Abstract
Evidence has shown that long non-coding RNAs (lncRNA) play pivotal roles in cancer promotion as well as suppression. But the molecular mechanism of lncRNA TMPO antisense transcript 1 (TMPO-AS1) in lung cancer (LC) remains unclear. This study mainly investigated the effect of TMPO-AS1 in LC treatment. TMPO-AS1 was tested by Kaplan-Meier method. Quantitative real time polymerase chain reaction (qRT-PCR) was employed to assess the expressions of TMPO-AS1, miR-143-3p, and CDK1 respectively in LC tissues and cell lines. TMPO-AS1, miR-143-3p and CDK1 expressions in LC cells were regulated through cell transfection, followed by MTT for cell viability detection. Besides, dual-luciferase reporter assays were performed to verify the interrelated microRNA of TMPO-AS1 and the target of miR-143-3p. Western blot experiments were used to examine the expressions of apoptosis-related proteins, and flow cytometry tested the cell apoptosis in treated cells. TMPO-AS1 and CDK1 were overexpressed in LC tissues and cells, while miR-143-3p level was suppressed. The decrease of TMPO-AS1 led to the increase of miR-143-3p, which further resulted in the reduction of CDK1. Down-regulating TMPO-AS1 reduced LC cell viability, motivated cell apoptosis, as well as promoted the expressions of Bcl and CCND1 and restrained Caspase-3 level, but all these consequences were abrogated by miR-143-3p inhibitor. Simultaneously, siCDK1 could reverse the anti-apoptosis and pro-activity functions of miR-143-3p inhibitor in LC cells. Down-regulation of TMPO-AS1 has the effects of pro-apoptosis in LC by manipulating miR-143-3p/CDK1, which is hopeful to be a novel therapy for LC patients.
Keywords: TMPO antisense transcript 1, lung cancer, cyclin-dependent kinase 1, miR-143-3p
Introduction
Lung cancer (LC) is still a form of cancer with high morbidity and mortality throughout the world, especially prevalent in most developed countries.1,2 Statistics has shown that in America 158,040 people died of lung cancer in 2015, which equaled to more than one quarter of all cancer-related deaths.3 Small-cell carcinoma (SCLC) and non-small-cell carcinoma (NSCLC) are 2 main subtypes of lung cancer classified by histopathology and clinical therapy.3-5 Between them, NSCLC accounts for the majority of all the cases and can be histologically further categorized into lung adenocarcinoma (LUAD) and lung squamous carcinoma (LUSC).6,7 Surgical resection, medication and radiation were the remedies most commonly used for LC, but the curative effects of improving survivals remain controversial.8 Thus, exploring new therapies of LC is of practical significance for LC patients.
Non-coding RNAs (ncRNA) are kinds of transcribed genomes that do not encode proteins,9 among which long non-coding RNAs (lncRNA) show great universality and functional multiplicity.9,10 Commonly, ncRNAs with more than 200 nt in length are defined as lncRNA, but Amaral et al.11 suggested that lncRNA should be initial or joint transcripts, differentiating from current-known other sorts of ncRNAs. Literature has shown that lncRNA does function in human cancers,12 for instance, TMPO antisense RNA 1 (TMPO-AS1) gene was found in chromosome 12, and it has been verified to promotes Prostate cancer (PCa) by inhibiting cell apoptosis, as well as motivating cell proliferation, migration and cycle process in PCa cells.13 Another class of ncRNAs is called microRNAs (miRNA), which interacts with lncRNA14 and also matters in human cancers.12 On the basis of current data, miR-143 locates on chromosome 5q32, decreasing in a large amount of tumors, such as ovarian cancer, breast cancer, colorectal cancer and so on.15-17 Evidence has shown the inhibitory effect of miR-143 works through pathway G1-S transition,18 with cyclin-dependent kinase 1 (CDK1) a regulatory factor in the process of cell cycle.19
TMPO-AS1 promotes the proliferation and viability of estrogen receptor (ER)-positive breast cancer cells in vitro and in vivo.20 Besides, little research has been done on TMPO-AS1, not to mention its potential effect in LC. Long noncoding RNA TMPO-AS1 promotes progression of non-small cell lung cancer through regulating its natural antisense transcript TMPO.21 However, the molecular mechanisms of miR-143-3p and CDK1 remain unclear in LC cells. Here, we set out to study the potential therapeutic effects of TMPO-AS1 in LC cells and its underlying working mechanisms. If the hypothesis were to hold, molecular-biological approaches are hopefully benefit large amounts of LC patients.
Materials and Methods
Clinical Specimen Collection
LC tissues were obtained from 50 numbers of patients including 27 males and 23 females with a median age of 60, who were diagnosed with the disease in Zhuji Affiliated Hospital of Shaoxing University from May, 2017 to May, 2018. Each group of specimens included cancerous tissue and corresponding adjacent normal lung tissue (5 cm from the margin of the tumor). All the patients did not undergo radiotherapy or chemotherapy before surgery. They had signed informed consent, and agreed that their tissues would be used for clinical research. The clinical trial program had been reviewed and approved by the Ethics Committee of Zhuji Affiliated Hospital of Shaoxing University (approval no.: AS20170245834). All patients provided written informed consent prior to enrollment in the study.
Data Source
Through starBase v3.0 project, the data of TMPO-AS1 and miR-143-3p expression in LUAD samples, LUSC samples and normal samples were collected and compared. Besides, the predictive target gene miR-143-3p and target protein CDK1 were locked on respectively by starBase v3.0 as well as TargetScan7.2 (http://www.targetscan.org/vert_72/).
Cell Culture
Human bronchial epithelial cell line 16HBE and 4 LC cell lines (H1299, A549, 95D, H125) were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were maintained in RPMI-1640 medium (Gibco, Rockville, MO), containing 10% fetal bovine serum (FBS, Gibco, USA), 100 units/ml penicillin (TargetMol, Boston, UK) and 100 µg/ml streptomycin (TargetMol, Boston, UK). The cell culture process took place in a humid incubator at 37°C with 5% CO2. After 24 h cells in logarithmic phase could be harvested for further experiment.
Cell Transfection
Modified plasmids siTMPO-AS1, siCDK1, and miR-143-3p inhibitor were commercially structured by BlueGene Biotech (http://www.elisakit.cc, Shanghai, China), and scrambled sequence was used as negative control (NC). Cultured cells H1299 and A549 were transfected with TMPO-AS1 small interfering RNA (siTMPO-AS1, sense: 5’-UUUAAACUGCGUUUCUACCUC-3’; antisense: 5’-GGUAGAAACGCAGUUUAAAAG-3’) or siNC, miR-143-3p inhibitor or inhibitor control, miR-143-3p mimics or mimics control, siTMPO-AS1 plus miR-143-3p inhibitor or inhibitor control, siCDK1 (sense: 5’-UAUUUUGGUAUAAUCUUCCAU-3’; antisense: 5’-GGAAGAUUAUACCAAAAUAGA -3’) or siNC, miR-143-3p inhibitor plus siNC or siCDK1, as well as by adding 0.15 µL of 15 times diluted transfection reagent Invitrogen Lipofectamine 3000 (Thermo Fisher Scientific, Massachusetts, US), followed by the measurement of transfection efficiency through quantitative real-time polymerase chain reaction (qRT-PCR).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNAs were extracted from LC tissues and cells with TRIzol Reagent (Thermo Fisher Scientific, Massachusetts, US), RNAs of nuclear and cytoplasm were isolated by Cytoplasmic and Nuclear RNA Purification Kit (Norgen, Thorold, ON, Canada). Then the concentration was tested by UV spectrophotometer DR3900 (HACH, NY, US) at a wavelength of 280 nm. By using TaqMan microRNA reverse transcription kit (Thermo Fisher Scientific, Massachusetts, US), reverse transcription was performed and the first-strand cDNA was synthesized. Then qRT-PCR was operated with BlazeTaq™ SYBR Green qRT-PCR Mix (BioCat, Heidelberg, Germany) in PCR machine CFX96 Touch (Bio-Rad, California, US) under following conditions: 2 minutes at 95°C, 45 cycles for 5 seconds at 94°C and 40 seconds at 60°C. Then the comparative cycle threshold (CT) method (2−ΔΔCT) was used to calculate the relative expression of each mRNA.22
The specific primers were as follows: TMPO-AS1: 5’-AGCCAGACCTCTACAATCGG-3’ (forward) and 5’-TTAGGATTCTTGCGGGTGGT-3’ (reverse); hsa-miR-143-3p: 5’-TGAGATGAAGCACTGTAGCTC-3’ (forward) and 5’-GAGCTACAGTGCTTCATCTCA-3’ (reverse); CDK1: 5’-TGGGGTCAGCTCGTTACTCA-3’ (forward) and 5’-CACTTCTGGCCACACTTCATTTA-3’ (reverse); U6: 5’-CTCGCTTCGGCAGCACA-3’ (forward) and 5’-AACGCTTCACGAATTTGCGT-3’ (reverse), GAPDH: 5’-CCTGCACCACCAACTGCTTA-3’ (forward) and 5’-GGCCATCCACAGTCTTCTGAG-3’ (reverse),23 among which GAPDH and U6 were performed as internal reference control.
Dual-Luciferase Reporter Assay
Partial sequences of the TMPO-AS1 and CDK1 3’ UTR containing putative pairing bases with miR-143-3p were inserted in the upstream of luciferase-coding sequence by pMIR-REPORT Luciferase vector (BioVecor, Beijing, China). Synthetic plasmids were integrated with miRNA-coupling sites by using the GeneArt Site-Directed Mutagenesis System (Thermo Fisher Scientific, Massachusetts, US). Next, miR-143-3p mimic, wild-type and mutant-type of TMPO-AS1 or CDK1 (TMPO-AS1-WT, TMPO-AS1-MUT, CDK1-WT and CDK1-MUT) commercially constructed by BlueGene Biotech (http://www.elisakit.cc, Shanghai, China) was transfected into LC cells, together with miR-143-3p inhibitors or miR-143-3p mimics. When cells lysed after 24 h, the relative luciferase activity was measured in the Dual-Luciferase Reporter Assay System (Promega, Madison, US).
MTT Assay
MTT assay was performed to monitor cell growth and cell viability at different time. After H1299 and A549 cells were cultured and transfected with siNC, siTMPO-AS1, siTMPO-AS1plus miR-143-3p inhibitor and siTMPO-AS1 plus inhibitor control respectively, 10 µL of MTT solution (5 mg/ml, SIGMA, Saint Louis, US) was added to each well at 24 h, 48 h and 72 h after transfection. Another set of experiments were performed by transfecting miR-143-3p inhibitor, inhibitor control, siNC plus inhibitor or siCDK1 plus inhibitor into LC cells, but MTT solution was added at 48 h after transfection only. Then cells were incubated at 37°C with 5% CO2 for another 4 h. Next, MTT solution was removed followed by adding 150 µL of dimethyl sulphoxide (DMSO, SIGMA, Saint Louis, US) to each well. Optical density (OD) was recorded at a wavelength of 570 nm in a microplate reader (Bio-Rad, California, US).
Flow Cytometry Analysis
H1299 and A549 cellular apoptosis was measured with Flow cytometry method by using Annexin V-FITC Apoptosis Detection Kit (SIGMA-ALDRICH, Cambridge, UK) in accordance with the specification. After transfection for 48 h at 37°C with 5% CO2, cells were rinsed with pre-cooled phosphate buffer sodium (PBS, Thermo Fisher Scientific, Massachusetts, US) and then re-suspended in buffer solution mixed with Annexin V-FITC and 50 mg/ml propidium iodide (PI) at 37°C for 1 h under light-proof conditions. The fluorescence was imaged and analyzed in flow cytometer CytoFLEX (Beckman Coulter, California, US).
Western Blot (WB) Assay
WB experiments were performed to detect the expression of proteins in LC cells transfected with molecules siNC, siCDK1, and siTMPO-AS1 with or without the combination of miR-134-3p inhibitor. Total proteins were extracted from cells and tissues with PIERCE RAPI Buffer (Thermo Fisher Scientific, MMAS, US) for 30 minutes on ice, followed by centrifugation with 14000rmp at 4°C for 10 minutes. Bicinchoninic Acid (BCA) Protein Quantitative Kit (SIGMA-ALDRICH, Cambridge, UK) was used to detect protein concentration according to manufacturers’ instructions. Then protein samples was separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, Solarbio, Beijing, China) in a boiling water bath for 3 minutes, after which transmembrane was conducted on polyvinylidene fluoride (PVDF). Further, 5% non-fat dried milk was used to block the transferred membrane for 1 h, and washed away with TBST. Next, membrane was incubated under the coaction of primary antibodies plus blocking buffer at 4°C overnight. Primary antibodies were CDK1 (1:1000, ab18, Abcam, US), GAPDH (1:20000, ab8245, Abcam, US), C caspase-3 (1:1000, ab2302, Abcam, US), CCND1 (1:2000, ab16663, Abcam, US) and Bcl-2 (1:1000, ab5934, Abcam, US), among which GAPDH acted as an internal reference. Though GAPDH might not be the best internal reference, the molecular weight of which was close to the target factors, it was commonly used and could be detected. After 3 washes with PBST, another incubation was conducted for 1 h with the secondary antibody Goat Anti-Mouse IgG H&L (1:2000, ab150113, Abcam, USA) and Goat Anti-Rabbit IgG H&L (1:1000, ab6702 Abcam, USA). Finally, FLoid™ Cell Imaging Station (Thermo Fisher Scientific, Massachusetts, US) was used to detect the specific protein brands after rinsing with TBST for 3 times. When the molecular weight of GAPDH was similar to target protein, the target protein and GAPDH could be obtained one by one. After obtaining target protein, the PVDF membrane was washed carefully, and primary antibody of GAPDH was incubated with membranes for 30 min at room temperature, a night at 4°C, and 30 min at room temperature to obtain GAPDH bands. It might be a limitation using GAPDH as a internal reference when the molecular weight of GAPDH was similar to target protein, which should be paid attention in future.
Statistical Analysis
Date from all experiments were analyzed by Statistical Product and Service Solutions (SPSS, NDtimes, Beijing, China) and expressed as the mean ± standard (mean ± SD). The student’s t-test were used to compare differences between 2 groups, while one-way analysis of variance (ANOVA) was used for data comparisons among multiple groups. Overall survival was assessed by the Kaplan-Meier method, and the log-rank test was used for conspicuous long-term discrepancies. P-values less than 0.05 were considered statistically significant.
Results
Up-Regulation of Survival-Related TMPO-AS1 and Down-Regulation of miR-143-3p in LC Tissues and LC Cells
According to analysis result of starBase, the expression level of TMPO-AS1 in LUAD and LUSC cancer samples both increased compared with normal ones, whereas the hsa-miR-143-3p expression oppositely decreased (P < 0.001; Figure 1A). Besides, through qRT-PCR assay (n = 50) we compared TMPO-AS1 and miR-143-3p expression levels in para-carcinoma tissues and LC tissues, finding the up-regulation of TMPO-AS1 and down-regulation of miR-143-3p in LC ones (P < 0.05; Figure 1B and C). The Kaplan-Meier survival curves indicated that patients with low TMPO-AS1 expression level (lower than that in corresponding adjacent normal lung tissues) had higher survival rate than those with high TMPO-AS1 expression level (higher than that in corresponding adjacent normal lung tissues) (P < 0.001; Figure 1D). QRT-PCR was also performed to detect the content of TMPO-AS1 and miR-143-3p in human bronchial epithelial cell lines 16HBE and 4 LC cell lines H1299, A549, 95D and H125. TMPO-AS1 expression level increased while miR-143-3p decreased in all LC cell lines (P < 0.05; Figure 1E and F), among which H1299 and A549 cell lines were selected to be representatives of LUAD and LUSC due to greatest expression differences.
Figure 1.
Survival-related TMPO-AS1 was up-regulated in LC tissues and LC cells, together with the down-regulation of miR-143-3p. (A) Box-plots showed the up-regulation of TMPO-AS1 and down-regulation of hsa-miR-143-3p levels in LUAD and LUSC samples. (B, C) TMPO-AS1 and miR-143-3p expression levels in 50 pairs of LC tissues were compared with adjacent normal tissues through qantitative real-time polymerase chain reaction (qRT-PCR) assay. (D) Kaplan-Meier method and log-rank test were used to test how TMPO-AS1 expression level affected survival rate among LC patients. Low: TMPO-AS1 expression in cancer tissue was lower than that in corresponding adjacent normal lung tissues; High: TMPO-AS1 expression in cancer tissue was higher than that in corresponding adjacent normal lung tissues. (E, F) TMPO-AS1 and miR-143-3p expression levels in human bronchial epithelial cells (16HBE) and LC cell lines (H1299, A549, 95D, and H125) were tested through qRT-PCR. ** P < 0.001 vs. normal, # P < 0.05 and ## P < 0.001 vs. 16HBE, n = 3.
Down-Regulation of TMPO-AS1 Promoted miR-143-3p Expression
Putative coupling locus of miR-143-3p on TMPO-AS1 was predicted through starBase v3.0 (Figure 2A). Further, dual-luciferase reporter assays were used for preliminary verification conjecture. Compared with control groups, relative luciferase activity of LC cells significantly increased under the action of TMPO-AS1-WT combined with miR-143-3p inhibitor, which was reserved by miR-143-3p mimic (P < 0.05; Figure 2B and C). Through qRT-PCR assay, expression levels of certain mRNA were measured and comparatively analyzed in subcellular fractions of LC cells. TMPO-AS1 expression in cytoplasm was much higher than that in nuclear (P < 0.001; Figure 2D and E). In addition, miR-143-3p expression level remarkably improved after transfecting siTMPO-AS1 into LC cells (P < 0.001; Figure 2F and G). Nevertheless, TMPO-AS1 expression remained unchanging in miR-143-3p inhibitor transfected LC cells (Figure 2H and I), which implied that TMPO-AS1 might be the upstream regulator of miR-143-3p.
Figure 2.
miR-143-3p was the target of TMPO-AS1. (A) Putative binding site of miR-143-3p on TMPO-AS1 was predicted by starBase v3.0. (B, C) Dual-luciferase reporter assays were performed to detect the H1299 cells and A549 cells co-transfected with wild-type TMPO-AS1 (TMPO-AS1-WT) or mutant-type TMPO-AS1 (TMPO-AS1-MUT), as well as miR-143-p inhibitor, miR-143-3p inhibitor control, miR-143-3p mimic, or miR-143-3p mimic control. (D, E) In LC cells, qantitative real-time polymerase chain reaction (qRT-PCR) assays were used to measure the expressions of GAPDH, U6 and TMPO-AS1 in nuclear and in cytoplasm respectively. (F, G) LC cells were transfected with siNC, siTMPO-AS1, (H, I) miR-143-3p inhibitor or miR-143-3p inhibitor control, followed by the respective detection of genetic expression for miR-143-3p and TMPO-AS1 through qRT-PCR. * P < 0.05 vs. inhibitor control, ^^P < 0.001 vs. mimic control, ## P < 0.001 vs. cytoplasm, △△ P < 0.001 vs. siNC, n = 3.
Down–Regulation of TMPO-AS1 in LC Cells Restrained Cell Viability and Motivated Apoptosis by Targeting miR-143-3p
By performing qRT-PCR assay, siTMPO-AS1 was proved to have the function of reducing the TMPO-AS1 expression in LC cells (P < 0.001; Figure 3A and B). It might be a limitation not showing the role of microRNA inhibitor on 16HBE cells, as the focus was on lung carcinoma cells. MTT assay clearly showed that siTMPO-AS1 decreased the LC cell viability by contrast with siNC, whereas miR-143-3p inhibitor dramatically reversed the inhibitory effect of siTMPO-AS1 (P < 0.05; Figure 3C and D). LC cells apoptosis then was detected through flow cytometry assay. SiTMPO-AS1 significantly promoted apoptosis, while miR-143-3p inhibitor protected cells form apoptosis (P < 0.001; Figure 3E-G). As the cells were in early apoptosis stage, the data of PI-positive cells were little. Proteins levels that related to apoptosis were assayed using WB method. Experiment phenomena demonstrated that siTMPO-AS1 down-regulated Bcl-2 and CCND1 contents, but up-regulated cleaved caspase3 expression. On the contrary, miR-143-3p inhibitor abrogated the function of siTMPO-AS1 to a large extent (P < 0.05; Figure 3H-J).
Figure 3.
TMPO-AS1 silencing in LC cells regulated cell viability and cell apoptosis, which could be reversed by miR-143-3p inhibitor. (A, B) TMPO-AS1 expression levels were assayed through qantitative real-time polymerase chain reaction (qRT-PCR) assays in LC cells transfected with siNC or siTMPO-AS1. After siNC, siTMPO-AS1, siTMPO-AS1 plus inhibitor, and siTMPO-AS1 plus inhibitor control were singly transfected in LC cells, (C, D) cell viability was tested through MTT, (E, F, G) apoptosis capability was measured through flow cytometry, (H, I, J) and apoptosis-related proteins were detected by western blot experiments. △△ P < 0.001 vs. siNC, * P < 0.05 and ** P < 0.001 vs. siTMPO-AS1, # P < 0.05 and ## P < 0.001 vs. siTMPO-AS1+Inhibitor control, n = 3.
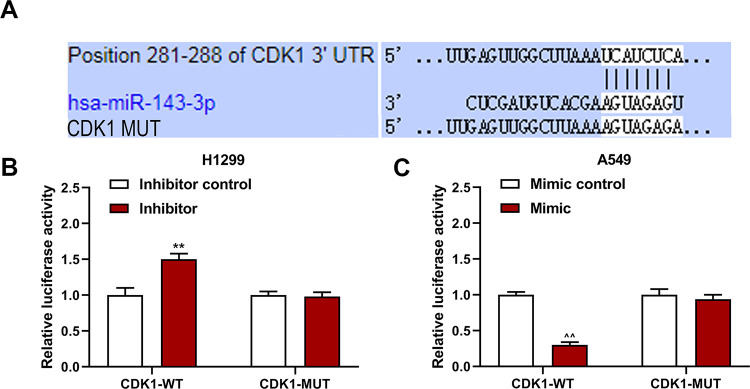
Carcinogenic Gene CDK1 in LC Cells Was Regulated by miR-143-3p and TMPO-AS1
Coactive base sequences of hsa-miR-143-3p and position 281-288 of CDK1 3’ UTR were predicted by TargetScan 7.2 (Figure 4A). Dual-luciferase reporter assays manifested that relative luciferase activity of LC cells was enhanced by miR-143-3p inhibitor in company with CDK1-WT, yet weakened by miR-143-3p mimic in comparison with CDK1-MUT and mimic control groups (P < 0.001; Figure 4B and C). Besides, CDK1 expression was measured by using qRT-PCR and WB assays. Consequently, CDK1 was reduced by siTMPO-AS1, but largely promoted under the coaction of siTMPO-AS1 and miR-143-3p inhibitor (P < 0.05; Figure 5A-E). Other qRT-PCR assays were performed for the detection of CDK1 expression in LC tissues and adjacent normal tissues. Not surprisingly, CDK1 expression level was much higher in LC tissues than that of normal ones (P < 0.001; Figure 5F).
Figure 4.
CDK1 was the target of miR-143-3p. (A) Position 281-288 of CDK1 3’ UTR was predicted as consequential pairing target region of hsa-miR-143-3p by TargetScan 7.2. (B, C) LC cells transfected with wild-type CDK1 (CDK1-WT) or mutant-type CDK1 (CDK1-MUT), together with inhibitor control, inhibitor, mimic control or mimic were then undergone dual-luciferase reporter assay. ** P < 0.001 vs. inhibitor control, ^^ P < 0.001 vs. mimic control, n = 3.
Figure 5.
TMPO-AS1 affected gene expressions of CDK1, but the effects were abrogate by miR-143-3p. LC cells were transfected with siNC, siTMPO-AS1, siTMPO-AS1 plus inhibitor control, or siTMPO-AS1 plus inhibitor, (A, B, C) followed by the measurement of apoptosis-related protein through western blot, (D, E) and the detection of CDK1 expression level through qantitative real-time polymerase chain reaction (qRT-PCR) assays. (F) CDK1 expression level was also tested through qRT-PCR in 50 pairs of LC and normal tissues respectively. △ P < 0.05 and △△ P < 0.001 vs. siNC, ** P < 0.001 vs. siTMPO-AS1, ## P < 0.001 vs. siTMPO-AS1+Inhibitor control, ‡‡ P < 0.001 vs. normal, n = 3.
MiR-143-3p Inhibitor Plays an Anti-Apoptotic Role by Targeting CDK1
In order to test whether miR-143-3p had regulatory effect on CDK1 in LC, qRT-PCR and WB were performed again. Overall analysis of experimental data showed that CDK1 was remarkably inhibited by siCDK1 alone (P < 0.001; Figure 6A-E). OD values of LC cells transfected with different plasmids were compared through MTT assay. In Figure 6F and G, LC cells’ activity was elevated by miR-143-3p inhibitor (P < 0.05). However, the combination of siCDK1 and miR-143-3p inhibitor led to a reversion. Further, flow cytometry contributed to apoptosis detection. Without interference, miR-143-3p inhibitor decreased apoptosis in LC cells. But synergy of siCDK1 and miR-143-3p inhibitor significantly promoted apoptosis (P < 0.05; Figure 6H-J).
Figure 6.
miR-143-3p inhibitor increased cell activity and decreased apoptosis rate in LC cells, which could be reversed by silencing CDK1. LC cells were transfected with siNC or siCDK1, (A, B, C) then apoptotic proteins were measured through western blot assays, (D, E) followed by testing CDK1 expression levels through qantitative real-time polymerase chain reaction (qRT-PCR) assays. Plasmids inhibitor control, inhibitor, inhibitor plus siCDK1, or inhibitor plus siNC were respectively transfected into LC cells, (F, G) after which cell activity was assayed through MTT, (H, I, J) while cell apoptosis was measured through flow cytometry. * P < 0.05 vs. inhibitor control, △△ P < 0.001 vs. siNC, # P < 0.05 and ## P < 0.001 vs. inhibitor, ^ P < 0.05 and ^^ P < 0.001 vs. inhibitor+siNC, n = 3.
Discussion
The study of lncRNA has been going on for a long time covering a large variety of species, and researchers endeavor to make a breakthrough in diseases treatment by probing into the loci of these transcriptome.10,11 Evidence has shown that lncRNAs play important roles in the progression of cancer and tumor,12 involving LC.24 LncRNA SNHG1 overexpression inhibits the NSCLC cells apoptosis both in vitro and in vivo by inhibiting miR-101-3p and activating Wnt/β-catenin signaling pathway, which highlights the carcinogenic effect of SNHG1.25 Besides, aberrant up-regulation of UCA1 also subserves the proliferation and colony formation in NSCLC tissues by sponging miR-193a-3p, indicating that UCA1 works as an oncogene.26 Moreover, lncRNA FEZF1-AS1 can be a tumor promoting regulator in NSCLC as well, enhancing epithelial-mesenchymal transition (EMT) through suppression of E-cadherin and regulation of WNT pathway.27 Over the study period, we proved that lncRNA TMPO-AS1 was abnormally expressed in LC tissues compared with adjacent normal ones, and LC patients with TMPO-AS1 low-expression were prone to live longer. Thus, TMPO-AS1 may function as an oncogene in the procession LC.
In Paraskevopoulou, M.D. and A.G. Hatzigeorgiou’s study, lncRNAs can work as sponges of miRNAs, reaching the purpose of interference and regulation.14 Primarily, starBase v3.0 was used to predict the potential target miRNAs for TMPO-AS1, and miR-143-3p was chosen for further investigation. Scholars have studied the functions of miR-143-3p by now, according to the literature, and verified that miR-143-3p is an essential molecular factor in cancers. In colorectal cancer tissues, miR-143-3p hindered cells proliferation, migration and invasion by targeting ITGA6 and ASAP3.15 Further, miR-143-3p acts as a suppressor gene in triple-negative breast cancer (TNBC), inhibiting the multiplication and wound healing function of MDA-MB-231 TNBC cells.16 MiR-143-3p also has the effect of anti-proliferation, anti-migration and ant-invasion in ovarian cancer tissues by suppressing the expression of transforming growth factor (TGF)-β-activated kinase 1 (TAK1).17 Currently, evidence indicated that miR-143-3p expression level is reduced in LC tissues,28 suggesting that miR-143-3p may also be an oncogene in LC.
In our study, we found that TMPO-AS1 expression level was abnormally higher in cancerous tissues than in adjacent normal tissues. Intriguingly, miR-143-3p content appeared to be totally opposite in the meantime. All the information indicated that up-regulated TMPO-AS1 expression and down-regulated miR-143-3p play important roles in the development of LC. So there was good reason to hypothesize that the regulatory effect of TMPO-AS1 in LC might be relevant to miR-143-3p. Further experimental results suggested that the suppression of TMPO-AS1 conduced to the up-regulation of miR-143-3p. But miR-143-3p inhibitor didn’t hamper the expression of TMPO-AS1 in LC cells, which indicating that TMPO-AS1 may be an upstream regulatory molecule of miR-143-3p. LncRNA were commonly reported regulating the expression of microRNAs, though the main mechanism might be not clear now. Besides, down-regulation of TMPO-AS1 was assayed to be effective on proliferation inhibition as well as apoptosis induction in LC cells, and miR-143-3p inhibitor can reverse the function of siTMPO-AS1, which further explained the regulatory relationship between TMPO-AS1 and miR-143-3p in the development of LC. MTT assay was applied to detect proliferation, and the expression of related marker CDK1 was measured. It might be a limitation not performing other related WB assay, which would be studied in future.
Different clusters of cyclin-dependent kinases (Cdks) regulate cell proliferation in different cell cycle stages, and a certain amount of CDK1 is the prerequisite for coordinating cell cycle transitions.29,30 Some experiments have demonstrated that miRNAs were hopeful to curb cancer through the inhibition of malignant proliferation. MiR-490-3p can be an effective molecule in impeding ovarian epithelial carcinoma tumorigenesis and progression by targeting CDK1, which arrests G1-S or G2-M and moderately reduces cell proliferation.31 In addition, miR-181a hampered the expression of CyclinB1 and CyclinD1 in NSCLC cells, so as to significantly suppress the capacities of cell proliferation.32 Consistent with these reports, we discovered that CDK1 expression level in LC cells was much higher than adjacent normal cells, with miR-143-3p expression strikingly low. Thus, CDK1 was used as the target for miR-143-3p, and its feasible coupling sequence was predicted by TargetScan7.2. The experimental results were encouraging. We discovered that the decrease of TMPO-AS1 resulted in the reduction of CDK1, but co-inhibition of TMPO-AS1 and miR-143-3p significantly promoted CDK1 expression, demonstrating CDK1 was a direct target of miR-143-3p. Through MTT, qRT-PCR, WB and flow cytometry assays, it was further proved that miR-143-3p hindered cell proliferation and motivated cell apoptosis in LC tissues by targeting CDK1.
Conclusion
So far we have verified that lncRNA TMPO-AS1, miR-143-3p and CDK1 play pivotal roles in LC procession, and this is the only study to our knowledge to dig into the carcinogenic factors of LC combining these 3 molecules. Down-regulation of TMPO-AS1 and up-regulation of miR-143-3p both inhibit LC cells multiplication and promote LC cells apoptosis, while the decrease of TMPO-AS1 stimulates the increase of miR-143-3p. Moreover, miR-143-3p takes effects by directly targeting CDK1. Thus, we concluded that down-regulation of TMPO-AS1 inhibits induces apoptosis in LC cells by regulating miR-143-3p/CDK1 axis. The findings of this study are hopeful to provide LC patients with novel and efficient treatments from molecular perspectives.
Footnotes
Authors’ Note: Our study was approved by Zhuji Affiliated Hospital of Shaoxing University. All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Qiu Li  https://orcid.org/0000-0003-1731-2337
https://orcid.org/0000-0003-1731-2337
References
- 1. Faure M. Lung cancer in Europe: turning the spotlight on the biggest cancer killer. Eur Respir J. 2016;47(1):42–44 doi:10.1183/13993003.01495-2015 [DOI] [PubMed] [Google Scholar]
- 2. Nanavaty P, Alvarez MS, Alberts WM. Lung cancer screening: advantages, controversies, and applications. Cancer Control. 2014;21(1):9–14 doi:10.1177/107327481402100102 [DOI] [PubMed] [Google Scholar]
- 3. Rodriguez-Canales J, Parra-Cuentas E, Wistuba II. Diagnosis and molecular classification of lung cancer. Cancer Treat Res. 2016;170:25–46 doi:10.1007/978-3-319-40389-2_2 [DOI] [PubMed] [Google Scholar]
- 4. Petersen I, Warth A. Lung cancer: developments, concepts, and specific aspects of the new WHO classification. J Cancer Res Clin Oncol. 2016;142(5):895–904 doi:10.1007/s00432-015-2004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am. 2016;25(3):447–468. doi:10.1016/j.soc.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 6. Li BQ, You J, Huang T, Cai YD. Classification of non-small cell lung cancer based on copy number alterations. PloS One. 2014;9(2):e88300 doi:10.1371/journal.pone.0088300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiu ZW, Bi JH, Gazdar AF, Song K. Genome-wide copy number variation pattern analysis and a classification signature for non-small cell lung cancer. Genes Chromosomes Cancer. 2017;56(7):559–569. doi:10.1002/gcc.22460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e278S–e313S. doi:10.1378/chest.12-2359 [DOI] [PubMed] [Google Scholar]
- 9. St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet. 2015;31(5):239–251. doi:10.1016/j.tig.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–933. doi:10.4161/rna.24604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res. 2011;39(Database issue): D146–D151. doi:10.1093/nar/gkq1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nature reviews. Cancer. 2018;18(1):5–18. doi:10.1038/nrc.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang W, Su X, Yan W, et al. Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate. 2018;78(16):1248–1261. doi:10.1002/pros.23700 [DOI] [PubMed] [Google Scholar]
- 14. Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol. 2016;1402:271–286. doi:10.1007/978-1-4939-3378-5_21 [DOI] [PubMed] [Google Scholar]
- 15. Guo L, Fu J, Sun S, et al. MicroRNA-143-3p inhibits colorectal cancer metastases by targeting ITGA6 and ASAP3. Cancer Sci. 2019;110(2):805–816. doi:10.1111/cas.13910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li D, Hu J, Song H, et al. miR-143-3p targeting LIM domain kinase 1 suppresses the progression of triple-negative breast cancer cells. Am J Transl Res. 2017;9(5):2276–2285. [PMC free article] [PubMed] [Google Scholar]
- 17. Shi H, Shen H, Xu J, Zhao S, Yao S, Jiang N. MiR-143-3p suppresses the progression of ovarian cancer. Am J Transl Res. 2018;10(3):866–874. [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou P, Chen WG, Li XW. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am J Cancer Res. 2015;5(6):2056–2063. [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia-Blanco N, Moreno S. Down-regulation of Cdk1 activity in G1 coordinates the G1/S gene expression programme with genome replication. Curr Genet. 2019;65(3):685–690. doi:10.1007/s00294-018-00926-y [DOI] [PubMed] [Google Scholar]
- 20. Mitobe Y, Ikeda K, Suzuki T, et al. ESR1-stabilizing long noncoding RNA TMPO-AS1 promotes hormone-refractory breast cancer progression. Mol Cell Biol. 2019;39(23). doi:10.1128/mcb.00261-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin Z, Zheng X, Fang Y. Long noncoding RNA TMPO-AS1 promotes progression of non-small cell lung cancer through regulating its natural antisense transcript TMPO. Biochem Biophys Res Commun. 2019;516(2):486–493. doi:10.1016/j.bbrc.2019.06.088 [DOI] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23. Qi H, Wang H, Pang D. miR-448 promotes progression of non-small-cell lung cancer via targeting SIRT1. Exp Ther Med. 2019;18(3):1907–1913. doi:10.3892/etm.2019.7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang J, Lin J, Liu T, et al. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer. 2014;85(2):110–115. doi:10.1016/j.lungcan.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 25. Cui Y, Zhang F, Zhu C, Geng L, Tian T, Liu H. Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8(11):17785–17794. doi:10.18632/oncotarget.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nie W, Ge HJ, Yang XQ, et al. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371(1):99–106. doi:10.1016/j.canlet.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 27. He R, Zhang FH, Shen N. LncRNA FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2017;95:331–338. doi:10.1016/j.biopha.2017.08.057 [DOI] [PubMed] [Google Scholar]
- 28. Li C, Yin Y, Liu X, Xi X, Xue W, Qu Y. Non-small cell lung cancer associated microRNA expression signature: integrated bioinformatics analysis, validation and clinical significance. Oncotarget. 2017;8(15):24564–24578. doi:10.18632/oncotarget.15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banyai G, Baidi F, Coudreuse D, Szilagyi Z. Cdk1 activity acts as a quantitative platform for coordinating cell cycle progression with periodic transcription. Nat Commun. 2016;7:11161. doi:10.1038/ncomms11161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao H, Ji F, Ying S. CDK1: beyond cell cycle regulation. Aging. 2017;9(12):2465–2466. doi:10.18632/aging.101348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer Lett. 2015;362(1):122–130. doi:10.1016/j.canlet.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 32. Shi Q, Zhou Z, Ye N, Chen Q, Zheng X, Fang M. MiR-181a inhibits non-small cell lung cancer cell proliferation by targeting CDK1. Cancer Biomark. 2017;20(4):539–546. doi:10.3233/cbm-170350 [DOI] [PubMed] [Google Scholar]