Abstract
Gossypol (Gos) is a natural polyphenolic compound that has shown a number of valuable biological properties such as antifertility, antioxidation, and antitumor activities. However, the clinical application of Gos has been hindered by its notable adverse effects such as hypokalemia, hemolytic anemia, and so on. Using sustained-release dosage form provides a hopeful solution to this problem. In this study, a gastric floating tablet for sustained-release of Gos (Gos-GFT) was developed using polyvinylpyrrolidone, hydroxypropyl methyl cellulose, ethyl cellulose, lactose, sodium bicarbonate, and magnesium stearate. Gos-GFT had an average weight of around 200 mg with a drug content percentage of around 13.66%. The physicochemical properties of Gos-GFT satisfied the pharmacopoeial requirements for tablets. Gos-GFT was able to float in an acidic medium and had a sustained drug release for over 12 h. In vivo studies showed that the relative bioavailability of Gos-GFT, as compared with Gos powders, was larger than that of a non-gastric floating tablet which was a dosage form used for comparison with Gos-GFT. Furthermore, compared with the Gos powders and the non-gastric floating Gos tablets, Gos-GFT could prolong the in vivo action time of Gos, and significantly relieve hypokalemia which is a major adverse effect of Gos. These properties made Gos-GFT a promising Gos preparation that warrants further investigation for more extensive clinical applications of this natural compound.
Keywords: Gastric floating tablet, Gossypol, Sustained release, Pharmacokinetics, Bioavailability, Adverse effect
1. Introduction
Gossypol (Gos) is a natural polyphenolic compound mainly extracted from cotton plants (Wang et al., 2019). It has been reported to have significant pharmacological properties, such as antioxidant, antitumor, antivirus, and antimicrobial properties (Keshmiri-Neghab and Goliaei, 2014). It was also found to inhibit the production and movement of sperms in males, thus it has been developed as a contraceptive for men. Despite its valuable biological properties, the clinical application of Gos has encountered considerable problems caused by its adverse effects. A major adverse effect of this drug was hypokalemia (i.e. an abnormally low potassium level in blood) which was related to the impacts of Gos on renal tubules (Liu and Lyle, 1987, Kumar et al., 1997). Other adverse effects of Gos included hemolytic anemia, dysgeusia, and diarrhea, etc (Van Poznak et al., 2001, Zbidah et al., 2012). To avoid or reduce these adverse effects, a possible approach is choosing low drug doses. For example, in some previous studies, low doses of Gos for male contraception were investigated to avoid the adverse effects (Gu et al., 2000, Yang et al., 2004, Chang et al., 2011). However, reduced drug doses could also lead to compromised drug efficacy. That is why the combined administration of Gos with some other contraceptive drugs such as steroid hormones was often required to obtain effective contraception (Yang et al., 2004, Chang et al., 2011).
It should be noted that, for the treatment of Gos in the previous studies, the experimental animals were mostly administered with pure Gos powder, and the human patients were treated with plain Gos tablets (Kumar et al., 1997, Gu et al., 2000, Van Poznak et al., 2001, Yang et al., 2004, Chang et al., 2011). Probably due to the lack of diversity in the dosage form, so far there were very few studies that have focused on the influences of dosage form on the changes (or the extent of fluctuation) in the plasma Gos concentration (Wang et al., 2018, Wen et al., 2018). Since neither the Gos powders nor the plain Gos tablets had controlled drug release, in our pilot studies considerable fluctuations of plasma drug concentration could be observed after drug administration. At higher Gos doses, such fluctuations may lead to long-term toxic drug concentration that consequently causes severe adverse effects. At reduced drug doses, a large fluctuation in plasma drug concentration may not lead to toxic drug concentration or keep toxic drug concentration for too long, but the time when the drug concentration is within the therapeutic window would also be shortened, resulting in compromised drug efficacy.
In order to solve the above conflict, a potential way is using controlled-release dosage forms to reduce the fluctuation in plasma drug concentration and to maintain the plasma drug level within the therapeutic window for a longer time. In an early study, Gos was incorporated into layer-by-layer (LBL) polyethylene glycol films for subcutaneous implantation (Wen et al., 2018). The LBL films had a zero-order release of Gos, which led to a minimized fluctuation in the plasma drug concentration and reduced side effects in the rats, indicating that controlled-release dosage form is promising for the development of Gos preparations. In our recent studies, a gastric floating tablet for sustained-release of dihydromyricetin, an anti-inflammatory natural compound, was investigated (Liu et al., 2019b). Typically, gastric floating preparations utilize the action of gastric juice to form gels and expand their volume, so that their densities become lower than the gastric contents (Anurag et al., 2017). Based on this, gastric floating preparations can float and have a sustained drug release in the stomach for a longer time, which may result in better control of the fluctuation in plasma drug concentration. Moreover, since the drug is released mostly in the upper gastrointestinal tract, the absorption of the drug can be maximized, thereby improving the drug bioavailability (Singh and Kim, 2000). So far, various gastric floating dosage forms, such as gastric floating tablets, microspheres, granules, and pills, have been investigated for the pharmaceutical development of different kinds of drugs (Pahwa et al., 2013, Namdev and Jain, 2019). Our previous study demonstrated that the gastric floating tablet could improve the bioavailability and drug efficacy of dihydromyricetin in vivo. These findings suggested that gastric floating dosage forms have a great potential to develop as suitable pharmaceutical preparations for oral administration of Gos.
In this study, a gastric floating sustained-release tablet for Gos (Gos-GFT) was prepared via direct powder compression. The properties of Gos-GFT, such as the weight, diameter, thickness, drug content, hardness, friability, and floating ability, were investigated. Moreover, the pharmacokinetics and the effects of Gos-GFT on the plasma K+ level were studied in rabbits.
2. Materials and methods
2.1. Materials and animals
Gossypol (2,2′-bis-(formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene), Gos, molecular weight 518 Da, yellow powder, purity > 98%), lactose, sodium bicarbonate (NaHCO3), ethyl cellulose (EC, viscosity 10 cP), starch (300 K Da on average), glycyrrhetinic acid (reference standard, purity > 98%), and sodium dodecyl sulfate (SDS) were supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd (Aladdin®, China). Polyvinylpyrrolidone (PVP) K30, hydroxypropyl methyl cellulose (HPMC) K4M, and magnesium stearate (Mg stearate) were supplied by MilliporeSigma Corporation (Sigma-Aldrich®, USA). The reagents above were used without further purification. New Zealand rabbits (2.1–2.3 kg, male) were supplied by the Laboratory Animal Center of Southwest Medical University.
2.2. Preparation of Gos-GFTs
Gos-GFTs were prepared via direct powder compression as described elsewhere (Liu et al., 2019b). For this purpose, Gos, lactose, EC, NaHCO3, Mg stearate, PVP, and HPMC (with the input weight ratios of 1:0.6:0.4:1.4:0.064–0.074:1.2–1.6:1.8–2.4) were ground together in a marble mortar to form a uniform mixture. Then 200 mg of the mixture was transferred into a ZP-12 tablet machine (Degong Machinery Equipment Co., Ltd, China) with a flat-faced round punch (8 mm in diameter), and was made into a tablet with the weight of around 200 mg and a diameter of around 8 mm (Fig. 1). For each batch, 40 tablets were prepared. The input amounts of PVP and HPMC were optimized according to the drug release rate and floating ability. The input weight ratios of the excipients to Gos in different formulations of Gos-GFTs were as listed in Table 1. The input amount of Mg stearate (the lubricant) in each formulation was about 1% of the total amount. To prepare a non-gastric floating tablet (i.e. plain Gos tablet) for comparative studies, Gos, lactose, EC, Mg stearate, PVP, and starch (with the input weight ratios of 1:0.6:0.4:0.072:1.4:3.8) were ground together in a marble mortar to form a uniform mixture. Then 200 mg of the mixture was made into a tablet similarly as described above.
Fig. 1.
Schematic illustration for the preparation of Gos-GFT.
Table 1.
The input weight ratios of the excipients to Gos in different formulations of Gos-GFTs.
| Formulation number | Gos | Lactose | EC | NaHCO3 | Mg stearate | PVP | HPMC | Theoretical drug content (%) |
|---|---|---|---|---|---|---|---|---|
| #1 | 1 | 0.6 | 0.4 | 1.4 | 0.064 | 1.2 | 1.8 | 15.47 |
| #2 | 1 | 0.6 | 0.4 | 1.4 | 0.067 | 1.2 | 2.1 | 14.78 |
| #3 | 1 | 0.6 | 0.4 | 1.4 | 0.07 | 1.2 | 2.4 | 14.14 |
| #4 | 1 | 0.6 | 0.4 | 1.4 | 0.066 | 1.4 | 1.8 | 15.00 |
| #5 | 1 | 0.6 | 0.4 | 1.4 | 0.069 | 1.4 | 2.1 | 14.35 |
| #6 | 1 | 0.6 | 0.4 | 1.4 | 0.072 | 1.4 | 2.4 | 13.75 |
| #7 | 1 | 0.6 | 0.4 | 1.4 | 0.068 | 1.6 | 1.8 | 14.56 |
| #8 | 1 | 0.6 | 0.4 | 1.4 | 0.071 | 1.6 | 2.1 | 13.95 |
| #9 | 1 | 0.6 | 0.4 | 1.4 | 0.074 | 1.6 | 2.4 | 13.38 |
2.3. Characterization of Gos-GFTs
To study the weight and the weight variation of the optimized Gos-GFTs, twenty randomly selected tablets were weighed individually (Kaleemullah et al., 2017). Then the weight of each tablet (wi) was compared with the average value (M) of the twenty tablets, and the weight variation was calculated according to the formula: weight variation (%) = |(M – wi)/M| × 100%.
To investigate the drug content percentage of the optimized Gos-GFTs, ten randomly selected tablets were weighed together and ground thoroughly to form a fine powder (Kesarla et al., 2015). Then 50 mg of the powder was weighed and extracted in 200 mL acetonitrile, followed by filtration using a 0.22 μm cellulose acetate membrane. The drug concentration of the extraction solution was determined via high-performance liquid chromatography (HPLC) on a 1260 Infinity Ⅱ HPLC system (Agilent Technologies, Inc., USA) with a G7129A automatic sampler and a G7114A variable wavelength detector (Liu et al., 2019b). For each measurement, 20 μL of the sample was separated on a C18 column (reversed-phase, 5 μm particle size, 4.6 × 250 mm). The temperature of the column was kept at 26 ℃. The mobile phase [acetonitrile-0.9% formic acid solution (77:23, v/v)] was kept at a flow rate of 0.8 mL/min. The separated samples were detected at the wavelength of 254 nm. The experiment for drug content percentage was done in triplicate. To investigate the drug content per tablet, one randomly selected tablet was weighed and ground thoroughly to form a fine powder. Then all the powder was extracted in 800 mL acetonitrile, followed by filtration using a 0.22 μm cellulose acetate membrane. The drug concentration of the extraction solution was determined via HPLC as described above. The average drug content per tablet was calculated based on the results of ten randomly selected tablets.
For the diameter and thickness of the optimized Gos-GFTs, ten randomly selected tablets were measured separately using a vernier caliper. To study the hardness of Gos-GFTs, ten randomly selected tablets were tested separately on a YD-3 hardness tester (Jingtuo Instrument Co., Ltd, China). The friability of Gos-GFTs was investigated using a CS-4 friability tester (Beijing Jinshisu Instrument Co., Ltd, China), which was conducted according to the pharmacopoeial requirements (Chinese Pharmacopoeia Commission, 2020). Briefly, thirty-three tablets were randomly selected and weighed. The tablets were then rolled together (25 rpm × 4 min) in the testing drum of the friability tester, followed by de-dusting using nitrogen flow and weighing again. The friability was calculated as the weight loss percentage of the tablets. The friability experiment was done in triplicate.
To investigate the floating ability of Gos-GFTs, one tablet was put into 900 mL hydrochloric acid solution (0.1 N, pH 1.2) (Gong et al., 2018a). The solution was stirred (100 rpm) at 37 ℃. Then the floating lag time and the floating duration of the tablet were recorded. The floating lag time is defined as the time needed to rise to the surface of the solution. The floating duration is defined as the duration for the tablet to keep buoyant. For the study of floating ability, five randomly selected tablets were tested individually.
2.4. In vitro drug release
To study the drug release of Gos-GFTs, one tablet was put into 900 mL hydrochloric acid solution (0.1 N, pH 1.2) that contained 5% (w/v) SDS for sink condition (Zeng et al., 2009, Gong et al., 2018a). The solution was stirred (100 rpm) at 37 ℃. At different time points, 1 mL solution was withdrawn for analysis, and the same amount of fresh hydrochloric acid solution was replenished. The withdrawn solution was added with 2 mL chloroform and was vortexed for 10 min, followed by centrifugation at 8000 rpm for 10 min. Then 1.6 mL of the clear chloroform layer was withdrawn and was evaporated to dryness using nitrogen flow. The extraction recovery of Gos from the release medium was about 92%. The dry residue was re-dissolved in 0.8 mL acetonitrile, followed by filtration through a 0.22 μm cellulose acetate membrane. The drug concentration of the acetonitrile solution was then determined via HPLC as described above. For the study of drug release, three randomly selected tablets were tested individually. The drug release of the plain Gos tablet (as a non-gastric floating tablet for comparative studies) was similarly investigated.
In order to investigate the release mechanism of Gos-GFTs, the Korsmeyer-Peppas and Kopcha release models were adopted (Acharya et al., 2014, Kesarla et al., 2015, Asadian-Ardakani et al., 2016). In the Korsmeyer-Peppas model, the drug release fraction (Mt/M∞) at time point “t” is fitted using the equation: Mt/M∞ =ktn, in which “k” is the release rate constant and the “n” value indicates the release mechanism (Liu et al., 2019b). In the Kopcha model, the amount of drug released (Mt) at time point “t” is fitted using the equation: Mt/t = α/t1/2 + β. The “α” value and the “β” value indicate the relative contributions of diffusion and erosion, respectively.
2.5. Swelling and erosion of Gos-GFTs
The swelling and erosion profiles of the optimized Gos-GFTs were studied as follows (Gong et al., 2018a). One tablet was weighed, and its initial weight was marked as “w1”. Then the tablet was put into 900 mL hydrochloric acid solution (0.1 N, pH 1.2). The solution was stirred (100 rpm) at 37 ℃. After the predetermined time, the tablet was withdrawn and placed on a piece of filter paper to take away the liquid on the surface, followed by weighing again to obtain its wet weight (w2). Then the tablet was dried at 55 ℃ in a vacuum oven until its weight did not change anymore. The dry weight of the tablet was marked as “w3”. The swelling and erosion degrees of the tablet were determined according to the formulae (Liu et al., 2019b): swelling percentage = (w2 – w1)/w1 × 100%; erosion percentage = (w1 – w3)/w1 × 100%.
2.6. Pharmacokinetic studies
The animal studies have been approved by the Ethics Committee of Southwest Medical University (approval No.20180391213). For the pharmacokinetic study of the optimized Gos-GFTs, the male rabbits (2.1–2.3 kg) were randomly allocated into three groups, six rabbits in each. The animals were treated with Gos-GFTs, plain Gos tablets, or Gos drug powder via oral administration, at the dose of 12.5 mg Gos/kg. At different time points post-administration, a blood sample (about 1.5 mL) of each rabbit was collected via the cardiac apex, followed by centrifugation (5000 rpm × 10 min) to obtain the plasma (Huang et al., 2016). To determine the concentration of Gos, 450 μL of the plasma was added with 50 μL glycyrrhetinic acid solution (16 μg/mL, dissolved in methanol) as the internal standard, and was vortexed for 5 min to mix. Then 500 µL acetonitrile was added to the mixture which was vortexed for another 5 min, followed by centrifugation at 12000 rpm for 10 min. After that, 800 µL of the supernatant was added with 200 µL acetonitrile and vortexed for 5 min, followed by centrifugation again at 12000 rpm for 10 min. Finally, 900 µL of the supernatant was filtered through a 0.22 μm membrane, and the drug concentration was analyzed via HPLC as described above. The plasma drug concentrations obtained from each animal were used to determine the pharmacokinetic parameters, according to the methods reported elsewhere (Liu et al., 2019b).
2.7. Studies on the plasma potassium (K+)
To study the changes in the plasma K+ level caused by Gos, the male rabbits (2.1–2.3 kg) were randomly allocated into three groups, six rabbits in each. The animals were treated with the optimized Gos-GFTs, plain Gos tablets, or Gos drug powder via oral administration, at the dose of 12.5 mg Gos/kg, once every two days. The day of the first administration was marked as day 1. The last administration was on day 39. On day 0 (i.e. the day before the first administration), 10, 20, 30, and 40, the body weight and plasma K+ level of each animal were recorded. To determine the plasma K+ concentration, a blood sample (about 1.5 mL) of each rabbit was collected via the cardiac apex, followed by centrifugation (5000 rpm × 10 min) to obtain the plasma (Huang et al., 2016). The concentration of plasma K+ was measured using an AU5800 automatic biochemistry analyzer (Beckman Coulter, Inc., USA) with the matched reagent and calibration solution provided by the company (Albert et al., 2011, Woolford et al., 2020). The measurements were done according to the standard protocol of the biochemistry analyzer.
2.8. Statistical analysis
The pharmacokinetic parameters were determined using the DAS 2.0 software (Chinese Pharmacological Society, China) based on non-compartmental methods (Liu et al., 2015, Zhou et al., 2018). The analysis of other data was carried out with the Prism 5 software (GraphPad, USA), using Student's t-test for comparison between two groups and one-way ANOVA for comparison among multiple groups (Liu et al., 2019a, Liu et al., 2020). P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Preparation of Gos-GFTs
In this study, Gos-GFTs were prepared via direct powder compression. For this purpose, excipients were carefully chosen to obtain a suitable formulation and the wanted physicochemical properties of the tablets. Lactose is a commonly used filler material that helps the formation of pharmaceutical tablets (Paul et al., 2019). EC is a hydrophobic polymer that can work as an adhesive for the tablets and can also form an insoluble matrix that adds to the resistance of tablets to shear forces (Tolia and Li, 2014). Mg stearate was used as the lubricant that can facilitate the preparation and avoid powder sticking to the punch during tableting (Dawes et al., 2012). NaHCO3 can generate carbon dioxide when reacted with the gastric acid, which speeds up the rising of the tablet to the liquid surface and thereby shortens the floating lag time. From our previous studies, the input of NaHCO3 can also prolong the floating duration of tablets (Liu et al., 2019b). However, the influence of NaHCO3 on the floating duration was much weaker than on the floating lag time. PVP is a hydrophilic polymer that can facilitate the hydration and erosion of the tablets, which accelerates the drug release and shortens both the floating lag time and the floating duration (Park et al., 2015). HPMC has multiple effects on Gos-GFTs too. It can form gel after hydration in the gastric juice, which makes the tablet swell and helps to keep its density lower than the gastric contents, thus prolonging the floating duration. Moreover, the HPMC gel formed in the tablet may also block up the diffusion path for both the inside components and the outside liquid, which can not only slow down the drug release but also hinder the reaction of NaHCO3 with the gastric acid and lead to prolonged floating lag time (Siepmann et al., 2017, Gong et al., 2018a).
The floating ability and the drug release are two basic and important properties for gastric floating dosage forms. In the previous studies on gastric floating tablets, the floating ability was usually evaluated based on the floating lag time and the floating duration (Kesarla et al., 2015, Gong et al., 2018b, Qin et al., 2018). Generally, the floating lag time should be as short as possible to ensure that the tablets can rise to the surface of the solution rapidly. Besides, for gastric floating tablets, a long floating duration can be more favorable. Particularly, the floating duration of the tablets should not be shorter than the time needed for the drug to be completely (or almost completely, e.g. > 95%) released, so that the release of the drug could be maximized in the upper gastrointestinal tract for better absorption of the drug (Singh and Kim, 2000). To determine the appropriate formulation, both the floating ability and the drug release should be considered. In this study, since PVP and HPMC have significant influences on the drug release and the floating ability of Gos-GFTs, their input amounts have been optimized. The investigated formulations for Gos-GFTs, with the numbers #1 to #9, were as listed in Table 1.
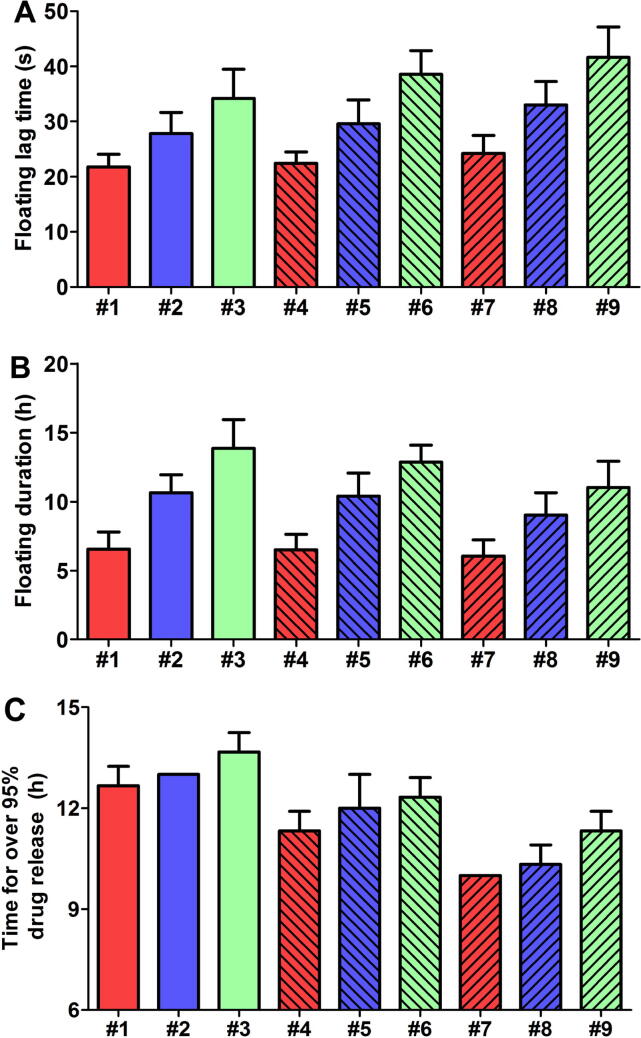
As shown in Fig. 2A, with fixed input amounts of the other components, increasing the amount of PVP or HPMC could prolong the floating lag time of tablets. It should be noted that increasing the amount of PVP should theoretically facilitate the reaction of NaHCO3 with the gastric acid and consequently lead to shortened floating lag time. However, changing the input amounts of PVP and HPMC could also result in the total amount changes at the same time. It is easy to see that, with fixed input amounts of NaHCO3 and the other components, increasing the amount of PVP alone could lead to a reduced concentration of NaHCO3 in the tablet. Therefore, prolonged floating lag time may be observed when the amount of PVP was increased. For all formulations, the average floating lag time was in the range of 21.8–41.6 s (Fig. 2A). According to some previous studies, a floating lag time of<1 min can be acceptable for gastric floating tablets (Gong et al., 2018a, Gong et al., 2018b). Since the floating lag time was adequately short (i.e. within 1 min) for all formulations, this parameter was considered as a secondary factor for comparing the floating ability among different formulations. In this study, the floating ability of different formulations was compared mainly based on the floating durations.
Fig. 2.
(A) Floating lag time and (B) floating duration of Gos-GFTs with different formulations (#1–9) in the acidic medium. Data were shown as mean ± SD (n = 5). (C) The time that different Gos-GFTs (#1–9) needed to almost complete (over 95%) the release of the drug. Data were shown as mean ± SD (n = 3).
As shown in Fig. 2B, decreasing the amount of PVP or increasing the amount of HPMC could prolong the floating duration. For all formulations, the average floating durations were in the range of 6.06–13.86 h. Particularly, the average floating durations for formulations #1, #2, #3, and #6 were over 12 h. These four formulations were considered for further screening.
Fig. 2C shows the time that each formulation needed to have over 95% drug release. Generally, decreasing the amount of PVP or increasing the amount of HPMC could slow down the drug release. For formulations #3 and #6, the time needed for over 95% drug release was near their average floating durations which were about 13.9 h and 12.9 h, respectively (Fig. 2B and 2C). However, for formulations #1 and #2, the floating durations were obviously shorter than the time needed for over 95% drug release. For example, the average floating duration of the formulations #1 was only about 6.6 h, while the time needed for over 95% drug release was about 12.7 h, which may probably lead to incomplete drug release in the upper gastrointestinal tract (Singh and Kim, 2000). Based on the above results, formulations #3 and #6 can be used for further consideration. Particularly, the formulation #6 had an average floating duration of about 12.9 h, and could almost complete (>95%) the drug release after about 12.3 h. Since both the floating duration and drug release of formulation #6 were near half a day, this formulation may be more suitable for twice-a-day dosing. Taken the floating ability and the drug release together, the formulation #6 was determined as the optimal formulation for Gos-GFT and was chosen for the consequent studies. Nevertheless, since the formulation #3 may also be worthy of study, investigations on the properties of this formulation will be done in near future.
It is also noteworthy that, since the total amount changed with changing the input amounts of PVP and HPMC, the theoretical drug content varied among different formulations; and determination of the optimal formulation may need to consider the drug content. The range of the theoretical drug contents was 13.38%-15.47% for all investigated formulations of Gos-GFT. Generally, higher drug content can be more favorable for the application of the tablets, because with higher drug content smaller number of tablets may be needed for drug administration. However, since the theoretical drug content did not vary too much among different formulations, in this study the formulation of Gos-GFT was not optimized in terms of the drug content of the tablets.
3.2. Characterization of Gos-GFTs
In this part, the optimized Gos-GFTs were investigated in terms of the weight, drug content, diameter, thickness, hardness, friability, and floating ability of the tablets. The results of characterization were exhibited in Table 2. The average weight of Gos-GFTs, as determined based on twenty randomly selected tablets, was 199.85 ± 1.13 mg. The weight variations of all measured tablets were<1.5% and were far below the pharmacopoeial limit (Chinese Pharmacopoeia Commission, 2020). The drug content of Gos-GFTs was about 13.66% by weight and was about 27.41 mg per tablet. These results were near the theoretical values (i.e. 13.75% by weight and 27.5 mg per tablet) as calculated according to the input amounts of the components (Table 1). In some other studies, due to considerable excipients (e.g. HPMC) needed for the desired floating ability of the tablets, the drug content of the tablets can be lower (Acharya et al., 2014, Gong et al., 2018b). However, although higher drug contents can be favorable for drug administration, the determination of acceptable drug contents for the tablets should be based on the dosage in practice. The average diameter and thickness of Gos-GFTs were 8.00 ± 0.03 mm and 3.58 ± 0.03 mm, respectively. The hardness of the tablet was about 60 N. The friability of Gos-GFTs was about 0.69%, which was notably below the pharmacopoeial limit of 1%. The floating ability of Gos-GFTs was studied in terms of the floating lag time and floating duration. The floating lag time and floating duration as determined based on five randomly selected tablets was 38.60 ± 4.28 s and 12.88 ± 1.20 h, respectively. The floating duration of about 12 h demonstrated the feasibility of Gos-GFTs for twice-a-day dosing.
Table 2.
The characterization results of Gos-GFT. Data are exhibited as mean ± SD.
| Evaluated items for the tablet | Measured valuesa | Theoretical values or pharmacopoeial requirements |
|---|---|---|
| Weight (mg) | 199.85 ± 1.13 | 200 |
| Weight variation (%) | < 1.5 | < 7.5 |
| Drug content percentage (%) | 13.66 ± 0.09 | 13.75 |
| Drug content per tablet (mg) | 27.41 ± 0.31 | 27.5 |
| Diameter (mm) | 8.00 ± 0.03 | 8 |
| Thickness (mm) | 3.58 ± 0.03 | Not specified |
| Hardness (N) | 60.10 ± 3.41 | Not specified |
| Friability (%) | 0.69 ± 0.08 | <1 |
| Floating lag time (s) | 38.60 ± 4.28 | Not specified |
| Floating duration (h) | 12.88 ± 1.20 | Not specified |
3.3. In vitro drug release and the mechanism
The reported solubility of Gos is<0.01 mg/mL in H2O at 37 ℃ (Tomoda et al., 2015). In this study, SDS has been added in the release medium to promote the solubilization of Gos. SDS is a commonly used surfactant to provide sink conditions for the release studies of hydrophobic drugs (Zeng et al., 2009, Zhang et al., 2012, Knoos et al., 2014). The choice of SDS concentration was based on whether the sink condition could be maintained. In this study, 5% (w/v) was the minimum concentration needed for SDS to maintain the sink condition for Gos during the experiment of drug release. The drug release profile of the optimized Gos-GFTs was shown in Fig. 3A. The drug release of Gos-GFTs was relatively slow for the first 4 h and then became faster for the next 6 h. After 12 h, most of the drug (about 95.3%) was released from the tablets. The plain Gos tablet, as a non-gastric floating preparation for comparison, was also investigated. For the preparation of plain Gos tablets, HPMC and NaHCO3 in Gos-GFTs were replaced by starch which is a commonly used disintegrant for tablets. As a result, the plain Gos tablets almost completed the release of the drug within 2 h (Fig. 3A). The fast drug release of the plain tablet was mainly due to its rapid disintegration which was observed to happen within the first 1.5 h (data not shown). It is noteworthy that, although sink condition was provided in the drug release study and the results can help to compare the drug release profiles of different formulations, these results may not reflect the actual drug release in the stomach. That is because the internal environment of the stomach can be more complex than the experimental conditions and may not provide sink condition for the drug release. Therefore, to clarify the actual drug release of the tablets in the stomach, in vivo investigations are necessary.
Fig. 3.
Profiles of the (A) cumulative drug release, (B) swelling, and (C) erosion of Gos-GFT. Data were exhibited as mean ± SD (n = 3).
To study the mechanism of in vitro drug release, the release curve of Gos-GFT was fitted using Korsmeyer-Peppas and Kopcha equations, and the swelling and erosion profiles of the tablets were investigated. It was found that in the aqueous medium the tablets had a rapid swelling in the first 4 h (Fig. 3B); meanwhile, the erosion of tablets was relatively slow (Fig. 3C). Interestingly, during this period the drug release of Gos-GFTs was also slow. The tablet swelling alone seemed not to significantly promote the diffusion of Gos which is hydrophobic with the Log P value of about 6.2 at 37 ℃. However, after 4 h the growth of swelling degree slowed down due to the increasing erosion degree, and the swelling degree started to decline at 6 h. Meanwhile, the drug release of Gos-GFTs became faster. At 12 h, the erosion degree was about 60%, and the drug release of Gos-GFTs was almost complete (Fig. 3). It is worth mentioning that, the medium for drug release contained 5% (w/v) SDS, while the medium used for swelling and erosion studies did not contain SDS. The swelling and erosion profiles of Gos-GFTs did not change significantly with or without the addition of SDS (data not shown). For drug release, SDS was added to provide sink condition. Without the addition of SDS, the released Gos from the tablets could not well dissolve in the medium and may form small drug particles suspended in the medium, which made it difficult to determine the amount of released Gos at different time points. It is noteworthy that, normally SDS is not added into real gastric juice in the animals or human patients during drug administrations. In order to make the conditions of in vitro experiments more consistent with in vivo conditions, the swelling and erosion profiles of Gos-GFTs in the medium without SDS were shown. However, since no significant difference was observed in the swelling and erosion profiles of Gos-GFTs with or without the addition of SDS, the analysis of the drug release results was not significantly affected.
The Korsmeyer-Peppas and Kopcha models are two commonly used mathematical models to understand the mechanism of drug release (Acharya et al., 2014, Kesarla et al., 2015, Asadian-Ardakani et al., 2016, Liu et al., 2021). In the equation of the Korsmeyer-Peppas model (i.e. Mt/M∞ =ktn), “Mt” is the amount of drug released at time point “t”, and “M∞” is the total amount of drug in the investigated preparation (i.e. Gos-GFTs for the present study), thereby “Mt/M∞” indicates the drug release fraction at time point “t”. The parameter “k” is the release rate constant which is related to the structural and geometric properties of the tablets. The parameter “n” is the release exponent that indicates the release mechanism (Narasimhan, 2001, Acharya et al., 2014). The “n” value of<0.5 indicates the release mechanism of Fickian diffusion. The “n” value of over 0.5 and below 1.0 indicates the release mechanism of anomalous (non-Fickian) diffusion. The “n” value equal to 1.0 indicates the release mechanism of case-II transport (zero-order drug release). The “n” value of > 1.0 indicates the release mechanism of super case-II transport. In this study, the fitted result using the Korsmeyer-Peppas model was Mt/M∞ = 0.083 t0.958 (R2 = 0.988) for the drug release. Therefore, the n value was 0.958, which indicates the mechanism of anomalous diffusion and that the drug release of Gos-GFTs may have been regulated by the joint action of erosion and diffusion (Acharya et al., 2014, Mahdizadeh Barzoki et al., 2016).
The Kopcha model is commonly used to specify the relative contributions of diffusion and erosion to drug release (Asadian-Ardakani et al., 2016, Rezk et al., 2019). In the equation of the Kopcha model (i.e. Mt/t = α/t1/2 + β), “Mt” is also the amount of drug released at time point “t”. The “α” value and the “β” value indicate the relative contributions of diffusion and erosion, respectively. If α is greater than β, the dominant drug release mechanism is diffusion. If α is smaller than β, then the drug release mechanism is dominantly matrix erosion. In this study, the fitted result using the Kopcha model was Mt/t = 0.346/t1/2 + 1.982 (R2 = 0.928). Therefore, the α value was much smaller than the β value, which demonstrated that erosion had a stronger impact on the drug release of Gos-GFTs than diffusion, even though both the mechanisms have affected (Rezk et al., 2019).
3.4. Gos-GFTs modified the pharmacokinetics of Gos in vivo
In some previous studies, the pharmacokinetic behaviors of Gos have been investigated on various species (Othman and Abou-Donia, 1988, Jia et al., 2008, Wang et al., 2018). In these studies, at the same dose levels the pharmacokinetic results varied notably among different formulations for Gos and routes of drug administration. For example, Gos-incorporated layer-by-layer (LBL) polyethylene glycol films have been investigated for subcutaneous implantation (Wen et al., 2018). The subcutaneously implanted LBL films had a zero-order release of Gos, which led to significantly reduced fluctuations in the plasma Gos concentration in rats as compared with the same drug administrated orally or via intravenous injection. In the present study, the pharmacokinetic behaviors of Gos in different formulations were studied in rabbits. The animals in each group were treated with Gos-GFTs, plain Gos tablets, or Gos powder, at the oral dose of 12.5 mg Gos/kg. The plasma concentration–time curves were shown in Fig. 4. The maximal drug concentration (Cmax) is an important parameter for the determination of a safe dose range, because theoretically the Cmax must be lower than the minimum toxic concentration of a drug, in order to avoid toxic reactions. Therefore, controlling the maximal drug concentration at a lower level can be favorable for avoiding or reducing the adverse effects of the drug. That is why in some previous studies, low doses of Gos have been investigated in order to avoid the adverse effects (Gu et al., 2000, Yang et al., 2004, Chang et al., 2011). However, although the Cmax may be lower at reduced drug doses, the time when the drug concentration is within the therapeutic window may also be shortened. Using controlled-release dosage forms can help to reduce the extent of fluctuation in plasma drug concentration and maintaining the plasma drug level within the therapeutic window for a longer time, thereby providing a potential way to reduce the adverse effects and maintain the drug efficacy of Gos at the same time. As shown in Fig. 4, the Cmax of Gos powder group was near 4 mg/L which appeared about 5 h after administration. Compared with Gos powder group, the Cmax of plain Gos tablet group appeared a little later, which was probably caused by the disintegration of tablets that took about 1.5 h on average. It is noteworthy that, since Gos is hydrophobic with very low solubility in water, the released drug may form a suspension in the gastric juice. However, there are various mechanisms that may promote the absorption of hydrophobic drugs in the body such as the bile salts-related transport (Chow and Nagwekar, 1993, Moghimipour et al., 2015). Therefore although Gos is hydrophobic, it could be absorbed in the gastrointestinal tract. The Cmax of plain Gos tablet was slightly lower than that of Gos powder group, but their difference was not statistically significant (P > 0.05). By contrast, Gos-GFT group had a much lower Cmax of about 2.31 mg/L appeared 10 h after administration. The delayed and lower Cmax of Gos-GFTs was mainly due to the sustained release of the drug. Compared with Gos powder and plain Gos tablet at the same dose of Gos, Gos-GFT could also considerably slow down the drop of plasma drug concentration after Cmax, which suggested prolonged retention time of the drug in vivo.
Fig. 4.
The plasma drug concentration of Gos powder group, plain tablet group, and Gos-GFT group at the same dose of Gos. Data were exhibited as mean ± SD (n = 6). Compared to the other two groups, Gos-GFT could considerably prolong the residence time of Gos in vivo.
More detailed pharmacokinetic parameters of each group were listed in Table 3. The time needed to reach Cmax (i.e. tmax) was 10 h for Gos-GFT group, which was significantly longer than that of Gos powder group (P < 0.001). The mean retention time (MRT) and the terminal elimination half-life (t1/2(T-∞)) that reflect the in vivo action time of the drug were also calculated for comparison. The MRT0-T and MRT0-∞ of Gos-GFT were 10.93 ± 0.11 h and 14.04 ± 0.99 h respectively and were significantly longer as compared with Gos powder (P < 0.001). The t1/2(T-∞) of Gos-GFT was 6.81 ± 1.63 h, also significantly longer than that of Gos powder (P < 0.01). These results demonstrated slower elimination and prolonged action time of Gos brought about by the sustained-release tablets. The area under the plasma concentration–time curve (AUC) reflects the bioavailability of the drug. In this study, the AUC0-T and AUC0-∞ of Gos-GFT were 23.78 ± 4.18 mg∙h/L and 28.03 ± 4.35 mg∙h/L respectively, and were significantly larger than those of Gos powder (P < 0.05 for AUC0-T, and P < 0.01 for AUC0-∞). For plain Gos tablet, the pharmacokinetic parameters were generally near those of Gos powder (Table 3), indicating that the plain tablet was not able to notably modify the pharmacokinetics of Gos in vivo. The relative bioavailability (FR) of Gos-GFT, as determined based on the average AUC0-∞ of Gos-GFT and Gos powder, was 131.45%, which was also larger than that of plain Gos tablet (only 110.64%). These results demonstrated that Gos-GFT as a novel sustained-release preparation for Gos could significantly enhance the drug bioavailability and prolong the in vivo action time of Gos. Besides, based on the pharmacokinetic parameters, lower fluctuation of plasma drug concentration may be obtained using Gos-GFT as compared with Gos powder and plain Gos tablet at the same dose of Gos, which needs further investigation to verify. Therefore, Gos-GFT was favorable for controlling the drug concentration within the therapeutic window for a longer period of time.
Table 3.
Pharmacokinetic parameters of Gos powder, plain Gos tablet, and Gos-GFT in rabbits. Data are exhibited as mean ± SD.
| Pharmacokinetic parameters # | Gos powder | Plain tablet | Gos-GFT |
|---|---|---|---|
| tmax (h) | 4.83 ± 0.41 | 5.67 ± 0.52 | 10.00 ± 0.00 *** |
| Cmax (mg/L) | 3.85 ± 0.33 | 3.58 ± 0.23 | 2.31 ± 0.32 |
| AUC0-T (mg∙h/L) | 18.63 ± 1.19 | 20.18 ± 1.96 | 23.78 ± 4.18 * |
| AUC0-∞ (mg∙h/L) | 21.32 ± 1.79 | 23.59 ± 3.01 | 28.03 ± 4.35 ** |
| MRT0-T (h) | 5.31 ± 0.17 | 6.74 ± 0.22 | 10.93 ± 0.11 *** |
| MRT0-∞ (h) | 6.89 ± 0.62 | 9.06 ± 0.76 | 14.04 ± 0.99 *** |
| t1/2(T-∞) (h) | 3.97 ± 0.94 | 5.53 ± 0.83 | 6.81 ± 1.63 ** |
| FR (%) | 110.64 | 131.45 |
P < 0.05, ** P < 0.01, *** P < 0.001 as compared with Gos powder group.
AUC0-T: AUC for 0 h to the last time point of measurement. AUC0-∞: the area under the whole curve. MRT0-T: MRT for 0 h to the last time point of measurement. MRT0-∞: MRT for the whole curve.
3.5. Gos-GFTs reduced the hypokalemia effect of Gos in vivo
The reported adverse effects of Gos include hypokalemia, hemolytic anemia, dysgeusia, and diarrhea, etc (Van Poznak et al., 2001, Zbidah et al., 2012). Although hypokalemia is not a unique adverse effect, it has been a major adverse effect of this drug. In previous studies, significantly reduced potassium levels in the blood have been observed in animals and human patients after repeated treatment using Gos (Liu and Lyle, 1987; Kumar et al., 1997, Van Poznak et al., 2001). In one of these studies, daily oral administration of Gos at about 0.6 mg/kg for 60 days has led to the decrease of plasma potassium by about 10% in langur monkeys (Kumar et al., 1997). Obvious reductions in plasma potassium may occur even at reduced Gos doses, probably because the traditional dosage forms that have been adopted in the previous studies could hardly avoid large fluctuation of plasma drug concentration that led to toxic concentrations (i.e. the concentrations higher than the minimum toxic concentration of the drug) from time to time. Therefore, maintaining the plasma drug level within the therapeutic window for a longer period using sustained-release dosage forms provides a promising way to reduce the side effects of Gos. In the present study, animals in each group were treated with Gos-GFTs, plain Gos tablets, or Gos powder, at the oral dose of 12.5 mg Gos/kg, once every two days. The treatment lasted 39 days. The changes in the plasma potassium concentration of each group were displayed in Fig. 5. The normal range of plasma potassium level may vary among different species. In this study, the initial concentration of plasma potassium was around 4 mmol/L for all groups, which was consistent with the results of a previous study using rabbits (Du et al., 2019). For the first 10 days, none of the three groups showed obvious changes in the plasma potassium concentration. After 10 days, however, the plasma potassium level started to drop in varying degrees. On day 40, the average plasma potassium of Gos powder group decreased to about 3.6 mmol/L (decreased by 10%). Compared with Gos powder, the plain Gos tablet group showed a little smaller change in the plasma potassium level; but the difference between the two groups was not statistically significant (P > 0.05). By contrast, Gos-GFT group showed a notably slower drop in the plasma potassium level. The average plasma potassium of Gos-GFT group was 3.84 ± 0.13 mmol/L on day 40, significantly higher than that of Gos powder group (P < 0.01). This result indicated the potential of Gos-GFT to relieve the hypokalemia caused by the drug.
Fig. 5.
Changes in the plasma potassium concentration of each group. The rabbits (with similar initial body weights of around 2.2 kg) were treated with 27.5 mg Gos powder, one plain Gos tablet (containing about 27.5 mg Gos), or one Gos-GFT (containing about 27.5 mg Gos), once every two days. Data were exhibited as mean ± SD (n = 6). **P < 0.01, as compared with Gos powder group.
The body weight changes that can reflect the systemic toxicity were also recorded, in order to better know the effects of Gos-GFT on reducing the adverse effects of Gos. As presented in Fig. 6, the average body weight of each group increased in varying degrees during the experiment. After 40 days, the body weight increment of Gos-GFT group was significantly larger as compared with Gos powder group (P < 0.01) and plain Gos tablet group (P < 0.05). The smaller body weight increment in Gos powder group and plain Gos tablet group may have been caused by Gos-related dysgeusia which is one of the reported adverse effects of this drug (Van Poznak et al., 2001). Indeed, during the experiment, less food intake has been observed in the two groups as compared with Gos-GFT group (data not shown). However, further investigation is needed to clarify the reasons for the different increments in body weight.
Fig. 6.
Changes in the body weight of different groups. The larger body weight increment of Gos-GFT group may reflect fewer adverse effects of Gos-GFT in vivo.
The results above demonstrated that Gos-GFT was able to relieve the hypokalemia caused by Gos, and may also relieve the drug-related dysgeusia, which was probably realized via relatively steady and lower plasma drug concentration brought about by sustained drug release. However, to further understand the influences of this dosage form on the side effects of Gos, including hypokalemia and the other reported side effects, a more systematic study is needed (Van Poznak et al., 2001, Zbidah et al., 2012). Moreover, it should be noted that the dosing regimen adopted in this study was simply utilized to compare the side effects among different groups. It may not be a suitable or even an effective dosing regimen for a certain purpose, because the therapeutic window varies for different biological properties of the drug and different species of experimental animals.
4. Conclusions
Gastric floating tablet as a novel sustained-release preparation for Gos (Gos-GFT) was prepared via direct powder compression. The physicochemical properties of Gos-GFT satisfied the pharmacopoeial requirements for tablets. Gos-GFT showed an average floating duration of about 12.9 h and had a sustained drug release for over 12 h in an acidic medium, suggesting that this Gos preparation is potential for twice-a-day dosing. The drug release of Gos-GFT was regulated by both the mechanisms of diffusion and erosion, but erosion had a stronger impact than diffusion. The pharmacokinetic studies demonstrated that Gos-GFT could significantly enhance the drug bioavailability and prolong the in vivo action time of the drug. Besides, based on the pharmacokinetic parameters, lower fluctuation of plasma drug concentration may be obtained using Gos-GFT as compared with Gos powder and plain Gos tablet at the same dose of Gos, which needs further investigation to verify. In addition, Gos-GFT was able to significantly reduce the hypokalemia effect of the drug as compared with the Gos powder and the plain Gos tablet, which was probably realized via relatively steady and lower plasma drug concentration brought about by sustained drug release. Taken together, Gos-GFT with the optimized formulation is a promising preparation for sustained release of Gos and warrants further investigation for more extensive clinical applications of this natural compound. Nevertheless, it should be noted that the formulation of Gos-GFT may be further optimized using some other possible polymers for even better results. Therefore, other commonly used polymers will also be investigated for the preparation of gastric floating sustained-release tablets for Gos in near future, in order for potentially greater ability to improve the bioavailability and reduce the side effects of the drug. Moreover, to further understand the influences of the dosage form on the side effects of Gos, including hypokalemia and the other reported side effects, a more systematic study is needed.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
This study was supported by Sichuan Science and Technology Program Project (2019YJ0489), the Joint Research Project of Southwest Medical University and the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University (2020XYLH-082), and the Research Project of National Undergraduate Innovation and Entrepreneurship Training Program of China (202010632003).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hao Liu, Email: h_lewis@126.com.
Weiling Zeng, Email: 1056231078@qq.com.
References
- Acharya S., Patra S., Pani N.R. Optimization of HPMC and carbopol concentrations in non-effervescent floating tablet through factorial design. Carbohyd. Polym. 2014;102:360–368. doi: 10.1016/j.carbpol.2013.11.060. [DOI] [PubMed] [Google Scholar]
- Albert V., Subramanian A., Rangarajan K., Pandey R.M. Agreement of two different laboratory methods used to measure electrolytes. J. Lab. Physicians. 2011;3(2):104–109. doi: 10.4103/0974-2727.86843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anurag V., Juhi D., Navneet V., Amit Kumar N. Chitosan-hydroxypropyl methylcellulose matrices as carriers for hydrodynamically balanced capsules of moxifloxacin HCl. Curr. Drug Deliv. 2017;14(1):83–90. doi: 10.2174/1567201813666160504100842. [DOI] [PubMed] [Google Scholar]
- Asadian-Ardakani V., Saber-Samandari S., Saber-Samandari S. The effect of hydroxyapatite in biopolymer-based scaffolds on release of naproxen sodium. J. Biomed. Mater. Res. A. 2016;104(12):2992–3003. doi: 10.1002/jbm.a.35838. [DOI] [PubMed] [Google Scholar]
- Chang Q., Liu Z., Ma W.Z., Hei C.C., Shen X.S., Qian X.J., Xu Z.L. Drug synergistic antifertility effect of combined administration of low-dose gossypol with steroid hormones in rats. Chinese Med. J. 2011;124(11):1678–1682. doi: 10.3760/cma.j.issn.0366-6999.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Chow S.L., Nagwekar J.B. Elucidation of the role of hydrophobic bonding in influencing intestinal absorption of model sulfonamides and revealing possible mechanism of drug absorption in rat model. J. Pharm. Sci. 1993;82(12):1221–1227. doi: 10.1002/jps.2600821208. [DOI] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2020. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- Dawes J., Gamble J.F., Greenwood R., Robbins P., Tobyn M. An investigation into the impact of magnesium stearate on powder feeding during roller compaction. Drug Dev. Ind. Pharm. 2012;38(1):111–122. doi: 10.3109/03639045.2011.594802. [DOI] [PubMed] [Google Scholar]
- Du Y., Mou Y., Liu J. Efficiency evaluation and safety monitoring of tailored rapid potassium supplementation strategy for fatal severe hypokalemia. Exp. Ther. Med. 2019;17(4):3222–3232. doi: 10.3892/etm.2019.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Sun Y., Yu M., Gao Y., Zou M., Cheng G. Development and evaluation of compression coating gastro-floating tablet of alfuzosin hydrochloride for zero-order controlled release. AAPS PharmSciTech. 2018;19(7):3277–3286. doi: 10.1208/s12249-018-1168-z. [DOI] [PubMed] [Google Scholar]
- Gong L., Yu M., Sun Y., Gao Y., An T., Zou M., Cheng G. Design and optimization of gastric floating sustained-release mini-tablets of alfuzosin hydrochloride based on a factorial design: in vitro/in vivo evaluation. Drug Dev. Ind. Pharm. 2018;44(12):1990–1999. doi: 10.1080/03639045.2018.1506473. [DOI] [PubMed] [Google Scholar]
- Gu Z.P., Mao B.Y., Wang Y.X., Zhang R.A., Tan Y.Z., Chen Z.X., Cao L., You G.D., Segal S.J. Low dose gossypol for male contraception. Asian J. Androl. 2000;2(4):283–287. [PubMed] [Google Scholar]
- Huang Y., Wei Y., Yang H., Pi C., Liu H., Ye Y., Zhao L. A 5-fluorouracil-loaded floating gastroretentive hollow microsphere: development, pharmacokinetic in rabbits, and biodistribution in tumor-bearing mice. Drug Des. Devel. Ther. 2016;10:997–1008. doi: 10.2147/DDDT.S97735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Coward L.C., Kerstner-Wood C.D., Cork R.L., Gorman G.S., Noker P.E., Kitada S., Pellecchia M., Reed J.C. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother. Pharmacol. 2008;61(1):63–73. doi: 10.1007/s00280-007-0446-3. [DOI] [PubMed] [Google Scholar]
- Kaleemullah M., Jiyauddin K., Thiban E., Rasha S., Al-Dhalli S., Budiasih S., Gamal O.E., Fadli A., Eddy Y. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage. Saudi Pharm. J. 2017;25(5):770–779. doi: 10.1016/j.jsps.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesarla R.S., Vora P.A., Sridhar B.K., Patel G., Omri A. Formulation and evaluation of floating tablet of H2-receptor antagonist. Drug Dev. Ind. Pharm. 2015;41(9):1499–1511. doi: 10.3109/03639045.2014.959969. [DOI] [PubMed] [Google Scholar]
- Keshmiri-Neghab H., Goliaei B. Therapeutic potential of gossypol: an overview. Pharm. Biol. 2014;52(1):124–128. doi: 10.3109/13880209.2013.832776. [DOI] [PubMed] [Google Scholar]
- Knoos P., Schulz C., Piculell L., Ludwig R., Gorton L., Wahlgren M. Quantifying the release of lactose from polymer matrix tablets with an amperometric biosensor utilizing cellobiose dehydrogenase. Int. J. Pharm. 2014;468(1–2):121–132. doi: 10.1016/j.ijpharm.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Kumar M., Sharma S., Lohiya N.K. Gossypol-induced hypokalemia and role of exogenous potassium salt supplementation when used as an antispermatogenic agent in male langur monkey. Contraception. 1997;56(4):251–256. doi: 10.1016/s0010-7824(97)00134-0. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang S.J., Zhang Q., Shao Y.X. Influence of three coccidiostats on the pharmacokinetics of florfenicol in rabbits. Exp. Anim. 2015;64(1):73–79. doi: 10.1538/expanim.14-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G.Z., Lyle, K.C.i. 1987. Clinical trial of gossypol as a male contraceptive drug. Part II. Hypokalemia study. Fertil. Steril. 48(3), 462-465. https://doi.org/10.1016/S0015-0282(16)59419-9. [DOI] [PubMed]
- Liu H., Gan C., Shi H., Qu K., Jing L., Lu M., Su B., Yu H., Yuan H., Chen J. Gastric floating pill enhances the bioavailability and drug efficacy of dihydromyricetin in vivo. J. Drug Deliv. Sci. Tec. 2021;61 doi: 10.1016/j.jddst.2020.102279. [DOI] [Google Scholar]
- Liu H., Marquez R.T., Wu X., Li K., Vadlamani S., Li S., Wang Y., Xu L., Wu D. A non-intrusive evaluation method for tumor-targeting characteristics of nanomedicines based on in vivo near-infrared fluorescence imaging. J. Mater. Chem. B. 2019;7(31):4751–4757. doi: 10.1039/c9tb00882a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang Y., Hu M., Wang S., Qu K., Shi X., Yuan H., Chen J., Qu X., Hu Y. Film-injection as a dosage form for etomidate: Enhancing the stability of nanomedicines using solid intermediate products. J. Drug Deliv. Sci. Tec. 2020;56 doi: 10.1016/j.jddst.2020.101541. [DOI] [Google Scholar]
- Liu H., Zhao W., Hu Q., Zhao L., Wei Y., Pi C., Yang Y., Yang X., Yuan H., Zhang Y. Gastric floating sustained-release tablet for dihydromyricetin: Development, characterization, and pharmacokinetics study. Saudi Pharm. J. 2019;27(7):1000–1008. doi: 10.1016/j.jsps.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdizadeh Barzoki Z., Emam-Djomeh Z., Mortazavian E., Akbar Moosavi-Movahedi A., Rafiee Tehrani M. Formulation, in vitro evaluation and kinetic analysis of chitosan-gelatin bilayer muco-adhesive buccal patches of insulin nanoparticles. J. Microencapsul. 2016;33(7):613–624. doi: 10.1080/02652048.2016.1234513. [DOI] [PubMed] [Google Scholar]
- Moghimipour E., Ameri A., Handali S. Absorption-enhancing effects of bile salts. Molecules. 2015;20(8):14451–14473. doi: 10.3390/molecules200814451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdev A., Jain D. Floating Drug Delivery Systems: An emerging trend for the treatment of peptic ulcer. Curr. Drug Deliv. 2019;16(10):874–886. doi: 10.2174/1567201816666191018163519. [DOI] [PubMed] [Google Scholar]
- Narasimhan B. Mathematical models describing polymer dissolution: consequences for drug delivery. Adv. Drug Deliv. Rev. 2001;48(2–3):195–210. doi: 10.1016/s0169-409x(01)00117-x. [DOI] [PubMed] [Google Scholar]
- Othman M.A., Abou-Donia M.B. Pharmacokinetic profile of (+/-)-gossypol in male Sprague-Dawley rats following single intravenous and oral and subchronic oral administration. Proc. Soc. Exp. Biol. Med. 1988;188(1):17–22. doi: 10.3181/00379727-188-42700. [DOI] [PubMed] [Google Scholar]
- Pahwa R., Bisht S., Kumar V., Kohli K. Recent advances in gastric floating drug delivery technology: a review. Curr. Drug Deliv. 2013;10(3):286–298. doi: 10.2174/1567201811310030005. [DOI] [PubMed] [Google Scholar]
- Park J.B., Park Y.J., Kang C.Y., Lee B.J. Modulation of microenvironmental pH and utilization of alkalizers in crystalline solid dispersion for enhanced solubility and stability of clarithromicin. Arch. Pharm. Res. 2015;38(5):839–848. doi: 10.1007/s12272-014-0471-9. [DOI] [PubMed] [Google Scholar]
- Paul S., Tajarobi P., Boissier C., Sun C.C. Tableting performance of various mannitol and lactose grades assessed by compaction simulation and chemometrical analysis. Int. J. Pharm. 2019;566:24–31. doi: 10.1016/j.ijpharm.2019.05.030. [DOI] [PubMed] [Google Scholar]
- Qin C., Wu M., Xu S., Wang X., Shi W., Dong Y., Yang L., He W., Han X., Yin L. Design and optimization of gastro-floating sustained-release tablet of pregabalin: In vitro and in vivo evaluation. Int. J. Pharm. 2018;545(1–2):37–44. doi: 10.1016/j.ijpharm.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Rezk A.I., Obiweluozor F.O., Choukrani G., Park C.H., Kim C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019;141:388–400. doi: 10.1016/j.ijbiomac.2019.09.013. [DOI] [PubMed] [Google Scholar]
- Siepmann F., Karrout Y., Gehrke M., Penz F.K., Siepmann J. Limited drug solubility can be decisive even for freely soluble drugs in highly swollen matrix tablets. Int. J. Pharm. 2017;526(1–2):280–290. doi: 10.1016/j.ijpharm.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Kim K.H. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J. Control. Release. 2000;63(3):235–259. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- Tolia G., Li S.K. Silicone adhesive matrix of verapamil hydrochloride to provide pH-independent sustained release. AAPS PharmSciTech. 2014;15(1):1–10. doi: 10.1208/s12249-013-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K., Chiang C., Kozak K.R., Kwon G.S. Examination of gossypol-pluronic micelles as potential radiosensitizers. AAPS J. 2015;17(6):1369–1375. doi: 10.1208/s12248-015-9809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poznak C., Seidman A.D., Reidenberg M.M., Moasser M.M., Sklarin N., Van Zee K., Borgen P., Gollub M., Bacotti D., Yao T.J. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res. Tr. 2001;66(3):239–248. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu Y., Zhang Y., Yasin A., Zhang L. Investigating stability and tautomerization of gossypol-A spectroscopy study. Molecules. 2019;24(7):1286. doi: 10.3390/molecules24071286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Wang J., Lei W., Li K., Wu D., Wang X. Pharmacokinetics, biodistribution, and bioavailability of gossypol-loaded Pluronic® F127 nanoparticles. J. Drug Deliv. Sci. Tec. 2018;45:388–396. doi: 10.1016/j.jddst.2018.04.002. [DOI] [Google Scholar]
- Wen N., Dong Y., Song R., Zhang W., Sun C., Zhuang X., Guan Y., Meng Q., Zhang Y. Zero-order release of gossypol improves its antifertility effect and reduces its side effects simultaneously. Biomacromolecules. 2018;19(6):1918–1925. doi: 10.1021/acs.biomac.7b01648. [DOI] [PubMed] [Google Scholar]
- Woolford L., Caraguel C.G.B., Taggart D.A., Lethbridge M., Strauss J., Andrews L., Sycamnias M., Boardman W.S.J. Serum biochemistry of free-ranging southern hairy-nosed wombats (Lasiorhinus latifrons) J. Zoo Wildl. Med. 2020;50(4):937–946. doi: 10.1638/2019-0001. [DOI] [PubMed] [Google Scholar]
- Yang Z.J., Ye W.S., Cui G.H., Guo Y., Xue S.P. Combined administration of low-dose gossypol acetic acid with desogestrel/mini-dose ethinylestradiol/testosterone undecanoate as an oral contraceptive for men. Contraception. 2004;70(3):203–211. doi: 10.1016/j.contraception.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Zbidah M., Lupescu A., Shaik N., Lang F. Gossypol-induced suicidal erythrocyte death. Toxicology. 2012;302(2–3):101–105. doi: 10.1016/j.tox.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Zeng A., Yuan B., Fu Q., Wang C., Zhao G. Influence of sodium dodecyl sulfate on swelling, erosion and release behavior of HPMC matrix tablets containing a poorly water-soluble drug. Pharm. Dev. Technol. 2009;14(5):499–505. doi: 10.1080/10837450902773592. http://doi.org/110.1080/10837450902773592. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhang H., Hu X., Bao S., Huang H. Synthesis and release studies of microalgal oil-containing microcapsules prepared by complex coacervation. Colloids Surf. B Biointerfaces. 2012;89:61–66. doi: 10.1016/j.colsurfb.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Zhou J., Li M., Jin W., Li X., Fan H., Zhang Y. Pharmacokinetic study on protocatechuic aldehyde and hydroxysafflor yellow A of Danhong Injection in rats with hyperlipidemia. Pharmacology. 2018;102(3–4):154–160. doi: 10.1159/000491020. [DOI] [PubMed] [Google Scholar]