Activating mutations in the KEAP1-NRF2 pathway are found in approximately 25% of lung tumors, where the hijacking of NRF2’s cytoprotective functions results in aggressive tumor growth, chemoresistance, and a poor prognosis for patients. There are currently no approved drugs which target aberrant NRF2 activation, which means that there is an urgent clinical need to target this orphan oncogenic pathway in human tumors. In this study, we used an isogenic pair of wild-type and Keap1 knockout cells to screen a range of chemotherapeutic and pathway-targeted anticancer drugs in order to identify compounds which display enhanced toxicity toward cells with high levels of Nrf2 activity.
KEYWORDS: NRF2, KEAP1, NFE2L2, oxidative stress, cancer, drug repositioning, concurrent synthetic lethality
ABSTRACT
Activating mutations in the KEAP1-NRF2 pathway are found in approximately 25% of lung tumors, where the hijacking of NRF2’s cytoprotective functions results in aggressive tumor growth, chemoresistance, and a poor prognosis for patients. There are currently no approved drugs which target aberrant NRF2 activation, which means that there is an urgent clinical need to target this orphan oncogenic pathway in human tumors. In this study, we used an isogenic pair of wild-type and Keap1 knockout cells to screen a range of chemotherapeutic and pathway-targeted anticancer drugs in order to identify compounds which display enhanced toxicity toward cells with high levels of Nrf2 activity. Through this approach, complemented by validation across a panel of eight human cancer cell lines from a range of different tissues, we identified the DNA-damaging agent mitomycin C to be significantly more toxic in cells with aberrant Nrf2 activation. Mechanistically, we found that the NRF2 target genes for cytochrome P450 reductase, NQO1, and enzymes in the pentose phosphate pathway are all responsible for the NRF2-dependent enhanced bioactivation of mitomycin C. As mitomycin C is already approved for clinical use, it represents as excellent drug repositioning candidate to target the currently untreatable NRF2 activation in human tumors.
INTRODUCTION
Activating mutations in the KEAP1-NRF2 pathway are found in 25% of lung squamous cell carcinomas, 23% of stomach and esophageal cancer, and 22% of human papillomavirus (HPV)-negative head and neck squamous cell carcinomas (1, 2). In patients, hijacking of the cytoprotective function of NRF2 results in resistance to DNA damage-inducing therapeutics, antimetabolites, and HER2-directed antibody therapy, which results in a poor prognosis and reduced overall survival (3–5). Despite the fact that NRF2 is a validated driver of aggressive cancer growth, the complete lack of approved drugs which can target oncogenic NRF2 signaling means that there is an urgent clinical need to identify compounds which display efficacy against NRF2-dependent tumors.
The KEAP1-NRF2 pathway regulates the inducible stress responses to oxidative and electrophilic stresses and, as such, plays an important role in human health and disease (6, 7). KEAP1 forms part of an E3-ubiquitin ligase which, under nonstressed conditions, targets the transcription factor NRF2 for constitutive ubiquitination and proteasome-dependent degradation (8, 9). The activity of KEAP1 can be modulated by a broad range of endogenous and exogenous inducers, including hydrogen peroxide, lipid oxidation products, and triterpenoids, which bind directly to sensor motifs within the KEAP1 protein (10–12). Inducer binding to these cysteine-based sensors inactivates KEAP1’s E3 ligase function, which allows NRF2 to escape proteasomal degradation and translocate to the nucleus, where it can activate its gene expression program (13–15). NRF2 is a member of the CNC family of transcription factors, which regulate transcription by heterodimerizing with members of the small Maf family of proteins (16). While all members of the CNC family bind to the same consensus TGACTCAGC enhancer motif, NRF2 uniquely regulates oxidative stress-associated cytoprotective gene expression (17, 18). Through this regulation of cytoprotective gene expression, activation of NRF2 confers a panoply of prosurvival characteristics upon tumorigenic cells, and as such, aberrant activation of NRF2 is commonly observed in human tumors (19, 20).
The incongruence between the medical importance of NRF2 activation and the therapeutic options available for clinicians is best exemplified in lung cancer. The KEAP1 gene is the third most commonly mutated gene in lung adenocarcinoma patients, where it functions as an initiating driver of carcinogenesis and is associated with reduced patient survival (20–22). Despite these facts, KEAP1-NRF2 mutations were excluded from a recent large-scale non-small cell lung carcinoma-stratified targeted therapy study due to the absence of drugs which can specifically target NRF2 (23). The complete lack of NRF2-directed therapies, coupled with a high incidence of cancer cell NRF2 activation and the associated poor prognosis for patients, means that there is a clear clinical need to identify compounds which can specifically target NRF2-dependent tumors.
Mitomycin C (MMC) is a natural product isolated from the fungus Streptomyces caespitosus, which received approval from the Food and Drug Administration (FDA) as a novel chemotherapeutic drug in 1974 (24). Mitomycin C functions as a DNA alkylating agent which induces DNA damage through the formation of interstrand cross-links (25, 26). A central feature of the pharmacodynamics of mitomycin C is the fact that it is administered as a prodrug which undergoes intracellular enzyme-mediated reductive activation in order to generate functional DNA alkylating compounds (27, 28). This bioactivation of mitomycin C preferentially occurs in hypoxic and low-pH environments, such as those found in solid tumors, as this favors the reductive processes required for the toxic metabolite generation (27, 28). Clinically, mitomycin C is efficacious against bladder and gastric tumors, head and neck squamous cell carcinoma, and non-small cell lung carcinoma, where it is administered as either a single agent or in combination therapies (24, 29).
As NRF2 is a transcription factor, it is challenging to identify compounds which directly inhibit its function. Initial attempts at inhibiting NRF2 activation focused on the use of translation inhibitors; however, this approach does not provide significant pathway specificity (30, 31). As NRF2 regulates the transcription of a broad range of xenobiotic-metabolizing enzymes, we hypothesized that the KEAP1-NRF2 pathway may be a good target for prodrug-induced synthetic lethality (32). In this way, administration of a prodrug which is specifically metabolized into a toxic product by an NRF2 target gene would provide specificity for NRF2-dependent cell death. Using this approach, we identified three geldanamycin-derived HSP90 inhibitors which are bioactivated by an NRF2 target gene, the NQO1 gene, resulting in NRF2-dependent cell death across a range of cell lines (32).
In the present study, we expanded upon our synthetic lethal screening approach and identified the DNA alkylating agent mitomycin C to be a compound which displays enhanced toxicity in cells with activated NRF2 signaling. Mechanistically, we found that a number of NRF2 target genes, including genes for CYPOR, NQO1, and enzymes in the pentose phosphate pathway, are all required for the intracellular bioactivation of mitomycin C. As mitomycin C is already approved for clinical use, we believe that it represents an excellent candidate for drug repositioning to target the currently untreatable aberrant NRF2 activation in human tumors.
RESULTS
Mitomycin C displays enhanced toxicity in Keap1 KO cells.
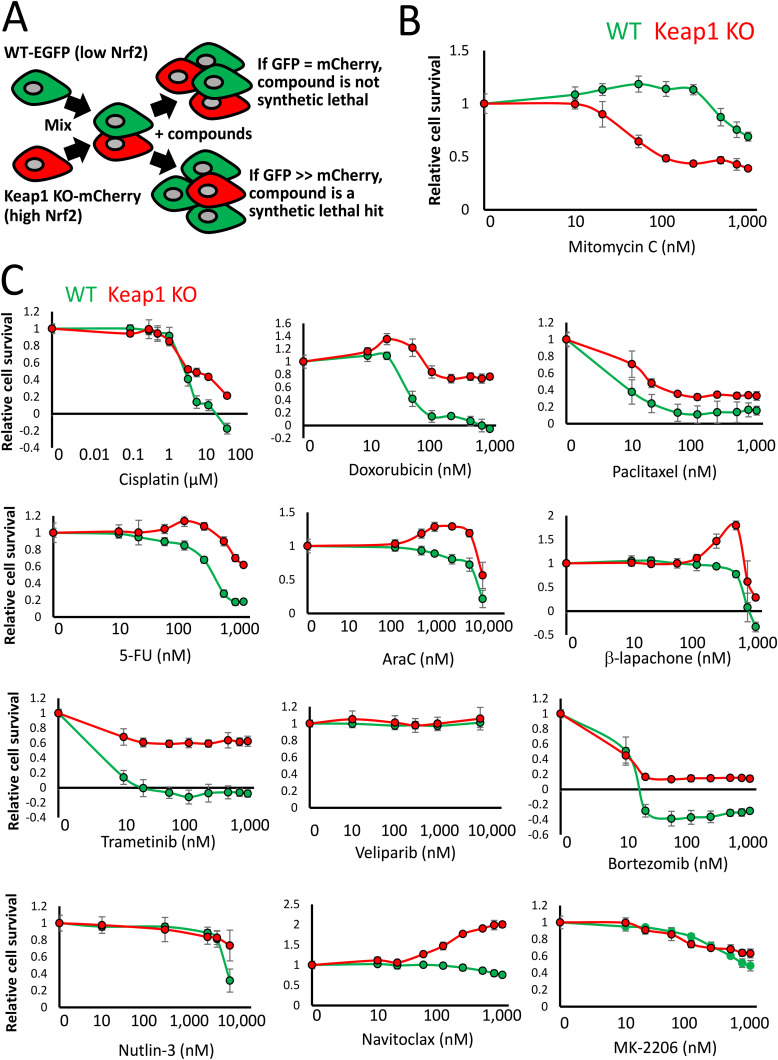
We developed a phenotypic screen using an isogenic pair of fluorescently labeled Hepa1 cells in order to identify compounds that are synthetic lethal with NRF2 activity (32). Stable clones of wild-type (WT) cells expressing enhanced green fluorescent protein (EGFP) and CRISPR-Cas9-mediated Keap1 knockout (KO) cells expressing mCherry were generated, which facilitates the tracking of the genetic identity of the cells throughout the screen due to the differential fluorophore expression (Fig. 1A). This feature enabled us to mix the cells in the same microplate well during the chemical compound treatment and, in doing so, generate a growth competition assay between the isogenic cell lines in response to the compounds. Using this approach, any compound which significantly decreases the mCherry/GFP ratio relative to the dimethyl sulfoxide (DMSO) control would be a candidate synthetic lethal hit with Nrf2.
FIG 1.
Mitomycin C displays enhanced toxicity in Keap1 KO cells. (A) Schematic showing an overview of the synthetic lethal screening strategy using isogenic WT and Keap1 KO Hepa1 cells. (B) Viabilities of cocultured WT-GFP and Keap1 KO-mCherry cells, determined by fluorescence intensity relative to the DMSO control, exposed to the indicated concentrations of mitomycin C for 8 days. (C) Viabilities of cocultured WT-GFP and Keap1 KO-mCherry cells, determined by fluorescence intensity relative to the DMSO control, exposed to the indicated concentrations of a broad range of anticancer drugs for 8 days.
Use of this screening system revealed that geldanamycin-derived HSP90 inhibitors are synthetic lethal with NRF2 activity (32). To expand upon these findings, we screened a diverse panel of molecular targeted anticancer drugs and chemotherapeutics across a broad range of concentrations in order to determine which additional compounds, if any, exhibit enhanced NRF2-dependent toxicity (Fig. 1B and C).
Through this approach, we identified the DNA alkylating agent mitomycin C to be significantly more toxic to the Keap1 KO cells than the cocultured isogenic WT cells (Fig. 1B). This result is in stark contrast to previous studies using other DNA-damaging agents, such as etoposide and carboplatin, where activation of NRF2 has been shown to be protective against their cytotoxic effects (33, 34).
Across the panel of anticancer drugs, we found that microtubule poisoning (paclitaxel), proteasome inhibition (bortezomib), antimetabolites (5-fluorouracil [5-FU], AraC), p53 activation (nutlin-3a), BCL2 inhibition (navitoclax), poly(ADP-ribose) polymerase (PARP) inhibition (veliparib), MEK inhibition (trametinib), AKT inhibition (MK-2206), topoisomerase inhibition (doxorubicin), platinum-based DNA damage induction (cisplatin), or redox-cycling reactive oxygen species (ROS) generation (β-lapachone) did not display enhanced NRF2-dependent toxicity (Fig. 1C). Indeed, Keap1 inactivation resulted in enhanced cellular survival in response to 10 of these 12 compounds, which is consistent with the cytoprotective role that NRF2 activation has been shown to confer on tumor cells (33, 35).
Mitomycin C toxicity is dependent on Nrf2 activity.
We next wished to elucidate the mechanism responsible for the increased survival of WT cells relative to the isogenic Keap1 KO cells upon treatment with mitomycin C. To ensure that the enhanced mitomycin C toxicity observed in Keap1 KO cells is due to decreased cell survival and not fluorophore quenching, we validated the results using a complementary assay which is independent of fluorescence intensity. These data from monocultured WT and Keap1 KO cells confirmed the concentration-dependent nanomolar range toxicity of mitomycin C in Keap1 KO cells (Fig. 2A). Furthermore, visualization of the cocultured cells revealed that in response to mitomycin C, the WT-GFP cells expand their domain within the microplate well, arguing against any mCherry fluorophore-quenching effects (Fig. 2B).
FIG 2.
Enhanced mitomycin C toxicity is dependent on the activity of Nrf2. (A) Viabilities of monococultured WT-GFP and Keap1 KO-mCherry cells, determined by total protein content relative to the DMSO control, exposed to the indicated concentrations of mitomycin C for 4 days. (B) Visualization of the cocultured WT-GFP and Keap1 KO-mCherry cells shows that in response to DMSO, the coculture is dominated by mCherry-expressing cells, while in mitomycin C-treated cells, the mCherry signal is significantly diminished, and the GFP-expressing cells expand their domain to fill the entire surface of the microplate well. Scale bars, 300 μm. (C) Fluorescence intensity of WT-GFP cells exposed to 0.1% DMSO or 50 nM mitomycin C, measured each day over a period of 6 days. (D) Fluorescence intensity of Keap1 KO-mCherry cells exposed to 0.1% DMSO or 50 nM mitomycin C, measured each day over a period of 6 days. The decrease in survival in response to mitomycin C is statistically significant from day 3. *, P < 0.001. (E) Fluorescence intensity of Keap1-Nrf2 DKO-mCherry cells exposed to 0.1% DMSO or 50 nM mitomycin C, measured each day over a period of 6 days. Note that the loss of Nrf2 completely rescues the phenotype. (F) Viabilities of cocultured WT-GFP and Keap1 KO-mCherry cells, determined by fluorescence intensity, exposed to 0.1% DMSO or 25 nM, 50 nM, or 100 nM mitomycin C, measured each day over a period of 7 days. The decrease in Keap1-mCherry cell survival is statistically significant for all mitomycin C concentrations from day 4. *, P < 0.001. (G) Viabilities of cocultured WT-GFP and Keap1-Nrf2 DKO-mCherry cells, determined by fluorescence intensity, exposed to 0.1% DMSO or 25 nM, 50 nM, or 100 nM mitomycin C, measured each day over a period of 7 days.
To ensure that the mitomycin C toxicity is a cell-intrinsic phenomenon, we carried out time course monoculture experiments using the isogenic Hepa1 cells. While the WT cells exhibited no toxic effects when cultured in 50 nM mitomycin C, the Keap1 KO cells displayed reduced cell growth, which was statistically significantly reduced from day 3 (Fig. 2C and D). Use of a third isogenic cell line in which Nrf2 is also knocked out in the Keap1 KO cells (DKO) completely rescued this phenotype, which strongly suggests that the enhanced toxicity is dependent on Nrf2 activity (Fig. 2E). This Nrf2-dependent effect was confirmed in the coculture system, where time course experiments revealed that increasing concentrations of mitomycin C displayed enhanced toxicity in Keap1 KO cells (Fig. 2F). For all three concentrations of mitomycin C tested (25, 50, and 100 nM), there was a statistically significant reduction in the survival of Keap1 KO cells from the fourth day after mitomycin C treatment. This mitomycin C-induced reduction in Keap1 KO cell survival was concentration dependent, with 100 nM treatment showing the largest inhibitory effect. Importantly, the cocultured WT cells displayed no change in survival in response to mitomycin C, confirming the requirement for Nrf2 activation at these concentrations. Consistent with this idea, the concentration-dependent effect of mitomycin C in Keap1 KO cells was completely rescued by the concomitant loss of Nrf2 in the DKO cells (Fig. 2G). Taken together, these data show that, unique among DNA-damaging agents, the alkylating agent mitomycin C is synthetic lethal with Nrf2 activity.
Aberrant NRF2 activation confers enhanced mitomycin C sensitivity in human cancer cell lines.
Activating mutations in the KEAP1-NRF2 pathway are commonly found in tumors of the liver, lung, and esophagus, where they are associated with poor patient survival (19, 20, 36). To determine whether mitomycin C also displays enhanced toxicity in NRF2-activated human cancer cells, we treated a panel of eight human cancer cell lines, four with aberrant NRF2 activation and four with a functional KEAP1-NRF2 axis, with mitomycin C and assayed cell survival (Fig. 3).
FIG 3.
Mitomycin C displays enhanced NRF2-dependent toxicity across a range of different human cancer cell lines. (A) Viabilities of monococultured Huh-1 (aberrant NRF2 activation) and Hep3B cells (normal NRF2 regulation). Cell viabilities were determined by total protein content, exposed to the indicated concentrations of mitomycin C (MMC) for 8 days. (B) Visualization of monococultured Huh-1 and Hep3B cells, treated with 0.1% DMSO or 50 nM mitomycin C, for 8 days. Scale bars, 300 μm. Note that only the Huh-1 cells display toxicity toward 50 nM mitomycin C. (C) Viabilities of monococultured JHH5 and JHH2 cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C for 8 days. (D) Visualization of monococultured JHH5 and JHH2 cells, treated with 0.1% DMSO or 50 nM mitomycin C, for 8 days. Scale bars, 300 μm. (E) Viabilities of monococultured A549 and COR-L105 cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C for 8 days. (F) Visualization of monococultured A549 and COR-L105 cells, treated with 0.1% DMSO or 100 nM mitomycin C, for 8 days. Scale bars, 300 μm. (G) Viabilities of monococultured KYSE70 and KYSE30 cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C for 8 days. (H) Visualization of monococultured KYSE70 and KYSE30 cells, treated with 0.1% DMSO or 50 nM mitomycin C, for 8 days. Scale bars, 300 μm. (I) Eleven additional KEAP1-NRF2 pathway-mutated nonsmall cell carcinoma cell lines, which have an MMC 50% inhibitory concentration (IC50) at least 5-fold lower than the median cell line within the Genomics of Drug Sensitivity in Cancer database. *, three of the seven most MMC sensitive cell lines within this database. (J) Viabilities of monococultured COR-L105 cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C, with or without cotreatment with the NRF2 inducer DEM (100 μM). *, P < 0.05; **, P < 0.01.
Within the hepatocellular carcinoma cell lines, Huh-1 and JHH5 cells, which both have activated NRF2 signaling (represented by orange lines in the graphs), were significantly more sensitive to mitomycin C than Hep3B and JHH2 cells, which have normal NRF2 regulation (Fig. 3A and C). This enhanced toxicity of mitomycin C was confirmed by visualization of the cell cultures after mitomycin C treatment, which clearly shows a significant NRF2-dependent reduction in cell survival (Fig. 3B and D).
Similarly, in the cell lines derived from lung cancer patients, hyperactivation of NRF2 in A549 cells conferred enhanced sensitivity to mitomycin C compared to the normal regulation of NRF2 found in COR-L105 cells (Fig. 3E and F). This NRF2-dependent toxicity was statistically significant from 10 nM mitomycin C treatment and throughout the range tested. Furthermore, aberrant NRF2 activation in the esophageal cancer-derived KYSE70 cells was also associated with increased mitomycin C-dependent cytotoxicity (Fig. 3G and H). As mitomycin C has been shown to be efficacious in the treatment of lung and esophageal tumors, these results suggest that it may be a good candidate for drug repositioning to target NRF2-dependent tumors in these tissues (24, 29).
To expand upon these findings, we used the Genomics of Drug Sensitivity in Cancer (GDSC) database, which contains data relating to cancer cell line sensitivity in response to a vast catalogue of anticancer drugs (37). This analysis identified 11 additional KEAP1-NRF2 pathway-mutated lung cancer cell lines which were at least 5-fold more sensitive to mitomycin C compared to the median cell line within this data set (Fig. 3I). Interestingly, three of the top seven most mitomycin C-sensitive cell lines (LC-2-ad, NCI-H2170, and NCI-H2228) contained mutations in the KEAP1-NRF2 pathway (38), which strongly supports our contention that NRF2 activation is a major determinant of mitomycin C efficacy.
To ascertain whether NRF2 target genes are required for the increased sensitivity to mitomycin C, we treated COR-L105 cells, which contain a functional KEAP1-NRF2 axis, with the KEAP1 Cys151-specific NRF2 inducer diethyl maleate (DEM), which has been shown to increase the expression of the NRF2-dependent gene signature in a range of model systems (39, 40). Cotreatment with DEM significantly increased the toxicity of 50 and 100 nM mitomycin C treatment, despite the fact that chemical activation of NRF2 produces a significantly reduced induction of the NRF2 transcription program compared to the genetic activation observed in human tumors (Fig. 3J) (41). Interestingly, prior to the discovery that the NRF2 gene functions as an oncogene, the NRF2 inducers DEM and 3H-1,2-dithiole-3-thione (D3T) were also shown to enhance mitomycin C-mediated toxicity (42). These findings suggest that NRF2 target genes play an important role in the cellular sensitivity to mitomycin C.
Taken together, these results strongly suggest that mitomycin C, a chemotherapeutic drug that has been used to treat patients for over 45 years and therefore is supported by a wealth of clinical efficacy and safety studies (29), is an excellent drug repositioning candidate to treat orphan KEAP1-NRF2 mutations in human cancer patients.
Multiple NRF2 target genes are responsible for the bioactivation of mitomycin C.
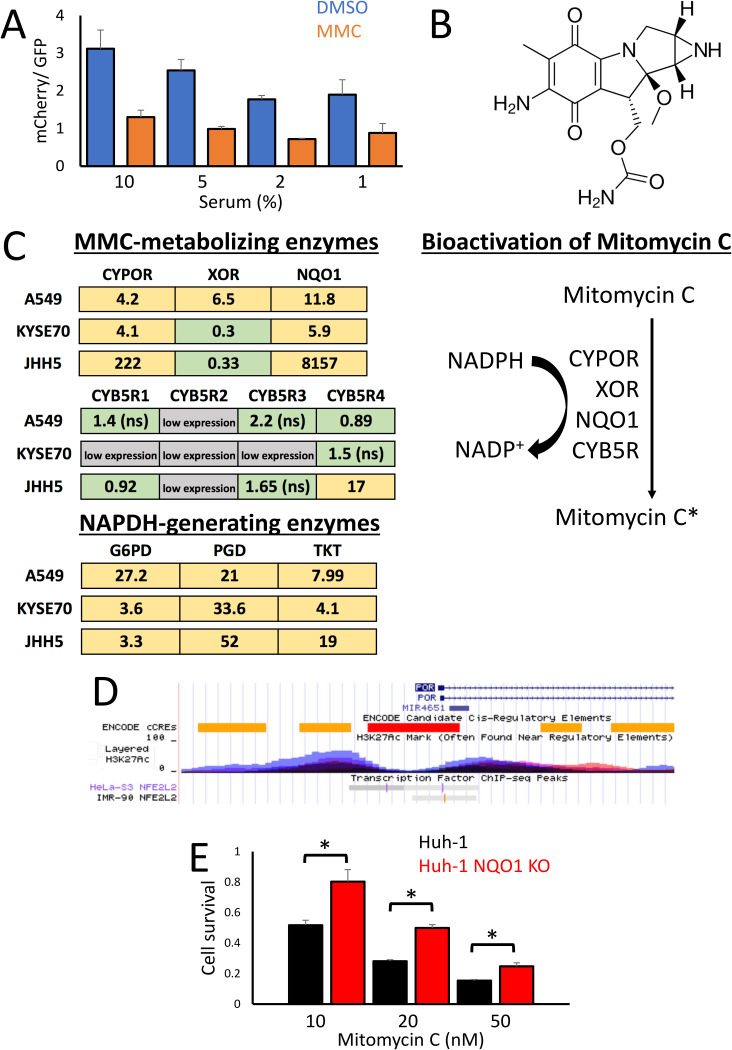
NRF2 activation results in increased cellular proliferation and, thus, an overall reduction in cell cycle time (32, 43). To explore whether this shortened cell cycle results in increased mitomycin C-mediated toxicity by reducing the amount of time available for DNA repair before the cells enter mitosis, we reduced the rate of cell proliferation by depleting the serum content of the culture media (Fig. 4A). However, while reducing the serum content did slow down cellular proliferation, mitomycin C was still significantly more toxic in Keap1 KO cells, which suggests that increased cellular proliferation is not responsible for the NRF2-dependent toxicity of mitomycin C.
FIG 4.
Multiple NRF2 target genes are responsible for the enhanced bioactivation of mitomycin C. (A) Ratio of mCherry/GFP fluorescence from cocultured WT-GFP and Keap1 KO-mCherry cells after 8 days treatment with either 0.1% DMSO or 100 nM mitomycin C, cultured in media containing the indicated percentages of growth serum. (B) Chemical structure of mitomycin C. (C) Fold induction of enzymes involved in the bioactivation of mitomycin C in NRF2-activated relative to wild-type cell lines. The enzymes CYPOR, XOR, NQO1, and CYB5R directly metabolize mitomycin C into the DNA-damaging agent mitomycin C*, with the pentose phosphate pathway generated NADPH providing the reducing equivalents for the bioactivation reactions. The data for A549 cells (NRF2 active) are shown as fold induction over COR-L105, KYSE70 as fold induction over KYSE30, and JHH5 as fold induction over JHH2 cells. The yellow color represents a statistically significant increase in gene expression, and green represents no significant change. Low expression, in gray, indicates a qPCR threshold cycle (CT) value of more than 15 above the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control. ns, not significant. (D) ChIP-Seq data of the human CYPOR (also named POR) locus on chromosome 7, taken from the UCSC genome browser. The ChIP-Seq tracks for NRF2 (also named NFE2L2) from HeLa-S3 and IMR-90 cells show binding sites in the histone H3K27Ac-marked enhancer regions adjacent to the CYPOR promoter. (E) Relative cell survival of Huh-1 cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C for 8 days, compared to the isogenic NQO1 KO cell line generated using CRISPR-Cas9.
As the increased proliferation of Keap1 KO cells is not responsible for the enhanced sensitivity to mitomycin C, we wished to explore alternative mechanisms which could explain the synthetic lethal interaction between NRF2 and mitomycin C. Mitomycin C is a quinone-containing compound which is known to be bioactivated intracellularly through a series of reduction reactions mediated by a range of enzymes, including cytochrome P450 reductase (CYPOR), xanthine oxidoreductase (XOR), cytochrome b5 reductases (CYB5R), and NADPH:quinone reductase (NQO1) (Fig. 4B) (28). Furthermore, these enzymes commonly use NADPH as a source of the reducing equivalents required to activate mitomycin C, which is generated predominantly through the pentose phosphate pathway (PPP) (44). In order to determine whether any of these enzymes are upregulated in an NRF2-dependent manner, we carried out quantitative PCR (qPCR) using pairs of human liver, lung, and esophageal cancer cell lines with aberrant and normal NRF2 activation (Fig. 4C). These experiments revealed that CYPOR, NQO1, and the PPP enzymes are consistently upregulated in cells with active NRF2 signaling, while XOR and CYB5R do not show a consistent NRF2-dependent expression pattern. The NQO1 gene and genes for the PPP enzymes are well-established NRF2 target genes, while the CYPOR gene represents a novel NRF2 target gene (44, 45). To confirm that CYPOR is a direct target of NRF2, we analyzed the NRF2 chromatin immunoprecipitation sequencing (ChIP-Seq) data sets in the UCSC genome browser to determine whether NRF2 binds to enhancer elements in the human CYPOR (also named POR) gene (46). In both HeLa-S3 and IMR-90 cells, NRF2 binding sites were found in histone H3K27Ac marked enhancer regions close to the CYPOR promoter (Fig. 4D). This strongly suggests that NRF2 directly regulates CYPOR expression in human cells.
Previously, we found that metabolism of the quinone in 17-allylamino-17-demethoxygeldanamycin (17-AAG) by the NRF2 target NQO1 gene is responsible for the synthetic lethal interaction between NRF2 and the geldanamycin-derived compounds such as 17-AAG (32). As mitomycin C also contains a quinone, we wished to determine whether a similar NQO1-dependent mechanism is required for bioactivation of mitomycin C. In contrast to 17-AAG, CRISPR-mediated knockout of NQO1 in Huh-1 cells was only able to partially rescue the lethality of mitomycin C (Fig. 4E). This finding is consistent with xenograft studies, which showed no correlation between NQO1 activity and mitomycin C toxicity (47). Together, these findings suggest that NQO1 plays a role in the bioactivation of mitomycin C but that other enzymes, such as CYPOR, are also required for the NRF2-dependent enhanced bioactivation phenotype.
NRF2-dependent bioactivation of mitomycin C is dependent on NADPH.
As aberrant NRF2 activation results in increased NADPH generation, which, in turn, can be used in the bioactivation of mitomycin C, we hypothesized that depleting cellular NADPH levels may rescue the NRF2-dependent synthetic lethal phenotype by reducing the efficiency of its bioactivation. To deplete the cellular NADPH pool, we cotreated cells with sublethal levels of the futile redox cycling compound β-lapachone (32, 48) (Fig. 5A). This cotreatment resulted in a concentration-dependent rescue of the lethal phenotype, with 100 nM β-lapachone completely rescuing the NRF2-dependent synthetic lethality (Fig. 5B). Visualization of the coculture of WT and Keap1 KO cells confirmed this result, as the GFP and mCherry distribution in the cotreated cells closely mimics that of the DMSO control, while the single treatment with mitomycin C is dominated by the GFP-expressing WT cells (Fig. 5C).
FIG 5.
Modulation of NADPH levels rescues the Nrf2-dependent enhanced lethality of mitomycin C. (A) Schematic of the NADPH-generating pentose phosphate pathway, with the activities of the NRF2-regulated enzymes glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (PGD) highlighted. Intracellular NADPH levels can be depleted by addition of the futile redox cycling compound β-lapachone, where β-lapachone* represents the semiquinone form. (B) Relative cell survival of cocultured WT-GFP and Keap1 KO-mCherry cells treated with 20 nM mitomycin C plus the indicated concentrations of β-lapachone. *, P < 0.005. (C) Visualization of the cocultures of WT-GFP and Keap1 KO-mCherry cells treated with β-lapachone and 20 nM mitomycin C as either single or cotreatments. Scale bars, 300 μm. (D) Relative cell survival of cocultured WT-GFP and Keap1 KO-mCherry cells treated with 20 nM mitomycin C plus the indicated concentrations of 6-aminonicotinamide (6-AN). *, P < 0.01. (E) Visualization of the cocultures of WT-GFP and Keap1 KO-mCherry cells treated with 6-aminonicotinamide and 20 nM mitomycin C as either single or cotreatments. Scale bar, 300 μm.
To confirm that the NRF2-dependent regulation of the pentose phosphate pathway is an important source of NADPH for the bioactivation of mitomycin C, we inhibited the metabolic flow through the PPP using 6-aminonicotinamide (6-AN). Similarly to β-lapachone, cotreatment with 6-AN resulted in a concentration-dependent rescue of the synthetic lethal phenotype, with a full rescue observed at 10 and 20 μM (Fig. 5D). Furthermore, visualization of the WT and Keap1 KO cocultures confirmed the enhanced survival of the mCherry-labeled Keap1 KO cells upon the cotreatment with 6-AN, producing a coculture which is predominantly red in color, similar to the DMSO control (Fig. 5E).
Taken together, these data strongly suggest that multiple NRF2 target genes function together in concert to enhance the bioactivation of mitomycin C and, as such, that NRF2 activity functions as a previously unrecognized sensitizer to mitomycin C. The NRF2-dependent regulation of the pentose phosphate pathway generates NADPH, which is directly used by the enzymes CYPOR and NQO1 in reduction reactions to bioactivate mitomycin C. As NADPH depletion through two distinct modalities is able to completely rescue this phenotype, these data strongly argue that the NADPH-dependent enzyme-mediated bioactivation mechanism is the main pathway through which mitomycin C becomes synthetic lethal with NRF2. Therefore, through this mechanism, the NRF2-dependent transcription program generates a potent DNA-damaging agent which can be utilized as a novel targeted cancer therapy for the orphan KEAP1-NRF2 pathway (Fig. 6).
FIG 6.

Model of the NRF2-dependent enhanced bioactivation of mitomycin C. Two products of NRF2 target genes, CYPOR and NQO1, are able to directly metabolize mitomycin C (MMC) into the bioactivated form MMC*, which is a potent DNA-damaging agent. These bioactivation reactions are dependent on the reducing power of NADPH, which is generated by a number of NRF2 target genes through the pentose phosphate pathway (PPP). Through this mechanism, a range of NRF2 target genes function in concert to bioactivate mitomycin C.
NRF2-dependent concurrent synthetic lethality.
Mitomycin C has commonly been used in combination therapies for the treatment of various tumor types, resulting in increased anticancer efficacy (24). Previously, we identified the geldanamycin-derived HSP90 inhibitors 17-AAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), and IPI-504, which have all been used in clinical trials, to be synthetic lethal with NRF2 (32). As mitomycin C and HSP90 inhibition induce cell death through distinct pathways (DNA damage and proteotoxic stress, respectively), we hypothesized that the combined treatment would result in an enhanced NRF2-specific cell death profile.
To test this hypothesis, we treated cocultures of isogenic WT and Keap1 KO Hepa1 cells with variable concentrations of mitomycin C and the HSP90 inhibitor 17-AAG. Consistent with our expectations, using this approach, we found that the cotreatment significantly enhanced NRF2-dependent cell death (Fig. 7A). While the single treatment with either mitomycin C or 17-AAG resulted in a maximal increase in the GFP/mCherry ratio of 3.17- and 4.21-fold, respectively, the cotreatment resulted in a synergistic effect, producing a maximum 9.90-fold increase in the survival of WT cells relative to Keap1 KO cells upon treatment with 50 nM MMC plus 200 nM 17-AAG (Fig. 7A, shown in brown).
FIG 7.
NRF2-dependent concurrent synthetic lethality enhances mitomycin C toxicity. (A) Viabilities of WT-GFP and Keap1 KO-mCherry Hepa1 cells, displayed as a ratio between GFP and mCherry fluorescence, in response to variable concentrations of cotreatments with mitomycin C and 17-AAG. (B to D) Comparison of the expected versus observed survival of Keap1 KO cells exposed to the indicated concentrations of mitomycin C, cotreated with 200 nM 17-AAG (B), 100 nM 17-AAG (C), and 50 nM 17-AAG. The expected cell death was calculated by multiplying together the Keap1 KO cell survival rates in response to the single treatment with each compound. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. (E) Viabilities of WT-GFP and Keap1 KO-mCherry cells, determined by fluorescence intensity relative to the DMSO control, exposed to the indicated concentrations for single treatments with mitomycin C or 17-AAG or the cotreatment of 200 nM 17-AAG with variable mitomycin C for 8 days. Note that the cotreatment depicted with the unbroken lines gives a significantly extended therapeutic window relative to either of the single treatments. (F) Viabilities of monococultured A549 and COR-L105 cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C plus fixed concentrations of 17-AAG. (G) Viabilities of monococultured Huh-1 and Hep3B cells, determined by total protein content, exposed to the indicated concentrations of mitomycin C, with or without 200 nM 17-AAG, for 8 days.
This synergistic killing of Keap1 KO cells by cotreatment with mitomycin C and 17-AAG can be most clearly revealed by comparing the additive effects of the single administration of each compound with the experimentally observed combination data. Multiplying together the survival data for the individual treatments will produce the expected Keap1 KO survival rate, which can then be compared to the observed cotreatment data to determine whether the cotreatment results in excessive cell death. For example, 200 nM 17-AAG treatment results in 31.3% survival, while 10 nM mitomycin C treatment results in 100% Keap1 KO cell survival. The additive effect of these drugs would be 31.3%, while the observed survival rate was significantly lower at just 17.6% (P < 0.0005) (Fig. 7B). Within the treatment range of 10 to 50 nM mitomycin C, we observed synergistic Keap1 KO cell death upon treatment with 50, 100, and 200 nM 17-AAG, which returned to the expected cell death rate at 100 nM mitomycin C treatment (Fig. 7B to D). When the data from the variable treatment with mitomycin C, coupled with the fixed addition of 200 nM 17-AAG, are presented graphically, they clearly show that the therapeutic window for the cotreatment of the two compounds is significantly increased relative to the administration of the single agents (Fig. 7E). Together, these data clearly show that cotreatment with mitomycin C and 17-AAG produces a synergistic NRF2-dependent cell death profile.
To confirm the generality of this enhanced synthetic lethality, we treated lung cancer cell lines with mitomycin C plus increasing concentrations of 17-AAG (Fig. 7F). These results clearly show that as the concentration of 17-AAG increases, enhanced toxicity is specifically observed in the NRF2-active A549 cells. This result was further confirmed in liver cancer cell lines, where cotreatment with 200 nM 17-AAG significantly enhanced the toxicity of MMC in an NRF2-dependent manner (Fig. 7G).
As NRF2 regulates the expression of a broad range of xenobiotic-metabolizing enzymes, we believe that it is an excellent target for prodrug bioactivation and synthetic lethal therapies. Furthermore, the combination of synthetic lethal treatments which target different cellular pathways can significantly enhance the NRF2-specific effects. As, to our knowledge, this is the first time that targeting multiple distinct synthetic lethal pathways has been used as an anticancer strategy, we have named this concept “concurrent synthetic lethality” (Fig. 8).
FIG 8.

Concurrent synthetic lethality. Schematic depicting the concept of concurrent synthetic lethality. NRF2 target genes are able to bioactivate both mitomycin C, to generate the potent DNA-damaging agent MMC*, and 17-AAG, to generate the proteotoxic stress-inducing HSP90 inhibitor 17-AAGH2. The parallel NRF2-dependent metabolism of prodrugs which target distinct cell death pathways increases the specificity and toxicity toward tumor cells with aberrant KEAP1-NRF2 pathway activation.
DISCUSSION
While NRF2 activation is a common event in human cancer and is associated with a poor prognosis for patients, there are currently no approved drugs which can target aberrant NRF2 activity in a clinical setting. Using an isogenic screening system, and validated across a panel of eight human cancer cell lines, we have found that mitomycin C, an anticancer drug which is already approved for clinical use, exhibits enhanced toxicity in cells with high levels of NRF2 activation. Mechanistically, we found that the activity of a multitude of NRF2 target genes results in the enhanced bioactivation of mitomycin C, generating a potent DNA-damaging agent in cells with high levels of NRF2 activity. As a result, we believe that mitomycin C is an excellent candidate for drug repositioning as a first-in-class compound to target NRF2-dependent tumors.
It is well established that mitomycin C induces cell death by functioning as a DNA-damaging agent (25). In contrast to our findings, NRF2 activation has been shown to be protective against other DNA-damaging agents, including cisplatin, carboplatin, etoposide, and doxorubicin, which suggests the existence of a special relationship between NRF2 activation and mitomycin C sensitivity that is unique among DNA damage-inducing compounds (33–35). Mitomycin C induces the formation of interstrand cross-links within DNA, which are normally repaired by the Fanconi anemia (FA) core complex (26). Cisplatin and carboplatin also induce interstrand cross-links, which suggests that this form of DNA damage per se is not responsible for the NRF2 selectivity (26). Consistent with this idea, NRF2 has not been shown to downregulate the transcription of the FA core complex genes, which suggests that the interaction between mitomycin C and NRF2 is not dependent on a DNA repair defect (40, 49, 50).
Mitomycin C is administered as a prodrug which is bioactivated intracellularly to generate the DNA-alkylating agents (27, 28). This bioactivation of mitomycin C can be mediated by multiple enzymes, including NQO1 and CYPOR, with NADPH providing the reducing equivalents required for the bioactivation reactions (51–53). The NQO1 gene is a well-characterized NRF2 target gene (8, 54), while we have found CYPOR to be specifically upregulated in human cancer cell lines with high levels of NRF2 activity. Furthermore, by regulating the transcription of the enzymes which regulate the pentose phosphate pathway, NRF2 activation also results in an increase in cellular NAPDH levels (43, 44, 55). As NRF2 regulates the expression of a broad range of xenobiotic-metabolizing enzymes, including members of the aldo-keto reductase family, we cannot exclude the possibility that additional NRF2 target genes are also involved in the bioactivation of mitomycin C (56). Xenograft experiments have revealed there to be no correlation between NQO1 activity alone and mitomycin C toxicity, which, when combined with our data, strongly suggest that multiple NRF2 target genes are required to function in concert to provide the specificity for enhanced mitomycin C bioactivation (47). Interestingly, cotreatment with the NRF2 inducers DEM and D3T have been shown to enhance the cytotoxic effects of mitomycin C, which provides complementary data to support our contention that NRF2 activity functions as a major sensitizer to mitomycin C toxicity (42, 54, 57). However, as these experiments were carried out before NRF2 activation was shown to be oncogenic, the relevance of these findings in relation to NRF2-dependent tumor therapy was not previously apparent.
Our data, when placed in the context of the literature concerning the bioactivation of mitomycin C, strongly suggest that mitomycin C is an excellent candidate for drug repositioning to target the orphan KEAP1-NRF2 pathway in human cancer. While other approaches, such as targeting glutaminase, may be promising future therapeutic strategies to target NRF2-dependent tumors, mitomycin C is already approved for human clinical use and thus can be quickly and cheaply repurposed to target aberrant NRF2 activation (58).
Mitomycin C has been used as an anticancer therapy for over 45 years and has been shown to be efficacious in the treatment of bladder and gastric tumors, head and neck squamous cell carcinoma (HNSCC), and non-small cell lung carcinoma (NSCLC) (24, 29, 59, 60). Interestingly, there is significant overlap between the tumor-specific efficacy of mitomycin C and the activation profile of NRF2. For example, positive NRF2 staining is associated with reduced overall survival in bladder cancer patients, while the expression of an NRF2-dependent gene signature is associated with reduced survival in HNSCC (61, 62). Furthermore, activating mutations in the KEAP1-NRF2 pathway are found in 22% of HPV-negative HNSCC tumors, which highlights the importance of NRF2 activation in these tumors (2).
Mitomycin C has an overall response rate of 25% in NSCLC patients, which is very similar to the mutation rate of KEAP1 in the same patient population (21, 29, 63). In light of these facts, coupled with our findings that multiple NRF2-dependent pathways can enhance the bioactivation of mitomycin C, we hypothesize that NRF2 activation is an unrecognized sensitizer to mitomycin C and that the lack of sequencing of mitomycin C-responsive tumors has hidden the significance of this drug-biomarker pair. Therefore, we believe that mitomycin C treatment in the context of aberrant NRF2 activation is an excellent candidate to be added to the ongoing National Lung Matrix Trial for molecularly targeted lung cancer therapies (23).
Cancer therapeutic strategies frequently include combination therapies in order to improve the rates of positive treatment outcomes (64). As we previously found that geldanamycin-derived HSP90 inhibitors such as 17-AAG are synthetic lethal with NRF2 activity, we hypothesized that the combination of 17-AAG plus mitomycin C would provide enhanced NRF2-dependent toxicity because these compounds induce cell death through different cellular pathways (32). Such dual synthetic lethality has previously been observed in BRCA-deficient cells, where PARP inhibition plus RAD52 inhibition results in enhanced synthetic lethality by simultaneously targeting multiple DNA damage response components (65). In the case of KEAP1-NRF2 activation, we observed synergistic NRF2-dependent cell death upon cotreatment with mitomycin C and 17-AAG, which provides proof of concept that a synthetic lethal strategy with targets distinct cellular pathways can be efficacious. This strategy has a significant advantage over synthetic lethality which targets a single cellular pathway, as it is extremely unlikely that a single novel mutation could rescue cell death which is simultaneously induced by proteotoxic stress and DNA damage. As to our knowledge, this is the first time such a parallel pathway approach has been taken, we have named it “concurrent synthetic lethality.” As mitomycin C and the 17-AAG family member IPI-504 have both shown efficacy in the treatment of non-small cell lung carcinoma, a tumor type in which NRF2 activation is both frequently observed and is associated with poor prognosis, we propose that this patient population is an ideal candidate for concurrent synthetic lethality treatment (20, 29, 66).
The concept of bioactivation of antineoplastic drugs has been actively pursued for over 40 years; however, the identification of tumors which express high levels of the activating enzymes has limited the selectivity of this therapeutic approach (67, 68). We have identified two distinct classes of compounds, mitomycin C and geldanamycin-derived HSP90 inhibitors, which are bioactivated in an NRF2-dependent manner and which promote cellular death through distinct pathways. Furthermore, previous reports have shown that an NRF2 target gene, the NQO1 gene, can activate a number of prodrugs, including apaziquone and tirapazamine, while another NRF2 target gene, the AKR1C3 gene, has been shown to bioactivate PR-104A (56, 68–70). In light of these findings, we propose that NRF2-positive tumors represent ideal molecular targets to provide specificity for prodrug bioactivation-based anticancer therapies, including the repositioning of existing drugs such as mitomycin C.
MATERIALS AND METHODS
Reagents.
Mitomycin C, 6-aminonicotinamide, and doxorubicin were purchased from Sigma-Aldrich (MO, USA). β-Lapachone, MK-2206, paclitaxel, bortezomib, nutlin-3, and navitoclax were purchased from Cayman Chemical (Ann Arbor, MI). 17-AAG was purchased from Selleck Chemicals (Houston, TX). Trametinib was purchased from Focus Biomolecules (PA). Veliparib was purchased from LKT Laboratories (MN).
Cell culture.
All cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM), except for the JHH2 and JHH5 cells, which were in maintained William’s E medium, supplemented with 10% fetal bovine serum (FBS) and antibiotics. All cells were cultured in a humidified atmosphere with 5% CO2 at 37°C.
Screening conditions and fluorescence intensity calculations.
For all experiments using cocultured WT-GFP and Keap1 KO-mCherry cells, on day −1, 1 × 103 WT and 2 × 103 Keap1 KO cells were mixed together in each individual well of a black flat-bottom 96-well plate (Corning; catalog no. 3904). On the following day (day 0), the compounds were added to the cells at the concentrations described above. Immediately after the addition of the compounds, the fluorescence intensities of GFP and mCherry were measured using a PHERAstar FS microplate reader (BMG Labtech, Ortenberg, Germany). The plates were then returned to the 37°C incubator until day 8, when the fluorescence intensity was measured again. The change in fluorescence for both GFP and mCherry during the growth period in response to the compounds was calculated as day 8 − day 0 and then normalized to the DMSO control. As the cell culture medium contains a background fluorescence signal which is highest on day 0, when the medium is fresh, and decreases with time as the medium components are metabolized by the cells, it is possible for the day 8 − day 0 calculation to provide a negative value. For example, if a compound such as bortezomib efficiently kills the WT-GFP cells, then no GFP fluorescence signal will be generated; however, the medium background fluorescence will reduce with time as the Keap1-mCherry cells metabolize the fluorescing culture medium components. By day 8, this combination may give a negative value in the GFP channel relative to day 0.
Cell survival assays for human cancer cells.
On day −1, 2 × 103 cells were plated into each individual well of a 96-well plate. On the following day (day 0), the compounds were added to the cell plates at the concentrations described above. The 96-well plates were then incubated at 37°C until day 8, when they were washed with phosphate-buffered saline (PBS) and lysed with 25 μl radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, 150 mM NaCl, 1% [vol/vol] NP-40, 0.5% (wt/vol) deoxycholic acid, 0.1% [wt/vol] SDS, pH 7.4), after which they were frozen at −30°C. Total protein concentrations for each well were determined using the bicinchoninic acid (BCA) protein assay (Pierce) following the manufacturer’s instructions and were normalized to the DMSO controls for comparisons between and within cell types.
Gene expression analysis by qPCR.
Total RNA was prepared from cell lysates using TRIzol reagent (Life Technologies, Carlsbad, CA) in accordance with the manufacturer’s instructions. A 1-μg aliquot of total RNA was reverse transcribed with ReverTra Ace (Toyobo, Osaka, Japan). The resultant cDNA was used as a temperate for quantitative reverse transcription-PCR (qRT-PCR) on an SYBR green 7300 real-time PCR analyzer (Life Technologies). The primers used during the qPCR analysis are available upon request.
ACKNOWLEDGMENTS
We thank all of the members of the Yamamoto lab for their thoughtful and stimulating discussions.
This research was partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from AMED under grant number JP17am0101001 (support number 1234), AMED-P-CREATE (JP19cm0106101 to M.Y.), JSPS KAKENHI 19H05649 (to M.Y.), and JSPS KAKENHI Grants-in-Aid for Early Career Scientists 19K16512 (to L.B.).
REFERENCES
- 1.Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, Chakravarty D, Daian F, Gao Q, Bailey MH, Liang WW, Foltz SM, Shmulevich I, Ding L, Heins Z, Ochoa A, Gross B, Gao J, Zhang H, Kundra R, Kandoth C, Bahceci I, Dervishi L, Dogrusoz U, Zhou W, Shen H, Laird PW, Way GP, Greene CS, Liang H, Xiao Y, Wang C, Iavarone A, Berger AH, Bivona TG, Lazar AJ, Hammer GD, Giordano T, Kwong LN, McArthur G, Huang C, Tward AD, Frederick MJ, McCormick F, Meyerson M, Van Allen EM, Cancer Genome Atlas Research Network, et al. 2018. Oncogenic signaling pathways in the cancer genome atlas. Cell 173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambardella V, Gimeno-Valiente F, Tarazona N, Martinez-Ciarpaglini C, Roda D, Fleitas T, Tolosa P, Cejalvo JM, Huerta M, Roselló S, Castillo J, Cervantes A. 2019. NRF2 through RPS6 activation is related to Anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res 25:1639–1649. doi: 10.1158/1078-0432.CCR-18-2421. [DOI] [PubMed] [Google Scholar]
- 4.Hu XF, Yao J, Gao SG, Wang XS, Peng XQ, Yang YT, Feng XS. 2013. Nrf2 overexpression predicts prognosis and 5-FU resistance in gastric cancer. Asian Pac J Cancer Prev 14:5231–5235. doi: 10.7314/apjcp.2013.14.9.5231. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki Y, Ishigami S, Arigami T, Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H, Nakajo A, Kijima Y, Maemura K, Natsugoe S. 2015. Clinicopathological significance of nuclear factor (erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer 15:5. doi: 10.1186/s12885-015-1008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kensler TW, Wakabayashi N, Biswal S. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Kensler TW, Motohashi H. 2018. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird L, Yamamoto M. 2020. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol 40:e00099-20. doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T, Muramatsu A, Saito R, Iso T, Shibata T, Kuwata K, Kawaguchi S-I, Iwawaki T, Adachi S, Suda H, Morita M, Uchida K, Baird L, Yamamoto M. 2019. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep 28:746–758.e4. doi: 10.1016/j.celrep.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 11.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. 2004. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J 378:373–382. doi: 10.1042/bj20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. 2005. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A 102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang DD, Hannink M. 2003. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23:8137–8151. doi: 10.1128/mcb.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon M, Lamont DJ, Beattie KA, Hayes JD. 2010. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A 107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. 1995. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol 15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baird L, Tsujita T, Kobayashi EH, Funayama R, Nagashima T, Nakayama K, Yamamoto M. 2017. A homeostatic shift facilitates endoplasmic reticulum proteostasis through transcriptional integration of proteostatic stress response pathways. Mol Cell Biol 37:e00439-16. doi: 10.1128/MCB.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsuoka F, Otsuki A, Takahashi M, Ito S, Yamamoto M. 2019. Direct and specific functional evaluation of the Nrf2 and MafG heterodimer by introducing a tethered dimer into small Maf-deficient cells. Mol Cell Biol 39:e00273-19. doi: 10.1128/MCB.00273-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata T, Kokubu A, Saito S, Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y, Kushima R, Kiyono T, Yamamoto M. 2011. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia 13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, Sasano H, Nakagawa T, Satoh K, Tanaka N, Kubo H, Motohashi H, Yamamoto M. 2012. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci 103:760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. 2014. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M, Horswell S, Escudero M, Matthews N, Rowan A, Chambers T, Moore DA, Turajlic S, Xu H, Lee S-M, Forster MD, Ahmad T, Hiley CT, Abbosh C, Falzon M, Borg E, Marafioti T, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Shah R, Joseph L, Quinn AM, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, TRACERx Consortium, et al. 2017. Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 23.Middleton G, Fletcher P, Popat S, Savage J, Summers Y, Greystoke A, Gilligan D, Cave J, O'Rourke N, Brewster A, Toy E, Spicer J, Jain P, Dangoor A, Mackean M, Forster M, Farley A, Wherton D, Mehmi M, Sharpe R, Mills TC, Cerone MA, Yap TA, Watkins TBK, Lim E, Swanton C, Billingham L. 2020. The National Lung Matrix Trial of personalized therapy in lung cancer. Nature 583:807–812. doi: 10.1038/s41586-020-2481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradner WT. 2001. Mitomycin C: a clinical update. Cancer Treat Rev 27:35–50. doi: 10.1053/ctrv.2000.0202. [DOI] [PubMed] [Google Scholar]
- 25.Iyer VN, Szybalski W. 1963. A molecular mechanism of mitomycin action: linking of complementary DNA strands. Proc Natl Acad Sci U S A 50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deans AJ, West SC. 2011. DNA interstrand crosslink repair and cancer. Nat Rev Cancer 11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz HS, Sodergren JE, Philips FS. 1963. Mitomycin C: chemical and biological studies on alkylation. Science 142:1181–1183. doi: 10.1126/science.142.3596.1181. [DOI] [PubMed] [Google Scholar]
- 28.Seow HA, Penketh PG, Baumann RP, Sartorelli AC. 2004. Bioactivation and resistance to mitomycin C. Methods Enzymol 382:221–233. doi: 10.1016/S0076-6879(04)82012-3. [DOI] [PubMed] [Google Scholar]
- 29.Sculier JP, Ghisdal L, Berghmans T, Branle F, Lafitte JJ, Vallot F, Meert AP, Lemaitre F, Steels E, Burniat A, Mascaux C, European Lung Cancer Working Party. 2001. The role of mitomycin in the treatment of non-small cell lung cancer: a systematic review with meta-analysis of the literature. Br J Cancer 84:1150–1155. doi: 10.1054/bjoc.2001.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. 2011. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A 108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchida K, Tsujita T, Hayashi M, Ojima A, Keleku-Lukwete N, Katsuoka F, Otsuki A, Kikuchi H, Oshima Y, Suzuki M, Yamamoto M. 2017. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic Biol Med 103:236–247. doi: 10.1016/j.freeradbiomed.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Baird L, Suzuki T, Takahashi Y, Hishinuma E, Saigusa D, Yamamoto M. 2020. Geldanamycin-derived HSP90 inhibitors are synthetic lethal with NRF2. Mol Cell Biol 40:e00377-20. doi: 10.1128/MCB.00377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. 2006. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. 2008. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. 2008. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res 68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Zhang C, Zhang L, Yang Q, Zhou S, Wen Q, Wang J. 2015. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer 15:531. doi: 10.1186/s12885-015-1541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, Bindal N, Beare D, Smith JA, Thompson IR, Ramaswamy S, Futreal PA, Haber DA, Stratton MR, Benes C, McDermott U, Garnett MJ. 2013. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res 41:D955–61. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saigusa D, Motoike IN, Saito S, Zorzi M, Aoki Y, Kitamura H, Suzuki M, Katsuoka F, Ishii H, Kinoshita K, Motohashi H, Yamamoto M. 2020. Impacts of NRF2 activation in non-small-cell lung cancer cell lines on extracellular metabolites. Cancer Sci 111:667–678. doi: 10.1111/cas.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, Iso T, Yamamoto H, Morita M, Baird L, Furusawa Y, Negishi T, Ichinose M, Yamamoto M. 2016. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol 36:271–284. doi: 10.1128/MCB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirotsu Y, Katsuoka F, Funayama R, Nagashima T, Nishida Y, Nakayama K, Engel JD, Yamamoto M. 2012. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res 40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, Liby KT, Sporn MB, Sutter TR, Kensler TW. 2009. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Doherty GP, Leith MK, Curphey TJ, Begleiter A. 1999. Enhanced cytotoxicity of mitomycin C in human tumour cells with inducers of DT-diaphorase. Br J Cancer 80:1223–1230. doi: 10.1038/sj.bjc.6690489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. 2012. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Wu KC, Cui JY, Klaassen CD. 2011. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 46.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. 2002. The human genome browser at UCSC. Genome Res 12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips RM, Burger AM, Loadman PM, Jarrett CM, Swaine DJ, Fiebig HH. 2000. Predicting tumor responses to mitomycin C on the basis of DT-diaphorase activity or drug metabolism by tumor homogenates: implications for enzyme-directed bioreductive drug development. Cancer Res 60:6384–6390. [PubMed] [Google Scholar]
- 48.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. 2000. NAD(P)H:quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem 275:5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 49.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. 2010. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. 2012. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel D, Beall H, Senekowitsch C, Kasai M, Arai H, Gibson NW, Ross D. 1992. Bioreductive activation of mitomycin C by DT-diaphorase. Biochemistry 31:7879–7885. doi: 10.1021/bi00149a019. [DOI] [PubMed] [Google Scholar]
- 52.Pan SS, Andrews PA, Glover CJ, Bachur NR. 1984. Reductive activation of mitomycin C and mitomycin C metabolites catalyzed by NADPH-cytochrome P-450 reductase and xanthine oxidase. J Biol Chem 259:959–966. [PubMed] [Google Scholar]
- 53.Iyer VN, Szybalski W. 1964. Mitomycins and porfiromycin: chemical mechanism of activation and cross-linking of DNA. Science 145:55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- 54.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. 2003. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 55.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. 2002. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62:5196–5203. [PubMed] [Google Scholar]
- 56.MacLeod AK, McMahon M, Plummer SM, Higgins LG, Penning TM, Igarashi K, Hayes JD. 2009. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis 30:1571–1580. doi: 10.1093/carcin/bgp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talalay P, De Long MJ, Prochaska HJ. 1988. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A 85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero R, Sayin VI, Davidson SM, Bauer MR, Singh SX, LeBoeuf SE, Karakousi TR, Ellis DC, Bhutkar A, Sánchez-Rivera FJ, Subbaraj L, Martinez B, Bronson RT, Prigge JR, Schmidt EE, Thomas CJ, Goparaju C, Davies A, Dolgalev I, Heguy A, Allaj V, Poirier JT, Moreira AL, Rudin CM, Pass HI, Vander Heiden MG, Jacks T, Papagiannakopoulos T. 2017. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med 23:1362–1368. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith PH. 1980. Cytotoxic chemotherapy in carcinoma of the bladder: a review. J R Soc Med 73:205–207. doi: 10.1177/014107688007300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preusser P, Achterrath W, Wilke H, Lenaz L, Fink U, Heinicke A, Meyer J, Bunte H. 1988. Chemotherapy of gastric cancer. Cancer Treat Rev 15:257–277. doi: 10.1016/0305-7372(88)90025-4. [DOI] [PubMed] [Google Scholar]
- 61.Hayden A, Douglas J, Sommerlad M, Andrews L, Gould K, Hussain S, Thomas GJ, Packham G, Crabb SJ. 2014. The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol Oncol 32:806–814. doi: 10.1016/j.urolonc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Namani A, Rahaman MM, Chen M, Tang X. 2018. Gene-expression signature regulated by the KEAP1-NRF2-CUL3 axis is associated with a poor prognosis in head and neck squamous cell cancer. BMC Cancer 18:46. doi: 10.1186/s12885-017-3907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cancer Genome Atlas Research Network. 2012. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, Zweibel J, Collins J, Doroshow JH. 2010. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov 9:843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan-Reed K, Bolton-Gillespie E, Dasgupta Y, Langer S, Siciliano M, Nieborowska-Skorska M, Hanamshet K, Belyaeva EA, Bernhardy AJ, Lee J, Moore M, Zhao H, Valent P, Matlawska-Wasowska K, Müschen M, Bhatia S, Bhatia R, Johnson N, Wasik MA, Mazin AV, Skorski T. 2018. Simultaneous targeting of PARP1 and RAD52 triggers dual synthetic lethality in BRCA-deficient tumor cells. Cell Rep 23:3127–3136. doi: 10.1016/j.celrep.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R, Hafeez N, Sweeney J, Walker JR, Fritz C, Ross RW, Grayzel D, Engelman JA, Borger DR, Paez G, Natale R. 2010. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol 28:4953–4960. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore HW. 1977. Bioactivation as a model for drug design bioreductive alkylation. Science 197:527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- 68.Wilson WR, Hay MP. 2011. Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 69.Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, Pullen SM, Hicks KO, Syddall SP, Atwell GJ, Yang S, Denny WA, Wilson WR. 2007. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res 13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 70.Guise CP, Abbattista MR, Singleton RS, Holford SD, Connolly J, Dachs GU, Fox SB, Pollock R, Harvey J, Guilford P, Doñate F, Wilson WR, Patterson AV. 2010. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res 70:1573–1584. doi: 10.1158/0008-5472.CAN-09-3237. [DOI] [PubMed] [Google Scholar]