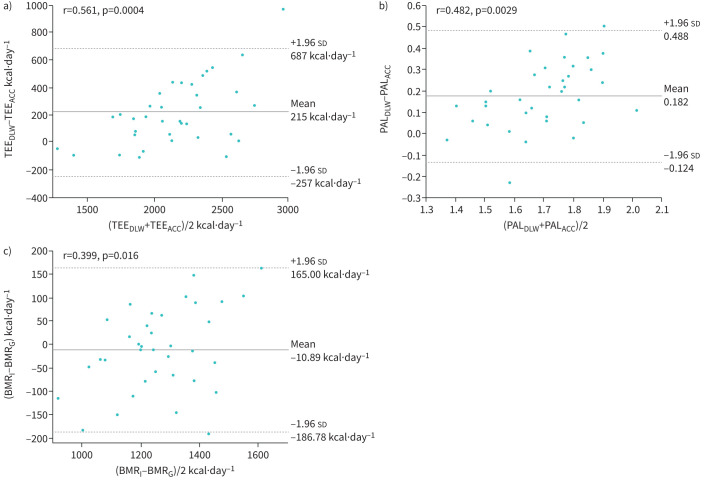
As shown in figure 2, fixed bias was observed in both the TEE (95% CI 133.5–296.6 kcal·day−1, p<0.0001) and PAL (95% CI 0.129–0.235, p<0.0001) between the two methods. Proportional bias was also observed in both TEE (r=0.561, p=0.0004) and PAL (r=0.482, p=0.0029). No fixed bias was seen in BMR (95% CI −25.8–4.1 kcal·day−1, nonsignificant), whereas slight proportional bias was observed (r=0.399, p=0.016).
FIGURE 2.
Bland–Altman plots for a) total energy expenditure (TEE) and b) physical activity level (PAL), used to compare the values determined by the doubly labelled water (DLW) and accelerometer (ACC) methods, and c) basal metabolic rate (BMR), to compare the values obtained by indirect calorimetry (BMRI) and the Ganpule (BMRG) equation. Mean values are depicted by solid lines, while dotted lines show ranges within the 95% confidence interval. The 95% confidence interval for the difference in TEE was 133.5–296.6 kcal·day−1 (p<0.0001) and that in PAL was 0.129–0.235 (p<0.0001) between the two methods. No difference in BMR (95% CI −25.8–4.1 kcal·day−1, nonsignificant) was seen between measurements and the predicted values.
When TEEACC and PALACC were calculated with BMRI instead of BMRG, the fixed and proportional biases to those values from DLW were still observed in TEE (mean 227 kcal·day−1, p<0.0001, r=0.461, p=0.0046) and PAL (mean 0.176, p<0.0001, r=0.451, p=0.0058), respectively, by Bland–Altman analyses.
Using AEEDLW (defined as 0.9×TEEDLW−BMRI), the difference between TEEDLW and TEEACC correlated well with AEEDLW (r=0.732, p<0.0001) and modestly with BMRI (r=0.488, p=0.0025). The difference between PALDLW and PALACC correlated well with AEEDLW (r=0.680, p<0.0001), but not with BMRI. A significant difference was apparent between AEEDLW (784±275 kcal·day−1) and AEEACC (580±178 kcal·day−1, p<0.0001).
Univariate regression analysis between energy expenditure, physical activity and clinical parameters
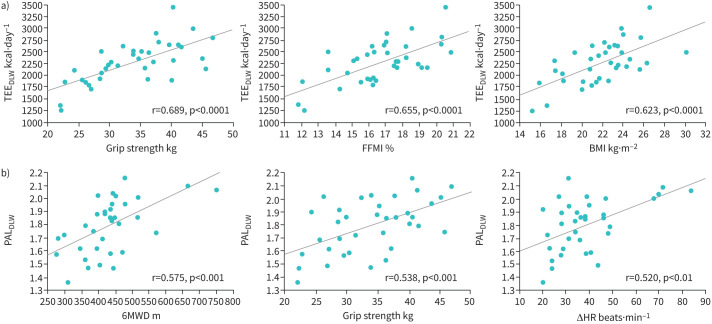
Various factors including age, BMI, fat-free mass index (FFMI), skeletal muscle mass index (SMI), grip strength, percentage vital capacity (%VC), %DLCO/alveolar volume (VA), low attenuation area (LAA)%, 6MWD, change in HR and leg fatigue during the 6MWT were associated with TEEDLW (table 3). In contrast, the factors related to PALDLW were limited to 6MWD, change in HR during 6MWT, grip strength and %FEV1. Figure 3 shows the association of the cardinal clinical parameters with TEEDLW (figure 3a) and PALDLW (figure 3b).
TABLE 3.
Relationships between energy expenditure/physical activity and clinical parameters
| TEEDLW | PALDLW | |||
| r | p-value | r | p-value | |
| Age years | -0.361 | <0.05 | 0.132 | ns |
| mMRC | 0.217 | ns | 0.276 | ns |
| CAT | 0.282 | ns | 0.213 | ns |
| BMI kg·m−2 | 0.623 | <0.0001 | 0.158 | ns |
| Fat mass % | 0.005 | ns | 0.092 | ns |
| FFMI kg·m−2 | 0.655 | <0.0001 | 0.226 | ns |
| SMI kg·m−2 | 0.593 | <0.001 | 0.156 | ns |
| Grip strength kg | 0.689 | <0.0001 | 0.538 | <0.001 |
| PEmax % | 0.255 | ns | 0.188 | ns |
| PImax % | 0.020 | ns | 0.280 | ns |
| Vital capacity % | 0.359 | <0.05 | 0.301 | ns |
| FEV1% | 0.313 | ns | 0.394 | <0.05 |
| FEV1/FVC % | 0.143 | ns | 0.286 | ns |
| Residual volume % | 0.165 | ns | 0.134 | ns |
| DLCO/VA% | 0.387 | <0.05 | 0.258 | ns |
| LAA % | -0.334 | <0.05 | -0.275 | ns |
| 6MWD m | 0.355 | <0.05 | 0.575 | <0.001 |
| ΔSpO2 % | 0.056 | ns | 0.142 | ns |
| ΔHR beats·min−1 | 0.330 | <0.05 | 0.520 | <0.01 |
| Dyspnoea | 0.048 | ns | 0.009 | ns |
| Leg fatigue | -0.322 | <0.05 | -0.269 | ns |
TEE: total energy expenditure; DLW: doubly labelled water; PALDLW: physical activity level using TEEDLW; mMRC: modified Medical Research Council dyspnoea scale; CAT: COPD Assessment Test; BMI: body mass index; FFMI: fat-free mass index; SMI: skeletal muscle mass index; PEmax: maximum expiratory pressure; PImax: maximum inspiratory pressure; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; VA: alveolar volume; LAA: low attenuation area; 6MWD: 6-min walk distance; SpO2: percutaneous oxygen saturation; HR: heart rate; ns: nonsignificant.
FIGURE 3.
Correlations between a) total energy expenditure determined using the doubly labelled water method (TEEDLW) or b) physical activity level determined using TEEDLW (PALDLW) and clinical parameters. There was a good correlation between the grip strength, fat-free mass index (FFMI) and body mass index (BMI) and the TEEDLW. 6-min walk distance (6MWD), grip strength, and the change in heart rate (ΔHR) during the 6-min walk test were well correlated with the PALDLW.
Relationships between energy expenditure, physical activity and the parameters of the accelerometer
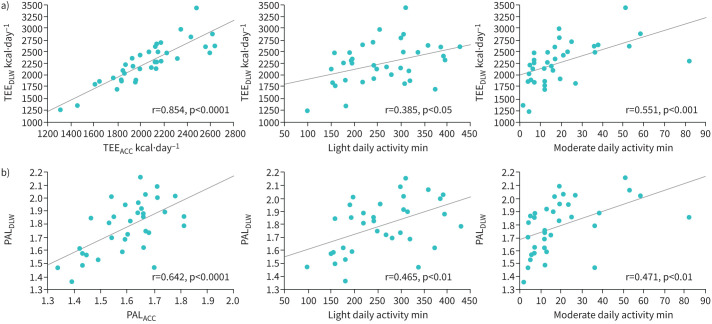
As shown in table 4, light and moderate daily activity times were correlated with both indices. Furthermore, there was a good correlation between TEEDLW and TEEACC. These associations are shown in figure 4a (TEEDLW) and 4b (PALDLW).
TABLE 4.
Relationships between energy expenditure/physical activity and parameters of the accelerometer
| TEEDLW | PALDLW | |||
| r | p-value | r | p-value | |
| Steps per day | 0.185 | ns | 0.377 | <0.05 |
| Sedentary time min | 0.022 | ns | 0.088 | ns |
| Light walking time min | 0.055 | ns | 0.191 | ns |
| Moderate walking time min | 0.205 | ns | 0.362 | <0.05 |
| Vigorous walking time min | 0.380 | <0.05 | 0.246 | ns |
| Light daily activity time min | 0.385 | <0.05 | 0.465 | <0.01 |
| Moderate daily activity time min | 0.551 | <0.001 | 0.471 | <0.01 |
| TEEACC kcal·day−1 | 0.854 | <0.0001 | 0.554 | <0.001 |
| PALACC | 0.531 | <0.001 | 0.642 | <0.0001 |
| AEEACC kcal·day−1 | 0.722 | <0.0001 | 0.635 | <0.0001 |
TEE: total energy expenditure; DLW: doubly labelled water; PALDLW: physical activity level using TEEDLW; ACC: accelerometer; AEE: activity energy expenditure; ns: nonsignificant.
FIGURE 4.
Correlations of a) total energy expenditure determined using the doubly labelled water method (TEEDLW) and b) physical activity level determined using TEEDLW (PALDLW) with the accelerometer (ACC) parameters. TEEDLW was strongly correlated with TEEACC (r=0.854, p<0.0001), while the association between PALDLW and PALACC was relatively modest (r=0.642, p<0.0001). Light (1.5–3.0 metabolic equivalents (METs)) and moderate (3.0–6.0 METs) daily activity times were correlated with both indices.
Prediction of TEEDLW and PALDLW according to the accelerometer and clinical parameters
Since TEEDLW correlated strongly with TEEACC, the following equation may predict TEEDLW using the accelerometer: TEEDLW=−210.2+1.207×TEEACC (r=0.854, corrected coefficient of determination=0.721). When this equation was used to predict TEEDLW based on TEEACC values in all 36 patients, the ratio of patients for whom predicted values were within ±10% of measured TEEDLW was 58%. In contrast, PALDLW correlated modestly with PALACC (r=0.642, corrected coefficient of determination=0.395). Multiple regression analyses including clinical parameters are shown in the supplementary material.
Discussion
The present study demonstrated that BMR, TEE and PAL as evaluated by indirect calorimetry and DLW methods were preserved as nearly normal among patients at risk for or with mild to moderate COPD. These three indices tended to decrease in patients with severe to very severe COPD. Yamada et al. [23] reported that elderly men in the Japanese community without any associated participation in sporting activities showed a mean BMI 23.6 kg·m−2, BMRI 1242 kcal·day−1, TEEDLW 2308 kcal·day−1 and PALDLW 1.85, similar to that found for COPD patients at GOLD 1 and 2 in this study. No significant difference in BMR was seen between measured values and prediction by the Ganpule equation even in COPD patients, although slight proportional bias was observed by Bland–Altman plots in the present study. The DLW method is the gold standard for measuring TEE [24]. However, since this method is difficult to apply during routine clinical practice, comparison of data from the DLW method and indirect calorimetry with that for the accelerometer and predicted BMR is important. The present study demonstrated that TEE and PAL by the DLW method correlated well with those from the accelerometer, but were underestimated by the accelerometer (fixed bias), as reported previously [14, 23]. In addition, in conjunction with the increases in TEE and PAL, the differences in TEE and PAL between the two methods also increased (proportional bias), respectively.
As stated in the results section, differences in TEE and PAL paralleled the AEE as evaluated by the DLW method. Since TEE and PAL are composed of BMR, AEE and DIT, these three factors should be tested as causes for biases between DLW and accelerometer-based methods. Bland–Altman analyses using measured BMR values did not significantly improve the biases, and a prior study did not show significant changes in DIT in patients with COPD [25]. Thus, underestimation of AEE by the accelerometer may be mainly responsible for the fixed and proportional biases between the two methods. One possibility is that energy expenditure by respiratory muscles that was not detected by the accelerometer could have been further increased during certain activities in COPD patients as compared to healthy subjects. The difference between AEEDLW (784 kcal·day−1) and AEEACC (580 kcal·day−1) nearly corresponded to that between TEEDLW (2273 kcal·day−1) and TEEACC (2058 kcal·day−1) in the total population. Figure 1a suggests that ∼200–300 kcal·day−1 should be added to the estimated energy expenditure by the accelerometer in COPD patients at GOLD 1 or 2.
As compared to the DLW method, accelerometers can provide additional information, such as steps per day and sedentary, walking and daily activity times. Of interest is the observation that the associations of light and moderate daily activity times with TEEDLW and PALDLW were greater than those observed for steps per day, sedentary time and light and moderate walking times. As the present results showed the best index was moderate daily activity time (3.0–6.0 METs), further analysis of daily activities is needed to clarify how best to maintain physical activity in COPD.
In 2005, Pitta et al. [26] reported that physical activity monitored by an accelerometer was significantly decreased in COPD patients versus healthy elderly subjects, in contrast to our findings. These differences may be partly attributable to the distinct study populations. Mean FEV1 was 43% in COPD patients and 111% in healthy subjects in the study by Pitta et al., while values in our study were 62% and 95%, respectively. In addition, all GOLD 0 patients were symptomatic, with most requiring bronchodilator treatment. Moreover, use of long-acting inhaled bronchodilator may have contributed to the differences in outcomes between the study by Pitta et al. and the present investigation, as only salmeterol was available before 2005, while various kinds of long-acting muscarinic antagonists and β2-agonists are now commonly available and in widespread use. In 2015, Waschki et al. [27] reported that PAL evaluated by an accelerometer decreased proportional to the extent of airflow limitation in GOLD grades 0–4. This trend was consistent with our observations that PAL tended to decrease in GOLD 3 or 4 as compared to that observed in GOLD 1 or 2. One of the most important findings in the study by Waschki et al. was that PAL was significantly decreased at 3 years after baseline measures in all groups classified by GOLD stage. Thus, inhibiting the progression of physical inactivity is important for every COPD patient, regardless of the degree of obstruction.
Analysis based on the DLW method demonstrated that TEE correlated modestly with %VC, % DLCO/VA and LAA% (p<0.05), but even better with grip strength and FFMI (p<0.0001). In addition, more significant correlations with PALDLW were seen for grip strength (p<0.001), 6MWD (p<0.001) and change in HR during 6MWT (p<0.01) as compared to %FEV1 (p<0.05). These observations imply that energy expenditure and physical activity in COPD patients were primarily determined by muscle strength in general and exercise capacity, while the contributions of pulmonary function were relatively small, based on TEEDLW. These findings were consistent with the notion that PALDLW correlated with changes in HR, but not with change in SpO2 or dyspnoea during the 6MWT. This demonstrates that dyspnoea on exertion may be unrelated to reduced physical activity in our study population. As expected, TEEDLW, which is a very important index for determining energy intake in COPD, was more significantly related to BMI, FFMI, SMI and grip strength than to the other factors.
Several limitations to this study need to be considered when interpreting the present findings. Due to the difficulties inherent in using the DLW method, the study population was relatively small. Furthermore, women were not included to avoid sex differences in TEE and PAL. In addition, the study population included only two patients with very severe COPD and excluded patients with diabetes mellitus who required medication.
In conclusion, TEE and PAL were preserved in patients with mild to moderate COPD, as well as those at risk of COPD. TEE and PAL values as estimated by the accelerometer were lower than those from the DLW method, but correlated with each other and showed differences related to activity energy expenditure. TEE and PAL can also be estimated by the accelerometer with clinical parameters including exercise capacity and muscle strength. These observations may promote better monitoring of the physical status in patients with mild to moderate COPD and could help prevent the development of future weight loss and poor prognosis.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00407-2020.SUPPLEMENT (131.5KB, pdf)
Acknowledgements
We thank Azusa Sasaki (National Institutes of Biomedical Innovation, Health and Nutrition, Tokyo, Japan) and Hitoshi Miyazawa (Saitama Medical University, Saitama, Japan) for their skillful technical assistance.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: H. Sato has nothing to disclose.
Conflict of interest: H. Nakamura reports grants from the Japan Agency for Medical Research and Development (AMED) during the conduct of the study.
Conflict of interest: Y. Nishida has nothing to disclose.
Conflict of interest: T. Shirahata has nothing to disclose.
Conflict of interest: S. Yogi has nothing to disclose.
Conflict of interest: T. Akagami has nothing to disclose.
Conflict of interest: M. Soma has nothing to disclose.
Conflict of interest: K. Inoue has nothing to disclose.
Conflict of interest: M. Niitsu has nothing to disclose.
Conflict of interest: T. Mio has nothing to disclose.
Conflict of interest: T. Miyashita has nothing to disclose.
Conflict of interest: M. Nagata has nothing to disclose.
Conflict of interest: S. Nakae has nothing to disclose.
Conflict of interest: Y. Yamada has nothing to disclose.
Conflict of interest: S. Tanaka reports grants from the Japan Agency for Medical Research and Development (AMED) during the conduct of the study.
Conflict of interest: F. Katsukawa reports grants from the Japan Agency for Medical Research and Development (AMED) during the conduct of the study.
Support statement: This work was supported by the Japan Agency for Medical Research and Development (grant numbers JP17ek0210045 and JP20ek0210112). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2020 Report. https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf
- 2.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 3.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140: 331–342. doi: 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 4.Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014; 44: 1504–1520. doi: 10.1183/09031936.00070914 [DOI] [PubMed] [Google Scholar]
- 5.Garvey C, Bayles MP, Hamm LF, et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines. J Cardiopulm Rehabil Prev 2016; 36: 75–83. doi: 10.1097/HCR.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 6.Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. doi: 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 7.Mantoani LC, Rubio N, McKinstry B, et al. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J 2016; 48: 69–81. doi: 10.1183/13993003.01744-2015 [DOI] [PubMed] [Google Scholar]
- 8.Farooqi N, Carlsson M, Håglin L, et al. Energy expenditure in women and men with COPD. Clin Nutr ESPEN 2018; 28: 171–178. doi: 10.1016/j.clnesp.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 9.Farooqi N, Slinde F, Håglin L, et al. Assessment of energy intake in women with chronic obstructive pulmonary disease: a doubly labeled water method study. J Nutr Health Aging 2015; 19: 518–524. doi: 10.1007/s12603-014-0575-4 [DOI] [PubMed] [Google Scholar]
- 10.Donahoe M, Rogers RM, Wilson DO, et al. Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 140: 385–391. doi: 10.1164/ajrccm/140.2.385 [DOI] [PubMed] [Google Scholar]
- 11.Hugli O, Schutz Y, Fitting JW. The daily energy expenditure in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153: 294–300. doi: 10.1164/ajrccm.153.1.8542132 [DOI] [PubMed] [Google Scholar]
- 12.Farooqi N, Slinde F, Carlsson M, et al. Predicting energy requirement with pedometer-determined physical-activity level in women with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10: 1129–1137. doi: 10.2147/COPD.S80616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayata A, Minakata Y, Matsunaga K, et al. Differences in physical activity according to mMRC grade in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 2203–2208. doi: 10.2147/COPD.S109694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinovich RA, Louvaris Z, Raste Y, et al. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J 2013; 42: 1205–1215. doi: 10.1183/09031936.00134312 [DOI] [PubMed] [Google Scholar]
- 15.Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 2014; 52: 242–250. doi: 10.1016/j.resinv.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 17.Tanabe N, Muro S, Sato S, et al. Fractal analysis of low attenuation clusters on computed tomography in chronic obstructive pulmonary disease. BMC Pulm Med 2018; 18: 144. doi: 10.1186/s12890-018-0714-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morino K, Kondo K, Tanaka S, et al. Total energy expenditure is comparable between patients with and without diabetes mellitus: Clinical Evaluation of Energy Requirements in Patients with Diabetes Mellitus (CLEVER-DM) Study. BMJ Open Diabetes Res Care 2019; 7: e000648. doi: 10.1136/bmjdrc-2019-000648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9. doi: 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganpule AA1, Tanaka S, Ishikawa-Takata K, et al. Interindividual variability in sleeping metabolic rate in Japanese subjects. Eur J Clin Nutr 2007; 61: 1256–1261. doi: 10.1038/sj.ejcn.1602645 [DOI] [PubMed] [Google Scholar]
- 21.Miyake R, Tanaka S, Ohkawara K, et al. Validity of predictive equations for basal metabolic rate in Japanese adults. J Nutr Sci Vitaminol 2011; 57: 224–232. doi: 10.3177/jnsv.57.224 [DOI] [PubMed] [Google Scholar]
- 22.Ohkawara K, Oshima Y, Hikihara Y, et al. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr 2011; 105: 1681–1691. doi: 10.1017/S0007114510005441 [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Hashii-Arishima Y, Yokoyama K, et al. Validity of a triaxial accelerometer and simplified physical activity record in older adults aged 64–96 years: a doubly labeled water study. Eur J Appl Physiol 2018; 118: 2133–2146. doi: 10.1007/s00421-018-3944-6 [DOI] [PubMed] [Google Scholar]
- 24.Hills AP, Mokhtar N, Byrne NM. Assessment of physical activity and energy expenditure: an overview of objective measures. Front Nutr 2014; 1: 5. doi: 10.3389/fnut.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doré MF, Laaban JP, Orvoën-Frija E, et al. Role of the thermic effect of food in malnutrition of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 155: 1535–1540. doi: 10.1164/ajrccm.155.5.9154854 [DOI] [PubMed] [Google Scholar]
- 26.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 972–977. doi: 10.1164/rccm.200407-855OC [DOI] [PubMed] [Google Scholar]
- 27.Waschki B, Kirsten AM, Holz O, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 295–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00407-2020.SUPPLEMENT (131.5KB, pdf)