Central Illustration
Key Words: blockchain, COVID-19, decentralized clinical trials, drug development, virtual clinical trials
The Covid-19 pandemic disrupted many clinical trials that were potentially bringing new therapeutics to market—an additional untallied cost of the pandemic in lives and quality of life owing to delays in releasing potentially beneficial therapeutics to patients in need. A separate side-effect of the pandemic has been swift adoption of virtual interactions between physicians and patients to provide continuity of care while maintaining social distancing. This comes at a time of rapid advancement of technology permitting those interactions, such as enhanced internet connectivity, electronic health records, real-time video conferencing, smartphone health applications, and remotely connectable health monitoring devices that are becoming both more accurate, practical, and affordable. Interest in decentralized clinical trials (DCTs) that use “virtual elements” like these has grown in parallel with acceptance of “virtual medicine,” accelerating shifts in clinical trial design that many feel are long overdue.
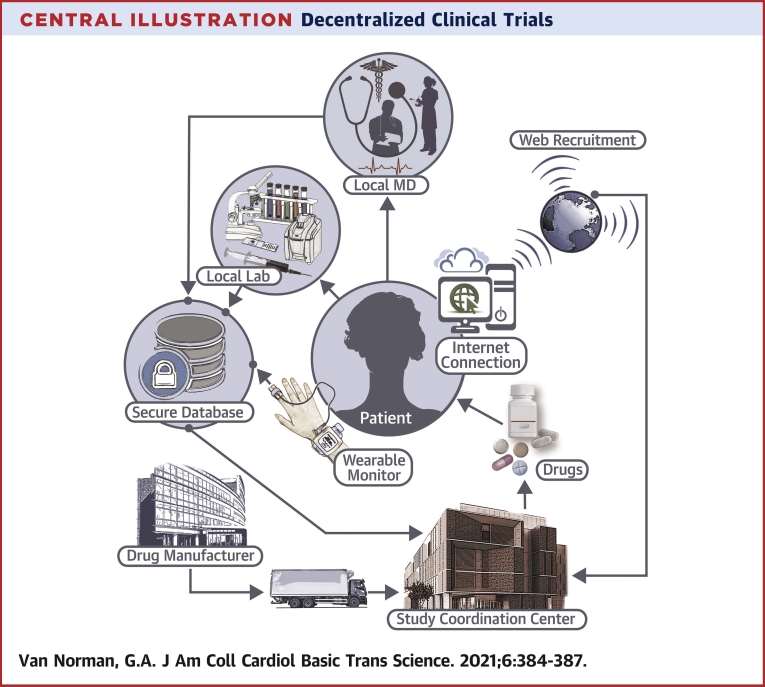
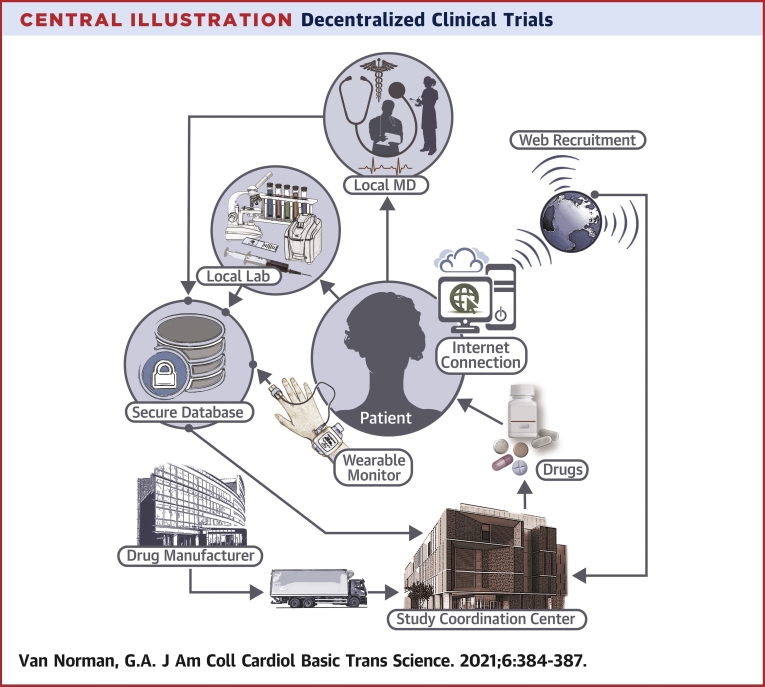
DCTs—also termed “direct-to-participant trials” or “virtual” studies— are characterized by less dependence on traditional research facilities or specialist intermediaries for data collection. DCTs leverage “virtual” tools, such as telemedicine, sensory-based technologies, wearable medical devices, home visits, patient-driven virtual health care interfaces, and direct delivery of study drugs and materials to patients’ homes. In a fully decentralized clinical trial, subject recruitment, delivery and administration of study medication, and acquisition of trial outcomes data all proceed without involving in-person contact between the study team and the patient/subject. Currently, clinical trials for drug approval often already include decentralized elements, and DCTs often incorporate traditional design with decentralization of the patient/subject interactions (Central Illustration).
Central Illustration.
Decentralized Clinical Trials
In decentralized clinical trials, patient visits for healthcare provider interaction and laboratory tests are localized in the patient’s community. Medications for the study are provided either directly to the patient or the local healthcare facility. Other interactions, including patient recruitment and monitoring occur via internet interactions, with data secured and maintained virtually.
Advantages of DCTs: Smaller, Fleeter, Cheaper, More Diverse?
DCTs may improve the logistics of conducting a clinical trial by improving recruitment and retention of subjects (1). Patients who would otherwise face daunting challenges from centralized study trials may be able or willing to enroll in DCTs, because remote monitoring and data collection minimize obstacles to participating, such as logistical difficulties in accessing the trial location—e.g., travel costs, nonacceptance of job absences for study activities, and mobility challenges posed by medical comorbidities. A collateral effect is improvement of trial access for participant populations that are currently most underrepresented in current traditional trials: the elderly, the poor, those living in remote locations, and many ethnic minorities. Recruitment times are also likely to improve, as are generalizability of results across diverse populations.
DCTs can potentially decrease trial sample size by enabling the development of individualized thresholds for measuring treatment effects. Digitalized tools such as biometric sensors may allow more objective methods of measuring pain, quality of life, functional status, and cognitive function, permitting better understanding of individual responses to treatment and individualized patient toxicities.
With fewer central research sites, DCTs reduce the number of institutional review boards and redundant applications, decreasing costs and site-specific inconsistencies. Fewer sites also means fewer resubmissions to multiple institutional review boards to institute changes, and better ability to pivot and make across-the-board protocol adjustments to meet evolving study parameters. Remote monitoring means fewer individual assessments, reduced variability of reporters, and potentially smaller studies. Remote patient/subject interactions can occur more frequently and at times and locations more convenient for the subject, thereby improving compliance and potentially enhancing both short- and long-term study safety.
Traditional clinical trials rely heavily on trained intermediaries belonging to the study team, e.g., study coordinators, research assistants, and nursing and physician staff, for data collection and compilation. With DCTs, this function becomes partially or fully virtual. A partially virtual intermediary is one that still requires some direct interaction with the patient, but not necessarily an investigator, such as having a patient enter daily details of medication side-effects in a portal of the research platform. An example of a fully virtual data collection system would be automatic cellular transmission of data at predetermined intervals from a wearable continuous glucose monitoring device to the research platform, requiring no interaction by the patient or a research intermediary (1). Studies that rely on such virtual tools and automation potentially require smaller investigative teams, and lower costs to sponsors, in both training and full-time employees. Fewer inconveniences to patients/subjects due to virtual data acquisition could potentially increase their willingness and ability to participate in a study, resulting in outcomes that better reflect the safety and efficacy behavior of the study drug in a “real world” environment.
Challenges for DCTs
Compared with DCTs, a major advantage to centralized studies is comparatively simple drug distribution and management. In centralized studies, drugs are shipped to trial centers that centrally manage and maintain them. DCTs require shipping to multiple coordinating sites, including potentially directly to patient homes. For this to occur, there must be assurance of drug stability and appropriate storage facilities in the patient’s home, as well as measures to prevent unauthorized access, methods to detect tampering, temperature tracking to assure appropriate drug storage, dosing diaries to record administration of the drug, and communication between the storage system and the drug source to provide timely refills and prevent study interruptions. In addition, local laws may need to be addressed that affect state-specific allowable parameters of drug dispensing. The complexity of decentralized drug shipping and management introduces potentially greater complexity and risk in clinical trials, both to the subjects themselves and to the integrity of the trial.
Technological advances are core elements that allow DCTs, but they are also a main challenge to adoption of DCT study design. Wearable biometric devices, for example, are still in early phases of development, and before these devices will be widely accepted in regulatory decisions, they require clinical validation. Operation of the devices themselves depend also on the availability of technical support and troubleshooting, batteries, transmission methods, and internet infrastructure, such as cellular towers in remote locations or hard-wired internet connectivity in homes currently without it.
Protecting patient privacy stored on connected devices, and the information transmitted through connection services is another problem. Reliable cybersecurity systems are a must, if private patient data is to be stored and transmitted in a DCT. Most traditional trials use local data systems that are firewalled and centrally managed. But in many studies, patients/subjects will interact with multiple health care providers using multiple electronic health record systems (EHRs), creating challenges for central data amalgamation from multiple EHRs that often do not communicate with each other efficiently, if at all.
One concept gaining traction in health care data management is “blockchain” technology—a form of decentralized data management. Blockchain, first associated with “bitcoin” commerce, is a decentralized framework for managing databases. In blockchain, 2 or more parties can exchange information without the need of a “trusted” centralized third party (such as a centralized server) to maintain the shared database. Blockchain technology replaces the authority of the “trusted third party” to resolve discrepancies between participating parties with a “consensus” mechanism among parties. The elimination of the centralized third party improves transaction speed, eliminates costs that are generated by the third party, and eliminates a significant single “point of failure” at which transactions can either be interrupted by a system malfunction, or experience malicious interference (i.e., hacking). Blockchain interactions are therefore also potentially more secure than centralized data management. In a health care blockchain, for example, patients would “own” their own data, which would be stored at multiple “nodes” (e.g., on the web of the health care organizations at which the patient receives care and at the same time on the patient’s private home network), and failure at any one node containing the patient’s data would not lead to significant data loss (2). The concept of blockchain technology for health care applications and clinical trial data management is a subject of intense current interest and investigation.
Initiatives
DCTs are gaining invigorated new support, but they are not entirely new with the pandemic. The first entirely web-based trial, under an Investigational New Drug application, was carried out by Pfizer in 2011 in the REMOTE (Research on Electronic Monitoring of Overactive Bladder Treatment Experience) trial. During REMOTE, no in-person site visits occurred at all and the study investigators used internet recruitment, online questionnaires, electronic diaries, and home delivery of the investigational drug (3). The Clinical Trials Transformation Initiative (CTTI), was cofounded by the Food and Drug Administration (FDA) and Duke University in 2007 to identify and promote quality and efficacy of clinical trials and has recently issued recommendations for DCTs (4). In 2015, the U.S. Congress passed the 21st Century Cures Act charged the FDA with developing a framework and guidance for novel trial designs and the use of evidence from sources other than traditional clinical trials to support drug approval. The FDA has now issued specific guidance on virtual study methods during the pandemic (5), which are likely to continue after the pandemic has passed. On December 10, 2020, the Decentralized Trials and Research Alliance (DTRA) was launched, bringing together more than 50 international organizations, including the FDA and patient advocacy groups, to promote DCT methods. With growing acceptance of virtual medicine and technology, there appears to be little doubt that DCTs have “arrived” and stand to change the face of human clinical trials in drug and therapeutic development both now and into the future.
Funding Support and Author Disclosures
Dr. Van Norman has received funding from the American College of Cardiology for this publication.
Footnotes
The author attests she is in compliance with human studies committees and animal welfare regulations of the author’s institution and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Khozin S., Coravox A. Decentralized trials in the age of real-world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther. 2019;106:25–27. doi: 10.1002/cpt.1441. [DOI] [PubMed] [Google Scholar]
- 2.Agbo C.C., Mahmoud Q.H., Eklund J.M. Blockchain technology in healthcare: a systematic review. Healthcare. 2019;7:56. doi: 10.3390/healthcare7020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orri M., Lispet C.H., Jacobs B.P. Web-based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials. 2014;38:190–197. doi: 10.1016/j.cct.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Clinical Trials Transformation Initiative CTTI recommendations: decentralized clinical trials. September 2018. https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/dct_recommendations_final.pdf Available at:
- 5.U.S. Food and Drug Administration FDA guidance on conduct of clinical trials of medical products during COVID-19 public health emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency Available at: