Abstract
Aquaporins (AQPs) are highly conserved small transmembrane proteins, which are responsible for the water transport across the cell membrane. AQPs are abundantly expressed in numerous types of cells such as epithelial and endothelial cells. The expression of AQP-1, -3, -4, -5, -8 and -9 were found in the digestive system, where these six AQP isoforms serve essential roles including mediating the transmembrane water transport and regulating the secretion of gastrointestinal (GI) fluids, consequently facilitating the digestion and absorption of GI contents. In addition, the expression levels of AQPs are controlled by various factors, and AQPs can stimulate numerous signaling pathways; however, aberrant expression of AQPs in the GI tracts are associated with the initiation and development of numerous diseases. Thus, this review provides an overview of the expression and functions of AQPs in the digestive system.
Keywords: Aquaporins, Digestive system, Distribution, Function, Water transfer
Introduction
Aquaporins (AQPs) consist of six transmembrane helices and two non-transmembrane helices.1 AQPs were discovered by Agre et al2 on the membranes of human red blood cells with the molecular weight of 28 kDa, and they are responsible for the transmembrane water transport. AQPs usually form tetramers, with each monomer contains an independent channels to allow the transmembrane water transport (Fig. 1A); when water pass through these narrow pores, the selectivity of AQPs was determined by the conformation and electrostatic force, thus, AQPs serve essential roles in maintaining water balance.3 AQPs in mammalians are divided into two major groups according to their osmotic permeabilities (i) the ‘classical’ water-permeable aquaporins with 30–50% conserved sequences at the amino-acid level (Fig. 1B), including AQP-1, -4 and -5, and (ii) water- and glycerol-permeable homologs referred to as aquaglyceroporins or glycerol facilitation-like proteins (GLP) with highly conserved amino-acid sequences, including AQP-3 and -9, which can also allow glycerol and urea to pass through.4,5 This review emphasizes the differential distribution of AQP-1, -3, -4, -5, -8 and -9 in the gastrointestinal (GI) tract and the effects of numerous AQPs on digestive diseases.
Figure 1.
Structure of AQPs (A) The three-dimensional structure of AQPs (B) AQPs-dependent water transport.
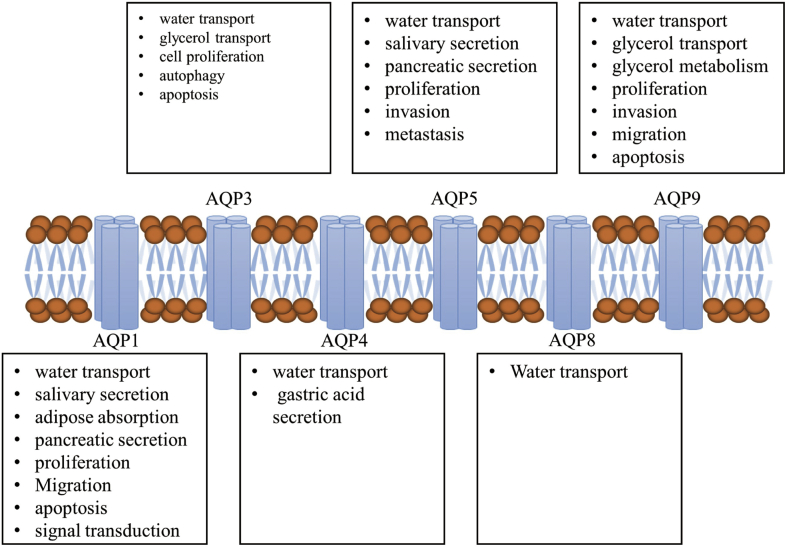
Distribution of AQPs in the digestive system
Tissue- and organ-specific expressions of AQPs are found in the digestive system (6). AQP-1, -8 and -9 are distributed in the salivary glands. AQP-1, -3, -4, -5 and -8 have been detected in the GI tract: AQP-1 is mainly expressed in the endothelial cells of the small blood vessels; AQP-3 and -4 are abundantly distributed in the basolateral membrane of epithelial cells in the GI tract, whereas AQP-5 is expressed in the apical membrane; AQP-7, -8, -10 and −11 are detected in the enterocytes in the intestines; AQP-1, -8 and -9 are abundantly expressed in the liver, bile duct and spleen.6 In summary, AQPs are differentially distributed in the digestive system (Fig. 2), where they exert numerous regulatory functions (Fig. 3).7
Figure 2.
Distribution of AQPs in the digestive system.
Figure 3.
Biological functions of AQPs in the digestive system.
The structure and distribution of AQP-1 in the digestive system
AQP-1 is a highly conserved membrane-integrated protein with a molecular weight of ∼28 kDa, and AQP-1 is located in 7p14; tetramers of AQPs can be found on the cell membrane, which consist of 269 amino acids. The main structure of AQP-1 is characterized by six α-helices transmembrane domains, which has been described in a hourglass model with each AQP-1 acts as an individual functional unit, consequently facilitating water to pass through the channels, which selectively allow water to be transported along the osmotic gradient.8 Furthermore, AQP-1 is also involved in the cell proliferation and migration, apoptosis and signal transduction. Previous studies have revealed that AQP-1 is widely distributed in the digestive system including salivary glands, stomach, liver, small and large intestine, bile duct and spleen, with abundant expression in the stomach, pylorus, duodenum and ascending colon.9,10 Expression of AQP-1 is detected in the epithelial cells, capillary endothelium and endothelial cells in the lymphatic vessel and GI tract. In addition, AQP-1 is predominantly expressed in the lobular and interlobular bile ducts, where it regulates the transmembrane water transport.11 In GI tract, AQP-1 is specifically detected in the endothelial cells such as the endothelium of capillary vessels in the ileum, but not in the epithelium or mucosa.
The association between AQP-1 and digestive diseases
Previous studies have demonstrated that aberrant expression or disrupted function of AQP-1 can affect the water transport, consequently resulting in numerous types of digestive diseases. The expression level of AQP-1 was downregulated by 38% in the labial gland of patients with Sjögren's syndrome, and AQP-1 expression was increased in the epithelial cells treated with rituximab monoclonal antibody, suggesting that AQP-1 could be involved in the secretion of saliva.12,13 AQP-1 is abundantly distributed in the digestive system and serves essential roles in the water transport between the mucosa of GI tract and blood. Additionally, AQP-1 is expressed in the endothelial cells of central lacteal in the small intestine, where it facilitates the formation of chylomicron, and impaired adipose absorption was detected in AQP-1 knockout mice, indicating that AQP-1 may be involved in the digestion of lipid.14 The regularory roles of AQP-1 in the digestive system as well as the underlying mechanisms require further investigation.
AQP-1 is widely distributed in the basolateral membrane of bile cyst, intrahepatic cholangiocyte, hepatic duct, hepatic endothelial barrier, pancreatic duct and centrocyte.15 A previous study revealed that the expression level of AQP-1 was significantly downregulated in the pancreatic tissue of rats with acute necrotizing pancreatitis.16 In addition, impaired secretion of pancreas was observed in the liver-X-receptor β knockout mice, and the AQP-1 expression in the epithelial cells of pancreatic ducts was also reduced in this model, suggesting that the levels of AQP-1 could be regulated by liver-X-receptor β.17 In comparison, upregulated expression of AQP-1 in the apical and lateral membranes of pancreatic ducts was detected in the patients with autoimmune pancreatitis. This variation could be due to the compensatory upregulation of AQP-1 stimulated by decreased secretion of the pancreatic juice.
Furthermore, previous studies demonstrated that the biological behaviour of cancer cells was closely associated with the transmembrane water transport, and the proliferation, migration and invasion of cancer cells relied on the rapid transport of water molecules across the membrane.18 Furthermore, AQP-1 was abundantly expressed in the entire GI system, which regulated the transmembrane transport of water and other small molecules and participate the cell proliferation and migration, consequently contributing to the initiation and development of numerous types of tumor.19
Compared with the loss-of function of AQP-1, cancer cells expressing AQP-1 exhibited migratory and invasive abilities, and the underlying molecular mechanisms require further investigation.20, 21, 22 Yamazato et al23 revealed that the expressed level of AQP-1 was increased in esophageal cancer, which could facilitate cell proliferation and migration by stimulating the death receptor-mediated signaling pathways and was associated with the prognosis of this disease. AQP-1 was broadly distributed in the endothelial cells of small blood vessels such as small intestines, and it was predominantly expressed in the stomach, indication that AQP-1 could be involved in the proliferation, differentiation and invasion of gastric adenocarcinoma cells. Upregulated expression of AQP-1 in cancer cells and blood vessels served essential roles on the development of gastric cancer.11 Quantitative polymerase chain reaction (qPCR) and immunohistochemistry analysis were performed on the tumor tissues from 109 patients with epithelial gastric neoplasms by Sun et al,24 and the results revealed that the mRNA and protein levels of AQP-1 were upregulated in epithelial gastric neoplasms. The elevated expression of AQP-1 was associated with the poorer prognosis and higher recurrence in the patients, and Cox's proportional hazards analysis suggested that AQP-1 is a potential prognostic biomarker of gastric cancer. In addition, knockout of AQP-1 in osteosarcoma cells could inhibit cell growth and induce of G1 phase cell cycle arrest, indicating that AQP-1 expression was involved in the initiation and development of tumor. Thus, AQP-1 could be a promising therapeutic target and prognostic marker in the treatment of cancer.
The structure and distribution of AQP-3 in the digestive system
AQP-3 gene is located in 9q13 and consists of four exons and three introns. AQP-3 protein contains 292 amino acids with a molecular weight of ∼32 kDa, and it can facilitate the transport of water and other small molecules.25 In the digestive system, AQP-3 is abundantly detected in the esophagus and colon, and selectively expressed in certain types of cells of the gastric antrum and mucosa. Tissue-specific expression of AQP-3 was found in the epithelial and goblet cells, and basolateral membrane of paneth cells in the small intestine.26 Immunohistochemistry study of rat GI tract tissues revealed that AQP-3 was also expressed in the basolateral membrane of mouth and perineum.27 However, the expression levels of AQP-3 in the aforementioned tissues still need to be evaluated and the regulatory functions of AQP-3 require further investigation.
The association between AQP-3 and digestive diseases
Colonic epithelial cells are able to absorb 1.5–2 L of water to maintain the osmotic gradient. Under the physiological and pathological conditions, colonic epithelial cells could secret water and electrolyte. The epithelial cells in colonic mucosa are tightly connected, and AQPs are involved in the transmembrane water transport. AQP-3 is abundantly expressed in the apical and lateral membranes of epithelial cells in the proximal colon, where it serves important roles in transmembrane water transport; it can assist the transfer of water and other small molecules from the lateral membranes to intercellular space and reduce the water level in colon.28 Thus, the expression level of AQP-3 in colonic mucosa is associated with the appearance of excrement.
A recently study reported that downregulated expression of AQP-3 was associated with numerous digestive diseases such as diarrhea; cholera toxin could reduce AQP-3-mediated water permeability in human small intestine and disrupt the water transport, subsequently resulting in the occurrence of diarrhea.29 Increased water content in the excrement or diarrhea was observed in mice treated with AQP-3 inhibitor. Furthermore, vasoactive intestinal peptide could regulate the water permeability and stimulate the secretion of water and electrolytes in the colon by altering the transmembrane AQPs levels via protein kinase A signalling pathway.30
AQP-3 is a target of the vasoactive intestinal peptide, and it can stimulate the secretion of water in the intestine and induce diarrhea by upregulating AQP-3.31 Additionally, the expression levels of AQP-3 are associated with constipation. Niu et al32 revealed that in rats treated with loperamide, which was used as a murine model of slow transit constipation, the excrement was extremely dry and the expression level of AQP-3 was increased in the colon. These results suggested that AQP-3 could stimulate the water absorption of colon and consequently resulted in the occurrence of constipation. Morphine is broadly used as an anti-inflammatory drug in clinic, with the side effects of reduced bowel movement and constipation. A recent study indicated that treatment with morphine could stimulate the expression of AQP-3.33 Thus, further investigation on the underlying mechanisms of AQP-3-mediated water transport may provide novel insight on the treatment of diarrhea and constipation.
A previous study revealed that dysregulated expression of AQP-3 served essential roles on the initiation and development of chronic gastritis, and the levels of AQP-3 were correlated with the severity and stage of this disease. In nitrobenzenesulfonic acid-induced colitis model, the expression levels of AQP-3 were reduced due to the increased inflammation and injury.27 Furthermore, it was reported that the absence of AQP-3 could stimulate the production of E. coli, disturb the integrity of intestinal barrier, which may be associated with the pathogenesis of inflammatory bowel diseases.34 AQP-3 is a member of the aquaglyceroporins family; it is involved in complex biological processes including cell proliferation, tumorgenesis, lipid metabolism and ATP synthesis by regulating the glycerol levels in keratinocytes, adipose and other tissues. A previous study demonstrated that AQP-3 served essential roles in the development of gastrointestinal tumors. Gao et al35 reported that knockdown of AQP-3 lead to elevated apoptosis and reduced proliferation in gastric cancer cells. Furthermore, downregulation of AQP-3 resulted in reduced autophagy, suggesting that AQP-3 knockdown may inhibit cell proliferation by suppressing autophagy in gastric cancer cells.
The structure and distribution of AQP-4 in the digestive system
AQP-4 is one of the most predominantly water permeable proteins in the AQPs family. Four monomers that are able to assist water transport are incorporated into a AQP-4 tetramer, with a molecular weight of ∼30 kDa. Human AQP-4 is located between 18q11.2 and q12.1 and contains four exons. AQP-4 protein contains two repeated membrane-spanning domains and two pore-forming loops with the signature Asn-Pro-Ala motif, which can overlap and form the pore to facilitate the transmembrane transport of a water molecule.36 AQP-4 is selectively expressed in the basolateral membrane of gastric epithelial cells and serves essential roles in the regulation of gastric acid secretion.37 In addition, AQP-4 is also distributed in the epithelial cells of intestinal crypt and colonic mucosa and the basolateral membrane of goblet cells, participating the transport of colonic fluid.
The association between AQP-4 and digestive diseases
AQP-4 was first detected in human stomach by Misaka et al in 1996. The secretion of gastric juice was not affected in AQP-4 knockout mice, which may be due to the compensatory overexpression of other AQPs caused by the absence of AQP-4.38 AQP-4 could serve important roles on the secretion of gastric juice by regulating the cell volume and maintaining the osmotic pressure. Thus, further investigation on the regulatory roles of AQP-4 in the secretion of gastric juice may provide novel insight on the discovery of novel gastric acid inhibitors. In addition, the water permeability was reduced in the proximal colon of AQP-4 knockout mice; however, water contents in the excrement remained unchanged. It has been reported that AQP-4 is able to facilitate water transport, increase the water permeability of cellular membrane and induce the migration of cancer cells, subsequently contributing to cell migration and invasion, tumor growth and angiogenesis.
The structure and distribution of AQP-5 in the digestive system
AQP-5 consists of 265 amino acids with a molecular weight of ∼28 kDa. Human AQP-5 gene is located in 12q13. AQP-5 retains the tetrameric structure, and each monomer is capable of facilitating transmembrane water transport independently. AQP-5 monomer consists of single peptide chain with intracellular N- and C-terminus, which contains six tandom transmembrane α-helices that are connected by five loops.39 AQP-5 was firstly isolated from the salivary glands and is abundantly expressed in numerous types of glands, including salivary and acrimal glands and pancreas; in the digestive system, AQP-5 is predominantly distributed in the stomach and duodenum.
The association between AQP-5 and digestive diseases
In the salivary gland, AQP-5 is expressed in the apical membrane of serous acinar cells, such as the intercellular secretory tubule of acinous cells.40 A previous study revealed that the secretion of saliva was notably reduced and the level of salivary hydrochloric acid was remarkably increased in AQP-5 knockout mice, suggesting that AQP-5 served essential roles in the regulation of saliva secretion.41 Furthermore, pilocarpine-stimulated secretion of saliva was reduced by 60% and the viscosity of the saliva was elevated in AQP-5 knockout mice.42 In rat lingual gland, AQP-5 participated in the regulation of acinar secretory granules.43 A recent study reported that AQP-5 expression in the saliva could be a promising diagnostic predictor of xerostomia-related diseases. Additionally, aberrant expression and impaired distribution of AQP-5, inflammatory infiltration and disrupted salivary gland vacuoles were observed in the salivary gland of patients and murine model with Sjögren's syndrome.44 Various factors including Toll-like receptors and TNF-α could affect the secretion of AQP-5 in salivary glands and xerostomia in the patients with Sjögren's syndrome.45 Thus, aberrant expression of AQP-5 could be involved in the pathogenesis of Sjögren's syndrome. Diabetes is also able to cause the xerostomia, the secretion of salivary gland was reduced in streptozotocin-stimulated type I diabetic mice; however, the levels of AQP-5 were not affected.46 The regulatory functions of AQP-5 in diabetes-induced xerostomia require further investigation.
In the pancreas, AQP-5 is distributed in the apical membrane of the centro-acinar and tubules cells, which may contribute to the occurrence of diabetes and pancreatitis.47 In the stomach, AQP-5 is expressed in the apical membrane of pyloric gland secretory cells, but not in the fundus gland; in the duodenum, AQP-5 is detected in the apical membrane of duodenal gland secretory cells. Furthermore, qPCR revealed that AQP-5 was not expressed in other tissues of the digestive system. AQP-5 could facilitate the rapid transmembrane water transport; AQP-5 also stimulated the migration of cancer cells and vascular endothelial cells, consequently lead to the invasion and metastasis of tumor, which was associated with the clinicopathological characteristics of patients with cancer.48 Additionally, Kang et al49 demonstrated that AQP-5 expression was increased in the patients with early stage colorectal cancer, and the upregulation of AQP-5 was more significant in the patients with advanced cancer and liver metastasis. Huang et al50 revealed that AQP-5 could induce the migratory and invasive abilities of gastric cancer cells, which may contribute to the initiation and development of gastric tumor, suggesting that AQP-5 was a potential therapeutic target in the treatment of this disease. Furthermore, overexpression of AQP-5 could induce the phosphorylation of retinoblastoma protein and activate Ras/ERK/Rb signaling pathway in colon cancer cells, subsequently contributing to the initiation and development of colonic carcinoma.
The structure and distribution of AQP-8 in the digestive system
AQP-8 was discovered in 1997 with the length of 1447 bp containing the coding region of 783 bp, encoding a protein with the molecular weight of ∼28 kDa that consists of 261 amino acids. There are six exons and five introns in AQP-8 gene, which is distinct from other members of the AQPs family.51 AQP-8 was first isolated from the pancreas, liver and testicle, and it transcripts are broadly distributed in the digestive system, including the salivary glands, small intestine, colon, pancreas and liver.
The association between AQP-8 and digestive diseases
In the digestive glands, AQP-8 is distributed in the epithelial cells of acinus and membrane duct. The levels of AQP-8 transcripts were increased in the salivary glands of AQP-8 knockout mice; however, the protein levels of AQP-8 and the secretion of saliva were not affected compared with the wild type. AQP-8 was predominantly expressed in the apical membrane of acinous cells in human and rat pancreas, where it is responsible for 90% of the water permeability;52 however, the pancreatic functions were not affected in AQP-8 knockout mice, which could be due to the compensatory secretion of membrane duct cells in the pancreas.
In the GI tract, AQP-8 was abundantly detected in the apical membrane of epithelial cells in the duodenum, jejunum and colon.53 AQP-8 facilitated the transport of water and small molecules on the apical membrane of rat small intestine; however, AQP-8 is rarely expressed in human proximal small intestine. Yang et al54 demonstrated that the appearance, growth, body weight, organ weight, urine concentrating ability, salivary gland secretion and intestinal fluid transport remained unchanged in AQP-8−/− mice compared with the wild type; however, the volume and weight of testicles and the metabolism after high-fat diet exhibited significant difference.
A previous study revealed that the effects of AQP-8 on the absorption and secretion in small intestine and colon were not remarkable. The secretion of cholera toxin was not affected in the small intestine of AQP-8 knock out mice; absence of AQP-8 exhibited no effects on the colonic absorption and water contents in the excrement. In the murine colonitis model, the expression level of AQP-8 was reversely correlated with the occurrence of inflammation and injury, suggesting that AQP-8 expression may be associated with the initiation and development of inflammatory bowel diseases.55
The structure and distribution of AQP-9 in the digestive system
AQP-9 is a type of glycoprotein with the molecular weight of ∼31 kDa that has been recently discovered. AQP-9 protein consists of four homologues monomers, and each monomer contains six transmembrane domains and 295 amino acids with one glycosylation site, three intracellular and four extracellular loops at the C- and N-terminus, respectively. AQP-9 gene contains five introns and six exons. It has been reported that, AQP-9 is expressed in the basolateral membrane of goblet cells in murine ileum, whereas in human digestive system, AQP-9 is distributed in the small intestine and liver, and is abundantly detected in the cytoplasmic surface of hepatocyte.
The association between AQP-9 and digestive diseases
AQP-9 is a member of the aquaglyceroporins family, which can assist the transmembrane transport of small molecules including water, glycerol and urea. AQP-9 was predominantly expressed in the basolacteral membrane of mammalian liver cells and served essential roles on the absorption of arsenite, whose accumulation could lead to damaged liver cells and hepatocellular carcinoma.56 A recent study revealed that upregulated expression of AQP-9 was detected in the patients with liver cancer, which may be associated with the poor prognosis; knockdown of AQP-9 induced the activation of PI3K/Akt and Caspase 3 signaling pathways, consequently suppressing the proliferation, migration and invasion and stimulating the apoptosis of cancer cells. Furthermore, the expression levels of AQP-9 in the basolacteral membrane of liver cells were reduced in extrahepatic cholestasis induced by bile duct ligation, indicating that AQP-9 could participate in the transport of bile.57 In addition, AQP-9 is involved in the metabolism of glycerol in the liver; glycerol is produced by triacylglycerols catabolism in the adipose tissue, and it enters the liver via portal vein and participates in glyconeogenesis.58 The expression levels of AQP-9 were increased after fasting and reduced following food intake; AQP-9 expression was closely correlated with the levels of glycerol kinase.59 In H4IIE cells, insulin was able to inhibit the expression of AQP-9 in a time- and dose-dependent manner. In addition, insulin could suppress the expression of AQP-9 by binding to the -496/-502 promoter region.60
Insulin negative regulatory element has been identified on the promoter of AQP-9. Additionally, the expression levels of AQP-9 were upregulated in the liver of insulin-resisteant mice despite of the hyperinsulinemia and high levels of plasma glycerol and blood sugar, indicating that AQP-9 could be involved in the metabolism of liver glycerol; AQP-9 levels were reduced in liver cells treated with insulin; the expression of AQP-9 was also affected by food intake; AQP-9−/− mice exhibited hypertriglyceridemia and high blood sugar compared with AQP-9+/− mice. AQP-9−/− mice were crossed with obese mice with type-II diabetes (Leprdb/Leprdb), and the blood sugar levels were reduced in Leprdb/Leprdb AQP9−/− compared with Leprdb/Leprdb AQP9+/− mice following 3 h of fasting; the amounts of plasma glycerol was decreased in AQP-9−/− mice compared with AQP-9+/- mice.56 In addition, treatment with streptozocin could stimulate the upregulation of plasma glycerol and liver AQP-9 and induce gluconeogenesis in the mice with insulin deficiency, suggesting that AQP-9 may serve essential roles on glucose metabolism and the glycerol uptake in the liver.
The discovery of aquaglyceroporins advanced our knowledge of associated physiological processes and pathogenesis of numerous diseases. Previous studies on the structures and functions of AQPs were performed, and the results revealed that AQPs assisted water and glycerol to transport across the membrane, which may help to understand the underlying mechanisms of metabolism. Furthermore, studies on AQPs-dependent glycerol metabolism may provide novel insight on the development of novel therapeutic strategies on the treatment of metabolism dysfunction; however, the association between AQPs expression and glycerol metabolism requires further investigation.
The roles of AQPs on water transport in the GI tract
Large amount of fluids are transported by the digestive system, and the epithelial cells of GI tract serve important roles on regulating the water and electrolytes balance.6 Water in the intestine is from food intake (∼2L/day) and the secretion of digestive juice (∼7L/day), which is absorbed by the small intestine and colon, and ∼100 mL was excreted every day. Intestinal epithelial cells allowed the fluids to pass through in both directions and participated in the biological processes including absorption and secretion. Following food intake, water and nutrient were absorbed in the upper GI tract, which induce the rapid osmotic balance of the intestinal contents and elevated gastrointestinal recirculation of the fluids. Water absorbed by the small and large intestine is isotonic and anti-osmotic, respectively; the fine-tuned balance is regulated by the neurotransmitter, gastrointestinal hormone and inflammatory factors, and AQPs are essential regulators of the fluid transport in GI tract.
AQPs facilitate the transmembrane water transport in the digestive system; investigation on the cellular and subcellular distribution of AQPs in the GI epithelium could provide novel insight on the diagnosis and treatment of digestive diseases. There are two pathways of AQPs-dependent water transport: (1) paracellular pathway that takes place in the intercellular space; (2) transcellular pathway, which is through the apical and basolateral membranes. These two pathways are not completely independent, lateral intercellular space is the barrier between them; water can only be transported by capillary blood vessels when the two pathways were entirely independent.4 Paracellular pathway has been considered as the main route of water transport and nutrient absorption; however, there is some limitation in paracellular water transport, especially in the epithelial cells of small intestine. There are three mechanisms of transcellular water transport via the apical and basolateral membranes: (1) passive diffusion by phospholipid bilayer; (2) co-transport with ion and nutrient; (3) AQPs-dependent diffusion.61 Inconsistent findings of AQPs were revealed in human and murine models; as numerous types of AQPs are often co-expressed, it is difficult to investigate the distribution and function of individual AQP in the GI tract. Gastric epithelial cells exhibited low permeability against water and small non-electrolyte and served protective roles when the cell volume suddenly increased; as the extracellular osmotic pressure changed, large amount of water pass through the gastric mucous to stimulate the secretion of gastric juice, consequently maintain the osmotic balance of the gastric contents.
Summary and prospects
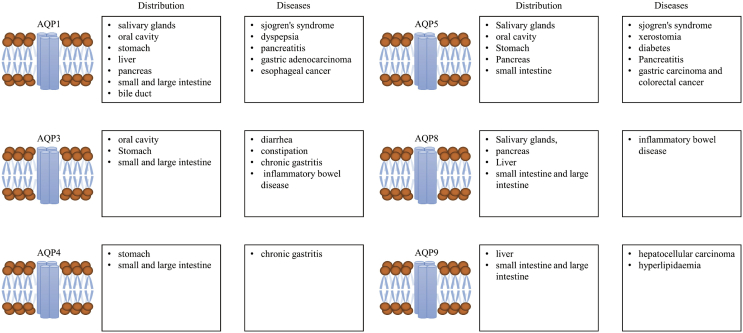
AQPs are widely expressed in the GI tract, and AQP-1, -3, -4, -5 and -9 are strongly associated with the initiation and development of various types of digestive diseases (Fig. 4). AQP-1 is involved in the digestion of adipose, secretion of saliva and is also associated with the pathogenesis of esophageal and gastric cancer. AQP3 is mainly responsible for the regulation of water transport in the digestive system, which is closely related to diarrhea, constipation, inflammatory bowel diseases and gastric tumor. AQP-4 is able to modulate the secretion of gastric juice and associated with the initiation and development of gastric cancer. AQP-5 can stimulate the secretion of saliva and is involved in the pathogenesis of diabetes, pancreatitis and colorectal cancer. AQP-8 can regulate the secretion of pancreatic juice and is associated with the development of inflammatory bowel diseases. AQP-9 facilitates the permeabilization of small molecules including water, glycerol and urea, and is involved in the lipid transport, insulin resistance, fatty liver, diabetes and liver cell carcinoma. The regulatory functions of AQPs in the digestive system remain unknown, investigation on the roles of AQPs could not only provide novel insights on the diagnosis and prognosis of digestive diseases, but also facilitate the development of potential therapeutic targets for the treatment of these diseases.
Figure 4.
AQPs-related diseases in the digestive system.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant number 81101827).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Lin Lv, Email: lin-miaomiao@cqmu.edu.cn.
Zhechuan Mei, Email: meizhechuan@cqmu.edu.cn.
References
- 1.Meli R., Pirozzi C., Pelagalli A. New perspectives on the potential role of aquaporins (AQPs) in the physiology of inflammation. Front Physiol. 2018;9 doi: 10.3389/fphys.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agre P., Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555(1):72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhu C., Ye J.L., Yang J. Differential expression of intestinal ion transporters and water channel aquaporins in young piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. 2017;95(12):5240–5252. doi: 10.2527/jas2017.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal R., Milatz S., Krug S.M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123(Pt 11):1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 5.Rojek A., Praetorius J., Frokiaer J. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 6.Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Asp Med. 2012;33(5–6):642–650. doi: 10.1016/j.mam.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Bottino C., Vazquez M., Devesa V. Impaired aquaporins expression in the gastrointestinal tract of rat after mercury exposure. J Appl Toxicol. 2016;36(1):113–120. doi: 10.1002/jat.3151. [DOI] [PubMed] [Google Scholar]
- 8.Benga G. The first discovered water channel protein, later called aquaporin 1: molecular characteristics, functions and medical implications. Mol Asp Med. 2012;33(5–6):518–534. doi: 10.1016/j.mam.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Pelagalli A., Squillacioti C., Mirabella N., Meli R. Aquaporins in health and disease: an overview focusing on the gut of different species. Int J Mol Sci. 2016;17(8) doi: 10.3390/ijms17081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu C., Chen Z., Jiang Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca A., Vassalotti G., Pelagalli A. Expression and localization of aquaporin-1 along the intestine of colostrum suckling buffalo calves. Anat Histol Embryol. 2015;44(5):391–400. doi: 10.1111/ahe.12157. [DOI] [PubMed] [Google Scholar]
- 12.Beroukas D., Goodfellow R., Hiscock J. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjogren's syndrome. Lab Investig. 2002;82(2):203–210. doi: 10.1038/labinvest.3780412. [DOI] [PubMed] [Google Scholar]
- 13.Ring T., Kallenbach M., Praetorius J. Successful treatment of a patient with primary Sjogren's syndrome with Rituximab. Clin Rheumatol. 2006;25(6):891–894. doi: 10.1007/s10067-005-0086-0. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki T., Tajika Y., Ablimit A. Aquaporins in the digestive system. Med Electron Microsc. 2004;37(2):71–80. doi: 10.1007/s00795-004-0246-3. [DOI] [PubMed] [Google Scholar]
- 15.Mobasheri A., Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol. 2004;286(3):C529–C537. doi: 10.1152/ajpcell.00408.2003. [DOI] [PubMed] [Google Scholar]
- 16.Feng D.X., Peng W., Chen Y.F. Down-regulation of aquaporin 1 in rats with experimental acute necrotizing pancreatitis. Pancreas. 2012;41(7):1092–1098. doi: 10.1097/MPA.0b013e318249938e. [DOI] [PubMed] [Google Scholar]
- 17.Gabbi C., Kim H.J., Hultenby K. Pancreatic exocrine insufficiency in LXRbeta-/- mice is associated with a reduction in aquaporin-1 expression. Proc Natl Acad Sci U S A. 2008;105(39):15052–15057. doi: 10.1073/pnas.0808097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribatti D., Ranieri G., Annese T. Aquaporins in cancer. Biochim Biophys Acta. 2014;1840(5):1550–1553. doi: 10.1016/j.bbagen.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Tan M., Shao C., Bishop J.A. Aquaporin-1 promoter hypermethylation is associated with improved prognosis in salivary gland adenoid cystic carcinoma. Otolaryngol Head Neck Surg. 2014;150(5):801–807. doi: 10.1177/0194599814521569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sreedharan S., Petros J.A., Master V.A. Aquaporin-1 protein levels elevated in fresh urine of renal cell carcinoma patients: potential use for screening and classification of incidental renal lesions. Dis Markers. 2014;2014 doi: 10.1155/2014/135649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X., Sun T., Yang M. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. BioMed Res Int. 2013;2013 doi: 10.1155/2013/206525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El H.N., Bankfalvi A., Herring A. Correlation of aquaporin-1 water channel protein expression with tumor angiogenesis in human astrocytoma. Anticancer Res. 2013;33(2):609–613. [PubMed] [Google Scholar]
- 23.Yamazato Y., Shiozaki A., Ichikawa D. Aquaporin 1 suppresses apoptosis and affects prognosis in esophageal squamous cell carcinoma. Oncotarget. 2018;9(52):29957–29974. doi: 10.18632/oncotarget.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun W.J., Hu D.H., Wu H. Expression of AQP1 was associated with apoptosis and survival of patients in gastric adenocarcinoma. Dig Surg. 2016;33(3):190–196. doi: 10.1159/000443843. [DOI] [PubMed] [Google Scholar]
- 25.Fujiyoshi Y. [Structure and function of water channels] Tanpakushitsu Kakusan Koso. 2005;50(10 Suppl):1278–1283. [PubMed] [Google Scholar]
- 26.Mobasheri A., Wray S., Marples D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J Mol Histol. 2005;36(1–2):1–14. doi: 10.1007/s10735-004-2633-4. [DOI] [PubMed] [Google Scholar]
- 27.Ikarashi N., Kon R., Sugiyama K. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int J Mol Sci. 2016;17(7) doi: 10.3390/ijms17071172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikarashi N., Kon R., Iizasa T. Inhibition of aquaporin-3 water channel in the colon induces diarrhea. Biol Pharm Bull. 2012;35(6):957–962. doi: 10.1248/bpb.35.957. [DOI] [PubMed] [Google Scholar]
- 29.Flach C.F., Qadri F., Bhuiyan T.R. Differential expression of intestinal membrane transporters in cholera patients. FEBS Lett. 2007;581(17):3183–3188. doi: 10.1016/j.febslet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Cooke H.J. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77–80. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 31.Itoh A., Tsujikawa T., Fujiyama Y. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol. 2003;18(2):203–210. doi: 10.1046/j.1440-1746.2003.02949.x. [DOI] [PubMed] [Google Scholar]
- 32.Niu T.H., Wu G.T., Chen Z.H. Expression of aquaporin-3 in colon mucosa of rats with slow transit constipation. J. gansu University of Chinese Medicine. 2017;34(2):7–10. [Google Scholar]
- 33.Dal Molin A., Mcmillan S.C., Zenerino F. Validity and reliability of the Italian constipation assessment scale. Int J Palliat Nurs. 2012;18(7):321–325. doi: 10.12968/ijpn.2012.18.7.321. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Xu Y., Chen Z. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett. 2011;585(19):3113–3119. doi: 10.1016/j.febslet.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 35.Gao L.B., Zhang Q., Chen L. Effects of inhibiting aquaporin-3 expression on the proliferation and apoptosis of gastric cancer cells. J of Wannan Medical College. 2017;36(6):516–520. [Google Scholar]
- 36.Kitchen P., Conner M.T., Bill R.M. Structural determinants of oligomerization of the aquaporin-4 channel. J Biol Chem. 2016;291(13):6858–6871. doi: 10.1074/jbc.M115.694729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuhara S., Matsuzaki J., Tsugawa H. Mucosal expression of aquaporin-4 in the stomach of histamine type 2 receptor knockout mice and Helicobacter pylori-infected mice. J Gastroenterol Hepatol. 2014;29(Suppl 4):53–59. doi: 10.1111/jgh.12771. [DOI] [PubMed] [Google Scholar]
- 38.Wang K.S., Komar A.R., Ma T. Gastric acid secretion in aquaporin-4 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2000;279(2):G448–G453. doi: 10.1152/ajpgi.2000.279.2.G448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Direito I., Madeira A., Brito M.A. Aquaporin-5: from structure to function and dysfunction in cancer. Cell Mol Life Sci. 2016;73(8):1623–1640. doi: 10.1007/s00018-016-2142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaki T., Ablimit A., Suzuki T. Changes of aquaporin 5-distribution during release and reaccumulation of secretory granules in isoproterenol-treated mouse parotid gland. J Electron Microsc. 2006;55(3):183–189. doi: 10.1093/jmicro/dfl023. [DOI] [PubMed] [Google Scholar]
- 41.Parvin M.N., Kurabuchi S., Murdiastuti K. Subcellular redistribution of AQP5 by vasoactive intestinal polypeptide in the Brunner's gland of the rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1283–G1291. doi: 10.1152/ajpgi.00030.2004. [DOI] [PubMed] [Google Scholar]
- 42.Krane C.M., Melvin J.E., Nguyen H.V. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276(26):23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 43.Matsuki M., Hashimoto S., Shimono M. Involvement of aquaporin-5 water channel in osmoregulation in parotid secretory granules. J Membr Biol. 2005;203(3):119–126. doi: 10.1007/s00232-005-0736-9. [DOI] [PubMed] [Google Scholar]
- 44.Soyfoo M.S., Konno A., Bolaky N. Link between inflammation and aquaporin-5 distribution in submandibular gland in Sjogren's syndrome? Oral Dis. 2012;18(6):568–574. doi: 10.1111/j.1601-0825.2012.01909.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu X., Ren C., Zhou H. Therapeutic effect of Zeng Ye decoction on primary Sjogren's syndrome via upregulation of aquaporin 1 and aquaporin 5 expression levels. Mol Med Rep. 2014;10(1):429–434. doi: 10.3892/mmr.2014.2208. [DOI] [PubMed] [Google Scholar]
- 46.Soyfoo M.S., Bolaky N., Depoortere I. Relationship between aquaporin-5 expression and saliva flow in streptozotocin-induced diabetic mice? Oral Dis. 2012;18(5):501–505. doi: 10.1111/j.1601-0825.2011.01902.x. [DOI] [PubMed] [Google Scholar]
- 47.Delporte C. Aquaporins in secretory glands and their role in Sjogren's syndrome. Handb Exp Pharmacol. 2009;190:185–201. doi: 10.1007/978-3-540-79885-9_9. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu H., Shiozaki A., Ichikawa D. The expression and role of Aquaporin 5 in esophageal squamous cell carcinoma. J Gastroenterol. 2014;49(4):655–666. doi: 10.1007/s00535-013-0827-9. [DOI] [PubMed] [Google Scholar]
- 49.Kang S.K., Chae Y.K., Woo J. Role of human aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 2008;173(2):518–525. doi: 10.2353/ajpath.2008.071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y.H., Zhou X.Y., Wang H.M. Aquaporin 5 promotes the proliferation and migration of human gastric carcinoma cells. Tumour Biol. 2013;34(3):1743–1751. doi: 10.1007/s13277-013-0712-4. [DOI] [PubMed] [Google Scholar]
- 51.Calvanese L., D'Auria G., Vangone A. Structural basis for mutations of human aquaporins associated to genetic diseases. Int J Mol Sci. 2018;19(6) doi: 10.3390/ijms19061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurley P.T., Ferguson C.J., Kwon T.H. Expression and immunolocalization of aquaporin water channels in rat exocrine pancreas. Am J Physiol Gastrointest Liver Physiol. 2001;280(4):G701–G709. doi: 10.1152/ajpgi.2001.280.4.G701. [DOI] [PubMed] [Google Scholar]
- 53.Zhao G.X., Dong P.P., Peng R. Expression, localization and possible functions of aquaporins 3 and 8 in rat digestive system. Biotech Histochem. 2016;91(4):269–276. doi: 10.3109/10520295.2016.1144079. [DOI] [PubMed] [Google Scholar]
- 54.Yang B., Song Y., Zhao D. Phenotype analysis of aquaporin-8 null mice. Am J Physiol Cell Physiol. 2005;288(5):C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- 55.Zhao G., Li J., Wang J. Aquaporin 3 and 8 are down-regulated in TNBS-induced rat colitis. Biochem Biophys Res Commun. 2014;443(1):161–166. doi: 10.1016/j.bbrc.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 56.Maeda N. Implications of aquaglyceroporins 7 and 9 in glycerol metabolism and metabolic syndrome. Mol Asp Med. 2012;33(5–6):665–675. doi: 10.1016/j.mam.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Calamita G., Ferri D., Gena P. Altered expression and distribution of aquaporin-9 in the liver of rat with obstructive extrahepatic cholestasis. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G682–G690. doi: 10.1152/ajpgi.90226.2008. [DOI] [PubMed] [Google Scholar]
- 58.Lebeck J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J Mol Endocrinol. 2014;52(2):R165–R178. doi: 10.1530/JME-13-0268. [DOI] [PubMed] [Google Scholar]
- 59.Kuriyama H., Shimomura I., Kishida K. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51(10):2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 60.Carbrey J.M., Gorelick-Feldman D.A., Kozono D. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci U S A. 2003;100(5):2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verkman A.S. Aquaporins at a glance. J Cell Sci. 2011;124(Pt 13):2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]