Abstract
Background:
Some evidence supports that the significance of inflammation is linked to a variety of tumors, including thyroid carcinoma. This work measured the preoperative serum inflammatory factors in thyroid tumors to explore their diagnostic values.
Material and Methods:
Altogether 487 thyroid tumor patients were recruited, their neutrophil (NE), white blood cell (WBC), monocyte (MO), lymphocyte (LY), platelet (PLT) counts, together with monocyte/lymphocyte ratio (MLR), neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), C-reactive protein (CRP), interleukin (IL)-1β, IL-2, IL-27, and tumor necrosis factor-α (TNF-α) levels were compared with controls. Afterward, the receiver operating characteristics (ROC) curve was plotted to further evaluate the values of these inflammatory markers in diagnosis. In addition, multivariable regression analysis was conducted to analyze all these inflammatory factors.
Results:
Serum PLR, NLR, CRP, and IL-27 levels in thyroid adenoma (TA) and differentiated thyroid carcinoma (DTC) patients were higher than those in controls. Only the areas under the curve (AUC) for CRP and IL-27 were significant in the context of DTC. Besides, the AUC for IL-27 was significant between papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) groups, while that for NLR+PLR was also significant between PTC and healthy control groups. According to multivariable logistic regression analysis, IL-27 and CRP were associated with DTC.
Conclusions:
Inflammation plays an important role in TA and DTC progression. Preoperative IL-27 and CRP levels help to differentially diagnose DTC. Moreover, IL-27 assists in distinguishing FTC from PTC, and NLR+PLR is important for the differential diagnosis of PTC.
Keywords: cancer, diagnosis, histopathology, inflammation, thyroid
Introduction
Thyroid cancer is mainly derived from thyroid follicular cells, one of the cell types distributed in thyroid parenchyma. These cells include interstitial and follicular cells, and mainly form the differentiated thyroid cancer (DTC).1 Related clinical studies show that, DTC, which can be classified as follicular and papillary cancers, accounts for 1-2% of all human malignancies and 80% of thyroid malignancies.2 DTC is an endocrine cancer that progresses slowly, with a 10-year survival rate of greater than 90%, but the incidences of capsule invasion, extra thyroidal extension, and lymphatic metastasis remain high, making it impedimental to further reduce its mortality and improve the survival rate.3 It is well known that, one of the important and efficient ways to reduce the mortality of DTC is to diagnose the cancer at the early stage. An increasing number of thyroid tumors are detected at the early stage thanks to the improvement of diagnostic technology and the gradual popularization of physical examinations. However, it still remains difficult to distinguish from benign thyroid tumors, papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC).
According to recent studies, inflammation exerts a vital part during cancer genesis and development,4 which may directly affect cancer cells, stimulate epithelial-to-mesenchymal transition (EMT), interact with chemokines, and augment cancer metastasis.5 A lot of inflammatory factors have been discovered, including CSCF, VEGF, NLR (neutrophil/lymphocyte ratio), PLR (platelet/lymphocyte ratio), MLR (monocyte/lymphocyte ratio), CRP (C-reactive protein), IL-6, IL-10, IL-12, IL-17, IL-23, TNF-α (tumor necrosis factor-α) and TGF-β.4,6-12 Growth of related inflammatory markers like LMR and IL-6 is shown to play an important role in PTC,13,14 but there is still little research on FTC, and some findings are controversial. Therefore, the present work aimed to systematically depict the heterogeneities in preoperative inflammatory factors between healthy control subjects, thyroid adenoma (TA) patients and DTC patients including PTC and FTC.
Materials and Methods
Patients and Normal Controls
Our study protocol was approved by the Institutional Ethics Committee of xxxx hospital. A total of 487 patients, including 104 with FTC, 200 with PTC and 183 with TA who received surgeries and were confirmed at xxxx Hospital were enrolled between June 1, 2018, and January 31, 2020 into the present work. Additionally, the medical records of 217 healthy individuals receiving annual healthy checkup at the same hospital were reviewed as healthy controls. The patient exclusion criteria were as follows: 1) those who had Hashimoto’s thyroiditis; 2) those who did previously undergo a thyroid operation; 3) those receiving acupuncture before surgery; botulinum toxin; hormone therapy (like glucocorticoids) or radiotherapy; 4) those with underlying hematologic disorders, hyperpyrexia, infectious disease, metabolic syndrome, diabetes mellitus, hypertension, severe liver or kidney dysfunction, severe heart disease, malignancies, inflammatory disorder, autoimmune disorder, or the use of medication associated with inflammation; 5) those with incomplete information related to inflammatory marker tests and routine blood tests before surgery. Each eligible patient provided the informed consent to participate in this study.
Data Extraction
The patient demographic and clinicopathological data, such as sex, age, and pathological type, were retrieved from medical records. After obtaining the informed consent from each subject, the peripheral blood samples were extracted within 1 week before surgery for inflammatory markers and routine blood tests, which served as one part of standard preoperative workup. For the normal subjects undergoing annual physical examination at this hospital, their peripheral blood was collected to test the same items. Then, each sample acquired was preserved under at lower than 20°C, and each test was carried out by the staff from the Clinical Laboratory Department in our hospital within 2 h. The white blood cell (WBC), neutrophil (NE), lymphocyte (LY), monocyte (MO), platelet (PLT) counts, and CRP levels were obtained from routine blood tests conducted using the normalized automatic counters. Then, the NLR, MLR, and PLR were calculated. Moreover, the levels of interleukin-2 (IL-2), interleukin-1β (IL-1β), interleukin-27 (IL-27) and TNF-α were determined using the enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, MN, USA) in accordance with manufacturer protocols.
Statistics Analyses
SPSS 24.0 (SPSS, Inc, Chicago, Illinois) was employed for data analysis. First of all, Kolmogorov-Smirnov test was applied to analyze whether the variables were normally distributed. Then, 1-way analysis of variance (ANOVA) or 2-tailed Student t-test was used to analyze data with normal distribution. Mann-Whitney U test was adopted to compare the nonparametric variables between 2 groups. Later, the receiver operating characteristic (ROC) curves were plotted, and the areas under the curve (AUC) were calculated to assess the accuracy of those inflammatory factors mentioned above in diagnosis. Following post hoc analysis, the threshold of outlier was obtained based on the maximum sum of specificity and sensitivity. Besides, univariate and multivariate logistic regression analyses were conducted to better analyze these inflammatory factors. A difference of P < 0.05 (2-tailed) indicated statistically significant.
Results
Characteristics of Study Populations and Preoperative Inflammatory factors Compared Among FTC, PTC, ta Patients and Normal Controls
Table 1 shows the details regarding the demographic information of study populations. According to Table 1, thyroid tumors showed remarkably higher levels of WBC, NE, PLT, PLR, CRP, IL-2, IL-1β, IL-27 and TNF-α, while thyroid carcinoma exhibited higher MO and NLR levels, while FTC showed higher LY level than normal controls. The WBC, NE, PLT, MO, NLR, CRP, IL-2, IL-1β, IL-27 and TNF-α levels in PTC and FTC patients were also higher than those in TA group. Compared with TA group, the PLR value in PTC group and the LY count in FTC group increased. Besides, the WBC and MO counts in FTC group markedly increased relative to those in PTC group. The PLR value and IL-27 level of FTC patients decreased relative to those in FTC group. DTC patients were also divided according to the tumor size and stage. When compared according to tumor size or stage, the WBC, NE, PLT, MO, NLR, CRP, IL-2, IL-1β, IL-27 and TNF-α levels in DTC patients (size ≥1 cm; stage III-IV) were higher than those in DTC patients (size <1 cm; stage I-II), with no significant difference among different groups (Table 2).
Table 1.
Demographic Details and Levels of Inflammatory Markers in Peripheral Blood of Patients With Follicular Thyroid Carcinoma, Papillary Thyroid Carcinoma, Thyroid Adenomas and Healthy Control.
| Variable | HC | TA | PTC | FTC |
|---|---|---|---|---|
| NO. | 217 | 183 | 200 | 104 |
| Age(years) | 45.83 ± 6.24 | 45.51 ± 6.79 | 45.87 ± 6.53 | 42.48 ± 4.57abc |
| Male/Female | 115/102 | 37/146a | 42/158a | 24/80a |
| WBC(109/L) | 6.16 ± 1.09 | 6.52 ± 1.40a | 6.74 ± 1.15ab | 7.13 ± 1.23abc |
| NE | 3.53 ± 0.99 | 3.86 ± 0.93a | 5.65 ± 2.65ab | 5.71 ± 2.63ab |
| PLT(109/L) | 224.62 ± 66.14 | 261.34 ± 39.99a | 347.11 ± 156.15ab | 318.80 ± 101.65ab |
| LY | 1.92 ± 0.53 | 1.96 ± 0.61 | 2.10 ± 0.80 | 2.18 ± 0.71ab |
| MO | 0.43 ± 0.10 | 0.45 ± 0.12 | 0.49 ± 0.13ab | 0.53 ± 0.18abc |
| NLR | 2.01 ± 0.83 | 2.17 ± 0.86 | 2.83 ± 1.05ab | 2.88 ± 1.53ab |
| PLR | 120.57 ± 40.77 | 146.71 ± 50.37a | 174.63 ± 61.28ab | 158.54 ± 61.52ac |
| MLR | 0.24 ± 0.09 | 0.25 ± 0.10 | 0.27 ± 0.13 | 0.26 ± 0.12 |
| CRP(mg/L) | 0.86 ± 0.72 | 0.94 ± 0.87a | 1.91 ± 1.52ab | 1.98 ± 1.30ab |
| TNF-α(pg/mL) | 21.45 ± 8.31 | 27.61 ± 13.69a | 39.13 ± 24.08ab | 43.23 ± 28.29ab |
| IL-1β(pg/mL) | 3.26 ± 1.33 | 3.63 ± 1.30a | 6.05 ± 3.52ab | 6.47 ± 3.92ab |
| IL-2(pg/mL) | 23.58 ± 3.79 | 22.50 ± 3.40a | 21.39 ± 3.47ab | 20.49 ± 3.77ab |
| IL-27(pg/mL) | 65.96 ± 45.27 | 80.49 ± 38.62a | 307.93 ± 134.36ab | 169.37 ± 105.73abc |
Abbreviations: HC, healthy control; TA, thyroid adenomas; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; WBC, white blood cell; NE, neutrophil; LY, lymphocyte; MO, monocyte; PLT, platelet; NLR, neutrophil/ lymphocyte ratio; PLR, platelet/ lymphocyte ratio; MLR, monocyte/ lymphocyte ratio; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-27, interleukin-27.
Data were expressed as mean ± standard deviation.
The significance level was set at p < 0.05. a p < 0.05 compared with healthy control group. b p < 0.05 compared with thyroid adenomas group. c p < 0.05 compared with PTC group.
Table 2.
Scrum Levels of Inflammatory Factors in Relation to Tumor Size and Stage in Patients With DTC.
| Variable | Size | P-value | Stage | P-value | ||
|---|---|---|---|---|---|---|
| <1cm | ≥1cm | I-II | III-IV | |||
| WBC(109/L) | 6.97 ± 1.18 | 6.98 ± 1.17 | 0.086 | 6.97 ± 1.17 | 6.97 ± 1.22 | 0.092 |
| NE(109/L) | 5.67 ± 2.65 | 5.67 ± 2.66 | 0.091 | 5.66 ± 2.65 | 5.67 ± 2.65 | 0.076 |
| PLT(109/L) | 336.57 ± 138.49 | 337.16 ± 141.37 | 0.083 | 335.92 ± 138.53 | 336.91 ± 139.16 | 0.095 |
| MO(109/L) | 0.50 ± 0.15 | 0.51 ± 0.14 | 0.074 | 0.50 ± 0.15 | 0.50 ± 0.15 | 0.093 |
| NLR | 2.85 ± 1.37 | 2.88 ± 1.39 | 0.085 | 2.84 ± 1.37 | 2.88 ± 1.41 | 0.071 |
| CRP(mg/L) | 1.94 ± 1.43 | 1.97 ± 1.44 | 0.059 | 1.95 ± 1.42 | 1.99 ± 1.47 | 0.054 |
| TNF-α(pg/mL) | 40.72 ± 25.13 | 40.75 ± 25.12 | 0.088 | 40.68 ± 25.11 | 41.20 ± 26.34 | 0.063 |
| IL-1β(pg/mL) | 6.36 ± 3.69 | 6.36 ± 3.71 | 0.093 | 6.35 ± 3.53 | 6.37 ± 3.72 | 0.075 |
| IL-2(pg/mL) | 21.01 ± 3.52 | 21.06 ± 3.55 | 0.069 | 21.03 ± 3.61 | 21.04 ± 3.66 | 0.087 |
| IL-27(pg/mL) | 256.81 ± 128.27 | 259.35 ± 129.31 | 0.061 | 256.17 ± 126.31 | 260.62 ± 129.42 | 0.053 |
Abbreviations: DTC, differentiated thyroid carcinoma; WBC, white blood cell; NE, neutrophil; PLT, platelet; MO, monocyte; NLR, neutrophil/lymphocyte ratio; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-27, interleukin-27.
Performances of Inflammatory Factors Together With Corresponding Combinations in the Diagnosis of FTC, PTC and TA Patients
Figure 1 exhibits the performances (ROC curves) of NE, WBC, PLT, MO, LY, MLR, PLR, NLR, CRP, IL-2, IL-1β, IL-27 and TNF-α, as well as their paired combinations like NLR+PLR, NLR+MLR and PLR+MLR in the diagnosis of thyroid tumor patients. Supplementary Table 1 presents the AUCs and corresponding 95% confidence intervals (CIs). According to the table, compared with normal controls, the AUCs (95% CIs; P) for WBC, NE, PLT, LY, MO, NLR, PLR, MLR, NLR+PLR, NLR+MLR, PLR+MLR, CRP, TNF-α, IL-1β, IL-2, IL-27 were 0.565 (0.508-0.622; P = 0.025), 0.571(0.515-0.627; P = 0.015), 0.666(0.613-0.718; P < 0.001), 0.509(0.452-0.566; P = 0.753), 0.534(0.477-0.590; P = 0.246), 0.541(0.485-0.598; P = 0.153), 0.649(0.596-0.703; P < 0.001), 0.529(0.472-0.586; P = 0.313), 0.649(0.596-0.703; P < 0.001), 0.510(0.443-0.557; P = 0.943), 0.649(0.595-0.702; P < 0.001), 0.570(0.514-0.627; P = 0.015), 0.635(0.580-0.691; P < 0.001), 0.583(0.527-0.639; P = 0.004), 0.429(0.373-0.485; P = 0.014) and 0.625(0.571-0.679; P < 0.001) in TA patients, respectively (supplementary Table 1).
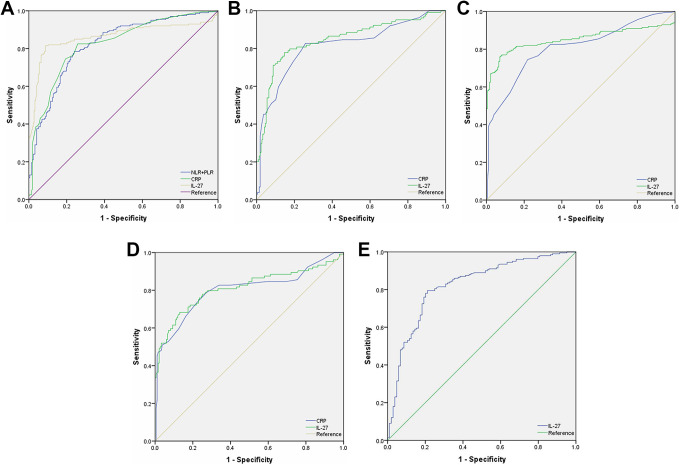
Figure 1.
Diagnostic significance of preoperative WBC (white blood cell), NE (neutrophil), PLT (platelet), LY (lymphocyte), MO (monocyte), NLR (neutrophil/lymphocyte ratio), PLR (platelet/lymphocyte ratio), MLR (monocyte/lymphocyte ratio), CRP (C-reactive protein), TNF-α (tumor necrosis factor-α), interleukin (IL)-1β, IL-2, IL-27 and their paired combinations like NLR+PLR, NLR+MLR and PLR+MLR for patients with FTC (follicular thyroid carcinoma), PTC (papillary thyroid carcinoma), TA (thyroid adenoma) and healthy controls. A, AUC (areas under the curve) 0.824 (95% CI, 0.784-0.864) for NLR+PLR, AUC 0.826 (95% CI, 0.786-0.866) for CRP, and AUC 0.864 (95% CI, 0.824-0.904) for IL-27 between PTC and healthy control group; B, AUC 0.815 (95% CI, 0.760-0.869) for CRP and AUC 0.846 (95% CI, 0.795-0.897) IL-27 between FTC and healthy control group; C, AUC 0.852 (95% CI, 0.809-0.895) for IL-27 and AUC 0.807 (95% CI, 0.764-0.851) for CRP between PTC group and TA group; D, AUC 0.811 (95% CI, 0.752-0.870) for IL-27 and AUC 0.801 (95% CI, 0.742-0.861) for CRP between FTC group and TA group; E, AUC 0.823 (95% CI, 0.773-0.874) for IL-27 between PTC group and FTC group.
Compared with TA or healthy controls, the performances of the above-mentioned inflammatory factors together with related combinations in the diagnosis of PTC or FTC patients were evaluated. It was obtained from ROC analysis in Figure 1A and supplementary Table 1 that, NLR+PLR (AUC, 0.824; 95% CI, 0.784-0.864; P < 0.001), CRP (AUC, 0.826; 95% CI, 0.786-0.866; P < 0.001), and IL-27 (AUC, 0.864; 95% CI, 0.824-0.904; P < 0.001) showed higher potential in predicting PTC patients than in healthy controls. In addition, CRP (AUC, 0.815; 95% CI, 0.760-0.869; P < 0.001) and IL-27 (AUC, 0.846; 95% CI, 0.795-0.897; P < 0.001) showed higher ability in predicting FTC patients than in healthy controls (Figure 1B, supplementary Table 1). Besides, IL-27 (AUC, 0.852; 95% CI, 0.809-0.895; P < 0.001) and CRP (AUC, 0.807; 95% CI, 0.764-0.851; P < 0.001) showed higher ability in predicting PTC patients than in TA patients (Figure 1C, supplementary Table 1). Moreover, IL-27 (AUC, 0.811; 95% CI, 0.752-0.870; P < 0.001) and CRP (AUC, 0.801; 95% CI, 0.742-0.861; P < 0.001) exhibited greater significance in predicting FTC patients than in TA patients (Figure 1D, supplementary Table 1). Compared with FTC patients, IL-27 (AUC, 0.823; 95% CI, 0.773-0.874; P < 0.001) had a higher ability in predicting and differentially diagnosing 2 DTC pathological subtypes (Figure 1E, supplementary Table 1). Table 3 displays the specificity, sensitivity, and threshold for every factor.
Table 3.
Sensitivity, Specificity and Thresholds for the Differential Diagnosis of Inflammatory Markers in Patient With TA, PTC and FTC.
| HC vs TA | HC vs PTC | HC vs FTC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| sensitivity | specificity | cutoff | sensitivity | specificity | cutoff | sensitivity | specificity | cutoff | |
| WBC | 0.585 | 0.535 | 6.095 | 0.755 | 0.535 | 6.095 | 0.827 | 0.618 | 6.335 |
| NE | 0.727 | 0.419 | 3.499 | 0.470 | 0.972 | 4.801 | 0.587 | 0.968 | 4.705 |
| PLT | 0.514 | 0.737 | 264.510 | 0.430 | 0.931 | 284.500 | 0.567 | 0.931 | 285.000 |
| LY | 0.120 | 0.931 | 2.650 | 0.470 | 0.65 | 2.050 | 0.558 | 0.650 | 2.050 |
| MO | 0.246 | 0.829 | 0.525 | 0.730 | 0.608 | 0.455 | 0.577 | 0.793 | 0.515 |
| NLR | 0.803 | 0.300 | 1.509 | 0.740 | 0.627 | 2.205 | 0.404 | 0.903 | 3.091 |
| PLR | 0.617 | 0.622 | 130.527 | 0.705 | 0.756 | 145.780 | 0.654 | 0.650 | 134.247 |
| MLR | 0.672 | 0.442 | 0.210 | 0.550 | 0.585 | 0.245 | 0.779 | 0.355 | 0.191 |
| NLR+PLR | 0.617 | 0.622 | 132.036 | 0.785 | 0.760 | 147.985 | 0.615 | 0.793 | 137.338 |
| NLR+MLR | 0.524 | 0.518 | 1.719 | 0.740 | 0.627 | 2.450 | 0.404 | 0.903 | 3.282 |
| PLR+MLR | 0.557 | 0.673 | 130.737 | 0.705 | 0.756 | 146.025 | 0.654 | 0.650 | 134.438 |
| CRP | 0.694 | 0.461 | 0.650 | 0.825 | 0.742 | 1.050 | 0.827 | 0.742 | 1.050 |
| TNF-α | 0.585 | 0.677 | 23.750 | 0.610 | 0.834 | 30.550 | 0.567 | 0.945 | 36.950 |
| IL-1β | 0.585 | 0.562 | 3.450 | 0.585 | 0.931 | 5.050 | 0.567 | 0.963 | 5.250 |
| IL-2 | 0.060 | 0.783 | 26.750 | 0.435 | 0.318 | 21.850 | 0.231 | 0.318 | 21.850 |
| IL-27 | 0.809 | 0.419 | 49.400 | 0.815 | 0.912 | 118.100 | 0.779 | 0.853 | 106.000 |
| TA vs PTC | TA vs FTC | PTC vs FTC | |||||||
| sensitivity | specificity | cutoff | sensitivity | specificity | cutoff | sensitivity | specificity | cutoff | |
| WBC | 0.725 | 0.464 | 6.175 | 0.808 | 0.552 | 6.385 | 0.731 | 0.465 | 6.525 |
| NE | 0.535 | 0.836 | 4.502 | 0.567 | 0.907 | 4.996 | 0.567 | 0.580 | 5.081 |
| PLT | 0.335 | 0.978 | 349.000 | 0.567 | 0.787 | 285.50 | 0.567 | 0.575 | 285.500 |
| LY | 0.470 | 0.623 | 2.050 | 0.433 | 0.749 | 2.250 | 0.490 | 0.640 | 2.150 |
| MO | 0.730 | 0.601 | 0.455 | 0.644 | 0.694 | 0.485 | 0.269 | 0.915 | 0.600 |
| NLR | 0.725 | 0.623 | 2.239 | 0.481 | 0.825 | 2.771 | 0.192 | 0.910 | 4.071 |
| PLR | 0.705 | 0.557 | 145.647 | 0.269 | 0.858 | 193.421 | 0.058 | 0.950 | 270.828 |
| MLR | 0.550 | 0.563 | 0.249 | 0.760 | 0.301 | 0.198 | 0.779 | 0.275 | 0.191 |
| NLR+PLR | 0.745 | 0.672 | 147.886 | 0.481 | 0.825 | 196.367 | 0.452 | 0.735 | 274.899 |
| NLR+MLR | 0.725 | 0.623 | 2.488 | 0.481 | 0.825 | 2.969 | 0.544 | 0.539 | 4.262 |
| PLR+MLR | 0.705 | 0.557 | 145.896 | 0.531 | 0.518 | 193.619 | 0.452 | 0.735 | 271.019 |
| CRP | 0.745 | 0.781 | 1.250 | 0.788 | 0.727 | 1.150 | 0.144 | 0.930 | 3.000 |
| TNF-α | 0.460 | 0.880 | 38.100 | 0.558 | 0.880 | 38.100 | 0.519 | 0.580 | 39.550 |
| IL-1β | 0.665 | 0.787 | 4.450 | 0.692 | 0.787 | 4.450 | 0.462 | 0.625 | 6.350 |
| IL-2 | 0.405 | 0.595 | 22.250 | 0.250 | 0.372 | 21.750 | 0.356 | 0.410 | 20.750 |
| IL-27 | 0.760 | 0.929 | 143.400 | 0.683 | 0.869 | 124.600 | 0.795 | 0.212 | 209.000 |
Abbreviations: HC, healthy control; TA, thyroid adenomas; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; WBC, white blood cell; NE, neutrophil; LY, lymphocyte; MO, monocyte; PLT, platelet; NLR, neutrophil/ lymphocyte ratio; PLR, platelet/ lymphocyte ratio; MLR, monocyte/ lymphocyte ratio; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-27, interleukin-27.
Inflammatory Factors Effects on DTC
The multivariable logistic regression model was constructed for DTC, as presented in Table 4. The results revealed that, WBC, NE, PLT, MO, CRP, IL-2, IL-27 and IL-1β were related to DTC. Meanwhile, the contents of WBC, NE, CRP, IL-1β, IL-2 and IL-27 were related to DTC after adjusting for Age. Furthermore, after adjusting for Age and Sex, the levels of CRP, IL-2 and IL-27 also showed significant association with DTC onset.
Table 4.
Impacts of Inflammatory Markers on DTC in Multivariate Model.
| Unadjusted | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| WBC | 1.542 (1.351-1.794) | 0.012 | 1.513 (1.327-1.781) | 0.019 | 2.237 (0.315-10.238) | 0.812 |
| NE | 2.393 (1.725-3.318) | <0.001 | 2.230 (1.547-3.526) | 0.001 | 2.901 (0.216-21.307) | 0.073 |
| PLT | 1.070 (1.034-1.108) | <0.001 | 1.013 (1.011-1.264) | 0.141 | 1.006 (0.847-3.571) | 0.271 |
| LY | 1.003 (0.994-1.009) | 0.377 | 1.002 (0.992-1.009) | 0.379 | 1.002 (0.491-2.218) | 0.746 |
| MO | 1.141 (1.113-1.231) | 0.004 | 1.084 (1.104-1.245) | 0.103 | 1.013 (0.346-1.715) | 0.417 |
| NLR | 1.018 (1.005-1.022) | 0.253 | 1.017 (1.004-1.019) | 0.257 | 1.287 (0.612-2.548) | 0.671 |
| PLR | 1.011 (1.002-1.017) | 0.184 | 1.010 (1.002-1.013) | 0.221 | 1.137 (0.014-17.422) | 0.628 |
| MLR | 1.004 (0.995-1.006) | 0.238 | 1.004 (0.998-1.006) | 0.562 | 1.001 (0.913-1.097) | 0.614 |
| CRP | 1.401 (1.210-1.721) | <0.001 | 1.392 (1.210-1.718) | <0.001 | 1.384 (1.208-1.715) | <0.001 |
| TNF-α | 1.136 (1.094-1.177) | 0.118 | 1.132 (1.091-1.172) | 0.124 | 1.088 (0.021-8.159) | 0.867 |
| IL-1β | 1.987 (1.264-3.124) | 0.003 | 1.875 (1.251-2.815) | 0.011 | 1.507 (0.133-6.143) | 0.242 |
| IL-2 | 0.588 (0.430-0.802) | 0.001 | 0.760 (0.599-0.973) | 0.014 | 0.829 (0.364-2.176) | 0.037 |
| IL-27 | 1.161 (1.134-1.189) | <0.001 | 1.158 (1.133-1.187) | <0.001 | 1.146 (1.128-1.185) | <0.001 |
Model I was adjusted for Age; Model II was adjusted for Age, Sex.
Abbreviations: CI, confidence interval; OR, odds ratio; WBC, white blood cell; NE, neutrophil; LY, lymphocyte; MO, monocyte; PLT, platelet; NLR, neutrophil/ lymphocyte ratio; PLR, platelet/ lymphocyte ratio; MLR, monocyte/ lymphocyte ratio; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-27, interleukin-27; DTC, differentiated thyroid carcinoma.
Discussion
The present study suggested that, chronic inflammation was significantly associated with carcinoma. As related research goes, factors that can serve as the simple inflammatory system indexes, such as NLR,15 PLT and leukocyte,16,17 have been widely used as biomarkers in detecting and monitoring malignancy. Nonetheless, little is known about the relationship of these inflammatory factors, like IL-6,14 IL-17, and IL-35 with thyroid cancer,18 together with their potential as biomarkers for thyroid cancer. Therefore, the present work further assessed the associations of other preoperative blood inflammatory factors with thyroid cancer.
According to our results of tests for peripheral blood inflammatory markers, the WBC, NE, PLT, MO, CRP, IL-1β, IL-27 and TNF-α levels were higher, whereas IL-2 level was lower in TA and DTC groups than those in other groups. This meant that TA and DTC might be associated with inflammation and inflammatory factors. NLR and PLR are valuable inflammatory factors, which reflect the balance between NE or PLT and LY immune response. As suggested in some recent studies, NLR and PLR, which are easily obtained from blood tests, significantly elevate in patients with PTC or FTC, and such results are consistent with our.19 Ari A et al. reported that NLR and PLR were higher in patients with papillary thyroid cancer than in healthy individuals, with statistically significant difference in PLR.20 Similarly, in the current study, PLR was significantly higher in patients with PTC than in healthy individuals, and NLR was also significantly higher. Such inconsistency might be because that, multiple parameters were associated with the study methodology and population. Cho JS et al. also reported that the proportions of PTC and FTC were not significant difference between the high and low NLR groups.21 The difference in baseline levels among the study populations, such as FTC (104vs34), may be another reason for the difference in research results. In addition, they found that the NLR was significant difference and high in poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC) as compared to DTC.
The increased IL-27 contents in serum are detected in lung cancer, esophageal cancer, breast cancer and gastro-esophageal cancer.22-25 However, the IL-27 role in cancer remains controversial. IL-27 can enhance the antitumor immunity to inhibit regulatory T cell differentiation and angiogenesis. It can also induce IL-10 to resist the proinflammatory immune reactions, thus favoring tumor development.26,27 In addition, the level of IL-27Rα in diverse cancer cell types may be related to effector response suppression and cancer growth promotion. These mechanisms have supported that, inflammation is related to thyroid cancer development and progression.
According to related epidemiological research, the increased CRP (a classical acute phase protein) contents in circulation, may mark the presence of prevalent tumor.28 It is also reported that, the higher preoperative levels of CRP are associated with DTC, and they may serve as the potential serum biomarker in PTC.29,30 However, the specific mechanism of circulating CRP levels and tumor microenvironment remains unknown at present. The Mendelian31 randomization design reported that, the CRP genotypes (4 SNPs) are not related to the risks of colorectal, lung, prostate, breast, or urinary tract and bladder cancers. The genetic variation outside the CRP locus, such as IL-6 receptor, may partially explain for the relationship between CRP and tumors in the presence of high IL-6 levels in most tumors including thyroid cancer.
Although most patients can be diagnosed by clinical manifestations and B-ultrasound, thyroid cancer, such as FTC, PTC and TA, can hardly be differentially diagnosed before operation. In our study, preoperative contents of NE, WBC, PLT, MO, LY, MLR, NLR, PLR, CRP, TNF-α, IL-1β, IL-2, IL-27 together with corresponding combinations were assessed using the ROC curves, and only CRP and IL-27 had the AUCs of greater than 0.8. In addition, the AUC value of IL-27 was higher than 0.8 between PTC and FTC groups, and that of NLR+PLR was also higher than 0.8 between PTC and healthy control groups. In the present work, the multivariable logistic regression model was also established for thyroid tumor; CRP, IL-2 and IL-27 were relevant to DTC after adjusting for the possible confounding factors. Based on those aforementioned findings, we speculated that CRP and IL-27 were closely related to DTC progression, and that NLR+PLR might also be tightly correlated with PTC progression. NLR+PLR is identified in prior work to exhibit predicting significance in a variety of cancer types. In thyroid cancer, NLR and PLR have diagnostic and predictive significance for advanced differentiated thyroid cancer and ATC, while the diagnostic and predictive significance of NMPLR is far superior to that of NLR or PLR.32 Similarly, in our study, NLR and PLR were significant for the diagnosis and prediction of PTC cases and healthy individuals, and the combination of NLR+PLR was also meaningful, which achieved far superior diagnostic effect than the individual one. Moreover, IL-27 rs17855750 and rs153109 have been proved to be associated with PTC occurrence, which is partially consistent with our results.33 The relationship of the FTC incidence with the gene polymorphisms of IL-27 remains unreported, and it may lead to the significantly different levels of IL-27 between PTC and FTC patients. Generally, FTC is identified as a comparatively aggressive subtype, which avoids the unnecessary overhand measures for thyroid tumors. As a result, it is necessary to identify the preoperative peripheral blood inflammatory factors to diagnose DTC. The different expressions of CRP and IL-27 may be potentially used to distinguish PTC and FTC from thyroid tumors. Our preliminary study found that inflammatory factors were not significantly correlated with the progression of thyroid tumors. We will continue to study them further in the later period. The research on the relationships between CRP, IL-27 and pathological tissue and prognosis of DTC is the specific direction of our follow-up further research. Nonetheless, some limitations should be noted in the present work. Firstly, this single-center study had small sizes of TA, PTC and FTC patients. Therefore, the prospective and multicenter studies with larger sample sizes should be performed for confirming these findings. Secondly, false positive results might be obtained when screening the asymptomatic populations due to conditions like systemic inflammation.
Conclusions
The present work indicates that, inflammation shows a close relationship with thyroid tumor based on our statistical results. The preoperative inflammatory markers, such as CRP and IL-27, may help us differentially diagnose DTC. In addition, IL-27 may partially assist in distinguishing FTC from PTC, and NLR+PLR may play a vital part during the differential diagnosis of PTC.
Supplemental Material
Supplemental Material, sj-docx-1-tct-10.1177_1533033821990055 for Relationship Between Serum Inflammatory Factor Levels and Differentiated Thyroid Carcinoma by Xue Zhang, Su Li, Jinhui Wang, Fubao Liu and Yong Zhao in Technology in Cancer Research & Treatment
Acknowledgments
Our thanks should go to all the staff for their assistance and suggestions on the present work.
Abbreviations
- AUC
areas under the curve
- CRP
C-reactive protein
- DTC
differentiated thyroid carcinoma
- FTC
follicular thyroid carcinoma
- IL-1β
interleukin-1β
- IL-2
interleukin-2
- IL-27
interleukin-27
- LY
lymphocyte
- MO
monocyte
- MLR
monocyte/lymphocyte ratio
- NE
neutrophil
- NLR
neutrophil/lymphocyte ratio
- PLT
platelet
- PLR
platelet/lymphocyte ratio
- PTC
papillary thyroid carcinoma
- ROC
receiver operating characteristics
- TNF-α
tumor necrosis factor-α
- TA
thyroid adenomas
- WBC
white blood cell.
Authors’ Note: Xue Zhang, Deparment of Surgical Oncology, Lu’an Hospital Affiliated to Anhui Medical University, Lu’an, China, 237000; Su Li, Department of Oncology Radiotherapy, Lu’an Hospital of Traditional Chinese Medicine, Lu’an, China, 237000; Jin-hui Wang, Deparment of Cardiothoracic Surgery, Lu’an Hospital Affiliated to Anhui Medical University y, Lu’an, China; Fu-bao Liu, Deparment of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China, 230022; Yong Zhao, Deparment of Surgical Oncology, Lu’an Hospital Affiliated to Anhui Medical University, Lu’an, China, 237000, e-mail ZYZY8151@sina.com.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Statement: The study design was approved by the Medical Ethics Committee of the Lu’an Hospital Affiliated to Anhui Medical University (Approval Number 2018YJ023) and informed consent was obtained from all participants.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xue Zhang, MM  https://orcid.org/0000-0001-8336-5256
https://orcid.org/0000-0001-8336-5256
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Shah JP. Thyroid carcinoma: epidemiology, histology, and diagnosis. Clin Adv Hematol Oncol. 2015;13(4 Suppl 4):3–6. [PMC free article] [PubMed] [Google Scholar]
- 2. American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 3. Lundgren CI, Hall P, Ekbom A, Frisell J, Zedenius J, Dickman PW. Incidence and survival of Swedish patients with differentiated thyroid cancer. Int J Cancer. 2003;106(4):569–573. [DOI] [PubMed] [Google Scholar]
- 4. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. [DOI] [PubMed] [Google Scholar]
- 5. Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43(3):374–379. [DOI] [PubMed] [Google Scholar]
- 6. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–84. [DOI] [PubMed] [Google Scholar]
- 7. Chang PH, Pan YP, Fan CW, et al. Pretreatment serum interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha levels predict the progression of colorectal cancer. Cancer Med. 2016;5(3):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohe Y, Fushida S, Yamaguchi T, et al. Peripheral blood platelet-lymphocyte ratio is good predictor of chemosensitivity and prognosis in gastric cancer patients. Cancer Manag Res. 2020;12:1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma Z, Qi Z, Shan Z, Li J, Yang J, Xu Z. The role of CRP and ATG9B expression in clear cell renal cell carcinoma. Biosci Rep. 2017;37(6):BSR20171082. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Wang M, Wu M, Yang T. The synergistic effect of sorafenib and TNF-alpha inhibitor on hepatocellular carcinoma. EBioMedicine. 2019;40:11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinagawa K, Yanamoto S, Naruse T, et al. Clinical roles of interleukin-6 and stat3 in oral squamous cell carcinoma. Pathol Oncol Res. 2017;23(2):425–431. [DOI] [PubMed] [Google Scholar]
- 13. Yokota M, Katoh H, Nishimiya H, et al. Lymphocyte-monocyte ratio significantly predicts recurrence in papillary thyroid cancer. J Surg Res. 2020;246:535–543. [DOI] [PubMed] [Google Scholar]
- 14. Kobawala TP, Trivedi TI, Gajjar KK, Patel DH, Patel GH, Ghosh NR. Significance of interleukin-6 in papillary thyroid carcinoma. J Thyroid Res. 2016;2016:6178921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilincalp S, Coban S, Akinci H, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015;24(4):328–333. [DOI] [PubMed] [Google Scholar]
- 16. Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res. 2018;78(13):3407–3412. [DOI] [PubMed] [Google Scholar]
- 17. Morrison L, Laukkanen JA, Ronkainen K, Kurl S, Kauhanen J, Toriola AT. Inflammatory biomarker score and cancer: a population-based prospective cohort study. BMC Cancer. 2016;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu Y, Yuan Y. Serum level of interleukin-17 and interleukin-35 as a biomarker for diagnosis of thyroid cancer. J Cancer Res Ther. 2015;11(Suppl 2):C209–211. [DOI] [PubMed] [Google Scholar]
- 19. Ceylan Y, Kumanlioglu K, Oral A, Ertan Y, Ozcan Z. The correlation of clinicopathological findings and neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in papillary thyroid carcinoma. Mol Imaging Radionucl Ther. 2019;28(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ari A, Gunver F. Comparison of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with thyroiditis and papillary tumors. J Int Med Res. 2019;47(5):2077–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cho JS, Park MH, Ryu YJ, Yoon JH. The neutrophil to lymphocyte ratio can discriminate anaplastic thyroid cancer against poorly or well differentiated cancer. Ann Surg Treat Res. 2015;88(4):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naumnik W, Naumnik B, Niewiarowska K, Ossolinska M, Chyczewska E. Novel cytokines: IL-27, IL-29, IL-31 and IL-33. Can they be useful in clinical practice at the time diagnosis of lung cancer? Exp Oncol. 2012;34(4):348–353. [PubMed] [Google Scholar]
- 23. Tao YP, Wang WL, Li SY, et al. Associations between polymorphisms in IL-12A, IL-12B, IL-12Rbeta1, IL-27 gene and serum levels of IL-12p40, IL-27p28 with esophageal cancer. J Cancer Res Clin Oncol. 2012;138(11):1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu D, Zhou X, Yao L, Liu C, Jin F, Wu Y. Clinical implications of the interleukin 27 serum level in breast cancer. J Investig Med. 2014;62(3):627–631. [DOI] [PubMed] [Google Scholar]
- 25. Diakowska D, Lewandowski A, Markocka-Maczka K, Grabowski K. Concentration of serum interleukin-27 increase in patients with lymph node metastatic gastroesophageal cancer. Adv Clin Exp Med. 2013;22(5):683–691. [PubMed] [Google Scholar]
- 26. Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37(6):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol. 2011;23(6):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–2418. [DOI] [PubMed] [Google Scholar]
- 29. Shimura T, Shibata M, Gonda K, et al. Prognostic impact of elevated preoperative C-reactive protein on patients with differentiated thyroid carcinoma. J Surg Res. 2018;231:338–345. [DOI] [PubMed] [Google Scholar]
- 30. Wen W, Wu P, Li J, Wang H, Sun J, Chen H. Predictive values of the selected inflammatory index in elderly patients with papillary thyroid cancer. J Transl Med. 2018;16(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a Mendelian randomization study. J Natl Cancer Inst. 2010;102(3):202–206. [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Luo H, Wang L, et al. Diagnostic and prognostic value of preoperative systemic inflammatory markers in anaplastic thyroid cancer. J Surg Oncol. 2020;122(5):897–905. [DOI] [PubMed] [Google Scholar]
- 33. Nie X, Yuan F, Chen P, et al. Association between IL-27 gene polymorphisms and risk of papillary thyroid carcinoma. Biomark Med. 2017;11(2):141–149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-tct-10.1177_1533033821990055 for Relationship Between Serum Inflammatory Factor Levels and Differentiated Thyroid Carcinoma by Xue Zhang, Su Li, Jinhui Wang, Fubao Liu and Yong Zhao in Technology in Cancer Research & Treatment