Growth hormone-activated STAT5b is an essential regulator of sex-differential gene expression in mouse liver; however, its impact on hepatic gene expression and epigenetic responses is poorly understood. Here, we found a substantial, albeit incomplete loss of liver sex bias in hepatocyte-specific STAT5a/STAT5b (collectively, STAT5)-deficient mouse liver.
KEYWORDS: RRBS, enhancers, CpG methylation, sex bias, DNase hypersensitive site, DHS, chromatin, liver
ABSTRACT
Growth hormone-activated STAT5b is an essential regulator of sex-differential gene expression in mouse liver; however, its impact on hepatic gene expression and epigenetic responses is poorly understood. Here, we found a substantial, albeit incomplete loss of liver sex bias in hepatocyte-specific STAT5a/STAT5b (collectively, STAT5)-deficient mouse liver. In male liver, many male-biased genes were downregulated in direct association with the loss of STAT5 binding; many female-biased genes, which show low STAT5 binding, were derepressed, indicating an indirect mechanism for repression by STAT5. Extensive changes in CpG methylation were seen in STAT5-deficient liver, where sex differences were abolished at 88% of ∼1,500 sex-differentially methylated regions, largely due to increased DNA methylation upon STAT5 loss. STAT5-dependent CpG hypomethylation was rarely found at proximal promoters of STAT5-dependent genes. Rather, STAT5 primarily regulated the methylation of distal enhancers, where STAT5 deficiency induced widespread hypermethylation at genomic regions enriched for accessible chromatin, enhancer histone marks (histone H3 lysine 4 monomethylation [H3K4me1] and histone H3 lysine 27 acetylation [H3K27ac]), STAT5 binding, and DNA motifs for STAT5 and other transcription factors implicated in liver sex differences. Thus, the sex-dependent binding of STAT5 to liver chromatin is closely linked to the sex-dependent demethylation of distal regulatory elements linked to STAT5-dependent genes important for liver sex bias.
INTRODUCTION
The mammalian STAT (signal transducer and activator of transcription) gene family encodes seven latent cytoplasmic transcription factors. STATs are activated by cell surface receptor-induced tyrosine phosphorylation, which stimulates STAT protein dimerization and nuclear translocation, followed by DNA binding and transcriptional activation of STAT target genes (1). STAT5a is highly expressed in mammary tissues, where it is activated by prolactin (2, 3), while the closely related (96% similar) STAT5b is particularly abundant in hepatocytes, where it is activated by growth hormone (GH) and regulates lipid metabolism (4–6) as well as body growth, in part through production of IGF-1 (7). STAT5b is also a key mediator of the sex differential transcriptional networks that GH regulates in the liver (8, 9), as seen in a STAT5b whole-body knockout mouse model (10). GH activates STAT5b signaling by binding to GH receptor on the liver cell surface. The GH receptor, in turn, activates the receptor-associated tyrosine kinase JAK2, stimulating phosphorylation of GH receptor on multiple intracellular tyrosine residues, thereby creating docking sites for downstream signaling proteins, including STAT5b. JAK2 phosphorylates STAT5b on Tyr-699, which enables STAT5b to dimerize and then translocate to the nucleus (11), where it binds to STAT5 motifs enriched at open chromatin regions in hepatocytes and stimulates gene transcription (12).
Liver STAT5 activity is highly responsive to GH stimulation (13). In many mammalian species, including humans (14), rats, and mice (8, 15), the temporal pattern of pituitary GH secretion differs between males and females. In male mice and rats, pituitary GH secretion is episodic, with strong pulses every 3 to 4 h, followed by a GH-free interval; whereas in females, pituitary GH secretion is more frequent, resulting in a persistent (near-continuous) plasma GH profile. Liver STAT5 activity mirrors the sex differences in circulating GH patterns, and consequently, it oscillates between active and inactive states in male liver, but it is more persistently active in female liver (12, 16, 17). These sex-dependent temporal differences in liver STAT5 activity are the dominant determinant of the sex-biased expression of hundreds of genes in mouse and rat liver, including many cytochromes P450 and other enzymes of steroid and drug metabolism, as was demonstrated in mice with a global deficiency in STAT5b (10); furthermore, they are an important factor in sex differences in drug and steroid metabolism and disease susceptibility (8, 18). STAT5a deficiency can also impact sex-biased gene expression in the liver, albeit to a much lesser extent than STAT5b, consistent with the much lower levels of STAT5a present in liver (19). Hypophysectomy (16, 20) and continuous GH infusion (21, 22) disrupt the sex-dependent patterns of circulating GH and liver STAT5 signaling, which in turn abolishes or dysregulates the expression of sex-dependent protein-coding genes, regulatory microRNAs (23), and long noncoding RNAs (lncRNAs) (24, 25). Importantly, while exogenous GH replacement can restore sex-specific expression of Cyp and other genes in the livers of hypophysectomized mice, this restoration is not achieved in the livers of hypophysectomized STAT5b knockout mice (22, 26). While these studies demonstrate the essential nature of STAT5b for sex-differentiated liver gene expression, they do not distinguish effects due to the loss of STAT5b in liver per se versus effects due to loss of STAT5 expression in other tissues, including the hypothalamus, which can impact plasma GH profiles and liver responses to GH (27). These issues are addressed here, where we use transcriptome sequencing (RNA-seq) to evaluate the impact of hepatocyte-specific deletion of the Stat5a/Stat5b locus (28) on the liver transcriptome in both male and female mice, and its relationship to sex-specific binding sites for STAT5 (12, 29) associated with regulation of sex-biased gene expression in the liver.
DNA methyltransferase-catalyzed cytosine methylation at the C-5 position is a heritable epigenetic mark in higher vertebrates and is key for many biological processes, including X chromosome inactivation and imprinting (30, 31). In mammalian somatic cells, a large majority of DNA methylation occurs in the context of CpG dinucleotides, with most CpG sites being highly methylated, except for CpGs within gene promoters. These patterns of DNA methylation are stably propagated in terminally differentiated cells throughout life; however, changes in CpG methylation can occur in response to hormonal and other environmental factors (32, 33) and diseased states (30). DNA methylation generally leads to gene silencing and chromatin condensation, although DNA methylation can also stimulate gene expression (34). Sex differences in DNA methylation have been reported for mouse liver (35–37) and may, in part, be determined by CpG demethylation associated with testosterone exposure at puberty (38–40). It is unclear, however, what role STAT5 might play in regulating these sex differences in CpG methylation or how they may relate to the STAT5-dependent regulation of sex-specific gene expression. Here, we use reduced representation bisulfite sequencing (41) to characterize the effects of STAT5 on CpG methylation, including sex differences in liver CpG methylation. Our findings establish that liver STAT5 deficiency abolishes the sex bias in DNA methylation at more than 1,300 genomic sites and that STAT5-dependent sex-biased genes are frequently controlled by STAT5-regulated demethylation associated with the activation of distal enhancers.
RESULTS
Impact of hepatocyte-specific STAT5 loss on sex-biased liver gene expression.
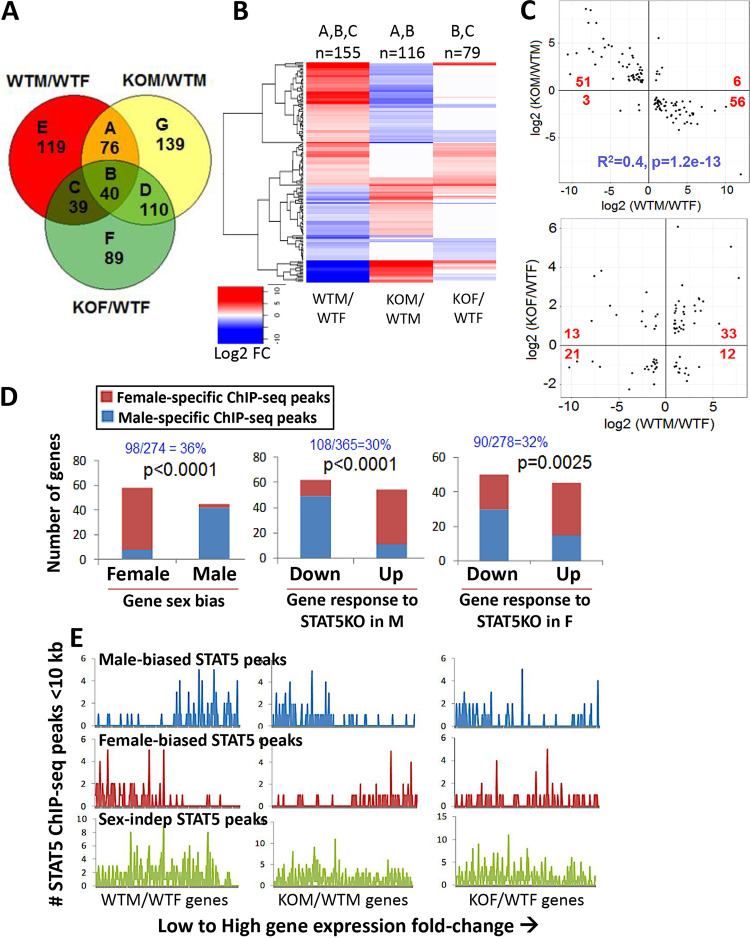
Global inactivation of the mouse Stat5b gene leads to widespread loss of sex-biased gene expression in the liver (10). As the observed changes in liver gene expression could be due, in part, to extrahepatic actions of STAT5b on pituitary GH secretion (26), which is a major regular of sex-specific gene expression in the liver (21), we used RNA-seq to determine the impact of hepatocyte-specific deletion of the Stat5a/Stat5b locus (STAT5 knockout [STAT5-KO]) (28, 42) on the expression of sex-biased genes in the liver. Differential expression analysis was conducted using liver RNA purified from wild-type male (WTM) and wild-type female (WTF) floxed control mice and from STAT5-KO male (KOM) and STAT5-KO female (KOF) mice. A total of 274 liver-expressed genes showed differential expression between male and female controls (WTM/WTF comparison, false-discovery rate [FDR] of <0.05): 132 male-biased and 142 female-biased genes. Genes that were responsive to STAT5-KO in male liver (365 genes) and in female liver (278 genes) were identified in two additional comparisons, KOM/WTM and KOF/WTF (see Table S1 in the supplemental material). The gene overlap across the three comparisons is presented in Fig. 1A. Of all 274 sex-biased genes, 155 (57%) responded to STAT5 deficiency in either male or female liver versus only 2.2% of stringently sex-independent genes (Table 1). Male-biased genes whose expression was altered were primarily downregulated in STAT5-KO male liver or were upregulated in STAT5-KO female liver. Conversely, female-biased genes whose expression was altered were primarily upregulated in STAT5-KO male liver or were downregulated in STAT5-KO female liver (Fig. 1B and Table 1). This relationship between sex specificity and response to STAT5 deficiency is visualized in quantitative scatterplots showing highly significant differences in the response of male-biased and female-biased genes to STAT5-KO loss in male liver, and to a lesser degree in female liver (Fig. 1C). The extent of sex bias and magnitude of response to STAT5 deficiency were significantly correlated in male liver (r2 = 0.4, P = 1.2E−13) but not in female liver. Thus, STAT5 acts at the level of the hepatocyte to regulate sex-biased gene expression via both activating and repressive mechanisms, strongly in male liver and weakly in female liver.
FIG 1.
Gene expression changes in STAT5a/STAT5b-deficient mouse liver. (A) Venn diagram showing 612 significantly changing genes (FDR < 0.05) identified in wild-type (WT) and STAT5 knockout (KO) male (M) and female (F) mouse liver for three comparisons: WTM/WTF (wild-type male versus wild-type female; sex-biased genes), KOM/WTM (knockout male versus wild-type male, i.e., STAT5-responsive genes in male liver), and KOF/WTF (knockout female versus wild-type female, i.e., STAT5-responsive genes in female liver). The number of differentially expressed genes for each Venn segment (labeled A through G) is indicated. See Table S1A and B in the supplemental material for gene lists. (B) Heat map representation of log2 fold change of 155 genes that showed sex-specific expression in wild-type liver and their corresponding expression changes in STAT5-KO male and STAT5-KO female livers. These genes correspond to segments A, B, and C in the Venn diagram, as indicated above the heatmap. n, number of genes. (C) Scatterplots of log2 expression fold change values of sex-biased genes (WTM/WTF) that responded significantly to STAT5 loss in male liver (top, 116 genes; Venn diagram segments A and B), or that responded significantly to STAT5 loss in female liver (bottom, 79 genes; Venn diagram segments B and C). The number of genes in each quadrant was analyzed by Fisher’s exact test to assess whether the proportions of male- and female-biased genes upregulated or downregulated by STAT5 loss are significantly different: P < 2.2E−16 (top) and P = 0.003 (bottom). Linear regression analysis of correlation between magnitude of sex bias and strength of response to STAT5 deletion: r2 = 0.40 and P = 1.2E−13 (top) and r2 = 0.07 and P = 0.01 (bottom). (D) Relationship between STAT5 transcription factor binding and liver gene expression. Genes with one or more sex-biased STAT5 ChIP-seq peaks within 10 kb were counted (y axis) and are indicated in blue text as a fraction of all genes in each gene set (top). (Left) In wild-type liver, female-biased genes were significantly enriched for having nearby female-biased STAT5 ChIP-seq peaks, and male-biased genes for having nearby male-biased STAT5 ChIP-seq peaks. (Middle) In male liver,genes downregulated by STAT5-KO were significantly enriched for nearby male-biased STAT5 ChIP-seq peaks, and genes upregulated by STAT5-KO were enriched for nearby female-biased STAT5 ChIP-seq peaks. P values for the sex difference in these response patterns are shown (Fisher’s exact test). (Right) In female liver, STAT5-responsive genes followed the same pattern as those in male liver but show less significant differential enrichment for sex-biased STAT5 ChIP-seq peaks (higher P value). (E) Shown are the number of STAT5 ChIP-seq peaks (male-biased, female-biased, or sex-independent STAT5 ChIP-seq peaks) within 10 kb of each individual gene that is differentially expressed at a FDR of <0.05 between WTM/WTF (274 genes, left), KOM/WTM (365 genes, middle), and KOF/WTF (278 genes, right), ranked from low to high gene expression fold change. This represents a per gene visual representation of the results calculated in panel D. See Table S1B for expression values of these 612 genes.
TABLE 1.
Liver-expressed genes showing significant expression changes in hepatocyte-specific STAT5-KO mouse livera
| Sex bias in wild-type liver |
Fraction of KO-responsive genes | Change in expression in STAT5-KO | Response to STAT5-KO in male liver |
Response to STAT5-KO in female liver |
||
|---|---|---|---|---|---|---|
| Gene count | % | Gene count | % | |||
| Male biased and KO responsive | 87/132 = 66% | Increase | 6 | 9.7 | 33 | 73.3 |
| Decrease | 56 | 90.3 | 12 | 26.7 | ||
| Female biased and KO responsive | 68/142 = 48% | Increase | 51 | 94.4 | 13 | 38.2 |
| Decrease | 3 | 5.6 | 21 | 61.8 | ||
| Sex independent and KO responsive | 140/6,306 = 2.2% | Increase | 65 | 61.3 | 58 | 66.7 |
| Decrease | 41 | 38.7 | 29 | 33.3 | ||
A total of 414 liver-expressed genes (FPKM > 1 in either wild-type male or female liver) were analyzed: 132 male-biased genes, 142 female-biased genes, and 140 stringently sex-independent genes whose expression changes significantly (FDR < 0.05) with STAT5 deficiency in either male or female mouse liver (Table S1A and B). Data are shown for 155 of the 274 sex-biased genes (57%; 87 of 132 male-biased genes and 68 of 142 female-biased genes) and for 140 of the 6,306 stringently sex-independent genes (2.2%) that are expressed in the liver and whose expression changes significantly with STAT5 deficiency in either male or female mouse liver (genes “responsive” to STAT5-KO). Percentage values indicate the distribution of each gene group between up- and downregulation in STAT5-deficient mouse liver. The patterns of response to STAT5 deficiency were significantly different (Fisher’s exact test) between male-biased and female-biased genes, between female-biased and sex-independent genes in male livers and in female livers, and between male-biased and sex-independent genes in male liver only. Response patterns were also significantly different for male-biased genes between male and female livers and for female-biased genes between male and female livers.
Sex-biased expression was lost in STAT5-KO liver for 168 (61%) of the 274 sex-biased genes, as judged by comparison of male and female STAT5-KO livers (KOM/KOF comparison [Table S1A]). Moreover, sex bias was reduced in the STAT5-KO strain for 80% of the 106 genes that did not meet our cutoff for loss of sex-biased expression. Further supporting our conclusion that STAT5 deficiency impacts the vast majority of sex-biased genes, we found that 81 of the 106 genes that were apparently unresponsive to STAT5-KO actually showed the same overall trend in their response to STAT5 deficiency in male liver as was seen for the 168 significantly responsive sex-biased genes, namely, 43 of 53 male-biased genes decreased in expression, and 38 of 53 female-biased genes increased in expression. Of note, strong female-specific expression was retained in STAT5-KO liver for the GH-regulated transcriptional repressor Cux2 (43, 44). The lack of an effect on Cux2 expression may in part explain the incomplete loss of sex-biased gene expression in STAT5-KO livers, given the role of CUX2 in GH regulation of a significant subset of sex-biased genes in mouse liver (43). Other highly female-biased genes (WTF/WTM > 4) that appeared to be truly unresponsive to the loss of STAT5 (KOM/WTM |fold change| < 1.2) include Cyp3a16, Hsd3b1, Ugt2b37, and Nipal1 (Table S1B).
STAT5 binding contributes to sex-biased gene expression and STAT5 response.
STAT5 binds to liver chromatin in a sex-differential manner near a subset of sex-biased genes, as determined by chromatin immunoprecipitation-sequencing (ChIP-seq) analysis (12). Here, we examined the set of 98 of 274 sex-biased genes (36%) with a nearby (within 10 kb of gene body) sex-biased STAT5 ChIP-seq peak (Table S1A). Female-biased genes were significantly associated with female-biased STAT5 ChIP-seq peaks, and male-biased genes were significantly associated with male-biased STAT5 ChIP-seq peaks (P < 0.0001, Fisher’s exact test) (Fig. 1D and E, left panels), as expected (12). These positive associations indicate that proximal sex-biased STAT5 binding primarily imparts sex-biased expression by activation of gene expression. Furthermore, genes downregulated in STAT5-KO male liver were significantly enriched for having proximal male-biased STAT5 ChIP-seq peaks in wild-type liver, consistent with gene downregulation being due to a direct loss of the transcriptional stimulatory effects of STAT5 (Fig. 1D and E, middle panels). In contrast, genes upregulated in STAT5-KO male liver were significantly enriched for proximal female-biased STAT5 ChIP-seq peaks, i.e., STAT5 binding near those genes is low in male liver compared to female liver. This indicates that the STAT5-dependent repression of female-biased genes in wild-type male liver is indirect, e.g., is mediated by a STAT5-dependent repressor whose loss in STAT5-KO liver leads to the observed gene derepression. The association between gene response to STAT5 deficiency and proximal sex-biased STAT5 ChIP-seq peaks was weaker in female liver (Fig. 1D and E, right panels). STAT5-dependent sex-biased genes that did not show proximal STAT5 binding (Table S1A) may be regulated by more-distal STAT5 binding sites (29). Finally, the much larger number of sex-independent STAT5 binding sites did not show any preference for binding nearby male-biased or female-biased genes and were not enriched for binding nearby genes dysregulated by STAT5 deficiency in either male or female liver (Fig. 1E, bottom row).
Sex-independent genes that respond to STAT5 deficiency.
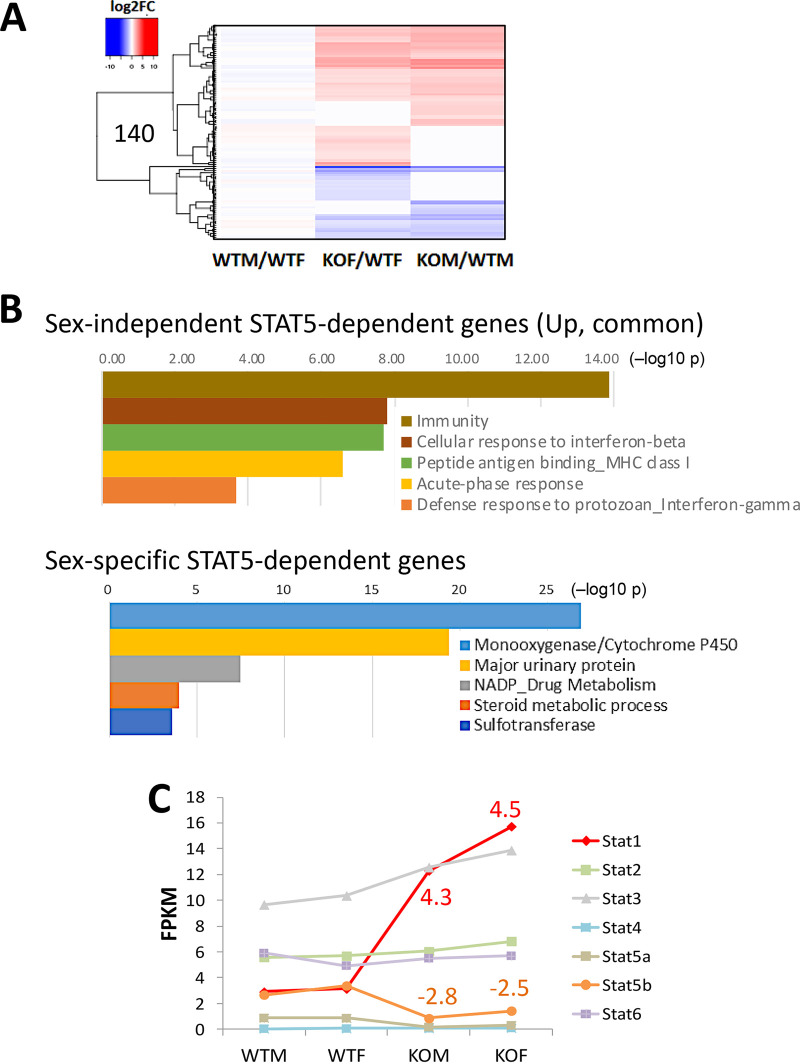
Analysis of sex-independent genes responsive to STAT5-KO gave additional insights into the biological changes occurring in this mouse model. We identified 338 sex-independent genes whose expression was significantly altered in STAT5-KO liver (Fig. 1A, Venn regions D, F, and G). Many of these genes responded to STAT5 deficiency in the same manner in both sexes, as seen in a heat map displaying 140 of the 338 genes, whose expression was stringently sex independent in wild-type liver (Fig. 2A). Examples include the classic STAT5 target genes Igf1, Socs2, and Onecut1 (HNF6), whose expression was downregulated in STAT5-KO livers of both sexes. Pathway analysis identified immunity, cellular response to beta interferon, and peptide antigen binding/major histocompatibility complex (MHC) as the top enriched pathways for 71 sex-independent genes that were induced in common in male and female STAT5-KO livers (Fig. 2B, top). This contrasts to the strong enrichment of cytochrome P450 and drug and steroid metabolism genes in the set of 155 STAT5-dependent sex-specific genes (Fig. 2B, bottom). The transcription factor STAT1 was among the sex-independent genes upregulated in both male and female STAT5-KO livers (Fig. 2C), consistent with reference 28. This increase in STAT1 may contribute to the observed increases in immune signaling and interferon response gene expression, given its essential role in the interferon-dependent antiviral defense, inflammation, and injury responses in the liver (45). Some residual expression of STAT5b was seen in STAT5-KO liver (Fig. 2C), reflecting its continued expression in nonparenchymal cells (42), where the Alb-Cre transgene used to excise the STAT5a-STAT5b locus is not active (46).
FIG 2.
Sex-independent genes responsive to STAT5 knockout (KO). (A) Heat map showing impact of STAT5 deficiency on 140 of the 6,306 stringent sex-independent genes whose expression was significantly increased (red) or decreased (blue) in either male or female STAT5-KO liver (Table S1A, column V; subset of Fig. 1A, Venn segments D plus F plus G). The log2 fold change (log2FC) is shown in the heat map. (B) DAVID (database for annotation, visualization, and integrated discovery) pathway analysis of 71 genes upregulated in common in STAT5-KO male and female liver (Table S1A, column W) (top) and of 155 sex-biased genes responsive to STAT5-KO in male or female mouse liver (Table S1A, column X) (bottom). Shown are the top five enriched clusters, with bars indicating −log10 P values (Benjamini-Hochberg corrected) for enrichment. Results are detailed in Table S1C and D. (C) Expression levels of seven STAT family members determined by RNA-seq, expressed as FPKM values. STAT1 was significantly upregulated in both male and female STAT5-KO liver. The incomplete loss of STAT5a and STAT5b is consistent with prior reports (42) and likely reflects STAT5 expressed in nonhepatocytes in mouse liver.
Sex differences in liver DNA methylation and impact of hepatic STAT5 loss.
The methylation state of CpG dinucleotides is a key epigenetic mark regulating chromatin structure and gene expression. Given the major impact of liver STAT5 deficiency on sex-biased gene expression shown above, we conducted reduced representation bisulfite sequencing (RRBS) to capture genomic regions with high CpG content and then identify sex differences in basal DNA methylation, as well as changes in DNA methylation upon loss of STAT5 in mouse liver. We used the same set of livers as in the RNA-seq analysis, but we analyzed a larger number of individual livers (biological replicates; n = 5 or 6) to increase statistical power. We identified genomic regions that show significantly differential CpG methylation (differentially methylated regions [DMRs]), either between male and female wild-type mouse liver (sex-biased DMRs) or between STAT5-KO and wild-type mouse liver (STAT5-dependent DMRs) (Table S2).
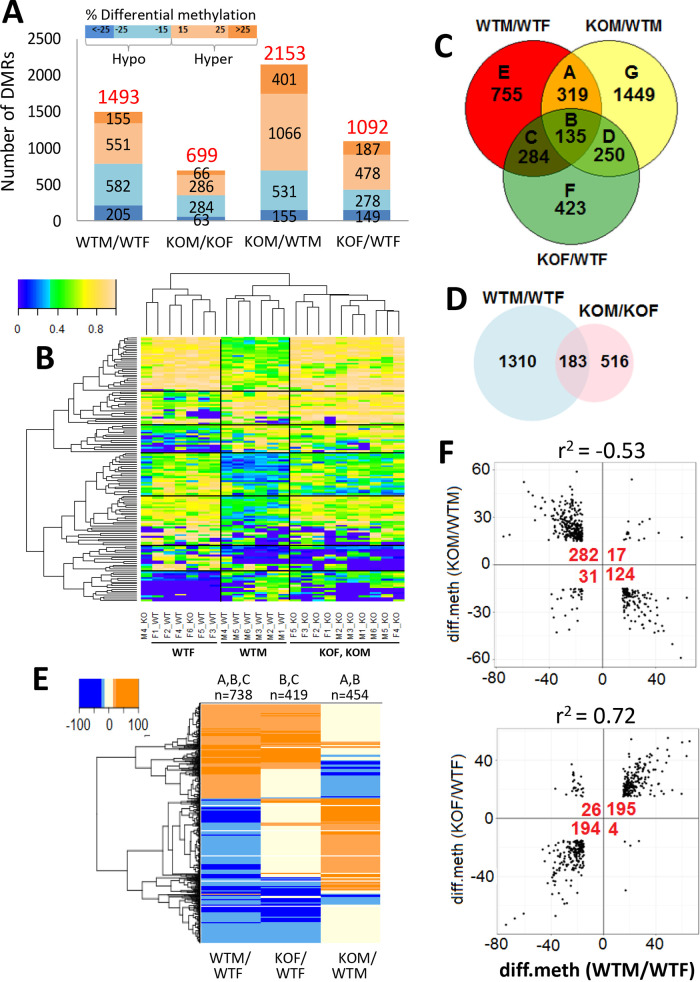
Each DMR covers a 100-bp genomic region (“tile”), which may be either hypermethylated or hypomethylated in the treatment sample compared to its control (e.g., male compared to female liver or STAT5-KO compared to wild-type liver). The extent of differential methylation was quantified as a differential methylation (diff.meth) value, corresponding to the difference in percent methylation between the samples being compared (Fig. 3A). Hypermethylation of the treatment relative to the control is indicated by a positive diff.meth value, and hypomethylation, where treatment is demethylated relative to the control, is indicated by a negative diff.meth value. DMRs with |diff.meth| between 15% and 25% at FDR < 0.05 were considered significant at standard stringency, and |diff.meth| > 25% at FDR < 0.05 identified robust DMRs, as shown in Fig. 3A for each of the four pairwise comparisons. DMRs sensitive to the loss of STAT5 were more frequently associated with hypermethylation than hypomethylation compared to wild-type liver, most notably in males (hypermethylation/hypomethylation ratio = 2.14 for KOM/WTM versus 0.9 to 1.01 for WTM/WTF and KOM/KOF). The increased hypermethylation upon STAT5 deletion was evidenced for both male and female liver by box plot analysis when comparing STAT5-KO to sex-matched controls, both for the set of 203 stringent sex-dependent tile-based DMRs (P < 0.05) and for a larger set of all 382 tile-based DMRs that respond to the loss of STAT5 in male mouse liver (P < 0.0005; Student’s t test). Furthermore, unsupervised (hierarchical) clustering of raw methylation values for all robust DMRs identified in the WTM/WTF comparison (Fig. 3B) correctly separated all of the male from female wild-type livers and a majority of the STAT5-KO from wild-type livers.
FIG 3.
Sex-specific DNA methylation and changes in STAT5-KO mouse liver. (A) Shown are the numbers of all DMRs (based on both tile statistic and individual CpG-based DMRs, i.e., the combined set in Table S2F) that were hypermethylated (orange) or hypomethylated (blue) at standard (|diff.meth| = 15 to 25%) or robust (|diff.meth| > 25%) thresholds for differential methylation in each of the four indicated comparisons. (B) Heat map showing raw methylation levels for the 203 tile-based DMRs that exhibited |diff.meth| of >25% and q value of <0.05 between wild-type male and wild-type female liver (Table S2D, column B). Data are shown for all 23 individual livers, with major clusters identified by hierarchical clustering separated by black lines. The colorbar shows that raw methylation levels range from 0% methylation (deep blue) to 100% methylation (tan). (C) Venn diagram showing the overlap between sex-biased DMRs in wild-type liver compared to the DMRs responsive to STAT5-KO in male and female liver, representing a total of 3,615 DMRs (|diff. meth| > 15%). The number of DMRs in each comparison (labeled A through G) is indicated. (D) Venn diagram overlap of sex-biased DMRs identified in wild-type liver compared to those found in STAT5-KO liver. Sex-dependent differential methylation is ∼88% lost in the knockout livers. Analyses shown in panels C and D are based on both tile statistics and individual CpG-based DMRs. (E) Heat map showing hypermethylated DMRs (orange) and hypomethylated DMRs (blue) from Venn diagram segments A, B, and C of panel C, as indicated at the top. (F) Scatterplots showing the relationship between sex specificity and STAT5 responsiveness of DMRs. In the top graph, 454 sex-biased DMRs (panel C, segments A plus B) that are responsive to loss of STAT5 in male liver are shown. Of the sex-biased DMRs that are hypomethylated in wild-type male compared to female liver, 282 show increased methylation and 31 show increased demethylation in STAT5-KO male liver, and of those that are hypermethylated in wild-type male compared to female liver, 124 become demethylated and 17 show increased methylation in STAT5-KO male liver. In the bottom graph, 419 sex-biased DMRs (panel C, segments B plus C) that are responsive to STAT5 loss in female liver are shown. STAT5 deficiency results in methylation and demethylation of these DMRs with response patterns opposite those shown in the top graph. Fisher’s exact test for DMR responses between the four quadrants: P < 2.2E−16 for both graphs. Linear regression analysis of |diff. meth| values showed significant correlations between the magnitude of sex specificity and response to STAT5-KO (r2 values).
Of the 1,493 sex-differential DMRs seen in wild-type liver, 738 (49%) showed significant differential methylation between STAT5-KO and wild-type livers, i.e., are STAT5-responsive DMRs, in either males or females (Fig. 3C, sections A, B, and C; Fig. 3E). Moreover, STAT5 deficiency caused a loss of significant sex differences in CpG methylation at 1,310 (88%) of the 1,493 sex-differential DMRs (WTM/WTF versus KOM/KOF comparison [Fig. 3D]). In male STAT5-KO liver, DMRs with a significantly lower methylation level in wild-type male than wild-type female liver (i.e., male hypomethylated DMRs) primarily became hypermethylated, while many male hypermethylated DMRs became hypomethylated (Fig. 3E and Table 2). In contrast, in female STAT5-KO liver, many female hypermethylated DMRs (i.e., male hypomethylated DMRs) became hypomethylated, and female hypomethylated DMRs became hypermethylated. Figure 3E shows that STAT5-KO induces a loss of sex-differential methylation at male hypomethylated sites by increasing their methylation in male liver or by decreasing methylation in female liver. In the case of female hypomethylated sites, STAT5-KO induces a loss of sex-differential methylation by increasing methylation in female liver or by decreasing methylation in male liver. Quantitative scatterplots illustrate this significant reversal of sex-biased differential DNA methylation upon loss of liver STAT5 (Fig. 3F). Further, the extent of methylation differences between the sexes correlated significantly with the level of DNA methylation changes in the STAT5-KO livers (Fig. 3F). Thus, liver STAT5 is required for sex-specific methylation and demethylation at several hundred genomic regions.
TABLE 2.
Differential methylated regions in hepatocyte-specific STAT5-KO livera
| DMR | KOM/WTM |
KOF/WTF |
||||
|---|---|---|---|---|---|---|
| Methylation change |
No. of DMRs |
% | Methylation change |
No. of DMRs |
% | |
| Male-hypomethylated DMRs (n = 787) |
Decrease | 31 | 3.9 | Decrease | 194 | 24.7 |
| Increase | 282 | 35.8 | Increase | 26 | 3.3 | |
| No change | 474 | 60.2 | No change | 567 | 72 | |
| Female-hypomethylated DMRs (n = 706) |
Decrease | 124 | 17.6 | Decrease | 4 | 0.6 |
| Increase | 17 | 2.4 | Increase | 195 | 27.6 | |
| No change | 565 | 80 | No change | 507 | 71.8 | |
A total of 1,493 sex-biased DMRs (Fig. 3C) were classified into DMRs that were more hypomethylated in male liver (787) or more hypomethylated in female liver (706). These DMRs were further sorted by their direction of change in STAT5-deficient compared to wild-type liver, as indicated, in both male KO liver (KOM/WTM) and in female KO liver (KOF/WTF). See also Fig. 3F.
Few DMRs are in promoter regions of sex-biased or STAT5-dependent genes.
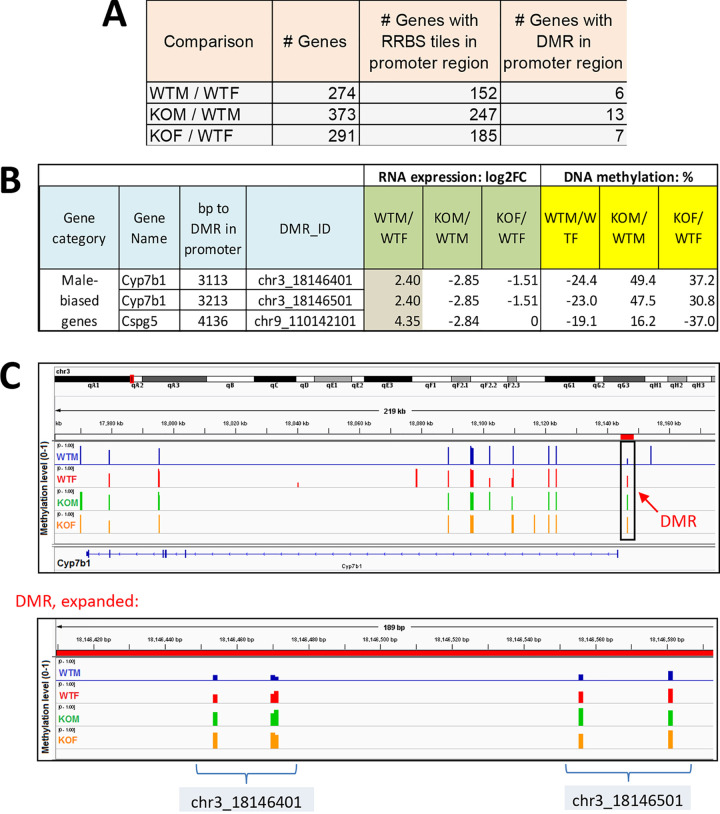
CpG islands, found at many gene promoters, generally exhibit low methylation levels and are correlated with active transcription (47). Further, hypermethylation of CpG islands is associated with stable silencing of genes (48). To determine whether changes in promoter CpG methylation are associated with the sex-biased and the STAT5-dependent gene expression changes we observed, we examined DMRs proximal (5 kb upstream or downstream) to the transcription start site of these genes. Only 4 to 5% of the genes with a promoter RRBS tile that are sex biased or liver STAT5 dependent have a DMR in their promoter region (Fig. 4A). Further, only two STAT5-dependent, male-biased genes, Cyp7b1 and Cspg5, showed significant promoter DNA methylation changes in STAT5-KO male liver that matched the general trends described above (Fig. 4B and C and Table S3A). For both genes, DNA methylation in the promoter region was significantly lower in wild-type male than wild-type female liver and was increased in STAT5-KO male liver in association with the decrease in gene expression. Thus, the large majority of sex-biased, STAT5-dependent genes do not display sex differences in promoter region DNA methylation. We also identified five examples of sex-independent STAT5-KO-responsive genes that showed STAT5-dependent changes in promoter region DNA methylation in a direction consistent with the change in their expression in STAT5-deficient male and/or female liver (Table S3B).
FIG 4.
Few DMRs are at promoters of sex-biased or STAT5-dependent genes. (A) Shown are the number of significant differentially expressed genes identified in each comparison (as in Fig. 1A), the number of those genes that have an RRBS-covered tile, and the number of those genes with a significant DMR tile < 5 kb upstream of the gene transcription start site (i.e., the extended promoter region). Analysis is based on data in Table S3A. (B) Differential expression and differential methylation data for the three promoter DMR/gene pairs that are consistently male biased at both the gene expression level and the promoter DMR level. FC, fold change. (C) Browser screen shot of genomic region encompassing Cyp7b1, with the methylation level of individual RRBS tiles (track height, scale 0 to 100) shown for male and female wild-type liver and for male and female STAT5-KO liver. Only the 3′-most RRBS tiles (boxed) correspond to significant DMRs. The latter region is expanded at the bottom and encompasses two adjacent DMRs, each containing four CpGs, whose percent methylation is indicated for each of the four conditions. The extent of differential methylation for these CpGs is shown in panel B.
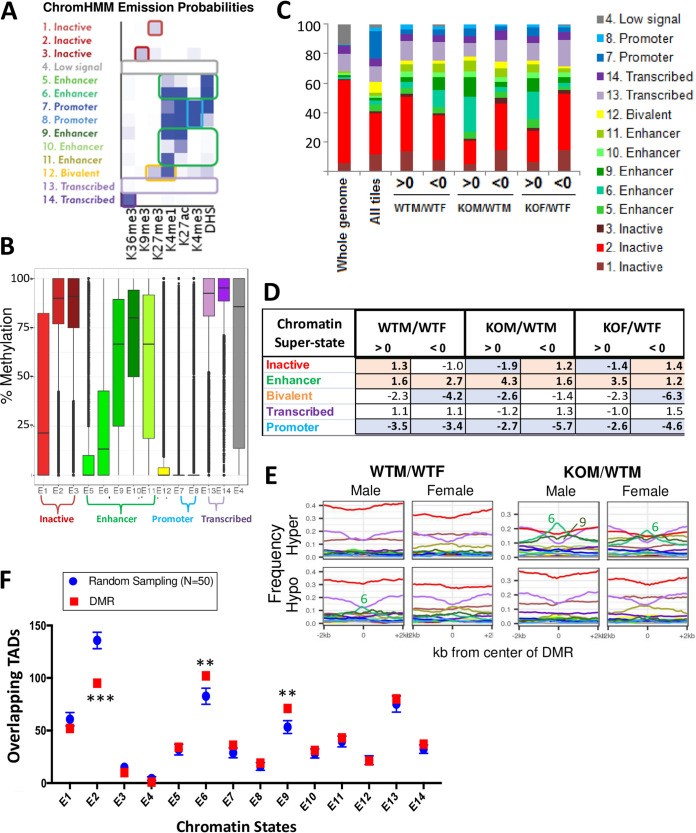
Chromatin states and chromatin accessibility at DMRs.
Given our finding that very few of the sex-dependent and STAT5-dependent DMRs are in the promoter region of differentially expressed genes (Fig. 4), we investigated DMR locations in terms of the local chromatin state. Specifically, we mapped each set of DMRs to 14 ChromHMM-derived chromatin states (Fig. 5A) that we previously learned (29) for both male and female mouse liver using genome-wide data sets for open chromatin regions (DNase I-hypersensitive sites [DHS]) and histone marks for each sex. A substantial fraction of the >1.3 million CpGs consistently captured by our RRBS samples were within a promoter state, reflecting the preferential capture of promoter CpG island sequences by the RRBS protocol. However, promoter states show very low CpG methylation overall, while the five different enhancer states identified showed a large range of CpG methylation levels (Fig. 5B, green bars). CpGs within sex-biased and STAT5-responsive DMRs were significantly depleted of promoter states and showed various levels of enrichment for enhancer states (Fig. 5C and D). Frequency profiles of each chromatin state in a 4-kb window surrounding each DMR revealed specific enrichment of enhancer state 6 for DMRs that were hypermethylated, but not hypomethylated, in the absence of STAT5 (KOM/WTM comparison) (Fig. 5E). A modest enrichment of enhancer state 6 was also seen in DMRs hypomethylated in wild-type male relative to wild-type female liver. Enhancer state 6 represents active enhancers characterized by high probabilities of histone H3 lysine 4 monomethylation (H3K4me1) and histone H3 lysine 27 acetylation (H3K27ac) histone marks and open chromatin (presence of a DHS) (Fig. 5A). Thus, loss of STAT5 leads to a preferential hypermethylation of genomic regions in an active enhancer state.
FIG 5.
Chromatin states at DMRs responsive to STAT5-KO in liver. (A) ChromHMM emission probabilities for the 14 chromatin states discovered in male and female mouse liver (data from reference 29). Each state is colored and given a descriptive name (left) based on the frequency of each of six chromatin marks or a DHS (darker color intensity, higher frequency), as marked at the bottom. (B) Box plots showing overall distribution of percent methylation values for individual CpGs in wild-type male liver for each of 14 chromatin states, labeled E1 to E14, and grouped into four “superstates,” plus a fifth state, bivalent state E12. Promoter states E7 and E8 are rich in CpGs (see panel C) but are largely methylation free, as was the bivalent chromatin state E12. In contrast, CpGs in transcribed chromatin states E13 and E14 were highly methylated. Large differences in CpG methylation were seen for the inactive states E1 to E3 and for the five enhancer states, E5, E6, and E9 to E11. (C) Cumulative distribution of chromatin states for the set of 232,882 tiles captured by RRBS for the 23 liver samples used in this study (all tiles) and for the subsets of tiles identified as DMRs in the WTM/WTF, KOM/WTM, and KOF/WTF comparisons. The first bar shows that the whole mouse genome is mostly covered (>60%) by chromatin state 2, an inactive state. The all tiles set is strongly enriched for promoter states (blue) and poised states (yellow) compared to the whole genome. DMRs identified in each of the three pairwise comparisons indicated at the bottom are marked >0 (hypermethylation) or <0 (hypomethylation) and show a relative depletion of promoter states and variable increases in the proportions in enhancer states (various shades of green) compared to all tiles. For example, hypermethylated DMRs in the KOM/WTM and KOF/WTF data sets showed an increased proportion of enhancer states. (D) Enrichment scores in each DMR group relative to the full set of tiles captured by RRBS, calculated separately for each of five indicated chromatin superstates (as in panel B). Significant enrichments and depletions (P value cutoff, 1E−5, two proportion z test) are shown in bold type on a colored background. (E) The frequency of occurrence of each of the 14 chromatin states in male and female wild-type liver and in male STAT5 knockout liver was calculated in 100-bp windows extending 2 kb upstream and 2 kb downstream of the sets of hypermethylated and hypomethylated DMRs for the WTM/WTF comparison (left) and for the KOM/WTM comparison (right). Chromatin states were identified for both male and female liver, as marked above each subpanel, and are colored as indicated in panel C. Chromatin state 6 showed a prominent peak in hypermethylated DMRs for the KOM/WTM comparison in both male and female liver, and to a lesser extent, in hypomethylated DMRs of the WTM/WTF comparison in male liver. Chromatin state 6 was also enriched at hypermethylated DMRs for the KOF/WTF comparison (not shown). (F) DMRs in enhancer states 6 and 9 show significant enrichment for TADs encompassing genes that are sex biased and/or STAT5 responsive. **, Z score > 2.5. Inactive chromatin state E2 shows significantly fewer overlapping TADs than a random sampling of CpG regions (see also Table S4C).
TAD-based association of sex-biased and STAT5-dependent genes with distal DMRs.
Next, we investigated whether the enhancer state-associated DMRs can be linked to STAT5-dependent transcriptional regulation. As it is difficult to directly link a given enhancer to its specific gene target(s) in the absence of chromatin interaction data (e.g., reference 49), we evaluated such interactions at the level of topologically associating domains (TADs), which are megabase-scale genomic regions with high propensity for intradomain contact in three-dimensional chromatin organization (50). Using TAD boundaries defined for mouse liver (51, 52), we investigated whether genes that are sex biased or STAT5 responsive are more likely to be in the same TAD as a sex-biased or STAT5-responsive DMR, compared to sets of random CpG regions. DMRs, grouped by chromatin state (Fig. 5A), were mapped to TADs, and their overlap with the set of 437 TADs containing at least one sex-biased gene or one STAT5-responsive gene was then determined (Table S4B). The statistical significance of TAD overlap was determined for each chromatin state by comparison to TAD overlap for a random sampling, performed 50 times, of comparably sized sets of 100-bp RRBS-captured CpG regions that did not show significant differential methylation. DMRs in chromatin states 6 and 9, but not DMRs in other chromatin states, showed significant enrichment for TADs containing sex-biased or STAT5-responsive genes than did random CpG regions (z scores of 2.54 and 2.98 and P values of 0.011 and 0.004 for chromatin states 6 and 9, respectively) (Fig. 5F and Table S4C). Chromatin states 6 and 9 are enhancer states with high levels of histone H3K4me1 and histone H3K27ac marks, either with (state 6) or without (state 9) a DNase hypersensitive site (Fig. 5A) and with the DHS-free enhancer state 9 showing much higher overall CpG methylation than enhancer state 6 (Fig. 5B). The observed enrichments support the proposal that the regulation of CpG methylation at these gene-distal enhancers plays a regulatory role for the sex-biased and STAT5-responsive genes within these TADs. Consistent with this proposal, the sex-biased, STAT5-responsive DMRs in chromatin states 6 and 9 were closer in median distance to sex-biased, STAT5-responsive genes than DMRs in other chromatin states (Table S4D).
Association of DMRs with nearby open chromatin regions.
Many DMRs are located near at least one liver DHS (Table S5). These DMR-proximal DHS include 308 male-biased DHS and 66 female-biased DHS identified previously (53). Gene set enrichment analysis showed that these 374 sex-biased DHS were nonrandomly distributed: the 308 male-biased DHS were associated with DMRs that were hypomethylated in wild-type male liver (Fig. 6A) (normalized enrichment score [NES] = −3.18, FDR = 0), and the 66 female-biased DHS were associated with DMRs hypermethylated in wild-type male liver (and consequently, hypomethylated in wild-type female liver) (NES = 1.57, FDR = 0.007) (Fig. 6B). Thus, sex-biased CpG demethylation is significantly associated with sex-biased chromatin opening in wild-type livers of both sexes. Moreover, in male liver, loss of STAT5 resulted in significant hypermethylation in genomic regions with a male-biased DHS, but not in regions with female-biased DHS (Fig. 6C [FDR = 0] versus Fig. 6D [FDR = not significant]), whereas the absence of STAT5 in female liver resulted in hypermethylation at sites near both male and female DHS (Fig. 6E and F). Thus, sex-biased chromatin opening is associated with sex-biased CpG demethylation, and loss of liver STAT5 enables hypermethylation of nearby sex-biased DHS, consistent with the enrichment of the DHS-containing enhancer state 6 seen in Fig. 5E.
FIG 6.
GSEA analysis of STAT5-responsive DMRs nearby sex-biased DHS. The three sets of DMRs indicated at the bottom were rank ordered from hypermethylation (red) to hypomethylation (blue). GSEA was then used to determine whether sex-biased DHS (Table S5) located within 5 kb of the DMRs in each set are significantly enriched at either the hypermethylated or hypomethylated end of the ranked DMR spectrum, as determined from the NES (normalized enrichment score), with a FDR of <0.05 considered significant. In panel A, male-biased DHS were significantly enriched for being nearby DMRs that were hypomethylated in male liver (right side of x axis), as indicated by the persistent decrease then steep rise in enrichment score going from left to right (NES, −3.18; FDR = 0), while in panel B, female-biased DHS were significantly enriched nearby DMRs hypermethylated in male liver (and thus hypomethylated in female liver) (NES = 1.57; FDR = 0.007). Also shown are the enrichments of male-biased and female-biased DHS for DMRs determined from the comparison of STAT5-KO and control male liver (C and D) and STAT5-KO and control female liver (E and F).
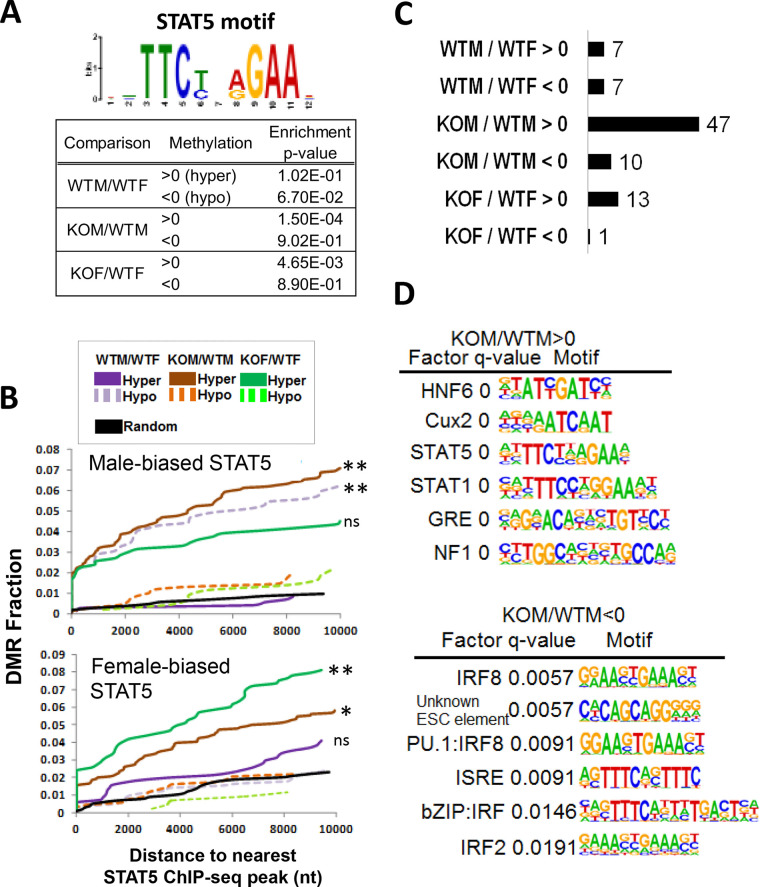
Association of STAT5 motifs and STAT5 binding with DMRs.
Given our finding that the loss of STAT5 in male liver increases methylation at male-biased DHS regions associated with hypomethylated DMRs, we scanned each set of DMRs (WTM/WTF, KOM/WTM, and KOF/WTF) for STAT5 motifs. We found significant enrichment of the canonical STAT5 motif at hypermethylated DMRs in STAT5-KO male liver (P = 0.00015), and to a lesser extent in STAT5-KO female liver (P = 0.00465) but not at the corresponding hypomethylated DMRs, consistent with hypermethylation occurring at or near STAT5 binding sites (Fig. 7A). A trend toward enrichment of the STAT5 motif at sites hypomethylated in male compared to female liver was also found (P = 0.067). Next, we used our prior STAT5 ChIP-seq binding site data for mouse liver (12) to determine whether the sites showing sex-dependent STAT5 binding are enriched in proximity to sex-specific DMRs or to DMRs hypermethylated in STAT5-KO liver. Cumulative frequency plots of distance from each DMR set to the nearest male-biased STAT5 binding site showed that male-biased STAT5 binding was significantly enriched at or near DMRs hypomethylated in male liver compared to DMRs that were hypermethylated (Fig. 7B, top, dashed versus solid purple curves). Moreover, DMRs hypermethylated in male STAT5-KO liver were significantly enriched for nearby male-biased STAT5 binding sites compared to hypomethylated DMRs (solid versus dashed brown curves), consistent with the STAT5 motif analysis in Fig. 7A. Finally, female-biased STAT5 binding was most significantly enriched at or near DMRs hypermethylated in STAT5-KO female liver (Fig. 7B, bottom). Thus, male-biased STAT5 binding is significantly associated with hypomethylation in wild-type male liver, and the loss of STAT5 increases CpG methylation in sex-dependent STAT5 binding regions in both male and female liver.
FIG 7.
Enriched motifs and STAT5 ChIP-seq peaks associated with DMRs. (A) Enrichment of STAT5 motifs at DMRs determined using the MEME Analysis of Motif Enrichment tool. (B) Cumulative frequency plots of hypermethylated and hypomethylated DMR (both sex-biased DMRs (purple) and STAT5-KO-reponsive DMRs (brown and green) as a function of distance to the nearest male-biased STAT5 binding site (top) or the nearest female-biased STAT5 binding site (bottom) (see Table S2F, last columns). Significance was determined by performing two-proportion Z-test between the hypermethylated and hypomethylated DMR sets for each of the three comparisons shown, with significance indicated as follows: *, P < 0.001; **, P < 0.00001; ns, not significant (P ≥ 0.001). These analyses used a set of 14,910 STAT5 ChIP-seq sites in mouse liver (12), of which 59% were within 5 kb of one of the CpG sites covered by our RRBS data set, and 6% were within 5 kb of one of the 3,620 DMRs showing significant differential methylation in one or more of these three comparisons: WTM/WTF, KOM/WTM, and KOF/WTF (Table S2F, column Y). (C) Number of enriched DNA motifs for each DMR set, as determined by Homer using a significance threshold of q value > 0.05. (D) Motif name, q value, and sequence logo for the top six de novo-discovered DNA motifs (lowest q values) for the indicated two sets of DMRs. See also Table S6.
Motifs enriched at hypermethylated DMRs.
Next, we used de novo motif discovery, implemented using Homer (54), to determine whether motifs for other transcription factors are enriched in the sets of sex-dependent and/or STAT5-dependent hypermethylated and hypomethylated DMRs described above. The largest number of significantly enriched motifs (n = 47, q value [FDR] < 0.05) was discovered in the set of hypermethylated DMRs from the KOM/WTM comparison, followed by 13 motifs for hypermethylated DMRs from the KOF/WTF comparison (Fig. 7C). A majority of the hypermethylated KOF/WTF motifs overlapped one of the hypermethylated KOM/WTM motifs (Table S6). The top motifs discovered are shown in Fig. 7D. Notably, motifs for STAT5, HNF6, and CUX2, all previously implicated in regulation of liver sex differences (29, 43, 44), were unique to the sets of hypermethylated DMRs common to the KOM/WTM and KOF/WTF comparisons. Thus, loss of STAT5 resulted in hypermethylation of regions enriched in DNA motifs for STAT5 and its coordinately regulating transcription factors. These hypermethylated regions are enriched for enhancer histone marks and chromatin states (Fig. 5), DHS (Fig. 6), and STAT5 binding (Fig. 7) in wild-type liver, and their presumed loss of activity upon hypermethylation (55, 56) is expected to contribute to the loss of sex-biased expression of their associated target genes.
DISCUSSION
We investigated the relationship between sex-biased DNA methylation and sex-biased liver gene expression in mice with a hepatocyte-specific deletion of STAT5, which plays a central role in GH regulation of adult liver sexual differentiation. Loss of STAT5 in hepatocytes led to a substantial, albeit incomplete loss of sex-biased liver gene expression. Our findings reveal that STAT5 induces male-biased genes in male liver via a positive, STAT5 binding-dependent regulatory mechanism, and that STAT5 represses female-biased genes in male liver by an indirect regulatory mechanism. Liver STAT5 deficiency also resulted in widespread changes in DNA methylation, abolishing the sex bias in DNA methylation at 88% of 1,493 sex-biased DMRs. Hypermethylated regions identified in male and female STAT5-KO liver were enriched for open chromatin regions (DHS), enhancer chromatin states, and STAT5 ChIP-seq peaks in wild-type liver and show significant enrichment for transcription factor motifs for STAT5 and other transcription factors previously implicated as regulators of liver sex bias. Thus, many DMRs that become hypermethylated in STAT5-KO liver are associated with STAT5-bound enhancers that require STAT5 to maintain a low DNA methylation state. As DNA methylation is often associated with condensed chromatin that is inaccessible to transcription factor binding (57), our findings suggest that the loss of STAT5 leads to silencing of these regulatory elements via CpG methylation. Proximal promoter regions of STAT5-responsive genes showed very few significant changes in DNA methylation in STAT5-KO liver, and correspondingly, promoter states were significantly underrepresented, especially in the sets of DMRs that become hypomethylated in the absence of STAT5. The later DMRs may represent latent regulatory elements that become activated in the absence of STAT5, e.g., due to the upregulation of STAT1 (Fig. 2), or by other compensatory mechanisms that have yet to be identified.
The present findings in hepatocyte-specific STAT5a/STAT5b-KO liver are fully consistent with our prior findings in whole-body STAT5b-KO liver based on microarray analysis (10), where liver sex bias was abolished for 78% of sex-biased genes versus 61% in the present study. Moreover, a full 80% of genes that apparently retain sex-biased expression in the present hepatocyte-specific STAT5-KO model nevertheless showed a decrease in the magnitude of sex bias upon loss of STAT5. Furthermore, and consistent with our prior work (10), the impact of hepatocyte-specific STAT5 loss was less pronounced in female liver, where fewer genes were dysregulated and where there was a weaker correlation between sex specificity and STAT5 response and also a weaker association with sex-biased proximal STAT5 binding. Nevertheless, hepatocyte-specific STAT5 deficiency did impact sex-biased gene expression in female liver, consistent with the female-biased binding of STAT5 to liver chromatin seen by ChIP-seq (12) and with the finding that STAT5a is specifically required for expression of 15% of female-specific genes in female liver (58).
One factor contributing to the more extensive loss of sex-biased gene expression in the whole-body STAT5b-KO mouse model (10) may be the loss of STAT5 throughout prenatal and postnatal development, as well as its absence in all liver cell types, including nonparenchymal cells. In contrast, in the present, hepatocyte-specific STAT5 knockout mouse model, STAT5 deletion was achieved by activation of a Cre transgene under the control of the Alb gene promoter (28), which does not occur in liver nonparenchymal cells, and consequently, there is significant residual expression of STAT5 (42). Another factor contributing to the less extensive loss of sex-biased gene expression in the hepatocyte-specific STAT5-KO mouse model may be CUX2, a female-specific transcriptional repressor that is rapidly induced by continuous GH infusion in male liver (21) and is derepressed in whole-body STAT5b-KO male liver (59), but not in hepatocyte-specific STAT5-KO mouse liver, as shown here. The induction of CUX2 seen in whole-body STAT5b-KO male liver is likely due to its direct, STAT5-independent induction by the persistent elevation of circulating GH found in that mouse model (42), which would enable CUX2 to contribute to a more complete feminization of male mouse liver through its widespread repression of male-biased genes (43). Other strongly female-biased genes that were not induced in hepatocyte STAT5-deficient male liver include the drug metabolizing genes Cyp3a16, Hsd3b1, and Ugt2b37. Given that these genes, as well as Cux2, are strongly induced in the livers of male mice treated with GH as a continuous infusion (female plasma profile) (21), it seems that their sex-biased expression is regulated by a GH-dependent but STAT5-independent signaling pathway.
CpG methylation at a specific promoter-proximal CpG dinucleotide has been implicated in the regulation of two sex-biased liver genes, Cyp2d9 and C4a/Slp (38, 60, 61). Our studies identified differential methylation proximal to C4a/Slp (see Table S2F in the supplemental material); however, our RRBS analysis did not identify CpGs near Cyp2d9, which could be due to insufficient CpG density in that region. While many sex-biased, STAT5-responsive gene promoters contain nearby CpGs captured by our RRBS analysis, very few differentially methylated CpG tiles were found at their gene promoters. Rather, we found that STAT5-dependent sex-biased genes are frequently associated with STAT5-regulated demethylation at or near distal enhancers. Thus, we observed a high correlation between the sex bias and STAT5 responsiveness of CpG methylation changes at sex-biased DMRs in both male and female liver. Loss of STAT5 preferentially increased CpG hypermethylation, with the sites that become hypermethylated in the absence of STAT5 being more strongly enriched for enhancer chromatin states, presence of open chromatin (DHS), and STAT5 binding compared to hypomethylated sites. This indicates that demethylation at regulatory elements is dependent on STAT5 and that loss of STAT5 leads to retention of DNA methylation at genomic loci that would otherwise be active, demethylated enhancers that bind STAT5 and undergo transactivation. This regulatory mechanism characterizes many distal enhancers, as indicated by the significant enrichment of DMRs in enhancer chromatin states for colocalization with sex-biased and/or STAT5-responsive genes within their TAD boundaries. Whether DNA demethylation plays a direct, instructive role in promoting STAT5 binding or is a passive consequence of binding of STAT5 or associated transcription factors is unknown.
The widespread effects of STAT5a/STAT5b-KO on sex differences in liver gene expression and DNA methylation described here can primarily be attributed to the loss of STAT5b, which is GH responsive and is the predominant liver STAT5 form. STAT5a is also GH responsive but is expressed in mouse liver at a much lower level than STAT5b, as confirmed here by RNA-seq; furthermore, its loss has much less impact on hepatic expression of sex-biased genes (58). STAT1, although classically activated by gamma interferon stimulation (62), can also be activated by GH in liver (63). STAT1 is upregulated in STAT5-KO liver (Fig. 2), perhaps as an adaptive response in the absence of competition by STAT5 for binding to their shared intracellular docking sites on the GH receptor (28). STAT1 has a DNA-binding motif distinct from that of STAT5 (63) and consequently has unique target genes, and thus is unable to compensate for the loss of STAT5-regulated sex-biased gene expression (as was seen here) or for other phenotypic consequences of liver STAT5 deficiency, including impaired cell proliferation and fatty liver development (28). Although STAT5-KO dysregulated only ∼2% of stringently sex-independent genes in liver, at least some of the sex-independent genes and DMRs responsive to STAT5-KO may reflect adaptive mechanisms in the liver. Indeed, the sex-independent genes induced by STAT5-KO in both male and female liver are enriched for immune-specific genes (Fig. 2), at least some of which may be STAT1 targets.
In the Alb-Cre-induced hepatocyte-specific STAT5-KO model used here, STAT5 deletion occurs during late gestation, when the Cre transgene is activated by the Alb gene promoter (46). Consequently, the gene expression and DNA methylation changes we observed could, in part, result from the loss of STAT5 during a critical late gestational or early postnatal period, when GH is proposed to have a programming effect on adult sex-specific hepatic gene expression (64). It is also true, however, that robust activation of STAT5 in male liver, by tyrosine phosphorylation, first becomes apparent around 4 weeks of age, triggered by the onset of pubertal plasma pulses of GH (65). Therefore, the effects of STAT5-KO described here, including its effects on CpG methylation, are most likely directly due to the loss of STAT5 function in pubertal and adult liver. Consistent with this possibility, although mammalian DNA methylation patterns are established at many sites during embryogenesis and then maintained throughout life (66, 67), DNA methylation continues to evolve postnatally at a subset of genomic regions in terminally differentiated cells (39, 40, 68). In mouse hepatocytes, demethylation at enhancer elements occurs after birth, when it is required to establish postnatal chromatin accessibility and gene transcription patterns (40). Our finding of enhancer hypermethylation in STAT5-KO liver indicates that STAT5 regulates demethylation of these genomic regions, which may contribute to chromatin opening and activation of enhancers critical for adult liver function. Our finding that these regions are also enriched for DNA motifs for other transcription factors important for sex-biased liver function (Fig. 7D) suggests that STAT5 may work with these other factors to recruit the TET demethylases (69) that actively demethylate these enhancer regions. Such demethylation events likely occur around puberty (39), when pituitary GH secretion becomes pulsatile in males, leading to intermittent activation of liver STAT5 (16, 17, 65), one of the key driving forces for the striking sex differences in liver gene expression that become widespread at that time (70). Further study is needed to determine whether CpG demethylation of these enhancers by STAT5 is a permanent, irreversible event (39) or whether the methylation status of these enhancers is dynamically responsive to the repeated activation of STAT5 in male liver by plasma GH pulses, as we recently found for other sex-biased epigenetic events (16).
One of the possible limitations of this study relates to our use of mixed mouse strains, including an FVB/N strain component in the Alb-Cre mice used to generate the STAT5-KO mice; these strain differences could, in principle, contribute to some of the differences in expression and DNA methylation that we report. Although this is certainly possible, it is unlikely to impact our major conclusions for several reasons. First, as discussed above, the overall effects of hepatocyte STAT5 deficiency on sex-specific genes described here are largely consistent with effects seen in a whole-body STAT5b mouse knockout model (10). Second, our findings linking sex-dependent sites of STAT5 binding in wild-type liver, determined by ChIP-seq, to genes that are differentially expressed in STAT5-KO liver (Fig. 1D and E) lend strong support to our conclusion that the STAT5-dependent genes we identified are dysregulated by the loss of STAT5 binding per se, and not as an artifact of the differentially mixed strains. Third, regarding the effects of STAT5-KO on sex-independent genes and their associated DMRs (e.g., genes represented by Venn diagram segment D in Fig. 1A), our finding that those genes are significantly enriched for immune/interferon response genes linked to the induction of STAT1 (Fig. 2) is consistent with earlier reports of robust STAT1 protein induction seen in two independent whole-body STAT5-KO mouse models (71, 72). Finally, our analysis of differential methylation sites was strictly limited to CpGs that are conserved across all 23 individual mice included in our study and were also present in the C57BL/6 reference mouse genome, thereby excluding strain-dependent variants at CpG sequences.
In contrast to STAT5-dependent enhancer DNA demethylation, we found that alteration of promoter DNA methylation is not a common mechanism for STAT5 regulation of sex-biased gene expression. Although CpG islands are frequently found at or near gene promoters, where they are mostly hypomethylated, even when the gene is transcriptionally silent (73), few promoters become activated in cells deficient in DNA methyltransferase activity, despite a 95% reduction in global DNA methylation and the creation of thousands of active enhancers (74–76). This suggests that DNA methylation does not drive promoter activation or repression but rather may be a more common regulatory mechanism at enhancers. The significance of DNA methylation at enhancer sites is apparent in cancer (74, 77, 78), where enhancer CpG methylation is more closely associated with changes in gene expression than is promoter methylation. Our finding that STAT5 primarily alters DNA methylation at enhancer elements is consistent with these findings and is reminiscent of our earlier observation that sex-biased histone marks are more pronounced at distal sex-biased DHS than at sex-biased gene promoters (29).
STAT5 can respond to many extracellular cytokines and growth factor signals, including GH, and is the direct downstream effector of the hypothalamo-pituitary-liver GH axis. Liver STAT5 signaling is susceptible to disruption by xenobiotics, which can dramatically alter hepatic transcriptomic profiles and impact the ability of liver to metabolize foreign chemicals, increasing susceptibility to liver diseases, including metabolic disorders, fatty liver, hepatocellular carcinoma, and obesity (4, 9). Our studies elucidating the interplay between STAT5-dependent DNA methylation and sex-biased gene expression have strong implications for understanding mechanisms underlying diseases associated with dysregulation of liver STAT5 function, which is a common occurrence (79, 80). Our findings suggest that STAT5 plays a critical role in shaping both sex-independent and sex-dependent enhancer elements via DNA methylation, which ultimately contributes to the control of proper RNA output for normal liver function. Given that DNA methylation is considered one of the more stable chromatin modifications that, once established, contributes to durable epigenetic memory through multiple cell divisions (81), dysregulation of STAT5 activity could lead to persistent effects on DNA methylation profiles at regulatory elements in the liver.
MATERIALS AND METHODS
Liver tissue.
Livers used for the analysis reported here were the same tissue samples used in our earlier work (42) and were originally obtained from Lothar Hennighausen (NIDDK, NIH). The livers were from 8- to 12-week-old hepatocyte STAT5-deficient males and females (KOM and KOF, respectively), and floxed controls (wild type with respect to STAT5, i.e., WTM and WTF, respectively). The hepatocyte-specific STAT5-deficient mouse line (STAT5-KO) was developed by mating albumin promoter-driven Cre transgenic mice (FVB/N) with C57BL/6 × 129J mice having a floxed Stat5a-Stat5b locus (28). All mouse work was performed according to the Animal Research Advisory Committee Guidelines of NIH (https://oacu.oir.nih.gov/animal-research-advisory-committee-guidelines) and was approved by the NIDDK Animal Care and Use Committee.
RNA-seq sample preparation and data analysis.
A portion of each frozen liver was used for RNA extraction with TRIzol reagent (Invitrogen Life Technologies Inc., Carlsbad, CA) following the manufacturer’s instructions. Total liver RNA was isolated from n = 5 or 6 individual livers per sex and genotype (WTM, WTF, KOM, and KOF). Two RNA-seq libraries (biological replicates) were prepared for each group, with each sequencing library comprised of a pool of RNAs obtained from n = 2 or 3 mouse livers. Libraries were prepared using the Illumina TruSeq RNA library preparation kit (Illumina catalog no. RS-122-2001) starting with total liver RNA depleted of rRNA using NEBNext rRNA Depletion kit. Paired-end sequence reads (50-nucleotide [nt] reads) were generated on an Illumina HiSeq instrument at the New York Genome Center (New York, NY). RNA-seq data were analyzed using a custom pipeline developed in our lab (16). Briefly, FASTQ files with raw sequence reads were aligned to mouse genome build mm9 (NCBI 37) by using Tophat (version 2.0.13) (82) and default parameters. FeatureCounts (83) was used to count sequence reads mapping to gene bodies of RefSeq genes, and EdgeR (84) was used to identify differentially expressed genes. A total of 9,175 genes (see Table S1A in the supplemental material) were identified as liver-expressed genes based on the criteria of fragments per kilobase per million (FPKM) >1 (average expression) in either male or female wild-type mouse liver. A FDR of <0.05 was used to identify differentially expressed genes between male and female liver (sex-specific genes; 132 male-biased and 142 female-biased genes, based on WTM/WTF comparison) and to identify genes responsive to STAT5 loss (KOM/WTM, n = 365 genes; KOF/WTF, n = 278 genes) (Table S1B). Genes with a FDR of >0.05 were deemed nonresponsive to STAT5 loss. Stringent sex-independent genes (n = 6,306) were identified by |fold difference| < 1.2 and FDR > 0.1 for the WTM/WTF comparison (Table S1A, column S). When calculating correlations between two conditions (Fig. 1C and 3F), fold change and differentially methylated values (see below) were first converted to absolute values to avoid artificial overestimation of correlation. Linear regression was performed in R, and R2 and P values are reported. To identify STAT5 ChIP-seq peaks near genes, genomic coordinates of male-biased, female-biased, and sex-independent STAT5 ChIP-seq peaks were downloaded from Supplemental file 5 of reference 12. STAT5 ChIP-seq peaks within 10 kb upstream or downstream to genes of interest were identified using the Bedtools (85) command: bedtools intersect -c -w 10000 and are shown in Table S1A.
Reduced representation bisulfite sequencing.
Reduced representation bisulfite sequencing (RRBS) libraries were prepared from frozen liver tissues as previously described with modifications (41). Briefly, ∼50 mg of frozen liver tissue was homogenized by vigorously pipetting up and down the sample in 370 μl TE buffer (10 mM Tris, 1 mM EDTA [pH 8]) containing 10 μl of proteinase K solution (10 mg/ml). Then 20 μl of 10% (vol/vol) sodium dodecyl sulfate (SDS) was quickly added to achieve 0.5% SDS final concentration. The homogenate was incubated at 50°C for 2 h. Genomic DNA (gDNA) was purified by extraction with 400 μl phenol chloroform with the addition of 32 μl of 5 M NaCl, using a large-bore pipette tip with gentle inversions and without vortexing. Intact gDNA was spooled from the aqueous phase following the addition of an equal volume pf 100% ethanol. The spooled gDNA was treated with RNase in phosphate-buffered saline (PBS) buffer (2 μl of 10 mg/ml RNase A in 298 μl of PBS) for 1 h at room temperature. The RNA-free gDNA was washed in ethanol and thoroughly resuspended in TE buffer for Qubit quantification of DNA, taking care to first sample a large volume (10 μl) and to then shear the DNA by vigorous pipetting to ensure that the subsequent 2-μl input for Qubit analysis is as homogenous as possible. Five hundred nanograms of intact gDNA was digested overnight with 2.5 μl of MspI (20,000 U/ml; New England Biolabs [NEB] catalog no. R0106S) in the manufacturer’s supplied buffer (40-μl total volume) at 37°C, with the lid heated to 40°C to ensure more complete digestion. The reaction was subjected to end repair in the same reaction by adding 1 μl of Klenow fragment (NEB catalog no. M0210S; 5,000 U/ml) and 1 μl of deoxynucleoside triphosphate (dNTP) mix (10 mM dA, 1 mM dG, and 1 mM dC). Samples were then incubated at 30°C for 20 min, followed by 37°C for 20 min, and then purified by phenol chloroform extraction. Four hundred fifty nanograms of MpsI-digested, end-repaired material (quantified by Nanodrop measurement) was ligated to Illumina adaptors supplied in the NEB DNA-seq kit (NEB catalog no. E7370S, using 1/20 diluted adaptor) and NEB Quick DNA ligase kit (NEB catalog no. E6056S) by incubation at 16°C overnight without a heated lid. After USER enzyme digestion and cleanup with Zymo’s DNA Clean and Concentrator-5 (Zymo catalog no. D4003T), the adaptor-ligated MspI fragments were treated with bisulfite for two consecutive rounds using Zymo's EZ DNA Methylation-Gold kit (Zymo catalog no. D5005) per the manufacturer’s instructions. A portion (5 μl) of each 12-μl bisulfite-treated DNA sample was amplified using the KAPA HiFi HS Uracil+ polymerase system (Kapa Biosytems/Roche catalog no. KM2800) for ∼12 to 15 PCR cycles, with the optimal cycle number determined empirically to find the lowest number of cycles that yielded sufficient material without overamplification. Finally, size selection of 160- to 340-bp fragments was accomplished by performing a double solid-phase reversible immobilization (SPRI) purification of 0.65× and 0.55×, as follows. SPRI beads (32.5 μl) were added to each 50-μl reaction mixture (0.65×). After the reaction mixtures were mixed well, the tubes were placed on a magnetic rack until the beads were separated from the supernatant. The supernatant was transferred to fresh tubes, to which fresh SPRI beads (27.5 μl; 0.55×) were then added. The beads were washed twice in 200 μl of 80% ethanol solution, air dried for 5 min, and eluted in 0.1× TE buffer. The final RRBS sequencing libraries were analyzed on a 1% agarose gel stained with SYBR green, quantified by Qubit analysis, and sequenced on an Illumina HiSeq instrument (paired-end, 50-bp reads) with no more than ∼30% of a sequencing lane being RRBS material. RRBS libraries were prepared and sequenced for the following samples with the indicated numbers of biological replicate livers: wild-type (WT) male (n = 6), WT female (n = 5), STAT5-KO male (n = 6), and STAT5-KO female (n = 6).
RRBS data processing.
About 15 million paired-end sequence reads were obtained for each RRBS sample. Raw FASTQ files were first processed by Trim Galore (version 0.4.3) using options: --rrbs --paired. The trimmed read1 and read2 files were mapped by Bismark (86) using default parameters. PileOMeth was used to extract and quantify all CpG dinucleotides in the bam files, outputting the location, total coverage, number of thymines, and number of cytosines of each CpG in MethylKit-compatible format (parameter: --methylKit), while avoiding any double counting of reads where paired-end reads for a given fragment overlap. The PileOMeth results were directly input into MethylKit, two sample groups at a time, for pairwise differential analysis. MethylKit (87) differential analysis was performed at the level of both 100-bp tiles and individual CpGs using default parameters combined with the following: context = “CpG.” filterByCoverage (count = 10), destrand = T. A total of four comparisons were performed in a treatment-versus-control configuration, yielding differential methylation values (diff.meth), where the absolute value indicates the magnitude of change, and positive or negative signs indicate hypermethylation or hypomethylation of the treatment group relative to the control group, respectively. Tiles and individual CpGs with a significant change in DNA methylation were defined using a threshold fold difference of 15% and a q value of <0.05 and were combined such that individual CpGs not captured at the tile level were included. Significant tiles and significant individual CpGs exhibit significant overlap with each other and are summarized in Table S2 (also see below). Here, differentially methylated regions (DMRs) refer to 100-bp genomic regions that fit one of the following criteria: (i) 100-bp tiles that were significantly differentially methylated at the level of overall tile statistics and also contain one or more CpGs that showed significant differences in methylation at the individual CpG level (this describes the majority of DMRs); (ii) 100-bp tiles that were significant in their overall tile statistics but did not show statistically significant differences in any individual CpGs; and (iii) 100-bp regions that were not significantly differentially methylated at the level of overall tile statistics but that contain at least one significant individual CpG, whose diff.meth values were averaged based on those CpGs that showed significant differences in methylation. The criteria by which the DMRs were identified is flagged in Table S2. In the majority of analyses, DMRs meeting any of the above three criteria were combined and analyzed as a single set.
A total 1,355,894 CpGs were captured by all 23 liver RRBS samples (File S4). To generate this set of individual CpGs, raw PileOmeth files were destranded using a custom Python script, script27_destranding_pileometh.py (see the supplemental material), which collapses CpG coordinates from opposite strands into a single coordinate, to facilitate downstream analyses, as follows. Each CpG is symmetrical in the genome, which means that each CpG will have two coordinates, e.g., chr1:100 (plus strand) and chr1:101 (minus strand). The destranding script aggregates the genomic position from the plus strand and from the minus strand into a single genomic position. Next, the frequency of occurrence of each CpG in each of the 23 RRBS-seq samples was determined. For downstream analysis, we considered only those CpGs that were covered by at least 10 sequence reads in each of the 23 liver samples analyzed and those CpGs whose CpG sequence is also conserved in the C57BL/6 reference mouse genome. By segmenting the mouse genome into 100-bp nonoverlapping windows, these 1,355,894 CpGs were aggregated into 232,882 windows (tiles) (File S1 complete_23STAT5_unique_tiles_u), many of which contain multiple CpGs.
Rationale for merging of tile and individual CpG statistics.
As described above, DMRs were determined by MethylKit pairwise comparison using a tile-based method and also an individual CpG method. The tile-based analysis gives higher statistical power due to the aggregation of signals from multiple CpGs within a 100-bp genomic region. For example, if a tile contains three CpGs, all of which are only slightly differential, the tile statistics may give a modest differential methylation value but with a low FDR, whereas individual CpG analysis would yield a high FDR for each CpG. In a different scenario, if a single CpG within a tile region is highly differentially methylated but is situated near other CpGs that are not differentially methylated, the signal from the highly differential CpG will be diluted by the averaging used in the tile analysis, causing the loss of a significantly differential CpG. Many tiles contain only one CpG, and results of the tile and individual CpG analyses were similar for the most part. The final list of differential CpGs includes 2,375 significant tiles, as well as 1,514 100-bp genomic regions with individual CpGs that were captured by the individual CpG analysis but not by the tile analysis, for a combined total of 3,889 differential 100-bp genomic regions, is presented and further explained in Table S2.
An example of the R code used for tile and individual CpG differential analysis follows:
#my.list refers to file locations for 11 PileOmeth output files
myobj_1 <-
methRead(my.list,sample.id=list('M1_WT','M2_WT','M3_WT','M4_WT','M5_WT','M6_WT','F1_WT','F2_WT','F3_WT','F4_WT','F5_WT'),assembly='mm9',context='CpG',treatment=c(1,1,1,1,1,1,0,0,0,0,0))
filtered.myobj_1<-filterByCoverage(myobj_1,lo.count = 10,lo.perc=NULL, hi.count=NULL, hi.perc = 99.9)
meth_1<-unite(myobj_1,destrand=T)
tiles_1<- tileMethylCounts(meth_1,win.size = 100,step.size = 100)
#calculate differential 100-bp tiles
myDiff_tiles_1<-calculateDiffMeth(tiles_1)
#calculate differential individual CpG
myDiff_cpg<-calculateDiffMeth(meth_1)
Generation of raw methylation heat maps.
An intermediate file containing raw methylation values for all 100-bp tiles was generated by inputting Raw PileOMeth files into MethylKit and using the following command: tileMethylCounts(pileometh_input, win.size = 100, step.size = 100). Raw methylation values were retrieved for only the differential tiles, which were visualized as a heat map using R. These raw methylation values in WTF, KOM, and KOF samples were tested against WTM and evaluated using two-tailed Student’s t test. For the heat maps shown in Fig. 3B, only DMRs determined by tile statistics with a stringent threshold (|diff.meth| > 25%, q value < 0.05) were included. The heat map in Fig. 3B presents raw methylation values of DMRs that were determined to be significantly differential by tile analysis between wild-type male liver and wild-type male liver when determined by tile analysis (Table S2D, column B), i.e., excluding DMRs identified by individual CpGs only.
DMRs in gene promoter regions.
Promoter regions were defined as 5 kb upstream of the gene transcription start site. Thus, for genes on the plus strand, the promoter coordinates were set as (start position − 5000, start position); for genes on the minus strand, coordinates were set as (end position, end position + 5000). DMRs positioned in a promoter region (minimum 1-nt overlap) (Table S3) were identified using the Bedtools windows command.
Distribution of CpGs across chromatin states and annotated genomic features.
To determine the distribution of chromatin states across all tiles (Fig. 5C), the 232,882 CpG-containing 100-bp tiles were mapped to 14 chromatin states previously defined in male mouse liver (29) using ChromHMM (88) and the Intersect command of Bedtools (85) (see Files S1 and S2, Male_14_Chromatin States and Female_14_Chromatin States, in the supplemental material). Custom Bash commands and R scripts were used to calculate the frequencies of the 14 chromatin states in each bin, and the results were plotted by ggplot. Chromatin state distributions were similarly determined for the sets of DMRs identified in the WTM/WTF, KOM/WTM, and KOF/WTF comparisons, using the full set of DMRs (i.e., DMRs identified by tiles and DMRs identified by individual CpGs) for each comparison (Table S2). The relative frequency of occurrence of each chromatin state was determined for each 100-bp bin across a 4-kb window surrounding each hypomethylated DMR, and separately, each hypermethylated DMR. To calculate enrichment scores, the 14 chromatin states were grouped into five superstates, designated inactive (states 1, 2, and 3), enhancer (states 5, 6, 9, 10, and 11), bivalent (state 12), transcribed (states 13 and 14), and promoter (states 7 and 8). Enrichment of DMRs for these five superstates relative to “all CpGs” (i.e., the set of 232,882 tiles covered by our RRBS samples) was calculated as follows: (number of differential DMRs in chromatin state X/total number of differential DMRs)/(number of all DMRs in chromatin state X/232,882). Statistical significance was determined in R by two proportion Z-test using the prop.test function.
TAD analysis.
DMRs were grouped by their chromatin states, as defined separately in wild-type male mouse liver and in wild-type female mouse liver (29). DMRs and genes were then mapped to topologically associating domains (TADs) defined for mouse liver (52), and the overlap of DMR-containing TADs and gene-containing TADs was analyzed using data sets provided in Table S4. As a control, for each chromatin state, comparably sized sets of RRBS tiles that did not show significant sex differences or a response to STAT5 loss were randomly sampled 50 times and mapped to TADs. The significance of DMRs occurring in TADs containing sex-biased or STAT5-responsive genes compared to the extent of overlap observed for the random controls was assessed by t test. In addition, the distance from the start of the DMR to the transcription start site of sex-biased and STAT5-responsive genes was determined and analyzed using box plots, where DMRs that are >106 bp away from genes were deemed outliers and excluded from the analysis.
Gene set enrichment analysis.
DMRs determined by tile statistics with a stringent threshold were used. DMRs were ranked from high (hypermethylated) to low (hypomethylated). This list of ranked DMRs as well as the list of DMRs with nearby (within 10 kb) male-biased and female-biased DHS (53) (Table S5), were input into the gene set enrichment analysis (GSEA) desktop application for preranked test analysis (89), with the number of permutations set at 1,000.
MEME DNA motif analysis.
The Analysis of Motif Enrichment (AME) algorithm of the MEME suite (90) was used to assess the enrichment of the STAT5 motif in the tile sequences. The following command was used: ame –control random_sequences.txt –evalue-report-threshold 1 –o comp1_N comp1_N_sequence.txt STAT5_meme_motif.txt, where random_sequences.txt is a FASTA file of 100-bp sequences from randomly selected genomic regions that are not covered by our RRBS data set; comp1_N_sequences.txt is a fasta file with the DMR tile sequences; and STAT5_meme_motif.txt is the MEME file with the STAT5 motif (see the supplemental material).
Cumulative frequency plots of STAT5 ChIP-seq peaks near DMRs.
DMRs based on both tiles and individual CpG statistics for three comparisons (WTM/WTF, KOM/WTM, and KOF/WTF) were separated into hypomethylated and hypermethylated DMRs. In addition, a file containing 1,000 randomly selected nondifferential methylated genomic regions was used. Male-biased and female-biased STAT5 ChIP-seq peaks were part of a list of 14,910 ChIP-seq peaks for STAT5 (see File S1, stat5b_merged_ss_sorted, in the supplemental material) (12) after excluding 184 peaks on chromosome X (ChrX) and chromosome M (ChrM) from the full set of 15,094 ChIP-seq peaks reported in that study. Male-biased STAT5 binding occurs at 1,661 of the 14,910 sites, and female-biased binding occurs at 1,782 sites. Forty seven percent of the male-biased STAT5 peaks contain one or more STAT5 motifs (12). The Bedtools command bedtools closest -d -t was used to find the distance to the nearest STAT5 peak for each DMR. For each of the seven sets of DMRs, DMRs were first ranked by distance to the nearest STAT5 peak from low to high, and then cumulative values (which range from 0 to 1) were assigned to the ranked DMRs as follows: (position in ranked list/total number in list). The cutoff for the distance to the nearest STAT5 peak was set at 10 kb. Distance (x axis) and cumulative frequency values (y axis) were plotted for each DMR set.
Homer DNA motif analysis.
The Homer motif analysis tool (54) was used to discover enriched motifs at DMRs relative to a background set comprised of a random selection of 1,000 100-bp regions that were detected by RRBS but not differentially methylated. Homer enrichment analysis of known motifs (Table S6) used a set of 332 motifs in the HOMER motif database that were mostly curated from published ChIP-seq data sets (http://homer.ucsd.edu/homer/motif/motifDatabase.html). The command for Homer motif analysis was as follows: findMotifsGenome.pl input.txt mm9 output_folder -size 100 -bg random_1000.txt, where input.txt contains the genomic coordinates of DMRs to be analyzed for enriched regulatory elements, mm9 specifies the genome, “-size 100” indicates that the genomic regions in input.txt are 100 bp long, and “-bg random_1000.txt” specifies the coordinates of background regions.
Data availability.
Raw and processed sequencing data are available at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE103885 (RNA-seq samples) and GSE103886 (RRBS samples).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by NIH grant DK121998 (to D.J.W.).
The funding agency had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Lothar Hennighausen, NIDDK, for originally providing the mouse liver tissue samples used in these analyses.
We state that we have no financial conflicts of interest to disclose.
P.H. and D.J.W. jointly conceptualized the project and designed the study. P.H. carried out all of the laboratory experiments, developed and implemented the methods for computational analysis, and carried out data analysis and figure preparation. D.J.W. obtained grant funding and supervised the study. P.H. and D.J.W. jointly drafted the initial manuscript, and D.J.W. revised and edited the text and figures for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Levy DE, Darnell JE, Jr. 2002. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 2.Furth PA, Nakles RE, Millman S, Diaz-Cruz ES, Cabrera MC. 2011. Signal transducer and activator of transcription 5 as a key signaling pathway in normal mammary gland developmental biology and breast cancer. Breast Cancer Res 13:220. doi: 10.1186/bcr2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes K, Watson CJ. 2012. The spectrum of STAT functions in mammary gland development. JAKSTAT 1:151–158. doi: 10.4161/jkst.19691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaltenecker D, Themanns M, Mueller KM, Spirk K, Suske T, Merkel O, Kenner L, Luis A, Kozlov A, Haybaeck J, Muller M, Han X, Moriggl R. 2019. Hepatic growth hormone - JAK2 - STAT5 signalling: metabolic function, non-alcoholic fatty liver disease and hepatocellular carcinoma progression. Cytokine 124:154569. doi: 10.1016/j.cyto.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Waters MJ. 2016. The growth hormone receptor. Growth Horm IGF Res 28:6–10. doi: 10.1016/j.ghir.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Dodington DW, Desai HR, Woo M. 2018. JAK/STAT—emerging players in metabolism. Trends Endocrinol Metab 29:55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Chia DJ. 2014. Minireview: mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol 28:1012–1025. doi: 10.1210/me.2014-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waxman DJ, Holloway MG. 2009. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichanska AM, Waters MJ. 2008. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet 24:41–47. doi: 10.1016/j.tig.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. 2006. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 11.Herrington J, Smit LS, Schwartz J, Carter-Su C. 2000. The role of STAT proteins in growth hormone signaling. Oncogene 19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Laz EV, Waxman DJ. 2012. Dynamic, sex-differential STAT5 and BCL6 binding to sex-biased, growth hormone-regulated genes in adult mouse liver. Mol Cell Biol 32:880–896. doi: 10.1128/MCB.06312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baik M, Yu JH, Hennighausen L. 2011. Growth hormone-STAT5 regulation of growth, hepatocellular carcinoma, and liver metabolism. Ann N Y Acad Sci 1229:29–37. doi: 10.1111/j.1749-6632.2011.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldhuis JD, Bowers CY. 2003. Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest 26:799–813. doi: 10.1007/BF03345229. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Bekaert AJ, Dupont J, Rouve S, Annesi-Maesano I, De Magalhaes Filho CD, Kappeler L, Holzenberger M. 2011. Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J Endocrinol 208:119–129. doi: 10.1677/JOE-10-0317. [DOI] [PubMed] [Google Scholar]
- 16.Connerney J, Lau-Corona D, Rampersaud A, Waxman DJ. 2017. Activation of male liver chromatin accessibility and STAT5-dependent gene transcription by plasma growth hormone pulses. Endocrinology 158:1386–1405. doi: 10.1210/en.2017-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannenbaum GS, Choi HK, Gurd W, Waxman DJ. 2001. Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology 142:4599–4606. doi: 10.1210/endo.142.11.8480. [DOI] [PubMed] [Google Scholar]
- 18.Seton-Rogers S. 2014. Hepatocellular carcinoma: gender differences. Nat Rev Cancer 14:578. doi: 10.1038/nrc3808. [DOI] [PubMed] [Google Scholar]
- 19.Park SH, Liu X, Hennighausen L, Davey HW, Waxman DJ. 1999. Distinctive roles of STAT5a and STAT5b in sexual dimorphism of hepatic P450 gene expression. Impact of STAT5a gene disruption. J Biol Chem 274:7421–7430. doi: 10.1074/jbc.274.11.7421. [DOI] [PubMed] [Google Scholar]
- 20.Wauthier V, Sugathan A, Meyer RD, Dombkowski AA, Waxman DJ. 2010. Intrinsic sex differences in the early growth hormone responsiveness of sex-specific genes in mouse liver. Mol Endocrinol 24:667–678. doi: 10.1210/me.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau-Corona D, Suvorov A, Waxman DJ. 2017. Feminization of male mouse liver by persistent growth hormone stimulation: activation of sex-biased transcriptional networks and dynamic changes in chromatin states. Mol Cell Biol 37:e00310-17. doi: 10.1128/MCB.00301-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloway MG, Laz EV, Waxman DJ. 2006. Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4alpha. Mol Endocrinol 20:647–660. doi: 10.1210/me.2005-0328. [DOI] [PubMed] [Google Scholar]
- 23.Hao P, Waxman DJ. 2018. Functional roles of sex-biased, growth hormone-regulated microRNAs miR-1948 and miR-802 in young adult mouse liver. Endocrinology 159:1377–1392. doi: 10.1210/en.2017-03109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melia T, Hao P, Yilmaz F, Waxman DJ. 2016. Hepatic long intergenic noncoding RNAs: high promoter conservation and dynamic, sex-dependent transcriptional regulation by growth hormone. Mol Cell Biol 36:50–69. doi: 10.1128/MCB.00861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melia T, Waxman DJ. 2019. Sex-biased lncRNAs inversely correlate with sex-opposite gene coexpression networks in diversity outbred mouse liver. Endocrinology 160:989–1007. doi: 10.1210/en.2018-00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ. 1999. STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J Biol Chem 274:35331–35336. doi: 10.1074/jbc.274.50.35331. [DOI] [PubMed] [Google Scholar]
- 27.Bennett E, McGuinness L, Gevers EF, Thomas GB, Robinson IC, Davey HW, Luckman SM. 2005. Hypothalamic STAT proteins: regulation of somatostatin neurones by growth hormone via STAT5b. J Neuroendocrinol 17:186–194. doi: 10.1111/j.1365-2826.2005.01296.x. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. 2007. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 29.Sugathan A, Waxman DJ. 2013. Genome-wide analysis of chromatin states reveals distinct mechanisms of sex-dependent gene regulation in male and female mouse liver. Mol Cell Biol 33:3594–3610. doi: 10.1128/MCB.00280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg MVC, Bourc’his D. 2019. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol 20:590–607. doi: 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- 31.da Rocha ST, Gendrel AV. 2019. The influence of DNA methylation on monoallelic expression. Essays Biochem 63:663–676. doi: 10.1042/EBC20190034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouzmenko A, Ohtake F, Fujiki R, Kato S. 2010. Hormonal gene regulation through DNA methylation and demethylation. Epigenomics 2:765–774. doi: 10.2217/epi.10.58. [DOI] [PubMed] [Google Scholar]
- 33.Singh S, Li SS. 2012. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci 13:10143–10153. doi: 10.3390/ijms130810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angeloni A, Bogdanovic O. 2019. Enhancer DNA methylation: implications for gene regulation. Essays Biochem 63:707–715. doi: 10.1042/EBC20190030. [DOI] [PubMed] [Google Scholar]
- 35.Ito S, Hirabayashi K, Moriishi K, Matsui Y, Moriya K, Koike K, Matsuura Y, Shiota K, Yagi S. 2015. Novel sex-dependent differentially methylated regions are demethylated in adult male mouse livers. Biochem Biophys Res Commun 462:332–338. doi: 10.1016/j.bbrc.2015.04.137. [DOI] [PubMed] [Google Scholar]
- 36.McCormick H, Young PE, Hur SSJ, Booher K, Chung H, Cropley JE, Giannoulatou E, Suter CM. 2017. Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. BMC Genomics 18:966. doi: 10.1186/s12864-017-4350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takasugi M, Hayakawa K, Arai D, Shiota K. 2013. Age- and sex-dependent DNA hypomethylation controlled by growth hormone in mouse liver. Mech Ageing Dev 134:331–337. doi: 10.1016/j.mad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Krebs CJ, Schultz DC, Robins DM. 2012. The KRAB zinc finger protein RSL1 regulates sex- and tissue-specific promoter methylation and dynamic hormone-responsive chromatin configuration. Mol Cell Biol 32:3732–3742. doi: 10.1128/MCB.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reizel Y, Spiro A, Sabag O, Skversky Y, Hecht M, Keshet I, Berman BP, Cedar H. 2015. Gender-specific postnatal demethylation and establishment of epigenetic memory. Genes Dev 29:923–933. doi: 10.1101/gad.259309.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reizel Y, Sabag O, Skversky Y, Spiro A, Steinberg B, Bernstein D, Wang A, Kieckhaefer J, Li C, Pikarsky E, Levin-Klein R, Goren A, Rajewsky K, Kaestner KH, Cedar H. 2018. Postnatal DNA demethylation and its role in tissue maturation. Nat Commun 9:2040. doi: 10.1038/s41467-018-04456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A. 2011. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc 6:468–481. doi: 10.1038/nprot.2010.190. [DOI] [PubMed] [Google Scholar]
- 42.Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. 2007. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology 148:1977–1986. doi: 10.1210/en.2006-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conforto TL, Zhang Y, Sherman J, Waxman DJ. 2012. Impact of CUX2 on the female mouse liver transcriptome: activation of female-biased genes and repression of male-biased genes. Mol Cell Biol 32:4611–4627. doi: 10.1128/MCB.00886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conforto TL, Steinhardt GF, IV, Waxman DJ. 2015. Cross talk between GH-regulated transcription factors HNF6 and CUX2 in adult mouse liver. Mol Endocrinol 29:1286–1302. doi: 10.1210/me.2015-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao B. 2005. Cytokines, STATs and liver disease. Cell Mol Immunol 2:92–100. [PubMed] [Google Scholar]
- 46.Weisend CM, Kundert JA, Suvorova ES, Prigge JR, Schmidt EE. 2009. Cre activity in fetal albCre mouse hepatocytes: utility for developmental studies. Genesis 47:789–792. doi: 10.1002/dvg.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeziorska DM, Murray RJS, De Gobbi M, Gaentzsch R, Garrick D, Ayyub H, Chen T, Li E, Telenius J, Lynch M, Graham B, Smith AJH, Lund JN, Hughes JR, Higgs DR, Tufarelli C. 2017. DNA methylation of intragenic CpG islands depends on their transcriptional activity during differentiation and disease. Proc Natl Acad Sci U S A 114:E7526–E7535. doi: 10.1073/pnas.1703087114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cedar H, Bergman Y. 2012. Programming of DNA methylation patterns. Annu Rev Biochem 81:97–117. doi: 10.1146/annurev-biochem-052610-091920. [DOI] [PubMed] [Google Scholar]
- 49.Matthews BJ, Waxman DJ. 2020. Impact of 3D genome organization, guided by cohesin and CTCF looping, on sex-biased chromatin interactions and gene expression in mouse liver. Epigenetics Chromatin 13:30. doi: 10.1186/s13072-020-00350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu M, Ren B. 2017. The three-dimensional organization of mammalian genomes. Annu Rev Cell Dev Biol 33:265–289. doi: 10.1146/annurev-cellbio-100616-060531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vietri Rudan M, Barrington C, Henderson S, Ernst C, Odom DT, Tanay A, Hadjur S. 2015. Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Rep 10:1297–1309. doi: 10.1016/j.celrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthews BJ, Waxman DJ. 2018. Computational prediction of CTCF/cohesin-based intra-TAD loops that insulate chromatin contacts and gene expression in mouse liver. Elife 7:e34077. doi: 10.7554/eLife.34077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling G, Sugathan A, Mazor T, Fraenkel E, Waxman DJ. 2010. Unbiased, genome-wide in vivo mapping of transcriptional regulatory elements reveals sex differences in chromatin structure associated with sex-specific liver gene expression. Mol Cell Biol 30:5531–5544. doi: 10.1128/MCB.00601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wade PA. 2001. Methyl CpG-binding proteins and transcriptional repression. Bioessays 23:1131–1137. doi: 10.1002/bies.10008. [DOI] [PubMed] [Google Scholar]
- 56.Cedar H, Bergman Y. 2009. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 57.Newell-Price J, Clark AJ, King P. 2000. DNA methylation and silencing of gene expression. Trends Endocrinol Metab 11:142–148. doi: 10.1016/s1043-2760(00)00248-4. [DOI] [PubMed] [Google Scholar]
- 58.Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, Ray WJ, Waxman DJ. 2007. Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics 31:63–74. doi: 10.1152/physiolgenomics.00055.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laz EV, Holloway MG, Chen CS, Waxman DJ. 2007. Characterization of three growth hormone-responsive transcription factors preferentially expressed in adult female liver. Endocrinology 148:3327–3337. doi: 10.1210/en.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yokomori N, Kobayashi R, Moore R, Sueyoshi T, Negishi M. 1995. A DNA methylation site in the male-specific P450 (Cyp 2d-9) promoter and binding of the heteromeric transcription factor GABP. Mol Cell Biol 15:5355–5362. doi: 10.1128/mcb.15.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokomori N, Moore R, Negishi M. 1995. Sexually dimorphic DNA demethylation in the promoter of the Slp (sex-limited protein) gene in mouse liver. Proc Natl Acad Sci U S A 92:1302–1306. doi: 10.1073/pnas.92.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Au-Yeung N, Mandhana R, Horvath CM. 2013. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAKSTAT 2:e23931. doi: 10.4161/jkst.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ram PA, Park SH, Choi HK, Waxman DJ. 1996. Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem 271:5929–5940. doi: 10.1074/jbc.271.10.5929. [DOI] [PubMed] [Google Scholar]
- 64.Das RK, Banerjee S, Shapiro BH. 2017. Growth hormone: a newly identified developmental organizer. J Endocrinol 232:377–389. doi: 10.1530/JOE-16-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi HK, Waxman DJ. 2000. Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology 141:3245–3255. doi: 10.1210/endo.141.9.7638. [DOI] [PubMed] [Google Scholar]
- 66.Smith ZD, Meissner A. 2013. DNA methylation: roles in mammalian development. Nat Rev Genet 14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 67.Weaver JR, Susiarjo M, Bartolomei MS. 2009. Imprinting and epigenetic changes in the early embryo. Mamm Genome 20:532–543. doi: 10.1007/s00335-009-9225-2. [DOI] [PubMed] [Google Scholar]
- 68.Cannon MV, Pilarowski G, Liu X, Serre D. 2016. Extensive epigenetic changes accompany terminal differentiation of mouse hepatocytes after birth. G3 (Bethesda) 6:3701–3709. doi: 10.1534/g3.116.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao H, Chen T. 2013. Tet family of 5-methylcytosine dioxygenases in mammalian development. J Hum Genet 58:421–427. doi: 10.1038/jhg.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conforto TL, Waxman DJ. 2012. Sex-specific mouse liver gene expression: genome-wide analysis of developmental changes from pre-pubertal period to young adulthood. Biol Sex Differ 3:9. doi: 10.1186/2042-6410-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. 1997. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A 94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. 1998. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850. doi: 10.1016/S0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 73.Deaton AM, Bird A. 2011. CpG islands and the regulation of transcription. Genes Dev 25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aran D, Sabato S, Hellman A. 2013. DNA methylation of distal regulatory sites characterizes dysregulation of cancer genes. Genome Biol 14:R21. doi: 10.1186/gb-2013-14-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blattler A, Yao L, Witt H, Guo Y, Nicolet CM, Berman BP, Farnham PJ. 2014. Global loss of DNA methylation uncovers intronic enhancers in genes showing expression changes. Genome Biol 15:469. doi: 10.1186/s13059-014-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charlet J, Duymich CE, Lay FD, Mundbjerg K, Dalsgaard Sorensen K, Liang G, Jones PA. 2016. Bivalent regions of cytosine methylation and H3K27 acetylation suggest an active role for DNA methylation at enhancers. Mol Cell 62:422–431. doi: 10.1016/j.molcel.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin W, Li QZ, Zuo YC, Cao YN, Zhang LQ, Hou R, Su WX. 2018. The relationship between DNA methylation in key region and the differential expressions of genes in human breast tumor tissue. DNA Cell Biol 38:49–62. doi: 10.1089/dna.2018.4276. [DOI] [PubMed] [Google Scholar]
- 78.Neiman D, Moss J, Hecht M, Magenheim J, Piyanzin S, Shapiro AMJ, de Koning EJP, Razin A, Cedar H, Shemer R, Dor Y. 2017. Islet cells share promoter hypomethylation independently of expression, but exhibit cell-type-specific methylation in enhancers. Proc Natl Acad Sci U S A 114:13525–13530. doi: 10.1073/pnas.1713736114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oshida K, Vasani N, Waxman DJ, Corton JC. 2016. Disruption of STAT5b-regulated sexual dimorphism of the liver transcriptome by diverse factors is a common event. PLoS One 11:e0148308. doi: 10.1371/journal.pone.0148308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cordoba-Chacon J, Sarmento-Cabral A, Del Rio-Moreno M, Diaz-Ruiz A, Subbaiah PV, Kineman RD. 2018. Adult-onset hepatocyte GH resistance promotes NASH in male mice, without severe systemic metabolic dysfunction. Endocrinology 159:3761–3774. doi: 10.1210/en.2018-00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim M, Costello J. 2017. DNA methylation: an epigenetic mark of cellular memory. Exp Mol Med 49:e322. doi: 10.1038/emm.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 84.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quinlan AR. 2014. BEDTools: the Swiss-Army tool for genome feature analysis. Curr Protoc Bioinformatics 47:11.12.1.1–11.12.34. doi: 10.1002/0471250953.bi1112s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krueger F, Andrews SR. 2011. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE. 2012. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13:R87. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ernst J, Kellis M. 2017. Chromatin-state discovery and genome annotation with ChromHMM. Nat Protoc 12:2478–2492. doi: 10.1038/nprot.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. 2007. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 90.McLeay RC, Bailey TL. 2010. Motif Enrichment Analysis: a unified framework and an evaluation on ChIP data. BMC Bioinformatics 11:165. doi: 10.1186/1471-2105-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and processed sequencing data are available at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE103885 (RNA-seq samples) and GSE103886 (RRBS samples).