Abstract
Effective interventions and treatments for complex diseases have been implemented globally, however, coverage in Africa has been comparatively lower due to lack of capacity, clinical applicability and knowledge on the genetic contribution to disease and treatment. Currently, there is a scarcity of genetic data on African populations, which have enormous genetic diversity. Pharmacogenomics studies have the potential to revolutionise treatment of diseases, therefore, African populations are likely to benefit from these approaches to identify likely responders, reduce adverse side effects and optimise drug dosing. This review discusses clinical pharmacogenetics studies conducted in African populations, focusing on studies that examined drug response in complex diseases relevant to healthcare. Several pharmacogenetics associations have emerged from African studies, as have gaps in knowledge.
Keywords: Africa, communicable diseases, genetic variation, noncommunicable diseases, pharmacogenetics, pharmacogenomics, precision medicine
There is huge genetic, cultural and lifestyle diversity among African populations that influence susceptibility to disease, disease progression and response to medical treatment employed against disease [1]. This applies to communicable and noncommunicable diseases, of which African countries face a huge burden of both. The challenges of the modern day patient compounded by the complexities associated with the poly-pharmacy requires careful management of dosages for the most optimal patient benefits while at the same time reducing the incidence of drug-induced adverse effects [1]. Infectious and parasitic diseases are highly prevalent and well-researched across Africa, however, there is growing interest in the increasing burden of noncommunicable diseases as well. Though effective interventions for these diseases have been implemented globally. Coverage in Africa has been comparatively low due to a lack of data, research and knowledge on African populations as well as funding, capacity and clinical applicability in this region [1,2]. Investigating the genetic influence of disease development, progression and treatment is further complicated in African populations, due to their great ethnolinguistic and genetic diversity [1,3].
The implementation of precision medicine in various medical fields has seen a global increase in recent times. Precision medicine describes a treatment approach which considers a patient’s genetics, behaviour, environment and lifestyle [4]. Pharmacogenomics, a branch of precision medicine, studies the influence of genomic variations on drug processing and response [5]. More specifically, pharmacogenetics looks specifically at the impact of variations in a single or few genes on drug response using genetic, epigenetic and nutrigenetic approaches [5]. For simplicity, we use these two terms interchangeably to refer to both single or multiple gene investigations. Studies in these fields have the potential to revolutionise the manner in which diseases are treated, emphasising the importance of studying these factors in African populations [3].
The use of pharmacogenetics as a tool for evidence-based medication management is gaining acceptance beyond academic research settings, with many users – individual patients, health professionals and medical plans – expressing interest in using pharmacogenetics tests to predict the efficacy and potential side effects of drug prescriptions. Unfortunately, pharmacogenetics and pharmacogenomics research in Africa is lagging behind international standards [5]. However, a few studies have been conducted and their outcomes are highlighted in this review. To implement pharmacogenetics into clinical practice in Africa, sharing of information and infrastructure support must be made available to researchers across the continent. Pharmacogenetics implementation requires digital storage and secure, prompt accessibility of information to authorised users, often with pharmacogenetics data embedded as part of an electronic health record system.
In this review, we investigated the scope of pharmacogenetics studies that have been conducted in African populations, and documented the relevant genotype–phenotype associations, focusing particularly on those that examined drug response in disease phenotypes relevant to African healthcare. The role of African genetic diversity and the opportunities for pharmacogenetics researchers are thus highlighted and will enable discovery of novel genetic mechanisms and validation of established markers. The review aimed to identify important genomic markers which can facilitate and guide precision medicine or precision public health in Africa. In addition, the review aimed to identify the current gaps in clinical pharmacogenetics and genomics research across the continent.
Surveying the literature on pharmacogenetics studies
A systematic literature review was performed using several databases, including OvidMEDLINE, PubMed, Cochrane Library via Wiley Online Library, clinicaltrials.gov, Sciencedirect, Google Scholar, Web of Science, WHO International Clinical Trials Registry Platform (ICTRP), patentscope, ARIPO and conference abstracts/proceedings. Search Terms included a combination of keywords such as: genomics, GWAS, pharmacogenomics, -genetics, -kinetics and -dynamics, precision medicine, genes, mutations, African populations, Africa, personalised medicine, and medical subject headings including: cardiovascular disease (CVD), metabolic syndrome (MetS), diabetes, obesity, cancer, neoplasms, obesity, infectious disease, malaria, tuberculosis, HIV, HCV, depression, mental disorder, kidney disease, sickle cell disease (SCD) and RD. A total of 520 papers were retrieved. Thereafter, a meticulous two-step filtration process was performed by at least two investigators assigned to each disease section. First, the titles and abstracts were analysed to retain the pertinent manuscripts. Subsequently, the complete texts of the remaining manuscripts were analysed to extract the information of the relevant variants.
Following filtration, only the pharmacogenetics studies found to have been conducted on African populations and diaspora remained. Subsequently, a template was designed to unify the information obtained from the retained studies. Whenever applicable, collected characteristics from each study included: variant ID, affected allele, gene name, key effect, ethnic group, sample size, drug family, variant frequency, allele frequency and p-values. The completed template was the base for the data presented in the current review. These key results from the extracted studies included in this review are summarised in Supplementary File 1.
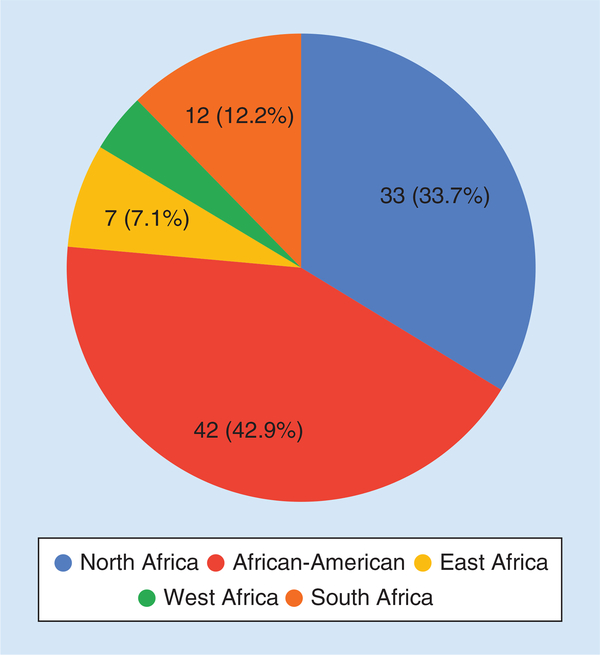
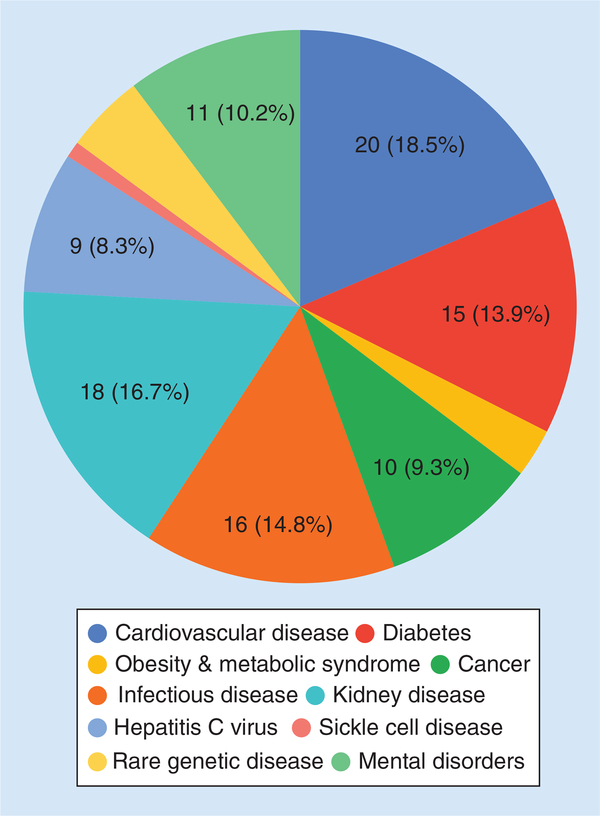
Within Africa specifically, the majority of studies were conducted in North Africa, followed by South, East and West Africa (Figure 1), while the most studied disease fields were CVDs, kidney diseases and infectious diseases (Figure 2). This review does not claim to be comprehensive as new studies are published regularly and some studies may have been missed, but it does represent a large proportion of published studies within the selected geographic regions and disease fields. Hereafter, summaries in each of the disease fields are provided, drugs and countries or populations where or in which the studies were conducted are highlighted in bold in each paragraph.
Figure 1.
Number of pharmacogenetics studies found to be conducted within various African regions.
Figure 2.
Number of pharmacogenetics studies found to be conducted within various disease fields.
CVDs
CVDs are defined as multi-factorial heart conditions and represent the leading cause of morbidity and mortality in both developed and developing countries [6,7]. CVDs are complex disorders, influenced by both genetic and environmental factors [7,8]. Precision medicine is not commonly employed for CVD management; however, 20 CVD-related pharmacogenetics studies were found to have been conducted on African populations.
The majority of these studies focused on warfarin, an anticoagulant that is commonly employed to treat CVD-related conditions. Response to warfarin has been associated with variation in two genes, CYP2C9 and VKORC1 [9]. Five studies conducted in Egypt were found. Exploring genetic variation in the genes encoding VKORC1, CYP3C9, CYP4F2, APOE and CALU, Shahin et al. discovered associations between reduced warfarin dose and genetic variants in VKORC1 (rs9923231), CYP2C9 (rs1799853, rs1057910) and APOE (rs429358, rs7412) [10]. These results were replicated in a separate Egyptian cohort by Bazan et al., who also found associations between rs9923231 (VKORC1), rs1799853 and rs1057910 (CYP2C9) and reduced warfarin dose [11]. In contrast, rs61742245, another variant in VKORC1, has been associated with higher warfarin dose requirements in Egyptian individuals [12]. Interestingly, when examined separately, genetic variants in ABCB1 (rs1045642), EPHX1 (rs2234922), and PZ (rs2273971) were not associated with warfarin dose, but when combined, these variants were found to influence warfarin dose requirements in the same population [13].
Few CVD pharmacogenetics studies were extracted from the East and South African regions. In Ethiopia, CYP2C9 haplotypes *1, *2 and *3 (encoded by rs1799853 and rs1057910) had variable frequency, at 94, 4 and 2%, respectively [14]. The effect of several CYP2C9 haplotypes (*2, *3, *5, *6, *8, *9, and *11), 20 VKORC1 polymorphisms and clinical covariates were comprehensively assessed in Sudanese patients treated with warfarin [15]. Patients with CYP2C9*2,*5,*6 or *11 haplotypes required reduced daily warfarin dose compared with CYP2C9*1/*1 homozygotes [15]. In contrast to Egyptian studies, no association was observed between rs61742245 in VKORC1 and warfarin dose in Sudanese individuals [11]. Similarly, rs61742245 was not associated with warfarin dose in a Kenyan cohort either [12]. Interestingly, the variant was not detected in the Ghanian cohort studied [12]. More recently, rs9923231 (VKORC1) has been associated with warfarin dose in Ghanians, with GA heterozygotes requiring higher warfarin dose compared with GG homozygotes [16]. In the same study, CYP2C9*2 and *3 haplotypes were not detected [16]. In South Africa, Mitchell et al. genotyped CYP2C9 and VKORC1 in black South Africans, observing 26 novel and 7 known CYP2C9 variants, as well as three known VKORC1 variants [17]. They demonstrated associations between both CYP2C9 (*8, g.16179 and g.46028) and VKORC1 variation (rs7200749 and rs7294) and warfarin dosage [17]. These, along with a small subset of environmental factors, explained 45% of warfarin dosage variability in the studied population [17].
The CVD pharmacogenetics has been widely investigated in African-American populations. Genetic variation in CYP2C9, VKORC1, CYP4F2 and APOE has previously been investigated, with observed associations between CYP2C9 haplotypes (*2, *3, *5, *6 *8, *11) and weekly warfarin dose [18]. The combination of CYP2C9 variants, VKORC1 rs9923231 and clinical variables explained 36% of inter-patient variability in warfarin dose requirements in the same study [18]. Additionally, rs339097 in CALU has previously been associated with increased warfarin dose in two replication cohorts [19] while novel polymorphisms in VKORC1 (rs61162043) and CYP2C9 (rs7089580) have also been associated with increased warfarin dose [19]. Moreover, polymorphisms in GGCX (rs10654848) and CYP2C19 (rs4244285) have been associated with higher warfarin dose requirements [20] and response to clopidogrel therapy [21], respectively. In addition, a genome-wide association study in African-Americans revealed an association between a novel SNP (rs12777823) in the CYP2C9 and warfarin dose [22].
Clopidogrel is an antiplatelet medication, prescribed to reduce risk of heart disease and stroke. Khalil et al. investigated the association between genetic variation in CYP2C19, ABCB1 and CES1 and clopidogrel response in Egyptian patients with acute coronary syndrome and/or percutaneous coronary intervention [23]. They identified CYP2C19 variants, age and body mass index as potential predictors associated with variable clopidogrel response [23]. More recently, rs2046934, in P2Y12, was associated with clopidogrel response in Moroccan patients with acute coronary syndrome [24]. In Tunisia, Charfi et al. investigated the association between the occurrence of adverse cardiac events in patients receiving clopidogrel treatment and CYP2C9*2, however no significant association was observed [25].
Two additional CVD drugs previously investigated are acenocoumarol and rosuvastatin. In Morocco, rs1799853 and rs1057910 (CYP2C9), as well as rs9923231 (VKORC1), have been associated with weekly acenocoumarol dose, while rs2108622 (CYP4F2) was not [26]. Majority of the patients with these variant genotypes were found to belong to a low acenocoumarol dose group [27]. In Tunisia, Ajmi et al. demonstrated an association between daily acenocoumarol dose and CYP2C9 (*2, *3) and VKORC1 (H1, H7) haplotypes [28].
In Zimbabwe, Soko et al. screened 785 individuals from nine ethnic African populations, discovering associations between rosuvastatin exposure and genetic variation in several genes, including the genes encoding SLCO1B1, ABCC2, SLC10A2, ABCB11, AHR, HNF4A, RXRA and FOXA3 [29]. Interestingly, interindividual differences in rosuvastatin pharmacokinetics appeared to be driven by a different set of variants [29].
Obesity & MetS
MetS is a cluster of multiple metabolic abnormalities that increase risk to CVDs and Type 2 Diabetes (T2D), including obesity, hypertension, dyslipidemia and insulin resistance [30]. The prevalence of these abnormalities in Africa has rapidly increased in the last decade [30]. Three MetS pharmacogenetics studies were extracted from North Africa.
In Egypt, El Sayed et al. associated promoter methylation in the genes encoding LEP and MMP2 with folic acid supplementation [31]. Obese children exhibited hypomethylation in this region prior to supplementation compared with post supplementation [32]. In Tunisia, Jmel et al. characterised the genetic variability of pharmacogenes previously shown to be involved in MetS drug response [33]. A total of 1056 variants on 24 pharmacogenes were identified in the Tunisian population, while several polymorphisms were associated with anticoagulant sensitivity, including rs3846662 (HMGGR) rs1045642 (ABCB1), rs7294 (VKORC1) and rs12255372 (TCF7L2) [34]. Additionally, rs776746 (CYP3A5) was also associated with hypolipidemic susceptibility, and rs729 (VKORC1) has been associated with warfarin dosage [33,34].
Diabetes
Diabetes mellitus represents a group of metabolic diseases characterized by abnormal, deficient or inadequate insulin secretion and/or action, resulting in chronic hyperglycemia [35]. Diabetes incidence in sub-Saharan Africa is rapidly rising, ranging from 1 to 15%, however no studies from this region were found [36–38]. Two drugs have been the primary focus of Diabetes pharmacogenetics studies, sulfonylureas and metformin. In the only study extracted from an African country, El-Sisi et al. associated genetic variation in IRS-1 and KCNJ11 (rs5219) with Sulfonylureas efficacy in an Egyptian cohort [39].
Several studies containing African-American individuals, explored the interaction between candidate genes and metformin as treatment or therapeutic intervention for T2D. The A allele of rs12943590 (SLC47A2) has previously been associated with increased renal and secretory clearance of metformin [40] as well as reduced metformin response in African-American T2D patients [41]. Similarly, SLC47A2 variation (rs2252281 and rs12943590) have also been associated with metformin efficacy and metabolism [40]. In addition, multiple variants in SP1 (rs784892, rs2683511, rs10747673 and rs784888) have been associated with metformin efficacy and metabolism, while rs149711321 (PPARA) has been associated with altered metformin response in T2D patients [42]. Moreover, renal clearance of metformin has been shown to be significantly greater in healthy African-American volunteers heterozygous for rs316019 (OCT2) than those homozygous for the reference allele [43]. Several additional candidate genes have been either nominally associated or not associated with metformin intervention in an African-American cohort included in the Diabetes Prevention Program [32,44–50]. These include the genes encoding SLC22A2, HNF1B, ABCC8, ENPP1, TCF7L2, WFS1, ATM, SLC30A8, PPARG, and more.
Cancer
The molecular landscape of cancer differs by geographical location and genetic ancestry; African-American individuals have been found to have 25% higher cancer mortality rates than Caucasian Americans [51]. A couple of African studies have been performed to link genetic data with response to drugs used for pain in cancer patients. Unlike previous studies conducted on Caucasian and Chinese populations, no significant associations were found between polymorphisms in OPRM1 (rs17174629, rs1799972 and rs1799971) and COMT (rs4680), and opioid treatment for pain in Tunisian cancer patients [52]. A study In Ethiopia revealed that a high proportion of the population are rapid codeine metabolisers due to CYP2D6 polymorphisms, resulting in rapid conversion of codeine to morphine and subsequent therapeutic overdoses [53].
There are a few African pharmacogenetics studies on chemotherapy drugs, with varying results observed. For example, in Tunisia, resistance to anthracycline-based chemotherapy was found to not be associated with variation in either genes encoding MDM2 (rs1196333) or TP53 (rs1042522) variation [54]. In African-Americans, carriers of DPYD variants have been shown to be predisposed to hematologic toxicities when treated with 5-fluorouracil compared with Caucasian-Americans, while Caucasian-Americans are more likely to suffer from diarrhea, nausea, vomiting and mucositis compared with African-Americans [55].
Several African studies have explored the pharmacogenetics of chronic myeloid leukemia (CML) treatment. In Tunisia, Ben Hassine et al. reported no significant association between imatinib therapy and ABCB1 in CML patients, neither at genetic variant nor transcriptional level [56]. In contrast, ABCB1 (rs1045642) has been associated with lower through plasma concentration of imatinib in Nigerian CML patients [57]. In Egypt, though no association was observed between SLCO1B3 and imatinib response, a CYP3A5 haplotype (*3) was associated with treatment failure [58]. In another Egyptian study, rs2032582 (in ABCB1) was associated with imatinib sensitivity and resistance [59]. Within the same gene, a haplotype was identified associated with lower probability of achieving optimal therapeutic response [60]. Interestingly, OCT1 expression has been suggested as a clinical biomarker for imatinib response, as the gene was significantly downregulated in Tunisian samples from the imatinib-resistant group compared with the imatinib-responder group [61]. In additiion, only one African study focused on breast cancer therapy; Abdeljaoued et al. showed that male Tunisian breast cancer patients with high FOXM1 expression exhibited significantly lower response rates to chemo- and hormone therapy than those with low FOXM1 expression [62].
Infectious diseases
Africa has a disproportionate burden of infectious disease, with the major killers being malaria, tuberculosis (TB) and HIV/AIDS. As an example, the continent has the highest proportion of individuals exposed to Plasmodium sp., with 81.7% of registered malaria cases and 92.6% of deaths in the world [63]. Yet only one study on malaria treatment study was found. In this study, genotyping was used to distinguish recrudescence from new malaria infection in Uganda. Efficacy, safety and risk of recurrent parasitemia was compared between artemether-lumefantrine treatment and dihydroartemisinin-piperaquine alternative therapy. Alternative therapy was described as highly efficacious and incorporated in the national antimalarial treatment policy [64].
Increased focus has been placed on the pharmacogenetics of TB and HIV. TB affects over 10 million people worldwide [65], with poor outcomes exacerbated by co-infection with HIV. HIV/AIDS remains a serious global health concern, with over 25.6 million cases in sub-Saharan Africa alone [66,67]. Efavirenz is commonly employed as HIV anti-retroviral, however, a genetic variant in CYP2B6 causes efavirenz to be metabolised at reduced rates. It has been estimated that up to 50% of individuals of African descent infected with HIV have this genetic variant [4]. Currently South Africa has the highest number of patients on antiretroviral therapy (ART) and thus, multiple studies have been conducted on ART’s efficacy. A recent study characterising HIV-infected children for CYP2B6 polymorphisms, identified a T-G-T haplotype which predicts efavirenz plasma concentration in black South African children [68]. Similarly, polymorphisms in ABCB1 (rs2032582 and rs1128503) have also been linked to efavirenz concentration [69]. These polymorphisms are also contained in an ABCB1 haplotype (T-G-T-A) associated with increased plasma efavirenz levels [69]. In Botswana, Gross et al. identified associations between CYP2B6 variants (rs3745274 and rs28399499) and efavirenz-based treatment outcomes among HIV-infected patients [70]. They identified slow metabolism alleles which were associated with reduced clearance but not with the treatment end points [70]. A study in Congo also investigated the distribution of rs3745274 genotypes in patients receiving efavirenz treatment. The CYP2B6*GG (rapid metabolizer) genotype was observed in 17% of Congolese individuals, while GT (intermediate metabolizer) and TT (poor metabolizers) were observed in 55 and 28% of individuals, respectively [71]. Recently the Clinical Pharmacogenetics Implementation Consortium published a comprehensive review on CYP2B6, providing dosing guidelines for different age groups based on genotype [72].
While studies in HIV have focused primarily on efavirenz pharmacogenetics [73,74], those in TB have looked mostly at isoniazid and rifampin. Ben Mahmoud et al. investigated the association between NAT2 haplotypes and antituberculosis hepatotoxicity induced by isoniazid in Tunisian patients [75]. They discovered the existence of slow acetylation profiles in southern Tunisia, exhibiting higher incidence of isoniazid-induced hepatotoxicity, while fast acetylation profiles were associated with treatment failure. These results were replicated In another Tunisian cohort, where NAT2 (*5,*6, *4,*12 and *7) haplotypes and CYP2E1 (rs2031920, rs3813867 and rs6413432) were associated with isoniazid treatment response [76]. In West Africa, Dompreh et al. examined the relationship between genetic variation in NAT2 and SLCO1B1, and isoniazid and rifampicin pharmacokinetics, respectively, in Ghanaian children with TB [77]. They discovered that NAT2 and SLCO1B1 genotyping had minimal clinical utility due to NAT2’s modest effect and the rarity of the SLCO1B1 polymorphisms in the population [77]. In east Africa, Weiner et al. discovered that rs11045819 in SLCO1B1 is associated with lower rifampin exposure in TB patients, while Chigutsa et al., reported that the rs4149032 (in the same gene) is common and also associated with low-level rifampicin exposure in TB patients from southern Africa [73,78]. However, these results were not replicated in Malawi [78].
With the frequency of TB-HIV co-infection, there is potential for interaction between TB therapies and ART. This is evidenced by the discovery of a modest decrease in mean efavirenz plasma exposure with rifampin co-administration in healthy African-American and Caucasion volunteers [79]. NAT2 genotypes have been associated with isoniazid hepatotoxicity, but also with fast, intermediate and slow acetylation of efavirenz in South Africa [79]. In Zimbabwe, a CYP2B6 haplotype (*18) was associated with reduced efavirenz clearance and elevated plasma concentration [80]. Similarly, the high frequency of this haplotype was associated with decreased metabolism of efavirenz in South African co-infected patients [81].
HCV is another major infectious disease with over 200 million cases worldwide [82]. HCV genotypes are widely distributed by region and ethnicity for example, HCV genotypes 1, 2, 3 and 4 are frequent in North Africa and the Middle-East [83], while HCV genotype 5 is prevalent in southern Africa [80]. Standard treatment for HCV consists of a combination of pegylated interferon (PEG-IFN) α and ribavirin (RBV) [84]. An association between HLA-A1 and susceptibility to viral clearance (SVR) following PEG-IFN/RBV therapy was previously reported in Egyptian patients [85]. Similarly, IL28B (rs12979860 and rs8099917) variants have also been associated with SVR rate following PEG-IFN/RBV therapy [86]. Derbala et al. also investigated the impact of IL28B polymorphisms (rs12979860, rs8099917 and rs11881222) in response to treatment in Egyptian patients with genotype 4 [87], suggesting them as pre-treatment biomarkers. Fathy et al. reported a 46% treatment response value for IL28B rs8099917 in predicting SVR among HCV-infected Egyptian patients treated with PEG-IFN/RBV [88]. In sub-Saharan Africa, an association between treatment response and rs12979860 was observed in HCV genotype 4 infected patients [89]. They showed that the treatment response rates among the different ethnic groups (Egyptian, European and sub-Saharan Africa) were 81.8, 46.5 and 29.4%, respectively [89].
In African-American samples, McCarthy et al. reported rs12979860 (IL28B) as the strongest PEG-IFN SVR pre-treatment predictor in HCV-infected patients [90]. Notably, Thomas et al. found that African-American patients displayed lower SVR rates than Caucasian patients despite having the same IL28B genotype [91]. Pagliaccetti and Robek further explained that IL28B polymorphisms (rs8099917 and rs12979860) are strongly associated with HCV clearance, noting that genotypes associated with poor response to therapy are found at higher frequency in African populations compared with European populations [92].
Kidney diseases
Kidney diseases are chronic or acute, involving damage to or disease of a kidney [93]. Majority of the pharmacogenetics studies have focused on drugs employed in kidney transplant procedures, such as cyclosporine. In Egypt, the rs4646437 (in CYP3A4) has been associated with cyclosporine therapy in individuals receiving kidney transplantation [94]. Similarly, rs2032582 (in ABCB1) has been associated with altered cyclosporine dosage requirements [95]. Notably, the variant was not associated with risk of transplant rejection during cyclosporine therapy [95].
Another drug used following kidney transplants is tacrolimus, an immunosuppressant. In Morocco, the rs776746 (in CYP3A5) has been associated with altered tacrolimus dose requirements in individuals receiving kidney transplantation compared with those with genotype CT [96]. Similarly, CYP3A5 haplotypes have been associated with decreased trough and dose-adjusted trough concentrations of tacrolimus in Tunisians receiving kidney transplantation [95].
Haplotypes in CYP3A4 and CYP3A5 have been extensively explored with regards to tacrolimus therapy in the USA. These include CYP3A5 haplotypes (*1, *3A, *6 and *7) which have been associated with decreased tacrolimus clearance, increased doses of tacrolimus, increased risk of transplant rejection and delayed graft function, and increased glomerular filtration rate in African-Americans receiving kidney transplantations [97–99]. Notably, these, along with an ABCB1 variant (rs1045642) were not associated with drug toxicity or concentration [97]. Notably, though several CYP3A4 and CYP3A5 variants have been investigated with regards to clinical outcomes of individuals receiving kidney transplants, no associations were observed (rs2740574, rs2246709 and rs776746) [6]. Two CYP3A4 variants (rs2246709 and rs2740574) have been associated with amlodipine efficacy in African-Americans with hypertensive renal disease [100]. Similarly, an ADRB2 variant (rs2053044) has been associated with ramipril efficacy in African-Americans with hypertensive renal disease [101].
Several additional studies, investigating tacrolimus therapy in kidney transplantations included African-American samples, however these numbers were either not defined or low compared with more focused investigations [101–104]. Two mixed ethnicity studies with African-American individuals associated rs776746 (in CYP3A5) with tacrolimus trough concentrations [101], and tacrolimus dose [102,103] in individuals receiving kidney transplantations. An additional mixed ethnicity study conducted by Pulk et al., associated CYP3A5 haplotypes with tacrolimus trough concentrations in kidney transplant recipients [104].
SCD
SCD is the most common recessive single gene disorder in the world, affecting the structure and function of hemoglobin [105]. Hydroxyurea is the only US FDA approved SCD treatment, however response has been associated with genetic variation [105]. Only one pharmacogenomics study was identified in Africa; in Egypt, no associations were found between genes encoding GSTM1, GSTT1 and GSTP1, and hydroxyurea treatment [106].
Rare genetic diseases
Rare diseases (RD) are life-threatening or chronically debilitating heterogeneous diseases which are of such low prevalence that there is often a lack of available knowledge and drugs developed to treat these disorders, and special combined efforts are required to address them [107]. RD of genetic origin constitute a serious health burden in developing countries, and little is known regarding their spectrum in African populations. In Tunisia, improved cochlear implant outcomes have been observed in deaf individuals for whom the aetiology of hearing loss is related to GJB2) mutations and who were implanted at an early age [108]. Similarly, primary hyperoxaluria type 1 patients that present with the Maghrebian founder mutation p.I244T have been shown to be pyridoxine non-responsive and therefore, the only therapeutic strategy is combined liver and kidney transplantation [109,110]. Lumacaftor, used for cystic fibrosis (CF) treatment, has also shown potential treatment benefit in African-American CF patients. For an African-American CF patient, with a variant in the gene encoding CFTR, Zhang et al. tested response in vitro [111]. The variant resulted in a significant increase in total CFTR protein expression and channel function [108]. Interestingly, in another African-American CF patient, CFTR-expressing cells also responded positively to the in vitro addition of lumacaftor [112].
Mental disorders
Mental disorders are characterised by behavioural or mental patterns that cause significant distress or impairment of personal functioning. In the absence of change in current prevalence rates, estimates suggest that sub-Saharan Africa will experience an increase in the burden of mental and substance use disorders of approximately 130% in the future [113].
Schizophrenia
Schizophrenia is a severe mental disorder which may lead to delusions, hallucinations and loss of reality, and is generally treated with anti-psychotics. No pharmacogenomics studies in this context were found in North, East and West Africa. The only study found was conducted in South African schizophrenia patients [114]. A frameshift variant, rs11368509, in UPP2 conferred to improved response to anti-psychotics in mixed ancestry and Xhosa populations [114].
A number of additional schizophrenia pharmacogenetics studies have been conducted in African-American individuals. A study assessing genetic variants in treatment-intolerant schizophrenia patients found that three DRD2 variants were associated with improved response to clozapine in African-American individuals [115]. Similarly, two variants (rs909706 and rs742105) in DTNBP1 have also been linked to clozapine and haloperidol response in a mixed cohort of schizophrenia patients [116]. Similar associations were observed between rs165599 (in COMT), rs724226 (in GRM3) and improved risperidone response in African-Americans compared with European populations [117].
Although individuals of African ancestry have exhibited improved response based on genetic factors, studies have indicated that these populations are at greater risk of antipsychotic-induced weight gain. Genetic variation in CNR1 (rs1049353) has been associated with higher percent weight gain in African-American patients, compared with European-Americans [118]. Other marginal findings include variants in PTPRD [119], IL1B [120] and INSIG2 [121].
Major depressive disorder
Major depressive disorder is a mental health disorder characterized by persistently depressed mood or loss of interest in activities, causing significant impairment in daily life [122]. No pharmacogenomics studies in this context were extracted from Africa. However, a recent review highlighted eight genes implicated in antidepressant treatment response in mixed populations (including African-Americans). These included CYP2D6, CYP2C19, SLC6A4, ABCB1 (rs2032583 and rs2235015), FKBP5 (rs1360780, rs3800373 and rs4713916); GNB3 (rs5443); BDNF (rs6265); and HTR2A (rs7997012 and rs6313) [122]. Another study used genome-wide single nucleotide polymorphism data to examine independent contributions of race and genetic ancestry to antidepressant response [123]. Genetic African ancestry predicted lower treatment response in all models [123]. Finally, rs10473984 in CRHBP has been associated with both remission and reduction in depressive symptoms in response to citalopram, African-American [124]. This association particularly pronounced in patients with features of anxious depression [124].
Discussion & future perspective
In recent times, precision medicine and pharmacogenomics have become cornerstones of healthcare in some developed countries, and an important avenue of research to improve the patient’s treatment and management. In this review, relevant pharmacogenetics studies on individuals of African ancestry were retrieved, however, a relative lack of information and data for many African populations were also revealed. Some regions are better studied than others, as are some disease treatments. Though this review does not claim to be comprehensive, several key points have emerged.
First, it is evident that studies on genetic associations with drug response in African populations are less abundant than for other populations, which may be due to limited funding for such studies in the generally more poorly resourced institutions in Africa, as well as overall lack of capacity. Of the >300 medicines with FDA pharmacogenetic product label information [125], and 100 medicines (small molecules) with clinical pharmacogenetics guidelines [126], this review shows that only 15 (warfarin, clopidogrel, acenocoumarol, rosuvastatin, anthracycline, codeine, 5-fluorouracil, imatinib, efavirenz, isoniazid, tacrolimus, clozapine, risperidone, haloperidol and citalopram) have been studied in African populations, and each, in an average of 1–3 studies in the vast continent of 54 countries, over 1 billion people and thousands of ethnically diverse populations. There is a lack of population-specific pharmacogenetics tests and dosing algorithms due to both limited capacity and cohesion of genomics research and clinical pharmacology expertise on the continent. Clinical pharmacogenetics tests are often too costly for low resource settings, and limited laboratory and health informatics infrastructure hamper the ability to offer these tests. The uneven geographic distribution of pharmacogenetics studies also reflects the unbalanced participation of African populations in clinical trials. If more clinical trials were run in low-to-middle-income countries with a high disease burden, there will be an increased likelihood of new therapies being appropriate for implementation in those countries. Additionally, if more clinical trials included a genetic component, our pharmacogenomics knowledge base would increase substantially.
Second, in addition to limited pharmacogenomics studies or clinical trials in Africa, we have a general lack of large-scale genetic studies which can contribute to data on background reference populations. More data are needed on which polymorphisms are truly novel, rare or common in some populations. There have been recent studies on variants in ADME (absorption, distribution, metabolism and excretion) genes in different healthy populations which are helping to identify variants and their frequencies that may be relevant in pharmacogenetics. In a study of sequence data from 40 South Africans of Bantu ancestry, 1662 variants were identified in 65 ADME genes, some of which were novel and a few were potential loss-of-function variants [127]. The novel variants may be important for moderating treatment outcome, but their effect still needs to be determined. The authors also highlight the need for a more comprehensive understanding of population-specific differences to implement pharmacogenetics approaches to treatment.
A third challenge is in identifying relevant studies through clarity on which ethnic group was involved in the study. Racial and ethnic categories are not always consistently reported in different studies [128]. Zhang and Finkelstein, in searching the literature for pharmacogenomics/pharmacogenetics papers, found heterogeneity in classification of ethnic categories with 62 different categories for ‘Black’ [128]. While classifying people into ethnic groups can raise racial issues, in pharmacogenetics, it is essential due to the vast differences in allele frequencies in highly relevant polymorphisms across different populations. Finally, there is still a lack of evidence-based clinical studies that are sufficiently powered (in terms of prevalence of pharmacogenetics variants or effect size of variants on clinical phenotypes) to detect and quantify clinical pharmacogenetics in African populations.
A fourth challenge is the lack of pharmacokinetics studies in people of African ancestry on both old and new medicines. It is important to note that the discovery of pharmacogenetics variability was driven by observations in variation in drug exposure pharmacokinetics studies (debrisoquine, isoniazid, etc) and/or associated with clinical responses (response, failure of response or drug toxicity). African institutions lack strong clinical pharmacology departments with bio-analytical and pharmacometric expertise to conduct such studies. Recent establishment of Phase I clinical trial facilities, some of them with strong bio-analytical capabilities should start addressing this gap [129]. This is because the current sequencing driven initiatives might continue to discover potentially functional genetic variants but with no phenotype (drug exposure and/or clinical effect) data to correlate with. Similar efforts in clinical pharmacology (pharmacokinetics and pharmacodynamics) studies need to be applied as those being put to population genetics/genomics work, only then can a meaningful pharmacogenomics outcome for precision medicine emerge.
A fifth issue that needs to be addressed as we go forward is the need to quantitatively evaluate the unexplained pharmacokinetic/pharmacodynamic variation gap when current European ancestry clinical pharmacogenetics tests are applied to African patients. This is against a current theoretical argument that the unique genetic variation continuously being discovered in African populations will affect clinical outcome significantly compared with outcomes observed in Caucasians. While it makes theoretical sense, given the controversies around the clinical impact/relevance of some pharmacogenetics markers even among the Caucasian populations [130], some observed genetic differences uniquely observed in African populations might easily disappear in the noise of the numerous factors (such as drug/food–drug interactions, disease–drug interactions, drug-biometric indices differences) that can affect clinical pharmacokinetic/pharmacodynamic outcomes. There is, therefore, a need to evaluate, in well powered studies, the currently used Clinical Pharmacogenetics Guidelines for selected drugs and apply them to African patient groups and determine their level of success or failure in predicting and/or reducing risk of adverse effects or treatment failure. Discovery genomics will then be driven by studying patients in whom the current Clinical Pharmacogenetics Guidelines will have failed to predict outcome in the African populations.
At last, but not least, issue to be addressed, is the need to continue applying for, and investing funds to prepare African scientists, healthcare personnel and specialized institutions for facing the challenge of transition from traditional to precision medicine approaches. As progress with regards to pharmacogenetics and precision medicine is taking confident steps all over the world, closely involved teams and facilities in Africa should be both vigilant and confident enough to actively participate in this era. There is a need to improve the knowledge scope and capabilities of both healthcare personnel and facilities concerned with genetics and genomics, population studies, precision medicine and well-designed clinical trials. This need will only be accomplished by integrative initiatives of African and International involved parties. To address these concerns, more synergy between African institutions in terms of African pharmacogenetics and precision medicine research is needed. Data and sample sharing are essential for accelerating scientific progress. Several ethical and socioeconomic challenges need to be resolved such as community engagement, informed consent, possibilities of genetic discrimination and stigmatisation and data security.
Fortunately, some of these challenges are being addressed through funding initiatives for genomics projects in Africa (e.g., H3Africa [131]), and the collaboration between cohorts, public data repositories and standards initiatives such as GA4GH [132], to encourage better curation of metadata associated with genomic and patient data. With the cost of genotyping and sequencing dropping, in 5 years it may be feasible, even in some African countries, to genotype all patients who are prescribed therapies with known pharmacokinetic or genetic variability prior to treatment. This will not only benefit the patient with more precise medication, but also save the healthcare system, the costs of inadequate treatment or the need to treat adverse side effects. In this way, the genetically diverse African populations would benefit from pharmacogenomics-based healthcare approaches to reduce drug side effects and optimize drug choices and doses for each patient. This will require several key components, including the implementation of policies that promote precision public health, such as ethical and legal procedures for the management of communicable and noncommunicable diseases. The policies must clearly articulate support for accurate diagnosis and treatment and should also include pharmacovigilance programs to ensure reporting of new cases of adverse drug reactions. Despite the dropping costs of sequencing, to achieve this in a shorter time frame, cost effective population-relevant panels for screening need to be developed, for which we need more data on pharmacogenetics of African populations. If pharma, research funders and governments are willing to invest in large-scale pharmacogenomic studies, possibly alongside clinical trials, then effective generic or disease-specific pharmacogenetics screening panels will be perfectly feasible in the near future even in resource limited countries.
Supplementary Material
Executive summary.
Pharmacogenetics studies in African populations are less abundant than for other populations.
Several studies are available on warfarin pharmacogenetics and dosing requirements.
There is a lack of pharmacogenetics studies for some diseases, such as sickle cell disease.
Pharmacogenetics studies for infectious diseases have focused on HIV (efavirenz) and TB (rifampin).
Disease-specific pharmacogenetics screening panels may be feasible in the future.
More precise medication for African patients can save the healthcare system the costs of inadequate treatment or the need to treat adverse side effects.
Acknowledgments
The authors would like to thank Chiamaka Okeke, Bilinga Tendwa and Ozlem Tastan Bishop from the Research Unit in Bioinformatics, Rhodes University, South Africa, for their contributions in summarizing the work.
No writing assistance was utilised in the production of this manuscript.
Financial & competing interests disclosure
Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number U24HG006941. H3ABioNet is an initiative of the Human Health and Heredity in Africa Consortium (H3Africa). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Mpye KL, Matimba A, Dzobo K, Chirikure S, Wonkam A, Dandara C. Disease burden and the role of pharmacogenomics in African populations. Glob. Health Epidemiol. Genomics 2, e1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulder N Development to enable precision medicine in Africa. Pers. Med. 14(6), 467–470 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Nordling L How the genomics revolution could finally help Africa. Nature 544(7648), 20–22 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Ramsay M Precision medicine for Africa: challenges and opportunities. Quest 14(3), 28–32 (2018). [Google Scholar]
- 5.Wilson H Pharmacogenomics failing to reach developing countries. Pharmacogenomics J. 15, 731–732 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 60(14), 1207–1216 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield GS, Baldridge A, Agarwal A et al. Disparities in cardiovascular research output and citations from 52 African countries: a time-trend, bibliometric analysis (1999–2008). J. Am. Heart Assoc. 4(4), e001606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geard A, Pule GD, Chelo D, Bitoungui VJN, Wonkam A. Genetics of sickle cell-associated cardiovascular disease: an expert review with lessons learned in Africa. Omics J. Integr. Biol. 20(10), 581–592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott SA, Edelmann L, Kornreich R, Desnick RJ. Warfarin pharmacogenetics: CYP2C9 and VKORC1 genotypes predict different sensitivity and resistance frequencies in the Ashkenazi and Sephardi Jewish populations. Am. J. Hum. Genet. 82, 495–500 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahin MHA, Khalifa SI, Gong Y et al. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet. Genomics 21(3), 130–135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazan NS, Sabry NA, Rizk A, Mokhtar S, Badary OA. Factors affecting warfarin dose requirements and quality of anticoagulation in adult Egyptian patients: role of gene polymorphism. Ir. J. Med. Sci. 183(2), 161–172 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Shahin MHA, Cavallari LH, Perera MA et al. VKORC1 Asp36Tyr geographic distribution and its impact on warfarin dose requirements in Egyptians. Thromb. Haemost. 109(6), 1045–1050 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Issac MSM, El-Nahid MS, Wissa MY. Is there a role for MDR1, EPHX1 and protein Z gene variants in modulation of warfarin dosage? a study on a cohort of the Egyptian population. Mol. Diagn. Ther. 18(1), 73–83 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Scordo MG, Aklillu E, Yasar U, Dahl ML, Spina E, Ingelman-Sundberg M. Genetic polymorphism of cytochrome P450 2C9 in a Caucasian and a black African population. Br. J. Clin. Pharmacol. 52(4), 447–450 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrif NEMA, Won H-H, Lee S-T et al. Evaluation of the effects of VKORC1 polymorphisms and haplotypes, CYP2C9 genotypes, and clinical factors on warfarin response in Sudanese patients. Eur. J. Clin. Pharmacol. 67(11), 1119–1130 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Kudzi W, Ahorhorlu SY, Dzudzor B, Olayemi E, Nartey ET, Asmah RH. Genetic polymorphisms of patients on stable warfarin maintenance therapy in a Ghanaian population. BMC Res. Notes. 9, 507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell C, Gregersen N, Krause A. Novel CYP2C9 and VKORC1 gene variants associated with warfarin dosage variability in the South African black population. Pharmacogenomics 12(7), 953–963 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Cavallari LH, Langaee TY, Momary KM et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 87(4), 459–464 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Voora D, Koboldt DC, King CR et al. A polymorphism in the VKORC1 regulator calumenin predicts higher warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 87(4), 445–451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavallari LH, Perera M, Wadelius M et al. Association of the GGCX (CAA)16/17 repeat polymorphism with higher warfarin dose requirements in African Americans. Pharmacogenet. Genomics 22(2), 152–158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuldiner AR, O’Connell JR, Bliden KP RB et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 26, 302(8), 849–857 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perera MA, Cavallari LH, Limdi NA et al. Genetic variants associated with warfarin dose in African-American individuals: a genome-wide association study. Lancet 382(9894), 790–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil BM, Shahin MH, Solayman MH et al. Genetic and nongenetic factors affecting clopidogrel response in the Egyptian population. Clin. Transl. Sci. 9(1), 23–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassani Idrissi H, Hmimech W, El Khorb N, Akoudad H, Habbal R, Nadifi S. Does i-T744C P2Y12 polymorphism modulate clopidogrel response among Moroccan acute coronary syndromes patients? Genet. Res. Int. 2017, 9532471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charfi RM, Khadija B, Miriam H et al. Response to clopidogrel and of the cytochrome CYP2C19 gene polymorphism. Tunis. Med. 96, 209–218 (2018). [PubMed] [Google Scholar]
- 26.Smires FZ, Moreau C, Habbal R et al. Influence of genetics and non-genetic factors on acenocoumarol maintenance dose requirement in Moroccan patients. J. Clin. Pharm. Ther. 37(5), 594–598 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Smires FZ, Habbal R, Moreau C, Assaidi A, Loriot MA, Nadifi S. Effect of different genetics variants: CYP2C9*2, CYP2C9*3 of cytochrome P-450 CYP2C9 and 1639G>A of the VKORC1 gene; On acenocoumarol requirement in Moroccan patients. Pathol. Biol. (Paris) 61(3), 88–92 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Ajmi M, Omezzine A, Achour S et al. Influence of genetic and non-genetic factors on acenocoumarol maintenance dose requirement in a Tunisian population. Eur. J. Clin. Pharmacol. 74(6), 711–722 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Soko ND, Masimirembwa C, Dandara C. Pharmacogenomics of rosuvastatin: a glocal (Global + Local) African perspective and expert review on a statin drug. Omics J. Integr. Biol. 20(9), 498–509 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Zhou K, Yee SW, Seiser EL et al. Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat. Genet. 48(9), 1055–1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Sayed S, Khairy E, Basheer AR, Zaki WS, Ahmad GF, Kassim SK. Evaluation of leptin and MMP2 genes methylation in childhood obesity. Gene Rep. 11, 79–86 (2018). [Google Scholar]
- 32.Moore AF, Jablonski KA, Mason CC et al. The association of ENPP1 K121Q with diabetes incidence is abolished by lifestyle modification in the diabetes prevention program. J. Clin. Endocrinol. Metab. 94(2), 449–455 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jmel H, Romdhane L, Ben Halima Y et al. Pharmacogenetic landscape of metabolic syndrome components drug response in Tunisia and comparison with worldwide populations. PloS ONE 13(4), e0194842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelhedi R, Bouayed NA, Alfadhli S, Abid L, Rebai A, Kharrat N. Characterization of drug-metabolizing enzymes CYP2C9, CYP2C19 polymorphisms in Tunisian, Kuwaiti and Bahraini populations. J. Genet. 94(4), 765–770 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J. Diabetes 6(6), 850–867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 11, 564 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastakia SD, Pekny CR, Manyara SM, Fischer L. Diabetes in sub-Saharan Africa - from policy to practice to progress: targeting the existing gaps for future care for diabetes. Diabetes Metab Syndr Obes Targets Ther. 10, 247–263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peer N, Kengne A-P, Motala AA, Mbanya JC. Diabetes in the Africa Region: an update. Diabetes Res. Clin. Pract. 103(2), 197–205 (2014). [DOI] [PubMed] [Google Scholar]
- 39.El-Sisi AE, Hegazy SK, Metwally SS, Wafa AM, Dawood NA. Effect of genetic polymorphisms on the development of secondary failure to sulfonylurea in egyptian patients with type 2 diabetes. Ther Adv Endocrinol Metab. 2(4), 155–164 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stocker SL, Morrissey KM, Yee SW et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin. Pharmacol. Ther. 93(2), 186–194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JH, Yee SW, Ramirez AH et al. A common 5’-UTR variant in MATE2-K is associated with poor response to metformin. Clin. Pharmacol. Ther. 90(5), 674–684 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goswami S, Yee SW, Stocker S et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin. Pharmacol. Ther. 96(3), 370–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Li S, Brown C et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet. Genomics 19(7), 497–504 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jablonski KA, McAteer JB, de Bakker PIW et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes 59(10), 2672–2681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Florez JC, Jablonski KA, Kahn SE et al. Type 2 diabetes-associated missense polymorphisms KCNJ11 E23K and ABCC8 A1369S influence progression to diabetes and response to interventions in the Diabetes Prevention Program. Diabetes 56(2), 531–536 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florez JC, Jablonski KA, McAteer J et al. Testing of diabetes-associated WFS1 polymorphisms in the Diabetes Prevention Program. Diabetologia 51(3), 451–457 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Florez JC, Jablonski KA, Taylor A et al. The C allele of ATM rs11212617 does not associate with metformin response in the Diabetes Prevention Program. Diabetes Care 35(9), 1864–1867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majithia AR, Jablonski KA, McAteer JB et al. Association of the SLC30A8 missense polymorphism R325W with proinsulin levels at baseline and after lifestyle, metformin or troglitazone intervention in the Diabetes Prevention Program. Diabetologia 54(10), 2570–2574 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Pawlikowski B, Schlessinger A et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet. Genomics 20(11), 687–699 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Florez JC, Jablonski KA, McAteer JB et al. Effects of genetic variants previously associated with fasting glucose and insulin in the Diabetes prevention program. PloS ONE 7(9), e44424 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baquet CR, Mishra SI, Commiskey P, Ellison GL, DeShields M. BrEast cancer epidemiology in blacks and whites: disparities in incidence, mortality, survival rates and histology. J. Natl Med. Assoc. 100(5), 480–488 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatti I, Creveaux I, Woillard J-B et al. Association of the OPRM1 and COMT genes’ polymorphisms with the efficacy of morphine in Tunisian cancer patients: impact of the high genetic heterogeneity in Tunisia? Therapie 71(5), 507–513 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Dean L Codeine therapy and CYP2D6 genotype. In: Medical Genetics Summaries. Dean L, Pratt V, McLeod H (Eds). National Center for Biotechnology Information (US), Bethesda (MD), USA: (2012). [PubMed] [Google Scholar]
- 54.Arfaoui A, Douik H, Kablouti G et al. MDM2 344T>A polymorphism; could it be a predictive marker of anthracycline resistance? J. BUON 21(3), 732–739 (2016). [PubMed] [Google Scholar]
- 55.O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 15(15), 4806–4814 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben Hassine I, Gharbi H, Soltani I et al. Molecular study of ABCB1 gene and its correlation with imatinib response in chronic myeloid leukemia. Cancer Chemother. Pharmacol. 80(4), 829–839 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Adeagbo BA, Bolaji OO, Olugbade TA, Durosinmi MA, Bolarinwa RA, Masimirembwa C. Influence of CYP3A5*3 and ABCB1 C3435T on clinical outcomes and trough plasma concentrations of imatinib in Nigerians with chronic myeloid leukaemia. J. Clin. Pharm. Ther. 41(5), 546–551 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Bedewy AM, El-Maghraby SM. Do SLCO1B3 (T334G) and CYP3A5*3 polymorphisms affect response in Egyptian chronic myeloid leukemia patients receiving imatinib therapy? Hematol. Amst. Neth. 18(4), 211–216 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Elghannam DM, Ibrahim L, Ebrahim MA, Azmy E, Hakem H. Association of MDR1 gene polymorphism (G2677T) with imatinib response in Egyptian chronic myeloid leukemia patients. Hematol. Amst. Neth. 19(3), 123–128 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Ali MA, Elsalakawy WA. ABCB1 haplotypes but not individual SNPs predict for optimal response/failure in Egyptian patients with chronic-phase chronic myeloid leukemia receiving imatinib mesylate. Med. Oncol. 31(11), 279–279 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Ben Hassine I, Gharbi H, Soltani I et al. hOCT1 gene expression predict for optimal response to Imatinib in Tunisian patients with chronic myeloid leukemia. Cancer Chemother. Pharmacol. 79(4), 737–745 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Abdeljaoued S, Bettaieb L, Nasri M et al. Forkhead box M1 (FOXM1) expression predicts disease free survival and may mediate resistance to chemotherapy and hormonotherapy in male brEast cancer. Breast Dis. 37(3), 109–114 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Moukam Kakmeni FM, Guimapi RYA, Ndjomatchoua FT, Pedro SA, Mutunga J, Tonnang HEZ. Spatial panorama of malaria prevalence in Africa under climate change and interventions scenarios. Int. J. Health Geogr. 17(1), 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeka A, Dorsey G, Kamya MR et al. Artemether-Lumefantrine versus Dihydroartemisinin–Piperaquine for treating uncomplicated Malaria: a randomized trial to guide policy in Uganda. PLoS ONE 3(6), e2390 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glaziou P, Floyd K, Raviglione MC. Global epidemiology of tuberculosis. Semin. Respir. Crit. Care Med. 39(3), 271–285 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat. Rev. Dis. Primer. 1, 15035 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Kharsany ABM, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 10, 34–48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reay R, Dandara C, Viljoen M, Rheeders M. CYP2B6 haplotype predicts efavirenz plasma concentration in black South African HIV-1-infected children: a longitudinal pediatric pharmacogenomic study. Omics J. Integr. Biol. 21(8), 465–473 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Swart M, Ren Y, Smith P, Dandara C. ABCB1 4036A>G and 1236C>T polymorphisms affect plasma efavirenz levels in South African HIV/AIDS patients. Front. Genet. 3, 236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gross R, Bellamy SL, Ratshaa B et al. CYP2B6 genotypes and early efavirenz-based HIV treatment outcomes in Botswana. AIDS Lond. Engl. 31(15), 2107–2113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peko SM, Gueye NSG, Vouvoungui C et al. Cytochrome P450 CYP2B6*6 distribution among Congolese individuals with HIV, tuberculosis and malaria infection. Int. J. Infect. Dis. 82, 111–116 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Desta Z, Gammal RS, Gong L, Whirl-Carrillo M, Gaur AH, Sukasem C. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2B6 and efavirenz-containing antiretroviral therapy. Clin. Pharmacol. Ther. 106(4), 726–733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chigutsa E, Visser ME, Swart EC et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob. Agents Chemother. 55(9), 4122–4127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhoro M, Zvada S, Ngara B et al. CYP2B6*6, CYP2B6*18, Body weight and sex are predictors of efavirenz pharmacokinetics and treatment response: population pharmacokinetic modeling in an HIV/AIDS and TB cohort in Zimbabwe. BMC Pharmacol. Toxicol. 16, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ben Mahmoud L, Ghozzi H, Kamoun A et al. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatotoxicity in Tunisian patients with tuberculosis. Pathol. Biol. (Paris) 60(5), 324–330 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Ben Fredj N, Gam R, Kerkni E et al. Risk factors of isoniazid-induced hepatotoxicity in Tunisian tuberculosis patients. Pharmacogenomics J. 17(4), 372–377 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Dompreh A, Tang X, Zhou J et al. Effect of genetic variation of NAT2 on isoniazid and SLCO1B1 and CES2 on rifampin pharmacokinetics in Ghanaian children with tuberculosis. Antimicrob. Agents Chemother. 62(3), e02099–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiner M, Peloquin C, Burman W et al. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob. Agents Chemother. 54(10), 4192–4200 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS Lond. Engl. 25(3), 388–390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilkins JJ, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson USH. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br. J. Clin. Pharmacol. 72(1), 51–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gengiah TN, Botha JH, Yende-Zuma N, Naidoo K, Abdool Karim SS. Efavirenz dosing: influence of drug metabolizing enzyme polymorphisms and concurrent tuberculosis treatment. Antivir. Ther. 20(3), 297–306 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Lavanchy D The global burden of hepatitis C. Liver Int. 29, 74–81 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Wantuck JM, Ahmed A, Nguyen MH. The epidemiology and therapy of chronic hepatitis C genotypes 4, 5 and 6. Aliment. Pharmacol. Ther. 39, 137–147 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Kamal SM. Hepatitis C virus genotype 4 therapy: progress and challenges. Liver Int. 31, 45–52 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Farag RE, Arafa MM, El-Etreby S et al. Human leukocyte antigen class I alleles can predict response to pegylated interferon/ribavirin therapy in chronic hepatitis C Egyptian patients. Arch. Iran. Med. 16(2), 68–73 (2013). [PubMed] [Google Scholar]
- 86.Ragheb MM, Nemr NA, Kishk RM et al. Strong prediction of virological response to combination therapy by IL28B gene variants rs12979860 and rs8099917 in chronic hepatitis C genotype 4. Liver Int. 34(6), 890–895 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Derbala M, Rizk NM, Al-Kaabi S et al. The predictive value of IL28B rs12979860, rs11881222 and rs8099917 polymorphisms and IP-10 in the therapeutic response of Egyptian genotype 4 patients. Virology 444(1–2), 292–300 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Fathy MM, Abo Taleb ME, El Hawary MS, Nabih MI, Aref WM, Makhlouf MM. Assessment of interleukin 28B genotype as a predictor of response to combined therapy with pegylated interferon plus ribavirin in HCV infected Egyptian patients. Cytokine 74(2), 268–272 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Asselah T, De Muynck S, Broët P et al. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J. Hepatol. 56(3), 527–532 (2012). [DOI] [PubMed] [Google Scholar]
- 90.McCarthy JJ, Li JH, Thompson A et al. Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology 138(7), 2307–2314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas DL, Thio CL, Martin MP et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461(7265), 798–801 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pagliaccetti NE, Robek MD. Interferon-lambda in the immune response to hepatitis B virus and hepatitis C virus. J. Interferon Cytokine Res. 585–590 doi: 10.1089/jir.2010.0060 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 19(1), 125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharaki O, Zeid M, Moez P, Zakaria NH, Nassar E. Impact of CYP3A4 and MDR1 gene (G2677T) polymorphisms on dose requirement of the cyclosporine in renal transplant Egyptian recipients. Mol. Biol. Rep. 42(1), 105–117 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Aouam K, Kolsi A, Kerkeni E et al. Influence of combined CYP3A4 and CYP3A5 single-nucleotide polymorphisms on tacrolimus exposure in kidney transplant recipients: a study according to the post-transplant phase. Pharmacogenomics 16(18), 2045–2054 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Elmachad M, Elkabbaj D, Elkerch F et al. Frequencies of CYP3A5*1/*3 variants in a Moroccan population and effect on tacrolimus daily dose requirements in renal transplant patients. Genet. Test Mol. Biomark. 16(6), 644–647 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Sanghavi K, Brundage RC, Miller MB et al. Genotype-guided tacrolimus dosing in African-American kidney transplant recipients. Pharmacogenomics J. 17(1), 61–68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trofe-Clark J, Brennan DC, West-Thielke P et al. Results of ASERTAA, a randomized prospective crossover pharmacogenetic study of immediate-release versus extended-release tacrolimus in African American kidney transplant recipients. Am. J. Kidney Dis. 71(3), 315–326 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Asempa TE, Rebellato LM, Hudson S, Briley K, Maldonado AQ. Impact of CYP3A5 genomic variances on clinical outcomes among African American kidney transplant recipients. Clin. Transplant. 32(1), e13162 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Bhatnagar V, Garcia EP, O’Connor DT, Brophy VH, Alcaraz J, Richard E et al. CYP3A4 and CYP3A5 polymorphisms and blood pressure response to amlodipine among African-American men and women with early hypertensive renal disease. Am. J. Nephrol. 31(2), 95–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anthony EG, Richard E, Lipkowitz MS, Bhatnagar V. Association of the ADRB2 (rs2053044) polymorphism and angiotensin-converting enzyme-inhibitor blood pressure response in the African American Study of Kidney Disease and Hypertension. Pharmacogenet. Genomics 25(9), 444–449 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Jacobson PA, Oetting WS, Brearley AM et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation 91(3), 300–308 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA. Dosing equation for tacrolimus using genetic variants and clinical factors. Br. J. Clin. Pharmacol. 72(6), 948–957 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pulk RA, Schladt DS, Oetting WS et al. Multigene predictors of tacrolimus exposure in kidney transplant recipients. Pharmacogenomics 16(8), 841–854 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wonkam A Is there a role for pharmacogenetics in the treatment of sickle cell disease? Pharmacogenomics 18(4), 321–325 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Shiba HF, El-Ghamrawy MK, Shaheen IAE-M, Ali RAE-G, Mousa SM. Glutathione S-transferase gene polymorphisms (GSTM1,GSTT1, and GSTP1) in Egyptian pediatric patients with sickle cell disease. Pediatr. Dev. Pathol. 17(4), 265–270 (2014). [DOI] [PubMed] [Google Scholar]
- 107.Pogue RE, Cavalcanti DP, Shanker S et al. Rare genetic diseases: update on diagnosis, treatment and online resources. Drug Discov. Today 23(1), 187–195 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Riahi Z, Zainine R, Mellouli Y et al. Compound heterozygosity for dominant and recessive GJB2 mutations in a Tunisian family and association with successful cochlear implant outcome. Int. J. Pediatr. Otorhinolaryngol. 77(9), 1481–1484 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Lorenzo V, Alvarez A, Torres A, Torregrosa V, Hernández D, Salido E. Presentation and role of transplantation in adult patients with type 1 primary hyperoxaluria and the I244T AGXT mutation: single-center experience. Kidney Int. 70(6), 1115–1119 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Nagara M, Tiar A, Ben Halim N et al. Mutation spectrum of primary hyperoxaluria type 1 in Tunisia: implication for diagnosis in North Africa. Gene 527(1), 316–320 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Zhang X, Hothi JS, Zhang YH, Srinivasan S, Stokes DC, Zhang W. c.3623G > A mutation encodes a CFTR protein with impaired channel function. Respir Res. 17, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arora K, Yarlagadda S, Zhang W et al. Personalized medicine in cystic fibrosis: genistein supplementation as a treatment option for patients with a rare S1045Y-CFTR mutation. Am. J. Physiol. Lung Cell. Mol. Physiol. 311(2), L364–374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Charlson FJ, Diminic S, Lund C, Degenhardt L, Whiteford HA. Mental and substance use disorders in Sub-Saharan Africa: predictions of epidemiological changes and mental health workforce requirements for the next 40years. PloS ONE 9(10), e110208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drogemöller BI, Niehaus DJH, Chiliza B et al. Patterns of variation influencing antipsychotic treatment outcomes in South African first-episode schizophrenia patients. Pharmacogenomics 15(2), 189–199 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Hwang R, Shinkai T, De Luca V et al. Association study of 12 polymorphisms spanning the dopamine D(2) receptor gene and clozapine treatment response in two treatment refractory/intolerant populations. Psychopharmacology (Berl). 181(1), 179–187 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Zuo L, Luo X, Krystal JH, Cramer J, Charney DS, Gelernter J. The efficacies of clozapine and haloperidol in refractory schizophrenia are related to DTNBP1 variation. Pharmacogenet. Genomics 19(6), 437–446 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fijal BA, Kinon BJ, Kapur S et al. Candidate-gene association analysis of response to risperidone in African-American and white patients with schizophrenia. Pharmacogenomics J. 9(5), 311–318 (2009). [DOI] [PubMed] [Google Scholar]
- 118.Tiwari AK, Zai CC, Likhodi O et al. A common polymorphism in the cannabinoid receptor 1 (CNR1) gene is associated with antipsychotic-induced weight gain in Schizophrenia. Neuropsychopharmacology 35(6), 1315–1324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maciukiewicz M, Gorbovskaya I, Tiwari AK et al. Genetic validation study of protein tyrosine phosphatase receptor type D (PTPRD) gene variants and risk for antipsychotic-induced weight gain. J. Neural. Transm. 126(1), 27–33 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Fonseka TM, Tiwari AK, Gonçalves VF et al. The role of genetic variation across IL-1β, IL-2, IL-6, and BDNF in antipsychotic-induced weight gain. World J. Biol. Psychiatry 16(1), 45–56 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Tiwari AK, Zai CC, Meltzer HY, Lieberman JA, Müller DJ, Kennedy JL. Association study of polymorphisms in insulin induced gene 2 (INSIG2) with antipsychotic-induced weight gain in European and African-American schizophrenia patients. Hum. Psychopharmacol. 25(3), 253–259 (2010). [DOI] [PubMed] [Google Scholar]
- 122.Barron CR, Tonarelli S, Delozier A et al. Pharmacogenetics of antidepressants, a review of significant genetic variants in different populations. Clin. Depress. 2, 109 (2017). [Google Scholar]
- 123.Murphy E, Hou L, Maher BS et al. Race, genetic ancestry and response to antidepressant treatment for major depression. Neuropsychopharmacology 38(13), 2598–2606 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Binder EB, Owens MJ, Liu W et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch. Gen. Psychiatry 67(4), 369–379 (2010). [DOI] [PubMed] [Google Scholar]
- 125.U.S Food & Drug Administration. Table of pharmacogenomic biomarkers in drug labeling (2019). http://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling
- 126.PharmGKB. Clinical Guideline Annotations. (2019). www.pharmgkb.org/guidelineAnnotations
- 127.Tshabalala S, Choudhury A, Beeton-Kempen N, Martinson N, Ramsay M, Mancama D. Targeted ultra-deep sequencing of a South African Bantu-speaking cohort to comprehensively map and characterize common and novel variants in 65 pharmacologically-related genes. Pharmacogenet. Genomics 29(7), 167 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang F, Finkelstein J. Inconsistency in race and ethnic classification in pharmacogenetics studies and its potential clinical implications. Pharmacogenomics Pers. Med. 12, 107–123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gutierrez MM, Pillai G, Felix S et al. Building capability for clinical pharmacology research in Sub-Saharan Africa. Clin. Pharmacol. Ther. 102(5), 786–795 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Cronin-Fenton DP, Damkier P, Lash TL. Metabolism and transport of tamoxifen in relation to its effectiveness: new perspectives on an ongoing controversy. Future Oncol. 10(1), 107–122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.H3Africa Consortium. Human Heredity and Health in Africa (2019). https://h3africa.org/
- 132.GA4GH. Global Alliance for Genomics and Health. (2019). www.ga4gh.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.